Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals

Mitochondria articles from across Nature Portfolio
Mitochondria are cytoplasmic organelles found in most eukaryotic cells. They are formed from an outer membrane, a highly invaginated inner membrane containing cristae, and a matrix. Mitochondria generate ATP through oxidative phosphorylation, and also have a central role in apoptosis.
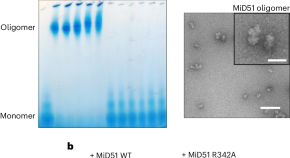
Regulation of mitochondrial fission by fatty acyl-coenzyme A
We show that the mitochondrial fission proteins MiD49 and MiD51 are activated by fatty acyl-coenzyme A (FA-CoA). FA-CoA binds in a previously identified pocket located within MiDs, inducing their oligomerization and ability to activate the dynamin DRP1, ultimately promoting mitochondrial fission. Activated MiDs synergize with mitochondrial fission factor (MFF) in stimulating DRP1 activity, leading us to hypothesize that MiDs act upstream of MFF during mitochondrial fission.
Related Subjects
- Energy metabolism
Latest Research and Reviews
Mitochondrial respiratory function is preserved under cysteine starvation via glutathione catabolism in NSCLC
The relevance of mitochondrial cysteine metabolism to ferroptosis is unknown. Here, Ward et al. show that mitochondrial Fe-S cluster synthesis persists under cysteine limitation via the catabolism of glutathione and at the expense of cell viability.
- Nathan P. Ward
- Sang Jun Yoon
- Gina M. DeNicola
Mitochondrial heterogeneity and adaptations to cellular needs
Granath-Panelo and Kajimura review emerging evidence of mitochondrial heterogeneity in different contexts and discuss how mitochondrial malleability contributes to cell fate determination and tissue remodelling.
- Melia Granath-Panelo
- Shingo Kajimura
Systematic analysis of NDUFAF6 in complex I assembly and mitochondrial disease
Sung et al. provide a powerful pipeline based on deep mutational scanning to elucidate the molecular mechanisms of mitochondrial complex I assembly and predict pathogenicity of mutations in complex I assembly factors.
- Andrew Y. Sung
- Rachel M. Guerra
- David J. Pagliarini
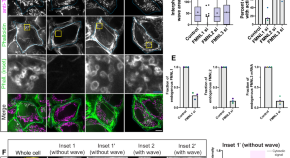
An interphase actin wave promotes mitochondrial content mixing and organelle homeostasis
A mitochondrial actin wave fragments mitochondria. Here, the authors find that the wave produces force that is resisted by mitochondrial tethering, inducing fission, with subsequent fusion promoting mitochondrial content mixing and mitochondrial homeostasis.
- Stephen M. Coscia
- Andrew S. Moore
- Erika L. F. Holzbaur
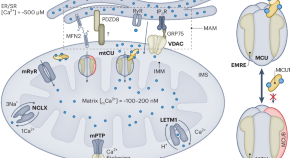
Mitochondrial calcium uniporter channel gatekeeping in cardiovascular disease
Stevens et al. review the current knowledge on the regulation of the mitochondrial calcium uniporter channel (mtCU) in cardiac physiology and disease.
- Tyler L. Stevens
- Henry M. Cohen
- John W. Elrod
Socialized mitochondria: mitonuclear crosstalk in stress
- Kyung Hwa Kim
News and Comment
Mitochondrial dynamics: updates and perspectives.
Mitochondria, the powerhouse and the vital signaling hub of the cell, participate in a variety of biological processes, such as apoptosis, redox responses, cell senescence, autophagy, and iron homeostasis. Mitochondria form a mostly tubular network, made up of an outer and a cristeae-forming inner membrane. The network undergoes dynamic fusion and fission that change its morphological structure according to the functional needs. Approximately 1500 mitochondrial proteins encoded by nuclear genome plus over 10 proteins encoded by mitochondrial DNA are folded and assembled in the mitochondria under a high-fidelity control system. These proteins are involved in oxidative phosphorylation, metabolism, network and cristae dynamics, mitophagy, import machinery, ion channels, and mitochondrial DNA maintenance. This Collection gathers original research that advances our understanding of the monitoring techniques and pathophysiological significance of mitochondrial dynamics in health and disease.
- Kezhong Zhang
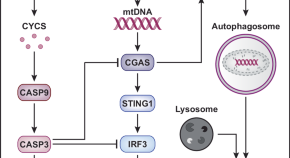
Inflammation and mitophagy are mitochondrial checkpoints to aging
Cellular and organismal aging have been consistently associated with mitochondrial dysfunction and inflammation. Accumulating evidence indicates that aging-related inflammatory responses are mechanistically linked to compromised mitochondrial integrity coupled with mtDNA-driven CGAS activation, a process that is tonically inhibited by mitophagy.
- Emma Guilbaud
- Kristopher A. Sarosiek
- Lorenzo Galluzzi
Neutrophil subsets
- Ioana Staicu
AMPK and insulin control the switch between mitochondrial hitchhiking of Pink1 mRNA and mitophagy in neurons
AMPK directly phosphorylates the mitochondrial protein SYNJ2BP to facilitate its interaction with the RNA-binding protein SYNJ2a, which transports Pink1 mRNA into neurites. AMPK inhibition downstream of insulin signalling untethers Pink1 mRNA from neuronal mitochondria and favours PINK1-dependent mitophagy in neurons. ApoE4-induced insulin receptor internalization reverses the process by stabilizing Pink1 mRNA binding to neuronal mitochondria.
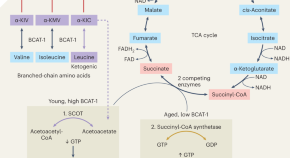
Slowing reproductive ageing by preserving BCAT-1
In this issue of Nature Metabolism , it is shown that the abundance of Caenorhabditis elegans branched-chain aminotransferase-1 (BCAT-1) — which catalyses the first step of branched-chain amino acid (BCAA) catabolism — declines sharply in aged wild-type nematodes but not in slowly ageing mutants, and that stimulating BCAA catabolism extends reproductive longevity.
- Leah E. Jamerson
- Patrick C. Bradshaw
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Suggestions or feedback?
MIT News | Massachusetts Institute of Technology
- Machine learning
- Social justice
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
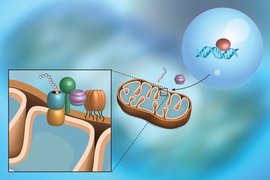
A “door” into the mitochondrial membrane
Press contact :.
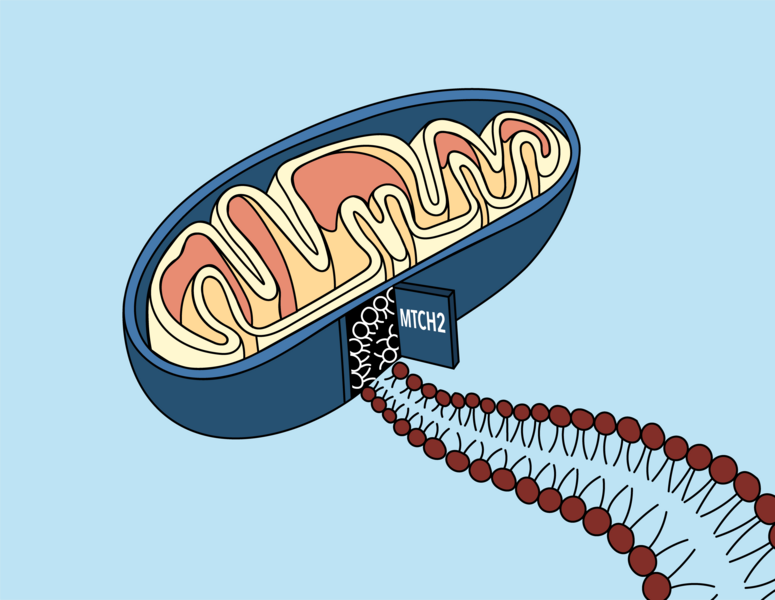
Previous image Next image
Mitochondria — the organelles responsible for energy production in human cells — were once free-living organisms that found their way into early eukaryotic cells over a billion years ago. Since then, they have merged seamlessly with their hosts in a classic example of symbiotic evolution, and now rely on many proteins made in their host cell’s nucleus to function properly.
Proteins on the outer membrane of mitochondria are especially important; they allow the mitochondria to communicate with the rest of the cell, and play a role in immune functions and a type of programmed cell death called apoptosis. Over the course of evolution, cells evolved a specific mechanism by which to insert these proteins — which are made in the cell’s cytoplasm — into the mitochondrial membrane. But what that mechanism was, and what cellular players were involved, has long been a mystery.
A new paper from the labs of MIT Professor Jonathan Weissman and Caltech Professor Rebecca Voorhees provides a solution to that mystery. The work, published Oct. 21 in the journal Science , reveals that a protein called mitochondrial carrier homolog 2, or MTCH2 for short, which has been linked to many cellular processes and even diseases such as cancer and Alzheimer’s, is responsible for acting as a “door” for a variety of proteins to access the mitochondrial membrane.
“Until now, no one knew what MTCH2 was really doing — they just knew that when you lose it, all these different things happen to the cell,” says Weissman, who is also member of the Whitehead Institute for Biomedical Research and an investigator of the Howard Hughes Medical Institute. “It was sort of a mystery why this one protein affects so many different processes. This study gives a molecular basis for understanding why MTCH2 was implicated in Alzheimer's and lipid biosynthesis and mitochondrial fission and fusion: because it was responsible for inserting all these different types of proteins in the membrane.”
“The collaboration between our labs was essential in understanding the biochemistry of this interaction, and has led to a really exciting new understanding of a fundamental question in cell biology,” Voorhees says.
The search for a door
In order to find out how proteins from the cytoplasm — specifically a class called tail-anchored proteins — were being inserted into the outer membranes of mitochondria, Weismann Lab postdoc and first author of the study Alina Guna, alongside Voorhees Lab graduate student Taylor Stevens and postdoc Alison Inglis, decided to use a technique called used the CRISPR interference (or CRISPRi) screening approach, which was invented by Weissman and collaborators.
“The CRISPR screen let us systematically get rid of every gene, and then look and see what happened [to one specific tail-anchored protein],” says Guna. “We found one gene, MTCH2, where when we got rid of it there was a huge decrease in how much of our protein got to the mitochondrial membrane. So we thought, maybe this is the doorway to get in.”
To confirm that MTCH2 was acting as a doorway into the mitochondrial membrane, the researchers performed additional experiments to observe what happened when MTCH2 was not present in the cell. They found that MTCH2 was both necessary and sufficient to allow tail-anchored membrane proteins to move from the cytoplasm into the mitochondrial membrane.
MTCH2’s ability to shuttle proteins from the cytoplasm into the mitochondrial membrane is likely due to its specialized shape. The researchers ran the protein’s sequence through Alpha Fold, an artificial intelligence system that predicts a protein’s structure through its amino acid sequence, which revealed that it is a hydrophobic protein — perfect for inserting into the oily membrane — but with a single hydrophilic groove where other proteins could enter.
“It's basically like a funnel,” Guna says. “Proteins come from the cytosol, they slip into that hydrophilic groove and then move from the protein into the membrane.”
To confirm that this groove was important in the protein’s function, Guna and her colleagues designed another experiment. “We wanted to play around with the structure to see if we could change its behavior, and we were able to do that,” Guna says. “We went in and made a single point mutation, and that point mutation was enough to really change how the protein behaved and how it interacted with substrates. And then we went on and found mutations that made it less active and mutations that made it super active.”
The new study has applications beyond answering a fundamental question of mitochondria research. “There's a whole lot of things that come out of this,” Guna says.
For one thing, MTCH2 inserts proteins key to a type of programmed cell death called apoptosis, which researchers could potentially harness for cancer treatments. “We can make leukemia cells more sensitive to a cancer treatment by giving them a mutation that changes the activity of MTCH2,” Guna says. “The mutation makes MTCH2 act more ‘greedy’ and insert more things into the membrane, and some of those things that have inserts are like pro-apoptotic factors, so then those cells are more likely to die, which is fantastic in the context of a cancer treatment.”
The work also raises questions about how MTCH2 developed its function over time. MTCH2 evolved from a family of proteins called the solute carriers, which shuttle a variety of substances across cellular membranes. “We're really interested in this evolution question of, how do you evolve a new function from an old, ubiquitous class of proteins?” Weissman says.
And researchers still have much to learn about how mitochondria interact with the rest of the cell, including how they react to stress and changes within the cell, and how proteins find their way to mitochondria in the first place. “I think that [this paper] is just the first step,” Weissman says. “This only applies to one class of membrane proteins — and it doesn't tell you all of the steps that happen after the proteins are made in the cytoplasm. For example, how are they ferried to mitochondria? So stay tuned — I think we'll be learning that we now have a very nice system for opening up this fundamental piece of cell biology.”
Share this news article on:
Related links.
- Voorhees Lab
- Weissman Lab
- Whitehead Institute
- Department of Biology
Related Topics
- Alzheimer's
- Collaboration
- Howard Hughes Medical Institute (HHMI)
Related Articles
New CRISPR-based map ties every human gene to its function

Pioneering researcher Jonathan Weissman joins Whitehead Institute and MIT
Countering mitochondrial stress
Previous item Next item
More MIT News
Janabel Xia: Algorithms, dance rhythms, and the drive to succeed
Read full story →

Jonathan Byrnes, MIT Center for Transportation and Logistics senior lecturer and visionary in supply chain management, dies at 75
Researchers develop a detector for continuously monitoring toxic gases

The beauty of biology

Navigating longevity with industry leaders at MIT AgeLab PLAN Forum

Jeong Min Park earns 2024 Schmidt Science Fellowship
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram
share this!
November 28, 2023
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Researchers uncover battery-like functions of mitochondria using super-resolution microscopes
by University of California, Irvine
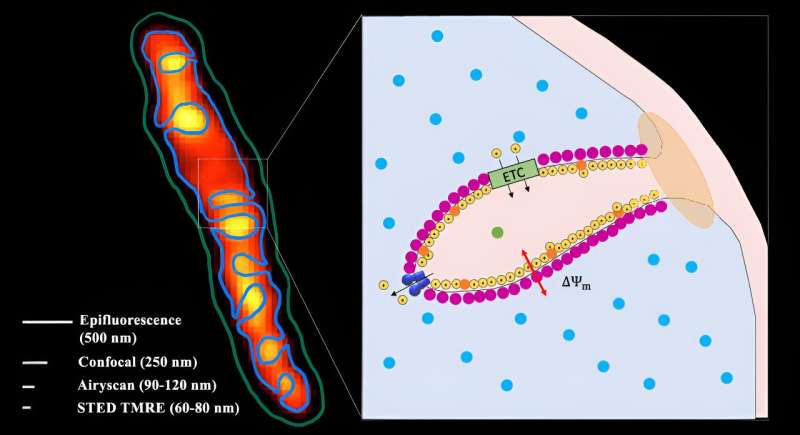
Using new super-resolution microscopes, researchers at the University of California, Irvine and the University of Pennsylvania have for the first time observed electrical charge and discharge functions inside mitochondria isolated from cells.
A mitochondrion is a structure within a cell that uses aerobic respiration to generate adenosine triphosphate , an organic compound that provides energy to support many processes in living tissues. Medical and biomedical engineering researchers have sought greater understanding of mitochondria, recognizing their importance in human health and disease.
While many past research projects have studied the physical characteristics of these components as they exist within living cells, the UCI-led project is the first to use super-resolution microscopes to study live, extracellular mitochondria. By observing the changes in the mitochondrial membranes under different metabolic states this way, the researchers were able to witness the electrophysiological functioning of these living organelles. The team's results were published in the journal ACS Nano .
"When we first started studying isolated mitochondria, we knew they behaved like a battery based on some work from the Tokyo Metropolitan Institute of Gerontology and UCLA, but we could not control them very well inside the cell to probe them," said co-author Peter Burke, UCI professor of electrical engineering and computer science. "Now we can control each individual electrical component and cause it to charge and discharge."
He said the work was made possible by a new generation of super-resolution microscopes. Team members used all three leading methods—Airy microscopy, stimulated emission depletion microscopy and lattice structured illumination microscopy—in their study.
This enabled them to examine cristae, repeating serpentine structures within mitochondria that measure about 100 nanometers. The shortest wavelength of visible light is violet at about 380 nanometers, Burke said, so they needed powerful instruments—super-resolution microscopes—to probe the voltage distribution of something less than a third of that size.
"Imagine trying to study how the battery pack in a Tesla works, but you can only do it by driving the car," he said. "You would not learn much about the battery pack inside the car."
By taking mitochondria out of the cell and keeping them alive, Burke and his collaborators— lead author ChiaHung Lee, UCI graduate student researcher in biomedical engineering, and Douglas Wallace of the University of Pennsylvania—were able to charge and discharge them.
"We could observe in detail how each individual part behaved as a single battery, much like how battery packs in drones and cars—which are many smaller batteries—individually combine to power the vehicle," Burke said. "Interestingly, we found that the batteries rearrange themselves when they charge and discharge, a feature not found in regular batteries."
He noted that his experiments proved what researchers had long thought while studying snapshots of frozen (dead) mitochondria: The internal structure changes in response to the metabolic needs of the cell. A mitochondrion can create and destroy its "batteries" (cristae) as needed. This shows that, unlike drones and Teslas, mitochondria can alter their internal shapes based on how much energy is needed by cells.
Burke said this work could have broad applications in human health, including studies on how humans age at the cellular level.
"Once we understand how they create energy, we can start to think of ways to modify this for improving human health and longevity," he said.
Journal information: ACS Nano
Provided by University of California, Irvine
Explore further
Feedback to editors
Composition of gut microbiota could influence decision-making
6 hours ago
Researchers realize multiphoton electron emission with non-classical light
7 hours ago

Saturday Citations: Mediterranean diet racks up more points; persistent quantum coherence; vegan dogs

Physicists propose path to faster, more flexible robots
13 hours ago

Scientists develop new geochemical 'fingerprint' to trace contaminants in fertilizer
May 17, 2024
Study reveals how a sugar-sensing protein acts as a 'machine' to switch plant growth—and oil production—on and off

Researchers develop world's smallest quantum light detector on a silicon chip

How heat waves are affecting Arctic phytoplankton

Horse remains show Pagan-Christian trade networks supplied horses from overseas for the last horse sacrifices in Europe
Ion irradiation offers promise for 2D material probing
Relevant physicsforums posts, dna-maternity test - could you see other relationship than mother, and now, here comes covid-19 version ba.2, ba.4, ba.5,..., is it usual for vaccine injection site to hurt again during infection.
May 16, 2024
A Brief Biography of Dr Virgina Apgar, creator of the baby APGAR test
May 12, 2024
Who chooses official designations for individual dolphins, such as FB15, F153, F286?
May 9, 2024
The Cass Report (UK)
May 1, 2024
More from Biology and Medical
Related Stories
Mitochondria work much like Tesla battery packs, study finds
Oct 15, 2019

Replacing tape used to make lithium-ion batteries could slow battery degradation and reduce self-discharge
Sep 29, 2023

Inside mitochondria and their fascinating genome
Sep 28, 2020

Investigating long-lived mitochondrial proteins
Aug 23, 2021
How mitochondria make the cut: When and where the powerhouse of the cell divides
May 5, 2021

Unexpected insights into the dynamic structure of mitochondria
Feb 18, 2020
Recommended for you

From fungi to fashion: Mushroom eco-leather is moving towards the mainstream

Researchers in Portugal develop an image analysis AI platform to boost worldwide research
Researchers use machine-learning modeling tools to improve zinc-finger nuclease editing technology
'Zombie cells' in the sea: Viruses keep the most common marine bacteria in check
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
May 13, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
New study reveals the key role of mitochondrial proteins in cardiac regeneration
by Centro Nacional de Investigaciones Cardiovasculares Carlos III (F.S.P.)
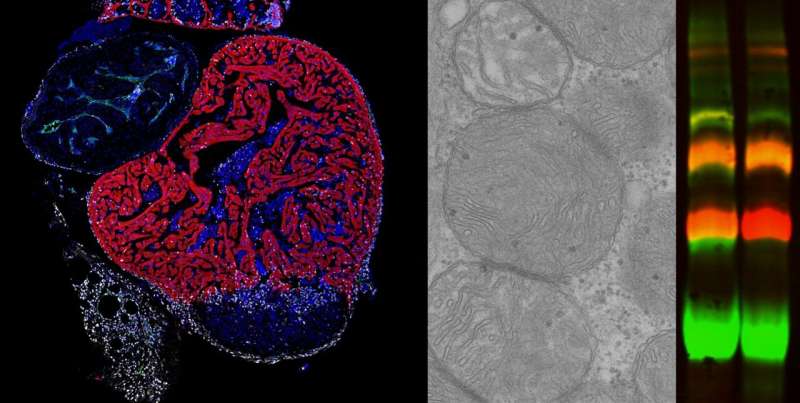
Mitochondria play an essential role in supplying the energy needed for correct cell function. Within mitochondria, energy production is generated by the respiratory chain, which is formed of five complexes called CI through CV. These complexes can assemble to form supercomplexes, but little is known about the role of this process or how it is controlled.
Now, a new study explores the mechanisms of supercomplex assembly and uncovers a major impact of mitochondrial assembly factors on cardiac regeneration. The study was led jointly by Dr. José Antonio Enríquez at the Centro Nacional de Investigaciones Cardiovasculares (CNIC) and Dr. Nadia Mercader, of the University of Berne in Switzerland, who is a visiting scientist at the CNIC.
The study, published in Developmental Cell , shows that a member of the Cox7a family of proteins plays a fundamental role in the assembly of CIV dimers and that this assembly is crucial for correct mitochondrial function, and thus for cellular energy production .
The Cox7a protein family includes three members: Cox7a1, Cox7a2, and Cox7a2l (also called SCAF1). Previous studies by both groups showed that when CIV contains SCAF1, it associates strongly with CIII to form a respiratory supercomplex known as the respirasome. In these previous studies, the authors postulated that inclusion of Cox7a2 would generate a CIV incapable of forming associations, while CIV molecules including Cox7a1 would associate together to form CIV homodimers. The new study demonstrates experimentally the role of Cox7a1 in the formation of these CIV homodimers.
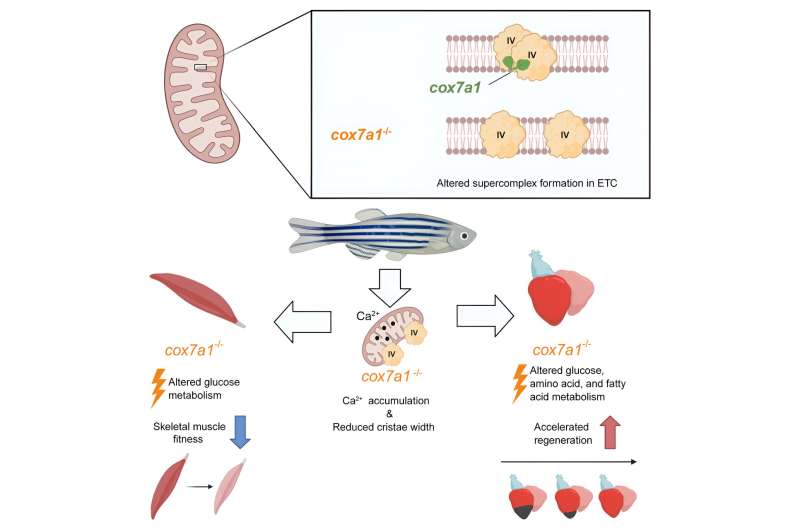
Working with the zebrafish model , the researchers found that the absence of Cox7a1 prevented the formation of CIV dimers, and the loss of these dimers influenced the weight and swimming ability of the affected fish.
"Cox7a1 is mainly expressed in striated muscle cells , and it is precisely skeletal muscle tissue that was most affected by the lack of Cox7a1 function. The other principal striated muscle type is the heart muscle, or myocardium," explained Dr. Enríquez.
But while the loss of Cox7a1 in skeletal muscle was detrimental, its loss in cardiac muscle improved the regenerative response of the heart to injury.
"This result shows that these proteins play a crucial role in the activation of the capacity for cardiac repair after injury," explained study first author Carolina García-Poyatos.
To explore the function of Cox7a1 in more depth, CNIC investigators Enrique Calvo and Jesús Vázquez performed a proteomic study of the skeletal muscle and myocardium of zebrafish lacking Cox7a1, and this analysis was expanded by a metabolomic study conducted by colleagues at the University of Berne. This collaborative analysis revealed profound differences from unaltered fish with intact Cox7a1 expression.
"These findings suggest that the molecules involved in mitochondrial supercomplex assembly can have a considerable effect on the control of metabolism, possibly opening the way to new treatments for cardiac diseases and other metabolic conditions," said Dr. Mercader.
According to the research team, this discovery represents "a significant advance in the understanding of the cellular mechanisms involved in cardiac regeneration and could point the way to the development of therapies aimed at promoting cardiac regeneration."
The authors conclude that mitochondrial assembly factors can significantly influence the control of metabolism.
Explore further
Feedback to editors

Modular communicative leadless ICD found to be safe and exceeds performance expectations
6 hours ago

Creativity and humor shown to promote well-being in older adults via similar mechanisms

Sweet taste receptor affects how glucose is handled metabolically by humans
8 hours ago

Better medical record-keeping needed to fight antibiotic overuse, studies suggest
14 hours ago

Repeat COVID-19 vaccinations elicit antibodies that neutralize variants, other viruses


A long-term ketogenic diet accumulates aged cells in normal tissues, new study shows
May 17, 2024

Gut bacteria enhance cancer immunotherapy in mouse study

Research finds the protein VISTA directly blocks T cells from functioning in immunotherapy
Study opens the door to designing therapies to improve lung development in growth-restricted fetuses
Researchers make strides in microbiome-based cancer therapies via iron deprivation in the tumor microenvironment
Related stories.
Scientists identify the mechanism that regulates mitochondrial energy production
Jun 24, 2020

Special cells contribute to regenerate the heart in zebrafish
Oct 24, 2019

Scientists describe the mechanism of heart regeneration in the zebrafish
Feb 12, 2018
Increasing skeletal muscle mitochondrial efficiency after weight loss as a novel mechanism for lower energy expenditure
Apr 18, 2023
GLA, the fatty acid that helps the heart to function properly after birth
May 24, 2023

Scientists identify targets to protect against anthracycline-induced cardiotoxicity
Apr 16, 2024
Recommended for you

New blood test for stroke detection combines blood-based biomarkers with a clinical score

Researchers reveal molecular mechanisms of different donor arteries for coronary artery bypass grafting
Consistent exercise changes how saturated fat is used by the body, study finds
May 16, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
- International edition
- Australia edition
- Europe edition
Can our mitochondria help to beat long Covid?
Mitochondria are the body’s power plants, fuelling our cells. New research shows they play a role in many aspects of keeping us healthy – and could be the key to unlocking treatments for chronic diseases, including Parkinson’s
A t Cambridge University’s MRC Mitochondrial Biology Unit, Michal Minczuk is one of a growing number of scientists around the world aiming to find new ways of improving mitochondrial health. This line of research could help provide much-needed treatments for people with long Covid, as well as revolutionising our understanding of everything from neurodegenerative illnesses such as Parkinson’s disease to the ageing process.
Mitochondria, tiny tube-shaped structures that are found in their hundreds, sometimes thousands, in nearly all of our cells, are best known as the body’s power plants, continuously converting the food we eat into ATP, a complex chemical that acts as a form of energy currency for cells. Without ATP, every one of our cells, from the brain to the muscles, would lack the fuel they need to keep churning away, and our organs would swiftly grind to a halt.
But while mitochondria are often typecast as energy factories, scientists have repeatedly discovered that they do far more than simply generate ATP. For one thing, they can help keep us warm when we are cold via an alternative form of heat generation to shivering, and studies have suggested that mitochondria in the eye even play a role in focusing light on to the retina , helping us perceive our environment.
In fact, the more we look, the more we find that they contribute to the many building blocks of life that keep us healthy, from synthesising the protein haemoglobin, which transports oxygen in the bloodstream, to storing calcium , and even the immune system response . While mitochondria sustain our cells, they also play a critical role in the natural process of cell death that occurs over and over again throughout our lives, identifying old and damaged cells which must be cleared away and destroyed.
Put simply they are vital to our survival, but like much of the body’s innate machinery, we only notice them when they start to go wrong. “Mitochondria are involved in many processes so when they don’t function well, this can precipitate different types of dysfunction in the human body leading to disease,” says Minczuk.

One of the unique complexities of mitochondria is that they have their own DNA, separate from the DNA stored in the nuclei of our cells, which comes from both parents. Mitochondrial DNA (mtDNA) is passed down from the mother only, and consists of fewer than 17,000 base pairs, compared with 3.3bn in the nucleus. But it still encodes specific instructions for a number of proteins, and over the past decade, scientists have found that mutations in mtDNA that prevent mitochondria from functioning normally can affect our health, contributing to a variety of chronic illnesses.
The most drastic cases are so-called mitochondrial diseases where mutations in mtDNA are acquired genetically. They affect around one in 4,300 people , and the consequences are grave. The life expectancy for most patients is between 10 and 35 years, with most dying from general body wasting owing to brain or muscle damage, or impairments to organs such as the heart and kidneys. But studies have also shown that mutations can accumulate in mtDNA as we age, and Minczuk’s research group at the University of Cambridge MRC mitochondrial biology unit is particularly interested in the role this might play in Parkinson’s.
It is thought that some Parkinson’s patients have genetic mutations that prevent damaged mitochondria being eliminated and replaced with healthy versions – a process called autophagy. As a result, the existing mitochondria in the body accrue more and more mutations, with damaging consequences for cells such as neurons, which rely heavily on the energy they supply.
But the rise of new gene-editing techniques may offer new treatment solutions in the years to come, initially for mitochondrial diseases but possibly for other illnesses too. This has been a challenge because Crispr technology – which uses a piece of RNA to guide an enzyme to a specific DNA location where it cuts out a mutation – cannot be used to tweak mitochondria, as it is not possible to deliver RNA into mtDNA.
However over the past few years, scientists including Minczuk have designed enzymes that can achieve the same effect as Crispr without requiring RNA. While studies are still being conducted on rodents, this offers enormous future potential.
“We’re slowly gathering the tools to be able to modify the mitochondrial genome in animal cells,” Minczuk says. “Right now we could eliminate existing mutations, changing the genetic make-up of mitochondria, but we also want to be able to trigger new mutations. This would allow us to study Parkinson’s in far more detail. We could take a healthy mouse, for example, and introduce mutations seen in Parkinson’s patients, and see what happens. Would that trigger the onset of symptoms?”
Treating long Covid
While hacking the mitochondrial genome could change healthcare in years to come, finding more immediate ways of improving mitochondrial health could help the millions of people with long Covid and chronic fatigue syndrome, also known as ME/CFS.
At Oxford University, cardiologist Betty Raman is currently in the middle of running a clinical trial to see whether an amino acid cocktail known as AXA1125, produced by Massachusetts-based biotech Axcella Therapeutics, can help long Covid patients where fatigue is by far the dominant symptom.

“The drug is a powdered drink, consumed three times a day along with meals, and we’re hoping that it will help people with their energy levels and fatigue,” she says. “The idea is that it can give the mitochondria additional fuel to produce energy, and help repair damaged mitochondria. Hopefully, by the end of July, we should have some top line results to report.”
The idea that mitochondria may be involved in the ailments of some of those with long Covid arises from research conducted by Raman and others on patients who find themselves chronically exhausted by exercise following Covid-19, despite showing no obvious heart or lung abnormalities. This symptom is often referred to as post-exertional malaise (PEM), and is also experienced by people with genetic mitochondrial diseases.
In long Covid patients with PEM, Raman has found that their muscles struggle to extract oxygen from the blood as efficiently as might be expected. After coming across research that showed that mitochondria in white blood cells were not as efficient in generating ATP in patients recovering from Covid-19, she concluded that this might be the root cause.
But why do the mitochondria of these patients become sluggish in generating ATP? David Systrom, a pulmonary and critical care doctor at Brigham & Women’s Hospital, Boston, believes he has found answers through studying patients with ME/CFS, an illness that in many cases is precipitated by viral infections such as Epstein-Barr and bears many similarities to long Covid.
When Systrom studied the mitochondrial DNA of these patients it appeared to be normal, but after taking a deep look and conducting muscle biopsies, he identified abnormalities at the electron level, deep within the mitochondria.
“In both ME/CFS and long Covid it’s most likely that these are acquired forms of mitochondrial dysfunction, perhaps related to the initial infection itself or an autoimmune response to a virus or both,” Systrom says. “This impedes the mitochondrial machinery, but doesn’t affect the DNA itself, and it means the mitochondria then fail to generate appropriate amounts of ATP to serve the needs of the muscles.”
Systrom is now running his own clinical trial in both ME/CFS and long Covid patients, in partnership with Japanese drug company Astellas, which has developed a drug that aims to restore normal mitochondrial metabolism.
Both Raman and Systrom agree that mitochondrial dysfunction is only likely to be a factor in a subset of long Covid and ME/CFS patients. However, because mitochondria are so ubiquitous throughout the body, damage inflicted to these structures across different organ types could contribute to the wide range of different symptoms that patients tend to report.
A common ailment reported by people with long Covid and ME/CFS is dysautonomia, a peculiar condition that causes a rapid increase in heartbeat and lightheadedness when patients attempt any form of activity. Raman says that this is often caused by damage to small sensory nerves in the skin, something that has been associated with mitochondrial dysfunction.
“There is a theory that the mitochondrial problem may come first,” she says. “And because nerves are high energy tissues, they are particularly dependent on normal mitochondrial function and ATP production.”
Learning from elite athletes
Different cell types have different numbers of mitochondria, owing to the varying energy requirements from one organ to the next. Organs with particularly high energy demands such as the brain, the heart and the pancreas tend to have more, which is why dysfunctional mitochondria have been linked to everything from cancer to type 2 diabetes and cardiovascular problems .
While mitochondria are not the main driving factor in any of these diseases, they are thought to be a key secondary factor. “The majority of heart failure or cardiac dysfunction is believed to be mediated by mitochondrial dysfunction involving the heart,” says Raman. “There’s a big metabolic component, and it has to do with the fact that the heart relies very much on continuous oxygen supply, but also that mitochondria are sensitive structures and can be affected by a number of risk factors.”
As a result, if mitochondrial drugs prove effective in long Covid and ME/CFS, they may have applications in other illnesses, while mitochondrial DNA editing to understand the effects of various mutations could shed further light on how the ageing process manifests in our cells.
Scientists are also taking some more left-field approaches to finding ways to improve mitochondrial health. At York University in Toronto, Chris Perry is looking at what we can learn from the mitochondria of elite athletes to help those with muscle diseases and even age-related sarcopenia.
As an example, Perry points out that endurance runners have high numbers of extra-efficient mitochondria which fuse to form extensive networks throughout their muscle tissues to deal with the stresses of prolonged exercise. Understanding the pathways that trigger mitochondria to adapt in this manner could lead to therapeutics to help people with different diseases, or keep us healthier in old age. This is already taking place in clinical trials, which have found that the dietary supplement urolithin A seems to improve mitochondrial health in older adults.
“When you get down to the cellular level, there are some surprising overlaps between exercise and disease, at least in the muscles,” says Perry. “Exercise creates enormous cellular stressors. It depletes ATP reserves, it causes physical strain on the cell membranes in the cytoskeleton, and it acidifies the muscle cells, which is exactly what happens in certain diseases.”
Studies have also shown that exercise itself can improve mitochondrial health in older adults who lead sedentary lifestyles, triggering proteins in mitochondria to cluster together in ways that allow them to pass electrons more efficiently.
“The basis of life is adaptation,” says Perry. “And that’s why exercise is good for us, because it exposes our cells to different stressors, which triggers these dedicated cellular feedback pathways to kick into action and regulate the situation. So when we exercise again, it is handled more efficiently. You slowly build those capacities as a result of that stress.”
- Medical research
- The Observer
- Coronavirus
- Infectious diseases
- Microbiology
Most viewed
Support Biology
Dei council and dei faculty committee, biology diversity community, mit biology catalyst symposium, honors and awards, employment opportunities, faculty and research, current faculty, in memoriam, areas of research, biochemistry, biophysics, and structural biology, cancer biology, cell biology, computational biology, human disease, microbiology, neurobiology, stem cell and developmental biology, core facilities, video gallery, faculty resources, undergraduate, why biology, undergraduate testimonials, major/minor requirements, general institute requirement, advanced standing exam, transfer credit, current students, subject offerings, research opportunities, biology undergraduate student association, career development, why mit biology, diversity in the graduate program, nih training grant, career outcomes, graduate testimonials, prospective students, application process, interdisciplinary and joint degree programs, living in cambridge, graduate manual: key program info, graduate teaching, career development resources, biology graduate student council, biopals program, postdoctoral, life as a postdoc, postdoc associations, postdoc testimonials, workshops for mit biology postdocs entering the academic job market, responsible conduct of research, postdoc resources, non-mit undergraduates, bernard s. and sophie g. gould mit summer research program in biology (bsg-msrp-bio), bsg-msrp-bio gould fellows, quantitative methods workshop, high school students and teachers, summer workshop for teachers, mit field trips, leah knox scholars program, additional resources, mitx biology, biogenesis podcast, biology newsletter, department calendar, ehs and facilities, graduate manual, resources for md/phd students, preliminary exam guidelines, thesis committee meetings, guidelines for graduating, mentoring students and early-career scientists, remembering stephen goldman (1962 – 2022), bringing new energy to mitochondria research.

Greta Friar | Whitehead Institute
September 17, 2020.
Tiny mitochondria in our cells turn oxygen and nutrients into usable energy in a process called respiration. This process is essential for powering our cells, and yet in spite of its importance many of the finer details of how it happens remain unknown. One long-standing mystery is how a molecule called nicotinamide adenine dinucleotide (NAD), which plays a big part in respiration and metabolism, gets into the mitochondria in humans and other animals. Mitochondria use NAD in order to produce adenosine triphosphate (ATP), the energy supply molecules used throughout the cell. Researchers knew the identities of the molecules that transport NAD from the wider cell into the mitochondria of yeast and plants, but had not found the animal equivalent—in fact, there was some debate over whether one even existed or whether animal cells used other methods altogether.
Now, research from postdoctoral researcher Nora Kory in Whitehead Institute Member David Sabatini’s lab may end the debate. In a paper published in Science Advances on September 9, the researchers show that the missing human NAD transporter is likely the protein MCART1. This discovery not only answers a longstanding question about a vital cellular process, but may contribute to research on aging—during which cells’ NAD levels drop—as well as research on diseases that involve certain mitochondrial dysfunctions, for which cells with broken NAD transporters could be an experimental model.
“I find it striking that mitochondria play such an important role in metabolism in the cell, which in turn plays a huge role in health and disease, but we still don’t understand how all of the molecules involved get in and out of mitochondria. It was exciting to fill in a piece of that puzzle.” Kory says.
AN UNEXPECTED DISCOVERY
Kory did not set out to find the long sought-after transport molecule. Rather, she was trying to better understand mitochondrial respiration by mapping the genes involved. She was comparing gene essentiality profiles, which show how important a gene is to different processes in a cell—the more co-essential two genes are, the more likely they are to be involved in the same cellular process—and one gene stood out: MCART1, also known as SLC25A51. It was highly correlated to other genes involved in mitochondrial respiration, and belonged to a family of genes known to code for transporters, yet its function was unknown. The protein coded for by MCART1 clearly played an important role, so Kory decided to figure out what that was; as her research progressed, she realized she had found the missing NAD transporter.
Kory and colleagues applied a common approach to determine MCART1’s function: inactivate the gene in cells, and see what breaks down in its absence. This approach is like troubleshooting a machine; if you cut a wire in your car and the headlights stop working, but everything else is fine, then that wire was probably linked to the headlights. When the researchers removed MCART1, the cells exhibited much lower oxygen consumption, reduced respiration and ATP production, and reliance on other, far less efficient means of ATP production—exactly what you’d expect to see if the inactivated gene was needed for respiration. Moreover, the biggest change that the researchers observed in cells without MCART1 was reduced levels of NAD in the mitochondria, while NAD levels in the wider cell remained the same, which they quantified using experiments previously developed in the lab. The researchers confirmed that MCART1 is essential for NAD transport into isolated mitochondria and overabundance of MCART1 caused an increased uptake.
“It’s very satisfying when our lab returns to the techniques that we have developed in order to make new findings such as identifying this important protein,” says Sabatini, who is also a professor of biology at Massachusetts Institute of Technology and an investigator with the Howard Hughes Medical Institute.
The evidence supports that the protein MCART1 is itself the transport channel. However, it is possible that the protein may play some other essential contributing role to transportation, or that it combines with other molecules to do its job. To strengthen the case for MCART1 as the transporter, the researchers showed that MCART1 and the known yeast NAD transport could be switched out for each other in both human and yeast cells, suggesting an equivalent function. Still, further experiments are needed to determine the precise mechanism of transport.
A serendipitous case of synchronous discovery reinforces Kory’s findings. A paper by other researchers published on the same day in the journal Nature also put forth that MCART1 is the missing NAD transporter, based on a completely different set of evidence. Combined, the papers provide an even more compelling case.
“It was nice to see how our different approaches complemented each other, and led to the same conclusion,” Kory says.
Understanding how NAD gets into the mitochondria opens up new questions about the details of mitochondrial respiration. Kory will shortly be leaving Sabatini’s lab to open her own lab at the Harvard T.H. Chan School of Public Health, where she intended to continue investigating the role of the mitochondria’s NAD supply in metabolism and signaling.
Written by Greta Friar
David Sabatini’s primary affiliation is with Whitehead Institute for Biomedical Research, where his laboratory is located and all his research is conducted. He is also a Howard Hughes Medical Institute investigator and a professor of biology at Massachusetts Institute of Technology.
Kory, N., et al. (2020). MCART1/SLC25A51 is required for mitochondrial NAD transport. Science Advances. doi:10.1126/sciadv.abe5310
Luongo, T. S., et al. (2020). SLC25A51 is a mammalian mitochondrial NAD+ transporter. Nature. doi:10.1038/s41586-020-2741-7
New Research to Plumb the Mysteries of Mitochondria, Yielding Insight into Evolution, Food Security and Climate Change
AMHERST, Mass. – The National Science Foundation (NSF) recently announced that it will support the efforts of a collaborative group of researchers, led by Elizabeth Vierling , Distinguished Professor of Biochemistry at the University of Massachusetts Amherst, who plan to spend the next four years investigating the role that mitochondria play in plant productivity . This research has immediate implications for ensuring that agriculture can meet the challenge of global warming.
“One of the things that fascinates me,” says Vierling, “is to see, at the cellular level, how much of life is the same, but also to see the tiny differences that give life on Earth such diversity.” One of those similarities is that the cells of every living organism more complex than bacteria contain mitochondria—the cell’s engine. Mitochondria are tiny, rod-shaped engines that convert oxygen and nutrients into a chemical known as ATP, which powers the cell.
Yet, while both plant and animal cells contain the mitochondria that power cells, the mitochondria in plants function differently than the mitochondria in animals. That’s because plants also have chloroplasts, which, Vierling says, “are the cell’s food factories” and are responsible for photosynthesis: the transformation of light into food that the cell can use. “The mitochondria in plants have to work with the chloroplasts—even though the mitochondria in both plants and animal cells do so many of the same things,” says Vierling. “How do mitochondria know what kind of cell they’re in? How do they know how to act? It’s an evolutionary mystery.”
To answer these unknowns, Vierling will be joining with colleagues at Dartmouth, San Diego State University, and the University of Georgia to investigate the role that a protein known as ATAD3 plays in the mitochondria.
ATAD3 is present in all higher forms of life, from fruit flies to tomatoes to humans, and, when it is defective, can cause a variety of serious health issues for humans. “Neither humans nor plants can live without ATAD3,” says Vierling, “and we think that it is one component that tells the mitochondrion whether it is inside a plant or animal cell.”
If it is indeed true that ATAD3 can tell the mitochondria when to act like an animal and when to act like a plant, then it is indeed one of the tiny differences responsible for life’s diversity.
In order to pinpoint the role of ATAD3, the project will rely on the evolutionary bioinformatics expertise of Elizabeth Waters at San Diego State University, and experiments with two plant “lab rats,” the flowering plant Arabidopsis thaliana, which will be the focus of the Vierling lab’s research and a moss, Physcomitrium patens, in the lab of Magdalena Bezanilla at Dartmouth. The group’s synergy is a major strength that led to the successful NSF application.
But there’s more. “It turns out that minor disruptions to ATAD3 somehow make plants more-heat tolerant,” says Vierling. “We don’t understand why you can disrupt the ATAD3’s function and get a better response to high temperatures, but that’s part of what we want to find out, and it may be a crucial trait for adapting to a warming world.”
A major component of the project includes developing and implementing a model program for enhancing STEM workforce diversity to train the next generation of biotech researchers. Each of the project’s primary investigators will hire students to help conduct the research—Vierling’s lab will take on 5 to 6 undergraduate students over the next four years. San Diego State’s Waters, along with Paula Lemons, a STEM education expert at the University of Georgia, will lead a program called “BioTech at San Diego State University”. All four senior researchers will work with more than 40 undergraduates from underrepresented backgrounds to train them in scientific research methods, link them to summer internships in biotech companies and engage them in a two-year learning community for professional development. Furthermore, the program can be a model for other institutions to enhance the diversity of the growing biotech workforce.
Articles on Mitochondria
Displaying 1 - 20 of 24 articles.

Why do people and animals need to breathe? A biologist explains why you need a constant source of oxygen
Christina S. Baer , Binghamton University, State University of New York

Hangry bacteria in your gut microbiome are linked to chronic disease – feeding them what they need could lead to happier cells and a healthier body
Christopher Damman , University of Washington

Alzheimer’s disease: problems with the brain’s energy supply could be a cause
Afshan Malik , King's College London

Cells routinely self-cannibalize to take out their trash, aiding in survival and disease prevention
Åsa Gustafsson , University of California, San Diego and Justin Quiles , University of California, San Diego

How COVID-19 damages lungs: The virus attacks mitochondria, continuing an ancient battle that began in the primordial soup
Stephen L Archer , Queen's University, Ontario

Alzheimer’s might not be primarily a brain disease. A new theory suggests it’s an autoimmune condition.
Donald Weaver , University of Toronto

Sea otters demonstrate that there is more to muscle than just movement – it can also bring the heat
Traver Wright , Texas A&M University ; Melinda Sheffield-Moore , Texas A&M University , and Randall Davis , Texas A&M University

Disputes over when life begins may block cutting-edge reproductive technologies like mitochondrial replacement therapies
Walter G. Johnson , Arizona State University and Diana Bowman , Arizona State University

Curious Kids: what are cells made out of?
Georgia Atkin-Smith , La Trobe University and Ivan Poon , La Trobe University

Light versus dark – the color of the turkey meat is due to the job of the muscle
Joshua Selsby , Iowa State University

3-parent IVF could prevent illness in many children (but it’s really more like 2. 002-parent IVF)
David Thorburn , Murdoch Children's Research Institute and John Christodoulou , Murdoch Children's Research Institute

Botswana is humanity’s ancestral home, claims major study – well, actually …
Isabelle Catherine Winder , Bangor University

Out of my wheelchair and back on my bike: why I’m putting MS diet to the test
Terry Wahls , University of Iowa

What the ban on gene-edited babies means for family planning
Marie Menke , University of Pittsburgh

Study shows mitochondrial DNA can be passed through fathers – what does this mean for genetics?
Michael Porter , University of Central Lancashire

Math shows how DNA twists, turns and unzips
Mariel Vazquez , University of California, Davis

Mitochondria mutation mystery solved: Random sorting helps get rid of duds
Arunas L. Radzvilavicius , University of Pennsylvania

The Force of biology is strong in Star Wars
Allison E. McDonald , Wilfrid Laurier University

It’s mostly mothers who pass on mitochondria – and a new theory says it’s due to the first sexual conflict

Explainer: what are mitochondria and how did we come to have them?
Steven Zuryn , The University of Queensland
Related Topics
- Mitochondrial disease
- Mitochondrial DNA
- Neurodegenerative disease
Top contributors
Postdoctoral Researcher of Evolutionary Biology, University of Pennsylvania
Emeritus Professor, University of Cape Town
The University of Adelaide Research Fellow, University of Adelaide
Professor, University of Oxford
Senior Research Fellow , University of Adelaide
Associate Professor , University of Adelaide
Clinical Professor of Internal Medicine, University of Iowa
Group Leader, The University of Queensland
Professor of Biology, Wilfrid Laurier University
MB BS Phase 1 Lead & Senior Lecturer in Medicine and Molecular Genetics, University of York
Professor of Mathematics, University of California, Davis
Research scientist, La Trobe University
Director of Translational Institute of Medicine (TIME), Queen's University, Ontario
Assistant Professor of Obstetrics, Gynecology & Reproductive Sciences, University of Pittsburgh
Chair in Zoology, University of Aberdeen
- X (Twitter)
- Unfollow topic Follow topic

- News Releases
New research to plumb the mysteries of mitochondria, yielding insight into evolution, food security and climate change
Researchers at UMass Amherst to lead collaborative, $2.3 million NSF-supported effort
University of Massachusetts Amherst
image: The mitochondria (green) and chloroplasts (pink) of the moss Physcomitrium patens. “Wildtype” is the control sample, while “atad3a” shows a mutant where ATAD3 has been disrupted, yielding enlarged, oddly shaped mitochondria. view more
Credit: Magdalena Benzanilla
AMHERST, Mass. – The National Science Foundation (NSF) recently announced that it will support the efforts of a collaborative group of researchers, led by Elizabeth Vierling , Distinguished Professor of Biochemistry at the University of Massachusetts Amherst, who plan to spend the next four years investigating the role that mitochondria play in plant productivity . This research has immediate implications for ensuring that agriculture can meet the challenge of global warming.
“One of the things that fascinates me,” says Vierling, “is to see, at the cellular level, how much of life is the same, but also to see the tiny differences that give life on Earth such diversity.” One of those similarities is that the cells of every living organism more complex than bacteria contain mitochondria—the cell’s engine. Mitochondria are tiny, rod-shaped engines that convert oxygen and nutrients into a chemical known as ATP, which powers the cell.
Yet, while both plant and animal cells contain the mitochondria that power cells, the mitochondria in plants function differently than the mitochondria in animals. That’s because plants also have chloroplasts, which, Vierling says, “are the cell’s food factories” and are responsible for photosynthesis: the transformation of light into food that the cell can use. “The mitochondria in plants have to work with the chloroplasts—even though the mitochondria in both plants and animal cells do so many of the same things,” says Vierling. “How do mitochondria know what kind of cell they’re in? How do they know how to act? It’s an evolutionary mystery.”
To answer these unknowns, Vierling will be joining with colleagues at Dartmouth, San Diego State University, and the University of Georgia to investigate the role that a protein known as ATAD3 plays in the mitochondria.
ATAD3 is present in all higher forms of life, from fruit flies to tomatoes to humans, and, when it is defective, can cause a variety of serious health issues for humans. “Neither humans nor plants can live without ATAD3,” says Vierling, “and we think that it is one component that tells the mitochondrion whether it is inside a plant or animal cell.”
If it is indeed true that ATAD3 can tell the mitochondria when to act like an animal and when to act like a plant, then it is indeed one of the tiny differences responsible for life’s diversity.
In order to pinpoint the role of ATAD3, the project will rely on the evolutionary bioinformatics expertise of Elizabeth Waters at San Diego State University, and experiments with two plant “lab rats,” the flowering plant Arabidopsis thaliana , which will be the focus of the Vierling lab’s research and a moss, Physcomitrium patens , in the lab of Magdalena Bezanilla at Dartmouth. The group’s synergy is a major strength that led to the successful NSF application.
But there’s more. “It turns out that minor disruptions to ATAD3 somehow make plants more-heat tolerant,” says Vierling. “We don’t understand why you can disrupt the ATAD3’s function and get a better response to high temperatures, but that’s part of what we want to find out, and it may be a crucial trait for adapting to a warming world.”
A major component of the project includes developing and implementing a model program for enhancing STEM workforce diversity to train the next generation of biotech researchers. Each of the project’s primary investigators will hire students to help conduct the research—Vierling’s lab will take on 5 to 6 undergraduate students over the next four years. San Diego State’s Waters, along with Paula Lemons, a STEM education expert at the University of Georgia, will lead a program called “BioTech at San Diego State University”. All four senior researchers will work with more than 40 undergraduates from underrepresented backgrounds to train them in scientific research methods, link them to summer internships in biotech companies and engage them in a two-year learning community for professional development. Furthermore, the program can be a model for other institutions to enhance the diversity of the growing biotech workforce.
Contacts: Elizabeth Vierling, [email protected]
Daegan Miller, [email protected]
Disclaimer: AAAS and EurekAlert! are not responsible for the accuracy of news releases posted to EurekAlert! by contributing institutions or for the use of any information through the EurekAlert system.
Additional Multimedia
Original Source
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

RECENT ADVANCES IN MITOCHONDRIAL RESEARCH
Bradford g. hill.
1 Institute of Molecular Cardiology, Department of Medicine, University of Louisville, Louisville, KY
2 Diabetes and Obesity Center, University of Louisville, Louisville, KY
3 Department of Biochemistry and Molecular Biology, University of Louisville, Louisville, KY
4 Department of Physiology and Biophysics, University of Louisville, Louisville, KY
Mitochondria are fundamental regulators of life and death. Not surprisingly, their role in the cardiovascular system is an intense area of research, with many important advances in the field published recently. Such articles have continued to enhance our appreciation of the importance of mitochondria in cardiovascular health and have further contributed to the idea that mitochondrial dysfunction is a sine qua non of cardiovascular disease (CVD).
A primary question of old that has been addressed anew relates to mitochondrial form. How do the shapes of mitochondria regulate their function? Do conditions associated with CVD alter mitochondrial dynamics? And does mitochondrial structure impact CVD? If so, how ? Over the past two years, there has been remarkable progress addressing these questions. Essential regulators of mitochondrial fission and fusion such as Mitofusins 1 and 2 (Mfn 1/2), Dynamin-related protein 1 (Drp1), and Optic atrophy 1 (Opa1) are proving to be important in cardiac development, vascular homeostasis, and cardiovascular disease. In the heart, Mfns have been shown to regulate permeability transition, cell death 1 , 2 and autophagy 3 , and Mfn2 was shown to be integral in the removal of damaged mitochondria 4 . Interestingly, loss of either Mfn1 2 or Mfn2 1 leads to increased tolerance to stress while loss of both is lethal after e9.5 of development 5 . It has also been reported that conditional ablation of both Mfn1 and Mfn2 in adult hearts causes mitochondrial fragmentation, respiratory dysfunction, and lethal dilated cardiomyopathy 5 . These effects of Mfn2 are likely related, in part, to its ability to tether mitochondria to sarco(endo)plasmic reticulum (SR), allowing for propagation of SR-mitochondrial Ca 2+ signaling and regulation of bioenergetic responses to stress 6 , 7 . Not unexpectedly, Mfn 1 and 2 are essential for postnatal metabolic remodeling in the heart 8 and cardiomyocyte differentiation 9 . Other regulators of mitochondrial fusion such as Opa1 have also been found to be critical for maintenance of cardiac function 10 . From a therapeutic angle, inhibition of mitochondrial fission has the potential to become a viable option to prevent cardiac dysfunction following myocardial infarction 11 .
Mitochondrial dynamics is proving to be a central regulator of vascular health as well. In smooth muscle, mitochondrial fission (via Drp1) was shown to be obligatory in the closure of the ductus arteriosus 12 , which is required for the transition from the fetal to the neonatal pattern of circulation. Hence, the physiological changes in circulation occurring upon first breath hinge upon the structure of our mitochondria! Mitochondrial fission, however, may be a double-edged sword in smooth muscle. It promotes a hyperproliferative phenotype that could be deleterious in the context of diseases such as pulmonary artery hypertension 13 , 14 . Furthermore, diabetes and hyperglycemia elevate the expression of fission-1 protein (Fis1) and Drp1, resulting in mitochondrial fragmentation 15 . Similar to smooth muscle cells, mitochondrial fragmentation in endothelial cells is associated with a hyperproliferative phenotype, which generates higher levels of ROS and is prone to senesce. Silencing of pro-fission proteins restores mitochondrial networks and diminishes reactive oxygen species (ROS) production while increasing the activation state of nitric oxide (NO) synthase 15 , the latter of which could have a direct role in adaptive mitochondrial dynamics 16 . In healthy endothelium, regulatory proteins such as uncoupling protein 2 (UCP2) appear to be integrated with pro-fission responses to protect endothelial cells from the damaging ROS and p53 activation associated with the fragmented mitochondrial phenotype 17 , 18 .
Such changes in mitochondrial structure are known to be intricately linked with their removal via mitophagy 19 , which appears to be essential for eliminating damaged mitochondria and preserving bioenergetic function 20 . Should damaged mitochondria fail to be removed, then cell death via necrosis or apoptosis is a likely fate 21 . Recent studies have shed further light into how the mitochondria regulate cell death and which specific pathways are activated by mitochondrial signaling. For example, loss of myeloid cell leukemia-1 (MCL-1), an anti-apoptotic BCL-2 protein, was found in two independent studies to impair mitochondrial respiration and lead to heart failure 22 , 23 . Results from the past two years have also imparted novel insights into the roles of Ca 2+ /calmodulin-dependent protein kinase II (CaMKII) 24 – 26 , G protein-coupled receptor kinase 2 (GRK2) 27 , and mitochondrial signal transducer and activator of transcription 3 (Stat3) 28 in myocardial cell death and mitochondrial stress in the heart. New advances were also made with respect to the well-studied protein targets, p53 and cyclophilin D (CypD): p53 was shown to bind to CypD, resulting in mitochondrial permeability transition pore (mPTP)-dependent necrosis 29 , 30 . While CypD is best known as a regulator of the mPTP, studies also suggest it modulates branched chain amino acid, pyruvate, and fatty acid metabolism 31 , which was could be related to its putative role in regulating the mitochondrial acetylome 32 , 33 . Interestingly, mitochondrial protein acetylation has also been suggested to play an important role in initiating mitophagy 34 .
While there is still much to learn about cell death and the role of post-translational modifications such as protein acetylation, it is becoming increasingly clear that both cell death pathways as well as protein acetylation are regulated by pyridine nucleotides. Nicotine adenine dinucleotides (NAD + /NADH) and their phosphorylated forms (NADP + /NADPH) are known to play central roles in energy production, ion channel regulation, and antioxidant defense in cardiovascular tissues 35 , 36 . Recent studies have revealed that they regulate the Na + /Ca 2+ exchanger to modulate Ca 2+ homeostasis in cardiac myocytes 37 , and a cytosolic form of the NAD + -utilizing enzyme ALDH2 (initially thought to be localized only in mitochondria) was shown to be important in the bioactivation of nitroglycerin 38 . The NAD + /NADH ratio also impacts the activity of the Sirtuin (Sirt) family of protein deacetylases, and this ratio is regulated by circadian rhythms 39 . Interestingly, circadian control of NAD + -dependent Sirt3 was shown to generate cadence in the acetylation of oxidative enzymes in mitochondria, linking respiratory activity with daily cycles of fasting and feeding 40 . Moreover, diminishing Complex I-supported mitochondrial respiration in the heart results in heightened NADH levels, elevated levels of protein acetylation, sensitization of mitochondria to PTP opening, and accelerated heart failure 41 . A deeper understanding of how pyridine nucleotides regulate energy metabolism could aid in the development of novel therapies for cardiovascular disease.
Recent work has also provided fresh insights into mechanisms underlying the salubrious effects of practical therapies such as caloric restriction and exercise. Caloric restriction is well-known to diminish symptoms of cardiovascular aging and increase longevity, in part due to its ability to not only regulate Sirt deacetylase activity, but mitophagy and PGC1-mediated processes as well 42 . During caloric restriction, deacetylation of specific subunits of the electron transport chain (educed via caloric restriction) was shown to be protective against ischemic stress 43 . Exercise functions as a calorie restriction mimetic of sorts and is one of the most robust activators of PGC1. Consistent with the idea that stimulation of PGC1 activity could prevent cardiovascular decline due to stress or aging, PGC1β was demonstrated to maintain mitochondrial function following pressure overload and to prevent oxidative stress 44 . Mitochondrial oxidative stress, in particular, could oxidize mtDNA, which is important in the fibrotic response that occurs following aortic constriction 45 . Exercise may be important in preventing such mitochondrial changes: it was shown to prevent mtDNA depletion and mutations, increase oxidative capacity and promote healthy aging 46 as well as attenuate doxorubicin-mediated cardiac injury 47 . A plausible idea concerning aging is that mitochondrial function and antioxidant capacity become mismatched over time, resulting in mitochondria-mediated oxidative stress, bioenergetic dysfunction, and cell death. This is supported by the fact that deletion of the regulator of mitochondrial biogenetic programming PGC1α can increase mitochondrial ROS production leading to vascular dysfunction and inflammation 48 and that aortic stiffening and cardiac deterioration occur in mice expressing lower levels of the mitochondrial form of superoxide dismutase (SOD2) 49 , 50 . How exercise may balance mitochondrial activity levels with antioxidant capacity and delineating approaches to increase exercise capacity in obese and older individuals are areas of inquiry that, once addressed, could diminish the burden of disease associated with aging. With regards to exercise, it appears that we may be forced to abandon our 1980s approaches, as deficiency in creatine—long regarded by the exercising community to have remarkable health and performance benefits—was shown in rodents to neither affect exercise capacity nor change responses to chronic myocardial stress 51 .
In summary, recent studies have addressed not only important, long-standing problems in mitochondrial research, but they have also led to unprecedented discoveries and novel insights into how mitochondria impact our cardiovascular health. Building on these discoveries, our search to identify the composition of mitochondria 52 , 53 and the mPTP 54 , to understand how metabolic enzymes regulate cardiovascular remodeling and function 55 – 61 , and to solidify the identity of the mitoK ATP channel 62 , 63 are likely to be active and contentious areas of mitochondrial research. However, such controversy is important and useful, as it fosters thoughtful and thorough testing of mitochondrial therapies targeting critical determinants of cardiovascular cell function 64 . We can rest assured that future findings regarding how mitochondria remodel, how miRNAs regulate mitochondria 65 , 66 and clarification of the actions and targets 67 of oxidants will further deepen our appreciation of the versatility of the mitochondrion and its critical roles in regulating cardiovascular physiology and disease.
Plants restrict use of 'Tipp-Ex proteins'
Molecules that modify copies of genes are only permitted in certain cell organelles.
Plants have special corrective molecules at their disposal that can make retrospective modifications to copies of genes. However, it would appear that these "Tipp-Ex proteins" do not have permission to work in all areas of the cell, only being used in chloroplasts and mitochondria. A study by the University of Bonn has now explained why this is the case. It suggests that the correction mechanism would otherwise modify copies that have nothing wrong with them, with fatal consequences for the cell. The findings have now been published in The Plant Journal .
Plant cells possess a whole host of specialized structures known as organelles, of which two particularly important ones are the chloroplasts and mitochondria. The former use light energy to convert carbon dioxide and water into oxygen and sugar, while the latter do more or less the same thing in reverse: they "burn" sugar and other compounds to generate the energy needed for numerous cellular processes.
The two organelles are unique in that they have their own genes. This genetic material works like sets of assembly instructions for key molecules that the organelles require for their work. If a chloroplast needs to make a certain protein, for instance, it first orders a copy of the relevant assembly instructions that it can then use to produce the protein.
Genes from chloroplasts and mitochondria often defective
"However, the genes in chloroplasts and mitochondria often contain defects," explains Elena Lesch, a doctoral student at the University of Bonn's Institute for Cellular and Molecular Botany. "So the copies have to be corrected, otherwise the proteins assembled based on their instructions won't work." For this, plants use a kind of Tipp-Ex -- special molecules that belong to the group of pentatricopeptide repeat (PPR) proteins.
Plants have at least a dozen and, in some cases, as many as several thousand of these special PPR proteins, each one of which corrects highly specific defects. It is as if every word in a newspaper had its own sub-editor. Rather than being made in the organelles in which they are used, however, the PPR proteins are manufactured outside of the organelles, within the cytosol.
The cytosol is also packed full of gene copies, although these come from the cell's nucleus, where most of the many thousands of the plant's genes are stored. By contrast, mitochondria and chloroplasts only contain a few dozen genes each. The "Tipp-Ex proteins" could theoretically correct the copies inside the cytosol too. "But they don't," Lesch says. "They only do their work in the organelles, and we wanted to know why."
Swamping the transportation mechanism into the organelles
One reason might be that the "molecular sub-editors" are simply moved too quickly from the cytosol into the organelles. To investigate this possibility, the researchers fitted a kind of molecular switch to PPR genes inside some of the moss Physcomitrium. This enabled them to make the cells produce very large quantities of PPR proteins virtually at the touch of a button. "We were able to demonstrate that this swamps the transportation mechanism," reveals Lesch's colleague Mirjam Thielen, who conducted many of the experiments. "It caused a pile-up of PPR proteins in the cytosol."
Once they had arrived in the cytosol, they began to modify copies from the nucleus. "We analyzed the changes they made and saw that the proteins had modified a great many sets of assembly instructions that would actually have been correct," Lesch says. "Incorrect interventions like these are counterproductive, of course, because they can put protein functions at risk." But why should this be happening in the first place? As well as detecting defects, the PPR proteins also bind to what are known as off-target sequences, areas that may look like a defective sequence but are actually perfectly fine. "With copies of tens of thousands of genes jostling for space inside the cytosol, the risk of these off-target sequences being corrected incorrectly would be high," Lesch notes.
Production of "Tipp-Ex" molecules subject to strict regulation
To prevent this, plants generally only ever make relatively low quantities of PPR proteins, which are then transported straight into the organelles before the molecular "Tipp-Ex" in the cytosol can do any harm. Because the number of genes -- and thus how many copies of them there are -- inside the chloroplasts and mitochondria is manageable, no such miscorrections tend to occur there.
The study is supplying new insights into how these corrective proteins identify their targets. In the future, therefore, it may be possible to use the findings to make highly targeted modifications to specific copies of genes inside mitochondria and chloroplasts and to investigate the effect of such modifications. Given the important roles that these organelles play in plants' energy metabolism, this also opens up scope for some interesting practical applications.
The work was funded by the German Research Foundation (DFG).
- Cell Biology
- Molecular Biology
- Endangered Plants
- Developmental Biology
- Biotechnology and Bioengineering
- Mitochondrion
- Natural killer cell
- Chloroplast
Story Source:
Materials provided by University of Bonn . Note: Content may be edited for style and length.
Journal Reference :
- Mirjam Thielen, Béla Gärtner, Volker Knoop, Mareike Schallenberg‐Rüdinger, Elena Lesch. Conquering new grounds: plant organellar C‐to‐U RNA editing factors can be functional in the plant cytosol . The Plant Journal , 2024; DOI: 10.1111/tpj.16804
Cite This Page :
Explore More
- Life Expectancy May Increase by 5 Years by 2050
- Toward a Successful Vaccine for HIV
- Highly Efficient Thermoelectric Materials
- Toward Human Brain Gene Therapy
- Whale Families Learn Each Other's Vocal Style
- AI Can Answer Complex Physics Questions
- Otters Use Tools to Survive a Changing World
- Monogamy in Mice: Newly Evolved Type of Cell
- Sustainable Electronics, Doped With Air
- Male Vs Female Brain Structure
Trending Topics
Strange & offbeat.

Research Offers New Ideas for Treating Alzheimer’s
“out-of-the-clump,” mitochondrial, and other theories offer hope on alzheimer’s..
Posted May 17, 2024 | Reviewed by Tyler Woods
- What Is Dementia?
- Find counselling to help with dementia
- The need for a new "out-of-the-clump" way of thinking about AD is emerging as a top priority in brain science.
- In Alzheimer’s, the brain’s immune system fails to differentiate between bacteria and brain cells.
- Probiotics may not only support a healthier gut, but a healthier brain as well.
- For the elderly, in particular those with cognitive impairment, good oral hygiene is essential.
Dementia refers to an array of symptoms characterized by failing short-term memory , confused thinking, and a decline in language skills. Of all the dementias, Alzheimer’s disease (AD) constitutes approximately 60 to 80 percent of cases.
Two drugs, Lecanemab and Donanemab, have been hailed as part of a new class of monoclonal antibody (MOA) drugs that could mark a turning point for Alzheimer’s (AZ) drug research. These drugs are incredibly expensive and carry risks of brain microbleeds and swelling. More importantly, they do not cure or even halt the disease, they delay it by about six months on average. At least 98 unique compounds tested in Phase 2 or 3 trials that pursued the various MOA classes have failed over the years. Howard Chertow, of McGill University, commented, “They’re not a home run.”
Personally, I think they’re more like a strike-out, in view of the fact that most neuroscientists and the drug companies employed by them may be looking in the wrong places in the wrong way.
In 2006, a research paper published in the highly regarded journal Nature asserted that the development of Alzheimer’s is caused by the formation in the brain of abnormally high levels of the naturally occurring protein beta-amyloid that clumps together to form plaques and the intracellular accumulations of neurofibrillary tangles of tau protein that disrupt cell function.
In 2023, a critical review in the journal Brain , collaboratively written by scientists from Denmark, the U.S., Italy, and Australia, stated that “Despite the importance of amyloid in the definition of Alzheimer's disease, we argue that the data point to Aβ playing a minor aetiological role.” They further asserted that the search for more effective ways to treat Alzheimer’s should involve more than amyloid as the single causative agent.
I propose to discuss the currently leading "out-of-the-clump" research, a term coined by Donald Weaver of the University of Toronto, that may eventually usher in new and better ways of dealing with Alzheimer’s.
One of the most auspicious of these novel directions comes from the above-mentioned Weaver, who found that significant resemblances between bacterial membranes and brain cell membranes exist. Beta-amyloid erroneously mistakes the brain cells for invading bacteria and attacks them. These brain cells gradually decay, ultimately leading to dementia. According to Weaver, Alzheimer’s is an autoimmune disease.
If this theory gains traction in the scientific world, treatments that are effective in autoimmune diseases such as celiac disease, Crohn’s disease, diabetes type 1, eczema, etc. may prove successful in the treatment of Alzheimer’s.
In addition to this autoimmune theory of Alzheimer’s, many other new and varied theories are appearing. John Mamo of Curtin University in Australia, demonstrated already in 2021 that the liver also makes amyloid protein.
It follows that finding ways to either prevent the liver from manufacturing the amyloid protein or destroying it before it enters the circulation ought to be explored.
A recent study from Portugal suggests that Alzheimer’s is a disease of the mitochondria . Mitochondria are tiny organelles (similar to organs like the heart or liver but much smaller inside cells) that generate most of the chemical energy required to power the cell's functions. The authors of this study reported positive outcomes in Alzheimer’s with animals fed a diet rich in antioxidants.
This is good news because we are in familiar territory here. We have known for a long time that antioxidants scavenge free radicals from the body cells and prevent or reduce the damage caused by oxidation. Of course, further research is necessary before it is proven that antioxidants in humans can lessen the risk of developing Alzheimer’s or benefit people in the early stages of Alzheimer’s. However, the consumption of antioxidants like vitamins A, C, and E, the minerals copper, zinc, and selenium, as well as nuts, fruits and vegetables, pecans, blueberries, and dark chocolate, seems well-proven to benefit the health of everyone, at any stage of life.
Scientists from the University of Bern, Switzerland, contend that Alzheimer’s is the end result of a brain infection, particularly with bacteria from the mouth. Since our hands and fingers swarm with viruses and bacteria, a recent paper that advanced the hypothesis that nose picking could play a role in increasing the risk of developing Alzheimer’s makes much sense. Digging around our noses is encouraging all those little critters to hop on the olfactory nerve train and take a vacation in our brains.
Recent research has focussed on probiotics as potentially beneficial in preventing the development or slowing the progression of Alzheimer’s. Probiotics are foods or supplements that contain live microorganisms that help to maintain or improve a diverse microflora in the gut. A systematic review of the literature on the effect of probiotics on Alzheimer’s by scientists from Malaysia in conjunction with researchers from Baghdad in 2022 write, “Probiotics are known to be one of the best preventative measures against cognitive decline in AD. Numerous in vivo trials and recent clinical trials have proven the effectiveness of selected bacterial strains in slowing down the progression of AD. It is proven that probiotics modulate the inflammatory process, counteract [with] oxidative stress , and modify gut microbiota.”

This and many other academic papers present robust evidence on the role of probiotics in alleviating the progression of Alzheimer’s.
As opposed to drugs, probiotics are readily available in foods such as yogurt, buttermilk, sauerkraut, pickles, and many others.
If we are going to make significant advances in the prevention and treatment of Alzheimer’s, we urgently require new approaches outside the old amyloid plaque box. Here I reviewed a number of such studies that promise to make a difference in the near future.
Understanding the condition, its origins, and effective strategies for prevention should be a top priority of our healthcare system.
Lee, Y. R., Ong, L., Gold, M., Kalali, A., & Sarkar, J. (2022). Alzheimer’s disease: key insights from two decades of clinical trial failures. Journal of Alzheimer's Disease, 87(1), 83-100.
Van Dyck, C. H., Swanson, C. J., Aisen, P., Bateman, R. J., Chen, C., Gee, M., ... & Iwatsubo, T. (2023). Lecanemab in early Alzheimer’s disease. New England Journal of Medicine, 388(1), 9-21.
Romanenko, M., Kholin, V., Koliada, A., & Vaiserman, A. (2021). Nutrition, gut microbiota, and Alzheimer's disease. Frontiers in psychiatry, 12, 712673
Prater, K. E., Green, K. J., Smith, C. L., ... & Jayadev, S. (2023). Human microglia show unique transcriptional changes in Alzheimer’s disease. Nature Aging, 3(7), 894-907.

Thomas R. Verny, M.D. , the author of eight books, including The Embodied Mind , has taught at Harvard University, University of Toronto, York University, and St. Mary’s University of Minnesota. His podcast, Pushing Boundaries , may be viewed on Youtube or listened to on Spotify and many other platforms.
- Find a Therapist
- Find a Treatment Center
- Find a Psychiatrist
- Find a Support Group
- Find Online Therapy
- International
- New Zealand
- South Africa
- Switzerland
- Asperger's
- Bipolar Disorder
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Self Tests NEW
- Therapy Center
- Diagnosis Dictionary
- Types of Therapy

At any moment, someone’s aggravating behavior or our own bad luck can set us off on an emotional spiral that threatens to derail our entire day. Here’s how we can face our triggers with less reactivity so that we can get on with our lives.
- Emotional Intelligence
- Gaslighting
- Affective Forecasting
- Neuroscience
Let your curiosity lead the way:
Apply Today
- Arts & Sciences
- Graduate Studies in A&S
Niemi Lab Seeks UG Researcher for FA24
We are seeking an undergraduate student in the Niemi Lab ( https://www.niemilab.com/ ) within the Department of Biochemistry and Molecular Biophysics on the medical campus. This undergraduate would start in the Fall 2024 Semester.
Our lab focuses on how mitochondria are built, regulated, and maintained across physiological contexts. Projects in the lab include understanding the mechanisms and regulation of mitochondrial biogenesis, determining how select mitochondrial proteins are targeted for degradation, and characterizing how post-translational modifications influence mitochondrial form and function. We work with cell culture systems, mouse-derived primary cells, and mouse model systems. Our long-term goal is to translate our discoveries into new therapeutics options that restore mitochondrial function in human disease.
As an undergraduate student under the supervision of Tessa Lochetto, a graduate student in the lab, you can expect your research activities to include genotyping of mouse strains, immunoblotting, quantitative real-time PCRs, mouse handling, mouse tissue histology, microscopic imaging, and more! Tessa’s projects focus on how regulated phosphorylation is imperative for the health, development, and physiology of skeletal and cardiac muscle.
The expected time commitment is approximately 10 hours/week. This is an excellent opportunity for students who are interested in immersing themselves in wet-lab, physiology-focused research with a plan of applying to graduate school, medical school, or for someone looking for a long-term position (e.g., research technician over a gap year). Lab work can be done for credit, work-study, or hourly pay (potentially).
Interested applicants should reach out to Tessa Lochetto ( [email protected] ) directly with:
- A resume/CV.
- A brief statement of why you’re interested in our lab and/or the described project(s).
- Would you be more interested in cardiac or skeletal muscle?
- Any prior research experiences?
- What is the time you can commit to during the semesters in the context of your other commitments?
All applications will be considered. Students will be encouraged to present their work at lab meetings, department seminars, and undergraduate research symposiums.
Header image by vecstock on Freepik.
in the news:

Apply for the WashU Research Ambassador Program (WRAP)
Summer Research with WashU Statistics and Data Science
MIT Postbacc: The Research Scholars Program in Brain and Cognitive Sciences

UG Research Poster Competition at BDN Western Regional Conference
Cornell Chronicle
- Architecture & Design
- Arts & Humanities
- Business, Economics & Entrepreneurship
- Computing & Information Sciences
- Energy, Environment & Sustainability
- Food & Agriculture
- Global Reach
- Health, Nutrition & Medicine
- Law, Government & Public Policy
- Life Sciences & Veterinary Medicine
- Physical Sciences & Engineering
- Social & Behavioral Sciences
- Coronavirus
- News & Events
- Public Engagement
- New York City
- Photos of the Week
- Big Red Sports
- Freedom of Expression
- Student Life
- University Statements
Around Cornell
- All Stories
- In the News
- Expert Quotes
- Cornellians

News directly from Cornell's colleges and centers
Research: Technology is changing how companies do business
By sarah mangus-sharpe.
A new study from the Cornell SC Johnson College of Business advances understanding of the U.S. production chain evolution amidst technological progress in information technology (IT), shedding light on the complex connections between business IT investments and organizational design. Advances in IT have sparked significant changes in how companies design their production processes. In the paper " Production Chain Organization in the Digital Age: Information Technology Use and Vertical Integration in U.S. Manufacturing ," which published April 30 in Management Science, Chris Forman , the Peter and Stephanie Nolan Professor in the Dyson School of Applied Economics and Management , and his co-author delved into what these changes mean for businesses and consumers.
Forman and Kristina McElheran, assistant professor of strategic management at University of Toronto, analyzed U.S. Census Bureau data of over 5,600 manufacturing plants to see how the production chains of businesses were affected by the internet revolution. Their use of census data allowed them to look inside the relationships among production units within and between companies and how transaction flows changed after companies invested in internet-enabled technology that facilitated coordination between them. The production units of many of the companies in their study concurrently sold to internal and external customers, a mix they refer to as plural selling. They found that the reduction in communication costs enabled by the internet shifted the mix toward more sales outside of the firm, or less vertical integration.
The research highlights the importance of staying ahead of the curve in technology. Companies that embrace digital technologies now are likely to be the ones that thrive in the future. And while there are still many unanswered questions about how these changes will play out, one thing is clear: The relationship between technology and business is only going to become more and more intertwined in the future.
Read the full story on the Cornell SC Johnson College of Business news site, BusinessFeed.
Media Contact
Media relations office.
Get Cornell news delivered right to your inbox.
You might also like

Gallery Heading

IMAGES
VIDEO
COMMENTS
Latest Research and Reviews. ... Mitochondria, the powerhouse and the vital signaling hub of the cell, participate in a variety of biological processes, such as apoptosis, redox responses, cell ...
An advanced imaging-based method from scientists at Scripps Research offers a new way of studying mitochondria, which are best known as the "powerhouses" of cells. In their report on February 14 ...
A "door" into the mitochondrial membrane. Study finds the protein MTCH2 is responsible for shuttling various other proteins into the membrane of mitochondria. The finding could have implications for cancer treatments and MTCH2-linked conditions. New research finds the protein MTCH2 acts as a doorway for proteins into the outer mitochondrial ...
February 15, 2023. LA JOLLA, CA— An advanced imaging-based method from scientists at Scripps Research offers a new way of studying mitochondria, which are best known as the "powerhouses" of cells. In their report on February 14, 2023, in the Journal of Cell Biology, the scientists described a set of techniques that enables the imaging and ...
DOI: 10.1021/acsnano.3c02768. Using new super-resolution microscopes, researchers at the University of California, Irvine and the University of Pennsylvania have for the first time observed ...
The study, published in Developmental Cell, shows that a member of the Cox7a family of proteins plays a fundamental role in the assembly of CIV dimers and that this assembly is crucial for correct ...
Mitochondrial biogenesis is the cellular process that produces new mitochondria. It determines both the quality and quantity of mitochondria in cells, both of which decline during normal aging. In ...
Mitochondria are the body's power plants, fuelling our cells. New research shows they play a role in many aspects of keeping us healthy - and could be the key to unlocking treatments for ...
September 17, 2020. Tiny mitochondria in our cells turn oxygen and nutrients into usable energy in a process called respiration. This process is essential for powering our cells, and yet in spite of its importance many of the finer details of how it happens remain unknown. One long-standing mystery is how a molecule called nicotinamide adenine ...
1. Introduction. Mitochondria play key roles in the energy supply, signaling, and apoptosis of cells [].The normal functions of mitochondria depend on the coordination of mitochondrial biogenesis, mitochondrial dynamics, and mitophagy [].A series of studies have shown that mitochondrial dysfunction is associated with numerous human conditions, such as cancer [], metabolic diseases ...
As research advances and synthetic biology opens up new possibilities, we may harness the power of custom-designed mitochondria to re-energize aging tissues and bolster our health over time. In the ever-evolving landscape of aging research, mitochondria must take center stage, offering the prospect of a healthier, more resilient journey through ...
Reviewed by Lily Ramsey, LLM Sep 19 2023. New research provides insight about the bedrock scientific principle that mitochondrial DNA -; the distinct genetic code embedded in the organelle that ...
The National Science Foundation (NSF) recently announced that it will support the efforts of a collaborative group of researchers, led by Elizabeth Vierling, Distinguished Professor of Biochemistry at the University of Massachusetts Amherst, who plan to spend the next four years investigating the role that mitochondria play in plant productivity.
Most of his research focuses on mitochondria and how they respond to various levels and types of exercise. "Recent scientific advancements reveal that mitochondria have functions even beyond ATP synthesis or oxygen consumption or energy production," Park said. "Now we understand that mitochondria control the life and death of the cell.
New research finds that 'leaky mitochondria' help keep sea otters warm. A computer illustration of a cross-section of a mitochondrion and its internal structure with DNA (gray), ribosomes ...
New research to plumb the mysteries of mitochondria, yielding insight into evolution, food security and climate change. Researchers at UMass Amherst to lead collaborative, $2.3 million NSF ...
Mitochondrial fusion critical for adult neurogenesis and brain circuit refinement. Nerve cells (neurons) are amongst the most complex cell types in our body. They achieve this complexity during ...
Essential regulators of mitochondrial fission and fusion such as Mitofusins 1 and 2 (Mfn 1/2), Dynamin-related protein 1 (Drp1), and Optic atrophy 1 (Opa1) are proving to be important in cardiac development, vascular homeostasis, and cardiovascular disease. In the heart, Mfns have been shown to regulate permeability transition, cell death 1, 2 ...
We here show that SNX10 localizes to endocytic compartments in a PtdIns3P-dependent manner and that mutations in the PX domain associated with autosomal recessive osteopetrosis prevent its endosomal recruitment. We demonstrate that SNX10 regulates endosomal trafficking but also interacts with mitochondrial proteins and shows dynamic interactions with mitochondria. Intriguingly, SNX10 and RAB5A ...
Your source for the latest research news. Follow: Facebook X/Twitter Subscribe: RSS Feeds. New! ... Genes from chloroplasts and mitochondria often defective "However, the genes in chloroplasts and ...
Two drugs, Lecanemab and Donanemab, have been hailed as part of a new class of monoclonal antibody (MOA) drugs that could mark a turning point for Alzheimer's (AZ) drug research. These drugs are ...
Research on the optimal combination approaches for venetoclax is ongoing, and new drugs are entering the market, such as the tyrosine kinase 3 inhibitor quizartinib. ... These studies revealed that while ONC201 activates TRAIL, the drug also activates a mitochondrial protein involved in cell death called ClpP. This dual activation shows that ...
The expected time commitment is approximately 10 hours/week. This is an excellent opportunity for students who are interested in immersing themselves in wet-lab, physiology-focused research with a plan of applying to graduate school, medical school, or for someone looking for a long-term position (e.g., research technician over a gap year).
The US Department of Health and Human Services on Wednesday suspended funding to EcoHealth Alliance, a virus research organization tied to controversy about the origins of the virus that causes ...
A new study from the Cornell SC Johnson College of Business advances understanding of the U.S. production chain evolution amidst technological progress in information technology (IT), shedding light on the complex connections between business IT investments and organizational design. Advances in IT have sparked significant changes in how companies design their production processes.
Friday, May 17, 2024. (U.S. Senate) - Following a push from U.S. Senator Jon Tester, the Department of Veterans Affairs' (VA) Office of Research and Development gave the VA Montana Health Care System approval this week to establish a VA research site in Montana. This will be the first VA research site in the state of Montana. "Veterans in ...