Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 19 August 2021

Towards the sustainable discovery and development of new antibiotics
- Marcus Miethke 1 , 2 ,
- Marco Pieroni 3 ,
- Tilmann Weber ORCID: orcid.org/0000-0002-8260-5120 4 ,
- Mark Brönstrup 2 , 5 ,
- Peter Hammann 6 ,
- Ludovic Halby 7 ,
- Paola B. Arimondo 7 ,
- Philippe Glaser 8 ,
- Bertrand Aigle ORCID: orcid.org/0000-0001-5266-5926 9 ,
- Helge B. Bode ORCID: orcid.org/0000-0001-6048-5909 10 , 11 ,
- Rui Moreira 12 ,
- Yanyan Li 13 ,
- Andriy Luzhetskyy 14 ,
- Marnix H. Medema ORCID: orcid.org/0000-0002-2191-2821 15 ,
- Jean-Luc Pernodet ORCID: orcid.org/0000-0002-6129-7093 16 ,
- Marc Stadler 2 , 17 ,
- José Rubén Tormo 18 ,
- Olga Genilloud 18 ,
- Andrew W. Truman ORCID: orcid.org/0000-0001-5453-7485 19 ,
- Kira J. Weissman ORCID: orcid.org/0000-0002-3012-2960 20 ,
- Eriko Takano ORCID: orcid.org/0000-0002-6791-3256 21 ,
- Stefano Sabatini ORCID: orcid.org/0000-0003-0971-3536 22 ,
- Evi Stegmann 2 , 23 ,
- Heike Brötz-Oesterhelt 2 , 23 ,
- Wolfgang Wohlleben 2 , 24 ,
- Myriam Seemann ORCID: orcid.org/0000-0002-2615-1574 25 ,
- Martin Empting 1 , 2 ,
- Anna K. H. Hirsch 1 , 2 ,
- Brigitta Loretz 1 ,
- Claus-Michael Lehr 1 ,
- Alexander Titz ORCID: orcid.org/0000-0001-7408-5084 1 , 2 ,
- Jennifer Herrmann 1 , 2 ,
- Timo Jaeger 2 ,
- Silke Alt 2 ,
- Thomas Hesterkamp 2 ,
- Mathias Winterhalter ORCID: orcid.org/0000-0002-1604-3318 26 ,
- Andrea Schiefer ORCID: orcid.org/0000-0002-9823-2090 2 , 27 ,
- Kenneth Pfarr ORCID: orcid.org/0000-0003-3096-2465 2 , 27 ,
- Achim Hoerauf 2 , 27 ,
- Heather Graz 28 ,
- Michael Graz ORCID: orcid.org/0000-0003-0818-2614 29 ,
- Mika Lindvall 30 ,
- Savithri Ramurthy 31 ,
- Anders Karlén 32 ,
- Maarten van Dongen 33 ,
- Hrvoje Petkovic 34 ,
- Andreas Keller ORCID: orcid.org/0000-0002-5361-0895 35 ,
- Frédéric Peyrane 36 ,
- Stefano Donadio 37 ,
- Laurent Fraisse 38 ,
- Laura J. V. Piddock ORCID: orcid.org/0000-0003-1460-473X 39 ,
- Ian H. Gilbert ORCID: orcid.org/0000-0002-5238-1314 40 ,
- Heinz E. Moser ORCID: orcid.org/0000-0002-8013-2139 41 &
- Rolf Müller ORCID: orcid.org/0000-0002-1042-5665 1 , 2
Nature Reviews Chemistry volume 5 , pages 726–749 ( 2021 ) Cite this article
74k Accesses
424 Citations
176 Altmetric
Metrics details
- Business strategy in drug development
- Drug therapy
An ever-increasing demand for novel antimicrobials to treat life-threatening infections caused by the global spread of multidrug-resistant bacterial pathogens stands in stark contrast to the current level of investment in their development, particularly in the fields of natural-product-derived and synthetic small molecules. New agents displaying innovative chemistry and modes of action are desperately needed worldwide to tackle the public health menace posed by antimicrobial resistance. Here, our consortium presents a strategic blueprint to substantially improve our ability to discover and develop new antibiotics. We propose both short-term and long-term solutions to overcome the most urgent limitations in the various sectors of research and funding, aiming to bridge the gap between academic, industrial and political stakeholders, and to unite interdisciplinary expertise in order to efficiently fuel the translational pipeline for the benefit of future generations.
Similar content being viewed by others

The global preclinical antibacterial pipeline
Alternative therapeutic strategies to treat antibiotic-resistant pathogens
Antibiotics in the clinical pipeline as of December 2022
Introduction.
This article is conceived as a general roadmap with the central aim of promoting and accelerating translational science in the early stages of novel antibiotic discovery towards lead candidate development. The overuse and misuse of antibiotics in healthcare and agriculture, together with inappropriate waste management and environmental transmission, have led to substantially increased antimicrobial resistance (AMR) 1 , 2 , 3 , 4 , 5 and associated bacterial persistence 6 , 7 . This is of major public concern, since most areas of modern medicine are inconceivable without access to effective antimicrobial treatment 8 . It is estimated that at least 700,000 people worldwide die each year as a result of drug-resistant infections, and this could rise to as much as 10 million by 2050 if the problem of AMR is not addressed 9 , 10 .
The anticipated death toll caused by drug-resistant infections over the next years and decades may be compared with the global fatality rate of the current SARS-CoV-2 (COVID-19) pandemic ( https://coronavirus.jhu.edu/ ), which has already led to multibillion-dollar investments in vaccine development, repurposing existing drugs and antiviral discovery. A perhaps overlooked aspect of concern with the COVID-19 pandemic is the high numbers of secondary infections, often associated with multidrug-resistant bacteria, which are observed especially in hospitalized patients and those with already compromised immune systems 11 , 12 . Associated with this problem is the massive use of antibiotics as a COVID-19 (co)treatment worldwide 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , which is predicted to add to the ongoing emergence of AMR 25 , 26 , 27 , 28 , 29 . This multiplying effect of COVID-19 on the spread of bacterial resistance will most likely have further negative clinical, economic and societal consequences in the near future 30 , 31 .
Unfortunately, the dramatic worldwide rise of bacterial pathogens resistant to antibacterial agents 32 cannot be counteracted by the current low development pace of therapeutics with new mode(s) of action (MoA(s)). While there are nearly 4,000 immuno-oncology agents in development 33 , only about 30–40 new antibacterial compounds are currently in the clinical trial phases of development, and, notably, those candidates targeting World Health Organization (WHO) priority pathogens are derivatives of existing classes 34 , 35 . Indeed, less than 25% of current drugs in the clinical development pipeline represent a novel class or act through a novel mechanism, and none of these are potentially active against Gram-negative ESKAPE or WHO critical threat pathogens 34 , 36 . In fact, only a small fraction of the antibiotics approved over the past 40 years represents new compound classes, while the majority were derived from already known chemical structures, and the most recent new class of antibiotics was discovered during the 1980s 37 .
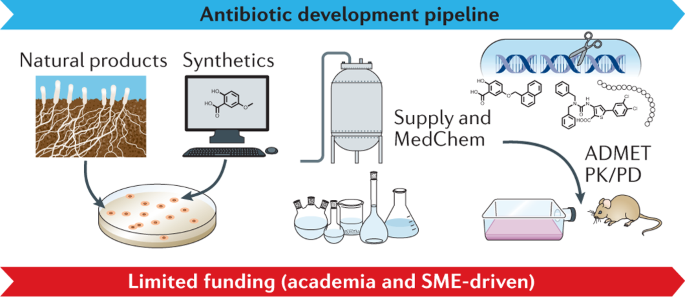
Thus, strategic investment in new therapeutic options to fight AMR is urgently required to address unmet patient need and, additionally, to counterbalance the exponentially increasing financial burden on global health systems 38 . Consequently, the research field should aim to leverage hit identification and hit-to-lead optimization programmes to ensure a sustainable flow of new antibacterial drug candidates into the development pipeline. For this purpose, the initial stages of drug discovery and development need to be strengthened, since they are essential to identify and validate novel therapeutic candidates effective to fight antibacterial resistance. However, for many years, such early-stage projects have been mainly conducted by academia and are generally underfunded, while increased allocation of funding into early-stage and mid-stage research and development (R&D) has been recommended to make the pipeline more robust 39 , 40 , 41 , 42 . Our network has identified major funding gaps especially within the academic sector, as well as for small and medium-sized enterprises (SMEs), where research is mainly associated with the early hit discovery and hit-to-lead phases, as well as with late lead optimization prior to preclinical candidate nomination (Fig. 1 ). Large pharmaceutical companies across the globe are extremely hesitant to fund early antibiotic R&D and, particularly, new classes of compounds, since the return on investment in this area is generally low or even negative. Further, the costs of developing entirely new scaffolds are much higher than for derivatives of established compound classes, while the attrition rate in antibacterial drug discovery has been particularly high in the recent decades, reflected by the fact that no new class of Gram-negative antibiotics has been launched for more than 50 years 43 , 44 . In the commercial sector, innovation has, thus, been left to SMEs, which must deal with high attrition associated with the early phases of discovery and optimization 39 , 43 , 45 , 46 , 47 , 48 , and the huge capital risks 49 , 50 .
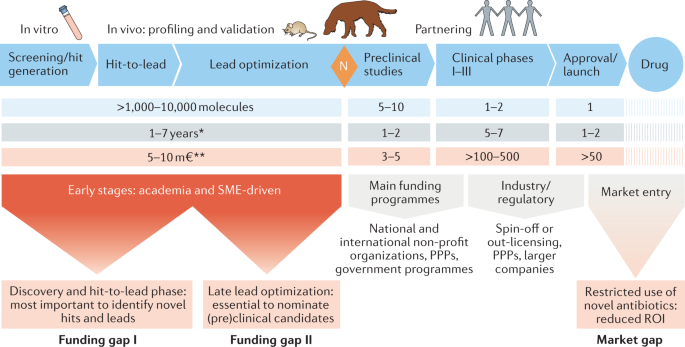
Large funding gaps can be seen in the early stages of hit discovery, as well as during hit and lead optimization, which are associated mainly with academic research and small and medium-sized enterprises (SMEs). Indicated figures are representative numbers of typical broad-spectrum antibiotic development programmes leading from several thousands of initial hits to the approval of at least one marketable candidate 72 , 318 , 319 , 320 , 321 . *Timings are dependent on a number of factors and can vary greatly. A minimum to maximum range for complete development (discovery to market) is 8–18 years (average 13–14 years). **The cost per molecule/candidate (in million euros, m€) does not include extended costs for attrition (failed programmes) and lost opportunities associated with increased cycle time until reaching the next development phase; such extensions can increase the required budget for the early stages up to 50–100 m€ (refs 39 , 48 , 322 ). N (orange diamond), nomination of (pre)clinical candidate(s); PPPs, public–private partnerships; ROI, return on investment.
New economic models for development specifically designed for this area are sorely needed to ensure future advancements 51 , 52 , 53 , 54 . A recent initiative that supports SMEs in the late-stage development of new antibiotics is the AMR Action Fund, which was launched by more than 20 leading biopharmaceutical companies to push mainly phase II and III trials of advanced candidates 55 . Unfortunately, the fund does not cater for the early stages of research. In addition, several countries are implementing new pull incentive programmes with different priorities. While the Swedish model aims at securing sustained access to relevant antibiotics that have already been approved 56 , plans in the UK 57 , 58 as well as in the USA (e.g. PASTEUR 59 and DISARM 60 acts) strive to stimulate the development of new antibacterial products by using subscription models or delinkage models 51 . Such initiatives are promising, as they introduce much-needed market entry rewards, but they might fall short on a global scale if they do not include the ‘critical mass’ of the world’s largest economies.
Innovation in the early stages of antibiotic drug discovery can also be driven by the academic sector. However, from the academic perspective, partnering with external funders such as the pharmaceutical industry is, in many cases, only realistic after the nomination of extensively validated preclinical candidates, and often even requires phase I clinical data. Typically, this cannot be achieved by research-driven funding and infrastructure alone. Several global health organizations and public–private partnerships (PPPs), including the Global Antibiotic Research and Development Partnership (GARDP), Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X), Innovative Medicines Initiative (IMI) and others, started to support, at least partially, the mid-to-late lead optimization through to clinical proof of concept 61 , 62 , 63 , possibly accompanied by stakeholders associated with the Biotech companies in Europe combating AntiMicrobial resistance (BEAM) Alliance or the Replenishing and Enabling the Pipeline for Anti-Infective Resistance (REPAIR) Impact Fund 64 , 65 . However, even the growing diversity of such push incentives are, in many cases, insufficient and primarily focused on companies. In addition to these approaches, a strategy is required that helps academic researchers to advance their project portfolio to a level that facilitates early interaction and possibly partnering with pharmaceutical companies in the interest of a successful, cross-sectoral development pipeline 66 . Hence, creating new incentive models in the field is an essential process that can only be moved forward if the public, academic and industrial sectors join forces 39 , 67 , 68 , 69 .
In this respect, our position paper provides an overview of the early phases of antibacterial drug discovery, including hit and lead identification, optimization and development to the (pre)clinical stages by summarizing current limitations, relevant approaches and future perspectives, as well as by presenting selected case studies. In terms of a principal guidance for researchers in the field, we suggest possible solutions for a number of obstacles to improve both quality and quantity of antibacterial hits and leads. To strengthen and emphasize these early stages as an absolute necessity for a sustained generation of novel antibiotics, we are recommending a new level of interaction between the various stakeholders and academic disciplines in the area of antibiotic drug research. The strengths and opportunities that small-molecule therapeutics offer can help address antibiotic resistance more successfully during the coming years, in the interests of both patients and investors, provided that the multiplicity of hurdles along the translational path will be overcome (Table 1 ). Altogether, our aims are in line with the ‘One Health Action Plan against Antimicrobial Resistance’ introduced by the European Commission 70 , as well as the WHO programme to fight the rising number of bacterial priority pathogens with steadily growing impact on global public health 71 .
Synthetic hit compounds
Here, we address the development of profitable strategies to identify and prioritize novel antibacterial hit compounds , with a particular focus on synthetic small molecules. As a foundation, we introduce three main pillars that represent core elements of fruitful hit discovery programmes.
Hit definition, chemical libraries and medicinal chemistry
The concept of ‘hit compound’ 72 as it is widely accepted today needs to be expanded to address the needs imposed by the threat of antibacterial resistance. In this context, a hit compound is a molecule with reproducible activity, with a defined chemical structure (or set of structures), against one or more bacterial target(s). Although the selectivity and cytotoxicity of initial hits are seen as important characteristics, their improvement should remain tasks for the hit-to-lead optimization phase (see below). The activity of hits against (selected) pathogens must be proven in relevant assays, initially in vitro (for example, using exposed/isolated targets or a whole-cell approach), which can be complemented later in the process by the use of animal models of infection to evaluate pharmacokinetic (PK) and pharmacodynamic (PD) properties. In any event, the chemical identity and integrity of a hit must be demonstrated, whereas the actual target and the precise MoA may remain unknown until a later stage. Thus, the initial activity readout for a hit can be on either the molecular or the cellular level (Box 1 ).
When considering the definition of valuable hits, it is important to look beyond the simple model of a single molecule addressing one particular target. Compounds that hit multiple defined targets (known as polypharmacology 73 ), or a combination therapy, in which the effects of several molecules are combined, can be equally valuable 74 . Depending on the target(s), hit combinations may act synergistically, preferably with different MoAs, or in an additive fashion. Such combinations can be useful in potentiating the activity of an existing antibiotic, slowing the onset of resistance and restoring the activity of antibiotics that have become inefficient because of resistance.
A major approach to identify novel hit compounds is by high-throughput screening of chemical libraries. It is important to select the correct set of compounds for each screen, for example, a (large) diverse set, a target-focused set or a fragment library. The make-up of a library should be based on specific characteristics or property space requirements, including chemical, structural and physicochemical aspects (Box 2 ); these may be tailored to a particular disease area 75 , 76 . We believe that carefully designed, and possibly even preselected (‘biased’), chemical libraries, which enable screening of a suitable chemical space against the bacterial target(s) of interest, represent an important first step to start a reliable hit identification campaign towards treatment of a specific bacterial infection. The design, assembly and curation of such libraries are costly processes that require the input of highly skilled practitioners. This frequently falls outside the funding range of most academic groups and, indeed, of many small companies. Models need to be found to grant access to the most useful libraries or compound collections for hit discovery, which should be facilitated at least for non-profit research entities.
Interactions and collaborations between academic researchers and pharmaceutical companies can accelerate hit discovery by, for example, using the high-throughput screening infrastructure of companies to interrogate novel targets. At the same time, pharmaceutical partners might search for close analogues of hits initially identified in academic labs, possibly together with existing biological and chemical property profiles. Such analogue series and accompanying data sets can be extremely valuable in enabling early improvement of antibacterial potency, as well as hit series validation. Pharmaceutical partners might also begin building profiles of absorption, distribution, metabolism, excretion and toxicity (ADMET) parameters, thus, accelerating the hit-to-lead transition. Sharing the relevant information will reinforce the efforts of medicinal chemistry and enhance its reliability and robustness. This, in turn, allows programmes to reach Go/No-Go decisions more quickly and can improve the chances of securing external funding or early partnering deals based on the impact of the medical need.
Notably, medicinal chemistry is the key discipline for the subsequent optimization of hits (see case studies in Boxes 1 – 4 ). A lack of sufficient funding and expertise to support medicinal chemistry at this early stage is highly detrimental for the entire translational process. Encouragingly, in 2016, a large number of pharmaceutical companies with interests in AMR signed the AMR Industry Declaration 77 , in which they jointly committed to support antibiotic R&D processes at virtually all stages. This has led to the formation of the AMR Industry Alliance ( https://www.amrindustryalliance.org/ ). Additionally, the implementation of new AMR-specific capital resources, for example, through the REPAIR Impact Fund and the AMR Action Fund, and the direct involvement of PPPs like CARB-X in hit-to-lead campaigns during recent years should lead to intensified collaborations between industry and academia as a near-term goal to drive the chemical optimization of hits and leads forward towards new preclinical candidates.
Those academic groups that have already built the capacity to carry out such optimization efforts, including broad know-how in medicinal chemistry, biological assays and ADMET studies , would still benefit greatly from early partnering with biopharmaceutical companies, particularly as their projects will stand a greater chance of attracting external investment. Both not-for-profit initiatives, like the European Research Infrastructure Consortium for Chemical Biology and early Drug Discovery (EU-OPENSCREEN; https://www.eu-openscreen.eu/ ), and collaborative PPP models as implemented by the European Lead Factory (ELF) 78 , 79 , allowing for open drug discovery programmes based on Europe-wide screening resources (for example, the Joint European Compound Library, JECL), could pave the way for such early cross-sectoral interactions and exchanges for the benefit of all involved partners 80 .
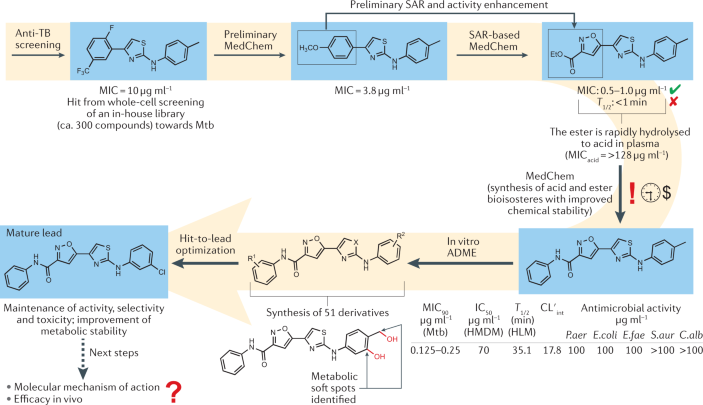
Box 1 Early-stage development of a synthetic antibiotic against Mycobacterium tuberculosis
Historically, the whole-cell assay has generally outclassed target-based methods as the main approach to discover novel antimicrobial drugs. This is particularly true for antitubercular drugs, where the peculiar cellular structure of Mycobacterium tuberculosis (Mtb) is responsible for a lack of correspondence between the biochemical and the phenotypic assays. In the case study pictured, a small in-house chemical library was evaluated using phenotypic screening against Mtb to identify novel antitubercular chemotypes. A few 2-aminothiazoles were found to be moderately active, and the initial hit series was expanded to investigate the structure–activity relationship (SAR) by iterative medicinal chemistry (MedChem) efforts 323 , leading to highly potent derivatives (minimum inhibitory concentrations (MICs) in the submicromolar range) towards susceptible Mtb. To further promote the advancement of these compounds, additional biological assays were carried out to investigate the activity against multidrug-resistant and extensively drug-resistant Mtb strains, the selectivity over other bacterial species and eukaryotic cells, and the susceptibility to the action of efflux pumps 324 . The next research step was focused on a hit-to-lead optimization based on the convergent analysis of the SAR and structure–metabolism relationship. Two metabolic soft spots were identified, and these findings were instrumental for the design of compounds that escaped rapid clearance by human liver microsomes and, at the same time, maintained good antitubercular activity against both drug-susceptible and drug-resistant strains. At this stage, determination of the mode of action at a molecular level and assays in animal model(s) of infection represent the next research progressions. Generally, academic drug discovery can suffer from long timescales and limited resources, which, in turn, make the research process difficult to move forward. For instance, academic chemical libraries are unlikely to yield a significant number of hits from a whole-cell screening, despite the intrinsic chemical novelty that characterizes their creation. Partnership with industrial stakeholders should fill the funding gap and add further expertise, for example, on advanced compound design and in vivo studies, to overcome the limitations mentioned above.
ADME, absorption, distribution, metabolism and excretion; C.alb , Candida albicans ; CL′ int , intrinsic clearance; E.coli , Escherichia coli ; E.fae , Enterococcus faecium ; HLM, human liver microsomes; HMDM, human monocyte-derived macrophages; IC 50 , half-maximal inhibitory concentration; MIC 90 , minimum concentration at which 90% of isolates were inhibited; P.aer ; Pseudomonas aeruginosa ; S.aur , Staphylococcus aureus ; T 1/2 , half-life; TB, tuberculosis.
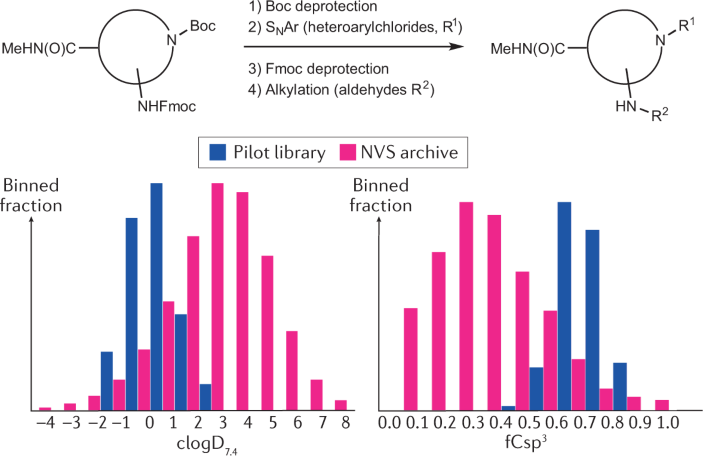
Box 2 Theoretical example of a focused library design generating new chemical entities within a preferred property space
The chemical drug space has been described as almost infinitely large, with an estimated 10 60 compounds 325 . To exemplify the ease of accessing novel chemical matter within a desired property space 75 , 76 , a focused small library could be based on commercially available building blocks. In the example shown, the central building block remains constant and two substituents are added, first by arylation of the Boc-deprotected secondary amine 326 , followed by alkylation of the Fmoc-deprotected primary amine following a reductive amination 327 . The in silico design is driven by diversity, clogD (pH 7.4) 328 between −2 and 2, molecular weight below 450 Da and increased sp 3 content (i.e. level of heavy atom saturation) 329 . This hypothetical pilot library represents 15 aldehydes and 15 heteroarylchlorides to provide a hypothetical 225-compound library, which is shown in comparison with the Novartis (NVS) archive based on polarity (clogD 7.4 ) and fraction of sp 3 hybridized carbon atoms (fCsp 3 ). All 225 compounds are yet unknown in the public domain (Reaxys, https://www.elsevier.com/solutions/reaxys ; last accessed May 2021) and absent from the Novartis archive (April 2018).
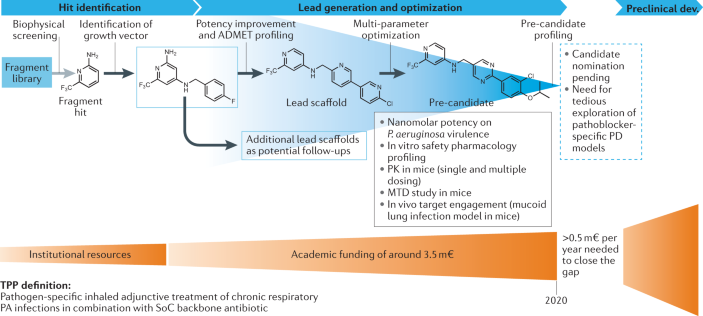
Box 3 Development of an anti-virulence therapeutic (‘pathoblocker’) against Pseudomonas aeruginosa
The concept of interfering with the Pseudomonas quinolone signal quorum sensing system for the discovery of pathoblockers against Pseudomonas aeruginosa (PA) has been explored in detail by multiple research groups 87 . Target validation of the bacterial signal molecule receptor PqsR, which functions as a global virulence regulator, has been achieved using mainly acute murine infection models. A target-driven medicinal chemistry campaign tackling this transcriptional regulator has achieved pre-candidate status starting from a fragment-based approach 330 , 331 , 332 . After biophysical screening, initial hit selection was guided by selection of enthalpy-driven binders (as determined by isothermal titration calorimetry). Successful growth vector identification enabled the detection of qualified hits with cellular anti-virulence activity and potential for advancement to the lead generation and optimization stages 331 . Hit identification was achieved with institutional resources. However, cost-intensive medicinal chemistry and compound profiling work towards a preclinical profiling candidate was only possible through non-dilutive joint funding, which amounted to approx. 3.5 million euros (m€). The chosen target product profile (TPP) is defined as a pathogen-specific inhaled adjunctive treatment of chronic respiratory PA infections in combination with a standard-of-care (SoC) backbone antibiotic. Resulting pre-candidates have nanomolar on-target and cellular efficacy, potentiate tobramycin efficacy against PA biofilms, show high exposures in vivo (various routes intratracheal, intravenous, subcutaneous, peroral) and no overt findings in safety pharmacology screens 333 . While demonstration of in vivo target engagement by means of signal molecule quantification was achieved swiftly in a mucoid acute murine lung infection model, assaying in vivo treatment efficacy related to the pathoblocker-specific activities remains a considerable challenge. Candidate nomination is, therefore, pending on tedious and expensive exploration of suitable pharmacodynamic (PD) models. Currently, this milestone is pursued through further public funding.
ADMET, absorption, distribution, metabolism, excretion and toxicity; MTD, maximum tolerated dose; PK, pharmacokinetics.
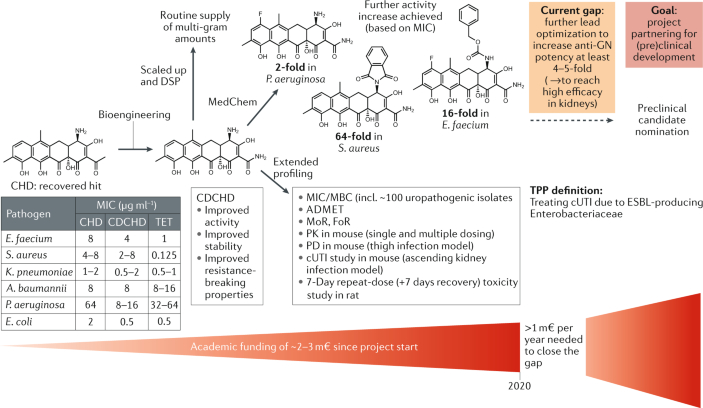
Box 4 Reassessing chelocardin for improved lead development towards complicated urinary tract infection therapy
The natural product chelocardin (CHD), a member of the atypical tetracyclines that was first described about 60 years ago 334 , 335 , has recently been recovered to generate a novel lead scaffold, amidochelocardin (2-carboxamido-2-deacetyl-chelocardin, CDCHD), by rational biosynthetic engineering 336 . For this purpose, the CHD biosynthetic gene cluster in Amycolatopsis sulphurea 337 was combined with genes from the oxytetracycline biosynthesis pathway of Streptomyces rimosus , and production peak titres of the novel hybrid compound CDCHD up to 400 mg l −1 were achieved 191 . CDCHD represents a new broad-spectrum antibiotic active against pathogens of the ESKAPE panel (including a large number of clinical isolates) 106 , which can be routinely supplied at the multi-gram scale with >95% purity by using large-scale in-house fermentation at the Helmholtz Centre for Infection Research (HZI) (~100-l batch cultures) and optimized downstream processing. Due to the lack of cross-resistance to known antibiotics (for example, preserved activity against pathogens carrying multiple tetracycline (TET) resistance determinants), the good production yield and the fact that efficacy for CHD treatment was already shown in a small phase II study 338 , CDCHD was chosen to enter a lead optimization programme (see Acknowledgements). Optimization of CDCHD includes further bioengineering and medicinal chemistry (MedChem) approaches for extensive structure–activity relationship profiling, which is currently based on >70 generated analogues with modifications achieved at about ten different scaffold positions 192 , 339 . Extended CDCHD profiling by absorption, distribution, metabolism, excretion and toxicity (ADMET), pharmacokinetics/pharmacodynamics (PK/PD), toxicity studies and validation of therapeutic efficacy in an ascending kidney infection model indicated the use of CDCHD for the treatment of complicated urinary tract infection (cUTI) caused by extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae according to the selected target product profile (TPP) 340 . However, further increase in potency is required to achieve higher efficacy in kidneys against clinically most relevant uropathogens, which is essential for preclinical candidate nomination in this project. To achieve this goal, funding limitations in the academic sector shall be overcome by partnering with an industrial stakeholder.
DSP, downstream processing; FoR, frequency of resistance; GN, Gram-negative; m€, million euros; MBC, minimum bactericidal concentration; MIC, minimum inhibitory concentration; MoR, mechanism of resistance.
Nature of the target
We recommend that hit identification against bacteria follows two convergent approaches: (i) identification of molecules active against molecular targets that are vital for all stages of the bacterial life cycle (‘essential targets’), thus, directly promoting clearance of the bacteria from the host/patient, and (ii) searching for molecules that inhibit so-called ‘non-essential targets’ 53 , 81 , 82 . The latter can be defined as bacterial structures that are not vital under standard laboratory growth conditions but become critical during processes of host colonization and infection, for example, by regulating virulence development, by evading host immune response or by triggering bacterial defence mechanisms 83 . Molecules hitting such targets may have weak or even no activity towards bacterial cells under non-infectious (in vitro) screening conditions, but might display highly synergistic or additive effects when tested in relevant in vivo infection models, either alone or in combination with antibacterial agents addressing essential targets. The latter molecules may be found among the current antibacterial arsenal or may be new chemical entities, identified as described above.
Compounds interacting with non-essential targets are usually classified as antibiotic adjuvants, potentiators or resistance breakers 84 , 85 . Examples of non-essential target inhibitors are represented by:
Inhibitors of virulence-conferring factors or pathways (also known as anti-virulence compounds or pathoblockers 86 that target, for example, quorum sensing mechanisms 87 , biofilm formation 88 , bacterial secretion systems 89 , 90 , enzymes for tissue penetration 91 or intracellular survival 92 ).
Efflux pump inhibitors 93 .
Suicide substrates such as β-lactamase inhibitors 94 , 95 .
Inhibitors of pathways serving as a mechanism of defence, e.g. glutathione biosynthesis 96 , 97 .
Modulators and inhibitors of energy metabolism 98 , 99 .
Host/pathogen epigenetic modulators 100 , 101 .
For some of the mentioned targets, such as efflux pumps, it has been demonstrated that their inhibition can reverse resistance to several antibacterials 102 . Therefore, an attractive therapeutic combination might be composed of a bactericidal agent and an adjuvant molecule, with the aim of potentiating the antibacterial effect(s) and significantly reducing resistance (either intrinsic or evolved) 103 . Since the pathoblocker approach is anticipated to be less susceptible towards resistance development and, in addition, to preserve the commensal bacteria of the microbiome 86 , it represents a non-traditional strategy for a focused disarming of resistant high-priority pathogens, most likely to be deployed as an adjunctive therapy in addition to antibiotic standard treatment 81 (Box 3 ).
Advanced screening and profiling based on standardized assays
There is a fundamental need for assays to identify hit compounds (both synthetic and natural-product-based hits, the latter are addressed below) specifically for the clinically most relevant indications. In addition to using focused libraries that cover desirable chemical diversity and property space, innovative screens are essential to increase the chances for identifying potent hits against most prevalent common infections associated with Gram-positive or Gram-negative pathogens, such as hospital-acquired pneumonia, community-acquired pneumonia, complicated urinary tract infection or complicated intra-abdominal infection 104 . To establish a reliable foundation for future development, both academia and industry must use state-of-the-art library screening procedures based on generally accepted rules and basic concepts of standardization.
It is important that a range of relevant assays is used to thoroughly select and profile novel hit compounds. These assays should have a high physiological significance, which may be applicable to biomimetic assays 105 , for example, by using defined culture media such as artificial urine for activity screens with uropathogens 106 , 107 , iron-depleted media that simulate bacterial growth conditions during bloodstream or wound infections 108 , 109 or assaying host–bacteria interactions 110 . Such schemes can further include the screening for new MoA(s), new drug sensitizing modes, non-killing mechanisms (e.g. anti-virulence factors like pathoblockers), compounds acting against biofilms and molecules acting synergistically with existing or new antimicrobials to overcome drug resistance 111 , 112 , 113 , 114 . Similarly, because hits generated by conventional biochemical assays or screens often fail to become whole-cell active leads, alternative phenotypic assays such as novel target-based whole-cell screening 115 are also a promising foundation for the identification of useful hits. Even known chemical libraries (including proprietary compound archives of pharmaceutical companies), which have failed to deliver antibacterial hits by simple growth inhibition measurement, might bear fruit if reassayed following these approaches. The way in which these innovative screens are envisaged could make them a more appropriate strategy to provide novel hits with a potential therapeutic impact compared with the molecular-target-based drug design approach 116 .
A further aim of the consortium is to design and develop informative assays that can provide information about the desired antibacterial effect, together with further characteristics such as target engagement, bacterial penetration characteristics (for example, kinetics of compound permeation through Gram-negative cell envelope models 117 , 118 ) and potential cytotoxicity.
In addition to devising standardized panels of assays according to contemporary technology, developing the respective standard operating procedures (SOPs) is mandatory to meet the requirements for good research practice, which facilitates the transfer of compounds with potential to become new drugs from academia to non-profit or private organizations for continued development. By using standardized proof-of-concept assays under predefined SOPs, more robust hit series will emerge, increasing their potential for late-stage development and minimizing reproducibility issues. For example, minimum inhibitory concentrations , and possibly also minimum bactericidal concentrations , should always be evaluated in a screening campaign, for example, by using the European Committee on Antimicrobial Susceptibility Testing (EUCAST) ( https://eucast.org/ ) or the Clinical and Laboratory Standards Institute (CLSI) ( https://clsi.org/meetings/ast/ ) guidelines. In addition, selected hits from standard screening panels should be consequently tested against contemporary clinical isolates to demonstrate that they overcome existing resistance mechanisms.
Owing to the high attrition rates from early hit discovery to advanced hits and leads, it is especially important in the field of antibacterials to diversify and generate multiple hit series, and to characterize them thoroughly regarding all features that appear relevant to the intended therapeutic use. This includes explorations to expand scaffold diversity in the context of understanding the target-based chemical and physicochemical requirements, as well as potential liabilities, like ADMET.
A summary of early target hit profiles is essential to nominate the most valuable hit series acting against the pathogen(s) or medical indication(s) of interest. The selection of hit series for lead generation follows the target candidate profile (TCP), which is predefined at the outset of the development programme according to the desired target product profile (TPP) (Fig. 2 ). Thus, the optimization of hits should generally be driven by TCPs and compound progression criteria that, in turn, are driven by chosen TPPs. If several TPPs have been selected or outlined for a campaign, for example, based on different indications, together with their corresponding TCPs, it has to be decided which TCP should be used as a base to aim at for a given chemical series or possibly natural-product-based hit that emerges from mining of biological sources (see below).
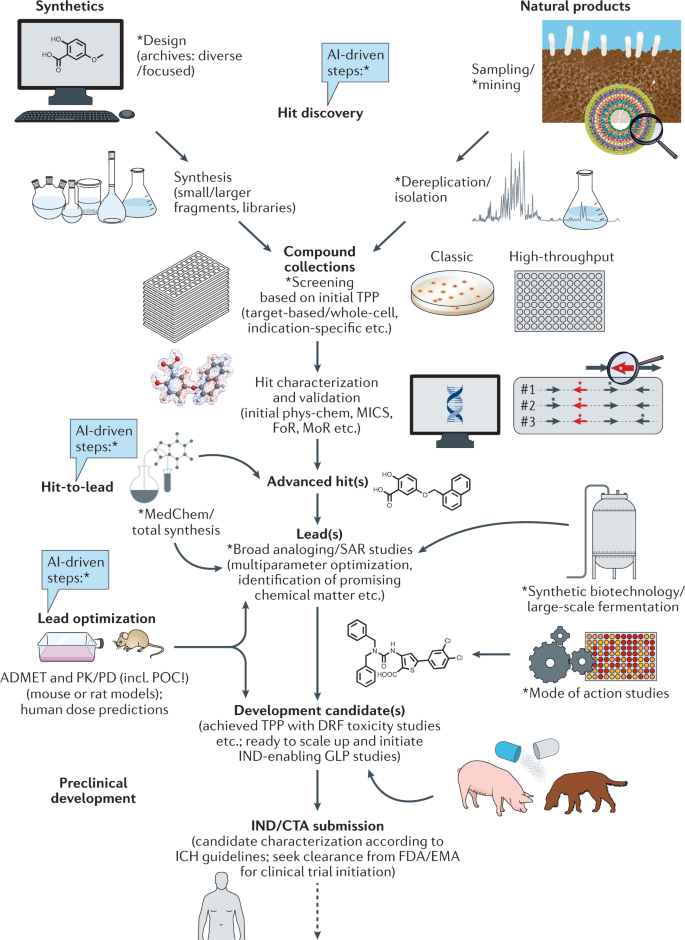
Approaches marked with * can be linked with emerging artificial intelligence (AI)-based technology, for example, for advanced data mining, screening or property predictions, to increase efficiency and outcome. ADMET, absorption, distribution, metabolism, excretion and toxicity; CTA, clinical trial application; DRF, dose range finding; EMA, European Medicines Agency; FDA, U.S. Food and Drug Administration; FoR, frequency of resistance; GLP, good laboratory practice; ICH, International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use; IND, investigational new drug; MedChem, medicinal chemistry; MICs, minimal inhibitory concentrations; MoR, mechanism of resistance; phys-chem, physicochemical properties; PK/PD, pharmacokinetics/pharmacodynamics; POC, proof of concept; SAR, structure–activity relationship; TPP, target product profile.
It is important to implement physicochemical and in vitro ADMET profiling at the start of hit optimization, to make sure that any PK issues are identified early and can be addressed through the entire chemistry programme. In this respect, a standardized list of essential compound properties is required for successful transfer of hits and early leads into the following discovery and development stages. Depending on the defined TPP, such a dossier on physicochemical and biological properties should comprise a set of minimal criteria for compound progression based on selected, standardized assays or attributes with clear benchmarks for transition to the next stages in the drug discovery pathway and for continued (pre)clinical development according to the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines ( https://www.ich.org/page/ich-guidelines ). This will help to ensure developable compounds of clinical relevance are produced, which are also attractive for potential industrial partners. Relevant parameters (depending on the particular stage of transition) may include:
Potency/cellular activity (e.g. based on minimum inhibitory concentrations and minimum bactericidal concentrations).
Chemical and metabolic stability, solubility, permeability (e.g. based on logP or, for ionizable compounds, logD, or complex membrane partitioning).
Distribution, efflux avoidance, selectivity/off-target avoidance (e.g. inhibition assays on receptor panels, hERG etc.).
Acid/base properties based on p K a .
Cytotoxicity (especially human cell lines).
Lack of reactive metabolites.
Phototoxicity.
Protein binding.
In vivo efficacy and human dose prediction.
(Oral) bioavailability.
Genotoxicity (e.g. based on Ames or mouse micronucleus tests).
Drug–drug interactions.
PK linearity.
Safety (in vivo toxicity).
Compound access (e.g. synthetic feasibility and scaling up to gram or kilogram).
Achievable degree of purity.
Formulation.
Once the hit discovery transitions into the hit-to-lead and lead optimization phases (see below), it is necessary to enlarge the scope of biological studies. These may include bacterial killing kinetics, MoA, frequency of resistance, mechanism of resistance and PK/PD analyses, which will deliver valuable parameters to assess a compound’s in vivo efficacy (assuming sufficient free drug exposure in a relevant animal model with acceptable tolerability). At this level, it is, once again, important to acquire information on a substantial number of structurally related analogues through extensive medicinal chemistry efforts (perhaps in collaboration with PPPs or the pharmaceutical industry, as suggested above) in order to establish clear and reliable dossiers of structure–activity relationship (SAR) and structure–property relationship. These data are essential to consistently improve all the required parameters as a basis for a continuous advancement of lead structures towards the selection of (pre)clinical candidates. Computational methods based on machine learning techniques like profile-quantitative structure–activity relationship (pQSAR) can help to build predictive models regarding activity, selectivity, toxicity, MoA and further parameters for specific compound classes, hence, providing valuable in silico input for more effective hit discovery and lead design 119 , 120 .
Natural-product-based hit compounds
Historically, microbial natural products have been the most important source of antibiotic lead compounds ; over the last 40 years, about 60% of all new chemical entities in the field of antibacterials were based on or derived from natural products 121 . Here, to complement the key aspects described above for synthetic hits, we outline the major requirements specific to the identification and prioritization of antibacterial natural product hits. We focus on efficiency and, particularly for the academic sector, achievability in terms of technological and financial demands.
Identification of new chemotypes from natural sources
The known antibiotic activity of natural products has, in general, been identified by phenotypic screening campaigns that determine activity against panels of test organisms in standardized assays. These screens, which constitute the basis for bioactivity-guided isolation of natural products from complex mixtures, efficiently retrieve bioactive compounds when libraries of crude extracts are evaluated. However, their ability to reveal useful novelty is limited by both a high rediscovery rate of already known molecules associated with pre-existing resistance mechanisms, as well as a substantial proportion of hits that show significant cytotoxicity or poor ADMET properties.
We emphasize that there is a general lack of efficient tools and strategies to increase the number of new chemotypes and to reduce the rediscovery rates in antibacterial screening approaches. Even on a global scale, the number of newly discovered chemotypes, especially novel scaffolds acting against Gram-negative bacteria, is consistently low. Several approaches are relevant to improve this situation:
One possibility to enforce the identification of new antibacterial chemistry is to limit screening of already broadly characterized groups of secondary metabolite producers, for example, actinomycetes, and to expand efforts on identifying new types of producers by extensive biodiversity mining. This can be achieved by focusing on the ~99.999% of microbial taxa of the Earth’s microbiome that remain undiscovered 122 , 123 , including the as yet underexplored taxa of human and animal microbiomes 124 , 125 , 126 , 127 . Emerging innovative isolation and cultivation techniques such as diffusion bioreactors (also carried out on the microscale as with the iChip 128 , 129 , 130 ), microfluidics 131 , 132 , 133 , elicitors 134 and various co-cultivations 135 , 136 will help to access and understand the rare and less-studied groups of microorganisms from diverse habitats 137 , 138 , 139 . Further, molecular (co-)evolution acting to generate novel metabolites for efficient microbial warfare could be exploited 140 , 141 , for example, by sampling from environments heavily contaminated with antibiotics (like sewage in Southeast Asia or South America), which are known to contain highly resistant microbes 142 , 143 . Complementarily, this can be achieved by laboratory exposure of potent producers to subinhibitory antibiotic concentrations 144 or by co-culturing them together with drug-resistant (pathogenic) strains 145 . Beyond microbial producers, a great variety of plants 146 , 147 , macroscopic filamentous fungi (e.g. Basidiomycota) 148 and animals 149 bear the potential to deliver useful compounds as a base for novel antimicrobials. Altogether, the exploration of untapped biological resources, which represent a major reservoir for future therapeutics, should generally be extended within the academic and industrial sector.
After genome mining of novel microbial isolates or metagenome-driven discovery of novel natural products 150 , 151 , 152 , 153 , selected biosynthetic gene clusters (BGCs) that potentially produce unknown secondary metabolites should be systematically expressed in specialized heterologous host strains 154 , 155 , 156 . This helps to facilitate a straightforward detection and isolation of the new compounds, particularly if their BGCs are ‘silent’ (i.e. not expressed under known conditions) in the native host. Such heterologous hosts or chassis strains can be based on microbial species that commonly produce a large variety of natural products, but have been made devoid of their own secondary metabolite BGCs and/or have been further optimized to efficiently express BGCs originating from ‘non-common’ sources (for example, rare actinomycetes or fungi) 154 , 157 , 158 . However, only a limited set of such specialized host strains is available so far, and a much more diverse array of microbial chassis needs to be developed to fit the demands of a growing arsenal of BGCs that potentially produce novel chemistry. BGC expression is often most successful in strains closely related to the native producer, and, thus, it is important to develop methods for standardized heterologous expression in selected host strains with desirable properties that have not yet been domesticated for the use as regular chassis 159 .
Chemical space can also be enlarged by using emerging synthetic biology approaches for medium-to-high-throughput genome editing and pathway engineering. These approaches, which are primarily based on CRISPR/Cas9 (refs 160 , 161 ) and diverse recombination, assembly and integrase systems 162 , 163 , 164 , can be followed up with advanced analytics and screening of the potentially modified natural products, which may be produced in only trace quantities. This technology involves the extensive use of information on genome sequences, enzyme activities and compound structures collected by publications, databases and web tools (such as MIBiG 165 , antiSMASH 166 and PRISM 167 ) over the past few decades. In many cases, the modularity of the BGC composition, which is found in gene clusters, for example, coding for polyketide synthases or non-ribosomal peptide synthetases, can be used to implement a bioinformatics-supported plug-and-play diversification strategy enabling the exchange and recombination of core units, as well as modifying enzymes 168 , 169 , 170 , 171 . A concomitant refactoring of BGCs, especially from rare microbial sources, often allows high-level heterologous production of the antibiotic compounds in suitable hosts 172 , 173 , 174 , 175 . However, these methods are still in their infancy and require wider testing with different classes of antimicrobials to define general principles of feasibility and scalability, which, furthermore, necessitates an improved understanding of the complex biosynthetic machineries and their modular evolution.
Advances in analytical chemistry techniques, for example, in mass-spectrometry-based metabolomics and its enhancement by molecular networking and the application of machine learning, support the process of dereplication 176 during (secondary) metabolome mining 177 , 178 , 179 , 180 , 181 . Known compounds produced in reasonably high yields can be rapidly identified via their high-resolution masses, tandem mass spectrometry fragmentation patterns or structural data in secondary metabolite databases 138 , 182 , 183 , 184 , 185 , 186 , 187 . However, the remaining bottleneck is to highlight and annotate novel antibiotic compounds, particularly those with low production titres, as early as possible in the discovery process (i.e. from crude extracts if possible, without the need for small-scale fractionation and enrichment). This objective can be supported by innovative extraction methods prior to bioactivity-guided isolation of novel compounds 188 .
In addition, revisiting known potent antibiotics, previously neglected as a result of unacceptable or non-addressable properties such cytotoxicity or lack of stability, can be a valuable strategy to provide novel leads and candidates. The reassessment of such scaffolds can be based on a variety of efforts, including the improvement of production and purification 189 , reconsideration of application and effective dose for natural derivatives 190 , or advantageous scaffold modification by biosynthetic engineering and semi-synthetic approaches 191 , 192 (Box 4 ).
Further opportunities remain to improve the discovery and development of agents for combination therapy as indicated above, i.e. compounds that act synergistically against multidrug-resistant and/or high-priority pathogens 193 , 194 . The discrimination of specific synergistic activities from non-specific antibiotic activities remains a challenge during the discovery process.
Improving bacterial target access, enhancing potency and broadening the antimicrobial spectrum of known and novel antibiotic scaffolds can be achieved by using drug-conjugate strategies, for example, linking of pathogen-specific antibodies 195 , 196 , siderophore moieties 197 , 198 or positively charged peptides 199 , 200 to the antibiotic core scaffold. Though these approaches have been proven effective in a number of cases, some of them may also have unintended effects, such as a spontaneously increasing frequency of resistance, which can be problematic, for example, in the case of the Trojan Horse approach 201 .
Overall, a variety of innovative and complementary technologies is required to improve access to novel natural product scaffolds. Computational methods can provide powerful assistance at different levels in many of the areas indicated above, as recent efforts show 202 , 203 . In this context, artificial intelligence might play a game-changing role in the future. The general power of neural networks for detecting new antimicrobial candidates has already been demonstrated 202 . By using a computational model that screens hundreds of millions of chemical compounds in a few days, potential antibiotics even with new MoA(s) could be proposed rapidly. Given the recent advances in artificial intelligence, these and other models will likely add to the future identification of new candidate drugs.
Interestingly, when looking at compound properties, it appears that there is often more flexibility in the selection of ‘successful’ natural product scaffolds compared with synthetics, for example, regarding Lipinski’s rule of five 204 , 205 , 206 , which natural products frequently ‘disobey’ (such as cyclosporine or macrolides like azithromycin). Thus, antimicrobial drug discovery in ‘beyond rule of five’ chemical space is an opportunity when using natural compound collections or when assembling libraries of de novo designed compounds 207 , 208 , 209 , though the general need for optimizing key pharmacological properties of such hits remains beyond question.
Another major challenge for natural products can be the generation of structurally diverse analogues (particularly if they are not accessible through biosynthesis). Many scaffold positions can be difficult to access by means of semi-synthesis and, thus, broad derivatization of natural-product-based hit and lead compounds is often much more labour-intensive, and establishing synthetic access to these scaffolds with a focus on the ability to systematically diversify their chemical space can require large amounts of resources 210 . Nevertheless, the modification of natural scaffolds with substituents that are often easier to incorporate by (semi-)synthetic or chemoenzymatic approaches, such as halogens that allow the modulation of solubility, permeability, selectivity, target affinity etc. 211 , 212 , proves that multiple opportunities arise when combining synthetic and biological chemistry.
Required access to biological and chemical material and data
Many scientists frequently experience difficulty in accessing and sharing research material from third parties, including microbial strains, cultivation extracts, pure compounds, genome or gene cluster sequences and further background data (of published or even unpublished results). For example, an interesting BGC is identified in publicly accessible databases, but the strain is not specified or not available from the indicated source. Similarly, access to industrial antibiotic overproducers can be impossible, even when a company no longer has a commercial interest in the resulting molecule. This phenomenon has several origins, including legal restraints (for example, imposed by the Nagoya Protocol 213 ) or intellectual property (IP) claims on strains, compounds, biologics or (re)profiling data of already known structures.
In the public interest, standardized procedures are necessary to facilitate access to research materials and to solve IP conflicts, at least within the field of academia, in which it is common practice to share research materials with colleagues by negotiating appropriate cooperation agreements.
Further, the access to in-house compound libraries of pharmaceutical companies (at least subsets of them and especially those that are not intended for antibiotic-related screening) could be very valuable for academic partners who are eager to identify novel antibacterial hits, which could lead to joint drug development programmes. Enabling access to materials can also be extended to strain collections, including clinical isolates representing the diversity of pathogens associated with a certain clinical indication, and advanced compound information based on pre-existing characterization and profiling campaigns. An increased availability of these resources will be of great benefit to the antimicrobial research community worldwide.
Furthermore, comprehensive databases and data-sharing platforms can provide another valuable resource for present and future antibiotic R&D projects and, hence, should be implemented and maintained with care 214 . There is a growing body of recently initiated and publicly available web-based tools and archives that support accumulation and exchange of data regarding antibacterial compounds in different stages of discovery or therapeutic development, known or predicted antibiotic targets and the diversity of antimicrobial resistance determinants (Box 5 ). Further connection and integration of such databases is desirable to optimize the output for a specific search request. In addition, initiatives comparable with the European Commission’s manifesto to maximize the public accessibility of research results in the fight against COVID-19 (ref. 215 ) are also highly recommended to support AMR-related scientific research at all levels, including facilitated access to online resources.
Box 5 Examples of public databases and tools related to antimicrobial compounds, targets and resistance
Discovery of antibacterial compounds and development into (potential) therapeutics:
https://db.co-add.org/downloads/
https://globalamrhub.org/dynamic-dashboard/
https://chemdb.niaid.nih.gov/DrugDevelopmentTB.aspx
https://coconut.naturalproducts.net/
https://zinc.docking.org/
https://revive.gardp.org/resources/
https://go.drugbank.com/
https://www.antibioticdb.com/
https://www.pewtrusts.org/en/research-and-analysis/articles/2018/09/21/the-shared-platform-for-antibiotic-research-and-knowledge
https://www.pewtrusts.org/en/research-and-analysis/data-visualizations/2014/antibiotics-currently-in-clinical-development
https://www.pewtrusts.org/en/research-and-analysis/data-visualizations/2017/nontraditional-products-for-bacterial-infections-in-clinical-development
https://www.who.int/observatories/global-observatory-on-health-research-and-development/monitoring/who-antibacterial-preclinical-pipeline-review
https://www.who.int/observatories/global-observatory-on-health-research-and-development/monitoring/antibacterial-products-in-clinical-development-for-priority-pathogens
Antimicrobial target search and prediction:
https://pypi.org/project/targetDB/
https://platform.opentargets.org/
https://arts.ziemertlab.com/
Antimicrobial resistance:
https://card.mcmaster.ca/
https://www.ncbi.nlm.nih.gov/pathogens/antimicrobial-resistance/
https://bench.cs.vt.edu/deeparg
https://github.com/abu034004/PARGT
Prediction of antimicrobial structure and function from genome sequence data
Driven by breakthroughs in sequencing technologies and genome mining, the identification of BGCs encoding the biosynthesis of natural products has matured to complement the chemistry-driven and bioactivity-driven screening processes for natural product hits. Computational methods are established and continuously improved to identify novel biosynthetic pathways in (meta)genomic sequence data 150 , 151 . Recently, third-generation genome sequencing techniques such as PacBio and Oxford Nanopore have been developed that provide high-quality full genome data even for complex microorganisms like filamentous fungi at reasonable cost, which is an ideal prerequisite for large-scale genome mining approaches 216 .
However, linking the obtained sequence information to possible structural or functional features of the encoded molecules remains a great challenge. Prediction of chemical structures directly from genome data would help to distinguish known from potentially novel scaffolds during a very early stage of dereplication; the training of machine learning algorithms with sufficient quantity of genome data from microbial producers could ultimately lead to fairly accurate predictions of chemical structures linked to specific BGCs and possibly even their biological activities 167 .
A successful strategy to decipher antibacterial targets of new natural products, without the need to isolate them, is a directed search for known resistance factors in the genomes of antibiotic-producing microbes 217 , 218 . These producers may code for resistant variants of the molecular target(s) that interact with the intrinsic antibiotic(s) without damaging the host or conserved class-specific transporters that release the compound(s) into the environment. This approach recently led to the discovery of novel antibiotic scaffolds 219 . However, most BGCs do not contain apparent or specific drug-resistance genes that could straightforwardly indicate a compound’s function. In the majority of cases, very limited predictions based on genomic data concerning function and potential target(s) of a natural product are currently possible, although advanced automated tools for target-directed genome mining are available 220 . Thus, there is a high demand for innovative methods to predict the molecular function or target of a natural compound based on genomic data. Such data would be extremely valuable in order to prioritize BGCs for experimental characterization. In the future, artificial intelligence approaches, based on either classical machine learning methods (extracting new knowledge from preprocessed data sets) or on deep learning (drawing conclusions from raw data such as representative examples, often by using multilayer neural networks), may deliver such predictions with increasing accuracy 221 . However, existing algorithms need to be improved, and new ones have to be developed to specifically address the question of how to assign target-based functions to natural products with confidence during the early stages of discovery and prioritization. These approaches also require a huge amount of validated training data 222 .
Advancing hits to (pre)clinical status
Regardless of whether antibacterial hits emerge from rationally designed synthetic molecules or from the pool of natural products, the subsequent hit-to-lead and lead-to-candidate optimization phases are very similar for compounds irrespective of origin (‘Y model’, see Fig. 2 ). We now discuss the most critical obstacles and requirements for delivering those advanced leads that may eventually become the next generation of (pre)clinical candidates.
Drug–target interaction studies as a base for hit development
For hits arising from phenotypic assays, cellular MoA(s) or specific molecular target(s) may not be known at the hit-to-lead stage, and, sometimes, the precise MoA is elucidated years after the approval of a drug, as in the case of daptomycin 223 . However, detailed insight into the mechanism(s) by which compounds exert their pharmacological activity is highly desirable for further rational optimization of chemical scaffolds, particularly when structurally enabled approaches can be used, for a convincing presentation of preclinical candidate dossiers and for regulatory requirements. Since universally applicable methods for characterizing the MoA(s) of antibiotics do not exist, a full suite of expertise in genetics, genomics, microbiology, chemical biology and biophysics is required. Identification of the molecular target can be achieved by targeted screens of indicator or mutant strains, whole-genome sequencing upon focused resistance development 224 , 225 , pattern recognition techniques based on transcriptomics 226 , imaging 227 , 228 , metabolomics 229 , macromolecular synthesis 230 , 231 or mutant fitness profiles 232 , 233 , which can be coupled with machine learning approaches for directed predictions 225 , 233 , or chemoproteomics 234 , 235 . The latter is specifically useful in the case of non-essential target inhibitors like pathoblockers, since these may not generate resistant mutants (at least under standard laboratory conditions). Additional techniques for MoA studies may include crystallography, a diverse set of spectroscopic and calorimetric analyses 236 , 237 , 238 , 239 , 240 , as well as the use of functionalized derivatives (‘tool compounds’) 241 , 242 , which can support both target identification and validation and may provide in-depth information of drug–target interactions to drive the rational hit-to-lead optimization process forward. Alternatively, identification of drug–target (or ligand–protein) interactions formed under native (unbiased) conditions by using specialized proteomic approaches is becoming increasingly successful 243 , 244 , 245 , 246 . Current bioinformatic tools can also combine genome-mining approaches with the prediction of potentially innovative MoA(s) based on the presence of resistant target genes in BGCs encoding novel antibiotics 220 . These and other examples illustrate how a diverse set of emerging learning methods is steadily enhancing the predictability of drug–target interactions 247 , 248 .
In addition to the specific molecular target(s), it is important to understand the impact of the antibiotic compound on the general physiology of the bacterial cell. This includes the sequence of events leading to bacterial death, the time point when killing occurs (based on either individual bacterial cells or their population/colonization level) and the conditions that might enhance or preclude it. Such characterizations may require the application or development of a range of secondary assays. For compounds acting on intracellular bacterial targets (i.e. targets located in the cytoplasm), the processes of compound influx and prevention of efflux (especially so for Gram-negative bacteria as a result of their complex cell envelope and presence of numerous multidrug efflux pumps) are both critical optimization parameters to ensure sufficient target engagement 249 , 250 , 251 , 252 , 253 . These factors can be addressed by suitable compound design, which generally remains rather empirical and challenging 254 , 255 , 256 , 257 . Other possibilities to address this key area would be to use these compounds in combination with outer membrane permeabilizing agents 258 , 259 or efflux inhibitors 93 , 260 . Alternative approaches targeting extracellular virulence factors, for example, extracellular lectins required for attachment and biofilm formation or secreted proteolytic enzymes, do not suffer from a possible lack of bacterial uptake 261 . Often, antibiotics, and particularly natural products, have more than one target and disturb bacterial physiology in several different pathways, a phenomenon referred to as polypharmacology 73 , 262 , 263 , which is beneficial for inflicting severe damage on the bacterial cell and slowing down target-mediated resistance development. Information related to such effects should be acquired for all bacterial species within the spectrum of activity of the potential drug, and it may diverge significantly across phylogenetically distant species.
Apart from the desired biological effects on bacterial pathogens, knowledge about undesired adverse effects on eukaryotic cells (‘off-target effects’ 264 , 265 , 266 , 267 , 268 , 269 ) should be acquired early on, since toxicity is a major contributor to attrition in the drug development process. However, whilst in vitro cytotoxicity screens are useful during the early discovery process, they are often not predictive of toxicological effects that can become most significant during in vivo studies. Furthermore, collateral damage to the microbiome needs to be considered 270 , 271 , 272 , 273 and can be modulated by selective drug design 274 . For compounds with a novel or particularly complex MoA, it often takes several years to achieve a detailed molecular understanding and the cellular consequences of exposure. Therefore, acquiring this knowledge as early as possible is a key aspect for further rational drug optimization, including SAR studies and structure-guided hit/lead optimization. We recommend investing resources into expanded MoA studies already during the initial stages of the drug development process and, furthermore, building a network of experts who can provide MoA analyses that fulfil the requirements of a preclinical candidate dossier. While these aspects are standard for drug development projects in the pharmaceutical industry, academia usually suffers from insufficient funding to appropriately address such requirements, and, therefore, additional resources need to be secured.
Limited resources to move from hit into lead stage
Once a hit validation has been accomplished, the resources needed to advance the selected compound series into hit-to-lead and lead optimization greatly increase. These stages require a diverse scientific team covering analytical, computational and medicinal chemistry, biochemistry, microbiology, bioinformatics (ideally including machine learning and artificial intelligence methods), drug metabolism and pharmacokinetics, as well as, specifically for natural-product-based compounds, biotechnology and genetic engineering. In industrial projects, typically 5–15 medicinal chemists work on the optimization of a hit (depending on how complex the chemistry of a certain compound is) to create promising leads or preclinical candidates, essentially by generating, testing and advancing SAR-based analogue series in an iterative manner. The challenge is to simultaneously optimize all properties necessary for the drug to be most effective and least toxic. This includes potency, selectivity, physicochemical parameters and cytotoxicity, as well as pharmacokinetics and pharmacodynamics (Fig. 2 ). The multi-parameter optimization can usually be achieved within a time frame of about 2–4 years, but remains dependent on the human, technological and financial capacities, as well as the particular challenges represented by the chemical series. Such resources are difficult to acquire through classical academic funding schemes, which usually reward new discoveries in fundamental science, rather than subsequent steps of time-consuming and resource-consuming optimization, where there is no guarantee of success.
Academia must, therefore, find new ways to provide suitable resources for early-stage translational research. Since few academic institutions possess the relevant expertise and facilities to carry out lead optimization, they usually require access to high-quality expertise and/or capacities in cooperation with pharmaceutical companies/SMEs or through contract research organizations (CROs), which can only be achieved through additional funding or partnerships. One possible strategy to acquire appropriate resources in future could be the application of alternative reward schemes for evaluation of academic project funding, which might not only be based on high-impact publications but also on verifiable commitment to health research, such as making dedicated contributions to a global antibacterial portfolio. The emergence of centres for translational science in many countries (for example, the German Center for Infection Research; https://www.dzif.de/en ) could be an opportunity to develop and implement such measures, possibly at an international level.
The bottleneck of compound supply
The enhanced biological profiling that is mandatory in hit and lead optimization programmes requires a considerable amount of sufficiently pure compounds to be tested. While this can be a problem for chemists in general even with respect to synthetic hits and leads (especially the massive scale-up of typical laboratory test reactions) 275 , 276 , the problem of supplying increasingly large quantities of natural products originating from bacteria, fungi or plants is particularly challenging. Indeed, academic projects are often concluded when natural compounds or biotechnologically generated variants thereof are identified at small scale (often <10 mg), with only rudimentary profiling. In many laboratories, there are no additional resources to increase the yields of natural product hits or initial leads, or to scale up production in a pre-pilot plant environment that is capable of carrying out the fermentation (possibly by using heterologous production hosts to achieve attractive yields 277 , 278 ). In addition, downstream processing has to be established and optimized for every new compound to ensure satisfactory purity at a sufficient quantity for the following stages, including scaffold optimization by medicinal chemistry or extended biological profiling. The fact that sufficient amounts of compounds (multigram-to-kilogram scale) cannot be produced in many cases severely decreases the chances of developing novel therapeutics from natural products. This is particularly unfortunate in the antibiotics field, because about two-thirds of all antibiotic drugs in therapeutic use are derived from natural products 44 , 121 . Regrettably, fermentation-independent supply, for example, through the total synthesis of complex natural compounds, can only be achieved for a low percentage of novel hits and leads and requires a tremendous amount of additional capacity and resources 279 , 280 , 281 , 282 .
Thus, suitable funding instruments are needed to cover the essential processes of natural compound scale-up and supply based on biotechnological methods, including large-scale fermentation and efficient downstream processing 283 , 284 , 285 , towards obtaining high-quality source material for semi-synthesis and further studies. In addition, a robust method for large-scale production and downstream processing of the candidate molecule is a prerequisite for process transfer to good manufacturing practice (GMP) production before entering (pre)clinical stages. Generally, further scientific and technological development is required to make the provision of compound material from various sources a more routine and affordable task, particularly in the non-industrial research environment.
Requirements for in vivo studies and project transfer
The primary assays in most discovery programmes usually address biochemical, biophysical and/or microbiological functionality of newly generated compounds. In order to convert a molecule with in vitro activity into a drug, sufficient exposure at the infection site in vivo must be achieved. To analyse this, a full suite of ADMET assays is required 286 , 287 , followed by pharmacokinetics experiments in animals (usually starting with rodent models) 288 , 289 , which can be combined with physiologically based PK modelling and in silico ADMET prediction 290 , 291 .
A sufficient correlation between in vitro and in vivo data, which is not always achievable for all antimicrobial compounds, should generally be pursued as early as possible in the programme, otherwise, continued lead design might be based on irrelevant or misleading data points (for example, see some case studies with LpxC inhibitors 292 , 293 ). Furthermore, the availability of PD models 294 , 295 of high translational relevance, i.e. reliably predicting a minimal efficacious dose in humans, is a critical factor of success in order to generate the optimal drug candidate during lead and lead-to-candidate optimization. In the field of antibiotics, in particular, preclinical PK/PD relationships are generally predictive and have a high relevance for regulatory dossiers 296 , 297 , for example, for human PK/PD target attainment at therapeutic doses and drug formulation development, and, as such, they have to be evaluated carefully at the earliest possible stages 298 , 299 , 300 . Typically, PK/PD target attainments for antibiotics require relatively high doses compared with other drug classes (particularly to achieve sufficient exposure at the site of infection), limiting the successful application of existing formulation and delivery technologies. This constraint is especially true for oral medications that may present further challenges, for example, to reach an adequate bioavailability of the drug. Hence, a broader array of potential delivery systems should be tested systematically, which may include conventional permeation enhancers 301 , as well as sophisticated nanoformulations, for example, liposome-based drug delivery systems 302 , 303 , 304 , 305 . The latter, however, can only be produced based on expert knowledge and infrastructure, which is, once again, not often available in academia, and, thus, specialized CROs or SMEs may be approached based on available funding.
A further obstacle is the need to perform (initially) rather extensive studies in laboratory animals to understand the PK/PD relationship of a novel compound, which, at subsequent stages, allows the number of animal experiments to be minimized according to the 3Rs principle 306 . However, these studies are generally associated with ethical concerns, high costs and administrative burden. Likewise, these matters are relevant for the in vivo evaluation of toxicology, toxicokinetics and safety pharmacology to cover safety aspects before entering clinical trials 307 , 308 . Here, exploratory or early-stage predictive assays using computational models, as well as in vivo systems with minimal ethical concerns, for example, in vertebrates like Danio rerio (zebrafish), insects like Galleria mellonella (the greater wax moth) or worms like Caenorhabditis elegans (a soil-dwelling nematode), are an opportunity to estimate both efficacy and potential toxicity risks before considering standard in vivo experiments in rodents and other mammals 309 , 310 , 311 .
Ultimately, the demonstration of efficacy in a relevant animal model, associated with convincing exposure at the site of infection and a rough estimation of a reasonable safety margin, is often a prerequisite to attract an investor’s interest; typical minimum requirements are a tolerance/dose range finding study in one or two animal species, as well as human dose prediction based on a solid set of PK/PD data, for example, by testing efficacy in the neutropenic thigh infection model in mice 312 .
Generally, TPPs and the corresponding TCPs should continue to be the base for all further optimization attempts, especially when including in vivo studies, and, hence, should be thoroughly compiled before the development programme starts, with the help of subject matter experts. These data will guide the strategies and decisions for all chemical and biological development processes during the optimization phases, mainly with respect to one (or more) clinical indication(s). In order to specify robust finishing lines, these documents should outline sets of minimum acceptable criteria for each phase, for example, for biochemical assays during early stages and (pre)clinical endpoints at later stages. Such compound progression criteria should be defined for a validated hit, entry into lead optimization, a late lead and a preclinical candidate. There are different TPPs for different bacterial infections. As projects evolve, they may encounter serendipitous discoveries, unsurmountable hurdles or important findings from other groups or competitors, which may affect the TPP that they target. Therefore, the TPP can be critically reviewed and possibly refined or adapted throughout the project, for example, at each transition into the next development stage. Ideally, a pool of commonly accepted TPPs (i.e. approved by the pharmaceutical industry as well as the public health sector) should be available for the multitude of clinical indications to serve as a base for each discovery and development programme of novel therapeutics. The WHO and the GARDP have already started to produce such TPPs for public health concerns, for example, in the field of sexually transmitted infections 313 . These TPPs need to be regularly reviewed, and, where necessary, updated, to make sure that they reflect the current clinical situation; for example, TPPs addressing indications caused by bacterial infections may be affected by the latest emerging (or anticipated) drug-resistant pathogens of critical relevance. It is important to note that only convincing TPPs together with comprehensive preclinical candidate dossiers (highly informative TCPs) and reliable SOPs for scalable compound supply will allow early partnering and a smooth transfer of the project to an industrial stakeholder to move into (pre)clinical development (Box 6 ).
Box 6 Late preclinical development of corallopyronin A to first-in-human trial
The bacterial DNA-dependent RNA polymerase inhibitor corallopyronin A (CorA), produced by the soil-dwelling myxobacterium Corallococcus coralloides , is active against Gram-negative and Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA) 341 , 342 , by targeting the hinge (switch) region of the holoenzyme 343 . Structure–activity relationships demonstrated that the initial natural product hit was the most effective compound 344 , allowing for its development without extensive medicinal chemistry. The essential intracellular Wolbachia symbionts (Gram-negative) of human filarial nematodes, which cause the neglected tropical diseases lymphatic filariasis and onchocerciasis (river blindness), are also targets of CorA 345 . Currently, the compound is being developed to clinical phase I to support elimination of these nematode infections 346 , 347 , 348 , an aim of the United Nations’ Sustainable Development Goal 3 (UN-SDG) 349 . CorA also has activity against the human pathogenic bacteria Chlamydia trachomatis , Neisseria gonorrhoeae , Rickettsia spp. and Orientia tsutsugamushi 350 , 351 , 352 , which are included in the UN-SDG or World Health Organization/Centers for Disease Control and Prevention (WHO/CDC) lists for priority antibiotic discovery and development. Heterologous expression of the CorA biosynthetic cluster in Myxococcus xanthus , yielding >100 mg l −1 (refs 277 , 278 , 353 ), allow consistent multi-gram-scale production with a purity of 90–95%. This preliminary product specification has been formally accepted by the German Federal Institute for Drugs and Medical Devices (BfArM). Most standard in vitro and in vivo non-GLP (good laboratory practice) absorption, distribution, metabolism, excretion and toxicity studies have been successfully completed. Compared with rifampicin, the expression of CYP450s is not altered and CYP3A4 induction is eightfold lower. CorA is stable in plasma >240 min. Its metabolism in human and dog microsomes is T 1/2 > 45 min, resulting in oxidation metabolites and minimal glucuronidation. Off-target profiling resulted in three hits (inhibition/activation), but the half maximal effective concentration (EC 50 ) are 170–1,500-fold higher than the in vitro EC 50 against Wolbachia . CorA does not inhibit hERG, and no genotoxicity was observed. These results indicate that CorA is non-toxic and pharmacologically safe (awaiting further in vivo validation). The project is publicly funded to finalize preclinical studies, including formulation development and in vivo toxicity in rodents and dogs (see Acknowledgements). In parallel, the manufacturing protocol for heterologous production and optimized, upscalable downstream processing (DSP) has been recently transferred to a good manufacturing practice (GMP)-certified contract manufacturing organization (CMO) to produce cGMP-grade material for the GLP and phase I studies. Completing the clinical studies will require a product development partnership. Provision of CorA to countries endemic for filarial infections is envisaged via public–private partnerships to achieve the United Nations’ Sustainable Development Goal 3. After regulatory approval, provision of CorA for treating sexually transmitted infections and as a reserve antibiotic for MRSA shall be achieved through licensing.
ADME, absorption, distribution, metabolism and excretion; m€, million euros; PK/PD, pharmacokinetics/pharmacodynamics.
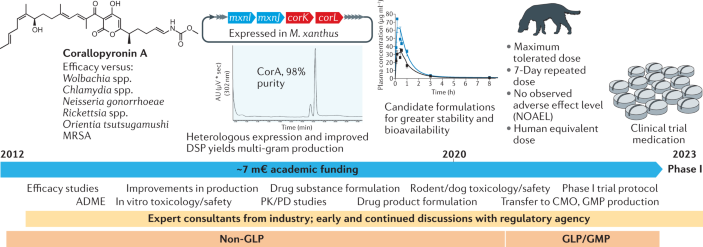
The management challenge in hit and lead optimization programmes
As the development of antibacterials requires a multidisciplinary approach, knowledge of a diverse set of techniques and domains (for example, assay development, high-throughput screening, medicinal and computational chemistry, ADMET, PK/PD, drug delivery, clinical background of disease processes etc.) is required in order to develop a compound to the level of a preclinical candidate. Single principal investigators (PIs) will usually not possess the broad base of expertise that is necessary, since academia largely focuses on early-stage discovery and compound optimization at the laboratory scale. Hence, research groups that do not possess the extensive skill set for drug development in its various stages should pursue a team approach by collaborating with organizations that have the relevant experience, be it within the academic or the industrial sector. There is also the possibility of calling on specialized consultancy or outsourcing packages of work (for example, ADMET) to CROs that possess relevant expertise and experimental capabilities. However, in addition to the relatively high costs of such services, PIs often struggle with remaining questions once a CRO assignment ends, and sufficient resources for tailor-made optimizations are often lacking. Moreover, the need to interpret results and devise a clear path forward towards the TPP from multiple data packages remains with the project teams. Hence, partnerships and collaborations are essential if relevant in-house expertise or infrastructure is missing. Therefore, we propose the following solutions for efficient translational project management:
Aligning and collaborating with suitable partners from various sectors or disciplines is crucial for groups with limited know-how in drug discovery and development. Generally, larger project teams can provide or identify expertise much more quickly to sufficiently resolve emerging knowledge gaps. Additionally, project consultants or CROs can be approached at different levels to fulfil remaining tasks, for example, data evaluation or processing defined and highly specific work packages, for example in PK and toxicological studies.
Databases of experts should be available for relevant research areas or services, and the various technical and IP-related aspects need to be elaborated on a case-by-case basis. Unbiased partners have to be identified to host and curate such databases on a regular basis, which could fall into the remit of non-profit health organizations such as the Joint Programming Initiative on Antimicrobial Resistance (JPIAMR) or the GARDP.
Training of PIs on a frequent basis is required to broaden their knowledge and to ensure a high-level understanding of potential barriers and pitfalls at least until projects reach the (pre)clinical stages. A number of renowned institutions already offer regular workshops and seminars (often as interactive webinars, for example, GARDP REVIVE; https://revive.gardp.org/ ), as well as extended training programmes (for example, the Interdisciplinary Course on Antibiotics and Resistance; https://www.icarecourse.org/ , hosted by Institut Pasteur, France), and these are increasingly popular. In addition to PIs from academia, non-academic experts from industry, health and political sectors should share their perspectives on current research and funding aspects more regularly within interdisciplinary settings.
Overall, it is important that the necessary financial and legal frameworks for efficiency-oriented project management are established as early as possible during the course of a development programme to avoid loss of time and resources. The required settings can be implemented either individually at a particular project level or existing management models (for example, available in the industrial sector or in translational research centres) could be used or adapted for the specific purpose.
Conclusions and outlook
To ensure a healthy and vibrant antimicrobial pipeline, considerable efforts are needed not only to develop the next generation of antibacterial drugs but also to safeguard and foster profound expertise in antibiotic drug discovery and development. In the short and medium term, such capacity-building must be performed as a collaborative and iterative process between academia and industry to ensure that the necessary skills are available to translate validated hits into potential drug products. The development of joint initiatives for education in translational sciences will require specific funding, as they are not part of most universities’ standard curricula. Many experienced scientists in the pharmaceutical industry are eager to share their translational and regulatory knowledge, often after retirement or due to change of operations. Thus, pharmaceutical companies could serve as a valuable training ground for acquiring and developing specific skills in the antimicrobial sector. However, limited funding (especially for SMEs) and economic uncertainties negatively affect this premise because it leads to business closures, high employee turnover rates, prevents the recruitment and training of inexperienced staff and deters scientists from embarking on a career in SMEs. In order to achieve transfer of vital expertise, workshops, symposiums and exchanges that foster academic–industrial interactions between students and advanced researchers are required and need financial support. Unfortunately, there are very few market-driven initiatives for such events and, therefore, a connection to already existing education and training programmes, for example, those supported by IMI, European Society of Clinical Microbiology and Infectious Diseases (ESCMID) or British Society for Antimicrobial Chemotherapy (BSAC), can be a valuable option as long as the transition into an era of mutually sustained knowledge transfer between industry and academia continues.
Another critical aspect for all future antibiotic R&D projects is the implementation of a legal framework for IP ownership at project commencement. The multidisciplinary and collaborative nature of antibiotic drug discovery often results in collaborations between different institutions on a national or international level. This creates challenging ownership structures with increasing complexity of such consortia, especially when an antibacterial programme is out-licensed, for example, to an SME. Negotiating ownership agreements among inventor institutions can be lengthy and may discourage industry from in-licensing valuable assets for further development. The increased collaboration between academia and industry requires fair and justifiable guidelines for knowledge and compound transfer outlined in appropriate agreements. The creation of such guidelines should be supported, for example, in the form of templates to settle ownership agreements between project partners or third parties, to facilitate processes for the benefit of researchers with limited experience in these matters. Such a framework will accelerate potential technology and compound transfer towards industrial drug developers, will make the relative commitment for each participant clearer and, thus, their gains more attractive.
Finally, we believe that AMR research requires diligent lobbying at the national and international levels to create entry points for large funders. Many scientists working on antimicrobials in either academia or SMEs are outside the few existing networks that involve decision makers within commercial funding sources, such as venture capitalists, including the newly announced AMR Action Fund, philanthropic organizations, national or regional governments or international bodies. This situation has resulted in an environment in which the challenges of antimicrobial drug developers are either not heard or are even ignored, even as public awareness of AMR steadily increases. It is evident that a strong lobbying position will lead to changes, which has recently been shown by the BEAM Alliance and their interaction and negotiations with diverse political bodies in Europe, leading to increased recognition of the challenges for antibacterial drug developers by the European Commission and Europe’s national governments 314 , 315 , 316 .
For the above reasons, we recommend that an international group of experienced AMR lobbyists should be formed that, together, can campaign for funding of early antibacterial drug discovery research along the principles set out in this article. Such a group should include national, regional and global scientific and industry associations that have practice in interacting with relevant stakeholders connected with national parliaments, EC, G7, G20 and further decision-making entities 317 . The Global AMR R&D Hub ( https://globalamrhub.org/ ) could be a crystallization point to pioneer such developments, which can be supported by various consortia, including the authors of this article: The International Research Alliance for Antibiotic Discovery and Development (IRAADD; https://www.iraadd.eu/ ), which we have recently established with the support of the JPIAMR Virtual Research Institute (JPIAMR-VRI; https://www.jpiamr.eu/jpiamr-vri/ ), identifies itself as a part of the mission that is addressed by the current roadmap. The IRAADD aims to improve the situation of novel antibiotic discovery and development by bringing together experts for early drug research from the academic and industrial sectors, who can provide knowledge and advice for diverse projects in the field. A recent example of our activities is the support of the JPIAMR-VRI to create a new online resource (the JPIAMR-VRI Digital Platform ‘DISQOVER’; https://www.jpiamr.eu/activities/jpiamr-vri/digital-platform/ ), serving as a comprehensive and interlinked database for AMR-related research at multiple levels. Although the IRAADD currently has only a short-term funding perspective, it is one of our main goals to help define and implement interdisciplinary innovative antibiotic development programmes based on sustainable research funding, in order to refill the translational pipeline with new drug candidates in the foreseeable future. In this respect, and as a possible long-term vision, the creation of internationally operating antibiotic research hubs, which may emerge from already existing pre-stage platforms such as the IRAADD, can be a major step forward to engage as many members as possible from academia, industry and public health organizations in antimicrobial R&D collaborations, and to create a strong and path-breaking position that cannot be overlooked. Only a responsible connection of thought leaders and dedicated experts from all relevant sectors of society, joining together now and for the future, will allow suitable rapid responses to globally emerging pathogens. Therefore, taking corrective and preventive action now through concerted and innovative approaches in the field of novel antibiotic drug discovery and development is the essential path forward to be prepared for future pandemics caused by multi-to-pan drug-resistant (so-called superbug) bacteria, which is an aim that deserves our undivided attention.
Ventola, C. L. The antibiotic resistance crisis: part 1: causes and threats. Pharm. Ther. 40 , 277–283 (2015). A comprehensive summary of the emergence of antibiotic resistance and the associated clinical and economic burden .
Google Scholar
Byrne, M. K. et al. The drivers of antibiotic use and misuse: the development and investigation of a theory driven community measure. BMC Public. Health 19 , 1425 (2019).
Article PubMed PubMed Central Google Scholar
Nadimpalli, M. L. et al. Urban informal settlements as hotspots of antimicrobial resistance and the need to curb environmental transmission. Nat. Microbiol. 5 , 787–795 (2020).
Article CAS PubMed Google Scholar
World Health Organization (WHO). WHO report on surveillance of antibiotic consumption 2016–2018 early implementation (WHO, 2018) https://www.who.int/medicines/areas/rational_use/oms-amr-amc-report-2016-2018/en/
Schrader, S. M., Vaubourgeix, J. & Nathan, C. Biology of antimicrobial resistance and approaches to combat it. Sci. Trans. Med. 12 , eaaz6992 (2020).
Article CAS Google Scholar
Conlon, B. P. et al. Persister formation in Staphylococcus aureus is associated with ATP depletion. Nat. Microbiol. 1 , 16051 (2016).
Article CAS PubMed PubMed Central Google Scholar
Harms, A., Maisonneuve, E. & Gerdes, K. Mechanisms of bacterial persistence during stress and antibiotic exposure. Science 354 , aaf4268 (2016).
Article PubMed CAS Google Scholar
Blaskovich, M. A. T. Antibiotics special issue: challenges and opportunities in antibiotic discovery and development. ACS Infect. Dis. 6 , 1286–1288 (2020). An excellent special issue combining viewpoints, perspectives, reviews and original research to provide a snapshot of the current state of antibiotic discovery and development .
O’Neill, J. Tackling drug-resistant infections globally: final report and recommendations. The Review on Antimicrobial Resistance https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (2016). Ground-breaking report and action plan to tackle present and future threats imposed by drug-resistant infections on a global scale .
AMR Industry Alliance. 2020 progress report (AMR Industry Alliance, 2020) https://www.amrindustryalliance.org/wp-content/uploads/2020/01/AMR-2020-Progress-Report.pdf
Zhou, P. et al. Bacterial and fungal infections in COVID-19 patients: A matter of concern. Infect. Control Hospital Epidemiol. 41 , 1124–1125 (2020).
Chen, X. et al. The microbial coinfection in COVID-19. Appl. Microbiol. Biotechnol. 104 , 7777–7785 (2020).
Article PubMed CAS PubMed Central Google Scholar
Zhou, F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395 , 1054–1062 (2020).
Nori, P. et al. Emerging co-pathogens: New Delhi metallo-beta-lactamase producing Enterobacterales infections in New York City COVID-19 patients. Int. J. Antimicrob. Agents 56 , 106179 (2020).
Getahun, H., Smith, I., Trivedi, K., Paulin, S. & Balkhy, H. H. Tackling antimicrobial resistance in the COVID-19 pandemic. Bull. World Health Organ. 98 , 442–442A (2020).
Oldenburg, C. E. & Doan, T. Azithromycin for severe COVID-19. Lancet 396 , 936–937 (2020).
Yates, P. A. et al. Doxycycline treatment of high-risk COVID-19-positive patients with comorbid pulmonary disease. Ther. Adv. Respir. Dis. 14 , 1753466620951053 (2020).
Sodhi, M. & Etminan, M. Therapeutic potential for tetracyclines in the treatment of COVID-19. Pharmacotherapy 40 , 487–488 (2020).
Baron, S. A., Devaux, C., Colson, P., Raoult, D. & Rolain, J.-M. Teicoplanin: an alternative drug for the treatment of COVID-19? Int. J. Antimicrob. Agents 55 , 105944 (2020).
Contou, D. et al. Bacterial and viral co-infections in patients with severe SARS-CoV-2 pneumonia admitted to a French ICU. Ann. Intensive Care 10 , 119 (2020).
Article PubMed PubMed Central CAS Google Scholar
Sharifipour, E. et al. Evaluation of bacterial co-infections of the respiratory tract in COVID-19 patients admitted to ICU. BMC Infect. Dis. 20 , 646 (2020).
Langford, B. J. et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin. Microbiol. Infect. 26 , 1622–1629 (2020).
Rawson, T. M. et al. Bacterial and fungal co-infection in individuals with coronavirus: A rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 71 , 2459–2468 (2020).
CAS PubMed Google Scholar
Vaughn, V. M. et al. Empiric antibacterial therapy and community-onset bacterial co-infection in patients hospitalized with COVID-19: a multi-hospital cohort study. Clin. Infect. Dis. 72 , e533–e541 (2020).
Article PubMed Central CAS Google Scholar
Hsu, J. How covid-19 is accelerating the threat of antimicrobial resistance. BMJ 369 , m1983 (2020).
Article PubMed Google Scholar
Rawson, T. M., Ming, D., Ahmad, R., Moore, L. S. P. & Holmes, A. H. Antimicrobial use, drug-resistant infections and COVID-19. Nat. Rev. Microbiol. 18 , 409–410 (2020).
Bengoechea, J. A. & Bamford, C. G. SARS-CoV-2, bacterial co-infections, and AMR: the deadly trio in COVID-19? EMBO Mol. Med. 12 , e12560 (2020).
Huttner, B. D., Catho, G., Pano-Pardo, J. R., Pulcini, C. & Schouten, J. COVID-19: don’t neglect antimicrobial stewardship principles! Clin. Microbiol. Infect. 26 , 808–810 (2020).
Buehrle, D. J. et al. Antibiotic consumption and stewardship at a hospital outside of an early coronavirus disease 2019 epicenter. Antimicrob. Agents Chemother. 64 , e01011–e01020 (2020).
Nieuwlaat, R. et al. COVID-19 and antimicrobial resistance: parallel and interacting health emergencies. Clin. Infect. Dis. 72 , 1657–1659 (2020).
Bassetti, M. & Giacobbe, D. R. A look at the clinical, economic, and societal impact of antimicrobial resistance in 2020. Expert Opin. Pharmacother. 21 , 2067–2071 (2020).
Magiorakos, A.-P. et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 18 , 268–281 (2012). This article presents the standardized international terminology to describe acquired resistance profiles in bacterial priority pathogens .
Xin Yu, J., Hubbard-Lucey, V. M. & Tang, J. Immuno-oncology drug development goes global. Nat. Rev. Drug Discov. 18 , 899–900 (2019).
The Pew Charitable Trusts . Tracking the global pipeline of antibiotics in development, April 2020 (The Pew Charitable Trusts, 2020) https://www.pewtrusts.org/en/research-and-analysis/issue-briefs/2020/04/tracking-the-global-pipeline-of-antibiotics-in-development
Beyer, P. & Paulin, S. The antibacterial research and development pipeline needs urgent solutions. ACS Infect. Dis. 6 , 1289–1291 (2020).
World Health Organization (WHO) . 2019 Antibacterial agents in clinical development: an analysis of the antibacterial clinical development pipeline. (WHO, 2019) https://apps.who.int/iris/bitstream/handle/10665/330420/9789240000193-eng.pdf
The Pew Charitable Trusts. A scientific roadmap for antibiotic discovery. (The Pew Charitable Trusts, 2016) https://www.pewtrusts.org/en/research-and-analysis/reports/2016/05/a-scientific-roadmap-for-antibiotic-discovery
OECD Publishing . Stemming the superbug tide: Just a few dollars more. (OECD, 2018) https://www.oecd.org/health/stemming-the-superbug-tide-9789264307599-en.htm
Årdal, C. et al. DRIVE-AB report: revitalizing the antibiotic pipeline: Stimulating innovation while driving sustainable use and global access. (DRIVE AB, 2018) http://drive-ab.eu/wp-content/uploads/2018/01/CHHJ5467-Drive-AB-Main-Report-180319-WEB.pdf
Kim, W., Prosen, K. R., Lepore, C. J. & Coukell, A. On the road to discovering urgently needed antibiotics: so close yet so far away. ACS Infect. Dis. 6 , 1292–1294 (2020).
Theuretzbacher, U., Outterson, K., Engel, A. & Karlén, A. The global preclinical antibacterial pipeline. Nat. Rev. Microbiol. 18 , 275–285 (2020). This review summarizes the most recent antibacterial discovery and preclinical development projects in academia and industry on a global scale .
O’Neill, J. Securing new drugs for future generations: the pipeline of antibiotics. (The Review on Antimicrobial Resistance, 2015) https://amr-review.org/sites/default/files/SECURING%20NEW%20DRUGS%20FOR%20FUTURE%20GENERATIONS%20FINAL%20WEB_0.pdf
Hutchings, M. I., Truman, A. W. & Wilkinson, B. Antibiotics: past, present and future. Curr. Opin. Microbiol. 51 , 72–80 (2019).
Lewis, K. The science of antibiotic discovery. Cell 181 , 29–45 (2020). Outstanding overview of past achievements as well as current perspectives and challenges in the field of antibiotic discovery .
Coates, A. R. M., Halls, G. & Hu, Y. Novel classes of antibiotics or more of the same? Br. J. Pharmacol. 163 , 184–194 (2011).
Hughes, D. & Karlén, A. Discovery and preclinical development of new antibiotics. Upsala J. Med. Sci. 119 , 162–169 (2014).
Jackson, N., Czaplewski, L. & Piddock, L. J. V. Discovery and development of new antibacterial drugs: learning from experience? J. Antimicrob. Chemother. 73 , 1452–1459 (2018).
Payne, D. J., Gwynn, M. N., Holmes, D. J. & Pompliano, D. L. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 6 , 29–40 (2007).
BEAM Alliance . Supporting financial investments on R&D to de-risk antimicrobial development. (BEAM Alliance, 2019) https://beam-alliance.eu/wp-content/uploads/2019/10/the-beam-push-memo.pdf
Shlaes, D. M. Antibacterial drugs: the last frontier. ACS Infect. Dis. 6 , 1313–1314 (2020).
Rex, J. H. & Outterson, K. Antibiotic reimbursement in a model delinked from sales: a benchmark-based worldwide approach. Lancet Infect. Dis. 16 , 500–505 (2016). This article describes how delinkage models should be built to ensure the development of antibiotics with the greatest possible innovative potential .
Outterson, K. A shot in the arm for new antibiotics. Nat. Biotechnol. 37 , 1110–1112 (2019).
Rex, J. H., Fernandez Lynch, H., Cohen, I. G., Darrow, J. J. & Outterson, K. Designing development programs for non-traditional antibacterial agents. Nat. Commun. 10 , 3416 (2019). This article discusses strategies focusing on how non-traditional antibacterial products can best be developed .
Årdal, C. et al. Antibiotic development - economic, regulatory and societal challenges. Nat. Rev. Microbiol. 18 , 267–274 (2020).
International Federation of Pharmaceutical Manufacturers & Associations (IFPMA) New AMR Action Fund steps in to save collapsing antibiotic pipeline with pharmaceutical industry investment of US$1 billion. (IFPMA, 2020) https://www.ifpma.org/resource-centre/new-amr-action-fund-steps-in-to-save-collapsing-antibiotic-pipeline/
The Public Health Agency of Sweden . Availability of antibiotics: Reporting of Government Commission. (The Public Health Agency of Sweden, 2017) https://www.folkhalsomyndigheten.se/contentassets/ab79066a80c7477ca5611ccfb211828e/availability-antibiotics-01229-2017-1.pdf
Mullard, A. UK outlines its antibiotic pull incentive plan. Nat. Rev. Drug Discov. 19 , 298–299 (2020).
PubMed Google Scholar
Mahase, E. UK launches subscription style model for antibiotics to encourage new development. BMJ 369 , m2468 (2020).
Bennet, S. M. & Young, S. T. The Pioneering Antimicrobial Subscriptions to End Up Surging Resistance Act of 2020. (The PASTEUR Act. Michael Bennet, U.S. Senator for Colorado); https://www.bennet.senate.gov/public/_cache/files/b/5/b5032465-bf79-4dca-beba-50fb9b0f4aee/C1FD8B8C4143F1332DC6A5E5EC09C83B.bon20468.pdf (2020).
Davis, D. K. Developing an Innovative Strategy for Antimicrobial Resistant Microorganisms Act of 2019. DISARM Act of 2019. (Congress.gov, 2019) https://www.congress.gov/116/bills/hr4100/BILLS-116hr4100ih.pdf (2019).
Balasegaram, M. & Piddock, L. J. V. The Global Antibiotic Research and Development Partnership (GARDP) not-for-profit model of antibiotic development. ACS Infect. Dis. 6 , 1295–1298 (2020).
Alm, R. A. & Gallant, K. Innovation in antimicrobial resistance: the CARB-X perspective. ACS Infect. Dis. 6 , 1317–1322 (2020).
Innovative Medicines Initiative (IMI) AMR Accelerator Programme . Collaboration for prevention and treatment of multi-drug resistant bacterial infections (COMBINE, 2019). https://amr-accelerator.eu/project/combine/
BEAM Alliance . BEAM Alliance and global partners call for urgent action on new reimbursement models for life-saving antibiotics. (BEAM Alliance, 2020) https://beam-alliance.eu/wp-content/uploads/2020/09/post-amr-conference-paper.pdf
Engel, A. Fostering antibiotic development through impact funding. ACS Infect. Dis. 6 , 1311–1312 (2020).
Zuegg, J., Hansford, K. A., Elliott, A. G., Cooper, M. A. & Blaskovich, M. A. T. How to stimulate and facilitate early stage antibiotic discovery. ACS Infect. Dis. 6 , 1302–1304 (2020).
van Hengel, A. J. & Marin, L. Research, innovation, and policy: an alliance combating antimicrobial resistance. Trends Microbiol. 27 , 287–289 (2019).
Simpkin, V. L., Renwick, M. J., Kelly, R. & Mossialos, E. Incentivising innovation in antibiotic drug discovery and development: progress, challenges and next steps. J. Antibiot. 70 , 1087–1096 (2017).
BEAM Alliance . Incentivising a sustainable response to the threat of AMR. (BEAM Alliance, 2019) https://beam-alliance.eu/wp-content/uploads/2019/11/the-beam-pull-memo.pdf
European Commission . A European One Health Action Plan against Antimicrobial Resistance. (AMR). (European Commission, 2017) https://ec.europa.eu/health/sites/default/files/antimicrobial_resistance/docs/amr_2017_action-plan.pdf
World Health Organization (WHO). Global action plan on antimicrobial resistance. (WHO, 2015) https://www.who.int/antimicrobial-resistance/publications/global-action-plan/en/
Hughes, J. P., Rees, S., Kalindjian, S. B. & Philpott, K. L. Principles of early drug discovery. Br. J. Pharmacol. 162 , 1239–1249 (2011).
Reddy, A. S. & Zhang, S. Polypharmacology: drug discovery for the future. Expert Rev. Clin. Pharmacol. 6 , 41–47 (2013). This review outlines the latest progress and challenges in polypharmacology studies .
Tyers, M. & Wright, G. D. Drug combinations: a strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 17 , 141–155 (2019).
O’Shea, R. & Moser, H. E. Physicochemical properties of antibacterial compounds: implications for drug discovery. J. Med. Chem. 51 , 2871–2878 (2008).
Reck, F., Jansen, J. M. & Moser, H. E. Challenges of antibacterial drug discovery. Arkivoc 2019 , 227–244 (2020). This study highlights challenges in the discovery of antibiotics, with a focus on physicochemical parameters and preferred property space .
AMR Industry Alliance . Declaration by the pharmaceutical, biotechnology and diagnostics industries on combating antimicrobial resistance. (AMR Industry Alliance, 2016) https://www.amrindustryalliance.org/wp-content/uploads/2017/12/AMR-Industry-Declaration.pdf
Karawajczyk, A., Orrling, K. M., Vlieger, J. S. B., de, Rijnders, T. & Tzalis, D. The European lead factory: a blueprint for public-private partnerships in early drug discovery. Front. Med. 3 , 75 (2016).
Morgentin, R. et al. Translation of innovative chemistry into screening libraries: an exemplar partnership from the European Lead Factory. Drug Discov. Today 23 , 1578–1583 (2018).
Besnard, J., Jones, P. S., Hopkins, A. L. & Pannifer, A. D. The Joint European Compound Library: boosting precompetitive research. Drug Discov. Today 20 , 181–186 (2015).
Theuretzbacher, U. & Piddock, L. J. V. Non-traditional antibacterial therapeutic options and challenges. Cell Host Microbe 26 , 61–72 (2019). Comprehensive overview of non-traditional approaches in antibacterial therapy .
Fleitas Martínez, O., Cardoso, M. H., Ribeiro, S. M. & Franco, O. L. Recent advances in anti-virulence therapeutic strategies with a focus on dismantling bacterial membrane microdomains, toxin neutralization, quorum-sensing interference and biofilm inhibition. Front. Cell. Infect. Microbiol. 9 , 74 (2019).
Kumar, A., Ellermann, M. & Sperandio, V. Taming the beast: Interplay between gut small molecules and enteric pathogens. Infect. Immun. 87 , e00131-19 (2019).
Laws, M., Shaaban, A. & Rahman, K. M. Antibiotic resistance breakers: current approaches and future directions. FEMS Microbiol. Rev. 43 , 490–516 (2019).
Annunziato, G. Strategies to overcome antimicrobial resistance (AMR) making use of non-essential target inhibitors: a review. Int. J. Mol. Sci. 20 , 5844 (2019).
Article CAS PubMed Central Google Scholar
Calvert, M. B., Jumde, V. R. & Titz, A. Pathoblockers or antivirulence drugs as a new option for the treatment of bacterial infections. Beilstein J. Org. Chem. 14 , 2607–2617 (2018).
Schütz, C. & Empting, M. Targeting the Pseudomonas quinolone signal quorum sensing system for the discovery of novel anti-infective pathoblockers. Beilstein J. Org. Chem. 14 , 2627–2645 (2018).
Sommer, R. et al. Glycomimetic, orally bioavailable LecB inhibitors block biofilm formation of pseudomonas aeruginosa. J. Am. Chem. Soc. 140 , 2537–2545 (2018). Example of synthetic pathoblockers acting against biofilm formation of Pseudomonas aeruginosa .
Boudaher, E. & Shaffer, C. L. Inhibiting bacterial secretion systems in the fight against antibiotic resistance. MedChemComm 10 , 682–692 (2019).
Duncan, M. C., Linington, R. G. & Auerbuch, V. Chemical inhibitors of the type three secretion system: disarming bacterial pathogens. Antimicrob. Agents Chemother. 56 , 5433–5441 (2012).
Schönauer, E. et al. Discovery of a potent inhibitor class with high selectivity toward clostridial collagenases. J. Am. Chem. Soc. 139 , 12696–12703 (2017).
Paolino, M. et al. Development of potent inhibitors of the Mycobacterium tuberculosis virulence factor Zmp1 and evaluation of their effect on mycobacterial survival inside macrophages. ChemMedChem 13 , 422–430 (2018).
Marshall, R. L. et al. New multidrug efflux inhibitors for Gram-negative bacteria. mBio 11 , e01340-20 (2020).
Bush, K. & Bradford, P. A. Interplay between β-lactamases and new β-lactamase inhibitors. Nat. Rev. Microbiol. 17 , 295–306 (2019).
Tooke, C. L. et al. β-Lactamases and β-lactamase inhibitors in the 21st century. J. Mol. Biol. 431 , 3472–3500 (2019).
Smirnova, G. V. & Oktyabrsky, O. N. Glutathione in bacteria. Biochemistry 70 , 1199–1211 (2005).
Chen, N. H. et al. A glutathione-dependent detoxification system is required for formaldehyde resistance and optimal survival of Neisseria meningitidis in biofilms. Antioxid. Redox Signal. 18 , 743–755 (2013).
Lu, P. et al. The anti-mycobacterial activity of the cytochrome bcc inhibitor Q203 can be enhanced by small-molecule inhibition of cytochrome bd. Sci. Rep. 8 , 2625 (2018).
Iqbal, I. K., Bajeli, S., Akela, A. K. & Kumar, A. Bioenergetics of Mycobacterium : an emerging landscape for drug discovery. Pathogens 7 , 24 (2018).
Fol, M., Włodarczyk, M. & Druszczyn´ska, M. Host epigenetics in intracellular pathogen infections. Int. J. Mol. Sci. 21 , 4573 (2020).
Ghosh, D., Veeraraghavan, B., Elangovan, R. & Vivekanandan, P. Antibiotic resistance and epigenetics: more to it than meets the eye. Antimicrob. Agents Chemother. 64 , e02225-19 (2020).
Venter, H. Reversing resistance to counter antimicrobial resistance in the World Health Organisation’s critical priority of most dangerous pathogens. Biosci. Rep. 39 , BSR20180474 (2019).
Rezzoagli, C., Archetti, M., Mignot, I., Baumgartner, M. & Kümmerli, R. Combining antibiotics with antivirulence compounds can have synergistic effects and reverse selection for antibiotic resistance in Pseudomonas aeruginosa . PLoS Biol. 18 , e3000805 (2020).
Strich, J. R. et al. Needs assessment for novel Gram-negative antibiotics in US hospitals: a retrospective cohort study. Lancet Infect. Dis. 20 , 1172–1181 (2020).
Lakemeyer, M., Zhao, W., Mandl, F. A., Hammann, P. & Sieber, S. A. Thinking outside the box — novel antibacterials to tackle the resistance crisis. Angew. Chem. Int. Ed. 57 , 14440–14475 (2018). Extensive and interdisciplinary overview of methods for mining novel antibiotics and strategies to unravel their modes of action .
Hennessen, F. et al. Amidochelocardin overcomes resistance mechanisms exerted on tetracyclines and natural chelocardin. Antibiotics 9 , 619 (2020).
Sarigul, N., Korkmaz, F. & Kurultak, I∙. A new artificial urine protocol to better imitate human urine. Sci. Rep. 9 , 20159 (2019).
Ganz, T. & Nemeth, E. Iron homeostasis in host defence and inflammation. Nat. Rev. Immunol. 15 , 500–510 (2015).
Martins, A. C., Almeida, J. I., Lima, I. S., Kapitão, A. S. & Gozzelino, R. Iron metabolism and the inflammatory response. IUBMB Life 69 , 442–450 (2017).
Krismer, B., Weidenmaier, C., Zipperer, A. & Peschel, A. The commensal lifestyle of Staphylococcus aureus and its interactions with the nasal microbiota. Nat. Rev. Microbiol. 15 , 675–687 (2017).
Ayaz, M. et al. Synergistic interactions of phytochemicals with antimicrobial agents: Potential strategy to counteract drug resistance. Chem. Biol. Interact. 308 , 294–303 (2019).
Mahomoodally, M. F. & Sadeer, N. B. Antibiotic potentiation of natural products: A promising target to fight pathogenic bacteria. Curr. Drug Targets 22 , 555–572 (2020).
Parkinson, E. I. et al. Deoxynybomycins inhibit mutant DNA gyrase and rescue mice infected with fluoroquinolone-resistant bacteria. Nat. Commun. 6 , 6947 (2015).
King, A. M. et al. Aspergillomarasmine A overcomes metallo-β-lactamase antibiotic resistance. Nature 510 , 503–506 (2014).
Park, S. W. et al. Target-based identification of whole-cell active inhibitors of biotin biosynthesis in Mycobacterium tuberculosis . Chem. Biol. 22 , 76–86 (2015).
Verma, S. & Prabhakar, Y. S. Target based drug design - a reality in virtual sphere. Curr. Med. Chem. 22 , 1603–1630 (2015).
Graef, F. et al. In vitro model of the Gram-negative bacterial cell envelope for investigation of anti-infective permeation kinetics. ACS Infect. Dis. 4 , 1188–1196 (2018).
Richter, R. et al. A hydrogel-based in vitro assay for the fast prediction of antibiotic accumulation in Gram-negative bacteria. Mater. Today Bio 8 , 100084 (2020).
Martin, E. J. et al. All-Assay-Max2 pQSAR: activity predictions as accurate as four-concentration IC 50 s for 8558 Novartis assays. J. Chem. Inf. Model. 59 , 4450–4459 (2019).
Durrant, J. D. & Amaro, R. E. Machine-learning techniques applied to antibacterial drug discovery. Chem. Biol. Drug Des. 85 , 14–21 (2015).
Newman, D. J. & Cragg, G. M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 83 , 770–803 (2020). Fundamental review addressing the role of natural products in drug discovery during the past 40 years .
Locey, K. J. & Lennon, J. T. Scaling laws predict global microbial diversity. Proc. Natl Acad. Sci. USA 113 , 5970–5975 (2016).
Thompson, L. R. et al. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 551 , 457–463 (2017).
Mousa, W. K., Athar, B., Merwin, N. J. & Magarvey, N. A. Antibiotics and specialized metabolites from the human microbiota. Nat. Prod. Rep. 34 , 1302–1331 (2017).
Zipperer, A. et al. Human commensals producing a novel antibiotic impair pathogen colonization. Nature 535 , 511–516 (2016). Research article describing the discovery of the novel antibiotic lugdunin produced by commensals of the human nasal microbiome .
Imai, Y. et al. A new antibiotic selectively kills Gram-negative pathogens. Nature 576 , 459–464 (2019). This study describes the discovery of the new antibiotic darobactin that is active against Gram-negative pathogens .
Adnani, N., Rajski, S. R. & Bugni, T. S. Symbiosis-inspired approaches to antibiotic discovery. Nat. Prod. Rep. 34 , 784–814 (2017).
Lodhi, A. F., Zhang, Y., Adil, M. & Deng, Y. Antibiotic discovery: combining isolation chip (iChip) technology and co-culture technique. Appl. Microbiol. Biotechnol. 102 , 7333–7341 (2018).
Kealey, C., Creaven, C. A., Murphy, C. D. & Brady, C. B. New approaches to antibiotic discovery. Biotechnol. Lett. 39 , 805–817 (2017).
Ling, L. L. et al. A new antibiotic kills pathogens without detectable resistance. Nature 517 , 455–459 (2015). An intriguing example of discovering a new antibiotic (teixobactin) from uncultured bacteria by using innovative cultivation techniques (iChip) .
Ma, L. et al. Gene-targeted microfluidic cultivation validated by isolation of a gut bacterium listed in Human Microbiome Project’s Most Wanted taxa. Proc. Natl Acad. Sci. USA 111 , 9768–9773 (2014).
Mahler, L. et al. Highly parallelized microfluidic droplet cultivation and prioritization on antibiotic producers from complex natural microbial communities. eLife 10 , e64774 (2021).
Jiang, C.-Y. et al. High-throughput single-cell cultivation on microfluidic streak plates. Appl. Environ. Microbiol. 82 , 2210–2218 (2016).
Seyedsayamdost, M. R. High-throughput platform for the discovery of elicitors of silent bacterial gene clusters. Proc. Natl Acad. Sci. USA 111 , 7266–7271 (2014).
Pishchany, G. et al. Amycomicin is a potent and specific antibiotic discovered with a targeted interaction screen. Proc. Natl Acad. Sci. USA 115 , 10124–10129 (2018).
Chaudhary, D. K., Khulan, A. & Kim, J. Development of a novel cultivation technique for uncultured soil bacteria. Sci. Rep. 9 , 6666 (2019).
Masschelein, J., Jenner, M. & Challis, G. L. Antibiotics from Gram-negative bacteria: a comprehensive overview and selected biosynthetic highlights. Nat. Prod. Rep. 34 , 712–783 (2017).
Hoffmann, T. et al. Correlating chemical diversity with taxonomic distance for discovery of natural products in myxobacteria. Nat. Commun. 9 , 803 (2018). This study underpins the ‘taxonomy paradigm’, suggesting to explore new genera of microorganisms to increase the chance of finding novel antibiotic chemotypes .
Hughes, C. C. & Fenical, W. Antibacterials from the sea. Chem. Eur. J. 16 , 12512–12525 (2010).
Abrudan, M. I. et al. Socially mediated induction and suppression of antibiosis during bacterial coexistence. Proc. Natl Acad. Sci. USA 112 , 11054–11059 (2015).
Granato, E. T., Meiller-Legrand, T. A. & Foster, K. R. The evolution and ecology of bacterial warfare. Curr. Biol. 29 , R521–R537 (2019).
Kraemer, S. A., Ramachandran, A. & Perron, G. G. Antibiotic pollution in the environment: from microbial ecology to public policy. Microorganisms 7 , 180 (2019).
Ju, F. et al. Wastewater treatment plant resistomes are shaped by bacterial composition, genetic exchange, and upregulated expression in the effluent microbiomes. ISME J. 13 , 346–360 (2019).
Sierra-Zapata, L. et al. Inducible antibacterial activity in the bacillales by triphenyl tetrazolium chloride. Sci. Rep. 10 , 5563 (2020).
Charusanti, P. et al. Exploiting adaptive laboratory evolution of Streptomyces clavuligerus for antibiotic discovery and overproduction. PLoS One 7 , e33727 (2012).
Cowan, M. M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 12 , 564–582 (1999).
Newman, D. J. & Cragg, G. M. Plant endophytes and epiphytes: Burgeoning sources of known and “unknown” cytotoxic and antibiotic agents? Planta Medica 86 , 891–905 (2020).
Hyde, K. D. et al. The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Divers. 97 , 1–136 (2019).
Article Google Scholar
Silva, O. N. et al. Repurposing a peptide toxin from wasp venom into antiinfectives with dual antimicrobial and immunomodulatory properties. Proc. Natl Acad. Sci. USA 117 , 26936–26945 (2020).
Medema, M. H. & Fischbach, M. A. Computational approaches to natural product discovery. Nat. Chem. Biol. 11 , 639–648 (2015).
Katz, M., Hover, B. M. & Brady, S. F. Culture-independent discovery of natural products from soil metagenomes. J. Ind. Microbiol. Biotechnol. 43 , 129–141 (2016).
Crits-Christoph, A., Diamond, S., Butterfield, C. N., Thomas, B. C. & Banfield, J. F. Novel soil bacteria possess diverse genes for secondary metabolite biosynthesis. Nature 558 , 440–444 (2018).
Milshteyn, A., Schneider, J. S. & Brady, S. F. Mining the metabiome: identifying novel natural products from microbial communities. Chem. Biol. 21 , 1211–1223 (2014).
Myronovskyi, M. et al. Generation of a cluster-free Streptomyces albus chassis strains for improved heterologous expression of secondary metabolite clusters. Metab. Eng. 49 , 316–324 (2018).
Gomez-Escribano, J. P. & Bibb, M. J. Engineering Streptomyces coelicolor for heterologous expression of secondary metabolite gene clusters. Microb. Biotechnol. 4 , 207–215 (2011).
Komatsu, M. et al. Engineered Streptomyces avermitilis host for heterologous expression of biosynthetic gene cluster for secondary metabolites. ACS Synth. Biol. 2 , 384–396 (2013).
Sherwood, E. J., Hesketh, A. R. & Bibb, M. J. Cloning and analysis of the planosporicin lantibiotic biosynthetic gene cluster of Planomonospora alba . J. Bacteriol. 195 , 2309–2321 (2013).
Ahmed, Y. et al. Engineering of Streptomyces lividans for heterologous expression of secondary metabolite gene clusters. Microb. Cell Fact. 19 , 5 (2020).
Wang, G. et al. CRAGE enables rapid activation of biosynthetic gene clusters in undomesticated bacteria. Nat. Microbiol. 4 , 2498–2510 (2019).
Tong, Y., Weber, T. & Lee, S. Y. CRISPR/Cas-based genome engineering in natural product discovery. Nat. Prod. Rep. 36 , 1262–1280 (2019). This review summarizes the current knowledge about the CRISPR/Cas system and how it became one of the most important tools for genome editing .
La Fuente-Núñez, C. de & Lu, T. K. CRISPR-Cas9 technology: applications in genome engineering, development of sequence-specific antimicrobials, and future prospects. Integr. Biol. 9 , 109–122 (2017).
Wang, H. et al. ExoCET: exonuclease in vitro assembly combined with RecET recombination for highly efficient direct DNA cloning from complex genomes. Nucleic Acids Res. 46 , e28 (2018).
Zeng, F. et al. AFEAP cloning: a precise and efficient method for large DNA sequence assembly. BMC Biotechnol. 17 , 81 (2017).
Snoeck, N. et al. Serine integrase recombinational engineering (SIRE): A versatile toolbox for genome editing. Biotechnol. Bioeng. 116 , 364–374 (2019).
Kautsar, S. A. et al. MIBiG 2.0: a repository for biosynthetic gene clusters of known function. Nucleic Acids Res. 48 , D454–D458 (2020). This article presents a major update of the ‘Minimum Information about a Biosynthetic Gene cluster’ (MIBiG) data repository .
Blin, K. et al. antiSMASH 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 47 , W81–W87 (2019). This article presents an entirely redesigned and extended version of the ‘antibiotics and secondary metabolite analysis shell’: antiSMASH .
Skinnider, M. A. et al. Comprehensive prediction of secondary metabolite structure and biological activity from microbial genome sequences. Nat. Commun. 11 , 6058 (2020).
Alanjary, M., Cano-Prieto, C., Gross, H. & Medema, M. H. Computer-aided re-engineering of nonribosomal peptide and polyketide biosynthetic assembly lines. Nat. Prod. Rep. 36 , 1249–1261 (2019).
Cummings, M. et al. Assembling a plug-and-play production line for combinatorial biosynthesis of aromatic polyketides in Escherichia coli . PLoS Biol. 17 , e3000347 (2019).
Hwang, S., Lee, N., Cho, S., Palsson, B. & Cho, B.-K. Repurposing modular polyketide synthases and non-ribosomal peptide synthetases for novel chemical biosynthesis. Front. Mol. Biosci. 7 , 87 (2020).
Bozhüyük, K. A. J. et al. Modification and de novo design of non-ribosomal peptide synthetases using specific assembly points within condensation domains. Nat. Chem. 11 , 653–661 (2019).
Luo, Y., Enghiad, B. & Zhao, H. New tools for reconstruction and heterologous expression of natural product biosynthetic gene clusters. Nat. Prod. Rep. 33 , 174–182 (2016).
Tan, G.-Y. & Liu, T. Rational synthetic pathway refactoring of natural products biosynthesis in actinobacteria. Metab. Eng. 39 , 228–236 (2017).
Zhang, J. J., Tang, X. & Moore, B. S. Genetic platforms for heterologous expression of microbial natural products. Nat. Prod. Rep. 36 , 1313–1332 (2019). This review highlights the present arsenal of genetic platforms to identify, evaluate and engineer biosynthetic gene clusters from diverse microbial sources .
Huo, L. et al. Heterologous expression of bacterial natural product biosynthetic pathways. Nat. Prod. Rep. 36 , 1412–1436 (2019).
Ito, T. & Masubuchi, M. Dereplication of microbial extracts and related analytical technologies. J. Antibiot. 67 , 353–360 (2014).
Quinn, R. A. et al. Molecular networking as a drug discovery, drug metabolism, and precision medicine strategy. Trends Pharmacol. Sci. 38 , 143–154 (2017).
Ernst, M. et al. MolNetEnhancer: Enhanced molecular networks by integrating metabolome mining and annotation tools. Metabolites 9 , 144 (2019).
Liebal, U. W., Phan, A. N. T., Sudhakar, M., Raman, K. & Blank, L. M. Machine learning applications for mass spectrometry-based metabolomics. Metabolites 10 , 243 (2020).
Hubert, J., Nuzillard, J.-M. & Renault, J.-H. Dereplication strategies in natural product research: How many tools and methodologies behind the same concept? Phytochem. Rev. 16 , 55–95 (2017).
Li, Y., Kuhn, M., Gavin, A.-C. & Bork, P. Identification of metabolites from tandem mass spectra with a machine learning approach utilizing structural features. Bioinformatics 36 , 1213–1218 (2020).
Zani, C. L. & Carroll, A. R. Database for rapid dereplication of known natural products using data from MS and Fast NMR experiments. J. Nat. Prod. 80 , 1758–1766 (2017).
van Santen, J. A. et al. The natural products atlas: an open access knowledge base for microbial natural products discovery. ACS Cent. Sci. 5 , 1824–1833 (2019).
Wang, M. et al. Sharing and community curation of mass spectrometry data with global natural products social molecular networking. Nat. Biotechnol. 34 , 828–837 (2016).
Guijas, C. et al. METLIN: a technology platform for identifying knowns and unknowns. Anal. Chem. 90 , 3156–3164 (2018).
Kuhn, S. & Schlörer, N. E. Facilitating quality control for spectra assignments of small organic molecules: nmrshiftdb2–a free in-house NMR database with integrated LIMS for academic service laboratories. Magn. Reson. Chem. 53 , 582–589 (2015).
López-Pérez, J. L., Therón, R., del Olmo, E. & Díaz, D. NAPROC-13: a database for the dereplication of natural product mixtures in bioassay-guided protocols. Bioinformatics 23 , 3256–3257 (2007).
Bader, C. D., Neuber, M., Panter, F., Krug, D. & Müller, R. Supercritical fluid extraction enhances discovery of secondary metabolites from myxobacteria. Anal. Chem. 92 , 15403–15411 (2020).
Nass, N. M. et al. Revisiting unexploited antibiotics in search of new antibacterial drug candidates: the case of γ-actinorhodin. Sci. Rep. 7 , 17419 (2017).
Ishikawa, M. et al. Lower ototoxicity and absence of hidden hearing loss point to gentamicin C1a and apramycin as promising antibiotics for clinical use. Sci. Rep. 9 , 2410 (2019).
Lešnik, U. et al. Construction of a new class of tetracycline lead structures with potent antibacterial activity through biosynthetic engineering. Angew. Chem. Int. Ed. 54 , 3937–3940 (2015).
Grandclaudon, C. et al. Semisynthesis and biological evaluation of amidochelocardin derivatives as broad-spectrum antibiotics. Eur. J. Med. Chem. 188 , 112005 (2020).
Omollo, C. et al. Developing synergistic drug combinations to restore antibiotic sensitivity in drug-resistant Mycobacterium tuberculosis . Antimicrob. Agents Chemother. 65 , e02554-20 (2021).
Article PubMed Central Google Scholar
Tan, J. H. et al. In vitro and in vivo efficacy of an LpxC inhibitor, CHIR-090, alone or combined with colistin against Pseudomonas aeruginosa biofilm. Antimicrob. Agents Chemother. 61 , e02223-16 (2017).
Mariathasan, S. & Tan, M.-W. Antibody–antibiotic conjugates: a novel therapeutic platform against bacterial infections. Trends Mol. Med. 23 , 135–149 (2017).
Koenig, S. G. & Pillow, T. H. in Complete Accounts of Integrated Drug Discovery and Development: Recent Examples from the Pharmaceutical Industry Volume 2 Vol. 1332 (eds Pesti, J. A., Abdel-Magid, A. F. & Vaidyanathan, R.) 85–105 (American Chemical Society, 2019).
Negash, K. H., Norris, J. K. S. & Hodgkinson, J. T. Siderophore–antibiotic conjugate design: New drugs for bad bugs? Molecules 24 , 3314 (2019).
Schalk, I. J. Siderophore–antibiotic conjugates: exploiting iron uptake to deliver drugs into bacteria. Clin. Microbiol. Infect. 24 , 801–802 (2018).
Blaskovich, M. A. T. et al. Protein-inspired antibiotics active against vancomycin- and daptomycin-resistant bacteria. Nat. Commun. 9 , 22 (2018). This study shows how positively charged effector sequences can ‘revitalize’ antibiotics that have lost effectiveness against recalcitrant bacteria .
Umstätter, F. et al. Vancomycin resistance is overcome by conjugation of polycationic peptides. Angew. Chem. Int. Ed. 59 , 8823–8827 (2020).
Kim, A. et al. Pharmacodynamic profiling of a siderophore-conjugated monocarbam in Pseudomonas aeruginosa : assessing the risk for resistance and attenuated efficacy. Antimicrob. Agents Chemother. 59 , 7743–7752 (2015).
Stokes, J. M. et al. A deep learning approach to antibiotic discovery. Cell 180 , 688–702.e13 (2020). This work demonstrates how deep learning approaches can expand the antibiotic arsenal through the discovery of structurally distinct hit compounds .
de la Fuente-Nunez, C. Toward autonomous antibiotic discovery. mSystems 4 , e00151-19 (2019).
Lipinski, C. A., Lombardo, F., Dominy, B. W. & Feeney, P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 23 , 3–25 (1997). This study presents, for the first time, ‘the rule of five’, defining five key physiochemical parameters for orally active drugs .
Lipinski, C. A. Rule of five in 2015 and beyond: Target and ligand structural limitations, ligand chemistry structure and drug discovery project decisions. Adv. Drug Deliv. Rev. 101 , 34–41 (2016).
Benet, L. Z., Hosey, C. M., Ursu, O. & Oprea, T. I. BDDCS, the Rule of 5 and drugability. Adv. Drug Deliv. Rev. 101 , 89–98 (2016).
DeGoey, D. A., Chen, H.-J., Cox, P. B. & Wendt, M. D. Beyond the rule of 5: lessons learned from AbbVie’s drugs and compound collection. J. Med. Chem. 61 , 2636–2651 (2018).
Doak, B. C., Over, B., Giordanetto, F. & Kihlberg, J. Oral druggable space beyond the rule of 5: insights from drugs and clinical candidates. Chem. Biol. 21 , 1115–1142 (2014).
Tyagi, M., Begnini, F., Poongavanam, V., Doak, B. C. & Kihlberg, J. Drug syntheses beyond the rule of 5. Chem. Eur. J. 26 , 49–88 (2020).
Wright, P. M., Seiple, I. B. & Myers, A. G. The evolving role of chemical synthesis in antibacterial drug discovery. Angew. Chem. Int. Ed. 53 , 8840–8869 (2014).
Agarwal, V. et al. Enzymatic halogenation and dehalogenation reactions: pervasive and mechanistically diverse. Chem. Rev. 117 , 5619–5674 (2017).
Wilcken, R., Zimmermann, M. O., Lange, A., Joerger, A. C. & Boeckler, F. M. Principles and applications of halogen bonding in medicinal chemistry and chemical biology. J. Med. Chem. 56 , 1363–1388 (2013).
Convention on Biological Diversity United Nations . Nagoya Protocol on access to genetic resources and the fair and equitable sharing of benefits arising from their utilization to the Convention on Biological Diversity. (Convention on Biological Diversity, 2011) https://www.cbd.int/abs/doc/protocol/nagoya-protocol-en.pdf
Sorokina, M. & Steinbeck, C. Review on natural products databases: where to find data in 2020. J. Cheminform. 12 , 20 (2020).
European Commission . Manifesto for EU COVID-19 research: maximising the accessibility of research results in the fight against COVID-19. (European Commission, 2020) https://ec.europa.eu/info/research-and-innovation/research-area/health-research-and-innovation/coronavirus-research-and-innovation/covid-research-manifesto_en
Wibberg, D. et al. High quality genome sequences of thirteen Hypoxylaceae (Ascomycota) strengthen the phylogenetic family backbone and enable the discovery of new taxa. Fungal Divers. 106 , 7–28 (2021).
Kling, A. et al. Antibiotics. targeting DnaN for tuberculosis therapy using novel griselimycins. Science 348 , 1106–1112 (2015). This study presents a primary example of discovering a new mode of action by self-resistant target identification .
Johnston, C. W. et al. Assembly and clustering of natural antibiotics guides target identification. Nat. Chem. Biol. 12 , 233–239 (2016).
Panter, F., Krug, D., Baumann, S. & Müller, R. Self-resistance guided genome mining uncovers new topoisomerase inhibitors from myxobacteria. Chem. Sci. 9 , 4898–4908 (2018).
Mungan, M. D. et al. ARTS 2.0: feature updates and expansion of the Antibiotic Resistant Target Seeker for comparative genome mining. Nucleic Acids Res. 48 , W546–W552 (2020). This study presents the update and expansion of the Antibiotic Resistant Target Seeker (ARTS) .
Emmert-Streib, F., Yang, Z., Feng, H., Tripathi, S. & Dehmer, M. An introductory review of deep learning for prediction models with big data. Front. Artif. Intell. 3 , 4 (2020). Comprehensive overview of deep learning models and future developments in artificial intelligence .
Schneider, P. et al. Rethinking drug design in the artificial intelligence era. Nat. Rev. Drug. Discov. 19 , 353–364 (2020).
Grein, F. et al. Ca 2+ -Daptomycin targets cell wall biosynthesis by forming a tripartite complex with undecaprenyl-coupled intermediates and membrane lipids. Nat. Commun. 11 , 1455 (2020). Describes the detailed mode of action of daptomycin for the first time .
Cardona, S. T., Selin, C. & Gislason, A. S. Genomic tools to profile antibiotic mode of action. Crit. Rev. Microbiol. 41 , 465–472 (2015).
van Camp, P.-J., Haslam, D. B. & Porollo, A. Bioinformatics approaches to the understanding of molecular mechanisms in antimicrobial resistance. Int. J. Mol. Sci. 21 , 1363 (2020).
O’Rourke, A. et al. Mechanism-of-action classification of antibiotics by global transcriptome profiling. Antimicrob. Agents Chemother. 64 , e01207–e01219 (2020).
Nonejuie, P., Burkart, M., Pogliano, K. & Pogliano, J. Bacterial cytological profiling rapidly identifies the cellular pathways targeted by antibacterial molecules. Proc. Natl Acad. Sci. USA 110 , 16169–16174 (2013).
Lamsa, A. et al. Rapid inhibition profiling in Bacillus subtilis to identify the mechanism of action of new antimicrobials. ACS Chem. Biol. 11 , 2222–2231 (2016).
Ribeiro da Cunha, B., Fonseca, L. P. & Calado, C. R. C. Metabolic fingerprinting with fourier-transform infrared (FTIR) spectroscopy: towards a high-throughput screening assay for antibiotic discovery and mechanism-of-action elucidation. Metabolites 10 , 145 (2020).
Eustice, D. C., Feldman, P. A. & Slee, A. M. The mechanism of action of DuP 721, a new antibacterial agent: effects on macromolecular synthesis. Biochem. Biophys. Res. Commun. 150 , 965–971 (1988).
Sugimoto, A., Maeda, A., Itto, K. & Arimoto, H. Deciphering the mode of action of cell wall-inhibiting antibiotics using metabolic labeling of growing peptidoglycan in Streptococcus pyogenes . Sci. Rep. 7 , 1129 (2017).
Cacace, E., Kritikos, G. & Typas, A. Chemical genetics in drug discovery. Curr. Opin. Syst. Biol. 4 , 35–42 (2017).
Santiago, M. et al. Genome-wide mutant profiling predicts the mechanism of a Lipid II binding antibiotic. Nat. Chem. Biol. 14 , 601–608 (2018). This study demonstrates how machine learning approaches can be used to predict the mode of action of antibiotics via mutant fitness profiles .
Wright, M. H. & Sieber, S. A. Chemical proteomics approaches for identifying the cellular targets of natural products. Nat. Prod. Rep. 33 , 681–708 (2016).
Drewes, G. & Knapp, S. Chemoproteomics and chemical probes for target discovery. Trends Biotechnol. 36 , 1275–1286 (2018).
Hoerr, V. et al. Characterization and prediction of the mechanism of action of antibiotics through NMR metabolomics. BMC Microbiol. 16 , 82 (2016).
Medeiros-Silva, J. et al. High-resolution NMR studies of antibiotics in cellular membranes. Nat. Commun. 9 , 3963 (2018).
Tietze, L. F., Krewer, B., Major, F. & Schuberth, I. CD-spectroscopy as a powerful tool for investigating the mode of action of unmodified drugs in live cells. J. Am. Chem. Soc. 131 , 13031–13036 (2009).
Altharawi, A., Rahman, K. M. & Chan, K. L. A. Towards identifying the mode of action of drugs using live-cell FTIR spectroscopy. Analyst 144 , 2725–2735 (2019).
Cirnski, K., Coetzee, J., Herrmann, J. & Müller, R. Metabolic profiling to determine bactericidal or bacteriostatic effects of new natural products using isothermal microcalorimetry. J. Vis. Exp. 164 , e61703 (2020).
Arrowsmith, C. H. et al. The promise and peril of chemical probes. Nat. Chem. Biol. 11 , 536–541 (2015).
Wang, Y. et al. Evidence-based and quantitative prioritization of tool compounds in phenotypic drug discovery. Cell Chem. Biol. 23 , 862–874 (2016).
Franken, H. et al. Thermal proteome profiling for unbiased identification of direct and indirect drug targets using multiplexed quantitative mass spectrometry. Nat. Protoc. 10 , 1567–1593 (2015).
Schopper, S. et al. Measuring protein structural changes on a proteome-wide scale using limited proteolysis-coupled mass spectrometry. Nat. Protoc. 12 , 2391–2410 (2017).
Piazza, I. et al. A map of protein-metabolite interactions reveals principles of chemical communication. Cell 172 , 358–372 (2018).
Dai, L. et al. Horizontal cell biology: monitoring global changes of protein interaction states with the proteome-wide cellular thermal shift assay (CETSA). Ann. Rev. Biochem. 88 , 383–408 (2019).
Bagherian, M. et al. Machine learning approaches and databases for prediction of drug–target interaction: a survey paper. Brief. Bioinform. 22 , 247–269 (2021).
Pliakos, K. & Vens, C. Drug-target interaction prediction with tree-ensemble learning and output space reconstruction. BMC Bioinform. 21 , 49 (2020).
Davin-Regli, A. et al. Membrane permeability and regulation of drug “influx and efflux” in enterobacterial pathogens. Curr. Drug Targets 9 , 750–759 (2008).
Louw, G. E. et al. A balancing act: efflux/influx in mycobacterial drug resistance. Antimicrob. Agents Chemother. 53 , 3181–3189 (2009).
Chitsaz, M. & Brown, M. H. The role played by drug efflux pumps in bacterial multidrug resistance. Essays Biochem. 61 , 127–139 (2017).
Weston, N., Sharma, P., Ricci, V. & Piddock, L. J. V. Regulation of the AcrAB-TolC efflux pump in Enterobacteriaceae. Res. Microbiol. 169 , 425–431 (2018).
Ropponen, H.-K., Richter, R., Hirsch, A. K. H. & Lehr, C.-M. Mastering the Gram-negative bacterial barrier – Chemical approaches to increase bacterial bioavailability of antibiotics. Adv. Drug Deliv. Rev. 172 , 339–360 (2021).
Richter, M. F. et al. Predictive compound accumulation rules yield a broad-spectrum antibiotic. Nature 545 , 299–304 (2017). This study highlights which physicochemical properties enforce the accumulation of small molecules in Gram-negative bacteria .
Jukic˅, M., Gobec, S. & Sova, M. Reaching toward underexplored targets in antibacterial drug design. Drug Dev. Res. 80 , 6–10 (2019).
Manchester, J. I., Buurman, E. T., Bisacchi, G. S. & McLaughlin, R. E. Molecular determinants of AcrB-mediated bacterial efflux implications for drug discovery. J. Med. Chem. 55 , 2532–2537 (2012).
Hevener, K. E. et al. Chapter Eighteen-Special challenges to the rational design of antibacterial agents. Ann. Rep. Med. Chem. 48 , 283–298 (2013).
CAS Google Scholar
Zavascki, A. P., Goldani, L. Z., Li, J. & Nation, R. L. Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review. J. Antimicrob. Chemother. 60 , 1206–1215 (2007).
Ferrer-Espada, R. et al. A permeability-increasing drug synergizes with bacterial efflux pump inhibitors and restores susceptibility to antibiotics in multi-drug resistant Pseudomonas aeruginosa strains. Sci. Rep. 9 , 3452 (2019).
Schweizer, H. P. Understanding efflux in Gram-negative bacteria: opportunities for drug discovery. Exp. Opin. Drug Discov. 7 , 633–642 (2012).
Wagner, S. et al. Novel strategies for the treatment of Pseudomonas aeruginosa infections. J. Med. Chem. 59 , 5929–5969 (2016).
Zhang, W., Bai, Y., Wang, Y. & Xiao, W. Polypharmacology in drug discovery: a review from systems pharmacology perspective. Curr. Pharm. Des. 22 , 3171–3181 (2016).
Proschak, E., Stark, H. & Merk, D. Polypharmacology by design: a medicinal chemist’s perspective on multitargeting compounds. J. Med. Chem. 62 , 420–444 (2019).
Cunningham, M. L., Kwan, B. P., Nelson, K. J., Bensen, D. C. & Shaw, K. J. Distinguishing on-target versus off-target activity in early antibacterial drug discovery using a macromolecular synthesis assay. J. Biomol. Screen. 18 , 1018–1026 (2013).
Singh, R., Sripada, L. & Singh, R. Side effects of antibiotics during bacterial infection: mitochondria, the main target in host cell. Mitochondrion 16 , 50–54 (2014).
Young, M. J. Off-target effects of drugs that disrupt human mitochondrial DNA maintenance. Front. Mol. Biosci. 4 , 74 (2017).
Moullan, N. et al. Tetracyclines disturb mitochondrial function across eukaryotic models: a call for caution in biomedical research. Cell Rep. 10 , 1681–1691 (2015).
Pasternak, B., Inghammar, M. & Svanström, H. Fluoroquinolone use and risk of aortic aneurysm and dissection: nationwide cohort study. BMJ 360 , k678 (2018).
Fief, C. A., Hoang, K. G., Phipps, S. D., Wallace, J. L. & Deweese, J. E. Examining the impact of antimicrobial fluoroquinolones on human DNA topoisomerase IIα and IIβ. ACS Omega 4 , 4049–4055 (2019).
Becattini, S., Taur, Y. & Pamer, E. G. Antibiotic-induced changes in the intestinal microbiota and disease. Trends Mol. Med. 22 , 458–478 (2016).
Bhalodi, A. A., van Engelen, T. S. R., Virk, H. S. & Wiersinga, W. J. Impact of antimicrobial therapy on the gut microbiome. J. Antimicrob. Chemother. 74 , i6–i15 (2019).
Wipperman, M. F. et al. Antibiotic treatment for Tuberculosis induces a profound dysbiosis of the microbiome that persists long after therapy is completed. Sci. Rep. 7 , 10767 (2017).
Xu, L. et al. The effect of antibiotics on the gut microbiome: a metagenomics analysis of microbial shift and gut antibiotic resistance in antibiotic treated mice. BMC Genomics 21 , 263 (2020).
Evans, L. E. et al. Exploitation of antibiotic resistance as a novel drug target: development of a β-lactamase-activated antibacterial prodrug. J. Med. Chem. 62 , 4411–4425 (2019).
Hong, J. Natural product synthesis at the interface of chemistry and biology. Chem. Eur. J. 20 , 10204–10212 (2014).
Bauer, A. & Brönstrup, M. Industrial natural product chemistry for drug discovery and development. Nat. Prod. Rep. 31 , 35–60 (2014).
Sucipto, H., Pogorevc, D., Luxenburger, E., Wenzel, S. C. & Müller, R. Heterologous production of myxobacterial α-pyrone antibiotics in Myxococcus xanthus . Metab. Eng. 44 , 160–170 (2017).
Pogorevc, D. et al. Production optimization and biosynthesis revision of corallopyronin A, a potent anti-filarial antibiotic. Metab. Eng. 55 , 201–211 (2019).
Sun, C. et al. A robust platform for the synthesis of new tetracycline antibiotics. J. Am. Chem. Soc. 130 , 17913–17927 (2008).
Hogan, P. C. et al. Large-scale preparation of key building blocks for the manufacture of fully synthetic macrolide antibiotics. J. Antibiot. 71 , 318–325 (2018).
Hüttel, S. et al. Discovery and total synthesis of natural cystobactamid derivatives with superior activity against Gram-negative pathogens. Angew. Chem. Int. Ed. 56 , 12760–12764 (2017).
Zong, Y. et al. Gram-scale total synthesis of teixobactin promoting binding mode study and discovery of more potent antibiotics. Nat. Commun. 10 , 3268 (2019).
Crater, J. S. & Lievense, J. C. Scale-up of industrial microbial processes. FEMS Microbiol. Lett. 365 , fny138 (2018).
Nielsen, J. et al. (eds) Metabolic Engineering. Advances in Biochemical Engineering/Biotechnology Vol 73 (Springer, 2001).
Pham, J. V. et al. A review of the microbial production of bioactive natural products and biologics. Front. Microbiol. 10 , 1404 (2019).
Balani, S. K., Miwa, G. T., Gan, L.-S., Wu, J.-T. & Lee, F. W. Strategy of utilizing in vitro and in vivo ADME tools for lead optimization and drug candidate selection. Curr. Top. Med. Chem. 5 , 1033–1038 (2005).
Chung, T. D. Y., Terry, D. B. & Smith, L. H. in Assay Guidance Manual (eds Markossian, S. et al.) (Eli Lilly & Company and the National Center for Advancing Translational Sciences, 2015).
Reichel, A. & Lienau, P. Pharmacokinetics in drug discovery: an exposure-centred approach to optimising and predicting drug efficacy and safety. Handb. Exp. Pharmacol. 232 , 235–260 (2016). This article highlights the central role of pharmacokinetics in drug discovery .
Singh, S. S. Preclinical pharmacokinetics: an approach towards safer and efficacious drugs. Curr. Drug Metab. 7 , 165–182 (2006).
Shi, J. & Zha, W. Predicting human pharmacokinetics: physiologically based pharmacokinetic modeling and in silico ADME prediction in early drug discovery. Eur. J. Drug. Metab. Pharmacokinet. 44 , 135–137 (2019).
Xiong, G. et al. ADMETlab 2.0: an integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 49 , W5–W14 (2021). Redesigned version of the widely used ADMETlab web server for predictions of pharmacokinetics and toxicity properties of chemicals .
Krause, K. M. et al. Potent LpxC inhibitors with in vitro activity against multidrug-resistant Pseudomonas aeruginosa . Antimicrob. Agents Chemother. 63 , e00977-19 (2019).
Cohen, F. et al. Optimization of LpxC inhibitors for antibacterial activity and cardiovascular safety. ChemMedChem 14 , 1560–1572 (2019).
Felmlee, M. A., Morris, M. E. & Mager, D. E. Mechanism-based pharmacodynamic modeling. Meth. Mol. Biol. 929 , 583–600 (2012).
Lepak, A. J. & Andes, D. R. in Antibiotic Pharmacodynamics Vol. 191 (eds Rotschafer, J. C., Andes, D. R. & Rodvold, K. A.) 59–87 (Springer, 2016).
van Peer, A., Snoeck, E., Huang, M. L. & Heykants, J. Pharmacokinetic-pharmacodynamic relationships in phase I/phase II of drug development. Eur. J. Drug Metab. Pharmacokinet. 18 , 49–59 (1993).
Zhao, M., Lepak, A. J. & Andes, D. R. Animal models in the pharmacokinetic/pharmacodynamic evaluation of antimicrobial agents. Bioorg. Med. Chem. 24 , 6390–6400 (2016).
McGinnity, D. F., Collington, J., Austin, R. P. & Riley, R. J. Evaluation of human pharmacokinetics, therapeutic dose and exposure predictions using marketed oral drugs. Curr. Drug Metab. 8 , 463–479 (2007).
Zou, P. et al. Applications of human pharmacokinetic prediction in first-in-human dose estimation. AAPS J. 14 , 262–281 (2012).
Page, K. M. Validation of early human dose prediction: a key metric for compound progression in drug discovery. Mol. Pharmaceutics 13 , 609–620 (2016).
Maher, S., Brayden, D. J., Casettari, L. & Illum, L. Application of permeation enhancers in oral delivery of macromolecules: an update. Pharmaceutics 11 , 41 (2019).
Ma, X. & Williams, R. O. Polymeric nanomedicines for poorly soluble drugs in oral delivery systems: an update. J. Pharm. Invest. 48 , 61–75 (2018).
Pridgen, E. M., Alexis, F. & Farokhzad, O. C. Polymeric nanoparticle technologies for oral drug delivery. Clin. Gastroenterol. Hepatol. 12 , 1605–1610 (2014).
Bassetti, M., Vena, A., Russo, A. & Peghin, M. Inhaled liposomal antimicrobial delivery in lung infections. Drugs 80 , 1309–1318 (2020).
Bulbake, U., Doppalapudi, S., Kommineni, N. & Khan, W. Liposomal formulations in clinical use: an updated review. Pharmaceutics 9 , 12 (2017).
Russell, W. M. S. & Burch, R. L. The Principles of Humane Experimental Technique (Methuen & Co. Limited, 1959). This work defines, for the first time, the 3Rs principle as the present ethical standard in animal research .
Jaeger, A., Sauder, P., Kopferschmitt & Dahlet, M. Toxicokinetics in clinical toxicology. Acta Clin. Belg. 45 , 1–12 (1990).
Goehl, T. J. Toxicokinetics in the national toxicology program. NIDA Res. Monogr. 173 , 273–304 (1997).
Low, Y. S., Sedykh, A. Y., Rusyn, I. & Tropsha, A. Integrative approaches for predicting in vivo effects of chemicals from their structural descriptors and the results of short-term biological assays. Curr. Top. Med. Chem. 14 , 1356–1364 (2014).
Cox, L. A. T., Popken, D. A., Kaplan, A. M., Plunkett, L. M. & Becker, R. A. How well can in vitro data predict in vivo effects of chemicals? Rodent carcinogenicity as a case study. Regul. Toxicol. Pharmacol. 77 , 54–64 (2016).
Zhang, X. et al. Zebrafish and Galleria mellonella : Models to identify the subsequent infection and evaluate the immunological differences in different Klebsiella pneumoniae intestinal colonization strains. Front. Microbiol. 10 , 2750 (2019).
Herlich, J. A. et al. The non-GLP toleration/dose range finding study: design and methodology used in an early toxicology screening program. Proc. West. Pharmacol. Soc. 52 , 94–98 (2009).
Alirol, E. et al. Multidrug-resistant gonorrhea: A research and development roadmap to discover new medicines. PLoS Med. 14 , e1002366 (2017).
BEAM Alliance . Position Paper 2017: Key Guidelines to implement effective measures toward SMEs to revive the antibacterial R&D field. (BEAM Alliance, 2017) https://beam-alliance.eu/wp-content/uploads/2019/03/2017-11-16-beam-alliance-position-paper.pdf
BEAM Alliance . A new vision for AMR innovation to support medical care. (BEAM Alliance, 2019) https://beam-alliance.eu/wp-content/uploads/2019/10/beam-alliance-a-new-vision-to-support-amr-innovation.pdf
BEAM Alliance . Reflexion paper on the EU pharmaceutical strategy roadmap. (BEAM Alliance, 2020) https://beam-alliance.eu/wp-content/uploads/2020/07/roadmap-reflexion-paper.pdf
ReAct Europe . AMR stakeholder mapping. (ReAct, 2016) https://www.reactgroup.org/uploads/Stakeholder%20Analysis_ReActForWHO.pdf
Fisher, J. F. & Mobashery, S. Endless resistance. Endless antibiotics? MedChemComm 7 , 37–49 (2016).
DiMasi, J. A., Grabowski, H. G. & Hansen, R. W. Innovation in the pharmaceutical industry: New estimates of R&D costs. J. Health Econ. 47 , 20–33 (2016).
The Boston Consulting Group . Breaking through the wall – A call for concerted action on antibiotics research and development. (Bundesministerium für Gesundheit, 2017) https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/5_Publikationen/Gesundheit/Berichte/GUARD_Follow_Up_Report_Full_Report_final.pdf
Access to Medicine Foundation . Antimicrobial resistance benchmark 2018. (Access to Medicine Foundation, 2018) https://accesstomedicinefoundation.org/media/uploads/downloads/5f292e3996b58_Antimicrobial-Resistance-Benchmark-2018.pdf
Paul, S. M. et al. How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat. Rev. Drug Discov. 9 , 203–214 (2010).
Pieroni, M., Wan, B., Cho, S., Franzblau, S. G. & Costantino, G. Design, synthesis and investigation on the structure–activity relationships of N-substituted 2-aminothiazole derivatives as antitubercular agents. Eur. J. Med. Chem. 72 , 26–34 (2014).
Azzali, E. et al. Substituted N -phenyl-5-(2-(phenylamino)thiazol-4-yl)isoxazole-3-carboxamides are valuable antitubercular candidates that evade innate efflux machinery. J. Med. Chem. 60 , 7108–7122 (2017).
Bohacek, R. S., McMartin, C. & Guida, W. C. The art and practice of structure-based drug design: a molecular modeling perspective. Med. Res. Rev. 16 , 3–50 (1996).
Hartwig, J. F. Evolution of a fourth generation catalyst for the amination and thioetherification of aryl halides. Acc. Chem. Res. 41 , 1534–1544 (2008).
Abdel-Magid, A. F. & Mehrman, S. J. A review on the use of sodium triacetoxyborohydride in the reductive amination of ketones and aldehydes. Org. Process. Res. Dev. 10 , 971–1031 (2006).
Gedeck, P. et al. Benefit of retraining p K a models studied using internally measured data. J. Chem. Inf. Model. 55 , 1449–1459 (2015).
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52 , 6752–6756 (2009).
Zender, M. et al. Discovery and biophysical characterization of 2-amino-oxadiazoles as novel antagonists of PqsR, an important regulator of Pseudomonas aeruginosa virulence. J. Med. Chem. 56 , 6761–6774 (2013).
Zender, M. et al. Flexible fragment growing boosts potency of quorum-sensing inhibitors against Pseudomonas aeruginosa virulence. ChemMedChem 15 , 188–194 (2020).
Ahmed, A. S. A. et al. PqsR inverse agonists. Patent WO2020007938A1 (2020).
Schütz, C. et al. A new PqsR inverse agonist potentiates tobramycin efficacy to eradicate Pseudomonas aeruginosa biofilms. Adv. Sci. 8 , 2004369 (2021). Demonstrates the synergistic effect of a quorum sensing-targeting pathoblocker with a standard-of-care antibiotic in a Pseudomonas aeruginosa lung infection mouse model .
Oliver, T. J. & Sinclair, A. C. Antibiotic M-319. US Patent US3155582A (1964).
Mitscher, L. A. et al. Structure of chelocardin, a novel tetracycline antibiotic. J. Am. Chem. Soc. 92 , 6070–6071 (1970).
Petkovic, H., Raspor, P. & Lesnik, U. Genes for biosynthesis of tetracycline compounds and uses thereof. US Patent US8361777B2 (2013).
Lukežic˅, T. et al. Identification of the chelocardin biosynthetic gene cluster from Amycolatopsis sulphurea : a platform for producing novel tetracycline antibiotics. Microbiology 159 , 2524–2532 (2013).
Molnar, V., Matković, Z., Tambić, T. & Kozma, C. Klinicko-farmakolosko ispitivanje kelokardina u bolesnika s infekcijom mokraćnih putova. Lijec. Vjesn. 99 , 560–562 (1977).
Lukežic˅, T. et al. Engineering atypical tetracycline formation in Amycolatopsis sulphurea for the production of modified chelocardin antibiotics. ACS Chem. Biol. 14 , 468–477 (2019).
Flores-Mireles, A. L., Walker, J. N., Caparon, M. & Hultgren, S. J. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 13 , 269–284 (2015).
Irschik, H., Jansen, R., Höfle, G., Gerth, K. & Reichenbach, H. The corallopyronins, new inhibitors of bacterial RNA synthesis from Myxobacteria. J. Antibiot. 38 , 145–152 (1985).
O’Neill, A. et al. RNA polymerase inhibitors with activity against rifampin-resistant mutants of Staphylococcus aureus . Antimicrob. Agents Chemother. 44 , 3163–3166 (2000).
Belogurov, G. A. et al. Transcription inactivation through local refolding of the RNA polymerase structure. Nature 457 , 332–335 (2008).
Schäberle, T. F. et al. Insights into structure–activity relationships of bacterial RNA polymerase inhibiting corallopyronin derivatives. J. Nat. Prod. 78 , 2505–2509 (2015).
Schiefer, A. et al. Corallopyronin A specifically targets and depletes essential obligate Wolbachia endobacteria from filarial nematodes in vivo. J. Infect. Dis. 206 , 249–257 (2012).
Pfarr, K. M. et al. Compounds for use in the treatment of filariasis. US Patent US9687470B2 (2017).
Pfarr, K. M. et al. Compounds for use in the treatment of filariasis. Patent EP2704708B1 (2017).
Schiefer, A. et al. Corallopyronin A for short-course anti-wolbachial, macrofilaricidal treatment of filarial infections. PLoS Negl. Trop. Dis. 14 , e0008930 (2020).
World Health Organization (WHO) . Ending the neglect to attain the Sustainable Development Goals – A road map for neglected tropical diseases 2021–2030 (WHO, 2020) https://www.who.int/neglected_diseases/resources/who-ucn-ntd-2020.01/en/
Loeper, N. et al. Elaborations on Corallopyronin A as a novel treatment strategy against genital chlamydial infections. Front. Microbiol. 10 , 943 (2019).
Kock, F. et al. Orientia tsutsugamushi is highly susceptible to the RNA polymerase switch region inhibitor corallopyronin A in vitro and in vivo . Antimicrob. Agents Chemother. 62 , e01732-17 (2018).
Shima, K. et al. Effective inhibition of rifampicin-resistant Chlamydia trachomatis by the novel DNA-dependent RNA polymerase inhibitor corallopyronin A. Int. J. Antimicrob. Agents 52 , 523–524 (2018).
Sucipto, H. et al. Production of myxopyronin and of its derivatives. Patent EP2994535A1 (2018).
Download references
Acknowledgements
We are grateful to Édith Brochu (JPIAMR-VRI, Canada) for helpful discussion and comments during manuscript preparation. Coordination of the IRAADD consortium is funded by the JPIAMR-VRI, including the publication of this article. The project on PqsR pathoblocker development acknowledges funding through the German Center for Infection Research (DZIF; projects TTU09.908 and TTU09.916), the Helmholtz Association (Helmholtz Validation Fund) and additional contributions by the associated academic institutes (HZI and HIPS). The development of chelocardins is supported by the DZIF (TTU09.814/09.821), the Helmholtz Innovation Fund (Pre-4D), by the Slovenian Research Agency, ARRS, grant no. J4-8226, and in collaboration with AciesBio, Slovenia. The corallopyronin project is funded by the DZIF (TTU09.807/09.816, TTU09.914), the German Federal Ministry of Education and Research (BMBF), the federal state of North Rhine-Westphalia (EFRE.NRW) and EU Horizon 2020. Eriko Takano was funded by the European Union’s Horizon 2020 research and innovation programme under grant agreement no. 720793 “TOPCAPI: Thoroughly Optimised Production Chassis for Advanced Pharmaceutical Ingredients”.
Author information
Authors and affiliations.
Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) - Helmholtz Centre for Infection Research (HZI), and Department of Pharmacy, Saarland University Campus E8.1, Saarbrücken, Germany
Marcus Miethke, Martin Empting, Anna K. H. Hirsch, Brigitta Loretz, Claus-Michael Lehr, Alexander Titz, Jennifer Herrmann & Rolf Müller
German Center for Infection Research (DZIF), Braunschweig, Germany
Marcus Miethke, Mark Brönstrup, Marc Stadler, Evi Stegmann, Heike Brötz-Oesterhelt, Wolfgang Wohlleben, Martin Empting, Anna K. H. Hirsch, Alexander Titz, Jennifer Herrmann, Timo Jaeger, Silke Alt, Thomas Hesterkamp, Andrea Schiefer, Kenneth Pfarr, Achim Hoerauf & Rolf Müller
Food and Drug Department, University of Parma, Parma, Italy
Marco Pieroni
The Novo Nordisk Foundation Center for Biosustainability, Technical University of Denmark, Lyngby, Denmark
Tilmann Weber
Department of Chemical Biology (CBIO), Helmholtz Centre for Infection Research (HZI), Braunschweig, Germany
Mark Brönstrup
Infectious Diseases & Natural Product Research at EVOTEC, and Justus Liebig University Giessen, Giessen, Germany
Peter Hammann
Epigenetic Chemical Biology, Department of Structural Biology and Chemistry, Institut Pasteur, UMR n°3523, CNRS, Paris, France
Ludovic Halby & Paola B. Arimondo
Ecology and Evolution of Antibiotic Resistance Unit, Microbiology Department, Institut Pasteur, CNRS UMR3525, Paris, France
Philippe Glaser
Université de Lorraine, INRAE, DynAMic, Nancy, France
Bertrand Aigle
Department of Biosciences, Goethe University Frankfurt, Frankfurt, Germany
Helge B. Bode
Max Planck Institute for Terrestrial Microbiology, Department of Natural Products in Organismic Interactions, Marburg, Germany
Faculty of Pharmacy, University of Lisbon, Lisbon, Portugal
Rui Moreira
Unit MCAM, CNRS, National Museum of Natural History (MNHN), Paris, France
Pharmaceutical Biotechnology, Saarland University, Saarbrücken, Germany
Andriy Luzhetskyy
Bioinformatics Group, Wageningen University and Research, Wageningen, Netherlands
Marnix H. Medema
Institute for Integrative Biology of the Cell (I2BC) & Microbiology Department, University of Paris-Saclay, Gif-sur-Yvette, France
Jean-Luc Pernodet
Microbial Drugs (MWIS), Helmholtz Centre for Infection Research (HZI), Braunschweig, Germany
Marc Stadler
Fundación MEDINA, Granada, Spain
José Rubén Tormo & Olga Genilloud
Department of Molecular Microbiology, John Innes Centre, Norwich, United Kingdom
Andrew W. Truman
Molecular and Structural Enzymology Group, Université de Lorraine, CNRS, IMoPA, Nancy, France
Kira J. Weissman
Manchester Institute of Biotechnology, Department of Chemistry, School of Natural Sciences, Faculty of Science and Engineering, University of Manchester, Manchester, United Kingdom
Eriko Takano
Department of Pharmaceutical Sciences, University of Perugia, Perugia, Italy
Stefano Sabatini
Department of Microbial Bioactive Compounds, Interfaculty Institute of Microbiology and Infection Medicine, University of Tübingen, Tübingen, Germany
Evi Stegmann & Heike Brötz-Oesterhelt
Department of Microbiology/Biotechnology, Interfaculty Institute of Microbiology and Infection Medicine, University of Tübingen, Tübingen, Germany
Wolfgang Wohlleben
Institute for Chemistry UMR 7177, University of Strasbourg/CNRS, ITI InnoVec, Strasbourg, France
Myriam Seemann
Life Sciences & Chemistry, Jacobs University Bremen, Bremen, Germany
Mathias Winterhalter
Institute of Medical Microbiology, Immunology and Parasitology (IMMIP), University Hospital Bonn, Bonn, Germany
Andrea Schiefer, Kenneth Pfarr & Achim Hoerauf
Biophys Ltd., Usk, Monmouthshire, United Kingdom
Heather Graz
School of Law, University of Bristol, Bristol, United Kingdom
Michael Graz
Recursion Pharmaceuticals, Salt Lake City, UT, USA
Mika Lindvall
Drug Discovery/Medicinal Chemistry, HiberCell, New York, NY, USA
Savithri Ramurthy
Department of Medicinal Chemistry, Uppsala University, Uppsala, Sweden
Anders Karlén
AMR Insights, Amsterdam, Netherlands
Maarten van Dongen
Department of Food Science and Technology, Biotechnical Faculty, University of Ljubljana, Ljubljana, Slovenia
Hrvoje Petkovic
Chair for Clinical Bioinformatics, Saarland University, University Hospital, Saarbrücken, Germany
Andreas Keller
BEAM Alliance, Paris, France
Frédéric Peyrane
NAICONS Srl, Milan, Italy
Stefano Donadio
Drugs for Neglected Diseases initiative (DNDi), Geneva, Switzerland
Laurent Fraisse
The Global Antibiotic Research and Development Partnership (GARDP), Geneva, Switzerland
Laura J. V. Piddock
Division of Biological Chemistry and Drug Discovery, University of Dundee, Dundee, United Kingdom
Ian H. Gilbert
Novartis Institutes for BioMedical Research (NIBR), Emeryville, CA, USA
Heinz E. Moser
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to researching data, discussion of content, writing of the article, as well as reviewing and editing the article.
Corresponding authors
Correspondence to Heinz E. Moser or Rolf Müller .
Ethics declarations
Competing interests.
M. H. Medema is a co-founder of Design Pharmaceuticals and a member of the scientific advisory board of Hexagon Bio, and S. Donadio is a co-founder and shareholder of NAICONS, owning intellectual property on antibacterial compounds. The remaining authors do not declare any competing interests.
Additional information
Peer review information.
Nature Reviews Chemistry thanks U. Theuretzbacher, G. Wright and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acronym of highly virulent and often (mainly in hospitals) multidrug-resistant bacterial priority pathogens, including Enterococcus faecium , Staphylococcus aureus , Klebsiella pneumoniae , Acinetobacter baumannii , Pseudomonas aeruginosa and Enterobacter spp.
Push incentives (for example, grants for the different phases of drug discovery or development) aim to generate and push a product (such as a new antibiotic) into the market.
Pull incentives ensure that a product is established at the market (for example, by defined payments to the manufacturer for a certain time after market release).
These models constitute that fixed prices will be paid at regular intervals for a certain period (for example, by governments) to the provider of a product to trigger (‘pull’) the development of therapeutics, such as novel antibiotics.
These models combine expanded government funding for drug development with cash reward incentives to drug developers in order to delink high innovation costs from high sales prices.
Molecules that show a desired type of activity in initial screening assay(s).
Absorption, distribution, metabolism, excretion and toxicity. A collection of experimental studies that determines the fate of a pharmaceutical compound in an organism.
A change in cellular function (referring to the bacterial cell throughout the article) that results from exposure to a drug.
Anti-virulence drugs, i.e. drugs acting against factors (usually non-essential targets) that are involved in the development of bacterial virulence, often combined with a regular antibiotic to provide a synergistic effect.
Bacteria that stain positive with Gram’s method by retaining the crystal violet dye in the thick peptidoglycan layer that composes their cell wall, together with the inner cytoplasmic cell membrane; the absence of an outer membrane often makes them more susceptible to cell wall targeting antibiotics and to influx of antibiotics into the cytoplasm.
Bacteria that stain negative (do not retain the crystal violet dye) when using Gram’s method for bacterial differentiation; their cell envelopes are composed of an inner cytoplasmic cell membrane and an outer membrane (containing amphiphilic lipopolysaccharides at the outer leaflet), which enclose the periplasmic space containing a thin peptidoglycan layer.
The minimum concentrations, usually expressed in units of mass per litre, that prevent visible growth of bacteria. Complementary to minimum bactericidal concentration.
The minimum concentrations, usually expressed in units of mass per litre, that result in death for ≥99.9% of bacteria. Complementary to minimum inhibitory concentration.
(TCP). Reflects how the chemical matter of an identified compound is optimized towards the target product profile by summarizing the desired chemical, physicochemical and biological characteristics of a preclinical drug candidate.
(TPP). Summarizes the key requirements for a new therapeutic that fulfils a priority medical need; thus, it identifies and outlines the critical attributes and (pre)clinical endpoints of a product as a guidance before development begins.
Native or acquired, this is the route by which a microorganism resists the action of a particular drug. This may include, for example, decreased influx, enhanced efflux, modification of the drug target and modification/inactivation of the drug.
Molecules with validated activity that serve as a basis for the development of a drug candidate.
(BGCs). Sets of genes, typically found close to one another in the genome, that code for the enzymes responsible for the synthesis of a particular secondary metabolite.
Clustered regularly interspaced short palindromic repeats–CRISPR-associated protein 9. Components of the immunological defence of certain bacteria against viruses and plasmids; used in molecular biology not only for genetic engineering of bacterial genomes.
Describes the addition of another chemical moiety (e.g. a siderophore) to a drug scaffold in order to facilitate the bacterial uptake of this drug conjugate (for example, by exploiting bacterial siderophore transporters).
(GMP). The set of minimum requirements that must be followed in manufacturing in order to satisfy the agencies responsible for licensing.
Inhibitors targeting the enzyme LpxC that catalyses the initial step in the biosynthesis of lipid A — a structural component of the lipopolysaccharide molecules in Gram-negative bacteria.
‘Replacement, reduction and refinement’; guiding principles defined for a more ethical approach to animal research.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Miethke, M., Pieroni, M., Weber, T. et al. Towards the sustainable discovery and development of new antibiotics. Nat Rev Chem 5 , 726–749 (2021). https://doi.org/10.1038/s41570-021-00313-1
Download citation
Accepted : 01 July 2021
Published : 19 August 2021
Issue Date : October 2021
DOI : https://doi.org/10.1038/s41570-021-00313-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Time series of chicken stool metagenomics and egg metabolomics in changing production systems: preliminary insights from a proof-of-concept.
- Michael E. G. Rosch
- Jacqueline Rehner
- Verena Keller
One Health Outlook (2024)
Antimicrobial resistance crisis: could artificial intelligence be the solution?
- Guang-Yu Liu
- Xiao-Fen Liu
Military Medical Research (2024)
Uncovering the potentiality of quinazoline derivatives against Pseudomonas aeruginosa with antimicrobial synergy and SAR analysis
- Rakshit Manhas
- Arti Rathore
- Avisek Mahapa
The Journal of Antibiotics (2024)
Tuning oxidant and antioxidant activities of ceria by anchoring copper single-site for antibacterial application
- Ludan Zhang
- Yuguang Wang
Nature Communications (2024)
Aerosolized delivery of ESKAPE pathogens for murine pneumonia models
- Katharina Rox
Scientific Reports (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
- See us on facebook
- See us on twitter
- See us on youtube
- See us on linkedin
- See us on instagram
Generative AI develops potential new drugs for antibiotic-resistant bacteria
Stanford Medicine researchers devise a new artificial intelligence model, SyntheMol, which creates recipes for chemists to synthesize the drugs in the lab.
March 28, 2024 - By Rachel Tompa
Acinetobacter baumannii infection is a leading cause of death related to antibiotic resistance. Stanford Medicine researchers employed artificial intelligence to provide recipes for drugs that can treat it. Kateryna Kon /Shutterstock.com
With nearly 5 million deaths linked to antibiotic resistance globally every year, new ways to combat resistant bacterial strains are urgently needed.
Researchers at Stanford Medicine and McMaster University are tackling this problem with generative artificial intelligence. A new model, dubbed SyntheMol (for synthesizing molecules), created structures and chemical recipes for six novel drugs aimed at killing resistant strains of Acinetobacter baumannii, one of the leading pathogens responsible for antibacterial resistance-related deaths.
The researchers described their model and experimental validation of these new compounds in a study published March 22 in the journal Nature Machine Intelligence .
“There’s a huge public health need to develop new antibiotics quickly,” said James Zou , PhD, an associate professor of biomedical data science and co-senior author on the study. “Our hypothesis was that there are a lot of potential molecules out there that could be effective drugs, but we haven’t made or tested them yet. That’s why we wanted to use AI to design entirely new molecules that have never been seen in nature.”
Before the advent of generative AI, the same type of artificial intelligence technology that underlies large language models like ChatGPT, researchers had taken different computational approaches to antibiotic development. They used algorithms to scroll through existing drug libraries, identifying those compounds most likely to act against a given pathogen. This technique, which sifted through 100 million known compounds , yielded results but just scratched the surface in finding all the chemical compounds that could have antibacterial properties.

Kyle Swanson
“Chemical space is gigantic,” said Kyle Swanson , a Stanford computational science doctoral student and co-lead author on the study. “People have estimated that there are close to 10 60 possible drug-like molecules. So, 100 million is nowhere close to covering that entire space.”
Hallucinating for drug development
Generative AI’s tendency to “hallucinate,” or make up responses out of whole cloth, could be a boon when it comes to drug discovery, but previous attempts to generate new drugs with this kind of AI resulted in compounds that would be impossible to make in the real world, Swanson said. The researchers needed to put guardrails around SyntheMol’s activity — namely, to ensure that any molecules the model dreamed up could be synthesized in a lab.
“We’ve approached this problem by trying to bridge that gap between computational work and wet lab validation,” Swanson said.
The model was trained to construct potential drugs using a library of more than 130,000 molecular building blocks and a set of validated chemical reactions. It generated not only the final compound but also the steps it took with those building blocks, giving the researchers a set of recipes to produce the drugs.
The researchers also trained the model on existing data of different chemicals’ antibacterial activity against A. baumannii . With these guidelines and its building block starting set, SyntheMol generated around 25,000 possible antibiotics and the recipes to make them in less than nine hours. To prevent the bacteria from quickly developing resistance to the new compounds, researchers then filtered the generated compounds to only those that were dissimilar from existing compounds.

“Now we have not just entirely new molecules but also explicit instructions for how to make those molecules,” Zou said.
A new chemical space
The researchers chose the 70 compounds with the highest potential to kill the bacterium and worked with the Ukrainian chemical company Enamine to synthesize them. The company was able to efficiently generate 58 of these compounds, six of which killed a resistant strain of A. baumannii when researchers tested them in the lab. These new compounds also showed antibacterial activity against other kinds of infectious bacteria prone to antibiotic resistance, including E. coli, Klebsiella pneumoniae and MRSA.
The scientists were able to further test two of the six compounds for toxicity in mice, as the other four didn’t dissolve in water. The two they tested seemed safe; the next step is to test the drugs in mice infected with A. baumannii to see if they work in a living body, Zou said.
The six compounds are vastly different from each other and from existing antibiotics. The researchers don’t know how their antibacterial properties work at the molecular level, but exploring those details could yield general principles relevant to other antibiotic development.
“This AI is really designing and teaching us about this entirely new part of the chemical space that humans just haven’t explored before,” Zou said.
Zou and Swanson are also refining SyntheMol and broadening its reach. They’re collaborating with other research groups to use the model for drug discovery for heart disease and to create new fluorescent molecules for laboratory research.
The study was funded by the Weston Family Foundation, the David Braley Centre for Antibiotic Discovery, the Canadian Institutes of Health Research, M. and M. Heersink, the Chan-Zuckerberg Biohub, and the Knight-Hennessy scholarship.
For more news about responsible AI in health and medicine, sign up for the RAISE Health newsletter.
Register for the RAISE Health Symposium on May 14.
- Rachel Tompa Rachel Tompa is a freelance science writer.
About Stanford Medicine
Stanford Medicine is an integrated academic health system comprising the Stanford School of Medicine and adult and pediatric health care delivery systems. Together, they harness the full potential of biomedicine through collaborative research, education and clinical care for patients. For more information, please visit med.stanford.edu .
Artificial intelligence
Exploring ways AI is applied to health care
- Share full article
Advertisement
Supported by
F.D.A. Approves Antibiotic for Increasingly Hard-to-Treat Urinary Tract Infections
Pivmecillinam, which has been used in Europe for decades, will become available next year to women 18 and older.

By Andrew Jacobs
The Food and Drug Administration on Wednesday approved the sale of an antibiotic for the treatment of urinary tract infections in women, giving U.S. health providers a powerful new tool to combat a common infection that is increasingly unresponsive to the existing suite of antimicrobial drugs.
The drug, pivmecillinam, has been used in Europe for more than 40 years, where it is often a first-line therapy for women with uncomplicated U.T.I.’s, meaning the infection is confined to the bladder and has not reached the kidneys. The drug will be marketed in the U.S. as Pivya and will be made available by prescription to women 18 and older.
It is the first time in two decades that the F.D.A. has approved a new antibiotic for U.T.I.s, which annually affect 30 million Americans. U.T.I.s are responsible for the single-greatest use of antibiotics outside a hospital setting.
“Uncomplicated U.T.I.s are a very common condition impacting women and one of the most frequent reasons for antibiotic use,” Dr. Peter Kim, director of the Division of Anti-Infectives at the F.D.A.’s Center for Drug Evaluation and Research, said in a statement . “The F.D.A. is committed to fostering new antibiotic availability when they prove to be safe and effective.”
Utility Therapeutics , the U.S. company that acquired the rights to pivmecillinam, said it would be available in 2025. The company is also seeking F.D.A. approval for an intravenous version of the drug that is used for more serious infections and is usually administered in a hospital setting.
Health practitioners said they were elated to have another tool in their arsenal given the growing challenge of antimicrobial resistance, which makes existing medications less effective as pathogens mutate in ways that allow them to survive a course of antibiotics.
The problem, largely an outgrowth of antibiotic overuse around the world, is associated with five million deaths, according to the World Health Organization .
“This is an exciting new possibility for treatment of lower urinary tract infections,” said Dr. Shruti Gohil, a professor of infectious diseases at the University of California, Irvine School of Medicine, and an author of a recent study in JAMA that focused on ways to reduce antibiotic overuse in hospitals. “But I would also say that it is going to be important that we use the drug responsibly in this country so that we don’t breed resistance against it.”
Most U.T.I.s occur when bacteria like E. coli travel from the rectum, genital area or vagina into the urethra and enter the bladder. As they multiply, the pathogens can cause abdominal cramping, burning and bloody urination.
More than half of all women in the United States will acquire a U.T.I. in their lifetime, compared with 14 percent of men. That is in large part because of the differing architecture of the urinary tract in the sexes: Women have shorter urethras than men, which makes it easier for bacteria to reach the urinary tract.
The majority of U.T.I.s are now resistant to one or more antibiotics; ampicillin, once a common treatment, has been largely abandoned. Infections that travel to the kidneys or that enter the bloodstream are more difficult to treat and more dangerous.
People with weakened immune systems or chronic medical conditions are usually the most vulnerable to drug-resistant infections. But U.T.I.s have a dubious distinction: They are the single biggest risk to healthy people from drug-resistant germs.
In the four decades since it was first approved for use in Europe, Pivmecillinam has been prescribed more than 30 million times, mostly in Nordic countries, with few reported complications.
The F.D.A. said that nausea and diarrhea were the most common side effects in the clinical trials that paved the way for pivmecillinam’s approval in the United States.
Tom Hadley, the president and chief operating officer of Utility Therapeutics, said his company moved to acquire the U.S. rights to pivmecillinam after Congress, in 2012, granted an additional five years of exclusivity to manufacturers of new antimicrobial drugs.
Henry Skinner, the chief executive at the AMR Action Fund , a venture capital fund that invested in Utility Therapeutics’ bid to bring pivmecillinam to the U.S., said he was gratified by the F.D.A.’s approval but said the long-term prognosis for new antimicrobial drugs remained grim. The $1 billion fund, financed by the pharmaceutical industry, invests in biotech start-ups working on promising antimicrobials.
Most of the nation’s biggest drug makers, unable to turn a profit on antibiotics, have long since abandoned the field, he said, and the dearth of investment has prompted an exodus of talented researchers.
A federal initiative that would create a subscription-based model for antibiotic development has been languishing in Congress. The $6 billion measure, the Pasteur Act , would provide pharmaceutical companies an upfront payment in exchange for unlimited access to a drug once it is approved by the F.D.A.
Mr. Skinner said he was haunted by one recent estimate suggesting that drug-resistant infections could claim 10 million lives by 2050.
“There are definitely bright spots,” he said. “But more people are dying today than ought to be because we are moving backward, and not delivering the physicians, drugs and diagnostics needed to address the crisis of antimicrobial resistance.”
Andrew Jacobs is a Times reporter focused on how healthcare policy, politics and corporate interests affect people’s lives. More about Andrew Jacobs
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
April 29, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
Study finds resistance to critically important antibiotics in uncooked meat sold for human and animal consumption
by European Society of Clinical Microbiology and Infectious Diseases

New research presented at the ESCMID Global Congress (formerly ECCMID) in Barcelona, Spain (27–30 April) has found substantial levels of resistance to critically-important antibiotics in meat sold for human and animal consumption. The study is by Dr. Jordan Sealey, Professor Matthew Avison and colleagues from the University of Bristol, UK.
Meat sold for consumption by humans and companion animals in the UK is regulated by the UK Government Food Standards Agency (FSA) to ensure it falls within bacterial limits deemed safe. However, while meat is tested for the types and amounts of pathogens present, it is not tested for resistant opportunistic pathogens (e.g. Escherichia coli).
Multiple studies have shown a strong association between feeding dogs a diet of uncooked meat (raw dog food) and an increased risk of excreting E. coli resistant to critically important antibiotics in their feces. It is possible that pet animals eating raw meat increases the risk of spreading resistant pathogens to their human owners in the household.
Here, the authors investigated 58 samples of uncooked meat (15 each of beef, chicken, lamb and 13 of pork) sold for human consumption after cooking (MHC) and 15 samples of chicken-based raw dog meat (RDM) for resistant E. coli.
Samples were enriched and plated onto agar containing common antibiotics—amoxicillin, amoxicillin-clavulanate, cefotaxime, ciprofloxacin, spectinomycin and streptomycin, of which some are considered critically important for human health, to test for resistant E. coli.
The team found the highest sample-level positivity rate for resistant E. coli in uncooked meat for human consumption was in chicken—100% of samples were positive for resistance to spectinomycin and streptomycin, and 47% of samples were positive for resistance to critically important fluoroquinolones. For lamb, pork and beef the values were 27%, 38% and 27% for spectinomycin, 40%, 38% and 47% for streptomycin and 7%, 8% and 13% for fluoroquinolones, respectively.
Similar levels of resistance were seen in chicken raw dog food samples as seen in chicken meat; 87% positive for spectinomycin and streptomycin resistance, and 47% for fluoroquinolones. Sample level positivity for resistance to critically important cefotaxime was 27% for raw dog food.
The authors say, "While most people know that if they don't use proper hygiene when handling uncooked meat they can pick up ' food poisoning ,' most people are not aware that you can also pick up antibiotic-resistant opportunistic pathogens. Owners who feed their dogs raw dog food perhaps don't realize this uncooked meat also contains these pathogens and maybe don't use proper hygiene after preparing the meat, and don't consider their dog as a potential source of these pathogens if it is raw fed."
The most common STs in raw dog food were ST10, ST162, ST744—in the authors' previous study on fluoroquinolone resistance in dogs, excretion of these STs was found to be strongly associated with dogs being fed a raw food diet.
The authors conclude, "This study confirms that uncooked meat carries multiple resistant E. coli, commonly including resistance to critically important antibiotics important for human health. If ingested, these bacteria can enter the gut, and may cause resistant opportunistic infections (e.g. urinary tract infections) in the future. It is therefore very important that people cook meat thoroughly before eating, and use appropriate hygiene practices during meat preparation.
"Our findings that raw dog food is similarly contaminated with resistant bacteria provides an explanation for why dogs fed raw meat are more likely to excrete these bacteria. Appropriate hygiene practices after handling raw dog food, and dogs that are fed such raw meat , are strongly advised."
Explore further
Feedback to editors
Microarray patches safe and effective for vaccinating children, trial shows
9 hours ago

Healthy lifestyle may offset effects of life-shortening genes by more than 60%

Fentanyl inhalation may cause potentially irreversible brain damage, warn doctors

Frequent teen vaping might boost risk of toxic lead and uranium exposure
Study in Haiti suggests early-onset heart failure is prevalent form of heart disease in low-income countries
12 hours ago

AI algorithms can determine how well newborns nurse, study shows

Kaposi sarcoma discovery and mouse model could facilitate drug development
Immune cell interaction study unlocks novel treatment targets for chikungunya virus
13 hours ago

New method rapidly reveals how protein modifications power T cells
Protein responsible for genetic inflammatory disease identified
14 hours ago
Related Stories

Feeding dogs raw meat increases the risk of antibiotic-resistant E. coli, finds study
Nov 20, 2023

Feeding dogs raw meat associated with increased presence of antibiotic-resistant bacteria
Jul 20, 2022

Multidrug-resistant bacteria found in 40% of supermarket meat samples
Apr 14, 2023

Antimicrobial-resistant E. coli found in dogs with diarrhea
Feb 29, 2024


UK and Portuguese study strongly suggests 'superbugs' are being passed from pets to owners
Apr 12, 2024

Fido's raw meat pet food may be loaded with harmful bacteria: study
Oct 16, 2019
Recommended for you

Study suggests that stevia is the most brain-compatible sugar substitute
17 hours ago

Pandemic fatigue and vaccine hesitancy continue to affect global public health, new 23-country study finds
16 hours ago

Nature's nudge: Study shows green views lead to healthier food choices
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Search form
Common antibiotic may be helpful in fighting respiratory viral infections.

(© stock.adobe.com)
A new, Yale-led study suggests that a range of respiratory viral infections — including COVID-19 and influenza — may be preventable or treatable with a generic antibiotic that is delivered to the nasal passageway.
A team led by Yale’s Akiko Iwasaki and former Yale researcher Charles Dela Cruz successfully tested the effectiveness of neomycin, a common antibiotic, to prevent or treat respiratory viral infections in animal models when given to the animals via the nose. The team then found that the same nasal approach — this time applying the over-the-counter ointment Neosporin — also triggers a swift immune response by interferon-stimulated genes (ISGs) in the noses of healthy humans.
The findings were published in the journal Proceedings of the National Academy of Sciences.
“ This is an exciting finding, that a cheap over-the-counter antibiotic ointment can stimulate the human body to activate an antiviral response,” said Iwasaki, the Sterling Professor of Immunobiology and professor of dermatology at Yale School of Medicine and co-senior author of the new study.
“ Our work supports both preventative and therapeutic actions of neomycin against viral diseases in animal models, and shows effective blocking of infection and transmission,” said Iwasaki, who is also professor of molecular, cellular, and developmental biology in Yale’s Faculty of Arts and Sciences, professor of epidemiology at Yale School of Public Health, and an investigator at the Howard Hughes Medical Institute.
Respiratory viruses affect millions of people each year. The global COVID-19 pandemic, caused by the coronavirus SARS-CoV-2, has led to 774.5 million cases worldwide as of February 2024, with global mortality of 6.9 million people. Influenza viruses account for up to 5 million cases of severe illness and 500,000 deaths annually worldwide.
Currently, most therapies used to fight respiratory viral infections — including antivirals, monoclonal antibodies, and convalescent plasma therapy — are delivered intravenously or orally. They focus on stopping the progression of existing infections.
A nasal-centered therapy has a much better chance of stopping infections before they can spread to the lower respiratory tract and cause severe diseases, the researchers said.
“ This collaborative multi-disciplinary work combined important insights from animal pulmonary infection modeling experiments with human study evaluation of this intranasal approach to stimulate antiviral immunity,” said Dela Cruz, former associate professor of pulmonary, critical care, and sleep medicine, and of microbial pathogenesis at Yale School of Medicine and former director of the Center for Pulmonary Infection Research and Treatment. Dela Cruz is currently at the University of Pittsburgh.
In their study, the researchers found that mice treated intranasally with neomycin showed a robust ISG line of defense against both SARS-CoV- 2 and a highly virulent strain of influenza A virus. The researchers also found that an intranasal treatment of neomycin strongly mitigated contact transmission of SARS-CoV-2 in hamsters.
In healthy humans, intranasal application of Neosporin (containing neomycin) also initiated a strong expression of ISGs in a subset of volunteers, the researchers said.
“ Our findings suggest that we might be able to optimize this cheap and generic antibiotic to prevent viral diseases and their spread in human populations, especially in global communities with limited resources,” Iwasaki said. “This approach, because it is host-directed, should work no matter what the virus is.”
The co-first authors of the new study, all from Yale, are Tianyang Mao, Jooyoung Kim, and Mario Peña-Hernández.
Health & Medicine
Media Contact
Fred Mamoun: [email protected] , 203-436-2643

Alison Cole ’99 named executive director of Yale Alumni Association

Tech tools and human values: Conference explores AI’s impact in government

Yale center makes ‘significant strides’ on quantum computing in chemistry

Preparing people who are sound-sensitive for noisy cicadas
- Show More Articles
New antibiotic class effective against multidrug-resistant bacteria
Scientists at Uppsala University have discovered a new class of antibiotics with potent activity against multi-drug resistant bacteria, and have shown that it cures bloodstream infections in mice. The new antibiotic class is described in an article in the scientific journal PNAS .
Antibiotics are the foundation of modern medicine and over the last century have dramatically improved the lives of people around the world. Nowadays we tend to take antibiotics for granted and rely heavily on them to treat or prevent bacterial infections, including for example, to reduce the risk of infections during cancer therapy, during invasive surgery and transplants, and in mothers and preterm babies. Increasingly though, the global rise in antibiotic resistance threatens their effectiveness. In order to ensure access to effective antibiotics in the future, development of novel therapeutics to which there is no existing resistance is essential.
Researchers at Uppsala University have recently published their work in the Proceedings of the National Academy of Sciences of the USA describing a new class of antibiotics developed as a part of multi-national consortia. The class of compounds they describe target a protein, LpxH, which is used in a pathway by Gram-negative bacteria to synthesize their outermost layer of protection from the environment, called lipopolysaccharide. Not all bacteria produce this layer, but those that do include the organisms that have been identified by the World Health Organization as being the most critical to develop novel treatments for, including Escherichia coli and Klebsiella pneumoniae that have already developed resistance to available antibiotics. The researchers were able to show that this new antibiotic class is highly active against multidrug-resistant bacteria and was able to treat bloodstream infections in a mouse model, demonstrating the promise of this class. Importantly, since this compound class is completely new and the protein LpxH has not yet been exploited as a target for antibiotics there is no pre-existing resistance to this class of compounds. This is in contrast to the many 'me-too' antibiotics of existing classes currently in clinical development. While the current results are very promising there will be considerable additional work required before compounds of this class will be ready for clinical trials.
The work to discover and develop this new class of antibiotics was supported by the EU project ENABLE which was funded through the Innovative Medicines Initiative's New Drugs 4 Bad Bugs program (ND4BB). The ENABLE project, led by researchers at Uppsala University and the pharmaceutical company GlaxoSmithKline, brought together stakeholders from across Europe representing academia and large and small pharmaceutical companies to pool resources and expertise to advance early-stage antibiotic development. This antibiotic class now continues to be developed in the follow-on project, ENABLE-2, an antibiotic drug discovery platform funded by Swedish Research Council, the National Research Programme on Antibiotic Resistance and Sweden's innovation agency Vinnova to continue the momentum generated by the original ENABLE project.
- Pharmaceuticals
- Infectious Diseases
- Pharmacology
- Wounds and Healing
- Microbes and More
- Biotechnology
- Antibiotic resistance
- Penicillin-like antibiotics
- Antiviral drug
- Drug discovery
- Rocky Mountain spotted fever
- Breastfeeding
- Pharmaceutical company
Story Source:
Materials provided by Uppsala University . Note: Content may be edited for style and length.
Journal Reference :
- Douglas L. Huseby, Sha Cao, Edouard Zamaratski, Sanjeewani Sooriyaarachchi, Shabbir Ahmad, Terese Bergfors, Laura Krasnova, Juris Pelss, Martins Ikaunieks, Einars Loza, Martins Katkevics, Olga Bobileva, Helena Cirule, Baiba Gukalova, Solveiga Grinberga, Maria Backlund, Ivailo Simoff, Anna T. Leber, Talía Berruga-Fernández, Dmitry Antonov, Vivekananda R. Konda, Stefan Lindström, Gustav Olanders, Peter Brandt, Pawel Baranczewski, Carina Vingsbo Lundberg, Edgars Liepinsh, Edgars Suna, T. Alwyn Jones, Sherry L. Mowbray, Diarmaid Hughes, Anders Karlén. Antibiotic class with potent in vivo activity targeting lipopolysaccharide synthesis in Gram-negative bacteria . Proceedings of the National Academy of Sciences , 2024; 121 (15) DOI: 10.1073/pnas.2317274121
Cite This Page :
Explore More
- Long Snouts Protect Foxes Diving Into Snow
- Promising Experimental Type 1 Diabetes Drug
- Giant, Prehistoric Salmon Had Tusk-Like Teeth
- Plants On the Menu of Ancient Hunter-Gatherers
- Unexpected Differences in Binary Stars: Origin
- Flexitarian: Invasive Species With Veggies
- T. Rex Not as Smart as Previously Claimed
- Asteroid Ryugu and Interplanetary Space
- Mice Think Like Babies
- Ancient Maya Blessed Their Ballcourts
Trending Topics
Strange & offbeat.
share this!
April 29, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Researchers discover new lantibiotic produced by staphylococci
by Inka Väth, University Hospital Bonn
Researchers at the University Hospital Bonn (UKB), the University of Bonn, and the German Center for Infection Research (DZIF) have discovered a new lantibiotic, namely epilancin A37. It is produced by staphylococci that colonize the skin and act specifically against their main competitor there, the corynebacteria.
This specificity is presumably mediated by a very special mechanism of action, which the researchers were able to decipher in detail. Their results have now been published in the ISME Journal .
Due to increasing antibiotic resistance in pathogens causing infections, the development of new antibacterial substances is important. Hopes are pinned on a new group of substances produced by gram-positive bacteria, the lantibiotics. These are antimicrobial peptides that often have a very narrow spectrum of activity.
"Such compounds are highly interesting from a medical point of view, as they could specifically attack individual groups of organisms without affecting the entire bacterial flora, as is the case with broad-spectrum antibiotics , for example," says corresponding author Dr. Fabian Grein, until recently head of the DZIF research group "Bacterial Interference" at the Institute of Pharmaceutical Microbiology at the UKB and member of the Transdisciplinary Research Area (TRA) "Life & Health" at the University of Bonn.
Essential competitive advantage over corynebacteria
The UKB research team led by Fabian Grein and Tanja Schneider, together with the team led by Ulrich Kubitscheck, Professor of Biophysical Chemistry at the University of Bonn, have now discovered a new lantibiotic, namely epilancin A37. It is produced by staphylococci, which are typical colonizers of the skin and mucous membranes. Little is known about these antimicrobial peptides.
"We were able to show that epilating is widespread in staphylococci, which underlines their ecological importance," says first author Jan-Samuel Puls, a doctoral student from the University of Bonn at the Institute of Pharmaceutical Microbiology at the UKB. This is because staphylococci and corynebacteria are important genera of the human microbiota—i.e., the totality of all microorganisms such as bacteria and viruses—in the nose and skin, which are closely linked to health and disease.
The need to produce such a compound indicates a pronounced competition between the species. The researchers were able to show that the newly discovered epilancin A37 acts very specifically against corynebacteria, which are among the main competitors of staphylococci within the skin microbiome.
New mode of action in the 'bacterial war' decoded
"This specificity is presumably mediated by a very special mechanism of action that we were able to decipher in detail," says Grein. Epilancin A37 penetrates the corynebacterial cell, initially without destroying it. The antimicrobial peptides accumulate in the cell and then dissolve the cell membrane from the inside, thus killing the corynebacterium.
Co-author Dr. Thomas Fließwasser from the Institute of Pharmaceutical Microbiology at the UKB, a postdoctoral researcher at the University of Bonn and acting head of the DZIF research group "Bacterial Interference" adds, "Our study shows how a specific mechanism of action can be used to combat a single bacterial species specifically. It, therefore, serves us as a 'proof of concept'."
Journal information: ISME Journal
Provided by University Hospital Bonn
Explore further
Feedback to editors

Unveiling nature's custodians: Study highlights crucial role of scavengers in wetlands
22 minutes ago

Too many vehicles, slow reactions and reckless merging: New math model explains how traffic and bacteria move
4 hours ago

Study says California's 2023 snowy rescue from megadrought was a freak event. Don't get used to it
11 hours ago

'Sour Patch' adults: 1 in 8 grown-ups love extreme tartness, study shows

Long snouts protect foxes when they dive headfirst into snow, study finds
12 hours ago
Laser imaging could offer early detection for at-risk artwork

Tibetan plateau had broader social dimensions than previously thought, suggests study
13 hours ago

Machine learning classifies 191 of the world's most damaging viruses

Theoretical biologists test two modes of social reasoning and find surprising truths in simplicity

It's all in the smile: New research finds politicians can influence voters with facial expressions
Relevant physicsforums posts, is 5 milliamps at 240 volts dangerous.
15 hours ago
The Cass Report (UK)
Apr 24, 2024
Major Evolution in Action
Apr 22, 2024
If theres a 15% probability each month of getting a woman pregnant...
Apr 19, 2024
Can four legged animals drink from beneath their feet?
Apr 15, 2024
Mold in Plastic Water Bottles? What does it eat?
Apr 14, 2024
More from Biology and Medical
Related Stories
New insights into how epilancin 15X kills bacteria
Feb 6, 2024

How blocking cell wall formation stops bacterial cell division
Mar 23, 2023
Antibiotic intercepts building blocks of the bacterial envelope
Mar 20, 2020

Study finds tomato juice's antimicrobial properties can kill Salmonella
Jan 30, 2024
A wound dressing that kills bacteria
Aug 11, 2020
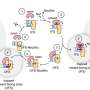
Researchers discover important membrane transport mechanism in pathogenic bacteria
Jan 8, 2024
Recommended for you

Quantum fiber optics in the brain enhance processing, may protect against degenerative diseases
16 hours ago
Novel mechanisms for cleavage-independent activation of gasdermins revealed
Vaccinia virus: New insights into the structure and function of the poxvirus prototype
Scientists reveal how SID-1 recognizes dsRNA and initiates systemic RNA interference
Cartilage healing discovery in animal models could lead to new human therapies
18 hours ago
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
‘Just in case’ antibiotics widely overused during COVID-19, says UN health agency
Facebook Twitter Print Email
Antibiotics saw “extensive overuse” globally among hospitalised COVID-19 patients during the pandemic without improving clinical outcomes, while also potentially increasing the already serious and growing threat of antimicrobial resistance from "superbugs" , the UN World Health Organization (WHO) said on Friday.
In an alert, WHO noted that although just eight per cent of hospitalised coronavirus patients also had bacterial infections which can be treated with antibiotics, a staggering three in four were given them on a “just in case” basis.
At no point during the global pandemic did the UN health agency recommend using antibiotics to treat COVID-19, insisted WHO spokesperson Dr. Margaret Harris.
Viral, not bacterial
“The advice was very clear from the start that this was a virus. So, it wasn’t that there was any guidance or any recommendation that that clinicians go in this direction, but perhaps because people were dealing with something completely new, they were looking for whatever they thought might be appropriate.”
According to the UN health agency, antibiotic use ranged from 33 per cent for patients in the Western Pacific region to 83 per cent in the Eastern Mediterranean and the African regions. Between 2020 and 2022, prescriptions decreased over time in Europe and the Americas, but they increased in Africa.
Data compiled by WHO also indicated that most antibiotics were given to critically ill COVID-19 patients, at a global average of 81 per cent. Antibiotic use in mild or moderate infections showed considerable variation across regions, with highest use in Africa, at 79 per cent.
Worryingly, the UN agency found that the most frequently prescribed bacteria-busting antibiotics globally were those with higher potential for antimicrobial resistance (AMR).
“When a patient requires antibiotics, the benefits often outweigh the risks associated with side effects or antibiotic resistance . However, when they are unnecessary, they offer no benefit while posing risks , and their use contributes to the emergence and spread of antimicrobial resistance,” said Dr. Silvia Bertagnolio, WHO unit head for surveillance, evidence and laboratory strengthening division for AMR.
No positive impact
The UN health agency report maintained that antibiotic use “did not improve clinical outcomes for patients with COVID-19” .
Instead, their systematic prescription “might create harm for people without bacterial infection, compared to those not receiving antibiotics,” WHO said in a statement.
“These data call for improvements in the rational use of antibiotics to minimise unnecessary negative consequences for patients and populations.”
The findings were based on data from the WHO Global Clinical Platform for COVID-19, a database of anonymous clinical data from patients hospitalised with the coronavirus. Data came from 450,000 patients in 65 countries from January 2020 to March 2023.
Antimicrobial resistance threatens the prevention and treatment of an ever-increasing range of infections caused by bacteria, fungi, parasites and viruses.
It occurs when these bacteria, fungi, parasites and viruses change over time and no longer respond to medicines, making infections harder to treat and increasing the risk of disease spread, severe illness and death. As a result, the medicines become ineffective, and infections persist in the body, increasing the risk of spread to others.
Antimicrobials – including antibiotics, antivirals, antifungals and antiparasitics – are medicines used to prevent and treat infections in humans, animals and plants. Micro-organisms that develop antimicrobial resistance are sometimes referred to as “superbugs”.
- antibiotic resistance
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.14(10); 2022 Oct

A Systematic Review of Antibiotic Resistance Trends and Treatment Options for Hospital-Acquired Multidrug-Resistant Infections
Walter y agyeman.
1 Internal Medicine, Piedmont Athens Regional Medical Center, Georgia, USA
2 Internal Medicine, California Institute of Behavioral Neurosciences & Psychology, Fairfield, USA
Aakash Bisht
3 Internal Medicine, Government Medical College, Amritsar, Amritsar, IND
Ankit Gopinath
4 Internal Medicine, Kasturba Medical College, Manipal, Manipal, IND
Ameer Haider Cheema
Keyur chaludiya, maham khalid, marcellina nwosu, srujana konka, safeera khan.
Antimicrobial resistance is a major public health challenge described by the World Health Organization as one of the top 10 public health challenges worldwide. Drug-resistant microbes contribute significantly to morbidity and mortality in the hospital, especially in the critical care unit. The primary etiology of increasing antibiotic resistance is inappropriate and excessive use of antibiotics. The alarming rise of drug-resistant microbes worldwide threatens to erode our ability to treat infections with our current armamentarium of antibiotics.
Unfortunately, the pace of development of new antibiotics by the pharmaceutical industry has not kept up with rising resistance to expand our options to treat microbial infections. The costs of antibiotic resistance include death and disability, extended hospital stays due to prolonged sickness, need for expensive therapies, rising healthcare expenditure, reduced productivity from time out of the workforce, and rising penury. This review sums up the common mechanisms, trends, and treatment options for hospital-acquired multidrug-resistant microbes.
Introduction and background
Hospital-acquired multidrug-resistant microbes are a significant cause of morbidity and mortality, especially in the critical care unit. It is a significant public health threat that prolongs hospital stays and increases healthcare costs. In 2019, an estimated 4.95 million deaths were associated with bacterial antimicrobial resistance alone [ 1 ]. Approximately, 1.2 million deaths per year are directly attributable to bacterial antimicrobial resistance alone [ 1 , 2 ].
Antibiotic resistance is classified into three broad groups according to the sensitivity pattern to the different antibiotic classes, namely, pan-drug-resistant (PDR), extensively drug-resistant (XDR), and multidrug-resistant (MDR) microbial infections. MDR microbes are resistant to at least one agent from three or more antibiotic classes. XDR microbes are resistant to at least one agent from each antibiotic class except two or fewer classes. Lastly, PDR microbes are resistant to an agent from all antibiotic classes [ 3 ].
The rising incidence of MDR microbes is a safety concern for patients, clinicians, and healthcare administrators. Risk factors for acquiring MDR infections are associated with medical treatment and healthcare facilities, i.e., recent antibiotic use (<90 days), catheter or medical device carriage, and prolonged stay in a healthcare facility [ 4 ]. Hospital-acquired or nosocomial infections occur at least 48 hours after admission in a healthcare delivery setting, including hospitals and long-term care facilities. They may also arise after discharge from a healthcare facility [ 5 ]. Nosocomial infections put patients and healthcare staff at risk. Long-term care facilities are a proposed connecting link in spreading MDR infections between the hospital and the community [ 6 ].
The emergence of antimicrobial resistance is a result of the indiscriminate use of antibiotics in the healthcare, veterinary, and agricultural industries. Wrong antibiotic choice, inadequate dosing, and unnecessarily extended treatment drive antibiotic resistance within hospitals and other healthcare settings, such as nursing homes and the community. Much work has been done to describe antimicrobial resistance genes. However, we need to interrogate the trends in antimicrobial resistance and treatment options for MDR infections to inform and guide public health policy, antimicrobial stewardship programs, and clinical treatment guidelines. This systematic review describes recent antibiotic resistance trends and treatment options for hospital-acquired MDR infections.
Reporting guideline
This systematic review was written according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines [ 7 ].
Databases and search strategy
A systematic search was conducted using PubMed, PubMed Central (PMC), ScienceDirect, and Cochrane Library register for all databases on January 16, 2022. The field search was done on PubMed using Medical Subject Headings (MeSH) and keywords. We searched the other databases using the keywords hospital-acquired infection, cross-infection, microbial drug resistance, and treatment. PubMed Search Builders were created using the Boolean scheme, as shown in Table Table1 1 .
We pooled the keywords using the Boolean term “OR” and combined their corresponding search builders that we obtained from PubMed using MeSH terms. In addition, we applied restrictions to MeSH-major topics. All concepts and keywords were combined into a final search strategy using the Boolean term “AND,” as shown in Table Table2 2 .
Inclusion and exclusion criteria
The population of interest includes patients admitted to the hospital for at least 48 hours with a culture or antigen/polymerase chain reaction (PCR)-confirmed microbial diagnosis. Our intervention is any novel antibiotic treatment. The comparator is the standard of care antibiotic treatment, and the outcome of interest is recovery as defined by clinical and microbiological cure-culture negative after antibiotic treatment.
The literature search was conducted to identify relevant studies that examine antibiotic resistance trends and treatment for hospital-acquired MDR infections. Inclusion criteria were studies conducted on the adult population and published in English as full-text papers in the past five years. Studies in the pediatric population, unpublished literature, papers older than 2016, irrelevant, non-full-text, gray, case reports, editorials, and non-English reports were excluded.
Screening of articles
After obtaining the relevant articles from the databases, we removed duplicates using Microsoft Excel. We subsequently screened the articles based on title, abstract, and reading full-text articles. Articles were screened based on their likelihood of yielding clinically significant practice changes as determined by the writing committee. Finally, we subjected all short-listed articles to a quality appraisal.
Quality appraisal
As displayed in Table Table3, 3 , we assessed the short-listed articles for quality and risk of bias using tools depending on the study type. Each assessment tool had its criteria and scoring. A score of at least 60% for each assessment tool was accepted.
A total of 2,895 articles were found upon employing the appropriate keywords. A total of 306 duplicates were filtered out before screening; 2,589 articles underwent the screening process, of which 2,432 articles were removed based on their titles and abstracts. The authors retrieved 157 articles to assess the full text for relevancy and screened 108 reports for eligibility. In total, 37 articles were finally included in the review upon an in-depth analysis of quality, inclusion/exclusion criteria, and study designs. The first two authors conducted the data extraction and appraised the studies independent of each other. Whenever there arose a difference of opinion, the writing committee settled the outcome. The search strategy and the process of selecting the final studies included in this review are depicted in Figure Figure1 1 .

PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Because of the variability, such as heterogeneity of participants, interventions, and outcome measures, between studies, this systematic review describes these trials and reviews based on their outcomes, applicability, and limitations on a narrative synthesis rather than conducting a meta-analysis.
Two independent investigators (the first and second author) performed article selection, assessment, and analyses in each step. If there was a contradictory result regarding an article’s eligibility, its full text was assessed by consensus within the group.
We evaluated randomized controlled trials (RCTs) in this study using the Cochrane Collaboration risk of bias tool. Seven RCTs were reviewed. Four were included, and three were rejected due to at least one substantial risk of bias in any domain. The results of the quality assessment are shown in Table Table4 4 .
We evaluated six systematic reviews using the Assessing the Methodological Quality of Systematic Reviews 2 (AMSTAR 2) criteria., We utilized a passing score of 60% as our cut-off for acceptance. Three articles were included, and three were rejected. The results are displayed in Table Table5 5 .
AMSTAR: Assessing the Methodological Quality of Systematic Reviews
We also reviewed 47 narrative review articles using the Scale for the Assessment of Narrative Review Articles 2 (SANRA 2). A passing score of 70% was utilized as the cut-off. A total of 26 articles were included while 21 narrative review articles were rejected. The results are summarized in Table Table6 6 .
0 (low standard), 1 (moderate standard), 2 (high standard).
SANRA: Scale for the Assessment of Narrative Review Articles
A total of 10 observational/cohort studies were reviewed using the Ottawa Quality Assessment Scale for Cohort Studies. We selected a passing score of 80% as the cut-off. Four studies were included in our final analysis and six studies were not accepted. The results are displayed in Table Table7 7 .
0 - 0 star, 1 - 1 star, and 2 - 2 stars according to Newcastle-Ottawa Scale.
Antibiotic Resistance Trends
The various mechanisms by which bacteria can become resistant to an antimicrobial agent due to the diverse resistance genes possessed by different microbial species are being elucidated [ 24 , 66 ]. Antibiotic therapy boosts the emergence of MDR strains through selection pressure and the transfer of genetic resistance elements. Drug resistance arises via de novo mutations during antibiotic use and horizontal transfer of genes via the acquisition of plasmids, transposons, and transferable genetic elements. These antibiotic resistance genes (ARGs) involve altered target binding sites, increased efflux pump activity, enzyme induction, and reduced porins. Extensive drug resistance and pan-drug resistance arise from accumulating multiple resistance gene elements [ 8 ]. The main antibiotic resistance mechanisms include active efflux pumps, beta-lactamases, carbapenemases, vancomycin resistance gene (Van A) ligases, and porin deficiency. Various gene families, such as the Ambler ampicillin hydrolyzing class C ( AmpC ), TEM-1, SHV-1, cefotaxime hydrolyzing gene( CTX-M ), and oxacillin hydrolyzing gene ( OXA ), encode beta-lactamases common in bacteria such as Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa , and Enterobacter spp . Staphylococcal cassette chromosome mecA ( SCCmecA ) and Mer7 are transferable genetic elements possessed by Staphylococcus aureus strains that encode methicillin resistance.
Enterococci are ubiquitous in the environment and present in the natural gut microbiome. Two clinically significant species are Enterococcus faecalis and Enterococcus faecium . Enterococcus faecium is more responsible for fatal invasive hospital-associated infections. Antimicrobial resistance is prevalent in 80-100% of E. faecium isolates compared to at most 16% of E. faecalis isolates [ 36 ]. Enterococci have obtained high-level β-lactam resistance through modification of the penicillin-binding protein gene, resulting in decreased β-lactam affinity and increased β-lactam tolerance due to upregulation of gene expression.
Glycopeptide-resistant enterococci spp . have become clinically significant due to the resistance conferred by gene resistance elements of the Van family. Risk factors for vancomycin-resistant enterococci infection include invasive gastrointestinal, pulmonary, and urologic procedures, indwelling medical devices, and exposure to fourth-generation cephalosporins. The vancomycin ( Van ) resistance gene family ( Van A, B, C, D, G, H, and L ) encodes enzymes that lead to decreased affinity at the glycopeptide binding site and substitution of the normal precursors, which end in D-Ala-D-Ala amino acid sequence [ 24 , 66 ]. The Van H gene, also possessed by vancomycin-resistant Staphylococcus aureus (VRSA), encodes a dehydrogenase that converts pyruvate to D-Lac . The Van A gene encodes a ligase which forms an ester bond between D-Ala and D-Lac. Vancomycin can only combine with the D-Ala-D-Ala binding site but not with the D-Ala-D-Lac binding site, thus leading to vancomycin resistance.
K. pneumoniae , belonging to the Enterobacteriaceae family, naturally inhabits the intestinal microbiome. In one study of MDR K. pneumoniae isolates in Eastern and South-Western Europe, 50-60% of isolates were resistant to fluoroquinolones, third-generation cephalosporins, and aminoglycosides [ 30 ]. The prevalence rate of resistance to commonly used antibiotics in K. pneumoniae was 40-80% of isolates in one study in Asia [ 19 , 22 ]. Colistin resistance was identified in 2.9% of K. pneumoniae isolates in Asia [ 19 ]. The global spread of hypervirulent K. pneumoniae strains with extensive antibiotic resistance is worrying. Half of hypervirulent K. pneumoniae infections affect patients who are non-elderly and do not have comorbidities, with a mortality rate of up to 40% [ 22 ].
K. pneumoniae resistance is driven by the accumulation of antibiotic resistance genes leading to XDR strains harboring a super resistome. A super resistome may encompass combinations of carbapenemase genes with aminoglycoside-modifying enzymes or association of CTX-M or New Delhi metalloproteinase( NDM ) carbapenemases, 16S ribosomal ribonucleic acid ( rRNA ) methylases together with porin deficiency and quinolone resistance chromosomal mutations [ 22 ]. K. pneumoniae can acquire or transfer mobile genetic elements such as transposons from other gram negatives, including E. coli and Serratia marcescens . Gene elements belonging to the NDM , VIM , IMP-1 ,and KPC ( K. pneumoniae carbapenemases) enzyme families encode carbapenemases that have an increased activity giving rise to extended-spectrum beta-lactamase (ESBL) Enterobacteriaceae. These enzymes can hydrolyze extended-spectrum cephalosporins. They confer resistance to commonly used beta-lactam antibiotics such as ceftazidime, ceftriaxone, and cefotaxime. Plasmid-mediated resistance genes of all classes have also been identified in K. Pneumoniae . The armA gene family encodes for enzymes that prevent aminoglycosides from binding to their 16S rRNA target. Other known plasmids gene-mediated 16S rRNA methylases include the Rmt family and NpmA gene families.
Chromosomal resistance mechanisms that have evolved against aminoglycosides in K. pneumoniae include alterations in AcrAB-TolC and KpnEF efflux pump systems and loss of porins. For fluoroquinolone resistance, the major mechanism is chromosomal mutations in the quinolone binding targets on DNA gyrase involving the gyrA-gyrB subunits and topoisomerase IV involving the parC-parE subunits. These mutations are also seen in other gram negatives such as P. aeruginosa [ 57 ]. Overall, fluoroquinolone resistance rates vary geographically but range from 30% to 40% in many countries [ 43 ]. Moreover, the K. pneumoniae plasmid encodes the aac and qnr subfamily of genes chromosomally encoded in other gram negatives such as Citrobacter spp., Stenotrophomonas maltophilia , and S. marcescens that confer resistance to aminoglycoside, fluoroquinolones, and beta-lactams [ 19 ].
Porin-mediated resistance in P. aeruginosa occurs through mechanisms that downregulate the transcription of the oprD gene leading to the deficiency of porins in the outer membrane resulting in decreased susceptibility. Hydrophilic antibiotics, such as β-lactams, tetracyclines, aminoglycosides, and some fluoroquinolones, have been shown to traverse the outer membrane via porins. On the other hand, a decrease in intracellular antibiotic concentration can occur via extrusion through efflux pumps on the membrane. Efflux pumps are classified into six superfamilies. The superfamilies contain (a) the ATP-binding cassette (ABC) superfamily, (b) the small multidrug resistance (SMDR) superfamily, (c) the major facilitator (MF) superfamily, (d) the resistance-nodulation-division (RND) superfamily, (e) the multidrug and toxic compound extrusion (MTCE) superfamily, and (f) the drug metabolite transporter (DMT) superfamily.
P. aeruginosa is widely prevalent in the hospital environment and usually causes colonization of the alimentary and respiratory tracts. A history of previous rectal colonization is typically present in most patients developing infections. Recent antibiotic therapy is a significant risk factor for rectal colonization by MDR P. aeruginosa in critically ill patients [ 39 , 59 ]. Intestinal colonization and previous use of antibiotics are key risk factors for P. aeruginosa infections. Pathogen-related factors that determine a worse outcome of P. aeruginosa infections include the presence of certain horizontally acquired genomic islands; infection by specific clonal lineages; and expression of virulence factors, such as elastase, type III secretion system (T3SS), and the production of cytotoxins [ 39 ]. Host factors such as age, immunosuppression, and underlying disease influence the outcome of Pseudomonas infections. Delayed adequate antimicrobial therapy is also independently associated with increased mortality. In one study, single-agent susceptibility rates for the 11,701 non-duplicate P. aeruginosa isolates ranged from 72.7% for fluoroquinolones and 85.0% for piperacillin-tazobactam [ 71 ]. Susceptibility rates were higher for blood isolates than for respiratory isolates [ 39 ]. The increasing prevalence of MDR or XDR P. aeruginosa isolates is associated with the spread of high-risk clones, such as ST175 [ 59 ].
Acinetobacter spp . is a gram-negative, non-fermenting coccobacillus strictly aerobic, oxidase-negative, catalase-positive, pleomorphic, and non-motile [ 41 ]. These bacteria are widespread in the environment in soil, water, and sewage. Acinetobacter baumannii causes opportunistic nosocomial infections involving patients on mechanical ventilation in intensive care units. A. baumannii can colonize new surfaces by the formation of a biofilm. Although polymyxin-resistant A. baumannii represents less than 1% of clinical isolates, its widespread dissemination, multidrug resistance, and multiple virulence factors make it a severe threat to public health worldwide [ 62 ]. It has been shown using molecular techniques that A. baumannii outbreaks have been primarily due to specific clones [ 41 ]. A. baumannii commonly has extensive resistance to penicillins, cephalosporins, tetracyclines, macrolides, chloramphenicol, fluoroquinolones, aminoglycosides, and carbapenems. Polymyxins are the antibiotic of last resort to treat infections caused by XDR A. baumannii . Polymyxin acts by disrupting membrane integrity through the displacement of divalent cations in the outer membrane by binding to the lipopolysaccharide (LPS) and causing cell lysis. Unfortunately, lineages with low sensitivity to polymyxins have increased in Europe, Asia, and South America [ 63 ].
Polymyxin resistance in A. baumannii is attributed to changes in the outer membrane through phosphoethanolamine addition, loss of LPS, changes in osmoprotective amino acids, and overexpression of efflux pumps. Inactivation of the lipid A biosynthetic genes, lpxA , lpxC , or lpxD , results in a complete loss of surface LPS. Thus, the loss of LPS prevents the essential interaction between it and polymyxins [ 25 , 63 ]. Mutations identified in the pmr family of genes are also associated with colistin resistance. This family of genes encodes enzymes involved in the synthesis of lipid A, a component of LPS. Modification of lipid A protects the outer membrane from the binding and action of polymyxins. A. baumannii also possesses genes expressing efflux pumps related to antibiotic resistance, including resistance-nodulation division (RND), major facilitator (MF), multidrug-toxic compound extrusion (MATE), and small multidrug resistance (SMR) families. The mcr -encoding plasmid found initially in E. coli in China has been subsequently reported worldwide in other gram-negative bacteria, including A. baumannii [ 25 ].
Fungal Antimicrobial Resistance
The growing incidence of fungal infections in the hospital environment is alarming. Fungi are normal commensals on the human body but can cause invasive infections, particularly in immunocompromised patients. Risk factors for invasive fungal infection include the presence of a central venous catheter, invasive catheterization, diabetes mellitus, immunosuppression, receiving total parenteral nutrition, recent surgery, extended hospital stay, prolonged admission to the ICU, and having received broad-spectrum antibiotics [ 29 , 38 ]. Nosocomial outbreaks due to relatively uncommon fungal species such as Exserohilum rostratum and Sarocladium kiliense have occurred following the contamination of medical products [ 49 ]. However, significant MDR fungemia is usually caused by Candida spp ., including Candida glabrata, Candida parapsilosis , and Candida auris .
C. auris , an MDR yeast species, is undoubtedly the most problematic species because of its ability to form a biofilm, colonize patients, and persist in the healthcare environment. First reported in 2009 in a Japanese patient, C. auris cases have since been reported, as of February 15, 2021, in 47 countries on all inhabited continents. C. auris isolates have been classified using whole-genome sequencing into four geographically distinct clonal populations [ 29 ]. C. auris is clinically significant because it has demonstrated resistance to multiple antifungal drugs, with some isolates resistant to all major antifungal classes (azoles, polyenes, and echinocandins). C. auris candidemia is associated with a 30-60% mortality rate. The transmissibility and extensive antifungal resistance characteristic of C. auris set it apart from other Candida species. In the United States, approximately 90% of isolates have been resistant to fluconazole, 30% to amphotericin B, and 5% to echinocandins compared to 10% of C. glabrata isolates exhibiting fluconazole resistance, and less than 10% exhibit echinocandin resistance [ 38 ].
In one study, 41% of patients received systemic antifungal therapy when C. auris was isolated. The median time from admission to infection was 19 days, 61% of patients had bloodstream infections, and 59% died. Interestingly, 41% of isolates were resistant to two antifungal classes, and 4% were resistant to three classes of antifungals [ 74 ]. In another study at a large tertiary hospital in China, the average detection rate was 0.29% over a decade. Non- Candida albicans was the main fungus, accounting for 62.5% of isolates. The drug resistance of non- Candida albicans was higher than that of C. albicans , among which C. glabrata had the highest resistance rate [ 38 ]. Molecular mechanisms underlying resistance in Candida species include Erg11 mutations, which mediate fluconazole resistance. Efflux pump activity also contributes to azole resistance. It is hypothesized that FKS mutations observed in C. auris isolates, such as the S639F mutation, are responsible for micafungin resistance. A mutation in a gene involved in ergosterol biosynthesis mediates resistance to amphotericin B via a reduction in ergosterol content in the fungal cell wall [ 29 ].
Biofilms are aggregates of microorganism communities that adhere irreversibly to abiotic or biotic surfaces through the production of extracellular polymeric material [ 40 ]. The self-produced polymeric matrix facilitates the formation of complex structures that promote antibiotic resistance through horizontal gene transfer and persister cells that result in chronic or recurring infections. Persister cells are dormant cells within biofilms that can tolerate high concentrations of antibiotic agents [ 45 ]. Biofilms play an essential role in healthcare-associated bacterial and fungal infections. They are more resistant to antimicrobials due to their (a) physiological state, (b) cell density, (c) quorum sensing abilities, (d) protective extracellular matrix, (e) upregulation of drug efflux pumps, (f) increased expression of resistance genes, and (g) presence of persister cells. The significance of the drug efflux pump mechanism in biofilms was observed in a study involving C. albicans that showed that strains lacking Cdr1p, Cdr2p , and Mdr1p pumps were more susceptible to fluconazole at the initial stages of biofilm formation compared to the wildtype. Again, the expression of the efflux pump, AfuMDR4 , was notably upregulated in vivo upon exposure to voriconazole [ 45 ].
Treatment options
The use of an antibiotic policy fosters improved prescribing practices and evidence-based antimicrobial use. An antimicrobial stewardship program involves a multifaceted and multidisciplinary approach to achieving the following goals: (a) controlling antimicrobial resistance, (b) improving clinical outcomes, and (c) reducing costs by improving antimicrobial use [ 56 ]. The core components of an antibiotic policy must include antimicrobial stewardship, especially the development of prescribing guidelines and standards of care, as well as infection prevention strategies such as hand hygiene, hospital cleaning, and disinfection. Active surveillance is required in outbreaks of MDR, XDR, and PDR infections. Antibiotic resistance surveillance and comparisons of prescribing practices are beneficial feedback activities once effectively communicated to healthcare practitioners [ 23 ].
One RCT identified high-certainty evidence that interventions in antimicrobial stewardship programs enabled physicians to improve their antibiotic prescribing practices, reduced the length of stay in hospitals by 1.12 days, and did not increase mortality [ 12 ]. Interventions were categorized into restrictive techniques, which incorporate policies to make physicians prescribe properly, and enablement techniques, which provide feedback and advice to help physicians prescribe properly. Enablement interventions were more effective in improving prescribing practices [ 12 ]. The best current intervention for optimizing antibiotic use is to have clear guidelines for using an antibiotic regimen. The antibiotic regimen selected should have the highest efficacy for a confirmed infection. The benefits of such intervention include improved clinical cure rates, less antibiotic toxicity, fewer Clostrididoides difficile infections, less disruption of the gut microbiome, and fewer MDR infections. The overarching goal is to provide timely, appropriate antibiotics while avoiding antimicrobial resistance. Timely initial appropriate antibiotics are a critical determining factor of outcomes in severe infections. Several studies have demonstrated that inappropriate initial antimicrobial therapy was independently associated with increased mortality and extended hospital stay [ 12 , 56 ].
Appropriate initial antimicrobial therapy can be achieved using a local antibiogram or rapid molecular identification methods. Keeping a local antibiogram is imperative to guide and periodically review antimicrobial stewardship programs. An antibiogram records the overall profile of antimicrobial susceptibility testing results of specific microbes to a battery of antimicrobial drugs. It helps to guide empiric treatment while microbiology culture and sensitivity results are pending. Additionally, they can be used to detect, monitor, and investigate trends in antimicrobial resistance. Rapid microbiological identification methods, such as PCR, are currently in clinical use to identify resistance genes and quickly guide initial targeted narrow-spectrum antibiotic treatment until final microbiological culture and sensitivity results are known. The ability to determine susceptibility patterns in hours rather than in days is handy to the clinician, especially in severely ill patients or those with bacteremia. The drawback is that these methods do not differentiate colonization from infection.
Non-antibiotic Measures
The healthy microbiota provides protective functions, including preventing colonization and infection via competitive pressure. Antibiotic exposure is associated with disrupting the microbiota that selects for resistance in the gut microbiome. Novel methods to exploit protective mechanisms provided by intact microbiota may provide the key to preventing the spread of MDR organisms in the healthcare setting [ 55 , 66 ]. It is hypothesized that probiotics may effectively decolonize and prevent MDR infections by promoting healthy intestinal microbiota. Some evidence-based analyses from various human studies and animal models have shown the clinical potential of probiotics against infectious diseases, diarrhea, intestinal infections, inflammatory diseases, and antibiotic-associated diarrhea. These studies suggest that it is possible to counteract microbial colonization and antimicrobial resistance spread [ 42 ]. However, this is yet to be proven by an RCT. In several RCTs, probiotic drugs were ineffective for decolonizing hospitalized patients harboring MDR gram-negative bacilli and preventing subsequent infections. They did not reduce the in-hospital length of stay, the incidence of adverse events, and in-hospital mortality rates [ 11 ].
Antibiotic Measures
Decolonization is a strategy to reduce the incidence of healthcare-associated infections. Decolonization involves the use of topical antimicrobial agents to reduce the bacterial burden on specific sites of the human body, including the nares and the skin. There have been only a few multicenter, randomized trials evaluating decolonization. Of the few that exist, even fewer have compared decolonizing agents head-to-head to determine the superiority of an agent or a decolonizing protocol [ 50 ]. The most robust evidence for decolonization is to prevent surgical site infections among surgical patients. The populations that benefit the most from decolonization are cardiac and orthopedic surgery patients. The common agents used for decolonization include chlorhexidine, mupirocin, and povidone-iodine [ 18 , 50 ]. Mupirocin is used for nasal decolonization for methicillin-resistant Staphylococcus aureus (MRSA). Chlorhexidine gluconate (CHG) is the decolonization agent with the most substantial evidence base for oral and skin cleansing. A meta-analysis revealed that 2% chlorhexidine bathing significantly reduced hospital-acquired infection incidence and MDR organisms in ICUs [ 18 ].
The reducing sensitivity of MRSA, coagulase-negative Staphylococcus spp. , and Enterococcus species to vancomycin is a worrisome threat [ 36 ]. The minimum inhibitory concentration (MIC) creep phenomenon is a notable cause of increasing resistance to vancomycin often occurring because of underdosing and excessive use. MIC creep refers to the gradual but steady increase in the levels of MIC standards for MRSA isolates. This results in poor clinical response, high relapse rates, and treatment failures. The two leading alternatives for vancomycin-resistant enterococci (VRE) and VRSA treatment are linezolid and daptomycin, with clinical success rates of 50-80% as a first-line drug and 50-59% as salvage therapy for VRE bacteremia, respectively [ 75 ].Enterococci spp. commonly have intrinsic resistance to penicillin monotherapy. However, susceptibility increases when antibiotics with activity against the bacterial cell wall, such as β-lactams, are used synergistically. Double β-lactam therapy is effective in enterococci endocarditis, although no studies have shown efficacy for such therapy in other sites such as deep-seated abscesses and osteomyelitis. Again, ampicillin, an aminopenicillin, is remarkably effective against enterococci infections when used in synergy with gentamicin, an aminoglycoside. Nephrotoxicity commonly limits the use of gentamicin.
For gram-negative bacteria, ceftolozane/tazobactam is especially active against P. aeruginosa (from the intrinsic activity of ceftolozane, a semi-synthetic fifth-generation cephalosporin). In contrast, the addition of tazobactam confers activity against most ESBL)producers. It is approved to treat complicated urinary tract infections, intra-abdominal infections, and nosocomial pneumonia. Avibactam is a novel β-lactamase inhibitor that inactivates class A [including K. pneumoniae carbapenemase ( KPC )], class C ( AmpC ), and some class D ( OXA ) β-lactamases. The combination of ceftazidime/avibactam inhibited 82% and 76% of MDR and XDR strains, respectively. The susceptibility of P. aeruginosa toward ceftazidime increases from 65% to 94% when used in combination with avibactam [ 59 ]. Another novel β-lactamase, relebactam, inhibits Ambler class A and class C cephalosporinases, effectively boosting imipenem activity against resistant K. pneumoniae carbapenemase (KPC) and P. aeruginosa [ 77 ]. The combination of imipenem, cilastatin, and relebactam is approved for the treatment of complicated intra-abdominal infections and complicated urinary tract infections.
Newer antibiotics discovered in the last decade include cefiderocol, plazomicin, and eravacycline. Cefiderocol is a novel siderophore cephalosporin that binds to ferric iron which is required for bacterial growth and virulence. Cefiderocol is actively transported across the outer membrane resulting in high concentrations in the periplasmic space, where it exerts a bactericidal effect by binding to penicillin-binding proteins and inhibiting cell wall synthesis. It has been shown to have potent in-vitro activity against MDR gram-negative bacteria including Enterobacterales (>90% of isolates), P. aeruginosa , A. baumannii, and Stenotrophomonas maltophilia [ 78 ]. However, cefiderocol has a label warning for higher all-cause mortality versus other antibiotics in critically ill patients with MDR gram-negative bacteria with a mortality rate of 34% for cefiderocol vs. 18% in the best-available therapy group [ 78 ]. Plazomicin is a synthetic aminoglycoside approved by the U.S. Food and Drug Administration (FDA) for complicated urinary tract infections active against >95% of Enterobacterales isolates. It is active against ESBL isolates and against 84.6% to 97.6% of carbapenem-resistant isolates. The presence of aminoglycoside-modifying enzymes does not inactivate plazomicin, and it is active against 52.2% of isolates that are resistant to three members of the aminoglycoside drug class [ 79 ]. Eravacycline is a fluorocycline of the tetracycline class. The FDA has approved eravacycline for the treatment of complicated intrabadominal infections. Eravacycline is active against ESBL E. coli and K. pneumoniae . It has activity against A. baumannii andcarbapenem-resistant Enterobacterales but has limited activity against P. aeruginosa . Eravacycline has been also investigated for complicated urinary tract infections but showed lower cure rates (84.8% vs. 94.8%) and (60.4% vs. 66.9%) than ertapenem and levofloxacin, respectively [ 79 ].
The overall incidence of non-ventilator hospital-acquired pneumonia was 1.6%, representing a rate of 3.63 per 1,000 patient days in the United States [ 34 ]. In one study, ceftazidime/avibactam was non-inferior to meropenem to treat healthcare-associated pneumonia/ventilator-associated bacterial pneumonia [ 53 ]. It is a valid option against carbapenem-resistant Enterobacteriaceae (CRE). Meropenem/vaborbactam, another novel therapeutic option, displayed a non-significant trend toward lower mortality in patients with CRE infections and penetrated well into the lung [ 53 ]. Vaborbactam inhibits class A and C β-lactamases but not class B or D lactamases. In one study, there was no statistical difference in all-cause mortality between monotherapy and combination therapy to treat people with ventilator-associated pneumonia (VAP) [ 10 ]. In VAP, a short treatment course of about seven days is validated, even though a longer treatment course may still be recommended for patients with a slower clinical response. Usually, carbapenem monotherapy is used for VAP, including MDR strains. However, there was no statistical difference in all-cause mortality between carbapenem and non-carbapenem therapies. However, carbapenems are associated with a statistically significant increase in clinical cures [ 10 ]. On the other hand, a meta-analysis identified significantly higher superinfection with imipenem than non-carbapenems. Superinfection is a new microbial infection occurring after or in addition to an earlier infection usually following treatment with broad-spectrum antibiotics. Superinfection was statistically higher when carbapenems were used compared to other antipseudomonal beta-lactams [ 20 ].
Drug concentrations in the airways can be 100-fold higher when antibiotics are administered through the aerosol route in mechanically ventilated patients. Several studies demonstrate a reduction of bacterial load and a good safety profile with aerosolized colistin, or aminoglycosides compared to the intravenous route [ 8 , 25 ]. Accordingly, aerosolized antibiotics are now increasingly used, especially in gram-negative VAP and, more specifically, with MDR strains. However, one rational approach for XDR strains, especially A. Baumannii is to consider combining colistin and carbapenem therapy, particularly when carbapenem MICs are elevated [ 25 ]. This approach attempts to supplement the therapeutic effect of the last-line polymyxin therapy with systemic therapy with a carbapenem or another agent.
In VAP caused by MDR A. baumannii , treatment with intravenous meropenem, colistin, and nebulized tobramycin was just as effective as intravenous meropenem, injectable colistin, and nebulized colistin, with no significant difference between clinical pulmonary infection score and creatinine level in both groups, suggesting that nebulized tobramycin is non-inferior to nebulized colistin [ 8 ]. Finally, in cases of non-bacteremic XDR Acinetobacter spp. pneumonia, the addition of inhaled colistin minimizes toxicity and maximizes levels delivered to the lung.
Treatment of Fungal Infections
C. auris infections pose a real treatment challenge due to the formation of biofilms and resistance mechanisms. Echinocandins are the recommended first-line treatment in adults. An alternative is liposomal amphotericin B. Antifungal susceptibility testing is required to inform targeted treatment. Outbreaks of Candida require hypervigilance, rapid diagnostic methods, and new molecular typing tools such as whole-genome sequencing (WGS), prompt investigation, and aggressive interventions, including notification of public health agencies [ 38 , 49 ]. For a suspected C. auris infection, the Centers for Disease Control and Prevention (CDC) recommends the identification of species from non-sterile sites when there is an invasive disease, colonization, or infection is detected in a unit or facility, or when a patient has had an overnight stay within the previous year in a healthcare facility in a country with documented C. auris transmission [ 29 ].
Alternative Treatments
Antimicrobial lock therapy (ALT) is an alternative therapy for biofilm-related infections associated with medical devices such as central vein catheters. ALT involves instilling antimicrobial agents, which exceed the MIC by 100- to 1,000-fold within an intravascular catheter lumen. The antibiotic will stay locked over a specific time, usually 24 hours for most agents [ 40 ]. Its efficacy is disputed, given the lack of source control. However, ALT is used when central catheters cannot be removed for clinical reasons. Several clinical studies indicate that ALT is an effective method for preventing Candida colonization without removing catheters but is yet to be confirmed by a large RCT [ 40 ]. In cases of Candida fungemia, however, removal of the catheter remains the standard of care.
Future directions
Antimicrobial coatings are promising options to eradicate biofilm-related infections. Medical devices typically associated with biofilm formation are coated with anti-biofilm layers to prevent the adherence of microbes. These coated surfaces serve as contact-killing surfaces preventing the formation of biofilms, as observed in cases of central line-associated bloodstream infections, catheter-associated urinary tract infections, and VAPs. Nanotechnology is a complementary therapeutic agent that employs quantum-dot, carbon nanotubes, and carbon-based nanoparticles (NPs) to disrupt biofilms and deliver antimicrobials directly to the targeted cells or pathogens without drug degradation. NPs are considered promising alternatives to antibiotics and effective against gram-positive and gram-negative bacteria. Natural NPs, polymer-based nanomaterials, and metallic NPs are cost-effective and may be exploited as antimicrobial coatings on the surface of medical devices for various biomedical applications [ 45 ].
Antimicrobial photodynamic therapy (aPDT), or photodynamic activation (PDI), is an alternative treatment modality for localized biofilm infections. Its mechanism of action results from synergism between non-toxic photosensitizer dye, molecular oxygen, and visible light. The principle behind aPDT is that exposure to a light source at a specific wavelength triggers the photosensitizer dyes to generate sufficient reactive oxygen species from molecular oxygen that cause damage and microbial cell death without exerting toxic effects in the host. These modalities are relatively low cost, and widespread adoption will further increase their cost-effectiveness.
Limitations
Although this review is based on a systematic analysis of the medical literature, it reflects the inherent biases of the writing team. We have summarized the most compelling areas of current investigations based on the literature and the experience of the writing committee. As new research becomes available, additions to the priorities of this research agenda should be considered.
Conclusions
Clinicians and scientists have long realized the remarkable ability of microorganisms to survive via evolving resistance to antibiotics. Infections caused by MDR/XDR strains are a cause of concern as they compromise the selection of appropriate empiric and definitive antimicrobial treatments. The knowledge about the variety of molecular mechanisms of antimicrobial resistance has expanded tremendously via advances in genomics and proteomics. Many molecular mechanisms that promote resistance have been elucidated; however, novel antibiotic drug development has not kept pace in tandem. The judicious use of antibiotics through antimicrobial stewardship, good clinical practice, and good public health practices are imperative to stem the tide of increasing drug resistance. There is a need for fundamental studies to answer questions regarding the development and use of new antibiotics and novel strategies for treating and preventing MDR/XDR/PDR bacterial infections.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.

IMAGES
VIDEO
COMMENTS
New class of antibiotics is a welcome discovery. Known as tethered macrocyclic peptides, a new class of antibiotics show potent activity against drug-resistant strains of Acinetobacter baumannii ...
To provide an overview of current research on optimised antimicrobial use in humans a core team (9 of the listed co-authors) gathered evidence through a narrative review of published and selected grey literature. ... The global antibiotic research and development partnership (GARDP): researching and developing new antibiotics to meet global ...
Antibacterial drugs launched from January 2013 to December 2022. In the last 10 years, 19 new small molecule antibacterial drugs (eight NP-derived and 11 synthetic-derived) and four new BL/BLI ...
A recent initiative that supports SMEs in the late-stage development of new antibiotics is the AMR Action Fund, which was launched by more than 20 leading biopharmaceutical companies to push ...
An estimated 0.9-1.7 million deaths per year are already attributable to antimicrobial resistance and, at the current rate of spreading resistance, this number could increase to as many as 10 million by 2050 (Antimicrobial Resistance Collaborators, 2022 ).
In its recent annual report on global risks, the World Economic Forum (WEF) concluded that "arguably the greatest risk . . . to human health comes in the form of antibiotic-resistant bacteria.
Predicting antimicrobial resistance (AMR) profiles from whole-genome sequences of pathogenic bacteria: current strategies, challenges, and future outlook. Provides a forum to explore solutions to antibiotic resistance, development and delivery to improve the health of the global population.
Infections caused by antibiotic-resistant bacteria (ARB) are one of the major global health challenges of our time. In addition to developing new antibiotics to combat ARB, sensitizing ARB, or pursuing alternatives to existing antibiotics are promising options to counter antibiotic resistance. This review compiles the most promising anti-ARB ...
The researchers described their model and experimental validation of these new compounds in a study published March 22 in the journal Nature Machine Intelligence. "There's a huge public health need to develop new antibiotics quickly," said James Zou, PhD, an associate professor of biomedical data science and co-senior author on the study ...
Discussion. The global burden associated with drug-resistant infections assessed across 88 pathogen-drug combinations in 2019 was an estimated 4·95 million (95% UI 3·62-6·57) deaths, of which 1·27 million (0·911-1·71) deaths were directly attributable to drug resistance.
Reading time: 2 min (429 words) WHO has published its first global research agenda for the world's scientists to address the most urgent human health priorities to combat antimicrobial resistance (AMR). It outlines 40 research topics on drug-resistant bacteria, fungi and Mycobacterium tuberculosis that must be answered by 2030, in line with ...
Credit: Nature Chemical Biology (2024). DOI: 10.1038/s41589-023-01525-w. Scientists at the University of Illinois Chicago and Harvard University have developed an antibiotic that could give ...
Antibiotics. , Volume 11, Issue 1 (January 2022) - 121 articles. Cover Story ( view full-size image ): Nosocomial and medical-device-induced biofilm infections affect millions of lives and urgently require innovative preventive approaches. Antimicrobial peptides (AMPs) are ideal candidates for this fight.
Bacterial vaccines, along with antibiotics, are a crucial tool in the fight against deadly microbes. In the latest paper, Huang announced several discoveries that will help the development of a ...
Current treatments often fail despite long bouts with three to four antibiotic combinations for 12 to 18 months -- more than half of the patients are not cured, yet more than 70% of the patients ...
New decade, new you: Encourage patients to follow these 10 health tips. Help patients kick the 2020s off to a roaring, healthy start with this timely advice from the AMA. Antibiotic resistance occurs when bacteria changes in response to antibiotic treatment. Stay current on antibiotic resistance with the latest articles on AMA.
Of all the inappropriately prescribed antibiotics dispensed in the last half of 2021, 15% were for a COVID-19 infection. And COVID-19 infections accounted for 2% of all antibiotic prescribing ...
Microbial biofilm formation creates a persistent and resistant environment in which microorganisms can survive, contributing to antibiotic resistance and chronic inflammatory diseases. Increasingly, biofilms are caused by multi-drug resistant microorganisms, which, coupled with a diminishing supply of effective antibiotics, is driving the search for new antibiotic therapies. In this respect ...
Introduction. In 2014, NIAID released a report, NIAID's Antibacterial Research Program: Current Status and Future Directions, emphasizing the Institute's commitment to addressing this urgent public health threat. Since that time, NIAID has made significant progress through a robust and diverse set of activities spanning basic, translational ...
In addition, research involving public investments aimed at the development of new antibiotics has increased in recent years: The Global Antibiotic Research and Development Partnership was created in 2016. Aware of the need to ensure the availability of antibiotics even for patients undergoing chemotherapy or organ transplants, many countries ...
By Andrew Jacobs. April 24, 2024. The Food and Drug Administration on Wednesday approved the sale of an antibiotic for the treatment of urinary tract infections in women, giving U.S. health ...
New research presented at the ESCMID Global Congress (formerly ECCMID) in Barcelona, Spain (27-30 April) has found substantial levels of resistance to critically-important antibiotics in meat ...
A new, Yale-led study suggests that a range of respiratory viral infections — including COVID-19 and influenza — may be preventable or treatable with a generic antibiotic that is delivered to the nasal passageway. A team led by Yale's Akiko Iwasaki and former Yale researcher Charles Dela Cruz successfully tested the effectiveness of ...
26 April 2024. News release. Geneva. Reading time: 2 min (501 words) New evidence from the World Health Organization (WHO) shows the extensive overuse of antibiotics during COVID-19 pandemic worldwide, which may have exacerbated "silent" spread of antimicrobial resistance (AMR). While only 8% of hospitalized patients with COVID-19 had bacterial ...
April 1, 2024. Source: Uppsala University. Summary: Scientists have discovered a new class of antibiotics with potent activity against multi-drug resistant bacteria, and have shown that it cures ...
Researchers at the University Hospital Bonn (UKB), the University of Bonn, and the German Center for Infection Research (DZIF) have discovered a new lantibiotic, namely epilancin A37. It is ...
26 April 2024 Health. Antibiotics saw "extensive overuse" globally among hospitalised COVID-19 patients during the pandemic without improving clinical outcomes, while also potentially increasing the already serious and growing threat of antimicrobial resistance from "superbugs", the UN World Health Organization (WHO) said on Friday. In an ...
In addition, we applied restrictions to MeSH-major topics. ... Recent antibiotic therapy is a significant risk factor for rectal colonization by MDR P. aeruginosa in critically ill patients [39,59]. ... As new research becomes available, additions to the priorities of this research agenda should be considered. ...