
- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.


2.5: Practice of Green Chemistry
- Last updated
- Save as PDF
- Page ID 284414

- Stanley E. Manahan
- University of Missouri
The limitations of a command and control system for environmental protection have become more obvious even as the system has become more successful. In industrialized societies with good, well-enforced regulations, most of the easy and inexpensive measures that can be taken to reduce environmental pollution and exposure to harmful chemicals have been implemented. Therefore, small increases in environmental protection now require relatively large investments in money and effort. Is there a better way? There is, indeed. The better way is through the practice of green chemistry.
Green chemistry can be defined as the practice of chemical science and manufacturing in a manner that is sustainable, safe, and non-polluting and that consumes minimum amounts of materials and energy while producing little or no waste material. This definition of green chemistry is illustrated in Figure \(\PageIndex{1}\). The practice of green chemistry begins with recognition that the production, processing, use, and eventual disposal of chemical products may cause harm when performed incorrectly. In accomplishing its objectives, green chemistry and green chemical engineering may modify or totally redesign chemical products and processes with the objective of minimizing wastes and the use or generation of particularly dangerous materials. Those who practice green chemistry recognize that they are responsible for any effects on the world that their chemicals or chemical processes may have. Far from being economically regressive and a drag on profits, green chemistry is about increasing profits and promoting innovation while protecting human health and the environment.
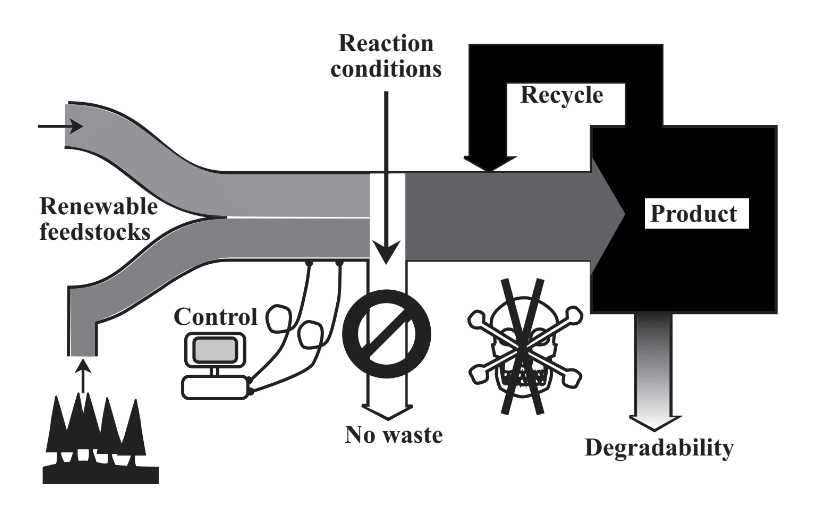
To a degree, we are still finding out what green chemistry is. That is because it is a rapidly evolving and developing subdiscipline in the field of chemistry. And it is a very exciting time for those who are practitioners of this developing science. Basically, green chemistry harnesses a vast body of chemical knowledge and applies it to the production, use, and ultimate disposal of chemicals in a way that minimizes consumption of materials, exposure of living organisms, including humans, to toxic substances, and damage to the environment. And it does so in a manner that is economically feasible and cost effective. In one sense, green chemistry is the most efficient possible practice of chemistry and the least costly when all of the costs of doing chemistry, including hazards and potential environmental damage are taken into account.
Green chemistry is sustainable chemistry. There are several important respects in which green chemistry is sustainable:
- Economic: At a high level of sophistication green chemistry normally costs less in strictly economic terms (to say nothing of environmental costs) than chemistry as it is normally practiced.
- Materials: By efficiently using materials, maximum recycling, and minimum use of virgin raw materials, green chemistry is sustainable with respect to materials.
- Waste: By reducing insofar as possible, or even totally eliminating their production, green chemistry is sustainable with respect to wastes

Key Elements of Green Chemistry
Lucian Lucia, North Carolina State University
Copyright Year: 2018
Publisher: North Carolina State University
Language: English
Formats Available
Conditions of use.
Learn more about reviews.
Reviewed by Debasish Bandyopadhyay, Assistant Professor, University of Texas Rio Grande Valley on 12/22/21
The book presents the major concepts of green chemistry in six chapters. read more
Comprehensiveness rating: 5 see less
The book presents the major concepts of green chemistry in six chapters.
Content Accuracy rating: 5
The content is accurate and to the point.
Relevance/Longevity rating: 5
All the materials presented in this book are updated.
Clarity rating: 5
The text is written in simple language that should be helpful for the readers to understand the topic.
Consistency rating: 4
The six chapters are well-aligned, and the whole concept of green chemistry has been thoughtfully presented in this book.
Modularity rating: 5
The text is easily and readily divisible into smaller reading sections that can be assigned at different points within the course. Under each chapter, there are several headings sections and subsections. The text is not overly self-referential and should be quickly reorganized and realigned with various subunits of a course without presenting much disruption to the reader.
Organization/Structure/Flow rating: 4
In each chapter, their sections and sub-sections have been organized stepwise.
Interface rating: 4
The text is free of significant interface issues, including navigation problems, distortion of images/charts, and any other display features that may distract or confuse the reader.
Grammatical Errors rating: 5
There are no significant grammatical errors.
Cultural Relevance rating: 5
The text is not culturally insensitive or offensive in any way.
Although the book's target readers are green chemists, it is an excellent reference book also for toxicologists, chemical engineers, pharmacologists, and biochemists. The author has successfully correlated the green chemistry parameters with the real world. For example, the author included the incidents of burning oil and debris that collected on the surface of the Cuyahoga River (Cleveland), the thalidomide issue etc. The discussion on the LCA software, the globally harmonized system (GHS) that attempts to categorize the general types of threats in society, specifically, is fascinating. The concept of different hazards such as carcinogens, mutagens, teratogens, tumor promoters, corrosives, neurotoxins, lachrymators has added special value to this book. The lethal dose of everyday things like water to Vit C provides real-world examples. The author has brilliantly correlated the chemistry world with the business world by the dual meaning of ‘solvent’. Discussion on alternative solvents like green biobased solvent methyl soyate, eutectic mixture, microemulsion, etc., are fascinating. Combinatorial chemistry and organic reaction mechanism part will undoubtedly draw attention to the organic chemistry students. In the last chapter, the classification of organic reactions and their correlation with green chemistry is eloquent. The images are significant. The author referenced each image so that curious readers could go more over it. At the end of each chapter, the review questions will insist the readers rethink the subject matter. A little comicalness throughout the book will keep the readers smiling.
Table of Contents
- Chapter 1: Principles Of Green Chemistry
- Chapter 2: Life-Cycle Analysis
- Chapter 3: Hazards
- Chapter 4: Alternative Solvents
- Chapter 5: Alternative Reagents
- Chapter 6: Reaction Types, Design, And Efficiency
- Index Of Terms
Ancillary Material
About the book.
Green chemistry, in addition to being a science, it is also a philosophy and nearly a religion. Attendance at American Chemical Society Green Chemistry & Engineering Conferences will instill such an ideal into any attendant because of the nearly universal appeal and possibilities in this novel approach to radicalizing the business of doing science and engineering.
About the Contributors
Lucian Lucia currently serves as an Associate Professor in the Departments of Forest Biomaterials and Chemistry and as a faculty in the programs of Fiber & Polymer Science and Environmental Sciences at North Carolina State University. His laboratory, The Laboratory of Soft Materials & Green Chemistry, probes fundamental materials chemistry of biopolymers. He received his Ph.D. in organic chemistry from the University of Florida under Professor Kirk Schanze for modeling photoinduced charge separation states of novel Rhenium (I)-based organometallic ensembles as a first order approximation of photosynthesis. He began his professional career as an Assistant Professor at the Institute of Paper Science and Technology at the Georgia Institute of Technology examining the mechanism of singlet oxygen’s chemistry with lignin & cellulose. A large part of his recent work has been focused on the chemical modification of cellulosics for biomedical applications. He teaches From Papyrus to Plasma Screens: Paper & Society (PSE 220), Principles of Green Chemistry (PSE / CH 335), and is the graduate supervisor for the Forest Biomaterials Seminar Series (WPS 590 / 790) while providing workshops in Wood Chemistry and Green Chemistry at Qilu University of Technology in PR China. He has co-founded and co-edits an open-access international research journal, BioResources, dedicated to original research articles, reviews, and editorials on the fundamental science & engineering and advanced applications of lignocellulosic materials.
Contribute to this Page

Green Chemistry: Principles and Case Studies
Green chemistry as a discipline is gaining increasing attention globally, with environmentally conscious students keen to learn how they can contribute to a safer and more sustainable world. Many universities now offer courses or modules specifically on green chemistry – Green Chemistry: Principles and Case Studies is an essential learning resource for those interested in mastering the subject.
Providing a comprehensive overview of the concepts of green chemistry this book engages students with a thorough understanding of what we mean by green chemistry and how it can be put into practice. Structured around the well-known 12 Principles, and firmly grounded in real-world applications and case-studies, this book shows how green chemistry is already being put into practice and prepare them to think about how they can be incorporated into their own work.
Targeted at advanced undergraduate and first-year graduate students with a background in general and organic chemistry, it is a useful resource both for students and for teachers looking to develop new courses.
- Cite Icon Cite
F. A. Etzkorn, Green Chemistry: Principles and Case Studies, The Royal Society of Chemistry, 2019.
Download citation file:
- Ris (Zotero)
- Reference Manager
Digital access
Print format, table of contents.
- Front Matter
- Acknowledgements
- The 12 Principles of Green Chemistry
- 1: Prevent Waste p1-22 Abstract Open the PDF Link PDF for 1: Prevent Waste in another window
- 2: Synthetic Efficiency p23-56 Abstract Open the PDF Link PDF for 2: Synthetic Efficiency in another window
- 3: Benign Synthesis p57-90 Abstract Open the PDF Link PDF for 3: Benign Synthesis in another window
- 4: Benign Products p91-124 Abstract Open the PDF Link PDF for 4: Benign Products in another window
- 5: Avoid Auxiliaries p125-168 Abstract Open the PDF Link PDF for 5: Avoid Auxiliaries in another window
- 6: Energy Efficiency p169-207 Abstract Open the PDF Link PDF for 6: Energy Efficiency in another window
- 7: Renewable Feedstocks p208-236 Abstract Open the PDF Link PDF for 7: Renewable Feedstocks in another window
- 8: Avoid Protecting Groups p237-270 Abstract Open the PDF Link PDF for 8: Avoid Protecting Groups in another window
- 9: Catalysis p271-298 Abstract Open the PDF Link PDF for 9: Catalysis in another window
- 10: Degradation or Recovery p299-320 Abstract Open the PDF Link PDF for 10: Degradation or Recovery in another window
- 11: Real-time Analysis p321-352 Abstract Open the PDF Link PDF for 11: Real-time Analysis in another window
- 12: Prevent Accidents p353-378 Abstract Open the PDF Link PDF for 12: Prevent Accidents in another window
- Appendix A: Organic Functional Groups p379-383 Open the PDF Link PDF for Appendix A: Organic Functional Groups in another window
- Appendix B: Organic Mechanism p384-408 Open the PDF Link PDF for Appendix B: Organic Mechanism in another window
- Appendix C: p K a Tables p409-415 Open the PDF Link PDF for Appendix C: p<em>K</em><sub>a</sub> Tables in another window
- Appendix D: Earth Abundance Periodic Table p416-417 Open the PDF Link PDF for Appendix D: Earth Abundance Periodic Table in another window
- Appendix E: Standard Reduction Potentials by Value p418-420 Open the PDF Link PDF for Appendix E: Standard Reduction Potentials by Value in another window
- Appendix F: Solvent Selection Guide p421-422 Open the PDF Link PDF for Appendix F: Solvent Selection Guide in another window
- Appendix G: Selected Bond Dissociation Energies p423-424 Open the PDF Link PDF for Appendix G: Selected Bond Dissociation Energies in another window
- Subject Index p425-447 Open the PDF Link PDF for Subject Index in another window
Advertisement
- Campaigning and outreach
- News and events
- Awards and funding
- Privacy policy
- Journals and databases
- Locations and contacts
- Membership and professional community
- Teaching and learning
- Help and legal
- Cookie policy
- Terms and conditions
- Get Adobe Acrobat Reader
- Registered charity number: 207890
- © Royal Society of Chemistry 2023
Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation

- Back to parent navigation item
- Primary teacher
- Secondary/FE teacher
- Early career or student teacher
- Higher education
- Curriculum support
- Literacy in science teaching
- Periodic table
- Interactive periodic table
- Climate change and sustainability
- Resources shop
- Collections
- Post-lockdown teaching support
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
- Nuffield practical collection
- Anecdotes for chemistry teachers
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Art analysis
- Pigments and colours
- Ancient art: today's technology
- Psychology and art theory
- Art and archaeology
- Artists as chemists
- The physics of restoration and conservation
- Ancient Egyptian art
- Ancient Greek art
- Ancient Roman art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Education in Chemistry
- Teach Chemistry
- On-demand online
- Live online
- Selected PD articles
- PD for primary teachers
- PD for secondary teachers
- What we offer
- Chartered Science Teacher (CSciTeach)
- Teacher mentoring
- UK Chemistry Olympiad
- Who can enter?
- How does it work?
- Resources and past papers
- Top of the Bench
- Schools' Analyst
- Regional support
- Education coordinators
- RSC Yusuf Hamied Inspirational Science Programme
- RSC Education News
- Supporting teacher training
- Interest groups

- More from navigation items
Inspirational chemistry book
- 1 Inspirational chemistry
- 2 Compounds and formula
- 3 Patterns in formula of compounds
- 4 Taboo – chemical reactions
- 5 Heating Group 1 metals in air and in chlorine
- 6 The extraction of copper: a microscale version
- 7 Extracting metals | words
- 9 Alloys | making an alloy
- 10 Alloys | modelling an alloy
- 11 Alloys of iron | steels
- 12 Electrolysis of molten zinc chloride
- 13 A colourful electrolysis
- 14 Cracking hydrocarbons | A microscale version
- 15 Nail varnish removal
- 16 Carbon monoxide
- 17 Polymers in everyday things
- 18 Monomer | Polymer card game
- 19 Changing the properties of polymers and plastics
- 20 Polythene bags
- 21 Using potato starch to make plastic
- 22 Investigating cross-linking | making slime
- 23 Cross-linking polymers | alginate worms
- 24 Polylactic acid
- 25 Emulsifiers
- 26 Textile conservation
- 27 Epoxy glues and the ATLAS project: the biggest experiment ever?
- 28 Cooking potatoes
- 29 Baking powder
- 30 Rates and rhubarb
- 31 The importance of structure: chocolate
- 32 Making new medicines | combinatorial chemistry
- 33 A composite material: concrete
- 34 Investigating a natural composite | chicken bone
- 35 Hydrogels | smart materials
- 36 Superconductors
- 37 Nanotechnology size and scale
- 38 Nanotechnology and smelly socks
- 39 Nanosilver in medicine
- 40 The surfaces of substances
- 41 Changing the surface
- 42 Plastics
- 43 Active and intelligent packaging
- 44 Disposable cups and the environment
- 45 Managing waste and rubbish
- 46 Nappy choice and the environment
- 47 Dry cleaning and green Chemistry
- 48 Feed the world: artificial nitrogen fertilisers
- 49 Green Chemistry, atom economy and sustainable development
- 50 Making oil from waste
- 51 Reactions of positive ions with sodium hydroxide
- 52 Testing for negative ions
- 53 Cold light
- 54 Spectroscopy
Green Chemistry, atom economy and sustainable development
- No comments
It’s not easy being green
The competing needs of development and environmental protection are often mentioned in the media and many students have opinions on the subject. Chemistry is seen as a ‘polluter,’ which partly accounts for its poor image among students and the general public.
This activity introduces the concept of atom economy in the context of sustainable development and Green Chemistry. It aims to show that development is necessary but can be achieved in a way which limits environmental damage.
Prior knowledge required
Students need to know/be able to:
- Calculate relative molecular mass (RMM or Mr)
- Know what a reversible reaction is and how the yield of a reaction might be affected by its reversible nature
- Calculate percentage yield – a section on this is included in the resource but it would be better if students had already covered it so that they do not get it mixed up with atom economy.
Further information
Further information on sustainable development is available on various websites, including: http://www.uyseg.org/sustain-ed/Index.htm – this website of the Chemical Industry Education Centre is a good introduction to why development is necessary and how it can be made more sustainable.
Students can calculate their personal sustainability and also how much carbon dioxide they produce in a year. Examples of chemical industries that are going greener are provided, along with links to several other sites.
A quarter of the world’s people have to survive on less than 70p a day. Millions have no health care and the world’s population is expected to increase by about another 3 billion over the next 50 years. Even in developed nations, poverty, education and healthcare could be improved. To help deal with this situation, the world’s economy needs to grow; in particular, the economies of developing nations need to expand. However, economic growth is often linked to environmental pollution problems.
The challenge is to develop in a way that meets the needs of the present generation without compromising the ability of future generations to meet their own needs – in other words, without causing a lot of environmental damage and wasting limited resources. This type of development is called ‘sustainable development’ and it will be more and more critical as the population of the world increases.
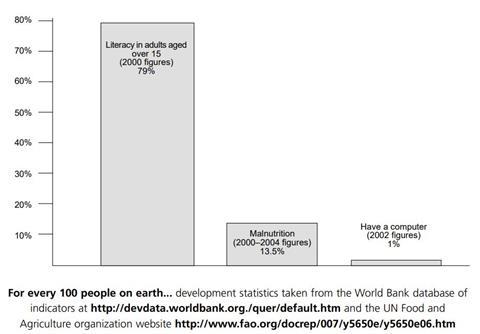
One of the ways in which the chemical industry is working towards sustainable development is by using ‘Green Chemistry.’ One of the basic ideas of Green Chemistry is to prevent pollution and the production of hazardous materials instead of producing them and then cleaning them up.
This means that Green Chemistry:
- Conserves raw materials and energy
- Is more cost-effective than conventional methods.
There are three main ways to make chemical processes ‘greener’:
- Redesign production methods to use different, less hazardous starting materials
- Use milder reaction conditions, better catalysts and less hazardous solvents
- Use production methods with fewer steps and higher atom economy.
Yield
Most of the chemical industry is concerned with turning one material (the raw material) into another one that is more useful and valuable (the product). This process may have several steps and is called a ‘chemical synthesis.’ All the designers of chemical processes want to make the maximum amount of product they can from a given raw material. It is possible to calculate how successful one of these processes is by using the idea of yield.
Atom economy
The idea of yield is useful, but from a Green Chemistry and sustainable development perspective, it is not the full picture. This is because yield is calculated by considering only one reactant and one product. One of the key principles of Green Chemistry is that processes should be designed so that the maximum amount of all the raw materials ends up in the product and a minimum amount of waste is produced.
A reaction can have a high percentage yield but also make a lot of waste product. This kind of reaction has a low atom economy. Both the yield and the atom economy have to be taken into account when designing a green chemical process.
Green chemistry
Additional information.
This resource is a part of our Inspirational chemistry collection.

Inspirational chemistry

Compounds and formula

Patterns in formula of compounds

Taboo – chemical reactions

Heating Group 1 metals in air and in chlorine

The extraction of copper: a microscale version

Extracting metals | words

Alloys | making an alloy

Alloys | modelling an alloy

Alloys of iron | steels

Electrolysis of molten zinc chloride

A colourful electrolysis

Cracking hydrocarbons | A microscale version

Nail varnish removal

Carbon monoxide

Polymers in everyday things

Monomer | Polymer card game

Changing the properties of polymers and plastics

Polythene bags

Using potato starch to make plastic

Investigating cross-linking | making slime
Cross-linking polymers | alginate worms

Polylactic acid

Emulsifiers

Textile conservation

Epoxy glues and the ATLAS project: the biggest experiment ever?

Cooking potatoes

Baking powder

Rates and rhubarb

The importance of structure: chocolate

Making new medicines | combinatorial chemistry

A composite material: concrete


Investigating a natural composite | chicken bone

Hydrogels | smart materials

Superconductors

Nanotechnology size and scale

Nanotechnology and smelly socks

Nanosilver in medicine

The surfaces of substances

Changing the surface

Active and intelligent packaging

Disposable cups and the environment

Managing waste and rubbish

Nappy choice and the environment

Dry cleaning and green Chemistry

Feed the world: artificial nitrogen fertilisers

Making oil from waste

Reactions of positive ions with sodium hydroxide

Testing for negative ions

Spectroscopy
- 11-14 years
- Investigation
- Problem solving
Related articles

Understanding how students untangle intermolecular forces
2024-03-14T05:10:00Z By Fraser Scott
Discover how learners use electronegativity to predict the location of dipole−dipole interactions

Chromatography challenge | 16–18 years
By Andy Markwick
Explore analytical techniques and their applications with a chromatography investigation and research activity

Boost maths skills to improve chemistry learning
2024-01-18T08:00:00Z By Fraser Scott
Use these evidence-based tips to help your learners get ahead with chemical calculations
No comments yet
Only registered users can comment on this article., more from resources.

Metallic bonding | Structure strip | 14–16
By Kristy Turner
Describe the metallic bonding model and explain how this leads to particular properties in metals, with this scaffolded writing activity

Ionic bonding | Structure strip | 14–16
Understand the models and diagrams used to represent ionic bonding and their limitations, with this scaffolded writing activity

Covalent bonding | Structure strip | 14–16
Understand covalent bonding diagrams and their limitations, with this scaffolded writing activity
- Contributors
- Email alerts
Site powered by Webvision Cloud
- Publications
ACS NETWORK
Chemistry community online.
- ACS Community
- Green Chemistry
- Green Chemistry Institute
- GCI Nexus Blog
- Green Chemistry Activities for ACS Student Chapter...
Green Chemistry Activities for ACS Student Chapters
- Subscribe to RSS Feed
- Printer Friendly Page
- Report Inappropriate Content
- Next Gen Green Chemistry
- acs student chapter
- green chemistry
- green chemistry student chapters
- virtual resources

Development of the Assessment of Student Knowledge of Green Chemistry Principles (ASK-GCP)

First published on 15th February 2022
As implementation of green chemistry into university-level courses increases, it is vital that educators have a tool to rapidly measure student knowledge of green chemistry principles. We report the development of the Assessment of Student Knowledge of Green Chemistry Principles (ASK-GCP) and evaluation of its sensitivity and effectiveness for measuring student knowledge of green chemistry. The 24-item true–false instrument was given to a total of 448 students to gather data on the reliability, validity, and sensitivity. The instrument proved to be sensitive for distinguishing known groups with various levels of green chemistry knowledge and instructional exposure. The instrument was able to detect gains in green chemistry knowledge in pre- and post- conditions. Psychometric analysis revealed that the item difficulty range matches the sample ability range. The findings verified that the ASK-GCP is an efficient and accurate instrument to measure student knowledge of green chemistry principles.
Introduction
True–false instruments for assessment of student knowledge in stem.
True–false instruments provide a variety of advantages over other testing formats because they promote quick and accurate scoring, provide reliable data, and require less reading than a comparable multiple choice question ( Frisbie and Becker, 1991 ). Additionally, true–false instruments can be rapidly administered and allow for at least 50% more questions than multiple choice instruments during the same time period ( Frisbie, 1974 ; Oosterhof and Glasnapp, 1974 ; Frisbie and Becker, 1991 ). Although true–false items have been shown to exhibit lower discrimination values than multiple choice items, the ability to utilize more items in the same time period compensates for this limitation ( Frisbie and Becker, 1991 ). Further, the reliability and validity of the data collected from true–false items have been shown to be higher when students are instructed not to guess on the questions ( Kinney and Eurich, 1933 ) providing evidence for incorporating an option for students who do not know the answer in formative assessments. Recently, it has been reported that the multiple true–false format that required students to independently evaluate each item in a multiple choice format provided a more accurate view of student knowledge of the topic than a traditional multiple choice question ( Couch et al. , 2018 ).
While true–false instruments provide many advantages, there are limitations that need to be considered. One limitation that has been widely discussed is the ability for student guessing to influence the final score. However, this impact greatly decreases as the length of the test increases ( Ebel, 1970 ). Additionally, this impact can be reduced through requiring students to correct false statements so that they are true ( McCullough, 1993 ) or through the use of confidence-weighted true–false tests ( Dutke and Barenberg, 2015 ).
Project goal and research question
While this assessment can be utilized in any course, it was particularly designed for students in organic chemistry because 53% of the green chemistry instructional activities have been implemented within organic chemistry ( Marques et al. , 2020 ). However, despite the frequency of reports on how green chemistry activities were perceived by students, very few report the assessment of student cognitive outcomes. An in-house designed assessment task remains the only way to assess an impact on student learning, weakening an overall inference about the effectiveness of numerous reported curriculum implementations. Therefore, this paper presents the development and evaluation of an assessment measure of green chemistry knowledge for students enrolled in organic chemistry courses. This instrument addressed an important, long-existing void of an easy to administer, score, and make inferences from assessment tool for numerous reported instructional innovations in green chemistry education. It was developed to be suitable for large enrollment classes, effective for all courses, and rapidly implemented and assessed so instructors can easily monitor student progress.
Instrument development
The initial set of 24 items were chosen from a list of 72 true–false items written by five individuals with experience in green and organic chemistry. Items were developed and formatted using recommendations for true–false items outlined by Thorndike and Thorndike-Christ (2010) . The selected items were chosen so that each green chemistry principle is assessed by one item whose correct response was true and one item whose correct response was false.
Students were administered the pilot version of the true–false instrument and subsequently asked to provide feedback using the prompt, “ Were any of the statements unclear or confusing to you? If yes, provide examples and explain in the space below. ” The use of student feedback to remove linguistically complicated wording is in accordance with findings by Lee and Orgill (2021) , which indicated that reducing linguistic complexity of assessment prompts can benefit all students, especially English language learners. Due to student feedback received and analysis of the results, the instrument was revised to remove the technical wording from items 3 and 24 to increase clarity in the statements. Item 3 originally stated, “reactions at elevated temperatures should be prioritized over reactions at ambient temperature,” but was revised to change the term “ambient temperature” to “room temperature.” The resulting item 3 was “reactions at elevated temperatures should be prioritized over reactions at room temperature.” Additionally, item 24 originally stated, “halogenated molecules are structural features that promote facile biodegradation.” It was revised to remove the phrases “facile biodegradation” and “halogenated molecules;” the revised version stated, “organic compounds containing chlorine are easily broken down.” Additionally, to limit the impact of student guessing due to only true and false options, the option “don’t know” was added for the revised version ( Schönborn et al. , 2015 ).
Participants, setting and data collection
Subsequently, the revised version of the instrument was administered the following year to students enrolled in either a general chemistry, hereafter referred to as GC ( N = 302), or an organic chemistry course, hereafter referred to as OC ( N = 61), which were taught by other faculty who were not members of the research team. To ease administration of the instrument, it was administered to students in the corresponding laboratory sections with a typical class size of 24 students. Neither the GC nor OC course explicitly integrated green chemistry instruction into the curriculum which allowed for comparisons between groups. Only students who responded to all questions of the ASK-GCP instrument were included in the analysis. Additionally, it was administered to students enrolled in two semesters of majors’ organic chemistry laboratory ( N = 14) to assess reliability and validity of repeated measures of the ASK-GCP data.
The instrument was administered electronically using Qualtrics. Students who completed the instrument were rewarded with nominal extra credit points. For both the initial and revised versions, student responses were scored as correct (1) or incorrect which included the option don’t know (0).
Data analysis
Confirmatory factor analysis was conducted using combined data from all three chemistry courses using StataIC 16. Due to the dichotomous nature of the data, response scores for the two questions for each green chemistry principle were summed and used in the analysis. Therefore, the model consisted of one factor, green chemistry knowledge, and 12 indicators representing each of the twelve principles. The model for CFA was identified using unit variance identification.
Item analysis was performed using jMetrik and StataIC 16 to determine item difficulty and discrimination indices. A detailed account describing item analysis in jMetrik is available ( Leontyev et al. , 2017 ). After confirming the unidimensionality of the data, Rasch analysis was performed on the combined data collected from the OC and GC course which were combined since neither explicitly taught about green chemistry. A Wright Map was generated in RStudio using the TAM and WrightMap packages. Rasch analysis was conducted using StataIC 16.
Results & discussion
Descriptive statistics.
Analysis of the distribution of student scores from all three cohorts ( Fig. 2 ) indicated that neither a ceiling effect nor floor effect was observed since few students scored either a 24 or 0, respectively. Additionally, the almost symmetrical distribution indicated that the data followed an approximately normal distribution. To further analyze the normality of the data, the kurtosis and skewness of the data were calculated. Kurtosis represents whether the data is heavy-tailed or light-tailed in relation to a normal distribution, whereas skewness represents any asymmetry in the distribution of the data. While a normal distribution has a skewness of 0, analysis indicated the data had a skewness of −0.407, indicating that it was slightly skewed to the left. Thus, the mean ( M = 12.61) was slightly less than the median (med = 13). Furthermore, a normal distribution would have a kurtosis value of 3, whereas our data indicated a kurtosis of 2.89, suggesting that it had a slightly lighter-tailed distribution than a normal distribution. However, overall, it followed an approximately normal distribution. Therefore, the mean and other descriptive statistics were representative of the data ( Mishra et al. , 2019 ). Similarly, Ferguson's δ was calculated for each group and is reported in Table 1 and was determined to be 0.981 overall. Ferguson's δ measures the discriminatory power of a test by comparing the range of observed student scores with the total possible range ( Ferguson, 1949 ; Ding and Beichner, 2009 ). Ferguson's δ ranges from 0 to 1 and instruments with values greater than 0.9 are generally considered to have good discriminatory power ( Ding and Beichner, 2009 ). The overall Ferguson's δ for all three groups was 0.981, indicating that the sample was distributed over 98.1% of the possible range of total scores ( Bretz and Linenberger, 2012 ).
Reliability
Furthermore, the percent of correct responses provided by students in G-OC and OC for each GCP was compared. As shown in Fig. 3 , G-OC outperformed OC on each of the GCP prompts, providing further evidence for the instrument's ability to distinguish student knowledge on green chemistry, not just on the overall score, but also on the individual scores for each of the GCP, again providing further evidence for its construct validity.
First, the goodness of fit for the model was established using model fit indices and their respective criteria. The comparative fit index (CFI), root mean square error of approximation (RMSEA), and standardized root mean squared residual (SRMR) were calculated. Overall, the one-factor CFA model was found to exhibit a good fit: χ 2 (54, N = 448) = 103.09 ( p < 0.001), χ 2 /d f = 1.91, CFI = 0.961, RMSEA = 0.045, SRMR = 0.037, therefore, establishing the construct validity of the scores obtained from the ASK-GCP.
After confirming a good model fit, the statistical significance and salience of each of the GCP was assessed and are illustrated in Table 3 . All items were found to exhibit statistical significance ( p < 0.001) with nine of the twelve GCP exhibiting salience and the remaining three GCP are near salience, thus indicating that most are sufficiently measuring the construct of interest. The three GCP (#9, 11, 12) that approached salience warrant future investigation.
A one-way repeated measures ANOVA was used to assess the statistical significance of the impact of the two interventions and the ability of the instrument to detect the change. While a statistically significant difference was observed, F (3, 40) = 8.62, p = 0.0016, the post hoc analysis indicated significant differences between pre-test 1 and post-test 2, pre-test 1 and pre-test 3, and pre-test 1 and post-test 4. There was no significant difference measured between pre-test 3 and post-test 4. Due to the small sample size consisting of less than 20 participants, Hedges g , which is the unbiased standardized mean difference between pre- and post-test, was used to calculate the effect size for each of the learning gains ( Lakens, 2013 ). The effect size of the learning gain for Intervention 1 ( g = 1.06) indicated that on average student scores increased by one standard deviation upon completion of the activity. However, the effect size of the learning gain for Intervention 2 ( g = 0.35) indicated that on average student scores only increased by approximately one-third of a standard deviation. Thus, the non-significance of the learning gain for Intervention 2 can be attributed to the smaller effect size which is likely due to the students having prior exposure to the green chemistry principles in the first semester.
Psychometric analysis using classical test theory
Item difficulty refers to the proportion of respondents who correctly answered the item. Thus, within CTT, items with high item difficulty values indicate an easy item since a larger proportion of respondents correctly answered the item, whereas items with low difficulty values indicate more difficult items. In terms of item difficulty, items with a difficulty of 0 or 1 exhibit no discrimination because all students either correctly or incorrectly answered the item ( Kline, 2005 ). This was observed for items 6 and 13 in the G-OC course because all students correctly answered those items, indicating a ceiling effect of those items after green chemistry instruction. Although a ceiling effect was observed, it indicated the ability of the instrument to measure student competencies upon completion of green chemistry instruction. Item difficulty indices under 0.24 can be considered as extremely difficult and item difficulty indices greater than 0.91 can be considered extremely easy ( Downing and Yudkowsky, 2009 ; Lahner et al. , 2018 ). While 11 items were classified as extremely easy for the G-OC course, no items were classified as extremely easy for the OC or GC courses. Similarly, no items were classified as extremely difficult for the students in the G-OC course; however, four items were classified as extremely difficult for students in the OC course and seven items were classified as extremely difficult for students in the GC course. Thus, the results of this psychometric analysis provided additional evidence for the instrument's construct validity.
The discrimination index refers to an item's ability to distinguish between individuals without reference to an external criterion ( Hankins, 2008 ). Item discrimination was traditionally calculated by comparing the item difficulty of the highest achieving students on the test to that of the lowest achieving students for each of the items. However, it is now typically calculated as a point-biserial correlation, which is the correlation between the item score and total test score ( Meyer, 2014 ). Items with higher discrimination indexes have a greater percentage of high achieving students than lower achieving students answering the question correctly. Likewise, items with a negative value for their discrimination value indicate a greater portion of lower achieving students than high achieving students correctly answering the item ( Kline, 2005 ). Values close to zero indicate no discrimination ( Meyer, 2014 ). A slight inversion was observed for items 9 and 11 for the G-OC course. However, while they were inverted both exhibited discrimination values close to zero indicating minimal discrimination and had a difficulty of greater than 0.9, indicating most students in the class answered the items correctly. Point-biserial correlations less than 0.20 indicate the item can benefit from revision, between 0.20 and 0.30 indicate the item is fair, and those between 0.40 and 0.70 are considered good ( McGahee and Ball, 2009 ). As shown in Table 5 , most of the discrimination indexes indicated fair to good items for the OC and GC courses. However, lower values were observed in the G-OC course due to the ceiling effect which limited their ability to psychometrically perform well.
Rasch analysis
For Rasch analysis to be performed, the items of the instrument must exhibit unidimensionality, local independence, and monotinicity ( Kean et al. , 2018 ). As shown through the CFA analysis, the instrument's items were found to be unidimensional, thus representing the construct of green chemistry. Local independence implies that the items of a test should be unrelated, which means that performance on the item indicates only the respondent's ability and the characteristics of the item ( Kean et al. , 2018 ). Monotinicity refers to the correlation between item response and ability, such that greater responses correlate to greater ability ( Kean et al. , 2018 ). Since these criteria have been met, Rasch analysis was appropriate for analyzing the data.
Rasch analysis creates a single linear scale, the logit, for both item difficulty and person ability. Based on the model, the logit is equal to B n − D i and is defined as the natural log of the odds ratio ( Ludlow and Haley, 2016 ). An item that has average difficulty will have a logit unit of zero since within the Rasch model the mean item difficulty is set to zero logit units ( Bond and Fox, 2013 ; Lutter et al. , 2019 ).
The ability of the model to predict student responses based on ability and difficulty estimates are described by the fit statistics. Through fit statistics, items that produce unexpected student responses or students who provide unexpected answers can be identified ( Wren and Barbera, 2014 ). Therefore, psychometric estimates of the items, including infit mean-square (MNSQ), outfit MNSQ, and item difficulty measures were calculated and are reported in Table 6 .
Infit and outfit were calculated to measure how well the data fit the Rasch model. Infit and outfit statistics have an expected value of 1 and are always positive in value ( Meyer, 2014 ). Values greater than one indicate underfit because the data contains more variation than expected by the Rasch model; likewise, items with infit and outfit less than one indicate that the responses are too consistent with what is expected from the model. Infit and outfit statistics between 0.5 and 1.5 are considered productive for measurement, whereas a range between 0.8 and 1.2 is recommended for high-stakes tests ( Bond and Fox, 2013 ; Meyer, 2014 ; Wren and Barbera, 2014 ). All items exhibited acceptable infit and outfit statistics with ranges of 0.87–1.10 and 0.81–1.22, respectively, thus indicating the items functioned well for students within the ability range of the item.
Furthermore, the item difficulty measure in Rasch analysis indicates how difficult an item is; the lower the negative number, the easier the item ( Wren and Barbera, 2014 ). Thus, the easiest three items were items 11 (GCP 12), 1 (GCP 4), and 19 (GCP 11), respectively, whereas the hardest three items were items 16 (GCP 11), 12 (GCP 9), and 2 (GCP 2), respectively. Surprisingly, GCP 11 was identified as having one of the easiest and hardest items.
Rasch analysis was also used to provide further validation of the ASK-GCP instrument through assessment of its item reliability and person reliability, which is illustrated in Table 7 . Item reliability indicates the extent to which items represent a range of difficulty for a single variable; whereas person reliability indicates whether the instrument is able to discriminate across the ability range of the participants ( Connor and Shultz, 2018 ). The item separation reliability (0.99) indicated that the instrument's items represented a single well-defined variable and also provided evidence for the reliability of the location of the items on the scale. Further, it indicated that the local independence assumption for the data was valid ( Arias González et al. , 2015 ). A lower person separation (separation index <2, reliability <0.8) was observed, indicating the instrument may not be sensitive enough to distinguish between high and low achievers. However, the reliability was greater than 0.5, indicating that it may be able to discriminate respondents into two levels. This differentiation between groups was observed in both the repeated measures evaluation and between known-groups validation. This lower person separation can be attributed to the selected sample of OC and GC students not being exposed to green chemistry and thus having a narrow range of abilities. However, a good item separation was observed (separation index >3, reliability >0.9), indicating that the person sample was large enough to evaluate the item difficulty hierarchy ( Linacre, 2021 ).
Within Rasch analysis, Wright maps are used to illustrate the relationship between student ability and item difficulty. A key feature of Wright maps is that item difficulty uses the same linear scale—logits—as the person measure of student ability ( Boone, 2016 ). Fig. 4 illustrates the Wright map generated for this study. The right side of the map illustrates the distribution of student ability, whereas the left side of the map illustrates the item difficulties. The items are arranged by GCP so that comparisons between the true and false item for each principle can be observed. The difficulty of items ranged between −2 and 2 logits, whereas the ability of respondents ranged between −3 and 2 logits. Items with difficulty measures that are close to the average student ability will have high item reliability estimates. Thus, items close to the center of the scale such as items 7, 23, and 20 will exhibit the most reliable measures when compared to items such as 11 and 16, which are located at the extremes of the scale ( Wren and Barbera, 2014 ). The Wright map provided a range of information about the data. First, it provided further evidence that the range of student ability was approximately normally distributed. Further, it provided evidence that item difficulty approximately matched student ability, thus indicating that the instrument is an appropriate tool for measuring student knowledge. Finally, it illustrated that the items with the correct answer of false are distributed in the higher ability region (upper region) whereas true items corresponded to lower ability (bottom region). This corresponded with the findings from the item difficulties calculated using CTT that indicated that the false items had greater difficulties.
Implications for practice
Because we have evaluated and provided difficulties for each item, instructors who are concerned with the ceiling effect can remove items 6 and 13 when utilizing the assessment or select items so that approximately two-thirds of the instrument have false as a correct response since the false items were found to be more difficult. This is supported by research that indicates that false items are more able to discriminate between students and should be included in a higher proportion of up to 67% of the items ( Frisbie, 1974 ; Frisbie and Becker, 1991 ).
This instrument has also been shown to be sensitive to student learning gains, even in two-fold interventions. Therefore, it is well-suited for use in measuring learning gains as a pre-post assessment or for measuring differences between treatment and control groups, including multi-tiered studies with various treatments. It can also be used to initially screen for student knowledge of green chemistry for qualitative studies to ensure students of diverse knowledge levels are selected.
This instrument was administered online, so has been shown to provide valid and reliable data through remote instruction. It has been demonstrated by Nissen et al. (2018) and Lewis (2020) that the method of administration method of administration may not impact the results of the assessment. Therefore, as we transition back into in-person instruction future work examining whether the instrument works consistently in person would be beneficial.
When evaluating the results of this instrument, one must remember that due to the nature of true–false items, it cannot provide complete insight into student conceptions, misconceptions, or reasoning. Furthermore, it aims to test student knowledge of green chemistry rather than the application of that knowledge. Therefore, other more open-ended questions on green chemistry, such as asking students to compare the greenness of two reactions, should be used in tandem with this prompt to gain further insights into student knowledge and reasoning structures.
Limitations
Impact of student guessing, differential item functioning, limited evidence for response process validity, single mode of administration, instrument assessed at only one institution, conclusions, conflicts of interest, acknowledgements.
- ACS Green Chemistry Institute, (2021), 12 Principles of Green Chemistry .
- AERA, APA, and NCME, (2014), Standards for Educational and Psychological Testing , American Educational Research Association.
- Andraos J. and Dicks A. P., (2012), Green chemistry teaching in higher education: A review of effective practices, Chem. Educ. Res. Pract. , 13 (2), 69–79.
- Arias González V. B., Crespo Sierra M. T., Arias Martínez B., Martínez-Molina A., and Ponce F. P., (2015), An in-depth psychometric analysis of the Connor–Davidson resilience scale: Calibration with Rasch–Andrich model, Health Qual. Life Outcomes , 13 (1).
- Arjoon J. A., Xu X. and Lewis J. E., (2013), Understanding the state of the art for measurement in chemistry education research: Examining the psychometric evidence, J. Chem. Educ. , 90 (5), 536–545.
- Armstrong L. B., Rivas M. C., Douskey M. C. and Baranger A. M., (2018), Teaching students the complexity of green chemistry and assessing growth in attitudes and understanding, Curr. Opin. Green Sustain. Chem. , 13 , 61–67.
- Armstrong L. B., Rivas M. C., Zhou Z., Irie L. M., Kerstiens G. A., Robak M. A. T., et al. , (2019), Developing a green chemistry focused general chemistry laboratory curriculum: What do students understand and value about green chemistry? J. Chem. Educ. , 96 (11), 2410–2419.
- Balabanoff M., Fulaiti H. Al, DeKorver B. K., Mack M. and Moon A., (2021), Development of the water instrument: A comprehensive measure of students’ knowledge of fundamental concepts in general chemistry, Chem. Educ. Res. Pract .
- Barbera J., (2013), A psychometric analysis of the chemical concepts inventory, J. Chem. Educ. , 90 (5), 546–553.
- Bichi A. A., Embong R., Talib R., Salleh S. and Ibrahim A. B., (2019), Comparative analysis of classical test theory and item response theory using chemistry test data, Int. J. Eng. Adv. Technol. , 8 (5), 2249–8958.
- Bond T. G. and Fox C. M., (2013), Applying the Rasch Model: Fundamental Measurement in the Human Sciences , Routledge.
- Boone W. J., (2016), Rasch analysis for instrument development: Why, when, and how? CBE Life Sci. Educ. , 15 (4).
- Brandriet A., Reed J. J. and Holme T., (2015), A historical investigation into item formats of acs exams and their relationships to science practices, J. Chem. Educ. , 92 (11), 1798–1806.
- Bretz S. L. and Linenberger K. J., (2012), Development of the enzyme–substrate interactions concept inventory, Biochem. Mol. Biol. Educ. , 40 (4), 229–233.
- Cannon A. S., Keirstead A. E., Hudson R., Levy I. J., MacKellar J., Enright M., et al. , (2020), Safe and sustainable chemistry activities: Fostering a culture of safety in K-12 and community outreach programs, J. Chem. Educ. , 98 (1), 71–77.
- Chen M., Jeronen E. and Wang A., (2020), What lies behind teaching and learning green chemistry to promote sustainability education? A literature review, Int. J. Environ. Res. Public Health , 17 (21), 1–24.
- Connor M. C. and Shultz G. V., (2018), Teaching assistants’ topic-specific pedagogical content knowledge in 1H NMR spectroscopy, Chem. Educ. Res. Pract. , 19 (3), 653–669.
- Couch B. A., Hubbard J. K. and Brassil C. E., (2018), Multiple-true–false questions reveal the limits of the multiple-choice format for detecting students with incomplete understandings, Bioscience , 68 (6), 455–463.
- Deng J. M., Streja N. and Flynn A. B., (2021), Response process validity evidence in chemistry education research, J. Chem. Educ. , 98 (12), 3656–3666.
- Ding L. and Beichner R., (2009), Approaches to data analysis of multiple-choice questions, Phys. Rev. Spec. Top.: Phys. Educ. Res. , 5 (2), 020103.
- Downing S. M. and Yudkowsky R., (2009), Assessment in health professions education , Routledge.
- Dutke S. and Barenberg J., (2015), Easy and informative: Using confidence-weighted true–false items for knowledge tests in psychology courses, Psychol. Learn. Teach. , 14 (3), 250–259.
- Ebel R. L., (1970), The case for true–false test items, Am. J. Educ. , 78 (3), 373–389.
- Fahmy A. and Lagowski J., (2012), Systemic assessment as a new tool for assessing students learning in chemistry using SATL methods: Systemic true false [STFQs] and systemic sequencing [SSQs] question types, Afr. J. Chem. Educ. , 2 (2), 66–78.
- Fan X., (1998), Item response theory and classical test theory: An empirical comparison of their item/person statistics, Educ. Psychol. Meas. , 58 (3), 357–381.
- Ferguson G. A., (1949), On the theory of test discrimination, Psychometrika , 14 (1), 61–68.
- Frisbie D. A., (1974), The effect of item format on reliability and validity: A study of multiple choice and true–false achievement tests, Educ. Psychol. Meas. , 34 (4), 885–892.
- Frisbie D. A. and Becker D. F., (1991), An analysis of textbook advice about true–false tests, Appl. Meas. Educ. , 4 (1), 67–83.
- Fritz M. F., (1927), Guessing in a true–false test, J. Educ. Psychol. , 18 (8), 558–561.
- Galgano P. D., Loffredo C., Sato B. M., Reichardt C. and Seoud O. A. E., (2012), Introducing education for sustainable development in the undergraduate laboratory: quantitative analysis of bioethanol fuel and its blends with gasoline by using solvatochromic dyes, Chem. Educ. Res. Pract. , 13 (2), 147–153.
- Green & Sustainable Chemistry Education Module Development Project , (2021).
- Grieger K. and Leontyev A., (2021), Student-generated infographics for learning green chemistry and developing professional skills, J. Chem. Educ. , 98 (9), 2881–2891.
- Grieger K. and Leontyev A., (n.d.), Teaching green chemistry though student-generated open educational resources, J. Coll. Sci. Teach. , in press.
- Hankins M., (2008), How discriminating are discriminative instruments? Health Qual. Life Outcomes , 6 , 36.
- Hays R. D. and Reeve B. B., (2008), Measurement and modeling of health-related quality of life, Int. Encycl. Public Heal. , 241–252.
- He P., Liu X., Zheng C. and Jia M., (2016), Using Rasch measurement to validate an instrument for measuring the quality of classroom teaching in secondary chemistry lessons, Chem. Educ. Res. Pract. , 17 (2), 381–393.
- Heaton A., Hodgson S., Overton T. and Powell R., (2006), The challenge to develop CFC (chlorofluorocarbon) replacements: A problem based learning case study in green chemistry, Chem. Educ. Res. Pract. , 7 (4), 280–287.
- Holme T. A., MacKellar J., Constable D. J. C., Michels O. R., Trate J. M., Raker J. R. and Murphy K. L., (2020), Adapting the anchoring concepts content map (ACCM) of ACS exams by incorporating a theme: Merging green chemistry and organic chemistry, J. Chem. Educ. , 97 (2), 374–382.
- Jabrayilov R., Emons W. H. M. and Sijtsma K., (2016), Comparison of classical test theory and item response theory in individual change assessment, Appl. Psychol. Meas. , 40 (8), 559–572.
- Kean J., Bisson E. F., Brodke D. S., Biber J. and Gross P. H., (2018), An introduction to item response theory and Rasch analysis: Application using the eating assessment tool (EAT-10), Brain Impair. , 19 (1), 91–102.
- Kendhammer L. K. and Murphy K. L., (2014), General statistical techniques for detecting differential item functioning based on gender subgroups: A comparison of the Mantel-Haenszel procedure, IRT, and logistic regression, ACS Symp. Ser. , 1182 , 47–64.
- Kinney L. B. and Eurich A. C., (1933), Studies of the true–false examination, Psychol. Bull. , 30 (7), 505–517.
- Kline T. J. B., (2005), Classical test theory: Assumptions, equations, limitations, and item analysis, in psychological testing: A practical approach to design and evaluation, Shaw L. C., Crouppen M., Hoffman C. A. and Weight B. (ed.), SAGE Publications, Inc.
- Krabbe P. F. M., (2017), Chapter 7 – Validity, in The Measurement of Health and Health Status , Academic Press, pp. 113–134.
- Lahner F.-M., Lörwald A. C., Bauer D., Nouns Z. M., Krebs R., Guttormsen S., et al. , (2018), Multiple true–false items: A comparison of scoring algorithms, Adv. Heal. Sci. Educ. , 23 (3), 455–463.
- Lakens D., (2013), Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t -tests and ANOVAs, Front. Psychol. , 4 (NOV), 863.
- Lasker G. A., (2019), Connecting systems thinking and service learning in the chemistry classroom, J. Chem. Educ. , 96 (12), 2710–2714.
- Lee E. N. and Orgill M., (2021), Toward equitable assessment of english language learners in general chemistry: Identifying supportive features in assessment items, J. Chem. Educ .
- Leontyev A., Pulos S. and Hyslop R., (2017), Making the most of your assessment: Analysis of test data in jMetrik, ACS Symp. Ser. , 1260 , 49–64.
- Lewis S. E., (2020), Chemistry assessments through the sudden implementation of online instruction, J. Chem. Educ. , 97 (9), 3418–3422.
- Linacre J. M., (2021), Reliability and separation of measures, Winsteps .
- Loevinger J., (1957), Objective tests as instruments of psychological theory: Monograph supplement 9, Psychol. Rep. , 3 (7), 694.
- Lu S. and Bi H., (2016), Development of a measurement instrument to assess students’ electrolyte conceptual understanding, Chem. Educ. Res. Pract. , 17 (4), 1030–1040.
- Lu H., Jiang Y. and Bi H., (2020), Development of a measurement instrument to assess students’ proficiency levels regarding galvanic cells, Chem. Educ. Res. Pract. , 21 (2), 655–667.
- Ludlow L. H. and Haley S. M., (2016), Rasch model logits: Interpretation, use, and transformation, Educ. Psychol. Meas. , 55 (6), 967–975.
- Lutter J. C., Hale L. V. A. and Shultz G. V., (2019), Unpacking graduate students’ knowledge for teaching solution chemistry concepts, Chem. Educ. Res. Pract. , 20 (1), 258–269.
- Magno C., (2009), Demonstrating the difference between classical test theory and item response theory using derived test data, Int. J. Educ. Psychol. Assess. , 1 (1), 1–11.
- Marques C. A., Marcelino L. V., Dias É. D. S., Rüntzel P. L., Souza L. C. A. B. and Machado A., (2020), Green chemistry teaching for sustainability in papers published by the Journal of Chemical Education, Quim. Nova , 43 (10), 1510–1521.
- McCullough T., (1993), A second look at true–false questions, J. Chem. Educ. , 70 (10), 829.
- McGahee T. W. and Ball J., (2009), How to read and really use an item analysis, Nurse Educ. , 34 (4), 166–171.
- Meyer J. P., (2014), Applied Measurement with jMetrik , Routledge.
- Mishra P., Pandey C. M., Singh U., Gupta A., Sahu C. and Keshri A., (2019), Descriptive statistics and normality tests for statistical data, Ann. Card. Anaesth. , 22 (1), 72.
- Nedungadi S., Paek S. H. and Brown C. E., (2019), Utilizing Rasch analysis to establish the psychometric properties of a concept inventory on concepts important for developing proficiency in organic reaction mechanisms, Chem. Teach. Int. , 2 (2).
- Nissen J. M., Jariwala M., Close E. W. and Dusen B. V., (2018), Participation and performance on paper- and computer-based low-stakes assessments, Int. J. STEM Educ. , 5 (1), 1–17.
- Oosterhof A. C. and Glasnapp D. R., (1974), Comparative reliabilities and difficulties of the multiple-choice and true—false formats, J. Exp. Educ. , 42 (3), 62–64.
- Pentecost T. C. and Barbera J., (2013), Measuring learning gains in chemical education: A comparison of two methods, J. Chem. Educ. , 90 (7), 839–845.
- Płotka-Wasylka J., Kurowska-Susdorf A., Sajid M., de la Guardia M., Namieśnik J. and Tobiszewski M., (2018), Green chemistry in higher education: State of the art, challenges, and future trends, ChemSusChem , 11 (17), 2845–2858.
- Polit D. F. and Beck C. T., (2006), The content validity index: Are you sure you know what's being reported? Critique and recommendations. Res. Nurs. Health , 29 (5), 489–97.
- Price R. M., Andrews T. C., McElhinny T. L., Mead L. S., Abraham J. K., Thanukos A. and Perez K. E., (2014), The Genetic Drift Inventory: A Tool for Measuring What Advanced Undergraduates Have Mastered about Genetic Drift, CBE Life Sci. Educ. , 13 (1), 65.
- Progar Š. and Sočan G., (2008), An empirical comparison of item response theory and classical test theory – PsycNET, Psihol. Obz./Horiz. Psychol. , 17 (3), 5–24.
- Şahin A. and Anıl D., (2017), The effects of test length and sample size on item parameters in item response theory, Educ. Sci. Theory Pract. , 17 (1), 321–335.
- Savec V. F. and Mlinarec K., (2021), Experimental work in science education from green chemistry perspectives: A systematic literature review using PRISMA, Sustain. 2021 , 13 , 12977.
- Schönborn K. J., Höst G. E. and Palmerius K. E. L., (2015), Measuring understanding of nanoscience and nanotechnology: development and validation of the nano-knowledge instrument (NanoKI). Chem. Educ. Res. Pract. , 16 (2), 346–354.
- Sorenson B. and Hanson K., (2021), Using classical test theory and rasch modeling to improve general chemistry exams on a per instructor basis, J. Chem. Educ. , 98 (5), 1529–1538.
- Taskin V., Bernholt S. and Parchmann I., (2015), An inventory for measuring student teachers’ knowledge of chemical representations: Design, validation, and psychometric analysis, Chem. Educ. Res. Pract. , 16 (3), 460–477.
- Thorndike R. M. and Thorndike-Christ T., (2010), Qualities Desired in Any Measurement Procedure: Reliability, in Measurement and Evaluation in Psychology and Education , Pearson Education, Inc., pp. 118–153.
- Wren D. and Barbera J., (2014), Psychometric analysis of the thermochemistry concept inventory, Chem. Educ. Res. Pract. , 15 (3), 380–390.
- Zamanzadeh V., Ghahramanian A., Rassouli M., Abbaszadeh A., Alavi-Majd H. and Nikanfar A.-R., (2015), Design and implementation content validity study: Development of an instrument for measuring patient-centered communication, J. Caring Sci. , 4 (2), 165.
- Zuin V. G., Eilks I., Elschami M. and Kümmerer K., (2021), Education in green chemistry and in sustainable chemistry: Perspectives towards sustainability. Green Chem. , 23 (4), 1594–1608.
- Environmental Chemistry
- What is Green Chemistry
What is Green Chemistry?
Green chemistry (sometimes referred to as sustainable chemistry) is the branch of chemistry that deals with the design and optimization of processes and products in order to lower, or remove altogether, the production and use of toxic substances. Green chemistry is not the same as environmental chemistry.
The former focuses on the environmental impact of chemistry and the development of sustainable practices that are environment-friendly (such as a reduction in the consumption of non-renewable resources and strategies to control environmental pollution ). The latter focuses on the effects that certain toxic or hazardous chemicals have on the environment.
The 12 Key Principles of Green Chemistry
The twelve principles put forward by the American chemists Paul Anastas and John Warner in the year 1998 to lay the foundation for green chemistry are listed below.
- Prevention of waste: Preventing the formation of waste products is always preferable to the clean-up of the waste once it is generated.
- Atom economy: The synthetic processes and methods that are devices through green chemistry must always try to maximise the consumption and incorporation of all the raw materials into the final product. This must strictly be followed in order to minimise the waste generated by any process.
- Avoiding the generation of hazardous chemicals: Reactions and processes that involve the synthesis of certain toxic substances that pose hazards to human health must be optimised in order to prevent the generation of such substances.
- The design of safe chemicals: During the design of chemical products that accomplish a specific function, care must be taken to make the chemical as non-toxic to humans and the environment as possible.
- Design of safe auxiliaries and solvents: The use of auxiliaries in processes must be avoided to the largest possible extent. Even in the circumstances where they absolutely need to be employed, they must be optimized to be as non-hazardous as possible.
- Energy efficiency: The amount of energy consumed by the process must be minimized to the maximum possible extent.
- Incorporation of renewable feedstock: The use of renewable feedstock and renewable raw materials must be preferred over the use of non-renewable ones.
- Reduction in the generation of derivatives: The unnecessary use of derivatives must be minimalized since they tend to require the use of additional reagents and chemicals, resulting in the generation of excess waste.
- Incorporation of Catalysis: In order to reduce the energy requirements of the chemical reactions in the process, the use of chemical catalysts and catalytic reagents must be advocated.
- Designing the chemicals for degradation: When designing a chemical product in order to serve a specific function, care must be taken during the design process to make sure that the chemical is not an environmental pollutant. This can be done by making sure that the chemical breaks down into non-toxic substances.
- Incorporating real-time analysis: Processes and analytical methodologies must be developed to the point that they can offer real-time data for their monitoring. This can enable the involved parties to stop or control the process before toxic/dangerous substances are formed.
- Incorporation of safe chemistry for the prevention of accidents: While designing chemical processes, it is important to make sure that the substances that are used in the processes are safe to use. This can help prevent certain workplace accidents, such as explosions and fires. Furthermore, this can help develop a safer environment for the process to take place in.
Examples of the Impact of Green Chemistry
Use of green solvents.
Many chemical synthesis reactions that are carried out on an industrial scale require large amounts of chemical solvents. Furthermore, these solvents are also used industrially for degreasing and cleaning purposes. However, many traditional solvents that have been used for such purposes in the past are known to be toxic to human beings. Some such solvents are also known to be chlorinated.
Click here to learn about the different examples of solvents .
The advancement of green chemistry has brought many alternatives to these toxic solvents. The green solvents that are coming up as alternatives are known to be derived from renewable sources and are also known to be biodegradable. Thus, green chemistry has great potential to lower the toxicity of certain industrial environments by developing safer alternatives.
Development of Specialised Synthetic Techniques
The development of specialised synthetic techniques can optimise processes in order to make them more environmentally friendly by making them adhere to the principles of green chemistry. An important example of such an enhanced synthetic technique is the development of the olefin metathesis reaction in the field of organic chemistry. This reaction, developed by Robert Grubbs, Richard Schrock, and Yves Chauvin, won the Nobel Prize for Chemistry in the year 2005.
Other notable developments brought forward by advancements in green chemistry include:
- The employment of supercritical carbon dioxide as a green solvent (as an alternative to other toxic solvents).
- Incorporating the use of hydrogen in enantioselective synthesis reactions (also known as asymmetric synthesis).
- Incorporating aqueous solutions of hydrogen peroxide (a chemical compound with the formula H 2 O 2 ) to drive relatively clean oxidation reactions.
Other notable applications of green chemistry include supercritical water oxidation (often abbreviated to SCWO), dry media reactions (also known as solid-state reactions and solvent fewer reactions), and on water reactions.
Production of Hydrazine
Initially, the most popular method for the production of hydrazine (an inorganic chemical compound with the chemical formula N 2 H 4 ) was the Olin Raschig process, which involved the use of ammonia and sodium hypochlorite. However, with the development of green chemistry, a more environment-friendly alternative to this process was discovered.
In the peroxide process for the production of hydrazine, ammonia is reacted with hydrogen peroxide. In this alternate method, water is produced as the only side product. It can also be noted that the peroxide process does not require any auxiliary extracting solvents.
To learn more about green chemistry and other important branches of chemistry such as stereochemistry , register with BYJU’S and download the mobile application on your smartphone.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Chemistry related queries and study materials
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
Good experience
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

Green Chemistry
Green chemistry, also known as sustainable chemistry, is a philosophy of chemical research and executive that encourages the structure of products as well as processes that minimize the use and generation of hazardous substances. Whereas environmental chemistry will be the chemistry of the natural environment, and of pollutant substances in nature, green chemistry seeks to relieve the negative impact of chemistry for the environment by avoiding pollution at its source and making use of fewer natural sources.
Named Reaction
Assignment on fragile x syndrome, assignment on market preparation of metformin hcl xr tablets, disorder of nervous system, biography of george wells beadle, job application and cover letter format for female secretary, about economic growth, annual report 2003-2004 of bajaj auto limited, biography of bob hayes, baaishakhi mela, latest post, mid-ocean ridge (mor), harnessing hydrogen at the genesis of life, ngc 5728’s faint characteristics are exposed, astronomers discover the oldest black hole ever observed, atomic hydrogen welding, variable-frequency transformer (vft).

IMAGES
VIDEO
COMMENTS
The present review work focuses on the importance and economic. development of green chemistry. It is new branch in chemistry dealing. with reduction of harmful a nd toxic chemicals in the ...
Designing Safer Chemicals. Chemists are molecular designers; they design new molecules and new materials. Green Chemists make sure that the things that we make not only do what they're supposed to do, but they do it safely. This means that it's not only important how chemists make something, it's also important that what they make isn't ...
As part of our ongoing efforts about organic solvent's toxicity [13], diversified use of cow related products [14,15], use of salts in synthesis [16], and green chemistry concept [17], we deducted ...
Protein Testing. Automated protein tagging technique. Tags amino acids commonly found in proteins. Employs non-toxic solutions. Generates no hazardous waste. Replaces hazardous materials and high temperatures in traditional methods. Applications in the food and pet food sectors. CEM Corporation.
The above statement defines the concept of "Green Chemistry.". The subject has now become a very well accepted and welcomed part of many chemistry curricula and industry philosophies. In fact, the concept of a triple bottom line, i.e., financial, social, and environmental owes its existence to green chemistry.
This guide provides simple steps that your ACS student chapter can take to. make safer, greener lab spaces while educating other students and faculty about green chemistry. and chemical engineering can be done. Over the years different principles have been proposed that can.
TECHNO WORLD. A T extbook of. Green Chemistr y. Sankar Prasad Dey, Ph.D. (J.U) Associate Professo r, Department of Chemistry (UG &PG), Behala College, Parnashree, Kolkata-700060, I ndia. Former ...
This definition of green chemistry is illustrated in Figure 2.5.1 2.5. 1. The practice of green chemistry begins with recognition that the production, processing, use, and eventual disposal of chemical products may cause harm when performed incorrectly. In accomplishing its objectives, green chemistry and green chemical engineering may modify ...
Green chemistry, in addition to being a science, it is also a philosophy and nearly a religion. Attendance at American Chemical Society Green Chemistry & Engineering Conferences will instill such an ideal into any attendant because of the nearly universal appeal and possibilities in this novel approach to radicalizing the business of doing science and engineering.
Green chemistry as a discipline is gaining increasing attention globally, with environmentally conscious students keen to learn how they can contribute to a safer and more sustainable world. Many universities now offer courses or modules specifically on green chemistry - Green Chemistry: Principles and Case Studies is an essential learning ...
The Twelve Principles of Green Chemistry, continued 6) Energy requirements should be recognized for their environmental and economic impacts and should be minimized. 7) A raw material feedstock should be renewable rather than depleting whenever technically and economically practical.
Green Chemistry. T.A. Lewandowski, in Encyclopedia of Toxicology (Third Edition), 2014 Abstract. The goal of green chemistry (GC) is the design (or redesign) of products and manufacturing processes to reduce their impact on human health and the environment. Fundamental to the GC concept is the idea of sustainability - reducing environmental impacts and conserving natural resources for future ...
The twelve principles of green chemistry provide a framework for scientists and engineers to follow when designing new products or improving existing materials and processes. Waste prevention: Instead of treating or cleaning up waste in the end, prevent it from being made. Atom economy: Incorporate as much of the starting materials (atoms) into ...
Chemistry is seen as a 'polluter,' which partly accounts for its poor image among students and the general public. This activity introduces the concept of atom economy in the context of sustainable development and Green Chemistry. It aims to show that development is necessary but can be achieved in a way which limits environmental damage.
In particular, the green chemistry activities from past ACS Program-in-a-Box or Chemists Celebrate Earth Week (CCEW) events are perfect options to do virtually with your Chapter mates. Take a look at the green chemistry activities available for the following past ACS events: The Evolving Periodic Table and the Future of Energy Storage.
The green chemistry moments included applications of green chemistry principles to core curriculum concepts of the organic chemistry course. A green chemistry moment was provided via lecture for each chapter of the textbook. An example of a green chemistry moment is a discussion on the replacement of diethyl ether with 2-methyltetrahydrofuran ...
Green chemistry is a newly emerging field to design, synthesize and implement chemical products by scientists and engineers that would protect and benefit the economy, people, and our planet by finding creative and innovative methods to reduce waste, conserve energy, and discover replacements for hazardous substances.
Green chemistry is the new and rapid emerging branch of chemistry. The beginning of green chemistry is considered as a response to the need to reduce the damage of the environment by man-made ...
Green chemistry (sometimes referred to as sustainable chemistry) is the branch of chemistry that deals with the design and optimization of processes and products in order to lower, or remove altogether, the production and use of toxic substances. Green chemistry is not the same as environmental chemistry. The former focuses on the environmental ...
Green Chemistry assignment week-Video#2 Design for Energy Efficiency youtu/obnN_gO8sbg In this video Julia and David explain the concept. Watch and answer the question below. 3. Why is energy a factor in chemical processes and how can synthetic processes achieve energy efficiency? Energy is a factor in chemical processes because energy ...
Green Chemistry Reaction Design. One very important component of green chemistry is the reaction design and the yield of product. Green chemistry aims to improve upon reactions in a positive, beneficial way, however if the reaction design is complicated or the yield is low, chemists and producers are unlikely to inherit the greener approach.
Assignment. Green chemistry, also known as sustainable chemistry, is a philosophy of chemical research and executive that encourages the structure of products as well as processes that minimize the use and generation of hazardous substances. Whereas environmental chemistry will be the chemistry of the natural environment, and of pollutant ...
Assignment - Green Chemistry - Free download as Word Doc (.doc), PDF File (.pdf), Text File (.txt) or read online for free. green chemistry and its influence on sustainable development