- BiologyDiscussion.com
- Follow Us On:
- Google Plus
- Publish Now


Essay on Nutritional Deficiency Diseases
ADVERTISEMENTS:
Some diseases are not spread from person to person or by microorganisms or directly or indirectly, these are called non-communicable diseases.
These diseases are caused by the deficiency of some vitamins or nutrients or due to malfunctioning of certain body organs.
Nutritional deficiency diseases:
When the supply of all nutrients is done in right amount and ratio according to body need, this is called balanced diet.
The lack of any of the required nutrients in the diet is called malnutrition (means faulty or inadequate diet). It leads to deficiency of specific nutrients which is the cause of some diseases called deficiency diseases.
Some nutritional deficiency diseases are as follows:
Protein deficiency:
Protein deficiency diseases affect the children from age group 1-5 years. The deficiency of proteins, fats and carbohydrates is called as protein energy malnutrition. It leads to two kinds of diseases -Kwashiorkor [Fig. 9.1 (a)] and Marasmus [Fig. 9.1 (b)]. When a child is getting a poor diet in protein, it results a disease known as Kwashiorkor, this disease retards the growth of children.
The children suffering from this problem show some symptoms such as protruding belly, mental retardation, bulging eyes, thin legs like stick and oedema means water retention. When a child suffers from protein and carbohydrate deficiency, it leads to a disease called marasmus. There is no oedema in the children suffering from marasmus, there is no change in skin colour, ribs look very prominent and limbs become very thin, this diseases occurs in infant of up to 1 year of age.
Vitamin deficiency :
Vitamins are very essential for the body although vitamins are not needed by the body in large quantity but required for proper growth and development of body. There are two types of vitamins, viz., fat soluble vitamins such as vitamins, A, D, E and K and water soluble vitamins such as B complex group and vitamin C.
Deficiency of vitamins causes diseases which are as follows:

Mineral deficiency :
The metals, non-metals and their salts are called minerals, because they are mined from the soil, ground or earth’s crust. Minerals are needed in smaller quantity for the growth and development of body, minerals do not supply any energy to the body. Our body can use minerals in the compound form and not as pure elements. Humans get most of the minerals from plant sources.
The following table shows the uses of some minerals in our body:
Related Articles:
- Essay on Plant Diseases: Classification of Plant Diseases
- Nutritional Problems due to Deficiency Disorders (With Diagram)
- Anybody can ask a question
- Anybody can answer
- The best answers are voted up and rise to the top
Forum Categories
- Animal Kingdom
- Biodiversity
- Biological Classification
- Biology An Introduction 11
- Biology An Introduction
- Biology in Human Welfare 175
- Biomolecules
- Biotechnology 43
- Body Fluids and Circulation
- Breathing and Exchange of Gases
- Cell- Structure and Function
- Chemical Coordination
- Digestion and Absorption
- Diversity in the Living World 125
- Environmental Issues
- Excretory System
- Flowering Plants
- Food Production
- Genetics and Evolution 110
- Human Health and Diseases
- Human Physiology 242
- Human Reproduction
- Immune System
- Living World
- Locomotion and Movement
- Microbes in Human Welfare
- Mineral Nutrition
- Molecualr Basis of Inheritance
- Neural Coordination
- Organisms and Population
- Photosynthesis
- Plant Growth and Development
- Plant Kingdom
- Plant Physiology 261
- Principles and Processes
- Principles of Inheritance and Variation
- Reproduction 245
- Reproduction in Animals
- Reproduction in Flowering Plants
- Reproduction in Organisms
- Reproductive Health
- Respiration
- Structural Organisation in Animals
- Transport in Plants
- Trending 14
Privacy Overview
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |

- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- Games & Quizzes
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center
- Introduction
Protein-energy malnutrition
- Carbohydrates
- Essential fatty acids
- Vitamin B 6
- Vitamin B 12
- Pantothenic acid
- Water deficiency (dehydration)
- Blood lipoproteins
- Dietary fat
- Dietary cholesterol
- Other dietary factors
- Other factors
- Dietary recommendations
- Hypertension
- Colorectal cancer
- Prostate cancer
- Breast cancer
- Diabetes mellitus and metabolic disorders
- Obesity and weight control
- Eating disorders
- Tooth decay
- Heartburn and peptic ulcer
- Bowel conditions and diseases
- Food-drug interactions
- Food allergies and intolerances
- Toxins in foods
- Foodborne illnesses
- Botanicals and functional foods

nutritional disease
Our editors will review what you’ve submitted and determine whether to revise the article.
- Kansas Department of Health and Environment - Nutrient Deficiency or Disease
- National Center for Biotechnology Information - PubMed Central - Nutrition and the science of disease prevention: a systems approach to support metabolic health
- Table Of Contents

nutritional disease , any of the nutrient-related diseases and conditions that cause illness in humans . They may include deficiencies or excesses in the diet, obesity and eating disorders , and chronic diseases such as cardiovascular disease , hypertension , cancer , and diabetes mellitus . Nutritional diseases also include developmental abnormalities that can be prevented by diet, hereditary metabolic disorders that respond to dietary treatment , the interaction of foods and nutrients with drugs , food allergies and intolerances, and potential hazards in the food supply. All of these categories are described in this article. For a discussion of essential nutrients , dietary recommendations, and human nutritional needs and concerns throughout the life cycle, see nutrition, human .
Nutrient deficiencies
Although the so-called diseases of civilization—for example, heart disease , stroke , cancer, and diabetes—will be the focus of this article, the most significant nutrition-related disease is chronic undernutrition, which plagues more than 925 million people worldwide . Undernutrition is a condition in which there is insufficient food to meet energy needs; its main characteristics include weight loss, failure to thrive, and wasting of body fat and muscle . Low birth weight in infants , inadequate growth and development in children , diminished mental function, and increased susceptibility to disease are among the many consequences of chronic persistent hunger , which affects those living in poverty in both industrialized and developing countries. The largest number of chronically hungry people live in Asia , but the severity of hunger is greatest in sub-Saharan Africa . At the start of the 21st century, approximately 20,000 people, the majority of them children, died each day from undernutrition and related diseases that could have been prevented. The deaths of many of these children stem from the poor nutritional status of their mothers as well as the lack of opportunity imposed by poverty .
Only a small percentage of hunger deaths is caused by starvation due to catastrophic food shortages. During the 1990s, for example, worldwide famine (epidemic failure of the food supply) more often resulted from complex social and political issues and the ravages of war than from natural disasters such as droughts and floods.

Malnutrition is the impaired function that results from a prolonged deficiency—or excess—of total energy or specific nutrients such as protein , essential fatty acids , vitamins , or minerals. This condition can result from fasting and anorexia nervosa ; persistent vomiting (as in bulimia nervosa ) or inability to swallow ; impaired digestion and intestinal malabsorption; or chronic illnesses that result in loss of appetite (e.g., cancer, AIDS ). Malnutrition can also result from limited food availability, unwise food choices, or overzealous use of dietary supplements .

Selected nutrient-deficiency diseases are listed in the table.
| disease (and key nutrient involved) | symptoms | foods rich in key nutrient |
|---|---|---|
| Source: Gordon M. Wardlaw, Perspectives in Nutrition (1999). | ||
| xerophthalmia (vitamin A) | blindness from chronic eye infections, poor growth, dryness and keratinization of epithelial tissues | liver, fortified milk, sweet potatoes, spinach, greens, carrots, cantaloupe, apricots |
| (vitamin D) | weakened bones, bowed legs, other bone deformities | fortified milk, fish oils, sun exposure |
| (thiamin) | nerve degeneration, altered muscle coordination, cardiovascular problems | pork, whole and enriched grains, dried beans, sunflower seeds |
| (niacin) | diarrhea, skin inflammation, dementia | mushrooms, bran, tuna, chicken, beef, peanuts, whole and enriched grains |
| (vitamin C) | delayed wound healing, internal bleeding, abnormal formation of bones and teeth | citrus fruits, strawberries, broccoli |
| (iron) | decreased work output, reduced growth, increased health risk in pregnancy | meat, spinach, seafood, broccoli, peas, bran, whole-grain and enriched breads |
| (iodine) | enlarged thyroid gland, poor growth in infancy and childhood, possible mental retardation, cretinism | iodized salt, saltwater fish |
Chronic undernutrition manifests primarily as protein -energy malnutrition (PEM), which is the most common form of malnutrition worldwide. Also known as protein-calorie malnutrition, PEM is a continuum in which people—all too often children—consume too little protein, energy, or both. At one end of the continuum is kwashiorkor , characterized by a severe protein deficiency, and at the other is marasmus, an absolute food deprivation with grossly inadequate amounts of both energy and protein.

An infant with marasmus is extremely underweight and has lost most or all subcutaneous fat. The body has a “skin and bones” appearance, and the child is profoundly weak and highly susceptible to infections. The cause is a diet very low in calories from all sources (including protein), often from early weaning to a bottled formula prepared with unsafe water and diluted because of poverty . Poor hygiene and continued depletion lead to a vicious cycle of gastroenteritis and deterioration of the lining of the gastrointestinal tract , which interferes with absorption of nutrients from the little food available and further reduces resistance to infection. If untreated, marasmus may result in death due to starvation or heart failure .
Kwashiorkor , a Ghanaian word meaning the disease that the first child gets when the new child comes, is typically seen when a child is weaned from high-protein breast milk onto a carbohydrate food source with insufficient protein. Children with this disease, which is characterized by a swollen belly due to edema (fluid retention), are weak, grow poorly, and are more susceptible to infectious diseases , which may result in fatal diarrhea . Other symptoms of kwashiorkor include apathy , hair discoloration, and dry, peeling skin with sores that fail to heal. Weight loss may be disguised because of the presence of edema, enlarged fatty liver , and intestinal parasites ; moreover, there may be little wasting of muscle and body fat.
Kwashiorkor and marasmus can also occur in hospitalized patients receiving intravenous glucose for an extended time, as when recovering from surgery , or in those with illnesses causing loss of appetite or malabsorption of nutrients. Persons with eating disorders, cancer, AIDS , and other illnesses where appetite fails or absorption of nutrients is hampered may lose muscle and organ tissue as well as fat stores.
Treatment of PEM has three components. (1) Life-threatening conditions—such as fluid and electrolyte imbalances and infections—must be resolved. (2) Nutritional status should be restored as quickly and safely as possible; rapid weight gain can occur in a starving child within one or two weeks. (3) The focus of treatment then shifts to ensuring nutritional rehabilitation for the long term. The speed and ultimate success of recovery depend upon the severity of malnutrition, the timeliness of treatment, and the adequacy of ongoing support. Particularly during the first year of life, starvation may result in reduced brain growth and intellectual functioning that cannot be fully restored.
Fact sheets
- Facts in pictures
- Publications
- Questions and answers
- Tools and toolkits
- Endometriosis
- Excessive heat
- Mental disorders
- Polycystic ovary syndrome
- All countries
- Eastern Mediterranean
- South-East Asia
- Western Pacific
- Data by country
- Country presence
- Country strengthening
- Country cooperation strategies
- News releases
Feature stories
- Press conferences
- Commentaries
- Photo library
- Afghanistan
- Cholera
- Coronavirus disease (COVID-19)
- Greater Horn of Africa
- Israel and occupied Palestinian territory
- Disease Outbreak News
- Situation reports
- Weekly Epidemiological Record
- Surveillance
- Health emergency appeal
- International Health Regulations
- Independent Oversight and Advisory Committee
- Classifications
- Data collections
- Global Health Observatory
- Global Health Estimates
- Mortality Database
- Sustainable Development Goals
- Health Inequality Monitor
- Global Progress
- World Health Statistics
- Partnerships
- Committees and advisory groups
- Collaborating centres
- Technical teams
- Organizational structure
- Initiatives
- General Programme of Work
- WHO Academy
- Investment in WHO
- WHO Foundation
- External audit
- Financial statements
- Internal audit and investigations
- Programme Budget
- Results reports
- Governing bodies
- World Health Assembly
- Executive Board
- Member States Portal
- Fact sheets /
Malnutrition
- Malnutrition, in all its forms, includes undernutrition (wasting, stunting, underweight), inadequate vitamins or minerals, overweight, obesity, and resulting diet-related noncommunicable diseases.
- In 2022, 2.5 billion adults were overweight, including 890 million who were living with obesity, while 390 million were underweight.
- Globally in 2022, 149 million children under 5 were estimated to be stunted (too short for age), 45 million were estimated to be wasted (too thin for height), and 37 million were overweight or living with obesity.
- Nearly half of deaths among children under 5 years of age are linked to undernutrition. These mostly occur in low- and middle-income countries. The developmental, economic, social and medical impacts of the global burden of malnutrition are serious and lasting, for individuals and their families, for communities and for countries.
- The developmental, economic, social and medical impacts of the global burden of malnutrition are serious and lasting, for individuals and their families, for communities and for countries.
Malnutrition refers to deficiencies, excesses, or imbalances in a person’s intake of energy and/or nutrients. The term malnutrition addresses 3 broad groups of conditions:
- undernutrition, which includes wasting (low weight-for-height), stunting (low height-for-age) and underweight (low weight-for-age);
- micronutrient-related malnutrition, which includes micronutrient deficiencies (a lack of important vitamins and minerals) or micronutrient excess; and
- overweight, obesity and diet-related noncommunicable diseases (such as heart disease, stroke, diabetes and some cancers).
Various forms of malnutrition
Undernutrition.
There are 4 broad sub-forms of undernutrition: wasting, stunting, underweight, and deficiencies in vitamins and minerals. Undernutrition makes children in particular much more vulnerable to disease and death.
Low weight-for-height is known as wasting. It usually indicates recent and severe weight loss because a person has not had enough food to eat and/or they have had an infectious disease, such as diarrhoea, which has caused them to lose weight. A young child who is moderately or severely wasted has an increased risk of death, but treatment is possible.
Low height-for-age is known as stunting. It is the result of chronic or recurrent undernutrition, usually associated with poor socioeconomic conditions, poor maternal health and nutrition, frequent illness, and/or inappropriate infant and young child feeding and care in early life. Stunting holds children back from reaching their physical and cognitive potential.
Children with low weight-for-age are known as underweight. A child who is underweight may be stunted, wasted or both.
Micronutrient-related malnutrition
Inadequacies in intake of vitamins and minerals often referred to as micronutrients, can also be grouped together. Micronutrients enable the body to produce enzymes, hormones and other substances that are essential for proper growth and development.
Iodine, vitamin A, and iron are the most important in global public health terms; their deficiency represents a major threat to the health and development of populations worldwide, particularly children and pregnant women in low-income countries.
Overweight and obesity
Overweight and obesity is when a person is too heavy for his or her height. Abnormal or excessive fat accumulation can impair health.
Body mass index (BMI) is an index of weight-for-height commonly used to classify overweight and obesity. It is defined as a person’s weight in kilograms divided by the square of his/her height in meters (kg/m²). In adults, overweight is defined as a BMI of 25 or more, whereas obesity is a BMI of 30 or more. Among children and adolescents, BMI thresholds for overweight and obesity vary by age.
Overweight and obesity result from an imbalance between energy consumed (too much) and energy expended (too little). Globally, people are consuming foods and drinks that are more energy-dense (high in sugars and fats) and engaging in less physical activity.
Diet-related noncommunicable diseases
Diet-related noncommunicable diseases (NCDs) include cardiovascular diseases (such as heart attacks and stroke, and often linked with high blood pressure), certain cancers, and diabetes. Unhealthy diets and poor nutrition are among the top risk factors for these diseases globally.
Scope of the problem
In 2022, approximately 390 million adults aged 18 years and older worldwide were underweight, while 2.5 billion were overweight, including 890 million who were living with obesity. Among children and adolescents aged 5-19 years, 390 million were overweight, including 160 million who were living with obesity. Another 190 million were living with thinness (BMI-for-age more than two standard deviations below the reference median).
In 2022, an estimated 149 million children under the age of 5 years were suffering from stunting, while 37 million were living with overweight or obesity.
Nearly half of deaths among children under 5 years of age are linked to undernutrition. These mostly occur in low- and middle-income countries.
Who is at risk?
Every country in the world is affected by one or more forms of malnutrition. Combating malnutrition in all its forms is one of the greatest global health challenges.
Women, infants, children, and adolescents are at particular risk of malnutrition. Optimizing nutrition early in life –including the 1000 days from conception to a child’s second birthday – ensures the best possible start in life, with long-term benefits.
Poverty amplifies the risk of, and risks from, malnutrition. People who are poor are more likely to be affected by different forms of malnutrition. Also, malnutrition increases health care costs, reduces productivity, and slows economic growth, which can perpetuate a cycle of poverty and ill-health.
The United Nations Decade of Action on Nutrition
On 1 April 2016, the United Nations (UN) General Assembly proclaimed 2016–2025 the United Nations Decade of Action on Nutrition. The Decade is an unprecedented opportunity for addressing all forms of malnutrition. It sets a concrete timeline for implementation of the commitments made at the Second International Conference on Nutrition (ICN2) to meet a set of global nutrition targets and diet-related NCD targets by 2025, as well as relevant targets in the Agenda for Sustainable Development by 2030 – in particular, Sustainable Development Goal (SDG) 2 (end hunger, achieve food security and improved nutrition and promote sustainable agriculture) and SDG 3 (ensure healthy lives and promote wellbeing for all at all ages).
Led by WHO and the Food and Agriculture Organization of the United Nations (FAO), the UN Decade of Action on Nutrition calls for policy action across 6 key areas:
- creating sustainable, resilient food systems for healthy diets;
- providing social protection and nutrition-related education for all;
- aligning health systems to nutrition needs, and providing universal coverage of essential nutrition interventions;
- ensuring that trade and investment policies improve nutrition;
- building safe and supportive environments for nutrition at all ages; and
- strengthening and promoting nutrition governance and accountability, everywhere.
WHO response
WHO aims for a world free of all forms of malnutrition, where all people achieve health and wellbeing. According to the 2016–2025 nutrition strategy, WHO works with Member States and partners towards universal access to effective nutrition interventions and to healthy diets from sustainable and resilient food systems. WHO uses its convening power to help set, align and advocate for priorities and policies that move nutrition forward globally; develops evidence-informed guidance based on robust scientific and ethical frameworks; supports the adoption of guidance and implementation of effective nutrition actions; and monitors and evaluates policy and programme implementation and nutrition outcomes.
This work is framed by the Comprehensive implementation plan on maternal, infant, and young child nutrition , adopted by Member States through a World Health Assembly resolution in 2012. Actions to end malnutrition are also vital for achieving the diet-related targets of the Global action plan for the prevention and control of noncommunicable diseases 2013–2020 , the Global strategy for women’s, children’s, and adolescent’s health 2016–2030 , and the 2030 Agenda for sustainable development .
- Breastfeeding
- The WHO Child Growth Standards
- Double burden of malnutrition
- Comprehensive implementation plan on maternal, infant and young child nutrition
- Global action plan for the prevention and control of NCDs 2013-2020
- Global nutrition targets for 2025
- Second International Conference on Nutrition (ICN2)
- UN Decade of Action on Nutrition 2016-2025
- Open access
- Published: 09 October 2021
The future of human malnutrition: rebalancing agency for better nutritional health
- Jonathan C. K. Wells ORCID: orcid.org/0000-0003-0411-8025 1 ,
- Akanksha A. Marphatia 2 ,
- Gabriel Amable 2 ,
- Mario Siervo 3 ,
- Henrik Friis 4 ,
- J. Jaime Miranda 5 , 6 ,
- Hinke H. Haisma 7 na1 &
- David Raubenheimer 8 na1
Globalization and Health volume 17 , Article number: 119 ( 2021 ) Cite this article
14k Accesses
25 Citations
23 Altmetric
Metrics details
The major threat to human societies posed by undernutrition has been recognised for millennia. Despite substantial economic development and scientific innovation, however, progress in addressing this global challenge has been inadequate. Paradoxically, the last half-century also saw the rapid emergence of obesity, first in high-income countries but now also in low- and middle-income countries. Traditionally, these problems were approached separately, but there is increasing recognition that they have common drivers and need integrated responses. The new nutrition reality comprises a global ‘double burden’ of malnutrition, where the challenges of food insecurity, nutritional deficiencies and undernutrition coexist and interact with obesity, sedentary behaviour, unhealthy diets and environments that foster unhealthy behaviour. Beyond immediate efforts to prevent and treat malnutrition, what must change in order to reduce the future burden? Here, we present a conceptual framework that focuses on the deeper structural drivers of malnutrition embedded in society, and their interaction with biological mechanisms of appetite regulation and physiological homeostasis. Building on a review of malnutrition in past societies, our framework brings to the fore the power dynamics that characterise contemporary human food systems at many levels. We focus on the concept of agency, the ability of individuals or organisations to pursue their goals. In globalized food systems, the agency of individuals is directly confronted by the agency of several other types of actor, including corporations, governments and supranational institutions. The intakes of energy and nutrients by individuals are powerfully shaped by this ‘competition of agency’, and we therefore argue that the greatest opportunities to reduce malnutrition lie in rebalancing agency across the competing actors. The effect of the COVID-19 pandemic on food systems and individuals illustrates our conceptual framework. Efforts to improve agency must both drive and respond to complementary efforts to promote and maintain equitable societies and planetary health.
Introduction
Until around 12,000 years ago, all human populations foraged for diets comprising wild foods. Nomadic foraging represented a broadly common social system, though subsistence practices varied by ecology and geography; and aside from the systematic use of tools and fire the basis of nutrition was not markedly different from that of other social primates. Since the beginning of the Holocene, however, human populations have to various extents undergone several cumulative revolutions, first in the emergence of different types of agriculture, then urbanization followed by industrialization and technological innovation, and finally globalization and the digitalization of many aspects of life. Throughout these revolutions, through which the overall human nutritional niche has been steadily reconstructed, the persistence and unequal distribution of malnutrition has remained a strong signal [ 1 ].
Scientific efforts to treat or prevent malnutrition have themselves evolved with the social priorities and dominant health challenges of the day. Early efforts targeted undernutrition, closely associated with poverty, infections and restricted diets. Today, however, the dominant manifestation comprises obesity, though undernutrition persists globally. The co-existence of these conditions, first observed at the population level, has been termed the ‘double burden of malnutrition’ (DBM) [ 2 ]. Recently, it has become apparent that many individuals also experience both nutritional extremes at different periods of the life-course, or even simultaneously as in the case of obesity and micronutrient deficiencies [ 3 ]. Ostensibly, the risk factors for undernutrition and obesity seem very different, but there are many common drivers [ 1 , 3 , 4 , 5 ].
Importantly, malnutrition in all its forms is increasingly linked with other major challenges facing our species. For example, at the population level there are common drivers of undernutrition, obesity and climate breakdown [ 5 ], hence human malnutrition is fundamentally linked with planetary dysfunction. A key issue, currently attracting substantial attention, is how we feed a projected global population of ~ 10 billion by 2050 in ways adequate for the health of both people and planet [ 6 ]. The DBM is also closely linked with many aspects of ongoing globalization and associated nutrition transition [ 3 , 4 ], which are likewise implicated in climate breakdown [ 5 ].
In the short-term, many different efforts have aimed to treat or prevent different forms of malnutrition, either through targeting malnourished individuals directly, or through preventive public health efforts that typically attempt to promote healthy diets and exercise while reducing environmental stresses such as infections. Here, we take a longer-term view, and consider what must be achieved if we are to see a substantial reduction in the global burden of malnutrition in all its forms in the future.
To develop this perspective, we articulate a conceptual framework that focuses on the deeper structural drivers of human malnutrition embedded in society. Whatever the contribution of ecological volatility, it has been recognized since Sen’s work in the 1980s that famines primarily represent the failure of societies to distribute food equitably [ 7 ]. We now need to reconsider Sen’s insight in the context of the DBM and globalized food systems. To promote healthy people, we need healthy societies, recognizing the primary role played by food systems in the construction and the functioning of all human communities [ 1 ]. This turns attention on the way that socio-economic systems and food systems are mutually embedded, with profound consequences for all aspects of food production, distribution and consumption. Although broader facets of the food system are widely understood to impact nutritional status and behavior at the individual level [ 1 , 2 , 4 , 5 ], research on the underlying physiological and behavioral mechanisms would benefit from better integration with our understanding of societal dynamics.
Our review therefore has five main aims. First, we set out a broader conceptual model of nutrition, that can provide a robust framework with which to imagine a better future. Second, we use this framework to critically examine how we got to where we are today, by looking at the long-term history of malnutrition. Third, we summarize the current manifestation of malnutrition and its associations with fundamental societal drivers. Fourth, we highlight the complex role of agency in malnutrition, focusing on how our biological drives are impacted by a ‘competition of agency’ between multiple actors. Using this approach, we highlight nutrition as a key pathway through which structural factors ‘get under the skin’ and damage health. We illustrate this framework by focusing on the COVID-19 pandemic. Finally, based on these insights, we review future opportunities to prevent and treat malnutrition.
A broad definition of nutrition
To underpin this discussion, our approach requires a broad definition of nutrition (Fig. 1 ).
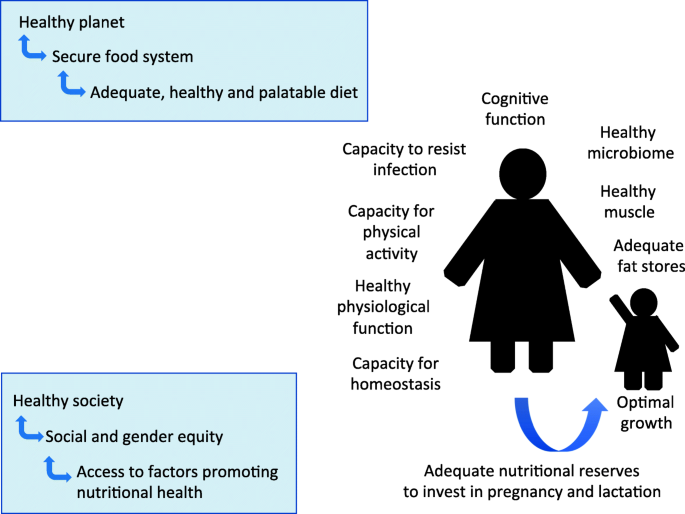
A broad conceptual model of the remit of human nutrition. In addition to dietary intake, nutritional health relates to functional capacities of the body, and a level of physical activity that maintains healthy metabolism. Healthy nutrition in one generation is essential for a healthy pattern of development in the next generation. Nutritional health at the individual level then depends on interacting with healthy societies that are compatible with planetary health
We need to go beyond the conventional remit of ‘what we eat’, to consider more broadly aspects of inequality in ‘how we are nourished’. This perspective allows us to consider what is needed from a society that would enable individuals to be free from all forms of malnutrition.
According to the Sustainable Development Goals, healthy societies may be considered to achieve each of ‘ecological health’, ‘wellbeing’, ‘social equity’, and ‘economic prosperity’ [ 5 ]. Nutrition is understood to be integral to each of these outcomes [ 1 , 5 , 8 , 9 , 10 , 11 , 12 , 13 ], but in this context means and ends are often confused [ 14 ], and the current role of nutrition in promoting economic prosperity works directly against its role in the other three dimensions.
At the level of the individual, we propose that nutritional health involves not only adequate quantity and quality of food intake, but also healthy physical activity levels, optimal growth from conception to adolescence, healthy body composition, the ability to maintain homeostasis and resist infections, and the capacity for women to adequately nourish the next generation during pregnancy and lactation, and thereafter.
Considering only this individual level, it is immediately clear that large numbers are unable to meet such a definition of health. In 2018, among children under 5 years of age, 150 million globally were stunted (low height for age), 50 million wasted (low weight for height), and 38 million had obesity, while over 2.1 billion adults had overweight or obesity [ 15 ]. From an evolutionary perspective, the human nutritional niche is impacting our survival, health and longevity, while also driving major inequalities in these outcomes.
Beyond the individual level, it is increasingly understood that malnutrition is embedded in unhealthy economies and societies, as well as planetary dysfunction [ 1 , 5 ]. To address this burden, we need to reframe the problem within an integrated scientific understanding of the full range of causal factors, and identify the subset that is most amenable to managing for change. We argue that the issue of ‘agency’ transcends all of these causes and opportunities.
Whatever form society takes, nutrition depends fundamentally on ‘agency’. At the level of the individual, we define agency as the capability of individuals to pursue their goals [ 16 , 17 ]. It is important to note that the range of ‘goals’ goes far beyond the simple relationship between dietary intake and personal health. Individuals maximize a wide range of goals related to food, including enjoyment, convenience, expression of identity, socializing and financial management. Moreover, choices related to food are often made in the context of ensuring the nutrition of others, such as younger and elderly age groups, or those with various forms of vulnerability and disease.
In the specific context of nutritional health, the expression of agency translates into the capability to obtain adequate quantities of a nutritious diet, while also having the physiological capacity and cognitive skillset to defend against societal and ecological causes of malnutrition. At the population level, collective agency should enable societies to create food environments that are sustainable for human and planetary health, and that protect against malnutrition [ 17 ]. As we show later in this article, however, many aspects of human food systems act directly and intentionally to distort or reduce agency at the level of individuals and populations, and are embedded in many forms of inequalities [ 1 ]. The collective agency of various organizations, including corporations, governments, and supranational institutions must therefore also be taken into account.
Using this broad model, we can revisit what is needed of human societies to reduce all forms of malnutrition. In order to improve understanding of where such efforts should be targeted for greatest efficacy, we first need to improve understanding of why malnutrition has persisted in different forms across time, geography and society. We show in the next section that the history of malnutrition is also fundamentally the history of constraints on individual agency.
The history of malnutrition
In non-hierarchical societies, nutrition is determined largely through individual agency, expressed as the interaction of appetite, social factors and environmental food availability. In the distant past, for example, Paleolithic foragers living in small social groups were able to achieve relatively large body size and nutritional health through consuming diverse diets of vegetables, tubers, fruits and meat, providing high intakes of protein, fiber and micronutrients [ 18 , 19 ]. Evidence that Paleolithic populations engaged in feasting reminds us that nutrition has long had a critical social dimension [ 20 , 21 ].
However, all human communities can potentially express common phenotypes of thinness and overweight, as indeed can many non-human primate species [ 22 ]. This indicates that ecological stresses were sufficiently common during primate evolution to have favored mechanisms of metabolic and behavioral plasticity. In any era, malnutrition emerges when adverse environments or food systems constrain these plastic mechanisms.
Beyond natural ecological volatility and the associated risk of food shortages, a new burden of human undernutrition emerged with the origins of agriculture. Over the past 12,000 years or so, the domestication of numerous species of plants and animals occurred independently and in different ways in different parts of the world, though a small proportion of humanity continues to practice hunting and gathering [ 23 ]. While the transition to agriculture may have increased the overall supply of food-energy, sedentary farmers were also inherently more susceptible than foragers to periodic undernutrition, being less mobile and dependent on a narrower range of foodstuffs, whilst also exposed to famines and higher pathogen burdens [ 23 ]. The skeletal record post-agriculture shows near-universal falls in linear bone lengths and increased markers of bone disease, indicating dietary inadequacies, repetitive physical activities related to growing and processing food, and elevated infectious burdens [ 23 , 24 , 25 , 26 ].
These stresses appear to have been most challenging when associated with the emergence of early states and hierarchical societies, which regulated access to the land and demanded from individual farmers a proportion of their harvest [ 27 ]. While foraging societies tend to constrain social differentiation, by pooling risks within and across social groups [ 28 ], farming allows new relations of inequality to emerge. At the level of individual households, early farmers were at risk of harvest failure, and of being unable to meet their obligations. The resulting debts often led to the loss of their land rights and agricultural capital. Over time, this led to the divergence of classes of landowners and disempowered tenant farmers, or peasants [ 29 ]. From a broader perspective, the emergence of differentiation in subsistence strategy is not unique to humans: many species display complementary strategies of ‘producing’ food, or ‘scrounging’ it from other producers [ 30 , 31 ]. Even if a strategy of ‘all producing’ generates the most equitable division and largest supply of food within a population, scrounging is predicted to emerge as soon as any individual producer can increase their returns by switching strategy [ 32 ]. For humans, this scenario generates a paradox that when farm productivity rises, egalitarian food production may inherently represent an unstable scenario. Consistent with that hypothesis, different forms of farming gave rise to many forms of social inequality [ 1 ].
In particular, early states sought to control large numbers of peasants, and across different global regions achieved this by converging on forms of grain agriculture [ 27 ]. Given intensive labor inputs, grains produce high yields and the harvests are easy to store and transport. This made them ideal for state taxation, but at the same time exposed their producers to high physical workloads in combination with diets low in protein and micronutrients, and hence increased the risk of chronic undernutrition [ 27 ]. Moreover, by controlling access to the land, elites and states proactively used the threat of hunger to coerce peasants to produce food for both landowners and peasants. To augment both the territory and the workers under their control, states also regularly invaded their neighbors [ 27 ], and deliberately used starvation in the form of sieges as a routine military strategy [ 1 ]. In these early forms of stratified societies, therefore, farming structurally connected the production of food with the control of large numbers of people through hierarchical relations.
In such societies, the primary defense against malnutrition comprised different ways of preserving or enhancing individual or collective agency. When the level of inequality and hierarchy became intolerable, or during periods of political instability, many farmers fled back to more marginal habitats and grew crops less amenable to taxation [ 27 ]. In ancient Greece, however, a different resolution emerged: competition between landowners and tenant farmers spurred the emergence of early democratic institutions, freeing the farmers from their obligations to provide food for the landlords, and recasting them as politically active citizens with new rights and social duties [ 33 ]. We highlight this as a way in which early state societies could reorganize themselves along more equitable lines, though it is important to note that these benefits did not reach all individuals, and that Greek society continued to use slave labor.
In ancient Rome, however, democratic institutions did not develop in this way, and the majority of citizens remained susceptible to economic uncertainty, hunger and debt. Roman agriculture remained fundamentally based on slave labor, and the expansion of the empire was explicitly driven by the aim of increasing the number of slaves. Instead of empowering its urban citizens, the Roman state simply provided food handouts during subsistence crises [ 25 ]. Roman law, with its emphasis on private property, has subsequently been influential in shaping global institutions, and has played a key role in underpinning restrictions on individual agency as market economies developed [ 33 ].
Even in the ancient world, food systems of different global regions were highly connected. Trade in luxuries such as spices was closely associated with trade in other commodities, including slaves [ 34 ]. From the medieval era onwards, food systems in different global regions began to become further inter-connected, and underwent a series of changes that cumulatively exacerbated both societal and geographical inequalities. A mercantile system, involving the import of tropical spices into Europe and the export of slaves from Africa to New World plantations, evolved into a system where Europe received large quantities of agricultural commodities produced in different global regions by European settlers, indentured laborers or farmers from colonized countries [ 35 , 36 ]. At every stage, the production of food continued to involve major constraints on the agency of those producing it.
Similarly, despite increasing food availability in wealthier countries as they began to industrialize, the threat of hunger continued to be used to coerce the new classes of industrial worker [ 37 ]. Access to the land was steadily reduced for rural populations, propelling them to rapidly-growing cities where they provided paid labor in the new factories. The provision of low wages by the new industrialists coerced these laborers to work long hours in order to earn enough to cover basic food requirements, and chronic undernutrition was widespread. By the late nineteenth century, it was increasingly recognized that this burden of undernutrition was itself undermining industrial productivity, and new public health efforts were introduced to improve working conditions and diets [ 38 , 39 ]. These efforts were consolidated in the aftermath of World War II, when it was clear that the entire global food system needed reconfiguration [ 40 ].
Despite these efforts, geographical inequalities persisted and took on new forms in the post-war era. The new international order initiated at the 1944 Bretton Woods conference aimed to stabilize the global economy, while ensuring that high-income countries (HICs) had access to the raw materials, markets and consumers that drive their national economies. To operationalize this system, new international financial institutions (IFIs) were created, such as the World Bank, International Monetary Fund (IMF) and World Trade Organization (WTO). These IFIs reduced the ability of governments of the formerly colonized nations to organize food production and consumption in the interests of their newly independent populations, while also empowering new transnational corporations (TNCs) [ 41 ]. Throughout these transformations, the agency of groups and organizations representing individual food producers and consumers was persistently subordinated to the interests of larger-scale corporate organizations. Renewed concern over global undernutrition in the 1970s stimulated the Green Revolution, applying new technologies to selected crops. This effort increased farm yields, but maintained structural inequalities [ 42 , 43 ].
Undernutrition remained the primary human nutritional stress for millennia, but there is also ancient evidence of corpulence. The earliest evidence relates to Venus figurines from the European Paleolithic [ 44 ], that provide sufficiently accurate depictions of the human body to indicate direct experience of female overweight in this era. These figurines are widely interpreted as expressing positive attitudes to large body size in women, though the specific reasons remain unclear, and there is no evidence of how this may have related to ill-health. However, by the early historical era, medical authorities in ancient Greece and Rome clearly recognized obesity as an undesirable condition that was detrimental to health, and developed treatments [ 45 ]. Overweight is generally considered to have remained relatively rare until recent centuries, and to have been restricted to elites, though relevant evidence remains scarce. Long-term systematic increases in average body mass index (BMI), and in the prevalence of overweight, are evident from the nineteenth century in HICs [ 46 ], and have accelerated in every global region during the last half-century [ 47 ]. The obesity epidemic initially affected wealthier groups but to varying degrees across countries is now increasing faster among poorer groups [ 48 , 49 ], reflecting a global shift towards unhealthy diets and sedentary behavior.
This brief history of malnutrition helps contextualize its current global manifestation. Given our unique agricultural niche, human nutrition is inherently sensitive to ecological shocks, but ever since the emergence of state societies the most powerful driver of malnutrition has been societal dynamics. At both local and global levels, the evolution of human food systems has always been fundamentally intertwined with the evolution of hierarchical politico-economic systems. These systems evolved to control populations as well as to feed them [ 1 ], and the primary change over time has been in how particular food systems achieve this control. Regarding both food production and consumption, the systematic suppression of individual agency underlies the persistence of malnutrition-inducing/enhancing environments. This relationship remains evident if we consider malnutrition in contemporary populations.
Contemporary manifestation of malnutrition
Contemporary malnutrition incorporates both deficiencies and excesses in diverse aspects of nutritional status including dietary intake, nutrient status, tissue masses, and physical activity [ 50 , 51 , 52 ]. Crucially, both extremes of malnutrition impact adversely across many different levels of biology (Fig. 2 ). Undernutrition remains a major risk for child mortality [ 37 ] and reduces human capital [ 43 ], while the DBM is the primary biological driver of the emerging global epidemic of non-communicable diseases (NCDs) [ 3 ]. The health penalties are exacerbated when the DBM manifests within individual life-courses, as the toxic effects of obesity on NCD risk are enhanced among those who also experienced undernutrition in early life [ 3 ]. Globally, the number of premature deaths per year attributable to dietary risk factors is estimated to be 11 million, and the number of ‘disability-adjusted life-years lost’ to be 255 million [ 53 ]. The prevalence of undernutrition is decreasing slowly, though large numbers of children remain affected, while that of overweight and obesity is rising among children and adults in every geographical region [ 15 ].
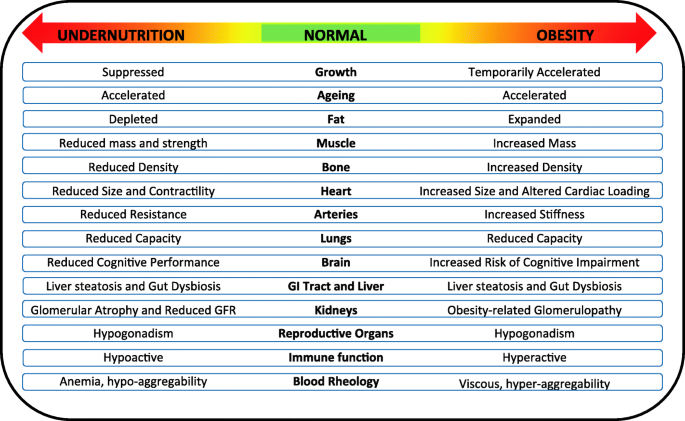
Undernutrition and obesity impact adversely at many biological levels. Both forms of malnutrition affect the morphology and functioning of many individual organs and tissues, as well as growth, ageing rate, and the composition and functioning of the microbiota
In settings where child undernutrition is common, a key proximate cause relates to monotonous diets based on starch-rich staples, that provide limited intakes of energy, micronutrients and protein. However, the broader environment is also important. Nutrient deficiencies and exposure to pathogens and toxins may in combination impair the absorptive capacity of the gut and cause intestinal and systemic inflammation [ 54 ]. Traditionally, conceptualization of the resulting child undernutrition differentiated ‘chronic’ versus ‘acute’ conditions. The latter, indicated by low tissue mass (wasting), implies a need for immediate nutritional rehabilitation, whereas linear growth retardation, eventually manifesting as ‘stunting’, was considered a marker of chronic undernutrition that would not respond to nutritional treatment. However, it is increasingly understood that the two forms are closely related [ 55 ], with each of wasting and stunting increasing the risk of the other developing over time [ 56 ]. Moreover, a recent study across 84 low- and middle-income countries (LMICs) found that 3% of young children are simultaneously wasted and stunted, resulting in particularly high mortality risk [ 57 ]. Precisely because it reflects exposures more distal than immediate food intake, the epidemiology and ontogenetic development of stunting provide unique insight into the broader causes of undernutrition.
Stunting emerges from composite ‘cycles of disadvantage’, bringing together several ecological and societal stresses that are embedded in social inequity and that propagate across generations [ 1 ]. These stresses impact nutrition and growth during the first ‘thousand days’ of life, and thereby shape adult size, body composition and health profile, as well as biological traits in the next generation [ 58 , 59 , 60 ].
In susceptible populations, growth faltering is typically already evident at birth, indicating undernutrition in utero [ 61 ]. From an evolutionary perspective, early growth faltering reflects both inadequate maternal nutrition, but also the diversion of nutritional resources away from growth to other biological functions. In post-natal life, for example, linear growth may be traded off first against immune function [ 62 ] and subsequently against earlier reproduction [ 63 ]. Those under-nourished in early life are prone to develop central adiposity if they subsequently gain excess weight [ 64 ], which may reflect the role of visceral fat in promoting immune function [ 65 , 66 ]. In a prospective Brazilian birth cohort, for example, a composite marker of low ‘maternal capital’ (incorporating education, height, BMI and family income) was associated with poor linear growth, higher BMI, more central fat distribution and early childbearing in the daughter [ 67 ]. These associations remind us that growth variability emerges as part of more comprehensive biological responses to prevailing ecological conditions.
At a global level, the geographical distribution of stunting closely replicates that of many specific markers of disadvantage (Fig. 3 ). Importantly, most of these markers reflect the dynamics and norms of human societies, all indicating reduced individual agency. However, LMIC populations with high levels of these challenges are now also increasingly exposed to the impacts of globalization and nutrition transition. This means that populations with high levels of undernutrition are now also experiencing an increased availability of cheap highly processed foods, alongside other unhealthy commodities and drivers of sedentary behavior [ 74 ].
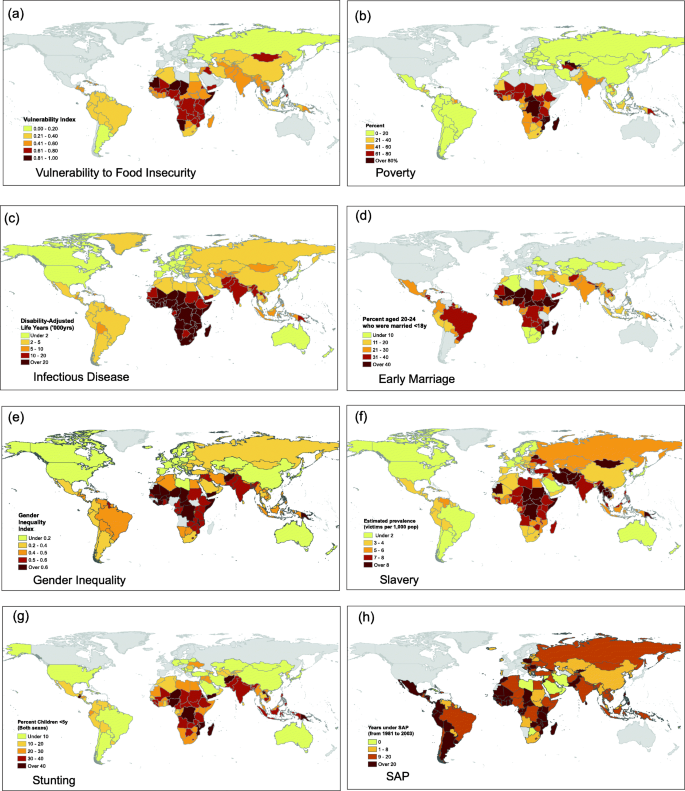
Multiple components of adversity are geographically clustered across low and middle-income countries. Persistent socio-ecological stresses include ( a ) food insecurity and vulnerability to climate change; ( b ) poverty measured as the proportion of the population living on <USD 3.1 per day; ( c ) infectious disease burden assessed as the disability-adjusted life years per 100,00 population attributable to communicable, maternal, neonatal, and nutritional diseases; ( d ) prevalence of marriage < 18 years among women aged 20–24 years; ( e ) women’s disadvantaged status in society, measured by the Gender Inequality Index; and ( f ) coerced labour, assessed as the estimated prevalence of slavery per 1000 population; Maps ( a ) to ( f ) show similarity to ( h ) the prevalence of stunting, a composite marker of undernutrition, categorised as height z-score < − 2. ( g ) The same countries have experienced exposure to economic liberalisation, assessed as the number of years subject to structural adjustment programs between 1981 and 2004. Data from ‘Our World in Data’ or [ 68 , 69 , 70 , 71 , 72 , 73 ]
Through nutrition transition, diets tend to increase in energy, refined carbohydrate and fat content, while lacking adequate protein, fiber or micronutrients [ 75 , 76 ]. These shifts may simultaneously drive excess energy consumption while maintaining nutrient deficiencies. There is growing evidence, for example, linking diets high in industrially-processed foods both with poor infant and child growth [ 77 , 78 , 79 , 80 ], and with obesity from childhood onwards [ 81 , 82 , 83 ]. Crucially, the global nutrition transition is rapidly outpacing public health success in resolving undernutrition, so that obesity is increasing faster than stunting is decreasing [ 15 ].
Exposure to heavily processed industrial foods is closely associated with international trade patterns and the activities of TNCs, which benefit from trade liberalization [ 84 , 85 ]. For many LMICs, trade liberalization was a key condition of receiving support from IFIs during economic crises [ 86 ]. Figure 3 highlights that the global regions prone to cycles of disadvantage are also those with long-term exposure to such conditionalities. This clustering of environmental, social and economic factors contributes to the speed of nutrition transition in many countries, as we discuss in more detail below.
Moreover, recent analyses show that the DBM is emerging at lower levels of economic development, both across and within countries, as processed foods become more widely available and cheaper [ 2 ]. This is causing rapid shifts in the population groups most affected by obesity, whereby it first emerges in wealthier group but then becomes most prominent in poorer groups [ 49 , 87 ]. Secular trends currently manifesting in LMICs are less in height, and more in BMI and, in females, earlier menarche [ 3 , 47 , 88 ]. Initially, obesity rates rose fastest in urban LMIC populations, but recently this shifted to rural populations [ 89 ], reflecting the growing penetration of nutrition transition into rural areas [ 10 ].
To understand why undernutrition and obesity increasingly co-exist not only within communities and households but within individuals through the life-course, and why obesity is increasing in prevalence faster than undernutrition is decreasing, we next develop our framework to demonstrate how the contemporary nutrition transition is related to the agency of both individuals and various types of organization.
Human nutrition is embedded in complex power dynamics operating at many levels of society, involving a ‘competition of agency’ between multiple actors [ 1 , 5 , 90 ]. To fully understand how these power dynamics drive the DBM, we need to consider how this competition of agency interacts with the physiological drives that underpin appetite and eating patterns. In setting out this conceptual model, we want to emphasize that a degree of agency pertains to each type of actor, and that no actor is entirely devoid of agency. At the same time, the notion of competition highlights the fact that the agency of any one type of actor may to varying degrees be constrained or manipulated by the agency of other types. We illustrate these issues in more detail below.
To illustrate these dynamics, we focus here primarily on the role of highly-processed industrial foods. These are not the only relevant dietary factors, but importantly, they have been linked with both extremes of malnutrition. If we conceive of the global food system as a ‘dynamic societal game’ [ 1 ] and the nutritional status of individuals as the key biological outcome, then our aim here is to understand the different actors involved, the ‘rules of the game’, and how and to what extent each type of actor can express agency. This will enable us to explore how broader structural factors ‘get under the skin’ to harm health, through the medium of different forms of malnutrition. We start with the component of agency that is embedded in our biology, our appetite systems.
Biological drives
At the level of physiology, individual agency is regulated through multiple components of homeostasis [ 91 ]. Physiological systems can be characterized as goal-directed entities organized to maintain or attain particular states in the face of external variation. Regarding nutrition, the key regulatory systems concern appetite. Across diverse species, including humans, the body satisfies its requirements for protein, fat, and carbohydrate (as well as some micronutrients) via specific appetites that detect deficiencies and surpluses and motivate feeding behavior accordingly [ 92 ].
In a balanced food environment these macronutrient-specific appetites can all achieve their target intakes. If balanced diets are unavailable, the nutrient-specific appetites come into conflict, because in such circumstances (by definition) all regulated nutrients cannot simultaneously be ingested at their respective target levels. The outcome of this conflict will be determined by the relative strength of different appetites, with the stronger appetites more closely reaching their target intakes than weaker appetites. Studies using the ‘nutritional geometry framework’ [ 93 ] have shown that in humans and some other primates, protein is regulated more strongly than carbohydrates and fats [ 94 , 95 ], and thus absolute protein intake remains relatively constant while fat and carbohydrate intake vary with the density of protein in the diet [ 92 ]. Accordingly, dilution of dietary protein by carbohydrate and fat results in the over-consumption of these nutrients, a scenario known as the ‘protein leverage’ of energy intake (Fig. 4 ) [ 92 , 96 ].

Schematic illustration of the protein leverage effect. The solid blue circle shows the bi-coordinate regulatory target for protein, non-protein energy (carbohydrate and fat) and total energy (the blue negative diagonal) in a hypothetical reference diet (Diet 1). When protein is diluted with carbohydrate and fat (solid red arrow), the strong protein appetite ensures that absolute protein intake remains constant (vertical black line). Consequently, fat and carbohydrate intake increases (dashed red arrow) as does total energy intake (dotted red arrow) as a passive consequence of strong protein regulation
Other biological mechanisms are also important. First, many foods and beverages incorporate psychoactive substances designed to tap into neurological ‘reward’ circuits that evolved in the context of much lower levels of stimulation [ 97 , 98 ]. Similarly, foods rich in both carbohydrate and fat [ 99 ] and sedentary behavior can also affect appetite regulation and promote over-consumption [ 100 ]. As we show below, this means that altering the composition of foods provides opportunities to influence human agency through the mediating pathway of appetite [ 1 , 101 , 102 ].
However, this scenario is not restricted to the composition of food itself, and is also relevant to broader factors that influence human behavior. We focus here on psychosocial stress, which can impact both eating behavior and metabolic processing of the diet (Fig. 5 ). Experimental studies of rodents and humans demonstrate that consuming a high fat diet dampens the stress response, though at a cost of elevated NCD risk markers [ 103 , 104 ]. These associations are attributed more strongly to the impact of the hormone cortisol on reward pathways and appetite centers in the brain, but there are many other components of signaling, including insulin, leptin, neuropeptide Y (NPY), endocannabinoids, gastrointestinal hormones and alterations of the microbiota [ 105 , 106 ]. Several cohort studies have reported that the level of perceived stress is associated prospectively with BMI increase [ 107 , 108 , 109 ].
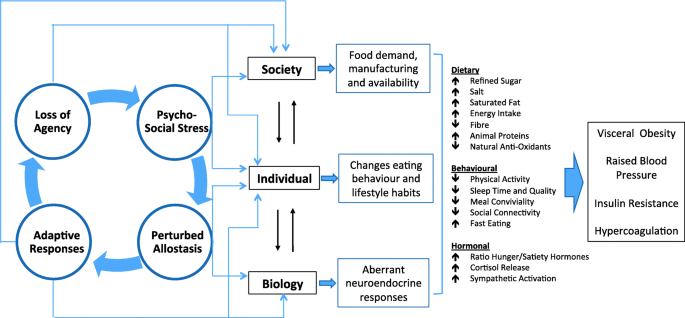
Impact of psychosocial stress on appetite, metabolism and the food system. Stressed individuals experience increased appetite, and consume high-energy palatable foods to dampen the stress response, under the influence of complex metabolic pathways involving the hormone cortisol and other signalling molecules. Within the body, these metabolic responses are associated with poorer cardio-metabolic profile, including insulin resistance, elevated blood pressure and greater susceptibility to blood clots (hypercoagulation). Insulin resistance and sustained increases in appetite also lead to excess weight gain, leading to chronically increased food intake. However, there are also many broader changes in behaviour, including perturbed sleep patterns and lower levels of physical activity, as well as faster eating behaviour and reduced sociality around meals. The interaction between stress and appetite generates an overall increased demand for high-energy palatable products, which drives greater supply, thus increasing the availability of unhealthy foods
Chronic activation of the stress response is therefore interacting in many settings with the plentiful availability of high-calorie foods, thereby contributing to the rise in obesity [ 110 , 111 ]. Importantly, stress is not experienced equally, and differential exposure to stress is a key factor mediating the relationship of societal inequities and inequalities with malnutrition. The role of stress biology is crucial for understanding both the etiology and the health impacts of the DBM, for through this medium the ‘competition of agency’ simultaneously structures the environments in which we eat, while also affecting how the body processes foods.
This nutritional ecology approach therefore frames nutrition as the interaction of biological mechanisms with the food environment, which sets the boundary conditions within which appetite systems operate [ 91 ]. This gives new insight into how societal factors drive malnutrition. For example, while overt dietary insufficiency drives weight loss, the ‘protein leverage’ hypothesis highlights how the aggressive marketing of highly processed foods can dilute protein intake, contributing to obesity [ 112 ]. Similarly, our appetite and metabolism respond to psychosocial stress, and to the social conditions in which we eat.
Individual agency
When it comes to behavior, individuals do not explicitly maximize nutritional health, and instead pursue proximate goals such as satisfying hunger, obtaining affordable and palatable foods that are convenient to prepare, and conducting desirable activities [ 113 , 114 , 115 ]. Their nutritional status is shaped both by their ability to pursue these goals, and by the environments to which they are exposed and which therefore impact their agency. Individuals may employ substantial creativity, to try to balance their competing goals [ 116 ]. Poverty exacerbates such trade-offs and drives more severe deficits in health, by forcing agency to be targeted at satisfying basic economic needs.
The fundamental association of poverty with food insecurity, food shortages and poor quality diets has been recognized for millennia, indicating reduced agency to access a healthy diet. However, beyond dietary intake itself, the constraints on agency driven by poverty and low education [ 117 , 118 ] also impact other stresses, such as exposure to pathogens and pollutants that impair growth and biological functions.
Structural drivers of malnutrition, such as poverty and inadequate education, inhibit both the agency and the means to improve household food insecurity and malnutrition. In turn, inadequate education (< 10 years) and in particular illiteracy, constrain women’s health, agency and opportunities to obtain better paid work which would enable the purchase of (typically costlier) healthier and diverse foods, and also to break out of poverty [ 119 , 120 ]. Working long hours in the informal economy, or returning to school/vocational training (for younger mothers) also means that women have limited contact with infants, limiting the opportunity to breastfeed [ 121 , 122 ]. Collectively, these structural factors not only maintain food insecurity, but also increase maternal stress and mental ill health and undermine their ability to fulfill their roles as mothers [ 123 ]. Left unaddressed, this cycle of disadvantage is likely to repeat across generations, whereby chronic malnutrition mediates the role of poverty in undermining physical health, cognitive development and academic ability [ 122 , 124 ]. In the most severe conditions, individuals may assert agency against such constraints through collective action. For example, increases in food prices that threaten food security often provoke riots, especially among urban groups and when society has broader discontent with the status quo [ 125 , 126 ].
Moreover, in contemporary populations these challenges are not experienced equally, and there are several groups whose agency over access to or selection of food is particularly prone to constraint. In poorer settings, for example, gender inequity may amplify these effects in women, who are often ascribed the most labor-demanding subsistence farming tasks [ 127 ], whilst being constrained in accessing adequately nourishing foods [ 128 ]. As an illustration of this, a study in Nepal identified gender differences in the household allocation of food, with men disproportionately consuming foods rich in animal proteins and important nutrients compared to women [ 129 ]. Another study in the same setting linked early marriage with shorter women’s height, suggesting a detrimental impact of psychosocial stress on linear growth [ 130 ]. The less women can meet the nutritional costs of reproduction, the more they transfer any nutritional insufficiencies to their offspring [ 131 ]. Consistent with that hypothesis, societal gender inequality assessed at the national level has been associated with higher rates of low birth weight and child wasting and stunting [ 132 ]. However, this scenario also relates to overweight as well as underweight, with women in countries with higher levels of gender inequality also at elevated risk of obesity [ 133 ].
At the level of geography, rural food producers tend to show higher rates of undernutrition than urban populations [ 51 ]. Farmers often lack agency over access to land, the ability to purchase agricultural inputs, and to decide on which crops or animals are farmed [ 43 ]. The returns on their labor are destabilized by ecological stresses and market volatility in commodity prices, both of which may demonstrate seasonal spikes [ 134 ]. Rwanda’s experience highlights how broader land consolidation and agricultural policies constrain the agency of poor households to obtain diverse nutritional diets [ 135 ]. Under this scheme, the government provides agrarian land for poor households to grow fruits and vegetables, and generate livestock products to sell at local rural markets. However, there is no mechanism to then facilitate the purchase of similar high nutrient foods for these households. Although poor households allocate 39% of their total monthly expenditure to food, the high price and poor availability of nutritious foods mean that they buy more of the cheaper low nutrient foods (e.g. high in starch/carbohydrates). Paradoxically, therefore, the greater proportion of time spent by women in growing healthy foods for markets, the higher the prevalence of child undernutrition [ 135 ]. Again, to overcome such challenges, food producers often mobilise collectively: the best-known example is the Via Campesina movement, an international alliance of peasant and family farmer organisations built from the bottom up. Via Campesina promotes ‘food sovereignty’ by make local agriculture and trade work more effectively in its members interests, improving outcomes for both food producers and consumers [ 136 ].
However, while rising incomes may increase agency over dietary intake by reducing the risk of food insecurity, they also increase exposure to commercial influences, and the resulting dietary shifts may lead to excess weight gain. Overweight typically first emerges among wealthier groups during nutrition transitions, but subsequently shifts to poorer groups, as cheap highly-processed foods are the most obesogenic [ 49 , 137 ]. This helps explain why, while urban populations may be less susceptible to undernutrition, they have also been more prone to overweight [ 138 ].
Figure 6 lists a range of properties of foods that are actively targeted through the expression of agency of individual food consumers, but also by the agency of corporate food vendors. However, for each individual food property, what consumers and vendors seek to gain from expressing their agency is very different. For example, a food corporation pursues its goal of maximizing sales by manipulating the taste of a food product to maximize palatability. The consumer in contrast maximizes the goal of enjoyment, which may have both personal and social components, by purchasing foods considered tasty or adding to a harmonious meal [ 139 , 140 ]. These contrasting approaches mans that although consumers may achieve a range of goals through their choice of foods, corporations are still able to influences these choices. The way in which they achieve this often steers the diet towards less healthy composition.
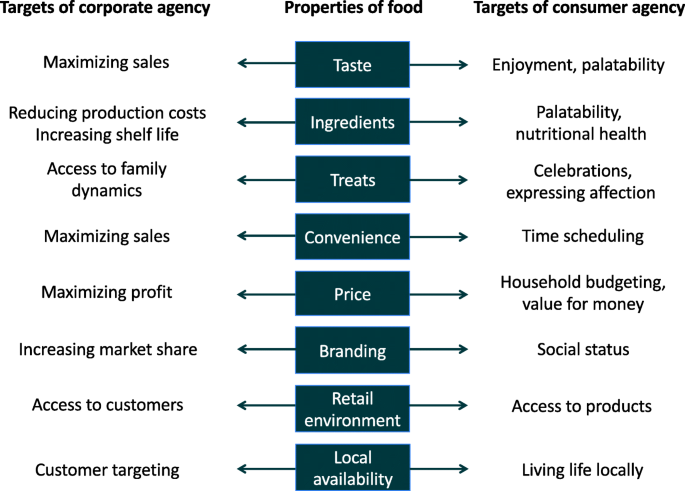
Differential targeting of food products by individual and corporate agency. Individuals express agency over their purchasing of food to satisfy a range of wants and needs, many of which are embedded in social or economic dynamics, and most of which do not relate directly to health. In contrast, corporate food producers maximise their agency over sales, and seek to manipulate a range of aspects of consumer behaviour to cut their production costs, increase their reach, and maximise their sales
Individual agency is also influenced by local social norms, relating to cultural valuations of body image and foods. This means that alongside biological drives (appetite), there are also social drives to eat. In settings with limited food availability, larger body size and plumpness are typically markers of status or beauty, and processed foods are often seen as desirable symbols of modernity [ 141 , 142 ]. The reverse pattern emerges in food-secure HICs, where slimness, leisure activities and the consumption of diverse fresh foods all signal status [ 143 ]. Changes in social norms therefore play a major role in dietary and behavioral change.
Corporate agency
Commerce is embedded in every component of the human food chain, from agricultural production, shipping, marketing and retailing, to consuming. Corporate agency acts deliberately to reduce or distort individual agency in order to stabilize and increase commercial profits, but this comes directly at the expense of health, as recognized through the lens of the ‘commercial determinants of health’ [ 144 ].
Food processing adds economic value along the whole food chain. The most profitable foods are highly processed products that are easy to manufacture, ship and store, and that stimulate consumption by targeting both appetite and social status through branding [ 145 ]. Corporations compete over market share, and the most efficient firms expand in size at the expense of others. Consequently, market share is increasingly dominated by a handful of large TNCs [ 127 ], however economies of scale mean that individual food items remain relatively cheap to consumers. In LMICs, these pressures steer local food companies towards the same business models.
Commercialization of the food supply has reshaped the entire mode of eating [ 36 ], reducing emphasis on major meals and promoting inter-meal snacking, in particular on processed foods and beverages as well as alcohol. Retailers and food venues deliberately target unhealthy but profitable processed foods at large susceptible communities whose agency is most readily manipulated, as discussed below. Fast food outlets are often clustered in deprived neighborhoods [ 146 , 147 ] and along school commuting routes [ 148 , 149 ], while poorer urban populations may also be exposed to forms of ‘food desert’, lacking adequate access to healthier items [ 150 ].
Norms of social status are actively targeted to change dietary behavior [ 151 ], the primary targets being poorer groups in HICs and wealthier groups in LMICs [ 1 ]. In LMICs, advertising plays a key role in driving nutrition transition by portraying ‘new kinds of consumer’, an aspiration that can seemingly then be realized by consuming the relevant products [ 1 ]. However, corporate agency is achieved in part by creating ‘illusory agency’ for consumers, who are bombarded with substantial ‘choice’ over individual products, whilst simultaneously offered a range of foodstuffs that have in common high processing, low nutrient content and high profitability. These products have been specifically designed to manipulate agency over what, how much, and when food is consumed, following decades of research on palatability (hence manipulating appetite) and desirability (hence manipulating cultural preferences) [ 1 , 101 , 102 ]. In this way, corporations simultaneously target both biological and social drives relating to eating. It is precisely because people pursue a wide range of goals relating to food and eating that the food industry has targeted a wide range of opportunities to manipulate individual agency in the interest of corporate profit.
Beyond diet itself, commercial factors drive many other aspects of lifestyle related to obesity, including sedentary behavior through labor-saving devices, mechanized transport, and addictive digital activities. Similarly, the commercialization of agricultural inputs has radically shifted control from individual farmers to large agritech businesses, that now sell coordinated packages of seeds, fertilizers and pesticides [ 43 ]. Agritech corporations thereby restrict the range of crops grown by small-scale farmers to a fraction of the possible varieties [ 43 ], which perpetuates fundamental asymmetries between HIC and LMIC food systems. Cheap grains from subsidized industrialized farms in HICs are dumped in LMICs, undermining local food production, which in turn drives LMIC consumption of imported foods [ 152 ].
Governments
Democratic governments should serve the interests of their voters by promoting health, through activities such as public health campaigns, taxing unhealthy foods, or regulating food composition, corporate advertising and nutrient-labeling [ 153 ]. However, their success in meeting these aims is limited by several factors. First, the financial resources available heavily favor corporations. In 2017, the leading 33 companies in the food, alcohol and tobacco sectors generated combined profits of USD 99 billion, an order of magnitude larger than the sum available globally for the prevention of undernutrition and NCDs [ 154 ]. Second, government agency is undermined by powerful corporate lobbying, and limited by the process of law, which gives many legal rights to corporations [ 155 ]. Third, corporations actively misinform and confuse consumers, thus negating education campaigns [ 156 ], and undermine public health research [ 157 , 158 ].
Even in democracies, governments can themselves contribute to malnutrition through providing inadequate safety nets. Recently, austerity policies have been associated with a rapid increase in food banks in the UK [ 159 ], and with increased rates of child malnutrition in Spain [ 160 ]. However, while overt hunger has often provoked food riots [ 25 ], citizens rarely protest in favor of healthy foods [ 161 ]. Instead, food corporations have ‘manufactured consent’ for unhealthy products by making them ultra-palatable and cheap [ 1 , 101 ]. This approach allows consumers to achieve agency over several goals – enjoying food, and obtaining value for money – though at a cost to their health. Consequently, the public often distrusts and resists public health campaigns, rejecting the assault on their agency to enjoy their diet and lifestyle. This is despite the fact that government regulation can cut NCDs substantially, as demonstrated by bans on transfats and smoking, and by salt reduction programs [ 162 , 163 , 164 ].
Non-democratic governments often use the medium of nutrition explicitly to control their populations. For example, autocratic regimes manipulate agricultural policy to maximize agricultural rents while minimizing the threat of unrest, and favor either urban populations, or landed elites and farmers, depending on where the threat is greatest [ 165 ]. Where such governments face active resistance, the resulting civil conflict may involve the deliberate imposition of food shortages to quell opposition [ 166 ]. Recent conflicts in Syria and Yemen highlight that sieges remain central to military strategy.
Neoliberalism
Conceptual frameworks for public health nutrition often treat government as the ‘uppermost’ or ‘outermost’ level, and consider that nutritional health emerges from dynamics between governments, their citizens and commercial actors. Crucially, however, governments themselves are subject to a broader economic system, which since WWII has been increasingly restructured in support of a broader ‘neoliberal’ approach centered around competitive capitalism, consumerism, free trade, rapid trade liberalization, and minimum government regulation, all of which are considered by economists such as Friedman to be key prerequisites for political freedom [ 167 ]. Whether ‘neoliberalism’ is a paradigm, a political-economic project or an ideology [ 168 , 169 , 170 ] continues to be debated [ 171 , 172 ], however as we show below, the specific policies supporting this approach have been widely implemented. The consequence has been to reshape the competition of agency amongst all the other actors.
In the 1970s, indebted LMICs undergoing economic crises were granted grants and loans by the World Bank and IMF with strict conditions coalescing around the central neoliberal principles. Since then, these practices have consolidated, and recent financial, food and fuel crises have resulted in the expansion of these ‘structural adjustment programs’ (SAPs), including to several European countries [ 173 , 174 ]. SAPs are designed to enable countries to achieve macroeconomic stabilisation through controlling inflation, servicing debts to foreign creditors and stimulating economic growth. SAPs generally comprise of six types of policies: monetary, fiscal, exchange rate, foreign trade, wages and prices [ 175 ]. Since economic crises are largely blamed on state intervention, protectionism and price subsidies that distort market forces and undercut economic growth, SAPs specifically aim to dismantle these policies. In the continual effort to improve the efficiency of the public sector, contemporary SAPs also explicitly promote the privatisation of state assets, property and public services, and, in an effort to open domestic markets to foreign investment, they aim to lower TNC taxes, deregulate financial markets and expand trade liberalisation [ 86 , 173 , 176 , 177 , 178 ].
Seminal research by Pinstrup-Andersen [ 175 ] and more recent work by Babu and colleagues [ 179 ] have highlighted multiple pathways through which macroeconomic policies fundamentally restructure national economies, including food and agricultural policy, that then adversely impact household food security, income and the nutritional status of the poor in particular. Cornia and colleagues, writing for UNICEF, provided early evidence of how these adjustment policies worsened children’s nutritional status in Ghana, Jamaica, Peru, Philippines, Sri Lanka and Bolivia in the 1980’s [ 180 ]. Since then, evidence of the adverse effects of SAPs on nutrition and health has increased substantially [ 174 ], as we summarise below.
At the national level, SAPs have transformed domestic food systems and production by facilitating TNCs in diverting LMIC farmers from growing food to growing cash crops for export, thereby increasing household food insecurity [ 85 , 181 ]. In order to drastically reduce inflation, repay debts and balance budgets in the short-term, countries have undergone stringent fiscal austerity. This has resulted in decreased government expenditure on welfare programs directly affecting nutritional status, such as food subsidies, and lowering or capping public sector wage bills, to the detriment of quality public health care and education provision, whilst simultaneously increasing the provision of these public services to either NGO or private providers [ 182 , 183 , 184 , 185 , 186 ]. Across LMICs, structural adjustment measures have been associated with falling real wages and increased household poverty, which, when coupled with rapidly increasing food prices, decrease purchasing power. As the overall consumption of food falls, households shift to purchasing cheaper, less nutritious foods, increasing risks of the DBM and infant and maternal mortality [ 180 , 187 , 188 , 189 ]. Seasonable food shortages and imbalanced dietary intake also increase susceptibility to infectious disease, which often remains untreated because of decreased access to healthcare due to poverty [ 190 ]. Importantly, SAPs have increased women’s undernutrition and ill-health by simultaneously increasing their workloads in the reproductive and (often informal, lower paid) economic spheres; at the same time, rampant privatisation has decreased their access to agrarian land and support systems such as cooperatives, and the removal of State produce subsidies has undermined their ability to source adequate food [ 191 , 192 , 193 ].
More recent work has explored in more detail the different pathways through which, collectively, these policies and the institutions that implement them have systematically undermined governments’ capacity and agency to promote nutrition and health [ 194 ]. For example, an analysis of 141 LMICs from 1985 to 2014 finds that specific conditions on privatization, price deregulation and public sector employment have had a negative effect on the capacity of the state to effectively implement development policies that in turn could sustain economic growth [ 195 ] – the latter ironically being a key goal of SAPs [ 182 , 183 , 184 , 185 , 186 , 195 ]. In contrast, Dollar and Svennson’s (2000) review of 182 World Bank adjustment loans suggested that the main reason for the 36% failure rate was due to the recipient country’s authoritarian political-economy. Hoey proposed that in Bolivia, efforts to decentralize and downsize government, and the rapid proliferation of NGOs delivering public provision, eroded state capacity to effectively deliver programs to reduce malnutrition [ 196 ]. In Fiji, trade and investment liberalization promoted by SAPs increased the power of multinational food and beverage companies to redefine the domestic food market. Initially, marketing regulations prohibiting the promotion of formula feeding led to companies retracting these products, which ironically led to consumer protest over reduced ‘choice’ [ 197 ]. The regulations were not only rescinded, but in 2016–17, the import of infant food became ‘duty free’ [ 197 ]. Through several different pathways, therefore, SAPs systematically undermine the capacity of states to promote the nutritional health of their citizens.
Within the neoliberal system, market and trade liberalization has been central to the LMIC ‘nutrition transition’ that has played a fundamental role in the emergence of the DBM [ 198 ]. Broadly, the WTO and other instruments such as Trade and Investment Agreements have reshaped the whole spectrum of food systems, impacting food production, manufacturing, distribution and marketing [ 199 , 200 ]. ‘Power hungry,’ a report by the NGO ActionAid International, documents several ways in which global food companies control domestic agrifood markets, from seed to supermarket, and remain unaccountable for their negative impacts on farmers’ livelihoods, the human right to food, and the environment. Their activities include raising the price of agricultural inputs; engaging in unfair buying practices, including price-fixing cartels; lowering prices for farmers’ goods, which decreases producer income whilst maintaining high retail prices, thereby increasing corporate revenue; and marginalizing poor farmers and rural workers from the supply chain and access to justice [ 127 ]. Food prices, supply and availability directly affect child nutritional status by decreasing the revenue of agricultural producers, thereby affecting household income and changing the types and amount of food purchased and consumed. In contrast, government assistance to tradable agriculture through reduced taxation was found to improve child nutritional status across 22 LMICS [ 201 ].
More specifically, trade liberalization has contributed directly to the escalating obesity pandemic, in part through expanding imports of highly-processed foods [ 198 , 202 ]. For example, WTO arrangements have been associated with substantially higher intakes of sweetened beverage across LMICs [ 10 , 203 , 204 ], while across African countries, the percentage of food that is imported correlates with adult obesity prevalence [ 205 ]. As with SAPs, the agency of LMIC governments to promote nutritional health and public health oversight is actively challenged by the legal rights accorded to TNCs to protect their investment in trade agreements [ 206 , 207 , 208 ]. For example, Thailand abandoned a proposal to initiate a healthy food labeling system [ 209 ], whilst Vietnam dropped a tax on sweetened beverages for fear that corporations could sue the government for potential loss of earnings [ 203 ]. Even when governments are able to defeat such lawsuits, they may be left with crippling legal costs [ 210 ].
Based on the evidence presented above, we conclude that there is a hegemonic neoliberal economic orthodoxy, which has been implemented in a concerted manner to restructure the world economy, most notably through technical policy mechanisms designed under SAPs of the IFIs, or through the WTO and trade agreements, which have transformed welfare states into competitive states [ 211 , 212 ]. Irrespective of the country, the neoliberal approach has exacerbated inequity in nutrition, health and educational outcomes, and has reduced the capacity of states to respond by fulfilling these basic rights more broadly. This overall reduction in human capital has on the one hand failed to achieve the central neoliberal goals of debt reduction and economic growth, but on the other hand succeeded in transferring wealth and capital to wealthy corporations and nations. Collectively, these examples show that neoliberalism functions at multiple levels and in multiple forms, involving global institutions, nation-states, and corporations [ 213 ]. We highlight that it is the way in which neoliberalism has reshaped the ‘competition of agency’ that accounts for its fundamental role in the emergence of the DBM (Fig. 7 ).

The political and commercial determinants of nutritional health. The nutritional status of individuals is strongly shaped by asymmetric power dynamics and financial flows among a set of actors, including corporations, governments and supranational organisation. In contemporary food systems, these dynamics drive the double burden of malnutrition. Black text – financial flows; blue text – power relations; red text – markers of ill-health
Perhaps most concerning is how the entry of cheaper, less nutritious foods has become a space of activism, with consumers paradoxically demanding their right to choose these less healthy products. These actions are in large part driven by lower income and limited control over food environments [ 197 ], and reflect the way that individual agency has been undermined by the manipulation of both purchasing power and appetite/palatability. It is precisely the way in which neoliberalism is increasingly embedded at every level of society and economy which makes it both difficult to address, but all the more important to challenge in order to achieve equity in nutritional health. This progressive erosion of social citizenship has provided the impetus for collective mobilization against neoliberalism over the past four decades [ 214 ].
The competition of agency
This review of agency as a ‘competition’ between multiple actors highlights how individuals are exposed to both commercial and political determinants of nutritional health [ 215 ]. Nutritionists have long gained insight from socio-ecological models that provide an individual-centric view [ 216 ], focusing primarily on behavioral interactions between individuals, corporations and governments. Following the logic of Swinburn and colleagues [ 5 ], we can gain a very different perspective by flipping the model, putting the politico-economic system center-stage (Fig. 8 ). Our alternative model contains more layers, and enables us to see malnutrition as the outcome of interactions between human metabolism and many forms of power dynamics deeply embedded in the global food system. The model also helps understand the many inequalities that produce contrasting nutritional outcomes across different social groups. The main value of our approach lies in the fact that, as we have highlighted above, there is now substantial evidence for interactions between all the different layers. We show below, moreover, how a novel ongoing stress is demonstrating in real time the sensitivity of human malnutrition to rapid changes in these dynamic relationships.
Contrasting socio-ecological models of nutrition and agency. ( a ) The individual-centric view emphasises the individual, whose behavioural agency drives interactions with the social community, corporations and government activities. ( b ) The system-centric view emphasises the food environment as a system shaped by the logic of market economics. The overall system shapes government and corporate activities, and generates structural associations between different socio-economic groups (shown by red or green filled circles), whose biological drives are exposed to contrasting nutritional experience through the life-course
By presenting this competition of agency, we also highlight the inadequacy of existing models of agency at the individual level. Growing understanding of how food composition impacts satiety, how eating patterns respond to social factors, and how psychosocial stress impacts both appetite and metabolism, forces us to question concepts of agency that define it in terms of other individual-level factors such as liberty and autonomy. Similar challenges relate to other aspects of behavior, such as physical activity and sedentary behavior. What are often portrayed as individual ‘choices’ are in reality better considered acts of behavior where choice is suppressed or manipulated. As we have argued previously, it is precisely because nutrition, metabolism and cognitive function represent an interface between corporate agency and individual agency that our understanding of individual agency in the context of nutrition is problematic [ 1 ]. The same scenario applies to the interactions of individuals with other actors, such as governments and IFIs. Without better understanding of this issue, we will struggle to promote individual level agency and empower public health programs, or curtail the agency of other actors central to the DBM and its associated effects on ill-health.
Lessons from the COVID-19 pandemic
The utility of our framework for understanding the future burden of malnutrition is supported by emerging evidence of the effects of the COVID-19 pandemic. The dynamic interactions of food and economic systems with individual agency and biology are giving rise in every global region to shifts in food insecurity, diets and eating behavior, however the effects are variable and depend on the local context. The pandemic highlights the inability of the global food system to protect populations either from hunger, or from diet-influenced NCDs, whilst also showing that malnutrition increases susceptibility to this disease [ 217 ].
Even in high-income countries, those who have experienced job loss or income insecurity have undergone increased exposure to food insecurity. Use of food banks has increased [ 217 , 218 ], while the practice of food hoarding temporarily reduced the availability of food for low-income families who cannot afford to buy in bulk [ 219 ]. Policies of isolation and ‘lockdown’ have in many cases increased the consumption of unhealthy foods, as well as unhealthy commodities such as alcohol and cigarettes, and some groups have experienced excess weight gain. Levels of physical activity have often declined, in association with limited access to recreational facilities. However, early data also indicate substantial heterogeneity in these responses, as some groups have experienced confinement without substantial change in economic circumstances.
Studies in European settings have shown that those already overweight tended to gain further weight, with psychosocial stress stimulating increased consumption of energy-dense ‘comfort foods’, whereas those already underweight have tended to lose weight [ 220 , 221 , 222 ]. In Italy and Spain, however, lockdown policies increased the prevalence of home cooking, promoting overall adherence to the healthy Mediterranean diet, while the consumption of savory snacks, processed meat, and carbonated/sugary drinks decreased [ 220 , 223 ]. Some, especially those already exercising, tended to adopt new exercise regimes, while those already sedentary tended not to change their status [ 224 ]. Similarly, some have quit smoking, whereas others have consumed more alcohol and increased smoking [ 222 , 224 ].
An international global survey in 7 languages, involving 1047 individuals primarily from Africa, Asia and Europe, found that home confinement reduced physical activity at every level of intensity, and led overall to less healthy patterns of food consumption and meals [ 225 ]. Another study of adolescents from Spain, Italy, Brazil, Colombia, and Chile found that confinement was associated on the one hand with more time for cooking and greater consumption of legumes, fruit, and vegetables, but also with higher sweet food consumption which was attributed to boredom and stress [ 226 ].
In lower income countries, there is risk of major disruption to many sectors fundamental to nutrition, including food supply chains and markets, income, social protection, health care services for women and children, and access to clean water and sanitation [ 227 , 228 ]. The effect of simultaneous loss of income with increases in food prices may be particularly detrimental to those working in urban informal economies. As yet, there are few published data on the numbers affected or the magnitude of the effects, but according to preliminary projections, UNICEF has predicted that ‘the COVID-19 pandemic may add an additional 83 to 132 million people to the ranks of the undernourished in 2020’ [ 229 ]. These stresses may generate particularly severe impacts on those already susceptible to food insecurity, for example migrant workers or those living with HIV [ 230 ].
The efforts of food corporations to manipulate individual agency in their own interest during the pandemic has also been observed. In India, for example, there is evidence of an infant formula manufacturer using social media to recommend the separation of mothers with COVID-19 from their infants for 72 h and to stop breast-feeding, despite this contradicting both Indian law and medical advice [ 231 ]. Corporations have used the pandemic and the rapid changes in eating patterns as a marketing opportunity [ 232 ], and among adolescents, the intake of highly processed foods was found to have increased in each of five countries surveyed in Europe and South America [ 233 ].
Finally, it should be noted that COVID-19 also has a bi-directional association with nutritional status. Obesity has already emerged as a strong risk factor for COVID severity and mortality [ 234 , 235 , 236 ], while impaired immunity associated with nutritional deficiencies may also increase the risk of infection and poor prognosis [ 237 ].
Overall, the impact of COVID-19 on food systems combined with lockdowns have greatly reduced individual agency for many, but the same factors have sometimes also reduced the agency of corporations in the short-term to vend unhealthy products or fast food. Of note, none of these events or policies deliberately targeted nutritional health, and yet changes in the prevalence of both undernutrition and overweight are likely to emerge as responses.
Future outlook
Over recent centuries the world has witnessed unprecedented economic growth, scientific progress and technological development. However, the benefits have not been shared equally, while the costs are manifesting as climate breakdown, environmental degradation and persisting malnutrition. The global food system developed in part through the function of maintaining societal inequalities, and the contemporary DBM reflects these dynamics. If we consider the global food system as a ‘game’ that requires solving, then there is a rapidly diminishing landing space for a solution that is simultaneously equitable and healthy for people and planet.
Sen’s insight that famines represent failures of society rather than food production [ 7 ] is highly relevant to the DBM. However, while progress has been made in increasing access to food, compromised agency at the level of the individual as well as the state continues to be crucial in understanding malnutrition, whatever its manifestation. How to maximize nutritional health therefore remains a major challenge.
Technical opportunities
Technical advances concern changes in the composition of food, and therefore either ignore individual agency, or seek actively to bypass it. This can potentially have advantages in certain situations, for example altering the composition of foods used to treat undernutrition may bypass poor appetite and accelerate nutritional recovery. The same approach could in theory also be used in reverse to combat obesity, however to date the food industry has shown very limited engagement with this opportunity, highlighting how their own agency and interests contribute to this health problem.
Regarding childhood undernutrition, the development of ready-to-use therapeutic foods (RUTFs) has played a key role in reducing mortality rates whilst also promoting weight recovery [ 238 ]. Containing no water, RUTFs prevent microbial growth and can be eaten directly from the sachet, allowing children without complications to be treated in their homes. However, the gut microbiota of severely undernourished children is immature, a scenario only transiently improved by RUTF treatment. Future work might identify complementary foods that could help ‘repair’ the immature microbiota and promote healthier growth [ 239 ]. Producing such RUTFs locally/regionally, and basing them on locally available microbiota-directed ingredients, may make them more efficient, culturally acceptable and sustainable. For less severely undernourished children, smaller amounts of RUTFs might also support a healthy gut microbiota, again benefitting growth. Recently developed food supplements for undernourished children primarily promote the accretion of lean mass rather than fat [ 240 ], reducing fears that treating short and underweight children might increase the risks of obesity and NCDs [ 241 ].
Regarding obesity, there is growing evidence that altering diet composition could potentially inhibit excess weight gain through effects on appetite. For example, the framework of nutritional geometry suggests that increasing dietary protein and fiber content could reduce passive energy consumption. To date, however, lifestyle interventions targeting individuals have generally had limited impact, largely because they are too easily countered by corporate agency. The food industry manufactures numerous ‘diet products’, but these do not lead to sustained changes in appetite, and are more effective in changing food purchase habits than in reducing body weight. Pharmacological solutions to obesity have also proven challenging, as there is no central metabolic pathway for drugs to target, though combination therapies are currently attracting interest [ 242 ]. The most effective therapies for significant prolonged weight loss are surgical operations [ 243 ], which cause unpleasant side effects and overwhelm health services.
In each case, technical development has primarily been directed to the treatment of malnutrition and its co-morbidities after the condition has developed. Progress in prevention remains relatively limited, the best example being micronutrient fortification programs that can reduce micronutrient deficiencies [ 244 ]. A criticism of this approach is that it provides opportunities to extract profit from the loss of individual agency over health, but from another perspective it could also be seen to improve agency over factors that promote nutritional deficiencies, and may be a very effective component of public health programs.
Societal opportunities
A rebalancing of agency represents the most powerful solution to the DBM. This requires that we elucidate in more detail the many components of power dynamics in the human food system, and clarify the conflicts of interest between the actors, in order to identify novel targets for intervention. As we have shown above, power dynamics at many levels play a central role in the rising prevalence and unequal distribution of all forms of malnutrition.
Hindering our ability to alter these power dynamics, however, are many ways in which the status quo is perpetuated. For example, within any country or community, gender inequality is underpinned by societal norms that must be actively challenged. Similarly, corporations have not only acquired legal protections similar to those of individual citizens, but also additional rights that render them largely autonomous from public control [ 245 ]. In the same vein, the larger economic system, including influential IFIs, is not subject to the kinds of scrutiny or regulatory mechanisms that pertain to governments, while TNCs are likewise unconstrained by international laws that protect vulnerable populations [ 215 ].
The level of IFIs is rarely considered as a potential target for intervention in nutrition policy, yet as we have reviewed above, evidence for its impact is now strong. Citing influential work by Farmer [ 246 , 247 , 248 ], Pfeiffer and Chapman argued that if ‘... social and economic rights are human rights, [then] the role of a robust public sector and government emerges as vital; not sufficient, but necessary to guarantee the right to survive. Viewed in this light, structural adjustment’s systematic dismantling of public services for health, education, agriculture, water, and safety nets is rightly seen as a war on the poor; its violence measured in increased morbidity, excess mortality, … and the harder-to quantify destruction of community … ’ [ 174 ]. Our argument is that malnutrition is a particularly sensitive lens through which such damage can be assessed.
Reflecting these power inequalities, there are increasing calls to address malnutrition by strengthening human rights [ 249 ]. In 1996, a UN charter outlawed the control of food distribution for political ends, yet the imposition of food restriction for political and military ends has continued in each continent in the early twenty-first century [ 166 ]. More generally, global food policies are deeply embedded in multiple economic activities that serve political ends. Moreover, although the ‘right to food’ is widely recognized, providing ‘adequate’ food represents a relative standard, open to debate and distortion [ 250 ]. The Convention of the Rights of the Child commits signatories to regulate unhealthy food advertising and promotion, but this may be undermined by cultural rights that protect ‘values, beliefs, convictions … and ways of life’, all of which may be targeted by corporate efforts to shape social norms [ 251 ]. The ‘capability approach’ of Sen and Nussbaum, already applied in other disciplines such as education and gender inequity, could also guide us in combatting the DBM [ 252 ] if we can identify effective strategies for transforming inequitable social power hierarchies [ 253 ]. The neoliberal system has narrowed our definition of human development, and has resulted in maximizing short-term economic growth at the cost of human and planetary health. Economic growth should not be considered an end in itself, rather as only a means towards increased wellbeing of populations and human development. This ‘means versus ends’ confusion has contributed to persisting inequalities, and the capability approach provides us with a legal framework that can guide us in combatting the DBM.
More broadly, existing rights frameworks are ill-equipped to deal with TNCs and IFIs, because government obligations are limited to their own territory. A new legal framework is needed on the extraterritorial application of human rights and specifically the obligations of supranational organizations.
However, we should also remember that power dynamics represent only one component of the human food system, and that other ecological, agricultural, biological, socio-cultural and economic factors are also relevant. Human nutrition represents a nexus of complex systems, in which there is no natural balance between the various hierarchical levels or actors, and where individual components may be connected by non-linear associations and by complex feedback loops. Systems theory may offer new ways to understand how changes in one aspect of the system are likely to affect others, and this concerns both power hierarchies and other ecological factors. For example, the association of dietary protein with appetite is expected to be sensitive to rising CO 2 levels, which have been found to dilute the protein, fiber and micronutrient content of vegetable crops with starches [ 254 , 255 ]. Better understanding of these inter-relations, including how the ‘competition of agency’ interacts with broader ecological factors, will facilitate interventions while avoiding unintended consequences, and may help identify how power dynamics may be targeted in order to achieve the greatest benefits.
Malnutrition, social inequity and inequality, and climate breakdown each manifests as an existential crisis for our species. Crucially, each is determined by a broad common set of political and commercial interests that override individual agency. However, to date scientists have struggled to develop broader conceptual models that are capable of expressing and exploring these interactions. This has hindered researchers and policymakers from gaining appropriate evidence on the deeper structural causes of these problems, and hence from developing appropriate policy responses. Existing physiological models of the causes and manifestation of malnutrition have remained largely disconnected from socio-ecological or economic models of food systems. In this paper, we aimed to address this lacuna, by setting out a new integrative conceptual framework that describes in detail how societal factors impact and interact with human physiology, thereby determining variability in nutritional status and the risk of all forms of malnutrition. For example, we are able to frame how international economic policies and corporate practices, mediated by food composition (eg protein content) and social factors (eg exposure to stress), shape dietary intake and nutritional status. This sheds new light on the international structural drivers of the global obesity epidemic and persisting under-nutrition.
This approach allows us to highlight a competition of agency between a range of different actors as the essential target of efforts to prevent malnutrition in the future. Given the foundational role of food systems in all human societies and ecosystems, the rebalancing of agency that we describe above must be central to tackling not only malnutrition, but also social inequality and climate breakdown. In the words of the former UN Secretary General, Ban Ki-moon, ‘Nutrition is both a maker and a marker of development. Improved nutrition is the platform for progress in health, education, empowerment of women and the reduction of poverty and inequality, and can lay the foundation for peaceful, secure and stable societies’ [ 256 ].
Availability of data and materials
Not applicable.
Abbreviations
Body mass index
Double burden of malnutrition
High-income country
International financial institution
Low- and middle-income country
Non-communicable disease
Non governmental organization
Neuropeptide Y
Ready-to-use therapeutic food
Structural adjustment programs’
Transnational corporations
World Trade Organization
Wells JC. The metabolic ghetto: an evolutionary perspective on nutrition, power relations and chronic disease. Cambridge: Cambridge University Press; 2016.
Book Google Scholar
Popkin BM, Corvalan C, Grummer-Strawn LM. Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet. 2020;395(10217):65–74.
Article PubMed Google Scholar
Wells JC, Sawaya AL, Wibaek R, Mwangome M, Poullas MS, Yajnik CS, et al. The double burden of malnutrition: etiological pathways and consequences for health. Lancet. 2020;395:75–88.
Scrinis G. Reframing malnutrition in all its forms: a critique of the tripartite classification of malnutrition. Global Food security. 2020;26:100396.
Article Google Scholar
Swinburn BA, Kraak VI, Allender S, Atkins VJ, Baker PI, Bogard JR, et al. The global Syndemic of obesity, undernutrition, and climate change: the lancet commission report. Lancet. 2019;393(10173):791–846. https://doi.org/10.1016/S0140-6736(18)32822-8 .
Willett W, Rockstrom J, Loken B, Springmann M, Lang T, Vermeulen S, et al. Food in the Anthropocene: the EAT-lancet commission on healthy diets from sustainable food systems. Lancet. 2019;393(10170):447–92. https://doi.org/10.1016/S0140-6736(18)31788-4 .
Sen A. Poverty and famines: an essay on entitlement and deprivation. Oxford: Oxford University Press; 1981.
Google Scholar
Marmot M. Social determinants of health inequalities. Lancet. 2005;365(9464):1099–104. https://doi.org/10.1016/S0140-6736(05)71146-6 .
Fogel RW. Health, nutrition, and economic growth. Economic Development and Cultural Change. 2004;52(3):643–58.
Stuckler D, McKee M, Ebrahim S, Basu S. Manufacturing epidemics: the role of global producers in increased consumption of unhealthy commodities including processed foods, alcohol, and tobacco. PLoS Med. 2012;9(6):e1001235. https://doi.org/10.1371/journal.pmed.1001235 .
Article PubMed PubMed Central Google Scholar
Stuckler D, Nestle M. Big food, food systems, and global health. PLoS medicine. 2012;9(6):e1001242. https://doi.org/10.1371/journal.pmed.1001242 .
Scaling Up Nutrition (SUN). (accessed 8 Oct 2020).
Ferrari M, Benvenuti L, Rossi L, de Santis A, Sette S, Martone D, et al. Could dietary goals and climate change mitigation be achieved through optimized diet? The experience of modeling the National Food Consumption data in Italy. Front Nutr. 2017;7:48. https://doi.org/10.3389/fnut.2020.00048 .
Article CAS Google Scholar
Sen A. Development as capability expansion. in: , et al. new delhi and new york: Oxford universit. In: Fukada-Parr S, Kumar AKS, editors. Readings in human development. (). Delhi and New York: Oxford University Press. New Delhi: Oxford University Press; 2003.
Global Nutrition Report. Shining a light to spur action on nutrition: globalnutritionreport.org , 2018.
Rapaport N, Overing J. Social and cultural anthropology: the key concepts. London: Routledge; 2007.
Crocker DA. Ethics of global development : agency, capability, and deliberative democracy. Cambridge: Cambridge University Press; 2008.
Gallagher A. Stature, body mass, and brain size: a two-million-year odyssey. Econ Hum Biol. 2013;11(4):551–62. https://doi.org/10.1016/j.ehb.2012.12.003 .
Cordain L, Miller JB, Eaton SB, Mann N, Holt SH, Speth JD. Plant-animal subsistence ratios and macronutrient energy estimations in worldwide hunter-gatherer diets. Am J Clin Nutr. 2000;71(3):682–92. https://doi.org/10.1093/ajcn/71.3.682 .
Article CAS PubMed Google Scholar
White R. Upper Palaeolithic land use in the Perigord. BAR international series no 253. Oxford: British Archaeological Reports; 1985.
Hayden B. Feasting in preshistoric and traditional societies. In: Wiessner P, Schiefenhovel W, editors. Food and the status quest: an interdisciplinary perspective. Providence, RI: Bergahn Books; 1997. p. 127–46.
Altmann J, Schoeller D, Altmann SA, Muruthi P, Sapolsky RM. Body size and fatness of free-living baboons reflect food availability and activity levels. AmJPrimatol. 1993;30:149–61.
Wells JC, Stock JT. Life history transitions at the origins of agriculture: a model for analysing how niche construction impacts human growth, demography and health. Front Endocrinol. 2020;11:325.
Cohen MN, Armelagos GJ. Palaeopathology and the origins of agriculture. Orlando,Florida: Academic Press; 1984.
Newman LF. Hunger in history: food shortage, poverty, and deprivation. Cambridge, MA: Blackwell Publishters; 1990.
Pomeroy E, Mushrif-Tripathy V, Cole TJ, Wells JCK, Stock JT. Ancient origins of low lean mass among south Asians and implications for modern type 2 diabetes susceptibility. Sci Rep. 2019;9(1):10515. https://doi.org/10.1038/s41598-019-46960-9 .
Article CAS PubMed PubMed Central Google Scholar
Scott JC. Against the grain: a deep history of the earliest states. New Haven, CT: Yale University Press; 2017.
Wiessner P. Levelling the hunter: constraints on the social quest in foraging soceties. In: Wiessner P, Schiefenhovel W, editors. Food and the status quest: an interdisciplinary perspective. Providence,RI: Bergahn Books; 1997. p. 171–91.
Graeber D. Debt: the first 5,000 years. London: Melville House; 2011.
Barnard CJ, Sibly RM. Producers and scroungers: a general model and its application to captive flocks of house sparrows. Anim Behav. 1981;29:543–50.
King AJ, Isaac NJ, Cowlishaw G. Ecological, social and reproductive factors shape producer-scrounger dynamics in baboons. Behav Ecol. 2009;20:1039–49.
Giraldeau LA, Caraco T. Social foraging theory. Princeton, NJ: Princeton University Press; 2000.
Roper B. The history of democracy: a Marxist interpretation. London: Pluto Press; 2013.
Bernstein W. A splendid exchange: how trade shaped the world. London: Atlantic Books; 2008.
Wolf E. Europe and the people without history Berkeley: University of California press; 1982.
Mintz SW. Sweetness and power: the place of sugar in modern history. New York: VikingPenguin Inc; 1985.
Townsend J. A dissertation on the poor Laws by a well-wisher to mankind. Berkeley, CA: University of California Press; 1786/1971.
Dwork D. War is good for babies and other young children: a history of the infant and child welfare movement in England 1898–1918. London: Tavistock Publications; 1987.
BMJ. Physical degeneration. Br Med J 1903/1904:1903: 338–341; 430–431; 471–474. 4: 86–88; 140–142.
Staples AL. The birth of development: how the World Bank, food and agriculture organization, and World Health Organization changed the world, 1945–1965. Kent OH: Kent State University Press; 2006.
Escobar A. Encountering development: the making an unmaking of the third world. Princeton, NJ: Princeton University Press; 1995.
Fowler C, Mooney P. The threatened gene : food, politics and the loss of genetic diversity Cambridge: Lutterworth; 1990.
Paul H, Steinbrecher R. Hungry corporations: transnational biotech companies colonise the food chain. London: Zed Books; 2003.
Conard NJ. A female figurine from the basal Aurignacian of Hohle Fels cave in southwestern Germany. Nature. 2009;459(7244):248–52. https://doi.org/10.1038/nature07995 .
Papavramidou N, Christopoulou-Aletra H. Greco-Roman and byzantine views on obesity. Obes Surg. 2007;17(1):112–6. https://doi.org/10.1007/s11695-007-9017-2 .
Komlos J, Brabec M. The trend of mean BMI values of US adults, birth cohorts 1882-1986 indicates that the obesity epidemic began earlier than hitherto thought. Am J Hum Biol. 2010;22(5):631–8. https://doi.org/10.1002/ajhb.21055 .
NCD Risk Factor Collaboration. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–42. https://doi.org/10.1016/S0140-6736(17)32129-3 .
Jones-Smith JC, Gordon-Larsen P, Siddiqi A, Popkin BM. Is the burden of overweight shifting to the poor across the globe? Time trends among women in 39 low- and middle-income countries (1991-2008). Int J Obes. 2012;36(8):1114–20. https://doi.org/10.1038/ijo.2011.179 .
Jaacks LM, Vandevijvere S, Pan A, McGowan CJ, Wallace C, Imamura F, et al. The obesity transition: stages of the global epidemic. Lancet Diabetes Endocrinol. 2019;7(3):231–40. https://doi.org/10.1016/S2213-8587(19)30026-9 .
James WP, Ferro-Luzzi A, Waterlow JC. Definition of chronic energy deficiency in adults. Report of a working party of the international dietary energy consultative group. Eur J ClinNutr. 1988;42(12):969–81.
CAS Google Scholar
Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382(9890):427–51. https://doi.org/10.1016/S0140-6736(13)60937-X .
Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U. Lancet physical activity series working G. global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. 2012;380(9838):247–57. https://doi.org/10.1016/S0140-6736(12)60646-1 .
Diet Collaborators GBD. Health effects of dietary risks in 195 countries, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2019;393(10184):1958–72. https://doi.org/10.1016/S0140-6736(19)30041-8 .
Harper KM, Mutasa M, Prendergast AJ, Humphrey J, Manges AR. Environmental enteric dysfunction pathways and child stunting: a systematic review. PLoS Negl Trop Dis. 2018;12(1):e0006205. https://doi.org/10.1371/journal.pntd.0006205 .
Wells JCK, Briend A, Boyd EM, Berkely JA, Hall A, Isanaka S, et al. Beyond wasted and stunted-a major shift to fight child undernutrition. Lancet Child Adolesc Health. 2019;3(11):831–4. https://doi.org/10.1016/S2352-4642(19)30244-5 .
Schoenbuchner SM, Dolan C, Mwangome M, Hall A, Richard SA, Wells JC, et al. The relationship between wasting and stunting: a retrospective cohort analysis of longitudinal data in Gambian children from 1976 to 2016. Am J Clin Nutr. 2019;110(2):498–507. https://doi.org/10.1093/ajcn/nqy326 .
Khara T, Mwangome M, Ngari M, Dolan C. Children concurrently wasted and stunted: a meta-analysis of prevalence data of children 6-59 months from 84 countries. Matern Child Nutr. 2018;14(2):e12516. https://doi.org/10.1111/mcn.12516 .
Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371(9609):340–57.
Martorell R. Improved nutrition in the first 1000 days and adult human capital and health. Am J Hum Biol. 2017;29(2):e22952. https://doi.org/10.1002/ajhb.22952 .
Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B. Group. ICDS. Developmental potential in the first 5 years foir children in developing countries. Lancet. 2007;369(9555):60–70.
Wells JCK, Devakumar D, Manandhar DS, Saville N, Chaube SS, Costello A, et al. Associations of stunting at 2 years with body composition and blood pressure at 8 years of age: longitudinal cohort analysis from lowland Nepal. Eur J Clin Nutr. 2019;73(2):302–10. https://doi.org/10.1038/s41430-018-0291-y .
Mera RM, Bravo LE, Goodman KJ, Yepez MC, Correa P. Long-term effects of clearing helicobacter pylori on growth in school-age children. Pediatr Infect Dis J. 2012;31(3):263–6. https://doi.org/10.1097/INF.0b013e3182443fec .
Brennan L, McDonald J, Shlomowitz R. Teenage births and final adult height of mothers in India, 1998-1999. J Biosoc Sci. 2005;37(2):185–91. https://doi.org/10.1017/s0021932003006515 .
Wells JC, Chomtho S, Fewtrell MS. Programming of body composition by early growth and nutrition. ProcNutrSoc. 2007;66(3):423–34.
Mathis D. Immunological goings-on in visceral adipose tissue. Cell Metab. 2013;17(6):851–9. https://doi.org/10.1016/j.cmet.2013.05.008 .
Wells JC. Ethnic variability in adiposity and cardiovascular risk: the variable disease selection hypothesis. IntJ Epidemiol. 2009;38:63–71.
Wells JCK, Cole TJ, Cortina-Borja M, Sear R, Leon DA, Marphatia AA, et al. Low maternal capital predicts life history trade-offs in daughters: why adverse outcomes cluster in individuals. Front Public Health. 2019;7:206. https://doi.org/10.3389/fpubh.2019.00206 .
Richardson KJ, Lewis KH, Krishnamurthy PK, Kent C, Wiltshire AJ, Hanlon HM. Food security outcomes under a changing climate: impacts of mitigation and adaptation on vulnerability to food security. Climactic Change. 2018;147(1–2):327–41.
UNICEF. Percentage of women aged 20 to 24 years who were first married or in union before age 15; percentage of women and percentage of men aged 20 to 24 years who were first married or in union before age 18. UNICEF, 2019.
Reports U-HD. Gender inequality index. UNDP, 2017.
Foundation WF. The global slavery index. Western Australia: Minderoo Foundation; 2018.
Abouharb MR, Cingranelli D. Methods. Edtion ed. Human Rights and Structural Adjustment. Cambridge, UK: Cambridge University Press; 2007. p. 81–104.
IMF. Monitoring of Fund Arrangements (MONA) database. 2002-Current Arrangements and Archived MONA data (1993-2003). Washington DC: IMF; 2019.
Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. 2012;70(1):3–21. https://doi.org/10.1111/j.1753-4887.2011.00456.x .
Baker P, Friel S. Processed foods and the nutrition transition: evidence from Asia. Obes Rev. 2014;15(7):564–77. https://doi.org/10.1111/obr.12174 .
Moubarac JC, Batal M, Louzada ML, Martinez Steele E, Monteiro CA. Consumption of ultra-processed foods predicts diet quality in Canada. Appetite. 2017;108:512–20. https://doi.org/10.1016/j.appet.2016.11.006 .
Aitsi-Selmi A. Households with a stunted child and obese mother: trends and child feeding practices in a middle-income country, 1992-2008. Matern Child Health J. 2015;19(6):1284–91. https://doi.org/10.1007/s10995-014-1634-5 .
Pries A. Snacks and nutrition during the complementary feeding period: a cross-sectional study among children 12–23 months of age in Kathmandu Valley. Nepal: London School of Hygiene & Tropical Medicine; 2019.
Sekiyama M, Roosita K, Ohtsuka R. Snack foods consumption contributes to poor nutrition of rural children in West Java, Indonesia. Asia Pac J Clin Nutr. 2012;21(4):558–67.
CAS PubMed Google Scholar
Ribas SA, de Rodrigues MC, Mocelin MC, Marques ES, da Rosa GP, Maganha CR. Quality of complementary feeding and its effect on nutritional status in preterm infants: a cross-sectional study. Journal of human nutrition and dietetics : the official journal of the British Dietetic Association 2020;in press.
Holmes MD, Dalal S, Sewram V, Diamond MB, Adebamowo SN, Ajayi IO, et al. Consumption of processed food dietary patterns in four African populations. Public Health Nutr. 2018;21(8):1529–37. https://doi.org/10.1017/S136898001700386X .
Zhou Y, Du S, Su C, Zhang B, Wang H, Popkin BM. The food retail revolution in China and its association with diet and health. Food Policy. 2015;55:92–100. https://doi.org/10.1016/j.foodpol.2015.07.001 .
Canhada SL, Luft VC, Giatti L, Duncan BB, Chor D, Fonseca M, et al. Ultra-processed foods, incident overweight and obesity, and longitudinal changes in weight and waist circumference: the Brazilian longitudinal study of adult health (ELSA-Brasil). Public Health Nutr. 2019:1–11. https://doi.org/10.1017/S1368980019002854 .
Baker P, Kay A, Walls H. Trade and investment liberalization and Asia's noncommunicable disease epidemic: a synthesis of data and existing literature. Global Health. 2014;10:66. https://doi.org/10.1186/s12992-014-0066-8 .
Labonte R, Mohindra KS, Lencucha R. Framing international trade and chronic disease. Global Health. 2011;7:21. https://doi.org/10.1186/1744-8603-7-21 .
Williamson J. What Washington means by policy reform Edtion ed. In: Williamson J, editor. Latin American adjustment: how much has happened? Washington DC: Institute for International Economics; 1990. p. 5–20.
Jiwani SS, Carrillo-Larco RM, Hernández-Vásquez A, Barrientos-Gutiérrez T, Basto-Abreu A, Gutierrez L, et al. The shift of obesity burden by socioeconomic status between 1998 and 2017 in Latin America and the Caribbean: a cross-sectional series study. Lancet Glob Health. 2019;7(12):e1644–e54.
NCD Risk Factor Collaboration. A century of trends in adult human height. Elife. 2016;5:e13410. https://doi.org/10.7554/eLife.13410 .
N. C. D. Risk Factor Collaboration. Rising rural body-mass index is the main driver of the global obesity epidemic in adults. Nature. 2019;569(7755):260–4. https://doi.org/10.1038/s41586-019-1171-x .
Foucault M. Discipline and punish: the birth of the prison. London: Allen Lane; 1975/1977.
Raubenheimer D, Simpson SJ, Tait AH. Match and mismatch: conservation physiology, nutritional ecology and the timescales of biological adaptation. Philos Trans R Soc Lond B Biol Sci. 2012;367(1596):1628–46. https://doi.org/10.1098/rstb.2012.0007 .
Raubenheimer D, Simpson SJ. Protein leverage: theoretical foundations and ten points of clarification. Obesity. 2019;27(8):1225–38. https://doi.org/10.1002/oby.22531 .
Simpson SJ, Raubenheimer D. The nature of nutrition: a unifying framework from animal adaptation to human obesity. Princeton university press: US; 2012.
Gosby AK, Conigrave AD, Raubenheimer D, Simpson SJ. Protein leverage and energy intake. Obes Rev. 2014;15(3):183–91. https://doi.org/10.1111/obr.12131 .
Felton AM, Felton A, Raubenheimer D, Simpson SJ, Foley WJ, Woods JT, et al. Protein content of diets dictates the daily energy intake of a free-ranging primate. Behavioral Ecology. 2009;20(4):685–90.
Simpson SJ, Raubenheimer D. Obesity: the protein leverage hypothesis. Obes Rev. 2005;6(2):133–42.
Keast RS, Swinburn BA, Sayompark D, Whitelock S, Riddell LJ. Caffeine increases sugar-sweetened beverage consumption in a free-living population: a randomised controlled trial. Br J Nutr. 2015;113(2):366–71. https://doi.org/10.1017/S000711451400378X .
Arora E, Shenoy S, Sandhu JS. Effects of resistance training on metabolic profile of adults with type 2 diabetes. Indian J Med Res. 2009;129(5):515–9.
Article PubMed CAS Google Scholar
DiFeliceantonio AG, Coppin G, Rigoux L, Edwin Thanarajah S, Dagher A, Tittgemeyer M, et al. Supra-additive effects of combining fat and carbohydrate on food reward. Cell Metab. 2018;28(1):33–44 e3. https://doi.org/10.1016/j.cmet.2018.05.018 .
Beaulieu K, Hopkins M, Blundell J, Finlayson G. Homeostatic and non-homeostatic appetite control along the spectrum of physical activity levels: an updated perspective. Physiol Behav. 2018;192:23–9. https://doi.org/10.1016/j.physbeh.2017.12.032 .
Moss M. Salt, sugar, fat: How the food giants hooked us: Random House; 2013.
Raubenheimer D, Simpson SJ. Eat like animals: what nature teaches us about the science of healthy eating. Boston: Houghton Mifflin Harcourt; 2020.
Silva FM, Giatti L, de Figueiredo RC, Molina M, de Oliveira CL, Duncan BB, et al. Consumption of ultra-processed food and obesity: cross sectional results from the Brazilian longitudinal study of adult health (ELSA-Brasil) cohort (2008-2010). Public Health Nutr. 2018;21(12):2271–9. https://doi.org/10.1017/S1368980018000861 .
Siervo M, Gan J, Fewtrell MS, Cortina-Borja M, Wells JCK. Acute effects of video-game playing versus television viewing on stress markers and food intake in overweight and obese young men: a randomised controlled trial. Appetite. 2018;120:100–8. https://doi.org/10.1016/j.appet.2017.08.018 .
Michels N. Biological underpinnings from psychosocial stress towards appetite and obesity during youth: research implications towards metagenomics, epigenomics and metabolomics. Nutr Res Rev. 2019;32(2):282–93.
Epel E, Lapidus R, McEwen B, Brownell KD. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26(1):37–49.
Harding JL, Backholer K, Williams ED, Peeters A, Cameron AJ, Hare MJ, et al. Psychosocial stress is positively associated with body mass index gain over 5 years: evidence from the longitudinal AusDiab study. Obesity. 2014;22(1):277–86. https://doi.org/10.1002/oby.20423 .
Block JP, He Y, Zaslavsky AM, Ding L, Ayanian JZ. Psychosocial stress and change in weight among US adults. Am J Epidemiol. 2009;170(2):181–92.
Brunner EJ, Chandola T, Marmot MG. Prospective effect of job strain on general and central obesity in the Whitehall II study. Am J Epidemiol. 2007;165(7):828–37.
Siervo M, Wells JC, Cizza G. The contribution of psychosocial stress to the obesity epidemic: an evolutionary approach. HormMetab Res. 2009;41(4):261–70.
Siervo M, Wells JCK, Cizza G. Evolutionary theories, psychosocial stress and the modern obesity epidemic. ObesMetab. 2008;4:131–42.
Martinez Steele E, Raubenheimer D, Simpson SJ, Baraldi LG, Monteiro CA. Ultra-processed foods, protein leverage and energy intake in the USA. Public Health Nutr. 2018;21(1):114–24. https://doi.org/10.1017/S1368980017001574 .
Visser SS, Hutter I, Haisma H. Building a framework for theory-based ethnographies for studying intergenerational family food practices. Appetite. 2016;97:49–57.
Perez-Leon S, Pesantes MA, Pastrana NA, Raman S, Miranda J, Suggs LS. Food perceptions and dietary changes for chronic condition Management in Rural Peru: insights for health promotion. Nutrients. 2018;10(11):1563.
Article PubMed Central Google Scholar
Sato PM, Couto MT, Wells J, Cardoso MA, Devakumar D, Scagliusi FB. Mothers' food choices and consumption of ultra-processed foods in the Brazilian Amazon: a grounded theory study. Appetite. 2020;148:104602.
Sato PM, Ulian MD, da Silva Oliveira MS, Cardoso MA, Wells J, Devakumar D, et al. Signs and strategies to deal with food insecurity and consumption of ultra-processed foods among Amazonian mothers. Glob Public Health. 2020;15(8):1130–43.
Atinmo T, Mirmiran P, Oyewole OE, Belahsen R, Serra-Majem L. Breaking the poverty/malnutrition cycle in Africa and the Middle East. Nutrition reviews. 2009;67(Suppl 1):S40–6. https://doi.org/10.1111/j.1753-4887.2009.00158.x .
Mahmudiono T, Segalita C, Rosenkranz RR. Socio-Ecological Model of Correlates of Double Burden of Malnutrition in Developing Countries: A Narrative Review. Int J Environ Res Public Health. 2019;16(19):3730. https://doi.org/10.3390/ijerph16193730 .
Begum S, Sen S. Maternal health, child well-being and chronic poverty: does Women’s agency matter? The Bangladesh Development Studies. 2009;32(4):69–93.
Kraamwinkel N, Ekbrand H, Davia S, Daoud A. The influence of maternal agency on severe child undernutrition in conflict-ridden Nigeria: modeling heterogeneous treatment effects with machine learning. PloS one. 2019;14(1):e0208937.
Horwood C, Haskins L, Alfers L, Masango-Muzindutsi Z, Dobson R, Rollins R. A descriptive study to explore working conditions and childcare practices among informal women workers in KwaZulu-Natal, South Africa: identifying opportunities to support childcare for mothers in informal work. BMC Pediatrics. 2019;19(1):1–11.
Kumeh OW, Fallah MP, Desai IK, Gilbert HN, Silverstein JB, Beste S, et al. Literacy is power: structural drivers of child malnutrition in rural Liberia. BMJ Nutr Prev Health. 2020;3:295–307.
Piperata BA, Salazar M, Schmeer KK, Rodríguez AH. Tranquility is a child with a full belly: pathways linking food insecurity and maternal mental distress in Nicaragua. Ecol Food Nutr. 2020;59(1):79–103.
Tanumihardjo SA, Anderson C, Kaufer-Horwitz M, Bode L, Emenaker NJ, Haqq AM, et al. Poverty, obesity, and malnutrition: an international perspective recognizing the paradox. J Am Diet Assoc. 2007;107(11):1966–72.
Bush R, Martiniello G. Food riots and protest: agrarian modernizations and structural crises. World Development. 2017;91:193–207.
Berazneva J, Lee DR. Explaining the African food riots of 2007–2008: an empirical analysis. Food policy. 2013;39:28–39.
Eagleton D. Power hungry: six reasons to regulate global food corporations. London: ActionAid International; 2005.
Neogy S. Gender inequality, mothers' health, and unequal distribution of food: experience from a CARE project in India. Gender ad Development. 2010;18(3):479–89.
Harris-Fry H, Paudel P, Shrestha N, Harrisson T, Beard BJ, Jha S, et al. Status and determinants of intra-household food allocation in rural Nepal. Eur J Clin Nutr. 2018;72(11):1524–36.
Marphatia AA, Saville NM, Manandhar DS, Cortina-Borja M, Reid AM, Wells JC. Independent associations of women's age at marriage and first pregnancy with their height in rural Nepal. Amercian Journal of Physical Anthropology 2020;in press.
Wells JC. Maternal capital and the metabolic ghetto: an evolutionary perspective on the transgenerational basis of health inequalities. Am J Hum Biol. 2010;22(1):1–17.
Marphatia AA, Cole TJ, Grijalva-Eternod CS, Wells JC. Associations of gender inequality with child malnutrition and mortality across 96 countries. Global Health Epidemiol Genom 2016;in press.
Wells JC, Marphatia AA, Cole TJ, McCoy D. Associations of economic and gender inequality with global obesity prevalence: understanding the female excess. Soc Sci Med. 2012;75(3):482–90. https://doi.org/10.1016/j.socscimed.2012.03.029 .
Madan EM, Haas JD, Menon P, Gillespie S. Seasonal variation in the proximal determinants of undernutrition during the first 1000 days of life in rural South Asia: a comprehensive review. Global Food Security. 2018;19:11–23.
Weatherspoon DD, Miller S, Chrysostome Ngabitsinze J, Weatherspoon LJ, Oehmke JF. Stunting, Food Security, Markets and Food Policy in Rwanda. 2019;19(1):882.
Martıinez-Torres ME, Rosset PM. La Vı’a Campesina: the birth and evolution of a transnational social movement. J Peasant Stud. 2010;37(1):149–75.
Monteiro CA, Conde WL, Lu B, Popkin BM. Obesity and inequities in health in the developing world. IntJ ObesRelat Metab Disord. 2004;28(9):1181–6.
Mascie-Taylor CG, Goto R. Human variaiton and body mass index: a review of the universality of BMI cut-offs, gender and urban-rural differences, and secular changes. J Physiol Anthropol. 2007;26(2):109–12.
Visser SS, Haisma H. Fulfilling food practices: applying the capability approach to ethnographic research in the northern Netherlands. Soc Sci Med. 2021;272:113701.
Pesantes MA, Tetens A, Del Valle A, Miranda JJ. “It is Not Easy Living with This Illness”: A Syndemic Approach to Medication Adherence and Lifestyle Change among Low-income Diabetes Patients in Lima, Peru. Hum Organ. 2019;78(1):85.
Brown PJ, Konner M. An anthropological perspective on obesity. AnnNYAcadSci. 1987;499:29–46.
Pettijohn TF 2nd, Jungeberg BJ. Playboy playmate curves: changes in facial and body feature preferences across social and economic conditions. Pers Soc Psychol Bull. 2004;30(9):1186–97. https://doi.org/10.1177/0146167204264078 .
Hallal PC, Wells JC, Bertoldi AD, Gazalle FK, Silva MC, Domingues MR, et al. A shift in the epidemiology of low body mass index in Brazilian adults. Eur J Clin Nutr. 2005;59(9):1002–6. https://doi.org/10.1038/sj.ejcn.1602204 .
Kickbusch I, Allen L, Franz C. The commercial determinants of health. Lancet Glob Health. 2016;4(12):e895–e6. https://doi.org/10.1016/S2214-109X(16)30217-0 .
Monteiro CA, Cannon G, Levy RB, Moubarac JC, Louzada ML, Rauber F, et al. Ultra-processed foods: what they are and how to identify them. Public Health Nutr. 2019;22(5):936–41. https://doi.org/10.1017/S1368980018003762 .
Kwate NO, Yau CY, Loh JM, Williams D. Inequality in obesigenic environments: fast food density in new York City. Health Place. 2009;15(1):364–73. https://doi.org/10.1016/j.healthplace.2008.07.003 .
Saunders P, Saunders A, Middleton J. Living in a 'fat swamp': exposure to multiple sources of accessible, cheap, energy-dense fast foods in a deprived community. Br J Nutr. 2015;113(11):1828–34. https://doi.org/10.1017/S0007114515001063 .
Day PL, Pearce J. Obesity-promoting food environments and the spatial clustering of food outlets around schools. Am J Prev Med. 2011;40(2):113–21. https://doi.org/10.1016/j.amepre.2010.10.018 .
Sturm R. Disparities in the food environment surrounding US middle and high schools. Public Health. 2008;122(7):681–90. https://doi.org/10.1016/j.puhe.2007.09.004 .
Cummins S, Smith DM, Aitken Z, Dawson J, Marshall D, Sparks L, et al. Neighbourhood deprivation and the price and availability of fruit and vegetables in Scotland. J Hum Nutr Diet. 2010;23(5):494–501. https://doi.org/10.1111/j.1365-277X.2010.01071.x .
McKee M, Stuckler D. Revisiting the corporate and commercial determinants of health. Am J Public Health. 2018;108(9):1167–70. https://doi.org/10.2105/AJPH.2018.304510 .
Murphy S, Hansen-Kuhn K. The true costs of US agriultural dumping. Renewable Agriculture and Food Systems 2019;in press.
Roberto CA, Swinburn B, Hawkes C, Huang TT, Costa SA, Ashe M, et al. Patchy progress on obesity prevention: emerging examples, entrenched barriers, and new thinking. Lancet. 2015;385(9985):2400–9. https://doi.org/10.1016/S0140-6736(14)61744-X .
Allen LN, Hatefi A, Feigl AB. Corporate profits versus spending on non-communicable disease prevention: an unhealthy balance. Lancet Glob Health. 2019;7(11):e1482–e3. https://doi.org/10.1016/S2214-109X(19)30399-7 .
Bowles S, Gintis H. Democracy and capitalism: property, community, and the contradictions of modern social thought. London: Routledge & Kegan Paul; 1986.
Nestle M. Food politics: how the food industry influences nutrition and health. Berkeley: University of California Press; 2007.
Steele S, Ruskin G, Sarcevic L, McKee M, Stuckler D. Are industry-funded charities promoting "advocacy-led studies" or "evidence-based science"?: a case study of the international Life Sciences Institute. Global Health. 2019;15(1):36. https://doi.org/10.1186/s12992-019-0478-6 .
Tanrikulu H, Neri D, Robertson A, Mialon H. Corporate political activity of the baby food industry: the example of Nestle in the United States of America. Int Breastfeed J. 2020;15(1):22.
Loopstra R, Reeves A, Taylor-Robinson D, Barr B, McKee M, Stuckler D. Austerity, sanctions, and the rise of food banks in the UK. BMJ. 2015;350:h1775. https://doi.org/10.1136/bmj.h1775 .
Rada AG. Child poverty and malnutrition rise in Spain as austerity measures bite. BMJ. 2013;347:5261.
Power SB. Dynamics in 21st-Century Food Systems. Nutrients. 2019;11(10):2544. https://doi.org/10.3390/nu11102544 .
He FJ, Pombo-Rodrigues S, MacGregor GA. Salt reduction in England from 2003 to 2011: its relationship to blood pressure, stroke and ischaemic heart disease mortality. BMJ open. 2014;4:e004549.
Meyers DG, Neuberger JS, He J. Cardiovascular effect of bans on smoking in public places: a systematic review and meta-analysis. J Am Coll Cardiol. 2009;54(14):1249–55. https://doi.org/10.1016/j.jacc.2009.07.022 .
Restrepo BJ, Rieger M. Trans fat and cardiovascular disease mortality: evidence from bans in restaurants in New York. J Health Econ. 2016;45:176–96. https://doi.org/10.1016/j.jhealeco.2015.09.005 .
Thomson H. Food and power: regime type, agricultural policy, and political stability. Cambridge: Cambridge University Press; 2019.
Action against hunger. The geopolitics of hunger, 2000–2001: hunger and power. Boulder, Colorado: Lynne Rienner Publishers, 2001.
Friedman M. Capitalism and freedom. Chicago: University of Chicago Press; 1962.
Saad-Filho A, Johnston D. Introduction. In: Saad-Filho A, Johnston D, editors. Neoliberalism – A Critical Reader. London: Pluto Press; 2005. p. 1–6.
Harvey D. A brief history of neoliberalism. Oxford: Oxford University Press; 2005.
Foucault M. The birth of biopolitics: lectures at the Collège de France, 1978–1979. New York: Palgrave Macmillan; 2008.
Thorsen DE, Lie A. What is neoliberalism. Oslo: University of Oslo, Department of Political Science; 2006.
Ferguson J. The uses of neoliberalism. Anitipode. 2010;41:166–84.
Labonté R, Stuckler D. The rise of neoliberalism: how bad economics imperils health and what to do about it. J Epidemiol Community Health. 2016;70(3):312–8.
Pfeiffer J, Chapman R. Anthropological perspectives on structural adjustment and public health. Ann Rev Anthropol. 2010;39:149–65.
Pinstrup-Andersen P. Macroeconomic adjustment policies and human nutrition: available evidence and research needs. Food Nutr Bull. 1987;9(1):1–19.
Summers L, Pritchett L. The structural-adjustment debate. Am Econ Rev. 1993;83:383–9.
Kentikelenis A, Stubbs T, King L. IMF conditionality and development policy space. Rev Int Polit Econ. 2016;23:543–82.
Babb S, Carruthers B. Conditionality: forms, function, and history. Annu Rev Law Soc Sci. 2008;4:13–29.
Babu S, Gajanan SN, Hallam JA. Nutrition economics: Principles and policy applications. London: Elsevier; 2016. 2016
Cornia GA, Deotti L, Sassi M. Food price volatility over the last decade in Niger and Malawi: extent, sources and impact on child malnutrition. Working paper WP 2012–002. New York: United Nations Development Programme; 2012.
Thow AM. Trade liberalisation and the nutrition transition: mapping the pathways for public health nutritionists. Public Health Nutr. 2009;12(11):2150–8. https://doi.org/10.1017/S1368980009005680 .
Pandolfelli L, Shandra J, Tyagi J. The international monetary fund, structural adjustment, and women's health: a cross-national analysis of maternal mortality in sub-Saharan Africa. Sociol Q. 2014;55:119–42.
Shandra C, Shandra J, London B. The international monetary fund, structural adjustment, and infant mortality: a cross-national analysis of sub-Saharan Africa. J Poverty. 2012;16:194–219.
Marphatia AA. The adverse effects of International Monetary Fund programs on the health and education workforce. Int J Health Serv. 2010;40(1):165–78. https://doi.org/10.2190/HS.40.1.j .
Forster T, Kentikelenis AE, Stubbs TH, King LP. Globalization and health equity: the impact of structural adjustment programs on developing countries. Soc Sci Med. 2019;267:112496. https://doi.org/10.1016/j.socscimed.2019.112496 .
Kentikelenis AE, Stubbs TH, King LP. Structural adjustment and public spending on health: Evidence from IMF programs in low-income countries. Soc Sci Med. 2015;126:169–76.
Ismi A. Impoverishing a continent: the World Bank and the IMF in Africa. Canadian Centre for Policy Alternatives: Halifax; 2004.
Thomson M, Kentikelenis A, Stubbs T. Structural adjustment programmes adversely affect vulnerable populations: a systematic-narrative review of their effect on child and maternal health. Public Health Rev. 2017;38:13. https://doi.org/10.1186/s40985-017-0059-2 .
Daoud A, Nosrati E, Reinsberg B, Kentikelenis AE, Stubbs TH, King LP. Impact of Internaitonal monetary fund programs on child health. PNAS. 2017;114(25):6492–7.
Pongou R, Salomon JA, Ezzati M. Health impacts of macroeconomic crises and policies: determinants of variation in childhood malnutrition trends in Cameroon. Int J Epidemiol. 2006;35(3):648–56. https://doi.org/10.1093/ije/dyl016 .
Pfeiffer J. Cash income, intrahousehold cooperative conflict, and child health in Central Mozambique. Medical Anthropol. 2003;22(2):87–130.
Due JM, Gladwin CH. Impacts of structural adjustment programs on African women farmers and female-headed households. Am J Agric Econ. 1991;73(5):1431–9.
Sparr P. Mortgaging women's lives: feminist critiques of structural adjustment. London: Zed Books; 1994.
Rowden R. The deadly ideas of neoliberalism: how the IMF has undermined public health and the fight against AIDS. London: Zed Books; 2013.
Reinsberg B, Kentikelenis A, Stubbs T, King L. The world system and the hollowing out of state capacity: How structural adjustment programs affect bureaucratic quality in developing countries. Am J Sociol. 2019;124(4):1222–57.
Hoey L. Reclaiming the authority to plan: how the legacy of structural adjustment affected Bolivia’s effort to recentralize nutrition planning. World Development. 2017;91:100–12.
Phillips T, Ravuvu A, McMichael C, Thow AM, Browne J, Waqa G, et al. Nutrition policy-making in Fiji: working in and around neoliberalisation in the global south. Critical Public Health. 2019;31(3):316–26.
Raschke V, Cheema B. Colonisation, the New World order, and the eradication of traditional food habits in East Africa: historical perspective on the nutrition transition. Public Health Nutr. 2008;11(7):662–74. https://doi.org/10.1017/S1368980007001140 .
Friel S, Ford L. Systems, food security and human health. Food Security. 2015;7(2):437–51.
McNeill D, Barlow P, Birkbech CD, Fukuda-Parr S, Grover A, Schrecker T, et al. Trade and investment agreements: implications for health protection. J World Trade. 2017;51(1):159–82.
Adjaye-Gbewonyo K, Vollmer S, Avendano M, Harttgen K. Agricultural trade policies and child nutrition in low-and middle-income countries: a cross-national analysis. Global Health. 2019;15(1):21.
Monteiro CA, Levy RB, Claro RM, de Castro IR, Cannon G. Increasing consumption of ultra-processed foods and likely impact on human health: evidence from Brazil. Public Health Nutr. 2011;14(1):5–13. https://doi.org/10.1017/S1368980010003241 .
Schram A, Labonte R, Baker P, Friel S, Reeves A, Stuckler D. The role of trade and investment liberalization in the sugar-sweetened carbonated beverages market: a natural experiment contrasting Vietnam and the Philippines. Globalization and health. 2015;11:41. https://doi.org/10.1186/s12992-015-0127-7 .
Baker P, Friel S, Schram A, Labonte R. Trade and investment liberalization, food systems change and highly processed food consumption: a natural experiment contrasting the soft-drink markets of Peru and Bolivia. Global Health. 2016;12(1):24. https://doi.org/10.1186/s12992-016-0161-0 .
Wells JC. Obesity as malnutrition: the dimensions beyond energy balance. Eur J Clin Nutr. 2013;67(5):507–12. https://doi.org/10.1038/ejcn.2013.31 .
Hawkes C. Uneven dietary development: linking the policies and processes of globalization with the nutrition transition, obesity and diet-related chronic diseases. Global Health. 2006;2:4. https://doi.org/10.1186/1744-8603-2-4 .
Eberhardt P, Olivet C. Modern pirates: How arbitration lawyers help corporations seize National Assets and limit state autonomy. Am J Econ Sociol. 2018;77:279–329.
Labonté R, Crosbie E, Gleeson D, McNamara C. USMCA (NAFTA 2.0): tightening the constraints on the right to regulate for public health. Global Health. 2019;15(1):1–15.
Rimpeekool W, Seubsman SA, Banwell C, Kirk M, Yiengprugsawan V, Sleigh A. Food and nutrition labelling in Thailand: a long march from subsistence producers to international traders. Food policy. 2015;56:59–66. https://doi.org/10.1016/j.foodpol.2015.07.011 .
Crosbie E, Sosa P, Glantz SA. Defending strong tobacco packaging and labelling regulations in Uruguay: transnational tobacco control network versus Philip Morris International. Tobacco control. 2018;27(2):185–94. https://doi.org/10.1136/tobaccocontrol-2017-053690 .
Collier S. The spatial forms and social norms of ‘actually existing neoliberalism’: toward a substantive analytics international affairs working paper; 2005.
Ruckert A, Labonté R. Health inequities in the age of austerity: the need for social protection policies. Soc Sci Med. 2017;187:306–11.
Larner W. Neoliberalism? Environ Plan D. 2003;21:509–12.
Almeida P. Unintended consequences of state-led development: a theory of collective opposition to neoliberalism. Sociol Dev. 2015;1(2):259–76.
Ottersen OP, Dasgupta J, Blouin C, Buss P, Chongsuvivatwong V, Frenk J, et al. The political origins of health inequity: prospects for change. Lancet. 2014;383(9917):630–67. https://doi.org/10.1016/S0140-6736(13)62407-1 .
Mozaffarian D, Angell SY, Lang T, Rivera JA. Role of government policy in nutrition-barriers to and opportunities for healthier eating. BMJ. 2018;361:k2426. https://doi.org/10.1136/bmj.k2426 .
Nestle M. A call for food system change. Lancet. 2020;395(10238):1685–6.
Article CAS PubMed Central Google Scholar
Huizar MI, Arena R, Laddu DR. The global food syndemic: the impact of food insecurity, malnutrition and obesity on the healthspan amid the COVID-19 pandemic. Prog Cardiovasc Dis. 2020. https://doi.org/10.1016/j.pcad.2020.07.002 .
Kinsey EW, Kinsey D, Rundle AG. COVID-19 and food insecurity: an uneven patchwork of responses. J Urban Health. 2020;97(3):332–5. https://doi.org/10.1007/s11524-020-00455-5 .
Scarmozzino F, Visioli F. Covid-19 and the Subsequent Lockdown Modified Dietary Habits of Almost Half the Population in an Italian Sample. Foods. 2020;9(5):675. https://doi.org/10.3390/foods9050675 .
Pellegrini M, Ponzo V, Rosato R, Scumaci E, Goitre I, Benso A, et al. Changes in Weight and Nutritional Habits in Adults with Obesity during the "Lockdown" Period Caused by the COVID-19 Virus Emergency. Nutrients. 2020;12(7):2016. https://doi.org/10.3390/nu12072016 .
Sidor A, Rzymski P. Dietary Choices and Habits during COVID-19 Lockdown: Experience from Poland. Nutrients. 2020;12(6):1657. https://doi.org/10.3390/nu12061657 .
Rodriguez-Perez C, Molina-Montes E, Verardo V, Artacho R, Garcia-Villanova B, Guerra-Hernandez EJ, et al. Changes in Dietary Behaviours during the COVID-19 Outbreak Confinement in the Spanish COVIDiet Study. Nutrients. 2020;12(6):1730. https://doi.org/10.3390/nu12061730 .
Di Renzo L, Gualtieri P, Pivari F, Soldati L, Attina A, Cinelli G, et al. Eating habits and lifestyle changes during COVID-19 lockdown: an Italian survey. J Transl Med. 2020;18(1):229. https://doi.org/10.1186/s12967-020-02399-5 .
Ammar A, Brach M, Trabelsi K, Chtourou H, Boukhris O, Masmoudi L, et al. Effects of COVID-19 Home Confinement on Eating Behaviour and Physical Activity: Results of the ECLB-COVID19 International Online Survey. Nutrients. 2020;12(6):1583. https://doi.org/10.3390/nu12061583 .
Ruiz-Roso MB, de Carvalho PP, Mantilla-Escalante DC, Ulloa N, Brun P, Acevedo-Correa D, et al. Covid-19 Confinement and Changes of Adolescent's Dietary Trends in Italy, Spain, Chile, Colombia and Brazil. Nutrients. 2020;12(6):1807. https://doi.org/10.3390/nu12061807 .
Franco JC. "If the virus doesn't kill me...": socioeconomic impacts of COVID-19 on rural working people in the Global South. Agric Human Values. 2020;12:1–2. https://doi.org/10.1007/s10460-020-10073-1 .
Akseer N, Kandru G, Keats EC, Bhutta ZA. COVID-19 pandemic and mitigation strategies: implications for maternal and child health and nutrition. Am J Clin Nutr. 2020;112(2):251–6. https://doi.org/10.1093/ajcn/nqaa171 .
FAO, IFAD, UNICEF, WFP, WHO. The state of food security and nutrition in the world 2020: transforming food systems for affordable healthy diets. FAO: Rome; 2020.
McLinden T, Stover S, Hogg RS. HIV and food insecurity: a Syndemic amid the COVID-19 pandemic. AIDS and behavior. 2020;24(10):2766–9. https://doi.org/10.1007/s10461-020-02904-3 .
van Tulleken C, Wright C, Brown A, McCoy D, Costello A. Marketing of breastmilk substitutes during the COVID-19 pandemic. Lancet. 2020;396:e58.
White M, Nieto C, Barquera S. Good deeds and cheap marketing: the food industry in the time of COVID-19. Obesity. 2020;28(9):1578–9.
Ruíz-Roso MB, de Carvalho PP, Matilla-Escalante DC, Brun P, Ulloa N, Acevedo-Correa D, et al. Changes of Physical Activity and Ultra-Processed Food Consumption in Adolescents from Different Countries during Covid-19 Pandemic: An Observational Study. Nutrients. 2020;12(8):2289.
Article PubMed Central CAS Google Scholar
Deng M, Qi Y, Deng L, Wang H, Xu Y, Li Z, et al. Obesity as a potential predictor of disease severity in young COVID-19 patients: a retrospective study. Obesity. 2020;28(10):1815–25. https://doi.org/10.1002/oby.22943 .
Cai Q, Chen F, Wang T, Luo F, Liu X, Wu Q, et al. Obesity and COVID-19 severity in a designated Hospital in Shenzhen. China. Diab Care. 2020;43(7):1392–8. https://doi.org/10.2337/dc20-0576 .
Lighter J, Phillips M, Hochman S, Sterling S, Johnson D, Francois F, et al. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin Infect Dis. 2020;71(15):896–7. https://doi.org/10.1093/cid/ciaa415 .
Briguglio M, Pregliasco FE, Lombardi G, Perazzo P, Banfi G. The Malnutritional status of the host as a virulence factor for new coronavirus SARS-CoV-2. Front Med. 2020;7:146. https://doi.org/10.3389/fmed.2020.00146 .
Briend A, Lacsala R, Prudhon C, Mounier B, Grellety Y, Golden MH. Ready-to-use therapeutic food for treatment of marasmus. Lancet. 1999;353(9166):1767–8. https://doi.org/10.1016/S0140-6736(99)01078-8 .
Gehrig JL, Venkatesh S, Chang HW, Hibberd MC, Kung VL, Cheng J, et al. Effects of microbiota-directed foods in gnotobiotic animals and undernourished children. Science. 2019;365(6449):eaau4732. https://doi.org/10.1126/science.aau4732 .
Fabiansen C, Yameogo CW, Iuel-Brockdorf AS, Cichon B, Rytter MJH, Kurpad A, et al. Effectiveness of food supplements in increasing fat-free tissue accretion in children with moderate acute malnutrition: a randomised 2 x 2 x 3 factorial trial in Burkina Faso. PLoS medicine. 2017;14(9):e1002387. https://doi.org/10.1371/journal.pmed.1002387 .
Fabiansen C, Phelan KPQ, Cichon B, Yameogo CW, Iuel-Brockdorff AS, Kurpad A, et al. Short Malnourished Children and Fat Accumulation With Food Supplementation. Pediatrics. 2018;142(3):e20180679. https://doi.org/10.1542/peds.2018-0679 .
Wilding JP. Combination therapy for obesity. J Psychopharmacol. 2017;31(11):1503–8. https://doi.org/10.1177/0269881117737401 .
Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934. https://doi.org/10.1136/bmj.f5934 .
Osendarp SJM, Martinez H, Garrett GS, Neufeld LM, De-Regil LM, Vossenaar M, et al. Large-scale food fortification and biofortification in low- and middle-income countries: a review of programs, trends, challenges, and evidence gaps. Food Nutr Bull. 2018;39(2):315–31. https://doi.org/10.1177/0379572118774229 .
Cerri L. Birth of the modern corporation: from servant of the state to semi-sovereign power. Am J Econ Sociol. 2018;77(2):239–77.
Farmer P. Infections and inequalities: the modern plagues. Berkeley: University of California Press; 2001.
Farmer P. Pathologies of power: human health rights and the new war on the poor. Berkeley: University of California Press; 2005.
Farmer P. Challenging orthodoxies: the road ahead for health and human rights. Health Human Rights. 2008;10(1):5–19.
Buse K, Patterson D, Magnusson R. Toebes B. BMJ: Urgent call for human rights guidance on diets and food systems; 2019.
Tomasevski K. The right to food: guide through applicable international law. Dordrecht: Martinus Nijhoff Publishers; 1987.
UN General Assembly. Convention on the Rights of the Child. New York: United Nations; 1989.
Haisma H, Pelto G, Venkatapuram S, Yousefzadeh S, Kramer L, Anand P. Towards a multidimensional index for child growth to combat the double burden of malnutrition. Annals of Nutrition Metabolism 2019;in press.
Haisma H, Yousefzadeh S, Boele Van Hensbroek P. Towards a capability approach to child growth: A theoretical framework. Matern Child Nutr. 2018;14(2):e12534. https://doi.org/10.1111/mcn.12534 .
Taub DR, Miller B, Allen H. Effects of elevated CO2 on the protein concentration of food crops: a meta-analysis. Global Change Biology. 2007;14(3):565–75.
Zhu C, Kobayashi K, Loladze I, Zhu J, Jiang Q, Xu X, et al. Carbon dioxide (CO2) levels this century will alter the protein, micronutrients, and vitamin content of rice grains with potential health consequences for the poorest rice-dependent countries. Science advances. 2018;4(5):eaaq1012. https://doi.org/10.1126/sciadv.aaq1012 .
Scaling Up Nutrition. (accessed 14 Nov 2020).
Download references
Acknowledgements
There was no specific funding for this manuscript.
Author information
Hinke H. Haisma and David Raubenheimer contributed equally to this work.
Authors and Affiliations
Childhood Nutrition Research Centre, Population Policy and Practice Research and Teaching Programme, UCL Great Ormond Street Institute of Child Health, 30 Guilford Street, London, WC1N 1EH, UK
Jonathan C. K. Wells
Department of Geography, University of Cambridge, Cambridge, UK
Akanksha A. Marphatia & Gabriel Amable
School of Life Sciences, University of Nottingham Medical School, Queen’s Medical Centre, Nottingham, UK
Mario Siervo
Department of Nutrition, Exercise and Sports, University of Copenhagen, Copenhagen, Denmark
Henrik Friis
CRONICAS Centre of Excellence in Chronic Diseases, Universidad Peruana Cayetano Heredia, Lima, Peru
J. Jaime Miranda
Department of Medicine, School of Medicine, Universidad Peruana Cayetano Heredia, Lima, Peru
Population Research Centre, Department of Demography, University of Groningen, Groningen, the Netherlands
Hinke H. Haisma
Charles Perkins Centre, University of Sydney, Sydney, Australia
David Raubenheimer
You can also search for this author in PubMed Google Scholar
Contributions
The paper was conceived by JW, DR and HH. JW, DR, HH, AM, JJM, HF and MS drafted the first manuscript. GA generated all the maps. All authors contributed to the subsequent write-up, and commented on and approved the final manuscript.
Corresponding author
Correspondence to Jonathan C. K. Wells .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
HF has received research grants from ARLA Food for Health Centre, and also has research collaboration with Nutriset. All other authors declare no competing interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Wells, J.C.K., Marphatia, A.A., Amable, G. et al. The future of human malnutrition: rebalancing agency for better nutritional health. Global Health 17 , 119 (2021). https://doi.org/10.1186/s12992-021-00767-4
Download citation
Received : 10 January 2021
Accepted : 15 September 2021
Published : 09 October 2021
DOI : https://doi.org/10.1186/s12992-021-00767-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Dual burden of malnutrition
- Undernutrition
- Food systems
- Social inequality
Globalization and Health
ISSN: 1744-8603
- General enquiries: [email protected]
Current Perspective on Malnutrition and Human Health
- First Online: 11 August 2023
Cite this chapter

- Alka Kurmi 11 , 12 ,
- D. K. Jayswal 13 ,
- Dharmendra Saikia 11 &
- Narayan Lal 14
Part of the book series: Sustainable Plant Nutrition in a Changing World ((SPNCW))
Malnutrition is a serious issue that affects the entire world and is brought on by a lack of dietary supplements (protein, vitamins, minerals and nutrients). It directly affects people’s lives and societies, contributing to a variety of health issues as well as reduced learning capacity, work capacity and earning potential. A lack of micronutrients, particularly Zn, Cu, I and Fe, affects about 75% of the world’s population, which can result in a number of health problems. Nutrition is a key variable in the environment that is adaptable and can be used to lower the burden of disease over the course of a person’s lifetime. In order to combat the issues related to malnutrition, numerous studies are being conducted in the fields of medical, agricultural and industrial sciences. These studies focus on improving human health through supplementation and providing the population with the right, sufficient amount of safe nutrients. A sustainable agricultural method known as biofortification is used to increase the concentration of specific micronutrients in staple foods and edible plant portions in order to lower the mortality and morbidity rates associated with malnutrition. According to certain findings, biofortification can also help to prevent micronutrient deficiencies and the risk of harmful metals in plants. Agronomic biofertilisation, conventional plant breeding, genetic engineering, gene modification or manipulation (CRISPR-Cas9) and increased micronutrient content can be achieved using biofortification techniques such as management of metal homeostasis and carrier proteins, which will increase nutrient concentration and production. Therefore, these approaches may be useful in eradicating malnutrition from society.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Similar content being viewed by others

Biofortification: A Remedial Approach Against Malnutrition in Rural and Tribal Population

Micronutrient Deficiencies in Humans and Animals: Strategies for Their Improvement

Role of the Food and Supplement Industries in Human Health
ACC/SCN. (2000). Fourth report on the world nutrition situation: Nutrition throughout the life cycle . ACC/SCN in Collaboration with IFPRI.
Google Scholar
Altarelli, M., Ben-Hamouda, N., Schneider, A., & Berger, M. M. (2019). Copper deficiency: Causes, manifestations, and treatment. Nutrition in Clinical Practice, 34 , 504–513.
Article CAS Google Scholar
Bailey, R. L., West, K. P., Jr., & Black, R. E. (2015). The epidemiology of global micronutrient deficiencies. Annals of Nutrition & Metabolism, 66 (supply 2), 22–33. https://doi.org/10.1159/000371618
Balamurugan, R., Mary, R. R., Chittaranjan, S., et al. (2010). Low levels of fecal lactobacilli in women with iron deficiency anaemia in South India. The British Journal of Nutrition, 104 , 931–934.
Bhutta, Z. A., Das, J. K., Rizvi, A., et al. (2013a). Evidence based interventions for improvement of maternal and child nutrition: What can be done and at what cost? Lancet, 382 , 452–477.
Article Google Scholar
Bhutta, Z. A., Das, J. K., Walker, N., et al. (2013b). Interventions to address deaths from childhood pneumonia and diarrhoea equitably: What works and at what cost? Lancet, 381 , 1417–1429.
Biesalski, H. K. (2014). Hidden hunger . Springer.
Biesalski, H. K. (2016, May). Nutrition meets the microbiome: Micronutrients and the microbiota. Annals of the New York Academy of Sciences, 1372 (1), 53–64. https://doi.org/10.1111/nyas.13145
Biesalski, H. K., & Nohr, D. (2004). New aspects in vitamin a metabolism: The role of retinyl esters as systemic and local sources for retinol in mucous epithelia. The Journal of Nutrition, 134 , 3453S–3457S.
Biesalski, H. K., Sobeck, U., & Weiser, H. (2001). Topical application of vitamin A reverses metaplasia of rat vaginal epithelium: A rapid and efficient approach to improve mucosal barrier function. European Journal of Medical Research, 6 , 391–398.
CAS Google Scholar
Brown, C. C., & Noelle, R. J. (2015). Seeing through the dark: New insights into the immune regulatory functions of vitamin A. European Journal of Immunology, 45 , 1287–1295.
Cantorna, M. T., McDaniel, K., & Bora, S. (2014). Vitamin D, immune regulation, the microbiota, and inflammatory bowel disease. Experimental Biology and Medicine, 239 , 1524–1530.
Cassani, B., Villablanca, E. J., & De Calisto, J. (2012). Vitamin A and immune regulation: Role of retinoic acid in gut associated dendritic cell education, immune protection and tolerance. Molecular Aspects of Medicine, 33 , 63–76.
Caulfield, L. E., Richard, S. A., Rivera, J. A., Musgrove, P., & Black, R. E. (2006). Stunting, wasting, and micronutrient deficiency disorders. In D. T. Jamison, J. G. Breman, & A. R. Measham (Eds.), Disease control priorities in developing countries (2nd ed., pp. 551–568). The International Bank for Reconstruction and Development/The World Bank; Oxford University Press.
Cave, D. P., Abbey, K. L., & Capra, S. M. (2019). Can foodservices in aged care homes deliver sustainable food fortification strategies? A review. International Journal of Food Sciences and Nutrition, 71 , 267–275. [CrossRef].
Cha, H. R., Chang, S., Chang, J., et al. (2010). Downregulation of Th17 cells in the small intestine by disruption of gut flora in the absence of retinoic acid. Journal of Immunology, 184 , 6799–6806.
Committee on Micronutrient Deficiencies, Board on International Health, Food and Nutrition Board, Howson, C. P., Kennedy, E. T., & Horwitz, A. (1998). Prevention of micronutrient deficiencies: Tools for policymakers and public health workers . National Academy Press.
Elia, M. (Ed.). (2000). Guidelines for detection and management of malnutrition. Malnutrition Advisory Group, Standing Committee of BAPEN . BAPEN.
FAO. (2021). The state of food security and nutrition in the world 2021. In Building climate resilience for food security and nutrition . Food and Agriculture Org.
Food and Agriculture Organization of the United Nations (FAO). (2017). The future of food and agriculture – Trends and challenges . FAO.
Forms of malnutrition* highlighted in this key findings report (UNICEF/WHO/World Bank Group Joint Child Malnutrition Estimates Key findings of the 2019 edition).
Frasca, L., & Lande, R. (2012). Role of defensins and cathelicidin LL37 in auto-immune and auto-inflammatory diseases. Current Pharmaceutical Biotechnology, 13 , 1882–1897.
Gibson, R. S. (1994). Zinc nutrition and public health in developing countries. Nutrition Research Reviews, 7 , 151–173.
Gibson, R. S. (2011). Strategies for preventing multi-micronutrient deficiencies: A review of experiences with food-based approaches in developing countries. In B. Thompson & L. Amoroso Wallingford (Eds.), Combating micronutrient deficiencies: Food-based approaches (pp. 7–27). CAB International.
Chapter Google Scholar
Global Hunger Index 2021 report, provided by Smriti Zubin Irani, the Union Minister for Women and Child Development.
Graham, R. D., & Welch, R. M. (1996). Breeding for staple food crops with high micronutrient density (Working papers on agricultural strategies for micronutrients NO 3). International Food policy Research Institute.
Grieger, J. A., & Nowson, C. A. (2009). Use of calcium, folate, and vitamin D3–fortified Milk for 6 months improves nutritional status but not bone mass or turnover, in a group of Australian aged care residents. Journal of Nutrition for the Elderly, 28 , 236–254. [CrossRef] [PubMed].
Hetzel, B. S. (1990). Iodine deficiency: An international public health problem. In M. L. Brown (Ed.), Present knowledge in nutrition (pp. 308–313). International Life Sciences Institute, Nutrition Foundation.
Joachimiak, M. P. (2021). Zinc against COVID-19? Symptom surveillance and deficiency risk groups. PLoS Neglected Tropical Diseases, 15 , e0008895. [CrossRef]).
Katona, P., & Katona-Apte, J. (2008). The interaction between nutrition and infection. Clinical Infectious Diseases, 46 , 1582–1588.
Keflie, T. S., Nolle, N., Lambert, C., et al. (2015). Vitamin D deficiencies among tuberculosis patients in Africa: A systematic review. Nutrition, 31 , 1204–1212.
Koch, M., Biesalski, H. K., Stofft, E., et al. (1990). Crystalloid lysozyme inclusions in Paneth cells of vitamin A deficient rats. Cell and Tissue Research, 260 , 625–628.
Kong, J., Zhang, Z., & Musch, M. W. (2008). Novel role of the vitamin D receptor inmaintaining the integrity of the intestinal mucosal barrier. The American Journal of Physiology-Gastrointestinal and Liver Physiology, 294 , G208–G216.
Lal, N. (2020). Bagging of Fruit Bunches: An eco-friendly approach for quality production and protection from physiological disorder in litchi. Agriculture & Food: E-Newsletter, 2 (11), 1–3.
Levrat, M. A., Remsey, C., & Demigne, C. (1991). High propionic acid fermentations and mineral accumulation in the cecum of rats adapted to different levels of inulin. The Journal of Nutrition, 121 , 1730–1737.
Liu, L., Johanson, H. L., Cousens, S., et al. (2012). Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet, 379 , 2151–2161.
Martorell, R. (2007). Effects of malnutrition on health and human development and effective strategies for its prevention. Public Health of Mexico, 49 , 151.
McDaniel, K. L., Restori, K. H., Dodds, J. W., et al. (2015). Vitamin A-deficient hosts become nonsymptomatic reservoirs of Escherichia coli-like enteric infections. Infection and Immunity, 83 , 2984–2991.
Meyer-Hoffert, U., et al. (2008). Secreted enteric antimicrobial activity localizes to the mucous surface layer. Gut, 57 , 764–771.
Mora, J. R., Iwata, M., & Andria, U. H. (2008). Vitamin effects on the immune system: Vitamins A and D on the center stage. Nature Reviews Immunology, 8 , 685–692.
Müller, O., & Krawinkel, M. (2005). Malnutrition and health in developing countries. Canadian Medical Association Journal, 173 , 279–286. [CrossRef].
Muthayya, S., Rah, J. H., Sugimoto, J. D., Roos, F. F., Kraemer, K., & Black, R. E. (2013). The global hidden hunger indices and maps: An advocacy tool for action. PLoS One , 8 , No. e67860.
Peumans, W. J., & Van Damme, E. (1995). Lectins as plant defense proteins. Plant Physiology, 109 , 347–352. [CrossRef].
Picciano, M. F. (2003). Pregnancy and lactation: Physiological adjustments, nutritional requirements and the role of dietary supplements. The Journal of Nutrition, 133 , 1997S–2002S.
Pinstrup-Andersen, P. (2007). Agricultural research and policy for better health and nutrition in developing countries: A food systems approach. Agricultural Economics, 37 , 187–198. [CrossRef].
Ramakrishna, B. S. (2013). Role of the gut microbiota in human nutrition and metabolism. Journal of Gastroenterology and Hepatology, 28 , 9–17.
Sirisinha, S. (2015). The pleiotropic role of vitamin A in regulating mucosal immunity. Asian Pacific Journal of Allergy and Immunology, 33 , 71–89.
Smith, L., & Haddad, L. (1999). Explaining child malnutrition in developing countries: A cross-country analysis (FCND discussion paper 1999 (60)). IFPRI.
Stoltzfus, R. J. (2012). Iron and malaria interactions: Programmatic ways forward. Advances in Nutrition, 3 , 579–582.
Stratton, R., Green, C. J., & Elia, M. (2003). Disease related malnutrition: An evidence-based approach to treatment . Cabi Publishing.
Book Google Scholar
United Nations Children’s Fund (UNICEF). (2013). Improving child nutrition: The achievable imperative for global Progress . UNICEF. http://www.unicef.org/infobycountry/indonesia_statistics.html#119 . Accessed 16 Apr 2014
United Nations Millennium Project: Millennium Development Goals. (2000). http://www.unmillenniumproject.org/goals/index.htm . Accessed 9 Apr 2014.
Vaishnava, S., Behrendt, C. L., & Ismail, A. S. (2008). Paneth cells directly sense gut commensals and maintain homoeostasis at the intestinal host–microbial interface. Proceedings. National Academy of Sciences. United States of America, 105 , 20858–20863.
Wisbaum, W. (2011). Child malnutrition. Causes, consequences and strategies for its prevention and treatment . UNICEF.
World Bank. India, Undernourished children: A call for reform and action . Available from: http://web.worldbank.org/WBSITE/EXTERNAL/COUNTRIES/SOUTHASIAEXT/0,contentMDK:20916955~pagePK:146736~piPK:146830~theSitePK:223547,00.html . Last accessed on 05 Apr 2014.
World Health Organization (WHO). (2018). The nutrition challenge: Food system solutions . WHO.
Zlotkin, S. (2011). Micronutrient deficiencies and effect of supplements on correcting them. Nestlé Nutrition Workshop Series. Paediatric Programme, 68 , 127–134; discussion 134–140.
Download references
Author information
Authors and affiliations.
Bioprospection and Product Development Division, CSIR-Central Institute of Medicinal and Aromatic Plants, Lucknow, Uttar Pradesh, India
Alka Kurmi & Dharmendra Saikia
Academy of Scientific and Innovative Research (AcSIR), Ghaziabad, Uttar Pradesh, India
ICAR-National Agricultural Higher Education Project, New Delhi, India
D. K. Jayswal
ICAR-Indian Institute of Soil Science, Bhopal, Madhya Pradesh, India
Narayan Lal
You can also search for this author in PubMed Google Scholar
Editor information
Editors and affiliations.
Academy of Biology and Biotechnology, Southern Federal University, Rostov-on-Don, Russia
Vishnu D. Rajput
Soil & Water Department Faculty of Agriculture, Kafrelsheikh University, Kafr El-Shiek, Egypt
Hassan El-Ramady
University Jaunpur, Veer Bahadur Singh Purvanchal, Jaunpur, Uttar Pradesh, India
Sudhir K. Upadhyay
Tatiana Minkina
School of Chemical Engineering, Yeungnam University, Gyeongsan, Korea (Republic of)
Bilal Ahmed
Saglara Mandzhieva
Rights and permissions
Reprints and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Kurmi, A., Jayswal, D.K., Saikia, D., Lal, N. (2023). Current Perspective on Malnutrition and Human Health. In: Rajput, V.D., El-Ramady, H., Upadhyay, S.K., Minkina, T., Ahmed, B., Mandzhieva, S. (eds) Nano-Biofortification for Human and Environmental Health. Sustainable Plant Nutrition in a Changing World. Springer, Cham. https://doi.org/10.1007/978-3-031-35147-1_9
Download citation
DOI : https://doi.org/10.1007/978-3-031-35147-1_9
Published : 11 August 2023
Publisher Name : Springer, Cham
Print ISBN : 978-3-031-35146-4
Online ISBN : 978-3-031-35147-1
eBook Packages : Biomedical and Life Sciences Biomedical and Life Sciences (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Nutrition and Immunity

During the flu season or times of illness, people often seek special foods or vitamin supplements that are believed to boost immunity. Vitamin C and foods like citrus fruits, chicken soup, and tea with honey are popular examples. Yet the design of our immune system is complex and influenced by an ideal balance of many factors, not just diet, and especially not by any one specific food or nutrient. However, a balanced diet consisting of a range of vitamins and minerals, combined with healthy lifestyle factors like adequate sleep and exercise and low stress, most effectively primes the body to fight infection and disease.
What Is Our Immune System?
On a daily basis, we are constantly exposed to potentially harmful microbes of all sorts. Our immune system, a network of intricate stages and pathways in the body, protects us against these harmful microbes as well as certain diseases. It recognizes foreign invaders like bacteria, viruses, and parasites and takes immediate action. Humans possess two types of immunity: innate and adaptive.
Innate immunity is a first-line defense from pathogens that try to enter our bodies, achieved through protective barriers. These barriers include:
- Skin that keeps out the majority of pathogens
- Mucus that traps pathogens
- Stomach acid that destroys pathogens
- Enzymes in our sweat and tears that help create anti-bacterial compounds
- Immune system cells that attack all foreign cells entering the body
Adaptive or acquired immunity is a system that learns to recognize a pathogen. It is regulated by cells and organs in our body like the spleen, thymus, bone marrow, and lymph nodes. When a foreign substance enters the body, these cells and organs create antibodies and lead to multiplication of immune cells (including different types of white blood cells) that are specific to that harmful substance and attack and destroy it. Our immune system then adapts by remembering the foreign substance so that if it enters again, these antibodies and cells are even more efficient and quick to destroy it.
Other conditions that trigger an immune response
Antigens are substances that the body labels as foreign and harmful, which triggers immune cell activity. Allergens are one type of antigen and include grass pollen, dust, food components, or pet hair. Antigens can cause a hyper-reactive response in which too many white cells are released. People’s sensitivity to antigens varies widely. For example, an allergy to mold triggers symptoms of wheezing and coughing in a sensitive individual but does not trigger a reaction in other people.
Inflammation is an important, normal step in the body’s innate immune response. When pathogens attack healthy cells and tissue, a type of immune cell called mast cells counterattack and release proteins called histamines, which cause inflammation. Inflammation may generate pain, swelling, and a release of fluids to help flush out the pathogens. The histamines also send signals to discharge even more white blood cells to fight pathogens. However, prolonged inflammation can lead to tissue damage and may overwhelm the immune system.
Autoimmune disorders like lupus, rheumatoid arthritis, or type 1 diabetes are partly hereditary and cause hypersensitivity in which immune cells attack and destroy healthy cells.
Immunodeficiency disorders can depress or completely disable the immune system, and may be genetic or acquired. Acquired forms are more common and include AIDS and cancers like leukemia and multiple myeloma. In these cases, the body’s defenses are so reduced that a person becomes highly susceptible to illness from invading pathogens or antigens.
What factors can depress our immune system?
- Older age: As we age, our internal organs may become less efficient; immune-related organs like the thymus or bone marrow produce less immune cells needed to fight off infections. Aging is sometimes associated with micronutrient deficiencies, which may worsen a declining immune function.
- Environmental toxins (smoke and other particles contributing to air pollution, excessive alcohol): These substances can impair or suppress the normal activity of immune cells.
- Excess weight: Obesity is associated with low-grade chronic inflammation. Fat tissue produces adipocytokines that can promote inflammatory processes. [1] Research is early, but obesity has also been identified as an independent risk factor for the influenza virus, possibly due to the impaired function of T-cells, a type of white blood cell. [2]
- Poor diet: Malnutrition or a diet lacking in one or more nutrients can impair the production and activity of immune cells and antibodies.
- Chronic diseases: Autoimmune and immunodeficiency disorders attack and potentially disable immune cells.
- Chronic mental stress: Stress releases hormones like cortisol that suppresses inflammation (inflammation is initially needed to activate immune cells) and the action of white blood cells.
- Lack of sleep and rest: Sleep is a time of restoration for the body , during which a type of cytokine is released that fights infection; too little sleep lowers the amount of these cytokines and other immune cells.
Does an Immune-Boosting Diet Exist?
Eating enough nutrients as part of a varied diet is required for the health and function of all cells, including immune cells. Certain dietary patterns may better prepare the body for microbial attacks and excess inflammation, but it is unlikely that individual foods offer special protection. Each stage of the body’s immune response relies on the presence of many micronutrients. Examples of nutrients that have been identified as critical for the growth and function of immune cells include vitamin C, vitamin D, zinc, selenium, iron, and protein (including the amino acid glutamine). [3,4] They are found in a variety of plant and animal foods.
The microbiome is an internal metropolis of trillions of microorganisms or microbes that live in our bodies, mostly in the intestines. It is an area of intense and active research, as scientists are finding that the microbiome plays a key role in immune function. The gut is a major site of immune activity and the production of antimicrobial proteins. [6,7] The diet plays a large role in determining what kinds of microbes live in our intestines. A high-fiber plant-rich diet with plenty of fruits, vegetables, whole grains, and legumes appear to support the growth and maintenance of beneficial microbes. Certain helpful microbes break down fibers into short chain fatty acids, which have been shown to stimulate immune cell activity. These fibers are sometimes called prebiotics because they feed microbes. Therefore, a diet containing probiotic and prebiotic foods may be beneficial. Probiotic foods contain live helpful bacteria, and prebiotic foods contain fiber and oligosaccharides that feed and maintain healthy colonies of those bacteria.
- Probiotic foods include kefir, yogurt with live active cultures, fermented vegetables, sauerkraut, tempeh, kombucha tea, kimchi, and miso.
- Prebiotic foods include garlic, onions, leeks, asparagus, Jerusalem artichokes, dandelion greens, bananas , and seaweed. However, a more general rule is to eat a variety of fruits, vegetables , beans , and whole grains for dietary prebiotics.

Chicken soup as medicine?
Do vitamin or herbal supplements help.
A deficiency of single nutrients can alter the body’s immune response. Animal studies have found that deficiencies in zinc , selenium , iron , copper, folic acid , and vitamins A , B6 , C , D , and E can alter immune responses. [8] These nutrients help the immune system in several ways: working as an antioxidant to protect healthy cells, supporting growth and activity of immune cells, and producing antibodies. Epidemiological studies find that those who are poorly nourished are at greater risk of bacterial, viral, and other infections.

Spotlight on vitamin D
Eating a good quality diet, as depicted by the Healthy Eating Plate, can prevent deficiencies in these nutrients. However, there are certain populations and situations in which one cannot always eat a variety of nutritious foods, or who have increased nutrient needs. In these cases a vitamin and mineral supplement may help to fill nutritional gaps. Studies have shown that vitamin supplementation can improve immune responses in these groups. [8-10] Low-income households, pregnant and lactating women, infants and toddlers, and the critically ill are examples of groups at risk.
The elderly are a particularly high-risk group. The immune response generally declines with increasing age as the number and quality of immune cells decreases. This causes a higher risk of poorer outcomes if the elderly develop chronic or acute diseases. In addition, about one-third of elderly in industrialized countries have nutrient deficiencies. [8] Some reasons include a poorer appetite due to chronic diseases, depression, or loneliness; multiple medications that can interfere with nutrient absorption and appetite; malabsorption due to intestinal issues; and increased nutrient needs due to hypermetabolic states with acute or chronic conditions. Diet variety may also be limited due to budget constraints or lower interest in cooking for one person; poor dentition; mental impairment; or lack of transportation and community resources to obtain healthy food.
A general multivitamin/mineral supplement providing the recommended dietary allowances (RDA) may be used in these cases, unless otherwise directed by one’s physician. Megadose supplements (many times the RDA) do not appear justified, and can sometimes be harmful or even suppress the immune system (e.g., as with zinc). Remember that vitamin supplements should not be considered a substitute for a good diet because no supplements contain all the benefits of healthful foods.
Several herbal supplements have been suggested to boost immune function. What does the research say?
- Echinacea: Cell studies have shown that echinacea can destroy influenza viruses, but limited research in humans has been inconclusive in determining echinacea’s active components. Taking echinacea after catching a cold has not been shown to shorten its duration, but taking it while healthy may offer a small chance of protection from catching a cold. [11,12]
- Garlic: The active ingredient in garlic, allicin sativum, is proposed to have antiviral and antimicrobial effects on the common cold, but high-quality clinical trials comparing garlic supplements to placebo are lacking. A Cochrane review identified only one trial of reasonable quality following 146 participants. Those taking the garlic supplement for 3 months had fewer occurrences of the common cold than those taking a placebo, but after contracting the cold virus, both groups had a similar duration of illness. [13] Note that these findings are from a single trial, which needs to be replicated.
- Tea catechins: Cell studies have shown that tea catechins such as those found in green tea can prevent flu and some cold viruses from replicating and can increase immune activity. Human trials are still limited. Two randomized controlled trials found that green tea capsules produced less cold/flu symptoms or incidence of flu than a placebo; however both studies were funded or had author affiliations with tea industries. [14]
8 Steps to Help Support a Healthy Immune System
- Eat a balanced diet with whole fruits, vegetables, lean proteins, whole grains, and plenty of water. A Mediterranean Diet is one option that includes these types of foods.
- If a balanced diet is not readily accessible, taking a multivitamin containing the RDA for several nutrients may be used.
- Don’t smoke (or stop smoking if you do).
- Drink alcohol in moderation .
- Perform moderate regular exercise .
- Aim for 7-9 hours of sleep nightly. Try to keep a sleep schedule, waking up and going to bed around the same time each day. Our body clock, or circadian rhythm, regulates feelings of sleepiness and wakefulness, so having a consistent sleep schedule maintains a balanced circadian rhythm so that we can enter deeper, more restful sleep.
- Aim to manage stress . This is easier said than done, but try to find some healthy strategies that work well for you and your lifestyle—whether that be exercise, meditation, a particular hobby, or talking to a trusted friend. Another tip is to practice regular, conscious breathing throughout the day and when feelings of stress arise. It doesn’t have to be long—even a few breaths can help. If you’d like some guidance, try this short mindful breathing exercise .
- Wash hands throughout the day: when coming in from outdoors, before and after preparing and eating food, after using the toilet, after coughing or blowing your nose.
Diet Review: Anti-Inflammatory Diet
A note on COVID-19
Food Safety, Nutrition, and Wellness during COVID-19
Ask the Expert: The role of diet and nutritional supplements during COVID-19
- Childs CE, Calder PC, Miles EA. Diet and Immune Function. Nutrients . 2019 Aug 16;11(8).
- Green WD, Beck MA. Obesity impairs the adaptive immune response to influenza virus. Annals of the American Thoracic Society . 2017 Nov;14(Supplement 5):S406-9.
- Guillin OM, Vindry C, Ohlmann T, Chavatte L. Selenium, selenoproteins and viral infection. Nutrients . 2019 Sep;11(9):2101.
- Wessels I, Maywald M, Rink L. Zinc as a gatekeeper of immune function. Nutrients . 2017 Dec;9(12):1286.
- Molendijk I, van der Marel S, Maljaars PW. Towards a Food Pharmacy: Immunologic Modulation through Diet. Nutrients . 2019 Jun;11(6):1239.
- Caballero S, Pamer EG. Microbiota-mediated inflammation and antimicrobial defense in the intestine. Annual review of immunology . 2015 Mar 21;33:227-56.
- Li XV, Leonardi I, Iliev ID. Gut mycobiota in immunity and inflammatory disease. Immunity . 2019 Jun 18;50(6):1365-79.
- Chandra RK. Nutrition and the immune system: an introduction. The American journal of clinical nutrition . 1997 Aug 1;66(2):460S-3S.
- Hemilä H, Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane database of systematic reviews . 2013(8).
- Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, Esposito S, Ganmaa D, Ginde AA, Goodall EC. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ . 2017 Feb 15;356:i6583.
- National Center for Complementary and Integrative Health. Echinacea. https://www.nccih.nih.gov/health/echinacea . Accessed 4/2/20.
- Karsch‐Völk M, Barrett B, Kiefer D, Bauer R, Ardjomand‐Woelkart K, Linde K. Echinacea for preventing and treating the common cold. Cochrane Database of Systematic Reviews . 2014(2).
- Lissiman E, Bhasale AL, Cohen M. Garlic for the common cold. Cochrane Database of Systematic Reviews . 2014(11).
- Furushima D, Ide K, Yamada H. Effect of tea catechins on influenza infection and the common cold with a focus on epidemiological/clinical studies. Molecules . 2018 Jul;23(7):1795.
Terms of Use
The contents of this website are for educational purposes and are not intended to offer personal medical advice. You should seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. The Nutrition Source does not recommend or endorse any products.
- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- News & Views
- Food and mood: how do...
Food and mood: how do diet and nutrition affect mental wellbeing?
Read our food for thought 2020 collection.
- Related content
- Peer review
This article has a correction. Please see:
- Food and mood: how do diet and nutrition affect mental wellbeing? - November 09, 2020
- Joseph Firth , research fellow 1 2 ,
- James E Gangwisch , assistant professor 3 4 ,
- Alessandra Borsini , researcher 5 ,
- Robyn E Wootton , researcher 6 7 8 ,
- Emeran A Mayer , professor 9 10
- 1 Division of Psychology and Mental Health, Faculty of Biology, Medicine and Health, Oxford Road, University of Manchester, Manchester M13 9PL, UK
- 2 NICM Health Research Institute, Western Sydney University, Westmead, Australia
- 3 Department of Psychiatry, Columbia University Vagelos College of Physicians and Surgeons, New York, USA
- 4 New York State Psychiatric Institute, New York, NY, USA
- 5 Section of Stress, Psychiatry and Immunology Laboratory, Institute of Psychiatry, Psychology and Neuroscience, Department of Psychological Medicine, King’s College London, London, UK
- 6 School of Psychological Science, University of Bristol, Bristol, UK
- 7 MRC Integrative Epidemiology Unit, Oakfield House, Bristol, UK
- 8 NIHR Biomedical Research Centre, University Hospitals Bristol NHS Foundation Trust and University of Bristol, Bristol, UK
- 9 G Oppenheimer Center for Neurobiology of Stress and Resilience, UCLA Vatche and Tamar Manoukian Division of Digestive Diseases, UCLA, Los Angeles, CA, USA
- 10 UCLA Microbiome Center, David Geffen School of Medicine, UCLA, Los Angeles, CA, USA
- Correspondence to: J Firth joseph.firth{at}manchester.ac.uk
Poor nutrition may be a causal factor in the experience of low mood, and improving diet may help to protect not only the physical health but also the mental health of the population, say Joseph Firth and colleagues
Key messages
Healthy eating patterns, such as the Mediterranean diet, are associated with better mental health than “unhealthy” eating patterns, such as the Western diet
The effects of certain foods or dietary patterns on glycaemia, immune activation, and the gut microbiome may play a role in the relationships between food and mood
More research is needed to understand the mechanisms that link food and mental wellbeing and determine how and when nutrition can be used to improve mental health
Depression and anxiety are the most common mental health conditions worldwide, making them a leading cause of disability. 1 Even beyond diagnosed conditions, subclinical symptoms of depression and anxiety affect the wellbeing and functioning of a large proportion of the population. 2 Therefore, new approaches to managing both clinically diagnosed and subclinical depression and anxiety are needed.
In recent years, the relationships between nutrition and mental health have gained considerable interest. Indeed, epidemiological research has observed that adherence to healthy or Mediterranean dietary patterns—high consumption of fruits, vegetables, nuts, and legumes; moderate consumption of poultry, eggs, and dairy products; and only occasional consumption of red meat—is associated with a reduced risk of depression. 3 However, the nature of these relations is complicated by the clear potential for reverse causality between diet and mental health ( fig 1 ). For example, alterations in food choices or preferences in response to our temporary psychological state—such as “comfort foods” in times of low mood, or changes in appetite from stress—are common human experiences. In addition, relationships between nutrition and longstanding mental illness are compounded by barriers to maintaining a healthy diet. These barriers disproportionality affect people with mental illness and include the financial and environmental determinants of health, and even the appetite inducing effects of psychiatric medications. 4

Hypothesised relationship between diet, physical health, and mental health. The dashed line is the focus of this article.
- Download figure
- Open in new tab
- Download powerpoint
While acknowledging the complex, multidirectional nature of the relationships between diet and mental health ( fig 1 ), in this article we focus on the ways in which certain foods and dietary patterns could affect mental health.

Mood and carbohydrates
Consumption of highly refined carbohydrates can increase the risk of obesity and diabetes. 5 Glycaemic index is a relative ranking of carbohydrate in foods according to the speed at which they are digested, absorbed, metabolised, and ultimately affect blood glucose and insulin levels. As well as the physical health risks, diets with a high glycaemic index and load (eg, diets containing high amounts of refined carbohydrates and sugars) may also have a detrimental effect on psychological wellbeing; data from longitudinal research show an association between progressively higher dietary glycaemic index and the incidence of depressive symptoms. 6 Clinical studies have also shown potential causal effects of refined carbohydrates on mood; experimental exposure to diets with a high glycaemic load in controlled settings increases depressive symptoms in healthy volunteers, with a moderately large effect. 7
Although mood itself can affect our food choices, plausible mechanisms exist by which high consumption of processed carbohydrates could increase the risk of depression and anxiety—for example, through repeated and rapid increases and decreases in blood glucose. Measures of glycaemic index and glycaemic load can be used to estimate glycaemia and insulin demand in healthy individuals after eating. 8 Thus, high dietary glycaemic load, and the resultant compensatory responses, could lower plasma glucose to concentrations that trigger the secretion of autonomic counter-regulatory hormones such as cortisol, adrenaline, growth hormone, and glucagon. 5 9 The potential effects of this response on mood have been examined in experimental human research of stepped reductions in plasma glucose concentrations conducted under laboratory conditions through glucose perfusion. These findings showed that such counter-regulatory hormones may cause changes in anxiety, irritability, and hunger. 10 In addition, observational research has found that recurrent hypoglycaemia (low blood sugar) is associated with mood disorders. 9
The hypothesis that repeated and rapid increases and decreases in blood glucose explain how consumption of refined carbohydrate could affect psychological state appears to be a good fit given the relatively fast effect of diets with a high glycaemic index or load on depressive symptoms observed in human studies. 7 However, other processes may explain the observed relationships. For instance, diets with a high glycaemic index are a risk factor for diabetes, 5 which is often a comorbid condition with depression. 4 11 While the main models of disease pathophysiology in diabetes and mental illness are separate, common abnormalities in insulin resistance, brain volume, and neurocognitive performance in both conditions support the hypothesis that these conditions have overlapping pathophysiology. 12 Furthermore, the inflammatory response to foods with a high glycaemic index 13 raises the possibility that diets with a high glycaemic index are associated with symptoms of depression through the broader connections between mental health and immune activation.
Diet, immune activation, and depression
Studies have found that sustained adherence to Mediterranean dietary patterns can reduce markers of inflammation in humans. 14 On the other hand, high calorie meals rich in saturated fat appear to stimulate immune activation. 13 15 Indeed, the inflammatory effects of a diet high in calories and saturated fat have been proposed as one mechanism through which the Western diet may have detrimental effects on brain health, including cognitive decline, hippocampal dysfunction, and damage to the blood-brain barrier. 15 Since various mental health conditions, including mood disorders, have been linked to heightened inflammation, 16 this mechanism also presents a pathway through which poor diet could increase the risk of depression. This hypothesis is supported by observational studies which have shown that people with depression score significantly higher on measures of “dietary inflammation,” 3 17 characterised by a greater consumption of foods that are associated with inflammation (eg, trans fats and refined carbohydrates) and lower intakes of nutritional foods, which are thought to have anti-inflammatory properties (eg, omega-3 fats). However, the causal roles of dietary inflammation in mental health have not yet been established.
Nonetheless, randomised controlled trials of anti-inflammatory agents (eg, cytokine inhibitors and non-steroidal anti-inflammatory drugs) have found that these agents can significantly reduce depressive symptoms. 18 Specific nutritional components (eg, polyphenols and polyunsaturated fats) and general dietary patterns (eg, consumption of a Mediterranean diet) may also have anti-inflammatory effects, 14 19 20 which raises the possibility that certain foods could relieve or prevent depressive symptoms associated with heightened inflammatory status. 21 A recent study provides preliminary support for this possibility. 20 The study shows that medications that stimulate inflammation typically induce depressive states in people treated, and that giving omega-3 fatty acids, which have anti-inflammatory properties, before the medication seems to prevent the onset of cytokine induced depression. 20
However, the complexity of the hypothesised three way relation between diet, inflammation, and depression is compounded by several important modifiers. For example, recent clinical research has observed that stressors experienced the previous day, or a personal history of major depressive disorders, may cancel out the beneficial effects of healthy food choices on inflammation and mood. 22 Furthermore, as heightened inflammation occurs in only some clinically depressed individuals, anti-inflammatory interventions may only benefit certain people characterised by an “inflammatory phenotype,” or those with comorbid inflammatory conditions. 18 Further interventional research is needed to establish if improvements in immune regulation, induced by diet, can reduce depressive symptoms in those affected by inflammatory conditions.
Brain, gut microbiome, and mood
A more recent explanation for the way in which our food may affect our mental wellbeing is the effect of dietary patterns on the gut microbiome—a broad term that refers to the trillions of microbial organisms, including bacteria, viruses, and archaea, living in the human gut. The gut microbiome interacts with the brain in bidirectional ways using neural, inflammatory, and hormonal signalling pathways. 23 The role of altered interactions between the brain and gut microbiome on mental health has been proposed on the basis of the following evidence: emotion-like behaviour in rodents changes with changes in the gut microbiome, 24 major depressive disorder in humans is associated with alterations of the gut microbiome, 25 and transfer of faecal gut microbiota from humans with depression into rodents appears to induce animal behaviours that are hypothesised to indicate depression-like states. 25 26 Such findings suggest a role of altered neuroactive microbial metabolites in depressive symptoms.
In addition to genetic factors and exposure to antibiotics, diet is a potentially modifiable determinant of the diversity, relative abundance, and functionality of the gut microbiome throughout life. For instance, the neurocognitive effects of the Western diet, and the possible mediating role of low grade systemic immune activation (as discussed above) may result from a compromised mucus layer with or without increased epithelial permeability. Such a decrease in the function of the gut barrier is sometimes referred to as a “leaky gut” and has been linked to an “unhealthy” gut microbiome resulting from a diet low in fibre and high in saturated fats, refined sugars, and artificial sweeteners. 15 23 27 Conversely, the consumption of a diet high in fibres, polyphenols, and unsaturated fatty acids (as found in a Mediterranean diet) can promote gut microbial taxa which can metabolise these food sources into anti-inflammatory metabolites, 15 28 such as short chain fatty acids, while lowering the production of secondary bile acids and p-cresol. Moreover, a recent study found that the ingestion of probiotics by healthy individuals, which theoretically target the gut microbiome, can alter the brain’s response to a task that requires emotional attention 29 and may even reduce symptoms of depression. 30 When viewed together, these studies provide promising evidence supporting a role of the gut microbiome in modulating processes that regulate emotion in the human brain. However, no causal relationship between specific microbes, or their metabolites, and complex human emotions has been established so far. Furthermore, whether changes to the gut microbiome induced by diet can affect depressive symptoms or clinical depressive disorders, and the time in which this could feasibly occur, remains to be shown.
Priorities and next steps
In moving forward within this active field of research, it is firstly important not to lose sight of the wood for the trees—that is, become too focused on the details and not pay attention to the bigger questions. Whereas discovering the anti-inflammatory properties of a single nutrient or uncovering the subtleties of interactions between the gut and the brain may shed new light on how food may influence mood, it is important not to neglect the existing knowledge on other ways diet may affect mental health. For example, the later consequences of a poor diet include obesity and diabetes, which have already been shown to be associated with poorer mental health. 11 31 32 33 A full discussion of the effect of these comorbidities is beyond the scope of our article (see fig 1 ), but it is important to acknowledge that developing public health initiatives that effectively tackle the established risk factors of physical and mental comorbidities is a priority for improving population health.
Further work is needed to improve our understanding of the complex pathways through which diet and nutrition can influence the brain. Such knowledge could lead to investigations of targeted, even personalised, interventions to improve mood, anxiety, or other symptoms through nutritional approaches. However, these possibilities are speculative at the moment, and more interventional research is needed to establish if, how, and when dietary interventions can be used to prevent mental illness or reduce symptoms in those living with such conditions. Of note, a recent large clinical trial found no significant benefits of a behavioural intervention promoting a Mediterranean diet for adults with subclinical depressive symptoms. 34 On the other hand, several recent smaller trials in individuals with current depression observed moderately large improvements from interventions based on the Mediterranean diet. 35 36 37 Such results, however, must be considered within the context of the effect of people’s expectations, particularly given that individuals’ beliefs about the quality of their food or diet may also have a marked effect on their sense of overall health and wellbeing. 38 Nonetheless, even aside from psychological effects, consideration of dietary factors within mental healthcare may help improve physical health outcomes, given the higher rates of cardiometabolic diseases observed in people with mental illness. 33
At the same time, it is important to be remember that the causes of mental illness are many and varied, and they will often present and persist independently of nutrition and diet. Thus, the increased understanding of potential connections between food and mental wellbeing should never be used to support automatic assumptions, or stigmatisation, about an individual’s dietary choices and their mental health. Indeed, such stigmatisation could be itself be a casual pathway to increasing the risk of poorer mental health. Nonetheless, a promising message for public health and clinical settings is emerging from the ongoing research. This message supports the idea that creating environments and developing measures that promote healthy, nutritious diets, while decreasing the consumption of highly processed and refined “junk” foods may provide benefits even beyond the well known effects on physical health, including improved psychological wellbeing.
Contributors and sources: JF has expertise in the interaction between physical and mental health, particularly the role of lifestyle and behavioural health factors in mental health promotion. JEG’s area of expertise is the study of the relationship between sleep duration, nutrition, psychiatric disorders, and cardiometabolic diseases. AB leads research investigating the molecular mechanisms underlying the effect of stress and inflammation on human hippocampal neurogenesis, and how nutritional components and their metabolites can prevent changes induced by those conditions. REW has expertise in genetic epidemiology approaches to examining casual relations between health behaviours and mental illness. EAM has expertise in brain and gut interactions and microbiome interactions. All authors contributed to, read, and approved the paper, and all the information was sourced from articles published in peer reviewed research journals. JF is the guarantor.
Competing interests: We have read and understood BMJ policy on declaration of interests and declare the following: JF is supported by a University of Manchester Presidential Fellowship and a UK Research and Innovation Future Leaders Fellowship and has received support from a NICM-Blackmores Institute Fellowship. JEG served on the medical advisory board on insomnia in the cardiovascular patient population for the drug company Eisai. AB has received research funding from Johnson & Johnson for research on depression and inflammation, the UK Medical Research Council, the European Commission Horizon 2020, the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust, and King’s College London. REW receives funding from the National Institute for Health Research Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol. EAM has served on the external advisory boards of Danone, Viome, Amare, Axial Biotherapeutics, Pendulum, Ubiome, Bloom Science, Mahana Therapeutics, and APC Microbiome Ireland, and he receives royalties from Harper & Collins for his book The Mind Gut Connection. He is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, and the US Department of Defense. The views expressed are those of the authors and not necessarily those of the organisations above.
Provenance and peer review: Commissioned; externally peer reviewed.
This article is part of series commissioned by The BMJ. Open access fees are paid by Swiss Re, which had no input into the commissioning or peer review of the articles. T he BMJ thanks the series advisers, Nita Forouhi, Dariush Mozaffarian, and Anna Lartey for valuable advice and guiding selection of topics in the series.
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
- Friedrich MJ
- Johnson J ,
- Weissman MM ,
- Lassale C ,
- Baghdadli A ,
- Siddiqi N ,
- Koyanagi A ,
- Gangwisch JE ,
- Salari-Moghaddam A ,
- Larijani B ,
- Esmaillzadeh A
- de Jong V ,
- Atkinson F ,
- Brand-Miller JC
- Seaquist ER ,
- Anderson J ,
- American Diabetes Association ,
- Endocrine Society
- Towler DA ,
- Havlin CE ,
- McIntyre RS ,
- Nguyen HT ,
- O’Keefe JH ,
- Gheewala NM ,
- Kastorini C-M ,
- Milionis HJ ,
- Esposito K ,
- Giugliano D ,
- Goudevenos JA ,
- Panagiotakos DB
- Teasdale SB ,
- Köhler-Forsberg O ,
- N Lydholm C ,
- Hjorthøj C ,
- Nordentoft M ,
- Yahfoufi N ,
- Borsini A ,
- Horowitz MA ,
- Kiecolt-Glaser JK ,
- Fagundes CP ,
- Andridge R ,
- Osadchiy V ,
- Martin CR ,
- O’Brien C ,
- Sonnenburg ED ,
- Sonnenburg JL
- Rampelli S ,
- Jeffery IB ,
- Tillisch K ,
- Kilpatrick L ,
- Walsh RFL ,
- Wootton RE ,
- Millard LAC ,
- Jebeile H ,
- Garnett SP ,
- Paxton SJ ,
- Brouwer IA ,
- MooDFOOD Prevention Trial Investigators
- Francis HM ,
- Stevenson RJ ,
- Chambers JR ,
- Parletta N ,
- Zarnowiecki D ,
- Fischler C ,
- Sarubin A ,
- Wrzesniewski A
- Harrington D ,
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- Diet & Nutrition
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
An Overview of Vitamin Deficiency
- Possible Deficiencies
- Complications
Vitamin deficiency can cause a range of symptoms such as fatigue, irritability, and changes to your skin and hair. The specific symptoms depend on which vitamin(s) are at low levels, as each one plays a different role in your body.
For example, a vitamin D deficiency can lead to weak bones and fractures, while a folate deficiency can lead to anemia, which causes fatigue and weakness.
You can develop a deficiency due to low vitamin intake or certain medical conditions. They're usually treated with vitamin supplements in either oral (by mouth) or injected forms.
This article covers the symptoms and complications common to vitamin deficiencies, the causes and risk factors, and how vitamin deficiencies are diagnosed and treated.
Aleksandr Zubkov / Getty Images
Vitamins You Might Be Lacking
Your body produces many of the vitamins it needs. But your health depends on 13 vitamins that your body can't make, so you need to get them through diet or supplements.
These essential vitamins are:
- Vitamin B1 (thiamine)
- Vitamin B2 (riboflavin)
- Vitamin B3 (niacin)
- Vitamin B5 (pantothenic acid)
- Vitamin B6 (pyroxidine)
- Vitamin B7 (biotin)
- Vitamin B9 (folate)
- Vitamin B12 (cobalamin)
Symptoms of Vitamin Deficiency
Vitamin deficiency can cause a number of symptoms, including fatigue, dry skin and hair, depression, poor wound healing, and more. While they can vary between deficiencies, many of them overlap.
Usually, noticeable effects don't begin to develop until you've had low levels for several months.
Fatigue and Weakness
If you feel sleepy or sluggish all the time, you may be deficient in vitamin D, any of the B vitamins, and/or vitamin C.
Vitamin D helps with bone and muscle strength, so when you don't have enough, you may feel weak and lack energy. Some research suggests that vitamin D deficiency is linked to fatigue and that taking vitamin D supplements can improve this symptom.
Vitamin C and all B vitamins except folate (B9) are involved in producing energy in your cells, so being deficient in any of them can leave you feeling wiped out. It can also have a major impact on your metabolism and overall health.
Folate deficiency can lead to fatigue and weakness by causing anemia. In that condition, your body doesn't have enough red blood cells, which carry oxygen to your tissues. That oxygen is essential for proper function and energy.
Dry Skin and Hair
Dry skin and hair are common symptoms of deficiencies in:
Some of these vitamins are commonly used in skincare and haircare products. Ask your healthcare provider whether dietary sources, supplements, or topical (on the skin) use is best for improving skin and hair health.
Use caution with vitamin A (retinol) products, however. High levels can actually harm your skin.
Vitamin deficiencies can sometimes be associated with clinical depression. They include:
- Vitamins B1, 3, 6, 9, 12
Supplements may help alleviate depression, but they're not a replacement for antidepressants. Don't stop taking your medication or add supplements to your regimen without first talking to your healthcare provider.
Easy Bruising or Bleeding
Easy bruising and bleeding can happen due to problems with blood clotting, poor healing, or collagen formation. (Collagen gives strength to the walls of blood vessels.)
Essential vitamin deficiencies that can contribute to easy bruising or bleeding are:
- Vitamin K, especially in newborn babies
Deficiencies of these vitamins are fairly uncommon, however. If you start bruising or bleeding easily, don't just assume a deficiency is causing it.
Poor Wound Healing
Poor wound healing means that your sores take especially long to heal. A lot of essential vitamins contribute to the healing process. Some aid in collagen creation, others help with re-building different types of cells or tissues, and others promote cellular health through antioxidant activity.
Vitamin deficiencies that can contribute to poor wound healing include:
- Vitamin D (when combined with zinc and arginine)
Drawbacks of Vitamin E
Vitamin E (a non-essential nutrient) might have a negative impact on wound healing. It can hamper collagen synthesis and antioxidant activity and may worsen inflammation. Research also suggests it may counter the benefits of vitamin A that's taken to help with wound healing.
Predisposition to Infections
Some vitamin deficiencies affect your immune system and can make you more likely to get infections and infectious diseases. These include:
A vitamin A deficiency is particularly dangerous when it comes to infection risk. It can predispose you to:
- Respiratory disease
- Chronic ear infections
Bone Fractures
Essential vitamins that keep your bones strong and healthy include vitamins A, B6, B9, B12, C, D, and K.
While research shows deficiencies in any of these vitamins can lower your bone density and lead to fractures, it's not clear whether nutritional supplements lower the fracture risk.
Talk to your healthcare provider about the best ways to protect your bone health.
While enough of Vitamins A, B6, and B12 is important for strong bones, too much may actually lower your bone density and increase your risk of breaking bones, according to research.
Skin Color Changes
Skin color changes due to vitamin deficiency can manifest in a few different ways: loss of pigmentation in spots, darker pigmentation in spots, or generally pale skin.
Loss of pigmentation and light-colored spots may result from deficiencies in:
- Vitamin D (in light-skinned people)
Darker pigmentation can be due to:
- Vitamin B12
- Vitamin D (in dark-skinned people)
Deficiencies that can cause generally pale skin include:
- Vitamin B6, 9, 12
Complications of Vitamin Deficiency
Prolonged vitamin deficiency can cause more serious health issues that may not improve, even with treatment. Severe vitamin deficiencies can cause:
- Decreased sensation of the hands and feet
- Weakness of the toes and fingers
- Vision loss
- Memory loss
- Behavioral changes
- Shortness of breath
- Tachycardia (a rapid heart rate)
Vitamin deficiency during pregnancy can be a serious problem, resulting in developmental problems that affect the growing baby. In fact, vitamin deficiency can have major effects during the first 10 weeks of pregnancy, when most people do not even know they are pregnant.
Rare Effects
A few uncommon symptoms may be associated with vitamin deficiency, including:
- Restless legs syndrome
- Insomnia (trouble sleeping)
- Increased stroke risk
- Spine disease
Causes of Vitamin Deficiency
Often, vitamin deficiency is related to your diet. Vitamins are complex molecules present in fruit, vegetables, grains, meat, poultry, and seafood. Each vitamin is found in more than one type of food, and some foods are fortified with vitamins. For example, milk naturally contains calcium (which is a mineral, not a vitamin) and it is fortified with vitamin D. Pasta, rice, and cereal are often fortified with a variety of vitamins.
In addition to dietary factors, medical conditions can affect your absorption of vitamins, even if your dietary vitamin intake is adequate.
Dietary Risk Factors
Some diets can make you prone to vitamin deficiency. Vitamin B12 is found in meats—a vegan or vegetarian diet can increase the risk of vitamin B12 and biotin deficiency. If you are dairy-free, then you may be at risk of becoming deficient in vitamin D.
A gluten-free diet is a diet low in grains, which are naturally rich in vitamins and are also often fortified with vitamins. So a gluten-free diet can make you deficient in many vitamins, including folate, and thiamine.
A diet that is high in processed foods and low in fresh fruits and vegetables can result in vitamin E and vitamin K deficiency.
It is absolutely possible to avoid vitamin deficiency if you are vegetarian, vegan, or gluten-free. However, this does require careful planning. Advice from a registered dietitian can help guide you.
Vitamin D is found in foods such as seafood, eggs, and dairy products. But sunlight is also an important source of vitamin D. And lack of sun exposure can result in vitamin D deficiency. In geographic regions that have a cold climate, this is fairly common during the winter,
Medical Illness
A number of medical problems make it hard to properly absorb and metabolize vitamins. This can lead to vitamin deficiencies.
Common medical causes of vitamin deficiency include:
- Alcoholic liver disease
- Liver failure
- Kidney disease
- Chronic diarrhea
- Malabsorption syndrome
- Gastric bypass
- Inflammatory bowel disease
- Crohn's disease
- Irritable bowel syndrome
- Pernicious anemia
What Is Pernicious Anemia?
Pernicious anemia is a type of autoimmune disease that affects the small intestine, decreasing absorption of vitamin B12. This results in an insufficient amount of healthy red blood cells.
Diagnosis of Vitamin Deficiency
Some vitamin deficiencies cause more than one symptom, and some symptoms (like sleepiness) can occur as a result of a few different vitamin deficiencies. Because symptoms do not always clearly correlate with the specific vitamin deficiency, diagnostic testing is the only way to confirm a vitamin deficiency.
The diagnosis of vitamin deficiencies can take some time. That is because it is not routine to test for vitamin levels. Your healthcare provider may consider testing if you have symptoms or if your physical examination identifies issues like bruises, wounds, skin discoloration, and neuropathy .
Neuropathy is a condition in which nerve function is impaired. It is associated with a lack of vitamin B12. It can cause you to have decreased sensation, diminished reflexes, and muscle weakness. Very early neuropathy might not cause these changes, but an electromyography (EMG) or nerve conduction study (NCV) can often detect early stages of neuropathy that have not yet caused signs or symptoms.
Blood Tests
Blood tests can show signs of vitamin deficiency and can be used to measure your vitamin levels. A complete blood count is the most common screening test. A low red blood cell count or a pattern of enlarged red blood cells (megaloblastic anemia) is a common sign of vitamin B12 deficiency.
In some instances, your vitamin levels may be measured with a blood test. Vitamins that can be measured with a blood test include folate (vitamin B9), vitamin D, and vitamin B12.
Interventional Tests
If there is a concern that you could have a digestive problem causing vitamin malabsorption, your healthcare provider may order a test to examine the internal appearance of your stomach or intestines.
An endoscopy is used to examine the appearance of your stomach and the upper portion of your small intestine using a camera that is inserted down your throat. A colonoscopy is used to examine the internal appearance of your large intestine using a camera that is inserted into the rectum.
These tests can be uncomfortable, so they are done with an anesthetic medication. Your healthcare provider can identify problems such as Crohn's disease and some types of malabsorptive syndromes with these interventional examinations.
Treatment of Vitamin Deficiency
Treatment for vitamin deficiency involves vitamin replacement. If a medical condition is the cause of your vitamin deficiency, then treatment of that condition is necessary as well.
Dietary Changes
In many instances, even if a medical condition is contributing to your vitamin deficiency, long term dietary changes can help correct and prevent the deficiency from worsening. You can learn which foods contain the vitamins you need so that you can pay attention to getting an adequate amount of these vitamins.
You may benefit from meeting with a dietitian, who can help you identify which foods you could consider including in your diet. You may also need help with creating a healthy meal plan.
Vitamin Replacement
There are several ways to make sure that you get adequate vitamins. Vitamin supplements can be an option. You may be given a recommendation for an over-the-counter (OTC) or prescription supplement. Sometimes when there is a problem with absorption, supplements such as vitamin B12 need to be injected instead of taken orally.
Nutritional supplements aren't safe for everyone. They may have side effects, negative effects on medical conditions, dangerous interactions with medications, or unwanted effects during pregnancy or breastfeeding. Always talk to your healthcare provider about a supplement before you start taking it.
Medical Management
If you have a medical condition such as irritable bowel syndrome or inflammatory bowel disease, you will benefit from getting treated for that condition. There are a number of medical and surgical treatments for gastrointestinal conditions.
Some illnesses, such as liver failure, may not be treatable at late stages. Long-term injected vitamin supplementation may be necessary.
A Word From Verywell
A healthy and varied diet is an important part of good health. Generally speaking, supplements are no substitute for good nutrition. Be sure to discuss any supplement use with your healthcare provider—they can help counsel you on appropriate use and dosage.
National Institutes of Health, U.S. National Library of Medicine: MedlinePlus. Vitamins .
Nowak A, Boesch L, Andres E, et al. Effect of vitamin D3 on self-perceived fatigue: A double-blind randomized placebo-controlled trial [published correction appears in Medicine (Baltimore). 2017 Jan 20;96(3):e6038]. Medicine (Baltimore) . 2016;95(52):e5353. doi:10.1097/MD.0000000000005353
Tardy AL, Pouteau E, Marquez D, Yilmaz C, Scholey A. Vitamins and minerals for energy, fatigue and cognition: A narrative review of the biochemical and clinical evidence . Nutrients . 2020;12(1):228. Published 2020 Jan 16. doi:10.3390/nu12010228
National Institutes of Health, U.S. National Library of Medicine: MedlinePlus. Folate-deficiency anemia .
VanBuren CA, Everts HB. Vitamin A in skin and hair: An update . Nutrients . 2022;14(14):2952. Published 2022 Jul 19. doi:10.3390/nu14142952
Rembe JD, Fromm-Dornieden C, Stuermer EK. Effects of vitamin B complex and vitamin C on human skin cells: is the perceived effect measurable? Adv Skin Wound Care. 2018;31(5):225-233. doi:10.1097/01.ASW.0000531351.85866.d9
Pullar JM, Carr AC, Vissers MCM. The roles of vitamin C in skin health . Nutrients . 2017;9(8):866. doi:10.3390/nu9080866
Umar M, Sastry KS, Al Ali F, Al-Khulaifi M, Wang E, Chouchane A.I. Vitamin D and the pathophysiology of inflammatory skin diseases . Skin Pharmacol Physiol . 2018;31(2):74-86. doi:10.1159/000485132
Oregon State University, Linus Pauling Institute: Micronutrient Information Center. Vitamin A and skin health .
Mikkelsen K, Stojanovska L, Apostolopoulos V. The effects of vitamin B in depression . Curr Med Chem . 2016;23(38):4317-4337. doi:10.2174/0929867323666160920110810
Pullar JM, Carr AC, Bozonet SM, Vissers MCM. High vitamin C status is associated with elevated mood in male tertiary students . Antioxidants (Basel). 2018;7(7). doi:10.3390/antiox7070091
Xie F, Huang T, Lou D, et al. Effect of vitamin D supplementation on the incidence and prognosis of depression: An updated meta-analysis based on randomized controlled trials . Front Public Health . 2022;10:903547. Published 2022 Aug 1. doi:10.3389/fpubh.2022.903547
Galimberti F, Mesinkovska NA. Skin findings associated with nutritional deficiencies . Cleve Clin J Med . 2016;83(10):731-739. doi:10.3949/ccjm.83a.15061
Wolpert K, Szadkowski M, Miescier M, Hewes HA. The presentation of a fussy infant with bruising: Late-onset vitamin K deficiency bleeding . Pediatr Emerg Care . 2019;35(4):e70-e71. doi:10.1097/PEC.0000000000001083
Harvard University Medical School, Harvard T.H. Chan School of Public Health. Vitamin K .
Barchitta M, Maugeri A, Favara G, et al. Nutrition and wound healing: An overview focusing on the beneficial effects of curcumin . Int J Mol Sci . 2019;20(5):1119. Published 2019 Mar 5. doi:10.3390/ijms20051119
Pazyar N, Houshmand G, Yaghoobi R, Hemmati AA, Zeineli Z, Ghorbanzadeh B. Wound healing effects of topical Vitamin K: A randomized controlled trial . Indian J Pharmacol . 2019;51(2):88-92. doi:10.4103/ijp.IJP_183_18
Katona P, Katona-Apte J. The interaction between nutrition and infection . Clin Infect Dis . 2008;46(10):1582-1588. doi:10.1086/587658
Oz HS. Nutrients, infectious and inflammatory diseases . Nutrients . 2017;9(10):1085. Published 2017 Sep 30. doi:10.3390/nu9101085
Burt LA, Billington EO, Rose MS, Raymond DA, Hanley DA, Boyd SK. Effect of high-dose vitamin D supplementation on volumetric bone density and bone strength: A randomized clinical trial [published correction appears in JAMA. 2019 Nov 19;322(19):1925]. JAMA . 2019;322(8):736-745. doi:10.1001/jama.2019.11889
Meyer HE, Willett WC, Fung TT, Holvik K, Feskanich D. Association of high intakes of vitamins B6 and B12 from food and supplements with risk of hip fracture among postmenopausal women in the Nurses' Health Study . JAMA Netw Open . 2019;2(5):e193591. Published 2019 May 3. doi:10.1001/jamanetworkopen.2019.3591
Yee MMF, Chin KY, Ima-Nirwana S, Wong SK. Vitamin A and bone health: A review on current evidence . Molecules . 2021;26(6):1757. Published 2021 Mar 21. doi:10.3390/molecules26061757
Scripps Health. What causes dry white patches on skin?
Kannan R, Ng MJ. Cutaneous lesions and vitamin B12 deficiency: an often-forgotten link . Can Fam Physician . 2008;54(4):529-532.
Aroni K, Anagnostopoulou K, Tsagroni E, Ioannidis E. Skin hyperpigmentation and increased angiogenesis secondary to vitamin B12 deficiency in a young vegetarian woman . Acta Derm Venereol . 2008;88(2):191-192. doi:10.2340/00015555-0377
Podiatry Today. When vitamin and nutritional deficiencies cause skin and nail changes .
National Institutes of Health, U.S. National Library of Medicine: MedlinePlus. Pellagra .
National Institutes of Health, National Institute of Neurological Disorders and Stroke. Restless Legs Syndrome Fact Sheet .
Vici G, Belli L, Biondi M, Polzonetti V. Gluten free diet and nutrient deficiencies: A review . Clin Nutr . 2016;35(6):1236-1241. doi:10.1016/j.clnu.2016.05.002
Wacker M, Holick MF. Sunlight and vitamin D: A global perspective for health . Dermatoendocrinol . 2013;5(1):51-108. doi:10.4161/derm.24494
Crohn’s & Colitis Foundation. Vitamin and mineral supplementation .
Foundation for Peripheral Neuropathy. Vitamin B12 deficiency neuropathy .
National Institutes of Health, U.S. National Library of Medicine: MedlinePlus. Vitamin B test .
International Foundation for Gastrointestinal Disorder. Malabsorption .
National Institutes of Health, U.S. National Library of Medicine: MedlinePlus. Vitamin D deficiency .
Diab L, Krebs NF. Vitamin excess and deficiency . Pediatr Rev . 2018 Apr;39(4):161-179. doi:10.1542/pir.2016-0068
Thomas-Valdés S, Tostes MDGV, Anunciação PC, da Silva BP, Sant'Ana HMP. Association between vitamin deficiency and metabolic disorders related to obesity . Crit Rev Food Sci Nutr . 2017 Oct 13;57(15):3332-3343. doi:10.1080/10408398.2015.1117413
By Brandon Peters, MD Dr. Peters is a board-certified neurologist and sleep medicine specialist and is a fellow of the American Academy of Sleep Medicine.
Nutritional deficiencies and their impact on health
- This person is not on ResearchGate, or hasn't claimed this research yet.

- University of the Punjab
Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations

- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up
- Search Menu
- Sign in through your institution
- Advance articles
- Editor's Choice
- Supplement Archive
- Article Collection Archive
- Author Guidelines
- Submission Site
- Open Access
- Call for Papers
- Why Publish?
- About Nutrition Reviews
- About International Life Sciences Institute
- Editorial Board
- Early Career Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Introduction, adhd: contributing influences, nutrition and adhd, food intolerance and adhd.
- < Previous
Nutritional and dietary influences on attention deficit hyperactivity disorder
- Article contents
- Figures & tables
- Supplementary Data
Natalie Sinn, Nutritional and dietary influences on attention deficit hyperactivity disorder, Nutrition Reviews , Volume 66, Issue 10, 1 October 2008, Pages 558–568, https://doi.org/10.1111/j.1753-4887.2008.00107.x
- Permissions Icon Permissions
An abundance of research has investigated causes and treatments for attention deficit hyperactivity disorder (ADHD). The research includes identification of suboptimal levels of nutrients and sensitivities to certain foods and food additives. This review gives an overview of this research and provides an up-to-date account of clinical trials that have been conducted with zinc, iron, magnesium, Pycnogenol, omega-3 fatty acids, and food sensitivities. A literature search was conducted using PubMed, ISI Web of Knowledge, and Google Scholar and included studies published before April 2008. Although further research is required, the current evidence supports indications of nutritional and dietary influences on behavior and learning in these children, with the strongest support to date reported for omega-3s and behavioral food reactions.
A vast body of literature and research has been focused on attention deficit hyperactivity disorder (ADHD), which is the most prevalent childhood disorder, estimated to affect 2–18% of children 1 depending largely on diagnostic criteria. Core symptoms associated with ADHD are developmentally inappropriate levels of hyperactivity, impulsivity, and inattention. ADHD has a high comorbidity rate with other mental health problems such as anxiety and mood disorders, including depression, suicidal ideation, 2 , 3 and bipolar disorder 4 ; it is often particularly associated with antisocial problems such as conduct disorder and oppositional defiant disorder. 3 , – 6 When combined with these problems, ADHD can lead to antisocial behavior, substance abuse, and borderline personality disorder in late adolescence and adulthood. 7 , – 10
In addition, ADHD is associated with cognitive deficits; it has been estimated that a quarter of these children have a specific learning disability in math, reading, or spelling. 11 Attention difficulties are associated with delays in general cognitive functioning, weak language skills, and poor adjustment in the classroom. 12 The disruptive behavior, poor self-discipline, distractibility, and problems with response inhibition, self-regulation, and emotional control that are associated with ADHD 13 can adversely impact families, relationships, social interactions, and children's self-esteem and school performance, presenting substantial personal, social, and economic burden for afflicted children, families, schools, and the broader community.
Prevalence of ADHD appears to be on the rise despite increased prescriptions of pharmaceutical medication, particularly methylphenidate and dextroamphetamine. Many parents are concerned about side effects of these medications, and a recent long-term follow-up of the Multimodal Treatment Study of Children with ADHD (MTA) study 14 found that children in their pre-teens who had been medicated with methylphenidate had stunted growth 15 as well as increased risk of juvenile behavior and, possibly, substance abuse. 16
The etiology of ADHD is complex and is associated with both genetic and environmental factors. 3 Studies of twins have provided strong evidence for a genetic component to the disorder, which, in combination with other biological factors, is likely to underlie the neurological deficits that are exacerbated over time by environmental influences. 17 Psychophysiological research has identified neurological abnormalities, particularly in the frontal lobes, in children with ADHD compared with controls. 18 , 19 Similarly, a number of studies have identified reduced blood flow to the frontal lobes in children with ADHD. 17 This is consistent with hypotheses that symptoms of ADHD are related to abnormalities in noradrenergic and dopaminergic systems in the frontal lobes. 7 The high comorbidity of ADHD with a variety of other psychopathologies suggests that these mental health problems share similar underlying neurological mechanisms. This notion is supported by the fact that children with ADHD often have family histories of neurodevelopmental and psychiatric disorders. 20
Biological influences that have been associated with ADHD, via their impact on brain development and neurological functioning, include exposure to lead, mercury, and pesticides as well as prenatal exposure to tobacco. 21 , 22 In many affected children, there are indications of suboptimal levels of various nutrients and evidence for behavioral reactions to certain foods and food additives. There is particularly compelling evidence that ADHD and other neurodevelopmental disorders such as dyspraxia, dyslexia, and autism may be associated with suboptimal levels of essential fatty acids. Therefore, it may be more prudent to address ADHD symptoms with a nutritional or dietary approach before prescribing medications. The present review evaluates the current state of evidence for the role of nutrients (following a brief overview of nutrition in brain development and function), Pycnogenol, and food sensitivities in ADHD.
Nutrition and the brain
The brain's critical need for adequate nutrition is demonstrated by effects of malnourishment on the developing brain, including reduced DNA synthesis, cell division, myelination, glial cell proliferation, and dendritic branching. The pathological manifestation of malnourishment will depend on the stage of brain development at the time of nutritional insult. 23 Effects of some nutrient deficiencies on development have become widely known and accepted; for instance, perinatal deficiencies in iodine – now considered the world's most preventable cause of mental retardation, 24 folate – related to spinabifida, and iron-related anemia. Severe deficiencies in omega-3 polyunsaturated fatty acids (PUFAs), particularly docosahexaenoic acid (DHA) can result in profound mental retardation associated with peroxisomal disorders. 25 , 26
Less extreme effects of suboptimal nutrient levels on brain development and ongoing function are not as well recognized.
Given the essentiality of an intricate interplay of macro- and micronutrients for optimal brain function, this could result in cognitive and behavioral problems for which the role of nutrition may be overlooked. Although the brain only accounts for 2–2.7% of body weight, it requires 25% of the body's glucose supply and 19% of the blood supply at rest; these requirements increase by 50% and 51%, respectively, in response to cerebral activity. 27 Glucose is required for the brain's metabolic activities and is its primary source of energy. The brain has very limited capacity for storing glucose, hence the essentiality of a continuous and reliable supply of blood. A number of nutrients appear to be involved in maintaining cerebral blood flow and the integrity of the blood-brain barrier, including folic acid, pyridoxine, colabamin, thiamine, 27 and omega-3 PUFA. 28 Neurotransmitters are also an integral component of the brain's communication system; various nutrients are required for monoamine metabolic pathways and act as essential cofactors for the enzymes involved in neurotransmitter synthesis. 27
As well as playing important roles in immune function, growth, development, and reproduction, zinc is required for the developing brain. It plays numerous roles in ongoing brain function via protein binding, enzyme activity, and neurotransmission. As an essential cofactor for over 100 enzymes, zinc is required for the conversion of pyridoxine (B 6 ) to its active form, which is needed to modulate the conversion of tryptophan to serotonin; zinc is involved in the production and modulation of melatonin, which is required for dopamine metabolism and is a cofactor for delta-6 desaturase, which is involved in essential fatty acid conversion pathways. 29
A comprehensive review of the role of zinc in brain function and in ADHD is provided by Arnold. 29 His review includes reports of nine studies conducted in various parts of the world, which all found lower zinc levels in children with ADHD as well as correlations between lower zinc levels and severity of symptoms. One avenue of zinc depletion in these children may be via reactions to synthetic chemicals found in food additives. Twenty hyperactive males who reacted to the orange food dye tartrazine were challenged in a double-blind, placebo-controlled trial with 50 mg of the food additive. Following the challenge, serum zinc levels decreased and urine levels increased in the hyperactive group compared with controls, suggesting that metabolic wastage of zinc occurs under chemical stress. Behavioral and emotional symptoms also deteriorated in hyperactive children in association with changes in zinc levels. 30
Two clinical zinc supplementation trials have been conducted in children with ADHD. One controlled study found significant improvements in hyperactivity, impulsivity, and socialization scores, but not inattention, after 12 weeks of supplementation with 150 mg zinc per day in children with ADHD compared with controls. It should be noted that this is a particularly high dose of zinc, and there was a high dropout rate in the study (although it was not significantly different between the active and placebo groups). 31 The other study allocated 44 children who were diagnosed with ADHD to methylphenidate along with either 55 mg zinc sulfate or placebo over 6 weeks to investigate adjunctive benefits of zinc. Scores on parent and teacher rating scales for the children improved in both groups, and these improvements were significantly greater in the zinc group. 32
It is interesting to note that both zinc and free serum fatty acid levels were found to be lower in a group of 48 children with ADHD compared with 45 controls, and that these levels were strongly correlated in the ADHD group. 33 In light of these studies and reports of other nutritional deficiencies in ADHD, the present author conducted a controlled trial (described below), that focused on omega-3 PUFAs and investigated additive benefits of a multivitamin/mineral tablet in conjunction with the PUFA supplement. 34 No additional benefits were found with the MVM supplement over and above the PUFA supplement; however, the supplement contained <2 mg zinc, which, when compared to the studies above, is likely to have provided inconclusive results regarding potentially additive benefits of zinc combined with PUFA.
Anemia from iron deficiency is estimated to affect 20–25% of infants, and many more are thought to suffer iron deficiencies without anemia, putting them at risk for delayed or impaired childhood development. Iron is important for the structure and function of the central nervous system and it plays a number of roles in neurotransmission. Iron deficiency has been associated with poor cognitive development and it has been proposed that iron deficiency may affect cognition and behavior via its role as a co-factor for tyrosine hydroxylase, the rate-limiting enzyme involved in dopamine synthesis. 35 , 36
Iron levels were found to be twice as low in 53 non-anemic children with ADHD compared to 27 controls with no other evidence of malnutrition; specifically, serum ferritin levels were abnormal (<30 ng/mL) in 84% of children with ADHD and 18% of controls ( p < 0.001). Furthermore, low serum ferritin levels were correlated with more severe ADHD symptoms measured with Conners' Parent Rating Scales (CPRS), particularly with cognitive problems and hyperactivity. 36 A recent study also found low iron levels in 52 non-anemic children with ADHD, and these were correlated with hyperactivity scores on CPRS, although not with a range of cognitive assessments. 37 It has been suggested that iron could play a role in ADHD due to its neuroprotective effect against lead exposure. 38 Iron deficiency is also associated with restless legs syndrome, which is a common comorbid condition in children with ADHD symptoms, and may, therefore, account for greater variance of symptoms in this subgroup of children. 39 Indeed, a recent study found that children with ADHD who suffered from restless legs had lower iron levels than those without restless legs. 40
An early, uncontrolled pilot study investigated effects of iron supplementation on ADHD symptoms in 14 non-anemic 7–11-year-old boys. After 30 days of daily supplementation with 5 mg/kg ferrous-calcium citrate (active elemental iron, 0.05 mg/kg daily), blood samples showed increases in serum ferritin levels and significant decreases were found on parent ratings of symptoms on Conners' Rating Scales. However, these improvements were not correlated with increased iron levels and no significant improvements were found on teacher ratings. It was concluded that iron supplementation may not be effective in non-iron-deficient children and that it should be investigated in iron-deficient children with ADHD. 41 It is also possible that 30 days may not have been long enough to observe any effects. One report of a case study outlined the effects of iron supplementation on a 3-year-old boy with diagnosed ADHD. This boy did have an iron deficiency and also displayed sleep problems (delayed sleep onset and excessive motility in sleep). After 4 months of iron supplementation, parents and teachers reported mild improvements in the child's symptoms, and marked improvements were reported after 8 months. He also showed enhanced quality of sleep. 42
These studies were followed up by a double-blind, placebo-controlled study with 23 non-anemic, 5–8-year-old iron-deficient children (serum ferritin levels <30 ng/mL) with ADHD. Following 12 weeks of supplementation with 80 mg ferrous sulfate per day or placebo, symptoms tended to improve in the treatment group on all ADHD scales and the improvements were significant on two outcome measures. Seventy five percent of children in the treatment group had diagnosed or possible restless leg syndrome and this condition improved in 12 of those 14 children following iron supplementation. These improvements were not seen in the placebo group ( n = 5). 43 This study supports indications that children with low iron levels who have both ADHD and restless legs may be more likely to benefit from iron supplementation.
Suboptimal magnesium (Mg) levels may impact brain function via a number of mechanisms including reduced energy metabolism, synaptic nerve cell signaling, and cerebral blood flow; it has also been suggested that its suppressive influence on the nervous system helps to regulate nervous and muscular excitability. 44 Low Mg levels have been reported in children with ADHD. In 116 children with diagnosed ADHD, 95% were found to have Mg deficiency (77.6% in hair; 33.6% in blood serum), and these occurred significantly more frequently than in a control group. Magnesium levels also correlated highly with a quotient of freedom from distractibility. 44 In 50 children aged 7–12 years with ADHD, Mg supplementation (200 mg/day) over 6 months resulted in significant reductions in hyperactivity and improved freedom from distractibility both compared with pre-test scores and with a control group of 25 children with ADHD who were not treated with magnesium. 45 Another open study also found lower Mg levels in 30 of 52 hyperactive children compared with controls, and improvements in symptoms of hyperexcitability following 1–6 months of supplementation with combined Mg/vitamin B 6 (100 mg/day). 46 A similar study by the same researchers 2 years later found lower Mg levels in 40 children with clinical symptoms of ADHD than in 36 healthy controls. Decreased Mg levels were also associated with increased hyperactivity and sleep disturbance and poorer school attention. After 2 months of Mg/vitamin B 6 supplementation for the 40 children with ADHD, hyperactive symptoms were reduced and school performance improved. 47 This work indicates the need for controlled studies in children with ADHD and magnesium deficiency.
Omega-3 fatty acids
Sixty percent of the dry weight of the brain is composed of fats, and the largest concentration of long-chain omega-3 PUFA docosahexaenoic acid (DHA) in the body is found in the retina, brain, and nervous system. 48 There is evidence that DHA is required for nerve cell myelination and is thus critical for neural transmission. 49 Importantly, DHA levels in neural membranes vary according to dietary PUFA intake. 49 , 50 DHA precursor eicosapentaenoic acid (EPA) is also believed to have important functions in the brain, 51 possibly via its role in synthesis of eicosanoids with anti-inflammatory, anti-thrombotic, and vasodilatory properties. Animal studies have associated omega-3 levels with levels of neurotransmitters dopamine and serotonin; 52 , 53 we have proposed that one of their primary influences on mental health may also be via improved cerebral vascular function. 28
In the 1980s, researchers observed signs of fatty acid deficiency in hyperactive children 54 ; thereafter, a number of studies found lower omega-3 PUFA levels in children with ADHD compared with controls. 55 , – 59 Randomized controlled trials have found equivocal results, which may be explained by variations in selection criteria, sample size, dosage and nature of the omega-3 PUFA supplement and length of supplementation. One study performed in the United States supplemented 6–12-year-old medicated boys with a “pure” ADHD diagnosis (without comorbidities) with 345 mg of algae-derived DHA per day for 16 weeks and found no significant improvements on outcome measures. 60 Another study in the United States gave 50 children aged 6–13 years with ADHD symptoms and skin and thirst problems 480 mg DHA and 80 mg EPA along with 40 mg arachidonic acid (AA; omega-6 PUFA) daily over 4 months. Significant improvements were only found in conduct problems rated by parents and attention problems rated by teachers; importantly, the latter was correlated with increases in erythrocyte DHA levels. 61 A study performed in Japan using both DHA and EPA found no significant treatment effects of bread enriched with fish oil (supplying 3600 mg DHA and 700 g EPA per week) on symptoms of ADHD in a 2-month, placebo-controlled, double-blind trial with 40 children aged 6–12 who were mostly drug-free (34/40). The placebo bread contained olive oil. 62 Blood samples were not taken, so it is not clear whether this sample had a baseline deficiency in fatty acids. Given that the study was conducted in Japan, a country known to have high fish consumption, it is possible that they did not. It is also possible that 2 months may not have been a sufficient length of time for effects to become observable. Another pilot study in the United Kingdom supplemented 41 non-medicated children aged 8–12 years who had literacy problems (mainly dyslexia) and ADHD symptoms above the population average with 186 mg EPA and 480 mg DHA along with 42 mg AA per day for 12 weeks; the results showed improvements in literacy and in ADHD symptoms evaluated using Conners' Rating Scales. 63
Since these small trials, the results of two large, randomized, placebo-controlled, double-blind interventions have been published. The first was conducted in the United Kingdom with 117 non-medicated children aged 5–12 years with developmental coordination disorder; a third of these children had ADHD symptoms above the 90 th percentile, placing them in the clinical range for a probable ADHD diagnosis. On average, these children were functioning a year behind their chronological age on reading and spelling. Following 3 months of daily supplementation with 552 mg EPA and 168 mg DHA with 60 mg gamma linolenic acid (GLA; omega-6 PUFA), children in the treatment group showed significant improvements in core ADHD symptoms, as rated by teachers on Conners' Rating Scales. The treatment groups also increased their reading age by 9.5 months (compared to 3.3 months in the placebo group) and their spelling age by 6.6 months (compared to 1.2 months in the placebo group). 64 A review of the above-mentioned studies was published following the latter trial. 65
The next study (conducted by the present author) investigated treatment with the same supplement in 132 non-medicated Australian children aged 7–12 years who all had ADHD symptoms in the clinical range for a probable diagnosis. This study also investigated additive benefits of a multivitamin/mineral (MVM) supplement. There were no differences between the PUFA groups with and without the MVM supplement. However, both of the PUFA groups showed significant improvements compared to placebo in core ADHD symptoms, as rated by parents on Conners' Rating Scales over 15 weeks. 34 Cognitive assessments found significant improvements in the children's ability to switch and control their attention, and in their vocabulary. Importantly, the latter improvements mediated parent-reported improvements in inattention, hyperactivity, and impulsivity. 66 The effect sizes of the UK and Australian studies are similar to those reported in a meta-analysis of stimulant medication trials.
Our group is currently following up on these studies by comparing EPA-rich and DHA-rich oils, each providing 1 g omega-3 PUFA per day, on ADHD symptoms and literacy in children with ADHD and learning difficulties; the aim is to identify whether this subgroup with learning difficulties may be more likely to respond to omega-3 supplementation. We are also measuring erythrocyte PUFA levels to gain further information regarding baseline levels, likely responders, and the relative importance of EPA and DHA versus sunflower oil (containing omega-6 PUFA).
Pycnogenol and ADHD
Antioxidants are receiving growing interest for their potential to reduce oxidative stress in the brain, which may contribute to a variety of psychiatric disorders including autism and ADHD. 67 Pycnogenol is the registered trademark for a potent antioxidant derived from maritime pine bark. It contains concentrated polyphenolic compounds, primarily procyanidins and phenolic acids (for a review of its pharmacology see Rohdewald 68 ). Pycnogenol may also increase nitric oxide production and has been reported to improve blood circulation. 69 , 70 Therefore, it may assist with cerebral blood flow, which is also thought to be impaired in ADHD.
Several anecdotal reports indicate successful treatment of ADHD symptoms with Pycnogenol. 71 , 72 In one case report, parents gave Pycnogenol to their 10-year-old boy with ADHD following unsuccessful response to stimulant medication. They noted significant improvements in target symptoms over 2 weeks. When they agreed to try him on stimulant medication without the Pycnogenol again, he reportedly became significantly more hyperactive and impulsive and received numerous demerits at school. When Pycnogenol supplementation was reinstated, he again improved within 3 weeks. 72
Only two controlled studies with Pycnogenol have been conducted. One compared Pycnogenol with methylphenidate and placebo in a three-way crossover trial with 24 adults aged 24–50 years who met the criteria for ADHD. They were all given 1 mg/lb body weight Pycnogenol per day, methylphenidate (increased gradually from 10 mg to 45 mg per day) and placebo for 3 weeks, each separated by a 1-week washout. No significant improvements were observed in the methylphenidate or the Pycnogenol groups compared with placebo. It is possible that there was no treatment effect in this group or, alternatively, that 3 weeks was not long enough and/or the sample was too heterogenous and the sample size too small. 73
In the other study, 61 children aged 9–14 years with ADHD symptoms [diagnosed as hyperkinetic disorder ( n = 44), hyperkinetic conduct disorder ( n = 11), or ADD ( n = 6)] were randomly allocated to receive 1 mg/kg body weight of Pycnogenol or placebo daily for 1 month and assessed again following an additional month of treatment washout. Significant improvements were observed in the treatment groups after 1 month, as measured by teacher ratings of hyperactivity and inattention, parent ratings of hyperactivity, and visual-motoric coordination and concentration. Symptoms tended to relapse following the 1-month washout. 74 Importantly, biomarkers of oxidative damage decreased in the treatment group compared with placebo, and this was associated with improvement in symptoms. 75 , – 77 Further controlled studies are clearly warranted to investigate effects of Pycnogenol on ADHD symptoms in children.
A summary of double-blind, randomized, placebo-controlled nutritional interventions for ADHD, including Pycnogenol, is provided in Table 1 .
Summary of double-blind, randomized, placebo-controlled trials of nutrition in ADHD evaluating zinc, iron, omega-3 fatty acids, and Pycnogenol.
| Reference . | Participants . | Daily dose . | Length of trial . | Measures . | Outcomes . |
|---|---|---|---|---|---|
| Bilici et al. (2004) | = 400; mean age 9.4, = 1.5 (78% boys). DSM-IV ADHD diagnosis, unmedicated | Zinc: 150 mg zinc sulfate | 12 weeks | ADHD Scale (ADHDS); ACTQ; DuPaul Parent Ratings of ADHD | > . Treatment = placebo on remaining measures |
| Akhondzadeh et al. (2004) | = 44; mean age 7.88, = 1.67 (59% boys); DSM-IV ADHD diagnosis, medicated | Zinc: 55 mg zinc sulfate | 6 weeks | Parent and Teacher ADHD Rating Scales | > |
| Konofal et al. (2008) | = 23; 5–8 year old (78% boys); non-anemic, iron-deficient (serum ferritin levels < 30 ng/mL); met DSM-IV criteria for ADHD; 16 had restless legs | Iron: 80 mg ferrous sulfate | 12 weeks | CPRS; CTRS; ADHD rating scale; CGI-S; restless legs | > ; Treatment > placebo on CPRS and CTRS; not significant; |
| Voigt et al. (2001) | = 54; 6–12 years old (78% boys); idiopathic ADHD diagnosis; were being treated successfully with medication | n-3 PUFA: 345 mg DHA | 16 weeks | CPRS; CBC; TOVA; CCT | Treatment = placebo on all measures |
| Stevens et al. (2003) | = 50; 6–13 years old (78% boys); ADHD diagnosis; high FADS ; some on medication (equally allocated to conditions) | n-3 and n-6 PUFA: 96 mg GLA, 40 mg AA, 80 mg EPA, 480 mg DHA, 24 mg Vit E | 16 weeks | DBD; ASQ; CPT; WJPEB-R; FADS | > . Other 14 outcome measures non-significant |
| Hirayama et al. (2004) | = 40; 6–12 years old (80% boys); ADHD diagnosis; 15% medicated; 82% comorbid conditions | n-3 PUFA: 100 mg EPA, 514 mg DHA | 8 weeks | DSMV-IV ADHD; DTVP; STM; CPT; Other | Treatment = placebo on all measures (except that placebo > treatment on CPT and STM) |
| Richardson et al. (2002) | = 29; 8–12 years old (62% boys); normal IQ; low reading ability; above-average ADHD scores on Conners' Index; no participants in treatment for ADHD | n-3 and n-6 PUFA: 864 mg LA, 42 mg AA, 96 mg LNA, 186 mg EPA, 480 mg DHA, 60 iµ Vit E | 12 weeks | CPRS | > |
| Richardson et al. (2005) | = 117; 5–12 years old (77% boys); developmental coordination disorder, 1/3 with ADHD symptoms in clinical range, not in treatment; IQ > 70 | n-3 and n-6 PUFA: 60 mg AA, 10 mg GLA, 558 mg EPA, 174 mg DHA, 9.6 mg Vit E | 12 weeks active vs placebo; one-way crossover to active treatment for 12 weeks | MABC; WORD; CTRS | > . Treatment = placebo: MABC |
| Sinn et al. (2007) | = 132 (questionnaire data available for 104); 7–12 years old (74% boys); ADHD symptoms in clinical range; unmedicated | n-3 and n-6 PUFA: 60 mg AA, 10 mg GLA, 558 mg EPA, 174 mg DHA, 9.6 mg Vit E | 15 weeks active vs placebo; one-way crossover to active treatment for 15 weeks | CPRS; CTRS | > . Treatment = placebo on other subscales and CTRS |
| Tenenbaum et al. (2002) | = 24; 24–50 years old (46% males); ADHD combined type | Pycnogenol: 1 mg/lb body weight | 3 weeks on Pycnogenol, methylphenidate and placebo separated by 1-week wash-out | Barkley's ADHD Scale; ADSA; BDI; BAI; clinical interviews; CSC for AADD; BIS; Brown ADD scales; CPT | Treatment = placebo on all measures |
| Trebatická et al. (2006) | = 61; 6–14 years old (82% boys); hyperkinetic disorder, hyperkenetic conduct disorder, attention deficit without hyperactivity | Pycnogenol: 1 mg/kg body weight | 1 month treatment or placebo; 1-month washout | CAP; CTRS; CPRS; PDW | > Treatment = placebo on remaining subscales |
| Reference . | Participants . | Daily dose . | Length of trial . | Measures . | Outcomes . |
|---|---|---|---|---|---|
| Bilici et al. (2004) | = 400; mean age 9.4, = 1.5 (78% boys). DSM-IV ADHD diagnosis, unmedicated | Zinc: 150 mg zinc sulfate | 12 weeks | ADHD Scale (ADHDS); ACTQ; DuPaul Parent Ratings of ADHD | > . Treatment = placebo on remaining measures |
| Akhondzadeh et al. (2004) | = 44; mean age 7.88, = 1.67 (59% boys); DSM-IV ADHD diagnosis, medicated | Zinc: 55 mg zinc sulfate | 6 weeks | Parent and Teacher ADHD Rating Scales | > |
| Konofal et al. (2008) | = 23; 5–8 year old (78% boys); non-anemic, iron-deficient (serum ferritin levels < 30 ng/mL); met DSM-IV criteria for ADHD; 16 had restless legs | Iron: 80 mg ferrous sulfate | 12 weeks | CPRS; CTRS; ADHD rating scale; CGI-S; restless legs | > ; Treatment > placebo on CPRS and CTRS; not significant; |
| Voigt et al. (2001) | = 54; 6–12 years old (78% boys); idiopathic ADHD diagnosis; were being treated successfully with medication | n-3 PUFA: 345 mg DHA | 16 weeks | CPRS; CBC; TOVA; CCT | Treatment = placebo on all measures |
| Stevens et al. (2003) | = 50; 6–13 years old (78% boys); ADHD diagnosis; high FADS ; some on medication (equally allocated to conditions) | n-3 and n-6 PUFA: 96 mg GLA, 40 mg AA, 80 mg EPA, 480 mg DHA, 24 mg Vit E | 16 weeks | DBD; ASQ; CPT; WJPEB-R; FADS | > . Other 14 outcome measures non-significant |
| Hirayama et al. (2004) | = 40; 6–12 years old (80% boys); ADHD diagnosis; 15% medicated; 82% comorbid conditions | n-3 PUFA: 100 mg EPA, 514 mg DHA | 8 weeks | DSMV-IV ADHD; DTVP; STM; CPT; Other | Treatment = placebo on all measures (except that placebo > treatment on CPT and STM) |
| Richardson et al. (2002) | = 29; 8–12 years old (62% boys); normal IQ; low reading ability; above-average ADHD scores on Conners' Index; no participants in treatment for ADHD | n-3 and n-6 PUFA: 864 mg LA, 42 mg AA, 96 mg LNA, 186 mg EPA, 480 mg DHA, 60 iµ Vit E | 12 weeks | CPRS | > |
| Richardson et al. (2005) | = 117; 5–12 years old (77% boys); developmental coordination disorder, 1/3 with ADHD symptoms in clinical range, not in treatment; IQ > 70 | n-3 and n-6 PUFA: 60 mg AA, 10 mg GLA, 558 mg EPA, 174 mg DHA, 9.6 mg Vit E | 12 weeks active vs placebo; one-way crossover to active treatment for 12 weeks | MABC; WORD; CTRS | > . Treatment = placebo: MABC |
| Sinn et al. (2007) | = 132 (questionnaire data available for 104); 7–12 years old (74% boys); ADHD symptoms in clinical range; unmedicated | n-3 and n-6 PUFA: 60 mg AA, 10 mg GLA, 558 mg EPA, 174 mg DHA, 9.6 mg Vit E | 15 weeks active vs placebo; one-way crossover to active treatment for 15 weeks | CPRS; CTRS | > . Treatment = placebo on other subscales and CTRS |
| Tenenbaum et al. (2002) | = 24; 24–50 years old (46% males); ADHD combined type | Pycnogenol: 1 mg/lb body weight | 3 weeks on Pycnogenol, methylphenidate and placebo separated by 1-week wash-out | Barkley's ADHD Scale; ADSA; BDI; BAI; clinical interviews; CSC for AADD; BIS; Brown ADD scales; CPT | Treatment = placebo on all measures |
| Trebatická et al. (2006) | = 61; 6–14 years old (82% boys); hyperkinetic disorder, hyperkenetic conduct disorder, attention deficit without hyperactivity | Pycnogenol: 1 mg/kg body weight | 1 month treatment or placebo; 1-month washout | CAP; CTRS; CPRS; PDW | > Treatment = placebo on remaining subscales |
Positive treatment effects are presented in italic.
Abbreviations : n-3 PUFA, omega-3 polyunsaturated fatty acids; n-6 PUFA, omega-6 polyunsaturated fatty acids; ACTQ, Turkish adaptation of Conners' Teacher Rating Scales; ADSA, Attention Deficit Scales for Adults; CPRS, Conners' Parent Rating Scales; ASQ, Conners' Abbreviated Symptom Questionnaires; BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; BIS, Barratt Impulsiveness Scale; Brown ADD Scales; CAP, Child Attention Problems, Teacher rating scale; CBC, Child Behavior Checklist; CCT, Children's Color Trails test; CGI-S, Clinical Global Impression-Severity; CPT, Continuous Performance Test; CPT, Conners' Continuous Performance Test; CSC for AADD, Copeland Symptom Checklist for Adult Attention Deficit Disorders; CTRS, Conners' Teacher Rating Scales; DBD, Disruptive Behavior Disorders rating scale; DTVP, Development Test of Visual Perception; FADS, fatty acid deficiency symptoms; MABC, Movement Assessment Battery for Children; Other, 2 questions assessing aggression and 2 questions assessing impulsivity; PDW, Prague Wechsler Intelligence Scale for Children (modified Wechsler Intelligence Scale for Children, WISC); RBPC, Revised Behavior Problem Checklist; STM, short-term memory; TOVA, Test of Variables of Attention; Vit E, vitamin E (α-tocopheryl acetate); WJPEB-R, Woodstock-Johnston Psycho-Educational Battery – Revised; WORD, Wechsler Objective Reading Dimensions.
In addition to nutritional influences, there is evidence that many of these children react to certain foods and/or food additives. Suggestions of links between diet and behavior go back to the 1920s; they became well-known in the 1970s with the Feingold diet, which focused on eliminating naturally occurring salicylates, artificial food colors, artificial flavors, and the preservative butylated hydroxytoluene. 78
Behavioral reactions to food substances are associated with pharmacological rather than allergic mechanisms, although it is possible that these reactions coexist. 79 Underlying mechanisms for behavioral food reactions are not entirely clear. Increased motor activity was identified in neonatal rats following ingestion of red food color; 80 other early animal studies linked reactions to the nervous system, e.g., similar hyperactive response was identified to dopamine depletion as well as administration of sulfanilic acid, an azo food dye metabolite, in developing rats; 81 dose-dependent increase in red food color may increase the release of acetylcholine into neuromuscular synapses; and colors may affect uptake of neurotransmitters. 82 In support of animal studies, EEG readings were reported to normalize in nearly 50% of children ( n = 20) with behavior disorders after starting an elimination diet. 83 Behavioral food reactions may be attributable to the presence of metals, including lead, mercury, and arsenic, in food colorings, 84 which warrants investigation.
Feingold reported that more than half of children who adhered to his elimination diet responded favorably and that many children's behavioral symptoms reached the normal range. It has since been discovered, however, that many of the foods in his diet contained salicylates, and that many of these children also react to other food components such as food coloring. 79 The complexities of dietary intervention, most notably the large variety of potentially suspect food substances and individual differences in the nature and dosage of the food intolerance, resulted in inconsistencies in subsequent research trials. Many of these studies also had interpretational issues 85 and methodological limitations involving the formulation of the intervention diet as well as the placebo diet and washout periods between them. Additionally, subsequent research has adapted to increasing knowledge on salicylates and potentially reactive food substances including amines. 86
Dietary interventions for ADHD and their inconsistent findings have generated a great deal of controversy, as have titles such as “Diet and child behavior problems: fact or fiction?” 87 However, despite methodological difficulties of measuring dietary complexities and individual variation, a recent review cited eight controlled studies that found either significant improvement following a “few-food” (oligoantigenic) diet compared with placebo or worsening of symptoms in placebo-controlled challenges of offending substances following an open challenge to identify the substance. 88
A meta-analysis of 15 double-blind, placebo-controlled trials focusing specifically on artificial food colors found that these food additives promoted hyperactive behavior in hyperactive children. 89 Following this meta-analysis a randomized, double-blind, placebo-controlled, crossover challenge trial with 153 children aged 3 years and 144 children aged 8/9 years from a general population of children reported significant effects of artificial colors and sodium benzoate preservative on hyperactive behavior. 90 It should be noted that the food colorings and preservative (or placebo) were delivered in fruit juice containing salicylates, which could have confounded the effects for the more hyperactive children at risk for salicylate sensitivity. It is interesting that this study demonstrated hyperactive effects of food colorings on healthy children from a general population, thus expanding the effects of food colorings beyond children with sensitivities.
Research to date indicates that nutrition and diet may have a role in the hyperactivity and concentration/attention problems associated with ADHD in children. In children with suboptimal levels of iron, zinc, and magnesium, there is some support for improvements being achieved with supplementation of these nutrients. There are also indications that supplementation with Pycnogenol might assist with symptoms. However, more well-controlled clinical trials are required. The strongest support so far is for omega-3 PUFA and behavioral reactions to food colorings. Research still needs to determine optimal levels of these nutrients for this group of children and markers of food sensitivity (currently requiring time-intensive dietary challenges) in order to inform clinical practice in the identification of potential deficiencies and/or behavioral food reactions. Suggestions that these children often react to inhaled environmental substances such as petrol fumes, perfumes, fly sprays, and felt pens, also require further investigation. 86
There are clearly multiple influences on ADHD, including genetic and environmental (parental, social) factors. Whether these constitute different groups of children or whether there is a common underlying component to some or all of these remains to be determined. A recent study found lower omega-3 PUFA levels in 35 young adults with ADHD than in 112 controls, but levels of iron, zinc, magnesium, or vitamin B 6 were not reduced. 91 However, since zinc is required for the metabolism of other nutrients, zinc deficiencies may contribute to suboptimal levels of nutrients such as omega-3 PUFA. In addition, a genetic problem with enzyme production or absorption of nutrients may predispose children to nutrient deficiencies and/or excessive oxidation, thus contributing concurrently to food sensitivities. Adverse genetic, environmental, and nutritional conditions may exacerbate psychosocial factors (e.g., it is easier to parent a child with an easygoing, undemanding personality). In order to provide optimal treatment for these children, all of these possibilities need to be explored in multidisciplinary, multimodal, research models that take all potential factors into consideration.
Declaration of interest . NS is the current recipient of an Australian Research Council Fellowship, with contributions by Novasel Australia, for the 3-year project “Cognitive and behavioral benefits of omega-3 fatty acids across the lifespan”.
Rowland AS Lesesne CA Abramowitz AJ . The epidemiology of attention-deficit/hyperactivity disorder: a public health view . Ment Retard Dev Disabil Res Rev . 2002 ; 8 : 162 – 170 .
Google Scholar
Birleson P Sawyer M Storm V . The mental health of young people in Australia: child and adolescent component of the national survey – a commentary . Australas Psychiatry . 2000 ; 8 : 358 – 362 .
Root RWI Resnick RJ . An update on the diagnosis and treatment of attention-deficit/hyperactivity disorder in children . Prof Psychol Res Pract . 2003 ; 34 : 34 – 41 .
Biederman J Faraone S Mick E , et al. Attention-deficit hyperactivity disorder and juvenile mania: an overlooked comorbidity? J Am Acad Child Adolesc Psychiatry . 1996 ; 35 : 997 – 1008 .
Colledge E Blair RJR . The relationship in children between the inattention and impulsivity components of attention deficit and hyperactivity disorder and psychopathic tendencies . Pers Individ Diff . 2001 ; 30 : 1175 – 1187 .
Crowley TJ Mikulich SK MacDonald M Young SE Zerbe GO . Substance-dependent, conduct-disordered adolescent males: severity of diagnosis predicts 2-year outcome . Drug Alcohol Depend . 1998 ; 49 : 225 – 237 .
Biederman J . ADHD across the lifecycle . Biol Psychiatry . 1997 ; 42 (Suppl): 146 .
Ingram S Hechtman L Morgenstern G . Outcome issues in ADHD: adolescent and adult long-term outcome . Ment Retard Dev Disabil . 1999 ; 5 : 243 – 250 .
Fossati A Novella L Donati D Donini M Maffei C . History of childhood attention deficit/hyperactivity disorder symptoms and borderline personality disorder: a controlled study . Compr Psychiatry . 2002 ; 43 : 369 – 377 .
Rey JM Morris-Yates A Singh M Andrews G Steward GW . Continuities between psychiatric disorders in adolescents and personality disorders in young adults . Am J Psychiatry . 1995 ; 152 : 895 – 900 .
Pliszka SR . Comorbidity of attention-deficit/hyperactivity disorder with psychiatric disorder: An overview . J Clin Psychiatry . 1998 ; 59 (Suppl 7 ): 50 – 58 .
Warner-Rogers J Taylor A Taylor E Sandberg S . Inattentive behavior in childhood: epidemiology and implications for development . J Learn Disabil . 2000 ; 33 : 520 – 536 .
Barkley RA . Issues in the diagnosis of attention-deficit/hyperactivity disorder in children . Brain Dev . 2003 ; 25 : 77 – 83 .
MTA Cooperative Group . National Institute of Mental Health Multimodal Treatment Study of ADHD Follow-up: changes in effectiveness and growth after the end of treatment . Pediatr . 2004 ; 113 : 762 – 769 .
Swanson JM Elliot GR Greenhill LL , et al. Effects of stimulant medication on growth rates across 3 years in the MTA follow-up . J Am Acad Child Adolesc Psychiatry . 2007 ; 46 : 1015 – 1027 .
Molina BSG Flory K Hinshaw SP , et al. Delinquent behavior and emerging substance use in the MTA at 36 months: prevalence, course, and treatment effects . J Am Acad Child Adolesc Psychiatry . 2007 ; 46 : 1028 – 1040 .
Bradley JDD Golden CJ . Biological contributions to the presentation and understanding of attention-deficit/hyperactivity disorder: a review . Clin Psychol Rev . 2001 ; 21 : 907 – 929 .
Mann CA Lubar JF Zimmerman AW Miler CA Muenchen RA . Quantitative analysis of EEG in boys with attention-deficit-hyperactivity disorder: controlled study with clinical implications . Pediatr Neurol . 1992 ; 8 : 30 – 36 .
Riccio CA Hynd GW Cohen MJ Gonzalez JJ . Neurological basis of attention deficit hyperactivity disorder . Except Child . 1993 ; 60 : 118 – 124 .
Richardson AJ . Clinical trials of fatty acid supplementation in ADHD . In: Glen AIM Peet M Horrobin DF , eds. Phospholipid Spectrum Disorders in Psychiatry and Neurology . Marius Press : Carnforth. 2003 : 529 – 541
Google Preview
Curtis LT Patel K . Nutritional and environmental approaches to preventing and treating autism and attention deficit hyperactivity disorder (ADHD): a review . J Altern Complement Med . 2008 ; 14 : 79 – 85 .
Braun JM Kahn RS Froehlich T Auinger P Lanphear BP . Exposures to environmental toxicants and attention deficit hyperactivity disorder in US children . Env Health Perspect . 2006 ; 114 : 1904 – 1909 .
Lecours AR Mandujano M Romero G . Ontogeny of brain and cognition: relevance to nutrition research . Nutr Rev . 2001 ; 59 (Suppl): S7 – S11 .
Hetzel BS . Iodine and neuropsychological development . J Nutr . 2000 ; 130 (Suppl): S493 – S495 .
Martinez M . Docosahexaenoic acid therapy in docosahexaenoic acid-deficient patients with disorders of peroxisomal biogenesis . Lipids . 1996 ; 31 (Suppl): S145 – S152 .
Uauy R Peirano P Hoffman D Mena P Birch D Birch E . Role of essential fatty acids in the function of the developing nervous system . Lipids . 1996 ; 31 (Suppl): S167 – S176 .
Haller J . Vitamins and brain function . In: Lieberman HR Kanarek RB Prasad C , eds. Nutritional Neuroscience . Taylor & Francis Group : Boca Raton. 2005 : 207 – 233 .
Sinn N Howe PRC . Mental health benefits of omega-3 fatty acids may be mediated by improvements in cerebral vascular function . Biosci Hypotheses . 2008 ; 1 : 103 – 108 .
Arnold LE DiSilvestro RA . Zinc in attention-deficit/hyperactivity disorder . J Child Adolesc Psychopharmacol . 2005 ; 15 : 619 – 627 .
Ward NI Soulsbury KA Zettel VH Colquhoun ID Bunday S Barnes B . The influence of the chemical additive tartrazine on the zinc status of hyperactive children – a double-blind placebo-controlled study . J Nutr Environ Med . 1990 ; 1 : 51 – 57 .
Bilici M Yildirim F Kandil S , et al. Double-blind, placebo-controlled study of zinc sulfate in the treatment of attention deficit hyperactivity disorder . Prog Neuropsychopharmacol Biol Psychiatry . 2004 ; 28 : 181 – 190 .
Akhondzadeh S Mohammadi M-R Khademi M . Zinc sulfate as an adjunct to methylphenidate for the treatment of attention deficit hyperactivity disorder in children: a double blind and randomized trial . BMC Psychiatry . 2004 ; 4 : 9
Bekaroglu M Aslan Y Gedik Y , et al. Relationships between serum free fatty acids and zinc, and attention deficit hyperactivity disorder: a research note . J Child Psychol Psychiatry . 1996 ; 37 : 225 – 227 .
Sinn N Bryan J . Effect of supplementation with polyunsaturated fatty acids and micronutrients on ADHD-related problems with attention and behavior . J Dev Behav Pediatr . 2007 ; 28 : 82 – 91 .
Black MM . Micronutrient deficiencies and cognitive functioning . J Nutr . 2003 ; 133 (Suppl): S3927 – S3931 .
Konofal E Lecendreux M Arnulf I Mouren MC . Iron deficiency in children with attention-deficit/hyperactivity disorder . Arch Pediatr Adolesc Med . 2004 ; 158 : 1113 – 1115 .
Oner O Alkar IY Oner P . Relation of ferritin levels with symptoms ratings and cognitive performance in children with attention deficit-hyperactivity disorder . Pediatr Int . 2008 ; 50 : 40 – 44 .
Konofal E Cortese S . Lead and neuroprotection by iron in ADHD . Environ Health Perspect . 2007 ; 115 : A398 – A399 .
Konofal E Cortese S Marchand M Mouren MC Arnulf I Lecendreux M . Impact of restless legs syndrome and iron deficiency on attention-deficit/hyperactivity disorder in children . Sleep Med . 2007 ; 8 : 711 – 715 .
Oner P Dirik EB Taner Y Caykoylu A Anlar O . Association between low serum ferritin and restless legs syndrome in patients with attention deficit hyperactivity disorder . Tohoku J Exp Med . 2007 ; 213 : 269 – 276 .
Sever Y Ashkenazi A Tyano S Weizman A . Iron treatment in children with attention deficit hyperactivity disorder. A preliminary report . Neuropsychobiol . 1997 ; 35 : 178 – 180 .
Konofal E Cortese S Lecendreux M Arnulf I Mouren MC . Effectiveness of iron supplementation in a young child with attention-deficit/hyperactivity disorder . Pediatrics . 2005 ; 116 : e732 – e734 .
Konofal E Lecendreux M Deron J , et al. Effects of iron supplementation on attention deficit hyperactivity disorder in children . Pediatr Neurol . 2008 ; 38 : 20 – 26 .
Kozielec T Starobrat-Hermelin B . Assessment of magnesium levels in children with attention deficit hyperactivity disorder (ADHD) . Magnes Res . 1997 ; 10 : 143 – 148 .
Starobrat-Hermelin B Kozielec T . The effects of magnesium physiological supplementation on hyperactivity in children with attention deficit hyperactivity disorder (ADHD). Positive response to magnesium oral loading test . Magnes Res . 1997 ; 10 : 149 – 156 .
Mousain-Bosc M Roche M Rapin J Bali J-P . Magnesium VitB6 intake reduces central nervous system hyperexcitability in children . J Am Coll Nutr . 2004 ; 23 (Suppl): S545 – S548 .
Mousain-Bosc M Roche M Polge A Pradal-Prat D Rapin J Bali J-P . Improvement of neurobehavioral disorders in children supplemented with magnesium-vitamin B6 . Magnes Res . 2006 ; 19 : 46 – 52 .
Salem N Jr Litman B Kim H-Y Gawrisch K . Mechanisms of action of docosahexaenoic acid . Lipids . 2001 ; 36 : 945 – 959 .
Youdim KA Martin A Joseph JA . Essential fatty acids and the brain: possible health implications . Int J Dev Neurosci . 2000 ; 18 : 383 – 399 .
Yehuda S Rabinovitz S Mostofsky DI . Essential fatty acids are mediators of brain biochemistry and cognitive functions . J Neurosci Res . 2000 ; 56 : 565 – 570 .
Hibbeln J Ferguson TA Blasbalg TL . Omega-3 fatty acid deficiencies in neurodevelopment, aggression and autonomic dysregulation . Int Rev Psychiatry . 2006 ; 18 : 107 – 118 .
Chalon S Vancassel S Zimmer L Guilloteau D Durand G . Polyunsaturated fatty acids and cerebral function: focus on monoaminergic neurotransmission . Lipids . 2001 ; 36 : 937 – 944 .
Chalon S . Omega-3 fatty acids and monoamine neurotransmission . Prostaglandins Leukot Essent Fatty Acids . 2006 ; 75 : 259 – 269 .
Colquhoun I Bunday S . A lack of essential fatty acids as a possible cause of hyperactivity in children . Med Hypotheses . 1981 ; 7 : 673 – 679 .
Burgess JR Stevens LJ Zhang W Peck L . Long-chain polyunsaturated fatty acids in children with attention-deficit hyperactivity disorder . Am J Clin Nutr . 2000 ; 71 : 327 – 330 .
Chen J-R Hsu S-F Hsu C-D Hwang L-H Yang S-C . Dietary patterns and blood fatty acid composition in children with attention-deficit hyperactivity disorder in Taiwan . J Nutr Biochem . 2004 ; 15 : 467 – 472 .
Mitchell EA Aman MG Tubott SH Manku M . Clinical characteristics and serum essential fatty acid levels in hyperactive children . Clin Pediatr . 1987 ; 26 : 406 – 411 .
Mitchell EA Lewis S Cutler DR . Essential fatty acids and maladjusted behaviour in children . Prostaglandins, Leukot Med . 1983 ; 12 : 281 – 287 .
Stevens LJ Zentall SS Deck JL , et al. Essential fatty acid metabolism in boys with attention-deficit hyperactivity disorder . Am J Clin Nutr . 1995 ; 62 : 761 – 768 .
Voigt RG Llorente AM Jensen CL Fraley JK Berretta MC Heird WC . A randomised, double-blind, placebo-controlled trial of docosahexaenoic acid supplementation in children with attention-deficit/hyperactivity disorder . J Pediatr . 2001 ; 139 : 189 – 196 .
Stevens LJ Zhang W Peck L , et al. EFA supplementation in children with inattention, hyperactivity, and other disruptive behaviors . Lipids . 2003 ; 38 : 1007 – 1021 .
Hirayama S Hamazaki T Terasawa K . Effect of docosahexaenoic acid-containing food administration on symptoms of attention-deficit/hyperactivity disorder – a placebo-controlled double-blind study . Eur J Clin Nutr . 2004 ; 58 : 467 – 473 .
Richardson AJ Puri BK . A randomised double-blind, placebo-controlled study of the effects of supplementation with highly unsaturated fatty acids on ADHD-related symptoms in children with specific learning difficulties . Prog Neuropsychopharmacol Biol Psychiatry . 2002 ; 26 : 233 – 239 .
Richardson AJ Montgomery P . The Oxford-Durham study: a randomised, controlled trial of dietary supplementation with fatty acids in children with developmental coordination disorder . Pediatrics . 2005 ; 115 : 1360 – 1366 .
Richardson AJ . Omega-3 fatty acids in ADHD and related neurodevelopmental disorders . Int Rev Psychiatry . 2006 ; 18 : 155 – 172 .
Sinn N Bryan J Wilson C . Cognitive effects of polyunsaturated fatty acids in children with attention deficit hyperactivity disorder symptoms: a randomised controlled trial . Prostaglandins Leukot Essent Fatty Acids . 2008 ; 78 : 311 – 326 .
Ng F Berk M Dean O Bush A . Oxidative stress in psychiatric disorders: evidence base and therapeutic implications . Int J Neuropsychopharmacol . 2008 ; 21 : 1 – 26 .
Rohdewald P . A review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology . Int J Clin Pharmacol Ther . 2002 ; 40 : 158 – 168 .
Fitzpatrick DF Bing B Rohdewald P . Endothelium-dependent vascular effects of Pycnogenol . J Cardiovasc Pharmacol . 1998 ; 32 : 509 – 515 .
Nishioka K Hidaka T Nakamura S , et al. Pycnogenol, French maritime pine bark extract, augments endothelium-dependent vasodilation in humans . Hypertens Res . 2007 ; 30 : 775 – 780 .
Greenblatt J . Nutritional supplements in ADHD . J Am Acad Child Adolesc Psychiatry . 1999 ; 38 : 1209 – 1210 .
Heimann SW . Pycnogenol for ADHD? J Am Acad Child Adolesc Psychiatry . 1999 ; 38 : 357 – 358 .
Tenenbaum S Paull JC Sparrow EP Dodd DK Green L . An experimental comparison of Pycnogenol and methylphenidate in adults with attention-deficit/hyperactivity disorder (ADHD) . 2002 ; 6 : 49 – 60 .
Trebatická J Kopasová S Hradečná Z , et al. Treatment of ADHD with French maritime pine bark extract, Pycnogenol . Eur Child Adolesc Psychiatry . 2006 ; 15 : 329 – 335 .
Chovanová Z Muchová J Sivoňová M , et al. Effect of polyphenolic extract, Pycnogenol, on the level of 8-oxoguanine in children suffering from attention deficit/hyperactivity disorder . Free Radical Res . 2006 ; 40 : 1003 – 1010 .
Dvořáková M Sivoňová M Trebatická J , et al. The effect of polyphenolic extract from pine bark, Pycnogenol, on the level of glutathione in children suffering from attention deficit hyperactivity disorder (ADHD) . Redox Report . 2006 ; 11 : 163 – 172 .
Dvořáková M Ježová D Blažíček P , et al. Urinary catecholamines in children with attention deficit hyperactivity disorder (ADHD): Modulation by a polyphenolic extract from pine bark (Pycnogenol) . Nutr Neurosci . 2007 ; 10 : 151 – 157 .
Feingold BF . Why is Your Child Hyperactive? 1975 , New York: Random House .
Swaine A Soutter V Loblay R Truswell AS . Salicylates, oligoantigenic diets, and behaviour . Lancet . 1985 ; 2 : 41 – 42 .
Shaywitz BA Goldenring JR Wool RS . The effects of chronic administration of food colorings on activity levels and cognitive performance in developing rat pups treated with 6-hydroxydopamine . Neurobehav Toxicol . 1979 ; 1 : 41 – 47 .
Goldenring JR Batter DK Shaywitz BA . Sulfanilic acid: behavioural change related to azo food dyes in developing rats . Neurobehav Toxicol Teratol . 1982 ; 4 : 43 – 49 .
Kaplita PV Triggle DJ . Food dyes: behavioural and neurochemical actions . Trends Pharmacol Sci . 1982 ; 3 : 70 – 71 .
Kittler FJ Baldwin DG . The role of allergic factors in the child with minimal brain dysfunction . Ann Allergy . 1970 ; 28 : 203 – 206 .
Food and Drug Administration . Food ingredients and packaging . Summary of color additives listed for use in the United States in food, drugs, cosmetics, and medical devices . 2007 : Available from: http://www.cfsan.fda.gov/~dms/opa-col2.html#table1A . Accessed July 25, 2008.
Weiss B . Food additives and environmental chemicals as sources of childhood behavior disorders . J Am Acad Child Psychiatry . 1982 ; 21 : 144 – 152 .
Breakey J Reilly C Connell H . The role of food additives and chemicals in behavioral, learning, activity, and sleep problems in children . In: Branen AL Davidson M Salminen S Thorngate JH III , eds. Food Additives . Second Edition. CRC Press . 2002 : 87 – 100 .
Cormier E . Diet and child behavior problems: fact or fiction? Pediatr Nurs . 2007 ; 33 : 138 – 143 .
Arnold LE . Treatment alternatives for attention-deficit/hyperactivity disorder . J Atten Disord . 1999 ; 3 : 30 – 48 .
Schab DW Trinh NH-T . Do artificial food colors promote hyperactivity in children with hyperactive syndromes? A meta-analysis of double-blind placebo-controlled trials . J Dev Behav Pediatr . 2004 ; 25 : 423 – 434 .
McCann D Barrett A Cooper A , et al. Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: a randomised, double-blinded, placebo-controlled trial . Lancet . 2007 ; 370 : 1560 – 1567 .
Antalis CJ Stevens LJ Campbell M Pazdro R Ericson K Burgess JR . Omega-3 fatty acid status in attention–deficit/hyperactivity disorder . Prostaglandins Leukot Essent Fatty Acids . 2006 ; 75 : 299 – 308 .
- omega-3 fatty acids
- attention-deficit/hyperactivity disorder
- science of nutrition
- adult attention deficit hyperactivity disorder
| Month: | Total Views: |
|---|---|
| January 2017 | 8 |
| February 2017 | 20 |
| March 2017 | 25 |
| April 2017 | 38 |
| May 2017 | 10 |
| June 2017 | 12 |
| July 2017 | 5 |
| August 2017 | 14 |
| September 2017 | 9 |
| October 2017 | 26 |
| November 2017 | 20 |
| December 2017 | 12 |
| January 2018 | 8 |
| February 2018 | 17 |
| March 2018 | 19 |
| April 2018 | 16 |
| May 2018 | 33 |
| June 2018 | 17 |
| July 2018 | 23 |
| August 2018 | 13 |
| September 2018 | 32 |
| October 2018 | 20 |
| November 2018 | 10 |
| December 2018 | 12 |
| January 2019 | 7 |
| February 2019 | 12 |
| March 2019 | 20 |
| April 2019 | 16 |
| May 2019 | 21 |
| June 2019 | 8 |
| July 2019 | 7 |
| August 2019 | 6 |
| September 2019 | 6 |
| October 2019 | 13 |
| November 2019 | 22 |
| December 2019 | 10 |
| January 2020 | 13 |
| February 2020 | 21 |
| March 2020 | 31 |
| April 2020 | 35 |
| May 2020 | 8 |
| June 2020 | 36 |
| July 2020 | 43 |
| August 2020 | 73 |
| September 2020 | 105 |
| October 2020 | 121 |
| November 2020 | 137 |
| December 2020 | 86 |
| January 2021 | 93 |
| February 2021 | 130 |
| March 2021 | 116 |
| April 2021 | 106 |
| May 2021 | 91 |
| June 2021 | 86 |
| July 2021 | 82 |
| August 2021 | 101 |
| September 2021 | 74 |
| October 2021 | 130 |
| November 2021 | 90 |
| December 2021 | 101 |
| January 2022 | 80 |
| February 2022 | 75 |
| March 2022 | 109 |
| April 2022 | 67 |
| May 2022 | 67 |
| June 2022 | 81 |
| July 2022 | 45 |
| August 2022 | 65 |
| September 2022 | 56 |
| October 2022 | 84 |
| November 2022 | 118 |
| December 2022 | 101 |
| January 2023 | 183 |
| February 2023 | 78 |
| March 2023 | 115 |
| April 2023 | 138 |
| May 2023 | 67 |
| June 2023 | 61 |
| July 2023 | 60 |
| August 2023 | 84 |
| September 2023 | 79 |
| October 2023 | 102 |
| November 2023 | 70 |
| December 2023 | 89 |
| January 2024 | 101 |
| February 2024 | 100 |
| March 2024 | 136 |
| April 2024 | 164 |
| May 2024 | 122 |
| June 2024 | 89 |
| July 2024 | 96 |
| August 2024 | 141 |
Email alerts
Citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 1753-4887
- Print ISSN 0029-6643
- Copyright © 2024 International Life Sciences Institute
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- PubMed/Medline
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Global burden of nutritional deficiencies among children under 5 years of age from 2010 to 2019.

1. Introduction
2. materials and methods, 2.1. data acquisition, 2.2. statistical analysis, 3.1. global burden of nutritional deficiencies, 3.1.1. incidence of nutritional deficiencies, 3.1.2. dalys of nutritional deficiencies, 3.2. main subcategories of nutritional deficiencies, 3.2.1. incidence of main subcategories of nutritional deficiencies, 3.2.2. dalys of main subcategories of nutritional deficiencies, 3.3. correlation between sdi and incidence or daly rate, 3.4. the influential factors for eapc, 4. discussion, 5. conclusions, supplementary materials, author contributions, institutional review board statement, informed consent statement, data availability statement, acknowledgments, conflicts of interest.
- Bhutta, Z.A.; Berkley, J.A.; Bandsma, R.H.J.; Kerac, M.; Trehan, I.; Briend, A. Severe childhood malnutrition. Nat. Rev. Dis. Primers 2017 , 3 , 17067. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- The burden of child and maternal malnutrition and trends in its indicators in the states of India: The Global Burden of Disease Study 1990–2017. Lancet Child Adolesc. Health 2019 , 3 , 855–870. [ CrossRef ] [ Green Version ]
- Kichloo, A.; Shaka, H.; El-Amir, Z.; Wani, F.; Singh, J.; Velazquez, G.R.; Edigin, E.; Dahiya, D. In-patient outcomes of patients with diabetic ketoacidosis and concurrent protein energy malnutrition: A national database study from 2016 to 2017. Postgrad. Med. 2021 , 133 , 854–859. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bailey, R.L.; West, K.P., Jr.; Black, R.E. The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab. 2015 , 66 (Suppl. 2), 22–33. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wells, J.C.; Sawaya, A.L.; Wibaek, R.; Mwangome, M.; Poullas, M.S.; Yajnik, C.S.; Demaio, A. The double burden of malnutrition: Aetiological pathways and consequences for health. Lancet 2020 , 395 , 75–88. [ Google Scholar ] [ CrossRef ]
- Mathur, A.; Tahilramani, G.; Makhija, S.; Devgan, V. Burden of Severe Acute Malnutrition in under-five Children (2–59 Months) Admitted in a Tertiary Care Hospital of Delhi. J. Trop. Pediatr. 2018 , 64 , 45–50. [ Google Scholar ] [ CrossRef ]
- Hassen, H.Y.; Ali, J.H.; Gebreyesus, S.H.; Endris, B.S.; Temesgen, A.M. National incidence, prevalence and disability-adjusted life years (DALYs) of common micronutrient deficiencies in Ethiopia from 1990 to 2017: Estimates from the global burden of diseases study. Glob. Health Action 2020 , 13 , 1776507. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Alderman, H.; Behrman, J.R.; Glewwe, P.; Fernald, L.; Walker, S. Evidence of Impact of Interventions on Growth and Development during Early and Middle Childhood. In Child and Adolescent Health and Development , 3rd ed.; Chapter 7, PMID: 30212122; Bundy, D.A.P., Silva, N.D., Horton, S., Jamison, D.T., Patton, G.C., Eds.; The International Bank for Reconstruction and Development/The World Bank: Washington, DC, USA, 2017. [ Google Scholar ]
- Mwene-Batu, P.; Bisimwa, G.; Baguma, M.; Chabwine, J.; Bapolisi, A.; Chimanuka, C.; Molima, C.; Dramaix, M.; Kashama, N.; Macq, J.; et al. Long-term effects of severe acute malnutrition during childhood on adult cognitive, academic and behavioural development in African fragile countries: The Lwiro cohort study in Democratic Republic of the Congo. PLoS ONE 2020 , 15 , e0244486. [ Google Scholar ] [ CrossRef ]
- Bringas Vega, M.L.; Guo, Y.; Tang, Q.; Razzaq, F.A.; Calzada Reyes, A.; Ren, P.; Paz Linares, D.; Galan Garcia, L.; Rabinowitz, A.G.; Galler, J.R.; et al. An Age-Adjusted EEG Source Classifier Accurately Detects School-Aged Barbadian Children That Had Protein Energy Malnutrition in the First Year of Life. Front. Neurosci. 2019 , 13 , 1222. [ Google Scholar ] [ CrossRef ]
- GBD. Available online: http://ghdx.healthdata.org/gbd-results-tool (accessed on 27 March 2022).
- Liu, J.; Qi, X.; Wang, X.; Qin, Y.; Jiang, S.; Han, L.; Kang, Z.; Shan, L.; Liang, L.; Wu, Q. Evolving Patterns of Nutritional Deficiencies Burden in Low- and Middle-Income Countries: Findings from the 2019 Global Burden of Disease Study. Nutrients 2022 , 14 , 931. [ Google Scholar ] [ CrossRef ]
- Georgieff, M.K.; Krebs, N.F.; Cusick, S.E. The Benefits and Risks of Iron Supplementation in Pregnancy and Childhood. Annu. Rev. Nutr. 2019 , 39 , 121–146. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lu, Z.; O’Dell, D.; Srinivasan, B.; Rey, E.; Wang, R.; Vemulapati, S.; Mehta, S.; Erickson, D. Rapid diagnostic testing platform for iron and vitamin A deficiency. Proc. Natl. Acad. Sci. USA 2017 , 114 , 13513–13518. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Sacri, A.S.; Bocquet, A.; de Montalembert, M.; Hercberg, S.; Gouya, L.; Blondel, B.; Ganon, A.; Hebel, P.; Vincelet, C.; Thollot, F.; et al. Young children formula consumption and iron deficiency at 24 months in the general population: A national-level study. Clin. Nutr. 2021 , 40 , 166–173. [ Google Scholar ] [ CrossRef ]
- Hombali, A.S.; Solon, J.A.; Venkatesh, B.T.; Nair, N.S.; Peña-Rosas, J.P. Fortification of staple foods with vitamin A for vitamin A deficiency. Cochrane Database Syst. Rev. 2019 , 5 , Cd010068. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Victora, C.G.; Christian, P.; Vidaletti, L.P.; Gatica-Domínguez, G.; Menon, P.; Black, R.E. Revisiting maternal and child undernutrition in low-income and middle-income countries: Variable progress towards an unfinished agenda. Lancet 2021 , 397 , 1388–1399. [ Google Scholar ] [ CrossRef ]
- Stevens, G.A.; Bennett, J.E.; Hennocq, Q.; Lu, Y.; De-Regil, L.M.; Rogers, L.; Danaei, G.; Li, G.; White, R.A.; Flaxman, S.R.; et al. Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: A pooled analysis of population-based surveys. Lancet Glob. Health 2015 , 3 , e528–e536. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- World Health Orgnization. Vitamin A Deficiency. Available online: https://www.who.int/data/nutrition/nlis/info/vitamin-a-deficiency (accessed on 4 March 2022).
- World Health Orgnization. WHO Guidance Helps Detect Iron Deficiency and Protect Brain Development. Available online: https://www.who.int/news/item/20-04-2020-who-guidance-helps-detect-iron-deficiency-and-protect-brain-development (accessed on 4 May 2022).
- Pasricha, S.R.; Tye-Din, J.; Muckenthaler, M.U.; Swinkels, D.W. Iron deficiency. Lancet 2021 , 397 , 233–248. [ Google Scholar ] [ CrossRef ]
- Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 2015 , 372 , 1832–1843. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Victora, C.G.; Adair, L.; Fall, C.; Hallal, P.C.; Martorell, R.; Richter, L.; Sachdev, H.S. Maternal and child undernutrition: Consequences for adult health and human capital. Lancet 2008 , 371 , 340–357. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- United Nations. 17 Goals to Transform Our World. Available online: https://www.un.org/sustainabledevelopment/ (accessed on 22 May 2022).
- Xu, Y.; Zeng, X.; Qiu, X.; Fang, L.; Wang, Z.; Zhou, M.; Wang, L. Burden of nutritional deficiencies of children under 5 years old in China, 1990–2015. Wei Sheng Yan Jiu 2021 , 50 , 237–241. [ Google Scholar ]
- Jiang, S.; Liu, J.; Qi, X.; Wang, R.; Wang, X.; Wang, K.; Xu, Q.; Chen, P.; Meng, N.; Wu, Q.; et al. Global, Regional, and National Estimates of Nutritional Deficiency Burden among Reproductive Women from 2010 to 2019. Nutrients 2022 , 14 , 832. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Diseases, G.B.D.; Injuries, C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020 , 396 , 1204–1222. [ Google Scholar ]
- Collaborators, G.B.D.O.C. The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020 , 5 , 582–597. [ Google Scholar ]
- Liu, W.; Liu, J.; Song, Y.; Zeng, X.; Wang, X.; Mi, L.; Cai, C.; Wang, L.; Ma, J.; Zhu, J.; et al. Burden of lymphoma in China, 2006–2016: An analysis of the Global Burden of Disease Study 2016. J. Hematol. Oncol. 2019 , 12 , 115. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Liu, Z.; Jiang, Y.; Yuan, H.; Fang, Q.; Cai, N.; Suo, C.; Jin, L.; Zhang, T.; Chen, X. The trends in incidence of primary liver cancer caused by specific etiologies: Results from the Global Burden of Disease Study 2016 and implications for liver cancer prevention. J. Hepatol. 2019 , 70 , 674–683. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Global, regional, and national progress towards Sustainable Development Goal 3.2 for neonatal and child health: All-cause and cause-specific mortality findings from the Global Burden of Disease Study 2019. Lancet 2021 , 398 , 870–905. [ CrossRef ]
- Hawkes, C.; Ruel, M.T.; Salm, L.; Sinclair, B.; Branca, F. Double-duty actions: Seizing programme and policy opportunities to address malnutrition in all its forms. Lancet 2020 , 395 , 142–155. [ Google Scholar ] [ CrossRef ]
- Ruel, M.T.; Quisumbing, A.R.; Balagamwala, M. Nutrition-sensitive agriculture: What have we learned so far? Global Food Secur. 2018 , 17 , 128–153. [ Google Scholar ] [ CrossRef ]
- Moller, A.B.; Petzold, M.; Chou, D.; Say, L. Early antenatal care visit: A systematic analysis of regional and global levels and trends of coverage from 1990 to 2013. Lancet Glob. Health 2017 , 5 , e977–e983. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Martínez-García, M.; Gutiérrez-Esparza, G.O.; Roblero-Godinez, J.C.; Marín-Pérez, D.V.; Montes-Ruiz, C.L.; Vallejo, M.; Hernández-Lemus, E. Cardiovascular Risk Factors and Social Development Index. Front. Cardiovasc Med. 2021 , 8 , 631747. [ Google Scholar ] [ CrossRef ]
- Camaschella, C. Iron deficiency: New insights into diagnosis and treatment. Hematol. Am. Soc. Hematol Educ Program. 2015 , 2015 , 8–13. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Alaofè, H.; Burney, J.; Naylor, R.; Taren, D. Prevalence of anaemia, deficiencies of iron and vitamin A and their determinants in rural women and young children: A cross-sectional study in Kalalé district of northern Benin. Public Health Nutr. 2017 , 20 , 1203–1213. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Zhao, T.; Liu, S.; Zhang, R.; Zhao, Z.; Yu, H.; Pu, L.; Wang, L.; Han, L. Global Burden of Vitamin A Deficiency in 204 Countries and Territories from 1990–2019. Nutrients 2022 , 14 , 950. [ Google Scholar ] [ CrossRef ]
- Kassie, G.W.; Workie, D.L. Determinants of under-nutrition among children under five years of age in Ethiopia. BMC Public Health 2020 , 20 , 399. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Amah, D.; van Biljon, A.; Brown, A.; Perkins-Veazie, P.; Swennen, R.; Labuschagne, M. Recent advances in banana (musa spp.) biofortification to alleviate vitamin A deficiency. Crit. Rev. Food Sci. Nutr. 2019 , 59 , 3498–3510. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Copenhagen Consensus Center. Malawi Priorities: Nutrition. Available online: https://www.copenhagenconsensus.com/publication/malawi-priorities-nutrition (accessed on 21 June 2022).
- Copenhagen Consensus Center. Ghana Priorities: Nutrition. Available online: https://www.copenhagenconsensus.com/publication/malawi-priorities-food-security (accessed on 21 June 2022).
- Coker, M.; Folayan, M.O.; Michelow, I.C.; Oladokun, R.E.; Torbunde, N.; Sam-Agudu, N.A. Things must not fall apart: The ripple effects of the COVID-19 pandemic on children in sub-Saharan Africa. Pediatr. Res. 2021 , 89 , 1078–1086. [ Google Scholar ] [ CrossRef ]
- Zarocostas, J. Hope for nutrition summit as global hunger spikes. Lancet 2021 , 398 , 2061–2062. [ Google Scholar ] [ CrossRef ]
Click here to enlarge figure
| Characteristics | Incidence | DALYs | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2010 | 2019 | 2010–2019 | 2010 | 2019 | 2010–2019 | |||||
| No. | IR/10 | No. | IR/10 | EAPC (%) (95%UI) | No. | DR/10 | No. | DR/10 | EAPC (%) (95%UI) | |
| 144,595,018 | 22,208.6 | 100,511,850 | 15,163.8 | −4.4 (−4.6 to −4.1) | 710,599 | 109.1 | 505,633 | 76.3 | −4.1 (−4.4 to −3.8) | |
| 395,255 | 60.7 | 312,948 | 47.2 | −3.1 (−3.5 to −2.6) | 18,759 | 2.9 | 12,113 | 1.8 | −5.4 (−6.4 to −4.4) | |
| 0 | 0 | 0 | 0 | 0 (0 to 0) | 5,424,687 | 833.2 | 5,179,895 | 781.5 | −0.7 (−0.7 to −0.7) | |
| 66,602,727 | 10,229.6 | 61,023,138 | 9206.3 | −1.7 (−2.4 to −1.0) | 16,518,390 | 2537.1 | 9,925,276 | 1497.4 | −5.6 (−5.8 to −5.4) | |
| 0 | 0 | 0 | 0 | 0 (0 to 0) | 602,624 | 92.6 | 381,462 | 57.5 | −5.0 (−5.3 to −4.7) | |
| 77,499,851 | 50,331.0 | 64,648,780 | 37,838.3 | −3.3 (−3.6 to −3.1) | 12,654,261 | 8218.1 | 9,152,867 | 5357.1 | −4.4 (−4.8 to −3.9) | |
| 63,374,480 | 35,451.1 | 40,781,384 | 23,669 | −4.9 (−5.4 to −4.4) | 7,258,770 | 4060.5 | 4,421,343 | 2566.1 | −5.1 (−5.2 to −4.9) | |
| 35,007,606 | 19,105.9 | 23,973,677 | 13,015.7 | −4.7 (−5.1 to −4.2) | 2,709,754 | 1478.9 | 1,913,652 | 1038.9 | −4.0 (−4.3 to −3.7) | |
| 8,219,025 | 10,221.1 | 6,475,620 | 7836.4 | −3.2 (−3.6 to −2.9) | 560,604 | 697.2 | 437,042 | 528.9 | −3.1 (−3.2 to −3.0) | |
| 2,021,301 | 3724.6 | 1,817,801 | 3466.7 | −1.0 (−1.3 to −0.7) | 78,265 | 144.2 | 69,522 | 132.6 | −1.0 (−1.0 to −0.9) | |
| 211,593,000 | 32,498.9 | 161,847,936 | 24,417.2 | −3.5 (−3.9 to −3.1) | 23,275,059 | 3574.9 | 16,004,379 | 2414.5 | −4.2 (−4.4 to −4.0) | |
| 744,525 | 12,984.9 | 585,325 | 9239.7 | −3.9 (−4.3 to −3.4) | 106,016 | 1849.0 | 74,227 | 1171.7 | −4.7 (−5.3 to −4.2) | |
| 46,207 | 2628.2 | 45,598 | 2506.5 | −0.5 (−0.7 to −0.4) | 4318 | 245.6 | 4120 | 226.5 | −0.9 (−1.0 to −0.8) | |
| 664,789 | 16,818.2 | 589,157 | 14,914.6 | −1.6 (−1.9 to −1.3) | 126,167 | 3191.8 | 108,517 | 2747.1 | −1.7 (−2.0 to −1.4) | |
| 1,333,242 | 15,680.6 | 1,200,248 | 12,538.6 | −2.6 (−3.0 to −2.2) | 98,809 | 1162.1 | 92,950 | 971.0 | −2.0 (−2.1 to −1.8) | |
| 1,030,090 | 16,774.7 | 802,792 | 14,203.2 | −1.9 (−2.0 to −1.8) | 27,267 | 444.0 | 22,435 | 396.9 | −1.1 (−1.2 to −1.1) | |
| 3,563,427 | 15,438.1 | 2,592,401 | 11,970.6 | −3.1 (−3.4 to −2.7) | 324,765 | 1407.0 | 212,374 | 980.7 | −3.9 (−4.3 to −3.6) | |
| 13,318,287 | 71,995.4 | 10,737,695 | 51,872.0 | −3.8 (−4.1 to −3.6) | 1,684,209 | 9104.4 | 820,961 | 3965.9 | −9.2 (−9.6 to −8.7) | |
| 8,087,107 | 11,137.3 | 7,265,853 | 8635.6 | −2.9 (−3.3 to −2.5) | 273,307 | 376.4 | 197,281 | 234.5 | −4.8 (−5.2 to −4.5) | |
| 784,037 | 6577.9 | 763,059 | 6185.4 | −1.1 (−1.6 to −0.6) | 33,719 | 282.9 | 29,051 | 235.5 | −2.1 (−2.2 to −2.0) | |
| 30,470,720 | 53,385.8 | 26,568,159 | 41,423.1 | −2.8 (−3.0 to −2.7) | 6,008,221 | 10,526.6 | 3,584,055 | 5588.0 | −6.7 (−7.4 to −6.0) | |
| 380,629 | 4738.8 | 319,033 | 4378.4 | −1.0 (−1.3 to −0.7) | 17,260 | 214.9 | 14,375 | 197.3 | −0.9 (−1.1 to −0.8) | |
| 539,065 | 2414.9 | 453,379 | 2160.6 | −1.4 (−1.7 to −1.1) | 13,619 | 61.0 | 13,813 | 65.8 | 0.9 (0.8 to 1.0) | |
| 15,029,443 | 25,188.1 | 12,214,970 | 20,453.8 | −2.6 (−3.0 to −2.1) | 1,018,213 | 1706.4 | 755,452 | 1265.0 | −3.3 (−3.3 to −3.2) | |
| 583,853 | 38,304.1 | 575,019 | 31,070.1 | −2.5 (−2.9 to −2.1) | 37,750 | 2476.6 | 37,476 | 2025.0 | −2.2 (−2.5 to −1.9) | |
| 80,495,955 | 45,888.8 | 53,510,848 | 32,548.3 | −4.3 (−4.9 to −3.7) | 7,805,120 | 4449.5 | 4,804,447 | 2922.3 | −4.7 (−4.9 to −4.5) | |
| 21,278,763 | 36,034.4 | 13,792,558 | 25,314.5 | −4.2 (−4.7 to −3.6) | 887,738 | 1503.3 | 606,269 | 1112.7 | −3.2 (−3.3 to −3.1) | |
| 769,282 | 15,784.7 | 612,018 | 12,607.6 | −2.6 (−2.8 to −2.3) | 30,155 | 618.8 | 25,864 | 532.8 | −1.6 (−2.2 to −1.1) | |
| 2,293,235 | 28,544.1 | 1,825,978 | 22,553.1 | −2.8 (−3.1 to −2.5) | 345,760 | 4303.7 | 331,076 | 4089.2 | −1.0 (−1.8 to −0.1) | |
| 3,130,047 | 18,153.3 | 2,310,875 | 14,324.3 | −2.7 (−3.2 to −2.2) | 290,412 | 1684.3 | 173,953 | 1078.3 | −5.0 (−5.7 to −4.2) | |
| 970,260 | 4242.0 | 869,238 | 3951.5 | −0.9 (−1.1 to −0.6) | 27,749 | 121.3 | 24,773 | 112.6 | −0.9 (−1.0 to −0.7) | |
| 26,080,037 | 41,552.9 | 24,213,730 | 33,291.8 | −2.6 (−2.9 to −2.2) | 4,114,484 | 6555.5 | 4,070,910 | 5597.1 | −1.0 (−1.7 to −0.3) | |
| MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
Share and Cite
Yue, T.; Zhang, Q.; Li, G.; Qin, H. Global Burden of Nutritional Deficiencies among Children under 5 Years of Age from 2010 to 2019. Nutrients 2022 , 14 , 2685. https://doi.org/10.3390/nu14132685
Yue T, Zhang Q, Li G, Qin H. Global Burden of Nutritional Deficiencies among Children under 5 Years of Age from 2010 to 2019. Nutrients . 2022; 14(13):2685. https://doi.org/10.3390/nu14132685
Yue, Tingting, Quanquan Zhang, Guangdi Li, and Hong Qin. 2022. "Global Burden of Nutritional Deficiencies among Children under 5 Years of Age from 2010 to 2019" Nutrients 14, no. 13: 2685. https://doi.org/10.3390/nu14132685
Article Metrics
Article access statistics, supplementary material.
ZIP-Document (ZIP, 1943 KiB)
Further Information
Mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
- India Today
- Business Today
- Harper's Bazaar
- Brides Today
- Cosmopolitan
- India Today Hindi
- Reader’s Digest
- Aaj Tak Campus
Download App

Why are Indians lacking iron, the nutrient needed for oxygen flow?
Nutrition-related illnesses are rapidly increasing worldwide. a study has shown that indians are deficient in several micronutrients, particularly, iron..
Listen to Story

- A significant portion of the Indian population faces iron deficiency
- Rising consumption of processed foods over fresh produce exacerbates the issue
- This purchasing pattern is leading to a 'double burden' of undernutrition and obesity
Nutrition-related illnesses have been rampantly surging worldwide. Chronic diseases like diabetes, heart disease and even cancers can stem from malnutrition, a highly prevalent health challenge in the world, including in India.
While proper nutrition is the basic right of a human being, billions cannot even afford a healthy diet. Over 2 billion people are micronutrient deficient.
According to the latest Household Consumption Expenditure Survey for 2022 to 2023, there is a continuing decline in the share of food items in the total spending basket of Indians.
A new study by the Lancet reveals that most Indians are deficient in several micronutrients particularly, iron, calcium and folate. Compared to men, more women were deficient in iodine.
These figures highlight a growing trend of people buying more processed and instant foods instead of fresh produce, including fruits and vegetables that could meet their micronutrient needs.

Iron deficiency is usually treated with dietary changes and iron supplements. Eating iron-rich foods, such as lean meats, beans, lentils, spinach, and fortified cereals, can help.
In some cases, vitamin C supplements are recommended to improve iron absorption.
Nutrition-related illnesses are rapidly increasing worldwide, with chronic diseases like diabetes and heart disease often linked to malnutrition.
In India, a significant portion of the population faces micronutrient deficiencies, particularly in iron. The rising consumption of processed foods over fresh produce exacerbates this issue, leading to a "double burden" where undernutrition and obesity coexist.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- PMC11346916

Zinc and thyroid cancer: A systematic review and meta-analysis protocol
Aline alves soares.
1 Postgraduating Program in Health Sciences, Federal University of Rio Grande do Norte, Natal, RN, Brazil
2 Department of Nutrition, Liga Contra o Câncer, Natal, RN, Brazil
Yasmin Guerreiro Nagashima
Camila xavier alves, kleyton santos de medeiros.
3 Pesquisa e Inovação, Liga Contra o Câncer, Instituto de Ensino, Natal, RN, Brazil
Márcia Marília Gomes Dantas Lopes
4 Department of Health Sciences, Federal University of Rio Grande do Norte, Natal, RN, Brazil
5 Department of Nutrition.Federal University of Rio Grande do Norte, Natal, RN, Brazil
José Brandão-Neto
6 Department of Internal Medicine, Federal University of Rio Grande do Norte, Natal, RN, Brazil
Associated Data
No datasets were generated or analysed during the current study. All relevant data from this study will be made available upon study completion.
Introduction
The thyroid cancer has the ninth larger incidence of cancer in the world. Investigations related to the exposure to metals have become important due to the sensibility of the thyroid gland to them. Studies reveal that carcinogenic progressions are associated to the deficiency of the essential trace elements. In this context, the zinc is highlighted, essential for the metabolism of the thyroidal hormone and has a potential relation with the pathogenesis of the thyroid cancer. The objective of this systematic review and meta-analysis is to evaluate the low serum zinc as a risk factor for thyroid cancer in adults.
Methods and analysis
PubMed/MEDLINE, Scopus, Embase and LILACS databases will be searched for observational studies investigating the low serum zinc as a risk factor for thyroid cancer in adults. No language or publication period restrictions will be imposed. The primary outcome will be that the low serum zinc is a risk factor for thyroid cancer. Three independent reviewers will select the studies and extract data from the original publications. The risk-of-bias will be assessed by using the Newcastle-Ottawa Quality Assessment Scale (NOS). Data synthesis will be performed using the R software (V.4.3.1) and to assess heterogeneity, we will compute the I2 statistic and the results will be based on either random-effects or fixed-effects models, depending on the heterogeneity. The Grading of Recommendations, Development, and Evaluation (GRADE) system will be used to evaluate the reliability and quality of evidence.
Prospero registration number
International Prospective Register of Systematic Reviews (PROSPERO) CRD42023463747 .
The thyroid cancer (TC) has the ninth larger incidence of cancer in the whole world [ 1 , 2 ]. And if the recent tendencies are maintained, it can become the fourth most common cancer until 2030 in the United States [ 3 ].
There is a number of reasons responsible for this high incidence, as the enhancement of access to diagnostic procedures more intensive and sensitive. Nevertheless, it has been suggested that diagnostic technologies may not totally explain the growth in TC frequency, arguing that the environmental factors, lifestyle and comorbidities may contribute with this phenomenon [ 4 – 6 ]. The previous irradiation in the head/neck, history of benign thyroid nodules, goiter and family history of proliferative thyroid disease are risk factors established for TC [ 7 , 8 ].
In addition, investigations related to exposure to metals have been becoming more important due to the sensibility of the thyroid gland to them. Studies reveal that carcinogenic progressions are associated to the excess of toxic metals (such as nickel, lead, cadmium), whereas the majority of the essential elements (selenium, zinc, magnesium) shows deficiency. This imbalance is capable of affecting the thyroid homeostasis because many of these trace elements are part of the metabolism of the thyroidal hormones, being an important risk factor in the development of TC [ 9 , 10 ].
In this context, considering the health of the thyroid gland, among the essential trace elements, zinc (Zn) is highlighted, defined as a regulator metal in a number of aspects concerning the cellular function and metabolism. With Zn deficiency, multiple nonspecific general changes in metabolism and function occur, including reductions in growth, as well as the impairment of reproductive function and neurobehavioral development [ 11 ]. In addition, Zn is essential for the metabolism of the thyroidal hormone and has a potential relation with the pathogenesis of the TC [ 12 ]. Studies reveal that the Zn serum concentration is significantly reduced in many malignant tumors [ 13 ], including the TC. Specifically in the papillary thyroid carcinoma (PTC) and medullary thyroid carcinoma (MTC), the levels of serum Zn are lower than the ones found in healthy individuals [ 13 , 14 ].
However, the results of studies concerning the Zn deficiency and TC are still inconsistent [ 13 , 15 , 16 ], showing that little is known about the role of Zn and the risk of progression of TC [ 9 ], preventing definitive recommendations.
In addition to the growing number of patients with TC 1 and the inconclusive results of studies on Zn deficiency and TC risk [ 13 , 15 , 16 ], a study exploring the serum status of this trace element with greater depth is useful, as it is considered a vital component for the proper functioning of thyroid hormone metabolism and its deficiency can have a detrimental effect on thyroid activity [ 17 ].
Research with this objective may help understand the possible biological mechanisms involved in the deficiency of Zn and the thyroid carcinogenesis, helping the diagnosis and handling of patients with the worst prognoses. With that said, the objective of this systematic review and meta-analysis is to evaluate the low serum Zn as a risk factor for TC in adults.
Materials and methods
The systematic review and meta-analysis will be conducted following the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines [ 18 ] and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [ 19 , 20 ]. This protocol is listed in the International Prospective Registry of Systematic Reviews (PROSPERO) (CRD42023463747).
Inclusion criteria
This systematic review and meta-analysis will include the following studies: observational studies (cohort, case-control, transversal) that evaluated the serum Zn a risk factor for TC; studies involving patients (age>18); with an apparently healthy population (in the controls for the case-control studies); studies without time restriction and studies published in any language.
Exclusion criteria
The studies will be excluded if they are case reports, meeting abstracts, review papers and commentaries. Children and adolescents under 18 years of age will be excluded.
The PECOT strategy
- Population: Adults (>18 years old)
- Exposure: Low serum zinc (12–16 μM Zn, equal to 0.785–1.046 mg/L) [ 21 ]
- Comparation: Adequate and/or elevated serum zinc
- Outcome: Thyroid Cancer
- Type of studies: Observational studies (cohort, case-control, transversal).
Search strategy
The following databases will be used: PubMed/MEDLINE, Scopus, Embase and LILACS. No language or publication period restrictions will be imposed.
The Medical Subject Headings (MeSH) terms will be: ((Zinc) AND (Thyroid Neoplasm OR Neoplasm, Thyroid OR Thyroid Carcinoma OR Carcinoma, Thyroid OR Cancer of Thyroid OR Thyroid Cancer OR Cancer, Thyroid OR Thyroid Adenoma OR Adenoma, Thyroid) AND (Observational Study OR Cohort Study OR Retrospective Study)) ( Table 1 ). The librarian participated in the development of the search strategy. The search strategy is shown in the S1 File .
| Pubmed/MEDLINE search strategy | |
|---|---|
| 1 | Zinc |
| 2 | 1/AND |
| 3 | Thyroid Neoplasm |
| 4 | Neoplasm, Thyroid |
| 5 | Thyroid Carcinoma |
| 6 | Carcinoma, Thyroid |
| 7 | Cancer of Thyroid |
| 8 | Thyroid Cancer |
| 9 | Cancer, Thyroid |
| 10 | Thyroid Adenoma |
| 11 | Adenoma, Thyroid |
| 12 | 3–11/OR |
| 13 | 1 AND 12 |
Other sources
The reference lists of the retrieved papers may also be used to choose appropriate research. In other words, the reference lists of the articles that were retrieved may allow the computerized literature search to be expanded. Identical strategies will be applied to other databases S1 File .
Selection of studies
With Rayyan ( https://www.rayyan.ai ), two authors, AAS and YGN, will independently filter the search results based on titles and abstracts. Reviews and duplicate entries will be eliminated from the database. There will be an Excel table with the articles in it (Google Drive). To ascertain whether the research satisfy the inclusion criteria, the same authors will examine the entire text. Any differences will be resolved by CXA, the third reviewer. A PRISMA flow diagram will be used to summarize the chosen studies Fig 1 .

Data extraction and management
In accordance with the Cochrane tool, a standardized data extraction form will be created and evaluated. Two reviewers (AAS and YGN) will extract data separately from each included study and any inconsistencies will be discussed and addressed with a third reviewer (CXA). The data extracted will include information as the name of the first author; year of publication; country; sample size; gender and age of participants; number of participants in the case group (if case-control study); number of participants in the control group (if case-control study); kind of study; follow-up period; eligibility criteria; serum zinc levels; zinc measurement methods; quality control procedure of the serum Zn measurement; quantitative method of variable analysis. Likewise, we will extract the odds ratio (OR) and the 95% confidence interval (CI) for TC risk.
Addressing missing data
Reviewers (AAS and YGN) will contact the authors or co-authors of the article if there are studies with missing, suppressed, or incomplete data. Communication will be via email. Additionally, supplementary documents related to the studies will be reviewed. If it is not feasible to obtain the necessary information, these studies will be addressed in the discussion section and excluded from the analysis.
Risk of bias assessment
The bias risks of the included researches will evaluated independently by two investigators (AAS and YGN). The Newcastle-Ottawa Quality Assessment Scale (NOS) [ 22 ] will be utilized to evaluate the methodological quality of the studies. This evaluation tool comprises eight criteria that are grouped into three overarching perspectives: choosing the study groups, group comparability, and exposures or outcomes of interest. All things on the scale are given one point, or one star, with the exception of the item "Comparability", which has a score between zero and two stars. A study that is considered high quality will receive a rating of at least six stars; a study that is considered moderate quality will receive four or five stars; and a study that is considered low quality will receive less than four stars [ 22 ].
Assessment of heterogeneity
A standard χ 2 test will assess the heterogeneity between the study outcomes at a significance threshold of p<0.1. We intended to compute the I2 statistic, a quantitative indicator of study inconsistency, to evaluate heterogeneity. Heterogeneity will only be assessed if a meta-analysis is warranted [ 23 ].
The I2 statistics <25% represented low heterogeneity, 25%-50%, moderate heterogeneity and >50%, high heterogeneity. In cases where there was substantial heterogeneity in the included studies (I2>50%), the random-effect model will be used, and when low heterogeneity exists in included studies the fixed-effects model will be used.
The R Software V.4.3.1 will be used to enter the data. The user can enter protocols, finish reviews, add text, research features, comparison tables, and study data, as well as carry out meta-analyses, with this software. The OR and 95% CI for each research will be extracted or computed for dichotomous data. The studies will be combined using the random-effects model in the event of heterogeneity (I2>50%), and the DerSimonian-Laird method will be used to get the OR and 95% CI. The robustness of the findings in relation to study quality and sample size will be investigated using sensitivity analysis. Only in the event that a meta-analysis is successful will this be feasible. In a summary table, the sensitivity analysis will be shown.
Considering the subgroup analyses, the assessment of serum Zn as a TC risk may be handled differently in the result analysis. The decision to perform subgroup analysis will take into account the heterogeneity and quantity of available studies. If a meta-analysis includes at least ten papers, we will attempt to perform subgroup analyses to account for any found heterogeneity among studies in order to provide for statistical power in these types of investigations. The nation, research type, age, gender, TC type, and Zn measuring techniques are the factors that will be taken into account.
If it is not possible to do a meta-analysis for all or part of the included studies, other research features and results will be narratively presented.
Grading quality of evidence
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) [ 24 ] method or a comparable approach that is properly stated and documented will be used to assess the degree of certainty in the evidence. The quality of evidence will be defined as “high”, “moderate”, “low,” and “very low” [ 24 ].
Ethics and dissemination
Since this review will rely on publicly available scientific literature, ethical approval is not necessary. The results of this systematic review and meta-analysis will be published in a peer-reviewed publication and if sufficient new evidence becomes available to warrant a revision in the review’s conclusions, updates will be carried out. Any modifications to the protocol made while the review was being conducted will be noted in the manuscript.
Considering that the metal ions assemble in the thyroid and some play an important part in the function and homeostatic mechanisms of the thyroid gland, Zhou et al . [ 12 ] explain that alterations in some serums may be related to the pathogenesis of the TC.
Zn is a crucial trace element in the link of triiodothyronine (T3) with the nuclear receptor and is involved in the conversion of the thyrotropin-releasing hormone (TRH) to produce TRH via proteolytic conversion by a carboxypeptidase enzyme. The most important way towards the metabolism of thyroxine (T4) is through monodeiodination to produce the active thyroidal hormone, T3. This reaction is catalyzed by deiodinases type I and II (DI and DII) that need Zn as cofactor [ 25 ]. Therefore, the decrease of the Zn serum level may have a harmful effect over the thyroid activity that may be involved in the carcinogenic activity [ 17 ].
In this case, to help understand the biological mechanisms involved in the thyroidal carcinogenesis, this study was based in the evaluation of Zn serums in patients with TC.
Findings in Stojsavljević A. et al . [ 15 ] studies have indicated that the Zn (1613 ng/g) concentration average was significantly reduced (p<0.05) in blood samples of patients with TC when compared to the ones of the control group (5147 ng/g), result that may have an important role from the clinical point of view, for the purposes of diagnostics and traces. Analyzing other studies, similar outcomes support hypothesis that low Zn serums are associated to TC [ 16 , 17 ].
The results of H. Al-Sayer et al . [ 16 ] and of Baltaci et al . [ 13 ] have discovered that the content of pre-operative Zn serum in patients with TC was significantly reduced when compared to a healthy one and that the surgical excision of the malignant thyroidal tissue has resulted in the restauration of the Zn content in regular amounts. Also, in the study by Baltaci et al . [ 13 ], measurements made immediately after the thyroid surgery have also shown lower levels of Zn serum in these patients (p<0.05). The surgical tissue though, indicated high average amounts of Zn. The fact that the same patients have presented lower zinc amounts in the serum samples indicates that this element is excessively withheld in the thyroidal tissue and can be related to the thyroid pathogenesis.
On the contrary, Rezaei M. et al . [ 26 ] couldn’t show any significant association between the Zn serum level and the risk of developing TC. The A. Emami et al . [ 14 ] study that sought to evaluate the status of micronutrients in Iranian patients with MTC before the thyroidectomy, has shown that the low Zn serum levels were not a risk factor for MTC.
Among types of TC, Bibi K and Shah MH [ 17 ] have compared the average Zn levels measured in the blood of various types of TC patients (anaplastic, follicular, medullary and papillary), identifying higher levels in anaplastic TC.
The results evidence the presence of altered Zn content in pathological blood samples in comparison to the control, indicating that the relation between Zn serum and TC is still controversial [ 13 , 15 , 16 ].
A systematic review and meta-analysis will help us to identify and synthesize the evidence of the association between Zn serum and TC. The results will also help us better understand the risk differences depending on gender, age, geographical location and types of TC. Also, a systematic review and meta-analysis about the matter will provide data about the methodology of different studies and the important points in published literature, which may help in the development of new experimental drawings, identifying the reasons of the discrepancies or contradictions between the results of the different investigations, encouraging the redrawing of the studies to improve the existing research methods.
The limitations of this review may involve the quality of primary studies, due to high methodological, clinical, and statistical heterogeneity among them. Especially, there is heterogeneity among the studies regarding Zn results and thyroid cancer risk, stemming from differences in social, demographic, and environmental factors, as well as variations in the types of TC among participants and characteristics of the measurement methods.
Supporting information
S1 checklist, acknowledgments.
The authors acknowledge the assistance provided by the Graduate Program in Health Sciences of the Federal University of Rio Grande do Norte (UFRN), the Liga Norte Riograndense Contra o Câncer and the librarian Rafaela Carla Melo de Paiva for the assistance with literary research.
Funding Statement
The author(s) received no specific funding for this work.
Data Availability

IMAGES
VIDEO
COMMENTS
Malnutrition is a condition that results from nutrient deficiency or overconsumption. Types of malnutrition include (1, 2):Undernutrition: This type of malnutrition results from not getting enough ...
Nutritional deficiencies often result in impaired function, and, conversely, intakes at recommended levels can resume or further enhance body functions. An increasing number of studies are revealing that diet and nutrition are critical not only for physiology and body composition, but also have significant effects on mood and mental well-being. ...
Iron deficiency is the most prevalent nutritional deficiency, with young children and premenopausal women at the highest risk of iron deficiency [21, 22]. Being iron a major contributor in hemoglobin synthesis, depletion of its reserves leads to microcytic hypochromic anemia, characterized by smaller-size red blood cells containing a smaller ...
Quality of Nutrition. Single nutrient interventions such as fortification of milk with vitamin D, cereal with iron, and table salt with iodine were effective in treating the corresponding nutrient deficiencies [].However, when applied to acquired metabolic syndromes that prevail in modern societies, the same approach has yielded inconclusive results [11,12].
Malnutrition. Malnutrition refers to deficiencies or excesses in nutrient intake, imbalance of essential nutrients or impaired nutrient utilization. The double burden of malnutrition consists of both undernutrition and overweight and obesity, as well as diet-related noncommunicable diseases. Undernutrition manifests in four broad forms: wasting ...
Nutritional deficiency diseases: When the supply of all nutrients is done in right amount and ratio according to body need, this is called balanced diet. The lack of any of the required nutrients in the diet is called malnutrition (means faulty or inadequate diet). It leads to deficiency of specific nutrients which is the cause of some diseases ...
The systematic review focused on nutritional diseases, nutrient toxicities, nutrients deficiency diseases, and the diets for health living. Nutritional diseases include obesity and eating ...
nutritional disease, any of the nutrient-related diseases and conditions that cause illness in humans.They may include deficiencies or excesses in the diet, obesity and eating disorders, and chronic diseases such as cardiovascular disease, hypertension, cancer, and diabetes mellitus.Nutritional diseases also include developmental abnormalities that can be prevented by diet, hereditary ...
Malnutrition refers to deficiencies, excesses, or imbalances in a person's intake of energy and/or nutrients. The term malnutrition addresses 3 broad groups of conditions: undernutrition, which includes wasting (low weight-for-height), stunting (low height-for-age) and underweight (low weight-for-age); micronutrient-related malnutrition, which includes micronutrient deficiencies (a lack of ...
Through nutrition transition, diets tend to increase in energy, refined carbohydrate and fat content, while lacking adequate protein, fiber or micronutrients [75, 76]. These shifts may simultaneously drive excess energy consumption while maintaining nutrient deficiencies.
In this essay, we will talk about both the concept of malnutrition and its effects. People of any age, race or condition who do not consume enough essential nutrients to maintain the health of their organs may experience serious health effects from nutrient shortage, including death. ... Oedemas: Nutrient deficiencies and electrolyte imbalances ...
A deficiency of single nutrients can alter the body's immune response. Animal studies have found that deficiencies in zinc, selenium, iron, copper, folic acid, and vitamins A, B6, C, D, and E can alter immune responses. [8] These nutrients help the immune system in several ways: working as an antioxidant to protect healthy cells, supporting ...
In the context of ageing, a major challenge to maintain health in old age is the imbalanced nutritional intake resulting into nutritional deficiency or malnutrition [11,12]. Among the various reasons for such a condition is the age-related decline in the digestive and metabolic activities, exacerbated by a reduced sense of taste and smell and ...
Poor nutrition may be a causal factor in the experience of low mood, and improving diet may help to protect not only the physical health but also the mental health of the population, say Joseph Firth and colleagues ### Key messages Depression and anxiety are the most common mental health conditions worldwide, making them a leading cause of disability.1 Even beyond diagnosed conditions ...
Diagnosis. Treatment. Vitamin deficiency can cause a range of symptoms such as fatigue, irritability, and changes to your skin and hair. The specific symptoms depend on which vitamin (s) are at low levels, as each one plays a different role in your body. For example, a vitamin D deficiency can lead to weak bones and fractures, while a folate ...
conclusion, nutritional deficiencies can have a significant impact on me ntal health as well as. physical health. By consuming a balanced diet that includes a variety of nutrient -dense foods ...
Two papers in this Special Issue focus on the impact of diet on chronic disease risk. ... Yet, it is well established that nutrient deficiencies or suboptimal nutritional status can contribute to the development of diseases and/or health problems (for example inadequate vitamin D and/or calcium status and their impact on bone development ...
The pathological manifestation of malnourishment will depend on the stage of brain development at the time of nutritional insult. 23 Effects of some nutrient deficiencies on development have become widely known and accepted; for instance, perinatal deficiencies in iodine - now considered the world's most preventable cause of mental ...
Under-five years of age is a critical period for children's growth and development. Nutritional deficiency during this period is associated with wasting, underweight and stunting. We aimed to conduct an epidemiological study using data derived from the GBD2019 to found the global distribution and changing trends of nutritional deficiencies among children under 5 years old, as well as the ...
Iron deficiency, also known as anemia, affects roughly 4 million to 5 million Americans every year, according to research published in the online journal PLOS One. Menstruating women are ...
A report, released in August, showed that there is an urgent need to make food systems more environmentally sustainable. India's apex health research body, the Indian Council of Medical Research (ICMR), released a set of dietary guidelines, written by researchers from the National Institute of Nutrition, highlighting that having a balanced diet prevents malnutrition.
Low intake of such nutrients could lead to nutritional deficiencies and impair an individual's health [70,72], with a negative impact on their QoL. Vitamin B12 deficiency should be highlighted, as this nutrient can only be found in animal-origin foods. Vegetarians (especially vegans) have been shown to have lower levels of serum vitamin B12.
In addition to the growing number of patients with TC 1 and the inconclusive results of studies on Zn deficiency and TC risk [13, 15, 16], a study exploring the serum status of this trace element with greater depth is useful, as it is considered a vital component for the proper functioning of thyroid hormone metabolism and its deficiency can ...