- Research article
- Open access
- Published: 05 October 2015

Physico-chemical and bacteriological quality of drinking water of different sources, Jimma zone, Southwest Ethiopia
- Mohammed Yasin 1 ,
- Tsige Ketema 1 &
- Ketema Bacha 1
BMC Research Notes volume 8 , Article number: 541 ( 2015 ) Cite this article
25k Accesses
43 Citations
4 Altmetric
Metrics details
The quality of drinking water has always been a major health concern, especially in developing countries, where 80 % of the disease cases are attributed to inadequate sanitation and use of polluted water. The inaccessibility of potable water to large segment of a population in the rural communities is the major health concern in most part of developing countries. This study was designed to evaluate the physico-chemical and bacteriological qualities of drinking water of different sources in the study area.
The study was conducted at Serbo town and selected kebeles around the same town in Kersa district of Jimma Zone, southwest Ethiopia. Socio-demographic characteristics of the study populations were gathered using structured and pre-tested questionnaires. Standard microbiological methods were employed for determination of bacterial load and detection of coliforms. Physico-chemical analyses [including total dissolved substances (TDS), total suspended substances (TSS), biological oxygen demand (BOD), nitrate and phosphate concentrations, turbidity and electrical conductivities] were conducted following guidelines of American Public Health Association and WHO. Correlations among measured parameters of water samples collected from different water sources were computed using SPSS software (version 20).
Only 18.1 % (43/237) of the study population had access to tap water in the study area. More than 50 % of the community relies on open field waste disposal. Members of the family Enterobacteriaceae, Bacillus and Pseudomonas were among dominant bacterial isolates in the water samples. All water samples collected from unprotected water sources were positive for total coliforms and fecal coliforms (FC). Accordingly, FC were detected in 80 % of the total samples with counts ranging between 0.67 and 266.67 CFU/100 ml although 66.67 % of tap water samples were negative for FC. The recorded temperature and pH ranged between 20.1–29.90 °C and 5.64–8.14, respectively. The lowest and highest mean TDS were 116 and 623 mg/l, respectively. Furthermore, the mean concentration of TSS ranged between 2.07 and 403.33 mg/l. Turbidity, electric conductivity, and nitrate concentration of the water samples ranged, respectively, between 0.01–65.4 NTU, 30.6–729 μS/cm, and below detection limit to 95.80 mg/l. In addition, the mean dissolved oxygen values were found to be between 1.62 and 10.71 mg/l; whereas BOD was within the range of 8–77 mg/l. In all water samples, the concentrations of zinc were within the WHO maximum permissible limits (3 mg/l) although the lead concentration in about 66.7 % of the samples exceeded the maximum permissible limit (0.01 mg/l).
The present study has revealed that some of the bacteriological data and physico-chemical parameters of the different water sources had values beyond the maximum tolerable limits recommended by WHO. Thus, it calls for appropriate intervention, including awareness development work and improving the existing infrastructure in order to minimize the potential health problems of those communities currently realizing of the available water sources.
Water-borne diseases are still major health burden in many parts of the world and reported to cause about 4 billion clinical cases of diarrhea per year, representing 5.7 % of the global disease burden in the year 2000 [ 1 ]. Water is a critical component of public health, and failure to supply safe water will place a heavy burden to humanity [ 2 ]. Although poor sanitation and food are the main sources for contamination with pathogen of gastrointestinal tract, drinking water is the major source of microbial pathogens in developing regions [ 3 ]. Furthermore, water may be contaminated by disease causing pathogens from landfills and septic systems, through careless disposal of hazardous household products, agricultural chemicals, and leaking of underground storage tanks.
According to WHO estimation, about 1.1 billion people globally drink unsafe water and the vast majority (88 %) of diarrheal disease reported across the globe is attributable to unsafe water, sanitation and hygiene [ 1 ]. Furthermore, around 250 million infections each year, which results in 10–20 million deaths world-wide, occur due to water-borne diseases [ 4 ]. The wide spread of a number of diseases such as cholera, dysentery and salmonellosis are mainly due to the lack of safe drinking water and adequate sanitation that ends up in death of millions of people in developing countries every year. Diarrhea is the major cause for the death of more than 2 million people per year world-wide, majority of which are children aged less than 5 years [ 1 ].
Prior to 2004, the majority of Ethiopia’s population does not have access to safe and reliable sanitation facilities besides insufficient hygienic practices related to food, water and personal hygiene. Accordingly, more than 75 % of the health problems in Ethiopia were due to infectious diseases attributed to unsafe and inadequate water supply, and unhygienic waste management, with human excreta being the major problem [ 5 ].
Some studies conducted on bacteriological qualities of drinking water in Akaki-Kalit sub-city of Addis Ababa, Ziway, Bahir Dar and Nazareth (Adama) towns showed contamination of the water samples with indicator bacteria including total coliforms (TTC) and faecal coliforms [ 6 ]. Besides microbial contaminants, contaminations of water resources with heavy metals have received particular concern because of their strong toxicity even at lower concentration [ 7 , 8 ]. Furthermore, heavy metals are not biologically degradable unlike the case of most organic pollutants, thus easily assimilated and can be bio-accumulated in the protoplasm of aquatic organisms [ 9 ]. The common heavy metals include iron, lead, arsenic, mercury, cadmium, chromium, nickel, zinc, cobalt, vanadium and copper [ 10 , 11 ]. Through food chain, those heavy metals potentially reach human posing health risk to the consumer.
It could be hypothesized that untreated water could be potential sources of health risk to the local community who heavily rely on those water sources for daily consumption. The risk could be even more pronounced among unprotected water including water from wells and springs. To this effect, this study was designed to evaluate the current safety status of different water sources being used for drinking in and around Serbo town, Jimma zone, southwest Ethiopia. The water sources included in this study were tap water, protected and unprotected wells, protected and unprotected springs. Although theoretically assumed to be safe, tap water samples were collected from point of disinfection, at household levels as well as points of public services to evaluate possible challenges on the route (such as leakage or mix with sewage line) and effect of poor handling at point of services. As majority of the local community rely on alternative water sources (springs and wells), the potential health risk because of heavy dependence on these water sources (protected and unprotected) were evaluated by including both unprotected wells and springs which were accessible to both human and animal use, and those water sources protected through fencing of the water environment to lower the external interferences, were included.
Study site and period
The study was conducted at Serbo town and the surrounding four kebeles (including Babo, Awaye sebu, Tikur balto and Tikur abulo) located in Kersa district, Jimma Zone, Southwest Ethiopia (Fig. 1 ). Serbo town is located about 332 km south of Addis Ababa, and 18 km from Jimma town, the Zonal capital. Geographically, the town is located between 7°35′–8°00′N latitudes, 36°46′–37°14′E longitude and altitude that ranges from 1740 to 2660 m above sea level. According to the 2006 census (CSA, 2006), the town has been inhabited with more than 11,855 people. The study was conducted from October, 2011 to May, 2012.
Map of the study sites, Jimma, Ethiopia, 2012
Socio-demograpphic data collection
Structured and pre-tested questionnaires were used to gather pertinent information on socio-demographic characteristics of the study population and their level of awareness about waterborne diseases. From among 2371 households in the study area [ 12 ] a total of 237 households were included in the study, representing about 10 % of the resident population. A systematic random sampling technique was used to address representative households during socio-demographic data collection.
Water sample collection
A total of 90 water samples were collected from five different water sources including tap water (n = 15), protected wells (n = 15), unprotected wells (n = 18), protected springs (n = 15) and unprotected springs (n = 27). Samples were aseptically collected from each sampling site in sterile glass bottles and transported to laboratory in ice box and analyzed within 6 h of sample collection. For the chlorinated water samples, about 2.5 ml sodium thiosulphate was added into each sampling bottle to stop the chlorination process during transportation.
Bacteriological analysis
Isolation and enumeration Ten ml of the water samples were separately transferred into 90 ml sterile peptone water. After thorough mixing and appropriate serial dilutions, 0.1 ml aliquot of each diluted sample was inoculated onto appropriate pre-sterilized and solidified growth medium in duplicates and spread plated on the surface of the solid agar media, incubated at appropriate temperature and time combination for the count of different microbial groups following standard procedure [ 13 ]. Accordingly, aerobic mesophilic microbes and aerobic spore formers were counted on plate count agar (PCA). MacConkey agar was used for the count of Enterobacteriaceae. For counts of coliforms and fecal coliforms, most probable number (MPN) method was employed using multiple fermentation tubes [ 14 ]. Further presumptive isolation of coliform bacteria was made on MacConkey broth. For water samples from unprotected spring, and open wells, 1, 0.1 and 0.01 ml samples were inoculated onto the first, second and third row of test tubes each containing 10 ml of single-strength MacConkey broth, respectively [ 15 ]. After incubation at 37 °C for 48 h, the tubes with acid and gas were considered positive for coliforms. From the distribution of these positive tubes, MPN of TTC was determined following standard probability table [ 16 ]. Furthermore, presence of Escherichia coli was confirmed by streaking loopful of broth culture onto Eosine Methylene Blue (EMB) agar and evaluating for the formation of metallic sheen color, a positive test for presence of E. coli [ 14 ].
Characterization of isolates About 10–15 colonies were randomly picked from countable plates of PCA and MacConkey agar and inoculated into 5 ml nutrient broth tubes followed by incubation at 30–35 °C for 24 h. Cultures were purified by repeated plating on nutrient agar and characterized to the genus level following standard microbiological methods. Gram reaction was determined using KOH test (test for lipopolysaccharide), the rapid method recommended by Gregerson [ 17 ]. Catalase test was performed by adding few drops of 3 % H 2 O 2 on an overnight grown culture plate for production of air bubbles. Cytochrome oxidase test was conducted as suggested earlier [ 18 ] using freshly prepared Kovac’s reagents for detection of a blue color on freshly activated colonies within 30 s to 2 min. The appearance of blue color within the set time was considered as a positive reaction.
Detection of Salmonella To test for the presence of Salmonella , 1 ml of each sample was aseptically inoculated into 10 ml of lactose broth (LB) and incubated at 37 °C for 24 h for recovery and proliferation of cells. After the pre-enrichment, 1 ml culture was transferred into 10 ml of secondary enrichment broth (selenite cystine broth) and incubated at 42 °C for 48 h. Loopful of culture from Rappaport-Vassiliadis broth was streaked onto Salmonella–Shigella agar, Xylose Lysine Deoxycholate agar and modified Brilliant Green agar followed by incubation at 37 °C for 18 h. Characteristic colonies were picked, further purified and tested biochemically. Suspected non-lactose fermenting bacterial colonies were further characterized having inoculated into the following biochemical tubes: Triple Sugar Iron (TSI) agar, Simmon’s Citrate agar, Sulfur Indole motility (SIM) medium, Lysine Iron agar, Urea agar, and fermentation tubes of glucose, sucrose and Mannitol. Finally, the proportions of Salmonella positive samples were determined based on the above biochemical results.
Physico-chemical analysis
Turbidity was measured using Wagtech International Turbidity Meter (Wag-WT3020, Halma PLC Company), whereas other physico-chemical parameters including pH, temperature, electrical conductivity, and dissolved oxygen were measured in situ using standard instruments (HQ 40d multi parameter meter, HQ 40d, HACH Company). Biological oxygen demand (BOD), total suspended substances (TSS), total dissolved substances (TDS), and phosphate and nitrate concentrations were measured in laboratory as suggested in APHA [ 19 ]. TSS, TDS, BOD and phosphate concentration were determined according to Standard Methods 2540 D, 2540 C, 5210 B and 4500-P D, respectively, whereas Nitrate concentration was determined by phenol disulphonic acid method [ 19 ].
Heavy metals (lead and zinc) determination
Water samples were analyzed for presence of heavy metals (lead and zinc) using Flame Atomic Absorption Spectrometer (FAAS) [ 19 ]. Accordingly, 100 ml of the different water samples were separately digested repeatedly in nitric acid and evaporated. After the content was rinsed with de-ionized water, the resulting digest was filtered to remove some insoluble particles. The filtrate was transferred into 100 ml volumetric flask and adjusted to 100 ml with de-ionized water. Corresponding blank samples were digested in the same manner. Finally, the concentration of lead and zinc in each sample was measured using Flame Atomic Absorption Spectrometer (FAAS).
Data analysis
Data were analyzed using SPSS statistical software (version 20). Results of physico-chemical analysis and mean microbial counts of the investigated water samples were compared with the set standards (WHO guide lines for drinking water quality) and interpreted as acceptable or unacceptable. The significances of differences within samples were determined based on calculated coefficient of variation (% CV). Mean separation between samples categories were computed using one-way ANOVA. The parameters were correlated against each other to determine their relationship using Pearson’s correlation. Variables were compared using Chi square test (χ 2 ). In all cases, significance was considered at 95 % confidence interval.
Socio-demographic characteristics of the study population
Of the total 237 respondents, the majority (32.1 %) have been using unprotected spring while equivalent proportion were relying on unprotected wells (18.6 %) and tap water (18.1 %) (Additional file 1 : Table S1). Very few of them (2.5 %) were practicing boiling of water before using for drink. Plastic pots are the most favored (86.5 %) material for water storage, making the heat treatment of facilities unlikely. About 43 % of the water sources were found at a distance of less than 20 m from latrine and 32.1 % of them were located in lower elevation with respect to the nearby toilet rooms. Waste management practices of the localities was found poor as more than 50 % of the respondents dispose waste materials on open field (Additional file 1 : Table S1). The Chi square test analysis revealed that, the type of water source had strong relationship with the quality of water (p < 005).
Microbial load of drinking water sources
The mean aerobic mesophilic count (AMC) (log CFU/ml) of tap water, protected wells, protected springs, unprotected wells and unprotected springs were 3.05, 3.53, 4.03, 4.39, and 5.25, respectively (Table 1 ). The highest mean Enterobacteriaceae count (4.38 ± 4.63 log CFU/ml), AMC (5.25 ± 5.84 log CFU/ml), aerobic spore formers (3.60 ± 3.49 log CFU/ml) and Fecal coliform (105.93 ± 94.92 log CFU/100 ml) were observed in unprotected springs. However, the lowest mean Enterobacteriaceae count (2.59 ± 2.65 log CFU/ml) and AMC (3.05 ± 3.12 log CFU/ml) were recorded from tap water. There were significant variations (CV > 10 %) in the count of the microbial groups within all samples and counts of both TTC and fecal coliform (FC), but the variation of TTC was not significant for unprotected spring water samples (Table 1 ).
Tap water sources had overall mean TTC and FC counts of 9.67 and 0.53 CFU/100 ml, respectively. Whereas, protected wells and protected springs had overall mean TTC counts of 33 and 30.6 CFU/100 ml, but FC counts of 6 and 3.4 CFU/100 ml, respectively (Table 1 ). Generally, analysis of tap water samples demonstrated that mean TTC bacterial count ranged from 2.00 ± 0.00 to 26.67 ± 19.40 CFU/100 ml, but FC ranged from 0 to 1.67 ± 0.58 CFU/100 ml. About 66.67 % of tap water samples were found to be negative for FC and E. coli were not detected in all the tap water samples. The entire samples from both unprotected wells and unprotected springs were positive for indicator organisms. Among the 15 protected well water samples analyzed, only 6 (40 %) had bacterial count below 10 CFU/100 ml and four (26.67 %) were negative for fecal coliforms. Sixty percent of protected springs were free from fecal coliforms and 46.67 % of these samples had TTC count less than 10 CFU/100 ml. Significant variations were observed for TTC and FC within water samples with % CV > 90 in both cases.
A total of 907 AMB were characterized to at least group/genus levels using different biochemical tests. Accordingly, the isolates were found dominated by Enterobacteriaceae (32 %), Bacillus (28.4 %) and Pseudomonas (17 %), followed by Micrococcus (6.9 %) and Staphylococcus (6.0 %). Unidentified Gram negative cocci (4.7 %) and Gram positive rods (5 %) were among the least encountered AMB in the water samples (Fig. 2 ). Furthermore, from a total of 90 samples examined, only 3 (3.33 %) water samples (one from unprotected well and two from unprotected springs) were found positive for Salmonella spp., but all samples were negative for Shigella (Additional file 2 : Table S2). Despite high counts of Enterobacteriaceae and coliforms in some of the water sample, the species of Salmonella and Shigella were found less prevalent.
Frequency distribution (%) of dominant aerobic mesophilic bacteria in drinking water samples, Serbo town and its surroundings, 2012
The recorded mean temperature of the water samples were 24.42 ± 1.15, 24.53 ± 1.23, 22.79 ± 1.03, 23.52 ± 1.93 and 23.37 ± 2.16 °C for tap water, protected wells, unprotected wells, protected spring and unprotected springs, respectively (Table 2 ). Of the total water samples (n = 90), the maximum temperature (25.80 °C) was recorded for tap water and the minimum (20.10 °C) for unprotected springs. There were no observable significant variations both within the samples (CV = 4.52–9.24 %) and among water samples collected from the five different sources (P = 0.34).
The mean pH of unprotected wells and springs were 6.48 (5.99–6.86) and 6.18 (5.64–6.75), respectively, whereas the protected wells and springs had mean pH of 6.8 (6.2–7.77) and 6.25 (5.79–6.62), respectively. Tap water samples had mean pH value around neutrality (pH = 7.85) ranging between 7.4 and 8.14. Statistically significant mean variations were observed among the water samples collected from five different sources (p < 0.05) although there was no significant differences within same sample source (CV < 10 %).
Mean electric conductivity (μS/cm) for tap water, protected wells, unprotected wells, protected spring and unprotected springs were 366.93 ± 5.24, 366.95 ± 262.65, 134.80 ± 126.41, 56.24 ± 19.98 and 46.42 ± 15.59, respectively (Table 2 ). There was a statistically significant difference (P < 0.05) among mean electric conductivities of different water samples and within samples (except for tap water).
The mean turbidity value of water samples was the highest (24.22 NTU) for unprotected wells and the least (1.87 NTU) for tap water. The high turbidity observed in some of the water sources did not agree with WHO standards (5 NTU). Variations were statistically significant within samples (CV > 10 %) and among means of different water samples (P = 0.03) (Table 2 ).
The mean valves of dissolved oxygen (DO) (mg/l) for tap water, protected wells, unprotected wells, protected spring and unprotected springs were 3.96 ± 1.00, 4.00 ± 0.94, 3.53 ± 0.83, 5.30 ± 0.36 and 5.90 ± 3.61, respectively (Table 2 ). There was no significant differences (P = 0.264) in DO among the assessed water samples. Similarly, mean phosphate concentration (mg/l) level recorded for tap water, protected wells, unprotected wells, protected spring and unprotected springs were 1.21 ± 0.38, 0.29 ± 0.12, 0.56 ± 0.42, 0.77 ± 0.26 and 0.76 ± 0.55, respectively (Table 2 ). Phosphate concentration did not show significant variations (p = 0.31) among water samples although highly variable within samples (CV > 10 %).
Mean nitrate concentration (mg/l) values of 1.92 ± 0.26, 42.39 ± 37.99, 8.48 ± 10.43, 5.60 ± 4.44 and 2.55 ± 1.30 were recorded, respectively, for tap water, protected wells, unprotected wells, protected spring and unprotected springs (Table 2 ). The maximum mean nitrate value of 95.80 ± 8.45 mg/l was recorded from protected well and the minimum from protected wells and protected spring with records below detection level of the instrument used (data not given). Variations were not statistically significant among means of different water samples (P = 0.09). Likewise, the mean TSS (mg/l) of tap water, protected wells, unprotected wells, protected spring and unprotected springs were 5.93 ± 2.25, 83.01 ± 112.76, 66.33 ± 25.90, 23.47 ± 10.08 and 101.08 ± 97.24, respectively (Table 2 ), the highest mean concentration being in unprotected spring and the least in tap water. The highest mean TSS concentration of 305.00 ± 14.14 (mg/l) was obtain from unprotected spring, whereas the lowest 2.67 ± 0.58 (mg/l) from tap water source. Statistically significant variations were not observed among mean values s of different water sampling sources (P = 0.25) but within all samples of the same source.
Unusually high TDS level (524.73 ± 51.25) was observed in tap water samples while relatively lowest level (137.19 ± 18.98) was encountered in unprotected well water. Variation in TDS within samples was not significant (% CV < 10). From the total sampling sites, 623.00 ± 10.54 mg/l was the highest total dissolved solids (TDS) recorded from one of the protected well while the lowest concentration (116.00 ± 12.00 mg/l) was recorded from unprotected spring (data not shown). Significant variations were noted among the five different water sample sources (P < 0.05) and variation within sample was not significant for tap water.
The observed BOD value (mg/l) was the highest in unprotected well (62.89 ± 11.93) followed by unprotected spring (35.33 ± 9.43), protected well (34.67 ± 7.15), protected spring (22.93 ± 1.98) and tap water (9.8 ± 1.21) (Table 2 ). The lowest mean BOD value was 8.33 ± 0.58 mg/l from private tap water sample, whereas the highest mean value (74.67 ± 2.52 mg/l) was recorded from unprotected well (detailed data not shown). There were statistically significant variations in BOD values among different water samples collected from the five sources (P = < 0.05).
In relation to the abundance and concentrations (mg/l) of the two heavy metals (lead and zinc) in the drinking water samples, relatively higher concentration was recorded in tap water and unprotected wells (0.03 ± 0.03 each) and almost similar concentration observed in protected wells (0.02 ± 0.01), protected spring (0.02 ± 0.01) and unprotected springs (0.02 ± 0.03) (Table 3 ). Maximum lead metal concentration of about 0.09 mg/l was observed in tap water. There was no statistically significant variations among the mean concentrations of the different water sampling sources (P = 0.644). Relatively higher zinc concentrations of about 0.41 and 0.27 mg/l were recorded from tap and protected well water samples, respectively, minimum values below detection level. Variations were statistically significant among means of different water sampling sources (P = 0.003).
Association between physico-chemical parameters and microbial loads
The correlation analysis indicated that AMC was positively correlated with turbidity, DO and total suspended solids (TSS) (r = 0.721, r = 0.626, and r = 0.718, respectively) (Additional file 3 : Table S3); and negatively correlated with pH, EC and TDS (p < 0.05) (r = −0.829, r = −0.845 and r = −0. 813, respectively) (Additional file 3 : Table S3). Temperature had negative correlation with turbidity and BOD (r = −0.987, p < 0.05 and r = −0.985, p < 0.05), respectively. The values of pH positively correlated with TDS and EC; p < 0.05, but negatively correlated with TSS. Furthermore, electric conductivity and turbidity values were positively correlated with total dissolved solids (TDS) (r = 0.831), and BOD (r = 0.860) (p < 0.05, in both cases).
The mean AMC of tap water (3.05 log CFU/ml) and protected well water (3.53 log CFU/ml) samples documented in this study, with about 70 % of the water samples having aerobic AMCs greater than 3 log CFU/ml, was in agreement with the earlier report from Nigeria [ 20 ]. Although the observed contamination level with regards to aerobic mesophilic bacteria was not significantly high, their very detection by itself is an indication of high vulnerability of the water sources to microbial contamination, including potential pathogens.
The predominant bacterial groups identified in the water samples were members of the family Enterobacteriaceae, Pseudomonas spp. and Bacillus spp. Similarly, other scholars [ 21 ] reported that the most prevalent bacterial species in well water sources from Rural Areas of Zimbabwe were members Gram negative, non-spore forming bacilli belonging the family Enterobacteriaceae. In agreement with the report made by earlier [ 22 ], Bacillus species were the second dominant bacterial groups in the current study. Few of the Bacillus species, including strains of Bacillus cereus , are pathogenic to humans and animals being responsible for food poisoning [ 23 ]. The incidence of Pseudomonas spp. as the third dominant bacteria in the current study was in agreement with report made elsewhere [ 24 , 25 ].
With 100 and 80 % detection rates of TTC and thermo-tolerant coliforms, respectively, about 76.67 % of the samples had TTC bacterial count beyond the Canadian acceptable level for drinking water (10 CFU/100 ml) [ 26 ] with all water samples having microbial counts above WHO recommendation (0 CFU/100 ml) [ 27 ]. According to WHO guidelines, E. coli or thermo-tolerant coliform bacteria should not be detectable in any water intended for drinking [ 15 , 28 ]. Results of this study were in agreement with the reported detection of coliforms from 75 % of unprotected well and spring samples from North-Gondar, Ethiopia [ 29 ] and the 90 % detection of the same microbial groups from protected spring samples of Uganda [ 30 ]. Similarly, 87.5 % of the water samples collected from other six protected wells and eighteen unprotected wells of Serbo town [ 31 ] revealed TTC count above the permissible limits for drinking water.
About 80 % of the water samples were positive for fecal coliforms (FC) and the highest observed mean coliform count was 266.67 CFU/100 ml. In contrary to our report, significantly high counts (1100 CFU/100 ml) of FC bacteria were reported from water samples collected from rural areas of Iran [ 32 ] and unprotected springs of central highlands of Ethiopia (741.7 CFU/100 ml) [ 33 ].
The prevalence of Salmonella was very low in the current study, with only two positive samples from unprotected springs and one from unprotected well water samples. In a related study, Shittu et al. [ 34 ] reported absence of Salmonella and Shigella in all well water samples examined in Nigeria although stream samples were positive. However, as long as the counts of fecal coliforms are high in most of the water samples examined for microbial load and safety, the absence of any Salmonella and Shigella in many of the samples could not qualify the water sources’ safety.
Temperature is one of the physico-chemical parameters used to evaluate quality of potable water. It affects many phenomena including the rate of chemical reactions in the water body, reduction in solubility of gases and amplifications of tastes and colours of water [ 35 ]. The highest (25.73 °C) and lowest (20.67 °C) temperature recorded from tap water and unprotected spring, respectively, were related to the 28 °C reported from different water source of Nigeria [ 13 ] but higher than the study conducted in Bahir Dar town (15–20 °C) [ 36 ]. Almost all the recorded water temperatures were above the WHO recommended level (<15 °C) and temperature optima of some aerobic mesophilic bacteria and fungi. The variations in temperature of the samples may be attributed to sampling locations as some of the water sources were collected from underground (including well water) while others were found partly on the surface exposed to direct sunlight. Richness in organic matter, hence microbial activities, could also contribute besides the geographic location of the study area (tropical zone). It is desirable to have the temperature of drinking water not exceeding 15 °C as the palatability of water is enhanced by its coolness [ 10 ].
With the overall mean pH value of 6.72 (ranged between 5.72 and 8.14), only about half (52.3 %) of the pH of water samples fall within WHO standard (6.5–8.5) [ 37 ]. Except tap water, the majority of other drinking water sources were slightly acidic (below pH of 7), whereas tap water sources had pH value greater than 7 (slightly alkaline). The pH values in most of the samples were found within the recommended standards of European Commission and WHO (ranges from 6.5 to 8.5) for potable waters. According to Byamukama et al. [ 38 ], the low pH values observed in most wells and springs could be associated with carbon dioxide saturation in the groundwater. In fact, the physico-chemical nature of the soil of sampling sites could partly contribute to the final pH of the samples. In related development, the pH of water samples collected and analyzed from Katanga, North of Kampala city, were found to be acidic [ 39 ] contributing to the final low pH of water samples analyzed from the same sites.
In this study, about 60 % of the samples had turbidity level above 5 NTU (beyond the acceptable standard) although all tap water and 80 % of the protected wells had values below 5 NTU. High turbidity is often associated with higher levels of suspended organic matter and microorganisms including bacteria and other parasites. Usually, the acceptable turbidity level is 5 NTU although it could vary with local circumstances [ 15 ]. The consumption of highly turbid water may constitute a health risk as excessive turbidity can protect pathogenic microorganisms from the effect of disinfectants, and also stimulate the growth of bacteria [ 40 ].
The highest conductivity recorded from tap and protected water sources could be due to the corrosion of metals that led to the accumulation of heavy metals. Even though conductivity values in the water samples ranged from 30.77 to 727.67 μS/cm, more than 93.33 % of the samples had electric conductivity (EC) value below 399 μS/cm, with the lowest conductivity values recorded from protected and unprotected springs. Actually all mean EC values were within WHO maximum recommended limit (1500 mg/l). Related results were reported from well water samples in Nigeria [ 13 ], where the EC levels ranged from 22 to 315 μS/cm. However, EC values greater than our finding was found in ground water sources of Turkey, where the lowest and highest conductivities were 463 and 1460 μS/cm, respectively [ 41 ].
The lowest total dissolved solid (TDS) (116 mg/l) recorded from unprotected spring and the highest value (623 mg/l) recorded from protected well were below the maximum allowable limit (1000 mg/l) recommended by WHO [ 37 ]. Total dissolved solid (TDS) are measures of the general nature of water quality [ 35 ]. The TDS include carbonate, bicarbonate, chloride, sulphate, phosphate, nitrate, calcium, magnesium, sodium, organic ions and other ions. TDS affect the taste of drinking water if present at levels above the WHO recommended level. Accordingly, the TDS values recorded in this study could be considered tolerable. On the other hands, the overall mean total soluble substances (TSS) recorded in the study ranged between 5.93 and 101.08 mg/l with the lowest and highest measurements being observed in tap water (2.67 mg/l) and the lowest in unprotected spring water samples (403.33 mg/l). The variability or range in the recoded TSS data was significantly high as compared to the earlier report (10–32.4 mg/l) made from Southern Rajasthan, India [ 42 ] from hand pump water sources and the 210.0 ± 127.7 mg/l from untreated tap water of Jimma town, Ethiopia [ 43 ]. Although there is no set guideline for the maximum permissible limit of TSS in drinking water, the TSS value recommended for fisheries and aquatic life in Ethiopia (25 mg/l) could be used as reference for this purpose [ 43 ]. Accordingly, the concentrations of TSS obtained from all unprotected wells, most of unprotected spring (85.2 %) and protected spring (80.0 %) water sources were above even the tolerable limits for maintenance of aquatic life and fisheries. The higher concentration of TSS in the water samples could be due to poor sanitation practice with possibility of contamination of the water sources with municipal wastes and plant debris.
The different water samples revealed mean dissolved oxygen (DO) values ranging between 3.53 and 5.9 mg/l although there were significant variations both within and among samples. About 93.3 % of the samples had mean DO ranging between 1.65 and 5.87 mg/l. As compared to the WHO acceptable standards for dissolved oxygen in fresh water (10–12 mg/l), the observed results were partly acceptable although significant number of individual records fall out of the range. Related observation was reported by Tenagne [ 44 ] from drinking water in Bahir Dar, Ethiopia, in which the mean DO concentration of the water samples were between 0.45 and 5.27 mg/l. Purushottam et al. [ 45 ], also reported DO values ranging from 1.2 to 4.6 mg/l from different lake water samples. Dissolved oxygen is an important water quality parameter and has special significance for aquatic organisms in natural waters [ 46 ]. Temperature of water influences the amount of dissolved oxygen with only lesser oxygen dissolved in warm water than cold water [ 44 ]. Therefore, high temperature of the water sources could be one of the factors for low DO values recorded in the current study.
The mean BOD after 5 days (BOD5) was found within the range of 8–77 mg/l. Although no guideline set for the maximum tolerable limit of BOD in drinking water, for fisheries and aquatic life, European Union and Ethiopia recommend 3–6 mg/l and less than 5 mg/l, respectively [ 43 ]. This suggests that drinking water sources were highly polluted by organic matter. Detection of phosphate in water sources (0.09–1.91 mg/l) usually indicates contamination of the water sources by run-off from agricultural farms using inorganic fertilizers [ 47 ]. Related result (0.27–1.41 mg/l) was also recorded from underground water samples from Ondo State, in the western part of Nigeria [ 48 ]. All the water samples assessed in this study were observed to have concentration of phosphate ions below the maximum permissible level (5 mg/l) set by European commission and WHO. The high phosphate concentrations in some of the water samples could be due to the presence of agricultural activities near the water sources, as most of the people in the study area were practicing farming. These observations indicate that the water from these sources could not be stored for long in open containers, as the presence of phosphate encourages the growth of algae and consequently cause adverse changes at least in colour and taste of the water sources [ 49 ].
The mean nitrate concentration in the samples varied from below detection limit to 102.11 mg/l. Accordingly, most of the water samples fall within the permissible limit (50 mg/l) set by the European commission [ 50 ] for drinkable water except for two of the protected wells with concentration above 50 mg/l. Study done on the quality of packaged water analyzed in Nigeria reported concentrations of 0.0–40.0 mg/l nitrate ions [ 51 ], while analysis on well water samples from the same country revealed nitrate concentration of about 50.6 mg/l [ 52 ]. Higher nitrate levels (>50 mg/l) were also previously reported [ 53 ]. These reports have conformity with the present findings. Similar observations have been reported from groundwater sources in Iganga, eastern Uganda, with nitrate levels ranged between 21 and 145 mg/l in protected springs. In another study done in Tanzania, nitrate levels ranging between 0 and 90.28 mg/l was recorded from different drinking water sources [ 54 ]. However, lower nitrate concentration was also reported from northeastern region of Buenos Aries Province, Argentina [ 55 ]. This variation may be explained by the differences in hydro-geological regimes and likely contaminant entry point. While nitrogen is a vital nutrient for plant growth, high concentrations are harmful to people and nature. The agricultural use of nitrates in organic and chemical fertilizers has been a major source of water pollution in Europe [ 50 ]. Generally, farming remains responsible for over 50 % of the total nitrogen discharge into surface waters. Thus, excessive nitrate concentrations in water are mainly related to pollution (with agriculture as the main source). Lifetime exposure to nitrite and nitrate at levels above the maximum acceptable concentration could cause such problems as diuresis, increased starch deposits and hemorrhaging of the spleen [ 53 ].
Because of their high toxicity to humans and aquatic life, some heavy metals have been used as indices of pollution [ 56 ]. The concentrations of metals ions, including lead, in the current water samples ranged from below detection level to 0.09 mg/l, with about 64.4 % of the water samples having lead concentration above the WHO maximum permissible level set for drinking waters [ 37 ]. Gebrekidan and Samuel [ 57 ] also reported Pb concentrations ranging from below detection level to 0.7 mg/l in ground drinking water in urban areas of Tigray, Ethiopia. Heavy metals have a marked effect on the aquatic flora and fauna which, through biomagnifications, enters the food chain and ultimately affect the human beings as well [ 58 ]. The heavy metals, in drinking water, are linked most often to human poisoning at larger dose are lead, iron, cadmium copper, zinc, chromium etc. The known fatal effects of heavy metal toxicity in drinking water include damaged or reduced mental and central nervous function and lower energy level.
Similar to the case of lead, zinc concentration ranging between below detection level to maximum of 0.27 mg/l were recorded from the different water samples. As compared to the maximum permissible level of the same in surface water (0.01 mg/l) and ground water (0.05 mg/l) [ 37 ], the observed zinc concentrations were significantly high with the concentration being much higher due to dissolution of zinc from the used pipes. However, the overall result recorded in this study showed that all the samples had Zn concentration within Ethiopian maximum permissible level (5 mg/l).
Bacteriological quality of most water samples analyzed in the current study did not meet the standards set for drinking water. From the quality and sanitary risk evaluation points of view, the studied water sources could be classified as grossly polluted and only very few of them had reasonable quality. Most of the physico-chemical data indicated marginally tolerable quality with respect to pH and TSS but poor quality in relation to turbidity, temperature, conductivity, BOD and nitrate concentration with values much in excess of the permissible standards. Excessive nitrate concentrations recoded from some water samples are mainly related to pollution (with agriculture as the main source). Lifetime exposure to nitrite and nitrate at levels above the maximum acceptable concentration could cause many health problems including increased starch deposits and hemorrhaging of the spleen. Lead concentrations recorded in most of water sources were above the permissible level stated in many guide lines. Thus, with the current high dependence on alternative water sources other than tap water, it calls for awareness development on hygienic handling of wells and springs besides designing protections and regular purification strategies by the concerned bodies.
WHO. The World Health Report. Switzerland: World Health Organization; 2002.
Google Scholar
Boe-Hansen R. Microbial growth in drinking water distribution systems. Ph.D. thesis, Lyngby, Denmark; 2001.
Ashbolt NJ. Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology. 2004;198:229–38.
Article CAS PubMed Google Scholar
Zamxaka M, Pironchev AG, Muyima NYO. Microbiological and physico-chemical assessment of the quality of domestic water sources in selected rural communities of the Eastern Cape Province, South Africa. Water SA. 2004;30:33–340.
WWAP. United Nations Educational, Scientific, and Cultural Organization World Water Assessment Program. National Water Development Report for Ethiopia, Addis Ababa, Ethiopia; 2004.
Desta K. Physico-chemical and bacteriological quality assessment of drinking water from source to households distribution Point in Debrezeit town. M.Sc. thesis, Addis Ababa University, Ethiopia; 2009.
Adepoju-Bello AA, Alabi OM. Heavy metals: a review. Niger J Pharm. 2005;37:41–5.
Marcovecchio JE, Botte SE, Freije RH. Heavy metals, major metals, trace elements. In: Nollet LM, editor. Handbook of water analysis. 2nd ed. London: CRC Press; 2007. p. 275–311.
Egborge ABM. Water pollution in Nigeria: biodiversity and chemistry of Warri River. Ben-Miller Books Nigeria Ltd; 1994. p. 27–59.
WHO. Technical Support Document for Ontario Drinking Water Standards, Objectives and Guidelines. Ministry of the Environment. 2003. http://www.ene.gov.on.ca/stdprodconsume/groups/lr/@ene/@resources/documents/resource/std01_079707.pdf .
Boustani F, Hojati M, Ebrahimzadeh S. Assessment of nickel concentration in surface and ground water of the Kowsar Dam Basin. World Acad Sci Eng Technol. 2012;63:37.
CSA. Ethiopia demographic and health survey. Addis Ababa: CSA; 2006.
Oparaocha ET, Iroegbu OC, Obi RK. Assessment of quality of drinking water sources in the Federal University of Technology, Owerri, Imo State, Nigeria. J Appl Biosci. 2010;32:1964–76.
Adetunde LA, Glover RLK. Evaluation of bacteriological quality of drinking water used by selected secondary schools in Navrongo in Kassena-Nankana district of upper east region of Ghana. Prime J Microbiol Res. 2011;1:47–51.
WHO. Guidelines for drinking-water quality, surveillance and control of community supplies. Switzerland: World Health Organization; 1997.
Al-Tomi AS. Manual of bacteriological examination of drinking water. Department of Microbiology, Biotechnology Research Center, Tripoli, Libya; 2007.
Gregersen T. Rapid method for distinction of Gram-negative from Gram-positive bacteria. Eur J Appl Microbiol. 1978;15:123–7.
Article Google Scholar
Kovacs N. Identification of Pseudomonas pyocyanea by the oxidase reaction. Nature. 1956;178:703.
APHA. Standard methods for the examination of water and wastewater. 19th ed. Washington DC: American Public Health Association; 1995.
Shamsuddeen U, Bukar A, Usman AD, Kabir MH, Abdulmalik SA. Bacteriological quality of water used for ice making in some parts of Kano metropolis, Nigeria. Bayero J Pure Appl Sci. 2010;3:199–201.
Zvidzai C, Mukurtiwa T, Mundembe R, Sithole-Niang L. Microbial community analysis of drinking water sources from rural areas of Zimbabwe. Afr J Microbiol Res. 2007;1:100–3.
Abed KF, Alwakeel SS. Mineral and microbial contents of bottled and tap water in Riyadh, Saudi Arabia. Middle East J Sci Res. 2007;2:151–6.
Jay JM, Loessner MJ, Golden DA. Modern food microbiology. 7th ed. New York: Springer; 2005. p. 619.
Geldreich EE. Characterizing microbial quality of water supply. In: Microbial quality of water supply in distribution systems. Boca Raton: CRC Press Inc; 1996. p. 236.
Chaidez C, Soto M, Martinez C, Keswick B. Drinking water microbiological survey of the Northwestern State of Sinaloa, Mexico. J Water Health. 2008;6(1):125–9.
Anon. Assessment studies of water and wastewater systems associated water management practices at selected first nation communities. Final report; 2002.
Chan CL, Zalifah MK, Norrakiah AS. Microbiological and physico-chemical quality of drinking water. Malays J Anal Sci. 2007;11:414–20.
Cheesbrough M. District laboratory practice in tropical countries. 2nd ed. New York: Cambridge University Press; 2006. p. 143–4.
Book Google Scholar
Mengesha A, Mamo W, Baye G. A survey of bacteriological quality of drinking water in North Gondar. Ethiop J Health Dev. 2004;18:112–5.
Haruna R, Ejobi F, Kabagambe E. The quality of water from protected springs in Katwe and Kisenyi parishes, Kampala city, Uganda. Afr Health Sci. 2005;5:14–20.
PubMed Central PubMed Google Scholar
Solomon A, Ahmed Z, Biruktawit K, Amare D, Solomon A, Endalew Z. Bacteriological analysis of drinking water sources. Afr J Microbiol Res. 2011;5:2638–41.
Ghaderpoori M, Hadi Dehghani M, Fazlzadeh M, Zarei A. Survey of microbial quality of drinking water in rural areas of Saqqez, Iran. Am Eurasian J Agric Environ Sci. 2009;5:627–32.
CAS Google Scholar
Birhanu M. Assessment of the contamination level of water at collection points and determination of the major sources of contaminants in the Central Highlands of Ethiopia (Yubdo-Legebatu PA). M.Sc. thesis, Addis Ababa University, Addis Ababa, Ethiopia; 2008.
Shittu OB, Olaitan JO, Amusa TS. Physico-chemical and bacteriological analyses of water used for drinking and swimming purposes in Abeokuta, Nigeria. Afr J Biomed Res. 2008;11:285–90.
Olajire AA, Imeokparia FE. Water quality assessment of Osun River: studies on inorganic nutrients. Environ Monit Assess. 2001;69:17–22.
Milkiyas T, Mulugeta K, Bayeh A. Bacteriological and physic-chemical quality of drinking water and hygiene-sanitation practices of the consumers in Bahir Dar city, Ethiopia. Ethiop J Health Sci. 2011;22:19–26.
WHO. Guidelines for drinking-water quality, health criteria and other supporting information. Switzerland: World Health Organization; 1996.
Byamukama D, Kansime F, Mach R, Farnleitner A. Determination of Escherichia coli contamination with Chromocult coliform agar showed a high level of discrimination efficiency for differing pollution levels in tropical waters of Kampala, Uganda. Appl Environ Microbiol. 1999;66:864–8.
Nshekanabo N, Wozei E. Spring water quality improvement in slums. Sanitation and water for all. In: Proceedings of 24th WEDC conference, Islamabad, Pakistan; 1997.
Zvikomborero H. An assessment of the water quality of drinking water in rural districts in Zimbabwe: the case of Gokwe South, Nkayi, Lupane and Mweezi districts. Phys Chem Earth. 2005;30:859–66.
Aydin A. The microbiological and physico-chemical quality of groundwater in West Thrace, Turkey. Pol J Environ Stud. 2006;16:377–83.
Sharma BK, Sharma LL, Durve VS. Assessment of hand pump waters in three tribal dominated districts of Southern Rajasthan, India. J Environ Sci Eng. 2008;50:133–6.
CAS PubMed Google Scholar
Israel D. Assessment of drinking water quality and pollution profiles along awetu stream Jimma. M.Sc. thesis, Addis Ababa University, Ethiopia; 2007.
Tenagne AW. The impact of urban storm water runoff and domestic waste effluent on water quality of Lake Tana and local groundwater near the city of Bahir Dar, Ethiopia. M.Sc. thesis, Cornell University, New York, USA; 2009.
Purushottam JP, Yenkie MKN, Battalwar DG, Nilesh VG, Dewanand BD. Study and interpretation of physico-chemical characteristic of lake water quality in Nagpur city, India. Rasayan J Chem. 2010;3:800–10.
Willock RJ, Stevenson CD, Robert CA. An inter-laboratory study of dissolve oxygen in water. Water Resh. 1981;15:321–5.
Taha GM, Younis M. Chemical and bacteriological evaluation of drinking water: a study case in Wadi El Saaida Hamlets in Aswan Governorate, Upper Egypt. J Int Appl Sci 2009;4:309–77.
Ololade IA, Adewunmi A, Ologundudu A, Adeleye A. Effects of household wastes on surface and underground waters. Int J Phys Sci. 2009;4:022–9.
Agunwamba JC. Water engineering systems. 2nd ed. Enugu: Immaculate Publications Limited; 2000. p. 33–139.
EC (European Commission). The EU Nitrate Directives. 2010. http://ec.europa . eu/environment/pubs/pdf/factsheets/nitrates.pdf.
Ajayi AA, Sridhar MKC, Adekunle Lola V, Oluwande PA. Quality of packaged waters sold in Ibadan, Nigeria. Afr J Biomed Res. 2008;11:251–8.
Adejuwon JO, Mbuk CJ. Biological and physiochemical properties of shallow wells in Ikorodu town, Lagos, Nigeria. J Geol Min Res. 2011;3:161–8.
Reimann C, Bjorvatn K, Frengstad B, Melaku Z, Teklehaimanot R, Siewers U. Drinking water quality in the Ethiopian section of the east African rift valley I—data and health aspects. Sci Total Environ. 2003;311:65–80.
Napacho ZA, Manyele SV. Quality assessment of drinking water in Temeke District (part II): characterization of chemical parameters. Afr J Environ Sci Technol. 2010;4:775–89.
Galindo G, Sainato C, Dapena C, Fernandez-Turiel JL, Gimeno D, Pomposiello MC, Panarello HO. Surface and groundwater quality in the northeastern region of Buenos Aries Province, Argentina. J S Am Earth Sci. 2007;23:136–45.
Omoigberale MO, Ogbeibu AE. Assessing the environmental impacts of oil exploration and production on the Osse river, Southern Nigeria, I. Heavy metals. Afr J Environ Pollut Health. 2005;4:27–32.
Gebrekidan M, Samuel Z. Concentration of heavy metals in drinking water from urban areas of the Tigray Region, Northern Ethiopia. MEJS. 2011;3:105–21.
Mohod CV, Dhote J. Review of heavy metals in drinking water and their effect on human health. Int J Innov Res Sci Eng Technol. 2013;2:2292–6.
Download references
Authors’ contributions
MY designed the study, involved in data collection, experimentation, data analysis and write up. KB supervised the study, involved in data analysis, and prepared the manuscript for publication. TK co-supervised the study, involved in project designing, data analysis and write-up of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to thank the study participants for provision of valuable information during the study; Department of Environmental Health Sciences and Technology, Jimma University, particularly Drs. Argaw Ambalu and Seid Tiku, for facilitation of access to laboratory facilities and the valuable comments during analysis of physico-chemical parameters; Jimma University sponsored the study.
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests.
Author information
Authors and affiliations.
Department of Biology, College of Natural Sciences, Jimma University, P. O. Box 378, Jimma, Ethiopia
Mohammed Yasin, Tsige Ketema & Ketema Bacha
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Ketema Bacha .
Additional files
13104_2015_1376_moesm1_esm.doc.
Additional file 1: Table S1 . The status and care being given to drinking water sources (n = 237) in Serbo town and its surroundings, 2012.
13104_2015_1376_MOESM2_ESM.doc
Additional file 2: Table S2 . The prevalence of Salmonella and Shigella in drinking water samples, Serbo town and its surroundings, 2012 (n = 3 for each sample sites).
13104_2015_1376_MOESM3_ESM.doc
Additional file 3: Table S3 . Correlations among measured parameters of water samples from five different water sources, Serbo town and its surroundings, 2012.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Yasin, M., Ketema, T. & Bacha, K. Physico-chemical and bacteriological quality of drinking water of different sources, Jimma zone, Southwest Ethiopia. BMC Res Notes 8 , 541 (2015). https://doi.org/10.1186/s13104-015-1376-5
Download citation
Received : 09 November 2014
Accepted : 24 August 2015
Published : 05 October 2015
DOI : https://doi.org/10.1186/s13104-015-1376-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Heavy metals
- Physico-chemical parameters
BMC Research Notes
ISSN: 1756-0500
- Submission enquiries: [email protected]
- General enquiries: [email protected]
PERSPECTIVE article
Microbial interaction as a determinant of the quality of supply drinking water: a conceptual analysis.

- Department of Microbiology, Jagannath University, Dhaka, Bangladesh
This conceptual analysis elucidates the microbial interaction inside municipal distribution pipes, subsequent deterioration in the quality of the supply water, and its impacts on public health. Literature review involved a total of 21 original reports on microbiological events inside the water distribution system were studied, summarizing the current knowledge about the build-up of microbes in treated municipal water at various points of the distribution system. Next, original reports from the microbiological analysis of supply water from Bangladesh were collected to enlist the types of bacteria found growing actively. A schematic diagram of microbial interaction among the genera was constructed with respect to the physical, chemical, and microbiological quality of the supply water. Finally latest guidelines and expert opinions from public health authorities around the world are reviewed to keep up with using cutting-edge molecular technology to ensure safe and good quality drinking water for municipal supply.
Introduction
Industrial revolution, urbanization, rapid increase in urban population, and increasing demand for expansion of public health infrastructures, increase in the demand for supply of drinking water from the municipality has led to complete change of the quality of lives in urban and sub-urban areas ( 1 ). Sustainable supply of safe drinking water is also a target to be achieved in the sustainable development goals 2030 ( 2 ). Therefore, maintaining the quality of the supply water is just as important as developing the infra-structures of water supply and conserving the sources of natural waters. The municipal water supply system acquires the surface water or aquifer water, treats the surface water physically (UV radiation or filtration) or chemically (chlorination), if necessary, and then distributes them through a network of pipes and overhead tanks to the points-of-use (homes, industries, public places, health care facilities, etc.). The quality and safety of the water at the receiving ends depend on the quality of the source from which it is acquired, the nature of treatment given in the municipal water treatment plant, the amount of residual disinfectant remaining in the water and the environments in the distribution network (pipes and overhead/underground reservoirs) ( 3 ). A good number of intrinsic and extrinsic factors determine the microbiological safety of the municipal (mains) water. Intrinsic factors include the length and duration of the treatment given to the source water, material, and length of the distribution pipes, total carbon, iron, lead, phosphate, and sulfate contents of the water, physical parameters of the water (pH, alkalinity, turbidity, hardness, conductivity), biological oxygen demand (BOD), chemical oxygen demand (COD), dissolved oxygen (DO), loose deposit accumulation inside the pipes, and the overhead tanks and microbes remaining in the supply water ( 4 ). All these factors might contribute to the quality deterioration potential (QDP) of the supply water as well ( 5 ). External factors also contribute to QDP. The deterioration of the quality of potable waters from distribution network emerged a decade ago when foul-odor or reddish color or incidences of waterborne diseases became a pressing issue ( 6 ). Subsequent investigation revealed that organisms from the natural water source, that survive the disinfection process, thrive inside the water distribution system, and interact among themselves as well as with the surface of the distribution pipes to form complex biofilms. This biofilm is able to deteriorate the safety and quality of the supplied water in more than one ways ( 7 ). Developed countries with full-scale mains distribution system employ task forces to study and control the in-process change of supply water throughout its passage in the distribution network.
Bangladesh, a nation of 170 million people, has 204 municipalities that supply treated or untreated surface water and groundwater in urban and sub-urban areas [Dhaka Water and Sewage Authority (DWASA) Annual Report 2015–16] 1 The capital Dhaka contains the oldest and the largest pipe network for water supply (DWASA Annual Report 2012–13) 2 Seasonal epidemics of waterborne diseases are common in Dhaka city ( 8 ). Few reports are found referring to the waterborne outbreaks at the beginning of monsoon and the foul quality of water, but the mechanism of water quality deterioration inside the supply network in Bangladesh is yet to find. This conceptual analysis summarizes information from the developed countries and predicts the possible events inside a Bangladesh supply network that poses health hazards to the consumers. The high incidences of morbidity and mortality from waterborne diseases call for re-evaluation of the surveillance, monitoring, and in-process control of the municipal water supply system. According to the annual report of Dhaka Water Supply and Sewage Authority (DWASA 2015–16), the organization supplied 2,450 million liters of water daily and in 2016 from four water treatment plants. There are 3,500 km of water lines connected to 361,938 household supplied from 38 overhead tanks, which also are points of biofouling. In addition, 1,643 hydrants moisturize the streets and highways. The Microbiology and Chemical Division of DWASA measures 50 parameters of the supply water to ensure safety and quality, but the emerging risks in mains water is not assayed (DWASA Annual Report 2012–13). DWASA follows the previous guidelines from WHO ( 4 ), which does not include the emerging biological and chemical risk factors known today. Despite the best efforts, risks are mounting from drinking water which common people consider safe to drink. This conceptual analysis brings to light the probable risk factors present in the water supplied by DWASA, so that modern techniques are introduced for water safety. The WHO puts emphasis on chemical residues and microbial interaction in drinking water ( 9 ) Risks associated with biofilm formation are enrichment of pathogens in the water, production of toxins, deterioration of the pipe material, release of antibiotic resistance genes, and supporting high-risk parasites such as Cryptosporidium and Naegleria fowlerii feeding off the biofilm ( 10 ).
This conceptual analysis attempts to predict the microbial interactions inside the water distribution pipelines, especially development of a biofilm consortium inside the pipes in Bangladesh so that an emerging risk to public health can be dealt with.
Theoretical Framework for the Conceptual Analysis
Every natural environment has its own microbial community, that plays characteristic function depending on the interaction between each species in the community. The bacteria present in tap water are likely to represent the species present in the biofilms inside the distribution system. The original reports on bacterial isolates discovered from tap water around the world (Table 1 ) and in Bangladesh were enlisted (Table 2 ). The interaction between each pair of species in biofilm were studied from original articles on dual-species biofilm formation experiments. A theoretical diagram was constructed to hypothesize the probable interaction of the reported bacterial species in the biofilm consortium (Figure 1 ). Interactions between the bacteria and other common members of the biofilm in water distribution system in other countries were studied (Table 1 ) and most common organisms associated with any given member of the hypothetical biofilm was included in Figure 1 because protozoa, viruses, and worms constitute matured biofilms inside the water supply pipes and pose considerable threat to consumers. The impact of the hypothetical biofilm on corrosion of the water distribution pipes and deterioration of water quality was studied from reports on water quality maintenance from around the world.
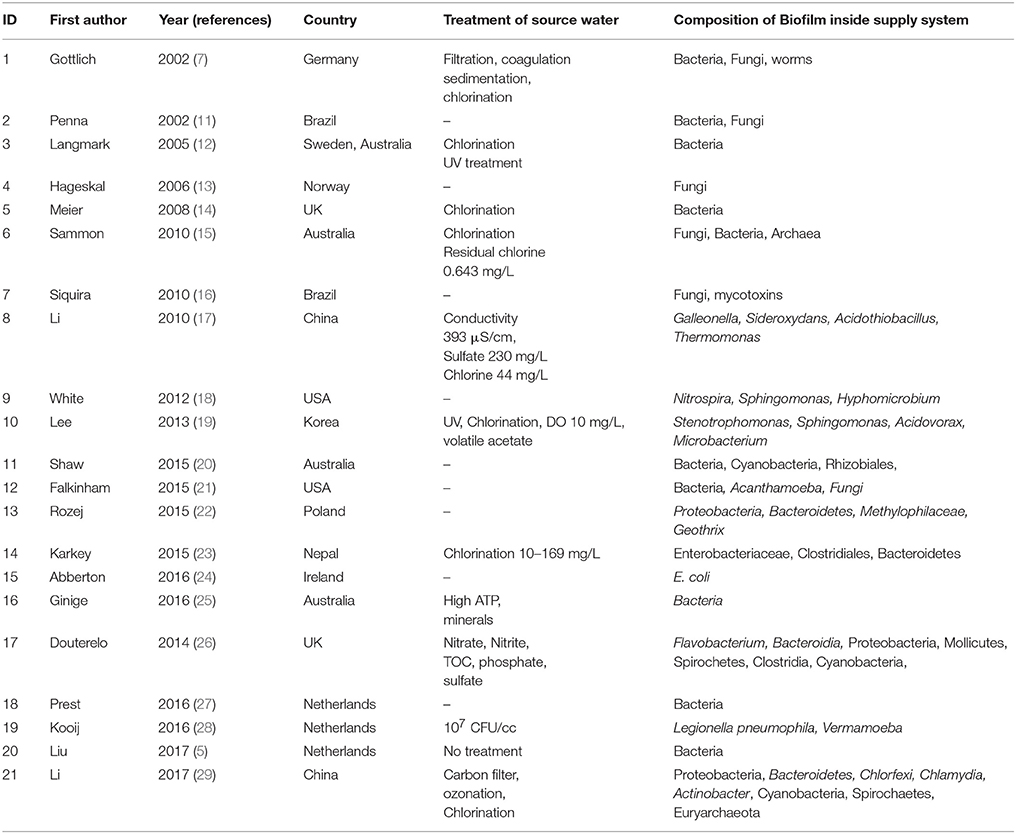
Table 1 . Original reports on biofilms inside municipal water distribution pipes.
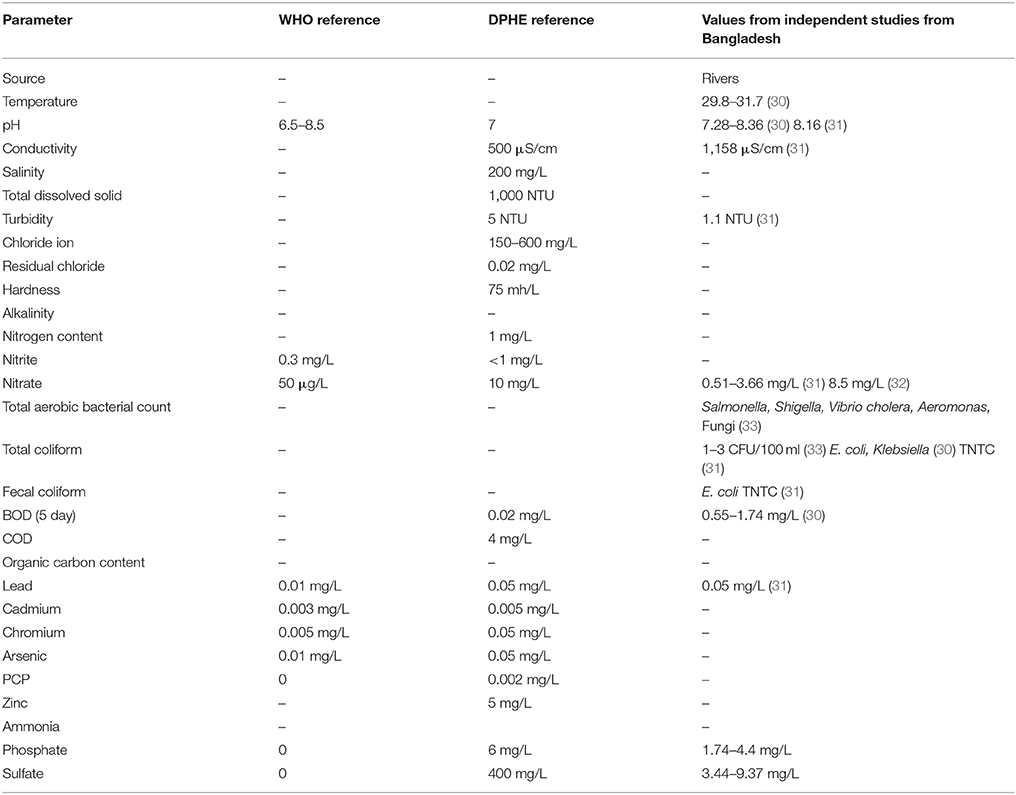
Table 2 . Water parameters from Municipal Supply Water in Bangladesh.
Literature Review and Inclusion-Exclusion Criteria
Literature review was done in three stages. First, Google Scholar, Pubmed, and the Cochrane Library were searched with keywords biofilm, water distribution system, municipality water pipes which returned 100 of results. Original reports that mentioned primary physico-chemical parameters of water and identified microbial species from biofilms developed inside municipal water supply pipes were included in this analytical report. Reports that did not mention the physico-chemical parameters or that did not identify biofilm members upto genus level were omitted. Gray literature was also omitted from literature review because information emerging from experiments done without strict adherence to established protocols might provide inaccurate information about biofilm composition. From the 21 relevant original reports on biofilms inside water distribution pipes listed in Table 1 , the patterns of biofilms formed in temperate regions could be outlined. These studies carried out between 2002 and 2017 in Europe, the US, Australia, China, Korea, and Brazil show varied types of microbial populations in biofilms. Secondly, original reports on identification of microbial genera from municipal supply water in Bangladesh were searched in Google Scholar, Pubmed, and the Cochrane Library using search words Bangladesh, WASA supply water, tap water, microorganisms. A total of 11 original reports from Bangladesh satisfied the inclusion-exclusion criteria mentioned above and were included for constructing the schematic diagram of biofilm inside water supply pipes in Bangladesh (Table 2 ). Thirdly, Google Scholar, Pubmed, and the Cochrane Library were searched using keywords interaction of microbial species in biofilm to retrieve original reports of pair-wise microbial interaction in biofilms. A total of 13 articles that explored metabolic interaction between two bacterial species in an experimental biofilm in the laboratory control environment were included. Articles that could not conclude the specific interaction between dual- species were excluded from this study.
Biofims Inside Water Supply Pipes
Microbial interactions differ depending on physico-chemical parameters of the natural waters. Temperature, pH, conductivity, turbidity, DO, type, and amounts of minerals, total organic Carbon (TOC), total Nitrogen, and biological/CODs are the non-biologic parameters that set the limits for microbial life ( 34 ). Different types of water treatment (desalination, decalcification, sedimentation) improve the physical quality of the source water ( 35 ). The distinct pattern of microbial interaction in aquatic environments is determined by the microbial population and the abiotic factors present. The microbes form and thrive in a biofilm through biomass transfer from the organic Carbon and microbial growth on any solid support ( 36 ). From the 21 relevant original reports on biofilms inside water distribution pipes listed in Table 1 , the patterns of biofilms formed in temperate regions could be outlined. These studies carried out between 2002 and 2017 in Europe, the US, Australia, China, Korea, and Brazil show varied types of microbial populations in biofilms. The summer temperature in Europe and the North America is around 15 to 25°C and the natural population of the surface waters is diverse genera of bacteria, fungi, molds, bacteriophages, aquatic viruses, parasites, and in some cases Archaea. Brazil and Australia have an average summer temperature of 30°C, closer to the summer temperature of Bangladesh. Turbidity of the natural waters in the studies ranged from 0.4 to 58 NTU, closer to the range of the turbidity of surface waters around Dhaka city. The pH of the waters in the studies also falls within the range of Bangladesh river waters. The TOC content was stated to be 8.5 mg/L in one of the reports ( 31 ). The water distribution pipes composed of a wide range of materials depending on the soil type, depth, water pressure, flow, and retention pattern, intended life of the pipes etc. The pipe materials include unplansticized polyvinyl chloride (PVCu), chlorinated polyvinylchloride (PVCc), polyethylene-100 (PE-100), steel, polypropylene (PP), latex, polybutyrate (PB), copper, and high-density polyethylene (HDPE), all providing appropriate surface for biofilms to thrive ( 19 ). The type and duration of water treatment for disinfection reduces the numbers of organisms present in the natural waters. Most municipalities use multistep water treatments for reduction of unwanted minerals through filtration, flocculation, sedimentation, and disinfection ( 35 ). Chlorination and UV radiation are the most widespread methods for supply water disinfection. While UV radiation does not produce residual effect, chlorination is allowed to leave a threshold of 5 mg/L residual free chlorine so that any remaining pathogens are gradually killed on their way to the receiving end ( 37 ). However, resistant microbes can survive chlorination and establish complex biofilms inside the supply pipes, deteriorating the quality of supply waters ( 38 ). As evident from Table 1 , the organisms that establish biofilms successfully inside a municipal distribution system range from pathogens ( Aeromonas hydrophila, Salmonella, Klebsiella, Pseudomonas aeruginosa, Legionella pneumophila, Escherichia coli ), opportunists ( Stenotrophomonas moltophilia, Mycobacterium avis complex), toxin producers (Cyanobacteria) to non-pathogens that destroy the pipe material and cause biofouling ( Galleonella, Siderooxydans, Geothrix, Nitrospira ). The reports from temperate weather shows enrichment of molds in the biofilm ( Penicillium, Alternaria, Fusarium, Aspergillus, Mucor, Geotrichum, Botrytis ) ( 13 ), whereas reports from Australia and Brazil with higher temperatures show biofilms that are dominated by heterotrophic bacterial species ( 11 , 16 , 20 , 25 ).
Microbiological Analysis of Tap Water from Bangladesh
The best-studied water supply system in Bangladesh is in the capital Dhaka, which acquires water from rivers Meghna, Buriganga, Sitalakshya, and Turag, treats them in water treatment plans in Gandharbapur, Saidabad, Rupganj, and Pagla ( 39 ). They treat water with sedimentation and chlorination, test the water for safety and drinking quality and supply it through the pipes to overhead tanks, from which water goes into points of use. Mahbub et al. ( 40 ) reported finding live bacteria in more than 60% of the sampled tap water in their study, which exceeds the Bangladesh Standards (BDS 1240:2001) for the microbiological quality of water. The Bangladesh Department of Public Health and Engineering (DPHE) has set different set of standards for potable water in Bangladesh, which varies from the universal standards set by the World Health Organization (WHO) in many parameters. Coliforms and E. coli are frequently reported in supply water ( 31 , 33 , 41 ). Acharjee et al. ( 33 , 42) had reported finding E. coli, Klebsiella, Salmonella, Shigella, Vibrio, Aeromonas , and fungi from supply water, indicating that these bacteria and molds survive disinfection procedures. When taken together with the quality of raw water with higher concentration of Iron and Arsenic, we find factors limiting certain kinds of microbes in the distribution system.
Theoretical Composition of Biofilm Inside the Wasa Water Supply Pipes in Bangladesh
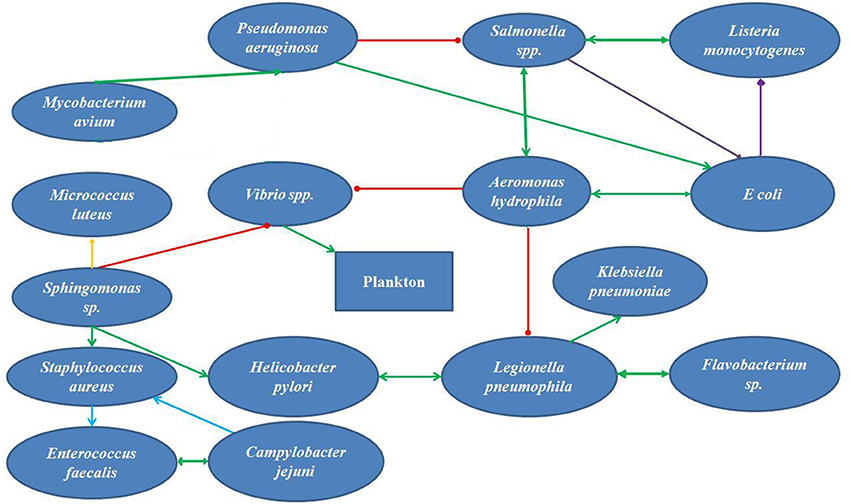
If we summarize the parameters reported from independent original studies, we can set the parameters of the supply water within the reported ranges. The temperature of the water in summer is around 30°C ( 30 ). The pH of the natural water varies between 7 and 8 and the DO ranges between 3 and 5 mg/L. The conductivity of the waters is 1,158 μS/cm, much higher than the reference value for natural waters ( 30 ). The turbidity of the water is 1.1 NTU. The nitrate concentrate was 8.5 mg/L, closer to the upper limit of nitrate concentration ( 33 ). The Iron concentration was 0.05 mg/L, and phosphate and sulfate concentrations were 4.4 and 9 mg/L, respectively. The tap water for domestic use contained 0.02 mg/L of residual Chlorine ( 43 ). Microbes that form biofilm within the water supply pipes must be organisms with their growth optimums within these ranges. According to Li et al. ( 29 ) the turbidity, ammonia concentration, nitrate content and TOC content of the water inside the supply system influence the nature and extend of biofilm formation. Pinto et al. ( 44 ) reported that the seasonal cycling of the biofilms inside the pipes correlated with seasonal temperature fluctuations. Bacteria that survive and develop biofilms under these conditions would be E. coli, Shigella, Vibrio, Klebsiella, Salmonella, L. pneumophila, Flavobacterium, Sphingomonas, P. aeruginosa, Nitrospira, Actinobacterium, Acidobacterium, Aeromonas, Sphingobacterium, Mycobacterium avium, Bacteroidium, Clostridia, Spirochaetes, Acremonium, Cladosporium, Fusarium, Microbacterium, Stenotrophomonas. Penicillium and Aspergillus are the most abundant molds in the natural waters in the tropics. In addition, Cyanobacteria could survive the parameters of Bangladeshi natural waters and contribute to the toxin production. The biofilm forming bacteria have different aspects regarding their roles on public health. Legionella pneumophila, Salmonella, E. coli, Shigella, Vibrio, Klebsiella , and Clostridia are pathogens that pose considerable threat to consumers. L. pneumophila causes life-threatening pneumonia and respiratory distress ( 28 ). Salmonella, Shigella, Vibrio, Klebsiella , and E. coli are waterborne agents of enteric diseases, often causing seasonal outbreaks in Dhaka city ( 8 ). Opportunistic waterborne pathogens include M. avium complex, Stenotrophomonas maltophilia , and A. hydrophila that can infect immune-compromised groups such as infants, adults, pregnant women, and people with underlying medical conditions (cancer, HIV/AIDS, etc.). M. avium causes pulmonary, soft tissue and lymph node infections ( 45 , 46 ). Stenotrophomonas maltophilia causes respiratory infection in cystic fibrosis patients ( 47 ). Aeromonas hydrophila is an enteric pathogen infecting children and immunocompromised people ( 48 ). The aquatic biofilms have also been implicated in spread of drug-resistance genes. Talukdar et al. ( 41 ) had shown presence of extended spectrum beta-lactam (ESBL) E. coli and qnrS elements for quinolone resistance from tap water in Dhaka city. Biofilm microbes also produce metabolites and components that change the quality of drinking water. Aspergillus spores are allergenic ( 49 ). The odor in tap water often results from dimethyl polysulfides, produced by Pseudomonas, Flavobacterium, Aeromonas , and Penicillium ( 50 ). Sulfur oxidizers like Acidobacterium change the pH of the water and produce foul odor and taste ( 51 ). Nitrospira spp. are nitrite-oxidizing autotrophs, colonizing plastic surfaces ( 52 ). Metal oxidizing bacteria corrode metal pipes ( 53 ). All these information stress on the development of robust and sensitive analytical techniques for evaluation of water quality as well as revised maintenance procedures that would help reduce formation of biofilms inside municipal water distribution pipes. Figure 1 presents a simplified hypothetical diagram of the microbial interactions inside a municipal water supply pipe.
Hypothetical Interaction of Microbial Isolates from Wasa Water in Bangladesh
Over the last decade, original report of microbiological analysis of tap water from Bangladesh mentioned isolating viable cells of coliforms and E. coli ( 40 ), Klebsiella, Salmonella, Shigella, Vibrio, Aeromonas , and fungi ( 33 ). Literature search on interaction of each pair of these microorganisms in a dual-species interaction helps to construct a hypothetical network of microbes in the water supplied by WASA (Figure 1 ). Aeromonas hydrophila showed positive interaction Salmonella spp. and Listeria monocytogenes ( 54 , 55 ) Vibrio spp. ( 56 ), but is inhibited by P. aeruginosa at the planktonic phase of biofilm development ( 57 ). Salmonella competes with E. coli in biofilm ( 58 ). E. coli maintains cooperative interaction with A. hydrophila ( 59 ) and P. aeruginosa ( 60 ) in a biofilm. P. aeruginosa and M. avium mutually benefit each other ( 21 ). Vibrio spp., the major recruiter of plankton in biofilms ( 61 ) are supported by A. hydrophila ( 56 ) Sphingomonas sp. enhanced sustainance of S. aureus ( 62 ) and Helicobacter pylori in biofilm ( 63 ). Sphingomonas coaggregates with Micrococus luteus ( 64 ). Biofilm-forming potential S. aureus and Enterococcus faecalis are enhanced by the presence of Campylobacter jejuni ( 65 ). Aeromonas hydrophila inhibited L. pneumophila ( 66 ), but maintained mutually beneficial interactions with H. pylori ( 63 ), Klebsiella pneumonia and Flavobacterium ( 67 , 68 ).
Recommendations for Risk Alleviation
Public health microbiologists and engineers put much emphasis on preventing formation of complex biofilms inside the municipal water supply network. WHO guidelines suggests that national priorities should be determined while designing the public health infra-structure. A maximum of one water-borne infection per 10,000 consumers per year is an acceptable level for quality drinking water. The first consideration for building a good quality water treatment and supply network starts from identifying the source of water, identifying hazards quantitatively, constructing the pipe network, a cleansing regime should be in practice devised according to the material and the longevity of the pipes. Safe water framework from the WHO constitutes of an iterative method where quantitative microbial risk assessment method outlines the hazard analysis. If the hazards are identified quantitatively, control measures are sought for log removal of microbial hazard and for reducing chemical hazard (carcinogen, irritant, nitrification, and biocorrosion) to an acceptable level. Water pressure inside the pipes and the possibility of leakage along the pipes must be monitored closely. Alternate source of supply water for non-potable purpose could reduce the cost of municipal water treatment. Husna and Rahman ( 69 ) re-evaluated the necessity of rainwater harvesting in Dhaka city for industrial purpose. Real-time monitoring with commercial sensors and microchip-based devices should be in place to assess the physical, chemical, and microbiological quality of water. There are critical values of fluoride, nitrate, lead, chromium, arsenic, and pesticide concentrations in drinking water that counter biofilm formation inside the pipe distribution network, but these are detrimental to health at higher concentrations. Silvestry-Rodriguez et al. ( 70 ) reported that 100 μg/L of Silver can prevent biofilm formation in PVC and steel pipes but affects of elemental silver on consumer health needs to be studied. Hitzfeld et al. ( 71 ) discussed the effectivity of 1.5 mg/L of ozonation for 30 min with a residual concentration of 0.6 mg/L sufficiently maintaining Cyanobacteria under control, saving the water system from toxins. The United States Environment Protection Agency ( 72 ) put forward experimental techniques to disrupt chlorine-resistant biofilms by using anti-quorum sensing molecules, such as UW85 from Bacillus cereus . Another approach to getting safe drinking water is the treatment of municipal water at point-of-use, such as microfiltered water dispensation system or reverse osmosis water dispensers installed at hospitals, households, or nurseries ( 73 ). Figure 2 summarizes current and proposed preventing measures for biofilm build-up inside the water distribution pipes. National water safety plans should combine total quality management (TQM) and ISO 14001 and ISO9001 to ensure certified standards approved by independent group.
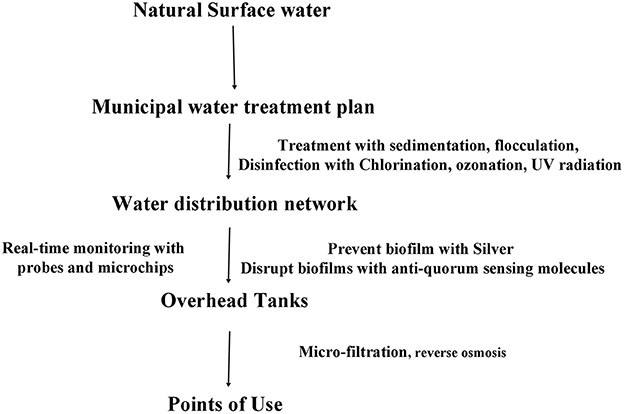
Figure 2 . Diagram of preventive and disruptive measures for biofilm control and elimination at different stages of the municipal water supply network.
The multi-faceted problem of development of biofilms inside the municipal water distribution pipe has not been addressed in Bangladesh yet. Microbial interactions get more complicated with virulence genes, especially antimicrobial resistance genes, that subjects consumers to even larger threat of antimicrobial resistance epidemic. The incidences of water-borne infections together with occasional deterioration in the quality of supply waters (odor, discoloration) calls for cutting-edge in-line real-time monitoring facilities with prompt interventions. Biological active Carbon (BAC) filters, granular active carbon (GAC) filter, UV lights, contact chlorine treatment, and an engineered storage and distribution might prove useful in improving water quality and safety.
Author Contributions
This manuscript enlists a single author, who reviewed scientific literature, formulated the study question and developed a theoretical model for the probable kind of microbial interaction inside water supply network in Bangladesh.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. ^ Dhaka Water and Sewage Authority (DWASA) Annual Report 2015–16 . Available online at: http://dwasa.org.bd/annual-reports/2015-16 .
2. ^ Dhaka Water and Sewage Authority (DWASA) Annual Report 2012–13 . Available online at: http://dwasa.org.bd/annual-reports/2012-13 .
1. WHO. World Bank documentation of Water safety and Distribution System (2014). Available online at: www.who.int/water_sanitation_health/publications/Water_safety_distribution_systems_2014v1.pdf
2. The United Nations Sustainable Development Goals (2015). Available online at: http://www.un.org/sustainabledevelopment
3. The World Health Organization. Guidelines for Drinking-Water Quality: Surveillance and Control of Community Supplies . 2nd Edn, Vol. 3 (1997), p 83–91. Available online at: http://www.who.int/water_sanitation_health/publications/small-water-supplies-guidelines/en/
4. The World Health Organization. Chapter 8 Guidelines for Drinking-Water Quality. 3rd Edn, Vol 1 (2004), p. 145–192.
5. Liu G, Tao Y, Zhang Y, Lut M, Knibbe WJ, Wielen P, et al. Hotspots for selected metal elements and microbes accumulation and the corresponding water quality deterioration potential in an unchlorinated drinking water distribution system. Water Res . (2017) 124:435–45. doi: 10.1016/j.watres.2017.08.002
PubMed Abstract | CrossRef Full Text | Google Scholar
6. Volk CJ, LeChevalier M. Impacts of the reduction of nutrient levels on bacterial water quality in distribution systems. Appl Environ Microbiol . (1999) 65:4957–66.
PubMed Abstract | Google Scholar
7. Göttlich E, van der Lubbe W, Lange B, Fiedler S, Melchert I, Reifenrath M, et al. Fungal flora in groundwater-derived public drinking water. Int. J. Hyg. Environ. Health (2002) 200:269–79. doi: 10.1078/1438-4639-00158
CrossRef Full Text | Google Scholar
8. Schwartz BS, Harris JB, Khan AI, Larocque RC, Sack DA, Malek MA, et al. Diarrheal epidemics in Dhaka, Bangladesh, during three consecutive floods: 1988:1998, and 2004. Am J Trop Med Hyg . (2006) 74:1067–73.
9. The World Health Organization. Chapter 11 Guidelines for Drinking-Water Quality. 4th Edn. (2011), p. 231–305.
10. Wang J, Stanford K, McAllister TA, Johnson RP, Chen J, Hou H, et al. Biofilm formation, virulence gene profile and anti-microbial resistance of non-O157 shiga toxin producing E . coli. Foodborne Pathogen Dis . (2016) 13:316–24. doi: 10.1089/fpd.2015.2099
CrossRef Full Text
11. Penna VTC, Martinus SAM, Mazzola PG. Identification of bacteria in drinking and purified water during the monitoring of a typical water purification system. BMC Public Health . (2002) 2:13. doi: 10.1186/1471-2458-2-13
12. Langmark J, Storey MV, Ashbolt NJ, Stenstrom TA. Accumulation and fate of microorganisms and microspheres in biofilms formed in a pilot-scale water distribution system. Appl Environ Microbiol . (2005) 71:706–12. doi: 10.1128/AEM.71.2.706-712.2005
13. Hageskal G, Knutsen AK, Gaustad P, Hoog GSD, Skaari I. Diversity and significance of mold species in norwegian drinking water. Appl Environ Microbiol . (2006) 72:7586–93. doi: 10.1128/AEM.01628-06
14. Meier TR, Maute CJ, Cadillac JM, Lee JY, Righter DJ, Hugunin KMS, et al. Quantification, distribution, and possible source of bacterial biofilm in mouse automated watering systems. J Am Assoc Lab Anim Sci . (2008) 47:63–70. doi: 10.1524/9783486843460.1
15. Sammon NB, Harrower KM, Fabbro LD, Reed RH. Incidence and distribution of microfungi in a treated municipal water supply system in sub-tropical Australia. Int J Environ Res Public Health (2010) 7:1597–611. doi: 10.3390/ijerph7041597
16. Siquira VM, Oliviera HMB, Santos C, Paterson RRM, Gusmao NB, Lima N. Filamentous fungi in drinking water, particularly in relation to biofilm formation. Int J Environ Res Public Health (2011) 8:456–69. doi: 10.3390/ijerph8020456
17. Li D, Li Z, Yu J, Cao N, Liu R, Yangi M. Characterization of bacterial community structure in a drinking water distribution system during an occurrence of red water. Appl Environ Microbiol . (2010) 76:7171–80. doi: 10.1128/AEM.00832-10
18. White CP, DeBry RW, Lytles DA. Microbial survey of a full-scale, biologically active filter for treatment of drinking water. Appl Environ Microbiol . (2012) 78:6390–4. doi: 10.1128/AEM.00308-12
19. Lee Y. An evaluation of microbial and chemical contamination sources related to the deterioration of tap water quality in the household water supply system. Int. J. Environ. Res. Public Health (2013) 10:4143–60. doi: 10.3390/ijerph10094143
20. Shaw JLA, Monis P, Weyrich LS, Sawade E, Drikas M, Cooper AJ. Using amplicon sequencing to characterize and monitor bacterial diversity in drinking water distribution systems. Appl Environ Microbiol. (2015) 81:6463–73. doi: 10.1128/AEM.01297-15
21. Falkinham JO, Pruden A, Edwards M. Opportunistic premise plumbing pathogens: increasingly important pathogens in drinking water. Pathogens (2015) 4:373–86. doi: 10.3390/pathogens4020373
22. Rozej A, Cydzik-Kwiatkowska A, Kowalska B, Kowalski D. Structure and microbial diversity of biofilms on different pipe materials of a model drinking water distribution systems. World J Microbiol Biotechnol. (2015) 31:37–47. doi: 10.1007/s11274-014-1761-6
23. Karkey A, Jombart T, Walker AW, Thomsons CN, Torres A, Dondo S, et al. The ecological dynamics of fecal contamination and Salmonella typhi and Salmonella paratyphi a in municipal kathmandu drinking water. PLoS Negl Trop Dis. (2015) 10:e0004346. doi: 10.1371/journal.pntd.0004346
24. Abberton CL, Bereschenko L, van der Wielen PWJJ, Smith CJ. Survival, biofilm formation, and growth potential of environmental and enteric Escherichia coli strains in drinking water microcosms. Appl Environ Microbiol . (2017) 82:5320–32. doi: 10.1128/AEM.01569-16
25. Ginige MP, Garbin S, Wylie J, Krishna KCB. Effectiveness of devices to monitor biofouling and metals deposition on plumbing. Materials exposed to a full-scale drinking water distribution system. PLoS ONE (2017) 12:e0169140. doi: 10.1371/journal.pone.0169140
26. Douterelo I, Sharpe R, Boxal E. Bacterial community dynamics during the early stages of biofilm formation in a chlorinated experimental drinking water distribution system: implications for drinking water discolouration. J Appl Microbiol . (2014) 117:286–301. doi: 10.1111/jam.12516
27. Prest EI, Weissbrodt DG, Hammes F, van Loosdrecht MCM, Vrouwenvelder JS. Long-term bacterial dynamics in a full-scale drinking water distribution system. PLoS ONE (2016) 11:e0164445. doi: 10.1371/journal.pone.0164445
28. Kooij DVD, Bakker GL, Italiaander R, Veenendaal HR, Wullings BA. Biofilm composition and threshold concentration for growth of Legionella pneumophila on surfaces exposed to flowing warm tap water without disinfectant. Appl Environ Microbiol . (2016) 83:e02737–16. doi: 10.1128/AEM.02737-16
29. Li Q, Xia PF, Tao ZY, Wang SG. Modeling biofilms in water systems with new variables: a review. Water (2017) 9:462. doi: 10.3390/w9070462
30. Real KH, Khanam N, Mia MY, Nasreen M. Assessment of water quality and microbial load of Dhaleshwari River Tangail, Bangladesh. Adv Microbiol. (2017) 7:523–33. doi: 10.4236/aim.2017.76041
31. Kormoker T, Proshad R, Khan MM. Analysis of water quality in urban water supply system of Bangladesh. J Env Analyt Toxicol . (2017) 7:4. doi: 10.4172/2161-0525.1000492
32. Janzon A, Sjöling A, Lothigius A, Ahmed D, Qadri F, Svennerholm AM. Failure to detect Helicobacter pylori DNA in drinking and environmental water in Dhaka, Bangladesh, using highly sensitive real-time PCR assays. Appl Environ Microbiol. (2009) 75:3039–44. doi: 10.1128/AEM.02779-08
33. Acharjee M, Rahman F, Beauty SA, Feroz F, Rahman MM, Noor R. Microbiological study on supply water and treated water in Dhaka City. Stamford J Microbiol . (2011) 1:42–5 doi: 10.3329/sjm.v1i1.9132
34. Andrew DR, Fitak RR, Munguia-Vega A, Racolta A, Martinson VG, Donstova K. Abiotic factors shape microbial diversity in Sonoran deserts soils. Appl Environ Microbiol . (2012) 78:7527–37. doi: 10.1128/AEM.01459-12
35. CDC. Center for Disease Control and Prevention Documentation on Water Treatment (2015). Available online at: https://www.cdc.gov/healthywater/drinking/public/water_treatment.html
36. Stewart PS. Diffusion in biofilms. J. Bacteriol. (2003) 185:1485–91. Available online at: http://jb.asm.org/content/185/5/1485
37. Kinsey GC, Paterson RR, Kelley J. Methods for the determination of filamentous fungi in treated and untreated waters. J Appl Microbiol SympSuppl. (1999) 85, 214S−24S.
38. Fish KE, Collins R, Green NH, Sharpe RS, Douterelo I, Osborn M.A, Boxall JB. Characterisation of the physical composition and microbial community structure of biofilms within a model full-scale drinking water distribution system. PLoS ONE (2016) 10:e0115824. doi: 10.1371/journal.pone.0115824
39. Khan TA. Dhaka water Supply and Sewerage Authority: Performance and Challenges. (2015). Available online at: http://app.dwasa.org.bd/admin/news/Dhaka%20WASA%20Article-for%20BOOK.pdf
40. Mahbub KR, Nahar A, Ahmed MM, Chakraborty A. Quality analysis of dhaka WASA drinking water: detection and biochemical characterization of the isolates. J Environ Sci Nat Res. (2011) 4:41–49. doi: 10.3329/jesnr.v4i2.10133
41. Talukdar PK, Rahman M, Rahman M, Nabi A, Islam Z, et al. Antimicrobial resistance, virulence factors and genetic diversity of Escherichia coli isolates from household water supply in Dhaka, Bangladesh. (2013) PLoS ONE 8:e61090. doi: 10.1371/journal.pone.0061090
42. Acharjee M, Rahman F, Jahan F, Noor R. Bacterial proliferation in municipal water supplied in mirpur locality of Dhaka City, Bangladesh. Clean Soil Air Water (2014) 42:434–41 doi: 10.1002/clen.201200618
43. Department of Public Health Engineering (DPHE) Water Quality parameters Bangladesh Standards and WHO Guidelines . (2011). Available online at: http://dphe.gov.bd/index.php?option-com_contentandview-articleandid-125&itemid-133
44. Pinto AJ, Schroeder J, Lunn M, Sloan W, Raskin L. Spatial-temporal survey and occupancy-abundance modeling to predict bacterial community dynamics in the drinking water microbiome. mBio (2014) 5:e01135–14. doi: 10.1128/mBio.01135-14
45. September SM, Brozel VS, Venter SN. Diversity of nontuberculoid mycobacterium species in biofilms of urban and semiurban drinking water distribution systems. Appl Environ Microbiol . (2004) 70:7571–3. doi: 10.1128/AEM.70.12.7571-7573.2004
46. Torvinen E, Lehtola MJ, Martikainen PJ, Miettinen IT. Survival of Mycobacterium avium in drinking water biofilms as affected by water flow velocity, availability of phosphorus, and temperature. Appl Environ Microbiol . (2007) 73:6201–7. doi: 10.1128/AEM.00828-07
47. Brooke J. Stenotrophomonas maltophilia : an emerging global opportunistic pathogens. Clin Microbiol Rev . (2012) 25:2–41. doi: 10.1128/CMR.00019-11
48. Igbinosa IH, Igumbor EU, Aghdasi F, Tom M, Okoh AI. Emerging Aeromonas species infections and their significance in public health. Sci W J . (2012) 12:625023. doi: 10.1100/2012/625023
49. Low SY, Dannmiller K, Yao M, Yamamoto N, Peccia J. The allergenicity of Aspergillus fumigatus conidia is influenced by growth temperature. Fungal Biol . (2011) 115:625–32 10.1016/j.funbio.2011.03.006
50. Schultz S, Dickschat JS. Bacterial volatiles: the smell of small organisms. Nat Prod Rep. (2006) 24:814–42. doi: 10.1039/b507392h
51. Luptakova A. Importance of sulphate-reducing bacteria in environment. Nova Biotechnol. (2007) 7:17–22. doi: 10.1080/01490450701672117
52. Yao Q, Peng DC. Nitrite oxidizing bacteria (NOB) dominating in nitrifying community in full-scale biological nutrient removal wastewater treatment plants. AMB Express . (2017) 7:25. doi: 10.1186/s13568-017-0328-y
53. Kip N, Veen JAV. The dual role of microbes in corrosion. ISME J . (2014) 9:542–51. doi: 10.1038/ismej.2014.169
54. Kostaki M, Chorianopoulos N, Braxou E, Nychas G-J, Giaouris E. Differential biofilm formation and chemical disinfection resistance of sessile cells of listeria monocytogenes strains under monospecies and dual-species (with Salmonella enterica ) conditions. Appl Environ Microbiol. (2012) 78:2586–95. doi: 10.1128/AEM.07099-11
55. Smith JN, Dyszel JL, Soares JA, Ellermeier CD, Altier C, et al. SdiA, an N-acylhomoserine lactone receptor, becomes active during the transit of Salmonella enterica through the gastrointestinal tract of turtles. PLoS ONE (2008) 3:e2826. doi: 10.1371/journal.pone.0002826
56. Shrout JD, Nerenberg R. Monitoring bacterial twitter: does quorum sensing determine the behavior of water and wastewater treatment biofilms? Environ Sci Technol . (2012) 46:1995–2005. doi: 10.1021/es203933h
57. Pang XY, Yang YS, Yuk HG. Biofilm formation and disinfectant resistance of Salmonella sp . in mono- and dual-species with Pseudomonas aeruginosa . J Appl Microbiol . (2017). 123:651–60. doi: 10.1111/jam.13521
58. Wang R, Kalchayanand N, King DA, Luedtke BE, Bosilevac JM, Arthur TM. Biofilm formation and sanitizer resistance of Escherichia coli O157:H7 strains isolated from “High Event Period” meat contamination. J Food Prot . (2014) 77:1982–7. doi: 10.4315/0362-028X
59. Banning N, Toze S, Mee BJ. Persistence of biofilm-associated Escherichia coli and Pseudomonas aeruginosa in groundwater and treated effluent in a laboratory model system. Microbiology (2003) 149(Pt 1):47–55. doi: 10.1099/mic.0.25938-0
60. Culotti A, Packman AI. Pseudomonas aeruginosa promotes Escherichia coli biofilm formation in nutrient-limited medium. PLoS ONE (2014) 9:e107186. doi: 10.1371/journal.pone.0107186
61. Haugo AJ, Watnick PI. Vibrio cholerae CytR is a repressor of biofilm development. Mol Microbiol . (2002) 45:471–83. doi: 10.1046/j.1365-2958.2002.03023.x
62. Simões LC, Simões M, Vieira MJ. Biofilm interactions between distinct bacterial genera isolated from drinking water. Appl Environ Microbiol . (2007) 73:6192–200. doi: 10.1128/AEM.00837-07
63. Gião MS, Azevedo NF, Wilks SA, Vieira MJ, Keevil CW. Interaction of Legionella pneumophila and Helicobacter pylori with bacterial species isolated from drinking water biofilms. BMC Microbiol. (2011). 11:57. doi: 10.1186/1471-2180-11-57
64. Min KR, Rickard AH. Coaggregation by the freshwater bacterium Sphingomonas natatoria alters dual-species biofilm formation. Appl Environ Microbiol . (2009) 75:3987–97. doi: 10.1128/AEM.02843-08
65. Teh KH, Flint S, French N. Biofilm formation by Campylobacter jejuni in controlled mixed-microbial populations. Int J Food Microbiol. (2010) 143:118–24. doi: 10.1016/j.ijfoodmicro.2010.07.037
66. Sulami AA, Al-Taee AMR, Juma's MG. Isolation and identification of Helicobacter pylori from drinking water in Basra governorate. Iraq Gastroenterol Res Pract. (2010) 16:920–5.
Google Scholar
67. Stewart CR, Muthye V, Cianciotto NP. Legionella pneumophila persists within biofilms formed by Klebsiella pneumoniae, Flavobacterium sp ., and Pseudomonas fluorescens under dynamic flow conditions. PloS ONE (2012) 7:e50560. doi: 10.1371/journal.pone.0050560
68. Whiley H, Keegan A, Fallowfield H, Bentham R. Detection of Legionella pneumophila and Mycobacterium avium Complex (MAC) along potable water distribution pipelines. Int J Environ Res Public Health . (2014) 11:7393–405. doi: 10.3390/ijerph110707393
69. Husna AU, Rahman MS. Potentials of rainwater harvesting as an alternative water supply system in dhaka city. J Sci Res Rep . (2017) 13:1–7. doi: 10.9734/JSRR/2017/31866
70. Silvestry-Rodriguez N, Bright KR, Slack DC, Uhlmann DR, Gerbia CP. Silver as a residual disinfectant to prevent biofilm formation in water distribution systems. Appl Environ Microbiol . (2008) 74:1639–41. doi: 10.1128/AEM.02237-07
71. Hitzfeld BC, Hoger SJ, Dietrich DR. Cyanobacterial toxins: removal during drinking water treatment, and human risk assessment.environ health Perspect (2000) 1:113–22 doi: 10.1289/ehp.00108s1113
72. United States Environmental Protection Agency Documents on Parameters of Water Quality: Interpretations Standards . (2011). Available online at: https://www.epa.ie/pubs/advice/water/quality/Water_Quality.pdf
73. Sacchetti R, De Luca G, Guberti E, Zanetti F. Quality of drinking water treated at point of use in residential healthcare facilities for the elderly. Int J Environ Res Public Health (2015) 12:11163–77. doi: 10.3390/ijerph120911163
Keywords: municipal water distribution, microbial biofilm, waterborne diseases, deterioration of water quality, biofouling
Citation: Towhid ST (2018) Microbial Interaction as a Determinant of the Quality of Supply Drinking Water: A Conceptual Analysis. Front. Public Health 6:184. doi: 10.3389/fpubh.2018.00184
Received: 31 January 2018; Accepted: 08 June 2018; Published: 26 June 2018.
Reviewed by:
Copyright © 2018 Towhid. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Syeda T. Towhid, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Advertisement
Scientific mapping of the research in microbial and chemical contamination of potable water in Bangladesh: A review of literature
- Review Article
- Published: 02 June 2023
- Volume 30 , pages 76421–76436, ( 2023 )
Cite this article

- Md Sharmon Hossain Arnob 1 ,
- Md Atif Arham 1 ,
- Rafszanul Islam 1 ,
- Nazratun Nawar 1 ,
- Sibgat Mehedi Hasan 1 ,
- Nusaiba Binte Saif 1 ,
- Asif Iqbal Arpon 1 &
- Md Abdullah Al Mamun ORCID: orcid.org/0000-0001-8490-5891 2
552 Accesses
Explore all metrics
Drinking water contamination is one of the most pressing concerns for the people of Bangladesh as they rely on groundwater to meet their water needs. The existing water sources of Bangladesh are losing potability due to natural, anthropogenic, and geogenic factors, resulting in acute to severe health consequences. To address the issue of safe drinking water, researchers are constantly examining potential sources that cause the pollution of drinking water. Through bibliometric and systematic research, the current work seeks to review the past research on microbiological and chemical contamination of drinkable water in Bangladesh. The bibliometric review provides insights into the research trends, notable authors, countries, and institutions, whereas the systematic review unfolds the key research areas, the contamination process, and the strategies used to mitigate the contamination process. The results show that arsenic and various coliform bacteria are the most commonly reported sources of chemical and microbiological contaminants that degrade water quality. The study demonstrates that the most crucial factors influencing arsenic mobilization include microbial decomposition of organic matter (biologically available organic matter, for example, peat), arsenic adsorption by metal-oxyhydroxides, Fe–Mn oxyhydroxide, chemical fertilizers, pond excavation, and altering of groundwater hydrology. The studies also indicated the sources that contribute to the microbiological quality decline. The current work has addressed the scope of future research.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
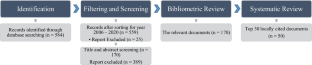
Similar content being viewed by others
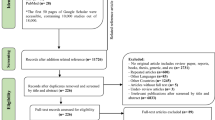
Systematic review and meta-analysis of arsenic concentration in drinking water sources of Iran
Reza Shokoohi, Mohammad Khazaei, … Zahra Torkshavand
Drinking and recreational water-related diseases: a bibliometric analysis (1980–2015)
Waleed M. Sweileh, Sa’ed H. Zyoud, … Naser Y. Shraim
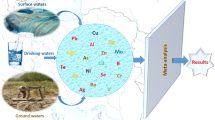
Presence of heavy metals in drinking water resources of Iran: a systematic review and meta-analysis
Masoumeh Ravanipour, Mahdi Hadi, … Simin Nasseri
Data availability
All data are available from the authors upon reasonable request.
Ahmad SA, Khan MH, Haque M (2018) Arsenic contamination in groundwater in Bangladesh: implications and challenges for healthcare policy. Risk management and healthcare policy 11:251–261. https://doi.org/10.2147/RMHP.S153188
Ahmed MF, Shamsuddin SA, Mahmud SG, Rashid H, Deere D, Howard G (2005) Risk Assessment of Arsenic Mitigation Options (RAAMO), APSU. Retrieved from https://www.ircwash.org/sites/default/files/Ahmed-2005-Risk.pdf
Ahamed S, Sengupta MK, Mukherjee SC, Pati S, Mukherjee A, Rahman MM, Hossain MA, Das B, Nayak B, Pal A, Zafar A, Kabir S, Banu SA, Morshed S, Islam T, Rahman MM, Quamruzzaman Q, Chakraborti D (2006) An eight-year study report on arsenic contamination in groundwater and health effects in Eruani village, Bangladesh and an approach for its mitigation. J Health, Popul Nutr 24(2):129–141 ( http://www.jstor.org/stable/23499352 )
Google Scholar
Al Mamun MA, Azad MAK, Al Mamun MA, Boyle M (2022) Review of flipped learning in engineering education: scientific mapping and research horizon. Educ Inf Technol 27(1):1261–1286. https://doi.org/10.1007/s10639-021-10630-z
Article Google Scholar
Anawar HM, Akai J, Mihaljevič M, Sikder AM, Ahmed G, Tareq SM, Rahman MM (2011) Arsenic contamination in groundwater of Bangladesh: perspectives on geochemical, microbial, and anthropogenic issues. Water 3(4):1050–1076. https://doi.org/10.3390/w3041050
Article CAS Google Scholar
Ayers JC, Goodbred S, George G, Fry D, Benneyworth L, Hornberger G, Akter F (2016) Sources of salinity and arsenic in groundwater in southwest Bangladesh. Geochem Trans 17(1):1–22. https://doi.org/10.1186/s12932-016-0036-6
Bashar MZI, Karim MR, Imteaz MA (2018) Reliability and economic analysis of urban rainwater harvesting: a comparative study within six major cities of Bangladesh. Resour Conserv Recycl 133:146–154. https://doi.org/10.1016/j.resconrec.2018.01.025
Begum YA, Talukder KA, Nair GB, Khan SI, Svennerholm A-M, Sack RB, Qadri F (2007) Comparison of enterotoxigenic Escherichia coli isolated from surface water and diarrhoeal stool samples in Bangladesh. Can J Microbiol 53(1):19–26. https://doi.org/10.1139/w06-098
Bhuiyan MAH, Rakib MA, Dampare SB, Ganyaglo S, Suzuki S (2011) Surface water quality assessment in the central part of Bangladesh using multivariate analysis. KSCE J Civ Eng 15(6):995–1003. https://doi.org/10.1007/s12205-011-1079-y
Bibi MH, Ahmed F, Ishiga H (2008) Geochemical study of arsenic concentrations in groundwater of the Meghna River Delta. Bangladesh J Geochem Explor 97(2–3):43–58. https://doi.org/10.1016/j.gexplo.2007.11.001
Chakraborti D, Rahman MM, Mukherjee A, Alauddin M, Hassan M, Dutta RN, Hossain MM (2015) Groundwater arsenic contamination in Bangladesh—21 years of research. J Trace Elem Med Biol 31:237–248. https://doi.org/10.1016/j.jtemb.2015.01.003
Charlet L, Polya DA (2006) Arsenic in shallow, reducing groundwaters in southern Asia: an environmental health disaster. Elements 2(2):91–96. https://doi.org/10.2113/gselements.2.2.91
Denyer D, Tranfield D (2009) Producing a systematic review. In D. A. Buchanan, & A. Bryman (Eds.), The Sage handbook of organizational research methods. Sage publications, p 671– 689
Díaz I, Cortey M, Olvera À, Segalés J (2016) Use of H-index and other bibliometric indicators to evaluate research productivity outcome on swine diseases. Plos One 11(3):e0149690. https://doi.org/10.1371/journal.pone.0149690
Donthu N, Kumar S, Pattnaik D (2020) Forty-five years of journal of business research: a bibliometric analysis. J Bus Res 109:1–14. https://doi.org/10.1016/j.jbusres.2019.10.039
Donthu N, Kumar S, Mukherjee D, Pandey N, Lim WM (2021) How to conduct a bibliometric analysis: an overview and guidelines. J Bus Res 133:285–296. https://doi.org/10.1016/j.jbusres.2021.04.070
Fawell J, Nieuwenhuijsen MJ (2003) Contaminants in drinking water Environmental pollution and health. Br Med Bull 68(1):199–208. https://doi.org/10.1093/bmb/ldg027
Feighery J, Mailloux BJ, Ferguson AS, Ahmed KM, van Geen A, Culligan PJ (2013) Transport of E. coli in aquifer sediments of Bangladesh: implications for widespread microbial contamination of groundwater. Water Resour Res 49(7):3897–3911. https://doi.org/10.1002/wrcr.20289
Ferguson AS, Layton AC, Mailloux BJ, Culligan PJ, Williams DE, Smartt AE, Sayler GS, Feighery J, McKay LD, Knappett PSK, Alexandrova E, Arbit T, Emch M, Escamilla V, Ahmed KM, Alam MJ, Streatfield PK, Yunus M, van Geen A (2012) Comparison of fecal indicators with pathogenic bacteria and rotavirus in groundwater. Sci Total Environ 431:314–322. https://doi.org/10.1016/j.scitotenv.2012.05.060
Ferguson AS, Mailloux BJ, Ahmed KM, van Geen A, McKay LD, Culligan PJ (2011) Hand-pumps as reservoirs for microbial contamination of well water. J Water Health 9(4):708–717. https://doi.org/10.2166/wh.2011.106
Fetscherin M, Heinrich D (2015) Consumer brand relationships research: a bibliometric citation meta-analysis. J Bus Res 68(2):380–390. https://doi.org/10.1016/j.jbusres.2014.06.010
Ganguli S, Rifat MAH, Das D, Islam S, Islam MN (2021) Groundwater pollution in Bangladesh: a review. Grassroots J Nat Resour 4(04):115–145. https://doi.org/10.33002/nr2581.6853.040409
Gu D, Li J, Li X, Liang C (2017) Visualizing the knowledge structure and evolution of big data research in healthcare informatics. Int J Med Informatics 98:22–32. https://doi.org/10.1016/j.ijmedinf.2016.11.006
Halim MA, Majumder RK, Nessa SA, Hiroshiro Y, Uddin MJ, Shimada J, Jinno K (2009) Hydrogeochemistry and arsenic contamination of groundwater in the Ganges Delta Plain. Bangladesh J Hazard Mater 164(2–3):1335–1345. https://doi.org/10.1016/j.jhazmat.2008.09.046
Halim MA, Majumder RK, Nessa SA, Oda K, Hiroshiro Y, Jinno K (2010) Arsenic in shallow aquifer in the eastern region of Bangladesh: insights from principal component analysis of groundwater compositions. Environ Monit Assess 161(1–4):453–472. https://doi.org/10.1007/s10661-009-0760-9
Harvey CF, Ashfaque KN, Yu W, Badruzzaman ABM, Ali MA, Oates PM, Michael HA, Neumann RB, Beckie R, Islam S, Ahmed MF (2006) Groundwater dynamics and arsenic contamination in Bangladesh. ChemGeol: 228(1–3):112–136. https://doi.org/10.1016/j.chemgeo.2005.11.025
Hasan MK, Shahriar A, Jim KU (2019) Water pollution in Bangladesh and its impact on public health. Heliyon 5(8):e02145. https://doi.org/10.1016/j.heliyon.2019.e02145
Heasley C, Sanchez JJ, Tustin J, Young I (2021) Systematic review of predictive models of microbial water quality at freshwater recreational beaches. Plos One 16(8):e0256785. https://doi.org/10.1371/journal.pone.0256785
Hirsch JE (2005) An index to quantify an individual’s scientific research output. Proc Natl Acad Sci USA 102(46):16569–16572. https://doi.org/10.1073/pnas.0507655102
Hossain MF (2006) Arsenic contamination in Bangladesh—an overview. Agr Ecosyst Environ 113(1–4):1–16. https://doi.org/10.1016/j.agee.2005.08.034
Hossain MG, Selim Reza AHM, Lutfun-Nessa M, Ahmed SS (2013) Factor and cluster analysis of water quality data of the groundwater wells of Kushtia, Bangladesh: implication for arsenic enrichment and mobilization. J Geol Soc India 81(3):377–384. https://doi.org/10.1007/s12594-013-0048-0
Howard G, Ahmed MF, Shamsuddin AJ, Mahmud SG, Deere D (2006) Risk assessment of arsenic mitigation options in Bangladesh. J Health Popul Nutr 24(3):346–355 ( https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3013255/ )
Howard G, Feroze Ahmed M, Gaifur Mahmud S, Teunis P, Davison A, Deere D (2007) Disease burden estimation to support policy decision-making and research prioritization for arsenic mitigation. J Water Health 5(1):67–81. https://doi.org/10.2166/wh.2006.056
Hussam A, Munir AKM (2007) A simple and effective arsenic filter based on composite iron matrix: development and deployment studies for groundwater of Bangladesh. J Environ Sci Health, Part A 42(12):1869–1878. https://doi.org/10.1080/10934520701567122
IAEA (2002) Arsenic contamination of groundwater in Bangladesh, International Atomic Energy Agency, Vienna. https://www.iaea.org/sites/default/files/arseniccontamination.pdf
Islam MM, Chou FN-F, Kabir MR, Liaw C-H (2010) Rainwater: a potential alternative source for scarce safe drinking and arsenic contaminated water in Bangladesh. Water Resour Manage 24(14):3987–4008. https://doi.org/10.1007/s11269-010-9643-7
Islam MA, Sakakibara H, Karim MR, Sekine M (2011a) Evaluation of risk communication for rural water supply management: a case study of a coastal area of Bangladesh. J Risk Res 14(10):1237–1262. https://doi.org/10.1080/13669877.2011.574315
Islam MA, Sakakibara H, Karim MR, Sekine M, Mahmud ZH (2011b) Bacteriological assessment of drinking water supply options in coastal areas of Bangladesh. J Water Health 9(2):415–428. https://doi.org/10.2166/wh.2011.114
Islam MA, Azad AK, Akber MA, Rahman M, Sadhu I (2015) Effectiveness of solar disinfection (SODIS) in rural coastal Bangladesh. J Water Health 13(4):1113–1122. https://doi.org/10.2166/wh.2015.186
Islam ARMT, Siddiqua MT, Zahid A, Tasnim SS, Rahman MM (2020) Drinking appraisal of coastal groundwater in Bangladesh: an approach of multi-hazards towards water security and health safety. Chemosphere 255:126933. https://doi.org/10.1016/j.chemosphere.2020.126933
Jebelli MA, Maleki A, Amoozegar MA, Kalantar E, Gharibi F, Darvish N, Tashayoe H (2018) Isolation and identification of the native population bacteria for bioremediation of high levels of arsenic from water resources. J Environ Manage 212:39–45. https://doi.org/10.1016/j.jenvman.2018.01.075
Jeelani G, Lone SA, Nisa AU, Mukherjee A, Deshpande RD (2020) Sources and processes of groundwater arsenic mobilization in upper Jhelum basin, western Himalayas. J Hydrol 591:125292. https://doi.org/10.1016/j.jhydrol.2020.125292
Jiang JQ, Ashekuzzaman SM, Jiang A, Sharifuzzaman SM, Chowdhury SR (2013) Arsenic contaminated groundwater and its treatment options in Bangladesh. Int J Environ Res Public Health 10(1):18–46. https://doi.org/10.3390/ijerph10010018
Johnston R, Hug SJ, Inauen J, Khan NI, Mosler H-J, Yang H (2014) Enhancing arsenic mitigation in Bangladesh: findings from institutional, psychological, and technical investigations. Sci Total Environ 488–489:477–483. https://doi.org/10.1016/j.scitotenv.2013.11.143
Kanel SR, Das TK, Varma RS, Kurwadkar S, Chakraborty S, Joshi TP, Bezbaruah AN, Nadagouda MN (2023) Arsenic Contamination in groundwater: geochemical basis of treatment technologies. ACS Environmental Au 3(3):135–152. https://doi.org/10.1021/acsenvironau.2c00053
Karim MR (2010) Microbial contamination and associated health burden of rainwater harvesting in Bangladesh. Water Sci Technol 61(8):2129–2135. https://doi.org/10.2166/wst.2010.031
Knappett PSK, Layton A, McKay LD, Williams D, Mailloux BJ, Huq MR, Alam MJ, Ahmed KM, Akita Y, Serre ML, Sayler GS, van Geen A (2011) Efficacy of hollow-fiber ultrafiltration for microbial sampling in groundwater. Ground Water 49(1):53–65. https://doi.org/10.1111/j.1745-6584.2010.00712.x
Knappett PSK, McKay LD, Layton A, Williams DE, Alam MJ, Huq MR, Mey J, Feighery JE, Culligan PJ, Mailloux BJ, Zhuang J, Escamilla V, Emch M, Perfect E, Sayler GS, Ahmed KM, van Geen A (2012a) Implications of fecal bacteria input from latrine-polluted ponds for wells in sandy aquifers. Environ SciTechnol 46(3):1361–1370. https://doi.org/10.1021/es202773w
Knappett PSK, McKay LD, Layton A, Williams DE, Alam MJ, Mailloux BJ, Ferguson AS, Culligan PJ, Serre ML, Emch M, Ahmed KM, Sayler GS, van Geen A (2012b). Unsealed tubewells lead to increased fecal contamination of drinking water. J Water Health : 10(4):565–578. https://doi.org/10.2166/wh.2012.102
Kurosawa K, Egashira K, Tani M, Jahiruddin M, Moslehuddin AZM, Zulfikar Rahman M (2008) Variation in arsenic concentration relative to ammonium nitrogen and oxidation reduction potential in surface and groundwater. Commun Soil Sci Plant Anal 39(9–10):1467–1475. https://doi.org/10.1080/00103620802004318
Leber J, Rahman MM, Ahmed KM, Mailloux B, van Geen A (2011) Contrasting influence of geology on E. coli and arsenic in aquifers of Bangladesh. Ground Water 49(1):111–123. https://doi.org/10.1111/j.1745-6584.2010.00689.x
Li H, An H, Wang Y, Huang J, Gao X (2016) Evolutionary features of academic articles co-keyword network and keywords co-occurrence network: based on two-mode affiliation network. Physica a: Stat Mech Appl 450:657–669. https://doi.org/10.1016/j.physa.2016.01.017
Liao VHC, Chu YJ, Su YC, Hsiao SY, Wei CC, Liu CW, Chang FJ (2011) Arsenite-oxidizing and arsenate-reducing acteria associated with arsenic-rich groundwater in Taiwan. J Contam Hydrol 123(1–2):20–29. https://doi.org/10.1016/j.jconhyd.2010.12.003
Liao H, Tang M, Luo L, Li C, Chiclana F, Zeng X (2018) A bibliometric analysis and visualization of medical big data research. Sustainability 10(2):166. https://doi.org/10.3390/su10010166
Luby SP, Gupta SK, Sheikh MA, Johnston RB, Ram PK, Islam MS (2008) Tubewell water quality and predictors of contamination in three flood-prone areas in Bangladesh. J Appl Microbiol 105(4):1002–1008. https://doi.org/10.1111/j.1365-2672.2008.03826.x
Meharg AA, Scrimgeour C, Hossain SA, Fuller K, Cruickshank K, Williams PN, Kinniburgh DG (2006) Codeposition of organic carbon and arsenic in Bengal delta aquifers. Environ Sci Technol 40(16):4928–4935. https://doi.org/10.1021/es060722b
Milton AH, Hore SK, Hossain MZ, Rahman M (2012) Bangladesh arsenic mitigation programs: lessons from the past. Emerg Health Threats J 5(1):7269. https://doi.org/10.3402/ehtj.v5i0.7269
Neumann RB, Ashfaque KN, Badruzzaman ABM, Ashraf Ali M, Shoemaker JK, Harvey CF (2010) Anthropogenic influences on groundwater arsenic concentrations in Bangladesh. Nat Geosci 3(1):46–52. https://doi.org/10.1038/ngeo685
Parvin F, Haque MM, Tareq SM (2022) Recent status of water quality in Bangladesh: a systematic review, meta-analysis and health risk assessment. Environ Challenges 6:100416. https://doi.org/10.1016/j.envc.2021.100416
Prasad G, Mamane H, Ramesh MV (2022) Geogenic and anthropogenic contamination of groundwater in a fragile eco-friendly region of southern Kerala, India. Agronomy Research 20(1):1072-1089. https://doi.org/10.15159/ar.22.010
Rahman M (2002) Arsenic and Contamination of Drinking-water in Bangladesh: A Public-health Perspective. J Health Popul Nutr 20(3):193–197
Rahaman MS, Mise N, Ichihara S (2022) Arsenic contamination in food chain in Bangladesh: a review on health hazards, socioeconomic impacts and implications. Hyg Environ Health Adv 2:100004. https://doi.org/10.1016/j.heha.2022.100004
Rahman MTU, Siddiqi UR, Kure S, Mano A, Udo K, Ishibashi Y (2015) Mobilization of high arsenic in the shallow groundwater of Kalaroa, south-western Bangladesh. Expo Health 8(2):159–175. https://doi.org/10.1007/s12403-015-0177-3
Raschid-Sally L (2000) Arsenic contamination of groundwater in Bangladesh. Discussion Paper iii. Colombo, Sri Lanka, International Water Management Institute. https://cgspace.cgiar.org/handle/10568/39146
Reyes-Gonzalez L, Gonzalez-Brambila CN, Veloso F (2016) Using co-authorship and citation analysis to identify research groups: a new way to assess performance. Scientometrics 108(3):1171–1191. https://doi.org/10.1007/s11192-016-2029-8
Riehmann P, Hanfler M, Froehlich B (2005) Interactive Sankey diagrams. IEEE Symposium on Information Visualization. INFOVIS 2005, Minneapolis, MN, USA 2005:233–240. https://doi.org/10.1109/INFVIS.2005.1532152
Sarkar AM, Rahman AKML, Samad A, Bhowmick AC, Islam JB (2019) Surface and groundwater pollution in Bangladesh: a review. Asian Rev Environ Earth Sci 6(1):47–69. https://doi.org/10.20448/journal.506.2019.61.47.69
Selim Reza AHM, Jean J-S, Lee M-K, Luo S-D, Bundschuh J, Li H-C, Yang H-J, Liu C-C (2010) Interrelationship of TOC, As, Fe, Mn, Al and Si in shallow alluvial aquifers in Chapai-Nawabganj, Northwestern Bangladesh: implication for potential source of organic carbon. Environ Earth Sci 63(5):955–967. https://doi.org/10.1007/s12665-010-0764-3
Shamsudduha M, Uddin A (2007) Quaternary shoreline shifting and hydrogeologic influence on the distribution of groundwater arsenic in aquifers of the Bengal Basin. J Asian Earth Sci 31(2):177–194. https://doi.org/10.1016/j.jseaes.2007.07.001
Shamsudduha M, Marzen LJ, Uddin A, Lee M-K, & Saunders JA (2008) Spatial relationship of groundwater arsenic distribution with regional topography and water-table fluctuations in the shallow aquifers in Bangladesh. Environ Geol 57(7). https://doi.org/10.1007/s00254-008-1429-3
Smith NM, Lee R, Heitkemper DT, Cafferky KD, Haque A, Henderson AK (2006) Inorganic arsenic in cooked rice and vegetables from Bangladeshi households. Sci Total Environ 370(2–3):294–301. https://doi.org/10.1016/j.scitotenv.2006.06.010
Some S, Mondal R, Mitra D, Jain D, Verma D, Das S (2021) Microbial pollution of water with special reference to coliform bacteria and their nexus with environment. Energy Nexus 1:100008. https://doi.org/10.1016/j.nexus.2021.100008
Sutton NB, van der Kraan GM, van Loosdrecht MCM, Muyzer G, Bruining J, Schotting RJ (2009) Characterization of geochemical constituents and bacterial populations associated with As mobilization in deep and shallow tube wells in Bangladesh. Water Res 43(6):1720–1730. https://doi.org/10.1016/j.watres.2009.01.006
Sweileh WM, Zyoud SH, Al-Jabi SW, Sawalha AF, Shraim NY (2016) Drinking and recreational water-related diseases: a bibliometric analysis (1980–2015). Ann Occup Environ Med 28(1):1–11. https://doi.org/10.1186/s40557-016-0128-x
Tareq SM, Maruo M, Ohta K (2013) Characteristics and role of groundwater dissolved organic matter on arsenic mobilization and poisoning in Bangladesh. Phys Chem Earth, Parts a/b/c 58–60:77–84. https://doi.org/10.1016/j.pce.2013.04.014
Tularam GA, Murali KK (2015) Water security problems in Asia and longer term implications for Australia. Sustainable Water Use and Management: Examples of New Approaches and Perspectives :119-149. https://doi.org/10.1007/978-3-319-12394-3_7
Van Geen A, Radloff K, Aziz Z, Cheng Z, Huq MR, Ahmed KM, Weinman B, Goodbred S, Jung HB, Zheng Y, Berg M, Trang PTK, Charlet L, Metral J, Tisserand D, Guillot S, Chakraborty S, Gajurel AP, Upreti BN (2008) Comparison of arsenic concentrations in simultaneously-collected groundwater and aquifer particles from Bangladesh, India, Vietnam, and Nepal. Appl Geochem 23(11):3244–3251. https://doi.org/10.1016/j.apgeochem.2008.07.005
WHO (2011) Guidelines for drinking-water quality: Fourth Edition. WHO chronicle, 38(4):104–108
Winkel L, Berg M, Amini M, Hug SJ, Annette Johnson C (2008) Predicting groundwater arsenic contamination in Southeast Asia from surface parameters. Nat Geosci 1(8):536–542. https://doi.org/10.1038/ngeo254
World Health Organization (2004) Meeting the MDG drinking water & sanitation target: A mid-term assessment of progress. A Report of the WHO-UNICEF Joint Monitoring Programme for Water Supply and Sanitation. Geneva, Switzerland. Retrieved from https://apps.who.int/iris/bitstream/handle/10665/43021/9241562781.pdf
Zahid A, Balke K-D, Hassan MQ, Flegr M (2006) Evaluation of aquifer environment under Hazaribagh leather processing zone of Dhaka city. Environ Geol 50(4):495–504. https://doi.org/10.1007/s00254-006-0225-1
Zahid A, Hassan MQ, Balke K-D, Flegr M, Clark DW (2008) Groundwater chemistry and occurrence of arsenic in the Meghna floodplain aquifer, southeastern Bangladesh. Environ Geol 54(6):1247–1260. https://doi.org/10.1007/s00254-007-0907-3
Download references
Author information
Authors and affiliations.
Department of Civil & Environmental Engineering, Islamic University of Technology, Gazipur, 1704, Bangladesh
Md Sharmon Hossain Arnob, Md Atif Arham, Rafszanul Islam, Nazratun Nawar, Sibgat Mehedi Hasan, Nusaiba Binte Saif & Asif Iqbal Arpon
Department of Technical and Vocational Education, Islamic University of Technology, Gazipur, 1704, Bangladesh
Md Abdullah Al Mamun
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to the study’s conception and design. Literature search and data collection were performed by Md Sharmon Hossain Arnob and Md Abdullah Al Mamun. Data analyses were performed by Md Sharmon Hossain Arnob, Md Atif Arham, Rafszanul Islam, Nazratun Nawar, Sibgat Mehedi Hasan, Nusaiba Binte Saif, and Asif Iqbal Arpon. The first draft of the manuscript was written by Md Sharmon Hossain Arnob, Md Atif Arham, and Rafszanul Islam. All authors commented on previous versions of the manuscript. Md Abdullah Al Mamun did the overall supervision, and corrected and edited the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Md Abdullah Al Mamun .
Ethics declarations
Ethical approval.
Not applicable.
Consent to participate
Consent for publication.
All authors agreed with the content and gave explicit consent to publish this article.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Philippe Garrigues
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Arnob, M.H., Arham, M., Islam, R. et al. Scientific mapping of the research in microbial and chemical contamination of potable water in Bangladesh: A review of literature. Environ Sci Pollut Res 30 , 76421–76436 (2023). https://doi.org/10.1007/s11356-023-27853-x
Download citation
Received : 20 September 2022
Accepted : 19 May 2023
Published : 02 June 2023
Issue Date : July 2023
DOI : https://doi.org/10.1007/s11356-023-27853-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Microbial contamination
- Chemical contamination
- Potable water
- Arsenic mobilization
- Find a journal
- Publish with us
- Track your research
Physical, chemical and microbial analysis of bottled drinking water
Affiliation.
- 1 Department of Agricultural Chemistry, Faculty of Agriculture, University of Jaffna, Sri Lanka. [email protected]
- PMID: 23086026
- DOI: 10.4038/cmj.v57i3.4149
Introduction: People rely on the quality of the bottled drinking water, expecting it to be free of microbial contamination and health hazards.
Objectives: To evaluate the quality of bottled drinking water sold in Jaffna peninsula by analysing the physical, chemical and microbial contents and comparing with the recommended Sri Lankan Standard (SLS) values.
Methods: All bottled water samples sold in Jaffna peninsula were collected. Electrical conductivity, total dissolved solid, pH, calcium, nitrate, total aerobic and anaerobic count, coliform bacterial count and faecal contamination were checked.
Results: These are 22 brands of bottled drinking water sold in Jaffna peninsula. The sample had very low electrical conductivity when compared with SLS (750 μS/ cm) and varied from 19 to 253 μS/cm with the mean of 80.53 (±60.92) μS/cm. The pH values of the bottled drinking water brands varied from 4.11 to 7.58 with a mean of 6.2 (±0.75). The total dissolved solid content of the bottled drinking water brands varied from 9 to 123.67 mg/l with a mean of 39.5 (±30.23) mg/l. The calcium content of the bottled drinking water brands varied from 6.48 to 83.77 mg/l with a mean of 49.9 (±25.09) mg/l. The nitrate content of the bottled drinking water brands varied from 0.21 to 4.19 mg/l with the mean of 1.26 (±1.08) mg/l. Aerobic bacterial count varied from 0 to 800 colony forming unit per ml (cfu/ml) with a mean of 262.6 (±327.50) cfu/ml. Among the 22 drinking bottled water brands 14 and 9% of bottled drinking water brands showed fungal and coliform bacterial contaminants respectively. The water brands which contained faecal contamination had either Escherichia coli or Klebsiella spp.
Conclusions: The bottled drinking water available for sale do not meet the standards stipulated by SLS.
Publication types
- Evaluation Study
- Bacterial Load
- Calcium / analysis
- Consumer Product Safety / standards
- Drinking Water / analysis
- Drinking Water / standards*
- Electric Conductivity
- Enterobacteriaceae / isolation & purification*
- Nitrates / analysis
- Water Microbiology / standards*
- Drinking Water
- Open access
- Published: 03 November 2021
Systematic review on the microbiological quality of fresh vegetables and ready-to-eat salad in Nigeria
- Igba Profit 1 ,
- Adebayo Sami’a Yunus 1 ,
- Gbonjubola Olusesan Adeshina 1 ,
- Babajide Akinyele Tytler 1 ,
- Ahmed Babangida Suleiman 2 &
- Busayo Olalekan Olayinka 1
Bulletin of the National Research Centre volume 45 , Article number: 185 ( 2021 ) Cite this article
2624 Accesses
Metrics details
The consumption of fresh vegetables and salads has become popular, and because of a greater understanding of health benefits, these are most often eaten raw or with minimal processing.
Main body of the abstract
The microbiological safety of these vegetables is necessary and the possible source of contamination includes microbial contamination of raw produce, workers hygiene and the condition of the environment and equipment used to process the salad and fresh vegetable for distribution. This article reviewed the previously published literature on the microbiological quality of fresh vegetable and salad. There was 100% isolation of bacteria in all of the studies review which include Escherichia coli , Aspergillus spp., Staphyloccocus aureus , Salmonella , Klebsiella spp., Actinomycetes , Bacillus subtilis , Pseudomonas aeroginosa , Staphyloccocus epidermidis , Bacillus spp., Shigella spp., Lactobacillus and Streptoccocus spp.
Short conclusion
The review study recommended that fresh vegetables and salad should be properly washed with clean water before preparing.
World Health Organization (WHO) estimated more than 500 children died daily from the consumption of contaminated food and water (WHO 2015 ). It was reported that illnesses due to contaminated foods are an important cause of reduced economic productivity (Okonko et al. 2008 ). The incidence rate of foodborne diseases is also rising in, both developed and developing nations due to problems compounded by poverty, inadequate sanitary conditions and poor general hygiene (Udo et al. 2009 ).
Foodborne illnesses are associated with significant morbidity and mortality rates worldwide (Scallan et al. 2011 ). Globally, an estimated 2 million people died from diarrheal diseases in 2011 and approximately 70% of these are foodborne. It is estimated 30% of the population in Nigeria are affected by foodborne disease annually (WHO 2011 ).
Also in Africa, it was estimated that 92 million people fall ill from consuming contaminated foods, resulting in 137,000 deaths each year (Narayan et al. 2017 ). Foodborne illnesses are major threat to health of people in Nigeria. In 1997, Local Government Health Systems profile for Nigeria reported leading causes of deaths in different geo-political zones to foodborne associated illnesses, which accounted for 25% of mortality followed by malaria (21%) and accidents (19%), while the Federal Ministry of Health in 2007, 90,000 cases of food poisoning was reported (FAO/WHO 2008 ).
Although the full extent of the burden and cost of unsafe food is unknown, the impact on global health and development are considered to be immense. The incidences of foodborne pathogens have been studied in Nigeria with more than 90% of annual cases of food poisoning reported to be caused by Escherichia coli, Salmonella spp., Shigella spp., Proteus spp., Bacillus cereus, (Enabulele et al. 2010 ; Eni et al. 2010 ; Onyeneho and Hedberg 2013 ; Adekanle et al. 2015 ; Ajayi et al. 2017 ; Negbenebor et al. 2019 ).
Fresh vegetables served as essential components of healthy diet whose, consumption rates increased in recent years (Sararaj et al. 2014 ). However, fresh vegetables are also associated with some risks to consumers (Soltan et al. 2015 ). Greater awareness and desire for healthier life style have led to increase consumption of fresh vegetable and fruits. Vegetables are recognized as an important source of micronutrients, carbohydrates, antioxidants, minerals, vitamins and fibers (Sararaj et al. 2014 ). Major human pathogens are recognized to be transmitted via uncooked vegetables (Gu et al. 2011 ).
The production process, use of poor quality in irrigation of farm plots, use of animal manure to fertilize soil and poor labourer hygiene have contributed to spreading of contaminants (Golly et al. 2016 ).
A number of studies have reported the isolation of pathogenic organisms from fresh vegetables from different points of the world as, enteric pathogens from wide variety of produce including Listeria monocytogenes , Salmonella spp, and Escherichia coli ( E. coli ), Staphylococcus aureus ( S. aureus ) , Clostridium perfringes , Campylobacter jejuni and Campylobacter coli were reported in different regions of the world (Bukar et al. 2010 ; Eni et al. 2010 ; Denis et al. 2016 ; Golly et al. 2016 ).
The aim of this study is to review published articles on the microbiological quality of fresh vegetables and salad.
Literature search
Articles published between January 2000 and September 2019 were retrieved from Medline via Pubmed, Biomed, Ajol and google scholar database using the following search terms; “fresh vegetables, microbial quality, salad and vegetables”. The review was performed using the preferred reporting items for systematic review and Meta-analysis (PRISMA) statement.
Articles selection and data extraction
The titles and abstracts of all potential papers were assessed to ensure all studies had been identified and duplicates removed. Relevant data were extracted using a database that listed the variables; author, year of study, location of study, methods of identification, commonest microorganism isolated, vegetables fresh produce components, quantitative indicators of fresh vegetables contamination, and microbial count were summarized.
Data synthesis
Using the PRISMA statement, data were extracted and reported as outlined by authors without any alteration following the search, a total of 325 articles were identified, duplicates were removed and 309 records excluded because the microbial quantity was not determined as well as incompatible title and abstract. One article was excluded due to inability to access the journal and irrelevant outcome compared to the objectives. Altogether, fifteen articles were included in the final data synthesis.
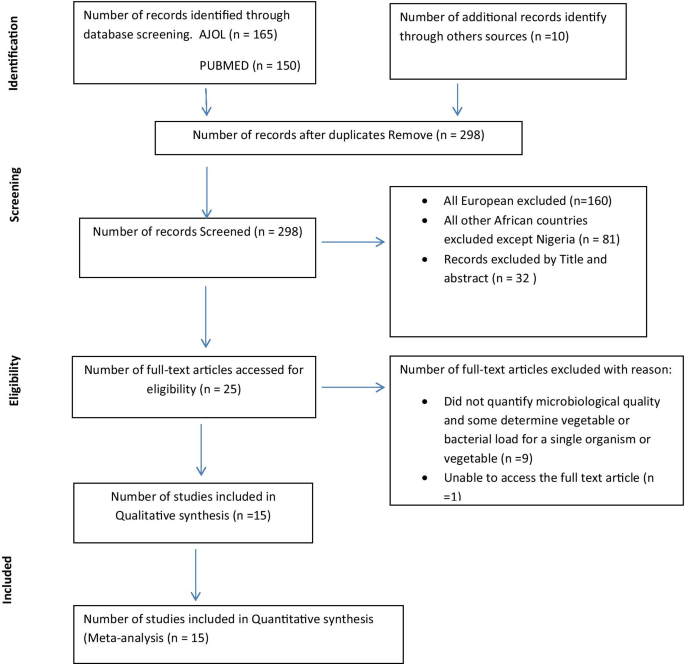
In seven previous studies, percentage of vegetable tested produced high bacterial load (Aboh et al. 2011 ; Eni et al. 2010 ; Adeshina et al. 2012 ; Owolabi 2013 ; Adekanle et al. 2015 ; Oji 2016 ; Negbenebor et al. 2019 ). Five studies indicated high bacterial load of contaminated vegetables without percentage of the microorganism (Uzeh et al. 2009 ; Abdullahi and Abdulkareem 2010 ; Oluwafemi et al. 2013 ; Nwankwo et al. 2015 ; Oji 2016 ). Only four studies analyzed in this review were able to isolate fungi (Uzeh et al. 2009 ; Oluwafemi et al. 2013 ; Adekanle et al. 2015 ; Nwankwo et al. 2015 ).
There was 100% isolation of bacteria in all of the studies analyzed which include Escherichia coli , Staphyloccocus aureus, Salmonella , Klebsiella spp., Bacillus subtilis, Actinomycetes , Pseudomonas aeroginosa , Staphyloccocus epidermidis , Bacillus spp., Shigella spp., Lactobacillus and Streptoccocus spp. Base on isolates obtained at individual fresh vegetables, cabbage (88.3%), cucumber (46.66%) and carrot (66.66%) from studies nine (Table 1 ).
Microorganisms found in salad and fresh vegetables explain the sanitary and hygienic quality of cultivation water, harvesting, transportation, storage and processing of products. All bacteria isolates reported in this review study have been previously isolated from salad vegetables and fresh vegetables in other studies basically in Nigeria. Various researches had different result methods documented; some quantify the bacterial load count in salad vegetables and fresh vegetables, while others quantify the microorganism isolated in percentage. Five studies of Uzeh et al. ( 2009 ), Abdullahi and Abdulkareem ( 2010 ), Eni et al. ( 2010 ), Oluwafemi et al. ( 2013 ) and Nwankwo et al. ( 2015 ) determined the bacterial load in each of these salads and fresh vegetables, i.e. lettuce, cabbage, cucumber and carrot. They only isolated the organism but did not determine the percentage. But Eni et al. ( 2010 ), Afolabi et al. ( 2011 ), Aboh et al. ( 2011 ), Adeshina et al. ( 2012 ), Osamwonyi et al. ( 2013 ), Wogu and Iwezeuna ( 2013 ), Owolabi ( 2013 ), Adekanle et al. ( 2015 ) and Negbenebor et al. ( 2019 ) determined the range of bacterial load and the percentage occurrence of the isolated microorganism which serves as an advantage over other studies.
The isolation of these organisms in the various studies is very disturbing as these samples were reported to be obtained from big fast food centre; most samples were supposedly ready to eat and others from the market. The high incidence of bacterial contamination of the ready to eat salad and fresh vegetables may be due to unhygienic practices. Restaurant staff may not observe basic sanitation requirement for processing products that required no pre-heating before consumption. Another reason may be the non-availability of water in good quantity and quality for washing of fresh vegetables and mass production of salad in big fast food centres. Based on Uzeh et al. ( 2009 ) and Oluwafemi et al. ( 2013 ), carrot was the more contaminated vegetable, followed by lettuce and cabbage was also high in three various studies (Abdullahi and Abdulkareem 2010 ; Eni et al. 2010 ; Nwankwo et al. 2015 ).
Conclusions
From the results obtained from the reviewed studies of the microbiological quality of fresh vegetables and ready-to-eat salad, it can be inferred that fresh vegetables and ready-to-eat salad may be contaminated with pathogenic or non-pathogenic microorganisms. Therefore, fresh vegetables and salad should be properly washed with clean water before preparing, maintenance of personnel and kitchen hygiene, during preparation of salad, fresh vegetables other food substance for meal.
Availability of data and materials
Not applicable in this section.
Abdullahi IO, Abdulkareem S (2010) Bcaterial uality of some ready-to-eat vegetable as retailed and consumed in Sabo-Gari, Zaria, Nigeria. Bayero J Pure Appl Sci 3(1):173–175
Google Scholar
Aboh MI, Oladosu P, Ibrahim K (2011) Bacterial contamination of salad vegetables in Abuja municipal area Council, Nigeria. Malays J Microb 7(2):111–114
Adekanle MA, Effedua HI, Oritogun KS, Adesiji YO, Ogunledum A (2015) A study of microbial analysis offresh fruit and vegetablein sagamu markets South-West, Nigeria. Agroserach 15:21–22
Adeshina GO, Samuel DJ, Victor EG (2012) Antibacterial susceptibility pattern of pathogenic bacteria isolates from vegetables salad sold in restaurant in Zaria. J Microb Res 2(2):5–11
Article Google Scholar
Afolabi OR, Oloyede AR, Ibrahim TA (2011) Evaluation of pathogenic bacteria associated with fresh produce obtained from selected market in Abeokuta. J Sci Sustain Dev 4:75–81
Ajayi OA, Amokeodo MI, Akinwunmi OO (2017) Microbial quality of selected ready-to eat vegetables from Iwo, Nigeria and effectiveness of rinsing agent. Appl Trop Agric 22(2):131–137
Bukar A, Uba A, Oyeyi TI (2010) Occurrence of some enteropathogenic bacteria in some minimally and fully processed ready-to-eat foods in kano metropolis, Nigeria. Afr J Food Sci 4(2):032–036
Denis N, Zhang H, Leroux A, Trudel R, Bietlot H (2016) Prevalence and trends of bacterial contamination in fresh fruits and vegetables sold at retail in Canada. Food Control 67:225–234
Enabulele SA, Amune PO, Aborisade TA (2010) Antibiograms of Salmonella isolates from poultry farms in Ovia North East Local Government Area, Edo State, Nigeria. Agric Biol J N Am 1:1287–1290
Eni OA, Oluwawemitan IA, Solomon OU (2010) African microbial quality of fruits and vegetables sold in Sango Ota, Nigeria. J Food Sci 4(5):291–296
Food and Agriculture Organization and World Health Organization (2008) The Nigerian experience on food safety regulations. In: FAO/WHO global forum of food safety regulations
Golly MK, Salifu PS, Mills-Robertson FC (2016) Resistance of bacteria isolates from cabbage ( Brassica oleracea ), carrots ( Daucus carota ) and lettuce ( Lactuca sativa ) in the Kumasi Metropolis of Ghana. Int J Nutr Food Sci 5:297–303
Article CAS Google Scholar
Gu G, Jiahuai HU, Juau MC, Susanna MR, Jerry A (2011) International colonization of Salmonella enterica serovar typhimurium tomatoes plant. PLoS ONE 6(11):e27340
Article ADS CAS Google Scholar
Narayan P, Victor BA, Djidjoh JH, Maitshwarelo M (2017) Prevalence of foodborne pathogens in food from selected African countries—a meta-analysis. Int J Food Microb 249:35–43
Negbenebor HE, Marami FM, Nura S (2019) Prevalence of bacterial load on some fruit and vegetables solg in Kaduna central Market, North-weastern Nigeria. J Appl Sci 19(1):20–24
Nwankwo IU, Eze VC, Onwuakor CE, Friday JU (2015) Evaluation of the degree of contamination of salad vegetable sold in Umuahia main market. Am J Microb Res 3:41–44
Oji PC (2016) Bacteriological analysis of salad vegetable in Eke Awka Mraket Anambra State, Nigeria. Int J Sci Res Publ 6:305–312
Oluwafemi F, Akisanya E, Odeniyi K, Salami W, Sharomi T (2013) Microbiologica quality of stree-vened food and ready-to-eat Vegetables in some Nigeria Cities, Africa. J Biomed Res 16:163–166
Okonko IO, Ogunjobi AA, Fajobi EA, Onoja BA, Babalola ET, Adedeji AO (2008) Comparative studies and microbial risk assessment of different ready-to-eat (RTE) frozen sea-foods processed in Ijora-Olopa, Lagos State, Nigeria. Afr J Biotechnol 7(16):2898–2901
Onyeneho SN, Hedberg CW (2013) An assessment of food safety needs of restaurants in Owerri, Imo State, Nigeria. Int J Environ Res Public Health 10:3296–3309
Osamwonyi OU, Obayagbona ON, Aborishade W, Folisaka. (2013) Bacteriological quality of vegetable salads sold at restaurant within Okada Town, Edo State, Nigeria. Afr J Basic Appl Sci 5(1):37–41
Owolabi OJ (2013) Active ileum relaxant fraction from the leaves of Ficus capensis Thunb (Moraceae), Nigeria. J Pharm Sci 12(1):1–7
MathSciNet Google Scholar
Sararaj P, Stella D, Reetha D (2014) Microbial spoilage of vegetable and its control measures. Int J Nat Prod Sci 2(2):1–12
Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL (2011) Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 17:7–15
Soltan MM, Dalla M, Shojaei MK, Sharifi Y, Vahedi S (2015) Microbial contamination of fresh vegetables and salad samples consumed in Tehran, Iran. J Food Qual Hazard Control 2:139–143
Udo S, Andy I, Umo A, Ekpo M (2009) Potential Human pathogen (Bacteria) and their antibiogram in ready to eat salad sold in Calabar, South-South, Nigeria. Internet J Trop Med 5(2):1
Uzeh RE, Alade FA, Bankole M (2009) THE Microbial quality of Pre-packed mixed vegetables salad in some retail outlets in Lagos, Nigeria. Afr J Food Sci 3(9):270–272
CAS Google Scholar
Wogu MD, Iwezeuna I (2013) Microbial quality of ready to eat salad sold Benni City, Souther Nigeria. Int J Sci Technol 2(2):26–38
World Health Organization [WHO] (2011) Initiative to estimate the Global Burden of Foodborne Diseases: Information and publications. Retrieved June 26, from http://www.who.int/foodsafety/foodborne_disease/ferg/en/index7.html (2011).
World Health Organization (WHO) (2015) Foodborne disease Fact sheet N°330". http://www.who.int/mediacentre/factsheets/fs330/en/ .
Download references

Acknowledgements
We wish to express our profound gratitude to the management of Ahmadu Bello University, Zaria for their unreserved support toward this research work and as well as the Ahmadu Bello University Pharmaceutical Microbiology staff for their support rendered.
Author information
Authors and affiliations.
Department of Pharmaceutics and Pharmaceutical Microbiology, Ahmadu Bello University, Zaria, Nigeria
Igba Profit, Adebayo Sami’a Yunus, Gbonjubola Olusesan Adeshina, Babajide Akinyele Tytler & Busayo Olalekan Olayinka
Department of Microbiology, Ahmadu Bello University, Zaria, Nigeria
Ahmed Babangida Suleiman
You can also search for this author in PubMed Google Scholar
Contributions
This review article intends to discuss on the potential risk of contamination of vegetables. IP—wrote the second and final draft, SY—wrote the first draft, GO—review manuscript, AB—review manuscript and BO—conceive the Idea and review the manuscript. All the authors’ general statement was good. All authors have read and approved the manuscript.
Corresponding author
Correspondence to Igba Profit .
Ethics declarations
Ethics approval and consent to participate.
Ethical clearance was obtained from Ahmadu Bello University Zaria, Kaduna State. (ABUCUHSR/2019/002). Name of ethical committee in Ahmadu Bello University Zaria, Nigeria. 1. Prof. I.H. Nock- Chairman of ABUCUHSR. 2. Dr. M.K. Lawan- Member of Committee. 3. Prof. G.O. Adeshina- Chairman of supervisor team and committee member.
Consent for publication
Competing interests.
The authors have no competing interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Profit, I., Yunus, A.S., Adeshina, G.O. et al. Systematic review on the microbiological quality of fresh vegetables and ready-to-eat salad in Nigeria. Bull Natl Res Cent 45 , 185 (2021). https://doi.org/10.1186/s42269-021-00633-8
Download citation
Received : 22 March 2021
Accepted : 05 October 2021
Published : 03 November 2021
DOI : https://doi.org/10.1186/s42269-021-00633-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Fresh vegetable and salad
- Microbiological quality
- Contamination
- Open access
- Published: 20 April 2024
Microbiological features of drowning-associated pneumonia: a systematic review and meta-analysis
- Vladimir L. Cousin 1 &
- Laure F. Pittet 2
Annals of Intensive Care volume 14 , Article number: 61 ( 2024 ) Cite this article
155 Accesses
2 Altmetric
Metrics details
Drowning-associated pneumonia (DAP) is frequent in drowned patients, and possibly increases mortality. A better understanding of the microorganisms causing DAP could improve the adequacy of empirical antimicrobial therapy. We aimed to describe the pooled prevalence of DAP, the microorganisms involved, and the impact of DAP on drowned patients.
Systematic review and meta-analysis of studies published between 01/2000 and 07/2023 reporting on DAP occurrence and microorganisms involved.
Of 309 unique articles screened, 6 were included, involving 688 patients. All were retrospective cohort studies, with a number of patients ranging from 37 to 270. Studies were conducted in Europe (France N = 3 and Netherland N = 1), United States of America (N = 1) and French West Indies (N = 1). Mortality ranged between 18 to 81%. The pooled prevalence of DAP was 39% (95%CI 29–48), similarly following freshwater (pooled prevalence 44%, 95%CI 36–52) or seawater drowning (pooled prevalence 42%, 95%CI 32–53). DAP did not significantly impact mortality (pooled odds ratio 1.43, 95%CI 0.56–3.67) but this estimation was based on two studies only. Respiratory samplings isolated 171 microorganisms, mostly Gram negative (98/171, 57%) and mainly Aeromonas sp. (20/171, 12%). Gram positive microorganisms represented 38/171 (22%) isolates, mainly Staphylococcus aureus (21/171, 12%) . Water salinity levels had a limited impact on the distribution of microorganisms, except for Aeromonas sp. who were exclusively found following freshwater drowning (19/106, 18%) and never following seawater drowning (0%) (p = 0.001). No studies reported multidrug-resistant organisms but nearly 30% of the isolated microorganisms were resistant to amoxicillin-clavulanate, the drug that was the most commonly prescribed empirically for DAP.
Conclusions
DAP are commonly caused by Gram-negative bacteria, especially Aeromonas sp. which is exclusively isolated following freshwater drowning. Empirical antimicrobial therapy should consider covering them, noting than amoxicillin-clavulanate may be inadequate in about one-third of the cases. The impact of DAP on patients’ outcome is still unclear.
Introduction
Drowning is defined as a respiratory impairment following immersion or submersion of the airways in a liquid, typically water [ 1 ]. The ensuing hypoxemia and cardiac arrest carry a high mortality rate even with a brief period of immersion [ 1 ]. In survivors, aspiration of water in the alveoli causes surfactant dysfunction and washout, leading to diffuse alveolar damage and pulmonary edema. In 12% to 51% of the cases [ 2 , 3 , 4 ], survivors develop drowning-associated pneumonia (DAP), following the inhalation of contaminated water, endogenous flora or gastric content [ 1 , 5 ]. DAP significantly impacts patient’s evolution, with prolonged mechanical ventilation and possibly higher mortality rate [ 2 ].
Limited data is available on microorganisms causing DAP; several factors can influence the microbial composition of contaminated water, including its chemical composition, geographic location and salinity level [ 6 ]. Ignoring the microorganisms causing DAP can adversely affect patients’ outcomes, through the administration of inappropriate empirical antimicrobial treatments. Indeed, a fair proportion of the microorganisms isolated in DAP are intrinsically resistant to the antimicrobials agents commonly recommended for community-acquired inhalation pneumonia. As suboptimal empirical antimicrobial therapy may lead to an unfavorable outcome, a better understanding of the causative microorganisms is needed [ 3 ].
The study aims to summarize the current knowledge on the ecology of microorganisms involved in DAP, and to assess the repercussion of DAP on patient outcomes.
Data sources and search strategy
Pubmed and EMBASE database were searched in August 2023 for relevant peer-reviewed articles, published in English or French, between January 2000 and July 2023, with no age restriction, in accordance with the PRISMA guidelines [ 7 ]. The following items were used for searches: (drowning associated pneumonia); (near-drowning associated pneumonia); (drowning AND pneumonia); (near-drowning AND pneumonia); (drowning AND microbiology). The references of all relevant publications were reviewed, and no further articles were identified.
One reviewer (VLC) screened the titles and abstracts to determine eligibility. Inclusion criteria were the following: studies reporting microbiological data on 10 humans or more who had survived drowning and later developed a DAP while being in intensive care units (ICU). Reviews and isolated case reports were excluded.
Data collection
The following baseline data was extracted: year of publication; geographical setting; severity of drowning (i.e. occurrence of pre-admission cardiac arrest, requirement of mechanical ventilation, occurrence of acute respiratory distress syndrome [ 8 ], and admission Simplified Acute Physiology Score II (SAPS II) [ 9 ] and/or Sequential Organ Failure Assessment (SOFA) [ 10 ]); patients outcome (i.e. mortality, and duration of mechanical ventilation); water location (i.e. sea, lake, river, damp, pond, swimming pool, or miscellaneous) and salinity (i.e. seawater or freshwater).
Confirmed DAP was established by microbiology. The variables of interest for DAP included: numbers of respiratory samples; proportion of positive samples; type of microorganisms isolated; number of positive individual microorganisms isolated in respiratory samples; technic of respiratory sampling (i.e. broncho-alveolar lavage (BAL), protected specimen brush, tracheal aspirates, or sputum); antimicrobials used for empirical therapy; antimicrobial resistance in the microorganisms isolated.
Statistics analysis
Descriptive statistics were used: continuous variables were reported as median (interquartile range IQR) and categorical variables as proportion (%). Microorganisms isolated from patients with seawater DAP were compared to those isolated from patients with freshwater DAP using a Fisher’s exact test. Stata v18 meta-analysis software pack was used to calculate the pooled prevalence of DAP (overall, following freshwater drowning and following seawater drowning) and to calculate the combined mortality odds ratio following DAP. Heterogeneity was assessed using the I 2 statistics. High heterogeneity was defined as a I 2 > 50%. In case of high heterogeneity, random-effect analyses were done and presented using forest plots. Stata v18 (StataCorp, College Station, TX, USA) was used for graphical and statistical analyses.
Review and population
Of 309 unique articles, 6 studies were included, with a period of publication ranging from 2012 to 2023 (Fig. 1 ) [ 2 , 3 , 11 , 12 , 13 , 14 ]. Their main characteristics are detailed in Table 1 . All were retrospective cohort studies, with a number of patients ranging from 37 to 270. Studies were conducted in Europe (France N = 3 and Netherland N = 1), United States of America (N = 1) and French West Indies (N = 1). Four studies included exclusively adult patients [ 2 , 3 , 11 , 14 ], one included a mixed population of adult and pediatric patients [ 12 ] and the last one included only pediatric patients [ 13 ].
Preferred reporting items for systematic reviews and meta-analyses flow diagram
A total of 688 patients were available for analysis. Location of drowning included sea (N = 393), swimming pool (N = 127), river (N = 55), pond (N = 30), bathtub (N = 19), other water source (N = 9), lake (N = 5) and swamp (N = 1). Location of drowning was not documented in 49 cases. As depicted in Table 1 , a high proportion of patients presented pre-admission cardiac arrest, ranging from 38 to 78%, and the overall outcome was poor with mortality rate ranging between 18 and 81%.
Drowning-associated pneumonia
Five of the 6 studies reported the prevalence of DAP, ranging between 24 to 51%; the criteria they used to diagnose DAP are summarized in Table 2 . The pooled prevalence of DAP was 39% (95% CI 29–48) (ι 2 0.01) (Figure 2 ) and was not influenced by the water salinity level, with similar pooled prevalence following freshwater DAP (44%, 95% CI 36–52) or seawater DAP (42%, 95% CI 32–53) (Additional file 1 : Figure S1).
Use of prophylactic antibiotics was not reported to be a routine procedure in any of the 6 studies. When DAP was suspected, various empirical antimicrobial therapies were used, predominately amoxicillin-clavulanate (Table 3 ). Only 3/6 studies evaluated the adequacy of the empirical antimicrobial therapy for the isolated microorganism, ranging from 50 to 89% [ 2 , 3 , 14 ] .
Forrest plot of included studies reporting prevalence of drowning associated pneumonia DAP drowning associated pneumonia
The impact of DAP on patient’s outcome was reported inconsistently across studies, with only 2 studies reporting individual-level data enabling to estimate the impact of DAP on patient outcome [ 2 , 12 ]. With a total of 414 patients and 136 DAP, the meta-analysis suggests a negative impact of DAP on survival, although not statistically significant (pooled odds ratio 1.43, 95% CI 0.56–3.67) (Fig. 3 ).
Forrest plot of included studies reporting impact of DAP on patients’ mortality rate DAP drowning associated pneumonia; Surv survivor
Isolated microorganisms
A total of 171 microorganisms were isolated from 167 respiratory samples (including bronchoalveolar lavage (30%), protected specimen brush (9%), tracheal aspirates (35%), sputum (3%), and 24% not documented), as detailed in Table 4 . Gram-negative were predominant (N = 98/171 (57%), primarily Aeromonas sp. (N = 20/171 (12%)), Haemophilus influenzae (N = 19/171 (11%)) and Pseudomonas aeruginosa (N = 12/171 (7%)). Gram positive followed with 38/171 (22%) isolates, mainly Staphylococcus aureus (N = 21/171 (12%)). Multiples germs were isolated in 10% of samples. Fungi were detected in a minority of samples with only 2/171 Candida sp. (1%) and 1/171 (0.5%) Aspergillus sp.
Microorganisms were compared according to the water salinity level (Table 4 ). The proportion of Gram positive and Gram negative was similar across both types of water. Aeromonas sp. was exclusively detected following freshwater drowning, with 19/106 (18%) of positive samples, and never following seawater drowning (0%) (p value 0.001). Enterobacter sp. were more frequently detected following seawater drowning (6/43) compared to freshwater drowning (3/106; p value 0.01). Fungi were exclusively isolated following freshwater drowning.
Antimicrobial therapy resistance and adequacy
Studies did not report systematically on antibiotic resistance. Three studies reported on the proportion of microorganisms being resistant to amoxicillin-clavulanate: 31% in Robert et al . [ 3 ], 36.4% in Reizine et al . [ 2 ] and 31.6% in Tadié et al . [ 14 ]. One study reported the prevalence of cefotaxime resistance to be 12% [ 2 ]. No studies reported on the presence of multidrug-resistant microorganisms.
Studies did not consistently report on the inadequacy of antimicrobial therapy and its consequences; however, some interesting results were mentioned by two studies. Reizine et al . reported a mortality rate of 7/10 (70%) among the patients who received inadequate antimicrobial therapy, whereas the global mortality rate in that study was 20% [ 2 ]. In the publication from Tadié et al . the mortality rate was 2/6 among the patients who received inadequate antimicrobial therapy, whereas the global mortality rate in that study was 81% [ 14 ].
In this systematic review, we assessed the impact of DAP on nearly 700 patients admitted to ICU following drowning. A variety of microorganisms were isolated, irrespective of the water salinity level, apart from Aeromonas sp. and fungi that were exclusively isolated following freshwater drowning. As the empirical antibiotic therapy used was usually not targeting the isolated microorganisms, our findings highlight the importance of early bacterial samplings in drowned patients, as inadequate treatment is likely to impact the patients’ outcome.
Drowning represents one of the leading causes of accidents worldwide and carries a high mortality rate [ 15 ]. Patients surviving the initial drowning event are often admitted to ICU and are at risk of secondary respiratory complications such as DAP. Historically, DAP prevalence was reported to be between 11% and 54% [ 4 , 16 , 17 , 18 ], in line with our updated estimate of 39%. Importantly, not all patients developed DAP, possibly owing to multiple factors, including: different microorganism load, varying immersive liquid chemical composition, occurrence of laryngospasm preventing aspiration, and the nature of drowning (i.e. primary or secondary to seizure, syncope, arrhythmia, or trauma) [ 1 , 6 ].
Whether DAP occurrence increases the mortality rate is still unknown. In the past, the mortality rate in patients with DAP ranged between 26% and 60%, whilst recent studies report a mortality rate of approximately 28% [ 2 , 4 , 6 , 12 ]. We found conflicting results in our review, with a non-significant trend for an impact of DAP on mortality rate. Cerland et al . reported similar mortality rates among patients with or without DAP, while Reizine et al . suggested a detrimental impact of DAP on patient outcome [ 2 , 12 ]. DAP can lead to hypotension, hypoxemia and temperature instabilities, all recognized as factors worsening patient outcome following a cardiac arrest [ 19 , 20 ]. Moreover, the intricated influence of inflammation triggered by DAP and the consequence of an inflamed lung may have on brain lesions might also play a significant role in affecting the patient outcome [ 20 , 21 ]. However, other factors may have more impact on patient’s outcome, such as pre-admission cardiac arrests, or patients’ comorbidities. Similarly, a study showed that the reduction of early ventilator-associated pneumonia occurrences in post-cardiac arrest patients did not improve the mortality rate or the duration of mechanical ventilation [ 22 ].
We underscore the high prevalence of Gram-negative bacteria, both in freshwater and seawater, as historically described [ 4 , 6 ]. The high incidence of Enterobacter sp. and other coliform bacteria could be explained by water fecal contamination [ 23 ]. Identification of those microorganisms strongly suggests the inhalation of contaminated water. In addition, a large number of samples suggest aspiration of oro-pharyngeal secretions ( Streptococcus pneumonia , Staphylococcus aureus , Haemophilus influenza ). Those results underline the role of aspiration, from both water or secretion, as a source for bacterial inoculum in DAP.
Importantly, Aeromonas sp . was the main germ isolated following freshwater drowning. This microorganism has several chromosomal beta-lactamases, which can impact DAP management trough reduced susceptibilities to antimicrobial agents, such as amoxicillin-clavulanate (only 16% susceptible isolates in a report), the most commonly used antimicrobial agent for empirical treatment [ 24 , 25 , 26 ]. However, most of Aeromonas sp. may remain susceptible to cefepime or piperacillin-tazobactam [ 25 ]. Despite its aquatic tropism, Pseudomonas aeruginosa was isolated in less than 10% of the samples. The density of Pseudomonas spp. colony in water is highly variable and may be very low in surface waters of natural water area, while contamination may be significant in recreational waters such as swimming pools [ 27 ]. Noticeably, fungal or anaerobic identification was rare. However, in special circumstances such as natural disasters, high incidence of Aspergillus sp. has been reported [ 28 ]. Considering those germs in these specific situations seems to be a practical approach to adopt [ 11 ].
The dilemma of whether empirical antimicrobial therapies are indicated at admission of drowned patient remains unresolved, but most guidelines discourage using them systematically [ 4 , 29 , 30 ]. A practical approach would be to restrain the use of such antimicrobial in drowned patients, with the exception of drowning occurring in highly contaminated environments (e.g. septic tank, manure pit) or in patients presenting severe lung lesions. As only a third of patients may develop a DAP, early respiratory sampling seems reasonable when DAP is suspected, as it has been shown to be effective to reduce antimicrobial prescription in patients with aspiration pneumonia and may help to guide antimicrobial therapy or help cease it [ 31 ]. Respiratory samplings will enable to rapidly identify the causative microorganisms and its antimicrobial susceptibilities. As the main isolated germs are Gram-negative, including Aeromonas spp. or Pseudomonas spp., close follow-up of antimicrobial susceptibilities is crucial as clinicians may encounter resistant microorganisms causing DAP. Antimicrobial resistance in the environment could be frequent through acquiring and sharing antibiotic resistance genes, in addition to natural resistance [ 32 ]. When antibiotic treatment cannot be delayed, piperacillin-tazobactam or a 4th generation cephalosporin could be suggested as first-line treatment, since inadequate antimicrobial therapy seems to carry a high risk of adverse outcome, as mentioned in the reviewed studies [ 2 , 3 , 11 , 14 ]. Importantly, antimicrobial therapy should always be tailored to local microbiological ecology and de-escalation performed as soon as possible.
It is important to note that diagnosis of DAP remains difficult as numerous criteria used for its definition can be confounded by concurring events, similarly to ventilator associated pneumonia [ 33 ]. The use of controlled temperatures after a cardiac arrest may mask any sign of hypo or hyperthermia linked to an infection, as illustrated by Reizine et al. who reported a median body temperature of 38.1 °C (IQR 35.6–38.7) at DAP diagnosis [ 2 ]. Interpreting radiological findings can be challenging in presence of lung damage and difficult to differentiate DAP from cardiogenic pulmonary edema, atelectasis and non-infective acute lung injury related to submersion [ 5 ]. In addition, inflammatory markers may be less useful following cardiac arrest as they will be deranged by the ischemia–reperfusion syndrome [ 34 ]. All these considerations highlight the importance of maintaining a low threshold for respiratory samplings in drowned patients, as it serves as a crucial criterion to initiating treatment and will be paramount to adjust the antimicrobial therapy.
Limitations
Our study has several limitations, including publication bias. All included studies were retrospectives; they differed in their methodology and their population in terms of proportion of pre-hospital cardiac arrests, severity score at admission, and whether they included drowned and/or nearly-drowned patients. The results may not apply to all geographic areas, especially tropical and/or warm temperature waters (only 1/6 studies take place in a tropical area, the one from the French West Indies). Moreover, meta-analyses on the impact of DAP on patients outcome could only be performed by 2 studies. Not all patients with DAP had a respiratory sampling done, and the microorganisms identified are not necessarily the causative agents of DAP. Finally, as mentioned above, diagnosis of pneumonia remains difficult in this population and some patients may have drowning-induced pulmonary damage misdiagnosed as DAP.
This study provides important information on DAP ecology, emphasizing the predominant role of Gram-negative bacteria and Aeromonas sp. who are commonly resistant to the antimicrobial frequently used empirically. As amoxicillin-clavulanate does not cover the microorganisms commonly isolated, piperacillin-tazobactam or a 4th generation cephalosporin could be more suitable for empirical treatment. When empirical therapy is required, respiratory sampling should be performed, and potential resistance should be investigated. Future studies are needed to investigate the impact of DAP on patient outcome and the role of an early antimicrobial therapy in drowned patients.
Availability of data and materials
Data are available on reasonable request.
Abbreviations
- Intensive care unit
Interquartile range
Simplified acute physiology score II
Sequential organ failure assessment
Szpilman D, Bierens JJ, Handley AJ, Orlowski JP. Drowning. N Engl J Med. 2012;366(22):2102–10.
Article CAS PubMed Google Scholar
Reizine F, Delbove A, Tattevin P, Santos AD, Bodenes L, Bouju P, et al. Clinical and microbiological features of drowning-associated pneumonia: a retrospective multicentre cohort study. Clin Microbiol Infect. 2023. https://doi.org/10.1016/j.cmi.2022.07.027 .
Article PubMed PubMed Central Google Scholar
Robert A, Danin PE, Quintard H, Degand N, Martis N, Doyen D, et al. Seawater drowning-associated pneumonia: a 10-year descriptive cohort in intensive care unit. Ann Intensive Care. 2017;7(1):45.
van Berkel M, Bierens JJ, Lie RL, de Rooy TP, Kool LJ, van de Velde EA, et al. Pulmonary oedema, pneumonia and mortality in submersion victims; a retrospective study in 125 patients. Intensive Care Med. 1996;22(2):101–7.
Article PubMed Google Scholar
Gregorakos L, Markou N, Psalida V, Kanakaki M, Alexopoulou A, Sotiriou E, et al. Near-drowning: clinical course of lung injury in adults. Lung. 2009;187(2):93–7.
Ender PT, Dolan MJ. Pneumonia associated with near-drowning. Clin Infect Dis. 1997;25(4):896–907.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–33.
Google Scholar
Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–63.
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996. https://doi.org/10.1007/BF01709751 .
Assink-de Jong E, Douma M, Beishuizen A, Hoogewerf M, Debets-Ossenkopp YJ, de Waard MC, et al. Microbiological findings and adequacy of antibiotic treatment in the critically ill patient with drowning-associated pneumonia. Intensive Care Med. 2014;40(2):290–1.
Cerland L, Megarbane B, Kallel H, Brouste Y, Mehdaoui H, Resiere D. Incidence and consequences of near-drowning-related pneumonia—a descriptive series from martinique. French West Indies: Int J Environ Res Public Health; 2017. https://doi.org/10.3390/ijerph14111402 .
Book Google Scholar
Moffett BS, Lee S, Woodend K, Sigdel B, Dutta A. Evaluation of antimicrobial utilization in the pediatric drowning population. J Pediatric Infect Dis Soc. 2021;10(2):179–82.
Tadie JM, Heming N, Serve E, Weiss N, Day N, Imbert A, et al. Drowning associated pneumonia: a descriptive cohort. Resuscitation. 2012;83(3):399–401.
Mortality GBD, Causes of Death C. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015. https://doi.org/10.1016/S0140-6736(14)61682-2 .
Article Google Scholar
Kennedy GA, Kanter RK, Weiner LB, Tompkins JM. Can early bacterial complications of aspiration with respiratory failure be predicted? Pediatr Emerg Care. 1992;8(3):123–5.
Lee KH. A retrospective study of near-drowning victims admitted to the intensive care unit. Ann Acad Med Singap. 1998;27(3):344–6.
CAS PubMed Google Scholar
Oakes DD, Sherck JP, Maloney JR, Charters AC 3rd. Prognosis and management of victims of near-drowning. J Trauma. 1982;22(7):544–9.
Hirsch KG, Abella BS, Amorim E, Bader MK, Barletta JF, Berg K, et al. Critical care management of patients after cardiac arrest: a scientific statement from the American heart Association and neurocritical care Society. Neurocrit Care. 2023. https://doi.org/10.1007/s12028-023-01871-6 .
Sandroni C, Cronberg T, Sekhon M. Brain injury after cardiac arrest: pathophysiology, treatment, and prognosis. Intensive Care Med. 2021;47(12):1393–414. https://doi.org/10.1007/s12028-023-01871-6 .
Article CAS PubMed PubMed Central Google Scholar
Mai N, Miller-Rhodes K, Knowlden S, Halterman MW. The post-cardiac arrest syndrome: a case for lung–brain coupling and opportunities for neuroprotection. J Cereb Blood Flow Metab. 2019;39(6):939–58.
Francois B, Cariou A, Clere-Jehl R, Dequin PF, Renon-Carron F, Daix T, et al. Prevention of early ventilator-associated pneumonia after cardiac arrest. N Engl J Med. 2019;381(19):1831–42.
Korajkic A, McMinn BR, Harwood VJ. Relationships between microbial indicators and pathogens in recreational water settings. Int J Environ Res Public Health. 2018. https://doi.org/10.3390/ijerph15122842 .
Janda JM, Abbott SL. The genus Aeromonas: taxonomy, pathogenicity, and infection. Clin Microbiol Rev. 2010;23(1):35–73. https://doi.org/10.3390/ijerph15122842 .
Aravena-Roman M, Inglis TJ, Henderson B, Riley TV, Chang BJ. Antimicrobial susceptibilities of Aeromonas strains isolated from clinical and environmental sources to 26 antimicrobial agents. Antimicrob Agents Chemother. 2012;56(2):1110–2.
Fernandez-Bravo A, Figueras MJ. An Update on the Genus Aeromonas : taxonomy, epidemiology, and pathogenicity. Microorganisms. 2020. https://doi.org/10.3390/microorganisms8010129 .
Mena KD, Gerba CP. Risk assessment of Pseudomonas aeruginosa in water. Rev Environ Contam Toxicol. 2009;201:71–115. https://doi.org/10.3390/microorganisms8010129 .
Benedict K, Park BJ. Invasive fungal infections after natural disasters. Emerg Infect Dis. 2014;20(3):349–55.
Wood C. Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary. BET 1: prophylactic antibiotics in near-drowning. Emerg Med J. 2010. https://doi.org/10.1136/emj.2010.094920 .
Thom O, Roberts K, Devine S, Leggat PA, Franklin RC. Treatment of the lung injury of drowning: a systematic review. Crit Care. 2021;25(1):253.
Lascarrou JB, Lissonde F, Le Thuaut A, Bachoumas K, Colin G, Henry Lagarrigue M, et al. Antibiotic therapy in comatose mechanically ventilated patients following aspiration: differentiating pneumonia from pneumonitis. Crit Care Med. 2017;45(8):1268–75.
Baquero F, Martinez JL, Canton R. Antibiotics and antibiotic resistance in water environments. Curr Opin Biotechnol. 2008;19(3):260–5.
Pouly O, Lecailtel S, Six S, Preau S, Wallet F, Nseir S, et al. Accuracy of ventilator-associated events for the diagnosis of ventilator-associated lower respiratory tract infections. Ann Intensive Care. 2020;10(1):6.
Mongardon N, Lemiale V, Perbet S, Dumas F, Legriel S, Guerin S, et al. Value of procalcitonin for diagnosis of early onset pneumonia in hypothermia-treated cardiac arrest patients. Intensive Care Med. 2010;36(1):92–9.
Download references
Acknowledgement
Not applicable.
Open access funding provided by University of Geneva.
Author information
Authors and affiliations.
Intensive Care Unit, Department of Pediatric, Gynecology and Obstetrics, University Hospital of Geneva, University of Geneva, Rue Gabrielle-Perret-Gentil 4, 1206, Geneva, Switzerland
Vladimir L. Cousin
Infectious Diseases, Immunology and Vaccinology Unit, Department of Pediatric, Gynecology and Obstetrics, University Hospital of Geneva, University of Geneva, Geneva, Switzerland
Laure F. Pittet
You can also search for this author in PubMed Google Scholar
Contributions
VLC and LFP conceived and designed the study, VLC collected the data, VLC and LFP interpreted the data, VLC and LFP wrote the first draft of the article. All authors revised the manuscript and approved the final version of the manuscript.
Corresponding author
Correspondence to Vladimir L. Cousin .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:.
Table. S1. Forrest plot of included studies reporting prevalence of drowning associated pneumonia, depending on the salinity of the water DAP drowning associated pneumonia
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Cousin, V.L., Pittet, L.F. Microbiological features of drowning-associated pneumonia: a systematic review and meta-analysis. Ann. Intensive Care 14 , 61 (2024). https://doi.org/10.1186/s13613-024-01287-1
Download citation
Received : 01 September 2023
Accepted : 02 April 2024
Published : 20 April 2024
DOI : https://doi.org/10.1186/s13613-024-01287-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Drowning associated pneumonia
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.6(8); 2020 Aug

Assessment of bacteriological quality and physico-chemical parameters of domestic water sources in Samaru community, Zaria, Northwest Nigeria
Taiwo adekanmi adesakin.
a Department of Biology, Faculty of Life Sciences, Ahmadu Bello University, Zaria, Nigeria
Abayomi Tolulope Oyewale
b Institute of Ecology and Environmental Studies, Faculty of Science, Obafemi Awolowo University, Ile-Ife, Nigeria
Umar Bayero
Abubakar ndagi mohammed, iduwo adedeji aduwo.
d Department of Zoology, Faculty of Science, Obafemi Awolowo University, Ile-Ife, Nigeria
Precious Zubeidat Ahmed
Niima dalhata abubakar, ibrahim balkisu barje.
The quality of water supply is assessed by its physico-chemical and bacteriological properties. This study was carried-out with the aim of determining the contamination level of domestic water sources of Samaru community, Zaria, Northcentral Nigeria in order to observed the trend of change in quality of these water sources, if any. This was with a view to safeguard the public health of the riparian users against a possible outbreak of water borne diseases. Water samples were collected and analyzed for bacteriological and physicochemical quality using standard procedures. The results showed that the mean values recorded for physico-chemical parameters among the domestic water sources were within stipulated limits of WHO for safe drinking water except for chloride mean value of 314 ± 142.4 mg/L recorded in borehole water. The total heterotrophic bacterial counts recorded in tap, borehole, well, reservoir and river water samples (3.67 × 10 6 ± 1.25 × 10 6 , 5.67 × 10 6 ± 8.49 × 10 5 , 2.60 × 10 7 ± 6.09 × 10 6 , 5.07 × 10 6 ± 1.59 × 10 6 and 6.02 × 10 7 ± 3.69 × 10 6 ) exceeded the WHO permissible limits for drinking water (<500 cfu/ml). High abundance of isolated bacteria genus such as Enterobacter , proteus , Escherichia , Salmonella and Shigella were recorded in well, river and reservoir water systems. There was a strong positive correlation between the total bacteria count and physico-chemical parameters, which suggested that the parameters influenced bacterial growth. The occurrence of these bacterial geniuses in the water sources are considered capable to cause potential health consequences for the consumers. Therefore, proper purification and treatment of domestic water sources of the Samaru community should be ensured before being used by the riparian users.
Domestic water sources; physico-chemical parameter; bacteriological quality; pathogen; contamination and treatment.
1. Introduction
The earth has an abundance of water but unfortunately, only about 0.3 % is usable by humans that comprise of freshwater and lakes (0.009%), inland seas (0.008%), soil moisture (0.005%), atmosphere (0.001%), rivers (0.0001%), groundwater (0.279%) and other composed of ocean (97.2%), glaciers and other ice (2.15%) ( Bibi et al. 2016 ). Water is an essential part of human nutrition either directly as drinking water or indirectly as constituent of food and served in various other applications of our daily life. Rapid growth of industrialization, urbanization and increase in human population around the globe has led to high demand for good quality water for domestic, recreational, industrial activities and other purposes have continuously threatened value of this resource ( Umeh et al. 2005 ). The vast majority of people living in undeveloped countries still rely on surface waters as their primary sources of water and simultaneously, as their means of waste disposal. A majority of this population depends on unprotected/or contaminated water sources as a means of drinking water which can cause outbreaks of waterborne diseases. A large percentage of the population in developing countries (majorly African countries) lack accessibility to potable water supply thus, they are compelled to use untreated water from other sources such as rivers, reservoir, springs, streams and groundwater for drinking and other domestic purposes ( Welch et al. 2000 ; Jamielson et al. 2004 ).
The provision of clean drinking water, especially in developing countries like Nigeria, has always been a major challenge ( Raji and Ibrahim, 2011 ). Based on an National Bureau of Statistics (2009) report, about 27 % of rural dwellers in Northcentral or far North of Nigeria, depend absolutely on springs, streams, ponds, rivers, dams and rainwater as main sources of water for their domestic uses due to lack of clean water ( Shittu et al. 2008 ; Taiwo et al. 2012 ). Water is not only essential for life; it also remains one of the most important vehicles of transmitting disease in humans and an important cause of infant mortality in many developing countries ( Ford, 1999 ). Water is contaminated by various pathogenic microorganisms such as bacteria, fungi, viral, protozoan and other biological organisms; these pathogenic agents have been implicated in various diseases that affect human health. The potential ability of water to transmit microbial pathogens to a great number of people causing subsequent illness is well document in many countries at all levels of economic development ( Dufour et al. 2003 ). Research has shown high prevalence of waterborne diseases such as cholera, diarrhea, dysentery, hepatitis in these regions, claims the lives of at least a hundred thousand of children and adults per year ( Raji and Ibrahim, 2011 ; Oguntoke et al. 2009 ). According to WHO about 80 % of diseases are cause by water borne due to drinking contaminated water in developing countries ( Khan et al. 2013 ) and about 3.1% deaths occur due to the unhygienic and poor quality of water ( Pawari and Gawande, 2015 ).
In recent years, the health concern because of poor water quality has gained public attention worldwide ( Jean, 1999 ; Barrell et al. 2000 ; Jean et al. 2006 ). Most of the microbes that grow in drinking water are heterotrophs requiring essential inorganic nutrients such as phosphate, nitrate and other organic matter that aids their growth under a favorable environmental condition ( Miettinen et al. 1996 ). The addition of nutrients to our drinking water greatly increases the growth of heterotrophic bacteria because this limiting nutrient such as phosphorous play a major ecological role in nature, it is an essential element for microbes growth and the least abundant element compared to carbon ( Ward et al. 1982 ). Water quality is a complex subject, which determines the quality of water and comprises of physical, chemical, hydrological and biological characteristics of water by which the user assessing the acceptability of water" ( Mauskar, 2008 ). The information concerning the water quality of particular waterbodies will provide a useful information for policy makers to formulate management strategy for control, abatement of water pollution and such reliable data can only be obtained through monitoring. Water quality monitoring is paramount especially in these parts of the country to safeguard the public health, to protect the water resources and fundamental tool necessary for the management of freshwater that are main sources of drinking water in the rural and some urban areas ( Adah and Abok, 2013 ). However, water quality monitoring becomes essential for identifying problems and formulating measures to minimize deterioration of water quality. The objective of this research was to provide information on the physico-chemical and bacteriological quality of domestic water sources as well as to discuss its suitability for human consumption based on water quality standards.
2. Materials and methods
2.1. the study area.
The study areas were located within Samaru, Zaria, Sabon Gari Local Government, Kaduna state, Nigeria. Samaru is located in the Northern Guinea Savannah zone of Nigeria falling within Longitude of 7° 37′ 60″ E and Latitude of 11° 10′00″ N with altitude of 763 m above sea level. It has a tropical climate with a well-defined rainy season, which occurs from May to October and the dry season from November to April. Mean monthly temperature ranges from 13.8 °C to 36.7 °C and annual rainfall of 1090 mm are characteristics of Zaria ( Swanta et al. 2013 ). Samaru as a climate similar to that of Zaria a whole with distinct variation in rainy and dry season. Samaru is a neighborhood in Kaduna state and situated in Zaria a major city in the state. It is predominantly residential area and located in close proximity to the Ahmadu Bello University community and Zaria Aviation School. According to National Population Commission (1991) , Samaru has 12, 978 people with 7,417 males and 5,561 females. Based on the 3.0 growth rate of the 1991 census, the population of Samaru was projected to about 18,039 by 2009. The resident of these area depend on water from the rivers, streams, ground waters and reservoirs as major domestic water sources due to lack of potable in this area. The map showing the different sampling location is presented in Figure 1 .

Map showing different domestic water sources location within Samaru community, Zaria.
2.2. Sample collection
A total number of five (5) sampling locations were randomly selected with the Global Positioning System (GPS) within Samaru and its environs namely: Hayin dogo (Tap water), Kubanni reservoir, also called ABU dam (Reservoir), Bomo River (River), ground water (Borehole and Well water) from Samaru market. Water samples were collected aseptically bi-monthly from five domestic water sources to covering both seasons. The samples were collected in sterilized plastic bottles of 1000 ml capacity for physico-chemical analysis while 100 ml sampling bottles were used for bacterial analysis. Water samples collected were properly labeled, stored in cooler containing an icebox to maintain stable temperature of 4 °C and immediately transported to the laboratory for further analysis. The water samples were analyzed with the holding time of the respective parameters using standard methods with adequate quality control measures.
Physico-chemical parameters (water temperature, pH and electrical conductivity) were measured in-situ using standard methods ( APHA, 2001 ) with a mercury-in-glass bulb thermometer was used measured water temperature (°C). Hanna Instrument meter (Model H19813-6) previously calibrated with buffer solutions were used for measuring pH while conductivity was measured with a conductivity meter calibrated with potassium chloride solution. The water samples for the determination of dissolved oxygen (DO) were collected in a 250/125ml capacity glass reagent bottles, fixed in the field using Winkler's A (manganous sulphate solution) and Winkler's B (alkali-iodide) reagents and brought to the laboratory for further processing. In the Laboratory, conc. Sulphuric acid was added to free the fixed oxygen inside water sample and they were titrated with sodium thiosulphate solution. Samples for (Biochemical oxygen demand) BOD5 determination were equally collected in glass reagent bottle but were not fixed. BOD water samples were kept in a dark cupboard at room temperature (25 °C) for five days after which its oxygen content was determined by the Winkler methods as described by APHA (2001) was used to determine the amount of dissolved oxygen at the end of the incubation period. Nitrate was determined using Brucine sulphanlic acid method ( Marczenko, 1986 ). Chloride was analyzed by mohr's titration method, spectrophotometric method was adopt in analyzed phosphate while total hardness was also determined by the tritimetic method using a dropper to add Ethylenediamine tetra-acetic acid (EDTA) solution to the water sample. Parameters obtained were compared with the limits set up by the World Health Organization (2011) and Standards Organization of Nigeria (SON, 2007) for drinking water.
2.3. Bacteriological analyses
The microbiological analysis included total heterotrophic bacterial count and total coliform using serial dilution method and pour plate techniques. Streaking method was used to obtained pure bacterial isolates by sub-culturing a previously incubated plate onto a freshly prepared sterile plate.
2.4. Isolation of Enterobacteriaceae using membrane filtration method
Phenol red indicator, purified by adsorption chromatography was incorporated into lauryl sulphate broth (LSB) used in the membrane filtration method for the detection of Escherichia coli and other coliform bacteria. Relative to LSB containing the impure dye or its major contaminant, the purified phenol red provided clear visualization of discrete yellow colonies observed against a white background. The colonies remained stable for at least 24 h at 25 degrees (ºC) under standard laboratory lighting conditions.
2.5. Pre-enrichment (non-selective enrichment)
Water samples (100 ml) were filtered through a sterile (0.45 μm) milipore membrane filter. The membrane filter was lifted with a blunt edge forceps and transferred into 90 ml of buffered peptone water and gently mixed then incubated for overnight at 37 °C.
2.6. Selective enrichment
A 1 ml volume of the pre-enrichment agar was transferred with a pipette into 10 ml Rappaport-Vassiliadis Soy Peptone (RVS) broth was incubated at 37 °C.
2.7. Serial dilution and selective plating
Serial dilution of 10 −6 was prepared using normal saline and a loopful of culture was streaked on selective agar Salmonella-shigella agar (SSA) and incubated at 37 °C overnight. Colonies on the Salmonella-shigella agar were then counted and subjected to biochemical test.
2.8. Coliform determination
The multiple tube fermentation method was used according to the methodology described in APHA (2001) beginning with 250 mL flasks and using lactose broth for the presumptive test and brilliant green and EC ( E. coli ) broth for the confirmation tests. The most probable number (MPN) of total coliform counts was calculated using the Hoskins table ( APHA, 2001 ). Aliquots of the positive tubes of brilliant green broth were collected and streaked onto MacConkey (MC) agar. Colonies with different morphotypes were collected and transferred into tubes containing tryptic soy agar (TSA) and incubated at 37 °C for 24–48 h for subsequent biochemical identification.
2.9. Biochemical identification
For biochemical identification, oxidase-negative bacteria were selected, and the colonies were subjected to biochemical tests using IMVIC characterization reaction from the nutrient slant used in completed test. The bacterial isolates were view microscopic or/macroscopic and characterized using colonial, morphological and biochemical identification methods that were further identified using Bergey's manual of Determinative Bacteriology.
2.10. Statistical analysis
Data collected were subjected to inferential statistical analysis and Analysis of variance (ANOVA) to determine the physico-chemial and bacteriological quality variations among the domestic water sources. Principal Component Analysis to compared relationship between physcio-chemical and bacteriological quality among domestic water sources by using SPSS 25, Past 3.0 software.
3.1. Physico-chemical parameters in domestic water sources
The physico-chemical parameters of water samples collected from five different domestic water sources from Samaru community during the study period are shown in Tables 1 and and2. 2 . The water quality observed during this study were compared with World Health Organization ( WHO, 2011 ) and Standard Organization of Nigeria ( SON, 2007 ) acceptable levels in the guidelines for drinking water is reported. The highest pH mean concentration was recorded from river water sample (7.28 ± 0.16) compared with the reservoir water sample (6.35 ± 0.13) and there was significant difference (p < 0.05) among the surface water samples. The overall water temperature observed at the period of study ranged from 26.1 - 31.5 (°C) while the highest mean water temperature was recorded from river water sample (28.07 ± 1.22 °C) and lowest was observed in reservoir water sample (27.93 ± 1.03 °C). The conductivity and TDS concentration ranged widely from 102- 484 (μS/cm) and 51–242 mg/L was recorded in surface water samples. Significantly, the mean values of BOD observed between reservoir and river water sample differ greatly and the highest mean was recorded in river water sample. The highest DO and phosphate mean concentrations (3.67 ± 0.61 mg/L and 0.049 ± 0.005 mg/L) were obtained from water sample collected from reservoir while higher mean value of sulphate (0.218 ± 0.07 mg/L) was recorded from river water sample. The highest mean concentration of total hardness was recorded in river water sample (422.67 ± 23.79 CaCO 3 mg/L) and there was highly significant difference (p < 0.01) between the mean value of total hardness obtained from river and reservoir water sample. The lowest mean value of alkalinity was obtained from reservoir water sample (8.0 ± 1.49 CaCO 3 mg/L) while highest was observed from river water sample (26.33 ± 2.25 CaCO 3 mg/L) and there was significant difference among the surface water samples. The highest chloride mean concentration was recorded from reservoir water sample while higher mean value of nitrate was obtained from river water sample. The maximum mean values of 6.40 ± 0.11 and 27.57 ± 0.81 °C were recorded for pH and water temperature from borehole water sample. The TDS and conductivity values ranged of 79.0–546 mg/L and 40.0–256 μS/cm were observed from ground water and there is high significant different (p < 0.001) among the mean value of TDS and conductivity recorded from groundwater samples. The highest BOD mean value was recorded from well water sample (1.7 ± 0.39 mg/L) while the maximum DO mean concentration was observed from tap water sample and phosphate mean concentration was higher in borehole water sample. High mean sulphate concentration was recorded from borehole water (0.325 ± 0.010 mg/L) while lower mean value was obtained from well water (0.034 ± 0.002 mg/L) and there significant differences (p < 0.001) between different ground water sources. Significantly, the mean concentration of total hardness obtained from the groundwater samples differ greatly. Maximum mean alkalinity concentration was observed at well water sample and there was high significant differences (p < 0.001) between different groundwater sources. Lowest chloride mean value was recorded from tap water sample (19.33 ± 3.40 mg/L) while highest value was observed from borehole water sample (314 ± 142.4 mg/L) and there was significant different (p < 0.01) among the various groundwater sources. The ranged of 0.2–3.2 mg/L was recorded from underground water for nitrate during the period study while 0.3–5.32 mg/L and 1.1–7.32 mg/L were recorded from reservoir and river water samples.
Table 1
Physico-chemical and bacteriological quality of Surface water samples.
∗significant difference (p < 0.05).
∗∗ High significant difference (p < 0.01).
∗∗∗Very high significant difference (p < 0.001).
Table 2
Physico-chemical and bacteriological quality of groundwater samples.
Seasonally, the highest pH mean concentration were recorded during rainy season in all domestic water sources compared with dry season as presented in Table 3 . The mean values recorded for water temperature were higher during the dry season than rainy season except for borehole water sample. Conductivity and TDS mean concentration were higher in the dry season among the surface and underground water samples but tap water sample was high in rainy season. The highest BOD mean values was recorded from underground water samples during rainy season while highest was recorded from surface water samples in dry season. The DO mean concentration was higher in rainy season among the various underground water samples while the highest was observed during rainy season for reservoir and in dry season for river water sample. High phosphate mean concentration was recorded among the domestic water samples during this study was higher in rainy season compared with dry season. The total hardness mean concentration recorded in underground water samples was higher in the dry season except for tap water while highest was recorded in the rainy season for reservoir water sample and during dry season in river water samples. Nitrate mean concentration was higher during rainy season among domestic water sample than dry season. The mean value of chloride was higher in all underground water samples during dry season but higher in surface water samples in rainy season.
Table 3
Seasonal variation of bacteriological quality and physico-chemical parameters of domestic water samples.
3.2. Bacteriological load in domestic water sources
The total heterotrophic bacteria counts recorded in this study varied widely from 1.2 × 10 7 - 9.0 × 10 7 cfu/ml with a significant difference (p < 0.001) between the surface water samples. The total heterotrophic bacteria counts ranged from 1.0 × 10 6 - 4.3 × 10 7 cfu/ml while the highest mean value of 2.6 × 10 7 ± 6.09 × 10 7 cfu/ml was recorded in well water sample which was highly significant differences (p < 0.001) between groundwater. The Enterobacter spp highest mean value was recorded in well and river water samples (1.08 × 10 7 ± 2.14 × 10 7 cfu/ml and 1.42 × 10 7 ± 3.17 × 10 7 cfu/ml) during the dry season while Proteus spp was observed in well and river water samples (3.75 × 10 6 ± 7.50 × 10 6 cfu/ml and 1.24 × 10 7 ± 2.77 × 10 7 cfu/ml) during rainy season ( Table 3 ). The highest mean for Escherichia coli and Salmonella typhi was observed in well and river water samples (33.75 ± 44.60 cfu/ml and 55.25 ± 96.69 cfu/ml) (1.06 × 10 7 ± 2.37 × 10 7 cfu/ml and 201 ± 446.66 cfu/ml) during rainy season. Shigella spp was recorded in well and river water samples (75 ± 9.57 cfu/ml and 811 ± 1782.83 cfu/ml) during dry season ( Table 3 ). The total heterotrophic bacteria counts mean values was higher during rainy season in all underground water samples but higher during dry season among the surface water samples ( Table 3 ). Five types of bacteria were identified during the period of study including Enterobacter Spp, Escherichia coli , Salmonella typhi , Shigella spp and Proteus Spp . Enterobacter spp had the highest frequency in river water followed by well water and least frequency in the tap water and highest occurrence of Proteus spp was observed in reservoir water, followed by borehole water and least in well water but Shigella spp , Salmonella typhi and Escherichia coli were high in river water ( Figure 2 ).

Abundances of bacterial counts observed from different domestic water.
3.3. Biochemical identification
Urea, H 2 S, indole, motile, MR were positive (+) for Proteus spp (tap water), borehole, reservoir water and Shigella spp (Reservoir) except for VP which was negative. Citrate is positive for Proteus in tap and borehole waters but negative for Salmonella, Shigella and Escherichia in river, well and reservoir waters. H 2 S, motile and VP showed positive for Enterobacter spp, Escherichia coli and Salmonella typhi in well and river water while biochemical parameters like urea, motile and MR showed negative as shown in Table 4 . There was a strong correlation between total heterotrophic bacteria counts and physico-chemical parameters ( Table 5 ). The variable score plot shows variable that cluster within close range with each other when a higher percentage of variable in the data explained. In the factor score plot it shows that there are clear differences between the well, borehole, river, reservoir and tap. The 1 st factor showed that there are close relationship between chloride, alkalinity, phosphate, TDS and electrical conductivity clustered in borehole and well water while 2 nd factor showed that total heterotrophic bacteria counts, pH and sulphate are related in River water. 3 rd factor showed that was association between water temperature, nitrate and total hardness in reservoir and 4 th factor showed BOD and DO clustering in Tap water but 2 nd factor are not seem to be good cluster when is compared with the 1 st factor, 3 rd factor and 4 th factor. It showed that there is a close relationship between borehole and well water, tap water and reservoir but river water is differ from the rest of domestic water sources ( Figure 3 ).
Table 4
Biochemical Test Analysis of different Domestic Water Sources.
Source by Donalson (1980) . + specie is present and − specie is absent.
Table 5
Correlation matrix showing the relationship between bacteriological quality and physico-chemical parameters of domestic water samples.

Principal component analysis (PCA) showing relationship between bacteriological load and physico-chemical quality of domestic water sources.
4. Discussion
4.1. physico-chemical parameters.
The physico-chemical parameters and bacteriological analysis for different domestic water source are discussed in relation to WHO and SON guidelines for drinking water quality. The importance of hydrogen ion concentration (pH) of water is evident in the manner by which it affects the chemical reactions and biological activities that occur only within a narrow range ( Kolawole et al. 2013 ). In this present study, the pH concentration trend observed in tap water is slightly acidic and pH range fell within standard acceptable range for drinking waters of 6.5–8.5 ( WHO, 2011 ). This finding is in agreement with Shittu et al. (2008) who reported a similar range for pH of water used for drinking and swimming purposes in Abeokuta, Nigeria. The mean pH values recorded lower than 6.5 are considered to be too acidic for human consumption and can cause health concern such as acidosis infections; and the low pH has synergistic effects on heavy metal toxicity in waterbodies. The pH value ranged of 6.2–6.7 (6.40 ± 0.11) indicating that the borehole water is slightly acidic. However, it falls within the range of pH of 5.5–9.0 of natural waters ( Hems, 1985 ). The pH value recorded for borehole water are similar with the results of Ogbonna et al. (2010) for various groundwater samples who reported that pH of groundwater could be determined by type of soil and free carbon (IV) oxide level in the water. The fluctuations in optimum pH ranges may result in increase or decrease in the toxicity of poisons in waterbodies ( Okonko et al. 2008 ). Well water had pH values varying between 6.24-6.40 (6.31 ± 0.03) which fell within stipulated permissible limit of WHO and SON for drinking water. This could be due to fluctuations in the carbon oxide/bicarbonate/carbonate equilibrium and consequently affect the bacterial counts. Brady (1998) reports that similar quality of water is mostly govern by function of the mineralogical and geochemical characteristics of the rocks underlying of that area. Most minerals in rocks are soluble under appropriate geochemical condition and ground water flows screens out most bacteria's through different types of soil layers. Many unseen dissolved minerals and organic constituents are present in ground water in various concentrations. Most are harmless or even beneficial while others are harmful and a few may be highly toxic. Sojobi et al. (2014) attributed the acidic nature to the geological formation of the area. The pH range of 6.24–6.40 and 7.02 to 7.73 observed for reservoir and river water sample in this present study was within the range reported for some Nigerian rivers such as the River Asa (6.8–8.9) ( Otobo, 1995 ) and the River Kaduna (6.4–7.2) ( Samuel et al. 2015 ). Similarly, the observed pH values fell within the range of Class II (Acceptable Quality) as classified by Prati et al. (1971) . The pH values were slightly acidic and alkaline. The variation of pH ranged observed in the reservoir can be explainable in terms of vegetation decay and higher influx into the basin ( Ikhile, 2004 ; Tyokumbur et al. 2002 ) (Awba stream and Reservoir); Ikomi et al. (2003 (River Adofi) . However, pH values obtained in surface waterbody could be linked to the predominant soil type in the area or possibly to the built-up of organic material from runoff. As organic substances decay, carbon dioxide is released and combines with water to produce weak acid “carbonic” acid.
Temperature is one of the major physico-chemical parameters used to assess quality of water for human consumption and control many activities in waterbody such as the rate of chemical reactions, reduction in solubility of gases and amplifications of tastes and colours of water have to be considered ( Olajire and Imeppeoria, 2001 ). The water temperature ranged from 26.0-29.1 °C, 26.0–29.8 °C and 26.2–29.7 °C (tap water, borehole and well) were recorded for underground water sources. Higher water temperature was recorded in borehole water might be as a result of factors such as climatic condition, geographical soil type, and depth of the ground water which may affect the biochemical and physiological activities of organisms found in the water sources ( Ekhaise and Anyansi, 2005 ). The mean water temperature observed during the period of study were within the standard permissible limit of WHO (2008) and SON (2007) . This is similar to Oparaocha et al. (2010) who reported the maximum water temperature of 28 °C from different water source in Nigeria but higher than the study conducted in Bahir Dar town (15–20 °C) ( Milkiyas et al. 2011 ). The water temperatures recorded in various underground waters were above the WHO recommended level (<15 °C) and temperature optimal ranged for some aerobic mesophilic bacteria and fungi. This result obtained could be attributed to time of sampling and geographic location of the study area. It is desirable to have the temperature of drinking water not exceeding 15 °C as the palatability of water is enhanced by its coolness WHO (2003) . The water temperature ranged obtained from surface water (reservoir and river) was similar to the report of Otobo (1995) ; Olobaniyi and Owoyemi (2004) ; Olobaniyi and Owoyemi (2006) who recorded water temperature ranged of 26–31 °C from surface waterbodies in Nigeria. Higher mean values of water temperature recorded in river could be attributed to high atmospheric temperature and exposed to direct solar radiation, low relative humidity and reduction in the amount of suspended particles which occurred as a result of high water transparency and heat from sunlight increasing the temperature of the surface water.
Electrical conductivity measures the degree of ions in water, which greatly affects taste and thus has a significant impact on the user's acceptance of the water. The mean value recorded for all underground water sample were within WHO permissible limit. Findings were related to report of Adetunde and Glover (2011) . Electrical conductivity levels varied between 22 to 315 μS/cm from well water samples in Nigeria. The groundwater in this area is suitable for domestic, irrigation and other purposes. The ranged of electrical conductivity values recorded from surface water are generally lower than the permissible limit by WHO for drinking water. Low conductivity indicates that the water receives low amount of dissolved inorganic substances in ionized form from their surface catchments ( Kidu et al., 2015 ). The reduction in conductivity observed in the study area could be attributed to the dilution effect of the increased water volume within waterbodies during the rainy season. Total dissolved solid (TDS) are measures of the general nature of water quality ( Olajire and Imeppeoria, 2001 ). The TDS is total sum of cations and anions in water including carbonate, bicarbonate, chloride, sulphate, phosphate, nitrate, calcium, magnesium, sodium, organic ions and other ions. TDS affect the taste of drinking water if present at levels above the WHO recommended level. The mean values of TDS recorded from underground water were below the desirable limits set by WHO standards and the range of values could be considered tolerable. The results obtained in this study indicate that total dissolved solid (TDS) is between 40.0-47.0 mg/l, 113–1500 mg/l and 224–2760 mg/l from tap water, borehole and well water samples. The present study showed that total dissolved solid values observed from surface water were below the permissible limits of WHO (2011) for drinking water. The high value of TDS recorded from river and reservoir water might be due to agricultural runoff, other human activities like washing. According to Otobo (1995) , the concentration and relative abundance of ions in waters is highly variable and depends mainly on the nature of the bedrock, precipitation and evaporation crystallization processes. Dissolved oxygen is one of the most important parameters of water quality that give direct and indirect information on nutrient availability, the level of pollution, metabolic activities of microorganisms, stratification, and photosynthesis in water body ( Premlata, 2009 ). The DO values ranging between 3.0-4.3 mg/L, 2.2–3.3 mg/L and 2.7–3.65 mg/L for tap water, borehole and well water were compared with WHO acceptable standards for drinking water. Temperature of water influences the amount of dissolved oxygen with only lesser oxygen dissolved in warm water than cold water ( Tenagne, 2009 ). Therefore, high temperature of the water sources could be one of the factors for low DO values recorded in the current study. Dissolved oxygen is of great significance to all living organisms; its presence in water bodies can result from direct diffusion from air or production by autotrophs through photosynthesis. The DO values observed during this study period from the surface water sources were within the WHO limits. Decreased in DO mean level observed in the river water samples may be indicative of too many bacteria that may use up the dissolved oxygen in it. Another likely reason for such decreased DO in this water sample may be fertilizer run offs from farmland and lawns. Nduka and Orish (2008) reported that DO oxidizes both organic and inorganic substances, thereby interfering with their capacity to constitute a nuisance to the consumer. Dissolved oxygen may not have a direct health hazard to humans, but it could have effects on other chemicals in the water ( Olajire and Imeppeoria, 2001 ). Dissolved oxygen is an important water quality parameter and has special significance for aquatic organisms in natural waters ( Willock et al., 1981 ). The BOD concentration recorded in underground water samples were within the range of 1.0–2.0 mg/L, 0.5–1.1 mg/L and 0.6–2.4 mg/L from tap water, borehole and well water. However, there ranged obtained from this study were below WHO guideline set for the maximum tolerable limit of BOD in drinking water, for fisheries and aquatic life. The BOD values recorded from surface water samples were within the recommended values of WHO (2008) . With the high range of BOD obtained from both river and reservoir, water suggests that drinking water sources were highly polluted by organic matter such as fecal matter and improper disposal domestic waste materials finding their way into waterbody through runoffs. Similarly, other findings also showed that a high level of BOD causes to decrease the value of dissolved oxygen ( WHO, 2008 ). BOD measures the amount of oxygen utilized by microorganisms such as bacterium oxidize organic matter available within the water ( Willock et al. 1981 ; Aniyikaiya et al., 2019 ; Rachna and Disha, 2016 ). Detection of phosphate in various groundwater ranging from 0.0035-0.078 mg/L (tap water), 0.036–0.255 mg/L (borehole) and 0.032–0.079 mg/L (well) could be because of geology or topography of that sampling location which contribute to amount of phosphate in this ground water. The ranged of phosphate observed in this study is low due to no seepage from run offs or sewage discharges, because it is a major constituent of fertilizers and detergents. The mean values of phosphate recorded in surface water (river and reservoir) is within acceptable limit of WHO and this indicates contamination of the water sources by run-off from agricultural farms using inorganic fertilizers as most of the people in the study area were practicing farming. These observations indicate that the water from these sources could not be stored for long in open containers, as the presence of phosphate encourages the growth of algae and consequently cause adverse changes at least in colour and taste of the water sources ( Taha and Younis, 2009 ; Agunwamba, 2000 ). However, the principal significance of high phosphate causes eutrophication, which is more common in lakes and sometimes rivers ( Abolude et al. 2016 ). Hardness is an important parameter in reducing the harmful effect of poisonous elements. The deposition of calcium and magnesium salts in water increases the hardness and pollution of the waters ( Bhatt et al. 1999 ). The soil composition of the sampling sites and lack of casting of the wall of well/or borehole may have contributed to the high total hardness mean recorded in well water sample. People with kidney diseases should avoid high content of calcium and magnesium in water. The value of total hardness recorded in ground water was similar to the reported by Ezeribe et al. (2012) but it is in consonance with the findings of Bello et al. (2013) . Total hardness mean observed from surface water sources fell within the maximum permissible limit by WHO for drinking water thus, the water will not precipitate soap, deposit scale and crust accumulation in containers will be highly minimized. The hard water does not pose a health hazard, but constitute a nuisance concerning its use for other domestic activities such as washing and household cleaning. This agrees with the results of Oladimeji and Kolo, 2004 from Shiroro Lake and Ufodike et al. (2001) from Dokowa mine lake. The range of sulphate concentration recorded from ground water samples (0.046–0.056 mg/L in tap water, 0.298–0.341 mg/L in borehole and 0.028–0.039 mg/L in well water) were significantly low comparable to the WHO and SON permissible limit for drinking water of 250 mg/L. The low level of sulphate can be attribute to the geological profile of the soil and the mineral constituent of the source of water sample. Sulphate naturally occur in groundwater by the dissolution of sulphides such as pyrite from the interstratified materials by percolating water producing sulphate ions ( Olobaniyi and Owoyemi, 2006 ). This study revealed a low mean sulphate values from surface water sources and were within the WHO and SON stipulated limits of 250 mg/L. The low concentration of sulphate could be due to the absence of anthropogenic activities that influence the concentration in waterbodies. The average nitrate concentrations recorded from tap water, borehole and well water sample (0.63 ± 0.15 mg/L, 1.3 ± 0.33 mg/L and 1.87 ± 20.62 mg/L) fall below the WHO standard limit for drinking water. The findings was similar to report of Adejuwon and Mbuk (2011) ; Reimann et al. (2003) who recorded high nitrate concentration of 50.6 mg/l in well water in Ikorodu. The nitrate levels ranging between 0.3-5.23 mg/L and 1.1–7.32 mg/L were recorded from surface water samples. The low variation recorded for nitrate concentration in this study may be due to differences in hydro-geological regimes and agricultural use of nitrates in organic and chemical fertilizers has been a major source of water pollution. Generally, farming remains responsible for over 50% of the total nitrogen discharge into surface waters. Lifetime exposure to nitrite and nitrate at levels above the maximum acceptable concentration could cause such problems as diuresis, increased starch deposits and hemorrhaging of the spleen ( Reimann et al., 2003 ). Antibacterial properties of nitrate may play a key role in protecting the gastrointestinal tract against a variety of gastrointestinal pathogens. The level of chlorine in the ground water samples collected ranging from10.0-26.0 mg/L (19.33 ± 3.40 mg/L), 70.0–219 mg/L (314 ± 142.4 mg/L) and 18.0–120 mg/L (172.67 ± 20.98 mg/L) were recorded from tap water, borehole and well water. The average chloride concentration obtained from borehole water was above the WHO and SON standard limit for drinking water. High concentration of chloride from this study could be due to uses of chlorine as a disinfectant in water purification for human consumption. The level of chloride is due to the natural occurrence of chlorides in the geological strata of borehole and it widely distributed element in all types of rocks in one or the other form ( Braide et al. 2004 ). The chloride range recorded from surface water sample were within stipulated limit by WHO for potable water. Although there were inputs of pollutants from municipal wastes into the river and reservoir but the level was not high, enough to have significantly increase chloride concentrations above the limit. The level of alkalinity values recorded from surface and underground water sources fell within the stipulated limit of 120 mg/L for portable water. The mean alkalinity agreed with the range documented by Moyle (2009) and Boyd (1981) for natural water. The low level of alkalinity indicates that the catchment geology as well as anthropogenic runoff are the main source of natural alkalinity, and probably contains low carbonate, bicarbonate, and hydroxide ( Dhameja, 2012 ).
The mean of alkalinity agreed with the range documented by Moyle (2009) for natural waterbodies. The level of alkalinity ranged recorded reservoir and river water samples were within the stipulated limit of 120 mg/L for portable water (WHO). The low level of the alkalinity indicates that the catchment geology as well as anthropogenic runoff are the main source of natural alkalinity, and probably contains low carbonate, bicarbonate, and hydroxide ( Dhameja, 2012 ). The seasonal fluctuation of physico-chemical parameters obtained in this study could depend on location of the sampling station as well as the activities that go on around the site. The mean values of pH, DO, and Alkalinity were higher in the rainy season maybe due to dilution of water while water temperature, conductivity and TDS were high during dry season. Seasonal variations showed that pH mean was low in the dry season and low pH is known to favour the solubility of ions associated with TDS. The high Electrical conductivity mean values was recorded in surface water sample during dry season when compared to the rainy season. High EC values are mostly associated with wastewater discharges from sewerage, agricultural runoff and industries.
4.2. Bacteriological quality
The mean total heterotrophic bacteria counts recorded in both reservoir and river were above WHO stipulated for drinking water. This study agrees with the report of Doughari et al. (2007) that extremely high total heterotrophic bacterial load in water suggested that the water has been contaminated by potentially dangerous microorganism and unfit for human consumption. Bacterial occasionally, find their way into ground water sometimes in dangerously high concentrations through runoffs or seepage. From this present study, it shows that borehole and well could be contaminated through floodwater forming after rainfall, depending on the depth of the groundwater or through broken underground pipes under this condition, the surrounding floodwater flows into the pipe through the cracks ( Nwachukwu and Otokunefor, 2006 ). The major diseases that could arise from bacteriological contamination of the groundwater include typhoid, diarrhea and cholera. The deeper ground water contains little or no presence of bacteria could have been removed by extensive filtration as water percolates through the soil ( Uzoigwe and Agwa, 2012 ). This was confirmed by the characterization of the isolates from the ground water samples from the sampling locations under study that were highly contaminated with one or more bacterial pathogens. The high bacterial load of genera like Enterobacter, Proteus, Escherichia, Salmonella and Shigella were isolated from well, borehole and tap water samples respectively. The high abundance of bacteria isolated in ground water sample as seen in this study indicate the presence of high feacal contamination and health risk for the human consumption due to high pathogens presence in the water sample ( Franciska et al. 2005 ). According to WHO recommendations, there should no fecal coliforms in 100 ml drinking water and the reason for the gross contamination of ground waters by pathogens as observed in this study may be due to openness and shallowness of this ground water that allows easy entrance of particles from the surroundings. It may also be due to poor sanitary condition around the areas where such wells are located.
Shittu et al. (2008) and Abednego et al. (2013) recorded high number of total coliform count exceeded the WHO permissible limit from water sources in river Ogun. The bacterial species identified from the water samples might be as a result of farming activities practices occurring near the surface water by habitat of the community living around this waterbody, which could result in open defecation along the farmland and there is tendency that the runoffs from these farmlands may be washed into the River. Contamination of surface water maybe due to human activities like bathing, farming, washing, and human or/animal feces seepage run-offs enters the waterbodies and are capable of transmitting a large number of infectious diseases ( Anyanwu and Okoli, 2012 ). The bacterial genus identified during this study can cause meningitis, pneumonia and urinary tract infections in consumers. The coliforms are the primary bacterial indicator for faecal pollution in water and they are most abundant bacteria in water responsible for waterborne diseases such as typhoid, dysentery, diarrhea and also been implicated in mortality across the world ( WHO, 2011 ). The high abundance of bacteria such as Enterobacter , Escherichia , Salmonella and Shigella that were recorded in river and reservoir water in this study could be related to one or to a combination of sewage effluents, such as agricultural run-off and direct fecal contamination from natural fauna ( Abulreesh, 2012 ). Surface water are particularly liable to pollution from animals and birds, and Salmonella spp . may be detected even when only a small number of indicator organisms are present, e.g. Escherichia coli . Additionally, some authors highlighted that the different rates of survival of Salmonella and E. coli in non-host environment suggest that E. coli may not be an appropriate indicator of Salmonella spp . contamination ( Polo et al. 1999 ). Salmonella spp. is a recognized human pathogen and its waterborne transmission has been well-documented Polo et al. 1999 ). Presence of Salmonella spp. in waterways indicates the spread of the agent in the environment, highlighting the importance of fecal contamination of the water environment in the spread of salmonellosis ( Polo et al. 1999 ; Cabral, 2010 ). There is a strong positive correlation between bacteriological and some physcio-chemical parameters such as nutrient compound, ions, pH and water temperature that indicate they have influence on the bacteria growth in water. The result obtained maybe caused due to abundance of these nutrients in soil composition or geology type of the sampling location anthropogenic going on around the area. Nutrients which support the growth of bacteria find the way into drinking water after disinfectant has been applied to it through the seepage and when the environmental condition favour their growth. Some disinfectant are selective in killing particular organisms while other survival or develop resistant towards it. Ward et al. (2006) reported that disinfection itself can be selective for a variety of bacteria has been demonstrated by the results of work of several researchers ( Armstrong et al., 1982 ; LeClerc and Mizon, 1978 ; Murray et al. 1984 who have indicated that chlorination of water supplies select for survivors which are multiply antibiotic resistant such as Flavobacterium strain was more sensitive to monochloramine than to free chlorine. The results indicate that selective pressures of water treatment can produce microorganisms with resistance mechanisms favoring survival in an otherwise restrictive environment. LeChevallier et al. (1993) suggested that the regrowth of coliform bacteria in chlorinated water may be limited by assimilable organic carbon (AOC) levels of less than 50–100 mg/L but heterotrophic bacterial levels in non-chlorinated systems did not increase when assimilable organic carbon (AOC) levels were lower than 10 mg/L.
High bacteria counts mean was recorded among the various ground water samples during the rainy season could be due to runoff from the environment as result of rainfall which can increased the microbial load especially coliforms in water. The finding is similar to that obtained by Esharegoma et al. (2018) who reported high microbial counts during the raining season compared to dry season. This could be attributed to high runoff which increased microbial load washed in from the soil by rainfall, and more nutrients are brought in by the rain through leaching of the soil. In addition, decaying organic matter from the top soil is washed into the water body by the rain, thereby increasing the substrate for organisms. The high mean of microbial was observed in surface water samples during dry season could be attributed to increased nutrient level occasioned by concentration of water through evaporation during dry season ( Ouma et al., 2016 ). This peculiar trend in the occurrence clearly shows that their proliferation is favoured by certain seasonal parameters in the tropics. The high microbial load could also be as a result of higher pH and increase biodegradable organics in the waterbody recorded during the dry season which favored an increase in the microbial population.
5. Conclusion
The findings of this study showed that physico-chemical properties of domestic water sources examined were within safe limits except for chloride concentration (borehole water) and total heterotrophic bacterial counts recorded in all water samples exceed WHO permissible limits for drinking water. All domestic water samples analyzed were contaminated with different types of Bacterial species such as Enterobacter , Escherichia , Salmonella, Shigella and Proteus indicated faecal pollution that can cause waterborne diseases.
6. Recommendation
The health concern of this community required serious attention since people use untreated water for a wide range of domestic purposes. Diseases related to contamination of drinking water constituent which are major burden on human health and intervention to improve the quality of drinking water provide significant benefits to humans health. Therefore, health authorities should make the public aware of potential danger in using untreated water as a source of drinking water and encourage in-house treatment of the raw water. In addition, continuous monitoring are highly recommended for the population before consumption of this domestic water to ensure maximum safety and a healthy living for all. It will be worthwhile to carry out further studies to determine the presence of other species of Enterobacteriacea in the study area.
Declarations
Author contribution statement.
Adesakin Taiwo: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Abayomi Oyewale: Analyzed and interpreted the data; Wrote the paper.
Precious Ahmed, Niima Abubakar, and Balkisu Barje: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Ndagi Mohammed, Umaru Bayero: Contributed reagents, materials, analysis tools or data.
Adedeji Adwuo: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
- Abednego M.M., Mbaruk A.S., John N.M., John M.M. Water-borne bacterial pathogens in surface waters of nairobi river and health implication to communities downstream athi river. World Appl. Sci. J. 2013; 3 (1) [ Google Scholar ]
- Abolude D.S., Edia-Asuke U.A., Aruta M., Ella E.E. Physicochemical and bacteriological quality of selected well water within Ahmadu Bello university community, Samaru, Zaria, Nigeria. Afr. J. Nat, Sci. 2016; 19 :1119-1104. [ Google Scholar ]
- Abulreesh H.H. Salmonellae in the environment. In: Annous B., Gurtler J.B., editors. Salmonella-Distribution, Adaptation, Control Measures and Molecular Technologies. InTech; 2012. pp. 19–50. [ Google Scholar ]
- Adah P.D., Abok G. Challenges of urban water management in Nigeria: the way forward. J. Env. Sci. Res. Manag. 2013; 5 (1):11–121. [ Google Scholar ]
- Adejuwon J.O., Mbuk C.J. Biological and physiochemical properties of shallow wells in Ikorodu town, Lagos, Nigeria. J. Geol. Min. Res. 2011; 3 :161–168. [ Google Scholar ]
- Adetunde L.A., Glover R.L.K. Evaluation of bacteriological quality of drinking water used by selected secondary schools in Navrongo in Kassena- Nankana district of upper east region of Ghana. Prime J. Microbiol. Res. 2011; 1 :47–51. [ Google Scholar ]
- Agunwamba J.C. second ed. Immaculate Publications Limited; Enugu: 2000. Water Engineering Systems; pp. 33–139. [ Google Scholar ]
- American Public Health Association (APHA) 2001. Standard Methods for Examination of 560 Water American Public Health Association (APHA), Standard Methods for Examination of Water, Washington, D.C. [ Google Scholar ]
- Anyanwu C.U., Okoli E.N. Evaluation of the bacteriological and physicochemical 674 quality of water supplies in Nsukka, Southeast, Nigeria. Afr. J. Biotechnol. 2012; 11 (48):10868–10873. [ Google Scholar ]
- Aniyikaiya T.E., Oluseyi T., Odiyo J.O., Edokpayi J.N. Physico-chemical analysis of wastewater discharge from selected paint industries in Lagos, Nigeria. Int. J. Environ. Res. Publ. Health. 2019; 16 (7):1235. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Armstrong J.L., Calomiris J.J., Seidler R.J. Selection of antibiotic-resistant standard plate count bacteria during water treatment. Appl. Environ. Microbiol. 1982; 44 :308–316. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Barrell R.A., Hunter P.R., Nichols G. Community Diseases and Public Health; 2000. Microbiological standards for water and their relationship to health risk; pp. 8–13. 3. [ PubMed ] [ Google Scholar ]
- Bello O.O., Osho A., Bankole S.A., Bello T.K. Bacteriological and physicochemical analyzes of borehole and well water sources in ijebu-ode, south western Nigeria. Journal of Pharmaceutical and Biological Research. 2013; 8 (2):18–25. [ Google Scholar ]
- Bhatt L.R., Lacoul H.D., Lekhak H., Jha P.K. Physicochemical characteristics and phytoplankton of taudaha lake, kathmandu. Pollut. Res. 1999; 18 (4):353–358. [ Google Scholar ]
- Bibi S., Khan R.L., Nazir R. Heavy metals in drinking water of lakki marwat district, KPK, Pakistan. World Appl. Sci. J. 2016; 34 (1):15–19. [ Google Scholar ]
- Boyd C.E. Ine. Oplika; Alabama: 1981. Water Quality in Warm Water Fishponds. Auburn University, 359 Craftmaster Printers. [ Google Scholar ]
- Brady K.B.C. Natural groundwater quality from unmined areas as a mine drainage quality prediction tool. In: Brady K.B.C., Smith M.W., Schueck J., editors. Coal Mine Drainage Prediction and Pollution Prevention in Pennsylvania. PA DEP; Harrisburg, PA: 1998. pp. 10.1–10.11. [ Google Scholar ]
- Braide S.A., Izonfuo W.A.L., Adiukwu P.U., Chindah A.C., Obunwo C.C. Water quality of Miniweja stream, a swamp forest stream receiving non-point source waste discharges in Eastern Niger Delta, Nigeria. Scientia Africana. 2004; 3 :1–8. [ Google Scholar ]
- Cabral J.P.S. Water microbiology. Bacterial pathogens and water. Int. J. Environ. Res. Publ. Health. 2010; 7 (10):3657–3703. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dhameja S.K. Kataria and Sons Pub; 2012. Environmental Engineering and Management S.K; pp. 51–218. [ Google Scholar ]
- Donalson W.E. Trace element toxicity. In: Hodgson E., Guthrie F.E., editors. Introduction to Biochemical Toxicology. Elsevier; New York: 1980. pp. 330–340. [ Google Scholar ]
- Doughari J.H., Elmahmood A.M., Manzara S. Studies on the antibacterial activity of root extracts of Carica papaya L. Afr. J. Microbiol. Res. 2007:37–41. [ Google Scholar ]
- Dufour A., Snozzi M., Koster W., Bartram J., Ronchi E. Assessing microbial safety of drinking water improving approaches and methods. J. Appl. Microbiol. 2003; 88 :1065–1165. [ Google Scholar ]
- Ekhaise F.O., Anyansi C.C. Influence of brewery effluent discharge on the Microbiological and physicochemical quality of Ikpoba River, Nigeria. Afr. J. Biotechnol. 2005; 4 (10):1062–1065. [ Google Scholar ]
- Esharegoma O.S., Awujo N.C., Jonathan I., Nkonye-asua I.P. Microbiological and physicochemical analysis of orogodo river, agbor, delta state, Nigeria. International Journal of Ecological Science and Environmental Engineering. 2018; 5 (2):34–42. [ Google Scholar ]
- Ezeribe A.I., Oshieke K.C., Jauro A. Physicochemical properties of well water 643 samples from some villages in Nigeria with cases of stained and mottled teeth. Sci. World J. 2012; 1 :7. [ Google Scholar ]
- Ford T.E. Microbiological safety of drinking water. United States and global perspectives. Environ. Health Perspect. 1999; 107 (1):191–206. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Franciska M.S., Marcel D., Rinald L.H. Escherichia coli 0157: H7 in drinking water from private supplies. Journal of Netherlands Water Resource. 2005; 39 :4485–4493. [ PubMed ] [ Google Scholar ]
- Hems J.D. US Geological Survey Water Suppl y ; 1985. Study and Interpretation of Chemical Characteristics of Natural Waters; pp. pp111–270. Paper 2254 http://www.ene.gov.on.ca/stdprodconsume/groups/lr/@ene/@resources/docu-ments/resource/std01-079707.pdf. [ Google Scholar ]
- Ikhile C.I. Water chemistry of some streams in tropical rainforest area of edo state, Nigeria. In: Ibitoye A. Scientific., editor. Environmental Issues in Population, Environment and Sustainable Development in Nigeria. Department of Geography and Regional Planning, University of Ado-Ekiti Research Group; 2004. [ Google Scholar ]
- Ikomi R.B., Iloba K.I., Ekure M.A. The physical and chemical hydrology of River Adofi at utagba-uno, delta state, Nigeria. Zoologica. 2003; 2 (2):84–95. [ Google Scholar ]
- Jamielson R., Gordon R., Joy D., Lee H. Assessing Microbial Pollution of rural surface waters: a review of current watershed scale modeling approaches. Agric. Water Manag. 2004; 70 :1–17. [ Google Scholar ]
- Jean J.S. Outbreak of enteroviruses and ground water contamination in Taiwan: concept of biomedical hydrogeology. Hydrogeol. J. 1999; 7 :339–340. [ Google Scholar ]
- Jean J.S., Guo H.R., Chen S.H., Liu C.C., Chang W.T., Yang Y.J., Huang M.C. The association between rainfall rate and occurrence of an enterovirus epidemic due to a contaminated well. J. Appl. Microbiol. 2006; 101 :1224–1231. [ PubMed ] [ Google Scholar ]
- Khan N., Hussain S.T., Saboor A. Physiochemical investigation of the drinking water sources from mardan, khyber pakhtunkhwa, Pakistan. Int. J. Phys. Sci. 2013; 8 (33):1661–1671. [ Google Scholar ]
- Kidu M., Abraha G., Hadera A., Yirgaalem W. Assessment of physico-chemical parameters of tsaeda agam river in mekelle city, tigray, Ethiopia. Bull. Chem. Soc. Ethiop. 2015; 29 (3):377. [ Google Scholar ]
- Kolawole O.M., Alamu F.B., Olayemi A.B., Adetitun D.O. Bacteriological analysis and effects of water consumption on the hematological parameters in rats. International Journal of Plant, Animal and Environmental Sciences. 2013; 3 (2):125–131. [ Google Scholar ]
- LeChevallier M.W., Shaw N.E., Kaplan L.A., Bott T.L. Development of a rapid assimilable organic carbon method for water. Appl. Environ. Microbiol. 1993; pp59 :1526–1531. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- LeClerc H., Mizon F. Eaux d'alimentationet bacte ries resistantes aux antibiotiques. Incidences sur les normes. Revision Epidemiological and Medical Society. Sante Publique. 1978; 26 :137–146. [ PubMed ] [ Google Scholar ]
- Marczenko Z. 2 nd 563. E. Horwood; Chichester: 1986. Separation and Spectrophotometric Determination of Elements; p. 678. [ Google Scholar ]
- Mauskar J.M. 2008. Control of Urban Pollution Series: CUPS/69/2008 Performance of Sewage Treatment Plants-Coliform Reduction. www.cpcb.mc.in/divisionsofheadoffice/pams/pstp-coliform.pd [ Google Scholar ]
- Miettinen I.T., Vartiainen T., Martikainen P.J. Contamination of drinking water. Nature. 1996; 381 :654–655. [ PubMed ] [ Google Scholar ]
- Milkiyas T., Mulugeta K., Bayeh A. Bacteriological and physic-chemical quality of drinking water and hygiene-sanitation practices of the consumers in Bahir Dar city, Ethiopia. Ethiopia Journal of Health Science. 2011; 22 :19–26. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Moyle J.B. Some indices of lake productivity. Trans. Am. Fish. Soc. 2009; 76 :322–334. [ Google Scholar ]
- Murray G.E., Tobin R.S., Junkins B., Kushner D.J. Effect of chlorination on antibiotic resistance profiles of sewage-related bacteria. Appl. Environ. Microbiol. 1984; 48 :73–77. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- National Bureau of Statistics (NBS) 2009. Annual Abstract of Statistics, Federal Republic of Nigeria. [ Google Scholar ]
- National Population Commission (NPC) National Population Commission; Abuja: 1991. Population Census of the Federal Republic 557 of Nigeria: Analytical Report at the National Level. [ Google Scholar ]
- Nduka J.K., Orish E.O. Some physicochemical parameters of potable water supply in Warri, Niger Delta area of Nigeria. Sci. Res. Essays. 2008; 3 (11):547–551. [ Google Scholar ]
- Nwachukwu C.I., Otokunefor T.V. Bacteriological quality of drinking water supplies in the University of Port Harcourt, Nigeria. Nigeria Journal of Microbiology. 2006; 20 (3):1383–1388. [ Google Scholar ]
- Ogbonna C.E., Njoku H.O., Onyeagba R.A., Nwaugo V.O. Effects of seepage from drilling burrow pit wastes on orashi river, egbema, rivers state, Nigeria. Nigerian Journal of Microbiology. 2010; 24 (1):193–200. [ Google Scholar ]
- Oguntoke O., Abodemi O.J., Bankole A.M. Association of waterborne diseases morbidity pattern and water quality in parts of Ibadan City, Nigeria. Tanzan. J. Health Res. 2009; 11 (4):189–195. ISSN: 182-6404. [ PubMed ] [ Google Scholar ]
- Okonko I.O., Adejoye O.D., Ogunnusi T.A., Fajobi E.A., Shittu O.B. Microbiological and physicochemical analysis of different water samples used for domestic purposes in Abeokuta and Ojota, Lagos State, Nigeria. Afr. J. Biotechnol. 2008; 7 (5):617–621. [ Google Scholar ]
- Oladimeji A.A., Kolo R.J. Water quality and some nutrient levels in Shiroro Lake Niger State. Nigeria. J. Aquat. Sci. 2004; 19 (2):99. [ Google Scholar ]
- Olajire A.A., Imeppeoria F.E. Water quality assessment of Osun River: studies on inorganic nutrients. Environ. Monit. Assess. 2001; 69 :17–28. [ PubMed ] [ Google Scholar ]
- Olobaniyi S.B., Owoyemi F.B. Quality of groundwater in the deltaic plain sand aquifer of warri and environs of delta state, Nigeria: water resource. Journal of the Nigeria, Association of Hydrogeologist. 2004; 15 :38–45. [ Google Scholar ]
- Olobaniyi S.B., Owoyemi F.B. Characterization by factor analysis of the chemical faces of groundwater in the deltaic plain sands aquifer of warri, western Niger delta, Nigeria. African Journal of Science and Technology (AJST), Science and Engineering Series. 2006; 7 (1):73–81. [ Google Scholar ]
- Oparaocha E.T., Iroegbu O.C., Obi R.K. Assessment of quality of drinking water sources in the federal university of technology, owerri, imo state, Nigeria. Journal of Applied Bioscience. 2010; 32 :1964–1976. [ Google Scholar ]
- Otobo A.J.T. University of Port Harcourt; 1995. The Ecology and Fishery of Them Pygmy Herring Sierratherissa Leonensis (Thys Van Dan Audenaerde, 1969) (Clupeidae) in the Nun River and Taylor 611 Creek of the Niger Delta; p. 221. Ph.D Thesis. [ Google Scholar ]
- Ouma S.O., Ngeranwa J.N., Juma K.K., Mburu D.N. Seasonal variation of the physicochemical and bacteriological quality of water from five rural catchment areas of lake victoria basin in Kenya. Journal of Environmental Analytical Chemistry. 2016; 3 :2–7. [ Google Scholar ]
- Pawari M.J., Gawande S. Ground water pollution and its consequence. International 536 Journal of Engineering Research and General Science. 2015; 3 (4):773–776. [ Google Scholar ]
- Polo F., Figueras M.J., Inza I., Sala J., Fleisher J.M., Guarro J. Prevalence of Salmonella serotypes in environmental waters and their relationships with indicator organisms. Antonie Leeuwenhoek. 1999; 75 (4):285–292. [ PubMed ] [ Google Scholar ]
- Prati L., Pavanello R., Pesarin F. Assessment of surface water quality by a single index of pollution. Water Res. 1971; 5 :741. [ Google Scholar ]
- Premlata V. Multivariant analysis of drinking water quality parameters of Lake Pichhola in Udaipur, India. Biological Forum. Int. J. 2009; 1 (2):97–102. [ Google Scholar ]
- Rachna B., Disha J. Water quality assessment of lake water: a review. Sustain. Water Resour. Manag. 2016; 2 :161–173. [ Google Scholar ]
- Raji M.I.O., Ibrahim Y.K.E. Prevalence of water-borne infections in North Western Nigeria: a retrospective study. J. Publ. Health Epidemiol. 2011; 3 (8) 382-512 385. [ Google Scholar ]
- Reimann C., Bjorvatn K., Frengstad B., Melaku Z., Teklehaimanot R., Siewers U. Drinking water quality in the Ethiopian section of the east African rift valley I-data and health aspects. Science and Total Environmental Journal. 2003; 311 :65–80. [ PubMed ] [ Google Scholar ]
- Samuel P.O., Adakole J.A., Suleiman B. Temporal spatial physico-chemical parameters of river galma, Zaria, Kaduna state, Nigeria. Research Environment. 2015; 5 (4):110–123. [ Google Scholar ]
- Shittu O.B., Olaitan J.O., Amusa T.S. Physico-chemical and bacteriological analyses of water used for drinking and swimming purposes in Abeokuta, Nigeria. Afr. J. Biomed. Res. 2008; 11 :285–290. [ Google Scholar ]
- Sojobi A.O., Owamah H.I., Dahunsi S.O. Comparative study of household water treatment in a rural community in Kwara State, Nigeria. Nigeria Journal of Technology. 2014; 33 (1):134–140. [ Google Scholar ]
- Standard Organization of Nigeria (SON) 2007. Nigerian Standard for Drinking Water Quality, SON, Abuja. Nigeria. [ Google Scholar ]
- Swanta A.A., Sonnie O., Mathias C. Domestic water quality assessment: microalgal and cyanobacterial contamination of stored water in plastic tanks of stored water in plastic tanks in Zaria, Nigeria. Eur. J. Sci. Res. 2013; 110 (4):501–510. [ Google Scholar ]
- Taha G.M., Younis M. Chemical and bacteriological evaluation of drinking water: a study case in wadi el saaida hamlets in aswan governorate, upper Egypt. Journal of International Applied Science. 2009; 4 :309–377. [ Google Scholar ]
- Taiwo A.M., Olujimi O.O., Bamgbose O., Arowolo T.A. In: Surface Water Quality Monitoring in Nigeria: Situational Analysis and Future Management Strategy, Water Quality Monitoring and Assessment. Voudoinis Dr., editor. InTech; 2012. Available from: http://www.intechopen-com/books/waterquailty-monitoring-and-assessment/surface-water-quailty-monitoring-in-nigeria-situation-analysis-and-futuremanagement-strategy . [ Google Scholar ]
- Tenagne A.W. Cornell University; New York, USA: 2009. The Impact of Urban Storm Water Runoff and Domestic Waste Effluent on Water Quality of Lake Tana and Local Groundwater Near the City of Bahir Dar, Ethiopia. M.Sc. thesis. [ Google Scholar ]
- Tyokumbur E.T., Okorie T.G., Ugumba O.A. Limnological assessment of the effects of effluents on macroinvertebrate fauna in Awba Stream and Reservoir, Ibadan, Nigeria. Zoologica. 2002; 1 (2):59–69. [ Google Scholar ]
- Ufodike E.B.C., Kwanasie A.S., Chude L.A. On-set of rain and its destabilizing effect on aquatic and physico-chemical parameters. J. Aquat. Sci. 2001; 16 (2):91–94. [ Google Scholar ]
- Umeh C.N., Okorie O.I., Emesiani G.A. University of Agriculture; Abeokuta: 2005. Towards the provision of safe drinking water: the bacteriological quality and safety of sachet water in Awka, Anambra State; pp. 14–17. (The Book of Abstract of the 29 th Annual Conference and General Meeting on Microbes as Agents of Sustainable Development Organized by Nigerian Society for Microbiology (NSM)). [ Google Scholar ]
- Uzoigwe C.I., Agwa K.O. Microbiological quality of water collected from boreholes sited near refuse dumpsites in Port Harcourt, Nigeria. Afr. J. Biotechnol. 2012; 11 (13):3135–3139. [ Google Scholar ]
- Ward N.R., Wolfe R.L., Means E.G., Olson B.H. Proceedings of the American Water Works Association Water Quality Technical Conference. American Water Works Association; Denver: 1982. The inactivation of total and selected gram-negative bacteria by inorganic monochloramine and dichloramines; pp. 81–97. [ Google Scholar ]
- Ward M.H., de Kok T.M., Levallois P., Brender J., Gulis G., VanDerslice J., Nolan B.T. Response to dietary nitrate: where is the risk? Environ. Health Perspect. 2006; 114 :A459–A460. [ Google Scholar ]
- Welch P., David J., Clarke T.A.P.D., Berston S., McDougall L., Adesiyin A.A. Microbial sanitation of water in rural communities of trinidad. Pan Am. J. Public Health. 2000; 8 (3):172–180. [ PubMed ] [ Google Scholar ]
- Willock R.J., Stevenson C.D., Robert C.A. An inter-laboratory study of dissolve oxygen in water. Water Res. 1981; 15 :321–325. [ Google Scholar ]
- World Health organization . Ministry of the Environment; 2003. Technical Support Document for Ontario Drinking Water Standards, Objectives and Guidelines. [ Google Scholar ]
- World Health Organization . 2008. Guidelines for Drinking Water Quality: Incorporating First Addendum, Vol. 1 Recommendations: 4 th Edition, Geneva. [ Google Scholar ]
- World Health Organization (WHO) Fourth 565 edition. WHO Library Cataloguing-in-Publication; Switzerland: 2011. Guidelines for Drinking-Water Quality. [ Google Scholar ]

IMAGES
VIDEO
COMMENTS
It was concluded that safe drinking water for all is one of the major challenges of the 21st century and that microbiological control of drinking water should be the norm everywhere. Routine basic microbiological analysis of drinking water should be carried out by assaying the presence of Escherichia coli by culture methods. Whenever financial ...
Methods for Microbiological Water Quality Analysis. ... This review investigated the most recent literature on water quality in the context of climate change, which leads to potential health impact. ... McGuigan KG, Conroy RM, Mosler H-J, du Preez M, Ubomba-Jaswa E, Fernandez-Ibañez P. Solar water disinfection (SODIS): a review from bench-top ...
Rapid and highly sensitive molecular diagnostics offer pivotal information needed for the quality control loop: monitoring-evaluation-action. In this review, the most frequently applied molecular methods for assessing water microbiology are addressed and discussed based on their intrinsic strengths and limitations. 2.
Here, we present a review on studies conducted on microbial water quality in surface waters. The review includes studies based on microbial monitoring (that explore correlation between environmental variables and FIB concentrations), modelling, future scenario analysis and health risk assessment. We focus on the differences in microbial ...
A systematic review of microorganisms as indicators of recreational water quality in natural and drinking water systems December 2020 Journal of Water and Health 19(6)
The microbiology of water systems is a rapidly develop-ing field. With increasingly affordable metagenomic analysis along with other meta-analysis, an explosion of microbial data are at researchers' fingertips like never before. This explo-sion of microbial data is well exemplified in the review article
Summary - What is needed to meet the water microbiology demands of the 21st century. Maintain water quality standards and public confidence. Apply chemistry metrology to microbiological AQC. Use new technology and methods to give faster, more reliable results. Microbiological CRMs from ISO17025 + Guide 34 accredited sources.
Microbiological Analysis of Water Used for Pharmaceutical Product Preparation DOI: 10.9790/3008-1504030710 www.iosrjournals.org 8 | Page usually drawn from a system on demand and is not subject to testing and batch or lot release before use. Assurance of quality to meet the on demand expectation is, therefore, essential 6 ...
A very important role in determining the quality of water is the assessment of its microbiological quality. Water quality control, which could pose a direct threat to human health and life, is performed in the case of water produced at water treatment plants, tap water, or water in swimming pools. However, these traditional methods used to assess its quality are laborious and time-consuming.
The Chi square test analysis revealed that, the type of water source had strong relationship with the quality of water (p < 005). Microbial load of drinking water sources The mean aerobic mesophilic count (AMC) (log CFU/ml) of tap water, protected wells, protected springs, unprotected wells and unprotected springs were 3.05, 3.53, 4.03, 4.39 ...
Literature review involved a total of 21 original reports on microbiological events inside the water distribution system were studied, summarizing the current knowledge about the build-up of microbes in treated municipal water at various points of the distribution system. ... Microbiological Analysis of Tap Water from Bangladesh. The best ...
The qualitative analysis was then used to give insights and understandings of the key themes and the root causes of microbiological and chemical contamination of potable water. Finally, co-citation analysis and bibliographic coupling are used to map the collaborative efforts and their impact in this area of research.
The book has three parts: an introduction to microbiology, microbial occurrence in ground-water systems, and biodegradation of contaminated ground water. The author's approach was to review ...
microbiological analysis. It was concluded that safe drinking water for all is one of the major challenges of the 21st century and that microbiological control of drinking water should be the norm everywhere. Routine basic microbiological analysis of drinking water should be carried out by assaying the presence of Escherichia coli by culture ...
Literature Review on Microbiological Analysis of Water - Free download as PDF File (.pdf), Text File (.txt) or read online for free. literature review on microbiological analysis of water
different physico-chemical parameters such as color, temperature, acidity, hardness, pH, sulphate, chloride, DO, BOD, COD, alkalinity used for testing of water quality. H eavy metals. like Pb, Cr ...
We have not carried out any microbiological assessments of the performance of the various tests. ... This was supplemented by a review of the literature and internet searches. ... Elliott J., Gundry S.W. The H2S test versus standard indicator bacteria tests for faecal contamination of water: systematic review and meta-analysis. Trop. Med. Int ...
The calcium content of the bottled drinking water brands varied from 6.48 to 83.77 mg/l with a mean of 49.9 (±25.09) mg/l. The nitrate content of the bottled drinking water brands varied from 0.21 to 4.19 mg/l with the mean of 1.26 (±1.08) mg/l. Aerobic bacterial count varied from 0 to 800 colony forming unit per ml (cfu/ml) with a mean of ...
World Health Organization (WHO) estimated more than 500 children died daily from the consumption of contaminated food and water (WHO 2015).It was reported that illnesses due to contaminated foods are an important cause of reduced economic productivity (Okonko et al. 2008).The incidence rate of foodborne diseases is also rising in, both developed and developing nations due to problems ...
Salt marshes play a critical role in supporting water quality, erosion control, flood protection, and carbon sequestration. Threats from climate change and human activities have prompted global restoration initiatives. We analyzed restoration efforts worldwide from 1978 to 2022, using the Web of Science database and SciMAT mapping tool. After a PRISMA screening to identify methodologies ...
Determination of coliform bacteria and E. coli in the water samples. EN ISO 9308-1 14 and EN ISO 9308-1/A1 15 were used for the enumeration of coliform bacteria and E. coli.For the enumeration of these bacteria, 100 mL of water samples were filtered with 0.45 µm diameter sterile membrane filters (Sartorius) and these filters were placed on Ready Plate Chromocult Coliform Agar (Merck).
We aimed to describe the pooled prevalence of DAP, the microorganisms involved, and the impact of DAP on drowned patients. Systematic review and meta-analysis of studies published between 01/2000 and 07/2023 reporting on DAP occurrence and microorganisms involved. Of 309 unique articles screened, 6 were included, involving 688 patients.
The purpose of the paper is to investigate the ethics of manufacturing and supplying bottled water. It uses a systematic review of the literature through the PRISMA method to determine the major ethical concerns surrounding these topics. A total of 107 articles were identified, and 31 were subjected to further reviews and analysis.
The samples were collected in sterilized plastic bottles of 1000 ml capacity for physico-chemical analysis while 100 ml sampling bottles were used for bacterial analysis. Water samples collected were properly labeled, stored in cooler containing an icebox to maintain stable temperature of 4 °C and immediately transported to the laboratory for ...
In the literature review analysis, a total of 84 interactions between lean construction and Sustainable Development Goals (SDGs) were detected. Through FGD, an additional 375 interactions were unveiled (previously unrecognized relationships between lean construction and SDGs). They are denoted in Table 4 as colored. In total, this study ...