How to write a research plan: Step-by-step guide
Last updated
30 January 2024
Reviewed by
Today’s businesses and institutions rely on data and analytics to inform their product and service decisions. These metrics influence how organizations stay competitive and inspire innovation. However, gathering data and insights requires carefully constructed research, and every research project needs a roadmap. This is where a research plan comes into play.
There’s general research planning; then there’s an official, well-executed research plan. Whatever data-driven research project you’re gearing up for, the research plan will be your framework for execution. The plan should also be detailed and thorough, with a diligent set of criteria to formulate your research efforts. Not including these key elements in your plan can be just as harmful as having no plan at all.
Read this step-by-step guide for writing a detailed research plan that can apply to any project, whether it’s scientific, educational, or business-related.
- What is a research plan?
A research plan is a documented overview of a project in its entirety, from end to end. It details the research efforts, participants, and methods needed, along with any anticipated results. It also outlines the project’s goals and mission, creating layers of steps to achieve those goals within a specified timeline.
Without a research plan, you and your team are flying blind, potentially wasting time and resources to pursue research without structured guidance.
The principal investigator, or PI, is responsible for facilitating the research oversight. They will create the research plan and inform team members and stakeholders of every detail relating to the project. The PI will also use the research plan to inform decision-making throughout the project.
- Why do you need a research plan?
Create a research plan before starting any official research to maximize every effort in pursuing and collecting the research data. Crucially, the plan will model the activities needed at each phase of the research project.
Like any roadmap, a research plan serves as a valuable tool providing direction for those involved in the project—both internally and externally. It will keep you and your immediate team organized and task-focused while also providing necessary definitions and timelines so you can execute your project initiatives with full understanding and transparency.
External stakeholders appreciate a working research plan because it’s a great communication tool, documenting progress and changing dynamics as they arise. Any participants of your planned research sessions will be informed about the purpose of your study, while the exercises will be based on the key messaging outlined in the official plan.
Here are some of the benefits of creating a research plan document for every project:
Project organization and structure
Well-informed participants
All stakeholders and teams align in support of the project
Clearly defined project definitions and purposes
Distractions are eliminated, prioritizing task focus
Timely management of individual task schedules and roles
Costly reworks are avoided
- What should a research plan include?
The different aspects of your research plan will depend on the nature of the project. However, most official research plan documents will include the core elements below. Each aims to define the problem statement, devising an official plan for seeking a solution.
Specific project goals and individual objectives
Ideal strategies or methods for reaching those goals
Required resources
Descriptions of the target audience, sample sizes, demographics, and scopes
Key performance indicators (KPIs)
Project background
Research and testing support
Preliminary studies and progress reporting mechanisms
Cost estimates and change order processes
Depending on the research project’s size and scope, your research plan could be brief—perhaps only a few pages of documented plans. Alternatively, it could be a fully comprehensive report. Either way, it’s an essential first step in dictating your project’s facilitation in the most efficient and effective way.
- How to write a research plan for your project
When you start writing your research plan, aim to be detailed about each step, requirement, and idea. The more time you spend curating your research plan, the more precise your research execution efforts will be.
Account for every potential scenario, and be sure to address each and every aspect of the research.
Consider following this flow to develop a great research plan for your project:

Define your project’s purpose
Start by defining your project’s purpose. Identify what your project aims to accomplish and what you are researching. Remember to use clear language.
Thinking about the project’s purpose will help you set realistic goals and inform how you divide tasks and assign responsibilities. These individual tasks will be your stepping stones to reach your overarching goal.
Additionally, you’ll want to identify the specific problem, the usability metrics needed, and the intended solutions.
Know the following three things about your project’s purpose before you outline anything else:
What you’re doing
Why you’re doing it
What you expect from it
Identify individual objectives
With your overarching project objectives in place, you can identify any individual goals or steps needed to reach those objectives. Break them down into phases or steps. You can work backward from the project goal and identify every process required to facilitate it.
Be mindful to identify each unique task so that you can assign responsibilities to various team members. At this point in your research plan development, you’ll also want to assign priority to those smaller, more manageable steps and phases that require more immediate or dedicated attention.
Select research methods
Research methods might include any of the following:
User interviews: this is a qualitative research method where researchers engage with participants in one-on-one or group conversations. The aim is to gather insights into their experiences, preferences, and opinions to uncover patterns, trends, and data.
Field studies: this approach allows for a contextual understanding of behaviors, interactions, and processes in real-world settings. It involves the researcher immersing themselves in the field, conducting observations, interviews, or experiments to gather in-depth insights.
Card sorting: participants categorize information by sorting content cards into groups based on their perceived similarities. You might use this process to gain insights into participants’ mental models and preferences when navigating or organizing information on websites, apps, or other systems.
Focus groups: use organized discussions among select groups of participants to provide relevant views and experiences about a particular topic.
Diary studies: ask participants to record their experiences, thoughts, and activities in a diary over a specified period. This method provides a deeper understanding of user experiences, uncovers patterns, and identifies areas for improvement.
Five-second testing: participants are shown a design, such as a web page or interface, for just five seconds. They then answer questions about their initial impressions and recall, allowing you to evaluate the design’s effectiveness.
Surveys: get feedback from participant groups with structured surveys. You can use online forms, telephone interviews, or paper questionnaires to reveal trends, patterns, and correlations.
Tree testing: tree testing involves researching web assets through the lens of findability and navigability. Participants are given a textual representation of the site’s hierarchy (the “tree”) and asked to locate specific information or complete tasks by selecting paths.
Usability testing: ask participants to interact with a product, website, or application to evaluate its ease of use. This method enables you to uncover areas for improvement in digital key feature functionality by observing participants using the product.
Live website testing: research and collect analytics that outlines the design, usability, and performance efficiencies of a website in real time.
There are no limits to the number of research methods you could use within your project. Just make sure your research methods help you determine the following:
What do you plan to do with the research findings?
What decisions will this research inform? How can your stakeholders leverage the research data and results?
Recruit participants and allocate tasks
Next, identify the participants needed to complete the research and the resources required to complete the tasks. Different people will be proficient at different tasks, and having a task allocation plan will allow everything to run smoothly.
Prepare a thorough project summary
Every well-designed research plan will feature a project summary. This official summary will guide your research alongside its communications or messaging. You’ll use the summary while recruiting participants and during stakeholder meetings. It can also be useful when conducting field studies.
Ensure this summary includes all the elements of your research project. Separate the steps into an easily explainable piece of text that includes the following:
An introduction: the message you’ll deliver to participants about the interview, pre-planned questioning, and testing tasks.
Interview questions: prepare questions you intend to ask participants as part of your research study, guiding the sessions from start to finish.
An exit message: draft messaging your teams will use to conclude testing or survey sessions. These should include the next steps and express gratitude for the participant’s time.
Create a realistic timeline
While your project might already have a deadline or a results timeline in place, you’ll need to consider the time needed to execute it effectively.
Realistically outline the time needed to properly execute each supporting phase of research and implementation. And, as you evaluate the necessary schedules, be sure to include additional time for achieving each milestone in case any changes or unexpected delays arise.
For this part of your research plan, you might find it helpful to create visuals to ensure your research team and stakeholders fully understand the information.
Determine how to present your results
A research plan must also describe how you intend to present your results. Depending on the nature of your project and its goals, you might dedicate one team member (the PI) or assume responsibility for communicating the findings yourself.
In this part of the research plan, you’ll articulate how you’ll share the results. Detail any materials you’ll use, such as:
Presentations and slides
A project report booklet
A project findings pamphlet
Documents with key takeaways and statistics
Graphic visuals to support your findings
- Format your research plan
As you create your research plan, you can enjoy a little creative freedom. A plan can assume many forms, so format it how you see fit. Determine the best layout based on your specific project, intended communications, and the preferences of your teams and stakeholders.
Find format inspiration among the following layouts:
Written outlines
Narrative storytelling
Visual mapping
Graphic timelines
Remember, the research plan format you choose will be subject to change and adaptation as your research and findings unfold. However, your final format should ideally outline questions, problems, opportunities, and expectations.
- Research plan example
Imagine you’ve been tasked with finding out how to get more customers to order takeout from an online food delivery platform. The goal is to improve satisfaction and retain existing customers. You set out to discover why more people aren’t ordering and what it is they do want to order or experience.
You identify the need for a research project that helps you understand what drives customer loyalty. But before you jump in and start calling past customers, you need to develop a research plan—the roadmap that provides focus, clarity, and realistic details to the project.
Here’s an example outline of a research plan you might put together:
Project title
Project members involved in the research plan
Purpose of the project (provide a summary of the research plan’s intent)
Objective 1 (provide a short description for each objective)
Objective 2
Objective 3
Proposed timeline
Audience (detail the group you want to research, such as customers or non-customers)
Budget (how much you think it might cost to do the research)
Risk factors/contingencies (any potential risk factors that may impact the project’s success)
Remember, your research plan doesn’t have to reinvent the wheel—it just needs to fit your project’s unique needs and aims.
Customizing a research plan template
Some companies offer research plan templates to help get you started. However, it may make more sense to develop your own customized plan template. Be sure to include the core elements of a great research plan with your template layout, including the following:
Introductions to participants and stakeholders
Background problems and needs statement
Significance, ethics, and purpose
Research methods, questions, and designs
Preliminary beliefs and expectations
Implications and intended outcomes
Realistic timelines for each phase
Conclusion and presentations
How many pages should a research plan be?
Generally, a research plan can vary in length between 500 to 1,500 words. This is roughly three pages of content. More substantial projects will be 2,000 to 3,500 words, taking up four to seven pages of planning documents.
What is the difference between a research plan and a research proposal?
A research plan is a roadmap to success for research teams. A research proposal, on the other hand, is a dissertation aimed at convincing or earning the support of others. Both are relevant in creating a guide to follow to complete a project goal.
What are the seven steps to developing a research plan?
While each research project is different, it’s best to follow these seven general steps to create your research plan:
Defining the problem
Identifying goals
Choosing research methods
Recruiting participants
Preparing the brief or summary
Establishing task timelines
Defining how you will present the findings
Get started today
Go from raw data to valuable insights with a flexible research platform
Editor’s picks
Last updated: 21 December 2023
Last updated: 16 December 2023
Last updated: 6 October 2023
Last updated: 5 March 2024
Last updated: 25 November 2023
Last updated: 15 February 2024
Last updated: 11 March 2024
Last updated: 12 December 2023
Last updated: 6 March 2024
Last updated: 10 April 2023
Last updated: 20 December 2023
Latest articles
Related topics, log in or sign up.
Get started for free
FLEET LIBRARY | Research Guides
Rhode island school of design, create a research plan: research plan.
- Research Plan
- Literature Review
- Ulrich's Global Serials Directory
- Related Guides
A research plan is a framework that shows how you intend to approach your topic. The plan can take many forms: a written outline, a narrative, a visual/concept map or timeline. It's a document that will change and develop as you conduct your research. Components of a research plan
1. Research conceptualization - introduces your research question
2. Research methodology - describes your approach to the research question
3. Literature review, critical evaluation and synthesis - systematic approach to locating,
reviewing and evaluating the work (text, exhibitions, critiques, etc) relating to your topic
4. Communication - geared toward an intended audience, shows evidence of your inquiry
Research conceptualization refers to the ability to identify specific research questions, problems or opportunities that are worthy of inquiry. Research conceptualization also includes the skills and discipline that go beyond the initial moment of conception, and which enable the researcher to formulate and develop an idea into something researchable ( Newbury 373).
Research methodology refers to the knowledge and skills required to select and apply appropriate methods to carry through the research project ( Newbury 374) .
Method describes a single mode of proceeding; methodology describes the overall process.
Method - a way of doing anything especially according to a defined and regular plan; a mode of procedure in any activity
Methodology - the study of the direction and implications of empirical research, or the sustainability of techniques employed in it; a method or body of methods used in a particular field of study or activity *Browse a list of research methodology books or this guide on Art & Design Research
Literature Review, critical evaluation & synthesis
A literature review is a systematic approach to locating, reviewing, and evaluating the published work and work in progress of scholars, researchers, and practitioners on a given topic.
Critical evaluation and synthesis is the ability to handle (or process) existing sources. It includes knowledge of the sources of literature and contextual research field within which the person is working ( Newbury 373).
Literature reviews are done for many reasons and situations. Here's a short list:
Sources to consult while conducting a literature review:
Online catalogs of local, regional, national, and special libraries
meta-catalogs such as worldcat , Art Discovery Group , europeana , world digital library or RIBA
subject-specific online article databases (such as the Avery Index, JSTOR, Project Muse)
digital institutional repositories such as Digital Commons @RISD ; see Registry of Open Access Repositories
Open Access Resources recommended by RISD Research LIbrarians
works cited in scholarly books and articles
print bibliographies
the internet-locate major nonprofit, research institutes, museum, university, and government websites
search google scholar to locate grey literature & referenced citations
trade and scholarly publishers
fellow scholars and peers
Communication
Communication refers to the ability to
- structure a coherent line of inquiry
- communicate your findings to your intended audience
- make skilled use of visual material to express ideas for presentations, writing, and the creation of exhibitions ( Newbury 374)
Research plan framework: Newbury, Darren. "Research Training in the Creative Arts and Design." The Routledge Companion to Research in the Arts . Ed. Michael Biggs and Henrik Karlsson. New York: Routledge, 2010. 368-87. Print.
About the author
Except where otherwise noted, this guide is subject to a Creative Commons Attribution license
source document
Routledge Companion to Research in the Arts
- Next: Literature Review >>
- Last Updated: Sep 20, 2023 5:05 PM
- URL: https://risd.libguides.com/researchplan
Students & Educators —Menu
- Educational Resources
- Educators & Faculty
- College Planning
- ACS ChemClub
- Project SEED
- U.S. National Chemistry Olympiad
- Student Chapters
- ACS Meeting Information
- Undergraduate Research
- Internships, Summer Jobs & Coops
- Study Abroad Programs
- Finding a Mentor
- Two Year/Community College Students
- Social Distancing Socials
- Planning for Graduate School
- Grants & Fellowships
- Career Planning
- International Students
- Planning for Graduate Work in Chemistry
- ACS Bridge Project
- Graduate Student Organizations (GSOs)
- Schedule-at-a-Glance
- Standards & Guidelines
- Explore Chemistry
- Science Outreach
- Publications
- ACS Student Communities
- You are here:
- American Chemical Society
- Students & Educators
Writing the Research Plan for Your Academic Job Application
By Jason G. Gillmore, Ph.D., Associate Professor, Department of Chemistry, Hope College, Holland, MI
A research plan is more than a to-do list for this week in lab, or a manila folder full of ideas for maybe someday—at least if you are thinking of a tenure-track academic career in chemistry at virtually any bachelor’s or higher degree–granting institution in the country. A perusal of the academic job ads in C&EN every August–October will quickly reveal that most schools expect a cover letter (whether they say so or not), a CV, a teaching statement, and a research plan, along with reference letters and transcripts. So what is this document supposed to be, and why worry about it now when those job ads are still months away?
What Is a Research Plan?
A research plan is a thoughtful, compelling, well-written document that outlines your exciting, unique research ideas that you and your students will pursue over the next half decade or so to advance knowledge in your discipline and earn you grants, papers, speaking invitations, tenure, promotion, and a national reputation. It must be a document that people at the department you hope to join will (a) read, and (b) be suitably excited about to invite you for an interview.
That much I knew when I was asked to write this article. More specifics I only really knew for my own institution, Hope College (a research intensive undergraduate liberal arts college with no graduate program), and even there you might get a dozen nuanced opinions among my dozen colleagues. So I polled a broad cross-section of my network, spanning chemical subdisciplines at institutions ranging from small, teaching-centered liberal arts colleges to our nation’s elite research programs, such as Scripps and MIT. The responses certainly varied, but they did center on a few main themes, or illustrate a trend across institution types. In this article I’ll share those commonalities, while also encouraging you to be unafraid to contact a search committee chair with a few specific questions, especially for the institutions you are particularly excited about and feel might be the best fit for you.
How Many Projects Should You Have?

While more senior advisors and members of search committees may have gotten their jobs with a single research project, conventional wisdom these days is that you need two to three distinct but related projects. How closely related to one another they should be is a matter of debate, but almost everyone I asked felt that there should be some unifying technique, problem or theme to them. However, the projects should be sufficiently disparate that a failure of one key idea, strategy, or technique will not hamstring your other projects.
For this reason, many applicants wisely choose to identify:
- One project that is a safe bet—doable, fundable, publishable, good but not earthshaking science.
- A second project that is pie-in-the-sky with high risks and rewards.
- A third project that fits somewhere in the middle.
Having more than three projects is probably unrealistic. But even the safest project must be worth doing, and even the riskiest must appear to have a reasonable chance of working.
How Closely Connected Should Your Research Be with Your Past?
Your proposed research must do more than extend what you have already done. In most subdisciplines, you must be sufficiently removed from your postdoctoral or graduate work that you will not be lambasted for clinging to an advisor’s apron strings. After all, if it is such a good idea in their immediate area of interest, why aren’t they pursuing it?!?
But you also must be able to make the case for why your training makes this a good problem for you to study—how you bring a unique skill set as well as unique ideas to this research. The five years you will have to do, fund, and publish the research before crafting your tenure package will go by too fast for you to break into something entirely outside your realm of expertise.
Biochemistry is a partial exception to this advice—in this subdiscipline it is quite common to bring a project with you from a postdoc (or more rarely your Ph.D.) to start your independent career. However, you should still articulate your original contribution to, and unique angle on the work. It is also wise to be sure your advisor tells that same story in his or her letter and articulates support of your pursuing this research in your career as a genuinely independent scientist (and not merely someone who could be perceived as his or her latest "flunky" of a collaborator.)
Should You Discuss Potential Collaborators?
Regarding collaboration, tread lightly as a young scientist seeking or starting an independent career. Being someone with whom others can collaborate in the future is great. Relying on collaborators for the success of your projects is unwise. Be cautious about proposing to continue collaborations you already have (especially with past advisors) and about starting new ones where you might not be perceived as the lead PI. Also beware of presuming you can help advance the research of someone already in a department. Are they still there? Are they still doing that research? Do they actually want that help—or will they feel like you are criticizing or condescending to them, trying to scoop them, or seeking to ride their coattails? Some places will view collaboration very favorably, but the safest route is to cautiously float such ideas during interviews while presenting research plans that are exciting and achievable on your own.
How Do You Show Your Fit?
Some faculty advise tailoring every application packet document to every institution to which you apply, while others suggest tweaking only the cover letter. Certainly the cover letter is the document most suited to introducing yourself and making the case for how you are the perfect fit for the advertised position at that institution. So save your greatest degree of tailoring for your cover letter. It is nice if you can tweak a few sentences of other documents to highlight your fit to a specific school, so long as it is not contrived.
Now, if you are applying to widely different types of institutions, a few different sets of documents will certainly be necessary. The research plan that you target in the middle to get you a job at both Harvard University and Hope College will not get you an interview at either! There are different realities of resources, scope, scale, and timeline. Not that my colleagues and I at Hope cannot tackle research that is just as exciting as Harvard’s. However, we need to have enough of a niche or a unique angle both to endure the longer timeframe necessitated by smaller groups of undergraduate researchers and to ensure that we still stand out. Furthermore, we generally need to be able to do it with more limited resources. If you do not demonstrate that understanding, you will be dismissed out of hand. But at many large Ph.D. programs, any consideration of "niche" can be inferred as a lack of confidence or ambition.
Also, be aware that department Web pages (especially those several pages deep in the site, or maintained by individual faculty) can be woefully out-of-date. If something you are planning to say is contingent on something you read on their Web site, find a way to confirm it!
While the research plan is not the place to articulate start-up needs, you should consider instrumentation and other resources that will be necessary to get started, and where you will go for funding or resources down the road. This will come up in interviews, and hopefully you will eventually need these details to negotiate a start-up package.
Who Is Your Audience?
Your research plan should show the big picture clearly and excite a broad audience of chemists across your sub-discipline. At many educational institutions, everyone in the department will read the proposal critically, at least if you make the short list to interview. Even at departments that leave it all to a committee of the subdiscipline, subdisciplines can be broad and might even still have an outside member on the committee. And the committee needs to justify their actions to the department at large, as well as to deans, provosts, and others. So having at least the introduction and executive summaries of your projects comprehensible and compelling to those outside your discipline is highly advantageous.
Good science, written well, makes a good research plan. As you craft and refine your research plan, keep the following strategies, as well as your audience in mind:
- Begin the document with an abstract or executive summary that engages a broad audience and shows synergies among your projects. This should be one page or less, and you should probably write it last. This page is something you could manageably consider tailoring to each institution.
- Provide sufficient details and references to convince the experts you know your stuff and actually have a plan for what your group will be doing in the lab. Give details of first and key experiments, and backup plans or fallback positions for their riskiest aspects.
- Hook your readers with your own ideas fairly early in the document, then strike a balance between your own new ideas and the necessary well referenced background, precedents, and justification throughout. Propose a reasonable tentative timeline, if you can do so in no more than a paragraph or two, which shows how you envision spacing out the experiments within and among your projects. This may fit well into your executive summary
- Show how you will involve students (whether undergraduates, graduate students, an eventual postdoc or two, possibly even high schoolers if the school has that sort of outreach, depending on the institutions to which you are applying) and divide the projects among students.
- Highlight how your work will contribute to the education of these students. While this is especially important at schools with greater teaching missions, it can help set you apart even at research intensive institutions. After all, we all have to demonstrate “broader impacts” to our funding agencies!
- Include where you will pursue funding, as well as publication, if you can smoothly work it in. This is especially true if there is doubt about how you plan to target or "market" your research. Otherwise, it is appropriate to hold off until the interview to discuss this strategy.
So, How Long Should Your Research Plan Be?

Learn more on the Blog
Here is where the answers diverged the most and without a unifying trend across institutions. Bottom line, you need space to make your case, but even more, you need people to read what you write.
A single page abstract or executive summary of all your projects together provides you an opportunity to make the case for unifying themes yet distinct projects. It may also provide space to articulate a timeline. Indeed, many readers will only read this single page in each application, at least until winnowing down to a more manageable list of potential candidates. At the most elite institutions, there may be literally hundreds of applicants, scores of them entirely well-suited to the job.
While three to five pages per proposal was a common response (single spaced, in 11-point Arial or 12-point Times with one inch margins), including references (which should be accurate, appropriate, and current!), some of my busiest colleagues have said they will not read more than about three pages total. Only a few actually indicated they would read up to 12-15 pages for three projects. In my opinion, ten pages total for your research plans should be a fairly firm upper limit unless you are specifically told otherwise by a search committee, and then only if you have two to three distinct proposals.
Why Start Now?
Hopefully, this question has answered itself already! Your research plan needs to be a well thought out document that is an integrated part of applications tailored to each institution to which you apply. It must represent mature ideas that you have had time to refine through multiple revisions and a great deal of critical review from everyone you can get to read them. Moreover, you may need a few different sets of these, especially if you will be applying to a broad range of institutions. So add “write research plans” to this week’s to do list (and every week’s for the next few months) and start writing up the ideas in that manila folder into some genuine research plans. See which ones survive the process and rise to the top and you should be well prepared when the job ads begin to appear in C&EN in August!

Jason G. Gillmore , Ph.D., is an Associate Professor of Chemistry at Hope College in Holland, MI. A native of New Jersey, he earned his B.S. (’96) and M.S. (’98) degrees in chemistry from Virginia Tech, and his Ph.D. (’03) in organic chemistry from the University of Rochester. After a short postdoctoral traineeship at Vanderbilt University, he joined the faculty at Hope in 2004. He has received the Dreyfus Start-up Award, Research Corporation Cottrell College Science Award, and NSF CAREER Award, and is currently on sabbatical as a Visiting Research Professor at Arizona State University. Professor Gillmore is the organizer of the Biennial Midwest Postdoc to PUI Professor (P3) Workshop co-sponsored by ACS, and a frequent panelist at the annual ACS Postdoc to Faculty (P2F) Workshops.
Other tips to help engage (or at least not turn off) your readers include:
- Avoid two-column formats.
- Avoid too-small fonts that hinder readability, especially as many will view the documents online rather than in print!
- Use good figures that are readable and broadly understandable!
- Use color as necessary but not gratuitously.
Accept & Close The ACS takes your privacy seriously as it relates to cookies. We use cookies to remember users, better understand ways to serve them, improve our value proposition, and optimize their experience. Learn more about managing your cookies at Cookies Policy .
1155 Sixteenth Street, NW, Washington, DC 20036, USA | service@acs.org | 1-800-333-9511 (US and Canada) | 614-447-3776 (outside North America)
- Terms of Use
- Accessibility
Copyright © 2024 American Chemical Society

- CREd Library , Planning, Managing, and Publishing Research
Developing a Five-Year Research Plan
Cathy binger and lizbeth finestack, doi: 10.1044/cred-pvd-path006.
The following is a transcript of the presentation videos, edited for clarity.
What Is a Research Plan, and Why Do You Need One?
Presented by Cathy Binger

First we’re going to talk about what a research plan is, why it’s important to write one, and why five years—why not one year, why not ten years. So we’ll do some of those basic things, then Liza is going to get down and dirty into the nitty-gritty of “now what” how do I go about writing that research plan.
First of all, what is a research plan? I’m sure some of you have taken a stab at these already. In case you haven’t, this is a real personalized map that relates your projects to goals. It’s exactly what it sounds like, it’s a plan of how you’re going to go about doing your research. It doesn’t necessarily just include research.
It’s something that you need to put a little time and effort into in the beginning. And then, if you don’t revisit it, it’s really a useless document. It’s something that you need to come back to repeatedly, at least annually, and you need to make it visible. So it’s not a document that sits around and once a year you pull it out and look at it.
It can and should be designed, especially initially, with the help of a mentor or colleague. And it does serve multiple purposes, with different lengths and different amounts of detail.
I forgot to say, too, getting started, the slides for this talk were started using as a jumping off point Ray Kent’s talk from last year. So some of the slides we’ve borrowed from him, so many thanks to him for that.

But why do we want to do a research plan? Well, to me the big thing is the vision. Dr. Barlow talked this morning about your line of research and really knowing where you want to go, and this is where that shows up with all the nuts and bolts in place.
What do you want to accomplish? What do you want to contribute? Most of you are at the stage in your career where maybe you have started out with that you want to change the world scenario and realized that whatever you wanted your first research project to be, really, is your entire career. You need to get that down to the point where it is manageable projects that you can do—this is where you map out what those projects are and set reasonable timelines for that.
You want to really demonstrate your independent thinking and your own creativity, whatever that is that you then establish as a PhD student, postdoc, and beyond—this is where you come back to, okay, here’s how I’m going to go about achieving all of that.
This next point, learning to realistically gauge how long it takes to achieve each goal, this for most of us is a phenomenally challenging thing to do. Most of us really overestimate what we can do in a certain amount of time, and we learn the hard way that you can’t, and that’s another reason why you keep coming back to these plans repeatedly and learning over time what’s really manageable, what’s really doable, so we can still reach our goals and be very strategic about how we do that.
When you’re not strategic, you just don’t meet the goals. Your time gets sucked into so many different things. We need to be really practical and strategic.
Everything we do is going to take longer than we think.
I think this last one is something that maybe we don’t talk about enough. Really being honest with ourselves about the role of research in our lives. Not all of you are at very high-level research universities. Some of you have chosen to go elsewhere, where research maybe isn’t going to be playing the same role as it is for other people. The research plan for someone at an R One research intensive university is going to look quite different from someone who is at a primary teaching university. We need to be open and practical about that.

Getting sidetracked. I love this picture, I just found this picture the other day. This feels like my life. You can get pulled in so many different directions once you are a professor. You will get asked to do a thousand different things. There are lots of great opportunities that are out there. Especially initially, it’s tempting to say yes to all of them. But if you’re going to be productive, you have to be very strategic. I’m going to be a little bit sexist against my own sex here for a minute, but my observation has been that women tend to fall into this a little bit more than men do in wanting to say yes and be people pleasers for everything that comes down the pike.
It is a professional skill to learn how to say no. And to do that in such a way that you are not burning bridges as you go down the path. That is a critical skill if you are going to be a successful researcher. I can’t tell you how many countless people I’ve seen who are very bright, very dedicated, have the skills that it takes in terms of doing the work—but then they are not successful because they’ve gotten sidetracked and they try to be too much of a good citizen, give too much service to the department, too much “sure I’ll take on that extra class” or whatever else comes down the line.
I just spoke with a professor recently who had something like five hours a week of office hours scheduled every single week for one class. Margaret is shaking her head like “are you kidding?” That’s crazy stuff. But he wanted to really support his students. His students loved him, but he was not going to get tenure. That’s the story.
So we have to be very thoughtful and strategic, and what can help you with this, and ASHA very firmly recognizes which is why we’re here—is that your mentors in your life should be there to help you learn these skills and learn what to say yes to, and learn what to say no to. I’ve learned to say things like, “Let me check with my mentor before I agree to that.” And it gives you a way out of that. The line that I use a lot is, “Let me check with my department head” or, I just said this to somebody last week, “I just promised my department head two weeks ago that I would only do X number of external workshops this year, so I’m going to have to turn this one down.” Those are really important skills to develop.
And having that research plan in place that you can go back to and say, know what, it’s not on my plan I can’t do it. If I do it—I have to go back to my research plan and figure out what I’m going to kick off in order to review this extra paper, in order to take on this extra task. The plan also helps me to know exactly what to say no to. And to be very direct and have a very strong visual.
I actually have my research plan up on a giant whiteboard in my office, so I can always go back to that and see where I am, and I can say, “Okay, what am I going to kick off of here? Nothing. Okay, I have to say no to whatever comes up.” Just be strategic. This is where I see most beginning professors really end up taking that wrong fork in the road—taking that right instead of that left, and ending up not being the successful researcher that they wanted to be.

What evidence supports research planning? This was something Ray Kent had found. That a recent analysis had found that postdoc scholars who developed a written plan with their postdoc advisers were much more productive than those who didn’t. And your performance during a postdoc—and I know many of you have either finished your postdoc or decided not to—so more simply, just during those first six years, the decisions you make really do establish the foundation for the rest of your professional life. It’s very important to get started and get off on the right foot.

I love this quote, I just found it the other day: “Productivity is never an accident. It is always the result of a commitment to excellence, intelligent planning, and focused effort.”
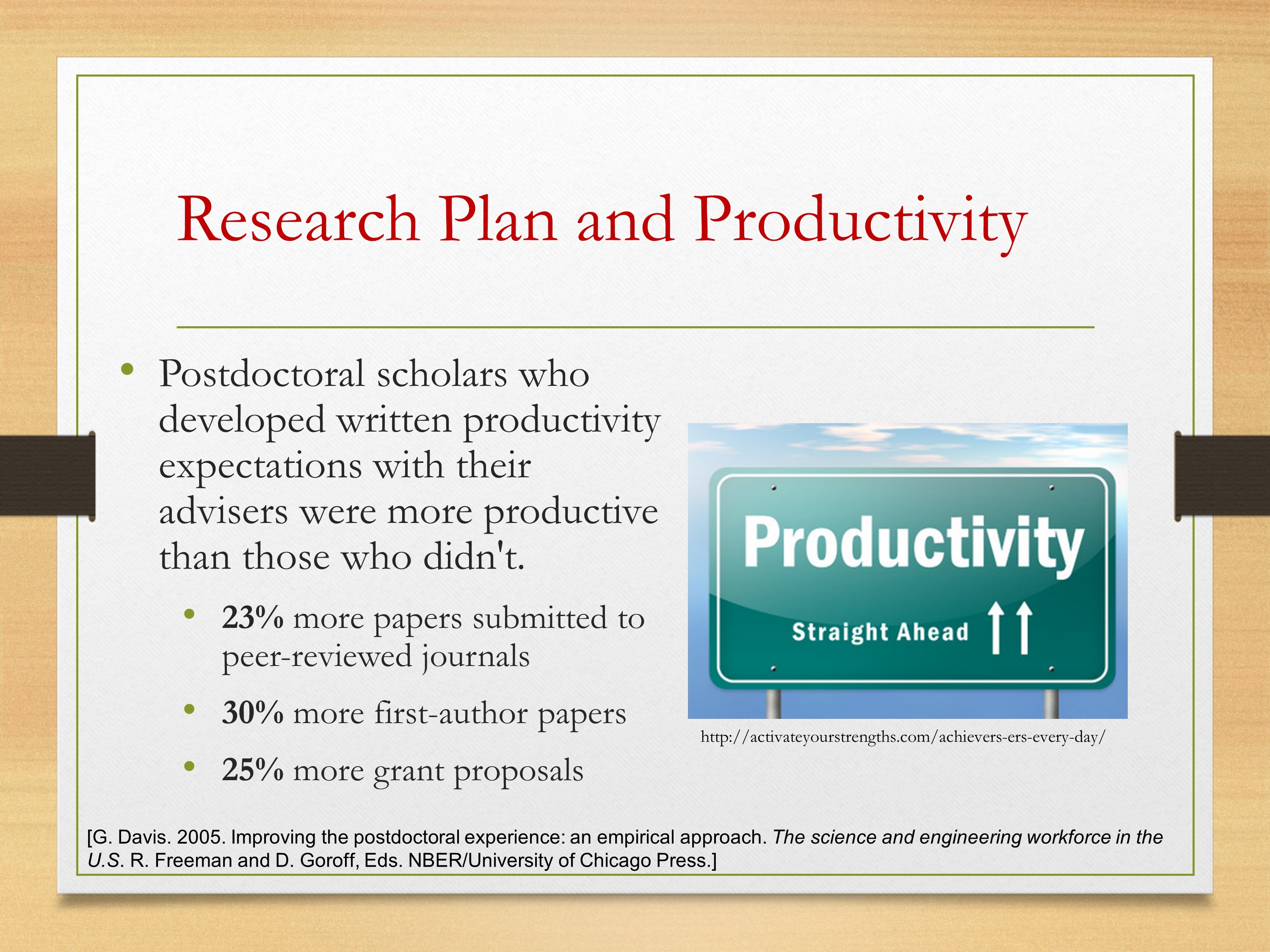
What we see with productivity is that postdoc scholars who developed written productivity expectations with their advisers were more productive than those who didn’t. You see 23% more papers submitted, 30% more first-author papers, and more grant proposals as well.

So why five years? I’m going to start with number 5. It’s long enough to build a program of research, but short enough to deal with changing circumstances. That’s really the long and the short of the matter. As well as these other things as well that I won’t take the time to go through point by point.
What Should a Five-Year Plan Include?
Presented by Lizbeth Finestack

So, thinking about a five-year research plan, I like to think about it like your major “To Do List.” It’s what you’re going to accomplish in five years. Start thinking: What is going to be on my to do list?

You can also think about it like: Okay, I have research. I’ve got to do research. Maybe think about this as one big bucket, or maybe one humongous silo. I have some farm themes going on. Cathy was just on a farm, so I thought I’d tie that in.
So here’s your big silo. You can call that your research silo.

But more realistically, you need to think about it like separate buckets, separate silos, where research is just one of those. Just like Cathy indicated, there’s going to be lots of other things coming up that you’re going to have to manage. They are going to have to be on your to do list, you need to figure out how to fit everything in.
What all those other buckets or silos are, are really going to depend on your job. And maybe the size of the silos, and the size of the buckets are going to vary depending on where you are, what the expectations are at your institution.
That’s important to keep in mind, and Cathy said this too, it’s not going to be the same for everyone. The five-year plan has to be your plan, your to do list.

Here are some buckets or some silos that I have on my list and the way that I break it up, this is just one example, take it or leave it.
The first three are all very closely related, right? Thinking about grants, thinking about research, thinking about publications. I’m going to define grants as actual writing, getting the grant, getting the money.
Research is what you’re going to do once you get that money. Steps you need to take before you are getting the money. Any sorts of projects, the lab work, that’s why I have the lab picture there. Of course, publications are part of the product—what’s coming out of the research—but it also cycles in because you need publications to support that you are a researcher to apply for funding and show you have this line of research that you’ve established and you’ll be able to continue. So, those first three are really closely related. And that’s where I’ll go next. And then have teaching and service you see here at the bottom.

So thinking about research, in that broad sense. As you’re writing your five-year plan you’re going to want to think of, “What’s my long-term goal?” There’s lots of ways to think of long-term goals. You could think, before I die, this is what I want to accomplish. For me I kind of have that. My long-term goal is that I’m going to find the most effective and efficient interventions for kids with language impairment. Huge broad goal. But within that I can start narrowing it down.
Where am I within that? Within the next five years or maybe the next ten years, what is it I want to accomplish towards that goal. Then start thinking about: In order to accomplish that goal, what are the steps I need to take? Starting to break it down a little bit. Then it’s also going to be really important to think: where are you going to start? Where are you now? What do you need to have happen? And is it reasonable to accomplish this goal within five years? Is it going to take longer? Maybe you could do it in a couple years? Start thinking about the timeline that’s going to work for you.
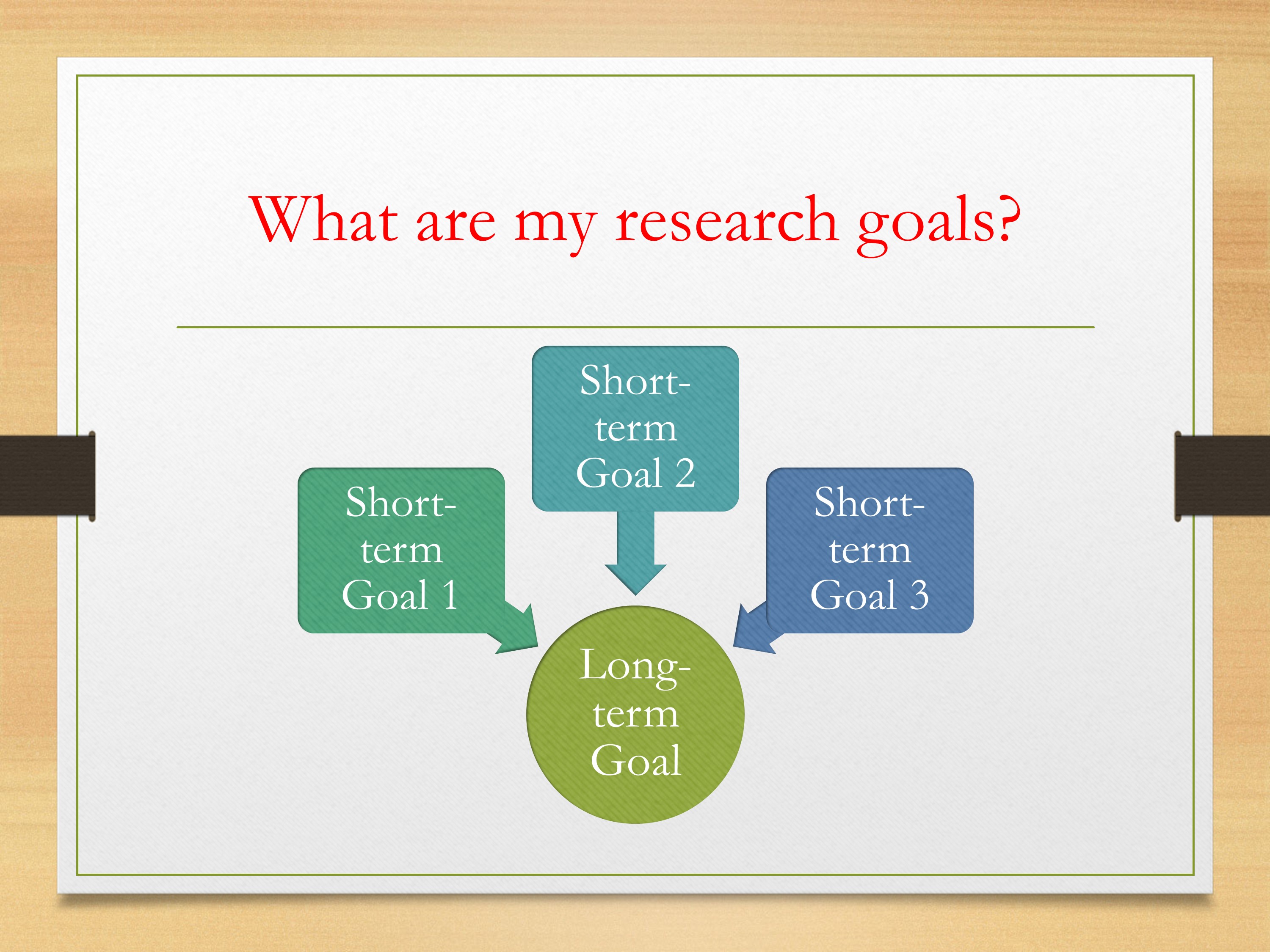
Then thinking about your goals—and everyone’s program is going to be different, like I said, there’s going to be a lot of individual needs, preferences. So it might be the case that you have this one long-term goal that you’re aiming for. Long-term goal in the sense of, maybe, what you want to study in your R01, perhaps something like that. But in order to get to that point, you’re going to have several short-term goals that need to be accomplished.
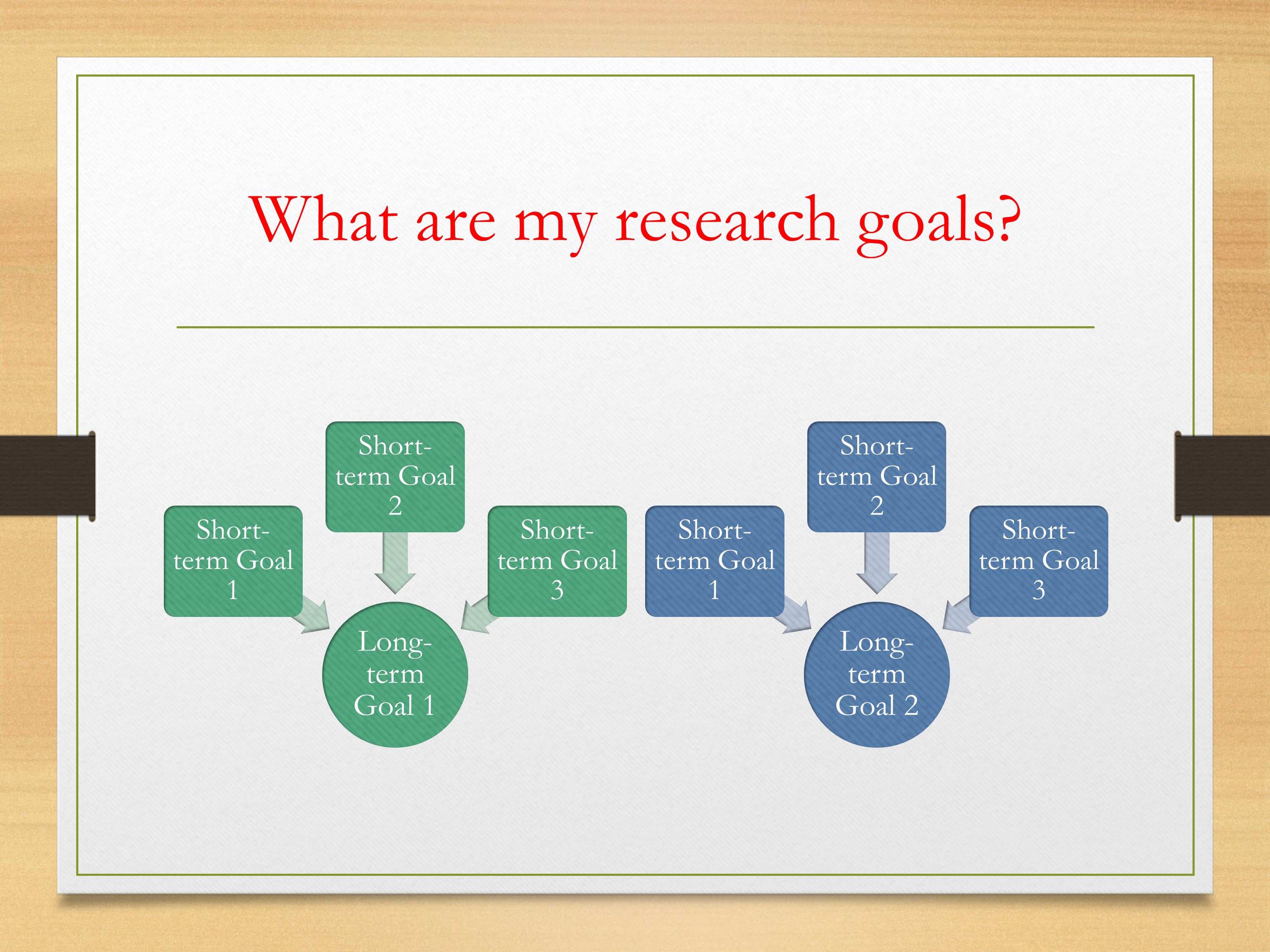
Or maybe it’s the case that you have two long-term goals. And with each of those you’re going to have multiple short-term goals that you’re working on. Maybe the scope of each of these long-term goals is a little bit less than in that first scenario.
Start thinking about my research, what I want to do, and how it might fit into these different circumstances.

Also thinking about your goals, this is a slide from Ray Kent from last year, was thinking about the different types of projects you might want to pursue, and thinking about ones that are definitely well on your way. They are safe bets. You have some funding. They are going to lead directly into your longer-term plan.
Those are going to be your front burner—things you can easily focus on. That said, don’t put everything there.
You can also have things on the back burner. Things that really excite you, might have huge benefits, big pay. But you don’t want to spend all of your time there because they could be pretty risky.
Start thinking about where you’re putting your time. Are you putting it all on this high-risk thing that if it doesn’t pan out you’re going to be in big trouble? Or balancing that somewhat with your front burner. Making that steady progress that will lead directly to help fund an R01 or whatever the mechanism that you’re looking for.
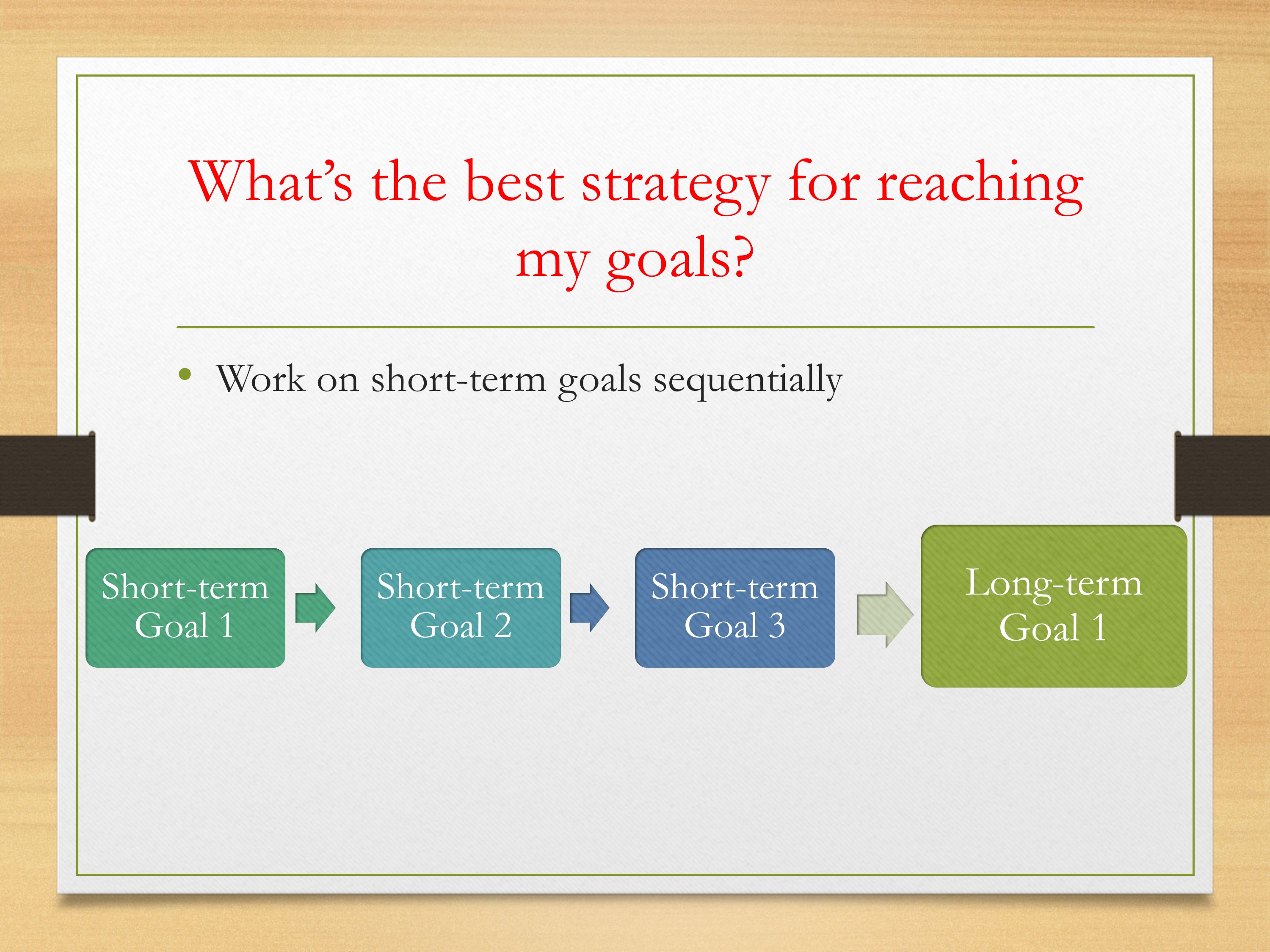
Then, thinking about your goals—if you have multiple long-term goals, or thinking about your short-term goals, you could think about your process. Is it something where you need to do study 1 then study 2, then study 3—each of those building on each other, that’s leading to that long-term goal. In many cases, that is the case, where you have to get information from the first study which is going to lead directly to the second study and so forth.
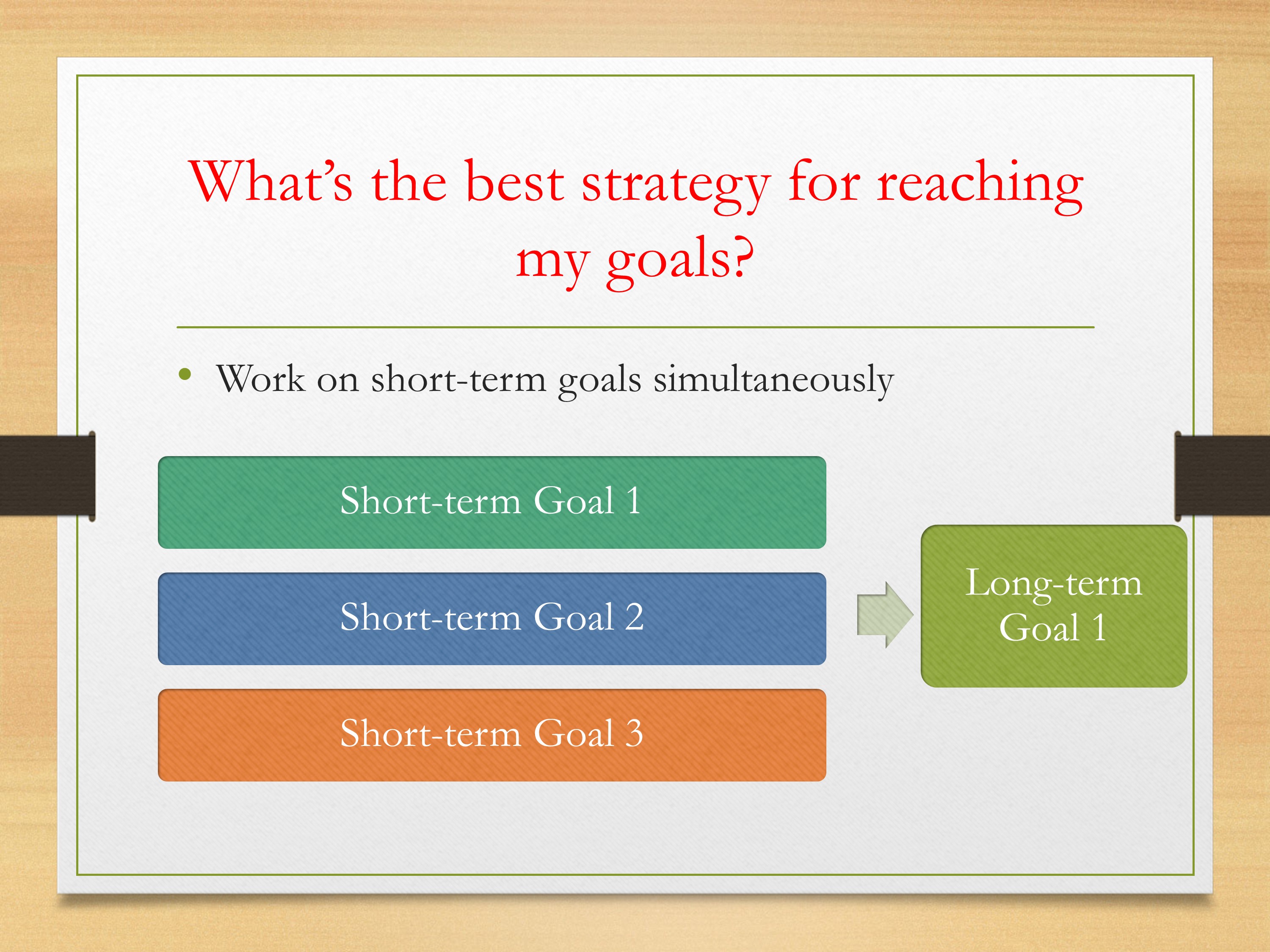
Or is it the case that you can be working on these three short-term goals simultaneously? Spreading your resources at the same time. Maybe it will take longer for any one study, but across a longer period of time you’ll get the information that you need to reach that long-term goal.
Lots and lots of different ways to go about it. The important thing is to think about what your needs are and what makes the most sense for you.
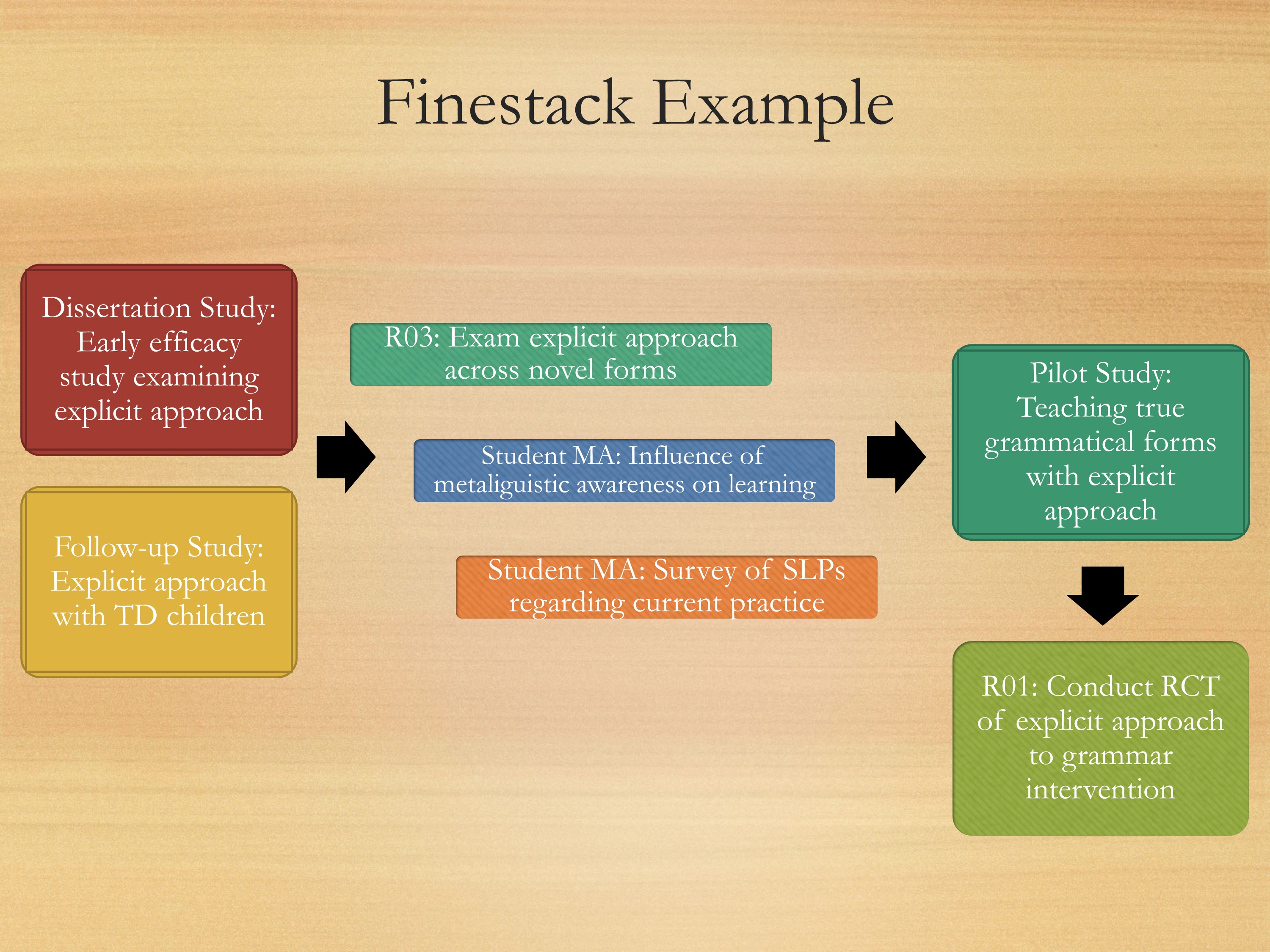
Here’s my own little personal example. Starting over here, I have my dissertation study. My dissertation study was this early efficacy study looking at one treatment approach using novel forms that really can’t generalize to anything too useful, but it was important.
Then I did a follow up study, where I was taking that same paradigm, looking to see where kids with typical development perform on the task. So I have these two studies, and they served as my preliminary studies for an R03. So I just finished an R03 where I was looking at different treatment approaching for kids with primary language impairment. At the same time, while conducting my R03, I’m also looking at some different approaches that might help with language development. Also conducting surveys to see what current practices are.
I have these three projects going on simultaneously, that are going to lead to a bigger pilot study that are going to feed directly into my R01. All of this will serve as preliminary data to go into an R01.
Start thinking about your projects, what you have. Maybe starting with your dissertation project or work that you’re doing as a postdoc as seeing how that can feed into your long-term goal. And really utilizing it, building on it, to your benefit.

That’s all fine and dandy. You can draw these great pictures. But you still have to break it down some more. It’s not like, “Oh, I’m just going to do this project.” There are other steps involved, and lots of the time these steps are going to be just as time consuming.
Starting to think about: well, if you have the funding. Saying, “I want to do this study, but I have no money to do it.” What are the steps in order to get the money to do it? Do you have a pilot study? What do you need?
Start thinking about the resources? Do you need to develop stimuli, protocols, procedures? Start working on that. All of these can be very time consuming, and if you don’t jump on that immediately, it’s going to delay when you can start that project.
Thinking about IRB. Relationships for recruitment, if you’re working with special populations especially? Do you have necessary personnel, grad students, people to help you with the project? Do you need to train them? What’s the timeline of the study?
Start thinking about all these pieces, and how they are going to fit in that timeline.
This is one way that might help you start thinking about the resources that you need. This is online—Ray Kent had it in his talk, and when I was doing my searches I came across it too and I have the website at the end. Just different ways to think about the resources you might need.
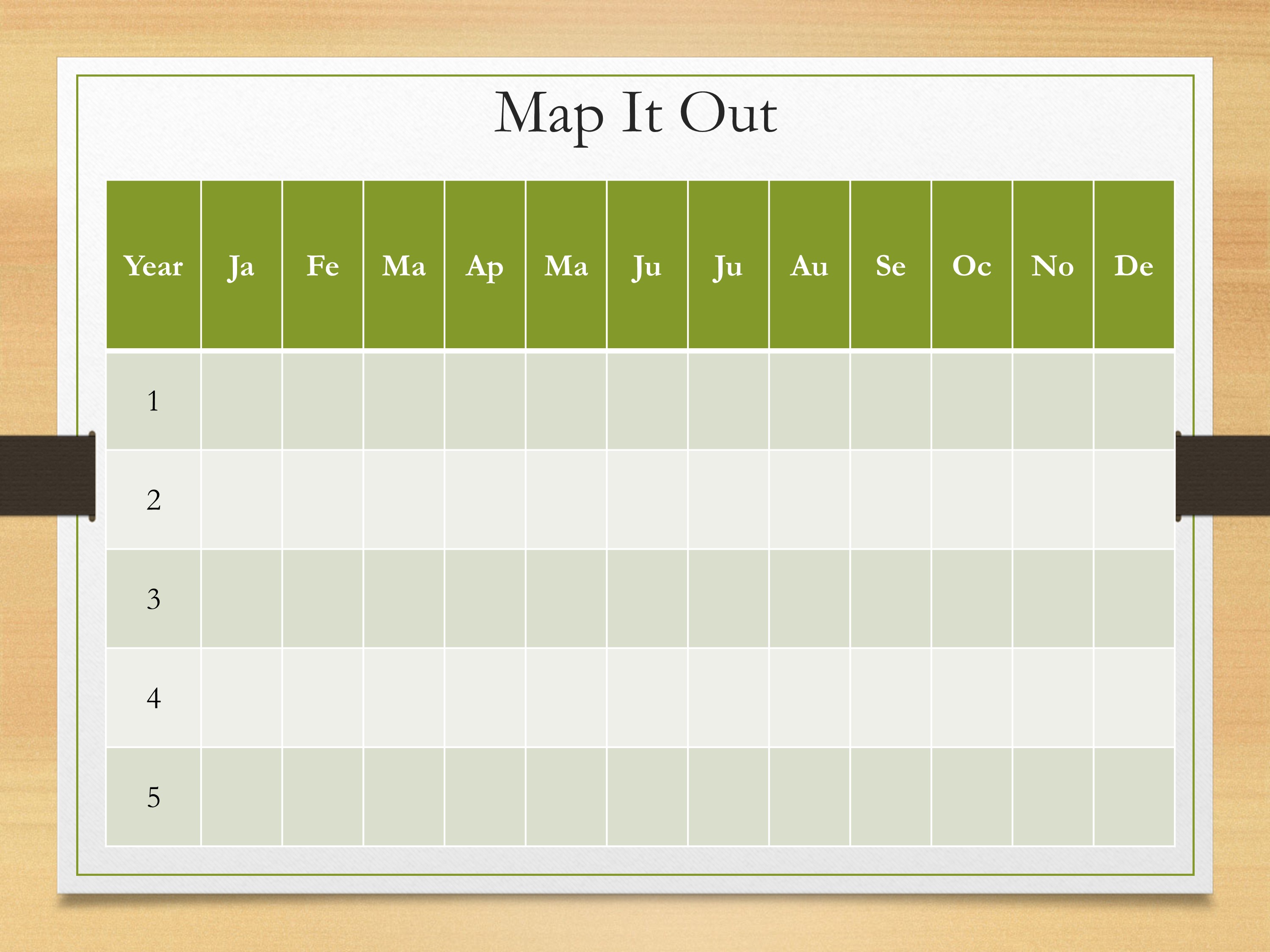
Let’s talk about mapping it out. You have your long-term goal. You have your short-term goals. You’re breaking it down thinking about all those little steps that you need to accomplish. We gotta put it on a calendar. When is it going to happen?
This is an example—you might have your five years. Each month plugging in what are you going to accomplish by that time. Maybe it’s when are grant applications due? It’s going to be important to put those on there to go what do I need to do to make that deadline. Maybe it’s putting when you’re going to get publications out. Things like that.
Honestly, looking at this drives me a little bit crazy, it seems a bit overwhelming. But it’s important to get to these details.
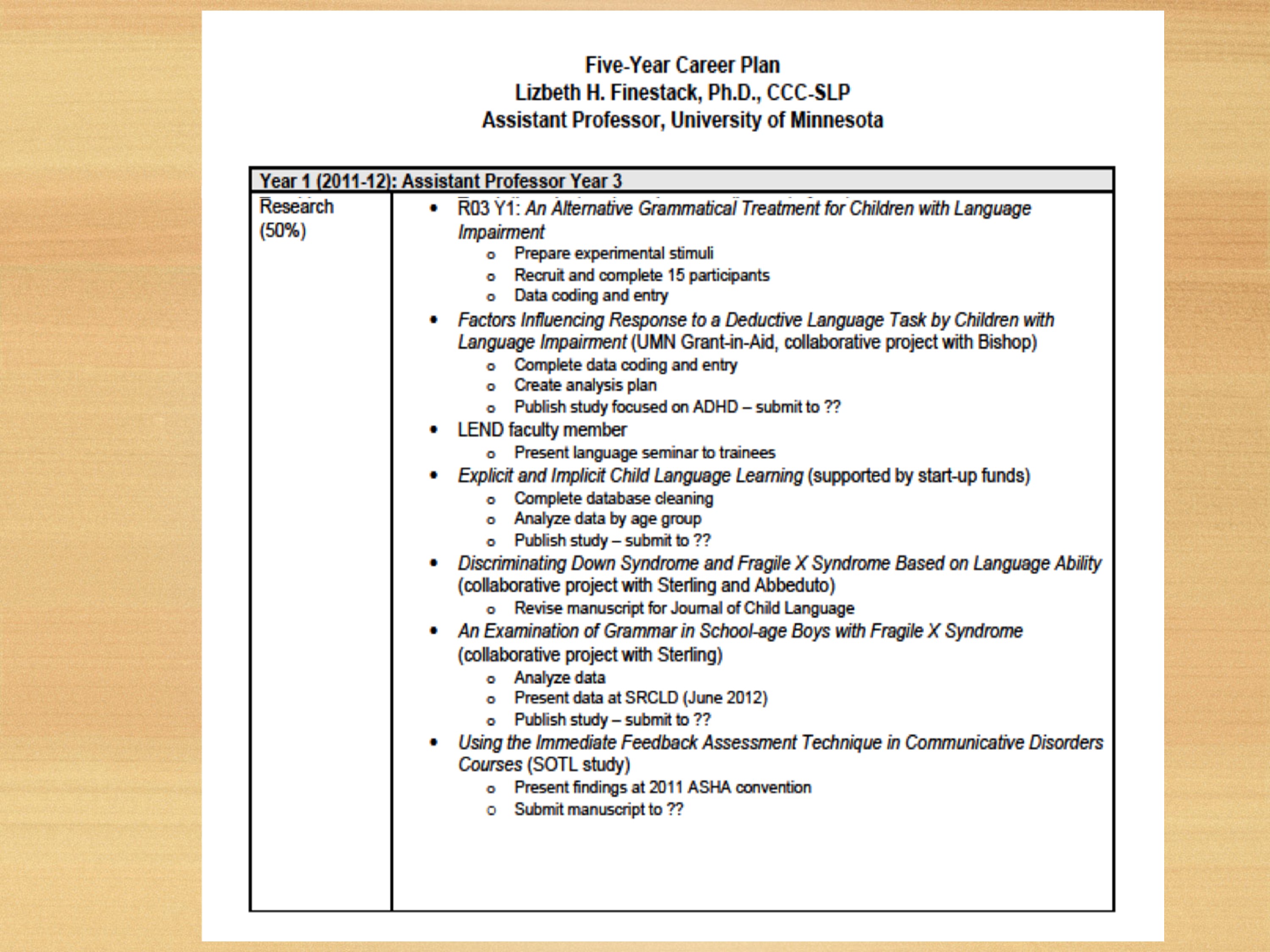
This is an example from, I did Lessons for Success a few years ago and they had their format for doing your plan. I wrote out all my projects, started thinking about all the different aspects. So if something like this works for you, by all means you could use that type of procedure.
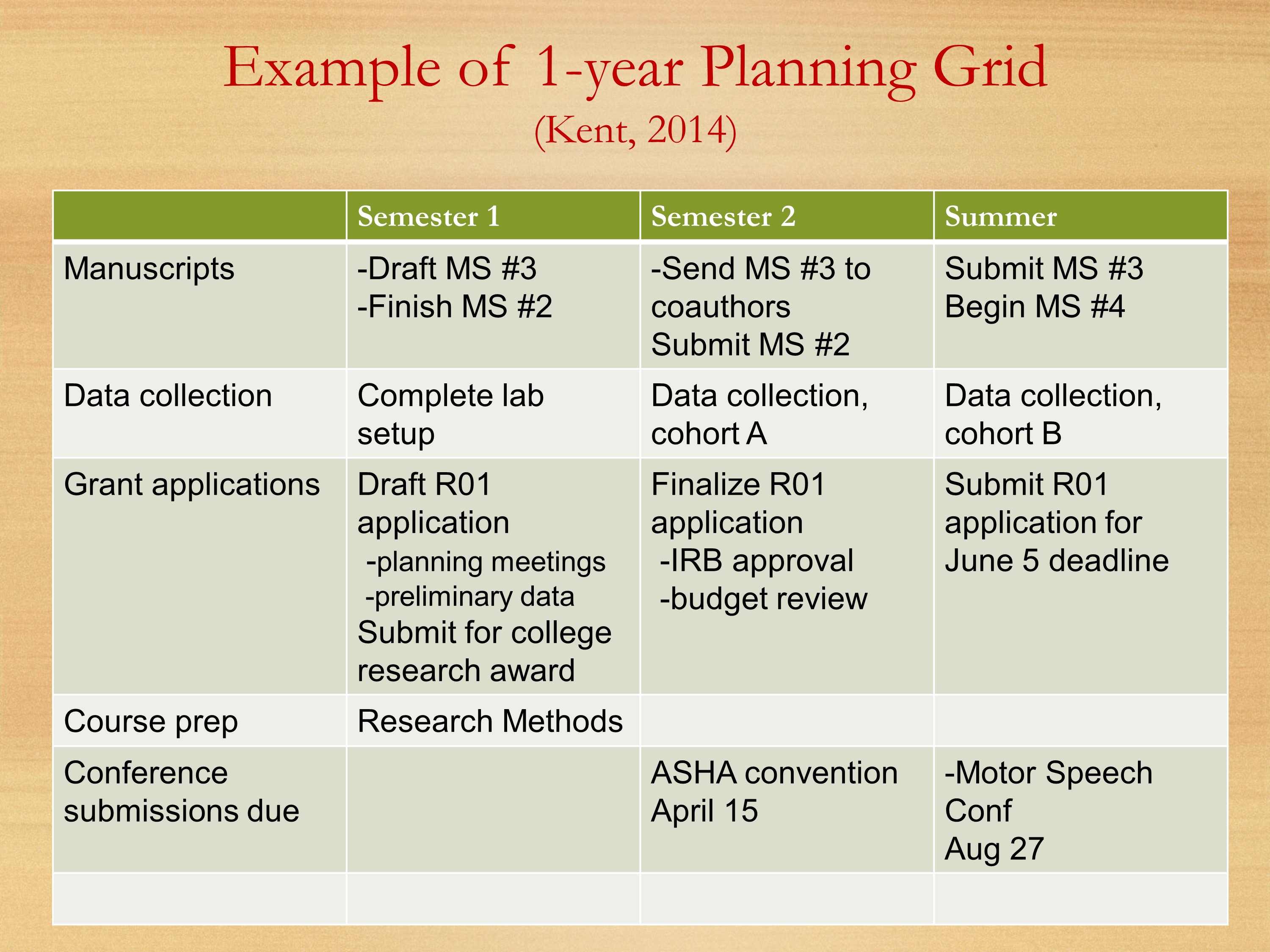
Here’s a grid that Ray Kent showed last year. We’re breaking it down by semester. Thinking about each of your semesters, what manuscripts you’re going to be working on, what data collection, your grant applications. Starting to get into some of those other buckets: course preparation, conference submissions.
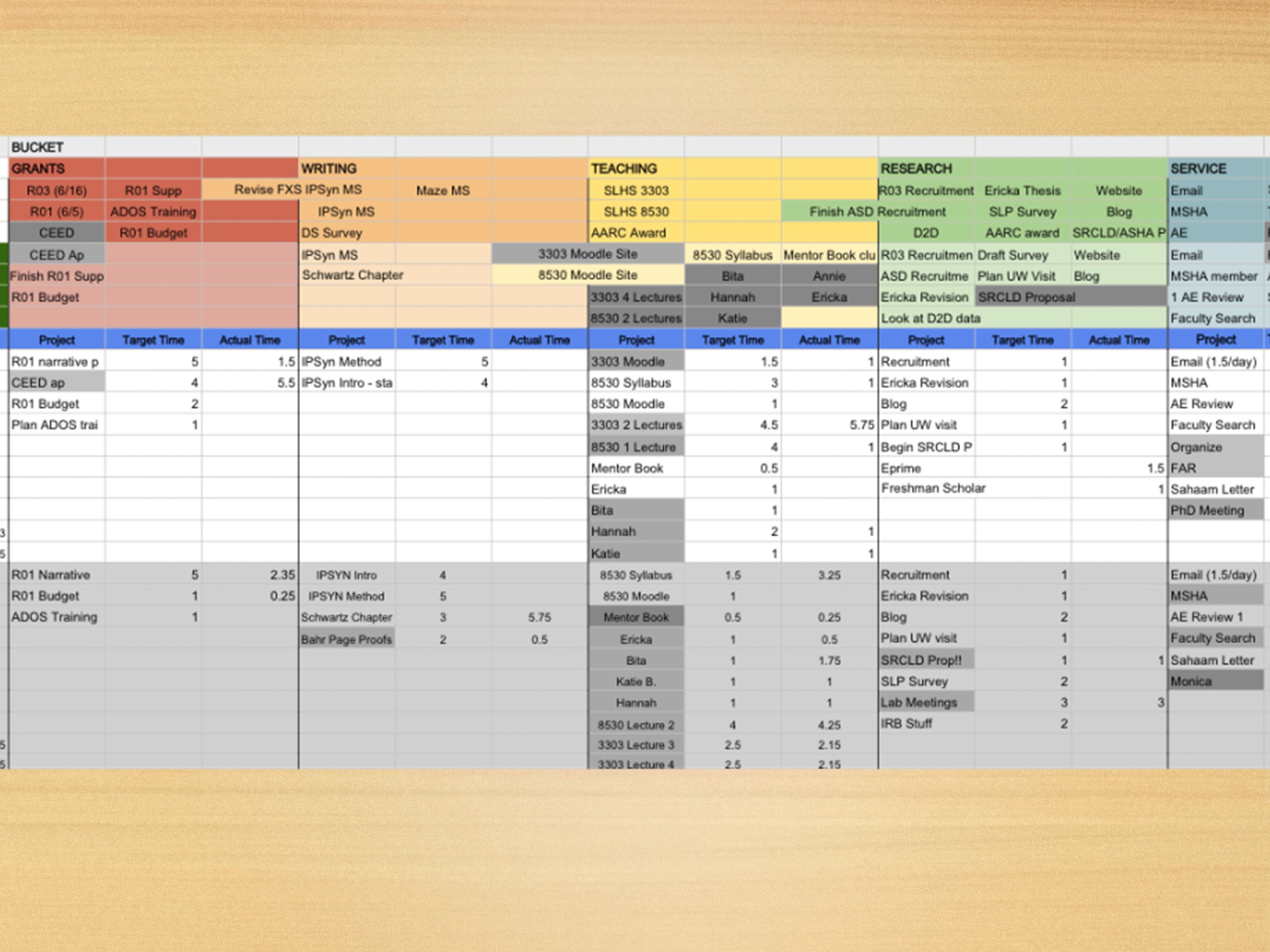
We also need to include teaching and service.
You probably can’t see this very well. This is similar to that last slide Ray Kent had used last year.
I have my five year plan: what studies I want to accomplish, start thinking about breaking it down.
Then at the beginning of each semester, I fill in a grid like this. Where at the top, I have each of my buckets. I have my grant bucket, my writing bucket which is going to include publications. I also include doing article reviews in my writing bucket, because that’s my writing time. My teaching bucket, my research bucket. Then at the end, my service bucket.
At the beginning of the semester, I think about the big things I want to accomplish. I list those at the top. Then at the beginning of each month, I say, okay what are the things I’m going to accomplish this month, write those in. Then at the beginning of each week, I start looking at whether I’m dedicating any time to the things I said I was going to do that month. I start listing those out saying, this is the amount of time I’m going to spend on that. Of course, I have to take data on what I actually do, so I plug in how much time I’m spending on each of the tasks. Then I graph it, because that’s rewarding to see how much time you’re spending on things, and I get a little side-tracked sometimes.
Think about a system that will help you keep on track, to make sure you’re meeting the goals that you want to meet in terms of your research. But also getting the other things done that you need to get done in terms of teaching and service.
Discussion and Questions
Compiled from comments made during the Pathways 2014 and 2015 conferences. (Video unavailable.)
Building Flexibility into Your Five-Year Plan Comments by Ray Kent, University of Wisconsin-Madison
The five-year plan is not a contract. It’s a map or a compass. A general set of directions to help you plan ahead. It’s not even a contract with yourself, because it will inevitably be revised in some ways.
Sometimes cool things land in your lap. Very often it turns out that through serendipity or whatever else, you find opportunities that are very enticing. Some of those can be path to an entirely new line of research. Some of them can be a huge distraction and a waste of time. It’s a really cool part of science that new things come along. If we put on blinders and say, “I’m committed to my research plan,” and we don’t look to the left or the right, we’re really robbing ourselves of much of the richness of the scientific life. Science is full of surprises, and sometimes those surprises are going to appear as research projects. The problem is you don’t want to redirect all your time and resources to those until you’re really sure they are going to pay off. I personally believe, some of those high risk but really appealing projects are things you can nurse along. You can devote some time and build some collaborations – far enough to determine how realistic and viable they are. That’s important because those things can be the core of your next research program.
It’s very easy to get overcommitted. We all know people who always say “yes”—and we know those people, and they are often disappointing because they can’t get things done. It’s important to have new directions, but limit them. Don’t say, “I’m going to have 12 new directions this year.” Maybe one or two. Weigh them carefully. Talk about them with other people to get a judgment about how difficult it might be to implement them. It enriches science: not only our knowledge, but the way we acquire new knowledge. A psychologist, George Miller—this is the guy with the magic number 7 +- 2—when we interviewed him years ago at Boystown, he said, “My conviction is that everybody should be able to learn a new area of study within three months.” That’s what he thought for a scientist was a goal.
The idea is that you can learn new things. And that’s very important because when you think of it in terms of a 30-year career, how likely is it that the project that you’re undertaking at age 28 is the same project you’ll be working on at age 68? Not very likely. You’re going to be reinventing yourself as a scientist. And reinventing yourself is one of the most important things you can do, because otherwise you’re going to be dead wood. Some projects aren’t worth carrying beyond five or ten years. They have an expiration date.
Building Risk into Your Five-Year Plan Comments by Ray Kent, University of Wisconsin-Madison
Your doctoral study should generally be low-risk research. As you move into a postdoctoral fellowship, think about having two studies—one low-risk, one high-risk with a potential for high impact. At this time you can begin to play the risk factor a little bit differently.
When you are tenure-track you can have a mix of significance with low-risk and high-risk studies. And when you are tenured, then you can go for high risk, clinical trials, and collaborations. Because you have established your independence, so you do not need to worry about losing your visibility. You can be recognized as a legitimate member of the team.
As you plan your career, you should take risk into account. Just as you manage your money taking risk into account, we should manage our careers taking risk into account. I have met people who did not really think about that, and they embarked on some very risky procedures and wasted a lot of time and resources with very little to show for it. For example, don’t put everything into an untested technology basket. You want to be using state of the art technology, but you want to be sure it is going to give you what you need.
Other Formats and Uses of Your Research Plan Audience Comments
- If you do your job right with your job talk, there’s a lot of cross-pollination between your job talk and your research plan. Ideally your job talk tells your colleagues that this is the long-term plan that you have. And they shouldn’t be surprised when you submit a more detailed research plan. They should say, “okay this is very consistent with the job talk.” In my view, the job talk should be a crystal summary of the major aspects of that research program. Of course, much of the talk will be about a specific project or two—but it should always be embedded within the larger program. That helps the audience keep sight of the fact that you are looking at the program. You can say that this is one project that I’ve done, and I plan to do more of these, and this is how they are conceptually related. That’s a good example of why the research plan has multiple purposes – it can be a research statement, it can be the core of your job talk, it can be the nature of your elevator message, and it can be a version of your research plan for a K award application or R01 application or anything else of that nature.
- I think what’s useful is to actually draft your NIH biosketch. The new biosketch has a section called “contributions to science.” It’s really helpful to think about all your projects. It’s hard to start with a blank sheet of paper. But to have it in the format of a biosketch can be really helpful.
Avoiding Overcommitment Audience Comments
- One of the things that is amazing about planning is that if you put an estimate on the level of effort for each part of your plan, you’ll quickly find that you are living three or four lives. Some 300% of your time is spent. It’s helpful for those of us who might share my lack of ability to see constraints or limitations to reel it back and say, “I have a lot on my plate.” Which allows you to say no—which is not something we all do very well when it comes to those nice colleagues and those people you want to impress nationally and connect with. But it allows you to look at what’s planned and go, “I don’t know where I’d find the time to do that.” Which will hopefully help you stay on track.
- I keep a to do list, but I also keep a “to not do” list. One of the things I will keep on my plan is the maximum number of papers I will review in a year. If I hit that number in March, that’s it. I say no to every other paper that comes down the pike. That’s something to work out with your mentor as far as what’s realistic and what’s okay for you. Every time I get a request, I think, “That’s my reading and writing time, so what am I willing to give up. If it means I won’t be able to write on my own paper this week, am I willing to do this?”
Staying on Schedule with Reading, Writing, and Reviewing Audience Comments
- You have to do what works for you. Some people do wait for big blocks of time for writing—which are hard to come by. But the most important thing is to block off your time. Put it on your schedule, or it is the first thing that will get pushed aside.
- Another thing I’ve done with some of my colleagues is writing retreats. So maybe once a year, twice a year, we’ll get together. Usually we’ll go to a hotel or somewhere, and we’re just writing. It’s a great way to get a jumpstart on a project. Like, I need to sit down and start this manuscript, and you can keep going once you’ve got that momentum.
- My input would be that you really have to write all the time, every day. It’s a skill. I’ve found that if I take time off, my writing deteriorates. It’s something you need to keep up with.
- I would look at it like a savings account that you put money into on a daily, weekly, monthly basis. The flip side of writing is reading. I would read constantly, widely, and not just in the discipline. That will give you not only a breadth in terms of your understanding of your field and the world around you, but it will also give you an incentive to make your own contributions. I think we don’t talk enough about the comprehensive side to this, and being receptive to the reading. I have a book, or something, by my bedside every night. And I read that until I fall asleep every night. And it’s done me in good stead over the years.
- Reviewing articles can help advance your career, but it is something you need to weigh carefully as a draw on your time. You get a lot from it. You get to see what’s out there. You get to see what’s coming down the pipe before publication. To me that’s a huge benefit. You get to learn from other people’s writing, and that’s part of your reading you get to do. But it is time consuming. And it depends on the kinds of papers you get. Sometimes you’re lucky and sometimes you’re not.
- If someone else is reviewing your grants and your articles, at some point you owe it back. You should at least be in break-even mode. Now, pre-tenure or postdoc your mentor should be doing that or senior faculty in the department. But there are so many articles to review. I review so many articles, but I am also at the tail end of my career. The bottom line is, if you don’t put on your schedule that if you don’t put time on your schedule for reading, reviewing articles forces you to look at and think about the literature, so you can be accomplishing what you owe back to the field—and at the same time, staying one step ahead knowledge wise. It forces you to do what you should be doing all along, which is keeping up with the literature.
Further Reading: Web Resources
Golash-Boza, T. (2014). In Response to Popular Demand, More on the 5-Year Plan. The Professor Is In . Available at http://theprofessorisin.com/2014/05/09/in-response-to-popular-demand-more-on-the-5-year-plan
Kelsky, K. (2010). The Five-Year Plan for Tenure-Track Professors. Get a life, PhD . Available at http://getalifephd.blogspot.com/2010/07/five-year-plan-for-tenure-track.html
National Association of Geoscience Teachers (NAGT). (2012). Planning Worksheets . Planning your Research Program (Available from the Science Education Resource Center at Carelton College Website at http://serc.carleton.edu/).
Pfirman, S., Bell, R., Culligan, P., Balsam, P. & Laird, J. (2008) . Maximizing Productivity and Recognition , Part 3: Developing a Research Plan. Science Careers. Available at http://sciencecareers.sciencemag.org/career_magazine/previous_issues/articles/2008_10_10/caredit.a0800148
Cathy Binger University of New Mexico
Lizbeth Finestack University of Minnesota
Based on a presentation and slides originally developed by Ray Kent, University of Wisconsin-Madison.
Presented at Pathways (2015). Hosted by the American Speech-Language-Hearing Association Research Mentoring Network.
Pathways is sponsored by the National Institute on Deafness and Other Communication Disorders (NIDCD) of the National Institutes of Health (NIH) through a U24 grant awarded to ASHA.
Copyrighted Material. Reproduced by the American Speech-Language-Hearing Association in the Clinical Research Education Library with permission from the author or presenter.

Clinical Research Education
More from the cred library, innovative treatments for persons with dementia, implementation science resources for crisp, when the ears interact with the brain, follow asha journals on twitter.

© 1997-2024 American Speech-Language-Hearing Association Privacy Notice Terms of Use
The Lesson Study Group
at Mills College

Develop a Research Theme
Access the teaching-learning plan, choose a topic.
10-30 minutes
- Develop or revisit your your long-term goals for student development
- Develop a draft of your “theory of action”—the approaches you will explore to build your long-term goals

Identify Your Long-Term Goals
The building blocks of your theme.
A research theme expresses the long-term goals of your work. If your team or school has already developed a research theme, revisit it now to refresh your memory about your long-term goals and ideas about how to get there.
Begin by having team members individually jot down qualities in response to the following prompt:
- Ideally, what qualities do we hope students will have when they graduate from our school? ( If we bumped into our students in 5-10 years, what qualities do we hope they would have?)
Now, again working individually, spend a few minutes jotting down a list of qualities in response to a second prompt:
- What are the current qualities of our students? (For example, what qualities of our students inspire us? Anything that concerns us?)
Again, share your individual lists and write all the qualities on a second list labeled “Current.”
Compare the two lists–ideal and current–and notice gaps that really speak to you as educators. Find one or two gaps where you would like to invest your time and energy.
Your research theme positively states the qualities you will work toward. Some examples follow.
- “For students to value friendship, develop their own perspectives and ways of thinking, and enjoy science.”
- “Develop social-emotional skills and…a deeper understanding of mathematics”
- “Across both math and language arts, develop our students’ abilities to use evidence and reasoning to support and critique arguments.”
- “…to take responsibility and initiative as learners.”
A lot of [U.S.] schools develop mission statements, but we don’t do anything with them. The mission statements get put in a drawer and then teachers become cynical…Lesson Study gives guts to a mission statement, makes it real, and brings it to life.
Develop a Theory of Action
Moving from the what to the how.
The second part of your research theme is a “theory of action”—how you will work toward your long-term goals and the specific research questions you will examine. What experiences in school help students move toward a goal such as “students have their own thoughts and can explain them logically?” Teachers addressing this research theme focused their initial theory of action on two classroom routines: students’ presentation of ideas at the board and their use of reflective journals. They actively tested strategies to improve these two classroom routines and posed questions about them. For example, they asked what the features are of effective student presentations and how teachers help students see the power of these strategies (such as using visual models). In order to strengthen the impact of reflective mathematics journals, teachers strategically selected several student journals from the prior day to be read aloud at the beginning of each mathematics lesson, which built students’ interest in each other’s ideas and helped them see the impact of well-explained ideas. The first part of your research theme—your overarching goal—is likely to stay the same for several years. The second part of your research theme—your theory of action—is likely to change as you incorporate effective ideas into your practice and go on to experiment with additional changes designed to achieve your long-term goals. For example, the group that experimented with changes to student presentations and reflective journals went on to experiment with routines for discussion and lesson summarization that further built students’ capacity “to have their own thoughts and explain them logically.”
Developing a Research Theme Presentation

Examples of Research Themes
- What is Lesson Study?
- Why Lesson Study?
- Teacher Learning
- Content Resources
- Teaching Through Problem-solving (TTP)
- School-wide Lesson Study
- U.S. Networks
- International Networks

The Academic Medicine Handbook pp 243–249 Cite as
How to Conceptualize a Research Project
- Shaili Jain M.D. 2 , 3 ,
- Steven E. Lindley MD, PhD 2 , 3 &
- Craig S. Rosen PhD 2 , 3 , 4
- First Online: 01 January 2013
3398 Accesses
1 Citations
The research process has three phases: the conceptual phase, the empirical phase, and the interpretative phase. In this chapter, we focus on the first phase: the conceptual phase—the part of the research process that determines which questions are to be addressed by the research and how research procedures are to be used as tools in finding the answers to these questions. Here we describe the various components of the conceptualization phase that need to be carefully considered before moving on to the empirical and interpretative phases of the research. Conceptualization involves simultaneously bringing together several considerations to identify a good research idea, i.e., an answerable research question that is worth answering. Components of this process include conducting a thorough search of the peer-reviewed literature, finding a research mentor and other collaborators, considering methodology and study design, and assessing feasibility. It should be noted that although we describe these various components in a linear fashion in the text, in reality, the conceptualization phase is not a linear process and will require consideration of these components to varying degrees at various stages depending upon evolving circumstances and the reader’s unique strengths and weaknesses. Even though careful attention to all these components will require considerable time and effort on the part of the physician scientist, we consider this to be time well spent as it will lay the ground for a successful research endeavor.
This is a preview of subscription content, log in via an institution .
Buying options
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
Tax calculation will be finalised at checkout
Purchases are for personal use only
Rose AM. Sociology: the study of human relations, 2nd ed. New York, NY: Alfred A. Knopf; 1965, p. 9
Google Scholar
Batey MV. Conceptualizing the research process. Nurs Res. 1971;20:296–301.
Article PubMed CAS Google Scholar
Sambunjak D, Straus SE, Marušic´ A. Mentoring in academic medicine: a systematic review. JAMA. 2006;296:1103–15.
Horn C, Plazas Snyder B, Coverdale JH, et al. Weiss Roberts L: educational research questions and study design. Acad Psychiatry. 2009;33:261–7.
Article PubMed Google Scholar
Additional Resources
Chapters 8, 10, 19, 20, and 24. In: Roberts LW, Hilty D, editors. Handbook of career development in academic psychiatry and behavioral sciences, 1st ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2006.
Hulley SB, Cumming CR, Warren S, et al. Designing clinical research. 3rd ed. Philadelphia, PA, USA: Lippincott Williams & Wilkins; 2007.
Kraemer HC, Kraemer KL, Kupfer DJ. To your health: how to understand what research tells us about risk. New York: Oxford University Press; 2005.
Motulsky H. Intuitive biostatistics: a nonmathematical guide to statistical thinking. New York: Oxford University Press; 2010.
Download references
Author information
Authors and affiliations.
VA Palo Alto Health Care System, National Center for Posttraumatic Stress Disorder, Menlo Park, CA, USA
Shaili Jain M.D., Steven E. Lindley MD, PhD & Craig S. Rosen PhD
Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Stanford, CA, USA
VA Sierra-Pacific Mental Illness Research, Education and Clinical Center, Menlo Park, CA, USA
Craig S. Rosen PhD
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Shaili Jain M.D. .
Editor information
Editors and affiliations.
, Department of Psychiatry and Behavioral, Stanford University School of Medicine, 450 Serra Mall, Stanford, 94305, California, USA
Laura Weiss Roberts
Rights and permissions
Reprints and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter.
Jain, S., Lindley, S.E., Rosen, C.S. (2013). How to Conceptualize a Research Project. In: Roberts, L. (eds) The Academic Medicine Handbook. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-5693-3_30
Download citation
DOI : https://doi.org/10.1007/978-1-4614-5693-3_30
Published : 22 February 2013
Publisher Name : Springer, New York, NY
Print ISBN : 978-1-4614-5692-6
Online ISBN : 978-1-4614-5693-3
eBook Packages : Medicine Medicine (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Copy/Paste Link Link Copied
Scientific Research Themes and Objectives
The NICHD Strategic Plan focuses the institute’s research and training efforts to understand human development, improve reproductive health, enhance the lives of children and adolescents, and optimize abilities for all. It presents the institute’s research goals and objectives under five broad research themes:
- Understanding the Molecular, Cellular, and Structural Basis of Development
- Promoting Gynecologic, Andrologic, and Reproductive Health
- Setting the Foundation for Healthy Pregnancies and Lifelong Wellness
- Improving Child and Adolescent Health and the Transition to Adulthood
- Advancing Safe and Effective Therapeutics and Devices for Pregnant and Lactating Women, Children, and People with Disabilities
These five themes will enable NICHD to focus its efforts on the most significant public health challenges and advance science in these areas of priority. The institute will measure progress toward these priorities; analyze the scientific, clinical, and public health impact of its research; and periodically report on metrics to stakeholders.
Cross-Cutting Topics
Several cross-cutting topics emerged during discussions of NICHD’s public health and scientific priorities. It became clear that the institute’s success in advancing its scientific goals and objectives will depend on its ability to integrate the following topics into each scientific research theme.
Health Disparities
Pervasive disparities exist in the health of racial/ethnic, rural, low-resource, sexual and gender minority, and other underrepresented populations. Understanding the contribution of social, economic, structural, and regional factors is vital to advancing preventive, diagnostic, and intervention efforts. These factors are particularly important in maternal health and mortality, birth outcomes, infant mortality, child development, and exposure to trauma and injury. Improving approaches in populations that experience specific cultural, social, or access issues will be an emphasis across the research themes.
Disease Prevention
Disease prevention and health promotion are central to efforts across NICHD. Advancing methods to identify at-risk populations or specific genetic, nutritional, medical, environmental, social, and behavioral risk factors will enhance the ability to target interventions. The institute will concentrate its efforts on prevention in maternal and infant mortality and morbidity, injuries in children, and risk-taking behaviors in adolescents. Promoting research to prevent adverse health outcomes, improve early detection, and understand the optimal timing of prevention efforts will be critical to success in this area.
Infectious Disease
Infectious agents, such as HIV, continue to pose a significant threat to maternal health and child development. At the same time, pathogens with previously unknown disease-causing properties constantly evolve and emerge. NICHD plans to improve the basic understanding of how infectious pathogens affect pregnant women and young children, address the impact of infections on reproductive and overall health in children and adolescents, and advance safe and effective treatments for pregnant and lactating women, children, and people with disabilities.
Nutrition is critical to growth and development. New technologies and methods allow scientists to characterize the complex biology of nutrition and the effects of nutritional status on health in a more systems-based approach. The recent revolution in ‘omics technologies, including metabolomics, and discoveries related to the interplay among diet, nutritional status, and the microbiome create important scientific opportunities. These new avenues for scientific discovery will allow NICHD to understand the lifetime impact of nutrition on reproductive health, fertility, pregnancy, and fetal, child, and adolescent growth and development. Of unique interest to the institute is a better understanding of the composition and function of human milk, the effect of maternal nutrition and length of gestation on human milk composition and lactation, and the optimal source of nutrition and mode of nutrient delivery to the infant, especially preterm infants.
Global Health
Disease and disability have no national borders. Because of investments made by NICHD and others, maternal and child survival is improving globally. There is now an opportunity to shift to research aimed at better understanding and addressing long-term health outcomes and chronic conditions of at-risk mothers and children. New interventions to improve pre-pregnancy health will help benefit pregnancy outcomes and prevent prematurity, malnutrition, childhood stunting, disease, and developmental delays. Studying populations globally will also improve domestic capabilities to address disease and risk factors among the culturally diverse U.S. population. Finally, new technologies are emerging to improve assessment and intervention for people worldwide with intellectual, developmental, and physical disabilities, and NICHD will support work in this area.
Chief Research Officer
- UTMB Research
Strategic Plan
Research Strategic Plan
Explore utmb's research strategic communities.
UTMB research is prioritized into six health communities that are identified in Experts with the graphic badges below. Researchers with 10 or more research outputs within a health community during the last 5 years receive a badge in that community. Researchers without 10 or more research outputs will receive a badge for the community in which they have the largest number of outputs.
MARCH 30 RESEARCH RETREAT

Project Updates * New Initiatives Funding Updates * Break-Out Discussions
8:30 a.m. -12:30 p.m. health education center 3.222, retreat agenda, register * discussion group questionnaire.

Our Strategic Research Plan has broad input and which has been used by leadership to develop a path forward through goals, objectives and tactics. UTMB will create six integrated health communities that bring together researchers, educators, clinicians and community members to use prevention and treatment to transform illness to health.
The six integrated health communities are:
- Infectious/Immunological Health
- Brain Health
- Metabolic Health
- Life Span Health
- Specialized Health
- Public (social/physical) Health
A team of more than 50 UTMB research leaders have been instrumental in the development of meaningful objectives that are specific, measurable, identify resource requirements and arrayed over no more than two academic years. It is the relationship among objectives, strategies and tactics that holds the potential to align individual behavior with actions that change the direction and acceleration of UTMB research.
Right-Side Nav

- Health Care
- UTMB Support Areas
Got any suggestions?
We want to hear from you! Send us a message and help improve Slidesgo
Top searches
Trending searches

suicide prevention
8 templates

46 templates

cybersecurity
6 templates
10 templates

biochemistry
37 templates

18 templates

Research Presentation templates
Customize our free themes and templates for google slides or powerpoint and explain what your research is about. these designs are easy to edit, so that will speed things up.
Premium template
Unlock this template and gain unlimited access
Project Research Infographics
Download the "Project Research Infographics" template for PowerPoint or Google Slides and discover the power of infographics. An infographic resource gives you the ability to showcase your content in a more visual way, which will make it easier for your audience to understand your topic. Slidesgo infographics like this set...

Research Methods Lesson
If you deal with Science, it’s important to learn more about research methods. Teach your students about them with this presentation full of illustrations and drawings related to labs. Use graphs, maps, tables and overview diagrams to support your lecture in a visual way!

Final Year Project Thesis Defense
Download the "Final Year Project Thesis Defense" presentation for PowerPoint or Google Slides. Congratulations, you have finally finished your research and made it to the end of your thesis! But now comes the big moment: the thesis defense. You want to make sure you showcase your research in the best...

Formal Research Paper Slideshow
Have you seen these slides? They are perfect for presenting your research paper! First of all, because we have included all the necessary sections of this type of work, such as hypothesis, objectives, methodology, analysis and the conclusions of the paper. The second reason is that the formal style will...
Economics Thesis
If numbers, exchange rates, money and trading are your forte, odds are you’re already working on an economics thesis for your master’s degree. Defending your dissertation is the last step and the most difficult one, but Slidesgo can help you. Here’s our new free presentation template with a focus on...

Research Project Proposal
Before embarking yourself on a new project, especially if it’s about research, you need to set out a proposal to explain its viability. Here at Slidesgo we’re offering this theme that you can actually use for any kind of project, regardless of the topic.

Pregnancy Breakthrough
Giving birth to a baby is a beautiful occasion, a manifestation of love between two people. Obstetrics are key during pregnancy, so how about giving a presentation about the latest breakthrough in this field? Our free medical template will come in handy.

Oral Cavity Diseases: Gingivitis
Download the "Oral Cavity Diseases: Gingivitis" presentation for PowerPoint or Google Slides. Taking care of yourself and of those around you is key! By learning about various illnesses and how they are spread, people can get a better understanding of them and make informed decisions about eating, exercise, and seeking...

AP Research Defense for High School
AP, or Advanced Placement, is a North American educational program that offers a rigorous course designed to challenge and prepare high school students for their future careers and academic pursuits. It requires students to conduct independent research, write a lengthy academic paper, and present their findings to a panel of...

Bariatric Surgery Breakthrough
Download the "Bariatric Surgery Breakthrough" presentation for PowerPoint or Google Slides. Treating diseases involves a lot of prior research and clinical trials. But whenever there’s a new discovery, a revolutionary finding that opens the door to new treatments, vaccines or ways to prevent illnesses, it’s great news. Should there be...

Nursing Capstone
In medical contexts, a capstone is often the final course in a nursing degree, a project of vital importance. It’s very demanding, so if you need help with the presentation, use this free professional template. Leave the design to us and focus on your data!
Oral Cavity Diseases: Periodontitis
Download the "Oral Cavity Diseases: Periodontitis" presentation for PowerPoint or Google Slides. Taking care of yourself and of those around you is key! By learning about various illnesses and how they are spread, people can get a better understanding of them and make informed decisions about eating, exercise, and seeking...

Paraphilias Causes and Symptoms
Download the "Paraphilias Causes and Symptoms" presentation for PowerPoint or Google Slides. Taking care of yourself and of those around you is key! By learning about various illnesses and how they are spread, people can get a better understanding of them and make informed decisions about eating, exercise, and seeking...

Biosafety Breakthrough
Download the "Biosafety Breakthrough" presentation for PowerPoint or Google Slides.Treating diseases involves a lot of prior research and clinical trials. But whenever there’s a new discovery, a revolutionary finding that opens the door to new treatments, vaccines or ways to prevent illnesses, it’s great news. Should there be a medical...

Elegant Black & White Thesis Defense
Present your research findings with grace and assertiveness through this template. Available for Google Slides and PowerPoint, this design set offers minimalistic charm with its simple, gray scale elegance. The template not only provides a polished platform to showcase your thesis but also ensures seamless and efficient delivery of your...

Medical Disease Explained With Cycle Diagrams
Download the "Medical Disease Explained With Cycle Diagrams" presentation for PowerPoint or Google Slides. Healthcare goes beyond curing patients and combating illnesses. Raising awareness about diseases, informing people about prevention methods, discussing some good practices, or even talking about a balanced diet—there are many topics related to medicine that you...

Data Analysis for Marketing Strategies
With the amount of data available through various digital platforms, it's easier than ever to determine the trends and preferences of your target audience. By collecting and analyzing data, marketers can create highly personalized campaigns that align with the exact needs and wants of their customers. If you're trying to...
SWOT Analysis Infographics
Discover the strengths, weaknesses, opportunities and threats of your own company performing a SWOT analysis. Use this basic strategic planning to evaluate your position with these new infographics created by Slidesgo.
- Page 1 of 79
New! Make quick presentations with AI
Slidesgo AI presentation maker puts the power of design and creativity in your hands, so you can effortlessly craft stunning slideshows in minutes.

Register for free and start editing online
This paper is in the following e-collection/theme issue:
Published on 16.4.2024 in Vol 26 (2024)
Adverse Event Signal Detection Using Patients’ Concerns in Pharmaceutical Care Records: Evaluation of Deep Learning Models
Authors of this article:

Original Paper
- Satoshi Nishioka 1 , PhD ;
- Satoshi Watabe 1 , BSc ;
- Yuki Yanagisawa 1 , PhD ;
- Kyoko Sayama 1 , MSc ;
- Hayato Kizaki 1 , MSc ;
- Shungo Imai 1 , PhD ;
- Mitsuhiro Someya 2 , BSc ;
- Ryoo Taniguchi 2 , PhD ;
- Shuntaro Yada 3 , PhD ;
- Eiji Aramaki 3 , PhD ;
- Satoko Hori 1 , PhD
1 Division of Drug Informatics, Keio University Faculty of Pharmacy, Tokyo, Japan
2 Nakajima Pharmacy, Hokkaido, Japan
3 Nara Institute of Science and Technology, Nara, Japan
Corresponding Author:
Satoko Hori, PhD
Division of Drug Informatics
Keio University Faculty of Pharmacy
1-5-30 Shibakoen
Tokyo, 105-8512
Phone: 81 3 5400 2650
Email: [email protected]
Background: Early detection of adverse events and their management are crucial to improving anticancer treatment outcomes, and listening to patients’ subjective opinions (patients’ voices) can make a major contribution to improving safety management. Recent progress in deep learning technologies has enabled various new approaches for the evaluation of safety-related events based on patient-generated text data, but few studies have focused on the improvement of real-time safety monitoring for individual patients. In addition, no study has yet been performed to validate deep learning models for screening patients’ narratives for clinically important adverse event signals that require medical intervention. In our previous work, novel deep learning models have been developed to detect adverse event signals for hand-foot syndrome or adverse events limiting patients’ daily lives from the authored narratives of patients with cancer, aiming ultimately to use them as safety monitoring support tools for individual patients.
Objective: This study was designed to evaluate whether our deep learning models can screen clinically important adverse event signals that require intervention by health care professionals. The applicability of our deep learning models to data on patients’ concerns at pharmacies was also assessed.
Methods: Pharmaceutical care records at community pharmacies were used for the evaluation of our deep learning models. The records followed the SOAP format, consisting of subjective (S), objective (O), assessment (A), and plan (P) columns. Because of the unique combination of patients’ concerns in the S column and the professional records of the pharmacists, this was considered a suitable data for the present purpose. Our deep learning models were applied to the S records of patients with cancer, and the extracted adverse event signals were assessed in relation to medical actions and prescribed drugs.
Results: From 30,784 S records of 2479 patients with at least 1 prescription of anticancer drugs, our deep learning models extracted true adverse event signals with more than 80% accuracy for both hand-foot syndrome (n=152, 91%) and adverse events limiting patients’ daily lives (n=157, 80.1%). The deep learning models were also able to screen adverse event signals that require medical intervention by health care providers. The extracted adverse event signals could reflect the side effects of anticancer drugs used by the patients based on analysis of prescribed anticancer drugs. “Pain or numbness” (n=57, 36.3%), “fever” (n=46, 29.3%), and “nausea” (n=40, 25.5%) were common symptoms out of the true adverse event signals identified by the model for adverse events limiting patients’ daily lives.
Conclusions: Our deep learning models were able to screen clinically important adverse event signals that require intervention for symptoms. It was also confirmed that these deep learning models could be applied to patients’ subjective information recorded in pharmaceutical care records accumulated during pharmacists’ daily work.
Introduction
Increasing numbers of people are expected to develop cancers in our aging society [ 1 - 3 ]. Thus, there is increasing interest in how to detect and manage the side effects of anticancer therapies in order to improve treatment regimens and patients’ quality of life [ 4 - 8 ]. The primary approaches for side effect management are “early signal detection and early intervention” [ 9 - 11 ]. Thus, more efficient approaches for this purpose are needed.
It has been recognized that patients’ voices concerning adverse events represent an important source of information. Several studies have indicated that the number, severity, and time of occurrence of adverse events might be underevaluated by physicians [ 12 - 15 ]. Thus, patient-reported outcomes (PROs) have recently received more attention in the drug evaluation process, reflecting patients’ real voices. Various kinds of PRO measures have been developed and investigated in different disease populations [ 16 , 17 ]. Health care authorities have also encouraged the pharmaceutical industry to use PROs for drug evaluation [ 18 , 19 ], and it is becoming more common to take PRO assessment results into consideration for drug marketing approval [ 20 , 21 ]. Similar trends can be seen in the clinical management of individual patients. Thus, health care professionals have an interest in understanding how to appropriately gather patients’ concerns in order to improve safety management and clinical decisions [ 22 - 24 ].
The applications of deep learning for natural language processing have expanded dramatically in recent years [ 25 ]. Since the development of a high-performance deep learning model in 2018 [ 26 ], attempts to apply cutting-edge deep learning models to various kinds of patient-generated text data for the evaluation of safety events or the analysis of unscalable subjective information from patients have been accelerating [ 27 - 31 ]. Most studies have been conducted to use patients’ narrative data for pharmacovigilance [ 27 , 32 - 35 ], while few have been aimed at improvement of real-time safety monitoring for individual patients. In addition, there have been some studies on adverse event severity grading based on health care records [ 36 - 39 ], but none has yet aimed to extract clinically important adverse event signals that require medical intervention from patients’ narratives. It is important to know whether deep learning models could contribute to the detection of such important adverse event signals from concern texts generated by individual patients.
To address this question, we have developed deep learning models to detect adverse event signals from individual patients with cancer based on patients’ blog articles in online communities, following other types of natural language processing–related previous work [ 40 , 41 ]. One deep learning model focused on the specific symptom of hand-foot syndrome (HFS), which is one of the typical side effects of anticancer treatments [ 42 ], and another focused on a broad range of adverse events that impact patients’ activities of daily living [ 43 ]. We showed that our models can provide good performance scores in targeting adverse event signals. However, the evaluation relied on patients’ narratives from the patients’ blog data used for deep learning model training, so further evaluation is needed to ensure the validity and applicability of the models to other texts regarding patients’ concerns. In addition, the blog data source did not contain medical information, so it was not feasible to assess whether the models could contribute to the extraction of clinically important adverse event signals.
To address these challenges, we focused on pharmaceutical care records written by pharmacists at community pharmacies. The gold standard format for pharmaceutical care records in Japan is the SOAP (subjective, objective, assessment, plan)-based document that follows the “problem-oriented system” concept proposed by Weed [ 44 ] in 1968. Pharmacists track patients’ subjective concerns in the S column, provide objective information or observations in the O column, give their assessment from the pharmacist perspective in the A column, and suggest a plan for moving forward in the P column [ 45 , 46 ]. We considered that SOAP-based pharmaceutical care records could be a unique data source suitable for further evaluation of our deep learning models because they contain both patients’ concerns and professional health care records by pharmacists, including the medication prescription history with time stamps. Therefore, this study was designed to assess whether our deep learning models could extract clinically important adverse event signals that require intervention by medical professionals from these records. We also aimed to evaluate the characteristics of the models when applied to patients’ subjective information noted in the pharmaceutical care records, as there have been only a few studies on the application of deep learning models to patients’ concerns recorded during pharmacists’ daily work [ 47 - 49 ].
Here, we report the results of applying our deep learning models to patients’ concern text data in pharmaceutical care records, focusing on patients receiving anticancer treatment.
Data Source
The original data source was 2,276,494 pharmaceutical care records for 303,179 patients, created from April 2020 to December 2021 at community pharmacies belonging to the Nakajima Pharmacy Group in Japan [ 50 ]. To focus on patients with cancer, records of patients with at least 1 prescription for an anticancer drug were retrieved by sorting individual drug codes (YJ codes) used in Japan (YJ codes starting with 42 refer to anticancer drugs). Records in the S column (ie, S records) were collected from the patients with cancer as the text data of patients’ concerns for deep learning model analysis.
Deep Learning Models
The deep learning models used for this research were those that we constructed based on patients’ narratives in blog articles posted in an online community and that showed the best performance score in each task in our previous work (ie, a Bidirectional Encoder Representations From Transformers [BERT]–based model for HFS signal extraction [ 42 ] and a T5-based model for adverse event signal extraction [ 43 ]). BERT [ 26 ] and T5 [ 51 ] both belong to a type of deep learning model that has recently shown high performance in several studies [ 29 , 52 ]. Hereafter, we refer to the deep learning model for HFS signals as the HFS model, the model for any adverse event signals as All AE (ie, all or any adverse events) model, and the model for adverse event signals limited to patients’ activities of daily living as the AE-L (adverse events limiting patients’ daily lives) model. It was also confirmed that these deep learning models showed similar or higher performance scores for the HFS, All AE, or AE-L identification tasks using 1000 S records randomly extracted from the data source of this study compared to the values obtained in our previous work [ 42 , 43 ] (the performance scores of sentence-level tasks from our previous work are comparable, as the mean number of words in the sentences in the data source in our previous work was 32.7 [SD 33.9], which is close to that of the S records used in this study, 38.8 [SD 29.4]). The method and results of the performance-level check are described in detail in Multimedia Appendix 1 [ 42 , 43 ]. We applied the deep learning models to all text data in this study without any adjustment in setting parameters from those used in constructing them based on patient-authored texts in our previous work [ 42 , 43 ].
Evaluation of Extracted S Records by the Deep Learning Models
In this study, we focused on the evaluation of S records that our deep learning models extracted as HFS or AE-L positive. Each positive S record was assessed as if it was a true adverse event signal, a sort of adverse event symptom, whether or not an intervention was made by health care professionals. We also investigated the kind of anticancer treatment prescription in connection with each adverse event signal identified in S records.
To assess whether an extracted positive S record was a true adverse event signal, we used the same annotation guidelines as in our previous work [ 43 ]. In brief, each S record was treated as an “adverse event signal” if any untoward medical occurrence happened to the patient, regardless of the cause. For the AE-L model only, if a positive S record was confirmed as an adverse event signal, it was further categorized into 1 or more of the following adverse event symptoms: “fatigue,” “nausea,” “vomiting,” “diarrhea,” “constipation,” “appetite loss,” “pain or numbness,” “rash or itchy,” “hair loss,” “menstrual irregularity,” “fever,” “taste disorder,” “dizziness,” “sleep disorder,” “edema,” or “others.”
For the assessment of interventions by health care professionals and anticancer treatment prescriptions, information from the O, A, and P columns and drug prescription history in the data source were investigated for the extracted positive S records. The interventions by health care professionals were categorized in any of the following: “adding symptomatic treatment for the adverse event signal,” “dose reduction or discontinuation of causative anticancer treatment,” “consultation with physician,” “others,” or “no intervention (ie, just following up the adverse event signal).” The actions categorized in “others” were further evaluated individually. For this assessment, we also randomly extracted 200 S records and evaluated them in the same way for comparison with the results from the deep learning model. Prescription history of anticancer treatment was analyzed by primary category of mechanism of action (MoA) with subcategories if applicable (eg, target molecule for kinase inhibitors).
Applicability Check to Other Text Data Including Patients’ Concerns
To check the applicability of our deep learning models to data from a different source, interview transcripts from patients with cancer were also evaluated. The interview transcripts were created by the Database of Individual Patient Experiences-Japan (DIPEx-Japan) [ 53 ]. DIPEx-Japan divides the interview transcripts into sections for each topic, such as “onset of disease” and “treatment,” and posts the processed texts on its website. Processing is conducted by accredited researchers based on qualitative research methods established by the University of Oxford [ 54 ]. In this study, interview text data created from interviews with 52 patients with breast cancer conducted from January 2008 to October 2018 were used to assess whether our deep learning models can extract adverse event signals from this source. In total, 508 interview transcripts were included with the approval of DIPEx-Japan.
Ethical Considerations
This study was conducted with anonymized data following approval by the ethics committee of the Keio University Faculty of Pharmacy (210914-1 and 230217-1) and in accordance with relevant guidelines and regulations and the Declaration of Helsinki. Informed consent specific to this study was waived due to the retrospective observational design of the study with the approval of the ethics committee of the Keio University Faculty of Pharmacy. To respect the will of each individual stakeholder, however, we provided patients and pharmacists of the pharmacy group with an opportunity to refuse the sharing of their pharmaceutical care records by posting an overview of this study at each pharmacy store or on their web page regarding the analysis using pharmaceutical care records. Interview transcripts from DIPEx-Japan were provided through a data sharing arrangement for using narrative data for research and education. Consent for interview transcription and its sharing from DIPEx-Japan was obtained from the participants when the interviews were recorded.
From the original data source of 2,180,902 pharmaceutical care records for 291,150 patients, S records written by pharmacists for patients with a history of at least 1 prescription of an anticancer drug were extracted. This yielded 30,784 S records for 2479 patients with cancer ( Table 1 ). The mean and median number of words in the S records were 38.8 (SD 29.4) and 32 (IQR 20-50), respectively. We applied our deep learning models, HFS, All AE, and AE-L, to these 30,784 S records for the evaluation of the deep learning models for adverse event signal detection.
For interview transcripts created by DIPEx-Japan, the mean and median number of words were 428.9 (SD 160.9) and 416 (IQR 308-526), respectively, in the 508 transcripts for 52 patients with breast cancer.
a SOAP: subjective, objective, assessment, plan.
b S: subjective.
Application of the HFS Model
First, we applied the HFS model to the S records for patients with cancer. The BERT-based model was used for this research as it showed the best performance score in our previous work [ 42 ].
S Records Extracted as HFS Positive
The S records extracted as HFS positive by the HFS model ( Table 2 ) amounted to 167 (0.5%) records for 119 (4.8%) patients. A majority of the patients had 1 HFS-positive record in their S records (n=91, 76.5%), while 2 patients had as many as 6 (1.7%) HFS-positive records. When we examined whether the extracted S records were true adverse event signals or not, 152 records were confirmed to be adverse event signals, while the other 15 records were false-positives. All the false-positive S records were descriptions about the absence of symptoms or confirmation of improving condition (eg, “no diarrhea, mouth ulcers, or limb pain so far” or “the skin on the soles of my feet has calmed down a lot with this ointment”). Some examples of S records that were predicted as HFS positive by the model are shown in Table S1 in Multimedia Appendix 2 .
The same examination was conducted with interview transcripts from DIPEx-Japan. Only 1 (0.2%) transcript was extracted as HFS positive by the HFS model, and it was a true adverse event signal (100%). The actual transcript extracted as HFS positive is shown in Table S2 in Multimedia Appendix 2 .
a S: subjective.
b HFS: hand-foot syndrome.
c All false-positive S records were denial of symptoms or confirmation of improving condition.
Interventions by Health Care Professionals
The 167 S records extracted as HFS positive as well as 200 randomly selected records were checked for interventions by health care professionals ( Figure 1 ). The proportion showing any action by health care professionals was 64.1% for 167 HFS-positive S records compared to 13% for the 200 random S records. Among the actions taken for HFS positives, “adding symptomatic treatment” was the most common, accounting for around half (n=79, 47.3%), followed by “other” (n=18, 10.8%). Most “other” actions were educational guidance from pharmacists, such as instructions on moisturizing, nail care, or application of ointment and advice on daily living (eg, “avoid tight socks”).

Anticancer Drugs Prescribed
The types of anticancer drugs prescribed for HFS-positive patients are summarized based on the prescription histories in Table 3 . For the 152 adverse event signals identified by the HFS model in the previous section, the most common MoA class of anticancer drugs used for the patients was antimetabolite (n=62, 40.8%), specifically fluoropyrimidines (n=59, 38.8%). Kinase inhibitors were next (n=49, 32.2%), with epidermal growth factor receptor (EGFR) inhibitors and multikinase inhibitors as major subgroups (n=28, 18.4% and n=14, 9.2%, respectively). The third and fourth most common MoAs were aromatase inhibitors (n=24, 15.8%) and antiandrogen or estrogen drugs (n=7, 4.6% each) for hormone therapy.
a EGFR: epidermal growth factor receptor.
b VEGF: vascular endothelial growth factor.
c HER2: human epidermal growth factor receptor-2.
d CDK4/6: cyclin-dependent kinase 4/6.
Application of the All AE or AE-L model
The All AE and AE-L models were also applied to the same S records for patients with cancer. The T5-based model was used for this research as it gave the best performance score in our previous work [ 43 ].
S Records Extracted as All AE or AE-L positive
The numbers of S records extracted as positive were 7604 (24.7%) for 1797 patients and 196 (0.6%) for 142 patients for All AE and AE-L, respectively. In the case of All AE, patients tended to have multiple adverse event positives in their S records (n=1315, 73.2% of patients had at least 2 positives). In the case of AE-L, most patients had only 1 AE-L positive (n=104, 73.2%), and the largest number of AE-L positives for 1 patient was 4 (2.8%; Table 4 ).
We focused on AE-L evaluation due to its greater importance from a medical viewpoint and lower workload for manual assessment, considering the number of positive S records. Of the 197 AE-L–positive S records, it was confirmed that 157 (80.1%) records accurately extracted adverse event signals, while 39 (19.9%) records were false-positives that did not include any adverse event signals ( Table 4 ). The contents of the 39 false-positives were all descriptions about the absence of symptoms or confirmation of improving condition, showing a similar tendency to the HFS false-positives (eg, “The diarrhea has calmed down so far. Symptoms in hands and feet are currently fine” and “No symptoms for the following: upset in stomach, diarrhea, nausea, abdominal pain, abdominal pain or stomach cramps, constipation”). Examples of S records that were predicted as AE-L positive are shown in Table S3 in Multimedia Appendix 2 .
The deep learning models were also applied to interview transcripts from DIPEx-Japan in the same manner. The deep learning models identified 84 (16.5%) and 18 (3.5%) transcripts as All AE or AE-L positive, respectively. Of the 84 All AE–positive transcripts, 73 (86.9%) were true adverse event signals. The false-positives of All AE (n=11, 13.1%) were categorized into any of the following 3 types: explanations about the disease or its prognosis, stories when their cancer was discovered, or emotional changes that did not include clear adverse event mentions. With regard to AE-L, all the 18 (100%) positives were true adverse event signals (Table S4 in Multimedia Appendix 2 ). Examples of actual transcripts extracted as All AE or AE-L positive are shown in Table S5 in Multimedia Appendix 2 .
b All AE: all (or any of) adverse event.
c AE-L: adverse events limiting patients’ daily lives.
d All false-positive S records were denial of symptoms or confirmation of improving condition.
Whether or not interventions were made by health care professionals was investigated for the 196 AE-L–positive S records. As in the HFS model evaluation, data from 200 randomly selected S records were used for comparison ( Figure 2 ). In total, 91 (46.4%) records in the 196 AE-L–positive records were accompanied by an intervention, while the corresponding figure in the 200 random records was 26 (13%) records. The most common action in response to adverse event signals identified by the AE-L model was “adding symptomatic treatment” (n=71, 36.2%), followed by “other” (n=11, 5.6%). “Other” included educational guidance from pharmacists, inquiries from pharmacists to physicians, or recommendations for patients to visit a doctor.

The types of anticancer drugs prescribed for patients with adverse event signals identified by the AE-L model were summarized based on the prescription histories ( Table 5 ). In connection with the 157 adverse event signals, the most common MoA of the prescribed anticancer drug was antimetabolite (n=62, 39.5%) and fluoropyrimidine (n=53, 33.8%), which accounted for the majority. Kinase inhibitor (n=31, 19.7%) was the next largest category with multikinase inhibitor (n=14, 8.9%) as the major subgroup. These were followed by antiandrogen (n=27, 17.2%), antiestrogen (n=10, 6.4%), and aromatase inhibitor (n=10, 6.4%) for hormone therapy.
b JAK: janus kinase.
c VEGF: vascular endothelial growth factor.
d BTK: bruton tyrosine kinase.
e FLT3: FMS-like tyrosine kinase-3.
f PARP: poly-ADP ribose polymerase.
g CDK4/6: cyclin-dependent kinase 4/6.
h CD20: cluster of differentiation 20.
Adverse Event Symptoms
For the 157 adverse event signals identified by the AE-L model, the symptoms were categorized according to the predefined guideline in our previous work [ 43 ]. “Pain or numbness” (n=57, 36.3%) accounted for the largest proportion followed by “fever” (n=46, 29.3%) and “nausea” (n=40, 25.5%; Table 6 ). Symptoms classified as “others” included chills, tinnitus, running tears, dry or peeling skin, and frequent urination. When comparing the proportion of the symptoms associated with or without interventions by health care professionals, a trend toward a greater proportion of interventions was observed in “fever,” “nausea,” “diarrhea,” “constipation,” “vomiting,” and “edema” ( Figure 3 , black boxes). On the other hand, a smaller proportion was observed in “pain or numbness,” “fatigue,” “appetite loss,” “rash or itchy,” “taste disorder,” and “dizziness” ( Figure 3 , gray boxes).

This study was designed to evaluate our deep learning models, previously constructed based on patient-authored texts posted in an online community, by applying them to pharmaceutical care records that contain both patients’ subjective concerns and medical information created by pharmacists. Based on the results, we discuss whether these deep learning models can extract clinically important adverse event signals that require medical intervention, and what characteristics they show when applied to data on patients’ concerns in pharmaceutical care records.
Performance for Adverse Event Signal Extraction
The first requirement for the deep learning models is to extract adverse event signals from patients’ narratives precisely. In this study, we evaluated the proportion of true adverse event signals in positive S records extracted by the HFS or AE-L model. True adverse event signals amounted to 152 (91%) and 157 (80.1%) for the HFS and AE-L models, respectively ( Tables 2 and 4 ). Given that the proportion of true adverse event signals in 200 randomly extracted S records without deep learning models was 54 (27%; categories other than “no adverse event” in Figures 1 and 2 ), the HFS and AE-L models were able to concentrate S records with adverse event mentions. Although 15 (9%) for the HFS model and 39 (19.9%) for the AE-L model were false-positives, it was confirmed all of the false-positive records described a lack of symptoms or confirmation of improving condition. We considered that such false-positives are due to the unique feature of pharmaceutical care records, where pharmacists might proactively interview patients about potential side effects of their medications. As the data set of blog articles we used to construct the deep learning models included few such cases (especially comments on lack of symptoms), our models seemed unable to exclude them correctly. Even though we confirmed that the proportion of true “adverse event” signals extracted from the S records by the HFS or AE-L model was more than 80%, the performance scores to extract true “HFS” or “AE-L” signals were not so high based on the performance check using 1000 randomly extracted S records ( F 1 -scores were 0.50 and 0.22 for true HFS and AE-L signals, respectively; Table S1 in Multimedia Appendix 1 ). It is considered that the performance to extract true HFS and AE-L signals was relatively low due to the short length of texts in the S records, providing less context to judge the impact on patients’ daily lives, especially for the AE-L model (the mean word number of the S records was 38.8 [SD 29.4; Table 1 ], similar to the sentence-level tasks in our previous work [ 42 , 43 ]). However, we consider a true adverse event signal proportion of more than 80% in this study represents a promising outcome, as this is the first attempt to apply our deep learning models to a different source of patients’ concern data, and the extracted positive cases would be worthy of evaluation by a medical professional, as the potential adverse events could be caused by drugs taken by the patients.
When the deep learning models were applied to DIPEx-Japan interview transcripts, including patients’ concerns, the proportion of true adverse event signals was also more than 80% (for All AE: n=73, 86.9% and for HFS and AE-L: n=18, 100%). The difference in the results between pharmaceutical care S records and DIPEx-Japan interview transcripts was the features of false-positives, descriptions about lack of symptoms or confirmation of improving condition in S records versus explanations about disease or its prognosis, stories about when their cancer was discovered, or emotional changes in interview transcripts. This is considered due to the difference in the nature of the data source; the pharmaceutical care records were generated in a real-time manner by pharmacists through their daily work, where adverse event signals are proactively monitored, while the interview transcripts were purely based on patients’ retrospective memories. Our deep learning models were able to extract true adverse event signals with an accuracy of more than 80% from both text data sources in spite of the difference in their nature. When looking at future implementation of the deep learning models in society (discussed in the Potential for Deep Learning Model Implementation in Society section), it may be desirable to further adjust deep learning models to reduce false-positives depending upon the features of the data source.
Identification of Important Adverse Events Requiring Medical Intervention
To assess whether the models could extract clinically important adverse event signals, we investigated interventions by health care professionals connected with the adverse event signals that are identified by our deep learning models. In the 200 randomly extracted S records, only 26 (13%) consisted of adverse event signals, leading to any intervention by health care professionals. On the other hand, the proportion of signals associated with interventions was increased to 107 (64.1%) and 91 (46.4%) in the S records extracted as positive by the HFS and AE-L models, respectively ( Figures 1 and 2 ). These results suggest that both deep learning models can screen clinically important adverse event signals that require intervention from health care professionals. The performance level in screening adverse event signals requiring medical intervention was higher in the HFS model than in the AE-L model (n=107, 64.1% vs n=91, 46.4%; Figures 1 and 2 ). Since the target events were specific and adverse event signals of HFS were narrowly defined, which is one of the typical side effects of some anticancer drugs, we consider that health care providers paid special attention to HFS-related signals and took action proactively. In both deep learning models, similar trends were observed in actions taken by health care professionals in response to extracted adverse event signals; common actions were attempts to manage adverse event symptoms by symptomatic treatment or other mild interventions, including educational guidance from pharmacists or recommendations for patients to visit a doctor. More direct interventions focused on the causative drugs (ie, “dose reduction or discontinuation of anticancer treatment”) amounted to less than 5%; 7 (4.2%) for the HFS model and 6 (3.1%) for the AE-L model ( Figures 1 and 2 ). Thus, it appears that our deep learning models can contribute to screening mild to moderate adverse event signals that require preventive actions such as symptomatic treatments or professional advice from health care providers, especially for patients with less sensitivity to adverse event signals or who have few opportunities to visit clinics and pharmacies.
Ability to Catch Real Side Effect Signals of Anticancer Drugs
Based on the drug prescription history associated with S records extracted as HFS or AE-L positive, the type and duration of anticancer drugs taken by patients experiencing the adverse event signals were investigated. For the HFS model, the most common MoA of anticancer drug was antimetabolite (fluoropyrimidine: n=59, 38.8%), followed by kinase inhibitors (n=49, 32.2%, of which EGFR inhibitors and multikinase inhibitors accounted for n=28, 18.4% and n=14, 9.2%, respectively) and aromatase inhibitors (n=24, 15.8%; Table 3 ). It is known that fluoropyrimidine and multikinase inhibitors are typical HFS-inducing drugs [ 55 - 58 ], suggesting that the HFS model accurately extracted HFS side effect signals derived from these drugs. Note that symptoms such as acneiform rash, xerosis, eczema, paronychia, changes in the nails, arthralgia, or stiffness of limb joints, which are common side effects of EGFR inhibitors or aromatase inhibitors [ 59 , 60 ], might be extracted as closely related expressions to those of HFS signals. When looking at the MoA of anticancer drugs for patients with adverse event signals identified by the AE-L model, antimetabolite (fluoropyrimidine) was the most common one (n=53, 33.8%), as in the case of those identified by the HFS model, followed by kinase inhibitors (n=31, 19.7%) and antiandrogens (n=27, 17.2%; Table 5 ). Since the AE-L model targets a broad range of adverse event symptoms, it is difficult to rationalize the relationship between the adverse event signals and types of anticancer drugs. However, the type of anticancer drugs would presumably closely correspond to the standard treatments of the cancer types of the patients. Based on the prescribed anticancer drugs, we can infer that a large percentage of the patients had breast or lung cancer, indicating that our study results were based on data from such a population. Thus, a possible direction for the expansion of this research would be adjusting the deep learning models by additional training with expressions for typical side effects associated with standard treatments of other cancer types. To interpret these results correctly, it should be noted that we could not investigate anticancer treatments conducted outside of the pharmacies (eg, the time-course relationship with intravenously administered drugs would be missed, as the administration will be done at hospitals). To further evaluate how useful this model is in side effect signal monitoring for patients with cancer, comprehensive medical information for the eligible patients would be required.
Suitability of the Deep Learning Models for Specific Adverse Event Symptoms
Among the adverse event signals identified by the AE-L model, the type of symptom was categorized according to a predefined annotation guideline that we previously developed [ 43 ]. The most frequently recorded adverse event signals identified by the AE-L model were “pain or numbness” (n=57, 36.3%), “fever” (n=46, 29.3%), and “nausea” (n=40, 25.5%; Table 6 ). Since the pharmaceutical care records had information about interventions by health care professionals, the frequency of the presence or absence of the interventions for each symptom was examined. A trend toward a greater proportion of interventions was observed in “fever,” “nausea,” “diarrhea,” “constipation,” “vomiting,” and “edema” ( Figure 3 , black boxes). There seem to be 2 possible explanations for this: these symptoms are of high importance and require early medical intervention or effective symptomatic treatments are available for these symptoms in clinical practice so that medical intervention is an easy option. On the other hand, a trend for a smaller proportion of adverse event signals to result in interventions was observed for “pain or numbness,” “fatigue,” “appetite loss,” “rash or itchy,” “taste disorder,” and “dizziness” ( Figure 3 , gray boxes). The reason for this may be the lack of effective symptomatic treatments or the difficulty of judging whether the severity of these symptoms justifies medical intervention by health care providers. In either case, there may be room for improvement in the quality of medical care for these symptoms. We expect that our research will contribute to a quality improvement in safety monitoring in clinical practice by supporting adverse event signal detection in a cost-effective manner.
Potential for Deep Learning Model Implementation in Society
Although we evaluated our deep learning models using pharmaceutical care records in this study, the main target of future implementation of our deep learning models in society would be narrative texts that patients directly write to record their daily experiences. For example, the application of these deep learning models to electronic media where patients record their daily experiences in their lives with disease (eg, health care–related e-communities and disease diary applications) could enable information about adverse event signal onset that patients experience to be provided to health care providers in a timely manner. Adverse event signals can automatically be identified and shared with health care providers based on the concern texts that patients post to any platform. This system will have the advantage that health care providers can efficiently grasp safety-related events that patients experience outside of clinic visits so that they can conduct more focused or personalized interactions with patients at their clinic visits. However, consideration should be given to avoid an excessive burden on health care providers. For instance, limiting the sharing of adverse event signals to those of high severity or summarizing adverse event signals over a week rather than sharing each one in a real-time manner may be reasonable approaches for medical staff. We also need to think about how to encourage patients to record their daily experiences using electronic tools. Not only technical progress and support but also the establishment of an ecosystem where both patients and medical staff can feel benefit will be required. Prospective studies with deep learning models to follow up patients in the long term and evaluate outcomes will be needed. We primarily looked at patient-authored texts as targets of implementation, but our deep learning models may also be worth using medical data including patients’ subjective concerns, such as pharmaceutical care S records. As this study confirmed that our deep learning models are applicable to patients’ concern texts tracked by pharmacists, it should be possible to use them to analyze other “patient voice-like” medical text data that have not been actively investigated so far.
Limitations
First, the major limitation of this study was that we were not able to collect complete medical information of the patients. Although we designed this study to analyze patients’ concerns extracted by the deep learning models and their relationship with medical information contained in the pharmaceutical care records, some information could not be tracked (eg, missing history of medical interventions or anticancer treatment at hospitals as well as diagnosis of patients’ primary cancers). Second, there might be a data creation bias in S records for patients’ concerns by pharmacists. For example, symptoms that have little impact on intervention decisions might less likely be recorded by them. It should be also noted that the characteristics of S records may not be consistent at different community pharmacies.
Conclusions
Our deep learning models were able to screen clinically important adverse event signals that require intervention by health care professionals from patients’ concerns in pharmaceutical care records. Thus, these models have the potential to support real-time adverse event monitoring of individual patients taking anticancer treatments in an efficient manner. We also confirmed that these deep learning models constructed based on patient-authored texts could be applied to patients’ subjective information recorded by pharmacists through their daily work. Further research may help to expand the applicability of the deep learning models for implementation in society or for analysis of data on patients’ concerns accumulated in professional records at pharmacies or hospitals.
Acknowledgments
This work was supported by Japan Society for the Promotion of Science, Grants-in-Aid for Scientific Research (KAKENHI; grant 21H03170) and Japan Science and Technology Agency, Core Research for Evolutional Science and Technology (CREST; grant JPMJCR22N1), Japan. Mr Yuki Yokokawa and Ms Sakura Yokoyama at our laboratory advised SN about the structure of pharmaceutical care records. This study would not have been feasible without the high quality of pharmaceutical care records created by many individual pharmacists at Nakajima Pharmacy Group through their daily work.
Data Availability
The data sets generated and analyzed during this study are available from the corresponding author on reasonable request.
Authors' Contributions
SN and SH designed the study. SN retrieved the subjective records of patients with cancer from the data source for the application of deep learning models and organized other data for subsequent evaluations. SN ran the deep learning models with the support of SW. SN, YY, and KS checked the adverse event signals for each subjective record that was extracted as positive by the models for hand-foot syndrome or adverse events limiting patients’ daily lives and evaluated the adverse event signal symptoms, details of interventions taken by health care professionals, and types of anticancer drugs prescribed for patients based on available data from the data source. HK and SI advised on the study concept and process. MS and RT provided pharmaceutical records at their community pharmacies along with advice on how to use and interpret them. SY and EA supervised the natural language processing research as specialists. SH supervised the study overall. SN drafted and finalized the paper. All authors reviewed and approved the paper.
Conflicts of Interest
SN is an employee of Daiichi Sankyo Co, Ltd. All other authors declare no conflicts of interest.
Performance evaluation of deep learning models.
Examples of S records and sample interview transcripts.
- Global cancer observatory: cancer over time. World Health Organization. URL: https://gco.iarc.fr/overtime/en [accessed 2023-07-02]
- Mattiuzzi C, Lippi G. Current cancer epidemiology. J Epidemiol Glob Health. 2019;9(4):217-222. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Montazeri F, Komaki H, Mohebi F, Mohajer B, Mansournia MA, Shahraz S, et al. Editorial: disparities in cancer prevention and epidemiology. Front Oncol. 2022;12:872051. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Lasala R, Santoleri F. Association between adherence to oral therapies in cancer patients and clinical outcome: a systematic review of the literature. Br J Clin Pharmacol. 2022;88(5):1999-2018. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Pudkasam S, Polman R, Pitcher M, Fisher M, Chinlumprasert N, Stojanovska L, et al. Physical activity and breast cancer survivors: importance of adherence, motivational interviewing and psychological health. Maturitas. 2018;116:66-72. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Markman M. Chemotherapy-associated neurotoxicity: an important side effect-impacting on quality, rather than quantity, of life. J Cancer Res Clin Oncol. 1996;122(9):511-512. [ CrossRef ] [ Medline ]
- Jitender S, Mahajan R, Rathore V, Choudhary R. Quality of life of cancer patients. J Exp Ther Oncol. 2018;12(3):217-221. [ Medline ]
- Di Nardo P, Lisanti C, Garutti M, Buriolla S, Alberti M, Mazzeo R, et al. Chemotherapy in patients with early breast cancer: clinical overview and management of long-term side effects. Expert Opin Drug Saf. 2022;21(11):1341-1355. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Cuomo RE. Improving cancer patient outcomes and cost-effectiveness: a Markov simulation of improved early detection, side effect management, and palliative care. Cancer Invest. 2023;41(10):858-862. [ CrossRef ] [ Medline ]
- Pulito C, Cristaudo A, La Porta C, Zapperi S, Blandino G, Morrone A, et al. Oral mucositis: the hidden side of cancer therapy. J Exp Clin Cancer Res. 2020;39(1):210. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Bartal A, Mátrai Z, Szûcs A, Liszkay G. Main treatment and preventive measures for hand-foot syndrome, a dermatologic side effect of cancer therapy. Magy Onkol. 2011;55(2):91-98. [ FREE Full text ] [ Medline ]
- Basch E, Jia X, Heller G, Barz A, Sit L, Fruscione M, et al. Adverse symptom event reporting by patients vs clinicians: relationships with clinical outcomes. J Natl Cancer Inst. 2009;101(23):1624-1632. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Basch E. The missing voice of patients in drug-safety reporting. N Engl J Med. 2010;362(10):865-869. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Fromme EK, Eilers KM, Mori M, Hsieh YC, Beer TM. How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the Quality-of-Life Questionnaire C30. J Clin Oncol. 2004;22(17):3485-3490. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Liu L, Suo T, Shen Y, Geng C, Song Z, Liu F, et al. Clinicians versus patients subjective adverse events assessment: based on patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). Qual Life Res. 2020;29(11):3009-3015. [ CrossRef ] [ Medline ]
- Churruca K, Pomare C, Ellis LA, Long JC, Henderson SB, Murphy LED, et al. Patient-reported outcome measures (PROMs): a review of generic and condition-specific measures and a discussion of trends and issues. Health Expect. 2021;24(4):1015-1024. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Pérez-Alfonso KE, Sánchez-Martínez V. Electronic patient-reported outcome measures evaluating cancer symptoms: a systematic review. Semin Oncol Nurs. 2021;37(2):151145. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Patient-reported outcome measures: use in medical product development to support labeling claims. U.S. Food & Drug Administration. 2009. URL: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-reported-outcome-measures-use-medical-product-development-support-labeling-claims [accessed 2023-11-26]
- Appendix 2 to the guideline on the evaluation of anticancer medicinal products in man—the use of patient-reported outcome (PRO) measures in oncology studies—scientific guideline. European Medicines Agency. 2016. URL: https://www.ema.europa.eu/en/appendix-2-guideline-evaluation-anticancer-medicinal-products-man-use-patient-reported-outcome-pro [accessed 2023-11-26]
- Weber SC. The evolution and use of patient-reported outcomes in regulatory decision making. RF Q. 2023;3(1):4-9. [ FREE Full text ]
- Teixeira MM, Borges FC, Ferreira PS, Rocha J, Sepodes B, Torre C. A review of patient-reported outcomes used for regulatory approval of oncology medicinal products in the European Union between 2017 and 2020. Front Med (Lausanne). 2022;9:968272. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Newell S, Jordan Z. The patient experience of patient-centered communication with nurses in the hospital setting: a qualitative systematic review protocol. JBI Database System Rev Implement Rep. 2015;13(1):76-87. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Yagasaki K, Takahashi H, Ouchi T, Yamagami J, Hamamoto Y, Amagai M, et al. Patient voice on management of facial dermatological adverse events with targeted therapies: a qualitative study. J Patient Rep Outcomes. 2019;3(1):27. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Giardina TD, Korukonda S, Shahid U, Vaghani V, Upadhyay DK, Burke GF, et al. Use of patient complaints to identify diagnosis-related safety concerns: a mixed-method evaluation. BMJ Qual Saf. 2021;30(12):996-1001. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Lauriola I, Lavelli A, Aiolli F. An introduction to deep learning in natural language processing: models, techniques, and tools. Neurocomputing. 2022;470:443-456. [ CrossRef ]
- Devlin J, Chang MW, Lee K, Toutanova K. BERT: pre-training of deep bidirectional transformers for language understanding. 2019. Presented at: 2019 Annual Conference of the North American Chapter of the Association for Computational Linguistics: Human Language Technologies; June 2-7, 2019;4171-4186; Minneapolis, MN, USA.
- Dreisbach C, Koleck TA, Bourne PE, Bakken S. A systematic review of natural language processing and text mining of symptoms from electronic patient-authored text data. Int J Med Inform. 2019;125:37-46. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Sim JA, Huang X, Horan MR, Stewart CM, Robison LL, Hudson MM, et al. Natural language processing with machine learning methods to analyze unstructured patient-reported outcomes derived from electronic health records: a systematic review. Artif Intell Med. 2023;146:102701. [ CrossRef ] [ Medline ]
- Weissenbacher D, Banda JM, Davydova V, Estrada-Zavala D, Gascó Sánchez L, Ge Y, et al. Overview of the seventh social media mining for health applications (#SMM4H) shared tasks at COLING 2022. 2022. Presented at: Proceedings of the Seventh Workshop on Social Media Mining for Health Applications, Workshop & Shared Task; October 12-17, 2022;221-241; Gyeongju, Republic of Korea. URL: https://aclanthology.org/2022.smm4h-1.54/
- Matsuda S, Ohtomo T, Okuyama M, Miyake H, Aoki K. Estimating patient satisfaction through a language processing model: model development and evaluation. JMIR Form Res. 2023;7(1):e48534. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Yu D, Vydiswaran VGV. An assessment of mentions of adverse drug events on social media with natural language processing: model development and analysis. JMIR Med Inform. 2022;10(9):e38140. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Liu X, Chen H. A research framework for pharmacovigilance in health social media: identification and evaluation of patient adverse drug event reports. J Biomed Inform. 2015;58:268-279. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Nikfarjam A, Sarker A, O'Connor K, Ginn R, Gonzalez G. Pharmacovigilance from social media: mining adverse drug reaction mentions using sequence labeling with word embedding cluster features. J Am Med Inform Assoc. 2015;22(3):671-681. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Kakalou C, Dimitsaki S, Dimitriadis VK, Natsiavas P. Exploiting social media for active pharmacovigilance: the PVClinical social media workspace. Stud Health Technol Inform. 2022;290:739-743. [ CrossRef ] [ Medline ]
- Bousquet C, Dahamna B, Guillemin-Lanne S, Darmoni SJ, Faviez C, Huot C, et al. The adverse drug reactions from patient reports in social media project: five major challenges to overcome to operationalize analysis and efficiently support pharmacovigilance process. JMIR Res Protoc. 2017;6(9):e179. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Young IJB, Luz S, Lone N. A systematic review of natural language processing for classification tasks in the field of incident reporting and adverse event analysis. Int J Med Inform. 2019;132:103971. [ CrossRef ] [ Medline ]
- Jacobsson R, Bergvall T, Sandberg L, Ellenius J. Extraction of adverse event severity information from clinical narratives using natural language processing. Pharmacoepidemiol Drug Saf. 2017;26(S2):37. [ FREE Full text ]
- Liang C, Gong Y. Predicting harm scores from patient safety event reports. Stud Health Technol Inform. 2017;245:1075-1079. [ CrossRef ] [ Medline ]
- Jiang G, Wang L, Liu H, Solbrig HR, Chute CG. Building a knowledge base of severe adverse drug events based on AERS reporting data using semantic web technologies. Stud Health Technol Inform. 2013;192(1-2):496-500. [ CrossRef ] [ Medline ]
- Usui M, Aramaki E, Iwao T, Wakamiya S, Sakamoto T, Mochizuki M. Extraction and standardization of patient complaints from electronic medication histories for pharmacovigilance: natural language processing analysis in Japanese. JMIR Med Inform. 2018;6(3):e11021. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Watanabe T, Yada S, Aramaki E, Yajima H, Kizaki H, Hori S. Extracting multiple worries from breast cancer patient blogs using multilabel classification with the natural language processing model bidirectional encoder representations from transformers: infodemiology study of blogs. JMIR Cancer. 2022;8(2):e37840. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Nishioka S, Watanabe T, Asano M, Yamamoto T, Kawakami K, Yada S, et al. Identification of hand-foot syndrome from cancer patients' blog posts: BERT-based deep-learning approach to detect potential adverse drug reaction symptoms. PLoS One. 2022;17(5):e0267901. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Nishioka S, Asano M, Yada S, Aramaki E, Yajima H, Yanagisawa Y, et al. Adverse event signal extraction from cancer patients' narratives focusing on impact on their daily-life activities. Sci Rep. 2023;13(1):15516. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Weed LL. Medical records that guide and teach. N Engl J Med. 1968;278(11):593-600. [ CrossRef ] [ Medline ]
- Podder V, Lew V, Ghassemzadeh S. SOAP Notes. Treasure Island, FL. StatPearls Publishing; 2023.
- Shenavar Masooleh I, Ramezanzadeh E, Yaseri M, Sahere Mortazavi Khatibani S, Sadat Fayazi H, Ali Balou H, et al. The effectiveness of training on daily progress note writing by medical interns. J Adv Med Educ Prof. 2021;9(3):168-175. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Grothen AE, Tennant B, Wang C, Torres A, Sheppard BB, Abastillas G, et al. Application of artificial intelligence methods to pharmacy data for cancer surveillance and epidemiology research: a systematic review. JCO Clin Cancer Inform. 2020;4:1051-1058. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Ohno Y, Kato R, Ishikawa H, Nishiyama T, Isawa M, Mochizuki M, et al. Using the natural language processing system MedNER-J to analyze pharmaceutical care records. medRxiv. Preprint posted online on October 2, 2023. [ CrossRef ]
- Ranchon F, Chanoine S, Lambert-Lacroix S, Bosson JL, Moreau-Gaudry A, Bedouch P. Development of artificial intelligence powered apps and tools for clinical pharmacy services: a systematic review. Int J Med Inform. 2023;172:104983. [ CrossRef ] [ Medline ]
- Nakajima Pharmacy. URL: https://www.nakajima-phar.co.jp/ [accessed 2023-12-07]
- Raffel C, Shazeer N, Roberts A, Lee K, Narang S, Matena M, et al. Exploring the limits of transfer learning with a unified text-to-text transformer. J Mach Learn Res. 2020;21(140):1-67. [ FREE Full text ]
- Pathak A. Comparative analysis of transformer based language models. Comput Sci Inf Technol. 2021.:165-176. [ FREE Full text ] [ CrossRef ]
- DIPEx Japan. URL: https://www.dipex-j.org/ [accessed 2024-02-04]
- Herxheimer A, McPherson A, Miller R, Shepperd S, Yaphe J, Ziebland S. Database of patients' experiences (DIPEx): a multi-media approach to sharing experiences and information. Lancet. 2000;355(9214):1540-1543. [ CrossRef ] [ Medline ]
- Lara PE, Muiño CB, de Spéville BD, Reyes JJ. Hand-foot skin reaction to regorafenib. Actas Dermosifiliogr. 2016;107(1):71-73. [ CrossRef ]
- Zaiem A, Hammamia SB, Aouinti I, Charfi O, Ladhari W, Kastalli S, et al. Hand-foot syndrome induced by chemotherapy drug: case series study and literature review. Indian J Pharmacol. 2022;54(3):208-215. [ FREE Full text ] [ CrossRef ] [ Medline ]
- McLellan B, Ciardiello F, Lacouture ME, Segaert S, Van Cutsem E. Regorafenib-associated hand-foot skin reaction: practical advice on diagnosis, prevention, and management. Ann Oncol. 2015;26(10):2017-2026. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Ai L, Xu Z, Yang B, He Q, Luo P. Sorafenib-associated hand-foot skin reaction: practical advice on diagnosis, mechanism, prevention, and management. Expert Rev Clin Pharmacol. 2019;12(12):1121-1127. [ CrossRef ] [ Medline ]
- Tenti S, Correale P, Cheleschi S, Fioravanti A, Pirtoli L. Aromatase inhibitors-induced musculoskeletal disorders: current knowledge on clinical and molecular aspects. Int J Mol Sci. 2020;21(16):5625. [ FREE Full text ] [ CrossRef ] [ Medline ]
- Lacouture ME, Melosky BL. Cutaneous reactions to anticancer agents targeting the epidermal growth factor receptor: a dermatology-oncology perspective. Skin Therapy Lett. 2007;12(6):1-5. [ FREE Full text ] [ Medline ]
Abbreviations
Edited by G Eysenbach; submitted 25.12.23; peer-reviewed by CY Wang, L Guo; comments to author 24.01.24; revised version received 14.02.24; accepted 09.03.24; published 16.04.24.
©Satoshi Nishioka, Satoshi Watabe, Yuki Yanagisawa, Kyoko Sayama, Hayato Kizaki, Shungo Imai, Mitsuhiro Someya, Ryoo Taniguchi, Shuntaro Yada, Eiji Aramaki, Satoko Hori. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 16.04.2024.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work, first published in the Journal of Medical Internet Research, is properly cited. The complete bibliographic information, a link to the original publication on https://www.jmir.org/, as well as this copyright and license information must be included.
The Federal Register
The daily journal of the united states government, request access.
Due to aggressive automated scraping of FederalRegister.gov and eCFR.gov, programmatic access to these sites is limited to access to our extensive developer APIs.
If you are human user receiving this message, we can add your IP address to a set of IPs that can access FederalRegister.gov & eCFR.gov; complete the CAPTCHA (bot test) below and click "Request Access". This process will be necessary for each IP address you wish to access the site from, requests are valid for approximately one quarter (three months) after which the process may need to be repeated.
An official website of the United States government.
If you want to request a wider IP range, first request access for your current IP, and then use the "Site Feedback" button found in the lower left-hand side to make the request.
College of Law

Research into action: Jonathan Picado leverages his legal education for environmental change
Why environmental is important to you.

In graduate school, I began researching environmental issues along the U.S.-Mexico border and worked with grassroots advocacy groups in the Paso del Norte region. I placed significant value on the narratives and stories of the families most affected in El Paso, and these families showed me first-hand the environmental injustices they were constantly exposed to in their schools, homes, parks, and workplaces within the Chamizal neighborhood of El Paso.
Through my research, my understanding of the problem became much more personal. As I was mapping out the wastewater treatment plants in El Paso, I noticed that a wastewater treatment plant was located right behind the church I attended growing up, and I thought back to the ever-present stench that filled the air, no matter what time of day it was. As a child, I never thought much of it, and the adults never treated it as anything more than a bad odor. Like Chamizal, the neighborhoods near my childhood church were home to lower-income, Latinx communities and were separated by train tracks from the wealthiest zip code in El Paso. Groups like Familias Unidas del Chamizal motivated and inspired me to pursue a legal career that focuses on these complex environmental issues.
How this fellowship funding made a difference in your education?
Having worked at a law firm prior to coming to law school, I wanted the opportunity to explore governmental and nonprofit work, and my HELI fellowship was truly the only reason I was able to take on the job for the summer.
At the Department of Justice Environment and Natural Resources Division , I was able to work on matters ranging from Fifth Amendment takings arising out of natural disasters to water rights disputes between states. My work with the DOJ felt exactly what I envisioned environmental advocacy to look like–one that is informed by both the law and the science surrounding many environmental issues.
How do you plan to use this education to create change?
I always think back to those families that showed me around the environmental injustices they were exposed to in El Paso and how they may see the racket and stench as the norm, just as I did growing up. But these seemingly minor inconveniences have consequences for the primarily lower-income, minority communities that are exposed to them.
Countless families in El Paso and across the country are subject to environmental injustices, and for many, one of the strongest forms of redress is through the legal system. Through my legal education and work on environmental matters, I hope to one day be able to amplify their voices through legal representation and to speak against this deadly injustice.

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
JavaScript appears to be disabled on this computer. Please click here to see any active alerts .
Tire Crumb Exposure Characterization Report (Volumes 1 and 2)
To help address concerns raised by the public about potential exposures and health effects due to the use of recycled tire rubber used on fields as infill material, the Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry (CDC/ATSDR) and the U.S. Environmental Protection Agency (EPA), in collaboration with the Consumer Product Safety Commission (CPSC), launched a multi-agency research effort in February 2016 called the Federal Research Action Plan on the Use of Tire Crumbs in Playing Fields and Playgrounds (FRAP).
In April 2024, EPA and CDC/ATSDR released the Synthetic Turf Field Recycled Tire Crumb Rubber Characterization Research Final Report: Part 2 -Tire Crumb Rubber Exposure Characterization. This report is part of the FRAP and is an effort to characterize the chemicals associated with tire crumb rubber and to identify the ways in which people may be exposed to those chemicals based on their activities on synthetic turf fields and playgrounds. The Part 1 Tire Crumb Rubber Characterization Research Report from EPA and CDC/ATSDR, which was released in 2019, summarizes results for the physical, chemical, and microbiological characterization for tire crumb rubber used on synthetic turf fields.
The Part 2 Tire Crumb Rubber Exposure Characterization from EPA and CDC/ATSDR summarizes an exposure characterization and pilot-scale exposure measurements study that included collection of both indoor and outdoor field, personal, and biomarker samples for indicators of exposures; questionnaire and video-based activity assessments; and exposure modeling assessments. CDC/ATSDR completed a supplemental biomonitoring study, which is part of the Tire Crumb Exposure Characterization Report. The Part 2 report consists of two volumes; Volume 1 contains the results from the pilot exposure characterization research study and Volume 2 contains the results from the supplemental biomonitoring study, quality control/quality assurance assessments, and information about methods.
Part 1 and Part 2 reports underwent a letter external peer review by independent experts. The response to external peer review comments can be found below. The supplemental biomonitoring study (Part 2, Appendix A) was separately peer reviewed through CDC/ATSDR.
Synthetic Turf Field Recycled Tire Crumb Rubber Research Under the Federal Research Action Plan Final Report Part 2 – Exposure Characterization
TCRS Exposure Characterization Volume 1 (pdf) (5.8 MB, 4/16/2024, EPA/600/R24/020.1)
TCRS Exposure Characterization Volume 2 (pdf) (48.7 MB, 4/16/2024, EPA/600/R24/020.2)
TCRS Responses to External Peer Review Comments (pdf) (1.1 MB, 4/16/2024)
- Chemical Safety Research Home
- Chemical Evaluation & Characterization
- Complex Systems Science
- Translation, Training, & Tools
- New Approach Methodologies Research
- Chemical Research to Inform Decision Making
- Collaborative Agreements
Numbers, Facts and Trends Shaping Your World
Read our research on:
Full Topic List
Regions & Countries
- Publications
- Our Methods
- Short Reads
- Tools & Resources
Read Our Research On:
Growing share of childless adults in U.S. don’t expect to ever have children
Birth rates in the United States dropped during the COVID-19 pandemic amid the twin public health and economic crises, lending evidence to predictions from early on in the outbreak that economic uncertainty might trigger a baby bust. This continued the downward trend in U.S. fertility rates, which were already at a record low before the pandemic began.
A new Pew Research Center survey finds that a rising share of U.S. adults who are not already parents say they are unlikely to ever have children, and their reasons range from just not wanting to have kids to concerns about climate change and the environment.
Pew Research Center conducted this study to learn more about Americans who don’t expect to have children in the future and the reasons they give.
This analysis is based on 3,866 U.S. adults ages 18 to 49, collected as a part of a larger survey conducted Oct. 18-24, 2021. Everyone who took part is a member of the Center’s American Trends Panel (ATP), an online survey panel that is recruited through national, random sampling of residential addresses. This way, nearly all U.S. adults have a chance of selection. The survey is weighted to be representative of the U.S. adult population by gender, race, ethnicity, partisan affiliation, education and other categories. Read more about the ATP’s methodology .
Here are the questions used for this post, along with responses, and its methodology .
Some 44% of non-parents ages 18 to 49 say it is not too or not at all likely that they will have children someday, an increase of 7 percentage points from the 37% who said the same in a 2018 survey. Meanwhile, 74% of adults younger than 50 who are already parents say they are unlikely to have more kids, virtually unchanged since 2018.
Among parents and non-parents alike, men and women are equally likely to say they will probably not have kids (or more kids) in the future. Perhaps not surprisingly, adults in their 40s are far more likely than younger ones to say they are unlikely to have children or to have more children in the future. Some 85% of non-parents 40 to 49 say this, compared with 37% of those younger than 40. And while 91% of older parents say they probably won’t have more kids, 60% of younger parents say the same.
A majority (56%) of non-parents younger than 50 who say it’s unlikely they will have children someday say they just don’t want to have kids. Childless adults younger than 40 are more likely to say this than those ages 40 to 49 (60% vs. 46%, respectively). There are no differences by gender.
Among childless adults who say they have some other reason for thinking they won’t have kids in the future, no single reason stands out. About two-in-ten (19%) say it’s due to medical reasons, 17% say it’s for financial reasons and 15% say it’s because they do not have a partner. Roughly one-in-ten say their age or their partner’s age (10%) or the state of the world (9%) is a reason they don’t plan to have kids. An additional 5% cite environmental reasons, including climate change, and 2% say their partner doesn’t want children.
When it comes to 18- to 49-year-old parents who say they are unlikely to have more children in the future, again a majority (63%) say it’s because they just don’t want to. Fathers (69%) are more likely to say this than mothers (59%), as are parents younger than 40 (71%) when compared with those 40 to 49 (57%).
Among those younger than 50 who say there was some other reason why they probably will not have more children, age (theirs or their partner’s) and medical reasons were among the top reasons why (29% and 23% of this group said so, respectively), followed by financial reasons (14%) and the fact that they already have kids (11%). Smaller shares cite the state of the world (4%), not having a partner (2%) or having a partner who doesn’t want kids (2%).
Mothers and fathers generally give similar reasons for saying it’s unlikely they will have more children, but mothers are more likely than fathers to mention medical reasons (27% vs. 16%), while fathers are more likely than mothers to cite already having kids (18% vs. 6%).
About a quarter of parents younger than 40 who don’t expect to have more children in the future cite financial reasons (26%), compared with 8% of those 40 to 49. The younger group is also more likely to mention not having a partner (6% vs. 1%), while older parents are more likely to say age is a reason why they don’t expect to have more kids (41% of those 40 to 49 vs. 5% of those younger than 40).
Note: Here are the questions used for this post, along with responses, and its methodology .
- Birth Rate & Fertility
- Family & Relationships
- Household Structure & Family Roles

Few East Asian adults believe women have an obligation to society to have children
A growing share of americans say they’ve had fertility treatments or know someone who has, key facts as india surpasses china as the world’s most populous country, key facts about china’s declining population, global population skews male, but un projects parity between sexes by 2050, most popular.
1615 L St. NW, Suite 800 Washington, DC 20036 USA (+1) 202-419-4300 | Main (+1) 202-857-8562 | Fax (+1) 202-419-4372 | Media Inquiries
Research Topics
- Age & Generations
- Coronavirus (COVID-19)
- Economy & Work
- Gender & LGBTQ
- Immigration & Migration
- International Affairs
- Internet & Technology
- Methodological Research
- News Habits & Media
- Non-U.S. Governments
- Other Topics
- Politics & Policy
- Race & Ethnicity
- Email Newsletters
ABOUT PEW RESEARCH CENTER Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
Copyright 2024 Pew Research Center
Terms & Conditions
Privacy Policy
Cookie Settings
Reprints, Permissions & Use Policy
An official website of the United States government
Here's how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS. A lock ( Lock Locked padlock ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
Dear Colleague Letter: Joint National Science Foundation and United States Department of Agriculture National Institute of Food and Agriculture Funding Opportunity: Supporting Foundational Research in Robotics (FRR)
April 18, 2024
Dear Colleague:
Recognizing the importance of use-inspired collaborations in promoting scientific discoveries, the National Science Foundation (NSF), in collaboration with United States Department of Agriculture National Institute of Food and Agriculture (USDA/NIFA), seeks proposals to advance foundational research in agricultural robotics. These proposals should be of mutual interest to the NSF Foundational Research in Robotics (FRR) program and to USDA/NIFA .
NSF's FRR program, jointly led by the Directorate for Engineering (ENG) and the Directorate for Computer and Information Science and Engineering (CISE), supports research to create innovative robots with unprecedented new functionality. USDA/NIFA has the mission to provide leadership and funding for programs that advance agriculture-related sciences. Proposals submitted under this Dear Colleague Letter (DCL) should present a compelling vision for pioneering robots with transformative potential in agricultural contexts. It is highly suggested that potential proposers contact the USDA/NIFA program director first (listed below) with a short narrative to determine project applicability for this program. If appropriate, an NSF program director will be further consulted.
PROPOSAL SUBMISSION REQUIREMENTS
NSF is the lead agency for this collaboration. Proposals to be considered under this Dear Colleague Letter should have a title prefixed by "NIFA:" and should be submitted to the FRR program. Submissions will be evaluated in FRR review panels, following the requirements of the NSF Proposal & Award Policies & Procedures Guide (PAPPG) ( https://new.nsf.gov/policies/pappg ), and the FRR Program Description ( https://new.nsf.gov/funding/opportunities/foundational-research-robotics-frr ). Proposals submitted under this Dear Colleague Letter must be clearly justified by important needs in agriculture and the agricultural sciences.
NSF will manage and conduct the review process of proposals submitted in accordance with NSF standards and procedures, as described in the PAPPG. USDA staff will participate in panels as observers during the discussion of USDA-focused proposals. Information about proposals and unattributed reviews of proposals will be shared with USDA staff. NSF and NIFA will meet as soon as possible after the proposals have been reviewed to formulate a set of funding recommendations consistent with the goals of this DCL. Note that if a proposal is selected for an award to be funded by NIFA, NSF will request the submitting institution withdraw their NSF proposal and submit to NIFA.
Recipients funded by NIFA will be encouraged to participate in annual FRR grantee meetings, along with recipients funded by NSF.
Interested parties are encouraged to contact the listed program directors at NSF and USDA/NIFA prior to submission.
TECHNICAL POINTS OF CONTACT
FRR Program Officers:
USDA/NIFA Program Officers:
Margaret Martonosi Assistant Director Directorate for Computer and Information Science and Engineering<
Susan Margulies Assistant Director Directorate for Engineering

IMAGES
VIDEO
COMMENTS
Start by defining your project's purpose. Identify what your project aims to accomplish and what you are researching. Remember to use clear language. Thinking about the project's purpose will help you set realistic goals and inform how you divide tasks and assign responsibilities.
If you want to learn how to write your own plan for your research project, consider the following seven steps: 1. Define the project purpose. The first step to creating a research plan for your project is to define why and what you're researching. Regardless of whether you're working with a team or alone, understanding the project's purpose can ...
A research plan is a framework that shows how you intend to approach your topic. The plan can take many forms: a written outline, a narrative, a visual/concept map or timeline. It's a document that will change and develop as you conduct your research. Components of a research plan. 1. Research conceptualization - introduces your research question.
Themes should be far away from the description of any facet of the context. Themes should be closer to explaining the endogenous constructs of a research. Further, often the contribution of a qualitative case study research (QCSR) emerges from the 'extension of a theory' or 'developing deeper understanding—fresh meaning of a phenomenon'.
When to use thematic analysis. Thematic analysis is a good approach to research where you're trying to find out something about people's views, opinions, knowledge, experiences or values from a set of qualitative data - for example, interview transcripts, social media profiles, or survey responses. Some types of research questions you might use thematic analysis to answer:
A research plan is a thoughtful, compelling, well-written document that outlines your exciting, unique research ideas that you and your students will pursue over the next half decade or so to advance knowledge in your discipline and earn you grants, papers, speaking invitations, tenure, promotion, and a national reputation.
Research proposal examples. Writing a research proposal can be quite challenging, but a good starting point could be to look at some examples. We've included a few for you below. Example research proposal #1: "A Conceptual Framework for Scheduling Constraint Management".
The research plan, however, serves another, very important function: It contributes to your development as a scientist. Your research plan is a map for your career as a research science professional. As will become apparent later in this document, one of the functions of a research plan is to demonstrate your intellectual vision and aspirations.
Review and Finalize Your Research Plan; Abstract and Narrative; Research Plan Overview and Your Approach. Your application's Research Plan has two sections: Specific Aims—a one-page statement of your objectives for the project. Research Strategy—a description of the rationale for your research and your experiments in 12 pages for an R01.
A research proposal is a written document, concerned with a comprehensive description of a proposed research plan or programme on a specific subject matter or topic to substantiate the need and relevance of carrying out the research [].Research proposals should draw attention to the proposed study's benefits and possible research outcomes, backed by informative and convincing evidence.
The Clinical Research Education Library is supported in part by the National Institute on Deafness and Other Communication Disorders (NIDCD) of the National Institutes of Health under award number U24-DC012078. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Internet Citation: Essentials of the Research Plan. Content last reviewed January 2017. Agency for Healthcare Research and Quality, Rockville, MD. The research plan is the main part of a grant application describing a principal investigator's proposed research, stating its importance and how it will be conducted.
Your research theme positively states the qualities you will work toward. Some examples follow. "For students to value friendship, develop their own perspectives and ways of thinking, and enjoy science.". "Develop social-emotional skills and…a deeper understanding of mathematics". "Across both math and language arts, develop our ...
The best way to remember the difference between a research plan and a research proposal is that they have fundamentally different audiences. A research plan helps you, the researcher, organize your thoughts. On the other hand, a dissertation proposal or research proposal aims to convince others (e.g., a supervisor, a funding body, or a ...
The success of any research study hinges on a well-crafted research plan, where the project background, research goals, and research questions serve as cornerstones. In this article, we will delve…
For a research project to successfully advance medical and scientific knowledge, each component of the entire research process must be clearly and rationally conceived before proceeding with active research steps such as data collection and analysis [ 1 ]. The research process has three phases: the conceptual phase the empirical phase, which ...
WRITING A RESEARCH PLAN. Essential components of a research plan. While there is no one approach to planni ng, there are a number of essential topics that you should at least. consider, if not f ...
The Research Plan comprises four intersecting themes, collectively building a powerful ecosystem of action towards our goals. In this 2022 - 2025 period, we aim to further deepen and extend our established excellence in research performance to ensure we are well positioned for
The NICHD Strategic Plan presents NICHD's research goals and objectives under five broad research themes that will enable NICHD to focus its efforts on the most significant public health challenges and advance science in these areas of priority.
Neither a research plan nor a research proposal is a "super specific thing you need to know about". A proposal, on any subject, generally includes things like objectives, background, potential challenges, and a vague schedule.
T. exas A&M University's Research Enterprise Strategic Plan for 2023-2030 identifies six strategic research themes and 21 sub-themes that leverage Texas A&M's strengths, capacity and capabilities across multiple disciplines. These themes and sub-themes provide opportunities for Texas A&M to i) strengthen its tripartite mission of research ...
Welcome and state of research update Summary and update on status of strategic priorities . 9-9:55 a.m. New Initiatives and Research Updates . 9:55 - 10:10 a.m. Break . 10:10 - 11:30 a.m. Breakout - Discussion Groups . Cores/Equipment Clinical Research Oncology Research Data Science Operations Training Grants (T, F, R) 11:30 a.m. - Noon ...
Here at Slidesgo we're offering this theme that you can actually use for any kind of project, regardless of the topic. Business. 16:9 / Like . Download ... Download the "Soil Mechanics Research Project Proposal" presentation for PowerPoint or Google Slides. A well-crafted proposal can be the key factor in determining the success of your project.
Background: Early detection of adverse events and their management are crucial to improving anticancer treatment outcomes, and listening to patients' subjective opinions (patients' voices) can make a major contribution to improving safety management. Recent progress in deep learning technologies has enabled various new approaches for the evaluation of safety-related events based on patient ...
The Workshop on the National Spectrum R&D Plan will be co-located with NSF Spectrum Week and will take place on May 17, 2024, from 8:00 a.m. to 3:30 p.m. (ET), at the Hilton Arlington National Landing, 2399 Richmond Hwy., Arlington, VA 22202. Instructions: Registration is required for in-person attendance; remote viewing will be available via ...
Through my research, my understanding of the problem became much more personal. As I was mapping out the wastewater treatment plants in El Paso, I noticed that a wastewater treatment plant was located right behind the church I attended growing up, and I thought back to the ever-present stench that filled the air, no matter what time of day ...
The Part 1 Tire Crumb Rubber Characterization Research Report from EPA and CDC/ATSDR, which was released in 2019, summarizes results for the physical, chemical, and microbiological characterization for tire crumb rubber used on synthetic turf fields. The Part 2 Tire Crumb Rubber Exposure Characterization from EPA and CDC/ATSDR summarizes an ...
Some 85% of non-parents 40 to 49 say this, compared with 37% of those younger than 40. And while 91% of older parents say they probably won't have more kids, 60% of younger parents say the same. A majority (56%) of non-parents younger than 50 who say it's unlikely they will have children someday say they just don't want to have kids.
April 18, 2024. Dear Colleague: Recognizing the importance of use-inspired collaborations in promoting scientific discoveries, the National Science Foundation (NSF), in collaboration with United States Department of Agriculture National Institute of Food and Agriculture (USDA/NIFA), seeks proposals to advance foundational research in agricultural robotics.