
Research Design 101
Everything You Need To Get Started (With Examples)
By: Derek Jansen (MBA) | Reviewers: Eunice Rautenbach (DTech) & Kerryn Warren (PhD) | April 2023

Navigating the world of research can be daunting, especially if you’re a first-time researcher. One concept you’re bound to run into fairly early in your research journey is that of “ research design ”. Here, we’ll guide you through the basics using practical examples , so that you can approach your research with confidence.
Overview: Research Design 101
What is research design.
- Research design types for quantitative studies
- Video explainer : quantitative research design
- Research design types for qualitative studies
- Video explainer : qualitative research design
- How to choose a research design
- Key takeaways
Research design refers to the overall plan, structure or strategy that guides a research project , from its conception to the final data analysis. A good research design serves as the blueprint for how you, as the researcher, will collect and analyse data while ensuring consistency, reliability and validity throughout your study.
Understanding different types of research designs is essential as helps ensure that your approach is suitable given your research aims, objectives and questions , as well as the resources you have available to you. Without a clear big-picture view of how you’ll design your research, you run the risk of potentially making misaligned choices in terms of your methodology – especially your sampling , data collection and data analysis decisions.
The problem with defining research design…
One of the reasons students struggle with a clear definition of research design is because the term is used very loosely across the internet, and even within academia.
Some sources claim that the three research design types are qualitative, quantitative and mixed methods , which isn’t quite accurate (these just refer to the type of data that you’ll collect and analyse). Other sources state that research design refers to the sum of all your design choices, suggesting it’s more like a research methodology . Others run off on other less common tangents. No wonder there’s confusion!
In this article, we’ll clear up the confusion. We’ll explain the most common research design types for both qualitative and quantitative research projects, whether that is for a full dissertation or thesis, or a smaller research paper or article.

Research Design: Quantitative Studies
Quantitative research involves collecting and analysing data in a numerical form. Broadly speaking, there are four types of quantitative research designs: descriptive , correlational , experimental , and quasi-experimental .
Descriptive Research Design
As the name suggests, descriptive research design focuses on describing existing conditions, behaviours, or characteristics by systematically gathering information without manipulating any variables. In other words, there is no intervention on the researcher’s part – only data collection.
For example, if you’re studying smartphone addiction among adolescents in your community, you could deploy a survey to a sample of teens asking them to rate their agreement with certain statements that relate to smartphone addiction. The collected data would then provide insight regarding how widespread the issue may be – in other words, it would describe the situation.
The key defining attribute of this type of research design is that it purely describes the situation . In other words, descriptive research design does not explore potential relationships between different variables or the causes that may underlie those relationships. Therefore, descriptive research is useful for generating insight into a research problem by describing its characteristics . By doing so, it can provide valuable insights and is often used as a precursor to other research design types.
Correlational Research Design
Correlational design is a popular choice for researchers aiming to identify and measure the relationship between two or more variables without manipulating them . In other words, this type of research design is useful when you want to know whether a change in one thing tends to be accompanied by a change in another thing.
For example, if you wanted to explore the relationship between exercise frequency and overall health, you could use a correlational design to help you achieve this. In this case, you might gather data on participants’ exercise habits, as well as records of their health indicators like blood pressure, heart rate, or body mass index. Thereafter, you’d use a statistical test to assess whether there’s a relationship between the two variables (exercise frequency and health).
As you can see, correlational research design is useful when you want to explore potential relationships between variables that cannot be manipulated or controlled for ethical, practical, or logistical reasons. It is particularly helpful in terms of developing predictions , and given that it doesn’t involve the manipulation of variables, it can be implemented at a large scale more easily than experimental designs (which will look at next).
That said, it’s important to keep in mind that correlational research design has limitations – most notably that it cannot be used to establish causality . In other words, correlation does not equal causation . To establish causality, you’ll need to move into the realm of experimental design, coming up next…
Need a helping hand?
Experimental Research Design
Experimental research design is used to determine if there is a causal relationship between two or more variables . With this type of research design, you, as the researcher, manipulate one variable (the independent variable) while controlling others (dependent variables). Doing so allows you to observe the effect of the former on the latter and draw conclusions about potential causality.
For example, if you wanted to measure if/how different types of fertiliser affect plant growth, you could set up several groups of plants, with each group receiving a different type of fertiliser, as well as one with no fertiliser at all. You could then measure how much each plant group grew (on average) over time and compare the results from the different groups to see which fertiliser was most effective.
Overall, experimental research design provides researchers with a powerful way to identify and measure causal relationships (and the direction of causality) between variables. However, developing a rigorous experimental design can be challenging as it’s not always easy to control all the variables in a study. This often results in smaller sample sizes , which can reduce the statistical power and generalisability of the results.
Moreover, experimental research design requires random assignment . This means that the researcher needs to assign participants to different groups or conditions in a way that each participant has an equal chance of being assigned to any group (note that this is not the same as random sampling ). Doing so helps reduce the potential for bias and confounding variables . This need for random assignment can lead to ethics-related issues . For example, withholding a potentially beneficial medical treatment from a control group may be considered unethical in certain situations.
Quasi-Experimental Research Design
Quasi-experimental research design is used when the research aims involve identifying causal relations , but one cannot (or doesn’t want to) randomly assign participants to different groups (for practical or ethical reasons). Instead, with a quasi-experimental research design, the researcher relies on existing groups or pre-existing conditions to form groups for comparison.
For example, if you were studying the effects of a new teaching method on student achievement in a particular school district, you may be unable to randomly assign students to either group and instead have to choose classes or schools that already use different teaching methods. This way, you still achieve separate groups, without having to assign participants to specific groups yourself.
Naturally, quasi-experimental research designs have limitations when compared to experimental designs. Given that participant assignment is not random, it’s more difficult to confidently establish causality between variables, and, as a researcher, you have less control over other variables that may impact findings.
All that said, quasi-experimental designs can still be valuable in research contexts where random assignment is not possible and can often be undertaken on a much larger scale than experimental research, thus increasing the statistical power of the results. What’s important is that you, as the researcher, understand the limitations of the design and conduct your quasi-experiment as rigorously as possible, paying careful attention to any potential confounding variables .

Research Design: Qualitative Studies
There are many different research design types when it comes to qualitative studies, but here we’ll narrow our focus to explore the “Big 4”. Specifically, we’ll look at phenomenological design, grounded theory design, ethnographic design, and case study design.
Phenomenological Research Design
Phenomenological design involves exploring the meaning of lived experiences and how they are perceived by individuals. This type of research design seeks to understand people’s perspectives , emotions, and behaviours in specific situations. Here, the aim for researchers is to uncover the essence of human experience without making any assumptions or imposing preconceived ideas on their subjects.
For example, you could adopt a phenomenological design to study why cancer survivors have such varied perceptions of their lives after overcoming their disease. This could be achieved by interviewing survivors and then analysing the data using a qualitative analysis method such as thematic analysis to identify commonalities and differences.
Phenomenological research design typically involves in-depth interviews or open-ended questionnaires to collect rich, detailed data about participants’ subjective experiences. This richness is one of the key strengths of phenomenological research design but, naturally, it also has limitations. These include potential biases in data collection and interpretation and the lack of generalisability of findings to broader populations.
Grounded Theory Research Design
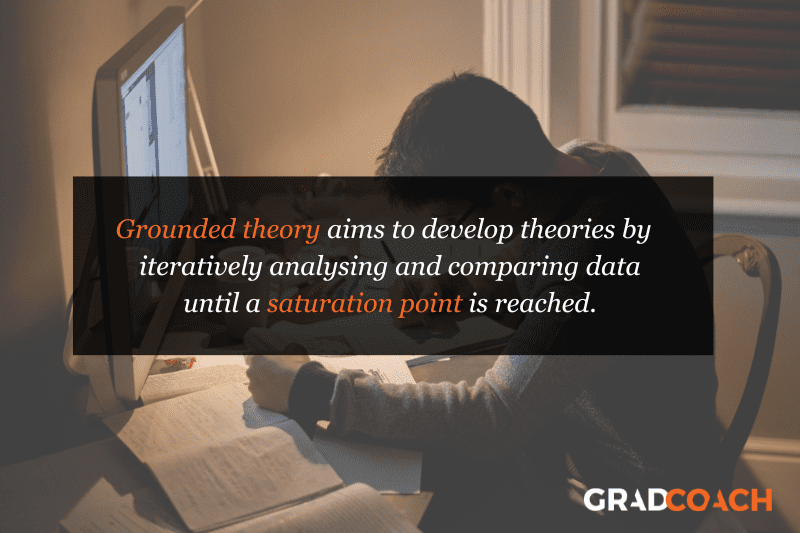
Grounded theory (also referred to as “GT”) aims to develop theories by continuously and iteratively analysing and comparing data collected from a relatively large number of participants in a study. It takes an inductive (bottom-up) approach, with a focus on letting the data “speak for itself”, without being influenced by preexisting theories or the researcher’s preconceptions.
As an example, let’s assume your research aims involved understanding how people cope with chronic pain from a specific medical condition, with a view to developing a theory around this. In this case, grounded theory design would allow you to explore this concept thoroughly without preconceptions about what coping mechanisms might exist. You may find that some patients prefer cognitive-behavioural therapy (CBT) while others prefer to rely on herbal remedies. Based on multiple, iterative rounds of analysis, you could then develop a theory in this regard, derived directly from the data (as opposed to other preexisting theories and models).
Grounded theory typically involves collecting data through interviews or observations and then analysing it to identify patterns and themes that emerge from the data. These emerging ideas are then validated by collecting more data until a saturation point is reached (i.e., no new information can be squeezed from the data). From that base, a theory can then be developed .
As you can see, grounded theory is ideally suited to studies where the research aims involve theory generation , especially in under-researched areas. Keep in mind though that this type of research design can be quite time-intensive , given the need for multiple rounds of data collection and analysis.
Ethnographic Research Design
Ethnographic design involves observing and studying a culture-sharing group of people in their natural setting to gain insight into their behaviours, beliefs, and values. The focus here is on observing participants in their natural environment (as opposed to a controlled environment). This typically involves the researcher spending an extended period of time with the participants in their environment, carefully observing and taking field notes .
All of this is not to say that ethnographic research design relies purely on observation. On the contrary, this design typically also involves in-depth interviews to explore participants’ views, beliefs, etc. However, unobtrusive observation is a core component of the ethnographic approach.
As an example, an ethnographer may study how different communities celebrate traditional festivals or how individuals from different generations interact with technology differently. This may involve a lengthy period of observation, combined with in-depth interviews to further explore specific areas of interest that emerge as a result of the observations that the researcher has made.
As you can probably imagine, ethnographic research design has the ability to provide rich, contextually embedded insights into the socio-cultural dynamics of human behaviour within a natural, uncontrived setting. Naturally, however, it does come with its own set of challenges, including researcher bias (since the researcher can become quite immersed in the group), participant confidentiality and, predictably, ethical complexities . All of these need to be carefully managed if you choose to adopt this type of research design.
Case Study Design
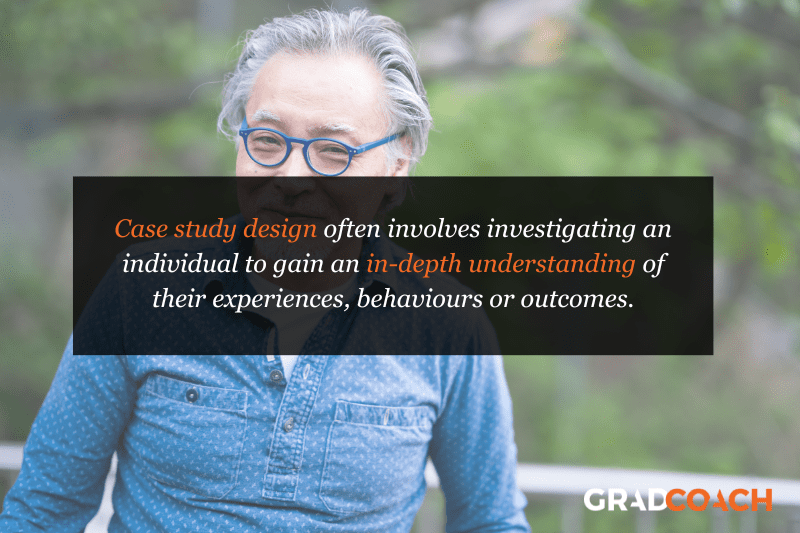
With case study research design, you, as the researcher, investigate a single individual (or a single group of individuals) to gain an in-depth understanding of their experiences, behaviours or outcomes. Unlike other research designs that are aimed at larger sample sizes, case studies offer a deep dive into the specific circumstances surrounding a person, group of people, event or phenomenon, generally within a bounded setting or context .
As an example, a case study design could be used to explore the factors influencing the success of a specific small business. This would involve diving deeply into the organisation to explore and understand what makes it tick – from marketing to HR to finance. In terms of data collection, this could include interviews with staff and management, review of policy documents and financial statements, surveying customers, etc.
While the above example is focused squarely on one organisation, it’s worth noting that case study research designs can have different variation s, including single-case, multiple-case and longitudinal designs. As you can see in the example, a single-case design involves intensely examining a single entity to understand its unique characteristics and complexities. Conversely, in a multiple-case design , multiple cases are compared and contrasted to identify patterns and commonalities. Lastly, in a longitudinal case design , a single case or multiple cases are studied over an extended period of time to understand how factors develop over time.
As you can see, a case study research design is particularly useful where a deep and contextualised understanding of a specific phenomenon or issue is desired. However, this strength is also its weakness. In other words, you can’t generalise the findings from a case study to the broader population. So, keep this in mind if you’re considering going the case study route.
How To Choose A Research Design
Having worked through all of these potential research designs, you’d be forgiven for feeling a little overwhelmed and wondering, “ But how do I decide which research design to use? ”. While we could write an entire post covering that alone, here are a few factors to consider that will help you choose a suitable research design for your study.
Data type: The first determining factor is naturally the type of data you plan to be collecting – i.e., qualitative or quantitative. This may sound obvious, but we have to be clear about this – don’t try to use a quantitative research design on qualitative data (or vice versa)!
Research aim(s) and question(s): As with all methodological decisions, your research aim and research questions will heavily influence your research design. For example, if your research aims involve developing a theory from qualitative data, grounded theory would be a strong option. Similarly, if your research aims involve identifying and measuring relationships between variables, one of the experimental designs would likely be a better option.
Time: It’s essential that you consider any time constraints you have, as this will impact the type of research design you can choose. For example, if you’ve only got a month to complete your project, a lengthy design such as ethnography wouldn’t be a good fit.
Resources: Take into account the resources realistically available to you, as these need to factor into your research design choice. For example, if you require highly specialised lab equipment to execute an experimental design, you need to be sure that you’ll have access to that before you make a decision.
Keep in mind that when it comes to research, it’s important to manage your risks and play as conservatively as possible. If your entire project relies on you achieving a huge sample, having access to niche equipment or holding interviews with very difficult-to-reach participants, you’re creating risks that could kill your project. So, be sure to think through your choices carefully and make sure that you have backup plans for any existential risks. Remember that a relatively simple methodology executed well generally will typically earn better marks than a highly-complex methodology executed poorly.

Recap: Key Takeaways
We’ve covered a lot of ground here. Let’s recap by looking at the key takeaways:
- Research design refers to the overall plan, structure or strategy that guides a research project, from its conception to the final analysis of data.
- Research designs for quantitative studies include descriptive , correlational , experimental and quasi-experimenta l designs.
- Research designs for qualitative studies include phenomenological , grounded theory , ethnographic and case study designs.
- When choosing a research design, you need to consider a variety of factors, including the type of data you’ll be working with, your research aims and questions, your time and the resources available to you.
If you need a helping hand with your research design (or any other aspect of your research), check out our private coaching services .

Psst... there’s more!
This post was based on one of our popular Research Bootcamps . If you're working on a research project, you'll definitely want to check this out ...
You Might Also Like:

10 Comments
Is there any blog article explaining more on Case study research design? Is there a Case study write-up template? Thank you.
Thanks this was quite valuable to clarify such an important concept.
Thanks for this simplified explanations. it is quite very helpful.
This was really helpful. thanks
Thank you for your explanation. I think case study research design and the use of secondary data in researches needs to be talked about more in your videos and articles because there a lot of case studies research design tailored projects out there.
Please is there any template for a case study research design whose data type is a secondary data on your repository?
This post is very clear, comprehensive and has been very helpful to me. It has cleared the confusion I had in regard to research design and methodology.
This post is helpful, easy to understand, and deconstructs what a research design is. Thanks
how to cite this page
Thank you very much for the post. It is wonderful and has cleared many worries in my mind regarding research designs. I really appreciate .
how can I put this blog as my reference(APA style) in bibliography part?
Submit a Comment Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
- Print Friendly
Quantitative study designs: Introduction
Quantitative study designs, introduction.
- Cohort Studies
- Randomised Controlled Trial
- Case Control
- Cross-Sectional Studies
- Study Designs Home
Email your Librarians
Related guides
- Study Design Basics
- Qualitative Study Designs
Throughout our lives, we are exposed to many factors that can affect our health and wellbeing. But what kinds of factors influence specific health outcomes? And what do we do when we become ill? Health researchers dedicate their working lives to answering a huge range of different clinical research questions and they do so by carrying out very specific studies. The findings from these studies eventually lead to the development of interventions that can help save lives.

Flowchart adapted from: Grimes, D. A., & Schulz, K. F. (2002). “ An overview of clinical research: The lay of the land ”. The Lancet, 359(9300), 57-61
- Accessibility document - study designs decision tree Text version of the decision tree illustration on identfiying a study's design.
So many study designs – what’s the difference?
In clinical research, a study design is a plan for selecting study subjects and for obtaining data. These study designs fall into two different categories:
- Experimental
- Observational
These categories are based on whether or not the investigators assign a particular exposure to a cohort.
Experimental trials
Investigators assign exposures (for example, a trial to test the effectiveness of a new medication) and these are categorised into randomized (studies with a control, or comparison, group) and non-randomized trials (those without a control group).
Observational studies
These studies focus on exposures that are already present in a population and assess the effects of the exposure on that cohort. These studies are further categorised into analytical and descriptive.
Analytical studies
- Include a control (or comparison group)
- In Cohort studies people are tracked forward in time from exposure to outcome.
- Case-control studies, by contrast, trace back from outcome to exposure.
- Cross-sectional studies are like a snapshot in time, measuring both exposure and outcome at a particular time point.
Descriptive studies
- Include case reports, case-series and case studies. Cross-sectional studies can also be descriptive.
- Do not have a control or comparison group
- Cannot examine associations between an exposure and an outcome.
Which study type will answer my clinical question?
Not all study types will be appropriate for answering a particular clinical question. For example, if you wanted to investigate the impact of maternal smoking on foetal development, then a randomized-controlled trial would not be appropriate as it is not ethical to assign a disease or potentially harmful exposure to an individual. In this case, an observational study would be more appropriate. This table illustrates the most appropriate study designs for answering specific types of clinical questions.
In this learning series, we will examine each study design type in more detail, the types of clinical questions they investigate and the methodologies applied in each study design.
- Next: Cohort Studies >>
- Last Updated: May 15, 2024 11:37 AM
- URL: https://deakin.libguides.com/quantitative-study-designs
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Indian J Crit Care Med
- v.23(Suppl 4); 2019 Dec
Understanding Research Study Designs
Priya ranganathan.
Department of Anesthesiology, Critical Care and Pain, Tata Memorial Hospital, Mumbai, Maharashtra, India
In this article, we will look at the important features of various types of research study designs used commonly in biomedical research.
How to cite this article
Ranganathan P. Understanding Research Study Designs. Indian J Crit Care Med 2019;23(Suppl 4):S305–S307.
We use a variety of research study designs in biomedical research. In this article, the main features of each of these designs are summarized.
TERMS USED IN RESEARCH DESIGNS
Exposure vs outcome.
Exposure refers to any factor that may be associated with the outcome of interest. It is also called the predictor variable or independent variable or risk factor. Outcome refers to the variable that is studied to assess the impact of the exposure on the population. It is also known as the predicted variable or the dependent variable. For example, in a study looking at nerve damage after organophosphate (OPC) poisoning, the exposure would be OPC and the outcome would be nerve damage.
Longitudinal vs Transversal Studies
In longitudinal studies, participants are followed over time to determine the association between exposure and outcome (or outcome and exposure). On the other hand, in transversal studies, observations about exposure and outcome are made at a single point in time.
Forward vs Backward Directed Studies
In forward-directed studies, the direction of enquiry moves from exposure to outcome. In backward-directed studies, the line of enquiry starts with outcome and then determines exposure.
Prospective vs Retrospective Studies
In prospective studies, the outcome has not occurred at the time of initiation of the study. The researcher determines exposure and follows participants into the future to assess outcomes. In retrospective studies, the outcome of interest has already occurred when the study commences.
CLASSIFICATION OF STUDY DESIGNS
Broadly, study designs can be classified as descriptive or analytical (inferential) studies.
Descriptive Studies
Descriptive studies describe the characteristics of interest in the study population (also referred to as sample, to differentiate it from the entire population in the universe). These studies do not have a comparison group. The simplest type of descriptive study is the case report. In a case report, the researcher describes his/her experience with symptoms, signs, diagnosis, or treatment of a patient. Sometimes, a group of patients having a similar experience may be grouped to form a case series.
Case reports and case series form the lowest level of evidence in biomedical research and, as such, are considered hypothesis-generating studies. However, they are easy to write and may be a good starting point for the budding researcher. The recognition of some important associations in the field of medicine—such as that of thalidomide with phocomelia and Kaposi's sarcoma with HIV infection—resulted from case reports and case series. The reader can look up several published case reports and case series related to complications after OPC poisoning. 1 , 2
Analytical (Inferential) Studies
Analytical or inferential studies try to prove a hypothesis and establish an association between an exposure and an outcome. These studies usually have a comparator group. Analytical studies are further classified as observational or interventional studies.
In observational studies, there is no intervention by the researcher. The researcher merely observes outcomes in different groups of participants who, for natural reasons, have or have not been exposed to a particular risk factor. Examples of observational studies include cross-sectional, case–control, and cohort studies.
Cross-sectional Studies
These are transversal studies where data are collected from the study population at a single point in time. Exposure and outcome are determined simultaneously. Cross-sectional studies are easy to conduct, involve no follow-up, and need limited resources. They offer useful information on prevalence of health conditions and possible associations between risk factors and outcomes. However, there are two major limitations of cross-sectional studies. First, it may not be possible to establish a clear cause–benefit relationship. For example, in a study of association between colon cancer and dietary fiber intake, it may be difficult to establish whether the low fiber intake preceded the symptoms of colon cancer or whether the symptoms of colon cancer resulted in a change in dietary fiber intake. Another important limitation of cross-sectional studies is survival bias. For example, in a study looking at alcohol intake vs mortality due to chronic liver disease, among the participants with the highest alcohol intake, several may have died of liver disease; this will not be picked up by the study and will give biased results. An example of a cross-sectional study is a survey on nurses’ knowledge and practices of initial management of acute poisoning. 3
Case–control Studies
Case–control studies are backward-directed studies. Here, the direction of enquiry begins with the outcome and then proceeds to exposure. Case–control studies are always retrospective, i.e., the outcome of interest has occurred when the study begins. The researcher identifies participants who have developed the outcome of interest (cases) and chooses matching participants who do not have the outcome (controls). Matching is done based on factors that are likely to influence the exposure or outcome (e.g., age, gender, socioeconomic status). The researcher then proceeds to determine exposure in cases and controls. If cases have a higher incidence of exposure than controls, it suggests an association between exposure and outcome. Case–control studies are relatively quick to conduct, need limited resources, and are useful when the outcome is rare. They also allow the researcher to study multiple exposures for a particular outcome. However, they have several limitations. First, matching of cases with controls may not be easy since many unknown confounders may affect exposure and outcome. Second, there may be biased in the way the history of exposure is determined in cases vs controls; one way to overcome this is to have a blinded assessor determining the exposure using a standard technique (e.g., a standardized questionnaire). However, despite this, it has been shown that cases are far more likely than controls to recall history of exposure—the “recall bias.” For example, mothers of babies born with congenital anomalies may provide a more detailed history of drugs ingested during their pregnancy than those with normal babies. Also, since case-control studies do not begin with a population at risk, it is not possible to determine the true risk of outcome. Instead, one can only calculate the odds of association between exposure and outcome.
Kendrick and colleagues designed a case–control study to look at the association between domestic poison prevention practices and medically attended poisoning in children. They identified children presenting with unintentional poisoning at home (cases with the outcome), matched them with community participants (controls without the outcome), and then elicited data from parents and caregivers on home safety practices (exposure). 4
Cohort Studies
Cohort studies resemble clinical trials except that the exposure is naturally determined instead of being decided by the investigator. Here, the direction of enquiry begins with the exposure and then proceeds to outcome. The researcher begins with a group of individuals who are free of outcome at baseline; of these, some have the exposure (study cohort) while others do not (control group). The groups are followed up over a period of time to determine occurrence of outcome. Cohort studies may be prospective (involving a period of follow-up after the start of the study) or retrospective (e.g., using medical records or registry data). Cohort studies are considered the strongest among the observational study designs. They provide proof of temporal relationship (exposure occurred before outcome), allow determination of risk, and permit multiple outcomes to be studied for a single exposure. However, they are expensive to conduct and time-consuming, there may be several losses to follow-up, and they are not suitable for studying rare outcomes. Also, there may be unknown confounders other than the exposure affecting the occurrence of the outcome.
Jayasinghe conducted a cohort study to look at the effect of acute organophosphorus poisoning on nerve function. They recruited 70 patients with OPC poisoning (exposed group) and 70 matched controls without history of pesticide exposure (unexposed controls). Participants were followed up or 6 weeks for neurophysiological assessments to determine nerve damage (outcome). Hung carried out a retrospective cohort study using a nationwide research database to look at the long-term effects of OPC poisoning on cardiovascular disease. From the database, he identified an OPC-exposed cohort and an unexposed control cohort (matched for gender and age) from several years back and then examined later records to look at the development of cardiovascular diseases in both groups. 5
Interventional Studies
In interventional studies (also known as experimental studies or clinical trials), the researcher deliberately allots participants to receive one of several interventions; of these, some may be experimental while others may be controls (either standard of care or placebo). Allotment of participants to a particular treatment arm is carried out through the process of randomization, which ensures that every participant has a similar chance of being in any of the arms, eliminating bias in selection. There are several other aspects crucial to the validity of the results of a clinical trial such as allocation concealment, blinding, choice of control, and statistical analysis plan. These will be discussed in a separate article.
The randomized controlled clinical trial is considered the gold standard for evaluating the efficacy of a treatment. Randomization leads to equal distribution of known and unknown confounders between treatment arms; therefore, we can be reasonably certain that any difference in outcome is a treatment effect and not due to other factors. The temporal sequence of cause and effect is established. It is possible to determine risk of the outcome in each treatment arm accurately. However, randomized controlled trials have their limitations and may not be possible in every situation. For example, it is unethical to randomize participants to an intervention that is likely to cause harm—e.g., smoking. In such cases, well-designed observational studies are the only option. Also, these trials are expensive to conduct and resource-intensive.
In a randomized controlled trial, Li et al. randomly allocated patients of paraquat poisoning to receive either conventional therapy (control group) or continuous veno-venous hemofiltration (intervention). Patients were followed up to look for mortality or other adverse events (outcome). 6
Researchers need to understand the features of different study designs, with their advantages and limitations so that the most appropriate design can be chosen for a particular research question. The Centre for Evidence Based Medicine offers an useful tool to determine the type of research design used in a particular study. 7
Source of support: Nil
Conflict of interest: None
Quantitative Research
- Reference work entry
- First Online: 13 January 2019
- Cite this reference work entry

- Leigh A. Wilson 2 , 3
4352 Accesses
4 Citations
Quantitative research methods are concerned with the planning, design, and implementation of strategies to collect and analyze data. Descartes, the seventeenth-century philosopher, suggested that how the results are achieved is often more important than the results themselves, as the journey taken along the research path is a journey of discovery. High-quality quantitative research is characterized by the attention given to the methods and the reliability of the tools used to collect the data. The ability to critique research in a systematic way is an essential component of a health professional’s role in order to deliver high quality, evidence-based healthcare. This chapter is intended to provide a simple overview of the way new researchers and health practitioners can understand and employ quantitative methods. The chapter offers practical, realistic guidance in a learner-friendly way and uses a logical sequence to understand the process of hypothesis development, study design, data collection and handling, and finally data analysis and interpretation.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Similar content being viewed by others

Writing Quantitative Research Studies

Qualitative Research Methods
Babbie ER. The practice of social research. 14th ed. Belmont: Wadsworth Cengage; 2016.
Google Scholar
Descartes. Cited in Halverston, W. (1976). In: A concise introduction to philosophy, 3rd ed. New York: Random House; 1637.
Doll R, Hill AB. The mortality of doctors in relation to their smoking habits. BMJ. 1954;328(7455):1529–33. https://doi.org/10.1136/bmj.328.7455.1529 .
Article Google Scholar
Liamputtong P. Research methods in health: foundations for evidence-based practice. 3rd ed. Melbourne: Oxford University Press; 2017.
McNabb DE. Research methods in public administration and nonprofit management: quantitative and qualitative approaches. 2nd ed. New York: Armonk; 2007.
Merriam-Webster. Dictionary. http://www.merriam-webster.com . Accessed 20th December 2017.
Olesen Larsen P, von Ins M. The rate of growth in scientific publication and the decline in coverage provided by Science Citation Index. Scientometrics. 2010;84(3):575–603.
Pannucci CJ, Wilkins EG. Identifying and avoiding bias in research. Plast Reconstr Surg. 2010;126(2):619–25. https://doi.org/10.1097/PRS.0b013e3181de24bc .
Petrie A, Sabin C. Medical statistics at a glance. 2nd ed. London: Blackwell Publishing; 2005.
Portney LG, Watkins MP. Foundations of clinical research: applications to practice. 3rd ed. New Jersey: Pearson Publishing; 2009.
Sheehan J. Aspects of research methodology. Nurse Educ Today. 1986;6:193–203.
Wilson LA, Black DA. Health, science research and research methods. Sydney: McGraw Hill; 2013.
Download references
Author information
Authors and affiliations.
School of Science and Health, Western Sydney University, Penrith, NSW, Australia
Leigh A. Wilson
Faculty of Health Science, Discipline of Behavioural and Social Sciences in Health, University of Sydney, Lidcombe, NSW, Australia
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Leigh A. Wilson .
Editor information
Editors and affiliations.
Pranee Liamputtong
Rights and permissions
Reprints and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this entry
Cite this entry.
Wilson, L.A. (2019). Quantitative Research. In: Liamputtong, P. (eds) Handbook of Research Methods in Health Social Sciences. Springer, Singapore. https://doi.org/10.1007/978-981-10-5251-4_54
Download citation
DOI : https://doi.org/10.1007/978-981-10-5251-4_54
Published : 13 January 2019
Publisher Name : Springer, Singapore
Print ISBN : 978-981-10-5250-7
Online ISBN : 978-981-10-5251-4
eBook Packages : Social Sciences Reference Module Humanities and Social Sciences Reference Module Business, Economics and Social Sciences
Share this entry
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Privacy Policy

Home » Quantitative Research – Methods, Types and Analysis
Quantitative Research – Methods, Types and Analysis
Table of Contents

Quantitative Research
Quantitative research is a type of research that collects and analyzes numerical data to test hypotheses and answer research questions . This research typically involves a large sample size and uses statistical analysis to make inferences about a population based on the data collected. It often involves the use of surveys, experiments, or other structured data collection methods to gather quantitative data.
Quantitative Research Methods

Quantitative Research Methods are as follows:
Descriptive Research Design
Descriptive research design is used to describe the characteristics of a population or phenomenon being studied. This research method is used to answer the questions of what, where, when, and how. Descriptive research designs use a variety of methods such as observation, case studies, and surveys to collect data. The data is then analyzed using statistical tools to identify patterns and relationships.
Correlational Research Design
Correlational research design is used to investigate the relationship between two or more variables. Researchers use correlational research to determine whether a relationship exists between variables and to what extent they are related. This research method involves collecting data from a sample and analyzing it using statistical tools such as correlation coefficients.
Quasi-experimental Research Design
Quasi-experimental research design is used to investigate cause-and-effect relationships between variables. This research method is similar to experimental research design, but it lacks full control over the independent variable. Researchers use quasi-experimental research designs when it is not feasible or ethical to manipulate the independent variable.
Experimental Research Design
Experimental research design is used to investigate cause-and-effect relationships between variables. This research method involves manipulating the independent variable and observing the effects on the dependent variable. Researchers use experimental research designs to test hypotheses and establish cause-and-effect relationships.
Survey Research
Survey research involves collecting data from a sample of individuals using a standardized questionnaire. This research method is used to gather information on attitudes, beliefs, and behaviors of individuals. Researchers use survey research to collect data quickly and efficiently from a large sample size. Survey research can be conducted through various methods such as online, phone, mail, or in-person interviews.
Quantitative Research Analysis Methods
Here are some commonly used quantitative research analysis methods:
Statistical Analysis
Statistical analysis is the most common quantitative research analysis method. It involves using statistical tools and techniques to analyze the numerical data collected during the research process. Statistical analysis can be used to identify patterns, trends, and relationships between variables, and to test hypotheses and theories.
Regression Analysis
Regression analysis is a statistical technique used to analyze the relationship between one dependent variable and one or more independent variables. Researchers use regression analysis to identify and quantify the impact of independent variables on the dependent variable.
Factor Analysis
Factor analysis is a statistical technique used to identify underlying factors that explain the correlations among a set of variables. Researchers use factor analysis to reduce a large number of variables to a smaller set of factors that capture the most important information.
Structural Equation Modeling
Structural equation modeling is a statistical technique used to test complex relationships between variables. It involves specifying a model that includes both observed and unobserved variables, and then using statistical methods to test the fit of the model to the data.
Time Series Analysis
Time series analysis is a statistical technique used to analyze data that is collected over time. It involves identifying patterns and trends in the data, as well as any seasonal or cyclical variations.
Multilevel Modeling
Multilevel modeling is a statistical technique used to analyze data that is nested within multiple levels. For example, researchers might use multilevel modeling to analyze data that is collected from individuals who are nested within groups, such as students nested within schools.
Applications of Quantitative Research
Quantitative research has many applications across a wide range of fields. Here are some common examples:
- Market Research : Quantitative research is used extensively in market research to understand consumer behavior, preferences, and trends. Researchers use surveys, experiments, and other quantitative methods to collect data that can inform marketing strategies, product development, and pricing decisions.
- Health Research: Quantitative research is used in health research to study the effectiveness of medical treatments, identify risk factors for diseases, and track health outcomes over time. Researchers use statistical methods to analyze data from clinical trials, surveys, and other sources to inform medical practice and policy.
- Social Science Research: Quantitative research is used in social science research to study human behavior, attitudes, and social structures. Researchers use surveys, experiments, and other quantitative methods to collect data that can inform social policies, educational programs, and community interventions.
- Education Research: Quantitative research is used in education research to study the effectiveness of teaching methods, assess student learning outcomes, and identify factors that influence student success. Researchers use experimental and quasi-experimental designs, as well as surveys and other quantitative methods, to collect and analyze data.
- Environmental Research: Quantitative research is used in environmental research to study the impact of human activities on the environment, assess the effectiveness of conservation strategies, and identify ways to reduce environmental risks. Researchers use statistical methods to analyze data from field studies, experiments, and other sources.

Characteristics of Quantitative Research
Here are some key characteristics of quantitative research:
- Numerical data : Quantitative research involves collecting numerical data through standardized methods such as surveys, experiments, and observational studies. This data is analyzed using statistical methods to identify patterns and relationships.
- Large sample size: Quantitative research often involves collecting data from a large sample of individuals or groups in order to increase the reliability and generalizability of the findings.
- Objective approach: Quantitative research aims to be objective and impartial in its approach, focusing on the collection and analysis of data rather than personal beliefs, opinions, or experiences.
- Control over variables: Quantitative research often involves manipulating variables to test hypotheses and establish cause-and-effect relationships. Researchers aim to control for extraneous variables that may impact the results.
- Replicable : Quantitative research aims to be replicable, meaning that other researchers should be able to conduct similar studies and obtain similar results using the same methods.
- Statistical analysis: Quantitative research involves using statistical tools and techniques to analyze the numerical data collected during the research process. Statistical analysis allows researchers to identify patterns, trends, and relationships between variables, and to test hypotheses and theories.
- Generalizability: Quantitative research aims to produce findings that can be generalized to larger populations beyond the specific sample studied. This is achieved through the use of random sampling methods and statistical inference.
Examples of Quantitative Research
Here are some examples of quantitative research in different fields:
- Market Research: A company conducts a survey of 1000 consumers to determine their brand awareness and preferences. The data is analyzed using statistical methods to identify trends and patterns that can inform marketing strategies.
- Health Research : A researcher conducts a randomized controlled trial to test the effectiveness of a new drug for treating a particular medical condition. The study involves collecting data from a large sample of patients and analyzing the results using statistical methods.
- Social Science Research : A sociologist conducts a survey of 500 people to study attitudes toward immigration in a particular country. The data is analyzed using statistical methods to identify factors that influence these attitudes.
- Education Research: A researcher conducts an experiment to compare the effectiveness of two different teaching methods for improving student learning outcomes. The study involves randomly assigning students to different groups and collecting data on their performance on standardized tests.
- Environmental Research : A team of researchers conduct a study to investigate the impact of climate change on the distribution and abundance of a particular species of plant or animal. The study involves collecting data on environmental factors and population sizes over time and analyzing the results using statistical methods.
- Psychology : A researcher conducts a survey of 500 college students to investigate the relationship between social media use and mental health. The data is analyzed using statistical methods to identify correlations and potential causal relationships.
- Political Science: A team of researchers conducts a study to investigate voter behavior during an election. They use survey methods to collect data on voting patterns, demographics, and political attitudes, and analyze the results using statistical methods.
How to Conduct Quantitative Research
Here is a general overview of how to conduct quantitative research:
- Develop a research question: The first step in conducting quantitative research is to develop a clear and specific research question. This question should be based on a gap in existing knowledge, and should be answerable using quantitative methods.
- Develop a research design: Once you have a research question, you will need to develop a research design. This involves deciding on the appropriate methods to collect data, such as surveys, experiments, or observational studies. You will also need to determine the appropriate sample size, data collection instruments, and data analysis techniques.
- Collect data: The next step is to collect data. This may involve administering surveys or questionnaires, conducting experiments, or gathering data from existing sources. It is important to use standardized methods to ensure that the data is reliable and valid.
- Analyze data : Once the data has been collected, it is time to analyze it. This involves using statistical methods to identify patterns, trends, and relationships between variables. Common statistical techniques include correlation analysis, regression analysis, and hypothesis testing.
- Interpret results: After analyzing the data, you will need to interpret the results. This involves identifying the key findings, determining their significance, and drawing conclusions based on the data.
- Communicate findings: Finally, you will need to communicate your findings. This may involve writing a research report, presenting at a conference, or publishing in a peer-reviewed journal. It is important to clearly communicate the research question, methods, results, and conclusions to ensure that others can understand and replicate your research.
When to use Quantitative Research
Here are some situations when quantitative research can be appropriate:
- To test a hypothesis: Quantitative research is often used to test a hypothesis or a theory. It involves collecting numerical data and using statistical analysis to determine if the data supports or refutes the hypothesis.
- To generalize findings: If you want to generalize the findings of your study to a larger population, quantitative research can be useful. This is because it allows you to collect numerical data from a representative sample of the population and use statistical analysis to make inferences about the population as a whole.
- To measure relationships between variables: If you want to measure the relationship between two or more variables, such as the relationship between age and income, or between education level and job satisfaction, quantitative research can be useful. It allows you to collect numerical data on both variables and use statistical analysis to determine the strength and direction of the relationship.
- To identify patterns or trends: Quantitative research can be useful for identifying patterns or trends in data. For example, you can use quantitative research to identify trends in consumer behavior or to identify patterns in stock market data.
- To quantify attitudes or opinions : If you want to measure attitudes or opinions on a particular topic, quantitative research can be useful. It allows you to collect numerical data using surveys or questionnaires and analyze the data using statistical methods to determine the prevalence of certain attitudes or opinions.
Purpose of Quantitative Research
The purpose of quantitative research is to systematically investigate and measure the relationships between variables or phenomena using numerical data and statistical analysis. The main objectives of quantitative research include:
- Description : To provide a detailed and accurate description of a particular phenomenon or population.
- Explanation : To explain the reasons for the occurrence of a particular phenomenon, such as identifying the factors that influence a behavior or attitude.
- Prediction : To predict future trends or behaviors based on past patterns and relationships between variables.
- Control : To identify the best strategies for controlling or influencing a particular outcome or behavior.
Quantitative research is used in many different fields, including social sciences, business, engineering, and health sciences. It can be used to investigate a wide range of phenomena, from human behavior and attitudes to physical and biological processes. The purpose of quantitative research is to provide reliable and valid data that can be used to inform decision-making and improve understanding of the world around us.
Advantages of Quantitative Research
There are several advantages of quantitative research, including:
- Objectivity : Quantitative research is based on objective data and statistical analysis, which reduces the potential for bias or subjectivity in the research process.
- Reproducibility : Because quantitative research involves standardized methods and measurements, it is more likely to be reproducible and reliable.
- Generalizability : Quantitative research allows for generalizations to be made about a population based on a representative sample, which can inform decision-making and policy development.
- Precision : Quantitative research allows for precise measurement and analysis of data, which can provide a more accurate understanding of phenomena and relationships between variables.
- Efficiency : Quantitative research can be conducted relatively quickly and efficiently, especially when compared to qualitative research, which may involve lengthy data collection and analysis.
- Large sample sizes : Quantitative research can accommodate large sample sizes, which can increase the representativeness and generalizability of the results.
Limitations of Quantitative Research
There are several limitations of quantitative research, including:
- Limited understanding of context: Quantitative research typically focuses on numerical data and statistical analysis, which may not provide a comprehensive understanding of the context or underlying factors that influence a phenomenon.
- Simplification of complex phenomena: Quantitative research often involves simplifying complex phenomena into measurable variables, which may not capture the full complexity of the phenomenon being studied.
- Potential for researcher bias: Although quantitative research aims to be objective, there is still the potential for researcher bias in areas such as sampling, data collection, and data analysis.
- Limited ability to explore new ideas: Quantitative research is often based on pre-determined research questions and hypotheses, which may limit the ability to explore new ideas or unexpected findings.
- Limited ability to capture subjective experiences : Quantitative research is typically focused on objective data and may not capture the subjective experiences of individuals or groups being studied.
- Ethical concerns : Quantitative research may raise ethical concerns, such as invasion of privacy or the potential for harm to participants.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

Questionnaire – Definition, Types, and Examples

Case Study – Methods, Examples and Guide

Observational Research – Methods and Guide

Qualitative Research Methods

Explanatory Research – Types, Methods, Guide

Survey Research – Types, Methods, Examples
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, automatically generate references for free.
- Knowledge Base
- Methodology
- What Is Quantitative Research? | Definition & Methods
What Is Quantitative Research? | Definition & Methods
Published on 4 April 2022 by Pritha Bhandari . Revised on 10 October 2022.
Quantitative research is the process of collecting and analysing numerical data. It can be used to find patterns and averages, make predictions, test causal relationships, and generalise results to wider populations.
Quantitative research is the opposite of qualitative research , which involves collecting and analysing non-numerical data (e.g. text, video, or audio).
Quantitative research is widely used in the natural and social sciences: biology, chemistry, psychology, economics, sociology, marketing, etc.
- What is the demographic makeup of Singapore in 2020?
- How has the average temperature changed globally over the last century?
- Does environmental pollution affect the prevalence of honey bees?
- Does working from home increase productivity for people with long commutes?
Table of contents
Quantitative research methods, quantitative data analysis, advantages of quantitative research, disadvantages of quantitative research, frequently asked questions about quantitative research.
You can use quantitative research methods for descriptive, correlational or experimental research.
- In descriptive research , you simply seek an overall summary of your study variables.
- In correlational research , you investigate relationships between your study variables.
- In experimental research , you systematically examine whether there is a cause-and-effect relationship between variables.
Correlational and experimental research can both be used to formally test hypotheses , or predictions, using statistics. The results may be generalised to broader populations based on the sampling method used.
To collect quantitative data, you will often need to use operational definitions that translate abstract concepts (e.g., mood) into observable and quantifiable measures (e.g., self-ratings of feelings and energy levels).
Prevent plagiarism, run a free check.
Once data is collected, you may need to process it before it can be analysed. For example, survey and test data may need to be transformed from words to numbers. Then, you can use statistical analysis to answer your research questions .
Descriptive statistics will give you a summary of your data and include measures of averages and variability. You can also use graphs, scatter plots and frequency tables to visualise your data and check for any trends or outliers.
Using inferential statistics , you can make predictions or generalisations based on your data. You can test your hypothesis or use your sample data to estimate the population parameter .
You can also assess the reliability and validity of your data collection methods to indicate how consistently and accurately your methods actually measured what you wanted them to.
Quantitative research is often used to standardise data collection and generalise findings . Strengths of this approach include:
- Replication
Repeating the study is possible because of standardised data collection protocols and tangible definitions of abstract concepts.
- Direct comparisons of results
The study can be reproduced in other cultural settings, times or with different groups of participants. Results can be compared statistically.
- Large samples
Data from large samples can be processed and analysed using reliable and consistent procedures through quantitative data analysis.
- Hypothesis testing
Using formalised and established hypothesis testing procedures means that you have to carefully consider and report your research variables, predictions, data collection and testing methods before coming to a conclusion.
Despite the benefits of quantitative research, it is sometimes inadequate in explaining complex research topics. Its limitations include:
- Superficiality
Using precise and restrictive operational definitions may inadequately represent complex concepts. For example, the concept of mood may be represented with just a number in quantitative research, but explained with elaboration in qualitative research.
- Narrow focus
Predetermined variables and measurement procedures can mean that you ignore other relevant observations.
- Structural bias
Despite standardised procedures, structural biases can still affect quantitative research. Missing data , imprecise measurements or inappropriate sampling methods are biases that can lead to the wrong conclusions.
- Lack of context
Quantitative research often uses unnatural settings like laboratories or fails to consider historical and cultural contexts that may affect data collection and results.
Quantitative research deals with numbers and statistics, while qualitative research deals with words and meanings.
Quantitative methods allow you to test a hypothesis by systematically collecting and analysing data, while qualitative methods allow you to explore ideas and experiences in depth.
In mixed methods research , you use both qualitative and quantitative data collection and analysis methods to answer your research question .
Data collection is the systematic process by which observations or measurements are gathered in research. It is used in many different contexts by academics, governments, businesses, and other organisations.
Operationalisation means turning abstract conceptual ideas into measurable observations.
For example, the concept of social anxiety isn’t directly observable, but it can be operationally defined in terms of self-rating scores, behavioural avoidance of crowded places, or physical anxiety symptoms in social situations.
Before collecting data , it’s important to consider how you will operationalise the variables that you want to measure.
Reliability and validity are both about how well a method measures something:
- Reliability refers to the consistency of a measure (whether the results can be reproduced under the same conditions).
- Validity refers to the accuracy of a measure (whether the results really do represent what they are supposed to measure).
If you are doing experimental research , you also have to consider the internal and external validity of your experiment.
Hypothesis testing is a formal procedure for investigating our ideas about the world using statistics. It is used by scientists to test specific predictions, called hypotheses , by calculating how likely it is that a pattern or relationship between variables could have arisen by chance.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the ‘Cite this Scribbr article’ button to automatically add the citation to our free Reference Generator.
Bhandari, P. (2022, October 10). What Is Quantitative Research? | Definition & Methods. Scribbr. Retrieved 14 May 2024, from https://www.scribbr.co.uk/research-methods/introduction-to-quantitative-research/
Is this article helpful?

Pritha Bhandari
To read this content please select one of the options below:
Please note you do not have access to teaching notes, testing a graduateness and employability skills model through the use of social media: findings from south africa.
Higher Education, Skills and Work-Based Learning
ISSN : 2042-3896
Article publication date: 17 May 2024
This study examined the influence of social media use on graduateness and the employability of exit students in South Africa.
Design/methodology/approach
The study used quantitative and descriptive research designs to test the proposed hypotheses. An online survey was used to collect the data from a study sample. A sample of 411 respondents was received, with structural equation modelling (SEM) being used to assess the model fit.
The study found that the direct effect of social media use on graduateness skills is significant. Secondly, the direct effect of graduateness skills on perceived employability is also significant. The results also showed existence of support for the mediation of graduateness skills on the relationship between social media use and perceived employability.
Research limitations/implications
The study provides empirical evidence to the proposed model and infers the potential role of social media in addressing issues related to graduateness and the employability of exit students.
Practical implications
In addressing the challenge of unemployment, the use of social media can potentially aid in matters of skills acquisition.
Originality/value
The results demonstrate how technology through the use of social media potentially fits within enhancing graduateness and employability skills.
- Social media
- Access to higher education
- Youth unemployment
Murire, O.T. , Cilliers, L. and Chinyamurindi, W. (2024), "Testing a graduateness and employability skills model through the use of social media: findings from South Africa", Higher Education, Skills and Work-Based Learning , Vol. ahead-of-print No. ahead-of-print. https://doi.org/10.1108/HESWBL-12-2023-0330
Emerald Publishing Limited
Copyright © 2024, Emerald Publishing Limited
Related articles
We’re listening — tell us what you think, something didn’t work….
Report bugs here
All feedback is valuable
Please share your general feedback
Join us on our journey
Platform update page.
Visit emeraldpublishing.com/platformupdate to discover the latest news and updates
Questions & More Information
Answers to the most commonly asked questions here
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Systematic Review
- Open access
- Published: 17 May 2024
Risk factors and incidence of central venous access device-related thrombosis in hospitalized children: a systematic review and meta-analysis
- Maoling Fu 1 , 2 ,
- Quan Yuan 2 ,
- Qiaoyue Yang 1 , 2 ,
- Yaqi Yu 1 , 2 ,
- Wenshuai Song 1 , 2 ,
- Xiuli Qin 1 ,
- Ying Luo 1 ,
- Xiaoju Xiong 1 &
- Genzhen Yu 1
Pediatric Research ( 2024 ) Cite this article
Metrics details
The risk factors for central venous access device-related thrombosis (CRT) in children are not fully understood. We used evidence-based medicine to find the risk factors for CRT by pooling current studies reporting risk factors of CRT, aiming to guide clinical diagnosis and treatment.
A systematic search of PubMed, Web of Science, Embase, Cochrane Library, Scopus, CNKI, Sinomed, and Wanfang databases was conducted. RevMan 5.4 was employed for data analysis.
The review included 47 studies evaluating 262,587 children with CVAD placement. Qualitative synthesis and quantitative meta-analysis identified D-dimer, location of insertion, type of catheter, number of lumens, catheter indwelling time, and central line-associated bloodstream infection as the most critical risk factors for CRT. Primarily due to observational design, the quality of evidence was regarded as low certainty for these risk factors according to the GRADE approach.
Because fewer high-quality studies are available, larger sample sizes and well-designed prospective studies are still needed to clarify the risk factors affecting CRT. In the future, developing pediatric-specific CRT risk assessment tools is important. Appropriate stratified preventive strategies for CRT according to risk assessment level will help improve clinical efficiency, avoid the occurrence of CRT, and alleviate unnecessary suffering of children.
This is the latest systematic review of risk factors and incidence of CRT in children.
A total of 47 studies involving 262,587 patients were included in our meta-analysis, according to which the pooled prevalence of CRT was 9.1%.
This study identified several of the most critical risk factors affecting CRT in children, including D-dimer, insertion location, type of catheter, number of lumens, catheter indwelling time, and central line-associated bloodstream infection (CLABSI).
Introduction
Central venous access device (CVAD) is an infusion device inserted through different parts to make the tip of the catheter to the vena cava. In the clinic, CVAD is mainly divided into the following four categories: tunneled central venous catheter (CVC), nontunneled CVC, peripherally inserted central catheter (PICC), and totally implantable venous access port (TIVAP). 1 Pediatric patients often require stable, multifunctional, and comfortable long-term vascular access due to factors such as poor puncture cooperation, small vessel diameter, poor peripheral venous visibility and tolerance, high water content in the body leading to easy dehydration, and easy changes in condition after diseases. 2 The application of CVAD can significantly reduce the frequency of venipuncture, relieve the stimulation of drugs on the venous blood vessels, alleviate the pain and fear of the children, improve their medication compliance, ensure the effectiveness of intravenous infusion, and improve the quality of disease treatment. 3 , 4 , 5 Therefore, CVAD is widely used in pediatric clinics and has become an indispensable aspect of complex medical care for children with severe and chronic diseases.
Although CVAD has become an important tool in the pediatric treatment and nursing process, there are also risks of complications related to it, including CVAD-related thrombosis (CRT), phlebitis, fluid and blood leakage at the puncture point, catheter displacement, catheter obstruction, central line-associated bloodstream infection (CLABSI) and so on. 6 , 7 Among these, CRT is one of the most common and serious complications. The prevalence of CRT in children varies significantly by country, age, disease, and medical institution, ranging from 2 to 81%, 4 , 8 , 9 , 10 while in Chinese children without prophylactic treatment ranges from 20 to 66%. 11 , 12 CRT has no obvious clinical symptoms in the early stage, but it may still cause serious side effects, not only increasing the patient pain and medical costs but also delaying treatment timing, affecting prognosis and quality of life, and in severe cases, may even lead to thromboembolism, endangering life. 13 , 14 , 15
Identifying risk factors and incidence of CRT facilitates clinical practitioners in the early identification of high-risk patients, designing specific preventive strategies, treatment regimens, and management plans, thereby effectively reducing the incidence of CRT in hospitalized children and alleviating unnecessary patient suffering. However, most current research on CRT involves only small-scale groups in isolated nursing units or specific disease types. To date, no up-to-date systematic review provides pooled estimates of the risk factors and prevalence of CRT in children. Therefore, this study had a dual purpose: 1. to explore potential risk factors for CRT in children and to determine a pooled level of CRT prevalence; and 2. to provide evidence-based recommendations to improve the recognition, control, and treatment of CRT in children, as well as better nursing management for CRT.
This review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. 16 The detailed research protocol can be accessed on the PROSPERO website (registration number: CRD42023421353).
Search strategy
Eight electronic databases were utilized to conduct a thorough literature search: PubMed, Web of Science, Embase, Cochrane Library, Scopus, China National Knowledge Infrastructure (CNKI), Sinomed, and Wanfang. The search in these databases was conducted from the earliest records available up to January 31st, 2024. The search strategy used a combination of Mesh terms and free words. The following Mesh terms and free words were mainly used: “child,” “children,” “adolescent,” “infant,” “pediatrics,” “central venous access device-related thrombosis,” “CRT,” “catheter-related thrombosis,” “catheter-related venous thrombosis,” “CVC-related thrombosis,” “risk factors,” “protective factors,” “predictors,” “causality,” “influencing factors”. The full search strategy for each database is available in the Supplementary Materials. In addition, we screened the reference lists of all included studies for relevant studies that met the criteria. Grey literature was searched as well. Some authors were contacted through email to gather more information or clarify any uncertainties.
Inclusion criteria
The study population was hospitalized children aged ≤18 years.
The primary research objective was to explore the risk factors for CRT.
The study results have at least one statistically significant predictor.
Case-control studies or cohort studies.
Published in English or Chinese.
Exclusion criteria
Catheter-related infection, catheter dysfunction, or other catheter complications as the primary outcome indicators.
Repeated published research.
Case reports, study designs, or clinical trials.
Reviews, editorials, letters, and conference abstracts.
In vitro or animal research.
Data were incomplete and could not be extracted.
Unable to find the original article.
Data extraction
Data from each eligible study were independently extracted by two reviewers using a pre-designed data collection form. Any disagreements were resolved by discussions among all authors. Data on the following characteristics were obtained from all included studies (see Supplementary Table S 1 for details):
Basic information: first author, country, year of publication, study duration, and study design.
Demographic characteristics: study population, sample size, number of CRT, and CRT rate.
Catheter-related features: catheter type, CRT type, and diagnostic method.
Potential risk factors for CRT: odds ratios (OR) or relative risks (RR) values and 95% confidence interval (CI) were extracted for each risk factor. If the study did not provide specific values, it was calculated by constructing a 2 × 2 contingency table.
Quality assessment
Two reviewers evaluated the quality of each study independently using the Risk of Bias Assessment for Nonrandomized Studies tool, 17 with any differences settled via group discussion. The tool assessed six domains of risk of bias: participant selection, confounding variables, exposure measurement, blinding of outcome assessment, incomplete outcome data, and selective outcome reporting. If all six domains were rated as low risk, the overall risk of bias for the study was low. The overall risk of bias was moderate if at least one domain was rated as unclear risk, and no domain was rated as high risk, and high if one or more domains were rated as high risk.
To ensure the accuracy of the assessment results, a third reviewer randomly selected five studies to check the data extraction and quality assessment.
Qualitative synthesis and quantitative meta-analysis
Qualitatively classify each risk factor as definite, likely, unclear, or not a risk factor based on the total number of studies with low and moderate bias risks and the proportion of studies demonstrating positive association (Box 1 in the supplementary material). If a risk factor was reported by more than two studies with low or moderate risk of bias, and the definition and reference range were sufficiently consistent, a quantitative meta-analysis was performed to estimate the combined OR.
Data were analyzed using Revman 5.4 software. In the meta-analysis of risk factors and CRT rate, the generic inverse variance method was applied, which only required effect estimate and standard error (SE). 18 The SE was obtained by inverse transforming the 95% CI applying the standard normal distribution. Heterogeneity tests were performed on the studies included in the Meta-analysis to examine for the combinability of the results of each independent study. P ≥ 0.05 and I-squared ( I 2 ) < 50% considered less heterogeneity between studies and therefore a fixed-effects model was chosen for the analysis, conversely, P < 0.05 or I 2 ≥ 50% considered greater heterogeneity, and a random-effects model was chosen.
Certainty of the evidence
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method was used to assess the certainty of the evidence. In this method, observational studies were initially classified as low-quality evidence and then downgraded and upgraded according to five downgrading and three upgrading principles. The 5 downgrading factors included risk of bias, inconsistency, indirectness, imprecision, and publication bias, and the 3 upgrading factors included the magnitude of an effect, dose-response gradient, and effect of plausible residual confounding. Based on these considerations, the overall certainty of each piece of evidence was rated as one of four levels: high, moderate, low, or very low.
The initial search of the databases extracted a total of 4193 articles, of which 1656 were duplicates and removed. The titles and abstracts of the remaining 2537 articles were screened according to the inclusion criteria and 142 were selected for full-text search. After a rigorous eligibility review, 45 articles met the inclusion criteria. In addition, two articles were found to meet the eligibility criteria in a search of the reference lists of the selected articles and grey literature. In the end, a total of 47 articles were included in this review, of which 43 contributed to the qualitative synthesis and quantitative meta-analysis (Fig. 1 ).
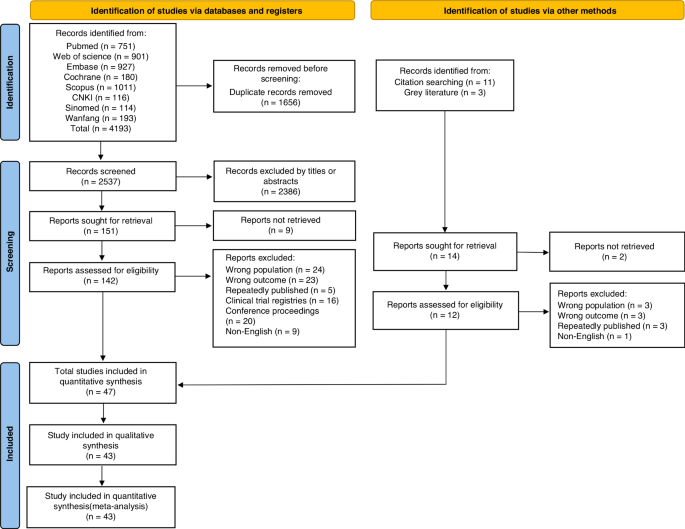
Demonstrate the screening and inclusion process for systematic literature search.
Of the 47 studies, 19 were prospective 4 , 13 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 and the rest were retrospective, 9 , 12 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 of which 10 were multicenter 4 , 9 , 13 , 21 , 23 , 26 , 27 , 28 , 49 , 59 and 37 were single-center. 12 , 19 , 20 , 22 , 24 , 25 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 60 , 61 The sample sizes ranged from 47 to 158,299, with the two largest being 71,782 13 and 158,299, 59 respectively. In addition, three studies constructed clinical prediction models. 22 , 28 , 47 Table 1 lists the summary characteristics of the included studies.
Study populations and CRT rates in included studies
These studies investigated a series of hospitalized children of different ages and departments, of which 12 studies with all hospitalized children as the study population, 12 studies with PICU hospitalized children as the study population, six studies with NICU hospitalized children as the study population, one study with all ICU hospitalized children as the study population, four studies with leukemia children as the study population, two studies with infants under 1-year-old as the study population, and the other ten studies with children with a specific disease as the study population.
The combined CRT rate was 9.1% (95% CI : 5.7–14.5%) with a high degree of heterogeneity ( I 2 = 100%). The combined CRT rate was 11.5% (95% CI : 5.7–23.1%; I 2 = 99%) in both male and female children. The frequency of CRT in PICU and NICU was available from 13 articles with 234,464 children and 7 articles with 6093 infants, which combined CRT rates were 10.7% (95% CI : 3.8–23.7%; I 2 = 100%), 2.9% (95% CI : 1.0–6.5%; I 2 = 96%), respectively. The combined CRT rate of children with leukemia was 13.0% (95% CI : 2.9–38.3%; I 2 = 98%) (Supplementary Material Figs. S 1 – 6 )
Quality of the CRT studies
The methodological quality of the included studies varied (Fig. 2 and Supplementary Material Fig. S 7 ). Nine studies had a low overall risk of bias, as all six domains were categorized as low risk. Four studies had a high overall risk of bias, three of which were associated with confounding variables and one to participant selection. The remaining 34 studies had a moderate overall risk of bias, with at least one of the six domains having an unclear risk.
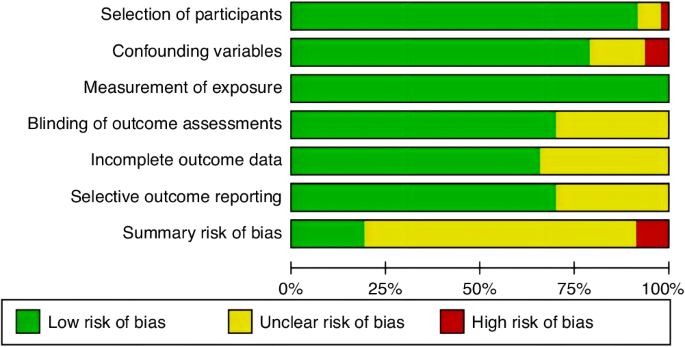
A summary presentation of the assessment results of risk of bias for the 47 studies.
Risk factors of CRT in included studies
The 47 included studies reported 61 statistically significant risk factors for CRT (Table 1 ). These factors were classified into three categories: patient-related risk factors (37.7%, 23/61); CVAD-related risk factors (34.4%, 21/61), and treatment-related risk factors (27.9%, 17/61).
Based on the qualitative synthesis, six variables were considered to be definite risk factors for CRT, including D-dimer, location of insertion, type of catheter, number of lumens, catheter indwelling time, and CLABSI. Eleven variables were considered likely associated with CRT, including gastrointestinal diseases, history of catheterization, thrombophilia, geographic location of line placement, catheter dysfunction, number of catheters, insertion length (cm), catheter to vein ratio, dialysis, hypertonic liquid, and cardiac catheterization. For 42 variables, the relationship with CRT was deemed unclear due to conflicting results from studies assessed as having low and moderate risk of bias, or because they were positively associated in only one study. Additionally, birth weight and gestational age were considered non-risk factors (Table 2 ).
Meta-analyses were implemented for risk factors that were reported by at least two low or moderate risk of bias studies with a consistent definition and reference range (Table 3 and Figs. 3 – 6 ).
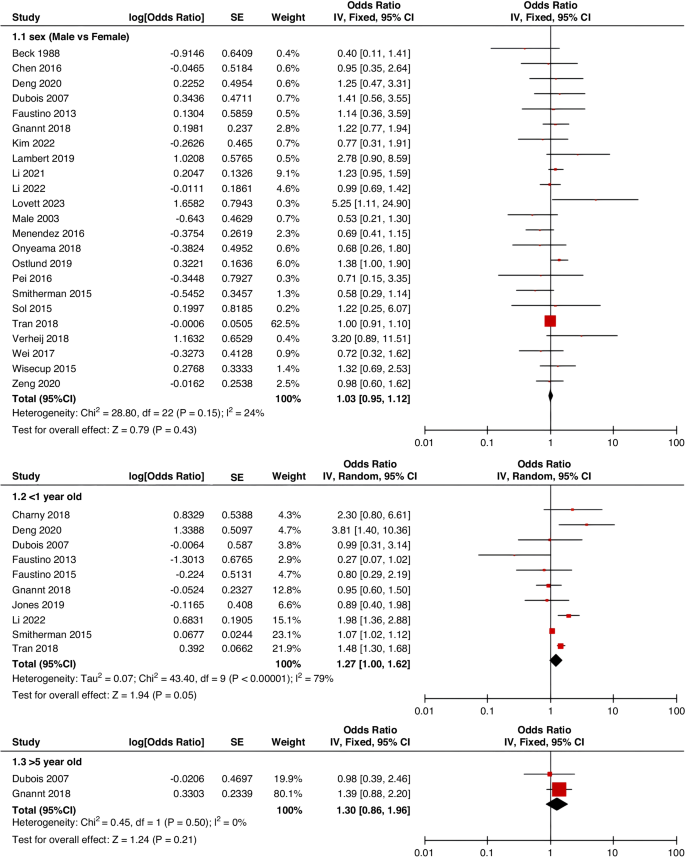
Forest plots of odds ratios (OR) that were included in the quantitative meta-analysis and the associated overall OR. For each OR, the size of the red square region is proportional to the corresponding study weight. Diamond shape intervals represent the overall OR. I 2 represents the fraction of variability among the individual OR that cannot be explained by sampling variability.
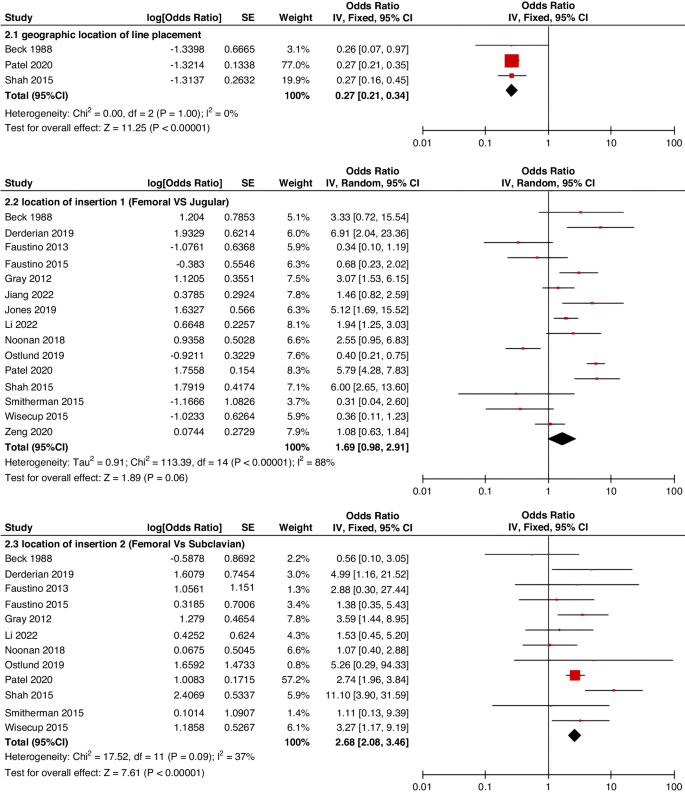
Forest plots of odds ratios (OR) that were included in the quantitative meta-analysis and the associated overall OR. For each OR, the size of the red square region is proportional to the corresponding study weight. Diamond shape intervals represent the overall OR. I 2 represents the fraction of variability among the individual OR that cannot be explained by sampling variability.
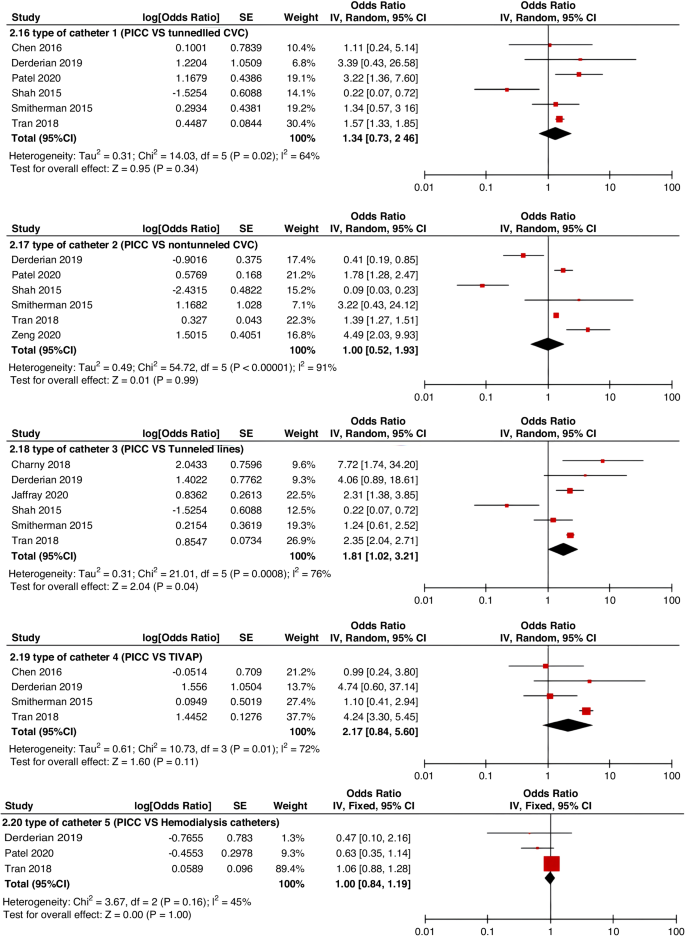
GRADE assessment of evidence
Supplementary Table S 2 shows GRADE assessments for the certainty of evidence. Due to the design of the observational studies, all evidence was initially rated as low certainty. Based on five downgrading and three upgrading principles, 17 pieces of evidence were still rated as low certainty, and the remaining 44 pieces of evidence were downgraded to very low certainty for serious inconsistency and imprecision.
Our study is the latest systematic review of risk factors and the incidence of CRT in hospitalized children. Based on 47 studies included in the current meta-analysis, which involved a total of 262,587 patients, the pooled prevalence of CRT is 9.1%. We conducted a qualitative synthesis analysis of 61 predictive factors and a quantitative meta-analysis of 38 factors, identifying six definite factors, 11 likely factors, and 42 unclear factors associated with CRT. Definite predictors included being of D-dimer, location of insertion, type of catheter, number of lumens, catheter indwelling time and CLABSI. The findings of our systematic review provide the latest comprehensive evidence summary that can inform the early identification of children at risk for CRT and the development of intervention measures to prevent and reduce CRT.
Implantable and temporary medical devices such as CVAD are exposed to blood for weeks to years depending on the type of CVAD in place. Since CVAD is an artificial surface and lacks an endothelial layer that inhibits platelet coagulation and adhesion, it is thought to potentially activate the contact pathways, ultimately leading to thrombosis. Assembly of artificial surface contact systems might be part of the host defense mechanism against foreign substances, but it can lead to kinin and thrombin generation, and complement activation. 62 This eventually promotes thrombosis and inflammation. The presence of CVAD is the most common risk factor for venous thromboembolism (VTE). CRT accounts for 10% of deep vein thrombosis (DVT) in adults and 50–80% in children. 10 , 55 , 63 The incidence of CRT in hospitalized children has increased significantly by 30–70% over the past 20 years, 64 , 65 which may cause serious medical complications besides increasing healthcare expenditures and length of stay.
We discover that a higher level of D-dimer is an independent risk factor for CRT in hospitalized children, consistent with the results of adult studies. 66 D-dimer is a soluble fibrin degradation product deriving from the plasmin-mediated degradation of cross-linked fibrin that is increased or positive in secondary hyperfibrinolysis, such as hypercoagulable states, disseminated intravascular coagulation, and thrombolytic therapy. 67 , 68 Increased D-dimer suggests an association with thrombotic disorders in the body of various origins and an increase in fibrinolytic activity. D-dimer has been extensively investigated for excluding the diagnosis of VTE and is used routinely for this indication. 67 , 69 Therefore, for early recognition and to reduce the incidence of CRT, D-dimer levels should be closely monitored before and after catheterization. However, the elevated D-dimer test results cannot fully explain the cause and location of CRT formation and must be analyzed in conjunction with clinical and other test results. Inherited thrombophilia, caused by genetic defects leading to a deficiency or abnormality in associated proteins, including protein C, protein S, antithrombin, the coagulation factor V Leiden mutation, and factor II mutation G20210A, 70 is considered a potential risk factor for CRT. The prevalence of thrombophilia varies widely among different populations, with a reported prevalence of 10% to 59% in pediatric VTE patients. 71 Children with gastrointestinal diseases like short bowel syndrome (SBS) and inflammatory bowel disease (IBD) have an increased risk of developing CRT during hospitalization. The precise mechanism behind this association is still uncertain according to current research. It may be attributed to the heightened inflammation levels during catheterization, particularly in patients with active IBD episodes or admissions during surgery, which leads to a period of increased inactivity. 55 This suggests that delaying placement during the most active period of inflammation may reduce the rate of thrombosis.
A narrative review pointed out that age is one of the most significant risk factors for VTE. In children, CRT shows a bimodal distribution, with the highest incidence rate in infancy and adolescence. 10 The higher incidence in infancy may be due in part to the smaller diameter of the vein, making insertion difficult and requiring multiple attempts. However, whether age is a risk factor for CRT is still highly controversial. The study by Chojnacka et al. did not find a statistically significant difference, 39 although a trend toward a similar bimodal distribution was found in the study population. Cancer, cardiovascular disease, sepsis, asphyxia, and neurological diseases are also considered unclear factors for CRT. Pediatric patients diagnosed with leukemia have multiple risk factors for VTE formation, such as the presence of hypercoagulable blast cells, the pro-thrombotic nature of the cancer itself, and treatment with steroids and L-asparaginase. Chen et al. 38 and Jaffray et al. 4 concluded that children with leukemia are more likely to develop CRT. Sepsis causes the coagulation mechanism to become fragile, which in turn activates the coagulation system and creates thrombosis. 72 However, a study by Onyeama et al. 52 showed that sepsis was significantly associated with a reduced incidence of CRT, and the exact mechanism is currently unknown.
The location of insertion and type of catheter are critical risk factors for CRT. The incidence of CRT is higher in femoral vein catheterizations compared to subclavian and jugular vein catheterizations in children, which is contrary to findings in adult patients. 73 The femoral location is a larger vessel and allows placement of a larger size catheter. Femoral CVAD is prioritized in urgent and emergency situations. In such cases, the patients tend to be more critically ill and often immobilized, further exacerbating the low-flow state. In addition, there may be vein compression and kinking beneath the inguinal ligament with leg movement, which may increase the risk of CRT. 27 PICC catheters provide a reliable medium to long-term route to intravenous therapy for children, but compared with other types of catheters, the risk of CRT is higher. We speculate that the long tunnel length and relatively large lumen size of the PICC, compared to the diameter of the vessel at the insertion site, may lead to increased blood flow obstruction. 52 Additionally, patients with PICC may be more likely to be diagnosed with symptomatic VTE than tunneled lines (TLs) because PICC is often placed in smaller vessels and journeys through the arm or leg causing limb pain and swelling, whereas TLs are located in the chest.
The risk of CRT increases with the number of lumens. A possible explanation for this finding is that multilumen catheters tend to have larger catheter sizes and thus occupy more area within the vessel lumen, leading to obstruction of normal blood flow within the veins. The relationship between CRT and CLABSI is bidirectional. Following catheter insertion, a fibrin sheath forms around the catheter. Microorganisms, especially staphylococcus aureus, easily adhere to the fibrin sheaths, and may lead to CLABSI. 74 Conversely, CLABSI can trigger inflammatory reactions, leading to further progression of thrombosis. CVAD duration is positively associated with the risk of CRT. Catheter placement may cause mechanical injury to the vein. As the indwelling duration increases, many damaged smooth muscle and endothelial cells become embedded within the fibrin, resulting in thrombus formation. In addition, prolonged indwelling increases the chance of platelet contact with the vessel lining, activating coagulation factors and thrombin, increasing the risk of thrombosis. 22 Therefore, nurses should perform routine maintenance of the catheter in children who require long-term CVAD indwelling. The duration of CVAD should be monitored, the necessity of its indwelling should be assessed daily, and the catheter should be removed as early as possible while ensuring treatment.
As obstruction of venous blood flow from the CVAD is considered an essential causative mechanism for the development of VTE, a high ratio between catheter size and vein diameter could be a risk factor for CRT. The 2012 international guidelines on pediatric CVC insertion recommend that the ratio between the catheter’s external diameter and the cannulated vein’s diameter should not exceed 0.33. 75 However, this suggestion is only based on expert opinions and currently lacks relevant clinical data support. Therefore, further research is still needed to verify it. Catheter dysfunction is mainly caused by small clots or fibrous sheaths wrapping around the tip of the catheter. Prolonged accumulation may lead to incomplete or complete blockage of blood vessels, becoming a gathering point for thrombosis. 74 Journeycake et al. observed that the risk of VTE was highest in pediatric cancer patients with multiple episodes of catheter dysfunction. 76 A study of pediatric brain tumor patients reported that VTE was more common in patients with catheter dysfunction. 77 Thus, these studies and the current data support the need to consider catheter dysfunction as a possible risk factor for CRT and to design further screening and intervention studies for early identification and prevention of catheter dysfunction.
The rationale for studying the relationship between the insertion side of CVAD and the risk of CRT is based on the anatomy of the upper body venous system. The left brachiocephalic vein is longer and courses more horizontally than the right side, thus entering the superior vena cava at a sharper angle. The right jugular vein is the most direct and shortest route for the CVAD to enter the heart. By contrast, the CVAD located in the left jugular vein has a greater distance to the heart and passes through 2 angles in the venous system, which may cause endothelial damage and increase the likelihood of blood flow obstruction and venous wall adhesion. 26 However, our meta-analysis did not find a statistically significant increase in the risk of CRT with left-sided placement compared to right-sided placement. The ideal location for the catheter tip is the junction of the superior vena cava and the right atrium. This location is preferred because of the higher blood flow rate, which may be protective against thrombosis. 43 Currently, the pediatric literature on the effect of optimal tip position on CRT is scarce and inconclusive. In addition, catheter tips do not always remain in that position after initial placement. Therefore, tip movement should be a significant concern in pediatric patients, especially active, growing, and requiring long-term catheter use.
Providing renal replacement therapy is a lifelong task for pediatric end-stage renal disease (ESRD) patients. Although successful transplantation can be achieved even in young patients, the lifespan of the graft is limited. Consequently, many transplant recipients may be put back on dialysis as part of their ESRD treatment. 78 CVC remains the main vascular access for hemodialysis in children. Long-term reliance on CVC is related to a high incidence of catheter dysfunction and failure. The frequent need for recurrent CVC placement in such patients leads to an elevated risk of central vein stenosis and CRT. Cardiac catheterization is also a possible risk factor for CRT. Appropriate anticoagulation is required during catheterization, without which the risk of thrombosis is up to 40%. However, the use of unfractionated heparin in pediatric patients is challenging because the coagulation system and heparin response are different from that of adults. 79 There’s a need for further research to determine if children are receiving adequate doses of heparin during cardiac catheterization to prevent thrombosis without increasing the risk of bleeding complications. The incidence of VTE in adult patients who are chronically bedridden and braked is 3.59 times higher than in patients with normal activity levels. 80 In critically ill or surgical children, mechanical ventilation is often performed in the early stages, requiring continuous use of multiple sedative or inotropic drugs to reduce cardiac load and protect pulmonary function. During sedation, the child is in a braked state, limb activity is reduced or even inactive, blood flow slows down, and blood stagnates in the veins, increasing the chance of platelet adhesion to the endothelium, which may increase the risk of CRT. Therefore, passive movements such as limb abduction, internal rotation, elbow flexion and elbow extension should be performed appropriately when the child’s condition permits.
Nutritional support is an important part of critical illness treatment, including enteral and parenteral nutrition (PN). CVAD is the supply channel for total parenteral nutrition (TPN), and some children may even need this method to provide calories for a long time. High glucose and calcium concentrations in PN are both possible triggers of CRT, and PN has been shown to upregulate the extrinsic coagulation cascade, especially with long-term use. 60 Diamanti et al. reported that the incidence rate of TPN complicated with CRT was 20%. 81 Mannitol or glycerol fructose are widely used as hypertonic drugs in clinical practice, which can increase plasma osmolality to dehydrate tissues after entering the body. At the same time, it may cause a cellular stress response, induce apoptosis, and can activate inflammatory cytokines and coagulation pathways to induce thrombosis. Jiang et al. 22 found vasoactive drugs to be a risk factor for CRT. The possible reason is that vasoactive drugs can cause strong vasoconstriction, endothelial function damage or impairment, and promote fibrinogen synthesis. However, this is contrary to the findings of Marquez et al. 28 and Faustino et al. 21 Therefore, larger prospective studies are still needed to assess this risk factor more precisely.
The strengths of this study include the systematic identification of all relevant studies of risk factors for CRT in hospitalized children and the classification of risk factors into three categories, patient-related risk factors, CVAD-related risk factors, and treatment-related risk factors, to offer a logical progression of the possible causes of CRT in children. However, several limitations of this systematic review should be stated. Firstly, as most of the studies originate from Western countries, extrapolating these results to Eastern populations is questionable. Second, significant heterogeneity was encountered in our analysis, potentially stemming from variations in regimen, duration, population enrolled, and center setting, among other factors. This diversity necessitates a cautious interpretation of the results. In addition, only a few high-quality studies with a low risk of bias, and many of the studies suffer from significant sources of bias. Furthermore, the effect in many occasions was assessed by very few studies. Therefore, the evidence to support it is low, which needs to be validated in future studies. Finally, risk factors for CRT could not be made causal assertions since the majority of studies were retrospective.
Conclusions
In conclusion, we have identified several critical factors that affect CRT, including D-dimer, location of insertion, type of catheter, number of lumens, catheter indwelling time, and CLABSI. Nevertheless, none of the included studies considered the impact of socio-demographic factors on CRT, such as parental education level, occupation, and family economic status. Therefore, larger sample sizes and well-designed prospective studies are still needed to clarify the predictors affecting CRT in the future. In addition, there is a lack of pediatric-specific CRT risk assessment tools, which need to be further developed and validated. Machine learning (ML), as a method for designing risk assessment models that help to efficiently explore and mine useful information, has been widely used in recent years to solve a variety of challenging medical problems. Likewise, the application of ML in CRT risk diagnosis may contribute to a more precise assessment. In clinical practice, it is necessary to take appropriate stratified preventive measures according to the level of CRT risk assessment of children, to improve the efficiency of clinical work, reduce the burden of clinical work, and minimize the occurrence of CRT under the premise of ensuring the safety of children.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Yeow, M. et al. A systematic review and network meta-analysis of randomized controlled trials on choice of central venous access device for delivery of chemotherapy. J. Vasc. Surg. Venous Lymphat. Disord. 10 , 1184–91.e8 (2022).
Article PubMed Google Scholar
Cellini, M. et al. Guidelines of the Italian Association of Pediatric Hematology and Oncology for the management of the central venous access devices in pediatric patients with onco-hematological disease. J. Vasc. Access 23 , 3–17 (2022).
Ares, G. & Hunter, C. J. Central venous access in children: indications, devices, and risks. Curr. Opin. Pediatr. 29 , 340–346 (2017).
Jaffray, J. et al. Peripherally inserted central catheters lead to a high risk of venous thromboembolism in children. Blood 135 , 220–226 (2020).
Article CAS PubMed Google Scholar
Zhang, J. J. et al. Factors affecting mechanical complications of central venous access devices in children. Pediatr. Surg. Int. 38 , 1067–1073 (2022).
Article PubMed PubMed Central Google Scholar
Akhtar, N. & Lee, L. Utilization and Complications of Central Venous Access Devices in Oncology Patients. Curr. Oncol. 28 , 367–377 (2021).
Ullman, A. J., Marsh, N., Mihala, G., Cooke, M. & Rickard, C. M. Complications of Central Venous Access Devices: A Systematic Review. Pediatrics 136 , e1331–e1344 (2015).
Östlund, Å. et al. Erratum to ‘Incidence of and risk factors for venous thrombosis in children with percutaneous non-tunnelled central venous catheters’ (Br J Anaesth 2019; 123: 316-24). Br. J. Anaesth. 123 , 918 (2019).
McLaughlin, C. M. et al. Symptomatic catheter-associated thrombosis in pediatric trauma patients: Choose your access wisely. Surgery 166 , 1117–1121 (2019).
Citla Sridhar, D., Abou-Ismail, M. Y. & Ahuja, S. P. Central venous catheter-related thrombosis in children and adults. Thromb. Res. 187 , 103–112 (2020).
Zhou, X. et al. A retrospective analysis of risk factors associated with catheter-related thrombosis: a single-center study. Perfusion 35 , 806–813 (2020).
Li, S. et al. Risk factors for central venous catheter-related thrombosis in hospitalized children: a single-center a retrospective cohort study. Transl. Pediatr. 11 , 1840–1851 (2022).
Patel, N., Petersen, T. L., Simpson, P. M., Feng, M. & Hanson, S. J. Rates of Venous Thromboembolism and Central Line-Associated Bloodstream Infections Among Types of Central Venous Access Devices in Critically Ill Children. Crit. Care Med. 48 , 1340–1348 (2020).
Timsit, J. F. et al. A state of the art review on optimal practices to prevent, recognize, and manage complications associated with intravascular devices in the critically ill. Intensive Care Med. 44 , 742–759 (2018).
Ullman, A. J. et al. Pediatric central venous access devices: practice, performance, and costs. Pediatr. Res. 92 , 1381–1390 (2022).
Hutton, B. et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern Med. 162 , 777–784 (2015).
Kim, S. Y. et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J. Clin. Epidemiol. 66 , 408–414 (2013).
Borenstein, M., Hedges, L. V., Higgins, J. P. & Rothstein, H. R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 1 , 97–111 (2010).
Beck, C., Dubois, J., Grignon, A., Lacroix, J. & David, M. Incidence and risk factors of catheter-related deep vein thrombosis in a pediatric intensive care unit: a prospective study. J. Pediatr. 133 , 237–241 (1998).
Dubois, J. et al. Incidence of deep vein thrombosis related to peripherally inserted central catheters in children and adolescents. Cmaj 177 , 1185–1190 (2007).
Faustino, E. V. et al. Incidence and acute complications of asymptomatic central venous catheter-related deep venous thrombosis in critically ill children. J. Pediatr. 162 , 387–391 (2013).
Jiang, W. et al. Construction and validation of a risk prediction model for central venous catheter-associated deep venous thromboses in children with congenital heart disease after surgery. Chin. J. Nurs. 57 , 2217–2224 (2022).
Google Scholar
Faustino, E. V. et al. Factor VIII May Predict Catheter-Related Thrombosis in Critically Ill Children: A Preliminary Study. Pediatr. Crit. Care Med. 16 , 497–504 (2015).
Jones, S., Butt, W., Monagle, P., Cain, T. & Newall, F. The natural history of asymptomatic central venous catheter-related thrombosis in critically ill children. Blood 133 , 857–866 (2019).
Kim, E. H. et al. Central venous catheter-related thrombosis in pediatric surgical patients: A prospective observational study. Paediatr. Anaesth. 32 , 563–571 (2022).
Male, C. et al. Central venous line-related thrombosis in children: association with central venous line location and insertion technique. Blood 101 , 4273–4278 (2003).
Male, C., Julian, J. A., Massicotte, P., Gent, M. & Mitchell, L. Significant association with location of central venous line placement and risk of venous thrombosis in children. Thromb. Haemost. 94 , 516–521 (2005).
Marquez, A., Shabanova, V. & Faustino, E. V. Prediction of Catheter-Associated Thrombosis in Critically Ill Children. Pediatr. Crit. Care Med. 17 , e521–e528 (2016).
Menéndez, J. J. et al. Incidence and risk factors of superficial and deep vein thrombosis associated with peripherally inserted central catheters in children. J. Thromb. Haemost. 14 , 2158–2168 (2016).
Rubio Longo, M. C. et al. Catheter-related deep vein thrombosis in newborn infants. Arch. Argent. Pediatr. 119 , 32–38 (2021).
PubMed Google Scholar
Östlund, Å. et al. Incidence of and risk factors for venous thrombosis in children with percutaneous non-tunnelled central venous catheters. Br. J. Anaesth. 123 , 316–324 (2019).
Sol, J. J. et al. Chronic Complications After Femoral Central Venous Catheter-related Thrombosis in Critically Ill Children. J. Pediatr. Hematol. Oncol. 37 , 462–467 (2015).
van Rooden, C. J. et al. Infectious complications of central venous catheters increase the risk of catheter-related thrombosis in hematology patients: a prospective study. J. Clin. Oncol. 23 , 2655–2660 (2005).
Zeng, X., Zhang, C. & Shi, Y. Analysis of risk factors for complicated catheter-related thrombosis in children. Chin. J. Emerg. Med. 29 , 719–723 (2020).
Wei, Y. et al. The incidence and risk factors of catheter-related-thrombosis during induction chemotherapy in acute lymphocytic leukemia children. Chin. J. Hematol. 38 , 313–317 (2017).
CAS Google Scholar
Deng, G. & Liao, Q. Analysis of risk factors for venous thrombosis after PICC placement in critically ill children. Int. I Nurs. 39 , 775–777 (2020).
Badheka, A. V. et al. Catheter related thrombosis in hospitalized infants: A neural network approach to predict risk factors. Thromb. Res. 200 , 34–40 (2021).
Chen, K. et al. Risk factors for central venous catheter-related thrombosis in children: a retrospective analysis. Blood Coagul. Fibrinol. 27 , 384–388 (2016).
Article Google Scholar
Chojnacka, K., Krasiński, Z., Wróblewska-Seniuk, K. & Mazela, J. Catheter-related venous thrombosis in NICU: A case-control retrospective study. J. Vasc. Access 23 , 88–93 (2022).
Derderian, S. C., Good, R., Vuille-Dit-Bille, R. N., Carpenter, T. & Bensard, D. D. Central venous lines in critically ill children: Thrombosis but not infection is site dependent. J. Pediatr. Surg. 54 , 1740–1743 (2019).
Diamond, C. E. et al. Catheter-Related Venous Thrombosis in Hospitalized Pediatric Patients with Inflammatory Bowel Disease: Incidence, Characteristics, and Role of Anticoagulant Thromboprophylaxis with Enoxaparin. J. Pediatr. 198 , 53–59 (2018).
Noailly Charny, P. A. et al. Increased Risk of Thrombosis Associated with Peripherally Inserted Central Catheters Compared with Conventional Central Venous Catheters in Children with Leukemia. J. Pediatr. 198 , 46–52 (2018).
Gnannt, R. et al. Increased risk of symptomatic upper-extremity venous thrombosis with multiple peripherally inserted central catheter insertions in pediatric patients. Pediatr. Radio. 48 , 1013–1020 (2018).
Gray, B. W. et al. Characterization of central venous catheter-associated deep venous thrombosis in infants. J. Pediatr. Surg. 47 , 1159–1166 (2012).
Haddad, H. et al. Routine surveillance ultrasound for the management of central venous catheters in neonates. J. Pediatr. 164 , 118–122 (2014).
Lambert, I., Tarima, S., Uhing, M. & Cohen, S. S. Risk Factors Linked to Central Catheter-Associated Thrombosis in Critically Ill Infants in the Neonatal Intensive Care Unit. Am. J. Perinatol. 36 , 291–295 (2019).
Li, H. et al. Prediction of central venous catheter-associated deep venous thrombosis in pediatric critical care settings. BMC Med. Inf. Decis. Mak. 21 , 332 (2021).
Article CAS Google Scholar
Lovett, M. E. et al. Catheter-associated deep vein thrombosis in children with severe traumatic brain injury: A single-center experience. Pediatr. Blood Cancer 70 , e30044 (2023).
MacLean, J. et al. Need for tissue plasminogen activator for central venous catheter dysfunction is significantly associated with thrombosis in pediatric cancer patients. Pediatr. Blood Cancer 65 , e27015 (2018).
Noonan, P. J., Hanson, S. J., Simpson, P. M., Dasgupta, M. & Petersen, T. L. Comparison of Complication Rates of Central Venous Catheters Versus Peripherally Inserted Central Venous Catheters in Pediatric Patients. Pediatr. Crit. Care Med. 19 , 1097–1105 (2018).
Pei, L. et al. Clinical characteristics and risk factors of symptomatic central venous catheter-related deep vein thrombosis in children. Chin. Pediatr. Emerg. Med. 23 , 450–454 (2016).
Onyeama, S. N. et al. Central Venous Catheter-associated Venous Thromboembolism in Children With Hematologic Malignancy. J. Pediatr. Hematol. Oncol. 40 , e519–e524 (2018).
Shah, S. H. et al. Clinical risk factors for central line-associated venous thrombosis in children. Front. Pediatr. 3 , 35 (2015).
Shin, H. S., Towbin, A. J., Zhang, B., Johnson, N. D. & Goldstein, S. L. Venous thrombosis and stenosis after peripherally inserted central catheter placement in children. Pediatr. Radio. 47 , 1670–1675 (2017).
Smitherman, A. B. et al. The incidence of catheter-associated venous thrombosis in noncritically ill children. Hosp. Pediatr. 5 , 59–66 (2015).
Steen, E. H. et al. Central Venous Catheter-Related Deep Vein Thrombosis in the Pediatric Cardiac Intensive Care Unit. J. Surg. Res 241 , 149–159 (2019).
Wang, J. & Ren, G. Peripherally inserted central catheter related venous thromboembolism in children with acute leukemia: a factorial analysis. Chin. J. Biomed. Eng. 27 , 288–293 (2021).
Dubbink-Verheij, G. H. et al. Femoral Vein Catheter is an Important Risk Factor for Catheter-related Thrombosis in (Near-)term Neonates. J. Pediatr. Hematol. Oncol. 40 , e64–e68 (2018).
Tran, M., Shein, S. L., Ji, X. & Ahuja, S. P. Identification of a “VTE-rich” population in pediatrics - Critically ill children with central venous catheters. Thromb. Res 161 , 73–77 (2018).
Wisecup, S., Eades, S. & Turiy, Y. Characterizing the Risk Factors Associated With Venous Thromboembolism in Pediatric Patients After Central Venous Line Placement. J. Pediatr. Pharm. Ther. 20 , 358–366 (2015).
Zhu, W., Zhang, H., Xing, Y. Clinical Characteristics of Venous Thrombosis Associated with Peripherally Inserted Central Venous Catheter in Premature Infants. Children 9 , https://doi.org/10.3390/children9081126 (2022).
Ekdahl, K. N. et al. Innate immunity activation on biomaterial surfaces: a mechanistic model and coping strategies. Adv. Drug Deliv. Rev. 63 , 1042–1050 (2011).
Article CAS PubMed PubMed Central Google Scholar
Takemoto, C. M. et al. Hospital-associated venous thromboembolism in children: incidence and clinical characteristics. J. Pediatr. 164 , 332–338 (2014).
Boulet, S. L. et al. Trends in venous thromboembolism-related hospitalizations, 1994-2009. Pediatrics 130 , e812–e820 (2012).
Raffini, L., Huang, Y. S., Witmer, C. & Feudtner, C. Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics 124 , 1001–1008 (2009).
Lin, S., Zhu, N., YihanZhang, Du, L. & Zhang, S. Development and validation of a prediction model of catheter-related thrombosis in patients with cancer undergoing chemotherapy based on ultrasonography results and clinical information. J. Thromb. Thrombolysis 54 , 480–491 (2022).
Johnson, E. D., Schell, J. C. & Rodgers, G. M. The D-dimer assay. Am. J. Hematol. 94 , 833–839 (2019).
Favresse, J. et al. D-dimer: Preanalytical, analytical, postanalytical variables, and clinical applications. Crit. Rev. Clin. Lab Sci. 55 , 548–577 (2018).
Weitz, J. I., Fredenburgh, J. C. & Eikelboom, J. W. A Test in Context: D-Dimer. J. Am. Coll. Cardiol. 70 , 2411–2420 (2017).
Darlow, J. & Mould, H. Thrombophilia testing in the era of direct oral anticoagulants. Clin. Med. 21 , e487–e491 (2021).
Monagle, P. et al. American Society of Hematology 2018 Guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv. 2 , 3292–3316 (2018).
Meziani, F., Gando, S. & Vincent, J. L. Should all patients with sepsis receive anticoagulation? Yes. Intensive Care Med 43 , 452–454 (2017).
Saber, W. et al. Risk factors for catheter-related thrombosis (CRT) in cancer patients: a patient-level data (IPD) meta-analysis of clinical trials and prospective studies. J. Thromb. Haemost. 9 , 312–319 (2011).
Journeycake, J. M. & Buchanan, G. R. Thrombotic complications of central venous catheters in children. Curr. Opin. Hematol. 10 , 369–374 (2003).
Lamperti, M. et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med 38 , 1105–1117 (2012).
Journeycake, J. M. & Buchanan, G. R. Catheter-related deep venous thrombosis and other catheter complications in children with cancer. J. Clin. Oncol. 24 , 4575–4580 (2006).
Deitcher, S. R., Gajjar, A., Kun, L. & Heideman, R. L. Clinically evident venous thromboembolic events in children with brain tumors. J. Pediatr. 145 , 848–850 (2004).
Mandel-Shorer, N., Tzvi-Behr, S., Harvey, E. & Revel-Vilk, S. Central venous catheter-related venous thrombosis in children with end-stage renal disease undergoing hemodialysis. Thromb. Res 172 , 150–157 (2018).
Chen, D., Långström, S., Petäjä, J., Heikinheimo, M. & Pihkala, J. Thrombin formation and effect of unfractionated heparin during pediatric cardiac catheterization. Catheter Cardiovasc Inter. 81 , 1174–1179 (2013).
Reynolds, P. M. et al. Evaluation of Prophylactic Heparin Dosage Strategies and Risk Factors for Venous Thromboembolism in the Critically Ill Patient. Pharmacotherapy 39 , 232–241 (2019).
Diamanti, A. et al. Prevalence of life-threatening complications in pediatric patients affected by intestinal failure. Transpl. Proc. 39 , 1632–1633 (2007).
Download references
This study was supported by the Fundamental Research Funds for the Central Universities [grant numbers YCJJ20230244] and Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology Research Fund [grant numbers 2022C09].
Author information
Authors and affiliations.
Department of Nursing, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Maoling Fu, Qiaoyue Yang, Yaqi Yu, Wenshuai Song, Xiuli Qin, Ying Luo, Xiaoju Xiong & Genzhen Yu
School of Nursing, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Maoling Fu, Quan Yuan, Qiaoyue Yang, Yaqi Yu & Wenshuai Song
You can also search for this author in PubMed Google Scholar
Contributions
GY and YL framed the review questions on the basis of input from MF and QY. YY and XQ conducted the literature search. MF, WS, and QY screened and evaluated the identified papers. GY and YY performed data extraction and analysis. MF, WS, XQ and QY prepared the initial manuscript with revisions and comments from GY, YL, and XX. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Corresponding author
Correspondence to Genzhen Yu .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary checklist, supplemental digital tables1, supplemental digital tables2, supplemental digital, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Fu, M., Yuan, Q., Yang, Q. et al. Risk factors and incidence of central venous access device-related thrombosis in hospitalized children: a systematic review and meta-analysis. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03225-0
Download citation
Received : 06 October 2023
Revised : 18 March 2024
Accepted : 25 March 2024
Published : 17 May 2024
DOI : https://doi.org/10.1038/s41390-024-03225-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

The copy number variant architecture of psychopathology and cognitive development in the ABCD study
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- ORCID record for Zhiqiang Sha
- For correspondence: [email protected]
- ORCID record for Ran Barzilay
- Info/History
- Preview PDF
Importance: Childhood is a crucial developmental phase for mental health and cognitive function, both of which are commonly affected in patients with psychiatric disorders. This neurodevelopmental trajectory is shaped by a complex interplay of genetic and environmental factors. While common genetic variants account for a large proportion of inherited genetic risk, rare genetic variations, particularly copy number variants (CNVs), play a significant role in the genetic architecture of neurodevelopmental disorders. Despite their importance, the relevance of CNVs to child psychopathology and cognitive function in the general population remains underexplored. Objective: Investigating CNV associations with dimensions of child psychopathology and cognitive functions. Design, Setting, and Participants: ABCD study focuses on a cohort of over 11,875 youth aged 9 to 10, recruited from 21 sites in the US, aiming to investigate the role of various factors, including brain, environment, and genetic factors, in the etiology of mental and physical health from middle childhood through early adulthood. Data analysis occurred from April 2023 to April 2024. Main Outcomes and Measures: In this study, we utilized PennCNV and QuantiSNP algorithms to identify duplications and deletions larger than 50Kb across a cohort of 11,088 individuals from the Adolescent Brain Cognitive Development study. CNVs meeting quality control standards were subjected to a genome-wide association scan to identify regions associated with quantitative measures of broad psychiatric symptom domains and cognitive outcomes. Additionally, a CNV risk score, reflecting the aggregated burden of genetic intolerance to inactivation and dosage sensitivity, was calculated to assess its impact on variability in overall and dimensional child psychiatric and cognitive phenotypes. Results: In a final sample of 8,564 individuals (mean age=9.9 years, 4,532 males) passing quality control, we identified 4,111 individuals carrying 5,760 autosomal CNVs. Our results revealed significant associations between specific CNVs and our phenotypes of interest, psychopathology and cognitive function. For instance, a duplication at 10q26.3 was associated with overall psychopathology, and somatic complaints in particular. Additionally, deletions at 1q12.1, along with duplications at 14q11.2 and 10q26.3, were linked to overall cognitive function, with particular contributions from fluid intelligence (14q11.2), working memory (10q26.3), and reading ability (14q11.2). Moreover, individuals carrying CNVs previously associated with neurodevelopmental disorders exhibited greater impairment in social functioning and cognitive performance across multiple domains, in particular working memory. Notably, a higher deletion CNV risk score was significantly correlated with increased overall psychopathology (especially in dimensions of social functioning, thought disorder, and attention) as well as cognitive impairment across various domains. Conclusions and Relevance: In summary, our findings shed light on the contributions of CNVs to interindividual variability in complex traits related to neurocognitive development and child psychopathology.
Competing Interest Statement
AFA-B receives consulting income from Octave Bioscience. AFA-B and JS hold equity in and serve on the board of Centile Bioscience.
Funding Statement
The research was funded by R01MH132934 and R01MH133843.
Author Declarations
I confirm all relevant ethical guidelines have been followed, and any necessary IRB and/or ethics committee approvals have been obtained.
The details of the IRB/oversight body that provided approval or exemption for the research described are given below:
The study used ONLY openly available human data that were originally located at https://abcdstudy.org/.
I confirm that all necessary patient/participant consent has been obtained and the appropriate institutional forms have been archived, and that any patient/participant/sample identifiers included were not known to anyone (e.g., hospital staff, patients or participants themselves) outside the research group so cannot be used to identify individuals.
I understand that all clinical trials and any other prospective interventional studies must be registered with an ICMJE-approved registry, such as ClinicalTrials.gov. I confirm that any such study reported in the manuscript has been registered and the trial registration ID is provided (note: if posting a prospective study registered retrospectively, please provide a statement in the trial ID field explaining why the study was not registered in advance).
I have followed all appropriate research reporting guidelines, such as any relevant EQUATOR Network research reporting checklist(s) and other pertinent material, if applicable.
Data Availability
All data produced are available online at https://abcdstudy.org/
https://nda.nih.gov/study.html?id=2589
View the discussion thread.
Thank you for your interest in spreading the word about medRxiv.
NOTE: Your email address is requested solely to identify you as the sender of this article.

Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager
- Tweet Widget
- Facebook Like
- Google Plus One
- Addiction Medicine (324)
- Allergy and Immunology (627)
- Anesthesia (163)
- Cardiovascular Medicine (2373)
- Dentistry and Oral Medicine (289)
- Dermatology (206)
- Emergency Medicine (379)
- Endocrinology (including Diabetes Mellitus and Metabolic Disease) (836)
- Epidemiology (11770)
- Forensic Medicine (10)
- Gastroenterology (702)
- Genetic and Genomic Medicine (3738)
- Geriatric Medicine (350)
- Health Economics (633)
- Health Informatics (2395)
- Health Policy (933)
- Health Systems and Quality Improvement (896)
- Hematology (341)
- HIV/AIDS (782)
- Infectious Diseases (except HIV/AIDS) (13310)
- Intensive Care and Critical Care Medicine (767)
- Medical Education (365)
- Medical Ethics (104)
- Nephrology (398)
- Neurology (3502)
- Nursing (198)
- Nutrition (525)
- Obstetrics and Gynecology (674)
- Occupational and Environmental Health (664)
- Oncology (1823)
- Ophthalmology (537)
- Orthopedics (219)
- Otolaryngology (287)
- Pain Medicine (232)
- Palliative Medicine (66)
- Pathology (446)
- Pediatrics (1033)
- Pharmacology and Therapeutics (426)
- Primary Care Research (420)
- Psychiatry and Clinical Psychology (3175)
- Public and Global Health (6139)
- Radiology and Imaging (1280)
- Rehabilitation Medicine and Physical Therapy (747)
- Respiratory Medicine (826)
- Rheumatology (379)
- Sexual and Reproductive Health (372)
- Sports Medicine (323)
- Surgery (402)
- Toxicology (50)
- Transplantation (172)
- Urology (145)

IMAGES
VIDEO
COMMENTS
Quantitative research example If you want to test the effectiveness of an online teaching method, a quantitative approach is most suitable. You can use this type of research to measure learning outcomes like grades and test scores. ... Type of design Purpose and characteristics; Case study: Detailed study of a specific subject (e.g., a place ...
Example: Quantitative research If you want to test the effectiveness of an online teaching method, a quantitative approach is most suitable. You can use this type of research to measure learning outcomes like grades and test scores. ... Type of design Purpose and characteristics; Case study: Detailed study of a specific subject (e.g., a place ...
Research design refers to the overall plan, structure or strategy that guides a research project, from its conception to the final analysis of data. Research designs for quantitative studies include descriptive, correlational, experimental and quasi-experimenta l designs. Research designs for qualitative studies include phenomenological ...
Consequently, these objectives determine the study design and research outcome. The development of research questions is a process based on knowledge of current trends, cutting-edge studies, and technological advances in the research field. ... Definitions and examples of quantitative research hypotheses. Quantitative research hypotheses ...
The study design used to answer a particular research question depends on the nature of the question and the availability of resources. In this article, which is the first part of a series on "study designs," we provide an overview of research study designs and their classification. The subsequent articles will focus on individual designs.
Large Sample Size: Quantitative research typically involves collecting data from a large sample size to increase statistical power and generalizability of the findings to a larger population. ... Case Study Design: Case studies involve in-depth examination of a specific individual, group, or phenomenon. They provide detailed and rich ...
We explain what research desig... Learn how to get started with research design for quantitative studies, including dissertations, theses and research projects.
In clinical research, a study design is a plan for selecting study subjects and for obtaining data. These study designs fall into two different categories: Experimental. Observational. These categories are based on whether or not the investigators assign a particular exposure to a cohort.
Quantitative . Research Methods. T. his chapter focuses on research designs commonly used when conducting . quantitative research studies. The general purpose of quantitative research is to investigate a particular topic or activity through the measurement of variables in quantifiable terms. Quantitative approaches to conducting educational ...
Quantitative research design is defined as a research method used in various disciplines, including social sciences, psychology, economics, and market research. It aims to collect and analyze numerical data to answer research questions and test hypotheses. Quantitative research design offers several advantages, including the ability to ...
Ranganathan P. Understanding Research Study Designs. Indian J Crit Care Med 2019;23 (Suppl 4):S305-S307. Keywords: Clinical trials as topic, Observational studies as topic, Research designs. We use a variety of research study designs in biomedical research. In this article, the main features of each of these designs are summarized. Go to:
Quantitative Research Excellence: Study Design and Reliable and Valid Measurement of Variables
Quantitative research methods. You can use quantitative research methods for descriptive, correlational or experimental research. In descriptive research, you simply seek an overall summary of your study variables.; In correlational research, you investigate relationships between your study variables.; In experimental research, you systematically examine whether there is a cause-and-effect ...
Quantitative research methods are concerned with the planning, design, and implementation of strategies to collect and analyze data. Descartes, the seventeenth-century philosopher, suggested that how the results are achieved is often more important than the results themselves, as the journey taken along the research path is a journey of discovery. . High-quality quantitative research is ...
Mixed-methods research is a flexible approach, where the research design is determined by what we want to find out rather than by any predetermined epistemological position. In mixed-methods research, qualitative or quantitative components can predominate, or both can have equal status. 1.4. Units and variables.
tive studies provoke the "why" questions of analytic (cause-and-effect) research. Two additional categories are epidemiologic and predictive correlational designs. When you read about designs in this chapter, examples of studies are given to illus-trate the design. The examples include some discussion of the results of statistical tests,
The study adopted a cross-sectional research design and quantitative research approach using a sample of 300 respondents from the six public hospitals in Tanzania.
For example, studying gender difference in learning mathematics is a comparative research. The correlational design is a study of relationships between two or more constructs. A positive correlation means that high values of a variable are associated with high values of another variable.
Experimental Quantitative Research Design. Experimental quantitative research design utilizes the scientific approach. It establishes procedures that allow the researcher to test a hypothesis and to systematically and scientifically study causal relationships among variables. All experimental quantitative research studies include three basic steps:
Here are some key characteristics of quantitative research: Numerical data: Quantitative research involves collecting numerical data through standardized methods such as surveys, experiments, and observational studies. This data is analyzed using statistical methods to identify patterns and relationships.
Quantitative research methods. You can use quantitative research methods for descriptive, correlational or experimental research. In descriptive research, you simply seek an overall summary of your study variables.; In correlational research, you investigate relationships between your study variables.; In experimental research, you systematically examine whether there is a cause-and-effect ...
Design/methodology/approach. The study used quantitative and descriptive research designs to test the proposed hypotheses. An online survey was used to collect the data from a study sample. A sample of 411 respondents was received, with structural equation modelling (SEM) being used to assess the model fit.
The risk factors for central venous access device-related thrombosis (CRT) in children are not fully understood. We used evidence-based medicine to find the risk factors for CRT by pooling current ...
Importance: Childhood is a crucial developmental phase for mental health and cognitive function, both of which are commonly affected in patients with psychiatric disorders. This neurodevelopmental trajectory is shaped by a complex interplay of genetic and environmental factors. While common genetic variants account for a large proportion of inherited genetic risk, rare genetic variations ...