July 26, 2011

The Science Behind Dreaming
New research sheds light on how and why we remember dreams--and what purpose they are likely to serve
By Sander van der Linden

Getty Images
For centuries people have pondered the meaning of dreams. Early civilizations thought of dreams as a medium between our earthly world and that of the gods. In fact, the Greeks and Romans were convinced that dreams had certain prophetic powers. While there has always been a great interest in the interpretation of human dreams, it wasn’t until the end of the nineteenth century that Sigmund Freud and Carl Jung put forth some of the most widely-known modern theories of dreaming. Freud’s theory centred around the notion of repressed longing -- the idea that dreaming allows us to sort through unresolved, repressed wishes. Carl Jung (who studied under Freud) also believed that dreams had psychological importance, but proposed different theories about their meaning.
Since then, technological advancements have allowed for the development of other theories. One prominent neurobiological theory of dreaming is the “activation-synthesis hypothesis,” which states that dreams don’t actually mean anything: they are merely electrical brain impulses that pull random thoughts and imagery from our memories. Humans, the theory goes, construct dream stories after they wake up, in a natural attempt to make sense of it all. Yet, given the vast documentation of realistic aspects to human dreaming as well as indirect experimental evidence that other mammals such as cats also dream, evolutionary psychologists have theorized that dreaming really does serve a purpose. In particular, the “threat simulation theory” suggests that dreaming should be seen as an ancient biological defence mechanism that provided an evolutionary advantage because of its capacity to repeatedly simulate potential threatening events – enhancing the neuro-cognitive mechanisms required for efficient threat perception and avoidance.
So, over the years, numerous theories have been put forth in an attempt to illuminate the mystery behind human dreams, but, until recently, strong tangible evidence has remained largely elusive.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Yet, new research published in the Journal of Neuroscience provides compelling insights into the mechanisms that underlie dreaming and the strong relationship our dreams have with our memories. Cristina Marzano and her colleagues at the University of Rome have succeeded, for the first time, in explaining how humans remember their dreams. The scientists predicted the likelihood of successful dream recall based on a signature pattern of brain waves. In order to do this, the Italian research team invited 65 students to spend two consecutive nights in their research laboratory.
During the first night, the students were left to sleep, allowing them to get used to the sound-proofed and temperature-controlled rooms. During the second night the researchers measured the student’s brain waves while they slept. Our brain experiences four types of electrical brain waves: “delta,” “theta,” “alpha,” and “beta.” Each represents a different speed of oscillating electrical voltages and together they form the electroencephalography (EEG). The Italian research team used this technology to measure the participant’s brain waves during various sleep-stages. (There are five stages of sleep; most dreaming and our most intense dreams occur during the REM stage.) The students were woken at various times and asked to fill out a diary detailing whether or not they dreamt, how often they dreamt and whether they could remember the content of their dreams.
While previous studies have already indicated that people are more likely to remember their dreams when woken directly after REM sleep, the current study explains why. Those participants who exhibited more low frequency theta waves in the frontal lobes were also more likely to remember their dreams.
This finding is interesting because the increased frontal theta activity the researchers observed looks just like the successful encoding and retrieval of autobiographical memories seen while we are awake. That is, it is the same electrical oscillations in the frontal cortex that make the recollection of episodic memories (e.g., things that happened to you) possible. Thus, these findings suggest that the neurophysiological mechanisms that we employ while dreaming (and recalling dreams) are the same as when we construct and retrieve memories while we are awake.
In another recent study conducted by the same research team, the authors used the latest MRI techniques to investigate the relation between dreaming and the role of deep-brain structures. In their study, the researchers found that vivid, bizarre and emotionally intense dreams (the dreams that people usually remember) are linked to parts of the amygdala and hippocampus. While the amygdala plays a primary role in the processing and memory of emotional reactions, the hippocampus has been implicated in important memory functions, such as the consolidation of information from short-term to long-term memory.
The proposed link between our dreams and emotions is also highlighted in another recent study published by Matthew Walker and colleagues at the Sleep and Neuroimaging Lab at UC Berkeley, who found that a reduction in REM sleep (or less “dreaming”) influences our ability to understand complex emotions in daily life – an essential feature of human social functioning. Scientists have also recently identified where dreaming is likely to occur in the brain. A very rare clinical condition known as “Charcot-Wilbrand Syndrome” has been known to cause (among other neurological symptoms) loss of the ability to dream. However, it was not until a few years ago that a patient reported to have lost her ability to dream while having virtually no other permanent neurological symptoms. The patient suffered a lesion in a part of the brain known as the right inferior lingual gyrus (located in the visual cortex). Thus, we know that dreams are generated in, or transmitted through this particular area of the brain, which is associated with visual processing, emotion and visual memories.
Taken together, these recent findings tell an important story about the underlying mechanism and possible purpose of dreaming.
Dreams seem to help us process emotions by encoding and constructing memories of them. What we see and experience in our dreams might not necessarily be real, but the emotions attached to these experiences certainly are. Our dream stories essentially try to strip the emotion out of a certain experience by creating a memory of it. This way, the emotion itself is no longer active. This mechanism fulfils an important role because when we don’t process our emotions, especially negative ones, this increases personal worry and anxiety. In fact, severe REM sleep-deprivation is increasingly correlated to the development of mental disorders. In short, dreams help regulate traffic on that fragile bridge which connects our experiences with our emotions and memories.
Are you a scientist who specializes in neuroscience, cognitive science, or psychology? And have you read a recent peer-reviewed paper that you would like to write about? Please send suggestions to Mind Matters editor Gareth Cook, a Pulitzer prize-winning journalist at the Boston Globe. He can be reached at garethideas AT gmail.com or Twitter @garethideas .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 19 October 2012
Scientists read dreams
- Mo Costandi
Nature ( 2012 ) Cite this article
4806 Accesses
1 Citations
733 Altmetric
Metrics details
- Brain imaging
- Computational neuroscience
- Neuroscience
Brain scans during sleep can decode visual content of dreams.
Scientists have learned how to discover what you are dreaming about while you sleep.
A team of researchers led by Yukiyasu Kamitani of the ATR Computational Neuroscience Laboratories in Kyoto, Japan, used functional neuroimaging to scan the brains of three people as they slept, simultaneously recording their brain waves using electroencephalography (EEG).

The researchers woke the participants whenever they detected the pattern of brain waves associated with sleep onset, asked them what they had just dreamed about, and then asked them to go back to sleep.
This was done in three-hour blocks, and repeated between seven and ten times, on different days, for each participant. During each block, participants were woken up ten times per hour. Each volunteer reported having visual dreams six or seven times every hour, giving the researchers a total of around 200 dream reports.
Perchance to dream
Most of the dreams reflected everyday experiences, but some contained unusual content, such as talking to a famous actor. The researchers extracted key words from the participants’ verbal reports, and picked 20 categories — such as 'car', 'male', 'female', and 'computer' — that appeared most frequently in their dream reports.
Kamitani and his colleagues then selected photos representing each category, scanned the participants’ brains again while they viewed the images, and compared brain activity patterns with those recorded just before the participants were woken up.
The researchers analysed activity in brain areas V1, V2 and V3, which are involved in the earliest stages of visual processing and encode basic features of visual scenes, such as contrast and the orientation of edges. They also looked at several other regions that are involved in higher order visual functions, such as object recognition.
In 2008, Kamitani and his colleagues reported that they could decode brain activity associated with the earliest stages of visual processing to reconstruct images shown to participants. Now, they have found that activity in the higher order brain regions could accurately predict the content of the participants’ dreams.
“We built a model to predict whether each category of content was present in the dreams,” says Kamitani. “By analysing the brain activity during the nine seconds before we woke the subjects, we could predict whether a man is in the dream or not, for instance, with an accuracy of 75–80%.”
The findings, presented at the annual meeting of the Society for Neuroscience in New Orleans, Louisiana, earlier this week, suggest that dreaming and visual perception share similar neural representations in the higher order visual areas of the brain.
“This is an interesting and exciting piece of work,” says neuroscientist Jack Gallant at the University of California, Berkeley, of the work presented at the meeting. “It suggests that dreaming involves some of the same higher level visual brain areas that are involved in visual imagery.”
“It also seems to suggest that our recall of dreams is based on short-term memory, because dream decoding was most accurate in the tens of seconds before waking,” he adds.
Kamitani and his colleagues are now trying to collect the same kind of data from the rapid eye movement (REM) stage of sleep, which is also associated with dreaming. “This is more challenging because we have to wait at least one hour before sleeping subjects reach that stage,” Kamitani says.
But the extra effort will be worth it, he says. “Knowing more about the content of dreams and how it relates to brain activity may help us to understand the function of dreaming.”
Miyawaki, Y. et al. Neuron 60 , 915-929 (2008).
Article CAS Google Scholar
Download references
You can also search for this author in PubMed Google Scholar
Related links
Related links in nature research.
Brain imaging: fMRI 2.0 2012-Apr-04
Voicegrams transform brain activity into words 2012-Jan-31
Mind-reading with a brain scan 2008-Mar-05
Related external links
Yukiyasu Kamitani
Society for Neuroscience annual meeting
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Costandi, M. Scientists read dreams. Nature (2012). https://doi.org/10.1038/nature.2012.11625
Download citation
Published : 19 October 2012
DOI : https://doi.org/10.1038/nature.2012.11625
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Subscribe or renew today
Every print subscription comes with full digital access
Science News
Here’s what lucid dreamers might tell us about our sleeping minds.
Dreams are one of the most universal yet elusive human experiences

Most people rarely lucid dream. But some people can do it regularly and even gain control over these alternate realities.
RUNE FISKER
Share this:
By Maria Temming
August 27, 2023 at 9:00 am
When Christopher Mazurek realizes he’s dreaming, it’s always the small stuff that tips him off.
The first time it happened, Mazurek was a freshman at Northwestern University in Evanston, Ill. In the dream, he found himself in a campus dining hall. It was winter, but Mazurek wasn’t wearing his favorite coat.
“I realized that, OK, if I don’t have the coat, I must be dreaming,” Mazurek says. That epiphany rocked the dream like an earthquake. “Gravity shifted, and I was flung down a hallway that seemed to go on for miles,” he says. “My left arm disappeared, and then I woke up.”
Most people rarely if ever realize that they’re dreaming while it’s happening, what’s known as lucid dreaming. But some enthusiasts have cultivated techniques to become self-aware in their sleep and even wrest some control over their dream selves and settings. Mazurek, 24, says that he’s gotten better at molding his lucid dreams since that first whirlwind experience, sometimes taking them as opportunities to try flying or say hi to deceased family members.
Other lucid dreamers have used their personal virtual realities to plumb their subconscious minds for insights or feast on junk food without real-world consequences. But now, scientists have a new job for lucid dreamers: to explore their dreamscapes and report out in real time.
Dream research has traditionally relied on reports collected after someone wakes up. But people often wake with only spotty, distorted memories of what they dreamed. The dreamers can’t say exactly when events occurred, and they certainly can’t tailor their dreams to specific scientific studies.
Gravity shifted, and I was flung down a hallway that seemed to go on for miles.… My left arm disappeared, and then I woke up. Christopher Mazurek

“The special thing about lucid dreaming is that you can get even closer to dream content and in a much more controlled and systematic fashion,” says Martin Dresler, a cognitive neuroscientist at the Donders Institute in Nijmegen, Netherlands.
Lucid dreamers who can perform assigned tasks and communicate with researchers during a dream open up tantalizing opportunities to study an otherwise untouchable realm. They are like the astronauts of the dream world, serving as envoys to the mysterious inner spaces created by slumbering minds.
So far, tests in very small groups of lucid dreamers suggest that the strange realities we visit in sleep may be experienced more like the real world than imagined ones. With more emissaries enlisted, researchers hope to probe how sleeping brains construct their elaborate, often bizarre plots and set pieces. Besides satisfying age-old curiosity, this work may point to new ways to treat nightmares. Lucid dream studies could also offer clues about how dreams contribute to creativity, regulating emotions or other cognitive jobs — helping solve the grand mystery of why we dream.
But there are still a lot of problems to solve before lucid dreaming research can really take off. Chief among them is that very few dreamers can become lucid on demand in the lab. Those who can often struggle to do scientists’ bidding or communicate with the waking world. Pinpointing the best techniques to give more people more lucid dreams may assuage those issues. But even if it does, not all scientists agree on what lucid dreams can tell us about the far more common, nonlucid kind.
Are lucid dreams real?
Tales of lucid dreams date back to antiquity. Aristotle may have been the first to mention them in Western literature in his treatise On Dreams . “Often when one is asleep,” he wrote, “there is something in consciousness which declares that what then presents itself is but a dream.”
If Aristotle had lucid dreams often, though, he was probably an outlier. Only about half of people say they’ve ever had a lucid dream , while a mere 1 percent or so say they lucid dream multiple times a week. Modern enthusiasts use various techniques to boost their likelihood of lucid dreaming — such as repeatedly telling themselves before bedtime that they will have a lucid dream, or making a habit of checking whether they’re awake several times a day in the hopes that this routine carries over into their dreams, where a self-check may help them realize they’re asleep. But those practices don’t guarantee lucidity.
The rarity of lucid dreaming may be why modern science took some convincing that it’s even real. For millennia, lucid dreamers’ own testimonies were the only evidence that someone could be self-aware while catching z’s. Some scientists wondered if so-called lucid dreams were just brief waking hallucinations between bouts of sleep.
But within the last few decades, experiments have offered proof that lucid dreams are truly what they seem. It turns out, when someone in a dream purposely sweeps their gaze all the way left, then all the way right, their eyes can match those movements behind closed lids in real life. These motions, measured by electrodes near the eyes, stand out from the smaller optical jitters typical of REM sleep, when most lucid dreams happen. This gives dreamers a crude way to signal they’ve become lucid or send other messages to the outside world ( SN: 9/19/81, p. 183 ). Meanwhile, brain waves and muscle paralysis throughout the rest of the body confirm that the dreamer is indeed asleep.
Eyes on eye movements
A person’s eyes can smoothly track left and right movements when they are awake or in a lucid dream. But when someone closes their eyes and tries to imagine tracking that motion, their eyes pan in small jumps, suggesting that lucid dreams are experienced more like waking perception.

Neuroscientists are just beginning to realize the potential of that line of communication. Lucid dream research “has been enjoying a renaissance over the last decade,” says neuroscientist Tore Nielsen. He directs the Dream & Nightmare Laboratory at the Center for Advanced Research in Sleep Medicine in Montreal. “This renaissance has made it one of the cutting-edge areas of dream study.”
One research team recently deployed experienced lucid dreamers to find out whether dream imagery is more like real-life visuals or imagined ones. While asleep, six lucid dreamers moved their thumbs in either a circle or a line (or both) and traced that motion with their eyes. Participants repeated the same task while awake with their eyes open and in their imaginations with their eyes closed. People’s gazes panned jerkily when they tracked the imagined movements, as though they were viewing something in low resolution. But in dreams, people’s eyes tracked the movements smoothly just as in real life, the team reported in 2018 in Nature Communications .
“It’s been debated really all the way back to the ancient Greeks, are dreams more like imagination, or is it more like perception?” says study coauthor Benjamin Baird, a cognitive psychologist and neuroscientist at the University of Texas at Austin. “The smooth tracking data suggests that, at least in that sense, the imagery is more like perception.”
This and other early experiments offer a taste of what dreamstronauts could teach us. But any conclusions based on just a handful of dreamers have to be taken with a grain of salt. “They’re more like proof-of-concept studies,” says Michelle Carr, a cognitive neuroscientist at the Center for Advanced Research in Sleep Medicine. “It needs to be studied in bigger samples.”
That means finding — or creating — more expert lucid dreamers.
Strategies for lucid dreaming
If you want to have a lucid dream, there are a few strategies you can use to up your chances. Besides regularly questioning whether you’re awake and setting an intention before bed to become lucid, you can keep a dream diary. Getting familiar with common characters, events or themes in your dreams may help you recognize when you’re dreaming. Some aspiring lucid dreamers also use a tactic called “wake-back-to-bed.” They wake up extremely early in the morning, stay up for a while, then get more shut-eye. That jolt of alertness right before tumbling back into REM sleep may help them become lucid in a dream.
Such techniques can be hit-or-miss, though. And data on their effectiveness are still pretty murky, Baird says. One study with about 170 Australians, for instance, suggested that checking if you’re awake, setting an intention to become lucid and doing wake-back-to-bed all together can increase your odds of lucid dreaming . But it wasn’t as clear if using just one or two of those practices worked.
Investigations by Baird and others have shown that the supplement galantamine promotes lucid dreaming , probably by fiddling with neurotransmitters involved in REM sleep. But galantamine can be saddled with side effects such as nausea. And although lucidity itself does not appear to spoil sleep quality , the long-term effects of using galantamine are not well-known. “Personally, I wouldn’t be mucking around with my neurotransmitters every night,” Baird says.
In 2020, Carr and colleagues reported that they’d coaxed 14 of 28 nappers to become lucid in the lab — including three people who’d never before lucid dreamed — no drugs necessary. Before falling asleep, participants learned to associate a cue, such as a series of beeps, with self-awareness. Hearing the same sound again while sleeping reminded them to become lucid. Carr is particularly interested in finding out whether lucid dreaming can help people conquer nightmares, but researchers at Northwestern use the sensory cue strategy to get more lucid emissaries to carry out dream tasks for their experiments.
Galantamine as a dream aid
For three nights, 121 people combined commonly used strategies for lucid dreaming with one of three doses of galantamine. Those who took higher doses of galantamine were more likely to have lucid dreams.
Effect of galantamine dose on likelihood of lucid dreaming

“Our method is kind of a shortcut,” says Northwestern cognitive neuroscientist Ken Paller. It doesn’t require a lot of mental training or the grueling sleep interruptions that some other lucid dreaming techniques do.
Another shortcut for researchers is to recruit dreamers from a special slice of the population: people with narcolepsy, who are liable to fall asleep suddenly during the day.
“They’re just champions at lucid dreams,” says Isabelle Arnulf, a sleep neurologist who heads the sleep disorders clinic at Pitie-Salpetriere University Hospital in Paris.
In 2018, Arnulf’s team reported a study where 18 of 21 narcolepsy patients signaled lucidity during lab naps . Even with those impressive numbers, a couple of lucid nappers still couldn’t control their dreams well enough to complete their assignment: to do something in a dream that made them briefly stop breathing, such as swimming underwater or speaking. One said after waking that they’d simply forgotten to stop breathing while diving off a cliff, while another said they tried to speak but couldn’t get any words out.
Staying lucid and successfully wrangling dream scenarios present challenges for lucid dreamers — and the scientists relying on them. In one study, lucid dreamers instructed to fill a dream room with objects, such as a clock and a rubber snake, ran into problems ; the clock spun wildly, or the snake slithered away. In another experiment, lucid dreamers asked to practice throwing darts were waylaid by only having pencils to throw or being pelted with darts by a nasty doll.
“It’s a lot harder than just passively lucid dreaming in your bed,” says Mazurek, who has participated in several lucid dream studies at Northwestern. “You realize, ‘OK, I have to stabilize the dream. I have to remember what the task is. I have to do the task without the dream falling apart.’ ”
Missions to the moon may be hard, but at least astronauts don’t have to worry about forgetting who or where they are, or their spaceship suddenly turning into a banana.
Despite these challenges, lucid dream expeditions are forging ahead — and fast. In fact, an international crew of dreamfarers, including Mazurek, recently embarked on their most ambitious mission yet.

Real-time dream science
When it comes to getting on-the-ground data, interviewing dreamers in real time is, well, the dream. Instead of just sitting back and watching dreamers do various activities, researchers could ask these agents about their experiences moment to moment, painting the realm of dreams in sharper detail than ever before.
“Reports of dreamed sensations, [such as] tasting certain foods, can be compared with those of actual sensations,” Nielsen says. “Similarly, one could test whether sexual pleasure, certain sounds or other types of experiences are accurately simulated.” These details, he says, might help “probe the limits and mechanisms of dream production.”
Karen Konkoly is especially excited about giving people assignments mid-dream. Say researchers want to know how much dreams help with creative problem-solving. If dreamers are assigned a problem before sleep, they’re liable to mull it over as they nod off. “Even if it feels like the lucid dream, maybe it’s really the time as you’re falling asleep that helped you solve the problem,” says Konkoly, a cognitive neuroscientist at Northwestern. Airdropping a puzzle straight into a dream could better isolate the usefulness of that specific part of sleep.
There’s a whole medley of theories about why people dream, from honing skills to tapping into creativity to processing memories or emotions. “But if you can’t control the dream in real time and then study the outcome, then you never know … if the dream is really doing anything,” Konkoly says. So a few years ago, she, Arnulf, Dresler and others decided to find out if people can receive and respond to outside input while dreaming.
Thirty-six people took snoozes at Northwestern, Arnulf’s lab, Dresler’s lab or another lab that was in Germany. Once sleepers signaled that they were lucid, researchers spoke yes-or-no questions or math problems in the sleepers’ ears. Or, for the Germans, lights flashing different colors conveyed math questions in Morse code. Before conking out, dreamers were told to answer whatever questions they received with eye signals or by smiling and frowning.
“Facial muscles are less inhibited than other muscles during REM sleep,” Arnulf explains. Someone smiling in a dream may not make that expression in real life, but electrodes on the face can register tiny corresponding muscle twitches.
Out of 158 attempts to interrogate lucid dreamers, 29 total correct responses came from six different people . Those six ranged from newbie to frequent lucid dreamers, including Mazurek, who heard scientists’ questions while dreaming he was in a Legend of Zelda game. The rest of the attempts yielded five wrong answers, 28 ambiguous ones and 96 nonresponses.
When Konkoly first saw someone correctly answer a question in their sleep, “my first reaction was to not believe it.” But for 26 of those 29 correct responses, a panel of independent sleep experts unanimously agreed that the dreamers were in the throes of REM sleep when they replied. Nearly 400 attempts to reach sleepers who hadn’t signaled lucidity netted a single correct response — bolstering the researchers’ confidence that correct answers from lucid dreamers weren’t flukes. The results appeared in 2021 in Current Biology .
Answering questions during a dream
While dreaming, Christopher Mazurek signaled the outside world by sweeping his eyes left and right. Electrodes on his face recorded those motions. On the graph below, Mazurek’s eye motions that indicate he is lucid appear as three big up-down sweeps. Eye signals answering “2” to researchers’ simple math question appear as two big up-down sweeps.
Lucid dreamer’s eye movements during a mid-dream conversation

“I was astonished,” says Robert Stickgold, a cognitive neuroscientist at Harvard Medical School who studies dreams but not lucid ones. “I had no question but that these people are in fact listening and are in fact having lucid dreams at the time of the communication — and that opens up all sorts of possibilities.”
Arnulf and others have since asked lucid dreamers to smile or frown as their dreams became more or less pleasant with the goal of understanding how dreamers experience emotion. Another study, not yet published, tracked when lucid dreamers answered or ignored researchers’ questions to see how people tuned in and out of the real world while dreaming. Knowing which signals break the dream-reality barrier could help “uncover the mechanism of the brain’s disconnection from the external world — which is huge,” Baird says. It could even be relevant for other states of unconsciousness, he adds, such as when someone is put under for surgery.
Limits of lucidity
Even if researchers get all the expert lucid dreamers they need to run all their desired experiments, there’s still one major sticking point to this whole field of study.
“The biggest issue is how far can you push these results to dreaming in general,” Stickgold says. Imagine, for instance, that lucid dreamers get better at a skill by practicing it in their dreams. It’s not clear that people who just happen to have normal dreams about doing those activities, without self-awareness, would reap the same rewards. “It’s a little bit like recruiting major league baseball players to give you some baseline data on how far people can throw balls,” Stickgold says.
Existing data do suggest that lucid dreamers may have access to parts of the brain that normal dreamers don’t. The lone case study comparing fMRIs of someone’s lucid and nonlucid REM sleep hints that brain areas linked with self-reflection and working memory are more active during lucidity. But those data come from just one person, and it’s not yet clear how such differences in brain activity would affect the outcomes of lucid dream experiments.
Brain clues to lucid dreams
Functional MRI scans of one sleeper’s brain during lucid and nonlucid sleep showed that some brain areas (highlighted) may be more active during lucid dreams than during normal sleep.
- The lateral parietal cortex is involved in working memory.
- The dorsolateral prefrontal cortex and frontopolar cortex are involved in working memory and introspection.
- Activity near the temporal cortex may make lucid dreams brighter and more detailed than normal dreams.

Some researchers, including Dresler, resist the idea that lucid dreams are profoundly different from nonlucid ones. “Lucid dreaming is not a strict all-or-nothing phenomenon,” he says, with people often fluttering in and out of awareness. “That suggests that lucid and nonlucid dreaming are in principle something very similar on the neural level and not two completely different animals.”
Perhaps lucidity affects some aspects of the dream experience but not all of them, Baird adds. In terms of how dreams look, he says, “it would be very, very surprising if it was somehow completely different when you become lucid.”
A more thorough inventory of the differences in brain activity between lucid and nonlucid dreams might help settle these questions. But even if lucid dreams don’t represent dreams in general, Nielsen still thinks they’re worth studying. “It is a type of consciousness that has intrigued and amused people for centuries,” he says. “It would be important for science to understand how and why humans have this extraordinary capacity for intentional world simulation.”
More Stories from Science News on Neuroscience

The heart plays a hidden role in our mental health

How smart was T. rex ?

Lampreys have ‘fight or flight’ cells, challenging ideas about nervous system evolution

Rat cells grew in mice brains, and helped sniff out cookies

These windpipe cells trigger coughs to keep water out of the lungs

Tiny treadmills show how fruit flies walk

In ‘Get the Picture,’ science helps explore the meaning of art

Chickadees use memory ‘bar codes’ to find their hidden food stashes
Subscribers, enter your e-mail address for full access to the Science News archives and digital editions.
Not a subscriber? Become one now .
Frontiers for Young Minds
- Download PDF
The Science of Dreams

Dreams are a common experience. Some are scary, some are funny. Recent research into how the brain works helps us understand why we dream. Strange combinations of ideas in our dreams may make us more creative and give us ideas that help us to solve problems. Or, when memories from the day are repeated in the brain during sleep, memories may get stronger. Dreams may also improve our moods. Together, these studies show that dreams and sleep are important for performing well when we are awake.
When she was 8, my daughter told me about one of her dreams. She was in a spaceship with some animals. Although she knew she was in a spaceship in her dream, when telling me about the dream, she realized the spaceship was actually a washing machine. At times, she and the animals would be out in space, but they also came back to earth. She told me the dream with a laugh and then moved on with her day, ignoring the crazy animals and spaceships that entertained her in her sleep.
Since we remember our dreams and then often forget them, what is their purpose? Why do we dream about the things we do? New research tools, particularly those that can be used to investigate the brain, are being used to answer these questions.
What Are Dreams?
Although it is hard to define what a dream is, for the purpose of this article, we will define dreams as our thoughts during sleep that we recall when we wake up. So, sleeping dreams are not the same as “daydreaming.” Dreams are mostly visual (made up of scenes and faces; sound, taste, and smell are rare in dreams [ 1 ]). Dreams can range from truly strange to rather boring, snapshots from a recent event.
To study dreams, scientists need a measure of dreaming. Most studies use dream reports (a person writes out her dreams when she wakes up) or questionnaires (a person answers questions like “How many dreams have you recalled in the past month?” [ 2 ]). Dreams are more likely to be recalled when a person is woken up from REM sleep. REM sleep is a type of sleep that is named for the rapid eye movements that can be measured during this stage of sleep. We do not dream as much in non-REM sleep, the sleep stages that make up the rest of the night, and dream reports from non-REM sleep are often less strange.
Dream frequency (how often dreams happen) and content (what dreams are about) is very different for everyone, and there are many reasons why this may be true. For example, you will remember dreams more if you are woken up by someone or by an alarm clock. This might be because you can still recall that dream memory while it is fresh but, if you wake up on your own, you will transition through a few sleep stages and possibly lose that dream memory. Dream recall changes with age, too. Older people are less likely to report dreaming. This could also be related to memory: since older people have weaker memories, it could be that they dream but cannot remember their dreams by the time they wake up. A brain area called the medial prefrontal cortex is also related to dream recall. If this brain area is damaged, the person recalls few dreams, which may mean the person dreams less (or not at all). Also, how tightly packed the brain cells are in the medial prefrontal cortex can vary from person to person, which may cause some healthy people to dream more or less than other healthy people. There are also genes that affect how much REM sleep people get. People with less REM sleep may not have the strange dreams that tend to come in REM. So, how long you sleep, your age, and your genetics may all explain why you dream more or less than someone else.
Do dreams actually happen while we sleep, or are they ideas that come to us when we wake up and we just “feel” like it happened during sleep? A recent study using a type of brain imaging called magnetic resonance imaging or (MRI: Read more in the Young Minds article “How Is Magnetic Resonance Imaging Used to Learn About the Brain?” [ 3 ]) helped answer this question ( Figure 1A ). The scientists made maps of the brain activity that occurred when people looked at pictures of things—keys, beds, airplanes. Later, the people in the study slept in the MRI machine. The scientists matched the pattern of brain activity from the people as they slept to brain activity patterns for the pictures they viewed earlier, and then chose the best match ( Figures 1B,C ). This match predicted what the person said they dreamed about 60% of the time. Although 60% is not perfect, it is better than guessing! [ 4 ]. This means that dreams are created in the brain during sleep.
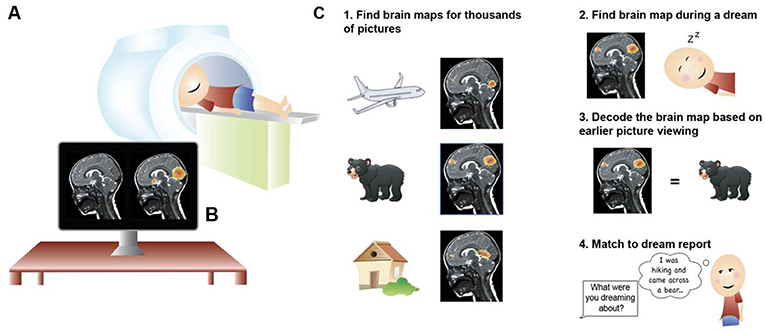
- Figure 1 - (A) Magnetic resonance imaging (MRI) is a way to investigate the brain.
- The person lies on a bed inside a giant magnet. (B) MRI can measure the structure of the brain and the areas of the brain that are active. (C) MRI was used to measure dreaming. First, while the participant was awake, they viewed thousands of pictures in the MRI. This told scientists the specific brain responses to specific pictures. Later, when the participant slept in the MRI, scientists measured the brain activity patterns and matched this to the brain responses to the pictures the participant saw when they were awake. Scientists guessed that the best match would tell them what the participant was dreaming about. By asking the participant about their dreams in the MRI, scientists found that the dreams did tend to match the pictures predicted by the brain activity.
Dreams Support Memories
What is the purpose of our dreams? Researchers have found that sleep is important for memory (see this Frontiers for Young Minds article ; “Thanks for the Memories…” [ 5 ]). Memories move from temporary storage in the hippocampus , a brain structure that is very important for short-term memory, to permanent storage in other parts of the brain. This makes the memories easier to remember later. Memories improve with sleep because the memories are replayed during sleep [ 6 ]. If you want to learn all the words to your favorite scene in a movie, you might re-watch that scene over and over again. The brain works the same way: neurons (brain cells) that are active with learning are active again and replay the learned material during sleep. This helps store the memory more permanently.
Memory replay may show up in our dreams. Dreams in non-REM sleep, when most memory replay happens, often contain normal people and objects from recent events. However, sleep switches between non-REM and REM sleep (see Figure 2 ). So, bizarre dreams in REM sleep may come from a combination of many different recent memories, which were replayed in non-REM sleep, and get jumbled up during REM sleep. If dreams help with memory processing, does that mean your memories are not being processed if you do not dream? No. Memories are moving to storage even if we do not dream.
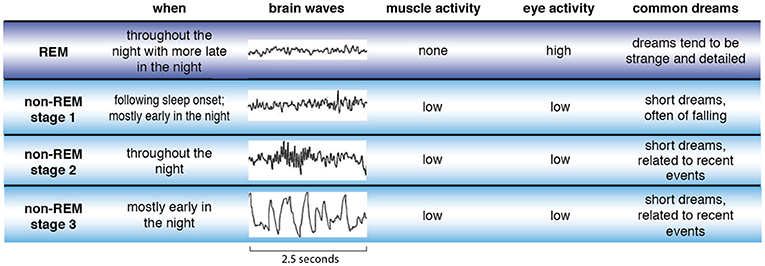
- Figure 2 - There are four types of sleep—REM sleep (purple) and three stages of non-REM sleep (blue).
- REM stands for rapid eye movements, which happen during this stage of sleep. During REM sleep, muscle and brain activity also differ from other sleep stages. Characteristics of dreams tend to be different for each of these sleep stages.
Dreams Improve Creativity and Problem Solving
My daughter’s dream of a spaceship made a great story that she recited to me, and later, to her classmates. The images were intense and interesting, inspiring her to draw scenes in a notebook and write about the dream for school. This is an example of how dreams can help make us more creative. Mary Shelley, the author of the book Frankenstein, got the idea for her book from a dream. Even scientists get ideas from dreams [ 7 ].
To measure creative problem solving, scientists used a remote associates task, in which three unrelated words are shown, and the person is to come up with a word they have in common. For instance, HEART, SIXTEEN, and COOKIES seem unrelated until you realize they all are related to SWEET (sweetheart, sweet sixteen, and cookies are sweet) ( Figure 3 ). The scientists wanted to see whether sleep helped people do better on this task. They found that people were better at thinking of the remote solution if they had a nap, particularly a nap with REM sleep. Given that REM is when most bizarre dreaming occurs, this supports the idea that these dreams might help us find creative solutions to problems [ 8 ].
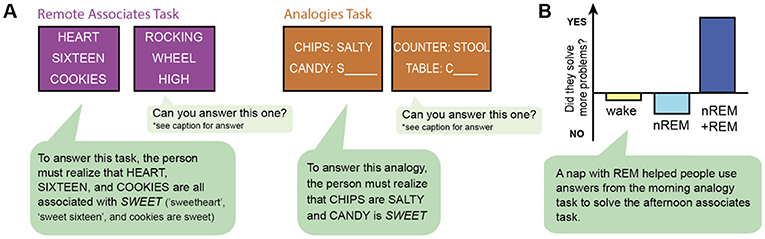
- Figure 3 - REM sleep helps people find creative solutions.
- In the morning, participants did two tasks to test creativity and problem solving (A) . They did one task again in the afternoon. In between, they either stayed awake (“wake” group) or took a nap. Those that took naps either did not have REM sleep in their nap (“nREM” group) or had both nREM and REM sleep (“nREM + REM” group). (B) If subjects stayed awake between the morning and afternoon tests (yellow bar), they did not improve on the task. They also did not improve if they had a nap that was only nREM sleep (light blue bar). But, if they had a nap with both nREM and REM sleep, they did better in the afternoon compared with when they did the task in the morning (dark blue bar). So, REM sleep must help us find creative solutions (from Cai et al. [ 8 ]).
This study and research like it gives us reason to believe that REM dreams may help us be more creative and solve problems. Many different memories may be activated at the same time and when these memories are mixed together, the result when we wake up may be both the memory of a strange dream and a unique perspective on problems.
Dreams Regulate Our Moods and Emotions
Dreams are usually emotional. One study found that most dreams are scary, angry, or sad.
Dreams might seem to be emotional simply because we tend to remember emotional things better than non-emotional things. For example, in waking life, the day you got a puppy is more memorable than a normal school day. So, dreams about emotional events might be remembered more easily than boring, non-emotional dreams. It is also possible that dreams are emotional because one job of dreams is to help us process emotions from our day [ 9 ]. This may be why the amygdala , an area of the brain that responds to emotions when we are awake, is active during REM sleep. If you had a sad day, you are more likely to have sad dreams. But, sleep also improves mood–sleep after a disagreement or sad event will make you happier.
Dreams could also help prepare us for emotional events, through something called threat simulation theory [ 10 ]. For example, when I dreamt that my young daughter, who could not swim, fell into a swimming pool, recall of that dream convinced me to sign her up for swim lessons. By simulating this fearful situation, I could prevent it by being prepared.
These studies show us that sleep and dreams are important for our emotions. By processing emotions in sleep, we may be better prepared and in a better mood the next day.
Conclusions
There are different ways scientists measure dreams—from asking questions to using MRI. These studies show us that activity in the brain while we sleep gives us the interesting dreams we recall when we wake up. These dreams help us remember things, be more creative, and process our emotions.
We know most kids do not get enough sleep. Some diseases (like Alzheimer’s disease) also make people sleep less, while others (like REM sleep behavior disorder and mood disorders) affect dreams directly. It is important to study sleep and dreams to understand what happens when we do not get enough sleep and how we can treat people with these diseases.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Rapid Eye Movement (REM) : ↑ A stage of sleep in which the eyes move rapidly and there is no muscle activity.
Medial Prefrontal Cortex : ↑ A specific area in the front of the brain that is associated with dream recall but also has a role in memory and decision-making.
Magnetic Resonance Imaging (MRI) : ↑ A tool used to take pictures of internal body parts (including the brain). MRI can also be used to measure the activity in the brain.
Hippocampus : ↑ An area in the brain that is thought to be important for short-term memory.
Neuron : ↑ A cell in the nervous system (brain and spinal cord) that can transmit information to other cells.
Amygdala : ↑ An area of the brain involved in the experience of emotions.
Threat Simulation Theory : ↑ A theory of dreaming that says that threats (things that could be bad) are simulated or practiced in your dreams to prepare you for those situations when you are awake.
1. ↑ Zandra, A. L., Nielsen, T. A., and Donderi, D. C. 1998. Prevalence of auditory, olfactory, and gustatory experiences in home dreams. Percept. Mot. Skills 87:819–26.
2. ↑ Schredl, M. 2002. Questionnaires and diaries as research instruments in dream research: methodological issues. Dreaming 12:17–26. doi: 10.1023/A:1013890421674
3. ↑ Hoyos, P., Kim, N., and Kastner, S. 2019. How Is Magnetic Resonance Imaging Used to Learn About the Brain? Front. Young Minds . 7:86. doi: 10.3389/frym.2019.00086
4. ↑ Horikawa, T., Tamaki, M., Miyawaki, Y., and Kamitani, T. 2013. Neural decoding of visual imagery during sleep. Science 340:639–42. doi: 10.1126/science.1234330
5. ↑ Davachi, L., and Shohamy, D. 2014. Thanks for the Memories.… Front. Young Minds. 2:23. doi: 10.3389/frym.2014.00023
6. ↑ O’Neill, J., Senior, T. J., Allen, K., Huxter, J. R., and Csicsvari, J. 2008. Reactivation of experience-dependent cell assembly patterns in the hippocampus. Nat. Neurosci . 11:209–15. doi: 10.1038/nn2037
7. ↑ Barrett, D. 2001. The Committee of Sleep: How artists, scientists, and athletes use dreams for creative problem-solving–and How You Can Too . New York, NY: Crown.
8. ↑ Cai, D. J., Mednick, S. A., Harrison, E. M., Kanady, J. C., and Mednick, S. C. 2009. REM, not incubation, improves creativity by priming associative networks. Proc. Natl. Acad. Sci. U.S.A . 106:10130–4. doi: 10.1073/pnas.0900271106
9. ↑ Cremone, A., Kurdziel, L. B. F., Fraticelli, A., McDermott, J., and Spencer, R. M. C. 2017. Napping reduces emotional attention bias during early childhood. Dev. Sci . 20:e12411. doi: 10.1111/desc.12411
10. ↑ Revonsuo, A. 2000. The reinterpretation of dreams: an evolutionary hypothesis of the function of dreaming. Behav. Brain Sci . 23:877–901. doi: 10.1017/s0140525x00004015
REVIEW article
Experimental research on dreaming: state of the art and neuropsychoanalytic perspectives.

- 1 INSERM U1028, Lyon Neuroscience Research Center, Brain Dynamics and Cognition Team, Lyon, France
- 2 CNRS UMR5292, Lyon Neuroscience Research Center, Brain Dynamics and Cognition Team, Lyon, France
- 3 University Lyon 1, Lyon, France
Dreaming is still a mystery of human cognition, although it has been studied experimentally for more than a century. Experimental psychology first investigated dream content and frequency. The neuroscientific approach to dreaming arose at the end of the 1950s and soon proposed a physiological substrate of dreaming: rapid eye movement sleep. Fifty years later, this hypothesis was challenged because it could not explain all of the characteristics of dream reports. Therefore, the neurophysiological correlates of dreaming are still unclear, and many questions remain unresolved. Do the representations that constitute the dream emerge randomly from the brain, or do they surface according to certain parameters? Is the organization of the dream’s representations chaotic or is it determined by rules? Does dreaming have a meaning? What is/are the function(s) of dreaming? Psychoanalysis provides hypotheses to address these questions. Until now, these hypotheses have received minimal attention in cognitive neuroscience, but the recent development of neuropsychoanalysis brings new hopes of interaction between the two fields. Considering the psychoanalytical perspective in cognitive neuroscience would provide new directions and leads for dream research and would help to achieve a comprehensive understanding of dreaming. Notably, several subjective issues at the core of the psychoanalytic approach, such as the concept of personal meaning, the concept of unconscious episodic memory and the subject’s history, are not addressed or considered in cognitive neuroscience. This paper argues that the focus on singularity and personal meaning in psychoanalysis is needed to successfully address these issues in cognitive neuroscience and to progress in the understanding of dreaming and the psyche.
The word “dream” is commonly used to express an unattainable ideal or a very deep and strong desire:
I have a dream that my four little children will one day live in a nation where they will not be judged by the color of their skin, but by the content of their character.
Martin Luther King
In dream reports, however, one often notices banal situations, strange scenes, or even frightening events. Why is there such a contrast between the popular meaning of the word “dream” and the content of dream reports? Why are some dream scenes so bizarre? Are dreams built from images that arise randomly from the sleeping brain? Or is the emergence and organization of dream images controlled by currently unknown parameters? Does dreaming have a function?
Answering these questions is not easy because dreaming is elusive. We still do not know when it happens during the night, how long it lasts, whether we can recall its entire content, or how to control it. For more than a century, such limited understanding of dreaming has seriously hampered experimental investigations. Nonetheless, scientific research has managed to produce considerable information about the phenomenology and physiology of dreaming and has improved our understanding of this fascinating phenomenon.
Experimental Research on Dreaming
Dreaming and experimental psychology, dream content.
Dreaming was first investigated on an experimental level in the nineteenth century. Calkins (1893) published the first statistical results about dreaming and argued that some aspects of dream content could be quantified. Later, questionnaires and automatic analysis of the lexical content of dream reports allowed psychologists to show that dream content has some precise phenomenological characteristics. According to psychological studies ( Hall and Van de Castle, 1966 ; Schwartz, 1999 ), visual imagery occurs more frequently in dreams than imagery of other senses (audition, olfaction, touch, and taste); the dream drama is mostly lived by the dreamer from a first-person perspective; some elements of real-life events previously experienced by the dreamer often contribute to the scene of the dream; most often, the dream sequence is not within the dreamer’s voluntary control (i.e., the dreamer may be convinced during the dream that the dream’s story is really happening); temporal and spatial incoherencies can occur in the dream story; the dream report is often full of people interacting with each other (e.g., discussions, fights, pursuit, sexuality); and finally, the dream report often contains strong emotions.
Substantial variability of content exists, however, among the same individual’s dreams and among the dreams of different individuals. Further, psychological studies have shown that many internal and external parameters can influence dream content. For example, males report more aggression and violence in their dreams than do females ( Nielsen et al., 2003 ; Schredl et al., 2004 ). External stimulation perceived by the dreamer can be incorporated into dreams ( Koulack, 1969 ; Saint-Denys, 1867; Hoelscher et al., 1981 ), as illustrated by the famous Dali painting Dream Caused by the Flight of a Bee around a Pomegranate a Second before Awakening . The current concerns of the subject may also be found in the content of his/her dreams ( Schwartz, 1999 ; Domhoff and Schneider, 2008 ), and many aspects of the subject’s daily life were found to influence dream content, including news events ( Bulkeley and Kahan, 2008 ), musical practice ( Uga et al., 2006 ), religious beliefs ( Domhoff and Schneider, 2008 ), chronic pain ( Raymond et al., 2002 ), mood ( Cartwright et al., 1998a ), or a violent living environment ( Valli et al., 2005 ). By contrast, congenital or acquired malformations do not seem to significantly influence dream content ( Voss et al., 2010 ; Saurat et al., 2011 ).
Based on these results, two opposing hypotheses were formulated: the continuity hypothesis ( Schredl and Hofmann, 2003 ) and the discontinuity hypothesis ( Rechtschaffen, 1978 ; Kahn et al., 1997 ; Stickgold et al., 2001 ). The former relies on results showing that the themes of an individual’s thoughts during waking life and dreaming are similar; the latter focuses on the fundamentally different structures of thoughts during waking life and dreaming. Voss et al. (2010) stressed in their recent paper that these hypotheses represent oversimplified approaches to dream analysis and argued that waking and dreaming thoughts were related but structurally independent; in other words, she argued in favor of merging the continuity and discontinuity hypotheses.
Dream report frequency
Dream report frequency (DRF) can vary within subjects and varies substantially among subjects. In a study of 900 German subjects with a large age range from various socioprofessional categories, the mean DRF was approximately 1 dream report per week ( Schredl, 2008 ). This result shows that the dream experience is common and familiar to everyone. Psychological studies have demonstrated that many parameters covary with DRF and may thus influence it.
Sleep parameters. First, DRF varies according to the sleep stage preceding awakening (e.g., Dement and Kleitman, 1957b ; Nielsen, 2000 , for a review). More dream reports are obtained after an awakening during rapid eye movement (REM) sleep than after an awakening during non-REM (NREM) sleep. These results inspired the REM sleep hypothesis of dreaming (see the section Dreaming and Neuroscience). Second, DRF increases with the number of awakenings during sleep, according to retrospective self-evaluations of awakenings ( Cory and Ormiston, 1975 ; Schredl et al., 2003 ). Such studies showed that the more the subjects tended to awaken during sleep, the higher their DRF. These results support the hypothesis of Koulack and Goodenough (1976) , which proposes that nocturnal awakenings facilitate the encoding of the dream in memory and thus facilitate dream recall upon awakening. However, this hypothesis has not been tested by measuring awakenings with polysomnographic recordings in healthy subjects with various DRFs. Finally, DRF varies according to the method of awakening. Abrupt awakenings lead to more dream reports than gradual awakenings ( Shapiro et al., 1963 , 1965 ; Goodenough et al., 1965 ).
Physiological and environmental parameters. Dream report frequency deceases with age (e.g., Schredl, 2008 ) and tends to be slightly higher among females than males (e.g., Schredl, 2008 ; Schredl and Reinhard, 2008 ). Remarkably, Schredl’s (2008) results revealed that DRF also varied according to the size of the subject’s place of residence.
Psychological parameters. First, increased professional stress or interpersonal stress resulted in an increase in DRF (for a review, see Schredl, 1999 ). Second, an interest in dreams or a positive attitude toward dreams clearly covaries with DRF ( Hill et al., 1997 ; Schredl, 1999 ; Schredl et al., 2003 ). The greater an individual’s interest in dreams, the higher his/her DRF. Third, several cognitive abilities have been found to covary with DRF. Contradictory results have been reported for the correlation between DRF and memory abilities (short-term, long-term, visual, verbal, implicit, and explicit; significant positive correlation: Cory and Ormiston, 1975 ; Belicki et al., 1978 ; Butler and Watson, 1985 ; Schredl et al., 1995 ; Solms, 1997 ; no significant correlation: Cohen, 1971 ; Belicki et al., 1978 ; Schredl et al., 1995 , 1997 , 2003 ; Solms, 1997 ) and the correlation between DRF and visual imagery ( significant positive correlation : Hiscock and Cohen, 1973 ; Richardson, 1979 ; Okada et al., 2000 ; no significant correlation : Hill et al., 1997 ; Okada et al., 2000 ). However, several studies have consistently shown that DRF is positively correlated with creativity ( Fitch and Armitage, 1989 ; Schredl, 1999 ; Schredl et al., 2003 ) and intelligence scales (multiple-choice vocabulary test, Schonbar, 1959 ; Shipley Intelligence Scale, Connor and Boblitt, 1970 ). Finally, many authors have reported a correlation between DRF and personality traits. Subjects with a high DRF are more likely to have a personality with thinner boundaries (Hartmann described people with thin boundaries as being open, trustworthy, vulnerable, and sensitive; Hartmann, 1989 ; Hartmann et al., 1991 ; Schredl et al., 2003 ), to be more anxious ( Schonbar, 1959 ; Tart, 1962 ), to have a higher level of absorption (the absorption scale measures the capacity to become absorptively involved in imaginative and esthetic experiences; Hill et al., 1997 ; Schredl, 1999 ; Schredl et al., 2003 ), to be more open to experience ( Hill et al., 1997 ; Schredl et al., 2003 ), and to be less alexithymic (alexithymia is a personality variable that incorporates difficulty identifying and describing feelings, difficulty distinguishing between feelings and the physical sensation of emotional arousal, limited imaginative processes, and an externally oriented cognitive style; De Gennaro et al., 2003 ; Nielsen et al., 2011 ) compared to subjects with a low dream recall frequency. However, those results have not always been reproducible (e.g., Schredl, 2002 for openness to experience; Cory and Ormiston, 1975 ; Hill et al., 1997 for anxiety; Nielsen et al., 1997 for alexithymia) and, according to the recent review by Blagrove and Pace-Schott (2010) , it is difficult to draw conclusions about a possible link between personality traits and DRF.
In conclusion, numerous parameters have been identified that covary with DRF. Schredl stressed in many of his papers that the studied parameters usually explain only a small percentage of the total variance (e.g., Schredl, 2008 ). Thus, the DRF variation profile suggests that the production, encoding and recall of dreams are influenced by numerous parameters that probably interact with each other.
Dreaming and Neuroscience
The neuroscientific approach to dreaming arose at the end of the 1950s with the discovery of REM during human sleep by the American physiologist Nathaniel Kleitman and his team ( Aserinsky and Kleitman, 1953 ; Dement and Kleitman, 1957a ). During these sleep episodes with saccades, the researchers noticed a decrease in voltage and an increase in frequency in the EEG, accompanied by an increase in cardiac frequency variability and a decrease in body movements. They concluded that these physiological modifications indicate a particular sleep stage, which they called REM sleep. A few years later, the French team led by neurobiologist Michel Jouvet discovered that the lack of movement during REM sleep in cats was due to a general muscular atonia, controlled notably by the locus coeruleus α in the brainstem ( Jouvet and Michel, 1959 ; Berger, 1961 later showed that muscular atonia during REM sleep also occurs in humans). Interestingly, the inability to move during REM sleep indicates deep sleep and paradoxically, the fast EEG activity of REM sleep resembles EEG activity in wakefulness. Jouvet concluded that this particular physiological state is associated with a “third state” of the brain (in addition to the brain states associated with wakefulness and NREM sleep) which he called “paradoxical sleep” instead of “REM sleep” ( Jouvet et al., 1959 ; Jouvet, 1992 ). Several years later, Fisher et al. (1965) discovered another physiological characteristic of REM sleep: the penile erection.
During the same period, the American team noticed that a subject awakened during REM sleep very often reported a dream (80% of awakenings in REM sleep vs. 6% of awakenings in NREM sleep are followed by a dream report, according to Dement and Kleitman, 1957b ). Researchers concluded that dreaming occurs during REM sleep. The eye movements of REM sleep would allow the dreamer to scan the imaginary scene of the dream (the scanning hypothesis); the cerebral cortex activation revealed by the rapid EEG would allow intense cognitive activity, creating the complex stories of a dream; and the lack of muscle tone would prevent the dreamer from acting out his dreams. From that time on, researchers investigated REM sleep to obtain answers about dreaming.
In the 1990s, researchers used functional neuroimaging techniques such as positron emission tomography (PET) to investigate brain activity during REM sleep in humans. This new approach enabled researchers to demonstrate that the functional organization of the brain during REM sleep is different from the functional organization of the brain during wakefulness ( Maquet et al., 1996 ; Braun et al., 1998 ). In comparison to wakefulness, brain activity during REM sleep is decreased in some brain regions (e.g., in the dorsolateral prefrontal cortex; Braun et al., 1998 ) and increased in other regions (e.g., in the occipital and temporal cortex, the hippocampus and parahippocampus, the anterior cingulate, the precentral and postcentral gyri, the superior parietal cortex, and the pons; Braun et al., 1998 ; Maquet et al., 2000 ). Looking more generally for brain activity correlating with REM sleep (the vigilance states considered included wakefulness, slow-wave sleep, and REM sleep), Maquet et al. (1996) found negative correlations in the precuneus, posterior cingulate cortex, temporoparietal junction, and dorsolateral prefrontal cortex and positive correlations in the amygdala, anterior cingulate, postcentral gyrus, thalamus, and pons (see Schwartz and Maquet, 2002 ; Maquet et al., 2005 ; Nir and Tononi, 2010 for reviews). Based on these results, researchers argued that the particular functional organization of the brain during REM sleep could explain the phenomenological characteristics of dream reports ( Hobson and Pace-Schott, 2002 ; Schwartz and Maquet, 2002 ; Maquet et al., 2005 ; Nir and Tononi, 2010 ). They considered that brain activity increases and decreases during REM sleep could be interpreted on the basis of what we know about brain activity during wakefulness. In this context, the increased occipital cortex activity during REM sleep could explain the visual component of dream reports because neuroimaging results during wakefulness showed that visual imagery with the eyes closed activates the occipital cortex ( Kosslyn and Thompson, 2003 ). The decreased activity in the temporoparietal junction during REM sleep may explain why dreams are mainly experienced in the egocentric coordinates of the first-person; indeed, during wakefulness, activity in the temporoparietal junction was reported to be greater for allocentric vs. egocentric representation (e.g., Ruby and Decety, 2001 ; Zacks et al., 2003 ) and for third- vs. first-person perspective (e.g., Ruby and Decety, 2003 , 2004 ). The increased activity in the hippocampus during REM sleep could explain why dreams are often composed of known images or characters, as the hippocampus is known to be associated with the encoding and retrieval of lived events during wakefulness (e.g., Piolino et al., 2009 ). The decreased activity in the lateral prefrontal cortex during REM sleep could explain why dream stories lack consistency, why the dreamer’s perception of time is altered, why the dream story is beyond the control of the dreamer and why the dreamer is convinced that the dream story is really happening. Indeed, during wakefulness, the lateral prefrontal cortex is involved in executive function, cognitive control, and working memory ( Petrides, 2005 ; Koechlin and Hyafil, 2007 ). The increased activity in the medial prefrontal cortex during REM sleep could explain the attribution of thoughts, beliefs, and emotions to the characters in the dream because, during wakefulness, the medial prefrontal cortex is known to participate in mind reading ( Ruby et al., 2007 , 2009 ; Legrand and Ruby, 2009 ). The increased activity in the motor cortex (precentral gyrus) during REM sleep could explain the movements of the characters’ bodies in the dream because, during wakefulness, motor imagery, and the imagination of someone’s action from the third-person perspective involve the precentral gyrus ( Decety et al., 1994 ; Ruby and Decety, 2001 ). Finally, the amygdala’s activity during REM sleep could explain why emotions, especially fear, are often mentioned in dream reports; indeed, the amygdala is involved in the processing of emotional stimuli during wakefulness ( Adolphs, 2008 ).
In conclusion, results from experimental psychology and neuroscience allow us to better understand the phenomenology of dreaming and the cerebral correlates of some characteristics of dream reports. Still, what do they tell us about the role of dreaming? What are the current hypotheses about dream function(s)?
Hypotheses about Dream Function(s)
No function.
At the end of the twentieth century, the neurologist Alan Hobson, who was profoundly anti-psychoanalysis, proposed a theory that deprived dreaming of any function. Hobson argued that dreaming is an epiphenomenon of REM sleep: “Because dreams are so difficult to remember, it seems unlikely that attention to their content could afford much in the way of high-priority survival value. Indeed, it might instead be assumed that dreaming is an epiphenomenon of REM sleep whose cognitive content is so ambiguous as to invite misleading or even erroneous interpretation” ( Hobson et al., 1998 ).
Psychological individualism
In contrast, other teams, like Michel Jouvet’s, believed that dreaming serves a vital function. In 1979, Jouvet’s team blocked muscular atonia during REM sleep in a cat by damaging the locus coeruleus α in its brainstem. This lesion resulted in the appearance of movements during REM sleep. Movies from the Jouvet lab show sleeping cats performing complex motor actions (with altered control and coordination) resembling those of wakefulness, such as fur licking, growling, chasing prey, mastication, and fighting. From these videos, the authors concluded that the cat was acting out its dream, and they called this non-physiological state “oneiric behavior” ( Sastre and Jouvet, 1979 ). These results led Jouvet to propose that dreaming plays a role in reinforcing a species’ typical behavior. Later in his career, Jouvet moved toward a hypothesis focusing on the role of dreaming in the individual dimension. He speculated that dreams (note that, for Jouvet, dreams and paradoxical sleep were equivalent) could be involved in psychological individualism and in the stability of the dreamer’s personality ( Jouvet, 1991 , 1992 , 1998 ). According to Jouvet, “the brain is the sole organ of homeotherms that do not undergo cell division. We thus have to explain how certain aspects of psychological heredity (found in homozygote twins raised in different surroundings) may persist for a whole life (psychological individuation). A definitive genetic programming during development (by neurogenesis) is unlikely due to the plasticity of the nervous system. That is why we have to consider the possibility of an iterative genetic programming. The internal mechanisms (synchronous) of paradoxical sleep (SP) are particularly adapted to such programming. This would activate an endogenous system of stimulation that would stimulate and stabilize receptors genetically programmed by DNA in some neuronal circuits. The excitation of these neurons during SP leads to oniric behaviors that could be experimentally revealed – the lists of these behaviors are specific to each individual and indirect data suggest a genetic component of this programming. Amongst the mechanisms allowing the iterative programming of SP, sleep is particularly important. Security – and hence the inhibition of the arousal system – is a sine qua non-condition for genetic programming to take place. In that sense, sleep could very well be the guardian of dreaming” ( Jouvet, 1991 ). In other words, Jouvet’s hypothesis is that paradoxical sleep restores neuronal circuitry that was modified during the day to preserve the expression of the genetic program that codes for psychological characteristics. This process would ensure the stability of personality across time.
The threat simulation theory
The Finnish psychologist Antti Revonsuo recently proposed a hypothesis called threat simulation theory, which explains the fearful characteristics of dream content ( Revonsuo, 2000 ; Valli and Revonsuo, 2009 ). According to this theory, dreams serve as virtual training places to improve threat avoidance or threat fighting ability. The theory postulates that such nocturnal training makes the dreamer more efficient at resolving threatening situations during wakefulness.

Emotional regulation
Cartwright et al. (1998a , b ) defended the idea that dreaming is involved in emotional regulation. Her team showed that, in healthy subjects, the depression level before sleep was significantly correlated with affect in the first REM report. Her team also observed that low scorers on the depression scale displayed a flat distribution of positive and negative affect in dreams, whereas those with a depressed mood before sleep showed a pattern of decreasing negative and increasing positive affect in dreams reported from successive REM periods ( Cartwright et al., 1998a ). These results led Cartwright’s team to suggest that dreaming may actively moderate mood overnight in normal subjects. The team strengthened this hypothesis by showing that among subjects who were depressed because of a divorce, those who reported more negative dreams at the beginning of sleep and fewer at the night’s end were more likely to be in remission 1 year later than subjects who had fewer negative dreams at the beginning of sleep and more at the end of the night ( Cartwright et al., 1998b ). The researchers concluded that negative dreams early in the night may reflect a within-sleep mood regulation process, whereas those that occur later may indicate a failure in the completion of this process.
Memory consolidation
Finally, a current mainstream hypothesis in cognitive neuroscience credits sleep and dreaming with a role in memory consolidation (for a recent review, see Diekelmann and Born, 2010 ). Numerous studies have shown that brain activity during training is replayed during post-training sleep (e.g., using a serial reaction time task Maquet et al., 2000 , demonstrated replay during REM sleep; using a maze exploration task Peigneux et al., 2004 , demonstrated replay during slow-wave sleep). Decreased performance during the post-training day in sleep-deprived subjects further suggested that the replay of brain activity at night contributes to memory consolidation (e.g., Maquet et al., 2003 ). Only recently, however, have experimental results in humans argued in favor of a role of dreaming per se in memory consolidation. In one study, subjects were trained on a virtual navigation task before taking a nap. Post-nap tests showed that subjects who dreamed about the task performed better than subjects who did not dream (note that only 4 out of 50 subjects dreamed about the task in this study; Wamsley et al., 2010 ). Using a different approach, Nielsen and colleagues provided additional arguments supporting a link between dreams and memory ( Nielsen et al., 2004 ; Nielsen and Stenstrom, 2005 ). This team demonstrated that dreams preferably incorporate events that the dreamer lived the day before and events that the dreamer lived 7 days before the dream (U shaped curve). Animal studies have shown that after associative learning, the excitability of hippocampal cells increases (which leads to an increase in neuronal plasticity) and then returns to baseline 7 days after training ( Thompson et al., 1996 ). The similarity between the delay of episodic event incorporation into dreams and the delay of post-training cellular plasticity in the hippocampus led the Canadian team to suggest a link between dreaming and episodic memory consolidation.
In summary, the preceding section describes the current state of the art on dreaming, its phenomenology and cerebral correlates and hypotheses about its functions. Some substantial advances have been made, but much remains to be understood.
Unresolved Issues
The link between oneiric behaviors and dream reports.
A piece of evidence in favor of a strong link between REM sleep and dreaming is the oneiric behavior (the appearance of complex motor behaviors when motor inhibition is suppressed during REM sleep) discovered by Sastre and Jouvet (1979) in cats and reproduced by Sanford et al. (2001) in rats. Researchers interpreted these results as the animal acting out its dream. However, as animals do not talk, the link between oneiric behavior and dream recall cannot be tested experimentally. This limitation seriously hampers our understanding of dreaming. In humans, complex motor behaviors (e.g., talking, grabbing, and manipulating imaginary objects, walking, and running) can also occur during REM sleep in a pathological context. This syndrome is called REM sleep behavior disorder (RBD). It can be caused by substance withdrawal (e.g., alcohol, Nitrazepam) or intoxication (e.g., caffeine, tricyclic antidepressants) or by various diseases (e.g., Parkinson’s and Alzheimer’s diseases, pontine neoplasms). According to physicians experts on this syndrome, some patients report dreams that are consistent with their behaviors in REM sleep ( Mahowald and Schenck, 2000 ). According to the literature, however, such matches seem to be loose and not systematic. Only one study has tested whether observers can link dream content to sleep behaviors in RBD ( Valli et al., 2011 ). In this study, each video recording of motor manifestations was combined with four dream reports, and seven judges had to match the video clip with the correctly reported dream content. The authors found that reported dream content can be linked to motor behaviors at a level better than chance. However, only 39.5% of video-dream pairs were correctly identified. Note, however, that because the authors obtained only movements and not behavioral episodes for many RBD patients, the link between videos and dream reports was unfairly difficult to make.
It is important to note that motor behavior during sleep can happen outside of REM sleep. Sleepwalking and sleep terrors, which occur during NREM sleep, are usually not considered dream enactments. However, we know that dreams can happen during NREM sleep, and many patients report dreamlike mentation after awakening from sleepwalking or sleep terrors (71%, according to Oudiette et al., 2009 ). In addition, Oudiette et al. (2009) reported that the dreamlike mentation can correspond with the sleep behavior in NREM sleep. Consequently, the authors concluded that sleepwalking may represent an acting out of corresponding dreamlike mentation.
Recent research suggests that any kind of motor behavior during sleep can be considered an oneiric behavior. One of the challenges for future research is to test the strength of the link between these oneiric behaviors and dream reports in a controlled and systematic way.
Neurophysiological Correlates of Dreaming
Despite the numerous neuroimaging studies of sleep in humans, the neurophysiological correlates of dreaming remain unclear.
Indeed, dreaming can happen during NREM sleep, and although NREM brain activity differs substantially from REM sleep brain activity ( Maquet et al., 2000 ; Buchsbaum et al., 2001 ), some NREM dreams are phenomenologically indistinguishable from REM dreams ( Hobson, 1988 ; Cavallero et al., 1992 ; Cicogna et al., 1998 ; Wittmann et al., 2004 ). This phenomenon is difficult to understand given what we currently know about the sleeping brain and about dreaming. One explanation may rely on the possibility that brain activity during sleep is not as stable as we think.
Brain activity during REM sleep in humans is considered to be well understood ( Hobson and Pace-Schott, 2002 ; Schwartz and Maquet, 2002 ; Nir and Tononi, 2010 ), but several results question this notion. First, contrary to the common belief that dorsolateral prefrontal cortex activity decreases during REM sleep, several studies have reported increased activity in the dorsolateral prefrontal cortex during REM sleep ( Hong et al., 1995 , 2009 ; Nofzinger et al., 1997 ; Kubota et al., 2011 ). Second, brain activity during REM sleep is heterogeneous. The mean regional cerebral blood flow during 1 min of REM sleep (e.g., as reported in Maquet et al., 1996 ) and the regional cerebral blood flow associated with the rapid eye movements of REM sleep ( Hong et al., 2009 ; Miyauchi et al., 2009 ) highlight different brain regions. Finally, few congruencies have been noted in the results of studies investigating brain activity during REM sleep ( Hong et al., 1995 , 2009 ; Maquet et al., 1996 , 2000 ; Braun et al., 1997 , 1998 ; Nofzinger et al., 1997 ; Peigneux et al., 2001 ; Wehrle et al., 2005 ; Miyauchi et al., 2009 ; Kubota et al., 2011 ), even between studies using the same technique and the same contrasts (e.g., Braun et al., 1998 ; Maquet et al., 2000 ), or between studies investigating the same REM event (e.g., brain activity associated with rapid eyes movements, as in Peigneux et al., 2001 ; Wehrle et al., 2005 ; Hong et al., 2009 ; Miyauchi et al., 2009 ). Furthermore, few brain regions are consistently reported across the majority of the studies. This inconsistency suggests great intra- and intersubject variability in brain activity during REM sleep in humans. A challenge for future research will be to find out whether the variability in brain activity during REM sleep can be explained by the variability in dream content.
Because dream reports can be collected after awakenings from any sleep stage, one may hypothesize that the brain activity that subserves dreaming (if such brain activity is reproducible across dreams) is quite constant throughout the night and can be observed during all sleep stages. Some results have supported this hypothesis and encouraged further attention in this direction. Buchsbaum et al. (2001) , for example, reported that metabolism in the primary visual areas and certain parts of the lateral temporal cortex does not fluctuate much across REM and slow-wave sleep. Similarly, Nielsen’s team found that dream recall (vs. no dream recall) was associated with decreased alpha (8–12 Hz) power in the EEG preceding awakening, regardless of the sleep stage (Stage 2 or REM sleep; Esposito et al., 2004 ). Interestingly, some authors have suggested that decreased power in the alpha band during wakefulness reflects search and retrieval processes in long-term memory (for a review, see Klimesch, 1999 ).
Processes of Selection and Organization of Dream Representations
Nielsen’s team found that episodic events from the 1, 7, and 8 days before a dream were more often incorporated into the dream than were events from 2 or 6 days before the dream ( Nielsen et al., 2004 ; results reproduced by Blagrove et al., 2011 ). This result tells us that internal processes control and shape dream content and thus help us to constrain and shape hypotheses about the function and biological basis of dreaming.
At the end of the nineteenth century, Saint-Denys (1867) showed that a sensory stimulus (e.g., the scent of lavender) presented to a sleeping subject without his or her knowledge could induce the incorporation of an event associated with the stimulus (e.g., holidays spent near a lavender field) into the dream, regardless of the delay between the dream and the association stimulus/events (lavender scent/holidays). The author demonstrated that the external world can influence dream content in a direct or indirect way.
Finally, it appears that both external and internal parameters can shape or govern dream content. Nonetheless, few of these parameters are known, and some regularities in the phenomenology of dreams suggest that more influencing parameters remain to be discovered. For example, some individuals experience recurring themes, characters, or places in their dreams. In line with this observation, Michael Schredl’s team showed that the content and style of a person’s life strongly influence dream content ( Schredl and Hofmann, 2003 ). However, the rule(s) governing which lived events are incorporated into dreams remain unknown. Do the representations constituting the dream emerge randomly from the brain, or do they surface according to certain parameters? Similarly, is the organization of the dream’s representations chaotic, or is it determined by rules? Does dreaming have a meaning? What is/are the function(s) of dreaming?
Dreaming, Psychoanalysis, and Neuropsychoanalysis
Psychoanalysis, which was developed by the neurologist Sigmund Freud in the beginning of the twentieth century, proposes answers to the questions raised above. Indeed, his theory of the human mind comprises hypotheses about the rules of selection and organization of the representations that constitute dreams.
At the beginning of the twentieth century, Freud presented the concept of the unconscious. He proposed that a part of our mind is made up of thoughts, desires, emotions, and knowledge that we are not aware of, but that nevertheless profoundly influence and guide our behaviors. In his books (e.g., Freud, 1900, 1920 ), Freud proposes that the unconscious mind comes out in slips and dreams. Its expression, however, is coded within dreams (the work of dream), and unconscious thoughts are distorted before they emerge in the conscious mind of the sleeping subject (manifest content of the dream). As a consequence, the dreamer is not disturbed by repressed and unacceptable thoughts (latent content of the dream) and can continue sleeping (this is the reason why Freud considered dreams the guardians of sleep). Hence, according to Freud, decoding dreams’ latent content provides an access to the unconscious mind.
In Freud’s theory of the mind, unconscious thoughts and feelings may cause the patient to experience life difficulties and/or maladjustment, and free unconscious thoughts can help the patient gain insight into his/her situation. As a consequence, Freud developed techniques to decode dreams and provide a way for an analyst to look inside the words and unconscious images of the patient, and to free them through patient insight. One of these techniques is called free association, and is regarded as an essential part of the psychoanalytic therapy process. In order for an analyst to get to the latent content of a dream, he requires the patient to discuss the dream’s manifest content and encourage free association about the dream. Free association is the principle that the patient is to say anything and everything that comes to mind. This includes decensoring his/her own speech so that he/she truly expresses everything. Over time, the therapist or analyst will draw associations between the many trains of uncensored speech the patient shares during each session. This can lead to patient insight into their unconscious thoughts or repressed memories, and the accomplishment of their ultimate goal of “freedom from the oppression of the unconscious” ( Trull, 2005 ).
Hence, Freud considered that dreams, as well as slips, have a meaning and can be interpreted, so that one is justified in inferring from them the presence of restrained or repressed intentions (Freud, 1900, 1920 ). Note that, in Freud’s theory of the mind, the words “meaning” and “intention” are closely linked: “Let us agree once more on what we understand by the ‘meaning’ of a psychic process. A psychic process is nothing more than the purpose which it serves and the position which it holds in a psychic sequence. We can also substitute the word ‘purpose’ or ‘intention’ for ‘meaning’ in most of our investigations” ( Freud, 1920 ).
In other words, according to Freud, decoding dreams with the free association method provides an access to what makes each of us so special, uncorvering the forces that guide one’s behavior. It gives access to an unknown dimension of ourselves that is fundamental in understanding who we are. It provides access to personal meaning.
This hypothesis, attributing significant importance and meaning to dreams, has rarely been considered by neuroscientists who often consider Freud’s work and theory unscientific.
However, this situation may change as the relationship between psychoanalysis and neuroscience evolves. The starting point was the creation of the International Society for Neuropsychoanalysis in 2000. It was founded by neuropsychologist and psychoanalyst Mark Solms with the intention to promote interactions and collaborations between psychoanalysis and neuroscience. The challenge was serious, as illustrated by neuroscientist Alan Hobson’s aggressiveness in the famous dream debate (Alan Hobson vs. Mark Solms) entitled “Should Freud’s dream theory be abandoned?” held in Tucson, Arizona, in 2006 during the Towards a Science of Consciousness meeting (scientific arguments can be found in Solms, 2000 and Hobson et al., 2000 ). Alan Hobson tried to convince the assembly that Freud was 100% wrong and that Freud’s dream theory was misguided and misleading and should be abandoned. He aimed to demonstrate that Freud’s dream theory is incompatible with what we know about how the brain works. He added that Freud’s dream theory was not scientific because it was not testable or falsifiable. Finally, he presented his model of dreaming, the activation-synthesis hypothesis ( Hobson and McCarley, 1977 ; Hobson et al., 2000 ): “The Activation-Synthesis model of dream construction proposed that the phasic signals arising in the pontine brainstem during REM sleep and impinging upon the cortex and limbic forebrain led directly to the visual and motor hallucinations, emotion, and distinctively bizarre cognition that characterize dream mentation. In doing so, these chaotically generated signals arising from the brain stem acted as a physiological Rorschach test, initiating a process of image and narrative synthesis involving associative and language regions of the brain and resulting in the construction of the dream scenarios.” In contrast, Mark Solms demonstrated that what is currently known about the dreaming brain is at least broadly consistent with Freud’s dream theory. He argued that it is generally accepted that brain stem activation is necessary, but not sufficient, to explain the particular characteristics of dream consciousness. What does explain the particular characteristics of dream consciousness, according to Solms, are the following features of brain activity during REM sleep ( Braun et al., 1997 ): the activation of core forebrain emotion and instinctual drive mechanisms, i.e., the limbic and paralimbic brain areas (the anterior cingulate, insula, hippocampus, parahippocampal gyrus, and temporal pole), and of the posterior perceptual system (the fusiform gyrus, superior, inferior and middle temporal gyrus, and angular gyrus) and the deactivation of executive dorsolateral frontal control mechanisms (the dorsolateral prefrontal cortex). He further argued that his lesion studies ( Solms, 1997 ) are congruent with neuroimaging results because they showed that a total cessation of dreaming results from lesions in the medial part of the frontal lobe and in the temporoparietal junction (whereas no cessation of dreaming was observed for core brainstem lesions or for dorsolateral prefrontal lesions). Finally he emphasized that the activation of motivational mechanisms (such as drives and basic emotions) and of posterior perceptual system associated with deactivation of the executive control (i.e., reality oriented regulatory mechanism) during REM sleep, is broadly consistent with Freud’s dream theory which claims that our instinctual drive states (notably appetitive and libidinal drive system) are relatively disinhibited during sleep. Note that experimental results demonstrating the existence of unconscious representations that guide behavior (e.g., Shevrin and Fritzler, 1968 ; Bunce et al., 1999 ; Arminjon, 2011 , for a review) could also have been cited in support of Freud’s dream theory. This debate was a success for Mark Solms and neuropsychoanalysis. Indeed, at the end of the debate, approximately 100 people voted “no” (i.e., “Freud’s dream theory should not be abandoned”), approximately 50 people voted “yes” and 50 voted “I don’t know”.
Solms’ (1997 , 2000 ) approach to dreaming and his experimental results fundamentally challenged our current understanding of dreaming. He proposes that dreaming and REM sleep are controlled by different brain mechanisms. According to Solms, REM sleep is controlled by cholinergic brain stem mechanisms, whereas dreaming is mediated by forebrain mechanisms that are probably dopaminergic. This implies that dreaming can be activated by a variety of NREM triggers. Several experimental results support this hypothesis.
First, behavioral studies have demonstrated that the link between REM sleep and dream reports is lax. Subjects awakened during NREM sleep can recall dreams at a high rate ( Foulkes, 1962 : 74% of awakenings in NREM sleep were followed by dream reports; Cavallero et al., 1992 : 64%; Wittmann et al., 2004 : 60%); dreams can be recalled after a nap consisting only of NREM sleep ( Salzarulo, 1971 ; Palagini et al., 2004 ); and some individuals never recall dreams, even when awakened from REM sleep ( Pagel, 2003 ). In addition, in healthy subjects with a normal dream recall frequency (around 1 dream recall per week, Schredl, 2008 ), dream recall after an awakening during REM sleep is not systematic: 5–30% of awakenings in REM sleep are not followed by a dream recall, according to the literature (e.g., Dement and Kleitman, 1957a , b ; Foulkes, 1962 ; Hobson, 1988 ). Finally, 5–10% of NREM dreams cannot be distinguished from REM dreams based on their content ( Hobson, 1988 ; Cavallero et al., 1992 ; Cicogna et al., 1998 ; Wittmann et al., 2004 ).
Second, as Solms (2000) argued, the amount of dream recall can be modulated by dopamine agonists ( Scharf et al., 1978 ; Nausieda et al., 1982 ) without concomitant modification of the duration and frequency of REM sleep ( Hartmann et al., 1980 ). Dream recall can be suppressed by focal brain lesions (at the temporo-parieto-occipital junction and ventromedial prefrontal cortex; Solms, 1997 , 2000 ). These lesions do not have any appreciable effects on REM frequency, duration, or density ( Kerr et al., 1978 ; Michel and Sieroff, 1981 ). Finally, some clinical studies suggest that a dream can be triggered by nocturnal seizures in NREM sleep, i.e., by focal brain stimulation. Some cases of recurring nightmares caused by epileptiform activity in the temporal lobe have indeed been reported ( Solms, 2000 ).
Conclusion: Collaboration between Neuroscience and Psychoanalysis Would Benefit Dream Research
Considering the issues that remain unresolved (e.g., neurophysiologic variability, parameter(s) influencing the emergence of representations in dreams, the meaning of dreams), a psychoanalytic perspective would certainly benefit dream research by providing new directions/leads and helping to reach a comprehensive understanding of dreaming.
On the one hand, psychological research has demonstrated that dream content is influenced by one’s personal life, especially personal concerns ( Schwartz, 1999 ; Schwartz and Maquet, 2002 ; Schredl and Hofmann, 2003 ), and some neuroscientists have hypothesized that dreaming is involved in psychological individualism. Thus, both psychology and neuroscience have provided results and hypotheses that validate the possibility that dreaming has something to do with personal and meaningful issues. On the other hand, Freud argued that the unconscious, which guides behaviors and desires, express itself during dreams. The two disciplines’ (cognitive neuroscience and psychoanalysis) convergence on dreaming thus seems obvious; however, very little collaboration has occurred to date.
Note that some experimental studies in psychology have considered the psychoanalytic perspective. For example, Greenberg et al. (1992) attempted “a research-based reconsideration of the psychoanalytical theory of dreaming.” They evaluated the presence of problems (defined as an expression of negative feeling or any situation evoking such feeling or requiring some change or adaptation) during dreaming and pre- and post-sleep wakefulness in two subjects. They showed that problems occurred very frequently in the manifest dream content and that these problems were nearly systematically related to the problems noted during pre-sleep wakefulness. In addition, they observed that effective dreams (i.e., dreams that presented some solution to the individuals’ problems) were followed by a waking state in which the impact of the problems was diminished, whereas ineffective dreams were followed by the persistence of the problems. This study thus confirmed that personal concerns influence dream content. In addition it provided new results suggesting that dreaming may have some psychological problem-solving function (this result recalls the neuroscientific findings that sleep has a cognitive problem-solving function associated with brain reorganization; e.g., Wagner et al., 2004 ; Darsaud et al., 2011 ). Greenberg et al.’s (1992) study managed to quantify personal issues and clearly broadened the cognitive neuroscience perspective on dreaming. To proceed further, approaches integrating psychoanalysis and neuroscience must now be developed. Several subjective issues at the core of the psychoanalytic approach, such as the concept of personal meaning, the concept of unconscious episodic memory and the subject’s history, are not addressed or considered in cognitive neuroscience. This limitation hampers the understanding of psychological and neurophysiological functioning in humans. These issues must be addressed, and the expertise of psychoanalysts in singularity and personal meaning is needed to do so in neuroscience and to further the understanding of dreaming and of the psyche.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Adolphs, R. (2008). Fear, faces, and the human amygdala. Curr. Opin. Neurobiol. 18, 166–172.
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text
Arminjon, M. (2011). The four postulates of Freudian unconscious neurocognitive convergences. Front. Psychol. 2:125. doi: 10.3389/fpsyg.2011.00125
CrossRef Full Text
Aserinsky, E., and Kleitman, N. (1953). Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science 118, 273–274.
Belicki, K., Hunt, H., and Kelly, P. (1978). The function of dream and dreamer variables in the question of dream recall. Sleep Res. 7, 167.
Berger, R. J. (1961). Tonus of extrinsic laryngeal muscles during sleep and dreaming. Science 134, 840.
Blagrove, M., Henley-Einion, J., Barnett, A., Edwards, D., and Heidi Seage, C. (2011). A replication of the 5-7 day dream-lag effect with comparison of dreams to future events as control for baseline matching. Conscious. Cogn. 20, 384–391.
Blagrove, M., and Pace-Schott, E. F. (2010). Trait and neurobiological correlates of individual differences in dream recall and dream content. Int. Rev. Neurobiol. 92, 155–180.
Braun, A. R., Balkin, T. J., Wesensten, N. J., Gwadry, F., Carson, R. E., Varga, M., Baldwin, P., Belenky, G., and Herscovitch, P. (1998). Dissociated pattern of activity in visual cortices and their projections during human rapid eye movement sleep. Science 279, 91–95.
Braun, A. R., Balkin, T. J., Wesenten, N. J., Carson, R. E., Varga, M., Baldwin, P., Selbie, S., Belenky, G., and Herscovitch, P. (1997). Regional cerebral blood flow throughout the sleep-wake cycle. An H215O PET study. Brain 120(Pt 7), 1173–1197.
Buchsbaum, M. S., Hazlett, E. A., Wu, J., and Bunney, W. E. Jr. (2001). Positron emission tomography with deoxyglucose-F18 imaging of sleep. Neuropsychopharmacology 25, S50–S56.
Bulkeley, K., and Kahan, T. L. (2008). The impact of September 11 on dreaming. Conscious. Cogn. 17, 1248–1256.
Bunce, S. C., Bernat, E., Wong, P. S., and Shevrin, H. (1999). Further evidence for unconscious learning: preliminary support for the conditioning of facial EMG to subliminal stimuli. J. Psychiatr. Res. 33, 341–347.
Butler, S. F., and Watson, R. (1985). Individual differences in memory for dreams: the role of cognitive skills. Percept. Mot. Skills 61, 823–828.
Calkins, M. W. (1893). Statistics of dreams. Am. J. Psychol. 5, 311–343.
Cartwright, R., Luten, A., Young, M., Mercer, P., and Bears, M. (1998a). Role of REM sleep and dream affect in overnight mood regulation: a study of normal volunteers. Psychiatry Res. 81, 1–8.
Cartwright, R., Young, M. A., Mercer, P., and Bears, M. (1998b). Role of REM sleep and dream variables in the prediction of remission from depression. Psychiatry Res. 80, 249–255.
Cavallero, C., Cicogna, P., Natale, V., Occhionero, M., and Zito, A. (1992). Slow wave sleep dreaming. Sleep 15, 562–566.
Pubmed Abstract | Pubmed Full Text
Cicogna, P. C., Natale, V., Occhionero, M., and Bosinelli, M. (1998). A comparison of mental activity during sleep onset and morning awakening. Sleep 21, 462–470.
Cohen, D. B. (1971). Dream recall and short-term memory. Percept. Mot. Skills 33, 867–871.
Connor, G. N., and Boblitt, W. M. E. (1970). Reported frequency of dream recall as a function of intelligence and various personality test factors. J. Clin. Psychol. 26, 438–439.
Cory, T. L., and Ormiston, D. W. (1975). Predicting the frequency of dream recall. J. Abnorm. Psychol. 84, 261–266.
Darsaud, A., Wagner, U., Balteau, E., Desseilles, M., Sterpenich, V., Vandewalle, G., Albouy, G., Dang-Vu, T., Collette, F., Boly, M., Schabus, M., Degueldre, C., Luxen, A., and Maquet, P. (2011). Neural precursors of delayed insight. J. Cogn. Neurosci. 23, 1900–1910.
De Gennaro, L., Ferrara, M., Cristiani, R., Curcio, G., Martiradonna, V., and Bertini, M. (2003). Alexithymia and dream recall upon spontaneous morning awakening. Psychosom. Med. 65, 301–306.
Decety, J., Perani, D., Jeannerod, M., Bettinardi, V., Tadary, B., Woods, R., Mazziotta, J. C., and Fazio, F. (1994). Mapping motor representations with positron emission tomography. Nature 371, 600–602.
Dement, W., and Kleitman, N. (1957a). Cyclic variations in EEG during sleep and their relation to eye movements, body motility, and dreaming. Electroencephalogr. Clin. Neurophysiol. 9, 673–690.
Dement, W., and Kleitman, N. (1957b). The relation of eye movements during sleep to dream activity: an objective method for the study of dreaming. J. Exp. Psychol. 53, 339–346.
Diekelmann, S., and Born, J. (2010). The memory function of sleep. Nat. Rev. Neurosci. 11, 114–126.
Domhoff, G. W., and Schneider, A. (2008). Studying dream content using the archive and search engine on DreamBank.net. Conscious. Cogn. 17, 1238–1247.
Esposito, M. J., Nielsen, T. A., and Paquette, T. (2004). Reduced alpha power associated with the recall of mentation from Stage 2 and Stage REM sleep. Psychophysiology 41, 288–297.
Fisher, C., Gorss, J., and Zuch, J. (1965). Cycle of penile erection synchronous with dreaming (Rem) sleep. Preliminary report. Arch. Gen. Psychiatry 12, 29–45.
Fitch, T., and Armitage, R. (1989). Variations in cognitive style among high and low frequency dream recallers. Pers. Individ. dif. 10, 869–875.
Foulkes, W. D. (1962). Dream reports from different stages of sleep. J. Abnorm. Soc. Psychol. 65, 14–25.
Freud, S. (1967). L’interprétation des rẽves (I. Meyerson, Trans.) . Paris: PUF. (Original work published 1900).
Freud, S. (1920). A General Introduction to Psychoanalysis . New York: Boni and Liveright publishers.
Goodenough, D. R., Lewis, H. B., Shapiro, A., Jaret, L., and Sleser, I. (1965). Dream reporting following abrupt and gradual awakenings from different types of sleep. J. Pers. Soc. Psychol. 56, 170–179.
Greenberg, R., Katz, H., Schwartz, W., and Pearlman, C. (1992). A research-based reconsideration of the psychoanalytic theory of dreaming. J. Am. Psychoanal. Assoc. 40, 531–550.
Hall, C. S., and Van de Castle, R. L. (1966). The Content Analysis of Dreams . New York: Appleton-Century-Crofts.
Hartmann, E. (1989). Boundaries of dreams, boundaries of dreamers: thin and thick boundaries as a new personality measure. Psychiatr. J. Univ. Ott. 14, 557–560.
Hartmann, E., Elkin, R., and Garg, M. (1991). Personality and dreaming: the dreams of people with very thick or very thin boundaries. Dreaming 1, 311–324.
Hartmann, E., Russ, D., Oldfield, M., Falke, R., and Skoff, B. (1980). Dream content: effects of l-DOPA. Sleep Res. 9, 153.
Hill, C. E., Diemer, R. A., and Heaton, K. J. (1997). Dream interpretation sessions: who volunteers, who benefits, and what volunteer clients view as most and least helpful. J. Couns. Psychol. 44, 53–62.
Hiscock, M., and Cohen, D. (1973). Visual imagery and dream recall. J. Res. Pers. 7, 179–188.
Hobson, J. A. (1988). The Dreaming Brain . New York: Basic Books.
Hobson, J. A., and McCarley, R. W. (1977). The brain as a dream-state generator: an activation-synthesis hypothesis of the dream process. Am. J. Psychiatry 134, 1335–1348.
Hobson, J. A., and Pace-Schott, E. F. (2002). The cognitive neuroscience of sleep: neuronal systems, consciousness and learning. Nat. Rev. Neurosci. 3, 679–693.
Hobson, J. A., Pace-Schott, E. F., and Stickgold, R. (2000). Dreaming and the brain: toward a cognitive neuroscience of conscious states. Behav. Brain Sci. 23, 793–842.
Hobson, J. A., Stickgold, R., and Pace-Schott, E. F. (1998). The neuropsychology of REM sleep dreaming. Neuroreport 9, R1–R14.
Hoelscher, T. J., Klinger, E., and Barta, S. G. (1981). Incorporation of concern- and nonconcern-related verbal stimuli into dream content. J. Abnorm. Psychol. 90, 88–91.
Hong, C. C., Gillin, J. C., Dow, B. M., Wu, J., and Buchsbaum, M. S. (1995). Localized and lateralized cerebral glucose metabolism associated with eye movements during REM sleep and wakefulness: a positron emission tomography (PET) study. Sleep 18, 570–580.
Hong, C. C., Harris, J. C., Pearlson, G. D., Kim, J. S., Calhoun, V. D., Fallon, J. H., Golay, X., Gillen, J. S., Simmonds, D. J., van Zijl, P. C., Zee, D. S., and Pekar, J. J. (2009). fMRI evidence for multisensory recruitment associated with rapid eye movements during sleep. Hum. Brain Mapp. 30, 1705–1722.
Jouvet, M. (1991). Le sommeil paradoxal: est-il le gardien de l’individuation psychologique? Can. J. Psychol. 45, 148–168.
Jouvet, M. (1992). Le sommeil et le rẽve . Paris: Odile Jacob.
Jouvet, M. (1998). Paradoxical sleep as a programming system. J. Sleep Res. 7(Suppl. 1), 1–5.
Jouvet, M., and Michel, F. (1959). Corrélations électromyographique du sommeil chez le Chat décortiqué et mésencéphalique chronique. C. R. Seances Soc. Biol. Fil. 153, 422–425.
Jouvet, M., Michel, F., and Courjon, J. (1959). Sur un stade d’activité électrique cérébrale rapide au cours du sommeil physiologique. C. R. Seances Soc. Biol. Fil. 153, 1024–1028.
Kahn, D., Pace-Schott, E. F., and Hobson, J. A. (1997). Consciousness in waking and dreaming: the roles of neuronal oscillation and neuromodulation in determining similarities and differences. Neuroscience 78, 13–38.
Kerr, N., Foulkes, D., and Jurkovic, G. (1978). Reported absence of visual dream imagery in a normally sighted subject with Turner’s syndrome. J. Ment. Imagery 2, 247–264.
Klimesch, W. (1999). EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Brain Res. Rev. 29, 169–195.
Koechlin, E., and Hyafil, A. (2007). Anterior prefrontal function and the limits of human decision-making. Science 318, 594–598.
Kosslyn, S. M., and Thompson, W. L. (2003). When is early visual cortex activated during visual mental imagery? Psychol. Bull. 129, 723–746.
Koulack, D. (1969). Effects of somatosensory stimulation on dream content. Arch. Gen. Psychiatry 20, 718–725.
Koulack, D., and Goodenough, D. R. (1976). Dream recall and dream recall failure: an arousal-retrieval model. Psychol. Bull. 83, 975–984.
Kubota, Y., Takasu, N. N., Horita, S., Kondo, M., Shimizu, M., Okada, T., Wakamura, T., and Toichi, M. (2011). Dorsolateral prefrontal cortical oxygenation during REM sleep in humans. Brain Res. 1389, 83–92.
Legrand, D., and Ruby, P. (2009). What is self-specific? Theoretical investigation, and critical review of neuroimaging results. Psychol. Rev. 116, 252–282.
Mahowald, M. W., and Schenck, C. H. (2000). “REM sleep parasomnias,” in Principles and Practice of Sleep Medicine , eds M. H. Kryger, T. Roth, and W. C. Dement (Philadelphia: W.B. Saunders), 724–741.
Maquet, P., Laureys, S., Peigneux, P., Fuchs, S., Petiau, C., Phillips, C., Aerts, J., Del Fiore, G., Degueldre, C., Meulemans, T., Luxen, A., Franck, G., Van Der Linden, M., Smith, C., and Cleeremans, A. (2000). Experience-dependent changes in cerebral activation during human REM sleep. Nat. Neurosci. 3, 831–836.
Maquet, P., Peters, J., Aerts, J., Delfiore, G., Degueldre, C., Luxen, A., and Franck, G. (1996). Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature 383, 163–166.
Maquet, P., Ruby, P., Maudoux, A., Albouy, G., Sterpenich, V., Dang-Vu, T., Desseilles, M., Boly, M., Perrin, F., Peigneux, P., and Laureys, S. (2005). Human cognition during REM sleep and the activity profile within frontal and parietal cortices: a reappraisal of functional neuroimaging data. Prog. Brain Res. 150, 219–227.
Maquet, P., Schwartz, S., Passingham, R., and Frith, C. (2003). Sleep-related consolidation of a visuomotor skill: brain mechanisms as assessed by functional magnetic resonance imaging. J. Neurosci. 23, 1432–1440.
Michel, F., and Sieroff, E. (1981). Une approche anatomo-clinique des déficits de l’imagerie onirique, est-elle possible? Sleep: Proceedings of an International Colloquium . Milan: Carlo Erba Formitala.
Miyauchi, S., Misaki, M., Kan, S., Fukunaga, T., and Koike, T. (2009). Human brain activity time-locked to rapid eye movements during REM sleep. Exp. Brain Res. 192, 657–667.
Nausieda, P., Weiner, W., Kaplan, L., Weber, S., and Klawans, H. (1982). Sleep disruption in the course of chronic levodopa therapy: an early feature of the levodopa psychosis. Clin. Neuropharmacol. 5, 183–194.
Nielsen, T. A. (2000). A review of mentation in REM and NREM sleep: “covert” REM sleep as a possible reconciliation of two opposing models. Behav. Brain Sci. 23, 851–866.
Nielsen, T. A., Kuiken, D., Alain, G., Stenstrom, P., and Powell, R. A. (2004). Immediate and delayed incorporations of events into dreams: further replication and implications for dream function. J. Sleep Res. 13, 327–336.
Nielsen, T. A., Levrier, K., and Montplaisir, J. (2011). Dreaming correlates of alexithymia among sleep-disordered patients. Dreaming 21, 16–31.
Nielsen, T. A., Ouellet, L., Warnes, H., Cartier, A., Malo, J. L., and Montplaisir, J. (1997). Alexithymia and impoverished dream recall in asthmatic patients: evidence from self-report measures. J. Psychosom. Res. 42, 53–59.
Nielsen, T. A., and Stenstrom, P. (2005). What are the memory sources of dreaming? Nature 437, 1286–1289.
Nielsen, T. A., Zadra, A. L., Simard, V., Saucier, S., Stenstrom, P., Smith, C., and Kuiken, D. (2003). The typical dreams of Canadian university students. Dreaming 13, 211–235.
Nir, Y., and Tononi, G. (2010). Dreaming and the brain: from phenomenology to neurophysiology. Trends Cogn. Sci. (Regul. Ed.) 14, 88–100.
Nofzinger, E. A., Mintun, M. A., Wiseman, M., Kupfer, D. J., and Moore, R. Y. (1997). Forebrain activation in REM sleep: an FDG PET study. Brain Res. 770, 192–201.
Okada, H., Matsuoka, K., and Hatakeyama, T. (2000). Dream-recall frequency and waking imagery. Percept. Mot. Skills 91(3 Pt 1), 759–766.
Oudiette, D., Leu, S., Pottier, M., Buzare, M. A., Brion, A., and Arnulf, I. (2009). Dreamlike mentations during sleepwalking and sleep terrors in adults. Sleep 32, 1621–1627.
Pagel, J. F. (2003). Non-dreamers. Sleep Med. 4, 235–241.
Palagini, L., Gemignani, A., Feinberg, I., Guazzelli, M., and Campbell, I. G. (2004). Mental activity after early afternoon nap awakenings in healthy subjects. Brain Res. Bull. 63, 361–368.
Peigneux, P., Laureys, S., Fuchs, S., Collette, F., Perrin, F., Reggers, J., Phillips, C., Degueldre, C., Del Fiore, G., Aerts, J., Luxen, A., and Maquet, P. (2004). Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron 44, 535–545.
Peigneux, P., Laureys, S., Fuchs, S., Delbeuck, X., Degueldre, C., Aerts, J., Delfiore, G., Luxen, A., and Maquet, P. (2001). Generation of rapid eye movements during paradoxical sleep in humans. Neuroimage 14, 701–708.
Petrides, M. (2005). Lateral prefrontal cortex: architectonic and functional organization. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360, 781–795.
Piolino, P., Desgranges, B., and Eustache, F. (2009). Episodic autobiographical memories over the course of time: cognitive, neuropsychological and neuroimaging findings. Neuropsychologia 47, 2314–2329.
Raymond, I., Nielsen, T. A., Lavigne, G., and Choinière, M. (2002). Incorporation of pain in dreams of hospitalized burn victims. Sleep 25, 765–770.
Rechtschaffen, A. (1978). The single-mindedness and isolation of dreams. Sleep 1, 97–109.
Revonsuo, A. (2000). The reinterpretation of dreams: an evolutionary hypothesis of the function of dreaming. Behav. Brain Sci. 23, 877–901; discussion 904–1121.
Richardson, A. (1979). Dream recall frequency and vividness of visual imagery. J. Ment. Imagery 3, 65–72.
Ruby, P., Collette, F., D’Argembeau, A., Peters, F., Degueldre, C., Balteau, E., Luxen, A., Maquet, P., and Salmon, E. (2009). Perspective taking to assess self personality: what is modified in Alzheimer’s disease? Neurobiol. Aging 30, 1637–1651.
Ruby, P., and Decety, J. (2001). Effect of subjective perspective taking during simulation of action: a PET investigation of agency. Nat. Neurosci. 4, 546–550.
Ruby, P., and Decety, J. (2003). What you believe versus what you think they believe: a neuroimaging study of conceptual perspective-taking. Eur. J. Neurosci. 17, 2475–2480.
Ruby, P., and Decety, J. (2004). How would you feel versus how do you think she would feel? A neuroimaging study of perspective-taking with social emotions. J. Cogn. Neurosci. 16, 988–999.
Ruby, P., Schmidt, C., Hogge, M., D’Argembeau, A., Collette, F., and Salmon, E. (2007). Social mind representation: where does it fail in frontotemporal dementia? J. Cogn. Neurosci. 19, 1–13.
Saint-Denys, H. (1977). Les Rẽves et les moyens de les diriger . Paris: D’Aujourd’hui. (Originally published in 1867).
Salzarulo, P. (1971). Electroencephalographic and polygraphic study of afternoon sleep in normal subjects. Electroencephalogr. Clin. Neurophysiol. 30, 399–407.
Sanford, L. D., Cheng, C. S., Silvestri, A. J., Mann, G. L., and Morrison, A. R. (2001). Sleep and behavior in rats with pontine lesions producing REM without atonia. Sleep Res. Online 4, 1–5.
Sastre, J. P., and Jouvet, M. (1979). Le comportement onirique du chat. Physiol. Behav. 22, 979–989.
Saurat, M. T., Agbakou, M., Attigui, P., Golmard, J. L., and Arnulf, I. (2011). Walking dreams in congenital and acquired paraplegia. Conscious. Cogn. 20, 1425–1432.
Scharf, B., Moskowitz, C., Lupton, M., and Klawans, H. (1978). Dream phenomena induced by chronic Levodopa therapy. J. Neural. Transm. 43, 143–151.
Schonbar, R. A. (1959). Some manifest characteristics of recallers and nonrecallers of dreams. J. Consult. Psychol. 23, 414–418.
Schredl, M. (1999). Dream recall: research, clinical implications and future directions. Sleep Hypn. 1, 72–81.
Schredl, M. (2002). Dream recall frequency and openness to experience: a negative finding. Pers. Individ. Dif. 33, 1285–1289.
Schredl, M. (2008). Dream recall frequency in a representative German sample. Percept. Mot. Skills 106, 699–702.
Schredl, M., Ciric, P., Gotz, S., and Wittmann, L. (2004). Typical dreams: stability and gender differences. J. Psychol. 138, 485–494.
Schredl, M., Frauscher, S., and Shendi, A. (1995). Dream recall and visual memory. Percept. Mot. Skills 81, 256–258.
Schredl, M., and Hofmann, F. (2003). Continuity between waking activities and dream activities. Conscious. Cogn. 12, 298–308.
Schredl, M., and Reinhard, I. (2008). Gender differences in dream recall: a meta-analysis. J. Sleep Res. 17, 125–131.
Schredl, M., Jochum, S., and Souguenet, S. (1997). Dream recall, visual memory, and absorption in imaginings. Pers. Individ. Dif. 2, 291–292.
Schredl, M., Wittmann, L., Ciric, P., and Gotz, S. (2003). Factors of home dream recall: a structural equation model. J. Sleep Res. 12, 133–141.
Schwartz, S. (1999). Exploration statistique et neuropsychologique des phénomènes oniriques au travers des textes et des images de rẽves . Ph.D. thesis, University of Lausanne, Lausanne, 375.
Schwartz, S., and Maquet, P. (2002). Sleep imaging and the neuro-psychological assessment of dreams. Trends Cogn. Sci. (Regul. Ed.) 6, 23–30.
Shapiro, A., Goodenough, D. R., and Gryler, R. B. (1963). Dream recall as a function of method of awakening. Psychosom. Med. 25, 174–180.
Shapiro, A., Goodenough, D. R., Lewis, H. B., and Sleser, I. (1965). Gradual arousal from sleep: a determinant of thinking reports. Psychosom. Med. 27, 342–349.
Shevrin, H., and Fritzler, D. E. (1968). Visual evoked response correlates of unconscious mental processes. Science 161, 295–298.
Solms, M. (1997). The Neuropsychology of Dreaming: A Clinico-Anatomical Study . Mahwah, NJ: Lawrence Erlbaum Associates.
Solms, M. (2000). Dreaming and REM sleep are controlled by different brain mechanisms. Behav. Brain Sci. 23, 843–850.
Stickgold, R., Hobson, J. A., Fosse, R., and Fosse, M. (2001). Sleep, learning, and dreams: off-line memory reprocessing. Science 294, 1052–1057.
Tart, C. T. (1962). Frequency of dream recall and some personality measures. J. Consult. Psychol. 26, 467–470.
Thompson, L. T., Moyer, J. R. Jr., and Disterhoft, J. F. (1996). Transient changes in excitability of rabbit CA3 neurons with a time course appropriate to support memory consolidation. J. Neurophysiol. 76, 1836–1849.
Trull, T. (2005). Clinical Psychology , 7th Edn. Belmont, CA: Thomson Wadsworth.
Uga, V., Lemut, M. C., Zampi, C., Zilli, I., and Salzarulo, P. (2006). Music in dreams. Conscious. Cogn. 15, 351–357.
Valli, K., Frauscher, B., Gschliesser, V., Wolf, E., Falkenstetter, T., Schönwald, S. V., Ehrmann, L., Zangerl, A., Marti, I., Boesch, S. M., Revonsuo, A., Poewe, W., and Högl, B. (2011). Can observers link dream content to behaviours in rapid eye movement sleep behaviour disorder? A cross-sectional experimental pilot study. J. Sleep Res. doi: 10.1111/j.1365-2869.2011.00938.x. [Epub ahead of print].
Valli, K., and Revonsuo, A. (2009). The threat simulation theory in light of recent empirical evidence: a review. Am. J. Psychol. 122, 17–38.
Valli, K., Revonsuo, A., Pälkäs, O., Ismail, K. H., Ali, K. J., and Punamäki, R. L. (2005). The threat simulation theory of the evolutionary function of dreaming: eidence from dreams of traumatized children. Conscious. Cogn. 14, 188–218.
Voss, U., Tuin, I., Schermelleh-Engel, K., and Hobson, A. (2010). Waking and dreaming: related but structurally independent. Dream reports of congenitally paraplegic and deaf-mute persons. Conscious. Cogn. 20, 673–687.
Wagner, U., Gais, S., Haider, H., Verleger, R., and Born, J. (2004). Sleep inspires insight. Nature 427, 352–355.
Wamsley, E. J., Tucker, M., Payne, J. D., Benavides, J. A., and Stickgold, R. (2010). Dreaming of a learning task is associated with enhanced sleep-dependant memory consolidation. Curr. Biol. 20, 850–855.
Wehrle, R., Czisch, M., Kaufmann, C., Wetter, T. C., Holsboer, F., Auer, D. P., and Pollmächer, T. (2005). Rapid eye movement-related brain activation in human sleep: a functional magnetic resonance imaging study. Neuroreport 16, 853–857.
Wittmann, L., Palmy, C., and Schredl, M. (2004). NREM sleep dream recall, dream report length and cortical activation. Sleep Hypn. 6, 53–57.
Zacks, J. M., Vettel, J. M., and Michelon, P. (2003). Imagined viewer and object rotations dissociated with event-related FMRI. J. Cogn. Neurosci. 15, 1002–1018.
Keywords: dream, neurophysiological correlates of dreaming, dream functions, unconscious, personal meaning, neuroimaging, psychoanalysis
Citation: Ruby PM (2011) Experimental research on dreaming: state of the art and neuropsychoanalytic perspectives. Front. Psychology 2 :286. doi: 10.3389/fpsyg.2011.00286
Received: 16 May 2011; Accepted: 10 October 2011; Published online: 18 November 2011.
Reviewed by:
Copyright: © 2011 Ruby. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Perrine M. Ruby, INSERM U1028, Lyon Neuroscience Research Center, Brain Dynamics and Cognition Team, Lyon F-69000, France. e-mail: perrine.ruby@inserm.fr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Greater Good Science Center • Magazine • In Action • In Education
Why Your Brain Needs to Dream
We often hear stories of people who’ve learned from their dreams or been inspired by them. Think of Paul McCartney’s story of how his hit song “Yesterday” came to him in a dream or of Mendeleev’s dream-inspired construction of the periodic table of elements.
But, while many of us may feel that our dreams have special meaning or a useful purpose, science has been more skeptical of that claim. Instead of being harbingers of creativity or some kind of message from our unconscious, some scientists have considered dreaming to be an unintended consequence of sleep—a byproduct of evolution without benefit.
Sleep itself is a different story. Scientists have known for a while now that shorter sleep is tied to dangerous diseases, like heart disease and stroke . There is mounting evidence that sleep deprivation leads to a higher risk of obesity and Alzheimer’s disease . Large population studies reflect a saddening truth—the shorter your sleep, the shorter your life . Not only that, sleep helps us to hold onto our memories and to learn facts and skills faster, making it important for everyone including infants, students, athletes, pilots, and doctors.

Much of this I outline in my new book, Why We Sleep: Unlocking the Power of Sleep and Dreams , which summarizes the many findings we have about sleep and its function in our lives.
But what about dreaming? Does it also have a purpose?
Recent work in my neuroscience lab and the work of other scientists has shown that dreams may have a very particular function important to our well-being. Here are the two main ways dreams help us.
Dreaming is like overnight therapy
It’s said that time heals all wounds, but my research suggests that time spent in dream sleep is what heals. REM-sleep dreaming appears to take the painful sting out of difficult, even traumatic, emotional episodes experienced during the day, offering emotional resolution when you awake the next morning.
REM sleep is the only time when our brain is completely devoid of the anxiety-triggering molecule noradrenaline. At the same time, key emotional and memory-related structures of the brain are reactivated during REM sleep as we dream. This means that emotional memory reactivation is occurring in a brain free of a key stress chemical, which allows us to re-process upsetting memories in a safer, calmer environment.
More on Sleep
Explore the neuroscience of sleep .
Learn how meditation can improve sleep .
Discover how sleeping poorly can cause conflict in your relationship .
Learn why sleep is key to peak performance .
How do we know this is so? In one study in my sleep center, healthy young adult participants were divided into two groups to watch a set of emotion-inducing images while inside an MRI scanner. Twelve hours later, they were shown the same emotional images—but for half the participants, the twelve hours were in the same day, while for the other half the twelve hours were separated by an evening of sleep.
Those who slept in between the two sessions reported a significant decrease in how emotional they felt in response to seeing those images again, and their MRI scans showed a significant reduction in reactivity in the amygdala, the emotional center of the brain that creates painful feelings. Moreover, there was a reengagement of the rational prefrontal cortex of the brain after sleep that helped maintain a dampening influence on emotional reactivity. In contrast, those who remained awake across the day showed no such dissolving of emotional reactivity over time.
That in itself doesn’t say anything about the role of dreaming. But we had recorded each participant’s sleep during the intervening night between the two test sessions, and we found that specific brain activity that reflected a drop in stress-related brain chemistry during the dream state determined the success of overnight therapy from one individual to the next.
Dreaming has the potential to help people de-escalate emotional reactivity, probably because the emotional content of dreams is paired with a decrease in brain noradrenaline. Support for this idea came from a study done by Murray Raskind on vets with PTSD, who often suffer debilitating nightmares. When given the drug Prazosin—a medication that lowers blood pressure and also acts as a blocker of the brain stress chemical noradrenaline—the vets in his study had fewer nightmares and fewer PTSD symptoms than those given a placebo. Newer studies suggest this effect can be shown in children and adolescents with nightmares, as well, though the research on this is still in its infancy.
The evidence points toward an important function of dreams: to help us take the sting out of our painful emotional experiences during the hours we are asleep, so that we can learn from them and carry on with our lives.
Dreaming enhances creativity and problem-solving
It’s been shown that deep non-REM sleep strengthens individual memories. But REM sleep is when those memories can be fused and blended together in abstract and highly novel ways. During the dreaming state, your brain will cogitate vast swaths of acquired knowledge and then extract overarching rules and commonalties, creating a mindset that can help us divine solutions to previously impenetrable problems.
How do we know dreaming and not just sleep is important to this process?
In one study , we tested this by waking up participants during the night—during both non-REM sleep and dreaming sleep—and gave them very short tests: solving anagram puzzles, where you try to unscramble letters to form a word (e.g., OSEOG = GOOSE). First, participants were tested beforehand, just to familiarize them with the test. Then, we monitored their sleep and woke them up at different points of the night to perform the test. When woken during non-REM sleep, they were not particularly creative—they could solve very few puzzles. But, when we woke up participants during REM sleep, they were able to solve 15-35 percent more puzzles than when they were awake. Not only that, participants woken while dreaming reported that the solution just “popped” into their heads, as if it were effortless.
In another study , I and my colleagues taught participants a series of relational facts—such as, A>B, B>C, C>D, and so on—and tested their understanding by asking them questions (e.g., Is B>D or not? ). Afterwards, we compared their performance on this test before and after a full night’s sleep, and also after they’d had a 60- to 90-minute nap that included REM sleep. Those who’d slept or had a long nap performed much better on this test than when they were awake, as if they’d put together disparate pieces of a jigsaw puzzle in their sleep.
Some may consider this trivial, but it is one of the key operations differentiating your brain from your computer. It also underlies the difference between knowledge (retention of individual facts) and wisdom (knowing what they all mean when you fit them together). The latter seems to be the work of REM-sleep dreaming.
“It’s said that time heals all wounds, but my research suggests that time spent in dream sleep is what heals”
Dreaming improves creative problem solving, too, according to another study . Participants learned to navigate a virtual maze using trial and error and aided by the placement of unique objects—like Christmas trees—at certain junctions in the maze. After this learning session, the group was split in two, with half napping and half watching a video for 90 minutes. Nappers were occasionally awoken to ask about the content of their dreams; those watching a video were also asked about thoughts going through their minds.
Afterwards, the participants again tried to solve the maze, and those who napped were significantly better at it than those who didn’t, as expected. But the nappers who reported dreaming about the maze were 10 times better at the task than those who napped and didn’t dream about the maze. There’s a reason you’ve never been told to stay awake on a problem.
Looking at the content of these dreams, it was clear that the participants didn’t dream a precise replay of the learning experience while awake. Instead, they were cherry-picking salient fragments of the learning experience and attempting to place them within the catalog of preexisting knowledge. This is how dreaming helps us be more creative.
While the benefits of dreaming are real, too many of us have problems getting a full eight hours of sleep and lose out on these advantages. Alternatively, we may think we’re the exception to the rule—that we’re one of those people who doesn’t happen to need a lot of sleep. But nothing could be further from the truth. Research clearly shows that people who overestimate their ability to get by on less sleep are sadly wrong.
Five ways to enhance your sleep
So how can we be sure to get enough sleep and experience a dream state? While we may be tempted to use sleeping pills to get to sleep, this has been shown to be detrimental to dreaming. Instead of taking pills, here are some simple ways to enhance your sleep:
1. Make sure your room is dark and that you are not looking at bright light sources—i.e., computer screens and cell phones—in the last hour or two before going to bed. You may even want to start dimming lights in your house in the earlier parts of the evening, which helps to stimulate sleepiness.
2. Go to bed and wake up at approximately the same time every day. This helps signal to your body a regular time for sleeping. It’s no use trying to sleep in a lot on weekends. There is no way to make up for regular sleep loss during the week.
3. Keep the temperature in your house cool at night—maybe even cooler than you think it should be, like around 65 degrees. Your body temperature needs to drop at night for sleep, and a lower room temperature helps signal your brain that it’s time to sleep.
4. If you have trouble falling asleep, or wake in the night feeling restless, don’t stay in bed awake. That trains the brain that your bed is not a place for sleeping. Instead, get up and read a book under dim light in a different room. Don’t look at your computer or cell phone. When sleepiness returns, then go back to bed. Or if you don’t want to get out of bed, try meditating. Studies suggest it helps individuals fall asleep faster, and also improves sleep quality.
5. Don’t have caffeine late in the day or an alcohol-infused nightcap. Both of these interfere with sleep—either keeping you awake or stimulating frequent wake-ups during the night.
Sleep is the single most effective thing we can do to rest our brain and physical health each day. Atop of sleep, dreaming provides essential emotional first aid and a unique form of informational alchemy. If we wish to be as healthy, happy, and creative as possible, these are facts well worth waking up to.
About the Author

Matthew Walker
Matthew Walker is a professor of psychology and neuroscience at the University of California, Berkeley, and the director of the university’s Center for Human Sleep Science .
You May Also Enjoy
Is Sleep the Most Important Happiness Habit?

Eight Ways to Help Teens Get More Sleep

Adventures in the Strange Science of Sleep

Does Sleeping Well Make Us More Socially Adept?

The Sleepless See Threats Everywhere

Tired Working Mother Sleep Saga

Nightmares, REM
Reviewed by Psychology Today Staff
Why humans dream remains one of behavioral science's great unanswered questions. Dreams have a purpose but it may not be to send us messages about self-improvement or the future, as many believe. Instead, many researchers now believe that dreaming mediates memory consolidation and mood regulation , a process a little like overnight therapy . But it's not a benefit all share equally: People who are sleep deprived also tend to be dream deprived, spending less time dreaming and perhaps not remembering dreams as well.
- What Dreams Mean
- Lucid Dreams

Dreams are the stories the brain tells during the REM (rapid eye movement) stage of sleep. People typically have multiple dreams each night that grow longer as sleep draws to a close. Over a lifetime, a person may dream for five or six full years. How best to examine all that content remains a source of debate.
Dreams typically involve elements from waking life , such as known people or familiar locations, but they also often have a fantastical feel. In dreams, people may live out scenarios that would never be possible in real life, although they aren’t always positive.
People have always tried to figure out the meaning of their dreams, but dream interpretation as a field of psychological study emerged in 1899, when Sigmund Freud published The Interpretation of Dreams . Today, most experts disagree with Freud’s conclusions, and some don’t believe dreams signify anything at all . But people continue to mine them for clues to their inner lives, creative insight, and even hints of the future.

Nightmares can create feelings of terror, anxiety , or despair, and lead to psychological distress or sleep problems like insomnia . Research has identified a range of causes for nightmares, including post- traumatic stress , anxiety—especially the presence of generalized anxiety disorder, dissociation, and physiological changes.
“Re-experiencing” is a common symptom of post-traumatic stress disorder, also known as flashbacks. These involuntary recollections often manifest in the form of nightmares that can cause significant emotional distress. Even when the dreams are not exact replays of a trauma, they may have a strong symbolic or indirect connection to the event.
Terrifying dreams that rouse people from sleep plague children more often than adults , and nightmares can be especially vivid for young children because they may have a harder time separating fantasy from reality. But at least half of grownups also have occasional nightmares, although fewer than 10 percent report frequent or recurring episodes.
Experts recommend that individuals experiencing nightmares tied to stress try to focus on positive elements of their day immediately before bed; catch themselves when they feel themselves ruminating or catastrophizing ; and train themselves not to dwell on disturbing images from nightmares. For nightmares tied to PTSD , visualization treatments in which patients replay traumatic memories in “safe” ways have shown potential to bring relief.
Not necessarily. Night terrors, which are primarily experienced by children, cause sleeping people to scream, bolt out of bed, or demonstrate symptoms similar to a panic attack. But night terrors tend to occur earlier in the sleep cycle , while nightmares take place primarily during REM sleep. And unlike nightmares, night terrors are usually not remembered by sufferers , even though they may appear to be awake during the experience.

During lucid dreaming, which most commonly occurs during late-stage REM sleep, a dreamer is aware that they’re asleep, but is able to control events within their dreams, to some extent. Lucid dreamers report willing themselves to fly, fight, or act out sexual fantasies . There are communities dedicated to learning how to lucid dream at will, although evidence that this is possible remains inconclusive.
Research suggests that the brain undergoes a physiological change during lucid dreaming. In fMRI studies, the prefrontal cortex and a cortical network including the frontal, parietal, and temporal zones have been shown to activate when the brain begins lucid dreaming. This appears related to the "waking consciousness” that characterizes lucidity.
Most people do not typically experience lucid dreaming, or do not realize they do, and those who do tend to experience it in a limited way, without full agency. But some experts, and advocates of the potential benefits of lucid dreaming for boosting creativity and confidence , and reducing stress, believe most people can train themselves to experience lucid dreams.
Advocates of lucid-dream training suggest starting with dedicated recording of one’s dreams to gain a greater awareness of the conscious roles they may already play in common scenarios . Another approach is waking up two hours earlier than normal, staying awake for a short time, and then going back to bed, with the goal of increasing awareness of fresh late-stage REM sleep dreams and eventually directing them.

The most powerful model for the future of dream interpretation will be a human-AI hybrid approach.

You will discover how getting stuck in a dream can promote movement in life.

A new study reveals the importance of idle periods for long-term memory formation.

Dream analysis involves finding the solution to a problem you are grappling with. Our unconscious proves sophisticated when a dream's location reveals the next step.

Common guidelines for sharing and interpreting dreams between people can be applied to AI systems, too. No need for reinventing the ethical wheel.
Daymares are negative, recurrent, and catastrophic fantasies. Here's a useful toolkit to stop them.

Every dream delivers a secret message. Apply these tools to find out what yours mean.

So often, we look for the personality traits that come to mind from a person's appearance in a dream; instead, try staying open to a specific "time in your life" they link you to.
Musings on babies, dreaming, meditating to slow down.

New advances in AI are making it possible to enhance, empower, and integrate Freudian and Jungian approaches to dream interpretation.
- Find a Therapist
- Find a Treatment Center
- Find a Psychiatrist
- Find a Support Group
- Find Online Therapy
- United States
- Brooklyn, NY
- Chicago, IL
- Houston, TX
- Los Angeles, CA
- New York, NY
- Portland, OR
- San Diego, CA
- San Francisco, CA
- Seattle, WA
- Washington, DC
- Asperger's
- Bipolar Disorder
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Self Tests NEW
- Therapy Center
- Diagnosis Dictionary
- Types of Therapy

At any moment, someone’s aggravating behavior or our own bad luck can set us off on an emotional spiral that threatens to derail our entire day. Here’s how we can face our triggers with less reactivity so that we can get on with our lives.
- Emotional Intelligence
- Gaslighting
- Affective Forecasting
- Neuroscience
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Environ Res Public Health

The Effects of Sleep Quality on Dream and Waking Emotions
Francesca conte.
1 Department of Psychology, University of Campania L. Vanvitelli, Viale Ellittico 31, 81100 Caserta, Italy; [email protected] (O.D.R.); [email protected] (M.L.R.); [email protected] (G.F.)
Nicola Cellini
2 Department of General Psychology, University of Padova, Via Venezia 8, 35131 Padova, Italy; [email protected]
3 Department of Biomedical Sciences, University of Padova, Via Ugo Bassi 58/B, 35131 Padova, Italy
4 Padova Neuroscience Center, University of Padova, Via Giuseppe Orus 2, 35131 Padova, Italy
5 Human Inspired Technology Center, University of Padova, Via Luzzatti 4, 35121 Padova, Italy
Oreste De Rosa
Marissa lynn rescott, serena malloggi.
6 Department NEUROFARBA, University of Firenze, Via di San Salvi 12, 50135 Firenze, Italy; [email protected] (S.M.); [email protected] (F.G.)
Fiorenza Giganti
Gianluca ficca, associated data.
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy reasons.
Despite the increasing interest in sleep and dream-related processes of emotion regulation, their reflection into waking and dream emotional experience remains unclear. We have previously described a discontinuity between wakefulness and dreaming, with a prevalence of positive emotions in wakefulness and negative emotions during sleep. Here we aim to investigate whether this profile may be affected by poor sleep quality. Twenty-three ‘Good Sleepers’ (GS) and 27 ‘Poor Sleepers’ (PS), identified through the Pittsburgh Sleep Quality Index (PSQI) cut-off score, completed three forms of the modified Differential Emotions Scale, assessing, respectively, the frequency of 22 emotions over the past 2 weeks, their intensity during dreaming and during the previous day. The ANOVA revealed a different pattern of emotionality between groups: GS showed high positive emotionality in wakefulness (both past 2 weeks and 24 h) with a significant shift to negative emotionality in dreams, while PS showed evenly distributed emotional valence across all three conditions. No significant regression model emerged between waking and dream affect. In the frame of recent hypotheses on the role of dreaming in emotion regulation, our findings suggest that the different day/night expression of emotions between groups depends on a relative impairment of sleep-related processes of affect regulation in poor sleepers. Moreover, these results highlight the importance of including sleep quality assessments in future dream studies.
1. Introduction
The interaction between sleep and affective brain function has received attention only in the last couple of decades. As pointed out by Walker and van der Helm [ 1 ], this delay appears surprising in light of two observations. On one hand, there is significant overlap between sleep physiology and the brain networks and neurochemical processes involved in affective modulation; in addition, sleep dysfunctions co-occur with remarkable frequency in most affective psychiatric disorders [ 1 ].
Despite the dearth of past research on the topic, recent work has begun to point out the importance of sleep for the regulation of emotions (see, e.g., [ 2 ] for a recent review). The role of sleep in affective processing is generally explained in light of the peculiar neurophysiology of sleep, and REM sleep in particular (see, e.g., [ 1 , 3 ]). In fact, this sleep state is associated with a relative deactivation of several areas of the neocortex [ 4 , 5 ], paralleling an increased activity in subcortical regions [ 4 , 6 ]. This pattern of activation, accompanied by the distinctive neurochemical balance occurring during REM sleep [ 7 , 8 ], is believed to provide optimal conditions for offline processing of emotional information.
In line with the prominent involvement hypothesized for REM sleep in emotional processing, the most recent theoretical approaches propose an important role of mental activity occurring during sleep (i.e., dreaming, according to Schredl and Wittman’s definition [ 9 ]) in these complex regulatory processes. At the biological level, it is supported by the existence of largely overlapping neural networks sustaining both (REM) dreaming and emotional processing (extensively reviewed in [ 10 ]). Indeed, several models propose that dreaming actively participates in the regulation of prior daytime emotions by facilitating the resolution of emotional conflicts [ 11 , 12 ], enhancing fear-extinction processes [ 3 ], and depotentiating the affective tone initially associated with waking events [ 1 ]. Another set of hypotheses focuses instead on the role of dreaming in optimizing affective reactions to future waking events: dreaming would allow an offline simulation of threatening or social episodes and a rehearsal of the corresponding threat- or social coping skills (respectively the “threat simulation theory” [ 13 ] and the “social simulation theory” [ 14 ]). Ultimately, both types of models converge in suggesting that waking and dream emotions are closely connected and that emotional processing occurring in dreams promotes adaptive behavioral responses to the challenges of waking life.
However, a clear understanding of the relationship between waking and dream emotions and their expression in subjective daytime consciousness and sleep mentation is still lacking. A recent study by our group [ 15 ] has addressed this issue in a sample of healthy adults: emotions of the last recalled dream, as well as those of the previous day and previous two weeks, were collected (through the modified Differential Emotions Scale, mDES [ 16 , 17 ]) and compared. Our findings mainly highlighted a discontinuity between waking and dream affect, with positive emotionality prevailing during the past two weeks as well as the day before the dream and reduced in the dream, while negative emotionality of the dream was similar to that of the preceding two weeks but significantly increased relative to the previous day. This interesting pattern of results opened the way to several hypotheses, such as the possibility that positive and negative emotions experienced in wakefulness may undertake different but parallel sleep-related regulation pathways.
As also suggested in the discussion of those findings [ 15 ], another intriguing hypothesis is that the relationships between waking and dream emotions (plausibly reflecting affective regulation processes) may be modulated by sleep quality. In fact, in the last couple of decades, a vast amount of research has focused on the effects of sleep disruption on several aspects of affective processing.
One night of sleep deprivation is sufficient to increase subjective reports of stress, anxiety, and anger in response to low-stress situations [ 18 ] and to increase impulsivity toward negative stimuli [ 19 ]. Moreover, after one night of sleep deprivation, subjects evaluated neutral pictures more negatively than control participants [ 20 , 21 ], independently of negative mood [ 20 ]. Impairments of emotion recognition [ 22 ] and expression [ 23 ] have been observed as well after single-night sleep deprivation.
Other studies provide evidence of emotional dysregulation following sleep deprivation using neural and physiological measures of emotionality. Enhanced amygdala reactivity in response to emotionally negative pictures, paralleled by a reduction of functional connectivity with medial prefrontal regions (believed to exert top-down regulatory control on the amygdala), has been detected after one night of sleep deprivation [ 24 ] as well as after five nights of sleep restriction [ 25 ]. Also, sleep loss has been shown to amplify pupil diameter responses during passive viewing of negative emotional pictures [ 26 ] and to increase sympathetic dominance of the autonomic nervous system, indexed by changes in heart rate variability [ 27 ].
An impact of sleep loss on affective processing has also been described in more ecologically relevant paradigms, i.e., based on cumulative sleep restriction protocols or on samples with impaired sleep quality. For instance, negative emotional changes have been reported in both adults [ 28 ] and adolescents [ 29 ] after several days of sleep restriction. Furthermore, poor subjective sleep quality has been associated with higher negative [ 30 , 31 ] and lower positive emotionality [ 30 , 31 , 32 ] and with decreased ability in cognitive reappraisal [ 33 ]. Habitual self-reported sleep quality has also been found to moderate the relationship between threat-related amygdala reactivity, negative affect, and perceived stress [ 34 ]. Furthermore, Tempesta et al. [ 21 ] showed that poor sleepers (classified through the Pittsburgh Sleep Quality Index, PSQI [ 35 ]) evaluated neutral pictures more negatively than good sleepers.
In sum, this brief review of data provides strong support to the idea that sleep disruption impairs affective regulation. In light of the aforementioned hypotheses on dreams as a reflection of ongoing emotional processing, dream emotions of individuals with disturbed sleep may represent an interesting object of study. The very few studies addressing this issue show that dreams of insomniacs [ 36 , 37 , 38 ] and narcolecptic subjects [ 39 ] are more negatively toned than those of good sleepers; also, nightmare frequency appears to be more elevated in individuals with poor sleep quality [ 40 , 41 , 42 , 43 ]. However, focusing exclusively on dream emotions, these studies do not allow the authors to make hypotheses on the possible differences between good and poor sleepers in emotion regulatory processes, which are probably better expressed in the relationships between waking and dream emotions rather than in dream emotions alone.
Indeed, several hypotheses on the presentation of waking and dream emotions in good and poor sleepers may be put forward. For instance, the profile of differences between daytime and dream emotionality observed in our previous study [ 15 ] could emerge in poor sleepers as well, indicating the presence of a similar pathway of affective processing notwithstanding the possible dysfunctionality of emotion regulation processes in poor sleepers observed in previous literature (e.g., [ 21 , 33 ]). Alternatively, poor sleepers could display an inverse pattern of emotionality in wakefulness and dreaming relative to good sleepers, with negative tone predominant in wakefulness and a positive rebound in sleep. Also, at variance with good sleepers, poor sleepers could manifest a more evenly distributed emotional tone (similar in both states of consciousness), and so on. The possibilities are multiplied when considering the time span over which these mechanisms unfold: for instance, each dream may process emotions experienced the day before, a few days before (in analogy with literature on the “dream lag” and “day-residue” effect [ 44 , 45 ]), or during wider daytime spans (e.g., the last few weeks, the general “time period”), etc.
Therefore, here we conduct an exploratory study to investigate the relationships between waking emotions and those of the subsequent night’s dreams in a sample of good and poor sleepers identified through the PSQI [ 35 ]. Specific aims of our study are:
- to compare, between good and poor sleepers, the prevalent emotional valence of the dream with that of the previous day and previous weeks;
- to assess the possibility that waking emotionality predicts dream emotionality in good and poor sleepers;
- to confirm findings from previous literature on dream emotional valence in good and poor sleepers using an instrument, the mDES [ 16 , 17 ], which addresses a repertoire of emotions broader than the ones commonly used in dream literature.
2. Materials and Methods
2.1. participants and procedure.
Figure 1 displays the recruitment and selection process. Four hundred volunteers from the cities of Naples and Caserta (Italy) were screened through a brief ad-hoc interview to collect general demographic data and information on medical conditions and life habits. The interview was conducted via telephone by a psychologist from the Sleep Lab of the University of Campania. Two hundred and twelve healthy participants (163 F, 49 M; mean age: 25 ± 8 years) were thus selected for the study, according to the following inclusion criteria: age between 18 and 65 years; absence of any relevant somatic or psychiatric disorder; absence of any sleep apnea or respiratory disorder symptoms; having a regular sleep–wake pattern; absence of sleep disorders; no history of drug or alcohol abuse; limited caffeine (no more than 150 mg caffeine per day, corresponding to about three cups of espresso or one cup of American coffee) and alcohol (no more than 250 mL per day) consumption.

Flowchart of the participant recruitment and selection process.
The whole selected sample ( N = 212) participated in a larger study [ 15 ], which included a validation of the Italian version of the mDES [ 16 , 17 ]. Thus, two forms of the questionnaire (WAKE-24 h and WAKE-2 weeks, assessing the frequency of specific emotions over the past 2 weeks and their intensity in the past 24 h, respectively) were administered to participants along with the Mannheim Dream Questionnaire (MADRE [ 46 ]) to collect data on dream recall frequency and several variables related to dreams, and the PSQI [ 35 ], in its Italian version [ 47 ], to assess habitual subjective sleep quality.
Of the 212 participants included in the validation study, 50 (38 F, 12 M; mean age: 24.6 ± 6.4 years) volunteered to take part in a second phase of the study, i.e., the assessment of relationships between waking and dream emotions. Participants received 10 copies of the WAKE-24 h mDES, with the instruction to complete one each night at bedtime, referring to the emotions experienced during that particular day. This had to be done until the day they recalled a dream. On the morning they recalled a dream, they had to fill in the DREAM mDES, specifically referring to the emotions experienced during the dream. Data collection was thus ended as soon as the mDES ratings of one dream were provided by each participant.
While our previous study [ 15 ] focused on differences between waking and dream emotions in the general sample, this study analyses the same dataset with regard to sleep quality, i.e., by dividing the final sample ( N = 50) into a group of ‘Good Sleepers’ and a group of ‘Poor Sleepers’ (GS and PS, respectively) based on the PSQI cut-off score (scores ≥ 5 indicate poor sleep quality [ 35 ]).
2.2. Instruments
- Italian version of the mDES: The original mDES [ 16 , 17 ] consists of 20 items corresponding to 20 different emotions (10 positive and 10 negative) whose intensity over the past 24 h is rated on a five-point Likert scale (from 0 = Not at all, to 4 = Extremely). Each category is described by three adjectives (e.g., “Grateful, appreciative, or thankful”): for clarity purposes, throughout the manuscript the noun referring to the first of the three adjectives will be used to identify specific emotion categories (e.g., “Gratefulness”). The Italian version [ 15 ] includes two additional positive emotions (“sexual/desiring/flirtatious” and “sympathy/concern/compassion”), which were included in the earlier version of the instrument [ 16 ]. In addition to this standard version (labeled WAKE-24 h mDES [ 15 ]), two other forms of the scale were developed in our previous study [ 15 ], assessing, respectively, the frequency of each emotion over the past two weeks (WAKE-2 weeks mDES) and the intensity of emotions experienced during the last recalled dream (DREAM mDES). The specific instructions provided in the DREAM and the WAKE-24 h mDES versions are: “Please think back to how you have felt during your last recalled dream/last 24 h. Using the 0–4 scale below, indicate the greatest amount that you’ve experienced each of the following feelings.” As for the WAKE-2 weeks form, the instructions are: “Please think back to how you have felt during the past two weeks. Using the 0–4 scale below, indicate the frequency with which you’ve experienced each of the following feelings.” (from 0 = Never, to 4 = Very frequently). The mDES also allows the use of aggregate measures of positive and negative emotionality (the Positive Affect (PA) and Negative Affect (NA) subscales, i.e., average scores of the positive and negative emotion items, respectively), which have shown to have high internal reliability, ranging from 0.82 to 0.94 [ 48 , 49 ]. The scale has been validated on the Greek [ 50 ] and Italian [ 15 ] populations and has shown to have good psychometric properties in its various translations [ 15 , 50 , 51 , 52 , 53 ].
- PSQI [ 35 ]: This questionnaire assesses sleep quality and disturbances over a 1-month time interval. It consists of 19 individual items which generate seven component scores: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication and daytime dysfunction. The sum of scores for these seven components yields one global score, ranging from 0 to 21, with 5 as a cut-off score which allows to differentiate good from poor sleepers [ 35 ] (higher scores indicate worse sleep quality). Here we use the Italian version of the PSQI [ 47 ], which has been validated on the Italian population [ 47 ].
- MADRE questionnaire [ 46 ]: This questionnaire measures several variables related to dreams such as frequency of dream recall, nightmares and lucid dreaming, attitude towards dreams and the effects of dreams on waking life. We report frequency of dreams, lucid dreams, and nightmares, as well as intensity of the dream experience, attitude towards dreams and correlates of dreams (the sum of items 13-14-15-16-17), all referring to how the contents of dreams are used in terms of problem solving and creativity (see [ 54 ]).
2.3. Data Analysis
Differences between GS and PS in age, gender distribution and MADRE scores were assessed using independent t -test, χ 2 (for categorical data) and Mann–Whitney test (for ordinal data). To assess the differences between groups in emotional valence of dreams and previous wakefulness, we conducted a 2 (Group: GS, PS) × 3 (Condition: WAKE-2 weeks, WAKE-24 h, DREAM) mixed ANOVA, with Δ mDES score (PA minus NA, i.e., an aggregate measure of valence, with positive values indicating positive valence and negative values indicating negative valence) as dependent variable. We used η 2 p as a measure of effect size and the Holm test for post-hoc analysis.
Also, in order to explore the potential predictors of dream emotions, we conducted, separately for GS and PS, a linear regression with DREAM Δ scores as dependent variables and WAKE-2 weeks and WAKE-24 h Δ scores as predictors. For each significant predictor, we reported the unstandardized (b) and the standardized (β) coefficient. All analyses were conducted using JAMOVI 1.2.27 and a p < 0.05 was considered statistically significant.
3.1. Descriptives
The sample was made up of 38 females (76%) and 12 males (24%), with an age range of 19 to 52 years.
A total of 50 DREAM mDES and 50 WAKE-2 weeks mDES (one per participant) were collected. As for the WAKE-24 h version, 84 scales were collected in total (50 referring to the day immediately preceding the dream and the remaining referred to the previous days); in fact, 30 participants (60%) recalled a dream after 1 night, 6 participants (12%) after 2 nights, and the remaining 14 (28%) after 3 nights. Only the 50 WAKE-24 h mDES scales (one per participant) referring to the day before the recalled dream were included in data analyses.
Twenty-seven participants (54% of the sample) reported a PSQI > 5 and were thus classified as PS [ 35 ], while the remaining 23 subjects (46%) made up the GS group. GS and PS were similar in terms of age (GS: 25.26 ± 7.39 vs. PS: 23.96 ± 5.04, t = 0.734, p = 0.466, Cohen’s d = 0.21) and gender distribution (GS: 5 M, 18 F vs. PS: 7 M, 20 F, χ 2 1 = 0.119, p = 0.730), while they significantly differed in PSQI scores (GS: 3.70 ± 0.92 vs. PS: 7.93 ± 1.83, t = −10.496, p < 0.001, Cohen’s d = −2.837).
3.2. MADRE Scores in Good and Poor Sleepers
The two groups showed similar dream frequency (median = 4, W = 301, p = 0.858), intensity of the dream experience (median = 2.5, W = 307.5, p = 0.959), attitude towards dreams (median = 2.4, W = 703.5, p = 0.770) and correlates of the dream experience (mean = 11, W = 286.5, p = 0.647). A significant difference was observed for the frequency of lucid dreams (W = 194.5, p = 0.023), with higher frequency in PS (median = 4) compared to GS (median = 3). The frequency of nightmares was nominally higher (W = 224.5, p = 0.091) in PS (median = 3) relative to GS (median = 4).
3.3. Characterisctics of Dream Emotions
3.3.1. good sleepers.
In GS, scores at the PA and NA subscales of the DREAM mDES showed a higher intensity of negative emotionality in the dream (PA: 0.80 ± 0.58 vs. NA: 1.40 ± 1.30; t22 = −2.29, p = 0.032, Cohens’ d = −0.48).
Looking at the specific emotions, all dreams contain at least 8 emotions and all of the 22 emotions are reported at least once. On average, GS reported 12.08 ± 4.79 dream emotions. As displayed in Figure 2 , the most frequent emotion is Sadness (reported by 91.3% of the participants), followed by Fear (82.6%) and Anger (78.3%), while the least frequent are Sensuality (30.4%) and Inspiredness (30.43%).

Proportion of Good Sleepers reporting each of the 22 emotions during the dream.
The most intensely experienced emotions during the dream were mostly negative ( Figure 3 ): Sadness (2.00 ± 0.28) was followed by Fear (1.87 ± 1.32), Stress (1.878 ± 1.28), Anger (1.70 ± 1.40), and Awe (1.61 ± 1.12).

Scores of each emotion in the WAKE-2 weeks, WAKE-24 h, and DREAM mDES in Good Sleepers. Panels ( a , b ) display positive and negative emotions, respectively. Error bars represent standard error of the means.
3.3.2. Poor Sleepers
Scores at the PA and NA subscales of the DREAM mDES did not differ (PA: 1.10 ± 0.76 vs. NA: 1.13 ± 0.81; t26 = −0.10, p = 0.925, Cohens’ d = −0.02), indicating an equal intensity of positive and negative emotionality in the dreams of PS.
As for specific emotions, all dreams contain at least 5 emotions and all of the 22 emotions are reported at least once. On average, PS reported 12.63 ± 4.39 dream emotions. As displayed in Figure 4 , the most frequent emotion is Awe (reported by 81.5% of the participants), followed by Pride (77.8%) and Solidarity (74.1%), while the least frequent are Gratefulness (37.04%) and Sensuality (29.6%).

Proportion of Poor Sleepers reporting each of the 22 emotions during the dream.
Although PA and NA scores did not differ, the most intensely experienced emotions during the dream were mostly negative ( Figure 5 ): Awe (1.74 ± 0.22) was followed by Anger (1.55 ± 0.25), Sadness (1.52 ± 0.26), Fear (1.48 ± 0.27), and Stress (1.41 ± 0.27).

Scores of each emotion in the WAKE-2 weeks, WAKE-24 h, and DREAM mDES in Poor Sleepers. Panels ( a , b ) display positive and negative emotions, respectively. Error bars represent standard error of the means.
3.4. Differences between Waking and Dream Emotions in Good and Poor Sleepers
The ANOVA on Δ mDES scores yielded a significant main effect of condition (F 2,96 = 15.41, p < 0.001, η p 2 = 0.24), with a decrease of delta scores (i.e., more negative emotionality) in the DREAM compared to WAKE-2 weeks and WAKE-24 h (all p holm ’s < 0.001), and no difference between WAKE-2 weeks and WAKE-24 h (p holm = 0.923). Although we did not find a main effect of Group (F 1,48 = 0.40, p = 0.528, η p 2 < 0.01), we observed a significant Group × Condition interaction (F 2,96 = 4.72, p = 0.011, η p 2 = 0.09, Figure 6 ): only GS displayed a reduction of delta scores from wakefulness to dream (WAKE-2 weeks vs. DREAM: p holm < 0.001; WAKE-24 h vs. DREAM: p holm < 0.001), while PS did not show any significant change (all p holm ’s > 0.644). No between-groups differences were observed in any of the three conditions (all p holm ’s > 0.643, Table 1 ).

Change in Δ mDES scores (PA minus NA) as a function of condition (WAKE-2 weeks, WAKE-24 h, and DREAM) in Good and Poor Sleepers. The orange area indicates positive affect and the blue area indicates negative affect. Error bars represent standard error of the means.
Mean and standard error of Δ scores in the two groups across conditions.
3.5. Predictors of Dream Emotional Valence (Δ mDES Scores) in Good and Poor Sleepers
In GS, linear regression analysis showed that neither WAKE-2 weeks nor WAKE-24 h Δ scores were predictive of DREAM Δ scores (F 2,20 = 0.04, p = 0.952, Adj. R2 < 0.01). The same result was observed in PS (F 2,24 = 0.99, p = 0.387, Adj. R2 < 0.01).
4. Discussion
This study investigated the relationships between dream emotions and those experienced during the previous days (both the day before the recalled dream and over the two weeks preceding it) in good and poor sleepers. In the frame of theoretical models on the role of dreaming in emotion regulation, postulating a close link between waking and dream emotionality, we aimed to assess the influence of poor sleep quality on this relationship. In fact, though previous literature has already shown the prevalence of negatively toned dreams in populations with disturbed sleep, we believe that affect regulation processes are plausibly better expressed in the interplay between waking and dream emotions rather than in dream emotions alone.
4.1. Proportion of Good and Poor Sleepers
Before discussing our main results, it is worth commenting on the high proportion of poor sleepers that emerged in our sample (54%). Considering that most of our participants were university students (mean age: 24.6 ± 6.4 years), this result is in line with those of several wide survey studies assessing the prevalence of poor sleep quality through the PSQI on similar populations and age groups. Indeed, the proportion of poor sleepers was over 40% in Mah et al. [ 55 ] and exceeded 60% in Lund et al. [ 56 ] and Becker et al. [ 57 ].
4.2. Results from the MADRE Questionnaire in Good and Poor Sleepers
Data from the MADRE questionnaire show that GS and PS are similar in most dream related variables, including dream frequency, intensity of dreams, attitude towards dreams, and perceived effects of dreams on waking life problem solving and creativity skills. However, PS show a higher frequency both of nightmares and lucid dreams. As for nightmares, this finding is consistent with previous studies showing increased nightmare frequency in poor sleepers [ 40 , 41 , 42 , 43 ]. Also, lucid dreaming has sometimes been associated with disrupted sleep [ 58 , 59 ]. Interestingly, nightmares and lucid dreaming have been conceptualized as belonging to a common domain involving unusual cognitions and perceptions in wakefulness and sleep [ 60 ], which would be linked to arousal and hypervigilance intruding in the sleep state [ 58 , 61 ] and thus could be viewed as indicators of poor sleep quality [ 58 ]. In other words, these hypotheses point to the existence of a close link between the quality of physiological sleep features and that of subjective sleep mentation.
4.3. Frequency and Valence of Dream Emotions in Good and Poor Sleepers
GS and PS reported on average a similar number of dream emotions (slightly more than 12), suggesting that the average amount of emotions (12.38) found in our previous study on the whole sample [ 15 ] was not affected by sleep quality. The number of emotions in our two samples is slightly higher than that reported in previous literature using the same self-report scale [ 62 ], probably because of methodological differences (see [ 15 ]).
As for emotional valence, GS displayed higher negative than positive emotionality (scores at the NA subscale) in the dream, whereas, in PS’ dreams, positive and negative emotionality appeared with equal intensity (no difference between PA and NA scores of the DREAM mDES). This finding well accounts for the one emerged in our previous study [ 15 ], in which NA scores were slightly higher than those at the PA subscale, but the difference failed to reach significance. The higher negative affect observed in GS is coherent with the finding that specific negative emotions were the most frequent as well as the most intense in this sample; also, it is in line with several previous studies showing a prevalence of negative emotions in dreams (e.g., [ 63 , 64 , 65 , 66 ], but see also [ 67 ] for a discussion on the differences between self and external ratings of dream emotions). Instead, the evenly distributed emotional tone observed in PS’ dreams apparently contradicts existing literature on populations with sleep impairments [ 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 ], which points to more negatively toned dreams in these individuals. However, our analysis of specific emotions showed that, although positive emotions were the most frequently reported by PS, their negative emotions were the most intense. In addition, it must be considered that: (a) the instrument we used is quite different from those commonly used in dream research, since it includes a much broader repertoire of emotions and a more balanced number of positive and negative items, thus reducing the risk of underestimating the presence of positive emotionality; (b) results obtained on sleep disordered populations [ 36 , 37 , 38 , 39 ] are not fully comparable to those observed in healthy samples reporting poor sleep quality; (c) the higher frequency of nightmares observed in individuals with poor sleep quality (both in our present study and in previous research [ 40 , 41 , 42 , 43 ]) does not necessarily imply that their dreams are generally more negatively valenced (in fact, emotionality may be viewed as a “tonic” feature of sleep mentation, while nightmares, or lucid dreams, may be better conceptualized as “phasic” events, although the notion of a continuum between bad dreams and nightmares is sustained by several authors [ 68 , 69 ]).
4.4. Relationships between Waking and Dream Emotions in Good and Poor Sleepers
The main finding of our study is the difference observed between GS and PS in the profile of waking and dream emotionality. While GS display a striking inflection of emotional tone from wakefulness to the dream (i.e., affective tone is prevalently positive both during the previous weeks and the previous day and becomes extremely negative in the dream), PS’ emotionality remains stable across conditions. Specifically, in PS, differences between positive and negative emotionality (i.e., delta values) are very close to zero in all three scales.
First of all, this pattern of data suggests that habitual sleep quality significantly affects the interplay of emotional expression across wakefulness and dreaming. This observation is particularly important in light of the numerous discrepancies existing in data on dream features and especially dream emotionality (see [ 62 , 67 , 70 ]). Controversial results in this field are usually explained through methodological biases as well as biases linked to the retrospective nature of dream descriptions [ 62 , 67 , 70 ]. Our data prompt us to consider sleep quality as an additional factor affecting dream emotional experience, and thus able to confound results when not controlled for. Therefore, we believe that future dream investigations should include assessments of sleep quality even when addressing nonclinical samples.
At the theoretical level, the differences observed between GS and PS appear to reflect a different functionality of sleep–wake emotion regulation processes, in line with the recent models on dream-related affect regulation [ 1 , 3 , 11 , 12 , 13 , 14 ]. In other words, the lack of oscillations in prevalent emotional valence of PS (expressed by their flattened curve of Δ mDES scores across daytime and sleep) may depend on a relative impairment of their emotion regulation processes, whose effectiveness would instead be expressed by the opposite emotional tone in wakefulness and dreams of GS. Specifically, GS display a prevalence of positive affect during daytime and negative affect during the dream. As suggested in Conte et al. [ 15 ], the negative emotions experienced more frequently or intensely in the general period in which the dream occurs would be those in need of regulation during sleep, whereas positive emotions, requiring less modulation, would be underrepresented in the dream. Also, the predominant positive affect observed during wakefulness in GS would at least partly depend on effective sleep-related modulation that occurred in previous dreams. As for PS, they showed lower positive emotionality than GS both during the two weeks and the day preceding the dream (although the differences between groups did not reach significance). This observation is in line with past literature showing lower well-being and positive affect in poor sleepers [ 30 , 31 , 32 , 71 ], which also is plausibly linked to less effective sleep-related affect modulation. Moreover, as suggested above, the fact that negative affect does not prevail in PS’ dreams could indicate poor functioning of sleep-related emotion regulation.
A complementary explanation may also be proposed, referring to the recent hypothesis of a dream rebound of thoughts suppressed during wakefulness [ 72 , 73 , 74 ], which, in turn, can be traced back to Freud’s idea [ 75 ] that dreams reflect the return of mental contents inhibited during the waking hours. This kind of mechanism was plausibly active in GS, whose negative emotions, excluded from waking consciousness in favor of positive ones, may have rebounded in the dream. The process of negative affect suppression could instead have been ineffective in PS, possibly due to the fact that disrupted sleep is linked to deficits in higher cognitive functions including inhibition (e.g., [ 19 , 76 , 77 ]). In line with Malinowski et al. [ 78 ], who showed that successful suppression of thoughts and their rebound in the dream benefit the emotional response to pleasant and unpleasant thoughts, it may be hypothesized that, in good sleepers, the dream rebound of negative emotions reflects their effective processing in sleep, irrespective of the specific episodic memories (thoughts, events, etc.) that generated them.
In sum, our findings are probably the result of two parallel mechanisms: a general day-night emotion regulation process (with prevalent negative emotions of daytime being processed in sleep and thus reappearing in dreaming) and the specific suppression (either deliberate or automatic) of certain negative emotions during wakefulness with consequent rebound in the dream for regulation purposes.
It must be acknowledged here that our regression analysis on waking and dream delta scores did not yield significant results. In fact, emotional tone of the previous day and previous two weeks did not predict that of the dream in either group of participants. This result is consistent with three other studies which found few [ 79 ], small [ 80 ], or no correlations [ 81 ] between corresponding dream and previous daytime emotions. The absence, to date, of data on direct relationships between waking and dream affect does not lend support to our main hypothesis, i.e., the interpretation of our data in the frame of theories on dream-related emotion regulation [ 1 , 3 , 11 , 12 , 13 , 14 ]. However, clearer associations between waking and dream affect could exist across different time spans and in different directions than those investigated here and in the abovementioned studies [ 79 , 80 , 81 ]. In fact, as pointed out in the introduction, each dream could process emotions experienced the day before, a few days before (in analogy with literature on the “dream lag” and “day-residue” effect [ 44 , 45 ]), or during wider daytime spans (e.g., the last few weeks, the general “time period”, etc.). Also, as predicted by the “simulation models” [ 13 , 14 ], dream emotionality could reveal stronger associations with future rather than past waking affect, a possibility to be investigated in forthcoming studies.
Furthermore, it may be speculated that poor sleepers rate their dreams as less negatively toned compared to good sleepers also because of a different general perception of the dreaming experience. In other words, they could retrospectively evaluate their dream experience as more positive than it actually was since the simple fact of having dreamed, per se, represents for them a sign of having slept well (good sleepers would obviously have no such bias). This interesting possibility could be usefully investigated in future research.
Finally, our findings allow us to extend the discussion of our previous work on the same sample [ 15 ], by underlining the influence exerted by poor habitual sleep quality on waking and dream emotional expression. In fact, here we observed that the opposite prevalent emotional tone of wakefulness and dreams, emerged in the previous study, well describes GS’ profile, while PS display an equal amount of positive and negative affect in both states. The hypotheses made on these findings are coherent with the main interpretations discussed in our previous work. However, the current data allow us to exclude a couple of alternative explanations advanced on those data [ 15 ]. Specifically, we proposed that participants may have undergone some sort of social desirability effect in compiling the scales (see, e.g., [ 82 ]); in other words, they would have more easily identified positive emotions (coherent with a positive image of the self) in wakefulness and negative emotions in the dream (which is experienced as “involuntary”). Similarly, we acknowledged a possible recall bias linked to the time frame of events to which the emotions refer. In the DREAM mDES, the participant is focusing on a much shorter time frame compared to those of the daytime scales (2 weeks and 24 h). Among this limited pool of memories, the negative ones could appear more salient and thus be more easily recognized (according to the widely held tenet in psychology that “bad is stronger than good” [ 83 ]). While these two hypotheses may have applied to our GS group, we see no reason why PS would not have equally undergone these types of biases: thus, the different emotional profile emerged in the latter group induces us to rule out these possibilities.
4.5. Limitations
Our results should be considered in light of some limitations to be overcome in future research. The main limitation is the use of a self-report measure of sleep quality rather than standard polysomnography for the identification of good and poor sleepers. However, it must be noted that groups of good and poor sleepers classified through the PSQI have been shown to significantly differ in polysomnographic sleep measures in several previous studies [ 35 , 84 , 85 ].
Furthermore, according to some authors [ 67 , 86 ], self-ratings of dream emotions based on emotion rating scales may be biased by demand characteristics of the rating task (i.e., individuals may be primed by answer options) or phenomena such as the positivity offset (i.e., the tendency to experience mildly positive mood most of the time); still, several authors argue that self-ratings more validly represent dream emotional experiences [ 65 , 87 ].
5. Conclusions
In conclusion, to the best of our knowledge, this is the first study to investigate differences between good and poor sleepers in the profile of emotionality across wakefulness and dreaming. Overall, our findings show that good sleepers experience a notable change in emotionality between wakefulness and dreaming, with a prevalence of positive affect during daytime and predominant negative affect during dreaming, whereas poor sleepers are characterized by equal intensity of positive and negative emotionality in both states. In the frame of recent theoretical models postulating a role of dreaming in affect regulation, the lack of changes in prevalent emotional valence across states observed in the latter group may be interpreted as reflecting ineffective sleep-related emotional processing. Furthermore, regardless of the theoretical framework, our results highlight that sleep quality is associated with notable differences in the expression of waking and dream emotions which should not be neglected in future dream research. Therefore, our findings definitely encourage researchers to include sleep quality assessments in dream studies (both on clinical and nonclinical samples) and prompt future investigations on sleep-impaired populations as a privileged object of study in the field of research on dreaming and emotion regulation processes.
Author Contributions
All authors contributed in a meaningful way to this manuscript. Conceptualization, F.C., F.G. and G.F.; Methodology, F.C. and G.F.; Formal analysis, N.C. and O.D.R.; Investigation, O.D.R. and S.M.; Writing—original draft preparation, F.C. and N.C.; Writing—review and editing, F.C., M.L.R. and G.F.; Visualization, F.C. and N.C.; Supervision, F.G. and G.F.; Project administration, F.C., F.G. and G.F. All authors have read and agreed to the published version of the manuscript.
This research received no external funding.
Institutional Review Board Statement
The study design was submitted to the Ethical Committee of the Department of Psychology, University of Campania “L. Vanvitelli”, which approved the research (code 1/2017) and certified that the involvement of human participants was performed according to acceptable standards.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

IMAGES
VIDEO
COMMENTS
The Science Behind Dreaming. New research sheds light on how and why we remember dreams--and what purpose they are likely to serve. For centuries people have pondered the meaning of dreams. Early ...
Contemporary dream research. Although dreams have fascinated us since the dawn of time, their rigorous, scientific study is a recent development[1-4] (Supplementary Fig. 1).In The interpretation of dreams [] Freud predicted that "Deeper research will one day trace the path further and discover an organic basis for the mental event."Recent work, which we review in this article, begins to ...
Dreams are one of the most fascinating and mystifying aspects of sleep. Since Sigmund Freud helped draw attention to the potential importance of dreams in the late 19th century, considerable research has worked to unravel both the neuroscience and psychology of dreams.
Dreaming is still a mystery of human cognition, although it has been studied experimentally for more than a century. Experimental psychology first investigated dream content and frequency. The neuroscientific approach to dreaming arose at the end of the 1950s and soon proposed a physiological substrate of dreaming: rapid eye movement sleep.
NREM dreams tend to be less emotional and visually vivid, as well as more thought-like (Cavallero, Cicogna, Natale, Occhionero and Zito, 1992; Hobson, Pace-Schott and Stickgold, 2000). Research suggests that lucid dreams, on the other hand, are predominantly a REM sleep phenomenon (LaBerge et al., 1986; LaBerge et al., 1981c). However, this ...
Dreaming is a multidisciplinary journal, the only professional journal devoted specifically to dreaming. The journal publishes scholarly articles related to dreaming from any discipline and viewpoint. This includes: biological aspects of dreaming and sleep/dream laboratory research; psychological articles of any kind related to dreaming;
Overall, both findings in subjects suffering from parasomnias and those related to "benign" phenomena (e.g., facial expressions, sleep talking), suggest that parasomnia-like episodes may open a new frontier in dream research making the oneiric production more accessible. 1.4.1 Nightmares
In line with this conceptualization of dreams, a large proportion of dream research 1,3,4,5,6,7 has been dedicated to quantifying various dimensions of people's dream reports and investigating ...
Abstract. This paper presents an evolutionary argument for the role of dreams in the development of human cognitive processes. While a theory by Revonsuo (2000) proposes that dreams allow for threat rehearsal and therefore provide an evolutionary advantage, the goal of this paper is to extend this argument by commenting on other fitness ...
Within the current clinical practice, the debate on the use of dream is still very topical. In this article, the author suggests to address this question with a notable scientific and cultural openness that embraces either the psychoanalytic approach (classical, modern and intersubjective), and the neurophysiological assumptions and both clinical research and cognitive hypotheses.
Abstract. The function of dreams is a longstanding scientific research question. Simulation theories of dream function, which are based on the premise that dreams represent evolutionary past ...
Theories of Dream Interpretation People have tried to decipher the meaning of dreams since the dawn of civilization, though scientific research on dreams is relatively new. The most prominent theories of dream interpretation include pioneers Trusted Source National Library of Medicine, Biotech Information The National Center for Biotechnology Information advances science and health by ...
Brain scans during sleep can decode visual content of dreams. Scientists have learned how to discover what you are dreaming about while you sleep. A team of researchers led by Yukiyasu Kamitani of ...
Lucid dream research "has been enjoying a renaissance over the last decade," says neuroscientist Tore Nielsen. He directs the Dream & Nightmare Laboratory at the Center for Advanced Research ...
Dreams are a common experience. Some are scary, some are funny. Recent research into how the brain works helps us understand why we dream. Strange combinations of ideas in our dreams may make us more creative and give us ideas that help us to solve problems. Or, when memories from the day are repeated in the brain during sleep, memories may get stronger. Dreams may also improve our moods ...
Conclusion: Collaboration between Neuroscience and Psychoanalysis Would Benefit Dream Research. Considering the issues that remain unresolved (e.g., neurophysiologic variability, parameter(s) influencing the emergence of representations in dreams, the meaning of dreams), a psychoanalytic perspective would certainly benefit dream research by ...
Research articles. Our dreams, our selves: automatic analysis of dream reports. Alessandro Fogli. ... It contains over 38 000 dream descriptions gathered from a variety of verified sources and research studies. Dream reports are annotated with their dates of recording, which span six decades (from 1960 to 2015), and are linked to free-text ...
Introduction. Whilst lucid dreaming (LD) is defined as being aware of dreaming whilst dreaming, a misconception exists in the public domain as a referral to controlling dream content and plot (Neuhäusler et al., 2018).This misconception reflects a number of widely-held beliefs about the nature of dreaming, which in part this commentary will seek to explain and rectify.
At the same time, key emotional and memory-related structures of the brain are reactivated during REM sleep as we dream. This means that emotional memory reactivation is occurring in a brain free of a key stress chemical, which allows us to re-process upsetting memories in a safer, calmer environment. Explore the neuroscience of sleep.
Dreams are the stories the brain tells during the REM (rapid eye movement) stage of sleep. People typically have multiple dreams each night that grow longer as sleep draws to a close. Over a ...
Most people dream 3-6 times per night, although many people will not remember dreaming at all. This article looks at some of the recent theories about why people dream, what causes them, what ...
1.1. The REM‐NREM sleep dichotomy. A classical view of the neurobiological basis of the oneiric activity postulates the existence of a close relationship between dream experience and REM sleep (Hobson et al., 2000; Nielsen, 2000).This hypothesis was based on early electroencephalographic (EEG) observations showing that >70% of individuals awakened during REM sleep reported dreams, while ...
1. Introduction. The interaction between sleep and affective brain function has received attention only in the last couple of decades. As pointed out by Walker and van der Helm [], this delay appears surprising in light of two observations.On one hand, there is significant overlap between sleep physiology and the brain networks and neurochemical processes involved in affective modulation; in ...