A Comprehensive Guide to Therapeutic Areas in Clinical Research
Clinical research plays a crucial role in advancing medical knowledge and improving patient outcomes. The field encompasses a wide range of therapeutic areas, each with its own unique challenges and opportunities. In this comprehensive guide, we will explore different therapeutic areas in clinical research and shed light on the latest advancements and breakthroughs.

Understanding Different Therapeutic Areas in Clinical Research
When it comes to clinical research, it's essential to have a deep understanding of the various therapeutic areas. This knowledge helps researchers design robust and effective studies that can address the specific needs of each medical specialty.
Therapeutic areas in clinical research encompass a wide range of medical specialties, each focusing on a specific aspect of human health. From oncology and hematology to dermatology, immunology, neurology, gastroenterology, cardiology, ophthalmology, rare diseases, endocrinology, gene therapy, medical devices, and digital therapeutics, researchers are constantly exploring new frontiers to improve patient outcomes and advance medical knowledge.
Exploring the World of Oncology and Hematology Research
Oncology and hematology research focus on understanding and treating cancer and blood disorders, respectively. With advancements in genomics and targeted therapies, these fields have witnessed remarkable progress in recent years. Researchers are constantly striving to develop more effective treatments and improve the quality of life for patients battling these diseases.
In oncology research, scientists investigate the underlying mechanisms of cancer development, progression, and metastasis. They explore different types of cancer, such as breast, lung, prostate, and colorectal cancer, to gain insights into their unique characteristics and develop tailored treatment approaches. Hematology research, on the other hand, delves into blood disorders like leukemia, lymphoma, and anemia, aiming to understand the causes and develop innovative therapies.
Unveiling the Latest Advancements in Dermatology Research
Dermatology research aims to enhance our understanding of skin conditions and develop innovative therapies to treat them. From studying the mechanisms behind various dermatological disorders to conducting clinical trials for breakthrough treatments, researchers in this field are making significant strides towards improving skin health.
Researchers in dermatology explore conditions such as acne, psoriasis, eczema, and skin cancer. They investigate the role of genetics, environmental factors, and immune responses in the development of these conditions. By uncovering the underlying causes, researchers can develop targeted treatments that address the specific needs of patients with different skin conditions.
The Role of Immunology in Clinical Trials
Immunology plays a crucial role in clinical trials by investigating the complex interactions between the immune system and diseases. With the rise of immunotherapies and personalized medicine, researchers are harnessing the power of the immune system to target and eradicate diseases like never before.
Immunology research focuses on understanding how the immune system functions, how it responds to pathogens and cancer cells, and how it can be modulated to enhance therapeutic outcomes. Scientists study immune cells, antibodies, cytokines, and other components of the immune system to develop novel immunotherapies that can effectively treat conditions such as autoimmune diseases, infectious diseases, and cancer.
Navigating the Complexities of Neurology and Central Nervous System Research
Neurology and central nervous system research delve into the intricate workings of the brain and the nervous system. From studying the causes and mechanisms of neurological disorders to testing potential treatments, researchers in this field are dedicated to improving the lives of patients with conditions such as Alzheimer's, Parkinson's, and multiple sclerosis.
Neurological research encompasses a wide range of conditions, including neurodegenerative diseases, movement disorders, epilepsy, and stroke. Scientists investigate the underlying mechanisms of these disorders, explore potential biomarkers for early diagnosis, and develop innovative therapies to slow down or halt disease progression. They also focus on neurorehabilitation techniques to improve the quality of life for individuals living with neurological conditions.
Investigating Breakthroughs in Gastroenterology Research
Gastroenterology research aims to advance our understanding of the digestive system and develop new treatments for conditions such as inflammatory bowel disease, gastroesophageal reflux disease, and liver diseases. With emerging technologies and innovative therapies, researchers are pushing the boundaries of what is possible in gastrointestinal care.
Gastrointestinal research covers a broad spectrum of diseases and disorders affecting the digestive tract, including the esophagus, stomach, intestines, liver, and pancreas. Scientists investigate the causes of conditions like Crohn's disease, ulcerative colitis, and liver cirrhosis, aiming to develop targeted therapies that can alleviate symptoms and improve long-term outcomes for patients.
Advancing Cardiology Research for Better Heart Health
Cardiology research focuses on preventing, diagnosing, and treating cardiovascular diseases, which remain one of the leading causes of death worldwide. Through clinical trials and groundbreaking studies, researchers are constantly striving to develop more effective interventions and therapies to improve heart health.
Cardiovascular research encompasses a wide range of conditions, including coronary artery disease, heart failure, arrhythmias, and congenital heart defects. Scientists investigate the risk factors, genetic predispositions, and lifestyle interventions that can prevent the development of cardiovascular diseases. They also explore innovative treatments such as minimally invasive procedures, drug therapies, and regenerative medicine approaches to repair damaged heart tissue.
Shedding Light on Ophthalmology Research and Innovations
Ophthalmology research aims to improve our understanding of eye diseases and develop innovative treatments to preserve vision. From investigating the causes of conditions like cataracts and glaucoma to exploring the potential of gene therapies and regenerative medicine, researchers in this field are at the forefront of eye care innovation.
Researchers in ophthalmology study a wide range of eye conditions, including age-related macular degeneration, diabetic retinopathy, and retinal detachment. They investigate the underlying mechanisms of these diseases, develop diagnostic tools for early detection , and explore novel treatment approaches such as gene therapies, stem cell therapies, and artificial retinas.
Uncovering the Challenges and Progress in Rare Disease Research
Rare disease research focuses on understanding and addressing conditions that affect a small percentage of the population. These diseases often present unique challenges due to their complexity and limited treatment options. Researchers in this field work tirelessly to unravel the mysteries of rare diseases and develop targeted therapies to improve the lives of affected individuals.
There are thousands of rare diseases, each with its own set of challenges and complexities. Researchers investigate the genetic and molecular basis of these diseases, explore potential biomarkers for early diagnosis, and develop innovative therapies tailored to the specific needs of patients. They also collaborate with patient advocacy groups and healthcare professionals to raise awareness and improve access to care for individuals with rare diseases.
Delving into the Realm of Endocrinology Research
Endocrinology research explores the hormonal imbalances that contribute to conditions such as diabetes, thyroid disorders, and hormonal cancers. Through clinical trials and innovative treatments, researchers are committed to improving our understanding of endocrine disorders and developing personalized approaches to management.
Endocrine disorders encompass a wide range of conditions, including diabetes mellitus, hypothyroidism, hyperthyroidism, and adrenal disorders. Researchers investigate the underlying causes of these disorders, explore the role of genetics and environmental factors, and develop novel therapies such as hormone replacement therapies, targeted drug therapies, and lifestyle interventions.
Gene Therapy: Revolutionizing the Future of Medicine
Gene therapy has the potential to revolutionize the treatment of various diseases by targeting the underlying genetic causes. From inherited disorders to certain types of cancer, researchers are harnessing the power of gene editing and delivery techniques to develop transformative therapies that could change the landscape of medicine.
Gene therapy research focuses on understanding the genetic basis of diseases, identifying specific gene mutations or dysregulations, and developing techniques to modify or replace faulty genes. Scientists explore various delivery methods, such as viral vectors and nanoparticles, to safely and effectively deliver therapeutic genes to the target cells or tissues. Gene therapy holds promise for conditions like cystic fibrosis, muscular dystrophy, and certain types of cancer.
Exploring the Intersection of Medical Devices and Clinical Trials
Medical devices play a crucial role in healthcare, and their impact is not limited to diagnosis and treatment. Clinical trials involving medical devices are essential to ensure safety, efficacy, and usability. Researchers in this field focus on developing and testing innovative devices that can improve patient outcomes and enhance the delivery of healthcare services.
Medical device research encompasses a wide range of technologies, including implantable devices, diagnostic tools, and therapeutic equipment. Scientists work on improving the design and functionality of devices, conducting preclinical and clinical trials to assess their performance, and collaborating with healthcare professionals to integrate devices into patient care pathways. The development of advanced medical devices has the potential to revolutionize healthcare delivery and improve patient outcomes.
Harnessing the Power of Digital Therapeutics in Clinical Research
Digital therapeutics leverage technology to deliver evidence-based interventions for the prevention, management, and treatment of various conditions. From smartphone apps to virtual reality-based therapies, researchers are exploring the potential of digital therapeutics to empower patients and revolutionize healthcare delivery.
Researchers in digital therapeutics investigate the effectiveness of digital interventions in improving patient outcomes, enhancing medication adherence, and promoting behavior change. They develop and test smartphone applications, wearable devices, and virtual reality platforms that can deliver personalized interventions, track patient progress, and provide real-time feedback. Digital therapeutics have the potential to increase access to healthcare, reduce healthcare costs, and empower patients to take control of their health.
The Global Landscape of Clinical Trials
As the field of clinical research continues to expand, it's essential to understand the global landscape of clinical trials. These trials play a critical role in bringing new treatments to patients and advancing medical knowledge.
Analyzing the Growth and Trends in the Global Clinical Trials Market
The global clinical trials market is witnessing significant growth, driven by factors such as increasing disease burden, technological advancements, and growing interest from pharmaceutical companies. Analyzing the trends and dynamics of this market provides valuable insights into the future of clinical research and the potential impact on patient care.
A Closer Look at Clinical Trials Across Different Therapeutic Areas
Clinical trials span across various therapeutic areas, with each specialty presenting its unique challenges and considerations. Understanding the nuances of conducting clinical trials within different therapeutic areas is crucial for researchers and stakeholders involved in these studies. It helps ensure that trials are designed and executed effectively, leading to reliable and meaningful results.
In conclusion, this comprehensive guide has provided an overview of the therapeutic areas in clinical research. From oncology and hematology to dermatology, immunology, and beyond, each field presents exciting opportunities for researchers to make a significant impact on patients' lives. By continually pushing boundaries, embracing innovation, and collaborating across disciplines, the clinical research community can drive advancements that transform healthcare and improve outcomes for patients worldwide.
If you're inspired by the potential of clinical research across diverse therapeutic areas and are seeking a partner to navigate the complexities of clinical trials, look no further than Lindus Health. Our comprehensive suite of services provides an all-in-one solution for your clinical trial needs, from protocol writing to data delivery, including site services and an innovative eClinical platform. To discover how Lindus Health can support your clinical research journey and drive your study towards success, book a meeting with our team today.

Download now
Related stories, speak with an expert about your study..

What can Lindus Health do for your study?
Quick links, your trial, solved..
THERAPEUTIC AREAS OF PRECLINICAL AND CLINICAL RESEARCH
Our expertise in many therapeutic areas of preclinical and clinical research ensures that we move your molecule forward, whether through our preclinical, clinical, or bioanalytical service offerings. As a full development program or a stand-alone service, we apply our expertise in therapeutic areas to everything we do. We will help seamlessly transition your molecule through the phases of development in any of our therapeutic specialties, helping you get your drug to market faster.
Share this page
Our experience in general toxicology, safety pharmacology, and clinical pharmacology spans a wide range of therapeutic specialties, both small and large molecules. Across the therapeutic areas of both preclinical and clinical research, we analyze numerous biomarkers, and are always adding new validated methods to our panels that cover preclinical and clinical matrices. Finally, we have extensive experience in designing and conducting proof-of-concept studies in healthy participants or patients.

Move your cardiology compound from preclinical to early clinical efficiently, with a CRO that has worked on 30 different cardiology compounds across more than 70 studies. We can quickly de-risk your molecule with preclinical cardiovascular studies and with early cardiac safety assessment in early clinical studies.
Our preclinical and clinical experience spans a wide range of cardiology indications, including arrhythmias, hypertension, dyslipidemias, and congestive heart failure. Preclinical programs can be conducted across different species with robust safety pharmacology cardiovascular studies. Our preclinical facility has a specially designed room for ECG monitoring by telemetry. Our clinics are equipped with telemetry and staffed by a trained cardiac team.
CLINICAL STUDIES
DIFFERENT COMPOUNDS STUDIED

Dermatology
Working with a dermatology expert from preclinical to proof of concept can help to speed up your development program. As one of our therapeutic specialties, Altasciences conducts specific safety assessments for locally acting dermatology therapies and proof of concept in locally or centrally acting therapies, including both small and large molecules.
We assess toxicology of topical therapies, including edema/erythema measurement and Draize scoring, using mini pigs or NHPs. In the clinic, we collaborate closely with our consulting dermatologists who perform specialized assessments, such as biopsies or scoring lesion severity. If your locally acting product requires irritation and sensitization studies, our dedicated clinic areas can offer different climates, and can enroll up to 200 participants in one day.
Consult our Topical Clinical Trials Fact Sheet and our Nonclinical Dermal Safety Assessment Fact Sheet .

Gastroenterology
We offer extensive preclinical and clinical expertise in the gastrointestinal therapeutic area, with over 100 Phase I/II PK or PD studies involving drugs acting systemically or locally in the GI tract. This includes general toxicology studies in a number of different species, as well as examining preclinical colitis and proctitis models. Our clinical pharmacology capabilities cover all study types, supported by a gastroenterologist Principal Investigator with experience in over 1,000 clinical studies.
Safety or efficacy monitoring is done via endoscopy, colonoscopy, intragastric pH monitoring, and X-ray imaging with barium. Additionally, we have experience with barostat model of colon pain and with PK sampling from ileostomy pouch.
Case Studies
- A Study to Evaluate the Effect of SYN-004 on the PK of IV Ceftriaxone in Adults With a Functioning Ileostomy
- An Interaction Study Evaluating the Effect of Esomeprazole on SYN-004 Degradation of Ceftriaxone In Adults With an Ileostomy
- A Study to Evaluate Safety, Tolerability and Pharmacokinetics of trimebutine 3-Thiocarbamoylbenzenesulfonate (GIC-1001) in a Randomized Phase I Integrated Design Study: Single and Multiple Ascending Doses and Effect of Food in Healthy Volunteers
- A Phase Ib Clinical Study on the Analgesic Effect of NCE on Visceral Pain under Rectal Distension and Rectal Sensory Threshold using the Barostat Method in Male and Female Healthy Volunteers
Consult our Gastroenterology Trials Fact Sheet
PHASE I/II PK/PD GI

Support your early phase hematology program by partnering with a CRO that has relevant nonclinical and clinical expertise, aided by efficacy measures using flow cytometry or coagulation measures, including thromboelastography (TEG) and bioanalytical methods for a number of biomarkers.
Both our preclinical and clinical facilities perform flow cytometry, including when methods require samples to be analyzed within four hours of collection. We investigate coagulation directly or look for changes in different components of the coagulation cascade. If the focus is on the immune system, we have validated methods for a number of steps in the complement pathway.

Support the development of your immunology compound by working with a CRO that has the relevant therapeutic area expertise — and the flexible, collaborative approach — you need. With over 250,000 square feet of analytical laboratories spanning both our preclinical and clinical sites, Altasciences’ experience in analyzing immune endpoints is best positioned to meet your molecule’s development needs. Our biomarker panels, including the complement pathway and flow cytometry assessments, will allow you to seamlessly translate your preclinical findings to the clinical phase. Whether small or large molecules, we have the experience in conducting many types of programs, with scientific and technical expertise in the preclinical setting, and clinics that are set up and staffed with expert teams to ensure the safety in first-in-human studies for immune modulators.

With a comprehensive understanding of FDA regulations and requirements for vaccine approval, and access to a large database of participants, we are uniquely positioned to streamline the clinical development of new vaccines and delivery systems. We have conducted vaccine trials for the following conditions:
- Seasonal influenza
- Pandemic influenza
- Hepatitis B
In-house vaccine development supported by:
- Respiratory panel testing through the multiplex PCR BIOFIRE® FILMARRAY® system to evaluate the presence of an infection in the respiratory tract of immuno-compromised patients
- Ability to isolate peripheral blood mononuclear cells (PBMCs) through multiple collection methods
- Biosafety Level 2 (BSL-2) laboratories with limited badge access, biosafety cabinets, biohazard-trained staff, cleaning protocols, and all required containment procedures
- Long-term sample storage
- Dedicated large molecule bioanalytical laboratory with capabilities and state of the art equipment for flow cytometry , cell-based functional assays, immunogenticity expertise, etc. to support all clinical activities.
Infectious Diseases
Maximize the efficiency of your infectious disease program by leveraging our significant preclinical, clinical, and bioanalytical therapeutic area expertise. We support the streamlined development of new vaccines, anti-viral therapies, delivery systems, and antimicrobial agents with our integrated, multi-disciplinary services. Partner with a leader that has a proven track record in infectious diseases and vaccine development.
Our preclinical site has the advantage of BSL-2 designation because of its NHP population. Our experts in infectious disease have conducted more than 220 clinical studies for acute or chronic infections, performed in healthy participants and patient populations.
Indications include:
- Bacterial infection
- Common cold
- Complicated skin infections
- Fungal infections
- Herpes zoster
We have performed clinical immune monitoring to establish proof-of-concept in diseases such as hepatitis C and influenza. Our experience in healthy participants includes antivirals, antifungals, antibiotics, and antiparasitics.
Infectious Diseases Case Studies
- A Phase Ib Study Evaluating GS-7340 in Treatment-Naive Adults with Chronic Hepatitis B (CHB) Infection
- A Phase Ib Study Evaluating GS-9620 in Virologically Suppressed Subjects with Chronic Hepatitis B Virus Infection
- A Double-Blind, Randomized, Placebo-Controlled, Single and Multiple-Dose Ranging, Adaptive Study Evaluating the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics, and Antiviral Activity of GS 9620 in Treatment-Naive Subjects with Chronic Hepatitis B Virus Infection
- A Single and Multiple Dose Study of MK-3682 (IDX21437) in Healthy and Hepatitis C Virus (HCV)-Infected participants (MK-3682-001)
Consult our Hepatitis Trials Fact Sheet Consult our Infectious Diseases Trials Fact Sheet
STUDIES FOR ACUTE OR CHRONIC INFECTIONS

Metabolism and Endocrinology
Beyond the standard clinical chemistry and lipid panels to assess disorders in this therapeutic specialty, our preclinical services include specialized assays such as A1C, and specialized tests such as glucose tolerance to further evaluate molecules in this area. In the clinic, we have conducted over 75 early stage trials involving antidiabetic and hypoglycemic agents in patients who are diabetic (Type I and II), obese, or have metabolic syndrome or NASH. These include a large number of proof-of-concept studies investigating different measures of glucose tolerance, including clamping studies using state-of-the-art YSI-2900 that can analyze multiple glucose samples per run.
Consult our Dyslipidemia Trials Fact Sheet Consult our Metabolic Disorders Trials Fact Sheet Consult our issue of The Altascientist on Metabolic Disorders
Read the results of a Phase I study conducted by Altasciences combining obesity medications with different mechanisms of actions to provide more efficient treatment options for weight management.
STUDIES INVOLVING ANTIDIABETIC AND HYPOGLYCEMIC AGENTS

Neurology is one of the key therapeutic areas of research conducted at Altasciences. Recognized globally as a CNS Center of Excellence, our scientific team has completed more than 200 preclinical and clinical neurology studies , in addition to providing formulation, manufacturing, and analytical testing services, as well as bioanalytical support. Whether large or small molecule, we can help move your compound through early development seamlessly and efficiently, from prototype formulation through commercialization.
Discover how working with a single partner for your end-to-end CNS drug development program can lead up to 40% in time savings .

Our preclinical oncology expertise can move your compound into patients, and we have clinical expertise with a number of non-cytotoxic oncology compounds. Drawing on a broad range of preclinical experience in oncology, we are accustomed to fast-tracking development so that you can proceed to patient studies as rapidly as possible. Our experience in oncology extends to the clinic, with experience testing nine different tyrosine kinase inhibitors in healthy participants.
DIFFERENT TYROSINE KINASE INHIBITORS STUDIED

Ophthalmology
As a leading partner in ocular therapy, our integrated CRO/CDMO solutions can support your ophthalmic drug development program from lead candidate selection to market. You will benefit from working with a single partner as your compound advances through each phase of drug development—from prototype formulation through preclinical testing, to early phase clinical trials, and manufacturing.
Discover our end-to-end ophthalmic drug development solutions that can lead up to 40% in time savings.

Orthopedics
Altasciences brings your orthopedics and musculoskeletal molecule through preclinical to proof of concept. Our experience with proof-of-concept studies in healthy participants and patient populations, such as those with osteoarthritis, includes models such as the 6-minute walk test or stair challenge, and imaging using X-ray, MRI, CT, or DEXA at our preclinical and clinical sites. Finally, our panels include a number of inflammatory biomarkers.
Consult our Pain Models Fact Sheet

Altasciences has therapeutic specialty expertise in psychiatric research, conducting studies from preclinical to clinical proof of concept in patients. Our preclinical expertise covers the complete range of general toxicology and safety pharmacology studies, and includes 24-hour video monitoring and performing functional observation battery tests to assess behavioral changes.
Our clinical expertise is augmented by two Principal Investigators who are psychiatrists, and our experience covers substance abuse, epilepsy, anxiety disorder, panic disorder, depression, ADHD, and sleep disorders. As your clinical development proceeds, we can conduct the human abuse potential testing and driving simulator studies that will likely be required, two areas where we have extensive expertise.
Consult our Central Nervous System Trials Fact Sheet
DECADES IN PSYCHIATRIC RESEARCH

Respirology
You can count on an efficient development of your compound to treat respiratory/pulmonary disorders, leveraging our preclinical and clinical experience with investigating both systemic and locally acting therapies. Our clinics have units with specialized air handling systems that quickly exchange air to minimize cross-contamination between dosed participants. Our staff consistently trains patients on using different pulmonary devices to ensure consistent dosing. We are adept at pulmonary function testing, and have pulmonologists and respiratory technicians on site to assist in study design or with assessments.
Consult our Pulmonary Trials Fact Sheet

Rheumatology
Altasciences can help support you in the development of your rheumatology compound, delivering the preclinical, clinical, and bioanalytical expertise and experience you need. We have extensive preclinical and clinical experience testing immune modulators backed up by a battery of biomarkers, including components of the complement pathway and cytokines.

Substance Abuse
Altasciences offers leading preclinical and clinical sites for substance use disorders, one of our key therapeutic areas of clinical trials, including expertise across a wide variety of mechanisms of action. Our experience in general toxicology, safety pharmacology, and clinical pharmacology covers a wide range of compounds, including oral, IM, IV, and implanted products. We also analyze a number of biomarkers and efficacy outcomes using visual analogue scales (VAS) and questionnaires.
Our robust database of patients combined with our upscale facilities enable us to deliver rapid enrolment and excellent retention rates. Our sites have controlled substance licenses, including a DEA Schedule I license to conduct research with tetrahydrocannabinol and cannabidiol in Kansas City. Cannabis study start times at our Montreal site have been positively impacted by the legalization of cannabis in Canada.
YEARS COMBINED EXPERIENCE BY OUR PRINCIPAL INVESTIGATORS

Leverage our preclinical and clinical expertise to help you tackle the development needs of your urology compound. Having conducted almost 30 studies, we are skilled in general toxicology, safety pharmacology, and clinical pharmacology of a wide range of compounds. Our experience covers treatments for urinary incontinence, erectile dysfunction, benign prostate hyperplasia, and prostate cancer. We can also analyze a number of different biomarkers. With our extensive patient database and outreach, we can help you establish proof of concept by recruiting patients across a range of urological conditions.
STUDIES COMPLETED

Women’s Health Clinical Trials
With extensive expertise, experience, and a multitude of solutions for sponsors developing therapeutic products and diagnostics in the area of women’s health, Altasciences is ideally positioned to attain your drug development goals.
Altasciences has conducted preclinical and clinical studies with products for breast cancer, oral contraception, fertility treatment, hot flashes, and hypoactive sexual disorder. Our experience includes a wide range of compounds delivered orally, vaginally, or by implant, and we have validated methods for the majority of marketed contraceptives, fertility treatments, and hormone replacement therapies.
Consult our women’s health fact sheet for more information on the specific therapeutic areas we have experience in.
Our clinical pharmacology units are located in densely populated areas, allowing access to a wide variety of ages, ethnicities, lifestyles, and health conditions. We have a diverse participant database of over 100,000 healthy pre- and postmenopausal women who are actively interested in participating in our clinical trials, as well as access to a number of patient populations through our own database and partnerships with local fertility clinics. This enables us to tailor your study and obtain the data you need to move your project forward.
We work closely with a network of local gynecologists for specialized exams and biopsies, and offer imaging service like transvaginal ultrasounds and DEXA.
Some of the study types we offer include:
- Alcohol interaction
- Comparative BA/BE
- Drug-drug interaction
- Driving impairment
- Proof of concept, efficacy
- Transdermal adhesion/HRIPT
- FIH (SAD/MAD)
- Food effect
- Genotyping PK
- Renal impairment
- Safety, PK, tolerability
In addition, Altasciences’ large and small molecule bioanalytical expertise and comprehensive research support services ensure an integrated, efficient, and holistic solution for your women’s health studies.
YEARS AS A LEADING CRO IN WOMEN'S HEALTH
Therapeutic - FAQs
Is your participant database searchable by therapeutic area.
Our database of over 400,000 participants contains comprehensive, detailed individual medical profiles and screening histories, searchable by therapeutic area. We also develop recruitment strategies specific to the therapeutic areas of clinical research required by our sponsors.
DOES ALTASCIENCES HAVE EXPERTISE IN THERAPEUTIC SPECIALTIES WITH BOTH LARGE AND SMALL MOLECULES?
The clinical trials we conduct include both large and small molecules, in therapeutic specialties ranging from CNS to immunology, dermatology to cardiology, and many other therapeutic areas. We also have manufacturing for small molecules and supporting services for all our preclinical and clinical therapeutic specialties.
WHICH THERAPEUTIC AREAS OF CLINICAL RESEARCH REQUIRE PURPOSE-BUILT FACILITIES?
Altasciences is proud of our specialized facilities, including 10 on-site driving simulators for CNS or other studies, negative pressure inhalation facilities for inhaled products, and secured pharmacies and clinical pharmacology units for controlled substance studies. Of course we also have the facilities (400 beds in two North American locations) to conduct clinical trials in a wide range of therapeutic areas not requiring specialized facilities.
DO YOU HAVE ACCESS TO PATIENTS OR SPECIAL POPULATIONS AS NECESSARY FOR SPECIFIC CLINICAL TRIALS BY THERAPEUTIC AREA?
We have relationships with local hospitals and clinics to ensure that we can conduct clinical trials with patients, or in specific population or age groups, as necessary for your projects. We can accommodate long or short stays in our upscale, well-appointed, North-American clinical pharmacology units.
Sign up for exclusive content
Join our list of 10,000+ VIP members and have access to our exclusive content.
Stay connected
2023 © Altasciences. All Rights Reserved.
Cookie Policy | Privacy Policy
I'd be happy to help.
- Definitive Healthcare (View)
- Monocl (ExpertInsight)
- Populi (Claims analytics)
- Carevoyance (Sales accelerator)
Most common clinical trial therapy areas
Share this post.
As of January 2024, there are 20,465 clinical trials recruiting patients in the U.S., according to the National Library of Medicine (NLM) . Worldwide, NLM reported 65,474 trials recruiting subjects.
While clinical trials are FDA-mandated for all new treatments—including drugs , medical devices , vaccines, and gene therapies —these studies also produce crucial data used in making other care decisions. In addition to evaluating the safety and efficacy of new treatment options, clinical trials can help both providers and manufacturers determine which medical approaches work best for certain illnesses or patient populations .
What are the different types of clinical trials?
Clinical studies are classified into two main groups based on the research protocol and whether or not participants receive medical interventions as part of their testing. These categories are defined as:
- Interventional studies (more commonly referred to as clinical trials) Investigators assess participant health outcomes based on specific interventions administered as part of the research protocol in order to test the safety and effectiveness of a candidate drug, therapy, or experimental treatment.
- Observational studies Investigators assess participant health outcomes as established in the research protocol without administering interventions or procedures.
Within this framework, clinical studies can be further segmented by subtype—including prevention, treatment, diagnostic , screening, genetic, epidemiological, or quality-of-life trials.
Definitive Healthcare tracks both interventional and observational trials across 14 different therapy areas . Data is compiled from The Clinical Trials Transformation Initiative (CTTI) and can be viewed in our HospitalView , PhysicianView , and PhysicianGroupView products. In this blog, we’ve compiled a list of the top 10 most common clinical trials by therapeutic area.
Cancer treatments have the highest clinical trial volume
With a projected global market value of $300 billion by 2026 , it’s no surprise that cancer drugs are among the most highly-tested therapies in clinical trials across the nation.
Top 10 clinical trial therapeutic areas
Fig 1 Data from the Definitive Healthcare Atlas Dataset and sourced from The Clinical Trials Transformation Initiative (CTTI) . The total number of clinical trials includes all historically reported. Accessed January 2024.
Treatments for cancer have the highest number of clinical trials by therapeutic area – 15.4% of all trials analyzed. With a projected global market value of $300 billion by 2026, it’s no surprise that cancer drugs are among the most highly-tested therapies in clinical trials across the nation. With an increasing number of new cancer drugs being developed each year, this clinical trial volume can be expected to grow.
Clinical trials for mental health and behavioral disorders represent 6.3% of the trials analyzed. From antidepressants and anti-anxiety medications to emerging psychedelic options , our mental health treatment choices continue to expand.
Next, trials for endocrinology and metabolic disease, including diabetes , and cardiovascular and circulatory diseases each represent more than five percent of the clinical trials on the list. The additional top therapeutic areas by clinical trial volume include:
- Nervous system diseases
- Musculoskeletal diseases
- Digestive diseases
- Infectious diseases
Where do clinical trials take place?
Below, we mapped the number of clinical trials by each state. The analysis includes 36,788 active clinical trials listed on ClinicalTrials.gov, which are those identified as “recruiting,” “not yet recruiting,” “active,” and “not recruiting.”
Clinical trial volume by state
Fig. 2 Data is sourced from the National Library of Medicine at clinicaltrials.gov and include trials with a status of recruiting, not yet recruiting, active, or not recruiting. Accessed January 2024.
Which states have the most clinical trials?
As expected, population density is a significant contributing factor in the geographic distribution of clinical trials across the U.S. Greater patient volumes, after all, increase the likelihood of finding eligible participants for clinical research. For this reason, we see a strong correlation between those states with the largest populations and the highest reported clinical trial volumes—including California , Texas , New York , and Florida .
Though it’s not explicitly represented on the above map, there also appears to be a strong correlation between high clinical trial volumes and facility types—where states with high-performing cancer centers , academic medical centers , or other research facilities report greater volumes of clinical trials than areas with fewer numbers of these facility types.
Massachusetts and Ohio , for instance, each have more than 5,500 active clinical trials despite their sizes. Both states are in the top five for most academic medical centers in the country.
Top hospitals by clinical trial volume
Fig 3 Data from the Definitive Healthcare Atlas Dataset and sourced from The Clinical Trials Transformation Initiative (CTTI) . The total number of clinical trials includes all historically reported. Accessed January 2024.
Clinical studies can be sponsored by a number of different agencies, ranging from industry organizations like pharmaceutical companies to academic medical centers or federal agencies—including the National Institutes of Health, the U.S. Department of Defense, and the U.S. Department of Veterans Affairs.
While clinical studies can be conducted in a variety of different locations, access to specific equipment, physician expertise, or sample patient populations quite often influence this decision. With greater access to these critical resources, places like cancer research facilities and university medical centers are frequently selected as locations for hosting clinical trial research.
In fact, all 10 of the top hospitals by historic clinical trial volume are cancer research centers, university hospitals, or another type of dedicated research facility, according to Definitive Healthcare data.
Learn more
Read our blogs to catch up on the latest clinical trial trends and learn more about recruiting the right patients for your studies. Or find out how the Atlas All-Payor Claims dataset and PhysicianView product can help you target the right physicians for your new therapies . Start a free trial today.
- Most Common Clinical Trial Therapy Areas
Therapeutic Areas
Focus and expertise.
Clinical trials present unique challenges for different therapeutic areas (TAs).
The complexity of trial design, the availability of patients, and the regulatory environment of each therapeutic area vary greatly and impact study success.
Our eSource technology combined with years of expertise in therapeutic areas—including neurology , immunology , infectious disease , oncology , Dermatology, Gastrointestinal, Rare Disease, Respiratory—ensure any TA-focused study achieves successful patient outcomes.

Patient-Centric Research, from the Ground Up
Patient-centric research is the Clinical ink commitment to focus on the needs and perspectives of patients throughout a clinical trial. We ensure research is relevant and meaningful, thus increasing engagement and participation of each patient.
Clinical ink delivers eSource technology solutions that go beyond EDC, integrating Direct Data Capture (DDC) with eCOA and ePRO modules, eConsent , sensors and wearables , and digital biomarkers .
Our therapeutic experience and pioneering patient-centric research approach translate into trials no others can deliver. Learn more about our therapeutic area-specific expertise.
Solutions for Therapeutic Areas
Diabetes trials are intricate due to the complexity of this metabolic condition. Measuring and quantifying symptoms can be challenging.
Discover how Clinical ink Continuous Glucose Monitoring and eSource technology offer a better patient experience and improve trial outcomes for diabetes research.
Encompassing a wide range of conditions from autoimmune disorders to allergies, the mechanisms of action of immunotherapies — and how to target them effectively — are complex.
Leverage Clinical ink technology and expertise to simplify challenging and complex clinical trials.
Infectious Disease
Caused by microorganisms such as bacteria, viruses, fungi, or parasites, infectious diseases range from mild, self-limiting infections to severe, life-threatening illnesses.
Learn how to speed up trial timelines and improve the quality of your data for faster, conclusive infectious disease studies.
Trials for neurological conditions such as Alzheimer’s disease or multiple sclerosis are complex, as symptoms are difficult to measure and quantify.
See how Clinical ink eSource technology, and particularly our digital biomarkers, helps improve results for trials involving central nervous system conditions.
Improve coordination across all of a cancer patient’s health care providers and supports personalized services that help patients navigate and manage their cancer care.
Deliver on the Enhancing Oncology Model and improve patient adherence throughout the trial.
Empower patients to power clinical discovery.
Discover eSource
Get to know us.
Sales and Solutions Direct 1.336.464.0697
Support Toll-Free 1.800.301.5033 Direct 1.336.464.0697
Insert HTML text here.
Therapeutic Areas in Drug Discovery & Clinical Research
COVID-19 Infectious Diseases/Vaccines Immuno-Oncology (IO)/Cancer Pain Auto-Immunity Heart Disease

The Carterra LSA is a real-time label-free biosensor unique from other technologies. The system is purpose built to characterize up to 384 samples with exquisite resolution. The capabilities of the LSA are well suited for long term infectious disease research objectives but with a throughput capability that allows the system to easily meet the limited timelines during rapidly evolving threats such as COVID-19. Additionally, the LSA’s patented microfluidics enable it to characterize epitope and affinity using sample quantities far below other technologies. Speed in conjunction with high resolution is vital towards making informed decisions on hundreds if not thousands of samples during viral pandemics.
- Publication: Jones et al, Science Magazine, The neutralizing antibody, LY-CoV555, protects against SARS-CoV-2 infection in non-human primates Supplementary Materials
- Publication: Schasfoort et al, Research Square 2020, Presence and strength of binding of IgM, IgG and IgA antibodies against SARS-CoV-2 during CoViD-19 infection
- White Paper: Addressing Viral Pandemics Such as COVID-19 Using the Carterra LSA
- Webinar: Antibody Discovery and Development for COVID-19 Diagnostic Testing
- Webinar: Rapid Discovery of Therapeutic Antibodies to Combat COVID-19 at Twist Bioscience
- Application Note: Discovery of Therapeutic Antibody Cocktails
- Press Release: Immunity Monitoring of COVID-19 Patients Using a SARS-CoV-2 Label-free Assay and Biosensor Chip
- Podcast: The Bamlanivimab story – how it reached the clinic in eight months
Infectious Diseases/Vaccines
Therapeutic cocktails comprise a collection of antibodies targeting several distinct epitopes to collectively achieve a broad neutralization of the virulence factor. Defeating an ever-mutating virus poses challenges in designing an effective medicine but targeting multiple epitopes at once can offset biological redundancy – when one pathway fails, another often compensates.
Carterra’s LSA facilitates epitope characterization on hundreds of antibodies in parallel, allowing you to quickly understand the epitope coverage of your antibody library and focus resources on studying the epitope clusters with the most biologically-relevant function. When epitope binning studies are performed on a large panel of antibodies, the competition picture gains resolution and nuance, allowing a deep appreciation of the epitope diversity contained in the antibody library. Developing a potently neutralizing therapeutic antibody cocktail relies on the proper selection of antibodies with complementary epitopes. The Carterra LSA accelerates the epitope characterization of an antibody library, such as one that is recovered by B-cell cloning of an Ebola survivor, to understand the immune response and guide the discovery of therapeutic antibody cocktails.
- Application Note: Multi-Parameter Epitope-Centric Data Used to Elucidate Mechanisms of Action of Antibodies
- Publication: Wec et al, PNAS 2020, Longitudinal dynamics of the human B cell response to the yellow fever 17D vaccine
- Publication: Cairnes et al, J Virol 2019, Surface plasmon resonance (SPR) reveals direct binding of HSV glycoproteins gH/gL to gD and locates a gH/gL binding site on gD.
- Publication: Awasthi et al, Vaccine 2019, Antibody responses to crucial functional epitopes as a novel approach to assess immunogenicity of vaccine adjuvants.
- Publication: Hook et al, PLoS Pathog 2018, Vaccine-induced antibodies to herpes simplex virus glycoprotein D epitopes involved in virus entry and cell-to-cell spread correlate with protection against genital disease in guinea pigs.
- Publication: Yeung et al, Nature Commun 2016, Germline-encoded neutralization of a Staphylococcus aureus virulence factor by the human antibody repertoire
- Publication: Chukwuma et al, PLoS One 2018, Increased breadth of HIV-1 neutralization achieved by diverse antibody clones each with limited neutralization breadth
Immuno-Oncology (IO)/Cancer
Immuno-oncology (IO) leverages the body’s own immune system to fight cancer and is revolutionizing the way we think about medicine because it is enabling long-term remission or in some cases “cures” to cancer patients. Common IO approaches involve antibody modulation of T-cells by various mechanisms, for example targeting T-cell checkpoints such as CTLA-4, PD-1, and PD-L1, redirecting T-cells via bispecifics antibodies or replenishing the immune system with “super-charged” T-cells, known as chimeric antigen receptor T-cellular (CAR-T) therapies. The clinical portfolio of antibodies in this space is diverse in format, engineering, and target, and requires deep knowledge of the mechanism of action (MOA) to ensure its safety and efficacy.
Carterra’s LSA provides a highly parallel way of screening hundreds of antibody binding interactions at once in terms of their binding kinetics, affinity, and epitope specificity. These binding properties underpin an understanding of antibody’s MOA and as such, are used as metrics to triage an antibody library to leads.
- Webinar: Discovery of Potent anti-PD-L1 Antibodies
- Publication: Brown ME, et al. PLoS One. 2020, Assessing the Binding Properties of the Anti-PD-1 Antibody Landscape Using Label-free Biosensors
- Poster: Use of High Throughput SPR to Characterize Antibodies To Common Cancer Biomarkers

Monoclonal antibodies are providing attractive alternatives to traditional small molecule therapies for treating chronic pain based on their high affinity and exquisite specificity that can minimize the unwanted off-target binding and adverse side-effects that seriously limit small molecule approaches. Due to their hydrophilicity and large molecular size, antibodies barely cross the BBB, so do not cause the lethal addictions of small molecule drugs. While the large size of antibodies limit their bioavailability via oral administration, necessitating injection via intravenous, intramuscular, or subcutaneous routes, this inconvenience to patients is somewhat offset by the naturally long serum exposure of antibodies, allowing for less frequent dosing, with some therapeutic antibodies requiring only a single injection per three months. From a practical perspective though, the high cost of developing and manufacturing therapeutic antibodies remains a major hurdle.
Therapeutic antibodies under clinical investigation and on the market aimed at treating chronic pain target a variety of molecules associated with pain pathways, including calcitonin gene-related peptide (CGRP), ion channels, nerve growth factor (NGF), tumor necrosis factor-alpha (TNF-alpha), and epidermal growth factor receptor (EGFR). Understanding how we can explore different mechanisms for treating pain is essential to bringing new medicines to market. Currently it takes about 12 years to progress a therapeutic antibody from bench to market, and antibodies for treating chronic pain are scrutinized in long and rigorous clinical trials.
The Carterra LSA is a high throughput screening and characterization tool for measuring antibody binding interactions, enabling the analysis of hundreds of interactions at once. By delivering high quality data quickly in the assessment of key binding parameters such as kinetics, affinity, and epitope specificity it can speed up research and ultimately help to converge upon clinically-ready leads faster.
Auto-Immunity
Therapeutic antibodies have proved highly successful in treating a variety of autoimmune diseases, as demonstrated by Humira (adalimumab), one of the biggest blockbuster drugs of all time. Like several other anti-inflammatory antibodies on the market, Humira targets and blocks tumor necrosis factor alpha (TNF-alpha), which relieves the pain and inflammation associated with some autoimmune diseases. Discovering “the next Humira” requires deep knowledge of biology to understand how to target other pathways relevant to autoimmune disease beyond TNF-alpha, such as T- and B-cell viability or activation, IL-1, IL-6, IL-17, and others. Understanding a drug’s mechanism of action (MOA) underpins the clinical success of any drug program.
Carterra’s LSA is a high throughput analytical platform that accelerates the screening and detailed characterization of large antibody libraries in terms of their binding kinetics, affinity and epitope specificity to provide crucial information about antibody binding properties that are used to assess and converge upon the most promising clinical candidates.
- Publication: Bednenko J, et al. MAbs. 2018, A multiplatform strategy for the discovery of conventional monoclonal antibodies that inhibit the voltage-gated potassium channel Kv1.3.
Heart Disease
When developing a new biotherapeutic, understanding the binding interactions that underpin its mechanism of action (MOA) are key to its success in the clinic. The Carterra LSA is a high throughput tool that enables interaction analysis measurements to be performed on hundreds of antibodies at once, allowing researchers to characterize the binding properties of antibody libraries quickly. The ability to generate kinetic, affinity and epitope specificity information at scale accelerates the library-to-lead triage, enabling the prioritization of resources to those antibodies with the most desirable binding properties.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Elsevier - PMC COVID-19 Collection

Therapeutic areas
Society and industry—still the only provider of approved medicine—share the desire to treat important diseases. Governments too, since some diseases’ high prevalence affect the economic output of society. Yet the decisions as to which drugs will be developed are strictly in private hands. The risks required to treat some really important, and often increasingly prevalent, diseases mean that the desires of society and the industry are diverging. This is not boding well for our society.
The understanding and willingness of governments to step in as a partner and financier is not apparent. Will governments recognize their role in this? Since the economic cost of these diseases, in terms of reduced productivity and medical costs to treat the disease, is enormous, understanding should be forthcoming. Government has invested for many decades in an enormous program of cancer research, which now shows dividends. The importance of further government investment in basic research is apparent when industry does not want to or cannot anymore afford it. As Big Pharma drops out of important therapeutic areas, it is not promising to assume that government can step in to the vacancy. There has to be a serious effort to find a formula by which the government gently or not so gently forces the industry to continue its efforts because it is so important.
Perhaps the most important of these neglected diseases is Alzheimer’s disease (AD) because of its dehumanizing nature and its predictably massively increasing prevalence. Yet, none of the currently approved Alzheimer drugs can be used to prevent, cure, or even significantly slow the progression of this disease. It is predicted that by 2030 50% of the over 80s will have the disease. Almost 100% of people with an inheritable trait will develop the disease unless we succeed in finding a preventive therapy. Only recently has a preventive trial been announced.
It is often thought that insulin-dependent diabetes is a well-treated disease and needs no further diagnostic and drug development efforts. This could not be further from the truth. The long-term complications of insulin-treated diabetes add to the economic burden, especially as the growth in prevalence of the disease expands with the waistlines of the world’s population. Three hundred million may have type 2 diabetes by 2030. Pharmaceutical control of weight loss has so far been vexed by serious side effects. When it comes to diabetes drugs, we start to recognize that the control of blood glucose can be further improved; the biggest contribution is not by drugs, but by self-diagnosis with point of care glucose measuring devices.
Morphine is still the most effective medicine against pain. It has been around for 5,000 years and we haven’t come up with anything better. But it can be ineffective against neuropathic pain and cancer pain. New pain medicines are difficult to find because of the subjective measurement and poor animal models. Since many current pain killers are abused, there are other hurdles. Despite the many difficulties, society needs new pain killers at the same time that notable pharma are pulling out, despite the economic opportunity.
Drugs against cancer have had many high-profile and expensive failures in recent years, with all major companies having at least one failed candidate. Biotech companies have made much progress, however, and they are either collaborating with or being taken over by Big Pharma. Oncology drug development has been crucially enabled by decades of government investment. The key is the many characterized drug targets that other disease areas do not have.
Government intervention in oncology and HIV provide the model for treatment of AD, neuropathic pain, and schizophrenia, among other therapeutic areas. Increasing governmental direct funding of translational research is not the solution many hope it will be.
Diverging views of society & industry
There are a few especially important diseases and therapeutic areas from the viewpoint of society, as well as from the viewpoint of the industry. These are especially important because these diseases affect a very large number of patients, have high prevalence, and thus, they affect the economic output of society and the distribution of expenditures within healthcare and, in a broader sense, within the whole society.
Some of these diseases are able to disrupt the workings of our society. Most countries recognize that epidemics are politically and legally highly destabilizing and cause huge economic losses. Even before modern globalization brought enormous and rapid flows of goods and people from all corners of the world together, epidemics of the plague, cholera, polio, and so forth showed the difficulties in isolating microbes, stopping outbreaks, and halting the spread of disease. Recently avian influenza (bird flu), severe acute respiratory syndrome (SARS), and the fights to restrict methicillin-resistant Staphylococcus aureus , and multidrug-resistant tuberculosis (MDR-TB), have confirmed modern vulnerability to outbreaks. The response to microbial epidemics needs to transcend national borders and ignore differences in the payer systems of national healthcare. This is clearly recognized, with the cooperation within the World Health Organization, with the setting up and use of the Global Fund, 76 but it is not sufficient just to share diagnostics and epidemiological data: we need vaccines and drugs developed for the whole world. Even with multinational cooperation, friendly countries such as the UK and the United States can be enveloped in arguments about delivery of paid-for flu vaccines.
Two large epidemics are slowly encompassing the whole world, not only Africa: obesity/type 2 diabetes and Alzheimer’s disease (AD). These epidemics threaten abrupt and almost unstoppable reorganization of the economy and societal structures unless we bring them under medical and pharmacological control. The obesity/diabetes epidemic is going to affect countries like China and India dramatically, while countries with an aging population like Japan, Germany, and the UK will be hit by the consequences of AD the hardest. Models of the consequences show that up to 15% of the work force will be involved in the 24/7 care of AD patients. These trends should be shaking governments into action.
The health economics that deal with the economic effects of disease burden and the effects of healthcare cost in society is, together with the study of public health, becoming increasingly important in political discourse in all countries; there are superb books on the topic from different political viewpoints. We will only deal with aspects of these important branches of the economy and of medicine that affect the choices of pharmaceutical companies.
Companies try to look forward 10–12 years and to foresee not only the biology and epidemiology, but also the economic and political landscape at the time when any new drug would enter clinical practice, would be paid for, and should start to produce revenue.
The development of drugs to prevent, or at least to treat, these strategically important diseases constitutes from society’s view the most important task for the industry, yet today in all countries’ pharmaceutical industry even vaccine production is in strictly private hands and the decisions are privately made. Governmental organizations weigh in on the safety of what is produced and, in cases of vaccines (and insulin and antipsychotics), about who should pay for the goods if proven safe and effective. Governments, by recognizing the societal importance of vaccine development and vaccination programs, have also influenced outcomes by limiting the liabilities of the private vaccine producers. Active involvement of nongovernmental organizations (NGOs) and governments in what drugs to develop is restricted to the trickle of funds devoted as charity to organizations working on diseases predominantly afflicting poor populations, such as malaria, leishmaniasis, sleeping sickness, and MDR-TB, which is now shared by all of Asia and prevalent in countries like Russia, China, and India.
The drugs that will treat these large diseases also potentially represent the largest incomes. Yet, because some of the diseases represent large drug development risks, even over the unusually high risk the whole industry carries, the big companies are abandoning work in some of these disease areas. The declining interest in AD and pain are examples. This does not bode well for our society.
The understanding and willingness of governments to step in as a partner and financier or limiter of liabilities is not apparent when it comes to the several large and potentially very profitable diseases we are discussing. Governments have always been involved as regulators and distributors, and have previously been involved in the development of some pediatric vaccines and drugs, for example, to control outbreaks or treat epidemics of infectious diseases. These increasingly neglected diseases are just as important for our society as are the infectious diseases.
The human & economic cost of major diseases
Among these large strategically important therapeutic areas/diseases that affect very large numbers of patients and have large human and economic costs are the following:
- 1. New antibiotics, especially for treatment-resistant Pseudomonas , Staphylococcus , and tuberculosis
- 3. Obesity and type 2 diabetes, and associated kidney and retinal complications
- 4. Neuropathic pain
- 5. Schizophrenia
- 6. Ovarian, prostate, lung, and gastric cancer, and glioblastoma
- 7. Hundreds of neurological diseases that collectively affect approximately 10 million patients, that are chronic, and where the scientific understanding of the pathophysiology is good but the market size is judged by the industry’s present standards as too small
Data are available in widespread sources for the estimated economic costs of these diseases. For example, the American Diabetes Association published 77 these data in 2008:
The total estimated cost of diabetes in 2007 is $174 billion, including $116 billion in excess medical expenditures and $58 billion in reduced national productivity. Medical costs attributed to diabetes include $27 billion for care to directly treat diabetes, $58 billion to treat the portion of diabetes-related chronic complications that are attributed to diabetes, and $31 billon in excess general medical costs
Table 4.1 and Figure 4.3Table 4.1Figure 4.3 describe the gap between the number of pharma-supported projects and the existing medical need. The list above is just a summary of the position in 2012. Most diseases are under-researched. Even though research is broad for many, it is just that it is not proportional to the need.
Of the diseases listed, only type 2 diabetes is being adequately addressed by resources of the industry. For the rest, we are not doing very well. While progress in the treatment of cancers, especially leukemias and breast cancer and in the past 2 years melanoma and non-small cell lung cancer, has been substantial, it is thanks to government-funded basic research since the 1960s. 78 Because of this large long-term investment, scientists are aware of many drug targets and thus the industry, which largely dropped its own basic research by the end of the 1990s, makes steady progress.
Each of these areas will be discussed in purely economic terms because, along with having disease-specific aspects, they also provide some very general points of view about the development of pharmaceuticals, about the interplay between government and private industry, and about the importance of government investment in basic research when industry does not want to or cannot anymore afford it.
AD: the most loomingly threatening disease
The most important example is AD. This neurodegenerative disease, which leads to cognitive decline, loss of memory, and inability to recognize faces or names, is, in short, one of the most horrible diseases in many senses. Many argue that all our humanity depends on our memories, and our memories determine who we are. When we lose these we are losing ourselves.
Although Alois Alzheimer diagnosed the first patient more than 100 years ago, AD has not been an important issue of research or of healthcare discussions, and certainly was not discussed in terms of a major threat to society as it is recognized today. The reason for this is that the major risk factor for AD is age. When the average life span of the population was 50–60 years, the frequency of AD was relatively low. Since definitive diagnosis still requires postmortem neuropathological examination of the brain, not many cases were identified. In contrast, the prediction for the United States is that in 2030 every second person aged 80 or above will have AD. The proportion of these people in Japan and the whole Western world is rapidly increasing.
What it really means is that up to 10% or more of the 2030 population in Japan, North America, and Europe may suffer from AD, and this is now being modeled by think tanks and insurers everywhere. Taking care of an Alzheimer patient, if they are taken care of individually, requires three full-time working persons each working 8 hours per day. This translates to a very large proportion of the productive labor force being devoted to taking care of patients with advanced AD, who cannot themselves be relied upon for the most elementary of functions. This is only the economic consideration. The societal implication is huge; there is currently a shortage of care givers. 79
The 2011 estimate is that $600 billion is being spent on taking care of AD patients. Yet there is no drug available that prevents or cures the disease, or even significantly modifies the rate of progression of the disease. The only drugs today approved are giving a relatively small—although in large trials statistically significant—slowing of the loss of cognitive function in mild to moderate AD and in one case are approved to treat moderate to severe AD. Memantine (Namenda from Forest Laboratories) is a 30-year-old German drug (from Merz) originally used for neuropathic pain treatment. It produces very small changes in the rate of decline in already seriously demented patients. It is liked because of its relative safety, not its efficacy. The total number of approved, available drugs is five and none of them can be used to prevent, cure, or even significantly slow the progression of this disease.
The number of diagnosed Alzheimer patients in 2011 in the United States was 5.3 million, and a similar number applies to Western Europe, but, including the nondiagnosed patients, the total is estimated at some 30% higher. Where do we stand with the development of drugs to be used in the therapy of AD patients? The scientific background is such that in this disease there are both sporadic forms, accounting for 99.8% of cases, and clear familial (or inherited) forms, which is to say, cases that have an early onset of disease at about 40 to 45 years based on a genetic predisposition; these account for 0.2% of cases. There are several other genetic vulnerabilities identified. A very frequently occurring vulnerability is being a carrier of a protein isoform called ApoE4. One can carry two of these gene copies when one is homozygotic. One can carry one ApoE4 and one ApoE3 , which is more common in the population, when one is heterozygotic. But, the vulnerability for AD is significantly higher in the ApoE4 homozygotes, who make up 2% of the U.S. population. 80
Incidentally, in 2007–2008, the first two full-genome sequences of notable individuals were published: those of Craig Venter, 81 who published “The Sequence of the Human Genome” 82 coincidently with the Human Genome Project’s parallel study, 83 and Jim Watson, 84 the co-discoverer of double helix. 85 In the first case, Venter’s sequence showed increased risk of AD 86 and in the second case the ApoE3/4 encoding sequence was omitted from Watson’s data at the specific request of Dr. Watson. He did not want to know if he were susceptible to an incurable disease to which a grandmother had succumbed. 87 Interestingly, the cost of the Human Genome Project was $3 billion over 13 years, Venter’s sequence cost some $100 million, and Watson’s was variously reported at $1–2 million in 2–4 months. 88 The costs are dropping in a dramatic manner: Complete Genomics, Life Technologies, and Illumina have disclosed that from January 2012 they will provide a full genome sequence for anyone for $1,000. We have yet to start a discussion about what cognitively happens to individuals carrying both the ApoE3 and ApoE4 genes—one from each parent—as their age increases.
There are more serious, but luckily less frequent, genetic causes of AD, such as the “Swedish” and “Dutch” mutations in the amyloid precursor protein. These mutations and mutations in another associated protein, presenilin, and a combination of these mutations lead, in a small group of people, to very early onset (about 40 years of age) rapid progression AD. These patients, who will have “familial forms of AD,” make up a small portion, 0.1–0.2%, of all AD patients. Most of the AD patients endure the sporadic form, yet the discovery of patients with familial AD suggests the possibility of highly focused trials for AD preventive medicines in a group whose risk is very high. Almost 100% will develop the disease unless we succeed in finding a preventive therapy.
It is worth explaining that in diseases where there is a large sporadic group of patients yet a small “familial group” or “genetically determined group” the understanding of the familial forms had great importance, in almost all cases, for finding treatments for the sporadic cases. This is because when people with familial disease are treated, we are fairly sure of the causative mechanism. It may be compared to the increasing introduction of companion diagnostics in oncology: treating those who have a genetic cause of the tumor improves responder rates from 5% to over 60%. 89 Yet we have great difficulties in finding any (preventive AD) trials involving family members of patients who have or have died from familial AD. The patient associations that “organize” these individuals have good lists of who would be eligible, but basic research and pharma have failed these people and missed this opportunity for two reasons. One is pure greed: “Why wait 4–5 years to show in familial cases that a therapy works, and then do another 4–5-year study in the real market representing sporadic patients?” goes the argument. It is added that “it is hard to recruit for familial case clinical trial,” which does not seem to be true, or it has to do with the second factor: the drugs we so far have put into trial are associated with such bad side effects that it is hard to convince people presently fully healthy, even if they know that they are likely to have early AD, to endure the side effects for an uncertain delay of the onset or for the prevention of the disease. We need to improve science and pharmacy, and we need governments, not NGOs, to pay for the more focused trials. 90
It is important that in the new healthcare legislation in the United States, upheld by the Supreme Court in June 2012, one cannot be excluded or penalized for a pre-existing condition or a genetic predisposition. This will become more and more important, especially as genome sequences are becoming affordable as part of diagnostics, not just as a research tool.
In general, the presence of genetic forms of the disease accelerates the understanding of the disease and indirectly accelerates the development of drugs to treat the disease. This is also the case with AD. The early onset disease has been linked to mutations in the metabolism of the amyloid precursor protein leading to the appearance of amyloid plaques. These are amorphous aggregates formed from several proteins. The major component is a fragment of the amyloid precursor protein. Research directed toward the proteolytic enzymes that produce this fragment of amyloid protein, the amyloid beta (or Aβ or Abeta) peptide, led to an investigation to dismantle the plaques formed from this peptide. The last 15 years have seen a tremendous effort by the industry to develop inhibitors for the two major APP-hydrolyzing proteases. Beta-secretase and gamma-secretase produce the amyloidogenic Abeta 1-40 or 1-42 peptides, but, so far, no peptidase inhibitor has reached clinical use. A gamma-secretase inhibitor (semagacestat or {"type":"entrez-nucleotide","attrs":{"text":"LY450139","term_id":"1258021836","term_text":"LY450139"}} LY450139 ) from Lilly came closest; it reached phase 3 trials and then was withdrawn in 2010 because of toxicities. This additionally shook the confidence of large pharma in Alzheimer therapies. 91 It is worth examining which companies try to develop AD drugs, the intensity of their effort, and the major issues that affect the intellectual and economic investment in the development of Alzheimer drugs.
Early diagnostic imperatives
The start of each treatment is diagnosis of the disease. Today, AD diagnosis heavily relies on neuropsychology test batteries, which have become better and better. They are now able to detect mild cognitive impairments and early AD. But nobody really knows how much damage has already occurred in the brain when cognitive impairment becomes obvious to relatives, friends, and colleagues. It is, therefore, very important that 2011 has seen the clinical entry of several imaging agents. 92 When working well, these permit the visualization of amyloid plaques in the living brain, and might, therefore, be important in establishing whether a drug aimed at inhibiting the production of amyloid peptide is really successful in reducing the plaque load. It is, however, evident that nobody is bothered by carrying amyloid plaques. When it is conclusively shown that the amyloid plaque load predicts cognitive impairment, we will then be able to convince people who exhibit no cognitive evidence of AD to take drugs that prevent the formation of amyloid peptide and amyloid plaques. But then these drugs will have to be very, very “clean” with no or a very low incidence of serious side effects or even annoying side effects.
If we had succeeded in producing a drug candidate with a strong scientific rationale suggesting it would prevent AD, without major and minor side effects, then which people would be convinced to take it? Firstly, those with a very high risk of developing familial AD would take it, preferably before showing any symptoms. Later, those who, through aging and some first signs of mild cognitive impairment, appear to have the risk of developing AD in a few years would take it. Some of these candidates would have to be prepared to test this drug in trials that will take 3–5 years. The principle is very similar to the way we have convinced millions and millions of people to take the cholesterol-lowering statins. High cholesterol per se is not a disease, but it is a harbinger of many cardiovascular diseases. If amyloid peptide and amyloid plaques can be detected and shown more rigorously to be a harbinger of AD, then volunteers would be easier to find. But for this to happen one will need much cleaner and much safer Alzheimer drugs than those we presently have.
Based on earlier neuropsychological diagnosis, patients are selected for the clinical trials of proposed Alzheimer drugs. These trials are becoming longer and longer. The reason for this is that we are entering into these trials based on the success of neuropsychological assessment that now captures early Alzheimer patients, in ever milder forms of the disease.
The first Alzheimer drug clinical trials in moderate to severe patients could show that the first class of drugs, the acetylcholine esterase inhibitors (AChEIs), could slow the decline of cognitive abilities significantly after a 6–12 month treatment (trial) period. The latest trials with AChEIs have, however, taken 18 months to show a significant difference in cognitive decline between drug-treated and placebo patients. The milder, and earlier the AD cases enrolled into the trials, the longer we have to dose the drug to show a significant difference in the cognitive performance between drug-treated and placebo patients. This is a clear recognition by pharma that, in order to show efficacy, they will need longer trials. These are a major expenditure, but, more importantly, the results are further and further away from what affects the stock price in the 2–3-year period upon which present pharma executives focus or are obliged to focus.
Another issue regarding Alzheimer patients and clinical trials is that the average age of these persons is above 65 and they suffer from a great number of other diseases, for which they are also being treated. This means that the drugs to be used in Alzheimer therapy have to be devoid of interactions with a very, very large number of drugs. It also means that because of age and other diseases, a relatively large number of these people will drop out of the trial or may die during the 18- or 24-month length of the trial. This means that these trials, at least at phase 3, will have to be very long and very large. The industry has conducted such long and large trials in cardiovascular disease and in type 2 diabetes. However, most large and long trials—usually post marketing, i.e., post approval, phase 4 trials—are carried out by governmental agencies in the UK, United States, and so forth. But the repeated failure of Alzheimer drugs over the last 10 years is making more and more companies silently withdraw from the effort to develop therapeutic agents for the treatment of AD, which would slow disease progression, prevent the disease, or cure the disease.
This is understandable from the point of view of profit within organizations, but it is not acceptable for our society. As we recognize AD as a serious risk for our society, we cannot afford to give up trying to make drugs that achieve effective treatment. Today, there are no seriously capable organizations except the pharma industry to develop such a drug. So, when they withdraw, openly or silently, and are permitted so to do, we will end up with the horrific prospect of every second over 80-year-old citizen suffering from AD, but not being offered any kind of treatment that would help the patient, the family, and society as a whole.
Translational research
When we say that the only seriously capable complex, sophisticated large organizations to develop therapeutics are the pharmaceutical companies, we are not disregarding the expensive efforts government-supported institutions, such as the National Institutes of Health (NIH) and corresponding European institutions, put into what they call translational research , that is, research that is directed toward affecting medical practice.
Translational research, which is the modern equivalent of applied research, is an increasingly important part of their task. However, in retrospect, one can today clearly state that these organizations have not discovered any major drugs in the past 40 years. They have, during these last 40 years, been the major contributors to the basic science breakthroughs that are the scientific basis of drug development in the world’s private pharmaceutical companies, which all abandoned in-house basic research from 1980 to 2000. The government-sponsored research institutions, together with some major NGO-sponsored research entities (e.g., the Wellcome Trust, the Howard Hughes Medical Institute, and the Bill and Melinda Gates Foundation), have introduced a multitude of extremely important tools to preclinical and clinical research. These permit the regulatory agencies and the industry to measure and improve the success of drug discovery, drug development, and the drug approval processes. Starting in 2012 the NIH collaborated officially with the Food and Drug Administration (FDA); in principle they are both government sponsored. This will assist the FDA, which has so far relied on ad hoc panels of academics for scientific advice, to lean more on scientists in government pay. NIH scientists have a much stronger set of rules to keep them from receiving payments from the industries that apply for drug approval. This will help dilute the requirement of Congressional Committees periodically to uncover high-profile examples of the FDA receiving advice from academics with a conflict of interest. Conflict of interest is a serious matter that can undermine all public trust in the impartiality of FDA decisions regarding drug approvals, no matter if the decision itself was impartial.
Thus, the recent, long-awaited official agreement, pushed by the Obama Administration, to improve the quality and image of FDA decisions by having two U.S. tax-payer financed organizations, the NIH and the FDA, sharing scientific competence, bodes well. The NIH is the world’s powerhouse for preclinical discoveries through its intramural and extramural programs, and it is important in academic medicine in small proof-of-concept trials; however, as a clinical research organization, it is small to medium sized. The roughly $5 billion spent by the NIH on clinical projects and the $700 million proposed for translational research in 2011–2012 is dwarfed by the estimated $92 billion research budgets of major pharma and major biotech spent in the same year on R&D, over 85% of which is translational research (see Table 5.1 , Table 5.2 ). The effort is also dwarfed by the collective experience that exists in these companies in the development and testing of clinically useful drugs.
Top 15 R&D budgets of the major pharma companies in 2009
Compare to the NIH budget of $29.5 billion in 2009. 1 Around 70% ($22B) of the NIH budget and 20% ($20B overall) of the companies’ budget is for preclinical research. This is not basic research per se, but research to select the drug candidate to enter into clinical trials. Almost all basic research is now left to government and NGO financing. Internationally, governments fund more than 60% of basic research in large national institutes and universities; the rest is from private sources to universities. NGOs fund 2% and industry the remaining approximately 30%. Eighty percent of all private investment is for translational research in the hope of developing diagnostics and drugs that will generate profit. We do not have precise figures, but the indications are that the budgets would have fallen by a collective 15% in the two subsequent years (2010 and 2011).
Top 15 R&D budgets of the major biotech companies in 2009
Around 80–90% of biotech budgets ($6.9B from the above) is allocated to translational research.
It is clear that the NIH and its international counterparts bear huge responsibility for the “real” science in AD. When one compares funds poured into HIV/AIDS and cancer research to those put into AD, one is shocked. It is clear that funding initiatives can bring large numbers of scientists to work on a problem, and that it is what we badly need. Instead, AD research is conducted by a small club; this we cannot afford.
Therefore, it is not a promising route to let private industry back out of trying to develop Alzheimer drugs, saying: “it’s too hard; it’s too risky; it’s too slow; it’s too expensive,” and assume that governments will step in to the vacancy. There has to be a serious effort to find a formula by which governments, gently or not so gently, force the industry to continue its effort in developing Alzheimer drugs. Intervention can come from tax breaks or other incentives, as well as somewhat punitive legislation for pharma to release data from programs they have dropped, in order to support this highly risky activity because it is so important . We have seen how U.S. and other governments can enhance and semi-force the development and production of antidotes to biological weapons, and how it can incentivize and encourage the production of vaccines.
AD is not as acute as bird flu or the risk of a bioterrorist attack, but it is as, or more, threatening for the individual and for our society.
Obesity & type 2 diabetes
The first biological to be used on a large scale was insulin, albeit preceded by some vaccines and antidotes to snake toxins, and so forth. The first clinical trial of insulin involved one patient: a child. We often forget this. As discussed earlier, production of insulin is now made with recombinant technology in bacteria or yeast. The three major producers, Novo Nordisk, Lilly, and Sanofi-Aventis, are producing both acutely active and delayed, slowly, or long-acting depot or retard insulin preparations.
One may think that insulin-dependent diabetes is, therefore, a well-treated disease and needs no further diagnostic and drug development efforts. This could not be further from the truth: up to 30% of patients are resistant to the most widely prescribed diabetes drug, metformin (see below); most patients are becoming increasingly resistant to insulin; and we have ongoing exacerbations with the patients who are not becoming resistant to either of these two drugs.
Even with well-controlled diabetic patients who, by judicious use of insulin, do not let their blood glucose rise, the development of renal illness, blindness resulting from retinal vascularization problems, diabetes-induced neuropathies, and pain in the extremities is very high. On top of this comes the world’s increasing obesity, the most obvious risk factor for type 2 diabetes. Thanks to a rising standard of living and global flows of food staples, fewer and fewer people in the world are starving, or, rather, more and more people are not starving. Obesity growth is linked with the change of diet in Asia. In India and China, where more bountiful food, coupled with wealth, is added to some genetic predisposition in these two huge countries, there has been a dramatic increase in obesity and in type 2 diabetes. It should be noted that not all obese people will develop type 2 diabetes, only about 20% do, but the cost of treatment for these people and the cost of treatment of the complications from diabetes is staggering and comparable to that of AD.
It is clear that it is in the interest of patients, society, and the pharmaceutical industry to find drugs that prevent the rapid increase in the number of obese people and, therefore, reduce the risk that 20% of these will develop type 2 diabetes. It is also clear that we have to develop better treatments for this very large group, which is estimated to include close to 300 million people by 2030, all of whom will have type 2 diabetes. Unfortunately, it is very difficult to develop safe drugs that prevent or cure obesity when the patients are unwilling or unable to switch diet and to exercise. Diet control and exercise are both becoming increasingly difficult in the stressful world of rising standards and rising expectations of work and other achievements.
The pharmaceutical industry has seen some spectacular successes in this area. Unfortunately, these then turned into spectacular failures, sometimes taking with them the entire company, no matter how big it was. Most in the industry clearly remember the successful development of Fen-Phen (fenfluramine + phentermine), a very active (amphetamine-like) mixture of appetite control drugs that has shown clearly visible, tangible results of large rapid weight loss. The beauty of trials on obesity drugs is that the patients can, by checking their waistlines or stepping on to a balance, see for themselves whether the drug is efficacious. The fallacies of these trials are many, however. While it is relatively easy to achieve a 5–10% weight loss in the first 6–8 weeks, it is very hard to keep it over 12 months. This is why the FDA now insists that drugs that might be approved achieve and maintain a 5% or higher weight loss over a 12-month treatment because less than that is not clinically meaningful. Also, drug treatment is not comparable to other ways of achieving dramatic weight loss such as bariatric surgery, itself not without dangers. Surgery is, however, very expensive and not acceptable or appropriate for every patient, but bariatric surgery can produce a 30% or higher weight loss that remains after 12 months.
Fen-Phen had been a spectacular success for the company American Home Products until it turned out that a large number of patients develop valvulopathy—changes in the heart valve—as a result of taking this drug. As this is an irreversible disease state leading to death in many cases, the subsequent lawsuits brought down this very large pharmaceutical conglomerate. History should record that Fen-Phen as a combined drug was never approved by the FDA and was the result of an off-label development at some U.S. academic institutions.
Similar amphetamine-based weight loss drugs by Servier were questioned and were recently withdrawn in France. Other weight-loss drugs have fallen on other safety issues. Some of the few approved and “safe” ones have very unpleasant side effects. For example, orlistat (Xenical) blocks uptake of fat by the intestine, which causes hard to control diarrhea with almost inevitable soiling. Despite this, GlaxoSmithKline entered the generic drug market with orlistat (Alli) as its first generic (see Chapter 04).
The development of weight loss drugs is very attractive but very difficult; the last 3 years have seen the industry bringing forward compounds or combinations of compounds that showed efficacy of 6–10% weight loss over 12 months, but which contributed to an increase in social isolation, suicidal ideation, and suicides. It forced the FDA to demand that companies specifically examine these side effects, and it finally asked its outside academic–clinical experts whether obesity is such a life-threatening condition that we should permit the taking of drugs that might significantly increase suicidality. The answer was no; we have to be able to find drugs that achieve the same weight loss without these serious side effects.
Other weight loss drugs have shown large increases in blood pressure and heart rate, and were deemed not safe. Presently, the industry is wondering if it is possible to make a truly safe obesity drug, while several biotechs are hampered by their financial inability to afford the long cardiovascular safety study rightly requested by the FDA.
But, unlike in the case of AD, the industry is not abandoning the area because it seems that the development of these drugs is easier in the sense that the scientific basis to fight obesity is better understood, and that the chance of proving that one has an efficacious safe drug is better than achieving the same in AD. In particular, the efficacy of the drug is shown and seen by the patients themselves within weeks. The other problem is maintaining this efficacy. It is so clear that there is a great desire in our world to achieve the ideal body weight determined by fashion and by health advisories of a body mass index (BMI) around 22–24. Despite a period of 3 years of efficacious drugs not being approved because they were not safe enough, it is less likely that the industry will abandon its search for obesity drugs.
When it comes to diabetes drugs, we start to recognize that the control of blood glucose can be further improved. The biggest contribution to improved control is not by drugs, but by continuous, daily, or repeated daily self-diagnosis of high blood glucose levels. These levels can be measured at home using the glucose meters developed in the last 30 years, which have become so cheap and reliable that all patients can use them. This so-called point of care device , of which we will see many in other indications, enables patients to control their own therapy, which in this case is of great importance. The first glucose meter developed by Boehringer Mannheim was as big as a refrigerator and required 2–4 ml blood. Today’s machines use just a drop of blood, are pocket-sized, and, in most countries, given free of charge. This locks the patients into buying the measuring strips, much like Kodak practically gave away the instamatic camera so you would buy its film. That this marketing works shows how widespread self-monitoring of blood glucose has become.
Secondly, we realize that there are drugs like the GLP-1 (glucagon-like peptide-1) analogs and inhibitors of the GLP-1 degrading enzyme DPP-4 (dipeptidyl peptidase-4), which can fine-tune glucose control and will postpone some of the diabetic complications.
The problem for those who are developing diabetes drugs is that we have a very large number of relatively efficacious, cheap, generic drugs such as metformin, 93 which have side effects but work quite well. For the largest number of patients these drugs will remain the mainstay of their diabetes treatment because they are well known and because they are cheap. The new drugs have to demonstrate significant improvements over the presently available insulin releasers, insulin sensitizers, and so on. In this quest for better control of blood glucose, we have also learned metabolic disease patients are accepting of injection, not only of insulin but also of other drugs. This opens the route for the use of biologicals in an area where this was not widely foreseen. Today, the GLP-1 analog exenatide (Amylin/Lilly’s Byetta—a synthetic version of exendin-4) and its competitor liraglutide (Novo Nordisk’s Victoza) are injectable biologicals generating revenues of more than a billion dollars each per annum. Together with insulin, we have now accepted in clinical practice other injectables to control blood glucose. In contrast, the inhibitors of DPP-4 are oral drugs: sitagliptin (Merck’s Januvia) and saxagliptin (BMS’s Onglyza).
In summary, it has to be said that the impact of the fight against obesity as a major risk factor for type 2 diabetes cannot be overstated. The problems of finding efficacious and safe obesity drugs are great, both practically and theoretically, because eating is so deeply and intricately programmed in our brain. So many processes of mental well-being are controlled by parts of the neuronal circuits that control repeated, pleasurable activities such as eating. Nevertheless, it is clear that here the industry continues to stand at the gates of the FDA with new drugs and drug combinations that can achieve significant weight loss.
Surgical versus drug interventions
A comparison of surgical procedures such as bariatric surgery to drug treatment is an important one. Those who work for insurance companies and major health plans calculate the risk–benefit ratios of major surgeries. One often finds that bariatric surgery is only recommended for those who are relatively young and who are enormously difficult to treat with drugs, diet, and exercise. Whether this will change with improvements in surgical procedures or not is hard to foresee. But it is clear that many patients will prefer any available oral or injectable drug if it comes close to the efficacy of the weight loss produced by a surgery where today mortality is over 1%.
Neuropathic pain
Morphine is one of our oldest drugs. The ancient Egyptians called it “God’s own medicine.” This was not because of its marvelous pain-killing properties, but because it effectively stops diarrhea, which is one of the great killers in the world. The existence and knowledge of how to dose morphine and other opiates has been the basis for the effective treatment of pain for a long time. Many thought that opiates could be useful in the treatment of all pain states. Neither the patients nor the anesthesiologists held this view and it is manifestly not the case. There is a very large group of pain syndromes—neuropathic pain resulting from neuronal injury and cancer pain—which are often not responsive to opiate painkillers. On top of this, opiates have huge safety problems that include constipation, alcohol interaction, and so forth, as well as their well-documented addiction potential. Relieving pain is one of the great dreams and aspirations of mankind. Yet the introduction of new efficacious pain medications is very, very slow. The inadequacy of all pain treatments is best illustrated by the following:
Most clinical trials for pain killers evaluate the efficacy of the medication using a visual analog scale. Patients judge for themselves the intensity of the pain on a linear analogue scale from “0” = no pain to “10” = excruciating pain. The gold standard of non-opiate, non-morphine-type pain drugs is gabapentin. Gabapentin is a more than $2 billion-selling drug that in its clinical trial reduced pain from an average of somewhat above “7” to an average of “4,” not to zero. The FDA suggests that patients may be recruited for pain trials if they assess their own pain to be above “4.” This means that even after having taken the best available drug many patients would still be eligible to enroll in a new trial for a painkiller.
Current practical treatment of pain may use multiple drugs. Physicians and patients learn to use several drugs to achieve some pain relief. The biggest problem is not that our basic pain research is lacking in effort, but that the animal models, in which we try to assess the efficacy of new pain medications, are not very reliable. Therefore, many clinical trials that were initiated after successful animal experiments have failed. There are additional big problems with developing new pain medications. This includes the potential for misuse of painkillers. People take them recreationally rather than because they have pain. Often these drugs, which were made for oral use, are being ground up and injected intravenously when their effect is much faster and much more robust in the brain, sometimes causing deadly accidents. So, whoever tries to introduce a novel serious painkiller has to prove or has to safeguard against abuse potential. This is difficult.
Painkillers are also the most often overdosed drugs. Patients in pain start taking the recommended dose then readily take more or more often when the pain does not abate. Therefore, these drugs have to be very safe even at 5 to 10 times the recommended dose. Another problem of many painkillers is their interaction with alcohol.
Despite these inherent difficulties, we cannot stop trying. In many ways painkiller development is ideal for the pharma companies as the trials are short: dental pain, 72 hours; postoperative pain, 3–7 days; and post-herpetic neuralgic pain, 7–14 days. It is just that what works in all the animal models consistently fails in patients, explaining why the industry has openly stopped trying in some very notable cases. Some of the largest pharmaceutical companies, such as Roche—the most profitable pharma company today—Novartis, Astra-Zeneca, and others, have openly closed their pain research units, saying that it is too difficult. This leaves the field to small companies or to companies such as Merck and Pfizer (previously active in COX-2 inhibitors, see Chapter 03) that are mostly only interested in one type of pain, inflammatory pain, for which we already have relatively good medication, as everybody who has been given ibuprofen by the dentist knows.
Pain is an example of an area of disease where the number of people affected is tremendous. The suffering is chronic. The economic opportunity therefore exists, yet the companies openly withdraw from competing in this area. This is calling for serious governmental entry into the field at the level of both basic and clinical research, which would help to define more valid, more reliable, and more translatable models, so that the existing argument of “whatever works in animals may or may not work in humans, and we will only know it $500 million dollars and 3 years later” will no longer be valid. Directive research by governments could change the situation and would, therefore, change the daily lives of tens of millions of people with chronic pain.
The huge medical need and market opportunity in neuropathic pain is thus dominated by gabapentin, a drug developed as the anti-epileptic Neurontin by Parke-Davis (now Pfizer), which is now more than 18 years old. It was approved in 1994 and has spawned many generic forms such as Fanatrex, Gabarone, Gralise, Nupentin, and so forth. Those who work in pain clinics are using a mixture of dedicated painkillers, anti-inflammatory drugs, tricyclic antidepressants, and anti-epileptic drugs to treat neuropathic pain, but their success is very limited.
In the 1970s and 1980s, the 20 large pharma companies, of which today exist less than 10 because of mergers and acquisitions, were not very interested in the area of oncology. The argument was that cancer, leukemias and solid tumors, are often discovered too late to do anything and that many cancers will not be treated easily because breast cancer, prostate cancer, lung cancer, renal carcinoma, pancreatic cancer, and so on, are clearly different diseases of different organs that will require completely different medications; therefore, the market is very fragmented. So, not only are the patients diagnosed late but there are also too few patients for each cancer form, maybe with the exception of some leukemias and lymphomas (see Table 5.3 ). However, it turns out we knew too little about some of the underlying cellular pathways and that certain controls of the cell division that runs amok in cancer are general in many cell types; thus, there will be carry over from developing a drug for one cancer indication (see the section “Effective governmental & societal intervention”). We were too organ focused, and not focused on cellular pathways.
Estimated cancer prevalence in the United States in 2007
Data include the 23 most prevalent cancers; totals include other less prevalent cancers, of which there are many.
In any clinical trial, the industry would not want to use, as the comparison, the most commonly used chemotherapy: the cytotoxic agents. These nucleotide analogs are still among the most efficacious cancer drugs, but with terrible side effects like hair loss, nausea, and weight loss. They are still used frequently, and these are drugs that are very cheap to make and very cheap to buy.
Big companies are not leaving the therapeutic area of oncology, although 2010–2011 was full of great disappointments when it came to clinical trials. This has shaken some of the best-selling cancer drugs such as bevacizumab (Roche-Genentech’s Avastin), which has been approved for treatment of non-small cell lung carcinoma and for colorectal cancer, but which has failed to show efficacy in breast cancer. This also may be initiating a possible re-evaluation of the original approval in non-small cell carcinoma, thereby endangering one of the best-selling cancer drugs from Roche.
Several of the spectacular clinical trial failures of 2010 were in oncology ( Table 5.4 ), but so were three of the most spectacular successes ( Table 5.5 ). Oncology remains a highly successful area, both medically and economically, for pharmaceutical companies. We also saw the first new therapeutic modalities such as the antibody-drug conjugate (ADC) brentuximab vedotin (Seattle Genetics’ Adcetris), approved in August 2011 for anaplastic large cell lymphoma (ALCL) and Hodgkin’s lymphoma; many others are in trials.
The top 10 phase 3 failures of 2010
Drug list is as reported by FierceBiotech. 1 The average phase 3 trial costs $400–750 million. All major pharma are present on the list. These companies have extensive drug development and clinical trial experience. The only small and new company is Novelos Therapeutics, Inc. of Madison, WI.
Advances in cancer therapies: new approvals 2009–2011
New drugs, new antibody-drug conjugates, and new orphan drug strategies by both small and large companies; phase 3; and approvals in 2009–2011. It is important to note three trends in this table: (1) companion diagnostics appearing and improving responder rate from 5–10% to 60%; (2) we have become—after 10 years and dozens of failures—good at designing protein kinase inhibitors that are less toxic than the first ones were (by not binding to the ATP-binding site of the enzyme), and we can often design a selective compound for the mutated protein kinase that leaves the non-mutated, non-cancer-related form free from inhibition; and (3) the therapeutic antibodies are coming in different formats and armed with different cell-killing mechanisms on top of selectively recognizing some marker of the cancer cell. Most of the entries in this table are first in class drugs, proving that we can, when we put the resources into it, innovate with cancer drugs. Why can we not in AD or pain?
Effective governmental & societal intervention
There are 40 times as many drugs being tested for cancers than for schizophrenia. Why? In short, the explanation is government-sponsored basic research. Today oncology 94 is the most drug target-rich area, whereas large chronic diseases, which affect up to 1–2% of the population over their entire lifetime, like schizophrenia, are extremely poor in drug targets.
The reason for the richness of drug targets in oncology is twofold. One is the decision by governments and NGOs, such as cancer societies, to engage large-scale sponsoring of basic research over several decades starting in the 1960s. Firstly, this research has shown that many molecular mechanisms are common in very different cancer forms. Therefore, even though on the surface prostate and ovarian cancer are clearly different and occurring in different genders, there are molecular pathway similarities. Effective drugs may affect basic underlying mechanisms of the repair of DNA, or the formation of vasculature, which is needed to supply the tumor with blood, and so on. These putative drugs might be used to treat more than one cancer form. Secondly, the discoveries of drug targets in leukemias and then in solid tumors have accelerated as a result of major public spending. When considering the importance of this, one has to remember that until the 1960s the pharma industry had been responsible for the basic research in most areas of life sciences (except embryology), which was the foundation for most of the drug developments. Since the 1960s, government-sponsored research in physical and life sciences in the United States and Western Europe has increased (the “Sputnik effect”) and has almost completely replaced the basic research efforts of big pharmaceutical companies. It is true that pharma spent $92 billion on research and development in 2009, but a very small portion, maybe $10 billion, of this was considered basic research. This is to be compared with the more than $60 billion spent annually on basic research by public and other sources. The NIH alone in the United States has a budget of over $30 billion.
The example of oncology shows that when politicians recognize the need either for popular or other reasons to support research, then they can initiate sustained and large-scale efforts that eventually produce scientific breakthroughs. HIV treatment is another area serving as a good example of the joint forces of patient interest groups, relatives of patients, celebrities, and governments to put pressure on a pharma industry that had not been in general very successful in developing antiviral agents. Yet, in the relatively short period of 20 years, HIV has switched from being a 100% deadly disease to a chronic disease that can be managed at several levels. This is one model, in which society can influence pharma by spending on basic research (to provide basic functional understanding and drug targets). Society would then rely on different sized companies, small ones first and bigger ones later, to pick up the scientific breakthroughs and apply them in the development of novel drugs. This is a very long-term, indirect way to find new drugs, but it has worked and it does not rely on specifying the commercial entities that will use the scientific results; instead it hopes that several will, and that they will do it in competition with one another.
There is also post-drug development encouragement and incentive by allocating NGO budgets to guarantee the purchase of a very large number of doses for developing countries. They exert a push–pull mechanism: the push on development and pull on production.
The examples of oncology and HIV cannot be underestimated. They are extremely important if we are not to give up work on Alzheimer therapies and on finding drugs against neuropathic pain and, for example, against schizophrenia, where the present drugs have relatively low response rates and are associated with severe metabolic side effects in many cases leading to early type 2 diabetes in young schizophrenics.
There are many good reasons to protect the freedom of researchers and not to direct all research, mostly because we really are not that good at predicting the truly innovative breakthroughs, many achieved through studying systems such as Escherichia coli , yeast, nematode worms, sea slugs, and fruit flies, and leading to cancer drugs. Nevertheless, one must not shy away from directing a much larger portion of the research budget to AD and antibiotics resistance than is happening presently. NIH and European research agencies already have large directed programs, but they are not large enough. Biotechs would be most helped if phase 2 and phase 3 clinical trials in AD were made possible with the partnership of government as an alternative to partnering to Big Pharma, which is currently not interested in these trials.
76 The Global Fund to Fight AIDS, Tuberculosis, and Malaria.
77 American Diabetes Association (2008) Economic costs of diabetes in the U.S. in 2007. Diabetes Care . Mar; 31(3):596–615.
78 See Figure 4.2.
79 See also Chapter 11.
80 See also Chapter 09.
81 Levy S et al. (2007) The diploid genome sequence of an individual human. PLoS Biol . Sep 4; 5(10):e254.
82 Venter JC et al. (2001) The sequence of the human genome. Science . Feb 16; 291(5507):1304–1351.
83 International Human Genome Sequencing Consortium; Lander, ES et al . (2001) Initial sequencing and analysis of the human genome. Nature . Feb 15; 409(6822):860–921.
84 Wheeler DA et al. (2008) The complete genome of an individual by massively parallel DNA sequencing. Nature . Apr 17; 452(7189):872–876.
85 Watson JD and Crick FHC (1953) A structure for deoxyribose nucleic acid. Nature . Apr 25; 171, 737–738.
86 In addition to antisocial behavior, cardiovascular disease, and wet ear wax (see http://en.wikipedia.org/wiki/Craig_Venter ).
87 See http://www.nature.com/news/2007/070528/full/news070528-10.html .
88 See http://www.nature.com/news/2008/080416/full/452788b.html .
89 See Chapter 08.
90 See also Chapter 11 where we may reveal that our argument is vindicated: one trial, designed in the way we recommend is now being conducted. For those readers who wish to “fast forward” you should look, for example, at http://www.multivu.com/mnr/56128-banner-alzheimer-s-institute-genentech-nih-prevention-trial-genetics .
91 Confidence has not been improved by the poor results from Lilly’s therapeutic antibody trial of solanezumab (see Chapter 06).
92 The approval for the imaging agent florbetapir (Amyvid) to image amyloid plaques from Avid-Lilly was, however, delayed over procedures of evaluation.
93 Metformin has had a long history. First synthesized in 1920, then trialled by a French physician in 1957, it was introduced in the UK in 1958 but not until 1995 in the United States. In 2010, 48 million generic prescriptions were filled. See http://en.wikipedia.org/wiki/Metformin .
94 perhaps together with inflammation.
Asia & Oceania
Middle east & africa.
- United States
- Asia Pacific
- Australia & NZ
- Southeast Asia
- Czech Republic
- Deutschland
- España
- Switzerland
- United Kingdom

EMEA Thought Leadership
Developing IQVIA’s positions on key trends in the pharma and life sciences industries, with a focus on EMEA.
- Middle East and Africa
- Research & Development
- Real World Evidence
- Commercialization
- Safety & Regulatory Compliance
- Technologies
LIFE SCIENCE SEGMENTS
- Pharmaceutical Manufacturers
- Emerging Biopharma
- Consumer Health
HEALTHCARE SEGMENTS
- Information Partner Services
- Financial Institutions
- Public Health and Government
- Patient Associations
THERAPEUTIC AREAS
Cardiovascular
- Cell and Gene Therapy
Central Nervous System
- GI & Hepatology
Infectious Diseases and Vaccines
Rare Diseases

Impacting People's Lives
"We strive to help improve outcomes and create a healthier, more sustainable world for people everywhere.

Harness the power to transform clinical development
Reimagine clinical development by intelligently connecting data, technology, and analytics to optimize your trials. The result? Faster decision making and reduced risk so you can deliver life-changing therapies faster.
Research & Development Quick Links
- Clinical Trials
- Functional Services
- Decentralized Trials
- Therapeutic Expertise
- Site and Investigators

Real World Evidence. Real Confidence. Real Results.
Generate and disseminate evidence that answers crucial clinical, regulatory and commercial questions, enabling you to drive smarter decisions and meet your stakeholder needs with confidence.
Real World Evidence Quick Links
- Real World & Health Data Sets
- Medical Affairs
- Health Data Apps & AI
- Health Data Transformation
- Study Design
- Evidence Networks
- Health Economics & Value
- Regulatory and Safety
- Natural Language Processing
- Real World Evidence Library

See markets more clearly. Opportunities more often.
Elevate commercial models with precision and speed using AI-driven analytics and technology that illuminate hidden insights in data.
Commercialization Quick Links
- COVID-19 & Commercialization
- Launch Strategy & Management
- Brand Strategy & Management
- Pricing & Market Access
- Healthcare Professional Engagement
- Patient Engagement
- Promotional Strategy

Service driven. Tech-enabled. Integrated compliance.
Orchestrate your success across the complete compliance lifecycle with best-in-class services and solutions for safety, regulatory, quality and medical information.
Safety & Regulatory Compliance Quick Links
- Safety Pharmacovigilance
- Regulatory Compliance
- Quality Compliance
- Medical Information
- Commercial Compliance

Intelligence that transforms life sciences end-to-end.
When your destination is a healthier world, making intelligent connections between data, technology, and services is your roadmap.
Technology Quick Links
- Orchestrated Clinical Trials
- Enterprise Information Management
- Performance Management & Insights
- Provider Reference Data Network
- Customer Engagement
- Safety, Regulatory, Quality Compliance
- Partner Programs
- Technology Insights
CLINICAL PRODUCTS
- Planning Suite
- Clinical Trial Payments
- Investigator Site Portal
- One Home for Sites
- Patient Engagement Suite
- Electronic Clinical Outcome Assessment (eCOA)
- Interactive Response Technology (IRT)
- Clinical Data Analytics Solutions
COMMERCIAL PRODUCTS
- Information Management
- Promotional Engagement
- Orchestrated Analytics
- Next Best Action
- Customer Engagement (OCE)
COMPLIANCE, SAFETY, REG PRODUCTS
- IQVIA Vigilance Platform
- SmartSolve eQMS
- Safety & Pharmacovigilance
REAL WORLD PRODUCTS
- Data Profiling
- Patient Profiling
- HCP Profiling
- Market Profiling

"Data in a Day
BLOGS, WHITE PAPERS & CASE STUDIES
Explore our library of insights, thought leadership, and the latest topics & trends in healthcare.
THE IQVIA INSTITUTE
An in-depth exploration of the global healthcare ecosystem with timely research, insightful analysis, and scientific expertise.

INSTITUTE REPORT
"Global Trends in R&D 2024

EMEA Insights
"IQVIA thought leadership with a focus on EMEA.

WHITE PAPER
"DCTs Deliver Big ROI

"Capturing value at scale: The $4 billion RWE imperative
FEATURED INNOVATIONS
- IQVIA Connected Intelligence™
- IQVIA Healthcare-grade AI™
- Human Data Science Cloud
- IQVIA Innovation Hub
- Patient Experience powered by Apple
- Commitment to Public Health
- Code of Conduct
- Environmental Social Governance
- Executive Team
NEWS & RESOURCES
- Events & Webinars
- Industry Analyst Reports
- COVID-19 Resources
- Our Locations

INVESTOR RELATIONS
"Visit our investor relations site for more information.

Unlock your potential to drive healthcare forward
By making intelligent connections between your needs, our capabilities, and the healthcare ecosystem, we can help you be more agile, accelerate results, and improve patient outcomes.

IQVIA AI is Healthcare-grade AI
Building on a rich history of developing AI for healthcare, IQVIA AI connects the right data, technology, and expertise to address the unique needs of healthcare. It's what we call Healthcare-grade AI.

Your healthcare data deserves more than just a cloud.
The IQVIA Human Data Science Cloud is our unique capability designed to enable healthcare-grade analytics, tools, and data management solutions to deliver fit-for-purpose global data at scale.

Innovations make an impact when bold ideas meet powerful partnerships
The IQVIA Innovation Hub connects start-ups with the extensive IQVIA network of assets, resources, clients, and partners. Together, we can help lead the future of healthcare with the extensive IQVIA network of assets, resources, clients, and partners.

Proven, faster DCT solutions
IQVIA Decentralized Trials deliver purpose-built clinical services and technologies that engage the right patients wherever they are. Our hybrid and fully virtual solutions have been used more than any others.

IQVIA Patient Experience Solutions powered by Apple
Empowering patients to personalize their healthcare and connecting them to caregivers has the potential to change the care delivery paradigm. IQVIA and Apple are collaborating to bring this exciting future of personalized care directly to devices patients already have and use.

WORKING AT IQVIA
Our mission is to accelerate innovation for a healthier world. Together, we can solve customer challenges and improve patient lives.
LIFE AT IQVIA
Careers, culture and everything in between. Find out what’s going on right here, right now.

WE’RE HIRING
"Improving human health requires brave thinkers who are willing to explore new ideas and build on successes. Unleash your potential with us.
Therapeutic & Specialty Expertise
The experts and expertise you need..
Therapeutic expertise isn’t just important. It’s mission-critical. Because to get to the right solutions for patients, you need to know what the problems are, and what questions to ask.
- Cell & Gene Therapy
GI and Hepatology
- Allergy & Respiratory
Dermatology
Reproductive Health
Rheumatology
Biosimilars

‘One size fits all’ has never applied in healthcare
And it never will. How you find, and treat, patients in oncology must be different than in infectious disease. Understanding regulatory and approval pathways is an entirely different journey in pediatrics than it is for Alzheimer’s treatments.
We’ve known this, but until today our ability to access, connect and leverage data, technology, and advanced analytics, and apply the critical lens of domain expertise has limited how precise we can be.
Explore IQVIA's deep domain expertise in the therapeutic and specialty areas you are committed to.
Translate science into new treatments
Through our Therapeutic Centers of Excellence, IQVIA connects your clinical program with strong scientific and medical expertise, deep therapeutic insights and unrivaled clinical trials experience. To help you find the most direct route from breakthrough science to new therapies for patients.
Ask the right questions
From the earliest planning stages of clinical development through launch and real world assessment, therapeutic expertise is key to ensuring we are asking the right questions.
- What is the real-world impact of innovation in treatments such as cell and gene therapies, and how does it differ by patient group?
- What is the right way to navigate reimbursement requirements, globally, and how do the strategies change based on therapeutic area?
- What is the potential for machine learning in finding patients, and how can we ensure the right assumptions and the right context is used to build the best algorithms?
- How can we be more proactive, more precise, and even more creative, in figuring out what works, what doesn’t, and why?

Flexible solutions for emerging biopharma companies
Wherever you are on your development journey, you need a partner who knows what comes next. Whose deep understanding of your therapeutic area means knowing what challenges lie ahead, and what it takes to succeed.
Making Human Data Science work for you
Building a predictive algorithm that can find Alzheimer's patients earlier, before they are even patients, doesn't just take data science. It requires deep domain expertise in the epidemiology, progression and experience of the disease to know what is clinically relevant. It takes unparalleled non-identified data, from a range of sources beyond EMRs. It takes analytics that can create the right composites and a well-taught machine learning system that will only get better. This is more than data science. This is Human Data Science.
As the leader in Human Data Science, IQVIA is at the forefront of integrating human science expertise with advances in analytics and technology to help customers ask better questions, and extract more meaningful insights.
Because to move healthcare forward, we must be able to respond to and reflect on the challenges, questions and concerns that are as diverse and as complex as the diseases themselves.
New thinking, new evidence driving therapeutic innovation
Strengthening pathways for cell and gene therapies: current state and future scenarios.
This report from the IQVIA Institute reflects on the current state of the end-to-end environment for cell and gene therapies
Global Trends in R&D 2024: Activity, productivity, and enablers
Annual trend report from the IQVIA Institute for Human Data Science
Changing of the guard: Immunology at an inflection point
Rewriting the code: gene-editing therapeutics, piping hot: a look at the state of cell, gene and rna therapies in early 2023, global oncology trends 2022.
Outlook to 2026
Assessing the Global Burden of Post-COVID-19 Conditions
Modeling the incidence of post-COVID conditions worldwide based on real-word evidence (RWE) and clinical literature review
Digital Health Trends 2021
Innovation, evidence, regulation, and adoption
The Use of Medicines in the U.S.
Spending and Usage Trends and Outlook to 2025
Global Medicine Spending and Usage Trends: Outlook to 2025
Immuno-oncology clinical development.
Moving novel therapies forward
Understanding Neuromuscular Disease Care
Related solutions.
Assess your protocol against real world evidence using advanced analytics, and reduce the risk of costly amendments.
Tap into evidence networks in oncology, neurology, immunology, and other therapy areas to enrich your studies.
Discover unrealized connections and manage your product across the entire lifecycle, from research and development, compliance to launch and commercialization.
Explore More
Cell & Gene Therapy
Allergy & Respiratory
For this browsing session please remember my choice and don't ask again.
Therapeutic Expertise - Embedded in Your Trial
Get early and ongoing insight from our collaborative medical team, spanning a broad range of therapeutic and crossover areas.
Helping You Push the Boundaries of Clinical Research
Biotech innovation is transforming the world. In parallel, clinical development is increasingly more complex and challenging–from scientific, operational, and regulatory perspectives. As drug and medical device development continues to become more complex and blurred across therapeutics areas—with advancements in immuno-therapies, cell and gene therapies, and microbiome therapeutics to name a few—our foundations in science, operations, and regulatory can be applied across:
- Core therapeutic areas and diseases
- Rare diseases and orphan indications
- Leading technologies and platforms such as cell and gene therapies

Therapeutic Depth
To give you the best possible experience, this site uses cookies and by continuing to use the site you agree that we can save them on your device.
Therapeutic Areas
Every therapeutic area comes with its own set of unique challenges and opportunities. At Biotrial we are committed to helping our partners develop innovative and effective therapies for a wide range of diseases and conditions. Our expertise lies in assisting sponsors across all stages of development, from early-stage proof-of-concept studies to late-stage pivotal trials.

With an established pedigree in Neuroscience, Biotrial provides translational solutions that closely link non-clinical and clinical development using in-house data validation and back translation. Biotrial’s excellence is driven by our team of in house and external neurologists’ experts as well as project management teams with significant expertise in Neuroscience trials. We offer an end-to-end solution for your Early Phase needs or bespoke services according to your needs.
We understand that the development of Neuroscience compounds is a complex and challenging process. That’s why we work closely with our sponsors to develop a tailored clinical development plan that meets their specific needs and goals. We offer a wide range of services, including:
- Preclinical services
- Clinical Development
- Biometrics services
- Core Lab services
- Bioanalysis services
We are committed to revolutionizing Oncology development by collaborating closely with a network of specialized researchers and clinicians. Our approach is tailored to the unique demands of the field, and we are dedicated to guiding our sponsors through every step of the process, from Non-clinical research, in-vitro and in vivo studies, to the initiation of Early Phase patient trials.
- In-vitro and in vivo studies
Our in-vitro and in vivo studies team uses a variety of state-of-the-art techniques to assess the safety and efficacy of new cancer therapies in a controlled setting. We also offer services to support the development of new preclinical models for cancer research.
- Early Phase patient trials
We work closely with our sponsors to develop trials that are tailored to their specific needs and that meet the highest ethical and regulatory standards.
- Customized packaged services
Each sponsor has distinct needs, so we offer a variety of customized packaged services to support our sponsors’ Oncology development needs. These services can be tailored to meet the specific requirements of each sponsor, and can include a combination of our Non-clinical research, in-vitro and in vivo studies, and Early Phase patient trials services.
At Biotrial, we offer a comprehensive end-to-end solution for your cardiology compound development, from preclinical to early clinical. Our experienced team of non-clinical and clinical experts is highly skilled in the scientific methodology and evolving regulatory requirements, ensuring that your studies are conducted to the highest standards and that your regulatory application is well-supported.
We understand that the development of cardiology compounds is a complex and challenging process. That’s why we work closely with our sponsors to develop a tailored clinical development plan that meets their specific needs and goals. We offer a wide range of services, including:
At Biotrial, we recognize that conducting clinical research studies for rare diseases presents unique challenges in terms of project management, site selection, and patient enrollment and retention. Given that children represent 50% of the rare disease population, the regulatory requirements specific to this age group represent an additional challenge that is mastered by our team of experts. We understand the urgent need to provide treatment options for patients living with rare diseases, and our full-service CRO offers innovative solutions to meet these challenges. Our services include:
- Physicians with pediatric experience who understand the complexities involved in rare disease study design and management.
- Experienced project team familiar with the challenges of rare disease and pediatrics clinical trials
- Customized use of patient innovation tools and tactics to enhance the patient and caregiver journey and improve study participation and retention.
- Data-driven insights and innovation applied throughout the study design and implementation process to ensure the highest quality data and results.
- Integration of evolving global regulatory landscapes to ensure compliance with all applicable regulations and requirements.
Non-Alcoholic Fatty Liver Disease (NAFLD) and non-alcoholic steatohepatitis (NASH)
Biotrial provides comprehensive solutions for Preclinical and Clinical studies. Our team of gastroenterologists has extensive experience in designing, planning, and executing clinical trials for a wide range of gastrointestinal (GI) disorders. We have established relationships with Key Opinion Leaders (KOLs) and sites ensuring access to specific patient population. Our medical expertise coupled with global regulatory knowledge and operational excellence allow Biotrial to deliver custom solutions for our clients for a broad spectrum of GI indications.
At Biotrial, we are committed to providing high-quality and comprehensive services adapted to the unique needs of each gastroenterology trial.
Gastroenterology encompasses many diseases and conditions, and our large experience includes constipation, diarrhea, gastroparesis, dyspepsia, Inflammatory Bowel Disease (IBD; Crohn’s disease, ulcerative colitis), antibiotic-induced dysbiosis, eosinophilic esophagitis, gastroesophageal reflux, irritable bowel syndrome (IBS), pancreatitis, short bowel syndrome, and more.
Biotrial has extensive experience and offers targeted solutions to support the development of programs that address high unmet needs across immunologic disorders. Sponsors face challenges when developing new medicines in a crowded and dynamic marketplace, and Biotrial guides them every step of the way to deliver clear and high-quality trial outcomes. What sets us apart is:
- A proactive approach to developing strategies that address evolving needs, such as diversity requirements in programs that meet FDA and EMA protocols.
- An understanding of the importance of data quality in immunology clinical trials, recognizing the need for high-quality data to produce robust clinical trial outcomes.
- Team of therapeutic experts and strong relationships with Key Opinion Leaders (KOLs) and sites ensuring access to specific patient population.
- A comprehensive expertise and experience in immunology clinical trials across different therapeutic areas, such as gout, rheumatoid arthritis, psoriasis, osteoarthritis, systemic sclerosis, lupus, and more.
Conducting metabolic studies can be a challenge due to difficulties in patient recruitment and ensuring study efficiency. It is critical to devote rigorous attention to regulatory strategies and safety data. At Biotrial, we have extensive experience conducting endocrine and metabolic clinical trials.
Our endocrine expertise spans: Type 1 and Type 2 diabetes, Dyslipidemia, Obesity, Metabolic syndrome, growth hormone disorders, and more.
At Biotrial, we have a wealth of expertise in bacterial, viral and fungal infectious diseases clinical trials. Our team includes medical, regulatory, operational, and laboratory experts who have extensive experience in infectious disease clinical research. What sets us apart is:
- Our broad access to patient populations , allows us to recruit diverse and representative study cohorts.
- Our team of health professionals provides expert care and oversight throughout the clinical trial process.
- Our global network of research sites , decentralized community outreach, outpatient clinics, hospitals, and critical-care specialty centers, which enables us to conduct studies in diverse settings and populations.
- Our data team ensures that the data collected is accurate, complete, and of the highest quality.
Biotrial possesses extensive knowledge and understanding of the regulatory drivers, treatment landscape, unmet needs, and recruitment strategies across a wide range of women’s health indications. We bring our clients significant experience in key areas of women’s health, including endometriosis, menopause-related disorders, contraceptives, uterine fibroids. Our dedication lies in providing:
- Clinical subject matter experts given that multiple disciplines intersect with women’s health clinical research.
- A team of experts with a wealth of expertise in women’s health development program and clinical trial operations.
- Effective site and patient solutions to overcome potential challenges in conducting women’s health studies.
- Prioritizing the patient’s well-being by utilizing targeted training.
Related Information
Participate in a clinical trial.

Clario accelerates your clinical trial from initiation to implementation. LEARN MORE →
- Home Therapeutic Areas
Therapeutic Areas
You need a partner with advanced technologies to power your clinical trials. you want a clinical trial research company with deep therapeutic expertise. with clario, you get both..

The complexity of today’s clinical therapeutic studies, and drug trials demands deep scientific, regulatory and therapeutic area expertise to help guide and support your research. From protocol design to regulatory submission, you need a clinical research company with a thorough understanding of the specific indication, the endpoints to target within that indication and the tools and technologies necessary to collect and harmonize endpoint data efficiently and securely.
Clario’s expertise extends across all major therapeutic areas, including cardiology, oncology, neuroscience, respiratory, musculoskeletal and gastroenterological indications. Our clinical research company pairs this deep expertise with our broad experience in protocol design, site management and patient support capabilities to provide you with powerful solutions to your study challenges.
Focus areas
Therapeutic Areas by Disease Area
Therapeutic Area User Guides (TAUGs) extend the Foundational Standards to represent data that pertains to specific disease areas. TAUGs include disease-specific metadata, examples and guidance on implementing CDISC standards for a variety of uses, including global regulatory submissions.
Cardiovascular
Gastrointestinal, mental health, rare diseases, respiratory.
Therapeutic Areas
Rare disease / orphan disease.
CTI is a leader in rare disease and orphan disease research with more than 3/4 of CTI’s active prospective studies in these therapeutic areas
Regenerative Medicine / Gene Therapy
With more the 50% of CTI's active prospective studies involving regenerative medicine, including contributing to the development program of several of the world's first approved gene therapy products, the company is a leader in the field.
With dozens of marketing approvals, we can support product development in immunology.
Transplantation
CTI is a leader in this field with contributions to nearly all transplant-related therapies on the market today. Approximately 50% of our active prospective studies involves transplantation, and of these, nearly half involve regenerative medicine.
With 20 years of experience in this area of research, we specialize in the unique challenges associated with nephrology populations.
Hematology / Oncology
CTI has unparalleled experience in working with complex and critically ill patients.
Neurology / Sleep Research
CTI has a long history of working in neurology including rare/orphan diseases, central nervous system (CNS) disorders, psychiatry, and sleep research. We've contributed to the development programs of 17 sleep focused drug approvals.
Infectious Diseases
Our experience in this area is specific to complex immunocompromised patient populations susceptible to opportunistic infections.
We specialize in the unique challenges associated with hepatic populations, from mild to moderate liver disease to end stage acute and chronic liver disease.
Cardiopulmonary
CTI’s expertise includes regulatory and clinical operations personnel who understand the intricate and interdependent characteristics of cardiopulmonary research.
Pediatric / Neonate Populations
The CTI team has diverse experience in pediatric and neonate populations in both clinical study management and regulatory consulting across a wide array of indications.
CTI has significant experience in device and in vitro diagnostic (IVD) development across a multiple therapeutic areas.

CTI Clinical Trial & Consulting Global Headquarters
Where life-changing therapies turn first®.
CTI is an innovative, international drug and device development organization that delivers a full spectrum of clinical trial and consulting services from bench to commercialization.
© 2020 CTI Clinical Trial and Consulting Services All rights reserved.
Privacy Policy

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 15 April 2024
Targeting aging and age-related diseases with vaccines
- Ruochen Wu ORCID: orcid.org/0009-0007-3379-8134 1 , 2 , 3 na1 ,
- Fei Sun 4 na1 ,
- Weiqi Zhang ORCID: orcid.org/0000-0002-8885-5104 3 , 5 , 6 , 7 , 8 ,
- Jie Ren ORCID: orcid.org/0000-0002-6450-3163 3 , 5 , 6 , 7 , 8 , 9 &
- Guang-Hui Liu ORCID: orcid.org/0000-0001-9289-8177 1 , 2 , 3 , 5 , 8 , 10 , 11 , 12
Nature Aging ( 2024 ) Cite this article
91 Accesses
24 Altmetric
Metrics details
Aging is a major risk factor for numerous chronic diseases. Vaccination offers a promising strategy to combat these age-related diseases by targeting specific antigens and inducing immune responses. Here, we provide a comprehensive overview of recent advances in vaccine-based interventions targeting these diseases, including Alzheimer’s disease, type II diabetes, hypertension, abdominal aortic aneurysm, atherosclerosis, osteoarthritis, fibrosis and cancer, summarizing current approaches for identifying disease-associated antigens and inducing immune responses against these targets. Further, we reflect on the recent development of vaccines targeting senescent cells, as a strategy for more broadly targeting underlying causes of aging and associated pathologies. In addition to highlighting recent progress in these areas, we discuss important next steps to advance the therapeutic potential of these vaccines, including improving and robustly demonstrating efficacy in human clinical trials, as well as rigorously evaluating the safety and long-term effects of these vaccine strategies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
111,21 € per year
only 9,27 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
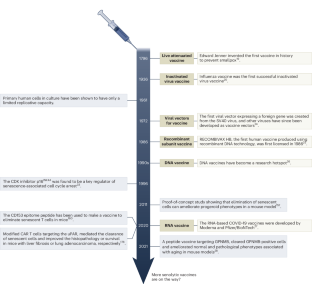
Similar content being viewed by others
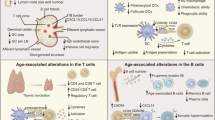
Insights into vaccines for elderly individuals: from the impacts of immunosenescence to delivery strategies
Yingying Hou, Min Chen, … Rongsheng Tong

Senolytic vaccination improves normal and pathological age-related phenotypes and increases lifespan in progeroid mice
Masayoshi Suda, Ippei Shimizu, … Tohru Minamino
mRNA booster vaccination protects aged mice against the SARS-CoV-2 Omicron variant
Etsuro Nanishi, Marisa E. McGrath, … David J. Dowling
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: an expanding universe. Cell 186 , 243–278 (2023).
Article CAS PubMed Google Scholar
Benz, C. C. & Yau, C. Ageing, oxidative stress and cancer: paradigms in parallax. Nat. Rev. Cancer 8 , 875–879 (2008).
Article CAS PubMed PubMed Central Google Scholar
Wu, Z., Qu, J., Zhang, W. & Liu, G. H. Stress, epigenetics, and aging: unraveling the intricate crosstalk. Mol. Cell 84 , 34–54 (2024).
Cai, Y. et al. The landscape of aging. Sci. China Life Sci. 65 , 2354–2454 (2022).
Article PubMed PubMed Central Google Scholar
Dehghan, A. et al. Risk of type 2 diabetes attributable to C-reactive protein and other risk factors. Diabetes Care 30 , 2695–2699 (2007).
Bussian, T. J. et al. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 562 , 578–582 (2018).
Yan, H. et al. Degeneration Directory: a multi-omics web resource for degenerative diseases. Protein Cell https://doi.org/10.1093/procel/pwad066 (2023).
Katz, D. L. & Meller, S. Can we say what diet is best for health? Annu. Rev. Public Health 35 , 83–103 (2014).
Newman, J. C. et al. Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell Metab. 26 , 547–557 (2017).
Hojman, P., Gehl, J., Christensen, J. F. & Pedersen, B. K. Molecular mechanisms linking exercise to cancer prevention and treatment. Cell Metab. 27 , 10–21 (2018).
Harrison, D. E. et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460 , 392–395 (2009).
Vaiserman, A., De Falco, E., Koliada, A., Maslova, O. & Balistreri, C. R. Anti-ageing gene therapy: not so far away? Ageing Res. Rev. 56 , 100977 (2019).
Article PubMed Google Scholar
Yan, P. et al. FOXO3-engineered human ESC-derived vascular cells promote vascular protection and regeneration. Cell Stem Cell 24 , 447–461 (2019).
Liu, F. et al. Identification of FOXO1 as a geroprotector in human synovium through single-nucleus transcriptomic profiling. Protein Cell https://doi.org/10.1093/procel/pwad060 (2023).
Huang, D. et al. CRL2 APPBP2 -mediated TSPYL2 degradation counteracts human mesenchymal stem cell senescence. Sci. China Life Sci. https://doi.org/10.1007/s11427-023-2451-3 (2023).
Jing, Y. et al. Single-nucleus profiling unveils a geroprotective role of the FOXO3 in primate skeletal muscle aging. Protein Cell 14 , 497–512 (2023).
PubMed Google Scholar
Yusheng Cai, Z. J., Si Wang. et al. Genetic enhancement: an avenue to combat aging-related diseases. Life Med. https://doi.org/10.1093/lifemedi/lnac054 (2022).
Rudin, C. M. et al. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin. Cancer Res. 18 , 3163–3169 (2012).
Riedel, S. Edward Jenner and the history of smallpox and vaccination. Proc. Bayl Univ. Med. Cent. 18 , 21–25 (2005).
Thomas, F. Jr & Magill, T. Vaccination of human subjects with virus of human influenza. Proc. Soc. Exp. Biol. Med. 33 , 604–606 (1936).
Article Google Scholar
Liu, J. et al. Cancer vaccines as promising immuno-therapeutics: platforms and current progress. J. Hematol. Oncol. 15 , 28 (2022).
Pulendran, B. & Ahmed, R. Immunological mechanisms of vaccination. Nat. Immunol. 12 , 509–517 (2011).
Pollard, A. J. & Bijker, E. M. A guide to vaccinology: from basic principles to new developments. Nat. Rev. Immunol. 21 , 83–100 (2021).
Jackson, D. A., Symons, R. H. & Berg, P. Biochemical method for inserting new genetic information into DNA of Simian Virus 40: circular SV40 DNA molecules containing lambda phage genes and the galactose operon of Escherichia coli . Proc. Natl Acad. Sci. USA 69 , 2904–2909 (1972).
McAleer, W. J. et al. Human hepatitis B vaccine from recombinant yeast. Nature 307 , 178–180 (1984).
Kutzler, M. A. & Weiner, D. B. DNA vaccines: ready for prime time? Nat. Rev. Genet. 9 , 776–788 (2008).
Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 383 , 2603–2615 (2020).
Suda, M. et al. Senolytic vaccination improves normal and pathological age-related phenotypes and increases lifespan in progeroid mice. Nat. Aging 1 , 1117–1126 (2021).
Aging Biomarker, C. et al. Biomarkers of aging. Sci. China Life Sci. 66 , 893–1066 (2023).
Aging Biomarker Consortium; Suo, J. et al. A framework of biomarkers for skeletal aging: a consensus statement by the Aging Biomarker Consortium. Life Med. https://doi.org/10.1093/lifemedi/lnad045 (2023).
Aging Biomarker Consortium; Zhang, L. et al. A framework of biomarkers for vascular aging: a consensus statement by the Aging Biomarker Consortium. Life Med. https://doi.org/10.1093/lifemedi/lnad033 (2023).
Aging Biomarker Consortium et al. A biomarker framework for cardiac aging: the Aging Biomarker Consortium consensus statement. Life Med. https://doi.org/10.1093/lifemedi/lnad035 (2023).
Alcorta, D. A. et al. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc. Natl Acad. Sci. USA 93 , 13742–13747 (1996).
Lu, H. et al. Aging hallmarks of the primate ovary revealed by spatiotemporal transcriptomics. Protein Cell https://doi.org/10.1093/procel/pwad063 (2023).
Boni-Schnetzler, M. et al. Increased interleukin (IL)-1beta messenger ribonucleic acid expression in beta -cells of individuals with type 2 diabetes and regulation of IL-1beta in human islets by glucose and autostimulation. J. Clin. Endocrinol. Metab. 93 , 4065–4074 (2008).
Valiukas, Z. et al. Immunotherapies for Alzheimer’s disease—a review. Vaccines https://doi.org/10.3390/vaccines10091527 (2022).
Pecchi, E. et al. Induction of nerve growth factor expression and release by mechanical and inflammatory stimuli in chondrocytes: possible involvement in osteoarthritis pain. Arthritis Res. Ther. 16 , R16 (2014).
Xia, X., Jiang, Q., McDermott, J. & Han, J. J. Aging and Alzheimer’s disease: Comparison and associations from molecular to system level. Aging Cell 17 , e12802 (2018).
McDade, E., Llibre-Guerra, J. J., Holtzman, D. M., Morris, J. C. & Bateman, R. J. The informed road map to prevention of Alzheimer disease: a call to arms. Mol. Neurodegener. 16 , 49 (2021).
Broussard, G. J., Mytar, J., Li, R. C. & Klapstein, G. J. The role of inflammatory processes in Alzheimer’s disease. Inflammopharmacology 20 , 109–126 (2012).
Zhang, Y., Chen, H., Li, R., Sterling, K. & Song, W. Amyloid beta-based therapy for Alzheimer’s disease: challenges, successes and future. Signal Transduct. Target. Ther. 8 , 248 (2023).
Ossenkoppele, R., van der Kant, R. & Hansson, O. Tau biomarkers in Alzheimer’s disease: towards implementation in clinical practice and trials. Lancet Neurol. 21 , 726–734 (2022).
Yamazaki, Y., Zhao, N., Caulfield, T. R., Liu, C. C. & Bu, G. Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nat. Rev. Neurol. 15 , 501–518 (2019).
Depp, C. et al. Myelin dysfunction drives amyloid-beta deposition in models of Alzheimer’s disease. Nature 618 , 349–357 (2023).
Gilman, S. et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology 64 , 1553–1562 (2005).
Reiss, A. B. et al. Alzheimer disease clinical trials targeting amyloid: lessons learned from success in mice and failure in humans. Neurologist 26 , 52–61 (2021).
Ketter, N. et al. A randomized, double-blind, phase 2 study of the effects of the vaccine vanutide cridificar with QS-21 adjuvant on immunogenicity, safety and amyloid imaging in patients with mild to moderate alzheimer’s disease. J. Prev. Alzheimers Dis. 3 , 192–201 (2016).
CAS PubMed Google Scholar
Plascencia-Villa, G. & Perry, G. Lessons from antiamyloid-beta immunotherapies in Alzheimer’s disease. Handb. Clin. Neurol. 193 , 267–292 (2023).
Hardy, J. & Allsop, D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol. Sci. 12 , 383–388 (1991).
Muhs, A. et al. Liposomal vaccines with conformation-specific amyloid peptide antigens define immune response and efficacy in APP transgenic mice. Proc. Natl Acad. Sci. USA 104 , 9810–9815 (2007).
Hickman, D. T. et al. Sequence-independent control of peptide conformation in liposomal vaccines for targeting protein misfolding diseases. J. Biol. Chem. 286 , 13966–13976 (2011).
Rafii, M. S. et al. Safety, tolerability, and immunogenicity of the ACI-24 vaccine in adults with Down syndrome: a phase 1b randomized clinical trial. JAMA Neurol. 79 , 565–574, (2022).
Wang, C. Y. et al. UB-311, a novel UBITh amyloid beta peptide vaccine for mild Alzheimer’s disease. Alzheimers Dement. 3 , 262–272 (2017).
Kwan, P., Konno, H., Chan, K. Y. & Baum, L. Rationale for the development of an Alzheimer’s disease vaccine. Hum. Vaccin. Immunother. 16 , 645–653 (2020).
Yu, H. J. et al. Safety, tolerability, immunogenicity, and efficacy of UB-311 in participants with mild Alzheimer’s disease: a randomised, double-blind, placebo-controlled, phase 2a study. EBioMedicine 94 , 104665 (2023).
Petrushina, I. et al. Characterization and preclinical evaluation of the cGMP grade DNA based vaccine, AV-1959D to enter the first-in-human clinical trials. Neurobiol. Dis. 139 , 104823 (2020).
Davtyan, H. et al. The MultiTEP platform-based Alzheimer’s disease epitope vaccine activates a broad repertoire of T helper cells in nonhuman primates. Alzheimers Dement. 10 , 271–283 (2014).
Lacosta, A. M. et al. Safety, tolerability and immunogenicity of an active anti-Abeta(40) vaccine (ABvac40) in patients with Alzheimer’s disease: a randomised, double-blind, placebo-controlled, phase I trial. Alzheimers Res. Ther. 10 , 12 (2018).
Neff, R. A. et al. Molecular subtyping of Alzheimer’s disease using RNA sequencing data reveals novel mechanisms and targets. Sci. Adv. https://doi.org/10.1126/sciadv.abb5398 (2021).
Theunis, C. et al. Efficacy and safety of a liposome-based vaccine against protein Tau, assessed in tau.P301L mice that model tauopathy. PLoS ONE 8 , e72301 (2013).
Song, C. et al. Immunotherapy for Alzheimer’s disease: targeting beta-amyloid and beyond. Transl. Neurodegener. 11 , 18 (2022).
Novak, P. et al. FUNDAMANT: an interventional 72-week phase 1 follow-up study of AADvac1, an active immunotherapy against tau protein pathology in Alzheimer’s disease. Alzheimers Res. Ther. 10 , 108 (2018).
Panza, F. & Logroscino, G. Anti-tau vaccine in Alzheimer’s disease: a tentative step. Lancet Neurol. 16 , 99–100 (2017).
Novak, P. et al. ADAMANT: a placebo-controlled randomized phase 2 study of AADvac1, an active immunotherapy against pathological tau in Alzheimer’s disease. Nat. Aging 1 , 521–534 (2021).
Sato, C. et al. Tau kinetics in neurons and the human central nervous system. Neuron 97 , 1284–1298 (2018).
Park, H. H. et al. Novel vaccine peptide GV1001 effectively blocks beta-amyloid toxicity by mimicking the extra-telomeric functions of human telomerase reverse transcriptase. Neurobiol. Aging 35 , 1255–1274 (2014).
Malonis, R. J., Lai, J. R. & Vergnolle, O. Peptide-based vaccines: current progress and future challenges. Chem. Rev. 120 , 3210–3229 (2020).
Frenkel, D., Maron, R., Burt, D. S. & Weiner, H. L. Nasal vaccination with a proteosome-based adjuvant and glatiramer acetate clears beta-amyloid in a mouse model of Alzheimer disease. J. Clin. Invest. 115 , 2423–2433 (2005).
Rafii, M. S. & Aisen, P. S. Detection and treatment of Alzheimer’s disease in its preclinical stage. Nat. Aging 3 , 520–531 (2023).
Baruch, K. et al. PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer’s disease. Nat. Med. 22 , 135–137 (2016).
Ogurtsova, K. et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 128 , 40–50 (2017).
DeFronzo, R. A. et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Prim. 1 , 15019 (2015).
Gallwitz, B. Clinical perspectives on the use of the GIP/GLP-1 receptor agonist tirzepatide for the treatment of type-2 diabetes and obesity. Front. Endocrinol. https://doi.org/10.3389/fendo.2022.1004044 (2022).
Vogt, A. S. et al. Anti-IAPP monoclonal antibody improves clinical symptoms in a mouse model of type 2 diabetes. Vaccines https://doi.org/10.3390/vaccines9111316 (2021).
Scarpa, E. S. et al. The combination of natural molecules naringenin, hesperetin, curcumin, polydatin and quercetin synergistically decreases sema3e expression levels and DPPIV activity in in vitro models of insulin resistance. Int. J. Mol. Sci. https://doi.org/10.3390/ijms24098071 (2023).
Voelker, J. et al. Anti-TGF-beta1 antibody therapy in patients with diabetic nephropathy. J. Am. Soc. Nephrol. 28 , 953–962 (2017).
Galicia-Garcia, U. et al. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci . https://doi.org/10.3390/ijms21176275 (2020).
Sandhu, H. et al. Glucagon-like peptide 1 increases insulin sensitivity in depancreatized dogs. Diabetes 48 , 1045–1053 (1999).
Pang, Z. et al. Therapeutic vaccine against DPP4 improves glucose metabolism in mice. Proc. Natl Acad. Sci. USA 111 , E1256–E1263 (2014).
Dinarello, C. A., Simon, A. & van der Meer, J. W. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 11 , 633–652 (2012).
Malozowski, S. & Sahlroot, J. T. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 357 , 302–303 (2007).
Cavelti-Weder, C. et al. Development of an interleukin-1beta vaccine in patients with type 2 diabetes. Mol. Ther. 24 , 1003–1012 (2016).
Dhimolea, E. Canakinumab. MAbs 2 , 3–13 (2010).
Zhang, Y. et al. Therapeutic vaccine against IL-1beta improved glucose control in a mouse model of type 2 diabetes. Life Sci. 192 , 68–74 (2018).
Ekoru, K. et al. Type 2 diabetes complications and comorbidity in sub-Saharan Africans. EClinicalMedicine 16 , 30–41 (2019).
Danser, A. H. & Deinum, J. Renin, prorenin and the putative (pro)renin receptor. Hypertension 46 , 1069–1076 (2005).
Kanda, A. & Ishida, S. Prorenin receptor: involvement in diabetic retinopathy and development of molecular targeted therapy. J. Diabetes Investig. 10 , 6–17 (2019).
Yokota, H. et al. Effect of prorenin peptide vaccine on the early phase of diabetic retinopathy in a murine model of type 2 diabetes. PLoS ONE 17 , e0262568 (2022).
Abdul-Ghani, M. A. & DeFronzo, R. A. Pathogenesis of insulin resistance in skeletal muscle. J. Biomed. Biotechnol. 2010 , 476279 (2010).
Shimizu, I. et al. Semaphorin3E-induced inflammation contributes to insulin resistance in dietary obesity. Cell Metab. 18 , 491–504 (2013).
Garay-Gutierrez, N. F., Hernandez-Fuentes, C. P., Garcia-Rivas, G., Lavandero, S. & Guerrero-Beltran, C. E. Vaccines against components of the renin–angiotensin system. Heart Fail. Rev. 26 , 711–726 (2021).
Downham, M. R. et al. Evaluation of two carrier protein-angiotensin I conjugate vaccines to assess their future potential to control high blood pressure (hypertension) in man. Br. J. Clin. Pharm. 56 , 505–512 (2003).
Article CAS Google Scholar
Ambuhl, P. M. et al. A vaccine for hypertension based on virus-like particles: preclinical efficacy and phase I safety and immunogenicity. J. Hypertens. 25 , 63–72 (2007).
Tissot, A. C. et al. Effect of immunisation against angiotensin II with CYT006-AngQb on ambulatory blood pressure: a double-blind, randomised, placebo-controlled phase IIa study. Lancet 371 , 821–827 (2008).
Brown, M. J. Success and failure of vaccines against renin–angiotensin system components. Nat. Rev. Cardiol. 6 , 639–647 (2009).
Chen, X. et al. Effectiveness and safety of a therapeutic vaccine against angiotensin II receptor type 1 in hypertensive animals. Hypertension 61 , 408–416 (2013).
Zhou, Y. et al. ATRQbeta-001 vaccine prevents atherosclerosis in apolipoprotein E-null mice. J. Hypertens. 34 , 474–485 (2016).
Pan, Y. et al. The ATRQbeta-001 vaccine improves cardiac function and prevents postinfarction cardiac remodeling in mice. Hypertens. Res. 42 , 329–340 (2019).
Ferrario, C. M. Importance of the renin-angiotensin-aldosterone system (RAS) in the physiology and pathology of hypertension. An overview. Drugs 39 , 1–8 (1990).
Li, C. et al. Vaccine targeted alpha 1D-adrenergic receptor for hypertension. Hypertension 74 , 1551–1562 (2019).
Tanoue, A. et al. The alpha 1D-adrenergic receptor directly regulates arterial blood pressure via vasoconstriction. J. Clin. Invest. 109 , 765–775 (2002).
Docherty, J. R. Subtypes of functional alpha1-adrenoceptor. Cell. Mol. Life Sci. 67 , 405–417 (2010).
Nordon, I. M., Hinchliffe, R. J., Loftus, I. M. & Thompson, M. M. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat. Rev. Cardiol. 8 , 92–102 (2011).
Greenhalgh, R. M. et al. Early elective open surgical repair of small abdominal aortic aneurysms is not recommended: results of the UK Small Aneurysm Trial. Steering Committee. Eur. J. Vasc. Endovasc. Surg. 16 , 462–464 (1998).
Kurashiki, T., Miyake, T., Nakagami, H., Nishimura, M. & Morishita, R. Prevention of progression of aortic aneurysm by peptide vaccine against Ang II (angiotensin II) in a rat model. Hypertension 76 , 1879–1888 (2020).
Xuan, H. et al. Inhibition or deletion of angiotensin II type 1 receptor suppresses elastase-induced experimental abdominal aortic aneurysms. J. Vasc. Surg. 67 , 573–584 (2018).
Zhang, H. et al. ATRQbeta-001 vaccine prevents experimental abdominal aortic aneurysms. J. Am. Heart Assoc. 8 , e012341 (2019).
Wang, J. C. & Bennett, M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ. Res. 111 , 245–259 (2012).
Kobiyama, K. & Ley, K. Atherosclerosis. Circ. Res. 123 , 1118–1120 (2018).
Hansson, G. K. & Nilsson, J. Developing a vaccine against atherosclerosis. Nat. Rev. Cardiol. 17 , 451–452 (2020).
Ait-Oufella, H. et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat. Med. 12 , 178–180 (2006).
Gistera, A. et al. Low-density lipoprotein-reactive T cells regulate plasma cholesterol levels and development of atherosclerosis in humanized hypercholesterolemic mice. Circulation 138 , 2513–2526 (2018).
Lichtman, A. H., Binder, C. J., Tsimikas, S. & Witztum, J. L. Adaptive immunity in atherogenesis: new insights and therapeutic approaches. J. Clin. Invest. 123 , 27–36 (2013).
Kimura, T. et al. Regulatory CD4 + T cells recognize major histocompatibility complex class II molecule-restricted peptide epitopes of apolipoprotein B. Circulation 138 , 1130–1143 (2018).
Momtazi-Borojeni, A. A., Jaafari, M. R., Badiee, A. & Sahebkar, A. Long-term generation of antiPCSK9 antibody using a nanoliposome-based vaccine delivery system. Atherosclerosis 283 , 69–78 (2019).
Ma, Z. et al. Peptide vaccine against ADAMTS-7 ameliorates atherosclerosis and postinjury neointima hyperplasia. Circulation 147 , 728–742 (2023).
Bourinbaiar, A. S. & Jirathitikal, V. Effect of oral immunization with pooled antigens derived from adipose tissue on atherosclerosis and obesity indices. Vaccine 28 , 2763–2768 (2010).
Bourinbaiar, A. S. & Jirathitikal, V. Safety and efficacy trial of adipose-tissue derived oral preparation V-6 Immunitor (V-6): results of open-label, two-month, follow-up study. Lipids Health Dis. 9 , 14 (2010).
Nilsson, J. & Hansson, G. K. Vaccination strategies and immune modulation of atherosclerosis. Circ. Res. 126 , 1281–1296 (2020).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377 , 1119–1131 (2017).
Loeser, R. F. The role of aging in the development of osteoarthritis. Trans. Am. Clin. Climatol. Assoc. 128 , 44–54 (2017).
PubMed PubMed Central Google Scholar
Shane Anderson, A. & Loeser, R. F. Why is osteoarthritis an age-related disease? Best. Pract. Res Clin. Rheumatol. 24 , 15–26 (2010).
von Loga, I. S. et al. Active immunisation targeting nerve growth factor attenuates chronic pain behaviour in murine osteoarthritis. Ann. Rheum. Dis. 78 , 672–675 (2019).
Lane, N. E. & Corr, M. Osteoarthritis in 2016: anti-NGF treatments for pain—two steps forward, one step back? Nat. Rev. Rheumatol. 13 , 76–78 (2017).
Chen, Y. et al. Aging reprograms the hematopoietic-vascular niche to impede regeneration and promote fibrosis. Cell Metab. 33 , 395–410 (2021).
Brack, A. S. et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317 , 807–810 (2007).
Sobecki, M. et al. Vaccination-based immunotherapy to target profibrotic cells in liver and lung. Cell Stem Cell 29 , 1459–1474 (2022).
Kang, H. M. et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 21 , 37–46 (2015).
Wu, D. et al. Vaccine against PCSK9 improved renal fibrosis by regulating fatty acid beta-oxidation. J. Am. Heart Assoc. 9 , e014358 (2020).
Melero, I. et al. Therapeutic vaccines for cancer: an overview of clinical trials. Nat. Rev. Clin. Oncol. 11 , 509–524 (2014).
Markowitz, L. E. et al. Human papillomavirus vaccine introduction—the first five years. Vaccine 30 , 139–148, (2012).
Crews, D. W., Dombroski, J. A. & King, M. R. Prophylactic cancer vaccines engineered to elicit specific adaptive immune response. Front. Oncol. 11 , 626463 (2021).
Karpanen, T. & Olweus, J. The potential of donor T-cell repertoires in neoantigen-targeted cancer immunotherapy. Front. Immunol. 8 , 1718 (2017).
Paston, S. J., Brentville, V. A., Symonds, P. & Durrant, L. G. Cancer vaccines, adjuvants, and delivery systems. Front. Immunol. 12 , 627932 (2021).
Giaccone, G. et al. A phase III study of belagenpumatucel-L, an allogeneic tumour cell vaccine, as maintenance therapy for non-small cell lung cancer. Eur. J. Cancer 51 , 2321–2329 (2015).
Verma, V. et al. PD-1 blockade in subprimed CD8 cells induces dysfunctional PD-1 + CD38 hi cells and anti-PD-1 resistance. Nat. Immunol. 20 , 1231–1243 (2019).
Saxena, M., van der Burg, S. H., Melief, C. J. M. & Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 21 , 360–378 (2021).
Lazaro, A. et al. Human leukocyte antigen (HLA) typing by DNA sequencing. Methods Mol. Biol. 1034 , 161–195 (2013).
Jennings, L. J. et al. Guidelines for validation of next-generation sequencing-based oncology panels: a joint consensus recommendation of the association for molecular pathology and college of american pathologists. J. Mol. Diagn. 19 , 341–365 (2017).
Duperret, E. K. et al. A Synthetic DNA, multi-neoantigen vaccine drives predominately MHC class I CD8 + T-cell responses, impacting tumor challenge. Cancer Immunol. Res. 7 , 174–182 (2019).
Liu, S. et al. A DNA nanodevice-based vaccine for cancer immunotherapy. Nat. Mater. 20 , 421–430 (2021).
Hammerich, L., Bhardwaj, N., Kohrt, H. E. & Brody, J. D. In situ vaccination for the treatment of cancer. Immunotherapy 8 , 315–330 (2016).
Chen, J. et al. In situ cancer vaccination using lipidoid nanoparticles. Sci. Adv. 7 , eadv.abf1244 (2021).
Chen, L. et al. Bacterial cytoplasmic membranes synergistically enhance the antitumor activity of autologous cancer vaccines. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.abc2816 (2021).
Lopez-Otin, C., Pietrocola, F., Roiz-Valle, D., Galluzzi, L. & Kroemer, G. Meta-hallmarks of aging and cancer. Cell Metab. 35 , 12–35 (2023).
Wells, D. K. et al. Key parameters of tumor epitope immunogenicity revealed through a consortium approach improve neoantigen prediction. Cell 183 , 818–834 (2020).
Liu, X. et al. Resurrection of endogenous retroviruses during aging reinforces senescence. Cell 186 , 287–304 (2023).
Xiaoqian Liu, H. J. et al. Migrasomes trigger innate immune activation and mediate transmission of senescence signals across human cells. Life Med. https://doi.org/10.1093/lifemedi/lnad050 (2023).
Marin, I. et al. Cellular senescence is immunogenic and promotes antitumor immunity. Cancer Discov. 13 , 410–431 (2023).
Sciorati, C. et al. Pharmacological blockade of TNF prevents sarcopenia and prolongs survival in aging mice. Aging 12 , 23497–23508 (2020).
Ridker, P. M. et al. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 390 , 1833–1842 (2017).
Kang, T. W. et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 479 , 547–551 (2011).
Chen, H. A. et al. Senescence rewires microenvironment sensing to facilitate antitumor immunity. Cancer Discov. 13 , 432–453 (2023).
Childs, B. G. et al. Senescent cells: an emerging target for diseases of ageing. Nat. Rev. Drug Discov. 16 , 718–735 (2017).
Childs, B. G. et al. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 354 , 472–477 (2016).
Jeon, O. H. et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 23 , 775–781 (2017).
Baker, D. J. et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479 , 232–236 (2011).
Baker, D. J. et al. Naturally occurring p16 Ink4a -positive cells shorten healthy lifespan. Nature 530 , 184–189 (2016).
Xue, W. et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445 , 656–660 (2007).
Yoshida, S. et al. The CD153 vaccine is a senotherapeutic option for preventing the accumulation of senescent T cells in mice. Nat. Commun. 11 , 2482 (2020).
Sharpless, N. E., Ramsey, M. R., Balasubramanian, P., Castrillon, D. H. & DePinho, R. A. The differential impact of p16 INK4a or p19 ARF deficiency on cell growth and tumorigenesis. Oncogene 23 , 379–385 (2004).
Xu, W. & Larbi, A. Markers of T cell senescence in humans. Int. J. Mol. Sci. https://doi.org/10.3390/ijms18081742 (2017).
Mittelbrunn, M. & Kroemer, G. Hallmarks of T cell aging. Nat. Immunol. 22 , 687–698 (2021).
Shirakawa, K. et al. Obesity accelerates T cell senescence in murine visceral adipose tissue. J. Clin. Invest. 126 , 4626–4639 (2016).
Smith, C. A. et al. CD30 antigen, a marker for Hodgkin’s lymphoma, is a receptor whose ligand defines an emerging family of cytokines with homology to TNF. Cell 73 , 1349–1360 (1993).
Blazar, B. R. et al. CD30/CD30 ligand (CD153) interaction regulates CD4 + T cell-mediated graft-versus-host disease. J. Immunol. 173 , 2933–2941 (2004).
Sato, Y. et al. CD153/CD30 signaling promotes age-dependent tertiary lymphoid tissue expansion and kidney injury. J. Clin. Invest. https://doi.org/10.1172/JCI146071 (2022).
Yazdanbakhsh, M., Kremsner, P. G. & van Ree, R. Allergy, parasites, and the hygiene hypothesis. Science 296 , 490–494 (2002).
Hansen, G., Berry, G., DeKruyff, R. H. & Umetsu, D. T. Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airway hyperreactivity but cause severe airway inflammation. J. Clin. Invest. 103 , 175–183 (1999).
Sallin, M. A. et al. Host resistance to pulmonary Mycobacterium tuberculosis infection requires CD153 expression. Nat. Microbiol. 3 , 1198–1205 (2018).
Vanhoutte, P. M. Endothelial dysfunction and atherosclerosis. Eur. Heart J. 18 , E19–E29 (1997).
Suda, M. et al. Glycoprotein nonmetastatic melanoma protein B regulates lysosomal integrity and lifespan of senescent cells. Sci. Rep. 12 , 6522 (2022).
Ogawa, T. et al. Osteoactivin upregulates expression of MMP-3 and MMP-9 in fibroblasts infiltrated into denervated skeletal muscle in mice. Am. J. Physiol. Cell Physiol. 289 , 697–707 (2005).
Saade, M., Araujo de Souza, G., Scavone, C. & Kinoshita, P. F. The role of GPNMB in inflammation. Front. Immunol. 12 , 674739 (2021).
Demaria, M. et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 31 , 722–733 (2014).
Hernandez-Segura, A. et al. Unmasking transcriptional heterogeneity in senescent cells. Curr. Biol. 27 , 2652–2660 (2017).
Van Damme, H. et al. Therapeutic depletion of CCR8 + tumor-infiltrating regulatory T cells elicits antitumor immunity and synergizes with anti-PD-1 therapy. J. Immunother. Cancer https://doi.org/10.1136/jitc-2020-001749 (2021).
Amor, C. et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 583 , 127–132 (2020).
Amor, C. et al. Prophylactic and long-lasting efficacy of senolytic CAR T cells against age-related metabolic dysfunction. Nat. Aging https://doi.org/10.1038/s43587-023-00560-5 (2024).
Grove, L. M. et al. Urokinase-type plasminogen activator receptor (uPAR) ligation induces a raft-localized integrin signaling switch that mediates the hypermotile phenotype of fibrotic fibroblasts. J. Biol. Chem. 289 , 12791–12804 (2014).
Elberling, C. et al. Urokinase receptor forms in serum from non-small cell lung cancer patients: relation to prognosis. Lung Cancer 74 , 510–515 (2011).
Li, Y. & Cozzi, P. J. Targeting uPA/uPAR in prostate cancer. Cancer Treatment Rev. 33 , 521–527, (2007).
Kiyan, J., Smith, G., Haller, H. & Dumler, I. Urokinase-receptor-mediated phenotypic changes in vascular smooth muscle cells require the involvement of membrane rafts. Biochem. J. 423 , 343–351 (2009).
Bajou, K. et al. Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat. Med. 4 , 923–928 (1998).
Sagiv, A. et al. NKG2D ligands mediate immunosurveillance of senescent cells. Aging 8 , 328–344 (2016).
Yang, D. et al. NKG2D-CAR T cells eliminate senescent cells in aged mice and nonhuman primates. Sci. Transl. Med. 15 , eadd1951 (2023).
Michieletto, D., Lusic, M., Marenduzzo, D. & Orlandini, E. Physical principles of retroviral integration in the human genome. Nat. Commun. 10 , 575 (2019).
Melenhorst, J. J. et al. Decade-long leukaemia remissions with persistence of CD4 + CAR T cells. Nature 602 , 503–509 (2022).
Hasegawa, T. et al. Cytotoxic CD4 + T cells eliminate senescent cells by targeting cytomegalovirus antigen. Cell 186 , 1417–1431 (2023).
Gasek, N. S., Kuchel, G. A., Kirkland, J. L. & Xu, M. Strategies for targeting senescent cells in human disease. Nat. Aging 1 , 870–879 (2021).
Wang, T. W. et al. Blocking PD-L1–PD-1 improves senescence surveillance and ageing phenotypes. Nature 611 , 358–364 (2022).
Lin, M. J. et al. Cancer vaccines: the next immunotherapy frontier. Nat. Cancer 3 , 911–926 (2022).
Kim, K. M. et al. Identification of senescent cell surface targetable protein DPP4. Genes Dev. 31 , 1529–1534 (2017).
Ambrosi, T. H. et al. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell 20 , 771–784 (2017).
Valencia, I. et al. DPP4 promotes human endothelial cell senescence and dysfunction via the PAR2–COX-2–TP axis and NLRP3 inflammasome activation. Hypertension 79 , 1361–1373 (2022).
Tian, X. L. & Li, Y. Endothelial cell senescence and age-related vascular diseases. J. Genet Genomics 41 , 485–495 (2014).
Mund, A. et al. Deep Visual Proteomics defines single-cell identity and heterogeneity. Nat. Biotechnol. 40 , 1231–1240 (2022).
Li, S. et al. Multiregional profiling of the brain transmembrane proteome uncovers novel regulators of depression. Sci. Adv. https://doi.org/10.1126/sciadv.abf0634 (2021).
Schmidt, J. et al. Prediction of neo-epitope immunogenicity reveals TCR recognition determinants and provides insight into immunoediting. Cell Rep. Med. 2 , 100194 (2021).
Sohail, M. S., Ahmed, S. F., Quadeer, A. A. & McKay, M. R. In silico T cell epitope identification for SARS-CoV-2: progress and perspectives. Adv. Drug Deliv. Rev. 171 , 29–47 (2021).
Chiang, E. Y. et al. Targeted depletion of lymphotoxin-alpha-expressing TH1 and TH17 cells inhibits autoimmune disease. Nat. Med. 15 , 766–773 (2009).
O’Connor, R. A. et al. Cutting edge: Th1 cells facilitate the entry of Th17 cells to the central nervous system during experimental autoimmune encephalomyelitis. J. Immunol. 181 , 3750–3754 (2008).
Liu, Z. et al. Immunosenescence: molecular mechanisms and diseases. Signal Transduct. Target. Ther. 8 , 200 (2023).
Goronzy, J. J. & Weyand, C. M. Understanding immunosenescence to improve responses to vaccines. Nat. Immunol. 14 , 428–436 (2013).
Pera, A. et al. Immunosenescence: implications for response to infection and vaccination in older people. Maturitas 82 , 50–55 (2015).
Weinberger, B. Adjuvant strategies to improve vaccination of the elderly population. Curr. Opin. Pharmacol. 41 , 34–41 (2018).
Mannick, J. B. et al. TORC1 inhibition enhances immune function and reduces infections in the elderly. Sci. Transl. Med . https://doi.org/10.1126/scitranslmed.aaq1564 (2018).
Akinc, A. et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol. Ther. 18 , 1357–1364 (2010).
Pardi, N., Hogan, M. J., Porter, F. W. & Weissman, D. mRNA vaccines - a new era in vaccinology. Nat. Rev. Drug Discov. 17 , 261–279 (2018).
Krizhanovsky, V. et al. Senescence of activated stellate cells limits liver fibrosis. Cell 134 , 657–667 (2008).
Meyer, K., Hodwin, B., Ramanujam, D., Engelhardt, S. & Sarikas, A. Essential role for premature senescence of myofibroblasts in myocardial fibrosis. J. Am. Coll. Cardiol. 67 , 2018–2028 (2016).
Ritschka, B. et al. The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes Dev. 31 , 172–183 (2017).
Jun, J. I. & Lau, L. F. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat. Cell Biol. 12 , 676–685 (2010).
Grosse, L. et al. Defined p16 high senescent cell types are indispensable for mouse healthspan. Cell Metab. 32 , 87–99 (2020).
Cai, Y. et al. Decoding aging-dependent regenerative decline across tissues at single-cell resolution. Cell Stem Cell 30 , 1674–1691 (2023).
Cullen, N. C. et al. Efficacy assessment of an active tau immunotherapy in Alzheimer’s disease patients with amyloid and tau pathology: a post hoc analysis of the “ADAMANT” randomised placebo-controlled double-blind multi-centre phase 2 clinical trial. eBioMedicine 99 , 104923 (2024).
Download references
Acknowledgements
We are grateful to Z. Wu for providing critical advice for this Review, and L. Bai for her administrative assistance. This work was supported by the National Natural Science Foundation of China (81921006 to G.-H.L., 92149301 to G.-H.L.), the National Key Research and Development Program of China (2020YFA0804000 to G.-H.L., 2020YFA0803401 to J.R., 2022YFA1103700 to W.Z. and 2019YFA0802202 to J.R.), the National Natural Science Foundation of China (92168201 to G.-H.L., 92049116 to W.Z., 32121001 to W.Z. and J.R., and 31970597 to J.R.), the Strategic Priority Research Program of the Chinese Academy of Science (XDB0570100 to J.R.), the CAS Project for Young Scientists in Basic Research (YSBR-076 to G.-H.L. and J.R., YSBR-012 to W.Z.) and the New Cornerstone Science Foundation through the XPLORER PRIZE (2021-1045 to G.-H.L.). All figures were created with or modified from BioRender.com .
Author information
These authors contributed equally: Ruochen Wu, Fei Sun.
Authors and Affiliations
State Key Laboratory of Membrane Biology, Institute of Zoology, Chinese Academy of Sciences, Beijing, China
Ruochen Wu & Guang-Hui Liu
Key Laboratory of Organ Regeneration and Reconstruction, Institute of Zoology, Chinese Academy of Sciences, Beijing, China
University of Chinese Academy of Sciences, Beijing, China
Ruochen Wu, Weiqi Zhang, Jie Ren & Guang-Hui Liu
Department of Cell Biology, Duke University Medical Center, Durham, NC, USA
Institute for Stem Cell and Regeneration, Chinese Academy of Sciences, Beijing, China
Weiqi Zhang, Jie Ren & Guang-Hui Liu
CAS Key Laboratory of Genomic and Precision Medicine, Beijing Institute of Genomics, Chinese Academy of Sciences, China National Center for Bioinformation, Beijing, China
Weiqi Zhang & Jie Ren
Sino-Danish College, School of Future Technology, University of Chinese Academy of Sciences, Beijing, China
Aging Biomarker Consortium, Beijing, China
Key Laboratory of RNA Science and Engineering, Beijing Institute of Genomics, Chinese Academy of Sciences and China National Center for Bioinformation, Beijing, China
Beijing Institute for Stem Cell and Regenerative Medicine, Beijing, China
Guang-Hui Liu
Advanced Innovation Center for Human Brain Protection, and National Clinical Research Center for Geriatric Disorders, Xuanwu Hospital, Capital Medical University, Beijing, China
Aging Translational Medicine Center, International Center for Aging and Cancer, Xuanwu Hospital, Capital Medical University, Beijing, China
You can also search for this author in PubMed Google Scholar
Contributions
G.-H.L., J.R. and W.Z. designed and supervised the review and reviewed the manuscript; R.W. and F.S. wrote the manuscript and constructed the figures and tables. All the authors read and approved the article.
Corresponding authors
Correspondence to Weiqi Zhang , Jie Ren or Guang-Hui Liu .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Aging thanks Tohru Minamino and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Wu, R., Sun, F., Zhang, W. et al. Targeting aging and age-related diseases with vaccines. Nat Aging (2024). https://doi.org/10.1038/s43587-024-00597-0
Download citation
Received : 28 July 2023
Accepted : 20 February 2024
Published : 15 April 2024
DOI : https://doi.org/10.1038/s43587-024-00597-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Therapeutically focused for greater quality and accuracy.

We focus our therapeutic efforts to create the best possible outcomes and experiences.
Although our individual sites have a wide breadth of experience in virtually every therapeutic indication, Atlas has identified four specific areas of focus where our network’s experience and expertise provide the greatest value to our sponsors and patients, namely our work in internal medicine, the central nervous system (CNS), infectious diseases, and gastrointestinal clinical research.
In support of these therapeutic areas, we provide access to diverse patient populations that target a wide variety of demographics, a network of experienced and knowledgeable healthcare providers and investigators, as well as accurate, reliable, and timely data gathering and reporting capabilities designed to get your treatment to market as swiftly and safely as possible.
Our clinical support team focuses on the following therapeutic areas and indications:

Internal Medicine
- Hypertension
- 24-hour ABPM
- Medical Devices / Screening Tests
- Rheumatology
- Dermatology
- High Cholesterol
- Smoking Cessation (move under CNS)
- Women and Men’s Health
- Respiratory
- Pain due to Osteoarthritis
- Adult Insomnia
- Migraine Headache
- Mild Cognitive Impairment
- Alzheimer’s Disease
- Obsessive Compulsive Disorder (OCD)
- Major Depressive Disorder (MDD)
- Treatment-Resistant Depression (TRD)
- Post Traumatic Stress Disorder (PTSD)
- Angelman Syndrome
- Fragile X Syndrome
- Schizophrenia
- Attention Deficit Hyperactivity Disorder
- Bipolar Disorder
- Acute Low Back Pain
- Chronic Back Pain
- Chronic Pain Syndromes
- Shoulder Pain

Gastrointestinal
- Diabetes Mellitus
- Dyslipidemia
- Dyslipidemia in NIDDM
- Hormone Replacement Therapy
- Obesity in NIDDM
- Duodenal Ulcer & Gastric Ulcer
- Erosive Esophagitis
- Gastric Protection
- Gastroesophageal Reflux Disease
- Irritable Bowel Syndrome
- Non-Ulcer Dyspepsia
- Overactive Bladder
Infectious Diseases
- Diagnostic Testing
- Clostridioides Difficile
- Epstein-Barr Virus
- Escherichia coli
- Hepatitis B
- Human Papillomavirus
- Lyme Disease
- Pneumococcal
- Yellow Fever
- Pediatric vaccines

Reach out to me regarding any questions you have about our therapeutic strongholds.

Louisa Khalil
Vp, business operations.
In my role as VP of Business Operations at Atlas Clinical Research, I am dedicated to driving our culture of quality, promoting our expertise, and connecting with our various stakeholders. If you have any questions about our work here, our Network approach to clinical trial research, or collaboration opportunities, please don’t hesitate to contact me. Thank you for your support as we advance clinical trial research.
" * " indicates required fields
Therapeutic Areas
- Hypertension
- Post-Stroke
- Alzheimer’s Disease
- Diabetic Peripheral Neuropathy
- Fibromyalgia
- Headaches/Migraines
- Post Herpetic Neuralgia
- Restless Leg Syndrome
- Sleep Disorders
- Smoking Cessation
- Glabellar Lines
- Hyperhidrosis
- Seborrheic Keratosis
- Actinic Keratosis
- Common Skin Warts
- Dyslipidemia
- Hyperlipidemia
- Constipation
- Osteoarthritis
- Chronic Lower Back Pain
- Rheumatoid Arthritis
- Benign prostatic hyperplasia (BPH)
- Erectile Dysfunction
- Interstitial Cystitis
- Hypogonadism
- Overactive Bladder
- Peyronie’s Disease
- Premature Ejaculation
- Prostatitis
- Prostate Cancer
- Rapid Ejaculation
- Urinary Incontinence
- Urinary Tract Infection
- Seasonal Flu
- Pandemic Flu
- Elderly RSV
- Staphylococcus aureus
- Meningitis B
- Dengue Fever
- Contraceptives
- Female Sexual Dysfunction
- Hypoactive Sexual Desire Disorder
- Hormone Replacement Therapy
- Osteoporosis
- Vasomotor Symptoms in postmenopausal and menopause
- Vulvovaginal Atrophy/Candidiasis
- Healthy Volunteers
- Nutraceuticals
- Ophthalmology
- Over the Counter and Consumer
- Pediatric trials

About AMR-Mobile
We are one of 16 locations across the United States. Our name has changed, but the mission remains the same- discover new treatments and impact lives along the Gulf Coast through clinical research trials.
CONNECT WITH US
- (251) 414-1984
- [email protected]

Comprehensive Therapeutic Areas of CRO Expertise
PRC’s CRO expertise span numerous therapeutic areas, from rare & orphan trials to regenerative medicine to first-in-human trials and beyond.
Ask us about our experience from the extensive indication listing below.

Over the last 10 years, regenerative medicine has been a major focus of PRC’s research efforts, with numerous trials conducted and supported by the organization. These trials have included stem cell and gene therapy trials, as well as our work supporting research funding though the California Institute of Regenerative Medicine (CIRM), and have covered a wide range of therapeutic indications.
PRC is also a member of the Alliance for Regenerative Medicine proudly supporting ARM events and their mission to bring the benefits of engineered cell therapies and genetic medicines to patients, healthcare systems and society.

We understand the unique challenges that come with rare and orphan disease trials, from diagnosis difficulties to participant enrollment and patient stress. We have also supported complex and rare pediatric, adolescent and infant trials.

PRC has a wealth of experience in first-in-human trials, providing support for pre-study development before the launch of a study drug or biologic product. With numerous therapeutic areas of CRO expertise, PRC will help you navigate the complex regulatory landscape and ensure that your product is ready for clinical trials. We can help you achieve success in your pre-study development efforts.
PRC Clinical is dedicated to providing exceptional results in various medical specialties. With therapeutic areas, indications & study types of CRO expertise that include:
- Amyotrophic Lateral Sclerosis – ALS
- Alzheimer’s disease
- Cardiovascular
- Dermatology
- Endocrinology
- Gastroenterology
- Genetic disorders
- Healthy volunteers
- Huntington’s disease
- Imaging studies
- Infectious disease
- Kidney disease
- Laboratory devices
- Men’s health
- Musculoskeletal
- Osteoarthritis (knee)
- Osteoporosis
- Ophthalmology
- Parkinson’s disease
- Pain — acute, chronic, surgical
- Psychedelics
- Spine studies
- Women’s health
Does Your Trial Need TLC From PRC?
Let’s Talk
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- News & Events for Human Drugs
- CDER Conversations
CDER Launches a Center for Clinical Trial Innovation
CDER provides scientific and regulatory advice to guide clinical trial development and implementation as a cornerstone in drug development, helping patients and consumers access the therapies they need. As part of an ongoing effort to innovate and enhance clinical trials, CDER is establishing the CDER Center for Clinical Trial Innovation (C3TI). C3TI will foster CDER’s innovation efforts and act as a central point for coordinating, sharing knowledge, and communicating with both internal and external parties.
In this CDER Conversation, Kevin Bugin, PhD, lead for C3TI and deputy director of operations in the Office of New Drugs (OND), explains the purpose of C3TI, the importance of clinical trial innovation, and ways in which C3TI can advance public health.
What is C3TI?

C3TI will be a central hub within CDER to support the implementation of innovative approaches to clinical trial design and conduct. C3TI’s mission is to promote existing and future CDER clinical trial innovation through enhanced communication and collaboration. This will ultimately improve the efficiency of drug development, bringing safe and effective drugs to patients as optimally as possible. C3TI will foster innovation across industry and therapeutic areas, and across new and ongoing initiatives within CDER. The goals of C3TI are to:
- Facilitate the sharing of lessons learned across CDER’s existing and new clinical trial innovation programs.
- Communicate and collaborate with external parties about innovative clinical trials.
- Manage a C3TI Demonstration Program that will expand opportunities for sponsors of drug development programs to work with CDER experts in enhanced ways to illustrate the value and impact of innovative approaches to the design and conduct of clinical trials.
Why did CDER create C3TI?
For years, CDER has supported new approaches in the design and conduct of clinical trials. However, drug development continues to evolve and now, more than ever, trials need to leverage the most fit-for-purpose designs and tools to efficiently and effectively generate evidence to support the regulatory decision-making for safe and effective new medicines. For this reason, CDER opened a public docket in late 2023 and held a two-day public workshop in March 2024, in collaboration with the Duke-Margolis Institute for Health Policy, to better understand opportunities that could improve the development and adoption of innovative approaches in clinical trials.
CDER heard that sponsors want our support integrating innovation into their trials for new drug development. CDER staff noted they would benefit from greater exposure to or experience with the use of innovations in their regular review work. There is a desire across the drug development “ecosystem” to integrate innovation, but there is a need for more direction on how to better bridge the gap between policy and implementation.
Based on this insight, C3TI will aspire to create more opportunities for staff to participate in development programs planning to implement novel trial approaches to gain experience with a given innovation, via a C3TI Demonstration Program. C3TI will also explore how to create new opportunities for CDER staff to receive training and exposure to new clinical trial methods, technologies, and tools. Lastly, C3TI intends to better capture and disseminate lessons learned from implementing a given clinical trial innovation through improved communication and coordination with colleagues (e.g., dedicated rounds to discuss clinical trial innovation) and with external parties (e.g., public meetings or webinars).
How can C3TI leverage opportunities in clinical trial innovation?
With advances in healthcare and technology, there are many ways to innovate clinical trials. C3TI can help us take advantage of these opportunities by fostering collaboration and knowledge sharing. For instance, while guidance documents have been critical to guide clinical trial innovation, real-world examples help us better understand how to implement these innovations. The experience from these examples, either identified by C3TI and its partners or generated through the Demonstration Program, can be shared broadly.
C3TI is also involved in planning public workshops, which can offer a unique forum to share and discuss case studies or examples. Our public workshop in March 2024 with Duke-Margolis identified several driving forces for clinical trial innovation, such as increasing the participation of diverse populations in clinical trials, increasing the number of clinical trials for rare diseases with participants with unique needs, and incorporating decentralized trial elements and digital health technologies. Furthermore, it was clear from this workshop that collectively we all benefit from discussing our real-world experience with implementing clinical trial innovation and should seek out future opportunities for similar collaborative discussions.
How does C3TI impact public health?
Clinical trials are a key step in the drug development process and for generating the evidence needed to support approving safe and effective drugs. By improving the efficiency and effectiveness of clinical trials, we hope to accelerate this process.
C3TI is very interested in increasing diversity and inclusion in clinical trials. This can potentially enhance the quality of clinical trial data and provide more reliable information on populations that may have been previously underrepresented in clinical trials such as racial and ethnic minority and indigenous populations, individuals with underlying genetic conditions, or those affected by environmental factors.
Rare diseases are another challenging area that C3TI will address. Many rare diseases have little or no approved therapies, which means there is rarely a clearly defined clinical roadmap for future drug development. Another potential obstacle is finding enough individuals to participate in rare disease clinical trials. C3TI will help in this area by supporting the broader understanding and adoption of the most fit-for-purpose clinical trial designs, ultimately accelerating the development and approval of new rare disease drugs.
What plans does C3TI have for the future?
As we look to the future, the vision of C3TI is to accelerate the pace of clinical trial innovation through collaboration and the promotion and adoption of useful innovations. In the near future, we’ll focus on our C3TI Demonstration Program , consisting of three initial project areas. The program is for selected sponsors of clinical trials in these specific project areas to serve as case examples. If selected, sponsors will have the opportunity for enhanced engagement with additional CDER staff within the context of their traditional interactions with the review teams. The goal of this program and selecting these case examples is to generate new lessons learned to share more broadly with the clinical trial community.
Using our experienced staff, working groups and public workshops, we will continue to provide insight and guidance on clinical trial design and execution for drug development. As C3TI is fully implemented, we hope to increase engagement with sponsors. We look forward to C3TI’s growth over the years to come and its positive impact on drug development.
Find a Trial
Below is a listing of therapeutic areas covered by clinical research. Click on one of these areas to learn more about it, as well as view the various conditions included in each therapeutic area. You can also browse clinical trials by condition or location by using our Clinical Trial Search feature. Also, you can always jump straight to our guide where we will walk you through the process and connect you with one of our clinical trial experts.
Cardiology / Vascular Diseases
Dental / Maxillofacial Surgery
Dermatology / Plastic Surgery
Endocrinology
Gastroenterology
Immunology / Infectious Diseases
Musculoskeletal
Nephrology / Urology
Ophthalmology
Otolaryngology
Pharmacology / Toxicology
Psychiatry / Psychology
Pulmonary / Respiratory Diseases
Reproductive
- Rheumatology
Select your condition
Common conditions.
- Atrial Fibrillation
- Breast Cancer
- Constipation
- Dermatology
- Fibromyalgia
- Healthy Studies
- High Blood Pressure (Hypertension)
- High Cholesterol
- Irritable Bowel Syndrome (IBS)
- Migraine Headaches
- Obesity Weight Loss
- Osteoarthritis (OA)
- Renal Impairment / Chronic Kidney Disease
- Skin Cancer
- Smoking Cessation
All Conditions
- Alzheimer Disease
- Athletes Foot
- Benign Prostate Hyperplasia
- Bipolar Disorder
- Bladder Cancer
- Blood Cancer
- Brain Cancer
- Cervical Cancer
- Chronic Obstructive Pulmonary Disease
- Chronic Pain
- Cognitive Studies
- Colorectal Cancer
- Contraception
- Contraception Birth Control
- Contraception Birth Control Patch
- Crohns Disease
- Diabetic Neuropathy
- Digestive Disease
- Diverticulitis
- Eating Disorder
- Endometrial Cancer
- Endometriosis
- Erectile Dysfunction
- Flu (Influenza)
- Food Studies
- Gastroesophageal Reflux Disease
- Gastrointestinal
- Genital Herpes
- Hemorrhoids
- Hepatitis C
- Infectious Disease
- Infertility
- Influenza Vaccine
- Insomnia Sleep Studies
- Iron Deficiency Anemia
- Kidney Cancer
- Liver Cancer
- Low Back Pain/Arthritis Pain
- Lung Cancer
- Major Depression Disorder (MDD)
- Menses Disorders
- Multiple Sclerosis
- Osteoporosis
- Other Indications
- Ovarian Cancer
- Overactive Bladder
- Pancreatic Cancer
- Parkinsons Disease
- Peripheral Vascular Disease
- Polycythemia Vera
- Post-Surgical Pain
- Postherpatic Neuralgia
- Postmenopausal Syndrome
- Premature Ejaculation
- Prostate Cancer
- Psychiatric
- Restless Leg Syndrome
- Rheumatoid Arthritis
- Schizophrenia
- Shingles Vaccine
- Skin and Soft Tissue Infections
- Thyroid Cancer
- Tobacco Consumers
- Tobacco Consumers: SNUS, Chew, Dip
- Urinary Tract Infections
- Women's Studies

- +1.919.321.3321
- [email protected]
put Our Experience to work for you
Expand the reach of your studies and find the right population using our global experience to help inform your portfolio and feasibility decisions.
we have a worldwide presence
Click on a highlighted country, state or pin to see details of our experience in that area, or to see our reach for a specific therapeutic area, select it from the dropdown menu.
Data current as of April 1, 2023. The boundaries and names on this map do not imply official endorsement or acceptance by FHI Clinical.

FHI Clinical Presence

FHI Clinical & FHI 360

FHI 360 Locations
We are localized
We know how to navigate location-specific challenges so you can be prepared for any circumstance regardless of study location.
FHI Clinical has the local resources and experience to keep your study moving forward: engage with the local communities, work with still-developing regulatory agencies, ramp up quickly for an outbreak, implement contingency plans for natural disasters or political unrest and more.

Successfully implementing studies in complex settings requires an understanding of and respect for the local communities.
Our far-reaching, in-country technical, regulatory and cultural knowledge has been developed over our long history of working globally.
Our experience helps us guide decisions about the best-fit countries and populations for your target indication.

Geographic Coverage
We’ve been involved in observational studies, behavioral studies and clinical trials for epidemiology, spatial repellents, vaccines and treatments in 32 African countries.

Local Resources
FHI Clinical offices and 361 staff members are located in 7 countries throughout Africa.
Priority Indications
Our research involvement has included a range of non-communicable diseases as well as infectious diseases.
Asia Pacific
We’ve been involved in observational and clinical trials for epidemiology, diagnostic tests, vaccines and disease treatment in 14 Asia Pacific countries.

FHI Clinical offices and resources are located in three countries throughout Asia Pacific.

Our research involvement has included acute respiratory infection, COVID-19, dengue, diabetes, HIV, HPV, influenza, Japanese encephalitis, lymphatic filariasis, malaria, melioidosis, oncology, polyvalent vaccines, rotavirus gastroenteritis, sepsis and tuberculosis.
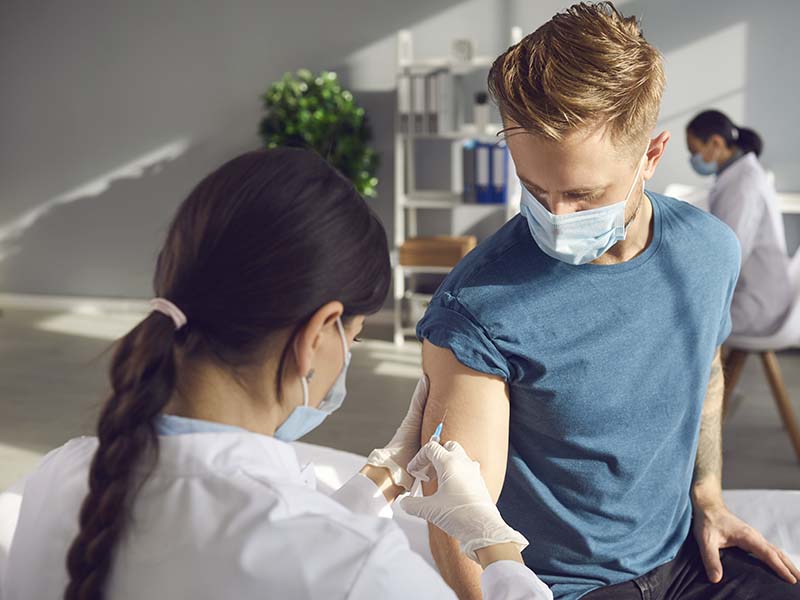
We’ve been involved in observational, epidemiological and clinical trials for diagnostic tests, vaccines and treatments in 12 European countries.

FHI Clinical has staff located in the United Kingdom.

Our research involvement has included studies for COVID-19, HIV, hormone replacement therapy (HRT), malaria and tuberculosis.
latin america and the caribbean
We’ve been involved in clinical trials for vaccines and treatments for diseases in 12 Latin American and Caribbean countries.

FHI Clinical has a resource located in Peru.
Our involvement in clinical trials has included Chikungunya, COVID-19, HIV, malaria, tuberculosis and Zika.
North America

FHI Clinical offices and resources are located in 17 locations throughout North America, including our headquarters in Durham, NC.

Our research involvement has included asthma, COVID-19, genital herpes, HIV, malaria, Nipah virus and Zika.
WE SUPPORT MANY THERAPEUTIC AREAS
We have a proven history of working to address challenges across nearly 20 therapeutic areas and 483 studies in both adult and pediatric populations:
SPECIFIC INFECTIOUS DISEASE EXPERTISE
Our team is particularly passionate about addressing infectious diseases, neglected tropical diseases and emerging and reemerging diseases in vulnerable populations. Our experience spans 308 studies across 37 indications:
we are globally connected
Access the skill sets necessary to quickly and efficiently combat disease.
Sustainable relationships with key partners ensure proactive disease management as well as rapid responses to immediate needs.
Reducing the global disease burden is only possible when we pool our resources and skills. We have collaborative partnerships with:
- Leading public and private medical research organizations
- Pharmaceutical and biotechnology companies
- Academic institutions
- United States National Institutes of Health (NIH)
- United States Centers for Disease Control and Prevention (CDC)
- United States Agency for International Development (USAID)
- Consultative partners
- World-renowned physicians and scientists
- Field-established thought leaders in multiple therapeutic areas
- Subject matter experts in regulatory authority guidance and support
- Experts in infectious diseases, tuberculosis, maternal and child health, family planning, HIV/AIDS and other sexually transmitted infections
Participation in research networks
Learn more about our specific experience through our case studies:.

Phase II Trial of a Chikungunya Vaccine in the Caribbean

Site Monitoring & Management in a Clinical Trial of a Spatial Repellent for Vector-Borne Disease Control

Rapid Site Assessment and Recommendations for Clinical Trials of a New Malaria Treatment

Providing Site Monitoring and Management Expertise in Malaria Vaccine Trials in Equatorial Guinea

Phase II/IIb Trial of a Zika Vaccine Spanning Nine Countries

Clinical Research Training to Support Capacity Building in Liberia

Building Capacity for Tuberculosis Research in China: The China TB Clinical Trials Consortium (CTCTC)

Rapid Study Start-Up for the Sierra Leone Trial to Introduce a Vaccine Against Ebola (STRIVE)

Rescue of an Ongoing Global, Multi-Site Oncology Trial

- Data Privacy Framework
- Terms of Use
- Cookie Notice
headquarters
FHI Clinical Headquarters 359 Blackwell Street, Suite 200 Durham, NC 27701 USA
get in touch
© fhi clinical inc. all rights reserved., our use of cookies, privacy overview.

Both countries identified a need to sustain the research capacity that was developed during their respective Ebola outbreaks. The partnerships were formed between the U.S. National Institute of Allergy and Infectious Diseases (NIAID) Division of Clinical Research (DCR) and the Ministry of Health (MOH) of each country. Their missions are to:
- Conduct and implement a program of high-quality national and international research focused on each country’s public health priorities
- Build and develop sustainable research capacity
In the PREGUI network, we have a role in the Finance Management Centers, where we:
- Provide audit-compliant, transparent management of project funds
- Assist with grant writing and management
- Pay staff salaries
- Provide accountable cash disbursement to research participants
- Coordinate international and local travel
- Arrange shipment of supplies and equipment
In the PREVAIL network, we provide full operational support:
- Sourcing, procuring and shipping
- Administrative and facilities staff hiring
- Staff training, including good clinical practice, laboratory practices, data management, computer skills, record keeping, etc.
- Facility management
- Renovation project management
- Planning and preparation for the conduct of clinical laboratory operations
- Data management, biostatistics and Data Safety Monitoring Board (DSMB)
- Social mobilization and community engagement, social analytics and participant biometrics for study enrollment

From its inception in 2013 through 2020, we provided technical support and guidance to the CTCTC to implement strategies for clinical trial capacity building and partnered with other organizations to participate in the CTCTC, including the University of North Carolina Institute for Global Health & Infectious Diseases.
The CTCTC provides mentoring and advice around the following topics to maintain a successful tuberculosis (TB) clinical trial network in China:
- Training and quality monitoring to follow Good Clinical Practice (GCP) guidelines
- Establishing and maintaining reliable, accurate TB laboratory services
- Conducting ethical, effective patient recruitment and retention
- Ensuring high-quality data collection and statistical analyses
Read more about FHI Clinical’s role in the CTCTC.

Therapeutic Areas of Expertise
- Neurosciences/CNS
- Pulmonary / Respiratory
- Gastroenterology
- Rare Diseases
- Ophthalmology
- Immunology / Autoimmune / Rheumatology
- Transplants
- Musculoskeletal Disorders
- Medical Aesthetics / Plastic Surgery
- Dermatology
- Medical Devices
- Infectious Diseases
- Endocrinology
- Cardiovascular Diseases
- Pain Management
- Women’s Health
- Reproductive Health
Are you a Site that works in these Therapeutic Areas?
Are you ready to get started, tell us about your next project.

IMAGES
VIDEO
COMMENTS
Therapeutic areas in clinical research encompass a wide range of medical specialties, each focusing on a specific aspect of human health. From oncology and hematology to dermatology, immunology, neurology, gastroenterology, cardiology, ophthalmology, rare diseases, endocrinology, gene therapy, medical devices, and digital therapeutics ...
Across the therapeutic areas of both preclinical and clinical research, we analyze numerous biomarkers, and are always adding new validated methods to our panels that cover preclinical and clinical matrices. Finally, we have extensive experience in designing and conducting proof-of-concept studies in healthy participants or patients.
Cancer treatments have the highest clinical trial volume. Treatments for cancer have the highest number of clinical trials by therapeutic area - 15.4% of all trials analyzed. With a projected global market value of $300 billion by 2026, it's no surprise that cancer drugs are among the most highly-tested therapies in clinical trials across ...
Priority Therapeutic Areas for Development. Standardizing key study data specific to therapeutic areas (TAs) will facilitate clinical research and the evaluation of medical products. In 2011, CDER ...
Therapeutic areas are the categories of diseases or medical conditions that clinical research focuses on. In this article, you will learn about the main therapeutic areas, their challenges, and their opportunities for innovation. Vial is a platform that helps clinical trial sponsors and sites manage their data and processes with interactive response technology (IRT).
We ensure research is relevant and meaningful, thus increasing engagement and participation of each patient. Clinical ink delivers eSource technology solutions that go beyond EDC, integrating Direct Data Capture (DDC) with eCOA and ePRO modules, eConsent , sensors and wearables, and digital biomarkers. Our therapeutic experience and pioneering ...
Whereas in a non-therapeutic clinical trial, the participant does not benefit from the drug. ... Among the various key application areas of clinical trial systems, the data analysis assumes increased significance. The clinical trial data collected at the site in the form of case record form is stored in the CDMS ensuring the errors with respect ...
Despite the progress made in the field of clinical research, unmet therapeutic needs are still identified in several clinical areas (Miller, 2009; Taiwo et al., 2010; Aceves, 2014; Markowitz, 2015; Morrow et al., 2017). It is notable, for instance, that multidrug-resistant infections are rapidly increasing worldwide, but very few antibiotics ...
Therapeutic Areas in Drug Discovery & Clinical Research. ... Therapeutic antibodies under clinical investigation and on the market aimed at treating chronic pain target a variety of molecules associated with pain pathways, including calcitonin gene-related peptide (CGRP), ion channels, nerve growth factor (NGF), tumor necrosis factor-alpha (TNF ...
The roughly $5 billion spent by the NIH on clinical projects and the $700 million proposed for translational research in 2011-2012 is dwarfed by the estimated $92 billion research budgets of major pharma and major biotech spent in the same year on R&D, over 85% of which is translational research (see Table 5.1, Table 5.2). The effort is also ...
These four therapeutic areas accounted for 79% of new trial starts in 2023. Obesity was by contrast a growth area, with a 68% increase in clinical trial starts in 2023. Over 120 drugs are now in ...
Explore IQVIA's deep domain expertise in the therapeutic and specialty areas you are committed to. Translate science into new treatments Through our Therapeutic Centers of Excellence, IQVIA connects your clinical program with strong scientific and medical expertise, deep therapeutic insights and unrivaled clinical trials experience.
As drug and medical device development continues to become more complex and blurred across therapeutics areas—with advancements in immuno-therapies, cell and gene therapies, and microbiome therapeutics to name a few—our foundations in science, operations, and regulatory can be applied across: Core therapeutic areas and diseases.
A comprehensive expertise and experience in immunology clinical trials across different therapeutic areas, such as gout, rheumatoid arthritis, psoriasis, osteoarthritis, systemic sclerosis, lupus, and more. Endocrinology Endocrine & Metabolic Disorders. Conducting metabolic studies can be a challenge due to difficulties in patient recruitment ...
With Clario, you get both. The complexity of today's clinical therapeutic studies, and drug trials demands deep scientific, regulatory and therapeutic area expertise to help guide and support your research. From protocol design to regulatory submission, you need a clinical research company with a thorough understanding of the specific ...
Therapeutic Area User Guides (TAUGs) extend the Foundational Standards to represent data that pertains to specific disease areas. TAUGs include disease-specific metadata, examples and guidance on implementing CDISC standards for a variety of uses, including global regulatory submissions.
CTI's focused therapeutic approach provides large and mid-size pharmaceutical and emerging biotechnology companies with clinical and disease area expertise. Toggle navigation. Contact; News & Events ... CTI is a leader in rare disease and orphan disease research with more than 3/4 of CTI's active prospective studies in these therapeutic areas.
Aging is a major risk factor for numerous chronic diseases. Vaccination offers a promising strategy to combat these age-related diseases by targeting specific antigens and inducing immune responses.
Although our individual sites have a wide breadth of experience in virtually every therapeutic indication, Atlas has identified four specific areas of focus where our network's experience and expertise provide the greatest value to our sponsors and patients, namely our work in internal medicine, the central nervous system (CNS), infectious diseases, and gastrointestinal clinical research.
To date Coastal Clinical Research Inc. has conducted over 600 industry-sponsored clinical trials. Our main areas of expertise include CARDIOVASCULAR Hypert. Menu. STUDIES ... Research Terminology; AMR Daphne (Baldwin County Location) SPONSORS/CRO . Sponsors/CRO; Therapeutic Areas; TEAM . About; Team; Facility; CONTACT . Contact Us; Apply Online ...
PRC has a wealth of experience in first-in-human trials, providing support for pre-study development before the launch of a study drug or biologic product. With numerous therapeutic areas of CRO expertise, PRC will help you navigate the complex regulatory landscape and ensure that your product is ready for clinical trials.
CDER is establishing the CDER Center for Clinical Trial Innovation (C3TI). C3TI will foster CDER's innovation efforts and act as a central point for coordinating, sharing knowledge, and ...
Below is a listing of therapeutic areas covered by clinical research. Click on one of these areas to learn more about it, as well as view the various conditions included in each therapeutic area. You can also browse clinical trials by condition or location by using our Clinical Trial Search feature.
Subject matter experts in regulatory authority guidance and support. Experts in infectious diseases, tuberculosis, maternal and child health, family planning, HIV/AIDS and other sexually transmitted infections. We also act as the coordinating and operations center for clinical research networks in Africa, Asia, Latin America and the United States.
Therapeutic Areas; Quality. Quality Management System; Technologies. EDC Systems; SAS and Other Statistical Analysis Tools; Phoenix WinNonlin and NONMEM; IRT (IWRS, eDiary, ePRO) Argus Safety Database; ... Everest Clinical Research 675 Cochrane Drive East Tower, 4th Floor Markham, Ontario
Clinical Operations SpecialistLocation - Hyderabad #LI HybridAbout the Role:Responsible to complete clinical services and meet planned deliverables in line with defined roles and responsibilities agreed with colleagues/customers.Key Responsibilities:Support set-up and maintenance of information in Clinical Trial Management Systems (CTMS) and other systems as applicable, under responsibility ...