- Patient Care & Health Information
- Diseases & Conditions
- Lung cancer
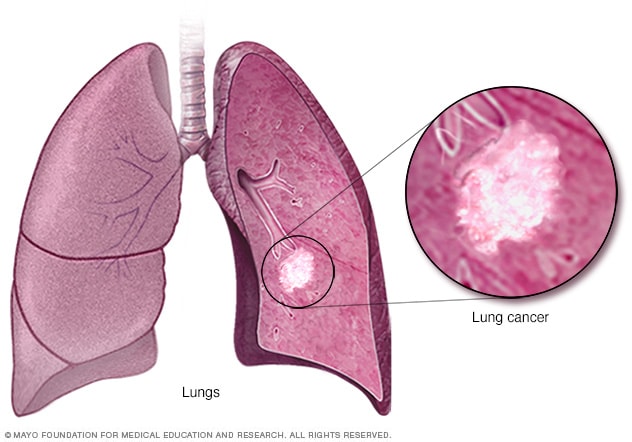
Lung cancer begins in the cells of the lungs.
Lung cancer is a kind of cancer that starts as a growth of cells in the lungs. The lungs are two spongy organs in the chest that control breathing.
Lung cancer is the leading cause of cancer deaths worldwide.
People who smoke have the greatest risk of lung cancer. The risk of lung cancer increases with the length of time and number of cigarettes smoked. Quitting smoking, even after smoking for many years, significantly lowers the chances of developing lung cancer. Lung cancer also can happen in people who have never smoked.

Products & Services
- A Book: Mayo Clinic Family Health Book
- Newsletter: Mayo Clinic Health Letter — Digital Edition
Lung cancer typically doesn't cause symptoms early on. Symptoms of lung cancer usually happen when the disease is advanced.
Signs and symptoms of lung cancer that happen in and around the lungs may include:
- A new cough that doesn't go away.
- Chest pain.
- Coughing up blood, even a small amount.
- Hoarseness.
- Shortness of breath.
Signs and symptoms that happen when lung cancer spreads to other parts of the body may include:
- Losing weight without trying.
- Loss of appetite.
- Swelling in the face or neck.
When to see a doctor
Make an appointment with your doctor or other healthcare professional if you have any symptoms that worry you.
If you smoke and haven't been able to quit, make an appointment. Your healthcare professional can recommend strategies for quitting smoking. These may include counseling, medicines and nicotine replacement products.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
Get Mayo Clinic cancer expertise delivered to your inbox.
Subscribe for free and receive an in-depth guide to coping with cancer, plus helpful information on how to get a second opinion. You can unsubscribe at any time. Click here for an email preview.
Error Select a topic
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing
Your in-depth coping with cancer guide will be in your inbox shortly. You will also receive emails from Mayo Clinic on the latest about cancer news, research, and care.
If you don’t receive our email within 5 minutes, check your SPAM folder, then contact us at [email protected] .
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
Lung cancer happens when cells in the lungs develop changes in their DNA. A cell's DNA holds the instructions that tell a cell what to do. In healthy cells, the DNA gives instructions to grow and multiply at a set rate. The instructions tell the cells to die at a set time. In cancer cells, the DNA changes give different instructions. The changes tell the cancer cells to make many more cells quickly. Cancer cells can keep living when healthy cells would die. This causes too many cells.
The cancer cells might form a mass called a tumor. The tumor can grow to invade and destroy healthy body tissue. In time, cancer cells can break away and spread to other parts of the body. When cancer spreads, it's called metastatic cancer.
Smoking causes most lung cancers. It can cause lung cancer in both people who smoke and in people exposed to secondhand smoke. But lung cancer also happens in people who never smoked or been exposed to secondhand smoke. In these people, there may be no clear cause of lung cancer.
How smoking causes lung cancer
Researchers believe smoking causes lung cancer by damaging the cells that line the lungs. Cigarette smoke is full of cancer-causing substances, called carcinogens. When you inhale cigarette smoke, the carcinogens cause changes in the lung tissue almost immediately.
At first your body may be able to repair this damage. But with each repeated exposure, healthy cells that line your lungs become more damaged. Over time, the damage causes cells to change and eventually cancer may develop.
Types of lung cancer
Lung cancer is divided into two major types based on the appearance of the cells under a microscope. Your healthcare professional makes treatment decisions based on which major type of lung cancer you have.
The two general types of lung cancer include:
- Small cell lung cancer. Small cell lung cancer usually only happens in people who have smoked heavily for years. Small cell lung cancer is less common than non-small cell lung cancer.
- Non-small cell lung cancer. Non-small cell lung cancer is a category that includes several types of lung cancers. Non-small cell lung cancers include squamous cell carcinoma, adenocarcinoma and large cell carcinoma.
Risk factors
A number of factors may increase the risk of lung cancer. Some risk factors can be controlled, for instance, by quitting smoking. Other factors can't be controlled, such as your family history.
Risk factors for lung cancer include:
Your risk of lung cancer increases with the number of cigarettes you smoke each day. Your risk also increases with the number of years you have smoked. Quitting at any age can significantly lower your risk of developing lung cancer.
Exposure to secondhand smoke
Even if you don't smoke, your risk of lung cancer increases if you're around people who are smoking. Breathing the smoke in the air from other people who are smoking is called secondhand smoke.
Previous radiation therapy
If you've had radiation therapy to the chest for another type of cancer, you may have an increased risk of developing lung cancer.
Exposure to radon gas
Radon is produced by the natural breakdown of uranium in soil, rock and water. Radon eventually becomes part of the air you breathe. Unsafe levels of radon can build up in any building, including homes.
Exposure to cancer-causing substances
Workplace exposure to cancer-causing substances, called carcinogens, can increase your risk of developing lung cancer. The risk may be higher if you smoke. Carcinogens linked to lung cancer risk include asbestos, arsenic, chromium and nickel.
Family history of lung cancer
People with a parent, sibling or child with lung cancer have an increased risk of the disease.
Complications
Lung cancer can cause complications, such as:
Shortness of breath
People with lung cancer can experience shortness of breath if cancer grows to block the major airways. Lung cancer also can cause fluid to collect around the lungs and heart. The fluid makes it harder for the affected lung to expand fully when you inhale.
Coughing up blood
Lung cancer can cause bleeding in the airway. This can cause you to cough up blood. Sometimes bleeding can become severe. Treatments are available to control bleeding.
Advanced lung cancer that spreads can cause pain. It may spread to the lining of a lung or to another area of the body, such as a bone. Tell your healthcare professional if you experience pain. Many treatments are available to control pain.
Fluid in the chest
Lung cancer can cause fluid to accumulate in the chest, called pleural effusion. The fluid collects in the space that surrounds the affected lung in the chest cavity, called the pleural space.
Pleural effusion can cause shortness of breath. Treatments are available to drain the fluid from your chest. Treatments can reduce the risk that pleural effusion will happen again.
Cancer that spreads to other parts of the body
Lung cancer often spreads to other parts of the body. Lung cancer may spread to the brain and the bones.
Cancer that spreads can cause pain, nausea, headaches or other symptoms depending on what organ is affected. Once lung cancer has spread beyond the lungs, it's generally not curable. Treatments are available to decrease symptoms and to help you live longer.
There's no sure way to prevent lung cancer, but you can reduce your risk if you:
Don't smoke
If you've never smoked, don't start. Talk to your children about not smoking so that they can understand how to avoid this major risk factor for lung cancer. Begin conversations about the dangers of smoking with your children early so that they know how to react to peer pressure.
Stop smoking
Stop smoking now. Quitting reduces your risk of lung cancer, even if you've smoked for years. Talk to your healthcare team about strategies and aids that can help you quit. Options include nicotine replacement products, medicines and support groups.
Avoid secondhand smoke
If you live or work with a person who smokes, urge them to quit. At the very least, ask them to smoke outside. Avoid areas where people smoke, such as bars. Seek out smoke-free options.
Test your home for radon
Have the radon levels in your home checked, especially if you live in an area where radon is known to be a problem. High radon levels can be fixed to make your home safer. Radon test kits are often sold at hardware stores and can be purchased online. For more information on radon testing, contact your local department of public health.
Avoid carcinogens at work
Take precautions to protect yourself from exposure to toxic chemicals at work. Follow your employer's precautions. For instance, if you're given a face mask for protection, always wear it. Ask your healthcare professional what more you can do to protect yourself at work. Your risk of lung damage from workplace carcinogens increases if you smoke.
Eat a diet full of fruits and vegetables
Choose a healthy diet with a variety of fruits and vegetables. Food sources of vitamins and nutrients are best. Avoid taking large doses of vitamins in pill form, as they may be harmful. For instance, researchers hoping to reduce the risk of lung cancer in people who smoked heavily gave them beta carotene supplements. Results showed the supplements increased the risk of cancer in people who smoke.
Exercise most days of the week
If you don't exercise regularly, start out slowly. Try to exercise most days of the week.
Lung cancer care at Mayo Clinic
- Non-small cell lung cancer. National Comprehensive Cancer Network. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450. Accessed Dec. 4, 2023.
- Small cell lung cancer. National Comprehensive Cancer Network. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1462. Accessed Dec. 4, 2023.
- Niederhuber JE, et al., eds. Cancer of the lung: Non-small cell lung cancer and small cell lung cancer. In: Abeloff's Clinical Oncology. 6th ed. Elsevier; 2020. https://www.clinicalkey.com. Accessed Dec. 4, 2023.
- Non-small cell lung cancer treatment (PDQ) – Patient version. National Cancer Institute. https://www.cancer.gov/types/lung/patient/non-small-cell-lung-treatment-pdq. Accessed Dec. 4, 2023.
- Small cell lung cancer treatment (PDQ) – Patient version. National Cancer Institute. https://www.cancer.gov/types/lung/patient/small-cell-lung-treatment-pdq. Accessed Dec. 4, 2023.
- Lung cancer – non-small cell. Cancer.Net. https://www.cancer.net/cancer-types/lung-cancer/view-all. Accessed Dec. 4, 2023.
- Lung cancer – small cell. Cancer.Net. https://www.cancer.net/cancer-types/33776/view-all. Accessed Dec. 4, 2023.
- Detterbeck FC, et al. Executive Summary: Diagnosis and management of lung cancer, 3rd ed.: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013; doi:10.1378/chest.12-2377.
- Palliative care. National Comprehensive Cancer Network. https://www.nccn.org/guidelines/guidelines-detail?category=3&id=1454. Accessed Dec. 4, 2023.
- Lung cancer. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/lung-cancer. Accessed Dec. 4, 2023.
- Cairns LM. Managing breathlessness in patients with lung cancer. Nursing Standard. 2012; doi:10.7748/ns2012.11.27.13.44.c9450.
- Warner KJ. Allscripts EPSi. Mayo Clinic. Jan. 13, 2020.
- Brown AY. Allscripts EPSi. Mayo Clinic. July 30, 2019.
- Searching for cancer centers. American College of Surgeons. https://www.facs.org/search/cancer-programs. Accessed Dec. 4, 2023.
- Temel JS, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. New England Journal of Medicine. 2010; doi:10.1056/NEJMoa1000678.
- Dunning J, et al. Microlobectomy: A novel form of endoscopic lobectomy. Innovations. 2017; doi:10.1097/IMI.0000000000000394.
- Leventakos K, et al. Advances in the treatment of non-small cell lung cancer: Focus on nivolumab, pembrolizumab and atezolizumab. BioDrugs. 2016; doi:10.1007/s40259-016-0187-0.
- Dong H, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nature Medicine. 1999;5:1365.
- Aberle DR, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. New England Journal of Medicine. 2011; doi:10.1056/NEJMoa1102873.
- Infographic: Lung Cancer
- Lung cancer surgery
- Lung nodules: Can they be cancerous?
- Super Survivor Conquers Cancer
Associated Procedures
- Ablation therapy
- Brachytherapy
- Bronchoscopy
- Chemotherapy
- Lung cancer screening
- Positron emission tomography scan
- Proton therapy
- Radiation therapy
- Stop-smoking services
News from Mayo Clinic
- Science Saturday: Study finds senescent immune cells promote lung tumor growth June 17, 2023, 11:00 a.m. CDT
- Era of hope for patients with lung cancer Nov. 16, 2022, 03:00 p.m. CDT
- Mayo Clinic Q&A podcast: Survivorship after surgery for lung cancer Nov. 15, 2022, 01:30 p.m. CDT
- Mayo Clinic Minute: Understanding lung cancer Nov. 02, 2022, 04:00 p.m. CDT
- Lung cancer diagnosis innovation leads to higher survival rates Nov. 02, 2022, 02:30 p.m. CDT
Mayo Clinic in Rochester, Minnesota, Mayo Clinic in Phoenix/Scottsdale, Arizona, and Mayo Clinic in Jacksonville, Florida, have been recognized among the top Pulmonology hospitals in the nation for 2024-2025 by U.S. News & World Report.
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
- Care at Mayo Clinic
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
5X Challenge
Thanks to generous benefactors, your gift today can have 5X the impact to advance AI innovation at Mayo Clinic.
Clinical Presentation of Lung Cancer
- First Online: 21 September 2023
Cite this chapter

- Pınar Akın Kabalak 5 &
- Ülkü Yılmaz 5
709 Accesses
Among lung cancer patients, 5–15% are asymptomatic despite experiencing serious morbidity and mortality [1]. Symptoms vary as a result of cell type, localization, diameter, stage, concomitant pulmonary disease, and concomitant paraneoplastic syndromes. The prognosis for patients who are symptomatic at the time of diagnosis has been shown to be worse than that for asymptomatic patients [2]. Therefore, to increase survival, it may be possible to reduce the cancer-related mortality at an early stage as a result of screening individuals in the high-risk group using low-dose tomography [3].
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
Institutional subscriptions
Similar content being viewed by others

Lung Cancer: Diagnosis and Treatment Approach

Update in the Diagnosis and Staging of Lung Cancer

Lung Cancer
Chute CG, Greenberg ER, Baron J, et al. Presenting conditions of 1539 population-based lung cancer patients by cell type and stage in New Hampshire and Vermont. Cancer. 1985;56(8):2107–11.
Article CAS PubMed Google Scholar
Santos-Martínez MJ, Curull V, Blanco ML, et al. Lung cancer at a university hospital: epidemiological and histological characteristics of a recent and a historical series. Arch Bronconeumol. 2005;41(6):307–12. https://doi.org/10.1016/s1579-2129(06)60230-9 .
Article PubMed Google Scholar
Field JK, Oudkerk M, Pedersen JH, et al. Prospects for population screening and diagnosis of lung cancer. Lancet. 2013;382(9893):732–41.
Ost DE, Yeung SC, Tanoue LT, et al. Clinical and organizational factors in the initial evaluation of patients with lung cancer. Chest. 2013;143(5 Suppl):e121S–41S.
Article CAS PubMed PubMed Central Google Scholar
Hyde L, Hayde CI. Clinical manifestations of lung cancer. Chest. 1974;65:299. PMID: 4813837.
Beckles MA, Spiro SG, Gen Colice GL, et al. Initial evaluation of the patient with lung cancer: symptoms, signs, laboratory tests, and paraneoplastic syndromes. Chest. 2003;123(1 Suppl):97S–104S.
Kvale PA, Simoff M, Prakash UBS, et al. Lung cancer palliative care. Chest. 2003;123(1 Suppl):284S–311S.
Athey VL, Walters SJ, Rogers TK. Symptoms at lung cancer diagnosis are associated with major differences in prognosis. Thorax. 2018;73(12):1177. Epub 2018 Apr 17
Chute CG, Greenberg ER, Baron J, et al. Presenting conditions of 1539 population-based lung cancer patients by cell type and stage in New Hampshire and Vermont. Cancer. 1985;56(8):2107.
Colice GL. Detecting lung cancer as a cause of hemoptysis in patients with a normal chest radiograph: bronchoscopy vs CT. Chest. 1997;111:877.
Abraham PJ, Capobianco DJ, Cheshire WP. Facial pain as the presenting symptom of lung carcinoma with Normal chest radiograph. Headache. 2003;43(5):499–504.
Farber SM, Mandel W, Spain DM. Diagnosis and treatment of tumors of the chest. New York: Grune & Stratton; 1960.
Google Scholar
Kuo CW, Chen YM, Chao JY, et al. Non-small cell lung cancer in very young and very old patients. Chest. 2000;117:354–7.
Buccheri G, Ferrigno D. Lung cancer: clinical presentation and specialist referral time. Eur Respir J. 2004;24:898–904.
Johnston WW. The malignant pleural effusion. A review of cytopathologic diagnoses of 584 specimens from 472 consecutive patients. Cancer. 1985;56(4):905–9.
Heffner JE, Klein JS. Recent advances in the diagnosis and management of malignant pleural effusions. Mayo Clin Proc. 2008;83(2):235–50.
Decker DA, Dines DE, Payne WS, et al. The significance of a cytologically negative pleural effusion in bronchogenic carcinoma. Chest. 1978;74(6):640.
Imazio M, Colopi M, De Ferrari GM. Pericardial diseases in patients with cancer: contemporary prevalence, management and outcomes. Heart. 2020;106(8):569–74.
Friedman T, Quencer KB, Kishore SA, et al. Malignant venous obstruction: superior vena cava syndrome and beyond. Semin Intervent Radiol. 2017;34(4):398–408.
Article PubMed PubMed Central Google Scholar
Gundepalli SG, Tadi P. Cancer, lung pancoast (superior sulcus tumour). Treasure Island: StatPearls Publishing; 2020.
Stankey RM, Roshe J, Sogocio RM. Carcinoma of the lung and dysphagia. Dis Chest. 1969;55(1):13–7. https://doi.org/10.1378/chest.55.1.13 .
Marmor S, Cohen S, Fujioka N, et al. Dysphagia prevalence and associated survival differences in older patients with lung cancer: a SEER-Medicare Population-Based Study. J Geriatr Oncol 2020;S1879-4068(19)30426-6.
Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80:1588–94.
Wang CY, Zhang XY. (99m)Tc-MDP wholebody bone imaging in evaluation of the characteristics of bone metastasis of primary lung cancer. Zhonghua Zhong Liu Za Zhi. 2010;32(5):382–6.
PubMed Google Scholar
Husaini HA, Price PW, Clemons M, et al. Prevention and management of bone metastases in lung cancer: a review. J Thorac Oncol. 2009;4:251–9.
Lassman AB, DeAngelis LM. Brain metastases. Neurol Clin. 2003;21(1):1.
Delattre JY, Krol G, Thaler HT, et al. Distribution of brain metastases. Arch Neurol. 1988;45(7):741.
Clouston PD, DeAngelis LM, Posner JB. The spectrum of neurological disease in patients with systemic cancer. Ann Neurol. 1992;31(3):268–73.
Nutt SH, Patchell RA. Intracranial haemorrhage associated with primary and secondary tumours. Neurosurg Clin N Am. 1992;3(3):591.
Grossman SA, Krabak MJ. Leptomeningeal carcinomatosis. Cancer Treat Rev. 1999;25:103–19.
Morris PG, Reiner AS, Szenberg OR, et al. Leptomeningeal metastasis from non-small cell lung cancer survival and the impact of whole brain radiotherapy. J Thorac Oncol. 2012;7:382–5.
Omuro AM, Lallana EC, Bilsky MH, et al. Ventriculoperitoneal shunt in patients with leptomeningeal metastasis. Neurology. 2005;64:1625–7.
Cheng H, Perez-Soler R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol. 2018;19(1):e43–55.
Lam KY, Lo CY. Metastatic tumours of the adrenal glands: a 30-year experience in a teaching hospital. Clin Endocrinol. 2002;56:95–101.
Article Google Scholar
Raz DJ, Lanuti M, Gaissert HC, et al. Outcomes of patients with isolated adrenal metastasis from non-small cell lung carcinoma. Ann Thorac Surg. 2011;92(5):1788–92.
Kagohashi K, Satoh H, Ishikawa H, et al. Liver metastasis at the time of initial diagnosis of lung cancer. Med Oncol. 2003;20(1):25–8.
https://www.cancer.ca/en/cancer-information/cancer-type/metastatic-cancer/liver-metastases/?region=on
Khaja M, Mundt D, Dudekula RA, et al. Lung cancer presenting as skin metastasis of the back and hand: a case series and literature review. Case Rep Oncol. 2019;12:480–7.
Joll CA. Metastatic tumours of bone. Br J Surg. 1923;11:38–72.
Afshar A, Farhadnia P, Khalkhali H. Metastases to the hand and wrist: an analysis of 221 cases. J Hand Surg Am. 2014;39(5):923–32.e17.
Flynn CJ, Danjoux C, Wong J, et al. Two cases of acrometastasis to the hands and review of the literature. Curr Oncol. 2008;15:51–8.
Mavrogenis AF, Mimidis G, Kokkalis ZT, et al. Acrometastases. Eur J Orthop Surg Traumatol. 2014;24:279–83.
Unsal M, Kutlar G, Sullu Y, et al. Tonsillar metastasis of small cell lung carcinoma. Clin Respir J. 2016;10(6):681–3.
Liyang Z, Yuewu L, Xiaoyi L, et al. Metastases to the thyroid gland. A report of 32 cases in PUMCH. Medicine. 2017;96(36):e7927.
Niu FY, Zhou Q, Yang JJ, et al. Distribution and prognosis of uncommon metastases from non-small cell lung cancer. BMC Cancer. 2016;16:149.
Kızılgöz D, Kabalak PA, Cengiz Tİ, et al. Splenic metastasis in lung cancer. Turk Klin Arch Lung. 2017;18(2):47–51.
Efthymiou C, Spyratos D, Kontakiotis T. Endocrine paraneoplastic syndromes in lung cancer. Hormones. 2018;17:351–8.
Anwar A, Jafri F, Ashraf S, et al. Paraneoplastic syndromes in lung cancer and their management. Ann Transl Med. 2019;7(15):359.
Rossato M, Zabeo E, Burei M, et al. Lung cancer and paraneoplastic neurologic syndromes. Case report and review of the literature. Clin Lung Cancer. 2013;14(3):301–9.
Pelosof LC, Gerber DE. Paraneoplastic syndromes: an approach to diagnosis and treatment. Mayo Clin Proc. 2010;85(9):838–54.
Thomas L, Kwok Y, Edelman MJ. Management of paraneoplastic syndromes in lung cancer. Curr Treat Options in Oncol. 2004;5(1):51–62.
Makiyama Y, Kikuchi T, Otani A, et al. Clinical and immunological characterization of paraneoplastic retinopathy. Invest Ophthalmol Vis Sci. 2013;54(8):5424–31.
Stoyanov GS, Dzhenkov DL, Tzaneva M. Thrombophlebitis Migrans (Trousseau syndrome) in pancreatic adenocarcinoma: an autopsy report. Cureus. 2019;11(8):e5528.
PubMed PubMed Central Google Scholar
Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362(9388):971–82.
Download references
Author information
Authors and affiliations.
Department of Pulmonology, Health Sciences University Atatürk Chest and Chest Surgery Hospital, Ankara, Turkey
Pınar Akın Kabalak & Ülkü Yılmaz
You can also search for this author in PubMed Google Scholar
Editor information
Editors and affiliations.
Department of Otorhinolaryngology, Eskişehir Osmangazi University, Eskisehir, Türkiye
Cemal Cingi
Department of Pulmonology, Celal Bayar University, Manisa, Türkiye
Arzu Yorgancıoğlu
Department of Otorhinolaryngology, Kırıkkale University, Kırıkkale, Türkiye
Nuray Bayar Muluk
Federal University of Bahia School of Medicine and ProAR Foundation, Salvador - Bahia, Brazil
Alvaro A. Cruz
Rights and permissions
Reprints and permissions
Copyright information
© 2023 Springer Nature Switzerland AG
About this chapter
Kabalak, P.A., Yılmaz, Ü. (2023). Clinical Presentation of Lung Cancer. In: Cingi, C., Yorgancıoğlu, A., Bayar Muluk, N., Cruz, A.A. (eds) Airway diseases. Springer, Cham. https://doi.org/10.1007/978-3-031-22483-6_60-1
Download citation
DOI : https://doi.org/10.1007/978-3-031-22483-6_60-1
Received : 11 November 2022
Accepted : 11 November 2022
Published : 21 September 2023
Publisher Name : Springer, Cham
Print ISBN : 978-3-031-22482-9
Online ISBN : 978-3-031-22483-6
eBook Packages : Medicine Reference Module Medicine
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 22 May 2019
Presentation of lung cancer in primary care
- D. P. Weller 1 ,
- M. D. Peake 2 &
- J. K. Field 3
npj Primary Care Respiratory Medicine volume 29 , Article number: 21 ( 2019 ) Cite this article
5498 Accesses
21 Citations
12 Altmetric
Metrics details
- Respiratory signs and symptoms
- Signs and symptoms
Survival from lung cancer has seen only modest improvements in recent decades. Poor outcomes are linked to late presentation, yet early diagnosis can be challenging as lung cancer symptoms are common and non-specific. In this paper, we examine how lung cancer presents in primary care and review roles for primary care in reducing the burden from this disease. Reducing rates of smoking remains, by far, the key strategy, but primary care practitioners (PCPs) should also be pro-active in raising awareness of symptoms, ensuring lung cancer risk data are collected accurately and encouraging reluctant patients to present. PCPs should engage in service re-design and identify more streamlined diagnostic pathways—and more readily incorporate decision support into their consulting, based on validated lung cancer risk models. Finally, PCPs should ensure they are central to recruitment in future lung cancer screening programmes—they are uniquely placed to ensure the right people are targeted for risk-based screening programmes. We are now in an era where treatments can make a real difference in early-stage lung tumours, and genuine progress is being made in this devastating illness—full engagement of primary care is vital in effecting these improvements in outcomes.
Similar content being viewed by others
A systematic review of interventions to recognise, refer and diagnose patients with lung cancer symptoms

Implications of incidental findings from lung screening for primary care: data from a UK pilot
The effect of comorbidities on diagnostic interval for lung cancer in England: a cohort study using electronic health record data
Introduction.
Lung cancer poses a significant public health burden around the world; it is the most common cause of cancer mortality in the UK and it accounts for >20% of cancer deaths. 1 There is significant variation in survival rates around the world and this has been largely attributed to the stage at which the cancer is diagnosed. 2 The International Cancer Benchmarking Partnership has demonstrated that survival rates in the UK lag behind those of other countries, and late diagnosis is thought to be a major underlying factor. 3 , 4 Importantly, patients with early-stage disease have a much better prognosis; stage 1 non-small-cell lung cancer can have a 5-year survival rate as high as 75%. 5 Even within the UK, however, there is wide variation in lung cancer survival rates and in the proportion of patients diagnosed with early-stage disease. 6
In the UK, most cancers present symptomatically in primary care (most commonly to a general practitioner, or ‘GP’, the medical lead of a primary care team), and the diagnosis is made after a referral for either investigations or directly to secondary care. 7 Many of the symptoms of lung cancer are very common but non-specific in primary care practice: these include chest pain, cough and breathlessness; 8 hence, lung cancer poses a very significant diagnostic challenge—a primary care practitioner (PCP) working full time is likely to only diagnose 1 or 2 cases per year. Further, lung cancer often emerges on a background of chronic respiratory disease and symptoms of chronic cough—typically in patients who smoke. It can be very difficult to identify changes in these chronic symptoms that might indicate the development of a lung tumour.
Smoking remains the principal aetiological factor and smoking cessation is the key public health initiative to reduce mortality from this disease; 9 indeed, at almost any age smoking cessation can produce health benefits. Hence, public health campaigns to promote smoking cessation, supplemented by strategies in primary care based on nicotine replacement therapies should be encouraged. 10 The role of e-cigarettes is not yet fully understood, 11 although any strategy that reduces exposure to tobacco smoke has a potential for producing significant benefits.
How do patients respond to lung cancer symptoms?
There is a significant body of research around patient response to symptoms that might potentially indicate lung cancer. Because symptoms often present within the context of chronic respiratory symptomatology, changes associated with the development of a tumour may go un-noticed or be dismissed. 8 It is known that patients often delay their help seeking through a range of psychological mechanisms including denial and nihilism—hence, there can often be significant delays before patients present to primary care. 12 , 13
There is evidence for variation in the timeliness of presentation of lung cancer in between countries; people with lung cancer often have symptoms for a considerable period of time before they present to primary care and this is a major source of delay in the diagnostic process with potential adverse impact on survival; 14 , 15 this patient interval does, however, vary between studies. It is important that PCPs understand some of the psychological mechanisms that either promote or inhibit early presentation among their patients.
Public awareness of lung cancer
Over the past few years, there have been campaigns run throughout the UK designed to make the public more aware of symptoms associated with lung cancer—for example the ‘Be clear on Cancer’ campaign run by Public Health England and ‘Diagnose Cancer Early’ in Scotland 16 , 17 (see Fig. 1 ). These campaigns have demonstrated an ability to diagnose additional cancers and effect modest increases in the proportion of patients having tumours diagnosed at stages where they are amenable to resection. 18 , 19

Posters used in the ‘Be Clear on Cancer’ campaign
Of course, lung cancer early detection programmes need to be focussed on the hard-to-reach population and those who will benefit most from involvement; there are often concerns expressed over burdening services with patients with insignificant symptoms 18 and an emerging consensus that all stakeholders should be closely engaged in the campaigns. Nevertheless, available evidence suggests that lung cancer could be diagnosed earlier through these public awareness campaigns, 19 particularly when associated with systems to help primary care physicians risk stratify their patients for lung cancer more effectively—indeed, further work to identify patients who might benefit from targeted interventions should be a priority.
Community-based social marketing interventions have a potential key role; 20 they can increase the likelihood of patients attending PCPs and increase primary care diagnostic activity (such as chest X-ray referrals)—as well as increases in lung cancer diagnostic rates. The level of suspicion at which PCPs consider a referral is a key factor in response to these campaigns—and there are concerns over ‘system overload’ through encouragement to present with symptoms. 13 Ideally, campaigns might preferentially target those at greater risk of lung cancer, such as people with significant smoking histories or occupational exposure.
Primary care response to lung cancer symptoms
In the UK, GPs will on average only diagnose one or two cases of lung cancer per year (if they are in full-time practice). 21 However, during that year, GPs will see hundreds of patients with common symptoms, such as cough, breathlessness and chest pain—hence, there are significant difficulties in identifying, diagnosing and referring these patients in a timely manner.
The 2015 NICE lung cancer guidelines on recognition and referral 22 have underpinned some important strategies to enhance timely lung cancer diagnosis; in many regions of the UK, there are now accelerated diagnostic pathways that assist GPs in identifying and referring patients appropriately. 23 Audit data demonstrate that there are typically several consultations prior to a diagnosis of lung cancer being made. 24 Evidence from significant event analysis in the UK has suggested that there is timely recognition and referral of symptoms in primary care; 25 longer intervals are typically attributed to factors such as X-rays being reported as normal, patient-mediated factors and presentations complicated by co-morbidity. The importance of safety netting has also been emphasised in presentations where a diagnosis of lung cancer is possible. 26
There needs to be continued work to counteract the ‘nihilism’ associated with lung cancer; PCPs are very well aware of patients who may suspect they have lung cancer but fail to present either because they blame themselves (through a history of smoking) or because they believe that if a cancer is diagnosed there is little that can be done about it. 27 This, coupled with the tendency for patients in the UK to be concerned about ‘bothering the doctor’, 28 can have detrimental effects on early diagnosis.
While public campaigns can do much to overcome barriers to presentation, it is vital that PCPs become more pro-active in achieving more timely diagnosis in their practice populations. It is been recommended that they should recognise the psychological mechanisms that might underlie patient delay and tackle nihilistic attitudes through educational and motivational strategies. 29 Indeed, there is cause for cautious optimism with new treatments, and this should be conveyed to patients; for example, the use of stereotactic radiotherapy and volume-sparing surgery means that patients who previously could not be offered curative treatment due to co-morbidities are often now eligible. 30
Audits that systematically identify at-risk patients who may be failing to present are a potential way forward; interventions which identify and target high-risk patients appear feasible in primary care. 31 Crucially, patients should be reassured that PCPs are always happy to see them if they are worried about potential cancer symptoms.
Risk assessment and lung cancer
It is vital in assessing lung cancer risk to look carefully at lifestyle factors and past medical history; only one in seven cases of lung cancer occur in people who have never smoked, and the presence of chronic obstructive pulmonary disease doubles the risk independent of smoking history. 32 A previous history of head and neck, bladder and renal cancers and other factors such as exposure to asbestos or living in high radon exposure areas are all important in lung cancer risk assessment. Family history produces an excess of risk and should be included in risk assessment—as should the symptom of fatigue, a common feature of lung cancer. Cancer decision support tools such as the ‘Caper’ instrument or ‘Q cancer’ have emerged in recent years in the UK, enabling GPs to make assessments of cancer risk based on presenting symptoms; 33 , 34 they have been incorporated into clinical systems in primary care with mixed results.
Beyond these symptom-based models, a number of lung cancer risk models have been developed based on validated epidemiological criteria—for example, the Liverpool Lung Project (LLP) risk model 35 ( www.MyLungRisk.org ), which was subsequently used in the UK Lung Cancer Screening Trial. 36 The LLP v2 risk model has also been used in the Liverpool Healthy Lung project, 37 which has accommodated the risk model within primary care practice and produced risk assessments that are useful in clinical decision making is now running into its third year. The Manchester lung cancer pilot study 38 has used the PLCO 2012 risk prediction model 39 and the recent Yorkshire Lung cancer screening trial 40 is using both the LLP v2 and the PLCO 2012 risk models. Models such as these provide a systematic way of assessing lung cancer risk, taking into account a range of factors, including smoking duration, previous respiratory disease, family history of lung cancer, age, previous history of malignancy and asbestos exposure.
Risk stratification in primary care is clearly a key priority. We need to look at instruments such as the LLP model and identify ways that lung cancer risk stratification can be made easy and convenient in primary care. At present, it is not possible to recommend a specific risk assessment tool for use in primary care; current ongoing research in primary care is externally validating existing tools and will compare their efficacy. 41 Acceptability and feasibility also need to be examined; complex algorithms that place extra burden on practitioners are unlikely to succeed. However, we do need to ensure that the basic risk prediction parameters are correctly documented in primary care, so they can be utilised in any future national lung cancer screening programme approved by the UKNSC. We also need a better understanding of ways to maximise benefits of these models—while minimising potential harms such as over-medicalisation, anxiety and false reassurance. 42 Machine learning or neuro-linguistic programming, whereby data from multiple practice-based and external sources might be examined to develop risk estimates, are also likely to play a significant role in the future. 43
Diagnostic pathways
Early diagnosis lung cancer clinics based on multi-disciplinary teams (MDTs) are an ideal option for expediting diagnosis—ideally with an urgent (2-week wait) referral; 44 there is good evidence that these specialist MDT clinics are associated with improved outcomes. Another important consideration is involving the whole primary care team and including other practitioners such as pharmacists who see a lot of patients with, for example, repeat purchases of cough medicine. There has been a push to change referral practices in some parts of the UK—for example, to lower the threshold that PCPs refer for chest X-ray 45 and to encourage practitioners to repeat the investigation after a few months if symptoms persist; critically a normal chest X-ray does not exclude diagnosis of lung cancer. One highly successful programme in Leeds included the option for people to self-refer for chest X-rays in walk-in clinics 19 —a crucial element was the engagement of primary care in the design and implementation of the programme.
Diagnostic pathways have been closely examined and tested over recent years, an example being CRUK’s ACE programme (accelerate, coordinate and evaluate) initiated in June 2014 in England and Wales. 23 Patients often have complex pathways that can lead to delays; important initiatives in the ACE programme and elsewhere include risk-stratified computed tomographic (CT) screening criteria for ‘straight to CT’ referrals following normal chest X-rays and a focus on diagnostic paths for patients with vague symptoms.
Work needs to continue on diagnostic pathways that might expedite lung cancer diagnosis. It is important, for example, that we get more evidence on the impact or potential impact of direct access to investigations such as spiral CT from primary care—at present, there is not sufficient evidence or resource to universally implement this strategy, and there is evidence that delays can occur in primary care (for example, through ordering too many chest X-rays. 46 Nevertheless, GPs in the UK often indicate that direct access to investigations would help streamline diagnosis. 7
Lung cancer screening
A major challenge for primary care is the lack of symptoms in very early stage lung cancer, highlighting the importance of examining the potential of screening. The US National Lung Cancer Screening Trial, which used low-dose CT scanning in high-risk patients, showed a 20% reduction in lung cancer-specific mortality and almost a 7% reduction in all-cause mortality—and the US Preventive Task Force on Lung cancer Screening recommended that lung cancer screening should be implemented in high-risk populations. 47 , 48 Accordingly, Medicare agreed to pay for lung cancer screening within certain criteria—however, the current uptake in the US is only ~2% of high-risk individuals.
The recent report on the NELSON trial at the World Lung Cancer Conference, Toronto 49 has demonstrated an encouragingly low rate of false positives and a mortality benefit of 26% in men and between 39% and 61% in women—depending on the number of years of follow-up (i.e. 8–10 years). These results provide further impetus for the introduction of spiral CT scanning for individuals at high risk of cancer in the UK. Figure 2 illustrates the process for identifying an appropriate screening population, recruiting them and implementing screening—in many ways more complex than existing cancer screening programmes where recruitment is based principally on age and gender.
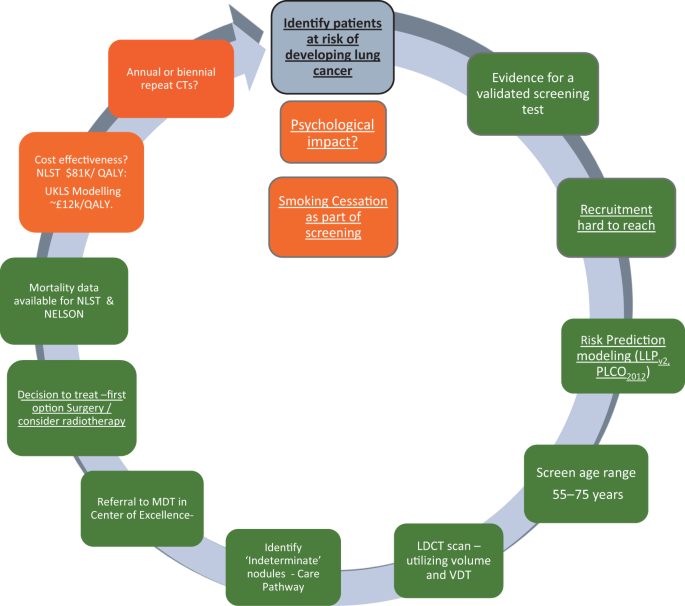
Levels of evidence for the implementation of lung cancer screening in Europe. The colour codes refer to the current status March 2019; traffic lights: green—ready, amber—borderline evidence. Underlined text indicates particular relevance for primary care 53
If we are, indeed, on the cusp of a new screening programme, there are important implications for primary care; the key issue in lung cancer screening is identifying the right patients to invite. This is a task that would involve primary care which currently lacks the systems and the processes to undertake the kind of population- based lung cancer risk assessment required. It is important, therefore, that we plan for an era where high-risk patients are screened for lung cancer (implemented, ideally, in tandem with smoking cessation programmes). We should be refining current strategies to risk stratify patients in primary care in preparation for this new era. 50 , 51 Screening alone, however, is not the total answer and a high level of awareness in both the public and the primary care community will remain vital elements in what needs to be a multi-pronged approach. 52
Conclusions and recommendations
Mortality rates for lung cancer remain stubbornly high; if we are to improve lung cancer outcomes, it is important that early diagnosis and screening efforts achieve their maximum potential. We need to:
identify ways of raising awareness of symptoms potentially associated with lung cancer in ways that encourage people at higher risk to come forward—this will require refinement of the messages delivered in awareness-raising strategies
counter the nihilistic beliefs often associated with lung cancer—early diagnosis CAN lead to improved outcomes
continually strive to improve the primary care response to patients with symptoms of lung cancer, supported by better diagnostic pathways and risk-based decision support
identify ‘fail-safe’ mechanisms by which patients advised to ‘watch and wait’ are not lost to follow-up; it is vital that patients understand these safety netting and follow-up advice
ensure that the basic risk prediction parameters are correctly documented in primary care, so they can be utilised in any future national lung cancer screening programme approved by the UKNSC
refine methods to implement lung cancer risk assessment model approaches; this is key to improving diagnosis of early lung cancer—and we should aim for risk estimates that can be readily incorporated into the various kinds of practice software used in primary care practices
continue to improve diagnostic pathways; at present, many different models are being evaluated, including those which give primary care more direct access to investigations such as spiral CT. The key task will be implementation and appropriate support once the best models are determined
fully engage primary care with the likely implementation of spiral CT lung cancer screening in the next few years—this will require the best possible risk-stratification approaches to ensure screening is directed at those who stand to benefit the most from it. It is vital that primary care rises to this challenge
Primary care needs to play a central role in efforts to diagnose lung cancer earlier, if there is to be an improvement in lung cancer outcomes in the years ahead. Research over the past decade gives us a much clearer idea of what needs to be done in refining primary care-based strategies; with adequate commitment and resources primary care will, in conjunction with other health care sectors, help reduce the burden from this disease.
Ferkol, T. & Schraufnagel, D. The global burden of respiratory disease. Ann. Am. Thorac. Soc. 11 , 404–406 (2014).
Article PubMed Google Scholar
Torre, L. A., Lindsey, A., Rebecca, L., Siegel, R. L. & Jemal, A. “Lung cancer statistics.” Lung cancer and personalized medicine. Adv. Exp. Med Biol. 893 , 1–19 (2016).
Coleman, M. P., Forman, D., Bryant, H., Butler, J. & Rachet, B. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet 377 , 127–138 (2011).
Tørring, M. L., Frydenberg, M., Hansen, R. P., Olesen, F. & Vedsted, P. Evidence of increasing mortality with longer diagnostic intervals for five common cancers: a cohort study in primary care. Eur. J. Cancer 49 , 2187–2198 (2013).
Walters, S. et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004–2007. Thorax 68 , 551–564 (2013).
Royal College of Physicians. National Lung Cancer Audit Annual report 2017. Available via www.nlcaudit.co.uk
Wagland, R. et al. Facilitating early diagnosis of lung cancer amongst primary care patients: the views of GPs. Eur. J. Cancer Care https://doi.org/10.1111/ecc.12704 (2017).
Article PubMed Central Google Scholar
Walter, F. M. et al. Symptoms and other factors associated with time to diagnosis and stage of lung cancer: a prospective cohort study. Br. J. Cancer 112 (suppl 1), S6–S13 (2015).
Article PubMed PubMed Central Google Scholar
Forman, D. et al. Time for a European initiative for research to prevent cancer: a manifesto for Cancer Prevention Europe (CPE). J. Cancer Policy 17, 15–23 (2018).
Article Google Scholar
Smith, S. S. et al. Comparative effectiveness of 5 smoking cessation pharmacotherapies in primary care clinics. Arch. Intern. Med. 14 (169), 2148–2155 (2009).
Brown, J., Beard, E., Kotz, D., Michie, S. & West, R. Real‐world effectiveness of e‐cigarettes when used to aid smoking cessation: a cross‐sectional population study. Addiction 109 , 1531–1540 (2014).
Hansen, R. P., Vedsted, P., Sokolowski, I., Søndergaard, J. & Olesen, F. Time intervals from first symptom to treatment of cancer: a cohort study of 2,212 newly diagnosed cancer patients. BMC Health Serv. Res. 11 , 284 (2011).
Niksic, M. et al. Cancer symptom awareness and barriers to symptomatic presentation in England—are we clear on cancer? Br. J. Cancer 28 (113), 533–542 (2015).
Biswas, M., Ades, A. E. & Hamilton, W. Symptom lead times in lung and colorectal cancers: what are the benefits of symptom based approaches to early diagnosis? Br. J. Cancer 112 , 271–277 (2015).
Article CAS PubMed Google Scholar
O’Dowd, E. L. et al. What characteristics of primary care and patients are associated with early death in patients with lung cancer in the UK? Thorax 70 , 161–168 (2015).
Peake, M. D. Be Clear on Cancer: regional and national lung cancer awareness campaigns 2011 to 2014, Final evaluation results. National Cancer Registration and Analysis Service, Public Health England, February 2018. Available via: http://www.ncin.org.uk/cancer_type_and_topic_specific_work/topic_specific_work/be_clear_on_cancer/
Calanzani, N., Weller, D. & Campbell, C. Development of a Methodological Approach to Evaluate the Detect Cancer Early Programme in Scotland Cancer Research UK Early Diagnosis Conference. https://www.cancerresearchuk.org/sites/default/files/edrc17_poster-42_nataliacalanzani_monteiro_detect_cancer_early_programme_in_scotland.pdf
Ironmonger, L. et al. An evaluation of the impact of large-scale interventions to raise public awareness of a lung cancer symptom. Br. J. Cancer 112 , 207 (2015).
Kennedy, M. P. T. et al. Lung cancer stage-shift following a symptom awareness campaign. Thorax 73 , 1128–1136 (2018).
Athey, V. L., Suckling, R. J., Tod, A. M., Walters, S. J. & Rogers, T. K. Early diagnosis of lung cancer: evaluation of a community-based social marketing intervention. Thorax 67 , 412–417 (2012).
Hamilton, W., Peters, T. J., Round, A. & Sharp, D. What are the clinical features of lung cancer before the diagnosis is made? A population based case-control study. Thorax 60 , 1059–1065 (2005).
Article CAS PubMed PubMed Central Google Scholar
NICE. Suspected Cancer: Recognition and Referral NICE Guideline [NG12] , National Centre for Health and Care Excellence, London (2015).
Fuller, E., Fitzgerald, K. & Hiom, S. Accelerate, Coordinate, Evaluate Programme: a new approach to cancer diagnosis. Br. J. Gen. Pract. 66 , 176–177 (2016).
Lyratzopoulos, G., Neal, R. D., Barbiere, J. M., Rubin, G. P. & Abel, G. A. Variation in number of general practioner consultations before hospital referral for cancer: findings from the 2010 National Cancer Patient Experience Survey in England. Lancet Oncol. 13 , 353–365 (2012).
Mitchell, E. D., Rubin, G. & Macleod, U. Understanding diagnosis of lung cancer in primary care: qualitative synthesis of significant event audit reports. Br. J. Gen. Pract. 63 , e37–e46 (2013).
Evans, J. et al. GPs’ understanding and practice of safety netting for potential cancer presentations: a qualitative study in primary care. Br. J. Gen. Pract. 68 , e505–e511 (2018).
Corner, J., Hopkinson, J., Fitzsimmons, D., Barclay, S. & Muers, M. Is late diagnosis of lung cancer inevitable? Interview study of patients’ recollections of symptoms before diagnosis. Thorax 60 , 314–319 (2005).
Forbes, L. J. et al. Differences in cancer awareness and beliefs between Australia, Canada, Denmark, Norway, Sweden and the UK (the International Cancer Benchmarking Partnership): do they contribute to differences in cancer survival? Br. J. Cancer 108 , 292–300 (2013).
Rubin, G. et al. The expanding role of primary care in cancer control. Lancet Oncol. 16 , 1231–1272 (2015).
Jones, G. S. & Baldwin, D. R. Recent advances in the management of lung cancer. Clin. Med. 18 (suppl 2), s41–s46 (2018).
Wagland, R. et al. Promoting help-seeking in response to symptoms amongst primary care patients at high risk of lung cancer: a mixed method study. PLoS ONE 11 , e0165677 (2016).
Ten Haaf, K., De Koning, H. & Field, J. Selecting the risk cut off for the LLP Model. J. Thorac. Oncol. 12 , S2174 (2017).
Hamilton, W. et al. Evaluation of risk assessment tools for suspected cancer in general practice: a cohort study. Br. J. Gen. Pract. 63 , e30–e36 (2013).
Hippisley-Cox, J. & Coupland, C. Identifying patients with suspected lung cancer in primary care: derivation and validation of an algorithm. Br. J. Gen. Pract. 61 , e715–e723 (2011).
Cassidy, A. et al. The LLP risk model: an individual risk prediction model for lung cancer. Br. J. Cancer 98 , 270–276 (2008).
Field, J. K. et al. The UK Lung Cancer Screening Trial: a pilot randomised controlled trial of low-dose computed tomography screening for the early detection of lung cancer. Health Technol. Assess. 20 , 1–146 (2016).
Field, J. K. et al. Abstract 4220: Liverpool Healthy Lung Project: a primary care initiative to identify hard to reach individuals with a high risk of developing lung cancer. Cancer Res . https://doi.org/10.1158/1538-7445.AM2017-4220 (2017).
Crosbie, P. A. et al. Implementing lung cancer screening: baseline results from a community-based ‘Lung Health Check’ pilot in deprived areas of Manchester. Thorax 0 , 1–5 (2018).
Google Scholar
Tammemagi, C. M. et al. Lung cancer risk prediction: prostate, lung, colorectal and ovarian cancer screening trial models and validation. J. Natl. Cancer Inst. 103 , 1058–1068 (2011).
ISRCTN Registry. The Yorkshire Lung Screening Trial ISRCTN42704678. (2019). https://doi.org/10.1186/ISRCTN42704678
Schmidt-Hansen, M., Berendse, S., Hamilton, W. & Baldwin, D. R. Lung cancer in symptomatic patients presenting in primary care: a systematic review of risk prediction tools. Br. J. Gen. Pract. 67 , e396–e404 (2017).
Usher-Smith, J., Emery, J., Hamilton, W., Griffin, S. J. & Walter, F. M. Risk prediction tools for cancer in primary care. Br. J. Cancer 113 , 1645 (2015).
Goldstein, B. A., Navar, A. M., Pencina, M. J. & Ioannidis, J. Opportunities and challenges in developing risk prediction models with electronic health records data: a systematic review. J. Am. Med. Inf. Assoc. 24 , 198–208 (2017).
Aldik, G., Yarham, E., Forshall, T., Foster, S. & Barnes, S. J. Improving the diagnostic pathway for patients with suspected lung cancer: analysis of the impact of a diagnostic MDT. Lung Cancer 115 , S12–S13 (2018).
Neal, R. D. et al. Immediate chest X-ray for patients at risk of lung cancer presenting in primary care: randomised controlled feasibility trial. Br. J. Cancer 116 , 293 (2017).
Guldbrandt, L. M., Rasmussen, T. R., Rasmussen, F. & Vedsted, P. Implementing direct access to low-dose computed tomography in general practice—method, adaption and outcome. PLoS ONE 9 , e112162 (2014).
Moyer, V. A. Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann. Intern. Med. 160 , 330–338 (2014).
PubMed Google Scholar
Cheung, L. C., Katki, H. A., Chaturvedi, A. K., Jemal, A. & Berg, C. D. Preventing lung cancer mortality by computed tomography screening: the effect of risk-based versus US Preventive Services Task Force eligibility criteria, 2005–2015. Ann. Intern. Med. 168 , 229–232 (2018).
IASLC. NELSON Study Shows CT Screening for Nodule Volume Management Reduces Lung Cancer Mortality by 26 Percent in Men. 9th World Congress on Lung Cancer . https://wclc2018.iaslc.org/media/2018%20WCLC%20Press%20Program%20Press%20Release%20De%20Koning%209.25%20FINAL%20.pdf
Field, J. K., Duffy, S. W. & Baldwin, D. R. Patient selection for future lung cancer computed tomography screening programmes: lessons learnt post National Lung Cancer Screening Trial. Transl. Lung Cancer Res. 7 (suppl 2), S114–S116 (2018).
Yousaf-Khan, U. et al. Risk stratification based on screening history: the NELSON lung cancer screening study. Thorax 72 , 819–824 (2017).
Peake, M. D., Navani, N. & Baldwin, D. The continuum of screening and early detection, awareness and faster diagnosis of lung cancer. Thorax 73 , 1097–1098 (2018).
Field, J. K., Devaraj, A., Duffy, S. W. & Baldwin, D. R. CT screening for lung cancer: is the evidence strong enough? Lung Cancer 91 , 29–35 (2016).
Download references
Author information
Authors and affiliations.
Usher Institute, University of Edinburgh, Edinburgh, UK
D. P. Weller
Centre for Cancer Outcomes, University College London Hospitals Cancer Collaborative, University of Leicester, NCRAS/PHE, London, UK
M. D. Peake
Roy Castle Lung Cancer Research Programme, Department of Molecular and Clinical Cancer Medicine, The University of Liverpool, Liverpool, UK
J. K. Field
You can also search for this author in PubMed Google Scholar
Contributions
D.P.W. led on literature searching and draft manuscript preparation. J.K.F. and M.D.P. provided input to early drafts and added text in their areas of expertise.
Corresponding author
Correspondence to D. P. Weller .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Weller, D.P., Peake, M.D. & Field, J.K. Presentation of lung cancer in primary care. npj Prim. Care Respir. Med. 29 , 21 (2019). https://doi.org/10.1038/s41533-019-0133-y
Download citation
Received : 22 November 2018
Accepted : 12 April 2019
Published : 22 May 2019
DOI : https://doi.org/10.1038/s41533-019-0133-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Anti-proliferative activity, molecular genetics, docking analysis, and computational calculations of uracil cellulosic aldehyde derivatives.
- Asmaa M. Fahim
- Sawsan Dacrory
- Ghada H. Elsayed
Scientific Reports (2023)
Lung cancer and Covid-19: lessons learnt from the pandemic and where do we go from here?
- Susanne Sarah Maxwell
- David Weller
npj Primary Care Respiratory Medicine (2022)
LncRNA CBR3-AS1 potentiates Wnt/β-catenin signaling to regulate lung adenocarcinoma cells proliferation, migration and invasion
Cancer Cell International (2021)
Is the combination of bilateral pulmonary nodules and mosaic attenuation on chest CT specific for DIPNECH?
- Bilal F. Samhouri
- Chi Wan Koo
Orphanet Journal of Rare Diseases (2021)
Evaluation of a national lung cancer symptom awareness campaign in Wales
- Grace McCutchan
- Stephanie Smits
British Journal of Cancer (2020)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

Small Cell Lung Cancer (SCLC) Clinical Presentation
- Author: Winston W Tan, MD, FACP; Chief Editor: Nagla Abdel Karim, MD, PhD more...
- Sections Small Cell Lung Cancer (SCLC)
- Practice Essentials
- Pathophysiology
- Epidemiology
- Patient Education
- Physical Examination
- Complications
- Approach Considerations
- Routine Laboratory Studies
- Thoracic Imaging Studies
- Brain and Spinal Cord Imaging Studies
- Skeletal Radionuclide Imaging
- PET Scanning
- Bronchoscopy and Fine Needle Aspiration
- Sputum Cytology
- Thoracentesis
- Histologic Findings
- Staging Overview
- VALSG and TNM Staging
- Combination Chemotherapy
- Chemotherapy Dose Intensity and Density
- Limited-Stage SCLC - Standard Management
- Extensive-Stage SCLC - Standard Management
- Management of Relapsed SCLC
- Management of Brain Metastases and Spinal Cord Compression
- Surgical Resection
- Management of Complications
- Consultations
- Long-Term Monitoring
- Guidelines Summary
- Treatment for Limited-Stage SCLC
- Treatment for Extensive-Stage SCLC
- Treatment of SCLC in the Elderly
- Medication Summary
- Antineoplastics, Alkylating
- Topoisomerase Inhibitors
- Antineoplastics, Anthracycline
- Antineoplastics, Vinca Alkaloid
- Antineoplastics, Antimicrotubular
- Antineoplastics, Antimetabolite
- Antineoplastics, Podophyllotoxin Derivatives
- Bispecific T-Cell Engager (BiTE) Antibodies
- PD-1/PD-L1 Inhibitors
- Corticosteroids
- Antiemetic Agents
- Questions & Answers
- Media Gallery
Fewer than 5% of patients with small cell lung cancer (SCLC) are asymptomatic at presentation. Common presenting signs and symptoms of the disease, which very often occur in advanced-stage disease, include the following:
- Shortness of breath
- Weight loss
- Neurologic dysfunction
Most patients with this disease present with a short duration of symptoms, usually only 8-12 weeks before presentation. The clinical manifestations of SCLC can result from local tumor growth, intrathoracic spread, distant spread, and/or paraneoplastic syndromes.
Local tumor growth
SCLCs are usually centrally located and may cause irritation and/or obstruction of the major airways. Common symptoms resulting from local tumor growth include cough, dyspnea, and hemoptysis. Squamous cell cancer also presents as a central lesion, but unlike SCLC, it frequently exhibits central cavitation.
Rapid tumor growth may lead to obstruction of major airways, with distal collapse leading to postobstructive pneumonitis, infection, and fever.
Intrathoracic spread
SCLCs usually grow rapidly and metastasize to mediastinal lymph nodes relatively early in the course of the disease. At presentation, patients may have very large intrathoracic tumors, and distinguishing the primary tumor from lymph node metastases may be impossible.
Pressure on mediastinal structures can cause various symptoms, including the following:
- Superior vena cava (SVC) obstruction
- Hoarseness - Due to compression of the recurrent laryngeal nerve
- Hemi-diaphragm paralysis - Due to phrenic nerve compression
- Dysphagia - Due to esophageal compression
- Stridor - Due to compression of the major airways
SCLC causes SVC obstruction more often than non-SCLC (NSCLC) . Patients present with swelling of the face and upper extremities, and can develop stridor due to laryngeal edema or headache, dizziness, and other neurologic symptoms due to cerebral edema. Hoarseness of recent onset can be caused by compression of the left recurrent laryngeal nerve by a mediastinal mass involving the aortopulmonary window (ie, primary tumor or lymph node metastasis).
Compression of the phrenic nerve causes paralysis of the ipsilateral hemidiaphragm, contributing to shortness of breath. In addition, esophageal compression can lead to dysphagia and odynophagia, and compression of the mainstem bronchi and trachea can cause severe shortness of breath and stridor or wheezing.
Symptoms from distant spread
Common sites of hematogenous metastases include the brain, bones, liver, adrenal glands, and bone marrow. The symptoms depend upon the site of spread.
Neurologic dysfunction can occur due to brain metastases or spinal cord compression. Patients with symptomatic brain metastases may have raised intracranial pressure secondary to mass lesions and vasogenic edema. Common symptoms include the following:
- Headache - Usually worse in the morning
- Blurred vision
- Photophobia
- Slurred speech
- Localizing symptoms - Such as extremity weakness
Suspected spinal cord compression is an oncologic emergency. Early recognition of vertebral and paraspinal metastases is important, because a delay in diagnosis and treatment frequently results in permanent loss of neurologic function. The initial symptom is usually back pain, with or without neurologic dysfunction. Once present, neurologic dysfunction can progress very rapidly (ie, within hours) to cause quadriplegia or paraplegia, depending upon the location of the lesion.
Other symptoms from distant metastasis may include pain from bone metastasis, as well as jaundice or abdominal/right upper quadrant pain due to liver metastasis.
Paraneoplastic syndromes
Paraneoplastic syndromes are rare disorders that are triggered by an altered immune system response to a neoplasm or ectopic production of a hormone or cytokine. Table 1, below, shows some examples of the paraneoplastic syndromes affecting the endocrine and neurologic systems in patients with SCLC.
See Paraneoplastic Diseases for more information.
Table 1. Paraneoplastic Syndromes Affecting Endocrine and Neurologic Function in SCLC (Open Table in a new window)
|
|
|
|
Endocrine | SIADH | Antidiuretic hormone | 15% ] |
Ectopic secretion of ACTH | ACTH | 2-5% ] | |
|
|
| |
Neurologic | Eaton-Lambert reverse myasthenic syndrome |
| 3% ] |
Subacute cerebellar degeneration |
|
| |
Subacute sensory neuropathy |
|
| |
Limbic encephalopathy | Anti-Hu, anti-Yo antibodies |
| |
ACTH = adrenocorticotropic hormone; SCLC = small cell lung cancer; SIADH = syndrome of inappropriate antidiuretic hormone. (1) Campling BG, Sarda IR, Baer KA, et al. Secretion of atrial natriuretic peptide and vasopressin by small cell lung cancer. May 15, 1995;75(10):2442-51 ] ; (2) Shepherd FA, Laskey J, Evans WK, et al. Cushing's syndrome associated with ectopic corticotropin production and small-cell lung cancer. Jan 1992;10(1):21-7 ] ; (3) Sher E, Gotti C, Canal N, et al. Specificity of calcium channel autoantibodies in Lambert-Eaton myasthenic syndrome. Sep 16, 1989;2(8664):640-3. ] | |||
Physical findings in small cell lung cancer (SCLC) depend upon the extent of local and distant spread and the organ system involved.
Respiratory system
Patients usually experience shortness of breath; physical examination may reveal use of the accessory muscles of respiration (scalene muscles, intercostal muscles) and flaring of the nasal alae. In addition, by virtue of a central tumor location, patients may develop distal atelectasis and postobstructive pneumonia. With pleural effusion , the examination reveals dullness to percussion and decreased or absent breath sounds on the side of the effusion.
Cardiovascular system
Pericardial effusions may be asymptomatic when small, or they may result in tamponade if they are large or accumulate over a short period. Patients are usually short of breath and their heart sounds may be distant on auscultation. Jugular venous pulsation is elevated, and, paradoxically, it rises with inspiration.
Tamponade is an emergency and requires immediate decompression of the pericardium. Pulsus paradoxus is a classic sign of pericardial tamponade . If tamponade is suspected, an echocardiogram should be performed. The definitive diagnosis is established with cardiac catheterization, which reveals equalization of pressures in cardiac chambers. Definitive management may include chemotherapy and/or surgical creation of a pleuropericardial window.
Examination of the extremities may reveal clubbing, cyanosis, or edema. In the presence of superior vena cava (SVC) obstruction, the right upper extremity is usually edematous.
Central nervous system
Asymptomatic brain metastases occur in 5-10% of patients with SCLC (see Workup). Patients with symptomatic brain metastases may have raised intracranial pressure secondary to mass lesions and surrounding brain edema. The physical findings depend on the site of the brain lesions.
Perform funduscopy to look for signs of raised intracranial pressure, as well as a thorough neurologic examination and an evaluation of cerebellar function, coordination, and gait.
Gastrointestinal system
The liver is a common site of metastatic spread. Physical examination may reveal icterus (secondary to widespread liver metastasis or obstruction of biliary outflow) and/or hepatomegaly. However, most patients do not have any specific finding related to the gastrointestinal (GI) tract on examination. Very often patients are asymptomatic but may have mild elevation of liver enzyme levels.
Lymphatic system
Carefully perform a lymph node examination. Currently, enlarged ipsilateral supraclavicular lymph nodes are included in limited-stage disease, but enlarged axillary lymph nodes upstage the diagnosis to extensive-stage disease.
Multiple complications may be noted, depending on the site of metastasis or the metabolic factor that the tumor affects. Hypercalcemia could initially be asymptomatic but in late stages could lead to weakness, fatigue, and sleepiness, and in extreme cases to severe constipation and lethargy.
Brain metastasis is often asymptomatic but could manifest as a unilateral eye abnormality, focal neurologic deficit, or at times with a new-onset headache that wakes the patient up. Seizures are a possible manifestation.
Basumallik N, Agarwal M. Small Cell Lung Cancer. 2024 Jan. [QxMD MEDLINE Link] . [Full Text] .
Malhotra J, Boffetta P, Mucci L. Cancer of the lung, larynx, and pleura. Adami H, Hunter D, Laglou P, Mucci L, eds. Textbook of Cancer Epidemiology . 3rd ed. New York, NY: Oxford University Press; 2018. 327-54.
Pietanza MC, Krug LM, Wu AJ, Kris MG, Rudin CM, Travis WD. Small Cell and Neuroendocrine Tumors of the lung. DeVita VT Jr, Lawrence TS, Rosenberg SA, eds. DeVita, Hellman, and Rosenberg's Cancer: Principles & Practice of Oncology . 10th ed. Philadelphia, Pa: Wolters Kluwer Health; 2015. 536-59.
Cascone T, Gold KA, Glisson BS. Small Cell Carcinoma of the Lung. Kantarjian H, Wolff R, eds. The MD Anderson Manual of Medical Oncology . 3rd ed. New York, NY: McGraw-Hill Education; 2016. 323-42.
Kalemkerian GP, Schneider BJ. Advances in Small Cell Lung Cancer. Hematol Oncol Clin North Am . 2017 Feb. 31 (1):143-156. [QxMD MEDLINE Link] .
Gay CM, Stewart CA, Park EM, et al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell . 2021 Mar 8. 39 (3):346-360.e7. [QxMD MEDLINE Link] . [Full Text] .
Wynder EL, Graham EA. Tobacco smoking as a possible etiologic factor in bronchiogenic carcinoma; a study of 684 proved cases. J Am Med Assoc . 1950 May 27. 143(4):329-36. [QxMD MEDLINE Link] .
Pesch B, Kendzia B, Gustavsson P, Jockel KH, Johnen G,et al. Cigarette smoking and lung cancer--relative risk estimates for the major histological types from a pooled analysis of case-control studies. Int J Cancer . 2012 Sep 1. 131(5):1210-9. [QxMD MEDLINE Link] . [Full Text] .
Parsons A, Daley A, Begh R, Aveyard P. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ . 2010 Jan 21. 340:b5569. [QxMD MEDLINE Link] . [Full Text] .
Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin . 2024 Jan-Feb. 74 (1):12-49. [QxMD MEDLINE Link] . [Full Text] .
Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol . 2006 Oct 1. 24(28):4539-44. [QxMD MEDLINE Link] .
American Cancer Society. Cancer Facts & Figures 2023. Available at https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdfer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf . Accessed: May 20, 2024.
Small Cell Lung Cancer Treatment (PDQ®): Health Professional Version. National Cancer Institute. Available at https://www.ncbi.nlm.nih.gov/books/NBK65909/#CDR0000062945__1 . March 1, 2024; Accessed: May 20, 2024.
Cancer Facts & Figures 2024. American Cancer Society. Available at https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2024/2024-cancer-facts-and-figures-acs.pdf . Accessed: May 20, 2024.
[Guideline] Dingemans AC, Früh M, Ardizzoni A, Besse B, Faivre-Finn C, Hendriks LE, et al. Small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up † . Ann Oncol . 2021 Apr 9. 24 Suppl 6:vi99-105. [QxMD MEDLINE Link] . [Full Text] .
Cancer fact sheet. World Health Organization. Available at https://www.who.int/mediacentre/factsheets/fs297/en/ . 3 February 2022; Accessed: May 20, 2024.
Jemal A, Miller KD, Ma J, Siegel RL, Fedewa SA, Islami F, et al. Higher Lung Cancer Incidence in Young Women Than Young Men in the United States. N Engl J Med . 2018 May 24. 378 (21):1999-2009. [QxMD MEDLINE Link] .
Jackman DM, Johnson BE. Small-cell lung cancer. Lancet . 2005 Oct 15-21. 366(9494):1385-96. [QxMD MEDLINE Link] .
Janne PA, Freidlin B, Saxman S, Johnson DH, Livingston RB, Shepherd FA, et al. Twenty-five years of clinical research for patients with limited-stage small cell lung carcinoma in North America. Cancer . 2002 Oct 1. 95(7):1528-38. [QxMD MEDLINE Link] .
Wu C, Xu B, Yuan P, Miao X, Liu Y, Guan Y, et al. Genome-wide interrogation identifies YAP1 variants associated with survival of small-cell lung cancer patients. Cancer Res . 2010 Dec 1. 70(23):9721-9. [QxMD MEDLINE Link] .
Xun WW, Brennan P, Tjonneland A, Vogel U, Overvad K, el at. Single-nucleotide polymorphisms (5p15.33, 15q25.1, 6p22.1, 6q27 and 7p15.3) and lung cancer survival in the European Prospective Investigation into Cancer and Nutrition (EPIC). Mutagenesis . 2011 Sep. 26(5):657-66. [QxMD MEDLINE Link] .
Hermes A, Waschki B, Reck M. Hyponatremia as prognostic factor in small cell lung cancer--a retrospective single institution analysis. Respir Med . 2012 Jun. 106(6):900-4. [QxMD MEDLINE Link] .
Campling BG, Sarda IR, Baer KA, Pang SC, Baker HM, Lofters WS, et al. Secretion of atrial natriuretic peptide and vasopressin by small cell lung cancer. Cancer . 1995 May 15. 75(10):2442-51. [QxMD MEDLINE Link] .
Shepherd FA, Laskey J, Evans WK, Goss PE, Johansen E, Khamsi F. Cushing's syndrome associated with ectopic corticotropin production and small-cell lung cancer. J Clin Oncol . 1992 Jan. 10(1):21-7. [QxMD MEDLINE Link] .
Sher E, Gotti C, Canal N, Scoppetta C, Piccolo G, Evoli A, et al. Specificity of calcium channel autoantibodies in Lambert-Eaton myasthenic syndrome. Lancet . 1989 Sep 16. 2(8664):640-3. [QxMD MEDLINE Link] .
[Guideline] Wolf AMD, Oeffinger KC, Shih TY, Walter LC, Church TR, Fontham ETH, et al. Screening for lung cancer: 2023 guideline update from the American Cancer Society. CA Cancer J Clin . 2023 Nov 1. 63 (2):107-17. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] Lung Cancer: Screening. U.S. Preventive Services Task Force. Available at https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/lung-cancer-screening . March 9, 2021; Accessed: May 20, 2024.
[Guideline] National Comprehensive Cancer Network. Lung Cancer Screening. NCCN. Available at https://www.nccn.org/professionals/physician_gls/pdf/lung_screening.pdf . Version 2.2024 — October 18, 2023; Accessed: May 20, 2024.
Katki HA, Kovalchik SA, Petito LC, Cheung LC, Jacobs E, Jemal A, et al. Implications of Nine Risk Prediction Models for Selecting Ever-Smokers for Computed Tomography Lung Cancer Screening. Ann Intern Med . 15 May 2018. [Full Text] .
[Guideline] NCCN Clinical Practice Guidelines in Oncology: Small Cell Lung Cancer. National Comprehensive Cancer Network. Available at https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf . Version 3.2023 — December 21, 2022; Accessed: April 10, 2023.
[Guideline] Detterbeck FC, Lewis SZ, Diekemper R, Addrizzo-Harris D, Alberts WM. Executive Summary: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest . 2013 May. 143 (5 Suppl):7S-37S. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] Ung YC, Maziak DE, Vanderveen JA, Smith CA, Gulenchyn K, Evans WK, for the Lung Cancer Disease Site Group. 18-fluorodeoxyglucose positron emission tomography in the diagnosis and staging of lung cancer: a clinical practice guideline. Toronto, Ontario: Cancer Care Ontario; 2007. [Full Text] .
Thomson D, Hulse P, Lorigan P, Faivre-Finn C. The role of positron emission tomography in management of small cell lung cancer. Lung Cancer . 2011 Aug. 73(2):121-6. [QxMD MEDLINE Link] .
American Joint Committee on Cancer. Lung. AJCC Cancer Staging Manual Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, et al, eds. AJCC Cancer Staging Manual . 8th edition. New York, NY: Springer; 2016.
Dresler CM, Olak J, Herndon JE 2nd, Richards WG, el at. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest . 2005 Mar. 127(3):909-15. [QxMD MEDLINE Link] .
Zakowski MF. Pathology of small cell carcinoma of the lung. Semin Oncol . 2003 Feb. 30(1):3-8. [QxMD MEDLINE Link] .
Small Cell Lung Cancer Stages. American Cancer Society. Available at https://www.cancer.org/cancer/types/lung-cancer/detection-diagnosis-staging/staging-sclc.htmlde/small-cell-lung-cancer-staging . January 29, 2024; Accessed: May 20, 2024.
Micke P, Faldum A, Metz T, Beeh KM, Bittinger F, Hengstler JG, et al. Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer--what limits limited disease?. Lung Cancer . 2002 Sep. 37(3):271-6. [QxMD MEDLINE Link] .
[Guideline] National Collaborating Centre for Cancer. Lung cancer. The diagnosis and treatment of lung cancer. Publication no. 121. London, UK: National Institute for Health and Clinical Excellence; 2011. [Full Text] .
Halvorsen TO, Sundstrøm S, Fløtten Ø, Brustugun OT, Brunsvig P, Aasebø U, et al. Comorbidity and outcomes of concurrent chemo- and radiotherapy in limited disease small cell lung cancer. Acta Oncol . 2016 Aug 23. 1-9. [QxMD MEDLINE Link] .
Spigel DR, Townley PM, Waterhouse DM, Fang L, Adiguzel I, et al. Randomized phase II study of bevacizumab in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer: results from the SALUTE trial. J Clin Oncol . 2011 Jun 1. 29(16):2215-22. [QxMD MEDLINE Link] .
Lally BE, Urbanic JJ, Blackstock AW, Miller AA, Perry MC. Small cell lung cancer: have we made any progress over the last 25 years?. Oncologist . 2007 Sep. 12(9):1096-104. [QxMD MEDLINE Link] .
Hanna NH, Einhorn LH. Small-cell lung cancer: state of the art. Clin Lung Cancer . 2002 Sep. 4(2):87-94. [QxMD MEDLINE Link] .
Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med . 2018 Dec 6. 379 (23):2220-2229. [QxMD MEDLINE Link] . [Full Text] .
Harrison P. After Decades, New Standard of Care in Small Cell Lung Cancer. Medscape Medical News. Available at https://www.medscape.com/viewarticle/902700 . September 28, 2018; Accessed: October 10, 2018.
Petty WJ, Paz-Ares L. Emerging Strategies for the Treatment of Small Cell Lung Cancer: A Review. JAMA Oncol . 2023 Mar 1. 9 (3):419-429. [QxMD MEDLINE Link] .
Amarasena IU, Walters JA, Wood-Baker R, Fong K. Platinum versus non-platinum chemotherapy regimens for small cell lung cancer. Cochrane Database Syst Rev . 2008 Oct 8. CD006849. [QxMD MEDLINE Link] .
Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med . 2002 Jan 10. 346(2):85-91. [QxMD MEDLINE Link] .
Jiang L, Yang KH, Guan QL, Mi DH, Wang J. Cisplatin plus etoposide versus other platin-based regimens for patients with extensive small-cell lung cancer: a systematic review and meta-analysis of randomised, controlled trials. Intern Med J . 2012 Dec. 42(12):1297-309. [QxMD MEDLINE Link] .
Hanna N, Bunn PA Jr, Langer C, Einhorn L, Guthrie T Jr, Beck T, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol . 2006 May 1. 24(13):2038-43. [QxMD MEDLINE Link] .
Ready NE, Pang HH, Gu L, Otterson GA, Thomas SP, Miller AA, et al. Chemotherapy With or Without Maintenance Sunitinib for Untreated Extensive-Stage Small-Cell Lung Cancer: A Randomized, Double-Blind, Placebo-Controlled Phase II Study-CALGB 30504 (Alliance). J Clin Oncol . 2015 May 20. 33 (15):1660-5. [QxMD MEDLINE Link] .
Schmittel A, Sebastian M, Fischer von Weikersthal L, et al. A German multicenter, randomized phase III trial comparing irinotecan-carboplatin with etoposide-carboplatin as first-line therapy for extensive-disease small-cell lung cancer. Ann Oncol . 2011 Aug. 22(8):1798-804. [QxMD MEDLINE Link] .
Rossi A, Di Maio M, Chiodini P, Rudd RM, Okamoto H, Skarlos DV, et al. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J Clin Oncol . 2012 May 10. 30(14):1692-8. [QxMD MEDLINE Link] .
Klasa RJ, Murray N, Coldman AJ. Dose-intensity meta-analysis of chemotherapy regimens in small-cell carcinoma of the lung. J Clin Oncol . 1991 Mar. 9(3):499-508. [QxMD MEDLINE Link] .
Arriagada R, Le Chevalier T, Pignon JP, Riviere A, Monnet I, Chomy P, et al. Initial chemotherapeutic doses and survival in patients with limited small-cell lung cancer. N Engl J Med . 1993 Dec 16. 329(25):1848-52. [QxMD MEDLINE Link] .
Takada M, Fukuoka M, Kawahara M, Sugiura T, Yokoyama A, Yokota S, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol . 2002 Jul 15. 20(14):3054-60. [QxMD MEDLINE Link] .
Turrisi AT 3rd, Kim K, Blum R, Sause WT, Livingston RB, Komaki R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med . 1999 Jan 28. 340(4):265-71. [QxMD MEDLINE Link] .
Slotman B, Faivre-Finn C, Kramer G, Rankin E, Snee M, Hatton M, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med . 2007 Aug 16. 357(7):664-72. [QxMD MEDLINE Link] .
Schild SE, Foster NR, Meyers JP, Ross HJ, Stella PJ, et al. Prophylactic cranial irradiation in small-cell lung cancer: findings from a North Central Cancer Treatment Group Pooled Analysis. Ann Oncol . 2012 Nov. 23(11):2919-24. [QxMD MEDLINE Link] .
Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet . 2019 Nov 23. 394 (10212):1929-1939. [QxMD MEDLINE Link] .
Natale R, Lara P, Chansky K, et al. A randomized phase III trial comparing irinotecan/cisplatin (IP) with etoposide/cisplatin (EP) in patients (pts) with previously untreated extensive stage small cell lung cancer (E-SCLC). J Clin Oncol. 2008;26 (suppl):400s.
Heigener D, Freitag L, Eschbach C et al. Topotecan/cisplatin (TP) compared to cisplatin/etoposide (PE) for patients with extensive disease-small cell lung cancer (ED-SCLC): final results of a randomised phase III trial. J Clin Oncol. 2008;26 (suppl):400s.
Slotman BJ, van Tinteren H, Praag JO, Knegjens JL, El Sharouni SY, Hatton M, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet . 2015 Jan 3. 385 (9962):36-42. [QxMD MEDLINE Link] . [Full Text] .
Takahashi T, Yamanaka T, Seto T, Harada H, Nokihara H, Saka H, et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol . 2017 May. 18 (5):663-671. [QxMD MEDLINE Link] .
Harris S, Chan MD, Lovato JF, Ellis TL, Tatter SB, Bourland JD, et al. Gamma knife stereotactic radiosurgery as salvage therapy after failure of whole-brain radiotherapy in patients with small-cell lung cancer. Int J Radiat Oncol Biol Phys . 2012 May 1. 83(1):e53-9. [QxMD MEDLINE Link] .
Ahn MJ, Cho BC, Felip E, and the, DeLLphi-301 Investigators. Tarlatamab for Patients with Previously Treated Small-Cell Lung Cancer. N Engl J Med . 2023 Nov 30. 389 (22):2063-2075. [QxMD MEDLINE Link] .
Jotte R, Conkling P, Reynolds C, Galsky MD, Klein L, Fitzgibbons JF, et al. Randomized phase II trial of single-agent amrubicin or topotecan as second-line treatment in patients with small-cell lung cancer sensitive to first-line platinum-based chemotherapy. J Clin Oncol . 2011 Jan 20. 29(3):287-93. [QxMD MEDLINE Link] .
Goto K, Ohe Y, Shibata T, Seto T, Takahashi T, Nakagawa K, et al. Combined chemotherapy with cisplatin, etoposide, and irinotecan versus topotecan alone as second-line treatment for patients with sensitive relapsed small-cell lung cancer (JCOG0605): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol . 2016 Aug. 17 (8):1147-1157. [QxMD MEDLINE Link] .
Zepzelca (lurbinectedin) [package insert]. Palo Alto, CA: Jazz Pharmaceuticals, Inc. June 2020. Available at [Full Text] .
Farago AF, Yeap BY, Stanzione M, et al. Combination Olaparib and Temozolomide in Relapsed Small-Cell Lung Cancer. Cancer Discov . 2019 Oct. 9 (10):1372-1387. [QxMD MEDLINE Link] . [Full Text] .
Pietanza MC, Waqar SN, Krug LM, Dowlati A, Hann CL, Chiappori A, et al. Randomized, Double-Blind, Phase II Study of Temozolomide in Combination With Either Veliparib or Placebo in Patients With Relapsed-Sensitive or Refractory Small-Cell Lung Cancer. J Clin Oncol . 2018 Aug 10. 36 (23):2386-2394. [QxMD MEDLINE Link] . [Full Text] .
Nivolumab Indication in Small Cell Lung Cancer Withdrawn in U.S. Market. The ASCO Post. Available at https://ascopost.com/issues/january-25-2021/nivolumab-indication-in-small-cell-lung-cancer-withdrawn-in-us-market/ . January 25, 2021; Accessed: March 23, 2021.
Merck Provides Update on KEYTRUDA® (pembrolizumab) Indication in Metastatic Small Cell Lung Cancer in the US. Merck. Available at https://www.merck.com/news/merck-provides-update-on-keytruda-pembrolizumab-indication-in-metastatic-small-cell-lung-cancer-in-the-us/ . March 1, 2021; Accessed: March 23, 2021.
Schreiber D, Rineer J, Vongtama D, et al. Surgery for limited-stage small cell lung cancer, should the paradigm shift? A SEER-based analysis. J Clin Oncol (Suppl) . 2008. 26:403s.
Fu Z, Li D, Deng C, Zhang J, Bai J, Li Y, et al. Excellent survival of pathological N0 small cell lung cancer patients following surgery. Eur J Med Res . 2023 Feb 21. 28 (1):91. [QxMD MEDLINE Link] . [Full Text] .
Anraku M, Waddell TK. Surgery for small-cell lung cancer. Semin Thorac Cardiovasc Surg . 2006 Fall. 18(3):211-6. [QxMD MEDLINE Link] .
Mazzone PJ, Silvestri GA, Patel S, Kanne JP, Kinsinger LS, Wiener RS, et al. Screening for Lung Cancer: CHEST Guideline and Expert Panel Report. Chest . 2018 Apr. 153 (4):954-985. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] Jaklitsch MT, Jacobson FL, Austin JH, Field JK, Jett JR, Keshavjee S, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg . 2012 Jul. 144 (1):33-8. [QxMD MEDLINE Link] .
[Guideline] Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest . 2013 May. 143 (5 Suppl):e142S-65S. [QxMD MEDLINE Link] . [Full Text] .
[Guideline] Rudin CM, Ismaila N, Hann CL, Malhotra N, Movsas B, Norris K, et al. Treatment of Small-Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians Guideline. J Clin Oncol . 2015 Dec 1. 33 (34):4106-11. [QxMD MEDLINE Link] . [Full Text] .
Moffat GT, Wang T, Robinson AG. Small Cell Lung Cancer in Light/Never Smokers - A Role for Molecular Testing?. J Natl Compr Canc Netw . 2023 Feb 15. 21 (4):336-339. [QxMD MEDLINE Link] .
- High-power photomicrograph of small cell carcinoma on the left side of the image with normal ciliated respiratory epithelium on the right side of the image.
- This coronal positron emission tomogram shows a large, focal, hypermetabolic area on the right that is consistent with a large mass in the central portion of the right upper pulmonary lobe. Multiple other smaller hypermetabolic areas suggest lymph-node metastatic disease in the chest, abdomen, and right supraclavicular region.
- Table 1. Paraneoplastic Syndromes Affecting Endocrine and Neurologic Function in SCLC
- Table 2. AJCC TNM Categories for Lung Cancer
- Table 3. AJCC Stage Groupings for Lung Cancer
|
|
|
|
Endocrine | SIADH | Antidiuretic hormone | 15% ] |
Ectopic secretion of ACTH | ACTH | 2-5% ] | |
|
|
| |
Neurologic | Eaton-Lambert reverse myasthenic syndrome |
| 3% ] |
Subacute cerebellar degeneration |
|
| |
Subacute sensory neuropathy |
|
| |
Limbic encephalopathy | Anti-Hu, anti-Yo antibodies |
| |
ACTH = adrenocorticotropic hormone; SCLC = small cell lung cancer; SIADH = syndrome of inappropriate antidiuretic hormone. (1) Campling BG, Sarda IR, Baer KA, et al. Secretion of atrial natriuretic peptide and vasopressin by small cell lung cancer. May 15, 1995;75(10):2442-51 ] ; (2) Shepherd FA, Laskey J, Evans WK, et al. Cushing's syndrome associated with ectopic corticotropin production and small-cell lung cancer. Jan 1992;10(1):21-7 ] ; (3) Sher E, Gotti C, Canal N, et al. Specificity of calcium channel autoantibodies in Lambert-Eaton myasthenic syndrome. Sep 16, 1989;2(8664):640-3. ] | |||
|
|
| |
| Primary tumor can’t be assessed, or sputum cytology reveals tumor cells but the tumor is not seen on radiologic or bronchoscopic evaluation | ||
| No evidence of a primary tumor | ||
| Carcinoma in situ | ||
| ≤3 cm in greatest dimension | Surrounded by lung or visceral pleura; no invasion more proximal than lobar bronchus | |
| ≤1 cm in greatest dimension | ||
| >1 cm but ≤2 cm in greatest dimension | ||
| >2 cm but ≤3 cm in greatest dimension | ||
| |||
| >3 cm but ≤4 cm in greatest dimension | ||
| >5 cm but ≤7 cm in greatest dimension | ||
| Direct invasion of: | ||
| Invasion of: | ||
|
| ||
| Regional lymph nodes cannot be assessed | ||
| No regional lymph node metastasis | ||
| |||
| Ipsilateral mediastinal and/or subcarinal lymph node(s) | ||
| Contralateral mediastinal, contralateral hilar, ipsilateral/contralateral scalene, or supraclavicular lymph node(s) | ||
|
| ||
| No distant metastasis | ||
| Distant metastasis | ||
| |||
| Single extrathoracic metastasis in a single organ and involvement of a single distant (nonregional) node | ||
| Multiple extrathoracic metastases in one or more organs | ||
AJCC = American Joint Committee on Cancer. (1) Edge SB, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer; 2010:299-330 ] ; (2) National Comprehensive Cancer Network. [serial online]. 2018;v.2. Available at: . ] | |||
|
|
| ||
| TX | N0 | M0 | |
| Tis | N0 | M0 | |
|
| T1a | N0 | M0 |
| T1b | N0 | M0 | |
| T1c | N0 | M0 | |
| T2b | N0 | M0 | |
| T1a,b,c | N1 | M0 | |
T2a,b | N1 | M0 | ||
T3 | N0 | M0 | ||
| T1a,b,c | N2 | M0 | |
T2a,b | N2 | M0 | ||
T3 | N1-2 | M0 | ||
T4 | N0-1 | M0 | ||
| T1a,b,c | N3 | M0 | |
T2a,b | N3 | M0 | ||
T3 | N2 | M0 | ||
T4 | N2 | M0 | ||
| T3-4 | N3 | M0 | |
| Any T | Any N | M1a,b | |
| Any T | Any N | M1c | |
AJCC = American Joint Committee on Cancer. (1) Edge SB, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2016 ] ; (2) National Comprehensive Cancer Network. [serial online]. 2018;v.2. Available at: . ] | ||||

Contributor Information and Disclosures
Winston W Tan, MD, FACP Associate Professor of Medicine, Mayo Medical School; Consultant and Person-in-Charge of Genitourinary Oncology-Medical Oncology, Division of Hematology/Oncology, Department of Internal Medicine, Mayo Clinic Jacksonville; Vice Chairman, Division of Hematology/Oncology Education, Chair, Cancer Survivorship Program, Associate Chair, Department of Medicine Faculty Development, Mayo Clinic Florida; Vice President, Florida Society of Clinical Oncology Winston W Tan, MD, FACP is a member of the following medical societies: American College of Physicians , American Society of Clinical Oncology , American Society of Hematology , Philippine Medical Association , Texas Medical Association Disclosure: Nothing to disclose.
Irfan Maghfoor, MD Consulting Oncologist, Department of Oncology, King Faisal Specialist Hospital and Research Center, Saudi Arabia Irfan Maghfoor, MD is a member of the following medical societies: American Society of Hematology Disclosure: Nothing to disclose.
Nagla Abdel Karim, MD, PhD Director of Early Therapeutics, Inova Schar Cancer Institute; Professor of Medicine, University of Virginia School of Medicine Nagla Abdel Karim, MD, PhD is a member of the following medical societies: American Medical Association , American Society of Clinical Oncology , Egyptian American Medical Association, Egyptian Cancer Society, International Association for the Study of Lung Cancer Disclosure: Nothing to disclose.
What would you like to print?
- Print this section
- Print the entire contents of
- Print the entire contents of article

- Small Cell Lung Cancer Staging
- Non-Small Cell Lung Cancer (NSCLC) Staging
- Small Cell Lung Cancer (SCLC)
- Small Cell Lung Cancer (SCLC) Imaging
- Non-Small Cell Lung Cancer (NSCLC)
- Genetics of Non-Small Cell Lung Cancer
- Non-Small Cell Lung Cancer (NSCLC) Imaging
- FDA Approves Tarlatamab for Extensive-Stage Small Cell Lung Cancer
- IASLC Issues Consensus on Neoadjuvant, Adjuvant Treatments in Non–Small Cell Lung Cancer
- Is Consolidation Osimertinib Superior to Durvalumab in EGFR-Mutated Non–Small Cell Lung Cancer?

- Drug Interaction Checker
- Pill Identifier
- Calculators

- 20022006716-overviewDiseases & Conditions Diseases & Conditions Small Cell Lung Cancer Staging

- 20022007813-overviewDiseases & Conditions Diseases & Conditions Non-Small Cell Lung Cancer (NSCLC) Staging

Non-Small Cell Lung Cancer Treatment (PDQ®)–Health Professional Version
General information about non-small cell lung cancer (nsclc).
NSCLC is any type of epithelial lung cancer other than small cell lung cancer (SCLC). The most common types of NSCLC are squamous cell carcinoma, large cell carcinoma, and adenocarcinoma, but there are several other types that occur less frequently, and all types can occur in unusual histological variants. Although NSCLCs are associated with cigarette smoke, adenocarcinomas may be found in patients who never smoked.
As a class, NSCLC is usually less sensitive to chemotherapy and radiation therapy than SCLC. Patients with resectable disease may be cured by surgery or surgery followed by chemotherapy. Local control can be achieved with radiation therapy in many patients with unresectable disease, but cure is seen in relatively few patients. Patients with locally advanced unresectable disease may achieve long-term survival with radiation therapy combined with chemotherapy. Patients with advanced metastatic disease may achieve improved survival and palliation of symptoms with chemotherapy, targeted agents, and other supportive measures.
Incidence and Mortality
Estimated new cases and deaths from lung cancer (NSCLC and SCLC combined) in the United States in 2024:[ 1 ]
- New cases: 234,580.
- Deaths: 125,070.
Lung cancer is the leading cause of cancer-related mortality in the United States. The 5-year relative survival rate from 2013 to 2019 for patients with lung cancer was 25%. The 5-year relative survival rate varies markedly for patients diagnosed at local stage (63%), regional stage (35%), or distant stage (8%).[ 1 ]
NSCLC arises from the epithelial cells of the lung of the central bronchi to terminal alveoli. The histological type of NSCLC correlates with site of origin, reflecting the variation in respiratory tract epithelium of the bronchi to alveoli. Squamous cell carcinoma usually starts near a central bronchus. Adenocarcinoma and bronchioloalveolar carcinoma usually originate in peripheral lung tissue.

Pathogenesis
Smoking-related lung carcinogenesis is a multistep process. Squamous cell carcinoma and adenocarcinoma have defined premalignant precursor lesions. Before becoming invasive, lung epithelium may undergo morphological changes that include:
- Hyperplasia.
- Metaplasia.
- Carcinoma in situ .
Dysplasia and carcinoma in situ are considered the principal premalignant lesions because they are more likely to progress to invasive cancer and less likely to spontaneously regress.
Risk Factors
Increasing age is the most important risk factor for most cancers. Other risk factors for lung cancer include the following:
- History of or current tobacco use: cigarettes, pipes, and cigars.[ 2 ]
- Exposure to cancer-causing substances in secondhand smoke.[ 3 , 4 ]
- Occupational exposure to asbestos, arsenic, chromium, beryllium, nickel, and other agents.[ 5 ]
- Radiation therapy to the breast or chest.[ 6 ]
- Radon exposure in the home or workplace.[ 7 ]
- Medical imaging tests, such as computed tomography (CT) scans.[ 8 ]
- Atomic bomb radiation.[ 9 ]
- Living in an area with air pollution.[ 10 - 12 ]
- Family history of lung cancer.[ 13 ]
- Human immunodeficiency virus infection.[ 14 ]
- Beta carotene supplements in heavy smokers.[ 15 , 16 ]
The single most important risk factor for the development of lung cancer is smoking. For a smoker, the risk of lung cancer is, on average, tenfold higher than in a lifetime nonsmoker (defined as a person who has smoked <100 cigarettes in his or her lifetime). The risk increases with the quantity of cigarettes, duration of smoking, and starting age.
Smoking cessation results in a decrease in precancerous lesions and a reduction in lung cancer risk. Former smokers continue to have an elevated risk of lung cancer for years after quitting. Asbestos exposure may exert a synergistic effect of cigarette smoking on lung cancer risk.[ 17 ]
In addition, after resection of a lung cancer, there is a 1% to 2% risk per patient per year that a second lung cancer will occur.[ 18 ]
A significant number of patients cured of their smoking-related lung cancer may develop a second malignancy. In the Lung Cancer Study Group trial of 907 patients with stage T1, N0 resected tumors, the rate was 1.8% per year for nonpulmonary second cancers and 1.6% per year for new lung cancers.[ 19 ] Other studies have reported even higher risks of second tumors in long-term survivors, including rates of 10% for second lung cancers and 20% for all second cancers.[ 20 ]
Because of the persistent risk of developing second lung cancers in former smokers, various chemoprevention strategies have been evaluated in randomized control trials. None of the phase III trials using the agents beta carotene, retinol, 13- cis -retinoic acid, [alpha]-tocopherol, N-acetylcysteine, or acetylsalicylic acid has demonstrated beneficial, reproducible results.[ 16 , 21 - 24 ][ Level of evidence A1 ] Chemoprevention of second primary cancers of the upper aerodigestive tract is undergoing clinical evaluation in patients with early-stage lung cancer.
For more information, see Lung Cancer Prevention .
In patients considered at high risk of developing lung cancer, the only screening modality for early detection that has been shown to alter mortality is low-dose helical CT scanning.[ 25 ] Studies have failed to demonstrate that screening with chest radiography and sputum cytology lowers lung cancer mortality rates.
For more information, see the Screening by low-dose computed tomography: benefit section in Lung Cancer Screening.
Clinical Presentation
Lung cancer may present with symptoms or be found incidentally on chest imaging. The most common symptoms at presentation include:
- Worsening cough.
- Chest pain.
- Hemoptysis.
- Weight loss.
- Hoarseness.
Symptoms may result from local invasion or compression of adjacent thoracic structures, such as compression involving the esophagus causing dysphagia, compression involving the laryngeal nerves causing hoarseness, or compression involving the superior vena cava causing facial edema and distension of the superficial veins of the head and neck.
Symptoms from distant metastases may also be present and include neurological defect or personality change from brain metastases or pain from bone metastases. Infrequently, patients may present with symptoms and signs of paraneoplastic diseases such as hypertrophic osteoarthropathy with digital clubbing or hypercalcemia from parathyroid hormone-related protein.
Physical examination may identify enlarged supraclavicular lymphadenopathy, pleural effusion or lobar collapse, unresolved pneumonia, or signs of associated disease such as chronic obstructive pulmonary disease or pulmonary fibrosis.
Investigations of patients with suspected NSCLC focus on confirming the diagnosis and determining the extent of the disease. Treatment options are determined by histology, stage, and general health and comorbidities of the patient.
The procedures used to determine the presence of cancer include:
- Physical examination.
- Routine laboratory evaluations.
- Chest x-ray.
- Chest CT scan with infusion of contrast material.
Before a patient begins lung cancer treatment, an experienced lung cancer pathologist must review the pathological material. This is critical because SCLC, which responds well to chemotherapy and is generally not treated surgically, can be confused on microscopic examination with NSCLC.[ 26 ] Immunohistochemistry and electron microscopy are invaluable techniques for diagnosis and subclassification, but most lung tumors can be classified by light microscopic criteria.
For more information on tests and procedures used for staging, see the General Staging Evaluation section.
Prognostic Factors
Multiple studies have attempted to identify the prognostic importance of a variety of clinicopathological factors.[ 20 , 27 - 30 ] Factors that have correlated with adverse prognosis include:
- Increasing stage.
- Presence of pulmonary or constitutional symptoms.
- Large tumor size (>3 cm).
- Metastases to multiple lymph nodes within a TNM-defined nodal station.[ 31 - 41 ] For more information, see the Evaluation of mediastinal lymph node metastasis section.
- Vascular invasion.[ 28 , 42 - 44 ]
For patients with inoperable disease, prognosis is adversely affected by poor performance status and weight loss of more than 10%. These patients have been excluded from clinical trials evaluating aggressive multimodality interventions.
In multiple retrospective analyses of clinical trial data, advanced age alone has not been shown to influence response or survival with therapy.[ 45 ]
Because treatment is not satisfactory for almost all patients with NSCLC, eligible patients should consider clinical trials. Information about ongoing clinical trials is available from the NCI website .
- American Cancer Society: Cancer Facts and Figures 2024. American Cancer Society, 2024. Available online . Last accessed June 21, 2024.
- Alberg AJ, Ford JG, Samet JM, et al.: Epidemiology of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 132 (3 Suppl): 29S-55S, 2007. [PUBMED Abstract]
- Tulunay OE, Hecht SS, Carmella SG, et al.: Urinary metabolites of a tobacco-specific lung carcinogen in nonsmoking hospitality workers. Cancer Epidemiol Biomarkers Prev 14 (5): 1283-6, 2005. [PUBMED Abstract]
- Anderson KE, Kliris J, Murphy L, et al.: Metabolites of a tobacco-specific lung carcinogen in nonsmoking casino patrons. Cancer Epidemiol Biomarkers Prev 12 (12): 1544-6, 2003. [PUBMED Abstract]
- Straif K, Benbrahim-Tallaa L, Baan R, et al.: A review of human carcinogens--part C: metals, arsenic, dusts, and fibres. Lancet Oncol 10 (5): 453-4, 2009. [PUBMED Abstract]
- Friedman DL, Whitton J, Leisenring W, et al.: Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst 102 (14): 1083-95, 2010. [PUBMED Abstract]
- Gray A, Read S, McGale P, et al.: Lung cancer deaths from indoor radon and the cost effectiveness and potential of policies to reduce them. BMJ 338: a3110, 2009. [PUBMED Abstract]
- Berrington de González A, Kim KP, Berg CD: Low-dose lung computed tomography screening before age 55: estimates of the mortality reduction required to outweigh the radiation-induced cancer risk. J Med Screen 15 (3): 153-8, 2008. [PUBMED Abstract]
- Shimizu Y, Kato H, Schull WJ: Studies of the mortality of A-bomb survivors. 9. Mortality, 1950-1985: Part 2. Cancer mortality based on the recently revised doses (DS86). Radiat Res 121 (2): 120-41, 1990. [PUBMED Abstract]
- Katanoda K, Sobue T, Satoh H, et al.: An association between long-term exposure to ambient air pollution and mortality from lung cancer and respiratory diseases in Japan. J Epidemiol 21 (2): 132-43, 2011. [PUBMED Abstract]
- Cao J, Yang C, Li J, et al.: Association between long-term exposure to outdoor air pollution and mortality in China: a cohort study. J Hazard Mater 186 (2-3): 1594-600, 2011. [PUBMED Abstract]
- Hales S, Blakely T, Woodward A: Air pollution and mortality in New Zealand: cohort study. J Epidemiol Community Health 66 (5): 468-73, 2012. [PUBMED Abstract]
- Lissowska J, Foretova L, Dabek J, et al.: Family history and lung cancer risk: international multicentre case-control study in Eastern and Central Europe and meta-analyses. Cancer Causes Control 21 (7): 1091-104, 2010. [PUBMED Abstract]
- Shiels MS, Cole SR, Kirk GD, et al.: A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr 52 (5): 611-22, 2009. [PUBMED Abstract]
- The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med 330 (15): 1029-35, 1994. [PUBMED Abstract]
- Omenn GS, Goodman GE, Thornquist MD, et al.: Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 334 (18): 1150-5, 1996. [PUBMED Abstract]
- Wingo PA, Ries LA, Giovino GA, et al.: Annual report to the nation on the status of cancer, 1973-1996, with a special section on lung cancer and tobacco smoking. J Natl Cancer Inst 91 (8): 675-90, 1999. [PUBMED Abstract]
- Johnson BE: Second lung cancers in patients after treatment for an initial lung cancer. J Natl Cancer Inst 90 (18): 1335-45, 1998. [PUBMED Abstract]
- Thomas P, Rubinstein L: Cancer recurrence after resection: T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 49 (2): 242-6; discussion 246-7, 1990. [PUBMED Abstract]
- Martini N, Bains MS, Burt ME, et al.: Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg 109 (1): 120-9, 1995. [PUBMED Abstract]
- van Boxem AJ, Westerga J, Venmans BJ, et al.: Photodynamic therapy, Nd-YAG laser and electrocautery for treating early-stage intraluminal cancer: which to choose? Lung Cancer 31 (1): 31-6, 2001. [PUBMED Abstract]
- Blumberg J, Block G: The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study in Finland. Nutr Rev 52 (7): 242-5, 1994. [PUBMED Abstract]
- Lippman SM, Lee JJ, Karp DD, et al.: Randomized phase III intergroup trial of isotretinoin to prevent second primary tumors in stage I non-small-cell lung cancer. J Natl Cancer Inst 93 (8): 605-18, 2001. [PUBMED Abstract]
- van Zandwijk N, Dalesio O, Pastorino U, et al.: EUROSCAN, a randomized trial of vitamin A and N-acetylcysteine in patients with head and neck cancer or lung cancer. For the EUropean Organization for Research and Treatment of Cancer Head and Neck and Lung Cancer Cooperative Groups. J Natl Cancer Inst 92 (12): 977-86, 2000. [PUBMED Abstract]
- Aberle DR, Adams AM, Berg CD, et al.: Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 365 (5): 395-409, 2011. [PUBMED Abstract]
- Travis WD, Colby TV, Corrin B, et al.: Histological typing of lung and pleural tumours. 3rd ed. Springer-Verlag, 1999.
- Albain KS, Crowley JJ, LeBlanc M, et al.: Survival determinants in extensive-stage non-small-cell lung cancer: the Southwest Oncology Group experience. J Clin Oncol 9 (9): 1618-26, 1991. [PUBMED Abstract]
- Macchiarini P, Fontanini G, Hardin MJ, et al.: Blood vessel invasion by tumor cells predicts recurrence in completely resected T1 N0 M0 non-small-cell lung cancer. J Thorac Cardiovasc Surg 106 (1): 80-9, 1993. [PUBMED Abstract]
- Ichinose Y, Yano T, Asoh H, et al.: Prognostic factors obtained by a pathologic examination in completely resected non-small-cell lung cancer. An analysis in each pathologic stage. J Thorac Cardiovasc Surg 110 (3): 601-5, 1995. [PUBMED Abstract]
- Fontanini G, Bigini D, Vignati S, et al.: Microvessel count predicts metastatic disease and survival in non-small cell lung cancer. J Pathol 177 (1): 57-63, 1995. [PUBMED Abstract]
- Sayar A, Turna A, Kiliçgün A, et al.: Prognostic significance of surgical-pathologic multiple-station N1 disease in non-small cell carcinoma of the lung. Eur J Cardiothorac Surg 25 (3): 434-8, 2004. [PUBMED Abstract]
- Osaki T, Nagashima A, Yoshimatsu T, et al.: Survival and characteristics of lymph node involvement in patients with N1 non-small cell lung cancer. Lung Cancer 43 (2): 151-7, 2004. [PUBMED Abstract]
- Ichinose Y, Kato H, Koike T, et al.: Overall survival and local recurrence of 406 completely resected stage IIIa-N2 non-small cell lung cancer patients: questionnaire survey of the Japan Clinical Oncology Group to plan for clinical trials. Lung Cancer 34 (1): 29-36, 2001. [PUBMED Abstract]
- Tanaka F, Yanagihara K, Otake Y, et al.: Prognostic factors in patients with resected pathologic (p-) T1-2N1M0 non-small cell lung cancer (NSCLC). Eur J Cardiothorac Surg 19 (5): 555-61, 2001. [PUBMED Abstract]
- Asamura H, Suzuki K, Kondo H, et al.: Where is the boundary between N1 and N2 stations in lung cancer? Ann Thorac Surg 70 (6): 1839-45; discussion 1845-6, 2000. [PUBMED Abstract]
- Riquet M, Manac'h D, Le Pimpec-Barthes F, et al.: Prognostic significance of surgical-pathologic N1 disease in non-small cell carcinoma of the lung. Ann Thorac Surg 67 (6): 1572-6, 1999. [PUBMED Abstract]
- van Velzen E, Snijder RJ, Brutel de la Rivière A, et al.: Lymph node type as a prognostic factor for survival in T2 N1 M0 non-small cell lung carcinoma. Ann Thorac Surg 63 (5): 1436-40, 1997. [PUBMED Abstract]
- Vansteenkiste JF, De Leyn PR, Deneffe GJ, et al.: Survival and prognostic factors in resected N2 non-small cell lung cancer: a study of 140 cases. Leuven Lung Cancer Group. Ann Thorac Surg 63 (5): 1441-50, 1997. [PUBMED Abstract]
- Izbicki JR, Passlick B, Karg O, et al.: Impact of radical systematic mediastinal lymphadenectomy on tumor staging in lung cancer. Ann Thorac Surg 59 (1): 209-14, 1995. [PUBMED Abstract]
- Martini N, Burt ME, Bains MS, et al.: Survival after resection of stage II non-small cell lung cancer. Ann Thorac Surg 54 (3): 460-5; discussion 466, 1992. [PUBMED Abstract]
- Naruke T, Goya T, Tsuchiya R, et al.: Prognosis and survival in resected lung carcinoma based on the new international staging system. J Thorac Cardiovasc Surg 96 (3): 440-7, 1988. [PUBMED Abstract]
- Thomas P, Doddoli C, Thirion X, et al.: Stage I non-small cell lung cancer: a pragmatic approach to prognosis after complete resection. Ann Thorac Surg 73 (4): 1065-70, 2002. [PUBMED Abstract]
- Macchiarini P, Fontanini G, Hardin MJ, et al.: Relation of neovascularisation to metastasis of non-small-cell lung cancer. Lancet 340 (8812): 145-6, 1992. [PUBMED Abstract]
- Khan OA, Fitzgerald JJ, Field ML, et al.: Histological determinants of survival in completely resected T1-2N1M0 nonsmall cell cancer of the lung. Ann Thorac Surg 77 (4): 1173-8, 2004. [PUBMED Abstract]
- Earle CC, Tsai JS, Gelber RD, et al.: Effectiveness of chemotherapy for advanced lung cancer in the elderly: instrumental variable and propensity analysis. J Clin Oncol 19 (4): 1064-70, 2001. [PUBMED Abstract]
Cellular and Molecular Classification of NSCLC
Malignant non-small cell epithelial tumors of the lung are classified by the World Health Organization (WHO)/International Association for the Study of Lung Cancer (IASLC). The three main subtypes of non-small cell lung cancer (NSCLC) include:
- Squamous cell carcinoma (25% of lung cancers).
- Adenocarcinoma (40% of lung cancers).
- Large cell carcinoma (10% of lung cancers).
Additional types include adenosquamous carcinoma, sarcomatoid carcinomas, salivary gland type tumors, carcinoid tumors, and other unclassified carcinomas. There are many subtypes in these categories.[ 1 ]
Tumor Types
Squamous cell carcinoma.
Most squamous cell carcinomas of the lung are located centrally, in the larger bronchi of the lung. Squamous cell carcinomas are linked more strongly with smoking than other forms of NSCLC. The incidence of squamous cell carcinoma of the lung has been decreasing in recent years.
Adenocarcinoma
Adenocarcinoma is now the most common histological subtype in many countries, and subclassification of adenocarcinoma is important. One of the biggest problems with lung adenocarcinomas is the frequent histological heterogeneity. Mixtures of adenocarcinoma histological subtypes are more common than tumors consisting purely of a single pattern of acinar, papillary, bronchioloalveolar, and solid adenocarcinoma with mucin formation.
Criteria for the diagnosis of bronchioloalveolar carcinoma have varied widely in the past. The current WHO/IASLC definition is much more restrictive than that previously used by many pathologists because it is limited to only noninvasive tumors.
If stromal, vascular, or pleural invasion are identified in an adenocarcinoma that has an extensive bronchioloalveolar carcinoma component, the classification would be an adenocarcinoma of mixed subtype with predominant bronchioloalveolar pattern and a focal acinar, solid, or papillary pattern, depending on which pattern is seen in the invasive component. However, the future of bronchioloalveolar carcinoma as a distinct clinical entity is unclear; a multidisciplinary expert panel representing the IASLC, the American Thoracic Society, and the European Respiratory Society proposed a major revision of the classification of adenocarcinomas in 2011 that entails a reclassification of what was called bronchioloalveolar carcinoma into newly defined histological subgroups.
The following variants of adenocarcinoma are recognized in the WHO/IASLC classification:
- Well-differentiated fetal adenocarcinoma.
- Mucinous (colloid) adenocarcinoma.
- Mucinous cystadenocarcinoma.
- Signet ring adenocarcinoma.
- Clear cell adenocarcinoma.
Large cell carcinoma
In addition to the general category of large cell carcinoma, several uncommon variants are recognized in the WHO/IASLC classification, including:
- Large cell neuroendocrine carcinoma (LCNEC).
- Basaloid carcinoma.
- Lymphoepithelioma-like carcinoma.
- Clear cell carcinoma.
- Large cell carcinoma with rhabdoid phenotype.
Basaloid carcinoma is also recognized as a variant of squamous cell carcinoma, and rarely, adenocarcinomas may have a basaloid pattern; however, in tumors without either of these features, they are regarded as a variant of large cell carcinoma.
Neuroendocrine tumors
LCNEC is recognized as a histologically high-grade non-small cell carcinoma. It has a very poor prognosis similar to that of small cell lung cancer (SCLC). Atypical carcinoid is recognized as an intermediate-grade neuroendocrine tumor with a prognosis that falls between typical carcinoid and high-grade SCLC and LCNEC.
Neuroendocrine differentiation can be demonstrated by immunohistochemistry or electron microscopy in 10% to 20% of common NSCLCs that do not have any neuroendocrine morphology. These tumors are not formally recognized within the WHO/IASLC classification scheme because the clinical and therapeutic significance of neuroendocrine differentiation in NSCLC is not firmly established. These tumors are referred to collectively as NSCLC with neuroendocrine differentiation.
Carcinomas with pleomorphic, sarcomatoid, or sarcomatous elements
This is a group of rare tumors. Spindle cell carcinomas and giant cell carcinomas comprise only 0.4% of all lung malignancies, and carcinosarcomas comprise only 0.1% of all lung malignancies. In addition, this group of tumors reflects a continuum in histological heterogeneity, as well as epithelial and mesenchymal differentiation. On the basis of clinical and molecular data, biphasic pulmonary blastoma is regarded as part of the spectrum of carcinomas with pleomorphic, sarcomatoid, or sarcomatous elements.
Molecular Features
The identification of mutations in lung cancer has led to the development of molecularly targeted therapy to improve the survival of subsets of patients with metastatic disease.[ 2 ] In particular, subsets of adenocarcinoma now can be defined by specific mutations in genes encoding components of the epidermal growth factor receptor (EGFR) and downstream mitogen-activated protein kinases (MAPK) and phosphatidylinositol 3-kinases (PI3K) signaling pathways. These mutations may define mechanisms of drug sensitivity and primary or acquired resistance to kinase inhibitors. Genomic alterations that can be targeted with approved therapies or for which treatments are under development include:
- NTRK1 , NTRK2 , and NTRK3 .
EGFR and ALK mutations predominate in adenocarcinomas that develop in nonsmokers, and KRAS and BRAF mutations are more common in smokers or former smokers. EGFR mutations strongly predict the improved response rate and progression-free survival of patients who take EGFR inhibitors. In a set of 2,142 lung adenocarcinoma specimens from patients treated at Memorial Sloan Kettering Cancer Center, EGFR exon 19 deletions and L858R were found in 15% of tumors from former smokers (181 of 1,218; 95% confidence interval [CI], 13%–17%), 6% from current smokers (20 of 344; 95% CI, 4%–9%), and 52% from never-smokers (302 of 580; 95% CI, 48%–56%; P < .001 for ever- vs. never-smokers).[ 3 ]
Fusions of ALK with EML4 genes form translocation products that occur in ranges from 3% to 7% in unselected NSCLC and are responsive to pharmacological inhibition of ALK by agents such as crizotinib. Sensitizing fusions of ALK with other genes have also been reported.
- Travis WD, Brambilla E, Nicholson AG, et al.: The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 10 (9): 1243-1260, 2015. [PUBMED Abstract]
- Pao W, Girard N: New driver mutations in non-small-cell lung cancer. Lancet Oncol 12 (2): 175-80, 2011. [PUBMED Abstract]
- D'Angelo SP, Pietanza MC, Johnson ML, et al.: Incidence of EGFR exon 19 deletions and L858R in tumor specimens from men and cigarette smokers with lung adenocarcinomas. J Clin Oncol 29 (15): 2066-70, 2011. [PUBMED Abstract]
Stage Information for NSCLC
General staging evaluation.
In non-small cell lung cancer (NSCLC), the determination of stage has important therapeutic and prognostic implications. Careful initial diagnostic evaluation to define the location and to determine the extent of primary and metastatic tumor involvement is critical for the appropriate care of patients.
In general, symptoms, physical signs, laboratory findings, and perceived risk of distant metastasis lead to an evaluation for distant metastatic disease. Additional tests such as bone scans and computed tomography (CT)/magnetic resonance imaging (MRI) of the brain may be performed if initial assessments suggest metastases or if patients with stage III disease are being evaluated for aggressive local and combined modality treatments.
Stage has a critical role in the selection of therapy. The stage of disease is based on a combination of clinical factors and pathological factors.[ 1 ] The distinction between clinical stage and pathological stage should be considered when evaluating reports of survival outcome.
Procedures used to determine stage include:
- Fluorine F 18-fludeoxyglucose positron emission tomography (18F-FDG PET) scanning.
Procedures used to obtain tissue samples include bronchoscopy, mediastinoscopy, or anterior mediastinotomy.
Pathological staging of NSCLC requires examination of the tumor, knowledge of resection margins, and determination of lymph node status.
At diagnosis, patients with NSCLC can be divided into the following three groups that reflect both the extent of the disease and the treatment approach:
- Has the best prognosis, which depends on a variety of tumor and host factors.
- Patients with resectable disease who have medical contraindications to surgery are candidates for curative radiation therapy.
- Postoperative cisplatin-based combination chemotherapy may provide a survival advantage for patients with resected stage II or stage IIIA NSCLC.
- Has a diverse natural history.
- Selected patients with locally advanced tumors may benefit from combined modality treatments.
- Patients with unresectable or N2–N3 disease are treated with radiation therapy in combination with chemotherapy.
- Selected patients with T3 or N2 disease can be treated effectively with surgical resection and either preoperative or postoperative chemotherapy or chemoradiation therapy.
- May be treated with systemic therapy (chemotherapy and/or immunotherapy or targeted therapy). Radiation therapy can be used for palliation.
Evaluation of mediastinal lymph node metastasis
Surgical evaluation.
Surgical staging of the mediastinum is considered standard if accurate evaluation of the nodal status is needed to determine therapy.
Accurate staging of the mediastinal lymph nodes provides important prognostic information.
Evidence (nodal status):
- Patients with five to eight lymph nodes examined during surgery had a modest but statistically significant increase in survival, with a proportionate hazard ratio (HR) of 0.90 (95% confidence interval [CI], 0.84–0.97).
- For patients with 9 to 12 examined lymph nodes, the HR was 0.86 (95% CI, 0.79–0.95).
- For patients with 13 to 16 examined lymph nodes, the HR was 0.78 (95% CI, 0.68–0.90).
- There appeared to be no incremental improvement after evaluating more than 16 lymph nodes.
The corresponding results for lung cancer–specific mortality and for patients who received radiation therapy were not substantially different.
- These results indicate that patient survival following resection for NSCLC is associated with the number of lymph nodes evaluated during surgery. Because this is most likely the result of a reduction-of-staging error, namely, a decreased likelihood of missing positive lymph nodes with an increasing number of lymph nodes sampled, it suggests that an evaluation of nodal status should include 11 to 16 lymph nodes.
CT scanning is primarily used for determining the size of the tumor. The CT scan should extend inferiorly to include the liver and adrenal glands. MRI scans of the thorax and upper abdomen do not appear to yield advantages over CT scans.[ 3 ]
Evidence (CT scan):
- The median prevalence of mediastinal metastasis was 28% (range, 18%–56%).
- The pooled estimates of sensitivity and specificity of CT scanning for identifying mediastinal lymph node metastasis were 51% (95% CI, 47%–54%) for sensitivity and 86% (95% CI, 84%–88%) for specificity. Corresponding positive (3.4%) and negative (0.6%) likelihood ratios were provided.
- The results from the systematic review are similar to those of a large meta-analysis that reported the median sensitivity and specificity of CT scanning for identifying malignant mediastinal nodes as 61% for sensitivity and 79% for specificity.[ 5 ]
- An earlier meta-analysis reported an average sensitivity rate of 64% and specificity rate of 74%.[ 6 ]
18F-FDG PET scanning
The wider availability and use of 18F-FDG PET scanning for staging has modified the approach to staging mediastinal lymph nodes and distant metastases.
Randomized trials evaluating the utility of 18F-FDG PET scanning in potentially resectable NSCLC patients reported conflicting results in terms of the relative reduction in the number of noncurative thoracotomies.
Although the current evidence is conflicting, 18F-FDG PET scanning may improve results of early-stage lung cancer by identifying patients who have evidence of metastatic disease that is beyond the scope of surgical resection and that is not evident by standard preoperative staging procedures.
Evidence (18F-FDG PET scan):
- 18F-FDG PET scanning appears to be superior to CT imaging for mediastinal staging in NSCLC.
- 18F-FDG PET scanning also appears to have high sensitivity and reasonable specificity for differentiating benign from malignant lesions as small as 1 cm.
- The median prevalence of mediastinal metastases was 29% (range, 5%–64%).
- Pooled estimates of sensitivity and specificity for identifying mediastinal metastasis were 74% (95% CI, 69%–79%) for sensitivity and 85% (95% CI, 82%–88%) for specificity.
- Corresponding positive (4.9%) and negative (0.3%) likelihood ratios were provided for mediastinal staging with 18F-FDG PET scanning.
- These findings demonstrated that 18F-FDG PET scanning is more accurate than CT scanning for staging of the mediastinum in patients with lung cancer.
Decision analyses demonstrate that 18F-FDG PET scanning may reduce the overall costs of medical care by identifying patients with falsely negative CT scans in the mediastinum or otherwise undetected sites of metastases.[ 8 - 10 ] Studies concluded that the money saved by forgoing mediastinoscopy in 18F-FDG PET-positive mediastinal lesions was not justified because of the unacceptably high number of false-positive results.[ 8 - 10 ] A randomized study found that the addition of 18F-FDG PET scanning to conventional staging was associated with significantly fewer thoracotomies.[ 11 ] A second randomized trial evaluating the impact of 18F-FDG PET scanning on clinical management found that 18F-FDG PET scanning provided additional information regarding appropriate stage but did not lead to significantly fewer thoracotomies.[ 12 ]
Combination of CT imaging and 18F-FDG PET scanning
The combination of CT imaging and 18F-FDG PET scanning has greater sensitivity and specificity than CT imaging alone.[ 13 ]
Evidence (CT/18F-FDG PET scan):
- If there is no evidence of distant metastatic disease on CT scan, 18F-FDG PET scanning complements CT scan staging of the mediastinum. Numerous nonrandomized studies of 18F-FDG PET scanning have evaluated mediastinal lymph nodes using surgery (i.e., mediastinoscopy and/or thoracotomy with mediastinal lymph node dissection) as the gold standard of comparison.
- The median sensitivity and specificity of 18F-FDG PET scans were reported as 100% for sensitivity and 78% for specificity in patients with enlarged lymph nodes.[ 5 ]
- 18F-FDG PET scanning is considered very accurate in identifying malignant nodal involvement when lymph nodes are enlarged. However, 18F-FDG PET scanning will falsely identify a malignancy in approximately one-fourth of patients with lymph nodes that are enlarged for other reasons, usually as a result of inflammation or infection.[ 14 , 15 ]
- The median sensitivity and specificity of 18F-FDG PET scanning in patients with normal-sized mediastinal lymph nodes were 82% for sensitivity and 93% for specificity.[ 5 ] These data indicate that nearly 20% of patients with normal-sized lymph nodes but with malignant involvement had falsely negative 18F-FDG PET scan findings.
For patients with clinically operable NSCLC, the evidence supports performing a biopsy of mediastinal lymph nodes that are found to be larger than 1 cm in shortest transverse axis on chest CT scan or are found to be positive on 18F-FDG PET scan. Negative 18F-FDG PET scanning does not preclude biopsy of radiographically enlarged mediastinal lymph nodes. Mediastinoscopy is necessary for the detection of cancer in mediastinal lymph nodes when the results of the CT scan and 18F-FDG PET scan do not corroborate each other.
Evaluation of brain metastasis
Patients at risk of brain metastases may be staged with CT or MRI scans.
Evidence (staging with CT or MRI):
- MRI showed a trend towards a higher preoperative detection rate than CT scan ( P = .069), with an overall detection rate of approximately 7% from pretreatment to 12 months after surgery.
- Patients with stage I or stage II disease had a detection rate of 4% (i.e., 8 detections out of 200 patients); however, individuals with stage III disease had a detection rate of 11.4% (i.e., 15 detections out of 132 patients).
- The mean maximal diameter of the brain metastases was significantly smaller in the MRI group.
Whether the improved detection rate of MRI translates into improved outcome remains unknown. Not all patients are able to tolerate MRI, and for these patients contrast-enhanced CT scan is a reasonable substitute.
Evaluation of distant metastasis to sites other than the brain
Numerous nonrandomized, prospective, and retrospective studies have demonstrated that 18F-FDG PET scanning offers diagnostic advantages over conventional imaging in staging distant metastatic disease; however, standard 18F-FDG PET scans have limitations. 18F-FDG PET scans may not extend below the pelvis and may not detect bone metastases in the long bones of the lower extremities. Because the metabolic tracer used in 18F-FDG PET scanning accumulates in the brain and urinary tract, 18F-FDG PET scanning is not reliable for detection of metastases in these sites.[ 16 ]
The Revised International System for Staging Lung Cancer
The Revised International System for Staging Lung Cancer, based on information from a clinical database of more than 5,000 patients, was adopted in 2010 by the American Joint Committee on Cancer (AJCC) and the Union Internationale Contre le Cancer.[ 17 , 18 ] These revisions provide greater prognostic specificity for patient groups; however, the correlation between stage and prognosis predates the widespread availability of PET imaging.
AJCC Stage Groupings and TNM Definitions
The AJCC has designated staging by TNM (tumor, node, metastasis) classification to define NSCLC.[ 18 ]
| T Criteria | Reprinted with permission from AJCC: Lung. In: Amin MB, Edge SB, Greene FL, et al., eds.: . 8th ed. New York, NY: Springer, 2017, pp. 431–56.||
|---|---|---|
| TX | Primary tumor cannot be assessed, or tumor proven by the presence of malignant cells in sputum or bronchial washings but not visualized by imaging or bronchoscopy. | |
| T0 | No evidence of primary tumor. | |
| Tis | Carcinoma ; SCIS =Squamous cell carcinoma ; AIS: Adenocarcinoma ; Adenocarcinoma with pure lepidic pattern, ≤3 cm in greatest dimension. | |
| T1 | Tumor ≤3 cm in greatest dimension, surrounded by lung or visceral pleura, without bronchoscopic evidence of invasion more proximal than the lobar bronchus (i.e., not in the main bronchus). | |
| T1mi | Minimally invasive adenocarcinoma: adenocarcinoma (≤3 cm in greatest dimension) with a predominantly lepidic pattern and ≤5 mm invasion in greatest dimension. | |
| T1a | Tumor ≤1 cm in greatest dimension. A superficial, spreading tumor of any size whose invasive component is limited to the bronchial wall and may extend proximal to the main bronchus also is classified as T1a, but these tumors are uncommon. | |
| T1b | Tumor >1 cm but ≤2 cm in greatest dimension. | |
| T1c | Tumor >2 cm but ≤3 cm in greatest dimension. | |
| T2 | Tumor >3 cm but ≤5 cm or having any of the following features: involves the main bronchus regardless of distance to the carina, but without involvement of the carina; invades visceral pleura (PL1 or PL2); associated with atelectasis or obstructive pneumonitis that extends to the hilar region, involving part or all of the lung. T2 tumors with these features are classified as T2a if ≤4 cm or if the size cannot be determined and T2b if >4 cm but ≤5 cm. | |
| T2a | Tumor >3 cm but ≤4 cm in greatest dimension. | |
| T2b | Tumor >4 cm but ≤5 cm in greatest dimension. | |
| T3 | Tumor >5 cm but ≤7 cm in greatest dimension or directly invading any of the following: parietal pleura (PL3), chest wall (including superior sulcus tumors), phrenic nerve, parietal pericardium; or separate tumor nodule(s) in the same lobe as the primary. | |
| T4 | Tumor >7 cm or tumor of any size invading one or more of the following: diaphragm, mediastinum, heart, great vessels, trachea, recurrent laryngeal nerve, esophagus, vertebral body, or carina; separate tumor nodule(s) in an ipsilateral lobe different from that of the primary. | |
| N Criteria | Reprinted with permission from AJCC: Lung. In: Amin MB, Edge SB, Greene FL, et al., eds.: . 8th ed. New York, NY: Springer, 2017, pp. 431–56.|
|---|---|
| NX | Regional lymph nodes cannot be assessed. |
| N0 | No regional lymph node metastasis. |
| N1 | Metastasis in ipsilateral peribronchial and/or ipsilateral hilar lymph nodes and intrapulmonary nodes, including involvement by direct extension. |
| N2 | Metastasis in ipsilateral mediastinal and/or subcarinal lymph node(s). |
| N3 | Metastasis in contralateral mediastinal, contralateral hilar, ipsilateral or contralateral scalene, or supraclavicular lymph node(s). |
| M Criteria | Reprinted with permission from AJCC: Lung. In: Amin MB, Edge SB, Greene FL, et al., eds.: . 8th ed. New York, NY: Springer, 2017, pp. 431–56.||
|---|---|---|
| M0 | No distant metastasis. | |
| M1 | Distant metastasis. | |
| M1a | Separate tumor nodule(s) in a contralateral lobe; tumor with pleural or pericardial nodules or malignant pleural or pericardial effusion. Most pleural (pericardial) effusions with lung cancer are a result of the tumor. In a few patients, however, multiple microscopic examinations of pleural (pericardial) fluid are negative for tumor, and the fluid is nonbloody and not an exudate. If these elements and clinical judgment dictate that the effusion is not related to the tumor, the effusion should be excluded as a staging descriptor. | |
| M1b | Single extrathoracic metastases in a single organ (including involvement of a single nonregional node). | |
| M1c | Multiple extrathoracic metastases in a single organ or in multiple organs. | |
| TNM Classification | Illustration | |
|---|---|---|
| aReprinted with permission from AJCC: Lung. In: Amin MB, Edge SB, Greene FL, et al., eds.: . 8th ed. New York, NY: Springer, 2017, pp. 431–56. | ||
| Occult carcinoma | TX, N0, M0 | |
| 0 | Tis, N0, M0 | |
| IA1 | T1mi, N0, M0 | |
| T1a, N0, M0 | ||
| IA2 | T1b, N0, M0 | |
| IA3 | T1c, N0, M0 | |
| IB | T2a, N0, M0 | |
| IIA | T2b, N0, M0 | |
| IIB | T1a, N1, M0 | |
| T1b, N1, M0 | ||
| T1c, N1, M0 | ||
| T2a, N1, M0 | ||
| T2b, N1, M0 | ||
| T3, N0, M0 | ||
| IIIA | T1a, N2, M0 | |
| T1b, N2, M0 | ||
| T1c, N2, M0 | ||
| T2a, N2, M0 | ||
| T2b, N2, M0 | ||
| T3, N1, M0 | ||
| T4, N0, M0 | ||
| T4, N1, M0 | ||
| IIIB | T1a, N3, M0 | |
| T1b, N3, M0 | ||
| T1c, N3, M0 | ||
| T2a, N3, M0 | ||
| T2b, N3, M0 | ||
| T3, N2, M0 | ||
| T4, N2, M0 | ||
| IIIC | T3, N3, M0 | |
| T4, N3, M0 | ||
| IV | Any T, Any N, M1 | |
| IVA | Any T, Any N, M1a | |
| Any T, Any N, M1b | ||
| IVB | Any T, Any N, M1c | |
- Pfister DG, Johnson DH, Azzoli CG, et al.: American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J Clin Oncol 22 (2): 330-53, 2004. [PUBMED Abstract]
- Ludwig MS, Goodman M, Miller DL, et al.: Postoperative survival and the number of lymph nodes sampled during resection of node-negative non-small cell lung cancer. Chest 128 (3): 1545-50, 2005. [PUBMED Abstract]
- Webb WR, Gatsonis C, Zerhouni EA, et al.: CT and MR imaging in staging non-small cell bronchogenic carcinoma: report of the Radiologic Diagnostic Oncology Group. Radiology 178 (3): 705-13, 1991. [PUBMED Abstract]
- Toloza EM, Harpole L, McCrory DC: Noninvasive staging of non-small cell lung cancer: a review of the current evidence. Chest 123 (1 Suppl): 137S-146S, 2003. [PUBMED Abstract]
- Gould MK, Kuschner WG, Rydzak CE, et al.: Test performance of positron emission tomography and computed tomography for mediastinal staging in patients with non-small-cell lung cancer: a meta-analysis. Ann Intern Med 139 (11): 879-92, 2003. [PUBMED Abstract]
- Dwamena BA, Sonnad SS, Angobaldo JO, et al.: Metastases from non-small cell lung cancer: mediastinal staging in the 1990s--meta-analytic comparison of PET and CT. Radiology 213 (2): 530-6, 1999. [PUBMED Abstract]
- Ung YC, Maziak DE, Vanderveen JA, et al.: 18Fluorodeoxyglucose positron emission tomography in the diagnosis and staging of lung cancer: a systematic review. J Natl Cancer Inst 99 (23): 1753-67, 2007. [PUBMED Abstract]
- Dietlein M, Weber K, Gandjour A, et al.: Cost-effectiveness of FDG-PET for the management of potentially operable non-small cell lung cancer: priority for a PET-based strategy after nodal-negative CT results. Eur J Nucl Med 27 (11): 1598-609, 2000. [PUBMED Abstract]
- Scott WJ, Shepherd J, Gambhir SS: Cost-effectiveness of FDG-PET for staging non-small cell lung cancer: a decision analysis. Ann Thorac Surg 66 (6): 1876-83; discussion 1883-5, 1998. [PUBMED Abstract]
- Gambhir SS, Hoh CK, Phelps ME, et al.: Decision tree sensitivity analysis for cost-effectiveness of FDG-PET in the staging and management of non-small-cell lung carcinoma. J Nucl Med 37 (9): 1428-36, 1996. [PUBMED Abstract]
- van Tinteren H, Hoekstra OS, Smit EF, et al.: Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet 359 (9315): 1388-93, 2002. [PUBMED Abstract]
- Viney RC, Boyer MJ, King MT, et al.: Randomized controlled trial of the role of positron emission tomography in the management of stage I and II non-small-cell lung cancer. J Clin Oncol 22 (12): 2357-62, 2004. [PUBMED Abstract]
- Vansteenkiste JF, Stroobants SG, De Leyn PR, et al.: Lymph node staging in non-small-cell lung cancer with FDG-PET scan: a prospective study on 690 lymph node stations from 68 patients. J Clin Oncol 16 (6): 2142-9, 1998. [PUBMED Abstract]
- Roberts PF, Follette DM, von Haag D, et al.: Factors associated with false-positive staging of lung cancer by positron emission tomography. Ann Thorac Surg 70 (4): 1154-9; discussion 1159-60, 2000. [PUBMED Abstract]
- Liewald F, Grosse S, Storck M, et al.: How useful is positron emission tomography for lymphnode staging in non-small-cell lung cancer? Thorac Cardiovasc Surg 48 (2): 93-6, 2000. [PUBMED Abstract]
- Yokoi K, Kamiya N, Matsuguma H, et al.: Detection of brain metastasis in potentially operable non-small cell lung cancer: a comparison of CT and MRI. Chest 115 (3): 714-9, 1999. [PUBMED Abstract]
- Mountain CF: Revisions in the International System for Staging Lung Cancer. Chest 111 (6): 1710-7, 1997. [PUBMED Abstract]
- Lung. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp. 431–56.
Treatment Option Overview for NSCLC
In non-small cell lung cancer (NSCLC), results of standard treatment are poor except for the most localized cancers. All newly diagnosed patients with NSCLC are potential candidates for studies evaluating new forms of treatment.
Treatment decisions are based on some of the following factors:
- Knowledge of histological type and molecular features.
- Tumor size and location.
- Involvement of pleura.
- Surgical margins.
- Status and location of lymph nodes by station.
- Tumor grade.
- Lymphovascular invasion.
Surgery is potentially the most curative therapeutic option for this disease. Postoperative chemotherapy may provide an additional benefit to patients with resected NSCLC. Radiation therapy combined with chemotherapy can produce a cure in a small number of patients and can provide palliation in most patients. Prophylactic cranial irradiation may reduce the incidence of brain metastases, but there is no evidence of a survival benefit and the effect of prophylactic cranial irradiation on quality of life is not known.[ 1 , 2 ] In patients with advanced-stage disease, chemotherapy or epidermal growth factor receptor (EGFR) kinase inhibitors offer modest improvements in median survival, although overall survival is poor.[ 3 , 4 ]
Chemotherapy has produced short-term improvement in disease-related symptoms in patients with advanced NSCLC. Several clinical trials have attempted to assess the impact of chemotherapy on tumor-related symptoms and quality of life. In total, these studies suggest that tumor-related symptoms may be controlled by chemotherapy without adversely affecting overall quality of life;[ 5 , 6 ] however, the impact of chemotherapy on quality of life requires more study. In general, medically eligible older patients with good performance status obtain the same benefits from treatment as younger patients.
The identification of gene mutations in lung cancer has led to the development of molecularly targeted therapy to improve the survival of subsets of patients with metastatic disease.[ 7 ] In particular, genetic abnormalities in EGFR , MAPK , and PI3K signaling pathways in subsets of NSCLC may define mechanisms of drug sensitivity and primary or acquired resistance to kinase inhibitors. EGFR mutations strongly predict the improved response rate and progression-free survival of inhibitors of EGFR. Fusions of ALK with EML4 and other genes form translocation products that occur in ranges from 3% to 7% in unselected NSCLC and are responsive to pharmacological inhibition of ALK by agents such as alectinib. The MET oncogene encodes hepatocyte growth factor receptor. Amplification of this gene has been associated with secondary resistance to EGFR tyrosine kinase inhibitors. Recurrent fusions involving the ROS1 gene are observed in up to 2% of NSCLCs and are responsive to treatment with crizotinib and entrectinib. NTRK gene fusions can occur in up to 1% of NSCLCs and can be treated with the TRK inhibitors, larotrectinib and entrectinib. For more information, see the Molecular Features section.
The treatment options for each stage of NSCLC are presented in Table 5 .
| Treatment Options | ||
|---|---|---|
| Occult NSCLC | ||
| Stage 0 NSCLC | ||
| Stages IA and IB NSCLC | ||
| Stages IIA and IIB NSCLC | ||
| Stage IIIA NSCLC | Resected or resectable disease | |
| Unresectable disease | ||
| Superior sulcus tumors | ||
| Tumors that invade the chest wall | ||
| Stages IIIB and IIIC NSCLC | ||
| Newly Diagnosed Stage IV, Relapsed, and Recurrent NSCLC | ||
| (for patients with stable or responding disease after four cycles of platinum-based combination chemotherapy) | ||
| (for patients with mutations) | ||
| (for patients with exon 20 insertion mutations) | ||
| (for patients with translocations) | ||
| (for patients with V600E mutations) | ||
| (for patients with rearrangements) | ||
| (for patients with fusions) | ||
| (for patients with fusions) | ||
| (for patients with exon 14 skipping mutations) | ||
| (for patients with unresectable, locally advanced or metastatic, progressive, well-differentiated, nonfunctional, neuroendocrine tumors) | ||
| Progressive Stage IV, Relapsed, and Recurrent NSCLC | ||
| (for patients with V600E mutations) | ||
| (for patients with fusions) | ||
| (for patients with fusions) | ||
| (for patients with exon 14 skipping mutations) | ||
| (for patients with G12C mutations) | ||
| (for patients with mutations) | ||
| (for patients with unresectable, locally advanced or metastatic, progressive, well-differentiated, nonfunctional, neuroendocrine tumors) | ||
In addition to the treatment options presented in Table 5, treatment options under clinical evaluation include:
- Combining local treatment (surgery).
- Regional treatment (radiation therapy).
- Systemic treatments (chemotherapy, immunotherapy, and targeted agents).
- Developing more effective systemic therapy.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
- Lester JF, MacBeth FR, Coles B: Prophylactic cranial irradiation for preventing brain metastases in patients undergoing radical treatment for non-small-cell lung cancer: a Cochrane Review. Int J Radiat Oncol Biol Phys 63 (3): 690-4, 2005. [PUBMED Abstract]
- Pöttgen C, Eberhardt W, Grannass A, et al.: Prophylactic cranial irradiation in operable stage IIIA non small-cell lung cancer treated with neoadjuvant chemoradiotherapy: results from a German multicenter randomized trial. J Clin Oncol 25 (31): 4987-92, 2007. [PUBMED Abstract]
- Chemotherapy for non-small cell lung cancer. Non-small Cell Lung Cancer Collaborative Group. Cochrane Database Syst Rev (2): CD002139, 2000. [PUBMED Abstract]
- Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ 311 (7010): 899-909, 1995. [PUBMED Abstract]
- Spiro SG, Rudd RM, Souhami RL, et al.: Chemotherapy versus supportive care in advanced non-small cell lung cancer: improved survival without detriment to quality of life. Thorax 59 (10): 828-36, 2004. [PUBMED Abstract]
- Clegg A, Scott DA, Hewitson P, et al.: Clinical and cost effectiveness of paclitaxel, docetaxel, gemcitabine, and vinorelbine in non-small cell lung cancer: a systematic review. Thorax 57 (1): 20-8, 2002. [PUBMED Abstract]
Treatment of Occult NSCLC
In occult lung cancer, a diagnostic evaluation often includes chest x-ray and selective bronchoscopy with close follow-up (e.g., computed tomography scan), when needed, to define the site and nature of the primary tumor; tumors discovered in this fashion are generally early stage and curable by surgery.
After discovery of the primary tumor, treatment involves establishing the stage of the tumor. Therapy is identical to that recommended for other non-small cell lung cancer (NSCLC) patients with similar-stage disease.
Treatment Options for Occult NSCLC
Treatment options for occult NSCLC include:
Treatment of Stage 0 NSCLC
Stage 0 non-small cell lung cancer (NSCLC) frequently progresses to invasive cancer.[ 1 - 3 ] Patients may be offered surveillance bronchoscopies and, if lesions are detected, potentially curative therapies.
Treatment Options for Stage 0 NSCLC
Treatment options for stage 0 NSCLC include:
- Endobronchial therapies , including photodynamic therapy, electrocautery, cryotherapy, and neodymium-doped yttrium aluminum garnet (Nd-YAG) laser therapy.
Segmentectomy or wedge resection are used to preserve maximum normal pulmonary tissue because patients with stage 0 NSCLC are at a high risk of second lung cancers. Because these tumors are by definition noninvasive and incapable of metastasizing, they should be curable with surgical resection; however, such lesions, when identified, are often centrally located and may require a lobectomy.
Endobronchial therapies
Patients with central lesions may be candidates for curative endobronchial therapy. Endobronchial therapies that preserve lung function include photodynamic therapy, electrocautery, cryotherapy, and Nd-YAG laser therapy.[ 3 - 6 ]
Evidence (endobronchial therapies):
- Small case series have reported high complete response rates and long-term survival in selected patients.[ 7 , 8 ][ Level of evidence C2 ]
Efficacy of these treatment modalities in the management of patients with early NSCLC remains to be proven in definitive randomized controlled trials.
A high incidence of second primary cancers develop in these patients.[ 1 , 2 ]
- Woolner LB, Fontana RS, Cortese DA, et al.: Roentgenographically occult lung cancer: pathologic findings and frequency of multicentricity during a 10-year period. Mayo Clin Proc 59 (7): 453-66, 1984. [PUBMED Abstract]
- Venmans BJ, van Boxem TJ, Smit EF, et al.: Outcome of bronchial carcinoma in situ. Chest 117 (6): 1572-6, 2000. [PUBMED Abstract]
- Jeremy George P, Banerjee AK, Read CA, et al.: Surveillance for the detection of early lung cancer in patients with bronchial dysplasia. Thorax 62 (1): 43-50, 2007. [PUBMED Abstract]
- Kennedy TC, McWilliams A, Edell E, et al.: Bronchial intraepithelial neoplasia/early central airways lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 132 (3 Suppl): 221S-233S, 2007. [PUBMED Abstract]
- Corti L, Toniolo L, Boso C, et al.: Long-term survival of patients treated with photodynamic therapy for carcinoma in situ and early non-small-cell lung carcinoma. Lasers Surg Med 39 (5): 394-402, 2007. [PUBMED Abstract]
- Deygas N, Froudarakis M, Ozenne G, et al.: Cryotherapy in early superficial bronchogenic carcinoma. Chest 120 (1): 26-31, 2001. [PUBMED Abstract]
- van Boxem TJ, Venmans BJ, Schramel FM, et al.: Radiographically occult lung cancer treated with fibreoptic bronchoscopic electrocautery: a pilot study of a simple and inexpensive technique. Eur Respir J 11 (1): 169-72, 1998. [PUBMED Abstract]
Treatment of Stages IA and IB NSCLC
Treatment options for stages ia and ib nsclc.
Treatment options for stages IA non-small cell lung cancer (NSCLC) and IB NSCLC include:
- Adjuvant chemotherapy (for patients with large stage IB tumors).
- Adjuvant targeted therapy (for patients with stage IB tumors with EGFR mutations).
- Adjuvant immunotherapy (for patients with stage IB tumors >4 cm).
- Radiation therapy (for patients who cannot have surgery or choose not to have surgery).
Chemotherapy and radiation therapy have not been shown to improve survival in patients with stage I NSCLC that has been completely resected.
Surgery is the treatment of choice for patients with stage I NSCLC. A lobectomy or segmental, wedge, or sleeve resection may be performed as appropriate. Patients with impaired pulmonary function are candidates for segmental or wedge resection of the primary tumor. Careful preoperative assessment of the patient’s overall medical condition, especially the patient’s pulmonary reserve, is critical in considering the benefits of surgery. The immediate postoperative mortality rate is age related, but a 3% to 5% mortality rate with lobectomy can be expected.[ 1 ]
Evidence (surgery):
- A reduction in local recurrence for patients treated with lobectomy compared with those treated with limited excision.
- No significant difference in overall survival (OS).
- A survival advantage was noted with lobectomy for patients with tumors larger than 3 cm but not for those with tumors smaller than 3 cm.
- The rate of locoregional recurrence was significantly less after lobectomy, regardless of primary tumor size.
- Those treated with wedge or segmental resections had a local recurrence rate of 50% (i.e., 31 recurrences out of 62 patients) despite having undergone complete resections.[ 4 ]
- After a median follow-up of 7 years, sublobar resection was noninferior to lobar resection for DFS (hazard ratio [HR], 1.01; 90% confidence interval [CI], 0.83–1.24; one-sided P = .02 for noninferiority).
- OS was similar after sublobar resection or lobar resection (HR, 0.95; 95% CI, 0.72–1.26).
- No substantial differences were noted in the incidence of locoregional or distant disease recurrence between the two groups.
- At 6 months after surgery, the magnitude of reduction from baseline in the percentage of predicted FEV 1 (forced expiratory volume in first second of expiration) was greater in the lobectomy group (-6%; 95% CI, -8% to -5%) versus the sublobar resection group (-4%; 95% CI, -5% to -2%). The magnitude of reduction in the percentage of predicted FVC (forced vital capacity) was also greater after lobectomy (-5%; 95% CI, -7% to -3%) than after sublobar resection (-3%; 95% CI, -4% to -1%).
These results suggest that sublobar resection by anatomical segmentectomy or wedge resection is effective for management of clinical stage T1a, N0 NSCLC when intraoperative sampling of hilar and mediastinal lymph nodes is negative.
- Four-year survival was superior in patients with resectable stage I, II, or IIIA NSCLC who underwent resection and complete ipsilateral mediastinal lymph node dissection (CMLND), compared with those who underwent resection and lymph node sampling; the HR was estimated to be 0.78 (95% CI, 0.65–0.93, P = .005).[ 6 ][ Level of evidence A1 ]
- There was a significant reduction in any cancer recurrence (local or distant) in the CMLND group (relative risk [RR], 0.79; 95% CI, 0.66–0.95; P = .01) that appeared mainly because of a reduction in the number of distant recurrences (RR, 0.78; 95% CI, 0.61–1.00; P = .05).
- There was no difference in operative mortality.
- Air leak lasting more than 5 days was significantly more common in patients assigned to CMLND (RR, 2.94; 95% CI, 1.01–8.54; P = .05).
- Preliminary analyses of operative morbidity and mortality showed comparable rates from the procedures.[ 7 , 8 ]
- There was no difference in OS, DFS, local recurrence, and regional recurrence.[ 8 ][ Level of evidence A1 ]
Current evidence suggests that lung cancer resection combined with CMLND is not associated with improvement in survival compared with lung cancer resection combined with systematic sampling of mediastinal lymph nodes in patients with stage I, II, or IIIA NSCLC.[ 8 ][ Level of evidence A1 ]
Conclusions about the efficacy of surgery for patients with local and locoregional NSCLC are limited by the small number of participants studied to date and the potential methodological weaknesses of the trials.
Adjuvant therapy
Many patients who have surgery subsequently develop regional or distant metastases.[ 9 ] Such patients are candidates for entry into clinical trials evaluating postoperative treatment with chemotherapy or radiation therapy following surgery. At present, neither chemotherapy nor radiation therapy has been found to improve survival in patients with stage I NSCLC that has been completely resected.
Adjuvant chemotherapy
Based on a meta-analysis, postoperative chemotherapy is not recommended outside of a clinical trial for patients with completely resected stage I NSCLC.[ 10 ] However, there may be some benefit of adjuvant chemotherapy in patients with stage IB tumors that are larger than 4 cm.
Evidence (adjuvant chemotherapy for patients with stage IB NSCLC):
- Survival was not significantly different (HR, 0.83; 90% CI, 0.64–1.08; P = .12) at a median follow-up of 74 months.
- Grades 3 to 4 neutropenia were the predominant toxicity; there were no treatment-related deaths.
- A post-hoc exploratory analysis demonstrated a significant survival difference in favor of postoperative chemotherapy for patients who had tumors 4 cm or larger in diameter (HR, 0.69; 90% CI, 0.48–0.99; P = .043).
Given the magnitude of observed survival differences, CALGB-9633 may have been underpowered to detect small but clinically meaningful improvements in survival. In addition, the use of a carboplatin versus a cisplatin combination might have affected the results. At present, there is no reliable evidence that postoperative chemotherapy improves survival of patients with stage IB NSCLC.[ 11 ][ Level of evidence A1 ]
Adjuvant targeted therapy (for patients with stage IB NSCLC with EGFR mutations)
Adjuvant targeted therapy with osimertinib for patients with EGFR -mutated NSCLC and resected stage IB to IIIA NSCLC was studied in a phase III clinical trial and showed improved OS.
Evidence (adjuvant targeted therapy with osimertinib for patients with stage IB EGFR -mutated NSCLC):
- In the overall population, the 5-year OS rate was 88% in the osimertinib group and 78% in the placebo group (overall HR death , 0.49; 95.03% CI, 0.34–0.70; P < .001).
- Among patients with stage II to IIIA disease, the 5-year OS rate was 85% in the osimertinib group and 73% in the placebo group (overall HR death , 0.49; 95.03% CI, 0.33–0.73; P < .001).
- The adverse event profile is consistent with other studies that used osimertinib except for pneumonia related to COVID-19, which was reported later.
The U.S. Food and Drug Administration (FDA) approved osimertinib as adjuvant therapy for patients with stage IB to IIIA NSCLC with EGFR exon 19 deletions or exon 21 L858R mutations.
Adjuvant immunotherapy
Evidence (adjuvant immunotherapy with pembrolizumab for patients with stage IB tumors >4 cm):
- In the overall study population, the median DFS was 53.6 months (95% CI, 39.2–not reached [NR]) in the pembrolizumab group and 42.0 months (95% CI, 31.3–NR) in the placebo group (HR, 0.76; 95% CI, 0.63–0.91; P = .0014).
- In the PD-L1 TPS ≥50% population, the median DFS was not reached with either pembrolizumab (95% CI, 44.3–NR) or placebo (95% CI, 35.8–NR) (HR, 0.82; 95% CI, 0.57–1.18; P = .14).
- OS data were immature at the time of the prespecified interim analysis.
- No new safety signals were identified in this study.
The FDA approved pembrolizumab as a single agent for adjuvant treatment following resection and platinum-based chemotherapy for patients with stage IB (T2a ≥4 cm), II, or IIIA NSCLC. Of note, the FDA label specifies that pembrolizumab can be used as adjuvant therapy after platinum-based chemotherapy. However, chemotherapy was not required in the overall study patient population evaluated in KEYNOTE-091.
Adjuvant external radiation therapy
The value of postoperative (adjuvant) radiation therapy (PORT) has been evaluated and has not been found to improve the outcome of patients with completely resected stage I NSCLC.[ 14 ]
Adjuvant brachytherapy
The value of intraoperative (adjuvant) brachytherapy applied to the suture line has been evaluated in patients undergoing sublobar resections for stage I NSCLC to improve local control; it has not been found to improve outcomes.
Evidence (adjuvant brachytherapy):
- No difference in the primary end point of local recurrence (5-year estimate, 14.0% vs. 16.7%; P = .59).
- No difference in OS rates (5-year estimate, 61.4% vs. 55.6%; P = .38).[ 15 ][ Level of evidence B1 ] vs. [ Level of evidence A1 ]
Radiation therapy
A substantial number of patients are ineligible for standard surgical resection because of comorbid conditions that are associated with unacceptably high perioperative risk. Patients with potentially resectable tumors with medical contraindications to surgery or those with inoperable stage I disease and with sufficient pulmonary reserve may be candidates for radiation therapy with curative intent.[ 16 - 18 ] Nonrandomized observational studies comparing treatment outcomes associated with resection, radiation therapy, and observation have demonstrated shorter survival times and higher mortality for patients who undergo observation only.[ 16 , 19 ]
Conventional radiation therapy
Historically, conventional primary radiation therapy consisted of approximately 60 Gy to 70 Gy delivered with megavoltage equipment to the midplane of the known tumor volume using conventional fractionation (1.8–2.0 Gy per day).
Improvements in radiation techniques include planning techniques to account for tumor motion, more conformal planning techniques (e.g., 3-D conformal radiation therapy and intensity-modulated radiation therapy), and image guidance during treatment. Modern approaches to delivery of external-beam radiation therapy (EBRT) include hypofractionated radiation therapy and stereotactic body radiation therapy (SBRT). However, there are limited reliable data from comparative trials to determine which approaches yield superior outcomes.[ 17 , 18 ]
Evidence (conventional radiation therapy):
- Patients achieved 5-year survival rates of 10% to 30%.[ 20 - 22 ]
- Several series demonstrated that patients with T1, N0 tumors had better outcomes, and 5-year survival rates of 30% to 60% were found in this subgroup.[ 20 , 21 , 23 ]
- However, local-only failure occurs in as many as 50% of patients treated with conventional radiation therapy to doses in the range of 60 Gy to 65 Gy.[ 24 , 25 ]
- Survival at 5 years after radiation therapy with curative intent was comparable with a historical control group of patients of similar age who were resected with curative intent.
- The addition of endobronchial brachytherapy improved local disease control compared with EBRT.[ 4 ][ Level of evidence C2 ]
Hypofractionated radiation therapy
Hypofractionated radiation therapy involves the delivery of a slightly higher dose of radiation therapy per day (e.g., 2.4–4.0 Gy) over a shorter period of time compared with conventionally fractionated radiation therapy. Multiple prospective phase I/II trials have demonstrated that hypofractionated radiation therapy to a dose of 60 Gy to 70 Gy delivered over 3 to 4 weeks with 2.4 Gy to 4.0 Gy per day resulted in a low incidence of moderate to severe toxicity, 2-year OS rates of 50% to 60%, and 2-year tumor local control of 80% to 90%.[ 26 - 28 ][ Level of evidence C1 ]
Stereotactic body radiation therapy (SBRT)
SBRT involves the delivery of highly conformal, high-dose radiation therapy over an extremely hypofractionated course (e.g., one to five treatments) delivered over 1 to 2 weeks. Commonly used regimens include 18 Gy × 3, 12 Gy to 12.5 Gy × 4, and 10 Gy to 12 Gy × 5, and deliver a substantially higher biologically effective dose compared with historic conventional radiation therapy regimens.
Multiple prospective phase I/II trials and institutional series have demonstrated that SBRT results in a low incidence of pulmonary toxicity (<10% risk of symptomatic radiation pneumonitis), 2-year OS rates of 50% to 60%, and 2-year tumor control of 90% to 95%.[ 29 - 35 ][ Level of evidence C1 ]
Evidence (SBRT):
- This regimen resulted in a 2-year OS rate of 55% and 2-year local tumor control of 95%.
- An unacceptably high incidence (8.6%) of grade 5 toxicity was observed in patients with central tumors (defined as within 2 cm of the tracheobronchial tree from the trachea to the level of the lobar bronchi).[ 30 ]
- This trial demonstrated a 3-year OS rate of 56% and 3-year primary tumor control of 98%.
- The incidence of moderate to severe toxicity was low, with grade 3 toxicity in 24% of patients, grade 4 toxicity in 4% of patients, and no grade 5 toxicity, with a 4% incidence of grade 3 radiation pneumonitis.[ 34 ]
- With a median follow-up of 32.9 months, the median OS was 40.7 months, and 2-year local tumor control was 95%.[ 35 ]
- While central location is a contraindication to three-fraction SBRT based on data from the Indiana phase II study, a subsequent systematic review of published reports of 315 patients with 563 central tumors demonstrated a much lower incidence of severe toxicity, including a 1% to 5% risk of grade 5 events with more protracted SBRT regimens (e.g., four to ten fractions).[ 36 ]
- A multicenter phase I/II trial ( RTOG-0813 [NCT00750269]) is ongoing to identify the maximum tolerated dose for a five-fraction SBRT regimen for central tumors.
A randomized trial of hypofractionated radiation therapy versus SBRT (LUSTRE [ NCT01968941 ]) is ongoing to determine the optimal radiation therapy regimen, but SBRT has been widely adopted for patients with medically inoperable stage I NSCLC.
- Ginsberg RJ, Hill LD, Eagan RT, et al.: Modern thirty-day operative mortality for surgical resections in lung cancer. J Thorac Cardiovasc Surg 86 (5): 654-8, 1983. [PUBMED Abstract]
- Ginsberg RJ, Rubinstein LV: Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 60 (3): 615-22; discussion 622-3, 1995. [PUBMED Abstract]
- Warren WH, Faber LP: Segmentectomy versus lobectomy in patients with stage I pulmonary carcinoma. Five-year survival and patterns of intrathoracic recurrence. J Thorac Cardiovasc Surg 107 (4): 1087-93; discussion 1093-4, 1994. [PUBMED Abstract]
- Mantz CA, Dosoretz DE, Rubenstein JH, et al.: Endobronchial brachytherapy and optimization of local disease control in medically inoperable non-small cell lung carcinoma: a matched-pair analysis. Brachytherapy 3 (4): 183-90, 2004. [PUBMED Abstract]
- Altorki N, Wang X, Kozono D, et al.: Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N Engl J Med 388 (6): 489-498, 2023. [PUBMED Abstract]
- Manser R, Wright G, Hart D, et al.: Surgery for early stage non-small cell lung cancer. Cochrane Database Syst Rev (1): CD004699, 2005. [PUBMED Abstract]
- Allen MS, Darling GE, Pechet TT, et al.: Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 81 (3): 1013-9; discussion 1019-20, 2006. [PUBMED Abstract]
- Darling GE, Allen MS, Decker PA, et al.: Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 141 (3): 662-70, 2011. [PUBMED Abstract]
- Pignon JP, Tribodet H, Scagliotti GV, et al.: Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 26 (21): 3552-9, 2008. [PUBMED Abstract]
- Strauss GM, Herndon JE, Maddaus MA, et al.: Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 26 (31): 5043-51, 2008. [PUBMED Abstract]
- Tsuboi M, Herbst RS, John T, et al.: Overall Survival with Osimertinib in Resected EGFR-Mutated NSCLC. N Engl J Med 389 (2): 137-147, 2023. [PUBMED Abstract]
- O'Brien M, Paz-Ares L, Marreaud S, et al.: Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol 23 (10): 1274-1286, 2022. [PUBMED Abstract]
- PORT Meta-analysis Trialists Group: Postoperative radiotherapy for non-small cell lung cancer. Cochrane Database Syst Rev (2): CD002142, 2005. [PUBMED Abstract]
- Fernando HC, Landreneau RJ, Mandrekar SJ, et al.: Impact of brachytherapy on local recurrence rates after sublobar resection: results from ACOSOG Z4032 (Alliance), a phase III randomized trial for high-risk operable non-small-cell lung cancer. J Clin Oncol 32 (23): 2456-62, 2014. [PUBMED Abstract]
- McGarry RC, Song G, des Rosiers P, et al.: Observation-only management of early stage, medically inoperable lung cancer: poor outcome. Chest 121 (4): 1155-8, 2002. [PUBMED Abstract]
- Lanni TB, Grills IS, Kestin LL, et al.: Stereotactic radiotherapy reduces treatment cost while improving overall survival and local control over standard fractionated radiation therapy for medically inoperable non-small-cell lung cancer. Am J Clin Oncol 34 (5): 494-8, 2011. [PUBMED Abstract]
- Grutters JP, Kessels AG, Pijls-Johannesma M, et al.: Comparison of the effectiveness of radiotherapy with photons, protons and carbon-ions for non-small cell lung cancer: a meta-analysis. Radiother Oncol 95 (1): 32-40, 2010. [PUBMED Abstract]
- Raz DJ, Zell JA, Ou SH, et al.: Natural history of stage I non-small cell lung cancer: implications for early detection. Chest 132 (1): 193-9, 2007. [PUBMED Abstract]
- Dosoretz DE, Katin MJ, Blitzer PH, et al.: Radiation therapy in the management of medically inoperable carcinoma of the lung: results and implications for future treatment strategies. Int J Radiat Oncol Biol Phys 24 (1): 3-9, 1992. [PUBMED Abstract]
- Gauden S, Ramsay J, Tripcony L: The curative treatment by radiotherapy alone of stage I non-small cell carcinoma of the lung. Chest 108 (5): 1278-82, 1995. [PUBMED Abstract]
- Sibley GS, Jamieson TA, Marks LB, et al.: Radiotherapy alone for medically inoperable stage I non-small-cell lung cancer: the Duke experience. Int J Radiat Oncol Biol Phys 40 (1): 149-54, 1998. [PUBMED Abstract]
- Noordijk EM, vd Poest Clement E, Hermans J, et al.: Radiotherapy as an alternative to surgery in elderly patients with resectable lung cancer. Radiother Oncol 13 (2): 83-9, 1988. [PUBMED Abstract]
- Dosoretz DE, Galmarini D, Rubenstein JH, et al.: Local control in medically inoperable lung cancer: an analysis of its importance in outcome and factors determining the probability of tumor eradication. Int J Radiat Oncol Biol Phys 27 (3): 507-16, 1993. [PUBMED Abstract]
- Kaskowitz L, Graham MV, Emami B, et al.: Radiation therapy alone for stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys 27 (3): 517-23, 1993. [PUBMED Abstract]
- Bradley J, Graham MV, Winter K, et al.: Toxicity and outcome results of RTOG 9311: a phase I-II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys 61 (2): 318-28, 2005. [PUBMED Abstract]
- Bogart JA, Hodgson L, Seagren SL, et al.: Phase I study of accelerated conformal radiotherapy for stage I non-small-cell lung cancer in patients with pulmonary dysfunction: CALGB 39904. J Clin Oncol 28 (2): 202-6, 2010. [PUBMED Abstract]
- Cheung P, Faria S, Ahmed S, et al.: Phase II study of accelerated hypofractionated three-dimensional conformal radiotherapy for stage T1-3 N0 M0 non-small cell lung cancer: NCIC CTG BR.25. J Natl Cancer Inst 106 (8): , 2014. [PUBMED Abstract]
- Timmerman R, Papiez L, McGarry R, et al.: Extracranial stereotactic radioablation: results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest 124 (5): 1946-55, 2003. [PUBMED Abstract]
- Timmerman R, McGarry R, Yiannoutsos C, et al.: Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 24 (30): 4833-9, 2006. [PUBMED Abstract]
- Lagerwaard FJ, Haasbeek CJ, Smit EF, et al.: Outcomes of risk-adapted fractionated stereotactic radiotherapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 70 (3): 685-92, 2008. [PUBMED Abstract]
- Baumann P, Nyman J, Hoyer M, et al.: Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 27 (20): 3290-6, 2009. [PUBMED Abstract]
- Fakiris AJ, McGarry RC, Yiannoutsos CT, et al.: Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys 75 (3): 677-82, 2009. [PUBMED Abstract]
- Timmerman R, Paulus R, Galvin J, et al.: Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 303 (11): 1070-6, 2010. [PUBMED Abstract]
- Senthi S, Lagerwaard FJ, Haasbeek CJ, et al.: Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol 13 (8): 802-9, 2012. [PUBMED Abstract]
- Senthi S, Haasbeek CJ, Slotman BJ, et al.: Outcomes of stereotactic ablative radiotherapy for central lung tumours: a systematic review. Radiother Oncol 106 (3): 276-82, 2013. [PUBMED Abstract]
Treatment of Stages IIA and IIB NSCLC
Treatment options for stages iia and iib nsclc.
Treatment options for stages IIA non-small cell lung cancer (NSCLC) and IIB NSCLC include:
- Surgery alone .
- Adjuvant chemotherapy .
- Adjuvant targeted therapy (for patients with EGFR mutations).
- Adjuvant immunotherapy .
- Adjuvant radiation therapy .
- Neoadjuvant chemotherapy .
- Nivolumab plus platinum-based chemotherapy .
- Perioperative pembrolizumab plus platinum-based chemotherapy .
- Perioperative durvalumab plus platinum-based chemotherapy .
- Perioperative nivolumab plus platinum-based chemotherapy .
- Perioperative toripalimab plus platinum-based chemotherapy .
- Radiation therapy (for patients who cannot have surgery).
- Clinical trials of radiation therapy after curative surgery.
Adjuvant radiation therapy has not been shown to improve outcomes in patients with stage II NSCLC.
Surgery with or without adjuvant or neoadjuvant therapy
Surgery alone.
Surgery is the treatment of choice for patients with stage II NSCLC. A lobectomy, pneumonectomy, segmental resection, wedge resection, or sleeve resection may be performed as appropriate. Careful preoperative assessment of the patient’s overall medical condition, especially the patient’s pulmonary reserve, is critical in considering the benefits of surgery. In addition to the immediate and age-related postoperative mortality rate, a 5% to 8% mortality rate with pneumonectomy or a 3% to 5% mortality rate with lobectomy can be expected.
- Four-year survival was superior in patients with resectable stage I, II, or IIIA NSCLC who underwent resection and complete ipsilateral mediastinal lymph node dissection (CMLND), compared with those who underwent resection and lymph node sampling; the hazard ratio (HR) was estimated to be 0.78 (95% confidence interval [CI], 0.65–0.93; P = .005).[ 1 ][ Level of evidence A1 ]
- There was a significant reduction in any cancer recurrence (local or distant) in the CMLND group (relative risk [RR], 0.79; 95% CI, 0.66–0.95; P = .01) that appeared mainly as the result of a reduction in the number of distant recurrences (RR, 0.78; 95% CI, 0.61–1.00; P = .05).
- Preliminary analyses of operative morbidity and mortality showed comparable rates from the procedures.[ 2 ]
- There was no difference in overall survival (OS), disease-free survival (DFS), local recurrence, and regional recurrence.[ 3 ][ Level of evidence A1 ]
Evidence suggests that lung cancer resection combined with CMLND is not associated with improvement in survival compared with lung cancer resection combined with systematic sampling of mediastinal lymph nodes in patients with stage I, II, or IIIA NSCLC.[ 3 ][ Level of evidence A1 ]
Conclusions about the efficacy of surgery for patients with local and locoregional NSCLC are limited by the small number of participants studied and potential methodological weaknesses of the trials.
The preponderance of evidence indicates that postoperative cisplatin combination chemotherapy provides a significant survival advantage to patients with resected stage II NSCLC. Preoperative chemotherapy may also provide survival benefit. The optimal sequence of surgery and chemotherapy and the benefits and risks of postoperative radiation therapy in patients with resectable NSCLC remain to be determined.
After surgery, many patients develop regional or distant metastases.[ 4 ] Several randomized controlled trials and meta-analyses have evaluated the use of postoperative chemotherapy in patients with stage I, II, and IIIA NSCLC.[ 5 - 11 ]
Evidence (adjuvant chemotherapy):
- With a median follow-up time of 5.2 years, the overall HR death was 0.89 (95% CI, 0.82–0.96; P = .005), corresponding to a 5-year absolute benefit of 5.4% from chemotherapy.
- The benefit varied with stage (test for trend, P = .04; HR for stage IA, 1.40; 95% CI, 0.95–2.06; HR for stage IB, 0.93; 95% CI, 0.78–1.10; HR for stage II, 0.83; 95% CI, 0.73–0.95; and HR for stage III, 0.83; 95% CI, 0.72–0.94).
- The effect of chemotherapy did not vary significantly (test for interaction, P = .11) with the associated drugs, including vinorelbine (HR, 0.80; 95% CI, 0.70–0.91), etoposide or vinca alkaloid (HR, 0.92; 95% CI, 0.80–1.07), or other drugs (HR, 0.97; 95% CI, 0.84–1.13).
- The greater effect on survival observed with the doublet of cisplatin plus vinorelbine compared with other regimens should be interpreted cautiously as the total dose of cisplatin received was significantly higher in patients treated with vinorelbine.
- Superior OS for the trial population and patients with stage II disease was reported for the Lung Adjuvant Cisplatin Evaluation (LACE) pooled analysis (pooled HR, 0.83; 95% CI, 0.73–0.95); the Adjuvant Navelbine International Trialist Association (ANITA) trial (HR, 0.71; 95% CI, 0.49–1.03); and the National Cancer Institute of Canada Clinical Trials Group JBR.10 trial (HR, 0.59; 95% CI, 0.42–0.85).
- Chemotherapy effect was higher in patients with better performance status.
- Type of surgery.
- Planned radiation therapy.
- Planned total dose of cisplatin.
- Chemotherapy significantly prolonged OS for patients older than 65 years (HR, 0.61; 95% CI, 0.38–0.98; P = .04).
- There were no significant differences in toxic effects, hospitalization, or treatment-related death by age group, although patients older than 65 years received less treatment.[ 13 ]
- Several other randomized controlled trials and meta-analyses have evaluated the use of postoperative chemotherapy in patients with stages I, II, and IIIA NSCLC.[ 5 - 11 ]
Based on these data, patients with completely resected stage II lung cancer may benefit from postoperative cisplatin-based chemotherapy.[ 13 ][ Level of evidence A1 ]
Adjuvant targeted therapy (for patients with EGFR mutations)
Evidence (adjuvant targeted therapy with osimertinib for patients with stages IIA and IIB EGFR -mutated NSCLC):
Adjuvant immunotherapy for patients with resected stage IB to IIIA NSCLC has been found to significantly increase DFS.[ 15 , 16 ]
Evidence (adjuvant immunotherapy with pembrolizumab for patients with stage IIA and IIB tumors >4 cm):
Evidence (adjuvant immunotherapy with atezolizumab for patients with stages IIA and IIB NSCLC):
- The primary end point was tested hierarchically, first in the stage II to IIIA population subgroup whose tumors expressed PD-L1 on at least 1% of tumor cells (using the SP263 antibody), then in all patients in the stage II to IIIA population, and finally in the intention-to-treat (ITT) population (stage IB to IIIA). Of the 882 patients who were randomly assigned and had stage II to IIIA disease, 476 had tumors expressing PD-L1 on at least 1% of tumor cells per SP263.[ 16 ][ Level of evidence B1 ]
- After a median follow-up of 32.2 months, atezolizumab treatment improved DFS compared with best supportive care in patients in the stage II to IIIA population whose tumors expressed PD-L1 on at least 1% of tumor cells (HR, 0.66; 95% CI, 0.50–0.88; P = .0039). At 24 months, the DFS rate was 74.6% for the atezolizumab group and 61.0% for the best supportive care group.
- Atezolizumab also improved DFS in all patients in the stage II to IIIA population (HR, 0.79; 95% CI, 0.64–0.96; P = .020). At 24 months, the DFS rate was 70.2% for the atezolizumab group and 61.6% for the best supportive care group.
- In the ITT population, which included patients with stage IB to IIIA disease, HR DFS was 0.81 (95% CI, 0.67–0.99; P = .040). However, the boundary for statistical significance for DFS was not crossed.
- OS data are immature.
- No new safety signals were noted.
The FDA approved atezolizumab for adjuvant treatment of patients with stage II to IIIA NSCLC whose tumors express PD-L1 on at least 1% of tumor cells.
Adjuvant radiation therapy
The value of postoperative (adjuvant) radiation therapy (PORT) has been evaluated.[ 17 ]
Evidence (adjuvant radiation therapy):
- An 18% relative increase in the risk of death for patients who received PORT compared with surgery alone (HR, 1.18; P = .002). This is equivalent to an absolute detriment of 6% at 2 years (95% CI, 2%–9%), reducing OS from 58% to 52%. Exploratory subgroup analyses suggested that this detrimental effect was most pronounced for patients with stage I/II, N0 to N1 disease, whereas for patients with stage III, N2 disease there was no clear evidence of an adverse effect.
- Results for local (HR, 1.13; P = .02), distant (HR, 1.14; P = .02), and overall (HR, 1.10; P = .06) recurrence-free survival similarly showed a detriment of PORT.[ 17 ][ Level of evidence A1 ]
Further analysis is needed to determine whether these outcomes can potentially be modified with technical improvements, better definitions of target volumes, and limitation of cardiac volume in the radiation portals.
Neoadjuvant chemotherapy
The role of chemotherapy before surgery was tested in clinical trials. The proposed benefits of preoperative chemotherapy include:
- A reduction in tumor size that may facilitate surgical resection.
- Early eradication of micrometastases.
- Better tolerability.
Preoperative chemotherapy may, however, delay potentially curative surgery.
Evidence (neoadjuvant chemotherapy):
- Preoperative chemotherapy provided an absolute benefit in survival of 6% across all stages of disease, from 14% to 20% at 5 years (HR, 0.82; 95% CI, 0.69–0.97; P = .022).[ 18 ][ Level of evidence A1 ]
- This analysis was unable to address questions such as whether particular types of patients may benefit more or less from preoperative chemotherapy.
- No survival advantage was seen.[ 19 ]
- Postoperative complications were similar between groups, and no impairment of quality of life was observed.
- There was no evidence of a benefit in terms of OS (HR, 1.02; 95% CI, 0.80–1.31; P = .86).
- Updating the systematic review by addition of the present result suggests a 12% relative survival benefit with the addition of neoadjuvant (preoperative) chemotherapy (1,507 patients; HR, 0.88; 95% CI, 0.76–1.01; P = .07), equivalent to an absolute improvement in survival of 5% at 5 years.
Neoadjuvant immunotherapy with chemotherapy
Nivolumab plus platinum-based chemotherapy.
The CheckMate 816 trial evaluated the combination of nivolumab (an anti-programmed death 1 antibody) and platinum-based chemotherapy as neoadjuvant therapy in patients with resectable (≥4 cm or node positive) NSCLC. Nivolumab therapy improved event-free survival (EFS) and pathological complete response rates compared with chemotherapy alone.
Evidence (nivolumab plus platinum-based chemotherapy):
- With a minimum follow-up of 21 months, the median EFS was 31.6 months (95% CI, 30.2–NR) in the nivolumab-plus-chemotherapy group and 20.8 months (95% CI, 14.0–26.7) in the chemotherapy-alone group (HR, 0.63; 97.38% CI, 0.43–0.91; P = .005).
- The estimated percentage of patients surviving without disease progression or disease recurrence at 1 year was 76.1% for patients who received nivolumab plus chemotherapy and 63.4% for patients who received chemotherapy alone. The magnitude of benefit was greater in 1) patients with stage IIIA disease versus patients with stage IB or II disease (HR, 0.54; 95% CI, 0.37–0.80 vs. HR, 0.87; 95% CI, 0.48–1.56), 2) patients with tumor PD-L1 expression ≥1% versus <1% (HR, 0.41; 95% CI, 0.24–0.70 vs. HR, 0.85; 95% CI, 0.54–1.32), and 3) patients with nonsquamous histology versus squamous histology.
- Pathological complete response was observed in 24% (95% CI, 18.0%– 31.0%) of patients who received nivolumab plus chemotherapy and 2.2% (95% CI, 0.6%–5.6%) of patients who received chemotherapy alone (odds ratio [OR], 13.94; 99% CI, 3.49–55.75; P < .001).
- Median OS was not reached in either group (HR death , 0.57; 99.67% CI, 0.30–1.07; P = .008).
- Grade 3 or 4 treatment-related adverse events occurred in 33.5% of patients in the nivolumab-plus-chemotherapy group and in 36.9% of patients in the chemotherapy-alone group. Treatment-related adverse events led to treatment discontinuation in 10.2% of patients in the nivolumab-plus-chemotherapy group and in 9.7% of patients in the chemotherapy-alone group.
The FDA approved nivolumab in combination with platinum-doublet chemotherapy for neoadjuvant treatment of patients with resectable (tumors ≥4 cm or node positive) NSCLC.
Perioperative (neoadjuvant and adjuvant) immunotherapy with chemotherapy
Several immune checkpoint inhibitors have been approved by the FDA for select patient populations with potentially resectable NSCLC, either in the neoadjuvant setting (nivolumab) or adjuvant setting (atezolizumab, durvalumab, or pembrolizumab). Ongoing phase III trials are evaluating the role of perioperative immune checkpoint inhibitors. These regimens for patients with potentially resectable stages II to III NSCLC include neoadjuvant immune checkpoint inhibitors with chemotherapy followed by surgery and adjuvant immune checkpoint inhibitors. Compared with neoadjuvant chemotherapy alone, early results from studies of perioperative immune checkpoint inhibitor regimens have shown improvements in several key outcomes including EFS, major pathological response, pathological complete response, and OS.
Perioperative pembrolizumab plus platinum-based chemotherapy
Evidence (neoadjuvant pembrolizumab plus chemotherapy and adjuvant pembrolizumab):
- The EFS benefit with pembrolizumab was generally consistent across all subgroups.
- The estimated 24-month OS rate was 80.9% in the pembrolizumab group and 77.6% in the placebo group ( P = .02, not meeting the significance criterion).
- Major pathological response occurred in 30.2% of patients in the pembrolizumab group and 11.0% of patients in the placebo group.
- Pathological complete response occurred in 18.1% of patients in the pembrolizumab group and 4.0% of patients in the placebo group.
- Immune-related adverse events of any grade were seen in 25.3% of patients in the pembrolizumab group (grade 3 or greater in 5.8% of patients) and 10.5% of patients in the placebo group (grade 3 or greater in 1.5% of patients).
Perioperative durvalumab plus platinum-based chemotherapy
Evidence (durvalumab plus platinum-based chemotherapy):
- At 12 months, the EFS rate was 73.4% for patients who received durvalumab (95% CI, 67.9%–78.1%), and 64.5% for patients who received chemotherapy alone (95% CI, 58.8%–69.6%).
- Pathological complete response was significantly higher with perioperative durvalumab (17.2%), compared with chemotherapy alone (4.3%, P < .001).
- The EFS and pathological complete response benefit were observed regardless of stage and PD-L1 expression.
- The safety profile was consistent with known profiles of durvalumab and chemotherapy.
Perioperative nivolumab plus platinum-based chemotherapy
Evidence (neoadjuvant nivolumab plus chemotherapy and adjuvant nivolumab):
- In a prespecified interim analysis for EFS, with a median follow-up of 25.4 months, the median EFS was significantly improved for patients who received nivolumab plus chemotherapy/nivolumab (NR; 95% CI, 28.9 months–NR) when compared with patients who received neoadjuvant chemotherapy alone (18.4 months; 95% CI, 13.6–28.1) (HR, 0.58; 97.36% CI, 0.42–0.81; P = .00025).
- Patients who received nivolumab plus chemotherapy/nivolumab also had higher rates of both pathological complete response (25.3% vs. 4.7%; OR, 6.64; 95% CI, 3.40–12.97) and major pathological response (35.4% vs. 12.1%; OR, 4.01; 95% CI, 2.48–6.49) when compared with patients who received chemotherapy alone.
- Definitive surgery rates were similar among groups (78% for nivolumab plus chemotherapy/nivolumab vs. 77% for chemotherapy alone).
- Grade 3 to 4 treatment-related and surgical adverse events were similar among the groups.
Perioperative toripalimab plus platinum-based chemotherapy
Evidence (toripalimab plus platinum-based chemotherapy):
- In a prespecified interim analysis of EFS in patients with stage III NSCLC (n = 404) after a median follow-up of 18.3 months (IQR, 12.7–22.5 months), the median EFS was not reached (95% CI, 24.4 months–NR) in the toripalimab group and was 15.1 months (95% CI, 10.6–21.9) in the placebo group (HR, 0.40; 95% CI, 0.28–0.57; P < .001).
- The 1- and 2-year EFS rates were 84.4% and 64.7%, respectively, in the toripalimab group and 57.0% and 38.7%, respectively, in the placebo group. A consistent effect on EFS, favoring toripalimab, was observed in all subgroups.
- After surgical resection, a major pathological response occurred in 98 patients (48.5%) in the toripalimab group and 17 patients (8.4%) in the placebo group (between group difference, 40.2%; 95% CI, 32.2%–48.1%; P < .001).
The FDA has not approved this drug for patients with lung cancer.
Patients with potentially operable tumors with medical contraindications to surgery or those with inoperable stage II disease and with sufficient pulmonary reserve are candidates for radiation therapy with curative intent.[ 25 ] Primary radiation therapy often consists of approximately 60 Gy delivered with megavoltage equipment to the midplane of the volume of the known tumor using conventional fractionation. A boost to the cone down field of the primary tumor is frequently used to enhance local control. Careful treatment planning with precise definition of target volume and avoidance of critical normal structures, to the extent possible, is needed for optimal results; this requires the use of a simulator.
Among patients with excellent performance status, a 3-year survival rate of 20% may be expected if a course of radiation therapy with curative intent can be completed.
Evidence (radiation therapy):
- A 5-year OS rate of 10%.
- Forty-four patients with T1 tumors achieved an actuarial DFS rate of 60%.
- This retrospective study also suggested that improved DFS was obtained with radiation therapy doses greater than 60 Gy.[ 26 ]
- Winton T, Livingston R, Johnson D, et al.: Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 352 (25): 2589-97, 2005. [PUBMED Abstract]
- Arriagada R, Bergman B, Dunant A, et al.: Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 350 (4): 351-60, 2004. [PUBMED Abstract]
- Scagliotti GV, Fossati R, Torri V, et al.: Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA non-small-cell Lung cancer. J Natl Cancer Inst 95 (19): 1453-61, 2003. [PUBMED Abstract]
- Hotta K, Matsuo K, Ueoka H, et al.: Role of adjuvant chemotherapy in patients with resected non-small-cell lung cancer: reappraisal with a meta-analysis of randomized controlled trials. J Clin Oncol 22 (19): 3860-7, 2004. [PUBMED Abstract]
- Edell ES, Cortese DA: Photodynamic therapy in the management of early superficial squamous cell carcinoma as an alternative to surgical resection. Chest 102 (5): 1319-22, 1992. [PUBMED Abstract]
- Douillard JY, Rosell R, De Lena M, et al.: Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 7 (9): 719-27, 2006. [PUBMED Abstract]
- Pepe C, Hasan B, Winton TL, et al.: Adjuvant vinorelbine and cisplatin in elderly patients: National Cancer Institute of Canada and Intergroup Study JBR.10. J Clin Oncol 25 (12): 1553-61, 2007. [PUBMED Abstract]
- Felip E, Altorki N, Zhou C, et al.: Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 398 (10308): 1344-1357, 2021. [PUBMED Abstract]
- Burdett SS, Stewart LA, Rydzewska L: Chemotherapy and surgery versus surgery alone in non-small cell lung cancer. Cochrane Database Syst Rev (3): CD006157, 2007. [PUBMED Abstract]
- Gilligan D, Nicolson M, Smith I, et al.: Preoperative chemotherapy in patients with resectable non-small cell lung cancer: results of the MRC LU22/NVALT 2/EORTC 08012 multicentre randomised trial and update of systematic review. Lancet 369 (9577): 1929-37, 2007. [PUBMED Abstract]
- Forde PM, Spicer J, Lu S, et al.: Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med 386 (21): 1973-1985, 2022. [PUBMED Abstract]
- Wakelee H, Liberman M, Kato T, et al.: Perioperative Pembrolizumab for Early-Stage Non-Small-Cell Lung Cancer. N Engl J Med 389 (6): 491-503, 2023. [PUBMED Abstract]
- Heymach JV, Harpole D, Mitsudomi T, et al.: Perioperative Durvalumab for Resectable Non-Small-Cell Lung Cancer. N Engl J Med 389 (18): 1672-1684, 2023. [PUBMED Abstract]
- Cascone T, Awad MM, Spicer JD, et al.: CheckMate 77T: Phase III study comparing neoadjuvant nivolumab (NIVO) plus chemotherapy (chemo) vs neoadjuvant placebo plus chemo followed by surgery and adjuvant NIVO or placebo for previously untreated, resectable stage II–IIIb NSCLC. [Abstract] Ann Oncol 34 (Suppl 2): A-LBA1, S1295, 2023.
- Lu S, Zhang W, Wu L, et al.: Perioperative Toripalimab Plus Chemotherapy for Patients With Resectable Non-Small Cell Lung Cancer: The Neotorch Randomized Clinical Trial. JAMA 331 (3): 201-211, 2024. [PUBMED Abstract]
- Komaki R, Cox JD, Hartz AJ, et al.: Characteristics of long-term survivors after treatment for inoperable carcinoma of the lung. Am J Clin Oncol 8 (5): 362-70, 1985. [PUBMED Abstract]
Treatment of Stage IIIA NSCLC
Patients with stage IIIA non-small cell lung cancer (NSCLC) are a heterogenous group. Patients may have metastases to ipsilateral mediastinal nodes, potentially resectable T3 tumors invading the chest wall, or mediastinal involvement with metastases to peribronchial or hilar lymph nodes (N1). Presentations of disease range from resectable tumors with microscopic metastases to lymph nodes to unresectable, bulky disease involving multiple nodal stations.
Patients with clinical stage IIIA N2 disease have a 5-year overall survival (OS) rate of 10% to 15%; however, patients with bulky mediastinal involvement (i.e., visible on chest radiography) have a 5-year survival rate of 2% to 5%. Depending on clinical circumstances, the principal forms of treatment that are considered for patients with stage IIIA NSCLC are radiation therapy, chemotherapy, surgery, and combinations of these modalities.
Treatment options vary according to the location of the tumor and whether it is resectable.
Treatment Options for Resected/Resectable Stage IIIA NSCLC
Treatment options for resected/resectable disease include:
- Surgery with neoadjuvant or adjuvant therapy .
- Neoadjuvant chemoradiation therapy .
- Adjuvant chemoradiation therapy .
Despite careful preoperative staging, some patients will be found to have metastases to mediastinal N2 lymph nodes at thoracotomy.
The preponderance of evidence indicates that postoperative cisplatin combination chemotherapy provides a significant survival advantage to patients with resected NSCLC with occult N2 disease discovered at surgery. The optimal sequence of surgery and chemotherapy and the benefits and risks of postoperative radiation therapy in patients with resectable NSCLC are yet to be determined.
If complete resection of tumor and lymph nodes is possible, such patients may benefit from surgery followed by postoperative chemotherapy. Current evidence suggests that lung cancer resection combined with complete ipsilateral mediastinal lymph node dissection (CMLND) is not associated with improvement in survival compared with lung cancer resection combined with systematic sampling of mediastinal lymph nodes in patients with stage I, II, or IIIA NSCLC.[ 1 ][ Level of evidence A1 ]
The addition of surgery to chemoradiation therapy for patients with stage IIIA NSCLC did not result in improved OS in a phase III trial but did improve progression-free survival (PFS) and local control.[ 2 ][ Level of evidence B1 ]
- Four-year survival was superior in patients with resectable stage I, II, or IIIA NSCLC who underwent resection and CMLND, compared with those who underwent resection and lymph node sampling; the hazard ratio (HR) was estimated to be 0.78 (95% confidence interval [CI], 0.65–0.93; P = .005).[ 3 ][ Level of evidence A1 ]
- There was no difference in OS, disease-free survival (DFS), local recurrence, and regional recurrence.[ 1 ][ Level of evidence A1 ]
Conclusions about the efficacy of surgery for patients with local and locoregional NSCLC are limited by the small number of participants studied to date and by the potential methodological weaknesses of the trials.
Neoadjuvant therapy
The role of chemotherapy before surgery in patients with stage IIIA NSCLC has been extensively tested in clinical trials. The proposed benefits of preoperative (neoadjuvant) chemotherapy include:
- Preoperative chemotherapy provided an absolute benefit in survival of 6% across all stages of disease, from 14% to 20% at 5 years (HR, 0.82; 95% CI, 0.69–0.97; P = .022).[ 5 ][ Level of evidence A1 ]
- This analysis was unable to address questions such as whether particular types of patients may benefit more or less from preoperative chemotherapy.[ 6 ]
- Updating the systematic review by addition of the present result suggests a 12% relative survival benefit with the addition of preoperative chemotherapy (1,507 patients; HR, 0.88; 95% CI, 0.76–1.01; P = .07), equivalent to an absolute improvement in survival of 5% at 5 years.[ 7 ]
Neoadjuvant chemoradiation therapy
Administering concurrent neoadjuvant chemotherapy and radiation therapy before surgery may intensify treatment and increase the likelihood of downstaging the tumor burden. Commonly used regimens that have been tested in the phase II setting include cisplatin/etoposide (EP5050) and weekly carboplatin/paclitaxel.[ 8 , 9 ] In a randomized trial of neoadjuvant chemoradiation therapy and surgery versus concurrent chemoradiation therapy alone, there was no difference in OS, but surgery improved PFS and local control.[ 2 ][ Level of evidence B1 ]
Evidence (neoadjuvant chemoradiation therapy):
- Surgery did not improve OS (5-year OS rate, 27% vs. 20%; HR, 0.87; 0.70–1.10; P = .24).
- Surgery improved PFS (5-year PFS rate, 22% vs. 11%; HR, 0.77; 0.62–0.96; P = .017) and decreased the risk of local recurrence (10% vs. 22%; P = .002).
- There was increased treatment mortality with neoadjuvant chemoradiation with surgery (8% vs. 2%), particularly in the subset of patients who underwent pneumonectomy.
A direct comparison of neoadjuvant chemotherapy versus neoadjuvant chemoradiation therapy using modern treatment regimens has not been performed to date; the optimal neoadjuvant approach remains unclear.
- With a minimum follow-up of 21 months, the median EFS was 31.6 months (95% CI, 30.2–not reached [NR]) in the nivolumab-plus-chemotherapy group and 20.8 months (95% CI, 14.0–26.7) in the chemotherapy-alone group (HR, 0.63; 97.38% CI, 0.43–0.91; P = .005).
- The estimated percentage of patients surviving without disease progression or disease recurrence at 1 year was 76.1% for patients who received nivolumab plus chemotherapy and 63.4% for patients who received chemotherapy alone. The magnitude of benefit was greater in 1) patients with stage IIIA disease versus patients with stage IB or II disease (HR, 0.54; 95% CI, 0.37–0.80 vs. HR, 0.87; 95% CI, 0.48–1.56), 2) patients with tumor programmed death-ligand 1 (PD-L1) expression ≥1% versus <1% (HR, 0.41; 95% CI, 0.24–0.70 vs. HR, 0.85; 95% CI, 0.54–1.32), and 3) patients with nonsquamous histology versus squamous histology.
- Pathological complete response was observed in 24% (95% CI, 18.0%– 31.0%) of patients who received nivolumab plus chemotherapy and 2.2% (95% CI, 0.6%–5.6%) of patients who received chemotherapy alone (odds ratio, 13.94; 99% CI, 3.49–55.75; P < .001).
- The OS rate at 36 months was 81.9% (95% CI, 66.8%–90.6%) in the ITT population and 91% (95% CI, 74.2%–97.0%) in the per-protocol population.[ 11 ][ Level of evidence A1 ]
- In the ITT population, the PFS rate was 69.6% (95% CI, 54.1%–80.7%) at 36 and 42 months. In the per-protocol population, the PFS rate was 81.1% (95% CI, 64.4%–90.5%) at 36 and 42 months.
- Major pathological response was defined as the presence of 10% or fewer tumor cells in the primary tumor. A total of 82.9% of patients had a major pathological response, including 63.4% of patients who had a complete pathological response. A total of 17.1% patients had an incomplete response.
Circulating tumor DNA (ctDNA) from plasma samples obtained before and after neoadjuvant treatment (but before surgery) was analyzed with the hybridization capture–based TruSight Oncology 500 ctDNA next-generation sequencing assay on a NovaSeq sequencer (Illumina).
- Low pretreatment levels of ctDNA were significantly associated with improved PFS (HR, 0.20; 95% CI, 0.06–0.63) and OS (HR, 0.07; 95% CI, 0.01–0.39).
- Undetectable ctDNA levels after neoadjuvant treatment were significantly associated with PFS (HR, 0.26; 95% CI, 0.07–0.93) and OS (HR, 0.04; 95% CI, 0.00–0.55).
The U.S. Food and Drug Administration (FDA) approved nivolumab in combination with platinum-doublet chemotherapy for neoadjuvant treatment of patients with resectable (tumors ≥4 cm or node positive) NSCLC.
Patients with completely resected stage IIIA NSCLC may benefit from postoperative cisplatin-based chemotherapy.[ 16 ][ Level of evidence A1 ]
Evidence from randomized controlled clinical trials indicates that when stage IIIA NSCLC is encountered unexpectedly at surgery, chemotherapy given after complete resection improves survival.
Several randomized, controlled trials and meta-analyses have evaluated the use of postoperative chemotherapy in patients with stages I, II, and IIIA NSCLC.[ 16 - 22 ]
- With a median follow-up of 5.2 years, the overall HR death was 0.89 (95% CI, 0.82–0.96; P = .005), corresponding to a 5-year absolute benefit of 5.4% from chemotherapy.
- The benefit varied with stage (HR for stage IIIA, 0.83; 95% CI, 0.72–0.94).
- The greater effect on survival observed with the doublet of cisplatin plus vinorelbine compared with other regimens should be interpreted with caution as the total dose of cisplatin received was significantly higher in patients treated with vinorelbine.
- For the subgroup of stage IIIA patients in the ANITA trial (n = 325), the HR was 0.69 (95% CI, 0.53–0.90), and the result for the FRE-IALT trial (n = 728) was HR, 0.79 (95% CI, 0.66–0.95).
- The chemotherapy effect was higher in patients with a better performance status.
- There were no significant differences in toxic effects, hospitalization, or treatment-related death by age group, although patients older than 65 years received less treatment.
Evidence (adjuvant targeted therapy with osimertinib for patients with stage IIIA EGFR -mutated NSCLC):
The FDA approved osimertinib as adjuvant therapy for patients with stage IB to IIIA NSCLC with EGFR exon 19 deletions or exon 21 L858R mutations.
Adjuvant immunotherapy for patients with resected stage IB to IIIA NSCLC has been found to significantly increase DFS.[ 25 , 26 ]
Evidence (adjuvant immunotherapy with pembrolizumab for patients with stage IIIA NSCLC):
- In the overall study population, the median DFS was 53.6 months (95% CI, 39.2 to NR) in the pembrolizumab group and 42.0 months (95% CI, 31.3–NR) in the placebo group (HR, 0.76; 95% CI, 0.63–0.91, P = .0014).
- OS data were immature at the time of prespecified interim analysis.
Evidence (adjuvant immunotherapy with atezolizumab for patients with resected stage IIIA NSCLC):
- The primary end point was tested hierarchically, first in the stage II to IIIA population subgroup whose tumors expressed PD-L1 on at least 1% of tumor cells (using the SP263 antibody), then in all patients in the stage II to IIIA population, and finally in the ITT population (stage IB to IIIA). Of the 882 patients who were randomly assigned and had stage II to IIIA disease, 476 had tumors expressing PD-L1 on at least 1% of tumor cells per SP263.[ 26 ][ Level of evidence B1 ]
Adjuvant chemoradiation therapy
Combination chemotherapy and radiation therapy administered before or following surgery should be viewed as investigational and requiring evaluation in future clinical trials.
Evidence (adjuvant chemoradiation therapy):
- Only one trial reported improved DFS, and no trial reported improved OS.
- Median OS was 16.4 months for patients assigned to resection versus 17.5 months for patients assigned to radiation therapy; the 5-year OS rate was 15.7% for patients assigned to resection versus 14% for patients assigned to radiation therapy (HR, 1.06; 95% CI, 0.84–1.35).[ 31 ]
- Rates of PFS were also similar in both groups. In view of its low morbidity and mortality, it was concluded that radiation therapy should be considered the preferred locoregional treatment for these patients.[ 31 ]
The value of PORT has been assessed.[ 27 ] Although some studies suggest that PORT can improve local control for node-positive patients whose tumors were resected, it remains controversial whether it can improve survival. The optimal dose of thoracic PORT is not known at this time. Most studies cited used doses ranging from 30 Gy to 60 Gy, typically provided in 2 Gy to 2.5 Gy fractions.[ 27 ]
As referred to in the National Cancer Institute of Canada (NCIC) Clinical Trials Group JBR.10 study ( NCT00002583 ), PORT may be considered in selected patients to reduce the risk of local recurrence, if any of the following are present:[ 23 ]
- Involvement of multiple nodal stations.
- Extracapsular tumor spread.
- Close or microscopically positive resection margins.
Evidence from one large meta-analysis, subset analyses of randomized trials, and one large population study suggest that PORT may reduce local recurrence. Results from these studies on the effect of PORT on OS are conflicting.
- No difference in OS for the entire PORT group or for the subset of N2 patients.[ 18 ][ Level of evidence A1 ]
- Higher survival rates in patients who received radiation therapy in the observation arm (21% in patients who received PORT vs. 17% in patients who did not receive PORT) and in the chemotherapy arm (47% with PORT vs. 34% without PORT); however, statistical tests of comparison were not conducted.[ 6 ]
- The large SEER retrospective study (N = 7,465) found superior survival rates associated with radiation therapy in N2 disease (HR, 0.855; 95% CI, 0.762–0.959).
There is benefit of PORT in stage IIIA (N2) disease, and the role of PORT in early stages of NSCLC should be clarified in ongoing phase III trials. Further analysis is needed to determine whether these outcomes can be modified with technical improvements, better definitions of target volumes, and limitation of cardiac volume in the radiation portals.[ 18 ]
Treatment Options for Unresectable Stage IIIA NSCLC
Treatment options for patients with unresectable stage IIIA NSCLC include:
- Chemoradiation therapy .
- Locally advanced unresectable tumors .
- Palliative treatment .
Chemoradiation therapy
The addition of sequential and concurrent chemotherapy to radiation therapy has been evaluated in prospective randomized trials and meta-analyses. Overall, concurrent treatment may provide the greatest benefit in survival with an increase in toxic effects.
Concomitant platinum-based radiation chemotherapy may improve survival of patients with locally advanced NSCLC. However, the available data are insufficient to accurately define the size of such a potential treatment benefit and the optimal schedule of chemotherapy.[ 32 ]
Evidence (chemoradiation therapy):
- Cisplatin-based combinations plus radiation therapy resulted in a 10% reduction in the risk of death compared with radiation therapy alone.[ 33 ][ Level of evidence A1 ]
- The addition of concurrent chemotherapy to radical radiation therapy reduced the risk of death at 2 years (relative risk [RR], 0.93; 95% CI, 0.88–0.98; P = .01).
- For the 11 trials with platinum-based chemotherapy, RR was 0.93 (95% CI, 0.87–0.99; P = .02).[ 34 ]
- The HR death among patients treated with radiation therapy and chemotherapy compared with radiation therapy alone was 0.89 (95% CI, 0.81–0.98; P = .02), corresponding to an absolute benefit of chemotherapy of 4% at 2 years.
- The combination of platinum with etoposide appeared to be more effective than platinum alone.
Concurrent versus sequential chemoradiation therapy
The results from two randomized trials (including RTOG-9410 [NCT01134861]) and a meta-analysis indicate that concurrent chemotherapy and radiation therapy may provide greater survival benefit, albeit with more toxic effects, than sequential chemotherapy and radiation therapy.[ 35 - 37 ][ Level of evidence A1 ]
Evidence (concurrent vs. sequential chemoradiation therapy):
- Five-year OS rates favored concurrent therapy (27% vs. 9%).
- Myelosuppression was greater among patients in the concurrent arm, but treatment-related mortality was less than 1% in both arms.[ 35 ]
- Median and 5-year survival were superior in the concurrent chemotherapy with daily radiation therapy arm (17 months vs. 14.6 months and 16% vs. 10% for sequential regimen [ P = .046]).[ 37 ]
- Two smaller studies also reported OS results that favored concurrent over sequential chemotherapy and radiation, although the results did not reach statistical significance.[ 36 , 38 ][ Level of evidence A1 ]
- The analysis indicated a significant benefit of concurrent over sequential treatment (RR, 0.86; 95% CI, 0.78–0.95; P = .003). All studies used cisplatin-based regimens and once-daily radiation therapy.[ 34 ]
- More deaths (3% OS rate) were reported in the concurrent arm, but this did not reach statistical significance (RR, 1.60; CI, 0.75–3.44; P = .2).
- There was more acute esophagitis (grade 3 or worse) with concurrent treatment (range, 17%–26%) compared with sequential treatment (range, 0%–4%; RR, 6.77; P = .001). Overall, the incidence of neutropenia (grade 3 or worse) was similar in both arms.

Radiation therapy dose escalation for concurrent chemoradiation
With improvement in radiation therapy–delivery technology in the 1990s, including tumor-motion management and image guidance, phase I/II trials demonstrated the feasibility of dose-escalation radiation therapy to 74 Gy with concurrent chemotherapy.[ 39 - 41 ] However, a phase III trial of a conventional dose of 60 Gy versus dose escalation to 74 Gy with concurrent weekly carboplatin/paclitaxel did not demonstrate improved local control or PFS, and OS was worse with dose escalation (HR, 1.38; 95% CI, 1.09–1.76; P = .004). There was a nonsignificant increase in grade 5 events with dose escalation (10% vs. 2%) and higher incidence of grade 3 esophagitis (21% vs. 7%; P = .0003). Thus, there is no clear benefit in radiation dose escalation beyond 60 Gy for stage III NSCLC.[ 42 ][ Level of evidence A1 ]
Consolidation therapy following concurrent chemoradiation
Evidence (consolidation therapy following concurrent chemoradiation):
- Arm A: Pemetrexed (500 mg/m 2 ) and cisplatin (75 mg/m 2 ) intravenously every 3 weeks for three cycles plus concurrent thoracic radiation therapy (60 to 66 Gy) followed by pemetrexed consolidation every 3 weeks for four cycles.
- Arm B: Standard therapy with etoposide (50 mg/m 2 ) and cisplatin (50 mg/m 2 ) intravenously every 4 weeks for two cycles plus concurrent thoracic radiation therapy (60 to 66 Gy) followed by two cycles of consolidation platinum-based doublet chemotherapy.
The primary objective was OS. The study was designed as a superiority trial with 80% power to detect an OS HR of 0.74 with a type 1 error of .05. This study randomly assigned 598 patients (arm A, 301; arm B, 297) and treated 555 patients (arm A, 283; arm B, 272).
- Enrollment was stopped early because of futility.
- OS in arm A was not superior to arm B (HR, 0.98; 95% CI, 0.79–1.20; median, 26.8 vs. 25.0 months; P = .831).
- Arm A had a significantly lower incidence of any drug-related grade 3 to 4 adverse events (64.0% vs. 76.8%; P = .001), including neutropenia (24.4% vs. 44.5%; P < .001), during the overall treatment period.
Consolidation immunotherapy
Durvalumab is a selective human IgG1 monoclonal antibody that blocks PD-L1 binding to programmed death 1 (PD-1) and CD80, allowing T cells to recognize and kill tumor cells.[ 44 ]
Evidence (durvalumab following concurrent chemoradiation):
- At a median follow-up of 34.2 months for all patients and 61.6 months for censored patients, the median OS was 47.5 months for all patients and 29.1 months for censored patients (stratified HR, 0.72; 95% CI, 0.59–0.89). The median PFS was 16.9 months in the durvalumab group and 5.6 months in the placebo group (stratified HR, 0.55; 95% CI, 0.45–0.68).
- The estimated 5-year OS rates were 42.9% (95% CI, 38.2%–47.4%) in the durvalumab group and 33.4% (95% CI, 27.3%–39.6%) in the placebo group.[ 45 ][ Level of evidence A1 ]
- The estimated 5-year PFS rates were 33.1% (95% CI, 28.0%–38.2%) in the durvalumab group and 19% (95% CI, 13.6%–25.2%) in the placebo group.
- Grade 3 or 4 adverse events occurred in 29.9% of patients treated with durvalumab and in 26.1% of patients treated with placebo. The most common adverse event of grade 3 or 4 was pneumonia in 4.4% of the patients who received durvalumab and in 3.8% of the patients who received placebo.
Osimertinib (for patients with EGFR mutations)
Evidence (osimertinib following concurrent chemoradiation therapy):
- The median PFS was 39.1 months with osimertinib and 5.6 months with placebo (HR disease progression or death , 0.16; 95% CI, 0.10–0.24; P < .001).
- At 36 months, the OS rate was 84% for patients in the osimertinib group (95% CI, 75%–89%) and 74% for patients in the placebo group (95% CI, 57%–85%) (HR death , 0.81; 95% CI, 0.42–1.56; P = .53) (at 20% data maturity).[ 46 ][ Level of evidence A1 ]
- Grade 3 or higher adverse events occurred in 35% of patients in the osimertinib group and 12% of patients in the placebo group. Radiation pneumonitis was reported in 48% of patients in the osimertinib group and 38% of patients in the placebo group.
Other systemic consolidation therapies
The addition of induction chemotherapy before concurrent chemotherapy and radiation therapy has not been shown to improve survival.[ 47 ][ Level of evidence A1 ]
Randomized trials of other consolidation systemic therapies, including docetaxel,[ 48 ] gefitinib,[ 49 ] and tecemotide (MUC1 antigen-specific immunotherapy) [ 50 ] have not shown an improvement in OS.[ Level of evidence A1 ]
Locally advanced unresectable tumors
Radiation therapy alone may provide benefit to patients with locally advanced unresectable stage IIIA NSCLC.
Radiation therapy with traditional dose and fractionation schedules (1.8–2.0 Gy per fraction per day to 60–70 Gy in 6–7 weeks) results in reproducible long-term survival benefit in 5% to 10% of patients and significant palliation of symptoms.[ 51 ]
Evidence (radiation therapy for locally advanced unresectable tumor):
- Radiation therapy given continuously (including weekends) as three daily fractions (continuous hyperfractionated accelerated radiation therapy) improved OS compared with radiation therapy given as one daily fraction.[ 52 ][ Level of evidence A1 ]
- Patterns of failure for patients treated with radiation therapy alone included both locoregional and distant failures.
Although patients with unresectable stage IIIA disease may benefit from radiation therapy, long-term outcomes have generally been poor because of local and systemic relapse.
Palliative treatment
Radiation therapy may be effective in palliating symptomatic local involvement with NSCLC, such as:
- Tracheal, esophageal, or bronchial compression.
- Vocal cord paralysis.
- Superior vena cava syndrome.
In some cases, endobronchial laser therapy and/or brachytherapy has been used to alleviate proximal obstructing lesions.[ 53 ]
Evidence (radiation therapy for palliative treatment):
- Better overall symptom palliation and fewer re-treatments were required in previously untreated patients using EBRT alone.[ 54 ][ Level of evidence A3 ]
- Although EBRT is frequently prescribed for symptom palliation, there is no consensus about when the fractionation scheme should be used.
- For EBRT, different multifraction regimens appear to provide similar symptom relief;[ 55 - 60 ] however, single-fraction radiation therapy may be insufficient for symptom relief compared with hypofractionated or standard regimens, as seen in the NCIC Clinical Trials Group trial ( NCT00003685 ).[ 57 ][ Level of evidence A3 ]
- Evidence of a modest increase in survival in patients with better performance status given high-dose EBRT is available.[ 55 , 56 ][ Level of evidence A1 ]
- HDREB provided palliation of symptomatic patients with recurrent endobronchial obstruction previously treated by EBRT, when it was technically feasible.
Treatment Options for Superior Sulcus Tumors
Treatment options for superior sulcus tumors include:
- Chemoradiation therapy followed by surgery .
- Radiation therapy alone .
NSCLC of the superior sulcus, frequently termed Pancoast tumors, occurs in less than 5% of patients.[ 61 , 62 ] Superior sulcus tumors usually arise from the apex of the lung and are challenging to treat because of their proximity to structures at the thoracic inlet. At this location, tumors may invade the parietal pleura, chest wall, brachial plexus, subclavian vessels, stellate ganglion, and adjacent vertebral bodies. However, Pancoast tumors are amenable to curative treatment, especially in patients with T3, N0 disease.
Adverse prognostic factors include the presence of mediastinal nodal metastases (N2 disease), spine or subclavian-vessel involvement (T4 disease), and limited resection (R1 or R2).
- Retrospective case series have reported that complete resection was achieved in only 64% of T3, N0 tumors and 39% of T4, N0 tumors.[ 63 ]
Chemoradiation therapy followed by surgery
- The induction regimen was well tolerated, and only five participants had grade 3 or higher toxic effects.
- Induction chemoradiation therapy could sterilize the primary lesion. Induction therapy was completed by 104 patients (95%). Of the 95 patients eligible for surgery, 88 (80%) underwent thoracotomy, two (1.8%) died postoperatively, and 83 (76%) had complete resections.
- Pathological complete response or minimal microscopic disease was seen in 61 (56%) resection specimens. Pathological complete response led to better survival than when any residual disease was present ( P = .02).
- Five-year survival was 44% for all patients and 54% after complete resection, with no difference between T3 and T4 tumors. Disease progression occurred mainly in distant sites.
- There were 12 patients with pathological complete response.
- Major postoperative morbidity, including chylothorax, empyema, pneumonitis, adult respiratory distress syndrome, and bleeding, was observed in eight patients. There were three treatment-related deaths.
- At 3 years, the DFS rate was 49%, and the OS rate was 61%; at 5 years, the DFS rate was 45%, and the OS rate was 56%.[ 64 ][ Level of evidence C2 ]
With improvement in radiation therapy–delivery technology in the 1990s, including tumor-motion management and image guidance, phase I/II trials demonstrated the feasibility of dose-escalation radiation therapy to 74 Gy with concurrent chemotherapy.[ 39 - 41 ] However, a phase III trial of a conventional dose of 60 Gy versus dose escalation to 74 Gy with concurrent weekly carboplatin/paclitaxel did not demonstrate improved local control or PFS, and OS was worse with dose escalation (HR, 1.38 [1.09–1.76]; P = .004). There was a nonsignificant increase in grade 5 events with dose escalation (10% vs. 2%) and higher incidence of grade 3 esophagitis (21% vs. 7%; P = .0003). Thus, there is no clear benefit in radiation dose escalation beyond 60 Gy for stage III NSCLC.[ 42 ][ Level of evidence A1 ]
Radiation therapy alone
While radiation therapy is an integral part of the treatment of Pancoast tumors, variations in dose, treatment technique, and staging that were used in various published series make it difficult to determine its effectiveness.[ 61 , 62 ]
Small, retrospective series of radiation therapy in patients who were only clinically staged have reported 5-year survival rates of 0% to 40%, depending on T stage, total radiation dose, and other prognostic factors. Induction radiation therapy and en bloc resection was shown to be potentially curative.
- In the preoperative setting, a dose of 45 Gy over 5 weeks is generally recommended, while a dose of approximately 61 Gy is required when using definitive radiation therapy as the primary modality.[ 61 , 62 ]
Treatment Options for Tumors That Invade the Chest Wall
Treatment options for tumors that invade the chest wall include:
- Surgery and radiation therapy.
- Radiation therapy alone.
- Chemotherapy combined with radiation therapy and/or surgery.
Selected patients with bulky primary tumors that directly invade the chest wall can obtain long-term survival with surgical management provided that their tumor is completely resected.
Evidence (radical surgery):
- In a small case series of 97 patients, the 5-year survival rate of patients who had completely resected T3, N0, M0 disease was 44.2%. For patients with completely resected T3, N1, M0 disease, the 5-year survival rate was 40.0%. In patients with completely resected T3, N2, M0 disease, the 5-year survival rate was 6.2%.[ 66 ][ Level of evidence C2 ]
- In a small case series of 104 patients, the 5-year survival rate of patients who had completely resected T3, N0, M0 disease was 67.3%. For patients with completely resected T3, N1, M0 disease, the 5-year survival rate was 100.0%. In patients with completely resected T3, N2, M0 disease, the 5-year survival rate was 17.9%.[ 67 ][ Level of evidence C2 ]
- In a case series of 309 patients treated at three centers, patients who underwent en bloc resection had superior outcomes compared with patients who underwent extrapleural resections (60.3% vs. 39.1%; P = .03).[ 68 ][ Level of evidence C2 ]
Adjuvant chemotherapy is recommended, and radiation therapy is reserved for cases with unclear resection margins. Survival rates were lower in patients who underwent incomplete resection and had mediastinal lymph node involvement. Combined-modality approaches have been evaluated to improve ability to achieve complete resection.
- Albain KS, Swann RS, Rusch VW, et al.: Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 374 (9687): 379-86, 2009. [PUBMED Abstract]
- Albain KS, Rusch VW, Crowley JJ, et al.: Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol 13 (8): 1880-92, 1995. [PUBMED Abstract]
- Suntharalingam M, Paulus R, Edelman MJ, et al.: Radiation therapy oncology group protocol 02-29: a phase II trial of neoadjuvant therapy with concurrent chemotherapy and full-dose radiation therapy followed by surgical resection and consolidative therapy for locally advanced non-small cell carcinoma of the lung. Int J Radiat Oncol Biol Phys 84 (2): 456-63, 2012. [PUBMED Abstract]
- Provencio M, Serna-Blasco R, Nadal E, et al.: Overall Survival and Biomarker Analysis of Neoadjuvant Nivolumab Plus Chemotherapy in Operable Stage IIIA Non-Small-Cell Lung Cancer (NADIM phase II trial). J Clin Oncol 40 (25): 2924-2933, 2022. [PUBMED Abstract]
- Lally BE, Zelterman D, Colasanto JM, et al.: Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the surveillance, epidemiology, and end results database. J Clin Oncol 24 (19): 2998-3006, 2006. [PUBMED Abstract]
- Johnstone DW, Byhardt RW, Ettinger D, et al.: Phase III study comparing chemotherapy and radiotherapy with preoperative chemotherapy and surgical resection in patients with non-small-cell lung cancer with spread to mediastinal lymph nodes (N2); final report of RTOG 89-01. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 54 (2): 365-9, 2002. [PUBMED Abstract]
- Taylor NA, Liao ZX, Cox JD, et al.: Equivalent outcome of patients with clinical Stage IIIA non-small-cell lung cancer treated with concurrent chemoradiation compared with induction chemotherapy followed by surgical resection. Int J Radiat Oncol Biol Phys 58 (1): 204-12, 2004. [PUBMED Abstract]
- van Meerbeeck JP, Kramer GW, Van Schil PE, et al.: Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst 99 (6): 442-50, 2007. [PUBMED Abstract]
- Aupérin A, Le Péchoux C, Pignon JP, et al.: Concomitant radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (NSCLC): a meta-analysis of individual data from 1764 patients. Ann Oncol 17 (3): 473-83, 2006. [PUBMED Abstract]
- Rowell NP, O'rourke NP: Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev (4): CD002140, 2004. [PUBMED Abstract]
- Furuse K, Fukuoka M, Kawahara M, et al.: Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol 17 (9): 2692-9, 1999. [PUBMED Abstract]
- Fournel P, Robinet G, Thomas P, et al.: Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d'Oncologie Thoracique-Groupe Français de Pneumo-Cancérologie NPC 95-01 Study. J Clin Oncol 23 (25): 5910-7, 2005. [PUBMED Abstract]
- Curran WJ, Paulus R, Langer CJ, et al.: Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 103 (19): 1452-60, 2011. [PUBMED Abstract]
- Zatloukal P, Petruzelka L, Zemanova M, et al.: Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non-small cell lung cancer: a randomized study. Lung Cancer 46 (1): 87-98, 2004. [PUBMED Abstract]
- Rosenman JG, Halle JS, Socinski MA, et al.: High-dose conformal radiotherapy for treatment of stage IIIA/IIIB non-small-cell lung cancer: technical issues and results of a phase I/II trial. Int J Radiat Oncol Biol Phys 54 (2): 348-56, 2002. [PUBMED Abstract]
- Socinski MA, Blackstock AW, Bogart JA, et al.: Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non-small-cell lung cancer: CALGB 30105. J Clin Oncol 26 (15): 2457-63, 2008. [PUBMED Abstract]
- Bradley JD, Bae K, Graham MV, et al.: Primary analysis of the phase II component of a phase I/II dose intensification study using three-dimensional conformal radiation therapy and concurrent chemotherapy for patients with inoperable non-small-cell lung cancer: RTOG 0117. J Clin Oncol 28 (14): 2475-80, 2010. [PUBMED Abstract]
- Bradley JD, Paulus R, Komaki R, et al.: Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 16 (2): 187-99, 2015. [PUBMED Abstract]
- Senan S, Brade A, Wang LH, et al.: PROCLAIM: Randomized Phase III Trial of Pemetrexed-Cisplatin or Etoposide-Cisplatin Plus Thoracic Radiation Therapy Followed by Consolidation Chemotherapy in Locally Advanced Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 34 (9): 953-62, 2016. [PUBMED Abstract]
- Antonia SJ, Villegas A, Daniel D, et al.: Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 377 (20): 1919-1929, 2017. [PUBMED Abstract]
- Spigel DR, Faivre-Finn C, Gray JE, et al.: Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J Clin Oncol 40 (12): 1301-1311, 2022. [PUBMED Abstract]
- Lu S, Kato T, Dong X, et al.: Osimertinib after Chemoradiotherapy in Stage III EGFR-Mutated NSCLC. N Engl J Med 391 (7): 585-597, 2024. [PUBMED Abstract]
- Vokes EE, Herndon JE, Kelley MJ, et al.: Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III Non-small-cell lung cancer: Cancer and Leukemia Group B. J Clin Oncol 25 (13): 1698-704, 2007. [PUBMED Abstract]
- Hanna N, Neubauer M, Yiannoutsos C, et al.: Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: the Hoosier Oncology Group and U.S. Oncology. J Clin Oncol 26 (35): 5755-60, 2008. [PUBMED Abstract]
- Kelly K, Chansky K, Gaspar LE, et al.: Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol 26 (15): 2450-6, 2008. [PUBMED Abstract]
- Butts C, Socinski MA, Mitchell PL, et al.: Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol 15 (1): 59-68, 2014. [PUBMED Abstract]
- Saunders M, Dische S, Barrett A, et al.: Continuous hyperfractionated accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small-cell lung cancer: a randomised multicentre trial. CHART Steering Committee. Lancet 350 (9072): 161-5, 1997. [PUBMED Abstract]
- Miller JI, Phillips TW: Neodymium:YAG laser and brachytherapy in the management of inoperable bronchogenic carcinoma. Ann Thorac Surg 50 (2): 190-5; discussion 195-6, 1990. [PUBMED Abstract]
- Ung YC, Yu E, Falkson C, et al.: The role of high-dose-rate brachytherapy in the palliation of symptoms in patients with non-small-cell lung cancer: a systematic review. Brachytherapy 5 (3): 189-202, 2006 Jul-Sep. [PUBMED Abstract]
- Sundstrøm S, Bremnes R, Aasebø U, et al.: Hypofractionated palliative radiotherapy (17 Gy per two fractions) in advanced non-small-cell lung carcinoma is comparable to standard fractionation for symptom control and survival: a national phase III trial. J Clin Oncol 22 (5): 801-10, 2004. [PUBMED Abstract]
- Lester JF, Macbeth FR, Toy E, et al.: Palliative radiotherapy regimens for non-small cell lung cancer. Cochrane Database Syst Rev (4): CD002143, 2006. [PUBMED Abstract]
- Bezjak A, Dixon P, Brundage M, et al.: Randomized phase III trial of single versus fractionated thoracic radiation in the palliation of patients with lung cancer (NCIC CTG SC.15). Int J Radiat Oncol Biol Phys 54 (3): 719-28, 2002. [PUBMED Abstract]
- Erridge SC, Gaze MN, Price A, et al.: Symptom control and quality of life in people with lung cancer: a randomised trial of two palliative radiotherapy fractionation schedules. Clin Oncol (R Coll Radiol) 17 (1): 61-7, 2005. [PUBMED Abstract]
- Kramer GW, Wanders SL, Noordijk EM, et al.: Results of the Dutch National study of the palliative effect of irradiation using two different treatment schemes for non-small-cell lung cancer. J Clin Oncol 23 (13): 2962-70, 2005. [PUBMED Abstract]
- Senkus-Konefka E, Dziadziuszko R, Bednaruk-Młyński E, et al.: A prospective, randomised study to compare two palliative radiotherapy schedules for non-small-cell lung cancer (NSCLC). Br J Cancer 92 (6): 1038-45, 2005. [PUBMED Abstract]
- Rusch VW: Management of Pancoast tumours. Lancet Oncol 7 (12): 997-1005, 2006. [PUBMED Abstract]
- Narayan S, Thomas CR: Multimodality therapy for Pancoast tumor. Nat Clin Pract Oncol 3 (9): 484-91, 2006. [PUBMED Abstract]
- Rusch VW, Parekh KR, Leon L, et al.: Factors determining outcome after surgical resection of T3 and T4 lung cancers of the superior sulcus. J Thorac Cardiovasc Surg 119 (6): 1147-53, 2000. [PUBMED Abstract]
- Kunitoh H, Kato H, Tsuboi M, et al.: Phase II trial of preoperative chemoradiotherapy followed by surgical resection in patients with superior sulcus non-small-cell lung cancers: report of Japan Clinical Oncology Group trial 9806. J Clin Oncol 26 (4): 644-9, 2008. [PUBMED Abstract]
- Rusch VW, Giroux DJ, Kraut MJ, et al.: Induction chemoradiation and surgical resection for superior sulcus non-small-cell lung carcinomas: long-term results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J Clin Oncol 25 (3): 313-8, 2007. [PUBMED Abstract]
- Matsuoka H, Nishio W, Okada M, et al.: Resection of chest wall invasion in patients with non-small cell lung cancer. Eur J Cardiothorac Surg 26 (6): 1200-4, 2004. [PUBMED Abstract]
- Facciolo F, Cardillo G, Lopergolo M, et al.: Chest wall invasion in non-small cell lung carcinoma: a rationale for en bloc resection. J Thorac Cardiovasc Surg 121 (4): 649-56, 2001. [PUBMED Abstract]
- Doddoli C, D'Journo B, Le Pimpec-Barthes F, et al.: Lung cancer invading the chest wall: a plea for en-bloc resection but the need for new treatment strategies. Ann Thorac Surg 80 (6): 2032-40, 2005. [PUBMED Abstract]
Treatment of Stages IIIB and IIIC NSCLC
On the basis of the Surveillance, Epidemiology, and End Results (SEER) Program registry, the estimated incidence of stage IIIB non-small cell lung cancer (NSCLC) is 17.6%.[ 1 ] The anticipated 5-year survival rate for most patients who present with clinical stage IIIB NSCLC is 3% to 7%.[ 2 ] In small case series, selected patients with T4, N0–1 disease, solely as the result of satellite tumor nodule(s) within the primary lobe, had 5-year survival rates of 20%.[ 3 , 4 ][ Level of evidence C1 ]
Treatment Options for Stages IIIB and IIIC NSCLC
Treatment options for stages IIIB NSCLC and IIIC NSCLC include:
- Radiation therapy dose escalation for concurrent chemoradiation .
- Consolidation immunotherapy .
- Other systemic consolidation therapies .
- For treatment of locally advanced unresectable tumor in patients who are not candidates for chemotherapy .
- For patients requiring palliative treatment .
- New fractionation schedules (under clinical evaluation).
- Radiosensitizers ( NCT02186847 ) (under clinical evaluation).
- Combined-modality approaches (under clinical evaluation).
- Incorporation of targeted agents into combined modality therapy in patients with EGFR -mutant or ALK -translocated tumors (RTOG-1306 [ NCT01822496 ]; 11-464 [ NCT01553942 ]) (under clinical evaluation).
- Adaptive radiation therapy using positron emission tomography–based response assessment during treatment ( RTOG-1106/ACRIN-6697 ) (under clinical evaluation).
In general, patients with stages IIIB and IIIC NSCLC do not benefit from surgery alone and are best managed by initial chemotherapy, chemotherapy plus radiation therapy, or radiation therapy alone, depending on:
- Sites of tumor involvement.
- The patient's performance status.
Most patients with excellent performance status are candidates for combined-modality chemotherapy and radiation therapy with the following exceptions:
- Selected patients with T4, N0 disease may be treated with combined-modality therapy and surgery similar to patients with superior sulcus tumors.
Patients with stages IIIB or IIIC NSCLC are candidates for clinical trials, which may lead to improvement in the control of disease.
Sequential or concurrent chemotherapy and radiation therapy
Many randomized studies of patients with unresectable stage III NSCLC show that treatment with preoperative or concurrent cisplatin-based chemotherapy and radiation therapy to the chest is associated with improved survival compared with treatment that uses radiation therapy alone. Although patients with unresectable stages IIIB or IIIC disease may benefit from radiation therapy, long-term outcomes have generally been poor, often the result of local and systemic relapse. The addition of sequential and concurrent chemotherapy to radiation therapy has been evaluated in prospective randomized trials.
Evidence (sequential or concurrent chemotherapy and radiation therapy):
- Cisplatin-based combinations plus radiation therapy resulted in a 10% reduction in the risk of death compared with radiation therapy alone.[ 5 ][ Level of evidence A1 ]
- The addition of concurrent chemotherapy to radical radiation therapy reduced the risk of death at 2 years (relative risk [RR], 0.93; 95% confidence interval [CI], 0.88–0.98; P = .01).
- For the 11 trials with platinum-based chemotherapy, RR was 0.93 (95% CI, 0.87–0.99; P = .02).[ 6 ]
- The hazard ratio (HR) death among patients treated with radiation therapy and chemotherapy compared with radiation therapy alone was 0.89 (95% CI, 0.81–0.98; P = .02) corresponding to an absolute benefit of chemotherapy of 4% at 2 years.
- The combination of platinum with etoposide appeared to be more effective than platinum alone. Concomitant platinum-based chemotherapy and radiation therapy may improve survival of patients with locally advanced NSCLC. However, the available data are insufficient to accurately define the size of such a potential treatment benefit and the optimal schedule of chemotherapy.[ 7 ]
- Five-year overall survival (OS) rates favored concurrent therapy (27% vs. 9%).
- Myelosuppression was greater among patients in the concurrent arm, but treatment-related mortality was less than 1% in both arms.[ 8 ]
- Median and 5-year survival were superior in the concurrent chemotherapy with daily radiation therapy arm (17 months vs. 14.6 months and 16% vs. 10% for sequential regimen [ P = .046]).
- Two smaller studies also reported OS results that favored concurrent over sequential chemotherapy and radiation, although the results did not reach statistical significance.[ 10 ][ Level of evidence A1 ]; [ 11 ]
- The analysis indicated a significant benefit of concurrent versus sequential treatment (RR, 0.86; 95% CI, 0.78–0.95; P = .003). All used cisplatin-based regimens and once-daily radiation therapy.[ 6 ]
- More deaths (3% overall) were reported in the concurrent arm, but this did not reach statistical significance (RR, 1.60; CI, 0.75–3.44; P = .2).
With improvement in radiation therapy–delivery technology in the 1990s, including tumor-motion management and image guidance, phase I/II trials demonstrated the feasibility of dose-escalation radiation therapy to 74 Gy with concurrent chemotherapy.[ 12 - 14 ] However, a phase III trial of a conventional dose of 60 Gy versus dose escalation to 74 Gy with concurrent weekly carboplatin/paclitaxel did not demonstrate improved local control or progression-free survival (PFS), and OS was worse with dose escalation (HR, 1.38 [1.09–1.76]; P = .004). There was a nonsignificant increase in grade 5 events with dose escalation (10% vs. 2%) and higher incidence of grade 3 esophagitis (21% vs. 7%; P = .0003).[ 15 ][ Level of evidence A1 ]
Systemic consolidation therapy before or after concurrent chemoradiation therapy
Durvalumab is a selective human IgG1 monoclonal antibody that blocks programmed death-ligand 1 (PD-L1) binding to programmed death 1 (PD-1) and CD80, allowing T cells to recognize and kill tumor cells.[ 16 ]
Evidence (durvalumab):
- At the interim analysis, the coprimary end point of PFS was met. The median PFS was 16.8 months with durvalumab versus 5.6 months with placebo (HR, 0.52; 95% CI, 0.42–0.65; P < .001).[ 16 ][ Level of evidence B1 ] The 18-month PFS rate was 44.2% with durvalumab versus 27% with placebo.
- PFS benefit was seen across all prespecified subgroups and was irrespective of PD-L1 expression before chemoradiation therapy or smoking status. EGFR mutations were observed in 6% of patients (29 treated with durvalumab vs. 14 treated with placebo). The unstratified HR for the EGFR -mutated subgroup was 0.76 (95% CI, 0.35–1.64).
- Grade 3 or 4 adverse events occurred in 29.9% of patients treated with durvalumab and in 26.1% of patients treated with placebo. The most common adverse event of grade 3 or 4 was pneumonia in 4.4% of patients treated with durvalumab and in 3.8% of patients treated with placebo.
- OS was not assessed at the interim analysis.
- At 36 months, the OS rate was 84% for patients in the osimertinib group (95% CI, 75%–89%) and 74% for patients in the placebo group (95% CI, 57%–85%) (HR death , 0.81; 95% CI, 0.42–1.56; P = .53) (at 20% data maturity).[ 17 ][ Level of evidence A1 ]
The addition of induction chemotherapy before concurrent chemotherapy and radiation therapy has not been shown to improve survival.[ 18 ][ Level of evidence A1 ]
Randomized trials of other consolidation systemic therapies, including docetaxel,[ 19 ] gefitinib,[ 20 ] and tecemotide (MUC1 antigen-specific immunotherapy) [ 21 ] have not shown an improvement in OS.[ Level of evidence A1 ]
The role of consolidation systemic therapy after concurrent chemotherapy and radiation therapy for unresectable NSCLC remains unclear. Phase III trials of consolidation systemic therapy including conventional chemotherapy (docetaxel),[ 19 ] tyrosine kinase inhibitors (gefitinib),[ 20 ] and immunotherapy (tecemotide: MUC1 antigen-specific immunotherapy) [ 21 ] have not shown an improvement in OS.[ Level of evidence A1 ]
For treatment of locally advanced unresectable tumor in patients who are not candidates for chemotherapy
Radiation therapy alone may provide benefit to patients with locally advanced unresectable stage III NSCLC.
Radiation therapy with traditional dose and fractionation schedules (1.8–2.0 Gy per fraction per day to 60–70 Gy in 6–7 weeks) results in reproducible long-term survival benefit in 5% to 10% of patients and significant palliation of symptoms.[ 22 ]
- Radiation therapy given as three daily fractions improved OS compared with radiation therapy given as one daily fraction.[ 23 ][ Level of evidence A1 ]
For patients requiring palliative treatment
In some cases, endobronchial laser therapy and/or brachytherapy has been used to alleviate proximal obstructing lesions.[ 24 ]
- Better overall symptom palliation and fewer re-treatments were required in previously untreated patients using EBRT alone.[ 25 ][ Level of evidence A3 ]
- Although different multifraction regimens appear to provide similar symptom relief,[ 26 - 31 ] single-fraction radiation may be insufficient for symptom relief compared with hypofractionated or standard regimens, as shown in the National Cancer Institute of Canada Clinical Trials Group trial ( NCT00003685 ).[ 28 ][ Level of evidence A3 ]
- Evidence of a modest increase in survival in patients with better performance status given high-dose radiation therapy is available.[ 26 , 27 ][ Level of evidence A1 ]
Patients with stages IIIB or IIIC disease with poor performance status are candidates for chest radiation therapy to palliate pulmonary symptoms (e.g., cough, shortness of breath, hemoptysis, or pain).[ 22 ][ Level of evidence C1 ] For more information, see Cardiopulmonary Syndromes and Cancer Pain .
- Wisnivesky JP, Yankelevitz D, Henschke CI: Stage of lung cancer in relation to its size: part 2. Evidence. Chest 127 (4): 1136-9, 2005. [PUBMED Abstract]
- Deslauriers J, Brisson J, Cartier R, et al.: Carcinoma of the lung. Evaluation of satellite nodules as a factor influencing prognosis after resection. J Thorac Cardiovasc Surg 97 (4): 504-12, 1989. [PUBMED Abstract]
- Urschel JD, Urschel DM, Anderson TM, et al.: Prognostic implications of pulmonary satellite nodules: are the 1997 staging revisions appropriate? Lung Cancer 21 (2): 83-7; discussion 89-91, 1998. [PUBMED Abstract]
- Langendijk JA, ten Velde GP, Aaronson NK, et al.: Quality of life after palliative radiotherapy in non-small cell lung cancer: a prospective study. Int J Radiat Oncol Biol Phys 47 (1): 149-55, 2000. [PUBMED Abstract]
Treatment of Newly Diagnosed Stage IV, Relapsed, and Recurrent NSCLC
Factors affecting treatment selection.
Forty percent of patients with newly diagnosed non-small cell lung cancer (NSCLC) have stage IV disease . Treatment goals are to prolong survival and control disease-related symptoms. Treatment options include cytotoxic chemotherapy, targeted agents, and immunotherapy. Factors influencing treatment selection include comorbidity, performance status, histology, and molecular and immunologic features of the cancer. Therefore, assessment of tumor-genomic changes and programmed death-ligand 1 (PD-L1) expression is critical before initiating therapy. Radiation therapy and surgery are generally used in selective cases for symptom palliation.
Factors that affect selection of treatment include:
- History and molecular features .
- Age and comorbidity .
- Performance status .
History and molecular features
Patients with nonsquamous cell histology, good performance status, no history of hemoptysis or other bleeding, or recent history of cardiovascular events may benefit from the addition of bevacizumab to paclitaxel and carboplatin. Patients with tumors harboring sensitizing mutations in exons 19 or 21 of EGFR , particularly those from East Asia, never smokers, and those with adenocarcinoma may benefit from epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) as an alternative to first- or second-line chemotherapy. Patients with tumors harboring ALK translocations, ROS1 rearrangements, or NTRK fusions may benefit from anaplastic lymphoma kinase (ALK), ROS1, or neurotrophic tyrosine kinase (NTRK) inhibitors as an alternative to first- or second-line chemotherapy.
Patients with tumors expressing PD-L1 (>50% by immunohistochemistry) have improved survival with pembrolizumab. The addition of pembrolizumab to carboplatin-plus-pemetrexed chemotherapy for nonsquamous advanced lung cancer improves survival irrespective of PD-L1 expression.[ 1 ][ Level of evidence A1 ] For patients with stage IV or recurrent NSCLC and PD-L1 expression on at least 1% of tumor cells, frontline combination immunotherapy with nivolumab and ipilimumab increases overall survival (OS).[ 2 ][ Level of evidence A1 ] Second-line systemic therapy with nivolumab, docetaxel, pemetrexed, or pembrolizumab for PD-L1−positive tumors also improves survival in patients with good performance status (who have not received the same or a similar agent in the first-line setting).[ 3 ][ Level of evidence A1 ]
The role of systemic therapy in patients with an Eastern Cooperative Oncology Group (ECOG) performance status below 2 is less certain.
Patients with adenocarcinoma may benefit from pemetrexed [ 4 ] and bevacizumab, as well as from combination chemotherapy with pembrolizumab. Patients with unresectable, locally advanced or metastatic, well-differentiated, nonfunctional, neuroendocrine tumors benefit from the mammalian target of rapamycin (mTOR) inhibitor, everolimus.
Age and comorbidity
Evidence supports the concept that older patients with good performance status and limited comorbidity may benefit from combination chemotherapy. Age alone should not dictate treatment-related decisions in patients with advanced NSCLC. Older patients with a good performance status enjoy longer survival and a better quality of life when treated with chemotherapy compared with supportive care alone. Caution should be exercised when extrapolating data for patients aged 70 to 79 years to patients aged 80 years or older because only a very small number of patients aged 80 years or older have been enrolled in clinical trials, and the benefit in this group is uncertain.[ 5 , 6 ]
Evidence (age and comorbidity):
- Platinum-containing combination chemotherapy regimens provide clinical benefit when compared with supportive care or single-agent therapy; however, such treatment may be contraindicated in some older patients because of the age-related reduction in the functional reserve of many organs and/or comorbid conditions. Approximately two-thirds of patients with NSCLC are aged 65 years or older, and approximately 40% are aged 70 years or older.[ 7 ] Surveillance, Epidemiology, and End Results (SEER) Program data suggest that the percentage of patients older than 70 years is closer to 50%.
- A review of the SEER Medicare data from 1994 to 1999 found a much lower rate of chemotherapy use than expected for the overall population.[ 8 ] The same data suggested that older patients may have more comorbidities or a higher rate of functional compromise that would make study participation difficult, if not contraindicated; lack of clinical trial data may influence decisions to treat individual patients with standard chemotherapy.
- Patients who were treated with vinorelbine had a 1-year survival rate of 32%, compared with 14% for those who were treated with supportive care alone. Quality-of-life parameters were also significantly improved in the chemotherapy arm, and toxic effects were acceptable.
- Response rates (22% vs. 10%) and progression-free survival (PFS) (5.4 months vs. 3.1 months) were significantly better with docetaxel, but median survival (14.3 months vs. 9.9 months) and 1-year survival rates (59% vs. 37%) did not reach statistical significance.
- Retrospective data analyzing and comparing younger (age <70 years) patients with older (age ≥70 years) patients who participated in large randomized trials of doublet combinations have also shown that older patients may derive the same survival benefit, but with a higher risk of toxic effects in the bone marrow.[ 5 , 6 , 11 - 14 ]
Performance status
Performance status is among the most important prognostic factors for survival of patients with NSCLC.[ 15 ] The benefit of therapy for this group of patients has been evaluated through retrospective analyses and prospective clinical trials.
The results support further evaluation of chemotherapeutic approaches for both metastatic and locally advanced NSCLC; however, the efficacy of current platinum-based chemotherapy combinations is such that no specific regimen can be regarded as standard therapy. Outside of a clinical trial setting, chemotherapy should only be given to patients with good performance status and evaluable tumor lesions, who desire this treatment after being fully informed of its anticipated risks and limited benefits.
Randomized controlled trials of patients with stage IV disease and good performance status have shown that cisplatin-based chemotherapy improves survival and palliates disease-related symptoms.[ 3 ][ Level of evidence A1 ]
Evidence (performance status):
- When compared with patients with a performance status of 0 to 1, who had a median survival of 8.8 months and a 1-year survival rate of 38%, the corresponding median survival figures for patients with a performance status of 2 were 3.0 months and a 1-year survival rate of 14%; this demonstrates the poor prognosis conferred by a lower performance status. These differences were statistically significant.
- When patients with a performance status of 2 were analyzed by treatment arm, those who received combination chemotherapy had a significantly higher response rate (24% vs. 10%), longer median survival (4.7 months vs. 2.4 months), and a superior 1-year survival rate (18% vs. 10%), compared with those who were treated with single-agent paclitaxel.[ 13 ]
- Median OS was 5.3 months for the pemetrexed-alone group and 9.3 months for the carboplatin-and-pemetrexed group (hazard ratio [HR], 0.62; 95% confidence interval [CI], 0.46–0.83; P = .001).
- Median PFS was 2.8 months for the pemetrexed-alone group and 5.8 months for the carboplatin-and-pemetrexed group ( P < .001).
- The response rates were 10.3% for the pemetrexed-alone group and 23.8% for the carboplatin-and-pemetrexed group ( P = .032).
- Side effects were more frequent in the combination arm, as expected.
This study, which was performed in eight centers in Brazil and one center in the United States, reported rates of OS and PFS that were higher than has historically been noted in most, although not all, other published studies. This may indicate differences in patient selection.
- Despite a high incidence of adverse events, including five deaths, the final analysis showed that the overall toxic effects experienced by patients with a performance status of 2 was not significantly different from that experienced by patients with a performance status of 0 to 1.
- An efficacy analysis demonstrated an overall response rate of 14%, median survival time of 4.1 months, and a 1-year survival rate of 19%; all were substantially inferior to the patients with performance status of 0 to 1.
- Response rates were 25% in the cisplatin-plus-gemcitabine arm and 16% in the carboplatin-plus-paclitaxel arm; median survival times were 6.8 months in the cisplatin-plus-gemcitabine arm and 6.1 months in the carboplatin-plus-paclitaxel arm; 1-year survival rates were 25% in the cisplatin-plus-gemcitabine arm and 19% in the carboplatin-plus-paclitaxel arm. None of these differences was statistically significant, but the survival figures were longer than expected, based on historical controls.
- Results from two trials suggest that patients with a performance status of 2 may experience symptom improvement.[ 18 , 19 ]
Treatment Options for Newly Diagnosed Stage IV, Relapsed, and Recurrent NSCLC (First-Line Therapy)
Treatment options for patients with newly diagnosed stage IV , relapsed, and recurrent disease include:
- Combination chemotherapy .
- Drug and dose schedule .
- Bevacizumab .
- Cetuximab .
- Necitumumab .
- Maintenance therapy following first-line chemotherapy.
- Pemetrexed following first-line platinum-based combination chemotherapy.
- Osimertinib alone .
- Osimertinib plus chemotherapy .
- Dacomitinib .
- Gefitinib .
- Erlotinib .
- Amivantamab .
- Alectinib .
- Lorlatinib .
- Crizotinib .
- Ceritinib .
- Brigatinib .
- Dabrafenib and trametinib .
- Entrectinib .
- Larotrectinib .
- Selpercatinib .
- Pralsetinib .
- Tepotinib .
- Capmatinib .
- Pembrolizumab plus chemotherapy .
- Pembrolizumab alone .
- Cemiplimab-rwlc plus chemotherapy .
- Cemiplimab-rwlc alone .
- Tremelimumab .
- Atezolizumab alone .
- Atezolizumab plus chemotherapy .
- Atezolizumab plus bevacizumab plus chemotherapy .
- Nivolumab plus ipilimumab .
- Everolimus .
- Endobronchial laser therapy and/or brachytherapy (for obstructing lesions).[ 20 ]
- External-beam radiation therapy (EBRT) (primarily for palliation of local symptomatic tumor growth).[ 21 - 23 ]
- Treatment of second primary tumor.
- Treatment of brain metastases.
- Clinical trials can be considered as first-line therapy.
Cytotoxic combination chemotherapy
Combination chemotherapy.
The type and number of chemotherapy drugs to be used for the treatment of patients with advanced NSCLC has been extensively evaluated in randomized controlled trials and meta-analyses.
Several randomized trials have evaluated various drugs combined with either cisplatin or carboplatin in previously untreated patients with advanced NSCLC. On the basis of meta-analyses of the trials, the following conclusions can be drawn:
- Certain three-drug combinations that add so-called targeted agents may result in superior survival.
- EGFR inhibitors may benefit selected patients with EGFR mutations.
- Maintenance chemotherapy after four cycles of platinum combination chemotherapy may improve PFS and OS.
- Platinum combinations with vinorelbine, paclitaxel, docetaxel, gemcitabine, irinotecan, protein-bound paclitaxel, and pemetrexed yield similar improvements in survival. Types and frequencies of toxic effects differ, and these may determine the preferred regimen for an individual patient. Patients with adenocarcinoma may benefit from pemetrexed.
- Cisplatin and carboplatin yield similar improvements in outcome with different toxic effects. Some, but not all, trials and meta-analyses of trials suggest that outcomes with cisplatin may be superior, although with a higher risk of certain toxicities such as nausea and vomiting.
- Nonplatinum combinations offer no advantage to platinum-based chemotherapy, and some studies demonstrate inferiority.
- Three-drug combinations of the commonly used chemotherapy drugs do not result in superior survival and are more toxic than two-drug combinations.
Evidence (combination chemotherapy):
- In the trials that compared a doublet regimen with a single-agent regimen, a significant increase was observed in tumor response (odds ratio [OR], 0.42; 95% CI, 0.37–0.47; P < .001) and 1-year survival (OR, 0.80; 95% CI, 0.70–0.91; P < .001) in favor of the doublet regimen. The absolute benefit in 1-year survival was 5%, which corresponds to an increase in 1-year survival from 30% with a single-agent regimen to 35% with a doublet regimen. The rates of grades 3 and 4 toxic effects caused by doublet regimens were statistically increased compared with rates after single-agent therapy, with ORs ranging from 1.2 to 6.2. Infection rates did not increase in doublet regimens.
- There was no increase in 1-year survival (OR, 1.01; 95% CI, 0.85–1.21; P = .88) for triplet regimens versus doublet regimens. The median survival ratio was 1.00 (95% CI, 0.94–1.06; P = .97).
- The objective response rate was higher for patients treated with cisplatin (30%) than for patients treated with carboplatin (24%); (OR, 1.37; 95% CI, 1.16–1.61; P < .001).
- Carboplatin treatment was associated with a nonstatistically significant increase in the hazard of mortality relative to treatment with cisplatin (HR, 1.07; 95% CI, 0.99–1.15; P = .100).
- In patients with nonsquamous cell tumors and in patients treated with third-generation chemotherapy, carboplatin-based chemotherapy was associated with a statistically significant increase in mortality (HR, 1.12; 95% CI, 1.01–1.23 in patients with nonsquamous cell tumors and HR, 1.11; 95% CI, 1.01–1.21 in patients treated with third-generation chemotherapy).
- Treatment-related toxic effects were also assessed in the meta-analysis. More thrombocytopenia was seen with carboplatin than with cisplatin (12% vs. 6%; OR, 2.27; 95% CI, 1.71–3.01; P < .001), but cisplatin caused more nausea and vomiting (8% vs. 18%; OR, 0.42; 95% CI, 0.33–0.53; P < .001) and renal toxic effects (0.5% vs. 1.5%; OR, 0.37; 95% CI, 0.15–0.88; P = .018).
- The authors concluded that treatment with cisplatin was not associated with a substantial increase in the overall risk of severe toxic effects. This comprehensive individual-patient meta-analysis is consistent with the conclusions of other meta-analyses that were based on essentially the same clinical trials, but which used only published data.
- A 62% increase in the OR for response was attributable to platinum-based therapy (OR, 1.62; 95% CI, 1.46–1.8; P < .001). The 1-year survival rate was increased by 5% with platinum-based regimens (34% vs. 29%; OR, 1.21; 95% CI, 1.09–1.35; P = .003).
- No statistically significant increase in 1-year survival was found when platinum therapies were compared with third-generation-based combination regimens (OR, 1.11; 95% CI, 0.96–1.28; P = .17).
- The toxic effects of platinum-based regimens was significantly higher for hematologic toxic effects, nephrotoxic effects, and nausea and vomiting but not for neurological toxic effects, febrile neutropenia rate, or toxic death rate. These results are consistent with the second literature-based meta-analysis.
- The use of platinum-based doublet regimens was associated with a slightly higher survival at 1 year (relative risk [RR], 1.08; 95% CI, 1.01%–1.16%; P = .03) and a better partial response (RR, 1.11; 95% CI, 1.02–1.21; P = .02), with a higher risk of anemia, nausea, and neurological toxic effects.
- In subanalyses, cisplatin-based doublet regimens improved survival at 1 year (RR, 1.16%; 95% CI, 1.06–1.27; P = .001), complete response (RR, 2.29; 95% CI, 1.08–4.88; P = .03), and partial response (RR, 1.19; 95% CI, 1.07–1.32; P = .002), with an increased risk of anemia, neutropenia, neurological toxic effects, and nausea.
- Conversely, carboplatin-based doublet regimens did not increase survival at 1 year (RR, 0.95; 95% CI, 0.85–1.07; P = .43).
- Patients treated with a platinum-based regimen benefited from a statistically significant reduction in the risk of death at 1 year (OR, 0.88; 95% CI, 0.78–0.99; P = .044) and a lower risk of being refractory to chemotherapy (OR, 0.87; CI, 0.73–0.99; P = .049).
- Forty-four (1.9%) toxic-related deaths were reported for platinum-based regimens and 29 (1.3%) toxic-related deaths were reported for nonplatinum regimens (OR, 1.53; CI, 0.96–2.49; P = .08). An increased risk of grade 3 to 4 gastrointestinal and hematologic toxic effects for patients treated with platinum-based chemotherapy was statistically demonstrated. There was no statistically significant increase in the risk of febrile neutropenia (OR, 1.23; CI, 0.94–1.60; P = .063).
Drug and dose schedule
Among the active combinations, definitive recommendations regarding drug dose and schedule cannot be made, except for carboplatin, pemetrexed, and pembrolizumab for patients with nonsquamous tumor histology.
Evidence (drug and dose schedule):
- The pooled estimate for OS showed an 11% improvement in favor of docetaxel (HR, 0.89; 95% CI, 0.82–0.96; P = .004). Sensitivity analyses that considered only vinorelbine as a comparator or only the doublet regimens showed similar improvements.
- Grade 3 to 4 neutropenia and grade 3 to 4 serious adverse events were less frequent with docetaxel-based regimens (OR, 0.59; 95% CI, 0.38–0.89; P = .013) versus vinca alkaloid-based regimens (OR, 0.68; 95% CI, 0.55–0.84; P < .001).
- Two randomized trials compared weekly versus every-3-week dosing of paclitaxel and carboplatin, which reported no significant difference in efficacy and better tolerability for weekly administration.[ 32 , 33 ] Although meta-analyses of randomized controlled trials suggest that cisplatin combinations may be superior to carboplatin or nonplatinum combinations, the clinical relevance of the differences in efficacy must be balanced against the anticipated tolerability, logistics of administration, and familiarity of the medical staff in making treatment decisions for individual patients.
- OS for cisplatin and pemetrexed (median survival, 10.3 months) was noninferior to cisplatin and gemcitabine (median survival, 10.3 months; HR, 0.94; 95% CI, 0.84%–1.05%).
- In patients with adenocarcinoma (n = 847), OS was statistically superior for cisplatin and pemetrexed (12.6 months) versus cisplatin and gemcitabine (10.9 months); in patients with large cell carcinoma (n = 153), OS was statistically superior for cisplatin and pemetrexed (10.4 months) versus cisplatin and gemcitabine (6.7 months).
- In contrast, in patients with squamous cell histology (n = 473), there was a significant improvement in survival with cisplatin and gemcitabine (10.8 months) versus cisplatin and pemetrexed (9.4 months). For cisplatin and pemetrexed, rates of grade 3 or 4 neutropenia, anemia, and thrombocytopenia ( P ≤ .001); febrile neutropenia ( P = .002); and alopecia ( P < .001) were significantly lower, whereas grade 3 or 4 nausea ( P = .004) was more common.
- The results of this study suggested that the cisplatin and pemetrexed doublet is another alternative doublet for first-line chemotherapy for advanced NSCLC. The results also suggested that there may be differences in outcome depending on histology.
Combination chemotherapy with monoclonal antibodies
Bevacizumab.
Evidence (bevacizumab):
- Median survival was 12.3 months in the group assigned to chemotherapy plus bevacizumab, as compared with 10.3 months in the chemotherapy-alone group (HR death , 0.79; P = .003).
- Median PFS was 6.2 months in the group assigned to chemotherapy plus bevacizumab (HR disease progression , 0.66; P < .001), with a 35% response rate ( P < .001), and 4.5 months in the chemotherapy-alone group (HR disease progression , 0.66; P < .001), with a 15% response rate ( P < .001).
- Rates of clinically significant bleeding were 4.4% in the group assigned to chemotherapy plus bevacizumab and 0.7% in the chemotherapy-alone group ( P < .001). There were 15 treatment-related deaths in the chemotherapy-plus-bevacizumab group, including five from pulmonary hemorrhage.
- For this subgroup of patients with NSCLC, the addition of bevacizumab to paclitaxel and carboplatin may provide survival benefit.[ 34 ][ Level of evidence A1 ]
- PFS was significantly prolonged with the addition of bevacizumab; the HRs for PFS were 0.75 in the low-dose group (median PFS, 6.7 months vs. 6.1 months for the placebo group; P = .03) and 0.82 in the high-dose group compared with the placebo group (median PFS, 6.5 months vs. 6.1 months for the placebo group; P = .03).[ 35 ][ Level of evidence A1 ]
- Objective response rates were also improved with the addition of bevacizumab, and they were 20.1% for placebo, 34.1% for low-dose bevacizumab, and 30.4% for high-dose bevacizumab plus cisplatin/gemcitabine.
- Incidence of grade 3 or greater adverse events was similar across arms.
- Grade 3 or greater pulmonary hemorrhage rates were 1.5% or less for all arms, despite 9% of patients receiving therapeutic anticoagulation.
- These results support the addition of bevacizumab to platinum-containing chemotherapy, but the results are far less impressive than when the carboplatin-paclitaxel combination was used.
- Furthermore, no significant difference in survival was shown in this study, as reported in abstract form.
- Altogether, these findings may suggest that the backbone of chemotherapy may be important when bevacizumab is added.
Evidence (cetuximab):
- The addition of cetuximab did not result in a statistically significant improvement in PFS, the primary study end point, or OS.
- Median PFS was 4.40 months for patients in the cetuximab-chemotherapy arm versus 4.24 months for patients in the taxane-carboplatin arm (HR, 0.902; 95% CI, 0.761–1.069; P = .236).
- Median OS was 9.69 months for patients in the cetuximab-chemotherapy arm versus 8.38 months for patients in the chemotherapy-alone arm (HR, 0.890; 95% CI, 0.754–1.051; P = .169).
- No significant associations were found between EGFR expression, EGFR mutation, EGFR copy number, or KRAS mutations and PFS, OS, and response in the treatment-specific analyses.[ 38 ]
- The primary study end point, OS, was longer for patients treated with cetuximab and chemotherapy (median 11.3 months vs. 10.1 months; HR death , 0.871; 95% CI, 0.762–0.996; P = .044).
- A survival benefit was seen in all histological subgroups; however, survival benefit was not seen in non-White or Asian patients. Only the interaction between the treatment and the ethnic origin was significant ( P = .011).
- The main cetuximab-related adverse event was acne-like rash (grade 3, 10%).
- It is not clear whether the differences in outcome in these two studies are the result of differences in the study populations, tumor characterization for EGFR expression, or chemotherapy regimens.
Necitumumab
Evidence (necitumumab):
- Median OS was prolonged with the addition of necitumumab (11.5 months vs. 9.9 months; P = .01).
- PFS was also prolonged with the addition of necitumumab (5.7 months vs. 5.5 months); however, the overall response rate was similar in both groups (31% vs. 28%).
- Grades 3 and 4 adverse events were higher in the necitumumab-containing arm (72% vs. 62%).
- Necitumumab is associated with higher toxicity and relatively modest benefit.
- This study showed no benefit from the addition of necitumumab to standard first-line chemotherapy for advanced nonsquamous NSCLC.
- OS was 11.3 months (95% CI, 9.5–13.4) for patients in the necitumumab-containing arm versus 11.5 months (95% CI, 10.1–13.1) for patients in the chemotherapy alone arm; P = .96. Similarly, there was no difference between the arms in terms of objective response or PFS.
- Serious adverse events and rates of grades 3 and 4 adverse events, including thromboembolic events, were higher in patients in the necitumumab-containing arm; the incidence of treatment-related deaths was also higher (5% vs. 3%).
- On the basis of these results, necitumumab is not recommended as combination therapy with standard first-line chemotherapy for patients with advanced nonsquamous NSCLC.
Maintenance therapy after first-line chemotherapy (for patients with stable or responding disease after four cycles of platinum-based combination chemotherapy)
One extensively investigated treatment strategy in NSCLC is maintenance therapy after initial response to chemotherapy. Options for maintenance therapy that have been investigated include:
- Continuing the initial combination chemotherapy regimen.
- Continuing only single-agent chemotherapy.
- Introducing a new agent as maintenance .
Multiple randomized trials have evaluated the efficacy of continuing first-line combination cytotoxic chemotherapy beyond three to four cycles.
Evidence (maintenance therapy following first-line chemotherapy):
- None of the trials of continued cytotoxic combinations showed a significant OS advantage with additional or longer durations beyond four cycles. For patients with nonsquamous NSCLC, two studies have demonstrated improved PFS and OS with either switch or continuous maintenance chemotherapy (e.g., maintenance pemetrexed after initial cisplatin and gemcitabine or maintenance pemetrexed after initial cisplatin and pemetrexed).[ 41 ]
- Three trials found statistically significantly improved PFS or time to progression with additional chemotherapy.[ 42 - 44 ]
- No consistent improvement in quality of life was reported.[ 43 , 45 , 46 ]
- Chemotherapy-related toxicities were greater with prolonged chemotherapy.[ 45 , 46 ]
These data suggest that PFS and OS for patients with nonsquamous NSCLC may be improved either by continuing an effective chemotherapy beyond four cycles or by immediate initiation of alternative chemotherapy. The improvement in PFS, however, is tempered by an increase in adverse events including additional cytotoxic chemotherapy and no consistent improvement in quality of life. For patients who have stable disease or who respond to first-line therapy, evidence does not support the continuation of combination cytotoxic chemotherapy until disease progression or the initiation of a different chemotherapy before disease progression. Collectively, these trials suggest that first-line cytotoxic combination chemotherapy should be stopped at disease progression or after four cycles in patients whose disease is not responding to treatment; it can be administered for no more than six cycles.[ 42 , 43 , 45 , 46 ] For patients with nonsquamous NSCLC who have a response or stable disease after four to six cycles of platinum combination chemotherapy, maintenance chemotherapy with pemetrexed should be considered.[ 41 ]
Evidence (first-line platinum-based combination chemotherapy followed by pemetrexed):
The findings of two randomized trials ( NCT00102804 and NCT00789373 ) have shown improved outcomes with the addition of pemetrexed after standard first-line platinum-based combination chemotherapy.[ 44 , 47 ]
- Both the primary end point of PFS and the secondary end point of OS were statistically significantly prolonged with the addition of maintenance pemetrexed (median PFS, 4.3 months vs. 2.6 months; HR, 0.50; 95% CI, 0.42–0.61; P < .0001; median OS, 13.4 months vs. 10.6 months; HR, 0.79; 95% CI, 0.65–0.95; P = .012).
- Benefit was not seen in patients with squamous histology.
- Higher than grade 3 toxicity and treatment discontinuations that resulted from drug-related toxic effects were higher in the pemetrexed group than in the placebo group.
- No pemetrexed-related deaths occurred.
- Relatively fewer patients in the pemetrexed group than in the placebo group received systemic postdiscontinuation therapy (227 [51%] vs. 149 [67%]; P = .0001).
- Quality of life during maintenance therapy with pemetrexed was similar to placebo, except for a small increase in loss of appetite and significantly delayed worsening of pain and hemoptysis as assessed using the Lung Cancer Symptom Scale.[ 48 ] The quality-of-life results require cautious evaluation because there was a high degree of censoring (>50%) with the primary quality-of-life end point, which was time to worsening of symptoms.
- Trials have not evaluated maintenance pemetrexed versus pemetrexed at progression.
- There was a statistically significant improvement in the primary end point of PFS (4.1 months vs. 2.8 months, HR, 0.62; 95% CI, 0.49–0.79) and in the secondary end point of OS (13.9 months vs. 11 months, HR, 0.78; 95% CI, 0.64–0.96).[ 41 , 44 ][ Level of evidence B1 ]
EGFR tyrosine kinase inhibitors (TKIs) with or without chemotherapy (for patients with EGFR mutations)
Select patients with activating mutations in EGFR may benefit from single-agent EGFR TKIs. Randomized controlled trials of patients with chemotherapy-naïve NSCLC and EGFR mutations have shown that EGFR inhibitors alone improved both PFS and OS and have favorable toxicity profiles compared with combination chemotherapy. The combination of EGFR TKIs with chemotherapy showed improved PFS compared with EGFR TKI monotherapy and represents another treatment option.
Osimertinib alone
Evidence (osimertinib alone):
- The primary end point of PFS was significantly longer with osimertinib (18.9 months vs. 10.2 months; HR, 0.46; 95% CI, 0.37–0.57, P < .001).[ 49 ][ Level of evidence B1 ]
- The objective response rate was similar for both groups (80% for the osimertinib group vs. 76% for the standard EGFR TKI group).
- Central nervous system (CNS) progression was observed less often in the osimertinib group compared with the standard EGFR TKI group (6% vs. 15%).
- The median duration of response (DOR) was 17.2 months (95% CI, 13.8–22.0) with osimertinib versus 8.5 months (95% CI, 7.3–9.8) with standard EGFR TKIs.
- OS was a key secondary end point. With a follow-up of at least 39 months in each group, the median OS was 38.6 months (95% CI, 34.5–41.8) in the osimertinib group and 31.8 months (95% CI, 26.6–36.0) in the standard EGFR TKI group (HR death 0.80; 95.05% CI, 0.64–1.00; P = .046).[ 50 ][ Level of evidence A1 ]
- The crossover rate from the standard EGFR TKI group to the osimertinib group was 31% (85 of 277) among patients assigned to the standard EGFR TKI group and 47% (85 of 180) among patients discontinuing the EGFR TKI. The authors noted that this crossover probably contributed to the duration of OS in the EGFR TKI (31.8 months).
- No new safety signals were observed. Rates of adverse events of grade 3 or higher and adverse events leading treatment discontinuations were similar between groups. Adverse events leading to dose interruptions, reductions, or permanent discontinuations were 43%, 5%, and 15%, respectively, in the osimertinib group and 41%, 4%, and 18%, respectively, in the EGFR TKI group.
The FDA approved osimertinib for first-line treatment of EGFR -mutant NSCLC (exon 19 deletion or L858R).
Longer PFS and OS, activity against the EGFR T790M mutation in addition to EGFR -TKI−sensitizing mutations, decreased frequency of CNS progression, and good tolerability make osimertinib the preferred choice for treatment of patients with advanced EGFR -mutated NSCLC compared with first- and second-generation EGFR TKIs.
Osimertinib plus chemotherapy
Evidence (osimertinib plus chemotherapy):
- PFS was 25.5 months (24.7–not calculable) for patients in the osimertinib-plus-chemotherapy group and 16.7 months (14.1–21.3) for patients in the osimertinib-monotherapy group (HR, 0.62; 95% CI, 0.49–0.79; P < .001).[ 51 ][ Level of evidence B1 ] PFS was assessed according to blinded independent central review and was consistent with the primary analysis (HR, 0.62; 95% CI, 0.48–0.80).
- At 24 months, 57% (95% CI, 50%–63%) of the patients in the osimertinib-plus-chemotherapy group and 41% (95% CI, 35%–47%) of those in the osimertinib-alone group were alive and progression-free.
- An objective response (complete or partial) occurred in 83% of patients who received osimertinib plus chemotherapy and 76% of patients who received osimertinib alone.
- The median DOR was 24.0 months (95% CI, 20.9–27.8) in the osimertinib-plus-chemotherapy group and 15.3 months (95% CI, 12.7–19.4), in the osimertinib-alone group.
- Grade 3 or higher adverse events from any cause were more common with the combination (64%) than with monotherapy (27%); this is consistent with known chemotherapy-related adverse events. Osimertinib plus pemetrexed with a platinum-based agent had a safety profile that was consistent with the established profiles of these agents.
Analysis of OS, a secondary end point, requires further follow-up (data maturity, 27%).
Dacomitinib
Evidence (dacomitinib):
- Median PFS was 14.7 months in the dacomitinib group and 9.2 months in the gefitinib group (HR, 0.59; 95% CI, 0.47−0.74; P < .0001).[ 52 ][ Level of evidence B1 ]
- The objective response rate was similar between the two groups (75% for the dacomitinib group vs. 72% for the gefitinib group; P = 0.42).
- The median DOR was longer in the dacomitinib group (14.8 months vs. 8.3 months; HR, 0.4; 95% CI, 0.31−0.53; P < .0001).
- The median OS was 34.1 months with dacomitinib vs. 26.8 months with gefitinib (HR, 0.76; 95% CI, 0.58−0.99; P = .44).[ 52 ]
- Grade 3 or higher adverse events of any cause occurred in 63% of patients who received dacomitinib and 41% of patients who received gefitinib. The most common grade 3 or 4 adverse events were dermatitis acneiform (14% in the dacomitinib group vs. none in the gefitinib group), diarrhea (8% vs. 1%), and raised alanine aminotransferase (ALT) levels (1% vs. 8%). Serious treatment-related adverse events were more frequent in the dacomitinib group (9% vs. 4%). Permanent discontinuation of the study drug because of treatment-related adverse events occurred more often in the dacomitinib group (10% vs. 7%). Dose reductions were also more frequent in the dacomitinib group (66% vs. 8%).
The FDA approved dacomitinib for first-line treatment of patients with metastatic NSCLC with EGFR exon 19 deletion or exon 21 L858R substitution mutations as detected by an FDA-approved test.
Evidence (gefitinib):
- The study met its primary objective of demonstrating the superiority of gefitinib compared with the carboplatin-paclitaxel combination for PFS (HR progression or death , 0.74; 95% CI, 0.65–0.85; P < .001).
- The median PFS was 5.7 months in the gefitinib group and 5.8 months in the carboplatin-paclitaxel group.[ 53 ][ Level of evidence B1 ]
- Following the time that chemotherapy was discontinued and while gefitinib was continued, the PFS curves clearly separated and favored gefitinib.
- The 12-month PFS rates were 24.9% with the gefitinib group and 6.7% with the carboplatin-paclitaxel group.
- More than 90% of the patients in the trial with mutations had either del19 or exon 21 L858R mutations, which have been shown to be sensitive to EGFR inhibitors. In the subgroup of patients with a mutation, PFS was significantly longer among those who received gefitinib (HR, 0.48; 95% CI, 0.36–0.64; P < .001); however, in the subgroup of patients who were negative for a mutation, PFS was significantly longer in those who received the carboplatin-paclitaxel combination (HR with gefitinib, 2.85; 95% CI, 2.05–3.98; P < .001). There was a significant interaction between treatment and EGFR mutation with respect to PFS ( P < .001).[ 53 ]
- OS was similar for patients who received gefitinib and carboplatin-paclitaxel, with no significant difference between treatments overall (HR, 0.90; 95% CI, 0.79–1.02; P = .109) or in EGFR mutation–positive (HR, 1.00; 95% CI, 0.76–1.33; P = .990) or EGFR mutation–negative (HR, 1.18; 95% CI, 0.86–1.63; P = .309; treatment by EGFR mutation interaction P = .480) subgroups. A high proportion (64.3%) of EGFR mutation–positive patients randomly assigned to the carboplatin-paclitaxel regimen received subsequent EGFR TKIs. PFS was significantly longer with gefitinib for patients whose tumors had both high EGFR gene copy number and EGFR mutation (HR, 0.48; 95% CI, 0.34–0.67) but significantly shorter when high EGFR gene copy number was not accompanied by EGFR mutation (HR, 3.85; 95% CI, 2.09–7.09).
- In the planned interim analysis of data for the first 200 patients, PFS was significantly longer in the gefitinib group than in the standard-chemotherapy group (HR death or disease progression with gefitinib, 0.36; P < .001), resulting in early termination of the study.
- The gefitinib group had a significantly longer median PFS (10.8 months vs. 5.4 months in the chemotherapy group; HR, 0.30; 95% CI, 0.22–0.41; P < .001).[ 54 ][ Level of evidence B1 ] The median OS was 30.5 months in the gefitinib group and 23.6 months in the standard chemotherapy group ( P = .31).
- Patients were randomly assigned to receive either gefitinib or cisplatin plus docetaxel (administered every 21 days for three to six cycles). The primary end point was PFS.
- The gefitinib group had significantly longer PFS than the cisplatin-plus-docetaxel group, with a median PFS of 9.2 months (95% CI, 8.0–13.9) versus 6.3 months (range, 5.8–7.8 months; HR, 0.489; 95% CI, 0.336–0.710, log-rank P < .0001).[ 55 ][ Level of evidence B1 ]
Evidence (erlotinib):
- Median PFS was significantly longer in erlotinib-treated patients than in patients treated with chemotherapy (13.1 months [95% CI, 10.58–16.53] vs. 4.6 months [range, 4.21–5.42 months]; HR, 0.16; 95% CI, 0.10–0.26; P < .0001).[ 56 ][ Level of evidence B1 ]
- In an interim analysis of the first 153 patients, PFS in the chemotherapy arm was 5.2 months (95% CI, 4.5–5.8) compared with 9.7 months (95% CI, 8.4–12.3) in the erlotinib arm (HR, 0.37; P < .0001). Median survival was 19.3 months in patients in the chemotherapy arm and 19.5 months in patients in the erlotinib arm (HR, 0.80; P = .42).[ 58 ][ Level of evidence B1 ]
Evidence (afatinib):
- The primary end point was PFS. In this study, the afatinib group had significantly longer PFS than the cisplatin-plus-pemetrexed group, with a median PFS of 11.1 months for afatinib and 6.9 months for chemotherapy (HR, 0.58; 95% CI, 0.43–0.78; P = .001).[ 59 ][ Level of evidence B1 ]
- With a median follow-up of 41 months, median OS was 28.2 months in patients in both arms (HR, 0.88; 95% CI, 0.66–1.17; P = .39).
- In patients harboring common EGFR mutations (i.e., exon 19 deletion and L858R), survival did not differ significantly between treatment arms (HR, 0.78; 95% CI, 0.58–1.06; P = .11). However, prespecified subgroup analyses demonstrated a survival advantage with afatinib compared with chemotherapy in patients with tumors harboring the EGFR del19 mutation (median OS, 33.3 months vs. 21.1 months; HR, 0.54; 95% CI, 0.36–0.79; P = .0015) but no significant difference between treatment arms in patients with tumors harboring the L858R mutation (median OS, 27.6 months vs. 40.3 months; HR, 1.30; 95% CI, 0.80–2.11; P = .29).
- First-line afatinib was associated with a significant survival advantage compared with chemotherapy in patients with NSCLC-harboring EGFR del19 mutations but not in patients with EGFR L858R mutations or in the overall EGFR –mutation-positive patient population.[ 60 ][ Level of evidence A1 ]
- The primary end point was PFS. Median PFS was significantly longer in the afatinib group (11.0 months; 95% CI, 9.7–13.7) than in the gemcitabine and cisplatin group (5.6 months, [range, 5.1–6.7 months]; HR, 0.28; 95% CI, 0.20–0.39; P < .0001).[ 61 ][ Level of evidence B1 ]
- With a median follow-up of 33 months, median OS was 23.1 months in patients in the afatinib arm and 23.5 months in patients in the chemotherapy arm (HR, 0.93; 95% CI, 0.72–1.22; P = .61).
- In patients harboring common EGFR mutations (i.e., exon 19 deletion and L858R), survival did not differ significantly between treatment arms (HR, 0.83; 95% CI, 0.62–1.09; P = .18). However, prespecified subgroup analyses demonstrated a survival advantage with afatinib compared with chemotherapy in patients with tumors harboring the EGFR del19 mutation (median OS, 31.4 months vs. 18.4 months; HR, 0.64; 95% CI, 0.44–0.94; P = .023), but no significant difference between treatment arms was seen in patients with tumors harboring the L858R mutation (median OS, 19.6 months vs. 24.3 months; HR, 1.22; 95% CI, 0.81–1.83; P = .34).
- First-line afatinib was associated with a significant survival advantage compared with chemotherapy in patients with NSCLC-harboring EGFR del19 mutations but not in patients with EGFR L858R mutations or in the overall EGFR -mutation-positive patient population.[ 60 ][ Level of evidence A1 ]
EGFR-directed therapy (for patients with EGFR exon 20 insertion mutations)
Amivantamab.
Amivantamab has been previously approved for patients with locally advanced or metastatic NSCLC harboring EGFR exon 20 insertion mutations whose disease has progressed on or after platinum-based chemotherapy.
Evidence (amivantamab plus chemotherapy):
- At 18 months, the PFS rate was 31% for patients who received amivantamab plus chemotherapy, and 3% for patients who received chemotherapy alone.
- The objective response rate was higher in the amivantamab-plus-chemotherapy group (73%) than the chemotherapy-alone group (47%).
- In an interim OS analysis, there was no statistically significant difference.
- The most common adverse events with amivantamab plus chemotherapy were hematologic toxicities, rash, and paronychia. Infusion reactions occurred in 42% of patients.
The study supports amivantamab plus chemotherapy as an effective first-line treatment option for patients with NSCLC and EGFR exon 20 insertions based on superior PFS when compared with chemotherapy alone.[ 62 ]
ALK inhibitors (for patients with ALK translocations)
Evidence (alectinib):
- The rate of PFS was significantly higher with alectinib than crizotinib; the 12-month event-free survival rate was 68.4% for the alectinib group (95% CI, 61.0%–75.9%) compared with 48.7% for the crizotinib group (95% CI, 40.4%–56.9%) (HR, 0.47; 95% CI, 0.34–0.65; P < .001). The median PFS was not reached with alectinib. The results of independent review committee-assessed PFS were consistent.[ 63 ][ Level of evidence B1 ]
- CNS progression events were less frequent with alectinib (12%) than with crizotinib (45%) (HR, 0.16; 95% CI, 0.10–0.28; P <.001).
- The response rate was similar for both groups, 82.9% for the alectinib group compared with 75.5% for the crizotinib group ( P = .09).
- Grade 3 to 5 adverse events were less frequent with alectinib (41%) than with crizotinib (50%).
- At data cutoff for the second primary interim analysis, the independent data monitoring committee determined that the primary end point was met (HR, 0.34; 99.7% CI, 0.17–0.71; P <.0001) and recommended immediate release of the data. Median PFS had not been reached with alectinib but was reached at 10.2 months with crizotinib.
- Grade 3 or 4 adverse events occurred less frequently with alectinib (26% occurrence rate) than with crizotinib (52% occurrence rate).
Evidence (lorlatinib):
- A planned interim analysis was conducted after approximately 75% of expected events of disease progression or death had occurred. The percentage of patients alive without progression at 12 months was 78% (95% CI, 70%–84%) in the lorlatinib group and 39% (95% CI, 30%–48%) in the crizotinib group. Objective responses occurred in 76% of patients who received lorlatinib and 58% of patients who received crizotinib.
- Post hoc analysis showed improved PFS for the lorlatinib group, compared with the crizotinib group in patients with and without brain metastases at baseline (12-month PFS rates: 78% vs. 22% and 78% vs. 45%, respectively).[ 66 ]
- There was a lower 12-month cumulative incidence of CNS progression with lorlatinib, compared with crizotinib in patients with (7% vs. 72%) and without (1% vs. 18%) brain metastases at baseline.
- Lorlatinib was associated with more grade 3 to 4 adverse events than crizotinib (72% vs. 56%), the most common being altered lipid levels. Treatment discontinuation occurred in 7% of patients who received lorlatinib and 9% of patients who received crizotinib.[ 65 ]
- Updated safety data in the post hoc analysis showed that 35% of patients had CNS adverse events with lorlatinib, most of grade 1 severity. These included memory impairment and mood effects, including anxiety, depression, and lability. The occurrence of CNS adverse events did not result in a clinically meaningful difference in patient-reported quality of life. At analysis, 56% of CNS adverse effects had resolved (33% without intervention; 17% with lorlatinib dose modification), and 38% were unresolved. Lorlatinib dose modification did not notably influence PFS.
The FDA approved lorlatinib for patients with metastatic NSCLC whose tumors are ALK -positive, as detected by an FDA-approved test.
Evidence (crizotinib):
- The study met its primary end point and demonstrated that crizotinib is superior to chemotherapy in prolonging PFS (median, 10.9 months vs. 7.0 months; HR, 0.454; 95% CI, 0.346–0.596; P < .0001).[ 68 ][ Level of evidence B1 ]
Evidence (ceritinib):
- Median PFS, assessed by blinded independent review, was 16.6 months in the ceritinib group and 8.1 months in the chemotherapy group (HR, 0.55; 95% CI, 0.42–0.73; P < .00001).
- The median OS was not reached with ceritinib, and it was 26.2 months with chemotherapy (HR, 0.73; 95% CI, 0.50–1.08; P = .056).[ 69 ][ Level of evidence B1 ]
Evidence (brigatinib):
- The primary end point assessed by the investigators was objective response rate. The objective response rate was 45% (97.5% CI, 34%–56%) for patients who received the 90 mg dose and 54% (97.5% CI, 43%–65%) for patients who received the 180 mg dose.
- Median PFS was 9.2 months (95% CI, 7.4–15.6) for patients who received the 90 mg dose and 12.9 months (95% CI, 11.1–not reached) for patients who received the 180 mg dose.
- At data cutoff, the median DOR was 13.8 months (95% CI, 5.6–13.8) for patients who received the 90 mg dose and 11.1 months (95% CI, 9.2–13.8) for patients who received the 180 mg dose.[ 70 ][ Level of evidence B3 ]
- The CNS objective response rate in patients with measurable CNS lesions was 42% in patients who received 90 mg every day (n = 26) and 67% in patients who received 180 mg every day (n = 18).
- Common adverse events, which were mainly grade 1 or 2 and occurred in 27% to 38% of patients at the higher dose, were nausea, diarrhea, headache, and cough. A subset of pulmonary adverse events with early onset (median onset, day 2) occurred in 14 of 219 treated patients (all grades, 6%; grade ≥3, 3%); none occurred after escalation to 180 mg. These events included dyspnea, hypoxia, cough, pneumonia, or pneumonitis. They were managed with dose interruption. Seven of the 14 patients were successfully retreated with brigatinib.
- The FDA-approved dose of brigatinib is 90 mg every day for 7 days; if tolerated, the dose is increased to 180 mg every day.
BRAF V600E and MEK inhibitors (for patients with BRAF V600E mutations)
BRAF V600E mutations occur in 1% to 2% of lung adenocarcinomas.
Dabrafenib and trametinib
Evidence (dabrafenib and trametinib):
- The overall response rate was 64% (95% CI, 46%–79%). Six percent of patients had a complete response, and 58% of patients had a partial response.
- The median investigator-assessed PFS was 10.9 months (95% CI, 7.0–16.6). The estimated median DOR was 10.4 months (95% CI, 8.3–17.9). At data cutoff, 47% of patients had died, and the median OS was 24.6 months (95% CI, 12.3–not estimable).
- Sixty-nine percent of patients had at least one grade 3 or 4 adverse event, of which the most common were pyrexia, ALT increase, hypertension, or vomiting. Adverse events led to permanent discontinuation in 22% of patients, dose interruption or delay in 75% of patients, and dose reduction in 39% of patients.[ 71 ][ Level of evidence C3 ]
The FDA approved the combination of dabrafenib and trametinib in the treatment of patients with NSCLC whose tumors harbor BRAF V600E mutations as detected by an FDA-approved test.
ROS1 inhibitors (for patients with ROS1 rearrangements)
ROS1 rearrangements occur in approximately 1% of patients with NSCLC.[ 72 ] The FDA approved crizotinib and entrectinib for use in patients with NSCLC and ROS1 rearrangements, with the latter appearing to have greater activity against intracranial disease.
Entrectinib
The FDA approved entrectinib for treatment of patients with metastatic NSCLC whose tumors are ROS1 -positive, regardless of the number of previous systemic therapies.
Evidence (entrectinib):
Seventeen (32%) patients had received no previous systemic therapy, 23 (43%) had received one previous therapy, and 13 (25%) had received two or more lines of treatment. CNS disease was present in 23 (43%) patients at baseline. Thirty-one (59%) patients were never-smokers and 52 (98%) patients had adenocarcinoma histology.
- The objective response rate in 53 efficacy-evaluable patients was 77% (95% CI, 64%−88%). Six percent of patients had a complete response and 72% had a partial response. Among patients with CNS disease at baseline, the objective response rate was 74% (95% CI, 52%−90%) and all patients had a partial response. Among patients without CNS disease at baseline, the overall response rate was 80% (95% CI, 61%−92%) (10% had a complete response and 70% had a partial response).[ 73 ][ Level of evidence C3 ]
- The median DOR was 24.6 months (95% CI, 11.4−34.8) in efficacy-evaluable patients; 12.6 months (95% CI, 6.5−not estimable) in patients with baseline CNS disease, and 24.6 months (95% CI, 11.4−34.8) in those without CNS disease at baseline.
- Treatment-related adverse events were assessed in 134 patients in the safety-evaluable population. Grade 1 or 2 treatment-related adverse events were observed in 79 patients (59%). Grade 3 or 4 treatment-related adverse events were observed in 46 patients (34%). Fifteen patients (11%) had serious treatment-related adverse events. There were no treatment-related deaths.
- The median PFS was 19 months (95% CI, 12.2−36.6) in efficacy-evaluable patients; 13.6 months (95% CI, 4.5−not estimable) in patients with baseline CNS disease, and 26.3 months (95% CI, 15.7−36.6) in patients with no baseline CNS disease.
Crizotinib was approved for patients with metastatic NSCLC whose tumors are ROS1 -positive, regardless of the number of previous systemic therapies.
- The overall response rate was 72% (95% CI, 58%–84%). Six percent of patients had a complete response, 66% had a partial response, and 18% had stable disease as their best response.
- Median PFS was 19.2 months (95% CI, 14.4–not reached). The estimated DOR was 17.6 months (95% CI, 14.5–not reached).[ 74 ][ Level of evidence C3 ]
- The objective response rate was 71.7% (95% CI, 63.0%–79.3%). Response rates were similar, irrespective of the number of previous therapies. Complete responses occurred in 13.4% of patients, while 58.3% of patients had partial responses, and 16.5% of patients had stable disease as their best response.[ 75 ][ Level of evidence C3 ]
- Median PFS was 15.9 months (95% CI, 12.9–24). The DOR was 19.7 months (95% CI, 14.1–not reached).
- OS was 32.5 months (95% CI, 32.5–not reached).
NTRK inhibitors (for patients with NTRK fusions)
Somatic gene fusions in NTRK occur across a range of solid tumors including in fewer than 0.5% of NSCLC tumors.[ 76 , 77 ] These fusions appear to occur more frequently in nonsmokers with lung adenocarcinoma.
Larotrectinib
Evidence (larotrectinib):
- The objective response rate was 75% (95% CI, 61%‒75%) and 73% of these responses lasted at least 6 months.[ 78 ][ Level of evidence C3 ]
- Treatment was well tolerated with 93% of adverse events being grade 1 to 2; the most common grade 3 to 4 adverse events were anemia (11% of patients), transaminitis (7%), and neutropenia (7%).
The FDA approved larotrectinib for the treatment of patients who have locally advanced or metastatic tumors that harbor an NTRK gene fusion without a known acquired resistance mutation, and who have no satisfactory alternative treatments or whose cancer has progressed following treatment.
The FDA granted accelerated approval to entrectinib for the treatment of solid tumors that have an NTRK gene fusion without a known acquired resistance mutation, are metastatic, have progressed after treatment, have no satisfactory alternative therapy, or for cases in which surgical resection is likely to result in severe morbidity.
Of 54 patients in the NTRK gene fusion-positive efficacy-evaluable population, 20 (37%) had received no previous systemic therapy, 11 (20%) had received one previous systemic therapy, and 23 (43%) had received two or more systemic therapies. Twelve (22%) patients had CNS disease at baseline. Ten (19%) patients had NSCLC. Fifty-two (96%) patients had an NTRK gene fusion detected by NGS and 2 (4%) had an NTRK gene fusion detected by other nucleic acid–based tests.
- The objective response rate in 54 patients was 57% (95% CI, 43.2%−70.8%). Seven percent of patients had a complete response and 50% had a partial response. In patients with baseline CNS disease, 50% achieved a response (all partial responses), whereas in patients without baseline CNS disease, 60% achieved a response (10% complete response; 50% partial response).[ 79 ][ Level of evidence C3 ]
- The median DOR in efficacy-evaluable patients was 10.4 months (95% CI, 7.1−not estimable). In patients with baseline CNS disease DOR was not estimable, and in patients with no baseline CNS disease it was 12.9 months (95% CI, 7.1−not estimable).
- Among 10 patients with NSCLC, the response rate was 70% (95% CI, 35%−93%) and DOR ranged between 1.9 months and 20.1 months. For more information, see the prescribing information .
- The safety-evaluable population consisted of 68 patients with NTRK fusion-positive tumors. Most treatment-related adverse events were grade 1 or 2 and reversible. The most frequent grade 3 or 4 treatment-related adverse events were increased weight gain (10%) and anemia (12%). Serious treatment-related adverse events were reported in 7 (10%) patients. Three (4%) patients had dose interruptions and 27 (40%) patients had dose reductions due to treatment-related adverse events. There were no treatment-related deaths.
- Median PFS was 11.2 months (95% CI, 8.0−14.9). In patients with baseline CNS disease, median PFS was 7.7 months (95% CI, 4.7−not estimable), and it was 12 months (95% CI, 8.7−15.7) in patients with no baseline CNS disease.
RET inhibitors (for patients with RET fusions)
Somatic gene fusions of RET occur in 1% to 2% of patients with NSCLC and in patients with thyroid cancer.[ 80 ]
Selpercatinib
Evidence (selpercatinib):
- Updated analysis was conducted in 316 patients with RET fusion–positive NSCLC.[ 81 ]
- Among the 69 treatment-naïve patients, the objective response rate was 84% (95% CI, 73%–92%), and 6% achieved complete responses. The median DOR was 20.2 months (95% CI, 13.0–could not be evaluated); 40% of responses were ongoing at the data cutoff (median follow-up, 20.3 months). The median PFS was 22.0 months; 35% of patients were alive and progression-free at the data cutoff (median follow-up, 21.9 months).
- Among the 247 patients who had received prior platinum-based chemotherapy, the objective response rate was 61% (95% CI, 55%–67%), and 7% achieved complete responses. The median DOR was 28.6 months (95% CI, 20.4–could not be evaluated); 49% of responses were ongoing (median follow-up, 21.2 months). The median PFS was 24.9 months; 38% of patients were alive and progression-free at the data cutoff (median follow-up, 24.7 months).
- Among the 26 patients with measurable baseline CNS metastasis by the independent review committee, the intracranial objective response rate was 85% (95% CI, 65%–96%), and 27% had complete responses.
- In the full safety population (n = 796), the median treatment duration was 36.1 months.
- There was no significant change in the safety profile. Most adverse events were grade 1 to 2. The most common adverse events were edema, diarrhea, fatigue, dry mouth, hypertension, increased ALT and aspartate aminotransferase (AST), and rash.
The FDA approved selpercatinib to treat adults with locally advanced or metastatic NSCLC with RET gene fusion, as detected by an FDA-approved test.
Pralsetinib
Evidence (pralsetinib):
- Ninety-two patients who had received platinum-based chemotherapy and 29 patients who were treatment-naive (and not candidates for standard platinum-based treatment) received pralsetinib before the efficacy enrollment cutoff (July 11, 2019). Eighty-seven previously treated patients and 27 treatment-naive patients had centrally adjudicated baseline measurable disease, and thus formed the efficacy cohort.
- The overall response rate was 61% (95% CI, 50%–71%) in the 87 patients who had received platinum-based chemotherapy, including complete responses in 6%. The median DOR was not reached (15.2 months–not estimable).
- The overall response rate was 70% (95% CI, 50%–86%) in the 27 treatment-naive patients, including complete responses in 11%. The median DOR was 9.0 months (6.3–not estimable).
- In the 233-patient safety cohort, 93% had treatment-related adverse events, including 48% with grade 3 or worse events. The most common grade 3 or worse treatment-related adverse events were neutropenia (18%), hypertension (11%), and anemia (10%). Dose reductions occurred in 38% of patients, and 6% discontinued treatment because of adverse events.
MET inhibitors (for patients with MET exon 14 skipping mutations)
Dysregulation of the MET proto-oncogene resulting from disruption of distinct splice sites leads to loss of MET exon 14 and enhanced MET signaling. These MET alterations drive tumor proliferation, survival, invasion, and metastasis, and occur in 3% to 4% of patients with NSCLC.[ 83 ]
Evidence (tepotinib):
- Among the 99 patients who had been followed for at least 9 months (i.e., the efficacy population), the objective response rate as assessed by independent review was 46% (95% CI, 36%–57%), with a median DOR of 11.1 months (95% CI, 7.2–not estimable). Response rates were similar in the liquid biopsy and tissue biopsy groups.
- Responses were similar regardless of prior therapy.
- Grade 3 or higher adverse events occurred in 28% of patients, including peripheral edema in 7% of patients. Adverse events led to therapy discontinuation in 11% of patients.
Evidence (capmatinib):
- Of the 69 patients with MET exon 14 skipping mutations who had received one or two prior lines of therapy, the overall response rate was 41% (95% CI, 29%–53%). The median DOR was 9.7 months (95% CI, 5.6–13.0).
- Of the 28 patients with MET exon 14 skipping mutations who had not received any prior treatment, the overall response rate was 68% (95% CI, 48%–84%). The median DOR was 12.6 months (95% CI, 5.6–not estimable).
- Response rates in patients with MET amplification without the exon 14 skipping mutation did not meet the prespecified threshold for clinically relevant activity.
- Grade 3 to 4 adverse events of occurred in 67% of patients. The most common events, regardless of causality, were peripheral edema, nausea, vomiting, and increased creatinine. Adverse events led to therapy discontinuation 11% of patients.
Immune checkpoint inhibitors with or without chemotherapy
Pembrolizumab is a humanized monoclonal antibody that inhibits the interaction between the programmed death protein 1 (PD-1) coinhibitory immune checkpoint expressed on tumor cells and infiltrating immune cells and its ligands, PD-L1 and PD-L2.[ 86 ]
Pembrolizumab plus chemotherapy
Evidence (pembrolizumab plus chemotherapy):
- In the 5-year updated analysis, the median time from random assignment to data cutoff was 64.6 months (range, 60.1–72.4).[ 87 ]
- After 5 years, pembrolizumab plus pemetrexed-platinum was associated with improved OS and PFS, compared with placebo plus pemetrexed-platinum in patients with metastatic nonsquamous NSCLC, regardless of PD-L1 expression. In the ITT population, 5-year OS rates were 19.4% in the pembrolizumab plus pemetrexed-platinum group, compared with 11.3% in the placebo plus pemetrexed-platinum group.
- Survival was higher in patients with a higher PD-L1 tumor proportion score (TPS), especially in the TPS >50% subgroup (29.6% vs. 21.4%).
- There were 57 patients who completed 35 cycles of pembrolizumab. For these patients, the objective response rate was 86.0% and the 3-year OS rate after completing 35 cycles (approximately 5 years after random assignment) was 71.9%.[ 87 ]
- Immune-mediated adverse events and infusion reactions occurred in 113 (27.9%) and 27 (13.4%) patients.
- Adverse events of grade 3 or higher occurred with similar frequency in both treatment groups (71.9% in the pembrolizumab combination group vs. 66.8% in the placebo combination group).
- The median time from random assignment to data cutoff was 56.9 months (range, 49.9–66.2). OS and PFS were improved with pembrolizumab-plus-chemotherapy versus placebo-plus-chemotherapy (HR, 0.71 [0.59–0.85] and 0.62 [0.52–0.74]), respectively; 95% CI). The 5-year OS rates were 18.4% and 9.7%, respectively.[ 88 ][ Level of evidence A1 ]
- A total of 55 patients completed 35 cycles of pembrolizumab. The objective response rate was 90.9% and the 3-year OS rate after completion of 35 cycles (approximately 5 years after random assignment) was 69.5%.
Pembrolizumab alone
Evidence (pembrolizumab alone):
- PD-L1 expression was centrally assessed using the PD-L1 immunohistochemistry 22C3 pharmDx assay. PD-L1 tumor expression of 50% or more was found in 30.2% of 1,653 patient samples that were examined.
- Pembrolizumab demonstrated significant improvement in median PFS (10.3 months vs. 6.0 months; HR, 0.50; 95% CI, 0.37–0.68; P < .001). The overall response rate (44.8% vs. 27.8%), the median DOR (not reached, [range, 1.9–14.5 months] vs. 6.3 months [range, 2.1–12.6 months]), and the estimated rate of OS at 6 months (80.2% vs. 72.4%; HR, 0.60; 95% CI, 0.41–0.89; P = .005) were all higher with pembrolizumab than with chemotherapy.
- Further follow-up of this study confirmed an OS advantage in favor of pembrolizumab; the median OS for patients who received pembrolizumab was 30 months (95% CI, 18.3 months–not reached) versus 14.2 months for patients who received chemotherapy, with a 75% crossover to immunotherapy afterwards, suggesting the crossover did not impact survival.[ 89 ]
- Grade 3 to 5 adverse events occurred in 26.6% of patients treated with pembrolizumab and 53.3% of patients treated with chemotherapy.
- Grade 3 or 4 immune-related events occurred in 9.7% of patients treated with pembrolizumab and 0.7% of patients treated with chemotherapy.
- The most common grade 3 or 4 immune-related events associated with pembrolizumab were severe skin reactions (3.9%), pneumonitis (2.6%), and colitis (1.3%).
- There were no grade 5 immune-related events.
- Pembrolizumab treatment demonstrated significant improvement in PFS, OS, and DOR with less frequent adverse events compared with chemotherapy treatment.[ 86 ][ Level of evidence B1 ]
- The median follow-up was 61.1 months (range, 50.0–76.3).
- The HR was 0.68 (95% CI, 0.57–0.81) for the TPS >50% group, 0.75 (95% CI, 0.64–0.87) for the TPS >20% group, and 0.79 (95% CI, 0.70–0.89) for the TPS >1% group.
- The OS rates for patients who received pembrolizumab were 21.9% (TPS >50%), 19.4% (TPS >1%), and 16.6% (TPS >1%).
- The most common adverse reactions reported in at least 10% of patients who received pembrolizumab as a single agent in KEYNOTE-042 included fatigue, decreased appetite, dyspnea, cough, rash, constipation, diarrhea, nausea, hypothyroidism, pneumonia, pyrexia, and weight loss.
The FDA approved pembrolizumab in combination with pemetrexed and carboplatin as first-line treatment of patients with metastatic nonsquamous NSCLC, regardless of PD-L1 expression. The FDA also approved pembrolizumab as a first-line monotherapy for patients with NSCLC whose tumors express PD-L1 (>1%) (staining as determined by an FDA-approved test). Patients with EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapies before receiving pembrolizumab (see the FDA label for pembrolizumab ).
Cemiplimab-rwlc plus chemotherapy
Evidence (cemiplimab-rwlc plus chemotherapy):
- The median OS was 21.9 months (95% CI, 15.5–not evaluable [NE]) in the cemiplimab-rwlc-plus-chemotherapy group and 13 months (95% CI, 11.9–16.1) in the placebo-plus-chemotherapy group (HR, 0.71; 95% CI, 0.53–0.93; P = .014).
- The secondary end point of median PFS was 8.2 months (95% CI, 6.4–9.3) in the cemiplimab-rwlc-plus-chemotherapy group and 5.0 months (95% CI, 4.3–6.2) in the placebo-plus-chemotherapy group (HR, 0.56; 95% CI, 0.44–0.70; P < .0001).
- Another secondary end point, the estimated proportion of patients alive at 12 months, was 65.7% (95% CI, 59.9%–70.9%) in the cemiplimab-rwlc-plus-chemotherapy group and 56.1% (95% CI, 47.5%–63.8%) in the placebo-plus-chemotherapy group.
- Grade 3 or greater adverse events occurred in 43.6% (136 of 312) of patients who received cemiplimab-rwlc plus chemotherapy and 31.4% (48 of 153) of patients who received placebo plus chemotherapy.
- The most common (≥15%) adverse reactions were alopecia, musculoskeletal pain, nausea, fatigue, peripheral neuropathy, and decreased appetite.
The FDA approved cemiplimab-rwlc in combination with platinum-based chemotherapy for adult patients with advanced NSCLC and no EGFR , ALK , or ROS1 aberrations.
Cemiplimab-rwlc alone
Evidence (cemiplimab-rwlc alone):
- At 35 months of follow-up, in patients with PD-L1 expression ≥50%, the median OS was 26.1 months with cemiplimab-rwlc and 13.3 months with chemotherapy (HR, 0.57; P < .0001).
- The median PFS was 8.1 months for patients who received cemiplimab-rwlc and 5.3 months for patients who received chemotherapy (HR, 0.51; P < .0001).
- The objective response rate was 46% for patients who received cemiplimab-rwlc and 21% for patients who received chemotherapy (OR, 3.264; P < .0001).
- Benefits were greater in patients with PD-L1 expression ≥90% versus 50% to 89%.
- Among 64 patients who received cemiplimab-rwlc plus chemotherapy after initial progression on cemiplimab-rwlc alone, the median PFS was 6.6 months, the objective response rate was 31%, and the OS was 15.1 months.
- The most common adverse reactions (>10%) with cemiplimab-rwlc were musculoskeletal pain, rash, anemia, fatigue, decreased appetite, pneumonia, and cough. The safety profile of cemiplimab-rwlc was consistent over longer follow-up.
The FDA approved cemiplimab-rwlc for patients with advanced NSCLC (locally advanced who are not candidates for surgical resection or definitive chemoradiation or metastatic) and PD-L1 tumor expression of at least 50% with no EGFR , anaplastic ALK , or ROS1 genomic aberrations.
Tremelimumab
Tremelimumab is a fully human monoclonal antibody against cytotoxic T-lymphocyte associated antigen 4 (CTLA-4). It is an immune checkpoint blocker.
Durvalumab plus tremelimumab plus chemotherapy
Evidence (durvalumab plus tremelimumab plus chemotherapy):
- Arm 1: Tremelimumab 75 mg plus durvalumab 1,500 mg with platinum-based chemotherapy for up to four 21-day cycles followed by durvalumab once every 4 weeks until progression and one additional tremelimumab dose at week 16.
- Arm 2: Durvalumab plus chemotherapy for up to four 21-day cycles followed by durvalumab once every 4 weeks until progression.
- Arm 3: Platinum-based chemotherapy for up to six 21-day cycles (with or without maintenance pemetrexed).
The following results were observed:
- Treatment with durvalumab significantly improved PFS compared with chemotherapy alone. The median PFS was 5.5 months for patients who received durvalumab plus chemotherapy and 4.8 months for patients who received chemotherapy alone (HR, 0.74; 95% CI, 0.62–0.89; P = .0009).
- A trend for improved OS did not reach statistical significance for patients in arms 2 and 3. The median OS was 13.3 months for patients who received durvalumab plus chemotherapy and 11.7 months for patients who received chemotherapy alone. (HR, 0.86; 95% CI, 0.72–1.02; P = .0758). The 24-month OS rate was 29.6% in the durvalumab-plus-chemotherapy arm and 22.1% in the chemotherapy-alone arm.
- Both PFS and OS were significantly improved when tremelimumab therapy was added to durvalumab and chemotherapy compared with chemotherapy alone. The median PFS was 6.2 months for patients who received tremelimumab plus durvalumab and chemotherapy and 4.8 months for patients who received chemotherapy alone (HR, 0.72; 95% CI, 0.60–0.86; P = .0003). The median OS was 14.0 months in the tremelimumab arm and 11.7 months in the chemotherapy-alone arm (HR, 0.77; 95% CI, 0.65–0.92; P = .0030). The 24-month OS rate was 32.9% in the tremelimumab-plus durvalumab-and-chemotherapy arm and 22.1% in the chemotherapy-alone arm. In addition, this combination demonstrated consistent OS results across levels of PD-L1 expression.
- Grade 3 to 4 treatment-related events occurred in 51.8% of patients who received tremelimumab plus durvalumab and chemotherapy, 14.1% of patients who received durvalumab and chemotherapy, and 9.9% of patients who received chemotherapy alone.
The FDA approved tremelimumab in combination with durvalumab and platinum-based chemotherapy for adult patients with metastatic NSCLC with no sensitizing EGFR or ALK genomic tumor aberrations. The approval is based on a comparison of treatment arms one and three.
Atezolizumab alone
Evidence (atezolizumab alone):
- PD-L1 expression was assessed by the SP142 immunohistochemical assay. High expression was defined as more than 50% of tumor cells or more than 10% of tumor-infiltrating immune cells expressing PD-L1.
- In the 205 patients with high PD-L1 expression, the median OS was 20.2 months for patients who received atezolizumab and 13.1 months for patients who received chemotherapy (HR death , 0.59; P = .01).
- Grade 3 to 4 adverse events occurred in 30.1% of patients who received atezolizumab and 52.5% of patients who received chemotherapy.
Atezolizumab monotherapy is approved for first-line treatment of patients with high PD-L1 expression (PD-L1 staining ≥50% of tumor cells or PD-L1 stained tumor-infiltrating immune cells covering ≥10% of the tumor area), as determined by an FDA-approved test, in the absence of EGFR or ALK genomic aberrations.
Atezolizumab plus chemotherapy
Evidence (atezolizumab in combination with carboplatin and nab-paclitaxel chemotherapy):
- In the ITT wild-type population, the median OS was 18.6 months (95% CI, 16.0–21.2) in the atezolizumab-plus-chemotherapy group and 13.9 months (95% CI, 12.0–18.7) in the chemotherapy group (stratified HR, 0.79; 95% CI, 0.64–0.98; P = .033).
- The median PFS was 7 months (95% CI, 6.2–7.3) in the atezolizumab-plus-chemotherapy group and 5.5 months (95% CI, 4.4–5.9) in the chemotherapy group (stratified HR, 0.64; 95% CI, 0.54–0.77; P < .0001).
- Subgroup analyses showed OS and PFS benefit with atezolizumab across several clinical subgroups, with the exception of patients with liver metastases where the additional of atezolizumab did not improve OS versus chemotherapy alone, and for patients with EGFR and ALK genomic alterations.
- OS and PFS benefit with atezolizumab was also observed in the ITT wild-type population independent of PD-L1 expression.
- Grade 3 or 4 adverse events occurred in 81% of patients who received atezolizumab plus chemotherapy versus 71% of patients who received chemotherapy alone. Immune-related adverse events occurred in 45% of patients treated with atezolizumab plus chemotherapy and most were grade 1 or 2 in severity. The most common immune-related adverse events were rash (24%), hypothyroidism (15%), and hepatitis (10%).
Atezolizumab in combination with nab-paclitaxel and carboplatin is approved for the first-line treatment of patients with metastatic nonsquamous NSCLC with no EGFR or ALK genomic aberrations.
Atezolizumab plus bevacizumab plus chemotherapy
Evidence (atezolizumab in combination with carboplatin, paclitaxel, and bevacizumab):
- Median PFS was longer in the ABCP group (8.3 months) than the BCP group (6.8 months) (HR, 0.62; 95% CI, 0.52–0.74; P < .001). In the Teff-high wild-type population, PFS was 11.3 months versus 6.8 months (HR, 0.51; 95% CI, 0.38–0.68; P .001). PFS was also longer in the ABCP group versus the BCP group in the ITT population with EGFR and ALK genomic alterations, among patients with low or negative PD-L1 expression, low Teff gene-signature expression, and in patients with liver metastases.
- Median OS among patients with wild-type tumors was longer in the ABCP group (19.2 months), compared with the BCP group (14.7 months) (HR, 0.78; 95% CI, 0.64–0.96; P = .02).
- Grade 3 or 4 treatment-related adverse events occurred in 56% of patients in the ABCP group versus 48% of patients in the BCP group. Most immune-related adverse events in the ABCP group were grade 1 or 2, and rash, hypothyroidism, hyperthyroidism, hepatitis, pneumonitis, and colitis were most common. Treatment-related deaths occurred in 11 patients (2.8%) in the ABCP group and 9 patients (2.3%) in the BCP group. Five deaths in the ABCP group were caused by pulmonary hemorrhage or hemoptysis, and four of five occurred in patients with high-risk features, including tumors infiltrating great vessels or tumor cavitation.
Atezolizumab in combination with bevacizumab, paclitaxel, and carboplatin is approved for the first-line treatment of patients with metastatic nonsquamous NSCLC with no EGFR or ALK genomic aberrations.
Nivolumab plus ipilimumab
Nivolumab, a fully human anti–PD-1 antibody, and ipilimumab, a fully human anti–CTLA-4 antibody, are immune checkpoint inhibitors with distinct but complementary mechanisms of action.[ 98 ]
Evidence (nivolumab plus ipilimumab):
- Among patients with tumor PD-L1 ≥1% (n = 1,189), the median OS was 17.1 months (95% CI, 15.0–20.2) with nivolumab-plus-ipilimumab and 14.9 months (95% CI, 12.7–16.7) with chemotherapy (HR, 0.77; 95% CI, 0.66–0.91; P = .007). Five-year outcomes with nivolumab-plus-ipilimumab versus chemotherapy showed durable clinical benefit, with an OS rate of 24% with nivolumab-plus-ipilimumab and 14% for chemotherapy alone.[ 98 ]
- In patients with TMB-high NSCLC, the median PFS was 7.2 months (95% CI, 5.5–13.2) with nivolumab-plus-ipilimumab, versus 5.5 months (95% CI, 4.4–5.8) with chemotherapy alone (HR, 0.58; 97.5% CI, 0.41–0.81; P < .001).
- Among patients with tumor PD-L1 <1% (n = 550), the median OS was 17.4 months (95% CI, 13.2–22.0) with nivolumab-plus-ipilimumab, and 12.2 months (95% CI, 9.2–14.3) with chemotherapy alone (HR, 0.65; 95% CI, 0.52–0.81). Five-year outcomes with nivolumab-plus-ipilimumab versus chemotherapy alone showed durable clinical benefit, with an OS rate of 19% for nivolumab-plus-ipilimumab and 7% for chemotherapy alone.[ 98 ]
- The frequency of grade 3 to 4 treatment-related adverse events was similar in both groups (32.8% with nivolumab-plus-ipilimumab vs. 36.0% with chemotherapy alone). Treatment-related adverse events leading to therapy discontinuation were more common with nivolumab-plus-ipilimumab than with chemotherapy alone (24.5% vs. 13.9%).
- Treatment-related deaths occurred in eight patients who received nivolumab-plus-ipilimumab (pneumonitis in four patients; shock, myocarditis, acute tubular necrosis, and cardiac tamponade in one patient each) and in six patients who received chemotherapy (sepsis in two patients; febrile neutropenia, multifocal brain infarctions, interstitial lung disease, and thrombocytopenia in one patient each).
The FDA approved nivolumab-plus-ipilimumab as first-line therapy for patients with advanced NSCLC with PD-L1 expression of at least 1% and no EGFR or ALK genomic aberrations. While this regimen is not FDA-approved for patients with PD-L1 expression less than 1%, these patients were noted to have durable clinical benefit in CheckMate 227.
mTOR inhibitors
Everolimus is used for patients with unresectable, locally advanced or metastatic, progressive, well-differentiated, nonfunctional, neuroendocrine tumors.
Everolimus, an oral mTOR inhibitor, is clinically active against advanced pancreatic and nonpancreatic neuroendocrine tumors.[ 100 ] Based on the results of the RADIANT-4 clinical trial,[ 100 ] the FDA approved everolimus for the treatment of adult patients with unresectable, locally advanced or metastatic, progressive, well-differentiated (low or intermediate grade), nonfunctional neuroendocrine tumors of lung or gastrointestinal origin.
Evidence (everolimus):
- Median PFS was 11.0 months in the everolimus arm and 3.9 months in the placebo group (HR, 0.48; 95% CI, 0.35−0.67; P < .00001).[ 100 ][ Level of evidence B1 ]
- In a post hoc analysis of the lung subgroup, median PFS by central review was 9.2 months in the everolimus arm and 3.6 months in the placebo arm (HR, 0.50; 95% CI, 0.28−0.88).[ 100 ]
- The objective response rate was 2% in patients who received everolimus and 1% in patients who received placebo. Disease stabilization was observed in 81% of patients in the everolimus arm and 64% of patients in the placebo arm.
- The median duration of treatment was longer in the patients who received everolimus compared with those who received placebo (40.4 weeks vs. 19.6 weeks).
- A planned interim analysis of OS showed a 36% reduction in the estimated risk of death with everolimus relative to placebo (HR, 0.64; 95% CI, 0.40−1.05). These results were not statistically significant.
- The most common treatment-related adverse events were stomatitis, diarrhea, fatigue, infections, rash, and peripheral edema. The most common drug-related grade 3 or 4 adverse events were stomatitis, diarrhea, infections, anemia, and fatigue. Grade 3 or 4 adverse events resulted in treatment discontinuation in 12% of patients in the everolimus group and 3% of patients in the placebo group.
Local therapies and special considerations
Endobronchial laser therapy and/or brachytherapy (for obstruction lesions).
Radiation therapy may be effective in palliating symptomatic patients with local involvement of NSCLC with any of the following:
In some cases, endobronchial laser therapy and/or brachytherapy have been used to alleviate proximal obstructing lesions.[ 20 ]
EBRT (primarily for palliation of local symptomatic tumor growth)
Although EBRT is frequently prescribed for symptom palliation, there is no consensus on which fractionation scheme should be used. Although different multifraction regimens appear to provide similar symptom relief,[ 101 - 106 ] single-fraction radiation may be insufficient for symptom relief compared with hypofractionated or standard regimens, as evidenced in the NCT00003685 trial.[ 21 ][ Level of evidence A3 ] Evidence of a modest increase in survival in patients with a better performance status given high-dose radiation therapy is available.[ 23 , 107 ][ Level of evidence A1 ] In closely observed asymptomatic patients, treatment may often be appropriately deferred until symptoms or signs of a progressive tumor develop.
- Better overall symptom palliation and fewer re-treatments were required in previously untreated patients using EBRT alone.[ 108 ][ Level of evidence A3 ]
Treatment of second primary tumor
A solitary pulmonary metastasis from an initially resected bronchogenic carcinoma is unusual. The lung is frequently the site of second primary malignancies in patients with primary lung cancers. Whether the new lesion is a new primary cancer or a metastasis may be difficult to determine. Studies have indicated that in most patients the new lesion is a second primary tumor, and after its resection, some patients may achieve long-term survival. Thus, if the first primary tumor has been controlled, the second primary tumor should be resected, if possible.[ 109 , 110 ]
Treatment of brain metastases
Patients who present with a solitary cerebral metastasis after resection of a primary NSCLC lesion and who have no evidence of extracranial tumor can achieve prolonged disease-free survival with surgical excision of the brain metastasis and postoperative whole-brain radiation therapy.[ 111 , 112 ] Unresectable brain metastases in this setting may be treated with stereotactic radiosurgery.[ 113 ]
Approximately 50% of patients treated with resection and postoperative radiation therapy will develop recurrence in the brain; some of these patients will be suitable for additional treatment.[ 114 ] In those selected patients with good performance status and without progressive metastases outside of the brain, treatment options include reoperation or stereotactic radiation surgery.[ 113 , 114 ] For most patients, additional radiation therapy can be considered; however, the palliative benefit of this treatment is limited.[ 115 ][ Level of evidence C2 ]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al.: Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 378 (22): 2078-2092, 2018. [PUBMED Abstract]
- Hellmann MD, Paz-Ares L, Bernabe Caro R, et al.: Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med 381 (21): 2020-2031, 2019. [PUBMED Abstract]
- Weick JK, Crowley J, Natale RB, et al.: A randomized trial of five cisplatin-containing treatments in patients with metastatic non-small-cell lung cancer: a Southwest Oncology Group study. J Clin Oncol 9 (7): 1157-62, 1991. [PUBMED Abstract]
- Scagliotti GV, Parikh P, von Pawel J, et al.: Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 26 (21): 3543-51, 2008. [PUBMED Abstract]
- Langer CJ, Vangel M, Schiller J, et al.: Age-specific subanalysis of ECOG 1594: fit elderly patients (70-80 YRS) with NSCLC do as well as younger pts (<70). [Abstract] Proceedings of the American Society of Clinical Oncology 22: A-2571, 2003.
- Langer CJ, Manola J, Bernardo P, et al.: Cisplatin-based therapy for elderly patients with advanced non-small-cell lung cancer: implications of Eastern Cooperative Oncology Group 5592, a randomized trial. J Natl Cancer Inst 94 (3): 173-81, 2002. [PUBMED Abstract]
- Ries LA: Influence of extent of disease, histology, and demographic factors on lung cancer survival in the SEER population-based data. Semin Surg Oncol 10 (1): 21-30, 1994 Jan-Feb. [PUBMED Abstract]
- Ramsey SD, Howlader N, Etzioni RD, et al.: Chemotherapy use, outcomes, and costs for older persons with advanced non-small-cell lung cancer: evidence from surveillance, epidemiology and end results-Medicare. J Clin Oncol 22 (24): 4971-8, 2004. [PUBMED Abstract]
- Effects of vinorelbine on quality of life and survival of elderly patients with advanced non-small-cell lung cancer. The Elderly Lung Cancer Vinorelbine Italian Study Group. J Natl Cancer Inst 91 (1): 66-72, 1999. [PUBMED Abstract]
- Takeda K, Kudoh S, Nakagawa K, et al.: Randomized phase III study of docetaxel (D) versus vinorelbine (V) for elderly patients (pts) with advanced non-small cell lung cancer (NSCLC): Results of a West Japan Thoracic Oncology Group trial (WJTOG9904). [Abstract] J Clin Oncol 23 (Suppl 16): A-7009, 2005.
- Schiller JH, Harrington D, Belani CP, et al.: Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346 (2): 92-8, 2002. [PUBMED Abstract]
- Belani CP, Fossella F: Elderly subgroup analysis of a randomized phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for first-line treatment of advanced nonsmall cell lung carcinoma (TAX 326). Cancer 104 (12): 2766-74, 2005. [PUBMED Abstract]
- Lilenbaum RC, Herndon JE, List MA, et al.: Single-agent versus combination chemotherapy in advanced non-small-cell lung cancer: the cancer and leukemia group B (study 9730). J Clin Oncol 23 (1): 190-6, 2005. [PUBMED Abstract]
- Hensing TA, Peterman AH, Schell MJ, et al.: The impact of age on toxicity, response rate, quality of life, and survival in patients with advanced, Stage IIIB or IV nonsmall cell lung carcinoma treated with carboplatin and paclitaxel. Cancer 98 (4): 779-88, 2003. [PUBMED Abstract]
- Sweeney CJ, Zhu J, Sandler AB, et al.: Outcome of patients with a performance status of 2 in Eastern Cooperative Oncology Group Study E1594: a Phase II trial in patients with metastatic nonsmall cell lung carcinoma . Cancer 92 (10): 2639-47, 2001. [PUBMED Abstract]
- Zukin M, Barrios CH, Pereira JR, et al.: Randomized phase III trial of single-agent pemetrexed versus carboplatin and pemetrexed in patients with advanced non-small-cell lung cancer and Eastern Cooperative Oncology Group performance status of 2. J Clin Oncol 31 (23): 2849-53, 2013. [PUBMED Abstract]
- Tester WJ, Stephenson P, Langer CJ, et al.: ECOG 1599: randomized phase II study of paclitaxel/carboplatin or gemcitabine/cisplatin in performance status (PS) 2 patients with advanced non-small cell lung cancer (NSCLC). [Abstract] J Clin Oncol 22 (Suppl 14): A-7055, 630s, 2004.
- Vansteenkiste JF, Vandebroek JE, Nackaerts KL, et al.: Clinical-benefit response in advanced non-small-cell lung cancer: A multicentre prospective randomised phase III study of single agent gemcitabine versus cisplatin-vindesine. Ann Oncol 12 (9): 1221-30, 2001. [PUBMED Abstract]
- Hickish TF, Smith IE, O'Brien ME, et al.: Clinical benefit from palliative chemotherapy in non-small-cell lung cancer extends to the elderly and those with poor prognostic factors. Br J Cancer 78 (1): 28-33, 1998. [PUBMED Abstract]
- Macbeth F, Toy E, Coles B, et al.: Palliative radiotherapy regimens for non-small cell lung cancer. Cochrane Database Syst Rev (3): CD002143, 2001. [PUBMED Abstract]
- Delbaldo C, Michiels S, Rolland E, et al.: Second or third additional chemotherapy drug for non-small cell lung cancer in patients with advanced disease. Cochrane Database Syst Rev (4): CD004569, 2007. [PUBMED Abstract]
- Ardizzoni A, Boni L, Tiseo M, et al.: Cisplatin- versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: an individual patient data meta-analysis. J Natl Cancer Inst 99 (11): 847-57, 2007. [PUBMED Abstract]
- Jiang J, Liang X, Zhou X, et al.: A meta-analysis of randomized controlled trials comparing carboplatin-based to cisplatin-based chemotherapy in advanced non-small cell lung cancer. Lung Cancer 57 (3): 348-58, 2007. [PUBMED Abstract]
- Hotta K, Matsuo K, Ueoka H, et al.: Meta-analysis of randomized clinical trials comparing Cisplatin to Carboplatin in patients with advanced non-small-cell lung cancer. J Clin Oncol 22 (19): 3852-9, 2004. [PUBMED Abstract]
- D'Addario G, Pintilie M, Leighl NB, et al.: Platinum-based versus non-platinum-based chemotherapy in advanced non-small-cell lung cancer: a meta-analysis of the published literature. J Clin Oncol 23 (13): 2926-36, 2005. [PUBMED Abstract]
- Rajeswaran A, Trojan A, Burnand B, et al.: Efficacy and side effects of cisplatin- and carboplatin-based doublet chemotherapeutic regimens versus non-platinum-based doublet chemotherapeutic regimens as first line treatment of metastatic non-small cell lung carcinoma: a systematic review of randomized controlled trials. Lung Cancer 59 (1): 1-11, 2008. [PUBMED Abstract]
- Pujol JL, Barlesi F, Daurès JP: Should chemotherapy combinations for advanced non-small cell lung cancer be platinum-based? A meta-analysis of phase III randomized trials. Lung Cancer 51 (3): 335-45, 2006. [PUBMED Abstract]
- Douillard JY, Laporte S, Fossella F, et al.: Comparison of docetaxel- and vinca alkaloid-based chemotherapy in the first-line treatment of advanced non-small cell lung cancer: a meta-analysis of seven randomized clinical trials. J Thorac Oncol 2 (10): 939-46, 2007. [PUBMED Abstract]
- Belani CP, Ramalingam S, Perry MC, et al.: Randomized, phase III study of weekly paclitaxel in combination with carboplatin versus standard every-3-weeks administration of carboplatin and paclitaxel for patients with previously untreated advanced non-small-cell lung cancer. J Clin Oncol 26 (3): 468-73, 2008. [PUBMED Abstract]
- Schuette W, Blankenburg T, Guschall W, et al.: Multicenter randomized trial for stage IIIB/IV non-small-cell lung cancer using every-3-week versus weekly paclitaxel/carboplatin. Clin Lung Cancer 7 (5): 338-43, 2006. [PUBMED Abstract]
- Sandler A, Gray R, Perry MC, et al.: Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355 (24): 2542-50, 2006. [PUBMED Abstract]
- Reck M, von Pawel J, Zatloukal P, et al.: Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 27 (8): 1227-34, 2009. [PUBMED Abstract]
- Lynch TJ, Patel T, Dreisbach L, et al.: Cetuximab and first-line taxane/carboplatin chemotherapy in advanced non-small-cell lung cancer: results of the randomized multicenter phase III trial BMS099. J Clin Oncol 28 (6): 911-7, 2010. [PUBMED Abstract]
- Pirker R, Pereira JR, Szczesna A, et al.: Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet 373 (9674): 1525-31, 2009. [PUBMED Abstract]
- Khambata-Ford S, Harbison CT, Hart LL, et al.: Analysis of potential predictive markers of cetuximab benefit in BMS099, a phase III study of cetuximab and first-line taxane/carboplatin in advanced non-small-cell lung cancer. J Clin Oncol 28 (6): 918-27, 2010. [PUBMED Abstract]
- Paz-Ares L, Mezger J, Ciuleanu TE, et al.: Necitumumab plus pemetrexed and cisplatin as first-line therapy in patients with stage IV non-squamous non-small-cell lung cancer (INSPIRE): an open-label, randomised, controlled phase 3 study. Lancet Oncol 16 (3): 328-37, 2015. [PUBMED Abstract]
- Thatcher N, Hirsch FR, Luft AV, et al.: Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol 16 (7): 763-74, 2015. [PUBMED Abstract]
- Paz-Ares LG, de Marinis F, Dediu M, et al.: PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol 31 (23): 2895-902, 2013. [PUBMED Abstract]
- Brodowicz T, Krzakowski M, Zwitter M, et al.: Cisplatin and gemcitabine first-line chemotherapy followed by maintenance gemcitabine or best supportive care in advanced non-small cell lung cancer: a phase III trial. Lung Cancer 52 (2): 155-63, 2006. [PUBMED Abstract]
- Park JO, Kim SW, Ahn JS, et al.: Phase III trial of two versus four additional cycles in patients who are nonprogressive after two cycles of platinum-based chemotherapy in non small-cell lung cancer. J Clin Oncol 25 (33): 5233-9, 2007. [PUBMED Abstract]
- Paz-Ares L, de Marinis F, Dediu M, et al.: Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol 13 (3): 247-55, 2012. [PUBMED Abstract]
- Socinski MA, Schell MJ, Peterman A, et al.: Phase III trial comparing a defined duration of therapy versus continuous therapy followed by second-line therapy in advanced-stage IIIB/IV non-small-cell lung cancer. J Clin Oncol 20 (5): 1335-43, 2002. [PUBMED Abstract]
- von Plessen C, Bergman B, Andresen O, et al.: Palliative chemotherapy beyond three courses conveys no survival or consistent quality-of-life benefits in advanced non-small-cell lung cancer. Br J Cancer 95 (8): 966-73, 2006. [PUBMED Abstract]
- Ciuleanu T, Brodowicz T, Zielinski C, et al.: Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 374 (9699): 1432-40, 2009. [PUBMED Abstract]
- Belani CP, Brodowicz T, Ciuleanu TE, et al.: Quality of life in patients with advanced non-small-cell lung cancer given maintenance treatment with pemetrexed versus placebo (H3E-MC-JMEN): results from a randomised, double-blind, phase 3 study. Lancet Oncol 13 (3): 292-9, 2012. [PUBMED Abstract]
- Soria JC, Ohe Y, Vansteenkiste J, et al.: Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 378 (2): 113-125, 2018. [PUBMED Abstract]
- Ramalingam SS, Vansteenkiste J, Planchard D, et al.: Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med 382 (1): 41-50, 2020. [PUBMED Abstract]
- Planchard D, Jänne PA, Cheng Y, et al.: Osimertinib with or without Chemotherapy in EGFR-Mutated Advanced NSCLC. N Engl J Med 389 (21): 1935-1948, 2023. [PUBMED Abstract]
- Wu YL, Cheng Y, Zhou X, et al.: Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 18 (11): 1454-1466, 2017. [PUBMED Abstract]
- Mok TS, Wu YL, Thongprasert S, et al.: Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 361 (10): 947-57, 2009. [PUBMED Abstract]
- Maemondo M, Inoue A, Kobayashi K, et al.: Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362 (25): 2380-8, 2010. [PUBMED Abstract]
- Mitsudomi T, Morita S, Yatabe Y, et al.: Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 11 (2): 121-8, 2010. [PUBMED Abstract]
- Zhou C, Wu YL, Chen G, et al.: Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 12 (8): 735-42, 2011. [PUBMED Abstract]
- Rosell R, Gervais R, Vergnenegre A, et al.: Erlotinib versus chemotherapy (CT) in advanced non-small cell lung cancer (NSCLC) patients (p) with epidermal growth factor receptor (EGFR) mutations: Interim results of the European Erlotinib Versus Chemotherapy (EURTAC) phase III randomized trial. [Abstract] J Clin Oncol 29 (Suppl 15): A-7503, 2011.
- Rosell R, Carcereny E, Gervais R, et al.: Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13 (3): 239-46, 2012. [PUBMED Abstract]
- Sequist LV, Yang JC, Yamamoto N, et al.: Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 31 (27): 3327-34, 2013. [PUBMED Abstract]
- Yang JC, Wu YL, Schuler M, et al.: Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 16 (2): 141-51, 2015. [PUBMED Abstract]
- Wu YL, Zhou C, Hu CP, et al.: Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 15 (2): 213-22, 2014. [PUBMED Abstract]
- Zhou C, Tang KJ, Cho BC, et al.: Amivantamab plus Chemotherapy in NSCLC with EGFR Exon 20 Insertions. N Engl J Med 389 (22): 2039-2051, 2023. [PUBMED Abstract]
- Peters S, Camidge DR, Shaw AT, et al.: Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 377 (9): 829-838, 2017. [PUBMED Abstract]
- Hida T, Nokihara H, Kondo M, et al.: Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet 390 (10089): 29-39, 2017. [PUBMED Abstract]
- Shaw AT, Bauer TM, de Marinis F, et al.: First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N Engl J Med 383 (21): 2018-2029, 2020. [PUBMED Abstract]
- Solomon BJ, Bauer TM, Ignatius Ou SH, et al.: Post Hoc Analysis of Lorlatinib Intracranial Efficacy and Safety in Patients With ALK-Positive Advanced Non-Small-Cell Lung Cancer From the Phase III CROWN Study. J Clin Oncol 40 (31): 3593-3602, 2022. [PUBMED Abstract]
- Solomon BJ, Mok T, Kim DW, et al.: First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 371 (23): 2167-77, 2014. [PUBMED Abstract]
- Mok T, Kim D, Wu Y, et al.: First-line crizotinib versus pemetrexed-cisplatin or pemetrexed-carboplatin in patients (pts) with advanced ALK-positive non-squamous non-small cell lung cancer (NSCLC): results of a phase III study (PROFILE 1014). [Abstract] J Clin Oncol 32 (Suppl 15): A-8002, 2014.
- Soria JC, Tan DSW, Chiari R, et al.: First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 389 (10072): 917-929, 2017. [PUBMED Abstract]
- Kim DW, Tiseo M, Ahn MJ, et al.: Brigatinib in Patients With Crizotinib-Refractory Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. J Clin Oncol 35 (22): 2490-2498, 2017. [PUBMED Abstract]
- Planchard D, Smit EF, Groen HJM, et al.: Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol 18 (10): 1307-1316, 2017. [PUBMED Abstract]
- Gainor JF, Shaw AT: Novel targets in non-small cell lung cancer: ROS1 and RET fusions. Oncologist 18 (7): 865-75, 2013. [PUBMED Abstract]
- Drilon A, Siena S, Dziadziuszko R, et al.: Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1-2 trials. Lancet Oncol 21 (2): 261-270, 2020. [PUBMED Abstract]
- Shaw AT, Ou SH, Bang YJ, et al.: Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 371 (21): 1963-71, 2014. [PUBMED Abstract]
- Wu YL, Yang JC, Kim DW, et al.: Phase II Study of Crizotinib in East Asian Patients With ROS1-Positive Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 36 (14): 1405-1411, 2018. [PUBMED Abstract]
- Farago AF, Le LP, Zheng Z, et al.: Durable Clinical Response to Entrectinib in NTRK1-Rearranged Non-Small Cell Lung Cancer. J Thorac Oncol 10 (12): 1670-4, 2015. [PUBMED Abstract]
- Gatalica Z, Xiu J, Swensen J, et al.: Molecular characterization of cancers with NTRK gene fusions. Mod Pathol 32 (1): 147-153, 2019. [PUBMED Abstract]
- Drilon A, Laetsch TW, Kummar S, et al.: Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med 378 (8): 731-739, 2018. [PUBMED Abstract]
- Doebele RC, Drilon A, Paz-Ares L, et al.: Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol 21 (2): 271-282, 2020. [PUBMED Abstract]
- Drilon A, Hu ZI, Lai GGY, et al.: Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol 15 (3): 151-167, 2018. [PUBMED Abstract]
- Drilon A, Subbiah V, Gautschi O, et al.: Selpercatinib in Patients With RET Fusion-Positive Non-Small-Cell Lung Cancer: Updated Safety and Efficacy From the Registrational LIBRETTO-001 Phase I/II Trial. J Clin Oncol 41 (2): 385-394, 2023. [PUBMED Abstract]
- Gainor JF, Curigliano G, Kim DW, et al.: Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol 22 (7): 959-969, 2021. [PUBMED Abstract]
- Awad MM, Oxnard GR, Jackman DM, et al.: MET Exon 14 Mutations in Non-Small-Cell Lung Cancer Are Associated With Advanced Age and Stage-Dependent MET Genomic Amplification and c-Met Overexpression. J Clin Oncol 34 (7): 721-30, 2016. [PUBMED Abstract]
- Paik PK, Felip E, Veillon R, et al.: Tepotinib in Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N Engl J Med 383 (10): 931-943, 2020. [PUBMED Abstract]
- Wolf J, Seto T, Han JY, et al.: Capmatinib in MET Exon 14-Mutated or MET-Amplified Non-Small-Cell Lung Cancer. N Engl J Med 383 (10): 944-957, 2020. [PUBMED Abstract]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al.: Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 375 (19): 1823-1833, 2016. [PUBMED Abstract]
- Garassino MC, Gadgeel S, Speranza G, et al.: Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non-Small-Cell Lung Cancer: 5-Year Outcomes From the Phase 3 KEYNOTE-189 Study. J Clin Oncol 41 (11): 1992-1998, 2023. [PUBMED Abstract]
- Novello S, Kowalski DM, Luft A, et al.: Pembrolizumab Plus Chemotherapy in Squamous Non-Small-Cell Lung Cancer: 5-Year Update of the Phase III KEYNOTE-407 Study. J Clin Oncol 41 (11): 1999-2006, 2023. [PUBMED Abstract]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al.: Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol 37 (7): 537-546, 2019. [PUBMED Abstract]
- de Castro G, Kudaba I, Wu YL, et al.: Five-Year Outcomes With Pembrolizumab Versus Chemotherapy as First-Line Therapy in Patients With Non-Small-Cell Lung Cancer and Programmed Death Ligand-1 Tumor Proportion Score ≥ 1% in the KEYNOTE-042 Study. J Clin Oncol 41 (11): 1986-1991, 2023. [PUBMED Abstract]
- Gogishvili M, Melkadze T, Makharadze T, et al.: Cemiplimab plus chemotherapy versus chemotherapy alone in non-small cell lung cancer: a randomized, controlled, double-blind phase 3 trial. Nat Med 28 (11): 2374-2380, 2022. [PUBMED Abstract]
- Sezer A, Kilickap S, Gümüş M, et al.: Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet 397 (10274): 592-604, 2021. [PUBMED Abstract]
- Özgüroğlu M, Kilickap S, Sezer A, et al.: First-line cemiplimab monotherapy and continued cemiplimab beyond progression plus chemotherapy for advanced non-small-cell lung cancer with PD-L1 50% or more (EMPOWER-Lung 1): 35-month follow-up from a mutlicentre, open-label, randomised, phase 3 trial. Lancet Oncol 24 (9): 989-1001, 2023. [PUBMED Abstract]
- Johnson ML, Cho BC, Luft A, et al.: Durvalumab With or Without Tremelimumab in Combination With Chemotherapy as First-Line Therapy for Metastatic Non-Small-Cell Lung Cancer: The Phase III POSEIDON Study. J Clin Oncol 41 (6): 1213-1227, 2023. [PUBMED Abstract]
- Herbst RS, Giaccone G, de Marinis F, et al.: Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N Engl J Med 383 (14): 1328-1339, 2020. [PUBMED Abstract]
- West H, McCleod M, Hussein M, et al.: Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 20 (7): 924-937, 2019. [PUBMED Abstract]
- Socinski MA, Jotte RM, Cappuzzo F, et al.: Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 378 (24): 2288-2301, 2018. [PUBMED Abstract]
- Brahmer JR, Lee JS, Ciuleanu TE, et al.: Five-Year Survival Outcomes With Nivolumab Plus Ipilimumab Versus Chemotherapy as First-Line Treatment for Metastatic Non-Small-Cell Lung Cancer in CheckMate 227. J Clin Oncol 41 (6): 1200-1212, 2023. [PUBMED Abstract]
- Hellmann MD, Ciuleanu TE, Pluzanski A, et al.: Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med 378 (22): 2093-2104, 2018. [PUBMED Abstract]
- Yao JC, Fazio N, Singh S, et al.: Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet 387 (10022): 968-977, 2016. [PUBMED Abstract]
- Danson S, Middleton MR, O'Byrne KJ, et al.: Phase III trial of gemcitabine and carboplatin versus mitomycin, ifosfamide, and cisplatin or mitomycin, vinblastine, and cisplatin in patients with advanced nonsmall cell lung carcinoma. Cancer 98 (3): 542-53, 2003. [PUBMED Abstract]
- Smit EF, van Meerbeeck JP, Lianes P, et al.: Three-arm randomized study of two cisplatin-based regimens and paclitaxel plus gemcitabine in advanced non-small-cell lung cancer: a phase III trial of the European Organization for Research and Treatment of Cancer Lung Cancer Group--EORTC 08975. J Clin Oncol 21 (21): 3909-17, 2003. [PUBMED Abstract]
- Kubota K, Watanabe K, Kunitoh H, et al.: Phase III randomized trial of docetaxel plus cisplatin versus vindesine plus cisplatin in patients with stage IV non-small-cell lung cancer: the Japanese Taxotere Lung Cancer Study Group. J Clin Oncol 22 (2): 254-61, 2004. [PUBMED Abstract]
- Georgoulias V, Ardavanis A, Agelidou A, et al.: Docetaxel versus docetaxel plus cisplatin as front-line treatment of patients with advanced non-small-cell lung cancer: a randomized, multicenter phase III trial. J Clin Oncol 22 (13): 2602-9, 2004. [PUBMED Abstract]
- Sandler AB, Nemunaitis J, Denham C, et al.: Phase III trial of gemcitabine plus cisplatin versus cisplatin alone in patients with locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 18 (1): 122-30, 2000. [PUBMED Abstract]
- Salerno TA, Munro DD, Blundell PE, et al.: Second primary bronchogenic carcinoma: life-table analysis of surgical treatment. Ann Thorac Surg 27 (1): 3-6, 1979. [PUBMED Abstract]
- Yellin A, Hill LR, Benfield JR: Bronchogenic carcinoma associated with upper aerodigestive cancers. J Thorac Cardiovasc Surg 91 (5): 674-83, 1986. [PUBMED Abstract]
- Patchell RA, Tibbs PA, Walsh JW, et al.: A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 322 (8): 494-500, 1990. [PUBMED Abstract]
- Mandell L, Hilaris B, Sullivan M, et al.: The treatment of single brain metastasis from non-oat cell lung carcinoma. Surgery and radiation versus radiation therapy alone. Cancer 58 (3): 641-9, 1986. [PUBMED Abstract]
- Loeffler JS, Kooy HM, Wen PY, et al.: The treatment of recurrent brain metastases with stereotactic radiosurgery. J Clin Oncol 8 (4): 576-82, 1990. [PUBMED Abstract]
- Arbit E, Wroński M, Burt M, et al.: The treatment of patients with recurrent brain metastases. A retrospective analysis of 109 patients with nonsmall cell lung cancer. Cancer 76 (5): 765-73, 1995. [PUBMED Abstract]
- Hazuka MB, Kinzie JJ: Brain metastases: results and effects of re-irradiation. Int J Radiat Oncol Biol Phys 15 (2): 433-7, 1988. [PUBMED Abstract]
Treatment of Progressive Stage IV, Relapsed, and Recurrent NSCLC
Treatment options for progressive stage iv, relapsed, and recurrent nsclc (second-line therapy).
Treatment options for patients with progressive stage IV , relapsed, and recurrent non-small cell lung cancer (NSCLC) (second-line therapy and beyond) include:
- Docetaxel .
- Docetaxel plus ramucirumab .
- Pemetrexed .
- Osimertinib .
- Mobocertinib .
- Adagrasib .
- Sotorasib .
- Trastuzumab deruxtecan .
- Nivolumab .
- Pembrolizumab .
- Atezolizumab .
- Clinical trials can be considered as second-line therapy.
Chemotherapy
The use of chemotherapy has produced objective responses and small improvement in survival for patients with metastatic disease.[ 1 ][ Level of evidence A1 ] In studies that have examined symptomatic response, improvement in subjective symptoms has been reported to occur more frequently than objective response.[ 2 , 3 ] Informed patients with good performance status and symptomatic recurrence can be offered treatment with a platinum-based chemotherapy regimen for palliation of symptoms. For patients who have relapsed after platinum-based chemotherapy, second-line therapy can be considered.
Evidence (docetaxel):
- Two prospective randomized studies have shown an improvement in survival with the use of docetaxel compared with vinorelbine, ifosfamide, or best supportive care;[ 4 , 5 ] however, criteria for the selection of appropriate patients for second-line treatment are not well defined.[ 6 ]
- Median survival was 27.4 weeks for patients treated every 3 weeks and 26.1 weeks for patients treated weekly (log-rank P = .24).
- Significantly less severe neutropenia and febrile neutropenia were reported with weekly docetaxel ( P < .001 for both); however, no significant differences were observed for anemia, thrombocytopenia, and nonhematologic toxic effects.
Docetaxel plus ramucirumab
Evidence (docetaxel plus ramucirumab):
- The addition of ramucirumab to docetaxel compared with placebo plus docetaxel led to an increase in median OS (10.5 months vs. 9.1 months; hazard ratio [HR], 0.86; 95% confidence interval [CI], 0.75–0.98), objective response rate (23% vs. 14%), and PFS (4.5 months vs. 3 months). The improvement in OS from the addition of ramucirumab appeared consistent across subgroups including squamous and nonsquamous histologies.
- Grade 3 to 4 treatment-related adverse events occurred in 79% of patients who received docetaxel and ramucirumab as compared with 71% of patients who received docetaxel and placebo. Febrile neutropenia, fatigue, and hypertension were among the toxicities that were more common with the addition of ramucirumab to docetaxel. There was no significant difference in the incidence of grades 3 to 4 hemorrhage between the groups.
- On the basis of this study, the addition of ramucirumab to docetaxel chemotherapy can be considered for patients with good performance status with advanced NSCLC who have progressive disease after first-line chemotherapy.
Evidence (pemetrexed):
- The trial showed no difference in response rates, PFS, or OS between treatments.[ 9 ][ Level of evidence A1 ]
- Of note, patients with squamous histology benefited from docetaxel, and those with nonsquamous histologies appeared to benefit more from pemetrexed.[ 10 ]
Epidermal growth factor receptor (EGFR)-directed therapy
Advanced NSCLC that contains characteristic mutations in EGFR , most commonly exon 19 deletions or exon 21 L858R mutations, is highly sensitive to EGFR TKIs. The standard approach to decide whether to use an EGFR TKI for the treatment of a patient with advanced NSCLC is to analyze the tumor for the presence or absence of a driver mutation in EGFR . EGFR exon 20 insertions are uncommon mutations that are not sensitive to the EGFR TKIs used for the treatment of NSCLC with EGFR -sensitizing mutations.
EGFR-directed therapy after first-line chemotherapy (for patients with EGFR -sensitizing mutations)
Two randomized placebo-controlled trials indicated that erlotinib prolongs survival and time to deterioration in symptoms in patients with NSCLC after first-line or second-line chemotherapy compared with placebo [ 11 , 12 ] but does not improve survival compared with standard second-line chemotherapy with docetaxel or pemetrexed.[ 13 ]
- OS was 6.7 months among those who had received two previous chemotherapy regimens and 4.7 months among those who had received platinum-based chemotherapy. The HR was 0.70 ( P < .001) in favor of erlotinib.[ 11 ][ Level of evidence A1 ]
- There was no difference in the primary end point of OS (median OS, 5.3 months vs. 5.5 months; HR, 0.96; 95% CI, 0.78–1.19).[ 13 ][ Level of evidence A1 ]
- Gefitinib did not improve OS.
- Median survival did not differ significantly between the groups in the overall population (5.6 months for gefitinib and 5.1 months for placebo; HR, 0.89; 95% CI, 0.77–1.02; P = .087) or among the 812 patients with adenocarcinoma (6.3 months vs. 5.4 months; HR, 0.84; CI, 0.68–1.03; P = .089).
- Preplanned subgroup analyses showed significantly longer survival in the gefitinib group than in the placebo group for never-smokers (n = 375; 95% CI, 0.67 [0.49–0.92]; P = .012; median survival 8.9 months vs. 6.1 months) and for patients of Asian origin (n = 342; 95% CI, 0.66 [0.48–0.91]; P = .01; median survival 9.5 months vs. 5.5 months).[ 14 ][ Level of evidence A1 ]
- Noninferiority of gefitinib compared with docetaxel was confirmed for OS (HR, 1.020; 95% CI, 0.905–1.150). However, superiority of gefitinib in patients with high EGFR gene copy number (85 patients vs. 89 patients) was not proven (HR, 1.09; 95% CI, 0.78–1.51; P = .62).
- In the gefitinib group, the most common adverse events were rash or acne (49% vs. 10%) and diarrhea (35% vs. 25%). In the docetaxel group, neutropenia (5% vs. 74%), asthenia (25% vs. 47%), and alopecia (3% vs. 36%) were most common.
- This trial established noninferior survival of patients treated with gefitinib compared with docetaxel, suggesting that gefitinib is a valid treatment for pretreated patients with advanced NSCLC.
Objective response to erlotinib and gefitinib is more likely in patients who have never smoked, are female, are of East Asian race, or have adenocarcinoma or bronchioloalveolar carcinoma.[ 16 - 22 ] Responses may be associated with the presence of sensitizing mutations in the tyrosine kinase domain of EGFR [ 17 - 19 , 21 , 22 ] and with the absence of KRAS mutations.[ 20 - 22 ][ Level of evidence C2 ] Survival benefit may be greater in patients with EGFR protein expression by immunohistochemistry or increased EGFR gene copy number by fluorescence in situ hybridization studies (FISH),[ 21 , 22 ] but the clinical utility of EGFR testing by immunohistochemistry has been questioned.[ 23 ]
- After a median follow-up of 6.7 months, the PFS was 2.4 months versus 1.9 months (HR, 0.82; 95% CI, 0.68–1.00).
- After a median follow-up of 18.4 months, the median OS was significantly longer in the afatinib arm (7.9 months vs. 6.8 months; HR, 0.81; 95% CI, 0.69–0.95; P = .007). Survival at 6 months (63.6% vs. 54.6%; P = .009), 12 months (36.4% vs. 28.2%; P = .015), and 18 months (22% vs. 14.4%; P = .013) were all significantly better in patients who received afatinib.
- There was no significant difference in response rate between the two arms (6% vs. 3%; P = .551).
- The frequency of adverse events was similar between the two groups with 57% of the patients experiencing a rate of grade 3 or higher adverse events. Grade 3 treatment-related diarrhea and stomatitis occurred more frequently with afatinib; however, grade 3 rash or acne were more common in patients who received erlotinib.
- Afatinib, as compared with erlotinib, represents another option for the second-line treatment of patients with stage IV squamous cell NSCLC.
EGFR-directed therapy (for patients with acquired EGFR T790M mutations after previous EGFR-directed therapy)
Osimertinib.
Evidence (osimertinib):
- Osimertinib was superior to chemotherapy in prolonging median PFS (10.1 months vs. 4.4 months; HR, 0.30; 95% CI, 0.23–0.41; P < .001).
- The objective response was 71% with osimertinib versus 31% with platinum therapy (odds ratio for objective response, 5.39; 95% CI, 3.47–8.48; P < .001).
- Among 144 patients with central nervous system (CNS) metastases, median PFS duration was 8.5 months with osimertinib versus 4.2 months with platinum therapy (HR, 0.32; 95% CI, 0.21–0.49).
- Adverse events of grade 3 or greater occurred in 23% of osimertinib-treated patients versus 47% of platinum-treated patients.[ 25 ][ Level of evidence B1 ]
EGFR-directed therapy after first-line chemotherapy (for patients with EGFR exon 20 insertion mutations)
Evidence (amivantamab):
- At the time of clinical data cutoff, 81 patients were evaluable with at least three disease assessments. The overall response rate was 40% (95% CI, 29%–51%), including three complete responses. The median duration of response (DOR) was 11.1 months (95% CI, 6.9–not reached). The clinical benefit rate (including patients with stable disease) was 74% (95% CI, 63%–83%).
- The median PFS was 8.3 months (95% CI, 6.5–10.9); OS data are not mature.
- The most common adverse events were rash (86%), infusion-related reactions (66%), and paronychia (45%). Patients experienced adverse events related to both EGFR inhibition (rash, paronychia, stomatitis, pruritus, diarrhea) and MET inhibition (hypoalbuminemia, peripheral edema). Grade 3 or greater adverse events occurred in 35% of patients, with rash (4%) and infusion reactions (3%) as the most common.
- The infusion reactions occurred primarily with the first exposure to amivantamab. The administration of the first dose was split over 2 days to minimize this toxicity.
The U.S. Food and Drug Administration (FDA) granted accelerated approval to amivantamab for the treatment of patients with EGFR exon 20 mutations whose disease has progressed on or after platinum-based therapy.
Mobocertinib
Evidence (mobocertinib):
- The overall response rate was 28% (95% CI, 20%–37%), with a median DOR of 17.5 months (95% CI, 7.4–20.3).
- The median PFS was 7.3 months (95% CI, 5.5–9.2), and the median OS was 24.0 months (95% CI, 14.6–28.8).
- The most common adverse events were diarrhea (91% any grade, 21% grade >3) and rash (45%). Most adverse events were grade 1 to 2 and controlled with dose modification, supportive care, or drug discontinuation. Treatment was discontinued by 17% of participants because of adverse events. One patient died of cardiac failure that was deemed to be treatment related.
The FDA granted accelerated approval to mobocertinib for the treatment of patients with EGFR exon 20 mutations whose disease has progressed on or after platinum-based therapy.
ALK-directed tyrosine kinase inhibitors (TKIs)
Alk-directed tkis after first-line chemotherapy.
- At a mean treatment duration of 6.4 months, the overall response rate was 57% (47 of 82 patients, with 46 confirmed partial responses, and one confirmed complete response); 27 patients (33%) had stable disease.[ 28 ][ Level of evidence C2 ]
- The estimated probability of 6-month PFS was 72%.
- The 1-year OS rate was 74% (95% CI, 63%–82%), and the 2-year OS rate was 54% (40%–66%).
- Survival in 30 ALK -positive patients who were given crizotinib in the second-line or third-line setting was significantly longer than in 23 ALK -positive controls identified from a different cohort given any second-line therapy (median OS not reached [95% CI, 14 months–not reached] vs. 6 months [95% CI, 4–17], 1-year OS rate, 70% [95% CI, 50%–83%] vs. 44% [95% CI, 23%–64%], and 2-year OS rate, 55% [33%–72%] vs. 12% [2%–30%]; HR, 0.36; 95% CI, 0.17–0.75; P = .004).[ 29 ][ Level of evidence C2 ]
- Common toxicities were grade 1 or 2 (mild) gastrointestinal side effects.
- Patients with ALK rearrangements tended to be younger than those without the rearrangements; most of the patients had little or no exposure to tobacco; and the patients had adenocarcinomas.
- The primary end point was PFS. Median PFS was significantly longer in favor of crizotinib (7.7 months vs. 3.0 months, P < .001).[ 30 ][ Level of evidence B1 ]
- OS, a secondary end point, was not significantly different, but there was significant crossover in the design.
ALK-directed TKIs after prior ALK TKI therapy
- The objective response rate by blinded independent review was 43.6% (95% CI, 36%–52%), and the median DOR was 7.1 months (range, 5.6–not estimable).[ 31 ][ Level of evidence C3 ]
- Of note, 38% of patients required dose modification because of gastrointestinal toxicity; elevation of alanine transaminase to more than five times the upper limit of normal occurred in 27% of patients.
- The primary end point was objective response according to RECIST (version 1.1). At the time of primary end point analysis of this ongoing study, 48% of patients (95% CI, 36%–60%) had a confirmed partial response, and 32% had stable disease by blinded independent review. The median DOR was 13.5 months (95% CI, 6.7–not estimable). The estimated median PFS was 8.1 months (95% CI, 6.2–12.6).[ 32 ][ Level of evidence C3 ]
- Sixteen patients had measurable CNS disease at baseline, of whom 11 had received prior radiation therapy. The CNS overall response rate was 75% (95% CI, 48%–93%), with 25% of the patients attaining complete response and 50% of the patients attaining partial response.
- The most common side effects were grade 1 or 2 in severity; the most frequent adverse events, occurring in 23% to 36% of patients, were constipation, fatigue, myalgia, and peripheral edema. Dose interruption was needed in 36% of patients, and dose reduction occurred in 16%.
- The primary end point was objective response rate by independent central review. The objective response rate was 50% (95% CI, 41%–59%). Median DOR was 11.2 months (95% CI, 9.6–not reached). Median PFS was 8.9 months (95% CI, 5.6–11.3).[ 33 ][ Level of evidence C3 ]
- CNS objective response rate in 35 patients with measurable CNS lesions was 57% (95% CI, 39%–74%).
- Common adverse events that were mainly grade 1 or 2, which occurred in 25% to 33% of patients, were constipation, fatigue, and peripheral edema.
- At data cutoff, the median DOR was 13.8 months (95% CI, 5.6–13.8) for patients who received the 90 mg dose and 11.1 months (95% CI, 9.2–13.8) for patients who received the 180 mg dose.[ 34 ][ Level of evidence B3 ]
- Objective response rate, 90.0% (95% CI, 73.5%‒97.9%).
- Intracranial response rate (n = 3), 66.7% (95% CI, 9.4%‒99.2%).
- Objective response rate, 69.5% (95% CI, 56.1%‒80.8%).
- Intracranial response rate (n = 23), 87.0% (95% CI, 66.4%‒97.2%).
- Objective response rate, 32.1% (95% CI, 15.9%‒52.4%).
- Intracranial response rate (n = 9), 55.6% (95% CI, 21.2%‒86.3%).
- Objective response rate, 38.7% (95% CI, 29.6%‒48.5%).
- Intracranial response rate (n = 49), 53.1% (95% CI, 38.3%‒67.5%).
- The median DOR has not been reached for any of the pooled cohorts.
- The most common adverse event was hypercholesterolemia (16% grade 3–4), and 3% of patients discontinued treatment due to adverse events.
- The overall response rate was 63.2% (95% CI, 49.3%–75.6%), as determined independently by investigator and independent review committee assessments. There were 2 out of 36 complete responses by investigator assessment; all responses were deemed partial by the independent review committee.
- The median investigator-assessed PFS was 9.7 months (95% CI, 6.9–19.6 months). The estimated median DOR was 9.0 months (95% CI, 6.9–18.3). The OS data are immature.
- Forty-nine percent of patients had at least one grade 3 or 4 adverse event, the most common of which were neutropenia, hyponatremia, and anemia.[ 36 ][ Level of evidence C3 ]
The FDA approved the combination of dabrafenib and trametinib for patients with NSCLC whose tumors harbor BRAF V600E mutations as detected by an FDA-approved test.
ROS1-directed therapy
ROS1 rearrangements occur in approximately 1% of patients with NSCLC.[ 37 ] Crizotinib and entrectinib are approved for use in patients with NSCLC with ROS1 rearrangements, with the latter appearing to have greater activity against intracranial disease.
Seventeen (32%) patients had received no previous systemic therapy, 23 (43%) had received one previous therapy, and 13 (25%) had received two or more lines of treatment. CNS disease was present in 23 (43%) patients at baseline. Thirty-one (59%) patients were never smokers and 52 (98%) patients had adenocarcinoma histology.
- The overall response rate in 53 efficacy-evaluable patients was 77% (95% CI, 64%−88%). Six percent of patients had a complete response and 72% had a partial response. Among patients with CNS disease at baseline, the overall response rate was 74% and all patients had a partial response. Among patients without CNS disease at baseline, the overall response rate was 80% (10% complete response rate; 70% partial response rate). [ 38 ][ Level of evidence C3 ]
- The median DOR was 24.6 months (95% CI, 11.4−34.8); 12.6 months (95% CI, 6.5−not estimable) in patients with baseline CNS disease, and 24.6 months (95% CI, 11.4−34.8) in those without CNS disease at baseline.
- Treatment-related adverse events were assessed in 134 patients in the safety-evaluable population. Grade 1 or 2 treatment-related adverse events were observed in 79 (59%) patients. Grade 3 or 4 treatment-related adverse events were observed in 46 (34%) patients. Fifteen (11%) patients had serious treatment-related adverse events. There were no treatment-related deaths.
- The median PFS was 19 months (95% CI, 12.2−36.6); 13.6 months (95% CI, 4.5−not estimable) in patients with baseline CNS disease, and 26.3 months (95% CI, 15.7−36.6) in patients with no baseline CNS disease.
- Median PFS was 19.2 months (95% CI, 14.4–not reached). The estimated DOR was 17.6 months (95% CI, 14.5–not reached).[ 39 ][ Level of evidence C3 ]
- The objective response rate was 71.7% (95% CI, 63.0%–79.3%). Response rates were similar, irrespective of the number of previous therapies. Complete responses occurred in 13.4% of patients, while 58.3% of patients had partial responses and 16.5% of patients had stable disease as their best response.[ 40 ][ Level of evidence C3 ]
Somatic gene fusions in NTRK occur across a range of solid tumors including in fewer than 0.5% of NSCLC tumors.[ 41 , 42 ] These fusions appear to occur more frequently in nonsmokers with lung adenocarcinoma.
- The objective response rate was 75% (95% CI, 61%‒75%) and 73% of these responses lasted at least 6 months.[ 43 ][ Level of evidence C3 ]
Of 54 patients in the NTRK gene fusion-positive efficacy-evaluable population, 20 (37%) had received no previous systemic therapy, 11 (20%) had received one previous systemic therapy, and 23 (43%) had received two or more systemic therapies. Twelve (22%) patients had CNS disease at baseline. Ten (19%) patients had NSCLC. Fifty-two (96%) patients had an NTRK gene fusion detected by NGS and two (4%) had an NTRK gene fusion detected by other nucleic acid–based tests.
- The objective response rate in 54 patients was 57% (95% CI, 43.2%−70.8%). Seven percent of patients had a complete response and 50% had a partial response. In patients with baseline CNS disease the overall response rate was 50% (all partial responses), whereas in patients without baseline CNS disease, the overall response rate was 60% (10% complete response; 50% partial response).[ 44 ][ Level of evidence C3 ]
- The median DOR was 10.4 months (95% CI, 7.1−not estimable). In patients with baseline CNS disease, the DOR was not estimable, whereas it was 12.9 months (95% CI, 7.1−not estimable) in patients with no baseline CNS disease.
- Among 10 patients with NSCLC, the overall response rate was 70% (95% CI, 35%−93%) and the DOR ranged between 1.9 months and 20.1 months. For more information, see the prescribing information .
Somatic gene fusions of RET occur in 1% to 2% of patients with NSCLC and in patients with thyroid cancer.[ 45 ]
- Updated analysis was conducted in 316 patients with RET fusion–positive NSCLC.[ 46 ]
- There was no significant change in the safety profile. Most adverse events were grade 1 to 2. The most common adverse events were edema, diarrhea, fatigue, dry mouth, hypertension, increased alanine aminotransferase (ALT) and aspartate aminotransferase (AST), and rash.
Dysregulation of the MET proto-oncogene resulting from disruption of distinct splice sites leads to loss of MET exon 14 and enhanced MET signaling. These MET alterations drive tumor proliferation, survival, invasion, and metastasis, and occur in 3% to 4% of patients with NSCLC.[ 48 ]
- Among the 99 patients who had been followed for at least 9 months (i.e., the efficacy population), the objective response rate as assessed by independent review was 46% (95% CI, 36%–57%), with a median DOR of 11.1 months (95% CI, 7.2 –not estimable). Response rates were similar in the liquid biopsy and tissue biopsy groups.
- Grade 3 to 4 adverse events occurred in 67% of patients. The most common events, regardless of causality, were peripheral edema, nausea, vomiting, and increased creatinine. Adverse events led to therapy discontinuation in 11% of patients.
KRAS G12C inhibitors (for patients with KRAS G12C mutations)
Activating mutations in KRAS are found in 25% to 30% of nonsquamous NSCLC, resulting in activation of downstream oncogenic pathways and uncontrolled growth. The G12C single-nucleotide variant, with glycine substituted by cysteine at codon 12, is the most frequent variant in NSCLC, occurring in approximately 13% of lung adenocarcinomas.[ 51 ]
Evidence (adagrasib):
- Among 112 evaluable patients, the confirmed objective response rate was 42.9% (95% CI, 33.5%–52.6%), including one complete response (0.9%). The median DOR was 8.5 months (95% CI, 6.2–13.8). Disease control occurred in 79.5% of patients (95% CI, 70.8%–86.5%).
- The median PFS was 6.5 months (95% CI, 4.7–8.4), and the median OS was 12.6 months (95% CI, 9.2–19.2).
- Among 33 patients with previously treated stable CNS metastases, the intracranial confirmed objective response rate was 33.3% (95% CI, 18.0%–51.8%).
- Grade 3 or higher adverse events occurred in 44.8% of patients and included two deaths. The most common adverse events were diarrhea, nausea, fatigue, vomiting, dyspnea, increased creatinine, increased ALT, increased AST, and decreased appetite. Adverse events led to therapy discontinuation in 6.9% of patients.
The FDA approved adagrasib for the treatment of adult patients with KRAS G12C-mutated locally advanced or metastatic NSCLC, as determined by an FDA-approved test, who received at least one prior systemic therapy.[ 53 ]
Evidence (sotorasib):
- For the 124 evaluable patients, the overall response rate was 37.1% (95% CI, 28.6%–46.2%), including complete responses in 3.2%. The median DOR was 11.1 months (95% CI, 6.9–could not be evaluated). Disease control occurred in 80.6% of patients (95% CI, 72.6%–87.2%).
- Median PFS was 6.8 months (95% CI, 5.1–8.2) and median OS was 12.5 months (95% CI, 10.0–could not be evaluated).
- Grade 3 to 4 adverse events occurred in 20.6% of patients. The most common adverse events that were considered treatment related were diarrhea, nausea, increases in ALT or AST, and fatigue. Adverse events led to therapy discontinuation in 7.1% of patients.
The FDA approved sotorasib for the treatment of adult patients with KRAS G12C-mutated locally advanced or metastatic NSCLC, as determined by an FDA-approved test, who have received at least one prior systemic therapy.
HER2-targeted therapy (for patients with HER2 -mutations)
Mutations in the human epidermal growth factor receptor 2 ( HER2 ) gene are found in 1% to 4% of patients with nonsquamous NSCLC. These mutations are associated with female sex, Asian ethnicity, never-smoking status, a higher incidence of brain metastasis, moderate to poorly differentiated adenocarcinoma histology, and poor prognosis.[ 54 , 55 ]
Trastuzumab deruxtecan
Trastuzumab deruxtecan is an antibody-drug conjugate consisting of a humanized anti-HER2 monoclonal antibody linked to a topoisomerase I inhibitor.
Evidence (trastuzumab deruxtecan):
- All 91 patients were evaluable for response. The confirmed objective response rate was 55% (95% CI, 44%–65%), including one complete response (1%). The median DOR was 9.3 months (95% CI, 5.7–14.7). Disease control was observed in 92% of patients (95% CI, 85%–97%).
- The median PFS was 8.2 months (95% CI, 6.0–11.9), and the median OS was 17.8 months (95% CI, 13.8–22.1).
- Among 33 patients with CNS metastases at baseline, 14 had previously received radiation therapy to the brain, and 19 had not. Of these patients, 8 (57%) and 10 (53%), respectively, had a partial response. The median PFS for patients with CNS metastases at baseline was 7.1 months (95% CI, 5.5–9.8), and the median OS was 13.8 months (95% CI, 9.8–20.9).
- Grade 3 or higher drug-related adverse events occurred in 46% of patients. The most common adverse events were nausea, fatigue, alopecia, vomiting, neutropenia, anemia, diarrhea, decreased appetite, leukopenia, and constipation. The most common grade 3 or higher drug-related adverse events were neutropenia (19%) and anemia (10%).
- Drug-related adverse events led to treatment discontinuation in 23 patients (25%) and included pneumonitis in 12 patients (13%) and interstitial lung disease in 5 patients (5%).
- There were two drug-related deaths caused by interstitial lung disease.
The FDA granted accelerated approval to trastuzumab deruxtecan for patients with unresectable or metastatic NSCLC whose tumors have activating HER2 mutations, as detected by an FDA-approved test, and who have received a prior systemic therapy. This approval was based on objective response rate and DOR.
Immunotherapy
Nivolumab is a fully human monoclonal antibody that inhibits the PD-1 coinhibitory immune checkpoint expressed on tumor cells and infiltrating immune cells.[ 56 , 57 ] Pembrolizumab is a humanized monoclonal antibody that inhibits the interaction between the PD-1 coinhibitory immune checkpoint expressed on tumor cells and infiltrating immune cells and its ligands, PD-L1 and PD-L2.[ 58 ] Atezolizumab is a PD-L1–blocking antibody.
Evidence (nivolumab):
In two phase III clinical trials, one conducted in patients with advanced platinum-pretreated squamous NSCLC and the other trial conducted in patients with nonsquamous NSCLC, nivolumab demonstrated a significant improvement in OS compared with the previous standard treatment of docetaxel chemotherapy.[ 56 , 57 ][ Level of evidence A1 ] In addition, the rates of grade 3 and 4 treatment-related toxicity in both trials were significantly lower with nivolumab than with docetaxel. Of note, all patients enrolled in phase III studies of nivolumab had an ECOG performance status of 0 or 1; patients with autoimmune disease, symptomatic interstitial lung disease, or those receiving systemic immunosuppression were excluded from enrollment.
- Nivolumab demonstrated a significant improvement in median OS compared with docetaxel (9.2 months vs. 6 months; P < .001). In addition, the response rate (20% vs. 9%; P = .008) and median PFS (3.5 months vs. 2.8 months; P < .001) favored nivolumab.
- Rates of treatment-related toxicity were significantly lower with nivolumab than with docetaxel (all grades, 58% for nivolumab vs. 86% for docetaxel; grades 3–4, 7% for nivolumab vs. 55% for docetaxel).
- Patients who received nivolumab had a significant improvement in median OS compared with patients who received docetaxel (12.2 months vs. 9.4 months; HR, 0.73; 96% CI, 0.59–0.89; P = .002). In this study, the response rate (19% vs. 12%; P = .02) but not median PFS (2.3 months for nivolumab vs. 4.2 months for docetaxel) favored nivolumab. The median DOR in patients was 17.2 months for nivolumab and 5.6 months for docetaxel.
- Rates of treatment-related toxicity were significantly lower with nivolumab than with docetaxel (all grades, 69% for nivolumab vs. 88% for docetaxel; grades 3–4, 10% for nivolumab vs. 54% for docetaxel).
Both of these trials demonstrated long-term clinical benefit at the 2-year outcomes. The OS rates for nivolumab at 2 years compared with docetaxel in squamous NSCLC were 23% (95% CI, 16%–30%) versus 8% (95% CI, 4%–13%), and OS rates in nonsquamous NSCLC were 29% (95% CI, 24%–34%) versus 16% (95% CI, 12%–20%).[ 59 ] Ongoing responses at 2 years were observed in 10 (37%) confirmed responders with squamous NSCLC and 19 (34%) of 56 responders with nonsquamous NSCLC. No patient treated with docetaxel in either study had an ongoing response.
Nivolumab is now considered a standard second-line therapy for patients with metastatic NSCLC with progression on or after first-line platinum-based chemotherapy and is associated with improved survival and lower rates of toxicity than docetaxel. However, clinical trials of nivolumab to date have not enrolled patients with a history of autoimmune disease, interstitial lung disease, or an ECOG performance status higher than 1. Patients with active autoimmune conditions cannot be treated with nivolumab. Closely monitoring all patients for autoimmune toxicities from treatment is required. Specific algorithms for the management of autoimmune toxicity are included in the FDA label for nivolumab .
Pembrolizumab
Evidence (pembrolizumab):
- In the study, 495 patients received either pembrolizumab 2 mg/kg every 3 weeks, 10 mg/kg every 3 weeks, or 10 mg/kg every 2 weeks. No significant differences were seen among the different treatment schedules. Key exclusion criteria were autoimmune disease, history of pneumonitis, requirement for systemic immunosuppressive therapy, and a performance status higher than 1. The objective response rate was 19.4% (95% CI, 16.0%–23.2%), which included a response rate of 18.0% (95% CI, 14.4%–22.2%) in 394 previously treated patients and 24.8% (95% CI, 16.7%–34.3%) in 101 previously untreated patients. Median PFS was 3.7 months (95% CI, 2.9–4.1) for all patients, 3.0 months (95% CI, 2.2–4.0) for previously treated patients, and 6.0 months (95% CI, 4.1–8.6) for previously untreated patients. The median DOR was 12.5 months (range, 1.0–23.3 months) in all patients.
- The study evaluated the efficacy of pembrolizumab in patients with high levels of PD-L1, as assessed by the anti-PD-L1 antibody clone 22C3. Using the cutoff of membranous staining in at least 50% of tumor cells in a validation group of 73 patients, the response rate was 45.2% (95% CI, 33.5%–57.3%), and the median PFS in this group was 6.3 months (95% CI, 2.9–12.5). Median OS was not reached at the time of publication.
- The estimated prevalence of PD-L1 tumor staining from 1,143 screened patients, of whom 824 had evaluable samples, is as follows: 23.2% had 50% or more tumor cells with staining; 37.6% had between 1% and 49% tumor cells with staining; and 39.2% had less than 1% of tumor cells with staining.
- The most common adverse events were fatigue, pruritus, and decreased appetite. Grade 3 or higher adverse events were reported in 9.5% of patients. Inflammatory and immune-mediated adverse events that occurred in more than 2% of patients were infusion-related reactions (3.0%), hypothyroidism (6.9%), and pneumonitis (3.6%).
- In the total population, median OS was 10.4 months with pembrolizumab (2 mg/kg), 12.7 months with pembrolizumab (10 mg/kg), and 8.5 months with docetaxel. OS was significantly longer for pembrolizumab (2 mg/kg) versus docetaxel (HR 0.71; 95% CI, 0.58–0.88; P = .0008) and for pembrolizumab (10 mg/kg) versus docetaxel (HR, 0.61; CI, 0.49–0.75; P < .0001).
- In the total population, PFS was not prolonged in the pembrolizumab groups compared with the docetaxel group.
- Among patients with at least 50% of tumor cells expressing PD-L1, OS was significantly longer with pembrolizumab (2 mg/kg) than with docetaxel (median, 14.9 months vs. 8.2 months; HR, 0.54; 95% CI, 0.38–0.77; P = .0002) and with pembrolizumab (10 mg/kg) than with docetaxel (median, 17.3 months vs. 8.2 months; HR, 0.50; CI, 0.36–0.70; P < .0001).
- In the group of patients with at least 50% of tumor cells expressing PD-L1, PFS was significantly longer with pembrolizumab (2 mg/kg) than with docetaxel (median, 5.0 months vs. 4.1 months; HR, 0.59; 95% CI, 0.44–0.78; P = .0001) and with pembrolizumab (10 mg/kg) than with docetaxel (median, 5.2 months vs. 4.1 months; HR, 0.59; CI, 0.45–0.78; P < .0001).
- Grade 3 to 5 treatment-related adverse events were less common with pembrolizumab than with docetaxel (43 [13%] of 339 patients given pembrolizumab (2 mg/kg), 55 [16%] of 343 patients given pembrolizumab (10 mg/kg), and 109 [35%] of 309 patients given docetaxel).
The FDA granted accelerated approval to pembrolizumab as a second-line therapy for patients with NSCLC whose tumors express PD-L1 (>50% staining as determined by an FDA-approved test) with progression on or after first-line chemotherapy. Patients with EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapies before receiving pembrolizumab (see the FDA label for pembrolizumab ).
Atezolizumab
Evidence (atezolizumab):
- In the OAK trial, the median OS was 13.8 months in the atezolizumab arm (95% CI, 11.8–15.7) compared with 9.6 months in the docetaxel arm (95% CI, 8.6–11.2) (HR, 0.74; 95% CI, 0.63–0.87; P = .0004).
- The median OS in the POPLAR trial was 12.6 months in the atezolizumab arm (95% CI, 9.7–16.0) and 9.7 months in the docetaxel arm (95% CI, 8.6–12.0) (HR, 0.69; 95% CI, 0.52–0.92).
- Although the magnitude of improvement correlated with PD-L1 immunohistochemistry expression on tumor cells and tumor-infiltrating immune cells, survival benefit with atezolizumab was seen in patients with tumors with and without PD-L1 expression.
- In the POPLAR trial, the most common (≥20%) adverse reactions were in patients treated with atezolizumab and included fatigue, decreased appetite, dyspnea, cough, nausea, musculoskeletal pain, and constipation.
- The most common (≥2%) grade 3 to 4 adverse events in patients treated with atezolizumab were dyspnea, pneumonia, hypoxia, hyponatremia, fatigue, anemia, musculoskeletal pain, ALT and AST increase, dysphagia, and arthralgia.
- Clinically significant immune-related adverse events for patients who received atezolizumab included pneumonitis, hepatitis, colitis, and thyroid disease.
Everolimus, an oral mTOR inhibitor, is clinically active against advanced pancreatic and nonpancreatic neuroendocrine tumors.[ 63 ] Based on the results of the RADIANT-4 clinical trial,[ 63 ] the FDA approved everolimus for the treatment of adult patients with unresectable, locally advanced or metastatic, progressive, well-differentiated (low or intermediate grade), nonfunctional neuroendocrine tumors of lung or gastrointestinal origin.
- Median PFS was 11.0 months in the everolimus arm and 3.9 months in the placebo group (HR, 0.48; 95% CI, 0.35−0.67; P < .00001).[ 63 ][ Level of evidence B1 ]
- In a post hoc analysis of the lung subgroup, median PFS by central review was 9.2 months in the everolimus arm and 3.6 months in the placebo arm (HR, 0.50; 95% CI, 0.28−0.88).[ 63 ]
- Souquet PJ, Chauvin F, Boissel JP, et al.: Polychemotherapy in advanced non small cell lung cancer: a meta-analysis. Lancet 342 (8862): 19-21, 1993. [PUBMED Abstract]
- Ellis PA, Smith IE, Hardy JR, et al.: Symptom relief with MVP (mitomycin C, vinblastine and cisplatin) chemotherapy in advanced non-small-cell lung cancer. Br J Cancer 71 (2): 366-70, 1995. [PUBMED Abstract]
- Girling DJ, et al.: Randomized trial of etoposide cyclophosphamide methotrexate and vincristine versus etoposide and vincristine in the palliative treatment of patients with small-cell lung cancer and poor prognosis. Br J Cancer 67 (Suppl 20): A-4;2, 14, 1993.
- Fossella FV, DeVore R, Kerr RN, et al.: Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol 18 (12): 2354-62, 2000. [PUBMED Abstract]
- Shepherd FA, Dancey J, Ramlau R, et al.: Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 18 (10): 2095-103, 2000. [PUBMED Abstract]
- Huisman C, Smit EF, Giaccone G, et al.: Second-line chemotherapy in relapsing or refractory non-small-cell lung cancer: a review. J Clin Oncol 18 (21): 3722-30, 2000. [PUBMED Abstract]
- Di Maio M, Perrone F, Chiodini P, et al.: Individual patient data meta-analysis of docetaxel administered once every 3 weeks compared with once every week second-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 25 (11): 1377-82, 2007. [PUBMED Abstract]
- Garon EB, Ciuleanu TE, Arrieta O, et al.: Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 384 (9944): 665-73, 2014. [PUBMED Abstract]
- Hanna N, Shepherd FA, Fossella FV, et al.: Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 22 (9): 1589-97, 2004. [PUBMED Abstract]
- Scagliotti G, Hanna N, Fossella F, et al.: The differential efficacy of pemetrexed according to NSCLC histology: a review of two Phase III studies. Oncologist 14 (3): 253-63, 2009. [PUBMED Abstract]
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al.: Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 353 (2): 123-32, 2005. [PUBMED Abstract]
- Bezjak A, Tu D, Seymour L, et al.: Symptom improvement in lung cancer patients treated with erlotinib: quality of life analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol 24 (24): 3831-7, 2006. [PUBMED Abstract]
- Ciuleanu T, Stelmakh L, Cicenas S, et al.: Efficacy and safety of erlotinib versus chemotherapy in second-line treatment of patients with advanced, non-small-cell lung cancer with poor prognosis (TITAN): a randomised multicentre, open-label, phase 3 study. Lancet Oncol 13 (3): 300-8, 2012. [PUBMED Abstract]
- Thatcher N, Chang A, Parikh P, et al.: Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 366 (9496): 1527-37, 2005 Oct 29-Nov 4. [PUBMED Abstract]
- Kim ES, Hirsh V, Mok T, et al.: Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet 372 (9652): 1809-18, 2008. [PUBMED Abstract]
- Miller VA, Kris MG, Shah N, et al.: Bronchioloalveolar pathologic subtype and smoking history predict sensitivity to gefitinib in advanced non-small-cell lung cancer. J Clin Oncol 22 (6): 1103-9, 2004. [PUBMED Abstract]
- Paez JG, Jänne PA, Lee JC, et al.: EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304 (5676): 1497-500, 2004. [PUBMED Abstract]
- Lynch TJ, Bell DW, Sordella R, et al.: Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350 (21): 2129-39, 2004. [PUBMED Abstract]
- Pao W, Miller V, Zakowski M, et al.: EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 101 (36): 13306-11, 2004. [PUBMED Abstract]
- Pao W, Wang TY, Riely GJ, et al.: KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med 2 (1): e17, 2005. [PUBMED Abstract]
- Tsao MS, Sakurada A, Cutz JC, et al.: Erlotinib in lung cancer - molecular and clinical predictors of outcome. N Engl J Med 353 (2): 133-44, 2005. [PUBMED Abstract]
- Hirsch FR, Varella-Garcia M, Bunn PA, et al.: Molecular predictors of outcome with gefitinib in a phase III placebo-controlled study in advanced non-small-cell lung cancer. J Clin Oncol 24 (31): 5034-42, 2006. [PUBMED Abstract]
- Clark GM, Zborowski DM, Culbertson JL, et al.: Clinical utility of epidermal growth factor receptor expression for selecting patients with advanced non-small cell lung cancer for treatment with erlotinib. J Thorac Oncol 1 (8): 837-46, 2006. [PUBMED Abstract]
- Soria JC, Felip E, Cobo M, et al.: Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol 16 (8): 897-907, 2015. [PUBMED Abstract]
- Mok TS, Wu Y-L, Ahn M-J, et al.: Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 376 (7): 629-640, 2017. [PUBMED Abstract]
- Park K, Haura EB, Leighl NB, et al.: Amivantamab in EGFR Exon 20 Insertion-Mutated Non-Small-Cell Lung Cancer Progressing on Platinum Chemotherapy: Initial Results From the CHRYSALIS Phase I Study. J Clin Oncol 39 (30): 3391-3402, 2021. [PUBMED Abstract]
- Zhou C, Ramalingam SS, Kim TM, et al.: Treatment Outcomes and Safety of Mobocertinib in Platinum-Pretreated Patients With EGFR Exon 20 Insertion-Positive Metastatic Non-Small Cell Lung Cancer: A Phase 1/2 Open-label Nonrandomized Clinical Trial. JAMA Oncol 7 (12): e214761, 2021. [PUBMED Abstract]
- Kwak EL, Bang YJ, Camidge DR, et al.: Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 363 (18): 1693-703, 2010. [PUBMED Abstract]
- Shaw AT, Yeap BY, Solomon BJ, et al.: Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol 12 (11): 1004-12, 2011. [PUBMED Abstract]
- Shaw AT, Kim DW, Nakagawa K, et al.: Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 368 (25): 2385-94, 2013. [PUBMED Abstract]
- Shaw AT, Kim DW, Mehra R, et al.: Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 370 (13): 1189-97, 2014. [PUBMED Abstract]
- Shaw AT, Gandhi L, Gadgeel S, et al.: Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol 17 (2): 234-42, 2016. [PUBMED Abstract]
- Ou SH, Ahn JS, De Petris L, et al.: Alectinib in Crizotinib-Refractory ALK-Rearranged Non-Small-Cell Lung Cancer: A Phase II Global Study. J Clin Oncol 34 (7): 661-8, 2016. [PUBMED Abstract]
- Solomon BJ, Besse B, Bauer TM, et al.: Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol 19 (12): 1654-1667, 2018. [PUBMED Abstract]
- Planchard D, Besse B, Groen HJM, et al.: Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol 17 (7): 984-993, 2016. [PUBMED Abstract]
- Skoulidis F, Li BT, Dy GK, et al.: Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N Engl J Med 384 (25): 2371-2381, 2021. [PUBMED Abstract]
- Jänne PA, Riely GJ, Gadgeel SM, et al.: Adagrasib in Non-Small-Cell Lung Cancer Harboring a KRASG12C Mutation. N Engl J Med 387 (2): 120-131, 2022. [PUBMED Abstract]
- U.S. Food and Drug Administration: FDA grants accelerated approval to adagrasib for KRAS G12C-mutated NSCLC. Food and Drug Administration, 2022. Available online . Last accessed August 30, 2024.
- Riudavets M, Sullivan I, Abdayem P, et al.: Targeting HER2 in non-small-cell lung cancer (NSCLC): a glimpse of hope? An updated review on therapeutic strategies in NSCLC harbouring HER2 alterations. ESMO Open 6 (5): 100260, 2021. [PUBMED Abstract]
- Li BT, Smit EF, Goto Y, et al.: Trastuzumab Deruxtecan in HER2-Mutant Non-Small-Cell Lung Cancer. N Engl J Med 386 (3): 241-251, 2022. [PUBMED Abstract]
- Brahmer J, Reckamp KL, Baas P, et al.: Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 373 (2): 123-35, 2015. [PUBMED Abstract]
- Borghaei H, Paz-Ares L, Horn L, et al.: Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 373 (17): 1627-39, 2015. [PUBMED Abstract]
- Garon EB, Rizvi NA, Hui R, et al.: Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372 (21): 2018-28, 2015. [PUBMED Abstract]
- Horn L, Spigel DR, Vokes EE, et al.: Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). J Clin Oncol 35 (35): 3924-3933, 2017. [PUBMED Abstract]
- Herbst RS, Baas P, Kim DW, et al.: Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387 (10027): 1540-50, 2016. [PUBMED Abstract]
- Rittmeyer A, Barlesi F, Waterkamp D, et al.: Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389 (10066): 255-265, 2017. [PUBMED Abstract]
- Fehrenbacher L, Spira A, Ballinger M, et al.: Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 387 (10030): 1837-46, 2016. [PUBMED Abstract]
Latest Updates to This Summary (08/30/2024)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Treatment of Stage IIIA Non-Small Cell Lung Cancer (NSCLC)
Added Osimertinib (for patients with EGFR mutations) as a new subsection.
This summary is written and maintained by the PDQ Adult Treatment Editorial Board , which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages.
About This PDQ Summary
Purpose of this summary.
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of non-small cell lung cancer. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board , which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Non-Small Cell Lung Cancer Treatment are:
- Janet Dancey, MD, FRCPC (Ontario Institute for Cancer Research & NCIC Clinical Trials Group)
- Meredith McAdams, MD (National Cancer Institute)
- Monaliben Patel, MD (University of Rochester Medical Center)
- Arun Rajan, MD (National Cancer Institute)
- Eva Szabo, MD (National Cancer Institute)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us . Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Non-Small Cell Lung Cancer Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/lung/hp/non-small-cell-lung-treatment-pdq . Accessed <MM/DD/YYYY>. [PMID: 26389304]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online , a collection of over 2,000 scientific images.
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us .
Lung Cancer Clinical Presentation
There are no specific signs and symptoms for lung cancer, and the clinical presentation of lung cancer may vary from patient to patient. At the time of diagnosis, the vast majority of patients with lung cancer have advanced disease. The frequent absence of symptoms until locally advanced or metastatic disease is present reveals the aggressive nature of lung cancer and also highlights the urgent need to expand efforts to routinely screen patients at high risk. Research indicates that many cases of lung cancer are often detected incidentally via chest imaging. In about 7% to 10% of cases, lung cancer is detected and diagnosed in asymptomatic patients when a chest radiograph is performed to diagnose other conditions. At initial diagnosis, 20% of patients have localized disease, 25% of patients have regional metastasis, and 55% of patients have distant disease spread. In some cases, high-risk patients may be diagnosed while asymptomatic through screening with low-dose computed tomography. About three-fourths of nonscreened patients with lung cancer present with one or more symptoms at the time of diagnosis. The most common symptoms include cough, dyspnea, and hemoptysis. Although the clinical presentation of lung cancer is not specific to the classification or histology of the cancer, certain obstacles may be more likely with different types. One study noted that the most common symptoms at presentation were cough (55%), dyspnea (45%), pain (38%), and weight loss (36%), as well as hemoptysis. The new onset of cough in a smoker or former smoker should raise suspicion for lung cancer. The clinical manifestations of lung cancer may be due to intrathoracic effects of the tumor (e.g., cough, hemoptysis, pleural disease), extrathoracic metastases (most commonly liver, bone, brain), or paraneoplastic phenomena (e.g., hypercalcemia, Cushing syndrome, hypercoagulability disorders, various neurologic syndromes). Squamous cell and small cell cancers usually cause a cough early due to the involvement of the central airways. Hemoptysis is a significant symptom in anyone with a history of smoking. Although bronchitis is the most frequent cause of hemoptysis, 20% to 50% of patients with underlying lung cancer present with hemoptysis. While rare, patients with lung cancer may present with shoulder pain, Horner syndrome, and hand-muscle atrophy, and this group of symptoms is referred to as Pancoast syndrome . Pancoast syndrome is most frequently due to lung cancers arising in the superior sulcus. Metastasis from lung cancer to bone is generally symptomatic, and pain in the back, chest, or extremity and elevated levels of serum alkaline phosphatase are frequently present in patients who have bone metastasis. Moreover, levels of serum calcium may be elevated due to extensive bone disease, and an estimated 20% of patients with non–small cell lung cancer have bone metastases at the time of diagnosis. The American Cancer Society (ACS) indicates that in addition to the signs and symptoms mentioned above, patients with lung cancer may also experience hoarseness, new-onset wheezing, and fatigue. The ACS also encourages individuals to seek medical care early if they are experiencing any symptoms, since early detection and treatment may improve overall clinical outcomes in some patients. The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.

August 2024

Related Content
Contemporary developments in met-selective kinase inhibitors in advanced non–small-cell lung cancer.

- Advertising Contacts
- Editorial Staff
- Professional Organizations
- Submitting a Manuscript
- Privacy Policy
- Classifieds
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
September 8, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
TROP2 expression a promising predictor of clinical outcomes in patients with advanced or metastatic NSCLC
by International Association for the Study of Lung Cancer
New data presented today demonstrate that TROP2 expression as measured by quantitative continuous scoring (QCS), a computational pathology approach, is a promising predictor of clinical outcomes in patients with advanced or metastatic non-small cell lung cancer (NSCLC) treated with the TROP2 antibody-drug conjugate (ADC) datopotamab deruxtecan (Dato-DXd). The data showed that patients with TROP2 positivity, as determined by QCS, experienced improved efficacy with Dato-DXd compared to patients receiving docetaxel in the TROPION-Lung01 phase III trial.
The study was presented today at the International Association for the Study of Lung Cancer (IASLC) 2024 World Conference on Lung Cancer by Dr. Marina Chiara Garassino, from The University of Chicago.
Dato-DXd is a TROP2-directed ADC designed with a unique plasma-stable linker that necessitates active internalization for effective payload release. Traditional methods of assessing TROP2 expression through visual scoring of immunohistochemical (IHC) assays have not been predictive of responses to TROP2-directed ADCs in NSCLC patients. To address this gap, the authors hypothesized that a more precise, quantitative measurement of TROP2 expression both on the cell membrane and in the cytoplasm would better predict therapeutic responses to Dato-DXd.
This analysis utilized digitalized TROP2 IHC-stained whole-slide images (WSI) from NSCLC patients to develop the QCS model. This deep learning algorithm , trained on pathologists' annotations, identifies tumor areas and cellular compartments (membrane and cytoplasm) within the WSI. The QCS model calculates TROP2 expression in the membrane relative to the cytoplasm of tumor cells, producing a normalized membrane ratio (NMR). Tumors were classified as TROP2 QCS-NMR+ if most tumor cells exhibited an NMR below a predetermined value.
The QCS-NMR was optimized for progression-free survival (PFS) in the biomarker-evaluable subgroup of nonsquamous (NSQ) NSCLC patients without actionable genomic alterations (non-AGA) in TROPION-Lung01, which compared Dato-DXd to docetaxel in second-line or later advanced or metastatic NSCLC. Clinical outcomes were assessed across all biomarker-evaluable patients.
Out of 604 patients randomized in TROPION-Lung01, 352 were biomarker-evaluable, with 221 in the NSQ/non-AGA subgroup. The baseline characteristics were consistent between randomized and biomarker-evaluable populations, showing similar overall PFS outcomes. Among the evaluable patients, 63% were classified as TROP2 QCS-NMR+. The highest prevalence of TROP2 QCS-NMR+ was observed in the NSQ/AGA subgroup (75%), followed by NSQ/non-AGA (63%) and squamous (SQ) (43%) subgroups.
Data indicated that the objective response rate (ORR) was higher and median PFS was longer with Dato-DXd compared to docetaxel in TROP2 QCS-NMR+ subgroups. The rates of overall and grade 3+ adverse events were similar regardless of TROP2 QCS-NMR status.
"It is important that research identifies how to optimize treatment options for patients with NSCLC. This exploratory analysis is going in this direction and reveals that Dato-DXd exhibits robust efficacy in patients with TROP2 QCS-NMR+ advanced non-small cell lung cancer , in the nonsquamous, non-AGA subgroup," Dr. Garassino reported.
The QCS-measured TROP2 NMR shows potential as a predictive biomarker for Dato-DXd response. Further studies are underway to validate this biomarker in the first-line advanced or metastatic NSCLC setting.
Explore further
Feedback to editors

Team discovers role of ferroptosis in combating breast cancer resistance
10 hours ago

Metformin found to reduce organ aging in male monkeys
11 hours ago

Researchers discover new target for treating heart failure: Protein kinase N

RNA-sequencing study provides novel insights into chronic lymphocytic leukemia
12 hours ago

Scientists discover potential cause of an enigmatic vascular disease primarily impacting women

Key factors identified that can impact long-term weight loss in patients with obesity prescribed GLP-1 RA medications

Study finds 'supercharging' T cells with mitochondria enhances their antitumor activity

Using AI, researchers find e-cigarette brands are skirting the rules about health warning labels on Instagram

New therapy that targets and destroys tau tangles: A promising Alzheimer's disease treatment

Neoself-antigens found to induce autoimmune response in lupus
Related stories.
Clinical trial: Combining durvalumab with novel agents increases pathological responses in resectable NSCLC
Sep 8, 2024

Clinical trial finds antibody-drug conjugate helps patients with metastatic non-small cell lung cancer live longer
Oct 26, 2023
Tumor mutational burden not significantly associated with efficacy of pembrolizumab
Sep 9, 2019

Ivonescimab outperforms pembrolizumab in study for first-line treatment of PD-L1-positive advanced NSCLC: Clinical trial

Researchers identify mechanisms of resistance to drug for triple-negative breast cancer
Aug 17, 2021

Tumor hyaluronan as a novel biomarker in non-small cell lung cancer: A retrospective study
Nov 9, 2022
Recommended for you

Antibody-drug conjugate found effective against brain metastases in patients with HER2-positive breast cancer
13 hours ago

How the immune system fails as cancer arises
Sep 12, 2024

Study identifies five key factors that predict response of cancer patients to immunotherapy
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Ready to start planning your care? Call us at 800-525-2225 to make an appointment.
A Phase 2 Study of Sotorasib to Treat Advanced KRAS G12C-Mutant Non-Small Cell Lung Cancer
The purpose of this study is to see how well sotorasib works in people with advanced lung cancer. The people in this study have non-small cell lung cancer (NSCLC) with a mutation (change) in the KRAS G12C gene. This mutation can cause cancer cells to grow. In addition, the people in this study have not received treatment for their cancer since it became advanced.
Sotorasib is a standard treatment for advanced NSCLC with a KRAS G12C mutation. However, doctors normally give this drug to people who have already received another medication for advanced NSCLC. Researchers are doing this study to assess sotorasib as initial therapy for advanced NSCLC with a KRAS G12C mutation.
Sotorasib targets and blocks the mutated KRAS G12C protein. By blocking this protein, sotorasib may slow or stop the growth of your cancer. It is taken orally (by mouth).
Who Can Join
To join this study, there are a few conditions. You must:
- Have NSCLC with a KRAS G12C mutation.
- Have not received anti-cancer medication for advanced NSCLC.
- Be well enough to walk and take care of yourself. You must be able to do activities such as office work or light housework.
- Be age 18 or older.
For more information or to see if you can join this study, please call Dr. Gregory Riely’s office at 646-608-3913 .
Disease Status
Investigator, co-investigators, clinicaltrials.gov id, clinicaltrials.gov.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Integration of clinical and blood parameters in risk prognostication for patients receiving immunochemotherapy for extensive stage small cell lung cancer: real-world data from two centers
Affiliations.
- 1 Department of Oncology, Beijing Chest Hospital, Capital Medical University, Beijing Tuberculosis and Thoracic Tumor Research Institute, Beijing, 101149, China.
- 2 Department of Oncology, Beijing Tuberculosis and Thoracic Tumor Research Institute, Beijing, 101149, China.
- 3 Cancer Center, Beijing Friendship Hospital, Capital Medical University, Beijing, 100050, China.
- 4 First Clinical Medical College, Shaanxi University of Traditional Chinese Medicine, Xianyang, 712000, Shaanxi, China.
- 5 Cancer Center, Beijing Friendship Hospital, Capital Medical University, Beijing, 100050, China. [email protected].
- 6 Department of Oncology, Beijing Chest Hospital, Capital Medical University, Beijing Tuberculosis and Thoracic Tumor Research Institute, Beijing, 101149, China. [email protected].
- 7 Department of Oncology, Beijing Chest Hospital, Capital Medical University, Beijing Tuberculosis and Thoracic Tumor Research Institute, Beijing, 101149, China. [email protected].
- 8 Laboratory for Clinical Medicine, Capital Medical University, Beijing, 100069, China. [email protected].
- PMID: 39256789
- PMCID: PMC11389556
- DOI: 10.1186/s12916-024-03612-8
Background: Immune checkpoint inhibitors (ICIs) had modest advances in the treatment of extensive-stage small cell lung cancer (ES-SCLC) in clinical trials, but there is a lack of biomarkers for prognosis in clinical practice.
Methods: We retrospectively collected data from ES-SCLC patients who received ICIs combined chemotherapy from two centers in China, integrated clinical and blood parameters, and constructed risk prognostication for immunochemotherapy. The population was divided into high- and low-risk groups, and the performance of the model was assessed separately in the training and validation cohorts.
Results: Two hundred and twenty and 43 patients were included in the training and validation groups, respectively. The important predictors were screened including body mass index, liver metastases, coefficient variation of red blood cell distribution width, lactate dehydrogenase, albumin, and C-reactive protein. Predicting 1-year overall survival (OS), the AUC values under ROC for the model under training, internal validation, and external validation were 0.760, 0.732, and 0.722, respectively, and the calibration curve and clinical decision curve performed well. Applied the model to divide patients into low-risk and high-risk groups, and the median OS was 23.7 months and 9.1 months, and the median progression-free survival was 8.2 months and 4.8 months, respectively; furthermore, this ability to discriminate survival was also observed in the validation cohort.
Conclusions: We constructed a novel prognostic model for ES-SCLC to predict survival employing baseline tumor burden, nutritional and inflammatory parameters, it is easily measured to screen high-risk patient populations.
Keywords: Biomarkers; Extensive stage small cell lung cancer; Immunochemotherapy; Prognostic models; Real-world studies.
© 2024. The Author(s).
PubMed Disclaimer
Conflict of interest statement
The authors declare no competing interests.
- Zou K, Sun P, Huang H, Zhuo H, Qie R, Xie Y, et al. Etiology of lung cancer: Evidence from epidemiologic studies. Journal of the National Cancer Center. 2022;2(4):216–25. 10.1016/j.jncc.2022.09.004. 10.1016/j.jncc.2022.09.004 - DOI - PMC - PubMed
- Micke P, Faldum A, Metz T, Beeh KM, Bittinger F, Hengstler JG, et al. Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer–what limits limited disease? Lung Cancer. 2002;37(3):271–6. 10.1016/s0169-5002(02)00072-7. 10.1016/s0169-5002(02)00072-7 - DOI - PubMed
- Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer. 2015;121(5):664–72. 10.1002/cncr.29098. 10.1002/cncr.29098 - DOI - PMC - PubMed
- Rudin CM, Brambilla E, Faivre-Finn C, Sage J. Small-cell lung cancer. Nat Rev Dis Primers. 2021;7(1):3. 10.1038/s41572-020-00235-0. 10.1038/s41572-020-00235-0 - DOI - PMC - PubMed
- Wang Q, Gümüş ZH, Colarossi C, Memeo L, Wang X, Kong CY, et al. SCLC: Epidemiology, Risk Factors, Genetic Susceptibility, Molecular Pathology, Screening, and Early Detection. J Thorac Oncol. 2023;18(1):31–46. 10.1016/j.jtho.2022.10.002. 10.1016/j.jtho.2022.10.002 - DOI - PMC - PubMed
Publication types
- Search in MeSH
Related information
Grants and funding.
- Z211100002921013/Beijing Municipal Science and Technology Commission
- YH201920/Tongzhou Lianggao Talents Project
- JYY2023-15/Beijing Municipal Public Welfare Development and Reform Pilot Project for Medical Research Institutes
LinkOut - more resources
Full text sources.
- BioMed Central
- MedlinePlus Health Information
Research Materials
- NCI CPTC Antibody Characterization Program

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- World J Clin Cases
- v.12(25); 2024 Sep 6
- PMC11263051
Returning from the afterlife? Sotorasib treatment for advanced KRAS mutant lung cancer: A case report
Ming-xing wang.
Department of Medical Oncology, Lu’an Hospital of Traditional Chinese Medicine Affiliated to Anhui University of Chinese Medicine, Lu’an 237000, Anhui Province, China
Department of Medical Oncology, Lu’an Hospital of Traditional Chinese Medicine, Lu’an 237000, Anhui Province, China
Qing-Ming Sun
Wan-hui dong.
Department of Medical Oncology, Lu’an Hospital of Traditional Chinese Medicine, Lu’an 237000, Anhui Province, China. nc.yyzsal@iuhnawgnod
Corresponding author: Wan-Hui Dong, MD, Chief Doctor, Department of Medical Oncology, Lu’an Hospital of Traditional Chinese Medicine, No. 76 Renmin Road, Lu’an 237000, Anhui Province, China. nc.yyzsal@iuhnawgnod
Lung cancer is increasing in incidence worldwide, and targeted therapies are developing at a rapid pace. Furthermore, the KRAS specific gene is strongly associated with non-small cell lung cancer (NSCLC). Adult patients with locally advanced or metastatic NSCLC who have tested positive for the KRAS G12C mutation and have progressed after at least one systemic treatment are treated with sotorasib.
CASE SUMMARY
In this study, we report on an advanced NSCLC with a KRAS G12C mutation. The histological diagnosis indicates stage IVB left lung adenocarcinoma with pelvic and bone metastases, identified as cT4N2bM1c. Using circulating tumor DNA analysis, it was possible to determine the mutation abundance of the KRAS gene exon 2, c.34G>Tp.G12C, which was 32.3%. The patient was advised to take sotorasib as part of their treatment. The imaging data were compared before and after treatment. Furthermore, clinical reassessments and regular serial blood testing were conducted. We found that the patient’s clinical symptoms significantly improved after receiving sotorasib medication, and there were no notable side effects, such as liver toxicity, during the treatment.
Sotorasib has shown promising clinical efficacy in patients with the KRAS G12c mutation and has no apparent toxic side effects.
Core Tip: In this study, we demonstrate the efficacy of sotorasib in treating advanced non-small cell lung cancer with the KRAS G12C mutation. A patient diagnosed with stage IVB adenocarcinoma, exhibiting pelvic and bone metastases, showed a significant improvement in clinical symptoms post-sotorasib treatment, with no severe side effects observed. This case highlights the potential of targeted therapies in personalized cancer treatment, emphasizing the importance of genomic profiling for therapeutic decision-making.
INTRODUCTION
Primary bronchogenic carcinoma, also known as lung cancer, primarily consists of two types: small cell lung cancer and non-small cell lung cancer (NSCLC), with the latter accounting for approximately 85% of all lung cancer cases[ 1 , 2 ]. Tobacco usage is the most common cause of lung cancer[ 3 ]. The International Agency for Research on Cancer of the World Health Organization has released its latest data on the global cancer burden. In 2020, there were 9.96 million cancer-related deaths worldwide, with 1.8 million of those fatalities attributed to lung cancer, which is significantly higher than any other type of cancer[ 4 ]. Currently, chemotherapy, targeted therapy, and immunotherapy are the primary treatments for non-small cell lung cancer worldwide.
As a form of systemic treatment, chemotherapy has significant toxic side effects on the entire body. In recent years, the effectiveness of immunotherapy and targeted therapy has been remarkable. Immunotherapy, which targets tumor cells or immune cells through immune checkpoint inhibitors to produce therapeutic effects, has demonstrated superior efficacy in treating esophageal cancer, lung cancer, and other tumors. This treatment can significantly extend the survival period, but can also increase the occurrence of adverse reactions, such as liver function abnormalities[ 5 - 8 ]. Consequently, targeted therapy is increasingly crucial in lung cancer treatment.
One of the frequently mutated genes in the RAS (rat sarcoma) gene family is KRAS (Kirsten-RAS), which regulates the signaling pathways responsible for cell development, differentiation, proliferation, and survival. The first small molecule chemical that directly suppresses RAS was discovered by Herbert Waldmann[ 9 ]. Since then, other RAS inhibitors have been discovered[ 10 ]. Twenty-three percent of patients with NSCLC have KRAS mutations, with the most common mutation being G12C. KRAS G12C mutants are associated with cancer due to their ability to maintain an active state[ 11 ]. The United States Food and Drug Administration authorized sotorasib, the first medication specifically designed to treat KRAS mutations, on 28 May 2021. This medication is a KRASG12C inhibitor developed by Amgen, which locks KRAS in an inactive conformation, thereby irreversibly inhibiting KRASG12C[ 12 ].
We present a case study of a 52-year-old female patient who was diagnosed with adenocarcinoma in her left lung. With cT4N2M1 characteristics, the tumor was classified as stage IV and was accompanied by metastases of the bone and pelvis. A G12C mutation was identified through genetic testing. Following comprehensive systemic targeted therapy and immunotherapy, the patient received sotorasib—a novel targeted medication—that resulted in substantial clinical benefits. We mention this case because the effectiveness of sotorasib, a targeted drug used in patients with G12C mutant NSCLC, has not been widely clinically validated in China. Although sotorasib has been approved in other countries for the treatment of certain types of advanced NSCLC, particularly those carrying the G12C gene mutation, the efficacy and safety data are insufficient in China due to a limited number of clinical trials or inadequate sample size. Therefore, this case provides practical clinical evidence of the positive therapeutic effect that sotorasib may have on Chinese patients. This will not only help promote further research and development of the drug in China but also provide additional reference information for doctors when selecting treatment options. This case may stimulate more clinical research interest and promote future larger-scale clinical trials in China to accurately evaluate the efficacy and safety of sotorasib in Chinese lung cancer patients.
CASE PRESENTATION
Chief complaints.
A 52-year-old female patient was admitted to our hospital after experiencing recurrent episodes of unexplained chest tightness.
History of present illness
At the beginning of September 2019, the patient experienced repeated chest distress without obvious inducement. During the course of the disease, there was no fever, hemoptysis, cough, or expectoration. The patient experienced occasional chest distress, shortness of breath, left chest pain, dizziness, headache, skin petechiae, ecchymosis, normal defecation, poor appetite, sleep, and weight loss of approximately 9 kg in the April prior.
History of past illness
The patient was in good health, and denied a history of hypertension, diabetes, coronary heart disease, hepatitis, tuberculosis, other infectious diseases, trauma and blood transfusion, surgery, and food allergies. She reported a history of penicillin allergy, and had no history of vaccination with the Xinguan vaccine.
Personal and family history
The patient denied ever smoking, having a family history of cancer, or being exposed to high-risk work environments.
Physical examination
The patient’s skin and mucosa showed no yellow staining or petechiae. The superficial lymph nodes were not enlarged. Respiratory sounds of both lungs included wet rales. Percussion revealed dullness. The heart rate was 90 beats/min, with a regular rhythm and no pathological murmur heard. The abdomen was soft, with the liver and spleen not palpable under the ribs. There was no tenderness or rebound pain, and the ascites sign was negative. Both lower limbs were not swollen, and there was no edema.
Laboratory examinations
Adenocarcinoma was revealed by a puncture biopsy of the left lung lesion (Figure (Figure1A). 1A ). Other results included the following IHC results: P40 negativity; CK5/6 negativity; P63 negativity; Ki-67 approximately 30% positivity; CK7 positivity; TTF-1 positivity; and NapsinA partial positivity. Genetic testing revealed a KRAS G12C mutation and wild-type epidermal growth factor receptor (EGFR). The following lung tumor markers were evaluated on 2 November 2019: CYFRA21-1 4.05 ng/mL (elevated); carbohydrate antigen 125 (CA125) 242.60 U/mL (elevated); neuron-specific enolase 17.54 ng/mL (elevated); and carcinoembryonic antigen (CEA) ≤ 1000 ng/mL. On 22 February 2023, an enzyme-linked immunosorbent assay enzyme substrate biopsy of the abdominal and pelvic masses revealed adenocarcinoma, suggesting lung cancer as a potential source (Figure (Figure1B 1B ).

Pathology of puncture. A: Left lung; B: Pelvic mass.
Imaging examinations
On 11 September 2019, thoracic and abdominal enhanced computed tomography (CT) showed the following: (1) Inflammation in the lower left lung, small pleural effusion on the left side, small nodules in the posterior segment of the upper lobe of the left lung, and enlarged hilar lymph nodes on the left lung; and (2) Occupying the uterus, full cervical volume.
A chest CT on 17 October 2019 revealed possible lung cancer in the lower lobe of the left lung, enlarged lymph nodes in the mediastinum and hilum of the lung, and multiple nodules on the left pleura, suggesting metastasis; left pleural effusion. A pelvic MRI conducted on 18 July 2022 revealed the presence of both bone and pelvic metastases. On 12 February 2023, a CT scan revealed multiple metastases in the abdomen and pelvis, along with abdominal pelvic effusion.
MULTIDISCIPLINARY EXPERT CONSULTATION
After consulting with pulmonary disease experts, the diagnosis was confirmed as adenocarcinoma of the left lung with a KRAS G12c gene mutation.
FINAL DIAGNOSIS
The patient was finally diagnosed with adenocarcinoma of the left lung, stage cT4N2bM1c.
In our department, we delivered four consecutive cycles of chemotherapy using a pemetrexed + cisplatin regimen. Eight sessions of oral maintenance chemotherapy using Tegio capsules as the sole medication followed this. We then treated the patient with a combination of pemetrexed + carboplatin + bevacizumab injections. On 9 February 2022, a single cycle of chemotherapy began with an injection of albumin paclitaxel, carboplatin, and bevacizumab. This was followed by three cycles of chemotherapy, including two cycles of bevacizumab, pemetrexed, and carboplatin, and a final adjustment to bevacizumab and pemetrexed due to bone marrow suppression.
Thereafter, recombinant human endostatin targeted therapy and tislelizumab immunotherapy were combined and administered for five courses. On 15 November 2022, the final course of treatment was administered, which included symptomatic measures such as bone protection. During the course of the illness, the patient’s Karnofsky Performance Status (KPS) score dropped below 50. She was unable to take care of herself, spent most days in bed, and her blood pressure decreased below the baseline level. Considering the patient’s frail constitution, high susceptibility to intravenous chemotherapy, immune-related factors, and the tumor’s ongoing progression, as well as the patient’s young age and refusal to discontinue treatment, it was ultimately recommended that the patient undergo independent sotorasib treatment. This recommendation is based on the presence of a KRASG12C target mutation. Due to the patient’s limited financial resources and poor physical condition, it was recommended that she be administered half the recommended dose.
The patient began taking sotorasib 240 mg twice a day on 15 March 2023. The whole treatment flow chart of this patient is shown in Figure Figure2 2 .

Treatment flow chart. A: September 2019: Diagnosis with lung cancer and type of gene mutation; B: November 2019: Four cycles of pemetrexed + cisplatin, followed by eight oral tegafur maintenance cycles; C: Adjustment of medication due to myelosuppression; D: Chemotherapy and targeted therapy on 9 February 2022; E: Received immunotherapy on 18 July 2022; F: Metastasis occurred again in February 2023; G: In July 2023, the tumor regressed and the patient improved; H: Sotorasib started on 15 March 2023.
OUTCOME AND FOLLOW-UP
After a week on sotorasib, the patient’s quality of life score gradually improved. After a 4-month observation period, a CT scan showed a decrease in the size of the left lung lesion, a reduction in pulmonary metastases, the absence of pelvic metastases, and the resolution of peritoneal effusion (Figure (Figure3). 3 ). Furthermore, the patient experienced a significant decrease in CEA and CA125 tumor markers (Figure (Figure4A 4A and Figure Figure4B 4B ).

Computed tomography tracking of changes in lung, peritoneal fluid, and pelvic mass before and after treatment with sotorasib. A: Lungs before treatment in the sotorasib group; B: Abdomen before sotorasib treatment; C: Pelvis before sotorasib treatment; D: Lungs after sotorasib treatment; E: Abdomen after sotorasib treatment; F: Pelvis after sotorasib treatment.

Changes in tumor markers. A: Carcinoembryonic antigen (CEA) values; B: Carbohydrate antigen 125 (CA125) values.
The patient is now fully self-sufficient, and the KPS score has increased from 50 to 90 points (Figure (Figure5). 5 ). The patient did not experience significant toxic side effects during the treatment with sotorasib, and her liver and kidney functions, as well as blood routine, were normal.

Changes in Karnofsky Performance Status score. KPS: Karnofsky Performance Status.
Significant progress has been made in the treatment of NSCLC over the last decade. This includes the development of immune checkpoint inhibitors, anti-angiogenic therapy, and personalized therapeutics for actionable genetic alterations[ 13 - 15 ]. The standard of care for patients with advanced illness and no modifiable changes is to administer immunotherapy either sequentially or simultaneously with platinum-based chemotherapy. However, there are few approved therapeutic options available as the cancer progresses, with docetaxel being a recommended choice[ 16 , 17 ]. There is a pressing need to develop safe and effective therapy options for patients with advanced NSCLC, as some patients may not be able to tolerate more aggressive treatments to achieve beneficial results.
Approximately 25%-39% of non-squamous NSCLCs have activating mutations in KRAS, while 13%-16% of lung adenocarcinomas have the KRAS G12C mutation[ 18 - 21 ]. With respect to well-established actionable driver genomic alterations (such as EGFR, ALK, ROS1, BRAF, MET, RET, NTRK, and HER2), the KRASG12C mutation is nearly always exclusive[ 22 - 24 ]. For over 40 years, KRAS has been considered undruggable, underscoring the unmet need for these patients to receive targeted therapy rather than chemotherapy. The KRAS G12C protein contains a targetable regulatory pocket, which was discovered in 2013[ 25 ]. Sotorasib, a small molecule, specifically and irreversibly inhibits the KRAS G12C protein. This action traps KRAS G12C in the inactive GDP-bound state, inhibits downstream signaling in cancer cells, and reduces oncogenesis (Appendix p4)[ 26 ].
The first randomized phase 3 trial for a KRAS G12C inhibitor, CodeBreaK 200, demonstrates that sotorasib significantly improved progression-free survival compared to docetaxel[ 27 ]. Sotorasib also decreased the relative risk of disease progression or death by 34%. When sotorasib was used instead of docetaxel, there was a significant increase in overall response, as well as a faster and more enduring response in the sotorasib group. The overall response rate and progression-free survival favored sotorasib in all subgroups. The experimental results sufficiently establish the basis for the clinical use of sotorasib.
Although sotorasib’s effectiveness in treating NSCLC has been recognized internationally, there is no substantial experimental data from China. A 52-year-old female patient diagnosed with stage IV cT4N2M1 lung adenocarcinoma, as well as pelvic and bone metastases, was selected by our department. Imaging results indicated that the largest diameter of pelvic lesions was approximately 126 mm. The tumor measured 85 mm before treatment with sotorasib. Following treatment, there was no evidence of pelvic metastasis, the left lung lesion decreased in size, the pulmonary metastasis reduced, and the peritoneal fluid disappeared. Leukocyte count, alanine aminotransferase, aspartate aminotransferase, creatinine, and estimated glomerular filtration rate were monitored during sotorasib therapy. The results showed that there was no noticeable decrease in the mentioned variables. In comparison to docetaxel, sotorasib was found to be better tolerated and had fewer major adverse events associated with treatment (grade 3 or worse) in the CodeBreaK 200 study. Hepatotoxicity was the most frequent treatment-related adverse event that resulted in the withdrawal of sotorasib. Patients who received immunotherapy less than 2.6 months before starting sotorasib treatment experienced a higher incidence of this side effect. On 15 November 2022, 5 months before receiving sotorasib, the selected case for this trial received the final dose of tirelizumab 200 mg q3w vaccination + Enduranti-vascular targeted therapy. So far, there have been no signs of hepatotoxicity, indicating that the patient is tolerating the medication well. Furthermore, the patient exhibited no obvious signs of medication side effects. Consequently, sotorasib may yield unexpected clinical outcomes without inducing severe side effects in patients undergoing multi-line therapy for advanced lung adenocarcinoma with a G12c mutation. However, sotorasib has not been extensively utilized in clinical trials and requires more data samples to support its usage due to its high cost and the fact that only 20% of KRAS protein Gly12 mutations in lung cancer are Cys12 (KRAS G12C).
Combined with the current research progress of targeted therapy, we believe that the reasons why sotorasib has not been widely expanded in China are as follows: sotorasib has not been marketed in China, and there is no relevant medication experience and guidance for such a large population, which is obviously not suitable. In addition, the cost of genetic testing is relatively high, and most Chinese patient’s families cannot afford the high cost of treatment and testing, which will affect the use of targeted drugs. Sotorasib, a novel anticancer drug, is increasingly used worldwide, especially in patients with advanced NSCLC specific gene mutations. However, sotorasib has not been officially launched in the Chinese market, which means that doctors and patients in China lack practical experience and professional guidance on this drug. Without sufficient clinical data to support it, blind promotion may pose unforeseen risks. In this context, reporting on the response and efficacy of sotorasib in Chinese patients is particularly important. By sharing specific cases, reports can provide valuable reference information for domestic counterparts to help them better understand the application prospects of sotorasib in Chinese individuals. Furthermore, this will encourage the relevant domestic authorities to expedite the approval process of sotorasib, enabling more patients to benefit from this innovative drug promptly. Therefore, it is important to report the efficacy and safety of sotorasib in Chinese patients. By gaining insight into the use of sotorasib in the Chinese population, physicians can better tailor treatment regimens to prolong patient survival and improve quality of life. In the next 5 years, as sotorasib expands in the Chinese market, the drug will be more widely used. If the effect is significant, the targeted drugs for the same site will be continuously updated to better serve patients.
There are limitations to this study. (1) Sample size limitations: Since this is a single case study, the results may not be generalized to all NSCLC patients with KRAS G12C mutations; (2) Individual differences in patients: A patient’s response may be influenced by their unique genetic background, lifestyle, environmental factors, and other health conditions that may influence the effectiveness and tolerability of treatment; (3) Under-observation of treatment duration: Case studies may not provide sufficient information to assess long-term efficacy and safety, including long-term side effects and the likelihood of disease recurrence; (4) Lack of control group: There was no control group to compare the effects of sotorasib treatment; and (5) Lack of statistical rigor: Due to the lack of statistical comparisons, it is difficult to determine whether the observed efficacy is statistically significant. To address these limitations, future work should include multicenter, large-scale clinical trials to improve the universality and reliability of results. Focus should be on individualized medicine, considering individual differences in patients, and personalize treatment protocols through genomics, biomarkers, and other clinical data. At the same time, more long-term follow-up studies should be carried out to evaluate the long-term efficacy and safety of sotorasib, to better improve clinical efficacy and enhance patient quality of life.
The patient’s distant metastases were significantly reduced with sotorasib in this case of stage IV left lung adenocarcinoma (T4N2M1). The patient, who had advanced lung cancer and was at risk of serious tumor-related complications, achieved a long-term remission because of this. Five months prior to starting sotorasib, the patient underwent immunotherapy and did not experience any serious side effects, such as hepatotoxicity, while receiving the medication. Sotorasib’s impact on quality of life in a first-line setting is currently being evaluated worldwide, either in combination with chemotherapy or as a precursor to immunotherapy prior to initiating treatment. This situation could inspire some thoughts. Additionally, many nations, such as China, have not yet adopted sotorasib. Clinicians may be encouraged by this example to identify and manage KRAS G12C.
ACKNOWLEDGEMENTS
The authors would like to express their sincere gratitude to the Lu’an Hospital of Traditional Chinese Medicine Affiliated to Anhui University of Chinese Medicine for their invaluable support and contributions throughout the course of this research. We extend our thanks to the hospital’s administration and staff for providing access to essential facilities and resources that have significantly aided our study.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: All authors declare that they have no conflict of interest to disclose.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade A, Grade C
Novelty: Grade A, Grade C
Creativity or Innovation: Grade A, Grade C
Scientific Significance: Grade A, Grade C
P-Reviewer: Ricci AD S-Editor: Liu JH L-Editor: Filipodia P-Editor: Yu HG
Contributor Information
Ming-Xing Wang, Department of Medical Oncology, Lu’an Hospital of Traditional Chinese Medicine Affiliated to Anhui University of Chinese Medicine, Lu’an 237000, Anhui Province, China.
Pei Zhu, Department of Medical Oncology, Lu’an Hospital of Traditional Chinese Medicine Affiliated to Anhui University of Chinese Medicine, Lu’an 237000, Anhui Province, China.
Yue Shi, Department of Medical Oncology, Lu’an Hospital of Traditional Chinese Medicine, Lu’an 237000, Anhui Province, China.
Qing-Ming Sun, Department of Medical Oncology, Lu’an Hospital of Traditional Chinese Medicine, Lu’an 237000, Anhui Province, China.
Wan-Hui Dong, Department of Medical Oncology, Lu’an Hospital of Traditional Chinese Medicine, Lu’an 237000, Anhui Province, China. nc.yyzsal@iuhnawgnod .
AMGEN PRESENTS NEW DATA FOR FIRST-IN-CLASS IMDELLTRA™ (TARLATAMAB-DLLE) IN SMALL CELL LUNG CANCER AT WCLC 2024
News provided by
Sep 09, 2024, 09:00 ET
Share this article
DeLLphi-303 Study Results Show Potential for IMDELLTRA in Combination with a PD-L1 Inhibitor as First-Line Maintenance Therapy in ES-SCLC
DeLLphi-301 Long-Term Follow-up Data Demonstrate Sustained Safety and Efficacy for IMDELLTRA
THOUSAND OAKS, Calif. , Sept. 9, 2024 /PRNewswire/ -- Amgen (NASDAQ: AMGN ) today announced the presentation of new data showcasing IMDELLTRA TM (tarlatamab-dlle), a first-in-class delta-like ligand 3 (DLL3)-targeting Bispecific T-cell Engager (BiTE ® ) molecule, at the 2024 World Conference on Lung Cancer (WCLC) in San Diego .
IMDELLTRA will be featured in two oral presentations at the "DLL3 Targeting BiTE Therapies in SCLC" session, taking place today at 2:00 p.m. PDT . New data from the global Phase 1b DeLLphi-303 study of IMDELLTRA combined with PD-L1 inhibitors in first-line maintenance extensive-stage small cell lung cancer (ES-SCLC) will be presented as a late-breaking abstract (#LBA OA10.04). Additionally, long-term results from the Phase 2 DeLLphi-301 study in previously treated ES-SCLC will be highlighted as an oral presentation (#OA10.03).
"Earlier this year, the FDA approved IMDELLTRA for patients with extensive-stage small cell lung cancer who progressed on or after platinum-based chemotherapy. Today, we are thrilled to share results showing long-term sustained benefit in this setting as well as initial evidence supporting a combination approach in front-line maintenance therapy," said Jay Bradner , M.D., executive vice president, Research and Development, and chief scientific officer at Amgen. "These data support our goal to deliver an effective targeted immunotherapy to more patients living with this aggressive cancer."
DeLLphi-303 Phase 1b Study Data in First-Line Maintenance Therapy IMDELLTRA combined with a PD-L1 inhibitor as first-line maintenance therapy in ES-SCLC demonstrated a manageable safety profile with sustained disease control and positive survival outcomes. Key findings include:
- IMDELLTRA plus a PD-L1 inhibitor: demonstrated a positive benefit: risk profile with no new or unexpected safety findings
- IMDELLTRA plus durvalumab: disease control rate (DCR) of 62.5% (95% CI: 45.8-77.3) and median duration of disease control (DoDC) that was Not Estimable (95% CI: 3.9, NE)
- IMDELLTRA plus atezolizumab: DCR of 62.5% (95% CI: 47.4-76.0) and median DoDC of 7.2 months (95% CI: 5.6, NE)
- IMDELLTRA plus durvalumab showed a 9-month overall survival (OS) of 91.8% (95% CI: 76.6-97.3) and median progression-free survival (mPFS) of 5.3 months (95% CI: 3.5-NE)
- IMDELLTRA plus atezolizumab showed a 9-month OS of 86.7% (95% CI: 70.3-94.4) and mPFS of 5.6 months (95% CI: 3.5-8.5)
"Tarlatamab has been a major breakthrough for patients with extensive-stage small cell lung cancer, who have had limited options for the past 30 years, and these data are impressive as a potential first-line maintenance treatment as well," said Sally Lau , M.D., oncologist and assistant professor of medicine, Perlmutter Cancer Center, NYU Grossman School of Medicine. "In particular, tarlatamab in combination with a PD-L1 inhibitor showed exciting safety and efficacy, which strongly supports continued evaluation in the ongoing Phase 3 DeLLphi-305 trial."
In patients receiving IMDELLTRA plus durvalumab, treatment-related adverse events (TRAEs) resulted in dose interruptions in 15% of cases and discontinuation in 8% of patients. In the IMDELLTRA plus atezolizumab treatment arm, TRAEs led to dose interruptions in 17% of cases and discontinuation of IMDELLTRA in 4% of patients. Cytokine release syndrome (CRS) was mostly grade 1-2, occurring primarily in cycle 1 and generally manageable with supportive care. Immune effector cell-associated neurotoxicity syndrome (ICANS) was infrequent overall, with a lower incidence and severity observed in the IMDELLTRA plus durvalumab treatment arm compared to IMDELLTRA plus atezolizumab treatment arm.
DeLLphi-301 Phase 2 Extended Follow-up Data in ES-SCLC Extended follow-up data from the DeLLphi-301 Phase 2 study demonstrated sustained anticancer activity and a manageable safety profile with IMDELLTRA in patients with ES-SCLC previously treated with platinum-based chemotherapy.
Among 100 patients treated with IMDELLTRA 10 mg biweekly, the objective response rate (ORR) was 40%, with nearly half of the responders maintaining their response at data cutoff. Stable disease was observed in 30% of the patients, and the median duration of disease control was 6.9 months (95% CI, 5.4-8.6). Median OS for this group was 15.2 months and was similar regardless of progression-free interval (<90 days or 90+ days) after first-line platinum-based chemotherapy. IMDELLTRA demonstrated long-term tolerability with no new safety concerns identified. These findings support the continued use of IMDELLTRA in this patient population, underscoring its clinical significance.
About DeLLphi-303 Study Preclinical studies indicated that IMDELLTRA upregulated PD-L1 expression and demonstrated increased cytotoxic activity when combined with a PD-L1 inhibitor. 1,2
The DeLLphi-303 study is a Phase 1b , multicenter, open-label study evaluating the safety and efficacy of first-line IMDELLTRA in combination with standard-of-care chemoimmunotherapy, followed by IMDELLTRA plus PD-L1 inhibitor, in patients with ES-SCLC.
DeLLphi-303 will also evaluate IMDELLTRA in combination with a PD-L1 inhibitor as first-line maintenance only following standard-of-care chemoimmunotherapy. This part of the study includes 88 patients assigned to receive either IMDELLTRA 10 mg administered intravenously (IV) every two weeks plus atezolizumab 1680 mg IV every four weeks (n=48), or IMDELLTRA 10 mg IV every two weeks plus durvalumab 1500 mg IV every four weeks (n=40). The study protocol allowed for switching of PD-L1 inhibitor for the maintenance treatment, from that received by the patient during initial first-line treatment with platinum-based chemotherapy.
The primary endpoint in DeLLphi-303 is safety and tolerability of IMDELLTRA in combination with a PD-L1 inhibitor, with or without chemotherapy. For the investigation of IMDELLTRA in front-line with chemoimmunotherapy followed by maintenance with a PD-L1 inhibitor, the secondary endpoints include ORR, duration of response (DoR), DCR, PFS and OS. For the investigation of IMDELLTRA in first-line maintenance following front-line standard-of-care chemoimmunotherapy, the secondary endpoints include DCR, PFS, and OS, beginning from the start of first-line maintenance.
About DeLLphi-301 Study The U.S. Food and Drug Administration accelerated approval of IMDELLTRA is based on results from the Phase 2 DeLLphi-301 clinical trial, in which IMDELLTRA at 10 mg or 100 mg dosed once every 2 weeks was evaluated in patients with SCLC who were refractory to or relapsed after one platinum-based regimen, with or without a checkpoint inhibitor, and at least one other line of therapy. The primary efficacy endpoint was ORR per RECIST 1.1 by blinded independent central review. In part 2 of the study, additional patients were enrolled at the 10 mg dose until 100 patients were reached, and Part 3 was a safety sub-study that evaluated a shortened monitoring period at a medical facility following administration of the first two doses of IMDELLTRA. Across all parts, patients received an initial 1 mg step up dose on day 1, followed by the 10 mg or 100 mg target doses on days 8 and 15 of cycle 1, and then every two weeks in 28-day cycles until disease progression.
About IMDELLTRA™ (tarlatamab-dlle) IMDELLTRA received accelerated approval from the U.S. Food and Drug Administration on May 16, 2024. IMDELLTRA is a first-in-class immunotherapy engineered by Amgen researchers that binds to both DLL3 on cancer cells and CD3 on T cells, creating a cytolytic synapse between T cells and cancer cells. The activated T cells then mediate lysis of DLL3-expressing SCLC cells. 1,3 DLL3 is a protein that is expressed on the surface of SCLC cells in ~85-96% of patients with SCLC, but is minimally expressed on healthy cells, making it an exciting target. 4,5
IMDELLTRA TM (tarlatamab-dlle) U.S. Indication IMDELLTRA™ (tarlatamab-dlle) is indicated for the treatment of adult patients with extensive-stage small cell lung cancer (ES-SCLC) with disease progression on or after platinum-based chemotherapy.
This indication is approved under accelerated approval based on overall response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
IMPORTANT SAFETY INFORMATION
WARNING: CYTOKINE RELEASE SYNDROME AND NEUROLOGIC TOXICITY including IMMUNE EFFECTOR CELL-ASSOCIATED NEUROTOXICITY SYNDROME
- Cytokine release syndrome (CRS), including serious or life-threatening reactions, can occur in patients receiving IMDELLTRA TM . Initiate treatment with IMDELLTRA TM using the step-up dosing schedule to reduce the incidence and severity of CRS. Withhold IMDELLTRA TM until CRS resolves or permanently discontinue based on severity.
- Neurologic toxicity, including immune effector cell-associated neurotoxicity syndrome (ICANS), including serious or life-threatening reactions, can occur in patients receiving IMDELLTRA TM . Monitor patients for signs and symptoms of neurologic toxicity, including ICANS, during treatment and treat promptly. Withhold IMDELLTRA TM until ICANS resolves or permanently discontinue based on severity.
WARNINGS AND PRECAUTIONS
- Cytokine Release Syndrome (CRS): IMDELLTRA TM can cause CRS including serious or life-threatening reactions. In the pooled safety population, CRS occurred in 55% of patients who received IMDELLTRA TM , including 34% Grade 1, 19% Grade 2, 1.1% Grade 3 and 0.5% Grade 4. Recurrent CRS occurred in 24% of patients, including 18% Grade 1 and 6% Grade 2. Most events (43%) of CRS occurred after the first dose, with 29% of patients experiencing any grade CRS after the second dose and 9% of patients experiencing CRS following the third dose or later. Following the Day 1, Day 8, and Day 15 infusions, 16%, 4.3% and 2.1% of patients experienced ≥ Grade 2 CRS, respectively. The median time to onset of all grade CRS from most recent dose of IMDELLTRA TM was 13.5 hours (range 1 to 268 hours). The median time to onset of ≥ Grade 2 CRS from most recent dose of IMDELLTRA TM was 14.6 hours (range: 2 to 566 hours). Clinical signs and symptoms of CRS included pyrexia, hypotension, fatigue, tachycardia, headache, hypoxia, nausea, and vomiting. Potentially life-threatening complications of CRS may include cardiac dysfunction, acute respiratory distress syndrome, neurologic toxicity, renal and/or hepatic failure, and disseminated intravascular coagulation (DIC). Administer IMDELLTRA TM following the recommended step-up dosing and administer concomitant medications before and after Cycle 1 IMDELLTRA TM infusions as described in Table 3 of the Prescribing Information (PI) to reduce the risk of CRS. Administer IMDELLTRA TM in an appropriate health care facility equipped to monitor and manage CRS. Ensure patients are well hydrated prior to administration of IMDELLTRA TM . Closely monitor patients for signs and symptoms of CRS during treatment with IMDELLTRA TM . At the first sign of CRS, immediately discontinue IMDELLTRA TM infusion, evaluate the patient for hospitalization and institute supportive care based on severity. Withhold or permanently discontinue IMDELLTRA TM based on severity. Counsel patients to seek medical attention should signs or symptoms of CRS occur.
- Neurologic Toxicity, Including ICANS : IMDELLTRA TM can cause serious or life-threatening neurologic toxicity, including ICANS. In the pooled safety population, neurologic toxicity, including ICANS, occurred in 47% of patients who received IMDELLTRA TM , including 10% Grade 3. The most frequent neurologic toxicities were headache (14%), peripheral neuropathy (7%), dizziness (7%), insomnia (6%), muscular weakness (3.7%), delirium (2.1%), syncope (1.6%), and neurotoxicity (1.1%). ICANS occurred in 9% of IMDELLTRA TM -treated patients. Recurrent ICANS occurred in 1.6% of patients. Most patients experienced ICANS following Cycle 2 Day 1 (24%). Following Day 1, Day 8, and Day 15 infusions, 0.5%, 0.5% and 3.7% of patients experienced ≥ Grade 2 ICANS, respectively. The median time to onset of ICANS from the first dose of IMDELLTRA TM was 29.5 days (range: 1 to 154 days). ICANS can occur several weeks following administration of IMDELLTRA TM . The median time to resolution of ICANS was 33 days (range 1 to 93 days). The onset of ICANS can be concurrent with CRS, following resolution of CRS, or in the absence of CRS. Clinical signs and symptoms of ICANS may include but are not limited to confusional state, depressed level of consciousness, disorientation, somnolence, lethargy, and bradyphrenia. Patients receiving IMDELLTRA TM are at risk of neurologic adverse reactions and ICANS resulting in depressed level of consciousness. Advise patients to refrain from driving and engaging in hazardous occupations or activities, such as operating heavy or potentially dangerous machinery, in the event of any neurologic symptoms until they resolve. Closely monitor patients for signs and symptoms of neurologic toxicity and ICANS during treatment. At the first sign of ICANS, immediately evaluate the patient and provide supportive therapy based on severity. Withhold IMDELLTRA TM or permanently discontinue based on severity.
- Cytopenias: IMDELLTRA TM can cause cytopenias including neutropenia, thrombocytopenia, and anemia. In the pooled safety population, decreased neutrophils occurred in 12% including 6% Grade 3 or 4 of IMDELLTRA TM -treated patients. The median time to onset for Grade 3 or 4 neutropenia was 29.5 days (range: 2 to 213). Decreased platelets occurred in 33% including 3.2% Grade 3 or 4. The median time to onset for Grade 3 or 4 decreased platelets was 50 days (range: 3 to 420). Decreased hemoglobin occurred in 58% including 5% Grade 3 or 4. Febrile neutropenia occurred in 0.5% of patients treated with IMDELLTRA TM . Monitor patients for signs and symptoms of cytopenias. Perform complete blood counts prior to treatment with IMDELLTRA TM , before each dose, and as clinically indicated. Based on the severity of cytopenias, temporarily withhold, or permanently discontinue IMDELLTRA TM .
- Infections: IMDELLTRA TM can cause serious infections, including life-threatening and fatal infections. In the pooled safety population, infections, including opportunistic infections, occurred in 41% of patients who received IMDELLTRA TM . Grade 3 or 4 infections occurred in 13% of patients. The most frequent infections were COVID-19 (9%, majority during the COVID-19 pandemic), urinary tract infection (10%), pneumonia (9%), respiratory tract infection (3.2%), and candida infection (3.2%). Monitor patients for signs and symptoms of infection prior to and during treatment with IMDELLTRA TM and treat as clinically indicated. Withhold or permanently discontinue IMDELLTRA TM based on severity.
- Hepatotoxicity: IMDELLTRA TM can cause hepatotoxicity. In the pooled safety population, elevated ALT occurred in 42%, with Grade 3 or 4 ALT elevation occurring in 2.1%. Elevated AST occurred in 44% of patients, with Grade 3 or 4 AST elevation occurring in 3.2%. Elevated bilirubin occurred in 15% of patients; Grade 3 or 4 total bilirubin elevations occurred in 1.6% of patients. Liver enzyme elevation can occur with or without concurrent CRS. Monitor liver enzymes and bilirubin prior to treatment with IMDELLTRA TM , before each dose, and as clinically indicated. Withhold IMDELLTRA TM or permanently discontinue based on severity.
- Hypersensitivity: IMDELLTRA TM can cause severe hypersensitivity reactions. Clinical signs and symptoms of hypersensitivity may include, but are not limited to, rash and bronchospasm. Monitor patients for signs and symptoms of hypersensitivity during treatment with IMDELLTRA TM and manage as clinically indicated. Withhold or consider permanent discontinuation of IMDELLTRA TM based on severity.
- Embryo-Fetal Toxicity: Based on its mechanism of action, IMDELLTRA TM may cause fetal harm when administered to a pregnant woman. Advise patients of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with IMDELLTRA TM and for 2 months after the last dose.
ADVERSE REACTIONS
- The most common (> 20%) adverse reactions were CRS (55%), fatigue (51%), pyrexia (36%), dysgeusia (36%), decreased appetite (34%), musculoskeletal pain (30%), constipation (30%), anemia (27%) and nausea (22%). The most common (≥ 2%) Grade 3 or 4 laboratory abnormalities were decreased lymphocytes (57%), decreased sodium (16%), increased uric acid (10%), decreased total neutrophils (6%), decreased hemoglobin (5%), increased activated partial thromboplastin time (5%), decreased potassium (5%), increased aspartate aminotransferase (3.2%), decreased white blood cells (3.8%), decreased platelets (3.2%), and increased alanine aminotransferase (2.1%).
- Serious adverse reactions occurred in 58% of patients. Serious adverse reactions in > 3% of patients included CRS (24%), pneumonia (6%), pyrexia (3.7%), and hyponatremia (3.6%). Fatal adverse reactions occurred in 2.7% of patients including pneumonia (0.5%), aspiration (0.5%), pulmonary embolism (0.5%), respiratory acidosis (0.5%), and respiratory failure (0.5%).
DOSAGE AND ADMINISTRATION: Important Dosing Information
- Administer IMDELLTRA TM as an intravenous infusion over one hour.
- Administer IMDELLTRA TM according to the step-up dosing schedule in the IMDELLTRA TM PI (Table 1) to reduce the incidence and severity of CRS.
- For Cycle 1, administer recommended concomitant medications before and after Cycle 1 IMDELLTRA TM infusions to reduce the risk of CRS reactions as described in the PI (Table 3).
- IMDELLTRA TM should only be administered by a qualified healthcare professional with appropriate medical support to manage severe reactions such as CRS and neurologic toxicity including ICANS.
- Due to the risk of CRS and neurologic toxicity, including ICANS, monitor patients from the start of the IMDELLTRA TM infusion for 22 to 24 hours on Cycle 1 Day 1 and Cycle 1 Day 8 in an appropriate healthcare setting.
- Recommend that patients remain within 1 hour of an appropriate healthcare setting for a total of 48 hours from start of the infusion with IMDELLTRA TM following Cycle 1 Day 1 and Cycle 1 Day 8 doses, accompanied by a caregiver.
- Prior to administration of IMDELLTRA TM evaluate complete blood count, liver enzymes, and bilirubin before each dose, and as clinically indicated.
- Ensure patients are well hydrated prior to administration of IMDELLTRA TM .
Please see IMDELLTRA™ full Prescribing Information , including BOXED WARNINGS
About Small Cell Lung Cancer SCLC is one of the most aggressive and devastating solid tumor malignancies, with a median survival of approximately 12 months following initial therapy and a 3% five-year relative survival rate for ES-SCLC. 6,7,8 Current second-line treatments impart a short duration of response (median DoR: 3.3–5.3 months) and limited survival (median OS: 5.8-9.3 months), while current third-line treatments for SCLC, which consist primarily of chemotherapy, yield a short median DoR of 2.6 months and a median OS of 4.4-5.3 months. 9,10,11,12,13 SCLC comprises ~15% of the 2.4 million plus patients diagnosed with lung cancer worldwide each year. 14,15,16 Despite initial high response rates to first-line platinum-based chemotherapy, most patients quickly relapse within months and require subsequent treatment options. 14
About IMDELLTRA (taralatamab-dlle) Clinical Trials Amgen's robust IMDELLTRA development program includes the DeLLphi clinical trials, which evaluate IMDELLTRA as both a monotherapy and in combination regimens in earlier lines of SCLC, and DeLLpro clinical trials, which evaluate the efficacy and safety of tarlatamab in neuroendocrine prostate cancer.
In the Phase 1 DeLLphi-300 study, IMDELLTRA showed responses in 23.4% of patients with encouraging durability in heavily pre-treated patients with SCLC. 17 In the Phase 2 DeLLphi-301 study, IMDELLTRA administered as 10 mg dose every two weeks demonstrated an ORR of 40% in patients with advanced SCLC who had failed two or more prior lines of treatment. In the DeLLphi-301 Phase 2 trial, the most frequent treatment-related adverse events seen with 10 mg Q2W dosing regimen were CRS (51%), pyrexia (32%), and decreased appetite (23%). CRS events were predominantly grade 1 or 2 and occurred most often after the first or second dose. 2 Treatment discontinuation for adverse events occurred in 4-7% of patients in the two trials. 4,17
Tarlatamab is being investigated in multiple studies including DeLLphi-303, a Phase 1b study investigating tarlatamab in combination with standard-of-care therapies in first-line ES-SCLC; DeLLphi-304, a randomized Phase 3 trial comparing tarlatamab monotherapy with standard-of-care chemotherapy in second-line treatment of SCLC; DeLLphi-305, a randomized Phase 3 trial comparing tarlatamab in combination with durvalumab versus durvalumab alone as first-line maintenance treatment in ES-SCLC; DeLLphi-306, a randomized placebo-controlled Phase 3 trial of tarlatamab following concurrent chemoradiotherapy in limited-stage SCLC; and DeLLpro-300, a Phase 1b study of tarlatamab in de novo or treatment-emergent neuroendocrine prostate cancer. 18
For more information, please visit https://tarlatamabclinicaltrials.com/ .
About Bispecific T-Cell Engager (BiTE ® ) Technology BiTE technology is a targeted immuno-oncology platform that is designed to engage a patient's own T cells to any tumor-specific antigen, activating the cytotoxic potential of T cells to eliminate detectable cancer. The BiTE immuno-oncology platform has the potential to treat different cancer types through tumor-specific antigens. The BiTE platform has a goal of leading to off-the-shelf solutions, which have the potential to make innovative T-cell treatment available to all providers when their patients need it. For more than a decade, Amgen has been advancing this innovative technology, which has demonstrated strong efficacy in hematological malignancies and now a solid tumor with the approval of IMDELLTRA. Amgen remains committed to progressing multiple BiTE molecules across a broad range of hematologic and solid tumor malignancies, paving the way for additional applications in more tumor types. Amgen is further investigating BiTE technology with the goal of enhancing patient experience and therapeutic potential. To learn more about BiTE technology, visit BiTE ® Technology 101 .
About Amgen Amgen discovers, develops, manufactures and delivers innovative medicines to help millions of patients in their fight against some of the world's toughest diseases. More than 40 years ago, Amgen helped to establish the biotechnology industry and remains on the cutting-edge of innovation, using technology and human genetic data to push beyond what's known today. Amgen is advancing a broad and deep pipeline that builds on its existing portfolio of medicines to treat cancer, heart disease, osteoporosis, inflammatory diseases and rare diseases.
In 2024, Amgen was named one of the "World's Most Innovative Companies" by Fast Company and one of "America's Best Large Employers" by Forbes, among other external recognitions . Amgen is one of the 30 companies that comprise the Dow Jones Industrial Average ® , and it is also part of the Nasdaq-100 Index ® , which includes the largest and most innovative non-financial companies listed on the Nasdaq Stock Market based on market capitalization.
For more information, visit Amgen.com and follow Amgen on X , LinkedIn , Instagram , TikTok , YouTube and Threads .
Amgen Forward-Looking Statements This news release contains forward-looking statements that are based on the current expectations and beliefs of Amgen. All statements, other than statements of historical fact, are statements that could be deemed forward-looking statements, including any statements on the outcome, benefits and synergies of collaborations, or potential collaborations, with any other company (including BeiGene, Ltd. or Kyowa Kirin Co., Ltd.), the performance of Otezla ® (apremilast) (including anticipated Otezla sales growth and the timing of non-GAAP EPS accretion), our acquisitions of Teneobio, Inc., ChemoCentryx, Inc., or Horizon Therapeutics plc (including the prospective performance and outlook of Horizon's business, performance and opportunities, any potential strategic benefits, synergies or opportunities expected as a result of such acquisition, and any projected impacts from the Horizon acquisition on our acquisition-related expenses going forward), as well as estimates of revenues, operating margins, capital expenditures, cash, other financial metrics, expected legal, arbitration, political, regulatory or clinical results or practices, customer and prescriber patterns or practices, reimbursement activities and outcomes, effects of pandemics or other widespread health problems on our business, outcomes, progress, and other such estimates and results. Forward-looking statements involve significant risks and uncertainties, including those discussed below and more fully described in the Securities and Exchange Commission reports filed by Amgen, including our most recent annual report on Form 10-K and any subsequent periodic reports on Form 10-Q and current reports on Form 8-K. Unless otherwise noted, Amgen is providing this information as of the date of this news release and does not undertake any obligation to update any forward-looking statements contained in this document as a result of new information, future events or otherwise.
No forward-looking statement can be guaranteed and actual results may differ materially from those we project. Discovery or identification of new product candidates or development of new indications for existing products cannot be guaranteed and movement from concept to product is uncertain; consequently, there can be no guarantee that any particular product candidate or development of a new indication for an existing product will be successful and become a commercial product. Further, preclinical results do not guarantee safe and effective performance of product candidates in humans. The complexity of the human body cannot be perfectly, or sometimes, even adequately modeled by computer or cell culture systems or animal models. The length of time that it takes for us to complete clinical trials and obtain regulatory approval for product marketing has in the past varied and we expect similar variability in the future.
Even when clinical trials are successful, regulatory authorities may question the sufficiency for approval of the trial endpoints we have selected. We develop product candidates internally and through licensing collaborations, partnerships and joint ventures. Product candidates that are derived from relationships may be subject to disputes between the parties or may prove to be not as effective or as safe as we may have believed at the time of entering into such relationship. Also, we or others could identify safety, side effects or manufacturing problems with our products, including our devices, after they are on the market.
Our results may be affected by our ability to successfully market both new and existing products domestically and internationally, clinical and regulatory developments involving current and future products, sales growth of recently launched products, competition from other products including biosimilars, difficulties or delays in manufacturing our products and global economic conditions. In addition, sales of our products are affected by pricing pressure, political and public scrutiny and reimbursement policies imposed by third-party payers, including governments, private insurance plans and managed care providers and may be affected by regulatory, clinical and guideline developments and domestic and international trends toward managed care and healthcare cost containment. Furthermore, our research, testing, pricing, marketing and other operations are subject to extensive regulation by domestic and foreign government regulatory authorities. Our business may be impacted by government investigations, litigation and product liability claims. In addition, our business may be impacted by the adoption of new tax legislation or exposure to additional tax liabilities. If we fail to meet the compliance obligations in the corporate integrity agreement between us and the U.S. government, we could become subject to significant sanctions. Further, while we routinely obtain patents for our products and technology, the protection offered by our patents and patent applications may be challenged, invalidated or circumvented by our competitors, or we may fail to prevail in present and future intellectual property litigation. We perform a substantial amount of our commercial manufacturing activities at a few key facilities, including in Puerto Rico , and also depend on third parties for a portion of our manufacturing activities, and limits on supply may constrain sales of certain of our current products and product candidate development. An outbreak of disease or similar public health threat, such as COVID-19, and the public and governmental effort to mitigate against the spread of such disease, could have a significant adverse effect on the supply of materials for our manufacturing activities, the distribution of our products, the commercialization of our product candidates, and our clinical trial operations, and any such events may have a material adverse effect on our product development, product sales, business and results of operations. We rely on collaborations with third parties for the development of some of our product candidates and for the commercialization and sales of some of our commercial products. In addition, we compete with other companies with respect to many of our marketed products as well as for the discovery and development of new products. Further, some raw materials, medical devices and component parts for our products are supplied by sole third-party suppliers. Certain of our distributors, customers and payers have substantial purchasing leverage in their dealings with us. The discovery of significant problems with a product similar to one of our products that implicate an entire class of products could have a material adverse effect on sales of the affected products and on our business and results of operations. Our efforts to collaborate with or acquire other companies, products or technology, and to integrate the operations of companies or to support the products or technology we have acquired, may not be successful. There can be no guarantee that we will be able to realize any of the strategic benefits, synergies or opportunities arising from the Horizon acquisition, and such benefits, synergies or opportunities may take longer to realize than expected. We may not be able to successfully integrate Horizon, and such integration may take longer, be more difficult or cost more than expected. A breakdown, cyberattack or information security breach of our information technology systems could compromise the confidentiality, integrity and availability of our systems and our data. Our stock price is volatile and may be affected by a number of events. Our business and operations may be negatively affected by the failure, or perceived failure, of achieving our environmental, social and governance objectives. The effects of global climate change and related natural disasters could negatively affect our business and operations. Global economic conditions may magnify certain risks that affect our business. Our business performance could affect or limit the ability of our Board of Directors to declare a dividend or our ability to pay a dividend or repurchase our common stock. We may not be able to access the capital and credit markets on terms that are favorable to us, or at all.
Any scientific information discussed in this news release relating to new indications for our products is preliminary and investigative and is not part of the labeling approved by the U.S. Food and Drug Administration for the products. The products are not approved for the investigational use(s) discussed in this news release, and no conclusions can or should be drawn regarding the safety or effectiveness of the products for these uses.
CONTACT: Amgen, Thousand Oaks Elissa Snook , 609-251-1407 (media) Justin Claeys , 805-313-9775 (investors)
- Giffin MJ, Cooke K, Lobenhofer EK, et al. AMG 757, a Half-Life Extended, DLL3-Targeted Bispecific T-Cell Engager, Shows High Potency and Sensitivity in Preclinical Models of Small-Cell Lung Cancer. Clin Cancer Res . 2021;27:1526-1537.
- You R, et al. Elucidating the effects of chemotherapy and immune checkpoint blockade on the activity of tarlatamab, a DLL3-targeting bispecific T cell engager molecule, in small cell lung cancer preclinical models. Presentation at SITC 2023; November 1-5, 2023 . San Diego, CA. Abstract #1189.
- Baeuerle PA, Kufer P, Bargou R. BiTE: Teaching antibodies to engage T-cells for cancer therapy. Curr Opin Mol Ther . 2009;11:22-30.
- Ahn MJ, Cho BC, Felip E, et al. Tarlatamab for Patients with Previously Treated Small-Cell Lung Cancer. N Engl J Med . 2023;389:2063-2075.
- Rojo F, Corassa M, Mavroudis D, et al. International real-world study of DLL3 expression in patients with small cell lung cancer. Lung Cancer . 2020;147:237-243.
- American Cancer Society. Lung Cancer Survival Rates. www.cancer.org/cancer/types/lung-cancer/detection-diagnosis-staging/survival-rates.html. Accessed on March 15, 2024 .
- Paz-Ares L, Chen Y, Reinmuth N, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN. ESMO Open . 2022;7:100408.
- Liu SV, Reck M, Mansfield AS, et al. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients With Extensive-Stage Small-Cell Lung Cancer Treated With Atezolizumab, Carboplatin, and Etoposide (IMpower133). J Clin Oncol . 2021;39:619-630.
- Trigo J, Subbiah V, Besse B, et al. Lurbinectedin as second-line treatment for patients with small-cell lung cancer: a single-arm, open-label, phase 2 basket trial. Lancet Oncol . 2020;21(5):645-654.
- Von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol . 1999;17(2):658-67.
- Von Pawel J, Jotte R, Spigel DR, et al. Randomized phase III trial of amrubicin versus topotecan as second-line treatment for patients with small-cell lung cancer. J Clin Oncol . 2014;32(35):4012-9.
- Coutinho AD, Shah M, Lunacsek OE, et al. Real-world treatment patterns and outcomes of patients with small cell lung cancer progression after 2 lines of therapy. Lung Cancer . 2019;127:53-58.
- Borghaei H, Pundole X, Anderson E, et al. Treatment patterns and outcomes in recent US clinical practice for SCLC patients after two prior lines of therapy. Presentation at World Conference on Lung Cancer 2023. September 9-12, 2023; Singapore, SGP. Poster #EP13.07-03.
- Oronsky B, Abrouk N, Caroen S, et al. A 2022 Update on Extensive Stage Small-Cell Lung Cancer (SCLC). J Cancer . 2022;13:2945-2953.
- World Health Organization. Lung. 2020. https://gco.iarc.who.int/media/globocan/factsheets/cancers/15-trachea-bronchus-and-lung-fact-sheet.pdf . Accessed on March 15, 2024 .
- Sabari JK, Lok BH, Laird JH, et al. Unravelling the biology of SCLC: implications for therapy. Nat Rev Clin Oncol . 2017;14:549-561.
- Paz-Ares L, Champiat S, Lai WV, et al. Tarlatamab, a First-in-Class DLL3-Targeted Bispecific T-Cell Engager, in Recurrent Small-Cell Lung Cancer: An Open-Label, Phase I Study. J Clin Oncol . 2023;41:2893-2903.
- Clinical Trials. Tarlatamab Clinical Trial Listings. www.clinicaltrials.gov. Accessed March 15, 2024.
SOURCE Amgen
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
Modal title
Also from this source.

AMGEN TO PRESENT AT THE 2024 WELLS FARGO HEALTHCARE CONFERENCE
Amgen (NASDAQ:AMGN) will present at the 2024 Wells Fargo Healthcare Conference at 9:30 a.m. ET on Thursday, Sept. 5, 2024. Peter Griffith, executive...

AMGEN TO PRESENT AT THE MORGAN STANLEY 22ND ANNUAL GLOBAL HEALTHCARE CONFERENCE
Amgen (NASDAQ:AMGN) will present at the Morgan Stanley 22nd Annual Global Healthcare Conference at 9:15 a.m. ET on Wednesday, Sept. 4, 2024. Robert A....

Pharmaceuticals

Biotechnology

Health Care & Hospitals

Medical Pharmaceuticals

IMAGES
VIDEO
COMMENTS
One study noted that the most common symptoms at presentation were cough (55 percent), dyspnea (45 percent), pain (38 percent), and weight loss (36 percent) (table 1) [3]. This discussion will present the clinical manifestations of non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). Screening and risk factors for lung cancer ...
Clinical Presentation and Diagnosis ... et al. Clinical and organizational factors in the initial evaluation of patients with lung cancer: diagnosis and management of lung cancer, 3rd ed: American ...
Musculoskeletal manifestations include the following: Bone pain (6-25%) Spinal cord impingement. Paraneoplastic syndromes occur in 10-20% of patients. Most paraneoplastic syndromes are caused by small cell lung cancer (SCLC). However many paraneoplastic syndromes also occur in non-small cell lung cancer (NSCLC) patients. Some examples include:
Lung cancer or bronchogenic carcinoma refers to tumors originating in the lung parenchyma or within the bronchi. It is one of the leading causes of cancer-related deaths in the United States. Since 1987, lung cancer has been responsible for more deaths in women than breast cancer. It is estimated that there are 225,000 new cases of lung cancer in the United States annually, and approximately ...
Clinical Presentation. Patients with lung cancer are almost always symptomatic ... The initial evaluation of a patient with suspected lung cancer begins with a history and physical examination;
Lung cancer typically doesn't cause symptoms early on. Symptoms of lung cancer usually happen when the disease is advanced. Signs and symptoms of lung cancer that happen in and around the lungs may include: A new cough that doesn't go away. Chest pain. Coughing up blood, even a small amount.
Treatment. Anaplastic lymphoma kinase (ALK)-positive advanced non-small cell lung cancer. Bronchoscopic laser in the management of airway disease in adults. Clinical presentation, diagnostic evaluation, and management of malignant central airway obstruction in adults. Endobronchial photodynamic therapy in the management of airway disease in adults.
Lung cancer remains the second most common cancer diagnosis, and top cancer killer, in the United States. Early diagnosis is key for patient survival, but only 16% of cases are currently caught in the early stages. The primary care provider is uniquely poised to intervene with high-risk patients through careful monitoring and screening of select patients. This article includes discussion of ...
The clinical manifestations and diverse initial symptoms of pulmonary malignant lesions remain a challenge in clinical practice. Although the presymptomatic detection of lung cancers has been emphasized, screening methods remain controversial, and many patients have advanced disease at the time of initial assessment. 1-4 The estimated doubling time or rate of growth of various lung tumors ...
Most patients with lung cancer present for diagnostic evaluation because of suspicious symptoms or an incidental finding on chest imaging. The goal of the initial evaluation is to obtain sufficient clinical and radiologic information to guide diagnostic tissue biopsy, staging, and treatment.
It is the clinical condition caused by superior sulcus tumors that are located in the apex of the upper lobes of the lung and present with pain in the shoulder, chest wall, and scapula. The cause of the pain is invasion to the chest wall, vertebrae, or costa. It constitutes 3-5% of lung cancer cases.
British Journal of Cancer (2020) Survival from lung cancer has seen only modest improvements in recent decades. Poor outcomes are linked to late presentation, yet early diagnosis can be ...
Shortness of breath. Cough. Bone pain. Weight loss. Fatigue. Neurologic dysfunction. Most patients with this disease present with a short duration of symptoms, usually only 8-12 weeks before presentation. The clinical manifestations of SCLC can result from local tumor growth, intrathoracic spread, distant spread, and/or paraneoplastic syndromes.
Pulmonary enteric adenocarcinoma (PEAC) is a rare variant of non-small cell lung cancer (NSCLC), which shows several morphological and immunohistochemical features in common with both pulmonary and colorectal adenocarcinomas. PEAC was first described by Tsao and Fraser in 1991 [1] but only in 2015 was this variant included in the World Health Organization (WHO) classification of lung tumors [2 ...
Second, COVID-19 clinical presentation in lung cancer patients can range from an asymptomatic condition to severe respiratory complications requiring intensive care (8,12,13). In the present study, we sought to compare the clinical presentation of patients with lung cancer and confirmed COVID-19 to patients with suspected COVID-19 with negative ...
Clinical Presentation. Lung cancer may present with symptoms or be found incidentally on chest imaging. The most common symptoms at presentation include: ... Overall survival and local recurrence of 406 completely resected stage IIIa-N2 non-small cell lung cancer patients: questionnaire survey of the Japan Clinical Oncology Group to plan for ...
Abstract. In the absence of screening, most patients with lung cancer are not diagnosed until later stages, when the prognosis is poor. The most common symptoms are cough and dyspnea, but the most specific symptom is hemoptysis. Digital clubbing, though rare, is highly predictive of lung cancer. Symptoms can be caused by the local tumor ...
The most common symptoms include cough, dyspnea, and hemoptysis. Although the clinical presentation of lung cancer is not specific to the classification or histology of the cancer, certain obstacles may be more likely with different types. One study noted that the most common symptoms at presentation were cough (55%), dyspnea (45%), pain (38% ...
We sought to compare the clinical presentation and prognosis of patients with lung cancer and confirmed COVID-19 infection to those with negative RT-PCR SARS-CoV-2 results. We included patients with confirmed lung cancer and suspected COVID-19 who presented to the emergency department. The primary o …
Clinical trial finds antibody-drug conjugate helps patients with metastatic non-small cell lung cancer live longer Oct 26, 2023 Tumor mutational burden not significantly associated with efficacy ...
Further clinical studies must be conducted to validate the effectiveness of anamorelin in the acute-phase treatment of patients with lung cancer. CRediT authorship contribution statement Haruka Fujioka: Conceptualization, Data curation, Formal analysis, Investigation, Resources, Methodology, Software, Visualization, Writing - original draft ...
This article includes discussion of the usual clinical presentation of lung cancer, and reviews the role of routine screening. A brief update in treatment advances in lung cancer is also included. ... nearly 85%, of patients diagnosed with lung cancer are experiencing some symptomatic sequelae at the time of diagnosis. 8 Symptoms vary widely ...
Poor outcomes are linked to late presentation, yet early diagnosis can be challenging as lung cancer symptoms are common and non-specific. In this paper, we examine how lung cancer presents in primary care and review roles for primary care in reducing the burden from this disease. Reducing rates of smoking remains, by far, the key strategy, but ...
Full Title A Phase 2 Study of First-line Sotorasib for Patients with Advanced KRAS G12C-mutant Non-Small Cell Lung Cancer Purpose The purpose of this study is to see how well sotorasib works in people with advanced lung cancer. The people in this study have non-small cell lung cancer (NSCLC) with a mutation (change) in the KRAS G12C gene. This mutation can cause cancer cells to grow.
An overview of the risk factors, pathology, and clinical manifestations of lung cancer is presented separately, as is an overview of the management of patients with advanced NSCLC, and a discussion of issues concerning lung cancer survivors. (See "Clinical manifestations of lung cancer" and "Overview of the initial treatment of advanced non ...
These results will be presented today during an oral presentation (OA08.03) ... AstraZeneca is working to bring patients with lung cancer closer to cure through the detection and treatment of early-stage disease, while also pushing the boundaries of science to improve outcomes in the resistant and advanced settings. ... Clinical impact of TROP2 ...
Lung cancer is the leading cause of cancer-related mortality worldwide. For advanced non-small-cell lung cancer (NSCLC) without targetable mutations, the combination of immune checkpoint inhibitor (ICI) and chemotherapy has become the standard first-line treatment, particularly in patients with programmed death receptor ligand 1 (PD-L1) expression below 50%.
Background: Immune checkpoint inhibitors (ICIs) had modest advances in the treatment of extensive-stage small cell lung cancer (ES-SCLC) in clinical trials, but there is a lack of biomarkers for prognosis in clinical practice. Methods: We retrospectively collected data from ES-SCLC patients who received ICIs combined chemotherapy from two centers in China, integrated clinical and blood ...
INTRODUCTION. Primary bronchogenic carcinoma, also known as lung cancer, primarily consists of two types: small cell lung cancer and non-small cell lung cancer (NSCLC), with the latter accounting for approximately 85% of all lung cancer cases[1,2].Tobacco usage is the most common cause of lung cancer[].The International Agency for Research on Cancer of the World Health Organization has ...
Rojo F, Corassa M, Mavroudis D, et al. International real-world study of DLL3 expression in patients with small cell lung cancer. Lung Cancer. 2020;147:237-243. American Cancer Society. Lung ...