- En español – ExME
- Em português – EME

An introduction to different types of study design
Posted on 6th April 2021 by Hadi Abbas

Study designs are the set of methods and procedures used to collect and analyze data in a study.
Broadly speaking, there are 2 types of study designs: descriptive studies and analytical studies.
Descriptive studies
- Describes specific characteristics in a population of interest
- The most common forms are case reports and case series
- In a case report, we discuss our experience with the patient’s symptoms, signs, diagnosis, and treatment
- In a case series, several patients with similar experiences are grouped.
Analytical Studies
Analytical studies are of 2 types: observational and experimental.
Observational studies are studies that we conduct without any intervention or experiment. In those studies, we purely observe the outcomes. On the other hand, in experimental studies, we conduct experiments and interventions.
Observational studies
Observational studies include many subtypes. Below, I will discuss the most common designs.
Cross-sectional study:
- This design is transverse where we take a specific sample at a specific time without any follow-up
- It allows us to calculate the frequency of disease ( p revalence ) or the frequency of a risk factor
- This design is easy to conduct
- For example – if we want to know the prevalence of migraine in a population, we can conduct a cross-sectional study whereby we take a sample from the population and calculate the number of patients with migraine headaches.
Cohort study:
- We conduct this study by comparing two samples from the population: one sample with a risk factor while the other lacks this risk factor
- It shows us the risk of developing the disease in individuals with the risk factor compared to those without the risk factor ( RR = relative risk )
- Prospective : we follow the individuals in the future to know who will develop the disease
- Retrospective : we look to the past to know who developed the disease (e.g. using medical records)
- This design is the strongest among the observational studies
- For example – to find out the relative risk of developing chronic obstructive pulmonary disease (COPD) among smokers, we take a sample including smokers and non-smokers. Then, we calculate the number of individuals with COPD among both.
Case-Control Study:
- We conduct this study by comparing 2 groups: one group with the disease (cases) and another group without the disease (controls)
- This design is always retrospective
- We aim to find out the odds of having a risk factor or an exposure if an individual has a specific disease (Odds ratio)
- Relatively easy to conduct
- For example – we want to study the odds of being a smoker among hypertensive patients compared to normotensive ones. To do so, we choose a group of patients diagnosed with hypertension and another group that serves as the control (normal blood pressure). Then we study their smoking history to find out if there is a correlation.
Experimental Studies
- Also known as interventional studies
- Can involve animals and humans
- Pre-clinical trials involve animals
- Clinical trials are experimental studies involving humans
- In clinical trials, we study the effect of an intervention compared to another intervention or placebo. As an example, I have listed the four phases of a drug trial:
I: We aim to assess the safety of the drug ( is it safe ? )
II: We aim to assess the efficacy of the drug ( does it work ? )
III: We want to know if this drug is better than the old treatment ( is it better ? )
IV: We follow-up to detect long-term side effects ( can it stay in the market ? )
- In randomized controlled trials, one group of participants receives the control, while the other receives the tested drug/intervention. Those studies are the best way to evaluate the efficacy of a treatment.
Finally, the figure below will help you with your understanding of different types of study designs.

References (pdf)
You may also be interested in the following blogs for further reading:
An introduction to randomized controlled trials
Case-control and cohort studies: a brief overview
Cohort studies: prospective and retrospective designs
Prevalence vs Incidence: what is the difference?
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
No Comments on An introduction to different types of study design
you are amazing one!! if I get you I’m working with you! I’m student from Ethiopian higher education. health sciences student
Very informative and easy understandable
You are my kind of doctor. Do not lose sight of your objective.
Wow very erll explained and easy to understand
I’m Khamisu Habibu community health officer student from Abubakar Tafawa Balewa university teaching hospital Bauchi, Nigeria, I really appreciate your write up and you have make it clear for the learner. thank you
well understood,thank you so much
Well understood…thanks
Simply explained. Thank You.
Thanks a lot for this nice informative article which help me to understand different study designs that I felt difficult before
That’s lovely to hear, Mona, thank you for letting the author know how useful this was. If there are any other particular topics you think would be useful to you, and are not already on the website, please do let us know.
it is very informative and useful.
thank you statistician
Fabulous to hear, thank you John.
Thanks for this information
Thanks so much for this information….I have clearly known the types of study design Thanks
That’s so good to hear, Mirembe, thank you for letting the author know.
Very helpful article!! U have simplified everything for easy understanding
I’m a health science major currently taking statistics for health care workers…this is a challenging class…thanks for the simified feedback.
That’s good to hear this has helped you. Hopefully you will find some of the other blogs useful too. If you see any topics that are missing from the website, please do let us know!
Hello. I liked your presentation, the fact that you ranked them clearly is very helpful to understand for people like me who is a novelist researcher. However, I was expecting to read much more about the Experimental studies. So please direct me if you already have or will one day. Thank you
Dear Ay. My sincere apologies for not responding to your comment sooner. You may find it useful to filter the blogs by the topic of ‘Study design and research methods’ – here is a link to that filter: https://s4be.cochrane.org/blog/topic/study-design/ This will cover more detail about experimental studies. Or have a look on our library page for further resources there – you’ll find that on the ‘Resources’ drop down from the home page.
However, if there are specific things you feel you would like to learn about experimental studies, that are missing from the website, it would be great if you could let me know too. Thank you, and best of luck. Emma
Great job Mr Hadi. I advise you to prepare and study for the Australian Medical Board Exams as soon as you finish your undergrad study in Lebanon. Good luck and hope we can meet sometime in the future. Regards ;)
You have give a good explaination of what am looking for. However, references am not sure of where to get them from.
Subscribe to our newsletter
You will receive our monthly newsletter and free access to Trip Premium.
Related Articles

Cluster Randomized Trials: Concepts
This blog summarizes the concepts of cluster randomization, and the logistical and statistical considerations while designing a cluster randomized controlled trial.

Expertise-based Randomized Controlled Trials
This blog summarizes the concepts of Expertise-based randomized controlled trials with a focus on the advantages and challenges associated with this type of study.

A well-designed cohort study can provide powerful results. This blog introduces prospective and retrospective cohort studies, discussing the advantages, disadvantages and use of these type of study designs.

- Hirsh Health Sciences
- Lilly Music
- Webster Veterinary
- Hirsh Health Sciences Library
Study Designs in the Health Sciences
Introduction.
- Case-Control
- Clinical Trials
- Randomized Controlled Trial (RCT)
- Systematic Reviews/Meta-Analysis
- Retrieving Articles by Study Design in PubMed
This guide was created to introduce you to the following study designs frequently used in the health sciences:
- Systematic reviews/Meta-Analysis
Each tab in this guide describes a particular study design and will include a definition of the design, why it is used, ts format and features, and an example.
How to Read and Understand the Literature
- Next: Case-Control >>

Desk Hours: M-F 7:45am-5pm
- Last Updated: Dec 26, 2019 11:38 AM
- URL: https://researchguides.library.tufts.edu/study_design
Cookies on this website
We use cookies to ensure that we give you the best experience on our website. If you click 'Accept all cookies' we'll assume that you are happy to receive all cookies and you won't see this message again. If you click 'Reject all non-essential cookies' only necessary cookies providing core functionality such as security, network management, and accessibility will be enabled. Click 'Find out more' for information on how to change your cookie settings.

Study designs
This short article gives a brief guide to the different study types and a comparison of the advantages and disadvantages.
See also Levels of Evidence
These study designs all have similar components (as we’d expect from the PICO):
- A defined population (P) from which groups of subjects are studied
- Outcomes (O) that are measured
And for experimental and analytic observational studies:
- Interventions (I) or exposures (E) that are applied to different groups of subjects
Overview of the design tree
Figure 1 shows the tree of possible designs, branching into subgroups of study designs by whether the studies are descriptive or analytic and by whether the analytic studies are experimental or observational. The list is not completely exhaustive but covers most basics designs.

Figure: Tree of different types of studies (Q1, 2, and 3 refer to the three questions below)
> Download a PDF by Jeremy Howick about study designs
Our first distinction is whether the study is analytic or non-analytic. A non-analytic or descriptive study does not try to quantify the relationship but tries to give us a picture of what is happening in a population, e.g., the prevalence, incidence, or experience of a group. Descriptive studies include case reports, case-series, qualitative studies and surveys (cross-sectional) studies, which measure the frequency of several factors, and hence the size of the problem. They may sometimes also include analytic work (comparing factors “” see below).
An analytic study attempts to quantify the relationship between two factors, that is, the effect of an intervention (I) or exposure (E) on an outcome (O). To quantify the effect we will need to know the rate of outcomes in a comparison (C) group as well as the intervention or exposed group. Whether the researcher actively changes a factor or imposes uses an intervention determines whether the study is considered to be observational (passive involvement of researcher), or experimental (active involvement of researcher).
In experimental studies, the researcher manipulates the exposure, that is he or she allocates subjects to the intervention or exposure group. Experimental studies, or randomised controlled trials (RCTs), are similar to experiments in other areas of science. That is, subjects are allocated to two or more groups to receive an intervention or exposure and then followed up under carefully controlled conditions. Such studies controlled trials, particularly if randomised and blinded, have the potential to control for most of the biases that can occur in scientific studies but whether this actually occurs depends on the quality of the study design and implementation.
In analytic observational studies, the researcher simply measures the exposure or treatments of the groups. Analytical observational studies include case””control studies, cohort studies and some population (cross-sectional) studies. These studies all include matched groups of subjects and assess of associations between exposures and outcomes.
Observational studies investigate and record exposures (such as interventions or risk factors) and observe outcomes (such as disease) as they occur. Such studies may be purely descriptive or more analytical.
We should finally note that studies can incorporate several design elements. For example, a the control arm of a randomised trial may also be used as a cohort study; and the baseline measures of a cohort study may be used as a cross-sectional study.
Spotting the study design
The type of study can generally be worked at by looking at three issues (as per the Tree of design in Figure 1):
Q1. What was the aim of the study?
- To simply describe a population (PO questions) descriptive
- To quantify the relationship between factors (PICO questions) analytic.
Q2. If analytic, was the intervention randomly allocated?
- No? Observational study
For observational study the main types will then depend on the timing of the measurement of outcome, so our third question is:
Q3. When were the outcomes determined?
- Some time after the exposure or intervention? cohort study (‘prospective study’)
- At the same time as the exposure or intervention? cross sectional study or survey
- Before the exposure was determined? case-control study (‘retrospective study’ based on recall of the exposure)
Advantages and Disadvantages of the Designs
Randomised Controlled Trial
An experimental comparison study in which participants are allocated to treatment/intervention or control/placebo groups using a random mechanism (see randomisation). Best for study the effect of an intervention.
Advantages:
- unbiased distribution of confounders;
- blinding more likely;
- randomisation facilitates statistical analysis.
Disadvantages:
- expensive: time and money;
- volunteer bias;
- ethically problematic at times.
Crossover Design
A controlled trial where each study participant has both therapies, e.g, is randomised to treatment A first, at the crossover point they then start treatment B. Only relevant if the outcome is reversible with time, e.g, symptoms.
- all subjects serve as own controls and error variance is reduced thus reducing sample size needed;
- all subjects receive treatment (at least some of the time);
- statistical tests assuming randomisation can be used;
- blinding can be maintained.
- all subjects receive placebo or alternative treatment at some point;
- washout period lengthy or unknown;
- cannot be used for treatments with permanent effects
Cohort Study
Data are obtained from groups who have been exposed, or not exposed, to the new technology or factor of interest (eg from databases). No allocation of exposure is made by the researcher. Best for study the effect of predictive risk factors on an outcome.
- ethically safe;
- subjects can be matched;
- can establish timing and directionality of events;
- eligibility criteria and outcome assessments can be standardised;
- administratively easier and cheaper than RCT.
- controls may be difficult to identify;
- exposure may be linked to a hidden confounder;
- blinding is difficult;
- randomisation not present;
- for rare disease, large sample sizes or long follow-up necessary.
Case-Control Studies
Patients with a certain outcome or disease and an appropriate group of controls without the outcome or disease are selected (usually with careful consideration of appropriate choice of controls, matching, etc) and then information is obtained on whether the subjects have been exposed to the factor under investigation.
- quick and cheap;
- only feasible method for very rare disorders or those with long lag between exposure and outcome;
- fewer subjects needed than cross-sectional studies.
- reliance on recall or records to determine exposure status;
- confounders;
- selection of control groups is difficult;
- potential bias: recall, selection.
Cross-Sectional Survey
A study that examines the relationship between diseases (or other health-related characteristics) and other variables of interest as they exist in a defined population at one particular time (ie exposure and outcomes are both measured at the same time). Best for quantifying the prevalence of a disease or risk factor, and for quantifying the accuracy of a diagnostic test.
- cheap and simple;
- ethically safe.
- establishes association at most, not causality;
- recall bias susceptibility;
- confounders may be unequally distributed;
- Neyman bias;
- group sizes may be unequal.
The Graduate Health & Life Sciences Research Library at Georgetown University Medical Center
Research in the health sciences.
- Qualitative
- Literature Review
- Data Analysis
- Writing & Publishing
- References & Bibliographies
Research Design: Overview
Generally, analytic research can be divided into categories of Observational and Experimental studies:
Observational Research: Observational studies do not allow for investigator allocation, sometimes due to ethical considerations. In these studies, the factors are self-selected, and so the empirical evidence is weaker due to possible confounding biases.
- Cohort Study
- Case Control Study
- Cross-Sectional Study
- Case Series
- Case Report
Experimental Research: In these studies, the investigator has control over allocation, and so randomization can be employed. For this reason, experimental studies tend to provide strong empirical evidence.
- Randomized Clinical Trial or Randomized Controlled Trial
- Controlled Clinical Trial
In addition, various filtered or secondary studies exist. They review and evaluate applicable studies, and sometimes perform additional statistical analysis on the cumulative data.
- Meta-Analysis
- Systematic Review
- Narrative Review
Research Design: Reference Shelf
- << Previous: Home
- Next: Qualitative >>
- Last Updated: Apr 2, 2024 9:22 AM
- URL: https://guides.dml.georgetown.edu/research
The Responsible Use of Electronic Resources policy governs the use of resources provided on these guides. © Dahlgren Memorial Library, Georgetown University Medical Center. Unless otherwise stated, these guides may be used for educational or academic purposes as long as proper attribution is given. Please seek permission for any modifications, adaptations, or for commercial purposes. Email [email protected] to request permission. Proper attribution includes: Written by or adapted from, Dahlgren Memorial Library, URL.

Types of Study Designs in Health Research: The Evidence Hierarchy
by guest contributer Leave a Comment
by Danielle Bodicoat
Statistics can tell us a lot about our data, but it’s also important to consider where the underlying data came from when interpreting results, whether they’re our own or somebody else’s.
Not all evidence is created equally, and we should place more trust in some types of evidence than others.
In medical research, there’s a well-known evidence hierarchy that ranks the main types of evidence. It looks like this:
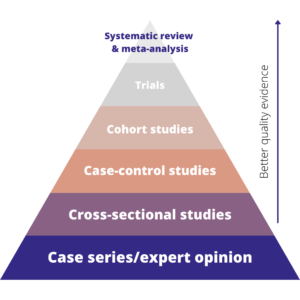
The hierarchy basically shows that the best quality evidence we have comes from systematic reviews, followed by trials, then observational studies. Expert opinion is the lowest form of evidence. Whilst this hierarchy, and some of the specific study types, are mostly used for medical research, the concept translates well to other disciplines.
Below, we’ll walk through each level of the hierarchy, what it is and how to analyze it.
But there’s a caveat!
The quality of the evidence will also depend on how well the study is conducted. So, for example, a large, well-conducted trial might be better than a poorly-conducted, biased systematic review.
For this article, we’ll assume everybody has done a great job and we’re talking about well-conducted studies.
Systematic reviews
Systematic reviews are a specialist type of literature review. We’re essentially trying to find all of the available evidence on a particular research question. The evidence might be published or unpublished (grey literature).
We then combine all of that evidence either qualitatively (narrative review) or quantitatively (meta-analysis) to get a definitive answer to our research question.
This type of evidence is top of the hierarchy because systematic reviews are:
- Objective – there should be no opinion or selection bias involved when choosing which evidence to include in a systematic review
- Comprehensive – includes all of the evidence on a topic
- Precise – a review should answer a very specific research question
- Reproducible – if somebody else followed the same methodology then they should get the exact same answer.
Trials are tests or experiments designed to answer a specific research question. They have an experimental and control group, and units of observation (such as people) are allocated randomly to each group. This random allocation, along with some other good practices, helps to keep trials unbiased and that’s why they appear second in the hierarchy.
As with any analysis that we do, lots of different things will affect the approach that we take . However, the design of trials means that often we can use fairly simple statistical methods since there may not be any confounders to adjust for.
The main exception to this is where the randomization has been stratified, in which case you will need to adjust for the stratification factors in your analysis.
We also have a known direction of effect because of the study design, which affects our choice of analysis . Based on all of this, trials will typically be analyzed using a generalized linear model .
Cohort studies
In this type of study, we take a group of people (or whatever else we’re interested in) with a characteristic or exposure that we’re interested in, and a group without that characteristic. We then follow them up for a period of time to see whether our outcome of interest develops more often in the exposed group than the unexposed group.
This is the strongest form of non-experimental evidence that we have, because we follow unbiased groups (i.e. when we start the study we have no idea who will develop the outcome of interest).
This design works best where you have a fairly common outcome, otherwise you wouldn’t have any events to analyze. It can also be a great design when you’ve got a rare exposure so that you can make sure you have plenty of exposed people in your study.
People will be followed for different lengths of time. Some will choose to withdraw from the study. You’ll lose touch with others and not be able to find out whether the outcome occurred. Some will develop the outcome of interest at which point you may stop following them up.
We need to account for these differences in follow-up time in our analysis, so we’ll typically use approaches that allow us to include it, such as survival analysis or a comparison of the incidence rates in the exposed and unexposed groups to estimate an incidence rate ratio .
Case-control studies
Case-control studies are sort of the opposite of cohort studies in that we select a group with our outcome of interest (cases), and a group without it (controls). We then look back to see whether the cases were more likely than controls to have been exposed to the potential causal factor that we’re interested in.
Case-control studies work best for rare outcomes and common exposures. Our outcome here is the binary case/control status so this type of study is typically analyzed using logistic regression .
Cross-sectional studies
In this type of study, we just look at a single-point in time to get a ‘snapshot’ of what is happening. This means that everything is measured at the same point in time, although we can ask about the past.
They are particularly useful for measuring prevalence, i.e. how common something is within a population of interest. There is no time element to include in the analyses so again they are typically analyzed using a generalized linear model , though as always your choice of analysis will depend on your research question.
Case-series and expert opinion
In case-series, everybody with an exposure, or outcome, of interest is included in a study. They are typically used in medical research and are often based on medical notes from one hospital.
Because everybody with the exposure or outcome is included, there is no comparator group, and so it isn’t possible to calculate a relative risk. Case-series are often described using a narrative review, rather than analytical methods.
Similarly, expert opinion papers often don’t include any analysis. Whilst they can be very helpful in terms of providing context, they are subjective in nature and so they don’t provide a strong form of evidence.
Danielle Bodicoat works with health researchers helping them to get confident with using statistics to analyze their data. She’s an escaped academic now working as a medical statistics consultant through her company, Simplified Data . She has spent nearly 15 years designing, conducting and supervising statistical analyses, and has 80+ peer-reviewed publications.
Reader Interactions
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Privacy Overview
Types of studies and research design
Affiliation.
- 1 Department of Anesthesiology, Max Smart Super Specialty Hospital, New Delhi, India.
- PMID: 27729687
- PMCID: PMC5037941
- DOI: 10.4103/0019-5049.190616
Medical research has evolved, from individual expert described opinions and techniques, to scientifically designed methodology-based studies. Evidence-based medicine (EBM) was established to re-evaluate medical facts and remove various myths in clinical practice. Research methodology is now protocol based with predefined steps. Studies were classified based on the method of collection and evaluation of data. Clinical study methodology now needs to comply to strict ethical, moral, truth, and transparency standards, ensuring that no conflict of interest is involved. A medical research pyramid has been designed to grade the quality of evidence and help physicians determine the value of the research. Randomised controlled trials (RCTs) have become gold standards for quality research. EBM now scales systemic reviews and meta-analyses at a level higher than RCTs to overcome deficiencies in the randomised trials due to errors in methodology and analyses.
Keywords: Clinical trials; evidence-based medicine; medical research; meta-analysis.
Publication types
- USC Libraries
- Research Guides
Organizing Your Social Sciences Research Paper
- Types of Research Designs
- Purpose of Guide
- Design Flaws to Avoid
- Independent and Dependent Variables
- Glossary of Research Terms
- Reading Research Effectively
- Narrowing a Topic Idea
- Broadening a Topic Idea
- Extending the Timeliness of a Topic Idea
- Academic Writing Style
- Applying Critical Thinking
- Choosing a Title
- Making an Outline
- Paragraph Development
- Research Process Video Series
- Executive Summary
- The C.A.R.S. Model
- Background Information
- The Research Problem/Question
- Theoretical Framework
- Citation Tracking
- Content Alert Services
- Evaluating Sources
- Primary Sources
- Secondary Sources
- Tiertiary Sources
- Scholarly vs. Popular Publications
- Qualitative Methods
- Quantitative Methods
- Insiderness
- Using Non-Textual Elements
- Limitations of the Study
- Common Grammar Mistakes
- Writing Concisely
- Avoiding Plagiarism
- Footnotes or Endnotes?
- Further Readings
- Generative AI and Writing
- USC Libraries Tutorials and Other Guides
- Bibliography
Introduction
Before beginning your paper, you need to decide how you plan to design the study .
The research design refers to the overall strategy and analytical approach that you have chosen in order to integrate, in a coherent and logical way, the different components of the study, thus ensuring that the research problem will be thoroughly investigated. It constitutes the blueprint for the collection, measurement, and interpretation of information and data. Note that the research problem determines the type of design you choose, not the other way around!
De Vaus, D. A. Research Design in Social Research . London: SAGE, 2001; Trochim, William M.K. Research Methods Knowledge Base. 2006.
General Structure and Writing Style
The function of a research design is to ensure that the evidence obtained enables you to effectively address the research problem logically and as unambiguously as possible . In social sciences research, obtaining information relevant to the research problem generally entails specifying the type of evidence needed to test the underlying assumptions of a theory, to evaluate a program, or to accurately describe and assess meaning related to an observable phenomenon.
With this in mind, a common mistake made by researchers is that they begin their investigations before they have thought critically about what information is required to address the research problem. Without attending to these design issues beforehand, the overall research problem will not be adequately addressed and any conclusions drawn will run the risk of being weak and unconvincing. As a consequence, the overall validity of the study will be undermined.
The length and complexity of describing the research design in your paper can vary considerably, but any well-developed description will achieve the following :
- Identify the research problem clearly and justify its selection, particularly in relation to any valid alternative designs that could have been used,
- Review and synthesize previously published literature associated with the research problem,
- Clearly and explicitly specify hypotheses [i.e., research questions] central to the problem,
- Effectively describe the information and/or data which will be necessary for an adequate testing of the hypotheses and explain how such information and/or data will be obtained, and
- Describe the methods of analysis to be applied to the data in determining whether or not the hypotheses are true or false.
The research design is usually incorporated into the introduction of your paper . You can obtain an overall sense of what to do by reviewing studies that have utilized the same research design [e.g., using a case study approach]. This can help you develop an outline to follow for your own paper.
NOTE : Use the SAGE Research Methods Online and Cases and the SAGE Research Methods Videos databases to search for scholarly resources on how to apply specific research designs and methods . The Research Methods Online database contains links to more than 175,000 pages of SAGE publisher's book, journal, and reference content on quantitative, qualitative, and mixed research methodologies. Also included is a collection of case studies of social research projects that can be used to help you better understand abstract or complex methodological concepts. The Research Methods Videos database contains hours of tutorials, interviews, video case studies, and mini-documentaries covering the entire research process.
Creswell, John W. and J. David Creswell. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches . 5th edition. Thousand Oaks, CA: Sage, 2018; De Vaus, D. A. Research Design in Social Research . London: SAGE, 2001; Gorard, Stephen. Research Design: Creating Robust Approaches for the Social Sciences . Thousand Oaks, CA: Sage, 2013; Leedy, Paul D. and Jeanne Ellis Ormrod. Practical Research: Planning and Design . Tenth edition. Boston, MA: Pearson, 2013; Vogt, W. Paul, Dianna C. Gardner, and Lynne M. Haeffele. When to Use What Research Design . New York: Guilford, 2012.
Action Research Design
Definition and Purpose
The essentials of action research design follow a characteristic cycle whereby initially an exploratory stance is adopted, where an understanding of a problem is developed and plans are made for some form of interventionary strategy. Then the intervention is carried out [the "action" in action research] during which time, pertinent observations are collected in various forms. The new interventional strategies are carried out, and this cyclic process repeats, continuing until a sufficient understanding of [or a valid implementation solution for] the problem is achieved. The protocol is iterative or cyclical in nature and is intended to foster deeper understanding of a given situation, starting with conceptualizing and particularizing the problem and moving through several interventions and evaluations.
What do these studies tell you ?
- This is a collaborative and adaptive research design that lends itself to use in work or community situations.
- Design focuses on pragmatic and solution-driven research outcomes rather than testing theories.
- When practitioners use action research, it has the potential to increase the amount they learn consciously from their experience; the action research cycle can be regarded as a learning cycle.
- Action research studies often have direct and obvious relevance to improving practice and advocating for change.
- There are no hidden controls or preemption of direction by the researcher.
What these studies don't tell you ?
- It is harder to do than conducting conventional research because the researcher takes on responsibilities of advocating for change as well as for researching the topic.
- Action research is much harder to write up because it is less likely that you can use a standard format to report your findings effectively [i.e., data is often in the form of stories or observation].
- Personal over-involvement of the researcher may bias research results.
- The cyclic nature of action research to achieve its twin outcomes of action [e.g. change] and research [e.g. understanding] is time-consuming and complex to conduct.
- Advocating for change usually requires buy-in from study participants.
Coghlan, David and Mary Brydon-Miller. The Sage Encyclopedia of Action Research . Thousand Oaks, CA: Sage, 2014; Efron, Sara Efrat and Ruth Ravid. Action Research in Education: A Practical Guide . New York: Guilford, 2013; Gall, Meredith. Educational Research: An Introduction . Chapter 18, Action Research. 8th ed. Boston, MA: Pearson/Allyn and Bacon, 2007; Gorard, Stephen. Research Design: Creating Robust Approaches for the Social Sciences . Thousand Oaks, CA: Sage, 2013; Kemmis, Stephen and Robin McTaggart. “Participatory Action Research.” In Handbook of Qualitative Research . Norman Denzin and Yvonna S. Lincoln, eds. 2nd ed. (Thousand Oaks, CA: SAGE, 2000), pp. 567-605; McNiff, Jean. Writing and Doing Action Research . London: Sage, 2014; Reason, Peter and Hilary Bradbury. Handbook of Action Research: Participative Inquiry and Practice . Thousand Oaks, CA: SAGE, 2001.
Case Study Design
A case study is an in-depth study of a particular research problem rather than a sweeping statistical survey or comprehensive comparative inquiry. It is often used to narrow down a very broad field of research into one or a few easily researchable examples. The case study research design is also useful for testing whether a specific theory and model actually applies to phenomena in the real world. It is a useful design when not much is known about an issue or phenomenon.
- Approach excels at bringing us to an understanding of a complex issue through detailed contextual analysis of a limited number of events or conditions and their relationships.
- A researcher using a case study design can apply a variety of methodologies and rely on a variety of sources to investigate a research problem.
- Design can extend experience or add strength to what is already known through previous research.
- Social scientists, in particular, make wide use of this research design to examine contemporary real-life situations and provide the basis for the application of concepts and theories and the extension of methodologies.
- The design can provide detailed descriptions of specific and rare cases.
- A single or small number of cases offers little basis for establishing reliability or to generalize the findings to a wider population of people, places, or things.
- Intense exposure to the study of a case may bias a researcher's interpretation of the findings.
- Design does not facilitate assessment of cause and effect relationships.
- Vital information may be missing, making the case hard to interpret.
- The case may not be representative or typical of the larger problem being investigated.
- If the criteria for selecting a case is because it represents a very unusual or unique phenomenon or problem for study, then your interpretation of the findings can only apply to that particular case.
Case Studies. Writing@CSU. Colorado State University; Anastas, Jeane W. Research Design for Social Work and the Human Services . Chapter 4, Flexible Methods: Case Study Design. 2nd ed. New York: Columbia University Press, 1999; Gerring, John. “What Is a Case Study and What Is It Good for?” American Political Science Review 98 (May 2004): 341-354; Greenhalgh, Trisha, editor. Case Study Evaluation: Past, Present and Future Challenges . Bingley, UK: Emerald Group Publishing, 2015; Mills, Albert J. , Gabrielle Durepos, and Eiden Wiebe, editors. Encyclopedia of Case Study Research . Thousand Oaks, CA: SAGE Publications, 2010; Stake, Robert E. The Art of Case Study Research . Thousand Oaks, CA: SAGE, 1995; Yin, Robert K. Case Study Research: Design and Theory . Applied Social Research Methods Series, no. 5. 3rd ed. Thousand Oaks, CA: SAGE, 2003.
Causal Design
Causality studies may be thought of as understanding a phenomenon in terms of conditional statements in the form, “If X, then Y.” This type of research is used to measure what impact a specific change will have on existing norms and assumptions. Most social scientists seek causal explanations that reflect tests of hypotheses. Causal effect (nomothetic perspective) occurs when variation in one phenomenon, an independent variable, leads to or results, on average, in variation in another phenomenon, the dependent variable.
Conditions necessary for determining causality:
- Empirical association -- a valid conclusion is based on finding an association between the independent variable and the dependent variable.
- Appropriate time order -- to conclude that causation was involved, one must see that cases were exposed to variation in the independent variable before variation in the dependent variable.
- Nonspuriousness -- a relationship between two variables that is not due to variation in a third variable.
- Causality research designs assist researchers in understanding why the world works the way it does through the process of proving a causal link between variables and by the process of eliminating other possibilities.
- Replication is possible.
- There is greater confidence the study has internal validity due to the systematic subject selection and equity of groups being compared.
- Not all relationships are causal! The possibility always exists that, by sheer coincidence, two unrelated events appear to be related [e.g., Punxatawney Phil could accurately predict the duration of Winter for five consecutive years but, the fact remains, he's just a big, furry rodent].
- Conclusions about causal relationships are difficult to determine due to a variety of extraneous and confounding variables that exist in a social environment. This means causality can only be inferred, never proven.
- If two variables are correlated, the cause must come before the effect. However, even though two variables might be causally related, it can sometimes be difficult to determine which variable comes first and, therefore, to establish which variable is the actual cause and which is the actual effect.
Beach, Derek and Rasmus Brun Pedersen. Causal Case Study Methods: Foundations and Guidelines for Comparing, Matching, and Tracing . Ann Arbor, MI: University of Michigan Press, 2016; Bachman, Ronet. The Practice of Research in Criminology and Criminal Justice . Chapter 5, Causation and Research Designs. 3rd ed. Thousand Oaks, CA: Pine Forge Press, 2007; Brewer, Ernest W. and Jennifer Kubn. “Causal-Comparative Design.” In Encyclopedia of Research Design . Neil J. Salkind, editor. (Thousand Oaks, CA: Sage, 2010), pp. 125-132; Causal Research Design: Experimentation. Anonymous SlideShare Presentation; Gall, Meredith. Educational Research: An Introduction . Chapter 11, Nonexperimental Research: Correlational Designs. 8th ed. Boston, MA: Pearson/Allyn and Bacon, 2007; Trochim, William M.K. Research Methods Knowledge Base. 2006.
Cohort Design
Often used in the medical sciences, but also found in the applied social sciences, a cohort study generally refers to a study conducted over a period of time involving members of a population which the subject or representative member comes from, and who are united by some commonality or similarity. Using a quantitative framework, a cohort study makes note of statistical occurrence within a specialized subgroup, united by same or similar characteristics that are relevant to the research problem being investigated, rather than studying statistical occurrence within the general population. Using a qualitative framework, cohort studies generally gather data using methods of observation. Cohorts can be either "open" or "closed."
- Open Cohort Studies [dynamic populations, such as the population of Los Angeles] involve a population that is defined just by the state of being a part of the study in question (and being monitored for the outcome). Date of entry and exit from the study is individually defined, therefore, the size of the study population is not constant. In open cohort studies, researchers can only calculate rate based data, such as, incidence rates and variants thereof.
- Closed Cohort Studies [static populations, such as patients entered into a clinical trial] involve participants who enter into the study at one defining point in time and where it is presumed that no new participants can enter the cohort. Given this, the number of study participants remains constant (or can only decrease).
- The use of cohorts is often mandatory because a randomized control study may be unethical. For example, you cannot deliberately expose people to asbestos, you can only study its effects on those who have already been exposed. Research that measures risk factors often relies upon cohort designs.
- Because cohort studies measure potential causes before the outcome has occurred, they can demonstrate that these “causes” preceded the outcome, thereby avoiding the debate as to which is the cause and which is the effect.
- Cohort analysis is highly flexible and can provide insight into effects over time and related to a variety of different types of changes [e.g., social, cultural, political, economic, etc.].
- Either original data or secondary data can be used in this design.
- In cases where a comparative analysis of two cohorts is made [e.g., studying the effects of one group exposed to asbestos and one that has not], a researcher cannot control for all other factors that might differ between the two groups. These factors are known as confounding variables.
- Cohort studies can end up taking a long time to complete if the researcher must wait for the conditions of interest to develop within the group. This also increases the chance that key variables change during the course of the study, potentially impacting the validity of the findings.
- Due to the lack of randominization in the cohort design, its external validity is lower than that of study designs where the researcher randomly assigns participants.
Healy P, Devane D. “Methodological Considerations in Cohort Study Designs.” Nurse Researcher 18 (2011): 32-36; Glenn, Norval D, editor. Cohort Analysis . 2nd edition. Thousand Oaks, CA: Sage, 2005; Levin, Kate Ann. Study Design IV: Cohort Studies. Evidence-Based Dentistry 7 (2003): 51–52; Payne, Geoff. “Cohort Study.” In The SAGE Dictionary of Social Research Methods . Victor Jupp, editor. (Thousand Oaks, CA: Sage, 2006), pp. 31-33; Study Design 101. Himmelfarb Health Sciences Library. George Washington University, November 2011; Cohort Study. Wikipedia.
Cross-Sectional Design
Cross-sectional research designs have three distinctive features: no time dimension; a reliance on existing differences rather than change following intervention; and, groups are selected based on existing differences rather than random allocation. The cross-sectional design can only measure differences between or from among a variety of people, subjects, or phenomena rather than a process of change. As such, researchers using this design can only employ a relatively passive approach to making causal inferences based on findings.
- Cross-sectional studies provide a clear 'snapshot' of the outcome and the characteristics associated with it, at a specific point in time.
- Unlike an experimental design, where there is an active intervention by the researcher to produce and measure change or to create differences, cross-sectional designs focus on studying and drawing inferences from existing differences between people, subjects, or phenomena.
- Entails collecting data at and concerning one point in time. While longitudinal studies involve taking multiple measures over an extended period of time, cross-sectional research is focused on finding relationships between variables at one moment in time.
- Groups identified for study are purposely selected based upon existing differences in the sample rather than seeking random sampling.
- Cross-section studies are capable of using data from a large number of subjects and, unlike observational studies, is not geographically bound.
- Can estimate prevalence of an outcome of interest because the sample is usually taken from the whole population.
- Because cross-sectional designs generally use survey techniques to gather data, they are relatively inexpensive and take up little time to conduct.
- Finding people, subjects, or phenomena to study that are very similar except in one specific variable can be difficult.
- Results are static and time bound and, therefore, give no indication of a sequence of events or reveal historical or temporal contexts.
- Studies cannot be utilized to establish cause and effect relationships.
- This design only provides a snapshot of analysis so there is always the possibility that a study could have differing results if another time-frame had been chosen.
- There is no follow up to the findings.
Bethlehem, Jelke. "7: Cross-sectional Research." In Research Methodology in the Social, Behavioural and Life Sciences . Herman J Adèr and Gideon J Mellenbergh, editors. (London, England: Sage, 1999), pp. 110-43; Bourque, Linda B. “Cross-Sectional Design.” In The SAGE Encyclopedia of Social Science Research Methods . Michael S. Lewis-Beck, Alan Bryman, and Tim Futing Liao. (Thousand Oaks, CA: 2004), pp. 230-231; Hall, John. “Cross-Sectional Survey Design.” In Encyclopedia of Survey Research Methods . Paul J. Lavrakas, ed. (Thousand Oaks, CA: Sage, 2008), pp. 173-174; Helen Barratt, Maria Kirwan. Cross-Sectional Studies: Design Application, Strengths and Weaknesses of Cross-Sectional Studies. Healthknowledge, 2009. Cross-Sectional Study. Wikipedia.
Descriptive Design
Descriptive research designs help provide answers to the questions of who, what, when, where, and how associated with a particular research problem; a descriptive study cannot conclusively ascertain answers to why. Descriptive research is used to obtain information concerning the current status of the phenomena and to describe "what exists" with respect to variables or conditions in a situation.
- The subject is being observed in a completely natural and unchanged natural environment. True experiments, whilst giving analyzable data, often adversely influence the normal behavior of the subject [a.k.a., the Heisenberg effect whereby measurements of certain systems cannot be made without affecting the systems].
- Descriptive research is often used as a pre-cursor to more quantitative research designs with the general overview giving some valuable pointers as to what variables are worth testing quantitatively.
- If the limitations are understood, they can be a useful tool in developing a more focused study.
- Descriptive studies can yield rich data that lead to important recommendations in practice.
- Appoach collects a large amount of data for detailed analysis.
- The results from a descriptive research cannot be used to discover a definitive answer or to disprove a hypothesis.
- Because descriptive designs often utilize observational methods [as opposed to quantitative methods], the results cannot be replicated.
- The descriptive function of research is heavily dependent on instrumentation for measurement and observation.
Anastas, Jeane W. Research Design for Social Work and the Human Services . Chapter 5, Flexible Methods: Descriptive Research. 2nd ed. New York: Columbia University Press, 1999; Given, Lisa M. "Descriptive Research." In Encyclopedia of Measurement and Statistics . Neil J. Salkind and Kristin Rasmussen, editors. (Thousand Oaks, CA: Sage, 2007), pp. 251-254; McNabb, Connie. Descriptive Research Methodologies. Powerpoint Presentation; Shuttleworth, Martyn. Descriptive Research Design, September 26, 2008; Erickson, G. Scott. "Descriptive Research Design." In New Methods of Market Research and Analysis . (Northampton, MA: Edward Elgar Publishing, 2017), pp. 51-77; Sahin, Sagufta, and Jayanta Mete. "A Brief Study on Descriptive Research: Its Nature and Application in Social Science." International Journal of Research and Analysis in Humanities 1 (2021): 11; K. Swatzell and P. Jennings. “Descriptive Research: The Nuts and Bolts.” Journal of the American Academy of Physician Assistants 20 (2007), pp. 55-56; Kane, E. Doing Your Own Research: Basic Descriptive Research in the Social Sciences and Humanities . London: Marion Boyars, 1985.
Experimental Design
A blueprint of the procedure that enables the researcher to maintain control over all factors that may affect the result of an experiment. In doing this, the researcher attempts to determine or predict what may occur. Experimental research is often used where there is time priority in a causal relationship (cause precedes effect), there is consistency in a causal relationship (a cause will always lead to the same effect), and the magnitude of the correlation is great. The classic experimental design specifies an experimental group and a control group. The independent variable is administered to the experimental group and not to the control group, and both groups are measured on the same dependent variable. Subsequent experimental designs have used more groups and more measurements over longer periods. True experiments must have control, randomization, and manipulation.
- Experimental research allows the researcher to control the situation. In so doing, it allows researchers to answer the question, “What causes something to occur?”
- Permits the researcher to identify cause and effect relationships between variables and to distinguish placebo effects from treatment effects.
- Experimental research designs support the ability to limit alternative explanations and to infer direct causal relationships in the study.
- Approach provides the highest level of evidence for single studies.
- The design is artificial, and results may not generalize well to the real world.
- The artificial settings of experiments may alter the behaviors or responses of participants.
- Experimental designs can be costly if special equipment or facilities are needed.
- Some research problems cannot be studied using an experiment because of ethical or technical reasons.
- Difficult to apply ethnographic and other qualitative methods to experimentally designed studies.
Anastas, Jeane W. Research Design for Social Work and the Human Services . Chapter 7, Flexible Methods: Experimental Research. 2nd ed. New York: Columbia University Press, 1999; Chapter 2: Research Design, Experimental Designs. School of Psychology, University of New England, 2000; Chow, Siu L. "Experimental Design." In Encyclopedia of Research Design . Neil J. Salkind, editor. (Thousand Oaks, CA: Sage, 2010), pp. 448-453; "Experimental Design." In Social Research Methods . Nicholas Walliman, editor. (London, England: Sage, 2006), pp, 101-110; Experimental Research. Research Methods by Dummies. Department of Psychology. California State University, Fresno, 2006; Kirk, Roger E. Experimental Design: Procedures for the Behavioral Sciences . 4th edition. Thousand Oaks, CA: Sage, 2013; Trochim, William M.K. Experimental Design. Research Methods Knowledge Base. 2006; Rasool, Shafqat. Experimental Research. Slideshare presentation.
Exploratory Design
An exploratory design is conducted about a research problem when there are few or no earlier studies to refer to or rely upon to predict an outcome . The focus is on gaining insights and familiarity for later investigation or undertaken when research problems are in a preliminary stage of investigation. Exploratory designs are often used to establish an understanding of how best to proceed in studying an issue or what methodology would effectively apply to gathering information about the issue.
The goals of exploratory research are intended to produce the following possible insights:
- Familiarity with basic details, settings, and concerns.
- Well grounded picture of the situation being developed.
- Generation of new ideas and assumptions.
- Development of tentative theories or hypotheses.
- Determination about whether a study is feasible in the future.
- Issues get refined for more systematic investigation and formulation of new research questions.
- Direction for future research and techniques get developed.
- Design is a useful approach for gaining background information on a particular topic.
- Exploratory research is flexible and can address research questions of all types (what, why, how).
- Provides an opportunity to define new terms and clarify existing concepts.
- Exploratory research is often used to generate formal hypotheses and develop more precise research problems.
- In the policy arena or applied to practice, exploratory studies help establish research priorities and where resources should be allocated.
- Exploratory research generally utilizes small sample sizes and, thus, findings are typically not generalizable to the population at large.
- The exploratory nature of the research inhibits an ability to make definitive conclusions about the findings. They provide insight but not definitive conclusions.
- The research process underpinning exploratory studies is flexible but often unstructured, leading to only tentative results that have limited value to decision-makers.
- Design lacks rigorous standards applied to methods of data gathering and analysis because one of the areas for exploration could be to determine what method or methodologies could best fit the research problem.
Cuthill, Michael. “Exploratory Research: Citizen Participation, Local Government, and Sustainable Development in Australia.” Sustainable Development 10 (2002): 79-89; Streb, Christoph K. "Exploratory Case Study." In Encyclopedia of Case Study Research . Albert J. Mills, Gabrielle Durepos and Eiden Wiebe, editors. (Thousand Oaks, CA: Sage, 2010), pp. 372-374; Taylor, P. J., G. Catalano, and D.R.F. Walker. “Exploratory Analysis of the World City Network.” Urban Studies 39 (December 2002): 2377-2394; Exploratory Research. Wikipedia.
Field Research Design
Sometimes referred to as ethnography or participant observation, designs around field research encompass a variety of interpretative procedures [e.g., observation and interviews] rooted in qualitative approaches to studying people individually or in groups while inhabiting their natural environment as opposed to using survey instruments or other forms of impersonal methods of data gathering. Information acquired from observational research takes the form of “ field notes ” that involves documenting what the researcher actually sees and hears while in the field. Findings do not consist of conclusive statements derived from numbers and statistics because field research involves analysis of words and observations of behavior. Conclusions, therefore, are developed from an interpretation of findings that reveal overriding themes, concepts, and ideas. More information can be found HERE .
- Field research is often necessary to fill gaps in understanding the research problem applied to local conditions or to specific groups of people that cannot be ascertained from existing data.
- The research helps contextualize already known information about a research problem, thereby facilitating ways to assess the origins, scope, and scale of a problem and to gage the causes, consequences, and means to resolve an issue based on deliberate interaction with people in their natural inhabited spaces.
- Enables the researcher to corroborate or confirm data by gathering additional information that supports or refutes findings reported in prior studies of the topic.
- Because the researcher in embedded in the field, they are better able to make observations or ask questions that reflect the specific cultural context of the setting being investigated.
- Observing the local reality offers the opportunity to gain new perspectives or obtain unique data that challenges existing theoretical propositions or long-standing assumptions found in the literature.
What these studies don't tell you
- A field research study requires extensive time and resources to carry out the multiple steps involved with preparing for the gathering of information, including for example, examining background information about the study site, obtaining permission to access the study site, and building trust and rapport with subjects.
- Requires a commitment to staying engaged in the field to ensure that you can adequately document events and behaviors as they unfold.
- The unpredictable nature of fieldwork means that researchers can never fully control the process of data gathering. They must maintain a flexible approach to studying the setting because events and circumstances can change quickly or unexpectedly.
- Findings can be difficult to interpret and verify without access to documents and other source materials that help to enhance the credibility of information obtained from the field [i.e., the act of triangulating the data].
- Linking the research problem to the selection of study participants inhabiting their natural environment is critical. However, this specificity limits the ability to generalize findings to different situations or in other contexts or to infer courses of action applied to other settings or groups of people.
- The reporting of findings must take into account how the researcher themselves may have inadvertently affected respondents and their behaviors.
Historical Design
The purpose of a historical research design is to collect, verify, and synthesize evidence from the past to establish facts that defend or refute a hypothesis. It uses secondary sources and a variety of primary documentary evidence, such as, diaries, official records, reports, archives, and non-textual information [maps, pictures, audio and visual recordings]. The limitation is that the sources must be both authentic and valid.
- The historical research design is unobtrusive; the act of research does not affect the results of the study.
- The historical approach is well suited for trend analysis.
- Historical records can add important contextual background required to more fully understand and interpret a research problem.
- There is often no possibility of researcher-subject interaction that could affect the findings.
- Historical sources can be used over and over to study different research problems or to replicate a previous study.
- The ability to fulfill the aims of your research are directly related to the amount and quality of documentation available to understand the research problem.
- Since historical research relies on data from the past, there is no way to manipulate it to control for contemporary contexts.
- Interpreting historical sources can be very time consuming.
- The sources of historical materials must be archived consistently to ensure access. This may especially challenging for digital or online-only sources.
- Original authors bring their own perspectives and biases to the interpretation of past events and these biases are more difficult to ascertain in historical resources.
- Due to the lack of control over external variables, historical research is very weak with regard to the demands of internal validity.
- It is rare that the entirety of historical documentation needed to fully address a research problem is available for interpretation, therefore, gaps need to be acknowledged.
Howell, Martha C. and Walter Prevenier. From Reliable Sources: An Introduction to Historical Methods . Ithaca, NY: Cornell University Press, 2001; Lundy, Karen Saucier. "Historical Research." In The Sage Encyclopedia of Qualitative Research Methods . Lisa M. Given, editor. (Thousand Oaks, CA: Sage, 2008), pp. 396-400; Marius, Richard. and Melvin E. Page. A Short Guide to Writing about History . 9th edition. Boston, MA: Pearson, 2015; Savitt, Ronald. “Historical Research in Marketing.” Journal of Marketing 44 (Autumn, 1980): 52-58; Gall, Meredith. Educational Research: An Introduction . Chapter 16, Historical Research. 8th ed. Boston, MA: Pearson/Allyn and Bacon, 2007.

Longitudinal Design
A longitudinal study follows the same sample over time and makes repeated observations. For example, with longitudinal surveys, the same group of people is interviewed at regular intervals, enabling researchers to track changes over time and to relate them to variables that might explain why the changes occur. Longitudinal research designs describe patterns of change and help establish the direction and magnitude of causal relationships. Measurements are taken on each variable over two or more distinct time periods. This allows the researcher to measure change in variables over time. It is a type of observational study sometimes referred to as a panel study.
- Longitudinal data facilitate the analysis of the duration of a particular phenomenon.
- Enables survey researchers to get close to the kinds of causal explanations usually attainable only with experiments.
- The design permits the measurement of differences or change in a variable from one period to another [i.e., the description of patterns of change over time].
- Longitudinal studies facilitate the prediction of future outcomes based upon earlier factors.
- The data collection method may change over time.
- Maintaining the integrity of the original sample can be difficult over an extended period of time.
- It can be difficult to show more than one variable at a time.
- This design often needs qualitative research data to explain fluctuations in the results.
- A longitudinal research design assumes present trends will continue unchanged.
- It can take a long period of time to gather results.
- There is a need to have a large sample size and accurate sampling to reach representativness.
Anastas, Jeane W. Research Design for Social Work and the Human Services . Chapter 6, Flexible Methods: Relational and Longitudinal Research. 2nd ed. New York: Columbia University Press, 1999; Forgues, Bernard, and Isabelle Vandangeon-Derumez. "Longitudinal Analyses." In Doing Management Research . Raymond-Alain Thiétart and Samantha Wauchope, editors. (London, England: Sage, 2001), pp. 332-351; Kalaian, Sema A. and Rafa M. Kasim. "Longitudinal Studies." In Encyclopedia of Survey Research Methods . Paul J. Lavrakas, ed. (Thousand Oaks, CA: Sage, 2008), pp. 440-441; Menard, Scott, editor. Longitudinal Research . Thousand Oaks, CA: Sage, 2002; Ployhart, Robert E. and Robert J. Vandenberg. "Longitudinal Research: The Theory, Design, and Analysis of Change.” Journal of Management 36 (January 2010): 94-120; Longitudinal Study. Wikipedia.
Meta-Analysis Design
Meta-analysis is an analytical methodology designed to systematically evaluate and summarize the results from a number of individual studies, thereby, increasing the overall sample size and the ability of the researcher to study effects of interest. The purpose is to not simply summarize existing knowledge, but to develop a new understanding of a research problem using synoptic reasoning. The main objectives of meta-analysis include analyzing differences in the results among studies and increasing the precision by which effects are estimated. A well-designed meta-analysis depends upon strict adherence to the criteria used for selecting studies and the availability of information in each study to properly analyze their findings. Lack of information can severely limit the type of analyzes and conclusions that can be reached. In addition, the more dissimilarity there is in the results among individual studies [heterogeneity], the more difficult it is to justify interpretations that govern a valid synopsis of results. A meta-analysis needs to fulfill the following requirements to ensure the validity of your findings:
- Clearly defined description of objectives, including precise definitions of the variables and outcomes that are being evaluated;
- A well-reasoned and well-documented justification for identification and selection of the studies;
- Assessment and explicit acknowledgment of any researcher bias in the identification and selection of those studies;
- Description and evaluation of the degree of heterogeneity among the sample size of studies reviewed; and,
- Justification of the techniques used to evaluate the studies.
- Can be an effective strategy for determining gaps in the literature.
- Provides a means of reviewing research published about a particular topic over an extended period of time and from a variety of sources.
- Is useful in clarifying what policy or programmatic actions can be justified on the basis of analyzing research results from multiple studies.
- Provides a method for overcoming small sample sizes in individual studies that previously may have had little relationship to each other.
- Can be used to generate new hypotheses or highlight research problems for future studies.
- Small violations in defining the criteria used for content analysis can lead to difficult to interpret and/or meaningless findings.
- A large sample size can yield reliable, but not necessarily valid, results.
- A lack of uniformity regarding, for example, the type of literature reviewed, how methods are applied, and how findings are measured within the sample of studies you are analyzing, can make the process of synthesis difficult to perform.
- Depending on the sample size, the process of reviewing and synthesizing multiple studies can be very time consuming.
Beck, Lewis W. "The Synoptic Method." The Journal of Philosophy 36 (1939): 337-345; Cooper, Harris, Larry V. Hedges, and Jeffrey C. Valentine, eds. The Handbook of Research Synthesis and Meta-Analysis . 2nd edition. New York: Russell Sage Foundation, 2009; Guzzo, Richard A., Susan E. Jackson and Raymond A. Katzell. “Meta-Analysis Analysis.” In Research in Organizational Behavior , Volume 9. (Greenwich, CT: JAI Press, 1987), pp 407-442; Lipsey, Mark W. and David B. Wilson. Practical Meta-Analysis . Thousand Oaks, CA: Sage Publications, 2001; Study Design 101. Meta-Analysis. The Himmelfarb Health Sciences Library, George Washington University; Timulak, Ladislav. “Qualitative Meta-Analysis.” In The SAGE Handbook of Qualitative Data Analysis . Uwe Flick, editor. (Los Angeles, CA: Sage, 2013), pp. 481-495; Walker, Esteban, Adrian V. Hernandez, and Micheal W. Kattan. "Meta-Analysis: It's Strengths and Limitations." Cleveland Clinic Journal of Medicine 75 (June 2008): 431-439.
Mixed-Method Design
- Narrative and non-textual information can add meaning to numeric data, while numeric data can add precision to narrative and non-textual information.
- Can utilize existing data while at the same time generating and testing a grounded theory approach to describe and explain the phenomenon under study.
- A broader, more complex research problem can be investigated because the researcher is not constrained by using only one method.
- The strengths of one method can be used to overcome the inherent weaknesses of another method.
- Can provide stronger, more robust evidence to support a conclusion or set of recommendations.
- May generate new knowledge new insights or uncover hidden insights, patterns, or relationships that a single methodological approach might not reveal.
- Produces more complete knowledge and understanding of the research problem that can be used to increase the generalizability of findings applied to theory or practice.
- A researcher must be proficient in understanding how to apply multiple methods to investigating a research problem as well as be proficient in optimizing how to design a study that coherently melds them together.
- Can increase the likelihood of conflicting results or ambiguous findings that inhibit drawing a valid conclusion or setting forth a recommended course of action [e.g., sample interview responses do not support existing statistical data].
- Because the research design can be very complex, reporting the findings requires a well-organized narrative, clear writing style, and precise word choice.
- Design invites collaboration among experts. However, merging different investigative approaches and writing styles requires more attention to the overall research process than studies conducted using only one methodological paradigm.
- Concurrent merging of quantitative and qualitative research requires greater attention to having adequate sample sizes, using comparable samples, and applying a consistent unit of analysis. For sequential designs where one phase of qualitative research builds on the quantitative phase or vice versa, decisions about what results from the first phase to use in the next phase, the choice of samples and estimating reasonable sample sizes for both phases, and the interpretation of results from both phases can be difficult.
- Due to multiple forms of data being collected and analyzed, this design requires extensive time and resources to carry out the multiple steps involved in data gathering and interpretation.
Burch, Patricia and Carolyn J. Heinrich. Mixed Methods for Policy Research and Program Evaluation . Thousand Oaks, CA: Sage, 2016; Creswell, John w. et al. Best Practices for Mixed Methods Research in the Health Sciences . Bethesda, MD: Office of Behavioral and Social Sciences Research, National Institutes of Health, 2010Creswell, John W. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches . 4th edition. Thousand Oaks, CA: Sage Publications, 2014; Domínguez, Silvia, editor. Mixed Methods Social Networks Research . Cambridge, UK: Cambridge University Press, 2014; Hesse-Biber, Sharlene Nagy. Mixed Methods Research: Merging Theory with Practice . New York: Guilford Press, 2010; Niglas, Katrin. “How the Novice Researcher Can Make Sense of Mixed Methods Designs.” International Journal of Multiple Research Approaches 3 (2009): 34-46; Onwuegbuzie, Anthony J. and Nancy L. Leech. “Linking Research Questions to Mixed Methods Data Analysis Procedures.” The Qualitative Report 11 (September 2006): 474-498; Tashakorri, Abbas and John W. Creswell. “The New Era of Mixed Methods.” Journal of Mixed Methods Research 1 (January 2007): 3-7; Zhanga, Wanqing. “Mixed Methods Application in Health Intervention Research: A Multiple Case Study.” International Journal of Multiple Research Approaches 8 (2014): 24-35 .
Observational Design
This type of research design draws a conclusion by comparing subjects against a control group, in cases where the researcher has no control over the experiment. There are two general types of observational designs. In direct observations, people know that you are watching them. Unobtrusive measures involve any method for studying behavior where individuals do not know they are being observed. An observational study allows a useful insight into a phenomenon and avoids the ethical and practical difficulties of setting up a large and cumbersome research project.
- Observational studies are usually flexible and do not necessarily need to be structured around a hypothesis about what you expect to observe [data is emergent rather than pre-existing].
- The researcher is able to collect in-depth information about a particular behavior.
- Can reveal interrelationships among multifaceted dimensions of group interactions.
- You can generalize your results to real life situations.
- Observational research is useful for discovering what variables may be important before applying other methods like experiments.
- Observation research designs account for the complexity of group behaviors.
- Reliability of data is low because seeing behaviors occur over and over again may be a time consuming task and are difficult to replicate.
- In observational research, findings may only reflect a unique sample population and, thus, cannot be generalized to other groups.
- There can be problems with bias as the researcher may only "see what they want to see."
- There is no possibility to determine "cause and effect" relationships since nothing is manipulated.
- Sources or subjects may not all be equally credible.
- Any group that is knowingly studied is altered to some degree by the presence of the researcher, therefore, potentially skewing any data collected.
Atkinson, Paul and Martyn Hammersley. “Ethnography and Participant Observation.” In Handbook of Qualitative Research . Norman K. Denzin and Yvonna S. Lincoln, eds. (Thousand Oaks, CA: Sage, 1994), pp. 248-261; Observational Research. Research Methods by Dummies. Department of Psychology. California State University, Fresno, 2006; Patton Michael Quinn. Qualitiative Research and Evaluation Methods . Chapter 6, Fieldwork Strategies and Observational Methods. 3rd ed. Thousand Oaks, CA: Sage, 2002; Payne, Geoff and Judy Payne. "Observation." In Key Concepts in Social Research . The SAGE Key Concepts series. (London, England: Sage, 2004), pp. 158-162; Rosenbaum, Paul R. Design of Observational Studies . New York: Springer, 2010;Williams, J. Patrick. "Nonparticipant Observation." In The Sage Encyclopedia of Qualitative Research Methods . Lisa M. Given, editor.(Thousand Oaks, CA: Sage, 2008), pp. 562-563.
Philosophical Design
Understood more as an broad approach to examining a research problem than a methodological design, philosophical analysis and argumentation is intended to challenge deeply embedded, often intractable, assumptions underpinning an area of study. This approach uses the tools of argumentation derived from philosophical traditions, concepts, models, and theories to critically explore and challenge, for example, the relevance of logic and evidence in academic debates, to analyze arguments about fundamental issues, or to discuss the root of existing discourse about a research problem. These overarching tools of analysis can be framed in three ways:
- Ontology -- the study that describes the nature of reality; for example, what is real and what is not, what is fundamental and what is derivative?
- Epistemology -- the study that explores the nature of knowledge; for example, by what means does knowledge and understanding depend upon and how can we be certain of what we know?
- Axiology -- the study of values; for example, what values does an individual or group hold and why? How are values related to interest, desire, will, experience, and means-to-end? And, what is the difference between a matter of fact and a matter of value?
- Can provide a basis for applying ethical decision-making to practice.
- Functions as a means of gaining greater self-understanding and self-knowledge about the purposes of research.
- Brings clarity to general guiding practices and principles of an individual or group.
- Philosophy informs methodology.
- Refine concepts and theories that are invoked in relatively unreflective modes of thought and discourse.
- Beyond methodology, philosophy also informs critical thinking about epistemology and the structure of reality (metaphysics).
- Offers clarity and definition to the practical and theoretical uses of terms, concepts, and ideas.
- Limited application to specific research problems [answering the "So What?" question in social science research].
- Analysis can be abstract, argumentative, and limited in its practical application to real-life issues.
- While a philosophical analysis may render problematic that which was once simple or taken-for-granted, the writing can be dense and subject to unnecessary jargon, overstatement, and/or excessive quotation and documentation.
- There are limitations in the use of metaphor as a vehicle of philosophical analysis.
- There can be analytical difficulties in moving from philosophy to advocacy and between abstract thought and application to the phenomenal world.
Burton, Dawn. "Part I, Philosophy of the Social Sciences." In Research Training for Social Scientists . (London, England: Sage, 2000), pp. 1-5; Chapter 4, Research Methodology and Design. Unisa Institutional Repository (UnisaIR), University of South Africa; Jarvie, Ian C., and Jesús Zamora-Bonilla, editors. The SAGE Handbook of the Philosophy of Social Sciences . London: Sage, 2011; Labaree, Robert V. and Ross Scimeca. “The Philosophical Problem of Truth in Librarianship.” The Library Quarterly 78 (January 2008): 43-70; Maykut, Pamela S. Beginning Qualitative Research: A Philosophic and Practical Guide . Washington, DC: Falmer Press, 1994; McLaughlin, Hugh. "The Philosophy of Social Research." In Understanding Social Work Research . 2nd edition. (London: SAGE Publications Ltd., 2012), pp. 24-47; Stanford Encyclopedia of Philosophy . Metaphysics Research Lab, CSLI, Stanford University, 2013.
Sequential Design
- The researcher has a limitless option when it comes to sample size and the sampling schedule.
- Due to the repetitive nature of this research design, minor changes and adjustments can be done during the initial parts of the study to correct and hone the research method.
- This is a useful design for exploratory studies.
- There is very little effort on the part of the researcher when performing this technique. It is generally not expensive, time consuming, or workforce intensive.
- Because the study is conducted serially, the results of one sample are known before the next sample is taken and analyzed. This provides opportunities for continuous improvement of sampling and methods of analysis.
- The sampling method is not representative of the entire population. The only possibility of approaching representativeness is when the researcher chooses to use a very large sample size significant enough to represent a significant portion of the entire population. In this case, moving on to study a second or more specific sample can be difficult.
- The design cannot be used to create conclusions and interpretations that pertain to an entire population because the sampling technique is not randomized. Generalizability from findings is, therefore, limited.
- Difficult to account for and interpret variation from one sample to another over time, particularly when using qualitative methods of data collection.
Betensky, Rebecca. Harvard University, Course Lecture Note slides; Bovaird, James A. and Kevin A. Kupzyk. "Sequential Design." In Encyclopedia of Research Design . Neil J. Salkind, editor. (Thousand Oaks, CA: Sage, 2010), pp. 1347-1352; Cresswell, John W. Et al. “Advanced Mixed-Methods Research Designs.” In Handbook of Mixed Methods in Social and Behavioral Research . Abbas Tashakkori and Charles Teddle, eds. (Thousand Oaks, CA: Sage, 2003), pp. 209-240; Henry, Gary T. "Sequential Sampling." In The SAGE Encyclopedia of Social Science Research Methods . Michael S. Lewis-Beck, Alan Bryman and Tim Futing Liao, editors. (Thousand Oaks, CA: Sage, 2004), pp. 1027-1028; Nataliya V. Ivankova. “Using Mixed-Methods Sequential Explanatory Design: From Theory to Practice.” Field Methods 18 (February 2006): 3-20; Bovaird, James A. and Kevin A. Kupzyk. “Sequential Design.” In Encyclopedia of Research Design . Neil J. Salkind, ed. Thousand Oaks, CA: Sage, 2010; Sequential Analysis. Wikipedia.
Systematic Review
- A systematic review synthesizes the findings of multiple studies related to each other by incorporating strategies of analysis and interpretation intended to reduce biases and random errors.
- The application of critical exploration, evaluation, and synthesis methods separates insignificant, unsound, or redundant research from the most salient and relevant studies worthy of reflection.
- They can be use to identify, justify, and refine hypotheses, recognize and avoid hidden problems in prior studies, and explain data inconsistencies and conflicts in data.
- Systematic reviews can be used to help policy makers formulate evidence-based guidelines and regulations.
- The use of strict, explicit, and pre-determined methods of synthesis, when applied appropriately, provide reliable estimates about the effects of interventions, evaluations, and effects related to the overarching research problem investigated by each study under review.
- Systematic reviews illuminate where knowledge or thorough understanding of a research problem is lacking and, therefore, can then be used to guide future research.
- The accepted inclusion of unpublished studies [i.e., grey literature] ensures the broadest possible way to analyze and interpret research on a topic.
- Results of the synthesis can be generalized and the findings extrapolated into the general population with more validity than most other types of studies .
- Systematic reviews do not create new knowledge per se; they are a method for synthesizing existing studies about a research problem in order to gain new insights and determine gaps in the literature.
- The way researchers have carried out their investigations [e.g., the period of time covered, number of participants, sources of data analyzed, etc.] can make it difficult to effectively synthesize studies.
- The inclusion of unpublished studies can introduce bias into the review because they may not have undergone a rigorous peer-review process prior to publication. Examples may include conference presentations or proceedings, publications from government agencies, white papers, working papers, and internal documents from organizations, and doctoral dissertations and Master's theses.
Denyer, David and David Tranfield. "Producing a Systematic Review." In The Sage Handbook of Organizational Research Methods . David A. Buchanan and Alan Bryman, editors. ( Thousand Oaks, CA: Sage Publications, 2009), pp. 671-689; Foster, Margaret J. and Sarah T. Jewell, editors. Assembling the Pieces of a Systematic Review: A Guide for Librarians . Lanham, MD: Rowman and Littlefield, 2017; Gough, David, Sandy Oliver, James Thomas, editors. Introduction to Systematic Reviews . 2nd edition. Los Angeles, CA: Sage Publications, 2017; Gopalakrishnan, S. and P. Ganeshkumar. “Systematic Reviews and Meta-analysis: Understanding the Best Evidence in Primary Healthcare.” Journal of Family Medicine and Primary Care 2 (2013): 9-14; Gough, David, James Thomas, and Sandy Oliver. "Clarifying Differences between Review Designs and Methods." Systematic Reviews 1 (2012): 1-9; Khan, Khalid S., Regina Kunz, Jos Kleijnen, and Gerd Antes. “Five Steps to Conducting a Systematic Review.” Journal of the Royal Society of Medicine 96 (2003): 118-121; Mulrow, C. D. “Systematic Reviews: Rationale for Systematic Reviews.” BMJ 309:597 (September 1994); O'Dwyer, Linda C., and Q. Eileen Wafford. "Addressing Challenges with Systematic Review Teams through Effective Communication: A Case Report." Journal of the Medical Library Association 109 (October 2021): 643-647; Okoli, Chitu, and Kira Schabram. "A Guide to Conducting a Systematic Literature Review of Information Systems Research." Sprouts: Working Papers on Information Systems 10 (2010); Siddaway, Andy P., Alex M. Wood, and Larry V. Hedges. "How to Do a Systematic Review: A Best Practice Guide for Conducting and Reporting Narrative Reviews, Meta-analyses, and Meta-syntheses." Annual Review of Psychology 70 (2019): 747-770; Torgerson, Carole J. “Publication Bias: The Achilles’ Heel of Systematic Reviews?” British Journal of Educational Studies 54 (March 2006): 89-102; Torgerson, Carole. Systematic Reviews . New York: Continuum, 2003.
- << Previous: Purpose of Guide
- Next: Design Flaws to Avoid >>
- Last Updated: Apr 24, 2024 10:51 AM
- URL: https://libguides.usc.edu/writingguide
- Basicmedical Key
Fastest Basicmedical Insight Engine
- BIOCHEMISTRY
- GENERAL & FAMILY MEDICINE
- HUMAN BIOLOGY & GENETICS
- MEDICAL DICTIONARY & TERMINOLOGY
- MICROBIOLOGY
- PATHOLOGY & LABORATORY MEDICINE
- PUBLIC HEALTH AND EPIDEMIOLOGY
- Abdominal Key
- Anesthesia Key
- Otolaryngology & Ophthalmology
- Musculoskeletal Key
- Obstetric, Gynecology and Pediatric
- Oncology & Hematology
- Plastic Surgery & Dermatology
- Clinical Dentistry
- Radiology Key
- Thoracic Key
- Veterinary Medicine
- Gold Membership
Chapter 2. Study Designs in Medical Research
Key Concepts Study designs in medicine fall into two categories: studies in which subjects are observed, and studies in which the effect of an intervention is observed. Observational studies may be forward-looking (cohort), backward-looking (case-control), or looking at simultaneous events (cross-sectional). Cohort studies generally provide stronger evidence than the other two designs. Studies that examine patient outcomes are increasingly published in the literature; they focus on specific topics, such as resource utilization, functional status, quality of life, patient satisfaction, and cost-effectiveness. Studies with interventions are called experiments or clinical trials. They provide stronger evidence than observational studies. The single best way to minimize bias is to randomly select subjects in observational studies or randomly assign subjects to different treatment arms in clinical trials. Bias occurs when the way a study is designed or carried out causes an error in the results and conclusions. Bias can be due to the manner in which subjects are selected or data are collected and analyzed. Clinical trials without controls (subjects who do not receive the intervention) are difficult to interpret and do not provide strong evidence. Each study design has specific advantages and disadvantages. Study Designs in Medical Research: Introduction This chapter introduces the different kinds of studies commonly used in medical research. Because we believe that knowing how a study is designed is important for understanding the conclusions that can be drawn from it, we have chosen to devote considerable attention to the topic of study designs. If you are familiar with the medical literature, you will recognize many of the terms used to describe different study designs. If you are just beginning to read the literature, you should not be dismayed by all the new terminology; there will be ample opportunity to review and become familiar with it. Also, the glossary at the end of the book defines the terms we use here. In the final chapter of this book, study designs are reviewed within the context of reading journal articles, and pointers are given on how to look for possible biases that can occur in medical studies. Bias can be due to the manner in which patients are selected, data are collected and analyzed, or conclusions are drawn. Classification of Study Designs There are several different schemes for classifying study designs. We have adopted one that divides studies into those in which the subjects were merely observed, sometimes called observational studies, and those in which some intervention was performed, generally called experiments. This approach is simple and reflects the sequence an investigation sometimes takes. With a little practice, you should be able to read medical articles and classify studies according to the outline in Table 2–1 with little difficulty. Table 2–1. Classification of Study Designs. Table 2–1. Classification of Study Designs. I. Observational studies A. Descriptive or case–series B. Case–control studies (retrospective) 1. Causes and incidence of disease 2. Identification of risk factors C. Cross-sectional studies, surveys ( prevalence) 1. Disease description 2. Diagnosis and staging 3. Disease processes, mechanisms D. Cohort studies (prospective) 1. Causes and incidence of disease 2. Natural history, prognosis 3. Identification of risk factors E. Historical cohort studies II. Experimental studies A. Controlled trials 1. Parallel or concurrent controls a. Randomized b. Not randomized 2. Sequential controls a. Self-controlled b. Crossover 3. External controls (including historical) B. Studies with no controls III. Meta-analyses Each study design in Table 2–1 is illustrated in this chapter, using some of the studies that are presenting problems in upcoming chapters. In observational studies, one or more groups of patients are observed, and characteristics about the patients are recorded for analysis. Experimental studies involve an intervention —an investigator-controlled maneuver, such as a drug, a procedure, or a treatment—and interest lies in the effect the intervention has on study subjects. Of course, both observational and experimental studies may involve animals or objects, but most studies in medicine (and the ones discussed most frequently in this text) involve people. Observational Studies Observational studies are of four main types: case–series, case–control, cross-sectional (including surveys), and cohort studies. When certain characteristics of a group (or series) of patients (or cases) are described in a published report, the result is called a case–series study; it is the simplest design in which the author describes some interesting or intriguing observations that occurred for a small number of patients. Case–series studies frequently lead to the generation of hypotheses that are subsequently investigated in a case–control, cross-sectional, or cohort study. These three types of studies are defined by the period of time the study covers and by the direction or focus of the research question. Cohort and case–control studies generally involve an extended period of time defined by the point when the study begins and the point when it ends; some process occurs, and a certain amount of time is required to assess it. For this reason, both cohort and case–control studies are sometimes also called longitudinal studies. The major difference between them is the direction of the inquiry or the focus of the research question: Cohort studies are forward-looking, from a risk factor to an outcome, whereas case–control studies are backward-looking, from an outcome to risk factors. The cross-sectional study analyzes data collected on a group of subjects at one time. Kleinbaum and colleagues (1997) describe a number of hybrids or combinations of these designs if you are interested in more detail than we give in this chapter. If you would like a more detailed discussion of study designs used in medicine, see the companion text on epidemiology by Greenberg and coworkers (2000) . A book by Hulley and Cummings (2001) is devoted entirely to the design of clinical research. Garb (1996) and Burns and Grove (2002) discuss study design in medicine and nursing, respectively. Case–Series Studies A case–series report is a simple descriptive account of interesting characteristics observed in a group of patients. For example, Alexandrov and coworkers (1997) presented information on a series of 40 patients who had been referred for evaluation of stroke, transient ischemic attack, or carotid bruit. The authors wanted to compare two methods to see which better predicted peak systolic velocity. They concluded that the relationship between both methods and peak systolic velocity was very strong. Case–series reports generally involve patients seen over a relatively short time. Generally case–series studies do not include control subjects, persons who do not have the disease or condition being described. Some investigators would not include case–series in a list of types of studies because they are generally not planned studies and do not involve any research hypotheses. On occasion, however, investigators do include control subjects. We mention case–series studies because of their important descriptive role as a precursor to other studies. Case–Control Studies Case–control studies begin with the absence or presence of an outcome and then look backward in time to try to detect possible causes or risk factors that may have been suggested in a case–series report. The cases in case–control studies are individuals selected on the basis of some disease or outcome; the controls are individuals without the disease or outcome. The history or previous events of both cases and controls are analyzed in an attempt to identify a characteristic or risk factor present in the cases’ histories but not in the controls’ histories. Figure 2–1 illustrates that subjects in the study are chosen at the onset of the study after they are known to be either cases with the disease or outcome (squares) or controls without the disease or outcome (diamonds). The histories of cases and controls are examined over a previous period to detect the presence (shaded areas) or absence (unshaded areas) of predisposing characteristics or risk factors, or, if the disease is infectious, whether the subject has been exposed to the presumed infectious agent. In case–control designs, the nature of the inquiry is backward in time, as indicated by the arrows pointing backward in Figure 2–1 to illustrate the backward, or retrospective, nature of the research process. We can characterize case–control studies as studies that ask “What happened?” In fact, they are sometimes called retrospective studies because of the direction of inquiry. Case–control studies are longitudinal as well, because the inquiry covers a period of time. Figure 2–1. Schematic diagram of case–control study design. Shaded areas represent subjects exposed to the antecedent factor; unshaded areas correspond to unexposed subjects. Squares represent subjects with the outcome of interest; diamonds represent subjects without the outcome of interest. (Adapted and reproduced, with permission, from Greenberg RS: Retrospective studies. In Kotz S, Johnson NL [editors]: Encyclopedia of Statistical Sciences, Vol 8. Wiley, 1988.) Olsen and colleagues (2003) compared patients who had a surgical site infection following laminectomy or spinal fusion (cases) with patients who developed no infection (controls). The investigators found that length of hospital stay and readmission rates were greater with patients with infections. Furthermore, postoperative incontinence was one of the risk factors associated with the development of infection. Investigators sometimes use matching to associate controls with cases on characteristics such as age and sex. If an investigator feels that such characteristics are so important that an imbalance between the two groups of patients would affect any conclusions, he or she should employ matching. This process ensures that both groups will be similar with respect to important characteristics that may otherwise cloud or confound the conclusions. Deciding whether a published study is a case–control study or a case–series report is not always easy. Confusion arises because both types of studies are generally conceived and written after the fact rather than having been planned. The easiest way to differentiate between them is to ask whether the author’s purpose was to describe a phenomenon or to attempt to explain it by evaluating previous events. If the purpose is simple description, chances are the study is a case–series report. Cross-Sectional Studies The third type of observational study goes by all of the following names: cross-sectional studies, surveys, epidemiologic studies, and prevalence studies. We use the term “cross-sectional” because it is descriptive of the time line and does not have the connotation that the terms “surveys” and “prevalence” do. Cross-sectional studies analyze data collected on a group of subjects at one time rather than over a period of time. Cross-sectional studies are designed to determine “What is happening?” right now. Subjects are selected and information is obtained in a short period of time ( Figure 2–2 ; note the short time line). Because they focus on a point in time, they are sometimes also called prevalence studies. Surveys and polls are generally cross-sectional studies, although surveys can be part of a cohort or case–control study. Cross-sectional studies may be designed to address research questions raised by a case–series, or they may be done without a previous descriptive study. Figure 2–2. Schematic diagram of cross-sectional study design. Squares represent subjects with the outcome of interest; diamonds represent subjects without the outcome of interest. Diagnosing or Staging a Disease In a presenting problem in Chapter 10, Soderstrom and his coinvestigators (1997) were interested in learning more about the relationship between demographic measures that might be helpful in identifying trauma patients who have an elevated blood alcohol concentration. They wanted to develop a simple scoring system that could be used to detect these patients when they come to an emergency department. These patients could be targeted for assessment of alcohol abuse and dependence and other possible substance abuse. They chose to look at the time of day (day or night), the day of the week (weekday or weekend), race (white or nonwhite), and age (40 years or older versus younger than 40). Using these four simple measures, the investigators were able to construct four models: for men whose injury was intentional, men whose injury was not intentional, women whose injury was intentional, and women whose injury was not intentional. Evaluating Different Methods of Doing the Same Thing A presenting problem in Chapter 5 is a cross-sectional study designed to examine the relationship between histology slides and magnetic resonance imaging (MRI) to study characteristics of diseased carotid arteries ( Yuan et al, 2001 ). The histology slides were evaluated by a pathologist who was blinded to the imaging results. It is important to establish the level of agreement between the MRI findings and histology, and the level of agreement was found to be relatively high. Cross-sectional studies are used in all fields of medicine, but they are especially common in examinations of the usefulness of a new diagnostic procedure. Establishing Norms Knowledge of the range within which most patients fit is very useful to clinicians. Laboratories, of course, establish and then provide the normal limits of most diagnostic tests when they report the results for a given patient. Often these limits are established by testing people who are known to have normal values. We would not, for example, want to use people with diabetes mellitus to establish the norms for serum glucose levels. The results from the people known to have normal values are used to define the range that separates the lowest 2½% of the values and the highest 2½% of the values from the middle 95%. These values are called normal values, or norms. Outside of the laboratory there are many qualities for which normal ranges have not been established. This was true for two measures of the autoimmune nervous system function. These two measures, heart variation to deep breathing and the Valsalva ratio, are noninvasive tests that can help clinicians evaluate patients with diabetes mellitus and other neuropathic disorders. Gelber and colleagues (1997) analyzed data from subjects recruited from 63 centers throughout North America to develop normative values for these two measurements. After comparing certain demographic groups, such as males versus females, the investigators established the normative values for heart rate variation to deep breathing and the Valsalva. Surveys Surveys are especially useful when the goal is to gain insight into a perplexing topic or to learn how people think and feel about an issue. Surveys are generally cross-sectional in design, but they can be used in case–control and cohort studies as well. Caiola and Litaker (2000) wanted to know the factors that influence fellows to select a specific general internal residency fellowship program. Because they did not know the names and addresses of the fellows, the authors sent a questionnaire to the program directors and asked them to distribute the questionnaires to the fellows. We examine this study in more detail in Chapter 11 and illustrate how the authors asked the questions on the survey. Many times investigators use preexisting surveys rather than creating their own, especially if good questionnaires already exist. Patenaude and colleagues (2003) asked medical students at a Canadian medical school to complete a questionnaire on moral reasoning (the Kohlberg Moral Judgment Interview). They wanted to learn how moral reasoning progressed over time, so they gave the questionnaire at the beginning of medical school and again at the end of the third year. They learned that the stage of moral development did not change in about 70% of the students, whereas it either decreased or increased in 15%. The authors had expected the level of moral reasoning to increase, and the results of the study prompted them to raise questions about the possible features of medical education that might inhibit its development. Interviews are sometimes used in surveys, especially when it is important to probe reasons or explanations more deeply than is possible with a written questionnaire. Kendler and colleagues (2003) wanted to investigate the role of genetic and environmental risk factors for substance abuse. They studied six classes of illicit substances to learn whether substance use disorders are substance-specific. After interviewing almost 1200 sets of adult male twins, they concluded that environmental experiences unique to a given individual are primarily responsible for whether the person misuses one class of psychoactive substances over another. Increasingly, surveys are performed using existing databases of information. As an illustration, Huang and Stafford (2002) used survey data from the National Ambulatory Medical Care Survey to examine the relationship between demographics and clinical characteristics of women who visit primary care physicians and specialists for urinary tract infection. Using preexisting databases can have a number of advantages, such as saving time and effort, but many national surveys use complicated designs; and it is important to know what these are, as we discuss when we explore this study in more detail in Chapter 11. Many countries and states collect data on a variety of conditions to develop tumor registries and databases of cases of infectious disease. Diermayer and colleagues (1999) , a presenting problem in Chapter 4, analyzed epidemiologic surveillance data from the State of Oregon and reported an increase in the overall incidence rate of meningococcal disease from 2 cases/100,000 population during 1987–1992 to 4.5 cases/100,000 in 1994. Epidemiologists from Oregon and the Centers for Disease Control in Atlanta, Georgia, wanted to know if the increased number of cases of meningococcal disease indicated a transition from endemic to epidemic disease. They also sought these other features of an epidemic: the predominance of a single bacterial strain rather than a heterogeneous mix of strains and a shift in age distri bution of cases toward older age groups. Cohort Studies A cohort is a group of people who have something in common and who remain part of a group over an extended time. In medicine, the subjects in cohort studies are selected by some defining characteristic (or characteristics) suspected of being a precursor to or risk factor for a disease or health effect. Cohort studies ask the question “What will happen?” and thus, the direction in cohort studies is forward in time. Figure 2–3 illustrates the study design. Researchers select subjects at the onset of the study and then determine whether they have the risk factor or have been exposed. All subjects are followed over a certain period to observe the effect of the risk factor or exposure. Because the events of interest transpire after the study is begun, these studies are sometimes called prospective studies. Figure 2–3.
Share this:
- Click to share on Twitter (Opens in new window)
- Click to share on Facebook (Opens in new window)
Related posts:
- Chapter 1. Introduction to Medical Research
- Chapter 5. Research Questions About One Group
- Chapter 3. Summarizing Data & Presenting Data in Tables & Graphs
- Chapter 7. Research Questions About Means in Three or More Groups
Stay updated, free articles. Join our Telegram channel
Comments are closed for this page.

Full access? Get Clinical Tree


Literature Reviews: Types of Clinical Study Designs
- Library Basics
- 1. Choose Your Topic
- How to Find Books
- Types of Clinical Study Designs
- Types of Literature
- 3. Search the Literature
- 4. Read & Analyze the Literature
- 5. Write the Review
- Keeping Track of Information
- Style Guides
- Books, Tutorials & Examples
Types of Study Designs
Meta-Analysis A way of combining data from many different research studies. A meta-analysis is a statistical process that combines the findings from individual studies. Example : Anxiety outcomes after physical activity interventions: meta-analysis findings . Conn V. Nurs Res . 2010 May-Jun;59(3):224-31.
Systematic Review A summary of the clinical literature. A systematic review is a critical assessment and evaluation of all research studies that address a particular clinical issue. The researchers use an organized method of locating, assembling, and evaluating a body of literature on a particular topic using a set of specific criteria. A systematic review typically includes a description of the findings of the collection of research studies. The systematic review may also include a quantitative pooling of data, called a meta-analysis. Example : Complementary and alternative medicine use among women with breast cancer: a systematic review. Wanchai A, Armer JM, Stewart BR. Clin J Oncol Nurs . 2010 Aug;14(4):E45-55.
Randomized Controlled Trial A controlled clinical trial that randomly (by chance) assigns participants to two or more groups. There are various methods to randomize study participants to their groups. Example : Meditation or exercise for preventing acute respiratory infection: a randomized controlled trial . Barrett B, et al. Ann Fam Med . 2012 Jul-Aug;10(4):337-46.
Cohort Study (Prospective Observational Study) A clinical research study in which people who presently have a certain condition or receive a particular treatment are followed over time and compared with another group of people who are not affected by the condition. Example : Smokeless tobacco cessation in South Asian communities: a multi-centre prospective cohort study . Croucher R, et al. Addiction. 2012 Dec;107 Suppl 2:45-52.
Case-control Study Case-control studies begin with the outcomes and do not follow people over time. Researchers choose people with a particular result (the cases) and interview the groups or check their records to ascertain what different experiences they had. They compare the odds of having an experience with the outcome to the odds of having an experience without the outcome. Example : Non-use of bicycle helmets and risk of fatal head injury: a proportional mortality, case-control study . Persaud N, et al. CMAJ . 2012 Nov 20;184(17):E921-3.
Cross-sectional study The observation of a defined population at a single point in time or time interval. Exposure and outcome are determined simultaneously. Example : Fasting might not be necessary before lipid screening: a nationally representative cross-sectional study . Steiner MJ, et al. Pediatrics . 2011 Sep;128(3):463-70.
Case Reports and Series A report on a series of patients with an outcome of interest. No control group is involved. Example : Students mentoring students in a service-learning clinical supervision experience: an educational case report . Lattanzi JB, et al. Phys Ther . 2011 Oct;91(10):1513-24.
Ideas, Editorials, Opinions Put forth by experts in the field. Example : Health and health care for the 21st century: for all the people . Koop CE. Am J Public Health . 2006 Dec;96(12):2090-2.
Animal Research Studies Studies conducted using animal subjects. Example : Intranasal leptin reduces appetite and induces weight loss in rats with diet-induced obesity (DIO) . Schulz C, Paulus K, Jöhren O, Lehnert H. Endocrinology . 2012 Jan;153(1):143-53.
Test-tube Lab Research "Test tube" experiments conducted in a controlled laboratory setting.
Adapted from Study Designs. In NICHSR Introduction to Health Services Research: a Self-Study Course. http://www.nlm.nih.gov/nichsr/ihcm/06studies/studies03.html and Glossary of EBM Terms. http://www.cebm.utoronto.ca/glossary/index.htm#top
Study Design Terminology
Bias - Any deviation of results or inferences from the truth, or processes leading to such deviation. Bias can result from several sources: one-sided or systematic variations in measurement from the true value (systematic error); flaws in study design; deviation of inferences, interpretations, or analyses based on flawed data or data collection; etc. There is no sense of prejudice or subjectivity implied in the assessment of bias under these conditions.
Case Control Studies - Studies which start with the identification of persons with a disease of interest and a control (comparison, referent) group without the disease. The relationship of an attribute to the disease is examined by comparing diseased and non-diseased persons with regard to the frequency or levels of the attribute in each group.
Causality - The relating of causes to the effects they produce. Causes are termed necessary when they must always precede an effect and sufficient when they initiate or produce an effect. Any of several factors may be associated with the potential disease causation or outcome, including predisposing factors, enabling factors, precipitating factors, reinforcing factors, and risk factors.
Control Groups - Groups that serve as a standard for comparison in experimental studies. They are similar in relevant characteristics to the experimental group but do not receive the experimental intervention.
Controlled Clinical Trials - Clinical trials involving one or more test treatments, at least one control treatment, specified outcome measures for evaluating the studied intervention, and a bias-free method for assigning patients to the test treatment. The treatment may be drugs, devices, or procedures studied for diagnostic, therapeutic, or prophylactic effectiveness. Control measures include placebos, active medicines, no-treatment, dosage forms and regimens, historical comparisons, etc. When randomization using mathematical techniques, such as the use of a random numbers table, is employed to assign patients to test or control treatments, the trials are characterized as Randomized Controlled Trials.
Cost-Benefit Analysis - A method of comparing the cost of a program with its expected benefits in dollars (or other currency). The benefit-to-cost ratio is a measure of total return expected per unit of money spent. This analysis generally excludes consideration of factors that are not measured ultimately in economic terms. Cost effectiveness compares alternative ways to achieve a specific set of results.
Cross-Over Studies - Studies comparing two or more treatments or interventions in which the subjects or patients, upon completion of the course of one treatment, are switched to another. In the case of two treatments, A and B, half the subjects are randomly allocated to receive these in the order A, B and half to receive them in the order B, A. A criticism of this design is that effects of the first treatment may carry over into the period when the second is given.
Cross-Sectional Studies - Studies in which the presence or absence of disease or other health-related variables are determined in each member of the study population or in a representative sample at one particular time. This contrasts with LONGITUDINAL STUDIES which are followed over a period of time.
Double-Blind Method - A method of studying a drug or procedure in which both the subjects and investigators are kept unaware of who is actually getting which specific treatment.
Empirical Research - The study, based on direct observation, use of statistical records, interviews, or experimental methods, of actual practices or the actual impact of practices or policies.
Evaluation Studies - Works consisting of studies determining the effectiveness or utility of processes, personnel, and equipment.
Genome-Wide Association Study - An analysis comparing the allele frequencies of all available (or a whole genome representative set of) polymorphic markers in unrelated patients with a specific symptom or disease condition, and those of healthy controls to identify markers associated with a specific disease or condition.
Intention to Treat Analysis - Strategy for the analysis of Randomized Controlled Trial that compares patients in the groups to which they were originally randomly assigned.
Logistic Models - Statistical models which describe the relationship between a qualitative dependent variable (that is, one which can take only certain discrete values, such as the presence or absence of a disease) and an independent variable. A common application is in epidemiology for estimating an individual's risk (probability of a disease) as a function of a given risk factor.
Longitudinal Studies - Studies in which variables relating to an individual or group of individuals are assessed over a period of time.
Lost to Follow-Up - Study subjects in cohort studies whose outcomes are unknown e.g., because they could not or did not wish to attend follow-up visits.
Matched-Pair Analysis - A type of analysis in which subjects in a study group and a comparison group are made comparable with respect to extraneous factors by individually pairing study subjects with the comparison group subjects (e.g., age-matched controls).
Meta-Analysis - Works consisting of studies using a quantitative method of combining the results of independent studies (usually drawn from the published literature) and synthesizing summaries and conclusions which may be used to evaluate therapeutic effectiveness, plan new studies, etc. It is often an overview of clinical trials. It is usually called a meta-analysis by the author or sponsoring body and should be differentiated from reviews of literature.
Numbers Needed To Treat - Number of patients who need to be treated in order to prevent one additional bad outcome. It is the inverse of Absolute Risk Reduction.
Odds Ratio - The ratio of two odds. The exposure-odds ratio for case control data is the ratio of the odds in favor of exposure among cases to the odds in favor of exposure among noncases. The disease-odds ratio for a cohort or cross section is the ratio of the odds in favor of disease among the exposed to the odds in favor of disease among the unexposed. The prevalence-odds ratio refers to an odds ratio derived cross-sectionally from studies of prevalent cases.
Patient Selection - Criteria and standards used for the determination of the appropriateness of the inclusion of patients with specific conditions in proposed treatment plans and the criteria used for the inclusion of subjects in various clinical trials and other research protocols.
Predictive Value of Tests - In screening and diagnostic tests, the probability that a person with a positive test is a true positive (i.e., has the disease), is referred to as the predictive value of a positive test; whereas, the predictive value of a negative test is the probability that the person with a negative test does not have the disease. Predictive value is related to the sensitivity and specificity of the test.
Prospective Studies - Observation of a population for a sufficient number of persons over a sufficient number of years to generate incidence or mortality rates subsequent to the selection of the study group.
Qualitative Studies - Research that derives data from observation, interviews, or verbal interactions and focuses on the meanings and interpretations of the participants.
Quantitative Studies - Quantitative research is research that uses numerical analysis.
Random Allocation - A process involving chance used in therapeutic trials or other research endeavor for allocating experimental subjects, human or animal, between treatment and control groups, or among treatment groups. It may also apply to experiments on inanimate objects.
Randomized Controlled Trial - Clinical trials that involve at least one test treatment and one control treatment, concurrent enrollment and follow-up of the test- and control-treated groups, and in which the treatments to be administered are selected by a random process, such as the use of a random-numbers table.
Reproducibility of Results - The statistical reproducibility of measurements (often in a clinical context), including the testing of instrumentation or techniques to obtain reproducible results. The concept includes reproducibility of physiological measurements, which may be used to develop rules to assess probability or prognosis, or response to a stimulus; reproducibility of occurrence of a condition; and reproducibility of experimental results.
Retrospective Studies - Studies used to test etiologic hypotheses in which inferences about an exposure to putative causal factors are derived from data relating to characteristics of persons under study or to events or experiences in their past. The essential feature is that some of the persons under study have the disease or outcome of interest and their characteristics are compared with those of unaffected persons.
Sample Size - The number of units (persons, animals, patients, specified circumstances, etc.) in a population to be studied. The sample size should be big enough to have a high likelihood of detecting a true difference between two groups.
Sensitivity and Specificity - Binary classification measures to assess test results. Sensitivity or recall rate is the proportion of true positives. Specificity is the probability of correctly determining the absence of a condition.
Single-Blind Method - A method in which either the observer(s) or the subject(s) is kept ignorant of the group to which the subjects are assigned.
Time Factors - Elements of limited time intervals, contributing to particular results or situations.
Source: NLM MeSH Database
- << Previous: How to Find Books
- Next: Types of Literature >>
- Last Updated: Dec 29, 2023 11:41 AM
- URL: https://research.library.gsu.edu/litrev
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Dtsch Arztebl Int
- v.106(15); 2009 Apr
Types of Study in Medical Research
Bernd röhrig.
1 MDK Rheinland-Pfalz, Referat Rehabilitation/Biometrie, Alzey
Jean-Baptist du Prel
2 Zentrum für Präventive Pädiatrie, Zentrum für Kinder- und Jugendmedizin, Mainz
Daniel Wachtlin
3 Interdisziplinäres Zentrum Klinische Studien (IZKS), Fachbereich Medizin der Universität Mainz
Maria Blettner
4 Institut für Medizinische Biometrie, Epidemiologie und Informatik (IMBEI), Johannes Gutenberg Universität Mainz
The choice of study type is an important aspect of the design of medical studies. The study design and consequent study type are major determinants of a study’s scientific quality and clinical value.
This article describes the structured classification of studies into two types, primary and secondary, as well as a further subclassification of studies of primary type. This is done on the basis of a selective literature search concerning study types in medical research, in addition to the authors’ own experience.
Three main areas of medical research can be distinguished by study type: basic (experimental), clinical, and epidemiological research. Furthermore, clinical and epidemiological studies can be further subclassified as either interventional or noninterventional.
Conclusions
The study type that can best answer the particular research question at hand must be determined not only on a purely scientific basis, but also in view of the available financial resources, staffing, and practical feasibility (organization, medical prerequisites, number of patients, etc.).
The quality, reliability and possibility of publishing a study are decisively influenced by the selection of a proper study design. The study type is a component of the study design (see the article "Study Design in Medical Research") and must be specified before the study starts. The study type is determined by the question to be answered and decides how useful a scientific study is and how well it can be interpreted. If the wrong study type has been selected, this cannot be rectified once the study has started.
After an earlier publication dealing with aspects of study design, the present article deals with study types in primary and secondary research. The article focuses on study types in primary research. A special article will be devoted to study types in secondary research, such as meta-analyses and reviews. This article covers the classification of individual study types. The conception, implementation, advantages, disadvantages and possibilities of using the different study types are illustrated by examples. The article is based on a selective literature research on study types in medical research, as well as the authors’ own experience.
Classification of study types
In principle, medical research is classified into primary and secondary research. While secondary research summarizes available studies in the form of reviews and meta-analyses, the actual studies are performed in primary research. Three main areas are distinguished: basic medical research, clinical research, and epidemiological research. In individual cases, it may be difficult to classify individual studies to one of these three main categories or to the subcategories. In the interests of clarity and to avoid excessive length, the authors will dispense with discussing special areas of research, such as health services research, quality assurance, or clinical epidemiology. Figure 1 gives an overview of the different study types in medical research.

Classification of different study types
*1 , sometimes known as experimental research; *2 , analogous term: interventional; *3 , analogous term: noninterventional or nonexperimental
This scheme is intended to classify the study types as clearly as possible. In the interests of clarity, we have excluded clinical epidemiology — a subject which borders on both clinical and epidemiological research ( 3 ). The study types in this area can be found under clinical research and epidemiology.
Basic research
Basic medical research (otherwise known as experimental research) includes animal experiments, cell studies, biochemical, genetic and physiological investigations, and studies on the properties of drugs and materials. In almost all experiments, at least one independent variable is varied and the effects on the dependent variable are investigated. The procedure and the experimental design can be precisely specified and implemented ( 1 ). For example, the population, number of groups, case numbers, treatments and dosages can be exactly specified. It is also important that confounding factors should be specifically controlled or reduced. In experiments, specific hypotheses are investigated and causal statements are made. High internal validity (= unambiguity) is achieved by setting up standardized experimental conditions, with low variability in the units of observation (for example, cells, animals or materials). External validity is a more difficult issue. Laboratory conditions cannot always be directly transferred to normal clinical practice and processes in isolated cells or in animals are not equivalent to those in man (= generalizability) ( 2 ).
Basic research also includes the development and improvement of analytical procedures—such as analytical determination of enzymes, markers or genes—, imaging procedures—such as computed tomography or magnetic resonance imaging—, and gene sequencing—such as the link between eye color and specific gene sequences. The development of biometric procedures—such as statistical test procedures, modeling and statistical evaluation strategies—also belongs here.
Clinical studies
Clinical studies include both interventional (or experimental) studies and noninterventional (or observational) studies. A clinical drug study is an interventional clinical study, defined according to §4 Paragraph 23 of the Medicines Act [Arzneimittelgesetz; AMG] as "any study performed on man with the purpose of studying or demonstrating the clinical or pharmacological effects of drugs, to establish side effects, or to investigate absorption, distribution, metabolism or elimination, with the aim of providing clear evidence of the efficacy or safety of the drug."
Interventional studies also include studies on medical devices and studies in which surgical, physical or psychotherapeutic procedures are examined. In contrast to clinical studies, §4 Paragraph 23 of the AMG describes noninterventional studies as follows: "A noninterventional study is a study in the context of which knowledge from the treatment of persons with drugs in accordance with the instructions for use specified in their registration is analyzed using epidemiological methods. The diagnosis, treatment and monitoring are not performed according to a previously specified study protocol, but exclusively according to medical practice."
The aim of an interventional clinical study is to compare treatment procedures within a patient population, which should exhibit as few as possible internal differences, apart from the treatment ( 4 , e1 ). This is to be achieved by appropriate measures, particularly by random allocation of the patients to the groups, thus avoiding bias in the result. Possible therapies include a drug, an operation, the therapeutic use of a medical device such as a stent, or physiotherapy, acupuncture, psychosocial intervention, rehabilitation measures, training or diet. Vaccine studies also count as interventional studies in Germany and are performed as clinical studies according to the AMG.
Interventional clinical studies are subject to a variety of legal and ethical requirements, including the Medicines Act and the Law on Medical Devices. Studies with medical devices must be registered by the responsible authorities, who must also approve studies with drugs. Drug studies also require a favorable ruling from the responsible ethics committee. A study must be performed in accordance with the binding rules of Good Clinical Practice (GCP) ( 5 , e2 – e4 ). For clinical studies on persons capable of giving consent, it is absolutely essential that the patient should sign a declaration of consent (informed consent) ( e2 ). A control group is included in most clinical studies. This group receives another treatment regimen and/or placebo—a therapy without substantial efficacy. The selection of the control group must not only be ethically defensible, but also be suitable for answering the most important questions in the study ( e5 ).
Clinical studies should ideally include randomization, in which the patients are allocated by chance to the therapy arms. This procedure is performed with random numbers or computer algorithms ( 6 – 8 ). Randomization ensures that the patients will be allocated to the different groups in a balanced manner and that possible confounding factors—such as risk factors, comorbidities and genetic variabilities—will be distributed by chance between the groups (structural equivalence) ( 9 , 10 ). Randomization is intended to maximize homogeneity between the groups and prevent, for example, a specific therapy being reserved for patients with a particularly favorable prognosis (such as young patients in good physical condition) ( 11 ).
Blinding is another suitable method to avoid bias. A distinction is made between single and double blinding. With single blinding, the patient is unaware which treatment he is receiving, while, with double blinding, neither the patient nor the investigator knows which treatment is planned. Blinding the patient and investigator excludes possible subjective (even subconscious) influences on the evaluation of a specific therapy (e.g. drug administration versus placebo). Thus, double blinding ensures that the patient or therapy groups are both handled and observed in the same manner. The highest possible degree of blinding should always be selected. The study statistician should also remain blinded until the details of the evaluation have finally been specified.
A well designed clinical study must also include case number planning. This ensures that the assumed therapeutic effect can be recognized as such, with a previously specified statistical probability (statistical power) ( 4 , 6 , 12 ).
It is important for the performance of a clinical trial that it should be carefully planned and that the exact clinical details and methods should be specified in the study protocol ( 13 ). It is, however, also important that the implementation of the study according to the protocol, as well as data collection, must be monitored. For a first class study, data quality must be ensured by double data entry, programming plausibility tests, and evaluation by a biometrician. International recommendations for the reporting of randomized clinical studies can be found in the CONSORT statement (Consolidated Standards of Reporting Trials, www.consort-statement.org ) ( 14 ). Many journals make this an essential condition for publication.
For all the methodological reasons mentioned above and for ethical reasons, the randomized controlled and blinded clinical trial with case number planning is accepted as the gold standard for testing the efficacy and safety of therapies or drugs ( 4 , e1 , 15 ).
In contrast, noninterventional clinical studies (NIS) are patient-related observational studies, in which patients are given an individually specified therapy. The responsible physician specifies the therapy on the basis of the medical diagnosis and the patient’s wishes. NIS include noninterventional therapeutic studies, prognostic studies, observational drug studies, secondary data analyses, case series and single case analyses ( 13 , 16 ). Similarly to clinical studies, noninterventional therapy studies include comparison between therapies; however, the treatment is exclusively according to the physician’s discretion. The evaluation is often retrospective. Prognostic studies examine the influence of prognostic factors (such as tumor stage, functional state, or body mass index) on the further course of a disease. Diagnostic studies are another class of observational studies, in which either the quality of a diagnostic method is compared to an established method (ideally a gold standard), or an investigator is compared with one or several other investigators (inter-rater comparison) or with himself at different time points (intra-rater comparison) ( e1 ). If an event is very rare (such as a rare disease or an individual course of treatment), a single-case study, or a case series, are possibilities. A case series is a study on a larger patient group with a specific disease. For example, after the discovery of the AIDS virus, the Center for Disease Control (CDC) in the USA collected a case series of 1000 patients, in order to study frequent complications of this infection. The lack of a control group is a disadvantage of case series. For this reason, case series are primarily used for descriptive purposes ( 3 ).
Epidemiological studies
The main point of interest in epidemiological studies is to investigate the distribution and historical changes in the frequency of diseases and the causes for these. Analogously to clinical studies, a distinction is made between experimental and observational epidemiological studies ( 16 , 17 ).
Interventional studies are experimental in character and are further subdivided into field studies (sample from an area, such as a large region or a country) and group studies (sample from a specific group, such as a specific social or ethnic group). One example was the investigation of the iodine supplementation of cooking salt to prevent cretinism in a region with iodine deficiency. On the other hand, many interventions are unsuitable for randomized intervention studies, for ethical, social or political reasons, as the exposure may be harmful to the subjects ( 17 ).
Observational epidemiological studies can be further subdivided into cohort studies (follow-up studies), case control studies, cross-sectional studies (prevalence studies), and ecological studies (correlation studies or studies with aggregated data).
In contrast, studies with only descriptive evaluation are restricted to a simple depiction of the frequency (incidence and prevalence) and distribution of a disease within a population. The objective of the description may also be the regular recording of information (monitoring, surveillance). Registry data are also suited for the description of prevalence and incidence; for example, they are used for national health reports in Germany.
In the simplest case, cohort studies involve the observation of two healthy groups of subjects over time. One group is exposed to a specific substance (for example, workers in a chemical factory) and the other is not exposed. It is recorded prospectively (into the future) how often a specific disease (such as lung cancer) occurs in the two groups ( figure 2a ). The incidence for the occurrence of the disease can be determined for both groups. Moreover, the relative risk (quotient of the incidence rates) is a very important statistical parameter which can be calculated in cohort studies. For rare types of exposure, the general population can be used as controls ( e6 ). All evaluations naturally consider the age and gender distributions in the corresponding cohorts. The objective of cohort studies is to record detailed information on the exposure and on confounding factors, such as the duration of employment, the maximum and the cumulated exposure. One well known cohort study is the British Doctors Study, which prospectively examined the effect of smoking on mortality among British doctors over a period of decades ( e7 ). Cohort studies are well suited for detecting causal connections between exposure and the development of disease. On the other hand, cohort studies often demand a great deal of time, organization, and money. So-called historical cohort studies represent a special case. In this case, all data on exposure and effect (illness) are already available at the start of the study and are analyzed retrospectively. For example, studies of this sort are used to investigate occupational forms of cancer. They are usually cheaper ( 16 ).

Graphical depiction of a prospective cohort study (simplest case [2a]) and a retrospective case control study (2b)
In case control studies, cases are compared with controls. Cases are persons who fall ill from the disease in question. Controls are persons who are not ill, but are otherwise comparable to the cases. A retrospective analysis is performed to establish to what extent persons in the case and control groups were exposed ( figure 2b ). Possible exposure factors include smoking, nutrition and pollutant load. Care should be taken that the intensity and duration of the exposure is analyzed as carefully and in as detailed a manner as possible. If it is observed that ill people are more often exposed than healthy people, it may be concluded that there is a link between the illness and the risk factor. In case control studies, the most important statistical parameter is the odds ratio. Case control studies usually require less time and fewer resources than cohort studies ( 16 ). The disadvantage of case control studies is that the incidence rate (rate of new cases) cannot be calculated. There is also a great risk of bias from the selection of the study population ("selection bias") and from faulty recall ("recall bias") (see too the article "Avoiding Bias in Observational Studies"). Table 1 presents an overview of possible types of epidemiological study ( e8 ). Table 2 summarizes the advantages and disadvantages of observational studies ( 16 ).
1 = slight; 2 = moderate; 3 = high; N/A, not applicable.
*Individual cases may deviate from this pattern.
Selecting the correct study type is an important aspect of study design (see "Study Design in Medical Research" in volume 11/2009). However, the scientific questions can only be correctly answered if the study is planned and performed at a qualitatively high level ( e9 ). It is very important to consider or even eliminate possible interfering factors (or confounders), as otherwise the result cannot be adequately interpreted. Confounders are characteristics which influence the target parameters. Although this influence is not of primary interest, it can interfere with the connection between the target parameter and the factors that are of interest. The influence of confounders can be minimized or eliminated by standardizing the procedure, stratification ( 18 ), or adjustment ( 19 ).
The decision as to which study type is suitable to answer a specific primary research question must be based not only on scientific considerations, but also on issues related to resources (personnel and finances), hospital capacity, and practicability. Many epidemiological studies can only be implemented if there is access to registry data. The demands for planning, implementation, and statistical evaluation for observational studies should be just as high for observational studies as for experimental studies. There are particularly strict requirements, with legally based regulations (such as the Medicines Act and Good Clinical Practice), for the planning, implementation, and evaluation of clinical studies. A study protocol must be prepared for both interventional and noninterventional studies ( 6 , 13 ). The study protocol must contain information on the conditions, question to be answered (objective), the methods of measurement, the implementation, organization, study population, data management, case number planning, the biometric evaluation, and the clinical relevance of the question to be answered ( 13 ).
Important and justified ethical considerations may restrict studies with optimal scientific and statistical features. A randomized intervention study under strictly controlled conditions of the effect of exposure to harmful factors (such as smoking, radiation, or a fatty diet) is not possible and not permissible for ethical reasons. Observational studies are a possible alternative to interventional studies, even though observational studies are less reliable and less easy to control ( 17 ).
A medical study should always be published in a peer reviewed journal. Depending on the study type, there are recommendations and checklists for presenting the results. For example, these may include a description of the population, the procedure for missing values and confounders, and information on statistical parameters. Recommendations and guidelines are available for clinical studies ( 14 , 20 , e10 , e11 ), for diagnostic studies ( 21 , 22 , e12 ), and for epidemiological studies ( 23 , e13 ). Since 2004, the WHO has demanded that studies should be registered in a public registry, such as www.controlled-trials.com or www.clinicaltrials.gov . This demand is supported by the International Committee of Medical Journal Editors (ICMJE) ( 24 ), which specifies that the registration of the study before inclusion of the first subject is an essential condition for the publication of the study results ( e14 ).
When specifying the study type and study design for medical studies, it is essential to collaborate with an experienced biometrician. The quality and reliability of the study can be decisively improved if all important details are planned together ( 12 , 25 ).
Acknowledgments
Translated from the original German by Rodney A. Yeates, M.A., Ph.D.
Conflict of interest statement
The authors declare that there is no conflict of interest in the sense of the International Committee of Medical Journal Editors.
- Open access
- Published: 22 April 2024
Training nurses in an international emergency medical team using a serious role-playing game: a retrospective comparative analysis
- Hai Hu 1 , 2 , 3 na1 ,
- Xiaoqin Lai 2 , 4 , 5 na1 &
- Longping Yan 6 , 7 , 8
BMC Medical Education volume 24 , Article number: 432 ( 2024 ) Cite this article
151 Accesses
Metrics details
Although game-based applications have been used in disaster medicine education, no serious computer games have been designed specifically for training these nurses in an IEMT setting. To address this need, we developed a serious computer game called the IEMTtraining game. In this game, players assume the roles of IEMT nurses, assess patient injuries in a virtual environment, and provide suitable treatment options.
The design of this study is a retrospective comparative analysis. The research was conducted with 209 nurses in a hospital. The data collection process of this study was conducted at the 2019-2020 academic year. A retrospective comparative analysis was conducted on the pre-, post-, and final test scores of nurses in the IEMT. Additionally, a survey questionnaire was distributed to trainees to gather insights into teaching methods that were subsequently analyzed.
There was a significant difference in the overall test scores between the two groups, with the game group demonstrating superior performance compared to the control group (odds ratio = 1.363, p value = 0.010). The survey results indicated that the game group exhibited higher learning motivation scores and lower cognitive load compared with the lecture group.
Conclusions
The IEMT training game developed by the instructor team is a promising and effective method for training nurses in disaster rescue within IEMTs. The game equips the trainees with the necessary skills and knowledge to respond effectively to emergencies. It is easily comprehended, enhances knowledge retention and motivation to learn, and reduces cognitive load.
Peer Review reports
Since the beginning of the twenty-first century, the deployment of international emergency medical teams in disaster-stricken regions has increased world wide [ 1 ]. To enhance the efficiency of these teams, the World Health Organization (WHO) has introduced the International Emergency Medical Team (IEMT) initiative to guarantee their competence. Adequate education and training play a vital role in achieving this objective [ 2 ].
Nurses play a vital role as IEMTs by providing essential medical care and support to populations affected by disasters and emergencies. Training newly joined nurses is an integral part of IEMT training.
Typical training methods include lectures, field-simulation exercises, and tabletop exercises [ 3 , 4 , 5 ]. However, lectures, despite requiring fewer teaching resources, are often perceived as boring and abstract. This may not be the most ideal method for training newly joined nurses in the complexities of international medical responses. However, simulation field exercises can be effective in mastering the knowledge and skills of disaster medicine responsiveness. However, they come with significant costs and requirements, such as extended instructional periods, additional teachers or instructors, and thorough preparation. These high costs make it challenging to organize simulation exercises repeatedly, making them less ideal for training newly joined nurses [ 6 ].
Moreover, classic tabletop exercises that use simple props, such as cards in a classroom setting, have limitations. The rules of these exercises are typically simple, which makes it challenging to simulate complex disaster scenarios. In addition, these exercises cannot replicate real-life situations, making them too abstract for newly joined nurses to fully grasp [ 7 , 8 ].
Recently, game-based learning has gained increasing attention as an interactive teaching method [ 9 , 10 ]. Previous studies have validated the efficacy of game-based mobile applications [ 11 , 12 ]. Serious games that align with curricular objectives have shown potential to facilitate more effective learner-centered educational experiences for trainees [ 13 , 14 ]. Although game-based applications have been used in disaster medicine education, no serious computer games have been designed specifically for training newly joined nurses in an international IEMT setting.
Our team is an internationally certified IEMT organization verified by the WHO, underscoring the importance of providing training for newly joined nurses in international medical responses. To address this need, we organized training courses for them. As part of the training, we incorporated a serious computer game called the IEMTtraining game. In this game, players assume the roles of IEMT nurses, assess patient injuries in a virtual environment, and provide suitable treatment options. This study aims to investigate the effectiveness of the IEMTtraining game. To the best of our knowledge, this is the first serious game specifically designed to train newly joined nurses in an IEMT setting.
The IEMTtraining game was subsequently applied to the training course for newly joined nurses, and this study aimed to investigate its effectiveness. To the best of our knowledge, this is the first serious game specifically designedto train newly joined nurses in an IEMT setting.
Study design
This study was conducted using data from the training records database of participants who had completed the training. The database includes comprehensive demographic information, exam scores, and detailed information from post-training questionnaires for all trainees. We reviewed the training scores and questionnaires of participants who took part in the training from Autumn 2019 to Spring 2020.
The local Institutional Review Committee approved the study and waived the requirement for informed consent due to the study design. The study complied with the international ethical guidelines for human research, such as the Declaration of Helsinki. The accessed data were anonymized.
Participants
A total of 209 newly joined nurses needed to participate in the training. Due to limitations in the size of the training venue, the trainees had to be divided into two groups for the training. All trainees were required to choose a group and register online. The training team provided the schedule and training topic for the two training sessions to all trainees before the training commenced. Each trainee had the opportunity to sign up based on their individual circumstances. Furthermore, the training team set a maximum limit of 110 trainees for each group, considering the dimensions of the training venue. Trainees were assigned on a first-come-first-served basis. In the event that a group reached its capacity, any unregistered trainees would be automatically assigned to another group.
In the fall of 2019, 103 newly joined nurses opted for the lecture training course (lecture group). In this group, instructors solely used the traditional teaching methods of lectures and demonstrations. The remaining 106 newly joined nurses underwent game-based training (game group). In addition to the traditional lectures and demonstrations, the instructor incorporated an IEMTtraining game to enhance the training experience in the game group.
The IEMTTraining game
The IEMTtraining game, a role-playing game, was implemented using the RPG Maker MV Version1.6.1 (Kadokawa Corporation, Tokyo, Tokyo Metropolis, Japan). Players assumed the roles of rescuers in a fictional setting of an earthquake (Part1 of Supplemental Digital Content ).
The storyline revolves around an earthquake scenario, with the main character being an IEMT nurse. Within the game simulation, there were 1000 patients in the scenario. The objective for each player was to treat as many patients as possible to earn higher experience points compared to other players. In addition, within the game scene, multiple nonplayer characters played the role of injured patients. The players navigate the movements of the main character using a computer mouse. Upon encountering injured persons, the player can view their injury information by clicking on them and selecting the triage tags. The player can then select the necessary medical supplies from the kit to provide treatment. Additionally, the player is required to act according to the minimum standards for IEMTs, such as registration in the IEMT coordination cell and reporting of injury information following the minimum data set (MDS) designed by the WHO [ 15 , 16 ]. This portion of the training content imposes uniform requirements for all IEMT members, hence it is necessary for IEMT nurses to learn it. All correct choices result in the accumulation of experience points. Game duration can be set by the instructor and the player with the highest experience points at the end of the game.
Measurement
We have collected the test scores of the trainees in our training database to explore their knowledge mastery. Additionally, we have collected post-training questionnaire data from the trainees to investigate their learning motivation, cognitive load, and technology acceptance.
Pre-test, post-test, and final test
All trainees were tested on three separate occasions: (1) a “pre-test”before the educational intervention, (2) a “post-test”following the intervention, and (3) a “final test”at the end of the term (sixweeks after the intervention). Each test comprised 20 multiple-choice questions (0.5 points per item) assessing the trainees’ mastery of crucial points in their knowledge and decision-making. The higher the score, the better the grade will be.
Questionnaires
The questionnaires used in this study can be found in Part 2 of the Supplemental Digital Content .
The learning motivation questionnaire used in this study was based on the measure developed by Hwang and Chang [ 17 ]. It comprises seven items rated on a six-point scale. The reliability of the questionnaire, as indicated by Cronbach’s alpha, was 0.79.
The cognitive load questionnaire was adapted from the questionnaire developed by Hwang et al [ 18 ]. It consisted of five items for assessing “mental load” and three items for evaluating “mental effort.” The items were rated using a six-point Likert scale. The Cronbach’s alpha values for the two parts of the questionnaire were 0.86 and 0.85, respectively.
The technology acceptance questionnaire, which was only administered to the game group, as it specifically focused on novel teaching techniques and lacked relevance tothe lecture group, was derived from the measurement instrument developed by Chu et al [ 19 ]. It comprised seven items for measuring “perceived ease of use” and six items for assessing “perceived usefulness.” The items were rated on a six-point Likert scale. The Cronbach’s alpha values for the two parts of the questionnaire were 0.94 and 0.95, respectively.
The lecture group received 4 hours of traditional lectures. Additionally, 1 week before the lecture, the trainees were provided with a series of references related to the topic and were required to preview the content before the class. A pre-test was conducted before the lecture to assess the trainees’ prior knowledge, followed by a post-test immediately after the lecture, and a final test 6 weeks after training.
In the game group, the delivery and requirements for references were the same as those in the lecture group. However, the training format differed. The game group received a half-hour lecture introducinggeneral principles, followed by 3 hours of gameplay. The last halfhour was dedicated to summarizing the course and addressing questions or concerns. Similar to the lecture group, the trainees in this group also completed pre-, post-, and final tests. Additionally, a brief survey ofthe teaching methods was conducted at the end of the final test (see Fig. 1 ).
General overview of the teaching procedure. Figure Legend: The diagram shows the teaching and testing processes for the two groups of trainees. Q&A: questions and answers
Data analysis
All data were analyzed using IBM SPSS Statistics (version 20.0;IBM Inc., Armonk, NY, USA). Only the trainees who participated in all three tests were included in the analysis. In total, there were 209 trainees, but 11 individuals (6 from the lecture group and 5 from the game group) were excluded due to incomplete data. Therefore, the data of 198 trainees were ultimately included in the analysis.
In addition, measurement data with a normal distribution were described as mean (standard deviation, SD). In contrast, measurement data with non-normal distributions were expressed as median [first quartile, third quartile]. Furthermore, enumeration data were constructed using composition ratios.
Moreover, a generalized estimating equation (GEE) was employed to compare the groups’ pre-, post-, and final test scores. The Mann–Whitney U test was used to compare the questionnaire scores between the two groups. The statistical significance was set at a level of 0.05.
Among the data included in the analysis, 97 (48.99%) participants were in the lecture group, and 101 (51.01%)were in the game group.
The number of male trainees in the lecture and game groups was 30 (30.93%) and 33 (32.67%), respectively. The mean age of participants in the lecture group was 27.44 ± 4.31 years, whereas that of the game group was 28.05 ± 4.29 years. There were no significant differences in sex or age (Table 1 ). Regarding the test scores, no significant differences were found between the two groups in the pre- and post-tests. However, a significant difference was observed in the final test scores conducted 6 weeks later (Table 1 ).
According to the GEE analysis, the overall scores for the post-test and final test were higher compared to the pre-test scores. Additionally, there was a significant difference in the overall test scores between the two groups, with the game group demonstrating superior performance compared to the control group (odds ratio = 1.363, p value = 0.010). Further details of the GEE results can be found in Part 3 of the supplementary materials .
Table 2 presents the results of the questionnaire ratings for the two groups. The median [first quartile, third quartile] of the learning motivation questionnaire ratings were 4 [3, 4] for the lecture group and 5 [4, 5] for the game group. There were significant differences between the questionnaire ratings of the two groups ( p < 0.001), indicating that the game group had higher learning motivation for the learning activity.
The median [first quartile, third quartile] of the overall cognitive load ratings were 3 [3, 4] and 4 [4, 5] for the game and lecture groups, respectively. There was a significant difference between the cognitive load ratings of the two groups ( p < 0.001).
This study further compared two aspects of cognitive load: mental load and mental effort. The median [first quartile, third quartile] for the mental effort dimension were 3 [2, 3] and 4 [4, 5] for the game and lecture groups, respectively (p < 0.001). For mental load, the median [first quartile, third quartile] were 4 [3, 4] and 4 [3, 4] for the game and lecture groups, respectively. There was no significant difference in the mental load ratings between the two groups ( p = 0.539).
To better understand the trainees’ perceptions of the use of the serious game, this study collected the feedback of the trainees in the game group regarding “perceived usefulness” and “perceived ease of use,” as shown in Table 2 . Most trainees provided positive feedback on the two dimensions of the serious game.
To the best of our knowledge, this IEMT training game is the first serious game intended for newly joined nurses of IEMTs. Therefore, this study presents an initial investigation into the applicability of serious games.
Both lectures and serious games improved post-class test scores to the same level, consistent with previous studies. Krishnan et al. found that an educational game on hepatitis significantly improved knowledge scores [ 20 ]. Additionally, our study showed higher knowledge retention in the game group after 6 weeks, in line with previous studies on serious games. In a study on sexually transmitted diseases, game-based instruction was found to improve knowledge retention for resident physicians compared to traditional teaching methods [ 21 ]. The IEMTtraining game, designed as a role-playing game, is more likely to enhance knowledge retention in newly joined nurses in the long term. Therefore, serious games should be included in the teaching of IEMT training.
This study demonstrated improved learning motivation in the game group, consistent with previous research indicating that game-based learning enhances motivation due to the enjoyable and challenging nature of the games [ 22 , 23 ]. A systematic review by Allan et al. further supports the positive impact of game-based learning tools on the motivation, attitudes, and engagement of healthcare trainees [ 24 ].
As serious games are a novel learning experience for trainees, it is worth investigating the cognitive load they experience. Our study found that serious games effectively reduce trainees’ overall cognitive load, particularly in terms of lower mental effort. Mental effort refers to the cognitive capacity used to handle task demands, reflecting the cognitive load associated with organizing and presenting learning content, as well as guiding student learning strategies [ 25 , 26 ]. This reduction in cognitive load is a significant advantage of serious gaming, as it helps learners better understand and organize their knowledge. However, our study did not find a significant difference in mental load between the two groups. Mental load considers the interaction between task and subject characteristics, based on students’ understanding of tasks and subject characteristics [ 18 ]. This finding is intriguing as it aligns with similar observations in game-based education for elementary and secondary school students [ 27 ], but is the first mention of game-based education in academic papers related to nursing training.
In our survey of the game group participants, we found that their feedback regarding the perceived ease of use and usefulness of the game was overwhelmingly positive. This indicates that the designed game was helpful to learners during the learning process. Moreover, the game’s mechanics were easily understood by the trainees without requiring them to investsignificant time and effort to understand the game rules and controls.
This study had some limitations. First, this retrospective observational study may have been susceptible to sampling bias due to the non-random grouping of trainees. It only reviewed existing data from the training database, and future research should be conducted to validate our findings through prospective studies. Therefore, randomized controlled trials are required. Second, the serious game is currently available only in China. We are currently developing an English version to better align with the training requirements of international IEMT nurses. Third, the development of such serious gamescan be time-consuming. To address this problem, we propose a meta-model to help researchers and instructors select appropriate game development models to implement effective serious games.
An IEMT training game for newly joined nurses is a highly promising training method. Its potential lies in its ability to offer engaging and interactive learning experiences, thereby effectively enhancing the training process. Furthermore, the game improved knowledge retention, increased motivation to learn, and reduced cognitive load. In addition, the game’s mechanics are easily understood by trainees, which further enhances its effectiveness as a training instrument.
Availability of data and materials
Availability of data and materials can be ensured through direct contact with the author. If you require access to specific data or materials mentioned in a study or research article, reaching out to the author is the best way to obtain them. By contacting the author directly, you can inquire about the availability of the desired data and materials, as well as any necessary procedures or restrictions for accessing them.
Authors are willing to provide data and materials to interested parties. They understand the importance of transparency and the positive impact of data sharing on scientific progress. Whether it is raw data, experimental protocols, or unique materials used in the study, authors can provide valuable insights and resources to support further investigations or replications.
To contact the author, one can refer to the email address provided in the article.
Abbreviations
World Health Organization
International Emergency Medical Team
Minimum Data Set
Generalized estimating eq.
Standard deviation
World Health Organization.Classification and minimum standards for emergency medical teams. https://apps.who.int/iris/rest/bitstreams/1351888/retrieve . Published 2021. Accessed May 6, 2023.
World Health Organization. Classification and Minimum Standards for Foreign Medical Teams in Sudden Onset Disasters. https://cdn.who.int/media/docs/default-source/documents/publications/classification-and-minimum-standards-for-foreign-medical-teams-in-suddent-onset-disasters65829584-c349-4f98-b828-f2ffff4fe089.pdf?sfvrsn=43a8b2f1_1&download=true . Published 2013. Accessed May 6, 2023.
Brunero S, Dunn S, Lamont S. Development and effectiveness of tabletop exercises in preparing health practitioners in violence prevention management: a sequential explanatory mixed methods study. Nurse Educ Today. 2021;103:104976. https://doi.org/10.1016/j.nedt.2021.104976 .
Article Google Scholar
Sena A, Forde F, Yu C, Sule H, Masters MM. Disaster preparedness training for emergency medicine residents using a tabletop exercise. Med Ed PORTAL. 2021;17:11119. https://doi.org/10.15766/mep_2374-8265.11119 .
Moss R, Gaarder C. Exercising for mass casualty preparedness. Br J Anaesth. 2022;128(2):e67–70. https://doi.org/10.1016/j.bja.2021.10.016 .
Hu H, Liu Z, Li H. Teaching disaster medicine with a novel game-based computer application: a case study at Sichuan University. Disaster Med Public Health Prep. 2022;16(2):548–54. https://doi.org/10.1017/dmp.2020.309 .
Chi CH, Chao WH, Chuang CC, Tsai MC, Tsai LM. Emergency medical technicians' disaster training by tabletop exercise. Am J Emerg Med. 2001;19(5):433–6. https://doi.org/10.1053/ajem.2001.24467 .
Hu H, Lai X, Li H, et al. Teaching disaster evacuation management education to nursing students using virtual reality Mobile game-based learning. Comput Inform Nurs. 2022;40(10):705–10. https://doi.org/10.1097/CIN.0000000000000856 .
van Gaalen AEJ, Brouwer J, Schönrock-Adema J, et al. Gamification of health professions education: a systematic review. Adv Health Sci Educ Theory Pract. 2021;26(2):683–711. https://doi.org/10.1007/s10459-020-10000-3 .
Adjedj J, Ducrocq G, Bouleti C, et al. Medical student evaluation with a serious game compared to multiple choice questions assessment. JMIR Serious Games. 2017;5(2):e11. https://doi.org/10.2196/games.7033 .
Hu H, Xiao Y, Li H. The effectiveness of a serious game versus online lectures for improving medical Students' coronavirus disease 2019 knowledge. Games Health J. 2021;10(2):139–44. https://doi.org/10.1089/g4h.2020.0140.E .
Pimentel J, Arias A, Ramírez D, et al. Game-based learning interventions to Foster cross-cultural care training: a scoping review. Games Health J. 2020;9(3):164–81. https://doi.org/10.1089/g4h.2019.0078 .
Hu H, Lai X, Yan L. Improving nursing Students' COVID-19 knowledge using a serious game. Comput Inform Nurs. 2021;40(4):285–9. https://doi.org/10.1097/CIN.0000000000000857 .
Menin A, Torchelsen R, Nedel L. An analysis of VR technology used in immersive simulations with a serious game perspective. IEEE Comput Graph Appl. 2018;38(2):57–73. https://doi.org/10.1109/MCG.2018.021951633 .
Kubo T, Chimed-Ochir O, Cossa M, et al. First activation of the WHO emergency medical team minimum data set in the 2019 response to tropical cyclone Idai in Mozambique. Prehosp Disaster Med. 2022;37(6):727–34.
Jafar AJN, Sergeant JC, Lecky F. What is the inter-rater agreement of injury classification using the WHO minimum data set for emergency medical teams? Emerg Med J. 2020;37(2):58–64. https://doi.org/10.1136/emermed-2019-209012 .
Hwang GJ, Chang HF. A formative assessment-based mobile learning approach to improving the learning attitudes and achievements of students. Comput Educ. 2011;56(4):1023–31. https://doi.org/10.1016/j.compedu.2010.12.002 .
Hwang G-J, Yang L-H. Sheng-yuan Wang.Concept map-embedded educational computer game for improving students’ learning performance in natural science courses. Comput Educ. 2013;69:121–30.
Chu HC, Hwang GJ, Tsai CC, et al. A two-tier test approach to developing location-aware mobile learning system for natural science course. Comput Educ. 2010;55(4):1618–27. https://doi.org/10.1016/j.compedu.2010.07.004 .
Krishnan S, Blebil AQ, Dujaili JA, Chuang S, Lim A. Implementation of a hepatitis-themed virtual escape room in pharmacy education: A pilot study. Educ Inf Technol (Dordr). 2023;5:1–13. https://doi.org/10.1007/s10639-023-11745-1 . Epub ahead of print. PMID: 37361790; PMCID: PMC10073791
Butler SK, Runge MA, Milad MP. A game show-based curriculum for teaching principles of reproductive infectious disease (GBS PRIDE trial). South Med J. 2020;113(11):531–7. https://doi.org/10.14423/SMJ.0000000000001165 . PMID: 33140104
Haruna H, Hu X, Chu SKW, et al. Improving sexual health education programs for adolescent students through game-based learning and gamification. Int J Environ Res Public Health. 2018;15(9):2027. https://doi.org/10.3390/ijerph15092027 .
Rewolinski JA, Kelemen A, Liang Y. Type I diabetes self-management with game-based interventions for pediatric and adolescent patients. Comput Inform Nurs. 2020;39(2):78–88. https://doi.org/10.1097/CIN.0000000000000646 .
Allan R, McCann L, Johnson L, Dyson M, Ford J. A systematic review of 'equity-focused' game-based learning in the teaching of health staff. Public Health Pract (Oxf). 2023;27(7):100462. https://doi.org/10.1016/j.puhip.2023.100462 . PMID: 38283754; PMCID: PMC10820634
Zumbach J, Rammerstorfer L, Deibl I. Cognitive and metacognitive support in learning with a serious game about demographic change. Comput Hum Behav. 2020;103:120–9. https://doi.org/10.1016/j.chb.2019.09.026 .
Chang C-C, Liang C, Chou P-N, et al. Is game-based learning better in flow experience and various types of cognitive load than non-game-based learning? Perspective from multimedia and media richness. Comput Hum Behav. 2017;71:218–27. https://doi.org/10.1016/j.chb.2017.01.031 .
Kalmpourtzis G, Romero M. Constructive alignment of learning mechanics and game mechanics in serious game design in higher education. Int J Serious Games. 2020;7(4):75–88. https://doi.org/10.17083/ijsg.v7i4.361 .
Download references
Acknowledgements
We would like to thank all the staffs who contribute to the database. We would like to thank Editage ( www.editage.cn ) for English language editing. We also would like to thank Dr. Yong Yang for statistics help. We would like to thank The 10th Sichuan University Higher Education Teaching Reform Research Project (No. SCU10170) and West China School of Medicine (2023-2024) Teaching Reform Research Project (No. HXBK-B2023016) for the support.
Author information
Both Hai Hu and Xiaoqin Lai contributed equally to this work and should be regarded as co-first authors.
Authors and Affiliations
Emergency Management Office of West China Hospital, Sichuan University, The street address: No. 37. Guoxue Road, Chengdu City, Sichuan Province, China
China International Emergency Medical Team (Sichuan), Chengdu City, Sichuan Province, China
Hai Hu & Xiaoqin Lai
Emergency Medical Rescue Base, Sichuan University, Chengdu City, Sichuan Province, China
Day Surgery Center, West China Hospital, Sichuan University, Chengdu City, Sichuan Province, China
Xiaoqin Lai
Department of Thoracic Surgery, West China Tianfu Hospital, Sichuan University, Chengdu City, Sichuan Province, China
West China School of Nursing, Sichuan University, Chengdu City, Sichuan Province, China
Longping Yan
West China School of Public Health, Sichuan University, Chengdu, Sichuan, China
West China Fourth Hospital, Sichuan University, Chengdu, Sichuan, China
You can also search for this author in PubMed Google Scholar
Contributions
HH conceived the study, designed the trial, and obtained research funding. XL supervised the conduct of the data collection from the database, and managed the data, including quality control. HH and LY provided statistical advice on study design and analyzed the data. All the authors drafted the manuscript, and contributed substantially to its revision. HH takes responsibility for the paper as a whole.
Corresponding author
Correspondence to Hai Hu .
Ethics declarations
Ethics approval and consent to participate.
The local institutional review committee approved the study and waived the need for informed consent from the participants owing to the study design.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Hu, H., Lai, X. & Yan, L. Training nurses in an international emergency medical team using a serious role-playing game: a retrospective comparative analysis. BMC Med Educ 24 , 432 (2024). https://doi.org/10.1186/s12909-024-05442-x
Download citation
Received : 05 November 2023
Accepted : 17 April 2024
Published : 22 April 2024
DOI : https://doi.org/10.1186/s12909-024-05442-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Rescue work
- Gamification
- Simulation training
BMC Medical Education
ISSN: 1472-6920
- Submission enquiries: [email protected]
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Types of study design. Medical research is classified into primary and secondary research. Clinical/experimental studies are performed in primary research, whereas secondary research consolidates available studies as reviews, systematic reviews and meta-analyses. Three main areas in primary research are basic medical research, clinical research ...
Introduction. In clinical research, our aim is to design a study, which would be able to derive a valid and meaningful scientific conclusion using appropriate statistical methods that can be translated to the "real world" setting. 1 Before choosing a study design, one must establish aims and objectives of the study, and choose an appropriate target population that is most representative of ...
Research study design is a framework, or the set of methods and procedures used to collect and analyze data on variables specified in a particular research problem. Research study designs are of many types, each with its advantages and limitations. The type of study design used to answer a particular research question is determined by the ...
We may approach this study by 2 longitudinal designs: Prospective: we follow the individuals in the future to know who will develop the disease. Retrospective: we look to the past to know who developed the disease (e.g. using medical records) This design is the strongest among the observational studies. For example - to find out the relative ...
A research design is a strategy for answering your research question using empirical data. Creating a research design means making decisions about: Your overall research objectives and approach. Whether you'll rely on primary research or secondary research. Your sampling methods or criteria for selecting subjects. Your data collection methods.
Study design is the procedure under which a study is carried out Study design is the procedure under which a study is. Two main categories. •Observation: •Identify subjects, then. •Observe and record characteristics. •Experiment. •Identify subjects, •Place in common context, •Intervene, then.
Introduction. This guide was created to introduce you to the following study designs frequently used in the health sciences: Case-Control. Clinical Trials. Randomized Controlled Trial (RCT) Cohort. Systematic reviews/Meta-Analysis. Each tab in this guide describes a particular study design and will include a definition of the design, why it is ...
Spotting the study design. The type of study can generally be worked at by looking at three issues (as per the Tree of design in Figure 1): Q1. What was the aim of the study? ... Qualitative Research Methods; Essential Medical Statistics; The History and Philosophy of Evidence-Based Health Care; Teaching Evidence-Based Practice;
Introduction. Study designs are frameworks used in medical research to gather data and explore a specific research question.. Choosing an appropriate study design is one of many essential considerations before conducting research to minimise bias and yield valid results.. This guide provides a summary of study designs commonly used in medical research, their characteristics, advantages and ...
Abstract. Background: The quality of scientific literature is judged by study design, validity, and applicability to unique patient populations. Methods: We searched the available literature to explore the hierarchy of evidence, explain research fundamentals such as sample size calculation, and discuss common study designs employed in surgical ...
Ranganathan P. Understanding Research Study Designs. Indian J Crit Care Med 2019;23 (Suppl 4):S305-S307. Keywords: Clinical trials as topic, Observational studies as topic, Research designs. We use a variety of research study designs in biomedical research. In this article, the main features of each of these designs are summarized. Go to:
Publication Date: 2022-05-19. Essential Concepts in Clinical Research by Kenneth Schulz; David A. Grimes. ISBN: 9780702073946. Publication Date: 2018-10-03. Introduction to Health Research Methods by Kathryn H. Jacobsen. Access Note: Concurrent User (s): 1. ISBN: 9781284197563. Publication Date: 2020-02-20.
to biological research, and is the substance of research design. Research design, above all else, must account for and maintain the role of chance in order to ensure validity. It is statistical methods which preserve the laws of probability in our inquiry, and allow proper analysis and interpretation of results. Statistics are the tool that permits
This type of evidence is top of the hierarchy because systematic reviews are: Objective - there should be no opinion or selection bias involved when choosing which evidence to include in a systematic review. Comprehensive - includes all of the evidence on a topic. Precise - a review should answer a very specific research question.
Medical research has evolved, from individual expert described opinions and techniques, to scientifically designed methodology-based studies. Evidence-based medicine (EBM) was established to re-evaluate medical facts and remove various myths in clinical practice. ... Types of studies and research design Indian J Anaesth. 2016 Sep;60(9):626-630 ...
This will guide your research design and help you select appropriate methods. Select a research design: There are many different research designs to choose from, including experimental, survey, case study, and qualitative designs. Choose a design that best fits your research question and objectives.
Observational research methods. Research design II: cohort, cross sectional, ... Clinical research is an alternative terminology used to describe medical research. Clinical research involves ...
This type of research design draws a conclusion by comparing subjects against a control group, in cases where the researcher has no control over the experiment. There are two general types of observational designs. ... Journal of the Medical Library Association 109 (October 2021): 643-647; Okoli, Chitu, and Kira Schabram. "A Guide to Conducting ...
Cohort studies are a powerful tool for conducting research in human populations. They are a type of longitudinal study design. Longitudinal studies follow participants over a period of time.
The STROBE statement for cross-sectional studies is a useful guideline for this design . Open in a separate window. FIG. 2. Cross-sectional study design ... du Prel JB, Wachtlin D, Blettner M. Types of study in medical research: part 3 of a series on evaluation of scientific publications. Dtsch Arzteblatt Int. 2009; 106:262-8. [PMC free ...
Observational studies are of four main types: case-series, case-control, cross-sectional (including surveys), and cohort studies.When certain characteristics of a group (or series) of patients (or cases) are described in a published report, the result is called a case-series study; it is the simplest design in which the author describes some interesting or intriguing observations that ...
Quantitative Studies - Quantitative research is research that uses numerical analysis. Random Allocation - A process involving chance used in therapeutic trials or other research endeavor for allocating experimental subjects, human or animal, between treatment and control groups, or among treatment groups. It may also apply to experiments on ...
The study type is a component of the study design (see the article "Study Design in Medical Research") and must be specified before the study starts. The study type is determined by the question to be answered and decides how useful a scientific study is and how well it can be interpreted.
Introduction. In clinical research, our aim is to design a study, which would be able to derive a valid and meaningful scientific conclusion using appropriate statistical methods that can be translated to the "real world" setting. 1 Before choosing a study design, one must establish aims and objectives of the study, and choose an appropriate target population that is most representative of ...
Methods. The design of this study is a retrospective comparative analysis. The research was conducted with 209 nurses in a hospital. The data collection process of this study was conducted at the 2019-2020 academic year. A retrospective comparative analysis was conducted on the pre-, post-, and final test scores of nurses in the IEMT.