- Open access
- Published: 22 August 2018

Health policy and systems research: the future of the field
- David H. Peters ORCID: orcid.org/0000-0001-8377-3444 1
Health Research Policy and Systems volume 16 , Article number: 84 ( 2018 ) Cite this article
15k Accesses
30 Citations
82 Altmetric
Metrics details
Health policy and systems research (HPSR) has changed considerably over the last 20 years, but its main purpose remains to inform and influence health policies and systems. Whereas goals that underpin health systems have endured – such as a focus on health equity – contexts and priorities change, research methods progress, and health organisations continue to learn and adapt, in part by using HPSR. For HPSR to remain relevant, its practitioners need to re-think how health systems are conceptualised, to keep up with rapid changes in how we diagnose and manage disease and use information, and consider factors affecting people’s health that go well beyond healthcare systems. The Sustainable Development Goals (SDGs) represent a shifting paradigm in human development by seeking convergence across sectors. They also offer an opportunity for HPSR to play a larger role, given its pioneering work on applying systems thinking to health, its focus on health equity, and the strength of its multi-disciplinary approaches that make it a good fit for the SDG era.
Globally, population health is being challenged in different ways, from climate change and growing air pollution and toxic environmental exposure to food insecurity, massive population migration and refugee crises, to emerging and re-emerging diseases. Each of these trends reinforce each other and concentrate their harms on the most vulnerable populations. Multi-level governance, together with novel regulatory strategies and socially oriented investments, are key to successful action against many of the new challenges, with HPSR guiding their design and evolution.
The HPSR community cannot be complacent about its successful, yet short, history. Tensions remain about how different stakeholders use HPSR such as the contrast between embedding research within government institutions versus independently evaluating and holding decision-makers accountable. Such tensions are inevitable in the boundary-spanning field that HPSR has become. We should strive to enhance the influence of HPSR by staying relevant in a changing world and embracing the strength of our diversity of disciplines, the range of problems addressed, and the opportunity of the SDGs to ensure that health and social benefits are more inclusive for people within and across countries.
Peer Review reports
In 1962, Burnet, the Nobel prize-winning immunologist, wrote that the twentieth century would be witness to “ the virtual elimination of infectious disease as a significant factor in social life ” [ 1 ] – a reminder to be humble when predicting the future effects of health research. Nonetheless, the last 20 years has brought impressive change in the growth of health policy and systems research (HPSR), and the settings in which it is applied. One safe prediction is that the HPSR landscape will continue to change and grow in complexity.
The value of HPSR
The central idea behind HPSR is that research should inform and influence policies and systems to pursue health goals [ 2 ]. Health systems goals and the values that underpin them are enduring and should continue to be examined through HPSR. Contexts will change and new challenges will emerge, but research will still be needed to inform how to achieve the multiple health systems goals – improving effectiveness, equity and efficiency, expanding health services coverage, and enhancing people’s financial protection, while minimising costs and improving accountability and trust. HPSR provides the tools for Ministries of Health and other health organisations to become learning organisations, serving to lead and adapt to changes in the health sector.
Re-thinking health systems in a changing context
The changing context will also challenge how we think about health systems; HPSR should have a central role in understanding change and how to intervene. The social, political and environmental conditions for healthy living are rapidly shifting, as are expectations about the role of the state, civil society and business. Information communications and other technologies are transforming the diagnosis and management of disease, as well as the collection, analysis and sharing of individual and population health data. Additionally, there are growing population pressures due to environmental degradation, urbanisation and aging along with new threats due to emerging diseases and the failure of poorly organised market systems for health services, technologies and financial products. Each condition is both a driver of change and an effect of another; they are interdependent issues in an increasingly interconnected world.
The Sustainable Development Goals (SDGs) represent a shifting paradigm in human development, moving from the building up of individual core sectors within countries to seeking convergence across co-influencing and co-dependent sectors. In a world where wealth inequality is escalating, the SDGs mark a shift from efforts to provide overall benefits to a nation to focusing on inclusive growth and tackling inequities as the core of development efforts. HPSR is well placed to take on these issues of the future since it has a traditional focus on understanding and addressing different types of disadvantage and inequity, taking advantage of various disciplines and approaches to address inequities, including social epidemiology, economics, participatory action research and ethics [ 3 ]. Further, HPSR has pioneered the application of systems thinking in health, providing a wide set of theories, frameworks and tools to examine and test how different elements of systems – actors, functions and their relationships – fit together to make an overall whole [ 4 ].
Efforts to strengthen health systems have been both facilitated and constrained by the dominance of the Health Systems Building Blocks model [ 5 ]. The model focuses on inputs and selected functions of a healthcare system, but was designed as a communication tool to indicate options for government investment, and not as an analytic or explanatory model of a complete health system. The building blocks model has especially neglected people (indeed the entire demand side of a health system) and institutions, the importance of dynamic linkages between stakeholders and functions in a health system, and connections between health systems and other related systems (e.g. education, economic development, ecology, etc.). To apply research to questions concerned with the linkages across sectors – as envisioned by the SDGs – it will be more important to consider the roles of people (as individuals, families, communities and larger populations) and the dynamic connections between policies and systems that affect people both inside the traditional health sector and through related sectors.
Growing challenges for HPSR
There are many new issues and evolving roles for different stakeholders in a health system, as well as novel ways in which we can study and influence health systems. Globally, population health is being challenged in different ways. Ambient air pollution in cities and indoor air pollution in rural homes have become important risk factors for chronic diseases. Food insecurity is again a critical public concern, as climate change is projected to decrease crop yields, particularly in South America, Africa, South Asia and Australia, while contributing to increased food price volatility [ 6 ]. Poor nutrition, exposure to environmental toxins and a resurgence of vector-borne diseases, such as malaria and dengue, are all consequences of environmental degradation. The poor are especially vulnerable, as they are most exposed to the direct and indirect shocks of environmental degradation, are more vulnerable because they lose relatively more wealth, and are less resilient because they do not have the financial and social safety nets required to manage them and recover [ 7 ].
The growing phenomenon of antimicrobial resistance is another major threat to global health that needs to be tackled in both the health and agricultural sectors at local, national and global levels. The failure to develop new antimicrobials or ensure equitable access to existing antibiotics, while counterfeit and substandard drugs flourish, represent major market failures [ 8 , 9 ]. New regulatory strategies, socially oriented investment and a realignment of incentives are needed at all levels. Multi-level governance is the key for successful action in containment strategies, supported by HPSR to assess how well they work and guide their evolution.
Population migration is another major social, political and health systems challenge. One billion individuals are now on the move globally, one-quarter of whom are crossing national borders. The estimated refugee population reached an unprecedented 19.6 million individuals worldwide in 2015, half of whom are children [ 10 ]. Health systems are at the forefront of the response to the ongoing crisis facing refugees and other migrants, both at first point of contact and later during resettlement. There is a need to develop more effective approaches that respond to the health needs of displaced populations, yet the evidence base regarding which interventions are effective is quite weak.
Opportunities for HPSR
HPSR has developed as a boundary-spanning field, not only crossing disciplinary lines, but also linking stakeholders with very different roles (e.g. policy-makers, health practitioners, researchers, civil society leaders, the media). As such, HPSR should continue to influence policy both within and across countries. HPSR has served in each of six types of research utilisation as described by Weiss [ 11 ], though in recent years it has tended to be used most directly in a problem-solving model (to facilitate decisions by policy-makers and managers) or to otherwise contribute to complex policy-making through an interactive model of health research. However, there is also a growing tension between new approaches that promote embedded and implementer-led research, which pursues problem-solving from ‘within’ [ 12 , 13 ] and research that takes an external perspective, seeking to independently evaluate policy effects, identify neglected problems or hold decision-makers accountable [ 14 , 15 ]. HPSR should be used to serve each of these perspectives, and not become captive to a single approach.
Encouraging diversity and equity has become part of the shared values of many practitioners and users of HPSR, crossing contexts and types of research utilisation. For example, the Alliance for Health Policy and Systems Research, along with many partners working in global health, have expressed a very clear set of values – “ to address problems of inequity, poverty and disadvantage ” [ 16 ] and to support partnerships and collaboration on an inclusive and participatory basis.
However, this set of values has come into conflict with recent policy and electoral decisions made around the world over the last few years. There has been a rise in electoral trends that seem to undermine the values of global citizenship and even the role of evidence in decision-making. Recent political events around the world, including in the Americas, Europe, Africa, and Asia, suggest that massive numbers of people are dissatisfied with incumbent policy-makers and their policies, and are voting for the politics of division. In contrast, HPSR can be a vehicle to learn from and promote the diversity of cultures, building of local capabilities and forging of international cooperation. The promise of research and its application to policy and public health practice can help people to overcome the divisions of nationalism, race, class, wealth and other obstacles to social justice and health equity.
Conclusions
This is a time when the technical skills, knowledge contributions and historical values of HPSR are needed more than ever. We need a HPSR agenda to better understand and meet peoples’ expectations, and to sharpen our science of communication, both issues within the remit of HPSR. However, the HPSR community cannot be complacent about its successful but short-lived history. We should strive to enhance the influence of HPSR by staying relevant in a changing world, embracing the strength of our diversity of disciplines, the range of problems addressed, and the opportunity of the SDGs to ensure that health and social benefits are more inclusive for people within and across countries. If we are to have policies and interventions that promote justice and good health whilst being grounded in evidence, then we must ensure that our thinking and practice of HPSR help us rise to these challenges.
Abbreviations
health policy and systems research
- Sustainable Development Goals
Burnet FM. Natural History of Infectious Disease. Cambridge: Cambridge University Press; 1962.
Google Scholar
Alliance for Health Policy and Systems Research. World Report on Health Policy and Systems Research. Geneva: WHO; 2017.
Gilson L, editor. Health Policy and Systems Research: A Methodology Reader. Geneva: Alliance for Health Policy and Systems Research, World Health Organization; 2012.
Peters DH. The application of systems thinking in health: why use systems thinking? Health Res Policy Syst. 2014;12:51.
Article PubMed PubMed Central Google Scholar
World Health Organization. Everybody Business: Strengthening Health Systems to Improve Health Outcomes - WHO’s Framework for Action. Geneva: WHO; 2007.
Havlik P, Valin H, Gusti M, Schmid E, Leclère D, Forsell N, Herrero M, Khabarov N, Mosnier A, Cantele M, Obersteiner M. Climate change impacts and mitigation in the developing world: an integrated assessment of the agriculture and forestry sectors. Policy Research Working Paper No. WPS 7477. 2015. http://pure.iiasa.ac.at/11657 . Accessed 14 Aug 2018.
Hallegatte S, Bangalore M, Bonzanigo L, Fay M, Kane T, Narloch U, Rozenberg J, Treguer D, Vogt-Schilb A. Shock Waves: Managing the Impacts of Climate Change on Poverty. Climate Change and Development. Washington, DC: World Bank; 2016.
Cars O, Högberg LD, Murray M, Nordberg O, Sivaraman S, Lundborg CS, So AD, Tomson G. Meeting the challenge of antibiotic resistance. BMJ. 2008;337:a1438.
Article PubMed Google Scholar
Peters DH, Bloom G. Developing world: bring order to unregulated health markets. Nature. 2012;487(7406):163–5.
Article PubMed CAS Google Scholar
United Nations Population Division. International Migrant Stock 2015. 2016. http://www.un.org/en/development/desa/population/migration/data/estimates2/estimates15.shtml . Accessed 14 Aug 2018.
Weiss CH. The many meanings of research utilization. Public Adm Rev. 1979;39:426–31.
Article Google Scholar
MacGregor H, Bloom G. Health systems research in a complex and rapidly changing context: ethical implications of major health systems change at scale. Dev World Bioeth. 2016;16(3):158–67.
Tran N, Langlois EV, Reveiz L, Varallyay I, Elias V, Mancuso A, et al. Embedding research to improve program implementation in Latin America and the Caribbean. Rev Panam Salud Publica. 2017;41:e75.
PubMed Google Scholar
Gaventa J, McGee R. The impact of transparency and accountability initiatives. Dev Policy Rev. 2013;31:s3–s28. https://doi.org/10.1111/dpr.12017 .
World Health Organization. Monitoring, Evaluation and Review of National Health Strategies: A Country-led Platform for Information and Accountability. Geneva: WHO; 2011.
Alliance for Health Policy and Systems Research. Investing in Knowledge for Resilient Health Systems: Strategic Plan 2016–2020. Geneva: WHO; 2016.
Download references
Author information
Authors and affiliations.
Department of International Health, Johns Hopkins University, Bloomberg School of Public Health, 615 N. Wolfe St, Suite E-8527, Baltimore, MD, 21205, United States of America
David H. Peters
You can also search for this author in PubMed Google Scholar
Contributions
DP was sole author. The author read and approved the final manuscript.
Corresponding author
Correspondence to David H. Peters .
Ethics declarations
Ethics approval and consent to participate.
Ethics approval was not required for this Commentary, as it does not involve human subjects research.
Consent for publication
Not applicable.
Competing interests
The author declares that he has no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Peters, D.H. Health policy and systems research: the future of the field. Health Res Policy Sys 16 , 84 (2018). https://doi.org/10.1186/s12961-018-0359-0
Download citation
Received : 24 January 2018
Accepted : 12 March 2018
Published : 22 August 2018
DOI : https://doi.org/10.1186/s12961-018-0359-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Health policy
- Health systems
- Health systems research
Health Research Policy and Systems
ISSN: 1478-4505
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Loading metrics
Open Access
Peer-reviewed
Research Article
Assessing the impact of healthcare research: A systematic review of methodological frameworks
Roles Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing
Affiliation Centre for Patient Reported Outcomes Research, Institute of Applied Health Research, College of Medical and Dental Sciences, University of Birmingham, Birmingham, United Kingdom
Roles Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Supervision, Validation, Writing – review & editing
* E-mail: [email protected]
Roles Data curation, Formal analysis, Methodology, Validation, Writing – review & editing
Roles Formal analysis, Methodology, Supervision, Validation, Writing – review & editing
- Samantha Cruz Rivera,
- Derek G. Kyte,
- Olalekan Lee Aiyegbusi,
- Thomas J. Keeley,
- Melanie J. Calvert

- Published: August 9, 2017
- https://doi.org/10.1371/journal.pmed.1002370
- Reader Comments
Increasingly, researchers need to demonstrate the impact of their research to their sponsors, funders, and fellow academics. However, the most appropriate way of measuring the impact of healthcare research is subject to debate. We aimed to identify the existing methodological frameworks used to measure healthcare research impact and to summarise the common themes and metrics in an impact matrix.
Methods and findings
Two independent investigators systematically searched the Medical Literature Analysis and Retrieval System Online (MEDLINE), the Excerpta Medica Database (EMBASE), the Cumulative Index to Nursing and Allied Health Literature (CINAHL+), the Health Management Information Consortium, and the Journal of Research Evaluation from inception until May 2017 for publications that presented a methodological framework for research impact. We then summarised the common concepts and themes across methodological frameworks and identified the metrics used to evaluate differing forms of impact. Twenty-four unique methodological frameworks were identified, addressing 5 broad categories of impact: (1) ‘primary research-related impact’, (2) ‘influence on policy making’, (3) ‘health and health systems impact’, (4) ‘health-related and societal impact’, and (5) ‘broader economic impact’. These categories were subdivided into 16 common impact subgroups. Authors of the included publications proposed 80 different metrics aimed at measuring impact in these areas. The main limitation of the study was the potential exclusion of relevant articles, as a consequence of the poor indexing of the databases searched.
Conclusions
The measurement of research impact is an essential exercise to help direct the allocation of limited research resources, to maximise research benefit, and to help minimise research waste. This review provides a collective summary of existing methodological frameworks for research impact, which funders may use to inform the measurement of research impact and researchers may use to inform study design decisions aimed at maximising the short-, medium-, and long-term impact of their research.
Author summary
Why was this study done.
- There is a growing interest in demonstrating the impact of research in order to minimise research waste, allocate resources efficiently, and maximise the benefit of research. However, there is no consensus on which is the most appropriate tool to measure the impact of research.
- To our knowledge, this review is the first to synthesise existing methodological frameworks for healthcare research impact, and the associated impact metrics by which various authors have proposed impact should be measured, into a unified matrix.
What did the researchers do and find?
- We conducted a systematic review identifying 24 existing methodological research impact frameworks.
- We scrutinised the sample, identifying and summarising 5 proposed impact categories, 16 impact subcategories, and over 80 metrics into an impact matrix and methodological framework.
What do these findings mean?
- This simplified consolidated methodological framework will help researchers to understand how a research study may give rise to differing forms of impact, as well as in what ways and at which time points these potential impacts might be measured.
- Incorporating these insights into the design of a study could enhance impact, optimizing the use of research resources.
Citation: Cruz Rivera S, Kyte DG, Aiyegbusi OL, Keeley TJ, Calvert MJ (2017) Assessing the impact of healthcare research: A systematic review of methodological frameworks. PLoS Med 14(8): e1002370. https://doi.org/10.1371/journal.pmed.1002370
Academic Editor: Mike Clarke, Queens University Belfast, UNITED KINGDOM
Received: February 28, 2017; Accepted: July 7, 2017; Published: August 9, 2017
Copyright: © 2017 Cruz Rivera et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the paper and supporting files.
Funding: Funding was received from Consejo Nacional de Ciencia y Tecnología (CONACYT). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript ( http://www.conacyt.mx/ ).
Competing interests: I have read the journal's policy and the authors of this manuscript have the following competing interests: MJC has received consultancy fees from Astellas and Ferring pharma and travel fees from the European Society of Cardiology outside the submitted work. TJK is in full-time paid employment for PAREXEL International.
Abbreviations: AIHS, Alberta Innovates—Health Solutions; CAHS, Canadian Academy of Health Sciences; CIHR, Canadian Institutes of Health Research; CINAHL+, Cumulative Index to Nursing and Allied Health Literature; EMBASE, Excerpta Medica Database; ERA, Excellence in Research for Australia; HEFCE, Higher Education Funding Council for England; HMIC, Health Management Information Consortium; HTA, Health Technology Assessment; IOM, Impact Oriented Monitoring; MDG, Millennium Development Goal; NHS, National Health Service; MEDLINE, Medical Literature Analysis and Retrieval System Online; PHC RIS, Primary Health Care Research & Information Service; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PROM, patient-reported outcome measures; QALY, quality-adjusted life year; R&D, research and development; RAE, Research Assessment Exercise; REF, Research Excellence Framework; RIF, Research Impact Framework; RQF, Research Quality Framework; SDG, Sustainable Development Goal; SIAMPI, Social Impact Assessment Methods for research and funding instruments through the study of Productive Interactions between science and society
Introduction
In 2010, approximately US$240 billion was invested in healthcare research worldwide [ 1 ]. Such research is utilised by policy makers, healthcare providers, and clinicians to make important evidence-based decisions aimed at maximising patient benefit, whilst ensuring that limited healthcare resources are used as efficiently as possible to facilitate effective and sustainable service delivery. It is therefore essential that this research is of high quality and that it is impactful—i.e., it delivers demonstrable benefits to society and the wider economy whilst minimising research waste [ 1 , 2 ]. Research impact can be defined as ‘any identifiable ‘benefit to, or positive influence on the economy, society, public policy or services, health, the environment, quality of life or academia’ (p. 26) [ 3 ].
There are many purported benefits associated with the measurement of research impact, including the ability to (1) assess the quality of the research and its subsequent benefits to society; (2) inform and influence optimal policy and funding allocation; (3) demonstrate accountability, the value of research in terms of efficiency and effectiveness to the government, stakeholders, and society; and (4) maximise impact through better understanding the concept and pathways to impact [ 4 – 7 ].
Measuring and monitoring the impact of healthcare research has become increasingly common in the United Kingdom [ 5 ], Australia [ 5 ], and Canada [ 8 ], as governments, organisations, and higher education institutions seek a framework to allocate funds to projects that are more likely to bring the most benefit to society and the economy [ 5 ]. For example, in the UK, the 2014 Research Excellence Framework (REF) has recently been used to assess the quality and impact of research in higher education institutions, through the assessment of impact cases studies and selected qualitative impact metrics [ 9 ]. This is the first initiative to allocate research funding based on the economic, societal, and cultural impact of research, although it should be noted that research impact only drives a proportion of this allocation (approximately 20%) [ 9 ].
In the UK REF, the measurement of research impact is seen as increasingly important. However, the impact element of the REF has been criticised in some quarters [ 10 , 11 ]. Critics deride the fact that REF impact is determined in a relatively simplistic way, utilising researcher-generated case studies, which commonly attempt to link a particular research outcome to an associated policy or health improvement despite the fact that the wider literature highlights great diversity in the way research impact may be demonstrated [ 12 , 13 ]. This led to the current debate about the optimal method of measuring impact in the future REF [ 10 , 14 ]. The Stern review suggested that research impact should not only focus on socioeconomic impact but should also include impact on government policy, public engagement, academic impacts outside the field, and teaching to showcase interdisciplinary collaborative impact [ 10 , 11 ]. The Higher Education Funding Council for England (HEFCE) has recently set out the proposals for the REF 2021 exercise, confirming that the measurement of such impact will continue to form an important part of the process [ 15 ].
With increasing pressure for healthcare research to lead to demonstrable health, economic, and societal impact, there is a need for researchers to understand existing methodological impact frameworks and the means by which impact may be quantified (i.e., impact metrics; see Box 1 , 'Definitions’) to better inform research activities and funding decisions. From a researcher’s perspective, understanding the optimal pathways to impact can help inform study design aimed at maximising the impact of the project. At the same time, funders need to understand which aspects of impact they should focus on when allocating awards so they can make the most of their investment and bring the greatest benefit to patients and society [ 2 , 4 , 5 , 16 , 17 ].
Box 1. Definitions
- Research impact: ‘any identifiable benefit to, or positive influence on, the economy, society, public policy or services, health, the environment, quality of life, or academia’ (p. 26) [ 3 ].
- Methodological framework: ‘a body of methods, rules and postulates employed by a particular procedure or set of procedures (i.e., framework characteristics and development)’ [ 18 ].
- Pathway: ‘a way of achieving a specified result; a course of action’ [ 19 ].
- Quantitative metrics: ‘a system or standard of [quantitative] measurement’ [ 20 ].
- Narrative metrics: ‘a spoken or written account of connected events; a story’ [ 21 ].
Whilst previous researchers have summarised existing methodological frameworks and impact case studies [ 4 , 22 – 27 ], they have not summarised the metrics for use by researchers, funders, and policy makers. The aim of this review was therefore to (1) identify the methodological frameworks used to measure healthcare research impact using systematic methods, (2) summarise common impact themes and metrics in an impact matrix, and (3) provide a simplified consolidated resource for use by funders, researchers, and policy makers.
Search strategy and selection criteria
Initially, a search strategy was developed to identify the available literature regarding the different methods to measure research impact. The following keywords: ‘Impact’, ‘Framework’, and ‘Research’, and their synonyms, were used during the search of the Medical Literature Analysis and Retrieval System Online (MEDLINE; Ovid) database, the Excerpta Medica Database (EMBASE), the Health Management Information Consortium (HMIC) database, and the Cumulative Index to Nursing and Allied Health Literature (CINAHL+) database (inception to May 2017; see S1 Appendix for the full search strategy). Additionally, the nonindexed Journal of Research Evaluation was hand searched during the same timeframe using the keyword ‘Impact’. Other relevant articles were identified through 3 Internet search engines (Google, Google Scholar, and Google Images) using the keywords ‘Impact’, ‘Framework’, and ‘Research’, with the first 50 results screened. Google Images was searched because different methodological frameworks are summarised in a single image and can easily be identified through this search engine. Finally, additional publications were sought through communication with experts.
Following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (see S1 PRISMA Checklist ), 2 independent investigators systematically screened for publications describing, evaluating, or utilising a methodological research impact framework within the context of healthcare research [ 28 ]. Papers were eligible if they included full or partial methodological frameworks or pathways to research impact; both primary research and systematic reviews fitting these criteria were included. We included any methodological framework identified (original or modified versions) at the point of first occurrence. In addition, methodological frameworks were included if they were applicable to the healthcare discipline with no need of modification within their structure. We defined ‘methodological framework’ as ‘a body of methods, rules and postulates employed by a particular procedure or set of procedures (i.e., framework characteristics and development)’ [ 18 ], whereas we defined ‘pathway’ as ‘a way of achieving a specified result; a course of action’ [ 19 ]. Studies were excluded if they presented an existing (unmodified) methodological framework previously available elsewhere, did not explicitly describe a methodological framework but rather focused on a single metric (e.g., bibliometric analysis), focused on the impact or effectiveness of interventions rather than that of the research, or presented case study data only. There were no language restrictions.
Data screening
Records were downloaded into Endnote (version X7.3.1), and duplicates were removed. Two independent investigators (SCR and OLA) conducted all screening following a pilot aimed at refining the process. The records were screened by title and abstract before full-text articles of potentially eligible publications were retrieved for evaluation. A full-text screening identified the publications included for data extraction. Discrepancies were resolved through discussion, with the involvement of a third reviewer (MJC, DGK, and TJK) when necessary.
Data extraction and analysis
Data extraction occurred after the final selection of included articles. SCR and OLA independently extracted details of impact methodological frameworks, the country of origin, and the year of publication, as well as the source, the framework description, and the methodology used to develop the framework. Information regarding the methodology used to develop each methodological framework was also extracted from framework webpages where available. Investigators also extracted details regarding each framework’s impact categories and subgroups, along with their proposed time to impact (‘short-term’, ‘mid-term’, or ‘long-term’) and the details of any metrics that had been proposed to measure impact, which are depicted in an impact matrix. The structure of the matrix was informed by the work of M. Buxton and S. Hanney [ 2 ], P. Buykx et al. [ 5 ], S. Kuruvila et al. [ 29 ], and A. Weiss [ 30 ], with the intention of mapping metrics presented in previous methodological frameworks in a concise way. A consensus meeting with MJC, DGK, and TJK was held to solve disagreements and finalise the data extraction process.
Included studies
Our original search strategy identified 359 citations from MEDLINE (Ovid), EMBASE, CINAHL+, HMIC, and the Journal of Research Evaluation, and 101 citations were returned using other sources (Google, Google Images, Google Scholar, and expert communication) (see Fig 1 ) [ 28 ]. In total, we retrieved 54 full-text articles for review. At this stage, 39 articles were excluded, as they did not propose new or modified methodological frameworks. An additional 15 articles were included following the backward and forward citation method. A total of 31 relevant articles were included in the final analysis, of which 24 were articles presenting unique frameworks and the remaining 7 were systematic reviews [ 4 , 22 – 27 ]. The search strategy was rerun on 15 May 2017. A further 19 publications were screened, and 2 were taken forward to full-text screening but were ineligible for inclusion.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pmed.1002370.g001
Methodological framework characteristics
The characteristics of the 24 included methodological frameworks are summarised in Table 1 , 'Methodological framework characteristics’. Fourteen publications proposed academic-orientated frameworks, which focused on measuring academic, societal, economic, and cultural impact using narrative and quantitative metrics [ 2 , 3 , 5 , 8 , 29 , 31 – 39 ]. Five publications focused on assessing the impact of research by focusing on the interaction process between stakeholders and researchers (‘productive interactions’), which is a requirement to achieve research impact. This approach tries to address the issue of attributing research impact to metrics [ 7 , 40 – 43 ]. Two frameworks focused on the importance of partnerships between researchers and policy makers, as a core element to accomplish research impact [ 44 , 45 ]. An additional 2 frameworks focused on evaluating the pathways to impact, i.e., linking processes between research and impact [ 30 , 46 ]. One framework assessed the ability of health technology to influence efficiency of healthcare systems [ 47 ]. Eight frameworks were developed in the UK [ 2 , 3 , 29 , 37 , 39 , 42 , 43 , 45 ], 6 in Canada [ 8 , 33 , 34 , 44 , 46 , 47 ], 4 in Australia [ 5 , 31 , 35 , 38 ], 3 in the Netherlands [ 7 , 40 , 41 ], and 2 in the United States [ 30 , 36 ], with 1 model developed with input from various countries [ 32 ].
https://doi.org/10.1371/journal.pmed.1002370.t001
Methodological framework development
The included methodological frameworks varied in their development process, but there were some common approaches employed. Most included a literature review [ 2 , 5 , 7 , 8 , 31 , 33 , 36 , 37 , 40 – 46 ], although none of them used a recognised systematic method. Most also consulted with various stakeholders [ 3 , 8 , 29 , 31 , 33 , 35 – 38 , 43 , 44 , 46 , 47 ] but used differing methods to incorporate their views, including quantitative surveys [ 32 , 35 , 43 , 46 ], face-to-face interviews [ 7 , 29 , 33 , 35 , 37 , 42 , 43 ], telephone interviews [ 31 , 46 ], consultation [ 3 , 7 , 36 ], and focus groups [ 39 , 43 ]. A range of stakeholder groups were approached across the sample, including principal investigators [ 7 , 29 , 43 ], research end users [ 7 , 42 , 43 ], academics [ 3 , 8 , 39 , 40 , 43 , 46 ], award holders [ 43 ], experts [ 33 , 38 , 39 ], sponsors [ 33 , 39 ], project coordinators [ 32 , 42 ], and chief investigators [ 31 , 35 ]. However, some authors failed to identify the stakeholders involved in the development of their frameworks [ 2 , 5 , 34 , 41 , 45 ], making it difficult to assess their appropriateness. In addition, only 4 of the included papers reported using formal analytic methods to interpret stakeholder responses. These included the Canadian Academy of Health Sciences framework, which used conceptual cluster analysis [ 33 ]. The Research Contribution [ 42 ], Research Impact [ 29 ], and Primary Health Care & Information Service [ 31 ] used a thematic analysis approach. Finally, some authors went on to pilot their framework, which shaped refinements on the methodological frameworks until approval. Methods used to pilot the frameworks included a case study approach [ 2 , 3 , 30 , 32 , 33 , 36 , 40 , 42 , 44 , 45 ], contrasting results against available literature [ 29 ], the use of stakeholders’ feedback [ 7 ], and assessment tools [ 35 , 46 ].
Major impact categories
1. primary research-related impact..
A number of methodological frameworks advocated the evaluation of ‘research-related impact’. This encompassed content related to the generation of new knowledge, knowledge dissemination, capacity building, training, leadership, and the development of research networks. These outcomes were considered the direct or primary impacts of a research project, as these are often the first evidenced returns [ 30 , 62 ].
A number of subgroups were identified within this category, with frameworks supporting the collection of impact data across the following constructs: ‘research and innovation outcomes’; ‘dissemination and knowledge transfer’; ‘capacity building, training, and leadership’; and ‘academic collaborations, research networks, and data sharing’.
1 . 1 . Research and innovation outcomes . Twenty of the 24 frameworks advocated the evaluation of ‘research and innovation outcomes’ [ 2 , 3 , 5 , 7 , 8 , 29 – 39 , 41 , 43 , 44 , 46 ]. This subgroup included the following metrics: number of publications; number of peer-reviewed articles (including journal impact factor); citation rates; requests for reprints, number of reviews, and meta-analysis; and new or changes in existing products (interventions or technology), patents, and research. Additionally, some frameworks also sought to gather information regarding ‘methods/methodological contributions’. These advocated the collection of systematic reviews and appraisals in order to identify gaps in knowledge and determine whether the knowledge generated had been assessed before being put into practice [ 29 ].
1 . 2 . Dissemination and knowledge transfer . Nineteen of the 24 frameworks advocated the assessment of ‘dissemination and knowledge transfer’ [ 2 , 3 , 5 , 7 , 29 – 32 , 34 – 43 , 46 ]. This comprised collection of the following information: number of conferences, seminars, workshops, and presentations; teaching output (i.e., number of lectures given to disseminate the research findings); number of reads for published articles; article download rate and number of journal webpage visits; and citations rates in nonjournal media such as newspapers and mass and social media (i.e., Twitter and blogs). Furthermore, this impact subgroup considered the measurement of research uptake and translatability and the adoption of research findings in technological and clinical applications and by different fields. These can be measured through patents, clinical trials, and partnerships between industry and business, government and nongovernmental organisations, and university research units and researchers [ 29 ].
1 . 3 . Capacity building , training , and leadership . Fourteen of 24 frameworks suggested the evaluation of ‘capacity building, training, and leadership’ [ 2 , 3 , 5 , 8 , 29 , 31 – 35 , 39 – 41 , 43 ]. This involved collecting information regarding the number of doctoral and postdoctoral studentships (including those generated as a result of the research findings and those appointed to conduct the research), as well as the number of researchers and research-related staff involved in the research projects. In addition, authors advocated the collection of ‘leadership’ metrics, including the number of research projects managed and coordinated and the membership of boards and funding bodies, journal editorial boards, and advisory committees [ 29 ]. Additional metrics in this category included public recognition (number of fellowships and awards for significant research achievements), academic career advancement, and subsequent grants received. Lastly, the impact metric ‘research system management’ comprised the collection of information that can lead to preserving the health of the population, such as modifying research priorities, resource allocation strategies, and linking health research to other disciplines to maximise benefits [ 29 ].
1 . 4 . Academic collaborations , research networks , and data sharing . Lastly, 10 of the 24 frameworks advocated the collection of impact data regarding ‘academic collaborations (internal and external collaborations to complete a research project), research networks, and data sharing’ [ 2 , 3 , 5 , 7 , 29 , 34 , 37 , 39 , 41 , 43 ].
2. Influence on policy making.
Methodological frameworks addressing this major impact category focused on measurable improvements within a given knowledge base and on interactions between academics and policy makers, which may influence policy-making development and implementation. The returns generated in this impact category are generally considered as intermediate or midterm (1 to 3 years). These represent an important interim stage in the process towards the final expected impacts, such as quantifiable health improvements and economic benefits, without which policy change may not occur [ 30 , 62 ]. The following impact subgroups were identified within this category: ‘type and nature of policy impact’, ‘level of policy making’, and ‘policy networks’.
2 . 1 . Type and nature of policy impact . The most common impact subgroup, mentioned in 18 of the 24 frameworks, was ‘type and nature of policy impact’ [ 2 , 7 , 29 – 38 , 41 – 43 , 45 – 47 ]. Methodological frameworks addressing this subgroup stressed the importance of collecting information regarding the influence of research on policy (i.e., changes in practice or terminology). For instance, a project looking at trafficked adolescents and women (2003) influenced the WHO guidelines (2003) on ethics regarding this particular group [ 17 , 21 , 63 ].
2 . 2 . Level of policy impact . Thirteen of 24 frameworks addressed aspects surrounding the need to record the ‘level of policy impact’ (international, national, or local) and the organisations within a level that were influenced (local policy makers, clinical commissioning groups, and health and wellbeing trusts) [ 2 , 5 , 8 , 29 , 31 , 34 , 38 , 41 , 43 – 47 ]. Authors considered it important to measure the ‘level of policy impact’ to provide evidence of collaboration, coordination, and efficiency within health organisations and between researchers and health organisations [ 29 , 31 ].
2 . 3 . Policy networks . Five methodological frameworks highlighted the need to collect information regarding collaborative research with industry and staff movement between academia and industry [ 5 , 7 , 29 , 41 , 43 ]. A policy network emphasises the relationship between policy communities, researchers, and policy makers. This relationship can influence and lead to incremental changes in policy processes [ 62 ].
3. Health and health systems impact.
A number of methodological frameworks advocated the measurement of impacts on health and healthcare systems across the following impact subgroups: ‘quality of care and service delivering’, ‘evidence-based practice’, ‘improved information and health information management’, ‘cost containment and effectiveness’, ‘resource allocation’, and ‘health workforce’.
3 . 1 . Quality of care and service delivery . Twelve of the 24 frameworks highlighted the importance of evaluating ‘quality of care and service delivery’ [ 2 , 5 , 8 , 29 – 31 , 33 – 36 , 41 , 47 ]. There were a number of suggested metrics that could be potentially used for this purpose, including health outcomes such as quality-adjusted life years (QALYs), patient-reported outcome measures (PROMs), patient satisfaction and experience surveys, and qualitative data on waiting times and service accessibility.
3 . 2 . Evidence-based practice . ‘Evidence-based practice’, mentioned in 5 of the 24 frameworks, refers to making changes in clinical diagnosis, clinical practice, treatment decisions, or decision making based on research evidence [ 5 , 8 , 29 , 31 , 33 ]. The suggested metrics to demonstrate evidence-based practice were adoption of health technologies and research outcomes to improve the healthcare systems and inform policies and guidelines [ 29 ].
3 . 3 . Improved information and health information management . This impact subcategory, mentioned in 5 of the 24 frameworks, refers to the influence of research on the provision of health services and management of the health system to prevent additional costs [ 5 , 29 , 33 , 34 , 38 ]. Methodological frameworks advocated the collection of health system financial, nonfinancial (i.e., transport and sociopolitical implications), and insurance information in order to determine constraints within a health system.
3 . 4 . Cost containment and cost-effectiveness . Six of the 24 frameworks advocated the subcategory ‘cost containment and cost-effectiveness’ [ 2 , 5 , 8 , 17 , 33 , 36 ]. ‘Cost containment’ comprised the collection of information regarding how research has influenced the provision and management of health services and its implication in healthcare resource allocation and use [ 29 ]. ‘Cost-effectiveness’ refers to information concerning economic evaluations to assess improvements in effectiveness and health outcomes—for instance, the cost-effectiveness (cost and health outcome benefits) assessment of introducing a new health technology to replace an older one [ 29 , 31 , 64 ].
3 . 5 . Resource allocation . ‘Resource allocation’, mentioned in 6frameworks, can be measured through 2 impact metrics: new funding attributed to the intervention in question and equity while allocating resources, such as improved allocation of resources at an area level; better targeting, accessibility, and utilisation; and coverage of health services [ 2 , 5 , 29 , 31 , 45 , 47 ]. The allocation of resources and targeting can be measured through health services research reports, with the utilisation of health services measured by the probability of providing an intervention when needed, the probability of requiring it again in the future, and the probability of receiving an intervention based on previous experience [ 29 , 31 ].
3 . 6 . Health workforce . Lastly, ‘health workforce’, present in 3 methodological frameworks, refers to the reduction in the days of work lost because of a particular illness [ 2 , 5 , 31 ].
4. Health-related and societal impact.
Three subgroups were included in this category: ‘health literacy’; ‘health knowledge, attitudes, and behaviours’; and ‘improved social equity, inclusion, or cohesion’.
4 . 1 . Health knowledge , attitudes , and behaviours . Eight of the 24 frameworks suggested the assessment of ‘health knowledge, attitudes, behaviours, and outcomes’, which could be measured through the evaluation of levels of public engagement with science and research (e.g., National Health Service (NHS) Choices end-user visit rate) or by using focus groups to analyse changes in knowledge, attitudes, and behaviour among society [ 2 , 5 , 29 , 33 – 35 , 38 , 43 ].
4 . 2 . Improved equity , inclusion , or cohesion and human rights . Other methodological frameworks, 4 of the 24, suggested capturing improvements in equity, inclusion, or cohesion and human rights. Authors suggested these could be using a resource like the United Nations Millennium Development Goals (MDGs) (superseded by Sustainable Development Goals [SDGs] in 2015) and human rights [ 29 , 33 , 34 , 38 ]. For instance, a cluster-randomised controlled trial in Nepal, which had female participants, has demonstrated the reduction of neonatal mortality through the introduction of maternity health care, distribution of delivery kits, and home visits. This illustrates how research can target vulnerable and disadvantaged groups. Additionally, this research has been introduced by the World Health Organisation to achieve the MDG ‘improve maternal health’ [ 16 , 29 , 65 ].
4 . 3 . Health literacy . Some methodological frameworks, 3 of the 24, focused on tracking changes in the ability of patients to make informed healthcare decisions, reduce health risks, and improve quality of life, which were demonstrably linked to a particular programme of research [ 5 , 29 , 43 ]. For example, a systematic review showed that when HIV health literacy/knowledge is spread among people living with the condition, antiretroviral adherence and quality of life improve [ 66 ].
5. Broader economic impacts.
Some methodological frameworks, 9 of 24, included aspects related to the broader economic impacts of health research—for example, the economic benefits emerging from the commercialisation of research outputs [ 2 , 5 , 29 , 31 , 33 , 35 , 36 , 38 , 67 ]. Suggested metrics included the amount of funding for research and development (R&D) that was competitively awarded by the NHS, medical charities, and overseas companies. Additional metrics were income from intellectual property, spillover effects (any secondary benefit gained as a repercussion of investing directly in a primary activity, i.e., the social and economic returns of investing on R&D) [ 33 ], patents granted, licences awarded and brought to the market, the development and sales of spinout companies, research contracts, and income from industry.
The benefits contained within the categories ‘health and health systems impact’, ‘health-related and societal impact’, and ‘broader economic impacts’ are considered the expected and final returns of the resources allocated in healthcare research [ 30 , 62 ]. These benefits commonly arise in the long term, beyond 5 years according to some authors, but there was a recognition that this could differ depending on the project and its associated research area [ 4 ].
Data synthesis
Five major impact categories were identified across the 24 included methodological frameworks: (1) ‘primary research-related impact’, (2) ‘influence on policy making’, (3) ‘health and health systems impact’, (4) ‘health-related and societal impact’, and (5) ‘broader economic impact’. These major impact categories were further subdivided into 16 impact subgroups. The included publications proposed 80 different metrics to measure research impact. This impact typology synthesis is depicted in ‘the impact matrix’ ( Fig 2 and Fig 3 ).
CIHR, Canadian Institutes of Health Research; HTA, Health Technology Assessment; PHC RIS, Primary Health Care Research & Information Service; RAE, Research Assessment Exercise; RQF, Research Quality Framework.
https://doi.org/10.1371/journal.pmed.1002370.g002
AIHS, Alberta Innovates—Health Solutions; CAHS, Canadian Institutes of Health Research; IOM, Impact Oriented Monitoring; REF, Research Excellence Framework; SIAMPI, Social Impact Assessment Methods for research and funding instruments through the study of Productive Interactions between science and society.
https://doi.org/10.1371/journal.pmed.1002370.g003
Commonality and differences across frameworks
The ‘Research Impact Framework’ and the ‘Health Services Research Impact Framework’ were the models that encompassed the largest number of the metrics extracted. The most dominant methodological framework was the Payback Framework; 7 other methodological framework models used the Payback Framework as a starting point for development [ 8 , 29 , 31 – 35 ]. Additional methodological frameworks that were commonly incorporated into other tools included the CIHR framework, the CAHS model, the AIHS framework, and the Exchange model [ 8 , 33 , 34 , 44 ]. The capture of ‘research-related impact’ was the most widely advocated concept across methodological frameworks, illustrating the importance with which primary short-term impact outcomes were viewed by the included papers. Thus, measurement of impact via number of publications, citations, and peer-reviewed articles was the most common. ‘Influence on policy making’ was the predominant midterm impact category, specifically the subgroup ‘type and nature of policy impact’, in which frameworks advocated the measurement of (i) changes to legislation, regulations, and government policy; (ii) influence and involvement in decision-making processes; and (iii) changes to clinical or healthcare training, practice, or guidelines. Within more long-term impact measurement, the evaluations of changes in the ‘quality of care and service delivery’ were commonly advocated.
In light of the commonalities and differences among the methodological frameworks, the ‘pathways to research impact’ diagram ( Fig 4 ) was developed to provide researchers, funders, and policy makers a more comprehensive and exhaustive way to measure healthcare research impact. The diagram has the advantage of assorting all the impact metrics proposed by previous frameworks and grouping them into different impact subgroups and categories. Prospectively, this global picture will help researchers, funders, and policy makers plan strategies to achieve multiple pathways to impact before carrying the research out. The analysis of the data extraction and construction of the impact matrix led to the development of the ‘pathways to research impact’ diagram ( Fig 4 ). The diagram aims to provide an exhaustive and comprehensive way of tracing research impact by combining all the impact metrics presented by the different 24 frameworks, grouping those metrics into different impact subgroups, and grouping these into broader impact categories.
NHS, National Health Service; PROM, patient-reported outcome measure; QALY, quality-adjusted life year; R&D, research and development.
https://doi.org/10.1371/journal.pmed.1002370.g004
This review has summarised existing methodological impact frameworks together for the first time using systematic methods ( Fig 4 ). It allows researchers and funders to consider pathways to impact at the design stage of a study and to understand the elements and metrics that need to be considered to facilitate prospective assessment of impact. Users do not necessarily need to cover all the aspects of the methodological framework, as every research project can impact on different categories and subgroups. This review provides information that can assist researchers to better demonstrate impact, potentially increasing the likelihood of conducting impactful research and reducing research waste. Existing reviews have not presented a methodological framework that includes different pathways to impact, health impact categories, subgroups, and metrics in a single methodological framework.
Academic-orientated frameworks included in this review advocated the measurement of impact predominantly using so-called ‘quantitative’ metrics—for example, the number of peer-reviewed articles, journal impact factor, and citation rates. This may be because they are well-established measures, relatively easy to capture and objective, and are supported by research funding systems. However, these metrics primarily measure the dissemination of research finding rather than its impact [ 30 , 68 ]. Whilst it is true that wider dissemination, especially when delivered via world-leading international journals, may well lead eventually to changes in healthcare, this is by no means certain. For instance, case studies evaluated by Flinders University of Australia demonstrated that some research projects with non-peer-reviewed publications led to significant changes in health policy, whilst the studies with peer-reviewed publications did not result in any type of impact [ 68 ]. As a result, contemporary literature has tended to advocate the collection of information regarding a variety of different potential forms of impact alongside publication/citations metrics [ 2 , 3 , 5 , 7 , 8 , 29 – 47 ], as outlined in this review.
The 2014 REF exercise adjusted UK university research funding allocation based on evidence of the wider impact of research (through case narrative studies and quantitative metrics), rather than simply according to the quality of research [ 12 ]. The intention was to ensure funds were directed to high-quality research that could demonstrate actual realised benefit. The inclusion of a mixed-method approach to the measurement of impact in the REF (narrative and quantitative metrics) reflects a widespread belief—expressed by the majority of authors of the included methodological frameworks in the review—that individual quantitative impact metrics (e.g., number of citations and publications) do not necessary capture the complexity of the relationships involved in a research project and may exclude measurement of specific aspects of the research pathway [ 10 , 12 ].
Many of the frameworks included in this review advocated the collection of a range of academic, societal, economic, and cultural impact metrics; this is consistent with recent recommendations from the Stern review [ 10 ]. However, a number of these metrics encounter research ‘lag’: i.e., the time between the point at which the research is conducted and when the actual benefits arise [ 69 ]. For instance, some cardiovascular research has taken up to 25 years to generate impact [ 70 ]. Likewise, the impact may not arise exclusively from a single piece of research. Different processes (such as networking interactions and knowledge and research translation) and multiple individuals and organisations are often involved [ 4 , 71 ]. Therefore, attributing the contribution made by each of the different actors involved in the process can be a challenge [ 4 ]. An additional problem associated to attribution is the lack of evidence to link research and impact. The outcomes of research may emerge slowly and be absorbed gradually. Consequently, it is difficult to determine the influence of research in the development of a new policy, practice, or guidelines [ 4 , 23 ].
A further problem is that impact evaluation is conducted ‘ex post’, after the research has concluded. Collecting information retrospectively can be an issue, as the data required might not be available. ‘ex ante’ assessment is vital for funding allocation, as it is necessary to determine the potential forthcoming impact before research is carried out [ 69 ]. Additionally, ex ante evaluation of potential benefit can overcome the issues regarding identifying and capturing evidence, which can be used in the future [ 4 ]. In order to conduct ex ante evaluation of potential benefit, some authors suggest the early involvement of policy makers in a research project coupled with a well-designed strategy of dissemination [ 40 , 69 ].
Providing an alternate view, the authors of methodological frameworks such as the SIAMPI, Contribution Mapping, Research Contribution, and the Exchange model suggest that the problems of attribution are a consequence of assigning the impact of research to a particular impact metric [ 7 , 40 , 42 , 44 ]. To address these issues, these authors propose focusing on the contribution of research through assessing the processes and interactions between stakeholders and researchers, which arguably take into consideration all the processes and actors involved in a research project [ 7 , 40 , 42 , 43 ]. Additionally, contributions highlight the importance of the interactions between stakeholders and researchers from an early stage in the research process, leading to a successful ex ante and ex post evaluation by setting expected impacts and determining how the research outcomes have been utilised, respectively [ 7 , 40 , 42 , 43 ]. However, contribution metrics are generally harder to measure in comparison to academic-orientated indicators [ 72 ].
Currently, there is a debate surrounding the optimal methodological impact framework, and no tool has proven superior to another. The most appropriate methodological framework for a given study will likely depend on stakeholder needs, as each employs different methodologies to assess research impact [ 4 , 37 , 41 ]. This review allows researchers to select individual existing methodological framework components to create a bespoke tool with which to facilitate optimal study design and maximise the potential for impact depending on the characteristic of their study ( Fig 2 and Fig 3 ). For instance, if researchers are interested in assessing how influential their research is on policy making, perhaps considering a suite of the appropriate metrics drawn from multiple methodological frameworks may provide a more comprehensive method than adopting a single methodological framework. In addition, research teams may wish to use a multidimensional approach to methodological framework development, adopting existing narratives and quantitative metrics, as well as elements from contribution frameworks. This approach would arguably present a more comprehensive method of impact assessment; however, further research is warranted to determine its effectiveness [ 4 , 69 , 72 , 73 ].
Finally, it became clear during this review that the included methodological frameworks had been constructed using varied methodological processes. At present, there are no guidelines or consensus around the optimal pathway that should be followed to develop a robust methodological framework. The authors believe this is an area that should be addressed by the research community, to ensure future frameworks are developed using best-practice methodology.
For instance, the Payback Framework drew upon a literature review and was refined through a case study approach. Arguably, this approach could be considered inferior to other methods that involved extensive stakeholder involvement, such as the CIHR framework [ 8 ]. Nonetheless, 7 methodological frameworks were developed based upon the Payback Framework [ 8 , 29 , 31 – 35 ].
Limitations
The present review is the first to summarise systematically existing impact methodological frameworks and metrics. The main limitation is that 50% of the included publications were found through methods other than bibliographic databases searching, indicating poor indexing. Therefore, some relevant articles may not have been included in this review if they failed to indicate the inclusion of a methodological impact framework in their title/abstract. We did, however, make every effort to try to find these potentially hard-to-reach publications, e.g., through forwards/backwards citation searching, hand searching reference lists, and expert communication. Additionally, this review only extracted information regarding the methodology followed to develop each framework from the main publication source or framework webpage. Therefore, further evaluations may not have been included, as they are beyond the scope of the current paper. A further limitation was that although our search strategy did not include language restrictions, we did not specifically search non-English language databases. Thus, we may have failed to identify potentially relevant methodological frameworks that were developed in a non-English language setting.
In conclusion, the measurement of research impact is an essential exercise to help direct the allocation of limited research resources, to maximise benefit, and to help minimise research waste. This review provides a collective summary of existing methodological impact frameworks and metrics, which funders may use to inform the measurement of research impact and researchers may use to inform study design decisions aimed at maximising the short-, medium-, and long-term impact of their research.
Supporting information
S1 appendix. search strategy..
https://doi.org/10.1371/journal.pmed.1002370.s001
S1 PRISMA Checklist. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist.
https://doi.org/10.1371/journal.pmed.1002370.s002
Acknowledgments
We would also like to thank Mrs Susan Bayliss, Information Specialist, University of Birmingham, and Mrs Karen Biddle, Research Secretary, University of Birmingham.
- View Article
- PubMed/NCBI
- Google Scholar
- 3. HEFCE. REF 2014: Assessment framework and guidance on submissions 2011 [cited 2016 15 Feb]. Available from: http://www.ref.ac.uk/media/ref/content/pub/assessmentframeworkandguidanceonsubmissions/GOS%20including%20addendum.pdf .
- 8. Canadian Institutes of Health Research. Developing a CIHR framework to measure the impact of health research 2005 [cited 2016 26 Feb]. Available from: http://publications.gc.ca/collections/Collection/MR21-65-2005E.pdf .
- 9. HEFCE. HEFCE allocates £3.97 billion to universities and colleges in England for 2015–1 2015. Available from: http://www.hefce.ac.uk/news/newsarchive/2015/Name,103785,en.html .
- 10. Stern N. Building on Success and Learning from Experience—An Independent Review of the Research Excellence Framework 2016 [cited 2016 05 Aug]. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/541338/ind-16-9-ref-stern-review.pdf .
- 11. Matthews D. REF sceptic to lead review into research assessment: Times Higher Education; 2015 [cited 2016 21 Apr]. Available from: https://www.timeshighereducation.com/news/ref-sceptic-lead-review-research-assessment .
- 12. HEFCE. The Metric Tide—Report of the Independent Review of the Role of Metrics in Research Assessment and Management 2015 [cited 2016 11 Aug]. Available from: http://www.hefce.ac.uk/media/HEFCE,2014/Content/Pubs/Independentresearch/2015/The,Metric,Tide/2015_metric_tide.pdf .
- 14. LSE Public Policy Group. Maximizing the impacts of your research: A handbook for social scientists. http://www.lse.ac.uk/government/research/resgroups/LSEPublicPolicy/Docs/LSE_Impact_Handbook_April_2011.pdf . London: LSE; 2011.
- 15. HEFCE. Consultation on the second Research Excellence Framework. 2016.
- 18. Merriam-Webster Dictionary 2017. Available from: https://www.merriam-webster.com/dictionary/methodology .
- 19. Oxford Dictionaries—pathway 2016 [cited 2016 19 June]. Available from: http://www.oxforddictionaries.com/definition/english/pathway .
- 20. Oxford Dictionaries—metric 2016 [cited 2016 15 Sep]. Available from: https://en.oxforddictionaries.com/definition/metric .
- 21. WHO. WHO Ethical and Safety Guidelines for Interviewing Trafficked Women 2003 [cited 2016 29 July]. Available from: http://www.who.int/mip/2003/other_documents/en/Ethical_Safety-GWH.pdf .
- 31. Kalucy L, et al. Primary Health Care Research Impact Project: Final Report Stage 1 Adelaide: Primary Health Care Research & Information Service; 2007 [cited 2016 26 Feb]. Available from: http://www.phcris.org.au/phplib/filedownload.php?file=/elib/lib/downloaded_files/publications/pdfs/phcris_pub_3338.pdf .
- 33. Canadian Academy of Health Sciences. Making an impact—A preferred framework and indicators to measure returns on investment in health research 2009 [cited 2016 26 Feb]. Available from: http://www.cahs-acss.ca/wp-content/uploads/2011/09/ROI_FullReport.pdf .
- 39. HEFCE. RAE 2008—Guidance in submissions 2005 [cited 2016 15 Feb]. Available from: http://www.rae.ac.uk/pubs/2005/03/rae0305.pdf .
- 41. Royal Netherlands Academy of Arts and Sciences. The societal impact of applied health research—Towards a quality assessment system 2002 [cited 2016 29 Feb]. Available from: https://www.knaw.nl/en/news/publications/the-societal-impact-of-applied-health-research/@@download/pdf_file/20021098.pdf .
- 48. Weiss CH. Using social research in public policy making: Lexington Books; 1977.
- 50. Kogan M, Henkel M. Government and research: the Rothschild experiment in a government department: Heinemann Educational Books; 1983.
- 51. Thomas P. The Aims and Outcomes of Social Policy Research. Croom Helm; 1985.
- 52. Bulmer M. Social Science Research and Government: Comparative Essays on Britain and the United States: Cambridge University Press; 2010.
- 53. Booth T. Developing Policy Research. Aldershot, Gower1988.
- 55. Kalucy L, et al Exploring the impact of primary health care research Stage 2 Primary Health Care Research Impact Project Adelaide: Primary Health Care Research & Information Service (PHCRIS); 2009 [cited 2016 26 Feb]. Available from: http://www.phcris.org.au/phplib/filedownload.php?file=/elib/lib/downloaded_files/publications/pdfs/phcris_pub_8108.pdf .
- 56. CHSRF. Canadian Health Services Research Foundation 2000. Health Services Research and Evidence-based Decision Making [cited 2016 February]. Available from: http://www.cfhi-fcass.ca/migrated/pdf/mythbusters/EBDM_e.pdf .
- 58. W.K. Kellogg Foundation. Logic Model Development Guide 2004 [cited 2016 19 July]. Available from: http://www.smartgivers.org/uploads/logicmodelguidepdf.pdf .
- 59. United Way of America. Measuring Program Outcomes: A Practical Approach 1996 [cited 2016 19 July]. Available from: https://www.bttop.org/sites/default/files/public/W.K.%20Kellogg%20LogicModel.pdf .
- 60. Nutley S, Percy-Smith J and Solesbury W. Models of research impact: a cross sector review of literature and practice. London: Learning and Skills Research Centre 2003.
- 61. Spaapen J, van Drooge L. SIAMPI final report [cited 2017 Jan]. Available from: http://www.siampi.eu/Content/SIAMPI_Final%20report.pdf .
- 63. LSHTM. The Health Risks and Consequences of Trafficking in Women and Adolescents—Findings from a European Study 2003 [cited 2016 29 July]. Available from: http://www.oas.org/atip/global%20reports/zimmerman%20tip%20health.pdf .
- 70. Russell G. Response to second HEFCE consultation on the Research Excellence Framework 2009 [cited 2016 04 Apr]. Available from: http://russellgroup.ac.uk/media/5262/ref-consultation-response-final-dec09.pdf .
- Program Finder
- Admissions Services
- Course Directory
- Academic Calendar
- Hybrid Campus
- Lecture Series
- Convocation
- Strategy and Development
- Implementation and Impact
- Integrity and Oversight
- In the School
- In the Field
- In Baltimore
- Resources for Practitioners
- Articles & News Releases
- In The News
- Statements & Announcements
- At a Glance
- Student Life
- Strategic Priorities
- Inclusion, Diversity, Anti-Racism, and Equity (IDARE)
- What is Public Health?
research@BSPH
The School’s research endeavors aim to improve the public’s health in the U.S. and throughout the world.
- Funding Opportunities and Support
- Faculty Innovation Award Winners
Conducting Research That Addresses Public Health Issues Worldwide
Systematic and rigorous inquiry allows us to discover the fundamental mechanisms and causes of disease and disparities. At our Office of Research ( research@BSPH), we translate that knowledge to develop, evaluate, and disseminate treatment and prevention strategies and inform public health practice. Research along this entire spectrum represents a fundamental mission of the Johns Hopkins Bloomberg School of Public Health.
From laboratories at Baltimore’s Wolfe Street building, to Bangladesh maternity wards in densely packed neighborhoods, to field studies in rural Botswana, Bloomberg School faculty lead research that directly addresses the most critical public health issues worldwide. Research spans from molecules to societies and relies on methodologies as diverse as bench science and epidemiology. That research is translated into impact, from discovering ways to eliminate malaria, increase healthy behavior, reduce the toll of chronic disease, improve the health of mothers and infants, or change the biology of aging.
120+ countries
engaged in research activity by BSPH faculty and teams.
of all federal grants and contracts awarded to schools of public health are awarded to BSPH.
citations on publications where BSPH was listed in the authors' affiliation in 2019-2023.
publications where BSPH was listed in the authors' affiliation in 2019-2023.
Departments
Our 10 departments offer faculty and students the flexibility to focus on a variety of public health disciplines
Centers and Institutes Directory
Our 80+ Centers and Institutes provide a unique combination of breadth and depth, and rich opportunities for collaboration
Institutional Review Board (IRB)
The Institutional Review Board (IRB) oversees two IRBs registered with the U.S. Office of Human Research Protections, IRB X and IRB FC, which meet weekly to review human subjects research applications for Bloomberg School faculty and students
Generosity helps our community think outside the traditional boundaries of public health, working across disciplines and industries, to translate research into innovative health interventions and practices
Introducing the research@BSPH Ecosystem
The research@BSPH ecosystem aims to foster an interdependent sense of community among faculty researchers, their research teams, administration, and staff that leverages knowledge and develops shared responses to challenges. The ultimate goal is to work collectively to reduce administrative and bureaucratic barriers related to conducting experiments, recruiting participants, analyzing data, hiring staff, and more, so that faculty can focus on their core academic pursuits.
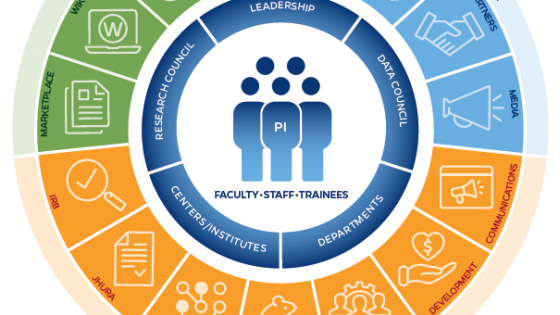
Research at the Bloomberg School is a team sport.
In order to provide extensive guidance, infrastructure, and support in pursuit of its research mission, research@BSPH employs three core areas: strategy and development, implementation and impact, and integrity and oversight. Our exceptional research teams comprised of faculty, postdoctoral fellows, students, and committed staff are united in our collaborative, collegial, and entrepreneurial approach to problem solving. T he Bloomberg School ensures that our research is accomplished according to the highest ethical standards and complies with all regulatory requirements. In addition to our institutional review board (IRB) which provides oversight for human subjects research, basic science studies employee techniques to ensure the reproducibility of research.
Research@BSPH in the News
Four bloomberg school faculty elected to national academy of medicine.
Considered one of the highest honors in the fields of health and medicine, NAM membership recognizes outstanding professional achievements and commitment to service.
The Maryland Maternal Health Innovation Program Grant Renewed with Johns Hopkins
Lerner center for public health advocacy announces inaugural sommer klag advocacy impact award winners.
Bloomberg School faculty Nadia Akseer and Cass Crifasi selected winners at Advocacy Impact Awards Pitch Competition
Stop COVID Cohort: An Observational Study of 3480 Patients Admitted to the Sechenov University Hospital Network in Moscow City for Suspected Coronavirus Disease 2019 (COVID-19) Infection
Collaborators.
- Sechenov StopCOVID Research Team : Anna Berbenyuk , Polina Bobkova , Semyon Bordyugov , Aleksandra Borisenko , Ekaterina Bugaiskaya , Olesya Druzhkova , Dmitry Eliseev , Yasmin El-Taravi , Natalia Gorbova , Elizaveta Gribaleva , Rina Grigoryan , Shabnam Ibragimova , Khadizhat Kabieva , Alena Khrapkova , Natalia Kogut , Karina Kovygina , Margaret Kvaratskheliya , Maria Lobova , Anna Lunicheva , Anastasia Maystrenko , Daria Nikolaeva , Anna Pavlenko , Olga Perekosova , Olga Romanova , Olga Sokova , Veronika Solovieva , Olga Spasskaya , Ekaterina Spiridonova , Olga Sukhodolskaya , Shakir Suleimanov , Nailya Urmantaeva , Olga Usalka , Margarita Zaikina , Anastasia Zorina , Nadezhda Khitrina
Affiliations
- 1 Department of Pediatrics and Pediatric Infectious Diseases, Institute of Child's Health, Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia.
- 2 Inflammation, Repair, and Development Section, National Heart and Lung Institute, Faculty of Medicine, Imperial College London, London, United Kingdom.
- 3 Soloviev Research and Clinical Center for Neuropsychiatry, Moscow, Russia.
- 4 School of Physics, Astronomy, and Mathematics, University of Hertfordshire, Hatfield, United Kingdom.
- 5 Biobank, Institute for Regenerative Medicine, Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia.
- 6 Institute for Regenerative Medicine, Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia.
- 7 Chemistry Department, Lomonosov Moscow State University, Moscow, Russia.
- 8 Department of Polymers and Composites, N. N. Semenov Institute of Chemical Physics, Moscow, Russia.
- 9 Department of Clinical and Experimental Medicine, Section of Pediatrics, University of Pisa, Pisa, Italy.
- 10 Institute of Social Medicine and Health Systems Research, Faculty of Medicine, Otto von Guericke University Magdeburg, Magdeburg, Germany.
- 11 Institute for Urology and Reproductive Health, Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia.
- 12 Department of Intensive Care, Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia.
- 13 Clinic of Pulmonology, Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia.
- 14 Department of Internal Medicine No. 1, Institute of Clinical Medicine, Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia.
- 15 Department of Forensic Medicine, Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia.
- 16 Department of Statistics, University of Oxford, Oxford, United Kingdom.
- 17 Medical Research Council Population Health Research Unit, Nuffield Department of Population Health, University of Oxford, Oxford, United Kingdom.
- 18 Centre for Tropical Medicine and Global Health, Nuffield Department of Medicine, University of Oxford, Oxford, United Kingdom.
- 19 Oxford University Hospitals NHS Foundation Trust, John Radcliffe Hospital, Oxford, United Kingdom.
- 20 Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia.
- PMID: 33035307
- PMCID: PMC7665333
- DOI: 10.1093/cid/ciaa1535
Background: The epidemiology, clinical course, and outcomes of patients with coronavirus disease 2019 (COVID-19) in the Russian population are unknown. Information on the differences between laboratory-confirmed and clinically diagnosed COVID-19 in real-life settings is lacking.
Methods: We extracted data from the medical records of adult patients who were consecutively admitted for suspected COVID-19 infection in Moscow between 8 April and 28 May 2020.
Results: Of the 4261 patients hospitalized for suspected COVID-19, outcomes were available for 3480 patients (median age, 56 years; interquartile range, 45-66). The most common comorbidities were hypertension, obesity, chronic cardiovascular disease, and diabetes. Half of the patients (n = 1728) had a positive reverse transcriptase-polymerase chain reaction (RT-PCR), while 1748 had a negative RT-PCR but had clinical symptoms and characteristic computed tomography signs suggestive of COVID-19. No significant differences in frequency of symptoms, laboratory test results, and risk factors for in-hospital mortality were found between those exclusively clinically diagnosed or with positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RT-PCR. In a multivariable logistic regression model the following were associated with in-hospital mortality: older age (per 1-year increase; odds ratio, 1.05; 95% confidence interval, 1.03-1.06), male sex (1.71; 1.24-2.37), chronic kidney disease (2.99; 1.89-4.64), diabetes (2.1; 1.46-2.99), chronic cardiovascular disease (1.78; 1.24-2.57), and dementia (2.73; 1.34-5.47).
Conclusions: Age, male sex, and chronic comorbidities were risk factors for in-hospital mortality. The combination of clinical features was sufficient to diagnose COVID-19 infection, indicating that laboratory testing is not critical in real-life clinical practice.
Keywords: COVID-19; Russia; SARS-CoV-2; cohort; mortality risk factors.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: [email protected].
Publication types
- Observational Study
- Research Support, Non-U.S. Gov't
- Hospitalization
- Middle Aged
Grants and funding
- 20-04-60063/Russian Foundation for Basic Research
- Reference Manager
- Simple TEXT file
People also looked at
Original research article, implementation of the electronic health record in the german healthcare system: an assessment of the current status and future development perspectives considering the potentials of health data utilisation by representatives of different stakeholder groups.

- Faculty of Health and Healthcare Sciences, University of Applied Sciences Zwickau, Zwickau, Saxony, Germany
Introduction: The digitalisation of the German healthcare system enables a wide range of opportunities to utilize healthcare data. The implementation of the EHR in January 2021 was a significant step, but compared to other European countries, the implementation of the EHR in the German healthcare system is still at an early stage. The aim of this paper is to characterise the structural factors relating to the adoption of the EHR in more detail from the perspective of representatives of stakeholders working in the German healthcare system and to identify existing barriers to implementation and the need for change.
Methods: Qualitative expert interviews were conducted with one representative from each of the stakeholder groups health insurance, pharmacies, healthcare research, EHR development and panel doctors.
Results: The interviews with the various stakeholders revealed that the implementation process of the EHR is being delayed by a lack of a viable basis for decision-making, existing conflicts of interest and insufficient consideration of the needs of patients and service providers, among other things.
Discussion: The current status of EHR implementation is due to deficiency in legal regulations as well as structural problems and the timing of the introduction. For instance, the access rights of various stakeholders to the EHR data and the procedure in the event of a technical failure of the telematics infrastructure are remain unclear. In addition, insufficient information and communication measures have not led to the desired acceptance of EHR use among patients and service providers.
Introduction
In order to achieve comprehensive digitalisation of the German healthcare system, all stakeholders must agree to implement this change together. In addition to hospitals, doctors' surgeries and health insurance companies, patients and politicians are also involved. This change includes the creation of digital infrastructures on the one hand and the digitalisation of procedures and processes in healthcare on the other ( 1 ). The digitalisation of the healthcare system offers a multitude of opportunities. In the German healthcare system alone, there is an economic potential of 42 billion euros per year ( 2 ). Other potentials include opportunities to develop new diagnostic and treatment options, improve communication between individual players in the healthcare system and between patients and service providers. The quality of care can also become better ( 3 ). One basis for realising the aforementioned potential is the use of healthcare data generated in everyday care. To effectively use this data, a platform is required where it can be digitally collated and then made accessible to selected groups of people. In this context, the electronic health record is a central and promising application. The contents of the record include data on diagnostics and medical treatment in the doctor's surgery and in hospitals, as well as data on the supply of medicines or from providers of remedies and aids.
With regard to the current status of digitalisation in various healthcare systems, it is clear that Germany still much ground to cover compared to other countries. This is reflected, among other things, in the fact that the implementation of electronic health records in everyday care in the German healthcare system has not yet been realised to the desired extent. In Europe, Estonia and Denmark in particular have comprehensively digitalised their healthcare systems ( 4 ). In Denmark, for example, the EHR was introduced as early as 2012 and in Estonia as early as 2008. In addition, almost the entire population in both countries currently has an EHR ( 3 ). They use the digital data of the healthcare system and provide patients and authorised service providers with additional information from the consolidation and analysis of a wide variety of data, in particular through the use of data from electronic health records.
In order to promote the further development of the German healthcare system with regard to electronic health records, it is necessary to identify the causal factors that have caused the delay this process thus far. Consequently, these factors should be analysed in a way that allows adjustment of these factors with the aim of achieving the objectives.
Definition of an electronic health record
The health record is a document to be kept by the service providers involved in the treatment of a patient. All essential medical data that arises during treatment must be documented in the record. This includes information from the medical history, the measures applied and their results, as well as diagnoses made by the service provider during the patient's treatment. The patient's explanations and consent are also recorded. A patient's entire medical history thus becomes part of the record ( 5 ).
In the context of this work, the term electronic health record refers to the integrative electronic health record for an electronic documentation system that can be viewed and edited by doctors and other service providers involved in the treatment across disciplines, institutions and sectors [( 3 ), p. 70].
In the EHR consent procedure, a basic distinction is made between the opt-out and opt-in procedure. While in the opt-out procedure, an EHR is set up for each patient or insured person as long as they do not expressly object, in an opt-in procedure, patients or insured persons must actively contact a corresponding authority in order to open a file.
Development and current status of implementation in the German healthcare system
The German healthcare system can be divided into three levels: the legal framework, self-administration and the individual players. The legal framework becomes the responsibility of the federal, state and local governments. The Federal Ministry of Health is in charge of health policy within the federal government. However, the organisation of healthcare is carried out by the self-administration. This means that the individual institutions organise themselves independently and thus guarantee the provision of medical care [( 6 ), p. 18]. The individual stakeholders who are ultimately directly involved in patient care include the medical profession and various healthcare professionals, hospitals and pharmacies. To enable them to represent their interests at the health policy level, the stakeholders are organised in professional organisations, as well as professional and business associations ( 6 ). Around 84.3 million citizens need to be cared for within the German healthcare system ( 7 ). The healthcare system is primarily financed by statutory and private health insurance. These in turn are financed by the contributions of their members.
gematik is one of the central institutions involved in the digitalisation of the healthcare system. The Federal Ministry of Health holds the largest share in gematik with 51%. gematik's tasks primarily include the introduction, operation and further development of the telematics infrastructure, the electronic health card and associated specialised applications, as well as the creation of an interoperability directory. It also assumes responsibilities in the area of data security ( 8 ). With regard to health data protection, the Bundesbeauftragter für den Datenschutz und die Informationsfreiheit [Federal Commissioner for Data Protection and Information Security] (BfDI) is a key authority. This is the public supervisory authority for all public bodies of the federal government as well as for certain social security organisations. Issues relating to data protection and data security must be coordinated with the BfDI ( 9 ). Each of the 16 federal states in Germany also has its own state data protection officer and corresponding supervisory authorities.
Several laws form the legal framework for the development and design of digitalisation in the German healthcare system, particulary for the implementation of the EHR and use of healthcare data. In addition, the requirements of the Datenschutz-Grundverordnung [General Data Protection Regulation] (GDPR) and the Bundesdatenschutzgesetz [Federal Data Protection Act] (BDSG) apply in Germany. The basis for digitalisation in the German healthcare system became law with the Act for Secure Digital Communication and Applications in Healthcare (E-Health Act), which came into force on 29 December 2015.
The EHR was introduced as a patient-moderated record in the German healthcare system in January 2021. From this point onwards, anyone with statutory health insurance can obtain an EHR on request from their health insurance provider. Private health insurers can offer an EHR on a voluntary basis; therefore, there is no legal obligation. The term patient-moderated means that the patient alone decides whether and to what extent they use the record and to whom they make which data available. Data that can be entered into the EHRs includes care and service data. Patients can also enter their own health data, such as that collected by wearable devices, into the record ( 10 – 12 ). Access becomes possible via an app provided by the respective health insurance company using a smartphone, tablet or computer. The prerequisite for using the EHR is prior registration with the respective health insurance company. EHR registration currently takes place either via the new electronic health card with NFC interface and a PIN applied for the card or alternatively via two-factor authentication. As of April 2023, around 667,449 people with statutory health insurance, which corresponds to around 1% of people with statutory health insurance, have an EHR ( 13 ). In addition, the records that currently exist are hardly filled with data. Due to the low number of existing records, utilisation by service providers is also at a very low level. Finally, data is exchanged between service providers via the telematics infrastructure. Access to the telematics infrastructure is via a connector ( 14 ). The use of EHR data, for example, for research projects, should be possible from 2023 for a selected group of authorised applicants. In this context, patients should be given the opportunity to voluntarily make their data available for research projects as part of a data donation through an opt-in procedure. Until now, using the data contained in EHRs for research purposes or merging data collected at different locations in the healthcare system has not been possible.
Most recently, in March 2023, the Federal Ministry of Health presented its digitalisation strategy for the healthcare and nursing sectors up to 2030. In this context, gematik is to become a digital health agency and will be tasked with defining comprehensive binding requirements for interoperability. The further development of the lead data protection supervisory authority is also planned. The aim is to become a standardised data protection supervisory practice in the health and care sector. One of the objectives of the strategy is to facilitate access to pseudonymised health data for researchers. The decisive interface in this context is the research data centre, through which the data is to be made available after an application has been submitted. In addition, around 80% of people with statutory health insurance should have an EHR by 2025. These goals are to be achieved, among other things, with the legislative proposals presented in this context for a Health Data Utilisation Act and a Digital Act. As part of a digital law, the consent procedure for the EHR is to become an opt-out procedure ( 15 ).
The aim of this study is to scrutinise the structural factors in the context of EHR implementation from the perspective of stakeholders working in the German healthcare system and, based on this, to identify existing barriers to implementation and the need for change. The aim is to answer the question of how relevant actors in the German healthcare system assess the current structural conditions regarding the implementation of her. Additionally, the study aims to explore existing implementation hurdles or areas that require change related to this topic and examine the causes contributing to the current status of EHR implementation, particularly regarding the potential use of health data in the German healthcare system. The focus here will be on the current legal regulations and the framework conditions of the healthcare system.
Methodological approach
The methodological approach was based on a qualitative research design. In view of the defined objectives of the work, the investigation using a qualitative method is suitable, as it offers the possibility of describing an object of investigation in detail and developing hypotheses and theories. The approach taken in the study is exploratory in character and is intended to provide initial insights into the research topic ( 16 ). In order to ensure the quality of this scientific work, the catalogue of criteria from Lincoln et al. ( 17 ) was used. Firstly, it is necessary to define individual specific subject areas in relation to the general survey topic. The specific interview topics were selected on the basis of expert opinions on the one hand and a selective literature review on the other. In addition to the access authorisations of various stakeholders to the data contained in the EHR, the topics also include the existence or expansion of corresponding infrastructures and technical requirements, data protection and information security as well as the needs of patients with regard to the design of the EHR. The development of the EHR since its introduction and the resulting potential are also discussed. With regard to the field of investigation, the decision was made to take a multi-perspective view of the various representatives of stakeholder groups in the German healthcare system ( 16 ). With regard to the existing structures in the German healthcare system, the central stakeholder groups can be identified as service providers, patients, health insurers, academic and other public research, developers and manufacturers as well as healthcare policy and authorities [( 18 ), p. 30]. However, the analysis should be limited exclusively to the perspectives of healthcare providers, patients, health insurers, developers and researchers. The views of health policy makers and authorities will deliberately not be included in the study, as both actors have a controlling or monitoring function regarding the implementation of the EHR and the use of health data. Consequently, they are not directly involved in the utilisation. However, the focus should be on these actors who are directly involved in the utilisation. The selection of these stakeholders can be justified by the fact that they formulate stakeholder-specific requirements and needs for the use of the EHR in everyday care and work as well as for the design of the record itself. These requirements and needs play a crucial role in the design of the legal framework and the EHR itself, so that it can be integrated seamlessly into care provision. The sample was then compiled from the previously defined field of investigation. This should be as heterogeneous a group of people as possible, with maximum contrast in the relevant characteristics and therefore informative for the study ( 19 ). With regard to representative of the health insurance stakeholder group, the analysis should focus on the level of statutory health insurance. Statutory health insurers cover around 90% of the German population ( 20 ). In addition, they have become legally obliged to provide an EHR for their policyholders with corresponding requirements. Private health insurers, on the other hand, cover a comparatively smaller proportion of the population. In addition, there are no precisely defined requirements for the provision of EHRs. In order to comprehensively reflect the perspective of the statutory health insurance funds, it is necessary for them to be represented by a high-ranking representative from the management level of a nationally active statutory health insurance fund. In addition, the representative must have extensive professional experience and appropriate specialist knowledge in the context of the EHR and its development. The service provider perspective is to be illuminated by doctors on the one hand and pharmacies on the other. The selection of pharmacies is justified by the fact that they have the role of supplying medicines within the healthcare system. By utilising the health data contained in the EHR, they would become empowered to actively improve the quality of the supply of medicines to patients. Both the representative of the medical profession and the representative of the pharmacy profession must be a person who acts within the framework of one of the corresponding nationwide interest groups. Only on this basis can the results be generalised beyond the specific case. In addition, it is necessary for the physician and pharmacy representative to have extensive professional experience and expertise in the context of the EHR and its development. For the area of research, the analysis should be carried out from the perspective of health services research, as EHR data in particular forms a central basis for their activities ( 11 ). The developer and manufacturer perspective is to be presented by a member of an EHR development team from the statutory health insurance funds and the patient perspective by a patient representative from a nationwide patient organisation. These representatives of the relevant stakeholder group must also hold a high-ranking position in their professional activities, have extensive professional experience and appropriate knowledge of the EHR and its development. Recruitment was carried out through the relevant contact persons of a nationwide association of pharmacies, patients and statutory health insurance physicians. The contact details of two other people were obtained from the contact database available as part of the work. The identified potential interviewees were then contacted by email and the subject of the research and the preliminary interview guidelines were briefly described. Finally, the interviewees were selected on the basis of predefined criteria such as length of professional experience, existing knowledge of the EHR and its development, and professional position. The result was a sample of five male interviewees ( Table 1 ). This is therefore a convenience sampling [( 16 ), p. 306]. The interviewees were selected for the study because they were easy to reach and met the predefined inclusion criteria. In accordance with the aforementioned requirements for the participants, this is not a random sample.
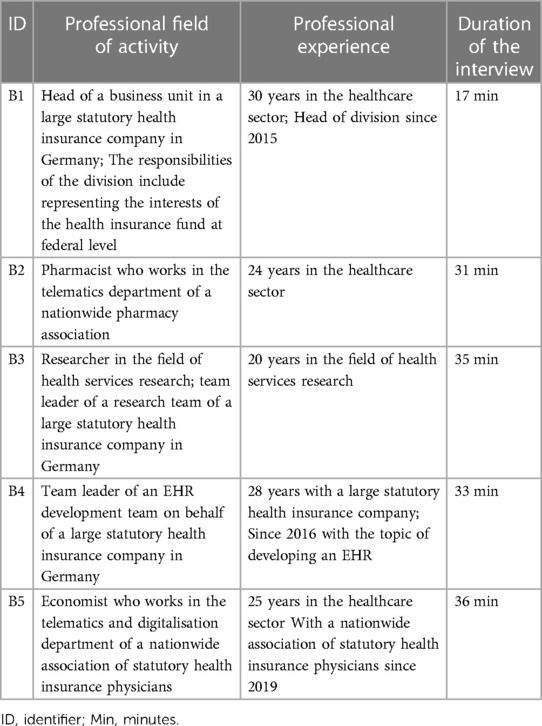
Table 1 . Composition of the sample.
The semi-structured interview method in the form of guided interviews was chosen to illustrate the structural conditions of EHR implementation from the perspective of the actors working in the German healthcare system. The reason for choosing this method is that the interviewees, who represent one of the previously defined actors, have a certain proximity to both the topic and the corresponding actor due to the nature of their work. They can describe their view of the situation subjectively. This variant of the guided interview is also referred to as an expert interview, as the interviewees are technical experts on a specific topic whose structural expertise is to be developed with the help of the interview [( 16 ), p. 375]. The semi-structured interview is based on an interview guideline, which roughly specifies and structures the questions to be answered by the interviewee. The guideline itself is considered flexible. Accordingly, additional or in-depth questions that arise during the interview can be asked [( 16 ), p. 358]. The data collection took place in the period from 1 March 2023 to 20 March 2023. The appointments were arranged in advance by email or in person. While one interview took place face-to-face, the other interviews were conducted as hybrid interviews. Before the interviews were conducted, all interviewees were informed about the use of the data and a verbal declaration of consent was obtained. In addition, all interviews were recorded. All interviews were based on the same interview guide.
The material obtained from the guided interviews was initially available in the form of audio recordings. These were then transcribed into a document with the help of AI-supported transcription software. Then the documents were then compared with the audio recordings and any content that was not correctly transferred by the software was corrected with the help of the audio recordings. The transcription became complete and was carried out without adapting the wording. Only dialect was not taken into account in this context and was translated into standardised German. Punctuation marks were used when revising the transcripts according to sound and not grammatical correctness. In addition, personal and company-related data became unrecognisable by replacing them with synonyms. Finally, the transcripts in Word documents were imported into the computer-aided analysis programme MAXQDA (version 2020). The data collected in the context of the interviews, which were subsequently transcribed, were available in qualitative form. This paper therefore uses the method of qualitative content analysis with the help of the computer-assisted analysis programme MAXQDA (Version 2020) [( 16 ), p. 602]. With regard to the content analysis process model, the material was first analysed comprehensively based on the research question and text passages that deemed relevant were then marked. This was followed by the development of thematic main categories, which should roughly structure the material. These became deductively derived from the research question and the thematic complexes of the individual questions of the interview guide. Finally, the entire material became coded with the help of the main categories. In the next step, the text passages that were coded with the same main category were compiled. Subcategories were then inductively derived from the material within the individual main categories and the entire material was coded using the differentiated category system [( 16 ), p. 557]. The final evaluation was carried out along the main categories. Only those categories that were classified as relevant to answering the research question were analysed. In addition, it was analysed whether there were correlations within a main category between the subcategories and between the main thematic categories. In addition, the statements of the interviewees were compared as part of the multi-perspective analysis.
In the following, the results of the evaluation of the qualitative interviews are presented along the main categories.
Development of the EHR
The development of the EHR became predominantly negative for all respondents. In this context, four interviewees refer to the fact that only a very small number of people with statutory health insurance have an EHR. The representative of the statutory health insurance physicians cites the fact that it has never been defined what is meant by a patient-moderated record and how the use of the EHR is intended for service providers and in which architectures.
However, the EHR developer states that he considers the development of the EHR to be positive in terms of the functionality of the record.
Access authorisations
The representative of the SHI-accredited physicians stated that a basis for the discussion on access authorisation must be created at the outset. This means that it must be determined for which group of people which information should be made accessible and for what reason.
In the context of access authorisations, three interviewees refer to the role of the record owners. The care researcher criticises the fact that the will of the record owners regarding the use of the data has not been sufficiently taken into account in the discussions on access authorisations to date. The representative of the SHI-accredited physicians emphasises the need to provide comprehensive information to record users on how the system works. Building on this basis, the record owners can ultimately become empowered to make informed decisions regarding access authorisations. The representative of the pharmacies is in favour of an opt-out procedure in order to give people who do not trust anonymisation the freedom to make their own decisions.
The EHR developer is in favour of access by all service providers involved in the treatment and justifies this with the fact that medicine depends on a holistic view of the state of health.
The representative of the health insurance company is in favour of health insurance companies having access and emphasises that they would thus become able to use the EHR data to support the insured person in their care. The representative of the pharmacies, on the other hand, emphasises that, in his view, the statutory health insurance funds should not be given access to the EHR data. The EHR developer states that if the health insurance funds are to be granted access, this must be regulated via an opt-in procedure, as in this case it is a matter of private interests. The EHR developer states that the release of data for research purposes should also be regulated via an opt-out objection procedure, as the data is used to further develop and improve medical care for the entire German population.
In this context, the representative of the health insurance company emphasised that research must be able to access the overall data of the healthcare system in anonymised form.
Infrastructure and technical requirements
With regard to infrastructures and technical requirements, various problems were mentioned by the interviewees. The representative of the SHI-accredited physicians criticised the fact that the current discussion is mainly about fictitious usage scenarios. The processes and their process steps and how the relevant players are involved in these are not currently defined in relation to the infrastructures. However, these would become the basis for formulating requirements for corresponding architectures. The representative of the health insurance company points out that the different structures of the data from the outpatient and inpatient areas are seen as problematic from the health insurance company's perspective. This means that the data from the outpatient sector is transmitted quarterly and is therefore available to the health insurance funds with a considerable delay, which makes it difficult to react promptly to acute events. In this context, the pharmacy representative cites time pressure when introducing new applications as a problem. In the past, this mistake had already been made with e-prescriptions and applications had been introduced that were not yet fully developed for use in practice . The EHR developer emphasises that there would be an implementation problem in Germany. In his view, the providers of practice management systems or hospital information systems must become active and implement the specifications published by gematik in 2019. A functioning system would already exist and therefore no new concepts would be necessary.
All interviewees emphasised the necessity for technical requirements and infrastructures to ensure that data is input into the EHR in a structured manner. The representative of the SHI-accredited physicians emphasised that the various EHR usage scenarios must be defined at the outset. In addition, priorities must be set as to which clinical pictures and scenarios are currently most important in order to plan the further procedure for expanding the corresponding infrastructures on this basis. The representative of the health insurance company states that the system needs to be converted to the use of cloud applications. The representative of the pharmacies also stated that appropriate lead times must be guaranteed regarding to the technical requirements. In addition, future users would have to be trained in advance.
Data protection and information security
In terms of data protection and information security, the EHR developer and the representative of the health insurance company consider the current design of the registration process for insured persons to be problematic. This is very complex, and it becomes an access barrier for users.
Two interviewees named specific requirements that they place on EHR use related to data protection and information security.
The representative of the statutory health insurance physicians points out that data protection and information security are a fundamental prerequisite for entry and should not be seen as a hurdle. The representative of the pharmacies highlights a further requirement that the possibility for patients to exercise their right to self-determination must be retained in the context of EHR use. In addition, the establishment of such a system should become possible in agreement with the BfDI.
Patient requirements for the EHR
The representative of the pharmacies emphasises that the EHR must become simple and user-friendly. There must also be options for using a proxy, for example for people who do not have their own end device or those who cannot use the functions of the record themselves due to other restrictions.
The representatives of statutory health insurance physicians and pharmacies emphasise the need for comprehensive information and communication measures aimed at patients in order to create a basic understanding among them of what the aim of the record is and the aim of its use. All stakeholders involved in the record as well as the Federal Ministry of Health must become involved in information and communication.
The supply researcher stated that a basis of trust must be created among users. They must be able to trust that it is a functioning and secure application in terms of data protection, information security and user-orientation.
Needs from the service provider perspective
The representative of the pharmacies addresses the needs from the perspective of the service providers and emphasises that additional costs incurred by the service providers through the use of the EHR should be remunerated. In this context, it must also be clarified which group of people checks that the records are up to date. In addition, the issue of liability for service providers must be clarified in advance. In particular, it must be clarified whether service providers are obliged to read all the data contained in the EHR. In addition, a regulation must be found on how to deal with technical problems or failures in day-to-day care.
The challenges
Two interviewees commented on the challenges that they believe the German healthcare system will have to overcome in the context of the introduction. The representative of the health insurance company and the healthcare researcher point out that it will be challenging to bring together the various data sets and formats of health data to subsequently use them in the direct context of healthcare or to further develop healthcare from a scientific perspective. The healthcare researcher emphasises that a regulation must be found on how to deal with data that can be set by the patient themselves, such as vital signs data collected by smart watches. The health insurance company representative also sees a challenge in the mandatory filling of EHRs by service providers. With the introduction of a writing obligation, it would also be necessary to discuss corresponding sanctions for service providers in the event of non-compliance with the requirements
The health insurance company representative sees a further challenge in dealing with the current structure of data protection regulations. He emphasises that an assessment must be made of how important data protection is to the system and its stakeholders compared to the benefits of health data. This requires committees to carry out this assessment.
The healthcare researcher, the health insurance company and pharmacy representatives emphasise the resulting benefits from an economic perspective for the entire German healthcare system. Due to the data situation, an optimisation of patient flows could be achieved. This would give practitioners the opportunity to deal with patients who actually fall within their area of specialisation. The pharmacy and health insurance company representatives emphasise that financial resources could be saved by avoiding incorrect treatment and duplicate examinations.
Respondents also mentioned improvements in the area of research and care. The EHR developer emphasises that improvements for research would be achieved through the availability of mass data on the health status of the entire German population. The German healthcare system would ultimately have to rely less on data from other countries. In this context, the representative of the statutory health insurance physicians criticised the fact that the focus is currently more on improving research opportunities. However, such improvements would become of little use to those who are currently ill. In his view, care must first become better and then improvements in the area of research can be considered. From the healthcare researcher's point of view, tangible added value would be available to patients if they were able to find their optimal level of care promptly and consequently receive targeted treatment in a timely manner. The representative of the health insurance company and the healthcare researcher highlight the aspect that patients can receive more quality-oriented support and guidance within the healthcare system, thanks to the improved possibilities of cross-sector treatment. Moreover, doctors would be able to incorporate the expertise of another doctor into their own decisions. In the context of follow-up treatment, health data would also be directly available and promptly accessible to doctors in private practice. The pharmacy representative emphasised that pharmacies could improve medication management using the available data.
The healthcare researcher states that, considering the enhancement in care, there would likely be an increase in satisfaction among service providers and patients with the German healthcare system.
Discussion of results
All respondents rated the development of the EHR as predominantly negative since its introduction in January 2021. One of the reasons for this is that only a very small number of people with statutory health insurance have an EHR and existing records are hardly ever used by them. In turn, the low use of the records also shows that patients do not recognise any added value in this functionality. On the one hand, the cause of this can be the fact that patients were not sufficiently involved in the type and scope of the information measures and the design of the EHR. On the other hand, the cause could also be on the patient side, them being closed to digital innovations or their insufficient individual resources for developing digital skills to cope with this change.
From the perspective of the service provider, the pharmacy representative points out that the EHR is currently not being used in pharmacies. There is also a conceptual problem with the introduction of new applications such as the e-prescription or the EHR. As an example of this, he brings up the fact that in the past, various applications were introduced at the same time. Both service providers and patients did not receive sufficient support and training. The EHR developer assesses the record itself as a functional product, which was simply unable to achieve the desired effect in the area with the objectives formulated for the record. According to this, the current status of target achievement is not because of the EHR itself, but because of a lack of communicative and informative measures. As a result, the experience gained so far and the causes identified as to why the objectives formulated for the record could not be achieved should be taken into account and be included in the future design of the further implementation procedure.
The low user numbers for the EHR in the German healthcare system and the poorly rated development of the EHR against this backdrop are due, among other things, to the choice of consent procedure. In comparison, Estonia and Denmark have at least relied on an opt-out procedure when implementing the EHR, which means that almost the entire population is provided with an EHR ( 3 , 4 ). In the German healthcare system, on the other hand, an opt-in procedure was used. The patient must therefore make a conscious decision in favour of an EHR and apply for it independently. The choice of consent procedure for the EHR is therefore a relevant factor in terms of successful implementation. The low availability of EHRs and their low utilisation in the German healthcare system can therefore be explained, among other things, by the choice of consent procedure. In order to achieve the widespread availability of EHRs in the German healthcare system, it is therefore necessary to rely on an opt-out procedure.
Furthermore, with regard to the process of implementing the EHR in Denmark and Estonia, it can be seen that both countries are pursuing a stand-alone digital health strategy ( 4 , 21 ). This forms the framework of a structured, holistic concept according to which the EHR was implemented. There was no digitalisation strategy for the German healthcare system until March 2023. Although the various laws enacted contained requirements and objectives for the introduction of an EHR and were intended to promote the use of the health data it contains, there was no holistic framework in the form of a strategy. The pursuit of a digitalisation strategy with its individual goals and measures serves as orientation for all representatives of stakeholders involved in a complex and lengthy process. For the implementation of an EHR in the German healthcare system, it is therefore crucial in future to be guided by the strategy defined by the Federal Ministry of Health ( 15 ). The strategy's goals and measures must be actively and gradually implemented. However, it is necessary to regularly evaluate the current status of target achievement during the course of the process. If, for example, it turns out that individual goals cannot be achieved by the specified date, they must therefore also be updated and adjusted as part of the digitalisation strategy.
The interviewees express various opinions regarding the access authorisations of different stakeholders. In particular, the interests behind the required access play a role here. Accordingly, it is decisive whether there are economic interests or interests in the public. According to the representative of the SHI-accredited physicians, the fundamental question that must be clarified is which interests exist for each actor regarding access to EHR data and what justifies the type and scope of access. Only with this understanding can the discussion about corresponding access options be conducted.
Both the representatives of statutory health insurance physicians and pharmacies and the healthcare researchers emphasise that the role of record owners has not yet been sufficiently taken into account in the discussions on access authorisations thus far. In this context, comprehensive communication and information measures towards patients would have to be implemented at the outset. As part of these measures, it should be demonstrated in a simple and understandable way how this system works and where the potential of file utilisation lies in terms of improving medical care. The aim should be to create a basis of trust and enable patients to make informed decisions based on the information provided. The representative of pharmacies is also in favour of the planned opt-out procedure, as this will allow patients who do not trust the system or do not wish to use it to retain their freedom of choice. The latter aspect in particular plays a decisive role in creating a basis of trust.
Access options for all service providers involved in the treatment are a crucial prerequisite, as only on this basis can be possible the simplified interdisciplinary and cross-sectoral exchange of data be made possible.
The opinions of the interviewees differed when it came to health insurance companies' access authorisations to EHR data. While the health insurance company representative is in favour of access options and argues that the health insurance companies can better support the insured persons in their care based on the data, the pharmacy representative rejects access options for statutory health insurance companies. From the EHR developer's point of view, the access options for health insurance companies should be regulated via an opt-in procedure, as there are no public interests involved here. As already explained, this also raises the question of the interests of the respective actor behind the corresponding demand. On the one hand, the statutory health insurance funds are pursuing the goal of improving the provision of customised care services. On the other hand, however, they also have economic interests, as the services provided can in turn be billed. The choice of an opt-in procedure for the access options of health insurance funds would therefore be a conceivable solution that would preserve the freedom of choice of the insured person. With the implementation of the EHR, its use became mandatory for all healthcare institutions in Estonia and Denmark. A debate on access authorisations comparable to that in the German healthcare system has therefore not been held in these countries. In Estonia, for example, patients are allowed to decide for themselves which service providers are authorised to view their data. The reason for the extensive discussion of access authorisations in the German healthcare system is partly due to the number of structures and players involved. Compared to Estonia and Denmark, the German healthcare system has many structures and actors that need to be taken into account when making political decisions. Bertram et al. ( 22 ) describes this aspect as an inhibiting factor with regard to the digitalisation of the healthcare system. The large number of actors in self-government significantly slows down the decision-making process in connection with existing conflicts of interest.
With regard to the release of data for research purposes, it is crucial that research institutes can access the overall data of the healthcare system in anonymised form to serve as a foundation for advancing healthcare from a scientific perspective. In this context, the EHR developer also proposes the release of data via an opt-out procedure. This regulation is one approach to generating the largest possible amount of data. However, it is questionable to what extent such regulations will be approved by patients. In both Estonia and Denmark, the release of data from the EHR for research purposes is permitted ( 4 ). However, the laws applicable in the respective countries specify that the data may only be made available for research purposes in anonymised form.
From the point of view of the representative of the SHI-accredited physicians, a corresponding basis for decision-making, which provides for the definition of usage scenarios for the EHR, would be missing—similar to the sub-item access authorisations. Here too, the political authorities had not fulfilled their tasks in a timely manner and to a sufficient extent. As a result, the various utilisation scenarios must first be defined. This includes the individual processes and process steps as well as the involvement of the various stakeholders. Based on this, there are requirements for the further development of the infrastructure and the associated technical requirements. In addition, all interviewees see a need to define specifications for the structure of the data. In particular, the aim is to ensure that relevant data can be retrieved by various service providers in a targeted manner and processed by the systems in a partially automated manner.
From a health insurance perspective, it is crucial that data from the outpatient sector is available promptly. In fact, this aspect is also essential for the exchange of data between the outpatient and inpatient sectors. As long as the data is not available on the same day, it becomes more difficult to react quickly to acute events. The gradual approach to the ideal of real-time availability of health and care data is one of the goals of the digitalisation strategy [( 15 ), p. 27]. The health insurance company representative suggests switching to cloud-based systems for this purpose. However, this requires an adjustment to the regulatory framework, as the use of such systems is currently only possible to a very limited extent in the German healthcare system. This changeover would facilitate the exchange of data across disciplines and sectors and would significantly increase the speed of the system. Cloud-based systems are already in use in Denmark and Estonia. In Denmark, for example, data from the individual databases of hospitals and general practitioners is transmitted to the national health portal sundhed.dk and can then be accessed by the various service providers and patients ( 3 ). Estonia also has a national health information exchange network called the Estonian National Health Information Service. Similar to Denmark, data is passed on to the health information portal by the service providers ( 4 ). The use of these systems is associated with a number of advantages. Among other things, they offer a high degree of flexibility and nationwide access options, as the data can be accessed by authorised persons at any time and from any location as long as there is an internet connection ( 3 ). The use of cloud-based systems in the German healthcare system could improve the timely availability of health data in the future.
From a service provider perspective, appropriate lead times and training for future users must be guaranteed when introducing new applications. This is a task for the health policy authorities and, according to the pharmacy representative, has not been sufficiently taken into account in the past. The latter point also highlights the conceptual problems in the German healthcare system when introducing new applications. From the healthcare researcher's point of view, it is crucial, especially when it comes to infrastructures, to involve the user stakeholders and to organise the further development in such a way that they experience added value as a result.
The EHR developer and the representative of the health insurance fund emphasise that the current design of the registration process would represent a barrier to access for insured persons and that a more user-friendly solution must be found here. In contrast, the representative of the statutory health insurance physicians emphasised that data protection requirements should not be put on the back burner. Compliance with data protection requirements is a prerequisite for handling of health data. Similar to the issue of access authorisations, a conflict of interest between various stakeholders can be identified here. The design of the registration process is particularly crucial for the statutory health insurance funds, as this has a significant influence on the number of users of the corresponding EHR checkout app. For the care itself, the design of the registration process plays a less decisive role, as the EPR, if the patient does not object as part of an opt-out procedure, is in the system as a pure service provider file and can be used by the service providers in the context of treatment. For the representative of the statutory health insurance fund, the focus is on increasing the number of users of the health insurance app on the part of the insured. In contrast, the representative of the statutory health insurance physicians prioritises the protection of doctor-patient confidentiality.
The pharmacies' representative mentiones the requirement that patients must be able to exercise their right to self-determination when using the EHR. According to the BfDI, the current design of the EHR for access management violates the GDPR, especially for people who do not have their own device or do not want to use one. These people would become restricted in their patient sovereignty and would not be able to exercise their rights to self-determination ( 23 ). It is important to establish regulations for this group of people that enable the uncomplicated use of a representative. In addition, from the perspective of pharmacists and statutory health insurance physicians, data protection and information security must become a matter of agreement with the BfDI. As announced when the Federal Ministry of Health published its digitalisation strategy, the BfDI's right of co-determination is to be changed from agreement to consultation.
With regard to data protection and information security, the requirements of the GDPR apply in Germany, Estonia and Denmark. However, it is up to each individual country to organise the corresponding measures to ensure that the requirements of the GDPR are met. With regard to the number of existing laws and the requirements formulated therein, the German healthcare system has a comparatively strict framework in terms of data protection and information security requirements. In addition, the requirements and interests of various stakeholders must be taken into account in Germany with regard to this topic. According to former Federal Data Protection Commissioner Peter Schaar, this federal system would secure nationwide healthcare provision, but at the same time make it more difficult to implement nationwide digital information structures ( 24 ). By comparison, in Estonia and Denmark, only individual institutions are entrusted with ensuring data protection requirements are met ( 25 , 26 ). This makes the decision-making process much simpler. At the same time, compliance with the requirements of the GDPR ensures that the handling of health data becomes more secure.
The healthcare researcher and the representatives of statutory health insurance physicians and pharmacies name specific requirements for the EHR that must be met from the patient's perspective. As already mentioned in the access authorisations sub-item, comprehensive information and communication measures for patients and the creation of a basis of trust are emphasised once again. Verifiable value propositions should form the basis of a foundation of trust and corresponding information and communication measures. Formulating these is a task for the political authorities. The EHR must also become user-friendly. Particularly, it should be determined how the patient side defines the criterion of user-friendliness. In this context, it must be taken into account that Germany is strongly affected by the effects of demographic change and that the population therefore has an increasing proportion of older people ( 27 ). The consequences of demographic change must be taken into account in corresponding communication and information measures by integrating the channels that older people prefer to use. As already shown in the subsection on data protection and information security, people become excluded from EHR use due to restrictions of any kind. This aspect must be viewed critically from an ethical perspective. In order to make the EHR user-friendly, the different needs of patients must be recorded and then implemented in the design of the EHR. This could be done as part of further research, as the needs of the different user groups are complex and also depend on the age group. In addition, a test phase in which a heterogeneous group of people goes through the registration process up to the actual use of the various EHR applications would be recommended.
Only the representative of pharmacies commented on needs that exist from the perspective of service providers. Here, he mentions, among other things, the remuneration of additional time expenditure incurred by service providers through the use of the EHR. This measure could increase the acceptance of service providers to use the EHR, as it would provide financial compensation for the increased workload. In addition, the issue of liability must become regulated before the introduction to create a secure basis for action and information for the service provider. The use of e-prescriptions and electronic certificates of incapacity for work in day-to-day care has recently led to an increase in technical faults in connection with the telematics infrastructure ( 28 ). As the use of digital applications is to be further expanded in the future, it is imperative to find a regulation for dealing with technical problems or failures. The representative of the statutory health insurance physicians does not comment on needs that exist from the point of view of service providers. This could be due to the fact that, unlike the representative of the pharmacies, he did not work in healthcare himself.
The healthcare researcher and the representative of the health insurance company see a challenge in bringing together the various data sets and formats so that they can be analysed together. A legal basis must first be created for this, as already explained in advance. Currently, data collected at different points in the healthcare system may neither be merged nor analysed together. The basis for this is expected to become part of the announced Health Data Utilisation Act.
In addition, a regulation must be found on how to deal with such data that was not collected in the context of medical treatment and can, for example, be set by the patient themselves. The problem here is that no clear statements can be made about how valid this data is. One possible approach would be to include data collected on a patient's exercise behaviour using appropriate wearables in the treatment of diabetes or obesity. However, this data should only be used to supplement the data collected by doctors.
The representative of the health insurance company identifies another challenge in the need to discuss corresponding sanctions alongside the introduction of a reporting obligation for service providers. The concern is that this might reduce the acceptance of using the EHR among service providers. Additionally, it entails increased bureaucratic effort, and relevant authorities must take responsibility for checking compliance with the obligations outlined in this context.
The care researcher, the representatives of health insurance company and the pharmacies emphasise the economic benefits that arise in relation to the successful implementation of the EHR. These include, in particular, cost and time savings resulting from the avoidance of incorrect treatment and duplicate examinations. These freed-up financial and time resources can ultimately be used for more effective and targeted patient care. In addition, the avoidance of incorrect treatment and duplicate examinations is also in the interests of patients.
Improvements in the areas of care and research are also mentioned. In this context, however, the representative of the SHI-accredited physicians points out that the priority should initially be on improving care. This includes ways to improve interdisciplinary and cross-sector treatment, as the data is available more quickly. During the COVID-19 pandemic, it became particularly clear how the rapid and structured exchange of health data can contribute to the early detection and thus containment of infections and improve care ( 29 ). In addition, patients can be guided through the healthcare system in a more targeted manner in order to find the optimal level of care for them in a timely manner.
At the research level, improvements can be achieved by making a very large amount of data on the health status of the German population accessible as soon as the corresponding access rights for research projects have been clarified. The findings gained here can then in turn be used to improve care. This process is also referred to by the German Advisory Council on Health and Care as a learning healthcare system ( 3 ). This opens up possibilities for developing new forms of care. In light of the expected improvements, satisfaction among service providers and patients can be expected to increase.
Discussion of methods and limitations
As part of the discussion of methods, we would first like to emphasise that the results, particularly due to the small sample size, are to be understood as a first step towards a deeper understanding of the introduction of the EHR and cannot cover the topic in its entirety.
A qualitative study design was consciously chosen for this study, as it allows for an in-depth exploration of the different perspectives and experiences of the actors involved in the EHR implementation process. Given the complexity of the topic and the need to develop a comprehensive understanding of the challenges and opportunities associated with the implementation of the EHR, a qualitative design provided the scope for a multi-perspective investigation. Building on the more exploratory findings of this thesis, a political science model that provides an appropriate framework in terms of decision-making, conflicts of interest and lobbying influence could now be applied to gain further insights in this area. The outcomes of our paper can provide a starting point for this. To ensure the quality of this work, the study became subject to the quality criteria proposed by Lincoln et al. ( 17 ). Trustworthiness was ensured by collecting data comprehensively and over a longer period of approximately two months in the field of investigation. The quality criterion of transferability was achieved through the dense description of the people and contextual conditions studied. In this context, personal data and information on professional development in the German healthcare system became part of the interviews. According to Lincoln et al. ( 17 ), the quality criteria of reliability and confirmability should be ensured with the help of a research audit. In the context of this work, a reviewer was used as an alternative. During data evaluation using qualitative content analysis, the data was coded in several passes and the finalised category system was discussed with the reviewer in order to avoid distortion of the results by the researcher's feelings, values, interests and motives.
As the selected sample is a convenience sample and therefore not a random sample, the sample is not fully representative of the population under investigation ( 16 ). Distortions in the study results could also be due to the composition of the sample, as three of the interviewees come from the working environment of a large statutory health insurance fund in Germany. In addition, one interviewee, who was to be interviewed as a representative of the patient side, cancelled at short notice. Therefore the patient perspective can not be analysed in this study. With regard to the survey instrument used, it must be mentioned that the guidelines contain some very large and complex subject areas. When analysing the data, it must be taken into account that the respondents' answers are subjective and depend on their professional activity and thus their proximity to one of the previously defined actors. The strengths of qualitative content analysis lie in the systematic and rule-based approach, which ensures a high level of transparency in the research process ( 16 ).
The current status of EHR implementation and the low utilisation of health data in the German healthcare system in this context can be attributed to a lack of legal regulations, structural problems and the timing of implementation. In order to catch up, it is essential to implement the measures and objectives set out in the digitalisation strategy promptly and with the involvement of all stakeholders. With regard to the implementation process, Germany can orientate itself on Estonia and Denmark, for example, as the EHR is implemented nationwide there and is used extensively within the healthcare systems.
With regard to the political authorities in the German healthcare system, they did not fulfil their tasks to a sufficient extent, as the basis for decision-making was lacking and the set time frame was not adhered to.
In the context of implementation, the focus must be on ensuring that the added value of the application becomes tangible for all those involved. Only with this foundation can acceptance of the introduction of new digital applications be increased. Attention should be paid to a user-friendly design for both patients and all other stakeholders.
From the perspective of the German healthcare sector, there are various changes and challenges that need to be addressed as part of the implementation process. With regard to the service provider perspective, for example, appropriate lead times should be guaranteed and liability issues clarified in advance. When it comes to the issue of access authorisations for various stakeholders to EHR data, there is still no viable basis for decision-making. The decision-making process becomes significantly more difficult in this context, as the needs of the various stakeholders must be taken into account and the interests behind the request for data access differ greatly. In order to improve the exchange of data across disciplines and sectors, a solution is needed to ensure that data becomes available in near real time. How the use of data for research purposes will develop against the background of a successful implementation depends largely on the design of the legal framework and consequently the choice of the consent procedure for data release as well as the will of the patients. The findings obtained in this thesis can be used as a basis for further investigations in order to take a more differentiated look at the individual problems that have been identified.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the West Saxon University of Applied Sciences Zwickau Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants in accordance with the national legislation and the institutional requirements.
Author contributions
ER: Writing – original draft, Conceptualization, Formal Analysis, Writing – review & editing. TT: Writing – original draft, Project administration, Visualization, Writing – review & editing. BM: Conceptualization, Methodology, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BDSG, Bundesdatenschutzgesetz [Federal Data Protection Act]; BfDI, Bundesbeauftragter für den Datenschutz und die Informationsfreiheit [Federal Commissioner for Data Protection and Information Security]; e-prescription, electronic prescription; EHR, electronic health record, GDPR, Datenschutz-Grundverordnung [General Data Protection Regulation].
1. Wissenschaftsrat. Digitalisierung und Datennutzung für Gesundheitsforschung und Versorgung—Positionen und Empfehlungen; Köln Digitisation and Data Use for Health Research and Care—Positions and Recommendations . Cologne: Wissenschaftsrat (2022). doi: 10.57674/bxkz-8407
Google Scholar
2. Biesdorf S, Niedermann F, Sickmüller K, Tuot K. Studie zu Digitalisierung—Digitalisierung im Gesundheitswesen: die 42-Milliarden- Euro-Chance für Deutschland. Study on digitalisation—digitalisation in healthcare: the 42 billion euro opportunity for Germany. Gesundheitsökonomie Qualitätsmanagement . (2022) 27(04):171–2. doi: 10.1055/a-1871-4664
Crossref Full Text | Google Scholar
3. Sachverständigenrat zur Begutachtung der Entwicklung im Gesundheitswesen (SVR-Gesundheit), Gerlach F, Greiner W, Jochimsen B, von Kalle C, Meyer G, et al. Digitalisierung für Gesundheit Ziele und Rahmenbedingungen eines dynamisch lernenden Gesundheitssystems Gutachten 2021 [Digitalisation for Health Objectives and Framework Conditions of a Dynamically Learning Healthcare System Expert Report 2021]. (2021). Available online at: https://www.svr-gesundheit.de/fileadmin/Gutachten/Gutachten_2021/SVR_Gutachten_2021.pdf (retrieved June 1, 2023).
4. Thiel R, Deimel L, Schmidtmann D, Piesche K, Hüsing T, Rennoch J, et al. #SmartHealthSystems. International Comparison of Digital Strategies . GüTersloh: Bertelsmann Stiftung (2018). Available online at: https://www.bertelsmann-stiftung.de/de/publikationen/publikation/did/smarthealthsystems
5. Patientenakte [Patient file]. gesund.bund.de (undated). Available online at: https://gesund.bund.de/patientenakte (retrieved April 4, 2023).
6. Bundesministerium für Gesundheit [Federal Ministry of Health Germany]. Das deutsche Gesundheitssystem—Leistungsstark. Sicher. Bewährt [The German healthcare system—Efficient. Safe. Proven]. (2., aktualisierte Auflage). Bundesministerium für Gesundheit Referat L 8—Öffentlichkeitsarbeit, Publications. (2022). Available online at: https://www.bundesgesundheitsministerium.de/fileadmin/user_upload/Das-deutsche-Gesundheitssystem_bf.pdf (retrieved May 15, 2023).
7. Statistisches Bundesamt. Bevölkerungsstand: Amtliche Einwohnerzahl Deutschlands [Population Status: Official Population of Germany]. (2023). Available online at: https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Bevoelkerung/Bevoelkerungsstand/_inhalt.html# (retrieved 3 June 2023).
8. gematik GmbH. Gesetzliche Grundlagen der gematik [Legal basis of gematik]. gematik. Available online at: https://www.gematik.de/ueber-uns/gesetzlichegrundlagen (retrieved March 5, 2023).
9. Der BfDI—Aufgaben und Befugnisse des BfDI [The BfDI—Tasks and Powers of the BfDI]. BfDI. (undated). Available online at: https://www.bfdi.bund.de/DE/DerBfDI/Inhalte/DerBfDI/AufgabenBFDI.html (retrieved on March 3, 2023).
10. Bundesministerium für Gesundheit [Federal Ministry of Health Germany]. Arzt, Krankenhaus, Apotheke—Wer macht was im deutschen Gesundheitswesen? Doctor, Hospital, Pharmacy—Who Does What in the German Healthcare System? Bonn: Bundesministerium für Gesundheit (undated). Available online at: https://www.bundesgesundheitsministerium.de/themen/internationale-gesundheitspolitik/migration-und-integration/fluechtlinge-und-gesundheit/online-ratgeber-fuer-asylsuchende/wer-macht-was.html (retrieved June 4, 2023).
11. Bundesministerium für Gesundheit [Federal Ministry of Health Germany]. Das E-Health-Gesetz The E-Health Act . Bonn: Bundesministerium für Gesundheit. (undated). Available online at: https://www.bundesgesundheitsministerium.de/service/begriffe-von-a-z/e/e-health-gesetz.html (retrieved April 3, 2023).
12. Bundesministerium für Gesundheit [Federal Ministry of Health Germany]. Elektronische Patientenakte. Electronic Health Record . Bonn: Bundesministerium für Gesundheit. (undated). Available online at: https://www.bundesgesundheitsministerium.de/elektronische-patientenakte.html (retrieved February 3, 2023).
13. gematik GmbH. Struktur der gematik Legal Basis of Gematik . Berlin: Gematik (undated). Available online at: https://www.gematik.de/ueber-uns/struktur (retrieved on March 5, 2023).
14. Kassenärztliche Bundesvereinigung (KBV). Telematikinfrastruktur: Notwendige technische Komponenten für Anschluss und Anwendungen. Telematics Infrastructure: Necessary Technical Components for Connection and Applications . Berlin: Kassenärztliche Bundesvereinigung (2022). Available online at: https://www.kbv.de/html/30722.php (retrieved June 1 2023).
15. Bundesministerium für Gesundheit Abteilung 5—Digitalisierung und Innovation [Federal Ministry of Health Department 5—Digitalisation and Innovation]. Digitalisierungsstrategie für das Gesundheitswesen und die Pflege [Digitisation strategy for healthcare and nursing]. Bundesministerium für Gesundheit. (2023). Available online at: https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/3_Downloads/D/Digitalisierungsstrategie/BMG_Broschuere_Digitalisierungsstrategie_bf.pdf (retrieved May 19, 2023).
16. Döring N, Bortz J, Pöschl S, Werner CS, Schermelleh-Engel K, Gerhard C, et al. Forschungsmethoden und Evaluation in den Sozial- und Humanwissenschaften (Springer-Lehrbuch) (5. Aufl.). Research Methods and Evaluation in the Social and Human Sciences (Springer Textbook) (5th Ed.) . Ilmenau: Springer (2015). ISBN: 978-3-642-41089-5.
17. Lincoln YS, Guba EG. Naturalistic Inquiry . Newbury Park: SAGE (1985).
18. Expertenkommission Forschung und Innovation (EFI), Bratan T, Schneider D, Helen N, Pullmann L, Friedewald M, et al. E-Health in Deutschland Entwicklungsperspektiven und internationaler Vergleich . E-Health in Germany Development Perspectives and International Comparison . Berlin: Expertenkommission Forschung und Innovation (2022). Available online at: https://www.e-fi.de/fileadmin/Assets/Studien/2022/StuDIS_12_2022.pdf (retrieved May 26, 2023).
19. Petrucci, Marco/Wortz, Marcus. Sampling und Stichprobe. QUASUS. Qualitatives Methodenportal zur Qualitativen Sozial-, Unterrichts- und Schulforschung. (2007). Available online at: https://www.ph-freiburg.de/quasus/was-muss-ich-wissen/daten-auswaehlen/sampling-und-stichprobe.html (retrieved May 20, 2023).
20. GKV-Spitzenverband. Die gesetzlichen Krankenkassen [The statutory health insurance funds]. GKV-Spitzenverband. (2023). Available online at: https://www.gkv-spitzenverband.de/krankenversicherung/kv_grundprinzipien/alle_gesetzlichen_krankenkassen/alle_gesetzlichen_krankenkassen.jsp#:~:text=Die%20gesetzlichen%20Krankenkassen-,Die%20gesetzlichen%20Krankenkassen,rund%2090%20Prozent%20der%20Bev%C3%B6lkerung (retrieved April 6, 2023).
21. Sundhedsdatastyrelsen. Core Tasks—Sundhedsdatastyrelsen. (undated). Available online at: https://sundhedsdatastyrelsen.dk/da/english/about/core_tasks (retrieved May 20, 2023).
22. Bertram N, Püschner F, Gonçalves A, Binder S, Amelung V. Einführung einer elektronischen Patientenakte in Deutschland vor dem Hintergrund der internationalen Erfahrungen . Introduction of an Electronic Health Record in Germany Against the Background of International Experience . Berlin: Klauber (2019).
23. BfDI. Die elektronische Patientenakte (ePA) . The Electronic Health Record (EHR) . Bonn: BfDI (undated). Available online at: https://www.bfdi.bund.de/DE/Buerger/Inhalte/GesundheitSoziales/eHealth/elektronischePatientenakte.html (retrieved on May 6, 2023).
24. Schaar P. Diagnose Digital-Disaster: Ist das Gesundheitswesen noch zu retten? Digital Disaster Diagnosis: Can the Healthcare System Still Be Saved? Stuttgart: Hirzel S. Verlag (2023).
25. Sundhedsdatastyrelsen. Digital health strategy. (undated). Available online at: https://sundhedsdatastyrelsen.dk/da/english/digital_health_solutions/digital_health_strategy (retrieved Mai 20, 2023).
26. TEHIK. About TEHIK. (undated). Available online at: https://www.tehik.ee/en/about (retrieved May 20, 2023).
27. Demografischer Wandel [Demographic change]. Statistisches Bundesamt. (undated). Available online at: https://www.destatis.de/DE/Themen/Querschnitt/Demografischer-Wandel/_inhalt.html;jsessionid=4AD17CFFFE956AF4F28E241E88F14901.internet8732#sprg239000 (retrieved May 6, 2023).
28. Ärzteblatt, D. Ä. G. R. D. Telematikinfrastruktur: Ärzte bemängeln technische Probleme bei eAU und E-Rezept. Deutsches Ärzteblatt. (2022). Available online at: https://www.aerzteblatt.de/archiv/225091/Telematikinfrastruktur-Aerzte-bemaengeln-technische-Probleme-bei-eAU-und-E-Rezept (retrieved May 25, 2023).
29. Sachverständigenrat zur Begutachtung der Entwicklung im Gesundheitswesen, Gerlach (SVR-Gesundheit), Greiner W, Jochimsen B, von Kalle C, Meyer G, Schreyögg J, et al. Resilienz im Gesundheitswesen: Wege zur Bewältigung künftiger Krisen Gutachten 2023 [Resilience in the healthcare sector: Ways to cope with future crises Expert opinion 2023]. (2023). Available online at: https://www.svr-gesundheit.de/fileadmin/Gutachten/Gutachten_2023/Gesamtgutachten_2023_barrierefrei.pdf (retrieved March 20, 2023).
Keywords: digital health, electronic health record (EHR), personal health records, health data use, digitalisation
Citation: Rau E, Tischendorf T and Mitzscherlich B (2024) Implementation of the electronic health record in the German healthcare system: an assessment of the current status and future development perspectives considering the potentials of health data utilisation by representatives of different stakeholder groups. Front. Health Serv. 4:1370759. doi: 10.3389/frhs.2024.1370759
Received: 15 January 2024; Accepted: 26 April 2024; Published: 10 May 2024.
Reviewed by:
© 2024 Rau, Tischendorf and Mitzscherlich. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tim Tischendorf, [email protected]
This article is part of the Research Topic
Digital Health Applications: Acceptance, Benefit Assessment, and Costs from the Perspective of Patients and Medical Professionals
Identifying Barriers and Facilitators to Accessing Care for Historically Marginalized Communities Affected by Parkinson Disease: A Qualitative Study
- Published: 08 May 2024
Cite this article

- Danielle Kipnis 1 ,
- Michele Lin 1 ,
- Alissa Pacheco 1 ,
- Nia Mensah 1 , 5 , 6 ,
- Chelsea E. Macpherson 1 ,
- Kelsey Kempner 1 ,
- Anita Parker 3 ,
- R. Bernard Coley 4 ,
- Denise Coley 4 ,
- Hiral Shah 2 &
- Lori Quinn ORCID: orcid.org/0000-0002-2982-923X 1
Introduction
Parkinson disease (PD) is the second most common neurodegenerative disease. Members of the Black Diaspora (MBD) and Hispanic/Latinx people are less likely to receive a timely diagnosis following the onset of symptoms and more likely to experience greater disease severity due to late diagnosis. Historically marginalized populations (i.e., MBD, Hispanic, and Latinx communities) are not accurately represented in research; this, along with many other barriers, compounds underreporting and lack of recognition of PD. It is important to understand barriers to early diagnosis and healthcare access for these historically marginalized populations from the community’s perspective.
Our team conducted two focus groups to identify barriers and facilitators to PD healthcare-seeking behavior. We sought to identify which barriers are modifiable to ultimately improve engagement in neurological care for MBD and Hispanic individuals affected by PD.
We enrolled 15 participants (13 female; African/African American/Black n = 10, Hispanic/Puerto Rican n = 3, other n = 2) for two focus groups. Discussions revealed sources of barriers to healthcare-seeking behavior in three main domains: legacy of racism in the United States, ancestral cultural environment, and healthcare system access. These sources influenced individuals’ PD knowledge and familiarity. Additionally, participants expressed a desire to know more about PD and called for increased community-based programming for education and awareness.
This paper uses a community-based participatory research approach to describe the experiences of MBD, Hispanic, and Latinx people in Manhattan and the surrounding areas in relation to possible sources of healthcare disparities and delayed PD diagnosis. These sources have broad implications and should be addressed through collaborative community programming.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Data Availability
De-identified data is available upon request to the authors.
Code Availability
Not applicable.
This term was decided on with our community partners. It is defined as the collection of individuals without regard to actual ethnicity or country of origin who share in the US Black experience, treatment, or targeting which results in inequitable or disparate treatment from the perceived normal treatment or experience.
When referring to the specific participants in our study, we will use this term to best reflect that participants in our study identified as Hispanic and Puerto Rican. We will use the term Hispanic or Latinx when referring to the population generally or to prior research reporting on those ethnicities.
LaHue SC, Comella CL, Tanner CM. The best medicine? The influence of physical activity and inactivity on Parkinson’s disease. Mov Disord. 2016;31:1444–54. https://doi.org/10.1002/mds.26728 .
Article PubMed PubMed Central Google Scholar
Wright Willis A, Evanoff BA, Lian M, Criswell SR, Racette BA. Geographic and ethnic variation in Parkinson disease: a population-based study of US Medicare beneficiaries. Neuroepidemiology. 2010;34:143–51. https://doi.org/10.1159/000275491 .
Dahodwala N, Karlawish J, Siderowf A, Duda JE, Mandell DS. Delayed Parkinson’s disease diagnosis among African-Americans: the role of reporting of disability. Neuroepidemiology. 2011;36:150–4. https://doi.org/10.1159/000324935 .
Ben-Joseph A, Marshall CR, Lees AJ, Noyce AJ. Ethnic variation in the manifestation of Parkinson’s disease: a narrative review. JPD. 2020;10:31–45. https://doi.org/10.3233/JPD-191763 .
Article PubMed Google Scholar
Cheng EM, Siderowf AD, Swarztrauber K, Lee M, Vassar S, Jacob E, Eisa MS, Vickrey BG. Disparities of care in veterans with Parkinson’s disease. Parkinsonism Relat Disord. 2008;14:8–14. https://doi.org/10.1016/j.parkreldis.2007.05.001 .
Article CAS PubMed Google Scholar
Rhee TG, Marottoli RA, Van Ness PH, Levy BR. Impact of perceived racism on healthcare access among older minority adults. Am J Prev Med. 2019;56:580–5. https://doi.org/10.1016/j.amepre.2018.10.010 .
Turner BE, Steinberg JR, Weeks BT, Rodriguez F, Cullen MR. Race/ethnicity reporting and representation in US clinical trials: a cohort study. Lancet Reg Health - Am. 2022;11:100252. https://doi.org/10.1016/j.lana.2022.100252 .
Kipnis D, Pacheco A, Delfing D, Toomer-Mensah N, Macpherson CE, Rieger J, Parker A, Coley RB, Coley D, Shah H, Quinn L. Community-based participatory research approach to address healthcare disparities confronting members of the Black Diaspora with Parkinson’s disease. Parkinsonism Relat Disord. 2024;119:105936. https://doi.org/10.1016/j.parkreldis.2023.105936 .
Heffernan ME, Barrera L, Guzman ZR, Golbeck E, Jedraszko AM, Hays PT, Herzog KA, D’Aquila RT, Ison MG, McColley SA. Barriers and facilitators to recruitment of underrepresented research participants: perspectives of clinical research coordinators. J Clin Trans Sci. 2023;7:e193. https://doi.org/10.1017/cts.2023.611 .
Article Google Scholar
Nuytemans K, Manrique CP, Uhlenberg A, Scott WK, Cuccaro ML, Luca CC, Singer C, Vance JM. Motivations for participation in Parkinson disease genetic research among Hispanics versus non-Hispanics. Front Genet. 2019;10:658. https://doi.org/10.3389/fgene.2019.00658 .
Saadi A, Himmelstein DU, Woolhandler S, Mejia NI. Racial disparities in neurologic health care access and utilization in the United States. Neurology. 2017;88:2268–75. https://doi.org/10.1212/WNL.0000000000004025 .
Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Crotty K. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155:97. https://doi.org/10.7326/0003-4819-155-2-201107190-00005 .
Kim R, Kim H-J, Jeon B. The good, the bad, and the ugly of medical information on the Internet. Mov Disord. 2018;33:754–7. https://doi.org/10.1002/mds.27324 .
Fitzsimmons P, Michael B, Hulley J, Scott G. A readability assessment of online Parkinson’s disease information. JRCPE. 2010;40:292–6. https://doi.org/10.4997/JRCPE.2010.401 .
Article CAS Google Scholar
Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28:1504–10. https://doi.org/10.1007/s11606-013-2441-1 .
Hall WJ, Chapman MV, Lee KM, Merino YM, Thomas TW, Payne BK, Eng E, Day SH, Coyne-Beasley T. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health. 2015;105:e60–76. https://doi.org/10.2105/AJPH.2015.302903 .
Dahodwala N, Xie M, Noll E, Siderowf A, Mandell DS. Treatment disparities in Parkinson’s disease. Ann Neurol. 2009;66:142–5. https://doi.org/10.1002/ana.21774 .
Pan S, Stutzbach J, Reichwein S, Lee BK, Dahodwala N. Knowledge and attitudes about Parkinson’s disease among a diverse group of older adults. J Cross Cult Gerontol. 2014;29:339–52. https://doi.org/10.1007/s10823-014-9233-x .
Xia R, Stone JR, Hoffman JE, Klappa SG. Promoting community health and eliminating health disparities through community-based participatory research. Phys Ther. 2016;96:410–7. https://doi.org/10.2522/ptj.20140529 .
Neubauer BE, Witkop CT, Varpio L. How phenomenology can help us learn from the experiences of others, Perspect. Med Educ. 2019;8:90–7. https://doi.org/10.1007/s40037-019-0509-2 .
Betancourt JR, Green AR, Carrillo JE, Ananeh-Firempong O. Defining cultural competence: a practical framework for addressing racial/ethnic disparities in health and health care. Public Health Rep. 2003;118:293–302. https://doi.org/10.1016/S0033-3549(04)50253-4 .
Yacoubian TA, Howard G, Kissela B, Sands CD, Standaert DG. Racial differences in Parkinson’s disease medication use in the reasons for geographic and racial differences in stroke cohort: a cross-sectional study. Neuroepidemiology. 2009;33:329–34. https://doi.org/10.1159/000254568 .
Social Determinants of Health - Healthy People 2030 health.gov. 2023. https://health.gov/healthypeople/priority-areas/social-determinants-health . Accessed 22 Jun 2023.
Canizares M, Gignac M, Hogg-Johnson S, Glazier RH, Badley EM. Do baby boomers use more healthcare services than other generations? Longitudinal trajectories of physician service use across five birth cohorts. BMJ Open. 2016;6:e013276. https://doi.org/10.1136/bmjopen-2016-013276 .
Lopez De Coca T, Moreno L, Alacreu M, Sebastian-Morello M. Bridging the generational digital divide in the healthcare environment. JPM. 2022;12:1214. https://doi.org/10.3390/jpm12081214 .
Ferdinand KC. Novel interventions in addressing racial disparities in blood pressure control: potential utilization of barbershops in Black men. Circulation. 2018;138:339–41. https://doi.org/10.1161/CIRCULATIONAHA.118.034869 .
Campbell MK, Hudson MA, Resnicow K, Blakeney N, Paxton A, Baskin M. Church-based health promotion interventions: evidence and lessons learned. Annu Rev Public Health. 2007;28:213–34. https://doi.org/10.1146/annurev.publhealth.28.021406.144016 .
Bernard Coley R, Coley D. Utilizing a variation of the train the trainer model to create best practice guidelines and to build a culturally sensitive and community educated team of ambassadors to increase diverse enrollment in clinical trial research studies. Barcelona, Spain: World Parkinson Congress; 2023;.
Download references

Acknowledgements
We would like to acknowledge Ms. Norma Husband, RN, who was a partner in our community-based participatory research project from 2018 to 2021. We would also like to acknowledge Elizabeth Delaney, MSW, LCSW, and Dr. Glenn Stebbins [GS], PhD, for their consultation throughout this project and Ashrita Satchida, MA, for her contributions to the literature review.
This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1TR001873.
Author information
Authors and affiliations.
Department of Biobehavioral Sciences, Teachers College, Columbia University, New York, USA
Danielle Kipnis, Michele Lin, Alissa Pacheco, Nia Mensah, Yu Gu, Chelsea E. Macpherson, Kelsey Kempner & Lori Quinn
Department of Neurology, Columbia University Irving Medical Center, New York, USA
St. Luke A.M.E. Church, New York, USA
Anita Parker
SIG-Black Diaspora, Morgan Hill, CA, USA
R. Bernard Coley & Denise Coley
Long Island University, Brooklyn, NY, USA
Kusudi International, New York, USA
You can also search for this author in PubMed Google Scholar
Contributions
Research project: A. conception, B. organization, C. execution.
Statistical analysis: A. design, B. execution, C. review and critique
Manuscript preparation: A. writing of the first draft, B. review and critique
Danielle Kipnis: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
Michele Lin: 2A, 2B, 2C, 3A, 3B
Alissa Pacheco: 1A, 1B, 1C, 2C, 3B
Nia Mensah: 1A, 1B, 2C, 3B
Yu Gu: 1A, 1B, 1C, 2C, 3B
Chelsea Macpherson: 1A, 1B, 2C, 3B
Kelsey Kempner: 3A, 3B
Bernard Coley: 2C, 3B
Denise Coley: 2C, 3B
Anita Parker: 1A, 1B, 1C, 2C, 3B
Hiral Shah: 1A, 1B, 1C, 2A, 2C, 3B
Lori Quinn: 1A, 1B, 1C, 2A, 2C, 3B
Corresponding author
Correspondence to Lori Quinn .
Ethics declarations
Ethics approval.
We obtained ethical approval to conduct research from the Teachers College and Columbia University Institutional Review Boards (IRB # 21–265 and IRB-AAAT5742).
Consent to Participate
Informed consent was obtained from all participants to participate in the study.
Consent for Publication
Consent for publication was obtained from all participants in the signed informed consent document.
Competing Interests
The authors declare no competing to disclose.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Kipnis, D., Lin, M., Pacheco, A. et al. Identifying Barriers and Facilitators to Accessing Care for Historically Marginalized Communities Affected by Parkinson Disease: A Qualitative Study. J. Racial and Ethnic Health Disparities (2024). https://doi.org/10.1007/s40615-024-02011-2
Download citation
Received : 18 October 2023
Revised : 07 April 2024
Accepted : 18 April 2024
Published : 08 May 2024
DOI : https://doi.org/10.1007/s40615-024-02011-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Parkinson disease
- Healthcare disparities
- Community-based participatory research
- Members of the Black Diaspora
- Hispanic people
- Puerto Rican people
- Qualitative research
- Find a journal
- Publish with us
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 09 May 2024
Skin-interfacing wearable biosensors for smart health monitoring of infants and neonates
- Lauren Zhou ORCID: orcid.org/0009-0008-0199-5296 1 , 2 ,
- Matthew Guess ORCID: orcid.org/0000-0002-7113-743X 1 , 2 ,
- Ka Ram Kim ORCID: orcid.org/0000-0001-9495-2392 1 , 2 &
- Woon-Hong Yeo ORCID: orcid.org/0000-0002-5526-3882 1 , 2 , 3 , 4
Communications Materials volume 5 , Article number: 72 ( 2024 ) Cite this article
3 Altmetric
Metrics details
- Biomedical engineering
- Engineering
Health monitoring of infant patients in intensive care can be especially strenuous for both the patient and their caregiver, as testing setups involve a tangle of electrodes, probes, and catheters that keep the patient bedridden. This has typically involved expensive and imposing machines, to track physiological metrics such as heart rate, respiration rate, temperature, blood oxygen saturation, blood pressure, and ion concentrations. However, in the past couple of decades, research advancements have propelled a world of soft, wearable, and non-invasive systems to supersede current practices. This paper summarizes the latest advancements in neonatal wearable systems and the different approaches to each branch of physiological monitoring, with an emphasis on smart skin-interfaced wearables. Weaknesses and shortfalls are also addressed, with some guidelines provided to help drive the further research needed.
Similar content being viewed by others

Skin-interfaced biosensors for advanced wireless physiological monitoring in neonatal and pediatric intensive-care units
Artificial intelligence-driven wearable technologies for neonatal cardiorespiratory monitoring: Part 1 wearable technology
An epidermal patch for the simultaneous monitoring of haemodynamic and metabolic biomarkers
Introduction.
The advancements in the miniaturization of electronics have allowed impressive achievements toward improving healthcare. Wearable sensing technology enables comfortable, continuous, and convenient alternatives to standard care while maintaining quality and accuracy. These systems are especially valuable for neonatal applications, where small footprints, gentle handling, and ease of use are essential. Traditional intensive care within the Neonatal Intensive Care Unit (NICU) involves a complicated mess of electrodes, tubing, and tape that is visually disturbing and makes it extremely difficult for kangaroo care, which is skin-to-skin swaddling reported to have numerous physiological, behavioral, and therapeutic benefits 1 . In addition, neonatal skin is thin and fragile, making it especially susceptible to irritation, stripping, and sores, which may result in permanent scarring and damage 2 . With improvements in miniaturization, material choices, fabrication methods, signal analysis techniques, and wireless communication, new wearable sensors and systems that alleviate many of the inconveniences of conventional care have been developed (Fig. 1 ). In this review, we will detail the material basis for wearable devices. Then, we will share the underlying principles and technological progress of essential branches of physiological monitoring, including biopotential, optical, temperature, electrochemical, and multi-signal sensing. Finally, we will identify deficiencies that should be addressed for future work to improve the equity and accessibility of quality healthcare.
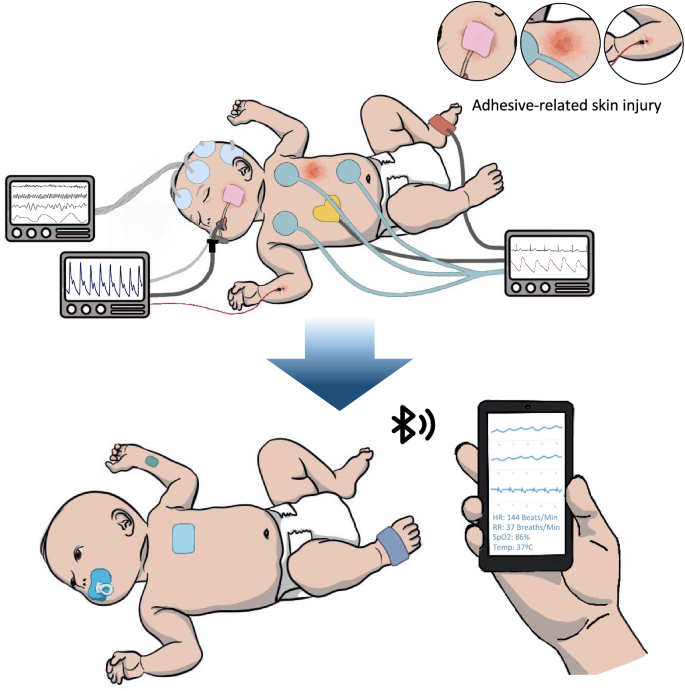
Methods include electronic miniaturization, soft and flexible materials, gentler adhesion mechanisms, designing all-in-one devices, prioritizing non-invasive monitoring and increased accessibility, wireless communication via Bluetooth, NFC, or radio, and cloud-based data processing. Stock image of infant outline adapted with permission from purchase by Getty Images iStock with color modifications and device adaptations added by the authors.
Material developments
Design considerations for neonates.
There are several nuances when designing for infant patient groups. First, infants are undeniably smaller than adults, with most high-risk patients suffering from prematurity (gestational age <37 weeks) and low birth weight (typically <2.5 kg) 3 . These patients undergo several monitoring options, including cardiac, respiratory, neurological, and general physiological monitoring 4 . Blood draws for diagnostic tests are also standard, subjecting neonates to an average of ~7.5–17.3 painful procedures per day 5 . These monitoring practices require expensive specialty equipment, with electrodes and tubing needing to be secured with adhesive tapes that are applied and removed multiple times daily. This can be especially damaging and dangerous to infant groups, as the stratum corneum and epidermis layers are 30% and 20% thinner than adult skin 6 , and the cohesion between the dermis and epidermis is much weaker 2 , 6 . This reduced skin function results in a high risk of epidermal stripping, contact dermatitis, pressure wounds, tension blisters, and burns (Fig. 2a, b ) 2 , 7 , and leads to a greater risk of infections due to percutaneous invasion of pathogens 8 . Thus, when it comes to modernizing this technology and making it wearable, the critical design considerations include small and conformal form factors, gentle and biocompatible adhesion to the skin, and making the design appear benign to caregivers, all while maintaining excellent signal quality and effective operation.
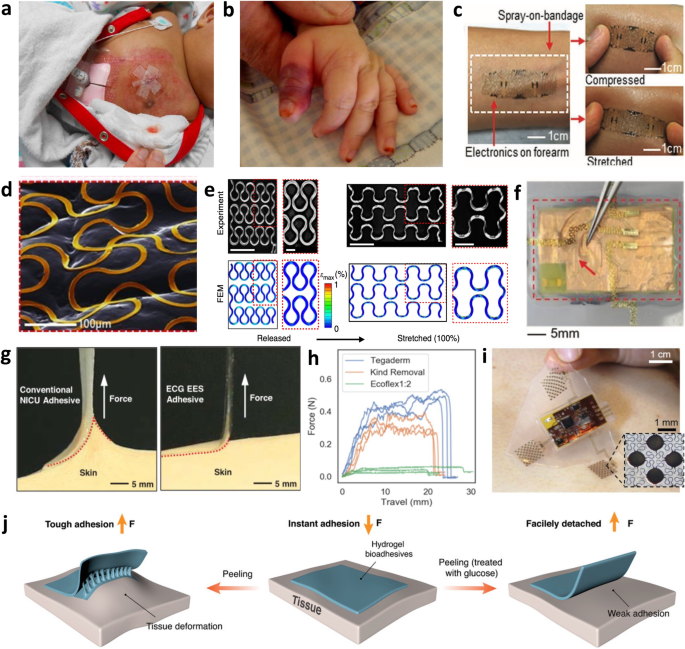
a Contact dermatitis from repeated adhesive wet electrode placement. Reproduced with permission 2 . Copyright 2014, Elsevier Inc. b Second-degree burn from pulse oximeter clip. Reproduced with permission 94 . Copyright 2012, Elsevier Inc. c Robust and conformal adhesion of EES via liquid bandage. Adapted with permission 11 . Copyright 2013, WILEY‐VCH Verlag GmbH. d High EES conformality to skin texture. Reproduced with permission 11 . Copyright 2013, WILEY‐VCH Verlag GmbH. e Geometric serpentine lines allowing linearly elastic response up to 100% strain. Reproduced with permission 13 . Copyright 2014, Springer Nature Limited. f Ionic liquid-filled decoupling layer within EES device. Reproduced under the terms of the Creative Commons CC BY license from ref. 12 . g Comparison of peel force between conventional NICU adhesive and elastomeric EES adhesive. Reproduced under the terms of the Creative Commons CC BY 4.0 license from ref. 33 . h Peel force comparison between Ecoflex and common NICU tapes Tegaderm and Kind Removal tape. Reproduced with permission 32 . Copyright 2020, IEEE. i Image demonstrating thicker elastomeric layers prevent irreparable EES deformation. Reproduced under the terms of the Creative Commons CC BY 4.0 license from ref. 12 . j Diagram illustrating glucose trigger-activated hydrogel adhesive that loses adhesion after wetting. Reproduced under the terms of the Creative Commons CC BY 4.0 license from ref. 18 .
Epidermal electronic systems
One of the primary technological techniques to combat these issues is called “epidermal electronic systems” (EES), where the material properties of the system match that of the epidermis 9 . The skin surface topology is imperfect, with creases, pores, and general surface texture forming gaps between the electrode-skin interface, resulting in increased impedance, motion artifacts, and worsened signal acquisition. EES systems are soft and flexible electronics that are fabricated using microelectromechanical systems (MEMS) fabrication techniques; however, while typical MEMS methods with silicon wafers produce delicate and brittle devices 10 , EES approaches allow robust flexibility. Employing thin film materials like polyimide (PI) (<100 μm thick) as a structural substrate and dielectric material and thin depositions of metals like Cr/Al/Cu/Au (<500 nm thick) to serve as the conductive layers 9 , 11 , 12 one can achieve ultrathin and flexible devices with sub-nanometer bending stiffnesses and effective moduli ~140 kPa 9 , preventing delamination from the skin. They can be easily attached to the skin with a thin adhesive transfer film like that of a temporary tattoo 9 or via liquid bandage (Fig. 2c ) 11 with very intimate contact with the skin’s surface (Fig. 2d ) 11 . Integrating modified geometric designs using curvilinear serpentine interconnects enables greater strain tolerance and stretchability as they act as pre-buckled lines. High amplitude serpentines are capable of 100% strain with minimal stress and maintain elastic responses (Fig. 2e ) 13 , surpassing the elastic behavior of skin where it is only linearly elastic up to tensile strains of 15% 14 . The high conformality allows the system to move dynamically with the skin, assures prominent signal quality, eliminates motion artifacts, and maintains continuous contact with the skin. The thin-film properties of these electronics allow comfortable and gentle physiological monitoring that protects the integrity of fragile infant skin while ensuring high-quality data recordings.
Thin film systems can have broader applications when interfaced with elastomers. More affordable than silicon wafer technology, they can have varying Young’s modulus depending on the amount of crosslinking allowed, and they are unique in that they can form tight seals with itself, silicon, and glass. Tight seals make the devices water resistant, which is necessary in high humidity NICU incubators, and they can be injected with ionic liquid to serve as a decoupling layer to reduce mechanical stresses within the device (Fig. 2f ) 12 . This quality also makes elastomers excellent for micro and nanofluidic applications for biofluid sensing which will be discussed later in this review. For applications with EES, elastomers are an excellent substrate to embed soft electronics within because they are biocompatible and naturally adhesive due to Van der Waals forces. Typical adhesive tapes used in the NICU to secure tubing and wires are pressure-based and have tackifiers derived from acrylate, resin, or petroleum. After applying pressure to the tape onto the skin, the adhesive flows into the creases and folds of the skin via wetting and form a bond, building strength over time 15 , 16 . Because the adhesive forms a strong bond with the skin, there is a very high likelihood that it is stronger than the bond between the skin cells, causing the epidermal layers to be stripped away with removal. This makes the elastomeric Van der Waals adhesion especially attractive because it can bond strongly to the skin (even wet) while allowing extremely gentle removal (Fig. 2g ). Compared to other standard adhesives used in the NICU designed to be delicate on the skin, Ecoflex has a peel force 15 and 10 times smaller than Tegaderm and Kind Removal tape, respectively (Fig. 2h ) 17 . In a clinical test on 50 neonates observing changes in skin condition (erythema, dryness, or breakdown) after 15 minutes of EES application, the average change was negligible to slightly improved 17 . Thicker layers of elastomer prevent EES disturbance (Fig. 2i ) and depending on the elastomer type, can be reusable, improving device longevity. Alternative adhesives that emphasize and improve on gentle removal processes are also being explored, like trigger-detachable hydrogels that swell and lose their adhesive energy once treated with glucose 18 (Fig. 2j ), water 19 , or shear force 20 , or thermally switchable copolymer tapes 21 and silicone-based adhesive with meltable oil crystallites 22 . These alternative and gentler removal processes may be better suited for infants with especially fragile skin, like premature infants.
All-in-one systems
Depending on the criticality of a patient, they may be connected to several monitors simultaneously, each with its own apparatus. These complicated setups form a perceived barrier of untouchability, curtailing skin-to-skin contact and exacerbating visual disturbance and stress. Elastomeric encapsulation is excellent for hybrid electronics, allowing the mix of flexible EES sensors, rigid passive and active electronics, wireless communication, and data processing to form all-in-one devices with real-time wireless monitoring. These self-sufficient devices are the basis for several systems described in this review.
Textile sensors
Textiles are a popular mechanism by which to integrate sensors for biosignal sensing. From a review of the literature for noninvasive infant monitoring, textile sensors were historically the preferred mechanism due to its perceived familiarity and simplicity. Integration methods of conductive materials include direct weaving of electrically conductive fibers 23 , 24 , sewing on pieces of Ag- or Au-coated nylon 25 or polyurethane 26 , or printing electric inks on the fibers directly 27 . These components can then be easily attached to items of clothing like onesies 24 , straps/bands 28 , jackets 29 , and more. Textile electrodes embedded within clothes eliminates the need for tapes and conductive gels, but at the cost of signal quality. Physiological metrics like respiration rate and motion can be measured by the resistive and capacitive strain response of conductive fibers 24 , 28 . Most of these works transfer data with wired connectors or bulky wireless transmitters; however, antennas can be knitted within clothing coupled with radio frequency identification (RFID) tags for battery-free wireless data transfer 30 . We found that as EES systems and flexible electronics were developed and advanced for adult monitoring, they had become the state of the art for infant monitoring as well; thus, it will be the main point of focus for this review.
Physiological monitoring
The measurement of physiological metrics can be performed over various modalities, including biopotential, optical, temperature, electrochemical, and multi-signal sensing. Here, we report gold standard of testing and non-invasive skin-interfaced alternatives applicable to neonatal patient groups, summarized in Table 1 .
Biopotential sensing
The human body functions via chemical reactions that generate action potentials to power and control the physiological processes within the body. Several of these biopotentials are measurable through the skin with electrodes that transduce the ionic current into assessable electric signals. Several biopotential signals that are significant for health monitoring including electrocardiograms (ECG) for heart activity, electromyograms for muscle activation, electroencephalograms (EEG) for brain activity, and electrooculograms for eye movement tracking, with ECG and EEG being most relevant for neonatal applications. Traditional wet Ag/AgCl electrodes use conductive gels (sometimes abrasive) to reduce the impedance made by the nonconformal gaps between the electrode and skin surface and improve electrical conductivity. However, these gels dry out over time degrading signal quality, irritating the skin, and still requiring the use of a strong adhesive to affix the electrode. Furthermore, conventional electrodes are wired to stationary monitors that restrict movement. Therefore, recent works to improve biopotential sensing have focused on using EES as dry electrodes that do not require the use of conductive gels, which mitigate and improve on these weaknesses.
Electrocardiography
Electrocardiography (ECG), which measures the electrical activity of the heart muscles, is a key signal used to monitor heart function by being able to derive heart rate (HR), heart rate variability (HRV), heart rhythms, and respiration rate (RR). To determine if the signal recorded is high quality, ECG fiducials and timings including the P, QRS, and T waves should be distinct and consistent without distortion. To minimize device footprints, lead placement is modified to place the electrodes closer to one another. With the adapted lead placement, it is important to ensure maintained accuracy. To extract real-time heart rate from the ECG signal, the Pan-Tompkins algorithm is a widely used method to identify the QRS complex which is then used by an automated algorithm to quantify HR, HRV, heart rhythm, and RR (Fig. 3a ) 31 , 32 . By recursively checking if the heart and respiratory rates are within normal range, emergency alarms can sound to alert the provider of abnormal behavior, thus meeting the standards of NICU monitoring. Chung et al. 33 were the first to apply an all-in-one EES system to mimic vital signs monitoring in the NICU (Fig. 3b ). Semiconductor fabrication steps form the circuital base of the device, including the electrodes, near field communication (NFC) coil, and sensor interconnects. Off-the-shelf active and passive components for biopotential amplification and filtering and an NFC System-on-a-Chip (SoC) allow wireless inductive power transfer and data sharing to a host reader platform that lays beneath the patient’s mattress. An ionic fluid injected into a microfluidic space beneath the circuitry maintains the resonant frequency and quality factor for the NFC antenna coil. In addition, the battery-free, open mesh design allows device usage during medical imaging (MRI and X-ray). Although only two electrodes are used, they had strong agreement in their HR and RR determination to the gold standard; however, they still required the use of a conductive gel. Weaknesses of this system is that it is mechanically fragile, collapsing on itself after extreme deformation during the removal process 9 , 17 . Due to it complicated fabrication steps requiring specialized facilities, these expensive systems are not suitable for popular and disposable use. In addition, the NFC communication protocol only has modest operating distances up to 25 cm, keeping the infant bedridden for continuous monitoring. Kim et al. 12 optimized the construction strategy for EES to preserve high conformality while improving the system hardiness, finding that a mixed elastomer with a 2:1 ratio of EcoFlex Gel to EcoFlex 0030 had the greatest adhesion force and conformability with robust manipulability. They applied this discovery to their own neonatal ECG monitoring device (Fig. 3c ) 32 , which had a removable lithium-ion polymer (LiPo) battery allowing hours of continuous monitoring and a Bluetooth low energy (BLE) SoC allowing long-range telemetry up to 15 meters. Using a modified Lead V2 configuration, they were able to easily distinguish the ECG fiducial PQRST waves and had an SNR over 40 dB without the use of conductive gels. This work also used semiconductor fabrication steps including spin coating, sputter deposition, chemical vapor deposition, wet/dry etching, and photolithography to form the device. Textile electrodes are also popular for ECG recording (Fig. 3d ) without conductive gels and have relatively good performance with low drift and distinguishable QRS complexes 29 . However, constant pressure needs to be applied, either by having the infant laying on the electrodes or with a tight-fitting jacket. With wireless communication and smart computation with machine learning, these device systems can perform real-time HR, RR, HRV, and heart rhythm determination. However, the real-time study of heart rhythms from wirelessly recorded systems should be taken fastidiously, as issues with inconsistent data transfer like data drop out and lagging can give artificial results.
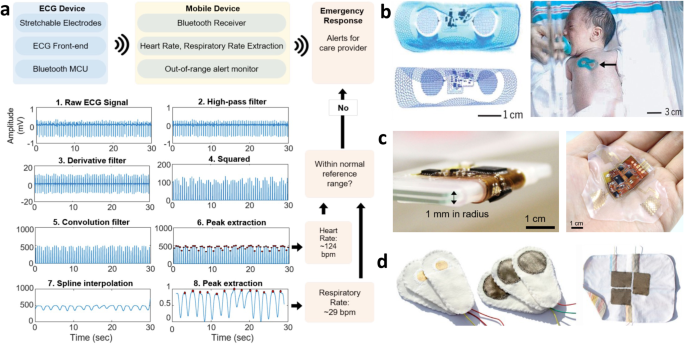
a Process diagram for heart rate and respiration rate determination from ECG waveform following Pan-Tompkins algorithm. The calculated values can be studied recursively for alert systems. Reproduced with permission 32 . Copyright 2020, IEEE. b First neonatal all-in-one EES system (left) for neonatal cardiovascular monitoring (right). Reproduced under the terms of the Creative Commons CC BY 4.0 license from 33 . c Thicker, more robust EES cardiovascular monitoring with V2 lead setup. Reproduced with permission 32 . Copyright 2020, IEEE. d Textile-based electrodes. Reproduced under the terms of the Creative Commons CC BY 4.0 license from ref. 29 .
Electroencephalography
Electroencephalography (EEG) measures the electrical activity of the brain with electrodes placed on the scalp, typically used for seizure detection. Common modes are single-channel amplitude integrated EEG (aEEG) and multichannel continuous EEG (cEEG), where cEEG is considered the diagnostic gold standard. aEEG can easily be performed on premature infants to observe abnormalities associated with brain injury 34 while cEEG, requiring ~10-20 electrodes, is more comprehensive and used for seizure detection and cortical function assessment 35 . Due to incubator limitation, head size constraints, and hair, placement and maintenance of the electrodes is difficult. In addition, EEG waveforms are very small in amplitude and susceptible to artifacts. A wireless communication device helps allow space-saving communication between the 23-lead system and a bedside laptop 36 , however limited research has been performed to materially redesign an EEG electrode system. Most developments involve designing textile caps or bands that improve the ease of electrode placement but still use traditional wet electrodes 37 , 38 , 39 . Although not designed for neonatal applications, Mullen et al. 40 designed a rigid cEEG headset using novel hybrid electrodes that have an ionic hydrogel sandwiched between a semi-permeable membrane and Ag/AgCl plates to transduce the electrical signal. This hybrid electrode design combines the improved conductivity and signal quality of wet electrodes with the skin biocompatibility of dry electrodes. Bristle electrodes, shaped like the bristles of a brush with a conductive tip, may also be a suitable dry electrode replacement, as they can part the hair for gentle conformal contact with the skin and record with high signal quality 41 . This design, however, has been reported as irritating after long term use as a constant pressure is required for low skin-electrode impedance.
Optical sensing
Optical sensing for medical use uses the principles of spectrophotometry to noninvasively observe physiological phenomena transcutaneously. Using pairs of light emitting diodes (LEDs) with corresponding photodetectors, the LED shines a wavelength with a known intensity into the tissue that then gets received by the photodetector. Based on how much light gets absorbed, one can form a representative relationship for key tissue metrics like blood oxygenation, hydration, and chemical composition 42 . There are two modes that exist: transmissive (LED and photodetector on opposite sides of the observed tissue) and reflective (LED and photodetector are side-by-side).
Photoplethysmography
Photoplethysmography (PPG) is an optical sensing technique used to estimate blood oxygen saturation (SpO2), also called pulse oximetry (POX). The gold standard of POX is with an invasive arterial line to find arterial oxygen saturation (SaO2), but optically derived values from POX are acceptable as well. POX is a dominant metric used for clinical decision making, including the decision to supply additional oxygen, the detection of early sepsis and cardiopulmonary complications, and is the sole screening metric for congenital heart defects (CHDs) 43 . POX can track respiration rate and indicate apnea events in premature infants, as cessations of breathing are accompanied by either bradycardia or oxygen desaturation (SpO2 < 80%) 44 . Using two sets of LED/photodetector pairs, one LED operates at a 640–660 nm wavelength (red) while the other at 880–940 nm (infrared) 45 to measure the differential light absorption through the observed skin tissue. This ratio is calibrated against SaO2 measurements to establish a measurement of SpO2. Typical POX probes for adults are designed for the finger and utilize the transmissive mode, often in a clip-style packaging. However, the strong forces of the clip can be especially damaging on fragile infant anatomy (Fig. 2b ). Thus, neonatal pulse oximeters operate with the reflective mode on the hand or foot and are often packaged within an adhesive wrap. POX is highly susceptible to motion artifacts, and because infants frequently swing around their limbs, packaging for these sensors are designed to induce a constant force and must be checked regularly to avoid pressure wounds and burns. In addition, due to their small anatomy and low body fat, neonates often have low blood perfusion in their limbs, which is necessary for accurate measurement. In these cases, clinicians will monitor from more proximal locations like the earlobe and forehead 46 . Grubb et al. 47 proposed forehead reflectance PPG for neonates in the NICU. Henry et al. 48 presented a wireless, cap-mounted device for measuring heart rate via PPG. The device was optimized for preterm infants by miniaturizing and ruggedizing the sensor, which consisted of four 525 nm LEDs arranged in pairs on either side of a photodetector. The sensor was encapsulated in silicone and wired to a computer for data recording. The sensor was held to the forehead using a specialized T-shaped cap (Fig. 4a ). They demonstrated the clinical capabilities of the device by comparing the heart rate from ECG. Chung et al. 33 developed a flexible PPG sensor for the foot that utilized ultra-thin electronics to allow a small bending radius of 5 mm to allow for conformality with the foot (Fig. 4b ). The thinness and design of the perforations of the device allowed for adhesion via only van der Waals forces. The PPG device offered on-board AC/DC calculation before transmitting data, reducing the bandwidth needed. The group built on this work in 2020 by introducing a system that improved on the limitations of the previous system (Fig. 4c ) 17 . This system improved the wireless data communication operating distance, the fragile nature of the designs, and specialized fabrication. The PPG unit was made with a flexible printed circuit board encapsulated with silicone, providing more stability. Due to the embedded battery, the full PPG waveform could be transmitted over the device with 95% limits of agreement (LOA) in HR of less than 4 BPM with standard clinical measurement. There exist global weaknesses with POX for infants that need to be addressed. First, skin color is a big factor, as individuals with darker skin absorb more light than lighter skin 49 . In an assessment among adult patients of different races and ethnicities, Black, Hispanic, and Asian patients experienced erroneous over-estimations by POX compared to SaO2 levels 50 . This is likely due to the historical limited inclusion of people of color in these formative studies, where calibration algorithms are derived from data sets primarily from individuals with lighter skin tones 51 . Additional research should also be conducted for modifying the calibration algorithms for infant patients, as a study found that conventional NICU pulse oximeters lose accuracy for hypoxic patients with blood saturations lower than 85%, often overestimating by more than 5% compared to arterial values 52 . Diseased neonatal patients that suffer from CHDs often have blood oxygen levels in the 75–85% region, therefore these overestimations are unacceptable for regular clinical care. Given POX’s clinical influence, these issues must be addressed with future research developments.
a Illustration of a cap-mounted device for measuring PPG at the forehead. The sensor sits above the eyebrow. Reproduced under the terms of the Creative Commons CC BY 4.0 license from ref. 48 . b Wireless PPG device bending around a glass cylinder. Reproduced under the terms of the Creative Commons CC BY 4.0 license from ref. 33 . c Schematic and photographs of a wireless limb unit for measuring PPG. Reproduced with permission 17 . Copyright 2020, Springer Nature.
Near-infrared spectroscopy
Complementary to POX is near-infrared spectroscopy (NIRS). NIRS is based on the near-infrared spectrum of wavelengths (700–1000 nm) and the absorption of chromophores such as myoglobin, hemoglobin, and cytochrome aa3 53 . Although similar in principle to POX, NIRS differs in that it represents the balance of local tissue oxygen supply and demand. Regional tissue oxygen saturation (rSO2) monitors discriminate light paths from different tissue depths, ergo measuring veinous as well as arterial hemoglobin oxygenation 54 . Therefore, NIRS can be more useful in measuring cerebral hemodynamics, which is helpful for seizure detection 55 , intraventricular hemorrhage 56 , and white matter injury 57 . Rwei et al. developed a soft, flexible, wireless system for monitoring infant cerebral hemodynamics 58 . The pair of LEDs emit at 740 nm and 850 nm wavelengths with four photodiodes at source-detector distances of 5, 10, 15, and 20 mm, allowing recording at different tissue depths for detection of both peripheral and cerebral hemodynamics, and was validated by Monte Carlo optical simulations and magnetic resonance imaging (MRI). Other wavelengths offer opportunities to measure different chromophores. If blue and green wavelengths of LEDs are used, the light absorption ratio of bilirubin can be monitored, and this principle was used by Inamori et al. 59 who reported a wearable device for jaundice detection. Calibrated by a commercial bilirubinometer, their device could successfully measure bilirubin concentration using the ratio of the reflected green and blue lights. Measuring NIRS offers challenges not found in photoplethysmography. The validation of NIRS is difficult because there is not a gold standard for tissue oxyhemoglobin content. Light absorption and scattering occurs from compounds other than hemoglobin, thus more wavelengths and source-detector distances are necessary for improved accuracy 60 .
Temperature sensing
Infants, due to their prematurity and lack of body fat, struggle to maintain a stable and normal body temperature between 36.5 and 37.5 °C 61 , thus are at extremely high risk of hypothermia. Premature infants are at even greater risks, which result in incubators stays tuned to the ideal temperature and levels of oxygen, humidity, and light. Skin and core temperatures are the two metrics studied. For skin temperature, single-measurement readings can be determined from a thermometer or human touch. For continuous readings, small flexible adhesive probes attached to the abdomen, chest, or back are used. Most wearable systems that have been developed for infants record skin temperature with off-the-shelf temperature gauges 17 , 19 , 62 . However, skin temperature is not very informative as a health metric because it cannot be used as a proxy for core temperature 63 , 64 . For core body temperature (CBT), common methods include measuring invasively from the pulmonary artery or semi-invasively with probes inserted into the esophagus, nasopharynx, tympanic membrane, or rectum 65 . However, these measurements still vary amongst each other depending on anatomical location because some organs produce heat (brain, liver) while others dissipate (lungs, skin) 66 . Nonetheless, except for the invasive line, the esophagus is considered the gold standard. Clinicians can also estimate temperature from the environment, where infants housed within an incubator have sensors that measure the ambient temperature. Little research has been conducted to develop a method to non-invasively and accurately measure neonatal core body temperature, but Atallah et al. 66 have succeeded with an unobtrusive method of continuous neonatal brain temperature (a proxy for CBT) monitoring with a zero-heat-flux (ZHF) sensor matrix placed beneath the head 67 . ZHF methodology assumes that if the heat loss from a surface is reduced to zero, the gradient between core and surface temperature will also reduce to zero. By placing thermistors on either side of a thermal resistance material, a control loop can control a heating pad beneath the matrix to heat one thermistor until it matches the skin-interfacing one, thus providing the cerebral temperature 68 , 69 . Clinically validated in comparison to esophageal measurements, they found moderately high correlations ( r > 0.5, p < 0.001) for most infants, with lower correlations likely due to poor head placement over the sensors. Alternatively, a miniaturized, skin-interfaced temperature sensing system usable by infants using two negative temperature coefficient (NTC) thermistors, one that is insulated within the foam and interfaces with the skin and the other that faces the ambient air 70 . Using single heat flux principles, they were able to estimate core body temperature with a mean difference of −0.05 °C with a 95% LOA of 0.24 °C in comparison to an ingestible temperature sensor. However, their estimation simply adds 1.2 °C to the measured skin temperature and was only tested on three adult subjects, thus further work is needed to observe if this system is usable for infants.
Electrochemical sensing
Detection of chemical or protein biomarkers from fluidic specimens by patients can provide progressive details for health pre-diagnosis, diagnosis, and prognosis. Typically, neonates admitted to the NICU are subjected to 7.5-17.3 painful procedures a day for laboratory tests 71 , with higher risk patients subjected to more frequent blood draws. Neonatal non-invasive thin film biomarker-sensing restricted to sweat, saliva, and urine can replace conventional invasive blood draws with the same sensitivity towards biomarker detection (e.g., electrolytes, metabolites, cell-secreted proteins). The electrochemical sensing principle relies on measuring the charge transfer of captured analyte reactions on a sensing electrode. These charges allow recordable changes in current (voltammetry/amperometry), conductivity (conductometry), and voltage/potential (potentiometry) for use with wearables. For saliva analysis, pacifiers can be an excellent platform for safe saliva sample-collecting (Fig. 5a ). Smart pacifiers can be used for detecting glucose 72 , sodium, and potassium 73 with amperometric and voltametric enzyme sensing. Glucose tracking is extremely important for neonatal monitoring, with admitted patients typically subjected to hourly glucose tests and contribute to more than 40% of all laboratory studies performed 71 . Garcia-Carmona, et al. 72 developed a pacifier with a rectifier system to enable forward saliva flow towards an electrochemical cell located in the back without backflow. Their system had excellent linearity ( R 2 = 0.994), sensitivity (0.69 ± 0.04 nA/mM), limits of detection (0.721 mg/dL), and limits of quantification (1.802 mg/dL) which are far below average glucose levels for neonates (70–150 mg/dL). The current prototype is limited by the biofouling characteristics of saliva affecting the long-term stability of the sensing electrode, but if addressed appropriately, can be effective for continuous glucose monitoring. Parilla et al. 74 designed a saliva-based wearable that aids in the monitoring of phenylalanine, the biomarker for phenylketonuria, a rare inheritable disorder that can be toxic to the nervous system and is tested for at birth. Their sensor has an affordable and user-friendly sampling strategy where saliva is absorbed by a filter paper pre-impregnated with a hydrogen carbonate buffer. The sample then gets placed onto a screen-printed electrode that is linked to a smart wristband that reports the phenylalanine levels after 15–240 s. This sensor has a dynamic range from 0.0004–18 mg/dL which spans below the normal levels (<2 mg/dL) to beyond the unhealthy limits (4 mg/mL) for neonates and can be a favorable alternative to conventional blood draws. For sweat analysis, patch-type sensors can be used to detect and analyze glucose. Open circuit potentiometry can identify the target ion concentration, and the monitoring of sodium and chloride contents trends can support the early detection of cystic fibrosis (Fig. 5b ) 75 . Although not a direct indicator of serum glucose levels, sweat glucose can track the tendency patterns, which could be beneficial for neonatal diabetes, where Lee et al. 76 developed a stretchable, ultra-thin, patch to facilitate drug delivery decisions. These devices utilize an enzymatic biosensing method within a chronoamperometric setup. From urine analysis, diapers are a simple wearable platform. Ning et al. 77 created a urea-based bilirubin detector for neonatal jaundice with hydrovoltaic-biosensing on a ZnO nanoarray (Fig. 5d ). It outputs a greater voltage the stronger the bilirubin concentration and proportionally powers a series of LEDs for visualization. The diaper has repeatable performance with multiple exposures spread over several hours and is expected to be discarded with the diaper. We expect much growth in wearable analyte-detection devices for neonatal applications, as there is an abundance of work being done with adult applications. However, there is a lack of defined relationships between these biofluids and serum ion concentrations, thus non-invasive diagnostic powers are limited.
a Electrochemical sensing pacifier that tracks electrolyte (sodium and potassium) levels from saliva. Reproduced with permission 73 . Copyright 2022, Elsevier B.V. All rights reserved. b Patch-style sweat sensor for sodium and chloride monitoring for cystic fibrosis monitoring. Reproduced with permission 95 . Copyright 2016, Springer Nature Limited. c Diaper-integrated urea sensing for jaundice monitoring using a ZnO nanoarray. Reproduced under the terms of the Creative Commons CC BY 4.0 license from ref. 77 .
Multi-signal systems
The synchronous recording of a multi-signal system being uploaded to a single data sink allows comparative relationships to form between the signals, driving new physiological metrics to be computed (Fig. 6a ). For example, the aforementioned work by Chung et al. 33 had a binodal ECG and PPG measuring system. Because both signals were recorded and saved concurrently, they were able to identify the time elapsed between the ECG R peak to the PPG valley fiducial to derive the pulse arrival time (PAT) (Fig. 6b ). They used the Moens-Korteweg equation to demonstrate a linear relationship between 1/PAT and systolic blood pressure (BP); however, their testing was conducted on one adult and with limited statistical validation compared to a cuff monitor. Improving upon their previous design, a year later, Chung et al. 17 developed a thicker, yet still conformal, binodal system with an added high-sensitivity tri-axial accelerometer for posture recognition and cry analysis (Fig. 6c ). From the accelerometer, they were also able to measure seismocardiograms (SCG), which is the recording of the physical vibrations of the heartbeat through the chest wall. SCG provides a new perspective to cardiovascular monitoring because it can distinguish key cardiological events including valve openings, closings, fillings, and ejections. The time delay between the tallest SCG peak representing aortic opening with the PPG valley provides the pulse transit time (PTT). Combined with a known distance between both sensors and vascular assumptions about arterial wall thickness, modulus, and blood density, a surrogate of systolic and diastolic blood pressure (BP) can be derived. Comparing their system to values from invasive arterial lines of two patients, they were able to generate their own calibration curves for BP with respect to pulse arrival/transit time. Testing with additional subjects showed their system performed within the ANSI/AAMI Sp10 requirements for blood pressure cuffs, which requires a mean difference <5 mmHg and standard deviation <8 mmHg. While the ability to determine BP noninvasively and continuously is incredibly beneficial, it is an extremely volatile metric depending on emotional and active states, body position, temperature, stress, and genetic history, thus requiring frequent calibration with a gold standard (invasive arterial line or oscillometric cuff) to maintain accuracy. Additionally, it is important to be cautious relying on metrics derived from timing differences between signals because data time synchronization can be unreliable. Lagging and imprecise time stamps can lead to poor relational data, which for BP estimation from PTT, even an erroneous hundredth of a second can be detrimental as the average PTT times of a 3-month-old is 140 ± 11 ms 78 (assumed even shorter for preterm neonates). Chung, et al. 17 navigated this issue by having their chest unit transmit its 16 MHz local clock information to the limb unit, eliminating drift to enable time syncing of less than 1 ms. The combination of ECG and SCG can also be used to estimate stroke volume of patients with congenital heart defects 79 . Yoo et al. 80 created a multi-signal system that combines an inertial measurement unit (IMU) with two microphones to acousto-mechanically monitor cardiorespiratory and gastrointestinal events. Combining the data recorded from each sensor allows spaciotemporal mapping of the lungs and bowels. The two microphones face opposite directions, one towards the body and other towards the environment, which allow sound separation and audio reconstruction of physiological sounds in noisy environments (Fig. 6d ). Simultaneous tracking from multiple of the same sensors can be advantageous as well. A four-IMU system applied to the wrists and ankles combined with machine learning has found high correspondence to cramped-synchronized general movements as compared to visually validated videos for cerebral palsy detection 81 . Additional IMUs can be used to reconstruct and track 3D body motions 82 (Fig . 6e ).
a Schematic diagram showing how concurrent and time-synced data uploading and processing allows new physiological phenomena to be monitored. b Systolic and diastolic blood pressure derivation from timing differences between fiducials from ECG, SCG, and PPG waveforms. Reproduced under the terms of the Creative Commons CC BY 4.0 license from ref. 96 . c Binodal multi-signal system that tracks ECG, PPG, SCG, and temperature. Reproduced with permission 17 . Copyright 2020, Springer Nature. d Dual-mic setup with one towards the body and another towards the environment allows real-time noise canceling and audio reconstruction (right) even in noisy environment (left). Reproduced with permission 80 . Copyright 2020, Springer Nature. e) Multi-device IMU system (left) that allows body position reconstruction (right). Reproduced with permission 82 . Copyright 2020, Springer Nature.
Future work
Most of the technology discussed in this review has been developed to simplify and improve treatment options within the NICU. Table 1 shows the comparative wearable methods to conventional physiological monitoring. Notably, there are many adult systems that we refrained from discussing because they had yet to be applied to neonatal and pediatric applications. However, we will share several developments that we find worthwhile but will need to be adapted to accommodate pediatric physiological differences. Wearable stethoscope technology 80 , 83 could be used to aid in asthma monitoring and continuous studying of cardiopulmonary sounds. Wearable dry cEEG, like the cap device by Mullen et al. 40 , could greatly improve ease of seizure monitoring for preterm infants. Wearable piezoelectric ultrasound patches could assist with complex and expensive internal imaging 84 . Use of continuous electrochemical sensing that are currently developed but not in a wearable form factor would be beneficial to help detect and monitor ailments like lactic acidosis 85 , neonatal sepsis 86 , and jaundice recovery 87 . The application of soft, flexible, and inexpensive passive sensors could help to monitor physiological metrics and movement 88 , 89 . It is also important to solve known deficiencies, like developing a more accurate pulse oximetry algorithm for hypoxic neonates and infants of various skin tone, as current commercial devices are often inaccurate for patients with blood saturation below 85% or dark skin.
Although the work on pediatric wearables is relatively new, with advanced multimodal systems being developed only within the past five years, it would be extremely valuable to integrate them into real hospital settings for monitoring. Though there is may exist a lack of trust in the efficacy in these devices, hesitating to implement them as tools for influential medical decision making, neonatal and infant patient groups are the most to benefit from this technology. With their fragile skin and risk for severe injury even from acrylic adhesive tapes with weak adhesion forces like paper tape and Tegaderm, the gentle adhesion from elastomers with Van der Waals forces ensure protected skin integrity. Wireless devices reduce the overwhelming visual stress of NICU monitoring systems making parents feel more comfortable to approach and hold their sick child. They also make it easier to transport patients between wards, hospitals, or even from the bed to their parent’s arms for kangaroo care 90 . Kangaroo care is critically important for newborn development, parent-baby bonding, and improving patient outcomes, especially for high-risk preterm neonates, with benefits for the infant including more stable physiological metrics, less pain, better sleep, improved weight gain, and earlier discharge 90 , 91 , 92 . Recent wearable technology has also been designed to account for sanitization in the hospital environment, either prepared for autoclave sterilization 17 or to be low cost for economic single-use 93 . The use of machine learning algorithms may aid in the detection of unusual behavior, with highly accurate systems being especially beneficial in remote or lower-resource areas with less-experienced physicians, increasing accessibility to quality healthcare. Combined with cloud-based processing and data storage, long-term patient-specific trend analysis could be manipulated to help clinicians recognize patient recovery/decline, discern abnormal deviations, and reduce misdiagnoses. Concerns for high-fidelity data transfer without dropouts or loss of connection are problems that should be addressed to improve trust in wireless systems, as they are highly likely in hospital environments with lots of devices communicating in the same frequency spectrum as BLE. However, operating with different communication protocols like ultra-wideband transmission may reduce signal interference from other devices while still operating at low power.
The wearable devices developed would provide even greater benefit to outpatient care and use in lower-resource communities. While still mostly in their exploratory and validations phases, the market lacks adequate and trustworthy home infant health monitoring systems. A drive for commercialization allowing normal use will be especially beneficial for recovery monitoring of post-surgical high-risk patients like interstage single-ventricle patients. At the same time, it is important to design user-friendly devices to be able to entrust nonmedically trained individuals to record high-quality data. Battery-free systems with passive sensors often use induction-based data transfer to receiver units. If embedded within clothes, it could standardize and improve ease of use by non-physician caretakers. With the rise of telehealth, low-cost reusable and disposable devices could improve healthcare accessibility, allowing remote or rural patients to conduct a pre-screening at home before deciding to travel long distances to hospitals with specialized care options. It could also reduce strain on hospital resources, allowing low-risk patients to be monitored remotely in the comfort of their own homes. These automated monitoring systems would be especially beneficial for diseased school-age children to improve independence, allowing them to be more self-sufficient and in tune with their medical needs during the long periods of the day they spend with reduced adult supervision. It is important to ensure proper and limited usage of these devices, as they can provide a false sense of safety and increase parental anxiety.
The development of flexible electronics and wearable health monitoring systems has been a growing field that greatly benefits a vulnerable and understudied population. They can assist in the measurement of key physiological metrics, including cardiovascular operation and rhythm, cerebral hemodynamics, blood oxygen saturation, temperature, and ion concentrations, replacing invasive procedures and improving patient outcomes. Other advanced signal processing allows further medical derivations including blood pressure, core body temperature, and reconstructive imaging of body movements. The information combined from these metrics can help with diagnostic practices and recovery monitoring; however, the work discussed has only brushed the surface of possible biomedical applications for neonatal populations. With further research and development tuned specifically for infants, this demographic may be the largest beneficiaries from what wearable biosensors and smart health monitoring systems have to offer.
Campbell-Yeo, M., Disher, T., Benoit, B. & Johnston, C. Understanding kangaroo care and its benefits to preterm infants. Pediatric Health Med. Therapeutics 15 , https://doi.org/10.2147/phmt.s51869 (2015).
Lund, C. Medical adhesives in the NICU. Newborn Infant Nursing Rev. 14 , 160–165 (2014).
Article Google Scholar
Low Birth Weight. Stanford Medicine Children’s Health - Lucile Packard Children’s Hospital. https://www.stanfordchildrens.org/en/topic/default?id=low-birthweight-90-P02382#:~:text=Low%20birth%20weight%20is%20a%20term%20used,than%205%20pounds%2C%208%20ounces%20 (2%2C500%20grams). (Accessed Nov 2023).
Finn, D., Boylan, G. B., Ryan, C. A. & Dempsey, E. M. Enhanced monitoring of the preterm infant during stabilization in the delivery room. Front. Pediatr. 4 , 30 (2016).
PubMed PubMed Central Google Scholar
Cruz, M. D., Fernandes, A. M. & Oliveira, C. R. Epidemiology of painful procedures performed in neonates: a systematic review of observational studies. Eur. J. Pain 20 , 489–498 (2016).
Stamatas, G. N., Nikolovski, J., Luedtke, M. A., Kollias, N. & Wiegand, B. C. Infant skin microstructure assessed in vivo differs from adult skin in organization and at the cellular level. Pediatr. Dermatol. 27 , 125–131 (2010).
Article PubMed Google Scholar
Lund, C. H. et al. Disruption of barrier function in neonatal skin associated with adhesive removal. J. Pediatrics 131 , 367–372 (1997).
Article CAS Google Scholar
Darmstadt, G. L. et al. Infection control practices reduce nosocomial infections and mortality in preterm infants in Bangladesh. J. Perinatol. 25 , 331–335 (2005).
Kim, D.-H. et al. Epidermal electronics. Science 333 , 838–843 (2011).
Article CAS PubMed Google Scholar
Chircov, C. & Grumezescu, A. M. Microelectromechanical systems (MEMS) for biomedical applications. Micromachines (Basel) 13 , 164 (2022).
Yeo, W. H. et al. Multifunctional epidermal electronics printed directly onto the skin. Adv. Mater. 25 , 2773–2778 (2013).
Kim, Y. S. et al. All‐in‐one, wireless, stretchable hybrid electronics for smart, connected, and ambulatory physiological monitoring. Adv. Sci. 6 , 1900939 (2019).
Fan, J. A. et al. Fractal design concepts for stretchable electronics. Nat. Commun. 5 , 3266 (2014).
Arumugam, V., Naresh, M. D. & Sanjeevi, R. Effect of strain rate on the fracture behaviour of skin. J. Biosci. 19 , 307–313 (1994).
Lund, C. & Tucker, J. A. in Neonatal Skin: Structure and Function 2nd edn (eds. Hoath, S. B. & Maibach, H. I.) 299–324 (Marcel Dekker, Inc., 2003).
Pocius, A. V. The relationship of Fundamental Forces of Adhesion and Practical Adhesion. In Adhesion and Adhesives Technology: An Introduction 3rd edn. 99–103 (Hanser Publications, Cincinnati, Ohio, 2012).
Chung, H. U. et al. Skin-interfaced biosensors for advanced wireless physiological monitoring in neonatal and pediatric intensive-care units. Nat. Med. 26 , 418–429 (2020).
Article CAS PubMed PubMed Central Google Scholar
Xue, Y. et al. Trigger‐detachable hydrogel adhesives for bioelectronic interfaces. Adv. Funct. Mater. 31 , 2106446 (2021).
Kwak, S. S. et al. Skin‐integrated devices with soft, holey architectures for wireless physiological monitoring, with applications in the neonatal intensive care unit. Adv. Mater. 33 , 2103974 (2021).
Wang, C. et al. Mussel inspired trigger-detachable adhesive hydrogel. Small 18 , 2200336 (2022).
Swanson, S. et al. Prototype development of a temperature-sensitive high-adhesion medical tape to reduce medical-adhesive-related skin injury and improve quality of care. Int. J.Mol. Sci. 23 , 7164 (2022).
Jinkins, K. R. et al. Thermally switchable, crystallizable oil and silicone composite adhesives for skin-interfaced wearable devices. Sci. Adv. 8 , eabo0537 (2022).
Jakubas, A., Łada-Tondyra, E. & Nowak, M. Textile sensors used in smart clothing to monitor the vital functions of young children. In: 2017 Progress in Applied Electrical Engineering (PAEE) 25–30 June 2017, 1–4. https://doi.org/10.1109/PAEE.2017.8008989 (2017).
Jakubas, A. & Łada-Tondyra, E. A study on application of the ribbing stitch as sensor of respiratory rhythm in smart clothing designed for infants. J. Textile Inst. 109 , 1208–1216 (2018).
Bouwstra, S., Chen, W., Feijs, L. & Oetomo, S. B. Smart jacket design for neonatal monitoring with wearable sensors. In: 2009 Sixth International Workshop on Wearable and Implantable Body Sensor Networks 3–5 June 2009, 162–167. https://doi.org/10.1109/BSN.2009.40 (2009).
Alzaidi, A. Bajwa, H. Patra, P. & Zhang, L. Noncontact Textile Electrodes for Wireless ECG System 1–5 (2013).
Linti, C., Horter, H. Osterreicher, P. & Planck, H. Sensory baby vest for the monitoring of infants. In: International Workshop on Wearable and Implantable Body Sensor Networks (BSN'06) 3–5 April 2006, 3–137 https://doi.org/10.1109/BSN.2006.49 (2006).
Cay, G. et al. Baby-guard: an IoT-based neonatal monitoring system integrated with smart textiles. In 2021 IEEE International Conference on Smart Computing (SMART COMP) . 129–136 (IEEE, 2023).
Chen, W. et al. Design of an integrated sensor platform for vital sign monitoring of newborn infants at neonatal intensive care units. J. Healthcare Eng. 1 , 124270 (2010).
Patron, D. et al. On the use of knitted antennas and inductively coupled RFID tags for wearable applications. IEEE Trans. Biomed. Circuits Syst. 10 , 1047–1057 (2016).
Pan, J. & Tompkins, W. J. A real-time QRS detection algorithm. IEEE Trans. Biomed. Eng. 32 , 230–236 (1985).
Kim, Y. S. et al. Wireless, skin-like membrane electronics with multifunctional ergonomic sensors for enhanced pediatric care. IEEE Trans. Biomed. Eng. 67 , 2159–2165 (2020).
Chung, H. U. et al. Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care. Science 363 , 6430 (2019).
Iyer, K. K. et al. Early detection of preterm intraventricular hemorrhage from clinical electroencephalography Crit. Care Med. 43 , [Online]. Available: https://journals.lww.com/ccmjournal/fulltext/2015/10000/early_detection_of_preterm_intraventricular.21.aspx (2015).
El-Dib, M. et al. Neuromonitoring in neonatal critical care part II: extremely premature infants and critically ill neonates. Pediatric Res. 94 , 55–63 (2023).
Ibrahim, Z. H. et al. Wireless multichannel electroencephalography in the newborn. J. Neonatal-Perinatal Med. 9 , 341–348 (2016).
Asayesh, A., Ilen, E., Metsäranta, M. & Vanhatalo, S. Developing disposable EEG cap for infant recordings at the neonatal intensive care unit. Sensors 22 , 7869 (2022).
Askari, S., Bastany, Z., Holsti, L. & Dumont, G. D. Lighting up babies’ brains: development of a combined NIRS/EEG system for infants. In Proc. SPIE 11638, Biophotonics in Exercise Science, Sports Medicine, Health Monitoring Technologies, and Wearables II (eds Shadgan, B. & Gandjbakhche, A. H.) 116380N (SPIE, 2021).
Wong, J. N. et al. A comprehensive wireless neurological and cardiopulmonary monitoring platform for pediatrics. PLOS Digital Health 2 , e0000291 (2023).
Article PubMed PubMed Central Google Scholar
Mullen, T. R. et al. Real-time neuroimaging and cognitive monitoring using wearable dry EEG. IEEE Trans. Biomed. Eng. 62 , 2553–2567 (2015).
Grozea, C., Voinescu, C. D. & Fazli, S. Bristle-sensors-low-cost flexible passive dry EEG electrodes for neurofeedback and BCI applications. J. Neural Eng. 8 , 025008 (2011).
Beć, K. B., Grabska, J. & Huck, C. W. Near-infrared spectroscopy in bio-applications. Molecules 25 , 2948 (2020).
Kumar, N., Akangire, G., Sullivan, B., Fairchild, K. & Sampath, V. Continuous vital sign analysis for predicting and preventing neonatal diseases in the twenty-first century: big data to the forefront. Pediatric Res. 87 , 210–220 (2020).
Lee, H. et al. A new algorithm for detecting central apnea in neonates. Physiol. Meas. 33 , 1–17 (2012).
Park, J., Seok, H. S., Kim, S.-S. & Shin, H. Photoplethysmogram analysis and applications: an integrative review. Front. Physiol . 12 , 808451 (2022).
Fernandez, M. et al. Evaluation of a new pulse oximeter sensor. Am. J. Crit. Care 16 , 146–152 (2007).
Grubb, M. R. et al. Forehead reflectance photoplethysmography to monitor heart rate: preliminary results from neonatal patients. Physiol. Meas. 35 , 881 (2014).
Henry, C. et al. Accurate neonatal heart rate monitoring using a new wireless, cap mounted device. Acta Paediatrica 110 , 72–78 (2021).
Zonios, G., Bykowski, J. & Kollias, N. Skin melanin, hemoglobin, and light scattering properties can be quantitatively assessed <em>in vivo</em> using diffuse reflectance spectroscopy. J. Investig. Dermatol. 117 , 1452–1457 (2001).
Gottlieb, E. R., Ziegler, J., Morley, K., Rush, B. & Celi, L. A. Assessment of racial and ethnic differences in oxygen supplementation among patients in the intensive care unit. JAMA Internal Med. 182 , 849–858, (2022).
Cabanas, A. M., Fuentes-Guajardo, M., Latorre, K., Leon, D. & Martin-Escudero, P. Skin pigmentation influence on pulse oximetry accuracy: a systematic review and bibliometric analysis. Sensors (Basel) 22 , https://doi.org/10.3390/s22093402 (2022).
Harris, B. U. et al. Accuracy of pulse oximeters intended for hypoxemic pediatric patients. Pediatric Crit. Care Med . 17 (2016). Available: https://journals.lww.com/pccmjournal/fulltext/2016/04000/accuracy_of_pulse_oximeters_intended_for_hypoxemic.6.aspx .
Sood, B. G., McLaughlin, K. & Cortez, J. Near-infrared spectroscopy: applications in neonates. Semin. Fetal Neonatal Med. 20 , 164–172 (2015).
Schober, P., Lust, E. J., Heunks, L. M. A. & Schwarte, L. A. Thinking out-of-the-box: a non-standard application of standard pulse-oximetry and standard near-infrared spectroscopy in a COVID-19 patient. Journal of Intensive Care Medicine 36 , 376–380 (2021).
Howard, R. et al. Optical monitoring in neonatal seizures (in eng). Cells 11 , https://doi.org/10.3390/cells11162602 (2022).
van Bel, F. & Mintzer, J. P. Monitoring cerebral oxygenation of the immature brain: a neuroprotective strategy? Pediatric Res. 84 , 159–164 (2018).
Lapointe, A. P. et al. Cerebral hemodynamics and microvasculature changes in relation to white matter microstructure after pediatric mild traumatic brain injury: an A-CAP pilot study (in eng). Neurotrauma Rep. 4 , 64–70 (2023).
Rwei, A. Y. et al. A wireless, skin-interfaced biosensor for cerebral hemodynamic monitoring in pediatric care. Proc. Natl Acad. Sci. 117 , 31674–31684 (2020).
Inamori, G. et al. Neonatal wearable device for colorimetry-based real-time detection of jaundice with simultaneous sensing of vitals. Sci. Adv. 7 , eabe3793 (2021).
Desmond, F. A. & Namachivayam, S. Does near-infrared spectroscopy play a role in paediatric intensive care? BJA Educ. 16 , 281–285 (2016).
World Health Organization. Thermal Protection of the Newborn: A Practical Guide (WHO, 1997).
Ma, Y. et al. Soft elastomers with ionic liquid‐filled cavities as strain isolating substrates for wearable electronics. Small 13 , 1602954 (2017).
Lodha, R., Mukerji, N., Sinha, N., Pandey, R. M. & Jain, Y. Is axillary temperature an appropriate surrogate for core temperature? Indian J. Pediatrics 67 , 571–574 (2000).
McCarthy, L. K. & O'Donnell, C. P. F. Comparison of rectal and axillary temperature measurements in preterm newborns. Arch. Dis. Childhood—Fetal Neonatal Edn 106 , 509 (2021).
Ji, Y., Han, D., Han, L., Xie, S. & Pan, S. The accuracy of a wireless axillary thermometer for core temperature monitoring in pediatric patients having noncardiac surgery: an observational study. J. PeriAnesthesia Nursing 36 , 685–689 (2021).
Atallah, L., Bongers, E., Lamichhane, B. & Bambang-Oetomo, S. Unobtrusive monitoring of neonatal brain temperature using a zero-heat-flux sensor matrix. IEEE J. Biomed. Health Informatics 20 , 100–107 (2016).
Mellergård, P. Intracerebral temperature in neurosurgical patients: Intracerebral temperature gradients and relationships to consciousness level. Surg. Neurol. 43 , 91–95 (1995).
Teunissen, L. P., Klewer, J., de Haan, A., de Koning, J. J. & Daanen, H. A. Non-invasive continuous core temperature measurement by zero heat flux. Physiol. Meas. 32 , 559–570 (2011).
Zeiner, A. et al. Non-invasive continuous cerebral temperature monitoring in patients treated with mild therapeutic hypothermia: an observational pilot study. Resuscitation 81 , 861–866 (2010).
Oh, S. et al. Simple, miniaturized biosensors for wireless mapping of thermoregulatory responses. Biosens. Bioelectron. 237 , 115545 (2023).
Klunk, C. J. et al. An initiative to decrease laboratory testing in a NICU. Pediatrics 148 (2021).
García-Carmona, L. et al. Pacifier biosensor: toward noninvasive saliva biomarker monitoring. Anal. Chem. 91 , 13883–13891 (2019).
Lim, H.-R. et al. Smart bioelectronic pacifier for real-time continuous monitoring of salivary electrolytes. Biosensors Bioelectron. 210 , 114329 (2022).
Parrilla, M., Vanhooydonck, A., Watts, R. & De Wael, K. Wearable wristband-based electrochemical sensor for the detection of phenylalanine in biofluids. Biosensors Bioelectron. 197 , 113764 (2022).
Emaminejad, S. et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl Acad. Sci. 114 , 4625–4630 (2017).
Lee, H., Hong, Y. J., Baik, S., Hyeon, T. & Kim, D. H. Enzyme‐based glucose sensor: from invasive to wearable device. Adv. Healthcare Mater. 7 , 1701150 (2018).
Ning, Z., Long, Z., Yang, G., Xing, L. & Xue, X. Self-powered wearable biosensor in a baby diaper for monitoring neonatal jaundice through a hydrovoltaic-biosensing coupling effect of ZnO nanoarray. Biosensors 12 , 164 (2022).
Galland, B. C., Tan, E. & Taylor, B. J. Pulse transit time and blood pressure changes following auditory-evoked subcortical arousal and waking of infants. Sleep 30 , 891–897 (2007).
Ganti, V. G. et al. Wearable seismocardiography‐based assessment of stroke volume in congenital heart disease. J. Am. Heart Assoc. 11 , https://doi.org/10.1161/jaha.122.026067 (2022).
Yoo, J.-Y. et al. Wireless broadband acousto-mechanical sensing system for continuous physiological monitoring. Nat. Med. https://doi.org/10.1038/s41591-023-02637-5 (2023).
Singh, M. & Patterson, D. J. Involuntary gesture recognition for predicting cerebral palsy in high-risk infants. In International Symposium on Wearable Computers (ISWC) 2010. 1–8 (IEEE, Seoul, Korea (South), 2010).
Jeong, H. et al. Miniaturized wireless, skin-integrated sensor networks for quantifying full-body movement behaviors and vital signs in infants. Proc. Natl Acad. Sci. 118 , e2104925118 (2021).
Lee S. H. et al. Fully portable continuous real-time auscultation with a soft wearable stethoscope designed for automated disease diagnosis. Sci. Adv . https://doi.org/10.1126/sciadv.abo5867 (2022).
Wang, C. et al. Monitoring of the central blood pressure waveform via a conformal ultrasonic device. Nat. Biomed. Eng. 2 , 687–695 (2018).
Malon, R. S., Chua, K. Y., Wicaksono, D. H. & Corcoles, E. P. Cotton fabric-based electrochemical device for lactate measurement in saliva. Analyst 139 , 3009–3016 (2014).
Balayan, S., Chauhan, N., Chandra, R. & Jain, U. Electrochemical based C-reactive protein (CRP) sensing through molecularly imprinted polymer (MIP) pore structure coupled with Bi-metallic tuned screen-printed electrode. Biointerface Res. Appl. Chem. 12 , 7697–7714 (2021).
Parnianchi, F. et al. Ultrasensitive electrochemical sensor based on molecular imprinted polymer and ferromagnetic nanocomposite for bilirubin analysis in the saliva and serum of newborns. Microchem. J. 179 , 107474 (2022).
Guess, M. et al. Wireless batteryless soft sensors for ambulatory cardiovascular health monitoring. Soft Sci. 3 , 24 (2023).
Ha, T. et al. A chest‐laminated ultrathin and stretchable E‐tattoo for the measurement of electrocardiogram, seismocardiogram, and cardiac time intervals. Adv. Sci. 6 , 1900290 (2019).
Bonner, O., Beardsall, K., Crilly, N. & Lasenby, J. There were more wires than him’: the potential for wireless patient monitoring in neonatal intensive care. BMJ Innovations 3 , 12–18 (2017).
Johnston, C. C. et al. Kangaroo care is effective in diminishing pain response in preterm neonates. Arch. Pediatrics Adolescent Med. 157 , 1084–1088 (2003).
Charpak, N. et al. Kangaroo mother care: 25 years after. Acta Paediatrica 94 , 514–522 (2005).
Ray, T. R. et al. Soft, skin-interfaced sweat stickers for cystic fibrosis diagnosis and management. Sci. Transl. Med. 13 , eabd8109 (2021).
Ceran, C. et al. Management of pulse oximeter probe–induced finger injuries in children: report of two consecutive cases and review of the literature. J. Pediatric Surg. 47 , e27–e29 (2012).
Gao, W. et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529 , 509–514 (2016).
Man, P.-K. et al. Blood pressure measurement: from cuff-based to contactless monitoring. Healthcare 10 , https://doi.org/10.3390/healthcare10102113 .
Download references
Acknowledgements
The authors acknowledge the support of the National Institutes of Health (Grant No. R21EB034893). This work was also supported by the Imlay Foundation – Innovation Fund.
Author information
Authors and affiliations.
George W. Woodruff School of Mechanical Engineering, Georgia Institute of Technology, Atlanta, GA, 30332, USA
Lauren Zhou, Matthew Guess, Ka Ram Kim & Woon-Hong Yeo
IEN Center for Wearable Intelligent Systems and Healthcare, Institute for Electronics and Nanotechnology, Georgia Institute of Technology, Atlanta, GA, 30332, USA
Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory University School of Medicine, Atlanta, GA, 30332, USA
Woon-Hong Yeo
Parker H. Petit Institute for Bioengineering and Biosciences, Institute for Robotics and Intelligent Machines, Georgia Institute of Technology, Atlanta, GA, 30332, USA
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization, L.Z. and W.-H.Y.; writing—original draft preparation, L.Z., M.G., K.R.K. and W.-H.Y.; writing—review and editing, L.Z., M.G., K.R.K. and W.-H.Y.; supervision, W.-H.Y.; project administration, W.-H.Y.; funding acquisition, W.-H.Y. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Correspondence to Woon-Hong Yeo .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Communications Materials thanks Liu Tao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Rona Chandrawati and John Plummer. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Peer review file, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Zhou, L., Guess, M., Kim, K.R. et al. Skin-interfacing wearable biosensors for smart health monitoring of infants and neonates. Commun Mater 5 , 72 (2024). https://doi.org/10.1038/s43246-024-00511-6
Download citation
Received : 05 December 2023
Accepted : 23 April 2024
Published : 09 May 2024
DOI : https://doi.org/10.1038/s43246-024-00511-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
May 7, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
Examining care and coverage in academic health systems
by Kathryn Ryan, Mary Ann Liebert, Inc
A new study published in Population Health Management , which builds on previous work in the journal, describes the Academic Payvider model, a joint approach to care and coverage aimed at reforming the relationship between payers and providers to enhance value-based care.
"There is an undeniable need for reformation of the relationship between health care payers and providers," states Erika Harness, MHA, from the Sidney Kimmel Medical College at Thomas Jefferson University, and co-authors of the study. The Payvider model is one promising approach, with the partnership model of shared ownership considered to be the most effective. The current study examines "Academic Payviders," a term that describes academic health systems that provide health plans to patients.
The investigators reported rapid growth of Academic Payvider systems within the last two decades. "The growth of Academic Payviders is stimulated by ongoing policy and market factors," stated the investigators.
"Ultimately, this shift in payment models can aid patients and providers alike. The patient experiences benefit from improved coordination and integration, reduced insurance hassles, and increased staff attention to ensuring optimal outcomes. Simultaneously, providers experience reduced administrative burden and burnout."
In an accompanying editorial , Josh Berlin, Chief Executive Officer of Rule of Three, LLC, writes, "Whether the Academic Payvider is the or an answer to an industry fraught with challenges remains to be seen.
"The underlying paper notes the sample size is still relatively small by comparison to the quantity of academic institutions serving health care overall. Nevertheless, these relationships bring inspiration for the art of what is possible to drive complex, higher cost care down through innovative payment and reimbursement structures more effectively and efficiently managed with the expertise of such unique collaborators."
Josh M. Berlin, The Academic Payvider Model: Commentary, Population Health Management (2024). DOI: 10.1089/pop.2024.0058
Explore further
Feedback to editors

New research traces the spread of HIV in and from Indonesia

Metabolism of autism reveals developmental origins

Under 4-minute milers' longevity shows that extreme exercise doesn't seem to curb lifespan
12 hours ago

Study shows how night shift work can raise risk of diabetes, obesity
14 hours ago

'Smart' contact lenses could someday enable wireless glaucoma detection
15 hours ago

Low-cost MRI paired with AI produces high-quality results

Navy Growler jet noise over Washington state's Whidbey Island could impact 74,000 people's health
16 hours ago

Human brain map contains never-before-seen details of structure

What makes a public health campaign successful?

High school student helps transform 'crazy idea' into a model that can predict neurotransmitters
17 hours ago
Related Stories

Opinion: Person-centered health care means ensuring that affected communities are leaders and partners in research
Apr 2, 2024
Importance of physician-led team-based care underscored in new ACP policy
Dec 27, 2023
Bundled payments with co-pay waivers creates substantial cost savings
Mar 1, 2021

Transcendental meditation helps to alleviate burnout in academic physicians
Feb 17, 2023

Burnout, lack of fulfillment linked to intention to leave among physicians
Dec 21, 2023

Medicaid coverage of physical, behavioral health together does not improve access, care
Dec 29, 2023
Recommended for you

Telehealth group sessions can benefit clinician-patient relationships
19 hours ago

New research reports on financial entanglements between FDA chiefs and the drug industry
May 8, 2024

Economists imagine an alternate universe where the opioid crisis peaked in '06, and then explain why it didn't
May 6, 2024

Online patient portal usage increasing, study shows

Companies may still buy consumer genetic information despite its modest predictive power
May 2, 2024

Study finds private equity expanding to mental health facilities
May 1, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

Hydroxychloroquine has no effect on SARS-CoV-2 load in nasopharynx of patients with mild form of COVID-19
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- For correspondence: [email protected]
- Info/History
- Preview PDF
Due to the constantly growing numbers of COVID-19 infections and death cases attempts were undertaken to find drugs with anti SARS-CoV-2 activity among ones already approved for other pathologies. In the framework of such attempts, in a number of in vitro, as well as in vivo, models it was shown that hydroxychloroquine (HCQ) has an effect against SARS-CoV-2. While there was not enough clinical data to support the use of HCQ, several countries including Russia have included HCQ in treatment protocols for infected patients and for prophylactic. Here, we evaluated the SARS-CoV-2 RNA in nasopharynx swabs from infected patients in mild conditions and compared the viral RNA load dynamics between patients receiving HCQ and control group without antiviral pharmacological therapy. We found statistically significant relationship between maximal RNA quantity and patients’ deteriorating medical conditions, as well as confirmed the arterial hypertension to be a risk factor for people with COVID-19. However, we showed that HCQ therapy neither shortened the viral shedding period nor reduced the virus RNA load.
Competing Interest Statement
The authors have declared no competing interest.
Funding Statement
The study was funded by Moscow Department of Healthcare and by the Russian Science Foundation grant, agreement #18-15-00420.
Author Declarations
I confirm all relevant ethical guidelines have been followed, and any necessary IRB and/or ethics committee approvals have been obtained.
The details of the IRB/oversight body that provided approval or exemption for the research described are given below:
Local ethics committees approved the study protocol and all participants provided their written consent.
All necessary patient/participant consent has been obtained and the appropriate institutional forms have been archived.
I understand that all clinical trials and any other prospective interventional studies must be registered with an ICMJE-approved registry, such as ClinicalTrials.gov. I confirm that any such study reported in the manuscript has been registered and the trial registration ID is provided (note: if posting a prospective study registered retrospectively, please provide a statement in the trial ID field explaining why the study was not registered in advance).
I have followed all appropriate research reporting guidelines and uploaded the relevant EQUATOR Network research reporting checklist(s) and other pertinent material as supplementary files, if applicable.
Two patients were additionally included into the Control group. Absolute SARS-CoV-2 RNA copy number was estimated using viral genomic RNA and the results were compared to synthetic DNA standards. Additional statistics were included into the results. Figures were edited. Manuscript text was edited.
Data Availability
All data generated and analysed during the study are included in the article. Any additional information is available from the corresponding author on reasonable request.
View the discussion thread.
Thank you for your interest in spreading the word about medRxiv.
NOTE: Your email address is requested solely to identify you as the sender of this article.

Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager
- Tweet Widget
- Facebook Like
- Google Plus One
Subject Area
- Infectious Diseases (except HIV/AIDS)
- Addiction Medicine (322)
- Allergy and Immunology (626)
- Anesthesia (162)
- Cardiovascular Medicine (2352)
- Dentistry and Oral Medicine (286)
- Dermatology (206)
- Emergency Medicine (377)
- Endocrinology (including Diabetes Mellitus and Metabolic Disease) (832)
- Epidemiology (11740)
- Forensic Medicine (10)
- Gastroenterology (698)
- Genetic and Genomic Medicine (3712)
- Geriatric Medicine (347)
- Health Economics (632)
- Health Informatics (2383)
- Health Policy (928)
- Health Systems and Quality Improvement (890)
- Hematology (340)
- HIV/AIDS (776)
- Infectious Diseases (except HIV/AIDS) (13293)
- Intensive Care and Critical Care Medicine (767)
- Medical Education (364)
- Medical Ethics (104)
- Nephrology (397)
- Neurology (3470)
- Nursing (197)
- Nutrition (521)
- Obstetrics and Gynecology (669)
- Occupational and Environmental Health (661)
- Oncology (1809)
- Ophthalmology (534)
- Orthopedics (218)
- Otolaryngology (286)
- Pain Medicine (232)
- Palliative Medicine (66)
- Pathology (445)
- Pediatrics (1026)
- Pharmacology and Therapeutics (426)
- Primary Care Research (417)
- Psychiatry and Clinical Psychology (3163)
- Public and Global Health (6116)
- Radiology and Imaging (1268)
- Rehabilitation Medicine and Physical Therapy (740)
- Respiratory Medicine (824)
- Rheumatology (379)
- Sexual and Reproductive Health (371)
- Sports Medicine (320)
- Surgery (398)
- Toxicology (50)
- Transplantation (171)
- Urology (145)
Purdue Online Writing Lab Purdue OWL® College of Liberal Arts
Welcome to the Purdue Online Writing Lab

Welcome to the Purdue OWL
This page is brought to you by the OWL at Purdue University. When printing this page, you must include the entire legal notice.
Copyright ©1995-2018 by The Writing Lab & The OWL at Purdue and Purdue University. All rights reserved. This material may not be published, reproduced, broadcast, rewritten, or redistributed without permission. Use of this site constitutes acceptance of our terms and conditions of fair use.
The Online Writing Lab at Purdue University houses writing resources and instructional material, and we provide these as a free service of the Writing Lab at Purdue. Students, members of the community, and users worldwide will find information to assist with many writing projects. Teachers and trainers may use this material for in-class and out-of-class instruction.
The Purdue On-Campus Writing Lab and Purdue Online Writing Lab assist clients in their development as writers—no matter what their skill level—with on-campus consultations, online participation, and community engagement. The Purdue Writing Lab serves the Purdue, West Lafayette, campus and coordinates with local literacy initiatives. The Purdue OWL offers global support through online reference materials and services.
A Message From the Assistant Director of Content Development
The Purdue OWL® is committed to supporting students, instructors, and writers by offering a wide range of resources that are developed and revised with them in mind. To do this, the OWL team is always exploring possibilties for a better design, allowing accessibility and user experience to guide our process. As the OWL undergoes some changes, we welcome your feedback and suggestions by email at any time.
Please don't hesitate to contact us via our contact page if you have any questions or comments.
All the best,
Social Media
Facebook twitter.

IMAGES
VIDEO
COMMENTS
Health system research is a part of health research. It. closely links eld evidences and applies its results for the. improvement of the health system. Initially, it was known as. health service ...
The WHO evidence synthesis, published as a HEN report [], provides a firm basis for decision-making by policy-makers and research leaders looking to strengthen the health research system in their country.It identifies five crucial policy approaches that can be applied as appropriate to the context of the country - conducting situation analyses, sustaining a comprehensive strategy, engaging ...
Health Services Research and Primary Care Research. AHRQ convened an interactive roundtable meeting of leaders in health systems, public health preparedness, and resiliency, facilitated by the Department of Health and Human Services Office of Climate Change and Health Equity interim director. The roundtable discussion laid the groundwork for advancing work on health system resilience, along ...
The focus of the research within a health system is explained more fully in the following sections.. The users of the research outputs (published results, findings, methodologies, etc.) fall broadly into three groups with operational research being predominantly, but not exclusively, of use to health care providers; implementation research predominantly of use to managers of programmes scaling ...
Health policy and systems research (HPSR) has changed considerably over the last 20 years, but its main purpose remains to inform and influence health policies and systems. Whereas goals that underpin health systems have endured - such as a focus on health equity - contexts and priorities change, research methods progress, and health organisations continue to learn and adapt, in part by ...
Health research is important for the achievement of the Sustainable Development Goals. However, there are many challenges facing health research, including securing sufficient funds, building capacity, producing research findings and using both local and global evidence, and avoiding waste. A WHO initiative addressed these challenges by ...
Introduction. The field of Health Policy and Systems Research (HPSR) is currently experiencing an unprecedented level of interest. The First Global Symposium on Health Systems Research, held in Montreux, Switzerland, in November 2010, is the most recent of a succession of conferences and task force deliberations that have spun off a series of debates about the nature of the field and the ...
Health systems resilience is key to learning lessons from country responses to crises such as coronavirus disease 2019 (COVID-19). In this perspective, we review COVID-19 responses in 28 countries ...
It is this importance of context in health system reform, that makes research papers based on specific experiences so valuable. In light of this, we have put together this white paper that summarises key articles published in the field of health systems strengthening. They represent work carried out in different regions,
Gilson L, ed. (2012). Health Policy and Systems Research: A Methodology Reader - The Abridged Version Alliance for Health Policy and Systems Research, World Health Organization 6 Part 2 Part 3 Part 4 Part 5 Doing HPSR: Key steps in the process Understanding Health Policy and Systems. References for empirical papers.....
History of Health Services Research. The history of HSR is generally considered to have begun in the 1950s and 1960s with the first funding of grants for health services research focused on the impact of hospital organizations. 19, 20 On the contrary, HSR began with Florence Nightingale when she collected and analyzed data as the basis for improving the quality of patient care and outcomes. 21 ...
Methods and findings. Two independent investigators systematically searched the Medical Literature Analysis and Retrieval System Online (MEDLINE), the Excerpta Medica Database (EMBASE), the Cumulative Index to Nursing and Allied Health Literature (CINAHL+), the Health Management Information Consortium, and the Journal of Research Evaluation from inception until May 2017 for publications that ...
Special Issue on Age-Friendly Health Systems Health Services Research and The John A. Hartford Foundation have partnered to publish a special issue on Age-Friendly Health Systems. Read Papers Health Services Research. Impacting Health Practice and Policy Through State-of-the-Art Research and Thinking. HSR is now online only. ...
Background Accelerated by the coronavirus disease 2019 (Covid-19) pandemic, major and lasting changes are occuring in healthcare structures, impacting people's experiences and value creation in all aspects of their lives. Information systems (IS) research can support analysing and anticipating resulting effects. Aim The purpose of this study is to examine in what areas health information ...
Research Goals. Current health challenges are often related to our modern way of living. High blood pressure, high glucose levels, and physical inactivity are all linked to a modern lifestyle characterized by sedentary living, chronic stress, or a high intake of energy-dense foods and recreational drugs [].Moreover, people usually make poor decisions related to their health for distinct ...
Overview. Health policy and systems research (HPSR) is an emerging field that seeks to understand and improve how societies organize themselves in achieving collective health goals, and how different actors interact in the policy and implementation processes to contribute to policy outcomes. By nature, it is inter-disciplinary, a blend of ...
The scope of a health system is so broad that it is more appropriate to model it as a system of systems, which has a diverse number of agents and uncountable interactions [44,45]. Similar to IOM [ 11 ], this paper defines a healthcare delivery system as activities that are directly involved in the provision, transaction, and consumption of ...
Systematic and rigorous inquiry allows us to discover the fundamental mechanisms and causes of disease and disparities. At our Office of Research (research@BSPH), we translate that knowledge to develop, evaluate, and disseminate treatment and prevention strategies and inform public health practice.Research along this entire spectrum represents a fundamental mission of the Johns Hopkins ...
10 Institute of Social Medicine and Health Systems Research, Faculty of Medicine, Otto von Guericke University Magdeburg, Magdeburg, Germany. 11 Institute for Urology and Reproductive Health, Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia.
Introduction: The digitalisation of the German healthcare system enables a wide range of opportunities to utilize healthcare data. The implementation of the EHR in January 2021 was a significant step, but compared to other European countries, the implementation of the EHR in the German healthcare system is still at an early stage. The aim of this paper is to characterise the structural factors ...
The research team underwent research training with a qualitative research expert to develop the data analysis plan. We organized the focus group question guide for the semi-structured interviews into system, provider, community, and individual level barriers, according to an existing healthcare disparities theory [ 21 ] (Table 1 ).
1. Introduction. Health information systems (HIS) are critical systems deployed to help organizations and all stakeholders within the healthcare arena eradicate disjointed information and modernize health processes by integrating different health functions and departments across the healthcare arena for better healthcare delivery [1,2,3,4,5,6].Over time, the HIS has transformed significantly ...
Wearable sensors have been widely studied, but research has tended to focus on their use in adults. This Review explores skin-interfacing smart health systems that are designed with infants and ...
More information: Erika D. Harness et al, The Academic Payvider Model: Care and Coverage, Population Health Management (2024).DOI: 10.1089/pop.2023.0300. Josh M ...
This report describes a multilevel city-wide profile of physical health in Moscow, examining individual and urban level factors. Objectives of the paper were to: (1) identify macro and micro risk factors for poor physical health in Moscow;(2) assess the effect of two dimensions of micro determinants — personal health habits and social connectivity, such as social cohesion, social support ...
The key objective of this research paper is to provide a comprehensive survey related to IoT-cloud-Artificial Intelligent-Machine Learning-Deep learning healthcare systems (i.e., smart healthcare). The rest of the study is organized as follows: Section 2 discusses related work. Section 3 explains background study.
Due to the constantly growing numbers of COVID-19 infections and death cases attempts were undertaken to find drugs with anti SARS-CoV-2 activity among ones already approved for other pathologies. In the framework of such attempts, in a number of in vitro, as well as in vivo, models it was shown that hydroxychloroquine (HCQ) has an effect against SARS-CoV-2.
The COVID-19 pandemic and related lockdowns around the world led to a general decline in physical and mental health because of isolation, lack of social interaction, restriction of movement and travel, and dramatic lifestyle changes [].The COVID-19 pandemic also demonstrated the importance of having access to green and blue spaces for human health and well-being during pandemics [2,3,4].
The Online Writing Lab at Purdue University houses writing resources and instructional material, and we provide these as a free service of the Writing Lab at Purdue.