
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

OHRP Guidance on Elimination of IRB Review of Research Applications and Proposals
NOTE : This guidance is consistent with the 2018 Requirements (i.e., the revised Common Rule).
Elimination of Institutional Review Board (IRB) Review of Research Applications and Proposals: 2018 Requirements
This guidance represents the Office for Human Research Protections’ (OHRP’s) current thinking on this topic. This guidance does not create or confer any rights for or on any person and does not operate to bind OHRP or the public.
OHRP guidance should be viewed as recommendations unless specific regulatory requirements are cited. The use of the word “must” in OHRP guidance means that something is required under the Department of Health and Human Services (HHS) regulations at 45 CFR part 46. The use of the word “should” in OHRP guidance means that something is recommended or suggested, but not required. An institution may use an alternative approach if the approach satisfies the requirements of 45 CFR part 46. OHRP is available to discuss alternative approaches by telephone at 240-453-6900 or 866-447-4777, or by email at [email protected] .
Date: July 20, 2020
Scope: This guidance document applies to nonexempt research involving human subjects that is conducted or supported by HHS. It provides guidance on the elimination of the requirement in section 46.103(f) of the pre-2018 Requirements that each application or proposal for research undergo IRB review and approval as part of the certification process. This guidance also addresses the requirement in the 2018 Requirements for certification of each proposed research study prior to initiation.
Target Audience : Institutions, IRBs, investigators, HHS funding agencies, and others that may be responsible for the review, conduct, or oversight of nonexempt research involving human subjects conducted or supported by HHS.
Regulatory Background
In this document, the term “pre-2018 Requirements” refers to subpart A of 45 CFR part 46 (i.e., the Common Rule) as published in the 2016 edition of the Code of Federal Regulations. The pre-2018 Requirements were originally promulgated in 1991, and subsequently amended in 2005. The pre-2018 Requirements may also be referred to as the “pre-2018 Common Rule.”
The term “2018 Requirements” refers to the Common Rule as published in the July 19, 2018 edition of the e-Code of Federal Regulations. The 2018 Requirements were originally published on January 19, 2017 and further amended on January 22, 2018 and June 19, 2018. The 2018 Requirements may also be referred to as the “revised Common Rule.”
Any study initiated [1] on or after January 21, 2019 is required to comply with the 2018 Requirements. Any study initiated before January 21, 2019 is required to comply with the pre-2018 Common Rule, unless an institution voluntarily instead elected to transition such studies to comply with the 2018 Requirements. That election to transition a study must be documented and dated by the institution or an IRB. (45 CFR 46.101( l) More information about implementing the revised Common Rule is available on the OHRP website.
The 2018 Requirements include several provisions pertinent to certification, including the following:
“Certification means the official notification by the institution to the supporting Federal department or agency component, in accordance with the requirements of this policy, that a research project or activity involving human subjects has been reviewed and approved by an IRB in accordance with an approved assurance.” (45 CFR 46.102(a))
Note: The Federalwide Assurance (FWA) is the only type of assurance that OHRP approves.
“Certification is required when the research is supported by a federal department or agency and not otherwise waived under 45 CFR 46.101(i) or exempted under 45 CFR 46.104. For such research, institutions shall certify that each proposed research study covered by the assurance and [45 CFR 46.103] has been reviewed and approved by the IRB. Such certification must be submitted as prescribed by the federal department or agency component supporting the research. Under no condition shall research covered by [45 CFR 46.103] be initiated prior to receipt of the certification that the research has been reviewed and approved by the IRB.” (45 CFR 46.103(d)).
Pre-2018 Requirements:
The pre-2018 Requirements at 45 CFR 46.103(f) require an institution with an approved assurance to certify to HHS that each application or proposal covered by an OHRP-approved assurance and by 45 CFR 46.103 has been reviewed and approved by the IRB: that is, the research grant application and/or proposal submitted to an HHS component. Such certifications must be submitted with the application or proposal or by such later date as may be prescribed by the department or agency to which the application or proposal is submitted (45 CFR 46.103(f) of the pre-2018 Requirements).
2018 Requirements:
The 2018 Requirements eliminate the requirement in the pre-2018 Requirements that grant applications or proposals for research undergo IRB review and approval for the purpose of certification. Experience suggests that review and approval of the application or proposal is not a productive use of IRB time. Elimination of that requirement is not expected to reduce protections for human subjects because the research study (e.g. a research protocol) would remain subject to the requirement for IRB review and approval, assuming that an HHS component funds the research.
The 2018 Requirements at 45 CFR 46.103(d) require certification when the research is supported by HHS, and applicability of the regulations is not otherwise waived under 45 CFR 46.101(i) or the study is not exempted under 45 CFR 46.104. For such research, institutions must certify that each proposed research study covered by an OHRP-approved assurance and by 45 CFR 46.103 has been reviewed and approved by an IRB. Such certification must be submitted as prescribed by the federal department or agency component supporting the research. Under no condition shall research covered by 45 CFR 46.103 be initiated prior to receipt by HHS of the certification that the research has been reviewed and approved by the IRB.
Thus, for research to which the 2018 Requirements apply, the IRB must review and approve such research (e.g., a research protocol) for certification; however, the IRB no longer is required to review and approve the research grant application or proposal under the 2018 Requirements.
If you have specific questions about how to apply this guidance, please contact OHRP by phone at (866) 447-4777 (toll-free within the United States) or (240) 453-6900, or by e-mail at [email protected] .
[1] OHRP interprets “initiated” to mean research (1) initially approved by an IRB, (2) for which IRB review is waived, or (3) determined to be exempt on or after January 21, 2019 consistent with 45 CFR 46.101( l ).
- Whitepapers
- Ethics in Clinical Research
- FDA & ICH
- Regulatory Compliance
Drafting a Research Plan for IRB Review and Research Conduct: Information That Must Be Included in a Clinical Trial Protocol

A clinical trial is an interventional clinical study, in which participants are recruited for the administration of a specific intervention (use of a study drug, a procedure with a medical device, an educational intervention, etc.). Planning for a clinical research trial includes the development of a clinical protocol document. There are several reasons to have a clear and well-written protocol:
- So that the research plan is clearly defined to everyone who will participate in conducting the study, and all processes and procedures are clearly and completely described to avoid variation in procedures and the introduction of assessment bias into the study conduct
- So that oversight committees and agencies (Institutional Review Boards (IRBs)/Research Ethics Committees, scientific review committees, radiation safety or other committees, and regulatory agencies such as the Food and Drug Administration (FDA)) can review the research proposal in enough detail to ensure that it meets regulatory requirements to grant approval for the research conduct
- So that the endpoints, study design, data collection parameters, and the data analysis plan are prospectively defined prior to the research being conducted, and bias cannot be introduced by changing the design or analysis plan during the study (for this reason, many journals now require public posting of the full research protocol along with the publication of study results).
There are many ways to outline and describe the necessary components of the research protocol and plan, and there are many protocol templates that are available to serve as guidelines for protocol development. In this paper, we are addressing these requirements from the perspective of the IRB. Federal regulations dictate specific criteria against which the IRB must review all protocol submissions , to make an independent determination regarding whether the prospective research plan can be approved. If the protocol (or other documents such as the IRB submission form and consent documents that accompany the protocol in the submission) does not provide adequate or complete information for the IRB to make a determination that all criteria have been met, the IRB cannot approve the research plan. This document is intended to assist investigators and research teams by providing a list of the necessary information that they can use as a writing guide, or use as a checklist against the protocol and submission document package, to ensure that all the necessary information is included prior to submitting the protocol and plan for review by an IRB.
TABLE 1 lists the criteria for the approval of research (paraphrased here from the full regulatory language in 21 CFR 56.111 ) by an IRB, and describes the information that is necessary for the clinical protocol (or elsewhere in the research plan submitted for review), for the IRB to be able to make determinations regarding whether a research plan can be approved.
TABLE 2 describes additional components or documents that may need to be included, based on specifics of the study product or the study plan.
In addition, all protocol documents should:
- Be final documents. Draft protocols or other study documents should not be submitted for IRB review as the IRB must review final and complete study plans. If edits or changes are made after IRB review, the amended protocol must be resubmitted for approval of the changes.
- Include page numbers and/or section or outline numbering so that the location of content can be identified
- Include a table of contents
- Include some kind of version control notation (either a version number or version date) so that revisions of the documents can be clearly tracked.
TABLE 1: Necessary Information for All Protocol Submissions
Criterion for approval of the research proposal:.
That risks to participants are minimized by using procedures that are consistent with sound research design and that do not unnecessarily expose subjects to risk, and whenever appropriate, by using procedures already being performed on the subjects for diagnostic or treatment purposes.
Corresponding Information required in the Protocol/Research Plan:
- A clear and detailed list of all study procedures, including screening and follow-up procedures (often in tabular format, describing the procedures to be conducted at each study visit). “Procedures” includes (but is not limited to) study visits; interviews for the collection of safety or other data; vital sign measurements; surveys, questionnaires, or diary completion; study drug administration or other investigational study intervention; invasive and non-invasive procedures including blood draws, radiologic tests or other diagnostic testing.
- The protocol should very clearly define what visits, assessments and procedures would be occurring as part of standard care
- If participants need to leave the study early or suddenly, the protocol should include any specific testing or follow-up that may need to occur for safety purposes.
That risks to subjects are reasonable in relation to anticipated benefits, if any, to subjects, and the importance of the knowledge that may reasonably be expected to result.
- the scientific knowledge expected to result from the research project
- the potential direct benefits to individual research participants in the study
- The protocol should also contain a clear and complete description of the study drugs/study intervention including (as appropriate) dosing information, storage information, and criteria for dose modification or dose adjustments.
That the selection of subjects is equitable.
- Eligibility criteria should be designed to make the study as inclusive as possible while excluding persons with factors or conditions that unacceptably increase the potential risks, or unreasonably confound the measurement of study endpoints
- Consider demographics, medical history and co-morbidities, concomitant medications, and whether the protocol must include testing for the presence or absence of conditions specified in the criteria
- It is not necessary to specify opposite factors in both lists, i.e., if inclusion criterion is age ≥ 18, the exclusion does not need to specify age < 18.
Informed consent will be sought from each prospective subject or the subject’s legally authorized representative.
Informed consent will be appropriately documented or appropriately waived.
- While the protocol submission must include a written informed consent document, it should also describe the process of obtaining informed consent from participants; how they will be identified and contacted, who will approach them about the study, and how they will be given adequate opportunity to ask questions during the consent discussion
- The written informed consent document must provide all of the elements required by federal regulations
- If potential participants may be incapable of providing informed consent and consent must be provided by legally authorized representatives, the consent process must describe this and the consent form must have appropriate signature spaces
- While there are circumstances in which the regulations allow either a waiver of documentation of consent (no signature) or a waiver of obtaining consent for standard or for emergency research , if a waiver is requested the submission should describe how the proposed research meets the specific requirements for these waivers, and how participants’ rights and privacy will be protected if the waiver is granted.
When appropriate, the research plan makes adequate provisions for monitoring the data collected to ensure the safety of subjects.
- The protocol should include an adequate description of the safety and efficacy data points that will be collected, including how adverse events will be collected, recorded, graded, and assessed
- How will safety data be monitored on an ongoing basis to identify any unanticipated safety issues during the study? Who will review the data, and will the study include a Data Safety Monitoring Committee, Data Monitoring Committee, or Adjudication Committee?
When appropriate, there are adequate provisions to protect the privacy of subjects and to maintain the confidentiality of data.
- The protocol or associated documents should describe how the privacy of participants will be protected (including if appropriate how people will be approached about the study and given the opportunity to ask questions in a private setting), as well as how the data being collected will be anonymized or de-identified, stored, transferred and analyzed to maintain confidentiality.
When some or all of the subjects are likely to be vulnerable to coercion or undue influence, such as children, prisoners, individuals with impaired decision making capacity, or economically or educationally disadvantaged persons, additional safeguards have been included in the study to protect the rights and welfare of these subjects.
- Note that federal regulations have specific sections for additional precautions required in the research when research is conducted on children , pregnant persons , and prisoners . Inclusion or exclusion of these groups should be specified in the eligibility criteria.
- Consideration should also be given to whether the protocol should include specific steps for the protection of participants who may be unable to make consent decisions (temporarily or permanently).
TABLE 2: Additional Components to Include when Appropriate
Circumstance or condition:.
If the protocol is evaluating the safety and/or efficacy of a drug, biologic or medical device (even if the drug, biologic or medical device is already FDA approved).
Additional information or documents that must be provided:
The regulatory status of the drug/device must be provided to the IRB, either via
- a description in the protocol,
- the submission form, or
- a copy of a letter from the FDA.
For drugs or biologics , the documentation must include an (Investigational New Drug (IND) number or a regulatory-based rationale for why the product is considered IND-exempt (21 CFR 312.2).
For medical devices , the documentation must specify whether the device is used ‘on label’ (for exactly the same indication and circumstances for which is has already been FDA-cleared or approved) or is investigational. If the device is investigational, the documentation must include an Investigational Device Exemption (IDE), an NSR justification, or a regulatory-based rationale for why the device is considered IDE-exempt.
When the protocol is evaluating interventions for foods , herbal products , dietary and other supplements, vitamins , and cosmetics , a regulatory-based justification for why that agent is not considered a drug or biologic as used in the research must be provided. Remember that foods that are being researched for possible use as drug products (looking for evidence of action in diagnosing, treating or mitigating a disease or condition) are regulated as drugs.
The IRB must ensure that research is being conducted in compliance with FDA regulations, so submitting appropriate documents or explanations may prevent questions during the review process. If the IRB cannot confirm documentation, or if the IRB is not completely sure that the FDA would agree with the justification for why certain research would not need FDA oversight (i.e., would be IND- or IDE- exempt), the IRB must ask the researcher/sponsor to consult with FDA and obtain documentation that FDA agrees that their oversight is not indicated.
Some or all of the subjects are likely to be vulnerable to coercion or undue influence, such as children, prisoners, pregnant persons, individuals with impaired decision-making capacity, or economically or educationally disadvantaged persons.
When persons from vulnerable populations may be enrolled in the research the research plan must always specify this and consideration should also be given to whether the protocol should include specific steps for the protection of participants who may be unable to make consent decisions (temporarily or permanently).
Note that federal regulations have specific sections for additional precautions required in the research when research is conducted on children , pregnant persons , and prisoners .
The study includes the use of surveys, questionnaires or other instruments (quality of life assessments, etc.).
If the protocol includes the use of surveys, all survey questions must be submitted as part of the protocol, an attachment to the protocol, or a supplemental document. If standardized questionnaires or instruments are being used, these must also be submitted. The IRB is required to review all participant-facing materials, including participant diaries and participant recruitment materials [note: some IRBs, including WCG IRB, do not require submission of common, standard instruments such as the SF-36, but if there is any doubt about whether submission is required it is better to add it and avoid possible delays].
Study participants must provide written informed consent.
The informed consent document must be provided for review and must have all the elements required by regulations .
A waiver of informed consent documentation is being requested.
In addition to the regulatory-based justification for the waiver (see Table 1), a Participant Information Sheet must be included in the submission.
A waiver or alteration of HIPAA requirements is being requested.
A regulatory-based justification must be provided specifying how the research is eligible for the waiver or alteration.
For researchers new at conducting clinical research or writing protocols, developing a new clinical trial protocol that is complete and sufficient for the purposes for which it will be needed is often a larger and more time-consuming project that was initially expected. While IRBs are generally happy to provide information and to answer specific regulatory questions in advance of a protocol being submitted, most do not have the resources or capacity to provide one-on-one guidance to researchers who have protocols that need major revisions to meet the criteria for approval.
Researchers may find it best to work with an experienced medical writer or clinical research consultant; while the cost of these services can be significant, this may be the most cost-effective plan when balanced against the time saved by the researcher and the facilitation of the review and approval process when guided by someone who is familiar with clinical research regulations.
Learn more about our ethical and scientific review solutions
Don't trust your study to just anyone.
WCG's IRB experts are standing by to handle your study with the utmost urgency and care. Contact us today to find out the WCG difference!
MAGI@home 2024
MAGI@home brings you and your team best-in-class, accredited training and education from the comfort of your home or office. Event Details
As the nation’s largest public research university, the Office of the Vice President for Research (OVPR) aims to catalyze, support and safeguard U-M research and scholarship activity.
The Office of the Vice President for Research oversees a variety of interdisciplinary units that collaborate with faculty, staff, students and external partners to catalyze, support and safeguard research and scholarship activity.
ORSP manages pre-award and some post-award research activity for U-M. We review contracts for sponsored projects applying regulatory, statutory and organizational knowledge to balance the university's mission, the sponsor's objectives, and the investigator's intellectual pursuits.
Ethics and compliance in research covers a broad range of activity from general guidelines about conducting research responsibly to specific regulations governing a type of research (e.g., human subjects research, export controls, conflict of interest).
eResearch is U-M's site for electronic research administration. Access: Regulatory Management (for IRB or IBC rDNA applications); Proposal Management (eRPM) for the e-routing, approval, and submission of proposals (PAFs) and Unfunded Agreements (UFAs) to external entities); and Animal Management (for IACUC protocols and ULAM).
Sponsored Programs manages the post-award financial activities of U-M's research enterprise and other sponsored activities to ensure compliance with applicable federal, state, and local laws as well as sponsor regulations. The Office of Contract Administration (OCA) is also part of the Office of Finance - Sponsored Programs.
Ethics & Compliance
- eResearch IRB NextGen Project
- Class Assignments & IRB Approval
- Operations Manual (OM)
- Authorization Agreement Process
- ORCR Policies and Procedures
- Self-Assessment Tools
- Resources and Web Links
- Single IRB-of-Record (sIRB) Process
- Certificate of Confidentiality Process
- HRPP Education Resources
- How to Register a Clinical Trial
- Maintaining and Updating ClinicalTrial.gov Records
- How to Report Clinical Trial Results
- Research Study Participation - FAQ
- International Research
- Coordinated Services & Practices (CSP)
- Collaborative Research: IRB-HSBS sIRB Process
- Data Security Guidelines
- Research Incentive Guidelines
- Routine fMRI Study Guidelines
- IRB-HSBS Website Directory and Guidance
- Waivers of Informed Consent Guidelines
IRB Review Process
- IRB Amendment Process
- Continuing Review Process
- Incident Reporting (AE/ORIO)
- IRB Repository Application
- IRB-HSBS Education
- Newsletter Archive
You are here
- Human Subjects
- IRB Health Sciences and Behavioral Sciences (HSBS)
U-M HRPP Operations Manual References
IRB approval criteria: Part 3, Section III, C 6
Regulated/not regulated research: Part 4, Section V
Exempt research policy: Part 4, Section VI
Using the U-M IRB System
IRB staff and board members have access to the IRB application and posted correspondence via the eResearch Regulatory Management (eRRM) system. The system facilitates the IRB review process by:
- Providing regulatory checklists that guide IRB staff review
- Routing submissions to ancillary committees (e.g., COI-UMOR), as applicable
- Re-routing submissions to a different U-M IRB, if applicable
IRB-HSBS Turnaround Times
"Turnaround" is the estimated time it takes to complete the IRB review and determination process.
Full-board : 4 - 8 weeks
Expedited : 2 - 4 weeks
Exempt : < 1 week
The U-M Institutional Review Boards (IRBs) fulfill their goals to protect human research participants and support the design and conduct of sound research by reviewing and approving IRB submissions for new applications, amendments, and continuing reviews.
All projects that meet the definition of research with human subjects ( 45 CFR 46.102 ) must be reviewed and approved by an IRB, or receive an exempt determination, prior to beginning the research. The IRB staff initially screens submissions to determine the completeness and the appropriate type of review. Submissions may be returned to the study team for changes before the review type is assigned. The review type may be reassessed at any time during the review process.
Types of IRB Review
The basic types of IRB Review are: Comprehensive , Exempt , and Not Regulated . The type of IRB review and the associated review process (e.g., full board , expedited , limited IRB review , system-generated ) are determined by the:
- Level of risk to research participants
- Type of research being conducted (e.g., an educational intervention, a survey, an ethnographic observation, etc.)
- Sensitivity of the research questions or complexity of the research design
- Involvement of vulnerable populations as research participants
- Use of identifiable information or indentifiable biospecimens
- Applicability of one or more of the criteria for exempt or expedited review
Research Requiring Comprehensive IRB Review
The IRB may conduct either an expedited or full board review for IRB-regulated research proposed in the Interaction/Intervention or Secondary Use application types to ensure:
- Risks to the subjects are minimal, and are reasonable in relation to anticipated benefits
- The subject selection is equitable
- Privacy and confidentiality are protected
- Informed consent processes meet federal regulatory and U-M requirements
Full Board Review
Federal regulations and institutional policy require a review by the IRB Full Board for applications where the research involves more than minimal risk to human subjects, does not meet the criteria for one of the categories of expedited review , or has been referred to the committee by an expedited reviewer or the Chair. Regardless of risk level, IRB-HSBS may require full board review when the research involves:
- Vulnerable populations, particularly prisoners
- Sensitive topics, including illegal behaviors which may require an NIH Certificate of Confidentiality (CoC) to protect subject data from compelled disclosure
- Research involving genetic/genomic analyses
- A complex research design requiring the expertise of multiple board members to evaluate
The IRB posts submission deadlines for upcoming IRB meeting dates. If an application is “board ready”, meaning that it contains all of the information and materials necessary for the full board to conduct its review, the application will be assigned to the next IRB meeting date (see Related Information to the right for schedule links), except where the agenda is already full or a reviewer with the necessary expertise is not available for that meeting. IRB staff assign submissions to a primary and secondary IRB reviewer for presentation at the full board meeting. Investigators may be invited to attend the meeting to answer questions from the board. At the conclusion of the meeting, the board votes and issues a determination for the submission.
IRB Full Board Determinations
Approved : the application is approved as submitted. The approval date is the date of the IRB review.
Approved with Contingencies : the application is approved, contingent on submission of specified changes to the protocol, informed consent document(s) and/or other supporting materials. Final approval status is granted when the IRB has reviewed and approved all requested changes. The date of the "approved with contingencies" determination is deemed the date of approval.
Action Deferred : the IRB needs additional information from the investigator before the IRB can make all of the determinations found at 45 CFR 46.111 necessary to approve the study. The principal investigator must submit the requested additional information before the IRB will consider the application for further review.
Disapproved : the protocol does not provide adequate protection to human participants, and it is unlikely that it can be modified to provide such protection. The IRB notifies the principal investigator of the disapproval in writing, including a statement of the reasons for its decision, and provides the opportunity for the investigator to respond to the IRB in person or in writing.
Tabled : the IRB full board did not have time to review the application at the convened board meeting. The application is placed on the agenda for the next convened meeting.
Expedited Review
Federal regulations ( 45 CFR 46.110 ) authorize the use of an expedited review process for:
- Minimal risk human research that meets one or more of the OHRP Expedited Review Categories
- Minor changes to research previously approved by the full board
Applications qualifying for expedited review are assigned to an expediting reviewer, an experienced IRB member appointed to the role by the IRB Chair. The expediting reviewer has the authority to make a determination or to refer a submission for full board review for multiple purposes (e.g., clarification, expertise), including in cases of disapproval. Only the full board has the authority to disapprove a study. Most studies that qualify for the expedited review process do not require annual Continuing Review .
IRB Expedited Review Determinations
In addition to the Approved and Approved with Contingencies determinations ( described above ) reviewer may issue a Changes Requested determination, when substantial changes to the application and/or materials are required before the expediting reviewer can approve the study.
Exempt Research Review
Per university policy, investigators must submit an IRB application for determination of exemption before research begins. Applications are routed for exempt review through the Interaction/Intervention application or the Secondary Use application types. IRB-HSBS recommends using the Brief Protocol for Exempt Research Projects (download) to provide an overview of you exempt project or as a data entry guide when completing the IRB application.
Projects that meet the criteria for a federal exemption category (45 CFR 46.104) or for a U-M exemption #5 may be granted a determination of exemption by the IRB, or where applicable, through the system-generated review process. The review determination, whether conducted by the IRB or system-generated, is limited in scope to the information necessary to determine if the proposed exemption applies. The IRB does not review informed consent documentation or recruitment materials for proposed exempt studies. Exemptions may be granted by the IRB Chair, expedited reviewers, or (in most cases) qualified IRB staff members.
Projects receiving an exempt determination are not subject to the Continuing Review process . Amendments are required only if the changes to the project would alter the exemption criteria. An exempt determination does not lessen the researcher's ethical obligations to participants as articulated in the Belmont Report or to the codes of conduct for specific disciplines.
Limited IRB Review
The Common Rule provides a Limited IRB Review process, which is a required expedited review of recruitment and consent materials as well as plans to maintain participant privacy and data confidentiality for exempt 2 and 3 projects that collect or use sensitive and identifiable data . An exempt determination is issued once the expediting reviewer confirms that these protections are acceptable.
Not Regulated Review
Not all research-related activities that involve people, their data, or their biospecimens are covered by the regulations governing human research. However, investigators may wish to submit a brief eResearch IRB application for a formal “not regulated” determination for funding or publication purposes; or, the investigator may be able to issue a system-generated determination letter without submission to the IRB.
Submission to the IRB is not required for the following activities:
- Case studies
- Class activities
- Journalism/documentary activities
- Oral history
- Quality assurance and quality improvement activities
- Research on organizations
- Research using deidentified data or biospecimens
- Research using publicly available data sets
Some categories require IRB review for the purpose of assessing compliance with HIPAA or other regulations. These include:
- Research involving existing information or biospecimens that have been coded before the researcher receives them, but identifiers exist
- Research involving deceased individuals only
- Pre-review of clinical data sets preparatory to research
- Standard public health surveillance or prevention activities
For a complete list of not regulated research activities, see the HRPP Operations Manual, Part 4 .
If you can answer "yes" to the following questions, you need to submit an IRB application in eResearch for IRB review:
1. Is it research?
Research is a systematic investigation (including research development, testing, and evaluation) designed to develop or contribute to generalizable knowledge ~ Federal definition, 45 CFR 46.102 (l)
- Activities such as the practice of public health, medicine, counseling, or social work are not research.
- Studies for internal management purposes (e.g., program evaluation, quality assurance, or quality improvement) are not research because the intent is not to provide generalizable knowledge but to apply findings only to the program or activity.
2. Does the research involve human subjects?
Human subjects research is a project that involves a living individual about whom the investigator (whether student or professional) (i) obtains information or biospecimens through interaction/intervention with the individual, and uses, studies, or analyzes the information or biospecimens; or (ii) obtains, uses studies, analyzes, or generates identifiable private information or indentifable biospecimens. ~ Federal definition 45 CFR 46.102 (e)(1)
3. Is the university engaged in the conduct of the research?
The university is "engaged" when the research is conducted by U-M faculty, staff, trainee, or other agent acting in connection to their university responsibilities. See OHRP's Guidance on Engagement of Institutions for more information and examples.
- Direct awards from federal sponsors that meet criteria #1 and #2 are always reviewed by a U-M IRB, whether or not the university is engaged in the research.
- If you answer "no" to any of these questions, you may have other obligations than IRB review. See the U-M HRPP Operations Manual Part 4, Section V for more information about regulated/non-regulated research.
IRB Health Sciences and Behavioral Sciences Phone: (734) 936-0933 Fax: (734) 936-1852 [email protected]
- Human Subjects Protections
- Request Info
- Faculty & Staff
- Job Seekers
- Scholarships & Aid
- Student Life
- Institutional Review Board
- Proposal Guidance
Research Proposal Guidance
Does your research require irb approval.
Research is defined as a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge. A project requires IRB review if it includes both research and human subjects. The IRB must make the final determination of whether or not a study requires review.
If you are wondering whether or not your research requires IRB oversight, the first question you should ask yourself is: Does your project involve human subjects? If your research does, then it requires IRB approval and you should continue reading this webpage. If it does not, then you do not need IRB approval.
Research That Involves Human Subjects
Below are a few examples of research that typically involve human subjects. This is not a comprehensive list and there are often exceptions to each research.
Generalizing Findings
Activities that obtain data about individuals, systematically performed with the intent to generalize findings.
Viewing Identifable Private Information
Identification of potential participants for a study or use of living individuals’ data for research purposes, whether or not the data will be recorded in an identifiable manner.
Survey, Interview, Observation
Collection of individuals’ data using surveys, interviews, or observation with the intent to generalize findings.
Audio or Videotaping
Taping individuals for study in situations not normally expected to be recorded or when individuals can be identified from recordings.
Research That Does Not Involve Human Subjects
Below are a few examples of research that typically does not involve human subjects. This is not a comprehensive list and there are often exceptions to each research.
Study or use of data that cannot be readily associated with the living individual about whom the information relates. There are some exceptions. Be sure to contact IRB for assistance.
Quality Improvement
Activities involving individuals intended solely for internal use, performed to improve services or develop new services or programs, (e.g., satisfaction surveys) without intent to generalize findings, even if results will be presented or published; audits (internal or external) performed as a part of organizational operations. There are some exceptions. Be sure to contact IRB for assistance.
Data Banking
Collection and storage of private information, if the data may be used in the future for research purposes, whether or not the data will be recorded in an identifiable manner.
Examples of Research & IRB Approval
Below are examples of research and whether or not they need IRB approval. Research that do need IRB approval also have levels of review.
Surveys, Questionnaires & Interviews with Adults
Not all survey, questionnaire, or interview research is minimal risk. For example, a survey or interview that asks questions about sensitive topics (childhood abuse, sexual functioning) likely to cause emotional stress or discomfort may require full IRB review. Some survey research may be classified as exempt from committee review if the information obtained is recorded in a way that the subject cannot be identified (either directly or through a code numbers or link); in other words, if the research data are anonymous.
A survey or interview study may also be considered exempt from committee review even when the data are not anonymous if the information being gathered could not reasonably place the subject at risk of criminal or civil liability or be damaging to the subject’s financial standing, employability, or reputation.
The most common classification for survey, questionnaire, or interview research is expedited approval. If the study is not anonymous and contains information that, if known, could be damaging as described above, but it does not rise to the level of more than minimal risk, it may be given expedited approval. Although the proposal application gives the investigator the opportunity to indicate a classification, the IRB makes the final determination as to the classification of exempt or expedited.
Normal Educational Practices
Normal education practices are considered exempt from committee review, but must still be reviewed and approved by the IRB office. Some examples of this could be a students’
- Curriculum-related written work, test scores, grades, artwork and other work samples produced by children
- Curriculum-related oral and non-verbal communicative responses individually, such as in an interview, in small groups and with the whole class
- Responses (written, oral or behavioral) to curriculum-related activities
- Level of active participation in curriculum-related activities
Please note: A “normal educational setting” means preschool, elementary, secondary, and higher educational facilities, and after-school programs (if the project relates to tutoring, or homework help). In Special Education, normal educational practices correspond to the Individualized Educational Program (IEP), which is tailored to each student with an identified disability and may be implemented in diverse settings (school, home, work, community).
Collection Methods
The following list outlines the ways in which a researcher may collect the information for their research.
- Videotapes and photographs of curriculum-related classroom activities
- Audio tapes of teacher-student and student-student discourse related to the assignment
- Teacher’s non-participant observation of curriculum-related activity of individual children or groups of children, noting what will be observed and how it will be analyzed, or whether it will be used as anecdotal evidence in the study
- Teacher’s commentary on students’ curriculum-related written work, artwork and other artifacts produced by children
- Student journals and communication books related to the curriculum
- Student grades and test scores
- Teacher journals, notes and reflective comments on student responses and participation in curriculum-related activities
- Questionnaires or interviews with students, parents and family members, teachers and administrators
- Non-participant classroom observations by colleagues, with the class teacher’s permission, stating what will be observed and how it will be used (i.e. How data will be analyzed or whether it will be used as anecdotal evidence)
Research for University Courses
Research conducted solely for pedagogical purposes may be excluded from IRB review, under the following conditions:
- The instructor’s intention is to teach professional research methods such as interviewing, surveying, or experimental design
- The data are gathered solely for the purposes of teaching how to analyze them
- The results will remain in the classroom
These data can be presented at the end of the semester within the confines of the institution, for instance, at Scholars Day. However, if the results will be published (including on Digital Commons), presented at a larger conference off-campus, or generalized in some other way, it will be necessary to obtain IRB approval.
If a class project evolves into a research project that the student/instructor wishes to publish or generalize, then the research will need to undergo IRB review. This should occur as soon as it is known that the data will be used for research. If this is not determined until after the research is completed, the investigator should submit a protocol to the IRB requesting permission to use existing data.
Professors, students, and research assistants are asked to submit the In-Class Research Form prior to beginning their in-class research projects to verify whether the proposed activity will require an IRB approval process.
Pilot Studies
Pilot studies with human research volunteers, no matter how small, must obtain IRB approval. You can include the pilot study as a smaller section of the complete protocol, or you can get approval for the pilot study first, then come through the IRB again for a review of the full “parent” study. At this stage, you may have modified your research to take into account the results of the pilot study. (For example, you may decide to change the survey questions as a result of the pilot study, or change inclusion/exclusion criteria.)
Oral History
The researcher’s intention plays a large part in determining whether research is an oral history or not. If the intention is to interview informants who have a unique perspective on a particular historical event or way of life, and the researcher also intends to let the informant’s stories stand on their own as a “testimonial” or in an archive, with no further analysis, the research is most likely oral history.
However, if the surveys or interviews are conducted with the intention of comparing, contrasting, or establishing commonalities between different segments or among members of the same segment, it is safe to say your research will be regular survey/interview procedures, because you will be generalizing the results.
Historians explain a particular past; they do not create general explanations about all that has happened in the past, nor do they predict the future.
Moreover, oral history narrators are not anonymous individuals, selected as part of a random sample for the purposes of a survey. Interviewees are selected because of their personal relationship to the topic under investigation. An oral history interview provides one person’s unique perspective. A series of oral history interviews offers up a number of particular, individual perspectives on the topic, not information that may be generalized to all research volunteers in the event or time under investigation.
Oral history interviews are not analyzed in the same way that qualitative data is generally analyzed. No content analysis, discourse analysis, coding for themes or other qualitative analysis methods of data analysis are performed on the interviews. They stand alone as unique perspectives.
It is primarily on the grounds that oral history interviews, in general, are not designed to contribute to “generalizable knowledge” that they are not subject to the requirements of 45 CFR part 46 and, therefore, can be excluded from IRB review.
Secondary Analysis of Existing Data
Research involving the secondary analysis of existing data must be reviewed by the IRB to ascertain whether or not it requires IRB oversight.
Such research will be considered exempt if one or more of the following are true:
- The sources of such data are publicly available
- The information is recorded by the investigator in such a manner that subjects cannot be identified, directly or through identifiers linked to the subjects
- The dataset has been stripped of all identifying information and there is no way that the data could be linked back to the subjects from whom it was originally collected
Such research will qualify for an expedited or full-board review if:
- The source of the data is not publicly available data and/or contains private identifiable information about living individuals
Content Experts/Consultants/Key Informant
It may not be necessary to get IRB approval if interview questions are with experts about a particular policy, agency, program, technology, technique, or best practice. The questions are not about the interviewee themselves, but rather about the external topic. For instance, questions will not include demographic queries about age, education, income or other personal information.
IRB review will be required when a researcher is interviewing individuals about content, but there is a research question or hypothesis involved and when a researcher intends to analyze and generalize the results–that look for common themes in the collected data, try to universalize the interviewees’ experiences, or quantify the results in some way.
Examples That May Be Excluded From IRB Review
In all the following examples, the questions are focused on the facts about the program, policy, software, curriculum, procedures or project. The researcher will simply report the facts as they are related by the content experts. You may not need to submit a protocol or an informed consent form for IRB approval if one or more of the following are true. You are,
- Interviewing managers in a company about their billing procedures, or their use of a particular software program
- Interviewing or surveying teachers about what should be included in the development of a particular curriculum unit
- Asking a panel of nurses and doctors to review your antismoking program for teens for correct medical content
- Interviewing social agency directors about their client intake procedures
Additional Resources
The following decision flowcharts can help you determine whether your project requires IRB oversight.
Is an Activity Research Involving Human Subjects?
Is the Human Subjects Research Eligible for Exemption?

Getting Started
Before you begin to set up an IRB, read and become familiar with the federal regulations that apply to research with human participants as specified in 45 CFR 46 and the " Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research " (National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, 1979). An essential resource is the 100-page "Institutional Review Board (IRB) Guidebook" published by the Office of Human Research Protections (OHRP). This guidebook is available for purchase or free download from the OHRP website. The OHRP website has everything you need for creating your IRB.
Keep in mind that the basic job of the IRB is to protect the rights and welfare of human research participants and facilitate research by using the basic ethical principles from the Belmont Report.
The Nuts and Bolts
This section summarizes some of the key federal regulations for establishing an IRB. Please keep in mind that this is a brief summary; be sure to refer to 45 CFR 46 for complete and specific information about these regulations.
Size of the IRB
According to federal regulations, the minimum number of people required for an IRB is five; however, you can certainly have more than five members. The number of members will most likely depend on the size of the institution and the IRB workload. The availability of potential members will also affect the number of members you choose to have on your IRB.
Your institution may have more than one IRB. Many universities have multiple IRBs that specialize in particular types of research. If you are working for a community college or other undergraduate college that has multiple campuses, it may be advisable to have multiple IRBs so that each campus has its own review panel. This may help speed up the review process for each individual proposal.
Composition
As noted above, an IRB must consist of a minimum of five members of varied backgrounds to facilitate diversity in its composition. Accordingly, if you are doing federally funded research, you will need to make sure that your IRB is composed of members who represent the following characteristics:
Scientific area. At least one member must work in science (e.g., biology, psychology, chemistry).
Nonscientific area. At least one member must work in a nonscience area (e.g., history, English, philosophy).
External to the institution. One member must come from outside the institution and not be affiliated with the institution.
Diversity of representation. An effort must be made to achieve diversity of representation, particularly if members of a “vulnerable population,” such as children or people with intellectual disabilities, are frequently a subject of study (see Definitions ). If such populations will be used, someone who has knowledge of or experience with those populations should participate as a member of the IRB.
Diversity of gender. The IRB should have both male and female representation.
Diversity of profession. The IRB should not have representation from just from one profession, such as psychology.
If you are not doing federally funded research, you have the freedom to choose alternative and sometimes more appropriate members for your IRB.
Other Considerations
- An IRB may not allow any member to participate in the review of any project in which the member has a conflicting interest. That would include researchers involved in the project and administrators involved in the grant applications.
- An IRB may invite individuals with expertise in specific areas to assist in the review of projects that require expertise that is not represented sufficiently on the IRB; however, they may not vote with the IRB.
- By definition, the IRB is a board, not a committee. As such, it means that members of an IRB are tasked with rendering decisions about research they review. In contrast, members of standing committees may or may not be tasked with rendering decisions—often, their purpose is to offer recommendations or organize information used to help others make decisions. The appointment process to an IRB often differs from the appointment process to other standing committees, as federal regulations include specific requirements about the membership of an IRB.
Your institution will need to provide adequate staffing for the IRB. You may be able to designate a current employee as the IRB staff person, depending on the person’s current duties and the expected workload for the IRB. Depending on the number of research projects, you may need a full-time staff person for the IRB. Key tasks for staff include:
- Answering questions regarding the IRB process.
- Assisting researchers in completing their IRB proposals.
- Tracking when ongoing research projects are due for their annual review.
- Communicating with the IRB regarding incoming proposals and/or other board responsibilities.
- Maintaining documentation of completed training for IRB members and principal investigators (PIs).
IRB Members
The members of the IRB that come from current faculty and staff may need release time to perform the functions of the IRB. In particular, the chairperson of the IRB will likely have some administrative functions for the IRB and may need the time to perform them. This release time needs to be taken into consideration when considering cost. Additionally, you may choose to provide a stipend or reimburse travel time or mileage to your community representative.
Procedures for IRBs That Meet Federal Requirements
Your IRB will need to establish written procedures so that it is clear how the IRB will function. Before the IRB creates these procedures, considering how the IRB will fulfill its duties will be helpful. The questions below will likely need to be addressed; the answers to the questions will be based on your institutional organization and the anticipated volume of research conducted at your institution that requires IRB review.
Members of an IRB will determine the level of IRB review required for submitted research proposals (e.g., “exempt,” “expedited,” or “full” IRB review). Studies that meet the definition of “research” and that involve human participants may be considered exempt if they meet certain requirements.
A “full” IRB review is required when the research is defined as (a) a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge (38 CFR 16.102d); (b) that involves human subjects (i.e., a living person about whom a researcher collects either identifiable private information OR data through an intervention or interaction); and (c) involves greater than minimal risk to those human subjects. A full IRB review usually requires attendance from a quorum of IRB-appointed members.
An “expedited” IRB review is selected when the research is defined as meeting the first two classifications noted above but involves no more than minimal risk to subjects OR is being reviewed strictly for minor changes to previously approved protocols in the research project. An expedited review procedure can be conducted by a subset of reviewers designated by the IRB chairperson from members of the IRB.
An “exempt” IRB review (see Criteria for Exempt Status ) is selected when the research falls into one of the six approved categories of exempt research (45 CFR 46.101 [b]) and is not applicable to research in a covered research category (e.g., FDA regulation - 21 CFR 50.20). Exempt research does not mean that a research project has no review. Rather, for studies that are determined to be exempt, it means that the exemption (and its corresponding category) is documented in the IRB records and that the decision is communicated in writing to the investigator.
Accordingly, one of the first questions to consider is who on the IRB makes the determination that a proposed study is exempt (see Criteria for Exempt Status ). Is the IRB chairperson solely responsible for that determination, or will a subcommittee screen all proposals for exemption?
- How will expedited or administrative review be conducted? Studies that pose minimal risk or proposals that are minor changes to studies that were previously approved by the IRB may not need to undergo a full IRB review.
- How will the IRB conduct initial and continuing review of research proposals? Studies that are ongoing (lasting more than 12 months) should have a follow-up review process at least once every 12 months.
- How will the IRB’s decision be communicated to the PI?
- How will changes in proposed research activity be communicated to the IRB? If the IRB has already approved a proposal, will changes to that proposal require new review?
- How will unanticipated problems that pose subsequent risks to human participants be reported to institutional officials?
- What are the deadlines for submissions, and how often will the IRB meet?
Be sure that you give these kinds of questions some thought up front and then solicit input from those people who are interested in either serving on the IRB or helping with the formation of the IRB. Most likely, you will also need to educate administrators at your institution about IRB regulations and procedures.
Educating IRB Members and Principal Investigators
IRB members and PIs need training and education in research ethics and current research regulations if they are going to be applying for federal funds. Most IRBs will choose to have some record of training, but it can be as innocuous as having researchers affirm that they have read the Belmont Report . If more extensive training is deemed necessary, it may be delivered a number of ways (e.g., a face-to-face class, an online class, a self-paced tutorial). The cost of providing training can vary widely. If you choose to have IRB members and PIs attend a face-to-face class, they need to have the time to participate, and you will need to provide a trainer. Online training costs also vary. There are online training modules that your institution can use for free or purchase and customize (see References and Resources for a list of inexpensive training options). As a psychologist, you may wish to review these training modules, as they are often quite naïve in their treatment of research methodology.
Setting up your own online training also has costs, such as the time of the person designing the website and the time of the experts needed to write the training modules. For institutions with limited time and/or budgets, the OHRP’s " Institutional Review Board Guidebook " is a good place to begin in terms of deciding what material to include in a training course. The key information that needs to be delivered includes:
- The basic ethical principles underlying research with human participants as elucidated in the Belmont Report.
- The federal regulations for the protection of research participants.
- The history and ethics of research with human participants.
Completion of training requirements should be documented and kept on file so that the institution can demonstrate that IRB members and PIs have been provided the relevant information. Although probably not necessary, this documentation can be acquired by requiring that IRB members and PIs take a test after reading all of the material that is provided to them.
Record Keeping
Federal records, whether in hard copy or electronic form, need to be maintained and easily accessible for at least 3 years after the research is completed. You may choose a significantly less cumbersome system for research that is not federally funded. Office space or computer space will need to be allocated for storage. These records include:
- Research proposals, sample consent documents, updates from the researchers, and documentation of unanticipated problems ( as described by OHRP ).
- Minutes of IRB meetings that document who attended; a record of voting; rationale for accepting, rejecting, or requiring changes to research proposals; and, where there is conflict among the IRB members, a summary of the issue and its resolution.
- Copies of communication, including email, between the IRB and researchers.
- List of IRB members, including their degrees, area they represent, relevant experience, and association with the college.
- IRB procedures and forms.
- Evidence of training completion.
How Much Will An IRB Cost?
The cost of the IRB depends on how much research the faculty, staff, and students at your institution are conducting and the nature of the research being conducted. Generally, faculty members serve on IRBs as part of their college service without additional compensation. If the volume of research is relatively low and/or most of the research qualifies as exempt (see Criteria for Exempt Status ), the cost may be minimal. If the volume of research is high and/or the research involves more than minimal risk to participants, the additional record keeping may create greater costs.
Does All Data Collection at Our Institution Require an IRB Review?
Much of the data collected within or on behalf of an institution does not meet the regulatory definition of “research” and, thus, would likely not require IRB review. For example, many institutions often engage in projects that are best defined as quality improvement initiatives or program evaluation. Such projects usually do not meet the regulatory definition of research and thus would not need IRB review. However, if (a) the data being collected meet the regulatory definition of research and (b) the research is done using human participants (see Definitions ), the study does require an IRB review. In addition, if one of the anticipated activities following the study is to disseminate the information, such as in a publication or conference presentation, the study may require an IRB review. When in doubt, the PI should submit an IRB proposal. Remember that the IRB is the institutional authority on research requirements, not the researcher or the institutional administration.
- The Institutional Review Board: A College Planning Guide
Contact Education
- Affiliated Professors
- Invited Researchers
- J-PAL Scholars
- Diversity, Equity, and Inclusion
- Code of Conduct
- Initiatives
- Latin America and the Caribbean
- Middle East and North Africa
- North America
- Southeast Asia
- Agriculture
- Crime, Violence, and Conflict
- Environment, Energy, and Climate Change
- Labor Markets
- Political Economy and Governance
- Social Protection
- Evaluations
Research Resources
- Policy Insights
- Evidence to Policy
- For Affiliates
- Support J-PAL
The Abdul Latif Jameel Poverty Action Lab (J-PAL) is a global research center working to reduce poverty by ensuring that policy is informed by scientific evidence. Anchored by a network of more than 900 researchers at universities around the world, J-PAL conducts randomized impact evaluations to answer critical questions in the fight against poverty.
- Affiliated Professors Our affiliated professors are based at 97 universities and conduct randomized evaluations around the world to design, evaluate, and improve programs and policies aimed at reducing poverty. They set their own research agendas, raise funds to support their evaluations, and work with J-PAL staff on research, policy outreach, and training.
- Board Our Board of Directors, which is composed of J-PAL affiliated professors and senior management, provides overall strategic guidance to J-PAL, our sector programs, and regional offices.
- Diversity, Equity, and Inclusion J-PAL recognizes that there is a lack of diversity, equity, and inclusion in the field of economics and in our field of work. Read about what actions we are taking to address this.
- Initiatives J-PAL initiatives concentrate funding and other resources around priority topics for which rigorous policy-relevant research is urgently needed.
- Events We host events around the world and online to share results and policy lessons from randomized evaluations, to build new partnerships between researchers and practitioners, and to train organizations on how to design and conduct randomized evaluations, and use evidence from impact evaluations.
- Blog News, ideas, and analysis from J-PAL staff and affiliated professors.
- News Browse news articles about J-PAL and our affiliated professors, read our press releases and monthly global and research newsletters, and connect with us for media inquiries.
- Press Room Based at leading universities around the world, our experts are economists who use randomized evaluations to answer critical questions in the fight against poverty. Connect with us for all media inquiries and we'll help you find the right person to shed insight on your story.
- Overview J-PAL is based at MIT in Cambridge, MA and has seven regional offices at leading universities in Africa, Europe, Latin America and the Caribbean, Middle East and North Africa, North America, South Asia, and Southeast Asia.
- Global Our global office is based at the Department of Economics at the Massachusetts Institute of Technology. It serves as the head office for our network of seven independent regional offices.
- Africa J-PAL Africa is based at the Southern Africa Labour & Development Research Unit (SALDRU) at the University of Cape Town in South Africa.
- Europe J-PAL Europe is based at the Paris School of Economics in France.
- Latin America and the Caribbean J-PAL Latin America and the Caribbean is based at the Pontificia Universidad Católica de Chile.
- Middle East and North Africa J-PAL MENA is based at the American University in Cairo, Egypt.
- North America J-PAL North America is based at the Massachusetts Institute of Technology in the United States.
- South Asia J-PAL South Asia is based at the Institute for Financial Management and Research (IFMR) in India.
- Southeast Asia J-PAL Southeast Asia is based at the Faculty of Economics and Business at the University of Indonesia (FEB UI).
- Overview Led by affiliated professors, J-PAL sectors guide our research and policy work by conducting literature reviews; by managing research initiatives that promote the rigorous evaluation of innovative interventions by affiliates; and by summarizing findings and lessons from randomized evaluations and producing cost-effectiveness analyses to help inform relevant policy debates.
- Agriculture How can we encourage small farmers to adopt proven agricultural practices and improve their yields and profitability?
- Crime, Violence, and Conflict What are the causes and consequences of crime, violence, and conflict and how can policy responses improve outcomes for those affected?
- Education How can students receive high-quality schooling that will help them, their families, and their communities truly realize the promise of education?
- Environment, Energy, and Climate Change How can we increase access to energy, reduce pollution, and mitigate and build resilience to climate change?
- Finance How can financial products and services be more affordable, appropriate, and accessible to underserved households and businesses?
- Firms How do policies affecting private sector firms impact productivity gaps between higher-income and lower-income countries? How do firms’ own policies impact economic growth and worker welfare?
- Gender How can we reduce gender inequality and ensure that social programs are sensitive to existing gender dynamics?
- Health How can we increase access to and delivery of quality health care services and effectively promote healthy behaviors?
- Labor Markets How can we help people find and keep work, particularly young people entering the workforce?
- Political Economy and Governance What are the causes and consequences of poor governance and how can policy improve public service delivery?
- Social Protection How can we identify effective policies and programs in low- and middle-income countries that provide financial assistance to low-income families, insuring against shocks and breaking poverty traps?
Introduction to randomized evaluations
The elements of a randomized evaluation
Teaching resources on randomized evaluations
Resources for researchers new to randomized evaluations
Formalize research partnership and establish roles and expectations
Assessing viability and building relationships
Checklist for launching a randomized evaluation in the United States
Administrative steps for launching a randomized evaluation in the United States
Ethical conduct of randomized evaluations
Institutional Review Board (IRB) proposals
Power calculations
Quick guide to power calculations
Grant proposals
Grant and budget management
Trial registration
Pre-analysis plans
Resources for conducting remote surveys
Introduction to measurement and indicators
Survey design
Repository of measurement and survey design resources
Design and iterate implementation strategy
Define intake and consent process
Randomization
Implementation monitoring
Real-time monitoring and response plans: Creating procedures
Increasing response rates of mail surveys and mailings
Survey programming
Data quality checks
Survey logistics
Surveyor hiring and training
Field team management
Working with a third-party survey firm
Questionnaire piloting
Using administrative data for randomized evaluations
Evaluating technology-based interventions
Data security procedures for researchers
Data cleaning and management
Data visualization
Data analysis
Conducting cost-effectiveness analysis (CEA)
Pre-publication planning and proofing
Data de-identification
Data publication
Communicating with a partner about results
Coding resources for randomized evaluations
Institutional Review Boards (IRBs) review research involving human subjects to ensure that participants are protected from potentially harmful research. This resource provides an overview of the roles of IRBs and ethics guidelines. It also includes practical tips for researchers preparing IRB proposals, including an annotated informed consent checklist, guidance on timeline planning and responding to common concerns of IRBs, and handling unanticipated events. Researchers who are already familiar with the roles of IRBs and obtaining human subjects training certificates may wish to begin at the section "Preparing the proposal."
Important terms
- Institutional Review Board (IRB): Ethics review committee. US requirements often govern the contents and elements of an IRB application, even if you also submit the application to an ethics board somewhere else. All J-PAL funded projects need to pass IRB review at MIT or cede authority to another host institution.
- Common Rule: Shorthand term for the Federal (US) Policy for the Protection of Human Subjects, which outlines the criteria and mechanisms for IRB review of human subjects research.
- Exempt research involves minimal risk and fits under one of the exempt review categories described in the Common Rule. Exempt status means the study is exempted from some (but not all) regulatory review, though not from ethics review, and IRBs may still place conditions on exempt research. It is up to the IRB, not the researcher, to determine whether the study qualifies for exempt status 1 . The revisions to the Common Rule include new categories and clarification of existing categories of exemption. See Human Subjects Decision Chart 2 : “Is the Research Involving Human Subjects Eligible for Exemption Under 45 CFR 46.104(d)?”
- Expedited review involves research with minimal risk and fulfills a set of other criteria listed by the US Department of Health and Human Services, Office of Human Research Protections (DHHS OHRP, 2017). Expedited review is carried out by one member of the IRB Committee.
- Full review is required if the protocol is greater than minimal risk and/or does not fall into one of the expedited or exempt review categories, according to both pre-2018 and 2018 regulations. This may happen, for example, when a study targets a vulnerable population. Full review requires that the protocol is discussed by the full Board, which requires a Board meeting (DHHS OHRP 2009 & 2018 ).
- Amendment : You should submit an amendment request to the IRB to make any change to the original protocol. This includes updates to personnel, consent scripts or processes, surveys questions, funding, and participating institutions.
- IRB Authorization Agreements (IAA): Agreements between two IRBs that one of them will take on all reviewing responsibilities for a project for both of them. For example, if a research team includes a principal investigator (PI) at Tufts University and one at Cornell, Cornell can cede reviewing responsibilities to Tufts. DHHS OHRP provides a sample IAA , though many IRBs now process IAAs online via Smart IRB . Please check with your IRB to see which is preferred.
- Adverse event: A violation of IRB protocols or an event during the research that carries the risk of potential harm to the subjects. This includes data breaches. Adverse events must be reported to the IRB.
Institutional Review Boards (IRBs) review research involving human subjects to ensure protection of research participants from potentially harmful research.
Research involving human subjects obtains information through interventions or generates identifiable private information (including bio-specimens). Identifiable information means that the identity of the subject may readily be ascertained or associated with an individual. Private information refers to behavior taking place in a context in which an individual can reasonably expect no observation, recording, or information that the individual provided for a specific purpose and can reasonably expect will not be made public (e.g., a medical record).
The "Common Rule" is the popular term for the Federal (US) Policy for the Protection of Human Subjects , 45 CFR part 46 , which outlines the criteria and mechanisms for IRB review of human subjects research. The revised Common Rule went into effect January 21, 2019. All new protocols submitted after January 21, 2019, are required to comply with the revised requirements. It is up to individual IRBs, however, whether to apply the previous or revised version of the regulations to ongoing studies that were submitted before that transition date (DHHS OHRP 2019a).
J-PAL IRB requirements
All research projects that are either funded or implemented by J-PAL must be reviewed by the IRB at the host institutions of all PIs on the project and adhere to all policies and protocols approved by the IRB. Effective all rounds of J-PAL funding starting October 1, 2022, and for all projects where data collection is supposed to start after January 1, 2023, at least one of the IRBs reviewing the project must have status as an IRB Organization (IORG). An IRB’s status can be found by consulting the database of IORGs .
Under the revised Common Rule, only one institution will be named the “IRB of Record,” and the other institutions will cede authority to it. If MIT is the IRB of Record, then all key personnel must have completed CITI training (see below). J-PAL also requires this of all J-PAL research staff. Field surveyors generally do not need CITI training but should be given training by the study staff on ethical research procedures and sign confidentiality agreements before contact with research participants 2 . Some IRBs may require CITI training for all staff; it is important to check specifics with your IRB.
CITI/Human Subjects training certification
- IRBs have different requirements as to which study personnel must receive CITI human subjects training and certificates , though requiring it of key personnel is common. As stated above, CITI training is required if MIT is the main IRB of record and of all J-PAL research staff. All US research institutions require human subjects training of their staff.
- The CITI training course on “Social and Behavioral Research Investigators” is preferred by MIT (and most US institutions) — you can find the course either via your host institution’s IRB or by going to the MIT COUHES website and following the links for MIT affiliates or unaffiliated personnel. See also this guide for instructions on navigating the CITI site. 3
- NIH training course recognized by MIT and the NBER: Protecting Human Research Participants (PHRP ) . This course now charges a fee for learners, but CITI can be taken for free via the MIT link above.
- Make sure to also keep your certificate updated ; it requires refreshing every three years (if you give CITI a permanent email address they will remind you). One way to complete this renewal requirement more efficiently is to take the refresher course in social and behavioral research ; these courses are designed to be shorter and test learners on the most important content. Note that the reviewing agency may need to have the original certification on file for these courses to be acceptable (CITI Program). See also this guide , particularly highlights on page 3, for instructions on enrolling in CITI courses, including refresher courses.
Ethics guidelines and principles for research
The principles of ethical research are detailed in IRB training. These principles, described below, are formulated in the Belmont report (DHHS OHRP 2017). For a more detailed overview of the role of ethics in social science research, see our resource on ethical conduct of randomized evaluations.
Respect for persons
Research subjects need to be given a chance to independently decide and volunteer to participate in the study given knowledge of all aspects of the study that concern them: procedures, costs, risks, and benefits. Derived from this principle is the obligation for researchers to:
- Carry out an informed consent procedure or an assent procedure (for a minor), and
- Protect vulnerable populations , such as prisoners and children.
Beneficence
Researchers should consider the burdens and risks of the research in all aspects, including the length and effort of the survey, possible embarrassment from answering questions, psychological burden on subjects and surveyors, and risk of personal data being exposed. In medical research, beneficence is interpreted very strictly; for example, researchers must end a trial and begin treating the whole study population if it becomes clear that the treatment significantly increases welfare.
Note: The rules of beneficence (not doing harm and minimizing possible harm) should be applied not just to the subjects of your research but all human actors involved, for example your surveyors (who need to be protected from, e.g., psychological harm from conducting very difficult interviews, say, with torture survivors). They apply to both the study procedures (e.g., how burdensome the interview process is) and any interventions you conduct (e.g., how burdensome the policy is).
Researchers should ensure that a just distribution of burdens and benefits governs the selection of the sample, subjects, and treatment recipients. The justice principle requires that the benefits of research don't go only to one group and the burdens to another .
Random assignment and the justice principle
It may appear that simply randomly assigning a program to some people but not others falls under the definition of "injustice" above. Do the phrases "when some benefit to which a person is entitled is denied without good reason or when some burden is imposed unduly" and "equals ought to be treated equally" rule out randomized designs? An article by Glennerster and Powers (2016) discusses this question in some detail. The relevant part starts on p. 12 of the ungated version .
Here are some additional thoughts:
- Suppose the intervention is part of your research and provides a benefit to some subjects but not all of them . These benefits do not represent an entitlement: if you do not conduct the research then no one will receive the benefit. The hypothetical alternative of giving the benefit to all study subjects (which makes the study worthless) is not relevant if the intervention is not implemented by the researchers.
- If the intervention is implemented by someone else , and the research is only measuring the effects of an existing program, the benefit or harm from the intervention should not be a factor in the decision if the research is ethical. We need to study programs that are conducted by governments or NGOs, especially if they may be unethical.
- The real ethical questions arise in the gray area in between . What if the researchers implement a program that poses a risk? What if the researchers get involved in the randomization or implementation of a program carried out by another organization? The latter is particularly problematic if a government program is tested that is in principle available to all study subjects: in this case, a benefit might be withheld as part of the research design to which the study subjects are actually entitled. In such cases, real ethical trade-offs need to be made between the inherent, general benefits of studying a program, and the potential for harm or injustice done to specific subjects. The benefits from learning may sometimes justify the disadvantages to the study subjects from receiving or not receiving a study treatment, but if they do not, the researchers may have to reconsider the randomized design. A possible alternative might be a so-called encouragement design, in which all study subjects have access to the program, but only a random subset is given additional information or "nudges" to encourage use.
Roles of researchers and the IRB
Weighing the general benefits of research against individual costs is the key task of an IRB. However, you should, of course, also ask yourself this question. You should only go ahead with study procedures after you have made every effort to minimize the burden on your subjects and any other affected parties, and made sure that you don't find your own research ethically questionable . A first check as to whether a given research protocol is problematic or not might be introspection: Would you feel upset, treated unfairly, or subjected to considerable potential for harm in your own study? Knowing all the details of the study, would you choose to participate?
More on ethics considerations are covered in the corresponding resource , and Glennerster & Powers (2016) discuss gaps in IRB review and ethics frameworks.
Only if you are sure that you can justify the research to yourself should you turn to the matter of describing your research protocol, and its acceptability from an ethical standpoint, to the IRB. Having thought through these questions will help you design procedures that truly minimize the burden on subjects and anticipate questions from the IRB. We will discuss some common concerns and issues that you should consider and be able to address further below in the section "Responding to common concerns of IRBs."
Choosing an IRB and planning a timeline
- Biomedical testing or any health procedures more invasive than measuring weight or height typically have to be reviewed by a medical review board. Social science research can go to a general IRB or a social and behavioral science IRB .
- An IRB at the institution of each PI must review the project, either as a full IRB application or as a cede to another institution.
- A local IRB (for J-PAL projects, typically at the host university of the J-PAL regional office) may need to review as well if the study is in a different country. Keep in mind that regulatory procedures, standards, and timelines can all vary substantially by country. For example, Zambia requires both IRB approval and research approval from the government; a research review and an ethics review would therefore be distinct processes there. If you are not sure which institution should be reviewing your project, especially in countries where J-PAL has not established a regional office, it may take some additional time to research and identify the appropriate institution to conduct a review. You might start this research process by consulting the OHRP's (incomplete) lists of general international standards and regulations or social and behavioral research standards , or searching the OHRP's database of registered IRBs & IORGs , but you should still confirm your specific project country's regulatory procedures with local staff or experts (Department of Health and Human Services, Office of Human Research Protections 2018).
- J-PAL-funded projects must be reviewed by MIT COUHES , either as a full IRB application or as a cede (ceding is preferable if the project has no MIT PIs involved).
- Many US institutions can sign IAAs between them, so only one US institution needs to review the project. Under the new Common Rule, IRB oversight for most federally-funded collaborative research projects located in the U.S. is required to use a single IRB (commercial, academic, or hospital-based).
Timeline planning (based on experience with US IRBs)
Plan when to submit your IRB application by calculating backwards from the piloting and study start. Typically, the procedure is that an IRB officer reviews the application first for completeness and then a Board member receives it. For full review, the proposal is then discussed at the next Board meeting; otherwise the decision is made by the Board reviewer. If you are not entirely sure if you will need a full review, plan for the full review and also budget for needing to resubmit.
- Find out when the relevant IRBs meet : some do not meet in the summer or winter break. Some US IRBs don't have more than one or two meetings between the Spring and Fall terms.
- Inquire about the turnaround times of the IRBs you can submit to - sometimes expedited reviews can take longer than submitting to a well-timed Board meeting (particularly if the IRB is busy around the Board meeting time).
- Some ethics boards outside the US may have different procedures, e.g. require a presentation of your project to the Board (e.g., the Comité National d'Ethique pour la Santé et les Sciences de la Vie, CNESS, in Mali).
- Some IRBs (e.g., at Brown University) use a " cultural consultant " to make sure the study protocols are culturally appropriate.
- Resubmission : The Board may give only conditional approval and/or request changes that require a second submission.
Some additional notes on the timing:
- Two IRBs: if one of two IRBs requests protocol changes you may need to resubmit to the other one. Make sure you plan for this.
- Initial submission plus amendment: Often, it is faster to get an amendment to an existing study approved rather than submitting a completely new protocol. Therefore, it may be better to submit an initial proposal that outlines the study and describes pilot procedures, for example, and then to submit protocol amendments as you get a better idea of the exact study procedures. In some cases, you need initial IRB approval for a study to receive funding so your study will typically later require an amendment (e.g., J-PAL funded projects require IRB approval before funds can be released; this is an MIT requirement).
Administrative data
Research involving administrative data is very likely to be considered human subjects research and thus subject to review by the IRB of the researcher’s home institution, particularly if the data contains identifiers. Beyond the purview of the IRB, an overlapping web of U.S. federal laws and regulations, state laws and regulations, and institutional restrictions and procedures governs access, storage, and use of administrative data.
Because compliance requirements and definitions of “identifiable” data differ by field, source, and geography, the guidance of an IRB and legal counsel at the researcher’s home institution can be critical in sorting through compliance and reporting requirements. Local regulations may apply as well (e.g., research involving the use of European data conducted by a US-based PI may involve IRB review by the PI’s home university, and would also be subject to the EU’s General Data Protection Regulation (GDPR)).
A Data Use Agreement (DUA) documents the terms under which a data provider shares data with a researcher’s home institution for use by the researcher. This agreement, which typically must be approved by legal counsel at the researcher’s home institution, contains a number of provisions that can significantly impact the underlying research. Many universities have a standard template that includes terms and conditions that are acceptable to the university, and were created with researchers’ needs in mind. More on DUAs is covered under the resource Using administrative data for randomized evaluations .
IRBs often require researchers to furnish signed DUAs before approving a study protocol, and data providers often require IRB approval before signing a DUA. Research teams may request provisional approval from one party, making clear to all parties the process and constraints, to find a path forward. Research teams can facilitate the process by proactively and frequently communicating with the IRB and data partners.
More information on working with IRBs and DUAs with administrative data can be found in the IDEA Initiative’s Handbook on Using Administrative Data for Research and Evidence-based Policy and in the resource Using administrative data for randomized evaluations .
Preparing the proposal
This section is based on experience with US IRBs. Check if your IRB has a submission form that pre-specifies the information they want to see. These are typically found on each IRB’s website along with templates for consent forms and other helpful documents. With the revised Common Rule, some universities such as MIT have changed the application and forms (e.g., closure, adverse event, etc.). Please download these forms directly from the y our IRB's site rather than using old versions you have on hand.
Contents of a proposal
The information required is very similar across most proposals.
- Research objectives and purpose : Be brief on the purpose or scientific background of the study. A comprehensive literature review is not required. Make this accessible to a well-educated lay person.
- Submit all the interview guides , survey instruments , and recruitment materials you want to use, and provide translations. Translations may need to be certified; ask the IRB about that.
- Describe the consent procedure in full and submit the consent form exactly as you plan on using it. Waivers or alterations of consent/assent may require additional materials. Once approved, the consent form cannot be changed without an amendment so it's worth putting some work into this (see next section and our resource on intake and consent processes ). You may need to use the IRB-stamped form.
- Data handling: An important risk of study participation for subjects is that their personal data is exposed in some way. Make sure you have good procedures for both physical and electronic data protection, including storing data in locked files and clearly stating the timeline and process for storage/destruction of personal information. Moreover, make sure you describe in the consent form to whom and how the subjects' data will be accessible. State clearly which specific information will not be shared with others. This is particularly important if you intend to publish the study data (which most researchers now want to do). Make sure you describe how you will de-identify the data and use language in the consent form that does not prevent you from publishing the de-identified data later on. See the “Permission to publish” section of our data publication resource for sample consent language that permits subsequent data publication.
- Deception and debriefing: The IRB will need a good explanation if you need to withhold information from subjects or deceive them. Some journal referees have rejected papers that they perceive to contain unnecessary deception. If you do use deception, the IRB will likely require that you debrief subjects afterwards and explain the true study purpose or procedures to them. A good rule of thumb is that the subjects will need enough information about the risks, costs, and benefits so that they can make an informed decision if they want to participate; everything else will require special reasons and/or a debriefing. 4
- Are you working with vulnerable populations (children, prisoners, pregnant, mentally disabled, or developmentally challenged individuals), and if so, why?
- Subject numbers: you cannot use more subjects than you originally planned to enroll unless you submit an amendment. If you are not sure of the final subject numbers, provide an upper bound estimate. There is no penalty for surveying fewer subjects than stated, but IRBs do not like to see approved subjects exceeded.
- For an RCT, describe the selection method for treatment and control groups.
- IRBs will read this very closely and they may come up with risks that you feel are remote. You may be required to safeguard in some way against such identified risks.
- Whatever risks and benefits you identify you need to mention in your consent form (see below and in our Define consent and intake process resource ).
Some additional hints, based on experience:
- There may be elements of your study that change later. If these changes are not substantial, you should write your protocol to allow for them . For example, you may want to adjust the amounts paid to subjects, change your estimate of how long it takes to conduct a survey, or rephrase some survey questions. If you get a reasonable range of options for these fields approved in your initial application then making the needed adjustments will be easy. For changes not written into the protocol always submit an amendment (and wait for approval) before implementing them in the field, even changes as small as the contact information on a consent form.
- Similarly, if you plan to choose between two different procedures , say, based on piloting results, you may request approval for both of them , and explain that you will choose one (and how). This can save you time later on.
- Note that even if you do not identify any risks , IRBs may still require you to mention risks and benefits in the consent form, e.g., by saying "there are no risks or benefits to you from this study." There is no need to try and bend over backwards to convince yourself, the IRB, or the subjects that there is absolutely zero risk. Depending on your study population and context, this can make subjects actually more suspicious or at least confused: if there are no risks, and so far the study sounded completely harmless, why is this explicitly mentioned? Thus, on balance it is actually not necessarily going to reduce participation or harm your relationship with the subjects if you mention small risks. It can help the subjects see that you thought carefully about this but that these are the only risks you came up with.
Procedure for informed consent
Normally, written, signed consent is required from all study subjects.
Consent procedures for research involving minors are more complicated, so you should check with your IRB on the specifics. Depending on the age of the child (typically seven or older) children have to give assent to study procedures that involve them, though the method of obtaining assent will vary depending on age. In addition, parent(s) have to consent to research involving their children. If the study involves more risk, both parents' consent may be required. MIT requires that research involving minors have written informed consent of a parent as well as the suitably documented assent of the minor, if the child is over seven years old.
The revised Common Rule includes a new "Key Elements" section and a rearrangement of content that is designed to facilitate a potential subject's decision of wheter to participate. Under the revised 2018 Common Rule, the requirements for informed consent have changed, with the addition of:
- "Key information" to be presented at the beginning of the consent form
- New consent elements
- Changes to waiver criteria and documentation (plus other process changes)
- A "broad consent" option for unspecified future use of identifiable data/bio-specimens
There are exceptions to the need for written consent . These may be important if the written consent procedure deters or prevents subjects from participating in the research. Examples could be subjects who cannot read, who do not want to be linked with their study answers, or who are in a rush and do not want to sign unfamiliar documents before studying them carefully.
Some of the alternatives available for such cases are:
- Oral consent with a witness (witness signature replaces subject signature)
- Waivers of written consent (replaced by an oral procedure), especially in the case of minimal risk
- Consent from only one or none of the child's parents (e.g., unavailable parents or risks from involving the parents, e.g., in cases of abuse)
- Waivers of assent (e.g., if the child is too young or incapable of assenting)
See Human Subjects Decision Chart 13 : “When Can Informed Consent Be Waived or Altered Under 45 CFR 46.116(f)?” for more details.
In our experience, for studies involving young students, where getting every parent to consent would be impossible, typically the head of school consents for all students. Examples of such scenarios include boarding schools or when it is likely parents will not all read or return the forms and when the study consists of non-invasive research such as math games conducted on school time. For high school students involved in educational interventions, both students and the head of school (or parent) provide legal binding consent.
An important provision of US law is a so-called Certificate of Confidentiality, which prevents law enforcement from accessing research data by subpoena for the purpose of criminal persecution. 5 The National Institutes of Health offers helpful resources on what a certificate of confidentiality is and answers some frequently asked questions about how it works. The law on certificates of confidentiality was originally conceived to allow research on illegal drug use by Vietnam veterans without compromising subjects or deterring them from participating. These certificates can help a researcher protect their subjects and assure subjects of the confidentiality of their responses. For some background, see for example Wolf et al. (2015, 76 pages) .
More details on informed consent are covered in J-PAL's Define intake and consent process resource.
Consent Forms
MIT has useful guidelines on providing and communicating adequate information and ensuring comprehension when obtaining informed consent. In particular, the language researchers use in their consent forms should be simple and clear. They include the following:
- Wording should be understandable to those with an 8th grade reading level.
- Avoid technical language or jargon.
- Use commonly recognizable terms.
- Explain terms that may not be easily understood.
- When minors are involved, use age-appropriate language.
- Note that one needs explicit permission from the IRB to obtain verbal consent, in place of the usual written consent, even if the survey is conducted over the phone. See Transitioning to CATI: Checklist and Resources for additional adaptations that should be made for phone surveys
Researchers should at a minimum include the following in their consent forms, even if an oral consent:
- A brief explanation of the purpose of the study
- That subjects can stop at any time or choose not to answer any questions they want
- A local contact and phone number/email for questions.
Subjects should get a copy of the consent form with this information. They should also receive a link to the local university's IRB office in case they have concerns they do not want to voice to the study researchers, e.g., “If you feel you have been treated unfairly, or you have questions regarding your rights as a research subject, you may contact the Chairman of the Committee on the Use of Humans as Experimental Subjects, MIT, Room E25-143b, 77 Massachusetts Ave, Cambridge, MA 02139, phone +1-617-253-6787."
MIT has consent templates on their website that researchers can use. The consent templates have also undergone changes with the Common Rule revisions. Check with your IRB with questions about modifying a consent template provided by MIT or another institution.
If you plan to collect personal data from participants residing in the European Union you should include additional language in your consent forms, in accordance with the European Union's General Data Protection Regulation ( GDPR). This should include information on how long you will retain subjects' information, whether their personal information will be transferred to the US (which may not offer the same level of protection as the EU), etc.
You should review the Annotated informed consent checklist , based on the OHRP checklist , to make sure you are including all elements of informed consent in your form. The checklist document contains information on:
- The various exceptions to written consent above,
- Elements of a written consent statement, and
- Sample language that makes sure J-PAL data management and data publication requirements are met and the study data can be published in de-identified form .
Broad Consent
The Revised Common Rule allows "Broad Consent" in lieu of informed consent. Broad consent is obtained only with respect to storage, maintenance, and secondary research use of individuals' identifiable data without obtaining additional consent. You are not required to obtain informed consent through broad consent; this is simply an option that is now available to researchers.
Broad consent under Common Rule has several mandated elements, including:
- A general description of the types of research that may be conducted with the identifiable private information.
- Whether sharing of identifiable private information might occur.
- Types of institutions or researchers that might conduct research with the identifiable private information.
- Description of the period of time that the identifiable private information may be stored and maintained and description of period of time that they may be used for research.
You can find the complete list of mandatory elements to broad consent on the HHS website . If an individual refuses to provide broad consent, the researcher can choose to de-identify the subjects' data to conduct further research. Your IRB can provide guidance on institutional policies regarding refusals, including on re-requesting broad consent or obtaining informed consent.
Not all IRBs have implemented use of Broad Consent. Be sure to check with your IRB to understand whether this option is available for your research. Implementing Broad Consent requires careful thought about the language of the consent form. When the IRB evaluates secondary research proposals, it must determine that the proposed secondary research is within the scope of the identified types of research in the original consent form.
Responding to common concerns of IRBs
Your IRB may ask you for clarification or flag things in your research protocols as problematic that may seem surprising to you. It is useful to know ahead of time what type of designs and issues can raise concerns or are particularly important to IRBs. Conversely, there are research design considerations you should keep in mind that may help you overcome some common concerns an IRB might have.
- Who is conducting the intervention? Recall the principle above: it is an injustice if "some benefit to which a person is entitled is denied without good reason or when some burden is imposed unduly." Researchers are not allowed to commit injustices in their research. You are still allowed to study programs, policies, or events that constitute an injustice by this definition. If an NGO carries out a questionable program (say, re-education camps for transgender youth) you can study the effects of these programs (and it may be a great service to humanity if you do). However you cannot run such a re-education camp, or help the NGO run it, or even select subjects from a potential pool for them. That would be unethical because there is good reason to think that these re-education efforts harm the children who are subjected to them. The more involved you are in the design, administration, targeting, or any other aspect of the intervention you are studying, the more the intervention itself falls under the scrutiny of the IRB and under your obligation as a researcher.
- The IRB will be particularly concerned if you get involved in a study of a benefit that people are legally entitled to receive . Note the phrasing "some benefit to which a person is entitled is denied." If part of your research plan is to suggest to the government to withhold an official program for research purposes, you will probably (rightfully) not be allowed to do that by your IRB. As another example, say there is a policy that could be harmful or unpleasant to some people. For example, suppose the government is choosing some households for tax audits. It is possible that the IRB will be concerned if you as the researcher get involved in the selection process (for example, by proposing a randomization strategy to pick households for auditing). It can help to make the argument very explicit that the government would have audited someone either way, and that without the randomization it may just have been a different, arbitrarily chosen person.
- Related to the argument above, IRBs are typically much more concerned if there is potential for undue burden or harm to the study participants or subjects, as opposed to potential benefits. Helping the government to randomly assign a benefit is often an easier case to make than helping to randomly assign a burden. This means, for example, if you are studying a demand elasticity and you want to vary prices, it will be looked upon more kindly by the IRB if you reduce prices (i.e., provide subsidies) to a random subset of people rather than increase prices.
- You may not always agree with the concerns that are raised by the IRB — take the example of the audit study. You may think, for example, that it is not unethical to audit people based on random selection rather than let the government choose by some other criteria that may actually be more questionable. If you truly think so, you should make your case to the IRB. In the author's experience, a request by the IRB for clarification is sometimes just that: there is not necessarily an issue, they just want clarity. Therefore, do not back away from protocol details that are important to your study unless you find that the IRB's concerns about the research design are justified. You may have to be prepared to explain your procedures at length. However, if you truly think that what you are doing is (a) ethical and (b) important research, do not self-censor in order to take the path of least resistance.
- IRBs can be particularly sensitive if the cultural context is unfamiliar to them or they feel that research interferes with traditions or deeply-held beliefs of subjects . Your IRB may, for example, worry about a special burden on women from conducting research on things like sexually transmitted diseases, gender empowerment, or fertility in a very patriarchal or religiously conservative society. In those cases, the IRB may consult a cultural advisor or require special protections for subjects. It often helps to demonstrate a deep understanding of the cultural context of your study and to have local researchers on the research team.
- Physical contact as part of your research is almost always subject to special scrutiny. For example, if you interview mothers about the health of their child, the child's assent is typically not required, but if you measure the kid's weight or even take their blood for a malaria test, not only do you need assent, you need the consent of one or both parents (and typically biomedical clearance). Relatedly, as soon as any body fluids are involved in your research procedures, biomedical IRBs will get involved. You need to think about things like how to safely dispose of any contaminated waste products. Note that some IRBs may go a bit overboard, and require for example that your medical testing is done by trained nurses, even if the procedures are designed to be safely carried out by lay persons (like a rapid detection test for malaria). In those cases, it is again sometimes worth pushing back and making your argument that it is not unethical to instead train your surveyors to carry out these procedures.
- Inclusiveness and screening : even after asking you a lot of questions about the potential harm that your study might do to subjects, some IRBs will then turn around and ask if your procedures are not unfairly excluding some people from the research. This is the flip side of fairness; if you are not sure that the research is harmful or beneficial, preventing someone from participating, say, based on their physical condition, age, gender, or race, or based on literacy or education, can be considered unjust. If you screen out some subjects from your study procedures make sure to carefully explain why.
- The general consensus within the ethical community is that payments are not “coercive” if the study passes the bar of risk to potential benefit (to an individual or society) assessment (with the payments not in the “benefit” side of the calculation).
- The concern over payments is particularly strong when it involves vulnerable populations. For example, IRBs typically do not allow you to pay mothers for the enrollment of their children in a study . Rather, you can reward study participation by giving a gift to the child. This is in order to prevent mothers from silencing their concerns over the study and agreeing to their child's participation in order to collect the payment.
- The IRB may also ask that the payment is not emphasized in the recruitment materials, and you cannot call payments a "benefit" of the study in the consent form.
- Both sides of this argument have some truth to them, and you should carefully weigh the costs and benefits of each approach. One way of dealing with the problem may be to not mention the full amount you are paying when you ask subjects if they agree to participate (to avoid coercion) but still pay an amount that fully compensates them for their time later (to avoid exploitation). This could be done by paying a baseline participation reward which everyone receives who "shows up" (essentially a token of appreciation for hearing you out, even if the subject later withdraws from the study or does not complete the survey). In addition, you can then pay an hourly fee at the end of a completed survey.
- A good approach may also be to first get approval from a local IRB and use their agreement when submitting the protocol to the US IRB, because they will often defer to what is considered appropriate by local ethics committees.
Handling IRB violations, unanticipated problems, and adverse events
After your study starts, you will need to remain aware of potential changes or issues with the study that require reporting and/or other processes. Researchers should ensure that there is a good communication system between field staff and PIs so that any changes or events are communicated to the PIs to then report to IRB.
It is the researchers' obligation to report the following to the IRB that approved the study:
- Violations of the IRB-approved study protocols.
- "Unanticipated problems" with the research.
Protocol violations or incidents
Protocol violations are any unapproved changes, deviations or departures from the study design or procedures of a research project that are under the investigator’s control and that have not been reviewed and approved by the IRB. Protocol violations are divided into two categories: major (reportable) or minor (non-reportable).
Changes to the study, such as to study team members, sample size, survey instrument, consent forms, etc. must be reviewed and approved by the IRB before implementation. Any changes in the research study design and/or procedures that are both within the investigator’s control and implemented prior to IRB approval, must be reported as protocol violations. Such changes concern the IRB because they may unintentionally affect the participant’s rights, safety, or well-being, or the completeness, accuracy, and reliability of the study data.
If the changes in the research study design and/or procedures do not have a major impact on either the participants' rights, safety or well-being, or the completeness, accuracy and reliability of the study data you do not need to report them to the IRB but should document them in the study files.
Any problematic or unanticipated events involving the conduct of the study or participant participation also need to be reported to the IRB. This could include, for example, breaches of study participants' privacy or confidentiality (see below), non-compliance, and changes to measures to mitigate risks to participants.
Data security and personally identifiable information
Potential breaches of privacy or confidentiality of study participants’ data are “major (reportable) incidents” that must be submitted to the IRB. The PI is responsible for ensuring that research data is secure when it is collected, stored, transmitted, or shared. All members of the research team should receive appropriate training about securing and safeguarding research data.
Note that IRB protocol violations often involve the exposure of confidential data, accidental or not (such as losing tablets, computers or cameras which have subjects’ data – whether or not it is encrypted). When personal or identifiable data is exposed, the IRB needs to make a determination on what risk of harm this exposure inflicts on the subjects, and what mitigating measures the research team needs to undertake in order to address these risks. They might decide, for example, that the researchers need to contact the study subjects to inform them of the breached data, or that additional psychological or legal assistance must be offered to subjects to deal with any consequences of the data breach. The IRB may also decide that data collected in violation of the study protocols or without full informed consent from the subjects cannot be used in research, though this is an extreme outcome that only rarely occurs. In most cases, the risk of harm is low, and the IRB will only request to halt data collection and amend the study protocols to ensure compliance with the original IRB proposal.
Unanticipated problems and adverse events
You must also report any unanticipated problem involving potential risk to participants or others. An unexpected, research-related event is one where the risk exceeds the nature, severity, or frequency described in the protocol, study consent form or other study information previously reviewed and approved by the IRB. OHRP gives further guidance on "unanticipated problems," including how and when researchers should report the problems to the IRB. Unanticipated problems that are serious adverse events should be reported to the IRB within one week of the investigator becoming aware of the event, while other unanticipated problems should be reported within two weeks. Adverse events encompass both physical and psychological harms. They occur most commonly in the context of biomedical research but can occasionally occur in the context of social and behavioral research. If an adverse event is an unanticipated problem, then it must be reported to IRB. Some examples of adverse events are listed below.
Emergencies/when do you have to report or stop?
Any time that there is a significant adverse event, the IRB of Record should be contacted immediately to see what needs to be done (e.g., does the study need to stop or do you just need to file an Adverse Event Form?). This is at the IRB’s discretion. Significant adverse events include:
- Non-consented surveying, sampling, or data use
- Participant deaths
- Work in dangerous situations
- Breaches by staff
For example, an unexpected death is a serious adverse event and must be reported to the IRB. Typically, unexpected deaths must be reported within 48 hours, although it is best to report as soon as possible. An unexpected death is defined as the death of a participant that was not related to a risk of participation listed in the protocol or consent document, and was more likely than not caused by the research procedures and/or study interventions.
Depending on the circumstances, the IRB may need to take immediate action to minimize further harm to participants, such as halting the enrollment of additional participants or suspending approval of the research.
Continuing review
The IRB is required to review and approve all non-exempt research projects at intervals appropriate to the degree of risk, but not less than once per year. This is called "continuing review."
Under the revised Common Rule, continuing review is not required for:
- Research that is eligible for expedited review,
- Exempt research conditioned on limited IRB review,
- Research that has completed all interventions and now only includes analyzing data, even if the information or biospecimens are identifiable,
- Research that has completed all interventions and now only includes accessing follow-up clinical data from clinical care procedures.
Under the new Common Rule, for the MIT IRB new protocols approved under expedited review (not going to the Board meeting) no longer require yearly renewals unless otherwise determined by COUHES. Instead, investigators must submit an annual progress report (very minor) and a Continuing Review Questionnaire every three years. Previously approved studies still need to be reviewed annually. MIT also requires annual review until the project is completely done with data analysis.
For continuing review, researchers must give a report to their IRB before the approval expires. MIT requires researchers to submit a Continuing Review Questionnaire, updating the IRB on the progress of their research, 60 days before the IRB approval expiration date. The form includes information on activities conducted in the past year, subject recruitment and attrition, adverse events, subject complaints and publications. If researchers do not submit the form by the required date, the study will be automatically terminated, research grants related to the study will be suspended, and no further research with human subjects can be conducted under the protocol.
The IRB makes its determination on whether to re-approve research based on whether there is any new information, especially with respect to potential benefits or risks to the subjects, that could alter its decision. Such information may also require the IRB to update its risk assessment for the protocol and its corresponding review category, so be aware that this is a possibility when budgeting the review time for your study's continuing review. The IRB also assesses whether there is any new information that would necessitate revision of the protocol and/or the informed consent document.
For more information, see Human Subjects Decision Chart 11 : “Is Continuing Review Required Under 45 CFR 46.109(f)?”
Reporting and publishing
Publishing: Researchers should report once a study has been published as a working paper or other publication in their yearly review form. Often IRB protocols are kept open for years after field work is complete, as multiple papers may come out of one study. MIT’s IRB allows you to close a study once “the study is closed to enrollment; all subjects have completed all study procedures; data collection is complete; AND data analysis is complete.” Researchers should report when de-identified data is made public in their IRB Closure.
Trial registry: Researchers should include their IRB information when registering their trial with the AEA RCT Registry . Specifically, they should state whether they received IRB approval (mandatory), the IRB name, approval date, and approval number.
For J-PAL projects: All researchers conducting research projects funded or implemented by J-PAL should submit IRB certificates and consent forms to J-PAL.
Last updated March 2023.
These resources are a collaborative effort. If you notice a bug or have a suggestion for additional content, please fill out this form .
We are grateful to Jack Cavanagh, Sarah Kopper, Jim Shen, Keesler Welch, and Evan Williams for review and copy-editing of this resource. Any errors are our own.
CITI Program, " Social-Behavioral-Educational (SBE) Refresher 1 " (last accessed September 2019).
Department of Health and Human Services, Office of Human Research Protections, 2017, " Institutional Review Board (IRB) Authorization Agreement " (last accessed Dec 2017). A sample IAA.
Murphy, Kathleen. 2020. “ Collaborating with the Institutional Review Board (IRB). ” In: Cole, Dhaliwal, Sautmann, and Vilhuber (eds), Handbook on Using Administrative Data for Research and Evidence-based Policy. Accessed at https://admindatahandbook.mit.edu/book/latest/irb.html on 2021-03-18.
O’Hara, Amy. 2020. “ Model Data Use Agreements: A Practical Guide. ” In: Cole, Dhaliwal, Sautmann, and Vilhuber (eds), Handbook on Using Administrative Data for Research and Evidence-based Policy. Accessed at https://admindatahandbook.mit.edu/book/latest/dua.html on 2021-03-18.
Staats, Alyssa, Kristianna Post, Lindsey Shaughnessy, and Anita Desai, 2019. " Human Subjects and IPA IRB. " Delivered at 2019 J-PAL/IPA Global Staff Training. An overview of the IRB process and how it applies to IPA/J-PAL projects.
Department of Health and Human Services, Office of Human Research Protections, 2019, " The Revised Common Rule Compliance Dates and Transition Provision (45 CFR 46.101(l)) " (last accessed September 2019).
Department of Health and Human Services, Office of Human Research Protections, 2018a, " Electronic Code of Federal Regulations " (last accessed September 2019).
Department of Health and Human Services, Office of Human Research Protections, 2018b, " Table 1: Social-Behavioral Research Standards: Description " (last accessed September 2019).
Department of Health and Human Services, Office of Human Research Protections, 2017, " OHRP Expedited Review Categories " (last accessed Dec 2017).
Department of Health and Human Services, Office of Human Research Protections, 2009, " Pre-2018 Requirements " (last accessed September 2019).
Department of Health and Human Services, Office of Human Research Protections, 2007, " Unanticipated Problems involving Risks & Adverse Events Guidance " (last accessed September 2019).
Glennerster, Rachel and Shawn Powers, 2016. " Balancing Risk and Benefit: Ethical Tradeoffs in Running Randomized Evaluations ". In: George DeMartino and Deirdre McCloskey, Oxford Handbook of Professional Economic Ethics, Oxford University Press.
MIT Committee on the Use of Human Subjects website, " Forms and Templates ," (last accessed January 18, 2019).
National Institutes of Health (NIH). " Certificates of Confidentiality: FAQs " (last accessed September 2019).
National Institutes of Health (NIH). " What is a Certificate of Confidentiality? " (last accessed September 2019).
Wolf, Leslie E., Patel, Mayank J., Williams Tarver, Brett A., Austin, Jeffrey L., Dame, Lauren A., and Laura M. Beskow, 2015, "Certificates of confidentiality: protecting human subjects research data in law and in practice" , Minn J Law Sci Technol. 14(1): 11–87.
In this resource
irb.richmond.edu web results only
Web Results
Directory results.
- Current Students
- Staff & Faculty
- Parents & Families
- Exchange email
- UR Talent Web
Info for...
www.richmond.edu web results only
Examples of Full Proposals
Proposals using the "full proposal" form.
Experiments in Virtual Reality (Crawford, Twedt, et al)
The full research proposal submission, Experiments in Virtual Reality , consists of four components: (1) the completed full proposal form , (2) the proposal consent form , (3) a debriefing form to be used with subjects after their participation in the study, and (4) recruiting materials which announce the study and solicit participants.
Mindsets Matter: An interdisciplinary approach for increasing female involvement and acheivement in STEM: Renewal (Burnette, Hoyt, Lawson et al)
The full research proposal submission, Mindsets Matter , consists of two components: (1) the full proposal form , which also includes information needed by the IRB for the renewal of the proposal, and (2) additional materials , which include the consent form, instruments to be used in the research, and other materials.
"Unique" Proposals That do not Fit the Full Proposal Form
Examples of approved unique full proposals from past years follow. Note that some of these proposals may not include all of the information currently required by the IRB.
Example of Unique Faculty Psychology Proposal .
Example of Unique Student Geography Proposal .
In some cases, a professor may want to submit a proposal that incorporates various components of separate student surveys or interviews. An example of such a classroom proposal can be downloaded HERE .
- Skip to content
- Skip to primary sidebar
- Skip to main content
- Request Info
- Search Search Site Faculty/Staff
- Open Navigation Menu Menu Close Navigation Menu
- IRB Review Process
III. IRB Review Process
To apply for IRB approval, the principal investigator must submit an application to the IRB. There are two types of application that may be submitted based on the categories of research determined by state and federal codes. Type 1 : If a researcher believes her/his project meets the requirements for exemption, s/he may submit an application for exemption. The Chair or designee of the IRB will review the application to make sure that it meets the criteria for exemption. Type 2 : If a project does not meet the criteria for exemption, an application for Expedited or Full Board Review must be submitted.
The Chair or designee makes one of three decisions regarding applications submitted for IRB review: (1) Approval, (2) Provisional Approval (pending required changes or the receipt of clarifying information or rationales, as specified on the review form), or (3) Hold for Committee. When the decision is made to refer the application to the full IRB, the Chair or designee gives the reason(s) on the review form.
A. Exempted from Review
Determinations regarding exemption from IRB review are made by the Chair or designee and not by investigators. Investigators can apply for an exemption by completing an exemption form. In making determinations on exemptions, the Chair or designee evaluates whether the narrative description of the research is consistent with the categories for exemption contained in federal and state regulations.
Exemptions are in force for a maximum of five years after approval. To continue research after that time period, investigators must apply for another exemption or complete a regular IRB application.
1. Exempt Categories
Category a. The research must be conducted in a commonly accepted educational setting (e.g., classroom, conference facility) as opposed to a laboratory or similar setting and involve commonly accepted educational practices (e.g., forms of instructional methods) as opposed to psychological manipulations or non-instructional interventions.
Category b . The research (educational tests, surveys, interviews, observations of public behavior) is conducted anonymously (names and identifying information are not recorded) and involves the collection of benign information that could not be damaging to a person. “Public behavior” is defined as behavior in places that any person can visit without special permission (e.g., street corners, parks, restaurants, theaters) or Internet communications in public forums. Observations of the public behavior of children do not qualify for exemption if the investigator interacts with them.
Category c. The research (educational tests, surveys, interviews, or observations of public behavior) is conducted among elected or appointed public officials or candidates for office.
Category d. The research involves the study of data (in existence prior to the research) that are publicly available or that are recorded in a manner in which they cannot be linked with individuals (e.g., there is not a master list of subjects that links participants to identification numbers).
Category e. The research to be conducted has been approved by federal departments or agency heads and determined to be exempt by those departments and agencies. To qualify for exemption under this category, investigators would be required to provide the IRB with appropriate documentation from the relevant federal departments or agencies.
Category f. Research involving food (taste, quality, or consumer acceptance) which is wholesome and contains no additives or foods with ingredients (type and amount) that meet federal safety standards.
2. Responsibilities of Investigators Conducting Exempt Research
Since exempted studies are still considered human subjects research, investigators are expected to adhere to ethical standards for the conduct of research. Specifically, investigators are expected to obtain Informed Consent Releases (when appropriate, given the nature of the research), take all necessary steps to minimize risks and maximize research benefits, and ensure the equitable selection of participants (when warranted). Investigators of exempt studies may not make substantive changes in the design of their studies, including changes involving investigators, research instruments and procedures, the location of their research, and sources of participants or data, without written approval by the IRB before any such changes are made. Changes must be requested in writing.
B. Expedited Review
Research activities that present no more than minimal risk may be eligible for expedited IRB approval. Determinations regarding expedited IRB approval are made by the Chair or his/her designee. The Chair or designee may seek the advice of another IRB member or consultant who has specific expertise in the area of research described in the application.
For purposes of Expedited Review, minimal risk refers to the risks of everyday life (e.g., providing information to others about one’s attitudes and other non-confidential issues) or those associated with routine medical and psychological tests. The expedited review procedure may not be used where identification of participants or their responses would reasonably place them at risk of criminal or civil liability or be damaging to their financial standing, employability, insurability, reputation, or be stigmatizing unless reasonable protections will be implemented to minimize these risks. Expedited approval may not be given for classified research.
1. Overview of Expedited Research
Research activities that (1) present no more than minimal risk to human participants and, (2) involve only procedures listed in one or more of the following seven categories may be reviewed by the IRB through the expedited review procedure. The activities listed are not deemed to be of minimal risk simply because they are included on this list; rather, inclusion on this list means that the activity is eligible for expedited review because the specific circumstances of the proposed research are seen to involve no more than minimal risk to human participants.
Categories 1 – 7 below apply regardless of the age of participants, except as noted. These seven categories pertain to both initial and continuing IRB review.
Standard requirements for informed consent (or its waiver, alteration, or exception) apply regardless of the type of review–Expedited or Full Board–utilized by the IRB.
2. Expedited Review Categories
Category 1. Clinical studies of drugs and medical devices only when condition (a) or (b) is met. (a) Research on drugs for which an investigational new drug application (21 CFR Part 312) is not required. ( Note : Research on marketed drugs that significantly increases the risks or decreases the acceptability of the risks associated with the use of the product is not eligible for Expedited Review.) (b) Research on medical devices for which (i) an investigational device exemption application (21 CFR Part 812) is not required; or (ii) the medical device is cleared/approved for marketing and the medical device is being used in accordance with its cleared/approved labeling.
Category 2. Collection of blood samples by finger stick, heel stick, ear stick, or venipuncture as follows: (a) from healthy, nonpregnant adults who weigh at least 110 pounds. For these participants, the amounts drawn may not exceed 550 ml in an eight (8) week period and collection may not occur more frequently than two (2) times per week; or (b) from other adults and children, considering the age, weight, and health of the participants, the collection procedure, the amount of blood to be collected, and the frequency with which it will be collected. For these participants, the amount drawn may not exceed the lesser of 50 ml or three (3) ml per kg in an eight (8) week period and collection may not occur more frequently than two (2) times per week.
Category 3. Prospective collection of biological specimens for research purposes by noninvasive means. Examples: (a) hair and nail clippings in a nondisfiguring manner; (b) deciduous teeth at time of exfoliation or if routine patient care indicates a need for extraction; (c) permanent teeth if routine patient care indicates a need for extraction; (d) excreta and external secretions (including sweat); (e) uncannulated saliva collected either in an unstimulated fashion or stimulated by chewing gumbase or wax or by applying a dilute citric solution to the tongue; (f) placenta removed at delivery; (g) amniotic fluid obtained at the time of rupture of the membrane prior to or during labor; (h) supra- and subgingival dental plaque and calculus, provided the collection procedure is not more invasive than routine prophylactic scaling of the teeth and the process is accomplished in accordance with accepted prophylactic techniques; (i) mucosal and skin cells collected by buccal scraping or swab, skin swab, or mouth washings; (j) sputum collected after saline mist nebulization.
Category 4. Collection of data through noninvasive procedures (not involving general anesthesia or sedation) routinely employed in clinical practice, excluding procedures involving x-rays or microwaves. Where medical devices are employed, they must be cleared/approved for marketing. (Studies intended to evaluate the safety and effectiveness of the medical device are not generally eligible for expedited review, including studies of cleared medical devices for new indications.) Examples : (a) physical sensors that are applied either to the surface of the body or at a distance and do not involve input of significant amounts of energy into the particpant or an invasion of the participant’s privacy; (b) determining body weight or testing sensory acuity; (c) magnetic resonance imaging; (d) electrocardiography, electroencephalography, thermography, detection of naturally occurring radioactivity, electroretinography, ultrasound, diagnostic infrared imaging, doppler blood flow, and echocardiography; (e) moderate exercise, muscular strength testing, body composition assessment, and flexibility testing where appropriate given the age, weight, and health of the individual.
Category 5. Research involving materials (data, documents, records, or specimens) that have been collected, or will be collected solely for nonresearch purposes (such as medical treatment or diagnosis).
Category 6. Collection of data from voice, video, digital, or image recordings made for research purposes.
Category 7. Research on individual or group characteristics or behavior (including, but not limited to, research on perception, cognition, motivation, identity, language, communication, cultural beliefs or practices, and social behavior) or research employing survey, interview, oral history, focus group, program evaluation, human factors evaluation, or quality assurance methodologies.
C. Full Board Review
Human research not eligible for Exemption or Expedited Review is evaluated by the IRB at a convened meeting. A majority of the IRB members, including at least one member whose primary concerns are not scientific, must be present to conduct a convened meeting. For research to be approved, it must receive the approval of a majority of those members present at the meeting where a quorum is in attendance.
Convened meetings are scheduled at least once a semester. The deadline for submission of materials for full IRB review is 10 workdays prior to the scheduled meeting. IRB members are sent applications and other materials for review immediately after the deadline for submission. Members are expected to review all materials prior to the meeting.
IRB meetings are conducted by the Chair or designee. The Chair presents an initial review of the research proposal, indicating preliminary questions or concerns, if any, about the proposal followed by a discussion among all IRB members present. In its review, the IRB considers the following review criteria: (1) adequacy and completeness of the application, (2) research design, (3) participant selection, (4) risks and benefits, (5) minimization of risks, (6) privacy and confidentiality, (7) additional safeguards for vulnerable participants, (8) consent, and (9) additional considerations. Whenever possible, the IRB attempts to reach consensus on reviewed research. IRB decisions are recorded as unanimous when consensus has been achieved. When members are unable to reach consensus on a decision, the decision is made by majority vote, with members voting for approval or disapproval (or abstaining), or required changes, as identified in the minutes.
The IRB makes one of four decisions on reviewed research: (1) Approval, (2) Provisional Approval (the IRB authorizes the Chair or another member to review and approve required revisions), (3) Postponed (tabled, pending further information), or (4) Withheld (disapproved). In a case where the research proposal is not fatally flawed or devoid of merit, but might be able to be strengthened, the IRB may provide recommendations or suggestions to investigators to improve their research design or methodology.
The Chair may permit persons not affiliated with the IRB (e.g., consultants) to attend meetings, upon request. Investigators or their collaborators are not permitted to be present at IRB meetings during actual deliberations on their research proposals. However as needed, the IRB may decide to invite investigators to the meeting to answer questions about their proposed research.
- Institutional Review Board
- IRB Reviewers by Department
- Meeting Dates
- IRB Administration at UMW
- Instructions and Forms
- IRB Training
- Public Lectures
- Faculty & Staff Site >>
Review of Graduate Student Research by the Institutional Review Board (IRB)
What is the irb.
The IRB is a committee of scientists, non-scientists and community members. At the UW and other universities, the IRB reviews research proposals to protect the rights and welfare of human research subjects who participate in research activities conducted under the auspices of the University.
When is IRB review required?
If the proposed study meets the federal definition of research…
“A systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge.”
If the proposed study involves “human subjects,” defined as “a living individual about whom an investigator (whether professional or student) conducting research obtains: 1) data through intervention or interaction with the individual; or 2) identifiable private information.”
Why is IRB review necessary?
IRB reviews help ensure the safety and protection of research subjects, as well as the ethical conduct of research that involves human subjects.
The IRB review must determine that all of the following requirements are satisfied.
- Risks to subjects are minimized.
- Risks to subjects are reasonable in relation to anticipated benefits.
- Selection of subjects is equitable.
- Informed consent will be sought from each prospective subject or the subject’s legally authorized representative.
- Informed consent will be appropriately documented, in accordance with, and to the extent required by HSS regulation 46.117.
- The research plan makes adequate provision for the monitoring of data collected to ensure the safety of subjects.
- There are adequate provisions to protect the privacy of subjects and to maintain the confidentiality of data. When some or all of the subjects are likely to be vulnerable to coercion or undue influence, such as children, prisoners, pregnant women, mentally disabled persons or economically disadvantaged persons, additional safeguards have been included in the study to protect the rights and welfare of these subjects.
How does the IRB evaluate research proposals?
The IRB reviews and responds to proposals at three levels, depending on the type of research proposed.
Exempt: Six categories of research involving human subjects qualify for exemption from federal regulations governing the protection of human subjects. A determination of eligibility for exemption must be made by the IRB or its designee. Exempt research must also comply with state laws, UW policy, and conform to sound research ethics/principles.
Expedited (minimal risk): An “expedited” review procedure can be used when research has been determined to be of “minimal risk” to subjects (i.e., “poses no more risk to subjects than would be encountered by the average person in his/her daily activities”) and involves only the procedures listed in the federally described categories of expedited review. All federal, state and local regulations must be taken into consideration. The standard requirements for informed consent (or its waiver or alteration) apply.
Expedited reviews may be carried out by the IRB chair, an IRB co-chair, or by one or more experienced reviewers designated by the chair from among members of the IRB.
Full IRB review: Research that does not qualify as exempt or for expedited review must undergo a full review by a quorum of IRB members. The application process is the same as for expedited review; however, it is recommended that researchers allow two to four months from the time of submission until approval. Researchers should also be aware that the initial full review process frequently does not result in an outright approval of the research; minor or major revisions and written clarifications are often requested.
How can I get help?
The Human Subjects Division (HSD) website is designed to help you decide whether or not you need review and has detailed instructions on how to apply.
If you have questions or need additional assistance, you can contact HSD directly .
This Mentor Memo was developed in collaboration with the Human Subjects Division.
The Three Types of IRB Review
IRB must review all projects that meet the definition of research and that involve human subjects prior to any data collection to determine the appropriate level of review, and, as appropriate, approve them. There are three major types of review: Exempt, Expedited, and Full.
Exempt Review
Studies that receive an exemption determination from IRB are exempt from the specific regulations and requirements in Title 45, Part 46 of the Code of Federal Regulations. Please note, however, that they are still considered human subject research.
If the proposed research involves no greater than minimal risk to participants and involves any of the following, it may qualify for exempt status in accordance with the revised Common Rule (effective January 21, 2019):
- Research, conducted in established or commonly accepted educational settings that specifically involves normal educational practices that are not likely to adversely impact students’ opportunity to learn required educational content or the assessment of educators who provide instruction. This includes most research on regular and special education instructional strategies, and research on the effectiveness of or the comparison among instructional techniques, curricula, or classroom management methods.
- The information obtained is recorded by the investigator in such a manner that the identity of the human subjects cannot readily be ascertained, directly or through identifiers linked to the subjects;
- Any disclosure of the human subjects’ responses outside the research would not reasonably place the subjects at risk of criminal or civil liability or be damaging to the subjects’ financial standing, employability, educational advancement, or reputation; or
- The information obtained is recorded by the investigator in such a manner that the identity of the human subjects can readily be ascertained, directly or through identifiers linked to the subjects, and an IRB conducts a limited IRB review to make the determination that there are adequate provisions to protect the privacy of subjects and to maintain the confidentiality of data.
- The identifiable private information or identifiable biospecimens are publicly available;
- Information, which may include information about biospecimens, is recorded by the investigator in such a manner that the identity of the human subjects cannot readily be ascertained directly or through identifiers linked to the subjects, the investigator does not contact the subjects, and the investigator will not re-identify subjects;
- The research involves only information collection and analysis involving the investigator’s use of identifiable health information when that use is regulated under HIPAA as “health care operations,” “research” or “public health”; or
- The research is conducted by, or on behalf of, a Federal department or agency using government-generated or government-collected information obtained for nonresearch activities and the information is subject to federal privacy standards and other requirements specified in the exemption [Refer to 45 CFR 46.104(d)(4) of the revised Common Rule]
- Research and demonstration projects that are conducted or supported by a Federal department or agency, or otherwise subject to the approval of department or agency heads (or the approval of the heads of bureaus or other subordinate agencies that have been delegated authority to conduct the research and demonstration projects), and that are designed to study, evaluate, improve, or otherwise examine public benefit or service programs, including procedures for obtaining benefits or services under those programs, possible changes in or alternatives to those programs or procedures, or possible changes in methods or levels of payment for benefits or services under those programs.
- Taste and food quality evaluation and consumer acceptance studies.
- Storage or maintenance of identifiable private information or identifiable biospecimens for potential secondary research use. The exemption can only be used when there is broad consent from the subjects for the storage, maintenance, and secondary research use of their identifiable materials. [Refer to 45 CFR 46.104(d)(7), 46.111(a)(8), and 46.116(d) of the revised Common Rule.]
- Broad consent is obtained from the subjects for the secondary research use of their identifiable materials,
- Documentation or waiver of documentation of informed consent is obtained,
- An IRB conducts a limited review to make certain determinations relating to privacy and confidentiality protections and broad consent, and
- The investigator does not include returning individual research results to subjects as part of the study plan. [Refer to sections 45 CFR 46.104(d)(8), 111(a)(7) and 46.116(d) of the revised Common Rule]
Expedited Review
Studies that involve no more than minimal risk but which do not meet any of the above criteria for exempt status may be eligible for Expedited Review. According to the CFR, “ minimal risk means that the probability and magnitude of harm or discomfort anticipated in the research are not greater in and of themselves than those ordinarily encountered in daily life or during the performance of routine physical or psychological examinations or tests.”
If the proposed research presents no more than minimal risk, does not involve any vulnerable populations (i.e., children, prisoners, individuals with impaired decision-making capacity, and/or economically or educationally disadvantaged persons), and involves any of the following, it may qualify for Expedited Review.
- Collection of data from voice, video, digital, or image recordings made for research purposes.
- Research on individual or group characteristics or behavior (including, but not limited to, research on perception, cognition, motivation, identity, language, communication, cultural beliefs or practices, and social behavior), or research employing survey, interview, oral history, focus group, program evaluation, human factors evaluation, or quality assurance methodologies.
- Research involving materials (data, documents, records, or specimens) that have been collected, or will be collected, solely for non-research purposes (such as for medical treatment or diagnosis).
- Collection of data through noninvasive procedures routinely employed in clinical practice, excluding procedures involving X-rays or microwaves.
- Clinical studies of drugs and medical devices only when condition (a) or (b) is met. (a) research on drugs for which an investigational new drug application (21 CFR Part 312) is not required. (b) research on medical devices for which (i) an investigational device exemption application (21CFR 812) is not required; or (ii) the medical device is cleared/approved for marketing and the medical device is being used in accordance with its cleared/approved labeling.
- Collection of blood samples by finger stick, heel stick, or venipuncture as follows: (a) from healthy, non-pregnant adults who weigh at least 110 pounds; or (b) from other adults and children, considering the age, weight, and health of the subjects, the collection procedure, the amount of blood to be collected, and the frequency with which it will be collected.
- Prospective collection of biological specimens for research purposes by noninvasive means.
- Minor changes to research previously approved by the Lafayette IRB may also qualify for expedited review.
Full Review
If the proposed research does not qualify for Exempt or Expedited Review as defined above, it will be subject to a Full Review. In addition, if the proposed research involves any of the following, it will be subject to Full Review.
- Children under the age of 18
- Individuals with impaired decision-making capacity
- Economically or educationally disadvantaged persons
- Procedures that might cause physical harm.
- Procedures that might cause significant psychological/emotional distress.
- Collection of information about highly sensitive topics.
- Collection of information about illegal behavior.
- Collection of information that could seriously harm the participant legally, socially, financially etc. if other people could identify them.
Protocols requiring Full Review are vetted by the entire IRB and discussed at a convened meeting. Please see our Timing of the IRB Process web page for submission deadlines and meeting dates for Full Review protocols.
If you have questions about what type of review may be appropriate, contact the Chair of IRB at [email protected] prior to submitting a proposal. IRB, however, makes all final determinations of what level of review is required.
OFFICE OF THE VICE PRESIDENT FOR Research and Innovation
Review types and requirements.
The Department of Health and Human Services federal regulations (45 CFR 46) define three types of IRB review. When submitting to the IRB, the VCU investigator makes the initial determination of what type of review a study may qualify for. Upon review of the research, the IRB makes the final determination.
All initial submissions are submitted in the RAMS-IRB electronic system. RAMS-IRB is available here . For guidance on navigating the RAMS-IRB system, see the “RAMS Gif Guides” on our blog , or watch these walkthrough videos , also available on our blog. For general guidance on getting started with an IRB submission, see our “Getting Started” webpage .
See below for information pertinent to specific study types.
Exempt review
The HHS and FDA regulations identify categories of research that are exempt from the regulations, although the ethical principles of study conduct still apply. Even though the research may be exempt, at VCU the determination of exemption must be made by the IRB . Exempt projects are different from expedited or full-board review in that they are not assigned an expiration date, do not have to undergo continuing review and are able to be modified in most cases without IRB approval.
To qualify for exemption, a research study must fall entirely within one or more of the six categories for exemption, and it cannot place subjects at greater than minimal risk. If the research involves prisoners, then it does not qualify for exemption from federal regulations and IRB review.
OHRP decision charts: Does my study qualify for exemption/which exempt category applies?
Category 1 - Education research
Research, conducted in established or commonly accepted educational settings, that specifically involves normal educational practices that are not likely to adversely impact students' opportunity to learn required educational content or the assessment of educators who provide instruction.
This includes most research on regular and special education instructional strategies, and research on the effectiveness of or the comparison among instructional techniques, curricula or classroom management methods.
Notes about involving children:
- Children can be included in this category of research.
- Evaluating a new curriculum or delivery methodology
- An evaluation of a continuing education workshop
Category 2 - Surveys, interviews, educational tests and public observations
Research that only includes interactions involving educational tests (cognitive, diagnostic, aptitude, achievement), survey procedures, interview procedures or observation of public behavior (including visual or auditory recording) if at least one of the following criteria is met:
- Data identifiability : Research data is anonymous (identifiers are never collected), and participants cannot be re-identified.
- Data identifiability : Research data may be identifiable, de-identified (data is linked to identifiers using a study ID) or anonymous (identifiers are never collected).
- Data identifiability : Research data is identifiable or de-identified (data is linked to identifiers using a study ID).
- Only criteria (1) and (2) can include children; criterion (3) cannot involve children.
- Children can be included when research will involve the use of educational tests (cognitive, diagnostic, aptitude or achievement) or when the investigator will observe public behavior but does not participate in that behavior or activity.
- Children cannot be included when research will involve the use of survey procedures, interview procedures or observation of public behavior when investigators will be involved in the activity.
- Conducting a quality of life survey
- Conducting a focus group about an experience or program
- Interviewing physicians about patient management practices
- Administering a numerical aptitude test and a working memory cognitive test to children
- Observing elementary children playground interactions, as long as the investigator has no involvement or does not manipulate the environment in any way
Category 3 - Benign behavioral interventions
Research involving benign behavioral interventions in conjunction with the collection of information from an adult subject through verbal or written responses (including data entry) or audiovisual recording if the subject prospectively agrees to the intervention and information collection and at least one of the following criteria is met:
- Data identifiability : Research data may be identifiable, de-identified (data is linked to identifiers using a study ID), or anonymous (identifiers are never collected).
Benign behavioral interventions are brief in duration, harmless, painless, not physically invasive, not likely to have a significant adverse lasting impact on the subjects, and the investigator has no reason to think the subjects will find the interventions offensive or embarrassing. If the research involves deceiving the subjects regarding the nature or purposes of the research, this exemption is not applicable unless the subject authorizes the deception through a prospective agreement to participate in research in circumstances in which the subject is informed that they will be unaware of or misled regarding the nature or purposes of the research.
- Children cannot be included in this category of research.
- Having subjects play an online game and then answer questions about it
- Video recording subjects solving puzzles under various noise conditions
- Having subjects decide how to allocate a nominal amount of received cash between themselves and someone else
Category 4 - Secondary data or specimen research that does not require consent
Secondary research for which consent is not required: Secondary research uses of identifiable private information or identifiable biospecimens, if at least one of the following criteria is met:
- VCU’s interpretation of publicly available : “Publicly available” refers to situations where a member of the general public could request information or biospecimens. Examples include data and specimens that are commercially available, available upon request or for a fee, or available under other conditions (such as having to register for an account or sign a privacy agreement)
- Data identifiability : Research data may be identifiable, de-identified (data is linked to identifiers using a study ID), or anonymous
- Data identifiability : Research data is anonymous, and participants cannot be re-identified. Neither identifiers nor a key linking study ID with identifiers may be retained at any point during the conduct of the research.
- Limitation : This category applies when information is obtained from an entity regulated by HIPAA and that information will continue to be maintained within either the same HIPAA-covered entity or another HIPAA-covered entity. If information is moved or shared to an outside entity NOT regulated by HIPAA the research will need expedited review.
- Data identifiability : Research data can be identifiable or de-identified (data is linked to identifiers using a study ID).
- Data identifiability : Research data may be identifiable, de-identified (data are linked to identifiers using a study ID) or anonymous.
- Analyzing publicly accessible Facebook posts (criteria i)
- An investigator obtains a list of MRN from the Informatics Core of patients records that meet the eligibility criteria. The investigator uses the MRN only to look up the relevant charts but does not write the MRN down anywhere in or connected to the research data. No other identifiers are written down in the research data. There is no way to go back to that particular patient’s chart later to collect additional information (criteria ii)
- An investigator receives an anonymous dataset from a registry (criteria ii)
- Analyzing anonymous waste tissue samples (criteria ii)
- Analyzing medical records of patients when the data are not shared outside the VCU HIPAA Covered Entity and when consent is not required (criteria iii)
- Conducting analyses of national student exam scores at the request of (or under contract to) the Department of Education (criteria iv)
Category 5 - Public benefit or service program research
Research and demonstration projects that are designed to study, evaluate, improve or otherwise examine public benefit or service programs, including:
- Procedures for obtaining benefits or services under those programs
- Possible changes in or alternatives to those programs or procedures
- Possible changes in methods or levels of payment for benefits or services under those programs
In addition, projects that are:
- Conducted by a federal department or agency
- Supported by a federal department or agency
- That are otherwise subject to the approval of (federal) department or agency heads (or the approval of the heads of bureaus or other subordinate agencies that have been delegated to conduct the project).
Such projects include, but are not limited to, internal studies by federal employees and studies under contracts or consulting arrangements, cooperative agreements, or grants. Exempt projects also include waivers of otherwise mandatory requirements using authorities such as sections 1115 and 1115A of the Social Security Act, as amended.
See OHRP guidance on types of programs that qualify for this exemption.
- Initiating and evaluating a program supported by a federal grant that administers financial or medical benefits under the Social Security Act.
- A state department received a federal award to administer a demonstration project with the state Medicaid services. The VCU investigator will evaluate the program as a consultant for the state department.
Category 6 - Taste and food quality evaluation and consumer acceptance studies
Taste and food quality evaluation and consumer acceptance studies, (1) if wholesome foods without additives are consumed or (2) if a food is consumed that contains a food ingredient at or below the level for a use found to be safe, or agricultural chemical or environmental contaminant at or below the level found to be safe, by the Food and Drug Administration or approved by the Environmental Protection Agency or the Food Safety and Inspection Service of the U.S. Department of Agriculture.
At this time, VCU is NOT implementing exemption categories 7 and 8.
Exempt research studies are reviewed to ensure that all research activities of the study fit into one or more of the exempt research categories (see above) and are no greater than minimal risk. Research that is determined to be exempt is not regulated by the DHHS or FDA regulations for conducting human subject research. However, all exempt studies should be conducted in accordance with the Belmont Principles for ethical research, and in accordance with institutional standards and requirements.
- Upload all supporting study documents such as:
- Information sheets to inform participants about the research activity
- Recruitment materials
- Survey instruments and interview questions
- Principal Investigator CV/Biosketch (and, if applicable, CVs/Biosketches for lead student investigators and/or medically/psychologically responsible investigators)
- Be sure all submitted documents include version numbers and/or dates in the footer. When documents are changed, a new version number and/or date should be applied. This helps the IRB track approved versions most efficiently.
Because exempt research is not regulated by federal requirements, informed consent is not required. VCU does, however, require some sort of information sheet be shared with prospective research participants. The information sheet may be brief and may or may not include all of the consent elements. At minimum, it should address:
- The activity involves research
- Participation is voluntary
- Brief description of what participants will be doing
- Whom to contact with questions (generally the principal investigator)
- Note any compensation to participants
Expedited review
Expedited review means that the review can be done by a single qualified IRB member rather than at a convened IRB meeting. Reviewers conducting an expedited review may exercise all of the authority of the IRB except that they may not disapprove a study. When reviewers cannot approve the research under expedited review, the study is referred for full board review.
To qualify for expedited review, research must be no greater than minimal risk and involve only procedures described by specific categories identified in the federal regulations.
Clinical studies of drugs and medical devices only when condition (a) OR (b) is met.
(a) Research on drugs for which an investigational new drug application (21 CFR 312) is not required. (Research on marketed drugs that significantly increases the risks or decreases the acceptability of the risks associated with the use of the product is not eligible for expedited review.)
(b) Research on medical devices for which (1) an investigational device exemption application (21 CFR 812) is not required; or (2) the medical device is cleared/approved for marketing and the medical device is being used in accordance with its cleared/approved labeling.
Collection of blood samples by finger stick, heel stick, ear stick or venipuncture as follows:
(a) From healthy, non-pregnant adults who weigh at least 100 pounds. For these participants, the amounts drawn may not exceed 550 ml in an eight-week period and collection may not occur more frequently than two times per week; OR
(b) From other adults and children, considering the age, weight, and health of the participants, the collection procedure, the amount of blood to be collected, and the frequency with which it will be collected. For these participants, the amount drawn may not exceed the lesser of 50 ml or 3 ml per kg in an eight-week period, and collection may not occur more frequently than two times per week.
Prospective collection of biological specimens for research purposes by noninvasive means. Examples include:
- Hair and nail clippings in a non-disfiguring manner
- Deciduous teeth at time of exfoliation or if routine patient care indicates a need for extraction
- Permanent teeth if routine patient care indicates a need for extraction
- Excreta and external secretions (including sweat)
- Uncannulated saliva collected either in an unstimulated fashion or stimulated by chewing gumbase or wax or by applying a dilute citric solution to the tongue
- Placenta removal at delivery
- Amniotic fluid obtained at the time of rupture of the membrane prior to or during labor
- Supra- and subgingival dental plaque and calculus, provided the collection procedure is not more invasive than routine prophylactic scaling of the teeth and the process is accomplished in accordance with accepted prophylactic techniques
- Mucosal and skin cells collected by buccal scraping or swab, skin swab, or mouth washings
- Sputum collected after saline mist nebulization
Note : On Oct. 4, 2010, OHRP clarified that it agrees with the FDA’s position that the following procedures are considered noninvasive:
- Vaginal swabs that do not go beyond the cervical os
- Rectal swabs that do not go beyond the rectum
- Nasal swabs that do not go beyond the nares
Collection of data through non-invasive procedures (not involving general anesthesia or sedation) routinely employed in clinical practice, excluding procedures involving X-rays or microwaves. Where medical devices are employed, they must be cleared/approved for marketing. Studies intended to evaluate the safety and effectiveness of the medical device are not generally eligible for expedited review, including studies of cleared medical devices for new indications. Examples include:
- Physical sensors that are applied either to the surface of the body or at a distance and do not involve input of significant amounts of energy into the participant or an invasion of the participant's privacy
- Weighing or testing sensory acuity
- Magnetic resonance imaging
- Electrocardiography, electroencephalography, thermography, detection of naturally occurring radioactivity, electroretinography, ultrasound, diagnostic infrared imaging, Doppler blood flow and echocardiography
- Moderate exercise, muscular strength testing, body composition assessment, and flexibility testing where appropriate given the age, weight, and health of the individual
Research involving materials (data, documents, records or specimens) that have been collected or will be collected solely for non-research purposes (such as medical treatment or diagnosis).
- Some research using only retrospective data may qualify for exempt category 4.
- This category is applicable to research involving the use of data obtained from medical charts when the investigator wishes to collect both retrospective and prospective data.
- This category includes materials that are collected for either non-research or research purposes, provided that any materials collected for research were not collected for the currently proposed research.
- The phrase " ...or will be collected solely for non-research purposes " pertains to the origin of the materials. For example, blood samples that were collected for a clinical test.
Collection of data from voice, video, digital, or image recordings made for research purposes.
Research on individual or group characteristics or behavior (including but not limited to research on perception, cognition, motivation, identity, language, communication, cultural beliefs or practices, and social behavior) or research employing survey, interview, oral history, focus group, program evaluation, human factors evaluation or quality assurance methodologies.
Note: Some research utilizing these procedures may qualify for exempt category 2.
Continuing review of research previously approved by the convened IRB as follows:
(1) Where (1) the research is permanently closed to the enrollment of new subjects; (2) all subjects have completed all research-related interventions and (3) the research remains active only for long-term follow-up of subjects; OR
(2) Where no subjects have been enrolled and no additional risks have been identified; OR
(3) Where the remaining research activities are limited to data analysis.
Note: Closure of enrollment only has to apply to the local site, not to all sites.
Continuing review of research, not conducted under an investigational new drug application or investigational device exemption where categories 2 through 8 do not apply but the IRB has determined and documented at a convened meeting that the research involves no greater than minimal risk and no additional risks have been identified.
Expedited research means a study may be reviewed by a single IRB member, rather than by the convened IRB, if all research activities of the study fit into one or more of the expedited research categories (see above) and are no greater than minimal risk. Expedited review does not necessarily mean the review will occur more quickly than other types of review.
- Informed consent form(s)
- Grant proposal, if applicable
Full board review
Full board review means that the research is reviewed at convened meetings at which a majority of the members of the IRB are present, including at least one member whose primary concerns are in nonscientific areas. This type of review applies to studies that are greater than minimal risk, or minimal risk but do not qualify for exempt or expedited review .
Full board review is conducted by the convened IRB. Studies that require full board review are those that are greater than minimal risk and/or do not qualify for an exempt or expedited research category.
Full board studies undergo an administrative pre-review process prior to IRB review. Following completion of pre-review, studies are forwarded to a convened panel for IRB review.
- Multi-center study protocol
- FDA regulatory documents, if applicable
- Skip to main content
- Skip to primary site menu
- Skip to primary site search

Research at Brown
Does my project need irb review, decision chart | comprehensive comparison table | definitions | public health surveillance.
Brown's IRB has assured federal regulatory agencies that the institution will review and approve all research that meets the federal definition of human subject research . Determining whether or not a project meets the federal definition of human subjects research is a two-step process. The investigator must first determine if the project meets the federal definition of research and, if so, then determine if the project includes human subjects.
In addition to the information below, the Brown HRPP has provided self-assessment resources for students and researchers to use to determine if their project requires IRB review:
- The Self-determination tools and checklists are guides to assist Brown researchers in complying with federal regulations and institutional policy.
- The NIH Decision Tool: Am I Doing Human Subjects Research? Developed by NIH to help you determine if your research involves human subjects, may be exempt from federal regulations, or is not considered human subjects research.
- Is it Human Subjects Research? Is IRB Review Required? educational video from HRPP's Learning Library .
Decision Chart
A decision chart to help guide researchers in making a determination of whether they're conducting human subject research and need to submit an IRB Application.
Step 1: Is your Project Considered Research?
Federal regulation defines "research" as a systematic investigation, including research development, testing, and evaluation, that is designed to develop or contribute to generalizable knowledge.
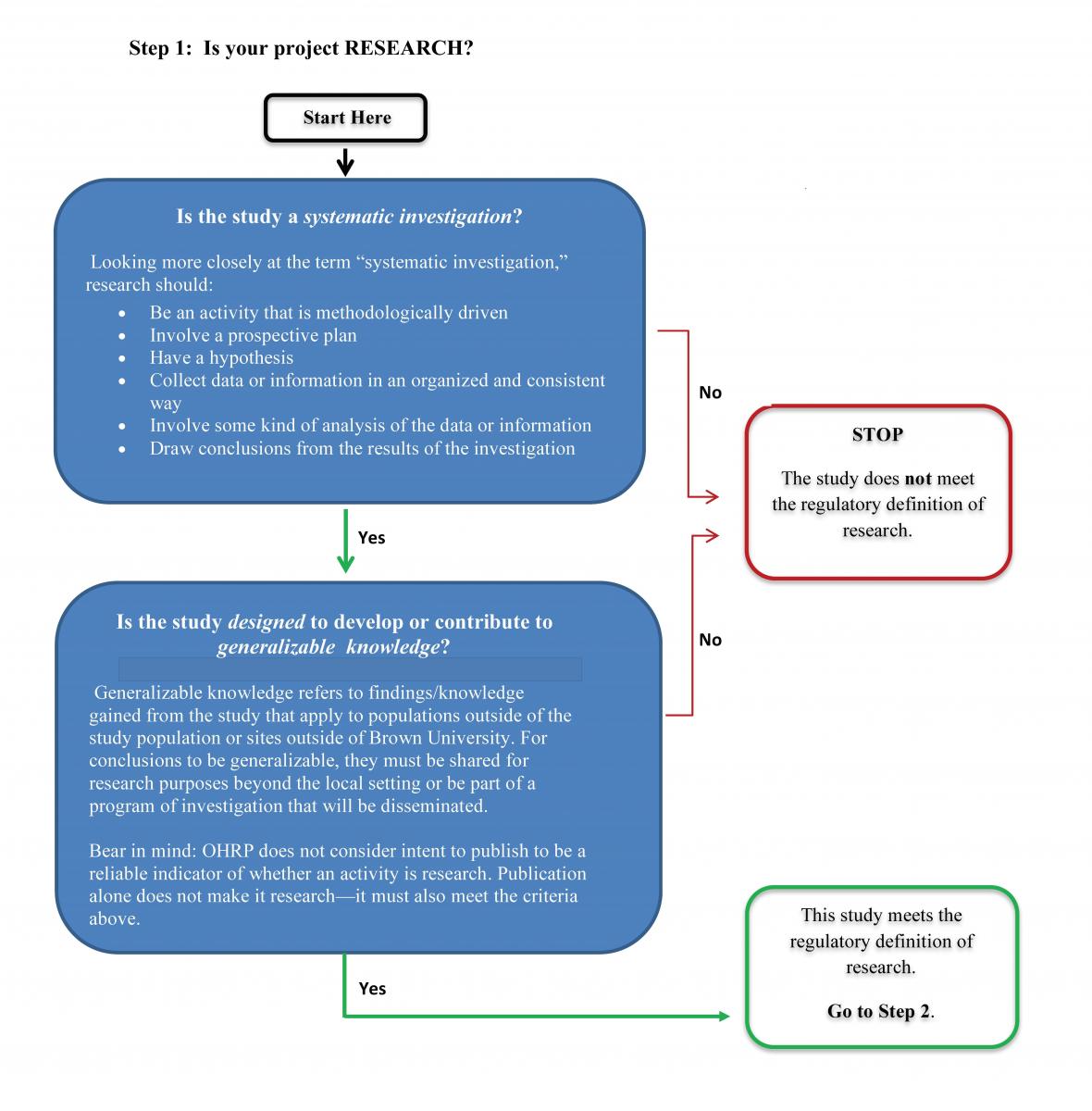
Systematic Investigation
A "systematic investigation" is a detailed or careful examination that has or involves a prospectively identified approach to studying a specific topic, answering a specific question(s), testing a specific hypothesis(es), or developing theory based on a system, method, or plan. Systematic investigations include observational studies, interview or survey studies, group comparison studies, test development, and interventional research. Projects that are not systematic investigations include, for example, oral histories, journalism, and phenomenological activities. Program evaluation is seen as a gray area and requires further assessment of design and intent.
Generalizable knowledge
Developing or contributing to "generalizable knowledge" means that the intent or purpose of the systematic investigation is to produce knowledge from which conclusions will be drawn that can be applied to populations outside of the specific study population. This usually includes one or more of the following concepts:
- knowledge that contributes to a theoretical framework of an established body of knowledge;
- the primary beneficiaries of the research are other researchers, scholars, and practitioners in the field of study;
- dissemination of the results is intended to inform the field of study (this alone does not make an activity constitute research “designed to contribute to generalizable knowledge”);
- the results are expected to be generalized to a larger population beyond the site of data collection;
- the results are intended to be replicated in other settings.
If your project is not considered research, you do not need to submit an IRB application. If your project does meet the definition of research, proceed to Step 2.
_______________________________________________________________________________________________________________________________________________________
Step 2: Does it involve human subjects?
The Federal Policy for the Protection of Human Subjects (Common Rule) defines a human subject as “…a living individual about whom an investigator (whether professional or student) conducting research obtains (1) data through intervention or interaction with the individual, or (2) identifiable private information.”
Examples that do not meet the definition of Human Subjects:
- Analysis of data about people who are deceased
- Secondary analysis of anonymous data
- Interviews with “key informants” about topics other than themselves
Note that the definition of human subject focuses on what information is obtained about people or material that is acquired from people. If either of the following is true, your research activity involves human subjects:
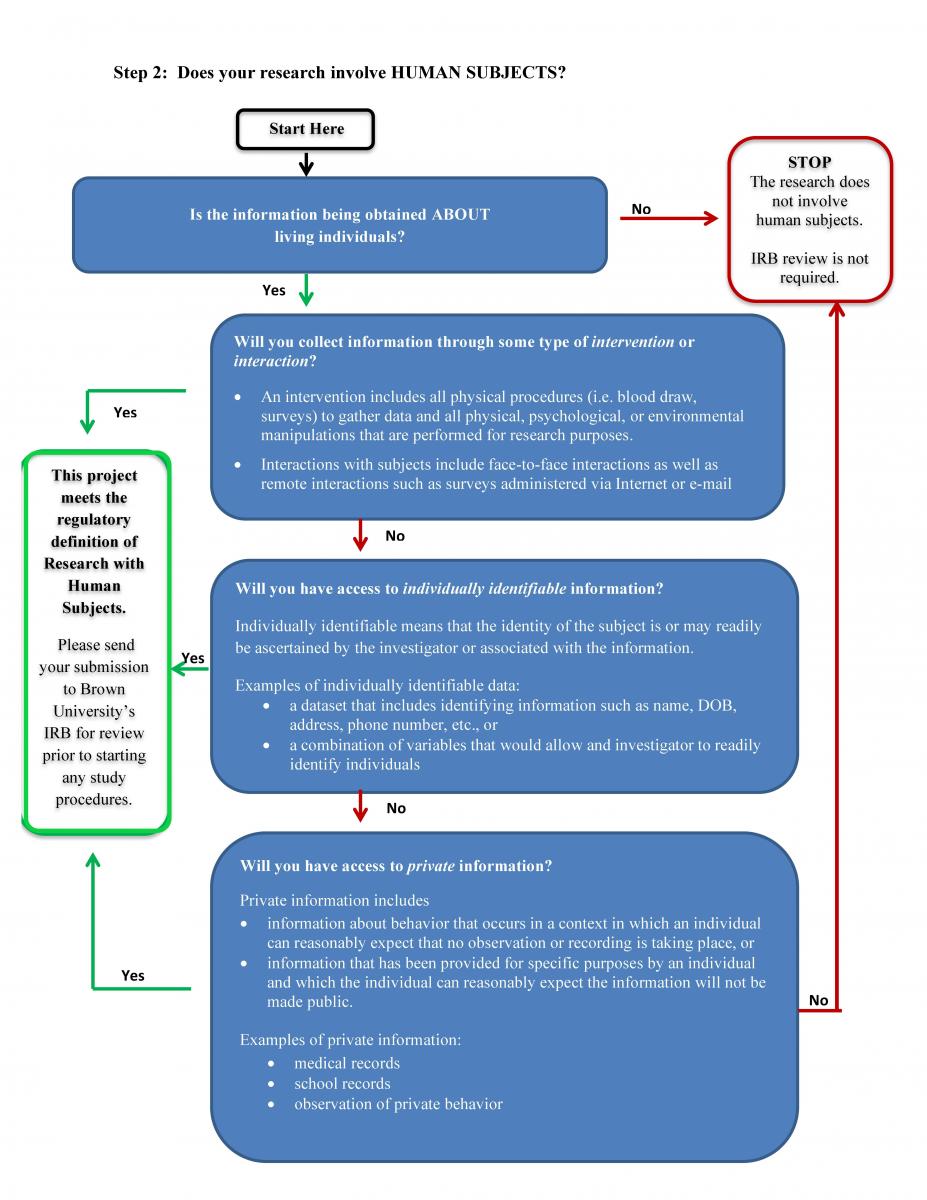
If your project does not include human subjects, you do not need to submit an IRB application.
Comprehensive Comparison Table
A comprehensive comparison table to assist students and researchers with distinguishing whether a project qualifies as human subject research, quality assurance/quality improvement, program evaluation, a student project or scholarly & journalistic activities.
| Common Rule: “Research means a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge.”
Belmont Report: “[T]he term ‘research’ designates an activity designed to test a hypothesis, permit conclusions to be drawn, and thereby to develop or contribute to generalizable knowledge (expressed, for example, in theories, principles, and statements of relationships). Research is usually described in a formal protocol that sets forth an objective and a set of procedures designed to reach that objective.”
OHRP: “The question "what is research" frequently arises in relation to an investigator or institutional activity being planned to gather data to evaluate a specific program, such as a QA/QI activity. Although the determination as to whether the activity will contribute to 'generalizable knowledge' is often based on whether the data will be disseminated by means of publication or presentation, this should not be the sole factor used to make the determination. In general, OHRP gives guidance that if the data will be used to draw conclusions related to a larger entity, then the activity is considered 'research'.” | Intent of the project is to improve a practice or process within a particular institution or ensure it conforms to expected norms. | Intent of the project is to evaluate a specific program, only to provide information for and about that program. | Intent of the project is to provide an educational experience about the research process or methods | This category of activities concerns certain activities in various fields that focus directly on the specific individuals about whom information are collected (e.g., oral history, journalism, biography, literary criticism, legal research, and historical scholarship).
Literary criticism …because while a piece of literary criticism might focus on information about the author(s), it would typically focus on the specific author(s) in view.
Legal research… because it would often focus on the circumstances of specific plaintiffs or parties involved in a case.
It is not the particular field that removes the activity from the definition [of HSR], but rather the particular activity's focus on specific individuals. | |
| Project occurs in large part as a result of individual professional goals and requirements (e.g., seeking tenure; obtaining grants; completing a thesis or dissertation). | Project occurs regardless of whether individual(s) conducting it may benefit professionally from conducting the project. | Project not initiated by the evaluator and occurs regardless of whether individual(s) conducting it may benefit professionally from conducting the project. | Project occurs as part of assigned course/class work or a requirement of an educational program in order to learn a new technique or pass a course/fulfill an assignment. | Project occurs to portray the individuals involved. | |
| Designed to develop or contribute to the scientific storehouse of knowledge; may involve randomization of individuals to different treatments, regimens, or processes; novel research ideas or experimental activities that are not yet known to be efficacious. May be designed to prove a relationship or correlation. | Not designed to develop or contribute to generalizable knowledge; generally does not involve randomization to different practices or processes. | Not designed to develop or contribute to generalizable knowledge; does not involve randomization of individuals, but may involve comparison of variations in programs | Not designed to develop or contribute to generalizable knowledge; design is often an example or template provided by a professor or course book. | Designed to collect and use information about specific individuals themselves, and not generalizing to other individuals. | |
| Activities not mandated by institution or program. | Activity mandated by the institution or clinic as part of its operations. | Activity mandated by the program, usually its funder, as part of its operations. | Activity mandated by regularly assigned coursework or educational program | Activities not necessarily mandated by institution or program | |
| Findings of the study are not expected to directly or immediately affect institutional or programmatic practice, although they may also be used for this purpose. Activity will be used to develop a problem statement, research questions, and/or theory-based hypotheses. | Findings of the study are expected to directly affect institutional practice and identify corrective action(s) needed. | Findings of the evaluation are expected to directly affect the conduct of the program and identify improvements. | Findings of project are not expected to directly affect the program; the project will mainly generate raw data, not generalizable knowledge. | N/A | |
| Human subject means a living individual about whom an investigator (whether professional or student) conducting research obtains (1) Data through intervention or interaction with the individual, or (2) Identifiable private information. May involve a subset of individuals; universal participation of an entire population (e.g., clinic, program, or department) is uncommon; generally, statistical justification for sample size is used to ensure endpoints can be met. | Information on all or most receiving a particular treatment or undergoing a particular practice or process expected to be included; exclusion of information from some individuals significantly affects conclusions | Information on participants receiving a particular treatment or undergoing a particular practice or process expected to be used; exclusion of information from some individuals significantly affects conclusions | Can either include all, most, or a subset of individuals; statistical justification may be used in the context to understand the process of subject selection; however, recruitment often utilizes convenience sampling. | Preamble to revised Common Rule: “… the focus is on the specific activities that collect and use information about specific individuals themselves, and not generalizing to other individuals, and that such activities occur in various fields of inquiry and methodological traditions… It is not the particular field that removes the activity from the definition, but rather the particular activity's focus on specific individuals.” | |
| Participants may or may not benefit directly – benefit, if any, to individuals is likely to be incidental or delayed. | Participants expected to benefit directly from the activities. | No benefit to participants expected; evaluation concentrates on program improvements or whether the program should continue. | Participants may or may not benefit directly; benefit is primarily for the student conducting the project for the fulfillment of educational requirements. | Individual benefit may or may not be anticipated. | |
| Intent to publish or present generally presumed at the outset of project as part of professional expectations, obligations; dissemination of information usually occurs in research/scientific publications, grant proposals, or other research/scientific forum; results expected to develop or contribute to generalizable knowledge by filling a gap in scientific knowledge or supporting, refining, or refuting results from other research studies. Results of the project will be disseminated outside the institution for the purpose of sharing the outcomes or implications of the project, not just the process. | Intent to publish or present generally not presumed at the outset of the project; dissemination of information often does not occur beyond the institution evaluated; dissemination of information may occur in quality improvement publications; when published or presented to a wider audience, the intent is to suggest potentially effective models, strategies, assessment tools or provide benchmarks or base rates rather than to develop or contribute to generalizable knowledge. | Intent to publish or present generally presumed at the outset of the project; dissemination of information to program stakeholders and participants; may be publicly posted (e.g., website) to ensure transparency of results; when published or presented to a wider audience, the intent is to suggest potentially effective models, strategies, assessment tools or provide benchmarks or base rates rather than to develop or contribute to generalizable knowledge. | Any presentations, posters, or publishing (including online) is simply to document the educational experience or completion of programmatic requirements. | Intent to publish or present generally presumed at the outset of the project; dissemination of information occurs to document a specific historical event or the experiences of individuals without intent to draw conclusions or generalize findings. |
Definitions
Data about living individuals through intervention or interaction.
An intervention may be physical procedures (e.g. venipuncture) or manipulations of living individuals or the living individuals’ environments.
An interaction may be communication or interpersonal contact between the investigator (or research team) and the living individual. Examples include interviews, questionnaires, surveys, observations, manipulations of subject behavior, diet, or environment, physical measurements, specimen collection (e.g. blood tissue), and administration of experimental drugs or devices.
Why “about whom” is key
Consider if the project focuses on the person or if the focus is on policies, practices or procedures about which the person is knowledgeable. Projects which collect information about policies, practices or procedures – even if the person who provided that information is identified – do not constitute human subject research. Asking a person about someone else does not make that person a human subject.
Identifiable private information about living individuals
Identifiable means 1) the identity of the individual from whom the information was obtained is ascertained or may be readily ascertained by the investigator; or 2) the identity of the individual from whom the information was obtained is associated or may be readily associated with the information.
Private Information is information about behavior that occurs in a context in which the individual can reasonably expect that no observation or recording is taking place or information that has been provided for specific purposes that the individual can reasonably expect will not be made public (e.g. medical record, employee or student records).
Examples of identifiable, private information include the subject’s name, address, phone number, social security number, medical record number, student or employee identification number, or in some cases, the combination of data such that they can identify a single individual through deductive reasoning. For example, data about employer, job title, age and gender may not individually identify a subject, but when combined, could in certain cases, identify a specific individual.
What is NOT considered identifiable, private information : If the information cannot be linked to a living individual, or is considered public or is given with the expectation that it will be made public and that it will be linked to the individual (e.g. biography or news story), then it would not be considered private identifiable information. For example, use of a publicly available data set that does not contain identifiers or codes linked to individuals does not involve human subjects research. However, use of a publicly available data set that does contain identifiers or codes linked to individuals does involve human subject research.
If you obtain/purchase/are given specimens/cells/material/data that has already been collected by someone else for some other purpose, and the specimens/cells/material/data are not linked to any identifiers that would make it reasonably possible to identify an individual, the activity is not considered research with human subjects.
If your activity does not involve human subjects as defined in the regulations, your activity does not fall under the purview of the IRB. You do not need to submit an application.
If you have determined that your research does meet the federal definition for human subjects research, you will need to apply for IRB review and approval before you begin ( the IRB can not review projects retrospectively ).
Still unsure or require documentation that your project does not need IRB review?
If you are unsure if your project meets the definition of research, or if you require documentation that your project does not require IRB review, please contact the HRPP at (401) 863-3050 or [email protected] to discuss. If you are a student, we strongly encourage you to first work with your advisor / mentor to discuss whether the proposed project meets the definition of human subjects research before contacting the HRPP.
If you have questions, please contact us at 401-863-3050 or [email protected] .
Explore Brown University
RSRB (IRB) Review Process
An Institutional Review Board (IRB) is a committee, constituted per federally-defined membership requirements, tasked with reviewing human subject research to ensure subject rights, safety and welfare are protected. The University of Rochester’s IRB is referred to as the Research Subjects Review Board (RSRB).
This page summarizes the RSRB’s review process for basic, single-site submissions, and highlights best practices for facilitating IRB review.
Important notes before getting started
The RSRB is just one component of the University’s Human Research Protection Program (HRPP) . The overarching aim of the HRPP is to ensure individuals involved in the conduct of human subject research understand and apply their obligation to protect the rights, dignity, welfare and privacy of research subjects.
- Based on the nature of a research proposal (e.g., the population under study, procedures and associated risks, and data collection methods), additional HRPP review processes may be required prior to initiation of the research (in addition to IRB review).
- Review our HRPP page and our Getting Started for additional information on HRPP roles/responsibilities and potential review requirements.
The review process described below may be altered when the research involves multiple sites and is federally-funded or industry-sponsored. In this circumstance, the RSRB may elect to: 1) defer to another IRB for the review and approval of the research; or 2) conduct IRB review for all participating sites engaged in the research.
For additional information on this process, reference OHSP Policy 504: IRB Reliance and Collaborative Research and its corresponding Guidelines for When the University of Rochester Relies on a Non-University IRB and When the University of Rochester is the Reviewing IRB . More details are also available in the ‘Overview of Multi-Site Study Review’ section of the Click IRB: Overview and Basic Navigation Guide .
The Click IRB Review System
RSRB review is facilitated through an online platform called the Integrated Online Research Administration (IORA) system; the system includes a module for conducting IRB reviews, which is referred to as Click IRB .
Step-by-step instructions for navigating the Click IRB system and submitting research for RSRB review are available in the Click IRB Study Staff Manual . New users are strongly encouraged to follow the instructions available within the Click IRB manual for various submissions until they become comfortable using the system.
Visit our Click IRB Resources page to learn more
Keys to facilitating review in Click IRB
- Submit documents as Microsoft Word documents, when feasible. Doing so allows IRB staff to easily monitor revisions to the document, as well as track revisions when necessary.
- Follow instructions for how to correctly upload and manage documents .
- Use the Principal Investigator (PI) Proxy designation to assist communications within Click IRB. Reference our Click IRB FAQs to learn more.
The RSRB review process
Following submission in the Click IRB system, proposals will undergo review as depicted in the image and drop-downs below.
All proposals are initially routed for departmental review and sign-off. Of note, some departments have their own procedures in place beyond the basic review conducted in Click IRB. As such, study team members are encouraged to contact their department reviewers early in the process to determine whether any additional procedures are required.
Following departmental approval, the submission will be assigned to any/all applicable ancillary committees (in accordance with OHSP Policy 503: Ancillary Committee Review ) for review. Next steps are dependent on whether ancillary committee review is required and when their approval is required. Some committees block the RSRB review process–meaning, the committee must provide approval before the IRB Coordinator can initiate the review process. For others, committee review can happen in tandem with RSRB review, though approval is required prior to RSRB approval . Alternately, other ancillary committees can conduct their review and provide approval at any time, without dependence on the timing of RSRB review and approval.
The IRB Coordinator’s role is to shepherd the submission through the review process. Once a proposal is assigned to an IRB Coordinator, they will conduct an initial pre-review, ensuring adequate information is provided for the board to make the requisite determinations about the research and that the research satisfies regulatory and institutional requirements. Typically, IRB Coordinators initiate their review with the study protocol, ensuring that all of the necessary elements are adequately addressed in a consistent manner. From there, they will review all supporting documentation (e.g., recruitment materials and consent forms) to ensure they are complete, consistent with the contents of the study protocol and written at an appropriate reading level (as applicable).
Following IRB Coordinator review, where the submission moves in the process depends on several factors. If it is unclear what the research entails or how regulatory and institutional requirements will be met (e.g., information is missing, incomplete or inconsistent), the submission will likely be returned to the Investigator for changes (see additional information in the section below). On the other hand, if the submission is in relatively good shape, the proposal can be moved forward via one of three review pathways, as summarized in Appendix 1 . Note, the review pathway is determined by IRB staff per the requirements set forth in federal regulations; Investigators and study staff cannot request a specific type of review.
The reviewing board or individual (based on the review pathway) will review the submission and make the requisite determinations, confirming whether: 1) the research meets exemption criteria or, for research undergoing expedited or convened board review, validate whether the criteria for IRB approval are met, as defined by the federal regulations; and 2) all applicable institutional policies have been met. In the event these criteria cannot be validated, based on the contents of the submission, the research will be returned for clarification/modification. Once the reviewing board/individual has made the necessary determinations, the submission will be returned to the IRB Coordinator for processing (submissions will either be returned for clarification/modification or processed for approval).
Key consideration
Although simplified for the purpose of this description, this process is complicated and involves multiple key players. Plan plenty of time for IRB review to be conducted; do not wait until the last minute if you have an anticipated start time for conducting the research. Turnaround times depend on multiple factors, including (but not limited to) submission quality, workload, and ancillary committee review requirements.
Monitoring review process
A workflow diagram is available in Click IRB to monitor where the submission is in the review process.
Study teams can identify where a proposal is in the review process via the study state and review workflow in the study workspace. When a submission is in Department Review, the study state will be noted as such.
Once the department has approved the proposal, both the study state and review workflow will update as follows:
- ‘Pre-Review’ corresponds to IRB Coordinator review;
- ‘IRB Review’ corresponds to IRB Board/Chair/Senior IRB Staff review;
- ‘Post-Review’ indicates that the IRB Board/Chair/Senior IRB Staff have completed their review and the submission has been sent back to the IRB Coordinator for processing.
Once an IRB Coordinator has been assigned to the proposal, their name will appear in the upper right corner of the study workspace. Contact information is available.
Responding to IRB requests for clarification/modification
Submissions can be returned to the Investigator for clarification/modification at any point during the review process. When revisions are required, the PI, PI Proxy and Primary Contact (as designated in the Click IRB system) will receive an email notification alerting them to the request (with a written description of the request provided directly in Click IRB).
Tips for responding to requests
- Make all necessary revisions to the applicable study documents first, prior to making revisions to the submission form in Click IRB: Revisions to one document often result in revisions to other supporting documents. Making multiple revisions back-and-forth between documents and uploading revised versions with each revision can easily result in errors. Once all documents have been revised and reviewed, and all issues addressed, the revised documents should then be uploaded into the submission form.
- If a tracked document (e.g., revised consent form) is provided by the RSRB, download and use the document provided rather than duplicating efforts in the document originally submitted: When a tracked document is provided, it is the Investigator’s responsibilities to review the changes made by the RSRB prior to accepting them; do not blindly accept changes as questions/comments are often included that need to be addressed prior to re-submission. Once the document has been reviewed and all requested clarifications addressed, the revised clean (unmarked) document with tracking turned off must be uploaded into the submission form. RSRB staff cannot replace or upload documents within a submission form on behalf of the study team.
- When uploading revised documents to the system, use the ‘Update’ button immediately to the left of the current file name: Do not use ‘Add’ to upload revised documents to the system; this eliminates the audit trail and complicates the IRB review process. ‘Add’ should only be used when new (i.e., not previously reviewed) documents are being provided. For additional information, reference our Document Management page .
- Ensure all requests are addressed prior to re-submission. Ensure all requested revisions/clarifications are made to all applicable submission documents and all applicable sections within any one document: Failing to address requests for revision/clarification or failing to revise all corresponding sections/documents to maintain consistency (e.g., correcting an error in one section but not all sections that the error appears) will only result in submissions being returned to the Investigator as the this leaves the reviewer with an unanswered question/issue and/or creates additional inconsistencies. If an Investigator does not agree with a request, provide rationale/justification with your re-submission.
- I f you have questions regarding a request , contact the IRB Coordinator assigned to the research; a discussion may be necessary in order to adequately address the issue prior to re-submission.
- Once all revisions/requests have been addressed, re-submit for continued review (only the PI and designated PI Proxies will have the ability to do so within the system). Similar to sending an email, the submission needs to be actively returned to the RSRB within Click IRB in order to the RSRB to continue the review process.

Post-IRB approval need-to-knows
Obtaining IRB approval provides approval for only the study procedures described in the IRB approved protocol, as well as the provisions identified in the IRB submission form (including all approved supporting documentation uploaded within the submission form). Updates or revisions to these documents (including adding additional study personnel) cannot be implemented until IRB-approval has been obtained .
IRB approval is only granted for the period determined by the IRB. If an expiration date is assigned to the research, a continuing review (i.e., progress report) must be submitted and approved for the research to continue. If the approval lapses (expires), all research activities must be stopped until re-approval is obtained. To avoid lapses in approval, submit continuing reviews well in advance of expiration (30-60 days). For additional information, see the links/resources provided below.
Appendix 1. IRB review pathways
Reference our Convened Board Guideline for full details
- Based on federal regulation, review by the convened board is the ‘default’ pathway. Meaning, any and all research can be reviewed by the convened board, though typically the convened board reviews research involving greater than minimal risk.
- The RSRB is comprised of 5 different IRB boards (4 biomedical boards and 1 social-behavioral board). Research is generally assigned to a board based on the PI’s department (primary appointment). Biomedical boards convene every 2 weeks and the social-behavioral board convenes once a month. The RSRB does not set submission deadlines for convened board review. Rather, submissions are assigned to convened meetings on a first come, first serve basis; workload can also affect the timing of board assignment. To allow the board time to review submissions and prepare for convened meetings, submissions are generally assigned to an agenda a week in advance of the meeting.
Reference our Expedited Review Guideline for full details
- If the research involves only minimal risk (as defined by federal regulations) and all study procedures involved in the research fall into one or more federally-defined expedited review categories, the board chair (or another experienced, designated board member) can review the research on behalf of the convened board. All research reviewed in this manner is reported back to the convened board.
- Note: The term ‘expedited’ is not meant to reflect turnaround time. Rather, the expedited process only reflects that the process does not require review by the convened board.
Reference our Exempt Status Determination Guideline for full details
- If the research involves only minimal risk (as defined by federal regulations) and all study procedures involved in the research fall into one of several federally-defined exemption categories, the research can be reviewed and deemed ‘exempt’ by a senior member of the RSRB staff (or by a board chair or designated member of the board).
- Note: The term ‘exempt’ does not mean that IRB review is not required. Rather, ‘exempt’ means that once the research is confirmed as meeting exemption criteria by the IRB, the research does not require further reporting to the IRB (provided there are no changes to the research nor reports of new information).
- Proposals that require formal determination by the RSRB but do not meet the definition of human subject research or those that involve human subject research but where the University of Rochester is not engaged in research activities follow a review pathway similar to exempt research.
IRB review process FAQs
Review our When Do I Need to get RSRB (IRB) Approval? page to learn when IRB approvals are needed.
When an IRB determines that a proposal is ‘not human research’, this means that the proposal does not involve ‘research’ and/or ‘human subjects’, as defined by federal regulation. This isn’t to say that aims of the project proposed are unimportant. Rather, the project just does not meet specific regulatory definitions and therefore review of the project falls out of IRB/RSRB scope (meaning, IRB/RSRB policies do not apply). If/when the aims or activities of the project evolve to include ‘research’ and ‘human subjects’ (as defined by federal regulation), the project would then be subject to IRB/RSRB policies, including IRB/RSRB review.
When an IRB determines that a proposal is ‘exempt’, this means the project: a) involves ‘research’ (as defined by federal regulation); b) involves ‘human subjects’ (as defined by federal regulation); and c) falls into a federally-defined exemption category . Once the IRB/RSRB confirms the research meets exemption criteria, further reporting to the IRB/RSRB in not required (provided there are no changes to the research, nor reports of new information).
For more information review the OHSP Guideline for Determining Human Subject Research ; Institutional Review Board Scope ; and IRB/RSRB Exemptions .
Exempt research involves very little risk and must fall into one or more federally-defined exemption categories .
The term ‘exempt’ does not mean that the research does not require IRB review. Rather, once it’s determined that the research falls into one of the exemption categories, it is exempt from further IRB reporting requirements provided there aren’t any revisions to the research.
Per institutional policy, the Research Subjects Review Board (RSRB) is responsible for making the determination of whether or not the research falls into one of the exemption categories; Investigators are not permitted to make this determination independently. For additional information reference our RSRB (IRB) Exemptions page .
Expedited research involves no more than minimal risk (meaning, the probability and magnitude of harm or discomfort anticipated in the proposed research are not greater, in and other themselves, than those ordinarily encountered in daily life or during the performance of routine physical or psychology examinations or tests) and must fall into one or more federally-defined expedited categories .
Whether a proposal’s review level is ‘exempt’ or ‘expedited’ affects the IRB review process. While both submissions are initially reviewed by an IRB coordinator, exempt submissions are confirmed by a secondary RSRB staff member (typically either a senior IRB coordinator or a director). Whereas expedited proposals, must be reviewed and approved by a board chair or the designated board member (i.e., the vice chair or another experienced board member). Based on this requirement, the term ‘expedited’ is not meant to reflect turnaround time; the term only reflects that the process does not require review by the convened board, which may only meet monthly or bi-monthly, depending on the board.
From the standpoint of the study team, whether the research falls into an exemption category or undergoes expedited review has very little effect on their IRB reporting requirements. In either case, following exempt confirmation or IRB approval, the study team is responsible for:
- submitting any revisions (modifications) to the research for review and confirmation of continued exemption (exempt) or IRB approval (expedited) prior to implementation; and
- reporting research events in accordance with OHSP Policy 801: Reporting Research Events .
The primary difference is the requirement for continuing review (i.e., submission of a progress report), which the IRB may require for expedited research. Circumstances where the IRB may require continuing review include (but are not limited to):
- research involving vulnerable populations;
- research conducted by Investigators with prior incidence of non-compliance; and
- research involving the collection of sensitive information.
When continuing review is required, the research will be assigned an expiration date (per federal regulations, this date cannot exceed a one-year approval period).
Reference our Submission Preparation FAQs for more information.
Reference our Deferred vs. Modifications page for more information.
As defined in OHSP Policy 501: Levels of RSRB Review , the approval date is the date the IRB decision was made to either approve or approve with modifications/stipulations the item under review (i.e., new study application, modification or continuing review). An effective date is the date IRB decision takes effect; it is the first possible date the research can be performed (or modification implemented) following notification from the IRB.
Depending on the determination the IRB makes, these dates may or may not be the same. When a submission is approved outright and no modifications/stipulations are required to secure approve, the approval and effective date will be the same. However, when modification/stipulations are required for approval, the effective date will be later; it will reflect the date the required modifications/stipulations were reviewed and accepted by the IRB.
Reference our Click IRB FAQs for more information.
The Research Subjects Review Board (RSRB) is frequently asked this question and the short and simple answer to this is everything!
Remember, IRB approval allows you to conduct the research as described in the approved IRB submission , including the approved version of the study protocol and all supporting documentation (i.e., the IRB submission form, consent forms, recruitment materials, study measures, etc.). Any modifications to those documents require the submission to, and re-review, by the Reviewing IRB. This review is required to ensure the research will continue to meet the criteria for IRB approval, given the proposed revisions.
This includes, but is not limited to, revisions regarding:
- Study personnel;
- Enrollment;
- Eligibility criteria;
- Study procedures;
- Data and safety monitoring procedures;
- Planned data analysis;
- Risks/benefits;
- Study measures;
- Recruitment methods/materials; and
- Informed consent processes or documentation.
Instructions for submitting a modification to the RSRB are available in the Click IRB Study Staff Guide .
Federal regulations ( 45 CFR 46 ; 21 CFR 56 ) require continued IRB review of some types of minimal risk ( expedited ) research and all greater than minimal risk human subject research at least annually. In order to maintain IRB approval of the research, it is the responsibility of the Principal Investigator (PI) to ensure timely submission of a continuing review (also referred to as a progress report) prior to a study’s expiration date. Timely submission is necessary in order to provide the Reviewing IRB adequate time to review the submission and request clarifications, if necessary. Based on the required review level, board scheduling, and workload, Reviewing IRBs cannot guarantee timely renewal of research for continuing reviews submitted on, or just shortly prior to, study expiration. If IRB approval of the research lapses, all research activities, including recruitment, enrollment, interventions, interactions and data analysis on current subjects must cease until re-approval is provided . Lapses in IRB approval are considered non-compliance and multiple incidents of lapses in approval may be considered continuing non-compliance.
For research reviewed and approved by the RSRB, study expiration dates (or approval end dates) can be found in the upper left corner of the STUDY workspace in Click IRB. Note that this field is blank when continuing review of the research is not required.
As a courtesy, reminder e-mails concerning approaching expiration of RSRB-approved studies are sent to the PI, PI Proxy, and Primary Contact at 30 and 60 days prior to expiration. To help ensure timely review, as a general rule of thumb, the RSRB recommends submitting continuing reviews no later than 45 days prior to expiration .
‘Suspending’ a study means that the study, or a specific component of the study (e.g., enrollment, a specific study procedure, or intervention administration) is halted. Routinely, this occurs because of a safety or non-compliance incident and the study sponsor, Reviewing IRB, data and safety monitoring committee, or PI determines that the study should be stopped for a period of time in order to investigate the incident and determine the best path forward.
When the RSRB is the Reviewing IRB and the PI, study sponsor or data and safety monitoring committee determine that the study should be suspended, it should be reported via a Reportable New Information (RNI) submission form in Click IRB. Suspensions should not be requested through a request for study closure via continuing review , as this does not allow the study to be re-opened and modified to reverse the study suspension. Instructions for submitting a RNI to the RSRB are available in the Click IRB Study Staff Guide .
Note: If the Reviewing IRB decides that a study needs to be suspended for any reason, the IRB is required by federal regulation ( 45 CFR 46.108 , 21 CFR 56.108 ) to report this determination to applicable institutional and regulatory authorities and the study sponsor.
Per OHSP Policy 502: Types of RSRB Submissions , when the RSRB is the Reviewing IRB, ‘a study is considered completed, and therefore may be closed with the RSRB, when:
- Subjects are no longer being recruited (or data/specimens being collected);
- Subjects are no longer being followed;
- Primary data analysis has been completed according to the protocol; and
- Identifiers have been removed from the analysis dataset (e.g., identifiers destroyed or identified maintain in a separate dataset).’
Exceptions are as follows:
- Multi-site studies where the University of Rochester is an enrolling/participating site only (i.e., the local site is not the coordinating center/lead site and is not responsible for data analysis): Enrolling site studies can be closed once the site’s participation in the research is complete (i.e., subjects are no longer being recruiting, no longer being following and all data collection and review [including data edits and queries] are complete) and the coordinating center/lead site has instructed the enrolling site to close the study with their Reviewing IRB.
- Multi-site studies where the University of Rochester is the coordinating center/lead site: When the University of Rochester acts as a coordinating center/lead site and is therefore responsible for the conduct of the research at each enrolling site, the lead site’s IRB cannot be closed until all enrolling site IRBs are closed and the primary data analysis is complete according to the protocol.
- Concept Studies: A ‘concept study’ is a type of IRB submission that allows for IRB review and approval of a protocol that is still in development. Protocols are only approved in this manner when the funding agency provides a period a time prior to the research for protocol development. Once the protocol is fully developed it must be submitted as a new IRB submission, following approval of which, the original concept study can be closed.
- Umbrella studies: An umbrella study provides funding for multiple studies, submitted under separate IRB submissions. Umbrella studies can be closed once all IRB submissions for each study falling under the umbrella study has been closed.
- Studies reviewed and approved by an External IRB: Studies undergoing review and approval by an IRB other than the RSRB (e.g., Advarra, WIRB, or another institution’s IRB), can be closed per the Reviewing IRB’s requirements.
To close a study with the RSRB, submit a final continuing review as described in the Click IRB Study Staff Guide .
Additional support
Getting started guide, click irb resources, irb submission checklist, policy documents, ohsp policy 402: rsrb meetings, ohsp policy 502: types of rsrb submissions, ohsp policy 901: investigator responsibilities, reach out for support, post-approval consultations, who’s my irb coordinator, contact ohsp.
Institutional Review Board
Health Sciences and Minimal Risk Research IRBs
When IRB Review May Not Be Required
April 26, 2022
This section of the Investigator Manual discusses program evaluation and quality improvement projects, case reports, oral history projects, and publicly available data.
The IRB reviews all activities that meet the federal definitions of human research and federal guidelines for engagement. Some activities involving human subjects or their data may not fall under these definitions and do not require IRB review. This section provides you with more details about these activities and when IRB input may be needed to assess whether IRB oversight is required. If you are unsure whether your project engages you in human subjects research or meets the criteria for not requiring IRB review, please contact the IRB office for assistance.
Note that even if your study does not constitute human research, you must still comply with relevant regulatory (e.g., HIPAA) and institutional requirements. For more information, see HRP 309-WORKSHEET-Ancillary Review Matrix .
Program Evaluation/Quality Improvement or Assurance Projects
Case reports, oral history, analysis of publicly available datasets.
Determining whether a project constitutes human subjects research rather than quality improvement or program evaluation involves multiple factors. The federal definition of research is “a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge. Activities which meet this definition constitute research for purposes of this policy, whether or not they are conducted or supported under a program which is considered research for other purposes.” This is an important distinction to make because it determines whether IRB review and oversight of a project is needed.
Often, determining whether a project constitutes research under federal and institutional regulations can be a complex process that involves assessing the project intent, design, mandates, expected outcomes, and dissemination of results. The QI/Program Evaluation Self-Certification Tool is designed to assist study teams in determining whether a project requires submission to the IRB. If the project involves some characteristics of a research project, the Tool will let you know that IRB review is required. If the project does qualify as program evaluation/QI, the Tool will provide you with a certification confirming this. This certification can be printed off and used as documentation that the project does not constitute research and further IRB review is not required.
NOTE: This tool is not designed to determine all cases when a project falls outside of the IRB’s purview. This tool is only for determining if a project is QI/Program Evaluation, rather than research. The tool should not be used for public health surveillance projects.
A journal or conference may not accept the certification as proof your project does not require IRB approval. In such cases, you will need to submit an application in ARROW to obtain a formal IRB determination.
Link to this section
Case reports of 3 or fewer individuals generally do not meet the regulatory definition of research because they would not qualify as a systematic investigation that contributes to generalizable knowledge. IRB review of such case reports is therefore not required. Some journal editors do require documentation from the IRB indicating that IRB review is not necessary to publish the case report. In such cases, you may submit an IRB application requesting a not research determination.
Even if IRB review is not required, case reports for publication must be prepared in accordance with the requirements of the HIPAA privacy regulations. This means that the case reports must be de-identified, i.e., the presentation or article must not contain any of the 18 identifiers for an individual that are described in the HIPAA Privacy Rule. For case reports that disclose PHI or where the health information described in conjunction with other characteristics could reasonably be used to identify the patient (e.g., a rare injury, disease or diagnosis in combination with age, gender, geographic region), patients (or their legally authorized representatives, e.g., parents, guardians, etc.) will need to sign a HIPAA-compliant authorization form. See the Office of Compliance Authorization for Disclosure of Medical Information for Publication or Conference Presentation form.
Case reports involving more than three patients are more likely to meet the criteria for research and require IRB Review. In addition, testing of a patient’s biospecimen (e.g., special stain, immunohistochemistry, molecular studies) is not typically permissible as part of a case report.
While oral history is typically not considered human research, the prospective intent of the investigator and the definition of “research” under the federal regulations needs to be taken into account. Per federal regulation, research is defined as “a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge.”
Specifically, the consideration of human research and the requirement for IRB review hinges upon whether:
- The activity involves a prospective research plan which incorporates data collection, including qualitative data, and data analysis to answer a research question; AND
- The activity is designed to draw general conclusions (i.e., knowledge gained from a study may be applied to populations outside of the specific study population), inform policy, or generalize findings.
General principles for evaluating whether oral history type activities are human research and require IRB review:
- Oral history activities, such as open-ended interviews, that ONLY document a specific historical event or the experiences of individuals without intent to draw conclusions or generalize findings would NOT constitute “research” as defined by federal regulation.
- Systematic investigations involving open-ended interviews that are designed to develop or contribute to generalizable knowledge (e.g., designed to draw conclusions, inform policy, or generalize findings) WOULD constitute “research” as defined by federal regulation.
- Oral historians and qualitative investigators may want to create archives for the purpose of providing a resource for others to do research. Since the intent of the archive is to create a repository of information for other investigators to conduct research as defined by 45 CFR part 46, the creation of such an archive WOULD constitute research under federal regulation.
Research projects involving analysis of secondary data will NOT require prior IRB approval in the following situations:
- The data set(s) is (are) published and publicly available without restriction (e.g., data are published by a reputable source in a publicly-available journal, textbook or web-site) and neither the UW researcher nor any collaborating researcher on the project(s) has access to links that would connect the data to the individuals from whom they were derived.
- The data set(s) are publicly available to researchers and others, but the data holder requires a “responsible use statement” or similar attestation to ensure appropriate use and protection of the data. Such an agreement or attestation may be automated. In this case, neither the UW researcher nor any collaborating researcher on the project can have access to any links that would connect the data to the individuals from whom they were derived, nor may any researcher on the project attempt to re-identify any person from whom the data were derived.
- The researcher will obtain a data set available from a Federal or State agency and will enter into an agreement with the data provider that includes language that: a) the data provided to the researcher does not contain any identifiers, including those specified under the HIPAA Privacy Rule; b) if the data are coded, the data provider will not release a link to the code to the researcher; and c) the researcher receiving the data set must agree to not attempt to re-identify any person from whom the data were derived.
- In all cases, the IRB expects that investigators have reviewed the data sources’ terms of service, responsible use statements, and data access agreements, if any. Questions about compliance with such terms should be directed to the UW Office of Legal Affairs.
West Liberty University
Institutional Review Board
Complete the initial Research Proposal Application FORM .
Preparing Your Proposal / FAQ
Preparing your irb proposal submission: helpful hints and special cases..
SCROLL DOWN FOR FAQ’s:
Step 1. Before submitting your research project to the IRB, determine if it meets the requirements of human subject research.
If yes, go to Step 2. If no, then the project does not have to be reviewed by the IRB. Ethical research practices should still be followed.
Step 2. Note submission deadlines for IRB committee reviews. See Timeline page for due dates and IRB meeting dates.
Step 3. Complete the required CITI training. Certificates are active for three years, after that a new or refresher course is required. Everyone that will be involved with human subjects as part of the study must complete the required training before the study can be reviewed.
Step 4. Read over the IRB initial submission form. This asks for details about your study. This is so the IRB can determine the level of review required, and the potential risks and benefits to human subjects.
Overview of what the IRB needs to know
Short version: your METHODS (proposed participants, materials (including informed consent/assent document, procedures)
Long version: who is your target population and how will people be sampled, is sampling equitable, do you have any needed research permissions (i.e. K-12 superintendent for children research), what will the people be asked to do (in detail, including any surveys or protocols), what are the benefits and risks beyond normal everyday life, what (if anything) do the people get out of participating, and how will the data be handled, stored, and used. The IRB wants to make sure that people can understand what is being asked of them, and that their safety and confidentiality (if applicable) is put first and foremost.
Overview of what the IRB does NOT need to know
Typically the IRB does not need any background literature review information, unless it pertains to supporting why certain materials or questionnaires are being used. The IRB does NOT review your application for spelling, grammar, or other non human subject safety content errors. The IRB is here to assist regarding the protection of human subjects, however, we will not write or revise your proposal for you.
Step 5. Make sure you have all the research study proposal information available BEFORE completing the application. Getting a study ready for IRB review can take a significant amount of time and effort. All study methodology including recruiting, sampling, and procedural details have to be determined BEFORE submitting your application. Missing or unclear information can lengthen the review process. Students should work closely with their faculty sponsors and have them review all materials before submission.
Step 6. Submit proposal to the IRB.
Step 7 . Check your email for any communications from the IRB. They may request additional information or clarification. Please respond to the IRB in an appropriate and timely manner. The IRB may request for you to attend the meeting to discuss your project.
Step 8. Notification of decisions. See due dates and IRB committee meeting times. The IRB meets one week after each submission due date. Typically you will get a response from the IRB within one week from that meeting date.
Step 9. Conduct your research as planned. Contact the IRB in a timely manner for any unanticipated aversive events related to human subjects. If you have ANY changes related to human subjects (i.e. have to add in a survey question), you must submit an Amendment form and receive approval BEFORE implementing any changes.
Why do we need an IRB?
Common question from students: “Why do I have to submit my research to the IRB? It seems like a lot of work, especially since all I want to do is ask a few anonymous survey questions”
Answer: As discussed in the CITI training, there was a need to ensure and provide guidance for the protection of human subjects. Although some research may be simplistic in nature, all human subjects research is still research and needs to adhere to the same guidelines. Ultimately this is to protect people, so when thinking about your research, take the perspective of a person who does not know anything about your project and may have little to no knowledge about university research.
Recruiting participants
Question: How do I recruit WLU people to participate in my study?
Answer: We want to avoid multiple mass emails to everyone at WLU. Each IRB proposal is required to include intended recruitment and sampling techniques. We are suggesting that people use “Hilltopper Headlines” to recruit people to participate in research studies. Include your contact information and deadlines for participating. Flyers can also be used, however, they should be removed at the end of the recruitment period.
Note that electronic recruiting and data collection pose some additional challenges to human protections, especially in the area of confidentiality.
Classroom projects & Program assessment – WHEN IN DOUBT…. ASK!
Question: Do classroom projects or program assessment require IRB review?
Certain activities have the characteristics of research but do not meet the regulatory definition of research needing IRB review. It is about the purpose and INTENT of the project. If the purpose is to teach about research and NOT contribute to generalizable knowledge then it is not considered research (for the IRB).
Examples of activities that may not need IRB review are:
- Data collection for internal departmental, school, or other college administrative purposes (e.g. teaching evaluations, course evaluations)
- If your research is a class project or term paper and will not be published in any form at any time.
- Reviews and searches of existing literature and research involving a living individual, such as a biography, that is not generalizable beyond that individual.
Use the following guidelines to determine if your activities in the classroom are subject to IRB review. IRB review is NOT required if all of the following are true:
1. The project is limited to surveys/questionnaires/interviews/observations of public behavior directly related to topics being studied in an official college course and will not be professionally published or presented outside the requirements of the course.
2. The above surveys/questionnaires/activities, etc. contain no sensitive personal questions (e.g., no questions about drug use, sexual behavior or attitudes, criminal activity, grades, medical history) or other personal information that could stigmatize an individual.
3. No identifying information is recorded to link a person with the data such that it could reasonably harm the individual’s reputation, employability, financial standing, or place them at risk for criminal or civil liability.
4. The participants in the project are not from a vulnerable or special population (e.g., pregnant women, prisoners, minors, cognitively impaired individuals).
5. The collected data does not leave the classroom setting, or if the project involves collecting data on an organization, agency or company, the data are shared only with that entity.
6. No WLU employee or student is receiving financial compensation for collecting, organizing, analyzing, or reporting the data.
If not all of these conditions are met, or if your project does not fall into any of these categories, your project will require IRB notification and may require formal IRB approval before you can start with your project.
When in doubt – email the irb and ask!
- Preparing Your Proposal
- IRB Forms – Information
WLU INSTITUTIONAL REVIEW BOARD West Liberty University College of Sciences 208 University Dr. West Liberty, WV 26074
- IRB Chair: Tifani Fletcher Email
- IRB Administrator: Karen Kettler Email
Office of the Vice President for Research
- Service Units
- Research Integrity & Compliance
- Human Subjects Protection Program (HSPP)
- Education and Resources
IRB Frequently Asked Questions
- uconn health
Questions Related to the Need for IRB Review
Q: Does a case study / case series require IRB review?
A: Research is defined as a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge. Consistent with many other institutions, UConn Health does not consider a write up of a single case, or two or three cases, as constituting research. The retrospective summary of such a few number of cases is not considered to meet the definition of research. A review of four or more cases is considered to meet the definition of research and prospective IRB review is required.
Q: When should I submit a Human Subject Research Determination Form?
A: The Human Subject Research Determination Form can be submitted when an individual is considering a project that s/he does not think constitutes human subject research, but would like an official determination made by the IRB prior to initiating the project. A representative of the IRB will review the form and make the final determination as to whether the proposed activity meets the definition of human subject research. If the reviewer determines the project does not constitute human subject research the IRB will have no further involvement. If the reviewer determines that the project does constitute human subject research a complete IRB application will be required. Determination are usually made within two weeks of the date the IRB received the request for determination.
Q: Does a student project involving humans require IRB review?
A: If the project meets both the definition of research and human subject IRB review is required. The definition of research is often key in determining if a student project needs IRB review. Research is defined as a systematic investigation designed to develop or contribute to generalizable knowledge . If a student project is not designed to develop or contribute to generalizable knowledge (e.g. it will be used only to satisfy a curricular requirement), it would not meet the definition of research. Master’s or Doctoral proposals are typically designed to develop or contribute to generalizable knowledge so IRB review for such is typically required if the project also involves human subjects. IRB approval cannot be granted retrospectively. Students are therefore encouraged to complete a Human Subject Research Determination Form prior to starting any activity for which a question exists as to whether it constitutes human subject research. That form can be used to self-determine whether IRB review is required; or it can be thoroughly completed and submitted to the IRB for a formal determination.
When students at UConn Health are involved in the conduct of human subjects research at an external institution, please consult this engagement guidance document to ensure any IRB requirements at UConn Health are addressed.
Q: What type of IRB review is required for a research project using existing specimens?
A: It depends on whether the existing specimens are identifiable. If not, and if the investigator will make no effort t re-identify the specimens, the specimens do not meet the definition of human subject and IRB review would not be required. If the specimens are identifiable, or efforts will be made to re-identify the samples, IRB review is required. Research that only involves the use of existing identifiable samples may qualify for exemption if one of the following is true:
- the specimens are publicly available
- information is recorded in such a manner that the identity of the human subject cannot readily be ascertained directly or through identifiers linked to the subjects, the investigator does not contact the subject and the investigator will not re-identify subjects.
Q: Does a pilot study have to be reviewed by the IRB?
A: Yes. Any study involving human subjects, regardless of the number of subjects to be involved, must be reviewed and approved by the IRB prior to initiation. Because the purpose of a pilot study is to test the feasibility of a design that is intended to be used on a larger scale a pilot study is considered be “designed to develop or contribute to generalizable knowledge”.
Q: If I am going to do research on excess specimen samples (i.e., urine, blood, skin, etc.) that are being collected for a clinical purpose and that are otherwise going to be thrown away, do I have to get IRB review?
A: If the samples are to be used for a research purpose, and if at any point in time the investigator can link a sample (directly or through codes) to the individual from whom it came, IRB review is required. If information is record in such a way that individuals from whom the sample came cannot be identified the research may qualify for exempt status.
Q: If I am doing research on cadavers or on samples obtained from cadavers, do I have to get IRB review?
A: IRB review is not needed as the definition of human subject pertains to living individuals. However, if personal identifying information is linked to the materials, then HIPAA must be appropriately addressed. The Assurance of Using Decedent Information form must be submitted to the IRB, which also acts as the HIPAA privacy board for research purposes.
Q: If I want to make a change to my study after I’ve received approval, do I need IRB approval again?
A: Yes. Any change to a previously approved non-exempt study must be reviewed and approved by the IRB prior to implementation. This includes changes to any document related to the study (e.g. informed consent form, surveys, advertisements, recruitment letters, etc.) You will need to submit the form to request a modification / addendum to a previously approved study. The only exception to this is that a change can be implemented if it is needed to eliminate immediate harm to subjects or others. Such changes must be reported to the IRB within 5 business day. Investigators are encouraged to seek approval for any modification to an exempt study to ensure that the change does not invalidate the exempt status of the study.
Q: How does the mandate for single IRB review for multi-site federally supported research apply?
A: The NIH has published a list of frequently asked questions pertaining to the requirement for single IRB review. Please review this list. If your question is not addressed by this list, you may contact the IRB. Please note, the UConn Health IRB does not intend to act as the single IRB for NIH funded multi-site research. IRB reliance agreements are in place between UConn Health and commercial IRBs that are willing to act as the single IRB, and additional IRB Reliance agreements can be entered into if needed.
Questions Related to IRB Submissions:
Q: Once I submit my application, how long does the IRB review process take?
A: Review of exempt and expedited applications occurs on an on-going basis. Screening of the application will generally occur within 7 to 10 business days of receipt. Any concerns will be communicated to the investigator through iRIS. Once all concerns have been addressed the application will be forwarded for official review. Studies requiring full board review must be submitted by the published deadline. This allows sufficient time for the committee to review the application prior to the meeting. After the meeting the minutes must be prepared and approved by the committee before letters are sent to the investigators. Therefore, investigators should not expect an official letter until 7 to 10 business days after the meeting. Note that you may not begin your research until you have received final, unconditional approval from the IRB.
Q: Can I e-mail my IRB materials to the IRB office?
A: IRB forms (applications, requests for continuation, requests for modifications, problem reports) and supporting documents should be submitted through the iRIS system. For UConn Health employees and students, your UConn Health username and password can be used to access iRIS.
Q: What is my IRB number?
A: After you have submitted your application to the IRB for review, the IRB will assign an IRB number to your study. The IRB number can be used to search for the study within iRIS. The first two digits represent the latter part of fiscal year that the study was submitted, the next three digits represent the sequential order of receipt, and the last digit represents the panel to which the study was assigned. If there is an X after the first two digits it indicates the research was determined to qualify for exemption. If there is a letter appended after the three sequential numbers the letter indicates that UConn Health is acting as the IRB for another institution. For example an IRB number of 19-001C-2 reflects receipt of the study in fiscal year 2018/2019, that it was the first study received within the fiscal year and that Connecticut Children’s Medical Center is relying on the UConn Health IRB for oversight of the research, and that the submission was reviewed by Panel 2.
Note that any e-mail written or phone inquiry should make reference to the IRB number of the study. This will enable the IRB to quickly locate any required information.
Q: Why should I have a version number on my protocol and consent form?
A: It should always be possible to quickly determine which version of the protocol was current when the ICF was signed. Investigators are encouraged to maintain a link between the consent document and current protocol. For example, if you submit a modification that affects the informed consent form, but not version 1.0 of the protocol, the informed consent version reference should be revised, by adding a number after the version reference, e.g., Version 1.1. This indicates that the informed consent form is still linked to version 1 of the protocol.
Revising the version number also helps investigators to differentiate between copies of the protocol that have been modified. For example, if a modification was made to procedures in the protocol but both versions were labeled as version 1 (as opposed to 1 and 2 or 1 and 1.1) it would be easier for the investigator to follow the incorrect version of the protocol.
Q: I am not sure how to answer some questions in the application; can I leave them blank?
A: The IRB expects the investigator to respond to relevant items on the IRB application. Providing complete responses will make it less likely that the application will be returned for corrections. If you have a question as to how to respond you are encouraged to contact a Regulatory Specialist within the IRB for guidance.
Q: Can I submit my application without the signature of the Principal Investigator?
A: No, the application must be signed by the Principal Investigator. With the implementation of the iRIS system, the PI can access the system from any place with internet access in order to sign the submission.
Q: What documents do I need to submit to the IRB for review of my study?
A: Because every study is unique it is not possible to provide directions that will address every situation. The relevant IRB application checklist should be used as guide in determining the submission requirements. You may also want to review the table “What Documents to Submit” at https://ovpr.uchc.edu//rcs/hspp/resources/expedited-exempt/ . The documents common to all new expedited and exempt submissions are noted in the first row and additional documents that are often associated with specific study designs follow. Some projects may combine characteristics of more than one study type.
Q: Are there any protocol templates in the IRB/HSPP web-site that can guide me in preparing my study protocol?
A: A protocol template is available on the IRB forms page.
Q: My study was deferred at the IRB meeting held on Monday night. Can I have an extension to the submission deadline so that my study can be reviewed at the next regularly scheduled meeting of that panel?
A: Yes, you can have a one week extension on the submission deadline. This is allowed for two reason. First because the IRB recognizes that the turn-around-time without the extension is quite difficult to meet. Second, because the reviewers have already seen the initial version of the protocol and discussed it at the board, allowing the reviewer one week to re-review is sufficient. Note that if you study was deferred it must be reviewed by the same IRB panel that conducted the initial review.
Q: Do I have to submit every single protocol deviation (e.g. instance of non-compliance with the protocol) to the IRB?
A: Yes, however the timing of when you report the non-compliance may differ. If the non-compliance was within the control of the research team (e.g. a follow-up appointment was scheduled by the research team outside of the study window) it should be reported within 5 business days of becoming aware of it using the problem report form available in iRIS. All reports should contain a description of the deviation, why and when it occurred, and corrective action implemented, if any, to prevent future occurrence. If the non-compliance was not within the control of the research team it and it does not place a subject at risk (e.g. the subject canceled a scheduled appointment and had to be rescheduled outside of the study window) the event can be reported at the time of continuation. However, if the event was not within the control of the research team but has in impact on subject safety or data integrity it should still be reported within 5 business days of becoming aware of it. If possible, investigators should allow for “windows” within the protocol design to reduce the instances of non-compliance. For example, rather than stating that a second visit will occur in 2 weeks state that second visits will occur within 10 to 18 days.
Q: How do I know which HIPAA form to submit?
A: You must address HIPAA within your application if your study will involve the use of protected health information. Protected health information means individually identifiable health information transmitted or maintained in any form (electronic means, on paper, or through oral communication) that relates to the past, present or future physical or mental health or conditions of an individual. In general,
- if you are obtaining informed consent you will also need to obtain a HIPAA Authorization, or
- if you are seeing, but not recording any identifiable information you will submit a Certification of Using De-Identified data form, or
- if you need to keep some identifiable information, and you cannot obtain an Authorization, you will apply for a Waiver of HIPAA Authorization.
Q: If the sponsor requires that a safety report be submitted to the IRB, but the adverse event reporting policy requires that it be reported only at the time of continuation (e.g., it is a non-serious event), should I still submit it to the IRB?
A: You should inform the sponsor that our reporting policy is that non-serious events will be submitted in summary format at the time of continuation. The IRB will not review individual non-serious reports.
Q: If my project will most likely qualify for exempt status do I still have to submit the entire IRB application, write a protocol, and make sure everyone has completed training in the protection of human subjects?
A: Yes. The IRB is the only office that can determine if a research project qualifies for exemption and must have sufficient information in order to make that determination; and training requirements pertain regardless of the type of review conducted.
Q: I submitted an application for expedited review three days ago. Why haven’t I heard from the IRB yet?
A: Expedited review does not mean a quicker review. It simply means that only the Chair or an IRB member designated by the Chair has to review the project. All of the same criteria for approval still apply and applications are reviewed with the same level of scrutiny as a full board review.
Q: I will be doing research that will involve individuals who speak a foreign language. If the IRB approved the English version of the consent form do I have to submit a translated version for approval?
A: Yes. The IRB must approve all translated documents that will be presented to subject, e.g. the informed consent form and surveys. Investigators may either use a professional translation service or the back translation process. The full details are available in the Human Subjects Protection Office/IRB operating policies. Information on companies that provide translation services is available on the Information for Investigators page.
Questions Related to Continuing Review:
Q: How do I know when my study is due for continuing review?
A: When the IRB grants you final approval the approval letter will include the date by which your request for continuation is due. The iRIS system should send 30, 60 and 90 day reminders to you to request continuing review. However, the investigator retains the responsibility for seeking continuation of an approved study and the IRB encourages you to add reminder notices to your outlook calendar.
Q: What happens if I miss the submission deadline for my continuation that requires full board review?
A: A lapse in IRB approval for the study will occur if continuing approval is not granted by the end of day on the expiration date of approval. For example if the expiration date of a study is May 1st, research activity could occur on May 1st, but could not occur on May 2nd unless continuing approval had been obtained. If a lapse in approval occurs, all research related activity must cease until approval for continuation is obtained. If a subject is on active treatment and will be exposed to harm if treatment is withheld during the lapse in approval, you must request in writing approval from the IRB Chair to continue to treat the subject. You must also confirm that you are seeking continuation and will meet the next submission deadline.
Q: Why must I request continuation in less than one year when my colleagues’ studies where given approval for a full year?
A: When continuing review is a requirement, regulations require that continuing review occur at least annually. However, the IRB has the authority to require that review occur more frequently. Example scenarios of when the IRB may exercise this option include studies that pose a high risk to subjects or studies for which the investigator does not have extensive experience in the field.
Q: Why do I have to submit the complete protocol again at the time of continuing review if I haven’t changed it since the initial approval?
A: Federal guidance states that in order for continuing review to be meaningful and substantive the IRB must review the complete protocol. Attaching the protocol at the time of continuing review includes it in a master pdf file that the iRIS system creates for the assigned IRB reviewer. This facilitates the review process for them.
Questions Related to Informed Consent:
Q: What is informed consent?
A: Informed consent reflects an individuals voluntary agreement to participate in a research study. In order to make this agreement the individual must have adequate knowledge and understanding of the relevant information (purpose of the study, risks and benefits of the study, methods to be used, etc.). the process of informing a subject about a study is documented using the informed consent form.
Q: Who can provide informed consent?
A: Individuals who have reached the age of majority and who are competent can provide informed consent to participate in a research study. If the subject is either a minor or lacks the capacity to provide informed consent, a surrogate person (i.e. a legally authorized representative) must provide consent and the subject will generally be asked to provide assent. The policy to provide surrogate consent for research studies follows the same standards as providing surrogate consent for clinical care.
Q: What does a research subject need to be informed of during the consent process?
A: The informed consent checklist outlines the regulatory requirements of the informed consent form and these requirements are incorporated into the IRB sample consent form. If these documents are used as a guideline, you should address all required elements within your consent document. However, consent is more than a document. Consent must be a process in which the research subject has the right to read and discuss the form and to ask any questions regarding the study and have them answered in a satisfactory manner.
Q: Is informed consent always required?
A: In the majority of non-exempt studies informed consent is required. However, there are situations in which the requirement to obtain informed consent may be waived or altered. A retrospective chart review study is an example of when the requirement to obtain consent may be waived. The form to request a waiver or alteration of informed consent must be completed and submitted to the IRB for review and approval. The form describes the criteria that must be satisfied in order for the IRB to grant approval to the request.
Questions Related to Closure of Human Subject Research Studies:
Q: When should I close my study?
A: A research study should be closed by the Principal Investigator (PI) once all human research activities are completed regardless of whether a study is subject to the continuing review requirement. To do so, a closure form should be submitted to the IRB through the iRIS submission system for each non-exempt human research study (e.g., studies approved under Expedited review or Full Board review). The closure form should be submitted before the expiration of IRB approval, however, the PI can also submit a closure form after they have received a notice of lapse of approval.
If the study is an exempt study, the PI may allow the IRB approval to expire. When approval of an exempt study expires, the IRB will administratively close the study, but this does not invalidate the exemption. The research, as proposed to the IRB, may continue; it is not necessary to keep the exemption actively registered with the IRB.
A closure form allows the IRB to have a summary of the following information:
- the findings of the study
- the final enrollment data
- whether the study met the recruitment goals, and
- subject complaints
- unanticipated problems involving risk to subjects or others,
- unexpected profile of adverse events in terms of frequency and/or severity,
- non-compliance with or deviation from the approved protocol or procedures,
- audits, inspections or monitoring visits by internal or external personnel.
- any publications, presentations, trademarks, patents, etc. related to the study.
The Guidance on Closure of Human Subject Research Studies .docx explains the circumstances in which a non-exempt human research study may be closed from IRB oversight, as well as ongoing researcher responsibilities that apply to closed studies.
- Submitting to the IRB
- Types of Reviews
Exempt Review
Updates to Exempt Studies
Restrictions on exemptions.
OHRP Exempt Categories
Exempt human subjects research is a specific sub-set of “research involving human subjects” that does not require ongoing IRB oversight. Research can qualify for an exemption if it is no more than minimal risk and all of the research procedures fit within one or more of the exemption categories in the federal IRB regulations. Studies that qualify for exemption must be submitted to the IRB for review before starting the research. Pursuant to NU policy, investigators do not make their own determination as to whether a research study qualifies for an exemption — the IRB issues exemption determinations. There is not a separate IRB application form for studies that could qualify for exemption – the appropriate protocol template for human subjects research should be filled out and submitted to the IRB in the eIRB+ system.
Although the HHS IRB regulations list eight exemption categories, NU has opted to implement six of those categories at this time (see the list below). Of the six exemption categories listed below, only exemption category 6 (for taste and food quality evaluation and consumer acceptance studies) applies to studies that are FDA-regulated.
The Belmont principle of Respect for Persons generally requires that subjects be given the opportunity to choose whether or not to participate in research. For this reason, voluntary informed consent should be obtained from participants for any exempt research where the investigator will be collecting data through interaction with participants. For exempt research studies that will collect data through interaction with participants, the NU IRB expects that researchers provide participants with consent information that includes, at a minimum:
- An explanation that they are being asked to participate in a research study.
- The identity and affiliation of the researcher.
- A clear description of the study procedures and how data will be used in the future.
- A statement that participation in the research is voluntary.
- Contact information for questions and concerns about the research.
Studies that qualify for an exemption do not undergo continuing review. Additionally, modifications do not need to be submitted for exempt studies so long as the research remains minimal risk and stays within the boundaries of the exemption categories that the IRB found were applicable to the research.
It is a best practice recommendation to create a note-to-file in your research record to document the changes you make and your determination that these updates did not change the scope of the study or risk to participants. You can download a template note-to-file template on the IRB Office’s Study Support Resources and Templates webpage.
If your proposed changes constitute any of the following, submit a track change version of the protocol you submitted to the IRB in a new IRB application using the “copy submission” function in eIRB+:
- add procedures that could affect risks to participants; or
- add procedures that do not fit within the exemption categories; or
- add new types of participants to your study that include vulnerable populations (e.g., adding children, individuals with cognitive impairments, prisoners, etc.)
- change of Principal Investigator
Examples of updates that would likely require IRB review:
- Removal of the consent process, or use of deception or incomplete disclosure.
- Significant changes to the recruitment procedures.
- Adding sensitive questions to a survey or interview process (e.g. questions regarding illegal activities; traumatic events such as childhood, sexual, or domestic abuse; suicide; or other probing questions that could reasonably place the subjects at risk of criminal or civil liability or be damaging to the subjects’ financial standing, employability, educational advancement, or reputation).
- Collection of new or additional identifiable information.
- Changes to the data storage plan which may affect confidentiality.
- Adding any new physiological measures that were not already determined to be exempt.
Exempt studies may also be subject to the HIPAA Privacy Rule. For instance, a study involving medical record review to gather a dataset that would be eligible for Exemption Category 4 involves access to Protected Health Information (PHI) and should request a waiver of HIPAA authorization.
- Studies that are greater than minimal risk do not qualify for exemption.
- Exemptions do not apply to research with prisoners , except for research aimed at involving a broader subject population that only incidentally includes prisoners. [45 CFR 46.104(b)(2)].
- Exemption 2(iii) and Exemption 3 do not apply to research with children.
- Exemptions other than Exemption Category 6 do not apply to FDA-regulated research.
OHRP Exempt Categories 45 CFR 46.104 – (HRP-312)
Research conducted in established or commonly accepted educational settings that specifically involves normal educational practices that are not likely to adversely impact students’ opportunity to learn required educational content or the assessment of educators who provide instruction. This includes most research on regular and special education instructional strategies, and research on the effectiveness of or the comparison among instructional techniques, curricula, or classroom management methods.
- Evaluating the use of accepted or revised standardized tests
- Testing or comparing a curriculum or lesson
- A program evaluation of pharmacy continuing education
Research that only includes interactions involving educational tests (cognitive, diagnostic, aptitude, achievement), survey procedures, interview procedures or observation of public behavior (including visual or auditory recording) if at least one of the following criteria is met:
- The information obtained is recorded by the investigator in such a manner that the identity of the Human Subjects cannot be readily ascertained, directly or indirectly through identifiers linked to the subjects; OR
- Any disclosure of Human Subjects’ responses outside the research would not reasonably place the subjects at risk of criminal or civil liability or be damaging to the subjects’ financial standing, employability, educational advancement, or reputation; OR
- The information obtained is recorded by the investigator in such a manner that the identity of the Human Subjects can be readily ascertained, directly or indirectly through identifiers linked to the subjects, AND an IRB conducts limited IRB review. (See “WORKSHEET: Limited IRB Review (HRP-319).”)
- Surveying teachers, nurses, or doctors about a technique or an outcome
- Interviewing managers about a management style or best practice
- Conducting a focus group about an experience or an opinion of a community program
Research involving benign behavioral interventions in conjunction with the collection of information from an adult subject through verbal or written responses (including data entry) or audiovisual recording if the subject prospectively agrees to the intervention and information collection and at least one of the following criteria is met:
- The information obtained is recorded by the investigator in such a manner that the identity of the Human Subjects cannot readily be ascertained, directly or indirectly, through identifiers linked to the subjects; OR
- Any disclosure of the Human Subjects’ responses outside the research would not reasonably place the subjects at risk of criminal or civil liability or be damaging to the subjects’ financial standing, employability, educational advancement, or reputation; OR
(i) For the purpose of this provision, benign behavioral interventions are brief in duration, harmless, painless, not physically invasive, not likely to have a significant adverse lasting impact on the subjects, and the investigator has no reason to think the subjects will find the interventions offensive or embarrassing. Provided all such criteria are met, examples of such benign behavioral interventions would include having the subjects play an online game, having them solve puzzles under various noise conditions, or having them decide how to allocate a nominal amount of received cash between themselves and someone else.
(ii) If the research involves deceiving the subjects regarding the nature or purposes of the research, this exemption is not applicable unless the subject authorizes the deception through a prospective agreement to participate in research in circumstances in which the subject is informed that he or she will be unaware of or misled regarding the nature or purposes of the research.
- Healthy adult subjects are asked to take part in two two-hour-long assessments of memory, attention and information processing speed before and after 1 hour of cognitive enhancement exercise using specially designed computer software. The procedures are conducted during a single visit, and subjects are encouraged to take breaks when desired.
Secondary research for which consent is not required: Secondary research uses of identifiable private information or identifiable biospecimens, if at least one of the following criteria is met:
- The identifiable private information or identifiable biospecimens are publicly available; OR
- Information, which may include information about biospecimens, is recorded by the investigator in such a manner that the identity of the human subjects cannot readily be ascertained directly or through identifiers linked to the subjects, the investigator does not contact the subjects, and the investigator will not re-identify subjects; OR
- The research involves only information collection and analysis involving the investigator’s use of identifiable health information when that use is regulated under 45 CFR parts 160 and 164 (HIPAA), subparts A and E, for the purposes of “health care operations” or “research” as those terms are defined at 45 CFR 164.501 or for “public health activities and purposes” as described under 45 CFR 164.512(b); OR
- The research is conducted by, or on behalf of, a Federal department or agency using government-generated or government-collected information obtained for non-research activities, if the research generates identifiable private information that is or will be maintained on information technology that is subject to and in compliance with section 208(b) of the E-Government Act of 2002, 44 U.S.C. 3501 note, if all of the identifiable private information collected, used, or generated as part of the activity will be maintained in systems of records subject to the Privacy Act of 1974, 5 U.S.C. 552a, and, if applicable, the information used in the research was collected subject to the Paperwork Reduction Act of 1995, 44 U.S.C. 3501 et seq.
Note: Exemption Category 4 only applies to the re-use of data and specimens that were or will be collected for non-research purposes or from research studies other than the proposed research study. The research materials typically will be publicly available materials, medical records, or existing repositories of clinical specimens. No contact between investigator and subject is allowed. If an investigator wants to collect information/specimens directly from research subjects, then another IRB review path will be required. Exemption Category 4(iii) only applies to the use of data (when HIPAA applies) and not to biospecimens.
- A researcher is given two datasets that contain private, identifiable information. The researcher uses the identifiers to merge the two datasets but strips the resulting (merged) data of identifiers immediately after the merge and before conducting data analysis. The resulting data used for the analysis is completely de-identified with no link to identifiers.
Research and demonstration projects which are conducted or supported by a Federal department or agency, or otherwise subject to the approval of department or agency heads (or the approval of heads of bureaus or other subordinate agencies that have been delegated authority to conduct the research and demonstration projects), and that are designed to study, evaluate, improve, or otherwise examine: public benefit or service programs, including procedures for obtaining benefits or services under those programs, possible changes in or alternatives to those programs or procedures, or possible changes in methods or levels of payment for benefits or services under those programs.
(i) Each Federal department or agency conducting or supporting the research and demonstration projects must establish, on a publicly accessible Federal Web site or in such other manner as the department or agency head may determine, a list of the research and demonstration projects that the Federal department or agency conducts or supports under this provision. The research or demonstration project must be published on this list prior to commencing the research involving human subjects.
Taste and food quality evaluation and consumer acceptance studies, (i) if wholesome foods without additives are consumed or (ii) if a food is consumed that contains a food ingredient at or below the level and for a use found to be safe, or agricultural chemical or environmental contaminant at or below the level found to be safe, by the Food and Drug Administration or approved by the Environmental Protection Agency or the Food Safety and Inspection Service of the Dept. of Agriculture
- Study protocol
- Open access
- Published: 28 June 2024
Comparative effectiveness of an individualized model of hemodialysis vs conventional hemodialysis: a study protocol for a multicenter randomized controlled trial (the TwoPlus trial)
- Mariana Murea 1 ,
- Jochen G. Raimann ORCID: orcid.org/0000-0002-8954-2783 2 ,
- Jasmin Divers 3 ,
- Harvey Maute 3 ,
- Cassandra Kovach 4 ,
- Emaad M. Abdel-Rahman 5 ,
- Alaa S. Awad 6 ,
- Jennifer E. Flythe 7 ,
- Samir C. Gautam 8 ,
- Vandana D. Niyyar 9 ,
- Glenda V. Roberts 10 ,
- Nichole M. Jefferson 11 ,
- Islam Shahidul 3 ,
- Ucheoma Nwaozuru 12 ,
- Kristie L. Foley 12 ,
- Erica J. Trembath 2 ,
- Merlo L. Rosales 2 ,
- Alison J. Fletcher 1 ,
- Sheikh I. Hiba 1 ,
- Anne Huml 4 ,
- Daphne H. Knicely 5 ,
- Irtiza Hasan 6 ,
- Bhaktidevi Makadia 6 ,
- Raman Gaurav 8 ,
- Janice Lea 9 ,
- Paul T. Conway 13 ,
- John T. Daugirdas 14 &
- Peter Kotanko 15
on behalf of the Two Plus Research Consortium
Trials volume 25 , Article number: 424 ( 2024 ) Cite this article
Metrics details
Most patients starting chronic in-center hemodialysis (HD) receive conventional hemodialysis (CHD) with three sessions per week targeting specific biochemical clearance. Observational studies suggest that patients with residual kidney function can safely be treated with incremental prescriptions of HD, starting with less frequent sessions and later adjusting to thrice-weekly HD. This trial aims to show objectively that clinically matched incremental HD (CMIHD) is non-inferior to CHD in eligible patients.
An unblinded, parallel-group, randomized controlled trial will be conducted across diverse healthcare systems and dialysis organizations in the USA. Adult patients initiating chronic hemodialysis (HD) at participating centers will be screened. Eligibility criteria include receipt of fewer than 18 treatments of HD and residual kidney function defined as kidney urea clearance ≥3.5 mL/min/1.73 m 2 and urine output ≥500 mL/24 h. The 1:1 randomization, stratified by site and dialysis vascular access type, assigns patients to either CMIHD (intervention group) or CHD (control group). The CMIHD group will be treated with twice-weekly HD and adjuvant pharmacologic therapy (i.e., oral loop diuretics, sodium bicarbonate, and potassium binders). The CHD group will receive thrice-weekly HD according to usual care. Throughout the study, patients undergo timed urine collection and fill out questionnaires. CMIHD will progress to thrice-weekly HD based on clinical manifestations or changes in residual kidney function. Caregivers of enrolled patients are invited to complete semi-annual questionnaires. The primary outcome is a composite of patients’ all-cause death, hospitalizations, or emergency department visits at 2 years. Secondary outcomes include patient- and caregiver-reported outcomes. We aim to enroll 350 patients, which provides ≥85% power to detect an incidence rate ratio (IRR) of 0.9 between CMIHD and CHD with an IRR non-inferiority of 1.20 ( α = 0.025, one-tailed test, 20% dropout rate, average of 2.06 years of HD per patient participant), and 150 caregiver participants (of enrolled patients).
Our proposal challenges the status quo of HD care delivery. Our overarching hypothesis posits that CMIHD is non-inferior to CHD. If successful, the results will positively impact one of the highest-burdened patient populations and their caregivers.
Trial registration
Clinicaltrials.gov NCT05828823. Registered on 25 April 2023.
Peer Review reports
Administrative information
| Comparative effectiveness of an individualized model of hemodialysis vs conventional hemodialysis: a study protocol for a multicenter randomized controlled trial (the Two Plus trial) |
|---|---|
| ClinicalTrials.gov identifier: NCT05828823 Registered on 25 April 2023 |
| Protocol version 0.5 before recruitment start-up Approved by central IRB on 31-01-2024 |
| Patient-Centered Outcomes Research Institute (PCORI) Award CER-2022C1-26300 |
| [SPIRIT guidance: Affiliations of protocol contributors.] 1. Wake Forest University School of Medicine 2. Renal Research Institute |
| Patient-Centered Outcomes Research Institute (PCORI) 1828 L Street NW, Suite 900 Washington, CD 20036 Phone: (202) 827-7700 Email: [email protected] |
| The study funder (PCORI) has no role in the design, execution, analyses, interpretation of data, manuscript writing, or decision to submit results for this study. |
Introduction
Background and rationale {6a}, central problem.
Patients diagnosed with kidney dysfunction requiring dialysis (KDRD), a condition widely labeled end-stage kidney disease (ESKD), are treated with a conventional “one-size-fits-all” strategy of chronic hemodialysis (HD). The regimen of thrice-weekly HD, known as “conventional HD” (CHD), has been the norm since the 1970s, yet it was implemented without the support of prospective studies to validate the adequacy of this treatment frequency and dose in all patients with new-onset KDRD [ 1 , 2 ]. Since then, CHD has been compared solely against more frequent HD [ 3 , 4 ]. Target HD dose per treatment was validated in clinical trials that studied only thrice-weekly CHD in patients with KDRD and no residual kidney function [ 5 , 6 ]; those results were viewed as “optimal” HD for all patients. Initiation of CHD is marked by a sudden rise in hospitalizations and mortality [ 7 ] that peak in the first 6 months of HD when adverse events are 3-fold higher than during later dialysis periods [ 8 ]. HD induces tissue damage in all organs—including the heart, brain, and gut [ 9 , 10 ]. The abrupt switch from pharmacologic-based treatment to “full-dose” thrice-weekly CHD is believed to cause systemic circulatory “stress” and multi-organ injury [ 11 ], which, in the context of preexisting comorbidities, contribute to proximate adverse outcomes [ 9 , 12 ]. An alternative to CHD consists of delivering HD at a dose no higher than would be needed to complement existing levels of endogenous residual kidney function while maintaining patient well-being; we call this approach “clinically matched incremental HD” (CMIHD). Patients who may benefit from CMIHD are those who have residual kidney function levels equivalent to one or two HD treatments in urea clearance metrics [ 13 ].
Patient-identified research priorities
A top priority of patients and other stakeholders is finding ways to reduce HD-related burden [ 14 ]. Starting dialysis is stressful to patients and their caregivers, with the initial months being highly critical for both adaptation and mortality [ 8 ]. From patients’ and caregivers’ perspectives, the reported stressors of HD are “uncertainty about the future,” “limits on physical activities,” “interference with social activities,” and “interference with job” [ 15 ]. Patients report that dialysis-associated fatigue impacts work, home responsibilities, and social participation [ 16 ]. In the Empowering Patients on Choices for Renal Replacement Therapy (EPOCH-RRT) study [ 17 ] and the Standardized Outcomes in Nephrology–Hemodialysis (SONG-HD) Initiative [ 18 , 19 ], patients receiving CHD reported that the largest negative impact on quality of life is how their HD schedule interferes with work and school, or how it can change life plans [ 20 ]. They felt that dialysis therapy should be more flexible to improve their functioning [ 20 ]. Furthermore, patients and caregivers valued the ability to travel, dialysis-free time, and not feeling “washed-out” after dialysis [ 21 ].
Growing evidence in support of CMIHD
A growing body of studies has shown that incremental transition from pre-dialysis to twice-weekly and then thrice-weekly hemodialysis is safe [ 22 ]. Observational studies in patients with incident KDRD and residual kidney function suggested a twice-weekly HD start, compared with thrice-weekly HD, yielding longer or similar [ 23 , 24 , 25 , 26 , 27 , 28 ] patient survival when adequate residual kidney function was present. One less HD treatment a week can offer more dialysis-free time, more flexible travel options, increased physical activity, and substantially better health-related quality of life [ 29 , 30 ]. In addition, residual kidney function, which may be better preserved with incremental HD, is associated with better health-related quality of life [ 31 ]. Despite the accumulation of clinical experience and research findings, these have yet to persuade most nephrology professional stakeholders, resulting in the underutilization of incrementally prescribed HD. Consequently, this treatment option remains inaccessible to most patients commencing chronic HD in the USA and numerous other nations, which represents a barrier to patient care choice.
Residual kidney function at HD Initiation national guidelines, updated in 2015, endorses twice-weekly HD for patients with kidney urea clearance (or urea elimination) levels ≥2.0 mL/min [ 32 ]. Kidney function at HD initiation, measured as average kidney urea and creatinine clearance from blood, ranges from 7.8 to 9.7 mL/min/1.73 m 2 ; >90% of patients [ 33 , 34 ] have ≥5 mL/min/1.73 m 2 , indicating that many who begin HD still have some residual kidney function [ 35 ]. Among patients with incident KDRD, studies showed that about 25–40% would be eligible for initial treatment with twice-weekly HD [ 34 , 36 , 37 , 38 , 39 , 40 ]. Yet, national registry data show CMIHD is provided to less than 1% of patients who start HD [ 24 , 41 ]. Lack of prospective studies to evaluate the clinical effectiveness and operationalization of CMIHD has hindered systematic incorporation of this treatment option into clinical practice.
Scope of the problem
Major gaps in CHD paradigm include: (a) it has not been tested in those with new-onset KDRD with residual kidney function [ 42 ]; (b) it is not tailored to each patient’s kidney function [ 43 , 44 , 45 ]; and (c) it unnecessarily subjects some patients to burdensome and costly dialysis overtreatment [ 46 , 47 ]. There is a need for rigorous, implementation-effectiveness controlled trials to compare CMIHD and CHD. In the TwoPlus trial, we will address 3 essential questions: (1) Can CMIHD treat clinical symptoms of advanced kidney failure in patients with residual kidney function? (2) Does CMIHD confer better quality of life? and (3) What factors mediate CMIHD implementation, according to different stakeholders?
Objectives {7}
Primary objective.
The primary objective is to compare the effectiveness of CMIHD with CHD in patients with new-onset KDRD who have residual kidney function. The primary outcome is the cumulative incidence rate of all-cause death, hospitalizations, or emergency department visits over an average period of follow-up of 2 years.
Secondary objective
The secondary objective is to compare patient- and caregiver-reported outcomes and other clinical effectiveness outcomes.
Tertiary objective
In parallel, we will characterize intervention implementation using process evaluation to identify contextual factors related to CMIHD implementation.
The primary hypothesis is that, compared to CHD, CMIHD will have similar effectiveness assessed as a composite of all-cause emergency department visits, hospitalizations, or death. After showing noninferiority, we will test whether there are fewer safety events in patients treated with CMIHD.
The secondary hypotheses are that, compared to patients treated with CHD, patients treated with CMIHD will:
Report better quality of life, and caregivers will report less caregiving burden
Experience better preservation of residual kidney function
Have similar control of anemia, bone mineral metabolism, and nutrition parameters
Have fewer vascular access complications
Have improved mental and health outcomes.
The tertiary hypothesis is that the intervention effect size on the primary and secondary outcomes will be moderated by effective implementation as measured by the process evaluation.
Trial design {8}
The TwoPlus trial is an individually randomized, parallel-group, type 1 hybrid effectiveness-implementation non-inferiority clinical trial with process evaluation (Fig. 1 ). The study has two concurrent activities:
Randomized controlled trial (RCT), to quantitatively assess and compare patient clinical outcomes, patient-reported outcomes, and caregiver-reported outcomes, using randomized treatment allocation, in a 1:1 ratio, to either CMIHD or CHD; and
Process Evaluation, to qualitatively assess stakeholders’ (patient participants, caregiver participants, dialysis treating providers, dialysis nurses, dialysis dieticians, and dialysis social workers) views on processes related to individualized HD during study conduct, using semi-structured interviews and focus group meetings.

The TwoPlus trial: study design and flowchart of recruitment, treatment allocation, and endpoints. Timepoint of assessment (months) is set from the month of baseline residual kidney function for patients, month of informed consent for caregivers, and Clinical Center recruitment start-up month for process evaluation
Patients who provide informed consent for study participation and who met all eligibility criteria will be randomized, in a 1:1 ratio, to one of two HD treatment models (Fig. 1 ):
CMIHD (intervention group): 2 HD sessions/week, ≥4 h per each HD session; and adjuvant pharmacotherapy (loop diuretics, potassium binder, and/or sodium bicarbonate). During follow-up, the HD schedule will migrate to 3 HD sessions per week when one or more Criteria for Progression are met.
CHD (control group): 3 HD sessions/week, as prescribed by the treating provider, typically ≥3 h per HD session.
Methods: participants, interventions, and outcomes
Study setting {9}.
The study will enroll 350 adult patients and 140 adult caregivers (of patient participants) in the USA of America (NCT05828823). Study participants will also include members of a Stakeholder Advisory Panel comprised of diverse stakeholders from all Clinical Centers. The target setting is community outpatient dialysis units and inpatient dialysis units at medical institutions caring for patients with new-onset KDRD [ESKD]. Enrollment will take place at 8 Clinical Centers represented by national healthcare institutions (Additional file 1, Figure S1) and their affiliated outpatient dialysis units. Study oversight will take place from the Clinical Coordinating Center (CCC) and Data Coordinating Center (DCC). Selection of Clinical Centers was determined by considering the patient population, ensuring an adequate volume and sociodemographic diversity for effective study recruitment. Selection criteria also considered the expertise of investigators in clinical trials, availability of research infrastructure, endorsement from regional and national leadership within the affiliated dialysis organization, and the demonstrated engagement of the study team in preparing for the implementation of the study.
Eligibility criteria {10}
Inclusion criteria are categorized into clinical inclusion criteria and residual kidney function inclusion criteria.
Clinical inclusion criteria
Age ≥ 18 years
New-onset kidney dysfunction requiring dialysis (also known as end-stage kidney disease [ESKD], end-stage renal disease [ESRD], or chronic kidney disease stage 5 on dialysis [CKD5D]) started on chronic, in-center HD, or anticipated to be started on chronic, in-center HD within the next 6 weeks
Has received ≤18 sessions of intermittent HD (i.e., on HD for ≤6 weeks) at the time patient is approached for potential study participation
Residual kidney function inclusion criteria
Kidney urea clearance ≥3.5 mL/min/1.73 m 2
Urine volume of ≥500 mL/24 h
Exclusion criteria
Serum potassium ≥5.8 mEq/L
Serum sodium ≤125 mEq/L
Serum bicarbonate level ≤17 mEq/L
Requirement or anticipated requirement of high-volume ultrafiltration
History of medical non-adherence that, in the opinion of the Site Investigators and/or treating provider, precludes safe study participation
A medical condition that, in the opinion of the Site Investigators and/or treating providers, would jeopardize the safety of the participant
Expected dialysis modality change (e.g., home HD, peritoneal dialysis) or kidney transplantation within the next 6 months
Estimated survival of <6 months, in the opinion of the Site Investigators and/or treating providers
Estimated transfer to a dialysis facility outside the care of the participating study team within the next 6 months
Known pregnancy, or positive serum pregnancy test, or planning to attempt to become pregnant in a woman of childbearing capacity
Unable or unwilling to follow the study protocol for any reason
Rationale for thresholds of residual kidney function
Based on a urea kinetics model, total stdKt/V urea clearance (total stdKt/V = dialysis stdKt/V + kidney stdKt/V) of ≥2.10 can be achieved with 2 HD sessions per week, each at a dose similar to thrice-weekly HD (i.e., dialysis spKt/V ≥1.20) when residual kidney urea clearance function is 3.5 mL/min/1.73 m 2 or above [ 47 ]. A more recent model of urea kinetics that gives more weight to residual kidney function indicates that kidney urea clearance levels ≥2.0 mL/min/1.73 m 2 are sufficient to complement twice-weekly HD [ 48 , 49 , 50 ]. Besides kidney urea clearance, 24-h urine volume is a requisite element of adequate residual kidney function [ 30 , 51 ]. For this study, we selected conservative thresholds for residual kidney function consisting of kidney urea clearance of ≥3.5 mL/min/1.73 m 2 and urine volume of ≥500 mL/24 h.
A patient-identified caregiver(s) will be eligible for study participation if they meet the following eligibility criteria:
Is of age ≥18 years
Has self-identified as caregiver (or care partner) for the respective patient participant
Has one of the following relationships with the patient participant, providing aid to the patient for activities related to daily care or health care:
Is a family relative of the patient: spouse, daughter, son, sister, brother, father, mother, grandchild, brother-in-law, sister-in-law, uncle, aunt); or
Is a close friend.
Does not have known psychiatric and neurologic disorders (through direct inquiry from the person)*
Is not a member of the medical or healthcare team
Does not provide care for another patient with chronic illness
Has not experienced severe life events within the 3 months prior to enrollment
To be randomized in this study, the candidate patients must meet all the inclusion criteria and none of the exclusion criteria. More information accompanying superscript characters to eligibility criteria is given in the Additional file 2.
Advisory panels
The members of the Advisory Panels will be selected to have the following characteristics:
Patient and Caregiver Advisory Panel will be comprised of enrolled patients and caregivers:
Patient enrolled and actively followed in the clinical trial;
Patient randomized to one of the treatment groups;
Caregiver enrolled and actively followed in the study.
Dialysis Treating Provider Advisory Panel will be comprised of treating nephrologists, dialysis medical directors, and/or advanced practice practitioners.
Treating nephrologists
Physician board-certified in Nephrology;
At least 1 year of clinical experience since Nephrology board certification; and
Treating adults with KDRD on HD at one or more dialysis facilities affiliated with the Clinical Center.
Dialysis medical directors
Served as Dialysis Medical Director for at least 1 year at one or more outpatient dialysis facilities affiliated with the Clinical Center.
Advanced practice practitioners (physician assistant, nurse practitioner)
Advanced Practice Practitioner credentialed in Nephrology;
At least 1 year of clinical experience in Nephrology-based practice since credentialed; and
Dialysis Nurse Advisory Panel will be comprised of dialysis nurses and/or nurse managers.
Licensed as a Registered Nurse or more advanced credentials; and
Employed as Dialysis Nurse, Dialysis Charge Nurse, and/or Dialysis Nurse Manager for ≥1 year at one or more outpatient dialysis facilities affiliated with the Clinical Center.
Dialysis Dietitian Advisory Panel
Licensed as a Registered Dietitian; and
Employed as a Dialysis Dietitian for ≥1 year at one or more outpatient dialysis facilities affiliated with the Clinical Center.
Dialysis Social Worker Advisory Panel
Licensed as Medical Social Worker; and
Employed as Dialysis Dietitian for ≥1 year at one or more outpatient dialysis facilities affiliated with the Clinical Center.
Who will take informed consent? {26a}
Informed consent will be obtained from patients, caregivers, and members of the advisory panels to participate in the TwoPlus trial. There will be three types of participant consent:
Patient informed consent for study participation: in-person and signed informed consent
Patient participant informed consent for limited data collection: in-person and signed informed consent
Caregiver consent: telephone consent; waiver of in-person, signed informed consent
Provider consent: electronic consent for survey, telephone consent for semi-structured interview; waiver of in-person, signed informed consent
More information regarding participants’ informed consent is available in Additional file 3.
Additional consent provisions for collection and use of participant data and biological specimens {26b}
In the consent form, participants will be requested to allow the utilization of their data in accordance with central and local Institutional Review Board (IRB) privacy policy. In the event of drop-out, data collected to the date of drop-out will be used in statistical analyses of study results. Additionally, participants will be asked for their consent to share pertinent data with the funder. For patients who cannot provide informed consent, the study team will not seek consent from an independent, legally designated representative; these patients will be deemed ineligible to participate in the study. At the time of this writing, the TwoPlus trial does not involve the collection and storage of biological samples.
Interventions
Explanation for the choice of comparators {6b}.
We will compare incrementally prescribed HD that starts with twice-weekly HD with adjuvant pharmacologic therapy and steps up to thrice-weekly hemodialysis as needed based on changes in residual kidney function levels (CMIHD or intervention group) with conventional HD that starts with thrice-weekly HD (CHD or control group). Adjuvant pharmacotherapy (e.g., loop diuretics, potassium binders, bicarbonate) can enhance residual kidney function and symptom management [ 34 , 52 , 53 , 54 ]. These drugs, ordinarily prescribed before HD initiation, are well tolerated. Based on international registry data, continuation of loop diuretics after HD initiation was associated with lower weight gain between HD treatments, twice the odds of retaining residual kidney function [ 55 ], and more stable blood pressure during HD [ 53 ]. Potassium binders can effectively enhance potassium excretion in patients with advanced kidney disease [ 56 ], and maintain potassium homeostasis in patients treated with less frequent HD [ 57 , 58 ] or those on CHD prone to hyperkalemia [ 59 ]. Thus, we will include adjuvant pharmacotherapy in the CMIHD group while the patient is on twice-weekly hemodialysis.
Intervention description {11a}
Hd prescription.
During the study, the HD dose per session will target spKt/V ≥1.20 in both treatment groups [ 32 , 47 , 48 ]. Excluding HD frequency and session duration allocation at randomization, all other HD treatment elements will be prescribed according to the treating providers (Table 1 ).
Blood tests and timed urine collection
These are recommended to be followed during the study in all participants. Per standard care, blood tests are done once or twice a month at outpatient dialysis units in all patients receiving HD. A blood test frequency of twice a month in patients randomized to CMIHD is advised; the lab tests and frequency can be adapted according to clinical judgment by the treating providers and Site Investigators (Additional file 1, Table S1). Timed urine collection will be obtained at a minimum frequency of every 3 months starting from the month of baseline residual kidney function assessment that was obtained at the time of screening and more often as deemed medically necessary by the treating providers and/or Site Investigators.
Adjuvant pharmacotherapy in the CMIHD group
The following medications have been termed “adjuvant medications”: diuretics, sodium bicarbonate, and potassium binder. These medications are Food and Drug Administration (FDA)-approved for the medication indication utilized during the study. Most patients are on one or more of these medications before HD treatment. The use of these medications is advised for patients assigned to CMIHD, the decision on the use and dose of these medications is guided by the Site Investigators according to their judgement (Table 2 ). These medications can also be used (and are often used) in patients treated with CHD according to the judgement of treating providers and/or Site Investigators. Loop diuretics [ 34 , 52 , 53 , 54 , 60 ], potassium binding agent, sodium bicarbonate [ 61 , 62 ], and/or a potassium binding agent [ 56 , 63 ] are recommended to those randomized to CMIHD to maintain volume, electrolyte, and acid-base homeostasis [ 64 ].
Loop diuretic
An oral loop diuretic will be prescribed to all patient participants in CMIHD during the period of twice-weekly HD, to be taken on non-HD days. For those on a loop diuretic before HD initiation, the same loop diuretic will be continued, and the dose will be doubled and administered on non-HD days. Those not on a loop diuretic before HD initiation will be started on a loop diuretic at a dose and frequency deemed medically appropriate; for example, Furosemide 80 mg per day, the dose of Furosemide is expected to range between 80 and 320 mg per day, in one or two divided doses, on non-HD days. Treating providers and Site Investigators may elect not to prescribe a loop diuretic, discontinue administration, or change dose during the study according to medical needs.
Bicarbonate buffer
Oral sodium bicarbonate will be prescribed to all patient participants in CMIHD during the period of twice-weekly HD, to be taken on non-HD days. Sodium bicarbonate will be prescribed on non-HD days for patient participants with pre-HD serum bicarbonate level ≤19 mEq/L. In clinical practice, sodium bicarbonate dose and frequency are adjusted based on pre-HD serum bicarbonate level, with a goal of 20–22 mEq/L. Treating providers and Site Investigators retain the discretion to opt against prescribing a bicarbonate buffer, halt its administration, or adjust the dosage as necessary based on medical requirements during the study.
Potassium-binding agents
Any potassium binder can be used as deemed medically necessary during the study and at the discretion of the Site Investigators and\or treating providers. It is recommended that, when administration of a potassium binder is deemed medically necessary, the dose and frequency will be adjusted for target pre-HD serum potassium level <5.5 mEq/L.
Criteria for discontinuing or modifying allocated interventions {11b}
Criteria for progression from twice-weekly to thrice-weekly hd.
All patient participants will be monitored according to standard care. The treating provider, in collaboration with the site investigators, will decide on progression from twice-weekly to thrice-weekly HD in the CMIHD treatment group. Criteria for Progression are summarized in Table 3 as a guidance to providers in decision-making process regarding progress from twice-weekly HD to thrice-weekly HD. Changes in HD prescription in both treatment groups will be at the discretion of the treating providers. The decision to transition from twice- to thrice-weekly HD will be made by the treating nephrologist and/or the site investigator, not by a Research Coordinator. No criterion, taken in isolation, is an absolute indication for transitioning from twice- to thrice-weekly HD. Each criterion will be judged in the overall clinical context for each individual to decide on medical necessity and timing of transition from twice- to thrice-weekly HD. More information accompanying superscript characters to Criteria for Progression is given in the Additional file 2.
Censoring events
The occurrence of any of the events below will lead to censoring of patient participation:
Participant withdraws consent and does not allow continued collection of limited data
Reason for consent withdrawal will be documented.
Withdrawal of HD by the treating team due to kidney function recovery
Reason for withdrawing the participant, i.e., kidney function recovery and dialysis is discontinued, will be documented.
Patient undergoes kidney transplantation, and hemodialysis is discontinued.
Transition to peritoneal dialysis and patient participant does not allow continued collection of limited data.
Transition to home HD and patient participant does not allow continued collection of limited data.
Diagnosis of pregnancy
Drop-out event
It is noted that “Withdrawal of HD by the treating team as an end-of-life event” is not a censoring event. If and when “Withdrawal of HD” represents a scenario of HD withdrawal as part of hospice care, data is collected until end-of-study event, such as death.
Drop-out events
The occurrence of any of the events below will be logged as drop-out event, and data collection will be censored at the time of event occurrence:
Transfer of nephrology care outside participating health system network
Lost to follow-up
End-of-study events
These events indicate a study finishing point with no further data collection. They include:
Participant death
Study end date
Temporary patient participant status change
For scenarios when the patient participant travels (e.g., vacation, visiting) and will be absent from receiving HD at the participating healthcare system, data collection will be temporarily suspended if the temporary status change is no longer than 6 weeks. Upon return to the dialysis unit/healthcare system participating in the study, the patient will continue to be a study participant, and data collection will be resumed. During the period of patient participant temporary status change, data collection from affiliated caregiver participant will be temporarily held and resumed as soon as patient participant data collection resumed.
Caregivers and duration of follow-up
Caregivers will be followed in the study until one of the following end-of-study events that pertain to caregiver participation:
Caregiver withdraws consent
Death of the caregiver
Caregiver is lost to follow-up
The affiliated patient participant had an end-of-study event, and data are no longer collected for the patient participant
Temporary caregiver participant status change
For scenarios when the caregiver participant travels (e.g., vacation, visiting), data collection will be temporarily suspended if the temporary status change is no longer than 6 weeks. Upon return, the caregiver will continue to be a study participant, and data collection will be resumed. During the caregiver participant temporary status change, data collection from affiliated patient participant will continue to be collected.
Strategies to improve adherence to interventions {11c}
Non-adherence events.
Non-adherence to serial urine collection, transitioning from twice- to thrice-weekly HD, and/or crossover between groups will be monitored. The following will be logged as non-adherence events:
Non-transition to thrice-weekly HD from twice-weekly HD in patients in the CMIHD treatment group when the treating providers make this recommendation to the patient.
Patient’s decision to get HD twice-weekly when they have been randomized to the CHD treatment group and the treating provider recommends thrice-weekly HD.
Patient has not submitted timed urine collection on one or more scheduled month(s) of assessment.
Missed HD treatments (not due to hospitalization)
Collected data will be summarized as:
N (%) patients in CMIHD who refused to progress to thrice-weekly HD when recommended
N (%) patients in CHD who crossed over to twice-weekly HD
Average percentage of missed HD treatments (not due to hospitalization) per month in the CMIHD group
Average percentage of missed HD treatments (not due to hospitalization) per month in the CHD group
Approach to optimizing adherence to study-related processes of care
To mitigate the potential for non-adherence events, the research team will apply the following measures: (i) training and education of the research team and local providers on the study score and study-related processes of care before study recruitment begins; (ii) thorough sharing with the prospective patient participant of information and expectations on adherence to the study protocol when obtaining informed consent for study participation; (iii) establishing close lines of communication between the local study teams and the treating providers teams and dialysis personnel; and (iv) providing quarterly Patient Participant Report.
Integration of CMIHD into usual care is a complex process, and thus, education of local providers is a prerequisite to initiating study enrollment. Training will occur in three levels, in a train-the-trainer format. First, level, the research staff of the CCC and DCC will conduct training modules to educate the Site Investigators and the Research Coordinators on study procedures, safety monitoring and adverse event adjudication, data collection, data entry, assembly of Advisory Panels during the study, local lines of communication, and local workflows and procedures to ensure study interventions are adequately implemented. Training of the Research Coordinators will also include acquisition of knowledge on HD, vascular access, residual kidney function, and workflows at outpatient dialysis units. Simulated case scenarios will be used to optimize the integration of residual kidney function-related tests into local workflow and usual blood tests. All training modules will be video recorded and centrally stored with access to research team members from all Clinical Centers. Second, the Site Investigators will educate the local treating providers (physicians and advanced practice practitioners), dialysis administration, and dialysis personnel on study-related procedures and patient monitoring. The Site Investigators will educate the Research Coordinators in navigating healthcare systems and dialysis electronic medical records (EMRs) to ensure all data needed for this study is optimally collected. Third, the local treating providers will establish their workflows and communication with dialysis personnel as it relates to study intervention implementation.
Lines of communication between the study team and treating providers and dialysis personnel
Following randomization, the assigned treatment group (CMIHD or CHD) will be conveyed by the study team to the patient participant, dialysis personnel, and the treating providers (physician and advanced practice practitioners). The communications will consist of elements related to the allocated treatment group, including HD frequency, HD treatment duration, and adjuvant pharmacotherapy, depending on the treatment group. During the study, the study teams will maintain continued communication with the treating providers and dialysis personnel to monitor residual kidney function tests, blood tests, and recommended changes in HD prescriptions and medications.
Thorough patient information on study-related processes of care
Prospective patient participants will be given thorough information, education, and expectations associated with study participation. Strong and kind emphasis will be placed on the expectation of serial timed urine collection, the temporary nature of twice-weekly HD, and importance of adherence to dialysis.
Relevant concomitant care permitted or prohibited during the trial {11d}
Participation in this study will not interfere with any medically indicated procedure or intervention during the study. The decisions and recommendations to start chronic HD will be made by the treating physicians, independent of this study. Participation in this study will not affect treating KDRD (or ESKD) associated comorbid conditions, including the management of anemia, mineral and bone disorders, vascular access, nutritional support, and others, at the discretion of the treating physicians. A patient or caregiver participant may be withdrawn from the study at any time without negative consequences to their clinical care. While an investigator may remove a study participant from study participation at any time, this will not mean that the clinical team will stop HD or clinical treatment of the patient.
Provisions for post-trial care {30}
There is no provision for post-trial care. The outcome will be determined at the end of the study. However, since participants are routine clinical patients at participating medical institutions, they will continue to receive dialysis treatments and all clinical care as medically required.
Outcomes {12}
Primary outcome.
The primary outcome will be a composite of all-cause ED visits, hospitalizations, or death over a 2-year average follow-up period (Table 4 ).
Secondary outcomes
The secondary outcomes will compare the effects of CMIHD and CHD on 13 domains: (i) patient-reported health-related quality of life; (ii) changes in residual kidney function; (iii) hospital-free days per 100 patient-days; (iv) caregiver burden; (v) functional status and fatigue; (vi) cognitive function; (vii) volume management; (viii) blood pressure control; (ix) solute clearance, electrolyte and acid-base homeostasis; (x) nutrition and inflammation; (xi) mineral metabolism; (xii) anemia management; and (xiii) vascular access complications. Domains (i) and (ii) are the main secondary outcomes (Table 4 ) because they are linked with our overarching hypothesis regarding CMIHD effectiveness on patient-centered outcomes and the primary outcome.
Patient- and caregiver-reported outcomes
We will capture patient-reported outcomes and caregiver burden using instruments validated in patients with KDRD. The following instruments will be completed at baseline (pre-randomization) and during follow-up (study month): Illness Intrusiveness Rating Scale (IIRS) [ 65 ] (monthly), EuroQOL-5 Dimensions-5 Level [ 66 ], and employment status [ 67 ] (months 6, 12, 18, and 24). All interviewers will be trained in proper questionnaire management and report collection. Baseline patient data will be collected in person (after obtaining informed consent for study participation and before randomization). During follow-up, trained research personnel will collect data in person (for cognitive and physical function assessment), in person, or by telephone (for questionnaires). Caregiver burden will be assessed using the Zarit Caregiver Burden Scale [ 68 ]. Baseline caregiver data will be collected at the time of informed consent and before patient randomization. Patient-reported functional status and fatigue will be assessed using the Time to recover from HD [ 69 ] and SONG-HD Fatigue [ 70 ] instruments.
Changes in residual kidney function
Urine volume and kidney urea and creatinine clearance will be measured no less often than every 3 months and within 2–4 weeks of hospitalization in both groups. At enrollment, dialysis personnel, treating providers, and/or trained research personnel will instruct patient participants on performing timed urine collections using uniform instructions. Urine collections will be analyzed at the lab used by the dialysis center. Results will be entered in the centralized database to calculate kidney urea clearance per week (i.e., kidney stdKt/V) [ 71 ] and automatically sent to the Site Investigators.
Hospital-free days per 100 patient days
We define hospital-free days as all days alive that are spent outside of an acute-care hospital, long-term acute-care hospital (LTACH), or in an emergency department (ED), including days spent wholly or in part under “observation” status. All other days, including days spent in a long- or short-stay nursing facility, inpatient hospice facility, or rehabilitation facility, count as hospital-free, as would all days at home, including those with home-based medical services. This definition aligns with how others have operationalized days alive and outside the hospital [ 72 , 73 ].
Other outcomes
Patient participants’ cognitive function will be assessed using change in Trail Making Test Part B score [ 74 ]. Volume management, solute clearance, electrolyte and acid-base homeostasis, and measurement related to those outcomes will be collected and analyzed. Conversion rates will be compared from in-center HD to home dialysis, kidney transplantation, and dialysis withdrawal. Components of the primary outcome will be compared as cause-specific and time-to-event outcomes between the groups.
Process evaluation
These are outcomes concerning the evaluation of processes related to this study (Table 5 ). Process evaluation will include (a) Intervention characteristics , to assess organizational readiness to change [ 75 ], intervention acceptability and appropriateness [ 76 ]; (b) Inner setting characteristics , to assess barriers and facilitators to the adoption of HD intervention at the partnering organizations [ 77 , 78 ]; (c) External factors [ 79 , 80 ] that mediate implementation; (d) Adoption ; (e) Reach ; (f) Fidelity , to assess adherence to serial timed urine collection and HD treatment schedule; and (g) Sustainability , to assess barriers and facilitators to maintaining intervention. To assess these processes, we will employ surveys and semi-structured interviews with patient participants, caregiver participants, and dialysis stakeholders.
The assessment timepoints for secondary outcomes and process evaluation are listed in Tables 4 and 5 , respectively.
Participant timeline {13}
A flowchart and a schematic diagram of the schedule are presented in Fig. 1 and Table 6 , respectively (information accompanying superscript characters in Table 6 and participant timeline is present in the Additional file 2). Semi-structured interviews with members of the advisory panels will occur on a pre-established timeline with months of interviews calculated from the enrollment start-up month for each Clinical Center.
Sample size {14}
Power considerations for the primary outcome.
The sample size of 350 total (175 per treatment group) was calculated under the following assumptions and conditions: (a) reported combined 2-year incidence of all-cause mortality, all-cause ED visits not leading to hospitalization, and all-cause hospital admissions from the ED is 2.954 per person-year for CHD [ 81 ]; (b) clinical trial participants tend to be healthier than the overall population with KDRD [ 82 ]; thus, our null safety rate will assume a conservative downward adjustment in all-cause mortality estimate by 50% and all-cause ED/hospitalization visit estimate by 30% to yield an incidence rate of 2.021 events per person-year with CHD; (c) a lower incidence rate with CMIHD, i.e., IRR equal to 0.9 [ 64 , 83 ]; (d) safety events follow a negative binomial distribution using a restricted likelihood estimation variance calculation method and overdispersion equal to 0.12 [ 57 ]; (e) 20% dropout rate across groups; (f) average of 2.06 years of HD per participant; (g) non-inferiority margin for IRR of 1.20, corresponding to an unacceptable worsening of safety event rate of 2.426 or higher per person-year in the CMIHD group; (h) ≥85% power; and (i) one-sided level of significance equal to 0.025. This sample size is conservatively adjusted for the healthier population incidence rate of 2.021 events per person-year as suggested in (b); if we use the unadjusted 2-year safety event rate of 2.954 per person-year in (a) for our null safety rate, our sample size of 350 would have >93% power to detect the same non-inferiority margin of 1.20 (Additional file 1, Table S2).
Power considerations for secondary outcomes
We calculated statistical power based on a 2-year average follow-up, assuming an analysis of covariance model (ANCOVA) [ 84 ], which provides an intuitive interpretation that is less dependent on the number of intermediary measurements. We also assume a correlation of 0.8 between baseline and follow-up values based on preliminary data [ 57 , 66 , 68 , 83 , 85 ]. Our calculations show detectable standardized effect sizes of 0.201 and 0.233 for 80 and 90% power, respectively, based on an average sample size of 280 participants across the five assessments (baseline and semi-annual for 2 years) (Additional file 1, Table S3). There is no consensus on the appropriate type I error level for secondary outcomes or even whether they should be tested when the primary endpoint is not statistically significant [ 86 , 87 , 88 ]. However, for completeness, with a strict Bonferroni correction for the two main secondary outcomes, the detectable effect sizes become 0.221 and 0.253 for 80 and 90% power, respectively, leading to slightly higher detectable average differences for the secondary outcomes.
Recruitment {15}
All patients initiating chronic HD and undergoing treatment at participating Clinical Centers and the associated outpatient dialysis facilities will undergo a three-stage evaluation to determine their eligibility for the study: prescreening, invitation to participate in the study, and screening (Additional file 1, Figure S2). During the prescreening stage, the research team will assess the patients’ EMRs to check eligibility against inclusion criteria 1 through 3, exclusion criteria 1 through 9, and exclusion criterion 10 when applicable. When needed, the team will consult the patients’ healthcare providers to ascertain eligibility based on exclusion criteria 4 through 9. Patients meeting prescreening conditions will be approached by a member of the study team and receive a comprehensive explanation of the study’s scope and procedures, followed by an assessment against exclusion criteria 10 (where relevant) and 11. During the informed consent process, the patients will be asked about their willingness to participate in semi-structured interviews should they meet all eligibility criteria and be enrolled in the study, and whether they have a caregiver that could be approached for study participation in the form of completing questionnaires and potentially engaging in semi-structured interviews. Should the patients demonstrate a clear understanding of the study’s requirements and provide their written informed consent, they will progress to the screening stage and will be asked to complete a baseline timed urine collection; a serum pregnancy test will be obtained when applicable. Once patients successfully clear the screening process, their caregivers will be approached to participate in the study (Additional file 2). These caregivers will be screened with targeted questions, and upon providing verbal informed consent, they will be asked to complete baseline questionnaires. This step will take place prior to the randomization of the associated patient.
For process evaluation , study participants include patients and caregivers enrolled in the study, as well as advisory panels. Patient and caregiver participants from each Clinical Center will form the Patient and Caregiver Advisory Panel ( n =4–8 patients and n =4–8 caregiver participants per interview meeting, from each Clinical Center). Dialysis stakeholders comprised of treating providers including nephrologists, dialysis medical directors, and advanced practice practitioners ( n =4 per interview meeting, from each Clinical Center), dialysis nurses including nurses and nurse managers ( n =4 per interview meeting, from each Clinical Center), dialysis dieticians ( n =2 per interview meeting, from each Clinical Center), and dialysis social workers ( n =2 per interview meeting, from each Clinical Center) will form Dialysis Treating Providers Advisory Panel, Dialysis Nurse Advisory Panel, Dialysis Dietician Advisory Panel, and Dialysis Social Worker Advisory Panel, respectively.
Sampling strategy for members of the advisory panels
For semi-structured interviews, we will aim equal engagement of patient participants from each treatment group and caregivers linked to patients assigned to each treatment group. To help ensure generalizability of experiences, we aim to engage stakeholders of different genders, age groups, races, and socioeconomic status.
We anticipate patient and caregiver enrollment period will span 2 years. The average follow-up period is estimated to be 2 years, with an anticipated range of 1 to 4 years. The unit of randomization will be eligible patients who consent to participate in the study. The unit of analysis will be all study participants.
Assignment of interventions: allocation
Sequence generation {16a}.
Randomization will be centralized using computer-generated randomly permuted blocks to ensure balance in treatment allocation within each stratum. Randomization will be stratified by clinical center and type of vascular access used (central venous catheter or arteriovenous access) at enrollment.
Concealment mechanism {16b}
Allocation concealment will be ensured. The study teams will not have access to the randomization until the patient has passed all eligibility criteria and completed all baseline measurements.
Implementation {16c}
Before proceeding with patient randomization, we will gather all baseline questionnaires from both patients and their caregivers. Once randomization is complete, the assigned treatment group will be communicated to the patient, dialysis staff, and the patient’s nephrology care team, including physicians and advanced practice practitioners, by the Site Investigator.
Screen failures of baseline residual kidney function
In the event that patients’ baseline residual kidney function does not satisfy inclusion criteria 4 and 5, they will be notified and excluded from taking part in the study. There are occasions where recorded levels of residual kidney function might be artifactually or temporarily low, such as instances where patients acknowledge incomplete urine collection or have experienced an acute illness. Under these circumstances, the Site Investigator has the discretion to authorize a repeat of the timed urine collection for screening purposes to determine whether the patient qualifies for inclusion based on their baseline residual kidney function.
Serum pregnancy test
Women ≤55 years old without a history of hysterectomy will undergo serum pregnancy testing as a component of their eligibility assessment for study participation. This test may be either qualitative or quantitative. Should the pregnancy test result positive, the participant will be ineligible for study participation. For women who become part of the study, routine and serial serum pregnancy tests will not be required by the protocol. Instead, future tests will be administered on an individual basis, aligning with usual care when clinical indications arise.
Assignment of interventions: blinding
Who will be blinded {17a}
This is an open-label study. Blinding of the participants, dialysis personnel, and clinicians is not feasible; however, data collection for the primary endpoint will be based on objective events. The data analysts in charge of statistical analyses will access to the database after data cleaning and database lock and perform analyses according to a predetermined statistical analysis plan.
Procedure for unblinding if needed {17b}
The study is open label. Therefore, unblinding is not required.
Data collection and management
Plans for assessment and collection of outcomes {18a}.
Data collection forms will be provided by the CCC to all participating Clinical Centers.
Data collection methods
All data variables related to prescreening, informed consent, screening, questionnaires, timed urine collection and related urine and blood tests, hospitalizations (detailed by reason, duration, dialysis treatments received, and any instances of mechanical ventilation), emergency department (ED) visits (including cause), and deaths (along with cause) will be prospectively and systematically collected on a monthly basis through reviews of healthcare system EMRs, dialysis EMRs, and participant reports, and will be entered in a centralized electronic data capture (EDC) TwoPlus study platform. Patient information routinely logged at outpatient dialysis units within the dialysis EMRs will undergo de-identification and be digitally transmitted to the DCC on a scheduled basis.
Semi-structured questionnaires will be guided by the CFIR (Consolidated Framework for Implementation Research) [ 89 ] and RE-AIM (Reach, Effectiveness, Adoption, Implementation, Maintenance) [ 90 , 91 ] and will be conducted by members of the Implementation Science Team (IST) with experience in qualitative research and conducting semi-structured interviews. These interviews will take place either via telephone or a video platform. The semi-structured interviews will consist of open-ended, qualitative questions that allow and encourage participants to elaborate as they like, which we expect to yield rich personal narratives and provide insight into processes of care and experiences related to the study intervention. The interviews will be audio recorded, professionally transcribed verbatim, and checked for accuracy against the corresponding recording. The IST will also hold periodic (about every 6 months) focus group meetings with Site Investigators and Stakeholder Partners to gather operational adaptations and other perspectives related to the implementation of incremental-start HD.
In events where a participating patient expresses the desire to withdraw from the study, they will be courteously inquired if they permit the continuation of data collection through the non-intrusive gathering of clinical events and relevant variables present within the healthcare system and dialysis EMRs up to the study’s conclusion. If a caregiver participant asks not to be contacted again regarding the study, this will be noted, and they will not be contacted again.
Plans to promote participant retention and complete follow-up {18b}
Pragmatic study design.
Research suggests that enhancing retention and reducing missing data can be achieved through several strategies: employing brief assessments, implementing reminders for participants, offering incentives, limiting the number of visits exclusive to the study, and making use of data collection reports [ 92 ]. We have integrated these effective methods into our existing strategy to optimize patient engagement and data quality. We will administer succinct participant-reported outcomes that patients complete in approximately 10 min monthly, while caregivers do so semi-annually, and an extended 25-min version for patients every 6 months. Both patients and caregivers can conveniently respond to the questionnaires via telephone. We will integrate semi-annual cognitive assessments into patients’ outpatient dialysis schedules, obtaining Trail Making Test B assessments before the afternoon or after the morning HD sessions. There will be no study-specific visits or needle sticks, integrating all necessary procedures with HD treatments. To optimize compliance with timed urine collections, patients will be given telephone reminders and will be remunerated to incentivize completion. Patients will be advised to bring their collected urine samples to their dialysis sessions for convenience. The use of EMR-extracted outcome data will reduce the risk of disproportionate data loss for participants who are minimally engaged. Disengaged patients wishing to withdraw will be queried for consent to continue collecting their EMR data for research purposes. Finally, the information we will obtain during the semi-structured interviews with the patient participants, caregivers, and dialysis personnel will be used to improve processes of care associated with this recruitment and retention in this study.
Additional strategies to improve or monitor adherence to the study protocol
These will include reports generated by DCC:
Monthly recruitment reports of patients screened, enrolled, and consented (accrual figures)
Prescreen, informed consent, and screen fails to assess for bias in inclusion/exclusion decisions
Monthly reports detailing data received at the data center, data consistency, missing data, performance measures, and adherence to the study protocol
Participant adherence to the assigned treatment group and HD prescription
Supplementary blinded reports requested by the study investigators or subcommittee that do not disclose allocation-group–specific outcomes (primary, secondary, or any safety outcomes)
Data management {19}
Centralized calculations of residual kidney function and dialysis clearance.
The centralized TwoPlus database platform is programmed with validated formulas [ 71 ] to calculate residual kidney function, kidney weekly urea clearance (i.e., kidney standard Kt/V [kidney stdKt/V]), dialysis single pool urea clearance (spKt/V), and dialysis weekly urea clearance (dialysis stdKt/V), ultrafiltration rate, residual weight, and inter-dialytic weight gain (Additional file 4). Data collected at the site level (for residual kidney function) and electronically transferred from dialysis EMR to the centralized study platform will be linked using participant identification number (PID), and results will be distributed to the study teams on a regular basis.
Data quality
The DCC and CCC will undertake data quality checks, supported by close liaison with the Site Investigators and Research Coordinators at each Clinical Center. Staff at each Clinical Center will be trained to ensure that data are captured reliably using Case Report Forms pro forma as mock data for data entry training in the EDC platform. Ranges for data variables will be placed in the EDC system to reduce the risk of inaccurate data entry. The DCC will issue monthly reports detailing data completeness and omissions, segmented by team and Clinical Center, to Site Investigators and Research Coordinators. These reports will inform biweekly discussions at research meetings aimed at monitoring follow-up rates and addressing data gaps. The DCC will alert the Research Coordinators to any data that are missing or inaccurate.
Data security
Wherever possible, all personal and research data will be entered and stored only in electronic and password-protected format. When it is necessary to store personal or research data in hard copies, for example, where there is no access to a laptop or where staff complete paper versions of an encounter, data will be stored at the Clinical Center in a designated base and a locked filing cabinet. Electronic copies of study participant’s personal and study data will be stored on secure shared drives at each Clinical Center working area of the study team. All study data will be password-protected using a password known only to the study team. No study participant’s personal or study data will be downloaded or stored on individual employee’s drives or desktops. Data will be entered into the TwoPlus EDC platform and REDCap. It is Good Clinical Practice (GCP) and 21 CFR Part 11 compliant, with a full audit trail and database lock functionality. The linkage between personal information and routine data collected from individual participants will be securely maintained in a separate, password-protected file to ensure privacy and confidentiality. Audio recordings of semi-structured interviews will be maintained by the Implementation Science Team on a secure, shared drive and stored in an anonymized and encrypted form. Data will not be shared with anyone outside of the study teams and organizations hosting the study. Research data will be archived at each clinical center and centrally for all centrally collated electronic data and stored for a 10-year duration in line with the funders’ and local regulatory policies. After 10 years, research data will be shredded, deleted, or destroyed using confidential data destruction measures in place for each organization of the TwoPlus Research Consortium.
Confidentiality {27}
The TwoPlus trial will be conducted in accordance with the principles of the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice (ICH GCP), applicable United States (US) Code of Federal Regulations (CFR), and institutional regulatory policies at each participating organization. To ensure privacy, a participant identification number (PID) in the form of an alpha-numeric ID for each patient will be assigned to preserve personal information and contacts. Participants’ data will be stored in a secure password-protected file at each clinical center. The drive will only be accessible to the study team members. In both hard and electronic versions, personal and study data will be kept separate. Study data will be identified using PID. This ID will be linked to the participant’s name in a master recruitment log file that will be password-protected, with password known only to the study team.
Plans for collection, laboratory evaluation, and storage of biological specimens for genetic or molecular analysis in this trial/future use {33}
As of the date of this document, there are no plans for storage of biological specimens.
Statistical methods
Statistical methods for primary and secondary outcomes {20a}, primary outcome analysis.
The primary outcome will be analyzed as the incidence rate of all-cause ED visits not leading to hospitalization, all-cause hospitalizations, or all-cause death for each treatment group. This incidence rate in each HD treatment group will be calculated as the total number of primary outcome events divided by the number of person-years in the study. A participant could have recurring ED visits, hospitalizations, and/or die during the study; all events will be included. Negative binomial regression will be used to model the number of events observed in each group using a dummy variable to denote treatment groups [ 93 ]. The time between randomization and the last assessment for each patient will be used as an offset. This model will be adjusted for sex, race, baseline comorbidities, and stratification variables (i.e., Healthcare System, vascular access, and age at enrollment). Adjustments will be made for the potential correlation between patients from the same Clinical Center using generalized estimating equations [ 94 , 95 , 96 ]. We will estimate the incidence rate ratio (IRR) in the CMIHD group vs the CHD group as a linear contrast on the log scale, which will then be exponentiated. A one-sided 97.5% confidence bound will be computed, and non-inferiority will be met if that bound is less than 1.20 times the incidence rate in the CHD group. If non-inferiority is established, we will test whether CMIHD is superior to CHD in reducing the IRR of the primary outcome using a fixed sequential approach. Superiority will be claimed if the upper limit of the same 97.5% confidence interval (CI) was <1, with a type I error rate of 0.05 for a two-sided test [ 97 ].
Secondary outcomes analysis
Changes from baseline in patient- and caregiver-reported outcomes, employment status, and lab values will be analyzed using repeated-measures analysis of covariance (RMANCOVA) [ 98 ], starting with an unstructured covariance matrix to account for measurements that are not independent. For the main secondary outcomes, measured monthly, we will use mixed-effects models using baseline value as a covariate and accounting for within-subject and within-clinic center correlation [ 99 , 100 ]. Taking advantage of the randomization, we will constrain the pre-randomization intervention-specific outcome means to be the same [ 101 ]. For randomized trials, constrained mixed-effects models can provide more efficient estimates of post-randomization treatment differences when either baseline or post-randomization measures are missing. In addition to the dummy variable to denote the intervention groups, the model will contain terms for recruitment center, vascular access, and baseline value of the outcome of interest. Interaction effects between the intervention and time will be tested using likelihood ratio tests, while treatment effects will be estimated and tested using linear contrasts. For outcomes collected from caregiver participants, we expect randomization of patient participants would balance caregiver characteristics between groups. A unique identification number that links caregiver data with patient participant data will be created.
The analysis for hospital-free days per 100 patient days will be performed under the intention-to-treat principle and will be measured from the date of randomization to the date of an end-of-study event or end of the study, whichever comes first. For each participant, the primary outcome will be the cumulative number of days that the patient was not hospitalized. We will develop an algorithm that gives different “weight” to a hospitalization event based on the presence or absence of mechanical ventilation and, in later events, based on the number of days of mechanical ventilation. We will compare outcomes between the treatment groups using Poisson regression, modeling the number of hospital-free days as the dependent variable with a log link, the treatment assignment as the predictor, and the natural log of the number of at-risk days as an offset. Covariate adjustment will be performed. Analyses will take into account events of recurring hospitalizations after hospitalization-free days. Deviance residuals and the overall deviance measure will be calculated to assess the overall goodness of fit of the model. If we observe overdispersion, we will explore the negative binomial models and conduct model diagnostics.
Potential occurrence of social desirability bias and its effect on patient-reported outcome measurements
Some may argue that since patients in either CMIHD or CHD group are not blinded to randomized intervention, patients in the CMIHD may give more favorable responses to survey questionnaires than patients in the CHD group. This phenomenon has been termed “social desirability bias.” Survey questionnaires will be administered pre-randomization at the baseline visit and then either monthly or semi-annually, depending on the instrument. Baseline responses will not be affected by the intervention. Surveys administered post-randomization could potentially be influenced by treatment assignment, especially the first questionnaire filled after randomization. However, it can be argued that the “optimism” effect related to CMIHD may subside over time. Similarly, patients who transitioned from twice- to thrice-weekly HD may become more pessimistic. To explore these effects, we will look for non-linear association between time on study and current intervention (CMIHD on twice-weekly HD, CMIHD transitioned to thrice-weekly HD or CHD) on the survey scores. We will also describe this as a potential limitation in our papers, as this potential for bias cannot eliminated from the study because blinding is not practical.
Analyses for components of primary outcome
When interpreting the findings from clinical trials with composite endpoints, it may be difficult to ascribe efficacy to individual components of the composite, even when overall findings are positive [ 102 ]. One way to address this situation is by using shared parameter models, in which a latent variable is included in the parameterization of event rates for individual conditions, and the intervention effect on this variable is assessed. A related approach involves multivariate survival models in which incidence times of multiple endpoints are assessed [ 103 ]. An alternative is to follow analyses of the composite with formal tests for heterogeneity in intervention effects on the individual components [ 104 ]. While power may be limited due to a small number of events, this approach is designed to detect situations when there are marked differences in how the intervention relates to the various components of the composite primary outcome.
Analysis populations
Primary and secondary outcomes.
Analyses of the primary and secondary outcomes will be conducted as intention-to-treat (ITT), i.e., all randomized participants will be included in primary analyses. This will include all the scenarios when patient participants provide signed informed consent for continued data collection of a limited set. Participant data will be analyzed according to their randomized treatment, regardless of adherence to the prescribed HD schedule in each treatment group.
Sensitivity analyses
Sensitivity analyses will be conducted in the per-protocol population set. These analyses will include participants who exhibited full adherence to as-recommended HD treatment schedules. The per-protocol analyses will censor the data on participants who did not adhere to conversion from twice- to thrice-weekly HD and patients with non-adherence and crossover from thrice- to twice-weekly HD. This censoring will apply at the time the first non-adherence is observed. Only data prior to non-adherence will be used in the per-protocol analysis. Analyses will be adjusted by the inverse probability of censoring. Zero-inflated mixed-effects negative binomial models will be fit to assess the effect of time‐varying non-adherence weights. We will (i) use information on recorded AEs and recommended HD prescription; (ii) collect interim-diagnosed comorbidities for those who are medically advised to transition from CMIHD to CHD; and (iii) define each participant when for the first time (in days after randomization), for how long, and how often they were nonadherent to the HD prescription. Propensity score weighted analyses (1/P(adherence)) will be performed.
Secondary analyses
These analyses will include an evaluation of crossovers from twice- to thrice-weekly dialysis and as-treated analyses.
Analysis plan to account for crossover timing from twice-weekly to thrice-weekly HD
An important analytic question is how to account for the transition (crossover) from twice- to thrice-weekly HD. To do so, we will collect the time of the transition and a second set of “baseline” characteristics data at the time of transition. Patients who transition to thrice-weekly HD will be followed until the end of the observation period. We note that transition would follow loss of residual kidney function (disease progression) such that a two-stage adjustment method can be applied in this context. However, unlike current practice in oncology trials where patients are transitioned from the standard of care to the experimental treatment (because of benefit), patients will be transitioned from the intervention to the “control,” CHD group. Under the two-stage model, treatment effects will be estimated separately for patients who remained on the twice-weekly HD, those who transitioned to thrice-weekly HD, and those who were initially randomized to thrice-weekly HD. These estimates will then be combined to generate an overall treatment effect [ 105 , 106 ].
As-treated analyses
The as-treated analysis will take into account randomized treatment allocation and time-varied receipt of the two HD treatment schedules. The as-treated population will comprise the ITT population and statistical modeling of HD schedule in each treatment group, thus adjusting for the effects of lack of adherence to conversion from twice- to thrice-weekly HD or presence of crossover from thrice- to twice-weekly HD. The as-treated analysis with time‐varying non-adherence weights will track and adjust for pre-randomization and post-randomization prognostic factors that can impact both, adherence to assigned treatment and the primary outcome.
Analysis of process evaluation
We will analyze results by thematic analysis [ 107 ]. Following the principle of constant comparison, transcripts will be analyzed as interviews are conducted. First-cycle codes will be derived directly from the data [ 107 ]. Codes will consist of a short phrase generated by the researcher that captures the essence or attributes of a data segment [ 107 ]. We will use open coding and will apply codes to data sections that the analyzing researcher deems appropriate. We will use constant comparison to examine the data, both within a given interview and across interviews. We will use NVIVO qualitative data analysis software to analyze the data and record codes. When a substantial number of interviews (~8) has been completed, we will begin searching for themes by analyzing the initial codes to determine how the codes can be grouped into themes. Themes are constructs that succinctly capture an important pattern in the data in relation to the research question [ 107 ]. We will review the candidate themes and read the data extracts for each theme to determine if the data fit the candidate themes. If not, we will then decide if certain data extracts fail to fit the theme or if the theme needs to be reworked. If the data do fit the candidate theme, then we will assess the accuracy with which the set of themes reflects the meanings and relationships of the whole data set [ 107 ]. We will identify and revise themes simultaneously as the interviews continue until data saturation. As the themes mature, we will determine how the themes fit together to tell the overall narrative [ 107 ]. We will ensure there is minimal overlap between themes and identify any sub-themes that may be contained within a given theme.
Triangulation and data synthesis
Our process evaluation is not designed to make immediate changes to the study unless ethical issues require them; early semi-structured interviews and intervention fidelity evaluations will be reported to the study teams and may be considered in intervention implementation. The triangulation protocol aims to produce meta-themes that cut across individual methods [ 108 ]. To enhance rigor and trustworthiness, we will conduct the study and report our findings in accordance with the Consolidated Criteria for Reporting Qualitative Research (COREQ) [ 109 ]. We will ensure credibility by attaining prolonged engagement in each interview and continuing interviews until data saturation is reached. We will repeat interviews if/as needed to clarify or expand upon any issues not completely explored in initial interviews. We will analyze interviews as they are completed and use constant comparison to test for data saturation. To increase internal validity, interviews will be audio recorded, professionally transcribed verbatim, and checked for accuracy against the corresponding recording. Each interview will be analyzed by at least 2 investigators. To maximize credibility, we will use analytic triangulation, whereby researchers analyze independently and discuss any discrepancies in their codes to arrive at an agreed-upon conclusion. To minimize bias, each interviewer will record field notes regarding details of the interview, the participant, the setting, and any thoughts or impressions before and after the interviews emphasizing self-reflexivity (the process by which investigators become aware of their own biases). To optimize confirmability, we will record memos to create an audit trail of all coding decisions.
Interim analyses {21b}
Not applicable. There are no interim analyses planned.
Methods for additional analyses (e.g., subgroup analyses) {20b}
We will perform subgroup analyses for the primary outcome and main secondary outcomes to explore whether the treatment effect is consistent across subgroups [ 110 ]. Using the ITT population set, we will employ the negative binomial model with terms for treatment group, subgroup variable, and treatment by subgroup variable to test for significance of the interaction terms. Models will be adjusted for stratification variables before treatment effects in subgroups are estimated. Forest plots will be generated displaying the estimated IRRs, and 95% CIs for each subgroup will be presented. For subgroups defined using continuous variables, analyses based on the continuous form will be considered primary, but these variables can also be categorized [ 111 ]. Non-linear effects will also be explored using splines [ 112 ]. The following subgroups determined at baseline will be examined: vascular access (ventral venous catheter and arteriovenous access); age (≥ 65 and <65 years); race categories (White, Black, and Asian; Hispanic ethnicity); diabetes mellitus (presence or absence); and anthropometric volume (Watson [ 113 ] volume < 35 L vs. ≥ 35 L). Likelihood ratio tests will be used to determine whether treatment effects vary across subgroups, followed by post hoc HTE analysis when interaction effects are significant. We will follow Wang et al.’s recommendation and calculate and report the overall probability of type I error given the number of subgroups tested [ 114 ].
Further details regarding other statistical analyses will be provided in the statistical analysis plan prior to commencing analysis.
Methods in analysis to handle protocol non-adherence and any statistical methods to handle missing data {20c}
To minimize missingness, we will follow standard methods for increasing response (e.g., good communication, reminders, and patient incentives for timed urine collection). Data monitoring will decrease the frequency of missing data and identify data management problems. Programmers and software developers at the DCC will develop a flexible and reliable data entry system. This system will provide multiple checks for data plausibility, including variable outliers and data completeness checks. Validation checks will be implemented to coincide with skip logic. Data entry personnel will receive ongoing feedback to facilitate complete data collection and reduce errors. Real-time reports will be incorporated into the web-based data management system to allow multiple quality control and performance monitoring reports. These include reports of missing data by Clinical Center and data entry personnel. Reasons for missing data will be collected and described. All patients will be accounted for in all analyses and presentations. To account for missingness, those discontinuing the study prematurely will be censored at the time of dropout. Sensitivity analyses will be undertaken [ 115 ], including inverse probability weighting (IPW) [ 116 , 117 ] under the missing at random assumption (MAR) [ 118 , 119 ] and pattern-mixture approach to explore the possible effect of deviations from MAR [ 120 , 121 ].
Plans to give access to the full protocol, participant-level data, and statistical code {31c}
Data and the protocol will be available upon request and permission of relevant authorities (e.g., Principal Investigators, dialysis organization leadership, and funding agency) after the trial.
Oversight and monitoring
Composition of the coordinating center and trial steering committee {5d}.
The study will be led by two main Principal Investigators (PIs). Wake Forest University School of Medicine (WFUSM) will be the prime institution of the TwoPlus Research Consortium with the role of CCC and will also include the Implementation Science Team (IST). The DCC will be located at New York University (NYU) Langone. Enrollment will take place at Clinical Centers of national healthcare systems and their affiliated dialysis organizations, under the leadership of Site PIs. An External Expert Advisor will provide guidance regarding the monitoring of residual kidney function and solute clearance. The TwoPlus Research Consortium has partnered two largest patient advocacy organizations in the USA to make certain the study is continuously informed by patient insights: the American Association of Kidney Patients and Home Dialyzors United. The TwoPlus Research Consortium Team has also partnered with stakeholders involved in providing hemodialysis care, i.e., clinicians, nurses, dietitians, and social workers. These Stakeholder Partners will represent the Stakeholder Advisory Panels across Clinical Centers (Additional file 1, Figure S1).
Steering committee
The Steering Committee will be the main governing body of the study. This committee will be comprised of the main PIs, Lead Biostatistician, Site PIs, Stakeholder Partners, External Expert Advisor, and Dissemination Committee Chair. The members of the Steering Committee will (i) be responsible for the overall study governance; (ii) hold regular meetings with Stakeholder Advisory Panels; (iii) review Safety Reports and manage communications with central IRB and PCORI Project Officials; (iv) report study results; and (v) oversee return of aggregated study results to the community, decision-makers, and policy-makers. The Steering Committee will receive input from the External Expert Advisor on matters associated with scientific developments during the study. The Steering Committee will convene on a regular basis through virtual meetings and correspond often via emails.
Dissemination committee
This committee will be formed by professional staff and organizational leaders of the American Association of Kidney Patients (AAKP). Together with the main PI, Site PIs, IST, this committee is tasked with the dissemination of study implementation, study progress, and study results. The main PIs and the Chair of the Dissemination Committee will meet on a regular basis with payor representatives of the Centers of the Medicare and Medicaid Services (CMS), primary payor for dialysis service in the USA.
Other committees
The Project Managers Committee is comprised of the project managers of the CCC, DCC, and IST and is tasked with overseeing study implementation at all Clinical Centers, monitoring of recruitment, study-specific activities, and periodic retraining at each Clinical Center; and reporting of protocol deviations and serious adverse events (SAEs) to regulatory bodies. The Publications and Presentations Committee and the Ancillary Studies Committee will develop ideas for future studies and manuscripts, and they will work with the Steering Committee to identify the investigators within the consortium that can lead these efforts.
Composition of the data monitoring committee, its role and reporting structure {21a}
An independent Data and Safety Monitoring Board (DSMB) will have the primary responsibility for monitoring the accumulating study data for signs of adverse trends in morbidity/mortality and reportable SAEs. This committee will be composed of six members, inclusive of the DSMB Chair. The DSMB includes one biostatistician, three nephrologists, one patient representative, and one caregiver representative. Meetings of the DSMB will be held periodically. Material for these meetings will be prepared by the DCC and distributed 2 weeks before the meetings via communication with the PCORI Team. The DSMB will have unlimited access to data upon request.
Adverse event reporting and harms {22}
The TwoPlus trial poses a level 3, moderate risk for patient participants, and a level 1, no more risk than expected in daily life for caregiver and the members of the advisory panels as defined in federal regulations at 45 CFR 46.102(i) and 21 CFR 50.3(k). Expected risks include electrolyte and acid-base imbalances and volume overload, each with attendant consequences (Additional file 1, Table S4).
Adverse events
An adverse event (AE) is any untoward medical occurrence in a participant, which does not necessarily have a causal relationship with the study intervention. In the context of the TwoPlus trial, an AE will be considered to be:
Any unintentional, unfavorable clinical sign or symptom, including complications of HD
Any new illness or disease or the deterioration of existing disease or illness
Any clinically significant deterioration in any laboratory assessments or clinical tests
In the context of the TwoPlus trial, the circumstances listed below will NOT be considered to be AEs:
A pre-existing condition (unless it worsened significantly during HD)
Routine diagnostic and therapeutic procedures for an incident or chronic conditions
AEs will be assessed for severity and relationship to the study.
Classification of an AE
All AEs will be classified using the current version of the Common Terminology Criteria for Adverse Events (CTCAE) developed and maintained by CTEP at National Cancer Institute, as follows:
Mild— awareness of sign, symptom or event, but easily tolerated; requires no special treatment and does not interfere with the participant’s daily activities.
Moderate— discomfort enough to cause interference with usual activity and may warrant intervention.
Severe— incapacitating with inability to do usual activities or significantly affects clinical status and warrants intervention.
Serious (SAEs)
These are AEs that meet any of the following criteria:
Results in death
Is life threatening, or places the participant at immediate risk of death from the event as it occurred
Requires or prolongs hospitalization
Causes persistent or significant disability or incapacity
Results in congenital anomalies or birth defects
Is another condition which investigators judge to represent significant hazards
Relationship to study intervention
The Site PIs will grade the degree of certainty about causality by using the categories below.
Definitely Related to the study intervention— There is clear evidence to suggest a causal relationship with the study intervention, i.e., randomization and assigned HD treatment group, and other possible contributing factors can be ruled out. The clinical event, including an abnormal laboratory test result, occurs in a clear, direct time relationship to study intervention administration and cannot be explained by concurrent disease or other drugs or chemicals.
Probably related to the study intervention —An event that follows a reasonable temporal association with the study intervention, i.e., randomization and ascribed HD treatment group, that is not easily explained by another cause such as known characteristics of the participant’s clinical state or other treatment.
Probably not related to the study intervention —An event that does not follow a reasonable temporal association with the study intervention, i.e., randomization and ascribed HD treatment group, that can be explained by another cause such as known characteristics of the participant’s clinical state or other treatment.
Definitely not related to the study intervention —There is clear evidence that there is no causal relationship with the study intervention, i.e., randomization and assigned HD treatment group, and other possible contributing factors have been ruled in. The clinical event, including an abnormal laboratory test result, occurs in a clear, direct time relationship with other etiology and/or can be explained by concurrent disease or other drugs or chemicals.
Causal relationship/relatedness to the study intervention is not assessable —There is insufficient or incomplete evidence to make a clinical judgement of the causal relationship.
Protection against risks
Residual kidney function.
The TwoPlus EDC platform will generate alerts when (a) data needed to monitor residual kidney function are missing and (b) residual kidney function metrics indicate a patient participant can transition from twice-weekly to thrice-weekly HD.
Summary data
These data will comprise participants’ biochemical laboratory values and volume status management. Summary data, for each Clinical Center, will be generated monthly by the DCC and will be reviewed by the Site Investigators who, in turn, will take appropriate measures and contact the treating providers.
Patient participant report
Patient participants will be provided with a report on a quarterly basis or more often, when timed urine collection and residual kidney function is assessed. These reports will include a summary of the last 2 measurements of residual kidney function, pre-HD serum potassium, pre-HD serum bicarbonate, and average interdialytic weight gain over the last 2 weeks. These reports will increase the interactions between study personnel and participants, giving an opportunity to the patients to ask questions pertaining to their study participation, and questions that can be relayed to the treating team and/or study team.
Frequency and plans for auditing trial conduct {23}
The CCC and DCC will perform audits periodically during the entire duration of the trial. At each audit, congruousness with respect to trial procedures and data collection will be evaluated. Any critical issues will be discussed by the steering committee and communicated to clinical center PIs and local study coordinators. Additionally, auditing may be performed, without anticipated communication, by the local regulatory bodies.
Plans for communicating important protocol amendments to relevant parties (e.g., trial participants, ethical committees) {25}
All modifications about protocol and procedures must first be approved, by amendment, by the funder, the DSMB and the Steering Committee, and then by the central IRB of the WFUSM. If approved, the changes will be communicated and reported in the national web-based platform of inter-institutional reliance exchange program.
Dissemination plans {31a}
The research team has partnered with the AAKP, the oldest and largest independent kidney patient organization in the USA. AAKP will participate in the dissemination of study results, carefully tailoring strategies to match program, research, and educational objectives and key milestones. Through its Centers for Patient Research & Education and Center Patient Engagement & Advocacy, AAKP is uniquely positioned to engage with a variety of key stakeholders, including patients, researchers, state elected, and appointed leaders as well the U. S. Congress and appointed federal officials at the White House and within federal health agencies. AAKP will use its comprehensive communications resources to achieve the highest levels of dissemination and impact possible.
The study’s findings will be distributed by its Site Investigators and Stakeholder Partners. These results are intended to be shared with the wider scientific community through publications in scholarly peer-reviewed journals and showcased at conferences via presentations and poster sessions. Following their official publication, a summary of the key findings may also be disseminated through targeted websites, forums, and various social media platforms. Neither the funder not any pharmaceutical company plays no part in the conduct, analysis, interpretation of the data, or in the sharing of the study’s outcomes.
Public health impact
KDRD is a burdensome condition at individual and societal levels, both in terms of the direct costs of treatment and the associated costs of disability, dependency, and unemployment. Every day, 350 people in the USA are diagnosed with KDRD [ 122 ]. The prevalence of this condition is expected to double by 2050, driven by an annual increase in incidence rates of 8%, heralding unmanageable costs against the backdrop of strained healthcare economies and dripping resources [ 123 ]. Over 90% of people diagnosed with KDRD are treated with the same regimen of thrice-weekly, CHD [ 124 ], regardless of the seriousness of their KDRD [ 33 , 34 ]. The 2019 Executive Order on Advancing American Kidney Health Initiative urged innovative interventions to transform kidney disease care and reduce costs [ 125 , 126 ]. Consequently, there is a compelling demand for HD treatment modalities that offer personalized care, alleviate the burden of treatment, and reduce healthcare resource utilization, all while maintaining the quality of health-related outcomes [ 45 ]. In pursuit of this goal, it is critical to conduct research that rigorously evaluates the effectiveness of CMIHD in comparison to CHD among appropriate patient populations. The findings from such research will be pivotal in endorsing the widespread adoption of CMIHD within common clinical settings and will also provide patients and advocacy organizations additional data on a treatment option that expands their patient care choice.
The overarching goal of the TwoPlus trial
CMIHD is a form of personalized, kidney function-coupled HD does not assume that all patients need the same minimum HD intensity conventionally delivered thrice-weekly at a minimum dialysis intensity with each treatment [ 5 , 6 ]. It further recognizes that the minimum CHD intensity is also a prescription of maximum effectiveness beyond which clinical trials have shown that a higher HD dose would add little if any clinical benefit [ 127 , 128 ]. Several observational studies and two pilot clinical trials indicated that CMIHD prescribed to patients with apposite levels of residual kidney function is similarly effective to CHD [ 22 ]. The absence of multicenter prospective and randomized studies to objectively assess the clinical effectiveness and practical implementation of CMIHD is contributing to the lack of systematic adoption of this treatment modality in clinical practice. Drawing from practical clinical insights [ 46 , 64 , 129 , 130 ] and pilot clinical trial findings [ 131 , 83 , 132 ], the TwoPlus trial is designed to conduct a fair and thorough evaluation of patient outcomes across diverse healthcare systems and dialysis organizations, based on the initial HD schedule. Should the trial demonstrate the effectiveness of CMIHD, we anticipate that its findings will catalyze advancements, paving the way for incremental-start HD to become a universally accessible option for all patients, in all countries.
Trial status
Protocol version 0.5, 31-01-2024
The recruitment for this study is anticipated to begin on 01 March 2024. Recruitment and data collection is anticipated to end on 01 March 2028.
Availability of data and materials {29}
The DCC tracks the life cycle for all data sets to be collected, processed, and transferred in the context of the TwoPlus Trial. Substantive metadata and source code will be generated and stored with the data during this process. For ease of access, codebooks for harmonized datasets will be generated and stored alongside data files. They will be shared with other funded centers through a shared file directory accessible through the study website. Study protocol, manual of operations, training manuals, and related documents are available behind the login wall on the study website. All completed analyses, derived datasets, and metadata will be digitally archived. For data sharing, the DCC will construct a public-use version of the final research, with the contents and corresponding metadata to be determined jointly by the PI and collaborating centers.
Abbreviations
American Association of Kidney Patients
Adverse event
Analysis of covariance
Clinical Coordinator Center
Code of Federal Regulations
Conventional hemodialysis
Clinically Matched Incremental HD
Centers of the Medicare and Medicaid Services
Common Terminology Criteria for Adverse Events
Data Coordinator Center
Data and Safety Monitoring Board
Emergency department
Electronic data capture
Empowering Patients on Choices for Renal Replacement Therapy
- End-stage kidney disease
- Hemodialysis
Heterogeneity of treatment effects
Institutional Review Boards
Implementation Science Team
Intention to treat
Kidney Dysfunction Requiring Dialysis
New York University
Patient-Centered Outcomes Research Institute
Principal Investigator
- Randomized controlled trial
Repeated-measures analysis of covariance
Serious adverse event
Standardized Outcomes in Nephrology–Hemodialysis
Wake Forest University School of Medicine
De Palma JR, Bolton CF, Baltzan MA, Baltzan RB. Adequate hemodialysis schedule. N Engl J Med. 1971;285(6):353–4.
Article PubMed Google Scholar
Scribner BH, Cole JJ, Ahmad S, Blagg CR. Why thrice weekly dialysis? Hemodial Int. 2004;8(2):188–92.
Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, et al. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363(24):2287–300.
Article CAS PubMed Google Scholar
Rocco MV, Lockridge RS Jr, Beck GJ, Eggers PW, Gassman JJ, Greene T, et al. The effects of frequent nocturnal home hemodialysis: the Frequent Hemodialysis Network Nocturnal Trial. Kidney Int. 2011;80(10):1080–91.
Article PubMed PubMed Central Google Scholar
Lowrie EG, Laird NM, Parker TF, Sargent JA. Effect of the hemodialysis prescription of patient morbidity: report from the National Cooperative Dialysis Study. N Engl J Med. 1981;305(20):1176–81.
Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347(25):2010–9.
Kassam H, Sun Y, Adeniyi M, Agaba EI, Martinez M, Servilla KS, et al. Hospitalizations before and after initiation of chronic hemodialysis. Hemodial Int. 2011;15(3):341–9.
Bradbury BD, Fissell RB, Albert JM, Anthony MS, Critchlow CW, Pisoni RL, et al. Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol. 2007;2(1):89–99.
Canaud B, Kooman JP, Selby NM, Taal MW, Francis S, Maierhofer A, et al. Dialysis-induced cardiovascular and multiorgan morbidity. Kidney Int Rep. 2020;5(11):1856–69.
McIntyre C, Crowley L. Dying to feel better: the central role of dialysis-induced tissue hypoxia. Clin J Am Soc Nephrol. 2016;11(4):549–51.
Article CAS PubMed PubMed Central Google Scholar
Hazara AM, Bhandari S. Can incremental haemodialysis reduce early mortality rates in patients starting maintenance haemodialysis? Curr Opin Nephrol Hypertens. 2019;28(6):641–7.
Kooman JP, Katzarski K, van der Sande FM, Leunissen KM, Kotanko P. Hemodialysis: A model for extreme physiology in a vulnerable patient population. Semin Dial. 2018;31(5):500–6.
Casino FG, Lopez T, Santarsia G, Mostacci SD, Sabato A, Di Carlo M, Procida C, Saracino A, Deira J, Basile C. Could incremental haemodialysis be a new standard of care? A suggestion from a long-term observational study. G Ital Nefrol. 2022;39(3):2022-vol3.
PubMed Google Scholar
Moore C, Carter LA, Mitra S, Skevington S, Wearden A. Quality of life improved for patients after starting dialysis but is impaired, initially, for their partners: a multi-centre, longitudinal study. BMC Nephrol. 2020;21(1):185.
Chen SS, Al Mawed S, Unruh M. Health-related quality of life in end-stage renal disease patients: how often should we ask and what do we do with the answer? Blood Purif. 2016;41(1–3):218–24.
Heiwe S, Clyne N, Dahlgren MA. Living with chronic renal failure: patients’ experiences of their physical and functional capacity. Physiother Res Int. 2003;8(4):167–77.
Dahlerus C, Quinn M, Messersmith E, Lachance L, Subramanian L, Perry E, et al. Patient perspectives on the choice of dialysis modality: results from the Empowering Patients on Choices for Renal Replacement Therapy (EPOCH-RRT) study. Am J Kidney Dis. 2016;68(6):901–10.
Tong A, Craig JC, Nagler EV, Van Biesen W. Composing a new song for trials: the Standardized Outcomes in Nephrology (SONG) initiative. Nephrol Dial Transplant. 2017;32(12):1963–6.
Tong A, Manns B, Hemmelgarn B, Wheeler DC, Evangelidis N, Tugwell P, et al. Establishing core outcome domains in hemodialysis: report of the Standardized Outcomes in Nephrology-Hemodialysis (SONG-HD) Consensus Workshop. Am J Kidney Dis. 2017;69(1):97–107.
Dąbrowska-Bender M, Dykowska G, Żuk W, Milewska M, Staniszewska A. The impact on quality of life of dialysis patients with renal insufficiency. Patient Prefer Adherence. 2018;19(12):577–83.
Article Google Scholar
Evangelidis N, Tong A, Manns B, Hemmelgarn B, Wheeler DC, Tugwell P, et al. Developing a set of core outcomes for trials in hemodialysis: an international delphi survey. Am J Kidney Dis. 2017;70(4):464–75.
Caton E, Sharma S, Vilar E, Farrington K. Impact of incremental initiation of haemodialysis on mortality: a systematic review and meta-analysis. Nephrol Dial Transplant. 2023;38(2):435–46.
Park JI, Park JT, Kim YL, Kang SW, Yang CW, Kim NH, Oh YK, Lim CS, Kim YS, Lee JP; CRC for ESRD Investigators. Comparison of outcomes between the incremental and thrice-weekly initiation of hemodialysis: a propensity-matched study of a prospective cohort in Korea. Nephrol Dial Transplant. 2017;32(2):355-363.
Mathew A, Obi Y, Rhee CM, Chen JL, Shah G, Lau WL, et al. Treatment frequency and mortality among incident hemodialysis patients in the United States comparing incremental with standard and more frequent dialysis. Kidney Int. 2016;90(5):1071–9.
Fernández-Lucas M, Teruel-Briones JL, Gomis-Couto A, Villacorta-Pérez J, Quereda-Rodríguez-Navarro C. Maintaining residual renal function in patients on haemodialysis: 5-year experience using a progressively increasing dialysis regimen. Nefrologia. 2012;32(6):767–76 (English, Spanish).
Vilar E, Wellsted D, Chandna SM, Greenwood RN, Farrington K. Residual renal function improves outcome in incremental haemodialysis despite reduced dialysis dose. Nephrol Dial Transplant. 2009;24(8):2502–10.
Obi Y, Streja E, Rhee CM, Ravel V, Amin AN, Cupisti A, et al. Incremental hemodialysis, residual kidney function, and mortality risk in incident dialysis patients: a cohort study. Am J Kidney Dis. 2016;68(2):256–65.
Obi Y, Rhee CM, Mathew AT, Shah G, Streja E, Brunelli SM, et al. Residual kidney function decline and mortality in incident hemodialysis patients. J Am Soc Nephrol. 2016;27(12):3758–68.
Rhee CM, Unruh M, Chen J, Kovesdy CP, Zager P, Kalantar-Zadeh K. Infrequent dialysis: a new paradigm for hemodialysis initiation. Semin Dial. 2013;26(6):720–7.
Kalantar-Zadeh K, Unruh M, Zager PG, Kovesdy CP, Bargman JM, Chen J, et al. Twice-weekly and incremental hemodialysis treatment for initiation of kidney replacement therapy. Am J Kidney Dis. 2014;64(2):181–6.
Shafi T, Jaar BG, Plantinga LC, Fink NE, Sadler JH, Parekh RS, et al. Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am J Kidney Dis. 2010;56(2):348–58.
KDOQI Clinical Practice Guideline for Hemodialysis Adequacy. 2015 update. Am J Kidney Dis. 2015;66(5):884–930.
Li Y, Jin Y, Kapke A, Pearson J, Saran R, Port FK, et al. Explaining trends and variation in timing of dialysis initiation in the United States. Medicine (Baltimore). 2017;96(20):e6911.
Chin AI, Appasamy S, Carey RJ, Madan N. Feasibility of incremental 2-times weekly hemodialysis in incident patients with residual kidney function. Kidney Int Rep. 2017;2(5):933–42.
Hsu CY, Parikh RV, Pravoverov LN, Zheng S, Glidden DV, Tan TC, et al. Implication of trends in timing of dialysis initiation for incidence of end-stage kidney disease. JAMA Intern Med. 2020;180(12):1647–54.
Hwang HS, Hong YA, Yoon HE, Chang YK, Kim SY, Kim YO, et al. Comparison of clinical outcome between twice-weekly and thrice-weekly hemodialysis in patients with residual kidney function. Medicine (Baltimore). 2016;95(7):e2767.
Merino JL, Domínguez P, Bueno B, Amézquita Y, Espejo B, Paraíso V. Application of a pattern of incremental haemodialysis, based on residual renal function, when starting renal replacement therapy. Nefrologia. 2017;37(1):39–46.
Kaja Kamal RM, Farrington K, Busby AD, Wellsted D, Chandna H, Mawer LJ, et al. Initiating haemodialysis twice-weekly as part of an incremental programme may protect residual kidney function. Nephrol Dial Transplant. 2019;34(6):1017–25.
Wolley MJ, Hawley CM, Johnson DW, Marshall MR, Roberts MA. Incremental and twice weekly haemodialysis in Australia and New Zealand. Nephrology (Carlton). 2019;24(11):1172–8.
Davenport A, Guirguis A, Almond M, Day C, Chilcot J, Wellsted D, et al. Comparison of characteristics of centers practicing incremental vs. conventional approaches to hemodialysis delivery - postdialysis recovery time and patient survival. Hemodial Int. 2019;23(3):288–96.
Hanson JA, Hulbert-Shearon TE, Ojo AO, Port FK, Wolfe RA, Agodoa LY, et al. Prescription of twice-weekly hemodialysis in the USA. Am J Nephrol. 1999;19(6):625–33.
Basile C, Casino FG. Incremental haemodialysis and residual kidney function: more and more observations but no trials. Nephrol Dial Transplant. 2019;34(11):1806–11.
Golper TA. Incremental hemodialysis: how i do it. Semin Dial. 2016;29(6):476–80.
Gedney N, Kalantar-Zadeh K. Dialysis patient-centeredness and precision medicine: focus on incremental home hemodialysis and preserving residual kidney function. Semin Nephrol. 2018;38(4):426–32.
Murea M. Precision medicine approach to dialysis including incremental and decremental dialysis regimens. Curr Opin Nephrol Hypertens. 2021;30(1):85–92.
Murea M, Kalantar-Zadeh K. Incremental and twice-weekly hemodialysis program in practice. Clin J Am Soc Nephrol. 2020;16(1):147–9.
Daugirdas JT. Solute solver “what if” module for modeling urea kinetics. Nephrol Dial Transplant. 2016;31(11):1934–7.
Casino FG, Basile C. The variable target model: a paradigm shift in the incremental haemodialysis prescription. Nephrol Dial Transplant. 2017;32(1):182–90.
CAS PubMed Google Scholar
Casino FG, Basile C. How to set the stage for a full-fledged clinical trial testing “incremental haemodialysis.” Nephrol Dial Transplant. 2018;33(7):1103–9.
Casino FG, Basile C. A user-friendly tool for incremental haemodialysis prescription. Nephrol Dial Transplant. 2018;33(6):1046–53.
Zhong J, Yang HC, Fogo AB. A perspective on chronic kidney disease progression. Am J Physiol Renal Physiol. 2017;312(3):F375-f84.
Obi Y, Kalantar-Zadeh K. Incremental and once- to twice-weekly hemodialysis: from experience to evidence. Kidney Int Rep. 2017;2(5):781–4.
Sibbel S, Walker AG, Colson C, Tentori F, Brunelli SM, Flythe J. Association of continuation of loop diuretics at hemodialysis initiation with clinical outcomes. Clin J Am Soc Nephrol. 2019;14(1):95–102.
Wang K, Bansal N. Diuretic use in incident ESKD: are we out of the loop? Clin J Am Soc Nephrol. 2019;14(1):13–5.
Bragg-Gresham JL, Fissell RB, Mason NA, Bailie GR, Gillespie BW, Wizemann V, et al. Diuretic use, residual renal function, and mortality among hemodialysis patients in the Dialysis Outcomes and Practice Pattern Study (DOPPS). Am J Kidney Dis. 2007;49(3):426–31.
Weir MR, Bakris GL, Bushinsky DA, Mayo MR, Garza D, Stasiv Y, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372(3):211–21.
Murea M, Patel A, Highland BR, Yang W, Fletcher AJ, Kalantar-Zadeh K, et al. Twice-weekly hemodialysis with adjuvant pharmacotherapy and transition to thrice-weekly hemodialysis: a pilot study. Am J Kidney Dis. 2022;80(2):227–240.e1.
De La Flor JC, Deira J, Marschall A, Valga F, Linares T, Monzon T, et al. Patiromer in a patient with severe hyperkalemia on incremental hemodialysis with 1 session per week: a case report and literature review. Case Rep Nephrol Dial. 2021;11(2):158–66.
Kovesdy CP, Rowan CG, Conrad A, Spiegel DM, Fogli J, Oestreicher N, et al. Real-World Evaluation of Patiromer for the Treatment of Hyperkalemia in Hemodialysis Patients. Kidney Int Rep. 2019;4(2):301–9.
Trinh E, Bargman JM. Are diuretics underutilized in dialysis patients? Semin Dial. 2016;29(5):338–41.
Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Association of serum bicarbonate levels with mortality in patients with non-dialysis-dependent CKD. Nephrol Dial Transplant. 2009;24(4):1232–7.
Wu DY, Shinaberger CS, Regidor DL, McAllister CJ, Kopple JD, Kalantar-Zadeh K. Association between serum bicarbonate and death in hemodialysis patients: is it better to be acidotic or alkalotic? Clin J Am Soc Nephrol. 2006;1(1):70–8.
Bakris GL, Pitt B, Weir MR, Freeman MW, Mayo MR, Garza D, et al. Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial. JAMA. 2015;314(2):151–61.
Murea M, Moossavi S, Fletcher AJ, Jones DN, Sheikh HI, Russell G, et al. Renal replacement treatment initiation with twice-weekly versus thrice-weekly haemodialysis in patients with incident dialysis-dependent kidney disease: rationale and design of the TWOPLUS pilot clinical trial. BMJ Open. 2021;11(5):e047596.
Devins GM. Using the illness intrusiveness ratings scale to understand health-related quality of life in chronic disease. J Psychosom Res. 2010;68(6):591–602.
Smyth B, van den Broek-Best O, Hong D, Howard K, Rogers K, Zuo L, et al. Varying association of extended hours dialysis with quality of life. Clin J Am Soc Nephrol. 2019;14(12):1751–62.
Nie Y, Witten B, Schatell D, Assari S, Ding X, Saran R, Bragg-Gresham JL. Changes in employment status prior to initiation of maintenance hemodialysis in the USA from 2006 to 2015. Clin Kidney J. 2019;13(3):434–41.
PubMed PubMed Central Google Scholar
Hoang VL, Green T, Bonner A. Informal caregivers of people undergoing haemodialysis: associations between activities and burden. J Ren Care. 2019;45(3):151–8.
Jaber BL, Finkelstein FO, Glickman JD, Hull AR, Kraus MA, Leypoldt JK, et al. Scope and design of the Following Rehabilitation, Economics and Everyday-Dialysis Outcome Measurements (FREEDOM) study. Am J Kidney Dis. 2009;53(2):310–20.
Ju A, Teixeira-Pinto A, Tong A, Smith AC, Unruh M, Davison SN, et al. Validation of a core patient-reported outcome measure for fatigue in patients receiving hemodialysis: the SONG-HD fatigue instrument. Clin J Am Soc Nephrol. 2020;15(11):1614–21.
Daugirdas JT, Depner TA, Greene T, Levin NW, Chertow GM, Rocco MV. Standard Kt/Vurea: a method of calculation that includes effects of fluid removal and residual kidney clearance. Kidney Int. 2010;77(7):637–44.
Lopes RD, Macedo AVS, de Barros ESPGM, Moll-Bernardes RJ, DosSantos TM, Mazza L, et al. Effect of Discontinuing vs Continuing Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers on Days Alive and Out of the Hospital in Patients Admitted With COVID-19: A Randomized Clinical Trial. JAMA. 2021;325(3):254–64.
Fanaroff AC, Cyr D, Neely ML, Bakal J, White HD, Fox KAA, et al. Days alive and out of hospital: exploring a patient-centered, pragmatic outcome in a clinical trial of patients with acute coronary syndromes. Circ Cardiovasc Qual Outcomes. 2018;11(12):e004755.
Yeudall LT, Reddon JR, Gill DM, Stefanyk WO. Normative data for the Halstead-Reitan neuropsychological tests stratified by age and sex. J Clin Psychol. 1987;43(3):346–67.
Helfrich CD, Li YF, Sharp ND, Sales AE. Organizational readiness to change assessment (ORCA): development of an instrument based on the Promoting Action on Research in Health Services (PARIHS) framework. Implement Sci. 2009;4:38.
Weiner BJ, Lewis CC, Stanick C, Powell BJ, Dorsey CN, Clary AS, et al. Psychometric assessment of three newly developed implementation outcome measures. Implement Sci. 2017;12(1):108.
Ehrhart MG, Aarons GA, Farahnak LR. Assessing the organizational context for EBP implementation: the development and validity testing of the Implementation Climate Scale (ICS). Implement Sci. 2014;9:157.
Aarons GA, Ehrhart MG, Farahnak LR. The Implementation Leadership Scale (ILS): development of a brief measure of unit level implementation leadership. Implement Sci. 2014;9(1):45.
Dückers ML, Spreeuwenberg P, Wagner C, Groenewegen PP. Exploring the black box of quality improvement collaboratives: modelling relations between conditions, applied changes and outcomes. Implement Sci. 2009;4:74.
Dückers ML, Wagner C, Groenewegen PP. Developing and testing an instrument to measure the presence of conditions for successful implementation of quality improvement collaboratives. BMC Health Serv Res. 2008;8:172.
Lovasik BP, Zhang R, Hockenberry JM, Schrager JD, Pastan SO, Mohan S, et al. Emergency department use and hospital admissions among patients with end-stage renal disease in the United States. JAMA Intern Med. 2016;176(10):1563–5.
Smyth B, Haber A, Trongtrakul K, Hawley C, Perkovic V, Woodward M, et al. Representativeness of randomized clinical trial cohorts in end-stage kidney disease: a meta-analysis. JAMA Intern Med. 2019;179(10):1316–24.
Vilar E, Kaja Kamal RM, Fotheringham J, Busby A, Berdeprado J, Kislowska E, Wellsted D, Alchi B, Burton JO, Davenport A, Farrington K. A multicenter feasibility randomized controlled trial to assess the impact of incremental versus conventional initiation of hemodialysis on residual kidney function. Kidney Int. 2022;101(3):615–25.
Rutherford, A. (2014) Introducing ANOVA and ancova: A GLM approach. SAGE Publications Ltd.
Chan CT, Greene T, Chertow GM, Kliger AS, Stokes JB, Beck GJ, et al. Effects of frequent hemodialysis on ventricular volumes and left ventricular remodeling. Clin J Am Soc Nephrol. 2013;8(12):2106–16.
Davis CE. Secondary endpoints can be validly analyzed, even if the primary endpoint does not provide clear statistical significance. Control Clin Trials. 1997;18(6):557–60.
Freemantle N. Interpreting the results of secondary end points and subgroup analyses in clinical trials: should we lock the crazy aunt in the attic? BMJ. 2001;322(7292):989–91.
Prentice RL. Discussion: On the role and analysis of secondary outcomes in clinical trials. Control Clin Trials. 1997;18(6):561–7.
Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50.
Gaglio B, Shoup JA, Glasgow RE. The RE-AIM framework: a systematic review of use over time. Am J Public Health. 2013;103(6):e38-46.
Holtrop JS, Estabrooks PA, Gaglio B, Harden SM, Kessler RS, King DK, Kwan BM, Ory MG, Rabin BA, Shelton RC, Glasgow RE. Understanding and applying the RE-AIM framework: Clarifications and resources. J Clin Transl Sci. 2021;5(1):e126.
Daykin A, Clement C, Gamble C, Kearney A, Blazeby J, Clarke M, et al. “Recruitment, recruitment, recruitment” - the need for more focus on retention: a qualitative study of five trials. Trials. 2018;19(1):76.
Ross GJS, Preece DA. The negative binomial distribution. J R Stat Soc. 1985;34(3):323–35.
Google Scholar
Kahan BC. Accounting for centre-effects in multicentre trials with a binary outcome - when, why, and how? BMC Med Res Methodol. 2014;14:20.
Kahan BC, Harhay MO. Many multicenter trials had few events per center, requiring analysis via random-effects models or GEEs. J Clin Epidemiol. 2015;68(12):1504–11.
Senn SJ, Lewis RJ. Treatment effects in multicenter randomized clinical trials. JAMA. 2019;321(12):1211–2.
Committee for Proprietary Medicinal Products. Points to consider on switching between superiority and non-inferiority. Br J Clin Pharmacol. 2001;52(3):223–8.
Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–74.
Moerbeek M, van Breukelen GJ, Berger MP. A comparison between traditional methods and multilevel regression for the analysis of multicenter intervention studies. J Clin Epidemiol. 2003;56(4):341–50.
Mallinckrodt CH, Lane PW, Schnell DJ, Peng Y, Mancuso JP. Recommendations for the primary analysis of continuous endpoints in longitudinal clinical trials. Drug Inf J. 2008;42:303–19.
Lu K. On efficiency of constrained longitudinal data analysis versus longitudinal analysis of covariance. Biometrics. 2010;66(3):891–6.
Freemantle N, Calvert M, Wood J, Eastaugh J, Griffin C. Composite outcomes in randomized trials: greater precision but with greater uncertainty? JAMA. 2003;289(19):2554–9.
Tibaldi F, Molenberghs G, Burzykowski T, Geys H. Pseudo-likelihood estimation for a marginal multivariate survival model. Stat Med. 2004;23(6):947–63.
Pogue J, Devereaux PJ, Thabane L, Yusuf S. Designing and analyzing clinical trials with composite outcomes: consideration of possible treatment differences between the individual outcomes. PLoS One. 2012;7(4):e34785.
Latimer NR, Henshall C, Siebert U, Bell H. Treatment switching: statistical and decision-making challenges and approaches. Int J Technol Assess Health Care. 2016;32(3):160–6.
Sullivan TR, Latimer NR, Gray J, Sorich MJ, Salter AB, Karnon J. Adjusting for treatment switching in oncology trials: a systematic review and recommendations for reporting. Value Health. 2020;23(3):388–96.
Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101.
O’Cathain A, Murphy E, Nicholl J. Three techniques for integrating data in mixed methods studies. BMJ. 2010;341:c4587.
Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–57.
Rothman KJ, Greenland S, Walker AM. Concepts of interaction. Am J Epidemiol. 1980;112(4):467–70.
Varadhan R, Segal JB, Boyd CM, Wu AW, Weiss CO. A framework for the analysis of heterogeneity of treatment effect in patient-centered outcomes research. J Clin Epidemiol. 2013;66(8):818–25.
Wand MP. A comparison of regression spline smoothing procedures. Comput Stat. 2000;15(4):443–62.
Watson PE, Watson ID, Batt RD. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr. 1980;33(1):27–39.
Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine–reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357(21):2189–94.
Liu Y, De A. Multiple imputation by fully conditional specification for dealing with missing data in a large epidemiologic study. Int J Stat Med Res. 2015;4(3):287–95.
Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22(3):278–95.
Seaman SR, White IR, Copas AJ, Li L. Combining multiple imputation and inverse-probability weighting. Biometrics. 2012;68(1):129–37.
van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1–67.
Ebrahim GJ. Missing data in clinical studies Molenberghs G. and Kenward M.G. J Trop Pediatr. 2007;53(4):294.
Little RJ. Pattern-mixture models for multivariate incomplete data. J Am Stat Assoc. 1993;88(421):125–34.
Little RJ, D’Agostino R, Cohen ML, Dickersin K, Emerson SS, Farrar JT, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med. 2012;367(14):1355–60.
Himmelfarb J, Vanholder R, Mehrotra R, Tonelli M. The current and future landscape of dialysis. Nat Rev Nephrol. 2020;16(10):573–85.
Renal Disease. Chapter 9. Healthcare Expenditures for Persons with ESRD:USRDS; 2020 Reviewed 2024 Feb 8. Available from: https://adr.usrds.org/2020/end-stage-renal-disease/9-healthcare-expenditures-for-persons-with-esrd .
United States Renal Data System. The United States Renal Data System 2020 Annual Data Report: USRDS; 2020. Chapter 8: Transition of Care in Chronic Kidney Disease. Reviewed 2024 Feb 8. Available from: https://adr.usrds.org/2020/chronic-kidney-disease/8-transition-of-care-in-chronic-kidney-disease .
Lin E, Ginsburg PB, Chertow GM, Berns JS. The “advancing american kidney health” executive order: challenges and opportunities for the large dialysis organizations. Am J Kidney Dis. 2020;76(5):731–4.
United States. Executive Office of the President. Executive Order on Advancing American Kidney Health: The White House; 2024. Available from: http://trumpwhitehouse.archives.gov/presidential-actions/executive-order-advancing-american-kidney-health .
Group TFT. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363(24):2287–300.
Deira J, Murea M, Kalantar-Zadeh K, Casino FG, Basile C. Does delivering more dialysis improve clinical outcomes? What randomized controlled trials have shown. J Nephrol. 2022;35(5):1315–27.
Torreggiani M, Fois A, Samoreau C, Santagati G, Piccoli GB. The ABCs of personalized incremental dialysis start Le Mans style. J Nephrol. 2022;35(9):2417–23.
Bowline IG, Russell GB, Bagwell B, Crossley B, Fletcher AJ, Murea M. Temporal trends in fluid management with incremental hemodialysis. Clin Nephrol. 2019;92(4):165–73.
Murea M, Patel A, Highland BR, Yang W, Fletcher AJ, Kalantar-Zadeh K, et al. Twice-weekly hemodialysis with adjuvant pharmacotherapy and transition to thrice-weekly hemodialysis: a pilot study. Am J Kidney Dis. 2022;80(2):227-40.e1.
Murea M, Highland BR, Yang W, Dressler E, Russell GB. Patient-reported outcomes in a pilot clinical trial of twice-weekly hemodialysis start with adjuvant pharmacotherapy and transition to thrice-weekly hemodialysis vs conventional hemodialysis. BMC Nephrol. 2022;23(1):322.
Download references
Acknowledgements
We express profound gratitude to the patients, caregivers, and healthcare professionals participating in the TwoPlus trial. We are grateful for the invaluable support from the leadership of all dialysis organizations and dialysis centers that are actively participated in this study. Our appreciation extends to the healthcare systems, dialysis clinics, and their dedicated medical teams for their invaluable care and support of the patients enrolled in the trial. We also extend our acknowledgment to the grant administration teams and regulatory personnel collaborating with the study teams. Sincere appreciation goes to all members of the Data and Safety Monitoring Board for their dedicated oversight of study conduct and commitment to monitoring the safety of the study participants.
The TwoPlus Research Consortium:
Denisse A. Funes, Jessica Guillaume, Victoria Shoyelu, Katherine Vergara, Lyn B. Lyman, Fatima Salmi, Erika Adams, Jessica Farrell, Nancy Ginsberg, Christa Howard, Suzanne Shabdue, Shawanna Jackson, Seth Johnson, Randall D. Blackie, Sheetal Chaudhuri, Priya Desai, Kristy Hamilton, Igor Shumilin, Diana Clynes, Valerie Gonzalez, Erin Kahle, Marie Mitchell, Jennifer Rate, Brindusa Burciu, Lilliana Serrano, Alexandra Peluso, Valeria G. Bittencourt, Zohreh Forghani, Elnaz R. Ghalechi, Allison Green, Marina Markovic, Debra Martin, Caroline Poulton, Simran Singh, Katlyn Stiles, Ashleigh Trapuzzano, Joni Baker, Susan Trynosky.
This research is funded through a Patient-Centered Outcomes Research Institute (PCORI) Award (CER-2022C1-26300). The views, statements, and opinions presented are solely the responsibility of the author(s) and do not necessarily represent the views of PCORI, its Board of Governors or Methodology Committee. The study sponsor (PCORI) does not play a role in study activities.
Author information
Authors and affiliations.
Department of Internal Medicine, Section on Nephrology, Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, NC, USA
Mariana Murea, Alison J. Fletcher & Sheikh I. Hiba
Renal Research Institute, New York, NY, USA
Jochen G. Raimann, Erica J. Trembath & Merlo L. Rosales
Department of Foundations of Medicine, Center for Population and Health Services Research, NYU Grossman Long Island School of Medicine, New York, NY, USA
Jasmin Divers, Harvey Maute & Islam Shahidul
Department of Nephrology and Hypertension, Glickman Urological and Kidney Institute, Cleveland Clinic, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland, OH, USA
Cassandra Kovach & Anne Huml
Division of Nephrology, University of Virginia Health System, Charlottesville, VA, USA
Emaad M. Abdel-Rahman & Daphne H. Knicely
Division of Nephrology, University of Florida, Jacksonville, FL, USA
Alaa S. Awad, Irtiza Hasan & Bhaktidevi Makadia
University of North Carolina (UNC) Kidney Center, Division of Nephrology and Hypertension, Department of Medicine, UNC School of Medicine, Chapel Hill, NC, USA
Jennifer E. Flythe
Department of Medicine, Division of Nephrology, Johns Hopkins School of Medicine, Baltimore, MD, USA
Samir C. Gautam & Raman Gaurav
Division of Nephrology, Department of Medicine, Emory University, Atlanta, GA, USA
Vandana D. Niyyar & Janice Lea
External Relations and Patient Engagement, Division of Nephrology, Department of Medicine, Kidney Research Institute and Center for Dialysis Innovation, University of Washington, Seattle, WA, USA
Glenda V. Roberts
Home Dialyzors United, Columbia, MO, USA
Nichole M. Jefferson
Department of Implementation Science, Wake Forest University School of Medicine, Winston-Salem, NC, USA
Ucheoma Nwaozuru & Kristie L. Foley
American Association of Kidney Patients, Tampa, FL, USA
Paul T. Conway
Division of Nephrology, Department of Medicine, University of Illinois College of Medicine, Chicago, IL, USA
John T. Daugirdas
Department of Internal Medicine, Section on Nephrology, LLC Icahn School of Medicine at Mount Sinai, New York, NY, USA
Peter Kotanko
You can also search for this author in PubMed Google Scholar
- Denisse A. Funes
- , Jessica Guillaume
- , Victoria Shoyelu
- , Katherine Vergara
- , Lyn B. Lyman
- , Fatima Salmi
- , Erika Adams
- , Jessica Farrell
- , Nancy Ginsberg
- , Christa Howard
- , Suzanne Shabdue
- , Shawanna Jackson
- , Seth Johnson
- , Randall D. Blackie
- , Sheetal Chaudhuri
- , Priya Desai
- , Kristy Hamilton
- , Igor Shumilin
- , Diana Clynes
- , Valerie Gonzalez
- , Erin Kahle
- , Marie Mitchell
- , Jennifer Rate
- , Brindusa Burciu
- , Lilliana Serrano
- , Alexandra Peluso
- , Valeria G. Bittencourt
- , Zohreh Forghani
- , Elnaz R. Ghalechi
- , Allison Green
- , Marina Markovic
- , Debra Martin
- , Caroline Poulton
- , Simran Singh
- , Katlyn Stiles
- , Ashleigh Trapuzzano
- , Joni Baker
- & Susan Trynosky
Contributions
MM conceived the study. MM, JGR, CK, EAR, JEF, SCG, ASA, VDN, GVR, NMJ, EJT, UN, KLF, JTD, PTC, and PK contributed to the study design and procedures for each aspect of the trial protocol. MM drafted the study protocol with significant contributions from all investigators and consortium members. MM, PK, and all investigators and consortium members contributed to the design of study procedures, which included recruitment strategies and data collection procedures. HM and all consortium members contributed to the design of centralized study platform for data entry. JD and IS contributed to the study design, sample size estimates, and analytic plan. All protocol authors reviewed, edited, and approved the final protocol. Authorship eligibility for this protocol and for subsequent publications will adhere to the International Committee of Medical Journal Editors (ICMJE) guidelines. The authors read and approved the final manuscript.
Corresponding authors
Correspondence to Mariana Murea or Peter Kotanko .
Ethics declarations
Ethics approval and consent to participate {24}.
Ethics review and approval was received by the Wake Forest University School of Medicine Institutional Review Board’s Committee on Human Research (IRB00092986). Informed consent to participate will be obtained from all participants.
Consent for publication {32}
Not applicable. No details, images, or videos relating to an individual person will be published as part of this study.
Competing interests {28}
PK, ET, LR, and JGR are employees of the Renal Research Institute, a wholly owned subsidiary of Fresenius Medical Care. PK and JGR own stock in Fresenius Medical Care. The remaining authors declare no completing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., supplementary material 2. , supplementary material 3. , supplementary material 4. , rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Murea, M., Raimann, J.G., Divers, J. et al. Comparative effectiveness of an individualized model of hemodialysis vs conventional hemodialysis: a study protocol for a multicenter randomized controlled trial (the TwoPlus trial). Trials 25 , 424 (2024). https://doi.org/10.1186/s13063-024-08281-9
Download citation
Received : 25 March 2024
Accepted : 20 June 2024
Published : 28 June 2024
DOI : https://doi.org/10.1186/s13063-024-08281-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Incremental
ISSN: 1745-6215
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
DHHS Regulations define research as a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge according to 45 CFR 46.102[1] Common Rule.. For purposes of IRB review, we further defines the following terms: A "systematic investigation" as an activity involving a prospective plan that incorporates:
Date: July 20, 2020 Scope: This guidance document applies to nonexempt research involving human subjects that is conducted or supported by HHS.It provides guidance on the elimination of the requirement in section 46.103(f) of the pre-2018 Requirements that each application or proposal for research undergo IRB review and approval as part of the certification process.
TABLE 1 lists the criteria for the approval of research (paraphrased here from the full regulatory language in 21 CFR 56.111) by an IRB, and describes the information that is necessary for the clinical protocol (or elsewhere in the research plan submitted for review), for the IRB to be able to make determinations regarding whether a research ...
Your research advisor will help you determine which IRB should review your research protocol. JAASEP FALL, 2011 85. Information specific to your research institution may also be available via the Internet. Additionally, you may find that your advisor has received prior IRB approval via a grant-writing process.
IRB-HSBS Turnaround Times. "Turnaround" is the estimated time it takes to complete the IRB review and determination process. Full-board: 4 - 8 weeks. Expedited: 2 - 4 weeks. Exempt: < 1 week. The U-M Institutional Review Boards (IRBs) fulfill their goals to protect human research participants and support the design and conduct of sound research ...
Research is defined as a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge. A project requires IRB review if it includes both research and human subjects. The IRB must make the final determination of whether or not a study requires review.
Studies that pose minimal risk or proposals that are minor changes to studies that were previously approved by the IRB may not need to undergo a full IRB review. How will the IRB conduct initial and continuing review of research proposals? Studies that are ongoing (lasting more than 12 months) should have a follow-up review process at least ...
Heather Paskalides, IRB/RCR Specialist, 401.874.4328. Lynnea Lau, Director, Research Integrity, 401.874.4813. or [email protected], 401.874.4328. The Research Resources page contains FORMS, Policies, Templates, Guidance, Tools, Instruction and Training Material, and other resources to support your research efforts by topic area ...
Institutional Review Boards (IRBs) review research involving human subjects to ensure that participants are protected from potentially harmful research. This resource provides an overview of the roles of IRBs and ethics guidelines. It also includes practical tips for researchers preparing IRB proposals, including an annotated informed consent checklist, guidance on timeline planning and ...
The full research proposal submission, Experiments in Virtual Reality, consists of four components: (1) the completed full proposal form, (2) the proposal consent form, (3) a debriefing form to be used with subjects after their participation in the study, and (4) recruiting materials which announce the study and solicit participants. Mindsets ...
IRB Review Process. To apply for IRB approval, the principal investigator must submit an application to the IRB. There are two types of application that may be submitted based on the categories of research determined by state and federal codes. Type 1: If a researcher believes her/his project meets the requirements for exemption, s/he may ...
Expedited reviews may be carried out by the IRB chair, an IRB co-chair, or by one or more experienced reviewers designated by the chair from among members of the IRB. Full IRB review: Research that does not qualify as exempt or for expedited review must undergo a full review by a quorum of IRB members. The application process is the same as for ...
Minor changes to research previously approved by the Lafayette IRB may also qualify for expedited review. Full Review. If the proposed research does not qualify for Exempt or Expedited Review as defined above, it will be subject to a Full Review. In addition, if the proposed research involves any of the following, it will be subject to Full Review.
Full board review is conducted by the convened IRB. Studies that require full board review are those that are greater than minimal risk and/or do not qualify for an exempt or expedited research category. Full board studies undergo an administrative pre-review process prior to IRB review. Following completion of pre-review, studies are forwarded ...
If you are unsure if your project meets the definition of research, or if you require documentation that your project does not require IRB review, please contact the HRPP at (401) 863-3050 or [email protected] to discuss.
The IRB Coordinator's role is to shepherd the submission through the review process. Once a proposal is assigned to an IRB Coordinator, they will conduct an initial pre-review, ensuring adequate information is provided for the board to make the requisite determinations about the research and that the research satisfies regulatory and institutional requirements.
The QI/Program Evaluation Self-Certification Tool is designed to assist study teams in determining whether a project requires submission to the IRB. If the project involves some characteristics of a research project, the Tool will let you know that IRB review is required. If the project does qualify as program evaluation/QI, the Tool will ...
An Institutional Review Board (IRB) is a committee set up by an organization to review, approve, and regulate research conducted by its members, on its premises, or under its sponsorship (Babie, 2001). The National Research Act, passed by Congress in 1974, directed all institutions receiving federal support for research and evaluation studies ...
The IRB is here to assist regarding the protection of human subjects, however, we will not write or revise your proposal for you. Step 5. Make sure you have all the research study proposal information available BEFORE completing the application. Getting a study ready for IRB review can take a significant amount of time and effort.
A: Yes. The IRB is the only office that can determine if a research project qualifies for exemption and must have sufficient information in order to make that determination; and training requirements pertain regardless of the type of review conducted. Q: I submitted an application for expedited review three days ago.
Contents. Exempt human subjects research is a specific sub-set of "research involving human subjects" that does not require ongoing IRB oversight. Research can qualify for an exemption if it is no more than minimal risk and all of the research procedures fit within one or more of the exemption categories in the federal IRB regulations.
Which would be included in a full review of a research study proposal by an Institutional Review Board (IRB) for a study that involves greater than minimal risk to participants? Select all that apply. a. Ensure minimal risks to participants b. Protect the privacy of research participants c. Determine that sample selection is fair and equal d.
Full Board Review Expedited Review Research Exemptions from IRB Review Full Board Review Researching involving more than minimal risk (deception, distress) Proposal is presented and discussed at a meeting at which all IRB members are present, and it must receive the approval of the majority to be approved
This worksheet is for use by Designated Reviewers (expedited reviews) and the Primary and Secondary Reviewers (convened IRB reviews) when preparing for the review of initial applications for a study and for non-UW sites that are reviewed by the UW IRB per a reliance or cooperative agreement, including deferral responses and conditional approval responses.
This document describes the policies and procedures the Human Subjects Division (HSD) follows under the Post Approval Verification and Education (PAVE) program to confirm by observation that University of Washington (UW) human subjects research is conducted in compliance with relevant federal regulations, state laws, UW institutional polices and Institutional Review Board (IRB)-approved ...
Approved by central IRB on 31-01-2024. Funding {4} Patient-Centered Outcomes Research Institute (PCORI) Award. CER-2022C1-26300. Author details {5a} [SPIRIT guidance: Affiliations of protocol contributors.] 1. Wake Forest University School of Medicine. 2. Renal Research Institute. Name and contact information for the trial sponsor {5b}