An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Singapore Med J
- v.60(3); 2019 Mar

Developmental delay: identification and management at primary care level
Ying ying choo.
1 SingHealth Polyclinics – Sengkang, Singapore
Pratibha Agarwal
2 Department of Paediatrics, Child Developmental Service, KK Women’s and Children’s Hospital, Singapore
Choon How How
3 Care and Health Integration, Changi General Hospital, Singapore
4 Family Medicine Academic Clinical Programme, SingHealth Duke-NUS Academic Medical Centre, Singapore
Sita Padmini Yeleswarapu
Associated data.
Developmental delays are common in childhood, occurring in 10%–15% of preschool children. Global developmental delays are less common, occurring in 1%–3% of preschool children. Developmental delays are identified during routine checks by the primary care physician or when the parent or preschool raises concerns. Assessment for developmental delay in primary care settings should include a general and systemic examination, including plotting growth centiles, hearing and vision assessment, baseline blood tests if deemed necessary, referral to a developmental paediatrician, and counselling the parents. It is important to follow up with the parents at the earliest opportunity to ensure that the referral has been activated. For children with mild developmental delays, in the absence of any red flags for development and no abnormal findings on clinical examination, advice on appropriate stimulation activities can be provided and a review conducted in three months’ time.
Jason, a three-year-old boy, was brought by his mother to your clinic to seek advice regarding his speech delay. She was concerned because his preschool teachers had told her that he seemed to be slow in speech compared to his classmates. Jason had a vocabulary of about 10–12 words and had not started speaking in phrases. He could respond to his name and followed simple instructions well. A physical examination found that he had no dysmorphic facial features and was in good health. He had attained all other developmental milestones appropriate to his age except for expressive language .
WHAT IS DEVELOPMENTAL DELAY?
Developmental delay occurs when a child does not achieve developmental milestones in comparison to peers of the same age range. The degree of developmental delay can be further classified as mild (functional age < 33% below chronological age), moderate (functional age 34%–66% of chronological age) and severe (functional age < 66% of chronological age).( 1 ) A significant delay is defined as performance that is two or more standard deviations below the mean on age-appropriate standardised norm-referenced testing (usually conducted in secondary or tertiary care settings).( 2 )
The delay can be in a single domain (i.e. isolated developmental delay) or more than one domain. A significant delay in two or more developmental domains affecting children under the age of five years is termed global developmental delay (GDD).( 3 ) Other patterns of abnormal development include: developmental disorder; developmental arrest and regression; and developmental disability. In developmental disorders, development does not follow the normal pattern, such as in a child with autism who has language abilities but is unable to use it for social interaction and communication purposes. Developmental arrest and regression refers to a normal developmental phase in a child that is followed by a failure to develop new skills or even loss of previously acquired skills. Regression is an unequivocal red flag and warrants an urgent referral to a specialist for further assessment and management. Not all children with developmental delay will have a developmental disability, which refers to severe, lifelong impairment in areas of development that affects learning, self-sufficiency and adaptive skills. Developmental delays can be transient, such as during a phase of prolonged illness, or persistent.
Variations in patterns of development
Some children may not follow the normal pattern of development. These variants include ‘bottom shufflers’, who do not crawl but shuffle around. These children tend to walk late and may be mildly hypotonic, especially in the lower limbs. Some ‘commando crawl’, while others do not go through the crawling phase at all. Children may exhibit variation in their rate of acquisition of language, social skills, play and behaviour, as they may follow a familial pattern (e.g. family history of speech delay) or be affected by environmental influences (e.g. not attending a preschool). There is a general belief that boys tend to acquire language later than girls, which has not been proven true.( 4 ) Children coming from bilingual families may seem to have delayed acquisition of one language, but they eventually catch up in the absence of any risk factors. Nevertheless, physicians should be aware of the red flags in the context of the child’s development when determining a further management plan.
HOW RELEVANT IS THIS TO MY PRACTICE?
Singapore does not have a national database to track the prevalence of developmental delays or disability. However, based on data from other developed countries, developmental delays are reported to occur in 10%–15%( 5 ) and GDD in 1%–3%( 1 , 3 ) of children under the age of five years. Various factors determine the prognosis or eventual outcome of children with developmental delay. These include the cause of the delay (i.e. is it a treatable cause, such as nutritional deficiencies), areas in which the child is delayed, how significant the delay is, the age at which the child commenced an intervention, and the extent of parental and caregiver involvement. If developmental delays are detected late, opportunities for early intervention are lost, resulting in poor outcomes such as learning difficulties, behaviour problems and functional impairments later on in life.( 6 ) There is strong research evidence suggesting that effective early identification of developmental delays and timely early intervention can positively alter a child’s long-term trajectory.( 7 )
Primary care physicians play a significant role in early identification of developmental delays, both through developmental screening and routine developmental surveillance. Hence, it is essential that they have the knowledge and skills to identify developmental delays and provide an appropriate management plan to the family, including counselling the parents if necessary.
WHAT CAUSES DEVELOPMENTAL DELAY?
Multiple causes or illnesses can contribute to developmental delay. The causes listed in Box 1 are not exhaustive but cover most of the common aetiologies. These can be broadly divided into four categories: prenatal; perinatal; postnatal; and other causes.

Common aetiologies of developmental delay:( 2 , 8 )
Studies evaluating the causes of GDD have indicated that in one-third of the cases, the cause can be established through history and examination alone, and in another one-third, through a thorough clinical evaluation prompting investigations. The remaining cases can be identified through investigations alone.( 9 )
HOW DO I IDENTIFY DEVELOPMENTAL DELAY?
In primary care settings, children with developmental delays are normally identified through three major channels: during routine developmental surveillance or screening; following parental concern; and after third parties such as preschool teachers or nursery care professionals raise concerns. The child’s health booklet is a useful resource that should be wisely utilised by parents and clinicians to monitor a child’s development. An important step to improve the early identification of developmental delay is educating the parent to make use of the health booklet’s developmental checklist. Other details regarding developmental surveillance and screening have been discussed in our previous article on developmental assessment.( 10 )
Common barriers to early identification
Apart from the aforementioned barriers of lack of time, resources and training, the primary care physician’s referral to a specialist for further assessment and management may not be activated by the parent. This could be due to parents disagreeing with the referral, denial, lack of understanding of the significance of the referral or the family’s social circumstances (e.g. single parenthood, lack of financial resources), preventing the children from accessing further care.
WHAT CAN I DO IN MY PRACTICE?
During each consultation, the primary care physician should encourage the parents to share any concerns they might have about their child’s development or behaviour, conduct an opportunistic evaluation (developmental surveillance) and ensure that the child has attended developmental screening at the prescribed touch points. Based on the consultation, a decision can be made to review again, refer further or discharge. For children presenting with mild developmental delay, in the absence of any red flags and no abnormality detected on clinical examination, parents can be advised about appropriate stimulation activities and a review conducted in three months’ time, especially if earlier milestones were achieved. For example, an 18-month-old child may present with concerns of expressive language delay, as he has only started saying a few single words with meaning. In the absence of any other concerns (e.g. the child has good eye contact and joint attention, with no behaviour concerns), advice on language stimulation activities could be given. In children presenting with significant developmental delays or with a history of regression in development, and those at risk for developmental delays, a prompt referral should be made to a developmental paediatrician.
In cases where delays have been identified, but there is parental denial, consider arranging a follow-up appointment to conduct a more detailed developmental assessment. The functional impact of the child’s developmental delay should be explained to the parents. For example, if a child is identified with a fine motor delay, the possible impact on adaptive skills should be explored. When a consultation is pitched at the parental level of understanding, there is a better chance of acceptance. A lower referral threshold is advisable for children who are at high risk for developmental problems, such as preterm children (without follow-up), children with chronic medical conditions, and children in challenging circumstances (e.g. being in the care system or having a main caregiver with mental health problems). Primary care physicians should follow up with the parents at the next visit to ensure that the referral has been activated.
Furthermore, consistent long-term emotional and practical support should be provided to the families of children with special needs. As this group is at high risk for caregiver stress, consider evaluating stress levels at each opportunistic visit, as well-being has an impact on their capability to look after their children.( 11 ) The family can be referred to a Family Service Centre for further support if necessary. Other assessments and investigations include:
- Head-to-toe examination, including plotting the child’s weight, height and occipitofrontal circumference;
- Hearing assessment if there are concerns about hearing (e.g. poor response to name when called) and language delay;
- Vision assessment if the child (≥ 6 weeks) is not fixing and following, has a history of frequent bumping into objects (for a mobile child), or may have delayed fine motor skills; and
- Full blood count (possible iron deficiency), bone mineral profile and vitamin D levels (if rickets are suggested), thyroid function tests (especially for children with GDD and growth problems), urea levels and electrolyte levels.
For preschoolers in Singapore, the two main Child Developmental Units (CDUs) are the Department of Child Development at KK Women’s and Children’s Hospital and the Child Development Unit at National University Hospital. If a child is referred to a CDU, a developmental assessment is conducted, and investigations are tailored based on the clinical evaluation. Apart from the aforementioned tests, these could include: genetic evaluation; creatine phosphokinase test; screening for inborn errors of metabolism; TORCH (toxoplasmosis, rubella cytomegalovirus, herpes simplex and HIV) screen; neuroimaging; and electroencephalography ( Box 2 ).

Additional tests for children referred to a Child Development Unit
Services for children presenting with developmental delays
Following further specialist assessment, children can be referred to appropriate therapies, such as speech language therapy, physiotherapy, occupational therapy and behavioural intervention (e.g. psychologist). Children who could benefit from intensive and long-term interventions, such as those presenting with GDD, are referred to EIPIC (Early Intervention Programme for Infants and Children) centres during their preschool years. Some children with developmental delays may require cognitive testing (e.g. IQ testing) and assessment of adaptive functioning at about six years of age. This would guide appropriate school placement if they are deemed more suitable for a special education school. Children in mainstream schools who continue to present with developmental delays may need ongoing therapy services provided in a hospital or private setting.
TAKE HOME MESSAGES
- Developmental delays are common and can involve either a single domain or multiple domains of the child’s functioning.
- Early identification of developmental delays and appropriate management can positively alter the child’s developmental trajectory.
- Primary care physicians play a pivotal role in early identification of developmental delays through developmental screening and surveillance.
- For children presenting with mild developmental delays and in the absence of any red flags, appropriate stimulation activities can be suggested, with close monitoring of the child.
- There should be a low threshold for specialist referral for children at high risk for developmental problems, such as those who are in care, have an underlying chronic medical condition, or have a primary caregiver with a mental health problem.
You obtained more relevant history from Jason's mother. She said he was full term and had an uneventful neonatal period. Jason had passed his newborn hearing test and developmental screening at the previous touch points. He had never been hospitalised or exposed to any ototoxic agents, and had no history of any significant infections such as meningitis. You explained that Jason's expressive language seemed to be delayed. At three ye rs of age, he should be able to speak in three- to four-word sentences and have speech that is intelligible to trangers. Because of the expressive language delay, you recommended a referral to a Child Developmental Unit for a hearing assessment as well as further evaluation of speech and language .

KIRSTEN VITRIKAS, MD, DILLON SAVARD, MD, AND MERIMA BUCAJ, DO
Am Fam Physician. 2017;96(1):36-43
Author disclosure: No relevant financial affiliations.
An estimated 15% of children in the United States have at least one developmental delay, yet less than one-fifth of those children receive early intervention services before three years of age. Many barriers exist to implementing initial screening and referral, but screening tools can be easily incorporated into the workflow of the primary care practice with preparation. The use of a validated screening tool at regular, repeated intervals, in addition to physician surveillance at well-child visits, may improve early detection. Early intervention is effective in high-risk children and associated with improvements in cognitive and academic performance. Parent-completed tools are preferable to directly administered tools in the primary care setting because of time constraints. The most extensively evaluated parent-completed tools are the Ages and Stages Questionnaire and the Parents' Evaluation of Developmental Status. Family physicians should be familiar with currently available screening tools and the limitations and strengths of these tools. Additional evaluations and referrals are recommended if screening suggests developmental delays are present.
The prevalence of any developmental delay is estimated at 15% in U.S. children three to 17 years of age. 1 Only 3% of all children received public early intervention services by three years of age in 2014. 2 The percentage of school-aged children receiving public intervention services reaches a peak of 12.5% between the ages of nine and 12 years. 2 Risk factors for developmental delay include male sex, lower socioeconomic status, perinatal risk factors, and lower level of maternal education. 1 , 3 , 4 Table 1 indicates the prevalence of delays in specific domains such as cognition and language. 4 , 5 Identification of developmental delays and their etiology allows for the implementation of interventions and treatment plans specific to the disorder.
Parental concern and surveillance alone are often inadequate for identifying children with developmental delays. One study from 1987 showed that without routine screening, only 29% of children with developmental issues were identified before kindergarten. 6 More recently, a randomized controlled trial found that children who underwent routine screening were more likely to have delays detected (23% to 26% vs. 13% of children not routinely screened; P < .001) and receive earlier referrals to early intervention and evaluation. 7 Early intervention is particularly effective for children who have risk factors for developmental delays. 8 – 10 Studies have shown that children who have received early intervention services experience improvements in cognitive and academic performance and engage less in risky behaviors such as alcohol, tobacco, and drug use, and high-risk sexual activity. 8 – 10
The U.S. Preventive Services Task Force (USPSTF) specifically addresses screening for autism and speech and language delays, but it does not address broader developmental screening. Its recommendations state that there is insufficient evidence to assess the balance of benefits and harms of screening for autism and speech and language delays in asymptomatic children younger than five years. 4 , 5 The American Academy of Family Physicians affirms both of the task force's recommendations. 3 , 11 The USPSTF did not find adequate evidence to support surveillance (i.e., active monitoring for concerns and identification of risk based on history and physical examination) by primary care physicians to identify whether further evaluation for speech and language delays and disorders is warranted, nor were there sufficient data that children who screen positive for autism or communication disorders in the primary care setting will benefit from interventions. 4 , 5 There is also some difficulty in distinguishing speech disorders from delays with available screening tools.
The Canadian Task Force on Preventive Health Care also recommends against screening for developmental delay using standardized tools in children one to four years of age when there are no signs of delay or concern on the part of the physician or parent. 12 In contrast, the American Academy of Pediatrics (AAP) recommends three developmental screenings (using standardized tools) by the age of three years (at nine, 18, and 24 or 30 months of age) in addition to surveillance at every well-child visit. 13 It also recommends autism screening at 18 and 24 months of age, with additional evaluation of motor development at 48 months. 14 All three U.S. organizations agree that when there is parental concern for developmental delay, a standardized tool should be used to assess the child.
Barriers to Screening
Developmental delay can be identified with reasonable accuracy using a validated screening tool. 15 However, in 2011 it was reported that only 48% of pediatricians were using a standardized developmental screening tool in practice. 16 According to a report from the Centers for Disease Control and Prevention, 52% of parents said they were informally asked about their child's development, and 21% reported filling out a questionnaire. 17
There are multiple challenges to screening for developmental delays in routine clinical practice. In one study, 82% of primary care physicians cited ongoing time constraints as the most prominent barrier. 18 Other barriers to screening include competing clinical demands, long waits for children to be seen by subspecialists, lack of available subspecialists for referral, staffing requirements, lack of consensus on the best screening tools, and lack of physician confidence in their training and ability to successfully manage children's behavioral and emotional issues. 19 – 25 Additional barriers noted were high staff turnover with subsequent need for training in administration of the tools and lack of reimbursement. 26
Tools for Developmental Screening
Developmental screening tests cannot be used to make a diagnosis of a developmental disorder; therefore, it is important to use a tool that is as accurate as possible to minimize underdetection and over-referrals. 27 No ideal initial screening tool has been identified by the literature. An ideal test would cover all areas of development, be equally applicable to all ages, have construct validity, and have a lower number of false-negatives and false-positives. 27 The AAP recommends broad screening tools that address the following developmental domains: fine and gross motor skills, language and communication, problem-solving and adaptive behavior, and personal-social skills. Screening tools should be culturally sensitive and in the native language of the patient being screened. 13 Table 2 lists commonly used developmental screening tools suitable for the typical busy primary care practice. 13 , 15 , 18 , 27 – 32 More details about a particular test, such as languages available or relevance to a specific culture, can be found at the various test websites.
Psychometrics
Sensitivity, specificity, validity, and reliability are measures that reflect the accuracy and potential usefulness of a particular tool. Table 2 includes psychometric values for four developmental delay screening tools. 13 , 15 , 18 , 27 – 32 Physicians must balance the sensitivity and specificity of available tests, ensuring that children with delays are not erroneously ruled out (false-negatives) while also minimizing the number of children who are misidentified as having a delay and subsequently referred for unnecessary evaluation (false-positives). Higher sensitivity tests result in greater false-positive rates, whereas those with higher specificity result in greater false-negative rates. An acceptable sensitivity for a developmental screening tool is 70% to 80%, and the accepted standard for specificity is approximately 80%. 18 , 27
Reliable developmental screening tools are those that have been tested on a large sample of children who have characteristics representative of the general child population or the population in which the test is being used. 33 It is important to know whether screening tools that are embedded in electronic health records (EHRs) are valid because a shortened version of a tool may degrade its validity and reliability.
Parent-Completed vs. Directly Administered Tools
There are two types of formal developmental screening tools: parent-completed (based on the parent's report alone) and directly administered (based on direct physician observation of the child). Directly administered tools are more comprehensive, but take longer to complete. They are best used as follow-up to an abnormal initial parent-completed screening test, and are typically conducted at a subspecialty consultation. 15 , 18 , 27
Parent-completed tools are an effective, efficient, relatively inexpensive, and practical way to screen for developmental delay in busy practices. 15 , 33 – 38 Parents can complete them online via the practice's web portal, by mail in advance, or in the waiting room before the appointment. 37 , 38 Several validated parent-completed tools have a sensitivity and specificity similar to those of directly administered tools. 15 , 20 These tools also meet two important elements of the patient-centered medical home: they engage parents as active participants in their child's health and facilitate the parent-child-physician relationship.
Specific Tools
Two of the most extensively evaluated parent-completed tools are the Parents' Evaluation of Developmental Status (PEDS) and the Ages and Stages Questionnaire (ASQ). 30 , 32 Both of these tools are available online. The PEDS tool can be used to assess infants and children up to eight years of age. It is comprised of eight yes or no questions and two open-ended questions written at a fourth- to fifth-grade reading level and takes two minutes for the parent to complete. An electronic version that can be integrated into the EHR is available at http://www.pedstest.com . This website also offers an electronic version of the Modified Checklist of Autism in Toddlers.
For all ages combined, the PEDS tool has a sensitivity of 75% and a specificity of 74%. 15 Psychometric properties are maintained across parental education level, socioeconomic status, and child-rearing experience. 15 There is no numeric scoring 18 ; children are instead placed in low-, medium-, and high-risk categories. In general, children found to be at medium or high risk require referral for further evaluation. In one study of urban pediatric clinics, physicians identified developmental problems more accurately and earlier during visits after implementing use of the PEDS tool. The physicians also reported that by using the tool, the efficiency of their visits and appropriate follow-up care improved. 36
The ASQ-3, the third edition of the questionnaire, includes a series of 21 age-specific questionnaires that cover ages one month through five and a half years. Five developmental domains are evaluated (i.e., fine motor; gross motor; language and communication; problem-solving and adaptive behavior; and personal and social performance), with six items to evaluate skills in each area. In addition, general parental concerns are assessed in a 10-question section. There is a pass/fail score to measure each domain, as well as an overall pass/fail score. The questionnaires are written at a fourth- to sixth-grade reading level and take 10 to 15 minutes for parents to complete. They also take one to three minutes to score. 30 The ASQ-3 is available at http://agesandstages.com .
The overall sensitivity of the ASQ-3 is 86%, with an average specificity of 85%. 30 Test-retest and inter-rater reliability are strong (r = 0.94). 18 One study (n = 334) directly compared the ASQ-3 with the PEDS and found sensitivities of 82% and 74% and specificities of 78% and 64%, respectively. ASQ-3 had moderate sensitivity and specificity across all age subgroups. The PEDS had either low sensitivity or low specificity in most of the age subgroups. 39 Studies looking at implementation of the ASQ in busy health care settings found it was feasible and inexpensive to incorporate into practice and did not impede workflow. 35 , 40 Other available parent-completed tools for developmental screening include the Infant Development Inventory and the Child Development Review–Parent Questionnaire. More information about these tools can be found in Table 2 . 13 , 15 , 18 , 27 – 32
The AAP recommends that, in addition to a general developmental screening tool, an autism-specific tool should be administered at 18- and 24-month visits for all children. 13 Neither the PEDS nor the ASQ screens specifically for autism. A resource that offers a suite of online screening tools including the ASQ and the Modified Checklist of Autism in Toddlers is Patient Tools ( http://www.patienttools.com ). A similar resource is the Child Health and Development Interactive System ( http://www.chadis.com/site ). The available online screening tools are made to integrate with and incorporate testing data into EHRs.
No screening tools have been well validated in children with gross and fine motor delays. 41 For this reason, the AAP published a guideline in 2013 specifically regarding evaluation for motor delays. 14 The guideline supports the use of a screening tool, but also recommends assessment of gross and fine motor function via a review of motor milestones at every preventive visit in the first four years. If there is concern for possible developmental delay, a detailed neurologic examination is recommended, including use of the scarf sign and popliteal angle maneuvers to assess for muscle tone. If muscle tone is high, magnetic resonance imaging of the brain is recommended. If muscle tone is low to normal, laboratory evaluation with creatine phosphokinase, thyroxine, and thyroid-stimulating hormone is recommended. Chromosome testing and subspecialist evaluation may also be advisable. The Harris Infant Neuromotor Test is another option for completing motor delay–specific screening. 42 , 43 This test combines aspects of the parent-completed questionnaire and specific examination elements performed at the office visit and covers many of the recommendations from the 2013 AAP guideline. 44 A resource for learning more about evaluating for motor delay is available at http://www.childmuscleweakness.org .
Evaluation and Referral
When a developmental delay is suspected or identified using a screening tool, further evaluation is necessary ( Figure 1 45 ) . A detailed developmental assessment and comprehensive medical evaluation should be scheduled in a timely fashion, in addition to referral for early developmental and intervention services. 6 Evaluation and referral patterns among physicians have been shown to be inconsistent because of the barriers noted previously. 33 , 35 Additionally, tracking of referrals to ensure that services are received can be complex.
A state-by-state listing of early intervention programs can be found at http://www.cdc.gov/ncbddd/actearly/parents/state-text.html . For children older than three years, a local public school should be contacted for evaluation services. For more extensive developmental testing, referral to a developmental pediatrician, child psychiatrist, or pediatric neurologist should be considered. Children who do not qualify for participation in state early intervention programs may have coverage through private insurance. For parents with concerns about speech or language delays, referral to speech therapy is indicated. Motor delays can be evaluated by one or more pediatric neurology, physical therapy, or occupational therapy subspecialists. Table 3 lists various evaluations, tests, and services that may be needed depending on the type of delay suspected, as well as referral options for subspecialists and programs. 13 , 27 , 28 Social workers or case workers may be helpful for families requiring assistance with transportation to therapies or service coordination. The Center for Parent Information and Resources ( http://www.parentcenterhub.org ) provides an extensive selection of resources on a variety of subjects affecting families. Resources regarding patient care are listed in Table 4 . Family physicians, as part of the patient-centered medical home, are integral to coordinating the evaluations of children in their practice.
This article updates a previous article on this topic by Mackrides and Ryherd. 28
Data Sources: A PubMed search was completed in Clinical Queries using the key terms developmental screening, developmental delay screening, and developmental screening tools. Also searched were the Agency for Healthcare Research and Quality, the Canadian Task Force on Preventive Health, the Cochrane database, Essential Evidence Plus, the Centers for Disease Control and Prevention, and UpToDate. Search dates: October 15, 2015, and December 23, 2016.
The views expressed in this material are those of the authors, and do not reflect the official policy or position of the U.S. government, the Department of Defense, or the Department of the Air Force.
Boyle CA, Boulet S, Schieve LA, et al. Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics. 2011;127(6):1034-1042.
U.S. Department of Education. IDEA section 618 data products: static tables. http://www2.ed.gov/programs/osepidea/618-data/static-tables/index.html . Accessed June 26, 2016.
American Academy of Family Physicians. Clinical preventive service recommendation: speech and language delay. https://www.aafp.org/patient-care/clinical-recommendations/all/speech-language-delay.html . Accessed February 12, 2016.
U.S. Preventive Services Task Force. Speech and language delay and disorders in children age 5 and younger: screening. July 2015. http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/speech-and-language-delay-and-disorders-in-children-age-5-and-younger-screening . Accessed February 12, 2016.
U.S. Preventive Services Task Force. Final recommendation statement. Autism spectrum disorder in young children: screening. http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/autism-spectrum-disorder-in-young-children-screening . Accessed June 26, 2016.
Palfrey JS, Singer JD, Walker DK, Butler JA. Early identification of children's special needs: a study in five metropolitan communities. J Pediatr. 1987;111(5):651-659.
Guevara JP, Gerdes M, Localio R, et al. Effectiveness of developmental screening in an urban setting. Pediatrics. 2013;131(1):30-37.
Roberts MY, Kaiser AP. Early intervention for toddlers with language delays: a randomized controlled trial. Pediatrics. 2015;135(4):686-693.
McCormick MC, Brooks-Gunn J, Buka SL, et al. Early intervention in low birth weight premature infants: results at 18 years of age for the Infant Health and Development Program. Pediatrics. 2006;117(3):771-780.
Spittle A, Orton J, Anderson PJ, Boyd R, Doyle LW. Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants. Cochrane Database Syst Rev. 2015(11):CD005495.
American Academy of Family Physicians. Clinical preventive service recommendation. Autism spectrum: children (aged 18 to 30 months). https://www.aafp.org/patient-care/clinical-recommendations/all/autism-children.html . Accessed January 4, 2017.
Canadian Task Force on Preventive Health Care. Recommendations on screening for developmental delay. CMAJ. 2016;188(8):579-587.
Council on Children With Disabilities; Section on Developmental Behavioral Pediatrics; Bright Futures Steering Committee; Medical Home Initiatives for Children With Special Needs Project Advisory Committee. Identifying infants and young children with developmental disorders in the medical home: an algorithm for developmental surveillance and screening [published correction appears in Pediatrics . 2006;118(4):1808–1809]. Pediatrics. 2006;118(1):405-420.
Noritz GH, Murphy NA Neuromotor Screening Expert Panel. Motor delays: early identification and evaluation. Pediatrics. 2013;131(6):e2016-e2027.
Hamilton S. Screening for developmental delay: reliable, easy-to-use tools. J Fam Pract. 2006;55(5):415-422.
Radecki L, Sand-Loud N, O'Connor KG, Sharp S, Olson LM. Trends in the use of standardized tools for developmental screening in early childhood: 2002–2009. Pediatrics. 2011;128(1):14-19.
Rice CE, Naarden Braun KV, Kogan MD, et al.; Centers for Disease Control and Prevention (CDC). Screening for developmental delays among young children—National Survey of Children's Health, United States, 2007. MMWR Suppl. 2014;63(2):27-35.
Rydz D, Shevell MI, Majnemer A, Oskoui M. Developmental screening. J Child Neurol. 2005;20(1):4-21.
Rydz D, Srour M, Oskoui M, et al. Screening for developmental delay in the setting of a community pediatric clinic: a prospective assessment of parent-report questionnaires. Pediatrics. 2006;118(4):e1178-e1186.
Oberklaid F, Efron D. Developmental delay—identification and management. Aust Fam Physician. 2005;34(9):739-742.
Nelson HD, Nygren P, Walker M, Panoscha R. Screening for speech and language delay in preschool children: systematic evidence review for the US Preventive Services Task Force [published correction appears in Pediatrics . 2006;117(6):2336–2337]. Pediatrics. 2006;117(2):e298-e319.
Cunningham PJ. Beyond parity: primary care physicians' perspectives on access to mental health care. Health Aff (Millwood). 2009;28(3):w490-w501.
Olson AL, Kelleher KJ, Kemper KJ, Zuckerman BS, Hammond CS, Dietrich AJ. Primary care pediatricians' roles and perceived responsibilities in the identification and management of depression in children and adolescents. Ambul Pediatr. 2001;1(2):91-98.
Horwitz SM, Kelleher KJ, Stein RE, et al. Barriers to the identification and management of psychosocial issues in children and maternal depression. Pediatrics. 2007;119(1):e208-e218.
Weitzman C, Wegner L Section on Developmental and Behavioral Pediatrics; Committee on Psychosocial Aspects of Child and Family Health; Council on Early Childhood; Society for Developmental and Behavioral Pediatrics; American Academy of Pediatrics. Promoting optimal development: screening for behavioral and emotional problems [published correction appears in Pediatrics . 2015;135(2):384–395]. Pediatrics. 2015;135(2):384-395.
Carroll AE, Bauer NS, Dugan TM, Anand V, Saha C, Downs SM. Use of a computerized decision aid for developmental surveillance and screening: a randomized clinical trial. JAMA Pediatr. 2014;168(9):815-821.
Glascoe FP. Screening for developmental and behavioral problems. Ment Retard Dev Disabil Res Rev. 2005;11(3):173-179.
Mackrides PS, Ryherd SJ. Screening for developmental delay. Am Fam Physician. 2011;84(5):544-549.
Squires J, Twombly J, Bricker D, Potter L. ASQ-3 User's Guide . Baltimore, Md.: Paul H. Brookes Pub.; 2009.
Ages and Stages Questionnaires. Commonly Used Parent-Report Developmental Screening Tools. http://agesandstages.com/wp-content/uploads/2015/03/Comparison-Chart1.pdf . Accessed February 14, 2016.
Child Development Review. IDI and CDR PQ research. http://childdevrev.com/idi-and-cdr-pq-research . Accessed February 14, 2016.
PEDSTest.com. Parents' Evaluation of Developmental Status (PEDS). http://www.pedstest.com/AboutOurTools/LearnAboutPEDS/IntroductiontoPEDS.aspx . Accessed February 14, 2016.
Aylward GP. Developmental screening and assessment: what are we thinking?. J Dev Behav Pediatr. 2009;30(2):169-173.
Earls MF, Hay SS. Setting the stage for success: implementation of developmental and behavioral screening and surveillance in primary care practice—the North Carolina Assuring Better Child Health and Development (ABCD) Project. Pediatrics. 2006;118(1):e183-e188.
King TM, Tandon SD, Macias MM, et al. Implementing developmental screening and referrals: lessons learned from a national project. Pediatrics. 2010;125(2):350-360.
Schonwald A, Huntington N, Chan E, Risko W, Bridgemohan C. Routine developmental screening implemented in urban primary care settings: more evidence of feasibility and effectiveness. Pediatrics. 2009;123(2):660-668.
Bergman DA, Beck A, Rahm AK. The use of internet-based technology to tailor well-child care encounters. Pediatrics. 2009;124(1):e37-e43.
Glascoe FP. Evidence-based early detection of developmental-behavioral problems in primary care: what to expect and how to do it. J Pediatr Health Care. 2015;29(1):46-53.
Limbos MM, Joyce DP. Comparison of the ASQ and PEDS in screening for developmental delay in children presenting for primary care. J Dev Behav Pediatr. 2011;32(7):499-511.
Marks K. Should general pediatricians not select the Ages & Stages Questionnaire in light of the Rydz et al study?. Pediatrics. 2007;120(2):457-459.
King-Dowling S, Rodriguez MC, Missiuna C, Cairney J. Validity of the Ages and Stages Questionnaire to detect risk of developmental coordination disorder in pre-schoolers. Child Care Health Dev. 2016;42(2):188-194.
Harris SR, Megens AM, Daniels LE. Harris Infant Motor Test. Test user's manual version 1.0 clinical edition (2009). http://thetimp.com/products-for-therapists#!/Harris-Infant-Neuromoto-HINT-Test-Manual/p/56137524/category=0 . Accessed January 4, 2017.
Harris SR. Early identification of motor delay: family-centered screening tool. Can Fam Physician. 2016;62(8):629-632.
Westcott McCoy S, Bowman A, Smith-Blockley J, Sanders K, Megens AM, Harris SR. Harris Infant Neuromotor Test: comparison of US and Canadian normative data and examination of concurrent validity with the Ages and Stages Questionaire. Phys Ther. 2009;89(2):173-180.
Centers for Disease Control and Prevention. Developmental monitoring and screening for health professionals. http://www.cdc.gov/ncbddd/childdevelopment/screening-hcp.html . Accessed September 23, 2016.
Continue Reading
More in afp, more in pubmed.
Copyright © 2024 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- For authors
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 102, Issue 11
- Current evidence-based recommendations on investigating children with global developmental delay
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Renuka Mithyantha 1 ,
- Rachel Kneen 2 , 3 ,
- Emma McCann 4 ,
- http://orcid.org/0000-0002-2579-9301 Melissa Gladstone 1 , 5
- 1 Department of Developmental Paediatrics , Alder Hey Children’s NHS Foundation Trust , Liverpool , UK
- 2 Department of Paediatric Neurology , Alder Hey Children’s NHS Foundation Trust , Liverpool , UK
- 3 Institute of Infection and Global Health, University of Liverpool , Liverpool , UK
- 4 Department of Clinical Genetics , Liverpool Women’s Hospital , Liverpool , UK
- 5 Department of Women and Children’s Health , Institute of Translational Medicine, University of Liverpool, Alder Hey Children’s NHS Foundation Trust , Liverpool , UK
- Correspondence to Dr Melissa Gladstone, Department of Women and Children’s Health, Institute of Translational Medicine, University of Liverpool, Alder Hey Children’s NHS Foundation Trust, Liverpool, L14 5AB, UK; M.J.Gladstone{at}liverpool.ac.uk
Introduction Global developmental delay (GDD) affects 1%–3% of the population of children under 5 years of age, making it one of the most common conditions presenting in paediatric clinics; causes are exogenous, genetic (non-metabolic) or genetic (metabolic). Recent advances in biotechnology and genetic testing mean that the investigations available to perform for children under 5 years are increasing and are more sensitive than previously. This change in availability and type of testing necessitates an update in the recommendations for investigating GDD.
Methods We conducted a review of the literature from 2006 to 2016 to identify articles with evidence relating to the investigation of developmental delay in children under the age of 5 years. We collated the evidence into first-line and second-line investigations and, where available, on their yield and cost implications.
Results We have provided up-to-date guidance for first-line and second-line investigations for children with GDD under the age of 5 years. Recent evidence demonstrates that genetic testing for all children with unexplained GDD should be first line, if an exogenous cause is not already established. Our review of the literature demonstrates that all patients, irrespective of severity of GDD, should have investigations for treatable conditions. Evidence demonstrates that the yield for treatable conditions is higher than previously thought and that investigations for these metabolic conditions should be considered as first line. Additional second-line investigations can be led by history, examination and developmental trajectories.
Discussion We may need to update present recommendations in the UK for investigation of developmental delay. This would include microarray testing as first line and a more thorough approach to investigations for metabolic disorders that can be treated. Clinical assessment remains vital for guiding investigations.
- neurodevelopment
- neurodisability
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
https://doi.org/10.1136/archdischild-2016-311271
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Introduction
Global developmental delay (GDD) is defined as a delay in two or more developmental domains of gross/fine motor, speech/language, cognition, social/personal and activities of daily living, affecting children under the age of 5 years. 1 2 The degree of developmental delay is further subclassified as: mild (functional age <33% below chronological age), moderate (functional age 34%–66% of chronological age) and severe (functional age <66% of chronological age). 1 GDD is considered significant when there is a deficit in performance of at least 2 SD below the age appropriate mean on accepted standardised assessment tests. 3 With a prevalence of 1%–3%, GDD is one of the the most common conditions encountered in paediatrics with genetic and structural brain abnormalities being the most frequent causes. 1 Establishing a diagnosis enables clinicians to define treatment options and conduct surveillance for known complications as well as provide prognosis and condition-specific family support (including family planning choices). This ensures the best overall outcomes for the child and their families/carers. 4 A diagnosis may also provide an explanation, a source of closure or acceptance to parents and stops clinicians advancing to potentially more expensive and invasive tests 5–7
Previous estimates for the yield of investigations for GDD are broad (10%–81%). 2 The variability may be due to differences in patient populations, clinical settings where tests are performed and the range of tests undertaken. 2 The last evidence-based UK guideline for investigation of developmental delay was published 10 years ago. 8 With the advent of more recent techniques in genetics and a recent burgeoning of guidelines in other countries, 4 9 10 there is a need to review our practice in the UK.
The primary objective of this paper is to provide (1) an update of the latest evidence for investigation of GDD, (2) recommendations for investigations and (3) evidence relating to yield and cost from literature presently available.
We conducted a systematic review of the literature relating to the investigation of GDD published in the last 10 years (since the McDonald review in 2006). We searched Pubmed, Google Scholar and Embase using the MESH terms: ‘developmental delay’, ‘developmental disorders’, ‘mental retardation’, ‘intellectual disability’, ‘learning disorders’ AND ‘guidelines’ AND ‘investigations’. ‘Cost’ and ‘yield’ were included along with the MESH terms. Papers included were reviews, consensus recommendations, retrospective or prospective studies. Relevant articles from reference lists were also included. We included papers published in English that were relevant to children that included investigations for GDD. We excluded papers that targeted specific metabolic, genetic or neurological conditions. We used the term GDD as meaning: delayed developmental domains in children under the age of 5 years and intellectual disability (ID) as the term used after this age when IQ can be reliably tested. 11
For this review, we discuss and categorise investigations into first-line and second-line tests and subcategorised them to genetics, metabolic and imaging. See table 1 for recommended first-line investigations to be considered prior to referral to specialist services. We show a flowchart and decision-making tree for investigations in figure 1 .
- Download figure
- Open in new tab
- Download powerpoint
Flow chart for decision making for investigations for global developmental delay in young children.
- View inline
Table demonstrating recommendations for first-line investigations for global developmental delay from four guidelines and our proposed recommendations
First-line assessment and investigations
History and examination.
Comprehensive clinical assessment remains the core to planning investigations in young children presenting with GDD. 4 8–10 Aetiology can be categorised into exogenous, genetic (non-metabolic) and genetic (metabolic). 11 The diagnosis of exogenous causes includes teratogenic agents (alcohol and drugs); prenatal, perinatal causes (prematurity, infections); and social causes often best assessed by history but must not be assumed.
Investigations following a thorough clinical history (including a family pedigree, pregnancy and birth history) and a detailed physical examination by a trained specialist lead to a higher diagnostic yield. 3 12 Identification and correction of sensory deficits are essential, while evaluating these children and may provide pointers to the underlying aetiology. 2 6
An examination of the child’s developmental status in all domains (gross motor, fine motor, language, socioemotional and cognitive skills) using a recognised tool to provide a normative comparison should also be conducted. Repeated clinical/dysmorphology and developmental assessments over time are more informative than one-off assessments in planning investigations and management.
It is important that the clinician consider investigations in all levels of developmental delay including those with persistent mild GDD, given the variable phenotypic presentations of genetic and metabolic conditions. Some studies, although from tertiary centres, have found that severity did not impact on the diagnostic rate of investigations, 12 while others report higher yield in patients with moderate-to-severe GDD. 13 Serial assessment enables clinicians to identify changing phenotypes over time. When metabolic conditions are clinically suspected, annual evaluation after the first year of life until school age is recommended. 14
Some studies have demonstrated that we can identify the cause of developmental or cognitive delay in a one-third of cases by history and examination alone. With clinical evaluation prompting investigations, we can identify another one-third. It is only the latter one-third that are identified by investigations only. 12 The presence of abnormal neurology, microcephaly, female gender, dysmorphism, abnormal prenatal or perinatal history and absence of autistic features are linked with higher aetiological yield of investigations. 15 Investigations following comprehensive clinical evaluation are also cost effective. 16
Genetic testing
First-line tests .
Genetic investigation by means of standard karyotyping was recommended as a first-line investigation in the UK guidance from 2006. 8 The implementation of ‘molecular karyotyping’ or chromosome microarray (array-based comparative genomic hybridisation (aCGH)) has changed the state of play. Recent evidence-based international guidelines promote the use of aCGH as a first-tier investigation for GDD if no aetiological indicators from history and examination are found. 4 9 10 The higher sensitivity that it has for identifying submicroscopic deletions and duplications (than standard karyotyping methods) and better definition of the breakpoints and size of imbalances all make microarray a suitable first-line test. 4 17 18
Chromosome microarray has been described to be the ‘single most efficient diagnostic test’ for GDD after history and examination. 4 A literature search of 33 studies that used this technique in nearly 22 000 patients has demonstrated that the diagnostic yield of aCGH is between 15% and 20%, while karyotyping is 3%. 18 The diagnostic yield of microarray is supported by a health economics report, which showed cost saving when comparing a National Health Service (NHS) clinical genetics service use of aCGH as a first-tier test while evaluating learning disability, compared with CGH as second line after negative karyotyping. 19
Molecular karyotyping will not detect conditions where structural changes in the chromosomes result in no loss or gain of genetic material such as balanced translocations or inversions, ring chromosomes and low-level mosaicism. 18 20 A standard karyotype is still required if such a disorder is suspected (eg, refractory epilepsy, if a family is known to have a balanced translocation associated with a phenotype, a history of multiple miscarriages or clinical features to suggest mosaicism). Syndromes caused by methylation defects (eg, Beckwith-Wiedemann, Angelman syndrome) or mutations in single genes will also go undetected unless specifically tested.
Fragile X syndrome affects approximately 1/5000 births, typically causing moderate ID in boys and a variable phenotype in girls (unaffected to significant). Phenotypic features evolve and are not as apparent in younger children. 9 The UK genetic testing network and international guidelines therefore do promote testing for fragile X for children with moderate-to-severe GDD, without profound physical disability, as an additional first-tier genetic investigation. 4 9 10 21 Testing criteria are available to help aid clinical decisions in older children. 21
Second-line tests
Clinical syndromes can present with variable phenotypes, and children who have a normal aCGH and FMR1 may be best assessed by a clinical geneticist to ensure that the most appropriate and cost-effective additional tests are undertaken. 22 Use of specific gene tests such as those for Rett syndrome (or its variants) or gene panels for ID has been proposed as second-line tests. 4 There is an increasing number of panels and exome sequencing tests available for ID (UK Genetic Testing Network; http://www.ukgtn.nhs.uk ) or private providers, but specialist services (clinical genetics or paediatric neurology) do most requests for these tests, although this is likely to change as mainstreaming of these investigations advances.
Metabolic and biochemical investigations
There is limited good quality evidence for first-line metabolic investigations. Recommendations from Ireland are based on evidence review by expert committee, 10 while those from Australia are based on a literature review, quoting grade III–IV evidence. 9
Inborn errors of metabolism (IEMs) are rare, their prevalence likely to vary in different populations. There is limited UK data on detecting metabolic disorders in patients with GDD. 14 IEMs are usually associated with systemic features, and previous guidelines recommend selective metabolic investigations. 2 8 Some IEMs are now (partially) treatable, and for others, treatment is in the research stages. Treatment includes dietary supplements (folinic acid for cerebral folate deficiency, pyridoxine or pyridoxal phosphate for B6-responsive epilepsy, creatine in creatine transporter deficiency, uridine in pyrimidine 5-nucleotidase super activity), dietary restriction (homocystinuria, glutaricacidaemia) and ketogenic diet (pyruvate dehydrogenase deficiency, Glut1 transporter deficiency). Other treatments include: haematopoietic stem cell transplantation (mucopolysaccharidoses, metachromatic leucodystrophy), enzyme replacement (Fabry’s disease, Gaucher’s disease, neuronal ceroid lipofuscinosis) or gene therapy (adrenoleucodystophy, lysosomal storage disorders). 23–25
A systematic review of literature by van Karnebeek et al identified 89 conditions presenting with ID as a major feature, which are susceptible to treatment. Of these, 60% could be identified by non-targeted urine and blood tests. Some of these conditions (eg, creatine transporter defects, mild homocystinuria, female ornithine transcarbamylase deficiency) can initially present as GDD alone. 25 26 While individual treatable IEMs are extremely rare in the general population, the prevalence will be higher in the at-risk population. Hence, though small in number, these treatable causes of GDD have been the focus of the more recent US guidance, with recommendations that screening for IEM should be used in all patients with GDD of unknown aetiology. 4 24 A list of tests with treatable conditions they identify is shown in table 2 .
Table demonstrating IEM tested for by first-line metabolic investigations 25
The neonatal screening programme in the UK (Guthrie test) currently includes six IEMs (phenyketonuria, medium-chain acyl-CoA dehydrogenase deficiency, maple syrup urine disease, isovaleric acidaemia, glutaricaciduria type 1, homocystinuria (pyridoxine unresponsive)) and congenital hypothyroidism. It is restricted when compared with other countries (eg, Canada, the USA, The Netherlands), which offer a wider range including urea cycle disorders, organic and some amino acid disorders. Testing for these is, therefore, more relevant in UK patients with GDD, and IEMs should be considered in symptomatic children. 14
There are also some conditions where early diagnosis can be made from simple and cheap biochemical screening tests. This includes creatine kinase and thyroid function tests as well as ferritin, vitamin B12 and lead on a selective basis when Pica, dietary restrictions (vegan diet in child/mother) or environmental exposure risk is possible. 9 While these tests seldom lead to a diagnosis, they also may add to a diagnosis (eg, macrocytic anaemia in organic acidaemias, abnormal triiodothyronine in Allan-Herdon-Dudley syndrome). 10 27
There is limited research on comprehensive metabolic evaluation in larger groups of individuals with GDD. It is, therefore, difficult to estimate the yield of many of the proposed first-line metabolic tests. A recent systematic review conducted for the American Academy of Neurology found that yield of metabolic investigations varied between 0.2% and 4.6%, based on clinical signs and range of tests undertaken in the studies (grade III evidence). 28 Second-line individually tailored testing in a tertiary setting in the Netherlands produced an overall yield of 2.8% for metabolic investigations. 11
Individually tailored second-line testing 4 14 26 and referral to a specialist service is recommended, 4 9 when clinical suspicion remains. An evidence-based, free web-based application ( http://www.treatable-id.org ) may be useful to tailor investigations for treatable IEMs not covered by first-line tests. 29
Neuroimaging
MRI of the brain has been used selectively and non-selectively in evaluating patients with GDD. The diagnostic yield of MRI is higher when used in patients where GDD is associated with clinical signs such as abnormal head circumference (microcephaly, non-familial macrocephaly, rapid change in head circumference), focal neurological signs or epilepsy. Targeted imaging was hence advocated by previous guidelines. 2 8 Previous studies have demonstrated abnormal results in targeted imaging in about 41% compared with 14% with non-selective screening. 3 Recent studies continue to demonstrate higher abnormality detection rates when MRI is performed in patients with GDD with additional clinical/neurological signs. 30 31 More complex MRI protocols (eg, proton magnetic resonance spectroscopy) are promising tools to investigate GDD and enable a non-invasive measure of brain metabolites such as lactate or white matter choline, 32 but studies have so far failed to show an increased diagnostic yield, 31 33 and hence these are best used as second line in selected patients.
MRI is a more sensitive test and has no radiation exposure, making it a preferred choice over CT. However, all children under 5 years will need sedation or a general anaesthetic, which has a slim risk attached, and some children will need further investigations including a lumbar puncture. There is an argument, therefore, that children requiring brain imaging should see a specialist prior to imaging, if an anaesthetic is required.
Special considerations
Ten most common causes of progressive intellectual and neurological deterioration.
10 most common causes of PIND reported in the PIND study in the UK ( www.rcpch.ac.uk/pind ) 34
NCL late infantile
Mucopolysaccharidosis IIIA (San Filippo)
Rett syndrome
Metachromatic leucodystrophy
Adrenoleucodystrophy
NCL juvenile
GM2 gangliosidosis type 1 (Tay-Sachs)
Niemann-Pick type C
GM2 gangliosidosis type 2 (Sandhoff)
NCL, neuronal ceroid lipofuscinosis; PIND, progressive intellectual and neurological deterioration.
Children that should be referred to a specialist in neurodisability or neurology are shown on table 3 . Investigations should be individualised and targeted as they can be invasive (eg, LP, muscle/skin biopsy) or painful (eg, nerve conduction studies and electromyography) and are expensive and time consuming for medical staff and families. Children with regression may also be referred to the clinical genetics team where specific next-generation sequencing panels can be undertaken and, at present, considered for the 100 000 Genome Project ( www.genomicsengland.co.uk/the-100000-genomes-project ).
Clinical pointers to consider referral to a specialist in neurodisability or neurology
Immigrant children
Immigrant children are exposed to a combination of biological, socioeconomical, emotional and environmental adverse events placing them at higher risk of developmental problems. This includes malnutrition and disability from trauma, overcrowding and toxin exposure and loss of parents or trauma from lack of stability. 35 Furthermore, children may have missed new-born screens and vaccinations and been exposed to infectious diseases. In these children, comprehensive clinical assessments should consider all these factors while planning individual investigations.
Despite new advances in technology, particularly in the realm of genetic investigation, clinical assessment continues to be vital in guiding investigation. Clues to investigation may lie in the history and examination with clinical judgement being essential to enabling the right pathways to be taken in making a diagnosis. A good history can help direct which route to take in terms of investigation, particularly when exogenous causes are identified. Assessment over a period will provide clarity as to whether a condition is resolving, static or deteriorating. Assessment over time enables the phenotype to evolve and more appropriate targeting of investigations.
It is clear that establishing a diagnosis enables us to answer questions on: why it has happened (aetiology), what does it mean for our child (prognosis), what treatments might be available (precision medicine) and whether it can be prevented in the future (prenatal testing and preimplantation genetic diagnosis).
In these recommendations, we have also highlighted the recent evidence that promotes metabolic screening tests to detect treatable conditions. This is a move away from older guidance where metabolic investigations were not recommended for children with no features/risk factors other than GDD. 2 Though rare, the possibility of presentation as stable developmental delay and potential for treatment merits their inclusion as first-line tests. Treatment outcomes vary but can potentially improve cognitive development, slow deterioration, prevent metabolic decompensation and improve seizure control and systemic manifestations. 25 26
GDD and ID affect 2%–3% of the worldwide population with a lifetime cost of up to US$1 million. 36 First-line metabolic investigations to identify treatable IEMs cost approximately $C568, 26 with costs in Ireland for all first-line tests at €1335. 10 Costs in the UK NHS laboratory for aCGH are not astronomical (£338–£350), 37 38 with the majority of combined metabolic tests costing under £1000. 38 Not all children will get a diagnosis and cost per diagnosis may be high, but there are obvious long-term cost savings if early diagnosis and treatment are possible. The options of genetic counselling and support for young families also make diagnosis invaluable.
Recent advances in genomic medicine are transforming the investigation of children with significant developmental delay and are likely to transform the way we assess and investigate children. Traditional models of care have relied on history and examination with broad and then specific investigations to funnel down to specific diagnoses. The advent of rapid genetic testing and ‘omic’ medicine is likely to turn this paradigm on its head with whole genome/exome sequencing identifying genes, which may be causing the phenotype in an individual. The clinician will then use knowledge of their patient to make a judgement about whether this is the cause for their patient—‘reverse dysmorphology’.
These advances in genomic medicine will lead to an increase in diagnoses that will modify how the individual is clinically cared for (precision medicine). The Deciphering Developmental Disorders study and the 100 000 Genome Project will both aid our understanding of disorders. We predict that, with time, whole genome sequencing/exome sequencing may become the first-line investigation of choice for all children with unexplained GDD and that other investigations will be secondary to this and used primarily for phenotyping. These will provide answers for families about the underlying cause of their child’s condition and will prevent further costly and potentially distressing investigations taking place.
Conclusions
In this paper, we have outlined the present evidence and recommendations for both first-line and second-line investigations for GDD in children in the UK. We have provided new evidence relating to the use of genetic testing techniques and have demonstrated that this should be a first-line investigation for all children with GDD. Second to this, any treatable metabolic conditions should be always considered. With time, it is likely that the investigation of children with developmental delay will be turned on its head and we will be going from genetic diagnosis to phenotypic diagnosis. Despite this, history and examination will always be crucial for defining the condition and the change over time.
- Majnemer A ,
- Shevell M ,
- Donley D , et al
- Shevell MI ,
- Rosenbaum P , et al
- Moeschler JB ,
- Shonkoff JP ,
- Hauser-Cram P
- McConachie H ,
- Hayeems RZ ,
- Babul-Hirji R ,
- Hoang N , et al
- McDonald L ,
- Tolmie J , et al
- Collins F ,
- O’Byrne JJ ,
- Treacy EP , et al
- Engbers HM ,
- van Hasselt P , et al
- van Karnebeek CD ,
- Scheper FY ,
- Abeling NG , et al
- Cleary MA ,
- Thomas RL , et al
- Miller DT ,
- Aradhya S , et al
- Kharbanda M ,
- Milunsky JM
- Corbella M ,
- Fons C , et al
- Stockler IS
- Zschocke J , et al
- Michelson DJ ,
- Sherr EH , et al
- Popurs MA ,
- Lafek M , et al
- Griffiths PD ,
- Warren D , et al
- Verbruggen KT ,
- Meiners LC ,
- Sijens PE , et al
- Maurits NM ,
- Meiners LC , et al
- Ritter S , et al
- Winstone AM ,
- Stellitano L , et al
- Davidson N ,
- Chaney G , et al
- ↵ Centers for Disease Control and Prevention (CDC) . Economic costs associated with mental retardation, cerebral palsy, hearing loss, and vision impairment--United States, 2003 . MMWR Morb Mortal Wkly Rep 2004 ; 53 : 57 – 9 . OpenUrl PubMed
- ↵ Great Ormond Street Hospial for Children NHS Foundation Trust,North East Thames Regional Genetics Service . Pricing , 2014 .
- ↵ Community Children’s Health Partnership . Investigations for developmental delay: blood tests costs (2014) and what results could indicate? 2014 .
Contributors RM, RK, EM and MG contributed to the initial idea for the paper, wrote and reviewed sections of the paper and approved the final version. RM conducted the literature review with the support of MG and wrote the first draft of the paper.
Funding None declared.
Competing interests None declared.
Provenance and peer review Commissioned; externally peer reviewed.
Linked Articles
- Original article Aetiological investigations in early developmental impairment: are they worth it? Anthony Richard Hart Ruchi Sharma Mark Atherton Samer Alabed Sally Simpson Stuart Barfield Judith Cohen Nicholas McGlashan Asha Ravi Michael James Parker Daniel JA Connolly Archives of Disease in Childhood 2017; 102 1004-1013 Published Online First: 22 Jul 2017. doi: 10.1136/archdischild-2017-312843
Read the full text or download the PDF:
- Introduction
- Conclusions
- Article Information
TMM BirThree indicates Tohoku Medical Megabank Project Birth and Three-Generation.
eTable. Results of the Complete Case Analysis (n = 6656)
Data Sharing Statement
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
Takahashi I , Obara T , Ishikuro M, et al. Screen Time at Age 1 Year and Communication and Problem-Solving Developmental Delay at 2 and 4 Years. JAMA Pediatr. 2023;177(10):1039–1046. doi:10.1001/jamapediatrics.2023.3057
Manage citations:
© 2024
- Permissions
Screen Time at Age 1 Year and Communication and Problem-Solving Developmental Delay at 2 and 4 Years
- 1 Graduate School of Medicine, Tohoku University, Sendai, Japan
- 2 Tohoku Medical Megabank Organization, Tohoku University, Sendai, Japan
- 3 Department of Pharmaceutical Sciences, Tohoku University Hospital, Sendai, Japan
- 4 United Graduate School of Child Development, Hamamatsu University School of Medicine, Hamamatsu, Japan
- 5 Research Center for Child Mental Development, Hamamatsu University School of Medicine, Hamamatsu, Japan
- 6 International Research Institute of Disaster Science, Tohoku University, Sendai, Japan
Question Is there a dose-response association between screen time for children aged 1 year and functional development at ages 2 and 4 years?
Findings In this cohort study including 7097 mother-child pairs, a dose-response association was observed between greater screen time at age 1 year and developmental delays in communication and problem-solving at ages 2 and 4 years.
Meaning These findings suggest that domains of developmental delay should be considered separately in future discussions on screen time and child development.
Importance Whether some domains of child development are specifically associated with screen time and whether the association continues with age remain unknown.
Objective To examine the association between screen time exposure among children aged 1 year and 5 domains of developmental delay (communication, gross motor, fine motor, problem-solving, and personal and social skills) at age 2 and 4 years.
Design, Participants, and Setting This cohort study was conducted under the Tohoku Medical Megabank Project Birth and Three-Generation Cohort Study. Pregnant women at 50 obstetric clinics and hospitals in the Miyagi and Iwate prefectures in Japan were recruited into the study between July 2013 and March 2017. The information was collected prospectively, and 7097 mother-child pairs were included in the analysis. Data analysis was performed on March 20, 2023.
Exposure Four categories of screen time exposure were identified for children aged 1 year (<1, 1 to <2, 2 to <4, or ≥4 h/d).
Main Outcomes and Measures Developmental delays in the 5 domains for children aged 2 and 4 years were assessed using the Japanese version of the Ages & Stages Questionnaires, Third Edition. Each domain ranged from 0 to 60 points. Developmental delay was defined if the total score for each domain was less than 2 SDs from its mean score.
Results Of the 7097 children in this study, 3674 were boys (51.8%) and 3423 were girls (48.2%). With regard to screen time exposure per day, 3440 children (48.5%) had less than 1 hour, 2095 (29.5%) had 1 to less than 2 hours, 1272 (17.9%) had 2 to less than 4 hours, and 290 (4.1%) had 4 or more hours. Children’s screen time was associated with a higher risk of developmental delay at age 2 years in the communication (odds ratio [OR], 1.61 [95% CI, 1.23-2.10] for 1 to <2 h/d; 2.04 [1.52-2.74] for 2 to <4 h/d; 4.78 [3.24-7.06] for ≥4 vs <1 h/d), fine motor (1.74 [1.09-2.79] for ≥4 vs <1 h/d), problem-solving (1.40 [1.02-1.92] for 2 to <4 h/d; 2.67 [1.72-4.14] for ≥4 vs <1 h/d), and personal and social skills (2.10 [1.39-3.18] for ≥4 vs <1 h/d) domains. Regarding risk of developmental delay at age 4 years, associations were identified in the communication (OR, 1.64 [95% CI, 1.20-2.25] for 2 to <4 h/d; 2.68 [1.68-4.27] for ≥4 vs <1 h/d) and problem-solving (1.91 [1.17-3.14] for ≥4 vs <1 h/d) domains.
Conclusions and Relevance In this study, greater screen time for children aged 1 year was associated with developmental delays in communication and problem-solving at ages 2 and 4 years. These findings suggest that domains of developmental delay should be considered separately in future discussions on screen time and child development.
Screen time is the amount of time that individuals spend watching television, playing video games, and using mobile phones, tablets, and other electronic devices. To ensure that children engage in physical activity and obtain adequate sleep for healthy growth and well-being, the World Health Organization 1 and the American Academy of Pediatrics 2 have issued guidelines that recommend limiting screen time for children, including a limit of 1 hour per day for children aged 2 to 5 years. 2 However, a recent meta-analysis reported that only a minority of children meet these guidelines. 3 In addition, children’s screen time has increased in recent years because of the rapid proliferation of digital devices and the COVID-19 pandemic. 4 - 6 Therefore, it is essential to consider how screen time affects child development.
Previous studies have reported associations between screen time and child development outcomes. These outcomes include communication, 7 , 8 daily living skills, 7 socialization, 7 gross and fine motor skills, 8 problem-solving skills, 8 personal and social skills, 8 developmental screening test total scores, 9 cognitive development, 10 , 11 socioemotional development, 9 language development, 11 - 13 attention problems, 14 behavioral problems, 15 , 16 and developmental disorders such as autism spectrum disorder. 17 , 18
Although several studies have examined the association between screen time and child development outcomes, 2 questions remain. The first is whether screen time is associated with child development domains and, if so, which ones. Because there are several domains of child development, its association with screen time may be domain specific. However, most previous studies examined a single measure as an outcome. 9 - 18 Only 2 studies 7 , 8 focused on multiple child development domains: one that considered several domains was cross-sectional, 8 and the other performed a longitudinal examination. 7 Therefore, further research focusing on several developmental domains is needed to clarify the association between screen time and individual child development domains.
The second question is whether the association between children’s screen time and developmental delay continues with age. To our knowledge, only 2 studies 9 , 16 have examined whether screen time is associated with child development outcomes at several later time points. Both studies used random-intercept cross-lagged panel models: one examined the association between screen time and developmental screening scores at ages 2, 3, and 5 years, 9 and the other examined the association between screen time and externalizing and internalizing behavior at ages 3, 5, 7, and 9 years. 16 The findings of both studies did not support an association between children’s screen time at a single point and child development outcomes at 2 or more later points. 9 , 16 These studies examined developmental and behavioral screening test scores 9 , 16 ; however, there are several phenotypic domains of child development.
Considering these findings, it is essential to examine whether screen time is continuously associated with child development domains at multiple time points and, if so, which ones. Therefore, this study examined the association between screen time exposure at age 1 year and 5 domains of developmental delay (communication, gross motor, fine motor, problem-solving, and personal and social skills) at ages 2 and 4 years among participants in the Tohoku Medical Megabank Project Birth and Three-Generation (TMM BirThree) Cohort Study, a representative population in Japan and one of the largest cohorts for this research area.
Details of the TMM BirThree cohort study are provided elsewhere. 19 - 22 The Tohoku Medical Megabank Organization Institutional Review Board reviewed and approved the study protocol. Pregnant women at 50 obstetric clinics and hospitals in the Miyagi and Iwate prefectures in Japan were recruited into the study between July 2013 and March 2017. 19 , 20 Trained genomic medical research coordinators explained the study details to all potential participants and obtained signed consent. 19 , 20 The study followed the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline.
Of the 23 130 mother-child pairs in the TMM BirThree cohort, 16 033 were excluded as follows: 505 withdrew informed consent, 875 participated in the study survey more than once, 8820 were missing information on screen time at age 1 year, and 2512 and 3321 were missing information on development outcomes at ages 2 and 4 years, respectively. Therefore, 7097 mother-child pairs were included in the analysis ( Figure ).
Children’s screen time at age 1 year was assessed using a questionnaire in which participants were asked the following: “On a typical day, how many hours do you allow your children to watch TV, DVDs, video games, internet games (including mobile phones and tablets), etc?” There were 5 response categories: none, less than 1, 1 to less than 2, 2 to less than 4, or 4 or more hours per day. We merged 2 categories (none and <1), resulting in 4 categories of screen time exposure (<1, 1 to <2, 2 to <4, or ≥4 h/d).
To assess developmental delay among children, we used the Ages & Stages Questionnaires, Third Edition (ASQ-3). 23 , 24 The ASQ-3 assesses child development from ages 1 to 66 months. In this study, parents responded to questions in the Japanese version of the ASQ-3 regarding their children aged 2 and 4 years. 24 The ASQ-3 comprised 6 questions divided into the following 5 domains: communication (babbling, vocalizing, and understanding), gross motor (arm, body, and leg movement), fine motor (hand and finger movement), problem-solving (learning and playing with toys), and personal and social skills (solitary social play and playing with toys and other children). The response options included “yes,” “sometimes,” or “not yet” (10, 5, or 0 points, respectively), and each domain was scored with a range of 0 to 60 points. 23 , 24 If 1 or 2 of the 6 questions were missed, the remaining total score was multiplied by 1.2 or 1.5, adjusted from 0 to 60, respectively. 23 , 24 One question in the gross motor domain for children aged 2 years asked about a possible behavior that they may have had previously but no longer did because they acquired more advanced skills. If parents answered “not yet” or “sometimes” on the easier item and “yes” on the more advanced item, the response on the earlier item was changed to “yes.” 23 , 24 A total score of each domain that was less than −2 SDs relative to the mean in reference indicated developmental delay and the need for further assessment. 23 , 24 A previous study showed that this cutoff point had moderate sensitivity and specificity to estimate any delay, severe delay, motor delay, and cognitive delay, 25 and it has also been used widely in the screening of Japanese children. 26 , 27
We selected covariates that may affect the association between children’s screen time and developmental delay based on previous studies. 7 - 18 Children’s sex was garnered from birth records. Information about maternal age at delivery and parity (nulliparous, or primiparous or multiparous) was gathered from medical records. We divided maternal age into 4 categories (<25, 25-29, 30-35, or >35 years). Information on annual household income (<¥4 000 000 [US <$28 400], ¥4 000 000-5 999 999 [US $28 400-$42 599], or ≥¥6 000 000 [US ≥$42 600]) was gathered from the midpregnancy questionnaire. Data on maternal educational attainment (high school graduate or less, junior college or vocational college graduate, university graduate or above, or other), child living with grandparents or other adults (yes or no), and maternal postpartum depression and maternal bonding disorder were gathered using the questionnaire at 1 year post partum. Maternal postpartum depression was assessed using the Japanese version of the Edinburgh Postnatal Depression Scale (EPDS). 28 - 30 In Japan, an EPDS score of 9 or higher is widely used as the cutoff point for screening of postpartum depression, with previous studies reporting sensitivity of 75% and 82% and specificity of 93% and 95% at 1 month post partum. 29 , 30 Maternal bonding disorder was assessed using the Japanese version of the Mother-to-Infant Bonding Scale 31 - 33 (MIBS-J), and the cutoff point was set at 5. A previous study of Japanese mothers with 1-month-old infants showed that an MIBS-J cutoff point of 4 or 5 correctly classified approximately 90% of pathological maternal bonding disorders. 33
Participant characteristics were described according to the 4 categories of child screen time at age 1 year (<1, 1 to <2, 2 to <4, or ≥4 h/d). Characteristics are presented as frequencies with percentages and as medians with IQRs. Associations between the 4 screen time categories at 1 year and the 5 ASQ-3 domains of developmental delay in children at 2 and 4 years were evaluated using multivariable logistic regression analysis to estimate odds ratios (ORs) and 95% CIs (with <1 h/d as the reference). Missing covariates were imputed through multiple imputations by chained equations using the exposure, outcome, and covariates in the main analysis. 34 Twenty sets of quasi-complete data were analyzed in the multivariable analyses independently and the estimates were integrated. 34 In addition, as a supplemental analysis, a complete case analysis was performed in which participants with at least 1 missing covariate were excluded. All statistical analyses were performed using R, version 4.0.2 (R Project for Statistical Computing), and 95% CIs not crossing 1.00 were considered statistically significant. Data analysis was performed on March 20, 2023.
Of the 7097 children included this study, 3674 were boys (51.8%) and 3423 were girls (48.2%). Table 1 presents participant characteristics according to the 4 categories of children’s screen time. In terms of screen time exposure per day, 3440 children (48.5%) had less than 1 hour, 2095 (29.5%) had 1 to less than 2 hours, 1272 (17.9%) had 2 to less than 4 hours, and 290 (4.1%) had 4 or more hours. At age 2 years, developmental delays were observed in the communication (361 [5.1%]), gross motor (400 [5.6%]), fine motor (329 [4.6%]), problem-solving (301 [4.2%]), and personal and social skills (387 [5.5%]) domains. At age 4 years, developmental delays were also observed in the communication (283 [4.0%]), gross motor (303 [4.3%]), fine motor (349 [4.9%]), problem-solving (269 [3.8%]), and personal and social skills (328 [4.6%]) domains. Mothers of children with high levels of screen time were characterized as being younger, having never given birth, and having a lower household income, lower maternal education level, and having postpartum depression.
Table 2 presents the association between the 4 screen time categories at age 1 year and each domain of developmental delay at ages 2 and 4 years through multivariable logistic regression (with <1 h/d as the reference). After adjusting for covariates, we observed an association between screen time at age 1 year and a higher risk of developmental delay at age 2 years in the communication (OR, 1.61 [95% CI, 1.23-2.10] for 1 to <2 h/d; 2.04 [1.52-2.74] for 2 to <4 h/d; 4.78 [3.24-7.06] for ≥4 vs <1 h/d), fine motor (1.74 [1.09-2.79] for ≥4 vs <1 h/d), problem-solving (1.40 [1.02-1.92] for 2 to <4 h/d; 2.67 [1.72-4.14] for ≥4 vs <1 h/d), and personal and social skills (2.10 [1.39-3.18] for ≥4 vs <1 h/d) domains. We also observed an association between screen time at age 1 year and developmental delay at age 4 years in the communication (OR, 1.64 [95% CI, 1.20-2.25] for 2 to <4 h/d; 2.68 [1.68-4.27] for ≥4 vs <1 h/d) and problem-solving (1.91 [1.17-3.14] for ≥4 vs <1 h/d) domains.
We conducted a supplemental analysis that excluded 19 children whose parents self-reported that their child had been diagnosed with autism spectrum disorder and cerebral palsy by age 4 years as a factor in the association between screen time and developmental delay. We observed that the estimates did not show any meaningful departure from the main results.
The eTable in Supplement 1 presents the results of the complete case analysis. No significant difference in interpretation due to the use of multiple imputations was observed.
The findings of this study support previous research showing an association between screen time among young children and subsequent developmental outcomes. 7 - 18 These results also suggest that there was a dose-response association between longer screen time at age 1 year and developmental delays in communication and problem-solving at ages 2 and 4 years. In particular, more than 4 hours of screen time per day was associated with developmental delays in communication and problem-solving across ages 2 and 4 years.
The association observed between screen time and developmental delay among young children was domain specific. For example, the associations between screen time of children aged 1 year and the communication and problem-solving domains were consistent across ages, although no association was observed in the gross motor domain at ages 2 and 4 years. Sugiyama et al 7 examined the association between screen time at age 2 years and 3 domains (communication skills, daily living skills, and social skills) at age 4 years. They found that screen time was associated with poorer communication and daily living skills and was not associated with social skills. 7 In terms of domain-specific associations, their results are consistent with ours. Here, associations were consistently observed in the communication and problem-solving domains for children aged 2 and 4 years and not in the personal and social skills domain at age 4 years. In addition, a meta-analysis 12 reported an association between screen time and language development, and a cross-sectional study 8 examining the association between screen time and the 5 domains of the ASQ-3 found associations in the domains of communication, problem-solving, and personal and social skills. The results of these previous studies and our study suggested an association between screen time and communication and problem-solving domains in young children, while results for personal and social skills were inconsistent across studies. Based on 2 longitudinal time points of outcomes for each developmental domain, this study emphasized that screen time was not associated with all developmental domains.
We observed that screen time for children aged 1 year was associated with the fine motor and personal and social skills domains at age 2 years; however, this association was not confirmed at age 4 years. There are 2 possible hypotheses for this finding. One hypothesis is that the developmental delay of fine motor and personal and social skills for children aged 2 years caught up with them at age 4 years. Further follow-up studies would be needed to verify whether this phenomenon is specific to the fine motor and personal and social skills domains or whether the association is not confirmed with age even in the communication and problem-solving domains. Another hypothesis is that reverse causation occurs, in which a developmental delay of fine motor or personal and social skills lengthens screen time. Previous studies have examined the association between screen time and the personal and social skills domain; although a cross-sectional study reported an association, 8 a prospective study found no association. 7 Application of the reverse causality described earlier would explain why the association was confirmed in the cross-sectional study and not in the prospective study. However, our prospective study confirmed an association between screen time at age 1 year and developmental delay of personal and social skills at age 2 years. Although this phenomenon is unknown, applying the reverse causality hypothesis may explain the confirmed association at age 2 years in our study by assuming that developmental delay in the personal and social skills domain at age 1 year leads to longer screen time at age 1 year, and developmental delay in the personal-social domain at age 1 year is reflected in the developmental delay in that domain at age 2 years.
Although screen time has been associated with developmental delay, it may have an educational aspect depending on the programs watched on electronic devices. In fact, a meta-analysis showed that greater screen use was associated with decreased language skills, whereas screen time spent on educational programs was associated with increased language skills. 12 In addition, the American Academy of Pediatrics recommends that high-quality (eg, educational) programs should be selected when introducing digital media to children aged 18 to 24 months. 2 Because it is difficult to limit screen time in general in today’s world of electronic devices, it may be beneficial to identify and limit the screen time aspects that are associated with developmental delays while taking advantage of the educational aspects.
This study has 2 strengths. First, developmental delay was measured using the ASQ-3, which has been validated worldwide and used in a variety of studies. 9 , 35 - 37 Although the ASQ-3 is not a diagnostic tool, it is an appropriate screening tool for examining developmental delays according to several developmental domains. Second, the analysis was conducted with one of the largest prospective cohorts of any study examining the association between screen time and child development outcomes.
A limitation is that the information we collected did not allow us to separate educational screen time from other types of screen time. Doing so may have helped us in examining the association between screen time and child development while considering both positive and negative aspects of screen time.
In this cohort study, greater screen time at age 1 year was associated in a dose-response manner with developmental delays in communication and problem-solving at ages 2 and 4 years. These findings suggest that domains of developmental delay should be considered separately in future discussions on screen time and child development.
Accepted for Publication: June 22, 2023.
Published Online: August 21, 2023. doi:10.1001/jamapediatrics.2023.3057
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2023 Takahashi I et al. JAMA Pediatrics .
Corresponding Author: Taku Obara, PhD, Tohoku Medical Megabank Organization, Tohoku University, 2-1 Seiryo-machi, Aoba-ku, Sendai 980-8573, Japan ( [email protected] ).
Author Contributions: Mr Takahashi and Dr Obara had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Takahashi, Obara, Tsuchiya.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Takahashi, Obara, Tsuchiya.
Critical review of the manuscript for important intellectual content: All authors.
Obtained funding: Obara, Kuriyama.
Administrative, technical, or material support: Takahashi, Obara, Kuriyama.
Supervision: Kuriyama.
Conflict of Interest Disclosures: Dr Kuriyama reported receiving grants from the Japanese government during the conduct of the study. No other disclosures were reported.
Funding/Support: The Tohoku Medical Megabank Project Birth and Three-Generation (TMM BirThree) Cohort Study was supported by grants JP17km0105001, JP21tm0124005, and JP19gk0110039 from the Japan Agency for Medical Research and Development.
Role of the Funder/Sponsor: The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement: See Supplement 2 .
Additional Contributions: We thank the participants in the TMM BirThree Cohort Study and the staff members of the Tohoku Medical Megabank Organization. The list of members is available at https://www.megabank.tohoku.ac.jp/english/a220901/ .
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Neuro-anatomic evidence for the maturational delay hypothesis of ADHD
Affiliation.
- 1 Department of Child Psychiatry/Medical Research Council Social, Genetic, and Developmental Psychiatry Research Center (SGDP), Institute of Psychiatry, 16 De Crespigny Park, SG DP PO46, London SE5 8AF, United Kingdom. [email protected]
- PMID: 18077397
- PMCID: PMC2148352
- DOI: 10.1073/pnas.0710329105
Publication types
- Attention Deficit Disorder with Hyperactivity / physiopathology*
- Developmental Disabilities / physiopathology*
- Nervous System / anatomy & histology*
- Nervous System / physiopathology*
Grants and funding
- G0300155/MRC_/Medical Research Council/United Kingdom
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 21 February 2020
Maturational delay and asymmetric information flow of brain connectivity in SHR model of ADHD revealed by topological analysis of metabolic networks
- Seunggyun Ha ORCID: orcid.org/0000-0003-2016-1373 1 , 2 ,
- Hyekyoung Lee 1 , 3 ,
- Yoori Choi 1 ,
- Hyejin Kang 1 , 4 ,
- Se Jin Jeon 5 ,
- Jong Hoon Ryu 5 , 6 ,
- Hee Jin Kim 7 ,
- Jae Hoon Cheong 7 ,
- Seonhee Lim 8 ,
- Bung-Nyun Kim 9 &
- Dong Soo Lee 1 , 10
Scientific Reports volume 10 , Article number: 3197 ( 2020 ) Cite this article
5085 Accesses
16 Citations
10 Altmetric
Metrics details
- Network models
Attention-deficit hyperactivity disorder (ADHD) is a complex brain development disorder characterized by hyperactivity/impulsivity and inattention. A major hypothesis of ADHD is a lag of maturation, which is supported mainly by anatomical studies evaluating cortical thickness. Here, we analyzed changes of topological characteristics of whole-brain metabolic connectivity in twelve SHR rats selected as ADHD-model rats by confirming behavior abnormalities using the marble burying test, open field test, and delay discounting task and 12 Wistar Kyoto rats as the control group, across development from 4 weeks old (childhood) and 6 weeks old (entry of puberty). A topological approach based on graph filtrations revealed a lag in the strengthening of limbic-cortical/subcortical connections in ADHD-model rats. This in turn related to impaired modularization of memory and reward-motivation associated regions. Using mathematical network analysis techniques such as single linkage hierarchical clustering and volume entropy, we observed left-lateralized connectivity in the ADHD-model rats at 6 weeks old. Our findings supported the maturational delay of metabolic connectivity in the SHR model of ADHD, and also suggested the possibility of impaired and compensative reconfiguration of information flow over the brain network.
Similar content being viewed by others

Autism spectrum disorder

Closed-loop recruitment of striatal interneurons prevents compulsive-like grooming behaviors

A brainstem–hypothalamus neuronal circuit reduces feeding upon heat exposure
Introduction.
Attention-deficit hyperactivity disorder (ADHD) is one of the most common brain developmental disorder characterized by typical symptoms of inattention, hyperactivity, and impulsivity. ADHD patients have not only problems with attention and executive function but also suffer from considerable memory impairment, especially for working memory and even long-term memory 1 , 2 . ADHD usually initiates during childhood or school-age 3 . Almost 35% of ADHD subjects become symptom-free within adolescence 4 . About the pathogenesis of ADHD, there has been controversy about whether ADHD is caused by permanent deviation from typical brain development or delayed normal maturation of the brain. The delayed maturation hypothesis was supported by structural evidence not only of longitudinal studies of the cortical thickening 5 , 6 , but also of a mega cross-sectional study of the volume of subcortical structures, including the amygdala, accumbens, and hippocampus 7 .
Meanwhile, ADHD subjects reveal not only these structural changes but also the dysfunction of interregional connectivity 8 . Pathophysiologic models of ADHD have suggested the dysfunction of the fronto-striatal circuit mediating executive functions or the insufficient suppression of the default mode network (DMN) during cognitively demanding situations 8 , 9 . However, regarding maturational changes of functional connectivity of ADHD, there is only a limited number of cross-sectional functional magnetic resonance imaging (fMRI) studies, which suggests developmental lag within DMN connectivity or in DMN connections with task-positive networks 10 . Considering the difficulty of repeated imaging in ADHD children, a preclinical longitudinal study in rats might help corroborate this ‘delayed maturation’ hypothesis or to discover more elaborate findings to be translated to explain the changes of brain networks in ADHD children.
In this investigation, we examined the developmental changes of whole-brain network longitudinally by acquiring brain F-18 fluorodeoxyglucose (FDG) positron-emission-tomography (PET) from ages of childhood (4 weeks old) to the period of ‘entry of puberty’ (6 weeks old) in a spontaneous hypertensive rat (SHR) model of ADHD and in its control that Wistar Kyoto rat (WKY). Because of the behavioral heterogeneity, we chose rats with ADHD-phenotype using three behavioral tests; marble burying test (MBT) and delay discounting task (DDT) as impulsivity tests and open field test (OFT) as a hyperactivity test. Burying of harmless objects has been considered compulsive-, anxiety-like, or impulsive behavior of rodents 11 . Even though it lacks illness specificity, MBT has been used as one of the impulsive burying indexes 12 , 13 . DDT was applied to compensate for the lack of specificity for impulsivity, which measures the tendency to prefer immediate rewards than larger but delayed rewards. Rats with impulsivity are intolerant to the forced waiting for a delayed reward 14 . OFT presents information about exploratory behavior in the new environment and often adopted as hyperactivity index in ADHD-model 15 .
One of most common approaches to assessing brain connectivity from brain imaging data is constructing a graph composed of nodes (i.e. voxels or brain regions) and edges representing connections among nodes. The connections are usually assessed based on statistical dependencies, (i.e. correlation) among neurophysiological signals of brain regions at a certain thresholding level 16 . The problem is that there is no general rule for the appropriate level of thresholding, and it affects to the results of graph parameters.
Topological data analysis is a relatively new mathematical framework based on the algebraic topology, which is useful to acquire intrinsic topological features 17 . Algebraic topology generally concerns the shape of topological spaces regardless of stretching and shrinking 18 . For example, the k -th Betti-number is topological information representing the number of k-dimensional holes in a topological space. The zeroth Betti-number ( β 0 ) represents the number of connected components in a given topological space. Creating a filtered graph from a weighted graph, which preserves the relational information of original weights and its ordering, is particularly useful to avoid arbitrariness of threshold 18 . The graph filtration was successfully applied to brain network analysis 19 , 20 , 21 .
The maturation status of brain connectivity over the age of the rat may be related to the integration of whole brain regions 22 . To evaluate the integration of brain networks, we used graph filtration 19 . During the graph filtration, we observed the change of the connected structure of a brain network by varying thresholds, which was related to hierarchical single linkage clustering. A barcode and persistence diagram are widely used in TDA. However, in this study, a β 0 -curve is used for quantifying and visualizing the change of connected structures in a brain network, because it has the same information as the barcode and persistence diagram of connected components in the graph filtration. We also quantified and visualized the local changes of brain network integration by single linkage matrix (SLM), and minimum spanning tree (MST).
The graph filtration showed the whole brain integration procedure; however, it did not show the network topology after all the brain regions were integrated. To solve this problem, we applied volume entropy and generalized Markov system (GMS) to a connected component that had all nodes in a network with the minimum number of edges, called the giant connected component (GCC) of the whole-brain metabolic network in this paper. The GMS with the volume entropy is a model for measuring information flow on a brain network. It found the volume entropy and edge capacity that measured network efficiency in the view of information flow and the amount and direction of information flow on edges, respectively 23 . Traditional approaches of directed brain networks have mainly considered effective connectivity where the direction of edges is estimated by causality between two nodes 16 . On the other hand, the direction in edge capacity was estimated from the local difference of topology in a network. In other words, while the traditional directed networks were mainly a method for network construction directly estimated from data, the proposed method applied to a network, not a data, in order to see the local difference of topology in a network.
The details of the experimental design (Fig. 1 ), image preprocessing method and topological network analysis are in the method section.
Scheme of rat experiments for FDG PET imaging and intervening behavioral tests. The overall scheme of the animal experiment was composed of two times of PET imaging at 4 weeks and 6 weeks of age and three behavioral tests, i.e., marble-burying test, open field test, and delay discounting task. Phenotypically positive ADHD-model rats were chosen later, with the behavioral tests blinded to the imaging results.
Behavioral characteristics of ADHD-model rats
The selected ADHD-model rats significantly buried more marbles than the control rats in the MBT at 5 weeks old (12.5 ± 2.6 vs . 2.8 ± 2.1; P < 0.001) (Fig. 2A ). The ADHD-model rats moved more than the control rats in the OFT at 8–9 weeks old with a borderline significance (5.0 m ± 1.5 m vs . 3.6 m ± 1.3 m; P = 0.079) (Fig. 2B ). In the DDT at 8–9 weeks old, the ADHD-model rats chose the immediate but small rewards more than the large but delayed rewards more frequently than the control rats (12.8% ± 8.2% vs . 35.8% ± 33.3%; P = 0.030) (Fig. 2C ).
Behavioral tests representing ADHD-phenotype. ADHD-phenotype was tested by three behavioral tests: marble burying test ( A ), open field test ( B ), and delay discounting test ( C ).
Delayed maturation of limbic-cortical/subcortical connections
Curves of β 0 which visualize the change in the number of connected components with filtration showed that GCC was made faster meaning stronger connections in the control rats than in the ADHD-model rats at the same ages. Also, GCC was made faster in older age than younger age in both groups of the control rats and ADHD-model rats (Fig. 3A ). In detail, the control rats at 4 weeks old showed that the frontal and somatosensory cortices were strongly connected first during filtration. Subsequently, other temporo-parieto-occipital cortices, striatum, and limbic regions including the entorhinal cortex and retrosplenial cortex (RSC) were joined to the fronto-somatosensory component. The thalamus (THA) and hippocampus were the last nodes to construct the GCC. When the control rats grew up to 6 weeks old, the GCC was made faster and kept the consistency of the hierarchy of whole brain integration.
Brain network analysis with graph filtration during maturation. The changes in the number of connected components with threshold filtration were shown ( A ). The sequence of integration of a GCC during filtration was visualized in each group ( B ). Maturational changes of SLDs between region pairs were shown in the form of SLMs ( C ). The SLDs between hippocampus-cortical/subcortical pairs of regions were significantly getting closer in the control rats (WKY) during growth (FDR < 0.05) ( D ). The SLDs between RSC-cortical/subcortical pairs and between left-sided cortices of the ADHD-model rats (SHR_ADHD) were significantly getting closer during growth (FDR < 0.05) ( E ). Abbreviations: AC, auditory cortex; ADH, anterodorsal hippocampus; F, frontal; FAC, frontal association cortex; FDR, false discovery rate; GCC, giant connected component; HIP, hippocampus; O, occipital; P, parietal; ParA, parietal association cortex; PVH, posteroventral hippocampus; RSC, retrosplenial cortex; SLD, single linkage distance; SLM, single linkage matrix; Ssc, somatosensory cortex; T, Temporal; VC, visual cortex; w. or w.o., weeks old.
The integrating hierarchy of the GCC in the ADHD-model rats at 4 weeks old was similar to that of the control rats even though the integration of the temporo-parieto-occipital cortices, striatum, and limbic regions was slower. In the ADHD-model rats at 6 weeks old, the connections among the left-sided fronto-parieto-temporo-occipital cortices were stronger than those among the right-sided cortices. The limbic regions were integrated faster than in the ADHD-model rats of 4 weeks of age but still the last part integrated into the GCC (Fig. 3B,C ). Dendrograms presenting a detailed connecting hierarchy were provided in Supplemental Fig. S1 .
The cortical/subcortical connections with the hippocampus in the control rats were significantly stronger at 6 weeks old compared with at 4 weeks old (false discovery rate [FDR] < 0.05 by paired permutation) (Fig. 3D ). Meanwhile, the ADHD-model rats at 6 weeks old had stronger connections among the left-sided cortices and between RSC and cortical/subcortical regions compared with at 4 weeks old (FDR < 0.05 by paired permutation) (Fig. 3E ).
Impaired modularization of memory and reward-motivation regions
The memory-related nodes (bilateral anterodorsal hippocampus [ADH], posteroventral hippocampus [PVH], THA, RSC, and INS) and the reward-motivation-related nodes (bilateral ACCmedial prefrontal cortex [mPFC], caudoputamen [CP]) were modularized on the MSTs of control rats during growth. The number of directly connected edges between the memory-related nodes on the MSTs significantly increased in the control rats from 4 weeks to 6 weeks old (5 vs . 8, P < 0.05 by paired permutation) (Fig. 4A,B ), but not in the ADHD-model rats (5 vs . 5, P > 0.10 by paired permutation) (Fig. 4C,D ). The modularization trend was also observed in the reward-motivation regions consisting of CP, mPFC and ACC in the control rats with borderline significance (3 vs . 5, P = 0.10 by paired permutation) (Fig. 4A,B ), not in the ADHD-model rats (3 vs . 3, P > 0.10 by paired permutation) (Fig. 4C,D ).
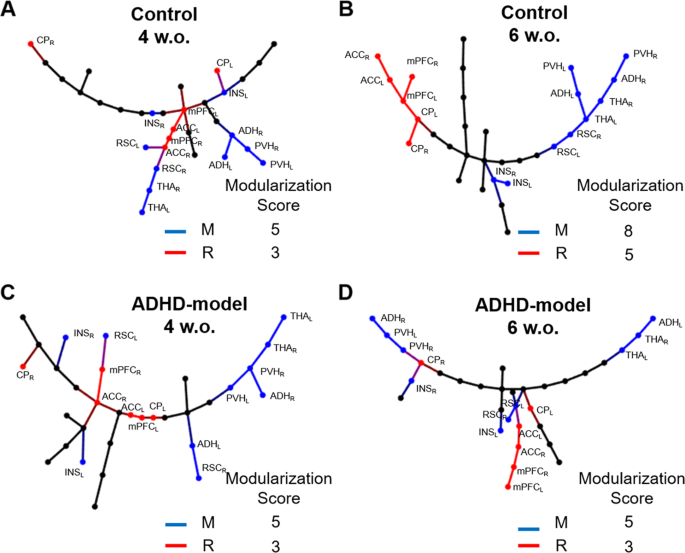
Modularization of memory and reward-motivation related regions on MSTs. The MSTs of control rats at the ages of 4 ( A ) and 6 weeks ( B ), and of ADHD-model rats at the ages of 4 ( C ) and 6 weeks ( D ) were visualized. The modularization score was counted as the number of directly connected edges between nodes belonging to the memory-related (blue) or the reward-motivation-related (red) regions. Abbreviations: ACC, anterior cingulate cortex; ADH, anterodorsal hippocampus; CP, caudoputamen; INS, insular cortex; L (small), left; mPFC, medial prefrontal cortex; MST, minimum spanning tree; M, memory-related regions; PVH, posteroventral hippocampus; R, reward-motivation-related regions; R (small), right; RSC (retrosplenial cortex); w.o., weeks old.
Global feature changes during development
There was an increasing trend of the volume entropy in the control rats during growth (P = 0.10 by paired permutation) but not in the ADHD-model rats (P > 0.10 by paired permutation) (Fig. 5A ). In contrast, both groups showed the trend of increasing global efficiency during growth with a borderline significance (all P < 0.10 by paired permutation, respectively), and the ADHD-model rats’ brain networks had lower global efficiency than the control rats’ ones. There was a significant difference in global efficiency between the ADHD-model rats at 4 weeks old and control rats at 6 weeks old (P < 0.05 by 10,000 permutation) (Fig. 5B ).
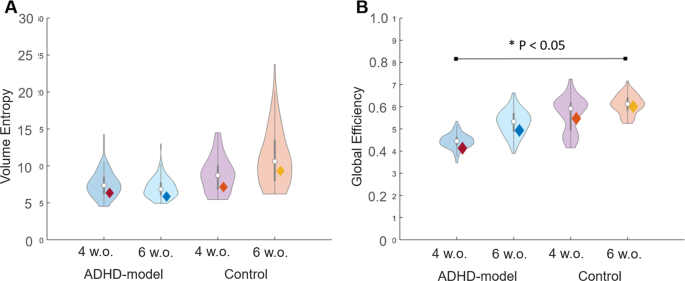
Volume entropy and global efficiency of brain graphs. The efficiency of brain graphs was assessed by volume entropy ( A ) and global efficiency ( B ). Rats with ADHD trait and younger age had less amount of volume entropy and less efficient brain graphs than control and older aged. Brain networks of both groups had been improved in regards to global efficiency, but only in control rats in regards to volume entropy. Note: Violin plots were results of 100-bootstrapping in each group. Abbreviation: w.o., weeks old.
Asymmetric information propagation
Mapping node capacity on a directed graph of edge capacities representing information propagation showed a pattern that positive (larger sum of afferent edge capacities than sum of efferent edge capacities at a node) at anterior and negative (larger sum of efferent edge capacities than sum of afferent edge capacities at a node) at posterior in the control rats (Fig. 6A , ‘Control’). Now we will call ‘afferent node capacity’ meaning a sum of afferent edge capacities for a node (Fig. 6B ) and ‘efferent node capacity’ meaning a sum of efferent edge capacities for a node (Fig. 6C ). Node capacity is simply the difference between afferent and efferent node capacities (afferent node capacity minus efferent node capacity).
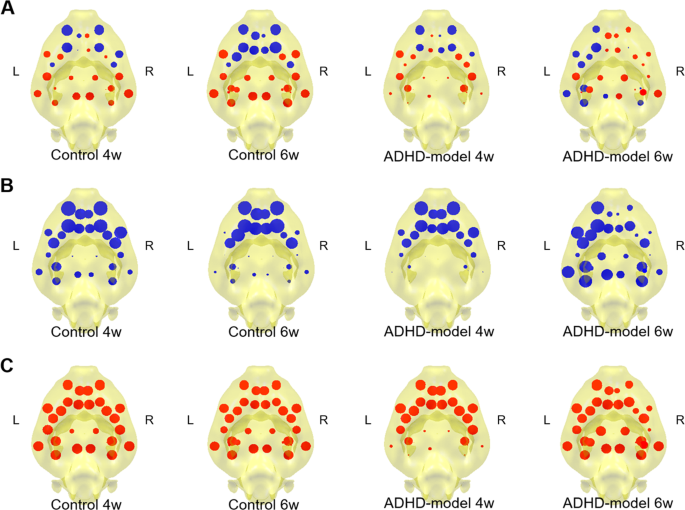
Mapping of stationary information flow at nodes. Node capacity maps of 4 groups that control rats of 4 and 6 weeks old and ADHD-model rats of 4 and 6 weeks old were visualized ( A ). Afferent node capacity (sum of afferent edge capacities to each node) ( B ) and efferent node capacity (sum of efferent edge capacities from each node) ( C ) were visualized as blue and red discs with variable size, respectivelyi. Thus, blue color in ( A ), means larger afferent node capacity than effent node capacity at a node and red color in ( A ) means larger efferent node capacity than afferent node capacity at a node. Abbreviations: L, left; R, right; w., weeks old.
During development from 4 weeks to 6 weeks of age, there were little changes in node capacity map. In the ADHD-model rats at 4 weeks of age, the afferent and efferent node capacities in the posterior/subcortical nodes were all small, which meant the least participation of the nodes to the information propagation (Fig. 6A–C , ‘ADHD-model 4w.’). The ADHD-model rats at 6 weeks of age had a hemispherical asymmetric pattern of left-deviated information propagation of the brain network. When compared afferent and efferent node capacity maps with ADHD-model rats at 4 weeks of age, the ADHD-model rats at 6 weeks of age had less participation of left frontal areas and relatively more participation of posterior/subcortical areas ( Fig. 6A–C , ‘ADHD-model 6w.’).
The ADHD-model rats at 6 weeks old had significantly larger afferent node capacity at the left auditory cortex (AC) (FDR < 0.05 by 10,000 permutation) and significantly smaller afferent node capacity at the right frontal association cortex (FAC), right orbitofrontal cortex (OFC) and left mPFC compared with those of the control rats of the same age (FDR < 0.05 by 10,000 permutation) (Fig. 7A ). The ADHD-model rats at 6 weeks old had significantly smaller efferent node capacities at right mPFC, OFC, and striatum than those of the control rats of 6 weeks age (FDR < 0.05 by 10,000 permutation) (Fig. 7B ).
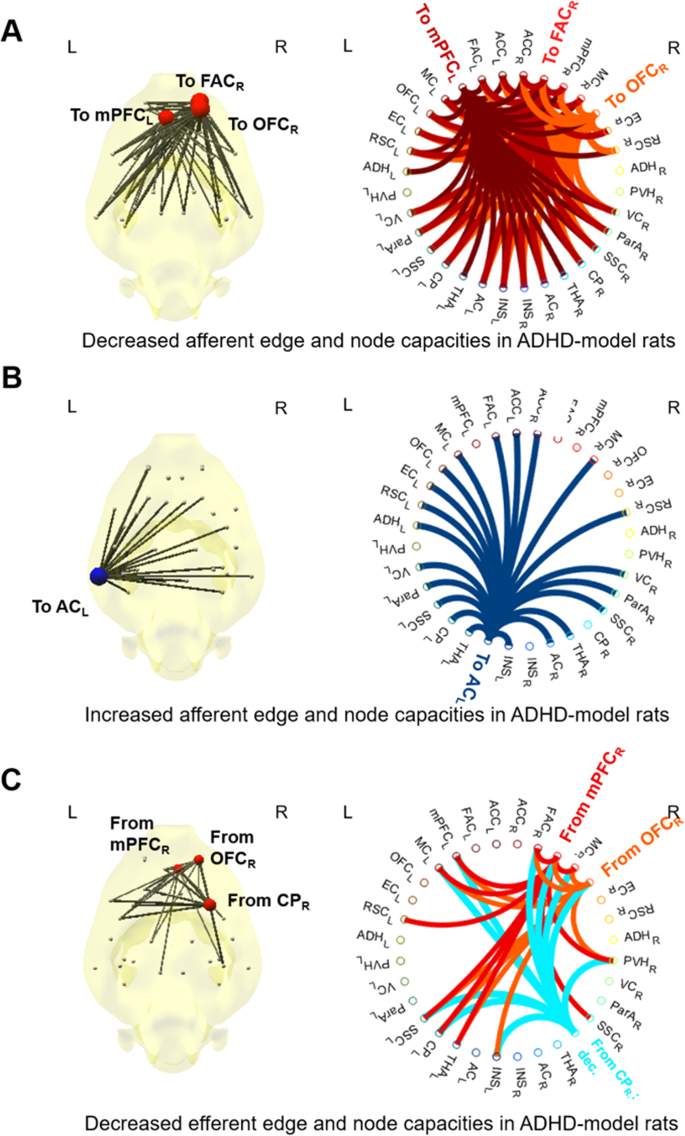
Nodes and edges with significantly different afferent or efferent capacities in the ADHD-model rats at 6 weeks old compared with the control rats at the same age. About afferent node capacity (sum of afferent edge capacities at a node), the ADHD-model rats’ was lower at right FAC, right OFC, and left mPFC ( A ), and higher at left AC significantly than the control rats at the same age (all FDR < 0.05) ( B ). About efferent node capacity (sum of efferent edge capacities at a node), the ADHD-model rats’ was lower at right mPFC, OFC and striatum (all FDR < 0.05) ( C ). Edges with significantly different edge capacities were also visualized (all FDR < 0.05) ( A – C ). Abbreviations: AC, auditory cortex; CP, caudoputamen; FDR, false discovery rate; mPFC, medial prefrontal cortex; OFC, orbitofrontal cortex. Note: See Supplemental Methods and Materials for details of regions of interest. The size and color (red/blue) of node disc on the brain diagram represented the amount of difference of afferent or efferent node capacity compared with the control rats. Each node had its color on circular plots, which indicated the destination of afferent edges in ( A,B ) or the starting points of efferent edges in ( C ).
Our results supported the delayed maturation hypothesis by metabolic connectivity with topology analysis in the ADHD-rat model. Within the observation period until early puberty age, the ADHD-model rats had the maturation pattern of the metabolic network architecture in a delayed way, especially in the limbic and cortical/subcortical connections as well as the global efficiency. The modularization problems were found for the memory-related and reward-motivation-related regions in the ADHD-model rats, which might be associated with ADHD-behavior. Noteworthy, the ADHD-model rats at 6 weeks old had asymmetry in both of the brain connectivity architecture by the filtration framework and the information flow by the mathematical modeling. We interpret this asymmetry due to the abnormal developmental circuit(s) dysfunction/compensation in the ADHD-model rats.
From the integration hierarchy of whole-brain network, we could find the trend of the delayed maturation of the cortico-striato-thalamo-cortical (CSTC) circuits and large-scale networks including the parieto-temporo-occipital cortices. This trend of integration hierarchy of whole-brain network was once identified in typically developing children evaluated with the graph-filtration framework, which revealed faster wiring between the frontal and parietal cortices, followed by other cortices and subcortical structures 19 . The CSTC circuits have extensive connections with the parieto-temporo-occipital cortices, which compose large-scale brain networks outside CSTC circuits. Several parallel CSTC circuits, including the motor, associative, and limbic circuits have been known to be associated with ADHD 24 . Less mature CSTC circuits and large scale network observed in the ADHD-model rats matched well with previous fMRI human studies, which reported a decrease of resting-state connectivity of ADHD in the fronto-striatal, fronto-parietal, and temporo-parietal networks 25 , 26 , 27 , 28 .
The finding of weaker limbic-cortical/subcortical connections in the ADHD-model SHR rats was concordant with the result of a preclinical study in another ADHD model, Naples-High-Excitability (NHE) rats, on the resting-state functional MRI (rsfMRI). In this previous study, connectivity decreased between the hippocampus and entire cortex in the NHE rats compared with that in the Naples-Random-Bred controls 29 . A human fMRI study reported that the immature frontal-limbic activation pattern was the most characteristic pattern for discriminating the controls and ADHD children, which furthermore correlated with symptom severity 30 .
The asymmetry of information flow which appeared at 6-weeks of age in the ADHD-model rats was thought to be the result of both abnormality and its compensation process. The right frontal cortices and striatum were considered to be impaired regions in the ADHD-model because those regions had both decreased afferent and efferent node capacities, which means less contribution of those nodes to the information flow. However, the left hemisphere with the rich afferent information flow had a similar level of efferent information flow compared with other nodes, which indicates a compensative process, not an impaired process. We interpreted this as representing the asymmetry in treating information flow.
The finding as hemispherical asymmetry is consistent with the previous studies reporting abnormal brain laterality with the right hemisphere deficit in ADHD children 31 , 32 , 33 , 34 . ADHD often have right-sided spatial attentional deficits, which could have resulted from dysfunction within the right frontal-parietal network 35 . However, there have been contradictory results emphasizing the left-sided impairment in ADHD 36 . Our data support the right frontal deficit in ADHD.
The left-lateralized connectivity with the rich information flow on the left AC might be compensatory changes to the developmental impairment of the right frontal cortex. A human fMRI study reported greater activation of left hemispheric linguistic processing areas during forward digit span task in adults with ADHD 37 . Another human fMRI study has reported the association of higher temporal cortex activations and lower variability for post-error trial with Go/No-Go task in ADHD children, suggesting the compensatory recruitment of temporal area 38 . The compensatory process is observed in various developmental disorders, including autism 39 or tic-disorders 40 as well as ADHD 41 .
SHR is used popularly as a rat model of ADHD-combined type, as they develop typical behavioral characteristics of ADHD such as hyperactivity and inattention at 5 to 9 weeks of age 42 . SHR rats had smaller striatal volumes than control WKY rats 43 , which was a consistent finding in human ADHD 7 . Individuals of SHR show the variability of behavioral characteristics 44 . The behaviorally homogeneous group with inattention and hyperactivity was used as the ADHD-model rats in our study. Meanwhile, a resting-state fMRI study with SHR rats at 6 weeks old without assessment of behavioral characteristics has reported variations in the DMN under the different depth of isoflurane anesthesia 45 . In our study, the rats were anesthetized during the injection of FDG and were awake for waiting and anesthetized again during PET/CT imaging. FDG was taken up in the rats’ brains for 35–40 minutes while they were awake. Therefore, the issue of behavioral heterogeneity in SHR rats and the effect of anesthesia did not compromise the findings of our study.
Our preclinical study has several limitations, such as small sample size, which would have affected statistical power and thus higher false non-discovery. As the first study of this kind with 12 rats per each group, we did not attend to type II error and instead carefully performed statistical tests to control type I error using FDR-correction. Our study provides information about brain network maturation of ADHD-rat model till entry of puberty age; however ADHD in humans has quite prominent symptoms after puberty and early adulthood ages. Though connectivity analysis for these ages might be helpful to understand ADHD pathophysiology more, SHR spontaneously develops hypertension after they are adults (10–12 weeks of age), which is a confounding factor in ADHD-model 46 . Further studies with ADHD children are needed to validate our findings. It might or might not corroborate our findings in rats, either of which will unravel the similarity between the rat model and human ADHD or discordance.
In conclusion, our preclinical longitudinal study validated that delayed maturation underlies in the wiring of the metabolic network in the SHR model of ADHD during development from childhood to entry of adolescence. Asymmetry of information propagation over the ADHD brain network might be a composite result of impairment in the right fronto-striatal regions, and compensatory reconfiguration of brain network in left-sided strengthened. Topological approaches with graph filtration and mathematical modeling estimating information flow might be useful for unveiling connectivity problems in brain disorders.
Animal models and experimental design
All animal care and experiments for this research were approved by the Seoul National University Institutional Animal Care and Use Committee and the Kyung Hee University Institutional Animal Care and Use Committee. All experiments were performed in accordance with relevant guidelines and regulations regarding the care and the use of animals for the experimental procedures. Thirty-four SHRs and 12 WKYs were raised in a laboratory cage with a standard condition (22–24 °C, 12-hours light and dark cycle) with no restriction of standard feeding and water-drinking. All the rats underwent two times of brain FDG PET scans at 4 weeks old and 6 weeks old, which represent childhood and entry of puberty, respectively 47 . Behaviors, including hyperactivity and impulsivity, were checked with MBT at 5 weeks old, and OFT and DDT at 8 to 9 weeks old (Fig. 1 ). Rats belong to the lower quartile per each behavioral test in SHRs were excluded from the ADHD-model rats, which led to twelve ADHD-model rats matching the number of WKY rats. Whole-brain connectivity based on brain metabolic activity was analyzed for 4 groups as follows: (1) ADHD-model rats at 4 weeks old, (2) ADHD-model rats at 6 weeks old, (3) control rats at 4 weeks old, and (4) control rats at 6 weeks old.
Behavioral tests
MBT was performed individually to reveal the rats’ degree of impulsivity. Rats were acclimated for 15 minutes before MBT. They were tested with 3 × 5 placed glass marbles for 15 minutes after the acclimatization. The number of buried marbles was counted after the removal of the rats from the cages. Burial of marble was determined when 50% or more of it was covered by bedding. OFT was performed individually to reveal rats’ hyperactivity by measuring the total distance of moving around for 30 minutes. The movement of rats was tracked by a video camera system installed above the open-field apparatus.
DDT measuring intolerance to delay was performed individually using previously described methods with minor changes 48 , 49 . Briefly, after habituation in the animal room without the restriction of feeding and sequential 2 days of food restriction, rats were trained for 5 days on two levers returning different amounts of food pellets per one press. A press on the right lever delivered a food pellet (about 45 mg) immediately (small and immediate reward), whereas a press on the left lever resulted in the delivery of five food pellets (large and delayed reward) later. After pellet delivery, the time-out period lasted 20 seconds, and the light was on during this period. During the testing phase for 4 days, a delay was sequentially increased for the large rewards over the test days (0, 10, 20, 30, and 40 seconds). Each test took 30 minutes. During adjustment of delay sessions, restricted feeding that 5 g of pellet per 100 g of body weight was allowed. The mean percentage of choice for the larger rewards with specific delays from 20 to 40 seconds was considered as the score of DDT.
Brain PET scanning and reconstruction
Brain PET images were obtained using a dedicated small animal PET/computed tomography (CT) scanner (eXplore VISTA, GE Healthcare, WI) after overnight fasting. Before brain PET/CT scanning, rats were anesthetized by 2% isoflurane at 1.5–2 L/min oxygen flow for 5 minutes before the injection of FDG (150–220 MBq/kg) via a tail vein. Rats were awake and took a rest in a dark room till brain PET/CT scanning. A static brain PET scan was acquired for 20 minutes, 45 minutes after FDG injection. The energy window of PET scanning was 250–700 keV. PET images were reconstructed using the three-dimensional ordered-subsets expectation maximum algorithm with the correction of attenuation, random, and scatter. The voxel size of reconstructed PET images was 0.3875 × 0.3875 × 0.775 mm 3 .
Preprocessing of brain FDG PET
Voxel size was rescaled by a factor of 10 in each dimension. The rescaled brain PET images were manually realigned to the Schiffer template of rat brain MRI T1 in PMOD2.7 (PMOD group, Zurich, Switzerland) 50 . Spatial alignment using non-linear registration on Statistical Parametric Mapping (SPM8, University College of London, London, UK) was applied with the Schiffer template of rat brain PET and binary brain mask. Global normalization of voxel counts was applied as the last step of preprocessing.
Brain parcellation and distance matrix computation
Among the 58 predefined ROIs on the Schiffer template, 32 ROIs, including the cortices and subcortical structures, were selected as nodes to construct a brain metabolic network in each group. See the Supplemental Methods and Materials for the details of the 32 ROIs. The Pearson correlation coefficient ( r ij , r ij > 0) between two nodes ( p i , p j ) was computed to obtain a positive correlation matrix. A distance ( d ij ) between two nodes ( p i , p j ) was defined as the following:
Graph filtration
We used a multiscale approach to analyze networks to avoid fixing the threshold of distance 18 . In this study, we performed graph filtration, which decomposed a weighted network into unweighted networks at many possible thresholds. We found the connected components and the number of connected components, denoted by β 0 of each unweighted network. The β 0 decreased from the number of nodes in a network to one by merging two connected components into a connected component by increasing the threshold. When β 0 = 1 at the minimum threshold, we called the connected component a GCC here. The change of β 0 with respect to threshold is visualized by β 0 -curve. The β 0 -curve had the same information as the barcode of connected components. All bars in the barcode of connected components always start from zero in a network, and only the end of bars which corresponds to the death of connected components has the information of the change of connected structures. The β 0 -curve is obtained by connecting the end of bars in the barcode.
The threshold of distance when two connected components were merged into a connected component during the graph filtration is called a single linkage distance (SLD) between the two connected components. If the two connected components are denoted by A and B ( \(A\cap B=\varnothing \) ), then, the SLD between A and B is defined by
The SLD between two nodes in A and B is defined by 51
When the number of nodes in a network is p , SLM is a p -by- p matrix of which element is an SLD. The β 0 was counted along the filtration to make a dendrogram, which is equivalent to an SLM. An example of counting β 0 and constructing an SLM is provided in Supplemental Fig. S2 .
Minimum spanning tree
Minimum spanning tree (MST) is a subset of a weighted network that has all nodes and the subset of edges which only connect \(\hat{x}\) and \(\hat{y}\) that satisfies
The MST of a weighted network is a network that has the minimum number of edges that have the same SLM of the weighted network 51 . Therefore, it is easier to see the modular structure of a weighted network.
We evaluated modularization of the reward-motivation system (CP, mPFC, and ACC) and memory system (anterodorsal hippocampus [ADH], posteroventral hippocampus [PVH], RSC, THA, and insula [INS]) during growth by counting the number of a direct connection between nodes included in each system 52 .
Volume entropy, and edge and node capacities
The graph filtration shows the change of the connected structure of a weighted network until constructing a GCC. To quantify the pattern of information flow of the weighted network constructing a GCC, we used the GMS of volume entropy.
The GMS is the generalization of the Markov chain defined on edges. In our GMS, an edge from v to w is different from an edge from w to v for any two nodes v and w in a network. Therefore, when the number of nodes is p , the number of all possible edges is q = p ( p − 1). Moreover, the GMS assumes that the sum of edge weights in a weighted network is equal to 2. Then, the edge-transition matrix is defined by
where a ef is 1 if an edge e is connected to an edge f in the network, 0, otherwise, h is a nonnegative constant, and l(f) is the weight of the edge f 23 , 53 .
Unlike random walk in Markov chain, the state transition by L(h) is from i + 1 to I such that
where z i shows the state of q edges at the i th step. When \(z={z}_{i}={z}_{i+1}\) , the GMS is stationary, and z , the eigenvector of L ( h ) corresponding to an eigen value of 1, is called the stationary state of q edges, and h satisfying \(z=L(h)z\) is called volume entropy.
The z can be represented in a matrix form such that \(Z=[{z}_{vw}]\) , where the ( v , w )th element is the stationary state of an edge from a node v to a node w in z . The Z is called an edge capacity matrix. The afferent node capacity of a node w is obtained by the sum of the column vectors of Z , i.e., \(\sum _{v}{z}_{vw}\) , and the efferent node capacity of a node v is obtained by the sum of the row vectors of Z , i.e., \(\sum _{w}{z}_{vw}\) . The node capacity of a node v is obtained by the difference between the afferent and efferent node capacities, i.e., \(\sum _{v}{z}_{wv}-\sum _{v}{z}_{vw}\) 23 .
To examine the statistical differences of the graph parameters, including SLD, modulation score, volume entropy, global efficiency, edge capacity, and afferent and efferent node capacities, we applied the permutation test, which is a subset of non-parametric tests. In the unpaired permutation tests between the ADHD-model and control groups, individual PET images were randomly shuffled from a single mixed data set to construct two pseudo-groups which had the same number of subjects to the original data sets ( n = 12) per each iteration. The graph parameters were calculated for the resampled pseudogroups in each iteration. The permuted distributions of the differences of the graph parameters between the pseudogroups were obtained via 10,000 times of iteration of the process, which were used to test the significance of the differences of the graph parameters between original groups with a two-tail P-value < 0.05 (Supplemental Fig. S3 ). In the paired permutation tests between 4 weeks old and 6 weeks old of the ADHD-model or control group, 12 times of random shuffling were available between paired data. Therefore, the paired permutation test with the enumeration of all the possible distinct 12-paired permutations (2 12 = 4,096 times) was done to test developmental changes of graph parameters of each group from 4 weeks old to 6 weeks old 54 . Multiple comparison problem was controlled as an FDR less than 0.05 using fdr_bh function with the Benjamini-Hochberg procedure in MATLAB 55 .
Rhodes, S. M., Park, J., Seth, S. & Coghill, D. R. A comprehensive investigation of memory impairment in attention deficit hyperactivity disorder and oppositional defiant disorder. Journal of Child Psychology and Psychiatry 53 , 128–137 (2012).
Article PubMed Google Scholar
Skowronek, J. S., Leichtman, M. D. & Pillemer, D. B. Long‐term episodic memory in children with Attention‐Deficit/Hyperactivity Disorder. Learning Disabilities Research & Practice 23 , 25–35 (2008).
Article Google Scholar
Tarver, J., Daley, D. & Sayal, K. Attention-deficit hyperactivity disorder (ADHD): an updated review of the essential facts. Child: Care, Health and Development 40 , 762–774 (2014).
CAS Google Scholar
Biederman, J. et al . Functional impairments in adults with self-reports of diagnosed ADHD: A controlled study of 1001 adults in the community. The Journal of Clinical Psychiatry 67 , 524–540 (2006).
Shaw, P. et al . Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proceedings of the National Academy of Sciences of the United States of America 104 , 19649–19654 (2007).
Article ADS CAS PubMed PubMed Central Google Scholar
Shaw, P. et al . Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Archives of General Psychiatry 63 , 540–549 (2006).
Hoogman, M. et al . Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis. The lancet Psychiatry 4 , 310–319 (2017).
Article PubMed PubMed Central Google Scholar
Cubillo, A., Halari, R., Smith, A., Taylor, E. & Rubia, K. A review of fronto-striatal and fronto-cortical brain abnormalities in children and adults with Attention Deficit Hyperactivity Disorder (ADHD) and new evidence for dysfunction in adults with ADHD during motivation and attention. Cortex; a journal devoted to the study of the nervous system and behavior 48 , 194–215 (2012).
Fassbender, C. et al . A lack of default network suppression is linked to increased distractibility in ADHD. Brain Research 1273 , 114–128 (2009).
Article CAS PubMed PubMed Central Google Scholar
Sripada, C. S., Kessler, D. & Angstadt, M. Lag in maturation of the brain’s intrinsic functional architecture in attention-deficit/hyperactivity disorder. Proceedings of the National Academy of Sciences of the United States of America 111 , 14259–14264 (2014).
Wolmarans, D. W., Stein, D. J. & Harvey, B. H. Of mice and marbles: Novel perspectives on burying behavior as a screening test for psychiatric illness. Cognitive, Affective, & Behavioral Neuroscience 16 , 551–560 (2016).
Schneider, T. & Popik, P. Attenuation of estrous cycle-dependent marble burying in female rats by acute treatment with progesterone and antidepressants. Psychoneuroendocrinology 32 , 651–659 (2007).
Article CAS PubMed Google Scholar
Llaneza, D. C. & Frye, C. A. Progestogens and estrogen influence impulsive burying and avoidant freezing behavior of naturally cycling and ovariectomized rats. Pharmacology Biochemistry and Behavior 93 , 337–342 (2009).
Article CAS Google Scholar
Koot, S., Adriani, W., Saso, L., Van Den Bos, R. & Laviola, G. Home cage testing of delay discounting in rats. Behavior Research Methods 41 , 1169–1176 (2009).
Zhuang, X. et al . Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proceedings of the National Academy of Sciences 98 , 1982–1987 (2001).
Article ADS CAS Google Scholar
Friston, K. J. Functional and effective connectivity: a review. Brain Connectivity 1 , 13–36 (2011).
Carlsson, G. Topology and data. Bulletin of the American Mathematical Society 46 , 255–308 (2009).
Article MathSciNet MATH Google Scholar
Sizemore, A. E., Phillips-Cremins, J. E., Ghrist, R. & Bassett, D. S. The importance of the whole: topological data analysis for the network neuroscientist. Network Neuroscience 3 , 656–673 (2019).
Lee, H., Kang, H., Chung, M. K., Kim, B. N. & Lee, D. S. Persistent brain network homology from the perspective of dendrogram. IEEE Transactions on Medical Imaging 31 , 2267–2277 (2012).
Kim, E. et al . Morphological brain network assessed using graph theory and network filtration in deaf adults. Hearing Research 315 , 88–98 (2014).
Kim, H. et al . Brain networks engaged in audiovisual integration during speech perception revealed by persistent homology-based network filtration. Brain Connectivity 5 , 245–258 (2015).
Choi, H. et al . Maturation of metabolic connectivity of the adolescent rat brain. Elife 4 , e11571 (2015).
Lee, H. et al . Volume entropy for modeling information flow in a brain graph. Scientific Reports 9 , 256 (2019).
Article ADS PubMed PubMed Central CAS Google Scholar
Jiang, X., Liu, L., Ji, H. & Zhu, Y. Association of affected neurocircuitry with deficit of response inhibition and delayed gratification in attention deficit hyperactivity disorder. Frontiers in Human . Neuroscience 12 , 506 (2018).
Google Scholar
Rubia, K. “Cool” inferior frontostriatal dysfunction in attention-deficit/hyperactivity disorder versus “hot” ventromedial orbitofrontal-limbic dysfunction in conduct disorder: a review. Biological Psychiatry 69 , e69–e87 (2011).
Cao, X. et al . Abnormal resting-state functional connectivity patterns of the putamen in medication-naive children with attention deficit hyperactivity disorder. Brain Research 1303 , 195–206 (2009).
Cao, Q. et al . Abnormal neural activity in children with attention deficit hyperactivity disorder: a resting-state functional magnetic resonance imaging study. Neuroreport 17 , 1033–1036 (2006).
Yu-Feng, Z. et al . Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain and Development 29 , 83–91 (2007).
Zoratto, F. et al . Enhanced limbic/impaired cortical-loop connection onto the hippocampus of NHE rats: Application of resting-state functional connectivity in a preclinical ADHD model. Behavioural Brain Research 333 , 171–178 (2017).
Hart, H. et al . Pattern classification of response inhibition in ADHD: toward the development of neurobiological markers for ADHD. Human Brain Mapping 35 , 3083–3094 (2014).
Carter, C. S., Krener, P., Chaderjian, M., Northcutt, C. & Wolfe, V. Asymmetrical visual-spatial attentional performance in ADHD: evidence for a right hemispheric deficit. Biological Psychiatry 37 , 789–797 (1995).
Casey, B. et al . Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry 36 , 374–383 (1997).
Castellanos, F. X. et al . Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Archives of General Psychiatry 53 , 607–616 (1996).
Chan, E. et al . Abnormal spatial asymmetry of selective attention in ADHD. Journal of Child Psychology and Psychiatry, and Allied Disciplines 50 , 1064–1072 (2009).
Johnson, K. et al . Right-sided spatial difficulties in ADHD demonstrated in continuous movement control. Neuropsychologia 48 , 1255–1264 (2010).
Hale, T. S. et al . Atypical EEG beta asymmetry in adults with ADHD. Neuropsychologia 48 , 3532–3539 (2010).
Hale, T. S., Bookheimer, S., McGough, J. J., Phillips, J. M. & McCracken, J. T. J. Atypical brain activation during simple & complex levels of processing in adult ADHD: an fMRI study. Journal of Attention Disorders 11 , 125–139 (2007).
Spinelli, S. et al . Variability in post‐error behavioral adjustment is associated with functional abnormalities in the temporal cortex in children with ADHD. Journal of Child Psychology and Psychiatry 52 , 808–816 (2011).
Hubl, D. et al . Functional imbalance of visual pathways indicates alternative face processing strategies in autism. Neurology 61 , 1232–1237 (2003).
Gates, L. et al . Neuroanatomy of coprolalia in Tourette syndrome using functional magnetic resonance imaging. Progress in Neuro-Psychopharmacology and Biological Psychiatry 28 , 397–400 (2004).
Konrad, K., Neufang, S., Hanisch, C., Fink, G. R. & Herpertz-Dahlmann, B. Dysfunctional Attentional Networks in Children with Attention Deficit/Hyperactivity Disorder: Evidence from an Event-Related Functional Magnetic Resonance Imaging Study. Biological Psychiatry 59 , 643–651 (2006).
Sontag, T. A., Tucha, O., Walitza, S. & Lange, K. W. Animal models of attention deficit/hyperactivity disorder (ADHD): a critical review. ADHD Attention Deficit and Hyperactivity Disorders 2 , 1–20 (2010).
Hsu, J.-W. et al . Striatal volume changes in a rat model of childhood attention-deficit/hyperactivity disorder. Psychiatry Research 179 , 338–341 (2010).
Johansen, E. B., Killeen, P. R. & Sagvolden, T. Behavioral variability, elimination of responses, and delay-of-reinforcement gradients in SHR and WKY rats. Behavioral and Brain Functions 3 , 60 (2007).
Huang, S. M. et al . Inter-Strain Differences in Default Mode Network: A Resting State fMRI Study on Spontaneously Hypertensive Rat and Wistar Kyoto Rat. Scientific Reports 6 , 21697 (2016).
Russell, V. A., Sagvolden, T. & Johansen, E. B. Animal models of attention-deficit hyperactivity disorder. Behavioral and Brain functions 1 , 9 (2005).
Article PubMed CAS Google Scholar
Sengupta, P. The Laboratory Rat: Relating Its Age With Human’s. International Journal of Preventive Medicine 4 , 624–630 (2013).
PubMed PubMed Central Google Scholar
Botanas, C. J. et al . Rearing in an enriched environment attenuated hyperactivity and inattention in the spontaneously hypertensive rats, an animal model of attention-deficit hyperactivity disorder. Physiology & Behavior 155 , 30–37 (2016).
dela Peña, I. et al . Methylphenidate and atomoxetine-responsive prefrontal cortical genetic overlaps in “impulsive” SHR/NCrl and Wistar rats. Behavior Genetics 47 , 564–580 (2017).
Schiffer, W. K. et al . Serial microPET measures of the metabolic reaction to a microdialysis probe implant. Journal of Neuroscience Methods 155 , 272–284 (2006).
Gower, J. C. & Ross, G. J. Minimum spanning trees and single linkage cluster analysis. Journal of the Royal Statistical Society: Series C (Applied Statistics) 18 , 54–64 (1969).
MathSciNet Google Scholar
Rogers, R. D. et al . Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biological Psychiatry 55 , 594–602 (2004).
Lim, S. Minimal volume entropy for graphs. Transactions of the American Mathematical Society 360 , 5089–5100 (2008).
Odiase, J. & Ogbonmwan, S. Exact permutation algorithm for paired observations: The challenge of RA Fisher. Journal of Mathematics and Statistics 3 , 116–121 (2007).
Article MathSciNet Google Scholar
Groppe, D. M., Urbach, T. P. & Kutas, M. Mass univariate analysis of event‐related brain potentials/fields I: A critical tutorial review. Psychophysiology 48 , 1711–1725 (2011).
Download references
Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIP) (No. 2015M3C7A1028926 and No. 2017M3C7A1048079), and NRF grant funded by the Korean Government (No. 2016R1D1A1A02937497, No. 2017R1A5A1015626, No. 2017R1E1A1A03070779, and No. 2018R1D1A1A02086383).
Author information
Authors and affiliations.
Department of Nuclear Medicine, Seoul National University College of Medicine, Seoul, Republic of Korea
Seunggyun Ha, Hyekyoung Lee, Yoori Choi, Hyejin Kang & Dong Soo Lee
Division of Nuclear Medicine, Department of Radiology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea
Seunggyun Ha
Biomedical Research Institute, Seoul National University Hospital, Seoul, Republic of Korea
Hyekyoung Lee
BK21 Plus Global Translational Research on Molecular Medicine and Biopharmaceutical Sciences, Seoul National University, Seoul, Republic of Korea
Hyejin Kang
Department of Oriental Pharmaceutical Science, College of Pharmacy, Kyung Hee University, Seoul, Republic of Korea
Se Jin Jeon & Jong Hoon Ryu
Department of Life and Nanopharmaceutical Science, College of Pharmacy, Kyung Hee University, Seoul, Republic of Korea
Jong Hoon Ryu
Department of Pharmacy, Uimyung Research Institute for Neuroscience, Sahmyook University, Seoul, Republic of Korea
Hee Jin Kim & Jae Hoon Cheong
Department of Mathematical Sciences, Seoul National University, Seoul, Republic of Korea
Seonhee Lim
Division of Child and Adolescent Psychiatry, Department of Psychiatry, Seoul National University College of Medicine, Seoul, Republic of Korea
Bung-Nyun Kim
Department of Molecular Medicine and Biopharmaceutical Sciences, Graduate School of Convergence Science and Technology, and College of Medicine or College of Pharmacy, Seoul National University, Seoul, Republic of Korea
Dong Soo Lee
You can also search for this author in PubMed Google Scholar
Contributions
B.N.K. and D.S.L. conceived the study and were in charge of overall direction and planning. S.H., Y.C. and S.J.J. collected data. S.H. analyzed the data and wrote the main text. J.H.R., H.J.K., J.H.C., H.K. and S.L. provided expert guidance. H.K., S.L. and D.S.L. revised the paper. All authors, S.H., H.L., Y.C., H.K., S.J.J., J.H.R., H.J.K., J.H.C., S.L., B.N.K. and D.S.L. reviewed and approved the paper.
Corresponding authors
Correspondence to Bung-Nyun Kim or Dong Soo Lee .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplemental figures., supplemental methods and materials., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions

About this article
Cite this article.
Ha, S., Lee, H., Choi, Y. et al. Maturational delay and asymmetric information flow of brain connectivity in SHR model of ADHD revealed by topological analysis of metabolic networks. Sci Rep 10 , 3197 (2020). https://doi.org/10.1038/s41598-020-59921-4
Download citation
Received : 21 August 2019
Accepted : 28 January 2020
Published : 21 February 2020
DOI : https://doi.org/10.1038/s41598-020-59921-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
In vivo symmetric multi-contrast mri brain templates and atlas for spontaneously hypertensive rats.
- Yingying Yang
- Zuojun Geng
Brain Structure and Function (2022)
Caffeine Improves GABA Transport in the Striatum of Spontaneously Hypertensive Rats (SHR)
- Regina Célia Cussa Kubrusly
- Thais da Rosa Valli
- Maurício dos Santos Pereira
Neurotoxicity Research (2021)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

Brain Maturation Delayed, Not Deviant, in Kids With ADHD
Cortical development in children with attention-deficit/hyperactivity disorder (ADHD) generally lags behind that in other children by several years, NIMH researchers reported recently.
Cortical development in children with attention-deficit/hyperactivity disorder (ADHD) generally lags behind that in other children by several years, NIMH researchers reported recently. 1 The greatest maturational delay occurs in prefrontal regions important for control of such cognitive processes as attention and working memory, they found.
There has been a long-standing debate as to whether ADHD is caused by a delay in brain development or is partly due to a complete deviation away from typical brain development, said Philip Shaw, MD, PhD, an NIMH staff clinician and leader of the research team.
To help resolve the controversy about the disorder that affects 3% to 5% of school-aged children, Shaw and his colleagues conducted a neuroanatomical MRI study and found evidence suggesting that ADHD is characterized by delay rather than deviance in cortical maturation.
"We looked at the development of the cortex, and we measured its thickness in 446 kids, half... with ADHD and half without the disorder," Shaw told Psychiatric Times.
The researchers scanned the brains of most of the study participants at least twice at about 3-year intervals. While the participants included preschoolers and young adults, most ranged in age from 7 to 16 years. Among the participants with ADHD, 92% had combined-type ADHD at baseline.
Using computational neuroanatomical techniques, the researchers estimated cortical thickness at more than 40,000 cerebral points from 824 MRI scans. They focused on the age of attaining peak cortical thickness-when cortex thickening during childhood gives way to thinning following puberty, as unused neural connections are pruned for optimal efficiency during the teen years.
"While healthy kids reached peak cortical thickness at age 7 or 8, the kids with ADHD reached... peak cortical thickness a few years later, around age 10," Shaw said.
The cortical maturation delay in ADHD was most prominent in the lateral prefrontal cortex, the region, according to the research team, that supports such cognitive functions as the ability to suppress inappropriate responses and thoughts, executive control of attention, evaluation of reward contingencies, and working memory. Delay was also found in the temporal cortex.
The only cortical area in which the ADHD group demonstrated slightly earlier maturation was the primary motor cortex.
"It is possible that the combination of early maturation of the primary motor cortex with late maturation of higher-order motor control regions may reflect or even drive the excessive and poorly controlled motor activity cardinal to the syndrome," the research team wrote.
Although there was a delay in the young people with ADHD, the order in which the different parts of the cortex matured was similar in both groups.
Shaw was asked whether the findings indicate that children will eventually grow out of ADHD. The study findings cannot be interpreted to mean that in ADHD the brain normalizes at age 10 or 12, he said.
"The delay we showed is carried forward into adolescence," he said. "Also we know from a host of other studies that there are very real persisting structural and functional differences between teenagers with ADHD and those who don't have the disorder." Frequently, he said, outcomes reported in previous studies depend on how ADHD is defined. If you use a strict definition, he explained, about one quarter of people who grow up with ADHD will still meet the definition in adulthood. If a broader definition is used, about two thirds of people with childhood ADHD will still have troublesome symptoms in adulthood.
Studies that measure brain volume or function also have detected differences between the brains of young people who have ADHD and those of individuals who do not have the disorder.
"One very striking thing about our findings is that they complement existing imaging studies from other groups that found structural and functional differences, and all of them are pointing to similar parts of the brain," Shaw said.
Why the delay?
Discussing factors that might underpin the delay, the research team mentioned psychostimulants and genetic factors. Most of those with ADHD in the study were receiving standard treatment with psychostimulants, but there were not enough medication-naive children to analyze them as a separate group, according to Shaw. In the published report, the research team wrote "trophic effects of treatment with psychostimulants in the ADHD group are possible, but unlikely, given our previous reports of no effect of psychostimulants on gray matter volume."
"Genetic factors will certainly play a role, with a perturbation in the developmental sequence of the activation and deactivation of genes that sculpt cortical architecture," the team wrote. "In this context, neurotrophins, essential for the proliferation, differentiation, and survival of neuronal and nonneuronal cells, emerge as promising candidates."
"The numbers needed to do genetic studies are enormous," Shaw said. "Of course, there are very good multisite collaborative studies going on, which are helping us identify the key genes."
There are a host of candidates and factors that could control neural growth, Shaw said, acknowledging that dopamine and other neurotransmitters in the brain also are important to the growth of the cortex.
While research continues on possible causes of ADHD, Shaw noted that his team would be using brain-imaging techniques to study what happens to children with ADHD as they grow older.
"There is a large cohort of children who have very persistent ADHD," he explained. "We want to compare them with the kids who get better from ADHD. That involves scanning the kids a little bit later when they are in their mid-teens."
Diagnosis and treatment
Brain imaging is not ready for use as a diagnostic tool in ADHD, Shaw said."It is still too early to use neuroanatomical scans for diagnosis," he said. "We had to scan hundreds of children to identify subtle differences. They [the differences] are very real, but they are subtle. So the scan of any one child will not tell you a great deal about whether [he or she has] ADHD or not. Currently, the diagnosis of ADHD remains clinical."
What's more, the brain imaging study was a "natural history study" and so it did not address treatment, he explained.
"We know the treatments that work for ADHD on the basis of very large clinical studies, including the Multimodal Treatment Study of Children With ADHD and the Treatment of Attention Deficit Hyperactivity Disorder in Preschool-Age Children study," he said.
While the Shaw et al study is not relevant to issues of diagnosis and treatment, it is nevertheless important in providing another facet of our increasing knowledge about the neurobiology of this disorder, said F. Xavier Castellanos, MD, Brooke and Daniel Neidich Professor of Child and Adolescent Psychiatry and director of research for the New York University Child Study Center.
In his own work, Castellanos said, his group is pursuing some novel methods of functional MRI that may well have diagnostic implications. 2,3
Also responding to the Shaw et al study was E. Clarke Ross, chief executive officer for Children and Adults With Attention Deficit/Hyperactivity Disorder, a national advocacy and support organization.
"In a time when a vocal minority denies the mountain of evidence showing ADHD to be a real disorder," he said, "it is nice to watch brain scans light up on televisions across the country with images actually showing the structural differences in the brains of those living with ADHD."
References:
Shaw P, Eckstrand K, Sharp W, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation.
Proc Natl Acad Sci U S A.
2007. Nov 16; [Epub ahead of print].
Castellanos FX, Margulies DS, Kelly C, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder.
Biol Psychiatry.
2007. Sep 20; [Epub ahead of print].
Margulies DS, Kelly C, Uddin LQ, et al. Mapping the functional connectivity of anterior cingulate cortex.
Neuroimage.
2007;37:579-588.

The 2024 APA Annual Meeting: Saturday, May 4

Treating ADHD in Children: Concerns, Controversies, Safety Measures

A Dynamic Model for the Clinical Diagnosis of Adult ADHD

ADHD in Older Adults

Opioid Use Disorder Research Roundup: April 19, 2024

Tris Digital Health Forms, Will Focus on Digital Diagnostic Platform for ADHD
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

- Reference Manager
- Simple TEXT file
People also looked at
Original research article, maturational delay in adhd: evidence from cpt.

- 1 Pediatric Division, The Neuro-Cognitive Center, Hadassah-Hebrew University Medical Center, Jerusalem, Israel
- 2 Faculty of Medicine, Hebrew University - Hadassah Medical Center, Jerusalem, Israel
- 3 Leumit HMO, Pediatric Neurology, Jerusalem, Israel
While data from behavioral, neuropsychological, and brain studies suggested that Attention-Deficit/Hyperactivity Disorder (ADHD) is related to a developmental lag that reduces with age, other studies have proposed that ADHD represents a deviant brain function. The present study used a cross-sectional approach to examine whether ADHD children show a developmental delay in cognitive performance measured by continuous performance test (CPT). We thus, compared six age groups of ADHD children ( N = 559) and their unaffected peers ( N = 365), aged 6–11, in four parameters of MOXO-CPT performance: Attention, Timing, Hyperactivity and Impulsivity. Results have shown that despite improvement in CPT performance with age, ADHD children continued to demonstrate impaired performance as compared to controls. In most parameters, CPT performance of ADHD children matched that of 1–3 years younger normal controls, with a delay most prominent in older children. However, in the Hyperactivity parameter, ADHD children's performance resembled that of much younger healthy children, with almost no evidence for a developmental catch up. This study suggests that while some cognitive functions develop slower but normally, other functions (e.g., inhibitory control) show a different trajectory.
Introduction
Attention-deficit hyperactivity disorder (ADHD) is the most common neurobehavioral disorders of childhood, characterized by inattention, impulsivity and hyperactivity. Using the DSM-IV criteria [American Psychiatric Association (APA), 2000 ], prevalence rates in the United States range from 7.4 to 9.9% ( Barkley, 2006 ). There is growing evidence that ADHD has important developmental aspects and its symptoms change considerably over time ( Greenberg and Waldman, 1993 ; Hart et al., 1995 ; Faraone et al., 2006 ). Leading researchers ( Barkley, 1990 , 1997 ; Gillberg, 2010 ; Sonuga-Barke and Halperin, 2010 ) have long argued that ADHD is a “developmental disorder” with early onset and that deficits in inhibition appear in early childhood leading to a cascade of other problems in self-regulation, encompassed under the rubric of executive functioning.
Many children with ADHD have been described as having co-morbid developmental problems in motor coordination, language, behavior, sleep, and mood ( Hartsough and Lambert, 1985 ; Gillberg and Kadesjo, 2003 ; Kalff et al., 2003 ; Gillberg, 2010 )
Although ADHD symptoms often persist over time ( Greydanus et al., 2007 ), maturation has a significant positive effect on ADHD symptoms in many children ( Faraone et al., 2000 ). These observations have given rise to the hypothesis that ADHD is related to a delay rather than a deviance of normal brain development ( Kinsbourne, 1973 ; Steffensson et al., 1999 ; El-Sayed, 2002 ).
According to the “maturational lag” model, ADHD children have neurodevelopment profiles representative of healthy children at younger ages ( Kinsbourne, 1973 ). As a child with ADHD gets older and “catches up” the developmental lag, the symptoms of ADHD might lessen. This model was initially based on the behavioral observation that children with ADHD often behave as younger children, who naturally have lesser ability to sustain attention, display impulse control, and sit still for a long time period.
In support of this model, two longitude studies using computational neuroanatomic techniques demonstrated that children with ADHD follow a similar sequential pattern of cortical development, yet were delayed by as much as 2–3 years, depending upon the specific cortical region ( Shaw et al., 2007 , 2012 ). Shaw et al. (2007) used the peak of cortical thickness as delineating a phase of childhood increase followed by adolescent decrease in cortical thickness. Results showed that while the peak in cortical thickness was attained in the cerebrum around 7 years in typically developing children, in children with ADHD, peak cortical thickness was reached around 10 years, with the delay most prominent in lateral prefrontal cortex. In the second longitudinal study, delayed brain maturation (of ~2 years) in ADHD children was reported in the cortical surface area ( Shaw et al., 2012 ). The authors concluded the congruent delay in both cortical thickness and surface area in ADHD represents a global perturbation in the mechanisms that guide cortical maturation.
Indirect neurobiological support to the maturation-lag model comes from cross-sectional structural imaging studies which yielded reduced size in cortico-striatal brain regions that are known to develop late in adolescence ( Krain and Castellanos, 2006 ). Additionally, research of brain activity demonstrated underactivation in those regions where function develops linearly with age between childhood and adulthood ( Krain and Castellanos, 2006 ; Rubia et al., 2006 ; Smith et al., 2006 ). Electroencephalography (EEG) studies have documented increased slow wave activity (mostly theta) ( Lazzaro et al., 2001 ; Clarke et al., 2002 ; El-Sayed et al., 2002 ; Yordanova et al., 2009 ) in preadolescent and adolescents with ADHD compared with normal controls. This finding has been interpreted as different arousal level in children with ADHD, which could be due to a delay in functional cortical maturation ( Mann et al., 1992 ).
Further evidence for the maturational lag model was found in neuropsychological functioning of ADHD children. ADHD children showed later development of executive functions, such as inhibitory self-control, attention, and temporal foresight, which are mainly dependent on circuits in the frontal lobes ( Barkley, 1997 ; Kalff et al., 2003 ; Rubia et al., 2007 ). For example, Shue and Douglas (1992) have demonstrated that on tests sensitive to frontal lobe functions (but not temporal lobe) ADHD children lagged 3–4 years behind their healthy peers. However, ADHD deficits in neuropsychological performance were not necessarily related to brain developmental delay. In order to test whether ADHD is related to a maturational lag in brain development, Doehnert et al. (2010) examined CPT performance and ERP (event related potentials) markers of attention and inhibitory control deficits in ADHD and non-ADHD children in three time points. Although CPT performance was consistent with the developmental lag model, ERP data did not support the developmental lag hypothesis for attentional dysfunction in ADHD. Results showed that ADHD effects may mimic age effects at the level of behavior or performance but these effects were unrelated to patterns of neural activation. Additional studies using ERP ( Johnstone et al., 2001 ; Smith et al., 2004 ), Magnetic Resonance Imaging (MRI) ( Castellanos et al., 2000 ) and functional Magnetic Resonance Imaging (fMRI) ( Mostofsky et al., 2006 ; Zhu et al., 2008 ) indicated that ADHD deficits shared little in common with the pattern of brain activity seen in younger control children, which suggests that ADHD children may have a deviant brain function rather than a maturation delay.
While ADHD symptoms and neuropsychological dysfunction are correlated ( Nigg, 2005 ; Seidman, 2006 ) it is still unclear to which degree neuropsychological functioning parallels the attenuation of ADHD symptoms over time. Evidence suggests that children with ADHD continued to exhibit impaired neuropsychological functioning despite clinical improvement of ADHD symptoms ( Fischer et al., 2005 ; Halperin et al., 2008 ; Hinshaw et al., 2007 ). For example, Hinshaw et al. (2007) found that commission errors in the Conners' CPT were not related to ADHD diagnostic status over a 5 year period (persisters and remitters did not differ on this outcome at follow up). In contrast, other studies ( Fischer et al., 2005 ; Halperin et al., 2008 ) reported that persisters, but not remitters were significantly differentiated from controls on commission errors on an identical pairs CPT task. To explain the association between behavioral and neuropsychological functioning of ADHD across the life-span, Halperin and Schulz (2006) argued that ADHD is caused by non-cortical neural dysfunction that is present early in ontogeny, remains relatively static throughout life, and is not associated with the reduction of symptoms typically seen over development. Age- related symptom reduction is attributed to prefrontally-mediated executive functions compensating for more primary and enduring subcortical deficits. According to this model, neuropsychological deficits on task measuring effortful controlled processing (e.g., commission errors on a go/no-go task) should decrease with maturation paralleling the reduction of ADHD symptomatology. On the other hand, neuropsychological deficits on tasks measuring automatic and less conscious control (e.g., reaction time variability) tend to persist over time remaining unrelated to ADHD symptom presentation.
Most of the longitudinal studies addressing ADHD manifestations over time examined ADHD symptoms dichotomously (i.e., either the patient meets ADHD criteria or not) ( Vaughn et al., 2011 ). Because the use of diagnostic stability is related to the definition of remission, it changes significantly between studies ( Biederman et al., 2000 ; Spencer et al., 2002 ; Faraone et al., 2006 ). For instance, when ADHD samples included only those who met full diagnostic criteria for ADHD the rate of persistence was ~15% at age of 25 years. However, when partial remission was also included, almost two thirds of ADHD cases suffered from significant clinical impairments in adulthood ( Faraone et al., 2006 ). Another problem with many longitudinal studies is that they use long follow up that may be insensitive to smaller changes in performance. Thus, Vaughn et al. (2011) highlightened the need to include more frequent assessments over a longer period of time, to fully map the likely non-linear developmental trajectories.
The present study used a cross-sectional approach in order to examine whether ADHD children show a developmental delay in CPT performance that mirrors the delayed maturation documented in brain development studies. We hypothesized that ADHD children will perform worse than normal controls in CPT and that their performance would consistently match that of younger typically developed children. We thus, compared six age groups of ADHD children and their unaffected peers (6–11 years) in four parameters of CPT performance to determine whether the disorder is characterized by a delay in cognitive development.
Materials and Methods
Participants.
Participants in this study were 924 children aged 6–11 years, of them 539 boys and 385 girls. The ADHD group included 559 children diagnosed with ADHD and the control group included 365 children without ADHD. The children were divided into six age categories (6–11 years). For example, the category of “8 years” included children who were equal or older than 8 years old, but younger than 9 years old. Background variables are presented in Table 1 . In the majority of age groups, the ADHD and control groups did not differ in age or gender distributions. In the group of 10 years, the control group were slightly older than the ADHD group (mean age of 10.60 vs., 10.45 years, respectively). The ADHD group included more boys relatively to the control group at ages 6 and 7.
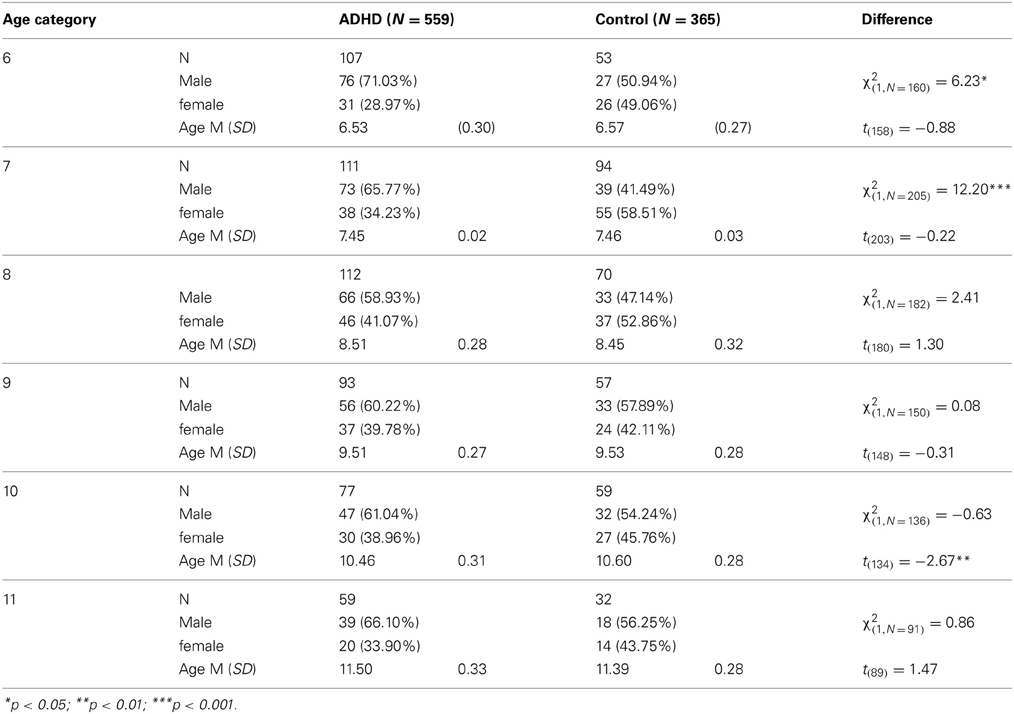
Table 1. participants' background variables .
Participants in the ADHD group were recruited from children referred to the out-patient paediatric clinics of a Neuro-Cognitive Center, based in a tertiary care university hospital. The children were referred through their paediatrician, general practitioner, teacher, psychologist, or directly by the parents.
Inclusion criteria for participants in the ADHD group were:
(1) Each child met the criteria for ADHD according to DSM-IV-TR criteria ( APA, 2000 ), as assessed by a certified paediatric neurologist. The diagnostic procedure included an interview with the child and parents, fulfilment of questionnaires, and medical/neurological examination that confirmed ADHD diagnosis.
(2) Each child scored above the standard clinical cut off values for ADHD symptoms on ADHD/DSM-IV Scales ( APA, 2000 ).
(3) All children were drug naïve.
Participants in the control group were randomly recruited from pupils in regular classes at primary schools. Inclusion criteria for participants in the control group were:
(1) Each child scored below the clinical cut off point for ADHD symptoms on ADHD/DSM-IV Scales ( APA, 2000 ).
(2) Absence of academic or behavioral problems, as reported by parents and teachers.
Exclusion criteria were intellectual disability, other chronic condition, chronic use of medications, and other primary psychiatric diagnosis (e.g., depression, anxiety, and psychosis). All participants agreed to participate in the study and their parents gave written informed consent to the study, approved by the Helsinki committee (IRB) of Hadassah-Hebrew University Medical Center (Jerusalem, Israel).
Measurement of child behavior
The parent and teacher forms of the Conner's ADHD/DSM-IV Scales were used to assess the level of children's ADHD behaviors ( Conners, 1997a , b ; APA, 2000 ).
The MOXO continuous performance test
This study employed the MOXO-CPT version 1 ( Berger and Goldzweig, 2010 ), which is a standardized computerized test designed to diagnose ADHD related symptoms. The test included visual and auditory stimuli that serve as distractors.
The total duration of the test was 15.2 min, and it is composed of eight levels (114.15 s, 53 trials each). In each trial a stimulus (target/non-target) was presented for 500, 1000, or 3000 ms and then followed by a “void” period of the same duration (Figure 1 ). The stimulus remained on the screen for the full duration no matter if a response was produced. This practice allowed the measuring response timing (whether the response occurred during stimulus presentation or the void period) as well as the accuracy of the response.
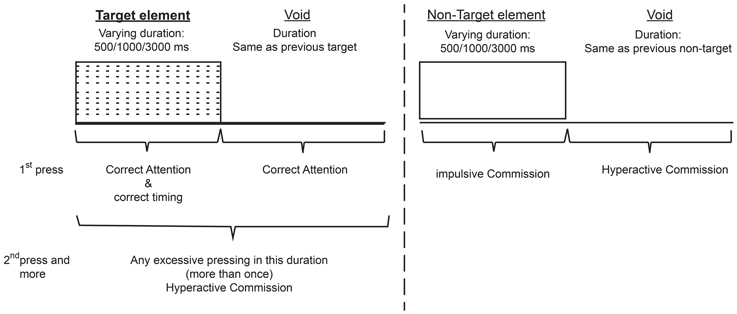
Figure 1. Definition of the time line (Target and non-target stimuli were presented for 500, 1000, or 3000 ms . Each stimulus was followed by a void period of the same duration. The stimulus remained on the screen for the full duration regardless the response. Distracting stimuli were not synchronized with target/non-target's onset and could be generated during target/non-target stimulus or during the void period).
In each level 33 target and 20 non-target stimuli were presented. Both target and non-target stimuli were cartoon pictures that do not include any letters. The absence of letters is important given the fact that ADHD patients tend to have learning difficulties e.g., dyslexia, dyscalculia) that may be confound with CPT performance ( Seidman et al., 2001 ). The stimuli were presented sequentially in the middle of a computer screen and the participant was instructed to respond as quickly as possible to target stimuli by pressing the space bar once, and only once. The participant was also instructed not to respond to any other stimuli except the target, and not to press any other key but the space bar.
Test level and distracting stimuli—In order to simulate everyday environment of children, the MOXO-CPT contained distracting stimuli. This feature is unique to this specific CPT. Distractors were short animated video clips containing visual and auditory features which can appear separately or together. This enabled to present three types of distractions that characterize everyday environment: (a) visual distractors (e.g., animated flying bird); (b) auditory distractors (e.g., bird singing); and (c) combination of both visual and auditory distractors (e.g., animated flying bird with the sound of a bird singing).
Overall, six different distractors were included, each of them could appear as pure visual, pure auditory or as a combination of them. Each distractor was presented for a different duration ranging from 3.5–14.8 s, with a fixed interval of 0.5 s between two distractors. Distractors' onset was not synchronized with target/non-target's onset and could be generated during target/non-target stimulus or during the void period. Visual distractors appeared at one of four spatial locations on the sides of the screen: down, up, left or right. Different levels of the MOXO-CPT were characterized by a different set of distractors: levels 1 and 8 did not include any distractors but only target and non-target stimuli, levels 2 and 3 contained pure visual stimuli, levels 4 and 5 contained pure auditory stimuli, and levels 6 and 7 contained a combination of visual and auditory stimuli. The sequence of distracters and their exact position on the display were constant for each level. The burden of the distracting stimuli increased at the odd number levels; in the 2nd, 4th, and 6th level only one distractor was presented at a time, while in the 3rd, 5th, and 7th level two distractors were presented simultaneously.
Performance indices. The MOXO-CPT included four performance indices: attention, Timing, Impulsivity, and Hyperactivity. For detailed description of performance indices see Supplementary A.
Attention. This index corresponded to the number of correct responses (a space bar keystroke in response to a target stimulus) performed during the stimulus presentation or the void period that followed it. This index was considered as a pure measure of sustained attention because it measured correct responses independently of the response time.
Timing. The timing index was the number of correct responses given only during the time in which the target stimulus was present on the screen.
Impulsivity. The impulsivity index was the number of commission responses performed only during the time in which a non-target stimulus was present on the screen.
Hyperactivity. The hyperactivity index was the total number of commission responses that were not coded as impulsive responses (e.g., multiple keystrokes in response to a target stimulus, responses performed in the void period after a non-target stimulus, random key pressing).
Data Analyses
All analyses were conducted with SAS software for Windows version 9.2. First, T -tests for independent samples and chi-square tests were used for examining group differences across demographic variables. Second, T -tests for independent samples were used to measure the effect of group on CPT indices. Then, each age category of ADHD children was matched to a group of typically developing children which had the closest mean value in the same parameter, by using Cohen's d measure (absolute difference in the mean values of the two groups divided by pooled standard deviation for each age.
First, differences in CPT performance parameters (Attention, Timing, Hyperactivity, and Impulsivity) between ADHD children and their age-matched healthy peers were examined by two tailed t -test analyses for independent samples.
As can be seen in Table 2 , in all age groups children with ADHD received significantly lower scores in the Attention and Timing parameters than normal controls. That is, ADHD children were less attended to the stimuli and performed less reactions on accurate time. In age groups 6, 7, and 10 ADHD children produced significantly more hyperactive and impulsive responses as compared to non-ADHD children. Marginally significant differences between the two groups were observed at ages 8 and 11 in hyperactivity responses ( p = 0.07 and p = 0.08, respectively) and at age 9 for impulsivity responses ( p = 0.06). The rest of the comparisons did not yield significant group differences.
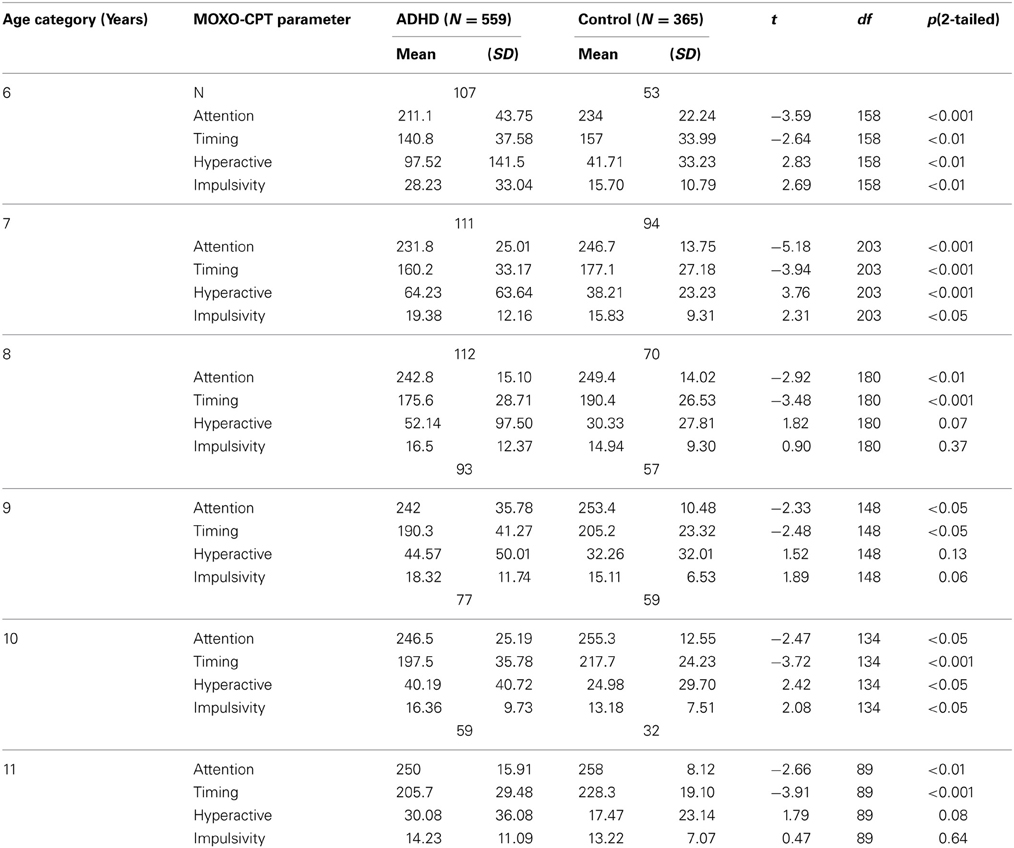
Table 2. Differences between ADHD children and their typically developed peers in MOXO-CPT performance .
In order to evaluate the developmental trajectories of the attention performance, each age category of ADHD children was matched to a group of typically developing children which had the closest mean value in the same parameter. The matched group was chosen by using Cohen's d measure (absolute difference in the mean values of the two groups divided by pooled standard deviation for each age) (Tables B1 – B4 , Appendix B). Results are shown in Figure 2 . As can be seen in the figures, both ADHD and control groups showed higher scores in Attention and Timing parameters and lower scores in Hyperactivity and Impulsivity with maturation, but the performance of ADHD children matched that of younger healthy controls. In the Attention parameter, the performance of 6–7 years old ADHD children closely resembled the performance of 6 years old typically developing children. Furthermore, the performance of 8–10, and 11 years old ADHD children closely resembled that of a 7 and 8 years old typically developing children, respectively. A very similar pattern was found for the Timing parameter: performance of 6–7 years ADHD children closely resembled the performance of 6 years old typically developing children. The performance of 8, 9–10, and 11 years old ADHD children closely resembled that of 7, 8, and 9 years old typically developing children, respectively. A slightly different, non-linear, pattern was obtained in the Impulsivity parameter, in which 6–7 and 9 years old ADHD children performed as 6 years old non-ADHD children, 8 and 10 ADHD children performed as 7 years old non-ADHD children, and 11 years old ADHD performed as 8 years old non-ADHD. In the Hyperactivity parameter, ADHD children aged 6–10 performed as 6 years old controls, whereas 11 years old ADHD children performed similar to 8 years old children.
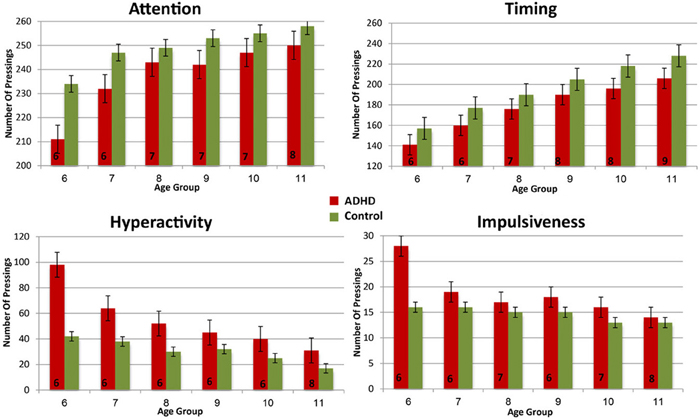
Figure 2. Performance in four CPT parameters among ADHD children and control group .
In most CPT indices, except Hyperactivity, ADHD children consistently lagged 1–3 years behind their typically developed peers. However, the delay was more prominent in older ages: while at ages 6–8, CPT performance of ADHD children resembled that of 6–7 years old controls, at ages 10-11, ADHD children were more likely to perform as 7–8 years old controls.
This paper examined CPT performance of ADHD and non-ADHD children, in order to determine whether the disorder is characterized by a delayed development of attentional functions. Consistent with previous literature ( Drechsler et al., 2005 ; Doehnert et al., 2010 ; Vaughn et al., 2011 ), our results have shown that ADHD children of all ages were significantly more inattentive and performed fewer reactions on accurate timing than the control group. In some age groups (6, 7, and 10 years), children with ADHD also produced significantly more hyperactive and impulsive responses than non-ADHD children, whereas in others (8, 9, and 11 years) only marginal or no group effects were found. This finding indicated that despite improvement in CPT performance, ADHD children continue to demonstrate impaired functioning as compared to healthy controls.
In line with findings from longitudinal studies ( Shaw et al., 2007 , 2012 ; Vaughn et al., 2011 ), our results revealed that ADHD and typically developing children showed a similar sequence of development in their attention capacities, but on a different time. In most CPT parameters, performance of ADHD children, delayed and matched that of 1–3 years younger healthy controls.
This pattern of maturation-lag in CPT performance mirrors the 2–3 delayed maturation of the brain in ADHD children ( Shaw et al., 2007 , 2012 ). In this context, the current study suggests that at least part of the difficulties of ADHD children could be explained by developmental delay that improves with time. Nevertheless, cautions should be taken when interpreting maturation lag in CPT performance as directly associated with a parallel lag in brain development. As reported previously, the two domains may not be directly linked ( Doehnert et al., 2010 ). More large scale longitudinal studies of brain structure and function are required to address this point ( Sonuga-Barke, 2010 ).
Inconsistent with Halperin and Schulz's (2006) hypothesis and with previous studies indicating that the decline in ADHD symptoms is most apparent for hyperactivity–impulsivity symptoms than in inattentiveness symptoms ( Biederman et al., 2000 ; Fischer et al., 2005 ; Vaughn et al., 2011 ), the current study did not identify different developmental patterns for inattentiveness vs. hyperactivity-impulsivity symptoms. Although hyperactive responses showed a slower pace of change relatively to other CPT indices, they had little in common with the developmental trajectory of impulsive responses. The discrepancy from studies mentioned above may be due to the cross-sectional design of the current study that does not detect within-subjects differences. In addition, our findings may be attributed to the type of neuropsychological task used. In contrast to other CPTs, the present CPT included environmental distracters that may increase the complexity of the task, especially for ADHD children. These higher cognitive demands may explain the lack of developmental catch up which is often observed in hyperactive and impulsive responses ( Biederman et al., 2000 ; Fischer et al., 2005 ; Vaughn et al., 2011 ).
Moreover, the majority of the behavioral studies is based on subjective measures of ADHD (e.g., parents rating, parent/children interview) and many of them included only boys ( Hart et al., 1995 ; Biederman et al., 2000 ). There is evidence to suggest that when including girls in a sample, the proportion of participants with ADHD decreases with age ( Cole et al., 2008 ). Finally, some longitudinal studies ( Vaughn et al., 2011 ) included children who were treated by psychostimulants, whereas our sample included only drug naïve children.
It is still unclear why the difference between ADHD and non-ADHD children was more pronounced in older than in younger children. First, this finding indicates that the test provided sufficient cognitive demands for all ages, especially for older children that often find CPT too easy ( Barkley, 1991 ; Robin, 1998 ; Uno et al., 2006 ). Second, it might also suggest that the detection of group differences may be more pronounced before adolescence than in early childhood. This finding is consistent with Drechsler et al. (2005) who found that differences between ADHD and non-ADHD children in reaction time variability and inhibitory tasks were most pronounced just before adolescence (mean age 12) than in younger children and tend to diminish into adolescence. Importantly, the increasing difference between the groups reduces the possibility of a developmental catch up before adolescence.
The findings reported here should be viewed against methodological limitations.
The most important shortcomings of this study are its relatively small sample and the imbalance of gender distribution in the younger age groups (6–7). Although CPT performance is often affected by gender ( Newcorn et al., 2001 ; Hasson and Fine, 2012 ), our results consistently showed that ADHD children performed as younger typically developed children at all ages and at all CPT parameters. Therefore, differences between the two groups could not be solely attributed to differences in gender distributions. In addition, all data in this study was limited to children between 6 and 11 years. We were able to draw a behavioral curve and describe milestones of attention performance but it is yet to be uncovered which pattern characterizes later stages of development. It was also impossible to determine whether the performance of 6 years old children with ADHD resembled that of younger typically developed children.
The fact that we used cross sectional design limits the test's power to detect within-subject changes in cognitive functions. In addition, because only clinically referred children participated in the study, our results may not generalize to ADHD in the community. Furthermore, participation in the study was based on a voluntary agreement of children and their parents. This self-selected sampling strategy tends to be biased toward favoring more cooperative and motivated individuals. Therefore, it is not possible to determine whether this sample also represents other children that were not recruited and whether cooperation is confounded with ADHD variables. This limitation is typical to most clinic-based ADHD studies around the world ( Lee and Ousley, 2006 ; Gau et al., 2010 ). Another limitation of the study is the exclusion of ADHD children with severe comorbidities. Since ADHD is associated with many psychiatric disorders ( Gentile et al., 2006 ) this exclusion limits the generalization of our results. Finally, more work is needed to determine if the normalization in some ADHD symptoms reflects true remission of ADHD symptoms or is due to the developmental insensitivity of the test.
This study shed light on the age -related CPT changes in both ADHD and non-ADHD children. Our results suggest that despite improvement in CPT across childhood, ADHD continue to demonstrate impaired cognitive functioning as compared to non-ADHD children. Importantly, this study suggests that while some cognitive functions develop slower but normally, other functions (e.g., inhibitory control) do not show a clear developmental trajectory. The cross-sectional approach chosen for this study allowed frequent evaluations of typically ADHD-related behavior, which is independent upon definition of remission and persistence. Thus, it was possible to trace small and non-linear changes in performance. One of the major difficulties in early diagnosis of ADHD is that decisions about the inappropriateness of behavior in young children are based on subjective judgments of the observers ( Rousseau et al., 2008 ; Berger and Nevo, 2011 ). Hence, our results highlight the importance of the CPT as an objective tool that is not affected by reporter's bias.
Future research is needed to investigate the course of ADHD symptoms in wider spectrum of age, in specific sub-types of ADHD, and in response to psychostimulants. Moreover, it is important to examine the clinical and behavioral implications of improvement in CPT performance.
Author Contributions
Itai Berger suggested the study. Itai Berger, Merav Aboud, Julia Melamed, and Hanoch Cassuto collected the data. Itai Berger, Ortal Slobodin, and Hanoch Cassuto designed the study with assistance from Merav Aboud and Julia Melamed. Ortal Slobodin, Itai Berger, and Hanoch Cassuto performed the statistical analysis. Itai Berger, Ortal Slobodin, and Hanoch Cassuto wrote the manuscript. All of the authors contributed to interpret the findings and writing the manuscript, and read and approved the final manuscript.
Conflict of Interest Statement
Itai Berger serves on the scientific advisory board of Neuro-Tech Solutions Ltd. All other authors declare no conflicts of interests.
Acknowledgments
The authors would like to thank the participating children and their families.
- ^ The term “MOXO” derives from the world of Japanese martial arts and means a “moment of lucidity.” It refers to the moments preceding the fight, when the warrior clears his mind from distracting, unwanted thoughts, and feelings.
American Psychiatric Association. (1994). Diagnostic and Statistical Manual of Mental Disorders . 4th Edn. Washington, DC: American Psychiatric Association.
American Psychiatric Association. (2000). Diagnostic and Statistical Manual of Mental Disorders Text Revision (DSM-IV-TR) . 4th Edn.-text revision. Washington, DC: American Psychiatric Association. doi: 10.1176/appi.books.9780890423349
CrossRef Full Text
Barkley, R. A. (1990). Attention Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment . New York, NY: Guilford Press.
Barkley, R. A. (1991). The ecological validity of laboratory and analogue assessment methods of ADHD symptoms. J. Abnorm. Child. Psychol . 19, 149–178. doi: 10.1007/BF00909976
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Barkley, R. A. (1997). Advancing age, declining ADHD. Am. J. Psychiatry 154, 1323–1325.
Pubmed Abstract | Pubmed Full Text
Barkley, R. A. (2006). Attention Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment. 3rd Edn. New York, NY: Guilford Press.
Berger, I., and Goldzweig, G. (2010). Objective measures of attention-deficit/hyperactivity disorder—a pilot study. Isrl. Med. Assoc. J . 12, 531–535.
Berger, I., and Nevo, Y. (2011). Early developmental cues for diagnosis of attention deficit/hyperactivity disorder in young children. Dev. Disabil. Res. Rev . 17, 170–179. doi: 10.1002/ddrr.1111
Biederman, J., Mick, E., and Faraone, S. V. (2000). Age-dependent decline of symptoms of attention deficit hyperactivity disorder: impact of remission definition and symptom type. Am. J. Psychiatry 157, 816–818. doi: 10.1176/appi.ajp.157.5.816
Castellanos, F. X., Lee, P. P., Sharp, W., Jeffries, N. O., Greenstein, D. K., Clasen, L. S., et al. (2000). Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA 288, 1740–1748. doi: 10.1001/jama.288.14.1740
Clarke, A. R., Barry, R. J., Bond, D., McCarthy, R., and Selikowitz, M. (2002). Effects of stimulant medications on the EEG of children with attentiondeficit/ hyperactivity disorder. Psychopharmacology 164, 277–284. doi: 10.1007/s00213-002-1205-0
Cole, R., Mostofsky, S. H., Larson, J. C. G., Denckla, M. B., and Mahone, E. M. (2008). Age-related changes in motor subtle signs among girls and boys with ADHD. Neurology 71, 1514–1520. doi: 10.1212/01.wnl.0000334275.57734.5f
Conners, C. K. (1997a). Conners' Parent Rating Scale—Revised (L) . New York, NY: Multi-Health Systems Inc.
Conners, C. K. (1997b). Conners' Teacher Rating Scale—Revised (L) . New York, NY: Multi-Health Systems Inc.
Doehnert, M., Brandeis, D., Imhof, K., Drechsler, R., and Steinhausen, H. C. (2010). Mapping attention-deficit/hyperactivity disorder from childhood to adolescence: no neurophysiologic evidence for a developmental lag of attention but some for inhibition. Biol. Psychiatry 67, 608–616. doi: 10.1016/j.biopsych.2009.07.038
Drechsler, R., Brandeis, D., Foldenyi, M., Imhof, K., and Steinhausen, H. C. (2005). The course of neuropsychological functions in children with attention deficit hyperactivity disorder from late childhood to early adolescence. J. Child. Psychol. Psychiatry 46, 824–836. doi: 10.1111/j.1469-7610.2004.00384.x
El-Sayed, E. (2002). Brain Maturation, Cognitive Tasks an Quantitative Electroencephalography: A Study in Children with Attention Deficit Hyperactive Disorder . Ph.D. thesis, Karolinska Institutet, Stockholm.
El-Sayed, E., Larsson, J. O., Persson, H. E., and Rydelius, P. A. (2002). Altered cortical activity in children with attention-deficit/hyperactivity disorder during attentional load task. J. Am. Acad. Child Adolesc. Psychiatry 41, 811–819. doi: 10.1097/00004583-200207000-00013
Faraone, S. V., Biederman, J., Spencer, T., Wilens, T., Seidman, L. J., Mick, E., et al. (2000). Attention-deficit/hyperactivity disorder in adults: an overview. Biol. Psychiatry 48, 9–20. doi: 10.1016/S0006-3223(00)00889-1
Faraone, S. V., Biederman, J., and Mick, E. (2006). Decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol. Med . 36, 159–165. doi: 10.1017/S003329170500471X
Fischer, M., Barkley, R. A., Smallish, L., and Fletcher, K. (2005). Executive functioning in hyperactive children as young adults: attention, inhibition, response perseveration, and the impact of comorbidity. Dev. Neuropsychol . 27, 107–133. doi: 10.1207/s15326942dn2701_5
Gau, S., Lin, Y., Shang, C., Liu, S., Chiu, Y., and Soong, W. (2010). Emotional/behavioral problems and functional impairment in clinic and community-based children with attention-deficit/hyperactivity disorder in Taiwan. J. Abnorm. Child. Psychol . 38, 521–532. doi: 10.1007/s10802-009-9381-6
Gentile, J., Atiq, R., and Gillig, P. M. (2006). Adult ADHD: diagnosis, differential diagnosis, and medication management. Psychiatry 3, 25–30.
Gillberg, C. (2010). The essence in child psychiatry: early symptomatic syndromes eliciting neurodevelopmental clinical examinations. Res. Dev. Disabil . 31, 1543–1551. doi: 10.1016/j.ridd.2010.06.002
Gillberg, C., and Kadesjo, B. (2003). Why bother about clumsiness? The implications of having developmental coordination disorder (DCD). Neural. Plast . 10, 59–68. doi: 10.1155/NP.2003.59
Greenberg, L. M. (1997). T.O.V.A Visual continuous performance 15. test. Los Alamitos, CA: Universal Attention Disorders Inc.
Greenberg, M., and Waldman, I. (1993). Developmental normative data on The test of variables of attention (T.O.V.A™). J. Child. Psychol. Psychiatry 34, 1019–1030. doi: 10.1111/j.1469-7610.1993.tb01105.x
Greydanus, D. E., Pratt, H. D., and Patel, D. R. (2007). Attention deficit hyperactivity disorder across the lifespan: the child, adolescent, and adult. Dis. Mon . 53, 70–131. doi: 10.1016/j.disamonth.2007.01.001
Halperin, J., and Schulz, K. (2006). Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychol. Bull . 132, 560–581. doi: 10.1037/0033-2909.132.4.560
Halperin, J. M., Trampush, J. W., Miller, C. J., Marks, D. J., and Newcorn, J. H. (2008). Neuropsychological outcome in adolescents/young adults with childhood ADHD: profiles of persisters, remitters and controls. J. Child. Psychol. Psychiatry 49, 958–966. doi: 10.1111/j.1469-7610.2008.01926.x
Hart, E. L., Lahey, B. B., and Loeber, R. (1995). Applegate B, Frick, PJ. Developmental change in attention-deficit hyperactivity disorder in boys: a four-year longitudinal study. J. Abnorm. Child. Psychol . 23, 729–749. doi: 10.1007/BF01447474
Hartsough, C. S., and Lambert, N. M. (1985). Medical factors in hyperactive and normal children: prenatal, developmental, and health history findings. Am. J. Orthopsychiatry 55, 190–201. doi: 10.1111/j.1939-0025.1985.tb03433.x
Hasson, R., and Fine, J.G. (2012). Gender differences among children with ADHD on continuous performance tests: a meta-analytic review. J. Atten. Disord . 16, 190–198. doi: 10.1177/1087054711427398
Hinshaw, S., Carte, E. T., Fan, C., Jassy, J. S., and Owens, E. B. (2007). Neuropsychological functioning of girls with attention-deficit/hyperactivity disorder followed prospectively into adolescence: evidence for continuing deficits? Neuropsychology 21, 263–273. doi: 10.1037/0894-4105.21.2.263
Johnstone, S. J., Barry, R. J., and Anderson, J. W. (2001). Topographic distribution and developmental timecourse of auditory event-related potentials in two subtypes of attention-deficit hyperactivity. Int. J. Psychophysiol . 42, 73–94. doi: 10.1016/S0167-8760(01)00135-0
Kalff, A. C., de Sonneville, L. M. J., Hurks, P. P. M., Hendriksen, J. G. M., Kroes, M., Feron, F. J. M., et al. (2003). Low- and high-level controlled processing in executive motor control tasks in 5 to 6-year-old children at risk of ADHD. J. Child Psychol. Psychiatry 44, 1049–1057. doi: 10.1111/1469-7610.00189
Kinsbourne, M. (1973). Minimal brain dysfunction as a neurodevelopmental lag. Ann. N.Y. Acad. Sci . 205, 268–273. doi: 10.1111/j.1749-6632.1973.tb43184.x
Krain, A. L., and Castellanos, F. X. (2006). Brain development and ADHD. Clin. Psychol. Rev . 26, 433–444. doi: 10.1016/j.cpr.2006.01.005
Lazzaro, I., Gordon, E., Whitmont, S., Meares, R., and Clarke, S. (2001). The modulation of late component event related potentials by pre-stimulus EEG theta activity in ADHD. Int. J. Neurosci . 107, 247–264. doi: 10.3109/00207450109150688
Lee, D. O., and Ousley, O. Y. (2006). Attention-deficit hyperactivity disorder symptoms in a clinic sample of children and adolescents with pervasive developmental disorders. J. Child. Adolesc. Psychopharmacol . 16, 737–746. doi: 10.1089/cap.2006.16.737
Mann, C. A., Lubar, J. F., Zimmerman, A. W., Miller, B. A., and Muenchen, R. A. (1992). Quantitative analysis of EEG in boys with attention deficit/hyperactivity disorder. A controlled study with clinical implications. Pediatr. Neurol . 8, 30–36. doi: 10.1016/0887-8994(92)90049-5
Mostofsky, S. H., Rimrodt, S. L., Schafer, J. G., Boyce, A., Goldberg, M. C., Pekar, J. J., et al. (2006). Atypical motor and sensory cortex activation in attention-deficit/hyperactivity disorder: a functional magnetic resonance imaging study of simple sequential finger tapping. Biol. Psychiatry 59, 48–56. doi: 10.1016/j.biopsych.2005.06.011
National institute of mental health. (2012). Attention Deficit Hyperactivity Disorder . Available online at: http://www.nimh.nih.gov/health/publications/attention-deficit-hyperactivity-disorder/complete-index.shtml
Newcorn, J. H., Halperin, J. M., Jensen, P. S., Abikoff, H. B., Arnold, L. E., Cantwell, D. P., et al. (2001). Symptom profiles in children with ADHD: effects of comorbidity and gender. J. Am. Acad. Child Adolesc. Psychiatry 40, 137–146. doi: 10.1097/00004583-200102000-00008
Nigg, J. T. (2005). Neuropsychologic theory and findings in attention-deficit/hyperactivity disorder: the state of the field and salient challenges for the coming decade. Biol. Psychiatry 57, 1424–1435. doi: 10.1016/j.biopsych.2004.11.011
Robin, A. L. (1998). ADHD in Adolescents: Diagnosis and Treatment . New York, NY: The Guilford Press.
Rousseau, C., Measham, T., and Bathiche-Suidan, M. (2008). DSM IV, culture and child psychiatry. J. Can. Acad. Child. Adolesc. Psychiatry 17, 69–75. doi: 10.1007/s00787-007-0640-1
Rubia, K., Smith, A. B., Woolley, J., Nosarti, C., Heyman, I., Brammer, M., et al. (2006). Progressive increase of fronto-striatal brain activation from childhood to adulthood during event related tasks of cognitive control. Hum. Brain. Mapp . 27, 973–993. doi: 10.1002/hbm.20237
Rubia, K., Smith, A. B., Taylor, E., and Brammer, M. (2007). Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Hum. Brain. Mapp . 28, 1163–1177. doi: 10.1002/hbm.20347
Seidman, L. J. (2006). Neuropsychological functioning in people with ADHD across the lifespan. Clin. Psychol. Rev . 26, 466–485. doi: 10.1016/j.cpr.2006.01.004
Seidman, L. J., Biederman, J., Monuteaux, M., Doyle, A. E., and Faraone, S. V. (2001). Learning disabilities and executive dysfunction in boys with attention deficit hyperactivity disorder. Neuropsychology 15, 544–556. doi: 10.1037/0894-4105.15.4.544
Shaw, P., Eckstrand, K., Sharp, W., Blumenthal, J., Lerch, J. P., Greenstein, D., et al. (2007). Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc. Natl. Acad. Sci. U.S.A . 104, 19649–19654. doi: 10.1073/pnas.0707741104
Shaw, P., Malek, M., Watson, B., Sharp, W., Evans, A., and Greenstein, D. (2012). Development of cortical surface area and gyrification in attention-deficit/hyperactivity disorder. Biol. Psychiatry 72, 191–197. doi: 10.1016/j.biopsych.2012.01.031
Shue, K. L., and Douglas, V. I. (1992). Attention deficit hyperactivity disorder and the frontal lobe syndrome. Brain Cogn . 20, 104–124. doi: 10.1016/0278-2626(92)90064-S
Smith, A. B., Taylor, E., Brammer, M., Toone, B., and Rubia, K. (2006). Task-specific hypoactivation in prefrontal and temporoparietal brain regions during motor inhibition and task switching in medication-naive children and adolescents with attention deficit hyperactivity disorder. Am. J. Psychiatry 163, 1044–1051. doi: 10.1176/appi.ajp.163.6.1044
Smith, J. L., Johnstone, S. J., and Barry, R. J. (2004). Inhibitory processing during the Go/NoGo task: an ERP analysis of children with attentiondeficit/hyperactivity disorder. Clin. Neurophysiol . 115. 1320–1331. doi: 10.1016/j.clinph.2003.12.027
Sonuga-Barke, E. J. (2010). Disambiguating inhibitory dysfunction in attention-deficit/hyperactivity disorder: toward the decomposition of developmental brain phenotypes. Biol. Psychiatry 67, 599–601. doi: 10.1016/j.biopsych.2010.01.017
Sonuga-Barke, E. J., and Halperin, J. M. (2010). Developmental phenotypes and causal pathways in attention deficit/hyperactivity disorder: potential targets for early intervention? J. Child Psychol. Psychiatry 51, 368–389. doi: 10.1111/j.1469-7610.2009.02195.x
Spencer, T. J., Biederman, J., Wilens, T. E., and Faraone, S. V. (2002). Overview and Neurobiology of attention-deficit/hyperactivity disorder. J. Clin. Psychiatry 63, 3–9.
Steffensson, B., Larsson, J. O., Fried, I., El-Sayed, E., Rydelius, P. A., and Lichtenstein, P. (1999). Genetic disposition for global maturity: an explanation for genetic effects on parental report on ADHD. Int. J. Behav. Dev . 23, 357–374. doi: 10.1080/016502599383865
Uno, M., Abe, J., Sawai, C., Sakaue, Y., Nishitani, A., Yasuda, Y., et al. (2006). Effect of additional auditory and visual stimuli on continuous performance test (noise-generated CPT) in AD/HD children—usefulness of noise-generated CPT. Brain. Dev . 28, 162–169. doi: 10.1016/j.braindev.2005.06.007
Vaughn, A., Epstein, J. N., Rausch, J., Altaye, M., Langberg, L., Newcorn, J. H., Hinshaw, S. P., et al. (2011). Relation between outcomes on a continuous performance test and ADHD symptoms over time. J. Abnorm. Child. Psychol . 39, 853–864. doi: 10.1007/s10802-011-9501-y
Yordanova, J., Kolev, V., and Rothenberger, A. (2009). Functional neuroelectric oscillations along the lifespan. J. Psychophys . 23, 153–156. doi: 10.1027/0269-8803.23.4.153
Zhu, C. Z., Zang, Y. F., Cao, Q. J., Yan, C. G., He, Y., Jiang, T. Z., et al. (2008). Fisher discriminative analysis of resting-state brain function for attention-deficit/hyperactivity disorder. Neuroimage 40, 110–120. doi: 10.1016/j.neuroimage.2007.11.029
Description of Performance Indices
This parameter included the number of correct responses (pressing the key in response to a target stimulus), which were performed either during the stimulus presentation on the screen or during the void period that followed. Thus, it was possible to evaluate whether the participant responded correctly to the target (was attentive to the target) independently of how fast he was. Knowing how many responses are expected, it was also possible to calculate the number of times the target was presented, but the participant did not respond to it (omission errors).
This parameter included the number of correct responses (pressing the key in response to a target stimulus) which were performed only while the target stimulus was still presented on the screen. This parameter did not include responses that were performed during the void period (after the stimulus has disappeared).
According to the National institute of mental health (2012) , inattention problems in ADHD may be expressed in “difficulties in processing information as quickly and accurately as others.” Traditionally, difficulties in timing at a CPT are evaluated by mean response time for correct responses to the target (which is interpreted as a measure of information processing and motor response speed) and by the standard deviation of response time for correct responses to the target (which is interpreted as a measure of variability or consistency) ( Greenberg, 1997 ). In these paradigms the stimulus is presented for short and fixed periods of time and the response occurs after the stimulus has disappeared. Given the short, fixed presentation, accurate but slow participants may be mistakenly diagnosed as inattentive. While a group of patients would respond correctly if allowed more time, inattentive patients would not respond at all because they were not alert to the target. Therefore, the measurement of response time per-se, addresses only the ability to respond quickly, but not the ability to respond accurately. By implanting a void period after each stimulus and using variable presentation durations of the elements, the MOXO-CPT could distinguish accurate responses performed in “good timing” (quick and correct responses to the target performed during stimulus presentation) from accurate but slow responses (correct responses to the target performed after the stimulus presentation; during the void period). These two aspects of timing correspond to the two different problems of ADHD described by the National institute of mental health (2012) ; responding quickly and responding accurately.
Impulsivity
This parameter included the number of commission errors (responses to a non-target stimulus), performed as responses to the non-target stimuli. Usually, commission errors are coded in any case of inappropriate response to the target (e.g., pressing a random key) ( Greenberg, 1997 ). In contrast, the MOXO-CPT's impulsivity parameter considered as impulsive behavior only the pressings on the keyboard's space–bar in response to non-target stimulus. All other non-inhibited responses (e.g., pressing the keyboard more than once) were not coded as impulsive responses (as will describe in the next paragraph).
Hyperactivity
This parameter included all types of commission responses that are not coded as impulsive responses. Several examples are: (1) Multiple responses- pressing the keyboard's space bar more than once (in response to target/non-target), which is commonly interpreted as a measure of motor hyper-responsivity ( Greenberg, 1997 ). The MOXO-CPT considered as multiple responses only the second press and above (the first response would be considered as correct response with good timing, as correct response with poor timing, or as impulsive response, depends on the type of element appearing on the screen). (2) Random key pressing—pressing any keyboard button other than the space bar. By separating commission errors due to impulsive behavior from commission errors due to motor hyper-responsivity, it was possible to identify the multiple sources of response inhibition problems. Thus, the MOXO- CPT was able to differentiate impulsive responses from hyperactive responses.
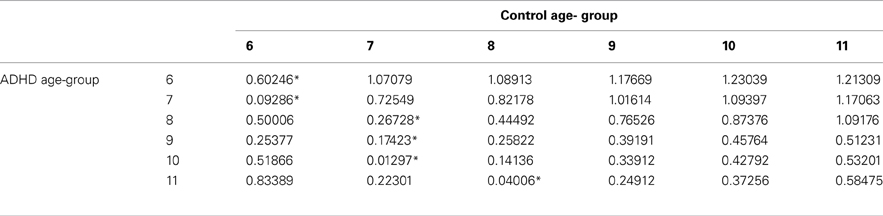
Table B1. ADHD and control group with the minimal difference in the attention parameter, using Cohen's D measure .
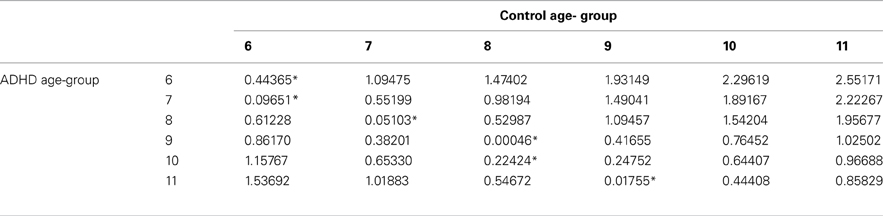
Table B2. ADHD and control group with the minimal difference in the timing parameter, using Cohen's D measure .
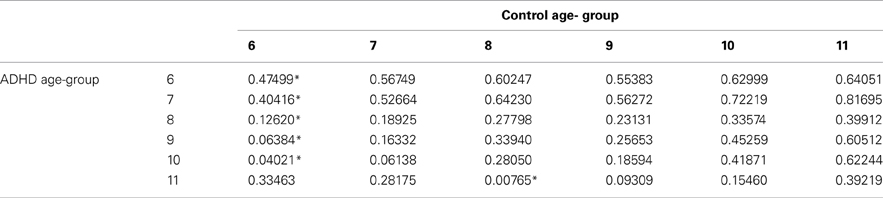
Table B3. ADHD and control groups with the minimal difference in the hyperactivity parameter, using Cohen's D measure .
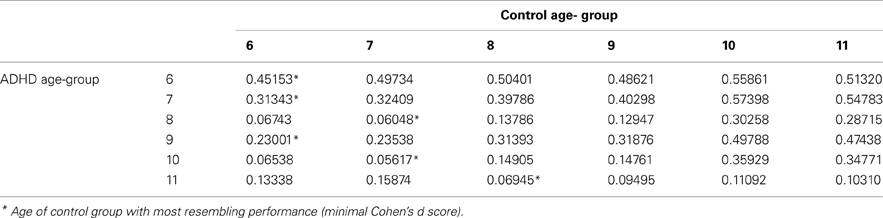
Table B4. ADHD and control groups with the minimal difference in the impulsivity parameter, using Cohen's D measure .
Keywords: ADHD, CPT, symptoms, maturation, delay, diagnosis
Citation: Berger I, Slobodin O, Aboud M, Melamed J and Cassuto H (2013) Maturational delay in ADHD: evidence from CPT. Front. Hum. Neurosci . 7 :691. doi: 10.3389/fnhum.2013.00691
Received: 01 September 2013; Accepted: 30 September 2013; Published online: 25 October 2013.
Reviewed by:
Copyright © 2013 Berger, Slobodin, Aboud, Melamed and Cassuto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Itai Berger, Pediatric Division, The Neuro-Cognitive Center, Hadassah-Hebrew University Medical Center, PO Box 24035, Mount Scopus, Jerusalem, 91240, Israel e-mail: [email protected]
This article is part of the Research Topic
Brain Development and the Attention Spectrum
- Open access
- Published: 18 December 2023
Gross motor developmental delay and associated factors among under-five children attending public health facilities of Dessie city, Ethiopia
- Kefale Mitiku 1 ,
- Tilaye Nega 2 ,
- Mastewal Arefaynie 3 ,
- Degalem Tilahun 4 ,
- Bereket Kefale 3 ,
- Yitayish Damtie 3 , 5 ,
- Bezawit Adane 6 &
- Melaku Yalew 3 , 6
BMC Pediatrics volume 23 , Article number: 638 ( 2023 ) Cite this article
1252 Accesses
Metrics details
Child psychomotor development and factors affecting it today is the subject of interest of many studies, in particular by the experts involved in the protection and improvement of children’s health. There is limited evidence on developmental delay among under-five children in low-income countries like Ethiopia. The aim of this study was to assess gross motor developmental delay and associated factors among under-five children attending public health facilities of Dessie city, Ethiopia.
Facility based cross sectional study design was used among under-five children attending under-five OPD in public health facilities of Dessie town from July 1, 2020 to August 15, 2021. A total of, 417 under-five children were systematically selected based on their average number of clients in a month. A pretested structured questionnaire was used for data collection, and data was entered into Epi-data 3.1 version and it was exported to STATA version 14 for analysis. Binary logistic regression analysis was used to identify factors associated with the outcome variable. Odds ratio with 95% confidence interval was used to show the strength and direction of association respectively and P -value less than 0.05 is used to declare statistical significance.
The overall proportion of gross motor developmental delay among under-five children attending health facilities of Dessie city, Ethiopia was 16.31%, 95% CI: (13.05, 20.19). Increased age of the child [AOR = 0.97, 95% CI: (0.96, 0.99)], increased gestational age during pregnancy [AOR = 0.47, 95% CI: (0.37, 0.65)], being male [AOR = 5.26, 95% CI: (1.76, 15.67)], having history of alcohol intake during pregnancy [AOR = 7.40, 95% CI: (2.36, 23.25)], taking iron during pregnancy [AOR = 0.04, 95% CI: (0.01, 0.15)], facing fetal and/or maternal complication [AOR = 4.98, 95% CI: (1.20, 20.62)], having instrumental delivery [AOR = 9.78, 95% CI: (2.48, 38.60)] were significantly associated with gross motor developmental delay.
Conclusions
The gross motor developmental delay among under-five children was higher as compared to other literatures. This study indicated that, age and sex of the child, iron and alcohol intake during pregnancy, gestational age, mode of delivery and any complication to her and or her neonate were independent variables which showed statistical significant association. The physicians should advise mothers to take iron-folic acid supplement properly and to avoid intake of alcohol during pregnancy. In addition, they should focus on those mothers who faced any complication to her and/or her neonate and better to discourage instrumental delivery unless there are no other options.
Peer Review reports
Child development can be defined as the process that begins in intrauterine life and involves physical growth, neurological maturation and the construction of behavior-related skills [ 1 ]. A process of change in which the child learns to master more complex levels of movement, thinking, feelings and relationships with others also can be taken as child development. It occurs when the child interacts with people, things, and other stimuli in their biophysical and social environment and learns from them [ 2 ]. The non-standardized definition also viewed it as a condition in which the child does not reach skills in accordance with the sequence of predetermined stages [ 3 ].
Based on theoretical perspectives, delays in the development of motor skills are interpreted from three different approaches, although they are not mutually exclusive. The comparative deficit approach, the social interaction approach, and the adaptive compensation approach [ 4 ].
Infant mortality, morbidity, prevalence of disability, living conditions and education of children, especially the under-fives are some of developmental indices which determine a country’s future human resource development. At early child development, an outcome of the survival and care practices adopted in a particular setting, is objectively reflected in the developmental status of children, any delay, dissociation or deviation in the development of children and its causes/contributory factors may be indicative of the need for strengthening the existing programs or the need for exploring and initiating new possibilities [ 5 ].
Worldwide, it is estimated that 200 million children younger than five years of age are at risk of not reaching their full development. Despite the prevalence of developmental delay is not certainly known, World Health Organization (WHO) indicate that 10% of the population of any country consists of individuals with some type of disability, with a rate of 4.5% among those younger than five years of age [ 3 ]. Psychomotor development and/or language disorders in children under the age of 6 years is estimated as 12–16% [ 6 ]. The United State of America survey revealed that, about 16% of children are affected by various disabilities caused by speech and language delay, mental retardation, learning disabilities and emotional/behavioral problems, however only 30% of such children were identified before school entrance age [ 7 ]. A preliminary assessment revealed that the prevalence of a psychomotor delay as determined by the global development quotient of children living in Mygoma orphanage was 25% in children under 16 months, and 19% in those age 16 to 31 months [ 8 ].
In the developed countries, there is a general consensus regarding the importance of monitoring children’s development through systematic screening. Developmental screening is a globally adopted measure by which children at various set ages (2 to 60 months) are routinely assessed to detect those at high risk for significant unsuspected deviation from the normal [ 7 ]. The necessity to screen a child’s development at a very young age is obvious since research has shown that the earlier the intervention the better the developmental outcome. Consequently, professional pediatric societies recommend the identification of those children with a developmental delay before the age of 6 years [ 6 ]. A number of studies have shown that early intervention programs are not only cost effective but they are also lifelong benefit and optimal developmental attainment. The earlier the intervention the greater the benefit will be [ 9 ]. In the first few years of life, growth and development is an important health indicator of children. Mortality and morbidity among children under the age of five years has strong associations with severe growth retardation [ 1 ], while impaired psycho-social and intellectual development and learning ability is strongly associated with developmental delay [ 10 ].
Gross motor developmental delays in early childhood are often associated different factors. For instance, child factors (age, sex, no of sibling, birth weight and nutritional status) [ 2 , 11 , 12 , 13 ], paternal factors (age of mother, marital status, mother education, father education, place of residence and ethnicity) [ 14 , 15 ], obstetric factors (gestational age, birth spacing, mode of delivery, complication during labor and or delivery) [ 6 , 16 , 17 ] and illness and behavioral factors (childhood exposure during child hood, childhood exposure to malaria, type of salt they used, mother take iron, r/ship b/n mother and child) [ 4 , 18 , 19 ].
Even though Ethiopia tried a lot to prevent child developmental delay by providing safety net, fortification of iodine in salt and the like and most of which would have been either preventable or manageable if it can detected early [ 7 ], it is still overlooked in determining its magnitude and associated factors. Since there is no similar study conducted in the study area and Ethiopia is diversified country and the problem varied, therefore, it will generate relevant information that will fill this gap. The aim of this study was to assess gross motor developmental delay and associated factors among under-five children attending public health facilities of Dessie city, Ethiopia.
Method and materials
Study area, period, design and population.
This study was conducted in public health facilities of Dessie town, Amhara Regional State, Ethiopia from June 1, 2020 to August 15, 2021. Dessie is the central town of South Wollo Zone, which is located 401 km to the North of Addis Ababa. According to the town administrative health office report the town had 10 urban and 6 rural kebeles and the estimated population size was 218,471 of which, 102,378 (46.86%) are males and 116,093 (53.14%) are females. The town is found at an altitude of 2470 to 2550 m above sea level and the temperature ranges from 15 to 17 oc. There are five hospitals and eight health centers and twenty seven private clinics, of which 10 are public health institutions.
Health facility based cross sectional study design was conducted. All under-five children attending under-five OPD in selected health facilities were the source population. All under-five children attending under-five OPD in selected health facilities and available during the study period was the study population.
Sample size determination and procedure
The sample size was determined by single population proportion formula by considering the following assumptions. Proportion of gross motor developmental delay in Ethiopia is 50%, since there is no any previous study, 95% of confidence level and allowed margin of error 5%.
After adding 10% non-response rates, the final sample size for this study is 424.
The calculated sample size was proportionately allocated based on the average number of client flow per month to each health facility and study participants were selected by systematically until reaching the final sample size (Fig. 1 ).
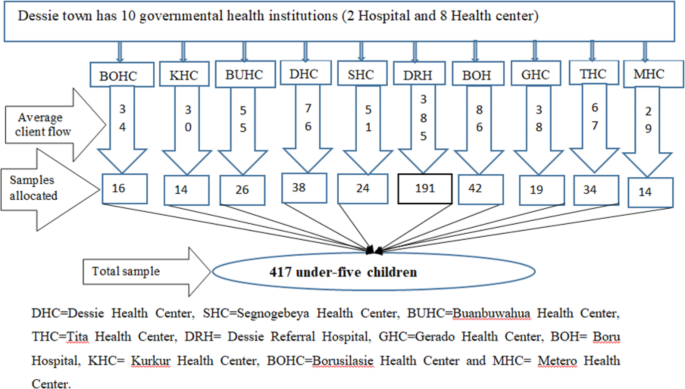
Schematic diagram of sampling procedure for gross motor developmental delay and associated factors among under-five children attending under-five OPD in public health facilities of Dessie city, Ethiopia
Outcome measurement
The care givers were interviewed and the outcome variable was measured based on Child Developmental Inventory (CDI) which consists 28 item checklists composed of yes/no statements about the child’s development. Each scale is scored by tallying the “yes” answers; a child who receives a score that is 2 SDs below the mean is graded as delayed otherwise normal [ 20 , 21 ].
Data collection tools procedures and quality control
Data were collected through interviewer administered questionnaire that were developed from previous done similar literatures [ 1 , 20 ]. The caregivers were asked for their valuable information after they have finished their primary intention to visit health facility in separate room and the data were filled in the questionnaire. Some of the questionnaires were also filled by observing child individual folder/card and height and weight of the child was also recorded. Two BSc public health officer supervisors and 10 BSc nurses who were working in another health facility were employed as data collectors.
The questionnaire was developed in English language and translated to Amharic again back translated to English to check its consistency by language experts. Supervisors and data collectors were trained on the objective of the study, how to approach participants, measure height and weight and take informed consent. Before entering to the actual data collection, the tool was pre-tested on 5% of the sample in Kombolcha health center and necessary modification was done according to the result of pretest. The data was checked by supervisors and principal investigators daily.
Data processing and analysis
After collecting the data, it was checked, coded, cleaned and entered in STATA version 14. The results were presented using texts, frequency, proportion, graphs and other summary measures were also computed to describe the study population. Binary logistic regression model was used to identify association between each independent variable and outcome variable and statistical significance was determined using odds ratios with the corresponding P -value.
Multi-colinearity between independent variables was checked using variance inflation factor as well as standard error and Hosmer- Lemeshow test was used to check model fitness. In the final model those variables with p -value less than 0.05 was considered as statistical significant and it was presented on odds ratio (OR), with 95% confidence interval (CI) to show the strength and direction of association respectively.
Socio-demographic characteristics of the respondents
Four hundred seventeen children with their caregivers were participated in this study and the response rate was 98.35%. The median (IQR) age of the child and the caregiver/mother was 30 ( ± 29) months and 30 ( ± 8) years respectively. Two hundred twenty two (53.24%) caregiver/mothers were educated to secondary and above whereas 100 (23.98%) were not formally educated. About one hundred fifty-nine (38.13%) children were females. In terms of ethnicity and occupation, 396 (94.96%) were Amhara and 262 (62.83%) mothers were house-wife (Table 1 ).
Obstetric, illness and behavioral factors
Out of all study participants, 262 (62.83%) children were delivered vaginal spontaneously. Two hundred eighty-seven (68.82%) mothers had history of antepartum and/or postpartum complication to the mother and/or the child. One hundred sixteen (23.60%) and two hundred thirty-four (56.12%) mothers didn’t take iron and take alcohol during their pregnancy respectively. Forty-one (10.33%) of the children had history of malaria exposure during pregnancy. The mean (SD) duration of pregnancy was 38.9 (1.39) weeks. The mean (SD) number of siblings was 1.35 (1.55) (Table 2 ).
Proportion of gross motor developmental delay
The overall proportion of gross motor developmental delay among under-five children attending health facilities of Dessie city, Ethiopia was demonstrated in figure below and its 95% CI: (13.05, 20.19) (Fig. 2 ).
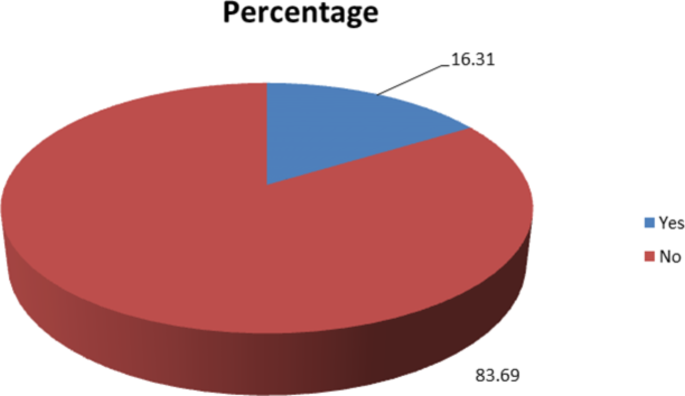
The overall proportion of gross motor developmental delay among under-five children attending health facilities of Dessie city, Ethiopia
Factors associated gross motor developmental delay
In Bivariable logistic regression, eleven variables (age of the child, gestational age, sex of the child, educational status of the mother, educational status of the father, mode of delivery, alcohol intake during pregnancy, iron intake during pregnancy, type of household salt used, family history of GMDD, and had history of complication to her and/or her child during pregnancy) with P -value less than 0.2 were considered in multi variable analysis. Multivariable analysis result showed that age of the child, gestational age, sex of the child, mode of delivery, alcohol intake during pregnancy, iron intake during pregnancy, and any fetal and/or maternal complication were found to have significant statistical association with GMDD.
As age of the child increased in one month, the probability of log of odds of gross motor developmental delay decreased by 4% [AOR = 0.97, 95% CI: (0.96, 0.99)]. Similarly, as the gestational age during pregnancy increased in one month, the probability of log of odds of gross motor developmental delay decreased by 66% [AOR = 0.47, 95% CI: (0.37, 0.65)].
The odds of gross motor developmental delay among males were five times more likely than females [AOR = 5.26, 95% CI: (1.76, 15.67)]. Those under-five children whose mothers took alcohol while she was pregnant were seven times more likely to develop gross motor developmental delay as compared to not [AOR = 7.40, 95% CI: (2.36, 23.25)]. However, those under-five children whose mothers took iron while she was pregnant were 99% less likely to develop gross motor developmental delay as compared to not [AOR = 0.04, 95% CI: (0.01, 0.15)].
Those under-five children whose mothers faced fetal and/or maternal complication while she gave birth were five times more likely to develop gross motor developmental delay as compared to not [AOR = 4.98, 95% CI: (1.20, 20.62)]. Lastly, the odds of gross motor developmental delay among children who were delivered through instrument were 10 times more likely than spontaneous vaginal delivery [AOR = 9.78, 95% CI: (2.48, 38.60)] (Table 3 ).
This facility based cross sectional-study was the first study done on gross motor developmental delay among under-five children in public health facilities of Dessie town. In this study, the proportion of gross motor developmental delay among under-five children was 16.31%. Variables like age and sex of the child, iron and alcohol intake during pregnancy, gestational age, mode of delivery and any complication to her and or her neonate were significantly associated to gross motor developmental delay.
The proportion of gross motor developmental delay among under-five children is comparable with a study conducted in India 19.80% [ 22 ]. In addiction, the finding of this study is higher than a study conducted in Ghana 6.7% [ 7 ]. In the contrary, it is lower than the studies conducted in Thailand (37.10%) [ 10 ] and Mexico (55.10%) [ 2 ]. The possible reason for this observed discrepancy may be due to the difference in the tools used to measure gross motor developmental delay and may be due to the difference in children health policy among countries.
As age of the child increased in one month, the probability of log of odds of gross motor developmental delay decreased by 4%. The result of this study is in agreement with a study conducted in France [ 6 ]. Nevertheless, the finding of this study is contrary to a study conducted in Indonesia which states that the probability of gross motor developmental delay decreased as the child goes above age two years [ 9 ]. The possible reason for this discrepancy may be due to the difference in the context of the country in which the research was undertaken.
Similarly, as the gestational age during pregnancy increased in one month, the probability of log of odds of gross motor developmental delay decreased by 66%. The result of this study is in agreement with a study conducted in Ghana [ 7 ]. The possible reason for this may be due to the fact that certain structural and functional cells and organs that have to be completed in uterus play a paramount role in child development.
The odds of gross motor developmental delay among males were five times more likely than females. The finding of this study is consistent with a study conducted in Baghdad [ 12 ]. The possible reason for this may be due to the fact that testosterone hormone in males affect development.
Those under-five children whose mothers took alcohol while they were pregnant were seven times more likely to develop gross motor developmental delay as compared to not. However, those under-five children whose mothers took iron while they were pregnant were 99% less likely to develop gross motor developmental delay as compared to not. The possible reason for this may be due to the fact that iron is very important in neurometabolism, myelination and neutransmitters function during brain development.
Those under-five children whose mothers faced fetal and/or maternal complication while they gave birth were five times more likely to develop gross motor developmental delay as compared to not. The finding of this study is consistent with a study conducted in Baghdad [ 12 ]. The possible reason for this may be due to the fact that any type of infection she/he faced during pregnancy or postpartum can cross the placenta barrier and it will cause brain cell damage that may predispose the child for gross motor developmental delay.
Lastly, the odds of gross motor developmental delay among children who were delivered through instrument were 10 times more likely than spontaneous vaginal delivery. The possible reason for this may be due to the fact that instrumental delivery may increase the risk of infection and usually cause head injury that may attribute for gross motor developmental delay.
Even if this study is the first in generating and testing hypothesis related to gross motor developmental delay in Ethiopia as strength, it is not without limitation. Firstly, the study was only on governmental health facilities that may not represent experience of private health facilities delivery care. Lastly, the study was conducted in health facility that may not truly represent the real community exposure.
The gross motor developmental delay among under-five children was higher as compared to other literatures. This study indicated that, age and sex of the child, iron and alcohol intake during pregnancy, gestational age, mode of delivery and any complication to her and or her neonate were independent variables which showed statistical significant association with gross motor developmental delay in the study area. The physicians should advise mothers to take iron-folic acid supplement properly and to avoid intake of alcohol during pregnancy. In addition, they should focus on those mothers who faced any complication to her ad/or her neonate and better to discourage instrumental delivery unless there is no other options. Further researchers better to study it in community based or prospective study design.
Data availability
The datasets used and/or analyzed during the current study are attached with the manuscript as supporting information (see supplementary file 1 ).
Zeppone SC, Volpon LC, Del Ciampo LA. Monitoring of child development held in Brazil. Revista Paulista De Pediatria. 2012;30:594–9.
Article Google Scholar
Rizzoli-Córdoba A, Campos-Maldonado MC, Vélez-Andrade VH, Delgado-Ginebra I, Baqueiro-Hernández CI, Villasís-Keever MÁ, et al. Diagnostic evaluation of the developmental level in children identified at risk of delay through the child development evaluation test. Boletín médico Del Hospital Infantil De México. 2015;72(6):397–408.
Article PubMed Google Scholar
Dornelas LF, Duarte NMC, Magalhães LC. Neuropsychomotor developmental delay: conceptual map, term definitions, uses and limitations. Revista Paulista De Pediatria. 2015;33:88–103.
Article PubMed PubMed Central Google Scholar
Brambring M. Divergent development of gross motor skills in children who are blind or sighted. J Visual Impairment Blindness. 2006;100(10):620–34.
Nair M, Rekha Radhakrishnan S. Early childhood development in deprived urban settlements. Indian Pediatr. 2004;41(3):227–38.
PubMed CAS Google Scholar
Duyme M, Zorman M, Tervo R, Capron C. French norms and validation of the Child Development Inventory (CDI): Inventaire Du Developpement De l’Enfant (IDE). Clin Pediatr. 2011;50(7):636–47.
Bello AI, Quartey JN, Appiah LA. Screening for developmental delay among children attending a rural community welfare clinic in Ghana. BMC Pediatr. 2013;13(1):1–7.
Espie E, Ouss L, Gaboulaud V, Candilis D, Ahmed K, Cohuet S, et al. Against the odds: psychomotor development of children under 2 years in a Sudanese orphanage. J Trop Pediatr. 2011;57(6):412–7.
Sitaresmi MN, Ismail D, Wahab A. Risk factors of developmental delay: a community-based study. Paediatr Indonesiana. 2008;48(3):161–5.
Jeharsae R, Sangthong R, Wichaidit W, Chongsuvivatwong V. Growth and development of children aged 1–5 years in low-intensity armed conflict areas in Southern Thailand: a community-based survey. Confl Health. 2013;7(1):1–8.
Davies L, Dunn M, Urban M, Chersich M, Chetty C, Olivier L, et al. Developmental delay of infants and young children with and without fetal alcohol spectrum disorder in the Northern Cape Province, South Africa: original. Afr J Psychiatry. 2011;14(4):298–305.
Article CAS Google Scholar
Al-Naddawi M, Ibraheem MF, Alwan SH. Causes of Global Developmental Delay in Children Welfare Teaching Hospital-Baghdad. Iraqi Postgrad Med J. 2013;12(3):383–9.
Google Scholar
Bliss SL, Test Reviews. Newborg, J.(2005). Battelle Developmental Inventory – Second Edition. Itasca, IL: Riverside. Journal of Psychoeducational Assessment. 2007;25(4):409 – 15.
McCoy DC, Waldman M, Team CF, Fink G. Measuring early childhood development at a global scale: evidence from the caregiver-reported early development instruments. Early Child Res Q. 2018;45:58–68.
Abubakar A, Holding P, Van Baar A, Newton C, van de Vijver FJ. Monitoring psychomotor development in a resourcelimited setting: an evaluation of the Kilifi Developmental Inventory. Ann Trop Paediatr. 2008;28(3):217–26.
Article PubMed PubMed Central CAS Google Scholar
Radmilović G, Matijević V, Zavoreo I. Comparison of psychomotor development screening test and clinical assessment of psychomotor development. Acta Clin Croatica. 2016;55(4):600–6.
Subasinghe S, Wijesinghe D. The effect of nutritional status on cognitive and motor development of pre-school children. 2006.
Shrestha RM. Effect of iodine and iron supplementation on physical, psychomotor and mental development in primary school children in Malawi. Wageningen University and Research; 1994.
Peyre H, Charkaluk M-L, Forhan A, Heude B, Ramus F, Group EMCCS. Do developmental milestones at 4, 8, 12 and 24 months predict IQ at 5–6 years old? Results of the EDEN mother–child cohort. Eur J Pediatr Neurol. 2017;21(2):272–9.
Rydz D, Srour M, Oskoui M, Marget N, Shiller M, Birnbaum R, et al. Screening for developmental delay in the setting of a community pediatric clinic: a prospective assessment of parent-report questionnaires. Pediatrics. 2006;118(4):e1178–e86.
Ireton H, Ireton H. Child development inventory. Behavior Science Systems Minneapolis, MN; 1992.
Ali SS. Assessment of growth and global developmental delay: a study among young children in a rural community of India. Int Multidisciplinary Res J. 2011;1(7).
Download references
Acknowledgements
Our special thanks go to study participants for providing the information for conducting the research.
No specific fund was received.
Author information
Authors and affiliations.
Department of Physiology, College of Medicine and Health Sciences, Injibara University, Injibara, Ethiopia
Kefale Mitiku
Department of Developmental Psychology, College Social Sciences, Wollo University, Dessie, Ethiopia
Tilaye Nega
Department of Reproductive and Family Health, School of Public Health, College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia
Mastewal Arefaynie, Bereket Kefale, Yitayish Damtie & Melaku Yalew
Department of Pediatrics, School of Medicine, College of Medicine and Health Sciences, Gondar University, Gondar, Ethiopia
Degalem Tilahun
Department of Reproductive Health, School of Public Health, College of Medicine and Health Sciences, Injibara University, Injibara, Ethiopia
Yitayish Damtie
Department of Epidemiology and Biostatistics, School of Public Health, College of Medicine and Health Sciences, Injibara University, Injibara, Ethiopia
Bezawit Adane & Melaku Yalew
You can also search for this author in PubMed Google Scholar
Contributions
KM, MY, TN, and MA: Analyzed the data and wrote the result, KM, DT, YD and BK: Drafted the manuscript, BA, KM, MY, and DT: Edited and revised the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Correspondence to Kefale Mitiku .
Ethics declarations
Ethical approval and consent to participate.
The actual data collection was carried out after getting ethical approval from the Ethical review committee of Wollo University, College of Medicine and Health Sciences. In addition, the official letter of cooperation was submitted to health facilities and informed written consent was obtained from their caregiver prior to enrollment. Each caregiver was informed about the aim and anyone who is not willing to participate in the study were not enforced and has full right to refuse or even withdraw from the study. They were also be informed that all data obtained from them would be kept confidential by assigned codes instead of using name and other personal identifiers and the information is used only for the purpose of the study. In this study, all of the methods were carried out in accordance with the relevant institutional guidelines and regulations. Again, all methods were conducted in accordance with the ethical standards of the declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Mitiku, K., Nega, T., Arefaynie, M. et al. Gross motor developmental delay and associated factors among under-five children attending public health facilities of Dessie city, Ethiopia. BMC Pediatr 23 , 638 (2023). https://doi.org/10.1186/s12887-023-04461-9
Download citation
Received : 15 August 2022
Accepted : 04 December 2023
Published : 18 December 2023
DOI : https://doi.org/10.1186/s12887-023-04461-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Gross motor
- Developmental delay
BMC Pediatrics
ISSN: 1471-2431
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Search Please fill out this field.
- Newsletters
- Sweepstakes
- Raising Kids
Major Domains in Child Development
There are four major developmental domains: physical, cognitive, language, and social-emotional. As children grow, they develop skills in all areas.
What Are Developmental Domains?
Physical developmental domain.
- Cognitive Developmental Domain
- Social and Emotional Developmental Domain
- Language Developmental Domain
Developmental Domain Delays
When to talk to a health care provider.
A child's development is a multi-faceted process comprised of growth, regression, and change in different domains. These domains include physical , cognitive, socio-emotional, and language development.
Acquiring and mastering skills in certain domains may appear more prominent during specific stages of life. Yet kids virtually always experience some degree of change in all domains as they grow.
Learn more about developmental domains, what to expect in the major areas of development, and when to contact a health care provider.
Phil Boorman / Getty Images
When discussing human development, the word "domain" refers to specific aspects of growth and change. The major domains of development are physical ( fine motor and gross motor skills ), cognitive, language, and social-emotional.
Children often experience a significant and obvious change in one domain at a time. For example, if a baby is focusing on learning to walk , which is in the physical domain, you may not notice as much language development or new words until they have mastered walking.
It might seem like a particular domain is the only one experiencing developmental change during different periods of a child's life. But change typically occurs in the other domains as well—just more gradually and less prominently.
The physical domain covers the development of physical changes, which includes the following:
- Growing in size and strength
Gross motor skills
Fine motor skills.
- Development of the senses
When young, children are learning how to perform different activities with their fingers in coordination with their eyes such as grasping , releasing, reaching, pinching, and turning their wrists. Because these small muscle movements take time to develop, they may not come easily at first.
What Are Fine Motor Skills?
These fine motor skills help kids perform tasks for daily living, like buttoning buttons, picking up finger foods , using a fork, pouring milk, going to the restroom, and washing their hands.
From an early age, give kids opportunities to use their hands and fingers. Give your baby rattles, plush balls, and other toys to grasp.
Later, toys that allow them to pick things up and fit them into slots are good for developing beginning skills. As they get older, teach them how to button buttons, use scissors, hold a pencil, and do other tasks with their fingers and hands.
In addition to these fine motor skills, kids also learn to use their larger muscles, like those in their arms, legs, back, and stomach. Walking, running, throwing, lifting, pulling, pushing, and kicking are all important skills that are related to body awareness, balance, and strength. These skills allow your child to control and move their body in different ways.
Parents can help their child's physical development by providing opportunities for age-appropriate activities . For instance, babies need regular tummy time to build their neck and upper body strength.
Preschoolers and school-aged children need plenty of opportunities to run around and play. Even tweens and teens need regular opportunities for physical activity.
Overall Health Impacts Physical Development
Physical development can also be influenced by nutrition and illness. So, make sure your kids have a healthy diet, regular exercise, and annual wellness check-ups to promote proper child development.
Cognitive Developmental Domain
The cognitive domain includes intellectual development and creativity. As they develop cognitively, kids gain the ability to do the following:
- Process thoughts
- Pay attention
- Develop memories
- Understand their surroundings
- Express creativity
- Make, implement, and accomplish plans
The child psychologist Jean Piaget outlined four stages of cognitive development, known as the Paigentian Theory. These stages are outlined below.
Sensorimotor stage (birth to age 2)
The sensorimotor stage involves learning about the environment through movements and sensations. Infants and toddlers use basic actions like sucking, grasping, looking, and listening to learn about the world around them.
Preoperational stage (ages 2 to 7)
During the preoperational stage, children learn to think symbolically as well as use words or pictures to represent things. Kids in this stage enjoy pretend play, but still struggle with logic and understanding another person's perspective.
Concrete operational stage (ages 7 to 11)
Once they enter the concrete operational stage, kids start to think more logically, but may still struggle with hypothetical situations and abstract thinking. Because they are beginning to see things from another person's perspective, now is a good time to start teaching empathy.
Formal operational stage (age 12 and up)
During the formal operational stage, a child develops an increase in logical thinking. They also develop an ability to use deductive reasoning and understand abstract ideas. As they become more adept at problem-solving, they also are able to think more scientifically about the world around them.
Even older kids and teens need play . You can help your child develop and hone their cognitive skills by giving them opportunities to play with blocks, puzzles, and board games.
You also should create an environment where your child feels comfortable asking questions about the world around them and has plenty of opportunities for free play.
Develop your child's desire to learn by helping them explore topics they are passionate about. Encourage thinking and reasoning skills by asking them open-ended questions and teaching them to expand on their thought processes. As they get older, teach them how to be critical consumers of media and where to find answers to things they don't know.
Social and Emotional Developmental Domain
The social-emotional domain includes a child's growing understanding and control of their emotions. They also begin to identify what others are feeling, develop the ability to cooperate, show empathy, and use moral reasoning.
Friendships
This domain includes developing attachments to others and learning how to interact with them. For instance, young children learn how to share , take turns, and accept differences in others. They also develop many different types of relationships, from parents and siblings to peers, teachers, coaches, and others in the community.
Key Skills of the Social-Emotional Stage
Children develop self-knowledge during the social-emotional stage. They learn how they identify with different groups and their innate temperament will emerge in their relationships.
Tweens, especially, demonstrate significant developments in the social-emotional domain as their peers become more central to their lives and they learn how to carry out long-term friendships . Typically, parents will notice major increases in social skills during this time.
To help your child develop socially and emotionally, look for opportunities for them to interact with kids their age and form relationships with both children and adults. You can arrange playdates, explore playgroups, and look into extracurricular activities. Also, encourage them to talk to their grandparents, teachers, and coaches.
Sense of self
To encourage a sense of self, ask your child about their interests and passions and encourage them to identify their strengths and weaknesses. Teach them about recognizing and managing feelings. As they get older, talk to them about healthy friendships and how to handle peer pressure.
You also should not shy away from challenging talks like those covering sex and consent . All of these different social and emotional facets play into your child's overall development.
Language Developmental Domain
Language development is dependent on the other developmental domains. The ability to communicate with others grows from infancy, but children develop these abilities at different rates. Aspects of language include:
- Phonology : Creating the sounds of speech
- Pragmatics : Communicating verbally and non-verbally in social situations
- Semantics : Understanding the rules of what words mean
- Syntax : Using grammar and putting sentences together
Read to kids
One of the most important things you can do with your child throughout their early life is to read to them —and not just at bedtime.
Make reading and enjoying books a central part of your day. Reading out loud to your kids from birth and beyond has a major impact on their emerging language and literacy skills.
Look for opportunities to read other things, too, like directions to a board game, letters from family members, holiday cards, online articles, and school newsletters. Hearing new words spoken expands a child's vocabulary and helps them identify unfamiliar words when used in context.
Talk to kids
In addition to reading, make sure you talk to your kids even before they can say their first word . Tell them about the things you are doing or what you're buying in the store. Point out different things and engage them in the world around them. Singing to your child is another excellent way to build your child's language skills.
As they get older, try holding regular conversations, answering questions, and asking for your child's ideas or opinions. All of these activities are an important part of their language development.
As children grow and learn, they will pass certain developmental milestones. While every child is different and progresses at a different rate, these milestones provide general guidelines that help parents and caregivers gauge whether or not a child is on track .
You can support your child's growth and development in each of these four areas by understanding these domains and supporting the work your child is doing. Watch the changes taking place in your child and supplement their learning with activities that support their efforts .
The exact time that a child reaches a particular milestone will vary significantly. However, missing one or two milestones can be a cause for concern.
Talk to a health care provider if you're ever worried your child is not meeting milestones in a particular area. If a delay is identified, they can evaluate your child and recommend different services.
Every state in the US offers an early intervention program to support children with developmental delays under the age of 3. Once they are over age 3, the community's local school district must provide programming.
So, don't delay determining whether your child needs assistance. There are resources out there to support them should they need it.
Principles of child development and learning and implications that inform practice . National Association for the Education of Young Children . n.d.
Toddlers (1-2 years old) . Centers for Disease Control and Prevention . 2021.
Transforming the workforce for children birth through age 8: a unifying foundation . Board on Children, Youth, and Families; Institute of Medicine; National Research Council. 2015.
Piagetian theory . American Psychological Association . 2018.
Social development in preschoolers . American Academy of Pediatrics . 2021.
Talking with your teen: Tips for parents . American Academy of Pediatrics . 2021.
Language in brief . American Speech-Language-Hearing Association . n.d.
Helping your child learn to read . American Academy of Pediatrics . 2015.
Is your toddler communicating with you? . American Academy of Pediatrics . 2021.
Related Articles
Advertisement
Supported by
News Analysis
Judge’s Decisions in Documents Case Play Into Trump’s Delay Strategy
Judge Aileen Cannon has given sober consideration to arguments that some experts say should have been promptly dispensed with, leaving a backlog of pretrial issues without a trial date in sight.
- Share full article

By Alan Feuer
The decision by Judge Aileen M. Cannon to avoid picking a date yet for former President Donald J. Trump’s classified documents trial is the latest indication of how her handling of the case has played into Mr. Trump’s own strategy of delaying the proceeding.
It is not impossible that the trial could still take place before Election Day, but the path is exceedingly narrow. And the question of when — or even whether — the charges against Mr. Trump will go before a jury will now largely hinge on how Judge Cannon handles an array of pretrial matters in the next few months, issues that many legal experts have said she could dispense with much more quickly.
Judge Cannon, who was appointed by Mr. Trump in his final days in office, has been on the bench for only four years. She has limited experience overseeing trials of any kind — let alone one involving explosive allegations that a former president and current candidate illegally took highly classified state secrets from the White House after he left office and then obstructed the government’s repeated efforts to retrieve them.
For months now, she has stood in the glare of the spotlight with each of her most minute decisions scrutinized by an often critical gallery of legal scholars and reporters.
Nancy Gertner, a former federal judge who was appointed to the bench by President Bill Clinton, said that rookie jurists handling prominent matters deserve some measure of leeway. But she added that Judge Cannon had put herself outside the normal boundaries with her languid pace and her willingness to grant a sober audience to several of Mr. Trump’s “meshuggeneh motions.”
“For a new judge in a big case, she could just be being careful, but the length of time all of this has taken and things she is allowing seems way beyond that,” Judge Gertner said. “She is treating everything the defense has done as if they all raise substantial and important issues, and that’s just not true.”
Throughout the case, Judge Cannon has shown herself willing to devote significant time to hearing legal motions in person that many federal judges would likely have rejected out of hand or at least decided more quickly on the merits of written filings.
In April, for example, she conducted a hearing to consider giving Mr. Trump’s two co-defendants, Walt Nauta and Carlos De Oliveira, what is known as a bill of particulars, a detailed recitation of the charges in the case supplementing those laid out in the indictment.
Such documents are almost never granted to criminal defendants. And while Judge Cannon ultimately denied the requests , her decision to open her courtroom to the issue in the first place was unusual.
Even more unusual perhaps was a separate decision, contained in her new scheduling order, to set a hearing in late June to decide what is known as the scope of the prosecution team working under Jack Smith, the special counsel appointed to oversee the federal prosecutions of Mr. Trump.
Prosecutors vehemently fought the move in March, telling Judge Cannon that no such proceeding had ever been held in the Southern District of Florida where she sits, and that there was neither case law nor any other legal authority to permit it.
But the hearing will now take place over the course of three full days. Her decision to hold it means Mr. Trump’s lawyers will presumably be able to explore a twisted question of the law: whether they are entitled to learn more from prosecutors about how they went about pulling together the evidence turned over to the defense team as part of the discovery process.
That issue began percolating four months ago when Mr. Trump’s lawyers told Judge Cannon in court papers that they needed more discovery. They asked specifically for information about how Mr. Smith may have worked with officials at the National Archives and with a vast swath of the U.S. national security establishment — including top intelligence, defense and Justice Department personnel — in bringing the case against Mr. Trump.
The lawyers want that information to bolster their claims that Mr. Smith worked hand in glove with the White House and other officials to prosecute Mr. Trump. And if Judge Cannon tells them they can have it, the case will be further delayed as prosecutors make their way through various federal agencies to collect it and turn it over to the defense.
More delays are likely to emerge from another of Judge Cannon’s decisions this week: to postpone until mid-June the deadline for Mr. Trump’s lawyers to submit a critical filing detailing an inventory of the classified materials they intend to use at trial.
The filing of that inventory is enormously important because it will begin a pitched and probably lengthy battle between the defense and prosecution over what sorts of classified materials the jury and the public will ultimately hear about.
Mr. Smith’s team had originally asked Judge Cannon to force the defense to submit the classified filing by mid-March. Mr. Trump’s lawyers, on the other hand, had initially pushed for June 17.
Appearing to split the difference last month, Judge Cannon set the deadline for May 9. But after last-minute pleas by Mr. Trump and Mr. Nauta to postpone it, she abruptly changed her mind on Tuesday and granted the defense’s original request.
“The way this is playing out makes it extraordinarily unlikely that there will be a trial before November,” said Brian Greer, a former lawyer for the C.I.A. who specializes in issues involving classified material.
Mr. Greer, who has followed the case closely, noted that Judge Cannon’s new date for the briefing was actually the second time she had changed the deadline, which had initially been set for November.
He also pointed out that the judge’s new calendar did not include all of the necessary filings concerning classified documents. The government will still have to file its own set of papers about what sorts of sensitive materials should be revealed at trial — a process that, of course, will take more time.
Complicating matters even further, Judge Cannon’s calendar said nothing at all about the deadlines to consider — let alone decide — some of Mr. Trump’s most difficult and potentially consequential motions. Two of those motions have been under seal for more than two months and have not even been placed on the public docket yet.
One of the sealed motions revolves around undisclosed claims by Mr. Trump that members of Mr. Smith’s team engaged in prosecutorial misconduct — an accusation that is sure to be hotly contested by the government.
The other sealed motion — also likely to result in a long fight — has challenged the legality of the F.B.I.’s search of Mar-a-Lago , Mr. Trump’s private club and residence in Florida. It also disputes the way in which the government pierced the normal protections of attorney-client privilege and obtained the audio notes of one of Mr. Trump’s former lawyers, M. Evan Corcoran.
Beyond all of that, there are more tough issues looming, which could add further layers of complexity and delay.
Just this week, for instance, Mr. Trump’s lawyers said they might soon file a motion accusing prosecutors of failing to preserve the integrity of the classified documents at the heart of the case. But it remains unclear, if the motion is filed, how seriously Judge Cannon would actually take it.
Mr. Greer said that her record in the case suggests she has been open to whatever the defense has chosen to send her.
“Certainly, her proclivity so far,” he said, “has been to listen to almost anything.”
Alan Feuer covers extremism and political violence for The Times, focusing on the criminal cases involving the Jan. 6 attack on the Capitol and against former President Donald J. Trump. More about Alan Feuer
Our Coverage of the Trump Documents Case
The justice department has filed federal criminal charges against former president donald trump over his mishandling of classified documents..
The Indictment: Federal prosecutors said that Trump put national security secrets at risk by mishandling classified documents and schemed to block the government from reclaiming the material. Here’s a look at the evidence .
The Co-Defendants: While Trump plays the leading role in the case, the narrative as laid out by prosecutors relies heavily on supporting characters like Carlos De Oliveira and Walt Nauta .
Obstruction: The Mueller report raised questions about whether Trump had obstructed the inquiry into the ties between the former president’s 2016 campaign and Russia. With prosecutors adding new charges in the documents case, the subject is back .
The Judge: Judge Aileen Cannon , a Trump appointee who showed favor to the former president earlier in the investigation, has scant experience running criminal trials. Can she prove her critics wrong ?
A Slow Pace: Cannon has allowed unresolved issues to build up on her docket, and that appears to have kept her from making a prompt decision on the timing of the case. It is one of several factors that have stirred concern about her decision-making .

IMAGES
VIDEO
COMMENTS
Delay in development is generally determined with a child does not attain developmental milestones as compared to peers from the same population. Statistical terms are often used to classify the degree of delay into mild (functional age (FA) <33% below chronological age (CA), moderate (FA 34% to 66% of CA), and severe (FA <66% of CA).
Abstract. Developmental delays are common in childhood, occurring in 10%-15% of preschool children. Global developmental delays are less common, occurring in 1%-3% of preschool children. Developmental delays are identified during routine checks by the primary care physician or when the parent or preschool raises concerns.
Global developmental delay (GDD) is defined in reference to infants and preschoolers, ages 0-5 years, who present with delays of 6 months or more, in two or more of the following developmental domains: gross/fine motor, speech/language, cognition, social/personal and daily living activities. ... Rice argument remains a working hypothesis as ...
In the UK, developmental disorders affect 1-3% of children under 5 years of age and are one of the most common causes for referral to community paediatricians. They are often detected early in childhood and can present with delay or abnormal progression of development in 2 or more domains (global) or affecting one domain (specific).
The prevalence of any developmental delay is estimated at 15% in U.S. children three to 17 years of age. 1 Only 3% of all children received public early intervention services by three years of age ...
Introduction Global developmental delay (GDD) affects 1%-3% of the population of children under 5 years of age, making it one of the most common conditions presenting in paediatric clinics; causes are exogenous, genetic (non-metabolic) or genetic (metabolic). Recent advances in biotechnology and genetic testing mean that the investigations available to perform for children under 5 years are ...
of the maturational delay hypothesis requires longitudinal imaging studies that map the developmental trajectories of brain maturation in healthy and ADHD children. In a recent issue of PNAS, Shaw et al. (11) study largely longitudinal data to provide direct neu-robiological evidence for the matura-tional delay hypothesis of ADHD. ADHD Is a ...
In addition, some babies and children have an increased risk of developmental delays as a result of environmental factors, including: Exposure to toxins before birth, such as alcohol, opioids or weed (marijuana). Exposure to toxins after birth, such as lead poisoning. Premature birth. Low birth weight.
One hypothesis is that the developmental delay of fine motor and personal and social skills for children aged 2 years caught up with them at age 4 years. Further follow-up studies would be needed to verify whether this phenomenon is specific to the fine motor and personal and social skills domains or whether the association is not confirmed ...
As the fronto-central NoGo P3 is considered to reflect the functioning of the frontal areas (which show a delayed maturation in ADHD), our findings are consistent with the ''last in, first out'' hypothesis (Douaud et al., 2014; Raz, 2005), which refers to a mirroring pattern of development and aging of the human brain.
Neuro-anatomic evidence for the maturational delay hypothesis of ADHD. Neuro-anatomic evidence for the maturational delay hypothesis of ADHD. Proc Natl Acad Sci U S A. 2007 Dec 11;104 (50):19663-4. doi: 10.1073/pnas.0710329105. Epub 2007 Dec 6.
A major hypothesis of ADHD is a lag of maturation, which is supported mainly by anatomical studies evaluating cortical thickness. ... Our findings supported the maturational delay of metabolic ...
Although there was a delay in the young people with ADHD, the order in which the different parts of the cortex matured was similar in both groups. Shaw was asked whether the findings indicate that children will eventually grow out of ADHD. The study findings cannot be interpreted to mean that in ADHD the brain normalizes at age 10 or 12, he ...
The present study used a cross-sectional approach to examine whether ADHD children show a developmental delay in cognitive performance measured by continuous performance test (CPT). ... These observations have given rise to the hypothesis that ADHD is related to a delay rather than a deviance of normal brain development (Kinsbourne, 1973 ...
Hypothesis. Motor delay in the context of global developmental delay is an initial "red flag" for ASD, with added risk in girls. ... A significant developmental delay in one area is defined by DQ below 70 (two standard deviations below the mean). For example, for a child achieving independent walking at 24 months, the calculated gross motor ...
Child psychomotor development and factors affecting it today is the subject of interest of many studies, in particular by the experts involved in the protection and improvement of children's health. There is limited evidence on developmental delay among under-five children in low-income countries like Ethiopia. The aim of this study was to assess gross motor developmental delay and ...
The general hypothesis is that just as there are clinical entities for idiopathic delays in other developmental domains (e.g., cognitive delay, language delay, speech delay) there is need in Speech Sound Disorders (SSD) for a classification entity for children with speech motor delay. ... Children with developmental delays in the acquisition of ...
A child's development is a multi-faceted process comprised of growth, regression, and change in different domains. These domains include physical, cognitive, socio-emotional, and language development.
The term developmental delay can refer to: Global developmental delay, an umbrella term used when children are significantly delayed in two or more areas of development. Specific developmental disorder, a classification of disorders characterized by delayed development. Intellectual disability, generalized neurodevelopmental disorder ...
More delays are likely to emerge from another of Judge Cannon's decisions this week: to postpone until mid-June the deadline for Mr. Trump's lawyers to submit a critical filing detailing an ...
The global economy has been resilient and appears headed for a soft landing. Inflation continues to recede and risks have become more balanced globally. Nonetheless, medium-term growth prospects remain at the lowest level in decades and a smooth completion of the disinflation process should not be taken for granted. While the outlook for low-income developing countries (LIDCs) is improving ...