Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 10 September 2020
- Ulcerative colitis
- Taku Kobayashi 1 ,
- Britta Siegmund 2 ,
- Catherine Le Berre 3 ,
- Shu Chen Wei 4 ,
- Marc Ferrante 5 ,
- Bo Shen 6 ,
- Charles N. Bernstein 7 ,
- Silvio Danese 8 ,
- Laurent Peyrin-Biroulet 3 &
- Toshifumi Hibi 1
Nature Reviews Disease Primers volume 6 , Article number: 74 ( 2020 ) Cite this article
20k Accesses
669 Citations
185 Altmetric
Metrics details
- Gastrointestinal diseases
Ulcerative colitis (UC) is a chronic inflammatory bowel disease of unknown aetiology affecting the colon and rectum. Multiple factors, such as genetic background, environmental and luminal factors, and mucosal immune dysregulation, have been suggested to contribute to UC pathogenesis. UC has evolved into a global burden given its high incidence in developed countries and the substantial increase in incidence in developing countries. An improved understanding of the mechanisms underlying UC has led to the emergence of new treatments. Since the early 2000s, anti-tumour necrosis factor (TNF) treatment has significantly improved treatment outcomes. Advances in medical treatments have enabled a paradigm shift in treatment goals from symptomatic relief to endoscopic and histological healing to achieve better long-term outcomes and, consequently, diagnostic modalities have also been improved to monitor disease activity more tightly. Despite these improvements in patient care, a substantial proportion of patients, for example, those who are refractory to medical treatment or those who develop colitis-associated colorectal dysplasia or cancer, still require restorative proctocolectomy. The development of novel drugs and improvement of the treatment strategy by implementing personalized medicine are warranted to achieve optimal disease control. However, delineating the aetiology of UC is necessary to ultimately achieve disease cure.
This is a preview of subscription content, access via your institution

Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
92,52 € per year
only 92,52 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Microscopic colitis

Crohn’s disease

Clinical factors associated with severity in patients with inflammatory bowel disease in Brazil based on 2-year national registry data from GEDIIB
Wilks, S. Morbid appearances in the intestine of Miss Bankes. Med. Gaz. 2 , 264–265 (1859). This paper is the first historical report of UC .
Google Scholar
Hibi, T. & Ogata, H. Novel pathophysiological concepts of inflammatory bowel disease. J. Gastroenterol. 41 , 10–16 (2006).
PubMed Google Scholar
Ungaro, R., Mehandru, S., Allen, P. B., Peyrin-Biroulet, L. & Colombel, J.-F. Ulcerative colitis. Lancet 389 , 1756–1770 (2017).
Rubin, D. T., Ananthakrishnan, A. N., Siegel, C. A., Sauer, B. G. & Long, M. D. ACG clinical guideline: ulcerative colitis in adults. Am. J. Gastroenterol. 114 , 384–413 (2019). This paper describes the clinical guidelines on the management of UC recommended by the American College of Gastroenterology .
Matsuoka, K. et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J. Gastroenterol. 53 , 305–353 (2018).
CAS PubMed PubMed Central Google Scholar
Magro, F. et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and Ileo-anal pouch disorders. J. Crohns Colitis 11 , 649–670 (2017). Consensus guidelines on the diagnosis and management of UC by the European Crohn’s and Colitis Organisation .
Truelove, S. C. & Witts, L. J. Cortisone in ulcerative colitis; final report on a therapeutic trial. BMJ 2 , 1041–1048 (1955). This paper describes the results from a clinical trial on the use of steroids in UC .
Gallo, G., Kotze, P. G. & Spinelli, A. Surgery in ulcerative colitis: When? How? Best Pract. Res. Clin. Gastroenterol. 32–33 , 71–78 (2018).
Ungaro, R., Colombel, J.-F., Lissoos, T. & Peyrin-Biroulet, L. A treat-to-target update in ulcerative colitis: a systematic review. Am. J. Gastroenterol. 114 , 874–883 (2019).
PubMed PubMed Central Google Scholar
Colombel, J.-F., D’haens, G., Lee, W.-J., Petersson, J. & Panaccione, R. Outcomes and strategies to support a treat-to-target approach in inflammatory bowel disease: a systematic review. J. Crohns Colitis 14 , 254–266 (2020).
Peyrin-Biroulet, L. et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am. J. Gastroenterol. 110 , 1324–1338 (2015). Consensus statements on treat-to-target strategy in the management of UC .
CAS PubMed Google Scholar
Ozaki, R. et al. Histological risk factors to predict clinical relapse in ulcerative colitis with endoscopically normal mucosa. J. Crohn’s Colitis 12 , 1288–1294 (2018).
Peyrin-Biroulet, L., Bressenot, A. & Kampman, W. Histologic remission: the ultimate therapeutic goal in ulcerative colitis? Clin. Gastroenterol. Hepatol. 12 , 929–934.e2 (2014).
Bernstein, C. N. et al. The epidemiology of inflammatory bowel disease in Canada: a population-based study. Am. J. Gastroenterol. 101 , 1559–1568 (2006).
Bitton, A. et al. Epidemiology of inflammatory bowel disease in Québec: recent trends. Inflamm. Bowel Dis. 20 , 1770–1776 (2014).
Leddin, D., Tamim, H. & Levy, A. R. Decreasing incidence of inflammatory bowel disease in Eastern Canada: a population database study. BMC Gastroenterol. 14 , 140 (2014).
Torabi, M. et al. Geographical variation and factors associated with inflammatory bowel disease in a Central Canadian province. Inflamm. Bowel Dis. 26 , 581–590 (2020).
Coward, S. et al. Past and future burden of inflammatory bowel diseases based on modeling of population-based data. Gastroenterology 156 , 1345–1353.e4 (2019).
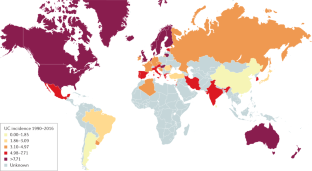
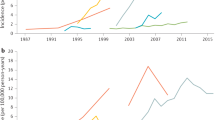
Ng, S. C. et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390 , 2769–2778 (2017). A systematic review providing an overview of the global epidemiology of IBD .
Ng, S. C. et al. Population density and risk of inflammatory bowel disease: a prospective population-based study in 13 countries or regions in Asia-Pacific. Am. J. Gastroenterol. 114 , 107–115 (2019).
Banerjee, R. et al. IBD in India: similar phenotype but different demographics than the west. J. Clin. Gastroenterol. https://doi.org/10.1097/MCG.0000000000001282 (2019).
Article Google Scholar
Kedia, S. & Ahuja, V. Epidemiology of inflammatory bowel disease in India: the Great Shift East. Inflamm. Intest. Dis. 2 , 102–115 (2017).
Perminow, G. et al. A characterization in childhood inflammatory bowel disease, a new population-based inception cohort from South-Eastern Norway, 2005–07, showing increased incidence in Crohn’s disease. Scand. J. Gastroenterol. 44 , 446–456 (2009).
Malmborg, P., Grahnquist, L., Lindholm, J., Montgomery, S. & Hildebrand, H. Increasing incidence of paediatric inflammatory bowel disease in northern Stockholm county, 2002–2007. J. Pediatr. Gastroenterol. Nutr. 57 , 29–34 (2013).
Benchimol, E. I. et al. Trends in epidemiology of pediatric inflammatory bowel disease in Canada: distributed network analysis of multiple population-based provincial health administrative databases. Am. J. Gastroenterol. 112 , 1120 (2017).
Muise, A. M., Snapper, S. B. & Kugathasan, S. The age of gene discovery in very early onset inflammatory bowel disease. Gastroenterology 143 , 285–288 (2012).
Shah, S. C. et al. Sex-based differences in incidence of inflammatory bowel diseases—pooled analysis of population-based studies from western countries. Gastroenterology 155 , 1079–1089.e3 (2018).
Benchimol, E. I. et al. Inflammatory bowel disease in immigrants to Canada and their children: a population-based cohort study. Am. J. Gastroenterol. 110 , 553–563 (2015).
Ananthakrishnan, A. N. Environmental triggers for inflammatory bowel disease. Curr. Gastroenterol. Rep. 15 , 302 (2013).
Bernstein, C. N. et al. World gastroenterology organisation global guidelines inflammatory bowel disease: update August 2015. J. Clin. Gastroenterol. 50 , 803–818 (2016).
Wang, P. et al. Smoking and inflammatory bowel disease: a comparison of China, India, and the USA. Dig. Dis. Sci. 63 , 2703–2713 (2018).
Amarapurkar, A. D. et al. Risk factors for inflammatory bowel disease: a prospective multi-center study. Indian J. Gastroenterol. 37 , 189–195 (2018).
Lerner, A. & Matthias, T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun. Rev. 14 , 479–489 (2015).
Wang, Y.-F. et al. Multicenter case-control study of the risk factors for ulcerative colitis in China. World J. Gastroenterol. 19 , 1827–1833 (2013).
Benchimol, E. I. et al. Rural and urban residence during early life is associated with risk of inflammatory bowel disease: a population-based inception and birth cohort study. Am. J. Gastroenterol. 112 , 1412–1422 (2017).
Ungaro, R. et al. Antibiotics associated with increased risk of new-onset Crohn’s disease but not ulcerative colitis: a meta-analysis. Am. J. Gastroenterol. 109 , 1728–1738 (2014).
Lewis, J. D. & Abreu, M. T. Diet as a trigger or therapy for inflammatory bowel diseases. Gastroenterology 152 , 398–414.e6 (2017).
Marrie, R. A. et al. Rising incidence of psychiatric disorders before diagnosis of immune-mediated inflammatory disease. Epidemiol. Psychiatr. Sci. 28 , 333–342 (2019).
Bonaz, B. L. & Bernstein, C. N. Brain-gut interactions in inflammatory bowel disease. Gastroenterology 144 , 36–49 (2013).
Myrelid, P., Landerholm, K., Nordenvall, C., Pinkney, T. D. & Andersson, R. E. Appendectomy and the risk of colectomy in ulcerative colitis: a national cohort study. Am. J. Gastroenterol. 112 , 1311–1319 (2017).
Parian, A. et al. Appendectomy does not decrease the risk of future colectomy in UC: results from a large cohort and meta-analysis. Gut 66 , 1390–1397 (2017).
Eaden, J. A., Abrams, K. R. & Mayberry, J. F. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 48 , 526–535 (2001).
Olén, O. et al. Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet 395 , 123–131 (2020).
Bernstein, C. N. et al. The impact of inflammatory bowel disease in Canada 2018: extra-intestinal diseases in IBD. J. Can. Assoc. Gastroenterol. 2 , S73–S80 (2019).
Desai, D. et al. Colorectal cancers in ulcerative colitis from a low-prevalence area for colon cancer. World J. Gastroenterol. 21 , 3644–3649 (2015).
Wang, Y. N. et al. Clinical characteristics of ulcerative colitis-related colorectal cancer in Chinese patients. J. Dig. Dis. 18 , 684–690 (2017).
Keller, D. S., Windsor, A., Cohen, R. & Chand, M. Colorectal cancer in inflammatory bowel disease: review of the evidence. Tech. Coloproctol. 23 , 3–13 (2019).
Moody, G. A., Jayanthi, V., Probert, C. S., Mac Kay, H. & Mayberry, J. F. Long-term therapy with sulphasalazine protects against colorectal cancer in ulcerative colitis: a retrospective study of colorectal cancer risk and compliance with treatment in Leicestershire. Eur. J. Gastroenterol. Hepatol. 8 , 1179–1183 (1996).
Eaden, J., Abrams, K., Ekbom, A., Jackson, E. & Mayberry, J. Colorectal cancer prevention in ulcerative colitis: a case-control study. Aliment. Pharmacol. Ther. 14 , 145–153 (2000).
Bye, W. A., Nguyen, T. M., Parker, C. E., Jairath, V. & East, J. E. Strategies for detecting colon cancer in patients with inflammatory bowel disease. Cochrane Database Syst. Rev. 9 , CD000279 (2017).
Turpin, W., Goethel, A., Bedrani, L. & Croitoru Mdcm, K. Determinants of IBD Heritability: Genes, Bugs, and More. Inflamm. Bowel Dis. 24 , 1133–1148 (2018).
Jostins, L. et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491 , 119–124 (2012). A study of genetics reporting 163 disease susceptibility loci for IBD .
Mirkov, M. U., Verstockt, B. & Cleynen, I. Genetics of inflammatory bowel disease: beyond NOD2. Lancet Gastroenterol. Hepatol. 2 , 224–234 (2017).
Spehlmann, M. E. et al. Risk factors in German twins with inflammatory bowel disease: results of a questionnaire-based survey. J. Crohns Colitis 6 , 29–42 (2012).
Halfvarson, J., Bodin, L., Tysk, C., Lindberg, E. & Järnerot, G. Inflammatory bowel disease in a Swedish twin cohort: a long-term follow-up of concordance and clinical characteristics. Gastroenterology 124 , 1767–1773 (2003).
Ng, S. C., Woodrow, S., Patel, N., Subhani, J. & Harbord, M. Role of genetic and environmental factors in British twins with inflammatory bowel disease. Inflamm. Bowel Dis. 18 , 725–736 (2012).
van Dongen, J., Slagboom, P. E., Draisma, H. H. M., Martin, N. G. & Boomsma, D. I. The continuing value of twin studies in the omics era. Nat. Rev. Genet. 13 , 640–653 (2012).
Cleynen, I. et al. Inherited determinants of Crohn’s disease and ulcerative colitis phenotypes: a genetic association study. Lancet 387 , 156–167 (2016).
Kredel, L. I. et al. T-cell composition in ileal and colonic creeping fat - separating ileal from colonic Crohn’s disease. J. Crohns Colitis 13 , 79–91 (2019).
Yoshitake, S., Kimura, A., Okada, M., Yao, T. & Sasazuki, T. HLA class II alleles in Japanese patients with inflammatory bowel disease. Tissue Antigens 53 , 350–358 (1999).
Inoue, N. et al. Lack of common NOD2 variants in Japanese patients with Crohn’s disease. Gastroenterology 123 , 86–91 (2002).
Martini, E., Krug, S. M., Siegmund, B., Neurath, M. F. & Becker, C. Mend your fences: the epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell Mol. Gastroenterol. Hepatol. 4 , 33–46 (2017).
Van Klinken, B. J., Van der Wal, J. W., Einerhand, A. W., Büller, H. A. & Dekker, J. Sulphation and secretion of the predominant secretory human colonic mucin MUC2 in ulcerative colitis. Gut 44 , 387–393 (1999).
Gitter, A. H., Wullstein, F., Fromm, M. & Schulzke, J. D. Epithelial barrier defects in ulcerative colitis: characterization and quantification by electrophysiological imaging. Gastroenterology 121 , 1320–1328 (2001).
Büning, C. et al. Increased small intestinal permeability in ulcerative colitis: rather genetic than environmental and a risk factor for extensive disease? Inflamm. Bowel Dis. 18 , 1932–1939 (2012).
Kaser, A., Adolph, T. E. & Blumberg, R. S. The unfolded protein response and gastrointestinal disease. Semin. Immunopathol. 35 , 307–319 (2013).
Grootjans, J., Kaser, A., Kaufman, R. J. & Blumberg, R. S. The unfolded protein response in immunity and inflammation. Nat. Rev. Immunol. 16 , 469–484 (2016).
Krug, S. M. et al. Tricellulin is regulated via interleukin-13-receptor α2, affects macromolecule uptake, and is decreased in ulcerative colitis. Mucosal Immunol. 11 , 345–356 (2018).
Neurath, M. F. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat. Immunol. 20 , 970–979 (2019).
Jorch, S. K. & Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 23 , 279–287 (2017).
Bennike, T. B. et al. Neutrophil extracellular traps in ulcerative colitis: a proteome analysis of intestinal biopsies. Inflamm. Bowel Dis. 21 , 2052–2067 (2015).
Dinallo, V. et al. Neutrophil extracellular traps sustain inflammatory signals in ulcerative colitis. J. Crohns Colitis 13 , 772–784 (2019).
Camoglio, L., Te Velde, A. A., Tigges, A. J., Das, P. K. & Van Deventer, S. J. Altered expression of interferon-gamma and interleukin-4 in inflammatory bowel disease. Inflamm. Bowel Dis. 4 , 285–290 (1998).
Heller, F. et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 129 , 550–564 (2005).
Heller, F., Fuss, I. J., Nieuwenhuis, E. E., Blumberg, R. S. & Strober, W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity 17 , 629–638 (2002).
Danese, S. et al. Tralokinumab for moderate-to-severe UC: a randomised, double-blind, placebo-controlled, phase IIa study. Gut 64 , 243–249 (2015).
Kindler, V., Sappino, A. P., Grau, G. E., Piguet, P. F. & Vassalli, P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell 56 , 731–740 (1989).
Lissner, D. et al. Monocyte and M1 macrophage-induced barrier defect contributes to chronic intestinal inflammation in IBD. Inflamm. Bowel Dis. 21 , 1297–1305 (2015).
Hanauer, S. B. et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 359 , 1541–1549 (2002).
Rutgeerts, P. et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 353 , 2462–2476 (2005). This study is the first phase III randomized, controlled clinical trial of an anti-TNF agent for UC .
Uhlig, H. H. et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity 25 , 309–318 (2006).
Kobayashi, T. et al. IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn’s disease. Gut 57 , 1682–1689 (2008).
Feagan, B. G. et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 375 , 1946–1960 (2016).
Sands, B. E. et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 381 , 1201–1214 (2019).
Gerlach, K. et al. TH9 cells that express the transcription factor PU.1 drive T cell-mediated colitis via IL-9 receptor signaling in intestinal epithelial cells. Nat. Immunol. 15 , 676–686 (2014).
Nishida, A. et al. Increased expression of interleukin-36, a member of the interleukin-1 cytokine family, in inflammatory bowel disease. Inflamm. Bowel Dis. 22 , 303–314 (2016).
Russell, S. E. et al. IL-36α expression is elevated in ulcerative colitis and promotes colonic inflammation. Mucosal Immunol. 9 , 1193–1204 (2016).
Scheibe, K. et al. Inhibiting interleukin 36 receptor signaling reduces fibrosis in mice with chronic intestinal inflammation. Gastroenterology 156 , 1082–1097.e11 (2019).
Gordon, I. O. et al. Fibrosis in ulcerative colitis is directly linked to severity and chronicity of mucosal inflammation. Aliment. Pharmacol. Ther. 47 , 922–939 (2018).
Harusato, A. et al. IL-36γ signaling controls the induced regulatory T cell-Th9 cell balance via NFκB activation and STAT transcription factors. Mucosal Immunol. 10 , 1455–1467 (2017).
Sonnenburg, J. L. & Bäckhed, F. Diet-microbiota interactions as moderators of human metabolism. Nature 535 , 56–64 (2016).
Machiels, K. et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 63 , 1275–1283 (2014).
Halfvarson, J. et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat. Microbiol. 2 , 17004 (2017).
Schulthess, J. et al. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity 50 , 432–445.e7 (2019).
Moayyedi, P. et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 149 , 102–109.e6 (2015).
Paramsothy, S. et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet 389 , 1218–1228 (2017).
Rossen, N. G. et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology 149 , 110–118.e4 (2015).
Zheng, L. et al. Microbial-derived butyrate promotes epithelial barrier function through IL-10 receptor-dependent repression of claudin-2. J. Immunol. 199 , 2976–2984 (2017).
Glauben, R. et al. Histone hyperacetylation is associated with amelioration of experimental colitis in mice. J. Immunol. 176 , 5015–5022 (2006).
Fischer, A. et al. Differential effects of α4β7 and GPR15 on homing of effector and regulatory T cells from patients with UC to the inflamed gut in vivo. Gut 65 , 1642–1664 (2016).
Feagan, B. G. et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 369 , 699–710 (2013).
Wei, S.-C. et al. Management of ulcerative colitis in Taiwan: consensus guideline of the Taiwan society of inflammatory bowel disease. Intest. Res. 15 , 266–284 (2017).
Fausel, R. A., Kornbluth, A. & Dubinsky, M. C. The first endoscopy in suspected inflammatory bowel disease. Gastrointest. Endosc. Clin. N. Am. 26 , 593–610 (2016).
American Society for Gastrointestinal Endoscopy Standards of Practice Committee. et al. The role of endoscopy in inflammatory bowel disease. Gastrointest. Endosc. 81 , 1101–1121.e1-13 (2015).
Chutkan, R. K., Scherl, E. & Waye, J. D. Colonoscopy in inflammatory bowel disease. Gastrointest. Endosc. Clin. N. Am. 12 , 463–483 (2002).
Langner, C. et al. The histopathological approach to inflammatory bowel disease: a practice guide. Virchows Arch. 464 , 511–527 (2014).
Su, H.-J. et al. Inflammatory bowel disease and its treatment in 2018: global and Taiwanese status updates. J. Formos. Med. Assoc. 118 , 1083–1092 (2019).
D’Haens, G. et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology 132 , 763–786 (2007).
Schroeder, K. W., Tremaine, W. J. & Ilstrup, D. M. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N. Engl. J. Med. 317 , 1625–1629 (1987).
Peyrin-Biroulet, L. et al. Defining disease severity in inflammatory bowel diseases: current and future directions. Clin. Gastroenterol. Hepatol. 14 , 348–354.e17 (2016).
Fagerhol, M. K., Dale, I. & Andersson, T. A radioimmunoassay for a granulocyte protein as a marker in studies on the turnover of such cells. Bull. Eur. Physiopathol. Respir. 16 , 273–282 (1980).
Guerrant, R. L. et al. Measurement of fecal lactoferrin as a marker of fecal leukocytes. J. Clin. Microbiol. 30 , 1238–1242 (1992).
van Rheenen, P. F., Van de Vijver, E. & Fidler, V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ 341 , c3369 (2010).
Walsham, N. E. & Sherwood, R. A. Fecal calprotectin in inflammatory bowel disease. Clin. Exp. Gastroenterol. 9 , 21–29 (2016).
Lin, W.-C. et al. Fecal calprotectin correlated with endoscopic remission for Asian inflammatory bowel disease patients. World J. Gastroenterol. 21 , 13566–13573 (2015).
Menees, S. B., Powell, C., Kurlander, J., Goel, A. & Chey, W. D. A meta-analysis of the utility of C-reactive protein, erythrocyte sedimentation rate, fecal calprotectin, and fecal lactoferrin to exclude inflammatory bowel disease in adults with IBS. Am. J. Gastroenterol. 110 , 444–454 (2015).
Poullis, A., Foster, R., Northfield, T. C. & Mendall, M. A. Review article: faecal markers in the assessment of activity in inflammatory bowel disease. Aliment. Pharmacol. Ther. 16 , 675–681 (2002).
Sipponen, T. et al. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn’s disease and histological findings. Aliment. Pharmacol. Ther. 28 , 1221–1229 (2008).
Holtman, G. A. et al. Evaluation of point-of-care test calprotectin and lactoferrin for inflammatory bowel disease among children with chronic gastrointestinal symptoms. Fam. Pract. 34 , 400–406 (2017).
D’Haens, G. et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm. Bowel Dis. 18 , 2218–2224 (2012).
Reenaers, C. et al. Expert opinion for use of faecal calprotectin in diagnosis and monitoring of inflammatory bowel disease in daily clinical practice. United European Gastroenterol. J. 6 , 1117–1125 (2018).
Ministro, P. & Martins, D. Fecal biomarkers in inflammatory bowel disease: how, when and why? Expert Rev. Gastroenterol. Hepatol. 11 , 317–328 (2017).
Tibble, J. A., Sigthorsson, G., Foster, R., Forgacs, I. & Bjarnason, I. Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease. Gastroenterology 123 , 450–460 (2002).
Hübenthal, M. et al. Sparse modeling reveals miRNA signatures for diagnostics of inflammatory bowel disease. PLoS ONE 10 , e0140155 (2015).
Zhang, H., Zeng, Z., Mukherjee, A. & Shen, B. Molecular diagnosis and classification of inflammatory bowel disease. Expert Rev. Mol. Diagn. 18 , 867–886 (2018).
Allocca, M. et al. Accuracy of Humanitas ultrasound criteria in assessing disease activity and severity in ulcerative colitis: a prospective study. J. Crohns Colitis 12 , 1385–1391 (2018).
Kinoshita, K. et al. Usefulness of transabdominal ultrasonography for assessing ulcerative colitis: a prospective, multicenter study. J. Gastroenterol. 54 , 521–529 (2019).
Sagami, S. et al. Transperineal ultrasound predicts endoscopic and histological healing in ulcerative colitis. Aliment. Pharmacol. Ther. 51 , 1373–1383 (2020).
Okabayashi, S. et al. A simple 1-day colon capsule endoscopy procedure demonstrated to be a highly acceptable monitoring tool for ulcerative colitis. Inflamm. Bowel Dis. 24 , 2404–2412 (2018).
Shi, H. Y. et al. A prospective study on second-generation colon capsule endoscopy to detect mucosal lesions and disease activity in ulcerative colitis (with video). Gastrointest. Endosc. 86 , 1139–1146.e6 (2017).
Li, J. & Leung, W. K. Colon capsule endoscopy for inflammatory bowel disease. J. Dig. Dis. 19 , 386–394 (2018).
Maeda, Y. et al. Fully automated diagnostic system with artificial intelligence using endocytoscopy to identify the presence of histologic inflammation associated with ulcerative colitis (with video). Gastrointest. Endosc. 89 , 408–415 (2019).
Ullman, T. A. & Itzkowitz, S. H. Intestinal inflammation and cancer. Gastroenterology 140 , 1807–1816 (2011).
Watanabe, T. et al. Comparison of targeted vs random biopsies for surveillance of ulcerative colitis-associated colorectal cancer. Gastroenterology 151 , 1122–1130 (2016).
Harbord, M. et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J. Crohns Colitis 11 , 769–784 (2017).
Van Assche, G., Vermeire, S. & Rutgeerts, P. Management of acute severe ulcerative colitis. Gut 60 , 130–133 (2011). Consensus guidelines on treatment of UC by the European Crohn’s and Colitis Organisation .
Nguyen, G. C. et al. Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian association of gastroenterology. Gastroenterology 146 , 835–848.e6 (2014).
Travis, S. P. et al. Predicting outcome in severe ulcerative colitis. Gut 38 , 905–910 (1996).
Higashiyama, M. et al. Management of elderly ulcerative colitis in Japan. J. Gastroenterol. 54 , 571–586 (2019).
Ruemmele, F. M. & Turner, D. Differences in the management of pediatric and adult onset ulcerative colitis — lessons from the joint ECCO and ESPGHAN consensus guidelines for the management of pediatric ulcerative colitis. J. Crohns Colitis 8 , 1–4 (2014).
Sturm, A. et al. European Crohn’s and Colitis organisation topical review on IBD in the elderly: table 1. J. Crohns Colitis https://doi.org/10.1093/ecco-jcc/jjw188 (2016).
Article PubMed Google Scholar
Marteau, P. et al. Combined oral and enema treatment with Pentasa (mesalazine) is superior to oral therapy alone in patients with extensive mild/moderate active ulcerative colitis: a randomised, double blind, placebo controlled study. Gut 54 , 960–965 (2005).
Sandborn, W. J. et al. Once-daily budesonide MMX® extended-release tablets induce remission in patients with mild to moderate ulcerative colitis: results from the CORE I study. Gastroenterology 143 , 1218–1226.e2 (2012).
Herfarth, H. et al. Methotrexate is not superior to placebo in maintaining steroid-free response or remission in ulcerative colitis. Gastroenterology 155 , 1098–1108.e9 (2018).
Nielsen, O. H., Steenholdt, C., Juhl, C. B. & Rogler, G. Efficacy and safety of methotrexate in the management of inflammatory bowel disease: a systematic review and meta-analysis of randomized, controlled trials. EClinicalMedicine 20 , 100271 (2020).
Colombel, J. F. et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology 141 , 1194–1201 (2011).
Panaccione, R. et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology 146 , 392–400.e3 (2014).
Sandborn, W. J. et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 142 , 257–265.e1–3 (2012).
Sandborn, W. J. et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 146 , 85–95 (2014).
Colombel, J.-F. et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut 66 , 839–851 (2017).
Sands, B. E. et al. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N. Engl. J. Med. 381 , 1215–1226 (2019).
Sandborn, W. J. et al. Efficacy and safety of vedolizumab subcutaneous formulation in a randomized trial of patients with ulcerative colitis. Gastroenterology 158 , 562–572.e12 (2020).
Sandborn, W. J. et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 376 , 1723–1736 (2017).
FDA. FDA approves boxed warning about increased risk of blood clots and death with higher dose of arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR). FDA https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-boxed-warning-about-increased-risk-blood-clots-and-death-higher-dose-arthritis-and (2019).
Sandborn, W. J. et al. Venous thromboembolic events in the tofacitinib ulcerative colitis clinical development programme. Aliment. Pharmacol. Ther. 50 , 1068–1076 (2019).
Samuel, S. et al. Cumulative incidence and risk factors for hospitalization and surgery in a population-based cohort of ulcerative colitis. Inflamm. Bowel Dis. 19 , 1858–1866 (2013).
Coakley, B. A., Telem, D., Nguyen, S., Dallas, K. & Divino, C. M. Prolonged preoperative hospitalization correlates with worse outcomes after colectomy for acute fulminant ulcerative colitis. Surgery 153 , 242–248 (2013).
Targownik, L. E., Singh, H., Nugent, Z. & Bernstein, C. N. The epidemiology of colectomy in ulcerative colitis: results from a population-based cohort. Am. J. Gastroenterol. 107 , 1228–1235 (2012).
Kaplan, G. G. et al. Decreasing colectomy rates for ulcerative colitis: a population-based time trend study. Am. J. Gastroenterol. 107 , 1879–1887 (2012).
Eriksson, C. et al. Changes in medical management and colectomy rates: a population-based cohort study on the epidemiology and natural history of ulcerative colitis in Örebro, Sweden, 1963–2010. Aliment. Pharmacol. Ther. 46 , 748–757 (2017).
Schineis, C. et al. Colectomy with ileostomy for severe ulcerative colitis-postoperative complications and risk factors. Int. J. Colorectal Dis. 35 , 387–394 (2020).
Gu, J., Stocchi, L., Remzi, F. & Kiran, R. P. Factors associated with postoperative morbidity, reoperation and readmission rates after laparoscopic total abdominal colectomy for ulcerative colitis. Colorectal Dis. 15 , 1123–1129 (2013).
Madbouly, K. M. et al. Perioperative blood transfusions increase infectious complications after ileoanal pouch procedures (IPAA). Int. J. Colorectal Dis. 21 , 807–813 (2006).
Zittan, E. et al. Preoperative anti-tumor necrosis factor therapy in patients with ulcerative colitis is not associated with an increased risk of infectious and noninfectious complications after ileal pouch-anal anastomosis. Inflamm. Bowel Dis. 22 , 2442–2447 (2016).
Mor, I. J. et al. Infliximab in ulcerative colitis is associated with an increased risk of postoperative complications after restorative proctocolectomy. Dis. Colon Rectum 51 , 1202–1207 (2008).
Selvasekar, C. R. et al. Effect of infliximab on short-term complications in patients undergoing operation for chronic ulcerative colitis. J. Am. Coll. Surg. 204 , 956–962 (2007).
Yung, D. E. et al. Systematic review and meta-analysis: vedolizumab and postoperative complications in inflammatory bowel disease. Inflamm. Bowel Dis. 24 , 2327–2338 (2018).
Gu, J., Remzi, F. H., Shen, B., Vogel, J. D. & Kiran, R. P. Operative strategy modifies risk of pouch-related outcomes in patients with ulcerative colitis on preoperative anti-tumor necrosis factor-α therapy. Dis. Colon Rectum 56 , 1243–1252 (2013).
Remzi, F. H. et al. Restorative proctocolectomy: an example of how surgery evolves in response to paradigm shifts in care. Colorectal Dis. 19 , 1003–1012 (2017).
Ahmed Ali, U. et al. Open versus laparoscopic (assisted) ileo pouch anal anastomosis for ulcerative colitis and familial adenomatous polyposis. Cochrane Database Syst. Rev. 1 , CD006267 (2009).
Shen, B., Remzi, F. H., Lavery, I. C., Lashner, B. A. & Fazio, V. W. A proposed classification of ileal pouch disorders and associated complications after restorative proctocolectomy. Clin. Gastroenterol. Hepatol. 6 , 145–158 (2008).
Garrett, J. W. & Drossman, D. A. Health status in inflammatory bowel disease. Biological and behavioral considerations. Gastroenterology 99 , 90–96 (1990).
De Dombal, F. T., Burton, I. & Goligher, J. C. The early and late results of surgical treatment for Crohn’s disease. Br. J. Surg. 58 , 805–816 (1971).
Guyatt, G. et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology 96 , 804–810 (1989).
Love, J. R., Irvine, E. J. & Fedorak, R. N. Quality of life in inflammatory bowel disease. J. Clin. Gastroenterol. 14 , 15–19 (1992).
Irvine, E. J., Zhou, Q. & Thompson, A. K. The short inflammatory bowel disease questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn’s relapse prevention trial. Am. J. Gastroenterol. 91 , 1571–1578 (1996).
Casellas, F., López-Vivancos, J., Vergara, M. & Malagelada, J. Impact of inflammatory bowel disease on health-related quality of life. Dig. Dis. 17 , 208–218 (1999).
Lönnfors, S. et al. IBD and health-related quality of life – discovering the true impact. J. Crohns Colitis 8 , 1281–1286 (2014).
U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research & U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual. Life Outcomes 4 , 79 (2006).
PubMed Central Google Scholar
WHO. International classification of functioning, disability and health (ICF). WHO http://www.who.int/classifications/icf/en/ (2018).
Cieza, A. & Stucki, G. The international classification of functioning disability and health: its development process and content validity. Eur. J. Phys. Rehabil. Med. 44 , 303–313 (2008).
Peyrin-Biroulet, L. et al. Disability in inflammatory bowel diseases: developing ICF core sets for patients with inflammatory bowel diseases based on the international classification of functioning, disability, and health. Inflamm. Bowel Dis. 16 , 15–22 (2010).
Ananthakrishnan, A. N. et al. Permanent work disability in Crohn’s disease. Am. J. Gastroenterol. 103 , 154–161 (2008).
Thompson, P. W. & Pegley, F. S. A comparison of disability measured by the stanford health assessment questionnaire disability scales (HAQ) in male and female rheumatoid outpatients. Br. J. Rheumatol. 30 , 298–300 (1991).
Dasgupta, B. et al. Patient and physician expectations of add-on treatment with golimumab for rheumatoid arthritis: relationships between expectations and clinical and quality of life outcomes. Arthritis Care Res. 66 , 1799–1807 (2014).
CAS Google Scholar
Noseworthy, J. H., Vandervoort, M. K., Wong, C. J. & Ebers, G. C. Interrater variability with the expanded disability status scale (EDSS) and functional systems (FS) in a multiple sclerosis clinical trial. Neurology 40 , 971–975 (1990).
Butzkueven, H. et al. Efficacy and safety of natalizumab in multiple sclerosis: interim observational programme results. J. Neurol. Neurosurg. Psychiatry 85 , 1190–1197 (2014).
Peyrin-Biroulet, L. et al. Development of the first disability index for inflammatory bowel disease based on the international classification of functioning, disability and health. Gut 61 , 241–247 (2012).
Gower-Rousseau, C. et al. Validation of the inflammatory bowel disease disability index in a population-based cohort. Gut 66 , 588–596 (2017).
Lee, Y. et al. Disability in restorative proctocolectomy recipients measured using the inflammatory bowel disease disability index. J. Crohns Colitis 10 , 1378–1384 (2016).
Lichtenstein, G. R., Cohen, R., Yamashita, B. & Diamond, R. H. Quality of life after proctocolectomy with ileoanal anastomosis for patients with ulcerative colitis. J. Clin. Gastroenterol. 40 , 669–677 (2006).
Peyrin-Biroulet, L., Germain, A., Patel, A. S. & Lindsay, J. O. Systematic review: outcomes and post-operative complications following colectomy for ulcerative colitis. Aliment. Pharmacol. Ther. 44 , 807–816 (2016).
Shafer, L. A. et al. Independent validation of a self-report version of the IBD disability index (IBDDI) in a population-based cohort of IBD patients. Inflamm. Bowel Dis. 24 , 766–774 (2018).
Chiricozzi, A. et al. Quality of life of psoriatic patients evaluated by a new psychometric assessment tool: PsoDisk. Eur. J. Dermatol. 25 , 64–69 (2015).
Mercuri, S. R., Gregorio, G. & Brianti, P. Quality of life of psoriasis patients measured by the PSOdisk: a new visual method for assessing the impact of the disease. G. Ital. Dermatol. Venereol. 152 , 424–431 (2017).
Ghosh, S. et al. Development of the IBD Disk: a visual self-administered tool for assessing disability in inflammatory bowel diseases. Inflamm. Bowel Dis. 23 , 333–340 (2017).
Levenstein, S. et al. Psychological stress and disease activity in ulcerative colitis: a multidimensional cross-sectional study. Am. J. Gastroenterol. 89 , 1219–1225 (1994).
Nahon, S. et al. Risk factors of anxiety and depression in inflammatory bowel disease. Inflamm. Bowel Dis. 18 , 2086–2091 (2012).
Mikocka-Walus, A. et al. Symptoms of depression and anxiety are independently associated with clinical recurrence of inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 14 , 829–835.e1 (2016).
Bitton, A. et al. Psychosocial determinants of relapse in ulcerative colitis: a longitudinal study. Am. J. Gastroenterol. 98 , 2203–2208 (2003).
Christensen, B. et al. Histologic normalization occurs in ulcerative colitis and is associated with improved clinical outcomes. Clin. Gastroenterol. Hepatol. 15 , 1557–1564.e1 (2017).
Colombel, J.-F. et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet 390 , 2779–2789 (2018).
Adedokun, O. J. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology 147 , 1296–1307.e5 (2014).
Kobayashi, T. et al. First trough level of infliximab at week 2 predicts future outcomes of induction therapy in ulcerative colitis-results from a multicenter prospective randomized controlled trial and its post hoc analysis. J. Gastroenterol. 51 , 241–251 (2016).
Papamichael, K. et al. Infliximab concentration thresholds during induction therapy are associated with short-term mucosal healing in patients with ulcerative colitis. Clin. Gastroenterol. Hepatol. 14 , 543–549 (2016).
Afif, W. et al. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am. J. Gastroenterol. 105 , 1133–1139 (2010).
Kobayashi, T. First trough level of infliximab at week 2 predicts future outcomes of induction therapy in ulcerative colitis—results from a multicenter prospective randomized controlled trial and its post hoc analysis. J. Gastroenterol. 51 , 241–251 (2016).
Vande Casteele, N. et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 148 , 1320–1329.e3 (2015).
Roda, G., Jharap, B., Neeraj, N. & Colombel, J.-F. Loss of response to anti-TNFs: definition, epidemiology, and management. Clin. Transl. Gastroenterol. 7 , e135 (2016).
Peyrin-Biroulet, L. et al. Loss of response to vedolizumab and ability of dose intensification to restore response in patients with Crohn’s disease or ulcerative colitis: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 17 , 838–846.e2 (2019).
Baker, K. F. & Isaacs, J. D. Novel therapies for immune-mediated inflammatory diseases: what can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn’s disease and ulcerative colitis? Ann. Rheum. Dis. 77 , 175–187 (2018).
Feagan, B. G. et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet 389 , 1699–1709 (2017).
Ma, C., Jairath, V., Khanna, R. & Feagan, B. G. Investigational drugs in phase I and phase II clinical trials targeting interleukin 23 (IL23) for the treatment of Crohn’s disease. Expert Opin. Investig. Drugs 27 , 649–660 (2018).
Vermeire, S. et al. Etrolizumab as induction therapy for ulcerative colitis: a randomised, controlled, phase 2 trial. Lancet 384 , 309–318 (2014).
Vermeire, S. et al. Anti-MAdCAM antibody (PF-00547659) for ulcerative colitis (TURANDOT): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 390 , 135–144 (2017).
Yoshimura, N. et al. Safety and efficacy of AJM300, an oral antagonist of α4 integrin, in induction therapy for patients with active ulcerative colitis. Gastroenterology 149 , 1775–1783.e2 (2015).
Langer-Gould, A., Atlas, S. W., Green, A. J., Bollen, A. W. & Pelletier, D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N. Engl. J. Med. 353 , 375–381 (2005).
Sandborn, W. J. et al. Ozanimod induction and maintenance treatment for ulcerative colitis. N. Engl. J. Med. 374 , 1754–1762 (2016).
Vermeire, S. et al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet 389 , 266–275 (2017).
D’Amico, F., Fiorino, G., Furfaro, F., Allocca, M. & Danese, S. Janus kinase inhibitors for the treatment of inflammatory bowel diseases: developments from phase I and phase II clinical trials. Expert Opin. Investig. Drugs 27 , 595–599 (2018).
Hansen, J. J. & Sartor, R. B. Therapeutic manipulation of the microbiome in IBD: current results and future approaches. Curr. Treat. Options Gastroenterol. 13 , 105–120 (2015).
Barber, G. E. et al. Genetic markers predict primary non-response and durable response to anti-TNF biologic therapies in Crohn’s disease. Am. J. Gastroenterol. 111 , 1816–1822 (2016).
Boyapati, R. K., Kalla, R., Satsangi, J. & Ho, G.-T. Biomarkers in search of precision medicine in IBD. Am. J. Gastroenterol. 111 , 1682–1690 (2016).
West, N. R. et al. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat. Med. 23 , 579–589 (2017).
Choung, R. S. et al. Serologic microbial associated markers can predict Crohn’s disease behaviour years before disease diagnosis. Aliment. Pharmacol. Ther. 43 , 1300–1310 (2016).
Doherty, M. K. et al. Fecal microbiota signatures are associated with response to Ustekinumab therapy among Crohn’s disease patients. mBio https://doi.org/10.1128/mBio.02120-17 (2018).
Article PubMed PubMed Central Google Scholar
Zhou, Y. et al. Gut microbiota offers universal biomarkers across ethnicity in inflammatory bowel disease diagnosis and infliximab response prediction. mSystems https://doi.org/10.1128/mSystems.00188-17 (2018).
Arijs, I. et al. Predictive value of epithelial gene expression profiles for response to infliximab in Crohn’s disease. Inflamm. Bowel Dis. 16 , 2090–2098 (2010).
Gamo, K. et al. Gene signature-based approach identified MEK1/2 as a potential target associated with relapse after anti-TNFα treatment for Crohn’s disease. Inflamm. Bowel Dis. 24 , 1251–1265 (2018).
Kaplan, G. G. et al. The impact of inflammatory bowel disease in Canada 2018: epidemiology. J. Can. Assoc. Gastroenterol. 2 , S6–S16 (2019).
Danese, S. New therapies for inflammatory bowel disease: from the bench to the bedside. Gut 61 , 918–932 (2012).
Download references
Acknowledgements
B.S. acknowledges the DFG (German Research foundation) for funding her research (CRC-TRR 241 and CRC1340).
Author information
Authors and affiliations.
Center for Advanced IBD Research and Treatment, Kitasato University Kitasato Institute Hospital, Tokyo, Japan
Taku Kobayashi & Toshifumi Hibi
Division of Gastroenterology, Infectiology and Rheumatology, Charite–Universitatsmedizin, Berlin, Germany
Britta Siegmund
Department of Gastroenterology, Nancy University Hospital, Inserm U1256 NGERE, Lorraine University, Lorraine, France
Catherine Le Berre & Laurent Peyrin-Biroulet
Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan
Shu Chen Wei
Department of Gastroenterology and Hepatology, University Hospitals Leuven, KU Leuven, Leuven, Belgium
Marc Ferrante
Center for Inflammatory Bowel Diseases, Columbia University Irving Medical Center-New York Presbyterian Hospital, New York, NY, USA
University of Manitoba IBD Clinical and Research Centre and Department of Internal Medicine, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, Canada
Charles N. Bernstein
Humanitas Clinical and Research Center - IRCCS - and Humanitas University, Department of Biomedical Sciences, Milan, Italy
Silvio Danese
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (T.H., T.K. and S.D.); Epidemiology (C.N.B.); Mechanisms/pathophysiology (B. Siegmund); Diagnosis, screening and prevention (S.C.W.); Management (M.F. and B. Shen); Quality of life (C.L.B. and L.P.-B.); Outlook (T.K.); Overview of Primer (T.K. L.P.-B and T.H.).
Corresponding authors
Correspondence to Taku Kobayashi or Toshifumi Hibi .
Ethics declarations
Competing interests.
T.K. receives research support from AbbVie GK, Alfresa Pharma, EA Pharma, Kyorin Pharmaceutical Co., Ltd, Mochida Pharmaceutical, Nippon Kayaku, Otsuka Holdings, Thermo Fisher Scientific and ZERIA; receives advisory fees from AbbVie GK, Activaid, Alfresa Pharma, Bristol-Myers Squibb, Celltrion, CovidienÐ, Eli Lilly, Ferring Pharmaceuticals, Gilead Sciences, Janssen, Kissei, Kyorin Pharmaceutical, Mochida Pharmaceutical, Nippon Kayaku, Pfizer, Takeda Pharmaceutical and Thermo Scientific and receives lecture fees from AbbVie GK, Astellas, Alfresa Pharma, Celltrion, EA Pharma, Gilead Sciences, Janssen, JIMRO, Kyorin Pharmaceutical, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, Nippon Kayaku, Takeda Pharmaceutical and ZERIA. B. Siegmund received speaker’s fees from Abbvie, CED Service GmbH, Falk, Ferring, Janssen, Novartis, and Takeda (B. Siegmund served as representative of the Charité) and has served as consultant for AbbVie, Boehringer, Celgene, Falk, Janssen, Lilly, Pfizer, Prometheus and Takeda. C.L.B. receives honoraria from AbbVie and Ferring. S.C.W. reports consultancy fees from AbbVie, AbGenomics, Celltrion, Ferring Pharmaceuticals Inc., Gilead, Janssen, Pfizer, Takeda, and Tanabe and receives lecture fees from AbbVie, Celltrion, Eisai, Excelsior Biopharma Inc., Ferring Pharmaceuticals Inc., Janssen, Takeda, Tanabe, Tillotts Pharma, and TSPC (Taiwan Specialty Pharma Corp.). M.F. receives research grants from Amgen, Biogen, Janssen, Pfizer, and Takeda and receives consultancy fees from AbbVie, Boehringer-Ingelheim, Janssen, MSD, Pfizer, Sandoz, and Takeda and receives speaker fees from AbbVie, Amgen, Biogen, Boehringer-Ingelheim, Falk, Ferring, Janssen, Lamepro, MSD, Mylan, Pfizer, and Takeda. B. Shen receives consultant fees for AbbVie, Takeda and Janssen. C.N.B. has received educational grants from AbbVie Canada, Janssen Canada, Pfizer Canada, Shire Canada, and Takeda Canada and a research grant from AbbVie Canada. C.N.B. has performed contract research for AbbVie, Boehringer Ingelheim, Celgene, Janssen, Pfizer and Roche. He is on the advisory boards for AbbVie Canada, Janssen Canada, Pfizer Canada, Takeda Canada, and Shire Canada and consulted to Mylan Pharmaceuticals. S.D. receives consultancy fees from AbbVie, Allergan, Amgen, AstraZeneca, Biogen, Boehringer Ingelheim, Celgene, Celltrion, Ely Lilly, Enthera, Ferring Pharmaceuticals Inc., Gilead, Hospira, Janssen, Johnson & Johnson, MSD, Mundipharma, Mylan, Pfizer, Roche, Sandoz, Sublimity Therapeutics, Takeda, TiGenix, UCB Inc., and Vifor. L.P.-B. receives research grants from AbbVie, MSD, and Takeda and reports personal fees from AbbVie, Allergan, Alma, Amgen, Applied Molecular Transport, Arena, Biogen, Boehringer Ingelheim, BMS, Celltrion, Celgene, Enterome, Enthera, Ferring, Fresenius, Genentech, Gilead, Hikma, Index Pharmaceuticals, Janssen, Lilly, MSD, Mylan, Nestle, Norgine, Oppilan Pharma, OSE Immunotherapeutics, Pfizer, Pharmacosmos, Roche, Samsung Bioepis, Sandoz, Sterna, Sublimity Therapeutics, Takeda, Vifor, and Tillots and stock options from CTMA. T.H. has received research grants from AbbVie, EA Pharma, JIMRO, Otuska Holdings, and Zeria Pharmaceuticals and lecture fees from Aspen Japan KK, AbbVie GK, Ferring, Gilead Sciences, Janssen, JIMRO, Kissei Pharmaceutical, Mitsubishi-Tanabe Pharma, Mochida Pharmaceutical, Nippon Kayaku Pfizer, Takeda Pharmaceutical, and Zeria Pharmaceutical and advisory or consultancy fees from AbbVie, Bristol-Myers Squibb, Celltrion, EA Pharma, Eli Lilly, Gilead Sciences, Janssen, Kyorin, Mitsubishi-Tanabe Pharma, Nichi-Iko Pharmaceutical, Pfizer, Takeda Pharmaceutical, and Zeria Pharmaceuticals.
Additional information
Peer review information.
Nature Reviews Disease Primers thanks O. H. Nielsen, D. Rubin, J. Schölmerich and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
An abnormal narrowing of the digestive tract.
An abnormal connection between two organs or spaces.
A medical sign of facial swelling with deposition of fat.
An abnormal feeling of incomplete defecation.
Dilation of the colon without any mechanical obstruction.
A repetitive invagination of colonic surface epithelium.
A collection of neutrophils in an intestinal crypt.
The presence of plasma cells beneath the base of the crypts.
A transformation of one differentiated cell type to another.
A type of colonic epithelial cell that secrete mucus.
An abnormally fragile surface of the intestine due to inflammation.
A surgically created opening of the small intestine in the abdominal wall.
A space or a cavity in a bone.
The process of formation of a kidney stone.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Kobayashi, T., Siegmund, B., Le Berre, C. et al. Ulcerative colitis. Nat Rev Dis Primers 6 , 74 (2020). https://doi.org/10.1038/s41572-020-0205-x
Download citation
Accepted : 21 July 2020
Published : 10 September 2020
DOI : https://doi.org/10.1038/s41572-020-0205-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Desulfovibrio vulgaris interacts with novel gut epithelial immune receptor lrrc19 and exacerbates colitis.
- Runxiang Xie
- Hailong Cao
Microbiome (2024)
YAP represses intestinal inflammation through epigenetic silencing of JMJD3
Clinical Epigenetics (2024)
Association between bowel movement frequency and erectile dysfunction in patients with ulcerative colitis: a cross-sectional study
- Shinya Furukawa
- Teruki Miyake
- Yoichi Hiasa
International Journal of Impotence Research (2024)
Endoscopic healing is associated with a reduced risk of biologic treatment failure in patients with ulcerative colitis
- Akira Komatsu
- Takahiko Toyonaga
- Masayuki Saruta
Scientific Reports (2024)
Longitudinal variation of serum PCSK9 in ulcerative colitis: association with disease activity, T helper 1/2/17 cells, and clinical response of tumor necrosis factor inhibitor
- Jialin Deng
- Yongqian Jiang
Irish Journal of Medical Science (1971 -) (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Ulcerative Colitis With an Unexpected Cause
— can the immune response to covid-19 trigger inflammatory bowel disease.
by Kate Kneisel , Contributing Writer, MedPage Today March 1, 2022

A 50-year-old male patient presented to an outpatient clinic in the spring of 2020 with fever and dyspnea; he told clinicians that the symptoms had persisted for the past 3 days.
Physical examination findings included a fever of 37.8°C (100°F), respiratory rate of 24 breaths/min, and heart rate of 105 beats/min. There was no organomegaly, and the patient was a non-smoker.
Initial laboratory test findings included:
- White blood cell count: 6.4 × 109/L
- C-reactive protein (CRP): 4.6 mg/L
- Ferritin: 162 ng/mL
- D-dimer: 842 ng/mL
Findings of a polymerase chain reaction (PCR) test for SARS-CoV-2 were negative. However, the patient's wife and two children had positive PCR test results; and the patient's CT chest scan revealed diffuse ground-glass opacities consistent with viral pneumonia. Clinicians diagnosed him with COVID-19, and he was started on a now-debunked 7-day regimen of hydroxychloroquine and azithromycin. Once he was clinically stable, he was released with instructions to return for a follow-up assessment.
He returned several weeks later with bloody diarrhea, which he explained had come on about 2 weeks after he completed COVID-19 treatment. Stool analysis revealed 10 to 12 erythrocytes and five to six leukocytes. However, there was no evidence of amoebas or Clostridium difficile A+B. Complete blood count and CRP were within normal ranges. Clinicians prescribed treatment with ciprofloxacin, metronidazole, and probiotics.
On follow-up assessment 1 week later, the patient reported no improvement in symptoms. His stool calprotectin level was 1800 μg/g (normal range: 0-50 μg/g). Endoscopy revealed a diffuse, micro-ulcerated, granulated appearance that clinicians noted continued uninterrupted from the dentate line to the sigmoid colon, as well as distortion of the submucosal vascularization.
Based on presumed diagnoses of infectious colitis and ulcerative colitis, biopsies were taken. Pathology findings included mucin loss and distortion in the colonic glands, as well as evidence of polymorphonuclear leukocytes (PMNL) and plasma cell infiltration. Clinicians also noted cryptitis and a crypt abscess between the glands; no granulomatous or specific micro-organisms were detected.
The patient was diagnosed with ulcerative colitis, which clinicians believed had been triggered by COVID-19. The patient was prescribed treatment with 5-aminosalicylic acid (5-ASA) therapy, initiated orally and by enema. After 3 days of this drug therapy, his bloody diarrhea and other symptoms resolved.
On testing, the patient's anti-SARS-CoV-2 antibodies were found to be IgG positive and IgM weak positive. A subsequent CT scan revealed significant improvement from the initial findings and evidence of a sequela lesion.
Clinicians presenting this case – which they believe is the second documentation of ulcerative colitis with COVID-19 in the literature – made the report "to show that COVID-19 can appear with other organ pathologies, in addition to upper and lower respiratory tract complaints."
The group noted that initial reports of COVID-19 from China around the time this patient was diagnosed focused only on its respiratory manifestations, so the absence of reports of diarrhea or other gastrointestinal complaints may have "led to under-recognition of these symptoms."
They noted that several studies have since reported the involvement of other organs and diarrhea symptoms. For example, a single-center study of 95 COVID-19 patients admitted to the hospital found GI symptoms in 61% (n=58) of patients overall. Of those symptomatic patients, about 12% were symptomatic on admission, and the remaining 49% developed symptoms (primarily elevated bilirubin and, to a lesser extent, diarrhea) during hospitalization, possibly aggravated by various medications, including antibiotics, researchers reported.
Those researchers found "no statistically significant difference in the general demographics or clinical outcomes between patients with and without GI symptoms." Of the 58 patients with GI manifestations , impaired hepatic function occurred in about 31% during hospitalization, compared with only 1% who were affected on initial presentation.
The next most common symptom, diarrhea (two to 10 loose or watery stools a day) was noted in 24% overall, followed by anorexia and nausea, each affecting 18%. Vomiting affected just 4% of patients.
The researchers noted that antibiotic treatment was associated with development of diarrhea ( P =0.034) and elevated bilirubin levels ( P =0.028) during hospitalization, effects that were not noted with antiviral treatment. Importantly, of the 11 patients with GI symptoms only, 12% had no evidence of COVID-19 pneumonia on imaging, that paper stated.
Authors of the current case report noted that while "COVID-19 RNA can be detected by PCR tests in the stool after respiratory samples become negative in some infected patients," it is not known how long the COVID-19 virus can remain viable in the stool.
They referred to a recent study conducted at China's Wuhan Inflammatory Bowel Disease (IBD) Center which suggested that the prompt measures taken to prevent the spread of the virus may explain why none of the 318 registered IBD patients developed COVID-19. While another case series noted diarrhea in just 3% to 5% of patients, authors of the current case report wrote that "clinicians have begun to question the prevalence of IBD as a symptom of COVID-19," citing another report in which 31% of 84 patients with COVID-19 pneumonia had diarrhea.
Case authors pointed to the other report of COVID-19 and ulcerative colitis in which a 19-year-old female from Italy "presented with fever, vomiting, bloody diarrhea, and loss of taste and smell ... a positive PCR test but no CT evidence of pneumonia, and contrast enhancement in the ileum and colon." She recovered fully, returned a negative PCR test, and was diagnosed with ulcerative colitis following an ultrasound of the small bowel on day 16, with no evidence of COVID-19 in stool samples.
Likewise, case authors noted that their patient also tested negative for COVID-19, despite CT evidence of diffuse ground-glass opacities, "the most common manifestation of COVID-19"; he also developed GI symptoms after finishing treatment for COVID-19, which improved on CT.
While noting that their patient's clinical picture was not compatible with ischemic colitis, case authors advised clinicians to also "consider ischemic colitis in the differential diagnosis of antibiotic-induced colitis." Regarding the latter, the group noted that while "late-onset antibiotic-induced colitis can occur on rare occasions," that did not apply in the current case, given his lack of symptoms for 2 weeks after antibiotic treatment, and the absence of amoeba and C. difficile toxins in stool analyses.
In this case, authors noted that their patient's clinical parameters, the presence of bloody diarrhea in the absence of a toxic condition (such as ischemia or necrosis), endoscopic and pathological findings, plus his "very rapid response to 5-ASA treatment for ulcerative colitis, and the onset of complaints after recovery from COVID-19" suggest his ulcerative colitis may have been due to an immune response triggered by COVID-19.
The group explained that the etiology of ulcerative colitis is unknown -- the disease may be induced by inflammation triggered by any condition. That their patient had no history of GI complaints; developed bloody diarrhea and abdominal pain shortly after the onset of COVID-19 symptoms; and had imaging and pathology findings compatible with ulcerative colitis "might suggest that the disease could be triggered by COVID-19," the group noted.
The high levels of angiotensin-converting enzyme-2 (ACE-2) and transmembrane serine protease required for the COVID-19 virus to enter cells are expressed by human intestines, they noted, citing emerging data suggesting the virus's effect on the GI system and liver may also be associated with hepatic cells' expression of ACE-2, "a major receptor for gastrointestinal epithelial cells and COVID-19."
Case authors observed that, while little is known about COVID-19 and inflammatory bowel disease (IBD), "the International Organization for the Study of Inflammatory Bowel Disease (IOIBD) ... recently recommended reducing corticosteroid therapy and maintaining thiopurines and biologics." In 2021, that group also released consensus recommendations regarding SARS-CoV-2 vaccination for IBD patients.
Given the dynamic course seen in COVID-19 and the increasing range of clinical symptoms being reported, "there is an urgent need to properly determine the clinical features of COVID-19," case authors wrote. They acknowledged that while the lack of PCR investigations in stool or tissue samples was a limitation in this case, there was considerable evidence to suggest that the patient did experience COVID-19.
They concluded by urging further study of the association between COVID-19 and IBD, especially ulcerative colitis, and COVID-19 testing in patients presenting with gastrointestinal complaints.
![case study of ulcerative colitis author['full_name']](https://clf1.medpagetoday.com/media/images/author/KKneisel_188.jpg)
Kate Kneisel is a freelance medical journalist based in Belleville, Ontario.
Disclosures
The case report authors noted no conflicts of interest.
Primary Source
Turkish Journal of Gastroenterology
Source Reference: Aydın MF, Taşdemir H "Ulcerative colitis in a COVID-19 patient: A case report" Turk J Gastroenterol 2021; DOI: 10.5152/tjg.2021.20851.
- Current Issue
- Supplements

Gastroenterology & Hepatology
October 2023 - volume 19, issue 10, case report: medical management of acute severe ulcerative colitis.
Sudheer Kumar Vuyyuru, DM Division of Gastroenterology, Department of Medicine, Western University, London, Ontario, Canada Alimentiv, London, Ontario, Canada
Vipul Jairath, MD, PhD Division of Gastroenterology, Department of Medicine, Western University, London, Ontario, Canada Alimentiv, London, Ontario, Canada Department of Epidemiology and Biostatistics, Western University, London, Ontario, Canada
Jurij Hanžel, MD, PhD Alimentiv, London, Ontario, Canada Faculty of Medicine, University of Ljubljana and Department of Gastroenterology, University Medical Center Ljubljana, Ljubljana, Slovenia
Christopher Ma, MD, MPH Alimentiv, London, Ontario, Canada Division of Gastroenterology and Hepatology, Departments of Medicine and Community Health Sciences, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada
Brian G. Feagan, MD Division of Gastroenterology, Department of Medicine, Western University, London, Ontario, Canada Alimentiv, London, Ontario, Canada Department of Epidemiology and Biostatistics, Western University, London, Ontario, Canada
A 29-year-old female patient presented 5 years ago with bloody diarrhea, fecal urgency, and crampy abdominal pain. A colonoscopy was performed, which revealed diffuse loss of vascular pattern and superficial ulcers throughout the colon with a normal terminal ileum. Histopathologic examination of colonic biopsies showed chronic inflammatory changes with cryptitis, crypt abscesses, and architectural distortion of crypts. On this basis, the patient was diagnosed with ulcerative colitis (UC) and began receiving 5-aminosalicylic acid (4.8 g/day). After an initial period of remission, she experienced flare-ups requiring oral corticosteroids, azathioprine (2 mg/kg), and then vedolizumab (Entyvio, Takeda; 300 mg intravenously at weeks 0, 2, and 6, followed by every 8 weeks).
The patient was in clinical remission for 2 years and then presented to the emergency department with an acute exacerbation of UC, with 20 bloody bowel movements per day. On physical examination, there was tachycardia (120 beats per minute), fever (38.3 °C), and mild tenderness in the left lower quadrant of the abdomen. Laboratory findings revealed a markedly elevated C-reactive protein (CRP; 104 mg/L) and fecal calprotectin (3000 µg/g), anemia (hemoglobin 90 g/L), leukocytosis (14,000 cells/mm 3 ), and hypoalbuminemia (28 g/L). She was hospitalized and received intravenous methylprednisolone (60 mg/day) along with other supportive measures, including thromboprophylaxis.
Despite 48 hours of intravenous corticosteroids, the patient continued to have bloody stools (15 per day) and her CRP concentration remained elevated (98 mg/L). Flexible sigmoidoscopy showed deep ulcers and spontaneous mucosal bleeding (modified Mayo endoscopic score of 3), and histopathology indicated features of severe colitis. There was no evidence of cytomegalovirus (CMV) inclusion bodies on immunohistochemistry, and stool culture and toxin testing for Clostridioides difficile were negative. Abdominal radiograph did not demonstrate colonic dilatation.
Infliximab (10 mg/kg) was administered as rescue therapy, and surgical consultation was obtained. Two days after the first dose of infliximab, stool frequency was still 12 times per day with blood and the CRP level was persistently high (98 mg/L). A second infusion of infliximab was administered on day 8 of hospitalization. On day 10, the patient experienced worsening abdominal pain without clinical improvement, and subtotal colectomy with temporary end ileostomy was performed. Following surgery, the patient recovered well and without any postoperative complications, and corticosteroids were rapidly tapered. Subsequently, she underwent surgery for ileal pouch formation and ileostomy closure.
Approximately one-fourth of patients diagnosed with UC will experience an acute exacerbation requiring hospital admission during their lifetime. 1 An episode of acute severe UC (ASUC) can be a life-threatening medical emergency with an overall mortality of 1%. 2 ASUC can lead to serious complications such as toxic megacolon and colonic perforation, and emergency colectomy may be needed in medically refractory cases. 3 Up to 20% of patients admitted with ASUC require a colectomy on their first admission, and this risk increases to 40% after 2 admissions. 1,4
Global hospitalization rates for UC have declined as a result of advanced biologic therapies, optimization of management algorithms, and shifting patterns in UC epidemiology. 5 Although hospitalization rates for UC are decreasing in Western nations, there has been an increase in hospitalizations in newly industrialized countries. 6 This could be attributed to increasing incidence of UC along with limited access to advanced therapies in developing countries. 7 Similarly, in a nationwide registry–based study, a declining trend in emergency colectomy rates was observed from 2000 to 2014 in the United States, while rates of elective ileoanal pouch surgery remained stable. 8 Rates of colectomy in patients with UC from 2007 to 2016 in the United States have decreased as the use of biologic drugs has increased, suggesting a potential association between advanced treatment and the reduction in need for colectomy. 9
Risk Stratification
In 1955, Truelove and Witts conducted a randomized controlled trial (RCT) of cortisone in hospitalized patients with UC in which patients with severe disease experienced worse outcomes than patients with moderate or mild disease. 10 Nearly 70 years later, the criteria that they developed to define severe disease are still commonly used. According to the Truelove and Witts definition, ASUC is characterized by the presence of 6 or more bloody stools per day and at least one of the following signs of systemic toxicity: tachycardia (mean pulse rate >90 beats per minute), fever (>37.8 °C), anemia (hemoglobin <105 g/L), and/or a raised erythrocyte sedimentation rate (>30 mm/hr). These criteria were later modified to include elevated CRP (>30 mg/L) (Table 1). 11
Initial Management
Patients with ASUC should be admitted urgently and treated according to a standardized management approach to prevent complications. Intravenous corticosteroids remain the gold standard for initial treatment. The pooled response rate following intravenous corticosteroids is reported to be 67% (95% CI, 65-69) with a colectomy rate of 27% (95% CI, 48-76). 12 In patients requiring nutritional support, enteral nutrition is preferred over parenteral nutrition because it is associated with fewer adverse events in ASUC. 13 All patients should receive thromboprophylaxis unless there is a clear contraindication. 14 Importantly, rectal bleeding associated with ASUC is generally not a contraindication to thromboprophylaxis. Stool cultures are essential to rule out C difficile and other bacterial infections. Although C difficile infection in patients with ASUC requires appropriate antibiotic therapy, routine antibiotics are not recommended in all patients. Colonic biopsy with immunohistochemistry should be performed to exclude active CMV infection, especially in patients with a history of corticosteroid dependency. 15 Performing CMV polymerase chain reaction analysis in peripheral blood and tissues is not routinely recommended, as sensitivity and specificity are suboptimal. 16
Predictors of Response to Corticosteroids
Corticosteroids (methylprednisolone 60 mg or hydrocortisone 300-400 mg intravenously) are generally administered for at least 3 to 5 days before proceeding to salvage therapy, as a longer course of corticosteroids is associated with increased morbidity 17 and higher doses are not superior to standard doses. 12,18 Approximately 40% of patients fail to respond to intravenous corticosteroids and are at an increased risk of complications. 3 Therefore, early identification of these patients and instituting appropriate salvage therapy are crucial.
A number of prognostic indices comprised of clinical, endoscopic, and biochemical parameters have been developed to predict corticosteroid therapy failure and subsequent colectomy (Table 2). CRP is one of the commonly monitored biomarkers, and a persistently high CRP on day 3 of corticosteroids has been associated with corticosteroid failure. 19 Criteria developed by Travis and colleagues based on a retrospective case series of 48 patients with ASUC demonstrated that elevated stool frequency (>8 per day) or between 3 and 8 stools per day along with a CRP concentration of greater than 45 mg/L on day 3 of admission predicted an 85% likelihood of colectomy. 20 Ho and colleagues formulated a risk score using stool frequency, colonic dilatation, and serum albumin levels on day 3 as predictive variables in which patients with a score of 4 or greater had a corticosteroid failure rate of 85%. 21 Similarly, the Seo index predicted a colectomy rate of 60% and 83% after 1 and 2 weeks of corticosteroids, respectively, in patients with a score of greater than 200. 22 In the index developed by Lindgren and colleagues, CRP and stool frequency were considered predictive factors of corticosteroid response (CRP mg/L × 0.14 + number of bowel movements). 19 A score of greater than 8 on day 3 of intravenous corticosteroids was associated with colectomy in 72% of patients within 30 days.
Some markers have been shown to be useful in predicting outcomes as early as day 1 of hospitalization. Notably, the number of systemic Truelove and Witts criteria present on admission, in addition to at least 6 bloody stools per day, has been correlated with colectomy (1 criterion: 8.5%; ≥3 criteria: 48%). 1 The Ulcerative Colitis Endoscopic Index of Severity (UCEIS) has been used to identify high-risk patients at the time of admission. A UCEIS score of 7 or greater was shown to be associated with the need for salvage therapy in 79% (n=11/14) of patients with ASUC. 23 In a subsequent study, 100% of patients with a UCEIS score of greater than 6 on admission day and a fecal calprotectin level greater than 1000 µg/g on day 3 did not respond to corticosteroid therapy. 24 Most recently, a predictive model composed of objective parameters (serum albumin, CRP, and UCEIS score) was developed in a patient cohort from Oxford, United Kingdom, and was externally validated in 2 additional cohorts. A score of 3 or greater on the day of admission had a predictive value of 84% for corticosteroid failure. 25
Medical Salvage Therapy
Patients who fail intravenous corticosteroids require either medical or surgical salvage therapy. Cyclosporine and infliximab have been systematically investigated in clinical trials and are recommended as medical salvage therapy in ASUC. In the open-label CYSIF trial, 115 patients with corticosteroid-refractory ASUC were randomized 1:1 to infliximab (5 mg/kg intravenously on days 0, 14, and 42) and cyclosporine (2 mg/kg intravenously per day for 1 week followed by oral cyclosporine). 26 The primary outcome was treatment failure (absence of clinical response at day 7, relapse between day 7 and day 98, absence of corticosteroid-free remission at day 98, a severe adverse event leading to treatment interruption, colectomy, or death). There was no statistically significant difference between infliximab and cyclosporine in treatment failure, adverse events, and colectomy-free survival at 1 year and 5 years. 27 In the subsequent open-label, pragmatic RCT CONSTRUCT, 270 patients were randomly allocated 1:1 to receive infliximab (5 mg/kg intravenously at weeks 0, 2, and 6) or cyclosporine (2 mg/kg intravenously for 7 days, followed by 5.5 mg/kg orally per day for 12 weeks). 28 No significant differences were found between the 2 drugs with respect to the primary endpoint of quality-adjusted survival, or the secondary endpoints of colectomy rates, time to colectomy, serious adverse events, and death. A meta-analysis of RCTs that investigated infliximab and cyclosporine as salvage therapy in corticosteroid-refractory UC also found no significant differences between infliximab and cyclosporine. 29 Although consensus guidelines do not favor either agent, infliximab is generally preferred at regular or accelerated dosing regimens because of ease of administration and concerns of cyclosporine-related nephrotoxicity and neurotoxicity, especially when associated with hypercholesterolemia and hypomagnesemia. Therefore, long-term use of cyclosporine is not recommended, and patients who responded to intravenous cyclosporine should be bridged to an alternative maintenance therapy such as thiopurines. 30 Thus, cyclosporine is not advisable for patients who have previously failed thiopurine therapy. 30 However, recent evidence for the use of biologics as maintenance therapies, including vedolizumab, ustekinumab (Stelara, Janssen), and ozanimod (Zeposia, Bristol Myers Squibb), following cyclosporine rescue therapy has emerged. 31-35 Additionally, there have been reports of sequential rescue therapy after failure of initial salvage therapy, but it is not recommended owing to increased risk of adverse events. 36
Accelerated Dosing of Infliximab
Increased clearance of infliximab in patients with ASUC, especially in those with high inflammatory burden, led to the hypothesis that higher induction doses of infliximab may be needed in this population. However, the data supporting this hypothesis are conflicting.
Several observational studies have assessed different accelerated induction regimens, including higher doses (10 mg/kg) and increased frequency of dosing than the standard dosing schedule (5 mg/kg intravenously at weeks 0, 2, and 6, followed by every 8 weeks). In a retrospective study by Kohn and colleagues, a statistically higher proportion of patients receiving a single infusion of infliximab underwent colectomy compared with patients who received more than 1 infusion (35% vs 5%; P =.001). 37 Gibson and colleagues significantly decreased colectomy rates with intensified infliximab dosing in patients with corticosteroid-refractory ASUC compared with historical controls (6.7% vs 40%; P =.039) during the induction period; however, longer-term colectomy rates were similar between standard and accelerated dosing regimens. 38 Conversely, a systematic review by Sebastian and colleagues that included 10 observational studies assessing a pooled population of 705 patients found no difference between accelerated and standard induction regimens associated with either short-term (17% vs 14.5%) or long-term (25% vs 30.7%) colectomy rates, and no significant difference in complication rates. 39 Although clinicians often use accelerated regimens as off-label therapy, the evidence supporting this practice is limited. RCTs exploring optimal dosing strategy for infliximab in ASUC (NCT02770040, 40 NCT03937609 41 ) are underway.
Factors Influencing Response to Salvage Therapy
Despite improved management protocols and availability of biologics, short- and long-term colectomy rates with medical salvage therapy remain high (26%-47% and 36%-58% for cyclosporine, and 0%-50% and 35%-50% for infliximab, respectively). 42 To date, no validated scores exist to predict medical salvage therapy response. Age over 40 years, high CRP and low serum albumin at the time of infliximab initiation, and severe endoscopic lesions have been shown to be predictive of salvage therapy failure. 42 These factors indirectly suggest that high inflammatory burden is associated with poor response, especially for infliximab, and could be a result of increased clearance of infliximab by several mechanisms. High mucosal and systemic levels of tumor necrosis factor (TNF), which is associated with severe disease, neutralize anti-TNF antibodies, acting as an “antigen sink.” 43 Intestinal losses owing to increased gut permeability secondary to mucosal ulceration also contribute to lower drug exposure. Last, observational studies have suggested that fecal loss of anti-TNF is associated with severe disease and lower serum drug concentrations. 44 These clearance mechanisms can result in subtherapeutic infliximab levels and may contribute to poor response. 45
Tofacitinib Salvage Therapy
Tofacitinib (Xeljanz, Pfizer) is a Janus kinase (JAK) inhibitor that blocks predominantly JAK1 and JAK3 at therapeutic doses. Phase 3 pivotal studies from the OCTAVE clinical program demonstrated the efficacy and safety of tofacitinib in moderate to severe UC, leading to approval by the US Food and Drug Administration (FDA) in 2018. 46 However, concerns were raised regarding an increased risk of major adverse cardiac events and thrombotic events in patients with rheumatoid arthritis exposed to tofacitinib. 47 Consequently, the FDA issued a black box warning for all currently approved JAK inhibitors, and guidelines now recommend tofacitinib as a second-line agent after failure of anti-TNF therapy in the United States.
Case reports and series have described off-label use of tofacitinib in patients with ASUC who did not respond to corticosteroid therapy. 48,49 Conceptually, several characteristics make tofacitinib an attractive candidate for inpatient induction therapy. First, the drug is readily absorbed and symptomatic improvement can be seen as early as day 3 in moderate to severe UC. 50 Second, as a small molecule, tofacitinib is less susceptible to intestinal loss than infliximab. Third, tofacitinib has been shown to be effective in patients with moderate to severe UC who have failed anti-TNF therapy. 51 Broad-spectrum immunosuppressive effects are important limitations among patients who are at substantial risk of life-threatening infections and thromboembolic disease.
A retrospective cohort study of tofacitinib in hospitalized pediatric patients with UC who had failed corticosteroids and infliximab demonstrated that 8 out of 11 (73%) patients were free of colectomy at 90 days and 6 (54%) were free of colectomy at 6 months. 52 In a case-control study, patients hospitalized with ASUC who received tofacitinib (n=40) were matched to controls with ASUC according to sex and date of admission (n=113). 53 The 90-day colectomy rate was significantly lower in patients managed with tofacitinib induction therapy in addition to intravenous corticosteroids (hazard ratio, 0.28; 95% CI, 0.10-0.81; P =.018) compared with patients in the control group when adjusted for disease severity covariables. Subgroup analyses showed that this benefit was statistically significant with tofacitinib doses of 10 mg 3 times daily, but not with twice-daily dosing. Although these data are interesting, they are largely limited to retrospective case series and should not be used to inform routine clinical practice. The efficacy and safety of tofacitinib for ASUC should be assessed rigorously in an RCT.
Patients who are refractory to medical therapy should be offered surgery. Subtotal colectomy is the surgery of choice in the emergent setting. Subsequently, ileal pouch–anal anastomosis performed in a staged manner is generally the preferred approach, although some patients choose completion proctectomy with permanent end ileostomy. Emergency colectomy is associated with higher morbidity and mortality rates than semi-elective procedures, 54 so controlling inflammation promptly with timely initiation of medical therapy is important. 55 Age, longer hospital stay, superimposed infections, prior admission owing to inflammatory bowel disease, and male sex are some of the factors associated with increased mortality after emergency colectomy. 56 However, delaying the decision for surgery can be associated with increased postoperative morbidity and mortality, especially in patients exposed to intravenous corticosteroids for longer than 7 days. 57 This underscores the importance of predicting response to medical therapy early in the course of ASUC and early decision-making.
ASUC is a potentially life-threatening condition that requires hospitalization and intensive medical and supportive management to prevent complications. Early identification of patients at a high risk for corticosteroid failure and timely initiation of salvage therapy are critical. The choice of therapy depends on several factors, including clinical, endoscopic, and laboratory parameters and prior treatment history, and should be a collective decision made by the patient and a multidisciplinary team of health care professionals comprised of the treating physician, gastroenterologists, and surgeons. Although a number of models have been developed to predict corticosteroid response in patients with ASUC, validated tools to predict the failure of medical salvage therapy are lacking.
Infliximab and cyclosporine are the only agents currently approved for medical salvage therapy, and off-label use of tofacitinib has been reported in case series. Although available data supporting use of tofacitinib in patients with ASUC are insufficient to make any recommendations, future clinical trials might shed light. Surgical decision-making should not be delayed while cycling through different agents; therefore, early prediction of response to medical therapy failure is crucial.
Disclosures
Dr Vuyyuru has no relevant conflicts of interest to disclose. Dr Jairath has received consulting/advisory board fees from AbbVie, Alimentiv, Arena Pharmaceuticals, Asahi Kasei Pharma, Asieris, AstraZeneca, Bristol Myers Squibb, Celltrion, Eli Lilly, Ferring, Flagship Pioneering, Fresenius Kabi, Galapagos, GlaxoSmithKline, Genentech, Gilead, Janssen, Merck, Metacrine, Mylan, Pandion, Pendopharm, Pfizer, Protagonist, Prometheus, Reistone Biopharma, Roche, Sandoz, Second Genome, Sorriso Pharmaceuticals, Takeda, Teva, TopiVert, Ventyx, and Vividion; and speaker’s fees from AbbVie, Ferring, Bristol Myers Squibb, Galapagos, Janssen, Pfizer, Shire, Takeda, and Fresenius Kabi. Dr Hanžel has received speaker’s fees from AbbVie, Janssen, and Takeda; and consulting fees from Alimentiv. Dr Ma has received consulting fees from AbbVie, Alimentiv, Amgen, AVIR Pharma, BioJAMP, Bristol Myers Squibb, Celltrion, Ferring, Fresenius Kabi, Janssen, McKesson, Mylan, Pendopharm, Pfizer, Prometheus Biosciences, Roche, Sanofi, Takeda, and Tillotts Pharma; speaker’s fees from AbbVie, Amgen, AVIR Pharma, Alimentiv, Bristol Myers Squibb, Ferring, Fresenius Kabi, Janssen, Organon, Pendopharm, Pfizer, and Takeda; royalties from Springer Publishing; and research support from Ferring and Pfizer. Dr Feagan has received grant/research support from AbbVie, Amgen, AstraZeneca/MedImmune, Atlantic Pharmaceuticals, Boehringer Ingelheim, Celgene, Celltech, Genentech/Hoffmann-La Roche, Gilead Sciences, GlaxoSmithKline, Janssen Research & Development, Pfizer, Receptos/Celgene International, Sanofi, Santarus, Takeda Development Center Americas, Tillotts Pharma, and UCB; consulting fees from Abbott/AbbVie, Akebia Therapeutics, Allergan, Amgen, Applied Molecular Transport, Aptevo Therapeutics, AstraZeneca, Atlantic Pharmaceuticals, AVIR Pharma, Biogen, BiomX, Boehringer Ingelheim, Bristol Myers Squibb, Calypso Biotech, Celgene, Elan/Biogen, EnGene, Ferring, Roche/Genentech, Galapagos, gIcare Pharma, Gilead Sciences, Gossamer Pharma, GlaxoSmithKline, Inception IBD, Johnson & Johnson/Janssen, Kyowa Kirin, Lexicon, Lilly, Lycera, Merck, Mesoblast Pharma, Millennium, Nestlé, Nextbiotix, Novo Nordisk, Pfizer, Prometheus Therapeutics and Diagnostics, Progenity, Protagonist, Receptos, Salix Pharmaceuticals, Shire, Sienna Biologics, Sigmoid Pharma, Sterna Biologicals, Synergy Pharma, Takeda, Teva, TiGenix, Tillotts Pharma, UCB, Vertex Pharmaceuticals, Vivelix Pharmaceuticals, VHsquared, and Zyngenia; speakers bureau fees from Abbott/AbbVie, Johnson & Johnson/Janssen, Lilly, Takeda, Tillotts Pharma, and UCB; is a scientific advisory board member for Abbott/AbbVie, Allergan, Amgen, AstraZeneca, Atlantic Pharmaceuticals, Avaxia Biologics, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Centocor, Elan/Biogen, Galapagos, Genentech/Roche, Johnson & Johnson/Janssen, Merck, Nestlé, Novartis, Novo Nordisk, Pfizer, Prometheus Laboratories, Protagonist, Salix Pharmaceuticals, Sterna Biologicals, Takeda, Teva, TiGenix, Tillotts Pharma, and UCB; and is the Senior Scientific Officer of Alimentiv.
1. Dinesen LC, Walsh AJ, Protic MN, et al. The pattern and outcome of acute severe colitis. J Crohns Colitis . 2010;4(4):431-437.
2. Dong C, Metzger M, Holsbø E, Perduca V, Carbonnel F. Systematic review with meta-analysis: mortality in acute severe ulcerative colitis. Aliment Pharmacol Ther . 2020;51(1):8-33.
3. Molnár T, Farkas K, Nyári T, Szepes Z, Nagy F, Wittmann T. Response to first intravenous steroid therapy determines the subsequent risk of colectomy in ulcerative colitis patients. J Gastrointestin Liver Dis . 2011;20(4):359-363.
4. Aratari A, Papi C, Clemente V, et al. Colectomy rate in acute severe ulcerative colitis in the infliximab era. Dig Liver Dis . 2008;40(10):821-826.
5. Tsai L, Nguyen NH, Ma C, Prokop LJ, Sandborn WJ, Singh S. Systematic review and meta-analysis: risk of hospitalization in patients with ulcerative colitis and Crohn’s disease in population-based cohort studies. Dig Dis Sci . 2022;67(6):2451-2461.
6. Buie MJ, Quan J, Windsor JW, et al; Global IBD Visualization of Epidemiology Studies in the 21st Century (GIVES-21) Research Group. Global hospitalization trends for Crohn’s disease and ulcerative colitis in the 21st century: a systematic review with temporal analyses. Clin Gastroenterol Hepatol . 2023;21(9):2211-2221.
7. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet . 2017;390(10114):2769-2778.
8. Kayal M, Saha A, Poojary P, et al. Emergent colectomy rates decreased while elective ileal pouch rates were stable over time: a nationwide inpatient sample study. Int J Colorectal Dis . 2019;34(10):1771-1779.
9. Barnes EL, Jiang Y, Kappelman MD, et al. Decreasing colectomy rate for ulcerative colitis in the United States between 2007 and 2016: a time trend analysis. Inflamm Bowel Dis . 2020;26(8):1225-1231.
10. Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. BMJ . 1955;2(4947):1041-1048.
11. Magro F, Gionchetti P, Eliakim R, et al; European Crohn’s and Colitis Organisation [ECCO]. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis . 2017;11(6):649-670.
12. Turner D, Walsh CM, Steinhart AH, Griffiths AM. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol . 2007;5(1):103-110.
13. González-Huix F, Fernández-Bañares F, Esteve-Comas M, et al. Enteral versus parenteral nutrition as adjunct therapy in acute ulcerative colitis. Am J Gastroenterol . 1993;88(2):227-232.
14. Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet . 2010;375(9715):657-663.
15. Sager K, Alam S, Bond A, Chinnappan L, Probert CS. Review article: cytomegalovirus and inflammatory bowel disease. Aliment Pharmacol Ther . 2015;41(8):725-733.
16. Mourad FH, Hashash JG, Kariyawasam VC, Leong RW. Ulcerative colitis and cytomegalovirus infection: from A to Z. J Crohns Colitis . 2020;14(8):1162-1171.
17. Meyers S, Lerer PK, Feuer EJ, Johnson JW, Janowitz HD. Predicting the outcome of corticoid therapy for acute ulcerative colitis. Results of a prospective, randomized, double-blind clinical trial. J Clin Gastroenterol . 1987;9(1):50-54.
18. Rosenberg W, Ireland A, Jewell DP. High-dose methylprednisolone in the treatment of active ulcerative colitis. J Clin Gastroenterol . 1990;12(1):40-41.
19. Lindgren SC, Flood LM, Kilander AF, Löfberg R, Persson TB, Sjödahl RI. Early predictors of glucocorticosteroid treatment failure in severe and moderately severe attacks of ulcerative colitis. Eur J Gastroenterol Hepatol . 1998;10(10):831-835.
20. Travis SP, Farrant JM, Ricketts C, et al. Predicting outcome in severe ulcerative colitis. Gut . 1996;38(6):905-910.
21. Ho GT, Mowat C, Goddard CJR, et al. Predicting the outcome of severe ulcerative colitis: development of a novel risk score to aid early selection of patients for second-line medical therapy or surgery. Aliment Pharmacol Ther . 2004;19(10):1079-1087.
22. Seo M, Okada M, Yao T, Matake H, Maeda K. Evaluation of the clinical course of acute attacks in patients with ulcerative colitis through the use of an activity index. J Gastroenterol . 2002;37(1):29-34.
23. Corte C, Fernandopulle N, Catuneanu AM, et al. Association between the ulcerative colitis endoscopic index of severity (UCEIS) and outcomes in acute severe ulcerative colitis. J Crohns Colitis . 2015;9(5):376-381.
24. Jain S, Kedia S, Bopanna S, et al. Faecal calprotectin and UCEIS predict short-term outcomes in acute severe colitis: prospective cohort study. J Crohns Colitis . 2017;11(11):1309-1316.
25. Adams A, Gupta V, Mohsen W, et al. Early management of acute severe UC in the biologics era: development and international validation of a prognostic clinical index to predict steroid response. Gut . 2023;72(3):433-442.
26. Laharie D, Bourreille A, Branche J, et al; Groupe d’Etudes Thérapeutiques des Affections Inflammatoires Digestives. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet . 2012;380(9857):1909-1915.
27. Laharie D, Bourreille A, Branche J, et al; Groupe d’Etudes Thérapeutiques des Affections Inflammatoires Digestives. Long-term outcome of patients with steroid-refractory acute severe UC treated with ciclosporin or infliximab. Gut . 2018;67(2):237-243.
28. Williams JG, Alam MF, Alrubaiy L, et al. Infliximab versus ciclosporin for steroid-resistant acute severe ulcerative colitis (CONSTRUCT): a mixed methods, open-label, pragmatic randomised trial. Lancet Gastroenterol Hepatol . 2016;1(1):15-24.
29. Narula N, Marshall JK, Colombel JF, et al. Systematic review and meta-analysis: infliximab or cyclosporine as rescue therapy in patients with severe ulcerative colitis refractory to steroids. Am J Gastroenterol . 2016;111(4):477-491.
30. Lamb CA, Kennedy NA, Raine T, et al; IBD guidelines eDelphi consensus group. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut . 2019;68(suppl 3):s1-s106.
31. Tarabar D, El Jurdi K, Traboulsi C, et al. A prospective trial with long term follow-up of patients with severe, steroid-resistant ulcerative colitis who received induction therapy with cyclosporine and were maintained with vedolizumab. Inflamm Bowel Dis . 2022;28(10):1549-1554.
32. Shaffer SR, Traboulsi C, Krugliak Cleveland N, Rubin DT. Combining cyclosporine with ustekinumab in acute severe ulcerative colitis. ACG Case Rep J . 2021;8(5):e00604.
33. Cohen NA, Dalal SR, Choi D, Rubin DT. Ozanimod maintenance therapy after cyclosporine induction in acute severe ulcerative colitis. ACG Case Rep J . 2022;9(7):e00832.
34. Pellet G, Stefanescu C, Carbonnel F, et al; Groupe d’Etude Thérapeutique des Affections Inflammatoires du tube Digestif. Efficacy and safety of induction therapy with calcineurin inhibitors in combination with vedolizumab in patients with refractory ulcerative colitis. Clin Gastroenterol Hepatol . 2019;17(3):494-501.
35. Ollech JE, Dwadasi S, Rai V, et al. Efficacy and safety of induction therapy with calcineurin inhibitors followed by vedolizumab maintenance in 71 patients with severe steroid-refractory ulcerative colitis. Aliment Pharmacol Ther . 2020;51(6):637-643.
36. Narula N, Fine M, Colombel J-F, Marshall JK, Reinisch W. Systematic review: sequential rescue therapy in severe ulcerative colitis: do the benefits outweigh the risks? Inflamm Bowel Dis . 2015;21(7):1683-1694.
37. Kohn A, Daperno M, Armuzzi A, et al. Infliximab in severe ulcerative colitis: short-term results of different infusion regimens and long-term follow-up. Aliment Pharmacol Ther . 2007;26(5):747-756.
38. Gibson DJ, Heetun ZS, Redmond CE, et al. An accelerated infliximab induction regimen reduces the need for early colectomy in patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol . 2015;13(2):330-335.e1.
39. Sebastian S, Myers S, Nadir S, Subramanian S. Systematic review: efficacy and safety of accelerated induction regimes in infliximab rescue therapy for hospitalized patients with acute severe colitis. Dig Dis Sci . 2019;64(5):1119-1128.
40. ClinicalTrials.gov. Optimising infliximab induction therapy for acute severe ulcerative colitis (PREDICT UC). https://classic.clinicaltrials.gov/show/NCT02770040. Identifier: NCT02770040. Accessed September 9, 2023.
41. ClinicalTrials.gov. TITRATE (inducTIon for acuTe ulceRATivE Colitis). https://classic.clinicaltrials.gov/show/NCT03937609. Identifier: NCT03937609. Accessed September 9, 2023.
42. Seah D, De Cruz P. Review article: the practical management of acute severe ulcerative colitis. Aliment Pharmacol Ther . 2016;43(4):482-513.
43. Yarur AJ, Jain A, Sussman DA, et al. The association of tissue anti-TNF drug levels with serological and endoscopic disease activity in inflammatory bowel disease: the ATLAS study. Gut . 2016;65(2):249-255.
44. Brandse JF, van den Brink GR, Wildenberg ME, et al. Loss of infliximab into feces is associated with lack of response to therapy in patients with severe ulcerative colitis. Gastroenterology . 2015;149(2):350-355.e2.
45. Seow CH, Newman A, Irwin SP, Steinhart AH, Silverberg MS, Greenberg GR. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut . 2010;59(1):49-54.
46. Sandborn WJ, Su C, Sands BE, et al; OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med . 2017;376(18):1723-1736.
47. Ytterberg SR, Bhatt DL, Mikuls TR, et al; ORAL Surveillance Investigators. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med . 2022;386(4):316-326.
48. Sedano R, Jairath V. High-dose rescue tofacitinib prevented inpatient colectomy in acute severe ulcerative colitis refractory to anti-TNF. Inflamm Bowel Dis . 2021;27(5):e59-e60.
49. Gilmore R, Hilley P, Srinivasan A, Choy M, De Cruz P. Sequential use of high-dose tofacitinib after infliximab salvage therapy in acute severe ulcerative colitis. J Crohns Colitis . 2022;16(1):166-168.
50. Hanauer S, Panaccione R, Danese S, et al. Tofacitinib induction therapy reduces symptoms within 3 days for patients with ulcerative colitis. Clin Gastroenterol Hepatol . 2019;17(1):139-147.
51. Sandborn WJ, Peyrin-Biroulet L, Sharara AI, et al. Efficacy and safety of tofacitinib in ulcerative colitis based on prior tumor necrosis factor inhibitor failure status. Clin Gastroenterol Hepatol . 2022;20(3):591-601.e8.
52. Constant BD, Baldassano R, Kirsch J, Mitchel EB, Stein R, Albenberg L. Tofacitinib salvage therapy for children hospitalized for corticosteroid- and biologic-refractory ulcerative colitis. J Pediatr Gastroenterol Nutr . 2022;75(6):724-730.
53. Berinstein JA, Sheehan JL, Dias M, et al. Tofacitinib for biologic-experienced hospitalized patients with acute severe ulcerative colitis: a retrospective case-control study. Clin Gastroenterol Hepatol . 2021;19(10):2112-2120.e1.
54. Nicholls RJ, Clark DN, Kelso L, et al. Nationwide linkage analysis in Scotland implicates age as the critical overall determinant of mortality in ulcerative colitis. Aliment Pharmacol Ther . 2010;31(12):1310-1321.
55. Singh S, Al-Darmaki A, Frolkis AD, et al. Postoperative mortality among patients with inflammatory bowel diseases: a systematic review and meta-analysis of population-based studies. Gastroenterology . 2015;149(4):928-937.
56. Bernstein CN, Ng SC, Lakatos PL, Moum B, Loftus EV Jr; Epidemiology and Natural History Task Force of the International Organization of the Study of Inflammatory Bowel Disease. A review of mortality and surgery in ulcerative colitis: milestones of the seriousness of the disease. Inflamm Bowel Dis . 2013;19(9):2001-2010.
57. Saha SK, Panwar R, Kumar A, et al. Early colectomy in steroid-refractory acute severe ulcerative colitis improves operative outcome. Int J Colorectal Dis . 2018;33(1):79-82.
58. Grant RK, Jones G-R, Plevris N, et al. The ACE (albumin, CRP and endoscopy) index in acute colitis: a simple clinical index on admission that predicts outcome in patients with acute ulcerative colitis. Inflamm Bowel Dis . 2021;27(4):451-457.
- Patient Care & Health Information
- Diseases & Conditions
- Ulcerative colitis
- What is ulcerative colitis? A Mayo Clinic expert explains
Listen to gastroenterologist William Faubion, M.D., walk through ulcerative colitis basics.
[Music playing]
William A. Faubion, Jr., M.D., Gastroenterology, Mayo Clinic I'm Dr. Bill Faubion, a gastroenterologist at Mayo Clinic. In this video, we'll cover the basics of ulcerative colitis. What is it? Who gets it? The symptoms, diagnosis, and treatment. Whether you're looking for answers for yourself or someone you love, we're here to give you the best information available.
Ulcerative colitis is an inflammatory bowel disease that causes chronic inflammation and ulcers in the superficial lining of the large intestine, also called the colon. And that includes the rectum. It's estimated that about a million Americans are living with ulcerative colitis, making it the most common form of inflammatory bowel disease. It can be painful and debilitating, occasionally leading to severe complications. It can also be emotionally stressful. And while there is no cure, once you've been diagnosed, treatment can help you get back to a much more normal and comfortable life.
Who gets it?
The exact cause of ulcerative colitis is unknown, but there are things that appear to trigger or aggravate it. It may involve an abnormal immune response against some microorganism in which your tissues are also attacked. Genetics might also play a role. You are at higher risk if a first-degree relative has it. There's also a correlation with age. Although it can show up at any stage of life, most people are diagnosed before the age of 30. And ethnicity is a risk factor. Whites have the highest risk, especially among people of Ashkenazi Jewish descent. While diet and stress don't cause ulcerative colitis, they are known to exacerbate symptoms.
What are the symptoms?
Most people have mild to moderate cases of ulcerative colitis. Although it can be more severe, you may also experience periods of remission when you have no issues at all. A person's symptoms depend on the severity of the case in the area of the colon that's involved. They usually develop over time, and they can include diarrhea, often with blood or pus, fever, fatigue, anemia, loss of appetite and weight loss, abdominal pain and cramping, rectal pain and bleeding, the need for a bowel movement, yet the inability to do so despite the urgency. And in children, delayed growth and development. Over time, ulcerative colitis can lead to other complications, such as severe dehydration, a perforated colon, bone loss, inflammation of your skin, joints and eyes. It can also increase your risk for blood clots and colon cancer. These symptoms don't automatically mean that you have ulcerative colitis. But if you're experiencing anything that concerns you, it's a good idea to make an appointment with your doctor.
How is it diagnosed?
The only way to definitively diagnose ulcerative colitis is with a biopsy after taking a tissue sample through an endoscopic procedure. But first, less invasive things can be done to rule out other causes. First, your doctor will consider your medical history. They may want to run a variety of tests or procedures. And at some point, your general practitioner may refer you to a specialist called a gastroenterologist like myself. A blood test can check for anemia and check for signs of infection. A stool study can test for white blood cells and other specific proteins that point to ulcerative colitis, as well as rule out certain pathogens. A colonoscopy may be needed. This allows your doctor to view the entirety of the large intestine using an endoscope, a small camera mounted on a thin flexible tube. They can take tissue samples for a biopsy at the same time. Or if your colon is extremely inflamed, they may do a flexible sigmoidoscopy, which only goes as far as the rectum and lower or sigmoid colon. If your symptoms are more severe, your doctor may want some imaging done. An abdominal x-ray can rule out serious complications, like a perforated colon. An MRI or CT scan can also be performed for a more detailed view of the bowel, as well as to reveal the extent of the inflammation.
How is it treated?
Although there is no cure for ulcerative colitis there are widely effective treatments, usually involving either drug therapy or surgery. Your doctor can work with you to find things that alleviate your symptoms and in some cases, even bring about long-term remission. Treatments may include anti-inflammatory drugs like corticosteroids and immune system suppressants. Certain targeted therapies directed against the immune system called biologics can help. Antidiarrheals, pain relievers, antispasmodics and iron supplements can help counter other symptoms. And surgery may be required to remove the damaged tissue. In extreme cases, the whole colon may be removed. Which sounds drastic, but this can sometimes be the best option for eliminating the pain and struggle of ulcerative colitis once and for all. Some of these therapies may have side effects themselves. So be sure to review the risks and benefits with your doctor.
Ulcerative colitis can be physically and emotionally challenging, but there are things that can help. Although there's no firm evidence that any foods cause ulcerative colitis, certain things seem to aggravate flare-ups. So a food diary can help you identify personal triggers. Beyond that, limit dairy products, eat small meals, stay hydrated, try to avoid caffeine and alcohol and carbonation. If you're concerned about weight loss or if your diet has become too limited, talk to a registered dietitian. It's important to take care of your mental health, too. Find ways to manage stress, like exercise, breathing and relaxation techniques or biofeedback. Some symptoms like abdominal pain, gas, and diarrhea can cause anxiety and frustration. That can make it difficult to be out in public for any amount of time. It can feel limiting and isolating and lead to depression. So learn as much as you can about ulcerative colitis. Staying informed can help a lot in feeling like you're in control of your condition. Talk to a therapist, especially one familiar with inflammatory bowel disease. Your doctor should be able to give you some recommendations. And you might want to find a support group for people going through the same thing that you are. Ulcerative colitis is a complex disease, but having expert medical care and developing a treatment strategy can make it more manageable and even help patients get back to the freedom of a normal life. Meanwhile, significant advances continue to be made in understanding and treating the disease and getting us closer to curing it or preventing it entirely. If you'd like to learn even more about ulcerative colitis, watch our other related videos or visit mayoclinic.org. We wish you well.
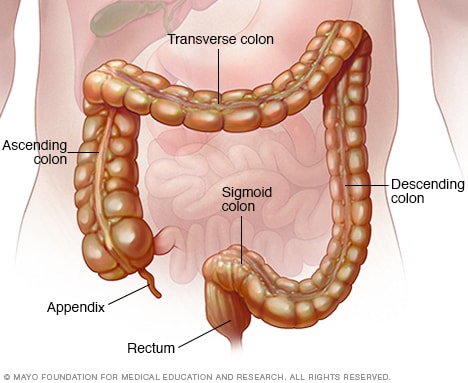
Colon and rectum
The colon is a long tube-like organ in the abdomen. It's the largest part of the large intestine. The colon carries waste to be expelled from the body. The rectum makes up the last several inches of the colon.
Gastroenterology & GI Surgery Blog
Ulcerative colitis (UL-sur-uh-tiv koe-LIE-tis) is an inflammatory bowel disease (IBD) that causes inflammation and ulcers (sores) in your digestive tract. Ulcerative colitis affects the innermost lining of your large intestine, also called the colon, and rectum. In most people, symptoms usually develop over time, rather than suddenly.
Ulcerative colitis can be draining and can sometimes lead to life-threatening complications. While it has no known cure, there are several new treatments that can greatly reduce signs and symptoms of the disease and bring about long-term remission.
Products & Services
- A Book: Mayo Clinic on Crohn’s Disease and Ulcerative Colitis
- A Book: Mayo Clinic on Digestive Health
- Available Digestive Health Products at Mayo Clinic Store
Ulcerative colitis symptoms can vary, depending on the severity of inflammation and where it occurs. Signs and symptoms may include:
- Diarrhea, often with blood or pus
- Rectal bleeding — passing small amount of blood with stool
- Abdominal pain and cramping
- Rectal pain
- Urgency to defecate
- Inability to defecate despite urgency
- Weight loss
- In children, failure to grow
Most people with ulcerative colitis have mild to moderate symptoms. The course of ulcerative colitis may vary, with some people having long periods when it goes away. This is called remission.
Health care providers often classify ulcerative colitis according to its location. Symptoms of each type often overlap. Types of ulcerative colitis include:
- Ulcerative proctitis. Inflammation is confined to the area closest to the anus, also called the rectum. Rectal bleeding may be the only sign of the disease.
- Proctosigmoiditis. Inflammation involves the rectum and sigmoid colon — the lower end of the colon. Symptoms include bloody diarrhea, abdominal cramps and pain, and an inability to move the bowels despite the urge to do so. This is called tenesmus.
- Left-sided colitis. Inflammation extends from the rectum up through the sigmoid and descending portions of the colon. Symptoms include bloody diarrhea, abdominal cramping and pain on the left side, and urgency to defecate.
- Pancolitis. This type often affects the entire colon and causes bouts of bloody diarrhea that may be severe, abdominal cramps and pain, fatigue, and significant weight loss.
When to see a doctor
See your health care provider if you experience a persistent change in your bowel habits or if you have signs and symptoms such as:
- Abdominal pain
- Blood in your stool
- Ongoing diarrhea that doesn't respond to nonprescription medications
- Diarrhea that awakens you from sleep
- An unexplained fever lasting more than a day or two
Although ulcerative colitis usually isn't fatal, it's a serious disease. In some cases, ulcerative colitis may cause life-threatening complications.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
Get the latest health information from Mayo Clinic delivered to your inbox.
Subscribe for free and receive your in-depth guide to digestive health, plus the latest on health innovations and news. You can unsubscribe at any time. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing
Your in-depth digestive health guide will be in your inbox shortly. You will also receive emails from Mayo Clinic on the latest health news, research, and care.
If you don’t receive our email within 5 minutes, check your SPAM folder, then contact us at [email protected] .
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
The exact cause of ulcerative colitis remains unknown. Previously, diet and stress were suspected. However, researchers now know that these factors may aggravate but don't cause ulcerative colitis.
One possible cause is an immune system malfunction. When your immune system tries to fight off an invading virus or bacterium, an irregular immune response causes the immune system to attack the cells in the digestive tract, too.
Heredity also seems to play a role in that ulcerative colitis is more common in people who have family members with the disease. However, most people with ulcerative colitis don't have this family history.
Risk factors
Ulcerative colitis affects about the same number of women and men. Risk factors may include:
- Age. Ulcerative colitis usually begins before the age of 30, but it can occur at any age. Some people may not develop the disease until after age 60.
- Race or ethnicity. Although white people have the highest risk of the disease, it can occur in any race. If you're of Ashkenazi Jewish descent, your risk is even higher.
- Family history. You're at higher risk if you have a close relative, such as a parent, sibling or child, with the disease.
Complications
Possible complications of ulcerative colitis include:
- Severe bleeding
- Severe dehydration
- A rapidly swelling colon, also called a toxic megacolon
- A hole in the colon, also called a perforated colon
- Increased risk of blood clots in veins and arteries
- Inflammation of the skin, joints and eyes
- An increased risk of colon cancer
- Bone loss, also called osteoporosis
Ulcerative colitis care at Mayo Clinic
- Feldman M, et al, eds. Epidemiology, pathogenesis, and diagnosis of inflammatory bowel diseases. In: Sleisenger and Fordtran's Gastrointestinal and Liver Disease: Pathophysiology, Diagnosis, Management. 11th ed. Elsevier; 2021. https://www.clinicalkey.com. Accessed July 22, 2020.
- Goldman L, et al., eds. Inflammatory bowel disease. In: Goldman-Cecil Medicine. 26th ed. Elsevier; 2020. https://www.clinicalkey.com. Accessed July 22, 2020.
- The facts about inflammatory bowel diseases. Crohn's and Colitis Foundation. https://www.crohnscolitisfoundation.org/. Accessed Sept. 6, 2022.
- Ulcerative colitis. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/digestive-diseases/ulcerative-colitis. Accessed Sept. 6, 2022.
- What is ulcerative colitis? Crohn's and Colitis Foundation. https://www.crohnscolitisfoundation.org/what-is-ulcerative-colitis. Accessed Sept. 6, 2022.
- Kliegman RM, et al. Inflammatory bowel diseases. In: Nelson Textbook of Pediatrics. 21st ed. Elsevier; 2020. https://www.clinicalkey.com. Accessed July 22, 2020.
- AskMayoExpert. Chronic ulcerative colitis. Mayo Clinic; 2019.
- Abraham B, et al. Antibiotics and probiotics in inflammatory bowel disease: When to use them? Frontline Gastroenterology. 2020; doi:10.1136/flgastro-2018-101057.
- What should I eat? Crohn's and Colitis Foundation. https://www.crohnscolitisfoundation.org/diet-and-nutrition/what-should-i-eat. Accessed Sept. 6, 2022.
- Mind-body therapies. Crohn's and Colitis Foundation. https://www.crohnscolitisfoundation.org/complementary-medicine/mind-body-therapies. Accessed Sept. 6, 2022.
- Nguyen H. Allscripts EPSi. Mayo Clinic. April 1, 2022.
- Special IBD diets. Crohn's and Colitis Foundation. https://www.crohnscolitisfoundation.org/diet-and-nutrition/special-ibd-diets. Accessed Sept. 6, 2022.
- Shergill A, et al. Surveillance and management of dysplasia in patients with inflammatory bowel disease. https://www.uptodate.com/contents/search. Accessed Sept. 6, 2022.
- Kashyap PC (expert opinion). Mayo Clinic. Aug. 13, 2020.
- Kane SV (expert opinion). Mayo Clinic. Sept. 12, 2020.
- Xeljanz, Xeljanz XR (tofacitinib): Drug safety communication — Initial safety trial results find increased risk of serious heart-related problems and cancer with arthritis and ulcerative colitis medicine. U.S. Food and Drug Administration. https://www.fda.gov/safety/medical-product-safety-information/xeljanz-xeljanz-xr-tofacitinib-drug-safety-communication-initial-safety-trial-results-find-increased?utm_medium=email&utm_source=govdelivery. Accessed Sept. 6, 2022.
- Khanna S (expert opinion). Mayo Clinic. Aug. 20, 2022.
- Cohen RD, et al. Management of moderate to severe ulcerative colitis in adults. https://www.uptodate.com/contents/search. Accessed Aug. 1, 2022.
- Ulcerative colitis flare-ups: 5 tips to manage them
Associated Procedures
- Acupuncture
- Barium enema
- Colonoscopy
- Flexible sigmoidoscopy
- Ileoanal anastomosis (J-pouch) surgery
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
- Care at Mayo Clinic
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Make twice the impact
Your gift can go twice as far to advance cancer research and care!
- Search Menu
- Advance articles
- Open Access
- Editor's Choice
- Supplements
- ECCO Guideline/Consensus Papers
- ECCO Topical Reviews
- ECCO Scientific Workshop Papers
- ECCO Position Statements
- Why Publish with JCC
- Author Guidelines
- Submission Site
- Order Offprints
- Advertising and Corporate Services
- Advertising
- Reprints and ePrints
- Sponsored Supplements
- Branded Books
- Journals Career Network
- About Journal of Crohn's and Colitis
- About the European Crohn's and Colitis Organisation
- Editorial Board
- Self-Archiving Policy
- Dispatch Dates
- Journals on Oxford Academic
- Books on Oxford Academic

- < Previous
Differences in Management and Outcomes of Older and Younger Adults with Acute Severe Ulcerative Colitis
- Article contents
- Figures & tables
- Supplementary Data
Taylor Boyd, Elizabeth Bonareri Araka, Bharati Kochar, Ashwin N Ananthakrishnan, Differences in Management and Outcomes of Older and Younger Adults with Acute Severe Ulcerative Colitis, Journal of Crohn's and Colitis , Volume 18, Issue 4, April 2024, Pages 570–577, https://doi.org/10.1093/ecco-jcc/jjad183
- Permissions Icon Permissions
Older adults with ulcerative colitis [UC] have greater morbidity than younger adults. The goal of this study was to investigate differences in the management and outcomes of older and younger patients hospitalised with severe UC.
We conducted a retrospective cohort study of patients hospitalised for acute severe ulcerative colitis requiring intravenous steroids. We compared outcomes of adults aged ≥65 years with outcomes of younger patients. Primary study outcomes included frequency and timing of medical and surgical rescue therapy during the hospitalisation, postoperative complications, frailty, and mortality outcomes up to 1 year following the hospitalisation.
Our cohort included 63 older adults [≥65 years] and 137 younger adults [14–64 years]. Despite similar disease severity at hospitalisation, older adults were half as likely to receive medical rescue therapy (odds ratio 0.45, 95% confidence interval [CI] 0.22–0.91). This difference was more striking among the frailest older adults. Older patients were similarly likely to undergo surgery but were more likely to undergo urgent or emergent procedures [50%] compared with younger patients [13%] [ p <0.004]. The fraction of older adults at high risk for frailty increased from 33% pre-hospitalisation to 42% post-hospitalisation. Nearly one-third [27.8%] of older adults died within 1 year of hospitalisation, with half the deaths among older adults being attributable to UC or complications of UC.
In comparison with younger patients, older adults had lower frequency use of medical rescue therapy, higher rates of emergency surgery, and increased mortality within 1 year. Further research is needed to optimise care pathways in this population.

- inflammatory bowel disease
- ulcerative colitis
- surgical procedures, operative
- older adult
- emergency surgical procedure
- severity of illness
Email alerts
Citing articles via.
- About Journal of Crohn's and Colitis
- Recommend to your Library
Affiliations
- Online ISSN 1876-4479
- Print ISSN 1873-9946
- Copyright © 2024 European Crohn's and Colitis Organisation (ECCO) Published by Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Ulcerative colitis.
Whitney D. Lynch ; Ronald Hsu .
Affiliations
Last Update: June 5, 2023 .
- Continuing Education Activity
Ulcerative colitis is an idiopathic inflammatory condition of the colon that results in diffuse friability and superficial erosions on the colonic wall and associated bleeding. It is the most common form of inflammatory bowel disease worldwide. Characteristically, it involves inflammation restricted to the mucosa and submucosa of the colon. Typically, the disease starts in the rectum and extends proximally in a continuous manner. In the United States, the disease accounts for a quarter-million provider visits annually. This activity reviews the evaluation and management of ulcerative colitis and highlights the role of the interprofessional team in evaluating and improving care for patients with this condition.
- Explain the history and physical examination of patients with ulcerative colitis.
- Outline evaluation of patients with ulcerative colitis.
- Describe treatment considerations for patients with ulcerative colitis.
- Identify interprofessional team strategies for improving care coordination and outcomes in patients with ulcerative colitis.
- Introduction
Ulcerative colitis is an idiopathic inflammatory condition of the colon that results in diffuse friability and superficial erosions on the colonic wall associated with bleeding. It is the most common form of inflammatory bowel disease worldwide. It characteristically involves inflammation restricted to the mucosa and submucosa of the colon. Typically, the disease starts in the rectum and extends proximally in a continuous manner. In the United States, the disease accounts for a quarter-million provider visits annually, and medical costs directly related to the disease are estimated to exceed four billion dollars annually. [1] [2] [3] Ulcerative has no cure and is a lifelong disorder with a significant impact on both physical and mental health.
The specific cause of inflammatory bowel disease is not known. There seems to be a primary genetic component since the most important independent risk factor is a family history of the disease (8% to 14% of patients). A first-degree relative of a patient with ulcerative colitis has a four times higher risk of developing the disease. Additionally, ulcerative colitis has a higher incidence in Jewish populations than other ethnicities.
Although there is little evidence to support this, it has been postulated that alterations in the composition of the gut microbiota and defects in mucosal immunity could lead to ulcerative colitis. Autoimmunity may also play an important role in the etiology of ulcerative colitis. [4] [5]
Some evidence suggests that smoking may be protective, but so far, no one has been able to confirm a direct relationship between the two.
- Epidemiology
Worldwide, the highest incidence and prevalence of inflammatory bowel diseases are seen in Northern Europe and North America. Inflammatory bowel disease is closely linked to a Westernized environment and lifestyle. Ulcerative colitis has an incidence of 9 to 20 cases per 100,000 persons per year. Its prevalence is 156 to 291 cases per 100,000 persons per year. Compared to Crohn disease, ulcerative colitis has a greater prevalence in adults. When considering the pediatric population, however, ulcerative colitis is less prevalent than Crohn disease.
Ulcerative colitis has a bimodal pattern of incidence. The main onset peaks between the age of 15 and 30 years. A second, and the smaller peak of incidence occurs between the age of 50 and 70 years. Though some studies show a slight predilection for men, most studies note no preference regarding sex. There is an increased prevalence of ulcerative colitis in nonsmokers or those who recently quit smoking. Additionally, smokers diagnosed with ulcerative colitis tend to have milder disease, fewer hospitalizations and need for less medication. There is evidence, though weak, that non-steroidal anti-inflammatory drug use is associated with the onset or relapse of ulcerative colitis.
There is also an association of inflammatory bowel disease with the removal of an inflamed appendix. Appendectomy before the age of twenty is associated with a decreased incidence of ulcerative colitis, whereas the opposite is true for Crohn disease. In fact, appendectomy has been shown to reduce the risk of developing ulcerative colitis by 69%. [6] [7]
- Pathophysiology
The pathophysiology of ulcerative colitis involves defects in the epithelial barrier, immune response, leukocyte recruitment, and microflora of the colon. [8] [9]
The epithelial barrier has a defect in colonic mucin and possibly tight junctions, leading to increased uptake of luminal antigens. The lamina propria of the mucosa also has an increased number of activated and mature dendritic cells, which include a large number of toll-like receptors (TLR), specifically TLR2 and TLR4. There also seems to be an atypical T-helper (Th) cell response in patients with ulcerative colitis, specifically Th2, which exerts a cytotoxic response against epithelial cells. Other immune-related factors that play a role in the pathophysiology of ulcerative colitis include tumor necrosis factor-alpha (TNF-alpha), interleukin 13, and natural killer T-cells. Levels of IgM, IgA, and IgG are elevated in inflammatory bowel disease; however, a disproportionate increase in IgG1 antibodies is found in patients diagnosed with ulcerative colitis.
Leukocyte recruitment is affected on two fronts. There is an upregulated release of the chemoattractant CXCL8 in ulcerative colitis so that leukocytes are recruited to the mucosa from systemic circulation. Additionally, there is an upregulation of mucosal cellular adhesion molecule-1 (Mad-CAM1) on the endothelium of mucosal blood vessels, which promotes leukocyte adhesion and extravasation into mucosal tissue.
Studies have shown that enteric microflora is important in the pathogenesis and severity of inflammation and disease phenotype. Ulcerative colitis seems also to result, in part, from a homeostatic imbalance between enteric microflora and the host's mucosal immunity. This results in an aberrant response to non-pathogenic bacteria.
- Histopathology
Histologically, the mucosal layer of the colon in a patient with ulcerative colitis includes infiltrates of varying density and composition, depending on the stage of the disease. These infiltrates primarily consist of lymphocytes, plasma cells, and granulocytes, with the latter being more prominent during acute flares of the disease. Additionally, goblet cell depletion, ulcerations, and alterations in mucosal crypts are sometimes apparent. These findings might also be present on histological evaluation of the distal ileum, even though ulcerative colitis is a disease confined to the colon. This is backwash ileitis.
- History and Physical
The main symptom of ulcerative colitis is bloody diarrhea, with or without mucus. Associated symptoms also include urgency or tenesmus, abdominal pain, malaise, weight loss, and fever, depending on the extent and severity of the disease. The onset of the disease is typically gradual, and patients will likely experience periods of spontaneous remission and subsequent relapses. Factors that typically exacerbate ulcerative colitis include smoking cessation and nonsteroidal anti-inflammatory drug use.
There are some extraintestinal manifestations (EIMs) that are also present in 10% to 30% of patients with ulcerative colitis. Extraintestinal manifestations associated with disease activity include episcleritis, scleritis, uveitis, peripheral arthropathies, erythema nodosum, and pyoderma gangrenosum. Extraintestinal manifestations independent of colitis activity include axial arthropathies, sacroiliitis, and ankylosing spondylitis. A significant hepatic extraintestinal manifestation of ulcerative colitis includes primary sclerosing cholangitis and is associated with a greater risk of colorectal cancer.
Diagnosis of ulcerative colitis is made clinically with supportive findings on endoscopy, biopsy, and by negative stool examination for infectious causes. Because colonic infection can produce clinical findings indistinguishable from idiopathic ulcerative colitis, microbiologic studies for bacterial infection and parasitic infestation should be included in the initial evaluation. [10] [11]
Radiologic examinations are not critical for the diagnosis but may be useful. Patients with longstanding ulcerative colitis may show a "stove-pipe" sign on double-contrast barium enema (DCBE).
Colonoscopy or proctosigmoidoscopy might reveal loss of typical vascular pattern, granularity, friability, and ulceration, which involve the distal rectum and proceed proximally in a symmetric, continuous, and circumferential pattern. The disease can range from isolated to the rectum and sigmoid colon (proctitis) to disease of the entire colon (pancolitis). Population-based studies show that, upon presentation, proctitis is found in 30% to 60% of patients, left-sided colitis is found in 16% to 45%, and pancolitis is found in 14% to 35%.
Laboratory evaluation will usually reveal an increase in inflammatory factors (ESR, CRP, leukocytosis), especially during an acute flare. Regardless of disease stage, 60% to 70% of ulcerative colitis patients are positive for perinuclear antineutrophil cytoplasmic antibodies (P-ANCA). P-ANCA is also found in a small number of patients with Crohn disease. In addition to P-ANCA, anti-saccharomyces cerevisiae antibodies (ASCA) are found in both Crohn disease and ulcerative colitis but are more prevalent in Crohn disease; therefore, testing for both P-ANCA and ASCA has some utility in distinguishing types. Testing for carcinoembryonic antigen (CEA) can also be helpful in ulcerative colitis, as higher levels can indicate a flare.
Testing for fecal calprotectin also has some utility in the diagnosis of ulcerative colitis, though it is nonspecific. Fecal calprotectin correlates with increased neutrophils in the intestine and, therefore, can be helpful in ruling out inflammatory bowel disease. Studies show that less than 1% of patients with low fecal calprotectin are likely to suffer from inflammatory bowel disease.
An endoscopy (colonoscopy) must be done at some point which will reveal:
- Fragile mucosa
- Granular mucosa
- Loss of vascular pattern
- Presence of erosions and pseudopolyposis
Multiple biopsies should be obtained to confirm the diagnosis.
When a diagnosis of ulcerative colitis is made, the most common classification system used to determine the extent and severity of the disease is the Montreal classification system. Extent (E) is determined by endoscopic evaluation and includes E1 (Proctitis), E2 (left-sided or distal colitis), and E3 (pancolitis). Symptoms and systemic findings determine severity (S). It ranges from S0 (remission) to S3 (severe).
- Treatment / Management
Treatment choice for patients with ulcerative colitis is based on both the extent of the disease and the severity. The prognosis during the first decade after diagnosis is often generally good, and most patients go into remission. Rectal application of medical therapy, via suppository or enema, is usually appropriate for isolated distal disease (proctitis); however, a rectal application is usually used in combination with systemic therapy to help target the distal colon and, therefore, decrease tenesmus. [6] [12] [13]
First-line treatment is sulfasalazine and 5-aminosalicylates, given orally or rectally, which have a remission rate of about 50%. Glucocorticoids, orally or rectally, can be added for those who fail to achieve remission within two weeks. Except for glucocorticoids, all of these medications can be used in the maintenance of remission. Additionally, there is some evidence that probiotics are helpful in attaining remission. Fecal microbiota transplantation also shows promise in the treatment of ulcerative colitis to help establish healthy gut microbiota.
If patients are refractory to glucocorticoids, thiopurines or biological drugs can be added to therapy. Thiopurines are immunosuppressants such as azathioprine or 6-mercaptopurine. Biological drugs include anti-TNF-alpha drugs, such as infliximab, adalimumab, and golimumab. Infliximab is the most widely used for ulcerative colitis and can be used in severe cases during hospital admissions. The newest class of biological drugs are anti-adhesion molecule inhibitors, such as vedolizumab, which blocks alpha-4-beta-7 integrin.
Since patients with ulcerative colitis have reduced expression of peroxisome proliferator-activated receptor-gamma (PPAR-gamma) in their colonocytes, future treatment may include (PPAR-gamma) agonistic activity. PPAR-gamma is a negative regulator of NF-KB-dependent inflammation. Novel 5-aminosalicylic acid (5-ASA) analogs are being developed that have greater PPAR-gamma activity. Cardiotoxicity and metabolic toxicity restrict the use of existing PPAR-gamma agonists.
Colectomy is curative in patients with ulcerative colitis since the disease is restricted to the colon. Indications for surgery are a failure of medical therapy, intractable fulminant colitis, toxic megacolon, perforation, uncontrollable bleeding, intolerable side effects of medications, strictures, unresectable high-grade or multifocal dysplasia, cancer, or growth retardation in children. The procedure of choice is proctocolectomy with ileal pouch-anal anastomosis (IPAA); however, in patients who are ineligible for IPAA, proctocolectomy with ileostomy is a viable alternative.
Because of the risk of colon cancer, colonoscopy is recommended at regular intervals.
All patients need maintenance therapy to prevent relapse. Oral aminosalicylates are the drugs of choice, but others may respond to azathioprine and 6-mercaptopurine.
There is no specific diet for patients with ulcerative colitis, but many develop lactose intolerance. Unlike Crohn disease, there is no role for elemental or parenteral nutrition.
- Differential Diagnosis
In patients presenting with lower abdominal pain and bloody diarrhea, the following differentials should be considered:
- Crohn disease
- Parasitic colitis
- Tuberculosis
- Radiation colitis
- Colon cancer
- Toxic megacolon
- Bacterial/viral gastroenteritis
The severity of ulcerative colitis is graded from mild to severe depending upon rectal bleeding:
- Mild: Fewer than four rectal bleeding episodes per day
- Moderate: More than four rectal bleeding episodes per day
- Severe: More than four rectal bleeding episodes per day, and the patient has systemic features of an illness combined with hypoalbuminemia
Ulcerative colitis is a lifelong illness, but the overall mortality is not greater than in the general population. However, mortality is increased in patients who develop shock and surgical complications. When the muscularis propria is involved, it may damage the nerves resulting in dilatation, aperistalsis, and ischemia (toxic megacolon). Today, toxic megacolon is the most common cause of death in ulcerative colitis. At least 5% of patients develop colon cancer, and this risk increases with the duration of the disease. Unlike Crohn disease, stricture formation is rare.
- Complications
Ulcerative colitis is a lifelong disease with periods of remission and relapse. Following complications can occur in patients suffering from ulcerative colitis:
- Leak from anastomosis
- Pelvic abscess
- Enterocutaneous fistulas
- Pouch prolapse,
- Pouchitis, acute is less than 4 weeks, chronic is more than 4 weeks
- Incontinence
- Sexual dysfunction
- Toxic megacolon
- Colon/rectal cancer
- Deterrence and Patient Education
American College of Gastroenterology has made guidelines on preventive care in patients with ulcerative colitis. These recommendations include:
- Screening for skin malignancies, irrespective of the use of biological agents
- Assessing bone mineral density
- Be vaccinated against Herpes zoster
- Vaccinated against Pneumococcus, H. influenzae , and the flu virus
- Should not travel to areas of yellow fever without first consulting with an infectious disease expert
- Be screened for depression and anxiety
- Women with ulcerative colitis should get annual cervical cancer screening
- Pearls and Other Issues
There is an increased risk of colorectal cancer in patients with ulcerative colitis. The risk is cumulative, with a 2% chance of colorectal cancer after ten years of diagnosis, 8% after 20 years, and 20% to 30% after 30 years. Two factors associated with increased risk of colorectal cancer are the duration and extent of the disease.
- Enhancing Healthcare Team Outcomes
Ulcerative colitis is a systemic disorder with no cure. The disorder has numerous extraintestinal involvement in addition to the colon. Thus, it is best managed by an interprofessional team. All patients with the disorder need lifelong monitoring. Because of the risk of colorectal cancer, surveillance colonoscopy should occur every 1-2 years. Further, since patients are often treated with biological agents, they need to undergo screening for melanoma and nonmelanoma skin cancer.
The pharmacists should assist the team by educating the patient on the importance of medication compliance to avoid relapse. The nurse should encourage regular vaccinations, hand washing, and cancer screening. A dietary consult should be obtained to educate the patient on foods to eat and what not to eat, especially if they have a stoma. In addition, a stoma nurse should be involved in the teaching of stoma care.
An infectious disease nurse should monitor the patient in the outpatient setting to ensure that they are not immunocompromised. Social workers should be involved to ensure that the patient has ample support and finances so that the treatments are not missed. Patients with risk factors for osteoporosis need screening for bone mineral density periodically. Patients should be encouraged to undergo annual vaccination against influenza and pneumococcus. Finally, many patients with ulcerative colitis develop depression and anxiety and should be referred to a mental health counselor. [14] [15]
Ulcerative colitis has no cure, and despite treatment, many continue to have increased bouts of stool frequency. An increase in mortality is usually seen in older patients, those with complications like infection, shock, and anemia, and those who require repeated surgical interventions. Data show that when the disease involves the muscularis propria, it can lead to bowel dysmotility, necrosis, and gangrene. A certain number of patients also develop toxic megacolon with poor outcomes. It is estimated that about 5% of patients will develop colorectal cancer over time. The risk of colon cancer is higher in patients with pancolitis and in patients whose disease started before the age of 15. Overall, the quality of life in patients with ulcerative colitis is poor. [16] [17] [Level 5]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Whitney Lynch declares no relevant financial relationships with ineligible companies.
Disclosure: Ronald Hsu declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Lynch WD, Hsu R. Ulcerative Colitis. [Updated 2023 Jun 5]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Review A comprehensive review and update on ulcerative colitis(). [Dis Mon. 2019] Review A comprehensive review and update on ulcerative colitis(). Gajendran M, Loganathan P, Jimenez G, Catinella AP, Ng N, Umapathy C, Ziade N, Hashash JG. Dis Mon. 2019 Dec; 65(12):100851. Epub 2019 Mar 2.
- Myositis as an Extraintestinal Manifestation of Ulcerative Colitis: A Case Report and Literature Review. [Cureus. 2023] Myositis as an Extraintestinal Manifestation of Ulcerative Colitis: A Case Report and Literature Review. Nagi TK, Gheit Y, Hernandez OL, Suarez ZK, Vallejo C, Haider MA, Zahra T. Cureus. 2023 Jul; 15(7):e42336. Epub 2023 Jul 23.
- Review Ulcerative colitis. [Lancet. 2012] Review Ulcerative colitis. Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Lancet. 2012 Nov 3; 380(9853):1606-19. Epub 2012 Aug 20.
- Review Inflammatory disease of the colon: ulcerative colitis and Crohn's colitis. [J Pediatr. 1975] Review Inflammatory disease of the colon: ulcerative colitis and Crohn's colitis. Ament ME. J Pediatr. 1975 Mar; 86(3):322-34.
- The Impact of Inflammatory Bowel Disease in Canada 2018: Indirect Costs of IBD Care. [J Can Assoc Gastroenterol. 2019] The Impact of Inflammatory Bowel Disease in Canada 2018: Indirect Costs of IBD Care. Kuenzig ME, Lee L, El-Matary W, Weizman AV, Benchimol EI, Kaplan GG, Nguyen GC, Bernstein CN, Bitton A, Lee K, et al. J Can Assoc Gastroenterol. 2019 Feb; 2(Suppl 1):S34-S41. Epub 2018 Nov 2.

Recent Activity
- Ulcerative Colitis - StatPearls Ulcerative Colitis - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

- Earn HPCSA and SACNASP CPD Points
- I have spots and my skin burns
- A case of a 10 year old boy with a 3 week history of diarrhoea, vomiting and cough
- A case of fever and general malaise
- A case of persistant hectic fever
- A case of sudden rapid neurological deterioration in an HIV positive 27 year old female
- A case of swollen hands
- An unusual cause of fulminant hepatitis
- Case of a right axillary swelling
- Case of giant wart
- Case of recurrent meningitis
- Case of repeated apnoea and infections in a premature infant
- Case of sudden onset of fever, rash and neck pain
- Doctor, my sister is confused
- Eight month old boy with recurrent infections
- Enlarged Testicles
- Failure to thrive despite appropriate treatment
- Right Axillary Swelling
- Severe anaemia in HIV positive child
- The case of a floppy infant
- Two year old with spiking fevers and depressed level of consciousness
- 17 year old male with fever and decreased level of consciousness
- 3 TB Vignettes
- A 10 year old girl with a hard palate defect
- A case of decreased joint function, fever and rash
- Keep up while the storm is raging
- Fireworks of autoimmunity from birth
- My hands swell and are painful
- My eyes cross at twilight
- A case of a 3 month old infant with bloody urine and stools
- A case of scaly annular plaques
- Case of eye injury and decreased vision
- My head hurts and I cannot speak?
- TB or not TB: a confusing case
- A 7 year old with severe muscle weakness and difficulty walking
- Why can I not walk today?
- 14 year old with severe hip pain
- A 9 year old girl presents with body swelling, shortness of breath and backache
- A sudden turn of events after successful therapy
- Declining CD4 count, despite viral suppression?
- Defaulted treatment
- 25 year old female presents with persistent flu-like symptoms
A case of persistent bloody diarrhoea
- I’ve been coughing for so long
- A case of acute fever, rash and vomiting
- Adverse event following routine vaccination
- A case of cough, wasting and lymphadenopathy
- A case of lymphadenopathy and night sweats
- Case of enlarged hard tongue
- A high risk pregnancy
- A four year old with immunodeficiency
- Young girl with recurrent history of mycobacterial disease
- Immunodeficiency and failure to thrive
- Case of recurrent infections
- An 8 year old boy with recurrent respiratory infections
- 4 year old boy with recurrent bacterial infections
- Is this treatment failure or malnutrition
- 1. A Snapshot of the Immune System
- 2. Ontogeny of the Immune System
- 3. The Innate Immune System
- 4. MHC & Antigen Presentation
- 5. Overview of T Cell Subsets
- 6. Thymic T Cell Development
- 7. gamma/delta T Cells
- 8. B Cell Activation and Plasma Cell Differentiation
- 9. Antibody Structure and Classes
- 10. Central and Peripheral Tolerance
- Immuno-Mexico 2024 Introduction
- Modulation of Peripheral Tolerance
- Metabolic Adaptation to Pathologic Milieu
- T Cell Exhaustion
- Suppression in the Context of Disease
- Redirecting Cytotoxicity
- Novel Therapeutic Strategies
- ImmunoInformatics
- Grant Writing
- Introduction to Immuno-Chile 2023
- Core Modules
- Gut Mucosal Immunity
- The Microbiome
- Gut Inflammation
- Viral Infections and Mucosal Immunity
- Colorectal Cancer
- Inflammatory Bowel Disease
- Equity, Diversity, Inclusion in Academia
- Immuno-India 2023 Introduction
- Principles of Epigenetic Regulation
- Epigenetics Research in Systems Immunology
- Epigenetic (De)regulation in Non-Malignant Diseases
- Epigenetic (De)regulation in Immunodeficiency and Malignant Diseases
- Immunometabolism and Therapeutic Applications of Epigenetic Modifiers
- Immuno-Morocco 2023 Introduction
- Cancer Cellular Therapies
- Cancer Antibody Therapies
- Cancer Vaccines
- Immunobiology of Leukemia & Therapies
- Immune Landscape of the Tumour
- Targeting the Tumour Microenvironment
- Flow Cytometry
- Immuno-Zambia 2022 Introduction
- Immunity to Viral Infections
- Immunity to SARS-CoV2
- Basic Immunology of HIV
- Immunity to Tuberculosis
- Immunity to Malaria
- Immunity to Schistosomiasis
- Immunity to Helminths
- Equity, Diversity and Inclusion in Academia
- Immuno-Argentina 2022 Introduction
- Dendritic Cells
- Trained Innate Immunity
- Gamma-Delta T cells
- Natural Killer Cell Memory
- Innate Immunity in Viral Infections
- Lectures – Innate Immunity
- T cells and Beyond
- Lectures – Cellular Immunity
- Strategies for Vaccine Design
- Lectures – Humoral Immunity
- Lectures – Vaccine development
- Lectures – Panel and Posters
- Immuno-Cuba 2022 Introduction
- Poster and Abstract Examples
- Immuno-Tunisia 2021 Introduction
- Basics of Anti-infectious Immunity
- Inborn Errors of Immunity and Infections
- Infection and Auto-Immunity
- Pathogen-Induced Immune Dysregulation & Cancer
- Understanding of Host-Pathogen Interaction & Applications (SARS-CoV-2)
- Day 1 – Basics of Anti-infectious Immunity
- Day 2 – Inborn Errors of Immunity and Infections
- Day 3 – Infection and Auto-immunity
- Day 4 – Pathogen-induced Immune Dysregulation and Cancer
- Day 5 – Understanding of Host-Pathogen Interaction and Applications
- Student Presentations
- Roundtable Discussions
- Orientation Meeting
- Poster Information
- Immuno-Colombia Introduction
- Core Modules Meeting
- Overview of Immunotherapy
- Check-Points Blockade Based Therapies
- Cancer Immunotherapy with γδ T cells
- CAR-T, armored CARs and CAR-NK therapies
- Anti-cytokines Therapies
- Tumor-infiltrating Lymphocytes (TIL)
- MDSC Promote Tumor Growth and Escape
- Immunological lab methods for patient’s follow-up
- Student Orientation Meeting
- Lectures – Week 1
- Lectures – Week 2
- Research Project
- Closing and Social
- Introduction to Immuno-Algeria 2020
- Hypersensitivity Reactions
- Immuno-Algeria Programme
- Online Lectures – Week 1
- Online Lectures – Week 2
- Student Presentations – Week 1
- Student Presentations – Week 2
- Introduction to Immuno-Ethiopia 2020
- Neutrophils
- Leishmaniasis – Transmission and Epidemiology
- Leishmaniasis – Immune Responses
- Leishmaniasis – Treatment and Vaccines
- Immunity to Helminth Infections
- Helminth immunomodulation on co-infections
- Malaria Vaccine Progress
- Immunity to Fungal Infections
- How to be successful scientist
- How to prepare a good academic CV
- Introduction to Immuno-Benin
- Immune Regulation in Pregnancy
- Immunity in infants and consequence of preeclampsia
- Schistosome infections and impact on Pregnancy
- Infant Immunity and Vaccines
- Regulation of Immunity & the Microbiome
- TGF-beta superfamily in infections and diseases
- Infectious Diseases in the Global Health era
- Immunity to Toxoplasma gondii
- A. melegueta inhibits inflammatory responses during Helminth Infections
- Host immune modulation by Helminth-induced products
- Immunity to HIV
- Immunity to Ebola
- Immunity to TB
- Genetic susceptibility in Tuberculosis
- Plant Extract Treatment for Diabetes
- Introduction to Immuno-South Africa 2019
- Models for Testing Vaccines
- Immune Responses to Vaccination
- IDA 2019 Quiz
- Introduction to Immuno-Jaipur
- Inflammation and autoinflammation
- Central and Peripheral Tolerance
- Autoimmunity and Chronic Inflammatory Diseases
- Autoimmunity & Dysregulation
- Novel Therapeutic strategies for Autoimmune Diseases
- Strategies to apply gamma/delta T cells for Immunotherapy
- Immune Responses to Cancer
- Tumour Microenvironment
- Cancer Immunotherapy
- Origin and perspectives of CAR T cells
- Metabolic checkpoints regulating immune responses
- Transplantation
- Primary Immunodeficiencies
- Growing up with Herpes virus
- Introduction to IUIS-ALAI-Mexico-ImmunoInformatics
- Introduction to Immunization Strategies
- Introduction to Immunoinformatics
- Omics Technologies
- Computational Modeling
- Machine Learning Methods
- Introduction to Immuno-Kenya
- Viruses hijacking host immune responses
- IFNs as 1st responders to virus infections
- HBV/HCV & Hepatocellular Carcinoma
- Cytokines as biomarkers for HCV
- HTLV & T cell Leukemia
- HCMV and Cancers
- HPV and Cancers
- EBV-induced Oncogenesis
- Adenoviruses
- KSHV and HIV
- Ethics in Cancer Research
- Sex and gender in Immunity
- Introduction to Immuno-Iran
- Immunity to Leishmaniasis
- Breaking Tolerance: Autoimmunity & Dysregulation
- Introduction to Immuno-Morocco
- Cancer Epidemiology and Aetiology
- Pathogens and Cancer
- Immunodeficiency and Cancer
- Introduction to Immuno-Brazil
- 1. Systems Vaccinology
- 2. Vaccine Development
- 3. Adjuvants
- 4. DNA Vaccines
- 5. Mucosal Vaccines
- 6. Vaccines for Neurodegenerative Diseases
- Introduction to Immuno-Gambia
- Immuno-Gambia Photos
- 1. Infant Immunity and Vaccines
- 2. Dendritic Cells
- 3. Conventional T Cells
- 4. gamma/delta T Cells
- 5. Immunity to Viral Infections
- 6. Immunity to Helminth Infections
- 7. Immunity to TB
- 8. Immunity to Malaria
- 9. Flow Cytometry
- Introduction to Immuno-South Africa
- 1. Introduction to Immunization Strategies
- 2. Immune Responses to Vaccination
- 3. Models for Testing Vaccines
- 4. Immune Escape
- 5. Grant Writing
- Introduction to Immuno-Ethiopia
- 1. Neutrophils
- 3. Exosomes
- 5. Immunity to Leishmania
- 6. Immunity to HIV
- 7. Immunity to Helminth Infections
- 8. Immunity to TB
- 9. Grant Writing
- Introduction to ONCOIMMUNOLOGY-MEXICO
- ONCOIMMUNOLOGY-MEXICO Photos
- 1. Cancer Epidemiology and Etiology
- 2. T lymphocyte mediated immunity
- 3. Immune Responses to Cancer
- 4. Cancer Stem Cells and Tumor-initiating cells.
- 5. Tumor Microenvironment
- 6. Pathogens and Cancer
- 7. Cancer Immunotherapy
- 8. Flow cytometry approaches in cancer
- Introduction to the Immunology Course
- Immuno-Tunisia Photo
- 1. Overview of the Immune System
- 2. Role of cytokines in Immunity
- 3. Tolerance and autoimmunity
- 4. Genetics, Epigenetics and immunoregulation
- 5. Microbes and immunoregulation
- 6. Inflammation and autoinflammation
- 7. T cell mediated autoimmune diseases
- 8. Antibody-mediated autoimmune diseases
- Introduction to the Immunology Symposium
- Immuno-South Africa Photo
- 1. Antibody Generation by B cells
- 2. Mucosal Immunity
- 3. Immunity to TB
- 4. Immunity to Malaria
- 5. Immunity to HIV
- 6. Defining a Biomarker
- 7. Grant Writing Exercise
- Immuno-Colombia Photo
- 1. Overview of Complement
- 2. Transplantation
- 3. Immune Regulation in Pregnancy
- 4. Breaking Tolerance: Autoimmunity & Dysregulation
- 5. Mucosal Immunity & Immunopathology
- 6. Regulation of Immunity & the Microbiome
- 7. Epigenetics & Modulation of Immunity
- 8. Primary Immunodeficiencies
- 9. Anti-tumour Immunity
- 10. Cancer Immunotherapy
- Introduction
- Immune Cells
- NCDs and Multimorbidity
- Mosquito Vector Biology
- Vaccines and Other Interventions
- Autoimmunity
- Career Development
- SUN Honours Introduction
- A Snapshot of the Immune System
- Ontogeny of the Immune System
- The Innate Immune System
- MHC & Antigen Presentation
- Overview of T Cell Subsets
- B Cell Activation and Plasma Cell Differentiation
- Antibody Structure and Classes
- Cellular Immunity and Immunological Memory
- Infectious Diseases Immunology
- Vaccinology
- Mucosal Immunity & Immunopathology
- Central & Peripheral Tolerance
- Epigenetics & Modulation of Immunity
- T cell and Ab-mediated autoimmune diseases
- Immunology of COVID-19 Vaccines
- 11th IDA 2022 Introduction
- Immunity to COVID-19
- Fundamentals of Immunology
- Fundamentals of Infection
- Integrating Immunology & Infection
- Infectious Diseases Symposium
- EULAR Symposium
- Thymic T Cell Development
- Immune Escape
- Genetics, Epigenetics and immunoregulation
- AfriBop 2021 Introduction
- Adaptive Immunity
- Fundamentals of Infection 2
- Fundamentals of Infection 3
- Host pathogen Interaction 1
- Host pathogen Interaction 2
- Student 3 minute Presentations
- 10th IDA 2021 Introduction
- Day 1 – Lectures
- Day 2 – Lectures
- Day 3 – Lectures
- Day 4 – Lectures
- Afribop 2020 Introduction
- WT PhD School Lectures 1
- EULAR symposium
- WT PhD School Lectures 2
- Host pathogen interaction 1
- Host pathogen interaction 2
- Bioinformatics
- Introduction to VACFA Vaccinology 2020
- Overview of Vaccinology
- Basic Principles of Immunity
- Adverse Events Following Immunization
- Targeted Immunization
- Challenges Facing Vaccination
- Vaccine Stakeholders
- Vaccination Questions Answered
- Malaria Vaccines
- IDA 2018 Introduction
- Vaccine Development
- Immune Escape by Pathogens
- Immunity to Viral Infections Introduction
- Flu, Ebola & SARS
- Antiretroviral Drug Treatments
- Responsible Conduct in Research
- Methods for Enhancing Reproducibility
- 6. B Cell Activation and Plasma Cell Differentiation
- 7. Antibody Structure and Classes
- CD Nomenclature
- 1. Transplantation
- 2. Central & Peripheral Tolerance
- 8. Inflammation and autoinflammation
- 9. T cell mediated autoimmune diseases
- 10. Antibody-mediated autoimmune diseases
- 1. Primary Immunodeficiencies
- Cancer Stem Cells and Tumour-initiating Cells
- 6. Tolerance and Autoimmunity
- Discovery of the Thymus as a central immunological organ
- History of Immune Response
- History of Immunoglobulin molecules
- History of MHC – 1901 – 1970
- History of MHC – 1971 – 2011
- SAIS/Immunopaedia Webinars 2022
- Metabolic control of T cell differentiation during immune responses to cancer
- Microbiome control of host immunity
- Shaping of anti-tumor immunity in the tumor microenvironment
- The unusual COVID-19 pandemic: the African story
- Immune responses to SARS-CoV-2
- Adaptive Immunity and Immune Memory to SARS-CoV-2 after COVID-19
- HIV prevention- antibodies and vaccine development (part 2)
- HIV prevention- antibodies and vaccine development (part 1)
- Immunopathology of COVID 19 lessons from pregnancy and from ageing
- Clinical representation of hyperinflammation
- In-depth characterisation of immune cells in Ebola virus
- Getting to the “bottom” of arthritis
- Immunoregulation and the tumor microenvironment
- Harnessing innate immunity from cancer therapy to COVID-19
- Flynn Webinar: Immune features associated natural infection
- Flynn Webinar: What immune cells play a role in protection against M.tb re-infection?
- JoAnne Flynn: BCG IV vaccination induces sterilising M.tb immunity
- IUIS-Immunopaedia-Frontiers Webinar on Immunology taught by P. falciparum
- COVID-19 Cytokine Storm & Paediatric COVID-19
- Immunothrombosis & COVID-19
- Severe vs mild COVID-19 immunity and Nicotinamide pathway
- BCG & COVID-19
- COVID-19 Vaccines
- Antibody responses and serology testing
- Flow Cytometry Part 1
- Flow Cytometry Part 2
- Flow Cytometry Part 3
- Lateral Flow
- Diagnostic Tools
- Diagnostic Tests
- HIV Life Cycle
- ARV Drug Information
- ARV Mode of Action
- ARV Drug Resistance
- Declining CD4 count
- Ambassador of the Month – 2024
- North America
- South America
- Ambassador of the Month – 2023
- Ambassador of the Month – 2022
- The Day of Immunology 2022
- AMBASSADOR SCI-TALKS
- The Day of Immunology 2021
- Ambassador of the Month – 2021
- Ambassador of the Month-2020
- Ambassador of the Month – 2019
- Ambassador of the Month – 2018
- Ambassador of the Month – 2017
- Host an IUIS Course in 2025
- Immuno-South Africa 2024
- COLLABORATIONS
We will not share your details

Patient presentation
Differential diagnosis, examination, investigations, special investigations, final outcome.
Patient is a 22 year old female who presented to the surgery department of a tertiary level hospital having been referred from a private clinic, with a two month history of severe abdominal cramps, persistent bloody and mucoid diarrhoea, weight loss and tiredness.
Acknowledgement This case study was kindly provided by Dr Monica Mercer from Immunopaedia
2 months ago: Symptoms began with abdominal cramps and an intense urge to pass stool after every meal. Her symptoms rapidly worsened with passage of stool becoming more frequent. Within two days she was passing persistently watery diarrhoea mixed with fresh blood and mucous. She was seen by her general practitioner who treated her for gastritis.
One week later she collapsed at home and was admitted to hospital for investigations. She was discharged two days later without a diagnosis.
1 month ago: Symptoms persisted and she experienced diarrhoea and vomiting after eating or drinking, which lasted for 10 days. She was admitted to hospital for rehydration and further investigations. No conclusive diagnosis was made.
Currently: Patient is passing 10-20 liquid stools per day. Diarrhoea is mucoid and bloody. Occurs day and night. Patient complains of malaise, lethargy and anorexia. She has lost 8 kg in the past 2 months.
No past surgical history No significant medical history
Family history: Mother – type 2 Diabetes Mellitus No other family members with chronic disease
No known allergies
- Cryptosporidium,
- Shigella,
- salmonella,
- E.coli,
- Campylobacter,
- Clostridium difficile
- If HIV positive consider- MAC, Isospera beli, cryptosporidium, TB
- Functional bowel syndromes e.g. irritable bowel syndrome (IBS)
- Malabsorbtion
- Coeliac disease
- Inflammatory bowel disease (IBD)
Thin ill looking young woman, conscious and alert, in obvious discomfort.
Vitals : Heart rate: 80bpm Respiratory rate: 18 bpm Blood pressure: 120/70 Temperature: 37˚C
Pale mucous membranes
Abdominal examination: Guarding and tenderness noted in the left iliac fossa and hypogastrium.
No results available from previous admissions. All results are from current admission.
Abdominal X-ray: No toxic megacolon
Gastroscopy Report: Oesophagus and gastro- oesopahageal junction were normal. Stomach mucosa was intact and normal. No gastritis, ulceration or blood was noted. Cardia was normal. Pylorus and duodenum normal.
Colonoscopy report: Very friable mucosa. Extensive ulceration with pseudopolyps, involving the rectum, entire sigmoid and left colon up to the transverse colon. Multiple biopsies of the colonic tissue were taken for histological analysis.
Histological Findings: Pathology is limited to the mucosa and submucosa. Intense infiltration of the mucosa and submucosa with neutrophils and crypt abscesses, lamina propria with lymphoid aggregates, plasma cells, mast cells and eosinophils, and shortening and branching of the crypts.
What is the Diagnosis?
Ulcerative Colitis, which is a chronic disease associated with diffuse mucosal inflammation of the colon, giving rise to significant morbidity and recurrent symptoms of intermittent bloody diarrhea, rectal urgency and tenesmus. Patients also present with fever, anemia, fatigue, weight loss, loss of appetite, loss of body fluids and nutrients, skin lesions, joint pain, and failure to grow. The latter is specifically seen in children. About half of the people diagnosed with ulcerative colitis have mild symptoms (Ulcerative colitis, no date). Onset of symptoms typically occurs between 15 and 40 years of age, with a second peak in incidence between 50 and 80 years of age.
Ulcerative colitis is closely related to another inflammatory intestinal condition called Crohn’s disease, which can lead to chronic inflammation in any part of the gastrointestinal tract. Together, these two conditions are collectively referred to as inflammatory bowel disease, or IBD. (Ulcerative colitis, no date).
Men and women are equally likely to develop ulcerative colitis. Extraintestinal manifestations may occur in up to 25% of patients. These include osteoporosis in 15%, oral ulcerations in 10%, arthritis in 5% to 10%, primary sclerosing cholangitis in 3%, uveitis in 0.5% to 3%, pyoderma gangrenosum in 0.5% to 2.0%, deep venous thrombosis in 0.3% and pulmonary embolism in 0.2%. Current cigarette smoking is associated with a reduction in the risk for ulcerative colitis, but former smokers have a higher risk of developing ulcerative colitis vs never smokers. Although the exact cause of ulcerative colitis is still unknown, there is strong evidence that primary dysregulation of the mucosal immune system causes an excessive immunologic response to normal microflora. Other contributing factors to ulcerative colitis are taken to be both genetic and environmental in nature (Richards, 2019).
What is the pathogenesis of ulcerative colitis?
What gene associations occur in ulcerative colitis?
The array of genetic polymorphisms associated with UC would point to the likelihood of abnormalities in the epithelial barrier contributing to the onset of this condition, one hypothesis supporting the presence of an epithelial cell defect that initiates the disease under pressure from the colonic microbiome (figure 4) (Fuss and Strober, 2015).
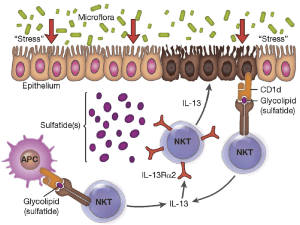
Figure 4:” Proposed mechanism of immune-mediated inflammation in UC. Inflammation in UC is initiated by release of glycolipid antigen(s) arising from genetically impaired epithelial cells under stress from exposure to components of the gut microbiome. These antigens are presented to and stimulate NK T cells in the context of CD1 on the surface of epithelial cells or on lamina propria dendritic cells. The NK T cells so stimulated cause epithelial cell damage by direct cytotoxic activity via interaction with CD1d loaded with glycolipid on the epithelial cell surface. Alternatively, the NK T cells cause epithelial apoptosis by release of IL-13 that then causes epithelial damage. Interleukin-13 also enhances inflammation by interacting with IL-13Rα2 on NK T cells, thereby inducing further NK T cell cytotoxic activity. Finally, epithelial ulceration resulting from these processes allows entry of bacterial components into the lamina propria that stimulates secondary inflammatory reactions.” Source: (Fuss and Strober, 2015)
What are Peyer’s patches?
Peyer’s patches are aggregations of lymphoid tissue, made up of lymphoid follicles located in the lamina propria of the mucosa. In adults, B lymphocytes are seen to predominate in the follicles’ germinal centers. T lymphocytes are found in the zones between follicles. With the lumen exposed to to the external environment, there are large numbers of potentially pathogenic microorganisms present. Peyer’s patches therefore carry out immune surveillance containing macrophages, dendritic cells, B-lymphocytes, and T-lymphocytes. The lymphoid tissue is covered by a special epithelium that contains specialized cells called M cells which sample antigen directly from the lumen and deliver it to antigen-presenting cells. These cells then pass to the mesenteric lymph nodes where the immune response is amplified.
How do you grade the severity of the disease?
Ulcerative Colits disease severity (based on Truelove and Witt classification):
- Symptoms Mild Severe Fulminant
- Stools per day 6 >10
- Hematochaezia Intermittent Frequent Continuous
- Temperature Normal >37.5 C
- Pulse Normal >90
- Haemoglobin Normal <75% of normal Transfusion
- ESR 30mm/hr
Download images for case
Ulcerative colitis.
Treatment and management: On admission patient was rehydrated and given Solucortef 100 mg IMI tds. She continued to pass 10 stools the following day.
Day 3: Patient continued to experience diarrhoea and unable to tolerate food or water. Transfused with 2 units of packed cells Prescribed: Asacol 1.2g po, tds ( mesalazine ) Asacol suppository PR bds Morphine 15mg IMI PRN Flagyl 500mg tds
Day 6: Patient has continued to experience diarrhoea of watery, bloody stools. Abdominal pain has decreased and abdomen is soft and undistended. It was decided to continue medical management for a further 7 days, with the addition of: Cyclosporine 80mg IVI, infused over 2hrs Losec 20 mg po daily ( omeprazol ) Slow K rider IVI bds Slow Magnesium IVI daily Clexane 40 mg S/C daily ( enoxaparin )
Day 13: It was decided that medical management had failed as no relief of symptoms was achieved. Surgical management was therefore required.
A laparoscopic total colectomy and ileostomy was performed. Three months post surgery the patient is scheduled to return for ileal-anal pouch surgery, to eliminate the need to wear a bag.
- Ulcerative colitis (no date) MedicineNet. Available at: https://www.medicinenet.com/ulcerative_colitis/article.htm (Accessed: November 14, 2022).
- Richards, M. (2019) Immunopathogenesis of Ulcerative Colitis. USA: Maureen Richards Immunology & Microbiology: YouTube channel. Available at: https://www.youtube.com/watch?v=FnahtfSmP60 .
- Fuss, I. J. and Strober, W. (2015) “Ulcerative Colitis,” in Mucosal Immunology. Elsevier, pp. 1573–1612.
Multiple Choice Questions
Earn 1 hpcsa or 0.25 sacnasp cpd points – online quiz.

© 2004 - 2024 Immunopaedia.org.za Sitemap - Privacy Policy - Cookie Policy - PAIA - Terms & Conditions
Website designed by Personalised Promotions in association with SA Medical Specialists .
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License .

- CLINICAL CASES
- ONLINE COURSES
- AMBASSADORS
- TREATMENT & DIAGNOSTICS

- Nutrition counsellor
- Food Allergy
- gastroenterology
- Heptologist In Delhi
- Liver transplant
- Case Study: Severe Ulcerative Colitis
Final diagnosis: Ulcerative Colitis
Patient: Female, 27-year-old
Ulcerative colitis signs and Symptoms: bloody diarrhoea, abdominal tenderness and crampy abdominal pain, and weight loss
Speciality: Gastroenterology and hepatology
Causes, symptoms, and treatment of severe ulcerative colitis
Ulcerative colitis is a chronic inflammatory disease of the colon and rectum. Going by the severe ulcerative colitis definition, it does not affect the upper gastrointestinal tract. It causes inflammation and ulcers in the digestive tract. It affects the inner lining of the large intestine and rectum. The symptoms of the disease develop over time; they do not appear suddenly. Although the disease can occur at any age, it is less common in children. ulcerative colitis symptoms in females
Case Review
In this case study on ulcerative colitis, a twenty seven-year-old girl was brought to the clinic with a history of prolonged diarrhoea that had lasted ten weeks and was progressive. The patient presented frequent passage of stool with small amounts of blood, abdominal pain and noticeable weight loss. She also reported bowel movements about ten times per day. On reviewing the patient, it was observed that there was fever, vomiting, jaundice, joint pains or mouth ulcers. She was suffering from crampy abdominal pain, which was neither relieved nor aggravated by any known factor. The symptoms were associated with noticeable weight loss despite a good appetite and sufficient diet. No other urinary or respiratory symptoms were reported. On physical examination, she was pale, weighed 19 kgs and was neither irritable nor dehydrated. The rectum appeared to be narrowed, and the examination finger had stains of blood. These findings suggested the presence of ulcerative colitis.
Case Discussion
Ulcerative colitis affects adults and children globally. Currently, there is an ulcerative colitis cure for the disease. The goal of the treatment is to control the symptoms with the least possible side effects of the medicines prescribed and enable the patient to function normally. A comprehensive approach is suggested for effective treatment and management of ulcerative colitis. She should be treated and followed up by a team consisting of a gastroenterologist, paediatrician and hepatologist. The line of treatment for severe ulcerative colitis treatment depends on the severity of the infection. The signs of abdominal cramps, bloody diarrhoea, the urgency to defecate but an inability to do so, weight loss, and abdominal tenderness were indicative of mild ulcerative colitis. Medicines were prescribed to reduce inflammation and patient-reported reduction of symptoms after a continual treatment. In some instances of acute colitis ulcerative colitis complications, the patient may require surgery. However, it is complementary to the medical procedures and is advised only for preventing complications.
Clinical Symptoms
Ulcerative colitis, an inflammatory bowel disease, causes inflammation and sores in the digestive tract. The symptoms can vary depending on the location of the inflammation and its severity. The symptoms do not happen overnight but usually develop over time. The inflammation damages the inner lining of the large intestine (colon) and rectum. Some common symptoms include diarrhoea often accompanied with pus or blood, pain in the abdomen, pain in the rectum area, rectal bleeding, or blood with stool, having a feeling to defecate but not being able to despite the urgency. In severe cases, the patient may experience fatigue, weight loss and fever.
The patient had mild to moderate symptoms with bleeding, pain, and problems in passing stool.
The type of treatment depends on the reasons behind ulcerative colitis. In most cases, the treatment option includes symptomatic care, medicines to regulate bowel movement. In some cases, patients who have acute colitis may need IV fluid to restore fluid balance. Blood tests and stool tests were advised to confirm the diagnosis of ulcerative colitis. Primarily, the treatment involves medications that reduce inflammation. The medicines that work on some people may not work for another person usually, as it takes some time to identify medicines that help relieve the symptoms. As drugs have side effects, the risks and benefits were weighed before prescribing the medicines and supporting disease management. When medicines do not seem to be effective, then surgery is an option. The process involves the removal of the rectum and colon. Though disease management is a challenge, making lifestyle and diet changes can help control the symptoms. Though there is no evidence that what you eat causes ulcerative colitis, certain foods are known to aggravate the symptoms and flare-ups. Here are some general suggestions for a severe ulcerative colitis diet:
- Restrict dairy products intake: Often, problems like diarrhoea, gas, and abdominal pain can improve by limiting or eliminating the dairy products.
- Eat small meals: The symptoms often abate by consuming five to six smaller meals than two to three larger ones.
- Keep hydrated: It is best to drink as much water as possible. Consuming drinks that contain caffeine can worsen the symptoms.
In this case, the patient was put on medicines as it was a mild case of ulcerative colitis. After a few months of therapy, the patient reported relief in the symptoms. She was advised to make lifestyle changes as complementary to medicine.
With routine check-ups and adequate treatment, ulcerative colitis can be cured. If you or someone you know is experiencing any of the symptoms above, consult the gastro & liver clinic Patna Bihar. It is best to consult gastroenterologists online free or online gastroenterologist doctors. Excellent medical assistance is available in several cities, including best physician in Jammu city, max hospital liver specialist, nor gastro liver clinic Gurgaon, the best doctor in Patna for stomach, best female gynaecologist in Jhansi, liver cirrhosis specialist doctor in India, and gastro surgeon in Delhi
1. Is the colon the same as the large intestine?
Yes, ulcerative colitis is the inflammation of the large intestine or the colon.
2. What is the most primary symptom of ulcerative colitis?
Rectal bleeding is a significant symptom. However, other symptoms presented with the disease are diarrhoea and cramping abdominal pain.
3. Can antibiotics help in curing ulcerative colitis?
Antibiotics cannot help in the cure of ulcerative antibiotics. They only help in the management of the disease.
Privacy Preference Center
Privacy preferences, nutrition counselling.
Nutrition counseling is the assessment of an individual’s dietary intake after which, they are helped set achievable goals and taught various ways of maintaining these goals. The nutrition counselor provides information, educational materials, support and follow-up care to help an individual make and maintain the needed dietary changes for problems like obesity.
Obesity/ Food allergy
I assist people dealing with weight-related health problems by evaluating the health risks and help in obesity management. I also help patients manage various food allergies.
As a hepatologist, I specialize in the treatment of liver disorders, pancreas, gallbladder, hepatitis C, jaundice and the biliary tree. I also see patients suffering from pancreatitis, liver cancers alcoholic cirrhosis and drug induced liver disease(DILI), which has affected the liver.
Gastroenterology
As a gastroenterologist, my primary focus is the overall health of the digestive system. I treat everything from acid reflux to ulcers, IBS, IBD: Crohns disease and ulcerative colitis, and colon cancer.
Endoscopy is a nonsurgical procedure to examine a person’s digestive tract. It is carried out with an endoscope, a flexible tube with a light and camera attached to it so that the doctor can see pictures of the digestive tract on a color TV monitor.
Medical Gastroenterology
Gastroenterology is a specialty that evaluates the entire alimentary tract from the mouth to anus and involves studying the diseases of the pancreas.
Liver Transplant Services
A liver transplant is a surgical procedure that removes a patient’s non-functioning liver and replaces it with a healthy liver from a deceased donor or a portion of healthy liver from a living donor. It is reserved as a treatment option for people who have significant complications due to end-stage chronic liver disease or in case of sudden failure of a previously healthy liver.
Exploring the Role of GDF-15 in Inflammatory Bowel Disease: A Case-Controlled Study Comparing Crohn's Disease and Ulcerative Colitis with Non-Inflammatory Controls
Affiliations.
- 1 Department of Medicine, First Faculty of Medicine, Charles University and Military University Hospital Prague, 169 02 Prague, Czech Republic.
- 2 Department of Military Internal Medicine and Military Hygiene, Military Faculty of Medicine, University of Defence, 500 02 Hradec Kralove, Czech Republic.
- 3 Department of Clinical Biochemistry, Military University Hospital, 169 02 Prague, Czech Republic.
- 4 Department of Food Chemistry and Analysis, University of Chemistry and Technology, 160 00 Prague, Czech Republic.
- PMID: 38668313
- PMCID: PMC11051727
- DOI: 10.3390/metabo14040185
Inflammatory bowel disease, encompassing Crohn's disease and ulcerative colitis, is a persistent immune-mediated inflammatory gastrointestinal disease. This study investigates the role of growth differentiation factor 15 in severe IBD cases, aiming to identify a reliable parameter to assess disease severity and monitor activity. We analyzed plasma samples from 100 patients undergoing biologic therapy for severe IBD and 50 control subjects. Our analysis included evaluations of GDF-15 levels, inflammatory markers, and clinical features. We employed statistical methods such as the Mann-Whitney U test, ANOVA, and Spearman's correlation for an in-depth analysis. Our results demonstrated consistently higher GDF-15 levels in patients with both Crohn's disease and ulcerative colitis compared to the control group, irrespective of the biologic treatment received. The correlation analysis indicated significant relationships between GDF-15 levels, patient age, fibrinogen, and IL-6 levels. This study positions GDF-15 as a promising biomarker for severe IBD, with notable correlations with age and inflammatory markers. These findings underscore GDF-15's potential in enhancing disease monitoring and management strategies in an IBD context and encourage further research to clarify GDF-15's role in the IBD pathophysiology.
Keywords: Crohn’s disease; GDF-15; Inflammatory Bowel Disease; ulcerative colitis.
Grants and funding
- Specific research SV/FVZ202201/Ministry of Education, Youth and Sports of the Czech Republic
- projects MO 1012 (Military University Hospital Prague, Czech Republic) and MO 1011 - Clinical Fields II (Military Faculty of Medicine, University of Defence, Hradec Kralove, Czech Republic)/Ministry of Defence of the Czech Republic

Is There a Link Between IBD and MS?
Prevalence of MS and IBD
“We don’t know how to predict who with IBD is going to get MS or vice versa, but it’s something doctors need to think about when we’re prescribing medications or treatments,” says Gil Melmed, MD , director of inflammatory bowel disease clinical research at Cedars-Sinai Medical Center in Los Angeles.
“While it’s not common to have both MS and IBD, having one of these conditions is a risk factor for having the other,” adds Brian Barry, MD , attending neurologist at MedStar Washington Hospital Center in Washington, DC. Dr. Barry notes that he has seen several patients with both conditions.
What Is IBD?
IBD is often referred to as an autoimmune disease, but Dr. Melmed prefers the term immune-mediated. “In IBD, the body is not attacking itself per se, but the immune system is being triggered by bacteria commonly found in the gut and going into overdrive,” he explains.
What Is MS?
In MS on the other hand, it’s generally thought that the immune system attacks the protective sheath, or myelin, around nerve fibers, which causes communication problems between your brain and the rest of your body. Over time, it can cause permanent damage to the nerve fibers.
IBD and MS: Some Overlapping Symptoms
How are ibd and ms connected.
Researchers don’t yet fully understand the exact connection between IBD and MS. “The gut plays a large role in the nervous system and the immune system, but we are not totally certain how that directly interacts with MS,” says Barry.
“Research has shown that the immune system is shaped by events in the gut and that immune cells in the gut can actually travel all the way to the central nervous system,” notes Carlos Camara-Lemarroy, MD , assistant professor at the University of Calgary’s Hotchkiss Brain Institute in Canada. “This is why the gut-brain axis may be important for development of MS and other inflammatory conditions.”
Whatever the exact nature of the connection between IBD and MS, there seems to be an elevated risk in people with one of the conditions to have the other as well.
The reason that’s important, Barry adds, is that anti-TNF therapy, also known as biologics — one of the most common classes of medication that’s prescribed for IBD — is associated with the potential worsening of MS, as well as other demyelinating neurological conditions like transverse myelitis and optic neuritis. “It’s not entirely clear, but that’s definitely a conversation that I as a neurologist would want to have with a patient’s gastroenterologist,” he says.
On the other hand, there are also classes of drugs that are effective in both IBD and MS. “For example, there’s a drug called natalizumab that’s approved for both MS and CD, and a newer drug called ozanimod that’s approved for MS and UC,” Melmed says. “So in instances where the two conditions overlap, those might be drugs to consider.”
“There would be an opportunity there to potentially treat the patient with one of these medications, as long as the gastroenterologist is in agreement as well,” adds Barry.
Melmed is quick to add that people who have IBD or MS shouldn’t necessarily rush to be tested for the other condition unless their symptoms clearly indicate that they might have both. “It’s not an easy, clear-cut diagnosis in someone who isn’t symptomatic, so it isn’t feasible to do MRI scans for MS in everyone with IBD.”
However, given the increased prevalence of MS in people who have IBD, Barry recommends that people with IBD get a referral to a neurologist if they experience weakness, tingling or numbness, or trouble walking.
Risk Factors and Prevention
- Higher socioeconomic status
- Vitamin D deficiency
- Cold climate
Other common risk factors include:
Editorial Sources and Fact-Checking
Everyday Health follows strict sourcing guidelines to ensure the accuracy of its content, outlined in our editorial policy . We use only trustworthy sources, including peer-reviewed studies, board-certified medical experts, patients with lived experience, and information from top institutions.
- Kadowaki A et al. The Gut-CNS Axis in Multiple Sclerosis. Trends in Neurosciences . August 2020.
- Alkhawajah M et al. Multiple Sclerosis and Inflammatory Bowel Diseases: What We Know and What We Would Need to Know! Multiple Sclerosis Journal . October 2012.
- Wang X et al. Multiple Sclerosis and Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Annals of Clinical and Translational Neurology . February 2022.
- Inflammatory Bowel Disease (Overview). Cleveland Clinic. May 3, 2021.
- Symptoms & Causes of Multiple Sclerosis. Mayo Clinic. December 24, 2022.
- Bourre B et al. Multiple Sclerosis and Bowel Symptoms: Frequency and Barriers to Their Management. Multiple Sclerosis and Related Disorders . October 2023.
- Bowel. MS Society UK.
- Morís G. Inflammatory Bowel Disease: An Increased Risk Factor for Neurologic Complications. World Journal of Gastroenterology . February 2014.
- Yang Y et al. Acute Transverse Myelitis in an Adult-Patient With Underlying Ulcerative Colitis: A Case Report. BMC Gastroenterology . April 2022.
- Transverse Myelitis. National Institute of Neurological Disorders and Stroke. February 22, 2024.
- Keogh C et al. Myelin as a Regulator of Development of the Microbiota-Gut-Brain Axis. Brain, Behavior, and Immunity . January 2021.
- Inflammatory Bowel Disease (IBD). Mayo Clinic. September 3, 2022.
Related Topics
- Ulcerative Colitis vs. Crohn's
- Omeprazole Side Effects
- Case report
- Open access
- Published: 01 May 2024
Mycophenolate-induced colitis in a patient with lupus nephritis: a case report and review of the literature
- Ziyad Alakkas 1 ,
- Abdulaziz M. Gari 2 ,
- Sara Makhdoum 3 &
- Eman A. AlSindi 2
Journal of Medical Case Reports volume 18 , Article number: 229 ( 2024 ) Cite this article
179 Accesses
3 Altmetric
Metrics details
Mycophenolate mofetil (MMF) is an immunosuppressive drug that is frequently prescribed to patients with rheumatological diseases. MMF’s side effects include abdominal discomfort, nausea, vomiting, and other gastro-intestinal side effects, which typically appear in the first few months of treatment. However, late-onset diarrhea does not rule out the presence of MMF-induced colitis, which can be misdiagnosed since it is linked to a broad range of histopathological characteristics, including alterations that resemble inflammatory bowel disease, graft-versus-host disease, and ischemia. The differences in treatment response may be explained by the complexity of the histopathologic characteristics.
Case presentation
Here we present a case of a 29-year-old Arabian female with lupus nephritis who started on MMF as induction therapy. In two months, the patient was presented to the Emergency Department with diarrhea and manifestations of severe dehydration. Infectious diseases and adverse drug events were suspected, so the patient was admitted for further workup, and MMF was stopped. The patient was diagnosed with MMF-induced colitis based on colonoscopy and histological findings. Fourteen days after stopping MMF, she was within her baseline.
The purpose of this paper is to report a case of early-onset MMF-induced colitis in a patient with lupus nephritis who had started MMF as induction therapy. A review of the available literature on this uncommon immunosuppressive effect is also presented.
Peer Review reports
Introduction
Mycophenolate mofetil (MMF) is widely used as an immunosuppressive agent for various inflammatory and/rheumatic conditions, including lupus nephritis and organ transplantation. Mycophenolic acid is an active metabolite of MMF that reversibly inhibits inosine monophosphate (IMP) dehydrogenase, preventing purine synthesis in T and B cells [ 1 , 2 ]. Dose modification or even discontinuation of MMF is quite common due to adverse effects, especially gastro-intestinal side effects, which occur in nearly 45% of cases [ 2 ]. Enterocytes are particularly susceptible to the antimetabolic effects of MMF due to their reliance on the de novo process of purine synthesis. This prevents the growth and reproduction of small bowel epithelial cells, which disrupts fluid absorption and causes diarrhea [ 3 , 4 ]. However, MMF is a well-tolerated therapy in general.
One of the major adverse effects of MMF is colitis, which can lead to serious complications that include perforation, bleeding, and hospitalization. In recent years, several studies have investigated the factors associated with MMF-induced colitis. One study evaluated the incidence of gastro-intestinal complications following kidney transplant and showed that MMF-induced colitis was the most common type of colitis, occurring in 6–9% of patients, and the most common symptom was diarrhea [ 5 , 6 ]. MMF is one of the common immunosuppression medications for rheumatological disease and has been used for the last two decades with very good outcomes in terms of different aspects and system involvement.
There are only a few reports of patients who developed MMF-related colitis (Table 1 ). A small retrospective study evaluated 11 patients with rheumatologic disease who had been treated with MMF and found that only one patient had medication-related colitis [ 7 ]. We report a case of a young female who had systemic lupus erythematosus and lupus nephritis and presented with abdominal pain and diarrhea. We also discuss the challenges in the diagnosis of MMF-induced colitis.
Written consent was obtained from the patient, and ethical approval was provided by the Institutional Review Board (IRB) of the Study and Research Department of King Fahad Hospital, Jeddah.
The patient was a 29-year-old Arabian woman with a known case of systemic lupus erythematosus, which had been diagnosed 5 years prior based on the criteria of the European League Against Rheumatism (EULAR)/American College of Rheumatology (ACR). She was started on hydroxychloroquine at 200 mg orally once per day, and she had no comorbidities except for hypothyroidism.
The patient had not been followed up due to the COVID-19 pandemic, but in January 2023, she presented to the clinic with an incidental lab result showing a creatinine level of 4.3 mg/dL. Thus, a renal biopsy was planned, and she was diagnosed with class IV lupus nephritis. She received pulse methylprednisolone therapy at 500 mg intravenously for 3 days, which was then switched to a tapering dose of prednisolone. Induction therapy using MMF was initiated at 500 mg orally twice daily then titrated up weekly until she was discharged to home on 1.5 g orally twice per day, which is the maximum recommended dose of induction phase for lupus nephritis.
After 2 months, the patient presented to the emergency department with complaints of nausea, vomiting, and left-sided abdominal pain associated with diarrhea 5–6 times per day, which was watery but contained no blood or mucus. Her symptoms started at just 2 weeks after starting MMF therapy and had progressed over the last month. She denied having fever, weight loss, or night sweats. Upon physical examination, she was alert and oriented but in pain. The abdomen was tender, but there were no signs of peritonitis. The rest of the physical examination was unremarkable apart from Cushingoid face. She was afebrile, and her blood pressure, heart rate, respiratory rate, and oxygen saturation were within normal ranges. Her weight was 90 kg.
Upon admission, MMF was promptly discontinued due to the possibility that it might have led to an infection. The immediate care involved the delivery of intravenous fluids, a low-residue diet, analgesic medications like intravenous acetaminophen, and antispastic treatments. Routine blood tests performed at admission indicated leukopenia 3.800 × 10 6 /L normal range (4–11 × 10 6 /L), elevated creatinine 6.3 mg/dL normal range (07–1.3 mg/dL) while her baseline of creatinine was 2 mg/dL and GFR 60 ml/min, and noticeably increased inflammatory markers (C-reactive protein 32 mg/dL normal range < 5 mg/dL, erythrocyte sedimentation rate 40 mm/hour normal range < 20 mm/hour). The C3 and C4 complement levels were 0.6 g/L (0.8–1.6 g/L) and C4 0.18 g/L (0.20–0.65 g/L), respectively. Stool analysis indicated + 2 pus cells and a negative culture. Urine analysis indicated a protein level of + 1 with no red blood cell crystals or casts. An abdominal ultrasound was performed, but the result was unremarkable.
Later, computed tomography scan was performed, which did not reveal any other abnormalities and confirmed the ultrasound results. A colonoscopy showed erythematous patches with few erosions and rectal-sparing colitis. Multiple biopsies been taken (Fig. 1 ). Infectious colitis, drug-induced colitis, newly diagnosed inflammatory bowel disease (IBD), gastro-intestinal involvement associated with systemic lupus erythematosus, and mesenteric ischemia were all considered in the differential diagnosis. Cytomegalovirus (CMV) infection is the main concern among infectious causes of colitis in patients with impaired immune systems, and its possible endoscopic findings include diffuse erythema, ischemia, erosions, and ulcers.
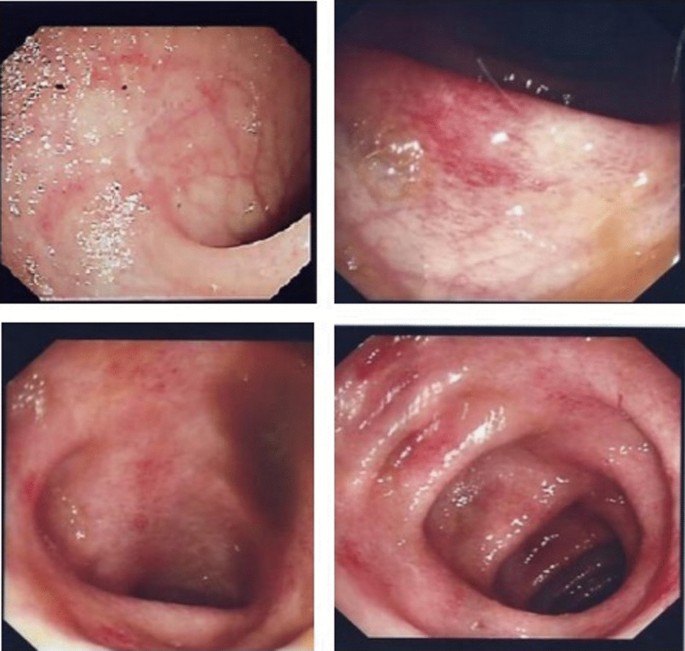
Colonoscopy shows hyperemic mucosa with some superficial ulcers from sigmoid up to the cecum, while the terminal ileum shows superficial ulceration with some area of inflammatory patches
The endoscopic appearance of drug-induced colitis can resemble that of ulcerative colitis, infectious colitis, and ischemic colitis. Non-steroidal anti-inflammatory drugs (NSAIDs) are the most common cause of drug-induced colitis, but MMF was probably involved in the present case. Rectal sparing almost always occurs in MMF-induced colitis. Microscopically, the colonic mucosa displayed a mild architectural distortion, with ruptures of few dilated glands. Mild cryptitis is observed. However, no obvious apoptosis seen as been previously described in few cases of MMF induced colitis. There were no evidence of viral cytopathic changes, granuloma, dysplasia or malignancy (Fig. 2 A–D),
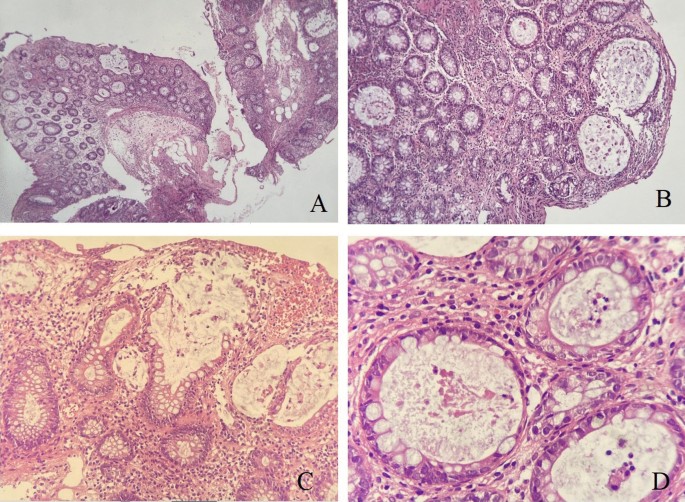
Mycophenolate Mofetil induced colitis. A Colonic biopsy with mild architectural distortion, crypt hyperplasia and lamina propria inflammation (HEx 4 ×). B Higher magnification (HE 20 ×) show dilated colonic glands. C Destructed ruptured colonic glands with mucin spillage (HE × 20 ×). D Acute inflammation within the glands (cryptitis), at high power magnification (HE x 40 ×)
Following the cessation of MMF, the gastro-intestinal symptoms and the biomarkers for systemic inflammation gradually subsided and returned to base line levels after 14 days without further therapy, thus supporting the suspicion of drug-induced colitis. The patient was discharged 24 days after admission, MMF was discontinued, and the Euro-Lupus protocol was started with cyclophosphamide as induction therapy for lupus nephritis. The patient has been followed up closely and has shown improvement of active and chronic issues with 6 doses of cyclophosphamide completed. Azathioprine with a low dose of steroid was initiated. There have been no other gastro-intestinal manifestations.
MMF is an immunosuppressive medication that was first used to decrease the risk of organ rejection after transplantation, but now, it is also being used to treat patients with autoimmune systemic disorders, including systemic lupus erythematosus. The target dose of MMF for the treatment of Lupus Nephritis is 2–3 g per day in combination with glucocorticoids especially for those high-risk patients for kidney failure including reduced GFR. Dosage may need to be adjusted according to adverse events, toxicity, efficacy and MPA blood level [according to 2019 European Leage Against Rheumatism and European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendation for the management of Lupus Nephritis]. General recommendation to not exceed 2 g in patients with chronic renal failure with GFR less than 25 mL/min.
As far as we are aware, there have been only a few documented cases of colitis caused by MMF in a patient with a rheumatological condition. The onset of MMF-induced colitis in a patient with lupus nephritis, sclerosis, mixed connective tissue disease (MCTD), and polymyositis has previously been documented by other authors (Table 1 ). In transplant recipients, however, MMF is a well-known trigger of drug-induced colitis [ 6 ].
Mycophenolate targets tissues with fast cell division and reliance on purine synthesis. Lymphocytes and gut cells are the two main organs in which regeneration is dependent on this system. Immunosuppression results from lymphocytes (B and T cells) being more dependent on this route (by 90%) [ 8 ]. The blood level of mycophenolic acid is directly inversely correlated with mycophenolate’s adverse effects [ 9 ]. Since 50% of enterocytes rely on the mycophenolate-targeting mechanisms, it is believed to explain why 45% of patients experience gastro-intestinal side effects, including simple diarrhea, esophagitis, gastroesophageal reflux disease, enteritis, and colitis, as in our patient [ 2 ]. The most typical gastro-intestinal mucosal pattern associated with MMF is mucosa that seems normal [ 10 ]. The histological changes in patients receiving MMF have mostly been classified in many studies as normal or near normal in around one-third of cases, followed by changes resembling IBD, graft-versus-host disease (GVHD), self-limited colitis, and ischemia [ 11 , 12 , 13 ]. Another study reported histological results that were in line with an acute colitis-like pattern in half of cases as being the most common, followed by IBD-like pathologic findings in 36% of cases, ischemia-like characteristics in 5.6% of cases, and GVHD-like abnormalities in 8.3% of cases [ 6 ]. Examples of specific histological characteristics of MMF-related colitis include crypt architectural disarray, increased lamina propria inflammation, dilated damaged crypts, increased crypt epithelial apoptosis, and GVHD-like alterations [ 14 ].
The wide morphological spectrum documented in MMF-induced colitis includes features that can lead to misdiagnosis and delayed intervention. Therefore, it is essential to discuss the clinical history of MMF therapy with pathologists and to take this diagnosis into consideration, regardless of the length of therapy, given the variations in the therapeutic management and prognosis of these disorders. The most frequent indication for a colonoscopy referral for patients on MMF medication is diarrhea. Nearly half of such patients have normal colonoscopy results. Other endoscopic findings include erythema (33%) and erosions/ulcers (19%), which indicate a need for routine biopsies to help with confirmation of the diagnosis [ 6 ].
Treatment options range from stopping MMF use to using specialized immunosuppressive medications to correct the histological pattern replicated by MMF-induced colitis. There are no recommendations available to help clinicians treat colitis induced by MMF. Case reports have frequently shown that after stopping MMF, diarrhea symptoms improve within a week. In another study, after unsuccessful attempts with MMF cessation, a patient was given 50 mg of intravenous steroids daily for two weeks and a single infusion of 5 mg/kg of infliximab, which led to decreased stool frequency within three days after infusion [ 28 ].
It is well known that MMF causes drug induced colitis with a variety of patterns and clinical manifestations. When caring for people with autoimmune systemic disorders, colitis should be recognized as a rare side effect of MMF therapy. It is necessary for physicians to be aware that discontinuing the medicine is typically effective without the need for extra treatments.
Availabilty of data and materials
Not applicable.
Abbreviations
- Mycophenolate mofetil
Mycophenolate sodium
Inosine monophosphate
Gastro-intestinal
Inflammatory bowel disease
Cytomegalovirus
Polyendocrinopathy-candidiasis-ectodermal dystrophy
Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47(2–3):85–118. https://doi.org/10.1016/s0162-3109(00)00188-0 .
Article CAS PubMed Google Scholar
Allison AC, Eugui EM. Purine metabolism and immunosuppressive effects of mycophenolate mofetil (MMF). Clin Transplant. 1996;10(1 Pt 2):77–84.
CAS PubMed Google Scholar
Tierce JC, Porterfield-Baxa J, Petrilla AA, Kilburg A, Ferguson RM. Impact of mycophenolate mofetil (MMF)-related gastrointestinal complications and MMF dose alterations on transplant outcomes and healthcare costs in renal transplant recipients. Clin Transplant. 2005;19(6):779–84. https://doi.org/10.1111/j.1399-0012.2005.00421.x .
Isaacs PE, Sladen GE, Filipe I. Mefenamic acid enteropathy. J Clin Pathol. 1987;40(10):1221–7. https://doi.org/10.1136/jcp.40.10.1221 .
Article CAS PubMed PubMed Central Google Scholar
Gioco R, Puzzo L, Patanè M, Corona D, Trama G, Veroux P, Veroux M. Post-transplant colitis after kidney transplantation: clinical, endoscopic and histological features. Aging (Albany NY). 2020;12(24):24709–20. https://doi.org/10.18632/aging.202345 .
Calmet FH, Yarur AJ, Pukazhendhi G, Ahmad J, Bhamidimarri KR. Endoscopic and histological features of mycophenolate mofetil colitis in patients after solid organ transplantation. Ann Gastroenterol. 2015;28(3):366–73.
PubMed PubMed Central Google Scholar
Bandelier C, Guerne PA, Genevay S, Finckh A, Gabay C. Clinical experience with mycophenolate mofetil in systemic autoimmune conditions refractory to common immunosuppressive therapies. Swiss Med Wkly. 2009;139(3–4):41–6. https://doi.org/10.4414/smw.2009.12441 .
Lamba V, Sangkuhl K, Sanghavi K, Fish A, Altman RB, Klein TE. PharmGKB summary: mycophenolic acid pathway. Pharmacogenet Genomics. 2014;24(1):73–9.
Md Dom ZI, Coller JK, Carroll RP, Tuke J, McWhinney BC, Somogyi AA, et al . Mycophenolic acid concentrations in peripheral blood mononuclear cells are associated with the incidence of rejection in renal transplant recipients. Br J Clin Pharmacol. 2018;84(10):2433–42.
Lee S, de Boer WB, Subramaniam K, Kumarasinghe MP. Pointers and pitfalls of mycophenolate-associated colitis. J Clin Pathol. 2013;66(1):8–11. https://doi.org/10.1136/jclinpath-2012-200888 .
Article PubMed Google Scholar
Histological spectrum of mycophenolate mofetil-related colitis: association with apoptosis.
Selbst MK, Ahrens WA, Robert ME, Friedman A, Proctor DD, Jain D. Spectrum of histologic changes in colonic biopsies in patients treated with mycophenolate mofetil. Mod Pathol. 2009;22(6):737–43. https://doi.org/10.1038/modpathol.2009.44 .
Mycophenolate mofetil-induced colitis with graft versus host disease-like features in a renal transplant recipient: case report and literature review.
Parfitt JR, Jayakumar S, Driman DK. Mycophenolate mofetil-related gastrointestinal mucosal injury: variable injury patterns, including graft-versus-host disease-like changes. Am J Surg Pathol. 2008;32(9):1367–72. https://doi.org/10.1097/pas.0b013e31816bf3fe .
Al Saadi W, Al Salmi I, Mohammed E, Al Ajmi Z, Hannawi S. Mycophenolate induced colitis: one-year post-kidney transplantation. Oman Med J. 2023;38(2): e489. https://doi.org/10.5001/omj.2023.14 .
Article PubMed PubMed Central Google Scholar
Schmitt MM, Ferré EMN, De Melo MS, Cooper MA, Quezado MM, Heller T, Lionakis MS. Mycophenolate-induced colitis in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy patients. JPGN Rep. 2021;2(4): e131. https://doi.org/10.1097/pg9.0000000000000131 .
Bani Fawwaz BA, Aldwairy A, Farooq A. Mycophenolate-induced colitis: a rare side effect. Cureus. 2021;13(9): e18250. https://doi.org/10.7759/cureus.18250 .
Farooqi R, Kamal A, Burke C. Mycophenolate-induced colitis: a case report with focused review of literature. Cureus. 2020;12(1): e6774. https://doi.org/10.7759/cureus.6774 .
Tayyem O, Saraireh H, Al Hanayneh M, Stevenson HL. Heart transplant recipient with mycophenolate mofetil-induced colitis: the great imitator. BMJ Case Rep. 2018;2018:bcr2017224035. https://doi.org/10.1136/bcr-2017-224035 .
Moroncini G, Benfaremo D, Mandolesi A, Gabrielli A. Mycophenolate mofetil-induced colitis in a patient with systemic sclerosis. BMJ Case Rep. 2018;2018:bcr2018224829. https://doi.org/10.1136/bcr-2018-224829 .
Sonoda A, Wada K, Mizukami K, Fukuda K, Shuto M, Okamoto K, Ogawa R, Okimoto T, Murakami K. Deep ulcers in the ileum associated with mycophenolate mofetil. Intern Med. 2017;56(21):2883–6. https://doi.org/10.2169/internalmedicine.8815-17 .
Dhakal P, Gami R, Giri S, Bhatt VR. Mycophenolate mofetil (MMF)-induced colitis. Blood. 2016;128:4795.
Article Google Scholar
Goyal A, Salahuddin M, Govil Y. A unique case of mycophenolate induced colitis after 10 years of use. Case Rep Gastrointest Med. 2016;2016:3058407. https://doi.org/10.1155/2016/3058407 .
Johal K, Ratuapli SK, Lam-Himlin DM, Gurudu SR. Mycophenolate mofetil-induced segmental colitis mimicking ischemic colitis. Case Rep Gastroenterol. 2014;8(1):95–100. https://doi.org/10.1159/000360847 .
Hamouda M, Mahmoudi H, Skhiri H, Elmay M. Mycophenolate mofetil-related pancolitis in a kidney transplant recipient [published correction appears in Exp Clin Transplant. 2012 Aug;10(4):418]. Exp Clin Transplant. 2012;10(5):501–5. https://doi.org/10.6002/ect.2011.0200 .
Patra S, Vij M, Sukanya B, Kapoor D. Mycophenolate mofetil-induced colitis with graft versus host disease-like features in a liver transplant recipient. Indian J Pathol Microbiol. 2012;55(4):506–8. https://doi.org/10.4103/0377-4929.107792 .
Gorospe EC. Chronic diarrhoea from mycophenolate mofetil-induced colitis. BMJ Case Rep. 2012;2012:bcr2220115344. https://doi.org/10.1136/bcr.12.2011.5344 .
Bouhbouh S, Rookmaaker MB. Rapid resolution of persistent mycophenolate mofetil-induced diarrhoea with a single dose of infliximab. Nephrol Dial Transplant. 2010;25(10):3437–8. https://doi.org/10.1093/ndt/gfq379 .
Download references
Acknowledgements
Guarantor of submission.
The corresponding author is the guarantor of submission.
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and affiliations.
Rheumatology Unit, Internal Medicine Department, King Abdul-Aziz Specialist Hospital, Taif, Saudi Arabia
Ziyad Alakkas
Rheumatology Unit, Internal Medicine Department, King Fahad Hospital, Jeddah, Saudi Arabia
Abdulaziz M. Gari & Eman A. AlSindi
Histopathology Department, King Fahad Hospital, Jeddah, Saudi Arabia
Sara Makhdoum
You can also search for this author in PubMed Google Scholar
Contributions
Ziyad Alakkas: Literature review and writing manuscript. Abdulaziz M. Gari: case review and got ethical approval. Sara Makhdoum: review histology slides and review literature. Eman A. AlSindi: mentor and most responsible physician (MRP) of the patient.
Corresponding author
Correspondence to Ziyad Alakkas .
Ethics declarations
Ethics approval and consent to participate.
This study complies with the Declaration of Helsinki and was approved by the local ethical research committee. Patient provided a written informed consent. All authors declare their participation.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that there is no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Alakkas, Z., Gari, A.M., Makhdoum, S. et al. Mycophenolate-induced colitis in a patient with lupus nephritis: a case report and review of the literature. J Med Case Reports 18 , 229 (2024). https://doi.org/10.1186/s13256-024-04539-7
Download citation
Received : 30 August 2023
Accepted : 05 April 2024
Published : 01 May 2024
DOI : https://doi.org/10.1186/s13256-024-04539-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Food & Function
6-gingerol ameliorates ulcerative colitis by inhibiting ferroptosis based on the integrative analysis of plasma metabolomics and network pharmacology.
6-Gingerol (6-G), an active ingredient of ginger with anti-inflammation and anti-oxidation properties, can treat ulcerative colitis (UC). However, its underlying mechanism is still unclear. In this study, the pharmacodynamic evaluation of 6-G for treating UC was performed, and the mechanism of 6-G in ameliorating UC was excavated by the plasma metabolomics and network pharmacology analysis, which was further validated by experimental and molecular docking. The results showed that 6-G could notably reduce diarrhea, weight loss, colonic pathological damage, and inflammation in UC mice. Plasma metabolomic results indicated that 6-G could regulate 19 differential metabolites, and its metabolic pathways mainly involved linoleic acid metabolism and arachidonic acid metabolism, which were tightly associated with ferroptosis. Moreover, 60 potential targets for 6-G intervention on ferroptosis in UC were identified by network pharmacology, and the enrichment analysis revealed that 6-G suppressed ferroptosis by modulating lipid peroxidation. Besides, the integration of metabolomics and network pharmacology showed that the regulation of 6-G on ferroptosis focused on 3 key targets, including ALOX5, ALOX15, and PTGS2. Further investigation indicated that 6-G significantly inhibited ferroptosis by decreasing iron load and malondialdehyde (MDA), and enhanced antioxidant capacity by reducing the content of glutathione disulfide (GSSG) and increasing the levels of superoxide dismutase (SOD) and glutathione (GSH) in UC mice and RSL3-induced Caco-2 cells. Furthermore, molecular docking showed the high affinity of 6-G with the identified 3 key targets. Collectively, this study elucidated the potential of 6-G in ameliorating UC by inhibiting ferroptosis. And the integrated strategy also provided a theoretical basis for 6-G in treating UC.
Supplementary files
- Supplementary information PDF (874K)
Article information
Download citation, permissions.
W. Li, Y. Zhang, Q. Wang, Y. Wang, Y. Fan, E. Shang, S. Jiang and J. Duan, Food Funct. , 2024, Accepted Manuscript , DOI: 10.1039/D4FO00952E
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page .
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page .
Read more about how to correctly acknowledge RSC content .
Social activity
Search articles by author.
This article has not yet been cited.
Advertisements

IMAGES
VIDEO
COMMENTS
A 37-year-old man with ulcerative colitis was admitted to the hospital because of abdominal cramping, diarrhea, hematochezia, fever to a peak temperature of 38.8°C, and drenching night sweats.
This month: A noteworthy case study. A 13-year-old girl with severe ulcerative colitis (UC) presented for assessment due to worsening symptoms. Two years earlier she had been diagnosed with severe ...
Source Reference: Cathomas M, et al "Herpes simplex virus colitis mimicking acute severe ulcerative colitis: A case report and review of the literature" J Surg Case Rep 2023; DOI: 10.1093/jscr ...
In this case, the patient nearly had a colectomy for refractory severe ulcerative colitis when the actual diagnosis was of cytomegalovirus (CMV)-related colitis. Second, this study revisits one of the common pitfalls of inflammatory bowel disease (IBD) management and the issue of CMV infection.
Case Report: Medical Management of Acute Severe Ulcerative Colitis. Sudheer Kumar Vuyyuru, DM, 1,2 Vipul Jairath, MD, PhD, 1,2,3 Jurij Hanžel, MD, PhD, 2,4 Christopher Ma, MD, MPH, 2,5 and Brian G. Feagan, MD 1,2,3. A 29-year-old female patient presented 5 years ago with bloody diarrhea, fecal urgency, and crampy abdominal pain. A colonoscopy ...
Introduction. Ulcerative colitis (UC) was first described in mid-1800s.1 It is an idiopathic, chronic inflammatory disorder of the colonic mucosa that commonly involves the rectum and may extend in a proximal and continuous fashion to involve other parts of the colon.2 The disease typically affects individuals in the second or third decade of life with hallmark clinical symptoms of bloody ...
Ulcerative colitis (UC) is a chronic inflammatory bowel disease of unknown aetiology affecting the colon and rectum. ... Wang, Y.-F. et al. Multicenter case-control study of the risk factors for ...
Source Reference: Aydın MF, Taşdemir H "Ulcerative colitis in a COVID-19 patient: A case report" Turk J Gastroenterol 2021; DOI: 10.5152/tjg.2021.20851. Share on Facebook. Opens in a new tab or ...
Despite improved management protocols and availability of biologics, short- and long-term colectomy rates with medical salvage therapy remain high (26%-47% and 36%-58% for cyclosporine, and 0%-50% and 35%-50% for infliximab, respectively). 42 To date, no validated scores exist to predict medical salvage therapy response.
Acute severe ulcerative colitis (ASUC) is a distinctive ulcerative colitis flare presentation characterised by the presence of systemic inflammation as well as bloody diarrhoea, and occurs at least once in 25% of patients with ulcerative colitis during their disease course. Each episode carries a risk of complications, need for colectomy, and mortality. Little is known about ASUC pathogenesis ...
Clinical trials. Explore Mayo Clinic studies testing new treatments, interventions and tests as a means to prevent, detect, treat or manage this condition.. Lifestyle and home remedies. Sometimes you may feel helpless when facing ulcerative colitis. But changes in your diet and lifestyle may help control your symptoms and lengthen the time between flare-ups.
Inflammatory bowel disease (IBD), both ulcerative colitis (UC) and Crohn's disease, has been termed a 'slow-motion' epidemic, with Western and recently industrialised countries seeing a continued rise in incidence. 1 Alongside genetic factors, it is thought that this trend is predominantly driven by environmental and lifestyle exposures, with dietary factors heavily implicated. 2 UC is a ...
Ulcerative colitis (UL-sur-uh-tiv koe-LIE-tis) is an inflammatory bowel disease (IBD) that causes inflammation and ulcers (sores) in your digestive tract. Ulcerative colitis affects the innermost lining of your large intestine, also called the colon, and rectum. In most people, symptoms usually develop over time, rather than suddenly.
Older adults with ulcerative colitis [UC] have greater morbidity than younger adults. The goal of this study was to investigate differe. ... Primary study outcomes included frequency and timing of medical and surgical rescue therapy during the hospitalisation, postoperative complications, frailty, and mortality outcomes up to 1 year following ...
P HARMACEUTICAL RESEARCH. www.wjcmpr.com ISSN: 2582-0222. Case report on ulcerative colitis in 16 year girl. MD.Salma, Y. Siva, J.Bhargava Narendra. Department of Pharmacy Practice, QIS College of ...
Ulcerative colitis is an idiopathic inflammatory condition of the colon that results in diffuse friability and superficial erosions on the colonic wall associated with bleeding. It is the most common form of inflammatory bowel disease worldwide. It characteristically involves inflammation restricted to the mucosa and submucosa of the colon. Typically, the disease starts in the rectum and ...
Case study of persistent bloody diarrhoea - investigation, diagnosis and treatment of Ulcerative Colitis plus discussion of the immunology of the disease. ... Gene association studies in patients with ulcerative colitis have identified the potential involvement of p40, IL-23R and STAT3 genes. These genes are involved in the signal transduction ...
Ulcerative colitis is a lifelong inflammatory disease affecting the rectum and colon to a variable extent. In 2023, the prevalence of ulcerative colitis was estimated to be 5 million cases around the world, and the incidence is increasing worldwide. Ulcerative colitis is thought to occur in people with a genetic predisposition following environmental exposures; gut epithelial barrier defects ...
In this case study on ulcerative colitis, a twenty seven-year-old girl was brought to the clinic with a history of prolonged diarrhoea that had lasted ten weeks and was progressive. The patient presented frequent passage of stool with small amounts of blood, abdominal pain and noticeable weight loss. She also reported bowel movements about ten ...
Inflammatory bowel disease, encompassing Crohn's disease and ulcerative colitis, is a persistent immune-mediated inflammatory gastrointestinal disease. This study investigates the role of growth differentiation factor 15 in severe IBD cases, aiming to identify a reliable parameter to assess disease severity and monitor activity.
Introduction. Ulcerative colitis (UC) is a chronic idiopathic inflammatory disease involving the colorectal segment in which lesions are mainly confined to the colonic mucosa and submucosa in a continuous diffuse distribution. 1 Recently, the prevalence of UC has risen dramatically worldwide, greatly affecting the quality of life. Recurrent diarrhea, abdominal pain, fever, weight loss, and ...
Case study Ulcerative colitis. 5 terms. matthew_brady75. Preview. Patho Unit 4 Disorders of the Large Intestine . 34 terms. nicole-eiden. Preview. ch. 6: green pages. 59 terms. yougotkaylee. Preview. Chapter 10: Nutrition during pregnancy and Lactation. Teacher 30 terms. aracelisvazquez. Preview. Cirrhosis HESI Case Study (evolve)
Studies have found that people with inflammatory bowel disease or multiple sclerosis ... Yang Y et al. Acute Transverse Myelitis in an Adult-Patient With Underlying Ulcerative Colitis: A Case ...
Image enhancement endoscopy techniques, such as linked color imaging (LCI) and autofluorescence imaging (AFI), have shown promise in diagnosing mucosal inflammation in ulcerative colitis (UC). However, no studies have directly compared the diagnostic efficacy of LCI and AFI.
The endoscopic appearance of drug-induced colitis can resemble that of ulcerative colitis, infectious colitis, and ischemic colitis. Non-steroidal anti-inflammatory drugs (NSAIDs) are the most common cause of drug-induced colitis, but MMF was probably involved in the present case. Rectal sparing almost always occurs in MMF-induced colitis.
6-Gingerol (6-G), an active ingredient of ginger with anti-inflammation and anti-oxidation properties, can treat ulcerative colitis (UC). However, its underlying mechanism is still unclear. In this study, the pharmacodynamic evaluation of 6-G for treating UC was performed, and the mechanism of 6-G in amelior