Progress in Lipid Research
Volume 4 • Issue 4
- ISSN: 0163-7827
- 5 Year impact factor: 15.7
- Impact factor: 14
- Journal metrics
Publishing Invited ReviewsThe importance of lipids as one of the fundamental classes of biological compounds is well established. The application of our of the biochemistry, ch… Read more


Subscription options
Institutional subscription on sciencedirect.
Publishing Invited Reviews
The importance of lipids as one of the fundamental classes of biological compounds is well established. The application of our of the biochemistry , chemistry and physiology of lipids to biotechnology, the fats and oils industry and medicine have continued to expand apace. In addition new dimensions such as lipid biophysics , especially with relevance to membranes and lipoproteins, and basic liposome research and applications have been added. To cope with all these advances in knowledge a journal is needed to review recent progress in particular fields and to set current research against its historical background. Progress in Lipid Research fulfils this role.
Each volume contains up-to-date surveys of special aspects of lipid research . The invited reviews are comprehensive enough to provide sufficient overview but concentrate on reporting and critically appraising the most recent data. Subjects are chosen for their timeliness or because major developments have taken place in the last few years. They include methodological reviews as well as chemical, biochemical and medical articles. All lipid compounds and derivatives are covered, ranging from fatty acids and other simple molecules, through steroids, terpenoids and phospho- or glycolipids to complex structures such as lipoproteins and biological membranes. We hope that those whose main interest is in lipid biophysics and liposome research will join as new readers, benefiting from the journal's classical aspects of lipid metabolism, lipids in signal transduction and lipid enzymology, and that current readers will benefit from the exposure to top quality research on the new aspects.
PLR solely publishes review articles and submissions are by invitation only. If you have not been invited, but would like to have a review article considered, please send your proposal to the Editorial Office (Ms. Carly Middendorp at [email protected] ), thereby indicating which editor has the most appropriate expertise to handle the manuscript.Proposals must include a short abstract, proposed table of contents/chapters, a representative figure (if relevant) and list of key references. If possible please supply a timeline for submission of your article. After assessment of the proposal by the Editors, we will let you know whether it is suitable for inclusion in the journal.
Editors and their expertise:
Makoto Arita : Polyunsaturated fatty acid (PUFA)-derived mediators; LC-MS/MS-based lipidomics; role of lipid mediators in inflammation and tissue homeostatis; eosinophils; cyclooxygenases, lipoxygenases, and cytochrome P450 monooxygenases. Kent Chapman : Plant lipid metabolism; plant lipid signaling; membranes; oilseeds; lipid storage; compartmentalization; organelle biogenesis; lipid analysis. John Harwood : Metabolism and function of acyl lipids; n-3 polyunsatruated fatty acids; oil accumulation in crops; algal lipids; regulation of metabolism; lipids in disease. Gabor Tigyi : Lysophospholipids, lysophosphatidic acid, sphingosine-1-phosphate, lipid signaling, radiation biology, drug discovery. Markus Wenk : Structure, function and metabolism of membrane lipids; application of lipidomics in drug and biomarker development; role of lipid metabolism in neurobiology.
Progress in Lipid Research

Subject Area and Category
- Biochemistry
- Cell Biology
Elsevier Ltd
Publication type
01637827, 18732194
Information
How to publish in this journal
The set of journals have been ranked according to their SJR and divided into four equal groups, four quartiles. Q1 (green) comprises the quarter of the journals with the highest values, Q2 (yellow) the second highest values, Q3 (orange) the third highest values and Q4 (red) the lowest values.
| Category | Year | Quartile |
|---|---|---|
| Biochemistry | 1999 | Q1 |
| Biochemistry | 2000 | Q1 |
| Biochemistry | 2001 | Q1 |
| Biochemistry | 2002 | Q1 |
| Biochemistry | 2003 | Q1 |
| Biochemistry | 2004 | Q1 |
| Biochemistry | 2005 | Q1 |
| Biochemistry | 2006 | Q1 |
| Biochemistry | 2007 | Q1 |
| Biochemistry | 2008 | Q1 |
| Biochemistry | 2009 | Q1 |
| Biochemistry | 2010 | Q1 |
| Biochemistry | 2011 | Q1 |
| Biochemistry | 2012 | Q1 |
| Biochemistry | 2013 | Q1 |
| Biochemistry | 2014 | Q1 |
| Biochemistry | 2015 | Q1 |
| Biochemistry | 2016 | Q1 |
| Biochemistry | 2017 | Q1 |
| Biochemistry | 2018 | Q1 |
| Biochemistry | 2019 | Q1 |
| Biochemistry | 2020 | Q1 |
| Biochemistry | 2021 | Q1 |
| Biochemistry | 2022 | Q1 |
| Biochemistry | 2023 | Q1 |
| Cell Biology | 1999 | Q1 |
| Cell Biology | 2000 | Q1 |
| Cell Biology | 2001 | Q1 |
| Cell Biology | 2002 | Q1 |
| Cell Biology | 2003 | Q1 |
| Cell Biology | 2004 | Q1 |
| Cell Biology | 2005 | Q1 |
| Cell Biology | 2006 | Q1 |
| Cell Biology | 2007 | Q1 |
| Cell Biology | 2008 | Q1 |
| Cell Biology | 2009 | Q1 |
| Cell Biology | 2010 | Q1 |
| Cell Biology | 2011 | Q1 |
| Cell Biology | 2012 | Q1 |
| Cell Biology | 2013 | Q1 |
| Cell Biology | 2014 | Q1 |
| Cell Biology | 2015 | Q1 |
| Cell Biology | 2016 | Q1 |
| Cell Biology | 2017 | Q1 |
| Cell Biology | 2018 | Q1 |
| Cell Biology | 2019 | Q1 |
| Cell Biology | 2020 | Q1 |
| Cell Biology | 2021 | Q1 |
| Cell Biology | 2022 | Q1 |
| Cell Biology | 2023 | Q1 |
The SJR is a size-independent prestige indicator that ranks journals by their 'average prestige per article'. It is based on the idea that 'all citations are not created equal'. SJR is a measure of scientific influence of journals that accounts for both the number of citations received by a journal and the importance or prestige of the journals where such citations come from It measures the scientific influence of the average article in a journal, it expresses how central to the global scientific discussion an average article of the journal is.
| Year | SJR |
|---|---|
| 1999 | 3.623 |
| 2000 | 3.210 |
| 2001 | 2.786 |
| 2002 | 2.874 |
| 2003 | 4.667 |
| 2004 | 4.609 |
| 2005 | 4.776 |
| 2006 | 5.773 |
| 2007 | 6.859 |
| 2008 | 6.329 |
| 2009 | 4.957 |
| 2010 | 3.740 |
| 2011 | 4.079 |
| 2012 | 4.518 |
| 2013 | 5.054 |
| 2014 | 5.088 |
| 2015 | 5.245 |
| 2016 | 4.726 |
| 2017 | 3.814 |
| 2018 | 4.204 |
| 2019 | 4.682 |
| 2020 | 3.634 |
| 2021 | 2.840 |
| 2022 | 3.158 |
| 2023 | 3.638 |
Evolution of the number of published documents. All types of documents are considered, including citable and non citable documents.
| Year | Documents |
|---|---|
| 1999 | 15 |
| 2000 | 18 |
| 2001 | 15 |
| 2002 | 19 |
| 2003 | 23 |
| 2004 | 20 |
| 2005 | 14 |
| 2006 | 22 |
| 2007 | 16 |
| 2008 | 26 |
| 2009 | 22 |
| 2010 | 34 |
| 2011 | 32 |
| 2012 | 24 |
| 2013 | 41 |
| 2014 | 22 |
| 2015 | 26 |
| 2016 | 43 |
| 2017 | 20 |
| 2018 | 19 |
| 2019 | 25 |
| 2020 | 30 |
| 2021 | 29 |
| 2022 | 32 |
| 2023 | 31 |
This indicator counts the number of citations received by documents from a journal and divides them by the total number of documents published in that journal. The chart shows the evolution of the average number of times documents published in a journal in the past two, three and four years have been cited in the current year. The two years line is equivalent to journal impact factor ™ (Thomson Reuters) metric.
| Cites per document | Year | Value |
|---|---|---|
| Cites / Doc. (4 years) | 1999 | 6.974 |
| Cites / Doc. (4 years) | 2000 | 7.352 |
| Cites / Doc. (4 years) | 2001 | 6.603 |
| Cites / Doc. (4 years) | 2002 | 7.823 |
| Cites / Doc. (4 years) | 2003 | 9.448 |
| Cites / Doc. (4 years) | 2004 | 10.747 |
| Cites / Doc. (4 years) | 2005 | 13.299 |
| Cites / Doc. (4 years) | 2006 | 13.711 |
| Cites / Doc. (4 years) | 2007 | 16.063 |
| Cites / Doc. (4 years) | 2008 | 14.597 |
| Cites / Doc. (4 years) | 2009 | 12.859 |
| Cites / Doc. (4 years) | 2010 | 12.640 |
| Cites / Doc. (4 years) | 2011 | 12.224 |
| Cites / Doc. (4 years) | 2012 | 12.728 |
| Cites / Doc. (4 years) | 2013 | 14.188 |
| Cites / Doc. (4 years) | 2014 | 12.809 |
| Cites / Doc. (4 years) | 2015 | 13.622 |
| Cites / Doc. (4 years) | 2016 | 12.814 |
| Cites / Doc. (4 years) | 2017 | 11.326 |
| Cites / Doc. (4 years) | 2018 | 12.486 |
| Cites / Doc. (4 years) | 2019 | 13.259 |
| Cites / Doc. (4 years) | 2020 | 16.084 |
| Cites / Doc. (4 years) | 2021 | 17.415 |
| Cites / Doc. (4 years) | 2022 | 17.262 |
| Cites / Doc. (4 years) | 2023 | 16.483 |
| Cites / Doc. (3 years) | 1999 | 6.974 |
| Cites / Doc. (3 years) | 2000 | 7.150 |
| Cites / Doc. (3 years) | 2001 | 5.957 |
| Cites / Doc. (3 years) | 2002 | 7.500 |
| Cites / Doc. (3 years) | 2003 | 10.269 |
| Cites / Doc. (3 years) | 2004 | 11.386 |
| Cites / Doc. (3 years) | 2005 | 12.242 |
| Cites / Doc. (3 years) | 2006 | 14.702 |
| Cites / Doc. (3 years) | 2007 | 15.429 |
| Cites / Doc. (3 years) | 2008 | 12.692 |
| Cites / Doc. (3 years) | 2009 | 12.750 |
| Cites / Doc. (3 years) | 2010 | 10.516 |
| Cites / Doc. (3 years) | 2011 | 12.390 |
| Cites / Doc. (3 years) | 2012 | 12.920 |
| Cites / Doc. (3 years) | 2013 | 13.411 |
| Cites / Doc. (3 years) | 2014 | 13.031 |
| Cites / Doc. (3 years) | 2015 | 13.161 |
| Cites / Doc. (3 years) | 2016 | 12.742 |
| Cites / Doc. (3 years) | 2017 | 10.165 |
| Cites / Doc. (3 years) | 2018 | 12.135 |
| Cites / Doc. (3 years) | 2019 | 14.756 |
| Cites / Doc. (3 years) | 2020 | 16.203 |
| Cites / Doc. (3 years) | 2021 | 16.176 |
| Cites / Doc. (3 years) | 2022 | 16.333 |
| Cites / Doc. (3 years) | 2023 | 15.352 |
| Cites / Doc. (2 years) | 1999 | 6.240 |
| Cites / Doc. (2 years) | 2000 | 6.034 |
| Cites / Doc. (2 years) | 2001 | 4.364 |
| Cites / Doc. (2 years) | 2002 | 8.394 |
| Cites / Doc. (2 years) | 2003 | 11.000 |
| Cites / Doc. (2 years) | 2004 | 9.619 |
| Cites / Doc. (2 years) | 2005 | 12.791 |
| Cites / Doc. (2 years) | 2006 | 14.118 |
| Cites / Doc. (2 years) | 2007 | 12.389 |
| Cites / Doc. (2 years) | 2008 | 12.711 |
| Cites / Doc. (2 years) | 2009 | 9.190 |
| Cites / Doc. (2 years) | 2010 | 10.521 |
| Cites / Doc. (2 years) | 2011 | 12.071 |
| Cites / Doc. (2 years) | 2012 | 11.515 |
| Cites / Doc. (2 years) | 2013 | 13.839 |
| Cites / Doc. (2 years) | 2014 | 11.108 |
| Cites / Doc. (2 years) | 2015 | 13.238 |
| Cites / Doc. (2 years) | 2016 | 11.396 |
| Cites / Doc. (2 years) | 2017 | 9.130 |
| Cites / Doc. (2 years) | 2018 | 13.159 |
| Cites / Doc. (2 years) | 2019 | 14.487 |
| Cites / Doc. (2 years) | 2020 | 14.795 |
| Cites / Doc. (2 years) | 2021 | 14.673 |
| Cites / Doc. (2 years) | 2022 | 14.881 |
| Cites / Doc. (2 years) | 2023 | 14.820 |
Evolution of the total number of citations and journal's self-citations received by a journal's published documents during the three previous years. Journal Self-citation is defined as the number of citation from a journal citing article to articles published by the same journal.
| Cites | Year | Value |
|---|---|---|
| Self Cites | 1999 | 2 |
| Self Cites | 2000 | 2 |
| Self Cites | 2001 | 5 |
| Self Cites | 2002 | 13 |
| Self Cites | 2003 | 7 |
| Self Cites | 2004 | 7 |
| Self Cites | 2005 | 11 |
| Self Cites | 2006 | 5 |
| Self Cites | 2007 | 3 |
| Self Cites | 2008 | 11 |
| Self Cites | 2009 | 17 |
| Self Cites | 2010 | 16 |
| Self Cites | 2011 | 13 |
| Self Cites | 2012 | 11 |
| Self Cites | 2013 | 29 |
| Self Cites | 2014 | 13 |
| Self Cites | 2015 | 15 |
| Self Cites | 2016 | 19 |
| Self Cites | 2017 | 9 |
| Self Cites | 2018 | 8 |
| Self Cites | 2019 | 14 |
| Self Cites | 2020 | 9 |
| Self Cites | 2021 | 10 |
| Self Cites | 2022 | 24 |
| Self Cites | 2023 | 17 |
| Total Cites | 1999 | 272 |
| Total Cites | 2000 | 286 |
| Total Cites | 2001 | 280 |
| Total Cites | 2002 | 360 |
| Total Cites | 2003 | 534 |
| Total Cites | 2004 | 649 |
| Total Cites | 2005 | 759 |
| Total Cites | 2006 | 838 |
| Total Cites | 2007 | 864 |
| Total Cites | 2008 | 660 |
| Total Cites | 2009 | 816 |
| Total Cites | 2010 | 673 |
| Total Cites | 2011 | 1016 |
| Total Cites | 2012 | 1137 |
| Total Cites | 2013 | 1207 |
| Total Cites | 2014 | 1264 |
| Total Cites | 2015 | 1145 |
| Total Cites | 2016 | 1134 |
| Total Cites | 2017 | 925 |
| Total Cites | 2018 | 1080 |
| Total Cites | 2019 | 1210 |
| Total Cites | 2020 | 1037 |
| Total Cites | 2021 | 1197 |
| Total Cites | 2022 | 1372 |
| Total Cites | 2023 | 1397 |
Evolution of the number of total citation per document and external citation per document (i.e. journal self-citations removed) received by a journal's published documents during the three previous years. External citations are calculated by subtracting the number of self-citations from the total number of citations received by the journal’s documents.
| Cites | Year | Value |
|---|---|---|
| External Cites per document | 1999 | 6.923 |
| External Cites per document | 2000 | 7.100 |
| External Cites per document | 2001 | 5.851 |
| External Cites per document | 2002 | 7.229 |
| External Cites per document | 2003 | 10.135 |
| External Cites per document | 2004 | 11.263 |
| External Cites per document | 2005 | 12.065 |
| External Cites per document | 2006 | 14.614 |
| External Cites per document | 2007 | 15.375 |
| External Cites per document | 2008 | 12.481 |
| External Cites per document | 2009 | 12.484 |
| External Cites per document | 2010 | 10.266 |
| External Cites per document | 2011 | 12.232 |
| External Cites per document | 2012 | 12.795 |
| External Cites per document | 2013 | 13.089 |
| External Cites per document | 2014 | 12.897 |
| External Cites per document | 2015 | 12.989 |
| External Cites per document | 2016 | 12.528 |
| External Cites per document | 2017 | 10.066 |
| External Cites per document | 2018 | 12.045 |
| External Cites per document | 2019 | 14.585 |
| External Cites per document | 2020 | 16.063 |
| External Cites per document | 2021 | 16.041 |
| External Cites per document | 2022 | 16.048 |
| External Cites per document | 2023 | 15.165 |
| Cites per document | 1999 | 6.974 |
| Cites per document | 2000 | 7.150 |
| Cites per document | 2001 | 5.957 |
| Cites per document | 2002 | 7.500 |
| Cites per document | 2003 | 10.269 |
| Cites per document | 2004 | 11.386 |
| Cites per document | 2005 | 12.242 |
| Cites per document | 2006 | 14.702 |
| Cites per document | 2007 | 15.429 |
| Cites per document | 2008 | 12.692 |
| Cites per document | 2009 | 12.750 |
| Cites per document | 2010 | 10.516 |
| Cites per document | 2011 | 12.390 |
| Cites per document | 2012 | 12.920 |
| Cites per document | 2013 | 13.411 |
| Cites per document | 2014 | 13.031 |
| Cites per document | 2015 | 13.161 |
| Cites per document | 2016 | 12.742 |
| Cites per document | 2017 | 10.165 |
| Cites per document | 2018 | 12.135 |
| Cites per document | 2019 | 14.756 |
| Cites per document | 2020 | 16.203 |
| Cites per document | 2021 | 16.176 |
| Cites per document | 2022 | 16.333 |
| Cites per document | 2023 | 15.352 |
International Collaboration accounts for the articles that have been produced by researchers from several countries. The chart shows the ratio of a journal's documents signed by researchers from more than one country; that is including more than one country address.
| Year | International Collaboration |
|---|---|
| 1999 | 6.67 |
| 2000 | 22.22 |
| 2001 | 0.00 |
| 2002 | 0.00 |
| 2003 | 4.35 |
| 2004 | 25.00 |
| 2005 | 21.43 |
| 2006 | 13.64 |
| 2007 | 6.25 |
| 2008 | 19.23 |
| 2009 | 22.73 |
| 2010 | 32.35 |
| 2011 | 12.50 |
| 2012 | 41.67 |
| 2013 | 31.71 |
| 2014 | 31.82 |
| 2015 | 42.31 |
| 2016 | 48.84 |
| 2017 | 50.00 |
| 2018 | 31.58 |
| 2019 | 52.00 |
| 2020 | 50.00 |
| 2021 | 34.48 |
| 2022 | 40.63 |
| 2023 | 45.16 |
Not every article in a journal is considered primary research and therefore "citable", this chart shows the ratio of a journal's articles including substantial research (research articles, conference papers and reviews) in three year windows vs. those documents other than research articles, reviews and conference papers.
| Documents | Year | Value |
|---|---|---|
| Non-citable documents | 1999 | 0 |
| Non-citable documents | 2000 | 0 |
| Non-citable documents | 2001 | 1 |
| Non-citable documents | 2002 | 1 |
| Non-citable documents | 2003 | 1 |
| Non-citable documents | 2004 | 0 |
| Non-citable documents | 2005 | 0 |
| Non-citable documents | 2006 | 0 |
| Non-citable documents | 2007 | 0 |
| Non-citable documents | 2008 | 0 |
| Non-citable documents | 2009 | 0 |
| Non-citable documents | 2010 | 0 |
| Non-citable documents | 2011 | 0 |
| Non-citable documents | 2012 | 2 |
| Non-citable documents | 2013 | 2 |
| Non-citable documents | 2014 | 2 |
| Non-citable documents | 2015 | 0 |
| Non-citable documents | 2016 | 0 |
| Non-citable documents | 2017 | 0 |
| Non-citable documents | 2018 | 0 |
| Non-citable documents | 2019 | 0 |
| Non-citable documents | 2020 | 0 |
| Non-citable documents | 2021 | 0 |
| Non-citable documents | 2022 | 0 |
| Non-citable documents | 2023 | 0 |
| Citable documents | 1999 | 39 |
| Citable documents | 2000 | 40 |
| Citable documents | 2001 | 46 |
| Citable documents | 2002 | 47 |
| Citable documents | 2003 | 51 |
| Citable documents | 2004 | 57 |
| Citable documents | 2005 | 62 |
| Citable documents | 2006 | 57 |
| Citable documents | 2007 | 56 |
| Citable documents | 2008 | 52 |
| Citable documents | 2009 | 64 |
| Citable documents | 2010 | 64 |
| Citable documents | 2011 | 82 |
| Citable documents | 2012 | 86 |
| Citable documents | 2013 | 88 |
| Citable documents | 2014 | 95 |
| Citable documents | 2015 | 87 |
| Citable documents | 2016 | 89 |
| Citable documents | 2017 | 91 |
| Citable documents | 2018 | 89 |
| Citable documents | 2019 | 82 |
| Citable documents | 2020 | 64 |
| Citable documents | 2021 | 74 |
| Citable documents | 2022 | 84 |
| Citable documents | 2023 | 91 |
Ratio of a journal's items, grouped in three years windows, that have been cited at least once vs. those not cited during the following year.
| Documents | Year | Value |
|---|---|---|
| Uncited documents | 1999 | 2 |
| Uncited documents | 2000 | 3 |
| Uncited documents | 2001 | 5 |
| Uncited documents | 2002 | 3 |
| Uncited documents | 2003 | 1 |
| Uncited documents | 2004 | 2 |
| Uncited documents | 2005 | 0 |
| Uncited documents | 2006 | 0 |
| Uncited documents | 2007 | 0 |
| Uncited documents | 2008 | 0 |
| Uncited documents | 2009 | 1 |
| Uncited documents | 2010 | 0 |
| Uncited documents | 2011 | 0 |
| Uncited documents | 2012 | 1 |
| Uncited documents | 2013 | 2 |
| Uncited documents | 2014 | 2 |
| Uncited documents | 2015 | 1 |
| Uncited documents | 2016 | 3 |
| Uncited documents | 2017 | 2 |
| Uncited documents | 2018 | 4 |
| Uncited documents | 2019 | 2 |
| Uncited documents | 2020 | 0 |
| Uncited documents | 2021 | 0 |
| Uncited documents | 2022 | 0 |
| Uncited documents | 2023 | 2 |
| Cited documents | 1999 | 37 |
| Cited documents | 2000 | 37 |
| Cited documents | 2001 | 42 |
| Cited documents | 2002 | 45 |
| Cited documents | 2003 | 51 |
| Cited documents | 2004 | 55 |
| Cited documents | 2005 | 62 |
| Cited documents | 2006 | 57 |
| Cited documents | 2007 | 56 |
| Cited documents | 2008 | 52 |
| Cited documents | 2009 | 63 |
| Cited documents | 2010 | 64 |
| Cited documents | 2011 | 82 |
| Cited documents | 2012 | 87 |
| Cited documents | 2013 | 88 |
| Cited documents | 2014 | 95 |
| Cited documents | 2015 | 86 |
| Cited documents | 2016 | 86 |
| Cited documents | 2017 | 89 |
| Cited documents | 2018 | 85 |
| Cited documents | 2019 | 80 |
| Cited documents | 2020 | 64 |
| Cited documents | 2021 | 74 |
| Cited documents | 2022 | 84 |
| Cited documents | 2023 | 89 |
Evolution of the percentage of female authors.
| Year | Female Percent |
|---|---|
| 1999 | 31.43 |
| 2000 | 29.79 |
| 2001 | 30.77 |
| 2002 | 31.25 |
| 2003 | 29.09 |
| 2004 | 20.34 |
| 2005 | 35.42 |
| 2006 | 32.76 |
| 2007 | 20.00 |
| 2008 | 33.82 |
| 2009 | 30.77 |
| 2010 | 39.68 |
| 2011 | 30.12 |
| 2012 | 42.17 |
| 2013 | 35.82 |
| 2014 | 28.24 |
| 2015 | 40.19 |
| 2016 | 33.54 |
| 2017 | 35.23 |
| 2018 | 31.96 |
| 2019 | 31.71 |
| 2020 | 39.81 |
| 2021 | 44.07 |
| 2022 | 35.16 |
| 2023 | 31.03 |
Evolution of the number of documents cited by public policy documents according to Overton database.
| Documents | Year | Value |
|---|---|---|
| Overton | 1999 | 0 |
| Overton | 2000 | 0 |
| Overton | 2001 | 0 |
| Overton | 2002 | 0 |
| Overton | 2003 | 0 |
| Overton | 2004 | 6 |
| Overton | 2005 | 3 |
| Overton | 2006 | 3 |
| Overton | 2007 | 0 |
| Overton | 2008 | 8 |
| Overton | 2009 | 8 |
| Overton | 2010 | 3 |
| Overton | 2011 | 7 |
| Overton | 2012 | 2 |
| Overton | 2013 | 2 |
| Overton | 2014 | 3 |
| Overton | 2015 | 2 |
| Overton | 2016 | 4 |
| Overton | 2017 | 1 |
| Overton | 2018 | 0 |
| Overton | 2019 | 0 |
| Overton | 2020 | 0 |
| Overton | 2021 | 2 |
| Overton | 2022 | 1 |
| Overton | 2023 | 0 |
Evoution of the number of documents related to Sustainable Development Goals defined by United Nations. Available from 2018 onwards.
| Documents | Year | Value |
|---|---|---|
| SDG | 2018 | 6 |
| SDG | 2019 | 11 |
| SDG | 2020 | 19 |
| SDG | 2021 | 10 |
| SDG | 2022 | 10 |
| SDG | 2023 | 13 |
Leave a comment
Name * Required
Email (will not be published) * Required
* Required Cancel
The users of Scimago Journal & Country Rank have the possibility to dialogue through comments linked to a specific journal. The purpose is to have a forum in which general doubts about the processes of publication in the journal, experiences and other issues derived from the publication of papers are resolved. For topics on particular articles, maintain the dialogue through the usual channels with your editor.

Follow us on @ScimagoJR Scimago Lab , Copyright 2007-2024. Data Source: Scopus®

Cookie settings
Cookie Policy
Legal Notice
Privacy Policy
Progress in Lipid Research
About the journal.
Publishing Invited Reviews The importance of lipids as one of the fundamental classes of biological compounds is well established. The application of our of the biochemistry , chemistry and physiology of lipids to biotechnology, the fats and oils industry and medicine have continued to expand …
View full aims & scope
Article publishing charge for open access
Compare APC with another journal
Editor-in-chief, makoto arita, phd.
RIKEN Center for Integrative Medical Sciences, Yokohama, Japan
Latest published
Articles in press, most downloaded, most popular, more from progress in lipid research, announcements, diversity & inclusion statement – progress in lipid research, special issues and article collections, boyhood in 21st century educative contexts, rethinking educational practices and responsibilities in the light of digitalisation, neoliberalism, education inequity and improvement, motivation of higher education faculty: theoretical approaches, empirical evidence, and future directions, partner journals.
The Progress in Lipid Research is a companion title of the Progress in Lipid Research is an open access, peer-reviewed journal which draws contributions from a wide community of international and interdisciplinary researchers …
Related journals
Educational Research...
Educational Research Review
Learning and Instruc...
Learning and Instruction
Teaching and Teacher...
Teaching and Teacher Education
International Journa...
Learning, Culture an...
Learning, Culture and Social Interaction
Copyright © 2024 Elsevier Ltd. All rights reserved
Progress in Lipid Research
Journal Abbreviation: PROG LIPID RES Journal ISSN: 0163-7827
| Year | Impact Factor (IF) | Total Articles | Total Cites |
| 2023 (2024 update) | 14.0 | 0 | 7497 |
| 2022 | 13.6 | - | 7560 |
| 2021 | 14.673 | - | 7982 |
| 2020 | 16.195 | 30 | 7328 |
| 2019 | 15.083 | 25 | 6139 |
| 2018 | 12.540 | 16 | 5839 |
| 2017 | 8.435 | 20 | 5302 |
| 2016 | 10.583 | 43 | 5097 |
| 2015 | 11.238 | 26 | 4814 |
| 2014 | 10.015 | 22 | 4825 |
| 2013 | 12.963 | 41 | 4382 |
| 2012 | 10.250 | 24 | 3893 |
| 2011 | 10.667 | 30 | 3761 |
| 2010 | 9.510 | 34 | 3265 |
You may also be interested in the following journals
- ► Journal of Lipid Research
- ► Lipids
- ► European Journal of Lipid Science and Technology
- ► Lipids in Health and Disease
- ► Prostaglandins & Other Lipid Mediators
- ► Cell Research
- ► Cell Metabolism
- ► Plos Computational Biology
- ► Nucleic Acid Therapeutics
- ► Pure and Applied Mathematics Quarterly
Top Journals in biology
- Nature Reviews Genetics
- Nature Reviews Microbiology
- Nature Genetics
- Nature Methods
- Cell Stem Cell
- Cell Metabolism
- Annual Review of Biochemistry
- Nature Cell Biology
- Annual Review of Plant Biology
- Trends in Cell Biology
- Cell Host & Microbe
Journal Impact
Search form

Progress In Lipid Research
You may order single or multiple copies of back and recent journal issues. If you are an Author wishing to obtain a printed copy of the journal issue featuring your article, or you require a printed copy for research, review or add to your library, the process is easy –
- Select your journal volume and issue.
- Select the required quantity in the Review cart page
- Provide the shipping details and process the payment.
- Average production time is approx. 2 weeks.
- Your shipping options and general shipping times are: DHL for international - 2-5 postal days and UPS for domestic – 1-6 business days depending on delivery address. . We can track your shipment status at any time.

PROGRESS IN LIPID RESEARCH
- Journal Search
- Journal Details
Note: The following journal information is for reference only. Please check the journal website for updated information prior to submission.
PROG LIPID RES
BIOCHEMISTRY & MOLECULAR BIOLOGY
NUTRITION & DIETETICS
| Category | Quartile | Rank |
|---|---|---|
| Biochemistry, Genetics and Molecular Biology - Biochemistry | Q1 | #7/438 |
| Biochemistry, Genetics and Molecular Biology - Cell Biology | Q1 | #14/285 |
| Science Citation Index Expanded (SCIE) | Social Sciences Citation Index (SSCI) |
|---|---|
| Indexed | - |
| Category (Journal Citation Reports 2024) | Quartile |
|---|---|
| BIOCHEMISTRY & MOLECULAR BIOLOGY | Q1 |
| NUTRITION & DIETETICS | Q1 |
- Popular journals in the same field
- Recent articles
Find Funding. Review Successful Grants.
Explore over 25,000 new funding opportunities and over 6,000,000 successful grants.
Create your own webinar
Interested in hosting your own webinar? Check the schedule and propose your idea to the Peeref Content Team.

- Featured Feed
- Collections
- Become A Contributor
- Account Settings
- My Public Profile
- Feed Subscriptions
- Change My Institution
Use the journals feature with a free QxMD account.
Use Read by QxMD to access full text via your institution or open access sources.
Read also provides personalized recommendations to keep you up to date in your field.
Existing User
New to Read
Sign up for a free QxMD account to keep track of pertinent research with access to hundreds of Journal Publications.
Progress in Lipid Research
All material on this website is protected by copyright, Copyright © 1994-2024 1994-{new Date().getFullYear()} by WebMD LLC. By using this service, you agree to our terms of use and privacy policy .
Save your favorite articles in one place with a free QxMD account.
Search tips.
Use Boolean operators: AND/OR
diabetic AND foot diabetes OR diabetic
Exclude a word using the 'minus' sign
Virchow -triad
Use Parentheses
water AND (cup OR glass)
Add an asterisk (*) at end of a word to include word stems
Neuro* will search for Neurology, Neuroscientist, Neurological, and so on
Use quotes to search for an exact phrase
"primary prevention of cancer" (heart or cardiac or cardio*) AND arrest -"American Heart Association"
We want to hear from doctors like you!
Take a second to answer a survey question.
VIVO Weill Cornell Medical College
This site uses HTML elements that are not recognized by Internet Explorer 8 and below in the absence of JavaScript. As a result, the site will not be rendered appropriately. To correct this, please either enable JavaScript, upgrade to Internet Explorer 9, or use another browser. Here are the instructions for enabling JavaScript in your web browser .
Progress in lipid research Journal
Publication venue for.
- Arachidonic acid metabolism in platelets and endothelial cells. . 20. 1981
- Lipid somersaults: Uncovering the mechanisms of protein-mediated lipid flipping. 2016
- Sphingomyelin and derivatives as cellular signals. 1991
ISO Abbreviation
- Prog Lipid Res
Linking ISSN
International standard serial number (issn), electronic international standard serial number (eissn).
- Search Menu
- Sign in through your institution
- Advance articles
- JALM Talk Podcasts
- Special Issues & Special Collections
- ADLM Guidance Documents
- Author Guidelines
- Submission Site
- Call for Papers
- Self-Archiving Policy
- Why Publish?
- Open Access
- About The Journal of Applied Laboratory Medicine
- Editorial Board
- Advertising & Corporate Services
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, the lipid panel, ldl cholesterol, lipoprotein(a), apolipoprotein b, apolipoprotein a-i, point-of-care lipid testing, ldl subfractions.
- < Previous
ADLM Guidance Document on the Measurement and Reporting of Lipids and Lipoproteins
This document was approved by the Academy Content Development Committee in January 2024, the Academy Council in February 2024, and the ADLM Board of Directors in April 2024.
- Article contents
- Figures & tables
- Supplementary Data
Jing Cao, Leslie Donato, Joe M El-Khoury, Anne Goldberg, Jeffrey W Meeusen, Alan T Remaley, ADLM Guidance Document on the Measurement and Reporting of Lipids and Lipoproteins, The Journal of Applied Laboratory Medicine , Volume 9, Issue 5, September 2024, Pages 1040–1056, https://doi.org/10.1093/jalm/jfae057
- Permissions Icon Permissions
The accurate measurement of blood lipids and lipoproteins is crucial for the clinical management of atherosclerotic disease risk. Despite progress in standardization, there are still significant variations in pre-analytical requirements, methods, nomenclature, and reporting work flows.
The guidance document aims to improve standardization of clinical lipid testing work flows. It provides recommendations for the components of the lipid panel, fasting requirements, reporting of results, and specific recommendations for non-high-density lipoprotein cholesterol (non-HDL-C), low-density lipoprotein cholesterol (LDL-C), lipoprotein(a) [Lp(a)], apolipoprotein B (apo B), point-of-care lipid testing, and LDL subfraction testing.
Lipid panels should always report non-HDL-C and LDL-C calculations if possible. Fasting is not routinely required except in specific cases. Modern equations should be utilized for LDL-C calculation. These equations allow for LDL-C reporting at elevated concentrations of triglycerides and obviate the need for direct measured LDL-C in most cases.
Modern clinical management of atherosclerotic cardiovascular disease (ASCVD) risk depends upon the accurate measurement of blood lipids and lipoproteins. Although great progress has been made in the standardization and harmonization of the methods for the measurement of lipids and lipoproteins, there remains significant heterogeneity in pre-analytical requirements, reference intervals, methods, nomenclature and ordering/reporting work flows. Low-density lipoprotein cholesterol (LDL-C), for example, can be assessed by a wide variety of analytical methods and calculations, each of which has unique limitations or biases. Unfortunately, ambiguous nomenclature in laboratory information systems (LISs) and electronic health records (EHRs) thwart interoperability of LDL-C and other lipid measurements. Some laboratories provide reference intervals as age- and sex-specific normal values, whereas other laboratories report clinical decision thresholds from various clinical guidelines. Finally, there is even less consensus on how to measure and report modern lipoprotein biomarkers like lipoprotein(a) [Lp(a)], apolipoproteinB (apo B), and lipoprotein particle number.
The purpose of this guidance document is to provide an evidence-based reference for use by clinical laboratories to improve standardization of clinical lipid testing work flows. It is our goal that adoption of these recommendations will not only facilitate communication and education among laboratorians but also improve clarity for clinicians and patients.
What Components Should Be Included in the Lipid Panel?
Measurement of blood lipids is essential in diagnosis and treatment of dyslipidemias and related diseases. Throughout this document, serum and plasma are used interchangably as both are acceptable and considered interchangable sample types for lipid and lipoprotein measurement. Due to the interdependence of serum total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG) in patient evaluation, serum lipids are measured as part of a “lipid panel” recognized by the American Medical Association (AMA). In addition to these 3 measures, it is best practice to also calculate and report LDL-C and non-high-density lipoprotein cholesterol (non-HDL-C) ( 1 ).
Multiple practice guidelines endorse the use of non-HDL-C ( 1–4 ), which is simply cholesterol contained in all lipoproteins except for HDL. Therefore, non-HDL-C is the cholesterol carried by all the atherogenic or potentially atherogenic lipoproteins that contribute to the development and progression of atherosclerotic plaques and can be calculated as the difference between TC and HDL-C (Eq. 1) .
In addition to non-HDL-C, calculated LDL-C should be reported if possible (more details below). Many studies have reported on the potential clinical utility of other calculated parameters based on TC, TG and HDL-C (e.g., ratios and lipoprotein fractions); however, to date, no clinical practice guidelines have endorsed the use of any calculations other than LDL-C and non-HDL-C. In summary, standard lipid panels should report 3 measured values: TC, TG, HDL-C, and 2 calculated parameters: LDL-C and non-HDL-C.
How Should the Lipid Panel Be Named?
Ideally, test names for the lipid panel and its components should indicate the specific analyte being measured, the sample obtained, and method used when appropriate ( 4 ). “Total cholesterol” is the preferred term when total serum or plasma cholesterol is measured. It is important to note that this includes both cholesteryl esters and free or non-esterified cholesterol, which are simultaneously measured by most enzymatic assays for total cholesterol. Cholesterol transported by HDL and LDL are best designated as HDL-cholesterol and LDL-cholesterol or abbreviated as HDL-C and LDL-C, respectively. Finally, because there now exists a wide variety of LDL-C methods in routine use, it is best practice to state either calculated or measured (also known as “direct LDL”) in the reporting name. As many estimations for LDL-C now exist (see below), the LDL-C calculation method should also be included in the report comment or laboratory test catalog.
Unlike many other laboratory methods, the reference values for lipids have been defined based on ASCVD outcomes. The concept of “desirable” lipid values was established by the National Cholesterol Education Program (NCEP) and carried forward by multiple medical societies ( 1 ). Reporting a table of lipid thresholds according to ASCVD risk has become common practice for clinical laboratories. However, this may lead to confusion and hinder identification of abnormal results. A single threshold for desirable values corresponding with reduced ASCVD risk is recommended to simplify reporting. However, it should be noted that desirable has been defined between the 50th and 75th percentiles of healthy populations ( Table 1 ), thus a relatively large proportion of patients will be abnormal.
Recommended reference intervals and commenting thresholds for basic lipid panel parameters.
| . | Adults . | Pediatrics . | . | ||
|---|---|---|---|---|---|
| Lipids and lipoproteins . | Reference value, mg/dL . | Population percentile . | Reference value, mg/dL . | Population percentile . | Reporting comments . |
| Total cholesterol | <200 | 50th | <170 | 75th | |
| HDL cholesterol | Female >50 Male >40 | 50th | >45 | 25th | <15 mg/dL refer to lipid specialist |
| Triglycerides | <150 | 50th | Age 2–9 years < 75 Age 10–18 years < 90 | 75th | |
| Non-HDL cholesterol | <130 | 50th | <110 | 75th | >220 consider inherited hyperlipidemia |
| LDL cholesterol | <100 | 50th | <120 | 90th | >190 consider familial hypercholesterolemia |
| Apolipoprotein B | <90 | 50th | <90 | 90th | |
| Lp(a) | <30 mg/dL < 75 nmol/L | NA | NA | ||
| . | Adults . | Pediatrics . | . | ||
|---|---|---|---|---|---|
| Lipids and lipoproteins . | Reference value, mg/dL . | Population percentile . | Reference value, mg/dL . | Population percentile . | Reporting comments . |
| Total cholesterol | <200 | 50th | <170 | 75th | |
| HDL cholesterol | Female >50 Male >40 | 50th | >45 | 25th | <15 mg/dL refer to lipid specialist |
| Triglycerides | <150 | 50th | Age 2–9 years < 75 Age 10–18 years < 90 | 75th | |
| Non-HDL cholesterol | <130 | 50th | <110 | 75th | >220 consider inherited hyperlipidemia |
| LDL cholesterol | <100 | 50th | <120 | 90th | >190 consider familial hypercholesterolemia |
| Apolipoprotein B | <90 | 50th | <90 | 90th | |
| Lp(a) | <30 mg/dL < 75 nmol/L | NA | NA | ||
NA, not applicable.
a Reference values established by NCEP and carried forward by US multi-society guidelines.
b Percentiles based on 2017 National Health and Nutrition Examination Survey (NHANES) data.
c HDL 2.5th percentile, non-HDL-C 95th percentile, LDL-C 95th percentile.
What Are the Expectations for Method Results Agreement and Allowable Error for Lipids?
Based on an expert consensus panel that considered what was analytically achievable and clinically needed, the NCEP established the following total error (bias ± 2SD) goals for the lipid panel tests: TC ≤9%, LDL-C to ≤12%, and HDL-C to ≤15% ( Table 2 ). There are also further recommendations for the individual bias and imprecision for each of these tests ( 5 , 6 ).
Recommended minimal analytical performance specifications for clinical lipid and lipoprotein methods.
| . | Total error, % . | Bias, % . | CV, % . |
|---|---|---|---|
| Cholesterol | ≤9 | ≤±3 | ≤3 |
| Triglycerides | ≤15 | ≤±5 | ≤5 |
| HDL cholesterol | ≤12 | ≤±5 | ≤4 |
| LDL cholesterol | ≤12 | ≤±4 | ≤4 |
| . | Total error, % . | Bias, % . | CV, % . |
|---|---|---|---|
| Cholesterol | ≤9 | ≤±3 | ≤3 |
| Triglycerides | ≤15 | ≤±5 | ≤5 |
| HDL cholesterol | ≤12 | ≤±5 | ≤4 |
| LDL cholesterol | ≤12 | ≤±4 | ≤4 |
According to accuracy-based proficiency surveys with fresh frozen plasma, most assays for TC and TG are within the total error limits recommended by the NCEP. Thus, test results from different laboratories that use different methods for TC and TG will usually not differ substantially and can be considered accurate. Direct homogenous assays for HDL-C and LDL-C also yield results within the total error limit set by the NCEP on most samples when lipids are within their typical range (normolipidemic) but can yield discrepant or inaccurate results on dyslipidemic samples ( 7 ). Depending on the direct assay, a significant bias (negative or positive) can exist due to lack of selectivity of the method for measuring cholesterol in the lipoprotein fraction of interest. Caution should be used when comparing HDL-C and LDL-C measured by different methods, particularly on samples with high TG ( 7 ). It is important to note that most commercial proficiency testing materials are not based on unmodified human plasma and therefore cannot be assumed to be commutable with clinical samples. These commercial proficiency testing results can only be used to compare performance to a peer group for a particular method and do not provide an adequate assessment of accuracy in terms of bias.
Is Fasting Required for Lipid Assessment?
Prior to the 2018 multi-society US guideline on the management of blood cholesterol ( 1 ), it was generally recommended that a fasting sample be obtained for lipid analysis. This was largely driven by the well-established post-prandial increase in TG ( 8–11 ). LDL-C calculated by the Friedewald equation significantly underestimates actual LDL-C when TG is elevated ( 12 ). Fortunately, as described below, modern equations for estimating LDL-C are less vulnerable to inaccuracy caused by elevated TG.
Recent clinical guidelines have lifted the fasting requirement for initial assessment of ASCVD risk based on multiple lines of evidence. First, large studies have repeatedly demonstrated that TC, HDL-C, and LDL-C are minimally affected by fasting, and that the post-prandial increases in TG are modest for most patients ( Table 3 ) ( 8–11 ). Furthermore, longitudinal studies have shown that lipids from a non-fasting blood sample improve ASCVD risk prediction ( 13 , 14 ). Finally, new LDL-C calculations are less affected by TG ( 15 , 16 ).
Maximal mean postprandial change in lipid and lipoproteins.
| Measure . | Pediatrics . | Adults . |
|---|---|---|
| Triglycerides | Increase 0–10 mg/dL | Men: increase 15–30 mg/dL Women: increase 0–20 mg/dL |
| Total cholesterol | No change | Decrease 3–8 mg/dL |
| HDL cholesterol | No change | No change |
| Non-HDL cholesterol | No change | Decrease 3–8 mg/dL |
| LDL cholesterol | Increase <7 mg/dL | Decrease 3–6 mg/dL |
| Apolipoprotein B | Not reported | Decrease <5 mg/dL |
| Measure . | Pediatrics . | Adults . |
|---|---|---|
| Triglycerides | Increase 0–10 mg/dL | Men: increase 15–30 mg/dL Women: increase 0–20 mg/dL |
| Total cholesterol | No change | Decrease 3–8 mg/dL |
| HDL cholesterol | No change | No change |
| Non-HDL cholesterol | No change | Decrease 3–8 mg/dL |
| LDL cholesterol | Increase <7 mg/dL | Decrease 3–6 mg/dL |
| Apolipoprotein B | Not reported | Decrease <5 mg/dL |
a Based on lipid panel data from 12 744 children, age 3 to 17 years ( 9 ).
b LDL calculated using the Friedewald formula.
c Data from general population observational studies including >100 000 subjects ( 8 , 11 , 13 ).
d Women have less impact <20 mg/dL ( 10 ).
Scenarios remain in which a fasting blood sample is required. It is recommended that adult patients with hypertriglyceridemia and pediatric patients with elevated non-HDL-C should be retested on a sample collected after fasting at least 8 h ( 1 , 17 ). Laboratories should work with their practice to build order sets, which allow for lipid panels to be collected both fasting or non-fasting to suit specific patient and provider needs. To prevent confusion in the mixed reporting of fasting and non-fasting results we recommend that the patient fasting status be documented at the time of collection and reported with lipids to aid interpretation ( Fig. 1 ).

Example of a basic lipid panel reporting layout in patient chart.
How Should Lipid Panel Test Results Be Reported by Clinical Laboratories?
Triglycerides.
Chylomicrons and very-low-density lipoproteins (VLDL) are the major carriers of TG. Elevated TG are associated with diabetes, metabolic syndrome, and obesity. Despite these associations, and several large epidemiology studies and Mendelian randomization studies showing associations between TG and ASCVD, a direct and causal relationship between TG and ASCVD is not yet fully established ( 18 ). Measurement of TG also helps differentiate certain types of hereditary dyslipidemias like familial chylomicronemia syndrome, familial combined hyperlipidemia, and familial hypertriglyceridemia. Furthermore, TG is also used by different equations for estimating LDL-C.
Reference intervals for triglycerides were defined in fasting samples based on age-specific percentiles with the 50th percentile defined as normal or acceptable ( Table 1 ). Persistently elevated TG ≥ 175 mg/dL are considered a risk-enhancing factor to be considered when managing ASCVD prevention ( 1 ). Severe hypertriglyceridemia (>500 mg/dL) raises risk for acute pancreatitis, which can be managed using TG-lowering drugs and or dietary interventions ( 19 ).
Fasting status can significantly affect triglyceride concentrations; higher results are observed from samples collected post-prandial compared to those obtained while fasting. It is, therefore, recommended when possible that an indication of fasting status be included with triglyceride test results. A comment suggesting repeat assessment after fasting is reasonable to include when non-fasting TG are >400 mg/dL ( 20 ).
Most widely used methods to quantify triglycerides start by enzymatically hydrolyzing triglycerides to fatty acids and glycerol, which is quantified. Free glycerol in most specimens is typically less than 1 mg/dL and therefore does not usually affect the TG result. Patients with deficiencies in glycerol metabolism, diabetes mellitus, or chronic kidney disease can have falsely high TG results ( 21 ).
Total Cholesterol
TC represents cholesterol from all lipoprotein particles. It includes cholesterol on chylomicrons and their remnants, VLDL, intermediate-density lipoproteins (IDL), LDL, HDL, Lp(a), and lipoprotein X (LpX). TC is minimally influenced by fasting status. The pooled cohort equation endorsed by the US multi-society guidelines on ASCVD risk assessment uses TC to calculate 10-year ASCVD risk in conjunction with HDL-C to compensate for the fact that some cholesterol in TC is not atherogenic ( 22 ). Universal TC screening to identify genetic or lifestyle-related pediatric dyslipidemia is recommended in 9 to 11 year-olds ( 1 , 2 ).
Reference intervals for total cholesterol are based on ASCVD risk with desirable concentrations set at the 50th percentile ( Table 1 ). Enzymatic assays for TC depend on the oxidation of the hydroxyl group on the A-ring of cholesterol and therefore can also detect other sterols like sitosterol. These other sterols, which are obtained from the diet, contribute only a small part of TC but can accumulate in some rare genetic disorders like sitosterolemia ( 23 ). Cholesterol can be differentiated from other sterols by the gas chromatography-based reference method for TC ( 24 ).
High-Density Lipoprotein Cholesterol
Unlike cholesterol on other lipoprotein fractions, HDL-C is inversely related to ASCVD risk. Individuals with low HDL-C appear to be at increased ASCVD risk but the cause–effect association is not fully understood. Nevertheless, HDL-C is a principal factor in ASCVD risk assessment. In general, higher HDL-C is better, however, recent studies have shown a more complicated U-shape relationship with ASCVD risk when HDL-C >100 mg/dL ( 25 ). Above a certain point, higher HDL-C levels may be pro-atherogenic, but this issue is not addressed by current guidelines. Markedly reduced HDL-C in the absence of liver disease suggests familial HDL deficiency such as Tangier disease or lecithin cholesterol acyltransferase (LCAT) deficiency ( 26 ).
Until the advent of direct homogeneous assays, HDL-C was quantified by measuring cholesterol in the supernatant after the manual precipitation of apo B lipoproteins. The fully automated direct HDL-C assays have largely replaced the precipitation methods, but direct HDL-C assays, much like direct LDL-C assays, can also suffer selectivity problems when measured in dyslipidemic samples ( 7 ). Reference intervals for HDL-C are based on age- and sex-specific 50th percentiles with lower values indicating higher risk ( Table 1 ). HDL-C <15 mg/dL (2.5th percentile) warrants a comment to investigate liver disease or potential genetic dyslipidemia ( 26 ).
Non-High Density Lipoprotein Cholesterol
Non-HDL-C encompasses cholesterol in all apo B-containing atherogenic lipoproteins: LDL, IDL, VLDL, Lp(a), and chylomicrons. Initially, non-HDL-C was proposed as an alternative measure of atherogenic cholesterol in cases when elevated TG prevented LDL-C estimation. However, concordance/discordance analyses have shown that non-HDL-C is as good or better at predicting ASCVD events compared with measured or calculated LDL-C in the general population and in patients on statin therapy.
Non-HDL-C is a simple calculation and should be reported in all lipid panels. Clinical practice guidelines recommend non-HDL-C values >190 mg/dL as a risk-enhancing factor indicative of primary hypercholesterolemia ( 1 ). Guideline-based non-HDL-C reference intervals have been somewhat arbitrarily defined at 30 mg/dL higher than LDL-C goals ( Table 1 ) and may benefit from further validation.
In addition to ASCVD management, non-HDL-C may help identify LpX, an abnormal lipoprotein that is found in patients with cholestatic or obstructive liver disease. LpX also forms in patients with LCAT deficiency and appears to be causally related to the development of end-stage renal disease in this disorder. Cholesterol in LpX contributes to non-HDL-C, and a pattern of acute onset elevated non-HDL-C and low HDL-C in a context of liver disease should prompt further investigation by lipoprotein electrophoresis to identify LpX.
Laboratories should program their LIS to calculate and report non-HDL-C and LDL-C for all lipid panels.
All clinical methods for TC, TG, and HDL-C should meet total error limits published by the CDC in normal and dyslipidemic samples ( Table 2 ).
Fasting is not routinely required for lipid panels except in cases of known hypertriglyceridemia.
Order sets should be created that allow for both fasting and non-fasting lipid collections.
Fasting status should be documented at time of collection and reported with any TG results.
What Is the Best Way to Calculate LDL-C?
Until recently, the Friedewald equation was the only calculation widely used for estimating LDL-C (Eq. 2) :
The Friedewald equation requires a fasting sample. The term TG/5 (when in mg/dL) provides an estimate of cholesterol contained in VLDL (VLDL-C). Thus, LDL-C is estimated by subtracting HDL-C and VLDL-C from total plasma cholesterol. A well-known limitation of this equation is that with hypertriglyceridemia VLDL-C is overestimated. The original publication set a hypertriglyceridemia limit of TG <400 mg/dL for use of the Friedewald equation ( 12 ); however, modern studies have demonstrated hypertriglyceridemia leads to LDL-C underestimation at TG >200 mg/dL ( 27 , 28 ).
Given this limitation, other equations have been developed that more accurately calculate LDL-C in samples with much wider ranges of TG concentrations. In 2013, the Martin equation was first described and later it was extended for higher TG samples ( 16 ). It is similar to the Friedewald equation but includes a variable denominator used for estimating VLDL-C (Eq. 3) .
The “X”-denominators are empirically defined and range from <4 to >11 depending on non-HDL-C and TG. In 2020, the Sampson equation was first described (Eq. 4) ( 15 ).
Like the Friedewald and Martin equations, the Sampson equation estimates LDL-C by subtracting the cholesterol contribution from HDL-C and VLDL-C from TC. Like the Martin equation, the Sampson equation adjusts the estimated VLDL-C using a weighted interaction between non-HDL-C and TG. Additionally, the Sampson equation corrects for the presence of chylomicrons with a negative TG 2 factor. It should also be noted that the Friedewald and Sampson equations were derived using the gold-standard beta-quantification LDL-C method as the reference method. The Martin equation was derived using LDL-C measured by vertical autocentrifugation profile as the reference method. Both the Martin and Sampson equations demonstrate improved comparability to measured LDL-C, particularly when LDL-C <70 mg/dL and when TG are elevated up to 800 mg/dL ( 16 , 28 , 29 ).
Based on recent proficiency test surveys, most clinical laboratories are still using the Friedewald equation, even though the Martin and Sampson equations have been shown to be more accurate, particularly on hypertriglyceridemic samples. The Sampson equation is easily implemented in the LIS, it does not require any additional parameters beyond the basic lipid panel, it is in the public domain, does not require a license fee and, when compared to the beta-quantification, it was shown to be the most accurate equation both when TG are high and LDL-C is low ( 15 ).
When Should LDL Cholesterol Be Measured and What Are the Limitations?
Many techniques have been developed for the direct measurement of LDL-C; however, the most widely used assay in clinical laboratories is the enzymatic/colorimetric method. The reason these methods are so popular is because they are commercially available as FDA-approved kits that can be loaded on automated analyzers that require minimal technical expertise or sample preparation and provide a rapid turnaround time. However, these assays are not standardized between different manufacturers and in some cases have been shown to be less reliable than calculating LDL-C ( 30 ). In one study that evaluated the performance of 7 of these direct methods, all methods failed the NCEP accuracy goals (total error goal of ≤12%) for dyslipidemic samples ( 7 ). For this reason, measuring LDL-C by these enzymatic/colorimetric methods is not universally recommended.
Historically, direct LDL-C was recommended in clinical scenarios where calculated LDL-C was less accurate. This included when LDL-C concentrations were <70 mg/dL or in samples with TG ≥400 mg/dL ( 27 , 31 ). However, the newer calculations (extended Martin or Sampson equations) have greater accuracy for patients with low LDL-C concentrations and can report results down to 20 mg/dL. Furthermore, these equations have a higher tolerance for hypertriglyceridemia up to 800 mg/dL with comparable performance to direct LDL-C methods ( 15 ). Considering the reported failure of direct LDL methods to meet recommended total error goals among individuals with dyslipidemia ( 7 ), modern LDL-C calculations may negate the need for direct LDL-C entirely.
None of the LDL-C equations (neither the modern equations nor the Friedewald equation) should be used to calculate LDL-C in patients with type III hyperlipidemia. This disorder affects around 0.6% of the general population and can be detected by calculating the VLDL-C to TG ratio (typically >0.30). The remnant lipoproteins formed in this condition contain an abnormal lipid composition in type III hyperlipidemia that cannot be accounted for by any of these equations. A modified form of the Sampson equation that includes apo B as variable has been shown to accurately estimate VLDL-C in type III hyperlipidemia and can be used to make the diagnosis with high sensitivity and specificity compared to ultracentrifugation ( 32 ).
Clinically available ultracentrifuge-based methods include the β-quantification reference method and the VAP method. VAP is a proprietary method with purpose-built hardware clinically performed by VAP Diagnostics Laboratory (formerly Atherotec). The β-quantification technique is considered the definitive reference method for measuring LDL-C and is the method used by the CDC for standardizing routine methods used by clinical laboratories. Because the β-quantification method involves an extensive ultra-centrifugation step (approximately 18 h), it is an unattractive option for routine clinical operations. On the other hand, the VAP method, despite being proprietary to a single reference lab, is attractive because it is much quicker, separating all lipoproteins in less than 1 h thanks to its vertical rotor ( 33 ). However, VAP also has an important limitation: it is known to be less accurate than β-quantification in hypertriglyceridemic samples, leading to an underestimation of VLDL-C level, which affects LDL-C calculation. This distinction is important for studies evaluating the accuracy of LDL-C calculations in hypertriglyceridemic samples, and why β-quantification is the preferred reference method for these studies ( 16 ).
Reporting names for LDL-C should state the method used (e.g., ß-quant, calculated, direct).
Calculated LDL-C methods should use a modern equation such as the Sampson or extended-Martin, which account for variations in non-HDL-C and TG.
Calculated LDL-C should have a lower reporting limit to prevent reporting of inappropriate negative results.
Evaluation of LDL-C methods should use ß-quantification as the reference method.
The extended-Martin or Sampson equations allow for accurate LDL estimation at TG concentrations up to 800 mg/dL.
It is reasonable to not use direct homogeneous methods, which do not always meet CDC guidelines for total allowable error, particularly at low LDL-C (<70 mg/dL) or elevated TG (>400 mg/dL).
What Is Lp(a)?
Lp(a) is an apo B-containing atherogenic lipoprotein that has a size, density, and structure that is very similar to LDL. The particle contains the covalent addition of apolipoprotein(a) [apo(a)] to apo B. The apo(a) protein is comprised of a series of Kringle domains including a variable number of type IV2 (KIV2) repeats followed by an inactive plasminogen-like protease domain. Many apo(a) alleles are present in the population differing in the number of KIV2 domain repeats (from 3 to >40), which result in variable lengths of the apo(a) protein and the subsequent size of the Lp(a) lipoprotein particles. Individuals expressing small apo(a) isoforms tend to have higher circulating concentrations compared to those expressing large isoforms. The population concentration of Lp(a) is skewed such that most individuals express low concentrations, while a minority of individuals express very high concentrations of Lp(a). High circulating concentrations of Lp(a) are associated with an increased risk of atherosclerosis and atherosclerotic cardiovascular events, as well as with calcific aortic stenosis.
How to Measure Lp(a)?
Traditionally, the concentration of circulating Lp(a) has been measured using immunoassays, employing antibodies specific for the apo(a) protein found in the particle. This allows for specific measurement of the concentration of this lipoprotein separate from all others in circulation. However, given the heterogeneity of Lp(a) size in the population, immunoassays using polyclonal antibodies with multiple binding sites per particle and optical interference-type detection mechanisms that can be altered by the size of the particle being measured suffer from inaccuracies in measuring particle concentration. While a reference standard exists for manufacturer calibration, commercially available assay results are not harmonized. Historically, immunoassays have been calibrated in mass units (mg/dL) and most Lp(a) risk cutpoints were derived from population studies measured using mass assays. More recently, assays with assigned calibrators in molar units have been developed and have been shown to be slightly more accurate than mass assays; however, none are currently FDA-approved in the United States. Importantly, it is not recommended to convert mass units to molar units because of the added inaccuracy those calculations contribute to the result. Clinical cutpoints for the Lp(a) test are another gray area. Statistically significant elevated ASCVD risk has been shown above 30 mg/dL, but clinical cutpoints in guidelines have suggested that clinically relevant increased risk starts at an Lp(a) concentration of 50 mg/dL or greater. Those risk cutpoints are higher (typically 75 nmol/L or 125 nmol/L, respectively) when using the molar units assay ( 3 , 34 ).
Does Lp(a) Influence the Results of the Basic Lipid Panel?
The basic lipid panel will not be useful to identify individuals that express high concentrations of Lp(a). However, because Lp(a) is the same size and density as LDL-C, the cholesterol carried in Lp(a) will be reported as part of the LDL-C measurement in nearly all methods (calculated, direct homogenous methods, and β-quantification). This can cause diagnostic confusion for physicians when investigating the etiology of dyslipidemia. Treatment plans, treatment goals, and cascade testing of family members may all be greatly influenced by knowing that a significant portion of reported LDL-C is coming from Lp(a). Testing for Lp(a)-cholesterol is not routinely available given the difficulty in separating LDL from Lp(a) but can be ordered from reference laboratories.
When Should Lp(a) Be Ordered and How Can It Be Treated?
Guidelines vary on when Lp(a) immunoassay testing should be ordered. European guidelines recommend that Lp(a) be checked at least once in everyone ( 35 ). The National Lipid Association has recommended that measurement would be reasonable in individuals with premature atherosclerosis, with a family history of premature atherosclerosis, with a family history of elevated Lp(a), with very high LDL-C levels or familial hypercholesterolemia, and individuals at very high ASCVD risk ( 34 ). It is generally recommended that patients with elevated Lp(a) be treated more aggressively by addressing their other risk factors, including elevated LDL-C. Similarly, the US multi-society guidelines include the measurement of Lp(a) as a risk-enhancing factor appropriate in patients at intermediate risk or with significant family history ( 1 ).
Lp(a) is genetically determined and circulating concentrations can increase in childhood but do not change much throughout adulthood. Traditional ASCVD risk-lowering lifestyle changes, such as improved diet and increased exercise, do not alter Lp(a) concentrations. Pharmacotherapies, such as statins and ezetimibe, are relatively ineffective and may raise Lp(a) to a small extent in some individuals. Niacin and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors lower Lp(a) by about 25%, but outcome studies looking at cardiovascular events in high Lp(a) expressers have shown mixed results. Niacin added to effective statin therapy does not provide additional cardiovascular outcome benefit ( 36 ). However, subgroup analyses of patients with increased Lp(a) in the Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER) trial showed improved outcomes with PCSK9 inhibition ( 37 ). There are also several new drugs in development that markedly lower Lp(a) by targeting the expression of apo(a), but they are still awaiting cardiovascular outcomes trial data to determine whether they will lower ASCVD events ( 38 ).
How Should Lp(a) Be Reported?
Lp(a) should be reported in molar units when possible. However, given the lack of FDA-approved assays, reporting in mass units is still acceptable. Despite known differences in Lp(a) expression in different ethnic groups, and poor standardization and harmonization of methods, multiple societies have endorsed specific clinical decision points. Flagging of results should start at 30 mg/dL when using mass assays or 75 nmol/L when using molar assays to be consistent with the 2018 American Heart Association multi-society guideline recommendations ( 1 ). Clinical decision limits used by physicians may vary based on the patient history and risk factor profile.
Lp(a) testing should be performed using an immunoassay that is minimally susceptible to inaccuracies caused by Lp(a) isoform size.
Lp(a) assays calibrated in molar units are most accurate, but traditional assays calibrated in mass units (mg/dL) are still acceptable.
Mathematical conversion of results generated using mass-based calibration to molar units should not be performed.
Flagging of results should start at ≥ 30 mg/dL if using as assay calibrated to mass units or ≥75 nmol/L if calibrated in molar units; however, patient care decisions may use different clinical cutpoints.
What Is apo B?
Apolipoprotein B is the core structural lipoprotein of all non-HDL lipoproteins. It is present in chylomicrons and VLDL, which are packaged and secreted with exactly one copy of apo B carried through the entire lipoprotein metabolism pathway. Thus, the concentration of apo B is directly proportional to the sum of all chylomicrons, VLDL, IDL, Lp(a), and LDL lipoprotein particles present. In most situations, LDL is the most prevalent non-HDL lipoprotein and as such apo B is highly correlated with both non-HDL-C and LDL-C. Despite this correlation, there is great variation in the particle size and lipid content that can result in the same level of LDL-C or non-HDL-C for any given apo B level. As a result, there can be discrepancies in risk classification when comparing these 3 measures.
When Should apo B Be Ordered?
Currently, most relevant societies recommend apo B as a useful biomarker of lipid-derived ASCVD risk but not in the initial screening of ASCVD risk ( 1 , 3 , 35 ). There is growing awareness that apo B may be superior to LDL-C and non-HDL-C as a univariate biomarker of ASCVD risk ( 1 ). apo B is especially preferred when TG are elevated, as elevated TG interfere with both estimated and direct homogeneous LDL-C methods. apo B does not have the same limitation as LDL-C, which typically decreases when TG levels rise, due to the production of small dense LDL particles that contain less cholesterol. This phenomenon likely explains why apo B is superior to LDL-C in predicting ASCVD risk when the 2 tests are discordant, which often occurs in diabetic and obese patients. apo B also likely accounts for much of the residual ASCVD risk after lipid-lowering therapy, which typically lowers large LDL particles more than smaller LDL subspecies and hence leads to greater lowering of LDL-C than apo B. Treatment goals based on apo B may, therefore, be better for monitoring lipid-lowering therapy, but at this time apo B is mostly recommended as a risk enhancer test for patients at intermediate 10-year risk.
How Should apo B Be Reported?
The IFCC in collaboration with the WHO maintains a standardized apo B reference material for vendor calibration of clinical methods. A new mass spectrometry reference method is being established for apo B, which will likely improve its standardization compared to LDL-C ( 39 ). The primary indication of apo B remains ASCVD risk assessment and as such it should be reported in a context of desirable vs at-risk concentrations. apo B percentiles include 65 mg/dL 10th, 100 mg/dL 75th, and 150 mg/dL 95th. A percentile conversion equation has been described for reporting apo B in LDL-C equivalent units to aid in the education of the value of apo B in ASCVD risk assessment and to allow the use of more familiar LDL-C cutpoints for managing patients.
apo B is present on all atherogenic lipoproteins as a single copy and thus provides an integrated measure of ASCVD risk.
apo B can be accurately measured by routine clinical methods and its standardization will likely further improve with the development of a new reference method.
When discordant with LDL-C or non-HDL-C, apo B has been shown to be a better predictor of ASCVD events.
Although not uniformly endorsed by all guidelines for initial risk assessment, greater use of apo B is warranted based on current evidence, particularly as a risk-enhancing factor when monitoring lipid-lowering therapy.
apolipoprotein A-I (apo AI) is the primary structural lipoprotein for HDL. Some studies have shown that blood concentrations of apo AI and the ratio apo B/apo AI can be predictive of atherosclerosis risk. However, apo AI clinical testing is not widely available, no consensus ASCVD risk thresholds have been established, and no clinical guidelines have endorsed its routine use. Interestingly, the most prominent and growing use of apo AI is as a biomarker of hepatocellular synthesis and functional capacity in liver disease ( 40 ).
apo AI methods are not harmonized or standardized.
Routine apo AI testing is not recommended.
What Are the Indications for Point-of-Care Lipid Testing?
Point-of-care testing (POCT) lipid methods have the advantage of short turnaround time and the use of whole blood as an acceptable specimen, which is especially helpful in the outpatient and field settings. Furthermore, POCT lipid testing enables the rapid communication of test results to patients. POCT tests should follow the same certification criteria from the Cholesterol Reference Method Laboratory Network (CRMLN) as serum/plasma-based lipid tests.
What Are the Limitations of Point-of-Care Lipid Testing?
Major limitations include higher imprecision compared to standard chemistry analyzers and narrow linearity ranges, which may result in the need for repeat testing on another analyzer. Another limitation is that typically only tests in the lipid panel are available for POCT testing and it can be challenging to report LDL-C results using one of the newer equations. Lastly, when using fingerstick whole blood, interferences from topical skin lotions can cause false results.
POCT lipid methods should be held to the same NCEP and CRMLN performance metrics as other methods.
POCT reporting should clearly describe the method, including any calculated LDL-C.
LDL is a heterogenous collection of different size lipoprotein particles with diameters between 19 and 22 nm. It is also heterogenous in terms of its density, protein content, and lipid composition. Using a variety of different techniques, such as those based on nuclear magnetic resonance (NMR), electrophoresis, density gradient centrifugation, gel filtration, and ion mobility, it is possible to separately measure the different subfractions of LDL. Because these different tests rely on different physical principles, the results of the different LDL subfraction tests are not interchangeable and often have different nomenclature ( 39 ). Some of the LDL subfractions like small dense LDL may be better predictors of ASCVD risk than total LDL-C. A fully automated direct assay for small dense LDL-C is available ( 41 ), and more recently, an equation estimating small dense LDL-C from lipid panel test results was developed ( 32 ). LDL subfraction testing, however, is not recommended by any current guidelines.
LDL subfraction methods are not harmonized or standardized.
Routine LDL subfraction testing is not recommended.
In conclusion, the recommendations outlined in this document provide guidance for standardizing lipid reports and improving the interpretation of lipid panel results ( Table 4 ). By emphasizing the reporting of specific measured values and calculated parameters, indicating test names with clarity, and specifying LDL-C methods, healthcare professionals can achieve greater consistency and accuracy in lipid reporting. We reinforce the importance of interpreting lipids within the context of healthy population percentiles and underscore the need for fasting samples only when adjusting medication dosage or in patients with hypertriglyceridemia. The recommendations also address the reporting and interpretation of Lp(a) results, the use of apo B as a biomarker, and the precautions necessary when using POCT lipid methods. It is our hope that these recommendations and subsequent revisions serve as a comprehensive resource to support clinicians and laboratories in lipid assessment and enhancing patient care.
Summary of recommendations to clinical laboratories and clinicians.
| Number . | Recommendation . | Laboratories . | Clinicians . |
|---|---|---|---|
| 1 | Standard lipid panels should report 3 measured values: TC, TG, HDL-C, and 2 calculated parameters: LDL-C and non-HDL-C. | ✅ | ✅ |
| 2 | Test names for the lipid panel and its components should indicate the specific analyte being measured, the sample obtained, and method used when appropriate. | ✅ | |
| 3 | There now exists a wide variety of LDL-C methods and calculations in routine use, so it is best practice to state either calculated or measured LDL-C in the reporting name. The LDL-C calculation method should also be included in the report comment or laboratory test catalog. | ✅ | |
| 4 | Lipids are best interpreted in a context of “desirable” vs “increased risk,” typically defined between the 50th and 75th percentiles of healthy populations. | ✅ | ✅ |
| 5 | Adult patients with hypertriglyceridemia and pediatric patients with elevated non-HDL-C should be retested on a fasting sample. | ✅ | |
| 6 | Order sets should allow for both fasting or non-fasting lipid panels, and fasting status should be documented at time of collection and indicated in the result report. | ✅ | |
| 7 | A comment suggesting repeat assessment after fasting is reasonable to include when non-fasting TG are >400 mg/dL. | ✅ | |
| 8 | Universal TC screening to identify genetic or lifestyle-related pediatric dyslipidemia is recommended in 9–11 year-olds. | ✅ | |
| 9 | HDL-C <15 mg/dL (2.5th percentile) warrants a comment to investigate liver disease or potential genetic dyslipidemia. | ✅ | |
| 10 | Cholesterol in LpX contributes to non-HDL-C, and a pattern of acute onset elevated non-HDL-C and low HDL-C in a context of liver disease should prompt further investigation by lipoprotein electrophoresis to identify LpX. | ✅ | |
| 11 | Switch from the Friedewald equation that calculates LDL-C to either the Sampson or Martin equations. Both the Martin and Sampson equations demonstrate improved comparability to measured LDL-C, particularly when LDL-C <70 mg/dL and when TG are elevated up to 800 mg/dL. | ✅ | ✅ |
| 12 | Measuring LDL-C by direct homogeneous methods is not universally recommended. Laboratories calculating LDL-C by the extended Martin or Sampson equations have reduced need for direct LDL-C because of improved estimation performance in samples with low LDL-C or high triglycerides (up to 800 mg/dL). Addressing triglyceride levels when >800 mg/dL should be the immediate focus before considering LDL-C management. | ✅ | |
| 13 | None of the LDL-C equations (neither the modern equations nor the Friedewald equation) should be used to calculate LDL-C in patients with type III hyperlipidemia. Laboratories are encouraged to add a comment in patient reports highlighting this drawback. | ✅ | ✅ |
| 14 | If LDL-C is necessary in patients with type III hyperlipidemia or TG >800 mg/dL, then β-quantification is the preferred method. | ✅ | ✅ |
| 15 | Lp(a) should be reported in molar units, when possible, but it is not recommended to mathematically convert mass units to molar units because of the added inaccuracy those calculations contribute to the result. | ✅ | |
| 16 | Lp(a) results >30 mg/dL when using mass assays or >75 nmol/L when using molar assays should be flagged as high. Clinical decision limits used by clinicians may vary based on the patient history and risk factor profile. | ✅ | ✅ |
| 17 | Measurement of Lp(a) would be reasonable in individuals with premature atherosclerosis, with a family history of premature atherosclerosis, with a family history of elevated Lp(a), with very high LDL-C levels or familial hypercholesterolemia, and individuals at very high ASCVD risk. European guidelines recommend measuring Lp(a) at least once in everyone. | ✅ | |
| 18 | Most relevant societies recommend apo B as useful biomarker of lipid-derived ASCVD risk but not in the initial screening of ASCVD risk. apo B may be superior to LDL-C and non-HDL-C as a univariate biomarker of ASCVD risk. apo B is especially preferred when TG are elevated, as elevated TG interfere with both estimated and direct homogeneous LDL-C methods. At this time, apo B is mostly recommended as a risk enhancer test for patients at intermediate 10-year risk. | ✅ | |
| 19 | Warning when using POCT lipid methods: when using fingerstick whole blood, interferences from topical skin lotions can cause false results. | ✅ | ✅ |
| 20 | Routine use of LDL subfraction testing is not recommended by any current guidelines. | ✅ | ✅ |
| Number . | Recommendation . | Laboratories . | Clinicians . |
|---|---|---|---|
| 1 | Standard lipid panels should report 3 measured values: TC, TG, HDL-C, and 2 calculated parameters: LDL-C and non-HDL-C. | ✅ | ✅ |
| 2 | Test names for the lipid panel and its components should indicate the specific analyte being measured, the sample obtained, and method used when appropriate. | ✅ | |
| 3 | There now exists a wide variety of LDL-C methods and calculations in routine use, so it is best practice to state either calculated or measured LDL-C in the reporting name. The LDL-C calculation method should also be included in the report comment or laboratory test catalog. | ✅ | |
| 4 | Lipids are best interpreted in a context of “desirable” vs “increased risk,” typically defined between the 50th and 75th percentiles of healthy populations. | ✅ | ✅ |
| 5 | Adult patients with hypertriglyceridemia and pediatric patients with elevated non-HDL-C should be retested on a fasting sample. | ✅ | |
| 6 | Order sets should allow for both fasting or non-fasting lipid panels, and fasting status should be documented at time of collection and indicated in the result report. | ✅ | |
| 7 | A comment suggesting repeat assessment after fasting is reasonable to include when non-fasting TG are >400 mg/dL. | ✅ | |
| 8 | Universal TC screening to identify genetic or lifestyle-related pediatric dyslipidemia is recommended in 9–11 year-olds. | ✅ | |
| 9 | HDL-C <15 mg/dL (2.5th percentile) warrants a comment to investigate liver disease or potential genetic dyslipidemia. | ✅ | |
| 10 | Cholesterol in LpX contributes to non-HDL-C, and a pattern of acute onset elevated non-HDL-C and low HDL-C in a context of liver disease should prompt further investigation by lipoprotein electrophoresis to identify LpX. | ✅ | |
| 11 | Switch from the Friedewald equation that calculates LDL-C to either the Sampson or Martin equations. Both the Martin and Sampson equations demonstrate improved comparability to measured LDL-C, particularly when LDL-C <70 mg/dL and when TG are elevated up to 800 mg/dL. | ✅ | ✅ |
| 12 | Measuring LDL-C by direct homogeneous methods is not universally recommended. Laboratories calculating LDL-C by the extended Martin or Sampson equations have reduced need for direct LDL-C because of improved estimation performance in samples with low LDL-C or high triglycerides (up to 800 mg/dL). Addressing triglyceride levels when >800 mg/dL should be the immediate focus before considering LDL-C management. | ✅ | |
| 13 | None of the LDL-C equations (neither the modern equations nor the Friedewald equation) should be used to calculate LDL-C in patients with type III hyperlipidemia. Laboratories are encouraged to add a comment in patient reports highlighting this drawback. | ✅ | ✅ |
| 14 | If LDL-C is necessary in patients with type III hyperlipidemia or TG >800 mg/dL, then β-quantification is the preferred method. | ✅ | ✅ |
| 15 | Lp(a) should be reported in molar units, when possible, but it is not recommended to mathematically convert mass units to molar units because of the added inaccuracy those calculations contribute to the result. | ✅ | |
| 16 | Lp(a) results >30 mg/dL when using mass assays or >75 nmol/L when using molar assays should be flagged as high. Clinical decision limits used by clinicians may vary based on the patient history and risk factor profile. | ✅ | ✅ |
| 17 | Measurement of Lp(a) would be reasonable in individuals with premature atherosclerosis, with a family history of premature atherosclerosis, with a family history of elevated Lp(a), with very high LDL-C levels or familial hypercholesterolemia, and individuals at very high ASCVD risk. European guidelines recommend measuring Lp(a) at least once in everyone. | ✅ | |
| 18 | Most relevant societies recommend apo B as useful biomarker of lipid-derived ASCVD risk but not in the initial screening of ASCVD risk. apo B may be superior to LDL-C and non-HDL-C as a univariate biomarker of ASCVD risk. apo B is especially preferred when TG are elevated, as elevated TG interfere with both estimated and direct homogeneous LDL-C methods. At this time, apo B is mostly recommended as a risk enhancer test for patients at intermediate 10-year risk. | ✅ | |
| 19 | Warning when using POCT lipid methods: when using fingerstick whole blood, interferences from topical skin lotions can cause false results. | ✅ | ✅ |
| 20 | Routine use of LDL subfraction testing is not recommended by any current guidelines. | ✅ | ✅ |
Nonstandard Abbreviations: non-HDL-C, non-high density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein(a); apo B, apolipoprotein B; apo(a), apolipoprotein(a); ASCVD, atherosclerotic cardiovascular disease; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglycerides; NCEP, National Cholesterol Education Program; VLDL, very-low-density lipoprotein; LpX, lipoprotein X; VLDL-C, very-low-density lipoprotein cholesterol; apo AI, apolipoprotein A-I; POCT, point-of-care testing.
Author Contributions: The corresponding author takes full responsibility that all authors on this publication have met the following required criteria of eligibility for authorship: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; (c) final approval of the published article; and (d) agreement to be accountable for all aspects of the article thus ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved. Nobody who qualifies for authorship has been omitted from the list.
Jeffrey Meeusen (Conceptualization-Equal, Data curation-Equal, Writing—original draft-Equal, Writing—review & editing-Equal), Leslie Donato (Conceptualization-Equal, Writing—original draft-Equal, Writing—review & editing-Equal), Anne Goldberg (Conceptualization-Equal, Writing—original draft-Equal, Writing—review & editing-Equal), Jing Cao (Conceptualization-Equal, Writing—original draft-Equal, Writing—review & editing-Equal), Joe El-Khoury (Conceptualization-Equal, Writing—original draft-Equal, Writing—review & editing-Equal), and Alan Remaley (Conceptualization-Equal, Writing—original draft-Equal, Writing—review & editing-Equal)
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form.
Research Funding: None declared.
Disclosures: A. Goldberg disclosed clinical trials—payment to institution from Novartis, Amgen, IONIS, Regeneron, Arrowhead, New Amsterdam, Sanofi, Bio89, and Esperion. A. Goldberg received consulting fees from New Amsterdam; honoraria from National Lipid Association, American College of Cardiology, and Preventive Cardiology Nurses Association; free registration to American Heart Association, Endocrine Society, and American College of Cardiology meetings when moderating or presenting; travel support from Austrian American Foundation; and medical writing assistance for group manuscripts from Esperion. A. Goldberg participated in advisory board meetings for AKCEA and Regeneron, data monitoring board for Lilly, and clinical trial steering committee for New Amsterdam, is country lead investigator for IONIS, and holds leadership roles with National Lipid Association and Foundation of the National Lipid Association. L. Donato received honoraria from Novartis and Helena. J.M. El-Khoury disclosed research grants to institution from Siemens Healthineers and Bioporto; consulting fees from Siemens Healthineers; honoraria, travel, and lodging support from ADLM; blood tubes for unrelated study from Becton-Dickinson; and a mass spectrometer for evaluation from Ionsense (now part of Bruker). J.M. El-Khoury serves on the Board of Directors of ADLM and is Associate Editor for Clinical Chemistry .
Role of Sponsor: No sponsor was declared.
Grundy SM , Stone NJ , Bailey AL , Beam C , Birtcher KK , Blumenthal RS , et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines . Circulation 2019 ; 139 : e1046 – 81 .
Google Scholar
Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute . Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report . Pediatrics 2011 ; 128 ( Suppl 5 ): S213 – 56 .
Wilson PWF , Jacobson TA , Martin SS , Jackson EJ , Le NA , Davidson MH , et al. Lipid measurements in the management of cardiovascular diseases: practical recommendations a scientific statement from the national lipid association writing group . J Clin Lipidol 2021 ; 15 : 629 – 48 .
White-Al Habeeb NMA , Higgins V , Venner AA , Bailey D , Beriault DR , Collier C , et al. Canadian society of clinical chemists harmonized clinical laboratory lipid reporting recommendations on the basis of the 2021 Canadian cardiovascular society lipid guidelines . Can J Cardiol 2022 ; 38 : 1180 – 8 .
Vesper HW , Wilson PWF , Rifai N . A message from the laboratory community to the National Cholesterol Education Program Adult Treatment Panel IV . Clin Chem 2012 ; 58 : 523 – 7 .
Centers for Disease Control and Prevention . CRMLN: Cholesterol Reference Method Laboratory Network. http://www.cdc.gov/labstandards/crmln.html/ (Accessed November 2020).
Miller WG , Myers GL , Sakurabayashi I , Bachmann LM , Caudill SP , Dziekonski A , et al. Seven direct methods for measuring HDL and LDL cholesterol compared with ultracentrifugation reference measurement procedures . Clin Chem 2010 ; 56 : 977 – 86 .
Langsted A , Nordestgaard BG . Nonfasting lipids, lipoproteins, and apolipoproteins in individuals with and without diabetes: 58 434 individuals from the Copenhagen General Population Study . Clin Chem 2011 ; 57 : 482 – 9 .
Steiner MJ , Skinner AC , Perrin EM . Fasting might not be necessary before lipid screening: a nationally representative cross-sectional study . Pediatrics 2011 ; 128 : 463 – 70 .
Mora S , Rifai N , Buring JE , Ridker PM . Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events . Circulation 2008 ; 118 : 993 – 1001 .
Sidhu D , Naugler C . Fasting time and lipid levels in a community-based population: a cross-sectional study . Arch Intern Med 2012 ; 172 : 1707 – 10 .
Friedewald WT , Levy RI , Fredrickson DS . Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge . Clin Chem 1972 ; 18 : 499 – 502 .
Mora S , Chang CL , Moorthy MV , Sever PS . Association of nonfasting vs fasting lipid levels with risk of major coronary events in the Anglo-Scandinavian cardiac outcomes trial-lipid lowering arm . JAMA Intern Med 2019 ; 179 : 898 – 905 .
Langsted A , Freiberg JJ , Nordestgaard BG . Fasting and nonfasting lipid levels: influence of normal food intake on lipids, lipoproteins, apolipoproteins, and cardiovascular risk prediction . Circulation 2008 ; 118 : 2047 – 56 .
Sampson M , Ling C , Sun Q , Harb R , Ashmaig M , Warnick R , et al. A new equation for calculation of low-density lipoprotein cholesterol in patients with normolipidemia and/or hypertriglyceridemia . JAMA Cardiol 2020 ; 5 : 540 – 8 .
Sajja A , Park J , Sathiyakumar V , Varghese B , Pallazola VA , Marvel FA , et al. Comparison of methods to estimate low-density lipoprotein cholesterol in patients with high triglyceride levels . JAMA Netw Open 2021 ; 4 : e2128817 .
Daniels SR , Gidding SS , de Ferranti SD ; National Lipid Association Expert Panel on Familial Hypercholesterolemia . Pediatric aspects of familial hypercholesterolemias: recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia . J Clin Lipidol 2011 ; 5 : S30 – 7 .
Gill PK , Dron JS , Hegele RA . Genetics of hypertriglyceridemia and atherosclerosis . Curr Opin Cardiol 2021 ; 36 : 264 – 71 .
Miller M , Stone NJ , Ballantyne C , Bittner V , Criqui MH , Ginsberg HN , et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association . Circulation 2011 ; 123 : 2292 – 333 .
Stone NJ , Grundy SM . The 2018 AHA/ACC/multi-society cholesterol guidelines: looking at past, present and future . Prog Cardiovasc Dis 2019 ; 62 : 375 – 83 .
Backes JM , Dayspring T , Moriarty PM . Pseudohypertriglyceridemia-Raising clinical awareness . J Clin Lipidol 2019 ; 13 : 855 – 6 .
Goff DC Jr , Lloyd-Jones DM , Bennett G , Coady S , D'Agostino RB Sr , Gibbons R , et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines . J Am Coll Cardiol 2014 ; 63 : 2935 – 59 .
Tada H , Nohara A , Inazu A , Sakuma N , Mabuchi H , Kawashiri MA . Sitosterolemia, hypercholesterolemia, and coronary artery disease . J Atheroscler Thromb 2018 ; 25 : 783 – 9 .
Cohen A , Hertz HS , Mandel J , Paule RC , Schaffer R , Sniegoski LT , et al. Total serum cholesterol by isotope dilution/mass spectrometry: a candidate definitive method . Clin Chem 1980 ; 26 : 854 – 60 .
Yang HS , Jeong HJ , Kim H , Hwang HK , Hur M , Lee S . Sex-specific U-shaped relationships between high-density lipoprotein cholesterol levels and 10-year major adverse cardiovascular events: a nationwide cohort study of 5.7 million South Koreans . Ann Lab Med 2022 ; 42 : 415 – 27 .
Bonilha I , Luchiari B , Nadruz W , Sposito AC . Very low HDL levels: clinical assessment and management . Arch Endocrinol Metab 2023 ; 67 : 3 – 18 .
Meeusen JW , Snozek CL , Baumann NA , Jaffe AS , Saenger AK . Reliability of calculated low-density lipoprotein cholesterol . Am J Cardiol 2015 ; 116 : 538 – 40 .
Martin SS , Blaha MJ , Elshazly MB , Toth PP , Kwiterovich PO , Blumenthal RS , Jones SR . Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile . JAMA 2013 ; 310 : 2061 – 8 .
Myers GL , Kimberly MM , Waymack PP , Smith SJ , Cooper GR , Sampson EJ . A reference method laboratory network for cholesterol: a model for standardization and improvement of clinical laboratory measurements . Clin Chem 2000 ; 46 : 1762 – 72 .
Mora S , Rifai N , Buring JE , Ridker PM . Comparison of LDL cholesterol concentrations by Friedewald calculation and direct measurement in relation to cardiovascular events in 27,331 women . Clin Chem 2009 ; 55 : 888 – 94 .
Whelton SP , Meeusen JW , Donato LJ , Jaffe AS , Saenger A , Sokoll LJ , et al. Evaluating the atherogenic burden of individuals with a Friedewald-estimated low-density lipoprotein cholesterol <70 mg/dL compared with a novel low-density lipoprotein estimation method . J Clin Lipidol 2017 ; 11 : 1065 – 72 .
Sampson M , Wolska A , Meeusen JW , Donato LJ , Jaffe AS , Remaley AT . Identification of dysbetalipoproteinemia by an enhanced Sampson-NIH equation for very low-density lipoprotein-cholesterol . Front Genet 2022 ; 13 : 935257 .
Kulkarni KR . Cholesterol profile measurement by vertical auto profile method . Clin Lab Med 2006 ; 26 : 787 – 802 .
Wilson DP , Jacobson TA , Jones PH , Koschinsky ML , McNeal CJ , Nordestgaard BG , Orringer CE . Use of lipoprotein(a) in clinical practice: a biomarker whose time has come. A scientific statement from the National Lipid Association . J Clin Lipidol 2019 ; 13 : 374 – 92 .
Mach F , Baigent C , Catapano AL , Koskinas KC , Casula M , Badimon L , et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk . Eur Heart J 2020 ; 41 : 111 – 88 .
AIM-HIGH Investigators ; Boden WE , Probstfield JL , Anderson T , Chaitman BR , Desvignes-Nickens P , et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy . N Engl J Med 2011 ; 365 : 2255 – 67 .
O'Donoghue ML , Fazio S , Giugliano RP , Stroes ESG , Kanevsky E , Gouni-Berthold I , et al. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk . Circulation 2019 ; 139 : 1483 – 92 .
Sosnowska B , Surma S , Banach M . Targeted treatment against lipoprotein (a): the coming breakthrough in lipid lowering therapy . Pharmaceuticals (Basel) 2022 ; 15 : 1573 .
Delatour V , Clouet-Foraison N , Gaie-Levrel F , Marcovina SM , Hoofnagle AN , Kuklenyik Z , et al. Comparability of lipoprotein particle number concentrations across ES-DMA, NMR, LC-MS/MS, immunonephelometry, and VAP: in search of a candidate reference measurement procedure for apoB and non-HDL-P standardization . Clin Chem 2018 ; 64 : 1485 – 95 .
Gurbuz B , Guldiken N , Reuken P , Fu L , Remih K , Preisinger C , et al. Biomarkers of hepatocellular synthesis in patients with decompensated cirrhosis . Hepatol Int 2023 ; 17 : 698 – 708 .
Tsai MY , Steffen BT , Guan W , McClelland RL , Warnick R , McConnell J , et al. New automated assay of small dense low-density lipoprotein cholesterol identifies risk of coronary heart disease: the Multi-ethnic Study of Atherosclerosis . Arterioscler Thromb Vasc Biol 2014 ; 34 : 196 – 201 .
Author notes
Email alerts, citing articles via.
- Recommend to Your Librarian
- Advertising and Corporate Services
- Journals Career Network
Affiliations
- Online ISSN 2475-7241
- Copyright © 2024 Association for Diagnostics & Laboratory Medicine
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Author Biographies
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
The role of selected lncrnas in lipid metabolism and cardiovascular disease risk, 1. introduction, 2. long noncoding rnas, 3. high-density lipoproteins, reverse cholesterol transport and cardiovascular risk, 4. lncrnas affecting lipoproteins, lipid metabolism, and atherosclerosis risk, 4.1. lncrna with beneficial effects, 4.1.1. nfia antisense rna 1.
- Name/organism: RP5-833A20.1/ NFIA antisense RNA 1/NFIA-AS1; Homo sapiens
- Databases &Code: Ensembl ENSG00000237853; HGNC: 40402; NCBI Gene: 645030
- Chromosomal location& size: 1p31.3: 61,248,945–61,253,510 reverse strand; 4 exons, is associated with 1718 variant alleles
- Interaction with other molecules: hsa-miR-382-5p
- Biological consequence of such interaction: Increases circulation of HDL-C, reduces levels of LDL-C, and VLDL-C
4.1.2. Liver-Expressed LXR-Induced Sequence
- Name/organism: LeXis/CT70 (cancer/testis associated transcript 70); Homo sapiens
- Databases &Code: Ensembl ENSG00000230013; HGNC:37195
- Chromosomal location& size: 9q31.1
- Interaction with other molecules: Raly
- Biological consequence of such interaction: Modulates expression of cholesterol biosynthetic genes
4.1.3. Macrophage-Expressed LXR-Induced Sequence
- Name/organism: MeXis/AI427809/LOC381524; Homo sapiens
- Databases &Code: Ensembl ENSMUSG00000086712.3
- Chromosomal location& size: Chromosome 4: 53,261,356–53,270,232 reverse strand.
- 4 transcripts (splice variants), 3 orthologues and is associated with 4 phenotypes
- Interaction with other molecules: Raly, DDX17
- Biological consequence of such interaction: Macrophage expressed LXRa (NR1H3)-dependent amplifier of Abca1 transcription lncRNA
4.1.4. LncRNA RP1-13D10.2
- Name/organism: RP1-13D10.2;
- Interaction with other molecules: LXR, SREBF2
- Biological consequence of such interaction: Regulates LDLR gene expression in a sterol-responsive and SNP genotype–dependent manner in vitro
4.1.5. LncLSTR
- Name/organism: LncLSTR (lncRNA liver-specific triglyceride regulator);
- Chromosomal location& size: Syntenic to human chromosome 1q25
- Interaction with other molecules: TDP-43
- Biological consequence of such interaction: Modulates bile acid composition to regulate APOC2 expression, via FXR,85 and to control serum triglyceride levels
4.1.6. Cholesterol-Induced Regulator of Metabolism RNA
- Name/organism: CHROME, PRKRA-AS1; Homo sapiens
- Databases &Code: Ensemble: ENSG00000223960.9; HGNC:54059
- Chromosomal location& size: 2q31.2: 178,413,635–178,440,243 forward strand; 35 transcripts (splice variants)
- Interaction with other molecules: miR-27b, miR-33a, miR-33b, and miR-128
- Biological consequence of such interaction: Promotes cholesterol secretion and HDL synthesis via suppressing the activity of specific miRNAs
4.1.7. Lipid-Droplet Transporter
- Name/organism: LIPTER/LINC00881; Homo sapiens
- Databases &Code: Ensemble: ENSG00000241135.8; HGNC: 48567; NCBI Gene: 100498859
- Chromosomal location& size: 3q25.31: 157,089,634–157,135,557 forward strand; 11 transcripts
- Interaction with other molecules: Phosphatidic acid, phosphatidylin-ositol 4-phosphate MYH10 motor protein
- Biological consequence of such interaction: Facilitates the connection between LDs and the cytoskeleton for intracellular transport
4.1.8. Regulator of Hyperlipidaemia Long Noncoding RNA
- Name/organism: lncRHPL; Mus musculus (house mouse)
- Databases &Code: NIH Gene ID: 105244982
- Chromosomal location& size: Chromosome 8
- Interaction with other molecules: hnRNPU, BMAL1
- Biological consequence of such interaction: Modulates hepatic VLDL secretion.
4.1.9. LncNONMUG027912
- Name/organism: LncNONMMUG027912/lnc027912
- Interaction with other molecules: AMPKα/mTOR signalling axis
- Biological consequence of such interaction: Upregulates p-AMPKα, reduces p-mTOR levels, suppresses nuclear expression of SREBP1C, and hinders the expression of lipid synthesis genes

4.1.10. Maternally Expressed 3
- Name/organism: MEG3/GTL2/LINC00023/NCRNA00023/ONCO-LNCRNA-83;
- Databases &Code: ENSG00000214548.18; HGNC (14575); NCBI Gene (55384); OMIM ® (605636); Open Targets Plat-form (ENSG00000214548)
- Chromosomal location& size: Chromosome 14: 100,779,410–100,861,031 forward strand;
- 50 transcripts and is associated with 6 phenotypes
- Interaction with other molecules: miR-21
- Biological consequence of such interaction: Modulates hepatic lipogenesis
4.1.11. Cyclin-Dependent Kinase Inhibitor 2B Antisense RNA 1
- Name/organism: ANRIL/CDKN2B-AS1/RP11-145E5.4/NCRNA00089/p15AS/PCAT12; Homo sapiens
- Databases &Code: HGNC: 34341; Ensembl: ENSG00000240498; NCBI Gene: 100048912; OMIM ® : 613149
- Chromosomal location& size: located within the CDKN2B-CDKN2A gene cluster at chromosome 9p21.3: 21,994,139–22,128,103 forward strand; 28 transcripts
- Interaction with other molecules: polycomb repressive complex-1 (PRC1) and -2 (PRC2),
- Biological consequence of such interaction: Epigenetic silencing of other genes in this cluster.
4.1.12. HOXC Cluster Antisense RNA 1
- Name/organism: HOXC-AS1/NONHSAG011268.2/HSALNG0091321; Homo sapiens
- Databases &Code: HGNC: 43749; NCBI Gene: 100874363; Ensembl: ENSG00000250451
- Chromosomal location& size: 12q13.13; Ch 12: 53,999,022–54,000,010 reverse strand; 2 transcripts
- Biological consequence of such interaction: Inhibition of intracellular lipid accumulation
4.2. LncRNA with Adverse Effects
4.2.1. ac068234.2–202 and ap001033.3–201.
- Name/organism: AC068234.2–202; Homo sapiens
- Databases &Code: AC068234.2
- Chromosomal location& size: Ch17:47,303,474–47,323,613 reverse strand; transcript with 3 exons, associated with 4518 variant alleles
- Interaction with other molecules: TBXA2R
- Biological consequence of such interaction: Possibly contribute to the trans-regulation of the protein-coding gene thromboxane A2 receptor (TBXA2R)
- Name/organism: AP001033.3–201; Homo sapiens
- Databases &Code: AP001033.3
- Chromosomal location& size: Ch18: 9,310,522–9,334,445 reverse strand; transcript with 3 exons and 5282 reported variant alleles
- Interaction with other molecules: antisense to ITGB3
- Biological consequence of such interaction: Acts a cis-regulator of the protein-coding gene integrin subunit beta 3 (ITGB3)
4.2.2. LncRNA ENST00000602558.1
- Name/organism: ENST00000602558.1; Homo sapiens
- Databases &Code: Ensembl: ENST00000602558.1
- Chromosomal location& size: Chromosome 12: 123,971,457-123,971,714 reverse strand;
- Exons: 1, Coding exons: 0, Transcript length: 258 bps; sense intronic to CCDC92
- Interaction with other molecules: p65
- Biological consequence of such interaction: Downregulates ABCG1 mRNA
4.2.3. Long Intergenic Non-Protein Coding RNA 1228
- Name/organism: LINCRNA-DYNLRB2-2/LINC01228;
- Databases &Code: Ensembl: ENST00000567966.1
- Chromosomal location& size: Chromosome 16: 79,798,050–79,827,150 reverse strand; Size: 623 bp
- Interaction with other molecules: GPR119
- Biological consequence of such interaction: Facilitates cholesterol efflux and diminishes neutral lipid accumulation
4.2.4. Taurine Upregulated Gene 1
- Name/organism: TUG1/FLJ20618/LINC00080/NCRNA00080; Homo sapiens
- Databases &Code: Ensembl: ENSG00000253352.10
- Chromosomal location& size: 22q12.2: 30,969,245–30,979,395 forward strand; 20 transcripts (splice variants) and 9 orthologues
- Interaction with other molecules: miR-92a, miR-133a
- Biological consequence of such interaction: Suppression of FGF1activation
4.2.5. Myocardial Infarction-Associated Transcript
- Name/organism: MIAT/RNCR2/GOMAFU/C22orf35/LINC00066/NCRNA00066/lncRNA-MIAT; Homo sapiens
- Databases &Code: HGNC: 33425; NCBI Gene: 440823; Ensembl: ENSG00000225783; OMIM ® : 611082
- Chromosomal location& size: 22q12.1: 26,646,411–26,676,475 forward strand; 30 transcripts (splice variants) and is associated with 1 phenotype
- Interaction with other molecules: PI3K/Akt signalling pathway
- Biological consequence of such interaction: May constitute a component of the nuclear matrix; enhances angiogenesis and increases the expression of inflammatory factors
4.2.6. LncRNA RP11-728F11
- Name/organism: LncRNA RP11-728F11;
- Interaction with other molecules: EWSR1 (Ewings sarcoma RNA binding protein-1)
- Biological consequence of such interaction: Induction of cholesterol uptake in monocytes-derived macrophages and proinflammatory cytokine production
4.2.7. lncRNA ENST00000416361
- Name/organism: ENST00000416361; Homo sapiens
- Databases &Code: Ensembl: ENST00000416361
- Chromosomal location& size: 2102 bp
- Interaction with other molecules: SREBP
- Biological consequence of such interaction: Affects the occurrence and development of CAD
4.2.8. LncRNA RAPIA
- Name/organism: RAPIA;
- Chromosomal location& size: 10,252 nucleotides
- Interaction with other molecules: miR-183-5p-ItgB1 (integrin β1)
- Biological consequence of such interaction: Coordination of proliferation and apoptosis of macrophages
4.2.9. Nuclear Paraspeckle Assembly Transcript 1
- Name/organism: NEAT1/LINC00084/MENEPSILON/BETA/NCRNA00084/TNCRNA/TP53LC15/VINC; Homo sapiens
- Databases &Code: HGNC: 30815; NCBI Gene: 283131; Ensembl: ENSG00000245532; OMIM ® : 612769
- Chromosomal location& size: 11q13.1; 11: 65,422,774–65,445,540 forward strand; 9 transcripts
- Interaction with other molecules: miR-342-3p
- Biological consequence of such interaction: Regulation of lipid droplet aggregation; affect TG metabolism
4.2.10. Nipsnap Homolog 3B
- Name/organism: NIPSNAP3B/FP944/LOC286367/FLJ11275, SNAP1
- Databases &Code: HGNC: 23641, NCBI Gene: 55335; Ensembl: ENSG00000165028; OMIM ® : 608872; UniProtKB/Swiss-Prot: Q9BS92
- Chromosomal location& size: 9q31.1; Ch9: 104,764,129–104,777,764 forward strand; 3 transcripts (splice variants), 160 orthologues and 3 paralogues
- Biological consequence of such interaction: Putative role in vesicular trafficking; promotion of intracellular lipid accumulation
4.2.11. Long Noncoding RNA Regulator of Akt Signalling Associated with HCC and RCC
- Name/organism: LNCARSR/ lnc-TALC
- Databases &Code: HGNC: 53864; NCBI Gene: 102723932; Ensembl: ENSG00000233086
- Chromosomal location& size: 9q21.31; Ch 9: 79,505,804–79,567,802 reverse strand; 10 transcripts (splice variants)
- Interaction with other molecules: SREBP-2
- Biological consequence of such interaction: Promotion of the expression of HMG-CoA reductase (HMGCR), enhancement of hepatic de novo cholesterol synthesis rate
4.2.12. LDLR Antisense RNA 1
- Name/organism: BM450697/LDLR-AS1
- Databases &Code: HGNC: 54407, NCBI Gene: 115271120
- Chromosomal location& size: 19p13.2; overlaps the 5′ UTR and coding sequence of the LDLR n the antisense orientation
- Interaction with other molecules: PolII and potentially SREBP1a
- Biological consequence of such interaction: Downregulation of the production of the low density lipoprotein receptor.
4.2.13. Long Non-Coding RNA Growth Arrest-Specific 5
- Name/organism: GAS5/NCRNA00030/SNHG2; Homo sapiens
- Databases &Code: HGNC: 16355; NCBI Gene: 60674; Ensembl: ENSG00000234741; OMIM ® : 608280
- Chromosomal location& size: 1q25.1; Ch 1: 173,858,559–173,868,882 reverse strand; 91 transcripts (splice variants)
- Interaction with other molecules: bind to the DNA binding domain of the glucocorticoid receptor (nuclear receptor subfamily 3, group C, member 1)
- Biological consequence of such interaction: blockage of the activation of glucocorticoid receptor, regulation of the transcriptional activity of other receptors, such as androgen, progesterone and mineralocorticoid receptors
4.3. LncRNA with Ambiguous Effects
4.3.1. apolipoprotein a1 and a4 antisense rnas.
- Name/organism: ApoA1-AS; Homo sapiens
- Databases &Code: GeneCaRNA, HGNC: 40079, NCBI Gene: 104326055, Ensembl: ENSG00000235910, OMIM ® : 620112
- Chromosomal location& size: 11q23.3, Size: 20,898 bases, Orientation: Plus strand
- Interaction with other molecules: SUZ12, a component of the polycomb repressive complex 2 (PRC2)
- Biological consequence of such interaction: Suppression of APOA1 expression
- Name/organism: ApoA4-AS; mouse
- Databases &Code: Ensembl and UCSC Genome Database
- Chromosomal location& size: ∼900-nt
- Interaction with other molecules: APOA4
- Biological consequence of such interaction: APOA4-AS may regulate the expression of APOA4
4.3.2. lncRNA Induced by HCV, Regulator of SREBF1
- Name/organism: LNCHR1; Homo sapiens
- Databases &Code: Ensemble: ENSG00000257400.1; HGNC:56254
- Chromosomal location& size: 12q22: 94,491,546–94,496,442 reverse strand; Size: 420 bp
- Interaction with other molecules: SREB-1c
- Biological consequence of such interaction: Regulation of the expression of SREBP-1-responsive genes
4.3.3. Solute Carrier Family 25 Member 15 (SLC25A15/lnc-HC)
- Name/organism: lnc-HC/SLC25A15/HHH/ORC1/ORNT1/D13S327
- Databases &Code: GenBank: MN026163.1
- Chromosomal location& size: 1063 bp, linear
- Interaction with other molecules: Coregulator: hnRNPA2B1
- Biological consequence of such interaction: Reduction of the stability of mRNAs encoding Cyp7a1 and Abca1 (critical enzymes that contribute to cholesterol catabolism).
4.3.4. Metastasis-Associated Lung Adenocarcinoma Transcript 1
- Name/organism: MALAT1; HCN, LINC00047, MASCRNA, NCRNA00047, NEAT2, PRO1073; Homo sapiens
- Databases &Code: Ensembl: ENSG00000251562.11; HGNC:29665
- Chromosomal location& size: 11q13.1: 65,497,640–65,508,073 forward strand; 66 transcripts (splice variants)
- Interaction with other molecules: miR-17-ABCA1; miRNA-124-3p (sponge)
- Biological consequence of such interaction: Contribution to cholesterol efflux, promotion of the upregulation of inflammatory CRP, modulation of PPARα expression.
4.3.5. A Novel Long Non-Coding RNA in Lipid Associated Single Nucleotide Polymorphism Gene Region
- Name/organism: LASER/ LINC02702; Homo sapiens
- Databases &Code: HGNC:54217; Ensembl: ENSG00000237937; NCBI Gene: 101929011
- Chromosomal location& size: 11q23.3; 11: 116,639,422–116,658,295 forward strand; 4 transcripts
- Interaction with other molecules: probably PCSK9
- Biological consequence of such interaction: Enhancement of the expression of cholesterol metabolism genes
5. LncRNAs as Diagnostic and Therapeutic Targets in Lipid Disorders
6. conclusions, institutional review board statement, informed consent statement, data availability statement, conflicts of interest.
- Zhang, X.; Price, N.L.; Fernández-Hernando, C. Non-coding RNAs in lipid metabolism. Vasc. Pharmacol. 2019 , 114 , 93–102. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- WHO. Cardiovascular Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 15 December 2023).
- Singh, D.D.; Kim, Y.; Choi, S.A.; Han, I.; Yadav, D.K. Clinical Significance of MicroRNAs, Long Non-Coding RNAs, and CircRNAs in Cardiovascular Diseases. Cells 2023 , 12 , 1629. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Poller, W.; Dimmeler, S.; Heymans, S.; Zeller, T.; Haas, J.; Karakas, M.; Leistner, D.-M.; Jakob, P.; Nakagawa, S.; Blankenberg, S. Non-coding RNAs in cardiovascular diseases: Diagnostic and therapeutic perspectives. Eur. Heart J. 2018 , 39 , 2704–2716. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Churov, A.; Summerhill, V.; Grechko, A.; Orekhova, V.; Orekhov, A. MicroRNAs as potential biomarkers in atherosclerosis. Int. J. Mol. Sci. 2019 , 20 , 5547. [ Google Scholar ] [ CrossRef ]
- Collins, L.; Binder, P.; Chen, H.; Wang, X. Regulation of Long Non-coding RNAs and MicroRNAs in Heart Disease: Insight Into Mechanisms and Therapeutic Approaches. Front. Physiol. 2020 , 11 , 798. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yoon, J.-H.; Abdelmohsen, K.; Srikantan, S.; Yang, X.; Martindale, J.L.; De, S.; Huarte, M.; Zhan, M.; Becker, K.G.; Gorospe, M. LincRNA-p21 suppresses target mRNA translation. Mol. Cell 2012 , 47 , 648–655. [ Google Scholar ] [ CrossRef ]
- Gong, C.; Maquat, L.E. lncRNAs transactivate STAU1-mediated mRNA decay by duplexing with 3′ UTRs via Alu elements. Nature 2011 , 470 , 284–288. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yin, J.; Zeng, X.; Ai, Z.; Yu, M.; Wu, Y.o.; Li, S. Construction and analysis of a lncRNA-miRNA-mRNA network based on competitive endogenous RNA reveal functional lncRNAs in oral cancer. BMC Med. Genom. 2020 , 13 , 84. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Paneru, B.; Ali, A.; Al-Tobasei, R.; Kenney, B.; Salem, M. Crosstalk among lncRNAs, microRNAs and mRNAs in the muscle ‘degradome’ of rainbow trout. Sci. Rep. 2018 , 8 , 8416. [ Google Scholar ] [ CrossRef ]
- Mercer, T.R.; Mattick, J.S. Structure and function of long noncoding RNAs in epigenetic regulation. Nat. Struct. Mol. Biol. 2013 , 20 , 300–307. [ Google Scholar ] [ CrossRef ]
- Das, S.; Shah, R.; Dimmeler, S.; Freedman, J.E.; Holley, C.; Lee, J.-M.; Moore, K.; Musunuru, K.; Wang, D.-Z.; Xiao, J.; et al. Noncoding RNAs in Cardiovascular Disease: Current Knowledge, Tools and Technologies for Investigation, and Future Directions: A Scientific Statement From the American Heart Association. Circ. Genom. Precis. Med. 2020 , 13 , e000062. [ Google Scholar ] [ CrossRef ]
- Harrow, J.; Frankish, A.; Gonzalez, J.M.; Tapanari, E.; Diekhans, M.; Kokocinski, F.; Aken, B.L.; Barrell, D.; Zadissa, A.; Searle, S. GENCODE: The reference human genome annotation for The ENCODE Project. Genome Res. 2012 , 22 , 1760–1774. [ Google Scholar ] [ CrossRef ]
- Zhao, Y.; Li, H.; Fang, S.; Kang, Y.; Wu, W.; Hao, Y.; Li, Z.; Bu, D.; Sun, N.; Zhang, M.Q. NONCODE 2016: An informative and valuable data source of long non-coding RNAs. Nucleic Acids Res. 2016 , 44 , D203–D208. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Kung, J.T.; Colognori, D.; Lee, J.T. Long noncoding RNAs: Past, present, and future. Genetics 2013 , 193 , 651–669. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Aryal, B.; Rotllan, N.; Fernández-Hernando, C. Noncoding RNAs and atherosclerosis. Curr. Atheroscler. Rep. 2014 , 16 , 1–11. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ma, L.; Bajic, V.B.; Zhang, Z. On the classification of long non-coding RNAs. RNA Biol. 2013 , 10 , 924–933. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Schmitz, S.U.; Grote, P.; Herrmann, B.G. Mechanisms of long noncoding RNA function in development and disease. Cell. Mol. Life Sci. 2016 , 73 , 2491–2509. [ Google Scholar ] [ CrossRef ]
- Wu, T.; Du, Y. LncRNAs: From basic research to medical application. Int. J. Biol. Sci. 2017 , 13 , 295. [ Google Scholar ] [ CrossRef ]
- Palazzo, A.F.; Lee, E.S. Non-coding RNA: What is functional and what is junk? Front. Genet. 2015 , 6 , 2. [ Google Scholar ] [ CrossRef ]
- Fang, Y.; Xu, Y.; Wang, R.; Hu, L.; Guo, D.; Xue, F.; Guo, W.; Zhang, D.; Hu, J.; Li, Y.; et al. Recent advances on the roles of LncRNAs in cardiovascular disease. J. Cell Mol. Med. 2020 , 24 , 12246–12257. [ Google Scholar ] [ CrossRef ]
- Hon, C.-C.; Ramilowski, J.A.; Harshbarger, J.; Bertin, N.; Rackham, O.J.; Gough, J.; Denisenko, E.; Schmeier, S.; Poulsen, T.M.; Severin, J. An atlas of human long non-coding RNAs with accurate 5′ ends. Nature 2017 , 543 , 199–204. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Liu, X.; Wang, T.-T.; Li, Y.; Shi, M.-M.; Li, H.-M.; Yuan, H.-X.; Mo, Z.-W.; Chen, J.; Zhang, B.; Chen, Y.-X.; et al. High density lipoprotein from coronary artery disease patients caused abnormal expression of long non-coding RNAs in vascular endothelial cells. Biochem. Biophys. Res. Commun. 2017 , 487 , 552–559. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Zeng, Y.; Ren, K.; Zhu, X.; Zheng, Z.; Yi, G. Long Noncoding RNAs: Advances in Lipid Metabolism. Adv. Clin. Chem. 2018 , 87 , 1–36. [ Google Scholar ] [ CrossRef ]
- Duan, J.; Huang, Z.; Nice, E.C.; Xie, N.; Chen, M.; Huang, C. Current advancements and future perspectives of long noncoding RNAs in lipid metabolism and signaling. J. Adv. Res. 2023 , 48 , 105–123. [ Google Scholar ] [ CrossRef ]
- Wang, K.C.; Chang, H.Y. Molecular mechanisms of long noncoding RNAs. Mol. Cell 2011 , 43 , 904–914. [ Google Scholar ] [ CrossRef ]
- Kino, T.; Hurt, D.E.; Ichijo, T.; Nader, N.; Chrousos, G.P. Noncoding RNA gas5 is a growth arrest–and starvation-associated repressor of the glucocorticoid receptor. Sci. Signal. 2010 , 3 , ra8. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ghafouri-Fard, S.; Dashti, S.; Taheri, M. The HOTTIP (HOXA transcript at the distal tip) lncRNA: Review of oncogenic roles in human. Biomed. Pharmacother. 2020 , 127 , 110158. [ Google Scholar ] [ CrossRef ]
- Lee, J.T. The X as model for RNA’s niche in epigenomic regulation. Cold Spring Harb. Perspect. Biol. 2010 , 2 , a003749. [ Google Scholar ] [ CrossRef ]
- Zhang, Y.; Du, W.; Yang, B. Long non-coding RNAs as new regulators of cardiac electrophysiology and arrhythmias: Molecular mechanisms, therapeutic implications and challenges. Pharmacol. Ther. 2019 , 203 , 107389. [ Google Scholar ] [ CrossRef ]
- Sallam, T.; Sandhu, J.; Tontonoz, P. Long noncoding RNA discovery in cardiovascular disease: Decoding form to function. Circ. Res. 2018 , 122 , 155–166. [ Google Scholar ] [ CrossRef ]
- Nelson, B.R.; Makarewich, C.A.; Anderson, D.M.; Winders, B.R.; Troupes, C.D.; Wu, F.; Reese, A.L.; McAnally, J.R.; Chen, X.; Kavalali, E.T. A peptide encoded by a transcript annotated as long noncoding RNA enhances SERCA activity in muscle. Science 2016 , 351 , 271–275. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Li, P.; Ruan, X.; Yang, L.; Kiesewetter, K.; Zhao, Y.; Luo, H.; Chen, Y.; Gucek, M.; Zhu, J.; Cao, H. A liver-enriched long non-coding RNA, lncLSTR, regulates systemic lipid metabolism in mice. Cell Metab. 2015 , 21 , 455–467. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wang, X.; Guo, S.; Hu, Y.; Guo, H.; Zhang, X.; Yan, Y.; Ma, J.; Li, Y.; Wang, H.; He, J.; et al. Microarray analysis of long non-coding RNA expression profiles in low high-density lipoprotein cholesterol disease. Lipids Health Dis. 2020 , 19 , 175. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Singh, A.K.; Aryal, B.; Zhang, X.; Fan, Y.; Price, N.L.; Suárez, Y.; Fernández-Hernando, C. Posttranscriptional regulation of lipid metabolism by non-coding RNAs and RNA binding proteins. In Seminars in Cell & Developmental Biology ; Academic Press: Cambridge, MA, USA, 2018; pp. 129–140. [ Google Scholar ]
- Chen, Z. Progress and prospects of long noncoding RNAs in lipid homeostasis. Mol. Metab. 2016 , 5 , 164–170. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999 , 340 , 115–126. [ Google Scholar ] [ CrossRef ]
- Glass, C.K.; Witztum, J.L. Atherosclerosis: The road ahead. Cell 2001 , 104 , 503–516. [ Google Scholar ] [ CrossRef ]
- Rosenson, R.S.; Brewer Jr, H.B.; Davidson, W.S.; Fayad, Z.A.; Fuster, V.; Goldstein, J.; Hellerstein, M.; Jiang, X.-C.; Phillips, M.C.; Rader, D.J. Cholesterol efflux and atheroprotection: Advancing the concept of reverse cholesterol transport. Circulation 2012 , 125 , 1905–1919. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Rader, D.J.; Hovingh, G.K. HDL and cardiovascular disease. Lancet 2014 , 384 , 618–625. [ Google Scholar ] [ CrossRef ]
- Linton, M.F.; Tao, H.; Linton, E.F.; Yancey, P.G. SR-BI: A multifunctional receptor in cholesterol homeostasis and atherosclerosis. Trends Endocrinol. Metab. 2017 , 28 , 461–472. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dikkers, A.; Tietge, U.J. Biliary cholesterol secretion: More than a simple ABC. World J. Gastroenterol. WJG 2010 , 16 , 5936. [ Google Scholar ]
- Nijstad, N.; Gautier, T.; Briand, F.; Rader, D.J.; Tietge, U.J. Biliary sterol secretion is required for functional in vivo reverse cholesterol transport in mice. Gastroenterology 2011 , 140 , 1043–1051. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Linsel-Nitschke, P.; Tall, A.R. HDL as a target in the treatment of atherosclerotic cardiovascular disease. Nat. Rev. Drug Discov. 2005 , 4 , 193–205. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Brewer, H.B. Focus on high-density lipoproteins in reducing cardiovascular risk. Am. Heart J. 2004 , 148 , S14–S18. [ Google Scholar ] [ CrossRef ]
- Chang, F.-J.; Yuan, H.-Y.; Hu, X.-X.; Ou, Z.-J.; Fu, L.; Lin, Z.-B.; Wang, Z.-P.; Wang, S.-M.; Zhou, L.; Xu, Y.-Q.; et al. High density lipoprotein from patients with valvular heart disease uncouples endothelial nitric oxide synthase. J. Mol. Cell. Cardiol. 2014 , 74 , 209–219. [ Google Scholar ] [ CrossRef ]
- Yuhanna, I.S.; Zhu, Y.; Cox, B.E.; Hahner, L.D.; Osborne-Lawrence, S.; Lu, P.; Marcel, Y.L.; Anderson, R.G.; Mendelsohn, M.E.; Hobbs, H.H.; et al. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat. Med. 2001 , 7 , 853–857. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Barter, P.J.; Caulfield, M.; Eriksson, M.; Grundy, S.M.; Kastelein, J.J.P.; Komajda, M.; Lopez-Sendon, J.; Mosca, L.; Tardif, J.-C.; Waters, D.D.; et al. Effects of Torcetrapib in Patients at High Risk for Coronary Events. N. Engl. J. Med. 2007 , 357 , 2109–2122. [ Google Scholar ] [ CrossRef ]
- Schwartz, G.G.; Olsson, A.G.; Abt, M.; Ballantyne, C.M.; Barter, P.J.; Brumm, J.; Chaitman, B.R.; Holme, I.M.; Kallend, D.; Leiter, L.A.; et al. Effects of Dalcetrapib in Patients with a Recent Acute Coronary Syndrome. N. Engl. J. Med. 2012 , 367 , 2089–2099. [ Google Scholar ] [ CrossRef ]
- HPS2-THRIVE randomized placebo-controlled trial in 25,673 high-risk patients of ER niacin/laropiprant: Trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur. Heart J. 2013 , 34 , 1279–1291. [ CrossRef ] [ PubMed ]
- Franceschini, G.; Sirtori, C.R.; Capurso, A., II; Weisgraber, K.H.; Mahley, R.W. A-IMilano apoprotein. Decreased high density lipoprotein cholesterol levels with significant lipoprotein modifications and without clinical atherosclerosis in an Italian family. J. Clin. Investig. 1980 , 66 , 892–900. [ Google Scholar ] [ CrossRef ]
- Speer, T.; Rohrer, L.; Blyszczuk, P.; Shroff, R.; Kuschnerus, K.; Kränkel, N.; Kania, G.; Zewinger, S.; Akhmedov, A.; Shi, Y.; et al. Abnormal high-density lipoprotein induces endothelial dysfunction via activation of Toll-like receptor-2. Immunity 2013 , 38 , 754–768. [ Google Scholar ] [ CrossRef ]
- Sorrentino, S.A.; Besler, C.; Rohrer, L.; Meyer, M.; Heinrich, K.; Bahlmann, F.H.; Mueller, M.; Horváth, T.; Doerries, C.; Heinemann, M.; et al. Endothelial-vasoprotective effects of high-density lipoprotein are impaired in patients with type 2 diabetes mellitus but are improved after extended-release niacin therapy. Circulation 2010 , 121 , 110–122. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ma, X.-Y.; Liu, J.-P.; Song, Z.-Y. Associations of the ATP-binding cassette transporter A1 R219K polymorphism with HDL-C level and coronary artery disease risk: A meta-analysis. Atherosclerosis 2011 , 215 , 428–434. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Li, D.; Cheng, M.; Niu, Y.; Chi, X.; Liu, X.; Fan, J.; Fan, H.; Chang, Y.; Yang, W. Identification of a novel human long non-coding RNA that regulates hepatic lipid metabolism by inhibiting SREBP-1c. Int. J. Biol. Sci. 2017 , 13 , 349. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Liu, C.; Yang, Z.; Wu, J.; Zhang, L.; Lee, S.; Shin, D.J.; Tran, M.; Wang, L. Long noncoding RNA H19 interacts with polypyrimidine tract-binding protein 1 to reprogram hepatic lipid homeostasis. Hepatology 2018 , 67 , 1768–1783. [ Google Scholar ] [ CrossRef ]
- Kumar, S.; Williams, D.; Sur, S.; Wang, J.-Y.; Jo, H. Role of flow-sensitive microRNAs and long noncoding RNAs in vascular dysfunction and atherosclerosis. Vasc. Pharmacol. 2019 , 114 , 76–92. [ Google Scholar ] [ CrossRef ]
- Hu, Y.-W.; Zhao, J.-Y.; Li, S.-F.; Huang, J.-L.; Qiu, Y.-R.; Ma, X.; Wu, S.-G.; Chen, Z.-P.; Hu, Y.-R.; Yang, J.-Y. RP5-833A20. 1/miR-382-5p/NFIA–dependent signal transduction pathway contributes to the regulation of cholesterol homeostasis and inflammatory reaction. Arterioscler. Thromb. Vasc. Biol. 2015 , 35 , 87–101. [ Google Scholar ] [ CrossRef ]
- Wang, B.; Tontonoz, P. Liver X receptors in lipid signalling and membrane homeostasis. Nat. Rev. Endocrinol. 2018 , 14 , 452–463. [ Google Scholar ] [ CrossRef ]
- Sallam, T.; Jones, M.C.; Gilliland, T.; Zhang, L.; Wu, X.; Eskin, A.; Sandhu, J.; Casero, D.; Vallim, T.Q.d.A.; Hong, C. Feedback modulation of cholesterol metabolism by the lipid-responsive non-coding RNA LeXis. Nature 2016 , 534 , 124–128. [ Google Scholar ] [ CrossRef ]
- Sallam, T.; Jones, M.; Thomas, B.J.; Wu, X.; Gilliland, T.; Qian, K.; Eskin, A.; Casero, D.; Zhang, Z.; Sandhu, J. Transcriptional regulation of macrophage cholesterol efflux and atherogenesis by a long noncoding RNA. Nat. Med. 2018 , 24 , 304–312. [ Google Scholar ] [ CrossRef ]
- Baggish, A.L.; Hale, A.; Weiner, R.B.; Lewis, G.D.; Systrom, D.; Wang, F.; Wang, T.J.; Chan, S.Y. Dynamic regulation of circulating microRNA during acute exhaustive exercise and sustained aerobic exercise training. J. Physiol. 2011 , 589 , 3983–3994. [ Google Scholar ] [ CrossRef ]
- Mitchel, K.; Theusch, E.; Cubitt, C.; Dosé, A.C.; Stevens, K.; Naidoo, D.; Medina, M.W. RP1-13D10.2 Is a Novel Modulator of Statin-Induced Changes in Cholesterol. Circ. Cardiovasc. Genet. 2016 , 9 , 223–230. [ Google Scholar ] [ CrossRef ]
- Zelcer, N.; Hong, C.; Boyadjian, R.; Tontonoz, P. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science 2009 , 325 , 100–104. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- de Aguiar Vallim, T.Q.; Tarling, E.J.; Edwards, P.A. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013 , 17 , 657–669. [ Google Scholar ] [ CrossRef ]
- Thomas, C.; Pellicciari, R.; Pruzanski, M.; Auwerx, J.; Schoonjans, K. Targeting bile-acid signalling for metabolic diseases. Nat. Rev. Drug Discov. 2008 , 7 , 678–693. [ Google Scholar ] [ CrossRef ]
- Lee, E.B.; Lee, V.M.-Y.; Trojanowski, J.Q. Gains or losses: Molecular mechanisms of TDP43-mediated neurodegeneration. Nat. Rev. Neurosci. 2012 , 13 , 38–50. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ye, W.C.; Huang, S.F.; Hou, L.J.; Long, H.J.; Yin, K.; Hu, C.Y.; Zhao, G.J. Potential Therapeutic Targeting of lncRNAs in Cholesterol Homeostasis. Front. Cardiovasc. Med. 2021 , 8 , 688546. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ghafouri-Fard, S.; Gholipour, M.; Taheri, M. The Emerging Role of Long Non-coding RNAs and Circular RNAs in Coronary Artery Disease. Front. Cardiovasc. Med. 2021 , 8 , 632393. [ Google Scholar ] [ CrossRef ]
- Hennessy, E.J.; van Solingen, C.; Scacalossi, K.R.; Ouimet, M.; Afonso, M.S.; Prins, J.; Koelwyn, G.J.; Sharma, M.; Ramkhelawon, B.; Carpenter, S.; et al. The long noncoding RNA CHROME regulates cholesterol homeostasis in primate. Nat. Metab. 2019 , 1 , 98–110. [ Google Scholar ] [ CrossRef ]
- Han, L.; Huang, D.; Wu, S.; Liu, S.; Wang, C.; Sheng, Y.; Lu, X.; Broxmeyer, H.E.; Wan, J.; Yang, L. Lipid droplet-associated lncRNA LIPTER preserves cardiac lipid metabolism. Nat. Cell Biol. 2023 , 25 , 1033–1046. [ Google Scholar ] [ CrossRef ]
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019 , 20 , 137–155. [ Google Scholar ] [ CrossRef ]
- Goldberg, I.J.; Reue, K.; Abumrad, N.A.; Bickel, P.E.; Cohen, S.; Fisher, E.A.; Galis, Z.S.; Granneman, J.G.; Lewandowski, E.D.; Murphy, R. Deciphering the role of lipid droplets in cardiovascular disease: A report from the 2017 national heart, lung, and blood institute workshop. Circulation 2018 , 138 , 305–315. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Listenberger, L.L.; Han, X.; Lewis, S.E.; Cases, S.; Farese, R.V., Jr.; Ory, D.S.; Schaffer, J.E. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. USA 2003 , 100 , 3077–3082. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Borradaile, N.M.; Schaffer, J.E. Lipotoxicity in the heart. Curr. Hypertens. Rep. 2005 , 7 , 412–417. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gao, Y.; Wang, Y.; Chen, X.; Peng, Y.; Chen, F.; He, Y.; Pang, W.; Yang, G.; Yu, T. MiR-127 attenuates adipogenesis by targeting MAPK4 and HOXC6 in porcine adipocytes. J. Cell. Physiol. 2019 , 234 , 21838–21850. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Geuens, T.; Bouhy, D.; Timmerman, V. The hnRNP family: Insights into their role in health and disease. Hum. Genet. 2016 , 135 , 851–867. [ Google Scholar ] [ CrossRef ]
- Shen, X.; Zhang, Y.; Ji, X.; Li, B.; Wang, Y.; Huang, Y.; Zhang, X.; Yu, J.; Zou, R.; Qin, D.; et al. Long Noncoding RNA lncRHL Regulates Hepatic VLDL Secretion by Modulating hnRNPU/BMAL1/MTTP Axis. Diabetes 2022 , 71 , 1915–1928. [ Google Scholar ] [ CrossRef ]
- Chu, K.; Zhao, N.; Hu, X.; Feng, R.; Zhang, L.; Wang, G.; Li, W.; Liu, L. LncNONMMUG027912 alleviates lipid accumulation through AMPKα/mTOR/SREBP1C axis in nonalcoholic fatty liver. Biochem. Biophys. Res. Commun. 2022 , 618 , 8–14. [ Google Scholar ] [ CrossRef ]
- Liu, Y.; Jia, L.; Min, D.; Xu, Y.; Zhu, J.; Sun, Z. Baicalin inhibits proliferation and promotes apoptosis of vascular smooth muscle cells by regulating the MEG3/p53 pathway following treatment with ox-LDL. Int. J. Mol. Med. 2019 , 43 , 901–913. [ Google Scholar ] [ CrossRef ]
- Yang, X.; Wang, C.C.; Lee, W.Y.W.; Trovik, J.; Chung, T.K.H.; Kwong, J. Long non-coding RNA HAND2-AS1 inhibits invasion and metastasis in endometrioid endometrial carcinoma through inactivating neuromedin U. Cancer Lett. 2018 , 413 , 23–34. [ Google Scholar ] [ CrossRef ]
- Zhu, X.; Li, H.; Wu, Y.; Zhou, J.; Yang, G.; Wang, W. lncRNA MEG3 promotes hepatic insulin resistance by serving as a competing endogenous RNA of miR-214 to regulate ATF4 expression. Int. J. Mol. Med. 2019 , 43 , 345–357. [ Google Scholar ] [ CrossRef ]
- Huang, P.; Huang, F.-Z.; Liu, H.-Z.; Zhang, T.-Y.; Yang, M.-S.; Sun, C.-Z. LncRNA MEG3 functions as a ceRNA in regulating hepatic lipogenesis by competitively binding to miR-21 with LRP6. Metabolism 2019 , 94 , 1–8. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Li, H.; Han, S.; Sun, Q.; Yao, Y.; Li, S.; Yuan, C.; Zhang, B.; Jing, B.; Wu, J.; Song, Y.; et al. Long non-coding RNA CDKN2B-AS1 reduces inflammatory response and promotes cholesterol efflux in atherosclerosis by inhibiting ADAM10 expression. Aging 2019 , 11 , 1695–1715. [ Google Scholar ] [ CrossRef ]
- Huang, C.; Hu, Y.-W.; Zhao, J.-J.; Ma, X.; Zhang, Y.; Guo, F.-X.; Kang, C.-M.; Lu, J.-B.; Xiu, J.-C.; Sha, Y.-H. Long noncoding RNA HOXC-AS1 suppresses Ox-LDL-induced cholesterol accumulation through promoting HOXC6 expression in THP-1 macrophages. DNA Cell Biol. 2016 , 35 , 722–729. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Papp, E.; Havasi, V.; Bene, J.; Komlosi, K.; Czopf, L.; Magyar, E.; Feher, C.; Feher, G.; Horvath, B.; Marton, Z. Glycoprotein IIIA gene (PIA) polymorphism and aspirin resistance: Is there any correlation? Ann. Pharmacother. 2005 , 39 , 1013–1018. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Morgan, E.A.; Schneider, J.G.; Baroni, T.E.; Uluçkan, Ö.; Heller, E.; Hurchla, M.A.; Deng, H.; Floyd, D.; Berdy, A.; Prior, J.L. Dissection of platelet and myeloid cell defects by conditional targeting of the β3-integrin subunit. FASEB J. 2010 , 24 , 1117. [ Google Scholar ] [ CrossRef ]
- Shao, J.; Fu, Y.; Yang, W.; Yan, J.; Zhao, J.; Chen, S.; Xia, W. Thromboxane A2 receptor polymorphism in association with cerebral infarction and its regulation on platelet function. Curr. Neurovasc. Res. 2015 , 12 , 15–24. [ Google Scholar ] [ CrossRef ]
- Cai, C.; Zhu, H.; Ning, X.; Li, L.; Yang, B.; Chen, S.; Wang, L.; Lu, X.; Gu, D. LncRNA ENST00000602558.1 regulates ABCG1 expression and cholesterol efflux from vascular smooth muscle cells through a p65-dependent pathway. Atherosclerosis 2019 , 285 , 31–39. [ Google Scholar ] [ CrossRef ]
- Ageeli Hakami, M. Diabetes and diabetic associative diseases: An overview of epigenetic regulations of TUG1. Saudi J. Biol. Sci. 2024 , 31 , 103976. [ Google Scholar ] [ CrossRef ]
- Yang, L.; Li, T. LncRNA TUG1 regulates ApoM to promote atherosclerosis progression through miR-92a/FXR1 axis. J. Cell. Mol. Med. 2020 , 24 , 8836–8848. [ Google Scholar ] [ CrossRef ]
- Zhang, L.; Cheng, H.; Yue, Y.; Li, S.; Zhang, D.; He, R. TUG1 knockdown ameliorates atherosclerosis via up-regulating the expression of miR-133a target gene FGF1. Cardiovasc. Pathol. 2018 , 33 , 6–15. [ Google Scholar ] [ CrossRef ]
- Zhu, X.H.; Yuan, Y.X.; Rao, S.L.; Wang, P. LncRNA MIAT enhances cardiac hypertrophy partly through sponging miR-150. Eur. Rev. Med. Pharmacol. Sci. 2016 , 20 , 3653–3660. [ Google Scholar ] [ PubMed ]
- Ye, Z.-M.; Yang, S.; Xia, Y.-P.; Hu, R.-T.; Chen, S.; Li, B.-W.; Chen, S.-L.; Luo, X.-Y.; Mao, L.; Li, Y. LncRNA MIAT sponges miR-149-5p to inhibit efferocytosis in advanced atherosclerosis through CD47 upregulation. Cell Death Dis. 2019 , 10 , 138. [ Google Scholar ] [ CrossRef ]
- Sun, G.; Li, Y.; Ji, Z. Up-regulation of MIAT aggravates the atherosclerotic damage in atherosclerosis mice through the activation of PI3K/Akt signaling pathway. Drug Deliv. 2019 , 26 , 641–649. [ Google Scholar ] [ CrossRef ]
- Li, H.; Zhang, Y.; Guo, Y.; Liu, R.; Yu, Q.; Gong, L.; Liu, Z.; Xie, W.; Wang, C. ALKBH1 promotes lung cancer by regulating m6A RNA demethylation. Biochem. Pharmacol. 2021 , 189 , 114284. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wu, L.; Pei, Y.; Zhu, Y.; Jiang, M.; Wang, C.; Cui, W.; Zhang, D. Association of N6-methyladenine DNA with plaque progression in atherosclerosis via myocardial infarction-associated transcripts. Cell Death Dis. 2019 , 10 , 909. [ Google Scholar ] [ CrossRef ]
- Sun, C.; Huang, L.; Li, Z.; Leng, K.; Xu, Y.; Jiang, X.; Cui, Y. Long non-coding RNA MIAT in development and disease: A new player in an old game. J. Biomed. Sci. 2018 , 25 , 1–7. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yan, B.; Yao, J.; Liu, J.-Y.; Li, X.-M.; Wang, X.-Q.; Li, Y.-J.; Tao, Z.-F.; Song, Y.-C.; Chen, Q.; Jiang, Q. lncRNA-MIAT regulates microvascular dysfunction by functioning as a competing endogenous RNA. Circ. Res. 2015 , 116 , 1143–1156. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dong, X.H.; Lu, Z.F.; Kang, C.M.; Li, X.H.; Haworth, K.E.; Ma, X.; Lu, J.B.; Liu, X.H.; Fang, F.C.; Wang, C.S.; et al. The Long Noncoding RNA RP11-728F11.4 Promotes Atherosclerosis. Arter. Thromb. Vasc. Biol. 2021 , 41 , 1191–1204. [ Google Scholar ] [ CrossRef ]
- Li, P.; Yan, X.; Xu, G.; Pang, Z.; Weng, J.; Yin, J.; Li, M.; Yu, L.; Chen, Q.; Sun, K. A novel plasma lncRNA ENST00000416361 is upregulated in coronary artery disease and is related to inflammation and lipid metabolism. Mol. Med. Rep. 2020 , 21 , 2375–2384. [ Google Scholar ] [ CrossRef ]
- Brown, M.S.; Goldstein, J.L. The SREBP Pathway: Regulation of Cholesterol Metabolism by Proteolysis of a Membrane-Bound Transcription Factor. Cell 1997 , 89 , 331–340. [ Google Scholar ] [ CrossRef ]
- Sato, R. Sterol metabolism and SREBP activation. Arch. Biochem. Biophys. 2010 , 501 , 177–181. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pérez-Belmonte, L.M.; Moreno-Santos, I.; Cabrera-Bueno, F.; Sánchez-Espín, G.; Castellano, D.; Such, M.; Crespo-Leiro, M.G.; Carrasco-Chinchilla, F.; Alonso-Pulpón, L.; López-Garrido, M.; et al. Expression of Sterol Regulatory Element-Binding Proteins in epicardial adipose tissue in patients with coronary artery disease and diabetes mellitus: Preliminary study. Int. J. Med. Sci. 2017 , 14 , 268–274. [ Google Scholar ] [ CrossRef ]
- Sun, C.; Fu, Y.; Gu, X.; Xi, X.; Peng, X.; Wang, C.; Sun, Q.; Wang, X.; Qian, F.; Qin, Z.; et al. Macrophage-Enriched lncRNA RAPIA. Arterioscler. Thromb. Vasc. Biol. 2020 , 40 , 1464–1478. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hu, S.; Liu, Y.; You, T.; Heath, J.; Xu, L.; Zheng, X.; Wang, A.; Wang, Y.; Li, F.; Yang, F. Vascular semaphorin 7A upregulation by disturbed flow promotes atherosclerosis through endothelial β1 integrin. Arterioscler. Thromb. Vasc. Biol. 2018 , 38 , 335–343. [ Google Scholar ] [ CrossRef ]
- Pan, Y.; Xin, W.; Wei, W.; Tatenhorst, L.; Graf, I.; Popa-Wagner, A.; Gerner, S.T.; Huber, S.E.; Kilic, E.; Hermann, D.M.; et al. Knockdown of NEAT1 prevents post-stroke lipid droplet agglomeration in microglia by regulating autophagy. Cell Mol. Life Sci. 2024 , 81 , 30. [ Google Scholar ] [ CrossRef ]
- Vlachogiannis, N.I.; Sachse, M.; Georgiopoulos, G.; Zormpas, E.; Bampatsias, D.; Delialis, D.; Bonini, F.; Galyfos, G.; Sigala, F.; Stamatelopoulos, K. Adenosine-to-inosine Alu RNA editing controls the stability of the pro-inflammatory long noncoding RNA NEAT1 in atherosclerotic cardiovascular disease. J. Mol. Cell. Cardiol. 2021 , 160 , 111–120. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wang, L.; Xia, J.W.; Ke, Z.P.; Zhang, B.H. Blockade of NEAT1 represses inflammation response and lipid uptake via modulating miR-342-3p in human macrophages THP-1 cells. J. Cell. Physiol. 2019 , 234 , 5319–5326. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Du, X.J.; Wei, J.; Tian, D.; Yan, C.; Hu, P.; Wu, X.; Yang, W.; Hu, X. NEAT1 promotes myocardial ischemia-reperfusion injury via activating the MAPK signaling pathway. J. Cell. Physiol. 2019 , 234 , 18773–18780. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and biological attributes of matrix metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017 , 147 , 1–73. [ Google Scholar ]
- Luo, M.; Sun, Q.; Zhao, H.; Tao, J.; Yan, D. Long noncoding RNA NEAT1 sponges miR-495-3p to enhance myocardial ischemia-reperfusion injury via MAPK6 activation. J. Cell. Physiol. 2020 , 235 , 105–113. [ Google Scholar ] [ CrossRef ]
- Fan, G.; Zhang, C.; Wei, X.; Wei, R.; Qi, Z.; Chen, K.; Cai, X.; Xu, L.; Tang, L.; Zhou, J. NEAT1/hsa-miR-372–3p axis participates in rapaMYCin-induced lipid metabolic disorder. Free Radic. Biol. Med. 2021 , 167 , 1–11. [ Google Scholar ] [ CrossRef ]
- Liu, X.; Liang, Y.; Song, R.; Yang, G.; Han, J.; Lan, Y.; Pan, S.; Zhu, M.; Liu, Y.; Wang, Y. Long non-coding RNA NEAT1-modulated abnormal lipolysis via ATGL drives hepatocellular carcinoma proliferation. Mol. Cancer 2018 , 17 , 1–18. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ma, X.; Wang, T.; Zhao, Z.-L.; Jiang, Y.; Ye, S. Propofol suppresses proinflammatory cytokine production by increasing ABCA1 expression via mediation by the long noncoding RNA LOC286367. Mediat. Inflamm. 2018 , 2018 , 8907143. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Joyce, C.W.; Amar, M.J.; Lambert, G.; Vaisman, B.L.; Paigen, B.; Najib-Fruchart, J.; Hoyt Jr, R.F.; Neufeld, E.D.; Remaley, A.T.; Fredrickson, D.S. The ATP binding cassette transporter A1 (ABCA1) modulates the development of aortic atherosclerosis in C57BL/6 and apoE-knockout mice. Proc. Natl. Acad. Sci. USA 2002 , 99 , 407–412. [ Google Scholar ] [ CrossRef ]
- Gao, J.-H.; He, L.-H.; Yu, X.-H.; Zhao, Z.-W.; Wang, G.; Zou, J.; Wen, F.-J.; Zhou, L.; Wan, X.-J.; Zhang, D.-W. CXCL12 promotes atherosclerosis by downregulating ABCA1 expression via the CXCR4/GSK3β/β-cateninT120/TCF21 pathway. J. Lipid Res. 2019 , 60 , 2020–2033. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Huang, J.; Chen, S.; Cai, D.; Bian, D.; Wang, F. Long noncoding RNA lncARSR promotes hepatic cholesterol biosynthesis via modulating Akt/SREBP-2/HMGCR pathway. Life Sci. 2018 , 203 , 48–53. [ Google Scholar ] [ CrossRef ]
- Cirera-Salinas, D.; Pauta, M.; Allen, R.M.; Salerno, A.G.; Ramírez, C.M.; Chamorro-Jorganes, A.; Wanschel, A.C.; Lasuncion, M.A.; Morales-Ruiz, M.; Suarez, Y. Mir-33 regulates cell proliferation and cell cycle progression. Cell Cycle 2012 , 11 , 922–933. [ Google Scholar ] [ CrossRef ]
- Go, G.-W.; Mani, A. Low-density lipoprotein receptor (LDLR) family orchestrates cholesterol homeostasis. Yale J. Biol. Med. 2012 , 85 , 19. [ Google Scholar ]
- Ray, R.M.; Hansen, A.H.; Slott, S.; Taskova, M.; Astakhova, K.; Morris, K.V. Control of LDL uptake in human cells by targeting the LDLR regulatory long non-coding RNA BM450697. Mol. Ther. -Nucleic Acids 2019 , 17 , 264–276. [ Google Scholar ] [ CrossRef ]
- Chen, L.; Yao, H.; Hui, J.Y.; Ding, S.H.; Fan, Y.L.; Pan, Y.H.; Chen, K.H.; Wan, J.Q.; Jiang, J.Y. Global transcriptomic study of atherosclerosis development in rats. Gene 2016 , 592 , 43–48. [ Google Scholar ] [ CrossRef ]
- Meng, X.-D.; Yao, H.-H.; Wang, L.-M.; Yu, M.; Shi, S.; Yuan, Z.-X.; Liu, J. Knockdown of GAS5 inhibits atherosclerosis progression via reducing EZH2-mediated ABCA1 transcription in ApoE−/− mice. Mol. Ther.-Nucleic Acids 2020 , 19 , 84–96. [ Google Scholar ] [ CrossRef ]
- Halley, P.; Kadakkuzha, B.M.; Faghihi, M.A.; Magistri, M.; Zeier, Z.; Khorkova, O.; Coito, C.; Hsiao, J.; Lawrence, M.; Wahlestedt, C. Regulation of the apolipoprotein gene cluster by a long noncoding RNA. Cell Rep. 2014 , 6 , 222–230. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Qin, W.; Li, X.; Xie, L.; Li, S.; Liu, J.; Jia, L.; Dong, X.; Ren, X.; Xiao, J.; Yang, C. A long non-coding RNA, APOA4-AS, regulates APOA4 expression depending on HuR in mice. Nucleic Acids Res. 2016 , 44 , 6423–6433. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lan, X.; Yan, J.; Ren, J.; Zhong, B.; Li, J.; Li, Y.; Liu, L.; Yi, J.; Sun, Q.; Yang, X. A novel long noncoding RNA Lnc-HC binds hnRNPA2B1 to regulate expressions of Cyp7a1 and Abca1 in hepatocytic cholesterol metabolism. Hepatology 2016 , 64 , 58–72. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lan, X.; Wu, L.; Wu, N.; Chen, Q.; Li, Y.; Du, X.; Wei, C.; Feng, L.; Li, Y.; Osoro, E.K. Long noncoding RNA lnc-HC regulates PPARγ-mediated hepatic lipid metabolism through miR-130b-3p. Mol. Ther.-Nucleic Acids 2019 , 18 , 954–965. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lv, F.; Liu, L.; Feng, Q.; Yang, X. Long non-coding RNA MALAT1 and its target microRNA-125b associate with disease risk, severity, and major adverse cardiovascular event of coronary heart disease. J. Clin. Lab. Anal. 2021 , 35 , e23593. [ Google Scholar ] [ CrossRef ]
- Guo, Y.; Luo, F.; Liu, Q.; Xu, D. Regulatory non-coding RNAs in acute myocardial infarction. J. Cell. Mol. Med. 2017 , 21 , 1013–1023. [ Google Scholar ] [ CrossRef ]
- Liu, L.; Tan, L.; Yao, J.; Yang, L. Long non-coding RNA MALAT1 regulates cholesterol accumulation in ox-LDL-induced macrophages via the microRNA-17-5p/ABCA1 axis. Mol. Med. Rep. 2020 , 21 , 1761–1770. [ Google Scholar ] [ CrossRef ]
- Liu, Z.; Liu, J.; Wei, Y.; Xu, J.; Wang, Z.; Wang, P.; Sun, H.; Song, Z.; Liu, Q. LncRNA MALAT1 prevents the protective effects of miR-125b-5p against acute myocardial infarction through positive regulation of NLRC5. Exp. Ther. Med. 2020 , 19 , 990–998. [ Google Scholar ] [ CrossRef ]
- Chao, C.T.; Yeh, H.Y.; Yuan, T.H.; Chiang, C.K.; Chen, H.W. MicroRNA-125b in vascular diseases: An updated systematic review of pathogenetic implications and clinical applications. J. Cell. Mol. Med. 2019 , 23 , 5884–5894. [ Google Scholar ] [ CrossRef ]
- Ding, X.-Q.; Ge, P.-C.; Liu, Z.; Jia, H.; Chen, X.; An, F.-H.; Li, L.-H.; Chen, Z.-H.; Mao, H.-W.; Li, Z.-Y. Interaction between microRNA expression and classical risk factors in the risk of coronary heart disease. Sci. Rep. 2015 , 5 , 14925. [ Google Scholar ] [ CrossRef ]
- Akhabue, E.; Thiboutot, J.; Cheng, J.-w.; Lerakis, S.; Vittorio, T.J.; Christodoulidis, G.; Grady, K.M.; Kosmas, C.E. New and emerging risk factors for coronary heart disease. Am. J. Med. Sci. 2014 , 347 , 151–158. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Li, C.; Hu, Z.; Zhang, W.; Yu, J.; Yang, Y.; Xu, Z.; Luo, H.; Liu, X.; Liu, Y.; Chen, C. Regulation of cholesterol homeostasis by a novel long non-coding RNA LASER. Sci. Rep. 2019 , 9 , 7693. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Seidah, N.G.; Awan, Z.; Chrétien, M.; Mbikay, M. PCSK9: A key modulator of cardiovascular health. Circ. Res. 2014 , 114 , 1022–1036. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lee, K.-H.; Hwang, H.-J.; Cho, J.-Y. Long Non-Coding RNA Associated with Cholesterol Homeostasis and Its Involvement in Metabolic Diseases. Int. J. Mol. Sci. 2020 , 21 , 8337. [ Google Scholar ] [ CrossRef ]
- Paredes, S.; Fonseca, L.; Ribeiro, L.; Ramos, H.; Oliveira, J.C.; Palma, I. Novel and traditional lipid profiles in metabolic syndrome reveal a high atherogenicity. Sci. Rep. 2019 , 9 , 11792. [ Google Scholar ] [ CrossRef ]
- Cox, R.; García-Palmieri, M. Cholesterol, Triglycerides, and Associated Lipoproteins. In Clinical Methods: The History, Physical, and Laboratory Examinations , 3rd ed.; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990. [ Google Scholar ]
- Wang, W.-T.; Sun, Y.-M.; Huang, W.; He, B.; Zhao, Y.-N.; Chen, Y.-Q. Genome-wide long non-coding RNA analysis identified circulating LncRNAs as novel non-invasive diagnostic biomarkers for gynecological disease. Sci. Rep. 2016 , 6 , 23343. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Pardini, B.; Sabo, A.A.; Birolo, G.; Calin, G.A. Noncoding RNAs in extracellular fluids as cancer biomarkers: The new frontier of liquid biopsies. Cancers 2019 , 11 , 1170. [ Google Scholar ] [ CrossRef ]
- Huang, S.F.; Peng, X.F.; Jiang, L.; Hu, C.Y.; Ye, W.C. LncRNAs as Therapeutic Targets and Potential Biomarkers for Lipid-Related Diseases. Front. Pharmacol. 2021 , 12 , 729745. [ Google Scholar ] [ CrossRef ]
- Pan, J.-X. LncRNA H19 promotes atherosclerosis by regulating MAPK and NF-kB signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2017 , 21 , 322–328. [ Google Scholar ]
- Tao, K.; Hu, Z.; Zhang, Y.; Jiang, D.; Cheng, H. LncRNA CASC11 improves atherosclerosis by downregulating IL-9 and regulating vascular smooth muscle cell apoptosis and proliferation. Biosci. Biotechnol. Biochem. 2019 , 83 , 1284–1288. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chen, L.; Yang, W.; Guo, Y.; Chen, W.; Zheng, P.; Zeng, J.; Tong, W. Exosomal lncRNA GAS5 regulates the apoptosis of macrophages and vascular endothelial cells in atherosclerosis. PLoS ONE 2017 , 12 , e0185406. [ Google Scholar ] [ CrossRef ]
- Li, F.-P.; Lin, D.-Q.; Gao, L.-Y. LncRNA TUG1 promotes proliferation of vascular smooth muscle cell and atherosclerosis through regulating miRNA-21/PTEN axis. Eur. Rev. Med. Pharmacol. Sci. 2018 , 22 , 7439–7447. [ Google Scholar ] [ PubMed ]
- Yao, X.; Yan, C.; Zhang, L.; Li, Y.; Wan, Q. LncRNA ENST00113 promotes proliferation, survival, and migration by activating PI3K/Akt/mTOR signaling pathway in atherosclerosis. Medicine 2018 , 97 , e0473. [ Google Scholar ] [ CrossRef ]
- Hu, Y.-W.; Guo, F.-X.; Xu, Y.-J.; Li, P.; Lu, Z.-F.; McVey, D.G.; Zheng, L.; Wang, Q.; John, H.Y.; Kang, C.-M. Long noncoding RNA NEXN-AS1 mitigates atherosclerosis by regulating the actin-binding protein NEXN. J. Clin. Investig. 2019 , 129 , 1115–1128. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Yao, Y.; Zhang, T.; Qi, L.; Zhou, C.; Wei, J.; Feng, F.; Liu, R.; Sun, C. Integrated analysis of co-expression and ceRNA network identifies five lncRNAs as prognostic markers for breast cancer. J. Cell. Mol. Med. 2019 , 23 , 8410–8419. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Park, J.G.; Kim, G.; Jang, S.Y.; Lee, Y.R.; Lee, E.; Lee, H.W.; Han, M.-H.; Chun, J.M.; Han, Y.S.; Yoon, J.S. Plasma long noncoding RNA LeXis is a potential diagnostic marker for non-alcoholic steatohepatitis. Life 2020 , 10 , 230. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mannu, G.; Zaman, M.; Gupta, A.; Rehman, H.; Myint, P. Evidence of lifestyle modification in the management of hypercholesterolemia. Curr. Cardiol. Rev. 2013 , 9 , 2–14. [ Google Scholar ]
- Chi, X.; Gatti, P.; Papoian, T. Safety of antisense oligonucleotide and siRNA-based therapeutics. Drug Discov. Today 2017 , 22 , 823–833. [ Google Scholar ] [ CrossRef ]
- Liu, S.J.; Horlbeck, M.A.; Cho, S.W.; Birk, H.S.; Malatesta, M.; He, D.; Attenello, F.J.; Villalta, J.E.; Cho, M.Y.; Chen, Y. CRISPRi-based genome-scale identification of functional long noncoding RNA loci in human cells. Science 2017 , 355 , eaah7111. [ Google Scholar ] [ CrossRef ]
- Zhang, H.; Zou, X.; Liu, F. Silencing TTTY15 mitigates hypoxia-induced mitochondrial energy metabolism dysfunction and cardiomyocytes apoptosis via TTTY15/let-7i-5p and TLR3/NF-κB pathways. Cell. Signal. 2020 , 76 , 109779. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Gong, N.; Teng, X.; Li, J.; Liang, X.-J. Antisense oligonucleotide-conjugated nanostructure-targeting lncRNA MALAT1 inhibits cancer metastasis. ACS Appl. Mater. Interfaces 2018 , 11 , 37–42. [ Google Scholar ] [ CrossRef ]
- Bernardo, B.C.; Ooi, J.Y.; Lin, R.C.; McMullen, J.R. miRNA therapeutics: A new class of drugs with potential therapeutic applications in the heart. Future Med. Chem. 2015 , 7 , 1771–1792. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lee, J.-S.; Mendell, J.T. Antisense-mediated transcript knockdown triggers premature transcription termination. Mol. Cell 2020 , 77 , 1044–1054.e1043. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wan, W.B.; Seth, P.P. The medicinal chemistry of therapeutic oligonucleotides. J. Med. Chem. 2016 , 59 , 9645–9667. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Dhuri, K.; Bechtold, C.; Quijano, E.; Pham, H.; Gupta, A.; Vikram, A.; Bahal, R. Antisense oligonucleotides: An emerging area in drug discovery and development. J. Clin. Med. 2020 , 9 , 2004. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Stein, C.A.; Castanotto, D. FDA-approved oligonucleotide therapies in 2017. Mol. Ther. 2017 , 25 , 1069–1075. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Bhat, S.A.; Ahmad, S.M.; Mumtaz, P.T.; Malik, A.A.; Dar, M.A.; Urwat, U.; Shah, R.A.; Ganai, N.A. Long non-coding RNAs: Mechanism of action and functional utility. Non-Coding RNA Res. 2016 , 1 , 43–50. [ Google Scholar ] [ CrossRef ]
- Bobbin, M.L.; Rossi, J.J. RNA interference (RNAi)-based therapeutics: Delivering on the promise? Annu. Rev. Pharmacol. Toxicol. 2016 , 56 , 103–122. [ Google Scholar ] [ CrossRef ]
- Winter, H.; Winski, G.; Busch, A.; Chernogubova, E.; Fasolo, F.; Wu, Z.; Bäcklund, A.; Khomtchouk, B.B.; Van Booven, D.J.; Sachs, N.; et al. Targeting long non-coding RNA NUDT6 enhances smooth muscle cell survival and limits vascular disease progression. Mol. Ther. 2023 , 31 , 1775–1790. [ Google Scholar ] [ CrossRef ]
Click here to enlarge figure
| lncRNA | Impact on Lipid Metabolism and Cardiovascular Risk | Refs. |
|---|---|---|
| lncRNA H19 | [ ] | |
| AC068234.2–202 | [ ] | |
| AP001033.3–201 | [ ] | |
| ApoA1-AS | [ ] | |
| ApoA4-AS | [ ] | |
| Overexpression of ENST00000602558.1 | [ ] | |
| RP5-833A20.1 | [ , ] | |
| LeXis | [ , ] | |
| MeXis | [ , , ] | |
| LncHR1 | [ ] | |
| RP1-13D10.2 | [ , ] | |
| LncLSTR | [ ] | |
| Lnc-HC | [ , , ] | |
| LincRNA-DYNLRB2-2 | [ ] | |
| CHROME (PRKRA-AS1) | [ , , ] | |
| lncRNA LIPTER | [ , ] | |
| lncRHPL | [ ] | |
| LncNONMMUG027912 | [ ] | |
| MALAT1 | [ , , , , , ] | |
| TUG1 | [ ] | |
| MIAT | [ , ] | |
| LncRNA RP11-728F11 | [ ] | |
| lncRNA RP5-833A20.1 | [ ] | |
| lncRNA ANRIL | [ ] | |
| LASER | [ ] | |
| lncRNA ENST00000416361 | [ , ] | |
| LncRNA RAPIA | [ , ] | |
| NEAT1 | [ , , , , ] | |
| LOC286367 | [ ] | |
| HOXC-AS1 | [ ] | |
| LncARSR | [ , ] | |
| BM450697 | [ ] | |
| GAS5 | [ , ] |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Gluba-Sagr, A.; Franczyk, B.; Rysz-Górzyńska, A.; Olszewski, R.; Rysz, J. The Role of Selected lncRNAs in Lipid Metabolism and Cardiovascular Disease Risk. Int. J. Mol. Sci. 2024 , 25 , 9244. https://doi.org/10.3390/ijms25179244
Gluba-Sagr A, Franczyk B, Rysz-Górzyńska A, Olszewski R, Rysz J. The Role of Selected lncRNAs in Lipid Metabolism and Cardiovascular Disease Risk. International Journal of Molecular Sciences . 2024; 25(17):9244. https://doi.org/10.3390/ijms25179244
Gluba-Sagr, Anna, Beata Franczyk, Aleksandra Rysz-Górzyńska, Robert Olszewski, and Jacek Rysz. 2024. "The Role of Selected lncRNAs in Lipid Metabolism and Cardiovascular Disease Risk" International Journal of Molecular Sciences 25, no. 17: 9244. https://doi.org/10.3390/ijms25179244
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals

Recent trends in the encapsulation of functional lipids: comprehensive review

First published on 2nd September 2024
Recently, the demand for natural foods with promising health benefits has increased daily. Functional lipids such as omega 3 fatty acids, omega 6 fatty acids, linoleic acid, conjugated linoleic acid, carotenoids, and other functional compounds have many beneficial effects on human health, such as cardiovascular diseases, mental disorders, and metabolic disorders such as diabetes. The application of such substances in food matrices is often hindered by their poor solubility in water, unpleasant flavor, low oral bioavailability and low stability during storage and gastrointestinal interactions. Several encapsulation techniques have been used to address these issues and make these compounds bioaccessible and bioavailable. In the present review, the current knowledge of encapsulation delivery systems with suitable wall materials for functional lipids and their production techniques and the mechanism and behavior of the wall and core matrix are discussed. Additionally, the impact of such encapsulation delivery systems on the stability of encapsulated functional lipids in storage as well as the gastrointestinal environment has been discussed. Furthermore, this review highlights the impact of encapsulated functional lipids on the fortification of staple foods in terms of enhanced physicochemical, functional and nutritional profiles. Finally, the review article concludes with the factors affecting the commercialization of these encapsulated functional lipids.
| In this extensive review, the current knowledge of encapsulation delivery systems with suitable wall materials for functional lipids and their production techniques and the mechanism and behavior of the wall and core matrix are discussed. Additionally, the impact of such encapsulation delivery systems on the stability of encapsulated functional lipids in storage as well as the gastrointestinal environment has been discussed. This work is related to UN's Sustainable Development, end hunger and ensure access by all people, in particular the poor and people in vulnerable situations, including infants, to safe, nutritious and sufficient food all year round as encapsulations have the following advantages: address formulation issues related to restricted chemical or physical stability of active ingredients overcome the incompatibility of active component and food matrix, regulate the release of a sensory active compound, help or enhance nutrition absorption. |
1. Introduction
Functional lipids such as omega 3 fatty acids, omega 6 fatty acids, linoleic acid, conjugated linoleic acid, carotenoids, and other functional compounds have many beneficial effects on human health, such as cardiovascular diseases, mental disorders, and metabolic disorders such as diabetes. 8 These compounds are available in a wide range of natural sources, such as vegetables, seeds, meat, fish, algae and microbes, and have tended to constitute an integral part of the human diet for many years. 9 However, several researchers have reported that the direct consumption of such functional lipids still does not satisfy the minimum dietary intake level, which can be a consequence of improper dietary patterns, the geographical distribution of sources, and the limited availability of sources. 10–13
Over the last few decades, researchers have developed various techniques and formulations to make functional lipids more accessible and convenient for consumers. 14–16 Among these, oils rich in functional lipids have become one of the most widely available and commonly used products. Oils extracted from plant sources such as walnut, linseed, canola and flaxseed are rich in α-linoleic acid. 8,17–19 Fish oils are rich sources of ω-3 fatty acids (O3FAs), especially eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), and have been used to make dietary supplements. 20–22 Various types of nonencapsulated delivery systems are available for the convenient supply of functional lipids as dietary supplements. 23 The most commonly used dietary supplements of functional lipids are O3FAs and ω-6 fatty acids (O6FAs). Fish oils entrapped by soft gels, flavored gummies and capsules are the most preferred options for the oral delivery of O3FA, which can mask the odd flavor and odor of the fish oil. 24,25 Plant-based oils, including flaxseed oil, primrose oil and pomegranate oils, are also entrapped in soft gels and provide a dietary supply of arachidonic acid (ARA), linoleic acid (LA), α-linoleic acid (ALA), γ-linoleic acid 26 and conjugated linoleic acid (CLA). 27,28 Recently, several manufacturers have targeted algal oils as sustainable sources of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). 29 Table 1 summarizes the commercially available functional lipid supplements and their nonencapsulated delivery systems.
| Product name | Source | Functional lipids | Delivery system | Reference |
|---|---|---|---|---|
| This table provides a selection of commercially available functional lipid supplements from various regions. It highlights key examples rather than offering an exhaustive list of all products available on the market. LA-linoleic acid, ALA-α-linoleic acid, EPA-eicosapentaenoic acid, DHA-docosahexaenoic acid, CLA-conjugated linoleic acid, GLA-γ-linoleic acid, ARA-arachidonic acid. | ||||
| Mar in Oil® | Salmon oil | EPA/DHA | Soft gels | |
| Nature's Bounty® | Herring, anchovy, mackerel, sardine oils | EPA/DHA | Gummies, capsules | |
| Jamieson® | Wild salmon fish oil complex | EPA/DHA | Gummies | |
| CLA One® | — | CLA | Capsules | |
| Nutra Vege® | Algal oil | DHA | Soft gels | |
| Nordic Naturals® | Plant based oil | ALA, ARA, LA | Soft gels | |
| Rx Omega3® | Flaxseed oil | LA, ALA | Soft gels | |
| Neptune Krill 1000® | Krill oil | EPA/DHA | Soft gels | |
| Source Naturals® Phytosterol complex | Plant based oil | β-Sterols and phytosterol complex | Tablets | |
| Clear Muscle® | — | ARA | Liquid caps | |
| Pometane® | Pomegranate oil | Punicic acid | Soft gels | |
| Deep blue® | Shark liver oil | Squalene | Capsules | |
| NOW® by Abbot Pharmaceuticals | Evening prime rose oil | ω-6 fatty acids | Soft gels | |
| Jarrow Formulas, Borage® | — | GLA | Soft gels | |
| NOW foods, astaxanthin | Fish and shellfish | Astaxanthin | Soft gels | |
| Fucothin® | Seaweed | Fucoxanthin | Capsules | |
Generally, marketed functional lipids are entrapped in gelatin-based capsules and soft gels and thus have poor GI stability and a shorter shelf life. 38–40 Moreover, functional lipids are unsaturated and hydrophobic in nature, so bioavailability and bioaccessibility can be major obstacles for oral delivery or food fortification. 4,5 Furthermore, commercially available dietary supplements contain synthetic antioxidants such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) to prevent the oxidation of functional lipids, which are associated with certain adverse health concerns. 41 In addition to these disadvantages, the “burp effect” is inconvenient for the user. Encapsulation techniques are thus designed to protect functional lipids from adverse environmental conditions in foods, enhance water dispersibility, improve food matrix compatibility, reduce unpleasant sensory attributes, and increase GI stability and bioavailability. 42–44 This review aims to provide a comprehensive overview of the current state of encapsulation technology for functional lipids by selecting and discussing seminal papers, key studies, and recent developments that have significantly impacted the field. Through this focused selection, we aim to highlight the most relevant and influential research, offering insights into the latest advancements, challenges, and future directions in the encapsulation of functional lipids. By doing so, we intend to support further innovation and application in this promising area of nutraceuticals and functional foods.
2. Conventional encapsulation strategies for functional lipids
Spray drying is one of the most commonly used encapsulation processes because of its low cost, simplicity, and flexibility. It yields high-quality powders and can preserve various vegetable and animal oils against oxidation as well as external deterioration influences such as humidity, light, and temperature. The processing time is only a few seconds, which is sufficient to preserve heat-sensitive components such as fatty acids. 50 Another benefit of encapsulation by spray drying is the capacity to decrease the amount of oil at the particle surface (nonencapsulated oil) and thus increase the encapsulation efficiency (EE).
Spray drying facilitates the preparation of the final product in powder form for better storage and transportation. The aqueous solution or dispersed lipids with wall materials are injected into the spray dryer in the form of sprayed particles, where the water is removed by the hot air in a fraction of time to obtain the powder form of the encapsulated particles. Spray drying provides a wide range of encapsulated functional lipids, including omega 3 fatty acids, EPA-rich oils, ALA-rich oils, and squalene. 51,52 Although spray drying is one of the most common methods for the encapsulation of functional lipids, some drawbacks have been linked to this process. For example, a major disadvantage is the use of hot air at high inlet temperatures, which can promote the volatilization and oxidation of some functional lipids. Several authors Encina et al. 51 have reported improvements in the oxidative stability of fish oil by spray drying with methanol (MeOH); Goyal et al. 53 reported the highest encapsulation efficiency and lowest peroxide values of flaxseed oil encapsulated via the spray drying process.
Recent advances in the encapsulation of essential fatty acids and other functional lipids through spray drying have been extensively reviewed. These reviews discuss challenges such as optimizing wall materials and process conditions to improve encapsulation efficiency and stability. 54 The detailed analysis of spray drying parameters highlights the impact of the inlet air temperature, total solids concentration, and wall materials on the encapsulation efficiency of oils. 52 Conventional and nanospray-drying technologies emphasize processing variables and their influence on powder characteristics, discussing advantages such as large yields in conventional spray drying and better preservation of active ingredients in nanospray drying. 55 Additionally, the encapsulation of various lipids, including essential oils, polyunsaturated fatty acids, and structured lipids, focuses on the selection of suitable encapsulating agents and the increasing trend of combining spray drying with other techniques to increase stability and bioavailability. 54
Encapsulation by freeze-drying is achieved by drying an aqueous solution or dispersion containing functional lipids as core and wall materials. This causes the two components to colyophilize, usually resulting in a porous, nonshrunken, complex structure. Minimizing thermal degradation reactions has been shown to be a highly suitable method for drying heat-sensitive substances. Rezvankhah et al. 56 and Hasani et al. 57 thoroughly reviewed the encapsulation of functional lipids, especially omega 3 fatty acid-rich fish oils, by means of freeze drying. However, the porous structure within the freeze-dried matrix may increase the exposure of the encapsulated core matrix to air if the final product is not packed under vacuum or an inert atmosphere. The major disadvantages of this technology are the high consumption of energy, the long time required for processing, and the higher costs than those of other encapsulation techniques.
3. Recent advancements in delivery systems for the encapsulation of functional lipids
| Techniques of encapsulation | Method used | Core material | Functional lipophilic compound | Carrier material/wall matrix | Encapsulation efficiency (%) | Particle size | In vitro digestion study | Heat stability study | Storage stability study | Remarks | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Antisolvent precipitation | Mechanical stirring at | Wakame algae oil | Fucoxanthin | Zein/casein | >85 | 100–130 nm | 29.02% oil release in simulated gastric fluid for 6 h | 100% stability after heating at 75 °C for 60 min | 72.32% retention after 16 days of storage at 25 °C | Static quenching, corresponding to the formation of complexes between fucoxanthin and casein or zein | |
| Mechanical stirring | Egg yolk | Lutein | Zein/soy protein | >80 | 14–200 nm | 33.94% oil release in simulated gastric fluid for 6 h | — | 96.27% retention after 15 days of storage at 25 °C | Zein–lutein complexes can be formed with the help of noncovalent interaction forces | ||
| Sonication from 200–800 W | — | Stigmasterol | Zein | 95.95 | 336.74 nm | — | — | — | Sonication improved the zeta potential of the encapsulated particles which might increase the stability of zein-stigmasterol complex | ||
| Emulsification solvent evaporation | Homogenization at 700 rpm for 15 min at 25 °C | Fish oil | O3FA | Zein | — | 73–265 nm | — | — | — | Oxidative gelation rate is reduced | |
| — | Fish oil | DHA | Zein | 98.8 | 150–200 nm | 3.3% DHA released in Phosphate buffered saline and with 2% Tween 80 as surfactant | — | — | Lower in vitro release proved that the zein and fish oil encapsulation has higher oxidative stability against the GI environment | ||
| Homogenization at 10 | Red palm oil | Carotenoids | Carboxy methyl cellulose | 83–96 | 600–2200 μm | >90% oil retained in the GI environment and >20% oil retained in the intestinal digestion | The oil loaded beads have shown lower weight loss up to 150 °C, after with increase in temperature the wight loss is increased | Lowest peroxide value of 25 meq. of O per kg of oil was found after 6 days of storage at 25 °C | Freeze-drying had diminished the migration of oil on to the surface of the beads as freezing temperature might have solidified the palm oil | ||
| Coacervation technique &ionic gelation | Emulsion obtained by mechanical stirring at 10 | Echium oil | Steariodonic acid and phytosterols | Protein–gelatin | 87 | — | — | — | 96% oil retention after 30 days storage at 37 °C | Gelatin and gum Arabic based coacervation entrapped the echium oil with higher storage stability and less oxidative degradation | |
| Poly saccharide–gum arabica | |||||||||||
| Emulsion obtained by mechanical stirring at 13 | Sacha inchi oil | PUFA | Protein–ovalbumin | 99.54 | — | 14.6% of oil release in simulated gastric digestion at pH 2.8 with the presence of pepsin enzyme | — | — | The reduced release under gastric conditions (low pH and presence of proteolytic enzymes) indicates that the ovalbumin and sodium alginate microcapsule protected the acyl in the omega-3 units | ||
| Polysaccharide–sodium alginate | |||||||||||
| Emulsion was made by mechanical stirring at 400 rpm at 40 °C and coacervation was made by pH shifting to 4 | Cod liver oil | PUFA: EPA and DHA | Protein–soy protein isolates | 94 | — | 80.54% oil stability at pH 5.5 | — | 72.24% oil retention at 90 °C for 30 min | Stable emulsion was carried out by the complex coacervation of inulin and soy protein isolates | ||
| Polysaccharide–inulin | |||||||||||
| Emulsion was obtained by stirring at 600 rpm at room temperature and coacervation complex was created by pH shifting at 3 | Algal oil | PUFA: O3FA and O6FA | Protein–soy protein isolates | 90.57 | — | — | — | — | The hexanal peak area is 23.34 which indicated the lowest oxidation | ||
| Polysaccharide–chitosan | |||||||||||
| Emulsion was prepared by mechanical stirring at 16 | Pomegranate seed oil | Punicic acid (omega 7 fatty acid) | Protein–whey protein | 67.40 | 8.36–10.96 μm | — | — | — | The complex coacervation provided minimum isomerization of pomegranate seed oil | ||
| Polysaccharide–gum arabica | |||||||||||
| Inclusion complex | Mixing followed by lyophilization | Perilla oil | ALA | γ-Cyclodextrin | — | — | — | 63.3% ALA retention after heating at 60 °C after 4 days | — | The perilla oil was more thermostable when included in the cavity of γ-CD than when placed at interspaces between pseudo rotaxane-type complexes | |
| Kneading method and crystallization method | Anchovy oil | EPA and DHA | β-Cyclodextrin | 74–99 | — | — | — | — | PUFA glycerides from the anchovy oil is poor encapsulated in β-cyclodextrin in controlled crystallization conditions, while the monounsaturated and especially saturated fatty acid glycerides were more appropriate for molecular encapsulation | ||
| Dextrinization method | Fish oil | O3FA | Amylose (maize starch) | 71.22 | — | — | — | — | Dextrinization improved dispersion stability of the complex particles | ||
| Supercritical fluid technique | CO pressure-8 M.Pa | Fish oil | EPA and DHA | Polycaprolactone | 38–43 | 6–73 nm | — | — | — | Supercritical fluid extraction successfully developed the nanoparticle s from liquid lipophilic compounds like fish oil | |
| Temperature of extractor 263 K | |||||||||||
| CO pressure- 80 bar | Shrimp oil | Astaxanthin | Ethyle cellulose | 84 | 363–370 nm | Almost 70% release of astaxanthin after 10 h in simulated intestinal fluid | — | — | The emulsified shrimp oil gets easily ionized in simulated intestinal fluid showed higher release of astaxanthin in intestinal tissues | ||
| Temperature of the extractor-38 °C. | |||||||||||
| CO pressure-9 M.Pa | — | Lycopene | n-Octenyl succinic anhydride (OSA)-modified starch | 64–89 | 345–366 nm | — | — | — | Supercritical extraction emulsion provided the stability of lycopene in aqueous media | ||
| Temperature- 353.15 K | |||||||||||
| Electrostatic nanoencapsulation | Electrospinning | Fish oil | O3FA | Zein fibers | 91 | 190 nm | — | — | Peroxide value of encapsulated complex remains below 200 μmol L for 14 days of storage at 25 °C | The distribution of fish oil in the electrospun materials, revealing that the lipid phase tended to concentrate at the core of the fibers and beads | |
| Coaxial electrospray | — | ARA | Zein | 77–95 | 1–7 μm | — | — | Peroxide value of encapsulated ARA is approximately 8.0 meq. per kg after 30 days of storage at room temperature | Coaxial electrospray technique to produce natural and edible microcapsules with core–shell structures and reduce the unpleasant flavor | ||
| Electro spraying assisted by pressurized gas | Fish oil | DHA | Zein | 84 | 2–3 μm | Peroxide value was 20 meq/kg oil after 30 days of storage at 23 °C | DHA was successfully stabilized in the zein microcapsules due to the low temperature and fast evaporation characteristics of electro spraying technique used | ||||
| Electro spraying at 20–25 kV voltage with flow rates ranging from 0.5 to 1 mL h | Fish oil | O3FA | Kafirin | 94 | 552–861 nm | — | — | — | The kafirin nano capsules loaded with fish oil obtained in this study (average diameter <1 μm) present a high surface-to-volume ratio which is desired for a better release of the encapsulated bioactive compound | ||
| Liposomes | Sonication of liposome suspension at 25 °C for 7 min (1 s on and 1 s off) with nominal frequency of 20 kHz at 80% of full power | Fish oil | EPA & DHA | Soybean lecithin | 73.5 | <200 nm | — | — | The TBA reactivity substance was 0.015 μmol MA equivalent after 90 days of storage in dark at 4 °C | The surface charge, physical stability and oxidative stability of liposomal PUFAs increased as the size of the liposomes decreased | |
| Ultrasonication (10 min; 1 s on and off pulse) at 25 °C using an ultrasonic processor at 80% amplitude | Shrimp oil | EPA & DHA, astaxanthin | Soybean lecithin | 93.64 | 40–284 nm | — | — | The peroxide value was approximately 5 meq. peroxide per kg of oil and TBARS approximately 50 malonaldehyde equivalent after 8 weeks of storage at 30 °C | Nanoliposomes produced using ultrasonication method were more stable, smaller in size and showed better nanoencapsulation efficiency | ||
| Thin film drying prior to ultrasonication for 10 min at 180 W in an ice-cold water bath with a cycle of 2 s sonication and 2 s standing | — | Astaxanthin | Egg yolk lecithin and lactoferrin | 71.92 | 190 nm | — | The rate of thermal degradation rate was approximately 0.7045 during the study from 0–70 °C | — | The lowest rate of thermal degradation was the result of antioxidant effect of lactoferrin coated with the liposomes | ||
| Thin film drying prior to sonication using a frequency of 20 kHz at 90% | Perilla oil | ALA and LA | Soybean lecithin and biopolymers | ALA-79.3 to 89.9, LA-72.6 to 85.6 | 120–300 nm | ∼10% release in simulated gastrointestinal conditions | — | The peroxide value was ∼40 meq peroxides/kg of oil after 30 days of storage at 45 °C | Liposomes crosslinked with biopolymers have more physical as well as gastrointestinal stability | ||
| Solid lipid micro/nanoparticles (SLNs) | Resveratrol-stearate and PUFA mixture were melted at 65 °C followed by cold homogenization at 8000 rpm for 15 min | Fish oil | ALA and DHA | Resveratrol | ALA-77, DHA-100 | ALA-842 nm, DHA-1000 nm | — | — | — | SLNs with resveratrol and PUFA omega-3 acted as anti-tumor for colon cancer and reduce the cell proliferation | |
| Oil phase (lipid careers and echium oil) and water phase (WPI solution) were homogenized at 15 | Echium oil | O3FA | Lauric acid, palmitic acid and stearic acid | 78–85 | ∼200 nm | — | — | Sample stabilized by lauric acid have less TBARS values after 21 days of storge as compare to other lipid careers | Different lipid carriers with different chain lengths affected the physicochemical properties of encapsulated echium oil | ||
| Supercritical carbon dioxide with 200 bar expansion pressure, 57 °C, and 50 μm nozzle diameters | Fish oil | O3FA | Fully hydrogenated soybean oil | 97.5 | 5–18 μm | — | — | Anisidine value for the particles with fish oil started to increase on day 9 and reached its maximum on day 15 (2840) while stored at 40 °C | The initial loading concentration of the fish oil have directly proportional to the thermal as well as storage stability of lipid particles |
3.1. Biopolymer-based delivery systems
| Antisolvent precipitation. | ||
Fucoxanthin, a functional lipid-soluble algal pigment, was entrapped in the zein and casein wall matrix by mechanical stirring to obtain nanoparticles with a 100–130 nm particle size. 86 They reported that static quenching between fucoxanthin and the wall material increased the encapsulation efficiency, i.e. , >85%, and increased the heat and storage stability ( Table 2 ). 59 developed a nanoencapsulated egg yolk pigment, lutein, via a similar technique with >80% encapsulation efficiency and a 140–200 nm particle size. They reported that the zein–lutein complexes formed noncovalent interactions via mechanical stirring, which increased the storage stability and release profile in gastric fluid ( Table 2 ). Recently, the sonication method replaced mechanical stirring for phase transition, which provides a uniform distribution of encapsulants and increases the encapsulation efficiency of drug delivery systems. Sonication improved the zeta potential of the encapsulated particles, which might increase the stability of the zein–stigmasterol complex. 60
The application of nanoparticles in food products is subject to stringent regulations, especially in Europe, where they are classified as novel foods. According to the European Food Safety Authority, 87 novel foods must undergo rigorous safety assessments that include evaluations of potential toxicity, absorption, distribution, metabolism, and excretion. 87 Products containing nanoparticles must be clearly labeled to inform consumers of their presence. 88 The authorization process requires companies to submit a detailed dossier with scientific evidence demonstrating the safety of the nanoparticle for its intended use, as reviewed by the EFSA. 89 Additionally, authorized novel foods are subject to ongoing monitoring to ensure safety and traceability, and environmental impact assessments must also be considered. 90 This regulatory framework ensures that the nanoparticles used in food products are safe for consumption and that consumers are well informed about their presence.
| Emulsification solvent evaporation. | ||
Recently, scientific research has explored the interaction between functional lipids and biopolymer composites such as zein and carboxy methyl cellulose (CMC) for the production of nanoparticles. For example, zein and fish oil-derived nanocomposites (100–120 nm) have higher encapsulation efficiency (98.8%), and high-pressure homogenization and solvent evaporation methods have been used to develop highly stable nanoparticles with better GI stability. 62 Furthermore, Soltani et al. 61 reported a reduction in oxidative gelation for zein-fish oil nanocomposites (73–265 nm). Similarly, carotenoids from red palm oil have been immobilized by CMC by high-pressure homogenization followed by freeze drying to achieve higher encapsulation efficiency (83–96%), better storage stability, enhanced GI stability and targeted drug delivery in the intestinal environment ( Table 2 ).
| Coacervation method (ionic gelation). | ||
Comunian et al. 64 reported that gelatin- and gum Arabic-based coacervation entrapped echium oil with high storage stability and 87% encapsulation efficiency.
The ovalbumin and sodium alginate microcapsule of sachainchi oil protected the acyl group in the omega-3 units, which ultimately reduced the rate of release of functional compounds in the GI tract and provided targeted drug delivery 65 . Rios-Mera et al. 66 developed a stable emulsion (94% encapsulation efficiency) consisting of cod liver oil by the complex coacervation of inulin and soy protein isolates, where they reported increased heat and GI stability at a simulated pH ( Table 2 ). The complex coacervation provided minimum isomerization of pomegranate seed oil in microcapsules (8.36–10.96 μm) developed by using whey protein and gum Arabic as the wall matrix. 68
| Inclusion complex. | ||
The perilla oil was more thermostable when it was included in the cavity of γ-CD than when it was placed in interspaces between pseudo rotaxane-type complexes. 69 However, the chemical affinity of functional compounds influences the encapsulation potential of inclusion complexes. For example, PUFA glycerides from anchovy oil are poorly encapsulated 93 in β-cyclodextrin under controlled crystallization conditions, whereas monounsaturated and especially saturated fatty acid glycerides are more appropriate for molecular encapsulation (99% EE). In addition to CDs, chemically modified biopolymers are also used as host compounds for inclusion complexes. Park et al. 71 developed a host compound by dextrinization by maize starch, which is used as a wall matrix for fish oil, where dextrinization improved the dispersion stability of the complex particles ( Table 2 ).
This technology has been applied to various functional lipids to form micro- or nanoencapsulations by using different biopolymers as wall materials. Santos et al. 74 applied ScCO 2 to encapsulate lycopene pigments with n -octenyl succinic anhydride 94 -modified starch and reported that a supercritical extraction emulsion provided stable lycopene in aqueous media. The importance of supercritical CO 2 encapsulation techniques was highlighted by Tirado et al. 73 for the emulsification of shrimp oil, where in vitro release profiles in simulated intestinal fluid (SIF) at pH 7.2 and 310 K revealed 70% release of the total encapsulated astaxanthin within 10 hours. Prieto et al. 95 successfully developed fish oil nanoparticles 6–73 nm in size from ScCO 2 with polycaprolactone as a wall matrix.
3.2. Electrohydrodynamic processing of encapsulation
| Electrohydrodynamic processing of encapsulation by electrospinning. | ||
Moomand et al. 75 reported the distribution of fish oil in electrospun zein fibers, revealing that the lipid phase tended to concentrate at the core of the fibers and beads. They reported that the applied technique developed spun nanofibers (190 nm) with an increased encapsulation efficiency of O3FA of up to 91% ( Table 2 ).
| Electrostatic encapsulation by electrosprying. | ||
Hu et al. 76 encapsulated (95% EE) ARA with a zein biopolymer via a coaxial spray technique, which produced natural and edible microcapsules (1–7 μm) with core–shell structures and reduced the unpleasant flavor. The electrospray technique provides low-temperature and fast evaporation characteristics and successfully stabilizes fish oil in zein microcapsules (2–3 μm) with an 84% EE of DHA. 72 Kafirin-based nanoencapsulated capsules (552–861 nm) loaded with fish oil (94% EE) obtained by electrospinning present a high surface-to-volume ratio, which is desirable for better release of the encapsulated bioactive compound. 77
3.3. Lipid-based delivery system for functional lipids
| Liposome production by thin film hydration. | ||
Rasti et al. 78 developed soybean lecithin-based liposomes containing fish oil by combining thin film hydration and ultrasonication and reported that ultrasonication reduced the size (<200 nm) of the liposomes and made them homogenous, which increased the stability of the nanoliposomes. Gulzar et al. 79 studied the impact of ultrasonication and microfluidization on the physicochemical properties of nanoliposomes containing shrimp oil and reported a greater encapsulation efficiency (93.64%) and smaller particle size (40 nm) of the nanoliposomes obtained via ultrasonication than via microfluidization.
| Solid lipid nanoparticles developed O3FA-rich resveratrol-based solid lipid nanoparticles by hot homogenization at 65 °C for the delivery of ALA (74% EE; 840 nm) and DHA (100% EE; 1000 nm). The encapsulation efficiency and particle size of solid lipid nanoparticles are affected by the chain length of lipid carriers. High-pressure homogenization, ultrasonication and supercritical CO are the most efficient methods for preparing solid lipid micro/nanoparticles for omega-3-rich oils with high encapsulation efficiency (95–99%) ( ). | ||
4. Impact of encapsulation techniques on the stability of functional lipids
Storage stability and heat stability are proposed considerations for the encapsulation of functional lipids. They are affected by various parameters, including the wall/carrier matrix, emulsifiers, wall matrix properties, glass transition temperature, crystallinity, chemical and physical interaction mechanisms, and processing conditions (temperature, pressure, ratio of wall to core material, particle size and surface area, and oil distribution within the particle). 114 Moreover, the amount of free surface oil on the surface of encapsulated particles is the most critical parameter, while considering the encapsulation strategies for functional lipids, as free surface oil is most prone to environmental stress. 115 Many researchers have successfully entrapped functional lipids with enhanced storage and heat stability, as shown in Table 2 .
Li et al. 86 reported 100% stability of fucoxanthin nanoparticles entrapped by a zein–casein wall matrix after heating at 75 °C. Static quenching, corresponding to the formation of complexes between fucoxanthin and casein–zein, also provided an oil retention of up to 72% after 16 days of storage at ambient temperature. Similarly, the zein–lutein complex formed by noncovalent bonding retained approximately 96% of the oil in egg yolk nanoparticles after storage at 25 °C for 15 days 59 . Sathasivam et al. 63 reported that freeze-drying diminished the migration of red palm oil to the surface of microbeads as the freezing temperature solidified the oil in the core of the carboxymethyl cellulose, which resulted in the lowest peroxide values (25 meq. of O 2 per kg of oil) after 6 days of storage at room temperature. In contrast, Anwar et al. 94 reported that freeze drying produced a porous powder of encapsulates, which allowed more oxygen to interact and generate higher peroxide concentrations.
Gelatin- and gum arabic-based coacervation entraps echium oil with greater storage stability and less oxidative degradation, which retains approximately 96% of the oil after 30 days of storage at 37 °C 64 . Rios-Mera et al. 66 developed a stable emulsion of cod liver oil by complex coacervation of inulin and soy protein, where approximately 72% oil retention was obtained after the emulsion was heated at 90 °C for 30 min. Hexanal is considered an end product of lipid oxidation, which impairs the sensorial attributes of fortified products. Yuan et al. 67 reported that hexanal production is reduced when algal oil is encapsulated in the complex coacervation of soy protein and chitosan. Researchers have also reported a decrease in peroxide concentrations during the storage of various encapsulated functional lipids via the use of electrostatic encapsulation techniques. 72,75,76 Certain antioxidants and biopolymers provide additional benefits in terms of enhancing the stability of functional lipids when combined with different encapsulation techniques. Liposomal nanoparticles of astaxanthin coated with lactoferrin enhance oxidative stability because of the antioxidant effect of lactoferrin 80 . Zamani-Ghaleshahi et al. 81 reported that perilla oil liposomes crosslinked with biopolymers have greater physical stability. Table 2 summarizes the effects of various encapsulation techniques and wall matrices on the storage and heat stability of encapsulated functional lipids.
5. Influence of encapsulation techniques on the gastrointestinal stability and bioavailability of functional lipids
The wakame algae oil entrapped in the zein–casein complex showed approximately 20% oil loss under simulated gastric conditions after 6 h, which might be due to strong static quenching between the zein–casein complex and the core compound. 86 Similarly, Li et al. 59 reported that the zein–lutein complex provided gastrointestinal stability to lutein nanoparticles through only 33% oil loss in gastric fluid after 6 h, which might be the result of strong noncovalent interactions between the wall and core material. Surfactants used in colloidal delivery systems, such as Tween 80, have also been shown to enhance the GI stability of DHA in fish oil-encapsulated nanoparticles. 62 The freeze-dried red palm oil-loaded microbeads retained approximately 90% of the oil in the simulated gastric environment due to the presence of less free surface oil, as discussed earlier. 63 The coacervation complex and ionic gelation technique also provided GI stability for various functional lipids by retaining up to 80% of the oil under simulated gastric conditions. 65,66 Tirado et al. 73 noted that the encapsulated structure of shrimp oil is easily ionized in simulated intestinal fluid, which increases the solubility of astaxanthin in the intestinal environment. Table 2 summarizes the effects of various encapsulation techniques and wall matrices on the gastrointestinal stability of encapsulated functional lipids.
The studies presented in Table 2 offer valuable insights into encapsulation techniques and the release behavior of functional lipids in simulated gastrointestinal environments. However, understanding bioavailability requires more comprehensive investigations, including studies that go beyond in vitro digestion and evaluate the actual absorption and efficacy of these encapsulated compounds in living systems. Several research groups have investigated the bioavailability of encapsulated lipids through cell culture and animal studies. For example, Serini et al. 82 investigated the antitumor efficacy of solid lipid nanoparticles (SLNs) encapsulating resveratrol and omega-3 fatty acids (ALA and DHA) in a colon cancer model. These findings demonstrated that these SLNs could reduce cell proliferation and exhibit antitumor activity, suggesting improved bioavailability and therapeutic potential in vivo . Similarly, Barbosa et al. 116 studied the stability and bioactivity of encapsulated echium oil in various lipid carriers via animal models. Research has shown that the chain length of lipid carriers affects the physicochemical properties and stability of the encapsulated oil, which in turn influences its bioavailability when it is administered to animals. In another study, Xie et al. 117 used supercritical carbon dioxide to encapsulate fish oil in fully hydrogenated soybean oil and evaluated its bioavailability in an animal model. This study revealed that the initial loading concentration of fish oil was directly proportional to its thermal and storage stability, which impacted its absorption and bioavailability in the tested animals.
These studies illustrate that while in vitro digestion studies provide preliminary insights, cell culture and animal studies are crucial for comprehensively evaluating the bioavailability of encapsulated functional lipids. These examples underscore the importance of moving beyond in vitro experiments to assess the true bioavailability and efficacy of encapsulated compounds in living systems.
6. Effect of encapsulation techniques on the fortification of functional lipids
| Fortified food products | Encapsulated functional lipids | Encapsulation technique used | Physicochemical properties of fortified products | Rheological properties of fortified products | Sensorial attributes of fortified products | References |
|---|---|---|---|---|---|---|
| Yogurt | Fish oil powder | Complex coacervate of gelatin/gum acacia | Acidity, and water holding capacity were increased; whey separation was decreased | Gel strength decreased and apparent viscosity increased | Fortified yogurt samples were more yellowish compared to control | |
| Fish oil microcapsules | Complex coacervate of gelatin/gum acacia | Fortified yogurt had higher apparent viscosity | Consistency coefficients of the enriched yogurt was 24.42–28.82 Pa s | — | ||
| Fish oil nanoliposomes | Liposomes by egg yolk lecithin and fish oil | Whey separation was deceased | — | Fish odor was eliminated | ||
| Cheese | Fish oil powder | Microencapsulation by freeze drying | Whey separation was deceased | Hardness, chewiness and gumminess was increased | — | |
| Fish oil powder | Microencapsulation by spray drying | Milk solid not fat was increased; pH level is maintained up to 30 days of storage | Hardness of enriched cheese is increased after 30 days of storage | Cheese color was changed to yellow after 60 days of storage | ||
| Bread | Fish oil nanoliposomes | Liposomes by sunflower oil and lecithin | Loaf volume was increased, improved crumb characteristics | Harness was reduced, decrease the level of chewiness and gumminess | Light browning in the crumb color | |
| Ice cream | Fish oil powder | Microencapsulation by freeze drying | Saturated fatty acids decreased, PUFA increased | — | — | |
| Flaxseed oil microcapsules | Microencapsulation by freeze drying | Free fatty acid content was increased, melt down rate was decreased | — | Off flavor was masked up to 30 days of storage | ||
| Frankfurter sausages | Fish oil microcapsules | Monolayer microencapsulation by spray drying | Lower down the pH values, MUFA and PUFA increased | — | — | |
| Chicken sausages | Fish oil powder | Microencapsulation by inclusion complex with gelatin wall material | pH was maintained up to 21 days of storage, water binding ability was increased | The sausages with microencapsulated oil showed better ability to accumulate elastic energy (G′); Hardness of sausage was increased | Fortified sausages were rated highest for their consistency (the thickest), especially when they were heated |
Over the past few decades, there has been a remarkable interest in fortifying milk and dairy products with functional lipids with the aim of increasing the fatty acid profile of such products. Yogurt, cheese and ice creams are the most popular dairy products, and various attempts have been made to fortify such products with various encapsulated functional lipids. The fortification of yogurt with fish oil powder increased its acidity, lowered its pH and increased its water holding capacity, which ultimately increased its shelf-life. 119,120 Moreover, yogurt tends to release whey during storage, which is called syneresis. The addition of fish oil powder can control whey separation due to the ability of the wall material to hold water and increase the stability of yogurt during storage 118,120 . Bermúdez-Aguirre et al. 121 reported a similar reduction in whey separation in fish oil microcapsule-fortified cheddar cheese. Moreover, the addition of functional lipids to cheese also increases its textural properties, increasing its shelf stability. 121,122 In addition to enhancing the physiochemical properties of emulsified dairy products, the fortification of functional lipids enhances their fatty acid profile by reducing the saturated fatty acids and increasing the PUFAs and MUFAs. The fatty acid profile of ice cream fortified with fish oil powder was greater than that of the control samples. 124 Furthermore, Gowda et al. 125 reported a lower melt-down rate in ice cream fortified with flaxseed oil microcapsules, which could be attributed to the encapsulated form of the fortified flaxseed oil, which might have increased flocculation and hence improved the structure of the ice cream.
Bread is another staple diet after milk and dairy products and has been popular among scientific communities for fortification with functional bioactive compounds. In addition to enhancing the fatty acid profile of breads, the encapsulated structure of functional lipids also improved the textural and sensorial attributes. Ojagh et al. 123 reported that the loaf volume in bread containing fish oil nanoliposomes increased, possibly due to the surface–active properties of lecithin, an emulsifier, and other ingredients within the liposomal system, which improved gas retention, bread volume, and dough stability. Additionally, lecithin reacts with linear amylose and external amylopectin branches and forms a complex that prevents hardening of the bread's crumb. In addition, some ready-to-eat meat products, such as frankfurt and sausages, have recently been fortified with fish oil encapsulates to enhance their fatty acid profile. 126,127
7. Factors affecting the industrialization and commercialization of encapsulated functional lipids
7.1. scalability of the encapsulation process, 7.2. stability and shelf life, 7.3. regulatory compliance, 7.4. cost-effectiveness, 7.5. consumer acceptance, 8. conclusion and future prospects.
In recent decades, researchers have developed certain encapsulation techniques involving the selection of suitable wall materials for functional lipids to increase bioavailability during oral delivery as well as the enrichment of food products. The mechanism of encapsulation of functional lipids within the wall/carrier matrix by various physical and chemical interactions affects the heat stability, storage stability and GI stability of encapsulates. Furthermore, encapsulated functional lipids tend to be more bioavailable within food systems and enhance the physicochemical and functional properties of food. Further studies are needed to address food safety concerns regarding fortified foods with encapsulated functional lipids, and a clear research gap was found in the utilization of novel sources of functional lipids such as algae, bacteria and fungi for the fortification of common staple foods by means of encapsulation techniques.
The successful industrialization and commercialization of encapsulated functional lipids depend on careful consideration of factors such as scalability, stability, regulatory compliance, cost-effectiveness, and consumer acceptance. Addressing these factors through research and innovation is essential for bringing effective and commercially viable functional lipid products to the market.
Data availability
Conflicts of interest.
- S. Chew, C. Tan, L. Pui, P. Chong, B. Gunasekaran and K. Nyam, Int. J. Innovative Technol. Explor. Eng. , 2019, 8 , 154–162 Search PubMed .
- S. Sabet, C. K. Seal, A. Akbarinejad, A. Rashidinejad and D. J. McGillivray, Food Hydrocolloids , 2020, 107 , 105922 CrossRef CAS .
- A. Patel and K. P. Velikov, LWT--Food Sci. Technol. , 2011, 44 , 1958–1964 CrossRef CAS .
- A. Araiza-Calahorra, M. Akhtar and A. Sarkar, Trends Food Sci. Technol. , 2018, 71 , 155–169 CrossRef CAS .
- A. Sarkar and A. R. Mackie, Curr. Opin. Colloid Interface Sci. , 2020, 48 , 40–52 CrossRef CAS .
- H. Pool, S. Mendoza, H. Xiao and D. J. McClements, Food Funct. , 2013, 4 , 162–174 RSC .
- A. Rezaei, M. Fathi and S. M. Jafari, Food Hydrocolloids , 2019, 88 , 146–162 CrossRef CAS .
- B. Alabdulkarim, Z. A. N. Bakeet and S. Arzoo, J. King Saud Univ. Sci. , 2012, 24 , 319–329 CrossRef .
- R. Katiyar and A. Arora, Algal Res. , 2020, 46 , 101800 CrossRef .
- E. Arab-Tehrany, M. Jacquot, C. Gaiani, M. Imran, S. Desobry and M. Linder, Trends Food Sci. Technol. , 2012, 25 , 24–33 CrossRef CAS .
- T. F. F. da Silveira, L. M. Cajaíba, L. Valentin, B. Baréa, P. Villeneuve and I. A. Castro, Food Chem. , 2020, 309 , 125586 CrossRef CAS PubMed .
- P. Karthik and C. Anandharamakrishnan, RSC Adv. , 2016, 6 , 3501–3513 RSC .
- Y. Wang, C. Li, L. Li, X. Yang, Y. Wu, Y. Zhao and Y. Wei, J. Aquat. Food Prod. Technol. , 2018 DOI: 10.1080/10498850.2018.1450573 .
- D. P. Killeen, S. N. Marshall, E. J. Burgess, K. C. Gordon and N. B. Perry, J. Agric. Food Chem. , 2017, 65 , 3551–3558 CrossRef CAS PubMed .
- E. C. Rizos, E. E. Ntzani, E. Bika, M. S. Kostapanos and M. S. Elisaf, Jama , 2012, 308 , 1024–1033 CrossRef CAS PubMed .
- X. Wan, Y. Zhang, P. Wang and M. Jiang, J. Microbiol. , 2011, 49 , 151–154 CrossRef CAS PubMed .
- A. N. Carey, D. R. Fisher, D. F. Bielinski, D. S. Cahoon and B. Shukitt-Hale, Inflammation , 2020, 43 , 241–250 CrossRef CAS PubMed .
- M. Garcia-Aloy, P. J. M. Hulshof, S. Estruel-Amades, M. C. J. Osté, M. Lankinen, J. M. Geleijnse, J. de Goede, M. Ulaszewska, F. Mattivi, S. J. L. Bakker, U. Schwab and C. Andres-Lacueva, Genes Nutr. , 2019, 14 , 7 CrossRef PubMed .
- A. Ashkar, S. Laufer, J. Rosen-Kligvasser, U. Lesmes and M. Davidovich-Pinhas, Food Hydrocolloids , 2019, 97 , 105218 CrossRef CAS .
- T. Sae-leaw and S. Benjakul, Eur. J. Lipid Sci. Technol. , 2017, 119 , 1700198 CrossRef .
- N. A. Irvine, B. Ruyter, T. K. Østbye, A. K. Sonesson, K. A. Lillycrop, G. Berge and G. C. Burdge, Lipids , 2019, 54 , 725–739 CrossRef CAS PubMed .
- J. A. Emery, F. Norambuena, J. Trushenski and G. M. Turchini, Lipids , 2016, 51 , 399–412 CrossRef CAS PubMed .
- F. Alghamdi, Analysis of the Omega-3 Fatty Acids Content in Commercial Omega-3 Supplements in Arab Gulf Countries , Southern Illinois University at Carbondale, 2019 Search PubMed .
- E. M. Tillman, C. M. Crill, D. D. Black, E. B. Hak, L. F. Lazar, M. L. Christensen, E. Y. Huang and R. A. Helms, Pharmacotherapy , 2011, 31 , 503–509 CrossRef CAS PubMed .
- M. A. Zulyniak, M. Perreault, C. Gerling, L. L. Spriet and D. M. Mutch, Metabolism , 2013, 62 , 1107–1113 CrossRef CAS PubMed .
- A. J. Buglass, Handbook of Alcoholic Beverages: Technical, Analytical and Nutritional Aspects , 2010 Search PubMed .
- A. Zargar and M. K. Ito, Metab. Syndr. Relat. Disord. , 2011, 9 , 255–271 CrossRef CAS PubMed .
- A. S. Gutstein and T. Copple, J. Am. Assoc. Nurse Pract. , 2017, 29 , 791–801 CrossRef PubMed .
- L. Oliver, T. Dietrich, I. Marañón, M. C. Villarán and R. J. Barrio, Producing omega-3 polyunsaturated fatty acids: A review of sustainable sources and future trends for the EPA and DHA market, Resources , 2020, 9 (12), 148 CrossRef .
- A. F. Cicero, M. Rosticci, M. Morbini, M. Cagnati, E. Grandi, A. Parini and C. Borghi, Arch. Med. Sci. , 2016, 12 , 507 CrossRef CAS PubMed .
- A. Gupta, C. K. Narkowicz, H. A. Al-Aubaidy, H. F. Jelinek, D. S. Nichols, J. R. Burgess and G. A. Jacobson, Phytosterol supplements do not inhibit dipeptidyl peptidase-4, Diabetes. Metab. Syndr. , 2020, 14 , 1475–1478 CrossRef PubMed .
- C. J. Mitchell, R. F. D'Souza, V. C. Figueiredo, A. Chan, K. Aasen, B. Durainayagam, S. Mitchell, A. J. Sinclair, I. M. Egner and T. Raastad, J. Appl. Physiol. , 2018, 124 , 1080–1091 CrossRef CAS PubMed .
- P. Mirmiran, M. R. Fazeli, G. Asghari, A. Shafiee and F. Azizi, Br. J. Nutr. , 2010, 104 , 402–406 CrossRef CAS PubMed .
- J. P. Dave, A. M. M. Ali and S. C. B. Bavisetty, An overview on recent advances in functional properties of dietary lipids, encapsulation strategies and applications, Nutr. Food Sci. , 2022, 52 (7), 1158–1180 CrossRef .
- R. R. Ambati, S. M. Phang, S. Ravi and R. G. Aswathanarayana, Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review, Mar. Drugs , 2014, 12 (1), 128–152 CrossRef PubMed .
- V. A. Ziboh, S. Naguwa, K. Vang, J. Wineinger, B. M. Morrissey, J. McIntyre, M. Watnik and M. E. Gershwin, Clin. Dev. Immunol. , 2004, 11 , 13–21 CAS .
- H. V. Chuyen and J.-B. Eun, Crit. Rev. Food Sci. Nutr. , 2017, 57 , 2600–2610 CrossRef CAS PubMed .
- X. Li, J. Liu, G. Chen, J. Zhang, C. Wang and B. Liu, Algal Res. , 2019, 43 , 101619 CrossRef .
- S. Liu, N. Low and M. T. Nickerson, J. Am. Oil Chem. Soc. , 2010, 87 , 809–815 CrossRef CAS .
- M. Ramos, A. Valdes, A. Beltran and M. C. Garrigós, Coatings , 2016, 6 , 41 CrossRef .
- A. Augustyniak, G. Bartosz, A. Čipak, G. Duburs, L. U. Horáková, W. Łuczaj, M. Majekova, A. D. Odysseos, L. Rackova and E. Skrzydlewska, Free Radic. Res. , 2010, 44 , 1216–1262 CrossRef CAS PubMed .
- C. Cheng, Z. Wu, D. J. McClements, L. Zou, S. Peng, W. Zhou and W. Liu, Colloids Surf. B Biointerfaces , 2019, 183 , 110460 CrossRef CAS PubMed .
- M. Fathi, A. Martin and D. J. McClements, Trends Food Sci. Technol. , 2014, 39 , 18–39 CrossRef CAS .
- K. Hu, X. Huang, Y. Gao, X. Huang, H. Xiao and D. J. McClements, Food Chem. , 2015, 182 , 275–281 CrossRef CAS PubMed .
- Q. Chen, D. McGillivray, J. Wen, F. Zhong and S. Y. Quek, J. Food Eng. , 2013, 117 , 505–512 CrossRef CAS .
- P. Pourashouri, B. Shabanpour, S. H. Razavi, S. M. Jafari, A. Shabani and S. P. Aubourg, J. Aquat. Food Prod. Technol. , 2014, 23 , 567–578 CrossRef CAS .
- S. Chatterjee and Z. M. Judeh, Carbohydrate Polym. , 2015, 123 , 432–442 CrossRef CAS PubMed .
- D. F. Keenan, V. C. Resconi, T. J. Smyth, C. Botinestean, C. Lefranc, J. P. Kerry and R. M. Hamill, Meat Sci. , 2015, 107 , 75–85 CrossRef CAS PubMed .
- A. L. Charles, A. A. Abdillah, Y. R. Saraswati, K. Sridhar, C. Balderamos, E. D. Masithah and M. A. Alamsjah, Food Hydrocolloids , 2021, 112 , 106281 CrossRef CAS .
- C. Chang and M. T. Nickerson, J. Food Sci. Technol. , 2018, 55 , 2850–2861 CrossRef CAS PubMed .
- C. Encina, C. Vergara, B. Giménez, F. Oyarzún-Ampuero and P. Robert, Trends Food Sci. Technol. , 2016, 56 , 46–60 CrossRef CAS .
- N. K. Mohammed, C. P. Tan, Y. A. Manap, B. J. Muhialdin and A. S. M. Hussin, Molecules , 2020, 25 , 3873 CrossRef CAS PubMed .
- A. Goyal, V. Sharma, M. K. Sihag, S. Arora, A. Singh and L. Sabikhi, Dry. Technol. , 2016, 34 , 810–821 CrossRef CAS .
- D. M. Sánchez-Osorno, M. C. López-Jaramillo, A. V. Caicedo Paz, A. L. Villa, M. S. Peresin and J. P. Martínez-Galán, Pharmaceutics , 2023, 15 , 1490 CrossRef PubMed .
- C. I. Piñón-Balderrama, C. Leyva-Porras, Y. Terán-Figueroa, V. Espinosa-Solís, C. Álvarez-Salas and M. Z. Saavedra-Leos, Processes , 2020, 8 , 889 CrossRef .
- A. Rezvankhah, Z. Emam-Djomeh and G. Askari, Dry. Technol. , 2020, 38 , 235–258 CrossRef CAS .
- M. Hasani, A. E. Rad, M. M. Hosseini and M. S. Noghabi, Biosci., Biotechnol. Res. Asia , 2015, 12 , 45–51 CrossRef .
- K. Li, A. J. Sinclair, F. Zhao and D. Li, Nutrients , 2018, 10 , 1559 CrossRef PubMed .
- H. Li, Y. Yuan, J. Zhu, T. Wang, D. Wang and Y. Xu, Food Hydrocolloids , 2020, 103 , 105715 CrossRef CAS .
- S. Feng, X. Zheng, D. Luan, P. Shao and P. Sun, LWT , 2019, 107 , 138–144 CrossRef CAS .
- S. Soltani and A. Madadlou, Food Hydrocolloids , 2015, 43 , 664–669 CrossRef CAS .
- J. Zeng, W. Yu, X. Dong, S. Zhao, Z. Wang, Y. Liu, M.-S. Wong and Y. Wang, Nanomed. Nanotechnol. Biol. Med. , 2019, 15 , 119–128 CrossRef CAS PubMed .
- T. Sathasivam, S. Muniyandy, L. H. Chuah and P. Janarthanan, J. Food Eng. , 2018, 231 , 10–21 CrossRef CAS .
- T. A. Comunian, M. Nogueira, B. Scolaro, M. Thomazini, R. Ferro-Furtado, I. A. de Castro and C. S. Favaro-Trindade, Food Chem. , 2018, 252 , 277–284 CrossRef CAS PubMed .
- B. da Silva Soares, R. P. Siqueira, M. G. de Carvalho, J. Vicente and E. E. Garcia-Rojas, Food Chem. , 2019, 298 , 125045 CrossRef CAS PubMed .
- J. D. Rios-Mera, E. Saldaña, Y. Ramírez, E. A. Auquiñivín, I. D. Alvim and C. J. Contreras-Castillo, LWT , 2019, 116 , 108555 CrossRef CAS .
- Y. Yuan, Z.-Y. Kong, Y.-E. Sun, Q.-Z. Zeng and X.-Q. Yang, Lwt , 2017, 75 , 171–179 CrossRef CAS .
- A. M. Costa, L. K. Moretti, G. Simões, K. A. Silva, V. Calado, R. V. Tonon and A. G. Torres, LWT , 2020, 131 , 109519 CrossRef CAS .
- K. Yoshikiyo, Y. Yoshioka, Y. Narumiya, S. Oe, H. Kawahara, K. Kurata, H. Shimizu and T. Yamamoto, Food Chem. , 2019, 294 , 56–59 CrossRef CAS PubMed .
- M. Ünlüsayin, N. G. Hădărugă, G. Rusu, A. T. Gruia, V. Păunescu and D. I. Hădărugă, LWT--Food Sci. Technol. , 2016, 68 , 135–144 CrossRef .
- E. Y. Park, S. M. Choi, S.-T. Lim and J.-Y. Kim, Food Hydrocolloids , 2018, 77 , 357–362 CrossRef CAS .
- M. Busolo, S. Torres-Giner, C. Prieto and J. Lagaron, Innovat. Food Sci. Emerg. Technol. , 2019, 51 , 12–19 CrossRef CAS .
- D. F. Tirado, I. Palazzo, M. Scognamiglio, L. Calvo, G. Della Porta and E. Reverchon, J. Supercrit. Fluids , 2019, 150 , 128–136 CrossRef CAS .
- D. T. Santos, Á. Martín, M. A. A. Meireles and M. J. Cocero, J. Supercrit. Fluids , 2012, 61 , 167–174 CrossRef CAS .
- K. Moomand and L.-T. Lim, Food Res. Int. , 2014, 62 , 523–532 CrossRef CAS .
- M. X. Hu, X. L. Chen, L. J. Song and F. He, J. Appl. Polym. Sci. , 2020, 50403 Search PubMed .
- T. Cetinkaya, A. C. Mendes, C. Jacobsen, Z. Ceylan, I. S. Chronakis, S. R. Bean and P. J. García-Moreno, LWT , 2021, 136 , 110297 CrossRef CAS .
- B. Rasti, S. Jinap, M. Mozafari and A. Yazid, Food Chem. , 2012, 135 , 2761–2770 CrossRef CAS PubMed .
- S. Gulzar and S. Benjakul, Food Chem. , 2020, 310 , 125916 CrossRef CAS PubMed .
- M. Qiang, X. Pang, D. Ma, C. Ma and F. Liu, Molecules , 2020, 25 , 610 CrossRef CAS PubMed .
- A. Zamani-Ghaleshahi, G. Rajabzadeh, H. Ezzatpanah and M. Ghavami, Food Biophys. , 2020, 1–15 Search PubMed .
- S. Serini, R. Cassano, P. A. Corsetto, A. M. Rizzo, G. Calviello and S. Trombino, Int. J. Mol. Sci.c , 2018, 19 , 586 CrossRef PubMed .
- M. Azizi, A. Kierulf, M. C. Lee and A. Abbaspourrad, Food Chem. , 2018, 246 , 448–456 CrossRef CAS PubMed .
- J. Yang and O. N. Ciftci, Food Chem. , 2017, 231 , 105–113 CrossRef CAS PubMed .
- C. Anandharamakrishnan, Techniques for Nanoencapsulation of Food Ingredients , Springer, 2014 Search PubMed .
- Z. Li, T. Meng, X. Ling, J. Li, C. Zheng, Y. Shi, Z. Chen, Z. Li, Q. Li and Y. Lu, J. Agric. Food Chem. , 2018, 66 , 5382–5391 CrossRef CAS PubMed .
- E. S. Committee, S. More, V. Bampidis, D. Benford, C. Bragard, T. Halldorsson, A. Hernández-Jerez, S. H. Bennekou, K. Koutsoumanis and C. Lambré, EFSA J. , 2021, 19 , e06769 Search PubMed .
- M. Correia, E. Verleysen and K. Loeschner, in Nanomaterials for Food Applications , Elsevier, 2019, pp. 273–311 Search PubMed .
- K. Rasmussen, H. Rauscher, S. Gottardo, E. Hoekstra, R. Schoonjans, R. Peters and K. Aschberger, in Nanomaterials for Food Applications , Elsevier, 2019, pp. 381–410 Search PubMed .
- E. Ververis, R. Ackerl, D. Azzollini, P. A. Colombo, A. de Sesmaisons, C. Dumas, A. Fernandez-Dumont, L. F. da Costa, A. Germini and T. Goumperis, Food Res. Int. , 2020, 137 , 109515 CrossRef CAS PubMed .
- A. F. Esfanjani and S. M. Jafari, Colloids Surf. B Biointerfaces , 2016, 146 , 532–543 CrossRef PubMed .
- A. Vaucher, P. C. M. Dias, P. T. Coimbra, I. Costa, R. N. Marreto, G. M. Dellamora-Ortiz, O. De Freitas and M. F. S. Ramos, J. Microencapsul. , 2019, 36 , 459–473 CrossRef CAS PubMed .
- C. Robert, L. Couëdelo, C. Knibbe, L. Fonseca, C. Buisson, E. Errazuriz-Cerda, E. Meugnier, E. Loizon, C. Vaysse and M. C. Michalski, J. Nutr. , 2020, 150 , 2900–2911 CrossRef PubMed .
- S. H. Anwar and B. Kunz, J. Food Eng. , 2011, 105 , 367–378 CrossRef CAS .
- C. Prieto and L. Calvo, J. Supercrit. Fluids , 2017, 128 , 227–234 CrossRef CAS .
- C. Anandharamakrishnan, 2017.
- P. Wen, Y. Wen, M.-H. Zong, R. J. Linhardt and H. Wu, J. Agric. Food Chem. , 2017, 65 , 9161–9179 CrossRef CAS PubMed .
- A. Moreira, D. Lawson, L. Onyekuru, K. Dziemidowicz, U. Angkawinitwong, P. K. Costa and G. R. Williams, Protein encapsulation by electrospinning and electrospraying, J. Controlled Release , 2021, 329 , 1172–1197 CrossRef CAS PubMed .
- C. M. Sabliov and C. E. Astete, Polymeric nanoparticles for food applications, Nanotechnology and functional foods: Effective delivery of bioactive ingredients , 2015, p. 272 Search PubMed .
- C. Jacobsen, P. J. García-Moreno, A. C. Mendes, R. V. Mateiu and I. S. Chronakis, Annu. Rev. Food Sci. Technol. , 2018, 9 , 525–549 CrossRef CAS PubMed .
- S. Chaudhary, T. Garg, R. Murthy, G. Rath and A. K. Goyal, J. Drug Target. , 2014, 22 , 871–882 CrossRef CAS PubMed .
- C. W. Pouton and C. J. Porter, Adv. Drug Deliv. Rev. , 2008, 60 , 625–637 CrossRef CAS PubMed .
- H. Bunjes, Curr. Opin. Colloid Interface Sci. , 2011, 16 , 405–411 CrossRef CAS .
- A. F. Esfanjani, E. Assadpour and S. M. Jafari, Trends Food Sci. Technol. , 2018, 76 , 56–66 CrossRef .
- A. Gasa-Falcon, I. Odriozola-Serrano, G. Oms-Oliu and O. Martín-Belloso, Foods , 2020, 9 , 325 CrossRef CAS PubMed .
- S. Peng, L. Zou, W. Liu, C. Liu and D. J. McClements, J. Agric. Food Chem. , 2018, 66 , 12421–12430 CrossRef CAS PubMed .
- C. Qian, E. A. Decker, H. Xiao and D. J. McClements, Food Res. Int. , 2013, 52 , 342–349 CrossRef CAS .
- V. da Silva Santos, A. P. B. Ribeiro and M. H. A. Santana, Food Res. Int. , 2019, 122 , 610–626 CrossRef PubMed .
- M. Azizi, Y. Li, N. Kaul and A. Abbaspourrad, J. Agric. Food Chem. , 2019, 67 , 671–679 CrossRef CAS PubMed .
- J. Weiss, E. A. Decker, D. J. McClements, K. Kristbergsson, T. Helgason and T. Awad, Food Biophys. , 2008, 3 , 146–154 CrossRef .
- F. Shahidi and Y. Zhong, Chem. Soc. Rev. , 2010, 39 , 4067–4079 RSC .
- F. Chen, G.-Q. Fan, Z. Zhang, R. Zhang, Z.-Y. Deng and D. J. McClements, Food Res. Int. , 2017, 100 , 387–395 CrossRef CAS PubMed .
- D. J. McClements and S. M. Jafari, in Nanoemulsions , Elsevier, 2018, pp. 3–20 Search PubMed .
- J. Velasco, S. Marmesat, C. Dobarganes and G. Márquez-Ruiz, J. Agric. Food Chem. , 2006, 54 , 1722–1729 CrossRef CAS PubMed .
- S. Sharma, S.-F. Cheng, B. Bhattacharya and S. Chakkaravarthi, Trends Food Sci. Technol. , 2019, 91 , 305–318 CrossRef CAS .
- R. de M. Barbosa, L. N. Ribeiro, B. R. Casadei, C. M. Da Silva, V. A. Queiróz, N. Duran, D. R. De Araújo, P. Severino and E. De Paula, Pharmaceutics , 2018, 10 , 231 CrossRef PubMed .
- D. Xie, M. Gong, W. Wei, J. Jin, X. Wang, X. Wang and Q. Jin, Compr. Rev. Food Sci. Food Saf. , 2019, 18 , 514–534 CrossRef PubMed .
- F. Tamjidi, A. Nasirpour and M. Shahedi, Food Sci. Technol. Int. , 2012, 18 , 381–390 CrossRef CAS PubMed .
- F. Tamjidi, A. Nasirpour and M. Shahedi, J. Agric. Sci. Technol. , 2014, 16 , 1073–1082 Search PubMed .
- T. Ghorbanzade, S. M. Jafari, S. Akhavan and R. Hadavi, Food Chem. , 2017, 216 , 146–152 CrossRef CAS PubMed .
- D. Bermúdez-Aguirre and G. V. Barbosa-Cánovas, LWT--Food Sci. Technol. , 2011, 44 , 1577–1584 CrossRef .
- F. Farbod, A. Kalbasi, S. Moini, Z. Emam-Djomeh, H. Razavi and A. Mortazavi, J. Food Sci. Technol. , 2015, 52 , 1372–1382 CrossRef CAS PubMed .
- S. M. Ojagh and S. Hasani, J. Food Meas. Char. , 2018, 12 , 1084–1092 CrossRef .
- P. T. Andajani, H. Purnomo and L. E. Radiati, Int. J. ChemTech Res. , 2015, 8 , 548–555 CAS .
- A. Gowda, V. Sharma, A. Goyal, A. Singh and S. Arora, J. Food Sci. Technol. , 2018, 55 , 1705–1715 CrossRef CAS PubMed .
- R. Domínguez, M. Pateiro, R. Agregán and J. M. Lorenzo, J. Food Sci. Technol. , 2017, 54 , 26–37 CrossRef PubMed .
- J. Stangierski, R. Rezler, K. Kawecki and B. Peplińska, J. Sci. Food Agric. , 2020, 100 , 2043–2051 CrossRef CAS PubMed .
- J. Xue, T. Wang, Q. Hu, M. Zhou and Y. Luo, Food Hydrocolloids , 2018, 79 , 110–116 CrossRef CAS .
- J. Sun, C. Bi, H. M. Chan, S. Sun, Q. Zhang and Y. Zheng, Colloids Surf. B Biointerfaces , 2013, 111 , 367–375 CrossRef PubMed .
- M. R. I. Shishir, L. Xie, C. Sun, X. Zheng and W. Chen, Trends Food Sci. Technol. , 2018, 78 , 34–60 CrossRef CAS .
- P. Ezhilarasi, S. Muthukumar and C. Anandharamakrishnan, RSC Adv. , 2016, 6 , 53784–53793 RSC .
- A. Guri, I. Gülseren and M. Corredig, Food Funct. , 2013, 4 , 1410–1419 RSC .
- Open access
- Published: 30 August 2024
Co-delivery of camptothecin and MiR-145 by lipid nanoparticles for MRI-visible targeted therapy of hepatocellular carcinoma
- Jing Rong 1 na1 ,
- Tongtong Liu 2 na1 ,
- Xiujuan Yin 1 ,
- Min Shao 1 ,
- Kun Zhu 1 ,
- Shiqi Wang 2 ,
- Yujie Zhu 1 ,
- Saisai Zhang 1 ,
- Likang Yin 1 ,
- Xiao Wang 1 &
- Lei Zhang 2
Journal of Experimental & Clinical Cancer Research volume 43 , Article number: 247 ( 2024 ) Cite this article
Metrics details
Camptothecin (CPT) is one of the frequently used small chemotherapy drugs for treating hepatocellular carcinoma (HCC), but its clinical application is limited due to severe toxicities and acquired resistance. Combined chemo-gene therapy has been reported to be an effective strategy for counteracting drug resistance while sensitizing cancer cells to cytotoxic agents. Thus, we hypothesized that combining CPT with miR-145 could synergistically suppress tumor proliferation and enhance anti-tumor activity.
Lactobionic acid (LA) modified lipid nanoparticles (LNPs) were developed to co-deliver CPT and miR-145 into asialoglycoprotein receptors-expressing HCC in vitro and in vivo. We evaluated the synergetic antitumor effect of miR-145 and CPT using CCK8, Western blotting, apoptosis and wound scratch assay in vitro, and the mechanisms underlying the synergetic antitumor effects were further investigated. Tumor inhibitory efficacy, safety evaluation and MRI-visible ability were assessed using diethylnitrosamine (DEN) + CCl 4 -induced HCC mouse model.
The LA modification improved the targeting delivery of cargos to HCC cells and tissues. The LA-CMGL-mediated co-delivery of miR-145 and CPT is more effective on tumor inhibitory than LA-CPT-L or LA-miR-145-L treatment alone, both in vitro and in vivo, with almost no side effects during the treatment period. Mechanistically, miR-145 likely induces apoptosis by targeting SUMO-specific peptidase 1 (SENP1)-mediated hexokinase (HK2) SUMOylation and glycolysis pathways and, in turn, sensitizing the cancer cells to CPT. In vitro and in vivo tests confirmed that the loaded Gd-DOTA served as an effective T1-weighted contrast agent for noninvasive tumor detection as well as real-time monitoring of drug delivery and biodistribution.
Conclusions
The LA-CMGL-mediated co-delivery of miR-145 and CPT displays a synergistic therapy against HCC. The novel MRI-visible, actively targeted chemo-gene co-delivery system for HCC therapy provides a scientific basis and a useful idea for the development of HCC treatment strategies in the future.
Hepatocellular carcinoma (HCC) is the third most frequent cause of cancer‑associated deaths worldwide, accounting for over 90% of liver cancer cases [ 1 ]. Systematic chemotherapy is currently the primary treatment for HCC [ 2 , 3 ]. Camptothecin (CPT), an inhibitor of topoisomerase I, is one of the frequently used small chemotherapy drugs for treating patients with solid malignant tumors, including HCC [ 4 , 5 ]. However, these drugs have poor solubility in water and generate a high amount of toxicity and side effects, compromising patients’ quality of life [ 6 , 7 ]. Moreover, HCC often develops resistance to these drugs due to the one-dimensional action mechanism of single drug therapy [ 8 , 9 ]. Therefore, there is an urgent need for the development an effective therapeutic strategy for clinical use.
MicroRNA (miRNA or miR) refers to a group of endogenous, noncoding small RNA that regulate a significant number of proteins at the translation level, making their use a popular gene therapy technique for cancer treatment [ 10 ]. Notably, miR-145, a member of the miRNA family, has been found to downregulate numerous malignant tumors and identified as a critical suppressor of cancer initiation, metastasis, and therapeutic resistance [ 11 , 12 , 13 , 14 ]. Moreover, overexpression of miR-145 can reduce the extracellular acidification rate (ECAR) and suppress glycolysis in various cancer cells by regulating the aerobic glucose metabolism [ 12 , 14 ]. ECAR are widely used proxies for glycolytic rate in cell metabolism studies and an increase in ECAR may indicate a greater reliance on glycolysis by the cells [ 15 ]. Despite the critical role of miR-145 in cancer cell regulation, it has not been sufficiently explored as a therapeutic target for HCC, either through monotherapy or combination therapy [ 12 , 16 ]. Therefore, it is necessary to explore the anti-HCC pathway of miR-145 and pay more attention to its potential for clinical application.
Combined chemo-gene therapy has been reported to be an effective strategy for counteracting drug resistance while sensitizing cancer cells to cytotoxic agents [ 7 , 17 , 18 , 19 ], thereby addressing the urgent need for nonsurgical therapy for liver cancer [ 6 , 20 ]. However, finding an effective and safe delivery strategy is the main challenge affecting the successful clinical translation of combined therapy [ 10 , 21 ]. Lipid nanoparticles (LNPs) containing an ionizable lipid (DLin-MC3-DMA, MC3) are currently among the most advanced oligonucleotide delivery systems [ 22 , 23 , 24 ]. MC3 is essential for RNA therapeutics protection and for driving lysosomal escape due to its ability to acquire charge after endocytosis [ 23 , 25 , 26 ]. In 2018, the first MC3-based siRNA therapeutic drug, Onpattro, was approved by the US Food and Drug Administration for the treatment of transthyretin-mediated amyloidosis [ 27 ]. More recently, a novel strategy named selective organ targeting (SORT) has been reported, which allows MC3-based LNPs containing four components to be systematically designed for the accurate delivery of diverse RNA to the livers of mice following intravenous (IV) administration [ 25 , 28 ]. Given the significant progress in the development of LNP-based nucleic acid delivery systems, we are positive that LNPs provide the most promising delivery platforms for the intravenous co-delivery of miRNA and small chemotherapeutics in HCC therapy [ 18 , 29 , 30 ].
Gd-DOTA is a rather safe MRI contrast agent that has been widely applied in clinical settings [ 31 , 32 ]. The introduction of Gd 3+ into nanosystems enables the MRI-visible delivery and biodistribution of drug carriers as well as cancer diagnosis [ 5 , 32 , 33 ]. In traditional medical care, diagnosis and therapy are considered two separate issues [ 2 , 34 ]. However, mounting evidence has shown that combining them mutually and synergically allows for achieving optimal personalized curative effects for many cancers [ 31 , 35 ]. Targeted delivery is particularly important in the treatment of HCC because it helps decrease systemic toxicity and off-target effects and promotes the accumulation of drugs and contrast agents in tumor cells and tissues [ 35 , 36 ]. Asialoglycoprotein receptors (ASGPRs) are cell membrane receptors that are overexpressed on the surface of liver cancer cells [ 6 , 37 ]. Lactobionic acid (LA), which comprises gluconic acid and Gal moiety, has been extensively investigated as a liver cancer-targeting ligand due to its specific affinity to ASGPRs [ 35 , 38 ]. Therefore, the incorporation of LA into LNPs would enhance the targeted delivery of cargos in liver cancer tissue via ASGPR-mediated endocytosis [ 39 ].
In this study, we fabricated LA-modified MC3-based LNPs for the co-delivery of CPT/miR-145 and Gd-DOTA to simultaneously achieve combined chemo-gene therapy for HCC and real-time MR imaging (Scheme 1 ). The LNPs protected miR-145 from degradation by endo/exonucleases and improved the water insolubility of CPT. After internalization via ASGPR-mediated endocytosis, the LA-modified CPT/miR-145 and Gd-DOTA coloaded lipid nanoparticles (LA-CMGL) disassembled rapidly in the acidic lysosomal environment and led to lysosomal escape due to the ion-pair mechanism [ 27 , 40 ]. The incorporated cargos, namely CPT/miR-145 and Gd 3+ , were then released into the cytoplasm. CPT/miR-145 in the LA-CMGL showed excellent synergetic antitumor effects both in vitro and in a diethylnitrosamine (DEN) + CCl 4 -induced HCC mouse model. The loaded Gd 3+ served as an effective T1-weighted contrast agent for noninvasive tumor detection and the real-time monitoring of drug delivery. In addition, a new mechanism for inhibition of HCC growth by LA-CMGL was demonstrated: specifically, miR-145 sensitizes cancer cells to CPT and promotes apoptosis by targeting the SENP1-mediated HK2 SUMOylation and glycolysis pathways. These findings open up a new avenue for the design of nano-based medicines for personalized cancer therapy.
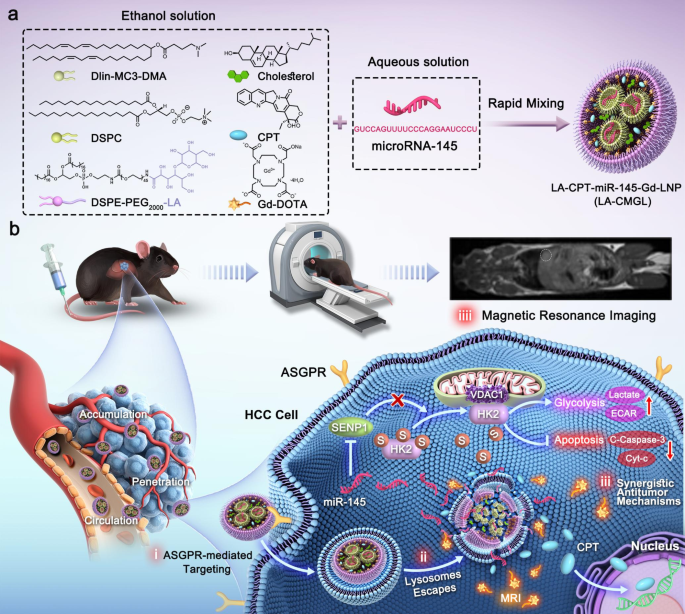
Schematic illustration for the design and application of LA-CMGL in HCC therapy. ( a ) Formulation of LA-CMGL by rapid pipette mixing of acidic aqueous solution and ethanol solution. ( b ) Schematic illustration of LA-CMGL co-delivery of CPT/miR-145 and Gd-DOTA to achieve MRI-visible targeted therapy of HCC (i) ASGPR-mediated targeting. (ii) Lysosome escape. (iii) Synergetic antitumor mechanism of CPT and miR-145, with the released CPT inducing the apoptosis of HepG2 cells by binding to chromosomal DNA and miR-145 promoting HepG2 cells apoptosis via the SENP1-mediated HK2 SUMOylation and glycolysis pathways. iiii) MR targeted imaging and real-time monitoring of drug delivery
DLin-MC3-DMA (heptatriaconta-6,9,28,31-tetraen-19-yl-4[dimethylamino]-butanoa-te), DSPC (1,2-distearoyl-sn-glycero-3-phosphocholine), cholesterol, LA (lactobio-nic acid), and DSPE-PEG2000-NH2 (1,2-distearoyl-sn-glycero-3-Phosphoethanola-mine-N-[methoxypolyethylene glycol-2000]) were obtained from Shanghai Yua Ye Bio-Technology Co., Ltd. CPT was purchased from Sigma-Aldrich. Cy5.5-miRNA-145 (abbreviated as miR-145 unless otherwise stated) was purchased from Shanghai GenePharma Co., Ltd. Gd-DOTA was purchased from Xi’an Kaixin Biotechnology Co., Ltd. FBS was obtained from Thermo Fisher Scientific, USA. All chemical reagents and solvents were of commercial special grade and were used without further purification.
Preparation of LA-CMGL
DSPE-PEG-LA was prepared by conjugating LA onto DSPE-PEG2000-NH2. Specifically, LA (53.7 mg, 150 µmol), EDC (43 mg, 225 µmol), and NHS (26 mg, 225 µmol) were dissolved in PBS (pH 7.4, 10 mM), and the mixture was stirred for 2 h at 0℃. DSPE-PEG2000-NH2 (140 mg, 50 µmol) was then added, and the mixture was stirred for another 12 h. After the reaction, the unreacted LA was removed via dialysis (MWCO = 3000 Da) against distilled water, and DSPE-PEG-LA was obtained by lyophilization. The successful synthesis of DSPE-PEG-LA was confirmed using 1 H NMR analysis.
LA-CPT-miR-145-Gd-LNP (abbreviated as LA-CMGL) was prepared using an ethanol dilution method. To this end, 2 mM ionizable lipid DLin-MC3-DMA was dissolved in ethanol with 1 mM DSPC, 1 mM Chol, and 0.5 mM DSPE-PEG-LA to prepare a lipid ethanol solution with the same molar composition (50/10/38.5/1.5, mol/mol) as the Onpattro formulation [ 27 , 28 ]. CPT and Gd-DOTA were also dissolved in ethanol, and miR-145 was dissolved in citrate buffer (10 mM, pH 4.0). The ethanol solution (40 µL) was then rapidly mixed with miR-145 solution (120 µL) at a volume ratio of 1:3 and a w/w ratio of 40:1 (lipid/miR-145). To achieve a final weight ratio of 40:1 for total lipid / total RNA, the concentration of miR-145 was set as 0.86 µg/µL. The dosages of CPT and Gd-DOTA were 15 µmol/kg and 40 µmol/kg, respectively. After 10 min of incubation at room temperature, the formulated LNPs were diluted with 1 × PBS for the in vitro studies. For in vivo studies, the LNPs were further purified by dialysis in sterile 1 × PBS dialysis tubes (MWCO = 3500 Da) for 2 h. Blank and single drug-loaded LNPs were prepared and modified using the same conditions as above and labeled blank L, CPT-L, miR-145-L, LA-CPT-L, and LA-miR-145-L. CMGL was prepared without any LA modification using the same method.
Characterization of lipid nanoparticles
Dynamic light scattering (DLS) was performed using the zetasizer Nano ZS (Malvern Panalytical, Malvern, UK) to determine the size distribution and zeta potential of LA-CMGL, CMGL, and blank L. The morphologies of the LNPs were observed using transmission electron microscopy (TEM, Thermo Fisher Scientific, USA). The encapsulation efficiencies (EE%) of CPT and miR-145 were determined using a fluorescence spectrophotometer (Spectrofluorometer FS5, Edinburgh Instruments Ltd, UK) [ 7 , 40 ]. The Gd 3+ content loaded in LA-CMGL was determined using an inductively coupled plasma-optical emission spectrometer (ICP-OES, PerkinElmer Optima 7000 DV, USA).
In vitro stability and drug release
To explore the stability of the LA-CMGL, their sizes and polydispersity indexes (PDIs) were monitored using DLS for one week, and they were stored in PBS (4 °C and 37 °C). To evaluate the protective ability of LA-CMGL against miR-145, free miR-145 and LA-CMGL were incubated in mouse serum for 2, 6, 8, 12, and 24 h, and the integrity of miR-145 was analyzed using a gel retardation assay.
The release profiles of LA-CMGL in PBS with different pH values (pH 4.5, pH 6.5, and pH 7.4) were evaluated using the dialysis method. Specifically, 1 mL samples of free CPT and LA-CMGL were dispersed separately and transferred immediately to a dialysis bag (MWCO = 5000 Da) against 20 mL of PBS at 37 °C. At predetermined time points (0, 2, 4, 6, 12, 24, 48, 72, and 96 h), 2 mL of the release medium was collected, and an equal volume of fresh PBS was added. The quantities of released CPT were measured using a fluorescence spectrophotometer.
Cellular uptake and lysosomal escape
For the cellular internalization study, we selected two cell lines: ASGPR-overexpressed HepG2 cancer cells and ASGPR-underexpressing HepaRG cells (Figure S1 ). HepaRG cells share some features and properties with adult liver cells, making them particularly useful for evaluating drugs [ 41 ]. Each cell type was seeded in 24-well plates (1 × 10 5 cells/well) and cultured overnight at 37 ℃. The culture medium was then refreshed with a serum-free Opti-MEM medium containing LA-CMGL or CMGL loaded with Cy5.5-miR-145 (50 nM) at 37 ℃. After 6 h of incubation, the cells were collected, washed with cold PBS thrice, and analyzed via confocal laser scanning microscopy (CLSM, Zeiss, LSM-700) and flow cytometry (Beckman, CytoFLEX, USA). For the competition assay, 1 mM of free LA was added to the incubation media prior to the addition of LA-CMGL, followed by the same steps described above. All experiments were performed in triplicate.
The intracellular delivery and distribution of Cy5.5-miR-145 were investigated through CLSM after cellular internalization. HepG2 cells were incubated with LA-CMGL for 1 h and 6 h. At the preassigned 30 min time point, 1 mL LysoTracker Green (1 mM) was added to stain the lysosomes, and lysosomal escape was observed using CLSM. The degree of Cy5.5-miR-145 and Lyso-Tracker Green colocalization was assessed by Image J.
In vitro cytotoxicity, apoptosis and cell migration assays
The cytotoxic effects of various formulations against HepG2 cells or HepaRG cells were assessed using a Cell Counting Kit-8 (CCK8, Biosharp, Japan). In brief, HepG2 or HepaRG cells were seeded in 96-well plates at a density of 1 × 10 4 per well at 37 °C. First, HepG2 cells were treated with different doses of CPT for 24 h. Second, HepG2 cells were treated with fixed concentrations of free drugs (CPT, miR-145, and CPT + miR-145) at 37 °C for 60 h; CPT and miRNA concentrations were 10 µg/mL and 100 nM, respectively. Third, HepG2 or HepaRG cells were treated with the same fixed concentrations of LA-NC-L, LA-CPT-L, LA-miR-145-L, LA-CMGL, and CMGL at 37 ℃ for 24 h. The cells treated with PBS were used as controls. Each section of the assay was performed in triplicate. Cell viability was determined using the CCK8 kit in accordance with the manufacturer’s suggestions.
To confirm the anticancer effect of the CPT/miR-145 coloaded LNPs, a cell apoptosis assay was performed using the annexin V-FITC/PI staining method. Briefly, HepG2 cells were seeded into six-well plates at a density of 1 × 10 5 cells per well and incubated overnight at 37 ℃. These cells were then treated with LA-NC-L, LA-CPT-L, LA-miR145-L, LA-CMGL, and CMGL in serum-free Opti-MEM at 37 ℃ for 48 h. Cells treated with PBS were used as controls. An Annexin V-FITC Apoptosis Detection Kit (Biosharp, China) was used to determine the apoptosis rate according to the manufacturer’s protocol.
To evaluate cell migration inhibition ability of LA-CMGL, the wound scratch assay was carried out. HepG2, Huh7 and Hep3B cells were seeded in 6-well plates at a density of 5 × 10 5 / well. When the cell reached 80–90% confluent monolayer, the cells were scratched off the plate with 200 µL pipette tip and washed with PBS. Then, the cells were treated with LA-NC-L, LA-CPT-L, LA-miR-145-L, CMGL and LA-CMGL. Cells without any treatment served as controls. Eventually, photographs were taken with a Zeiss microscope (Oberkochen, German) at 0 h and 24 h. The areas of the scratch were quantified by the Image J software and the wound closure rate = (0 h area − 24 h area/ 0 h area × 100%.
In vitro MRI
The longitudinal relaxation rate (1/T1) values for LA-CMGL and Gd-DOTA were measured using an Ingenia 1.5T MRI and calculated in line with our previous report [ 5 ]. Next, HepG2 cells and HepaRG cells were incubated in LA-CMGL with various Gd 3+ concentrations at 37 ℃ for 12 h. After washing them in a plate with 5 mL of PBS three times, the cells were transferred to microtubes for cellular MRI.
Antitumor effects and safety evaluation of LA-CMGL in vivo
To evaluate the antitumor effects of LA-CMGL, the DEN + CCl 4 -induced HCC mouse model was established as described [ 42 , 43 ]. The mice were randomly divided into six groups ( n = 12 per group) and received intravenous administration of PBS (control group), LA-NC-L, LA-CPT-L, LA-miR-145, CMGL, or LA-CMGL with the final CPT concentration of 10 µg/mL and miR-145 concentration of 100 mM. The different formulations (100 µL) were administered intravenously twice per week from week 28 to 36. The mice’s body weights were recorded every other day since the treatment began. In the 36th week after treatment, blood was collected, the alanine transaminase (ALT) and aspartate aminotransferase (AST) levels were analyzed to assess liver toxicity with the different formulations. The ALT and AST levels of healthy mice treated with PBS were used as controls ( n = 3). The model mice were sacrificed, and the highest tumor volume and tumor number were recorded ( n = 5 per group). For histological examinations, the tumor tissues and main organs (heart, liver, spleen, lung, and kidney) were removed. The biggest tumor tissue was selected and fixed in 4% paraformaldehyde at 4 °C overnight and later embedded in paraffin. Tumor sections of 5 μm were then prepared for H&E or TUNEL staining and microscopic observation. Part of the tumor tissue was homogenized in RIPA buffer (Thermo Scientific), and proteins were extracted for a WB analysis of apoptotic protein expression, as described above. The major organs were also stained with H&E for pathological analysis. The survival times of the mice were recorded daily from the beginning of treatment to the day of death and analyzed via a Kaplan − Meier survival study ( n = 7 per group).
MRI-visible targeted delivery of LA-CMGL in vivo
DEN + CCl 4 -induced HCC model mice were studied to evaluate the in vivo biodistribution and tumor-targeting MR imaging capabilities of LA-CMGL ( n = 3). Free Gd-DOTA, CMGL, or LA-CMGL were injected at a dose of 40 µmoL Gd/kg through the tail veins of the HCC model mice. The mice were anesthetized with an intraperitoneal injection of 1% sodium pentobarbital at a dose of 50 mg/kg. Precontrast and postcontrast T1-weighted magnetic resonance images were acquired using a 3.0T scanner (GE Signa Horizon) with a small animal coil and a fast spin-echo pulse sequence. The parameters were as follows: TR/TE = 400/12 ms, FOV 8 × 6.4 cm 2 , matrix 256 × 192, slices/space 1.0/0.5 mm, and NEX 6–8. To quantitatively analyze the biodistribution of LA-CMGL, the contrast-to-noise ratio (CNR) of a specific organ was computed using the following equation: CNR = Sp-S0/Sn, where Sp (post-injection) and S0 (pre-injection) denote the signal intensity in the region of interest (ROI), and Sn is the standard deviation of noise estimated from the background air. In addition, the HCC-targeting MRI capability of LA-CMGL was evaluated by calculating the T/N ratio using the same experimental procedure as described above. T/N ratios represent the signal intensities of various Gd 3+ preparations within the regions of interest of tumor and normal liver tissues.
Results and discussion
Synthesis and characterization of dspe-peg-la and la-cmgl.
To improve the liver-targeting ability of LNPs, DSPE-PEG-LA was generated by conjugating LA onto DSPE-PEG2000-NH2 through a one-step reaction (Figure S2 a). The successful synthesis of DSPE-PEG-LA was confirmed using a 1 H NMR analysis (Figure S2 b). LA-CMGL was then prepared using the thin-film evaporation ethanol dilution method. The size, morphology, and zeta potential of LA-CMGL and CMGL are given in Fig. 1 a–d and Table S1 . The results showed that the LA-CMGL and CMGL were about 160–170 nm in size on average, with low PDI < 0.36, an analogous spherical shape, and compact structure. LA-CMGL also exhibited a negative surface charge with a zeta potential of -3.5 mV, enhancing the stability of LA-CMGL via electrostatic repulsion [ 40 ]. Next, we investigated the stability of LA-CMGL and found that LA-CMGL remained stable in the 10% FBS condition at 4 °C and 37 °C for seven days (Fig. 1 e), indicating the good stability of the LNPs. We then used a gel retardation assay to evaluate the protective ability of LA-CMGL against miR-145. The results showed that LA-CMGL could protect miR-145 from degradation in mouse serum within 24 h, whereas free miR-145 was almost fully degraded within 6 h (Fig. 1 f). Thus, it was inferred that LA-CMGL has a superb ability to protect miR-145 from degradation by serum nucleases, which is beneficial for prolonged blood circulation in vivo.
The encapsulation rates (EE%) of CPT and miR-145 were measured through fluorescence spectrophotometry. As shown in Table S1 , LA-CMGL demonstrated high EE% for both CPT and miR-145, at about 85% and 81%, respectively. The Gd 3+ content in LA-CMGL was determined to be 2.6 wt%. In our previous work, Gd 3+ content of 1.8 ~ 2.64 wt% was shown to result in a favorable MR enhancement effect [ 5 , 34 ]. We hypothesized that high miR-145 and CPT encapsulation rates and a moderate level of Gd 3+ content would lead to effective synergetic antitumor properties and superb MRI efficacy. The drug release behavior of CPT in LA-CMGL was then assessed under different conditions, including a normal physiological environment (pH 7.4), an acidic tumor environment (pH 6.5), and a lysosomal environment (pH 4.5). As presented in Fig. 1 g, a burst release of free CPT was observed independent of the pH value, with a 60% CPT release in the first 4 h. The release rate of CPT in LA-CMGL was faster at pH 4.5 than at pH 6.5 and pH 7.4 within the first 4 h, indicating that CPT is rapidly released from LA-CMGL in lysosomal environments. The amount of CPT released in LA-CMGL was approximately 80% at pH 4.5 within 96 h, whereas it was less than 50% and 40% at pH 6.5 and pH 7.4, respectively (Fig. 1 h). Thus, we concluded that the pH-responsive drug release pattern of CPT contributes to the effective delivery of LA-CMGL to HCC cells, which is essential for further therapy.
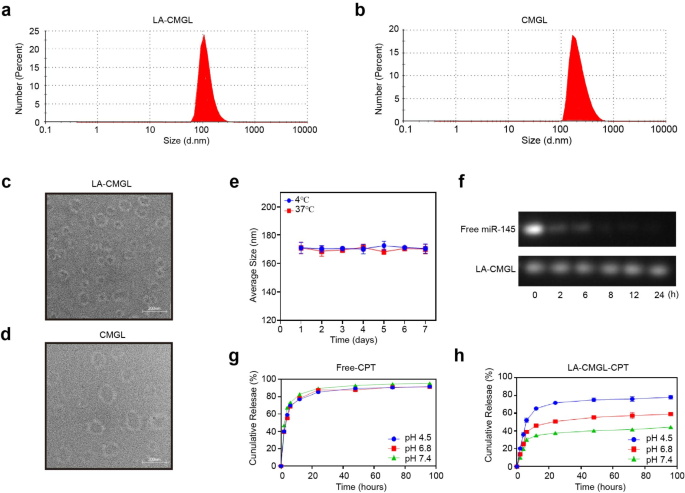
Characterizations of LA-CMGL. ( a , b ) The LA-CMGL and CMGL showed 160–170 nm average particle sizes, and similar morphology ( c , d ). ( e ) The stability of LA-CMGL during 7 days incubation with 10% FBS at 4 ℃ and 37 ℃. ( f ) Gel retardation assay after incubation with mouse serum for 2, 6, 8, 12 and 24 h. ( g , h ) In vitro release profile of free CPT and CPT in LA-CMGL at pH 4.5, 6.5 and 7.4. Data are mean ± standard deviation (SD)
Targeted delivery of LA-CMGL in vitro
Confocal laser scanning microscopy (CLSM) was conducted to assess the ability of LA-CMGL to target HepG2 cells; miR-145 was labeled with Cy5.5 (red), while CPT could emit blue fluorescence by itself. As shown in Fig. 2 a, the ASGPR-overexpressed HepG2 cells treated with LA-CMGL showed stronger red and blue fluorescence than those treated with CMGL, and this effect could be inhibited by preincubating the cells with extra free LA. Notably, the blue fluorescence found in the nucleus and the red fluorescence in the cytoplasm were not affected by each other in the cells treated with LA-CMGL, indicating the successful release of CPT and miR-145 from LA-CMGL. The accumulation of CPT in the nucleus was necessary for these small chemotherapy drugs to bind with DNA to show anticancer activity [ 3 , 18 ]. Additionally, a quantitative analysis involving flow cytometry (FCM) showed that LA-CMGL outperformed its nontargeted counterpart CMGL in delivering miR-145 to HepG2 cells. However, compared to CMGL, LA-CMGL did not increase the uptake of miR-145 in HepaRG cells (Figure S3 ). These results suggest that the modification of the LA ligand promoted cellular uptake through LA-receptor-mediated endocytosis, thus facilitating the efficient and simultaneous targeted delivery of CPT and miR-145 to HCC cells.
To further investigate the uptake process of LA-CMGL by HepG2 cells, CLSM was used to observe the escape process of LA-CMGL from lysosomes. We performed the colocalization analysis of Cy5.5-miR-145 in lysosome and cytoplasm using Manders’ colocalization coefficients. As illustrated in Fig. 2 b-c, upon co-incubation with LA-CMGL for 1 h, substantial cellular uptake into HepG2 cells and a higher colocalization ratio (73.4%) between miR-145 and lysosomes was observed, suggesting 73.4% of miR-145 is located in lysosomes and the remaining is in the cytoplasm. Extending the incubation time to 6 h, the colocalization ratio decreased to (33.3%), indicating the successful lysosome escape of miR-145 (66.7% of miR-145 is located in the cytoplasm). It is possible that the mechanism for this process involved the ionizable cationic lipid DLin-MC3-DMA acquiring a charge in the acidified lysosomes, thereby promoting lysosome destabilization and cargo release into the cytoplasm [ 26 , 27 , 40 ]. This critical lysosome-escaping ability of LA-CMGL guaranteed the intracellular release of miR-145 in the cytoplasm and CPT in the cell nucleus, enhancing synergetic antitumor efficacy.
Next, since 3D multicellular tumor spheroids (MCSs) have been reported to recapitulate critical physiological tumor parameters in vivo and simulate various aspects of human tumor environments [ 32 , 44 ], we focused on the delivery of LA-CMGL into MCSs derived from HepG2 cells. As shown in Fig. 2 d, LA-CMGL penetrated entire spheroids within 6 h, which was tracked using Cy5.5 fluorescence. In contrast, CMGL was unable to penetrate the center and was limited to the cell layers outside the spheroids. These results indicate the remarkable penetration and internalization of LA-CMGL into HCC spheroids, which holds promise for inflicting synergetic cytotoxicity on HCC cells.
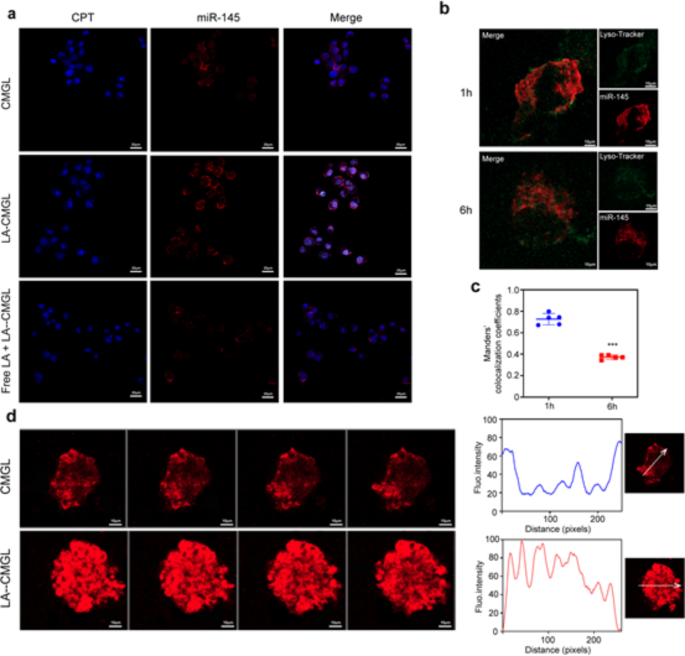
Targeting delivery and penetration ability of LA-CMGL in vitro. ( a ) Targeting delivery of CPT and miR-145 in HepG2 cells observed by CLSM (scale bar = 20 μm). ( b ) The escape ability of LA-CMGL from lysosomes in HepG2 cells at 1 h and 6 h evaluated by CLSM (scale bar = 10 μm). ( c ) Co-localization analysis of miR-145 in lysosome and cytoplasm in HepG2 cells. ( d ) Penetration of LA-CMGL (red) in HepG2 MCSs imaged by CLSM Z-stack scanning after 6 h. The MCSs surface was settled at 0 μm. Scale bar = 10 μm
Synergetic antitumor effect of LA-CMGL in vitro
To explore the synergetic cytotoxic effects of CPT and miR-145 against HepG2 cells, a CCK8 assay was performed using free CPT and miR-145 drugs without a nanocarrier. As shown in Fig. 3 a, the upregulation of CPT inhibited the survival rate of HepG2 cells in a slight dose–effect relationship. The peak inhibitory effect of CPT on HepG2 cell viability was seen at a CPT concentration of about 10 µg/mL, so this was chosen as a favorable concentration. Meanwhile, the concentration of 100 nM miR-145 was selected for miR-145 application according to previous reports [ 8 , 23 ]. We found that treating the HepG2 cells with free CPT + free miR-145 resulted in cell viability rates of 57.37% and 48.25% after 24 h and 48 h of incubation, respectively, which were higher than the viability rates resulting from treatment with free CPT (79.86% and 71.53%) (Fig. 3 b). In addition, CCK8 assay against Huh7 and Hep3B cells showed similar results with those of HepG2 cells (Figure S4 ). A Western blotting (WB) analysis showed that the levels of protein SENP1 and HK2 decreased, the apoptotic proteins cleaved-caspase3 (C-caspase3) and cytochrome-c (Cyt-c) increased in the HepG2, Huh7 and Hep3B cells after treatment with CPT + free miR-145, compared to treatment with CPT alone (Figure S5 ). These data confirmed clearly that the ability of miR-145 to significantly enhance the chemotherapeutic efficacy of CPT.
We then performed a CCK8 assay to investigate whether the co-delivery of CPT and miR-145 by LNPs provides better results. As shown in Fig. 3 c, the inhibitory effects of the LA-miR-145 LNPs (abbreviated as LA-miR-145-L) and LA-CPT LNPs (LA-CPT-L) on the viability of HepG2 cells were comparable to that of free CPT + miR-145 ( p > 0.05). Most importantly, a striking decrease in HepG2 cell viability was observed with the use of LA-CMGL (55.31% viability), compared to the use of LA-CPT-L (72.34%), LA-miR-145-L (64.79%), or CMGL (60.26%) (all p < 0.01). Additionally, the survival rate of HepG2 cells treated with blank LA LNPs (LA-NC-L) exhibited no significant difference from the control groups ( p > 0.05), indicating the biocompatibility of the drug carrier. Next, we used FCM to compare the apoptosis ratios of HepG2 treated with different LNP formulations (Fig. 3 d). Our data revealed that HepG2 cells treated with LA-CMGL exhibited a significantly higher apoptosis ratio (33.64%) than those treated with LA-CPT-L (6.2%), LA-miR-145-L (9.14%), or CMGL (16.95%) (all p < 0.01), which correlated well with the CCK8 assay results. Moreover, a WB analysis confirmed that, compared to LA-CPT-L and LA-miR-145-L, LA-CMGL significantly increased the levels of C-caspase3 and cyt-c in the HepG2 cells (Fig. 3 e).
Next, to investigate anti-cell migration activity of LA-CMGL against HepG2, the wound scratch assay was performed. As shown in Fig. 3 f, all drug formulations presented obviously inhibitory effect in comparison with the control and LA-NC-L groups after 24 h. The CMGL groups showed slightly higher inhibitory effect than LA-CPT-L and LA-miR-145-L groups, while LA-CMGL groups exhibited obviously stronger inhibit migration activity. The wound closure rate of LA-CMGL, LA-CPT-L and LA-miR-145-L was 4.6%, 22.3% and 14.7%, respectively. Similar trends were also observed in Huh7 and Hep3B cells (Figure S6 ). Taken together, these results suggest that LA-CMGL-mediated co-delivery of miR-145 and CPT is more effective than LA-CPT-L or LA-miR-145-L treatment, as it enhances the antiproliferation effect, cell apoptosis and anti-cell migration activity.
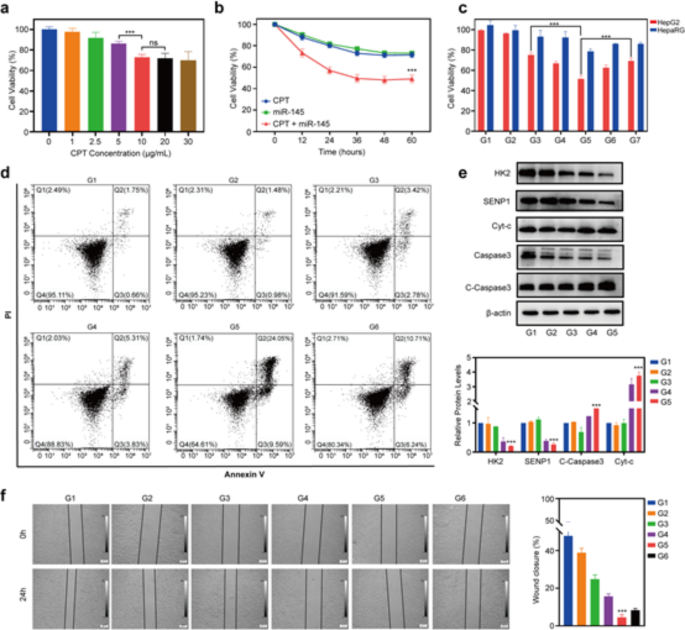
In vitro antitumor effect of LA-CMGL. ( a ) Viability of HepG2 cells treated with different dose levels of CPT for 24 h ( n = 3). ( b ) Viability of HepG2 cells incubated with CPT, miR-145, CPT + miR-145 from 12 to 60 h ( n = 3). ( c ) The proliferation inhibition of HepG2 cells and HepaRG cells treated with different formulations (G1-7) with the final miR-145 concentration of 100 nM and CPT concentration of 10 µg/mL. G1, G2, G3, G4, G5, G6 and G7 represented PBS, LA-NC-L, LA-CPT-L, LA-miR-145-L, LA-CMGL, CMGL and CPT + miR-145 groups, respectively ( n = 3). ( d ) Flow cytometry analysis of the apoptosis ratios in HepG2 cells treated with different formulations ( n = 3). ( e ) WB analysis showed LA-CMGL significantly increased the expression of C-caspase3 and Cyt-c protein. β-actin was used as an internal control. ( f ) In vitro wound scratch assay of the HepG2 cells ( n = 3). Quantitative analysis was performed using Image J software ( n = 3). Data are mean ± standard deviation (SD). Statistical significances in ( a ), ( c ), ( e ) and ( f ) were calculated via the Student’s t test (*** p < 0.001). Statistical significances in ( b ) were calculated via the one-way ANOVA with Tukey’s post hoc test (*** p < 0.001)
Synergetic antitumor mechanism of CPT and miR-145
Next, we explored the mechanisms underlying the synergetic cytotoxic effects of CPT and miR-145 in LA-CMGL. As a promising antitumor drug target, HK2 has dual regulatory effects on the metabolic and proliferative activities of cancer cells [ 45 , 46 ]. HK2 binds to voltage-dependent anion-selective channel protein 1 (VDAC1) on the mitochondrial surface, which contributes to the inhibition of apoptosis by closing the mitochondrial permeability transition pores and preventing cyt-c release [ 46 ]. We found that miR-145 mimics significantly decreased the colocation of HK2 and VDAC1 in HepG2 cells (Fig. 4 a). Moreover, the GPS-SUMO database (Figure S7 ) and co-inmunoprecipitation (Co-IP) assays (Fig. 4 b) showed that HK2 could be modified by SUMOylation (Fig. 4 c). Since SUMOylation (a reversible posttranslational modification) reportedly disrupts the binding of HK2 to VDAC1 [ 47 , 48 ], we attempted to explore whether miR-145 would alter the binding of HK2 to VDAC1 by affecting HK2 SUMOylation. SENP1, an important de-sumo protein, is largely responsible for the deconjugation of SUMO1 modifications [ 42 , 48 ]. As show in Figure S8 , the expression of SENP1 was markedly increased in HCC tumors and cells (HepG2, Huh7, and Hep3B). Altering SENP1 expression significantly affected the SUMOylation level of HK2 and it’s binding to VDAC1 (Figure S9 , S10 ). Now, the results showed that miR-145 inhibitor promoted the expression of SENP1 (Figure S12 c), while miR-145 mimics inhibited the expression of SENP1 (Fig. 4 d-e). Moreover, both ENCORI database predictions (Figure S11 ) and Dual-Luciferase Reporter results (Figure S12 a) showed that SENP1 was one of the targets regulated by miR-145. Additionally, the Co-IP results showed that miR-145 robustly increased the SUMOylated HK2 levels (Fig. 4 f), whereas co-transfection miR-145 mimics and SENP1 plasmid decreased the SUMOylated HK2 levels (Fig. 4 g). Taken together, these results indicate that SENP1 functions as the key HK2 deSUMOylase.
We then evaluated how miR-145 promotes the apoptosis of HepG2 cells. As expected, miR-145 decreased the binding of HK2 to VDAC1 and the mitochondrial membrane potential (Fig. 4 h). In addition, miR-145 increased the expression of C-caspase3 and cyt-c (Fig. 4 i-j) and the apoptosis rate of HepG2 cells (Fig. 4 k) which were accompanied by decreased levels of ECAR (Fig. 4 l) and extracellular lactate (Fig. 4 m). The simultaneous overexpression of miR-145 and SENP1 reversed the above effects of miR-145 (Figure S13 a–d). Thus, the above results strongly suggest that miR-145 promotes the apoptosis of HepG2 cells and then sensitize cancer cells to CPT by targeting the SENP1-mediated HK2 SUMOylation and glycolysis pathways.
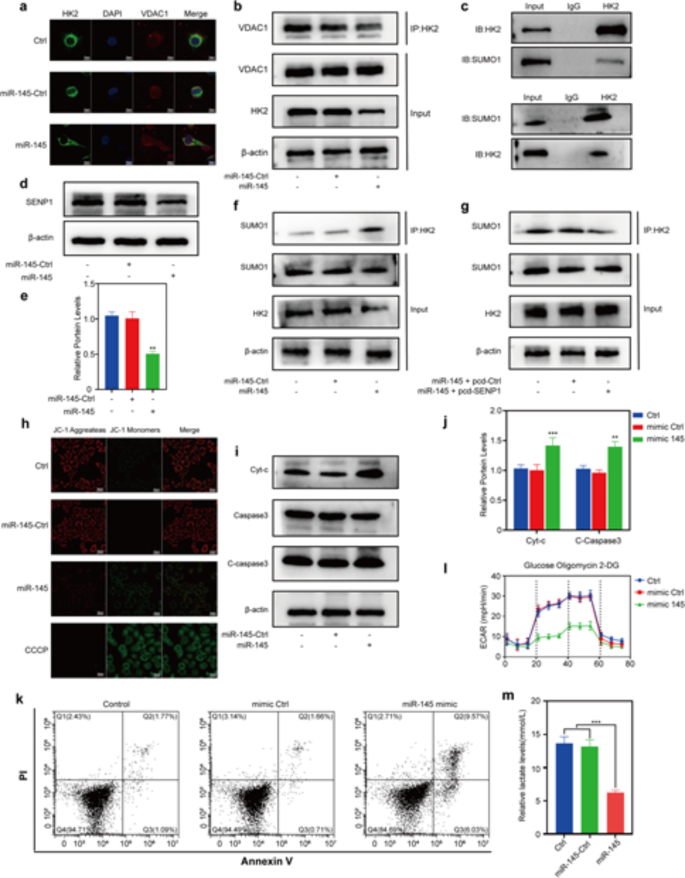
Synergetic antitumor mechanism of CPT and miR-145. ( a ) Immunofluorescence staining of HK2 (green) and VDAC1 (red) co-localization after HepG2 transfection with miR-145. ( b ) Co-IP detection of HK2 binding to VDAC1 after miR-145 transfection. ( c ) Immunoprecipitation of HK2 and SUMO1. ( d ) Expression and ( e ) quantification of SENP1 protein and after miR-145 transfection. ( f ) Co-IP of HK2 with SUMO1 after miR-145 transfection. ( g ) Co-IP detection SUMOylation of HK2 after cotransfection. ( h ) Detection of mitochondrial membrane potential after miR-145 transfection. ( i ) Protein expression and ( j ) quantification of Cyt-c, C-Caspase3 and Caspase3 after miR-145 transfection. ( k ) Detection of apoptosis after miR-145 transfection by flow cytometry. ( l ) HepG2 extracellular acidification rate after miR-145 transfection. ( m ) Lactate content of medium after miR-145 transfection or co-transfection. Statistical significance in ( e ), ( j ) and ( m ) was calculated via the Student’s t test (** p < 0.01)
Tumor inhibitory efficacy and safety evaluations in vivo
To evaluate the antitumor effect of LA-CMGL in an actual liver environment, a DEN + CCl 4 -induced HCC mouse model was established, and hepatocellular tumors were initiated and promoted via a two-stage application of DEN and CCl 4 [ 43 ]. This model is relevant for understanding human HCCs associated with chronic liver injury, inflammation, and fibrosis/cirrhosis [ 43 , 49 ], so we utilized it in the present study to simulate a difficult delivery challenge with a late-stage disease. This model was first used to evaluate inhibitory efficacy, followed by systemic toxicity.
We initiated a therapeutic regimen from week 28 after introducing HCC into the mouse model, which involved the administration of different LNP formulations containing 10 µg/mL CPT and/or 100 nmol/L miR-145 twice a week (Fig. 5 a). In the 36th week, ex vivo liver imaging (Fig. 5 b) confirmed that the mice that received LA-CMGL treatment had minimal tumor volumes and tumor numbers compared to the single drug-loaded groups (Fig. 5 c-d). More importantly, LA-CMGL significantly prolonged the survival time of tumor-bearing mice to 121 days, whereas the other LNPs were not able to extend survival past 90 days (Fig. 5 e). A WB analysis showed that the expression levels of the apoptosis-related proteins C-caspase3 and cyt-c were significantly higher in the LA-CMGL group than in the other groups (Fig. 5 f). Hematoxylin and eosin (H&E) or terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining further confirmed these results (Fig. 5 g). Thus, LA modification played a crucial role in the targeted co-delivery of CPT and miR-145, resulting in synergetic tumor inhibition and improved therapeutic effects.
We subsequently estimated the systemic toxicity levels of different LNPs formulations. As depicted in Fig. 5 h, the body weights of all the LNP-treated mice kept growing slowly or remained unchanged, indicating that negligible systemic side effects were generated during the co-delivery of CPT and miR-145 by LA-CMGL. Moreover, no histopathological alterations were observed in the major organs of the LA-CMGL-treated mice (Figure S14 ). We also performed a blood study to evaluate the potential clinical translation of the LA-CMGL. Due to liver injury induced by DEN + CCl 4 , ALT and AST levels increased sharply in the HCC model mice, as compared to the healthy mice, and LA-CMGL treatment slightly decreased these levels (Fig. 5 i, j). There were no obvious differences between the PBS- and LA-CMGL-treated mice with respect to the liver damage induced by DEN + CCl 4 . These findings highlight the excellent biocompatibility and biosafety of LA-CMGL.
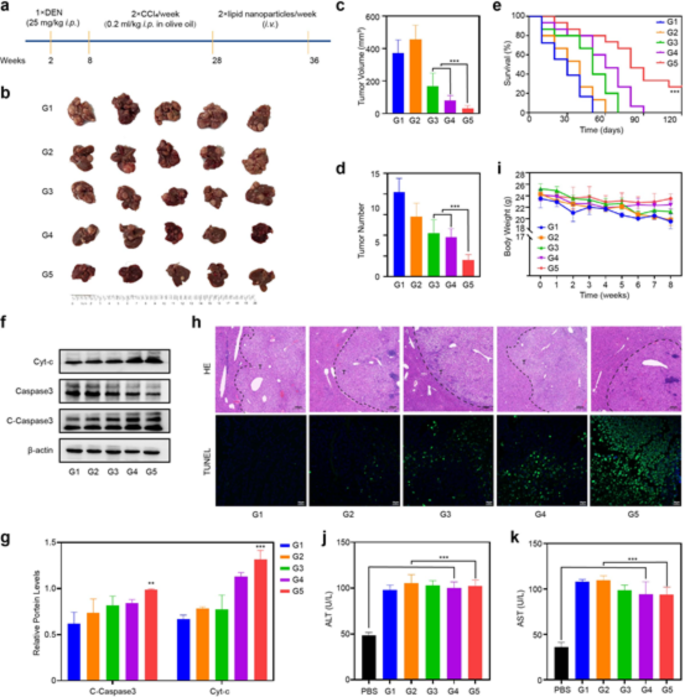
In vivo antitumor efficacy and safety evaluation. ( a ) Schemetic illustration of DEN + CCl 4 -induced HCC mouse model and administration regimen for systemic therapy. ( b ) The representative gross images of HCC tumor masses in different groups at 36th week after treatment ( n = 5). ( c , d ) Largest tumor volume and the number of tumor masses of model mice treated with different formulations ( n = 5). ( e ) Survival curves of model mice after different treatments ( n = 7). ( f ) The WB results showed LA-CMGL significantly increased the expression of C-Caspase3 and cyt-c protein in tumor tissue. ( g ) Quantification of WB by Image J software ( n = 3). ( h ) H&E and TUNEL staining of tumor sections ( n = 5) after different treatments. T indicate tumor tissue. Scale bars are 200 μm. ( i ) Weight changes of model mice after different treatments ( n = 5). ( j , k ) Serum ALT and AST levels in model mice ( n = 5). Healthy mice treated with PBS were used as control ( n = 3). Data are mean ± standard deviation (SD). Statistical significances in ( e ), and ( i ) were calculated via the one-way ANOVA with Tukey’s post hoc test (*** p < 0.001). Statistical significance in ( c ), ( d ), ( g ), ( j ) and ( k ) was calculated via the Student’s t test (** p < 0.01, *** p < 0.001)
In vitro and in vivo MRI analyses
To investigate the possibility of using LA-CMGL as a T1-weighted MRI contrast agent, the relaxivities (r1) of LA-CMGL and Gd-DOTA were evaluated. The r1 value for LA-CMGL was 11.379 mM − 1 S − 1 , which was nearly four times the 2.825 mM − 1 S − 1 of Gd-DOTA (Fig. 6 a). Furthermore, in a 1.5T MRI, LA-CMGL exhibited much better signal contrast and brightness than Gd-DOTA with the same Gd 3+ concentration (Fig. 6 b). This may be attributed to an increase in the local concentration of Gd 3+ and a decrease in the rate of molecular tumbling [ 32 , 33 ]. In addition, as the concentration of Gd 3+ gradually increased, the T1 signal improved in both HepG2 and HepaRG cell lines. Compared to the HepaRG cells, the HepG2 cells exhibited a much higher signal under the same Gd 3+ conditions (Fig. 6 c). This result strongly confirmed the site-specific accumulation and superior intracellular MRI contrasting effect of LA-CMGL due to ASGPR-mediated endocytosis [ 5 ].
Based on the preceding cellular MRI results, we further investigated the tumor-targeting properties and biodistribution of LA-CMGL in vivo. As shown in Fig. 6 d, Gd-DOTA was almost nonexistent in the cancerous tissue 180 min after injection, but LA-CMGL were found in local tumors. This prolonged enhancement of LA-CMGL in tumor environments would be beneficial for constant drug release. In addition, after injecting Gd-DOTA for 5 min, the enhancement of MRI signals could be identified. However, there was little distinction between the MRI signals for the tumor tissue and the adjacent normal liver tissue, and the tumor boundary was blurred. This can be explained by extracellular imaging property of Gd-DOTA rather than specific intracellular accumulation. In contrast, the tumor boundary was distinct after LA-CMGL injection compared with those of Gd-DOTA and CMGL at 5 min and particularly apparent at 30 min. This may be attributed to the targeted delivery of LA-CMGL into tumor cells/tissues, resulting in intracellular imaging.
For quantitative analysis, the tumor-to-normal ratio (T/N) of LA-CMGL was calculated, where T and N denote the signal intensities of the different Gd 3+ preparations in the region of tumor and the normal brain tissue, respectively. As depicted in Fig. 6 e, LA-CMGL was stronger than those of Gd-DOTA and CMGL (all p < 0.05) 15 min post injection, allowing for HCC to be identified at an early stage based on the clear boundary and shape of the tumor. Furthermore, to monitor the distribution of LA-CMGL in tumor-bearing mice, contrast-to-noise ratio (CNR) measurements were taken. As anticipated, LA-CMGL had a higher CNR in the liver than in the other organs (Fig. 6 f). This result aligned with the in vivo and ex vivo fluorescence imaging findings (Figure S15 ), indicating that the LA-CMGL composed of four components in a fixed ratio were optimal for liver delivery, despite the introduction of CPT/miR-145 and Gd-DOTA. Most importantly, LA-CMGL had a higher CNR in cancerous tissues than that in other organs at any time point ( p < 0.01), demonstrating that the LA-CMGL possessed good tumor-targeting accumulation abilities; this finding is consistent with the reports of previous cellular studies. Considering that Gd 3+ , CPT, and miR-145 were coloaded in LA-CMGL, it is likely that the MRI signal intensity of LA-CMGL in the major organs reflected the concentrations of the drugs distributed in these organs. Therefore, it is reasonable to assume that this quantitative analysis would be an efficient, real-time approach for noninvasively monitoring drug distribution. In sum, these results clearly show the strengths of using LA-CMGL for HCC target imaging and the real-time visualization of drug delivery and biodistribution in vivo, which is of great importance for tumor monitoring and treatment guidance.
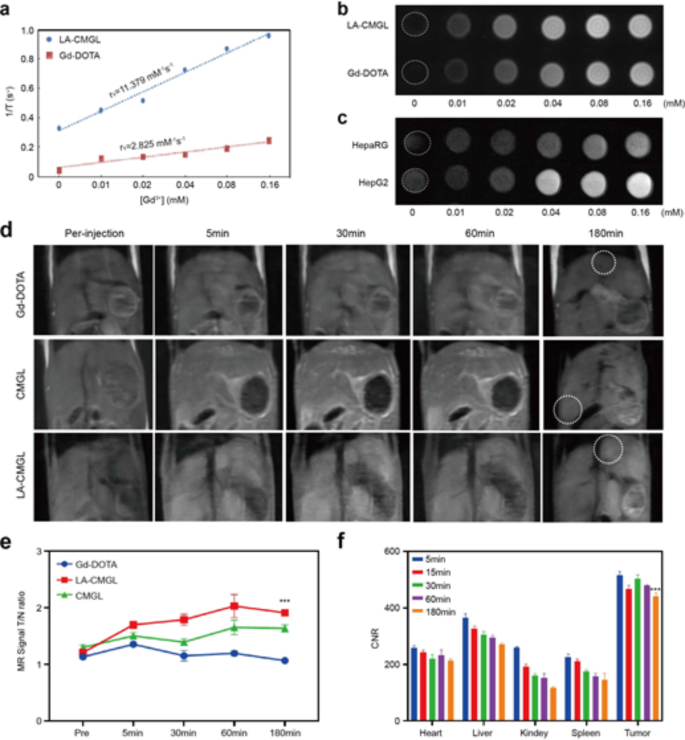
MR imaging relaxivity and in vivo MR imaging of LA-CMGL. ( a ) Longitudinal relaxation rate (1/T1) of Gd-DOTA and LA-CMGL in an aqueous solution as a function of the Gd 3+ concentration. ( b ) T1-weighted MR images of Gd-DOTA and LA-CMGL at different Gd 3+ concentrations. ( c ) T1-weighted MR images of HepG2 and HepaRG cells incubated with LA-CMGL at different concentrations. ( d ) T1-weighted MR enhancement of HCC model mice at various time points following the intravenous injection of Gd-DOTA, CMGL, and LA-CMGL. ( e ) Tumor and normal ratio (T/N) for the liver of HCC mice at pre-injection, 5, 30, 60 and 180 min after intravenous injection of the solution of LA-CMGL ( n = 3). ( f ) Contrast-to-noise ratio (CNR) of the heart, liver, kidney, spleen, and tumor in the HCC mice before injection and at 5, 15, 30, 60 and 180 min after intravenous injection of the solution of LA-CMGL ( n = 3). Data are mean ± standard deviation (SD). Statistical significances in ( e ) were calculated via the one-way ANOVA with Tukey’s post hoc test (*** p < 0.001). Statistical significance in ( f ) was calculated via the Student’s t test (** p < 0.01)
In this study, we designed LA-modified LNPs (LA-CMGL) with coloaded CPT/miR-145 and Gd-DOTA for simultaneous targeted therapy and MRI contrast enhancement for HCC. The results showed that the LA modification enabled LA-CMGL to precisely deliver CPT/miR-145 into tumor cells and tissues. In vitro and in vivo antitumor analyses demonstrated that the LA-CMGL were more effective than free drugs or single drug-loaded LNPs. Mechanistically, miR-145 may sensitize cancer cells to CPT and promote apoptosis by targeting SENP1-mediated HK2 SUMOylation and glycolysis pathways. Moreover, in vitro and in vivo tests confirmed that the loaded Gd-DOTA could serve as an effective T1-weighted contrast agent for tumor detection and drug delivery monitoring. In sum, the LA-modified chemo-gene co-delivery system developed in this work shows great potential as a theranostic system for personalized cancer therapy.
Data availability
The data of this study is available from the corresponding authors on reasonable request.
Abbreviations
Camptothecin
- Hepatocellular carcinoma
Lactobionic acid
- Lipid nanoparticles
Diethylnitrosamine
SUMO-specific peptidase 1
Extracellular acidification rate
DLin-MC3-DMA
Asialoglycoprotein receptors
Dynamic light scattering
Transmission electron microscopy
Encapsulation efficiencies
OES-Inductively coupled plasma-optical emission spectrometer
Polydispersity indexes
Molecular weight cut off
Confocal laser scanning microscopy
Cell Counting Kit-8
Western blotting
Contrast-to-noise ratio
Region of interest
Multicellular tumor spheroids
caspase3-Cleaved-caspase3
c-Cytochrome-c
Voltage-dependent anion-selective channel protein 1
IP-Co-inmunoprecipitation
Hematoxylin and eosin
Terminal deoxynucleotidyl transferase dUTP nick end labeling
Alanine transaminase
Aspartate aminotransferase
Sun Z, Tan Z, Peng C, Yi W: [Corrigendum] HK2 is associated with the Warburg effect and proliferation in liver cancer: Targets for effective therapy with glycyrrhizin. Mol Med Rep 2021;24.
Xiao Y, Liu Y, Yang S, Zhang B, Wang T, Jiang D, Zhang J, Yu D, Zhang N: Sorafenib and gadolinium co-loaded liposomes for drug delivery and MRI-guided HCC treatment. Colloids Surf B Biointerfaces 2016;141:83–92.
Chen F, Wang H, Zhu J, Zhao R, Xue P, Zhang Q, Bud NM, Qu W, Feng B, Pi J: Camptothecin suppresses NRF2-ARE activity and sensitises hepatocellular carcinoma cells to anticancer drugs. Br J Cancer 2017;117:1495–1506.
Li Y, Miao Y, Chen M, Chen X, Li F, Zhang X, Gan Y: Stepwise targeting and responsive lipid-coated nanoparticles for enhanced tumor cell sensitivity and hepatocellular carcinoma therapy. Theranostics 2020;10:3722–3736.
Wang X, Liu G, Chen N, Wu J, Zhang J, Qian Y, Zhang L, Zhou D, Yu Y: Angiopep2-Conjugated Star-Shaped Polyprodrug Amphiphiles for Simultaneous Glioma-Targeting Therapy and MR Imaging. ACS Appl Mater Interfaces 2020;12:12143–12154.
Wang H, Ellipilli S, Lee WJ, Li X, Vieweger M, Ho YS, Guo P: Multivalent rubber-like RNA nanoparticles for targeted co-delivery of paclitaxel and MiRNA to silence the drug efflux transporter and liver cancer drug resistance. J Control Release 2021;330:173–184.
Ji D, Wang Q, Zhao Q, Tong H, Yu M, Wang M, Lu T, Jiang C: Co-delivery of miR-29b and germacrone based on cyclic RGD-modified nanoparticles for liver fibrosis therapy. J Nanobiotechnology 2020;18:86.
Xu F, Liao JZ, Xiang GY, Zhao PX, Ye F, Zhao Q, He XX: MiR-101 and doxorubicin codelivered by liposomes suppressing malignant properties of hepatocellular carcinoma. Cancer Med 2017;6:651–661.
Bao MH, Wong CC: Hypoxia, Metabolic Reprogramming, and Drug Resistance in Liver Cancer. Cells 2021;10.
He W, Turkeshi A, Li X, Zhang H: Progress in systemic co-delivery of microRNAs and chemotherapeutics for cancer treatment by using lipid-based nanoparticles. Ther Deliv 2020;11:591–603.
Xu W, Hua Y, Deng F, Wang D, Wu Y, Zhang W, Tang J: MiR-145 in cancer therapy resistance and sensitivity: A comprehensive review. Cancer Sci 2020;111:3122–3131.
Hu C, Liu T, Zhang W, Sun Y, Jiang D, Zhang X, Liu Y, Mao S, Xu Y, Pan J, et al: miR-145 inhibits aerobic glycolysis and cell proliferation of cervical cancer by acting on MYC. FASEB J 2023;37: e22839.
Tao J, Ding WF, Che XH, Chen YC, Chen F, Chen XD, Ye XL, Xiong SB: Optimization of a cationic liposome-based gene delivery system for the application of miR-145 in anticancer therapeutics. Int J Mol Med 2016;37:1345–1354.
Qu H, Li X, Chen F, Zhang M, Lu X, Gu Y, Lv M, Lu C: LncRNA PVT1 influences breast cancer cells glycolysis through sponging miR-145-5p. Genes Genomics 2023;45:581–592.
Obeidat YM, Cheng MH, Catandi G, Carnevale E, Chicco AJ, Chen TW: Design of a multi-sensor platform for integrating extracellular acidification rate with multi-metabolite flux measurement for small biological samples. Biosens Bioelectron 2019;133:39–47.
Zhao S, Zhang Y, Pei M, Wu L, Li J: miR-145 inhibits mitochondrial function of ovarian cancer by targeting ARL5B. J Ovarian Res 2021;14:8.
Li W, Chen L, Gu Z, Chen Z, Li H, Cheng Z, Li H, Zou L: Co-delivery of microRNA-150 and quercetin by lipid nanoparticles (LNPs) for the targeted treatment of age-related macular degeneration (AMD). J Control Release 2023;355:358–370.
Butowska K, Han X, Gong N, El-Mayta R, Haley RM, Xue L, Zhong W, Guo W, Wang K, Mitchell MJ: Doxorubicin-conjugated siRNA lipid nanoparticles for combination cancer therapy. Acta Pharm Sin B 2023;13:1429–1437.
Ning Q, Liu YF, Ye PJ, Gao P, Li ZP, Tang SY, He DX, Tang SS, Wei H, Yu CY: Delivery of liver-specific miRNA-122 using a targeted macromolecular prodrug toward synergistic therapy for hepatocellular carcinoma. ACS Appl Mater Interfaces 2019;11:10578–10588.
Wang L, Hao Y, Li H, Zhao Y, Meng D, Li D, Shi J, Zhang H, Zhang Z, Zhang Y: Co-delivery of doxorubicin and siRNA for glioma therapy by a brain targeting system: angiopep-2-modified poly (lactic-co-glycolic acid) nanoparticles. J Drug Target 2015;23:832–846.
Li T, Liu Z, Fu X, Chen Y, Zhu S, Zhang J: Co-delivery of Interleukin-12 and doxorubicin loaded Nano-delivery system for enhanced immunotherapy with polarization toward M1-type Macrophages. Eur J Pharm Biopharm 2022;177:175–183.
Sabnis S, Kumarasinghe ES, Salerno T, Mihai C, Ketova T, Senn JJ, Lynn A, Bulychev A, McFadyen I, Chan J, et al: A novel amino lipid series for mRNA delivery: Improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol Ther 2018;26:1509–1519.
Han X, Gong N, Xue L, Billingsley MM, El-Mayta R, Shepherd SJ, Alameh MG, Weissman D, Mitchell MJ: Ligand-tethered lipid nanoparticles for targeted RNA delivery to treat liver fibrosis. Nat Commun 2023;14:75.
Zhang J, Shen H, Xu J, Liu L, Tan J, Li M, Xu N, Luo S, Wang J, Yang F, et al: Liver-targeted sirna lipid nanoparticles treat hepatic cirrhosis by dual antifibrotic and anti-inflammatory activities. ACS Nano 2020;14:6305–6322.
Cheng Q, Wei T, Farbiak L, Johnson LT, Dilliard SA, Siegwart DJ: Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat Nanotechnol 2020;15:313–320.
Dilliard SA, Siegwart DJ: Passive, active and endogenous organ-targeted lipid and polymer nanoparticles for delivery of genetic drugs. Nat Rev Mater 2023;8:282–300.
Akinc A, Maier MA, Manoharan M, Fitzgerald K, Jayaraman M, Barros S, Ansell S, Du X, Hope MJ, Madden TD, et al: The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat Nanotechnol 2019;14:1084–1087.
Wang X, Liu S, Sun Y, Yu X, Lee SM, Cheng Q, Wei T, Gong J, Robinson J, Zhang D, et al: Preparation of selective organ-targeting (SORT) lipid nanoparticles (LNPs) using multiple technical methods for tissue-specific mRNA delivery. Nat Protoc 2023;18:265–291.
Fei Q, Shalosky EM, Barnes R, Shukla VC, Xu S, Ballinger MN, Farkas L, Lee RJ, Ghadiali SN, Englert JA: Macrophage-targeted lipid nanoparticle delivery of microRNA-146a to mitigate hemorrhagic shock-induced acute respiratory distress syndrome. ACS Nano 2023;17:16539–16552.
Zhou T, Liang X, Wang P, Hu Y, Qi Y, Jin Y, Du Y, Fang C, Tian J: A hepatocellular carcinoma targeting nanostrategy with hypoxia-ameliorating and photothermal abilities that, combined with immunotherapy, inhibits metastasis and recurrence. ACS Nano 2020;14:12679–12696.
Ren L, Chen S, Li H, Zhang Z, Ye C, Liu M, Zhou X: MRI-visible liposome nanovehicles for potential tumor-targeted delivery of multimodal therapies. Nanoscale 2015;7:12843–12850.
Xie X, Chen Y, Chen Z, Feng Y, Wang J, Li T, Li S, Qin X, Wu C, Zheng C, et al: Polymeric hybrid nanomicelles for cancer theranostics: An efficient and precise anticancer strategy for the codelivery of doxorubicin/mir-34a and magnetic resonance imaging. ACS Appl Mater Interfaces 2019;11:43865–43878.
Andreozzi E, Wang P, Valenzuela A, Tu C, Gorin F, Dhenain M, Louie A: Size-stable solid lipid nanoparticles loaded with Gd-DOTA for magnetic resonance imaging. Bioconjug Chem 2013;24:1455–1467.
Li X, Qian Y, Liu T, Hu X, Zhang G, You Y, Liu S: Amphiphilic multiarm star block copolymer-based multifunctional unimolecular micelles for cancer targeted drug delivery and MR imaging. Biomaterials 2011;32:6595–6605.
Yao W, Liu C, Wang N, Zhou H, Chen H, Qiao W: An MRI-guided targeting dual-responsive drug delivery system for liver cancer therapy. J Colloid Interface Sci 2021;603:783–798.
Zhang L, Zhang M, Zhou L, Han Q, Chen X, Li S, Li L, Su Z, Wang C: Dual drug delivery and sequential release by amphiphilic Janus nanoparticles for liver cancer theranostics. Biomaterials 2018;181:113–125.
Qi C, Wang D, Gong X, Zhou Q, Yue X, Li C, Li Z, Tian G, Zhang B, Wang Q, et al: Co-Delivery of Curcumin and Capsaicin by Dual-Targeting Liposomes for Inhibition of aHSC-Induced Drug Resistance and Metastasis. ACS Appl Mater Interfaces 2021;13:16019–16035.
Zou Y, Song Y, Yang W, Meng F, Liu H, Zhong Z: Galactose-installed photo-crosslinked pH-sensitive degradable micelles for active targeting chemotherapy of hepatocellular carcinoma in mice. J Control Release 2014;193:154–161.
Wei X, Yang D, Xing Z, Cai J, Wang L, Zhao C, Wei X, Jiang M, Sun H, Zhou L, et al: Hepatocyte-targeted delivery using oleanolic acid-loaded liposomes for enhanced hepatocellular carcinoma therapy. Biomater Sci 2023;11:3952–3964.
Wang Q, Tian Y, Liu L, Chen C, Zhang W, Wang L, Guo Q, Ding L, Fu H, Song H, et al: Precise targeting therapy of orthotopic gastric carcinoma by siRNA and chemotherapeutic drug codelivered in ph-sensitive nano platform. Adv Healthc Mater 2021;10: e2100966.
Andersson TB, Kanebratt KP, Kenna JG: The HepaRG cell line: a unique in vitro tool for understanding drug metabolism and toxicology in human. Expert Opin Drug Metab Toxicol 2012;8:909–920.
Brand M, Bommeli EB, Rütimann M, Lindenmann U, Riedl R: Discovery of a Dual SENP1 and SENP2 Inhibitor. Int J Mol Sci 2022;23.
Fu Y, Mackowiak B, Feng D, Lu H, Guan Y, Lehner T, Pan H, Wang XW, He Y, Gao B: MicroRNA-223 attenuates hepatocarcinogenesis by blocking hypoxia-driven angiogenesis and immunosuppression. Gut 2023;72:1942–1958.
Zhang D, Wang G, Yu X, Wei T, Farbiak L, Johnson LT, Taylor AM, Xu J, Hong Y, Zhu H, Siegwart DJ: Enhancing CRISPR/Cas gene editing through modulating cellular mechanical properties for cancer therapy. Nat Nanotechnol 2022;17:777–787.
DeWaal D, Nogueira V, Terry AR, Patra KC, Jeon SM, Guzman G, Au J, Long CP, Antoniewicz MR, Hay N: Hexokinase-2 depletion inhibits glycolysis and induces oxidative phosphorylation in hepatocellular carcinoma and sensitizes to metformin. Nat Commun 2018;9:446.
Liao W, Du J, Wang Z, Feng Q, Liao M, Liu H, Yuan K, Zeng Y: The role and mechanism of noncoding RNAs in regulation of metabolic reprogramming in hepatocellular carcinoma. Int J Cancer 2022;151:337–347.
Zheng X, Pan Y, Yang G, Liu Y, Zou J, Zhao H, Yin G, Wu Y, Li X, Wei Z, et al: Kaempferol impairs aerobic glycolysis against melanoma metastasis via inhibiting the mitochondrial binding of HK2 and VDAC1. Eur J Pharmacol 2022;931:175226.
Shangguan X, He J, Ma Z, Zhang W, Ji Y, Shen K, Yue Z, Li W, Xin Z, Zheng Q, et al: SUMOylation controls the binding of hexokinase 2 to mitochondria and protects against prostate cancer tumorigenesis. Nat Commun 2021;12:1812.
Uehara T, Ainslie GR, Kutanzi K, Pogribny IP, Muskhelishvili L, Izawa T, Yamate J, Kosyk O, Shymonyak S, Bradford BU, et al: Molecular mechanisms of fibrosis-associated promotion of liver carcinogenesis. Toxicol Sci 2013;132:53–63.
Download references
This work is supported by the Research Improvement Program of Anhui Medical University (2021xkjT105, China), the Major Project of University Nature Science Research in Anhui Province (2023AH040370) and the National Natural Science Foundation of China (82070629 and 82270660).
Author information
Jing Rong and Tongtong Liu contributed equally to this work.
Authors and Affiliations
Department of Radiology, The First Affiliated Hospital of Anhui Medical University, Anhui Medical University, Hefei, 230022, China
Jing Rong, Xiujuan Yin, Min Shao, Kun Zhu, Bin Li, Yujie Zhu, Saisai Zhang, Likang Yin & Xiao Wang
School of Pharmacy, Key Laboratory of Anti-inflammatory of Immune Medicines of Ministry of Education, Inflammation and Immune Mediated Diseases Laboratory of Anhui Province, Anhui Institute of Innovative Drugs, Anhui Medical University, Hefei, 230032, China
Tongtong Liu, Shiqi Wang, Qi Liu & Lei Zhang
You can also search for this author in PubMed Google Scholar
Contributions
JR, TTL designed the experiments, performed all experimental work and analyzed the data. XJY, MS, KZ, BL, SQW, YJZ, SSZ, and LKY supported administration, technique and materials. QL, XW, and LZ designed and supervised the study, and edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Qi Liu , Xiao Wang or Lei Zhang .
Ethics declarations
Ethical approval.
Animal protocols were approved by Animal Experiment Ethics Review of Anhui Medical University (NO: LLSC20221110), which are consistent with AAALAS guidelines. Liver tumor tissues and non-tumor tissues were procured from the tissue biorepository through the department of Pathology, the first affiliated hospital of Anhui Medical University (AHMU). Following guidelines described by the Declaration of Helsinki, Institutional Review Board approval (NO: 2023 − 467) from the first affiliated hospital of AHMU was obtained for experimental use of specimens.
Consent for publication
All authors agreed to submit this manuscript.
Competing interests
The authors declare no competing financial interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Rong, J., Liu, T., Yin, X. et al. Co-delivery of camptothecin and MiR-145 by lipid nanoparticles for MRI-visible targeted therapy of hepatocellular carcinoma. J Exp Clin Cancer Res 43 , 247 (2024). https://doi.org/10.1186/s13046-024-03167-9
Download citation
Received : 03 April 2024
Accepted : 17 August 2024
Published : 30 August 2024
DOI : https://doi.org/10.1186/s13046-024-03167-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Combination therapy
- Target delivery
- Magnetic resonance imaging
Journal of Experimental & Clinical Cancer Research
ISSN: 1756-9966
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
In addition new dimensions such as lipid biophysics, especially with relevance to membranes and lipoproteins, and basic liposome research and applications have been added. To cope with all these advances in knowledge a journal is needed to review recent progress in particular fields and to set current research against its historical background.
2024 — Volumes 93-96. Volume 96. In progress (November 2024) Volume 95. July 2024. Volume 94. April 2024. Volume 93. January 2024.
Progress in Lipid Research is a quarterly peer-reviewed scientific journal that covers research on all aspects of research on lipids.The journal was established in 1973 with Ralph Holman (University of Minnesota) as founding editor-in-chief and is published by Elsevier.The current editors-in-chief are Makoto Arita (), Kent Chapman (University of North Texas), John Harwood (Cardiff University ...
Progress in Lipid Research. Volume 4 • Issue 4. ISSN: 0163-7827. 5 Year impact factor: 15.7. Impact factor: 14. Journal metrics. Request a sales quote. Publishing Invited Reviews. The importance of lipids as one of the fundamental classes of biological compounds is well established.
Lipid homeostasis and mevalonate pathway in COVID-19: Basic concepts and potential therapeutic targets. Maria Chiara Proto, Donatella Fiore, Chiara Piscopo, Cristina Pagano, ... Maurizio Bifulco. Article 101099. View PDF. Article preview. Read the latest articles of Progress in Lipid Research at ScienceDirect.com, Elsevier's leading platform ...
Progress in Lipid Research fulfils this role. Each volume contains up-to-date surveys of special aspects of lipid research. The invited reviews are comprehensive enough to provide sufficient overview but concentrate on reporting and critically appraising the most recent data. Subjects are chosen for their timeliness or because major ...
Progress in Lipid Research. Supports open access. 24.4 CiteScore. 13.6 Impact Factor. Articles & Issues ... Compare APC with another journal 5 days. Acceptance to publication. 120 days. Submission to acceptance ...
Progress in Lipid Research | Citations: 4,825 | The importance of lipids as one of the fundamental classes of biological compounds is well established. The application of our knowledge of the ...
The impact factor of Progress in Lipid Research, and other metrics like the H-Index and TQCC, alongside relevant research trends, citation patterns, altmetric scores, Twitter account and similar journals. ... (Based on citations to the other journals in the most recent 30 papers in this journal, at least if metadata about citations were ...
The Journal of Lipid Research focuses on the science of lipids in health and disease. The journal emphasizes lipid function and the biochemical and genetic regulation of lipid metabolism and aims to be on the forefront of the emerging areas of genomics, proteomics, metabolomics and lipidomics as they relate to lipid metabolism and function. More.
Progress in Lipid Research Impact Factor, IF, number of article, detailed information and journal factor. ISSN: 0163-7827. ... issn or abbr in this box to search. Progress in Lipid Research. Journal Abbreviation: PROG LIPID RES Journal ISSN: 0163-7827. Year: Impact Factor (IF) Total Articles: Total Cites: 2023 (2024 update) 14.0: 0: 7497: 2022 ...
In addition new dimensions such as lipid biophysics, especially with relevance to membranes and lipoproteins, and basic liposome research and applications have been added. To cope with all these advances in knowledge a journal is needed to review recent progress in particular fields and to set current research against its historical background.
Progress In Lipid Research. You may order single or multiple copies of back and recent journal issues. If you are an Author wishing to obtain a printed copy of the journal issue featuring your article, or you require a printed copy for research, review or add to your library, the process is easy -. Select your journal volume and issue ...
The journal addresses concerns in Cholesterol which are intertwined with other disciplines, such as Receptor, Steroid biosynthesis, ABCA1 and Pharmacology. Progress in Lipid Research holds forums on Lipid metabolism that merges themes from other disciplines such as Lipotoxicity, Virus, Immunology, Fatty acid and Adipose tissue.
To cope with all these advances in knowledge a journal is needed to review recent progress in particular fields and to set current research against its historical background. Progress in Lipid Research fulfils this role. Each volume contains up-to-date surveys of special aspects of lipid research. The invited reviews are comprehensive enough to ...
The imperative of arachidonic acid in early human development. Michael A. Crawford, Andrew J. Sinclair, Barbara Hall, Enitan Ogundipe, ... Mark R. Johnson. Article 101222. View PDF. Article preview. Read the latest articles of Progress in Lipid Research at ScienceDirect.com, Elsevier's leading platform of peer-reviewed scholarly literature.
Lipid oxidation in emulsions: New insights from the past two decades. Marie Hennebelle, Pierre Villeneuve, Erwann Durand, Jérôme Lecomte, John van Duynhoven, Anne Meynier, Betül Yesiltas, Charlotte Jacobsen, Claire Berton-Carabin
Progress in lipid research Journal Overview publication venue for . Arachidonic acid metabolism in platelets and endothelial cells.. 20. 1981; Lipid somersaults: Uncovering the mechanisms of protein-mediated lipid flipping. 2016; Sphingomyelin and derivatives as cellular signals. 1991; Identity ISO Abbreviation . Prog Lipid Res Linking ISSN
2-Arachidonoylglycerol (2-AG) is a signaling lipid in the central nervous system that is a key regulator of neurotransmitter release. 2-AG is an endocannabinoid that activates the cannabinoid CB1 receptor. It is involved in a wide array of (patho)physiological functions, such as emotion, cognition, energy balance, pain sensation and ...
The guidance document aims to improve standardization of clinical lipid testing work flows. It provides recommendations for the components of the lipid panel, fasting requirements, reporting of results, and specific recommendations for non-high-density lipoprotein cholesterol (non-HDL-C), low-density lipoprotein cholesterol (LDL-C), lipoprotein(a) [Lp(a)], apolipoprotein B (apo B), point-of ...
Lipid disorders increase the risk for the development of cardiometabolic disorders, including type 2 diabetes, atherosclerosis, and cardiovascular disease. Lipids levels, apart from diet, smoking, obesity, alcohol consumption, and lack of exercise, are also influenced by genetic factors. Recent studies suggested the role of long noncoding RNAs (lncRNAs) in the regulation of lipid formation and ...
The mean age of the participants at baseline was 54.7 years. During the 30-year follow-up, 3662 first major cardiovascular events occurred. Quintiles of increasing baseline levels of high ...
Corrigendum to "A novel regulatory facet for hypertriglyceridemia: The role of microRNAs in the regulation of triglyceride-rich lipoprotein biosynthesis" [Prog Lipid Res. 2023 Jan;89:101197. doi: 10.1016/j.plipres.2022.101197] Masoomeh Khalifeh, Raul D. Santos, Reza KazemiOskuee, Ali Badiee, ... Amirhossein Sahebkar.
The lipid optimisation pathway developed (Figure 3) integrates both the individual clinical expertise gathered in the lipid clinic with the best available external evidence, while staying within the confines of individual reimbursement thresholds for each available LLT.
Lipid-based delivery systems are wide-ranging designs for formulations containing active or functional compounds in dissolved or suspended forms in lipidic cores. 101 The melting range, solubilizing capacity and miscibility properties of the wall material depend on the fatty acid chain length and degree of unsaturation. 102 Lipid-based delivery ...
He W, Turkeshi A, Li X, Zhang H: Progress in systemic co-delivery of microRNAs and chemotherapeutics for cancer treatment by using lipid-based nanoparticles. Ther Deliv 2020;11:591-603. Xu W, Hua Y, Deng F, Wang D, Wu Y, Zhang W, Tang J: MiR-145 in cancer therapy resistance and sensitivity: A comprehensive review. Cancer Sci 2020;111:3122-3131.
Oxidative lipid damage by naringenin selectively sensitizes chronic myeloid leukemia cell lines and patient samples to Bcr-Abl tyrosine kinase inhibitors ... and the inability of Bcr-Abl TKIs to eliminate leukemia stem/progenitor cells may explain why patients progress to blast crisis [16]. ... This work was supported by a research grant ...