If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.

High school chemistry
Course: high school chemistry > unit 2.
- Quiz 1 Chemical bonding

- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- Science Experiments for Kids
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
Covalent Bond Definition and Examples

A covalent bond is a chemical bond between two atoms where they share one or more pairs of electrons. Usually, sharing electrons gives each atom a full valence shell and makes the resulting compound more stable than its constituent atoms are on their own. Covalent bonds usually form between nonmetals . Examples of covalent compounds include hydrogen (H 2 ), oxygen (O 2 ), carbon monoxide (CO), ammonia (NH 3 ), water (H 2 O), and all organic compounds . There are compounds that contain both covalent and ionic bonds , such as potassium cyanide (KCN) and ammonium chloride (NH 4 Cl).
What Is a Covalent Bond?
Covalent bonding is one of the main types of chemical bonds , along with ionic and metallic bonds. Unlike these other bonds, covalent bonding involves the sharing of electron pairs between atoms. These shared electrons exist in the outer shell of the atom, the so-called valence shell .
The water molecule (H 2 O) is an example of compound with covalent bonds. The oxygen atom shares one electron with each of the two hydrogen atoms, forming two covalent bonds.
Octet Rule and Covalent Bonding
The concept of covalent bonding ties in with the octet rule . This rule states that atoms combine in such a way that each atom has eight electrons in its valence shell, resembling the electronic configuration of a noble gas . By sharing electrons through covalent bonding, atoms effectively fill their outer shells and satisfy the octet rule.
Covalent Bond vs Ionic and Metallic Bonds
Covalent bonds differ significantly from ionic and metallic bonds . Ionic bonds form when one atom gives up one or more electrons to another atom, forming ions that attract each other due to their opposite charges. Sodium chloride (NaCl) is an example of a compound with ionic bonds.
Metallic bonds, on the other hand, form between metal atoms. In these bonds, electrons are not shared or transferred between atoms but instead move freely in what is sometimes referred to as an “electron sea”. This fluidity of electrons gives metals their unique properties, such as electrical conductivity and malleability.
Types of Covalent Bonds
Covalent bonds are either polar covalent bonds or nonpolar covalent bonds.
A nonpolar covalent bond forms when two atoms with the same electronegativity share electrons equally, as in a molecule of hydrogen gas (H 2 ).
A polar covalent bond, on the other hand, forms when the atoms involved in the bond have different electronegativities, resulting in unequal sharing of electrons. The atom with the higher electronegativity pulls the shared electrons closer, creating a region of slight negative charge, while the other atom becomes slightly positive. An example is water (H 2 O), where the oxygen atom is more electronegative than the hydrogen atoms.
Electronegativity and the Type of Bonding
Electronegativity is a measure of an atom’s tendency for attracting a bonding pair of electrons. The electronegativity values, proposed by Linus Pauling, range from around 0.7 to 4.0. The higher the electronegativity, the greater an atom’s attraction for bonding electrons.
When considering whether a bond is ionic or covalent, the difference in electronegativity between the two atoms a helpful guideline.
- If the electronegativity difference is greater than 1.7, the bond is ionic. This is because the more electronegative atom attracts the electron(s) so strongly that it effectively “steals” them from the other atom.
- If the electronegativity difference is less than 1.7 but greater than 0.5, the bond is polar covalent. The atoms do not share electrons equally. The more electronegative atom attracts the electron pair. This leads to a separation of charge, with the more electronegative atom carrying a slight negative charge and the other atom a slight positive charge.
- If the electronegativity difference is less than 0.5, the bond is nonpolar covalent. The atoms share the electron pair more or less equally.
However, these are just guidelines and there is no absolute cut-off value that cleanly separates ionic and covalent bonds. In reality, many bonds falling somewhere in between. Also, electronegativity is not the only factor that determines the type of bond formed. Other factors also play a role, including the size of the atoms, the lattice energy, and the overall structure of the molecule.
Single, Double, and Triple Bonds
Covalent bonds exist as single, double, or triple bonds. In a single covalent bond, two atoms share one pair of electrons. Hydrogen gas (H 2 or H-H) has a single covalent bond, where each hydrogen atom shares its single electron with the other.
In a double bond, atoms share two pairs of electrons. A typical example is oxygen gas (O 2 or O=O), where each oxygen atom shares two electrons with the other. A double bond is stronger than a single bond, but less stable.
Triple bonds involve the sharing of three pairs of electrons, as seen in nitrogen gas (N 2 or N≡N). The triple bond is strongest, yet least stable.
Properties of Covalent Compounds
Compounds that have covalent bonds often share several common properties .
- Low Melting and Boiling Points : Covalent compounds generally have lower melting and boiling points than ionic bonds due to the weaker forces of attraction between molecules.
- Poor Conductivity : Most covalent compounds do not conduct electricity because they lack free-moving charges (such as ions or delocalized electrons) that are necessary for the flow of electrical current. There are exceptions, such as graphite, which conducts electricity due to the delocalization of its electrons. Thermal conductivity varies widely among covalent compounds. For example, diamond, a form of carbon with each carbon atom covalently bonded to four other carbon atoms, is one of the best known thermal conductors. In contrast, many other covalently bonded substances, like water or polymers, are relatively poor thermal conductors.
- Insolubility in Water : Many covalent compounds are nonpolar and are not soluble in water. Water and ethanol are examples of polar covalent compounds that do dissolve ionic compounds and other polar compounds.
- Solubility in Organic Solvents : While nonpolar covalent compounds don’t dissolve well in water, they often dissolve well in organic solvents like benzene or in nonpolar solvents such as carbon tetrachloride. This is due to the ‘like dissolves like’ principle, where polar substances dissolve polar substances, and nonpolar substances dissolve nonpolar substances.
- Lower Density : Covalent compounds generally have lower densities than ionic compounds. This is because the atoms in covalently bonded substances are not packed as closely together as in ionic substances. As a result, they are lighter for their size.
- Brittle Solids : When covalent compounds do form solids, they are generally brittle. They are not ductile or malleable. This is due to the nature of their bonds. If a layer of atoms gets shifted, it disrupts the network of covalent bonds and the substance breaks.
- Atkins, Peter; Loretta Jones (1997). Chemistry: Molecules, Matter and Change . New York: W.H. Freeman & Co. ISBN 978-0-7167-3107-8.
- Langmuir, Irving (1919). “The Arrangement of Electrons in Atoms and Molecules”. Journal of the American Chemical Society . 41 (6): 868–934. doi: 10.1021/ja02227a002
- Lewis, Gilbert N. (1916). “The Atom and the Molecule”. Journal of the American Chemical Society . 38 (4): 772. doi: 10.1021/ja02261a002
- Pauling, Linus (1960). The Nature of the Chemical Bond and the Structure of Molecules and Crystals: An Introduction to Modern Structural Chemistry . ISBN 0-801-40333-2. doi: 10.1021/ja01355a027
- Weinhold, F.; Landis, C. (2005). Valency and Bonding . Cambridge University Press. ISBN 0521831288.
Related Posts
- Life at WOU
Home » Student Resources » Online Chemistry Textbooks » CH104: Chemistry and the Environment » CH104: Chapter 4 – Covalent Bonds and Molecular Compounds
- CH104: Chemistry and the Environment
Chapter 4 – Covalent Bonds and Molecular Compounds
4.1 introduction to covalent molecules and compounds, how to recognize covalent bonds, 4.2 electron sharing, single covalent bonds between the same atoms, single covalent bonds between different atoms, multiple covalent bonds, coordinate covalent bonds, 4.3 electronegativity and bond polarity, 4.4 properties of molecular compounds, 4.5 naming binary molecular compounds, 4.6 focus on the environment – the love canal, 4.7 chapter summary, 4.8 references.
Chemical bonds are generally divided into two fundamentally different types: ionic and covalent. In reality, however, the bonds in most substances are neither purely ionic nor purely covalent, but lie on a spectrum between these extremes. Although purely ionic and purely covalent bonds represent extreme cases that are seldom encountered in any but very simple substances, a brief discussion of these two extremes helps explain why substances with different kinds of chemical bonds have very different properties. Ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally consist of molecules, which are groups of atoms in which one or more pairs of electrons are shared between bonded atoms. In a covalent bond, atoms are held together by the electrostatic attraction between the positively charged nuclei of the bonded atoms and the negatively charged electrons they share. This chapter will focus on the properties of covalent compounds.
Just as an atom is the simplest unit that has the fundamental chemical properties of an element, a molecule is the simplest unit that has the fundamental chemical properties of a covalent compound. Thus, the term molecular compound is used to describe elements that are covalently bonded and to distinguish the compounds from ionic compounds. Some pure elements exist as covalent molecules. Hydrogen, nitrogen, oxygen, and the halogens occur naturally as the diatomic (“two atoms”) molecules H 2 , N 2 , O 2 , F 2 , Cl 2 , Br 2 , and I 2 (part (a) in Figure 4.1 ). Similarly, a few pure elements exist as polyatomic (“many atoms”) molecules, such as elemental phosphorus and sulfur, which occur as P 4 and S 8 (part (b) in Figure 4.1 ).
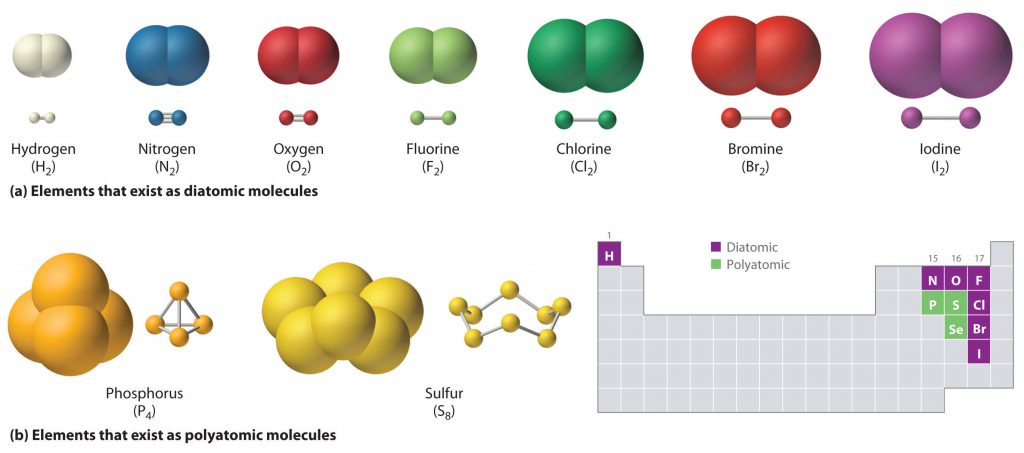
Figure 4.1 Elements That Exist as Covalent Molecules. (a) Several elements naturally exist as diatomic molecules, in which two atoms (E) are joined by one or more covalent bonds to form a molecule with the general formula E2. (b) A few elements naturally exist as polyatomic molecules, which contain more than two atoms. For example, phosphorus exists as P4 tetrahedra—regular polyhedra with four triangular sides—with a phosphorus atom at each vertex. Elemental sulfur consists of a puckered ring of eight sulfur atoms connected by single bonds. Selenium is not shown due to the complexity of its structure.
Each covalent compound is represented by a molecular formula, which gives the atomic symbol for each component element, in a prescribed order, accompanied by a subscript indicating the number of atoms of that element in the molecule. The subscript is written only if the number of atoms is greater than 1. For example, water, with two hydrogen atoms and one oxygen atom per molecule, is written as H 2 O. Similarly, carbon dioxide, which contains one carbon atom and two oxygen atoms in each molecule, is written as C O 2 .

Covalent compounds that predominantly contain carbon and hydrogen are called organic compounds . The convention for representing the formulas of organic compounds is to write carbon first, followed by hydrogen and then any other elements in alphabetical order (e.g., CH 4 O is methyl alcohol, a fuel). Compounds that consist primarily of elements other than carbon and hydrogen are called inorganic compounds ; they include both covalent and ionic compounds. The convention for writing inorganic compounds, involves listing the component elements beginning with the one farthest to the left in the periodic table, as in CO 2 or SF 6 . Those in the same group are listed beginning with the lower element and working up, as in ClF. By convention, however, when an inorganic compound contains both hydrogen and an element from groups 13–15, hydrogen is usually listed last in the formula. Examples are ammonia (NH 3 ) and silane (SiH 4 ). Compounds such as water, whose compositions were established long before this convention was adopted, are always written with hydrogen first: Water is always written as H 2 O, not OH 2 . Typically this distinguishes when hydrogen is participating in a covalent bond rather than an ionic interaction, as seen in many of the inorganic acids, such as hydrochloric acid (HCl) and sulfuric acid (H 2 SO 4 ), as described in chapter 3 .
In Chapter 3, we saw that ionic compounds are composed predominantly of a metal + a nonmetal. Covalent molecules, on the otherhand, are typically composed of two nonmetals or a nonmetal and a metalloid. This is an initial screening method that you can use to categorize compounds into the ionic or the covalent cagetogy.
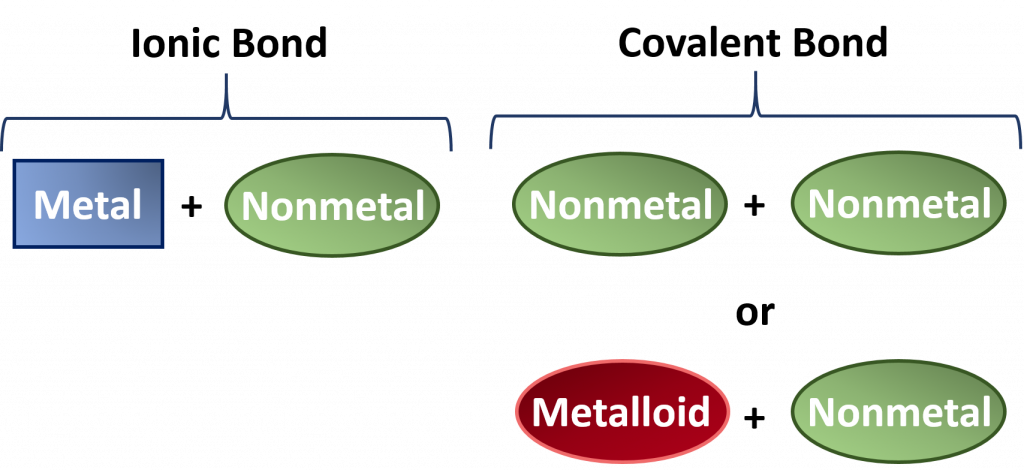
Figure 4.2 Recognizing Ionic vs Covalent Compounds. Typically compounds that are formed from a combination of a metal with a nonmetal have more ionic bond character whereas compounds formed from two nonmetals or a metalloid and a nonmetal show more covalent character. Although compounds usually lie on a spectrum somewhere between fully ionic and fully covalent character, for naming purposes, this guideline works well.
Chapter 3 described how electrons can be transferred from one atom to another so that both atoms have an energy-stable outer electron shell following the octet rule . However, there is another way an atom can achieve a full valence shell: atoms can share electrons to reach the octet state (or the duet state in the case of hydrogen).
This concept can be illustrated by using two hydrogen atoms, each of which has a single electron in its valence shell. (For small atoms such as hydrogen atoms, the valence shell will be the first shell, which holds only two electrons.) We can represent the two individual hydrogen atoms as follows:
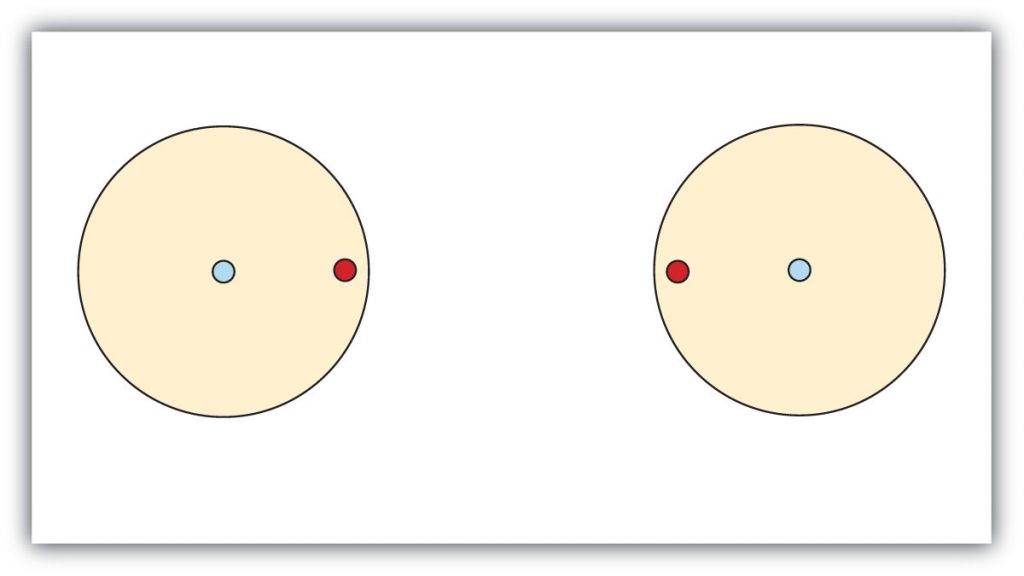
In this situation neither hydrogen can reach the preferred duet state. In contrast, when two hydrogen atoms get close enough together to share their electrons, they can be represented as follows:
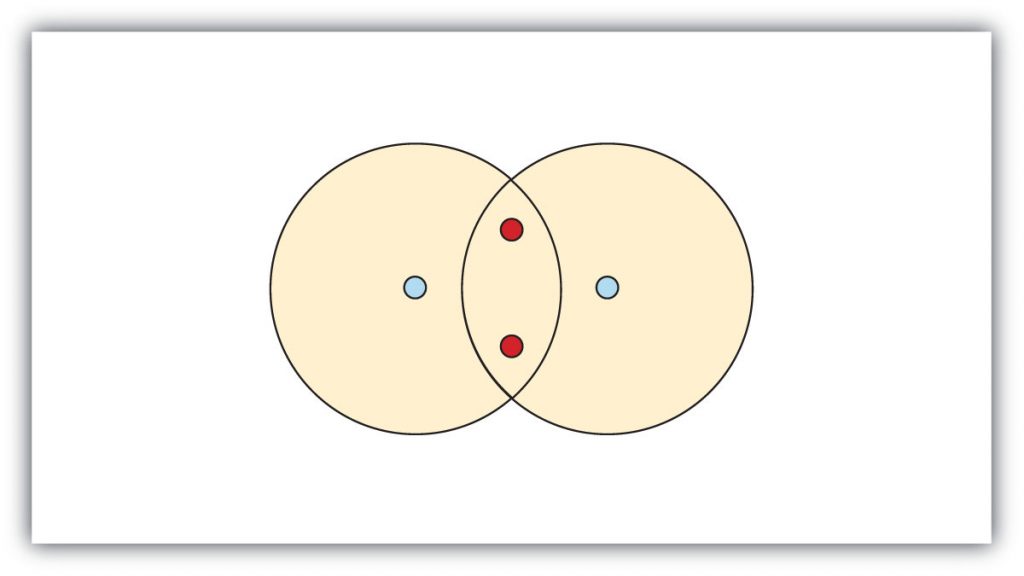
By sharing their valence electrons, both hydrogen atoms now have two electrons in their respective valence shells. Because each valence shell is now filled, this arrangement is more stable than when the two atoms are separate. In this configuration, each hydrogen has an electron configuration equivalent to that of the noble gas, helium. The sharing of electrons between atoms is called a covalent bond , and the two electrons that join atoms in a covalent bond are called a bonding pair of electrons . A discrete group of atoms connected by covalent bonds is called a molecule —the smallest part of a compound that retains the chemical identity of that compound. For example, one molecule of water would contain two hydrogen atoms and one oxygen atom (H 2 O).
Chemists frequently use Lewis electron dot diagrams to represent covalent bonding in molecular substances. For example, the Lewis diagrams of two separate hydrogen atoms are as follows:
The Lewis diagram of two hydrogen atoms sharing electrons looks like this:
This depiction of molecules is simplified further by using a dash to represent a covalent bond. The hydrogen molecule is then represented as follows:
Remember that the dash, also referred to as a single bond, represents a pair of bonding electrons.
The bond in a hydrogen molecule, measured as the distance between the two nuclei, is about 7.4 × 10 −11 m, or 74 picometers (pm; 1 pm = 1 × 10 −12 m). This particular bond length represents a balance between several forces: (1) the attractions between oppositely charged electrons and nuclei, (2) the repulsion between two negatively charged electrons, and (3) the repulsion between two positively charged nuclei. If the nuclei were closer together, they would repel each other more strongly; if the nuclei were farther apart, there would be less attraction between the positive and negative particles.
Fluorine is another element whose atoms bond together in pairs to form diatomic (two-atom) molecules. Two separate fluorine atoms have the following electron dot diagrams:

Each fluorine atom contributes one valence electron, making a single bond and giving each atom a complete valence shell, which fulfills the octet rule:

The circles show that each fluorine atom has eight electrons around it. As with hydrogen, we can represent the fluorine molecule with a dash in place of the bonding electrons:
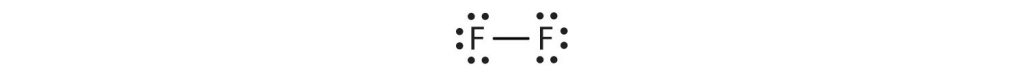
Each fluorine atom has six electrons, or three pairs of electrons, that are not participating in the covalent bond. Rather than being shared, they are considered to belong to a single atom. These are called nonbonding pairs (or lone pairs) of electrons.
Now that we have looked at electron sharing between atoms of the same element, let us look at covalent bond formation between atoms of different elements. Consider a molecule composed of one hydrogen atom and one fluorine atom:

Each atom needs one additional electron to complete its valence shell. By each contributing one electron, they make the following molecule:
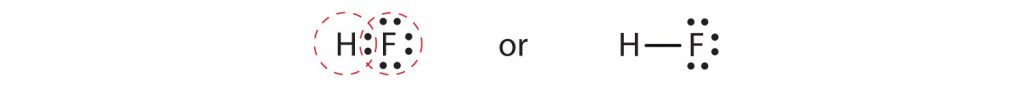
In this molecule, the hydrogen atom does not have nonbonding electrons, while the fluorine atom has six nonbonding electrons (three lone electron pairs). The circles show how the valence electron shells are filled for both atoms (recall that hydrogen is filled with two electrons).
Larger molecules are constructed in a similar fashion, with some atoms participating in more than one covalent bond. For example, water, with two hydrogen atoms and one oxygen atom, and methane (CH 4 ), with one carbon atom and four hydrogen atoms, can be represented as follows:

Atoms typically form a characteristic number of covalent bonds in compounds. Figure 4.3 shows valence electron configurations of each element family (or column).
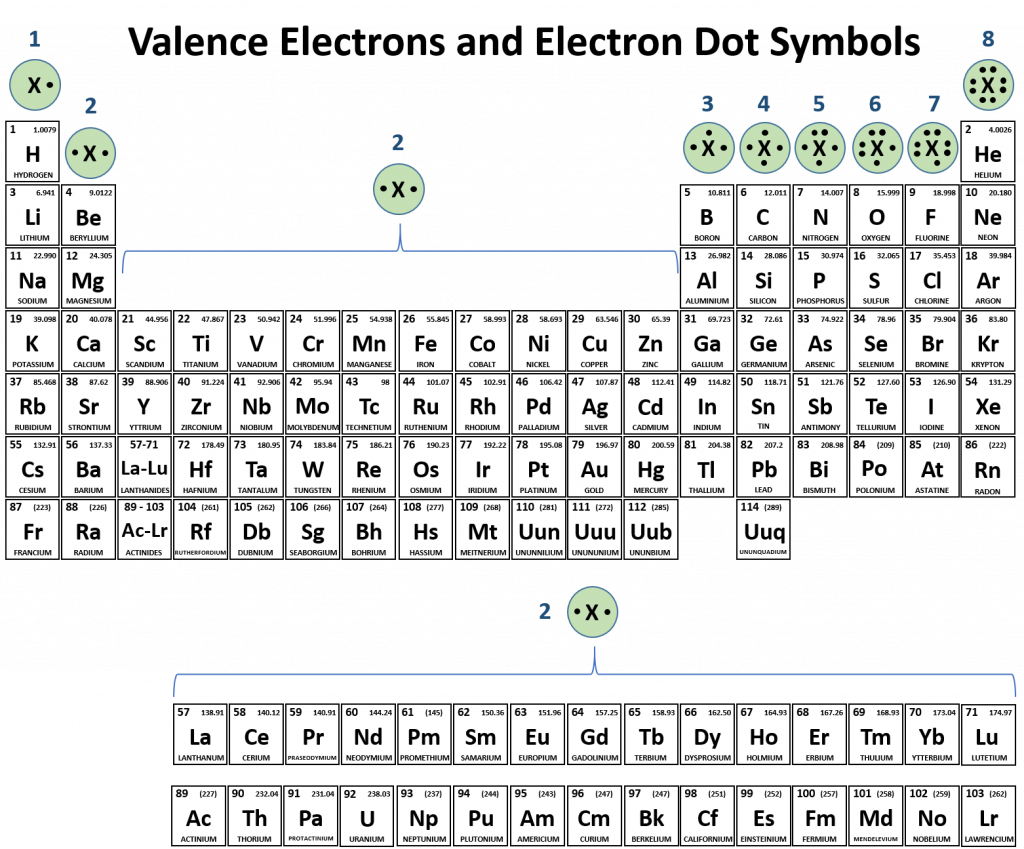
Fig 4.3 Periodic Table with Lewis Structures. Each family shows a representative lewis structure for that group of elements. For the nonmetals (Families 4A, 5A, 6A, and 7A) they can accept a complementary number of shared bonds to reach the octet state. Family 4A can share 4 covalent bonds (4 + 4 = 8), whereas Families 5A, 6A, and 7A can share 3, 2, and 1 covalent bond(s), respectively, to achieve the octet state. Exceptions to the octet rule do exist. For example, hydrogen can be considered to be in Group 1 or Group 7A because it has properties similar to both groups. Hydrogen can participate in either ionic or covalent bonding. When participating in covalent bonding, hydrogen only needs two electrons to have a full valence shell. As it has one electron to start with, it can only make one covalent bond. Similarly, boron has 3 electrons in its outer shell. This nonmetal typically forms 3 covalent bonds, having a maximum of 6 electrons in its outer shell. Thus, boron can never reach the octet state. Other atoms can have expanded orbitals and accept additional covalent bonds. Two of these that are important for living systems are sulfur and phosphorus. By the octet rule, sulfur can make 2 covalent bonds and phosphorus 3 covalent bonds. Sulfur can also have expanded orbitals to accept 4 or 6 covalent bonds, and phosphorus can expand to 5 covalent bonds.

In many molecules, the octet rule would not be satisfied if each pair of bonded atoms shares only two electrons. Consider carbon dioxide (CO 2 ). If each oxygen atom shares one electron with the carbon atom, we get the following:
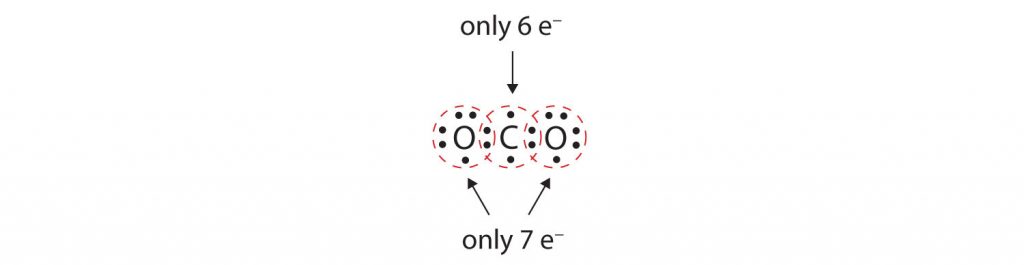
This does not give either the carbon or oxygen atoms a complete octet; The carbon atom only has six electrons in its valence shell and each oxygen atom only has seven electrons in its valence shell. Thus, none of the atoms can reach the octet state in the current configuration. As written, this would be an unstable molecular conformation.
Sometimes more than one pair of electrons must be shared between two atoms for both atoms to have an octet. In carbon dioxide, a second electron from each oxygen atom is also shared with the central carbon atom, and the carbon atom shares one more electron with each oxygen atom:
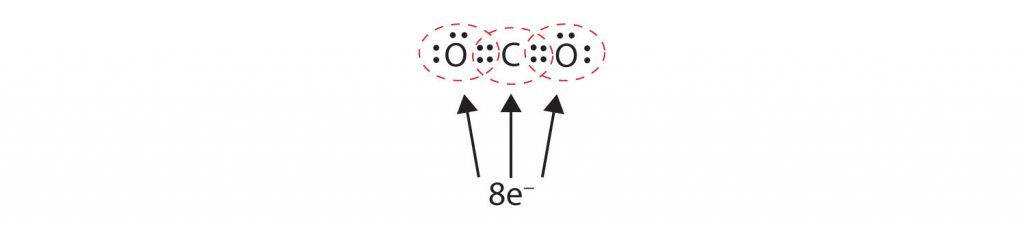
In this arrangement, the carbon atom shares four electrons (two pairs) with the oxygen atom on the left and four electrons with the oxygen atom on the right. There are now eight electrons around each atom. Two pairs of electrons shared between two atoms make a double bond between the atoms, which is represented by a double dash:
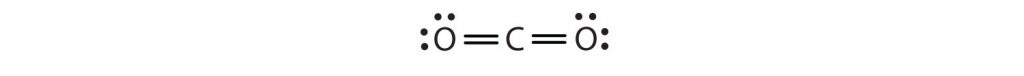
Some molecules contain triple bonds, covalent bonds in which three pairs of electrons are shared by two atoms. A simple compound that has a triple bond is acetylene (C 2 H 2 ), whose Lewis diagram is as follows:

A coordinate bond (also called a dative covalent bond) is a covalent bond (a shared pair of electrons) in which both electrons come from the same atom. A covalent bond is formed by two atoms sharing a pair of electrons. The atoms are held together because the electron pair is attracted by both of the nuclei. In the formation of a simple or ordinary covalent bond, each atom supplies one electron to the bond – but that does not have to be the case. In the case of a coordinate covalent bond, one atom supplies both of the electrons and the other atom does not supply any of the electrons. The following reaction between ammonia and hydrochloric acid demonstrates the formation of a coordinate covalent bond between ammonia and a hydrogren ion (proton).
The reaction between ammonia and hydrochloric acid
If these colorless gases are allowed to mix, a thick white smoke of solid ammonium chloride is formed.
Video provided by North Carolina School of Science and Mathematics
The overall reaction is
Ammonium ions, NH 4 + , are formed by the transfer of a hydrogen ion (a proton) from the hydrochloric acid molecule to the lone pair of electrons on the ammonia molecule. To visualize this reaction, we can use electron dot configurations to observe the electron movement during the reaction. First recall the valence electron states for all of the atoms involved in the reaction:
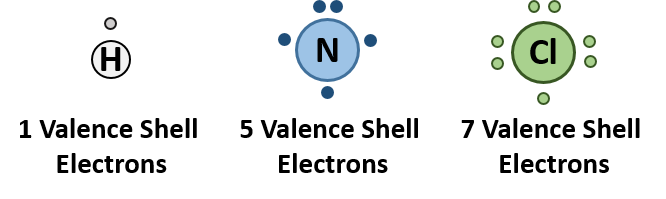
On the left side of the equation (to the left of the arrow) are the reactants of the reaction (ammonia and hydrochloric acid). On the right side of the reaction (to the right of the arrow) is the product of the reaction, the ionic compound – ammonium chloride. The diagram below shows the electron and proton movement during the reaction.
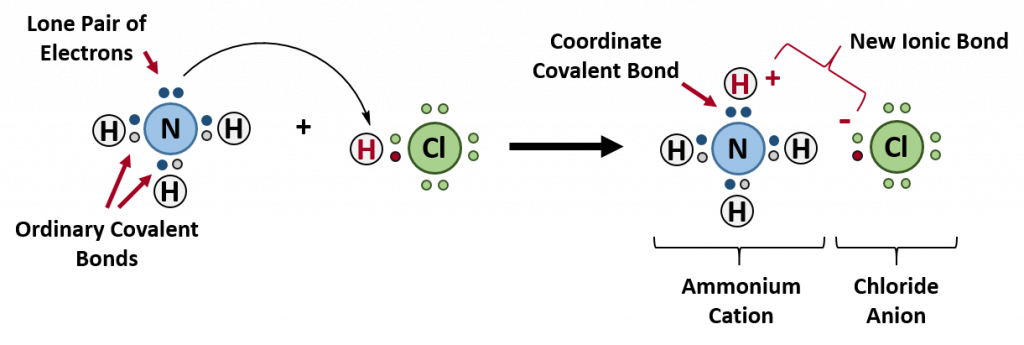
Figure 4.4 Formation of Ammonium Chloride. When the ammonium ion, NH 4 + , is formed, the fourth hydrogen (shown in red) is attached by a coordinate covalent bond, because only the hydrogen’s nucleus is transferred from the chlorine to the nitrogen. The hydrogen’s electron is left behind on the chlorine to form a negative chloride ion. Once the ammonium ion has been formed it is impossible to tell any difference between the coordinate covalent and the ordinary covalent bonds, all of the hydrogens are equivalent in the molecule and the extra positive charge is distributed throughout the molecule. Although the electrons are shown differently in the diagram, there is no difference between them in reality. In simple diagrams, a coordinate bond is shown by a curved arrow. The arrow points from the atom donating the lone pair to the atom accepting it.
Although we defined covalent bonding as electron sharing, the electrons in a covalent bond are not always shared equally by the two bonded atoms. Unless the bond connects two atoms of the same element, there will always be one atom that attracts the electrons in the bond more strongly than the other atom does, as shown in Figure 4.5. When such an imbalance occurs, there is a resulting buildup of some negative charge (called a partial negative charge and designated δ−) on one side of the bond and some positive charge (designated δ+) on the other side of the bond. A covalent bond that has an unequal sharing of electrons, as in part (b) of Figure 4.5, is called a polar covalent bond . A covalent bond that has an equal sharing of electrons (part (a) of Figure 4.5) is called a nonpolar covalent bond .
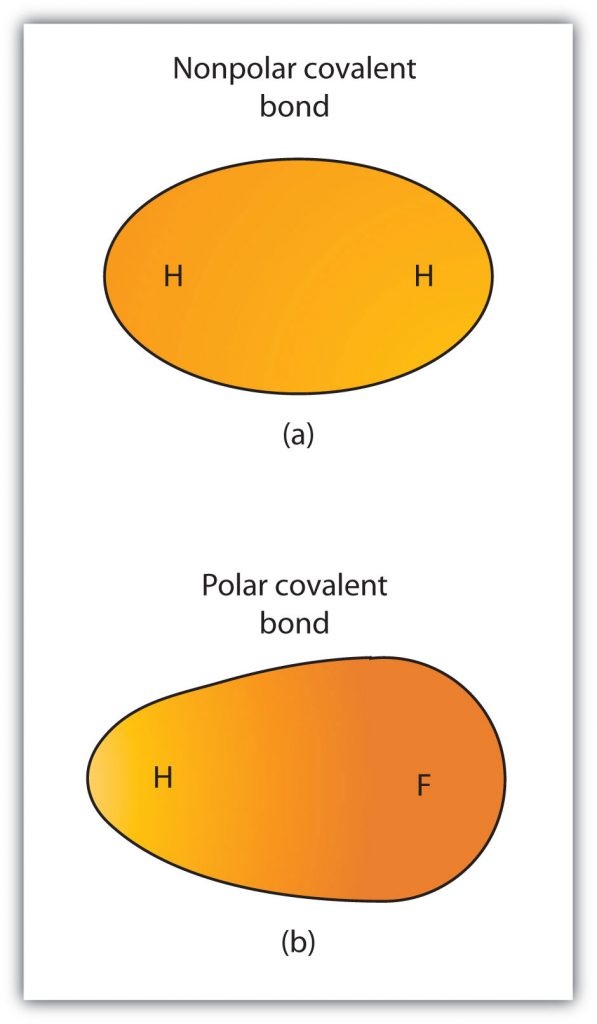
Figure 4.5 Polar versus Nonpolar Covalent Bonds. (a) The electrons in the covalent bond are equally shared by both hydrogen atoms. This is a nonpolar covalent bond. (b) The fluorine atom attracts the electrons in the bond more than the hydrogen atom does, leading to an imbalance in the electron distribution. This is a polar covalent bond.
Any covalent bond between atoms of different elements is a polar bond, but the degree of polarity varies widely. Some bonds between different elements are only minimally polar, while others are strongly polar. Ionic bonds can be considered the ultimate in polarity, with electrons being transferred completely rather than shared. To judge the relative polarity of a covalent bond, chemists use electronegativity , which is a relative measure of how strongly an atom attracts electrons when it forms a covalent bond.
There are various numerical scales for rating electronegativity. Figure 4.6 shows one of the most popular— the Pauling scale . The polarity of a covalent bond can be judged by determining the difference in the electronegativities between the two atoms making the bond. The greater the difference in electronegativities, the greater the imbalance of electron sharing in the bond.
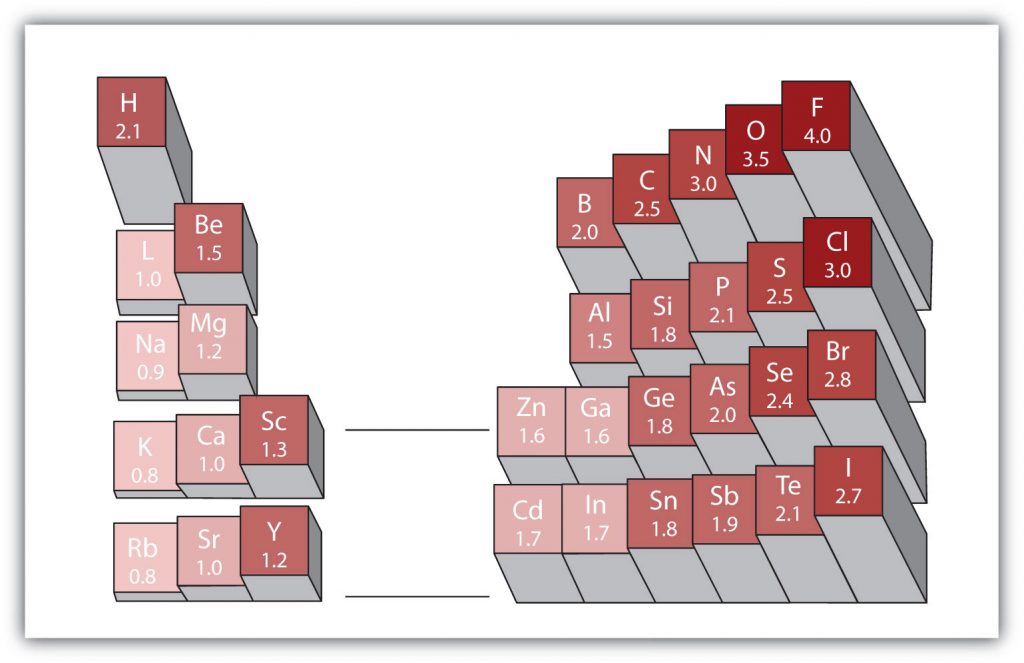
Figure 4.6 Electronegativities of Various Elements. The Pauling Scale for electronegativities has the value for fluorine atoms set at 4.0, the highest value.

Although there are no hard and fast rules, the general rule is that a difference in electronegativity less than 0.4 indicates the bond is nonpolar; when the difference is greater than 0.4, the bond is considered polar. When the difference in electronegativities is large enough (generally greater than about 1.8), the resulting compound is considered ionic rather than covalent. An electronegativity difference of zero, of course, indicates a nonpolar covalent bond. Examples of electronegativity difference are shown in Figure 4.7.
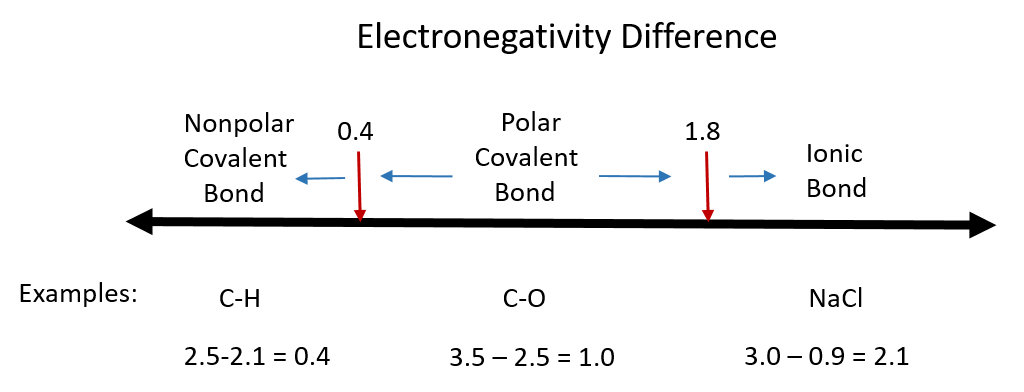
Figure 4.7 Electronegativity Difference Diagram. The diagram above is a guide for discerning what type of bond forms between two different atoms. By taking the difference between the electronegativity values for each of the atoms involved in the bond, the bond type and polarity can be predicted. Note that full ionic character is rarely reached, however when metals and nonmetals form bonds, they are named using the rules for ionic bonding.
When a molecule’s bonds are polar, the molecule as a whole can display an uneven distribution of charge, depending on how the individual bonds are oriented. For example, the orientation of the two O–H bonds in a water molecule (Figure 4.8) is bent: one end of the molecule has a partial positive charge, and the other end has a partial negative charge. In short, the molecule itself is polar. The polarity of water has an enormous impact on its physical and chemical properties. (For example, the boiling point of water [100°C] is high for such a small molecule and is due to the fact that polar molecules attract each other strongly.) In contrast, while the two C=O bonds in carbon dioxide are polar, they lie directly opposite each other in the molecule and so cancel each other’s effects. Thus, carbon dioxide molecules are nonpolar overall. This lack of polarity influences some of carbon dioxide’s properties. (For example, carbon dioxide becomes a gas at −77°C, almost 200° lower than the temperature at which water boils.)
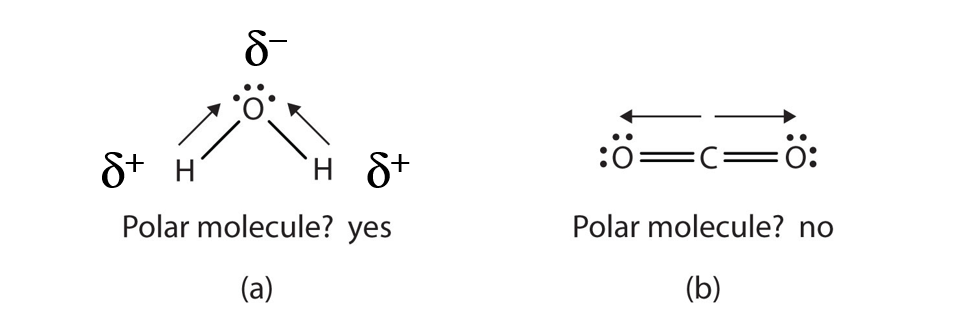
Figure 4.8 Physical Properties and Polarity. The physical properties of water (a) and carbon dioxide (b) are affected by their molecular polarities. Note that the arrows in the diagram always point in the direction where the electrons are more strongly attracted. In this diagram, the delta symbol (δ) is used with a (+) or (-) symbol to represent partial positive and partial negative charge distribution in polar covalent bonds. Note that the electrons shared in polar covalent bonds will be attracted to and spend more time around the atom with the higher electronegativity value. When the polarity is equal and directly opposing, as in the case of carbon dioxide (b), the overall molecule will have no overall charge.

(BACK TO THE TOP)
Molecular compounds have many properties that differ from ionic compounds. Some of the generalizations for this group include much lower melting and boiling points when compared with their ionic counterpoints. For example, water (H 2 O) has a melting point of 4 o C and a boiling point of 100 o C compared with NaCl that has a melting point of 801 o C and a boiling point of 1,413 o C. This is because the full charges created in ionic bonds have much stronger attractive force than the comparatively weak partial charges created in covalent molecules. thus, ionic compounds tend to form very strong crystalline lattice structures due to the repeating charges of the cation and anion components. Covalent compounds, on the otherhand, do not typically have such well-structured 3-dimensional shapes. Thus they tend to be more brittle and break more easily when in solid form, and many are found in liquid and gas phases. In addition, due to their lack of charges, they tend to be poor electrical and thermal conductors. Many are also insoluble in water due to their nonpolar nature (ie oil and water don’t mix).
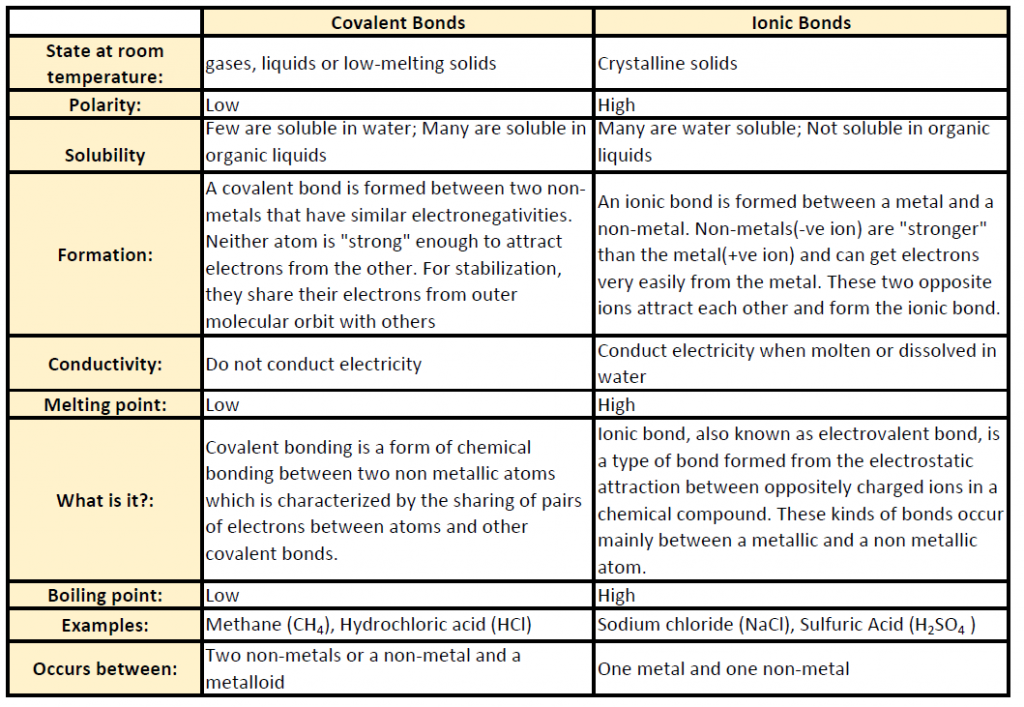
Table 4.1 shows common differences between covalent and ionic compounds.
Table 4.1 Comparison of Ionic and Covalent Compounds
Recall that a molecular formula shows the number of atoms of each element that a molecule contains. A molecule of water contains two hydrogen atoms and one oxygen atom, so its formula is H 2 O . A molecule of octane, which is a component of gasoline, contains 8 atoms of carbon and 18 atoms of hydrogen. The molecular formula of octane is C 8 H 18 . When writing the chemical formula the element that is the least electronegative (the element that is farther left or further down within the same family group) is written first while the more electronegative element is written second. You will be required to know how to name simple binary covalent compounds (compounds composed of two different elements)

Figure 4.9 Nitrogen dioxide ( NO 2 ) is a reddish-brown toxic gas that is a prominent air pollutant produced by internal combustion engines.
The elements that combine to form binary molecular compounds are both nonmetal atoms or they are a combination of a nonmetal and a metalloid. This contrasts with ionic compounds, which were formed from a metal ion and a nonmetal ion. Therefore, binary molecular compounds are different because ionic charges cannot be used to name them or to write their formulas. Another difference is that two nonmetal atoms will frequently combine with one another in a variety of ratios. Consider the elements nitrogen and oxygen. They combine to make several compounds including
NO , NO 2 , and N 2 O
They all can’t be called nitrogen oxide. How would someone know which one you were talking about? Each of the three compounds has very different properties and reactivity. A system to distinguish between compounds such as these is necessary.
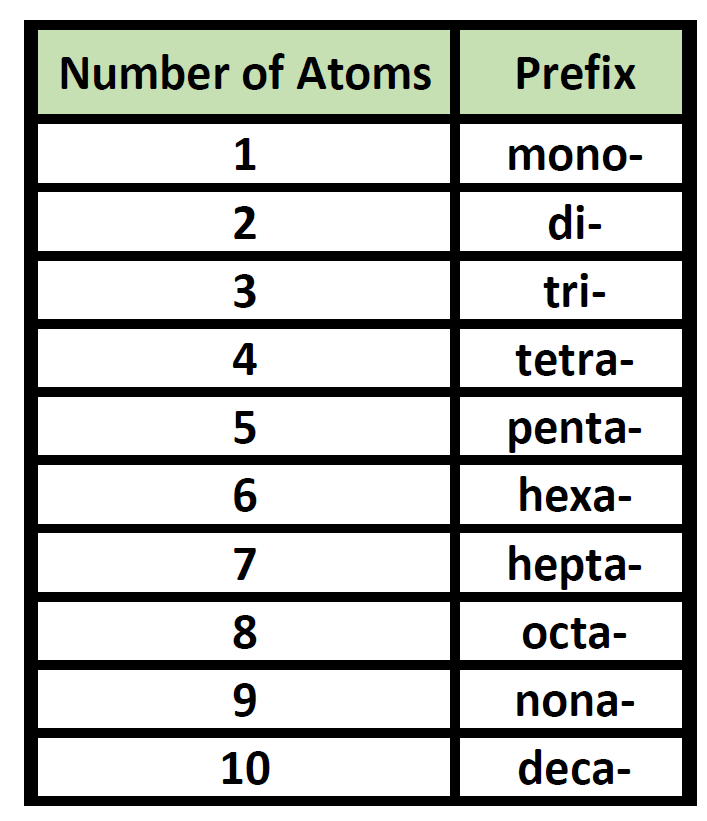
Prefixes are used in the names of binary molecular compounds to identify the number of atoms of each element. The table below shows the prefixes up to ten.
Table 4.2 Prefixes used for Nomenclature of Binary Covalent Molecules
The rules for using the prefix system of nomenclature of binary compounds can be summarized as follows.
- Generally, the less-electronegative element is written first in the formula, though there are a few exceptions. Exception 1 : Carbon is always first in a formula. Exception 2: When hydrogen is participating in a covalent bond, it is typically written in the second postion (For example: hydrogen is after nitrogen in a formula such as NH 3 ) Overall, t he order of common nonmetals in binary molecular compounds is C , P , N , H , S , I , Br , Cl , O ,
- When naming the first element, use the full name of the element and the appropriate prefix if there are more than one atom of that element in the formula. If there is only one atom for the first element, the term mono- is NOT used, but is implied. For example, CO is carbon monoxide, not monocarbon monoxide.
- For the second element the ending of the element’s name is typically changed to ‘ -ide’ and the appropriate prefix is always used for the second element.
Note: the a or o at the end of a prefix is usually dropped from the name when the name of the element begins with a vowel. As an example, four oxygen atoms, is tetroxide instead of tetraoxide. Some examples of molecular compounds are listed in Table 4 .3 .
Table 4.3 Examples of Naming Covalent Molecules
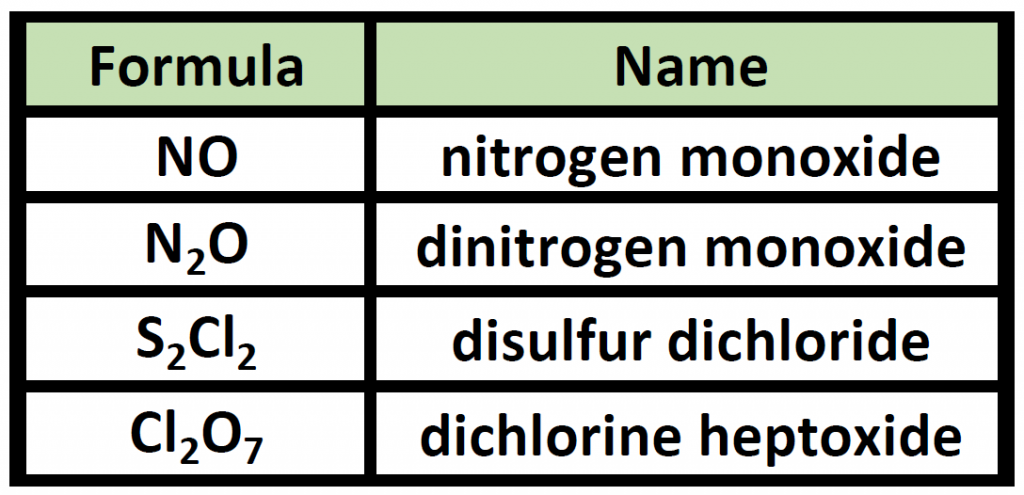
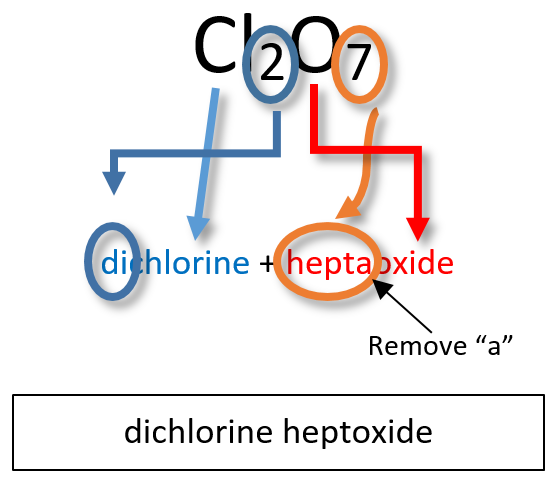
Notice that the mono- prefix is not used with the nitrogen in the first compound, but is used with the oxygen in both of the first two examples. The S 2 Cl 2 emphasizes that the formulas for molecular compounds are not reduced to their lowest ratios. The o of the mono- and the a of hepta- are dropped from the name when paired with oxide. For example:
The Environmental Protection Agency
On December 3rd of 1970 the United States Environmental Protection Agency (EPA) was proposed by President Richard Nixon to be an agency of the federal government charged with protecting human health and the environment. The agency was charged with writing and enforcing regulations based on laws passed by Congress.
The main focus of the EPA as an agency is to conduct research, make environmental assessments, and provide educational materials for use by Congress and the American people regarding environmental and health concerns. The EPA also enforces national standards under a variety of environmental laws, such as the clean air act, and can levy fines, sanctions, and other measures on violators. Within this section, we will learn about creation of the Comprehensive Environmental Response, Compensation, and Liability Act, that is also known as ‘Superfund ‘. The Superfund program was instituted in 1980 and designed to identify and clean up the worst of the hazardous chemical waste sites in the U.S. The first of these sites was the Love Canal described in the next section.
The Love Canal
One of the most famous and important examples of groundwater pollution in the U.S. is the Love Canal tragedy in Niagara Falls, New York (Figure 4.10). It is important because the pollution disaster at Love Canal, along with similar pollution calamities at that time (Times Beach, Missouri and Valley of Drums, Kentucky), helped to create Superfund .

Figure 4.10. Love Canal. Source: US Environmental Protection Agency
Love Canal is a neighborhood in Niagara Falls named after a large ditch (approximately 15 m wide, 3–12 m deep, and 1600 m long) that was dug in the 1890s for hydroelectric power. The ditch was abandoned before it actually generated any power and went mostly unused for decades, except for swimming by local residents. In the 1920s Niagara Falls began dumping urban waste into Love Canal, and in the 1940s the U.S. Army dumped waste from World War II there, including waste from the frantic effort to build a nuclear bomb. Hooker Chemical Company purchased the land in 1942 and lined it with clay. Then, the company put into Love Canal an estimated 21,000 tons of hazardous chemical waste, including the carcinogens benzene, dioxin, and polychlorobisphenols (PCBs) in large metal barrels and covered them with more clay (Figure 4.11).
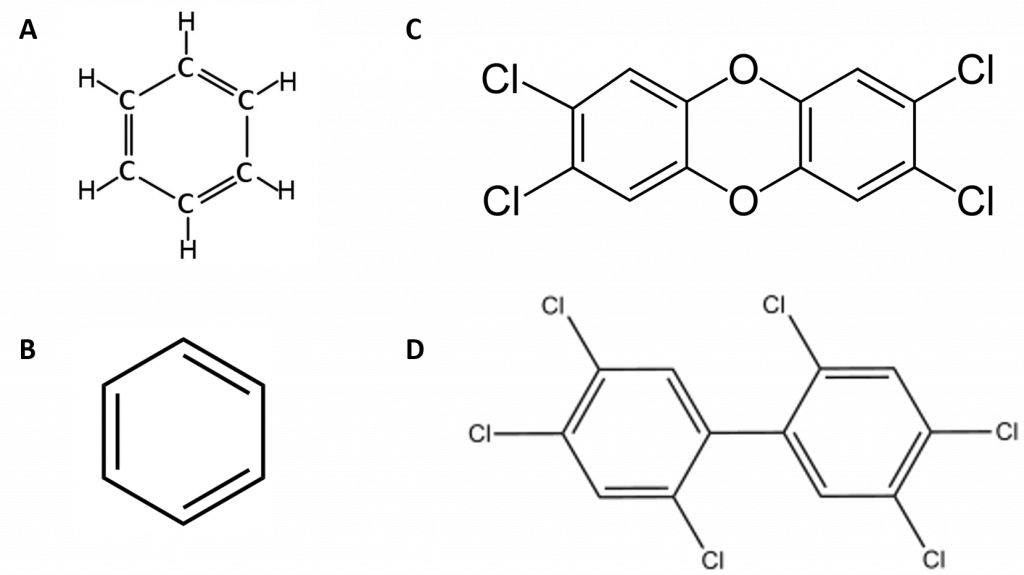
Figure 4.11 Examples of Carcinogens disposed of in the Love Canal. (A) and (B) are representations of Benzene. In (A) all of the atoms in benzene are shown. (B) is a representative line structure of benzene where carbon atoms are indicated at every bend in every line and hydrogen atoms are implied. (C) line structure of dioxin, and (D) is a line diagram of a representative PCB.
In 1953, Hooker sold the land to the Niagara Falls school board for $1, and included a clause in the sales contract that both described the land use (filled with chemical waste) and absolved them from any future damage claims from the buried waste. The school board promptly built a public school on the site and sold the surrounding land for a housing project that built 200 or so homes along the canal banks and another 1,000 in the neighborhood (Figure 4.10). During construction, the canal’s clay cap and walls were breached, damaging some of the metal barrels.
Eventually, the chemical waste seeped into people’s basements, and the metal barrels worked their way to the surface. Trees and gardens began to die; bicycle tires and the rubber soles of children’s shoes disintegrated in noxious puddles. From the 1950s to the late 1970s, residents repeatedly complained of strange odors and substances that surfaced in their yards. Eckardt Beck, the EPA administrator for the region from 1977-1979 recalled in the EPA Journal, “I visited the canal area at that time. Corroding waste-disposal drums could be seen breaking up trough the grounds of backyards. Trees and gardens were turning black and dying. One entire swimming pool had been popped up from its foundation, afloat now on a small sea of chemicals. Puddles of noxious substances were pointed out to be by the residents. Some of these puddles were in their yards, some were in their basements, others yet on the school grounds. Everywhere the air had a faint, choking smell. Children returned from play with burns on their hands and faces. And then there were the birth defects. The New York State Health Department is continuing an investigation into a disturbingly high rate of miscarriages, along with five birth-defect cases detected thus far in the area.” 10
In 1978 President Carter declared a state of emergency at Love Canal, making it the first human-caused environmental problem to be designated that way. The Love Canal incident became a symbol of improperly stored chemical waste. Clean up of Love Canal, which was funded by Superfund and completely finished in 2004, involved removing contaminated soil, installing drainage pipes to capture contaminated groundwater for treatment, and covering it with clay and plastic. In 1995, Occidental Chemical (the modern name for Hooker Chemical) paid $102 million to Superfund for cleanup and $27 million to Federal Emergency Management Association for the relocation of more than 1,000 families. New York State paid $98 million to EPA and the US government paid $8 million for pollution by the Army. The total clean up cost was estimated to be $275 million.
The Love Canal tragedy helped to create Superfund, which has analyzed tens of thousands of hazardous waste sites in the U.S. and cleaned up hundreds of the worst ones. Nevertheless, over 1,000 major hazardous waste sites with a significant risk to human health or the environment are still in the process of being cleaned.
Suggested Assignment:
1. Explore the EPA Superfund Website.
2. Scroll down to the bottom of the page and select ‘Sites Where You Live’
3. Select ‘Your State’ and Show ‘All Sites’.
4. Select a Superfund site within your state and write a 500 word summary describing your chosen site. In the first paragraph, describe the type of toxic waste that is found at the site and what the health and environmental impacts are due to the pollution. In a second paragraphs, describe who is responsible for generating the pollution and what has or is currently being done to restore and clean up the site. Be sure to include the monetary cost of the clean up in your response if it is disclosed and what parties are paying for the clean up.
Atoms can share pairs of valence electrons to obtain a valence shell octet. This sharing of electrons is a covalent bond . A species formed from covalently bonded atoms is a molecule and is represented by a molecular formula , which gives the number of atoms of each type in the molecule. The two electrons shared in a covalent bond are called a bonding pair of electrons . The electrons that do not participate in covalent bonds are called nonbonding pairs (or lone pairs ) of electrons . A covalent bond consisting of one pair of shared electrons is called a single bond .
Covalent bonds occur between nonmetal atoms. Naming simple covalent compounds follows simple rules similar to those for ionic compounds. However, for covalent compounds, numerical prefixes are used as necessary to specify the number of atoms of each element in the compound.
In some cases, more than one pair of electrons is shared to satisfy the octet rule. Two pairs of electrons are shared by two atoms to make a double bond . Three pairs of atoms are shared to make a triple bond . Single, double, and triple covalent bonds may be represented by one, two, or three dashes, respectively, between the symbols of the atoms. In the case of a coordinate covalent bond , one atom supplies both of the electrons and the other atom does not supply any of the electrons.
To judge the relative polarity of a covalent bond, chemists use electronegativity , which is a relative measure of how strongly an atom attracts electrons when it forms a covalent bond. The greater the electronegativity difference between the atoms involved in the covalent bond, the more polarity the bond displays.
In comparison to ionic compounds, covalent molecules tend to have lower melting and boiling points, are less soluble in water, and are poor conductors of electricity. These major differences are largely due to increased polarity of ionic bonds when compared with covalent bonds.
Chapter 4 materials have been adapted from the following creative commons resources unless otherwise noted:
1. Organic Chemistry Portal. WikiUniversity. Available at: https://en.wikiversity.org/wiki/Portal:Organic_chemistry
2. Anonymous. (2012) Introduction to Chemistry: General, Organic, and Biological (V1.0). Published under Creative Commons by-nc-sa 3.0. Available at: http://2012books.lardbucket.org/books/introduction-to-chemistry-general-organic-and-biological/index.html
3. Poulsen, T. (2010) Introduction to Chemistry. Published under Creative Commons by-nc-sa 3.0. Available at: http://openedgroup.org/books/Chemistry.pdf
4. Molecules and Molecular Compounds. (2017) Libretexts. Available at: https://chem.libretexts.org/Textbook_Maps/General_Chemistry_Textbook_Maps/Map%3A_Chemistry%3A_The_Central_Science_(Brown_et_al.)/02._Atoms,_Molecules,_and_Ions/2.6%3A_Molecules_and_Molecular_Compounds
5. Clark, J. (2017) ‘General Principles of Chemical Bonding’ Published by Libretexts. Available at: https://chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Chemical_Bonding/General_Principles_of_Chemical_Bonding/Coordinate_(Dative_Covalent)_Bonding
6. OpenStax (2015) Atoms, Isotopes, Ions, and Molecules: The Building Blocks. OpenStax CNX.Available at: http://cnx.org/contents/be8818d0-2dba-4bf3-859a-737c25fb2c99@12 .
7. Wikipedia, Ionic Compound. Available at: https://en.wikipedia.org/wiki/Ionic_compound
8. Physical and Theoretical Chemistry (2017) Libretexts. Available at: https://chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Chemical_Bonding/General_Principles_of_Chemical_Bonding/Covalent_Bonds_vs_Ionic_Bonds .
9. Lois Gibbs. (1998) Love Canal the story continues. Published by the Center for Health, Environment and Justice. Available at: http://chej.org/wp-content/uploads/Love-Canal-PDF-v1.pdf
10. Beck, E.C. (1979) The Love Canal Tragedy. EPA Journal. Available at: https://archive.epa.gov/epa/aboutepa/love-canal-tragedy.html
- CH104 – Chapter 1: Measurements in Chemistry
- CH104 – Chapter 2: Atoms and The Periodic Table
- CH104: Chapter 3 – Ions and Ionic Compounds
- CH104: Chapter 4 – Covalent Bonds and Molecular Compounds
- CH104: Chapter 5 – Chemical Reactions
- CH104: Chapter 6 – Quantities in Chemical Reactions
- CH104: Chapter 7 – Solutions

WESTERN OREGON UNIVERSITY 345 Monmouth Ave. N. Monmouth OR 97361
503-838-8000 | 1-877-877-1593
Campus Maps Canvas Find People Portal Virtual Tour WOU Email Technical Support
A-Z Index Accessibility Academic Calendar Class Schedule Jobs at WOU Partnerships Student Services
Western Oregon University’s Land Acknowledgement Western Oregon University in Monmouth, OR is located within the traditional homelands of the Luckiamute Band of Kalapuya. Following the Willamette Valley Treaty of 1855 (Kalapuya etc. Treaty), Kalapuya people were forcibly removed to reservations in Western Oregon. Today, living descendants of these people are a part of the Confederated Tribes of Grand Ronde Community of Oregon and the Confederated Tribes of the Siletz Indians .
Accessibility Public Records Privacy Student Consumer Information
WOU prohibits discrimination on the basis of race, color, sex, national or ethnic origin, age, religion, marital status, disability, veteran status, sexual orientation, gender identity, and gender expression in all programs, activities and employment practices as required by Title IX, other applicable laws, and policies. Retaliation is prohibited by WOU.

- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- Games & Quizzes
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center
- Introduction

Lewis formulation of a covalent bond
- Hypervalence

covalent bond
Our editors will review what you’ve submitted and determine whether to revise the article.
- Open Library Publishing Platform - Introductory Chemistry- 1st Canadian Edition - Covalent Bonds
- Chemguide - Covalent Bonding - Single Bonds
- Khan Academy - Covalent bond
- Chemistry LibreTexts - Ionic Bond
- The University of Hawaiʻi Pressbooks - Covalent Bonding
- UEN Digital Press with Pressbooks - The Covalent Bond
- Table Of Contents

covalent bond , in chemistry , the interatomic linkage that results from the sharing of an electron pair between two atoms. The binding arises from the electrostatic attraction of their nuclei for the same electrons. A covalent bond forms when the bonded atoms have a lower total energy than that of widely separated atoms.
A brief treatment of covalent bonds follows. For full treatment, see chemical bonding: Covalent bonds .
Molecules that have covalent linkages include the inorganic substances hydrogen, nitrogen, chlorine, water, and ammonia (H 2 , N 2 , Cl 2 , H 2 O, NH 3 ) together with all organic compounds . In structural representations of molecules, covalent bonds are indicated by solid lines connecting pairs of atoms; e.g.,

A single line indicates a bond between two atoms (i.e., involving one electron pair), double lines (=) indicate a double bond between two atoms (i.e., involving two electron pairs), and triple lines (≡) represent a triple bond , as found, for example, in carbon monoxide (C≡O). Single bonds consist of one sigma (σ) bond, double bonds have one σ and one pi (π) bond, and triple bonds have one σ and two π bonds.
Covalent bonds are directional, meaning that atoms so bonded prefer specific orientations relative to one another; this in turn gives molecules definite shapes, as in the angular (bent) structure of the H 2 O molecule. Covalent bonds between identical atoms (as in H 2 ) are nonpolar—i.e., electrically uniform—while those between unlike atoms are polar—i.e., one atom is slightly negatively charged and the other is slightly positively charged. This partial ionic character of covalent bonds increases with the difference in the electronegativities of the two atoms. See also ionic bond .
When none of the elements in a compound is a metal, no atoms in the compound have an ionization energy low enough for electron loss to be likely. In such a case, covalence prevails. As a general rule, covalent bonds are formed between elements lying toward the right in the periodic table (i.e., the nonmetals). Molecules of identical atoms, such as H 2 and buckminsterfullerene (C 60 ), are also held together by covalent bonds.
The idea that two electrons can be shared between two atoms and serve as the link between them was first introduced in 1916 by the American chemist G.N. Lewis , who described the formation of such bonds as resulting from the tendencies of certain atoms to combine with one another in order for both to have the electronic structure of a corresponding noble-gas atom.
In Lewis terms a covalent bond is a shared electron pair. The bond between a hydrogen atom and a chlorine atom in hydrogen chloride is formulated as follows:

In a Lewis structure of a covalent compound, the shared electron pair between the hydrogen and chlorine ions is represented by a line. The electron pair is called a bonding pair; the three other pairs of electrons on the chlorine atom are called lone pairs and play no direct role in holding the two atoms together.
Each atom in the hydrogen chloride molecule attains a closed-shell octet of electrons by sharing and hence achieves a maximum lowering of energy. In general, an incomplete shell means that some attracting power of a nucleus may be wasted, and adding electrons beyond a closed shell would entail the energetic disadvantage of beginning the next shell of the atom concerned. Lewis’s octet rule is again applicable and is seen to represent the extreme means of achieving lower energy rather than being a goal in itself.
A covalent bond forms if the bonded atoms have a lower total energy than the widely separated atoms. The simplest interpretation of the decrease in energy that occurs when electrons are shared is that both electrons lie between two attracting centres (the nuclei of the two atoms linked by the bond) and hence lie lower in energy than when they experience the attraction of a single centre.
Lewis structures of more complex molecules can be constructed quite simply by extending the process that has been described for hydrogen chloride. First, the valence electrons that are available for bonding are counted (2 × 1 + 6 = 8 in H 2 O, for example, and 4 + 4 × 7 = 32 in carbon tetrachloride , CCl 4 ), and the chemical symbols for the elements are placed in the arrangement that reflects which are neighbours:

Next, one bonding pair is added between each linked pair of atoms:

The remaining electrons are then added to the atoms in such a way that each atom has a share in an octet of electrons (this is the octet-rule part of the procedure):

Finally, each bonding pair is represented by a dash:

(Note that Lewis structures do not necessarily show the actual shape of the molecule, only the topological pattern of their bonds.)
In some older formulations of Lewis structures, a distinction was made between bonds formed by electrons that have been supplied by both atoms (as in H―Cl, where one shared electron can be regarded as supplied by the hydrogen atom and the other by the chlorine atom) and covalent bonds formed when both electrons can be regarded as supplied by one atom, as in the formation of OH − from O 2− and H + . Such a bond was called a coordinate covalent bond or a dative bond and symbolized O → H − . However, the difficulties encountered in the attempt to keep track of the origin of bonding electrons and the suggestion that a coordinate covalent bond differs somehow from a covalent bond (it does not) have led to this usage falling into disfavour.
2.7 Chemical Nomenclature
Learning objectives.
By the end of this section, you will be able to:
- Derive names for common types of inorganic compounds using a systematic approach
Nomenclature , a collection of rules for naming things, is important in science and in many other situations. This module describes an approach that is used to name simple ionic and molecular compounds, such as NaCl, CaCO 3 , and N 2 O 4 . The simplest of these are binary compounds , those containing only two elements, but we will also consider how to name ionic compounds containing polyatomic ions, and one specific, very important class of compounds known as acids (subsequent chapters in this text will focus on these compounds in great detail). We will limit our attention here to inorganic compounds, compounds that are composed principally of elements other than carbon, and will follow the nomenclature guidelines proposed by IUPAC. The rules for organic compounds, in which carbon is the principle element, will be treated in a later chapter on organic chemistry.
Ionic Compounds
To name an inorganic compound, we need to consider the answers to several questions. First, is the compound ionic or molecular? If the compound is ionic, does the metal form ions of only one type (fixed charge) or more than one type (variable charge)? Are the ions monatomic or polyatomic? If the compound is molecular, does it contain hydrogen? If so, does it also contain oxygen? From the answers we derive, we place the compound in an appropriate category and then name it accordingly.
Compounds Containing Only Monatomic Ions
The name of a binary compound containing monatomic ions consists of the name of the cation (the name of the metal) followed by the name of the anion (the name of the nonmetallic element with its ending replaced by the suffix – ide ). Some examples are given in Table 2.6 .
| NaCl, sodium chloride | Na O, sodium oxide |
| KBr, potassium bromide | CdS, cadmium sulfide |
| CaI , calcium iodide | Mg N , magnesium nitride |
| CsF, cesium fluoride | Ca P , calcium phosphide |
| LiCl, lithium chloride | Al C , aluminum carbide |
Compounds Containing Polyatomic Ions
Compounds containing polyatomic ions are named similarly to those containing only monatomic ions, i.e. by naming first the cation and then the anion. Examples are shown in Table 2.7 .
| KC H O , potassium acetate | NH Cl, ammonium chloride |
| NaHCO , sodium bicarbonate | CaSO , calcium sulfate |
| Al (CO ) , aluminum carbonate | Mg (PO ) , magnesium phosphate |
Chemistry in Everyday Life
Ionic compounds in your cabinets.
Every day you encounter and use a large number of ionic compounds. Some of these compounds, where they are found, and what they are used for are listed in Table 2.8 . Look at the label or ingredients list on the various products that you use during the next few days, and see if you run into any of those in this table, or find other ionic compounds that you could now name or write as a formula.
| Ionic Compound | Use |
|---|---|
| NaCl, sodium chloride | ordinary table salt |
| KI, potassium iodide | added to “iodized” salt for thyroid health |
| NaF, sodium fluoride | ingredient in toothpaste |
| NaHCO , sodium bicarbonate | baking soda; used in cooking (and as antacid) |
| Na CO , sodium carbonate | washing soda; used in cleaning agents |
| NaOCl, sodium hypochlorite | active ingredient in household bleach |
| CaCO calcium carbonate | ingredient in antacids |
| Mg(OH) , magnesium hydroxide | ingredient in antacids |
| Al(OH) , aluminum hydroxide | ingredient in antacids |
| NaOH, sodium hydroxide | lye; used as drain cleaner |
| K PO , potassium phosphate | food additive (many purposes) |
| MgSO , magnesium sulfate | added to purified water |
| Na HPO , sodium hydrogen phosphate | anti-caking agent; used in powdered products |
| Na SO , sodium sulfite | preservative |
Compounds Containing a Metal Ion with a Variable Charge
Most of the transition metals and some main group metals can form two or more cations with different charges. Compounds of these metals with nonmetals are named with the same method as compounds in the first category, except the charge of the metal ion is specified by a Roman numeral in parentheses after the name of the metal. The charge of the metal ion is determined from the formula of the compound and the charge of the anion. For example, consider binary ionic compounds of iron and chlorine. Iron typically exhibits a charge of either 2+ or 3+ (see Figure 2.29 ), and the two corresponding compound formulas are FeCl 2 and FeCl 3 . The simplest name, “iron chloride,” will, in this case, be ambiguous, as it does not distinguish between these two compounds. In cases like this, the charge of the metal ion is included as a Roman numeral in parentheses immediately following the metal name. These two compounds are then unambiguously named iron(II) chloride and iron(III) chloride, respectively. Other examples are provided in Table 2.9 .
| Compound | Name |
|---|---|
| FeCl | iron(II) chloride |
| FeCl | iron(III) chloride |
| Hg O | mercury(I) oxide |
| HgO | mercury(II) oxide |
| SnF | tin(II) fluoride |
| SnF | tin(IV) fluoride |
Out-of-date nomenclature used the suffixes – ic and – ous to designate metals with higher and lower charges, respectively: Iron(III) chloride, FeCl 3 , was previously called ferric chloride, and iron(II) chloride, FeCl 2 , was known as ferrous chloride. Though this naming convention has been largely abandoned by the scientific community, it remains in use by some segments of industry. For example, you may see the words stannous fluoride on a tube of toothpaste. This represents the formula SnF 2 , which is more properly named tin(II) fluoride. The other fluoride of tin is SnF 4 , which was previously called stannic fluoride but is now named tin(IV) fluoride.
Ionic Hydrates
Ionic compounds that contain water molecules as integral components of their crystals are called hydrates . The name for an ionic hydrate is derived by adding a term to the name for the anhydrous (meaning “not hydrated”) compound that indicates the number of water molecules associated with each formula unit of the compound. The added word begins with a Greek prefix denoting the number of water molecules (see Table 2.10 ) and ends with “hydrate.” For example, the anhydrous compound copper(II) sulfate also exists as a hydrate containing five water molecules and named copper(II) sulfate pentahydrate. Washing soda is the common name for a hydrate of sodium carbonate containing 10 water molecules; the systematic name is sodium carbonate decahydrate.
Formulas for ionic hydrates are written by appending a vertically centered dot, a coefficient representing the number of water molecules, and the formula for water. The two examples mentioned in the previous paragraph are represented by the formulas
| Number | Prefix | Number | Prefix |
|---|---|---|---|
| 1 (sometimes omitted) | mono- | 6 | hexa- |
| 2 | di- | 7 | hepta- |
| 3 | tri- | 8 | octa- |
| 4 | tetra- | 9 | nona- |
| 5 | penta- | 10 | deca- |
Example 2.13
Naming ionic compounds.
(a) Fe 2 S 3
(d) MgSO 4 ·7H 2 O
(e) Ti 2 (SO 4 ) 3
(a) iron(III) sulfide
(b) copper(II) selenide
(c) gallium(III) nitride
(d) magnesium sulfate heptahydrate
(e) titanium(III) sulfate
Check Your Learning
(a) chromium(III) phosphide
(b) mercury(II) sulfide
(c) manganese(II) phosphate
(d) copper(I) oxide
(e) iron(III) chloride dihydrate
(a) CrP; (b) HgS; (c) Mn 3 (PO 4 ) 2 ; (d) Cu 2 O; (e) FeCl 3 ·2H 2 O
Erin Brockovich and Chromium Contamination
In the early 1990s, legal file clerk Erin Brockovich ( Figure 2.32 ) discovered a high rate of serious illnesses in the small town of Hinckley, California. Her investigation eventually linked the illnesses to groundwater contaminated by Cr(VI) used by Pacific Gas & Electric (PG&E) to fight corrosion in a nearby natural gas pipeline. As dramatized in the film Erin Brockovich (for which Julia Roberts won an Oscar), Erin and lawyer Edward Masry sued PG&E for contaminating the water near Hinckley in 1993. The settlement they won in 1996—$333 million—was the largest amount ever awarded for a direct-action lawsuit in the US at that time.
Chromium compounds are widely used in industry, such as for chrome plating, in dye-making, as preservatives, and to prevent corrosion in cooling tower water, as occurred near Hinckley. In the environment, chromium exists primarily in either the Cr(III) or Cr(VI) forms. Cr(III), an ingredient of many vitamin and nutritional supplements, forms compounds that are not very soluble in water, and it has low toxicity. But Cr(VI) is much more toxic and forms compounds that are reasonably soluble in water. Exposure to small amounts of Cr(VI) can lead to damage of the respiratory, gastrointestinal, and immune systems, as well as the kidneys, liver, blood, and skin.
Despite cleanup efforts, Cr(VI) groundwater contamination remains a problem in Hinckley and other locations across the globe. A 2010 study by the Environmental Working Group found that of 35 US cities tested, 31 had higher levels of Cr(VI) in their tap water than the public health goal of 0.02 parts per billion set by the California Environmental Protection Agency.
Molecular (Covalent) Compounds
The bonding characteristics of inorganic molecular compounds are different from ionic compounds, and they are named using a different system as well. The charges of cations and anions dictate their ratios in ionic compounds, so specifying the names of the ions provides sufficient information to determine chemical formulas. However, because covalent bonding allows for significant variation in the combination ratios of the atoms in a molecule, the names for molecular compounds must explicitly identify these ratios.
Compounds Composed of Two Elements
When two nonmetallic elements form a molecular compound, several combination ratios are often possible. For example, carbon and oxygen can form the compounds CO and CO 2 . Since these are different substances with different properties, they cannot both have the same name (they cannot both be called carbon oxide). To deal with this situation, we use a naming method that is somewhat similar to that used for ionic compounds, but with added prefixes to specify the numbers of atoms of each element. The name of the more metallic element (the one farther to the left and/or bottom of the periodic table) is first, followed by the name of the more nonmetallic element (the one farther to the right and/or top) with its ending changed to the suffix – ide . The numbers of atoms of each element are designated by the Greek prefixes shown in Table 2.10 .
When only one atom of the first element is present, the prefix mono - is usually deleted from that part. Thus, CO is named carbon monoxide, and CO 2 is called carbon dioxide. When two vowels are adjacent, the ending vowel in the Greek prefix is sometimes omitted in common practice, though IUPAC guidelines only permit this for the duplicate letters o in monooxide , which is correctly written as monoxide . For purposes of the nomenclature exercises in this text, students may choose to follow either approach. Some examples demonstrating this Some other examples are shown in Table 2.11 .
| Compound | Name | Compound | Name |
|---|---|---|---|
| SO | sulfur dioxide | BCl | boron trichloride |
| SO | sulfur trioxide | SF | sulfur hexafluoride |
| NO | nitrogen dioxide | PF | phosphorus pentafluoride |
| N O | dinitrogen tetroxide | P O | tetraphosphorus decaoxide |
| N O | dinitrogen pentoxide | IF | iodine heptafluoride |
There are a few common names that you will encounter as you continue your study of chemistry. For example, although NO is often called nitric oxide, its proper name is nitrogen monoxide. Similarly, N 2 O is known as nitrous oxide even though our rules would specify the name dinitrogen monoxide. (And H 2 O is usually called water, not dihydrogen monoxide.) You should commit to memory the common names of compounds as you encounter them.
Example 2.14
Naming covalent compounds.
(b) N 2 O 3
(c) Cl 2 O 7
(d) P 4 O 6
(a) sulfur hexafluoride
(b) dinitrogen trioxide
(c) dichlorine heptoxide
(d) tetraphosphorus hexoxide
(a) phosphorus pentachloride
(b) dinitrogen monoxide
(c) iodine heptafluoride
(d) carbon tetrachloride
(a) PCl 5 ; (b) N 2 O; (c) IF 7 ; (d) CCl 4
Link to Learning
The following website provides practice with naming chemical compounds and writing chemical formulas. You can choose binary, polyatomic, and variable charge ionic compounds, as well as molecular compounds.
Binary Acids
Some compounds containing hydrogen are members of an important class of substances known as acids. The chemistry of these compounds is explored in more detail in later chapters of this text, but for now, it will suffice to note that many acids release hydrogen ions, H + , when dissolved in water. To denote this distinct chemical property, a mixture of water with an acid is given a name derived from the compound’s name. If the compound is a binary acid (comprised of hydrogen and one other nonmetallic element):
- The word “hydrogen” is changed to the prefix hydro-
- The other nonmetallic element name is modified by adding the suffix - ic
- The word “acid” is added as a second word
For example, when the gas HCl (hydrogen chloride) is dissolved in water, the solution is called hydrochloric acid . Several other examples of this nomenclature are shown in Table 2.12 .
| Name of Gas | Name of Acid |
|---|---|
| HF( ), hydrogen fluoride | HF( ), hydrofluoric acid |
| HCl( ), hydrogen chloride | HCl( ), hydrochloric acid |
| HBr( ), hydrogen bromide | HBr( ), hydrobromic acid |
| HI( ), hydrogen iodide | HI( ), hydroiodic acid |
| H S( ), hydrogen sulfide | H S( ), hydrosulfuric acid |
Many compounds containing three or more elements (such as organic compounds or coordination compounds) are subject to specialized nomenclature rules that you will learn later. However, we will briefly discuss the important compounds known as oxyacids , compounds that contain hydrogen, oxygen, and at least one other element, and are bonded in such a way as to impart acidic properties to the compound (you will learn the details of this in a later chapter). Typical oxyacids consist of hydrogen combined with a polyatomic, oxygen-containing ion. To name oxyacids:
- Omit “hydrogen”
- Start with the root name of the anion
- Replace – ate with – ic , or – ite with – ous
For example, consider H 2 CO 3 (which you might be tempted to call “hydrogen carbonate”). To name this correctly, “hydrogen” is omitted; the – ate of carbonate is replace with – ic ; and acid is added—so its name is carbonic acid. Other examples are given in Table 2.13 . There are some exceptions to the general naming method (e.g., H 2 SO 4 is called sulfuric acid, not sulfic acid, and H 2 SO 3 is sulfurous, not sulfous, acid).
| Formula | Anion Name | Acid Name |
|---|---|---|
| HC H O | acetate | acetic acid |
| HNO | nitrate | nitric acid |
| HNO | nitrite | nitrous acid |
| HClO | perchlorate | perchloric acid |
| H CO | carbonate | carbonic acid |
| H SO | sulfate | sulfuric acid |
| H SO | sulfite | sulfurous acid |
| H PO | phosphate | phosphoric acid |
As an Amazon Associate we earn from qualifying purchases.
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution License and you must attribute OpenStax.
Access for free at https://openstax.org/books/chemistry-2e/pages/1-introduction
- Authors: Paul Flowers, Klaus Theopold, Richard Langley, William R. Robinson, PhD
- Publisher/website: OpenStax
- Book title: Chemistry 2e
- Publication date: Feb 14, 2019
- Location: Houston, Texas
- Book URL: https://openstax.org/books/chemistry-2e/pages/1-introduction
- Section URL: https://openstax.org/books/chemistry-2e/pages/2-7-chemical-nomenclature
© Jan 8, 2024 OpenStax. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution License . The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.

IMAGES
VIDEO
COMMENTS
Identify arrows pointing to bonding electrons. a, b, d. Identify arrows pointing to nonbonding electrons. c. Identify arrows pointing to structures containing sigma bonds. a, b, d. Identify arrows pointing to structures containing pi bonds. a. Check all features below that you included in your Lewis structure.
Identify arrows pointing to bonding electrons. a,b,d. Identify arrows pointing to nonbonding electrons. c. Identify arrows pointing to structures containing sigma bonds. a,b,d. Identify arrows pointing to structures containing pi bonds. a. On a sheet of paper, draw the Lewis structure for the following compound.
Study with Quizlet and memorize flashcards containing terms like A solid compound in a sealed container was kept at a very low temperature in a freezer. When placed at room temperature, the substance quickly turned into a liquid. This compound is most likely which of the following?, Which type of bond will form between two chlorine atoms?, Which compound contains only nonpolar covalent bonds ...
Student Exploration: Covalent Bonds. Vocabulary: covalent bond, diatomic molecule, Lewis diagram, molecule, noble gases, nonmetal, octet rule, shell, valence, valence electron. Prior Knowledge Questions (Do these BEFORE using the Gizmo.) There are eight markers in a full set, but Flora and Frank each only have seven markers.
Formation of Covalent Bonds. Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. For example, the hydrogen molecule, H 2, contains a covalent bond between its two hydrogen atoms. Figure 7.4 illustrates why this bond is formed. Starting on the far right, we have two separate hydrogen atoms with a particular potential energy ...
High school chemistry. Course: High school chemistry > Unit 2. Quiz 1. Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Khan Academy is a nonprofit with the mission of providing a free, world-class education for anyone, anywhere.
Study with Quizlet and memorize flashcards containing terms like Propane, the gas used in barbecue grills, is made of carbon and hydrogen. Will the atoms that make up propane from covalent bonds? Why or why not?, Which of these atoms is most likely to share electrons with other atoms?, Chlorine, which is used to clean swimming pools, exist as two chlorine atoms covalently bonded to each other.
Covalent bonds usually form between nonmetals. Examples of covalent compounds include hydrogen (H 2 ), oxygen (O 2 ), carbon monoxide (CO), ammonia (NH 3 ), water (H 2 O), and all organic compounds. There are compounds that contain both covalent and ionic bonds, such as potassium cyanide (KCN) and ammonium chloride (NH 4 Cl).
Chapter 4 - Covalent Bonds and Molecular Compounds. Chemical bonds are generally divided into two fundamentally different types: ionic and covalent. In reality, however, the bonds in most substances are neither purely ionic nor purely covalent, but lie on a spectrum between these extremes. Although purely ionic and purely covalent bonds ...
delta bond. covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. The binding arises from the electrostatic attraction of their nuclei for the same electrons. A covalent bond forms when the bonded atoms have a lower total energy than that of widely separated atoms.
The distance between two bonded atoms at their minimum potential energy. The energy required to break the bonds in one mole of a chemical compound. A covalent bond in the bonding electrons are equally attracted to both atoms. A covalent bond in which a shared pair of electrons is held more closely by one of the atoms.
Derive names for common types of inorganic compounds using a systematic approach. Nomenclature, a collection of rules for naming things, is important in science and in many other situations. This module describes an approach that is used to name simple ionic and molecular compounds, such as NaCl, CaCO 3, and N 2 O 4.
An electronic structure of a molecule or ion in which electrons are shown by dashes or dots. Covalent bond. A chemical link between two atoms produced by sharing electrons in the region between the atoms. Unshared electron pair. A pair of electrons that "belongs" to a single atom and is not involved in bonding. Single bond.
A covalent bond is the force of attraction that holds together two atoms that share a pair of valence electrons. Covalent bonds form only between atoms of nonmetals. The two atoms that are held together in a covalent bond may be atoms of the same element or different elements. When atoms of different elements bond together, it forms a covalent ...
Study with Quizlet and memorize flashcards containing terms like Which statement best describe the structure of a carbonate anion? (Outcome # 12) (DOK 3) a. The electron geometry of carbon in a carbonate anion is trigonal planar with a sp2 hybridization and bond angles are 120°. There is a π-bond formed between a non-hybridized "C" p-orbital overlapping with an "O" p-orbital. b. The electron ...