Disclaimer » Advertising
- HealthyChildren.org

- Previous Article
- Next Article

Methods and Literature Search
Food insecurity and obesity, food insecurity and bmi, food insecurity and bmi z-score, results synthesis, limitations, conclusions, acknowledgments, food insecurity and childhood obesity: a systematic review.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- CME Quiz Close Quiz
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
Christine St. Pierre , Michele Ver Ploeg , William H. Dietz , Sydney Pryor , Chioniso S. Jakazi , Elizabeth Layman , Deborah Noymer , Tessa Coughtrey-Davenport , Jennifer M. Sacheck; Food Insecurity and Childhood Obesity: A Systematic Review. Pediatrics July 2022; 150 (1): e2021055571. 10.1542/peds.2021-055571
Download citation file:
- Ris (Zotero)
- Reference Manager
Video Abstract
Addressing food insecurity while promoting healthy body weights among children is a major public health challenge. Our objective is to examine longitudinal associations between food insecurity and obesity in US children aged 1 to 19 years.
Sources for this research include PubMed, CINAHL, and Scopus databases (January 2000 to February 2022). We included English language studies that examined food insecurity as a predictor of obesity or increased weight gain. We excluded studies outside the United States and those that only considered the unadjusted relationship between food security and obesity. Characteristics extracted included study design, demographics, methods of food security assessment, and anthropometric outcomes.
Literature searches identified 2272 articles; 13 met our inclusion criteria. Five studies investigated the relationship between food insecurity and obesity directly, whereas 12 examined its relationship with body mass index or body mass index z-score. Three studies assessed multiple outcomes. Overall, evidence of associations between food insecurity and obesity was mixed. There is evidence for possible associations between food insecurity and obesity or greater weight gain in early childhood, for girls, and for children experiencing food insecurity at multiple time points. Heterogeneity in study methods limited comparison across studies.
Evidence is stronger for associations between food insecurity and obesity among specific subgroups than for children overall. Deeper understanding of the nuances of this relationship is critically needed to effectively intervene against childhood obesity.
The United States faces 2 important public health challenges in reducing childhood obesity while ensuring that children and their families have enough nutritious food for an active, healthy life. From 2017 to 2018, ∼20% of US children aged 2 to 19 were estimated to have obesity, a prevalence level that has increased by nearly 40% over the past 20 years. 1 In 2020, the first year of the COVID-19 pandemic, food insecurity among US households with children was 14.8%, an increase over the 2019 level of 13.6% and a reversal of the declining trend observed over the previous decade. 2
Childhood obesity and food insecurity are more prevalent in lower-income households, 2 , 3 suggesting a potentially simultaneous occurrence of both under- and over-nutrition. Despite almost 3 decades since these dual problems were first observed, 4 no consensus exists about the underlying mechanisms of their relationship. The increases in food insecurity 2 , 5 and accelerated weight gain 6 – 8 observed among children during the COVID-19 pandemic indicate that greater understanding of how these 2 issues interact is of great importance for child health, particularly in terms of associations between food insecurity episodes and weight status over the long term.
In this systematic review, we examined longitudinal associations between food insecurity and obesity in US children aged 1 to 19 years. The review summarizes the overall evidence, then discusses differences in the evidence according to relevant demographics and the experience of multiple food insecurity episodes.
This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis. Searches were performed in December 2020 in PubMed, CINHAL, and Scopus databases and restricted to English language studies published between January 1, 2000, and November 30, 2020. Studies published before 2000 were excluded to maximize homogeneity in food security and child weight status assessment tools. Results were uploaded to the Covidence systematic review tool (Covidence, Melbourne, Australia) for screening.
Studies eligible for inclusion were those with participants who were infants or children in the United States from 1 to 19 years of age; that assessed food security or insufficiency at the household or child level; compared outcomes by food security status; and examined obesity or body mass index (BMI) as the primary outcome of interest (see full search strategy Supplemental Table 3 ). Included studies were limited to those conducted in the United States to reduce heterogeneity in the food security assessment tools employed. Studies were excluded if they only reported unadjusted relationships between food security and obesity, or if the study population was limited to only youth with overweight or obesity at baseline. Studies with less than 30 participants, the traditional minimum in statistics for a reliable confidence interval, were also excluded. Title and abstract screening, full-text screening and data extraction were performed by 2 independent reviewers; conflicts were resolved by consultation between researchers. Database searches were re-run in September 2021 and February 2022, and results were hand-searched to add relevant studies published between December 2020 and February 2022.
Study quality was assessed using the National Institutes of Health (NIH) quality assessment tool for observational cohort and cross-sectional studies (NIH, Bethesda, Maryland). Although quality assessment tools for clinical trials are well established, there is no consensus on the best methods to assess the quality of observational nutrition studies. 9 We selected the NIH tool because it could be applied consistently to all included studies. Two researchers applied the assessment tool independently. Disagreements were resolved via discussion among the research team.
The database search yielded 2272 studies after duplicates were removed. Following title and abstract review, 91 papers were retained for full-text screening. Forty-two were excluded for 1 of the following: methods (no adjusted estimate of the relationship between food security and anthropometrics); outcome (did not assess likelihood of obesity or a continuous BMI-related outcome); population (participants over 19 years old); or location (outside the United States). A total of 41 studies met the inclusion criteria after the initial screening, 4 studies were subsequently added following the search and screening process in September 2021, and no additional studies meeting the inclusion criteria were identified in the February 2022 search ( Fig 1 ).

Preferred Reporting Items for Systematic Reviews and Meta-Analysis flow diagram detailing review search process. PRISMA statement distributed under the terms of the Creative Commons Attribution License. Original source: Mohr D, Liberati A, Tetzlaff J, Altmann DC, the PRISMA Group. Preferred reporting items for systematic reviews and meta-analysis: the PRISMA statement. PLoS Med . 2009;6(7);e1000098. 37
A total of 45 papers were originally included for data extraction. Data on sample size, demographic characteristics of study participants, nutrition assistance program participation (when available), food security assessment methods, outcomes measured, method of analysis, covariates included in analysis, adjusted results, and tests for interaction with any subsequent stratified results, were extracted using a piloted, standardized extraction spreadsheet. Although both longitudinal and cross-sectional studies were initially included, only the longitudinal studies ( n = 16) were ultimately retained for evidence analysis because of their overall higher study quality and ability to provide insight into potential relationships between food insecurity and obesity over time.
In the quality analysis, all longitudinal studies were rated “good” or “fair” ( Supplemental Table 4 ). Thirteen studies 10 – 22 used either the “gold standard” USDA 18-item Household Food Security Survey Module or a shorter subset derived directly from the full module to assess food security, whereas 3 studies used single-item measures. These 3 studies were excluded from the overall results synthesis because they lacked a standardized food security assessment instrument.
In the final 13 studies, variation in outcomes measured, food security categorization (eg, binary vs multilevel and categorical versus continuous), and analysis methods prevented us from conducting a meta-analysis. We instead present the results according to the 3 different outcomes analyzed in the included studies. Our primary interest was examining the relationship between food insecurity and obesity. We focused on obesity rather than both overweight and obesity because obesity has a greater sensitivity and specificity for identifying excess body fat and carries a higher risk for adverse health outcomes. 23 To examine potential differences in trajectories of BMI growth, we also synthesized the evidence for associations between food insecurity and changes in BMI and BMI z-score. Both variables present interpretation challenges as longitudinal outcomes. BMI changes are not indexed to age and sex-specific references in studies and may not account for the normative dip in BMI that occurs in early childhood. 24 BMI z-score changes are smaller at higher levels of adiposity and do not adequately reflect large changes in weight and adiposity. 25 Despite these limitations, the growth trajectories identified in these analyses can illuminate associations between food insecurity and excess weight gain in children. When included studies measured multiple outcomes, we included the findings in each of the applicable syntheses. Table 1 summarizes the characteristics of all included studies.
Characteristics of Included Studies
Only analyses meeting our inclusion criteria are presented in the table; studies may have analyzed other outcomes that did not fit our criteria
Five studies examined the association between food insecurity and obesity. 12 , 14 , 19 – 21 Among a cohort of participants in the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) in Massachusetts, an association was found between low food security (worried about food but no disruption to eating patterns, as opposed to very low food security, which also includes disruption of eating patterns) when present in both infancy and preschool and greater odds of obesity in preschool. 20 This relationship was also moderated by maternal prepregnancy weight status, with greater odds of obesity for children in households with low food security at both time points and whose mothers were either overweight, had obesity, or were underweight prepregnancy. An analysis of the Early Childhood Longitudinal Study (ECLS) birth cohort using structural equation modeling found no direct association between food insecurity and obesity, but in mediation analysis, food insecurity affected parenting and infant feeding behaviors, which ultimately affected weight. 12 The 3 remaining studies found no significant associations between food insecurity and obesity; 2 used data from the ECLS-K 21 and ECLS-K: 2011 19 cohorts, and the third used findings from a cohort of Hispanic mothers and their children in California (CHAMACOS). 14
Four studies analyzed the relationship between food insecurity and changes in BMI over time. Of the 2 studies using ECLS-K data, 1 found a greater increase in BMI among children whose households were food insecure at 2 time points, 17 and both found higher BMI increases for girls from food insecure households but not for boys. 13 , 17 In a third study using ECLS-K data, the authors reported no significant associations but did not test for interactions by sex or consider food insecurity at multiple time points. 11 The CHAMACOS study found lower BMI gains among children whose households changed from highly food secure to marginally food secure or food insecure across 2 time points. 14
Nine studies investigated associations between food insecurity and changes in BMI z-score. The ECLS-K: 2011 study found an association between food insecurity in first grade and an increased BMI z-score in third grade, but no association between kindergarten food insecurity and third grade BMI z-score. 19 Likewise, a fourth ECLS-K study found an association between fifth grade food insecurity and a higher eighth grade BMI z-score but no significant associations when food insecurity occurred in younger grades. 22 In a Head Start preschool cohort, an association was found between food insecurity and increased BMI z-score for girls, but participants were only followed for an average of 6 months. 16 A birth cohort following infants through 12 months found an association between very low food security and a higher BMI z-score, 10 whereas the WIC cohort found no association in the main analysis but an association between food insecurity in infancy and an increased BMI z-score in early childhood if the mother was overweight or had obesity prepregnancy. 20 In the CHAMACOS cohort, food insecurity at age 9 was associated with a decrease in BMI z-score from ages 9 to 10.5, and food insecurity across 2 time points or changing from food secure to food insecure were also associated with decreased BMI z-score. 14 The remaining 3 studies found no significant associations. 11 , 15 , 18
The studies are categorized by outcome and findings in Table 2 . An association between food insecurity and obesity was found only in early childhood, 20 whereas 6 additional studies found evidence of associations between food insecurity and increases in BMI or BMI z-score in limited age groups or sex-specific analyses. 10 , 13 , 16 , 17 , 19 , 22 One study of an exclusively Hispanic population found evidence of an association between food insecurity and decreases in BMI z-score or BMI, limited to a specific age group or changes in food security status. 14 Although all studies assessed food security based on standardized US Department of Agriculture assessment tools, comparison is challenging because of differences in food security categorization. Most studies categorized participants as either food secure (high or marginal food security according to survey responses) or food insecure (low or very low food security). However, 2 studies combined marginal food security with low and very low food security, 14 , 17 3 studies used more than 2 categories for food security status, 10 , 12 , 20 and 1 used a continuous variable. 11 Studies also differed in the covariates used in their analyses. Child age, sex, race or ethnicity, household income, and parent or maternal education were consistently included as control variables, but other predictors of obesity, such as physical activity level, child birth weight, and maternal BMI, were included in no more than half of the studies. The variations in both the food security variable and covariates may help explain the mixed results observed across studies.
Findings by Outcome
The number of studies does not sum to 13 because some studies investigated multiple outcomes.
Our findings corroborate the previously published literature, indicating that potential relationships between food insecurity and childhood obesity and child weight changes are complex. Although the evidence did not allow us to draw broad conclusions about the relationship between food insecurity and obesity in children, we were nevertheless able to gain deeper insight and identify directions for further research by synthesizing results according to age, sex, and multiple experiences of food insecurity.
We observed associations between food insecurity and increases in BMI or BMI z-score among infants, 10 preschoolers, 16 , 20 elementary students, 17 , 19 and middle school students. 13 , 22 Although 5 of the studies with evidence of higher BMIs among food insecure youth were highly powered cohorts with large samples, 13 , 17 , 19 , 20 , 22 findings were limited to specific age ranges or subgroups within the sample, with the exception of the preschool study. 20 In the localized CHAMACOS cohort, food insecurity was associated with decreased BMI z-scores during mid- to late elementary years. 14 Thus, the mixed evidence is in agreement with the 2015 Dietary Guidelines Advisory Committee (DGAC) conclusion that limited evidence supports an association between food insecurity and higher anthropometric measurements in early childhood. 26 It is also particularly noteworthy that none of the studies followed children beyond eighth grade. Eight of the 13 studies in our review were published after the 2015 DGAC identified the need for additional study of food insecurity and weight changes into the adolescent years, 26 but none provided evidence of potential associations beyond middle school.
Three of the studies in the review presented results stratified by child sex, and all found an association between food insecurity and increased BMI or BMI z-score for girls but not for boys. 13 , 16 , 17 Three additional studies tested for interaction by sex but found no associations. 14 , 20 , 22 Associations between food insecurity and higher BMI for preschool girls have also been found cross-sectionally. 27 , 28 Potential explanations for this association in girls but not in boys could include differential parent feeding practices by gender, 29 or different responses to stress, including the experience of food insecurity. 17 Lack of testing for interaction by sex in many studies could also be masking associations in 1 of the groups, even if a relationship is not found in the overall population. 27 Following youth into adolescence and early adulthood could also help clarify differences in the interactions between food insecurity and weight by sex, particularly given recent evidence among adults that food insecurity was more prevalent among women with greater adiposity. 30
Several of the longitudinal studies in the review categorized food security across multiple time points to examine how changes in food security status or multiple episodes of food insecurity were related to obesity and BMI. 13 , 14 , 16 , 17 , 20 In 3 large studies, food insecurity at multiple time points was associated with obesity or greater BMI growth relative to food security at all time points, 13 , 17 , 20 and 1 preschool study found that for girls, higher BMI z-scores were associated with the household changing from food secure to food insecure over the course of 1 school year. 16 One smaller study found an association with decreased BMI z-scores when food insecurity occurred at multiple time points or when households transitioned from food security to food insecurity, 14 but more evidence points to a potential cumulative positive effect of multiple experiences of food insecurity on weight gain, an effect also observed by the 2015 DGAC. 26 The effects of the duration and episodic nature of food insecurity may be of particular relevance to the increase in childhood obesity that has occurred during the COVID-19 pandemic. 6 , 7
Potential differences by age range, sex, and the unknown effects of fluctuations in food security status over time indicate that a systems or structural modeling approach may provide better insight into how food insecurity and child weight status are related to one another through indirect channels. Household stress may play an important mediating role in the relationship between food insecurity and weight outcomes. One longitudinal study with a small sample size found an association between food insecurity and increased BMI when high stress was present at the child level, 31 and multiple studies have examined how interactions between maternal stress and food insecurity may affect child weight status. 32 – 35 Two of the studies included in this review included structural models that accounted for parental feeding practices, 12 , 18 and another structural model includes child dietary intake and both child and parent physical activity levels. 36 Further research can build on such models to better understand the complex mechanisms that affect the relationship between food insecurity and child weight status. Irrespective of any future conclusive evidence on the relationship mechanisms between food insecurity and childhood obesity, however, effective interventions against child food insecurity should be a public health priority to promote the physical, emotional, and cognitive wellbeing of children and parents.
Although limiting our analysis to longitudinal studies strengthens the evidence relative to cross-sectional findings, following low-income populations over long periods is a challenging endeavor. The ECLS-K studies did not remain nationally representative over the follow-up periods, and 1 of them specifically noted that participants excluded because of missing data were more likely to be of lower socioeconomic status. 19 Recent evidence indicates that racial or ethnic disparities in childhood obesity have increased since the COVID-19 pandemic, 6 but our ability to explore potential differences by race or ethnicity in the food insecurity-childhood obesity relationship was limited by lack of testing for interaction by race or ethnicity. Two of the 3 studies that followed youth into puberty omitted any discussion of pubertal status, despite connections between puberty and anthropometric measurements that could have affected study findings. Differences in covariates, most notably omission of control variables for physical activity in most studies and for dietary quality in all studies, may contribute to the inconsistent findings. Finally, we were limited in our ability to assess the relationship between food insecurity and obesity by the diverse outcomes measured in the included studies. A greater proportion of studies used continuous BMI outcomes relative to weight categories. Although these studies showed changes in BMI trajectories, it was not apparent whether these changes indicated movement across weight categories.
We observed mixed evidence of associations between food insecurity and childhood obesity, but the mechanisms of their relationship remain difficult to ascertain. This review highlights the importance of understanding the many nuances of how food insecurity and childhood obesity interact with one another, which is even more critical as we have observed increased child food insecurity and widening disparities in the prevalence of obesity amid the COVID-19 pandemic. Ongoing and future studies need to consider interactions between food insecurity and salient demographics and the broader context of the household environment to enable us to meet the dual challenges of reducing childhood obesity and ensuring food security for all families.
We thank the anonymous reviewers for their thoughtful and insightful feedback on this paper.
Ms St. Pierre and Dr Ver Ploeg conceptualized and designed the study, coordinated and supervised data collection, drafted the initial manuscript, and reviewed and revised the manuscript; Drs Dietz and Sacheck conceptualized and designed the study and critically reviewed the manuscript for important intellectual content; Ms Pryor, Ms Jakazi, Ms Layman, Ms Noymer, and Ms Coughtrey-Davenport collected data, conducted the initial analyses, and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FUNDING: This research was supported by Healthy Eating Research, a national program of the Robert Wood Johnson Foundation.
CONFLICT OF INTEREST DISCLOSURES: The authors have indicated they have no conflicts of interest to disclose.
body mass index
Dietary Guidelines Advisory Committee
Early Childhood Longitudinal Study
National Institutes of Health
Supplemental Nutrition Assistance Program
Special Supplemental Nutrition Program for Women, Infants, and Children
Supplementary data
Advertising Disclaimer »
Citing articles via
Email alerts.

Affiliations
- Editorial Board
- Editorial Policies
- Journal Blogs
- Pediatrics On Call
- Online ISSN 1098-4275
- Print ISSN 0031-4005
- Pediatrics Open Science
- Hospital Pediatrics
- Pediatrics in Review
- AAP Grand Rounds
- Latest News
- Pediatric Care Online
- Red Book Online
- Pediatric Patient Education
- AAP Toolkits
- AAP Pediatric Coding Newsletter
First 1,000 Days Knowledge Center
Institutions/librarians, group practices, licensing/permissions, integrations, advertising.
- Privacy Statement | Accessibility Statement | Terms of Use | Support Center | Contact Us
- © Copyright American Academy of Pediatrics
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Review of Childhood Obesity: From Epidemiology, Etiology, and Comorbidities to Clinical Assessment and Treatment
Affiliations.
- 1 Division of Pediatric Endocrinology and Metabolism, Mayo Clinic, Rochester, MN. Electronic address: [email protected].
- 2 Department of Pediatrics and Department of Medicine, University of Minnesota, Minneapolis.
- PMID: 28065514
- DOI: 10.1016/j.mayocp.2016.09.017
Childhood obesity has emerged as an important public health problem in the United States and other countries in the world. Currently 1 in 3 children in the United States is afflicted with overweight or obesity. The increasing prevalence of childhood obesity is associated with emergence of comorbidities previously considered to be "adult" diseases including type 2 diabetes mellitus, hypertension, nonalcoholic fatty liver disease, obstructive sleep apnea, and dyslipidemia. The most common cause of obesity in children is a positive energy balance due to caloric intake in excess of caloric expenditure combined with a genetic predisposition for weight gain. Most obese children do not have an underlying endocrine or single genetic cause for their weight gain. Evaluation of children with obesity is aimed at determining the cause of weight gain and assessing for comorbidities resulting from excess weight. Family-based lifestyle interventions, including dietary modifications and increased physical activity, are the cornerstone of weight management in children. A staged approach to pediatric weight management is recommended with consideration of the age of the child, severity of obesity, and presence of obesity-related comorbidities in determining the initial stage of treatment. Lifestyle interventions have shown only modest effect on weight loss, particularly in children with severe obesity. There is limited information on the efficacy and safety of medications for weight loss in children. Bariatric surgery has been found to be effective in decreasing excess weight and improving comorbidities in adolescents with severe obesity. However, there are limited data on the long-term efficacy and safety of bariatric surgery in adolescents. For this comprehensive review, the literature was scanned from 1994 to 2016 using PubMed using the following search terms: childhood obesity, pediatric obesity, childhood overweight, bariatric surgery, and adolescents.
Copyright © 2016 Mayo Foundation for Medical Education and Research. Published by Elsevier Inc. All rights reserved.
Publication types
- Bariatric Surgery / methods
- Bariatric Surgery / standards
- Body Mass Index
- Child, Preschool
- Comorbidity
- Diet / adverse effects
- Diet / standards
- Drug-Related Side Effects and Adverse Reactions
- Energy Intake / physiology*
- Exercise / physiology*
- Genetic Predisposition to Disease
- Pediatric Obesity* / complications
- Pediatric Obesity* / epidemiology
- Pediatric Obesity* / etiology
- Pediatric Obesity* / therapy
- Sedentary Behavior
- Sleep Wake Disorders / complications
- United States / epidemiology
- Weight Reduction Programs / methods
- Weight Reduction Programs / standards*
- Reference Manager
- Simple TEXT file
People also looked at
Review article, childhood and adolescent obesity: a review.

- 1 Division of Endocrinology, Diabetes and Metabolism, Department of Pediatrics, Medical College of Wisconsin, Milwaukee, WI, United States
- 2 Division of Adolescent Medicine, Department of Pediatrics, Medical College of Wisconsin Affiliated Hospitals, Milwaukee, WI, United States
- 3 Division of Adolescent Medicine, Department of Pediatrics, Medical College of Wisconsin, Milwaukee, WI, United States
Obesity is a complex condition that interweaves biological, developmental, environmental, behavioral, and genetic factors; it is a significant public health problem. The most common cause of obesity throughout childhood and adolescence is an inequity in energy balance; that is, excess caloric intake without appropriate caloric expenditure. Adiposity rebound (AR) in early childhood is a risk factor for obesity in adolescence and adulthood. The increasing prevalence of childhood and adolescent obesity is associated with a rise in comorbidities previously identified in the adult population, such as Type 2 Diabetes Mellitus, Hypertension, Non-alcoholic Fatty Liver disease (NAFLD), Obstructive Sleep Apnea (OSA), and Dyslipidemia. Due to the lack of a single treatment option to address obesity, clinicians have generally relied on counseling dietary changes and exercise. Due to psychosocial issues that may accompany adolescence regarding body habitus, this approach can have negative results. Teens can develop unhealthy eating habits that result in Bulimia Nervosa (BN), Binge- Eating Disorder (BED), or Night eating syndrome (NES). Others can develop Anorexia Nervosa (AN) as they attempt to restrict their diet and overshoot their goal of “being healthy.” To date, lifestyle interventions have shown only modest effects on weight loss. Emerging findings from basic science as well as interventional drug trials utilizing GLP-1 agonists have demonstrated success in effective weight loss in obese adults, adolescents, and pediatric patients. However, there is limited data on the efficacy and safety of other weight-loss medications in children and adolescents. Nearly 6% of adolescents in the United States are severely obese and bariatric surgery as a treatment consideration will be discussed. In summary, this paper will overview the pathophysiology, clinical, and psychological implications, and treatment options available for obese pediatric and adolescent patients.
Introduction
Obesity is a complex issue that affects children across all age groups ( 1 – 3 ). One-third of children and adolescents in the United States are classified as either overweight or obese. There is no single element causing this epidemic, but obesity is due to complex interactions between biological, developmental, behavioral, genetic, and environmental factors ( 4 ). The role of epigenetics and the gut microbiome, as well as intrauterine and intergenerational effects, have recently emerged as contributing factors to the obesity epidemic ( 5 , 6 ). Other factors including small for gestational age (SGA) status at birth, formula rather than breast feeding in infancy, and early introduction of protein in infant's dietary intake have been reportedly associated with weight gain that can persist later in life ( 6 – 8 ). The rising prevalence of childhood obesity poses a significant public health challenge by increasing the burden of chronic non-communicable diseases ( 1 , 9 ).
Obesity increases the risk of developing early puberty in children ( 10 ), menstrual irregularities in adolescent girls ( 1 , 11 ), sleep disorders such as obstructive sleep apnea (OSA) ( 1 , 12 ), cardiovascular risk factors that include Prediabetes, Type 2 Diabetes, High Cholesterol levels, Hypertension, NAFLD, and Metabolic syndrome ( 1 , 2 ). Additionally, obese children and adolescents can suffer from psychological issues such as depression, anxiety, poor self-esteem, body image and peer relationships, and eating disorders ( 13 , 14 ).
So far, interventions for overweight/obesity prevention have mainly focused on behavioral changes in an individual such as increasing daily physical exercise or improving quality of diet with restricting excess calorie intake ( 1 , 15 , 16 ). However, these efforts have had limited results. In addition to behavioral and dietary recommendations, changes in the community-based environment such as promotion of healthy food choices by taxing unhealthy foods ( 17 ), improving lunch food quality and increasing daily physical activity at school and childcare centers, are extra measures that are needed ( 16 ). These interventions may include a ban on unhealthy food advertisements aimed at children as well as access to playgrounds and green spaces where families can feel their children can safely recreate. Also, this will limit screen time for adolescents as well as younger children.
However, even with the above changes, pharmacotherapy and/or bariatric surgery will likely remain a necessary option for those youth with morbid obesity ( 1 ). This review summarizes our current understanding of the factors associated with obesity, the physiological and psychological effects of obesity on children and adolescents, and intervention strategies that may prevent future concomitant issues.
Definition of Childhood Obesity
Body mass index (BMI) is an inexpensive method to assess body fat and is derived from a formula derived from height and weight in children over 2 years of age ( 1 , 18 , 19 ). Although more sophisticated methods exist that can determine body fat directly, they are costly and not readily available. These methods include measuring skinfold thickness with a caliper, Bioelectrical impedance, Hydro densitometry, Dual-energy X-ray Absorptiometry (DEXA), and Air Displacement Plethysmography ( 2 ).
BMI provides a reasonable estimate of body fat indirectly in the healthy pediatric population and studies have shown that BMI correlates with body fat and future health risks ( 18 ). Unlike in adults, Z-scores or percentiles are used to represent BMI in children and vary with the age and sex of the child. BMI Z-score cut off points of >1.0, >2.0, and >3.0 are recommended by the World Health Organization (WHO) to define at risk of overweight, overweight and obesity, respectively ( 19 ). However, in terms of percentiles, overweight is applied when BMI is >85th percentile <95th percentile, whereas obesity is BMI > 95th percentile ( 20 – 22 ). Although BMI Z-scores can be converted to BMI percentiles, the percentiles need to be rounded and can misclassify some normal-weight children in the under or overweight category ( 19 ). Therefore, to prevent these inaccuracies and for easier understanding, it is recommended that the BMI Z-scores in children should be used in research whereas BMI percentiles are best used in the clinical settings ( 20 ).
As BMI does not directly measure body fat, it is an excellent screening method, but should not be used solely for diagnostic purposes ( 23 ). Using 85th percentile as a cut off point for healthy weight may miss an opportunity to obtain crucial information on diet, physical activity, and family history. Once this information is obtained, it may allow the provider an opportunity to offer appropriate anticipatory guidance to the families.
Pathophysiology of Obesity
The pathophysiology of obesity is complex that results from a combination of individual and societal factors. At the individual level, biological, and physiological factors in the presence of ones' own genetic risk influence eating behaviors and tendency to gain weight ( 1 ). Societal factors include influence of the family, community and socio-economic resources that further shape these behaviors ( Figure 1 ) ( 3 , 24 ).
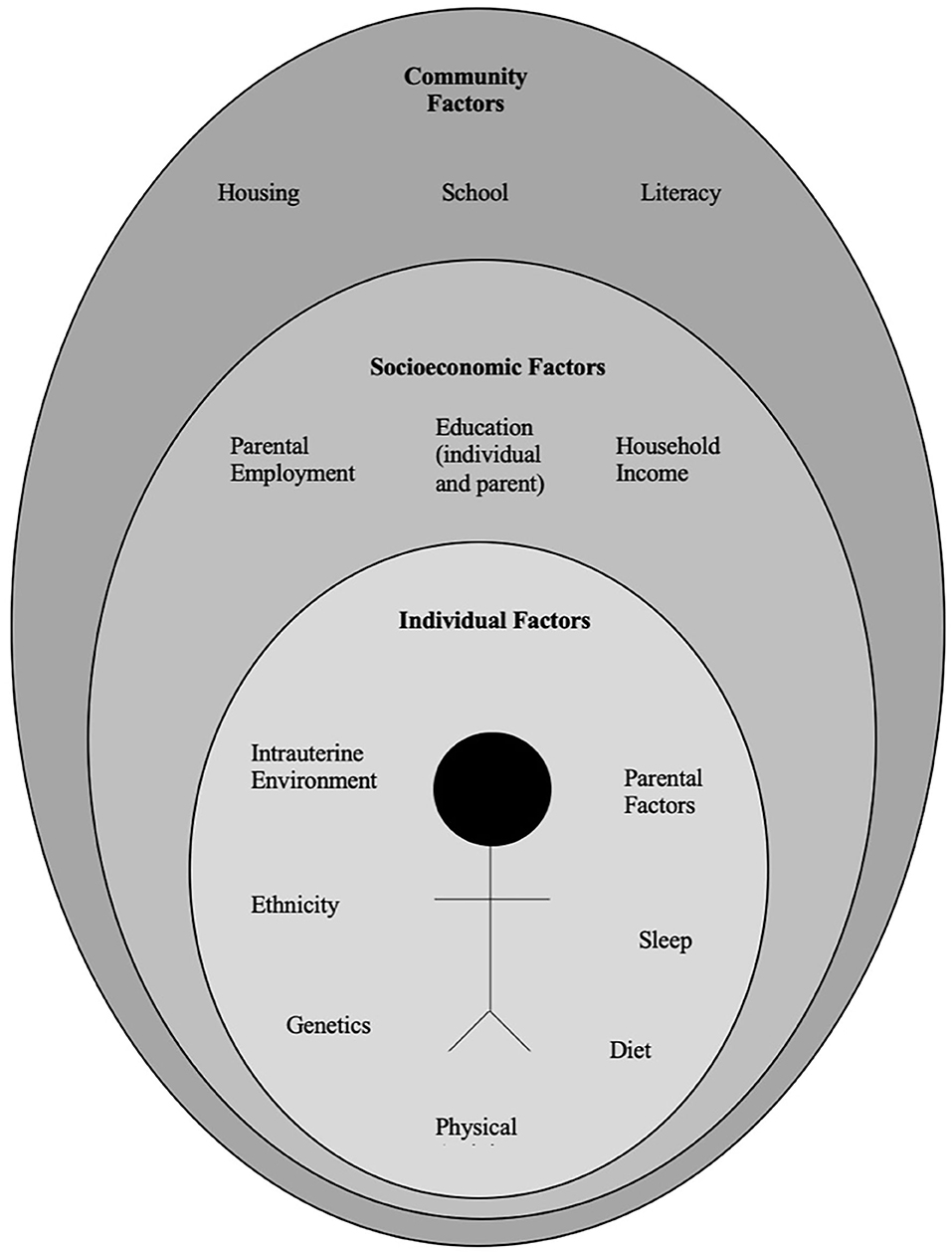
Figure 1 . Multidimensional factors contributing to child and adolescent obesity.
Biological Factors
There is a complex architecture of neural and hormonal regulatory control, the Gut-Brain axis, which plays a significant role in hunger and satiety ( Figure 2 ). Sensory stimulation (smell, sight, and taste), gastrointestinal signals (peptides, neural signals), and circulating hormones further contribute to food intake ( 25 – 27 ).
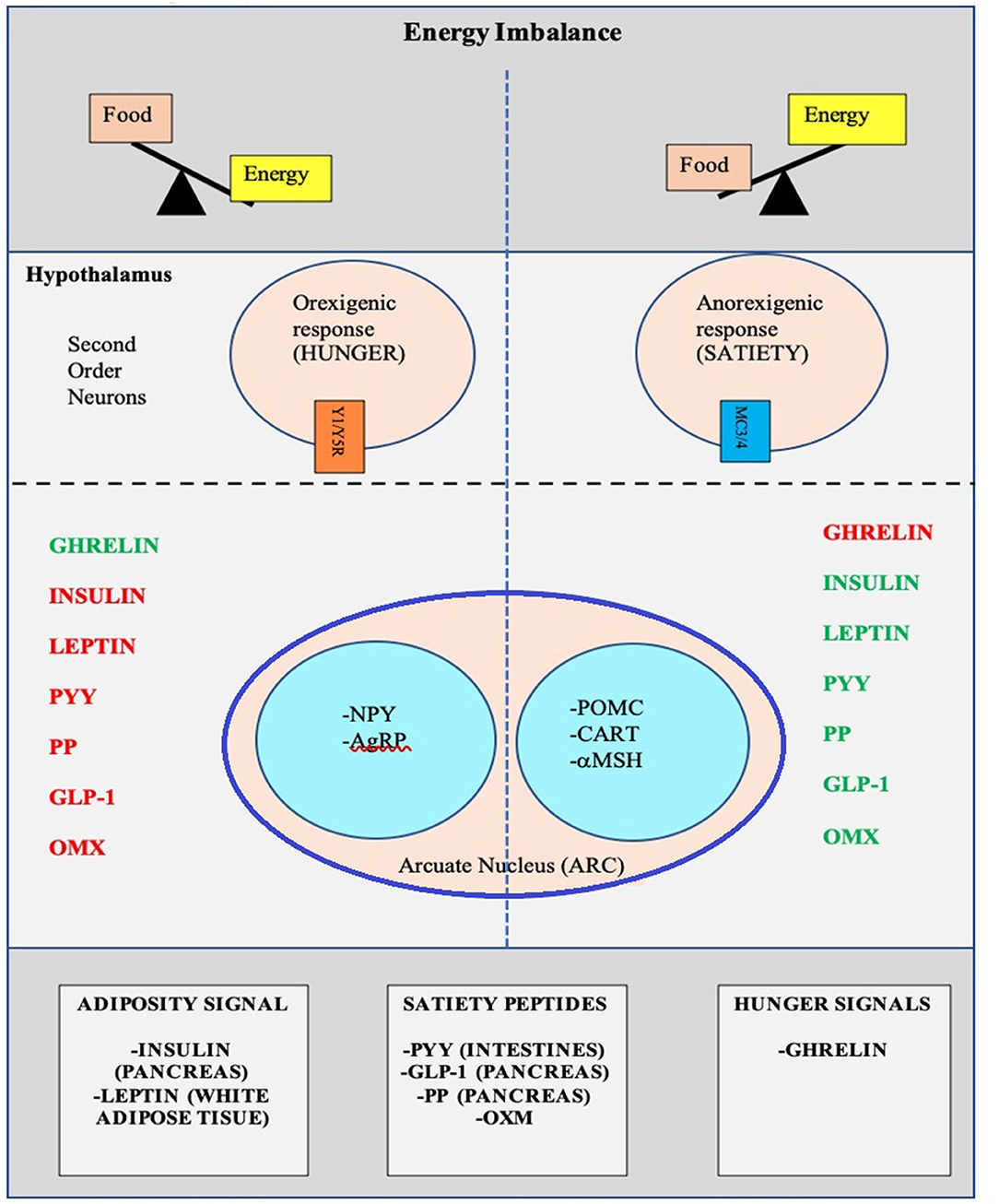
Figure 2 . Pictorial representation of the Hunger-Satiety pathway a and the various hormones b involved in the pathway. a, Y1/Y5R and MC3/4 are second order neuro receptors which are responsible in either the hunger or satiety pathway. Neurons in the ARC include: NPY, Neuropeptide Y; AgRP, Agouti-Related Peptide; POMC, Pro-Opiomelanocortin; CART, Cocaine-and Amphetamine-regulated Transcript; α-MSH, α-Melanocyte Stimulating Hormone. b, PYY, Peptide YY; PP, Pancreatic Polypeptide; GLP-1, Glucagon-Like Peptide- I; OMX, Oxyntomodulin.
The hypothalamus is the crucial region in the brain that regulates appetite and is controlled by key hormones. Ghrelin, a hunger-stimulating (orexigenic) hormone, is mainly released from the stomach. On the other hand, leptin is primarily secreted from adipose tissue and serves as a signal for the brain regarding the body's energy stores and functions as an appetite -suppressing (anorexigenic) hormone. Several other appetite-suppressing (anorexigenic) hormones are released from the pancreas and gut in response to food intake and reach the hypothalamus through the brain-blood barrier (BBB) ( 28 – 32 ). These anorexigenic and orexigenic hormones regulate energy balance by stimulating hunger and satiety by expression of various signaling pathways in the arcuate nucleus (ARC) of the hypothalamus ( Figure 2 ) ( 28 , 33 ). Dysregulation of appetite due to blunted suppression or loss of caloric sensing signals can result in obesity and its morbidities ( 34 ).
Emotional dysfunction due to psychiatric disorders can cause stress and an abnormal sleep-wake cycles. These modifications in biological rhythms can result in increased appetite, mainly due to ghrelin, and can contribute to emotional eating ( 35 ).
Recently, the role of changes in the gut microbiome with increased weight gain through several pathways has been described in literature ( 36 , 37 ). The human gut serves as a host to trillions of microorganisms, referred to as gut microbiota. The dominant gut microbial phyla are Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria, and Verrucomicrobia, with Firmicutes and Bacteroidetes representing 90% of human gut microbiota ( 5 , 38 ). The microbes in the gut have a symbiotic relationship within their human host and provide a nutrient-rich environment. Gut microbiota can be affected by various factors that include gestational age at birth, mode of infant delivery, type of neonatal and infant feeding, introduction of solid food, feeding practices and external factors like antibiotic use ( 5 , 38 ). Also, the maturation of the bacterial phyla that occurs from birth to adulthood ( 39 ), is influenced by genetics, environment, diet, lifestyle, and gut physiology and stabilizes in adulthood ( 5 , 39 , 40 ). Gut microbiota is unique to each individual and plays a specific role in maintaining structural integrity, and the mucosal barrier of the gut, nutrient metabolism, immune response, and protection against pathogens ( 5 , 37 , 38 ). In addition, the microbiota ferments the indigestible food and synthesizes other essential micronutrients as well as short chain fatty acids (SCFAs') ( 40 , 41 ). Dysbiosis or imbalance of the gut microbiota, in particularly the role of SCFA has been linked with the patho-physiology of obesity ( 36 , 38 , 41 , 42 ). SCFAs' are produced by anaerobic fermentation of dietary fiber and indigestible starch and play a role in mammalian energy metabolism by influencing gut-brain communication axis. Emerging evidence has shown that increased ratio of Firmicutes to Bacteroidetes causes increased energy extraction of calories from diets and is evidenced by increased production of short chain fatty acids (SCFAs') ( 43 – 45 ). However, this relationship is not affirmed yet, as a negative relationship between SCFA levels and obesity has also been reported ( 46 ). Due to the conflicting data, additional randomized control trials are needed to clarify the role of SCFA's in obese and non-obese individuals.
The gut microbiota also has a bidirectional interaction with the liver, and various additional factors such as diet, genetics, and the environment play a key role in this relationship. The Gut- Liver Axis is interconnected at various levels that include the mucus barrier, epithelial barrier, and gut microbiome and are essential to maintain normal homeostasis ( 47 ). Increased intestinal mucosal permeability can disrupt the gut-liver axis, which releases various inflammatory markers, activates an innate immune response in the liver, and results in a spectrum of liver diseases that include hepatic steatosis, non-alcoholic steatohepatitis (NASH), cirrhosis, and hepatocellular carcinoma (HCC) ( 48 , 49 ).
Other medical conditions, including type 2 Diabetes Mellitus, Metabolic Syndrome, eating disorders as well as psychological conditions such as anxiety and depression are associated with the gut microbiome ( 50 – 53 ).
Genetic Factors
Genetic causes of obesity can either be monogenic or polygenic types. Monogenic obesity is rare, mainly due to mutations in genes within the leptin/melanocortin pathway in the hypothalamus that is essential for the regulation of food intake/satiety, body weight, and energy metabolism ( 54 ). Leptin regulates eating behaviors, the onset of puberty, and T-cell immunity ( 55 ). About 3% of obese children have mutations in the leptin ( LEP ) gene and the leptin receptor (LEPR) and can also present with delayed puberty and immune dysfunction ( 55 , 56 ). Obesity caused by other genetic mutations in the leptin-melanocortin pathway include proopiomelanocortin (POMC) and melanocortin receptor 4 (MC4R), brain-derived neurotrophic factor (BDNF), and the tyrosine kinase receptor B (NTRK2) genes ( 57 , 58 ). Patients with monogenic forms generally present during early childhood (by 2 years old) with severe obesity and abnormal feeding behaviors ( 59 ). Other genetic causes of severe obesity are Prader Willi Syndrome (PWS), Alström syndrome, Bardet Biedl syndrome. Patients with these syndromes present with additional characteristics, including cognitive impairment, dysmorphic features, and organ-specific developmental abnormalities ( 60 ). Individuals who present with obesity, developmental delay, dysmorphic features, and organ dysfunction should receive a genetics referral for further evaluation.
Polygenic obesity is the more common form of obesity, caused by the combined effect of multiple genetic variants. It is the result of the interplay between genetic susceptibility and the environment, also known as the Gene-Environment Interaction (GEI) ( 61 – 64 ). Genome-wide association studies (GWAS) have identified gene variants [single nucleotide polymorphism (SNPs)] for body mass index (BMI) that likely act synergistically to affect body weight ( 65 ). Studies have identified genetic variants in several genes that may contribute to excessive weight gain by increasing hunger and food intake ( 66 – 68 ). When the genotype of an individual confers risk for obesity, exposure to an obesogenic environment may promote a state of energy imbalance due to behaviors that contribute to conserving rather than expending energy ( 69 , 70 ). Research studies have shown that obese individuals have a genetic variation that can influence their actions, such as increased food intake, lack of physical activity, a decreased metabolism, as well as an increased tendency to store body fat ( 63 , 66 , 67 , 69 , 70 ).
Recently the role of epigenetic factors in the development of obesity has emerged ( 71 ). The epigenetic phenomenon may alter gene expression without changing the underlying DNA sequence. In effect, epigenetic changes may result in the addition of chemical tags known as methyl groups, to the individual's chromosomes. This alteration can result in a phenomenon where critical genes are primed to on and off regulate. Complex physiological and psychological adjustment occur during infancy and can thereafter set the stage for health vs. disease. Developmental origins of health and disease (DOHaD) shows that early life environment can impact the risk of chronic diseases later in life due to fetal programming secondary to epigenetic changes ( 72 ). Maternal nutrition during the prenatal or early postnatal period may trigger these epigenetic changes and increase the risk for chronic conditions such as obesity, metabolic and cardiovascular disease due to epigenetic modifications that may persist and cause intergenerational effect on the health children and adults ( 58 , 73 , 74 ). Similarly, adverse childhood experiences (ACE) have been linked to a broad range of negative outcomes through epigenetic mechanisms ( 75 ) and promote unhealthy eating behaviors ( 76 , 77 ). Other factors such as diet, physical activity, environmental and psychosocial stressors can cause epigenetic changes and place an individual at risk for weight gain ( 78 ).
Developmental Factors
Eating behaviors evolve over the first few years of life. Young children learn to eat through their direct experience with food and observing others eating around them ( 79 ). During infancy, feeding defines the relationship of security and trust between a child and the parent. Early childhood eating behaviors shift to more self-directed control due to rapid physical, cognitive, communicative, and social development ( 80 ). Parents or caregivers determine the type of food that is made available to the infant and young child. However, due to economic limitations and parents having decreased time to prepare nutritious meals, consumption of processed and cheaper energy-dense foods have occurred in Western countries. Additionally, feeding practices often include providing large or super-sized portions of palatable foods and encouraging children to finish the complete meal (clean their plate even if they do not choose to), as seen across many cultures ( 81 , 82 ). Also, a segment of parents are overly concerned with dietary intake and may pressurize their child to eat what they perceive as a healthy diet, which can lead to unintended consequences ( 83 ). Parents' excessive restriction of food choices may result in poor self-regulation of energy intake by their child or adolescent. This action may inadvertently promote overconsumption of highly palatable restricted foods when available to the child or adolescent outside of parental control with resultant excessive weight gain ( 84 , 85 ).
During middle childhood, children start achieving greater independence, experience broader social networks, and expand their ability to develop more control over their food choices. Changes that occur in the setting of a new environment such as daycare or school allow exposure to different food options, limited physical activity, and often increased sedentary behaviors associated with school schedules ( 24 ). As the transition to adolescence occurs, physical and psychosocial development significantly affect food choices and eating patterns ( 25 ). During the teenage years, more independence and interaction with peers can impact the selection of fast foods that are calorically dense. Moreover, during the adolescent years, more sedentary behaviors such as video and computer use can limit physical exercise. Adolescence is also a period in development with an enhanced focus on appearance, body weight, and other psychological concerns ( 86 , 87 ).
Environmental Factors
Environmental changes within the past few decades, particularly easy access to high-calorie fast foods, increased consumption of sugary beverages, and sedentary lifestyles, are linked with rising obesity ( 88 ). The easy availability of high caloric fast foods, and super-sized portions, are increasingly common choices as individuals prefer these highly palatable and often less expensive foods over fruits and vegetables ( 89 ). The quality of lunches and snacks served in schools and childcare centers has been an area of debate and concern. Children and adolescents consume one-third to one-half of meals in the above settings. Despite policies in place at schools, encouraging foods, beverages, and snacks that are deemed healthier options, the effectiveness of these policies in improving children's dietary habits or change in obesity rate has not yet been seen ( 90 ). This is likely due to the fact that such policies primarily focus on improving dietary quality but not quantity which can impact the overweight or obese youth ( 91 ). Policies to implement taxes on sugary beverages are in effect in a few states in the US ( 92 ) as sugar and sugary beverages are associated with increased weight gain ( 2 , 3 ). This has resulted in reduction in sales of sugary drinks in these states, but the sales of these types of drinks has risen in neighboring states that did not implement the tax ( 93 ). Due to advancements in technology, children are spending increased time on electronic devices, limiting exercise options. Technology advancement is also disrupting the sleep-wake cycle, causing poor sleeping habits, and altered eating patterns ( 94 ). A study published on Canadian children showed that the access to and night-time use of electronic devices causes decreased sleep duration, resulting in excess body weight, inferior diet quality, and lower physical activity levels ( 95 ).
Infant nutrition has gained significant popularity in relation to causing overweight/obesity and other diseases later in life. Breast feeding is frequently discussed as providing protection against developing overweight/obesity in children ( 8 ). Considerable heterogeneity has been observed in studies and conducting randomized clinical trials between breast feeding vs. formula feeding is not feasible ( 8 ). Children fed with a low protein formula like breast milk are shown to have normal weight gain in early childhood as compared to those that are fed formulas with a high protein load ( 96 ). A recent Canadian childbirth cohort study showed that breast feeding within first year of life was inversely associated with weight gain and increased BMI ( 97 ). The effect was stronger if the child was exclusively breast fed directly vs. expressed breast milk or addition of formula or solid food ( 97 ). Also, due to the concern of poor growth in preterm or SGA infants, additional calories are often given for nutritional support in the form of macronutrient supplements. Most of these infants demonstrate “catch up growth.” In fact, there have been reports that in some children the extra nutritional support can increase the risk for overweight/obesity later in life. The association, however, is inconsistent. Recently a systemic review done on randomized controlled trials comparing the studies done in preterm and SGA infants with feeds with and without macronutrient supplements showed that macronutrient supplements may increase weight and length in toddlers but did not show a significant increase in the BMI during childhood ( 98 ). Increased growth velocity due to early introduction of formula milk and protein in infants' diet, may influence the obesity pathways, and can impact fetal programming for metabolic disease later in life ( 99 ).
General pediatricians caring for children with overweight/obesity, generally recommend endocrine testing as parents often believe that there may be an underlying cause for this condition and urge their primary providers to check for conditions such as thyroid abnormalities. Endocrine etiologies for obesity are rarely identified and patients with underlying endocrine disorders causing excessive weight gain usually are accompanied by attenuated growth patterns, such that a patient continues to gain weight with a decline in linear height ( 100 ). Various endocrine etiologies that one could consider in a patient with excessive weight gain in the setting of slow linear growth: severe hypothyroidism, growth hormone deficiency, and Cushing's disease/syndrome ( 58 , 100 ).
Clinical-Physiology of Pediatric Obesity
It is a well-known fact that early AR(increased BMI) before the age of 5 years is a risk factor for adult obesity, obesity-related comorbidities, and metabolic syndrome ( 101 – 103 ). Typically, body mass index (BMI) declines to a minimum in children before it starts increasing again into adulthood, also known as AR. Usually, AR happens between 5 and 7 years of age, but if it occurs before the age of 5 years is considered early AR. Early AR is a marker for higher risk for obesity-related comorbidities. These obesity-related health comorbidities include cardiovascular risk factors (hypertension, dyslipidemia, prediabetes, and type 2 diabetes), hormonal issues, orthopedic problems, sleep apnea, asthma, and fatty liver disease ( Figure 3 ) ( 9 ).
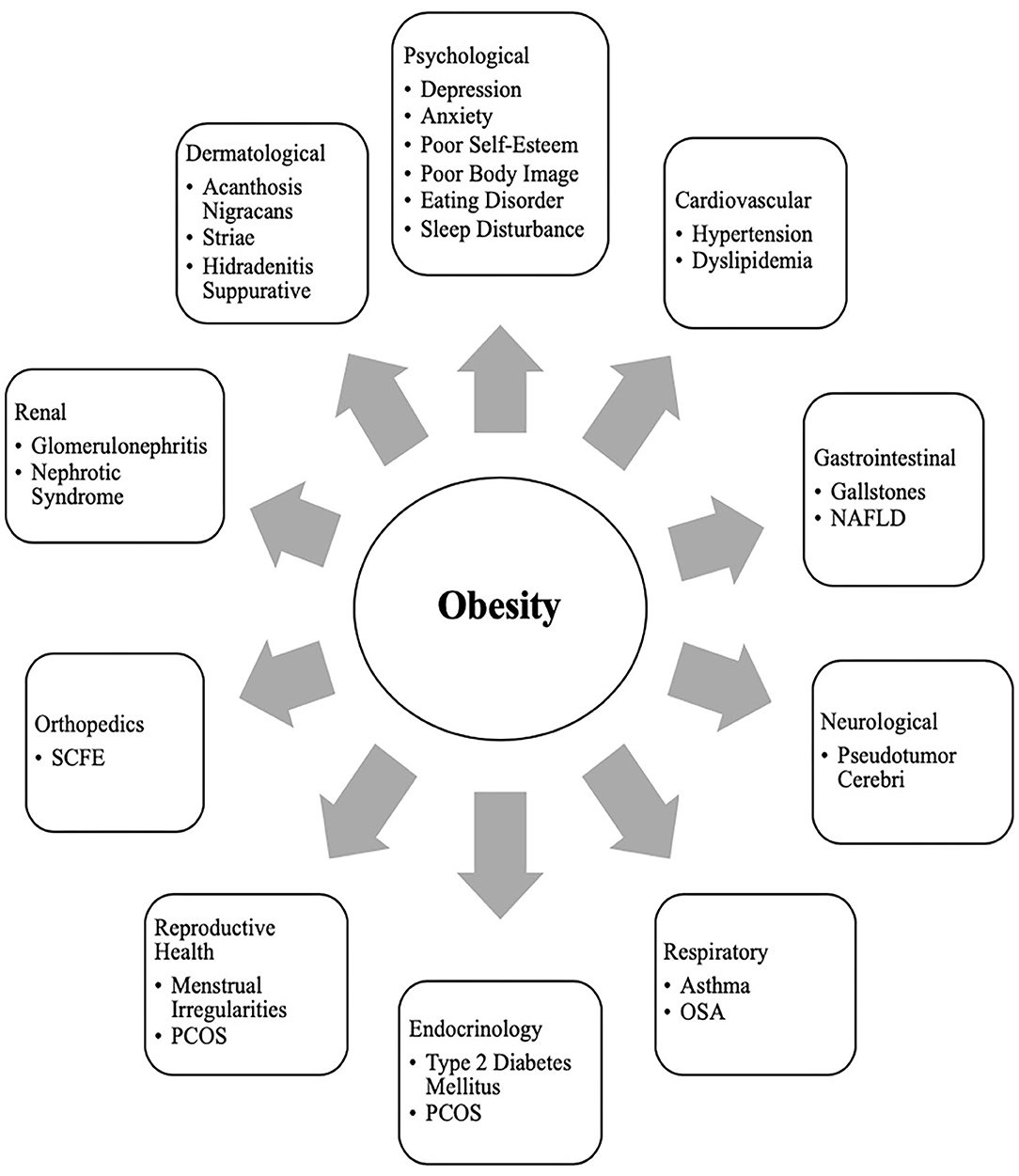
Figure 3 . Obesity related co-morbidities a in children and adolescents. a, NAFLD, Non-Alcoholic Fatty Liver Disease; SCFE, Slipped Capital Femoral Epiphysis; PCOS, Polycystic Ovary Syndrome; OSA, Obstructive Sleep Apnea.
Clinical Comorbidities of Obesity in Children
Growth and puberty.
Excess weight gain in children can influence growth and pubertal development ( 10 ). Childhood obesity can cause prepubertal acceleration of linear growth velocity and advanced bone age in boys and girls ( 104 ). Hyperinsulinemia is a normal physiological state during puberty, but children with obesity can have abnormally high insulin levels ( 105 ). Leptin resistance also occurs in obese individuals who have higher leptin levels produced by their adipose tissue ( 55 , 106 ). The insulin and leptin levels can act on receptors that impact the growth plates with a resultant bone age advancement ( 55 ).
Adequate nutrition is essential for the typical timing and tempo of pubertal onset. Excessive weight gain can initiate early puberty, due to altered hormonal parameters ( 10 ). Obese children may present with premature adrenarche, thelarche, or precocious puberty (PP) ( 107 ). The association of early pubertal changes with obesity is consistent in girls, and is well-reported; however, data is sparse in boys ( 108 ). One US study conducted in racially diverse boys showed obese boys had delayed puberty, whereas overweight boys had early puberty as compared to normal-weight boys ( 109 ). Obese girls with PP have high leptin levels ( 110 , 111 ). Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) is a cross-sectional study and suggested an indirect relationship between elevated leptin levels, early puberty, and cardiometabolic and inflammatory markers in obese girls ( 112 ). Additionally, obese girls with premature adrenarche carry a higher risk for developing polycystic ovary syndrome (PCOS) in the future ( 113 , 114 ).
Sleep Disorders
Obesity is an independent risk factor for obstructive sleep apnea (OSA) in children and adolescents ( 12 , 115 ). Children with OSA have less deleterious consequences in terms of cardiovascular stress of metabolic syndrome when compared to adolescents and adults ( 116 , 117 ). In children, abnormal behaviors and neurocognitive dysfunction are the most critical and frequent end-organ morbidities associated with OSA ( 12 ). However, in adolescents, obesity and OSA can independently cause oxidative systemic stress and inflammation ( 118 , 119 ), and when this occurs concurrently, it can result in more severe metabolic dysfunction and cardiovascular outcomes later in life ( 120 ).
Other Comorbidities
Obesity is related to a clinical spectrum of liver abnormalities such as NAFLD ( 121 ); the most important cause of liver disease in children ( 122 – 124 ). NAFLD includes steatosis (increased liver fat without inflammation) and NASH (increased liver fat with inflammation and hepatic injury). While in some adults NAFLD can progress to an end-stage liver disease requiring liver transplant ( 125 , 126 ), the risk of progression during childhood is less well-defined ( 127 ). NAFLD is closely associated with metabolic syndrome including central obesity, insulin resistance, type 2 diabetes, dyslipidemia, and hypertension ( 128 ).
Obese children are also at risk for slipped capital femoral epiphysis (SCFE) ( 129 ), and sedentary lifestyle behaviors may have a negative influence on the brain structure and executive functioning, although the direction of causality is not clear ( 130 , 131 ).
Clinical Comorbidities of Obesity in Adolescents
Menstrual irregularities and pcos.
At the onset of puberty, physiologically, sex steroids can cause appropriate weight gain and body composition changes that should not affect normal menstruation ( 132 , 133 ). However, excessive weight gain in adolescent girls can result in irregular menstrual cycles and puts them at risk for PCOS due to increased androgen levels. Additionally, they can have excessive body hair (hirsutism), polycystic ovaries, and can suffer from distorted body images ( 134 , 135 ). Adolescent girls with PCOS also have an inherent risk for insulin resistance irrespective of their weight. However, weight gain further exacerbates their existing state of insulin resistance and increases the risk for obesity-related comorbidities such as metabolic syndrome, and type 2 diabetes. Although the diagnosis of PCOS can be challenging at this age due to an overlap with predictable pubertal changes, early intervention (appropriate weight loss and use of hormonal methods) can help restore menstrual cyclicity and future concerns related to childbearing ( 11 ).
Metabolic Syndrome and Sleep Disorders
Metabolic syndrome (MS) is a group of cardiovascular risk factors characterized by acanthosis nigricans, prediabetes, hypertension, dyslipidemia, and non-alcoholic steatohepatitis (NASH), that occurs from insulin resistance caused by obesity ( 136 ). Diagnosis of MS in adults requires at least three out of the five risk factors: increased central adiposity, hypertension, hyperglycemia, hypertriglyceridemia, or low HDL level. Definitions to diagnose MS are controversial in younger age groups, and many definitions have been proposed ( 136 ). This is due to the complex physiology of growth and development during puberty, which causes significant overlap between MS and features of normal growth. However, childhood obesity is associated with an inflammatory state even before puberty ( 137 ). In obese children and adolescents, hyperinsulinemia during puberty ( 138 , 139 ) and unhealthy sleep behaviors increase MS's risk and severity ( 140 ). Even though there is no consensus on diagnosis regarding MS in this age group, when dealing with obese children and adolescents, clinicians should screen them for MS risk factors and sleep behaviors and provide recommendations for weight management.
Social Psychology of Pediatric Obesity in Children and Adolescents
Obese children and adolescents may experience psychosocial sequelae, including depression, bullying, social isolation, diminished self-esteem, behavioral problems, dissatisfaction with body image, and reduced quality of life ( 13 , 141 ). Compared with normal-weight counterparts, overweight/obesity is one of the most common reasons children and adolescents are bullied at school ( 142 ). The consequence of stigma, bullying, and teasing related to childhood obesity are pervasive and can have severe implications for emotional and physical health and performance that can persist later in life ( 13 ).
In adolescents, psychological outcomes associated with obesity are multifactorial and have a bidirectional relationship ( Figure 4 ). Obese adolescents due to their physique may have a higher likelihood of psychosocial health issues, including depression, body image/dissatisfaction, lower self-esteem, peer victimization/bullying, and interpersonal relationship difficulties. They may also demonstrate reduced resilience to challenging situations compared to their non-obese/overweight counterparts ( 9 , 143 – 146 ). Body image dissatisfaction has been associated with further weight gain but can also be related to the development of a mental health disorder or an eating disorder (ED) or disorder eating habits (DEH). Mental health disorders such as depression are associated with poor eating habits, a sedentary lifestyle, and altered sleep patterns. ED or DEH that include anorexia nervosa (AN), bulimia nervosa (BN), binge-eating disorder (BED) or night eating syndrome (NES) may be related to an individual's overvaluation of their body shape and weight or can result during the treatment for obesity ( 147 – 150 ). The management of obesity can place a patient at risk of AN if there is a rigid focus on caloric intake or if a patient overcorrects and initiates obsessive self-directed dieting. Healthcare providers who primarily care for obese patients, usually give the advice to diet to lose weight and then maintain it. However, strict dieting (hypocaloric diet), which some patients may later engage in can lead to an eating disorder such as anorexia nervosa ( 151 ). This behavior leads to a poor relationship with food, and therefore, adolescents perseverate on their weight and numbers ( 152 ).
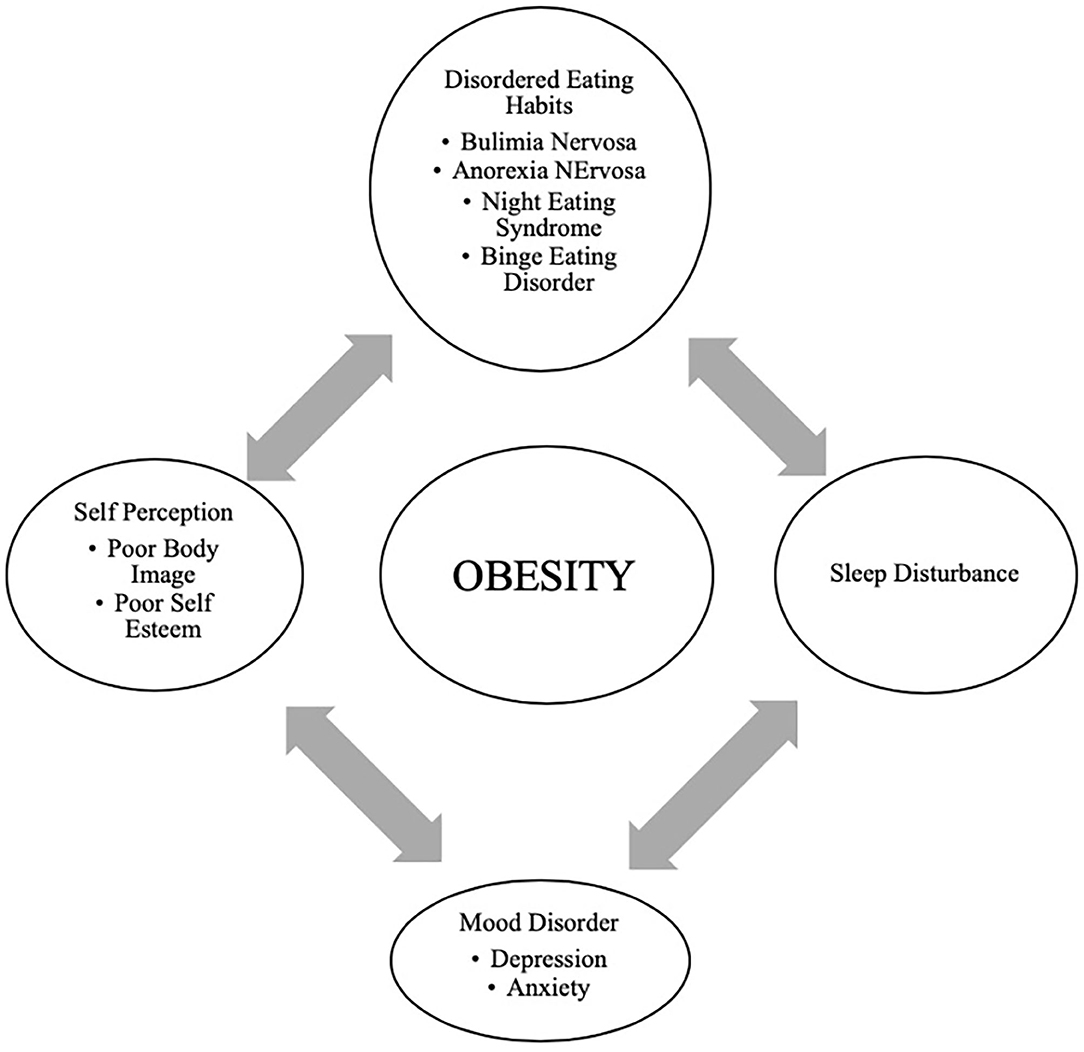
Figure 4 . Bidirectional relationship of different psychological outcomes of obesity.
Providers may not recognize DEHs when a morbidly obese patient loses the same weight as a healthy weight individual ( 149 ). It may appear as a positive result with families and others praising the individual without realizing that this youth may be engaging in destructive behaviors related to weight control. Therefore, it is essential to screen regarding the process of how weight loss was achieved ( 144 , 150 ).
Support and attention to underlying psychological concerns can positively affect treatment, overall well-being, and reduce the risk of adult obesity ( 150 ). The diagram above represents the complexity of the different psychological issues which can impact the clinical care of the obese adolescent.
Eating family meals together can improve overall dietary intake due to enhanced food choices mirrored by parents. It has also may serve as a support to individuals with DEHs if there is less attention to weight and a greater focus on appropriate, sustainable eating habits ( 148 ).
Prevention and Anticipatory Guidance
It is essential to recognize and provide preventive measures for obesity during early childhood and adolescence ( 100 , 153 , 154 ). It is well-established that early AR is a risk factor for adult obesity ( 66 – 68 ). Therefore, health care providers caring for the pediatric population need to focus on measures such as BMI but provide anticipatory guidance regarding nutritional counseling without stigmatizing or judging parents for their children's overweight/obesity ( 155 ). Although health care providers continue to pursue effective strategies to address the obesity epidemic; ironically, they frequently exhibit weight bias and stigmatizing behaviors. Research has demonstrated that the language that health care providers use when discussing a patient's body weight can reinforce stigma, reduce motivation for weight loss, and potentially cause avoidance of routine preventive care ( 155 ). In adolescents, rather than motivating positive changes, stigmatizing language regarding weight may negatively impact a teen and result in binge eating, decreased physical activity, social isolation, avoidance of health care services, and increased weight gain ( 156 , 157 ). Effective provider-patient communication using motivational interviewing techniques are useful to encourage positive behavior changes ( 155 , 158 ).
Anticipatory guidance includes educating the families on healthy eating habits and identifying unhealthy eating practices, encouraging increased activity, limiting sedentary activities such as screen time. Lifestyle behaviors in children and adolescents are influenced by many sectors of our society, including the family ( Figure 1 ) ( 3 , 24 ). Therefore, rather than treating obesity in isolation as an individual problem, it is crucial to approach this problem by focusing on the family unit. Family-based multi-component weight loss behavioral treatment is the gold standard for treating childhood obesity, and it is having been found useful in those between 2 and 6 years old ( 150 , 159 ). Additionally, empowering the parents to play an equal role in developing and implementing an intervention for weight management has shown promising results in improving the rate of obesity by decreasing screen time, promoting healthy eating, and increasing support for children's physical activity ( 160 , 161 ).
When dietary/lifestyle modifications have failed, the next option is a structured weight -management program with a multidisciplinary approach ( 15 ). The best outcomes are associated with an interdisciplinary team comprising a physician, dietician, and psychologist generally 1–2 times a week ( 15 , 162 ). However, this treatment approach is not effective in patients with severe obesity ( 122 ). Although healthier lifestyle recommendations for weight loss are the current cornerstone for obesity management, they often fail. As clinicians can attest, these behavioral and dietary changes are hard to achieve, and all too often is not effective in patients with severe obesity. Failure to maintain substantial weight loss over the long term is due to poor adherence to the prescribed lifestyle changes as well as physiological responses that resist weight loss ( 163 ). American TV hosts a reality show called “The Biggest Loser” that centers on overweight and obese contestants attempting to lose weight for a cash prize. Contestants from “The Biggest Loser” competition, had metabolic adaptation (MA) after drastic weight loss, regained more than they lost weight after 6 years due to a significant slow resting metabolic rate ( 164 ). MA is a physiological response which is a reduced basal metabolic rate seen in individuals who are losing or have lost weight. In MA, the body alters how efficient it is at turning the food eaten into energy; it is a natural defense mechanism against starvation and is a response to caloric restriction. Plasma leptin levels decrease substantially during caloric restriction, suggesting a role of this hormone in the drop of energy expenditure ( 165 ).
Pharmacological Management
The role of pharmacological therapy in the treatment of obesity in children and adolescents is limited.
Orlistat is the only FDA approved medication for weight loss in 12-18-year-olds but has unpleasant side effects ( 166 ). Another medicine, Metformin, has been used in children with signs of insulin resistance, may have some impact on weight, but is not FDA approved ( 167 ). The combination of phentermine/topiramate (Qsymia) has been FDA approved for weight loss in obese individuals 18 years and older. In studies, there has been about 9–10% weight loss over 2 years. However, caution must be taken in females as it can lead to congenital disabilities, especially with use in the first trimester of pregnancy ( 167 ).
GLP-1 agonists have demonstrated great success in effective weight loss and are approved by the FDA for adult obesity ( 168 – 170 ). A randomized control clinical trial recently published showed a significant weight loss in those using liraglutide (3.0 mg)/day plus lifestyle therapy group compared to placebo plus lifestyle therapy in children between the ages of 12–18 years ( 171 ).
Recently during the EASL conference, academic researchers and industry partners presented novel interventions targeting different gut- liver axis levels that include intestinal content, intestinal microbiome, intestinal mucosa, and peritoneal cavity ( 47 ). The focus for these therapeutic interventions within the gut-liver axis was broad and ranged anywhere from newer drugs protecting the intestinal mucus lining, restoring the intestinal barriers and improvement in the gut microbiome. One of the treatment options was Hydrogel technology which was shown to be effective toward weight loss in patients with metabolic syndrome. Hydrogel technology include fibers and high viscosity polysaccharides that absorb water in the stomach and increasing the volume, thereby improving satiety ( 47 ). Also, a clinical trial done in obese pregnant mothers using Docosahexaenoic acid (DHA) showed that the mothers' who got DHA had children with lower adiposity at 2 and 4 years of age ( 172 ). Recently the role of probiotics in combating obesity has emerged. Probiotics are shown to alter the gut microbiome that improves intestinal digestive and absorptive functions of the nutrients. Intervention including probiotics may be a possible solution to manage pediatric obesity ( 173 , 174 ). Additionally, the role of Vitamin E for treating the comorbidities of obesity such as diabetes, hyperlipidemia, NASH, and cardiovascular risk, has been recently described ( 175 , 176 ). Vitamin E is a lipid- soluble compound and contains both tocopherols and tocotrienols. Tocopherols have lipid-soluble antioxidants properties that interact with cellular lipids and protects them from oxidation damage ( 177 ). In metabolic disease, certain crucial pathways are influenced by Vitamin E and some studies have summarized the role of Vitamin E regarding the treatment of obesity, metabolic, and cardiovascular disease ( 178 ). Hence, adequate supplementation of Vitamin E as an appropriate strategy to help in the treatment of the prevention of obesity and its associated comorbidities has been suggested. Nonetheless, some clinical trials have shown contradictory results with Vitamin E supplementation ( 177 ). Although Vitamin E has been recognized as an antioxidant that protects from oxidative damage, however, a full understanding of its mechanism of action is still lacking.
Bariatric Surgery
Bariatric surgery has gained popularity since the early 2000s in the management of severe obesity. If performed earlier, there are better outcomes for reducing weight and resolving obesity-related comorbidities in adults ( 179 – 182 ). Currently, the indication for bariatric in adolescents; those who have a BMI >35 with at least one severe comorbidity (Type 2 Diabetes, severe OSA, pseudotumor cerebri or severe steatohepatitis); or BMI of 40 or more with other comorbidities (hypertension, hyperlipidemia, mild OSA, insulin resistance or glucose intolerance or impaired quality of life due to weight). Before considering bariatric surgery, these patients must have completed most of their linear growth and participated in a structured weight-loss program for 6 months ( 159 , 181 , 183 ). The American Society for Metabolic and Bariatric Surgery (AMBS) outlines the multidisciplinary approach that must be taken before a patient undergoing bariatric surgery. In addition to a qualified bariatric surgeon, the patient must have a pediatrician or provider specialized in adolescent medicine, endocrinology, gastroenterology and nutrition, registered dietician, mental health provider, and exercise specialist ( 181 ). A mental health provider is essential as those with depression due to obesity or vice versa may have persistent mental health needs even after weight loss surgery ( 184 ).
Roux-en-Y Gastric Bypass (RYGB), laparoscopic Sleeve Gastrectomy (LSG), and Gastric Banding are the options available. RYGB and LSG currently approved for children under 18 years of age ( 166 , 181 , 185 ). At present, gastric banding is not an FDA recommended procedure in the US for those under 18y/o. One study showed some improvements in BMI and severity of comorbidities but had multiple repeat surgeries and did not believe a suitable option for obese adolescents ( 186 ).
Compared to LSG, RYGB has better outcomes for excess weight loss and resolution of obesity-related comorbidities as shown in studies and clinical trials ( 183 , 184 , 187 ). Overall, LSG is a safer choice and may be advocated for more often ( 179 – 181 ). The effect on the Gut-Brain axis after Bariatric surgery is still inconclusive, especially in adolescents, as the number of procedures performed is lower than in adults. Those who underwent RYGB had increased fasting and post-prandial PYY and GLP-1, which could have contributed to the rapid weight loss ( 185 ); this effect was seen less often in patients with gastric banding ( 185 ). Another study in adult patients showed higher bile acid (BA) subtype levels and suggested a possible BA's role in the surgical weight loss response after LSG ( 188 ). Adolescents have lower surgical complication rates than their adult counterparts, hence considering bariatric surgery earlier rather than waiting until adulthood has been entertained ( 180 ). Complications after surgery include nutritional imbalance in iron, calcium, Vitamin D, and B12 and should be monitored closely ( 180 , 181 , 185 ). Although 5-year data for gastric bypass in very obese teens is promising, lifetime outcome is still unknown, and the psychosocial factors associated with adolescent adherence post-surgery are also challenging and uncertain.
Obesity in childhood and adolescence is not amenable to a single easily modified factor. Biological, cultural, and environmental factors such as readily available high-density food choices impact youth eating behaviors. Media devices and associated screen time make physical activity a less optimal choice for children and adolescents. This review serves as a reminder that the time for action is now. The need for interventions to change the obesogenic environment by instituting policies around the food industry and in the schools needs to be clarified. In clinical trials GLP-1 agonists are shown to be effective in weight loss in children but are not yet FDA approved. Discovery of therapies to modify the gut microbiota as treatment for overweigh/obesity through use of probiotics or fecal transplantation would be revolutionary. For the present, ongoing clinical research efforts in concert with pharmacotherapeutic and multidisciplinary lifestyle programs hold promise.
Author Contributions
AK, SL, and MJ contributed to the conception and design of the study. All authors contributed to the manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Gurnani M, Birken C, Hamilton. J. Childhood obesity: causes, consequences, and management. Pediatr Clin North Am. (2015) 62:821–40. doi: 10.1016/j.pcl.2015.04.001
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Sahoo K, Sahoo B, Choudhury AK, Sofi NY, Kumar R, Bhadoria. AS. Childhood obesity: causes and consequences. J Family Med Prim Care. (2015) 4:187–92. doi: 10.4103/2249-4863.154628
3. Brown CL, Halvorson EE, Cohen GM, Lazorick S, Skelton JA. Addressing childhood obesity: opportunities for prevention. Pediatr Clin North Am. (2015) 62:1241–61. doi: 10.1016/j.pcl.2015.05.013
4. Qasim A, Turcotte M, de Souza RJ, Samaan MC, Champredon D, Dushoff J, et al. On the origin of obesity: identifying the biological, environmental, and cultural drivers of genetic risk among human populations. Obes Rev. (2018) 19:121–49. doi: 10.1111/obr.12625
5. Rinninella E, Raoul P, Cintoni M, Fransceschi F, Miggiano GAD, Gasbarrini A, et al. What is the healthy gut microbiota composition? a changing ecosystem across age, environment, diet, and diseases. Microorganisms. (2019) 7:14. doi: 10.3390/microorganisms7010014
6. Indrio F, Martini S, Francavilla R, Corvaglia L, Cristofori F, Mastrolia SA, et al. Epigenetic matters: the link between early nutrition, microbiome, and long-term health development. Front Pediatr. (2017) 5:178. doi: 10.3389/fped.2017.00178
7. Marcovecchio ML, Gorman S, Watson LPE, Dunger DB, Beardsall K. Catch-up growth in children born small for gestational age related to body composition and metabolic risk at six years of age in the UK. Horm Res Paediatr. (2020) 93:119–27. doi: 10.1159/000508974
8. Koletzko B, Fishbein M, Lee WS, Moreno L, Mouane N, Mouzaki M, et al. Prevention of childhood obesity: a position paper of the global federation of international societies of paediatric gastroenterology, hepatology nutrition (FISPGHAN). J Pediatr Gastroenterol Nutr. (2020) 70:702–10. doi: 10.1097/MPG.0000000000002708
9. Pulgarón ER. Childhood obesity: a review of increased risk for physical and psychological comorbidities. Clin Ther. (2013) 35:A18–32. doi: 10.1016/j.clinthera.2012.12.014
10. De Leonibus C, Marcovecchio ML, Chiarelli F. Update on statural growth and pubertal development in obese children. Pediatr Rep. (2012) 4:e35. doi: 10.4081/pr.2012.e35
11. Witchel SF, Burghard AC, Tao RH, Oberfield SE. The diagnosis and treatment of PCOS in adolescents. Curr Opin Pediatr . (2019) 31:562–9. doi: 10.1097/MOP.0000000000000778
12. Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics . (2012) 130:e714–55. doi: 10.1542/peds.2012-1672
CrossRef Full Text | Google Scholar
13. Rankin J, Matthews L, Cobley S, Han A, Sanders R, Wiltshire HD, et al. Psychological consequences of childhood obesity: psychiatric comorbidity and prevention. Adolesc Health Med Ther . (2016) 7:125–46. doi: 10.2147/AHMT.S101631
14. Topçu S, Orhon FS, Tayfun M, Uçaktürk SA, Demirel F. Anxiety, depression, and self-esteem levels in obese children: a case-control study. J Pediatr Endocrinol Metabol. (2016) 29:357–61. doi: 10.1515/jpem-2015-0254
15. Katzmarzyk PT, Barlow S, Bouchard C, Catalano PM, Hsia DS, Inge TH, et al. An evolving scientific basis for the prevention and treatment of pediatric obesity. Int J Obes. (2014) 38:887–905. doi: 10.1038/ijo.2014.49
16. Brown T, Moore TH, Hooper L, Gao Y, Zayegh A, Ijaz S, et al. Interventions for preventing obesity in children. Cochrane Database Syst Rev . (2019) 7:CD001871. doi: 10.1002/14651858.CD001871.pub4
17. Smith E, Scarborough P, Rayner M, Briggs ADM. Should we tax unhealthy food and drink? Proc Nutr Soc. (2019) 77:314–20. doi: 10.1017/S0029665117004165
18. Adab P, Pallan M, Whincup PH. Is BMI the best measure of obesity? BMJ. (2018) 360:k 1274. doi: 10.1136/bmj.k1274
19. Anderson LN, Carsley S, Lebovic G, Borkhoff CM, Maguire JL, Parkin PC, et al. Misclassification of child body mass index from cut-points defined by rounded percentiles instead of Z-scores. BMC Res Notes. (2017) 10:639. doi: 10.1186/s13104-017-2983-0
20. Must A, Anderson SE. Body mass index in children and adolescents: consideration for population-based applications. Int J Obes. (2006) 30:590–4. doi: 10.1038/sj.ijo.0803300
21. Flegal KM, Wei R, Ogden C. Weight-for-stature compared with body mass index-for-age growth charts for the United States from the centers for disease control and prevention. Am J Clin Nutr. (2002) 75:761–6.22. doi: 10.1093/ajcn/75.4.761
22. Himes JH, Dietz WH. Guidelines for overweight in adolescent preventive services: recommendations from an expert committee. The expert committee on clinical guidelines for overweight in adolescent preventive services. Am J Clin Nutr. (1994) 59:307–16. doi: 10.1093/ajcn/59.2.307
23. Lazarus R, Baur L, Webb K, Blyth F. Body mass index in screening for adiposity in children and adolescents: systematic evaluation using receiver operating characteristic curves. Am J Clin Nutr. (1996) 63:500–6. doi: 10.1093/ajcn/63.4.500
24. McGinnis JM, Gootman JA. Food Marketing to Children and Youth: Threat or Opportunity? Institute of Medicine of the National Academies. Washington, DC: The National Academies Press. (2006).
Google Scholar
25. Chaudhri OB, Salem V, Murphy KG, Bloom SR. Gastrointestinal satiety signals. Annu Rev Physiol. (2008) 70:239–55. doi: 10.1146/annurev.physiol.70.113006.100506
26. Scaglioni S, De Cosmi V, Ciappolino V, Parazzini F, Brambilla P, Agostoni C. Factors influencing children's eating behaviours. Nutrients. (2018) 10:706. doi: 10.3390/nu10060706
27. Ahima RS, Antwi DA. Brain regulation of appetite and satiety. Endocrinol Metab Clin North Am. (2008) 37:811–23. doi: 10.1016/j.ecl.2008.08.005
28. Niswender KD, Baskin DG, Schwartz MW. Review insulin and its evolving partnership with leptin in the hypothalamic control of energy homeostasis. Trends Endocrinol Metab. (2004) 15:362–9. doi: 10.1016/j.tem.2004.07.009
29. Niswender KD, Schwartz MW. Review insulin and leptin revisited: adiposity signals with overlapping physiological and intracellular signaling capabilities. Front Neuroendocrinol. (2003) 24:1–10. doi: 10.1016/S0091-3022(02)00105-X
30. Amitani M, Asakawa A, Amitani H, Inui. A. The role of leptin in the control of insulin-glucose axis. Front Neurosci. (2013) 7:51. doi: 10.3389/fnins.2013.00051
31. Cowley MA, Smith RG, Diano S, Tschöp M, Pronchuk N, Grove KL, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. (2003) 37:649–61. doi: 10.1016/S0896-6273(03)00063-1
32. Buhmann H, le Roux CW, Bueter M. The gut–brain axis in obesity. Best Prac Res Clin Gastroenterol. (2014) 28:559–71. doi: 10.1016/j.bpg.2014.07.003
33. Cone RD. Review anatomy and regulation of the central melanocortin system. Nat Neurosci. (2005) 8:571–8. doi: 10.1038/nn1455
34. Timper K, Brüning JC. Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis Model Mech. (2017) 10:679–89. doi: 10.1242/dmm.026609
35. Labarthe A, Fiquet O, Hassouna R, Zizzari P, Lanfumey L, Ramoz N, et al. Ghrelin-derived peptides: a link between appetite/reward, gh axis, and psychiatric disorders? Front Endocrinol. (2014) 5:163. doi: 10.3389/fendo.2014.00163
36. Hills R. D Jr, Pontefract BA, Mishcon HR, Black CA, Sutton SC, Theberge CR. Gut microbiome: profound implications for diet and disease. Nutrients. (2019) 11:1613. doi: 10.3390/nu11071613
37. Torres-Fuentes C, Schellekens H, Dinan TG, Cryan JF. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol Hepatol. (2017) 2:747–56. doi: 10.1016/S2468-1253(17)30147-4
38. Gérard P. Gut microbiota and obesity. Cell Mol Life Sci. (2016) 73:147–62. doi: 10.1007/s00018-015-2061-5
39. Derrien M, Alvarez AS, de Vos WM. The gut microbiota in the first decade of life. Trends Microbiol. (2019) 27:997–1010.40. doi: 10.1016/j.tim.2019.08.001
40. Dao MC, Clément K. Gut microbiota and obesity: concepts relevant to clinical care. Eur J Intern Med . (2018) 48:18–24.41. doi: 10.1016/j.ejim.2017.10.005
41. Kim KN, Yao Y., Ju SY. Short chain fatty acids and fecal microbiota abundance in humans with obesity: a systematic review and meta-analysis. Nutrients. (2019) 11:2512. doi: 10.3390/nu11102512
42. Castaner O, Goday A, Park YM, Lee SH, Magkos F, Shiow STE, et al. The gut microbiome profile in obesity: a systematic review. Int J Endocrinol. (2018) 2018:4095789. doi: 10.1155/2018/4095789
43. Riva A, Borgo F, Lassandro C, Verduci E, Morace G, Borghi E, et al. Pediatric obesity is associated with an altered gut microbiota and discordant shifts in firmicutes populations. Enviroin Microbiol. (2017) 19:95–105. doi: 10.1111/1462-2920.13463
44. Fernandes J, Su W, Rahat-Rozenbloom S, Wolever TMS, Comelli EM. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes . (2014) 4:e121. doi: 10.1038/nutd.2014.23
45. Rahat-Rozenbloom S, Fernandes J, Gloor GB, Wolever TMS. Evidence for greater production of colonic short-chain fatty acids in overweight than lean humans. Int J Obes . (2014) 38:1525–31. doi: 10.1038/ijo.2014.46
46. Barczyńska R, Litwin M, Slizewska K, Szalecki M, Berdowska A, Bandurska K, et al. Bacterial microbiota fatty acids in the faeces of overweight obese children. Pol. J. Microbiol. (2018) 67:339–45. doi: 10.21307/pjm-2018-041
47. Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. (2020) 72:558–77. doi: 10.1016/j.jhep.2019.10.003
48. Yu EL, Golshan S, Harlow KE, Angeles JE, Durelle J, Goyal NP, et al. Prevalence of nonalcoholic fatty liver disease in children with obesity. J Pediatr. (2019) 207:64–70. doi: 10.1016/j.jpeds.2018.11.021
49. Ranucci G, Spagnuolo MI, Iorio R. Obese children with fatty liver: Between reality and disease mongering. World J Gastroenterol. (2017) 23:8277–82. doi: 10.3748/wjg.v23.i47.8277
50. Cox AJ, West NP, Cripps A. W. Obesity, inflammation, and the gut microbiota. Lancet Diabet Endocrinol. (2015) 3:207–15. doi: 10.1016/S2213-8587(14)70134-2
51. Seitz J, Trinh S, Herpertz-Dahlmann B. The microbiome and eating disorders. Psychiatr Clin North Am. . (2019) 42:93–103. doi: 10.1016/j.psc.2018.10.004
52. Deans E. Microbiome and mental health in the modern environment. J Physiol Anthropol. (2016) 36:1. doi: 10.1186/s40101-016-0101-y
53. Peirce JM, Alviña K. The role of inflammation and the gut microbiome in depression and anxiety. J Neurosci Res . (2019) 97:1223–41. doi: 10.1002/jnr.24476
54. Ranadive SA, Vaisse C. Lessons from extreme human obesity: monogenic disorders. Endocrinol Metab Clin North Am. (2008) 37:733–51. doi: 10.1016/j.ecl.2008.07.003
55. Soliman AT, Yasin M, Kassem A. Leptin in pediatrics: a hormone from adipocyte that wheels several functions in children. Indian J Endocrinol Metab . (2012) 16(Suppl. 3):S577–87. doi: 10.4103/2230-8210.105575
56. Farooqi IS, Wangensteen T, Collins S, Kimber W, Matarese G, Keogh JM, et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med. (2007) 356:237–47. doi: 10.1056/NEJMoa063988
57. Mutch DM, Clément K. Unraveling the genetics of human obesity. PLoS Genet. (2006) 2:e188. doi: 10.1371/journal.pgen.0020188
58. Crocker MK, Yanovski JA. Pediatric obesity: etiology and treatment. Endocrinol Metab Clin North Am. (2009) 38:525–48. doi: 10.1016/j.ecl.2009.06.007
59. Huvenne H, Dubern B, Clément K, Poitou C. Rare genetic forms of obesity: clinical approach and current treatments in 2016. Obes Facts. (2016) 9:158–73. doi: 10.1159/000445061
60. Stefan M, Nicholls RD. What have rare genetic syndromes taught us about the pathophysiology of the common forms of obesity? Curr Diab Rep. (2004) 4:143–50. doi: 10.1007/s11892-004-0070-0
61. Hetherington MM, Cecil JE. Gene-Environment interactions in obesity. Forum Nutr. (2009) 63:195–203. doi: 10.1159/000264407
62. Reddon H, Guéant JL, Meyre D. The importance of gene-environment interactions in human obesity. Clin Sci. (2016) 130:1571–97. doi: 10.1042/CS20160221
63. Castillo JJ, Orlando RA, Garver WS. Gene-nutrient interactions and susceptibility to human obesity. Genes Nutr. (2017) 12:29. doi: 10.1186/s12263-017-0581-3
64. Heianza Y, Qi L. Gene-Diet interaction and precision nutrition in obesity. Int J Mol Sci. (2017) 18:787. doi: 10.3390/ijms18040787
65. Goodarzi MO. Genetics of obesity: what genetic association studies have taught us about the biology of obesity and its complications. Lancet Diabetes Endocrinol. (2018) 6:223–36. . doi: 10.1016/S2213-8587(17)30200-0
66. Bouchard L, Drapeau V, Provencher V, Lemieux S, Chagnon Y, Rice T, et al. Neuromedin beta: a strong candidate gene linking eating behaviors and susceptibility to obesity. Am J Clin Nutr. (2004) 80:1478–86. . doi: 10.1093/ajcn/80.6.1478
67. Grimm ER, Steinle NI. Genetics of eating behavior: established and emerging concepts. Nutr Rev. (2011) 69:52–60. . doi: 10.1111/j.1753-4887.2010.00361.x
68. van der Klaauw AA, Farooqi IS. The hunger genes: pathways to obesity. Cell. (2015) 161:119–32. . doi: 10.1016/j.cell.2015.03.008
69. Martinez JA. Bodyweight regulation causes of obesity. Proc Nutr Soc. (2000) 59:337–45. Review. doi: 10.1017/S0029665100000380
70. Rask-Andersen M, Karlsson T, Ek WE, Johansson Å. Gene-environment interaction study for BMI reveals interactions between genetic factors and physical activity, alcohol consumption and socioeconomic status. PLoS Genet. (2017) 5:1. doi: 10.1371/journal.pgen.1006977
71. Xulong S, Pengzhou L, Xiangwu Y, Weizheng L, Xianjie Q, Shaihong Z, et al. From genetics and epigenetics to the future of precision treatment for obesity. Gastroenterol Rep. (2017) 5:266–70. doi: 10.1093/gastro/gox033
72. Bianco-Miotto T, Craig JM, Gasser YP, van dijk SJ, Ozanne SE. Epigenetics and DOHaD: from basics to birth and beyond. J Dev Orig Health Dis. (2017) 8:513–9. doi: 10.1017/S2040174417000733
73. van Dijk SJ, Molloy PL, Varinli H, Morrison JL, Muhlhausler BS, Members of EpiSCOPE. Epigenetics and human obesity. Int J Obes . (2015) 39:85–97. doi: 10.1038/ijo.2014.34
74. Li Y. Epigenetic mechanisms link maternal diets and gut microbiome to obesity in the offspring. Front Genet . (2018) 9:342. doi: 10.3389/fgene.2018.00342
75. Kaufman J, Montalvo-Ortiz JL, Holbrook H, O'Loughlin K, Orr C, Kearney C, et al. Adverse childhood experiences, epigenetic measures, and obesity in youth. J Pediatr. (2018) 202:150–6.76. doi: 10.1016/j.jpeds.2018.06.051
76. May Gardner R, Feely A, Layte R, Williams J, McGavock J. Adverse childhood experiences are associated with an increased risk of obesity in early adolescence: a population-based prospective cohort study. Pediatr Res. (2019) 86:522–28. doi: 10.1038/s41390-019-0414-8
77. Cheon BK„ Hong YY. Mere experience of low subjective socioeconomic status stimulates appetite food intake. Proc Natl Acad Sci USA . (2017) 114:72–7. doi: 10.1073/pnas.1607330114
78. Alegría-Torres JA, Baccarelli A, Bollati V. Epigenetics lifestyle. Epigenomics . (2011) 3:267-77. doi: 10.2217/epi.11.22
79. Birch LL, Fisher JO. Development of eating behaviors among children and adolescents. Pediatrics . (2011) 101:539–49.
PubMed Abstract | Google Scholar
80. Birch L, Savage JS, Ventura A. Influences on the development of children's eating behaviours: from infancy to adolescence. Can J Diet Pract Res. (2007) 68:s1–s56.
81. Nielsen SJ, Popkin BM. Patterns and trends in food portion sizes, 1977- 1998. JAMA. (2003) 289:450–53. . doi: 10.1001/jama.289.4.450
82. Munoz KA, Krebs-Smith SM, Ballard-Barbash R, Cleveland LE. Food intakes of US children and adolescents compared with recommendations. Pediatrics. (1997) 100:323–29. doi: 10.1542/peds.100.3.323
83. Fisher JO, Birch LL. Restricting access to palatable foods affects children's behavioral response, food selection, and intake. Am J Clin Nutr. (1999) 69:1264–72. doi: 10.1093/ajcn/69.6.1264
84. Faith MS, Scanlon KS, Birch LL, Francis LA, Sherry B. Parent-child feeding strategies and their relationships to child eating and weight status. Obes Res. (2004) 12:1711–22. . doi: 10.1038/oby.2004.212
85. Smith AD, Sanchez N, Reynolds C, Casamassima M, Verros M, Annameier SK, et al. Associations of parental feeding practices and food reward responsiveness with adolescent stress-eating. Appetite. (2020) 152:104715. doi: 10.1016/j.appet.2020.104715
86. Lowe CJ, Morton JB, Reichelt AC. Adolescent obesity and dietary decision making-a brain-health perspective. Lancet Child Adolesc Health. (2020) 4:388–96. doi: 10.1016/S2352-4642(19)30404-3
87. Goran MI, Treuth MS. Energy expenditure, physical activity, and obesity in children. Pediatr Clin North Am. (2001) 48:931–53. doi: 10.1016/S0031-3955(05)70349-7
88. Romieu I, Dossus L, Barquera S, Blottière HM, Franks PW, Gunter M, et al. Energy balance and obesity: what are the main drivers? Cancer Causes Control. (2017) 28:247–58. doi: 10.1007/s10552-017-0869-z
89. Mattes R, Foster GD. Food environment and obesity. Obesity. (2014) 22:2459–61. doi: 10.1002/oby.20922
90. Ickovics JR, O'Connor Duffany K, Shebl FM, Peters SM, Read MS, Gilstad-Hayden KR, et al. Implementing school-based policies to prevent obesity: cluster randomized trial. Am J Prev Med. (2019) 56:e1–11. doi: 10.1016/j.amepre.2018.08.026
91. Micha R, Karageorgou D, Bakogianni I, Trichia E, Whitsel LP, Story M, et al. Effectiveness of school food environment policies on children's dietary behaviors: A systematic review and meta-analysis. PLoS ONE. ( 2018 ) 13:e0194555. doi: 10.1371/journal.pone.0194555
92. Cawley J, Frisvold D, Hill A, Jones DJ. The impact of the philadelphia beverage tax on purchases and consumption by adults and children. Health Econ. (2019) 67:102225. doi: 10.1016/j.jhealeco.2019.102225
93. John Cawley J, Thow AM, Wen K, Frisvold D. The economics of taxes on sugar-sweetened beverages: a review of the effects on prices, sales, cross-border shopping, and consumption. Annu Rev Nutr. (2019) 39:317–38. doi: 10.1146/annurev-nutr-082018-124603
94. Fuller C, Lehman E, Hicks S, Novick MB. Bedtime use of technology and associated sleep problems in children. Glob Pediatr Health. (2017) 4:2333794X17736972. doi: 10.1177/2333794X17736972
95. Chahal H, Fung C, Kuhle S, Veugelers PJ. Availability and night-time use of electronic entertainment and communication devices are associated with short sleep duration and obesity among Canadian children. Pediatr Obes. (2012) 8:42–51. doi: 10.1111/j.2047-6310.2012.00085.x
96. Minghua T. Protein intake during the first two years of life and its association with growth and risk of overweight. Int J Environ Res Public Health. ( 2018 ) 15:1742. doi: 10.3390/ijerph15081742
97. Azad MB, Vehling L, Chan D, Klopp A, Nickel NC, McGavock JM, et al. Infant feeding and weight gain: separating breast milk from breastfeeding and formula from food. Pediatrics. (2018) 142:e20181092. doi: 10.1542/peds.2018-1092
98. Lin L, Amissah E, Gamble GD, Crowther CA, Harding JE. Impact of macronutrient supplements on later growth of children born preterm or small for gestational age: a systematic review and meta-analysis of randomised and quasirandomised controlled trials. PLoS Med. (2020) 17:e1003122. . doi: 10.1371/journal.pmed.1003122
99. Rzehak P, Oddy WH, Mearin ML, Grote V, Mori TA, Szajewska H, et al. Infant feeding and growth trajectory patterns in childhood and body composition in young adulthood. Am J Clin Nutr. (2017) 106:568–80. doi: 10.3945/ajcn.116.140962
100. Styne DM, Arslanian SA, Connor EL, Farooqi IS, Murad MH, Silverstein JH. Pediatric obesity-assessment, treatment, and prevention: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2017) 102:709–57. doi: 10.1210/jc.2016-2573
101. Whitaker RC, Pepe MS, Wright JA, Seidel KD, Dietz WH. Early adiposity rebound and the risk of adult obesity. Pediatrics . (1998) 101:E5. doi: 10.1542/peds.101.3.e5
102. Geserick M, Vogel M, Gausche R, Lipek T, Spielau U, Keller E, et al. Acceleration of BMI in early childhood and risk of sustained obesity. N Engl J Med. (2018) 379:1303–12. doi: 10.1056/NEJMoa1803527
103. Jabakhanji SB, Boland F, Ward M, Biesma RJ. Body mass index changes in early childhood. Pediatrics. (2018) 202:106–14. doi: 10.1016/j.jpeds.2018.06.049
104. Chung S. Growth and puberty in obese children and implications of body composition. J Obes Metab Syndr. (2017) 26:243–50. doi: 10.7570/jomes.2017.26.4.243
105. Tagi VM, Giannini C, Chiarelli F. Insulin resistance in children. Front Endocrinol. (2019) 10:342. doi: 10.3389/fendo.2019.00342
106. Kelesidis I, Mantzoros CS. Leptin and its emerging role in children and adolescents. Clin Pediatr Endocrinol . (2006) 15:1–14. doi: 10.1297/cpe.15.1
107. Burt Solorzano CM, McCartney CR, Obesity and the pubertal transition in girls and boys. Reproduction . (2010) 140:399–410. doi: 10.1530/REP-10-0119
108. Li W, Liu Q, Deng X, Chen Y, Liu S, Story M. Association between obesity and puberty timing: a systematic review and meta-analysis. Int J Environ Res Public Health. (2017) 14:1266. doi: 10.3390/ijerph14101266
109. Lee JM, Wasserman R, Kaciroti N, Gebremariam A, Steffes J, Dowshen S, et al. Timing of puberty in overweight vs. obese boys. Pediatrics. (2016) 137:e20150164. doi: 10.1542/peds.2015-0164
110. He J, Kang Y, Zheng L. Serum levels of LH, IGF-1 and leptin in girls with idiopathic central precocious puberty (ICPP) and the correlations with the development of ICPP. Minerva Pediatr . (2018). doi: 10.23736/S0026-4946.18.05069-7
111. Kang MJ, Oh YJ, Shim YS, Baek JW, Yang S, Hwang IT. The usefulness of circulating levels of leptin, kisspeptin, and neurokinin B in obese girls with precocious puberty. Gynecol Endocrinol. (2018) 34:627–30. doi: 10.1080/09513590.2017.1423467
112. Rendo-Urteaga T, Ferreira de Moraes AC, Torres-Leal FL, Manios Y, Gottand F, Sjöström M, et al. Leptin and adiposity as mediators on the association between early puberty and several biomarkers in European adolescents: the helena study. J Pediatr Endocrinol Metab. (2018) 31:1221–29. doi: 10.1515/jpem-2018-0120
113. Franks S. Adult polycystic ovary syndrome begins in childhood. Best Pract Res Clin Endocrinol Metab. (2002) 16:263–72. doi: 10.1053/beem.2002.0203
114. Franks S. Polycystic ovary syndrome in adolescents. Int J Obes. (2008) 32:1035–41. doi: 10.1038/ijo.2008.61
115. Jehan S, Zizi F, Pandi-Perumal SR, Wall S, Auguste E, Myers K, et al. Obstructive sleep apnea and obesity: implications for public health. Sleep Med Disord. (2017) 1:00019.
116. Patinkin ZW, Feinn R, Santos M. Metabolic consequences of obstructive sleep apnea in adolescents with obesity: a systematic literature review and meta-analysis. Childhood Obes. (2017) 13:102–10. doi: 10.1089/chi.2016.0248
117. Kaditis A. From obstructive sleep apnea in childhood to cardiovascular disease in adulthood: what is the evidence? Sleep. (2010) 33:1279–80. doi: 10.1093/sleep/33.10.1279
118. Marseglia L, Manti S, D'Angelo G, Nicotera A, Parisi E, Di Rose G, et al. Oxidative stress in obesity: a critical component in human diseases. Int J Mol Sci . (2014) 16:378–400. doi: 10.3390/ijms16010378
119. Eisele HJ, Markart P, Schulz R. Obstructive sleep apnea, oxidative stress, and cardiovascular disease: evidence from human studies. Oxid Med Cell Longev . (2015) 2015:608438. doi: 10.1155/2015/608438
120. Hui W, Slorach C, Guerra V, Parekh RS, Hamilton J, Messiha S, et al. Effect of obstructive sleep apnea on cardiovascular function in obese youth. Am J Cardiol. (2019) 123:341–7. doi: 10.1016/j.amjcard.2018.09.038
121. Matteoni CA, Younossi Z .m., Gramlich T, Boparai N, Liu YC, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. (1999) 1999:116:1413. doi: 10.1016/S0016-5085(99)70506-8
122. Lavine JE, Schwimmer JB. Nonalcoholic fatty liver disease in the pediatric population. Clin Liver Dis. ( 2004 ) 8:549. doi: 10.1016/j.cld.2004.04.010
123. Huang JS, Barlow SE, Quiros-Tejeira RE, Scheimann A, Skelton J, Suskind D, et al. Childhood obesity for pediatric gastroenterologists. J Pediatr Gastroenterol Nutr. (2013) 2013:56:99. doi: 10.1097/MPG.0b013e31826d3c62
124. Anderson EL, Howe LD, Jones HE, Higgins JPT, Lawlor DA, Fraser A. The prevalence of non-alcoholic fatty liver disease in children and adolescents: a systematic review and meta-analysis. PLoS ONE. ( 2015 ) 10:e0140908. doi: 10.1371/journal.pone.0140908
125. Nobili V, Alisi A, Newton KP, Schwimmer JB. Comparison of the phenotype and approach to pediatric vs adult patients with nonalcoholic fatty liver disease. Gastroenterology. (2016) 150:1798–810. doi: 10.1053/j.gastro.2016.03.009
126. Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, et al. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. (2009) 7:234–38. doi: 10.1016/j.cgh.2008.11.005
127. Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angula P. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut. (2009) 58:1538. doi: 10.1136/gut.2008.171280
128. Schwimmer JB, Pardee PE, Lavine JE, Blumkin AK, Cook S. Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation . (2008) 118:277. doi: 10.1161/CIRCULATIONAHA.107.739920
129. Perry DC, Metcalfe D, Lane S, Turner S. Childhood obesity and slipped capital femoral epiphysis. Pediatrics. (2018) 142:e20181067. doi: 10.1542/peds.2018-1067
130. Zavala-Crichton JP, Esteban-Cornejo I, Solis-Urra P, Mora-Gonzalez J, Cadenas-Sanchez C, Rodriguez-Ayllon M, et al. Association of sedentary behavior with brain structure and intelligence in children with overweight or obesity: Active Brains Project . (2020) 9:1101. doi: 10.3390/jcm9041101
131. Ronan L, Alexander-Bloch A, Fletcher PC. Childhood obesity, cortical structure, and executive function in healthy children. Cereb Cortex. (2019) 30:2519–28. doi: 10.1093/cercor/bhz257
132. Baker ER. Body weight and the initiation of puberty. Clin Obstetr Gynecol. (1985) 28:573–9. doi: 10.1097/00003081-198528030-00013
133. Siervogel RM, Demerath EW, Schubert C, Remsberg KE, Chumlea WM, Sun S, et al. Puberty and body composition. Horm Res. (2003) 60:36–45. doi: 10.1159/000071224
134. Sadeeqa S, Mustafa T, Latif S. Polycystic ovarian syndrome- related depression in adolescent girls. J Pharm Bioallied Sci. (2018) 10:55–9. doi: 10.4103/JPBS.JPBS_1_18
135. Himelein MJ, Thatcher SS. Depression and body image among women with polycystic ovary syndrome. J Health Psychol . (2006) 11:613–25. doi: 10.1177/1359105306065021
136. Magge SN, Goodman E, Armstrong SC. The metabolic syndrome in children and adolescents: shifting the focus to cardiometabolic risk factor clustering. Pediatrics. (2017) 140:e20171603. doi: 10.1542/peds.2017-1603
137. Mauras N, Delgiorno C, Kollman C, Bird K, Morgan M, Sweeten S, et al. Obesity without established comorbidities of the metabolic syndrome is associated with a proinflammatory and prothrombotic state, even before the onset of puberty in children. J Clin Endocrinol Metab. (2010) 95:1060–8. doi: 10.1210/jc.2009-1887
138. Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. (2004) 350:2362–74. doi: 10.1056/NEJMoa031049
139. Erdmann J, Kallabis B, Oppel U, Sypchenko O, Wagenpfeil S, Schusdziarra V. Development of hyperinsulinemia and insulin resistance during the early stage of weight gain. Am J Physiol Endocrinol Metabol. (2008) 294:e568–75. . doi: 10.1152/ajpendo.00560.2007
140. Pulido-Arjona L, Correa-Bautista JE, Agostinis-Sobrinho C, Mota J, Santos R, Correa-Rodrigues M, et al. Role of sleep duration and sleep- related problems in the metabolic syndrome among children and adolescents. Ital J Pediatr. (2018) 44:9. doi: 10.1186/s13052-018-0451-7
141. Harriger JA, Thompson JK. Psychological consequences of obesity: weight bias and body image in overweight and obese youth. Int Rev Psychiatry. (2012) 24:247–53. . doi: 10.3109/09540261.2012.678817
142. Bacchini D, Licenziati MR, Garrasi A, Corciulo N, Driul D, Tanas R, et al. Bullying and victimization in overweight and obese outpatient children and adolescents: an italian multicentric study. PLoS ONE. (2015) 10:e0142715. doi: 10.1371/journal.pone.0142715
143. Loth KA, Watts AW, Berg PVD, Neumark-Sztainer D. Does body satisfaction help or harm overweight teens? A 10-year longitudinal study of the relationship between body satisfaction and body mass index. J Adolesc Health. (2015) 57:559–61. doi: 10.1016/j.jadohealth.2015.07.008
144. Gowey MA, Lim CS, Clifford LM, Janicke DM. Disordered eating and health-related quality of life in overweight and obese children. J Pediatr Psychol. (2014) 39:552–61. doi: 10.1093/jpepsy/jsu012
145. Mannan M, Mamun A, Doi S, Clavarino A. Prospective associations between depression and obesity for adolescent males and females- a systematic review and meta-analysis of longitudinal studies. PLoS ONE. (2016) 11:e0157240. doi: 10.1371/journal.pone.0157240
146. Ruiz LD, Zuelch ML, Dimitratos SM, Scherr RE. Adolescent obesity: diet quality, psychosocial health, and cardiometabolic risk factors. Nutrients. (2019) 12:43. doi: 10.3390/nu12010043
147. Goldschmidt AB, Aspen VP, Sinton MM, Tanofsky-Kraff M, Wilfley DE. Disordered eating attitudes and behaviors in overweight youth. Obesity. (2008) 16:257–64. doi: 10.1038/oby.2007.48
148. Golden NH, Schneider M, Wood C. Preventing obesity and eating disorders in adolescents. Pediatrics. (2016) 138:e1–e12. doi: 10.1542/peds.2016-1649
149. Rastogi R, Rome ES. Restrictive eating disorders in previously overweight adolescents and young adults. Cleve Clin J Med. (2020) 87:165–71. doi: 10.3949/ccjm.87a.19034
150. Hayes JF, Fitzsimmons-Craft EE, Karam AM, Jakubiak JL, Brown ME, Wilfley D. Disordered eating attitudes and behaviors in youth with overweight and obesity: implications for treatment. Curr Obes Rep. (2018) 7:235. doi: 10.1007/s13679-018-0316-9
151. Goldschmidt AB, Wall MM, Loth KA, Neumark-Sztainer D. Risk factors for disordered eating in overweight adolescents and young adults: Table I. J Pediatr Psychol. (2015) 40:1048–55. doi: 10.1093/jpepsy/jsv053
152. Follansbee-Junger K, Janicke DM, Sallinen BJ. The influence of a behavioral weight management program on disordered eating attitudes and behaviors in children with overweight. J Am Diet Assoc. (2010) 110:653–9. doi: 10.1016/j.jada.2010.08.005
153. Blake-Lamb TL, Locks LM, Perkins ME, Woo Baidal JA, Cheng ER, Taveras EM. Interventions for childhood obesity in the first 1,000 days a systematic review. Am J Prev Med. (2016) 50:780–9. doi: 10.1016/j.amepre.2015.11.010
154. McGuire S. Institute of Medicine (IOM). Early childhood obesity prevention policies. Washington, DC: The National Academies Press. Adv Nutr . (2011) 3:56–7. doi: 10.3945/an.111.001347
155. Pont SJ, Puhl R, Cook SR, Slusser W. Stigma experienced by children and adolescents with obesity. Pediatrics. (2017) 140:e20173034. doi: 10.1542/peds.2017-3034
156. Puhl R, Suh Y. Health consequences of weight stigma: implications for obesity prevention and treatment. Curr Obes Rep. (2015) 4:182–90. doi: 10.1007/s13679-015-0153-z
157. Schwimmer JB, Burwinkle TM, Varni JW. Health-related quality of life of severely obese children and adolescents. JAMA. (2003) 289:1813–9. doi: 10.1001/jama.289.14.1813
158. Carcone AI, Jacques-Tiura AJ, Brogan Hartlieb KE, Albrecht T, Martin T. Effective patient-provider communication in pediatric obesity. Pediatr Clin North Am. (2016) 63:525–38. doi: 10.1016/j.pcl.2016.02.002
159. Coppock JH, Ridolfi DR, Hayes JF, Paul MS, Wilfley DE. Current approaches to the management of pediatric overweight and obesity. Curr Treat Options Cardiovasc Med. (2014) 16:343. doi: 10.1007/s11936-014-0343-0
160. Davison KK, Jurkowski JM, Li K, Kranz S, Lawson HA. A childhood obesity intervention developed by families for families: results from a pilot study. Int J Behav Nutr Phys Act. (2013) 10:3. doi: 10.1186/1479-5868-10-3
161. Krystia O, Ambrose T, Darlington G, Ma DWL, Buchholz AC, Haines J. A randomized home- based childhood obesity prevention pilot intervention has favourable effects on parental body composition: preliminary evidence from the guelph family health study. BMC Obes. (2019) 6:10. doi: 10.1186/s40608-019-0231-y
162. Skjåkødegård HF, Danielsen YS, Morken M, Linde SRF, Kolko RP, Balantekin KN, et al. Study protocol: a randomized controlled trial evaluating the effect of family-based behavioral treatment of childhood and adolescent obesity–The FABO-study. BMC Public Health. (2016) 16:1106. doi: 10.1186/s12889-016-3755-9
163. Hall KD, Kahan S. Maintenance of lost weight and long-term management of obesity. Med Clin North Am. (2018) 102:183–97. doi: 10.1016/j.mcna.2017.08.012
164. Hall KD. Diet vs. exercise in “the biggest loser” weight loss competition. Obesity. (2013) 21:957–9. doi: 10.1002/oby.20065
165. Lecoultre V, Ravussin E, Redman LM. The fall in leptin concentration is a major determinant of the metabolic adaptation induced by caloric restriction independently of the changes in leptin circadian rhythms. J Clin Endocrinol Metabol. (2011) 96:E1512–E516. doi: 10.1210/jc.2011-1286
166. Kaur KK, Allahbadia G, Singh M. Childhood obesity: a comprehensive review of epidemiology, aetiopathogenesis and management of this global threat of the 21st century. Acta Sci Paediatr. (2019) 2:56–66. doi: 10.31080/ASPE.2019.02.0132
167. Crimmins NA, Xanthakos SA. Obesity. in Neinstein's Adolescent and Young Adult Health , Guide. Philadelphia, PA: Wolters Kluwer (2016). p. 295–300.
168. Astrup A, Rossner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, et al. Effects of liraglutide in the treatment of obesity: a randomized, double-blind, placebo-controlled study. Lancet. (2009) 374:1606–16. doi: 10.1016/S0140-6736(09)61375-1
169. Monami M, Dicembrini I, Marchionni N, Rotella CM, Mannucci E. Effects of glucagon-like peptide-1 receptor agonists on body weight: a meta-analysis. Exp Diabetes Res. (2012) 2012:672658. doi: 10.1155/2012/672658
170. Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. (2015) 373:11–22 . doi: 10.1056/NEJMoa1411892
171. Kelly AS, Auerbach P, Barrientos-Perez M, Gies I, Hale PM, Marcus C, et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N Engl J Med. (2020) 382:2117–28. doi: 10.1056/NEJMoa1916038
172. Foster BA, Escaname E, Powell T, Larsen B, Siddiqui SK, Menchaca J, et al. Randomized controlled trial of DHA supplementation during pregnancy: child adiposity outcomes. Nutrients . (2017) 9:566. doi: 10.3390/nu9060566
173. Abenavoli L, Scarpellini E, Colica C, Boccuto L, Salehi B, Sharifi-Rad J, et al. Gut microbiota and obesity: a role for probiotics. Nutrients. (2019) 11:2690. doi: 10.3390/nu11112690
174. Vajro P, Mandato C, Veropalumbo C, De Micco I. Probiotics: a possible role in treatment of adult and pediatric nonalcoholic fatty liver disease. Ann Hepatol. (2013) 12:161–63. doi: 10.1016/S1665-2681(19)31401-2
175. Zhao L, Fang X, Marshall M, Chung S. Regulation of obesity and metabolic complications by gamma and delta tocotrienols. Molecules. (2016) 21:344. doi: 10.3390/molecules21030344
176. Wong SK, Chin K-Y, Suhaimi FH, Ahmad F, Ima-Nirwana S. Vitamin E as a potential interventional treatment for metabolic syndrome: evidence from animal and human studies. Front Pharmacol. (2017) 8:444. doi: 10.3389/fphar.2017.00444
177. Galli F, Azzi A, Birringer A, Cook-Mills JM, Eggersdorfer M, Frank J, et al. Vitamin E: Emerging aspects and new directions. Free Radic Biol Med. (2017) 102:16–36. doi: 10.1016/j.freeradbiomed.2016.09.017
178. Galmés S, Serra F, Palou A. Vitamin E metabolic effects and genetic variants: a challenge for precision nutrition in obesity and associated disturbances. Nutrients . (2018) 10:1919. doi: 10.3390/nu10121919
179. Ahn SM. Current issues in bariatric surgery for adolescents with severe obesity: durability, complications, and timing of intervention. J. Obes Metabol Syndrome. (2020) 29:4–11. doi: 10.7570/jomes19073
180. Lamoshi A, Chernoguz A, Harmon CM, Helmrath M. Complications of bariatric surgery in adolescents. Semin Pediatr Surg. (2020) 29:150888. doi: 10.1016/j.sempedsurg.2020.150888
181. Weiss AL, Mooney A, Gonzalvo JP. Bariatric surgery. Adv Pediatr. (2017) 6:269–83. doi: 10.1016/j.yapd.2017.03.005
182. Stanford FC, Mushannen T, Cortez P, Reyes KJC, Lee H, Gee DW, et al. Comparison of short and long-term outcomes of metabolic and bariatric surgery in adolescents and adults. Front Endocrinol. (2020) 11:157. doi: 10.3389/fendo.2020.00157
183. Inge TH, Zeller MH, Jenkins TM, Helmrath M, Brandt ML, Michalsky MP, et al. Perioperative outcomes of adolescents undergoing bariatric surgery: the teen-longitudinal assessment of bariatric surgery (Teen-LABS) study . JAMA Pediatr . (2014) 168:47–53. doi: 10.1001/jamapediatrics.2013.4296
184. Järvholm K, Bruze G, Peltonen M, Marcus C, Flodmark CE, Henfridsson P, et al. 5-year mental health and eating pattern outcomes following bariatric surgery in adolescents: a prospective cohort study. Lancet Child AdolescHealth . (2020) 4:210–9. doi: 10.1016/S2352-4642(20)30024-9
185. Xanthakos SA. Bariatric surgery for extreme adolescent obesity: indications, outcomes, and physiologic effects on the gut–brain axis. Pathophysiology. (2008) 15:135–46. doi: 10.1016/j.pathophys.2008.04.005
186. Zitsman JL, Digiorgi MF, Kopchinski JS, Sysko R, Lynch L, Devlin M, et al. Adolescent Gastric Banding: a five-year longitudinal study in 137 individuals. Surg Obes Relat Dis. (2018) 14. doi: 10.1016/j.soard.2018.09.030
187. Inge TH, Jenkins TM, Xanthakos SA, Dixon JB, Daniels SR, Zeller MH, et al. Long-term outcomes of bariatric surgery in adolescents with severe obesity (FABS-5+). A prospective follow-up analysis. Lancet Diabet Endocrinol . (2017) 5:165–73. doi: 10.1016/S2213-8587(16)30315-1
188. Kindel TL, Krause C, Helm MC, Mcbride CL, Oleynikov D, Thakare R, et al. Increased glycine-amidated hyocholic acid correlates to improved early weight loss after sleeve gastrectomy. Surg Endosc. (2017) 32:805–12. doi: 10.1007/s00464-017-5747-y
Keywords: obesity, childhood, review (article), behavior, adolescent
Citation: Kansra AR, Lakkunarajah S and Jay MS (2021) Childhood and Adolescent Obesity: A Review. Front. Pediatr. 8:581461. doi: 10.3389/fped.2020.581461
Received: 08 July 2020; Accepted: 23 November 2020; Published: 12 January 2021.
Reviewed by:
Copyright © 2021 Kansra, Lakkunarajah and Jay. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alvina R. Kansra, akansra@mcw.edu
This article is part of the Research Topic
Pediatric Obesity: From the Spectrum of Clinical-Physiology, Social-Psychology, and Translational Research
Socioeconomic Status and Childhood Obesity: a Review of Literature from the Past Decade to Inform Intervention Research
- Etiology of Obesity (M Rosenbaum, Section Editor)
- Published: 12 August 2020
- Volume 9 , pages 562–570, ( 2020 )
Cite this article
- Christian E. Vazquez ORCID: orcid.org/0000-0002-3792-9150 1 &
- Catherine Cubbin 1
7640 Accesses
78 Citations
18 Altmetric
Explore all metrics
Purpose of Review
This is a review of the patterns, conceptualization, and suggested mechanisms underlying the relationship of socioeconomic status (SES) to obesity in childhood and the implications of these data for interventions going forward.
Recent Findings
Adiposity and SES are negatively associated in high-income countries and positively associated in medium to low-income countries. Several mechanisms, such as early introduction of solid food and parental behaviors, which may explain the association of SES and adiposity, have been identified. Parental education and adiposity and early pediatric nutrition appear to be particularly salient SES-related effectors on adiposity.
There is a clear association of SES and adiposity which is affected by population affluence. Evaluation of the relationship of SES and obesity in children are complicated by the complexity of SES and lack of common definition. A number of SES-related interventional targets have been identified. Intervention research should ensure they are addressing SES-associated issues in the study population.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
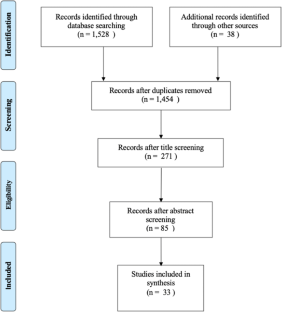
Similar content being viewed by others
Does Household Income Affect children’s Outcomes? A Systematic Review of the Evidence
Kerris Cooper & Kitty Stewart
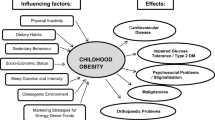
Risk Factors and Implications of Childhood Obesity
Susann Weihrauch-Blüher & Susanna Wiegand

The Importance of Parenting in Influencing the Lives of Children
Papers of particular interest, published recently, have been highlighted as: • of importance •• of major importance.
Pratt CA, Loria CM, Arteaga SS, Nicastro HL, Lopez-Class M, de Jesus JM, et al. A systematic review of obesity disparities research. Am J Prev Med. 2017;53(1):113–22.
PubMed Google Scholar
Trust for America’s Health. The state of obesity: better policies for a healthier America. [Internet]. 2019. Available from: https://www.tfah.org/wp-content/uploads/2019/09/2019ObesityReportFINAL-1.pdf
UNICEF. The state of the world’s children 2019. Children, food and nutrition: growing well in a changing world. [Internet]. New York: UNICEF; 2019. Available from: https://www.unicef.org/reports/state-of-worlds-children-2019
Google Scholar
Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. J Health Econ. 2012;31(1):219–30.
Finkelstein EA, Graham WKC, Malhotra R. Lifetime direct medical costs of childhood obesity. Pediatr. 2014;133(5):854–62.
Hamilton D, Dee A, Perry IJ. The lifetime costs of overweight and obesity in childhood and adolescence: a systemic review. Obes Rev. 2018;19(4):452–63.
CAS PubMed Google Scholar
Sonntag D, Ali S, De Bock F. Lifetime indirect costs of childhood overweight and obesity: a decision analytic model. Obesity. 2016;24(1):200–6.
Gebremariam M, Lien N, Nianogo R, Arah O. Review of mediators of socioeconomic differences in adiposity among youth: a systematic review. Obes Rev. 2017;18(8):880–98.
Vallgarda S. Childhood obesity policies - mighty concerns, meek reactions. Obes Rev. 2018;19(3):295–301.
The World Bank. World bank country and lending groups. [Internet]. 2019. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
Safron M, Cislak A, Gaspar T, Luszczynska A. Effects of school-based interventions targeting obesity-related behaviors and body weight change: a systematic umbrella review. J Behav Med. 2011;37(1):15–25.
Cislak A, Safron M, Pratt M, Gaspar T, Luszczynska A. Family-related predictors of body weight and weight-related behaviours among children and adolescents: a systematic umbrella review. Child Care Health Dev. 2012;38(3):321–31.
Rajjo T, Mohammed K, Alsawas M, Ahmed AT, Farah W, Asi N, et al. Treatment of pediatric obesity: an umbrella systematic review. J Clin Endocrinol Metab. 2017;102(3):763–75.
Kobes A, Kretschmer T, Timmerman G, Schreuder P. Interventions aimed at preventing and reducing overweight/obesity among children and adolescents: a meta-synthesis. Obes Rev. 2018;19(8):1065–79.
Ells LJ, Rees K, Brown T, Mead E, Al-Khudairy L, Azevedo L, et al. Interventions for treating children and adolescents with overweight and obesity: an overview of Cochrane reviews. Int J Obes. 2018;42(11):1823–33.
Jang M, Chao A, Whittemore R. Evaluating intervention programs targeting parents to manage childhood overweight and obesity: a systematic review using the re-aim framework. J Pediatr Nurs. 2015;30(6):877–87.
Ash T, Agaronov A, Young T, Aftosmes-Tobio A, Davison KK. Family-based childhood obesity prevention interventions: a systematic review and quantitative content analysis. Int J Behav Nutr Phys Act. 2017;14(1):113.
PubMed PubMed Central Google Scholar
Ling J, Robbins L, Wen F, Zhang N. Lifestyle interventions in preschool children: a meta-analysis of effectiveness. Am J Prev Med. 2017;53(1):102–12.
•• Brown T, Moore T, Hooper L, Gao Y, Zayegh A, Ijaz S, et al. Interventions for preventing obesity in children. Cochrane Database Syst Rev. 2019;7:CD001871 Most recent (at time of publication) systematic review of interventions that reports SES differences.
Wang Y, Lim H. The global childhood obesity epidemic and the association between socio-economic status and childhood obesity. Int Rev Psychiatry. 2012;24(3):176–88.
Schroeder K, Kulage KM, Lucero R. Beyond positivism: understanding and addressing childhood obesity disparities through a critical theory perspective. J Spec Pediatr Nurs. 2015;20(4):259–70.
Grant-Guimaraes J, Feinstein R, Laber E, Kosoy J. Childhood overweight and obesity. Gastroenterol Clin N Am. 2016;45(4):715–28.
Sobal J, Stunkard A. Socioeconomic status and obesity: a review of the literature. Psychol Bull. 1989;105:260–75.
Wu S, Ding Y, Wu F, Li R, Hu Y, Hou J, et al. Socio-economic position as an intervention against overweight and obesity in children: a systematic review and meta-analysis. Sci Rep. 2015;5(1):11354.
CAS PubMed PubMed Central Google Scholar
Shrewsbury V, Wardle J. Socioeconomic status and adiposity in childhood: a systematic review of cross-sectional studies 1990-2005. Obesity. 2008;16(2):275–84.
El-Sayed AM, Scarborough P, Galea S. Socioeconomic inequalities in childhood obesity in the United Kingdom: a systematic review of the literature. Obes Facts. 2012;5:671–92.
Barriuso L, Miqueleiz E, Albaladejo R, Villanueva R, Santos JM, Regidor E. Socioeconomic position and childhood-adolescent weight status in rich countries: a systematic review, 1990-2013. BMC Pediatr. 2015;15.
Stamatakis E, Wardle J, Cole TJ. Childhood obesity and overweight prevalence trends in England: evidence for growing socioeconomic disparities. Int J Obes. 2010;34(1):41–7.
CAS Google Scholar
Olds T, Maher C, Zumin S, Peneau S, Lioret S, Castetbon K, et al. Evidence that the prevalence of childhood overweight is plateauing: data from nine countries. Int J Pediatr Obes. 2011;6(5–6):342–60.
Frederick CB, Snellman K, Putnam RD. Increasing socioeconomic disparities in adolescent obesity. Proc Natl Acad Sci U S A. 2014;111(4):1338–42.
Robert Wood Johnson Foundation. Declining childhood obesity rates - where are we seeing signs of progress? [Internet]. Health Policy Snapshot - Childhood Obesity. 2013; Available from: https://www.issuelab.org/resources/15536/15536.pdf .
Ogden CL, Carroll MD, Lawman HG, Fryar C, Kruszon-Moran D, Kit B, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 through 2013-2014. JAMA. 2016;315(21):2292–9.
Chung A, Backholer K, Wong E, Palermo C, Keating C, Peeters A. Trends in child and adolescent obesity prevalence in economically advanced countries according to socioeconomic position: a systematic review. Obes Rev. 2016;17(3):276–95.
Dinsa GD, Goryakin Y, Fumagalli E, Suhrcke M. Obesity and socioeconomic status in developing countries: a systematic review. Obes Rev. 2012;13(11):1067–79.
Byrne M, Schwartz O, Simmons J, Sheeber L, Whittle S, Allen N. Duration of breastfeeding and subsequent adolescent obesity: effects of maternal behavior and socioeconomic status. J Adolesc Health. 2018;62(4):471–9.
Mech P, Hooley M, Skouteris H, Williams J. Review of parent-related mechanisms underlying the social gradient of childhood overweight and obesity: a systematic review. Child Care Health Dev. 2016;42(5):603–24.
• Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, et al. Socioeconomic status in health research one size does not fit all. JAMA. 2005;294(22):2879–88 Critical analysis of the problems with measuring socioeconomic status and suggested improvements that are reiterated in the current review .
Hillier-Brown FC, Bambra CL, Cairns J, Kasim A, Moore HJ, Summerbell CD. A systematic review of the effectiveness of individual, community and societal level interventions at reducing socioeconomic inequalities in obesity amongst children. BMC Public Health. 2014;14(1):834.
Silventoinen K, Huppertz C, van Beijsterveldt C, Bartels M, Willemsen G, Boomsma D. The genetic architecture of body mass index from infancy to adulthood modified by parental education. Obesity. 2016;24(9):2004–11.
Shackleton N. Is there a link between low parental income and childhood obesity? J Early Child Res. 2017;15(3):238–55.
Powell LM, Wada R, Krauss RC, Wang Y. Ethnic disparities in adolescent body mass index in the United States: the role of parental socioeconomic status and economic contextual factors. Soc Sci Med. 2012;75(3):469–76.
Laws R, Campbell KJ, van der Pligt P, Russell G, Ball K, Lynch J, et al. The impact of interventions to prevent obesity or improve obesity related behaviours in children (0-5 years) from socioeconomically disadvantaged and/or indigenous families: a systematic review. BMC Public Health. 2014;14(1):779.
Morgan EH, Schoonees A, Sriram U, Faure M, Seguin-Fowler RA. Caregiver involvement in interventions for improving children’s dietary intake and physical activity behaviors. Cochrane Database of Syst Rev. 2020;1:CD012547.
Gibbs B, Forste R. Socioeconomic status, infant feeding practices and early childhood obesity. Pediatr Obes. 2014;9(2):135–46.
Chatham RE, Mixer SJ. Cultural influences on childhood obesity in ethnic minorities: a qualitative systematic review. J Transcult Nurs. 2019;31(1):87–99.
Yan J, Liu L, Zhu Y, Huang G, Wang P. The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health. 2014;14(1):1267.
Kaur J, Lamb M, Ogden C. The association between food insecurity and obesity in children - the National Health and Nutrition Examination Survey. J Acad Nutr Diet 2015:115(5):751–758.
Jones KM, Power ML, Queenan JT, Schulkin J. Racial and ethnic disparities in breastfeeding. Breastfeed Med. 2015;10(4):186–96.
Tremblay MS, Gray CE, Akinroye K, Harrington DM, Katzmarzyk PT, Lambert EV, et al. Physical activity of children: a global matrix of grades comparing 15 countries. J Phys Act Health. 2014;11(suppl 1):S113–25.
Brown T, Smith S, Bhopal R, Kasim A, Summerbell C, Brown T. Diet and physical activity interventions to prevent or treat obesity in South Asian children and adults: a systematic review and meta-analysis. Int J Environ Res Public Health. 2015;12(1):566–94.
Andrea S, Hooker E, Messer L, Tandy T, Boone-Heinonen J. Does the association between early life growth and later obesity differ by race/ethnicity or socioeconomic status? A systematic review. Ann Epidemiol. 2017;27(9):583–92.
Whitaker R, Wright J, Pepe M, Seidel K, Dietz W. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med. 1997;337(13):869–73.
Reinehr T, Kleber M, Lass N, Toschke A. Body mass index patterns over 5 y in obese children motivated to participate in a 1-y lifestyle intervention: age as a predictor of long-term success. Am J Clin Nutr. 2010;91(5):1165–71.
Singh AS, Mulder C, Twisk JW, van Mechelen W, Chinapaw MJ. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev. 2008;9(5):474–88.
Fakhouri T, Hughes J, Brody D, Kit B, Ogden C. Physical activity and screen-time viewing among elementary school-aged children in the United States from 2009 to 2010. JAMA Pediatr. 2013;167(3):1–7.
Mcgoron L, Hvizdos E, Bocknek E, Montgomery E, Ondersma S. Feasibility of internet-based parent training for low-income parents of young children. Child Youth Serv Rev. 2018;84:198–205.
de Niet J, Timman R, Jongejan M, Passchier J, van den Akker E. Predictors of participant dropout at various stages of a pediatric lifestyle program. Pediatrics. 2011;127(1):e164–70.
Cui Z, Seburg E, Sherwood N, Faith M, Ward D. Recruitment and retention in obesity prevention and treatment trials targeting minority or low-income children: a review of the clinical trials registration database. Trials. 2015;16(1):564.
Shabbir S, Kwan D, Wang MC, Shih M, Simon PA. Asians and Pacific Islanders and the growing childhood obesity epidemic. Ethn Dis. 2010;20(2):129–35.
Bullock A, Sheff K, Moore K, Manson S. Obesity and overweight in American Indian and Alaska Native children, 2006-2015. Am J Public Health. 2017;107(9):1502–7.
Ligthart KAM, Buitendijk L, Koes BW, van Middelkoop M. The association between ethnicity, socioeconomic status and compliance to pediatric weight-management interventions - a systematic review. Obes Res Clin Pract. 2017;11(5 Suppl 1):1–51.
Barkin SL, Gesell SB, Po’e EK, Escarfuller J, Tempesti T. Culturally tailored, family-centered, behavioral obesity intervention for Latino-American preschool-aged children. Pediatrics. 2012;130(3):445–56.
Perez LG, Arredondo EM, Elder JP, Barquera S, Nagle B, Holub CK. Evidence-based obesity treatment interventions for Latino adults in the US: a systematic review. Am J Prev Med. 2013;44(5):550–60.
Gassman-Pines A, Skinner AT. Psychological acculturation and parenting behaviors in Mexican-immigrant families. J Fam Issues. 2018;39(5):1139–64.
Peeters A, Backholer K. Prioritising and tackling socio-economic inequalities in obesity. BMC Obesity. 2014;1.
Lin B, Smith T, Lee J, Hall K. Measuring weight outcomes for obesity intervention strategies: the case of a sugar-sweetened beverage tax. Econ Hum Biol. 2011;9(4):329–41.
Briggs A, Mytton O, Kehlbacher A, Tiffin R, Rayner M, Scarborough P. Overall and income specific effect on prevalence of overweight and obesity of 20% sugar sweetened drink tax in UK: econometric and comparative risk assessment modelling study. BMJ. 2013;347.
Ruff RR, Zhen C. Estimating the effects of a calorie-based sugar-sweetened beverage tax on weight and obesity in New York city adults using dynamic loss models. Ann Epidemiol. 2015;25(5):350–7.
• Nakhimovsky SS, Feigl AB, Avila C, O’Sullivan G, Macgregor-Skinner E, Spranca M. Taxes on sugar-sweetened beverages to reduce overweight and obesity in middle-income countries: a systematic review. PLoS ONE. 2016;11(9):e0163358 This recent systematic review, based on mostly estimates, suggests taxing SSBs will reduce net energy intake by enough to prevent further growth in obesity prevalence, but not to reduce population weight permanently .
Nakamura R, Mirelman A, Cuadrado C, Silva-Illanes N, Dunstan J, Suhrcke M. Evaluating the 2014 sugar-sweetened beverage tax in Chile: an observational study in urban areas. PLoS Med. 2018;15(7):e1002596.
Gortmaker S, Wang Y, Long M, Giles C, Ward Z, Barrett J, et al. Three interventions that reduce childhood obesity are projected to save more than they cost to implement. Health Aff. 2015;34(11):1932–9.
• Jones A. Race, socioeconomic status, and health during childhood: a longitudinal examination of racial/ethnic differences in parental socioeconomic timing and child obesity risk. Int J Environ Res Public Health. 2018;15(4) Strong recent study suggesting SES, particularly mother’s employment and father’s education, is linked to weight gain differently for White, Black, and Hispanic children at specific ages .
CONSORT. Transparent Reporting of Trials. [Internet]. 2020. Available from: http://www.consort-statement.org/
Hoffmann T, Glasziou P, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687.
Download references
Author information
Authors and affiliations.
Steve Hicks School of Social Work, The University of Texas at Austin, 1925 San Jacinto Blvd, Austin, TX, 78712, USA
Christian E. Vazquez & Catherine Cubbin
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Christian E. Vazquez .
Ethics declarations
Conflict of interest.
No conflicts of interest are declared for study authors.
Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Etiology of Obesity
Rights and permissions
Reprints and permissions
About this article
Vazquez, C.E., Cubbin, C. Socioeconomic Status and Childhood Obesity: a Review of Literature from the Past Decade to Inform Intervention Research. Curr Obes Rep 9 , 562–570 (2020). https://doi.org/10.1007/s13679-020-00400-2
Download citation
Published : 12 August 2020
Issue Date : December 2020
DOI : https://doi.org/10.1007/s13679-020-00400-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Socioeconomic status
- Health disparities
- Interventions
Advertisement
- Find a journal
- Publish with us
- Track your research
- Search Menu
- Advance articles
- Editor's Choice
- Supplements
- Author Guidelines
- Submission Site
- Open Access
- About Journal of Public Health
- About the Faculty of Public Health of the Royal Colleges of Physicians of the United Kingdom
- Editorial Board
- Self-Archiving Policy
- Dispatch Dates
- Advertising and Corporate Services
- Journals Career Network
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Introduction, results and review of papers.
- < Previous
Preventing obesity in pre-school children: a literature review
- Article contents
- Figures & tables
- Supplementary Data
Karen L. Saunders, Preventing obesity in pre-school children: a literature review, Journal of Public Health , Volume 29, Issue 4, December 2007, Pages 368–375, https://doi.org/10.1093/pubmed/fdm061
- Permissions Icon Permissions
Obesity in children is increasing worldwide, impacting on both long- and short-term health. Obesity prevention is an important contemporary public health priority and is firmly on the Government's agenda in the UK. Prevention involves addressing the main risk factors of diet and physical inactivity and also involves a wide range of environmental factors including access to sport and leisure, family life, diet, education and information.
A literature review undertaken on preventing obesity in children aged <5.
The review confirms that there is a limited and immature evidence and lack of comprehensive evidence on effective strategies to prevent obesity in younger children. The overall quality of studies is poor.
The need remains for structured, focused and systematic research on child obesity prevention. Well-designed studies examining a range of interventions remain a priority. The findings in this review support the recommendations in the National Institute for Health and Clinical Excellence (NICE) guidelines on obesity.
Child obesity will continue to be a problem without improved understanding of key factors operative during early childhood and identification of effective interventions. 1 The UK Government has responded to rising childhood obesity with a Public Service Agreement target to ‘halt the year on year rise in obesity among children aged under 11 by 2010’. 2
Primary Care Trusts (PCTs) now measure primary school children in the reception year (age, 4–5 years) and year 6 (age, 10–11 years). 3 Many Local Authorities (LAs) have incorporated targets to reduce childhood obesity into Local Area Agreements. 4
Strategic Health Authorities (SHAs), PCTs and LAs are expected to use the best available evidence in establishing plans to tackle child obesity. 5 The Wanless Report identified that the evidence base was particularly weak on interventions to reduce health inequalities due to obesity. 6 The recent NICE guidance reinforces that ‘ for children and young people, it is accepted that the evidence base is far from complete’. 7
At regional and local level, further information is needed on effective interventions. This review of the prevention of obesity in pre-school children has been conducted to inform such policy.
A broad scoping search was undertaken to identify key terms, to assess the breadth and depth of the literature and to establish a broad structure for the review. The search strategy was refined and candidate studies identified by searching PubMed (restricted to reviews), Cochrane and the Department of Health (DH) library catalogue. Inclusion criteria were obesity defined by body mass index (BMI), weight-for-height index and/or skinfold thickness, <5 years, some form of intervention and some assessment of effectiveness. Drug therapy was excluded and searches were restricted to English language. Titles and abstracts were assessed for relevance and where abstracts were unavailable and/or the relevance of the paper uncertain from the title, the full paper was obtained. Further candidate articles were identified from citations and review articles that specifically addressed obesity prevention in pre-school children. Assessment of studies was undertaken with an academic colleague from the University of Birmingham with experience in systematic reviewing.
Given the relatively underdeveloped field of work, with a small crop of peer reviewed papers, Internet searches using the ‘Google’ search engine were undertaken using the term ‘Interventions Preventing Obesity in Preschool Children’. There were 35 100 hits. Following discussions with West Midlands Health Technology Assessment Collaboration, a pragmatic approach was adopted and the first 200 results reviewed.
A data extraction form was developed by the author and was applied to all included papers. Data extracted included the objective and type of study, the setting, sample, intervention undertaken, measures used to evaluate impact, results and the author's comments on the studies. The types of studies ranged from simple observational methods to higher order studies—one randomized control trial (RCT) and two cohort studies.
The literature search identified 832 papers. Six papers met the inclusion criteria (Fig. 1 ). Excluded papers after full review and reasons for rejection are available from the author.
Schematic of literature survey.
The interventions identified in included papers were grouped around themes developed from the scoping search viz breastfeeding, 8 physical activity, 9 , 10 family-based interventions 11 , 12 and professional support. 13 Characteristics of the included studies are given in Table 1 . The quality of each study was assessed in terms of study design including subject numbers, randomization, control for confounding and minimization of bias.
Characteristics of included studies
Armstrong and Reilly 8 tested the hypothesis that breastfeeding is associated with a reduced risk of child obesity in a large, well-conducted cohort study in Scotland, using a population-based sample of 32 000 children. The authors examined the health records of children born in 1995 and 1996 who had undergone routine health screenings as part of the ‘Child Health Surveillance Programme’. During a screening at 6–8 weeks, the health worker asked the mother whether the baby was breastfed only, formula-fed only or fed both breast milk and formula. During a similar screening at 39–42 months, the health worker measured the child's height and weight and calculated the BMI. The prevalence of obesity was significantly lower among breastfed children compared with formula-fed children. This association persisted after adjustment for deprivation, birth weight and sex. The adjusted odds ratio (OR) for obesity was 0.70 (95% CI, 0.61–0.80). The findings suggest that breastfeeding is associated with a modest, but significant, reduction in childhood obesity risk. The authors also suggest that the reduction in risk is present in early childhood. There are limitations to the study given lack of information on other risk factors for obesity, including diet (once children began eating food), parental weight and physical activity.
Mo-suwan et al. 9 evaluated the effect of a school-based aerobic exercise programme on the obesity indexes of pre-school children in Thailand in a RCT. A total of 292 second-year elementary school pupils from two nursery schools were included: 147 (34 from school 1 and 113 from school 2) in the exercise group and 145 (45 from school 1 and 100 from school 2) in the control group. The mean age of the children was 4.5 years. Trained staff encouraged children in the exercise group to take part in a specially designed 30-week exercise programme. One school provided extra swimming for 1 h a week. Weight, height and triceps skinfold (TSF) thickness were measured four times throughout the study. Prevalence of obesity in both the exercise and control groups decreased. The exercise group decreased from 12.2% at baseline to 8.8% (Wilcoxon signed-rank test, P = 0.058), whereas the control group decreased from 11.7 to 9.7% (Wilcoxon signed-rank test, P = 0.179). The reduction in obesity in the exercise group was not significant, but was greater than the control group. A gender difference in the response of BMI to exercise was observed. Girls in the exercise group had a significantly lower likelihood of having an increasing BMI slope than the control girls (OR, 0.32; 95% CI, 0.18–0.56). The effect in boys had the opposite direction of study intention.
Daily dietary intake was not recorded and control of dietary intake may have added benefit. Parental guidance may also have reinforced activity. The study was relatively short and may reflect short-term change. The process of randomization was unclear, as was the sample size calculation and blinding was impossible with intervention and control conducted in each setting leading to the possibility of ‘contamination’. In school 1, there was an extra swimming class and it is not clear how this was controlled for. Other confounders could have been explored including ethnicity and parents' BMI. There are potential biases including children being recruited on teacher's advice, some family reported baseline data and measurement bias (adapted measures used and TSF measurement may not be accurate). There were 104 non-participating children, and it would be interesting to see whether their characteristics differed from those included.
Moore et al. 10 examined the effect of pre-school physical activity on the change in body fatness from pre-school to first grade in the USA in a longitudinal study. This study was part of the ‘Framingham Children's Study’ looking at childhood cardiovascular risk behaviours and began in 1987 with 106 children aged 3–5 years (mean, 4 years) and their parents. The authors analysed 97 healthy children with complete data from study entry into first grade. Physical activity of children and parents was assessed twice per year for five consecutive years using an electronic motion sensor. Each child also had yearly measurements of TSF thickness. Active girls i.e. those with above-median activity levels, had a better outcome and gained 1.0 mm in their TSF thickness from baseline to first grade, whereas inactive girls gained 1.75 mm TSF thickness. Active boys lost an average of 0.75 mm in their TSF thickness, whereas inactive boys gained 0.25 mm in their TSF thickness. Inactive pre-schoolers were almost four times as likely to have larger triceps during follow-up. Inactive preschoolers who were initially fatter were nearly six times as likely to have larger triceps during follow-up.
When age, television viewing, energy intake, baseline triceps and parents' BMI were controlled for, inactive pre-schoolers were 3.8 (95% CI, 1.4–10.6) times as likely as active pre-schoolers to have an increasing triceps slope during follow-up, rather than a stable or decreasing slope. This relative risk estimate was slightly higher for children with more body fat at baseline.
The study suggests that physical activity can affect obesity early in life and found a strong effect of low levels of physical activity on body fatness. Limitations include the small number of subjects and possible measurement bias.
Drucker et al. 11 examined the relationship between maternal parenting style, maternal eating cues and a child's eating behaviour during mealtime in the USA in an observational study using data collected as part of the ‘Stanford Infant Growth Study’, an ongoing longitudinal study. Seventy-seven children (mean age, 3.5 years) were included and visited the laboratory with their mothers for a videotaped lunch. Videotapes were coded for the child's eating rate and maternal parenting style, measured as the level of maternal control and support and the number and type of eating prompts given during a meal. The number and rate of verbal and physical encouragements and discouragements were significantly related to measures of general maternal parenting style and meal duration.
The rates of food offers, food presentations and total prompts were significantly positively related to the child's rate of calorie intake. A mother's level of support or control was not related to the child's eating behaviour. Although general maternal parenting style did not predict the child's eating behaviour, these behaviours were related to the frequency of maternal prompts, which, in turn, were significantly related to the number of calories eaten and the time spent eating. Children who ate fast had mothers who delivered eating prompts more frequently. The authors suggest that children's BMI was significantly and negatively correlated with total discouragement per minute ( r = 0.23, P ≤ 0.05) but not with other maternal prompts. Limitations include the representativeness of the study sample with participants being mainly white, older, working and well-educated mothers. The mother's prompts may also be in response to the child's behaviour rather than encouraging or discouraging certain eating behaviours. The total number of calories consumed was imputed from one meal in a laboratory setting and may not be representative of everyday eating behaviour. The setting may also have influenced behaviours.
In a large observational study, Baughcum et al. 12 developed and analysed two new instruments to assess maternal feeding practices and beliefs. The ‘Infant Feeding Questionnaire’ (IFQ) assessed feeding during the first year of life and was administered to 453 mothers of children aged 11–23 months. The ‘Pre-Schooler Feeding Questionnaire’ (PFQ) assessed feeding of young children between ages of 2–5 years (mean age of children, 39.5 months). Scores were calculated and linked with the children's measured and mothers' self-reported weight and height. Scores from the IFQ and PFQ were compared between obese and non-obese mothers, between those who did and did not have an overweight child and between those who had low and high incomes. There was no significant difference between boys and girls in the prevalence of overweight (20 versus 22%, P = 0.53). Within the low-income group mothers, the prevalence of maternal obesity was higher (27 versus 11%, P < 0.001), and their children were more often overweight (26 versus 13%, P = 0.001). However, overall obese mothers were no more likely than non-obese mothers to have overweight children (26 versus 20%, P = 0.19), and this was true when high and low income groups were examined separately.
Mothers who breast-fed for longer than 3 months were no less likely to have an overweight children than other breastfeeding mothers (14 versus 18%, P = not significant, data not shown). Low-income group mothers introduced solids earlier, but there was no evidence that early introduction of solids or the practice of adding cereal to the bottle was associated with overweight beyond infancy. After controlling for family income, there was no evidence that obese mothers had a different feeding style and the study did not suggest that there is a particular feeding style associated with overweight in young children. The only suggested difference in feeding style for obese mothers was the tendency to give children less control over feeding.
Harvey-Berino and Rourke conducted a low-powered observational study to determine whether maternal participation in a home-visiting obesity prevention plus parenting support (OPPS) intervention would reduce the prevalence of obesity in high-risk Native-American children compared with a parenting support (PS) only intervention. 13 Forty-three mother/child pairs were recruited. Mothers were 26.5 ± 5 years old with a mean BMI of 29.9 ± 3. Children (23 males) were 22 ± 8 months old with mean weight-for-height z- scores (WHZ) of 0.73 ± 1.4. Mothers were randomly assigned to a 16-week OPPS intervention or PS alone. The only difference was the focus of the lessons. The intervention was delivered one-on-one in homes by an indigenous peer educator.
Baseline and 16 week assessments included weight and height dietary intake, physical activity, parental feeding style and maternal outcome expectations, self-efficacy and intention to change diet and exercise behaviours. Children in the OPPS group gained less weight over 4 months than those in PS, but differences were not significant. WHZ scores decreased in the PS condition and increased among the OPPS group (−0.27 ± 1.1 versus 0.31 ± 1.1, P = 0.06), although this is not significant. Children in the OPPS condition significantly decreased energy intake (−316 ± 835 versus 197 ± 608 kcal/day, P < 0.05). Scores on the Child Feeding Questionnaire decreased significantly in the OPPS condition (−0.22 ± 0.42 versus 0.08 ± 0.63, P < 0.05), indicating that mothers in the OPPS group were engaging in less restrictive child-feeding practices over time. The authors considered a home-visiting programme focused on changing lifestyle behaviours and improving parenting skills, which showed promise for obesity prevention in high-risk children. Limitations include small sample size, short duration and the representativeness of the sample, including maternal age, education, employment, breast-feeding rates and childcare.
What is already known on this topic
NICE guidance reinforces that the pre-school years are a key time for shaping attitudes and behaviours; that opportunities for children to be active and to develop healthy eating habits are important as well as the need to involve parents and carers. 7
While there is a need for policy and practice to be evidence based, the review has considered some interventions that are good public health practice per se and should be encouraged in any case, for example, breast feeding and physical activity. As Wanless stated ‘the need for action is too pressing for the lack of a comprehensive evidence-base to be used as an excuse for inertia. Instead, current public health policy and practice, which includes a multitude of promising initiatives, should be evaluated as a series of natural experiments’. 14

What this study adds
The review confirms that there is a limited and immature evidence and a lack of comprehensive evidence on effective strategies to prevent obesity in younger children. There are some interesting individual studies that enhance and support recent NICE guidance around activity, family-based interventions and breast-feeding. The study reinforces that prevention of child obesity requires comprehensive, sustained and evidence-based action. Improvements in the evidence base are needed looking at points of intervention, such as those identified in this review, along with evaluation of those interventions. The evidence that childhood obesity persists into adulthood may justify shorter term monitoring at this age.
Limitations of this study
The review shows that the overall quality of studies is poor, there is no consistent research theme, inconsistent results across studies and compared with clinical decision-making where the evidence base is dominated by RCTs with high internal validity, the evidence base for child obesity prevention is poor. 15 The better quality studies tend to show small, but significantly beneficial, effects particularly for physical activity and breast-feeding suggesting that research should be focused in these areas. The lower order RCT by Mo-suwan 9 shows promise and results suggesting swimming may be effective in preventing obesity are worthy of follow-up. The longitudinal study by Armstrong et al. 8 indicates that breast-feeding is potentially useful for population-based strategies aimed at obesity prevention in children aged <5 years. However, caution is required in translating this research into local practice, given the different settings of the studies and the challenges in applying research including RCTs back into a community setting.
With family-based interventions the need exists for good quality longitudinal studies that carefully assess child growth as well as parental control over infant feeding practices and activity levels. The preliminary results of an unpublished RCT on the effectiveness of a multi-component family-based intervention suggest significant improvements in moderately obese older children (P.M. Sacher, unpublished results).
The findings from some studies suggest there are implications for the development of obesity in children and a correlation is evident between certain parental–child interactions and the relative weight and activity levels of the children. Future research should investigate the types of food being encouraged or discouraged and the intensity of children's activity levels. If findings are replicated in different settings it may, for example, explain the equivocal literature on the influence of children's physical activity on weight. Overweight children may in fact engage in equal frequency of activity, but less intensely.
Child obesity will continue to be a problem without improved understanding of key factors likely to be operative during very early childhood and without identification of those where intervention would have the greatest effect. 1
Greater effort is still required to establish an evidence-based approach to issues surrounding obesity in children.
Google Scholar
- childhood obesity
- overweight and obesity prevention
Email alerts
Citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 1741-3850
- Print ISSN 1741-3842
- Copyright © 2024 Faculty of Public Health
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Prevention and Management of Childhood Obesity and its Psychological and Health Comorbidities
Justin d. smith.
1 Department of Psychiatry and Behavioral Sciences, Department of Preventive Medicine, and Department of Pediatrics, Northwestern University Feinberg School of Medicine, Chicago, 750 N. Lake Shore Drive, Illinois, 60611, USA
2 Department of Psychiatry and Behavioral Sciences, Northwestern University Feinberg School of Medicine, 750 N. Lake Shore Drive, Chicago, Illinois, 60611, USA
Marissa Kobayashi
3 Department of Public Health Sciences, University of Miami Miller School of Medicine, 1120 NW 14th Street, Suite 1009, Miami, FL 33136. Phone: (305) 972-9961
Childhood obesity has become a global pandemic in developed countries, leading to a host of medical conditions that contribute to increased morbidity and premature death. The causes of obesity in childhood and adolescence are complex and multifaceted, presenting researchers and clinicians with myriad challenges in preventing and managing the problem. This chapter reviews the state-of-the-science for understanding the etiology of childhood obesity, the preventive interventions and treatment options for overweight and obesity, and the medical complications and co-occurring psychological conditions that result from excess adiposity, such as hypertension, non-alcoholic fatty liver disease, and depression. Interventions across the developmental span, varying risk levels, and service contexts (e.g., community, school, home, and healthcare systems) are reviewed. Future directions for research are offered with an emphasis on translational issues for taking evidence-based interventions to scale in a manner that reduce the public health burden of the childhood obesity pandemic.
1.0. INTRODUCTION
Influenced by genetics, biology, psychosocial factors, and health behaviors, overweight and obesity (OW/OB) in childhood is a complex public health problem affecting the majority of developed countries worldwide. Additionally, the key contributors to obesity—poor diet and physical inactivity—are among the leading causes of preventable youth deaths, chronic disease, and economic health burden ( Friedemann et al 2012 , Hamilton et al 2018 ). Despite the remarkable need to prevent childhood obesity and to intervene earlier to prevent excess weight gain in later developmental periods, few interventions have demonstrated long-lasting effects or been implemented at such a scale to have an appreciable public health impact ( Hales et al 2018 ).
In this review, we describe the extent and nature of the childhood obesity pandemic, present conceptual and theoretical models for understanding its etiology, and take a translational-developmental perspective in reviewing intervention approaches within and across developmental stages and in the various contexts in which childhood OW/OB interventions are delivered. We pay particular attention to co-occurring psychological conditions intertwined with OW/OB for children, adolescents, and their families as they relate to both development/etiology and to intervention. For this reason, our review begins with interventions aimed at prevention and moves to management and treatment options for obesity and its psychological and medical comorbidities. Then, we discuss the state-of-the-science and expert recommendations for interventions to prevent and manage childhood OW/OB and what it would take to implement current evidence-based programs at scale. Last, we end by discussing identified gaps in the literature to inform future directions for research and the translation of research findings to real-world practice that can curb the pandemic. For readability, we use the term “interventions for the prevention and management of childhood OW/OB” to capture an array of approaches referred to by a variety of monikers in the literature, including primary prevention, prevention of excess weight gain, weight loss intervention, weight management, and treatment of obesity. More specific labels are used when needed.
2.0. EPIDEMIOLOGY OF CHILDHOOD OBESITY
Childhood OW/OB is determined by the child’s height and weight to calculate body mass index (BMI), which is adjusted according to norms based on the child’s age and gender. BMI between the 85th and 94th percentile is in the “overweight” range, whereas BMI ≥ 95 th percentile for age and gender is in the “obese” range ( Centers for Disease Control and Prevention [CDC] 2018 ). Rates of obesity among children and adolescents in developed countries worldwide, collected in 2013, were 12.9% for boys and 13.4% for girls ( Ng et al 2014 ). In the United States (US) from 1999–2016, 18.4% of children ages 2–19 years had obesity, and 5.2% had severe obesity, defined as BMI ≥120% of the 95th percentile for age and gender ( Skinner et al 2018 ). The prevalence of obesity has increased between 2011–2012 and 2015–2016 in children ages 2–5 and 16–19 years ( Hales et al 2018 ). Being in the obese range during childhood or adolescence makes the youth five times more likely to be obese in adulthood compared to peers who maintain a healthy weight ( Simmonds et al 2016 ). Compared to obesity, severe obesity is strongly linked with greater cardiometabolic risk, adult obesity, and premature death ( Skinner et al 2015 ).
OW/OB and its health consequences are disproportionately distributed across the US, with a higher prevalence among children of disadvantaged racial and socioeconomic backgrounds. Rates of OW/OB are significantly higher among Non-Hispanic black and Hispanic children compared to Non-Hispanic White children (e.g., Hales et al 2018 ). Such disparities are particularly pronounced among severe obesity, where 12.8% of African American children, and 12.4% of Hispanic children have severe obesity compared to 5.0% of Non-Hispanic White children ( Hales et al 2018 ). Youth in low socioeconomic households are more likely to develop OW/OB compared to their counterparts in high socioeconomic households. In 2011–2014, 18.9% of children ages 2–19 living in the lowest income group (≤130% of Federal Poverty Line) had obesity, whereas 10.9% of children in the highest income group (>350% Federal Poverty Line) had obesity ( Ogden et al 2018 ). Influences on multiple socioecological levels put racially diverse children of low socioeconomic status (SES) at higher risk of developing OW/OB, which is further exacerbated by limited access to health services that can prevent excess weight gain and its sequelae.
3.0. ETIOLOGY OF CHILDHOOD OBESITY
At the most basic level, childhood OW/OB emerges from consuming more calories than expended, resulting in excess weight gain and an excess body fat. Caloric imbalance is the result of, and can be further exacerbated by, a range of obesogenic behaviors. That is, behaviors that are highly correlated with excess weight gain. The most common obesogenic behaviors are high consumption of sugar sweetened beverages and low-nutrient, high saturated fat foods, low levels of physical activity and high levels of sedentary behaviors, and shortened sleep duration (e.g., Sisson et al 2016 ). Diet, physical activity, screen time, and sleep patterns are influenced by a myriad of factors and interactions involving genetics, interpersonal relationships, environment, and community (e.g., Russell & Russell 2019 , Smith et al 2018d ). Children living in the United States commonly consume the “Western Diet,” known as a diet high in calories, rich in sugars, trans and saturated fats, salt and food additives, and low in complex carbohydrates, and vitamins. Poor sleep patterns, defined as short duration and late timing, can contribute to obesity through changing levels of appetite-regulating hormones, and irregular eating patterns including late night snacking and eating ( Miller et al 2015 ). Children who experience shortened night time sleep from infancy to school age are at increased risk of developing OW/OB compared to same-aged children sleeping average, age-specific hours (e.g., Taveras et al 2014 ). Research indicates that children with higher rates of screen time also consume high levels of energy-dense snacks, beverages, and fast food, and fewer fruits and vegetables, and screen time is hypothesized to affect food and beverage consumption through distracted eating, reducing feelings of satiety or fullness, and exposure to advertisements for junk food (sweet and salty, calorically-dense foods) ( Robinson et al 2017 ). Screen time can also negatively affect children’s sleeping patterns, and is correlated with sedentary behaviors (e.g., watching television, playing video games) ( Hale & Guan 2015 ).
3.1. Conceptual Models for Understanding and Addressing Childhood OW/OB
Conceptualizing development of childhood OW/OB requires consideration of interplay of genetic, biological, psychological, behavioral, interpersonal, and environment factors ( Kumar & Kelly 2017 ). OW/OB interventions are typically designed to account for these multilevel factors to assist children in achieving expert recommendations for physical activity and fruit and vegetable consumption, while limiting sugar sweetened beverages intake and screen time, and regulating sleep patterns ( Kakinami et al 2019 ). Creating behavioral change requires understanding of the multi-level interactions to identify opportunities for intervention to prevent excess weight gain long-term. A variety of conceptual models exist to explain potential interactions and individual influences leading to obesogenic behaviors and development of childhood OW/OB, and targets for improving health behaviors and routines. Importantly, basic science and conceptual models can be translated to develop effective, targeted intervention programs for prevention of excess weight gain.
3.1.1. Biopsychosocial model
The biopsychosocial model combines biological foundations in child development with environmental and psychosocial influences to identify and address mechanisms and processes to prevent and manage development of childhood OW/OB ( Russell & Russell 2019 ). This model features biological factors, such as genetics, alongside environmental, psychosocial, and behavioral risk factors (e.g., family disorganization, parenting skills, feeding practices, child appetite, temperament), and the development of self-regulation. Such an approach can illustrate developmental processes interacting with biological underpinnings that can be targeted in prevention and management interventions for OW/OB. Intervening from a biopsychosocial model involves cognitive behavioral and behavioral therapy to reframe thoughts and replace unhealthy eating behaviors with new habits.
3.1.2. Ecological systems theory (EST)
EST embeds individual development and change within multiple proximal and distal contexts and emphasizes the need to understand how an “ecological niche” can contribute to the development of specific characteristics, and how such niches are embedded in more distal contexts ( Davison & Birch 2001 ). For example, a child’s ecological niche can be the family or school, which are embedded in larger social contexts, such as the community and society. Individual child characteristics, such as gender and age, interact within and between the family and community context levels, which all influence development of OW/OB. The EST model presents various predictors of childhood OW/OB through identifying risk factors moderated by intraindividual child characteristics. The structure of the EST is present in various studies examining influences of community exposures and children’s individual attributes on weight outcomes.
3.1.3. The Six C’s Model
The Six-C’s is a developmental ecological model that includes environmental (family, community, country, societal), personal, behavioral, and hereditary influences, and a system for categorizing environmental influences, all of which can be adapted to each stage of child development from infancy to adolescence ( Harrison et al 2011 ). The Six C’s stand for: cell, child, clan, community, country, and culture, which represent biology/genetics, personal behaviors, family characteristics, factors outside of the home including peers and school, state and national-level institutions, and culture-specific norms, respectively. Each C includes factors that contribute to child obesity that occur and interact simultaneously throughout child development. For example, among preschool age children, obesity-predisposing genes (cell), excessive media exposure (child), parent dietary intake (clan), unhealthful peer food choices (community), national economic recession, (country) and oversized portions (culture), are all factors associated with obesity that can occur simultaneously and interact during this developmental stage.
3.1.3. The developmental cascade model of pediatric obesity
The model described in the Smith et al. (2018b) article offers a longitudinal framework to elucidate the way cumulative consequences and spreading effects of multiple risk and protective factors, across and within biopsychosocial spheres and phases of development, can propel children towards OW/OB outcomes. The cascade model of pediatric obesity ( Figure 1 ) was developed using a theory-driven model-building approach and a search of the literature to identify paths and relationships in the model that were empirically based. The model allows for different pathways and interactions between different combinations of variables and constructs that contribute to pediatric obesity (equifinality), identifying multi-level risk and protective factors spanning from the prenatal stage to adolescence stage. The complete model can, but has yet to, be tested. The model focuses on intra- and inter-individual child processes and mechanisms (e.g., parenting practices), while acknowledging that individuals are embedded within the broader ecological systems. St. George et al (in press) then conducted a systematic review of the intervention literature to elucidate the ways in which the developmental cascade model of childhood obesity can inform and is informed by intervention approaches for childhood OW/OB.

Note. Bold text indicates strongest support based on our review of the literature. Reprinted with permission from Taylor and Francis Group: Originally published in Smith JD, Egan KN, Montaño Z, Dawson-McClure S, Jake-Schoffman DE, et al. 2018. A developmental cascade perspective of paediatric obesity: Conceptual model and scoping review. Health Psychology Review 12: 271–293.
3.2. Psychosocial Contributors
3.2.1. maternal mental and physical health.
An emerging body of literature has shown a significant relationship between higher levels of parental stress and youths’ higher weight status and unhealthy lifestyle behaviors ( Tate et al 2015 ). In a prospective study, Stout et al (2015) found that fetal exposure to stress, as evidenced by elevated maternal cortisol and corticotropin-releasing hormone, was related to patterns of increasing BMI over the first 24 months of life. Children of mothers experiencing psychological distress and anxiety during pregnancy had higher fat mass, BMI, subcutaneous and visceral fat indices, liver fat fraction, and risk of obesity at age 10 years compared to those whose mothers did not ( Vehmeijer et al 2019 ). Early stress can have long-lasting effects, and studies from a nationally-representative cohort study have shown that postnatal maternal stress during the first year has a positive longitudinal relationship with the child’s BMI up to age 5 ( Leppert et al 2018 ), and psychological distress at age 5 was associated with risk of obesity at age 11 in another nationally-representative cohort ( Hope et al 2019 ). Among Hispanic children and adolescents whose caregivers reported ≥ 3 chronic stressors, Isasi et al (2017) found an increased likelihood of childhood obesity when compared to those whose parents reported no chronic stressors. In a systematic review assessing the impact of maternal stress on children’s weight-related behaviors, O’Connor et al (2017) found mixed evidence for the relationship specific to dietary intake; however, researchers found consistent evidence for the detrimental impact on youths’ physical activity and sedentary behavior, which was often conceptualized as screen time. Understandably, highly stressed parents may have an increased reliance on convenient fast-food options versus grocery shopping and preparing fresh and healthy meals for their children and may not have the energy or wherewithal to support their youths’ physical activity, nor engage in limit-setting behaviors specific to their children’s screen time.
One of the few studies using a longitudinal design did not replicate the relationship between high parental stress and lower levels of youth physical activity, but the relationship held for high levels of parental stress and increased fast food consumption ( Baskind et al 2019 ). Interestingly, this study observed an interaction effect on the relationship of high parental stress and childhood obesity by only low-income households and among ethnic minority children, specifically non-Hispanic black children—explaining one of the factors that contributes to healthy disparities for childhood obesity rates in the US. In another study using a large, prospective cohort, Shankardass et al (2014) found a significant effect of parental stress on BMI. The researchers also observed a significantly larger effect among Hispanics versus the total sample population, further noting that the relationship was weaker and not statistically significant among non-Hispanic children. Due to the salient role of caregiver stress on child health behaviors, it seems that interventions for childhood OW/OB should incorporate stress reduction strategies for parents while simultaneously focusing efforts on reaching racial/ethnic minority families and the economically disadvantaged.
Maternal mental health, most commonly operationalized as depressive symptoms and diagnosis, relate to children’s risk for OW/OB. The longitudinal effects of postnatal maternal depressive symptoms predicted obesity risk in preschool-age children, and unhealthier lifestyle behaviors, such as high TV viewing time and low levels of physical activity ( Benton et al 2015 ). Children of mothers with severe depression were more likely to be obese compared to children of mothers with fewer symptoms ( Marshall et al 2018 ). Maternal mental health could negatively affect child feeding behaviors such that elevated depressive symptoms in low-income mothers have been associated with increased use of feeding to soothe children ( Savage & Birch 2017 ). Few interventions for childhood obesity to date specifically target caregiver depression, but some protocols provide guidance to engage caregivers in services to manage depression and related stressors ( Smith et al 2018c ).
3.2.2. Child mental health
Poor self-regulation and related constructs such as reactivity and impulsivity, are prospective obesogenic risk factors ( Bergmeier et al 2014 , Smith et al 2018d ). A child’s temperament describes behavioral tendencies in reactivity and self-regulation. Negative reactivity is characterized by a quick response with intense negative affect, and is difficult to soothe. Infants and children with negative reactivity are at high risk of excess weight gain, and developing obesity later on and toddlers with low self-regulation and inability to control impulses or behavior are at increased risk for obesity and rapid weight over the subsequent nine years compared to toddlers with higher self-regulation abilities ( Graziano et al 2013 ). Poorer emotional self-regulation at age 3 is an independent predictor of obesity at age 11 ( Anderson et al 2017 ). On the other hand, the ability to delay gratification at age 4 is associated with lower BMI 30 years later ( Schlam et al 2013 ). It is possible that parents of children with difficult temperament experience challenges effectively managing children’s behaviors and setting limits, leading to irregular health routines and increased obesity risk ( Bergmeier et al 2014 , Smith et al 2018d ). Further, parents could overuse food and feeding to soothe children ( Anzman-Frasca et al 2012 ). Throughout childhood, emotional regulation deficits and other mental health disorders continue to predict obesity and weight gain. Emotional regulation in conjunction with stress during childhood is highly linked to low physical activity, emotional eating, irregular and disrupted sleep, and later development of obesity ( Aparicio et al 2016 ). A longitudinal study examining emotional psychopathology in preadolescence saw that boys diagnosed with a social phobia, panic disorder or dysthymia (persistent depressive disorder) had higher waist circumference and/or BMI, and girls diagnosed with dysthymia had increased waist circumference at the three-year follow-up ( Aparicio et al 2013 ). In a prospective study, overweight children who reported binge eating at ages 6–12 years gained 15% more fat mass over a period of four years compared to overweight children with no binge eating ( Tanofsky-Kraff et al 2006 ). The predictive role of mental health on physical health conditions and subsequent comorbidities can be costly and burdensome. Children with obesity-related health conditions (e.g., type 2 diabetes, metabolic syndrome) and a comorbid psychiatric diagnosis (e.g., depressive mood disorder, bipolar disorder, attachment disorder) have higher healthcare utilization and costs per year compared to children without a comorbid psychiatric diagnosis ( Janicke et al 2009a )
There is an association between OW/OB and depression in childhood and adolescence, but there is mixed evidence of the directionality of this effect among children and adolescents. A review of high quality studies by Mühlig et al (2016) saw that among nine studies examining the influence of depression on weight status, six found no significant influence. Of the studies that reported significant associations, one study saw effects only among female adolescents, another only for male adolescents, and a third showed effects of adolescent depressive symptoms on adult obesity at age 53 years only in women. Conversely, OW/OB status can have significant influences on risk of low self-esteem and depressive symptoms/diagnosis in adolescence, as discussed later in this paper.
3.2.3. Stigma/bullying
Weight-related stigma, defined as subtly or overtly having discriminatory actions against individuals with obesity, toward children with obesity can impair quality of life, and contributes to unhealthy behaviors that can worsen obesity such as social isolation, decreased physical activity, and avoidance of health care services ( Pont et al 2017 ). Unfortunately, stigma is widespread and tolerated in society, furthering the reach of negative harm. Children with obesity face explicit weight bias and stigma from multiple environments including from parents, obesity researchers, clinical settings, and school. Parents not only demonstrate implicit bias against childhood obesity, but also implicit and explicit biases against children with obesity ( Lydecker et al 2018 ). Even among obesity researchers and health professionals, significant implicit and explicit anti-fat bias, and explicit anti-fat attitudes increased between 2001–2013 ( Tomiyama et al 2015 ). Exposure to stigma and weight bias can have damaging psychosocial effects on children, such that stigma can mediate the relationship between BMI, depression, and body dissatisfaction ( Stevens et al 2017 ).
Weight stigma can also initiate bullying and weight related teasing, which can have serious psychological consequences such as depression among children, further weight gain and lessen motivation to change. A nationally representative sample of children ages 10–17 years saw that OW/OB adolescents were at higher odds of being a victim of bullying, and also higher odds of perpetrating bullying and victimizing others ( Rupp & McCoy 2019 ). The children at higher odds of engaging in bullying, or being bullied were also at significantly higher odds of having depression, difficulty making friends, and conduct problems compared to OW/OB adolescents who were not bullies or victims of bullying. The relationship between obesity and bullying needs to be addressed through bullying engagement, and coping skills for victimization to prevent and manage associated behavioral and depressive symptoms.
3.2.4. Family functioning and home environment
Evidence suggests a link between general family functioning, parent–child relationships, communication, and use of positive behavior support strategies and childhood OW/OB (see Smith et al 2017a ). Influence of general parenting styles, as opposed to the more specific feeding styles, have been extensively studied and linked to children’s diet, physical activity, and weight ( Shloim et al 2015 ). Children raised with an authoritative (warm and demanding) parenting style had healthier diet, higher physical activity levels, and lower BMI’s than those raised with the other styles ( Sleddens et al 2011 ). Parents proactively structuring home environments to support and positively reinforce healthy dietary and physical activity behaviors also play a key role in children’s healthy lifestyles ( Smith et al 2017b ). Children exposed to less supportive environments consisting of family stress, father absence, maternal depression, confinement, and unclean home environments at 1 year of age has been associated with high BMI at age 21 ( Bates et al 2018 ). Taken together, family participation and building parenting skills can play a salient role in the prevention of childhood OW/OB ( Pratt & Skelton 2018 , Wen et al 2011 ).
4.0. PREVENTION AND MANAGEMENT OF OVERWEIGHT AND OBESITY
This section discusses the state-of-the-science in childhood OW/OB prevention and management along with salient factors related to their implementation in varied healthcare delivery systems. The current climate is being shaped by the position of the American Medical Association. In 2013, the Board voted to classify obesity as a disease that requires medical attention. This classification aimed to emphasize health risks of obesity, remove individual blame, and create new implications and opportunities for intervention. This classification can help to further: 1) a broader public understanding of the obesity condition and associated stigma; 2) prevention efforts; 3) research for treatment and management; 4) insurance reimbursement for intervention; and 5) medical education ( Kyle et al 2016 ). In primary healthcare settings specifically, the US Preventive Services Task Force (USPSTF) gave childhood obesity screening and family-based intervention a “B” grade for evidence of effectiveness ( US Preventive Services Task Force 2017 ), which is sufficient to open insurance reimbursement streams for activities related to the prevention and management of childhood OW/OB that did not exist before. Reimbursement has been a significant barrier to uptake of effective interventions and the impact of the USPSTF in removing this impediment is not yet fully known.
A number of high-quality systematic reviews and meta-analyses have been published in recent years, which provide the most contemporary perspective of the effectiveness of interventions for prevention and management, as well as revealing wide variability and inconsistent findings. For example, Peirson et al (2015a) saw that prevention interventions were associated with slightly improved weight outcomes compared to control groups in mixed-weight children and adolescents. However, intervention effects were not consistent among each intervention strategy tested, suggesting that specific characteristics of the interventions, such as setting, participants, dose, and tailoring, should be examined to determine what is and is not effective in achieving desired outcomes.
Intervention strategies for the prevention and management of child OW/OB occur in various contexts and within, and in coordination with, multiple service delivery systems. This is due in large part to the risk factors inherent to familial, school, and community/societal levels. Relatedly, for prevention in particular, there is some correspondence between the sample being targeted and the context, such that community and school-based interventions are far more likely to be universal (sample does not consider weight status) or selective (target sample is overweight or specifically targeted due to being at-risk for obesity; e.g., ethnic minority, low income) compared to the indicated (majority of target sample is in the obese range) models more commonly found in primary and specialty healthcare systems. Unsurprisingly, the specific intervention targets and behavior change strategies align with the context and approach ( St. George et al in press ).
4.1. Community Interventions
Community interventions are defined as incorporating policies and strategies aimed at reducing the population risk of obesity through legislation, modifications to the built environment, provision of accessible resources, and changes in economic/pricing/food subsidies ( Bleich et al 2013 ). Community interventions can involve the use of media, businesses (e.g., restaurants), community health services, community gardens, community or recreational centers, city planning, and the local governments ( Karacabeyli et al 2018 ). Interventions delivered in community settings have the ability to provide high degrees of access and exposure to strategies and programs to racially diverse, low-income children, who are at the highest risk of OW/OB. Interventions delivered in community settings can be effective, but the impact could be diminished through the lower likelihood of intervention completion due to living in lower socioeconomic circumstances and other obstacles ( Fagg et al 2015 ).
In comparison to other settings, such as the school and family level, there were fewer studies conducted at the community level in a recent review ( Bleich et al 2018 ). This may be due to the numerous challenges and complications involved in building community capacity and engaging community leaders, stakeholders, community agencies, and city organizations. Alternatively, it could reflect a greater focus to date on other contexts and intervention targets, which we discuss in the following sections. To address effectiveness and sustainability, a combined clinical and community intervention could hold promise, especially for racially diverse children living in a low-income community, who are most at-risk. A study by Hoffman et al (2018) showed that an integrated clinic-community model is feasible and improves physical activity and quality of life when compared to multidisciplinary treatment only in clinical care settings.
To summarize, there is promise in community-based interventions that involve either the health clinic and community partnerships or community and school partnerships. Interventions using a community-based participatory approach and a strong quasi-experimental design could achieve the long term goal of reducing both child BMI, the prevalence of OW/OB in childhood, and remission of obesity in children ( Economos & Hammond 2017 ).
4.2. School-Based Interventions
School-based interventions are defined as taking place during school hours or after-school hours for children in kindergarten through high school, and being focused exclusively in the school or delivered primarily in the school setting with secondary settings of family/home, primary care, or community ( Bleich et al 2018 ). Considering that the majority of children spend a significant amount of their day in school, many preventive interventions have leveraged schools as an entry point to improve the obesogenic environment by promoting more physical activity in physical education classes and recess, improving school playgrounds and nutritional options in school cafeterias, and providing healthy lifestyle education in classes or other school policies ( Ickes et al 2014 ). Previous reviews recommend using multi-component interventions targeting two or more health behaviors (i.e., physical activity, dietary outcomes, sedentary behavior) to improve adiposity outcomes when compared to single-component interventions (e.g., Wang et al 2015 ). Interestingly, well-designed school-based studies are effective in improving dietary behavior, but typically do not see statistically significant differences in child BMI between intervention and control schools, except for among children who are already in the obese range ( Bogart et al 2016 ). While increasing fruit, vegetable and water consumption are important, the health behavior modifications are not sufficient for significant long-term obesity management. A way this has been addressed is partnerships between schools and community-based interventions which also engage parents. In a review, Ickes et al (2014) found that less than half of childhood obesity interventions incorporated parents; of those studies involving parents, 75% demonstrated positive outcomes in reducing BMI or weight status. In a synthesis of systematic reviews and meta-analyses of school-based interventions, long-term interventions with a combination of diet and physical activity components and family or parental involvement significantly reduced weight among children ( Khambalia et al 2012 ). Aligned with previous research, Bleich et al (2018) found that school-based interventions that used a multi-component approach of both physical activity and nutrition with some intervention with families in the home had the largest effects. A systematic review and meta-analysis by Wang et al (2015) observed that strength of evidence of obesity prevention programs for children ages 2–18 years was dependent on intervention type, and delivery setting(s). Strength of evidence was high for physical activity-only interventions delivered in school settings with home involvement, or combined diet and physical activity interventions delivered in school settings with home and community involvement. They also found moderately strong evidence when delivering combined interventions in school-based settings alone, in schools with home or community component, or in community with a school component.
Bleich et al (2018) also reviewed a smaller number of pre-school interventions and found some promise in both single component interventions—focusing solely on physical activity—and multi-component interventions. In two other reviews evaluating early child care center-based interventions, both found promising evidence for multi-component interventions and multiple levels influencing the child, parent, teachers/staff, and class ( Sisson et al 2016 , Ward et al 2017 ). An exemplar study, Natale et al (2017) conducted an early childhood multi-level obesity intervention, which included menu modifications at the child care center, a nutrition and physical activity educational curriculum for preschoolers, and a healthy meal preparation and role modeling curriculum for parents. At two-years follow-up, the researchers observed significantly less increase in BMI percentile among the intervention group versus controls. Overall, strong obesity prevention interventions in early care and education settings were associated with healthy eating and anthropometric outcomes, which was further improved by parental engagement. In sum, the preschool and school contexts hold promise for improving weight-related behaviors and adiposity outcomes; however, evidence is clear that parents should be engaged in the process of supporting and reinforcing their children’s health behaviors for these programs to be maximally effective ( Ward et al 2017 ).
4.3. Family-Based Interventions
The home environment (e.g., family routines, limit setting, household chaos, crowding) has long been considered one of the most powerful influences on children’s healthy behaviors and OW/OB outcomes ( Bates et al 2018 ). Playing an integral role in physical activity, diet, screen time, and sleep, parents can exhibit positive parenting practices (e.g., limit-setting, role modeling) and provide a healthy, supportive environment (e.g., provisions of fresh fruits and vegetables), thereby shaping their children’s lifelong habits and preventing the onset of childhood obesity (for a review see Smith et al 2018d ). Family-based interventions are defined as involving either passive or active parental involvement, often with parents viewed as the primary or sole agents of change ( Sung-Chan et al 2013 ). Active parental involvement entails repeated engagement, such as participation in workshops, counseling, or educational sessions; passive involvement does not integrally involve the parent or guardian (e.g., brochures, newsletters).
In a review evaluating family-based interventions for OW/OB prevention, Ash et al (2017) found a significant increase in the number of family-based interventions with just six studies published in 2008 compared to 35 studies in 2013. The majority of studies employed rigorous RCT study designs (73%), but almost two thirds of the studies were short-term and implemented for less than a year. A fraction of studies occurred in multiple settings and over half targeted multiple components beyond diet and physical activity, such as screen time or sleep. Many preventive studies targeting young children (pre-natal to five years old) tend to use home or primary-care based settings with parental involvement, whereas interventions targeting older children tended to take place in community- and school-based settings. These findings are commensurate with the review of St. George et al (in press) , which showed a decrease in parental involvement and family-based intervention strategies with child age. This dovetails with the conclusions of Kothandan (2014) that family-based interventions demonstrated effectiveness for children younger than twelve, but for children twelve and up, school-based interventions were most effective in the short-term.
Regarding preventive interventions specifically, the majority of interventions have been tested among low SES families and predominantly white families ( Ash et al 2017 ). Hispanics/Latinx have been well-represented in US intervention studies in comparison to other ethnic minorities (i.e., African Americans, Asians, and indigenous groups). Latinx are particularly well-suited to participate in family-based interventions given their cultural emphasis on familial values; however, a recent meta-analysis noted diminishing intervention effects with a higher proportion of Hispanic children ( Ling et al 2016 ), which was attributed to a lack of culturally competent interventions to address language barriers and dietary preferences. In addition to incorporating other ethnic minorities and culturally appropriate interventions, Ash et al (2017) suggested that preventive family-based interventions should account for non-traditional families and their different needs and family dynamics.
In regard to family dynamics and interactions, poor family functioning has been linked with an increased risk of obesity, obesogenic behaviors, and adverse health outcomes (e.g., Pratt & Skelton 2018 ). Family-based care for childhood OW/OB involves targeting dietary and physical activity behaviors along with the rules of the family unit, family health routines, communication, and dynamics ( Pratt & Skelton 2018 ). Existing protocols involve family counseling for diet and physical activity change in the home environment, with some approaches also targeting more general parenting and family management skills that have been found to impact OW/OB status of the child ( Smith et al 2018a , Smith et al 2018b , Smith et al 2017b ). Interventions including both parents and children have shown more positive short and long-term effects on child weight when compared to parent-only interventions and controls in some studies ( Yackobovitch-Gavan et al 2018 ), whereas others have found comparable effects for parent-only and child-involved family-based approaches ( Boutelle et al 2017 ). Further, parent-only interventions have been shown to be more cost-effective ( Janicke et al 2009b ). In a meta-analysis evaluating comprehensive behavioral family lifestyle interventions treating pediatric obesity, Janicke et al (2014) found an overall standardized effect size of 0.47, which indicates a small-to-moderate effect on BMI. The dose of treatment (i.e., number of intervention sessions, minutes spent in treatment) was positively related to the treatment effect, which provides support for the notion that more intense and longer interventions are associated with better outcomes, a conclusion also made by ( Whitlock et al 2010 ). In addition, age was a significant moderator for weight outcomes indicating that older children had larger and more beneficial intervention effects than younger children.
Specifically, family-based interventions targeting positive behavior support have been used to address key mechanisms of change specific to promoting children’s healthy lifestyle behaviors ( Smith et al 2017b ). Positive behavior support has been identified as a way to reduce weight gain through improving the caregiver’s ability to support and work with the child toward a healthier diet and improved physical activity. Long-term prevention trials using family-based intervention to target positive behavior support found that children randomized to the intervention had lower BMI in the years following participation ( Smith et al 2015 ). This finding was particularly promising given that these trials did not explicitly focus on child weight in any way; thus, prevention of childhood OW/OB was a spillover effect.
Given the various ways individual, interpersonal, and family health behaviors contribute to child obesity, a tailored family-based intervention could be effective in identifying specific family needs and providing appropriate resources. In a family-based tailored intervention, Taylor et al (2015) saw that the children of families randomized to the tailored treatment had significantly lower BMI compared to families in the usual care group. Additionally, children in the tailored treatment had better dietary behaviors and were more physically active than children in the treatment as usual group. Smith, Berkel et al. (2018b) adapted the highly effective and well-known individually-tailored family-based prevention program called the Family Check-Up ® ( Dishion et al 2008 ) to specifically target obesogenic behaviors with the aim of preventing obesity and excess weight gain in children ages 2 to 12 years. This adaptation is referred to as the Family Check-Up ® 4 Health and is being tested in two large RCTs in coordination with pediatric primary care ( Smith et al 2018a ) and with community-based family resource centers and public schools ( Berkel et al 2019 ) in low-income neighborhoods with racially/ethnically-diverse families at highest risk for childhood OW/OB.
4.4. Primary Healthcare
Primary care interventions are defined as health promotion or weight management programs conducted within or in close coordination with the primary healthcare system. Primary care is viewed as an ideal, real world environment for weight management interventions because of accessibility and frequency of visits (i.e., routine well-child visits) ( Davis et al 2007 ). In a meta-analysis evaluating weight management interventions delivered in primary-care settings, Mitchell et al (2016) found an overall effect size of 0.26, indicating a small treatment effect, and a smaller effect than has been found in broader meta-analytic reviews (e.g., Janicke et al 2014 , Whitlock et al 2010 ). The dose-response relationship was significant, where the number of treatment contacts, length of treatment in months, and the number of visits with the pediatrician was associated with larger treatment effects.
A systematic review examining randomized control trials targeting obesity management in children ages 2–5 years saw five of six interventions, all in ambulatory healthcare settings, had significant decreases in child weight, with sustained intervention effects through follow-up ( Ling et al 2016 ). The effective interventions actively involved parents in health education, group meetings, physical activity sessions, or behavioral therapy.
4.5. Interventions by Developmental Period
In a review of interventions of OW/OB from birth to age 18, St. George et al (in press) identified 74 distinct interventions reported across the 141 included articles. They were categorized based on the child’s age at entry into the intervention: prenatal/infancy (< 2 years; n = 4), early childhood (2–5 years; n = 11), childhood (6–11 years; n = 38), early adolescence (12–15 years; n = 18), and late adolescence (16–18 years; n = 3). Developmental stage of the child has also been found to align with the strategy, such that interventions in the prenatal and infancy periods are nearly all universal, whereas during childhood and adolescence, as compared to early childhood, the burden of disease is larger and intervention strategies more often target selected and indicated samples with greater intensity ( St. George et al in press ).
5.0. EXPERT RECOMMENDATIONS
5.1. youth health behaviors.
It is recommended that children and adolescents aged 6–17 years should achieve ≥ 60 minutes of physical activity each day ( Piercy et al 2018 ). The 2015–2020 Dietary Guidelines for Americans recommend consuming a variety of fruits and vegetables, whole grains, proteins, low-fat dairy products, and limiting intake of sodium, solid fats and added sugars beginning at age 2 years ( DeSalvo et al 2016 ). Unfortunately, only 21.6% of children 6–19 years reach the recommended 60 minutes of physical activity at least five days per week ( Alliance 2016 ). Dietary quality impacts weight gain and OW/OB, and it is estimated that the obesity epidemic largely contributed to statistics showing a declining life expectancy, which occurred in 2015 for the first time in 30 years ( Ludwig 2016 ).
The American Academy of Pediatrics (AAP) recommends that children under 18 months should have no screen time aside from video-chatting, and children ages 2–5 years engage in one hour of screen time per day of high-quality programs with parents. Children ages 6 and above should have limited media exposure, ≤ 2 hours per day, which should not interfere with sleep, physical activity, or other health behaviors. The AAP recommends that families should have “media-free” time together, and “media-free” locations such as in the dining room or bedroom to avoid interfering with meals and sleep duration ( American Academy of Pediatrics Council on Communications and Media 2016 ). The World Health Organization asserts that screen time brings no benefit to children, and infants younger than one year should have no electronic screen exposure, and children age 2–4 years should not have more than one hour of daily “sedentary screen time.” In recent years, the portability of screen devices has led to an overall increase in screen time, with the majority of US youth exceeding screen time guidelines by a wide margin (averaging more than 7 hours daily) ( Barnett Tracie et al 2018 ).
The most recent AAP guidelines recommend that children ages 1–2 years sleep 11–14 hours per 24 hours, children 3–5 sleep 10–13 hours, children 6–12 sleep 9–12 hours, and teenagers ages 13–18 should regularly sleep 8–10 hours ( Paruthi et al 2016 ). Certain behaviors such as a regular routine, avoiding large meals close to bedtime, being physically active during the day time, and eliminating electronic devices in the bedroom are associated with better sleep ( Irish et al 2015 ). According to the CDC, 60% of middle schoolers and 70% of high schoolers do not meet regular sleep recommendations.
5.2. Behavioral Intervention
Family-based intervention is recommended by The National Academy of Medicine, the American Academy of Pediatrics, and the Endocrine Society, among others, as the preferred approach for the management of OW/OB from infancy to adolescence. Based on a systematic review, the USPSTF concluded that lifestyle-based weight loss interventions (not necessarily family-based) consisting of 26 or more hours of intervention engagement are likely to assist children and adolescents in weight management ( O’Connor et al 2017 ). Recommendations from a number of expert committees and task forces support targeting the following behaviors for prevention and management of childhood OW/OB: limiting consumption of sugar sweetened beverages, consuming daily recommended fruit and vegetables, limiting screen time, increasing physical activity, eating breakfast, limiting eating out at restaurants, encouraging family meals, and limiting portion sizes. The majority of existing interventions target multiple behaviors, but some have been designed for discrete behaviors.
5.3. Pharmacologic Intervention
Orlistat is the only FDA-approved medication for treating obesity for pediatric patients ages 12 years and older. Side effects in the gastrointestinal area are common in children, and further clinical trials are needed to evaluate medication risk and benefits among pediatric patients ( Chao et al 2018 ). Expert opinion states that Orlistat, in conjunction to lifestyle changes, leads to modest weight loss and could benefit children in the indicated age range with obesity but tolerability limits its use ( Kelly & Fox 2018 ). And results are not unequivocal. In a meta-analysis looking at primary-care based interventions, Peirson et al (2015b) found a medium effect (standardized effect size [ES] = −0.54) favoring behavioral interventions when compared to Orlistat plus behavioral intervention components (ES = −0.43). Additional research is needed on both effectiveness and tolerability in youth. Additionally, new pharmacologic options continue to be developed and tested and could reach the market in the next few years if approval is granted ( Kelly & Fox 2018 ).
5.4. Surgical Intervention
The American Society for Metabolic and Bariatric Surgery Pediatric Committee’s best practice guidelines selection criteria are based on systematic reviews of co-morbidities, risks and outcomes, important team members, and patient selection. They recommend that adolescents being considered for a bariatric procedure should have a BMI of ≥35 kg/m 2 with major co-morbidities such as type-2 diabetes mellitus, moderate to severe sleep apnea, or severe nonalcoholic steatohepatitis ( Michalsky et al 2012 ). Data show that bariatric surgery in morbidly obese adolescents can greatly impact weight loss, and attenuate or resolve associated chronic disease. However, adolescents undergoing bariatric surgery should be assessed for capability to adhere to follow-up care regimens to ensure proper nutrition intake and care. The committee also recommends a multidisciplinary team for adolescents undergoing bariatric surgery, which could include an experienced bariatric surgeon, pediatric specialist, registered dietitian, mental health specialist, care coordinator, and exercise physiologist.
6.0. CLINICAL IMPLICATIONS OF CO-OCCURRING MEDICAL AND PSYCHOLOGICAL CONDITIONS
6.1. co-occurring medical conditions.
The pro-inflammatory disease nature of obesity and contributing health behaviors affects normal physiology and metabolism, and can cause many associated diseases ( Gonzalez-Muniesa et al 2017 ). If left untreated, obesity can lead to serious health conditions including type-2 diabetes, cardiovascular disease, asthma, obstructive sleep apnea, high blood pressure/hypertension, non-alcoholic fatty liver disease, hepatocellular carcinoma, and psychosocial problems (e.g., Nobili et al 2015 ). Recent research indicates increased risk of cardiovascular disease incidence, morbidity (ischemic heart disease, stroke), and mortality in adulthood associated with being in the obese BMI range in childhood or adolescence ( Sommer & Twig 2018 ). Obesity prevention and management interventions in childhood are imperative for averting the burden of associated comorbidities.
6.1.1. Type-2 diabetes
Children with obesity are four times as likely to develop type-2 diabetes compared to children with a normal BMI ( Abbasi et al 2017 ). Ethnic minority children of low income are at increased risk, and have limited maintenance and glycemic control, furthering the probability of developing additional health complications down the line ( Pulgaron & Delamater 2014 ). Metformin is the main treatment of type-2 diabetes in youth and adults, though emerging evidence implicates a role in treating children with obesity and a family history of type-2 diabetes (e.g., Warnakulasuriya et al 2018 ). Exercise and lifestyle interventions have had significantly positive health effects in adults, however trials evaluating effects in youth with type-2 diabetes are limited. Given the data from adult trials, the American Diabetes Association recommends that youth with type-2 diabetes meet the 1-hour per day physical activity goal to manage symptoms and decrease health risks ( Colberg et al 2016 ).
6.1.2. Obstructive sleep apnea
Pediatric obstructive sleep apnea (OSA) involves a child having disrupted breathing due to partially or completely blocked upper airways during sleep ( Narang & Mathew 2012 ). Obesity confers the most significant risk for OSA. As many as 60% of children and adolescents with obesity have OSA, or some sort of disrupted breathing during sleep ( Narang & Mathew 2012 ). Obesity and OSA have additional comorbidities and impairments including excessive daytime sleepiness, neurocognitive function, reduced physical activity, cardiovascular burden, and hypertension, further complicating quality life of children with obesity ( Blechner & Williamson 2016 ). Obesity management such as increased physical activity and a healthy diet are recommended for OSA treatment, as well as surgical procedures, if appropriate.
6.1.3. Asthma
Asthma is one of the most common chronic diseases among children and adolescents: 10.1% of children ages 5–14 years had asthma in 2016 ( National Center for Health Statistics 2019 ). Although both obesity and asthma rates have been increasing, it does not appear that obesity has been contributing to the increased asthma prevalence rate ( Akinbami et al 2018 ). This does not discount the risks of obesity on asthma and its unique effects on asthma symptoms. OW/OB children have been observed to have higher prevalence of asthma, and exacerbation as early as preschool age compared to normal weight children ( Lang et al 2018 ). Additionally, OW/OB children have reported distinct asthma symptoms, such as greater shortness of breath, reduced airway hyperresponsiveness, and loss of asthma control, compared to normal weight children ( Lang et al 2015 ). The relationship between asthma and OW/OB should be further investigated.
6.1.4. Hypertension
Hypertension, like obesity, has been increasing among youth and is associated with increased cardiovascular disease risk throughout the lifetime ( May et al 2012 ). The greatest risk factor for pediatric hypertension is elevated BMI ( Falkner et al 2006 ). About 3% of children in the general population have hypertension, compared to about 25% of obese children ( Shatat & Brady 2018 ). In a meta-analysis examining cardiovascular risk factors, compared with normal weight children, systolic blood pressure was higher by 4.54 mm Hg (n=12169, 8 studies) in overweight children, and by 7.49 mm Hg (n=8074, 15 studies) in obese children ( Friedemann et al 2012 ). A study examining childhood hypertension and OW/OB in school children saw that 2.2% of the sample had hypertension, and 37% of those cases could be attributed to OW/OB status ( Chiolero et al 2007 ). A review shows that children with obesity-related hypertension are at increased risk of cardiovascular morbidity and mortality ( Wuhl 2019 ). About 3.8%–24.8% of children with OW/OB have hypertension, though these rates could be higher due to inconsistences and challenges with diagnoses ( Flynn et al 2017 ). The risks of hypertension on children’s lifetime health emphasize the importance of preventing obesity early on.
6.1.5. Nonalcoholic fatty liver disease (NAFLD)
NAFLD is the leading cause of liver disease, leading to a shorter life expectancy due to associated comorbidities; one of which, non-alcoholic steatohepatitis, is projected to be the leading indication for pediatric liver transplant by 2025 ( Charlton et al 2011 ). Epidemiological studies consistently show associations between NAFLD and adiposity, unhealthy diet, and sedentary behavior ( Dunn & Schwimmer 2008 ). Prevalence of NAFLD is especially high in young people who have obesity such that 22.5%–52.8% of children with obesity have NAFLD compared to 2.6% of all children ( Anderson et al 2015 ). Child obesity has the highest risk in the development of NAFLD during childhood ( Hays & McGinnis 2018 ). A longitudinal study of participants ages 3–18 years were followed for 31 years, and saw that child OW/OB was associated with increased risk for adult NAFLD ( Cuthbertson et al 2018 ). The associated risk was removed if participants obtained a normal range BMI by adulthood, emphasizing the salient role of weight management. The high prevalence of NAFLD among children with obesity, and effectiveness of weight change in treating this condition, emphasizes the need for prevention and management of obesity. Smith et al (2017a) found that among children who had NAFLD, poorer family functioning was significantly related to higher BMI, elevated levels of cholesterol, HbA1c, and glucose. Their study exposes the critical role of family functioning on child health, and the importance of using targeted intervention to prevent, and manage obesity and associated disease using a family-centered approach. Weight being the most modifiable factor, the mainstay of NAFLD treatment is lifestyle behavior modifications aimed at weight loss ( Marchesini et al 2015 ).
6.2. Co-Occurring Psychological Conditions
6.2.1. self-esteem/depression.
Children with OW/OB are more likely to experience low self-esteem, and develop depressive symptoms during adolescence compared to normal weight peers (e.g., Mühlig et al 2016 ). This relationship can be attributed to multi-level factors including health behaviors, parenting styles, and family functioning. A review by Hoare et al (2014) suggests that obesogenic risk factors, such as infrequent physical activity, sedentary behavior, poor diet quality, and adiposity were associated with depressive symptoms in adolescents. Conversely, healthier eating patterns were associated with decreased depressive symptoms. Child eating disorder pathology, emotionally-manipulative parenting style, and lower child social status have been associated with depressive symptomatology among children with OW/OB ( Sheinbein et al 2019 ). Children in poorly functioning families with low self-esteem participating in weight loss interventions have been observed to have poor 6-month outcomes, suggesting that multiple social-ecological factors need to be addressed when targeting depressive symptoms in children with OW/OB ( Taylor et al 2017 ). Further, negative psychological experiences more generally, such as trauma and stigma, trigger emotional eating, leading to an ongoing obesity-depression cycle ( Milaneschi et al 2019 ).
6.2.2. Eating disorders
Children with OW/OB have a high prevalence of disordered eating attitudes and behaviors, which can increase risk of developing eating disorders in adulthood. A high proportion of adolescents with restrictive eating disorders report a history of OW/OB ( Lebow et al 2015 ). Additionally, it is estimated that over a quarter of youth with OW/OB have binge and loss of control eating ( He et al 2017 ). Adolescent girls with OW/OB experiencing overvaluation of weight—so concerned with weight that self-evaluation is influenced—are at higher risk of starting to binge eat weekly 2 years later, have more severe depressive symptoms, and continuous overvaluation ( Sonneville et al 2015 ). The bidirectional relationship of obesity and eating disorders, including eating disorder psychopathology, should be properly evaluated during treatment planning.
7.0. IMPLEMENTATION AND RESEARCH TRANSLATION CHALLENGES
One of the abundant challenges for the field is the translation and implementation of effective interventions to the real-world service delivery systems that can reach those most in need. This so-called research-practice gap is pronounced in obesity prevention and management given the preponderance of untested, usual care approaches currently in use; the persistence of debunked myths about causes and effective intervention approaches (e.g., fad diets); and the incongruence between what is being developed by experts and what is acceptable, feasible, and sustainable in existing systems given the constraints of the workforce, space, and funding. This says nothing about the consumer of evidence-based interventions, who historically have had only cursory involvement in the design and deployment of interventions. This has contributed to low engagement rates and high attrition from more intensive OW/OB interventions ( Lydecker & Grilo 2016 ). Raising public and caregiver concern about the risks posed by OW/OB in childhood and adolescence would also facilitate engagement and retention. Currently, many parents with children with obesity underestimate their children’s weight ( Lydecker & Grilo 2016 ) and are thus unlikely to seek intervention or to follow through with a referral for intervention. Add the stigma in society surrounding obesity and the shame parents experience concerning their child’s weight, and traditional approaches to care will continue to be underutilized.
While many of the aforementioned conceptual models encapsulate the multiple levels contributing to childhood obesity, researchers are trying to elucidate which combination of levels and service contexts have greatest effectiveness, and which implementation strategies best address the complexity at levels of the community, school, family, and primary care. Implementation strategies are defined as the methods or techniques used to enhance the adoption, implementation, and sustainability of a clinical program or practice ( Proctor et al 2013 ). They are the actions taken on agents in the system of care itself, and rarely only on the patient or client that is the recipient of the clinical program or practice. The first iteration of the Childhood Obesity Research Demonstration Projects (CORD 1.0), a program of research administered by the CDC, examined multi-sector intervention implementation in schools, community centers, early care and health centers, and pediatric primary care practices. The three projects around the US, identified the facilitators and barriers of implementing multi-setting interventions targeting levels of the socioecologial model in racially diverse, lower-income communities ( Dooyema et al 2017 ). CORD 1.0 projects identified common implementation barriers in schools, rural communities and community centers, including staff turnover, limited resources, and competing needs for existing requirements (such as standardized testing in schools) ( Chuang et al 2016 , Ganter et al 2017 ). Interventions in rural communities and multiple settings benefited from engaging parents and obtaining support from organization members and leadership ( Chuang et al 2016 , Ganter et al 2017 ). Facilitators of school interventions included using the principal as a champion and using students to engage other students ( Blaine et al 2017 ). Low-income primary care settings showed that only about 27% of referred patients enrolled in the intervention ( Barlow et al 2017 ). Such knowledge assists in the design of future studies to develop effective, accessible, and acceptable interventions for those needing it most.
These implementation challenges are not unique to childhood obesity but the complexity of the problem will require more rapid translation of discoveries in research with bidirectional input from successes and failures in practice back to researchers. Last, improving the packaging of evidence-based programs can provide potential implementers with a “ready off the shelf” product that requires less involvement by the intervention developers, which is a primary contributor to the high cost of adopting a new program ( Jordan et al 2019 ), and can arguably aid implementers in delivering interventions with fidelity. This is the goal of the CDC’s Childhood Obesity Research Demonstration (CORD) 3.0 Project ( https://www.cdc.gov/obesity/strategies/healthcare/cord3.html ). However, the scale up penalty—reduced effects as interventions are widely disseminated and adopted—has been shown in the childhood obesity literature to be about 75% of efficacy studies ( McCrabb et al 2019 ), but implementation scientists have argued for dynamic adaptation that retains effectiveness while also increasing sustainability (e.g., Chambers et al 2013 ). This is an area in need of attention as interventions are taken to scale.
8.0. RECOMMENDATIONS FOR FUTURE RESEARCH
Reviews of interventions for childhood OW/OB show variability in effectiveness, often changing health behaviors but not weight, thus exposing the difficulties of addressing and managing this public health crisis. There are a number of directions for future research to improve outcomes and address the challenges of wide-scale implementation.
1) Interventions need to be integrated across systems.
Given the multifaceted, multilevel, and interrelated nature of OW/OB development, if interventions are to be maximally effective there needs to be an integration of multiple service systems (primary care, schools, communities, child care, the home) for the delivery of multicomponent interventions that utilize behavioral, structural, environmental, policy, and biomedical approaches.
2) There is no “one size fits all.”
More complex, individual child and family interventions need to be tailored both in terms of content and implementation strategy to best align with the personal needs of those involved. This means flexible, adaptive, or modularized intervention protocols addressing the cadre of potential health behaviors and related individual and familial risk factors of OW/OB present, and getting the intervention to families in a manner that is engaging, accessible, and has wide reach.
3) Consider implementation earlier.
Researchers developing interventions for childhood OW/OB ought to consider their implementability from the beginning using the framework of “designing for dissemination and implementation” ( Dearing et al 2013 ), which considers the capacities, needs, and preferences of the end users (service delivery systems, children/families, funding mechanisms) during design and testing. Another method for speeding translation is to adapt existing programs for new service contexts and new populations, rather than following the traditional pipeline of treating something different as “new” and having to establish efficacy and effectiveness before moving to implement. This concept has been referred to as “scaling out” ( Aarons et al 2017 ) and it has been applied in childhood OW/OB prevention and management ( Smith et al 2018b ). Scaling out is a critical method for implementation research to address the health inequities and disparities of childhood obesity ( McNulty et al 2019 ).
4) Engage the community to enhance scalability and sustainability.
Berkel et al (in press) engaged a diverse group of stakeholders, including payors, in the adaptation and delivery processes of a recent trial of the Family Check-Up ® 4 Health as a means of increasing the likelihood of sustained adoption beyond the funded trial. Economos and Hammond (2017) suggest that community-level research should employ novel techniques of systems mapping and causal loop diagramming, which can help stakeholders to visualize the interrelated processes and elements that are relevant to the intervention. They also suggest using agent-based modeling and other simulation methods to help encapsulate the complex dynamics involved in implementing successful community-based interventions. Tailoring strategies to local communities and deepening engagement holds promise in enhancing sustainability and scalability of community-based interventions.
5) Research rigor—scale up balance.
Future directions should address the shortcomings of less rigorous study designs, which inherently increases the risk of confounding and presents challenges in attributing changes in the outcome to intervention effects, but as research translation moves toward scaling up after establishing effectiveness, this tradeoff is both expected and encouraged to increase external validity. Additionally, research is needed to determine the appropriate length and dosage of interventions, along with clear reporting of outcomes, consistency of measures, and long-term follow ups ( Bleich et al 2018 , Ickes et al 2014 , St. George et al in press ). Echoing Karacabeyli et al (2018) , we also recommend collecting process evaluation and outcome data in order to understand the complex causal chain and to help bolster inferences in regard to the effectiveness and implementation of the intervention using hybrid designs .
6) Engagement and participation are critical challenges.
Large community trials in particular often suffer high attrition rates because of mobile populations who move to different residences, which can impact the ability to track and communicate with participants. And this relates to effectiveness. Children completing >75% of a community-based intervention program experienced beneficial change in BMI as well as associated health behaviors (physical activity, screen time, unhealthy food consumption) compared with children completing <75% of the program ( Hardy et al 2015 ). A way to attenuate attrition in research on community-level interventions could be through adjusting study intervention design. The majority of community-based interventions used a quasi-experimental design, which is often attributed to practicality and sustainability ( Bleich et al 2018 , Karacabeyli et al 2018 ). Interestingly, less rigorous study designs (e.g., quasi-experimental vs. RCTs) demonstrated significant reductions in child weight ( Karacabeyli et al 2018 ). By removing randomization, the authors reported that communities with the resources, engagement/buy-in, and capacity could be selected to participate, which optimized community support for the obesity intervention efforts through both sustainable partnerships and buy-in from the community and its champions. This participatory approach could potentially lead to lasting positive health changes that extend beyond the study period. In addition, Karacabeyli et al (2018) described the benefits of a quasi-experimental design which lends itself to selecting at-risk communities that could greatly benefit from intervention efforts. For example, using a stepped wedge or randomized rollout trial design where all at-risk communities selected would eventually receive the intervention at different time periods but none serve as “no intervention” controls (see Landsverk et al 2017 ).
9. CONCLUSIONS
There are signs that progress is being made in stemming the tide of childhood obesity and evidence-based interventions are available across development and for various contexts and systems that affected and at-risk children routinely encounter. Tremendous challenges remain in connecting the dots between etiology, development, and intervention targets, as well as when and where to intervene. There needs to be a push to scale up effective interventions as even small changes in weight can yield significant impact on multiple cardiometabolic indices ( Lloyd-Jones et al 2010 ) that can improve quality and length of life. Clinical health psychologists are ideally suited to conduct research on this complex problem but transdisciplinary teams will be needed to increasingly move the dial.
SUMMARY POINTS
- Childhood obesity is a complex, multidetermined, preventable chronic disease that increases risk for premature death and psychological problems.
- Evidence-based interventions for obesity are available for all stages of development from birth to 18 years.
- Specific interventions can be delivered in community, school, home, and healthcare settings depending on the type of strategy and risk level of the targeted population.
- Associated co-occurring medical and psychological conditions of childhood obesity present an opportunity for clinical and health psychology researchers and practitioners.
FUTURE ISSUES
- Future research ought to focus on translational considerations from the start and ways to scale up delivery of effective interventions.
- Research is needed on interventions and their implementation to more effectively reach minority and underserved populations at greatest risk for obesity.
- Increasing engagement and retention in childhood obesity interventions is a promising focus for future research.
ACKNOWLEDGEMENTS
The authors wish to thank Sara St. George for feedback on an earlier version of this review and to acknowledge support of this work from the Centers for Disease Control and Prevention (grant U18DP006255) and the United States Department of Agriculture (grant 2018-68001-27550), awarded to Justin Smith and Cady Berkel; and the National Institute on Drug Abuse (grant P30 DA027828), to C. Hendricks Brown, in support of Justin Smith.
DISCLOSURE STATEMENT
Justin D. Smith is co-developer of the Family Check-Up ® 4 Health intervention for childhood obesity. The authors are not aware of any other affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Aarons GA, Sklar M, Mustanski B, Benbow N, Brown CH. 2017. “Scaling-out” evidence-based interventions to new populations or new health care delivery systems . Implementation Science 12 : 111. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Abbasi A, Juszczyk D, van Jaarsveld CHM, Gulliford MC. 2017. Body Mass Index and Incident Type 1 and Type 2 Diabetes in Children and Young Adults: A Retrospective Cohort Study . Journal of the Endocrine Society 1 : 524–37 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Akinbami LJ, Rossen LM, Fakhouri THI, Fryar CD. 2018. Asthma prevalence trends by weight status among US children aged 2–19 years, 1988–2014 . Pediatric Obesity 13 : 393–96 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Alliance NPAP. 2016. 2016 US report card on physical activity for children and youth
- American Academy of Pediatrics Council on Communications and Media. 2016. Media and Young Minds . Pediatrics 138 : e20162591. [ PubMed ] [ Google Scholar ]
- Anderson EL, Howe LD, Jones HE, Higgins JP, Lawlor DA, Fraser A. 2015. The Prevalence of Non-Alcoholic Fatty Liver Disease in Children and Adolescents: A Systematic Review and Meta-Analysis . PLoS One 10 : e0140908. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Anderson SE, Sacker A, Whitaker RC, Kelly Y. 2017. Self-regulation and household routines at age three and obesity at age eleven: longitudinal analysis of the UK Millennium Cohort Study . International Journal Of Obesity 41 : 1459. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Anzman-Frasca S, Stifter CA, Birch LL. 2012. Temperament and childhood obesity risk: A review of the literature . Journal of Developmental & Behavioral Pediatrics 33 : 732–45 [ PubMed ] [ Google Scholar ]
- Aparicio E, Canals J, Arija V, De Henauw S, Michels N. 2016. The role of emotion regulation in childhood obesity: implications for prevention and treatment . Nutrition Research Reviews 29 : 17–29 [ PubMed ] [ Google Scholar ]
- Aparicio E, Canals J, Voltas N, Hernandez-Martinez C, Arija V. 2013. Emotional psychopathology and increased adiposity: Follow-up study in adolescents . Journal of Adolescence 36 : 319–30 [ PubMed ] [ Google Scholar ]
- Ash T, Agaronov A, Young T, Aftosmes-Tobio A, Davison KK. 2017. Family-based childhood obesity prevention interventions: a systematic review and quantitative content analysis . The international journal of behavioral nutrition and physical activity 14 : 113. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Barlow SE, Butte NF, Hoelscher DM, Salahuddin M, Pont SJ. 2017. Strategies to Recruit a Diverse Low-Income Population to Child Weight Management Programs From Primary Care Practices . Preventing chronic disease 14 : E138–E38 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Barnett Tracie A, Kelly Aaron S, Young Deborah R, Perry Cynthia K, Pratt Charlotte A, et al. 2018. Sedentary Behaviors in Today’s Youth: Approaches to the Prevention and Management of Childhood Obesity: A Scientific Statement From the American Heart Association . Circulation 138 : e142–e59 [ PubMed ] [ Google Scholar ]
- Baskind MJ, Taveras EM, Gerber MW, Fiechtner L, Horan C, Sharifi M. 2019. Parent-Perceived Stress and Its Association With Children's Weight and Obesity-Related Behaviors . Prev Chronic Dis 16 : E39. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bates CR, Buscemi J, Nicholson LM, Cory M, Jagpal A, Bohnert AM. 2018. Links between the organization of the family home environment and child obesity: A systematic review . Obesity Reviews 19 : 716–27 [ PubMed ] [ Google Scholar ]
- Benton PM, Skouteris H, Hayden M. 2015. Does maternal psychopathology increase the risk of pre-schooler obesity? A systematic review . Appetite 87 : 259–82 [ PubMed ] [ Google Scholar ]
- Bergmeier H, Skouteris H, Horwood S, Hooley M, Richardson B. 2014. Child temperament and maternal predictors of preschool children's eating and body mass index. A prospective study . Appetite 74 : 125–32 [ PubMed ] [ Google Scholar ]
- Berkel C, Rudo-Stern J, Villamar JA, Wilson C, Flanagan E, et al. in press. Recommendations from community partners to promote sustainable implementation of evidence-based programs in primary care . Journal of Community Psychology [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Berkel C, Smith JD, Bruening MM, Jordan N, Grimm K, et al. 2019. The Family Check-Up 4 Health: A Health Maintenance Approach to Improve Nutrition and Prevent Early Childhood Obesity . Presented at Society for Nutrition Education and Behavior Annual Conference Orlando, FL [ Google Scholar ]
- Blaine RE, Franckle RL, Ganter C, Falbe J, Giles C, et al. 2017. Using School Staff Members to Implement a Childhood Obesity Prevention Intervention in Low-Income School Districts: the Massachusetts Childhood Obesity Research Demonstration (MA-CORD Project), 2012-2014 . Prev Chronic Dis 14 : E03. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Blechner M, Williamson AA. 2016. Consequences of Obstructive Sleep Apnea in Children . Current Problems in Pediatric and Adolescent Health Care 46 : 19–26 [ PubMed ] [ Google Scholar ]
- Bleich SN, Segal J, Wu Y, Wilson R, Wang Y. 2013. Systematic Review of Community-Based Childhood Obesity Prevention Studies . Pediatrics 132 : e201. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bleich SN, Vercammen KA, Zatz LY, Frelier JM, Ebbeling CB, Peeters A. 2018. Interventions to prevent global childhood overweight and obesity: a systematic review . The lancet. Diabetes & endocrinology 6 : 332–46 [ PubMed ] [ Google Scholar ]
- Bogart LM, Elliott MN, Cowgill BO, Klein DJ, Hawes-Dawson J, et al. 2016. Two-Year BMI Outcomes From a School-Based Intervention for Nutrition and Exercise: A Randomized Trial . Pediatrics 137 : e20152493. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Boutelle KN, Rhee KE, Liang J, Braden A, Douglas J, et al. 2017. Effect of Attendance of the Child on Body Weight, Energy Intake, and Physical Activity in Childhood Obesity Treatment: A Randomized Clinical Trial . JAMA Pediatrics 171 : 622–28 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Centers for Disease Control and Prevention. 2018. Defining Childhood Obesity . [ Google Scholar ]
- Chambers DA, Glasgow R, Stange K. 2013. The dynamic sustainability framework: addressing the paradox of sustainment amid ongoing change . Implement Sci 8 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chao AM, Wadden TA, Berkowitz RI. 2018. The safety of pharmacologic treatment for pediatric obesity . Expert Opinion on Drug Safety 17 : 379–85 [ PubMed ] [ Google Scholar ]
- Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. 2011. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States . Gastroenterology 141 : 1249–53 [ PubMed ] [ Google Scholar ]
- Chiolero A, Cachat F, Burnier M, Paccaud F, Bovet P. 2007. Prevalence of hypertension in schoolchildren based on repeated measurements and association with overweight . Journal of Hypertension 25 : 2209–17 [ PubMed ] [ Google Scholar ]
- Chuang E, Brunner J, Moody J, Ibarra L, Hoyt H, et al. 2016. Factors Affecting Implementation of the California Childhood Obesity Research Demonstration (CA-CORD) Project, 2013 . Prev Chronic Dis 13 : E147. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, et al. 2016. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association . Diabetes Care 39 : 2065. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cuthbertson DJ, Brown E, Koskinen J, Magnussen CG, Hutri-Kähönen N, et al. 2018. Longitudinal analysis of risk of non-alcoholic fatty liver disease in adulthood . Liver International 0 [ PubMed ] [ Google Scholar ]
- Davis MM, Gance-Cleveland B, Hassink S, Johnson R, Paradis G, Resnicow K. 2007. Recommendations for prevention of childhood obesity . Pediatrics 120 : S229–S53 [ PubMed ] [ Google Scholar ]
- Davison KK, Birch LL. 2001. Childhood overweight: A contextual model and recommendations for future research . Obesity Reviews 2 : 159–71 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dearing JW, Smith DK, Larson RS, Estabrooks CA. 2013. Designing for Diffusion of a Biomedical Intervention . American Journal of Preventive Medicine 44 : S70–S76 [ PubMed ] [ Google Scholar ]
- DeSalvo KB, Olson R, Casavale KO. 2016. Dietary Guidelines for AmericansDietary Guidelines for AmericansDietary Guidelines for Americans . JAMA 315 : 457–58 [ PubMed ] [ Google Scholar ]
- Dishion TJ, Shaw DS, Connell A, Gardner FEM, Weaver C, Wilson M. 2008. The Family Check-Up with high-risk indigent families: Preventing problem behavior by increasing parents' positive behavior support in early childhood . Child Development 79 : 1395–414 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dooyema CA, Belay B, Blanck HM. 2017. Implementation of Multisetting Interventions to Address Childhood Obesity in Diverse, Lower-Income Communities: CDC's Childhood Obesity Research Demonstration Projects . Prev Chronic Dis 14 : E140. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dunn W, Schwimmer J. 2008. The obesity epidemic and nonalcoholic fatty liver disease in children . Curr Gastroenterol Rep 10 : 67–72 [ PubMed ] [ Google Scholar ]
- Economos CD, Hammond RA. 2017. Designing effective and sustainable multifaceted interventions for obesity prevention and healthy communities . Obesity 25 : 1155–56 [ PubMed ] [ Google Scholar ]
- Fagg J, Cole TJ, Cummins S, Goldstein H, Morris S, et al. 2015. After the RCT: who comes to a family-based intervention for childhood overweight or obesity when it is implemented at scale in the community? Journal of Epidemiology and Community Health 69 : 142. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Falkner B, Gidding SS, Ramirez-Garnica G, Wiltrout SA, West D, Rappaport EB. 2006. The relationship of body mass index and blood pressure in primary care pediatric patients . J Pediatr 148 : 195–200 [ PubMed ] [ Google Scholar ]
- Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, et al. 2017. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents . Pediatrics 140 : e20171904. [ PubMed ] [ Google Scholar ]
- Friedemann C, Heneghan C, Mahtani K, Thompson M, Perera R, Ward AM. 2012. Cardiovascular disease risk in healthy children and its association with body mass index: systematic review and meta-analysis . BMJ : British Medical Journal 345 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ganter C, Aftosmes-Tobio A, Chuang E, Kwass JA, Land T, Davison KK. 2017. Lessons Learned by Community Stakeholders in the Massachusetts Childhood Obesity Research Demonstration (MA-CORD) Project, 2013-2014 . Prev Chronic Dis 14 : E08. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gonzalez-Muniesa P, Martinez-Gonzalez MA, Hu FB, Despres JP, Matsuzawa Y, et al. 2017. Obesity . Nature reviews. Disease primers 3 : 17034 [ PubMed ] [ Google Scholar ]
- Graziano PA, Kelleher R, Calkins SD, Keane SP, Brien MO. 2013. Predicting weight outcomes in preadolescence: the role of toddlers' self-regulation skills and the temperament dimension of pleasure . International journal of obesity (2005) 37 : 937–42 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hale L, Guan S. 2015. Screen time and sleep among school-aged children and adolescents: a systematic literature review . Sleep medicine reviews 21 : 50–8 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. 2018. Trends in obesity and severe obesity prevalence in us youth and adults by sex and age, 2007-2008 to 2015-2016 . JAMA 319 : 1723–25 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hamilton D, Dee A, Perry IJ. 2018. The lifetime costs of overweight and obesity in childhood and adolescence: a systematic review . Obesity Reviews 19 : 452–63 [ PubMed ] [ Google Scholar ]
- Hardy LL, Mihrshahi S, Gale J, Nguyen B, Baur LA, O’Hara BJ. 2015. Translational research: are community-based child obesity treatment programs scalable? BMC Public Health 15 : 652. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Harrison K, Bost KK, McBride BA, Donovan SM, Grigsby-Toussaint DS, et al. 2011. Toward a developmental conceptualization of contributors to overweight and obesity in childhood: The Six-Cs model . Child Development Perspectives 5 : 50–58 [ Google Scholar ]
- Hays SM, McGinnis C. 2018. Nonalcoholic Fatty Liver Disease in Children: Beyond Metabolic Syndrome . The Journal for Nurse Practitioners 14 : 725–31 [ Google Scholar ]
- He J, Cai Z, Fan X. 2017. Prevalence of binge and loss of control eating among children and adolescents with overweight and obesity: An exploratory meta-analysis . Int J Eat Disord 50 : 91–103 [ PubMed ] [ Google Scholar ]
- Hoare E, Skouteris H, Fuller-Tyszkiewicz M, Millar L, Allender S. 2014. Associations between obesogenic risk factors and depression among adolescents: a systematic review . Obesity Reviews 15 : 40–51 [ PubMed ] [ Google Scholar ]
- Hoffman J, Frerichs L, Story M, Jones J, Gaskin K, et al. 2018. An Integrated Clinic-Community Partnership for Child Obesity Treatment: A Randomized Pilot Trial . Pediatrics 141 : e20171444. [ PubMed ] [ Google Scholar ]
- Hope S, Micali N, Deighton J, Law C. 2019. Maternal mental health at 5 years and childhood overweight or obesity at 11 years: evidence from the UK Millennium Cohort Study . International Journal of Obesity 43 : 43–52 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ickes MJ, McMullen J, Haider T, Sharma M. 2014. Global school-based childhood obesity interventions: a review . Int J Environ Res Public Health 11 : 8940–61 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Irish LA, Kline CE, Gunn HE, Buysse DJ, Hall MH. 2015. The role of sleep hygiene in promoting public health: A review of empirical evidence . Sleep Medicine Reviews 22 : 23–36 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Isasi CR, Hua S, Jung M, Carnethon MR, Perreira K, et al. 2017. The Association of Parental/Caregiver Chronic Stress with Youth Obesity: Findings from the Study of Latino Youth and the Hispanic Community Health Study/Study of Latinos Sociocultural Ancillary Study . Childhood obesity (Print) 13 : 251–58 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Janicke DM, Harman JS, Kelleher KJ, Zhang J. 2009a. The Association of Psychiatric Diagnoses, Health Service Use, and Expenditures in Children with Obesity-related Health Conditions . Journal of Pediatric Psychology 34 : 79–88 [ PubMed ] [ Google Scholar ]
- Janicke DM, Sallinen BJ, Perri MG, Lutes LD, Silverstein JH, Brumback B. 2009b. Comparison of Program Costs for Parent-Only and Family-Based Interventions for Pediatric Obesity in Medically Underserved Rural Settings . The Journal of Rural Health 25 : 326–30 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Janicke DM, Steele RG, Gayes LA, Lim CS, Clifford LM, et al. 2014. Systematic review and meta-analysis of comprehensive behavioral family lifestyle interventions addressing pediatric obesity . Journal of Pediatric Psychology [ PubMed ] [ Google Scholar ]
- Jordan N, Graham AK, Berkel C, Smith JD. 2019. Budget impact analysis of preparing to implement the Family Check-Up 4 Health in primary care to reduce pediatric obesity . Prevention Science 20 : 655–64 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kakinami L, Houle-Johnson SA, Demissie Z, Santosa S, Fulton JE. 2019. Meeting fruit and vegetable consumption and physical activity recommendations among adolescents intending to lose weight . Preventive Medicine Reports 13 : 11–15 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Karacabeyli D, Allender S, Pinkney S, Amed S. 2018. Evaluation of complex community-based childhood obesity prevention interventions . Obes Rev 19 : 1080–92 [ PubMed ] [ Google Scholar ]
- Kelly AS, Fox CK. 2018. Role of Pharmacotherapy in the Treatment of Pediatric Obesity and Its Comorbidities In Pediatric Obesity: Etiology, Pathogenesis and Treatment , ed. Freemark MS, pp. 613–27. Cham: Springer International Publishing [ Google Scholar ]
- Khambalia AZ, Dickinson S, Hardy LL, Gill T, Baur LA. 2012. A synthesis of existing systematic reviews and meta-analyses of school-based behavioural interventions for controlling and preventing obesity . Obes Rev 13 : 214–33 [ PubMed ] [ Google Scholar ]
- Kothandan SK. 2014. School based interventions versus family based interventions in the treatment of childhood obesity- a systematic review . Archives of public health = Archives belges de sante publique 72 : 3. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kumar S, Kelly AS. 2017. Review of Childhood Obesity: From Epidemiology, Etiology, and Comorbidities to Clinical Assessment and Treatment . Mayo Clinic Proceedings 92 : 251–65 [ PubMed ] [ Google Scholar ]
- Kyle TK, Dhurandhar EJ, Allison DB. 2016. Regarding Obesity as a Disease: Evolving Policies and Their Implications . Endocrinology and metabolism clinics of North America 45 : 511–20 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Landsverk J, Brown CH, Smith JD, Chamberlain P, Palinkas LA, et al. 2017. Design and analysis in dissemination and implementation research In Dissemination and implementation research in health: Translating research to practice , ed. Brownson RC, Colditz GA, Proctor EK, pp. 201–27. New York: Oxford University Press [ Google Scholar ]
- Lang JE, Fitzpatrick AM, Mauger DT, Guilbert TW, Jackson DJ, et al. 2018. Overweight/obesity status in preschool children associates with worse asthma but robust improvement on inhaled corticosteroids . Journal of Allergy and Clinical Immunology 141 : 1459–67.e2 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lang JE, Hossain MJ, Lima JJ. 2015. Overweight children report qualitatively distinct asthma symptoms: analysis of validated symptom measures . The Journal of allergy and clinical immunology 135 : 886–93.e3 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lebow J, Sim LA, Kransdorf LN. 2015. Prevalence of a History of Overweight and Obesity in Adolescents With Restrictive Eating Disorders . Journal of Adolescent Health 56 : 19–24 [ PubMed ] [ Google Scholar ]
- Leppert B, Junge KM, Röder S, Borte M, Stangl GI, et al. 2018. Early maternal perceived stress and children’s BMI: longitudinal impact and influencing factors . BMC Public Health 18 : 1211. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ling J, Robbins LB, Wen F. 2016. Interventions to prevent and manage overweight or obesity in preschool children: A systematic review . International Journal of Nursing Studies 53 : 270–89 [ PubMed ] [ Google Scholar ]
- Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, et al. 2010. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s strategic impact goal through 2020 and beyond . Circulation 121 : 586–613 [ PubMed ] [ Google Scholar ]
- Ludwig DS. 2016. Lifespan Weighed Down by DietDiet and Decreasing LifespanDiet and Decreasing Lifespan . JAMA 315 : 2269–70 [ PubMed ] [ Google Scholar ]
- Lydecker JA, Grilo CM. 2016. The apple of their eye: Attitudinal and behavioral correlates of parents’ perceptions of child obesity . Obesity 24 : 1124–31 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lydecker JA, O’Brien E, Grilo CM. 2018. Parents have both implicit and explicit biases against children with obesity . Journal of Behavioral Medicine 41 : 784–91 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Marchesini G, Petta S, Dale Grave R. 2015. Diet, Weight Loss, and Liver Health in NAFLD: Pathophysiology, Evidence and Practice . Hepatology [ PubMed ]
- Marshall SA, Ip EH, Suerken CK, Arcury TA, Saldana S, et al. 2018. Relationship between maternal depression symptoms and child weight outcomes in Latino farmworker families . Maternal & Child Nutrition 14 : e12614. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- May AL, Kuklina EV, Yoon PW. 2012. Prevalence of cardiovascular disease risk factors among us adolescents, 1999–2008 . Pediatrics 129 : 1035–41 [ PubMed ] [ Google Scholar ]
- McCrabb S, Lane C, Hall A, Milat A, Bauman A, et al. 2019. Scaling-up evidence-based obesity interventions: A systematic review assessing intervention adaptations and effectiveness and quantifying the scale-up penalty . Obesity Reviews 20 : 964–82 [ PubMed ] [ Google Scholar ]
- McNulty M, Smith JD, Villamar J, Burnett-Zeigler I, Vermeer W, et al. 2019. Implementation Research Methodologies for Achieving Scientific Equity and Health Equity . In Ethnicity & disease , pp. 83–92 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Michalsky M, Reichard K, Inge T, Pratt J, Lenders C. 2012. ASMBS pediatric committee best practice guidelines . Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery 8 : 1–7 [ PubMed ] [ Google Scholar ]
- Milaneschi Y, Simmons WK, van Rossum EFC, Penninx BWJH. 2019. Depression and obesity: evidence of shared biological mechanisms . Molecular Psychiatry 24 : 18–33 [ PubMed ] [ Google Scholar ]
- Miller AL, Lumeng JC, LeBourgeois MK. 2015. Sleep patterns and obesity in childhood . Current Opinion in Endocrinology Diabetes and Obesity 22 : 41–47 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mitchell TB, Amaro CM, Steele RG. 2016. Pediatric Weight Management Interventions in Primary Care Settings: A Meta-Analysis . Health Psychol [ PubMed ]
- Mühlig Y, Antel J, Föcker M, Hebebrand J. 2016. Are bidirectional associations of obesity and depression already apparent in childhood and adolescence as based on high-quality studies? A systematic review . Obesity Reviews 17 : 235–49 [ PubMed ] [ Google Scholar ]
- Narang I, Mathew JL. 2012. Childhood obesity and obstructive sleep apnea . J Nutr Metab 2012 : 134202–02 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Natale RA, Messiah SE, Asfour LS, Uhlhorn SB, Englebert NE, Arheart KL. 2017. Obesity Prevention Program in Childcare Centers: Two-Year Follow-Up . American journal of health promotion : AJHP 31 : 502–10 [ PubMed ] [ Google Scholar ]
- National Center for Health Statistics. 2019. National Asthma Data (2017 National Health Interview Survey) , Centers for Disease Control and Prevention [ Google Scholar ]
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, et al. 2014. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013 . The Lancet 384 : 766–81 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nobili V, Alkhouri N, Alisi A, Della Court C, Fitzpatrick E, et al. 2015. Nonalcoholic fatty liver disease: A challenge for pediatricians . JAMA Pediatrics 169 : 170–76 [ PubMed ] [ Google Scholar ]
- O'Connor SG, Maher JP, Belcher BR, Leventhal AM, Margolin G, et al. 2017. Associations of maternal stress with children's weight-related behaviours: a systematic literature review . Obesity Reviews 18 : 514–25 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- O’Connor EA, Evans CV, Burda BU, Walsh ES, Eder M, Lozano P. 2017. Screening for Obesity and Intervention for Weight Management in Children and Adolescents: Evidence Report and Systematic Review for the US Preventive Services Task ForceUSPSTF Evidence Report: Screening for Obesity in Children and YouthUSPSTF Evidence Report: Screening for Obesity in Children and Youth . JAMA 317 : 2427–44 [ PubMed ] [ Google Scholar ]
- Ogden CL, Carroll MD, Fakhouri TH, Hales CM, Fryar CD, et al. 2018. Prevalence of obesity among youths by household income and education level of head of household—United States 2011–2014 . Morbidity and Mortality Weekly Report 67 : 186. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Paruthi S, Brooks LJ, D'Ambrosio C, Hall WA, Kotagal S, et al. 2016. Recommended Amount of Sleep for Pediatric Populations: A Consensus Statement of the American Academy of Sleep Medicine . J Clin Sleep Med 12 : 785–86 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Peirson L, Fitzpatrick-Lewis D, Morrison K, Ciliska D, Kenny M, et al. 2015a. Prevention of overweight and obesity in children and youth: a systematic review and meta-analysis . CMAJ open 3 : E23 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Peirson L, Fitzpatrick-Lewis D, Morrison K, Warren R, Usman Ali M, Raina P. 2015b. Treatment of overweight and obesity in children and youth: a systematic review and meta-analysis . CMAJ open 3 : E35–E46 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, et al. 2018. The Physical Activity Guidelines for AmericansPhysical Activity Guidelines for AmericansPhysical Activity Guidelines for Americans . JAMA 320 : 2020–28 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pont SJ, Puhl R, Cook SR, Slusser W. 2017. Stigma Experienced by Children and Adolescents With Obesity . Pediatrics 140 : e20173034. [ PubMed ] [ Google Scholar ]
- Pratt KJ, Skelton JA. 2018. Family Functioning and Childhood Obesity Treatment: a Family Systems Theory-Informed Approach . Academic Pediatrics [ PMC free article ] [ PubMed ]
- Proctor E, Powell BJ, McMillen JC. 2013. Implementation strategies: recommendations for specifying and reporting . Implement Sci 8 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pulgaron ER, Delamater AM. 2014. Obesity and type 2 diabetes in children: epidemiology and treatment . Current diabetes reports 14 : 508. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Robinson TN, Banda JA, Hale L, Lu AS, Fleming-Milici F, et al. 2017. Screen Media Exposure and Obesity in Children and Adolescents . Pediatrics 140 : S97–S101 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rupp K, McCoy SM. 2019. Bullying Perpetration and Victimization among Adolescents with Overweight and Obesity in a Nationally Representative Sample . Childhood Obesity [ PMC free article ] [ PubMed ]
- Russell CG, Russell A. 2019. A biopsychosocial approach to processes and pathways in the development of overweight and obesity in childhood: Insights from developmental theory and research . Obesity Reviews 20 : 725–49 [ PubMed ] [ Google Scholar ]
- Savage JS, Birch LL. 2017. WIC mothers' depressive symptoms are associated with greater use of feeding to soothe, regardless of perceived child negativity . Pediatr Obes 12 : 155–62 [ PubMed ] [ Google Scholar ]
- Schlam TR, Wilson NL, Shoda Y, Mischel W, Ayduk O. 2013. Preschoolers' delay of gratification predicts their body mass 30 years later . J Pediatr 162 : 90–3 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Shankardass K, McConnell R, Jerrett M, Lam C, Wolch J, et al. 2014. Parental stress increases body mass index trajectory in pre-adolescents . Pediatr Obes 9 : 435–42 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Shatat IF, Brady TM. 2018. Editorial: Pediatric Hypertension: Update . Frontiers in Pediatrics 6 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sheinbein DH, Stein RI, Hayes JF, Brown ML, Balantekin KN, et al. 2019. Factors associated with depression and anxiety symptoms among children seeking treatment for obesity: A social-ecological approach . Pediatr Obes : e12518. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Shloim N, Edelson LR, Martin N, Hetherington MM. 2015. Parenting Styles, Feeding Styles, Feeding Practices, and Weight Status in 4-12 Year-Old Children: A Systematic Review of the Literature . Front Psychol 6 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Simmonds M, Llewellyn A, Owen CG, Woolacott N. 2016. Predicting adult obesity from childhood obesity: a systematic review and meta-analysis . Obesity Reviews 17 : 95–107 [ PubMed ] [ Google Scholar ]
- Sisson SB, Krampe M, Anundson K, Castle S. 2016. Obesity prevention and obesogenic behavior interventions in child care: A systematic review . Preventive Medicine 87 : 57–69 [ PubMed ] [ Google Scholar ]
- Skinner AC, Perrin EM, Moss LA, Skelton JA. 2015. Cardiometabolic Risks and Severity of Obesity in Children and Young Adults . New England Journal of Medicine 373 : 1307–17 [ PubMed ] [ Google Scholar ]
- Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. 2018. Prevalence of Obesity and Severe Obesity in US Children, 1999–2016 . Pediatrics 141 : e20173459. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sleddens EFC, Gerards SMPL, Thijs C, de Vries NK, Kremers SPJ. 2011. General parenting, childhood overweight and obesity-inducing behaviors: A review . International Journal of Pediatric Obesity 6 : e12–e27 [ PubMed ] [ Google Scholar ]
- Smith JD, Berkel C, Jordan N, Atkins DC, Narayanan SS, et al. 2018a. An individually tailored family-centered intervention for pediatric obesity in primary care: Study protocol of a randomized type II hybrid implementation-effectiveness trial (Raising Healthy Children study) . Implementation Science 13 : 1–15 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Smith JD, Berkel C, Rudo-Stern J, Montaño Z, St George SM, et al. 2018b. The Family Check-Up 4 Health (FCU4Health): Applying implementation science frameworks to the process of adapting an evidence-based parenting program for prevention of pediatric obesity and excess weight gain in primary care . Frontiers in public health 6 : 293. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Smith JD, Berkel C, Rudo-Stern J, Montaño Z, St. George SM, et al. 2018c. The Family Check-Up 4 Health (FCU4Health): Applying implementation science frameworks to the process of adapting an evidence-based parenting program for prevention of pediatric obesity and excess weight gain in primary care . Frontiers in Public Health [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Smith JD, Egan KN, Montaño Z, Dawson-McClure S, Jake-Schoffman DE, et al. 2018d. A developmental cascade perspective of paediatric obesity: Conceptual model and scoping review . Health Psychology Review 12 : 271–93 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Smith JD, Montaño Z, Dishion TJ, Shaw DS, Wilson MN. 2015. Preventing weight gain and obesity: Indirect effects of a family-based intervention in early childhood . Prevention Science 16 : 408–19 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Smith JD, Montaño Z, Maynard A, Miloh T. 2017a. Family Functioning Predicts Body Mass Index and Biochemical Levels of Youths with Nonalcoholic Fatty Liver Disease . Journal of Developmental & Behavioral Pediatrics 38 : 155–60 [ PubMed ] [ Google Scholar ]
- Smith JD, St. George SM, Prado G. 2017b. Family-centered positive behavior support interventions in early childhood to prevent obesity . Child Development 88 : 427–35 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sommer A, Twig G. 2018. The Impact of Childhood and Adolescent Obesity on Cardiovascular Risk in Adulthood: a Systematic Review . Curr Diab Rep 18 : 91. [ PubMed ] [ Google Scholar ]
- Sonneville KR, Grilo CM, Richmond TK, Thurston IB, Jernigan M, et al. 2015. Prospective association between overvaluation of weight and binge eating among overweight adolescent girls . The Journal of adolescent health : official publication of the Society for Adolescent Medicine 56 : 25–9 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- St. George SM, Agosto Y, Rojas L, Soares M, Bahamon M, et al. in press. A developmental cascade perspective of pediatric obesity: A systematic review of preventive interventions from infancy through late adolescence . Obesity Reviews [ PMC free article ] [ PubMed ]
- Stevens SD, Herbozo S, Morrell HE, Schaefer LM, Thompson JK. 2017. Adult and childhood weight influence body image and depression through weight stigmatization . Journal of health psychology 22 : 1084–93 [ PubMed ] [ Google Scholar ]
- Stout SA, Espel EV, Sandman CA, Glynn LM, Davis EP. 2015. Fetal programming of children's obesity risk . Psychoneuroendocrinology 53 : 29–39 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sung-Chan P, Sung YW, Zhao X, Brownson RC. 2013. Family-based models for childhood-obesity intervention: A systematic review of randomized controlled trials . Obesity Reviews 14 : 265–78 [ PubMed ] [ Google Scholar ]
- Tanofsky-Kraff M, Cohen ML, Yanovski SZ, Cox C, Theim KR, et al. 2006. A Prospective Study of Psychological Predictors of Body Fat Gain Among Children at High Risk for Adult Obesity . Pediatrics 117 : 1203–09 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tate EB, Wood W, Liao Y, Dunton GF. 2015. Do stressed mothers have heavier children? A meta-analysis on the relationship between maternal stress and child body mass index . Obesity reviews : an official journal of the International Association for the Study of Obesity 16 : 351–61 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Taveras EM, Gillman MW, Peña M-M, Redline S, Rifas-Shiman SL. 2014. Chronic Sleep Curtailment and Adiposity . Pediatrics 133 : 1013–22 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Taylor JH, Xu Y, Li F, Shaw M, Dziura J, et al. 2017. Psychosocial predictors and moderators of weight management programme outcomes in ethnically diverse obese youth . Pediatric Obesity 12 : 453–61 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Taylor RW, Cox A, Knight L, Brown DA, Meredith-Jones K, et al. 2015. A Tailored Family-Based Obesity Intervention: A Randomized Trial . Pediatrics 136 : 281. [ PubMed ] [ Google Scholar ]
- Tomiyama AJ, Finch LE, Belsky ACI, Buss J, Finley C, et al. 2015. Weight bias in 2001 versus 2013: Contradictory attitudes among obesity researchers and health professionals . Obesity 23 : 46–53 [ PubMed ] [ Google Scholar ]
- US Preventive Services Task Force. 2017. Screening for Obesity in Children and Adolescents: US Preventive Services Task Force Recommendation Statement . JAMA 317 : 2417–26 [ PubMed ] [ Google Scholar ]
- Vehmeijer FOL CV Silva C, Derks IPM, El Marroun H, Oei EHG, et al. 2019. Associations of Maternal Psychological Distress during Pregnancy with Childhood General and Organ Fat Measures . Childhood Obesity [ PubMed ] [ Google Scholar ]
- Wang Y, Cai L, Wu Y, Wilson RF, Weston C, et al. 2015. What childhood obesity prevention programmes work? A systematic review and meta-analysis . Obesity Reviews 16 : 547–65 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ward DS, Welker E, Choate A, Henderson KE, Lott M, et al. 2017. Strength of obesity prevention interventions in early care and education settings: A systematic review . Preventive Medicine 95 : S37–S52 [ PubMed ] [ Google Scholar ]
- Warnakulasuriya LS, Fernando MMA, Adikaram AVN, Thawfeek ARM, Anurasiri WL, et al. 2018. Metformin in the Management of Childhood Obesity: A Randomized Control Trial . Childhood obesity (Print) 14 : 553–65 [ PubMed ] [ Google Scholar ]
- Wen M, Simpson JM, Baur LA, Rissel C, Flood VM. 2011. Family functioning and obesity risk behaviors: Implications for early obesity intervention . Obesity 19 : 1252–58 [ PubMed ] [ Google Scholar ]
- Whitlock EP, O'Connor EA, Williams SB, Beil TL, Lutz KW. 2010. Effectiveness of weight management interventions in children: A targeted systematic review for the USPSTF . Pediatrics 125 : e396–e418 [ PubMed ] [ Google Scholar ]
- Wuhl E 2019. Hypertension in childhood obesity . Acta paediatrica (Oslo, Norway : 1992) 108 : 37–43 [ PubMed ] [ Google Scholar ]
- Yackobovitch-Gavan M, Wolf Linhard D, Nagelberg N, Poraz I, Shalitin S, et al. 2018. Intervention for childhood obesity based on parents only or parents and child compared with follow-up alone . Pediatric Obesity 13 : 647–55 [ PubMed ] [ Google Scholar ]

IMAGES
VIDEO
COMMENTS
Abstract. Several dietary interventions have been conducted to prevent/reduce childhood obesity, but most of them are known to have failed in tackling the obesity epidemic. This study aimed to review the existing literature on dietary interventions for the prevention of childhood obesity and their effectiveness.
The prevalence of childhood obesity continues to rise despite decades of clinical and public health efforts. Early identification of children at risk of developing obesity is essential using newer electronic health systems, which move beyond traditional growth charts to provide a wealth of information about body mass index and other relevant parameters such as social determinants of health and ...
The present study conducted a systematic literature review to examine obesity research and machine learning techniques for the prevention and treatment of obesity from 2010 to 2020. Accordingly, 93 papers are identified from the review articles as primary studies from an initial pool of over 700 papers addressing obesity.
Childhood obesity has emerged as an important public health problem in the United States and other countries in the world. Currently 1 in 3 children in the United States is afflicted with overweight or obesity. ... For this comprehensive review, the literature was scanned from 1994 to 2016 using PubMed using the following search terms ...
10.1542/6305605698112Video AbstractPEDS-VA_2021-0555716305605698112BACKGROUND AND OBJECTIVES. Addressing food insecurity while promoting healthy body weights among children is a major public health challenge. Our objective is to examine longitudinal associations between food insecurity and obesity in US children aged 1 to 19 years.METHODS. Sources for this research include PubMed, CINAHL, and ...
This Review describes current knowledge on the epidemiology and causes of child and adolescent obesity, considerations for assessment, and current management approaches. Before the COVID-19 pandemic, obesity prevalence in children and adolescents had plateaued in many high-income countries despite levels of severe obesity having increased. However, in low-income and middle-income countries ...
Childhood obesity remains a serious public health problem affecting all ages of the pediatric life span. Despite increases in interventions and research, the prevalence of childhood obesity continues to rise. The National Center for Health Statistics 2015-2016 data report an overall childhood obesity rate of 18.5%, with variation between age groups: 13.9% among 2-5 years old, 18.4% among 6 ...
Childhood obesity has emerged as an important public health problem in the United States and other countries in the world. Currently 1 in 3 children in the United States is afflicted with overweight or obesity. ... For this comprehensive review, the literature was scanned from 1994 to 2016 using PubMed using the following search terms ...
Early childhood overweight and obesity is a major health concern affecting nearly a quarter of children in the United States, with similar rates in Europe, Canada and Australia (Morris et al., 2015), with Shakleton et al. (2018) reporting that New Zealand has among the highest rates in the world.Rudolf et al. (2019) has reported that as many as 10% of children in the United Kingdom are obese ...
Obesity is a complex condition that interweaves biological, developmental, environmental, behavioral, and genetic factors; it is a significant public health problem. The most common cause of obesity throughout childhood and adolescence is an inequity in energy balance; that is, excess caloric intake without appropriate caloric expenditure. Adiposity rebound (AR) in early childhood is a risk ...
Childhood obesity has become a public health problem worldwide. The prevalence of children who are either overweight or obese is increasing each year. In England, the National Child Measurement ... study period.33 Andela et al.34 published a literature review to eval-uate the effect and safety of a very‐low‐energy diet (VLED) in chil-dren ...
Introduction. Childhood and adolescent obesity have reached epidemic levels in the United States, affecting the lives of millions of people. In the past 3 decades, the prevalence of childhood obesity has more than doubled in children and tripled in adolescents. 1 The latest data from the National Health and Nutrition Examination Survey show that the prevalence of obesity among US children and ...
Purpose of Review This is a review of the patterns, conceptualization, and suggested mechanisms underlying the relationship of socioeconomic status (SES) to obesity in childhood and the implications of these data for interventions going forward. Recent Findings Adiposity and SES are negatively associated in high-income countries and positively associated in medium to low-income countries ...
Several dietary interventions have been conducted to prevent/reduce childhood obesity, but most of them are known to have failed in tackling the obesity epidemic. This study aimed to review the existing literature on dietary interventions for the prevention of childhood obesity and their effectiveness. A literature search was conducted using PubMed Central®. Only articles published between ...
Obesity in children is increasing worldwide, impacting on both long- and short-term health. Obesity prevention is an important contempo. ... Preventing obesity in pre-school children: a literature review, Journal of Public Health, Volume 29, Issue 4, December 2007, Pages 368-375, ...
Temperament and childhood obesity risk: A review of the literature. Journal of Developmental & Behavioral Pediatrics 33: 732-45 [Google Scholar] Aparicio E, Canals J, Arija V, De Henauw S, Michels N. 2016. The role of emotion regulation in childhood obesity: implications for prevention and treatment.
Article PDF Available Literature Review. Childhood and Adolescent Obesity: A Review ... The most common cause of obesity throughout childhood and adolescence is an inequity in energy balance; that ...