How to write a research article to submit for publication
Pharmacists and healthcare professionals who are undertaking research should have an understanding of how to produce a research article for publication, and be aware of the important considerations relating to submission to a peer-reviewed journal.

Viennaslide / Alamy Stock Photo
Undertaking and performing scientific, clinical and practice-based research is only the beginning of the scholarship of discovery [1] . For the full impact of any research to be achieved and to have an effect on the wider research and scientific community, it must be published in an outlet accessible to relevant professionals [2] .
Scientific research is often published in peer-reviewed journals. Peer review is defined as the unbiased, independent, critical assessment of scholarly or research manuscripts submitted to journals by experts or opinion leaders [3] . The process and requirements of reviewers has been covered recently [4] . On account of this rigorous process, peer-reviewed scientific journals are considered the primary source of new information that impacts and advances clinical decision-making and practice [5] , [6] .
The development of a research article can be helpful for the promotion of scientific thinking [7] , [8] and the advancement of effective writing skills, allowing the authors to participate in broader scientific discussions that lie beyond their scope of practice or discipline [2] .
This article aims to provide pharmacists and healthcare professionals who are undertaking research with an understanding of how to produce a research article for publication, as well as points to consider before submission to a peer-reviewed journal.

Importance of the research question
This article will not go into detail about forming suitable research questions, however, in principle, a good research question will be specific, novel and of relevance to the scientific community (e.g. pharmacy – pharmacists, pharmaceutical scientists, pharmacy technicians and related healthcare professionals). Hulley et al . suggest using the FINER criteria (see ‘Figure 1: FINER criteria for a good research question’) to aid the development of a good research question [9] .
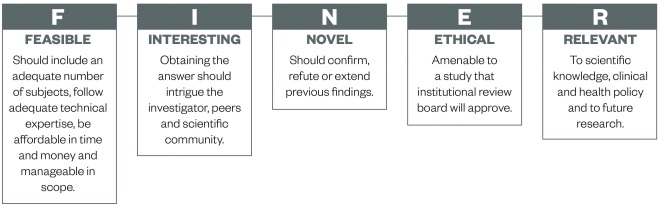
Figure 1: FINER criteria for a good research question
Source: Hulley S, Cummings S, Browner W et al . [9]
The FINER criteria highlight useful points that may generally increase the chances of developing a successful research project. A good research question should specify the population of interest, be of interest to the scientific community and potentially to the public, have clinical relevance and further current knowledge in the field.
Having a clear research question that is of interest to those working in the same field will help in the preparation of an article because it can be used as the central organising principle – all of the content included and discussed should focus on answering this question.
Preparing a first draft
Before writing the article, it is useful to highlight several journals that you could submit the final article to. It also helps to familiarise yourself with these journals’ styles, article structures and formatting instructions before starting to write. Many journals also have criteria that research articles should be able to satisfy. For example, all research article submissions to Clinical Pharmacist must demonstrate innovative or novel results that are sustainable, reproducible and transferable [10] .
Having researched potential target journals, you should have a clear idea about your target audience, enabling you to pitch the level of the article appropriately [11] (see ‘Box 1: Top tips to prepare for writing’).
Box 1: Top tips to prepare for writing
- Know the focus of the paper – identify two or three important findings and make these the central theme of the article;
- Gather important data, perform any analyses and make rough data plots and tables beforehand. These can then be refined for inclusion or submitted as supplementary information if needed;
- Organise your results to flow in a logical sequence;
- Know the structure and requirements of your target journals (check websites and author guidelines, as well as published articles);
- Think about the style of the piece and look to pitch the article at the level of the intended audience;
- Clarity should be your guiding principle.
Structuring a research article
Most research articles follow a similar structure and format that includes an abstract, introduction, methods, results and discussion, as well as a summary of the key points discussed in the article.
One approach is to start with the methods section, which can be derived from the protocol and any pilot phase. Many of the figures and tables can be constructed in advance, which will help with writing the results section. The questions addressed by the study can be used alongside the results to formulate the introduction, which can guide how the discussion is written [11] .
Clinical Pharmacist , like other peer-reviewed journals, has specific author guidelines and formatting instructions to help authors prepare their articles [10] , [12] , [13] . The following sections will discuss the required sections and important considerations for authors when writing.
Title, abstract and keywords
The title, abstract and keywords are essential to the successful communication of research. Most electronic search engines, databases (e.g. PubMed/MEDLINE) and journal websites extract words from them to determine whether your article will be displayed to interested readers [14] , [15] , [16] , [17] , enabling accurate dissemination and leading to future citations.
In addition, the title and abstract are usually freely available online. If an article is not published in an ‘open access’ format, (i.e. it is free and immediately available online and access is combined with the rights to use these articles fully in the digital environment) [18] , or if the reader does not have a subscription to the journal, they will have to decide on whether to pay to access the full article to continue reading. Therefore, it is imperative that they are informative and accurate.
The title should accurately reflect the research, identify the main issue and begin with the subject matter, while being both simple and enticing enough to attract the audience [19] . Authors should avoid using ‘a study of’, ‘investigations into’ and ‘observations on’ in titles. It is also worth remembering that abstracting and indexing services, such as MEDLINE, require accurate titles, because they extract keywords from them for cross-referencing [19] .
Many journals require the abstract to be structured in the same way as the main headings of the paper (e.g. introduction, methods, results, discussion and conclusion) and to be around 150–300 words in length [10] . In general, references should not be cited in the abstract.
Introduction
The introduction should provide the background and context to the study. Two or three paragraphs can be dedicated to the discussion of any previous work and identification of gaps in current knowledge. The rest of the introduction should then outline what this piece of work aims to address and why this is important, before stating the objectives of the study and the research question [20] .
The methods section should provide the reader with enough detail for them to be able to reproduce the study if desired [3] . The context and setting of the study should be described and the study design specified. The section should further describe the population (including the inclusion and exclusion criteria), sampling strategy and the interventions performed. The main study variables should be identified and the data collection procedures described [3] .
Authors should provide specific, technical and detailed information in this section. Several checklists and guidelines are available for the reporting of specific types of studies:
- CONSORT is used for developing and reporting a randomised controlled trial [21] ;
- The STARD checklist can help with designing a diagnostic accuracy study [22] ;
- The PRISMA checklist can be used when performing a metaâ€analyses or systematic review, but can also help with compiling an introduction [23] .
For the reporting of qualitative research studies, authors should explain which research tradition the study utilises and link the choice of methodological strategies with the research goals [24] .
For studies describing the development of new initiatives or clinical services, authors should describe the situation before the initiative began, the establishment of priorities, formulation of objectives and strategies, mobilisation of resources, and processes used in the methods section [10] .
The final portion of the methods section will include the statistical methods used to analyse the data [25] . The statistical methods employed should be described with enough detail to enable a knowledgeable reader with access to the original data to be able to judge its appropriateness for the study and verify the results [3] . For survey-based studies and information on sampling frame, size and statistical powers, see ‘When to use a survey in pharmacy practice research’ [26] .
Findings should be quantified and presented with appropriate indicators of measurement error or uncertainty (e.g. confidence intervals). Authors should avoid relying solely on statistical hypothesis testing, such as P values, because these fail to convey important information about effect size and precision of estimates [3] . Statistical terms, abbreviations and most symbols should be defined, and the statistical software package and versions used should be specified. Authors should also take care to distinguish prespecified from exploratory analyses, including subgroup analyses [3] .
The results section should be straightforward and factual and all of the results that relate to the research question should be provided, with detail including simple counts and percentages [27] . Data collection and recruitment should be commented on and the participants described. Secondary findings and the results of subgroup analyses can also be presented [27] .
Figures, schemes and tables
To present data and results of the research study, figures, schemes and tables can be used. They should include significant digits, error bars and levels of statistical significance.
Tables should be presented with a summary title, followed by caption, a sentence or two that describes the content and impact of the data included in the table. All captions should provide enough detail so that the table or figure can be interpreted and understood as stand-alone material, separate from the article.
Figures should also be presented with a summary title, a caption that describes the significant result or interpretation that can be made from the figure, the number of repetitions within the experiment, as well as what the data point actually represents. All figures and tables should be cited in the manuscript text [11] .
When compiling tables and figures, important statistics, such as the number of samples (n), the index of dispersion (standard deviation [SD], standard error of the mean [SEM]), and the index of central tendency (mean, median or mode), must be stated. The statistical analysis performed should also be included and specific statistical data should be indicated (e.g. P values) [11] .
Discussion and conclusions
The discussion section should state the main findings of the study. The main results should be compared with reference to previous research and current knowledge, and where this has been extended it should be fully described [2] , [11] , [25] . For clinical studies, relevant discussion of the implications the results may have on policy should be included [10] . It is important to include an analysis of the strengths and limitations of the study and offer perspectives for future work [2] . Excessive presentation of data and results without any discussion should be avoided and it is not necessary to cite a published work for each argument presented. Any conclusions should include the major findings, followed by a brief discussion of future perspectives and the application of this work to other disciplines [10] .
The list of references should be appropriate; important statements presented as facts should be referenced, as well as the methods and instruments used. Reference lists for research articles, however, unlike comprehensive reviews of a topic, do not necessarily have to be exhaustive. References to unpublished work, to documents in the grey literature (technical reports), or to any source that the reader will have difficulty finding or understanding should be avoided [27] . Most journals have reference limits and specific formatting requirements, so it is important to check the journal’s author guidelines [10] , [11] , [12] , [13] , [19] .
Authorship and acknowledgements
Determining contributors who qualify as authors and those who should be acknowledged can be difficult. Clinical Pharmacist follows guidance from the International Committee of Medical Journal Editors, which recommends that authorship be based on the following four criteria:
- Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND
- Drafting the work or revising it critically for important intellectual content; AND
- Final approval of the version to be published; AND
- Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved [3] .
Therefore, only individuals who meet all four criteria should be identified as authors [3] . The contribution of individuals who do not meet all four criteria should instead be included in the acknowledgements.
In addition, a statement that recognises any funding sources for the work should be added to the acknowledgements. This statement should adhere to the guidelines provided by the funding institution [11] .
Supplementary and supporting information
A key principle of research publication is that others should be able to replicate and build upon an author’s published claims. Therefore, submitted manuscripts should contain the necessary detail about the study and analytical design, and the data must be available for editors and peer-reviewers to allow full evaluation to take place. This is now commonplace and is seen as best practice. Author guidelines now include sections related to misconduct and falsification of data [28] . By participating in self-archiving practices and providing full data sets, authors can play their part in transparency.
The Royal Pharmaceutical Society website hosts a database to help share data from research studies. The map of evidence collates existing evidence and ongoing initiatives that can ultimately inform policy and practice relating to pharmacy; enables the sharing and showcasing of good pharmacy practice and innovation; and aims to increase the knowledge exchange and learning in pharmacy and pharmaceutical sciences [29] .
Revising your article prior to submission
Once a draft research article has been prepared, it should be shared among all of the co-authors for review and comments. A full revision of the draft should then take place to correct grammar and check flow and logic before journal submission. All authors will have to agree on the authenticity of the data and presentation before formal submission can take place [3] (see ‘Box 2: Common mistakes and reasons why research articles are rejected for publication’).
Box 2: Common mistakes and reasons why research articles are rejected for publication
- Lack of novelty and importance of the research question;
- Poor study design;
- Methods not accurately and adequately described;
- Results poorly reported, along with little analysis of data;
- Lack of statistical analysis;
- Not acknowledging the study’s limitations;
- Providing unsupported conclusions or overstating the results of the study;
- Poor writing;
- Not following the journal’s style and formatting guidance;
- Submitting a manuscript that is incomplete or outside of the aims and scope.
Selecting a journal and submitting your manuscript
It is important to select a journal for submission wisely because this choice can determine the impact and dissemination of your work [13] . Impact factor (a measure of the frequency with which the average article in a journal has been cited in a particular year), the scope and readership of a title may also influence your choice.
Furthermore, approval and adequate disclosures must be obtained from all co-authors. A conflict of interests form is also completed as part of the submissions process (normally completed by the lead author on behalf of all authors).
Many journals now request that a cover letter is also submitted to the editor, putting the study in context and explaining why the research is of importance to their audience and why it should be considered for publication in their journal.
Once this is all completed, the article can be formally submitted (usually via email or an online submission system). Figure 2 provides a sample process for a manuscript once submitted to a journal for consideration for publication.
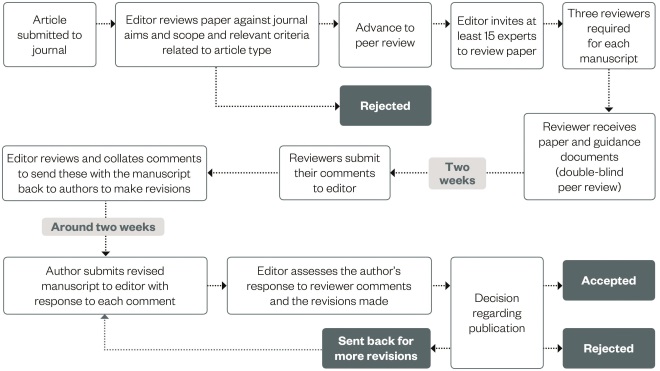
Figure 2: Sample process for a submitted manuscript
Source: The Pharmaceutical Journal
All journals follow a similar process for article submissions, whether they use a formal online submissions system or simply email. Clinical Pharmacist uses a process similar to this and it is useful for authors to be aware of how their submission may progress once submitted to a journal for publication.
Useful Links
- The EQUATOR Network
- Research4Life – Authorship skills modules
- Pharmacy Research UK
Reading this article counts towards your CPD
You can use the following forms to record your learning and action points from this article from Pharmaceutical Journal Publications.
Your CPD module results are stored against your account here at The Pharmaceutical Journal . You must be registered and logged into the site to do this. To review your module results, go to the ‘My Account’ tab and then ‘My CPD’.
Any training, learning or development activities that you undertake for CPD can also be recorded as evidence as part of your RPS Faculty practice-based portfolio when preparing for Faculty membership. To start your RPS Faculty journey today, access the portfolio and tools at www.rpharms.com/Faculty
If your learning was planned in advance, please click:
If your learning was spontaneous, please click:
[1] Boyer E. Scholarship reconsidered: Priorities for the professoriate . 1990. Princeton, NJ: The Carnegie Foundation for the Advancement of Teaching.
[2] Hoogenboom BJ & Manske RC. How to write a scientific article. Int J Sports Phys Ther . 2012;7(5):512–517. PMCID: PMC3474301
[3] International Committee of Medical Journal Editors. Recommendations for the conduct, reporting, editing, and publication of scholarly work in medical journals. 2014. Available at: http://www.icmje.org/icmje-recommendations.pdf (accessed November 2016).
[4] Dowdall M. How to be an effective peer reviewer. Clinical Pharmacist 2015;7(10). doi: 10.1211/CP.2015.20200006
[5] Nahata MC. Tips for writing and publishing an article. Ann Pharmaco . 2008;42:273–277. doi: 10.1345/aph.1K616
[6] Dixon N. Writing for publication: A guide for new authors. Int J Qual Health Care . 2001;13:417–421. doi: 10.1093/intqhc/13.5.417
[7] Keys CW. Revitalizing instruction in scientific genres: Connecting knowledge production with writing to learn in science. Sci Educ . 1999;83:115–130.
[8] Gopen G & Swan J. The science of scientific writing. Am Sci . 1990;78:550–558. Available at: http://www.americanscientist.org/issues/pub/the-science-of-scientific-writing (accessed November 2016)
[9] Hulley S, Cummings S, Browner W et al . Designing clinical research. 3rd ed. Philadelphia (PA): Lippincott Williams and Wilkins; 2007.
[10] The Pharmaceutical Journal and Clinical Pharmacist. Author Guidance (2015). Available at: http://www.pharmaceutical-journal.com/for-authors-and-referees/article-types/#Practice_reports (accessed November 2016)
[11] Fisher JP, Jansen JA, Johnson PC et al . Guidelines for writing a research article for publication. Mary Ann Liebert Inc. Available at: https://www.liebertpub.com/media/pdf/English-Research-Article-Writing-Guide.pdf (accessed November 2016)
[12] Nature. Author Resources: How to write a paper. Available at: http://www.nature.com/authors/author_resources/how_write.html (accessed November 2016)
[13] Wiley Online Library. Resources for authors and reviewers: preparing your article. Available at: http://olabout.wiley.com/WileyCDA/Section/id-828006.html (accessed November 2016)
[14] SAGE Publications. Help readers find your article. Available at: http://www.uk.sagepub.com/journalgateway/findArticle.htm (accessed November 2016)
[15] Bem DJ. Writing the empirical journal article. In: MP Zanna & JM Darley (Eds.), The complete academic: a practical guide for the beginning social scientist (pp. 171–201). New York: Random House; 1987.
[16] Fathalla M & Fathalla M. A practical guide for health researchers. Available at: http://www.emro.who.int/dsaf/dsa237.pdf (accessed November 2016)
[17] Coghill A & Garson L (Eds.). Scientific Papers. In: A Coghill & L Garson (Eds.), The ACS Style Guide, 3 rd Edition (pp. 20–21). New York: Oxford University Press, 2006.
[18] The Scholarly Publishing and Academic Resources Institute. Available at: http://sparcopen.org/open-access/ (accessed November 2016).
[19] Elsevier. Understanding the publishing process: how to publish in scholarly journals. Available at: https://www.elsevier.com/__data/assets/pdf_file/0003/91173/Brochure_UPP_April2015.pdf (accessed November 2016).
[20] SciDevNet. How do I write a scientific paper? 2008. Available at: http://www.scidev.net/global/publishing/practical-guide/how-do-i-write-a-scientific-paper-.html (accessed November 2016)
[21] Moher D, Schultz KR & Altman DG. CONSORT GROUP (Consolidatied Standards of Reporting Trials). The CONSORT statement: Revised recommendations for improving the quality of reports of parallelâ€group randomized controlled trials. Ann Intern Med . 2001;134:657–662. PMID: 11304106
[22] Bossuyt PM, Reitsma JB, Bruns DE et al . Towards complete and accurate reporting of studies of diagnostic accuracy: The STARD Initiative. Ann Int Med 2003;138:40–44. PMID: 12513043
[23] Moher D, Liberati A, Tetzlaff J et al . The PRISMA Group (2009). Preferred reporting items for systematic reviews and metaâ€analyses: the PRISMA statement. PLoS Med 6(6):e1000097. doi: 10.1371/journal.pmed1000097
[24] Devers KJ & Frankel RM. Getting qualitative research published. Educ Health 2001;14:109–117. doi: 10.1080/13576280010021888
[25] Van Way CW. Writing a scientific paper. Nutr Clin Pract 2007;22:636–640. PMID: 18042951
[26] Kishore V. When to use a survey in pharmacy practice research. The Pharmaceutical Journal 296(7886). doi: 10.1211/PJ.2016.20200700
[27] Perneger PV & Hudelson PM. Writing a research article: advice to beginners . Int J Qual Health Care 2004;16(3):191–192. doi: 10.1093/intqhc/mzh053
[28] World Association of Medical Editors. Professionalism Code of Conduct. 2016. Available at: http://www.wame.org/News/Details/16 (accessed November 2016)
[29] Royal Pharmaceutical Society. Map of Evidence. Available at: http://www.rpharms.com/support/map-of-evidence.asp (accessed November 2016)
You might also be interested in…

Working to improve our digital archive
The Pharmaceutical Journal is moving forward into a digital future

The launch of our new digital platform is just the beginning of our plans for the future of PJ
Accessibility Links
- Skip to content
- Skip to search IOPscience
- Skip to Journals list
- Accessibility help
- Accessibility Help
Click here to close this panel.
Purpose-led Publishing is a coalition of three not-for-profit publishers in the field of physical sciences: AIP Publishing, the American Physical Society and IOP Publishing.
Together, as publishers that will always put purpose above profit, we have defined a set of industry standards that underpin high-quality, ethical scholarly communications.
We are proudly declaring that science is our only shareholder.
Building IT-based Pharmacy: Computerized Pharmacy Management
B Kurniawan 1 and M Ikhsan 1
Published under licence by IOP Publishing Ltd IOP Conference Series: Materials Science and Engineering , Volume 407 , International Conference on Informatics, Engineering, Science and Technology (INCITEST) 9 May 2018, Bandung, Indonesia Citation B Kurniawan and M Ikhsan 2018 IOP Conf. Ser.: Mater. Sci. Eng. 407 012020 DOI 10.1088/1757-899X/407/1/012020
Article metrics
1076 Total downloads
Share this article
Author e-mails.
Author affiliations
1 Departement Teknik dan Ilmu Komputer, Universitas Komputer Indonesia, Bandung, Indonesia
Buy this article in print
This study aims to produce computer-based systems that allow pharmacists to perform their work in pharmaceutical management. This research used the descriptive method to analyze how pharmacies Cibadak Farma handled the drug supplied management activities, sales transactions, purchasing drugs, and reporting manually. Data collection methods used consisted of field research conducted by observation, questionnaires, and interviews. The method used prototyping system development. The results show that building computer-based management system pharmacies Cibadak Farma able to handle the processing of drug supplies, processing transactions and making transaction reports based on a particular period to be effective and efficient work.
Export citation and abstract BibTeX RIS
Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence . Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
- Open access
- Published: 18 April 2024
Research ethics and artificial intelligence for global health: perspectives from the global forum on bioethics in research
- James Shaw 1 , 13 ,
- Joseph Ali 2 , 3 ,
- Caesar A. Atuire 4 , 5 ,
- Phaik Yeong Cheah 6 ,
- Armando Guio Español 7 ,
- Judy Wawira Gichoya 8 ,
- Adrienne Hunt 9 ,
- Daudi Jjingo 10 ,
- Katherine Littler 9 ,
- Daniela Paolotti 11 &
- Effy Vayena 12
BMC Medical Ethics volume 25 , Article number: 46 ( 2024 ) Cite this article
1145 Accesses
6 Altmetric
Metrics details
The ethical governance of Artificial Intelligence (AI) in health care and public health continues to be an urgent issue for attention in policy, research, and practice. In this paper we report on central themes related to challenges and strategies for promoting ethics in research involving AI in global health, arising from the Global Forum on Bioethics in Research (GFBR), held in Cape Town, South Africa in November 2022.
The GFBR is an annual meeting organized by the World Health Organization and supported by the Wellcome Trust, the US National Institutes of Health, the UK Medical Research Council (MRC) and the South African MRC. The forum aims to bring together ethicists, researchers, policymakers, research ethics committee members and other actors to engage with challenges and opportunities specifically related to research ethics. In 2022 the focus of the GFBR was “Ethics of AI in Global Health Research”. The forum consisted of 6 case study presentations, 16 governance presentations, and a series of small group and large group discussions. A total of 87 participants attended the forum from 31 countries around the world, representing disciplines of bioethics, AI, health policy, health professional practice, research funding, and bioinformatics. In this paper, we highlight central insights arising from GFBR 2022.
We describe the significance of four thematic insights arising from the forum: (1) Appropriateness of building AI, (2) Transferability of AI systems, (3) Accountability for AI decision-making and outcomes, and (4) Individual consent. We then describe eight recommendations for governance leaders to enhance the ethical governance of AI in global health research, addressing issues such as AI impact assessments, environmental values, and fair partnerships.
Conclusions
The 2022 Global Forum on Bioethics in Research illustrated several innovations in ethical governance of AI for global health research, as well as several areas in need of urgent attention internationally. This summary is intended to inform international and domestic efforts to strengthen research ethics and support the evolution of governance leadership to meet the demands of AI in global health research.
Peer Review reports
Introduction
The ethical governance of Artificial Intelligence (AI) in health care and public health continues to be an urgent issue for attention in policy, research, and practice [ 1 , 2 , 3 ]. Beyond the growing number of AI applications being implemented in health care, capabilities of AI models such as Large Language Models (LLMs) expand the potential reach and significance of AI technologies across health-related fields [ 4 , 5 ]. Discussion about effective, ethical governance of AI technologies has spanned a range of governance approaches, including government regulation, organizational decision-making, professional self-regulation, and research ethics review [ 6 , 7 , 8 ]. In this paper, we report on central themes related to challenges and strategies for promoting ethics in research involving AI in global health research, arising from the Global Forum on Bioethics in Research (GFBR), held in Cape Town, South Africa in November 2022. Although applications of AI for research, health care, and public health are diverse and advancing rapidly, the insights generated at the forum remain highly relevant from a global health perspective. After summarizing important context for work in this domain, we highlight categories of ethical issues emphasized at the forum for attention from a research ethics perspective internationally. We then outline strategies proposed for research, innovation, and governance to support more ethical AI for global health.
In this paper, we adopt the definition of AI systems provided by the Organization for Economic Cooperation and Development (OECD) as our starting point. Their definition states that an AI system is “a machine-based system that can, for a given set of human-defined objectives, make predictions, recommendations, or decisions influencing real or virtual environments. AI systems are designed to operate with varying levels of autonomy” [ 9 ]. The conceptualization of an algorithm as helping to constitute an AI system, along with hardware, other elements of software, and a particular context of use, illustrates the wide variety of ways in which AI can be applied. We have found it useful to differentiate applications of AI in research as those classified as “AI systems for discovery” and “AI systems for intervention”. An AI system for discovery is one that is intended to generate new knowledge, for example in drug discovery or public health research in which researchers are seeking potential targets for intervention, innovation, or further research. An AI system for intervention is one that directly contributes to enacting an intervention in a particular context, for example informing decision-making at the point of care or assisting with accuracy in a surgical procedure.
The mandate of the GFBR is to take a broad view of what constitutes research and its regulation in global health, with special attention to bioethics in Low- and Middle- Income Countries. AI as a group of technologies demands such a broad view. AI development for health occurs in a variety of environments, including universities and academic health sciences centers where research ethics review remains an important element of the governance of science and innovation internationally [ 10 , 11 ]. In these settings, research ethics committees (RECs; also known by different names such as Institutional Review Boards or IRBs) make decisions about the ethical appropriateness of projects proposed by researchers and other institutional members, ultimately determining whether a given project is allowed to proceed on ethical grounds [ 12 ].
However, research involving AI for health also takes place in large corporations and smaller scale start-ups, which in some jurisdictions fall outside the scope of research ethics regulation. In the domain of AI, the question of what constitutes research also becomes blurred. For example, is the development of an algorithm itself considered a part of the research process? Or only when that algorithm is tested under the formal constraints of a systematic research methodology? In this paper we take an inclusive view, in which AI development is included in the definition of research activity and within scope for our inquiry, regardless of the setting in which it takes place. This broad perspective characterizes the approach to “research ethics” we take in this paper, extending beyond the work of RECs to include the ethical analysis of the wide range of activities that constitute research as the generation of new knowledge and intervention in the world.
Ethical governance of AI in global health
The ethical governance of AI for global health has been widely discussed in recent years. The World Health Organization (WHO) released its guidelines on ethics and governance of AI for health in 2021, endorsing a set of six ethical principles and exploring the relevance of those principles through a variety of use cases. The WHO guidelines also provided an overview of AI governance, defining governance as covering “a range of steering and rule-making functions of governments and other decision-makers, including international health agencies, for the achievement of national health policy objectives conducive to universal health coverage.” (p. 81) The report usefully provided a series of recommendations related to governance of seven domains pertaining to AI for health: data, benefit sharing, the private sector, the public sector, regulation, policy observatories/model legislation, and global governance. The report acknowledges that much work is yet to be done to advance international cooperation on AI governance, especially related to prioritizing voices from Low- and Middle-Income Countries (LMICs) in global dialogue.
One important point emphasized in the WHO report that reinforces the broader literature on global governance of AI is the distribution of responsibility across a wide range of actors in the AI ecosystem. This is especially important to highlight when focused on research for global health, which is specifically about work that transcends national borders. Alami et al. (2020) discussed the unique risks raised by AI research in global health, ranging from the unavailability of data in many LMICs required to train locally relevant AI models to the capacity of health systems to absorb new AI technologies that demand the use of resources from elsewhere in the system. These observations illustrate the need to identify the unique issues posed by AI research for global health specifically, and the strategies that can be employed by all those implicated in AI governance to promote ethically responsible use of AI in global health research.
RECs and the regulation of research involving AI
RECs represent an important element of the governance of AI for global health research, and thus warrant further commentary as background to our paper. Despite the importance of RECs, foundational questions have been raised about their capabilities to accurately understand and address ethical issues raised by studies involving AI. Rahimzadeh et al. (2023) outlined how RECs in the United States are under-prepared to align with recent federal policy requiring that RECs review data sharing and management plans with attention to the unique ethical issues raised in AI research for health [ 13 ]. Similar research in South Africa identified variability in understanding of existing regulations and ethical issues associated with health-related big data sharing and management among research ethics committee members [ 14 , 15 ]. The effort to address harms accruing to groups or communities as opposed to individuals whose data are included in AI research has also been identified as a unique challenge for RECs [ 16 , 17 ]. Doerr and Meeder (2022) suggested that current regulatory frameworks for research ethics might actually prevent RECs from adequately addressing such issues, as they are deemed out of scope of REC review [ 16 ]. Furthermore, research in the United Kingdom and Canada has suggested that researchers using AI methods for health tend to distinguish between ethical issues and social impact of their research, adopting an overly narrow view of what constitutes ethical issues in their work [ 18 ].
The challenges for RECs in adequately addressing ethical issues in AI research for health care and public health exceed a straightforward survey of ethical considerations. As Ferretti et al. (2021) contend, some capabilities of RECs adequately cover certain issues in AI-based health research, such as the common occurrence of conflicts of interest where researchers who accept funds from commercial technology providers are implicitly incentivized to produce results that align with commercial interests [ 12 ]. However, some features of REC review require reform to adequately meet ethical needs. Ferretti et al. outlined weaknesses of RECs that are longstanding and those that are novel to AI-related projects, proposing a series of directions for development that are regulatory, procedural, and complementary to REC functionality. The work required on a global scale to update the REC function in response to the demands of research involving AI is substantial.
These issues take greater urgency in the context of global health [ 19 ]. Teixeira da Silva (2022) described the global practice of “ethics dumping”, where researchers from high income countries bring ethically contentious practices to RECs in low-income countries as a strategy to gain approval and move projects forward [ 20 ]. Although not yet systematically documented in AI research for health, risk of ethics dumping in AI research is high. Evidence is already emerging of practices of “health data colonialism”, in which AI researchers and developers from large organizations in high-income countries acquire data to build algorithms in LMICs to avoid stricter regulations [ 21 ]. This specific practice is part of a larger collection of practices that characterize health data colonialism, involving the broader exploitation of data and the populations they represent primarily for commercial gain [ 21 , 22 ]. As an additional complication, AI algorithms trained on data from high-income contexts are unlikely to apply in straightforward ways to LMIC settings [ 21 , 23 ]. In the context of global health, there is widespread acknowledgement about the need to not only enhance the knowledge base of REC members about AI-based methods internationally, but to acknowledge the broader shifts required to encourage their capabilities to more fully address these and other ethical issues associated with AI research for health [ 8 ].
Although RECs are an important part of the story of the ethical governance of AI for global health research, they are not the only part. The responsibilities of supra-national entities such as the World Health Organization, national governments, organizational leaders, commercial AI technology providers, health care professionals, and other groups continue to be worked out internationally. In this context of ongoing work, examining issues that demand attention and strategies to address them remains an urgent and valuable task.
The GFBR is an annual meeting organized by the World Health Organization and supported by the Wellcome Trust, the US National Institutes of Health, the UK Medical Research Council (MRC) and the South African MRC. The forum aims to bring together ethicists, researchers, policymakers, REC members and other actors to engage with challenges and opportunities specifically related to research ethics. Each year the GFBR meeting includes a series of case studies and keynotes presented in plenary format to an audience of approximately 100 people who have applied and been competitively selected to attend, along with small-group breakout discussions to advance thinking on related issues. The specific topic of the forum changes each year, with past topics including ethical issues in research with people living with mental health conditions (2021), genome editing (2019), and biobanking/data sharing (2018). The forum is intended to remain grounded in the practical challenges of engaging in research ethics, with special interest in low resource settings from a global health perspective. A post-meeting fellowship scheme is open to all LMIC participants, providing a unique opportunity to apply for funding to further explore and address the ethical challenges that are identified during the meeting.
In 2022, the focus of the GFBR was “Ethics of AI in Global Health Research”. The forum consisted of 6 case study presentations (both short and long form) reporting on specific initiatives related to research ethics and AI for health, and 16 governance presentations (both short and long form) reporting on actual approaches to governing AI in different country settings. A keynote presentation from Professor Effy Vayena addressed the topic of the broader context for AI ethics in a rapidly evolving field. A total of 87 participants attended the forum from 31 countries around the world, representing disciplines of bioethics, AI, health policy, health professional practice, research funding, and bioinformatics. The 2-day forum addressed a wide range of themes. The conference report provides a detailed overview of each of the specific topics addressed while a policy paper outlines the cross-cutting themes (both documents are available at the GFBR website: https://www.gfbr.global/past-meetings/16th-forum-cape-town-south-africa-29-30-november-2022/ ). As opposed to providing a detailed summary in this paper, we aim to briefly highlight central issues raised, solutions proposed, and the challenges facing the research ethics community in the years to come.
In this way, our primary aim in this paper is to present a synthesis of the challenges and opportunities raised at the GFBR meeting and in the planning process, followed by our reflections as a group of authors on their significance for governance leaders in the coming years. We acknowledge that the views represented at the meeting and in our results are a partial representation of the universe of views on this topic; however, the GFBR leadership invested a great deal of resources in convening a deeply diverse and thoughtful group of researchers and practitioners working on themes of bioethics related to AI for global health including those based in LMICs. We contend that it remains rare to convene such a strong group for an extended time and believe that many of the challenges and opportunities raised demand attention for more ethical futures of AI for health. Nonetheless, our results are primarily descriptive and are thus not explicitly grounded in a normative argument. We make effort in the Discussion section to contextualize our results by describing their significance and connecting them to broader efforts to reform global health research and practice.
Uniquely important ethical issues for AI in global health research
Presentations and group dialogue over the course of the forum raised several issues for consideration, and here we describe four overarching themes for the ethical governance of AI in global health research. Brief descriptions of each issue can be found in Table 1 . Reports referred to throughout the paper are available at the GFBR website provided above.
The first overarching thematic issue relates to the appropriateness of building AI technologies in response to health-related challenges in the first place. Case study presentations referred to initiatives where AI technologies were highly appropriate, such as in ear shape biometric identification to more accurately link electronic health care records to individual patients in Zambia (Alinani Simukanga). Although important ethical issues were raised with respect to privacy, trust, and community engagement in this initiative, the AI-based solution was appropriately matched to the challenge of accurately linking electronic records to specific patient identities. In contrast, forum participants raised questions about the appropriateness of an initiative using AI to improve the quality of handwashing practices in an acute care hospital in India (Niyoshi Shah), which led to gaming the algorithm. Overall, participants acknowledged the dangers of techno-solutionism, in which AI researchers and developers treat AI technologies as the most obvious solutions to problems that in actuality demand much more complex strategies to address [ 24 ]. However, forum participants agreed that RECs in different contexts have differing degrees of power to raise issues of the appropriateness of an AI-based intervention.
The second overarching thematic issue related to whether and how AI-based systems transfer from one national health context to another. One central issue raised by a number of case study presentations related to the challenges of validating an algorithm with data collected in a local environment. For example, one case study presentation described a project that would involve the collection of personally identifiable data for sensitive group identities, such as tribe, clan, or religion, in the jurisdictions involved (South Africa, Nigeria, Tanzania, Uganda and the US; Gakii Masunga). Doing so would enable the team to ensure that those groups were adequately represented in the dataset to ensure the resulting algorithm was not biased against specific community groups when deployed in that context. However, some members of these communities might desire to be represented in the dataset, whereas others might not, illustrating the need to balance autonomy and inclusivity. It was also widely recognized that collecting these data is an immense challenge, particularly when historically oppressive practices have led to a low-trust environment for international organizations and the technologies they produce. It is important to note that in some countries such as South Africa and Rwanda, it is illegal to collect information such as race and tribal identities, re-emphasizing the importance for cultural awareness and avoiding “one size fits all” solutions.
The third overarching thematic issue is related to understanding accountabilities for both the impacts of AI technologies and governance decision-making regarding their use. Where global health research involving AI leads to longer-term harms that might fall outside the usual scope of issues considered by a REC, who is to be held accountable, and how? This question was raised as one that requires much further attention, with law being mixed internationally regarding the mechanisms available to hold researchers, innovators, and their institutions accountable over the longer term. However, it was recognized in breakout group discussion that many jurisdictions are developing strong data protection regimes related specifically to international collaboration for research involving health data. For example, Kenya’s Data Protection Act requires that any internationally funded projects have a local principal investigator who will hold accountability for how data are shared and used [ 25 ]. The issue of research partnerships with commercial entities was raised by many participants in the context of accountability, pointing toward the urgent need for clear principles related to strategies for engagement with commercial technology companies in global health research.
The fourth and final overarching thematic issue raised here is that of consent. The issue of consent was framed by the widely shared recognition that models of individual, explicit consent might not produce a supportive environment for AI innovation that relies on the secondary uses of health-related datasets to build AI algorithms. Given this recognition, approaches such as community oversight of health data uses were suggested as a potential solution. However, the details of implementing such community oversight mechanisms require much further attention, particularly given the unique perspectives on health data in different country settings in global health research. Furthermore, some uses of health data do continue to require consent. One case study of South Africa, Nigeria, Kenya, Ethiopia and Uganda suggested that when health data are shared across borders, individual consent remains necessary when data is transferred from certain countries (Nezerith Cengiz). Broader clarity is necessary to support the ethical governance of health data uses for AI in global health research.
Recommendations for ethical governance of AI in global health research
Dialogue at the forum led to a range of suggestions for promoting ethical conduct of AI research for global health, related to the various roles of actors involved in the governance of AI research broadly defined. The strategies are written for actors we refer to as “governance leaders”, those people distributed throughout the AI for global health research ecosystem who are responsible for ensuring the ethical and socially responsible conduct of global health research involving AI (including researchers themselves). These include RECs, government regulators, health care leaders, health professionals, corporate social accountability officers, and others. Enacting these strategies would bolster the ethical governance of AI for global health more generally, enabling multiple actors to fulfill their roles related to governing research and development activities carried out across multiple organizations, including universities, academic health sciences centers, start-ups, and technology corporations. Specific suggestions are summarized in Table 2 .
First, forum participants suggested that governance leaders including RECs, should remain up to date on recent advances in the regulation of AI for health. Regulation of AI for health advances rapidly and takes on different forms in jurisdictions around the world. RECs play an important role in governance, but only a partial role; it was deemed important for RECs to acknowledge how they fit within a broader governance ecosystem in order to more effectively address the issues within their scope. Not only RECs but organizational leaders responsible for procurement, researchers, and commercial actors should all commit to efforts to remain up to date about the relevant approaches to regulating AI for health care and public health in jurisdictions internationally. In this way, governance can more adequately remain up to date with advances in regulation.
Second, forum participants suggested that governance leaders should focus on ethical governance of health data as a basis for ethical global health AI research. Health data are considered the foundation of AI development, being used to train AI algorithms for various uses [ 26 ]. By focusing on ethical governance of health data generation, sharing, and use, multiple actors will help to build an ethical foundation for AI development among global health researchers.
Third, forum participants believed that governance processes should incorporate AI impact assessments where appropriate. An AI impact assessment is the process of evaluating the potential effects, both positive and negative, of implementing an AI algorithm on individuals, society, and various stakeholders, generally over time frames specified in advance of implementation [ 27 ]. Although not all types of AI research in global health would warrant an AI impact assessment, this is especially relevant for those studies aiming to implement an AI system for intervention into health care or public health. Organizations such as RECs can use AI impact assessments to boost understanding of potential harms at the outset of a research project, encouraging researchers to more deeply consider potential harms in the development of their study.
Fourth, forum participants suggested that governance decisions should incorporate the use of environmental impact assessments, or at least the incorporation of environment values when assessing the potential impact of an AI system. An environmental impact assessment involves evaluating and anticipating the potential environmental effects of a proposed project to inform ethical decision-making that supports sustainability [ 28 ]. Although a relatively new consideration in research ethics conversations [ 29 ], the environmental impact of building technologies is a crucial consideration for the public health commitment to environmental sustainability. Governance leaders can use environmental impact assessments to boost understanding of potential environmental harms linked to AI research projects in global health over both the shorter and longer terms.
Fifth, forum participants suggested that governance leaders should require stronger transparency in the development of AI algorithms in global health research. Transparency was considered essential in the design and development of AI algorithms for global health to ensure ethical and accountable decision-making throughout the process. Furthermore, whether and how researchers have considered the unique contexts into which such algorithms may be deployed can be surfaced through stronger transparency, for example in describing what primary considerations were made at the outset of the project and which stakeholders were consulted along the way. Sharing information about data provenance and methods used in AI development will also enhance the trustworthiness of the AI-based research process.
Sixth, forum participants suggested that governance leaders can encourage or require community engagement at various points throughout an AI project. It was considered that engaging patients and communities is crucial in AI algorithm development to ensure that the technology aligns with community needs and values. However, participants acknowledged that this is not a straightforward process. Effective community engagement requires lengthy commitments to meeting with and hearing from diverse communities in a given setting, and demands a particular set of skills in communication and dialogue that are not possessed by all researchers. Encouraging AI researchers to begin this process early and build long-term partnerships with community members is a promising strategy to deepen community engagement in AI research for global health. One notable recommendation was that research funders have an opportunity to incentivize and enable community engagement with funds dedicated to these activities in AI research in global health.
Seventh, forum participants suggested that governance leaders can encourage researchers to build strong, fair partnerships between institutions and individuals across country settings. In a context of longstanding imbalances in geopolitical and economic power, fair partnerships in global health demand a priori commitments to share benefits related to advances in medical technologies, knowledge, and financial gains. Although enforcement of this point might be beyond the remit of RECs, commentary will encourage researchers to consider stronger, fairer partnerships in global health in the longer term.
Eighth, it became evident that it is necessary to explore new forms of regulatory experimentation given the complexity of regulating a technology of this nature. In addition, the health sector has a series of particularities that make it especially complicated to generate rules that have not been previously tested. Several participants highlighted the desire to promote spaces for experimentation such as regulatory sandboxes or innovation hubs in health. These spaces can have several benefits for addressing issues surrounding the regulation of AI in the health sector, such as: (i) increasing the capacities and knowledge of health authorities about this technology; (ii) identifying the major problems surrounding AI regulation in the health sector; (iii) establishing possibilities for exchange and learning with other authorities; (iv) promoting innovation and entrepreneurship in AI in health; and (vi) identifying the need to regulate AI in this sector and update other existing regulations.
Ninth and finally, forum participants believed that the capabilities of governance leaders need to evolve to better incorporate expertise related to AI in ways that make sense within a given jurisdiction. With respect to RECs, for example, it might not make sense for every REC to recruit a member with expertise in AI methods. Rather, it will make more sense in some jurisdictions to consult with members of the scientific community with expertise in AI when research protocols are submitted that demand such expertise. Furthermore, RECs and other approaches to research governance in jurisdictions around the world will need to evolve in order to adopt the suggestions outlined above, developing processes that apply specifically to the ethical governance of research using AI methods in global health.
Research involving the development and implementation of AI technologies continues to grow in global health, posing important challenges for ethical governance of AI in global health research around the world. In this paper we have summarized insights from the 2022 GFBR, focused specifically on issues in research ethics related to AI for global health research. We summarized four thematic challenges for governance related to AI in global health research and nine suggestions arising from presentations and dialogue at the forum. In this brief discussion section, we present an overarching observation about power imbalances that frames efforts to evolve the role of governance in global health research, and then outline two important opportunity areas as the field develops to meet the challenges of AI in global health research.
Dialogue about power is not unfamiliar in global health, especially given recent contributions exploring what it would mean to de-colonize global health research, funding, and practice [ 30 , 31 ]. Discussions of research ethics applied to AI research in global health contexts are deeply infused with power imbalances. The existing context of global health is one in which high-income countries primarily located in the “Global North” charitably invest in projects taking place primarily in the “Global South” while recouping knowledge, financial, and reputational benefits [ 32 ]. With respect to AI development in particular, recent examples of digital colonialism frame dialogue about global partnerships, raising attention to the role of large commercial entities and global financial capitalism in global health research [ 21 , 22 ]. Furthermore, the power of governance organizations such as RECs to intervene in the process of AI research in global health varies widely around the world, depending on the authorities assigned to them by domestic research governance policies. These observations frame the challenges outlined in our paper, highlighting the difficulties associated with making meaningful change in this field.
Despite these overarching challenges of the global health research context, there are clear strategies for progress in this domain. Firstly, AI innovation is rapidly evolving, which means approaches to the governance of AI for health are rapidly evolving too. Such rapid evolution presents an important opportunity for governance leaders to clarify their vision and influence over AI innovation in global health research, boosting the expertise, structure, and functionality required to meet the demands of research involving AI. Secondly, the research ethics community has strong international ties, linked to a global scholarly community that is committed to sharing insights and best practices around the world. This global community can be leveraged to coordinate efforts to produce advances in the capabilities and authorities of governance leaders to meaningfully govern AI research for global health given the challenges summarized in our paper.
Limitations
Our paper includes two specific limitations that we address explicitly here. First, it is still early in the lifetime of the development of applications of AI for use in global health, and as such, the global community has had limited opportunity to learn from experience. For example, there were many fewer case studies, which detail experiences with the actual implementation of an AI technology, submitted to GFBR 2022 for consideration than was expected. In contrast, there were many more governance reports submitted, which detail the processes and outputs of governance processes that anticipate the development and dissemination of AI technologies. This observation represents both a success and a challenge. It is a success that so many groups are engaging in anticipatory governance of AI technologies, exploring evidence of their likely impacts and governing technologies in novel and well-designed ways. It is a challenge that there is little experience to build upon of the successful implementation of AI technologies in ways that have limited harms while promoting innovation. Further experience with AI technologies in global health will contribute to revising and enhancing the challenges and recommendations we have outlined in our paper.
Second, global trends in the politics and economics of AI technologies are evolving rapidly. Although some nations are advancing detailed policy approaches to regulating AI more generally, including for uses in health care and public health, the impacts of corporate investments in AI and political responses related to governance remain to be seen. The excitement around large language models (LLMs) and large multimodal models (LMMs) has drawn deeper attention to the challenges of regulating AI in any general sense, opening dialogue about health sector-specific regulations. The direction of this global dialogue, strongly linked to high-profile corporate actors and multi-national governance institutions, will strongly influence the development of boundaries around what is possible for the ethical governance of AI for global health. We have written this paper at a point when these developments are proceeding rapidly, and as such, we acknowledge that our recommendations will need updating as the broader field evolves.
Ultimately, coordination and collaboration between many stakeholders in the research ethics ecosystem will be necessary to strengthen the ethical governance of AI in global health research. The 2022 GFBR illustrated several innovations in ethical governance of AI for global health research, as well as several areas in need of urgent attention internationally. This summary is intended to inform international and domestic efforts to strengthen research ethics and support the evolution of governance leadership to meet the demands of AI in global health research.
Data availability
All data and materials analyzed to produce this paper are available on the GFBR website: https://www.gfbr.global/past-meetings/16th-forum-cape-town-south-africa-29-30-november-2022/ .
Clark P, Kim J, Aphinyanaphongs Y, Marketing, Food US. Drug Administration Clearance of Artificial Intelligence and Machine Learning Enabled Software in and as Medical devices: a systematic review. JAMA Netw Open. 2023;6(7):e2321792–2321792.
Article Google Scholar
Potnis KC, Ross JS, Aneja S, Gross CP, Richman IB. Artificial intelligence in breast cancer screening: evaluation of FDA device regulation and future recommendations. JAMA Intern Med. 2022;182(12):1306–12.
Siala H, Wang Y. SHIFTing artificial intelligence to be responsible in healthcare: a systematic review. Soc Sci Med. 2022;296:114782.
Yang X, Chen A, PourNejatian N, Shin HC, Smith KE, Parisien C, et al. A large language model for electronic health records. NPJ Digit Med. 2022;5(1):194.
Meskó B, Topol EJ. The imperative for regulatory oversight of large language models (or generative AI) in healthcare. NPJ Digit Med. 2023;6(1):120.
Jobin A, Ienca M, Vayena E. The global landscape of AI ethics guidelines. Nat Mach Intell. 2019;1(9):389–99.
Minssen T, Vayena E, Cohen IG. The challenges for Regulating Medical Use of ChatGPT and other large Language models. JAMA. 2023.
Ho CWL, Malpani R. Scaling up the research ethics framework for healthcare machine learning as global health ethics and governance. Am J Bioeth. 2022;22(5):36–8.
Yeung K. Recommendation of the council on artificial intelligence (OECD). Int Leg Mater. 2020;59(1):27–34.
Maddox TM, Rumsfeld JS, Payne PR. Questions for artificial intelligence in health care. JAMA. 2019;321(1):31–2.
Dzau VJ, Balatbat CA, Ellaissi WF. Revisiting academic health sciences systems a decade later: discovery to health to population to society. Lancet. 2021;398(10318):2300–4.
Ferretti A, Ienca M, Sheehan M, Blasimme A, Dove ES, Farsides B, et al. Ethics review of big data research: what should stay and what should be reformed? BMC Med Ethics. 2021;22(1):1–13.
Rahimzadeh V, Serpico K, Gelinas L. Institutional review boards need new skills to review data sharing and management plans. Nat Med. 2023;1–3.
Kling S, Singh S, Burgess TL, Nair G. The role of an ethics advisory committee in data science research in sub-saharan Africa. South Afr J Sci. 2023;119(5–6):1–3.
Google Scholar
Cengiz N, Kabanda SM, Esterhuizen TM, Moodley K. Exploring perspectives of research ethics committee members on the governance of big data in sub-saharan Africa. South Afr J Sci. 2023;119(5–6):1–9.
Doerr M, Meeder S. Big health data research and group harm: the scope of IRB review. Ethics Hum Res. 2022;44(4):34–8.
Ballantyne A, Stewart C. Big data and public-private partnerships in healthcare and research: the application of an ethics framework for big data in health and research. Asian Bioeth Rev. 2019;11(3):315–26.
Samuel G, Chubb J, Derrick G. Boundaries between research ethics and ethical research use in artificial intelligence health research. J Empir Res Hum Res Ethics. 2021;16(3):325–37.
Murphy K, Di Ruggiero E, Upshur R, Willison DJ, Malhotra N, Cai JC, et al. Artificial intelligence for good health: a scoping review of the ethics literature. BMC Med Ethics. 2021;22(1):1–17.
Teixeira da Silva JA. Handling ethics dumping and neo-colonial research: from the laboratory to the academic literature. J Bioethical Inq. 2022;19(3):433–43.
Ferryman K. The dangers of data colonialism in precision public health. Glob Policy. 2021;12:90–2.
Couldry N, Mejias UA. Data colonialism: rethinking big data’s relation to the contemporary subject. Telev New Media. 2019;20(4):336–49.
Organization WH. Ethics and governance of artificial intelligence for health: WHO guidance. 2021.
Metcalf J, Moss E. Owning ethics: corporate logics, silicon valley, and the institutionalization of ethics. Soc Res Int Q. 2019;86(2):449–76.
Data Protection Act - OFFICE OF THE DATA PROTECTION COMMISSIONER KENYA [Internet]. 2021 [cited 2023 Sep 30]. https://www.odpc.go.ke/dpa-act/ .
Sharon T, Lucivero F. Introduction to the special theme: the expansion of the health data ecosystem–rethinking data ethics and governance. Big Data & Society. Volume 6. London, England: SAGE Publications Sage UK; 2019. p. 2053951719852969.
Reisman D, Schultz J, Crawford K, Whittaker M. Algorithmic impact assessments: a practical Framework for Public Agency. AI Now. 2018.
Morgan RK. Environmental impact assessment: the state of the art. Impact Assess Proj Apprais. 2012;30(1):5–14.
Samuel G, Richie C. Reimagining research ethics to include environmental sustainability: a principled approach, including a case study of data-driven health research. J Med Ethics. 2023;49(6):428–33.
Kwete X, Tang K, Chen L, Ren R, Chen Q, Wu Z, et al. Decolonizing global health: what should be the target of this movement and where does it lead us? Glob Health Res Policy. 2022;7(1):3.
Abimbola S, Asthana S, Montenegro C, Guinto RR, Jumbam DT, Louskieter L, et al. Addressing power asymmetries in global health: imperatives in the wake of the COVID-19 pandemic. PLoS Med. 2021;18(4):e1003604.
Benatar S. Politics, power, poverty and global health: systems and frames. Int J Health Policy Manag. 2016;5(10):599.
Download references

Acknowledgements
We would like to acknowledge the outstanding contributions of the attendees of GFBR 2022 in Cape Town, South Africa. This paper is authored by members of the GFBR 2022 Planning Committee. We would like to acknowledge additional members Tamra Lysaght, National University of Singapore, and Niresh Bhagwandin, South African Medical Research Council, for their input during the planning stages and as reviewers of the applications to attend the Forum.
This work was supported by Wellcome [222525/Z/21/Z], the US National Institutes of Health, the UK Medical Research Council (part of UK Research and Innovation), and the South African Medical Research Council through funding to the Global Forum on Bioethics in Research.
Author information
Authors and affiliations.
Department of Physical Therapy, Temerty Faculty of Medicine, University of Toronto, Toronto, Canada
Berman Institute of Bioethics, Johns Hopkins University, Baltimore, MD, USA
Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, USA
Department of Philosophy and Classics, University of Ghana, Legon-Accra, Ghana
Caesar A. Atuire
Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford, Oxford, UK
Mahidol Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand
Phaik Yeong Cheah
Berkman Klein Center, Harvard University, Bogotá, Colombia
Armando Guio Español
Department of Radiology and Informatics, Emory University School of Medicine, Atlanta, GA, USA
Judy Wawira Gichoya
Health Ethics & Governance Unit, Research for Health Department, Science Division, World Health Organization, Geneva, Switzerland
Adrienne Hunt & Katherine Littler
African Center of Excellence in Bioinformatics and Data Intensive Science, Infectious Diseases Institute, Makerere University, Kampala, Uganda
Daudi Jjingo
ISI Foundation, Turin, Italy
Daniela Paolotti
Department of Health Sciences and Technology, ETH Zurich, Zürich, Switzerland
Effy Vayena
Joint Centre for Bioethics, Dalla Lana School of Public Health, University of Toronto, Toronto, Canada
You can also search for this author in PubMed Google Scholar
Contributions
JS led the writing, contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper. JA contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper. CA contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper. PYC contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper. AE contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper. JWG contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper. AH contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper. DJ contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper. KL contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper. DP contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper. EV contributed to conceptualization and analysis, critically reviewed and provided feedback on drafts of this paper, and provided final approval of the paper.
Corresponding author
Correspondence to James Shaw .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Shaw, J., Ali, J., Atuire, C.A. et al. Research ethics and artificial intelligence for global health: perspectives from the global forum on bioethics in research. BMC Med Ethics 25 , 46 (2024). https://doi.org/10.1186/s12910-024-01044-w
Download citation
Received : 31 October 2023
Accepted : 01 April 2024
Published : 18 April 2024
DOI : https://doi.org/10.1186/s12910-024-01044-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Artificial intelligence
- Machine learning
- Research ethics
- Global health
BMC Medical Ethics
ISSN: 1472-6939
- General enquiries: [email protected]
News and updates
Keep up to date with our latest news, articles and events.
Read the latest news from the GPhC
Click the news articles below to read them in full.
Contact centre closure - Thursday 2 May 2024
Our contact centre is closed all day on Thursday 2 May for an all-staff training event. We will be monitoring [email protected] for urgent enquiries. The contact centre will reopen on Friday 3 May at 9am .
GPhC response to Healthwatch England report
In response to the Healthwatch England report, ‘Pharmacy: what people want’ , Gisela Abbam, Chair of the General Pharmaceutical Council, said: “This important new research report shines a light on people’s views and experiences of pharmacy services...
GPhC Chief Executive examines meaning of good clinical governance at Pharmacy Business Conference
GPhC Chief Executive, Duncan Rudkin, told the Pharmacy Business Conference on Sunday that good clinical governance is key to pharmacies providing safe, effective, and good quality services to patients and the public. It comes after the GPhC conducted...
GPhC launches consultation on the quality assurance of pharmacy education and training
The General Pharmaceutical Council is seeking views on changing its approach to the quality assurance of pharmacy education and training . Pharmacy education and training providers have to meet GPhC standards through a quality assurance process. This...

Catch up on the latest guidance and important information in our e-bulletin.
See the latest Regulate editions
This is our BETA website where we're testing a new design and layout.
We need your feedback! Click here to send us your views
Main Navigation
- Contact NeurIPS
- Code of Ethics
- Code of Conduct
- Create Profile
- Journal To Conference Track
- Diversity & Inclusion
- Proceedings
- Future Meetings
- Exhibitor Information
- Privacy Policy
NeurIPS 2024
Conference Dates: (In person) 9 December - 15 December, 2024
Homepage: https://neurips.cc/Conferences/2024/
Call For Papers
Author notification: Sep 25, 2024
Camera-ready, poster, and video submission: Oct 30, 2024 AOE
Submit at: https://openreview.net/group?id=NeurIPS.cc/2024/Conference
The site will start accepting submissions on Apr 22, 2024
Subscribe to these and other dates on the 2024 dates page .
The Thirty-Eighth Annual Conference on Neural Information Processing Systems (NeurIPS 2024) is an interdisciplinary conference that brings together researchers in machine learning, neuroscience, statistics, optimization, computer vision, natural language processing, life sciences, natural sciences, social sciences, and other adjacent fields. We invite submissions presenting new and original research on topics including but not limited to the following:
- Applications (e.g., vision, language, speech and audio, Creative AI)
- Deep learning (e.g., architectures, generative models, optimization for deep networks, foundation models, LLMs)
- Evaluation (e.g., methodology, meta studies, replicability and validity, human-in-the-loop)
- General machine learning (supervised, unsupervised, online, active, etc.)
- Infrastructure (e.g., libraries, improved implementation and scalability, distributed solutions)
- Machine learning for sciences (e.g. climate, health, life sciences, physics, social sciences)
- Neuroscience and cognitive science (e.g., neural coding, brain-computer interfaces)
- Optimization (e.g., convex and non-convex, stochastic, robust)
- Probabilistic methods (e.g., variational inference, causal inference, Gaussian processes)
- Reinforcement learning (e.g., decision and control, planning, hierarchical RL, robotics)
- Social and economic aspects of machine learning (e.g., fairness, interpretability, human-AI interaction, privacy, safety, strategic behavior)
- Theory (e.g., control theory, learning theory, algorithmic game theory)
Machine learning is a rapidly evolving field, and so we welcome interdisciplinary submissions that do not fit neatly into existing categories.
Authors are asked to confirm that their submissions accord with the NeurIPS code of conduct .
Formatting instructions: All submissions must be in PDF format, and in a single PDF file include, in this order:
- The submitted paper
- Technical appendices that support the paper with additional proofs, derivations, or results
- The NeurIPS paper checklist
Other supplementary materials such as data and code can be uploaded as a ZIP file
The main text of a submitted paper is limited to nine content pages , including all figures and tables. Additional pages containing references don’t count as content pages. If your submission is accepted, you will be allowed an additional content page for the camera-ready version.
The main text and references may be followed by technical appendices, for which there is no page limit.
The maximum file size for a full submission, which includes technical appendices, is 50MB.
Authors are encouraged to submit a separate ZIP file that contains further supplementary material like data or source code, when applicable.
You must format your submission using the NeurIPS 2024 LaTeX style file which includes a “preprint” option for non-anonymous preprints posted online. Submissions that violate the NeurIPS style (e.g., by decreasing margins or font sizes) or page limits may be rejected without further review. Papers may be rejected without consideration of their merits if they fail to meet the submission requirements, as described in this document.
Paper checklist: In order to improve the rigor and transparency of research submitted to and published at NeurIPS, authors are required to complete a paper checklist . The paper checklist is intended to help authors reflect on a wide variety of issues relating to responsible machine learning research, including reproducibility, transparency, research ethics, and societal impact. The checklist forms part of the paper submission, but does not count towards the page limit.
Supplementary material: While all technical appendices should be included as part of the main paper submission PDF, authors may submit up to 100MB of supplementary material, such as data, or source code in a ZIP format. Supplementary material should be material created by the authors that directly supports the submission content. Like submissions, supplementary material must be anonymized. Looking at supplementary material is at the discretion of the reviewers.
We encourage authors to upload their code and data as part of their supplementary material in order to help reviewers assess the quality of the work. Check the policy as well as code submission guidelines and templates for further details.
Use of Large Language Models (LLMs): We welcome authors to use any tool that is suitable for preparing high-quality papers and research. However, we ask authors to keep in mind two important criteria. First, we expect papers to fully describe their methodology, and any tool that is important to that methodology, including the use of LLMs, should be described also. For example, authors should mention tools (including LLMs) that were used for data processing or filtering, visualization, facilitating or running experiments, and proving theorems. It may also be advisable to describe the use of LLMs in implementing the method (if this corresponds to an important, original, or non-standard component of the approach). Second, authors are responsible for the entire content of the paper, including all text and figures, so while authors are welcome to use any tool they wish for writing the paper, they must ensure that all text is correct and original.
Double-blind reviewing: All submissions must be anonymized and may not contain any identifying information that may violate the double-blind reviewing policy. This policy applies to any supplementary or linked material as well, including code. If you are including links to any external material, it is your responsibility to guarantee anonymous browsing. Please do not include acknowledgements at submission time. If you need to cite one of your own papers, you should do so with adequate anonymization to preserve double-blind reviewing. For instance, write “In the previous work of Smith et al. [1]…” rather than “In our previous work [1]...”). If you need to cite one of your own papers that is in submission to NeurIPS and not available as a non-anonymous preprint, then include a copy of the cited anonymized submission in the supplementary material and write “Anonymous et al. [1] concurrently show...”). Any papers found to be violating this policy will be rejected.
OpenReview: We are using OpenReview to manage submissions. The reviews and author responses will not be public initially (but may be made public later, see below). As in previous years, submissions under review will be visible only to their assigned program committee. We will not be soliciting comments from the general public during the reviewing process. Anyone who plans to submit a paper as an author or a co-author will need to create (or update) their OpenReview profile by the full paper submission deadline. Your OpenReview profile can be edited by logging in and clicking on your name in https://openreview.net/ . This takes you to a URL "https://openreview.net/profile?id=~[Firstname]_[Lastname][n]" where the last part is your profile name, e.g., ~Wei_Zhang1. The OpenReview profiles must be up to date, with all publications by the authors, and their current affiliations. The easiest way to import publications is through DBLP but it is not required, see FAQ . Submissions without updated OpenReview profiles will be desk rejected. The information entered in the profile is critical for ensuring that conflicts of interest and reviewer matching are handled properly. Because of the rapid growth of NeurIPS, we request that all authors help with reviewing papers, if asked to do so. We need everyone’s help in maintaining the high scientific quality of NeurIPS.
Please be aware that OpenReview has a moderation policy for newly created profiles: New profiles created without an institutional email will go through a moderation process that can take up to two weeks. New profiles created with an institutional email will be activated automatically.
Venue home page: https://openreview.net/group?id=NeurIPS.cc/2024/Conference
If you have any questions, please refer to the FAQ: https://openreview.net/faq
Ethics review: Reviewers and ACs may flag submissions for ethics review . Flagged submissions will be sent to an ethics review committee for comments. Comments from ethics reviewers will be considered by the primary reviewers and AC as part of their deliberation. They will also be visible to authors, who will have an opportunity to respond. Ethics reviewers do not have the authority to reject papers, but in extreme cases papers may be rejected by the program chairs on ethical grounds, regardless of scientific quality or contribution.
Preprints: The existence of non-anonymous preprints (on arXiv or other online repositories, personal websites, social media) will not result in rejection. If you choose to use the NeurIPS style for the preprint version, you must use the “preprint” option rather than the “final” option. Reviewers will be instructed not to actively look for such preprints, but encountering them will not constitute a conflict of interest. Authors may submit anonymized work to NeurIPS that is already available as a preprint (e.g., on arXiv) without citing it. Note that public versions of the submission should not say "Under review at NeurIPS" or similar.
Dual submissions: Submissions that are substantially similar to papers that the authors have previously published or submitted in parallel to other peer-reviewed venues with proceedings or journals may not be submitted to NeurIPS. Papers previously presented at workshops are permitted, so long as they did not appear in a conference proceedings (e.g., CVPRW proceedings), a journal or a book. NeurIPS coordinates with other conferences to identify dual submissions. The NeurIPS policy on dual submissions applies for the entire duration of the reviewing process. Slicing contributions too thinly is discouraged. The reviewing process will treat any other submission by an overlapping set of authors as prior work. If publishing one would render the other too incremental, both may be rejected.
Anti-collusion: NeurIPS does not tolerate any collusion whereby authors secretly cooperate with reviewers, ACs or SACs to obtain favorable reviews.
Author responses: Authors will have one week to view and respond to initial reviews. Author responses may not contain any identifying information that may violate the double-blind reviewing policy. Authors may not submit revisions of their paper or supplemental material, but may post their responses as a discussion in OpenReview. This is to reduce the burden on authors to have to revise their paper in a rush during the short rebuttal period.
After the initial response period, authors will be able to respond to any further reviewer/AC questions and comments by posting on the submission’s forum page. The program chairs reserve the right to solicit additional reviews after the initial author response period. These reviews will become visible to the authors as they are added to OpenReview, and authors will have a chance to respond to them.
After the notification deadline, accepted and opted-in rejected papers will be made public and open for non-anonymous public commenting. Their anonymous reviews, meta-reviews, author responses and reviewer responses will also be made public. Authors of rejected papers will have two weeks after the notification deadline to opt in to make their deanonymized rejected papers public in OpenReview. These papers are not counted as NeurIPS publications and will be shown as rejected in OpenReview.
Publication of accepted submissions: Reviews, meta-reviews, and any discussion with the authors will be made public for accepted papers (but reviewer, area chair, and senior area chair identities will remain anonymous). Camera-ready papers will be due in advance of the conference. All camera-ready papers must include a funding disclosure . We strongly encourage accompanying code and data to be submitted with accepted papers when appropriate, as per the code submission policy . Authors will be allowed to make minor changes for a short period of time after the conference.
Contemporaneous Work: For the purpose of the reviewing process, papers that appeared online within two months of a submission will generally be considered "contemporaneous" in the sense that the submission will not be rejected on the basis of the comparison to contemporaneous work. Authors are still expected to cite and discuss contemporaneous work and perform empirical comparisons to the degree feasible. Any paper that influenced the submission is considered prior work and must be cited and discussed as such. Submissions that are very similar to contemporaneous work will undergo additional scrutiny to prevent cases of plagiarism and missing credit to prior work.
Plagiarism is prohibited by the NeurIPS Code of Conduct .
Other Tracks: Similarly to earlier years, we will host multiple tracks, such as datasets, competitions, tutorials as well as workshops, in addition to the main track for which this call for papers is intended. See the conference homepage for updates and calls for participation in these tracks.
Experiments: As in past years, the program chairs will be measuring the quality and effectiveness of the review process via randomized controlled experiments. All experiments are independently reviewed and approved by an Institutional Review Board (IRB).
Financial Aid: Each paper may designate up to one (1) NeurIPS.cc account email address of a corresponding student author who confirms that they would need the support to attend the conference, and agrees to volunteer if they get selected. To be considered for Financial the student will also need to fill out the Financial Aid application when it becomes available.
Cookie Settings
On the Navigraph website we use cookies and similar methods to improve user experience, analyze website traffic, measure ad campaign performance, and target ads.
By clicking “Accept all cookies” you consent to the use of these methods by Navigraph and third parties. You can always change your cookie settings later.
Manage your cookies
Some cookies are essential to use our services. To continue to the website, review the available cookie controls and make any optional selections you’d like before accepting below.
One of the ways we use cookies is to show you useful and relevant ads on and off social media platforms. You can read more about the ways we use cookies in our Privacy Policy
What cookie settings can I control?
Accept cookies that come from our third party partners, which are used to optimize services including personalizing content (including ads), measuring ads, producing analytics and providing a safer experience?
If you don’t want cookies at all
Without cookies you will not be able to log in to your account, manage or buy subscriptions or access any data or content that is not plain text or images.
Other ways you can control your information
Follow the instructions provided by your browser (usually located within the "Help", "Tools" or "Edit" facility) to modify your cookie settings. Please note that if you set your browser to disable cookies or other technologies, you may not be able to access certain parts of our Service and other parts of our Service may not work properly.
To learn more about the choices that advertisers provide generally for individuals to influence how information about their online activities over time and across third-party Web sites or online services is collected and used, visit the Network Advertising Initiative at http://www.networkadvertising.org/managing/opt_out.asp , the Digital Advertising Alliance at http://www.aboutads.info , or the European Digital Advertising Alliance at http://youronlinechoices.eu
In addition, your browser or device may offer settings that allow you to choose whether browser cookies are set and to delete them. These controls vary by browser, and manufacturers may change both the settings they make available and how they work at any time.
- Google Chrome
- Internet Explorer
- Safari Mobile
Start typing to search
Navigate with ease using Navigraph airport charts: high-resolution Jeppesen charts, real-time weather updates, SID/STAR procedures, and airport terrain mapping for 7000+ airports globally.
URKK Charts Pashkovskiy
Navigraph Charts is powered by data from Jeppesen, and contains the largest database of aeronatical charts for flight simulation use. Read more on our Charts products page!
URKK Airport Charts

URKK Departure Charts

URKK Arrival Charts

URKK Approach Charts

URKK Reference Charts


IMAGES
VIDEO
COMMENTS
Various classifications for research designs and methods used in pharmacy practice have been used in the literature. The following are some of the approaches for the classification of research designs: 1. Classification based on time orientation: Retrospective vs. prospective designs. a.
We have some exciting research coming up in 2021, but in case you missed them the first time around, here are the top five most popular research articles of 2020: 5. Misuse of prescription and over-the-counter drugs to obtain illicit highs: how pharmacists can prevent abuse. Use of prescription and over-the-counter drugs for recreational ...
Anticancerous Drugs (B Pharm Project Report) November 2006. Thesis for: B Pharm. Advisor: Dr. Govind Pandey. Authors: Shipra Soni. Govind Pandey. SELF-RELIANT POSITON and Formerly at Nanaji ...
Journal of Pharmacy Practice. The Journal of Pharmacy Practice (JPP) is a peer-reviewed journal that was established in 1988 and is published 6 times per year. The journal is read and cited both nationally and … | View full journal description. This journal is a member of the Committee on Publication Ethics (COPE).
The Bachelor of Pharmacy (B.Pharm) ... project on a chosen and agreed-upon topic of the research problem and received a minimum of "C" grade in their research paper. 17 In all pharmacy schools, advisors are assigned to supervise undergraduate research projects. The advisors are expected to help the students with problem identification all ...
The Journal of Pharmacy Practice and Research (JPPR) publishes high-quality evidence to promote excellence in medicines management for better health outcomes through cutting-edge practice and research. JPPR is the official journal of the Society of Hospital Pharmacists of Australia (SHPA) and offers the option to publish open access. The journal's scope includes evaluations of current ...
Sharad V. Kuberkar. September 2013 View PDF. More opportunities to publish your research: Browse open Calls for Papers. Read the latest articles of Journal of Pharmacy Research at ScienceDirect.com, Elsevier's leading platform of peer-reviewed scholarly literature.
An IUPHAR-affiliated journal, International Union of Basic and Clinical Pharmacology. Pharmacological Research publishes cutting-edge articles in biomedical sciences to cover a broad range of topics that move the pharmacological field forward. We provide a venue through …. View full aims & scope.
The book is aimed at B. Pharm, Pharm D, Pharm D (PB), M. Pharm, allied course students, researchers at the academic and industry levels,Ph. ... Emphasized the various parts of a research paper ...
Following special issues within this section are currently open for submissions: Pharmaceutical Care Services in Pharmacy Practice II (Deadline: 1 May 2024) Medication Use and Patient Safety in Clinical Pharmacy (Deadline: 15 June 2024) Optimizing Hormonal Contraception: The Pharmacist's Guide to Patient-Centred Care (Deadline: 31 July 2024)
Hot Topics in Pharmaceutical Research. In this virtual issue, we highlight some of the most impactful recent articles in the journal as reflected by citations in 2022. Highly cited articles provide insight into which research topics are attracting the most attention and reflect innovative new discoveries, or timely reviews and perspectives on ...
Pharmaceutical Research is an official journal of the American Association of Pharmaceutical Scientists, covering innovative research in drug discovery, development, evaluation, and regulatory approval.. Current emphasis of the journal includes: preformulation; drug delivery and targeting; formulation design, engineering, and processing; pharmacokinetics, pharmacodynamics, and pharmacogenomics ...
When to use a survey in pharmacy practice research. The Pharmaceutical Journal 296(7886). doi: 10.1211/PJ.2016.20200700. Perneger PV & Hudelson PM. Writing a research article: advice to beginners. Int J Qual Health Care 2004;16(3):191-192. doi: 10.1093/intqhc/mzh053. World Association of Medical Editors. Professionalism Code of Conduct. 2016.
Drug development and formulation. | Explore the latest full-text research PDFs, articles, conference papers, preprints and more on PHARMACEUTICS. Find methods information, sources, references or ...
Objective The study quantitatively investigated the related research progress in pharmaceutical sciences/pharmacy education from a bibliometric angle and provided feasible suggestions to facilitate the development of pharmaceutical sciences/pharmacy postgraduate education. Methods Bibliometric analysis was conducted using the database of Web of Science Core Collection. The literature published ...
The coronavirus disease 2019 pandemic has prompted the exploration of new response strategies for such health contingencies in the near future. Over the last 15 years, several pharmacy-based immunization (PBI) strategies have emerged seeking to exploit the potential of pharmacies as immunization, medication sale, and rapid test centers.
Paper • The following article is Open access. Building IT-based Pharmacy: Computerized Pharmacy Management. B Kurniawan 1 and M Ikhsan 1. Published under licence by IOP Publishing Ltd ... This research used the descriptive method to analyze how pharmacies Cibadak Farma handled the drug supplied management activities, sales transactions ...
Search 218,118,443 papers from all fields of science. ... This research makes a case to consider actions to shift the monitoring focus from community pharmacists to community pharmacies for managing patient safety, and demonstrated individual and systemic factors predicting the well-being and turnover intention of community pharmacy ...
B PHARMA VIII SEM BIOSTATISTICS AND RESEARCH METHODOLOGY QUESTION BANK LONG ESSAYS 1. Explain the measures of central tendency Calculate the mean and standard deviation for the following data on systolic BP of volunteers - Systolic BP(mmHg) 91-100 101-110 111-120 121-130 131-140 141-150 Frequency 08 14 20 26 24 18 2.
The ethical governance of Artificial Intelligence (AI) in health care and public health continues to be an urgent issue for attention in policy, research, and practice. In this paper we report on central themes related to challenges and strategies for promoting ethics in research involving AI in global health, arising from the Global Forum on Bioethics in Research (GFBR), held in Cape Town ...
In response to the Healthwatch England report, 'Pharmacy: what people want' , Gisela Abbam, Chair of the General Pharmaceutical Council, said: "This important new research report shines a light on people's views and experiences of pharmacy services...
Call For Papers Abstract submission deadline: May 15, 2024 01:00 PM PDT or Full paper submission deadline, including technical appendices and supplemental material (all authors must have an OpenReview profile when submitting): May 22, 2024 01:00 PM PDT or Author notification: Sep 25, 2024
S G Kanorskiĭ's 59 research works with 80 citations and 983 reads, including: Reversion and remission are promising targets for patients with type 2 diabetes mellitus
Accept cookies that come from our third party partners, which are used to optimize services including personalizing content (including ads), measuring ads, producing analytics and providing a safer experience?
PDF | B. Pharmacy Project Report (Pharmacology), Shri Ram Institute of Technology Pharmacy, Jabalpur, RGPV, Bhopal, MP, India | Find, read and cite all the research you need on ResearchGate
N. P. Berezina's 78 research works with 1,553 citations and 3,933 reads, including: Conductometric and computational study of cationic polymer membranes in H+ and Na+-forms at various hydration levels
I. M. Bykov's 45 research works with 135 citations and 1,255 reads, including: The effect of ethyl alcohol solution with a reduced content of deuterium on the development of chronic alcohol ...