- Open access
- Published: 16 August 2021

Estimating case fatality risk of severe Yellow Fever cases: systematic literature review and meta-analysis
- Joseph L. Servadio 1 ,
- Claudia Muñoz-Zanzi 1 &
- Matteo Convertino 2 , 3
BMC Infectious Diseases volume 21 , Article number: 819 ( 2021 ) Cite this article
6606 Accesses
11 Citations
11 Altmetric
Metrics details
Case fatality risk (CFR), commonly referred to as a case fatality ratio or rate, represents the probability of a disease case being fatal. It is often estimated for various diseases through analysis of surveillance data, case reports, or record examinations. Reported CFR values for Yellow Fever vary, offering wide ranges. Estimates have not been found through systematic literature review, which has been used to estimate CFR of other diseases. This study aims to estimate the case fatality risk of severe Yellow Fever cases through a systematic literature review and meta-analysis.
A search strategy was implemented in PubMed and Ovid Medline in June 2019 and updated in March 2021, seeking reported severe case counts, defined by fever and either jaundice or hemorrhaging, and the number of those that were fatal. The searches yielded 1,133 studies, and title/abstract review followed by full text review produced 14 articles reporting 32 proportions of fatal cases, 26 of which were suitable for meta-analysis. Four studies with one proportion each were added to include clinical case data from the recent outbreak in Brazil. Data were analyzed through an intercept-only logistic meta-regression with random effects for study. Values of the I 2 statistic measured heterogeneity across studies.
The estimated CFR was 39 % (95 % CI: 31 %, 47 %). Stratifying by continent showed that South America observed a higher CFR than Africa, though fewer studies reported estimates for South America. No difference was seen between studies reporting surveillance data and studies investigating outbreaks, and no difference was seen among different symptom definitions. High heterogeneity was observed across studies.
Conclusions
Approximately 39 % of severe Yellow Fever cases are estimated to be fatal. This study provides the first systematic literature review to estimate the CFR of Yellow Fever, which can provide insight into outbreak preparedness and estimating underreporting.
Peer Review reports
Evaluations of infectious disease severity often account for both morbidity and mortality. The latter can be represented by the case fatality risk (CFR), defined as the probability of a case of a disease being fatal [ 1 , 2 ]. It is, in its simplest form, estimated by a quotient of the number of fatal cases and the total number of cases, which has been reported for disease outbreaks [ 3 , 4 , 5 ]. The CFR can be used in these contexts to understand the severity of a disease and implement appropriate policy in the event of an outbreak [ 6 ].
Case fatality risks have also been described as case fatality ratios or case fatality rates without difference in definition [ 7 , 8 ]. Case fatality risk can differ from case fatality rate in that the case fatality risk does not explicitly specify a time period, whereas a case fatality rate implies a period of time [ 2 , 9 ]. It is common, however, for the three terms to be used interchangeably, regardless of whether time periods are taken into consideration [ 9 ].
An estimate for the CFR of a disease based on observed fatal cases is typically included when reporting results from an outbreak investigation [ 4 , 10 ]. Outbreak investigations for various diseases tend to report the CFR during the time period of observation, though this is not often their primary study aim. Other works aiming to estimate CFR have done so by observing hospital records for the proportion of fatal cases [ 11 ] or by tracking outcomes for confirmed cases of diseases [ 7 , 12 ]. Many of these studies aimed not only to estimate CFR for various diseases, but also to examine risk factors for fatality in order to identify individuals most at risk [ 8 , 11 ] or examine changes in CFR over time [ 13 ]. While valuable for the certainty of information among those recruited, such studies can experience limitations such as ascertaining only the most severe cases or not observing fatal cases who died after data collection [ 14 ].
In ascertaining only the most severe cases, the denominator used to calculate the CFR is not representative of all cases. If more severe cases are more likely to be seen in the denominator of the CFR calculation, then the CFR will likely be overestimated due to the denominator representing a subset of cases. Another limitation seen in studies reporting CFRs is the use of unconfirmed cases. Reported CFRs of suspected cases without confirmation may lead to a CFR for a disease that also included non-cases in its calculation. Depending on the proportion of such cases that are fatal, this could lead to an overestimate or an underestimate of the CFR.
Other studies have aimed to estimate CFR by collecting data through literature review [ 1 , 15 ] and, in many cases, meta-analysis. Some articles have estimated an overall CFR by pooling numerators and denominators across studies [ 16 ], pooling study results with a random effects meta analytic method [ 17 , 18 , 19 ], or generalized linear models [ 20 ], though not all reviews combined results [ 21 ].
Yellow Fever (YF), a Flavivirus which is spread by multiple genera of mosquito [ 22 ], is endemic in sub-Saharan Africa and South America [ 23 ], with the ability for even higher burden worldwide due to increased global travel and reemerging outbreaks [ 24 , 25 ]. An estimated 200,000 global cases are seen per year, with reportedly high case fatality [ 26 ]. Future outbreaks of YF have potential to cause major morbidity and mortality; a review evaluating the reproductive number of YF estimated a reproductive number of approximately 4.2, with other estimates ranging between one and 11 [ 27 ]. The disease is asymptomatic in a large number of cases, and symptomatic cases present with flu-like symptoms such as fever and body aches [ 23 ]. In a smaller number of cases, severe disease develops within a few days, with more severe symptoms including jaundice and hemorrhaging. Estimates of fatality in severe cases commonly used in reports or cited in publications include 50 % [ 23 ], 30–60 % [ 28 ], and 20–50 % [ 26 ]. However, little work exists aiming to evaluate or update these estimates. A 2014 study by Johansson et al. aimed to estimate the proportions of cases that are asymptomatic, mild, and severe, and also estimated a CFR among severe cases which aligned with the WHO estimate of 50 % [ 15 ]. This was done through a literature review, where studies were selected through expert knowledge rather than a systematic search strategy [ 15 ]. No other works exist that offer an update of this estimate, and none use a systematic method for a literature review.
This study aims to estimate the CFR among severe YF cases through a systematic literature review [ 29 ] and meta-analysis. Through literature review, articles were found that contained denominators of severe YF cases and numerators of those cases that were fatal. The CFR was estimated among all studies and then stratified by continent, symptom definitions, and study type, which was based on whether an outbreak investigation was described or cases were reported without known data collection methods. The results of this study offer an estimate of the CFR for severe YF using a comprehensive search of relevant literature.
In conducting the literature review, parts of the search strategy aimed to estimate not only the proportion of fatal cases, but also the proportion of severe and mild cases to estimate burden of disease through proportions of asymptomatic, mild, and severe cases. There were insufficient studies for reliable estimates for disease burden; only methods and results for estimating the CFR among severe cases are presented here.
A systematic literature review [ 29 ] was conducted to collect relevant data to estimate the CFR of severe YF cases. Severe cases in this study are defined as cases that present with fever along with either jaundice or hemorrhaging, which is consistent with the World Health Organization’s definition [ 30 ]. The aim of the literature review was to find articles containing proportions of observed severe YF cases that are fatal, including a numerator and denominator.
Literature review
This study adhered to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [ 31 ]. The PRISMA checklist [ 32 ] for this review can be found in Additional file 1 : Table S1.
The search strategy, as run in PubMed, was as follows: (“Yellow Fever” in the title, abstract, or a medical subject heading) AND (fatal*, severe, severit*, mortality, asymptomatic, symptomatic, diagnosis, misdiagnosis, outbreak, or cases in multiple places) AND NOT (“Vaccine” in title or abstract) with asterisks denoting wildcards. “Vaccine” was excluded because of the high number of studies that aimed to study vaccine efficacy or describe vaccination campaigns, which are outside the aims of this study. A selection of studies excluded due to the “vaccine” criterion were examined and found not to lie within the scope of the analyses for the study aims. The search strategy was run in June 2019, and yielded a total of 485 articles from Ovid Medline and 605 articles from PubMed. After removing duplicates, 842 unique articles underwent title and abstract screening. The search strategy was rerun in March 2021 to add recent studies published between January 2019 and February 2021; 291 articles underwent title and abstract screening from this update.
Articles found via the database search were screened by title and abstract to remove those without relevant information. Articles were excluded if the topic was a disease other than YF or if the title and abstract did not mention investigating or reporting cases of YF. Excluded articles primarily focused on other diseases, laboratory diagnosis methods, or vaccine efficacy research. Following title and abstract screening, 164 articles remained for full text review from the original search, and 47 articles remained for full text review from the updated search.
In full text review, remaining articles were screened for relevant information for the purpose of this study. Articles were included if full text was available in English and contained both a denominator of total severe YF cases and a numerator of deaths among the severe cases. Articles that did not report a numerator and denominator, but did report a denominator and fatality proportion for YF cases, were included. While some studies reported that YF cases were laboratory confirmed, others did not report laboratory confirmation and classified identified YF cases by symptoms. Articles that were not included most commonly included editorials, single case reports, reporting of cases in nonhuman primates, reports of capacity building, or laboratory detection methods. Of the articles undergoing full text review from the original search, 14 were not available in full text, seven were not available in English, and 117 focused on YF topics, but did not include a specific denominator of cases or numerator of fatal cases. During full text review from the original search, four articles were added from the references of the articles read, yielding 30 articles in total. From the updated search, one article was retained from full text review, and another was added from references of the 47 articles. Following these steps, the 32 total articles were reread to validate the data extracted.
Following data extraction from the 32 articles, those that did not specifically report severe cases were removed. In this study, severe YF cases were defined by having a fever and at least one of jaundice or hemorrhaging. Studies that did not state that YF cases were defined by having fever as well as at least one of jaundice of hemorrhaging were removed. During the updated search, this criterion was applied during full text review. These symptoms were typically reported at the study level, where the authors of the studies stated broadly the symptoms used in identifying YF cases in the main text. Many studies only included fever and jaundice in the definition of severe YF, while others included hemorrhaging and other symptoms including organ failure (Additional file 2 : Table S2). A total of 14 studies contained explicit numbers for fatal and nonfatal severe cases through these definitions (Fig. 1 ; Table 1 ). In order to incorporate data from the recent YF outbreak in Brazil, four studies from the updated search that specified severe YF cases through healthcare use, but did not explicitly state the symptom definition outlined previously as inclusion criteria, were added. These studies stated that some patients showed symptoms such as fever, jaundice, and hemorrhaging, but the numbers of fatal and total cases did not exclusively consist of cases meeting the symptom definition.
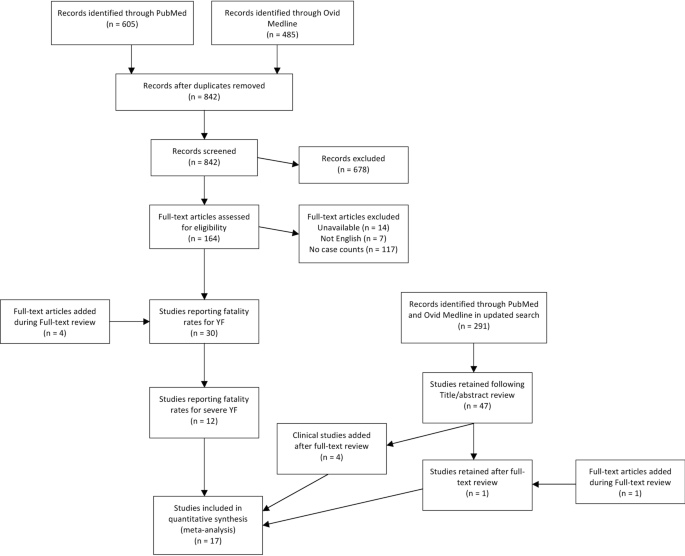
Flow diagram for screening and including articles in systematic literature review and meta-analysis for case fatality risk of severe Yellow Fever cases. Publication years of articles ranged between 1972 and 2020
In addition to the numbers of total and fatal severe cases, descriptive information was collected for each study. Data collected included country, continent, year, symptoms, applied case definitions, and research methods for each study. Case definition and symptoms in the main text were used to confirm that cases listed were severe cases. While, for the purposes of this study, fever as well as either jaundice or hemorrhaging were required for inclusion as severe YF cases, some included studies considered other symptoms as well in their case definitions, including abdominal pain and organ failure (Additional file 2 : Table S2). It also was noted whether the authors were active in recruiting YF patients or stating numbers from reported case data. If the authors reported specifying a case definition, being present in data collection, or stating details of an outbreak investigation, then the study was classified as an “investigative” study. Reports in which case counts were reported without specifically describing active measures to identify cases were classified as “reporting” studies. All studies were classified as either an investigative or reporting study (Table 1 ).
Also collected was whether the cases in each study were confirmed or suspected for YF. Cases were considered confirmed if the articles stated laboratory diagnostic confirmation for YF. Studies that specified a specific laboratory diagnosis, such as through polymerase chain reaction (PCR), or stated lab confirmed cases without specifying a specific type of test, were included as laboratory confirmed cases. Cases were considered suspect if the article explicitly stated cases were suspect. Probable cases, where symptoms are observed without a guaranteed laboratory result, were considered suspect cases in this study. If the article did not state whether cases were confirmed or suspect, then cases were assumed to be suspect (Table 1 ).
All included studies list total and fatal counts of severe YF cases without the CFR being the primary focus of the study. Therefore, the sources of bias in the individual studies remained consistent. Two possible major sources of bias are underreporting of cases within studies and deaths occurring after followup [ 14 ]. Underreported cases, if nonfatal, would lead to a smaller denominator and an overestimated CFR. If the underreported cases were as fatal as reported cases, no bias would be observed. Deaths occurring after followup would lead to fatal cases being classified as nonfatal, and lead to an underestimate of CFR.
Despite these two potential sources of bias, publication bias, where tests of statistical significance affect reporting of results [ 33 ], is not likely to be present in this study. Because the primary focus of the studies was not to estimate the CFR of YF, issues of publication bias are less likely since no included articles used statistical significance tests for CFR estimates. Therefore, the proportions of fatal cases within studies are unlikely to affect whether the studies were published.
Collected data were inputted into a Microsoft Excel (2013) spreadsheet during full text review, with information to be collected determined prior to data collection.
Data analysis
Case fatality risk was estimated using a meta-analysis for proportions. The metaprop() function from the ‘meta’ R package [ 34 ] was used to run an intercept-only logistic meta-regression with random effects for study. Model inputs included the observed proportion of fatal cases for each study, \(\widehat{p}\) , as well as its standard error, estimated by \(\sqrt{\frac{\widehat{p}(1- \widehat{p})}{n}}\) , where \(n\) is the denominator of the study. Only proportions not equal to zero or one were included in the meta-analysis, as these would produce standard errors of zero. They are, however, included in Table 1 . Estimates for CFR were found for laboratory confirmed, suspected, and all severe YF cases. Stratified CFRs were estimated for differences by continent (South America or Africa), by study type (investigative or reporting, as defined previously) and by symptoms reported (fever and jaundice or fever, jaundice, and other severe symptoms). Values of the I 2 statistic were calculated to describe heterogeneity [ 35 ]. Analyses were run separately to include and exclude the four recent studies added to show whether results are sensitive to the inclusion of studies containing data likely to be useful, but not meeting the strict inclusion criteria.
Article inclusion
A total of 18 studies were found through the literature review reporting a CFR for severe YF, three of which were added from the references of the 211 articles that underwent full-text screening and four of which underwent full text screening and were included due to their relevance to the recent Brazilian outbreak. The 18 papers contained a total of 36 proportions of fatal severe cases; 30 of these, present in 17 studies, were not equal to zero or one (Table 1 ) and therefore included in the meta-analysis. The six proportions that equaled zero or one were instances where only one severe YF case was reported in the denominator. Of the 30 proportions included in the meta-analysis, 14 articles provided 16 proportions of CFR among confirmed severe cases of YF, and another three articles provided 14 proportions among severe suspected cases.
Articles included in analyses reported cases in both Africa and South America (Fig. 2 ). The countries with more than one study or more than one fatality proportion found through the literature review were Brazil (7 papers, 8 proportions), Nigeria (3 papers, 9 proportions), Ghana (1 paper, 6 proportions), Cameroon (2 papers, 2 proportions), and Democratic Republic of Congo (2 papers, 2 proportions) (Fig. 3 ). Articles reporting multiple proportions in the same country during the same year(s) reported different proportions for different locations within countries.
Articles from the initial search ranged in year between 1942 and 2019, with 544 (64 %) published in 2000 or after. Articles from the updated search ranged between 2019 and 2021, with the article added from references in full text review, which did not appear in the initial search, published in 2017. Among the 18 final articles with severe YF fatality proportions, publication years ranged between 1972 and 2020, with 12 (66 %) published in 2000 or after (Table 1 ). Of these, four papers are clustered between 1984 and 1993, another four are clustered between 2007 and 2012, seven are clustered between 2017 and 2020, and the remaining three are interspersed outside these time clusters.
Among the 36 fatality proportions for severe YF found in literature, 21 represented fatality proportions among laboratory confirmed severe cases (Table 1 ), though the laboratory test used was not always specified in the text. The remaining 15 represented proportions from suspect severe cases, where laboratory confirmation was not stated. From assessing the study methods, 22 proportions were found from investigative studies as described previously, with the remaining 14 presented in reporting studies. All proportions, with the exception of those from recent clinical investigations in Brazil, explicitly stated use of both fever and jaundice in their case definitions, and some studies also included other symptoms as well in YF case diagnosis. These included hemorrhaging (15 proportions), abdominal pain (13 proportions), or organ failure (2 proportions) (Additional file 2 : Table S2). Travel to a YF endemic region was a criterion included in eight proportions.
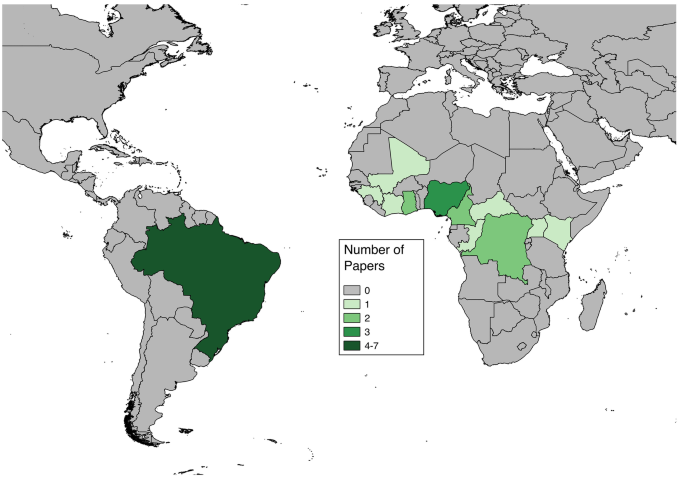
Numbers of articles found through systematic literature review reporting case fatality risk data for severe Yellow Fever cases for each nation. Some articles contained data for multiple nations
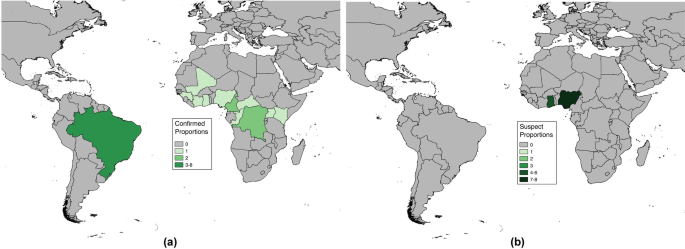
Numbers of proportions for case fatality risk among severe Yellow Fever cases found through systematic literature review by nation. Numbers of proportions are separated by ( a ) confirmed and ( b ) suspect severe Yellow Fever cases
Estimates of CFR
Forest plots are shown in Fig. 4 , separated by confirmed and suspect YF cases. The individual numerators and denominators in each study are shown in Table 1 .
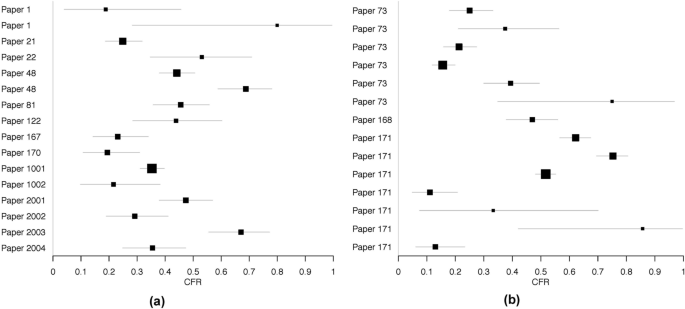
Forest plots of case fatality risk estimates among severe ( a ) laboratory confirmed and ( b ) suspect Yellow Fever cases found through literature review. Only risk estimates not equal to zero are included
The estimated CFR among all severe cases was 39 %, with a 95 % confidence interval of [31 %, 47 %]. Separating CFR by case confirmation yielded no substantial differences in CFR. Including or excluding the four recent clinical studies from Brazil also did not lead to substantial differences in CFR estimates (Table 2 ). Much heterogeneity was seen among studies, as indicated by I 2 values.
Stratified CFR
The CFRs for severe YF cases were stratified by characteristics of the articles to account for potential heterogeneities in either YF dynamics or data collection and reporting. First, CFRs were stratified by continent. Combining proportions of both confirmed and suspected severe cases, the estimated CFR among severe cases in African countries is 36 % (95 % CI: [27 %, 45 %], n = 22), and the estimated CFR among South American countries is 47 % (95 % CI: [38 %, 57 %], n = 8). Among investigative studies, the estimated CFR was 39 % (95 % CI: [30 %, 48 %], n = 22), and among reporting articles, the estimated CFR was 39 % (95 % CI: [27 %, 53 %], n = 8). Among studies that reported severe cases with only fever and jaundice, the estimated CFR was 38 % (95 % CI: [24 %, 53 %], n = 13), and among studies with cases showing other symptoms beyond fever and jaundice, the estimated CFR was 39 % (95 % CI: [32 %, 48 %], n = 17).
This study aimed to systematically evaluate the CFR for severe YF cases, defined as cases showing fever with either jaundice or hemorrhaging. A systematic literature review was conducted in order to find reported proportions of fatal cases of severe YF. Using 30 proportions recorded from 17 articles in a meta-analysis, the estimated CFR was 39 %, which was consistent among both confirmed and suspected severe cases. Separating the CFR by continent showed a notably higher CFR in South America compared to Africa, separating the CFR among severe cases by article type showed no difference in CFR between investigative studies and passively reported cases, and separating by inclusion of symptoms beyond fever and jaundice showed no difference in CFR estimates. The estimated CFR of 39 % is lower than the common estimate of half of severe cases being fatal [ 23 ] but consistent with other fatality ranges reported [ 26 , 28 ]. While there is uncertainty around all estimates, a difference of approximately 10 percentage points between this study’s estimate and the statement of half of cases being fatal may be notable for clinical decision-making and perceived mortality of YF.
The drastic difference in estimated CFR between South America and Africa may potentially result from differences in data collection as well as differences in YF dynamics. Other systematic reviews estimating CFR have seen geographic differences, including separating Hong Kong and other regions from mainland China in estimating CFR for hand foot and mouth disease [ 54 ] and separating WHO world regions to stratify CFR for Salmonella infections [ 55 ]. The differences seen across geographic locations in these studies were less pronounced than the difference in CFR between continents found in this study.
Different strains of YF are found between South America and Africa [ 56 , 57 ] as well as different primary mosquito vectors and nonhuman primate reservoirs [ 58 ]. Clinical care also differs across countries. Though International Health Regulations require reporting of YF cases [ 59 ], implementation and surveillance quality may differ between the two continents, as well as differences in healthcare seeking behaviors, which can lead to differences in severe cases represented across each continent. The small number of proportions representing CFR in South America may also account for the difference in estimated CFR across continents, as having eight South American proportions makes the CFR estimate more sensitive to any single study providing a non-representative sample of severe YF cases.
Across studies, there was significant heterogeneity among the CFRs reported (Fig. 4 ; Table 2 ). Among other reviews estimating CFR for other infectious diseases, high heterogeneities have also been seen [ 55 , 60 , 61 ]. Potential sources of heterogeneity across the different studies include differences in surveillance resources as well as differences in healthcare infrastructure across the various settings of the studies.
The results of this study show a reported CFR that is notably lower than the estimate of 50 % reported by the WHO [ 23 ] and by Johansson et al. [ 15 ]. This does not, however, suggest that the estimated CFR from this study will apply to every outbreak situation. The CFRs of other diseases have been seen to change over time [ 13 ] and may differ in relation to industrialization [ 62 ]. The estimate yielded in this study should be used as an average CFR and broad recommendation.
It is important to note that the interpretation of the estimated CFR in this study is based on the case definition used. This study produced estimates for the CFR among severe YF cases rather than among all YF cases, with severe YF cases defined by symptoms as described previously. A CFR among all YF cases, which would commonly include all symptomatic cases beyond the definition imposed in this study, would be lower. Further, an estimate of the infection fatality ratio (IFR) would represent risk of fatality among all infections, which includes asymptomatic infections, and be even lower than the CFR for all YF cases. The CFR of 39 % should only be applied to YF cases with fever and jaundice or hemorrhaging rather than to all infections or cases outside this definition; severe YF cases comprise approximately 15 % of all YF cases [ 63 ], and an even lower percentage of infections, so the IFR would be expected to be notably lower than 39 %. Relaxing the symptomatic definition to include cases of confirmed YF presenting in healthcare settings did not lead to notable differences in CFR estimates (Table 2 ).
This study’s literature review yielded a total of 18 relevant studies, which provided 36 proportions of fatal severe YF cases, 30 of which were included in meta-analysis. Other studies using systematic review methods to estimate the CFR of other diseases commonly have more available papers relevant to the study aims [ 16 , 55 , 60 ], though others have had similarly lower article counts [ 54 , 61 , 64 ]. Many of these studies also used the I 2 statistic to consider heterogeneity, with many of them similarly showing high heterogeneity [ 20 , 21 , 22 ]. Stratification by geography was also seen in other studies [ 54 , 55 ].
This study benefits from the use of a comprehensive strategy for literature review, which maximizes the completeness of data available for analysis. Conducting a literature review rather than estimating CFR solely from surveillance data allowed multiple outbreak investigations to contribute to the data analysis. As a result, studies with researchers playing a more active role in surveillance of YF cases, which may have greater accuracy, were included.
This study also stratified CFR by the methods of the individual studies into investigative and reporting studies. Separating the studies by the researchers’ involvement in patient recruitment and assessing symptoms of cases, however, showed no difference in CFR between studies with researchers involved in the investigation and studies reporting surveillance statistics. Prior to updating the search strategy, however, studies reporting surveillance statistics had a higher CFR (44 %, 95 % CI: [28 %, 61 %]). Both types of studies could experience different limitations to accuracy. Following the updated search strategy results, the similarity between the two estimates can demonstrate consistency, and potentially validity, in these two types of surveillance.
The analyses in this study included CFR estimates for confirmed and suspected severe YF cases separately as well as combined. Because YF can present similar symptoms and be misdiagnosed for other diseases such as dengue [ 65 ], there is less certainty of whether suspected YF cases in this study are true YF cases. However, the similarity in stratified results comparing laboratory confirmed and suspected YF cases shows that excluding the suspected cases from analyses would not lead to a substantial change in conclusions.
Use for estimating burden
An initial aim of this study was to use the systematic review to also collect data to estimate total cases through estimating proportions of cases that are asymptomatic and mild, similarly to the 2014 study by Johansson et al. [ 15 ]. There were insufficient studies from the literature review to reliably generate these estimates due to inconsistency of study results and few studies reporting such information. This is evidence of the challenges inherent to collecting highly detailed data, particularly in less affluent nations, which typically experience higher burdens of YF and other vector-borne diseases. However, having a reliable estimate for CFR, as generated in this study, can prove useful for attempting to quantify underreporting of YF cases. Case reports with higher proportions of fatal cases may suffer from underreporting under the assumption that the CFR found in this study is broadly applicable to other incidence of YF. For example, using data provided from the Pan American Health Organization and the Brazilian Ministry of Health, 157 fatal among 327 confirmed cases of YF were reported via surveillance in Brazil between 2000 and 2014, which may include non-severe cases. Under the assumptions that the 39 % CFR found in this study is applicable to these data, no fatal cases were unreported, and only severe cases become fatal, estimates of actual case counts can be produced. By multiplying the 157 fatal cases by the inverse of the estimated CFR, an estimate of 403 severe YF cases is obtained. If all 327 reported cases were severe cases, then approximately 19 % of severe cases were undetected. This serves as a minimum proportion of underreported cases rather than an estimate [ 15 ] since this assumes all fatal cases were observed and all reported cases were severe.
Limitations and future directions
Through the literature search, 14 studies were identified to fit the defined criteria for severe YF, 13 of which were suitable to be used in meta-analysis. The requirement that studies must indicate that cases present fever as well as either jaundice or hemorrhaging for inclusion in this study led to several studies to be excluded. Many studies stated numbers of cases and fatalities without specifying symptoms [ 44 , 66 , 67 , 68 , 69 , 70 , 71 ], which included studies from the recent outbreak in Angola [ 66 , 67 ]. Studies representing cases from the recent YF outbreak in Brazil, though not stating that all cases reported showed the symptoms, were added to the analyses to show whether inclusion of confirmed cases from a clinical setting might impact substantive results [ 50 , 51 , 52 , 53 ]. While the symptom requirements in this study led to potentially useful sources of information to be excluded from analysis, they do increase confidence that the CFR estimated applies directly to severe YF cases by not including potentially mild cases. Similarity in results when including four studies of YF cases seeking healthcare increase confidence that the results may be more broadly applicable.
The results of this study rely on the reported data from the 17 studies used in the meta-analysis. Because the purpose of these articles was not necessarily to offer estimates of the CFR for YF, it is possible that maximizing the accuracy of fatal and nonfatal case counts was not the highest priority. The studies detailing outbreak investigations do report numbers of severe and fatal cases, but the purpose was not to assure generalizable accuracy of the CFR. This possibility is even stronger among reporting studies. Since underreporting of infectious disease cases is a well established issue [ 72 ], it is likely that the proportions used in this analysis may also be subject to issues of data quality.
Within this review, the results are limited by the heterogeneity in studies and the assumption that different world regions are expected to have similar CFRs. In combining the studies across nations in South America and Africa, where stratified CFRs differed notably, it is assumed that the differences observed are artifacts of the individual studies rather than indicative of actual differences in CFR across the two continents. Heterogeneity likely exists within continents as well, as the nations represented in this study include both East and West Africa (Fig. 2 ). This heterogeneity may result from actual differences in probability of fatality; risk of fatality may differ by population demographics [ 1 , 73 ] or national industrialization [ 62 ], as seen in other diseases.
Among severe cases of YF, the CFR is estimated to be approximately 39 % based on the results of a systematic literature review. This is lower than the frequently cited CFR for severe cases, indicating that the previous estimate is either a cautious estimate or based on underreported data. However, these results indicate high fatality among severe YF cases, demonstrating the public health importance of this disease. Preventative measures such as vaccination and diagnosis methods are of importance for reducing deaths from YF.
Use of systematic reviews for estimating CFR has been seen for other diseases, and this method can be extended to further characteristics of various diseases beyond CFR. Further research is needed to distinguish among asymptomatic, mild, and severe YF infections in order to most accurately estimate the total burden of disease. The estimate of CFR found in this study can be used to estimate potential mortality in future YF outbreaks.
Availability of data and materials
The data analyzed within this study are shown in Table 1 .
Abbreviations
- Yellow Fever
Case fatality risk
Preferred reporting items for systematic reviews and meta-analyses
Polymerase chain reaction
Wong JY, Kelly H, Ip DKM, Wu JT, Leung GM, Cowling BJ. Case fatality risk of influenza a (H1N1pdm09): a systematic review. Epidemiology. 2013;24(6):830–41.
Article PubMed Google Scholar
Kelly H, Cowling BJ, Case Fatality. Epidemiology. 2013;24(4):622–3.
Salama M, Amitai Z, Lustig Y, Mor Z, Weiberger M, Chowers M, et al. Outbreak of West Nile Virus disease in Israel (2015): A retrospective analysis of notified cases. Travel Med Infect Dis. 2019;1:41–5.
Article Google Scholar
Kim KH, Tandi TE, Choi JW, Moon JM, Kim MS. Middle East respiratory syndrome coronavirus (MERS-CoV) outbreak in South Korea, 2015: epidemiology, characteristics and public health implications. J Hosp Infect. 2017;95(2):207–13.
Article CAS PubMed Google Scholar
Self JL, Conrad A, Stroika S, Jackson A, Whitlock L, Jackson KA, et al. Multistate outbreak of listeriosis associated with packaged leafy green salads, United States and Canada, 2015–2016. Emerg Infect Dis. 2019;25(8):1461–8.
Article PubMed PubMed Central Google Scholar
Cromer D, Van Hoek AJ, Jit M, Edmunds WJ, Fleming D, Miller E. The burden of influenza in England by age and clinical risk group: a statistical analysis to inform vaccine policy. J Infect. 2014;68(4):363–71.
Yu AT, Amin N, Rahman MW, Gurley ES, Rahman KM, Luby SP. Case-fatality ratio of blood culture-confirmed typhoid fever in Dhaka, Bangladesh. J Infect Dis. 2018;218(suppl_4):S222-6.
Reich NG, Lessler J, Cummings DAT, Brookmeyer R. Estimating absolute and relative case fatality ratios from infectious disease surveillance data. Biometrics. 2012;68(2):598–606.
Elandt-Johnson RC. Definition of rates: some remarks on their use and misuse. Am J Epidemiol. 1975;102(4):267–71.
Debnath F, Ponnaiah M, Acharya P. Dengue fever in a municipality of West Bengal, India, 2015: an outbreak investigation. Indian J Public Health. 2017;61(4):239–42.
Jia N, Feng D, Fang LQ, Richardus JH, Han XN, Cao WC, et al. Case fatality of SARS in mainland China and associated risk factors. Trop Med Int Heal. 2009;14(SUPPL. 1):21–7.
Yu H, Cowling BJ, Feng L, Lau EHY, Liao Q, Tsang TK, et al. Human infection with avian influenza A H7N9 virus: an assessment of clinical severity. Lancet. 2013;13(9887):138–45.
Gao Z, Parhar A, Gallant V, Heffernan C, Ahmed R, Egedahl ML, et al. Apopulation-based study of tuberculosis case fatality in Canada: do aboriginal peoples fare less well? Int J Tuberc Lung Dis. 2015;19(7):772–9.
Lipsitch M, Donnelly CA, Fraser C, Blake IM, Cori A, Dorigatti I, et al. Potential biases in estimating absolute and relative case-fatality risks during outbreaks. PLoS Negl Trop Dis. 2015;9(7):e0003846.
Article PubMed PubMed Central CAS Google Scholar
Johansson MA, Vasconcelos PFC, Staples JE. The whole iceberg: estimating the incidence of yellow fever virus infection from the number of severe cases. Trans R Soc Trop Med Hyg. 2014;108(8):482–7.
Uche IV, MacLennan CA, Saul A. A systematic review of the incidence, risk factors and case fatality rates of Invasive Nontyphoidal Salmonella (iNTS) disease in Africa (1966 to 2014). PLoS Negl Trop Dis. 2017. https://doi.org/10.1371/journal.pntd.0005118 .
Tan B, Wong JJM, Sultana R, Koh JCJW, Jit M, Mok YH, et al. Global case-fatality rates in pediatric severe sepsis and septic shock: a systematic review and meta-analysis. JAMA Pediatr. 2019;173(4):352–62.
Kenmoe S, Demanou M, Bigna JJ, Nde Kengne C, Fatawou Modiyinji A, Simo FBN, et al. Case fatality rate and risk factors for Nipah virus encephalitis: a systematic review and meta-analysis. J Clin Virol. 2019;117:19–26.
Woldeamanuel YW, Andemeskel AT, Kyei K, Woldeamanuel MW, Woldeamanuel W. Case fatality of adult tetanus in Africa: systematic review and meta-analysis. J Neurol Sci. 2016;368:292–9.
Portnoy A, Jit M, Ferrari M, Hanson M, Brenzel L, Verguet S. Estimates of case-fatality ratios of measles in low-income and middle-income countries: a systematic review and modelling analysis. Lancet Glob Heal. 2019;7(4):e472-81.
Szabo SM, Samp JC, Walker DR, Lane S, Cline SK, Gooch KL, et al. Liver-specific case fatality due to chronic hepatitis C virus infection: a systematic review. Ann Hepatol. 2015;14(5):618–30.
Couto-Lima D, Madec Y, Bersot MI, Campos SS, de Motta MA, dos Santos FBDFB, et al. Potential risk of re-emergence of urban transmission of Yellow Fever virus in Brazil facilitated by competent Aedes populations. Sci Rep. 2017;7(1):4848.
World Health Organization. Yellow fever [Internet]. Fact Sheets. 2018. https://doi.org/http://www.who.int/en/news-room/fact-sheets/detail/yellow-fever. Accessed 21 Jul 2018.
Gubler DJ. Pandemic yellow fever: a potential threat to global health via travelers. J Travel Med. 2018. https://doi.org/10.1093/jtm/tay097 .
Wilder-Smith A, Leong WY. Importation of yellow fever into China: assessing travel patterns. J Travel Med. 2017. https://doi.org/10.1093/jtm/tax008 .
Centers for Disease Control and Prevention. Yellow Fever [Internet]. Global Health - Newsroom. 2018. https://doi.org/https://www.cdc.gov/globalhealth/newsroom/topics/yellowfever/index.html. Accessed 9 Sep 2020.
Liu Y, Rocklöv J. What is the reproductive number of yellow fever? J Travel Med. 2020. https://doi.org/10.1093/jtm/taaa156 .
Centers for Disease Control and Prevention. Yellow Fever [Internet]. https://doi.org/https://www.cdc.gov/yellowfever/symptoms/index.html. Accessed 25 Feb 2019.
Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Heal Inf Libr J. 2009;1(2):91–108.
World Health Organization. Yellow fever [Internet]. 2019. https://doi.org/https://www.who.int/news-room/fact-sheets/detail/yellow-fever. Accessed 17 Apr 2021.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009. https://doi.org/10.1371/journal.pmed.1000100 .
PRISMA Checklist [Internet]. 2015. https://doi.org/http://prisma-statement.org/PRISMAStatement/Checklist.aspx. Accessed 1 May 2020.
Devito NJ, Goldacre B. Catalogue of bias: publication bias. BMJ Evidence-Based Med. 2019;24(1):53–4.
Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–60.
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;15(11):1539–58.
World Health Organization. Yellow fever in Africa and South America. Relev Epidemiol Hebd 2008. 2006;83(8):60–76.
Google Scholar
Wamala JF, Malimbo M, Okot CL, Atai-Omoruto AD, Tenywa E, Miller JR, et al. Epidemiological and laboratory characterization of a yellow fever outbreak in northern Uganda, October 2010-January 2011. Int J Infect Dis. 2012;16(7):e536-42.
de Filippis AMB, Nogueira RMR, Schatzmayr HG, Tavares DS, Jabor AV, Diniz SCM, et al. Outbreak of jaundice and hemorrhagic fever in the Southeast of Brazil in 2001: detection and molecular characterization of yellow fever virus. J Med Virol. 2002;68(4):620–7.
Article PubMed CAS Google Scholar
Tuboi SH, Costa ZGA, da Costa Vasconcelos PF, Hatch D. Clinical and epidemiological characteristics of yellow fever in Brazil: analysis of reported cases 1998–2002. Trans R Soc Trop Med Hyg. 2007;101(2):169–75.
Jones EM, Wilson DC. Clinical features of yellow fever cases at Vom Christian Hospital during the 1969 epidemic on the Jos Plateau, Nigeria. Bull World Health Organ. 1972;46(5):653–7.
CAS PubMed PubMed Central Google Scholar
World Health Organization. Weekly epidemiological record. 1993;68(11):77–8.
Ingelbeen B, Weregemere NA, Noel H, Tshapenda GP, Mossoko M, Nsio J, et al. Urban yellow fever outbreak-Democratic Republic of the Congo, 2016: Towards more rapid case detection. PLoS Negl Trop Dis. 2018;12(12):e0007029.
Monath TP, Craven RB, Adjukiewicz A, Germain M, Francy DB, Ferrara L, et al. Yellow fever in the Gambia, 1978–1979: Epidemiologic aspects with observations on the occurrence of Orungo virus infections. Am J Trop Med Hyg. 1980;29(5):912–28.
Yellow fever in Africa and Central and South America, 2008–2009. Relev Epidemiol Hebd. 2011;86(4):25–36.
Agadzi VK, Boatin BA, Appawu MA, Mingle JA, Addy PA. Yellow fever in Ghana, 1977-80. Bull World Health Organ. 1984;62(4):577–83.
De Cock KM, Monath TP, Nasidi A, Tukei PM, Enriquez J, Lichfield P, et al. Epidemic yellow fever in eastern Nigeria, 1986. Lancet (London, England). 1988;1(8586):630–3.
Nasidi A, Monath TP, DeCock K, Tomori O, Cordellier R, Olaleye OD, et al. Urban yellow fever epidemic in western Nigeria, 1987. Trans R Soc Trop Med Hyg. 1989;83(3):401–6.
Cunha MDP, Duarte-Neto AN, Pour SZ, Ortiz-Baez AS, Černý J, de Pereira BB, et al. Origin of the São Paulo Yellow Fever epidemic of 2017–2018 revealed through molecular epidemiological analysis of fatal cases. Sci Rep. 2019;9(1):20418.
Article CAS PubMed PubMed Central Google Scholar
Otshudiema JO, Ndakala NG, Mawanda EK, Tshapenda GP, Kimfuta JM, Nsibu L-RN, et al. Yellow Fever Outbreak—Kongo Central Province, Democratic Republic of the Congo, August 2016. MMWR Morb Mortal Wkly Rep. 2017;31(12):335–8.
de Ávila RE, José Fernandes H, Barbosa GM, Araújo AL, Gomes TCC, Barros TG, et al. Clinical profiles and factors associated with mortality in adults with yellow fever admitted to an intensive care unit in Minas Gerais, Brazil. Int J Infect Dis. 2020;93:90–7.
Ribeiro AF, Cavalin RF, Abdul Hamid Suleiman JM, Alves da Costa J, Januaria de Vasconcelos M, Sant’Ana Málaque CM, et al. Yellow Fever: Factors Associated with Death in a Hospital of Reference in Infectious Diseases, São Paulo, Brazil, 2018. Am J Trop Med Hyg. 2019;101(1):180–8.
Ho Y-L, Joelsons D, Leite GFC, Malbouisson LMS, Song ATW, Perondi B, et al. Severe Yellow Fever in Brazil: clinical characteristics and management. J Travel Med. 2019. https://doi.org/10.1093/jtm/taz040 .
Kallas EG, D’Elia Zanella LGFAB, Moreira CH V, Buccheri R, Diniz GBF, Castiñeiras ACP, et al. Predictors of mortality in patients with yellow fever: an observational cohort study. Lancet Infect Dis. 2019;19(7):750–8.
Zhao YY, Jin H, Zhang XF, Wang B. Case-fatality of hand, foot and mouth disease associated with EV71: A systematic review and meta-analysis. Epidemiol Infect. 2015;143(14):3094–102.
Pieters Z, Saad NJ, Antillón M, Pitzer VE, Bilcke J. Case Fatality Rate of Enteric Fever in Endemic Countries: A Systematic Review and Meta-analysis. Clin Infect Dis. 2018;67(4):628–38.
Mutebi JP, Barrett ADT. The epidemiology of yellow fever in Africa. Microbes Infect. 2002;4(14):1459–68.
Barrett ADT, Higgs S. Yellow Fever: a disease that has yet to be conquered. Annu Rev Entomol. 2007;52(1):209–29.
Auguste AJ, Lemey P, Pybus OG, Suchard MA, Salas RA, Adesiyun AA, et al. Yellow Fever virus maintenance in Trinidad and its dispersal throughout the Americas. J Virol. 2010;84(19):9967–77.
Gardner CL, Ryman KD. Yellow fever: a reemerging threat. Clin Lab Med. 2010;30(1):237–60.
Jin H, Zhao Y, Zhang X, Wang B, Liu P. Case-fatality risk of pregnant women with acute viral hepatitis type E: a systematic review and meta-analysis. Epidemiol Infect. 2016;144(10):2098–106.
Nyakarahuka L, Kankya C, Krontveit R, Mayer B, Mwiine FN, Lutwama J, et al. How severe and prevalent are Ebola and Marburg viruses? A systematic review and meta-analysis of the case fatality rates and seroprevalence. BMC Infect Dis. 2016;16(1):708.
Rota PA, Moss WJ, Takeda M, De Swart RL, Thompson KM, Goodson JL. Measles. Nat Rev Dis Prim. 2016 Jul 14;2(1):1–16.
Symptoms, diagnosis, & treatment [Internet]. https://doi.org/https://www.cdc.gov/yellowfever/symptoms/index.html. Accessed 16 Apr 2021.
Woldeamanuel YW, Girma B. A 43-year systematic review and meta-analysis: case-fatality and risk of death among adults with tuberculous meningitis in Africa. J Neurol. 2014;261(5):851–65.
Rogers DJ, Wilson AJ, Hay SI, Graham AJ. The global distribution of yellow fever and dengue. Adv Parasitol. 2006;62:181–220.
World Health Organization. Situation report: Yellow Fever. Geneva: World Health Organization; 2016.
Yellow fever in Africa and the Americas, 2016. Relev Epidemiol Hebd. 2017;92(32):442–52.
Vasconcelos PFC, Rodrigues SG, Degallier N, Moraes MAP, Travassos Da Rosa JFS, Rosa Travassos Da, et al. An epidemic of sylvatic yellow fever in the southeast region of Maranhao State, Brazil, 1993–1994: epidemiologic and entomologic findings. Am J Trop Med Hyg. 1997;57(2):132–7.
Yellow fever in Africa and South America, 2015. Relev Epidemiol Hebd. 2016;91(32):381–8.
Pinheiro FP, Travassos da Rosa AP, Moraes MA, Almeida Neto JC, Camargo S, Filgueiras JP. An epidemic of yellow fever in central Brazil. 1972–1973. I. Epidemiological studies. Am J Trop Med Hyg. 1978;27(1 Pt 1):125–32.
Anonymous. Yellow fever in 1989 and 1990. Relev Epidemiol Hebd. 1992;67(33):245–51.
Shearer FM, Longbottom J, Browne AJ, Pigott DM, Brady OJ, Kraemer MUGG, et al. Existing and potential infection risk zones of yellow fever worldwide: a modelling analysis. Lancet Glob Heal. 2018;6(3):e270-8.
Karlberg J, Chong DSY, Lai WYY. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am J Epidemiol. 2004;159(3):229–31.
Download references
Acknowledgements
Not applicable.
This study was funded by the University of Minnesota Doctoral Dissertation Fellowship. The funding source had no contribution in the study design, analyses, manuscript preparation, or decision to publish. MC acknowledges the SOUSEI funding program and GSB Gi-CORE funding at Hokkaido University, JP.
Author information
Authors and affiliations.
Division of Environmental Health Sciences, University of Minnesota School of Public Health, 420 Delaware St SE, Minneapolis, 55401, MN, USA
Joseph L. Servadio & Claudia Muñoz-Zanzi
Nexus Group and Gi-CORE, Graduate School of Information Science and Technology, Hokkaido University, Sapporo, Hokkaido, Japan
Matteo Convertino
Institute of Environment and Ecology, Tsinghua Shenzhen International Graduate School, Tsinghua University, Shenzhen, China
You can also search for this author in PubMed Google Scholar
Contributions
JLS conceptualized the study. JLS, CM, and MC developed the search strategy and analytic plan. JLS analyzed the data. JLS drafted the manuscript. JLS, CM and MC revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Joseph L. Servadio .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors have no competing interests to report.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: table s1..
Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) checklist for systematic review and meta-analysis for case fatality risk of severe Yellow Fever cases.
Additional file 2: Table S2.
Laboratory confirmation and symptom definitions used by included articles. Marked symptoms were required for case inclusion, “or__” indicates a set of symptoms where at least one from the set was required, and “some” indicates that some, but not all, cases showed the symptom.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Servadio, J.L., Muñoz-Zanzi, C. & Convertino, M. Estimating case fatality risk of severe Yellow Fever cases: systematic literature review and meta-analysis. BMC Infect Dis 21 , 819 (2021). https://doi.org/10.1186/s12879-021-06535-4
Download citation
Received : 15 January 2021
Accepted : 03 August 2021
Published : 16 August 2021
DOI : https://doi.org/10.1186/s12879-021-06535-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- case fatality risk
- systematic review
- meta-analysis
BMC Infectious Diseases
ISSN: 1471-2334
- General enquiries: [email protected]
Europe PMC requires Javascript to function effectively.
Either your web browser doesn't support Javascript or it is currently turned off. In the latter case, please turn on Javascript support in your web browser and reload this page.

Systematic review and meta-analysis of yellow fever vaccine in elderly population
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- ORCID record for Ariane de Jesus Lopes de Abreu
- For correspondence: [email protected]
- ORCID record for José Ueleres Braga
- Info/History
- Preview PDF
We conducted a systematic review and meta-analysis to assess the risk of serious adverse events in the elderly after yellow fever vaccination compared to the non-elderly population. We searched multiple databases and grey literature and selected research without language and publication date restriction. Studies were analysed in a descriptive way, meta-analysed and expressed in terms of prevalence ratio and risk ratio with a 95% confidence interval, depending on the degree of heterogeneity found. A total of 18 studies were included, of which 10 were meta-analysed. Results obtained through the meta-analysis showed that the risk of serious adverse events after yellow fever vaccination is three times higher for elderlies when compared to non-elderly population and even five times higher for persons >70 years of age. Also, in relation adverse event type a greater risk was for viscerotropic disease associated with yellow fever vaccine up to six times higher when compared to the population under 60 years. The evidence found in this review supports that the vaccine indication in individuals over 60 years of age should be based on a careful analysis of the individual benefit-risk assessment. Results found suggest a higher risk of SAE for individuals over 70 years, especially for viscerotropic and neurotropic disease associated with YFV contraindicating the use of the YFV in this age group.
Competing Interest Statement
The authors have declared that no competing interests exist.
Funding Statement
The authors received no specific funding for this work.
Author Declarations
I confirm all relevant ethical guidelines have been followed, and any necessary IRB and/or ethics committee approvals have been obtained.
Not Applicable
The details of the IRB/oversight body that provided approval or exemption for the research described are given below:
This study is a systematic literature review. Following the declaration of Helsinki and local guidelines no IRB approval is required for this study method.
I confirm that all necessary patient/participant consent has been obtained and the appropriate institutional forms have been archived, and that any patient/participant/sample identifiers included were not known to anyone (e.g., hospital staff, patients or participants themselves) outside the research group so cannot be used to identify individuals.
I understand that all clinical trials and any other prospective interventional studies must be registered with an ICMJE-approved registry, such as ClinicalTrials.gov. I confirm that any such study reported in the manuscript has been registered and the trial registration ID is provided (note: if posting a prospective study registered retrospectively, please provide a statement in the trial ID field explaining why the study was not registered in advance).
I have followed all appropriate research reporting guidelines and uploaded the relevant EQUATOR Network research reporting checklist(s) and other pertinent material as supplementary files, if applicable.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
View the discussion thread.
Thank you for your interest in spreading the word about medRxiv.
NOTE: Your email address is requested solely to identify you as the sender of this article.

Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager
- Tweet Widget
- Facebook Like
- Google Plus One
Subject Area
- Epidemiology
- Addiction Medicine (342)
- Allergy and Immunology (665)
- Anesthesia (180)
- Cardiovascular Medicine (2625)
- Dentistry and Oral Medicine (314)
- Dermatology (222)
- Emergency Medicine (397)
- Endocrinology (including Diabetes Mellitus and Metabolic Disease) (930)
- Epidemiology (12175)
- Forensic Medicine (10)
- Gastroenterology (756)
- Genetic and Genomic Medicine (4064)
- Geriatric Medicine (384)
- Health Economics (676)
- Health Informatics (2625)
- Health Policy (997)
- Health Systems and Quality Improvement (979)
- Hematology (360)
- HIV/AIDS (845)
- Infectious Diseases (except HIV/AIDS) (13659)
- Intensive Care and Critical Care Medicine (790)
- Medical Education (398)
- Medical Ethics (109)
- Nephrology (430)
- Neurology (3832)
- Nursing (209)
- Nutrition (570)
- Obstetrics and Gynecology (734)
- Occupational and Environmental Health (690)
- Oncology (2008)
- Ophthalmology (581)
- Orthopedics (238)
- Otolaryngology (304)
- Pain Medicine (250)
- Palliative Medicine (73)
- Pathology (471)
- Pediatrics (1107)
- Pharmacology and Therapeutics (459)
- Primary Care Research (447)
- Psychiatry and Clinical Psychology (3400)
- Public and Global Health (6499)
- Radiology and Imaging (1390)
- Rehabilitation Medicine and Physical Therapy (806)
- Respiratory Medicine (869)
- Rheumatology (400)
- Sexual and Reproductive Health (407)
- Sports Medicine (338)
- Surgery (441)
- Toxicology (52)
- Transplantation (185)
- Urology (165)
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Environ Health

Potential impacts of synthetic food dyes on activity and attention in children: a review of the human and animal evidence
Mark d. miller.
1 Office of Environmental Health Hazard Assessment, California Environmental Protection Agency, 1515 Clay St, Oakland CA, and 1001 I St, Sacramento, California, USA
Craig Steinmaus
Mari s. golub, rosemary castorina.
2 Center for Environmental Research and Community Health, School of Public Health, University of California, 2121 Berkeley Way, Berkeley, California, USA
Ruwan Thilakartne
Asa bradman.
3 Department of Public Health, School of Social Sciences, Humanities and Arts, University of California, Merced, 5200 N Lake Road, Merced, CA USA
Melanie A. Marty
Associated data.
As this is a review, data sharing is not applicable to this article as no datasets were generated during the current study. Details of the studies we reviewed are contained in the supplementary tables. The study quality review and coding are available in the supplementary files. Exposure estimates were based on the National Health and Nutrition Examination Survey conducted in 2015 and 2016: CDC. 2017. NHANES 2015–2016 Demographics Data. Available: https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Demographics & CycleBeginYear = 2015: CDC. 2018. NHANES Dietary Data. Available: https://wwwn.cdc.gov/nchs/nhanes/Search/DataPage.aspx?Component=Dietary . CDC. 2019. National Health and Nutrition Examination Survey. Available: https://www.cdc.gov/nchs/nhanes/index.htm .
Concern that synthetic food dyes may impact behavior in children prompted a review by the California Office of Environmental Health Hazard Assessment (OEHHA). OEHHA conducted a systematic review of the epidemiologic research on synthetic food dyes and neurobehavioral outcomes in children with or without identified behavioral disorders (particularly attention and activity). We also conducted a search of the animal toxicology literature to identify studies of neurobehavioral effects in laboratory animals exposed to synthetic food dyes. Finally, we conducted a hazard characterization of the potential neurobehavioral impacts of food dye consumption. We identified 27 clinical trials of children exposed to synthetic food dyes in this review, of which 25 were challenge studies. All studies used a cross-over design and most were double blinded and the cross-over design was randomized. Sixteen (64%) out of 25 challenge studies identified some evidence of a positive association, and in 13 (52%) the association was statistically significant. These studies support a relationship between food dye exposure and adverse behavioral outcomes in children. Animal toxicology literature provides additional support for effects on behavior. Together, the human clinical trials and animal toxicology literature support an association between synthetic food dyes and behavioral impacts in children. The current Food and Drug Administration (FDA) acceptable daily intakes are based on older studies that were not designed to assess the types of behavioral effects observed in children. For four dyes where adequate dose-response data from animal and human studies were available, comparisons of the effective doses in studies that measured behavioral or brain effects following exposure to synthetic food dyes indicate that the basis of the ADIs may not be adequate to protect neurobehavior in susceptible children. There is a need to re-evaluate exposure in children and for additional research to provide a more complete database for establishing ADIs protective of neurobehavioral effects.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12940-022-00849-9.
Concerns about possible associations between exposure to synthetic food dyes and the exacerbation of symptoms of Attention Deficit/Hyperactivity Disorder (ADHD) in children have surfaced periodically since the 1970s. The concern prompted the California legislature to request a review by the California Environmental Protection Agency’s Office of Environmental Health Hazard Assessment (OEHHA) of available studies to evaluate whether the synthetic food dyes currently allowed in foods and medications in the United States impact neurobehavior in children [ 1 ]. This paper provides an overview of key portions of OEHHA’s peer-reviewed assessment, specifically the evaluation of the clinical trials of synthetic food dyes in children and available animal toxicology studies, as well as discussion of our hazard characterization and the possible public health implications of our findings.
Our evaluation focused on seven of the nine food dyes subject to FD&C batch certification by the US Food and Drug Administration (FDA) and approved for general use in food in the US (Table 1 ). These seven dyes contribute nearly all of the exposure to synthetic food dyes for the general US public [ 1 ]. The term “FD&C batch-certified” refers to the Food Drug and Cosmetic Act requirements for chemical analysis of each manufactured batch of food dye to ensure that specific contaminants are present below legal limits. OEHHA evaluated the literature to determine whether there is any evidence supporting the association of exposure to synthetic food dyes with adverse neurobehavioral impacts in children in the general population with or without a diagnosis of ADHD.
US FDA batch-certified food colors addressed in OEHHA’s report
| Food Dye | Common Synonym | CAS # |
|---|---|---|
| FD&C Blue No. 1 | Brilliant Blue | 3844-45-9 |
| FD&C Blue No. 2 | Indigo Carmine, Indigotine | 860–22-0 |
| FD&C Green No. 3 | Fast Green | 2353-45-9 |
| FD&C Red No. 3 | Erythrosine | 16,423–68-0 |
| FD&C Red No. 40 | Allura Red | 25,956–17-6 |
| FD&C Yellow No. 5 | Tartrazine | 1934-21-0 |
| FD&C Yellow No. 6 | Sunset Yellow | 2783-94-0 |
The literature review methods were designed to identify all the literature most relevant to the assessment of evidence on the neurological or neurobehavioral effects of the synthetic food dyes listed in Table Table1. 1 . The search was executed to identify peer-reviewed open-source and proprietary journal articles, print and digital books, reports, and gray literature that potentially reported relevant toxicological and epidemiological information. We also included Citrus Red No. 2 and Orange B/CI Acid Orange in the search terms since these food dyes are part of an overlapping literature that might contain information on the commonly used FD&C synthetic food dyes. PubMed MeSH browser (PubMed MeSH browser) and PubChem ( PubChem ) were used to identify subject headings, other index terms and synonyms for the food dyes of interest and their metabolites, as well as for the concepts related to exposure, food, mechanisms of action, and neurological outcomes. Preliminary searches were run and results reviewed to identify additional terms. The concepts were combined in the following manner:
((food/dietary terms) AND (specific food dye terms)) OR ((specific food dye terms) AND (neurological outcome terms) OR (general exposure terms) OR (mechanisms of action terms))
The detailed search strategy executed in PubMed on November 26, 2018 is summarized in the additional information (Table A.1). This search was run again to capture literature updates, on March 8, 2019 and April 22, 2019, and again in October 2020.
Additional databases (PubMed, Embase, Scopus) and other data sources (European Food Safety Authority (EFSA) Journal, EFSA Scientific Output, US FDA Safety Information Office, University of California, San Francisco Food Industry Documents Archive, and Dyes and Pigments Journal) were also searched; strategies were tailored according to the search features unique to each database and data source. Relevant literature was also identified from citations in individual articles. In addition, we searched NIH RePort to identify additional unpublished clinical trials or animal research. In our systematic review of the epidemiologic research on synthetic food dyes and neurobehavioral outcomes in children, we summarized the major strengths and weaknesses of each study, described any consistencies across study results, and if heterogeneity exists, identified its sources as far as possible [ 1 ].
Our epidemiologic review focused on clinical trials. A major advantage of this type of study is that investigators generally have control over the exposure which can help reduce bias and confounding compared to other study designs. Next, we conducted systematic evaluations of study methods and quality to ensure an emphasis on the high quality studies for our conclusions. In evaluating study quality, we utilized criteria based on the National Toxicology Program’s OHAT Risk of Bias Rating Tool [ 2 ]. We modified these to be specific to randomized clinical trials (RCT) on artificial food dyes and childhood neurobehavior. We examined several key characteristics of each study to assess study quality including design, participant selection, exposure levels, age groups, washout period, infractions, outcome metric, and funding (Table A.2). This table also includes key information on results including statistical significance, effect size, dose-response, and subgroups. The coding used in our statistical analyses and quality scoring is provided in Tables A.3 and A.4. These tables show the criteria used to evaluate study quality, which included randomization, placebo use, dropout rate, blinding, whether dose-response was assessed, outcome metric validation, replication, and adequate washout. All this information was considered in making our overall conclusions about the human study results.
In determining whether the study reported an association, we define association as either a statistically significant outcome ( p value <.05 or 95% confidence intervals that excluded 1.0 for relative risk estimates or 0 for mean differences) or an effect size ≥20% or standardized effect size ≥0.20. Most studies involved small sample sizes and thus may not have had sufficient statistical power to identify effects that are relatively small but still of public health importance. Because of this, in addition to statistical significance, bias and effect size were also considered in our evaluations of association and causal inference. There are several arguments against solely using statistical significance to identify associations [ 3 , 4 ].
We searched the animal toxicology literature and identified numerous studies of neurobehavioral effects in laboratory animals exposed to synthetic food dyes. These included studies of exposures during prenatal, infant, and juvenile development, examining neurobehavioral effects in the offspring manifest during development and/or later in adult animals. The availability of studies at different developmental stages allowed a comprehensive review of adverse developmental effects, although it limited the ability to compare results across study designs, as exposures during different developmental stages may manifest differently later in life. The OEHHA report reviewed all available studies and provided strengths and limitations for the individual studies [ 1 ].
Acceptable Daily Intakes (ADIs) for synthetic food dyes were established by the US FDA between the 1960s and the1980s based on general toxicology studies. OEHHA therefore also evaluated whether newer studies that included neurobehavioral assessment would be useful for developing updated acceptable exposure levels that explicitly account for and protect against neurobehavioral effects of individual food dyes. OEHHA compared the results of those specific studies to the existing US FDA ADIs, as well as ADIs developed by the Joint FAO/WHO Expert Committee on Food Additives (JECFA).
Review of clinical trial studies
In total, 27 clinical trials were identified that met each of the following criteria:
- Human study
- Clinical trial design
- Participants were given a known quantity of synthetic food dyes or a diet low in or eliminating synthetic food dyes
- A neurobehavioral outcome related to hyperactivity or inattention was assessed
- The majority of participants were children ≤19 years of age
- The effects of an active ingredient or elimination diet were compared to those of a placebo
Studies were excluded if they were:
- Studies involving cohort, case-control, or cross-sectional designs
- Studies that assessed the effects of a broad range of food groups, including elimination studies, and did not specifically evaluate synthetic food dyes. Any effect identified in such studies would be difficult to ascribe specifically to synthetic food dyes.
No exclusions were made based on the number of participants, participation rates, blinding, randomization, or source (e.g., government reports), although each of these factors was considered in our review of study quality and in our overall conclusions.
Figure 1 presents the results of our literature search as the number of clinical studies reporting adverse neurobehavioral outcomes by key study variables. Of the 27 studies meeting our criteria for inclusion, 25 involved challenge studies, which we consider most relevant as they directly challenge children with food dyes, and two involved diet elimination studies. Detailed descriptions of the 25 included challenge studies are provided in Table A.2. Table 2 below summarizes the characteristics and overall findings of the reviewed challenge studies. Several studies of exposure to dye mixtures also included other dyes not used in the US.

Number of clinical studies reporting positive associations by key study variables
Clinical trials of synthetic food dyes and neurobehavioral outcomes in children: summary of study results
| Total | No association | Association identified | Large effect size | Statistically significant | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | N | N | % | N | % | N | % | N | % | |
| All studies | 25 | 9 | 36.0 | 16 | 64.0 | 3 | 12.0 | 13 | 52.0 | |
| Group results | ||||||||||
| Parent | 14 | 7 | 50.0 | 7 | 50.0 | 1 | 7.1 | 6 | 42.9 | Ref |
| Teacher | 7 | 6 | 85.7 | 1 | 14.3 | 0 | 0.0 | 1 | 14.3 | 0.11 |
| Other | 14 | 9 | 64.3 | 5 | 35.7 | 2 | 14.3 | 3 | 21.4 | 0.45 |
| Individual results | ||||||||||
| Parent | 12 | 3 | 25.0 | 9 | 75.0 | 4 | 33.3 | 5 | 41.7 | Ref |
| Teacher | 2 | 1 | 50.0 | 1 | 50.0 | 1 | 50.0 | 0 | 0.0 | 0.12 |
| Other | 5 | 1 | 20.0 | 4 | 80.0 | 3 | 60.0 | 1 | 20.0 | 0.91 |
| Study quality | ||||||||||
| Higher | 12 | 4 | 33.3 | 8 | 66.7 | 2 | 16.7 | 6 | 50.0 | Ref |
| Lower | 13 | 5 | 38.5 | 8 | 61.5 | 1 | 7.7 | 7 | 53.8 | 0.79 |
| Publication year | ||||||||||
| Before 1990 | 19 | 8 | 42.1 | 11 | 57.9 | 3 | 15.8 | 8 | 42.1 | Ref |
| 1990 and later | 6 | 1 | 16.7 | 5 | 83.3 | 0 | 0.0 | 5 | 83.3 | 0.26 |
| Location | ||||||||||
| United States | 10 | 4 | 40.0 | 6 | 60.0 | 2 | 20.0 | 4 | 40.0 | Ref |
| Elsewhere | 15 | 5 | 33.3 | 10 | 66.7 | 1 | 6.7 | 9 | 60.0 | 0.73 |
| In hyperactive only | ||||||||||
| Yes | 12 | 5 | 41.7 | 7 | 58.3 | 1 | 8.3 | 6 | 50.0 | Ref |
| No | 13 | 4 | 30.8 | 9 | 69.2 | 2 | 15.4 | 7 | 53.8 | 0.57 |
| Prior responders only | ||||||||||
| Yes | 14 | 7 | 50.0 | 7 | 50.0 | 1 | 7.1 | 6 | 42.9 | Ref |
| No | 11 | 2 | 18.2 | 9 | 81.8 | 2 | 18.2 | 7 | 63.6 | 0.10 |
| No. of participants | ||||||||||
| < 20 | 15 | 7 | 46.7 | 8 | 53.3 | 2 | 13.3 | 6 | 40.0 | Ref |
| 20–100 | 7 | 1 | 14.3 | 6 | 85.7 | 1 | 14.3 | 5 | 71.4 | 0.14 |
| ≥ 100 | 3 | 1 | 33.3 | 2 | 66.7 | 0 | 0.0 | 2 | 66.7 | 0.67 |
| RCDP | ||||||||||
| Yes | 16 | 6 | 37.5 | 10 | 62.5 | 2 | 12.5 | 8 | 50.0 | Ref |
| No | 9 | 3 | 33.3 | 6 | 66.7 | 1 | 11.1 | 5 | 55.6 | 0.83 |
| Challenge agents | ||||||||||
| Multiple dyes | 19 | 7 | 36.8 | 12 | 63.2 | 3 | 15.8 | 9 | 47.4 | Ref |
| Tartrazine only | 6 | 2 | 33.3 | 4 | 66.7 | 0 | 0.0 | 4 | 66.7 | 0.88 |
| Daily dose (mg) | ||||||||||
| ≤ 10 | 4 | 2 | 50.0 | 2 | 50.0 | 0 | 0.0 | 2 | 50.0 | Ref |
| 11–35 | 7 | 4 | 57.1 | 3 | 42.9 | 0 | 0.0 | 3 | 42.9 | 0.82 |
| 36–99 | 8 | 2 | 25.0 | 6 | 75.0 | 2 | 25.0 | 4 | 50.0 | 0.39 |
| ≥ 100+ | 3 | 1 | 33.3 | 2 | 66.7 | 0 | 0.0 | 2 | 66.7 | 0.66 |
| Unclear | 3 | 0 | 0.0 | 3 | 100.0 | 1 | 33.3 | 2 | 66.7 | 0.15 |
| Washout > 2 days | ||||||||||
| Yes | 11 | 6 | 54.5 | 5 | 45.5 | 1 | 9.1 | 4 | 36.4 | Ref |
| No | 14 | 3 | 21.4 | 11 | 78.6 | 2 | 14.3 | 9 | 64.3 | 0.09 |
| Food dyes only | ||||||||||
| Yes | 22 | 9 | 40.9 | 13 | 59.1 | 3 | 13.6 | 10 | 45.5 | Ref |
| Additional agent | 3 | 0 | 0.0 | 3 | 100.0 | 0 | 0.0 | 3 | 100.0 | 0.17 |
| Validated | ||||||||||
| Yes | 17 | 5 | 29.4 | 12 | 70.6 | 2 | 11.8 | 10 | 58.8 | Ref |
| No | 8 | 4 | 50.0 | 4 | 50.0 | 1 | 12.5 | 3 | 37.5 | 0.32 |
| Outcome timing | ||||||||||
| Hourly | 9 | 4 | 44.4 | 5 | 55.6 | 2 | 22.2 | 3 | 33.3 | Ref |
| Daily | 6 | 0 | 0.0 | 6 | 100.0 | 0 | 0.0 | 6 | 100.0 | 0.06 |
| Several per week | 3 | 1 | 33.3 | 2 | 66.7 | 1 | 33.3 | 1 | 33.3 | 0.74 |
| Weekly | 3 | 1 | 33.3 | 2 | 66.7 | 0 | 0.0 | 2 | 66.7 | 0.74 |
| Greater than weekly | 1 | 0 | 0.0 | 1 | 100.0 | 0 | 0.0 | 1 | 100.0 | 0.39 |
| Unclear | 3 | 3 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0.09 |
| Full results | ||||||||||
| Yes | 12 | 5 | 41.7 | 7 | 58.3 | 2 | 16.7 | 5 | 41.7 | Ref |
| No | 13 | 4 | 30.8 | 9 | 69.2 | 1 | 7.7 | 8 | 61.5 | 0.57 |
| Low infractions | ||||||||||
| Yes | 16 | 7 | 43.8 | 9 | 56.3 | 3 | 18.8 | 6 | 37.5 | Ref |
| No or unknown | 9 | 2 | 22.2 | 7 | 77.8 | 0 | 0.0 | 7 | 77.8 | 0.28 |
Abbreviations: RCDP , studies that are randomized crossover design, double blinded, and placebo controlled; Ref, reference category
Only includes studies involving an active challenge i.e. diet elimination trials were not included in this table
a Studies that did not report an association that was statistically significant, an effect size ≥20%, or standardized effect size ≥0.20
b Studies that reported a statistically significant association, an effect size ≥20%, or standardized effect size ≥0.20. This category combines the studies listed under the “Large effect size” and “Statistically significant” columns. The “Statistically significant” column includes any study reporting a statistically significant association, regardless of effect size. The “Large effect size” column includes studies that reported an effect size ≥20% or a standardized effect size ≥0.20 but the results were not statistically significant
c Chi-square p -value comparing proportion of studies finding no association (i.e. those in the “No association” column) to the proportion of studies finding an association (i.e. those in the “Association identified” column)
d In studies that presented group means, provides results by the source of the outcome information (Parent, Teacher, or Other). The number of studies listed here is greater than the total number of studies since several studies presented results for more than one outcome source
e In studies that presented results for individual participants, provides results by the source of the outcome information (Parent, Teacher, or Other). Several studies presented results for more than one outcome source
f Divides studies by quality scores above (“Higher”) or below (“Lower”) the median score of 10
g “Yes” if the study only included participants who were previously reported to have some condition related to hyperactivity
h “Yes” if the study only included participants who were previously reported to have had some behavioral improvements on a synthetic food dye elimination diet
i Typically a preservative like benzoic acid
j “Yes” if the average number of dietary infractions was low (e.g., < 2 per week)
The most frequent study locations were in the US (44%), followed by the UK (22%), and Australia and Canada (15% each). The mean number of participants was 44 (range 1–297). All studies used cross-over designs. In the cross-over design, each subject receives each treatment (including placebo) and, thus, the subjects serve as their own controls, which minimizes bias and confounding. Most challenge studies were double-blinded and the cross-over design was randomized, although in two studies the use of blinding was unclear. Randomization was either not done or was unclear in seven studies. Six studies assessed tartrazine only, whereas the rest studied mixtures of common dyes. The average dose assessed was 55.8 mg/day (range 1.2 to 250 mg/day, doses relevant to children’s exposure in the US). In all but one challenge study, participants were placed on an elimination diet during the study. Most studies (70%) used a validated or otherwise commonly accepted metric to assess neurobehavioral outcomes, with the most common being the Conners Parent scale.
Sixteen (64%) out of 25 challenge studies identified some evidence of an association and in 13 (52%), the association was statistically significant (Fig. 1 and Table Table2; 2 ; Table A.2). Associations (either large effect sizes or statistically significant results) were most commonly identified in studies that assessed neurobehavioral outcomes using information from the child’s parents. Out of eight challenge studies that provided results for both parents and teachers, four found associations only when examining parent reports [ 5 – 8 ], one found associations for both parent and teacher reports [ 9 ], two did not report an association for any outcome metric [ 10 , 11 ], and one found an association only for another metric [ 12 ].
Positive associations were also more frequently reported in studies published after the year 1990 (83.3 vs. 57.9%, p = 0.26), in studies that used validated metrics for assessing outcome (70.6 vs. 50.0%, p = 0.17) and in studies with larger numbers of participants (see Fig. Fig.1 1 and Table Table2). 2 ). The reason why more recent studies tended to report associations compared to earlier studies is unclear.
While two positive studies tested mixes of dyes plus preservatives [ 13 , 14 ], the large majority did not include preservatives and many of these (59.1% overall), identified associations between these dyes and adverse effects on neurobehavior with 10 of them reporting associations that were statistically significant [ 5 , 7 , 15 – 17 ].
Rowe and Rowe [ 17 ] saw a dose-response pattern between increasing doses of 1, 2, 5, 10, 20, and 50 mg of Yellow No. 5 (tartrazine) per day and worsening behavioral scores. Only two other studies reported information on dose-response, one using multiple dyes and one with Yellow No. 5 alone, with neither finding a clear dose-response pattern [ 18 , 19 ] However, Rowe and Rowe used many more doses and had a larger sample size than the other two studies. These differences and other study design issues may have affected whether a dose-response could be seen.
We could not divide studies based solely on age as there was a wide range of ages studied with broad overlap across studies reviewed. However, based on sensitivity analyses examining age, in three studies, results varied minimally [ 11 , 17 , 20 ], while in three others, greater effects were seen in younger participants [ 5 , 14 , 21 ].

Nigg et al., 2012 meta-analysis
A high-quality meta-analysis [ 22 ] is supportive of the hypothesis that synthetic food dye exposures is associated with adverse behavioral effects in children. This study identified statistically significant summary associations for findings based on parent reports or on attention tests, with effect sizes about one-sixth to one-third of those seen for improvements from ADHD medications. Nigg et al. estimated that 8% of children with ADHD may have symptoms related to synthetic food dyes. Our report evaluated the same studies used in the Nigg et al. meta-analysis as well as two pilot or preliminary reports [ 7 , 19 ], two studies with only 1–2 participants [ 8 , 16 ], and a study published after the meta-analysis was published [ 10 ]. These five studies reported mixed results. It is unlikely their inclusion in a meta-analysis would dramatically affect its results because most of these studies had small sample sizes. Additionally, the Lok et al. study [ 23 ] did not present means and standard deviations for analyses comparing placebo to artificial food dyes, and as such would be difficult to include in meta-analysis with most other studies.
Bias and confounding
As documented in Tables S.2-S.4 we performed extensive evaluations of quality for each study. One strength of our findings is that they are based on clinical trials with cross-over designs and placebo control. Non-compliance can lead to exposure misclassification in clinical trials, but we found that infraction rates were generally low in the studies when they were reported. Potential confounding can be markedly reduced with the use of cross-over designs since subjects are being compared to themselves. Bias that may be introduced by the expectations of the researchers and participants is minimized by use of blinding and placebo control. We performed a sensitivity analysis in which we only included studies that were double-blinded and had the cross-over randomized, and found that our conclusions were similar to that of our analysis that included all studies (Table (Table2, 2 , rows for RCDP).
Recruitment strategies and participation rates were not always clearly described in the studies, and most seemed to involve convenience samples. The use of convenience samples or low participation rates can introduce bias. However, in studies in which the participants, parents, and others were blinded, we found no clear evidence or obvious reason that convenience sampling or low participation might cause false positive results. While convenience sampling and low participation rates might affect the generalizability of some studies, we see no reason why they would affect the ability of a study to examine whether at least some children might be adversely affected by synthetic food dyes, especially given the cross-over design.
Adjustments for publication bias by Nigg et al. [ 22 ] attenuated summary effect sizes in the meta-analysis, although several remained statistically significant. However, these adjustment methods are imperfect. In addition, given the widespread interest in the potential health effects of synthetic food dyes, it seems unlikely that well-conducted clinical trials would remain unpublished resulting in publication bias.
Susceptibility
From the studies reviewed, it appears that not all children react to the dyes with adverse behavioral outcomes. Possible explanations for this sensitivity are not clear. Studies that included only children who were previously diagnosed with hyperactivity were not more likely to report positive associations between synthetic food dye exposure and poorer behavioral outcomes. Stevenson et al. [ 24 ] found that children (both 3 year-olds and 8/9 year-olds) with certain polymorphisms in histamine degradation genes had greater adverse responses to synthetic food dyes. In addition, gene polymorphisms in the dopamine transporter gene in 8/9 year-old children moderated the effects of the food dyes. Since histamine plays a role as a neurotransmitter in the brain and is involved in wakefulness, polymorphisms in the histamine degradation genes are a plausible basis for varied behavioral sensitivity to dyes associated with histamine release. Replication of this study and further research of the impacts of gene polymorphisms on response to food dyes are needed.
Review of animal toxicology studies
Animal toxicology studies were used by FDA as the basis for regulatory risk assessments of food dyes [ 25 ]. All current dye registrations were made between 1969 and 1986 based on studies performed 35 to 50 years ago. These studies were not designed to assess neurobehavioral endpoints. Dye registration was accompanied by derivation of an “acceptable daily intake” (ADI) based on these studies. FDA ADIs have not been updated since original dye registration, although there have been several reviews of specific effects since then, the latest in 2011 [ 25 ].
Our review of animal toxicology studies was intended to examine neurobehavioral toxicity of food dyes and included any study administering one or more of the FDA registered food dyes and measuring a behavioral endpoint. We obtained 25 reports from the peer-reviewed literature. Two reports could not be reviewed due to lack of study information. The 23 studies reviewed had the following characteristics:
- Rodent models (rats or mice)
- Oral administrations (diet or gavage)

Number of animal developmental neurobehavioral toxicity studies by dye and year
- Dosing included at or below that in studies used to establish FDA ADIs

Experimental designs of developmental neurotoxicity studies in animals with synthetic food dye exposures
- Behavioral endpoints including preweaning motor development, spontaneous motor activity and/or learning and memory tests
- Comparison of dosed and control groups
The study designs varied (Fig. (Fig.3) 3 ) and included exposures during prenatal, infant, juvenile and adult life stages, and examined neurobehavioral effects during development and/or adulthood. Due to the wide range of designs, an overall integration of findings was not possible but a broader picture of the potential for food dye neurobehavioral toxicity is seen. Details of the studies are presented in Table A.5 and A.6. Detailed evaluation and interpretation of each study is reported in the OEHHA document [ 1 ]. First author and dates of publication are shown.
Details from all the studies reviewed in this section are shown in Table A.5.
Findings from these studies have greatly advanced our knowledge of neurobehavioral effects of synthetic food dyes:
- Long term consequences of exposure during pregnancy [ 26 – 29 ]. This is the first research using the classical developmental neurotoxicology (DNT) design where exposure begins during pregnancy to identify long-term effects of perinatal exposure. Prior regulatory developmental toxicology studies have been limited to effects on mortality, malformation, and growth. There are no studies in humans using exposure in pregnancy.
- Effects of synthetic food dyes on behavior in adult rats after a single administration [ 30 , 31 ]. These are the only available animal studies measuring behavior shortly after a single dye administration.
- Behavioral effects when synthetic food dyes are administered at juvenile/adolescent life stages [ 32 , 33 ]
- Effects on behavior in adult rodents with chronic exposures [ 31 , 34 – 36 ]. Due to the emphasis on behavioral effects in children, more general studies of neurobehavioral toxicity in adults have been lacking but have recently been undertaken in animal models.
- Prevention of effects of synthetic food dyes on behavior by antioxidants [ 35 , 36 ]. This line of investigation has also been pursued for other aspects of dye toxicity [ 37 – 45 ]
- Brain changes associated with behavioral effects [ 26 , 29 – 31 , 34 – 36 ]. Emerging research in the last 10 years has begun to explore effects of synthetic food dye exposures on the brain at doses that affect behavior.
Individual dye studies
A series of neurobehavioral studies of individual dyes has been performed by one laboratory in Japan for 5 of the 7 food dyes approved for use in the US [ 46 – 51 ]. These studies used lifetime exposure beginning prior to parental mating. Details of the studies can be found in Table A.5. For three of the dyes, behavioral effects were identified at doses below those producing the toxicological effects used to establish the FDA ADIs. Several considerations limit the use of these studies in assessing food dye risk to children, including reproductive toxicity in the studies, multiple life stage exposure, dosing both before and during testing, and lack of litter-based statistics for preweaning endpoints.
Eight studies were from US laboratories, all published prior to 1987. We did not find any programmatic investigator-initiated research on neurobehavioral effects of food dyes currently being performed in the US.
The US FDA supported early studies of three synthetic food dyes (Yellow No. 5, Red No. 3, Red No. 40) that also used lifetime exposures beginning prior to or shortly after conception and continuing through adult animal testing [ 52 – 54 ]. Dosing was based on known non-behavioral toxicity of the dyes. Behavioral effects were reported for Red No. 3 [ 54 ] and Red No. 40 [ 53 ] using an extensive test battery.
The other 5 studies of individual dyes were conducted more recently and administered individual dyes to postpubertal (adolescent and adult) rodents [ 30 , 31 , 34 – 36 ]. These investigators were interested in specific hypotheses about food dye mechanism of action and included brain assays: brain microhistomorphometry [ 35 , 36 ]; measures of oxidative stress [ 34 ]; and measures of influence on the serotonin system [ 30 , 31 ].
Mixture studies
Early studies used dye mixtures designed to parallel US food dye exposure at that time [ 33 , 55 – 58 ]. More recent studies used dosing based on a multiple of regulatory ADIs [ 27 – 29 , 32 ]. Study details are presented in Table A.6. Animal mixture studies, like children’s mixture studies, are valuable for hazard identification, but not for ADI development, which is based on each dye individually.
The two studies using synthetic food dye mixtures that are most relevant to the studies in children reported behavioral effect during dye administration to immature rats [ 32 , 33 ]. Both studies reported effects on regulation of spontaneous motor activity. Shaywitz et al. [ 33 ] used a mixture based on human exposure and found greater activity in rats dosed at twice the estimated average exposure at that time in children. Erickson et al. [ 32 ] found increased movement time using a mixture of dyes in drinking water, each dye at a dose less than 2 times the FDA ADI.
One series of studies examined exposure to a mixture of synthetic food dyes during pregnancy [ 27 – 29 ]. The 9 dyes were administered at either the JECFA ADI [ 28 ] or 100 times the JECFA ADI [ 27 , 29 ]. Six of the seven FDA registered food dyes were included. Effects on activity and emotionality were reported with testing of one-month old (early adolescence) and three-month old (adulthood) offspring, but learning and memory tests were not affected.
Behavioral endpoints
Behavioral assessments were primarily conducted in a few domains: preweaning motor development (4 studies), spontaneous motor activity (21 studies), and trial-based learning and memory tests (18 studies). Some recent studies included emotionality tests [ 27 – 29 , 32 ].
Spontaneous motor activity, a sensitive and widely used test in developmental neurotoxicology, was the most frequently used test in animal dye studies because of the findings of hyperactivity in children’s studies. While the test apparatus and specific endpoints affected (vertical/horizontal activity, speed, distance, duration) varied, altered regulation of activity was seen in 17 of the 21 of the studies.
Sensitivity of learning and memory tests in developmental neurotoxicology is less consistent [ 59 , 60 ]. For the food dye studies we examined, tests included shock-motivated avoidance, food motivated mazes, and water mazes, with 12 of 18 studies reporting dye effects. Our review found that many of the test results could not be used for risk assessment due to design and statistical issues. For example, some studies did not use litter-based statistics.
Brain assays in behavioral studies
Many animal studies we reviewed conducted brain assays that evaluated a number of parameters with a focus on neurotransmitter systems. Early studies did not identify effects of synthetic food dye exposures on tissue catecholamine neurotransmitter concentrations [ 33 , 55 – 57 ]. More recent studies identified effects on gene receptor expression, enzyme activity in neurotransmitter systems, and localized changes in neurotransmitter levels [ 26 , 29 – 31 ]. Also, brain histomorphology assessed with contemporary methods has identified effects (decreased medial prefrontal cortex volume, decreased numbers of glia and neurons, changes in dendritic morphology) of the two most used food dyes, Red No. 40 and Yellow No. 5 [ 35 , 36 ]. Protective effects of antioxidants [ 35 , 36 ], as well as changes in brain anti-oxidant defense systems [ 34 ] provide evidence for oxidative stress as a mechanism of toxicity. Two other papers with no behavioral measures found markers of oxidative stress in the brain after in vivo treatment of rats with Yellow No. 5 [ 61 , 62 ].
Data from a human study provide evidence for a mechanism involving the neurotransmitter histamine. The investigators demonstrated that polymorphisms in the histamine degradation gene for histamine-N-methyltransferase influences response to a dye mixture [ 24 ]. In addition to its role in the inflammatory process, histamine is recognized for its role in regulating synaptic transmission alone and in concert with other neurotransmitters [ 2 ].
Considering both in vivo and in vitro research, other potential pathways for food dye neurotoxicity have been suggested [ 63 – 65 ].
- Endocrine (thyroid, estrogen) mediated effects
- Interference with neuronal proliferation and differentiation
- Effects secondary to general physiological toxicity
- Immune mediated effects
- Interference with nutrient bioavailability
The relevance of the animal toxicology findings to humans ingesting synthetic food dyes in food and medications would be better understood with more information about food dye toxicokinetics. In particular, the breakdown of azo dyes in the gut prior to absorption requires toxicological examination of metabolites. Future studies should evaluate whether the parent compounds act on the gut to influence behavior via the gut-brain axis [ 66 ].
Hazard characterization
The studies that form the basis of the FDA (and JECFA) ADIs are many decades old and as such were not capable of detecting the types of neurobehavioral outcomes measured in later animal studies, or in clinical trials in children consuming synthetic food dyes.
Nonetheless, OEHHA first compared the US FDA ADIs and the No-Observed-Adverse-Effect Levels (NOAELs) from which they were derived to NOAELs from the animal toxicology studies that were reviewed [ 1 ]. Next, we compared the estimated food dye exposures (mg/kg/d) from food consumption to available regulatory benchmarks in a traditional Hazard Index approach for noncancer health effects. The Hazard Index approach divides estimated exposures by a toxicity benchmark. If that ratio is greater than 1, then it is indicative of a possible risk of adverse noncancer effects. Finally, we compared the ADIs to NOAELs and Lowest-Observed-Adverse-Effect Levels (LOAELs) observed in the few key animal and human studies of sufficient quality. This comparison should help inform future revisions of the ADIs aimed at protecting children from neurobehavioral effects.
Comparing neurobehavioral effect levels to FDA ADI NOAELs
To derive the ADI for each dye, US FDA divided NOAELs reported by investigators from animal studies by a factor of 100. While reviewing animal neurobehavioral toxicology studies, we compared the effective doses (LOAELs) to animal NOAELs used by US FDA to derive human ADIs (hereinafter referred to as ADI NOAELs). The purpose of this comparison was to see if neurobehavioral effects were found at doses that FDA determined were not causing effects in the older general toxicology studies . Tables 3 and and4 4 presents these comparisons for both developmental and adult neurotoxicology studies where a single dye was administered.
Comparison of US FDA ADI and effective oral doses from developmental studies with individual dyes
| Vorhees et al., 1983a | Tanaka 2001 | Vorhees et al.,1983b | Tanaka 1994 | Tanaka et al., 2012 | Sobotka et al., 1977 | Tanaka et al., 2006 | Tanaka et al., 2008 | Tanaka 1996 | |
|---|---|---|---|---|---|---|---|---|---|
| Red No. 3 | Red No. 3 | Red No. 40 | Red No. 40 | Blue No. 1 | Yellow No. 5 | Yellow No. 5 | Yellow No. 5 | Yellow No. 6 | |
| 2.5 | 2.5 | 7.0 | 7.0 | 12.0 | 5.0 | 5.0 | 5.0 | 3.75 | |
| 250 | 250 | 700 | 700 | 1200 | 500 | 500 | 500 | 375 | |
| 0, 0.25 , 0.5, 1.0 | 0, 0.005 0.015 , 0.045 | 0, 2.5 , 5.0, 10.0 | 0, 0.42, 0.84, 1.68 | 0, 0.08 , 0.24, 0.72 | 0, 1.0, 2.0 | 0, 0.05, 0.15, 0.45 | 0, 0.05, 0.15, 0.45 | 0 0.15, 0.30, 0.60 | |
| LOAEL 125 | NOAEL 24 | LOAEL 1250 | NOAEL 3534 | LOAEL 127 | NOAEL 1000 | NOAEL 841 | Significant trend tests only | NOAEL 1146 | |
Effective doses are those at which statistically significant differences between dose group and control group were reported by authors . Endpoints are behavior or brain measures
a NOAEL used to derive FDA ADI
b NOAEL for study
c LOAEL for study
d For studies from the Tokyo Metropolitan Laboratory of Public Health, for NOAELS without LOAELS, the mean value for males and females were used. For LOAELs and NOAELs with LOAELs, the value for the sex affected at the LOAEL was used
e Calculated by OEHHA using standard assumptions about food intake and body weight
Comparison of US FDA ADI and effective oral dose from adult studies with individual dyes
| Tanaka 2001 | Dalal and Poddar 2009 | Dalal and Poddar 2010 | Noorafshan et al., 2018 | Tanaka et al., 2012 | Tanaka et al., 2006 | Tanaka et al., 2008 | Gao et al., 2011 (rats) | Gao et al., 2011 (mice) | Rafati et al., 2017 | Tanaka 1996 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Red No. 3 | Red No. 3 | Red No. 3 | Red No. 40 | Blue No. 1 | Yellow No. 5 | Yellow No. 5 | Yellow No. 5 | Yellow No. 5 | Yellow No. 5 | Yellow No. 6 | |
| 2.5 | 2.5 | 2.5 | 7.0 | 12 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 3.75 | |
| 250 | 250 | 250 | 700 | 1200 | 500 | 500 | 500 | 500 | 500 | 375 | |
| 0, 0.005, 0.015 , 0.045 % diet | 0, 1 , 10 , 100, 200 mg/kg/d | 0, 1 , 10 , 100, mg/kg/d | 0, 7 , 70 mg/kg/d | 0, 0.08 , 0.24, 0.72% diet | 0, 0.05 , 0.15 , 0.45% diet | 0, 0.05, 0.15, 0.45 diet | 0, 175 350 700 mg/kg/d | 0, 125 250 500 mg/kg/d | 0, 5 , 50 mg/kg/d | 0, 0.15, 0.30, 0.60 % diet |
NOAEL 28 | NOAEL 1.0 | NOAEL 1 | LOAEL 7.0 | LOAEL 122 | NOAEL 73 | NOAEL 824 | NOAEL 175 | NOAEL 125 | LOAEL 5 & 50 | NOAEL 1052 | |
Effective doses are those at which statistically significant differences between dose group and control group were reported by authors. Endpoints are behavior or brain measures
d For studies from the Tokyo Metropolitan Laboratory of Public Health (Tanaka studies), for NOAELS without LOAELS, the mean value for males and females were used. For LOAELs and NOAELs with LOAELs, the value for the sex affected at the LOAEL was used
e For studies using % diet as dosing metric, doses in mg/kg/d were calculated by OEHHA from data on food consumption and body weight provided in the paper
Comparing food dye exposures to available regulatory benchmarks
OEHHA [ 1 ] derived exposure estimates based on NHANES 2015–2016 Dietary Interview data, and information on food dye concentration data sourced from Doell et al. [ 67 ]. We calculated single-day and two-day average cumulative daily synthetic food dye intake estimates (mg/person/day) for the following demographic categories:
- Pregnant women 18 years and older
- Women of childbearing age (18–49 years)
- Children: 0- < 2 years, 2- < 5 years, 5- < 9 years, 9- < 16 years, and 16–18 years
We estimated daily synthetic food dye intakes (mg/person/day) for
- The typical-exposure scenario , which represents exposure to a given FD&C batch-certified synthetic food dye for a typical consumer, an individual who may not always eat products with the lowest or highest levels of that food dye but some combination of both.
- The high-exposure scenario , which represents the highest exposure where the individual is only consuming products with the highest levels of that food dye.
We divided each individual’s FD&C batch-certified synthetic food dye intake estimate (mg/person/day) by their body weight (kg) reported in NHANES 2015–16 [ 68 ] to produce synthetic food dye dose estimates in units of mg/kg/day. The most commonly consumed dyes for the various age ranges of children expressed as the mean of typical-exposure scenario estimates were Red No. 40 (ranged from 0.11 to 0.3 mg/kg-day), Red No. 3 (ranged 0.02 to 0.54 mg/kg-d), Yellow No. 5 (ranged from 0.05 to 0.19 mg/kg-d) and Yellow No. 6. (ranged from 0.05–0.20 mg/kg-d) [ 1 ]. The 95th percentile of the high-exposure scenario estimates ranged from about 1 to 8 mg/kg-day for these four dyes. Children’s exposures tended to be higher than adult women.
We compared the synthetic food dye dose estimates to the US FDA and JECFA ADIs (Table 5 ) by calculating the ratio of the dose estimates to the established ADIs [ 25 , 69 – 72 ] as the Hazard Index. Hazard index > 1 signifies that the food dye exposure estimates (mg/kg/day) exceeded the established ADI.
ADIs in mg/kg/day from US FDA and JECFA
| US FDA | JECFA (WHO) | |
|---|---|---|
| Yellow 5 | 5.0 | 0–10 |
| Yellow 6 | 3.75 | 0–4 |
| Red 3 | 2.5 | 0–.1 |
| Red 40 | 7.0 | 0–7 |
| Blue 1 | 12.0 | 0–6 |
| Blue 2 | 2.5 | 0–5 |
| Green 3 | 2.5 | 0–25 |
a JECFA presents their ADIs as a range from 0 to a positive value
With the exception of FD&C Red No. 3, all exposure estimates (mg/kg/day) from foods were below the US FDA or JECFA ADIs. The Hazard Indices (HI) that exceeded 1 for Red No. 3 are bolded in Table 6 . Children’s single day mean FD&C Red No. 3 exposure estimates for typical- and high-exposure scenarios ranged from 0.01 to 0.60, not exceeding the FDA ADI of 2.5 mg/kg-day. The 95th percentile exposure estimates ranged up to 3.16 (although it represents few children). For several age categories the mean single day typical- and high-exposure scenarios exceeded the JECFA ADI of 0.1 mg/kg-day, with HI ranging from 0.21 to 15; the 0 < 2 year age category had the highest HI.
Ratios of the FD&C Red No. 3 intake compared with US FDA and JECFA ADIs
| FD&C Red No. 3 | Typical-exposure scenario | High-exposure scenario | ||||||
|---|---|---|---|---|---|---|---|---|
| FDA Ratio Mean | FDA Ratio 95th% | JECFA Ratio Mean | JECFA Ratio 95th% | FDA Ratio Mean | FDA Ratio 95th% | JECFA Ratio Mean | JECFA Ratio 95th% | |
| Pregnant women | ||||||||
| Day 1 | 0.01 | 0.09 | 0.29 | 0.02 | 0.27 | 0.60 | ||
| Day 2 | 0.008 | 0.02 | 0.20 | 0.41 | 0.01 | 0.02 | 0.23 | 0.54 |
| 2 -Day average | 0.008 | 0.05 | 0.20 | 0.01 | 0.13 | 0.35 | ||
| Women 18–49 years | ||||||||
| Day 1 | 0.01 | 0.03 | 0.27 | 0.78 | 0.02 | 0.03 | 0.38 | 0.81 |
| Day 2 | 0.01 | 0.04 | 0.29 | 0.02 | 0.04 | 0.38 | ||
| 2 -Day average | 0.01 | 0.03 | 0.18 | 0.72 | 0.01 | 0.03 | 0.24 | 0.80 |
| Children (0- < 2 years) | ||||||||
| Day 1 | 0.01 | 0.04 | 0.28 | 0.90 | 0.01 | 0.04 | 0.32 | |
| Day 2 | 0.21 | 0.60 | ||||||
| 2-Day average | 0.07 | 0.03 | 0.68 | 0.19 | 0.03 | 0.68 | ||
| Children (2- < 5 years) | ||||||||
| Day 1 | 0.08 | 0.07 | 0.19 | 0.08 | ||||
| Day 2 | 0.02 | 0.06 | 0.56 | 0.03 | 0.07 | 0.84 | ||
| 2-Day average | 0.03 | 0.04 | 0.70 | 0.90 | 0.07 | 0.04 | 0.90 | |
| Children (5- < 9 years) | ||||||||
| Day 1 | 0.03 | 0.04 | 0.64 | 0.04 | 0.06 | |||
| Day 2 | 0.04 | 0.08 | 0.07 | 0.09 | ||||
| 2-Day average | 0.02 | 0.05 | 0.62 | 0.04 | 0.09 | 0.98 | ||
| Children (9- < 16 years) | ||||||||
| Day 1 | 0.03 | 0.06 | 0.87 | 0.08 | 0.13 | |||
| Day 2 | 0.03 | 0.06 | 0.87 | 0.06 | 0.06 | |||
| 2-Day average | 0.02 | 0.06 | 0.55 | 0.04 | 0.17 | |||
| Youth (16–18 years) | ||||||||
| Day 1 | 0.02 | 0.09 | 0.49 | 0.03 | 0.09 | 0.69 | ||
| Day 2 | 0.007 | 0.02 | 0.17 | 0.57 | 0.01 | 0.03 | 0.21 | 0.80 |
| 2-Day average | 0.007 | 0.02 | 0.18 | 0.54 | 0.01 | 0.02 | 0.24 | 0.62 |
US Food and Drug Administration (US FDA ADI = 2.5 mg/kg/day)
JECFA Joint FAO/WHO Expert Committee on Food Additives (JECFA ADI = 0.1 mg/kg/day)
Comparing US FDA ADIs to key neurobehavioral studies
There are several animal studies and one human study that could be used to evaluate whether existing ADIs are protective of neurobehavioral effects for Red No. 3, Red No. 40, Yellow No. 5 and Yellow No. 6. No suitable studies of green or blue dyes were found for this comparison.
Tanaka et al. [ 48 ] conducted a developmental toxicity study of Red No. 3 where various doses were administered via diet from preconception through PND 63 and reported increased activity measurements in female offspring. For adult female dams, more turning was reported in the high-dose group than in controls. Activity in male offspring was affected at 3 weeks of age ( p < 0.01 for linear dose trend), but not at 8 weeks of age. In the female offspring at 8 weeks, statistically significant dose-dependent dye-induced increases in activity were seen, but not at 3 weeks of age. These included number of activity bouts, distance traveled in each bout, greater speed, total time moving and total distance. This interesting finding of greater activity is particularly valuable because of the absence of more severe developmental toxicity.
The NOAEL was 24 mg/kg/day for the female offspring. This NOAEL is a factor of 10 higher than the FDA ADI of 2.5 mg/kg/day. If one were to apply the same methodology as US FDA (dividing the NOAEL by a factor of 100) to derive an ADI, the resulting ADI would be a factor of 10 lower.
The studies by Dalal and Poddar [ 30 , 31 ] (Table A.5) provide unique information on brain serotonin pathway changes, and on behavioral changes in young adult animals either following single gavage administration or following 15 or 30 day exposures to Red No. 3. In their first study, the investigators measured activity (vertical rearing frequency detected automatically) for 5 min at 30 to 60 min intervals up to 9 h post-dosing after single gavage doses of 0, 1, 10, 100 or 200 mg/kg. A dose-dependent pattern of diminished activity was observed that reached a low at 2 h after dye administration and then returned to baseline by 7 h (Fig. (Fig.1 1 in Dalal and Poddar (2009)). The effect of diminished activity was replicated in an experiment demonstrating reversal of this effect by inhibitors of monoamine oxidase (MAO), the enzyme that metabolizes serotonin. In the second report, the investigators administered the same doses daily for a period of 15 or 30 days and activity was measured following the last administration. Following the 15 or 30 day treatments, activity was increased rather than decreased in a dose-dependent fashion (Fig. (Fig.1 1 in Dalal and Poddar (2010)). One explanation for these contrasting results is the role of two neuronal corticotrophin releasing factor (CRF) receptors that determine an active versus passive response to stress [ 73 ]. The NOAEL from these studies is 1 mg/kg/day based on changes in vertical activity in male rats, on increased serotonin levels in specific brain regions, and increased plasma cortisone levels. The NOAEL of 1 mg/kg/day in these studies is lower than the FDA ADI of 2.5 mg/kg/day. If one were to use a 100-fold safety factor with this NOAEL, the ADI would be 0.01 mg/kg/day.
Red no. 40 and yellow no. 5
Noorafshan et al. [ 35 ] administered Red No. 40 to adult male rats ( N = 10 per dose group) at doses of 0, 7, or 70 mg/kg/day (Table A.6) with and without 200 mg/kg/day of the anti-inflammatory molecule taurine, by gavage for 6 weeks. Both Red No. 40 treated groups performed more reference memory errors and working memory errors in the radial arm maze than controls ( p < 0.01). Taurine administration mitigated this effect. Histomorphology and stereology found that, in the high dose Red No. 40 group, the medial prefrontal cortex volume was smaller, and there were fewer neurons and glial cells in this brain area. Interpretation of these results is somewhat complicated by the lack of information on body weight and brain weight. The LOAEL is 7 mg/kg/day for this study, which is the same as the US FDA and JECFA ADI of 7 mg/kg/day.
These investigators used the same protocol to evaluate the effect of another azo dye, Yellow No. 5 [ 36 ]. Adult male rats ( N = 10 per dose group) were gavaged with Yellow No. 5 at 0, 5, or 50 mg/kg/day for 7 weeks with and without vitamin E. Exploration time in the novel object test was decreased at the high dose (p < 0.01). More days were required for Yellow No. 5 treated rats (low- and high-dose groups were combined) to reach the learning criterion in the radial arm maze test, and more errors occurred during the learning and retention phases. The brain assays demonstrated a smaller volume of the medial prefrontal cortex in the high-dose group, and lower cell count and shorter dendrites with lower spine density at both doses; qualitative alterations in cell shape were described. These effects were ameliorated by concomitant administration of the antioxidant vitamin E. The LOAEL was 5 mg/kg/day, based on morphometry, the same as the US FDA ADI of 5 mg/kg/day and lower than the JECFA ADI of 10 mg/kg/day. If this study were to be used as the basis for setting an ADI, the resulting ADI would be considerably lower than the existing ADI. Changes in the medial prefrontal cortex can be directly related to the cognitive performance of the animals, as this part of the rodent brain is involved in spatial memory, decision-making and attention [ 35 , 74 ], and may predict similar effects in children.
One study in children used several doses and demonstrated a dose response effect on behavioral scores for Yellow No. 5 [ 17 ]. For this study, the investigators recruited 34 children whose parents had brought them to the Royal Children’s Hospital in Melbourne to be evaluated for hyperactivity and 20 children whose parents had no concern about behavior. The children, ranging in age from 2 to 14 years, were enrolled in a double blind, placebo-controlled repeated measures study of the effects of Yellow No. 5 on behavioral score. The investigators developed a Behavioral Rating Inventory for this study that included 11 items measuring irritability, 9 items that measured sleep disturbance, 4 items that measured restlessness, 3 items that measured aggression and 3 items that measured attention span. In addition, the investigators also used the Conners 10-item Abbreviated Parent-Teacher Questionnaire to assess behavior, which focuses on attention related problems. Children were placed on a dye-free diet for at least 6 weeks before the trial, and then given doses (randomly) of 0, 1, 2, 5, 10, or 20 mg Yellow No. 5 with 2 days in between each dosing. Parents rated the behavior daily using the two instruments.
The investigators found 24 children who had significant behavioral responses to dye challenge, based on ranking the behavioral scores for the six dye-challenge days paired with a set of placebo days; these children were labelled as reactors. The mean behavioral scores on dye-challenge days were significantly different than the scores for the placebo (day before) challenge for all dose/placebo pairs ( p < 0.05) in the reactors, while the nonreactors showed random fluctuations in behavioral scores. Using repeated measures ANOVA on the six dye-challenge scores with reactors and nonreactors as the between-groups factor, the authors report a significant between-groups effect ( p < 0.001). There was a dose-dependent effect and the mean score difference between the reactor and the nonreactor groups were significant at doses of 2 mg and higher (p < 0.05). There were no significant differences in mean behavioral rating between the groups on the placebo days. OEHHA identifies 1 mg tartrazine as a NOAEL. The children ranged from 2 to 14 years, with a mean of 7 years. To determine a NOAEL dosage, OEHHA divided the NOAEL of 1 mg by a reference body weight of 25.5 kg for the mean age of 7 years (US EPA, 2011, Table 8–10, based on NHANES 1988–1994); a NOAEL dosage of 0.04 mg/kg/day is obtained. This NOAEL is more than 100-fold lower than the US FDA ADI for Yellow No. 5 of 5 mg/kg/day.
While not all of the human trials demonstrated effects of mixtures of food dyes or of Yellow No. 5 on behavior, the findings of Rowe and Rowe [ 17 ] are supported by some of the other clinical trials in children (Table 7 ). Note that in all these studies, effects were observed at estimated doses lower than the US FDA ADI for Yellow No. 5 of 5 mg/kg/day. One study [ 9 ] reports that in a six-week open trial of the Feingold diet in 55 subjects, ages 3 to 15 years, who had been suspected of reacting to food dyes, 40 children demonstrated improvement when on the Feingold diet, based on assessment of attention span, activity level, distractability, frustration tolerance, and social and manipulative skills by therapists, and teacher and parent questionnaires. In the same study, 8 of the children were challenged with Yellow No. 5 using a double-blinded cross-over design, and two of these children were observed to exhibit strong behavioral responses to the dye. Based on reference body weights for children ages 3 to 15 years, the dosages employed in that study [ 9 ] would have been 0.9–2.7 mg/kg/day. In a double-blind crossover study of 22 children, 4 to 8 years of age, both objective tests for attention and parent and teacher ratings (Conners Parent Teacher Rating Scale) were administered before and after a 4 week dye-free diet, after a 2 week Yellow No. 5 (5 mg daily) challenge and after a 4 week washout dye-free diet [ 7 ]. The investigators report statistically significant effects of Yellow No. 5 based on parental ratings in a subgroup of children whose mothers had reported improved behavior while on the elimination diet. The dose for this range of ages and body weights to the children would be 0.2 to 0.3 mg/kg/day. Levy and Hobbs [ 75 ] reported that mothers’ ratings using the Conners scale were an average of 13% lower when children ( N = 8) ate placebo cookies compared to those containing Yellow No. 5, in a 2 week crossover trial with daily ratings by parents for a 3 h period after eating the cookies. While there were no statistically significant differences noted, the authors reported that this effect “just failed to reach the .05 level of significance”. The dose of Yellow No. 5 in this study was about 0.1 to 0.2 mg/kg/day.
Doses of Yellow No. 5 that elicited effects in children’s clinical trials
| Study | Rowe and Rowe (1994) | Rowe (1988) | Levy et al. 1978 | Levy and Hobbs (1978) |
|---|---|---|---|---|
| 0, 1, 2, 5, 10, or 20 mg | 50 mg | 5 mg | 4 mg | |
| 0.04 | 0.9–2.7 | 0.2–0.3 | 0.1–0.2 |
a LOAEL dose estimated for the mean age of 7 years
b single dose studies, dose estimated for reported range of ages of children
Taken together, these studies provide support for an effect of Yellow No. 5 on behavior and for use of a neurobehavioral endpoint to determine a safe level of exposure for Yellow No. 5 to protect children who respond to this food dye.
Yellow no. 6
There is only one study of Yellow No. 6 with neurobehavioral endpoints [ 47 ]. Some neurobehavioral effects in offspring were reported for preweaning development and maze learning, but it was not possible to draw firm conclusions due to the statistical approach and varying group sizes in the study.
Goldenring et al. demonstrated that sulfanilic acid (1 mg/kg/day I.p.), a common metabolite of the azo food dyes Yellow No. 5 and Yellow No. 6, increased activity in pups following direct administration assessed three times during a treatment extending throughout juvenile development [ 55 ].
Honohan et al. reported gastrointestinal absorption of sulfanilic acid of 37.4% [ 76 , 77 ]. The 1 mg/kg intraperitoneal dose of sulfanilic acid used by Goldenring et al. would be equivalent to 2.7 mg/kg produced in the gastrointestinal tract, which in turn would result from metabolism of 7 mg/kg of orally administered Yellow No. 5. Thus, one could view 7 mg/kg−/day of Yellow No. 6 to be a free-standing LOAEL. This LOAEL is about twice the FDA (3.75 mg/kg/day) and JECFA (4 mg/kg/day) ADIs for Yellow No. 6. The study by Goldenring et al. [ 55 ] indicates the ADIs for Yellow No. 6 may not be adequately protective of neurobehavioral effects.
Current evidence from studies in humans, largely from controlled exposure studies in children, supports a relationship between food dye exposure and adverse behavioral outcomes in children, both with and without pre-existing behavioral disorders. There appears to be considerable interindividual variability in the sensitivity to synthetic food dyes. While there were a range of results in the studies we identified, the majority reported at least some evidence of an association, including higher quality studies. Importantly, none of the factors we examined (e.g., parent vs teacher report, publication year, validated outcome metric) explained the majority of the heterogeneity seen across the study results. For example, although a large fraction of the studies published since 1990 reported statistically significant results (5 of 6 challenge studies), many studies published before 1990 also reported statistically significant results (8 of 19). And, while studies using a validated outcome metric were more likely to report associations, several studies without validated outcome metrics reported similar associations. Despite the various study limitations, we were unable to identify strong evidence for any apparent biases or other factors that invalidated the positive results reported in the literature.
Studies of Yellow No. 5 alone provide evidence that this dye affects children’s behavior. Most of the challenge studies involved administering multiple dyes at the same time so no single offending agent could be identified from those studies. Regardless, studies involving mixtures more closely represent real-life scenarios, where most children are exposed to multiple dyes in a single day.
Importantly, impacts on behavior and/or neurotransmitter systems or cellular architecture in the brain have been observed in animal studies. Several studies examining exposures during development, during pregnancy only, or as adolescents or adults reported changes in activity using a variety of metrics either in the offspring or in the adolescent or adult animals. In utero exposure was observed to have behavioral effects in the adult offspring. Thus, the animal literature provides support for behavioral effects of synthetic food dyes, including those most often consumed.
Taken together, the scientific literature supports an effect of synthetic food dye exposures on neurobehavior in children at environmentally relevant exposure levels.
Comparing estimated exposures we derived from the 2015–16 NHANES dietary interview to the FDA and JECFA ADIs revealed that for most dyes we analyzed, exposures do not exceed the ADIs. The exception is Red No. 3, where the Hazard Index based on the mean ranged up to 15 for the youngest age groups (Table (Table6 6 ).
Comparisons of the effective doses in some of the animal studies that measured behavioral or brain effects following exposure to synthetic food dyes indicates that the basis of the FDA ADIs are not adequate to protect neurobehavior in susceptible children. Three of the studies using developmental exposures reported LOAELS that were below the NOAEL that was used for the FDA ADI. Almost all studies in mature animals that measured behavioral changes and/or changes in the brain found effects of the synthetic food dyes at doses lower than the NOAELs used by the US FDA for the derivation of the ADIs. Several studies observe effects on behavior in animals at doses close to or even lower than the existing FDA ADIs. As noted above, the animal studies that form the basis of the FDA ADIs were not capable of detecting the types of neurobehavioral outcomes observed in many human challenge studies.
For four of the dyes with adequate animal studies explicitly reporting neurobehavioral effects, applying results from these studies would result in lower ADIs and likely exceedances of those ADIs from typical food consumption by children. Consumption of over-the-counter medications and vitamins adds to the exposure from foods [ 78 , 79 ].
If the ADI for Yellow No. 5 were based on the one study that evaluated a dose-response in children for behavioral effects, the ADI would be considerably lower. The human challenge studies provide support for an effect of Yellow No. 5 on behavior and for use of a neurobehavioral endpoint to determine a safe level of exposure for Yellow No. 5 to protect children who respond to this food dye.
It is not possible to compare the results of the animal or human mixtures studies to an ADI for a single dye. However, Erikson et al. [ 32 ] reported increased activity in male rats administered synthetic food dye mixtures where each dye was given at less than twice the ADI NOAEL. Shaywitz et al. [ 33 ] and Goldenring et al. [ 56 ] found greater activity and decreased habituation in a rodent model following administration of mixtures at doses near the ADIs. These mixture doses are in the range of doses in human mixture studies. Doses used in the human mixture studies were designed to mimic actual exposures in children.
A broad range of potential mechanisms by which the synthetic food dyes may impact behavior in susceptible children have been proposed. Additional research is warranted including:
- Animal testing in immature animals that includes a within-subjects design and measures of neurobehavior more similar to those in the human studies.
- Studies of the toxicokinetics of food dyes in humans and animals using modern techniques and including exposures during different life stages.
- Mechanistic studies and studies of underlying genetic susceptibility.
- Additional adequately powered clinical trials in children of the FD&C batch-certified synthetic food dyes with a cross-over, placebo-controlled, double blinded design utilizing validated outcome measures, inclusion of behavioral assessments by parents, and objective tests of attention and other behavioral measures by trained psychometricians. Such studies should attempt to evaluate whether the response differs by age, gender, ethnicity, race, or socioeconomic status through a design that evaluates dosing on a mg/kg/day basis.
- Studies that evaluate the potential long-term impacts of repeated exposures to food dyes in children.
Such research would provide additional data to inform appropriate acceptable daily intakes that explicitly protect children from neurobehavioral effects. In the short-term, the neurobehavioral effects of synthetic food dyes in children should be acknowledged and steps taken to reduce exposure to these dyes in potentially susceptible children.
Acknowledgements
The authors would like to acknowledge Marjannie Akintunde, Ph.D. for help organizing information from available animal toxicology studies for the OEHHA (2021) review, and Nancy Firchow for library services.
Abbreviations
| ADHD | Attention deficit hyperactivity disorder |
| ADI | Acceptable daily intake |
| CRF | Corticotrophin releasing factor |
| DNT | Developmental neurotoxicology |
| FDA | US Food and Drug Administration |
| FDA ADI NOAEL | The NOAEL used by FDA to derive the current FDA ADI |
| FD&C | Food Drug and Cosmetic Act, referring to dyes that must be batch-certified per FDA regulations |
| FAO | Food and Agriculture Organization of the World Health Organization |
| JECFA | Joint FAO/WHO Expert Committee on Food Additives |
| LOAEL | lowest-observed-adverse-effect level in a study |
| Mg | Milligrams |
| Mg/kg/day | Mg of substance per kg body weight per day |
| MAO | Monoamine oxidase |
| NIH | National Institutes of Health |
| NTP | National Toxicology Program |
| NHANES | National Health and Nutrition Examination Survey |
| NOAEL | No-observed-adverse-effect level in a study |
| OEHHA | Office of Environmental Health Hazard Assessment, California Environmental Protection Agency |
| OHAT | Office of Health Assessment and Translation |
| PND | Postnatal day |
| RCT | Randomized clinical trial |
| RCDP | Clinical trials that are randomized cross-over design, double-blinded and placebo controlled |
| UK | United Kingdom |
| US FDA | United States Food and Drug Administration |
Authors’ contributions
MDM and MAM were involved in conception, interpretation of results, and substantially drafted, reviewed and edited the paper. CS designed and conducted the review of the clinical trials of food dyes in children. MSG designed and conducted the review of animal toxicology studies. RC, RT, and AB conducted the exposure assessment and subsequent calculations of hazard index. All authors reviewed the paper.
The California state legislature appropriated funding to conduct this review. The legislature had no input into or control over the design of the study, collection, analysis, or interpretation of the data, or writing, reviewing or editing the manuscript.
Availability of data and materials
Declarations.
Not applicable.
AB is a volunteer member of the Board of Trustees for The Organic Center, a non-profit organization addressing scientific issues about organic food and agriculture, and is a member of the USDA National Organic Standards Board. The rest of the authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rothwell, Gnonto, Aaronson - Leeds United's strongest starting-XI after Georginio Rutter exit
Leeds United book medical for Almeria winger Largie Ramazani as Whites prepare permanent transfer
- Traffic and Travel
- Leeds United
- Food & Drink
- Homes & Gardens
- Submit Your Story
- Arts and Entertainment
- Things To Do
- Advertise My Business
- Place Announcement
- Place A Public Notice
- Advertise A Job
Disciplinary news as verdicts reached on Leeds Rhinos yellow cards: 8 players charged by match review panel
Two Leeds Rhinos players were among nine sin-binned during the two days of action at Elland Road. The review panel have now studied every tackle in all six games and charged eight players, with six of those being suspended.
Players have until noon tomorrow (Tuesday) to challenge the findings and any appeals will be heard at a tribunal the same afternoon. In some cases, players have received a sterner punishment than others for a similar grade of charge. That is based on their previous record. Here’s who has been charged and some players who weren’t.

1 . Disciplinary news
Here's the match review panel's verdicts after Magic Weekend. Photo: Olly Hassell/SWpix.com

2 . Sam Luckley (Hull FC)
Grade B dangerous contact (late contact on passer): £250 fine. Photo: John Clifton/SWpix.com

3 . Ligi Sao (Hull FC)
Grade B dangerous contact (late contact on passer): one-match penalty notice. Photo: Olly Hassell/SWpix.com

4 . Denive Balmforth (Hull FC)
Grade C dangerous contact (following sin-binning): one-match penalty notice. Photo: Olly Hassell/SWpix.com

5 . Shane Wright (Salford Red Devils)
Grade B dangerous contact (late contact on kicker): £250 fine. Photo: Olly Hassell/SWpix.com

6 . Oliver Partington (Salford Red Devils)
Grade B dangerous contact (late contact on kicker): one-match penalty notice. Photo: Olly Hassell/SWpix.com
Comment Guidelines
National World encourages reader discussion on our stories. User feedback, insights and back-and-forth exchanges add a rich layer of context to reporting. Please review our Community Guidelines before commenting.
Sign up for all of the latest Leeds Rhinos news, features and exclusive interviews.
Thank you for signing up.
Did you know with a Digital Subscription to Yorkshire Evening Post, you can get unlimited access to the website including our premium content, as well as benefiting from fewer ads, loyalty rewards and much more.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Evaluation of patient reporting of adverse drug reactions to the UK 'Yellow Card Scheme': literature review, descriptive and qualitative analyses, and questionnaire surveys
Affiliation.
- 1 University of Nottingham, Nottingham, UK.
- PMID: 21545758
- DOI: 10.3310/hta15200
Background: The monitoring of adverse drug reactions (ADRs) through pharmacovigilance is vital to patient safety. Spontaneous reporting of ADRs is one method of pharmacovigilance, and in the UK this is undertaken through the Yellow Card Scheme (YCS). Yellow Card reports are submitted to the Medicines and Healthcare products Regulatory Agency (MHRA) by post, telephone or via the internet. The MHRA electronically records and reviews information submitted so that important safety issues can be detected. While previous studies have shown differences between patient and health-care professional (HCP) reports for the types of drugs and reactions reported, relatively little is known about the pharmacovigilance impact of patient reports. There have also been few studies on the views and experiences of patients/consumers on the reporting of suspected ADRs.
Objectives: To evaluate the pharmacovigilance impact of patient reporting of ADRs by analysing reports of suspected ADRs from the UK YCS and comparing reports from patients and HCPs. To elicit the views and experiences of patients and the public about patient reporting of ADRs.
Design: (1) Literature review and survey of international experiences of consumer reporting of ADRs; (2) descriptive analysis of Yellow Card reports; (3) signal generation analysis of Yellow Card reports; (4) qualitative analysis of Yellow Card reports; (5) questionnaire survey of patients reporting on Yellow Cards; (6) qualitative analysis of telephone interviews with patient reporters to the scheme; (7) qualitative analysis of focus groups and usability testing of the patient YCS; and (8) national omnibus telephone survey of public awareness of the YCS.
Participants: Patients (n = 5180) and HCPs (n = 20,949) submitting Yellow Card reports from October 2005 to September 2007. Respondents to questionnaire survey (n = 1362). Participants at focus groups and usability testing sessions (n = 40). National omnibus telephone survey (n = 2028).
Setting: The literature review included studies in English from across the world. All other components included populations from the UK; the omnibus survey was restricted to Great Britain.
Interventions: None.
Main outcome measures: Characteristics of patient reports: types of drug and suspected ADR reported; seriousness of reports; and content of reports. The relative contributions of patient reports and of HCP reports to signal generation. Views and experiences of patient reporters. Views of members of the public about the YCS, including user-friendliness and usability of different ways of patient reporting. Public awareness of the YCS. Suggestions for improving patient reporting to the YCS.
Results: Compared with HCPs, patient reports to the YCS contained a higher median number of suspected ADRs per report, and described reactions in more detail. The proportions of reports categorised as 'serious' were similar; the patterns of drugs and reactions reported differed. Patient reports were richer in their descriptions of reactions than those from HCPs, and more often noted the effects of ADRs on patients' lives. Combining patient and HCP reports generated more potential signals than HCP reports alone; some potential signals in the 'HCP-only' data set were lost when combined with patient reports, but fewer than those gained; the addition of patient reports to HCP reports identified 47 new 'serious' reactions not previously included in 'Summaries of Product Characteristics'. Most patient reporters found it fairly easy to make reports, although improvements to the scheme were suggested, including greater publicity and the redesign of web- and paper-based reporting systems. Among members of the public, 8.5% were aware of the YCS in 2009.
Conclusions: Patient reporting of suspected ADRs has the potential to add value to pharmacovigilance by reporting types of drugs and reactions different from those reported by HCPs; generating new potential signals; and describing suspected ADRs in enough detail to provide useful information on likely causality and impact on patients' lives. These findings suggest that further promotion of patient reporting to the YCS is justified, along with improvements to existing reporting systems. In order of priority, future work should include further investigation of (1) the pharmacovigilance impact of patient reporting in a longer-term study; (2) the optimum approach to signal generation analysis of patient and HCP reports; (3) the burden of ADRs in terms of impact on patients' lives; (4) the knowledge and attitudes of HCPs towards patient reporting of ADRs; (5) the value of using patient reports of ADRs to help other patients and HCPs who are seeking information on patient experiences of ADRs; and (6) the impact of increasing publicity and/or enhancements to reporting systems on the numbers and types of Yellow Card reports from patients.
Funding: The National Institute for Health Research Health Technology Assessment programme.
PubMed Disclaimer
Similar articles
- How do patients contribute to signal detection? : A retrospective analysis of spontaneous reporting of adverse drug reactions in the UK's Yellow Card Scheme. Hazell L, Cornelius V, Hannaford P, Shakir S, Avery AJ; Yellow Card Study Collaboration. Hazell L, et al. Drug Saf. 2013 Mar;36(3):199-206. doi: 10.1007/s40264-013-0021-2. Drug Saf. 2013. PMID: 23444232
- Adverse drug reaction reporting in the UK: a retrospective observational comparison of yellow card reports submitted by patients and healthcare professionals. McLernon DJ, Bond CM, Hannaford PC, Watson MC, Lee AJ, Hazell L, Avery A; Yellow Card Collaboration. McLernon DJ, et al. Drug Saf. 2010 Sep 1;33(9):775-88. doi: 10.2165/11536510-000000000-00000. Drug Saf. 2010. PMID: 20701410
- How patient reporters identify adverse drug reactions: a qualitative study of reporting via the UK Yellow Card Scheme. Krska J, Anderson C, Murphy E, Avery AJ. Krska J, et al. Drug Saf. 2011 May 1;34(5):429-36. doi: 10.2165/11589320-000000000-00000. Drug Saf. 2011. PMID: 21513365
- The value of patient reporting to the pharmacovigilance system: a systematic review. Inácio P, Cavaco A, Airaksinen M. Inácio P, et al. Br J Clin Pharmacol. 2017 Feb;83(2):227-246. doi: 10.1111/bcp.13098. Epub 2016 Oct 12. Br J Clin Pharmacol. 2017. PMID: 27558545 Free PMC article. Review.
- Patient versus healthcare professional spontaneous adverse drug reaction reporting: a systematic review. Inch J, Watson MC, Anakwe-Umeh S. Inch J, et al. Drug Saf. 2012 Oct 1;35(10):807-18. doi: 10.1007/BF03261977. Drug Saf. 2012. PMID: 22928729 Review.
- A real-world disproportionality analysis of cyclosporine from the FDA Adverse Event Reporting System (FAERS) database. Cui S, Li L, Liu W, Zhao B, Zhong X. Cui S, et al. Braz J Med Biol Res. 2024 Jul 29;57:e13392. doi: 10.1590/1414-431X2024e13392. eCollection 2024. Braz J Med Biol Res. 2024. PMID: 39082578 Free PMC article.
- Patients' Identification, Management and Prevention of Adverse Drug Reactions: A Cross-Sectional Survey of Patients with Severe Adverse Drug Reactions. Srisuriyachanchai W, Cox AR, Jarernsiripornkul N. Srisuriyachanchai W, et al. J Clin Med. 2024 Jul 16;13(14):4165. doi: 10.3390/jcm13144165. J Clin Med. 2024. PMID: 39064204 Free PMC article.
- Burden of non-serious infections during biological use for rheumatoid arthritis. Bergmans B, Jessurun N, van Lint J, Murk JL, van Puijenbroek E, de Vries E. Bergmans B, et al. PLoS One. 2024 Feb 20;19(2):e0296821. doi: 10.1371/journal.pone.0296821. eCollection 2024. PLoS One. 2024. PMID: 38377117 Free PMC article.
- Sociodemographic Characteristics of Adverse Event Reporting in the USA: An Ecologic Study. Muñoz MA, Dal Pan GJ, Wei YJ, Xiao H, Delcher C, Giffin A, Sadiq N, Winterstein AG. Muñoz MA, et al. Drug Saf. 2024 Apr;47(4):377-387. doi: 10.1007/s40264-024-01397-6. Epub 2024 Feb 14. Drug Saf. 2024. PMID: 38353883 Free PMC article.
- Quantitative and qualitative analysis of individual experiences post botulinum toxin injection - United Kingdom Survey. Zargaran D, Zargaran A, Sousi S, Knight D, Cook H, Woollard A, Davies J, Weyrich T, Mosahebi A. Zargaran D, et al. Skin Health Dis. 2023 Jul 3;3(5):e265. doi: 10.1002/ski2.265. eCollection 2023 Oct. Skin Health Dis. 2023. PMID: 37799369 Free PMC article.
Publication types
- Search in MeSH
Related information
- Cited in Books
LinkOut - more resources
Full text sources.
- National Institute for Health and Care Research Journals Library
Other Literature Sources
- The Lens - Patent Citations
- MedlinePlus Health Information
Research Materials
- NCI CPTC Antibody Characterization Program
Miscellaneous
- NCI CPTAC Assay Portal

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.

IMAGES
COMMENTS
Reported CFR values for Yellow Fever vary, offering wide ranges. Estimates have not been found through systematic literature review, which has been used to estimate CFR of other diseases. This study aims to estimate the case fatality risk of severe Yellow Fever cases through a systematic literature review and meta-analysis.
1. Introduction. Yellow fever (YF) is a mosquito-borne viral illness caused by an arbovirus of the family Flaviviridae, genus Flavivirus, encompassing positive-single-stranded RNA viruses.The virus was isolated for the first time in 1927 in a male patient [].Transmission is primarily by mosquitoes [].After an incubation period of 3-6 days, YF infection can cause the onset of different ...
Go to: We conducted a systematic review and a meta-analysis to assess the risk of serious adverse events in the elderly after yellow fever vaccination compared to the non-elderly population. We searched multiple databases and grey literature, and we selected research without language and publication date restrictions.
Reported CFR values for Yellow Fever vary, offering wide ranges. Estimates have not been found through systematic literature review, which has been used to estimate CFR of other diseases. This study aims to estimate the case fatality risk of severe Yellow Fever cases through a systematic literature review and meta-analysis.
Conclusions: Approximately 39 % of severe Yellow Fever cases are estimated to be fatal. This study provides the rst systematic literature review to estimate the CFR of Yellow Fever, which can provide insight into outbreak preparedness and estimating underreporting. Keywords: Yellow Fever, case fatality risk, systematic review, meta‑analysis
Flowchart of the evidence selection process in the different stages of the review on yellow fever vaccine failure and associated factors. Regarding the study design, 33 were clinical trials, 10 were analytical cohorts, and three were cross-sectional analytical studies. ... The articles obtained via the literature search captured diverse study ...
Flow diagram for screening and including articles in systematic literature review and meta-analysis for case fatality risk of severe Yellow Fever cases. Publication years of articles ranged ...
Abstract. The duration of protection after a single dose of yellow fever vaccine is a matter of debate. To summarize the current knowledge, we performed a systematic literature review and meta-analysis. Studies on the duration of protection after 1 and ≥2 vaccine doses were reviewed. Data were stratified by time since vaccination.
Case fatality rate associated with yellow fever outbreaks ranged from 10% in Ghana to 86% in Nigeria. The mortality. rate ranged from 0.1/100,000 in Nigeria to 2200/100,000 in Ghana. Conclusion ...
We describe 5 cases of yellow fever vaccine-associated viscerotropic disease (YEL-AVD) in 2 familial clusters during the 2017-2018 yellow fever (YF) vaccination campaign in São Paulo state, Brazil.
The literature search was conducted using a combination of the search terms 'yellow fever', 'environment*' and either 'waste or container or water storage or house or screen or source reduction or habitat or elimination or breeding site' in the following databases: Cochrane Library (Reviews and Trials), the Global Index Medicus ...
Reported CFR values for Yellow Fever vary, offering wide ranges. Estimates have not been found through systematic literature review, which has been used to estimate CFR of other diseases. This study aims to estimate the case fatality risk of severe Yellow Fever cases through a systematic literature review and meta-analysis.
MATERIALS AND METHODS. A literature review of AEs occurring after YFV in high-risk groups was conducted by searching the public database PubMed for the words "yellow fever," "vaccine," "adverse events," and "contraindication" as well as the following MeSH terms: "yellow fever vaccine/ adverse effects" and "yellow fever ...
The gathered evidence suggests that a single dose of yellow fever vaccination provides lifelong protection in travellers. However, in people living with HIV and children (younger than 2 years), booster doses might still be required because lower proportions of vaccinees were seroprotected 10 or more years post-vaccination. Lower observed seroprotection rates among residents of endemic areas ...
Europe PMC is an archive of life sciences journal literature.
Kassy WC, et al - Yellow Fever Outbreaks and Public Health responses in Nigeria 430 Niger Med J 2023; 64(4):427 - 447 July -August, 2023 Figure 1: Flow Chart selection of relevant documents for review of literature. From our review of the literature, the Yellow fever outbreak occurred 23 times from the years 1864 to 2020 under review.
Introduction: Considering that vaccination with yellow fever vaccine (YFV) is the most important method to prevent and control yellow fever (YF), this study synthesized evidence on factors associated with YFV failure. Methods: A systematic review (SR) was performed in the PubMed, Cochrane CENTRAL, Embase, and LILACS databases up to November 2020.
We conducted a systematic review and meta-analysis to assess the risk of serious adverse events in the elderly after yellow fever vaccination compared to the non-elderly population. We searched multiple databases and grey literature and selected research without language and publication date restriction. Studies were analysed in a descriptive way, meta-analysed and expressed in terms of ...
Literature Review of Yellow Fever - Free download as PDF File (.pdf), Text File (.txt) or read online for free. This document discusses the challenges of writing a literature review on Yellow Fever, including: 1) Sifting through a vast amount of literature from diverse fields like epidemiology, virology, and public health to find the most relevant sources.
Methods A literature review was conducted by searching PubMed for "yellow fever vaccine" and "adverse events" (AEs); 207 studies were found, and 43 of them met the inclusion criteria and ...
Yellow fever (YF) is one of the most important mosquito-borne viral haemorrhagic diseases transmitted to humans. It is mostly vectored by the genus Aedes in Africa, and it is a serious public health problem in Africa, as well as in the South and Central Americas. In the past few decades, despite a highly effective vaccine, YF is re-emerging in ...
The literature review methods were designed to identify all the literature most relevant to the assessment of evidence on the neurological or ... identify strong evidence for any apparent biases or other factors that invalidated the positive results reported in the literature. Studies of Yellow No. 5 alone provide evidence that this dye affects ...
The RFL's match review panel has revealed its findings following Super League's Magic Weekend. Two Leeds Rhinos players were among nine sin-binned during the two days of action at Elland Road.
Design: (1) Literature review and survey of international experiences of consumer reporting of ADRs; (2) descriptive analysis of Yellow Card reports; (3) signal generation analysis of Yellow Card reports; (4) qualitative analysis of Yellow Card reports; (5) questionnaire survey of patients reporting on Yellow Cards; (6) qualitative analysis of ...