Jump to content

NIST Chemistry WebBook , SRD 69
- IUPAC identifier
- More options
- SRD Program
- Science Data Portal
- Office of Data and Informatics
- More documentation
- Formula : C 8 H 8
- Molecular weight : 104.1491

- IUPAC Standard InChIKey: PPBRXRYQALVLMV-UHFFFAOYSA-N Copy
- CAS Registry Number: 100-42-5
- Other names: Benzene, ethenyl-; Bulstren K-525-19; Cinnamene; Phenethylene; Phenylethene; Phenylethylene; Styrol (German); Styrole; Styrolene; Styropol SO; Vinylbenzene; Vinylbenzol; Ethenylbenzene; Cinnaminol; Cinnamol; Styrol; Benzene, vinyl-; Cinnamenol; Ethylene, phenyl-; NCI-C02200; Stirolo; Styreen; Styren; Styrene monomer; Vinylbenzen; Annamene; NSC 62785; ethenylbenzene (styrene); Vinylbenzene (styrene)
- Permanent link for this species. Use this link for bookmarking this species for future reference.
Infrared Spectrum
- Gas phase thermochemistry data
- Condensed phase thermochemistry data
- Phase change data
- Reaction thermochemistry data
- Henry's Law data
- Gas phase ion energetics data
- Ion clustering data
- IR Spectrum
- Mass spectrum (electron ionization)
- UV/Visible spectrum
- Gas Chromatography
- Computational Chemistry Comparison and Benchmark Database
- Gas Phase Kinetics Database
- NIST Polycyclic Aromatic Hydrocarbon Structure Index
- Switch to calorie-based units
Data at NIST subscription sites:
- NIST / TRC Web Thermo Tables, professional edition (thermophysical and thermochemical data)
NIST subscription sites provide data under the NIST Standard Reference Data Program , but require an annual fee to access. The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage.
Go To: Top , References , Notes
Data compilation copyright by the U.S. Secretary of Commerce on behalf of the U.S.A. All rights reserved.
Data compiled by: Coblentz Society, Inc.
Condensed Phase Spectrum
Notice: This spectrum may be better viewed with a Javascript and HTML 5 enabled browser.
- Help / Software credits
The interactive spectrum display requires a browser with JavaScript and HTML 5 canvas support.
Select a region with data to zoom. Select a region with no data or click the mouse on the plot to revert to the orginal display.
The following components were used in generating the plot:
- Resize (distributed with Flot)
- Selection (distributed with Flot)
- Axis labels
- Labels ( Modified by NIST for use in this application )
Additonal code used was developed at NIST: jcamp-dx.js and jcamp-plot.js .
Use or mention of technologies or programs in this web site is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that these items are necessarily the best available for the purpose.
Notice: Except where noted, spectra from this collection were measured on dispersive instruments, often in carefully selected solvents, and hence may differ in detail from measurements on FTIR instruments or in other chemical environments. More information on the manner in which spectra in this collection were collected can be found here.
Notice: Concentration information is not available for this spectrum and, therefore, molar absorptivity values cannot be derived.
Additional Data
View scan of original (hardcopy) spectrum .
View image of digitized spectrum (can be printed in landscape orientation).
View spectrum image in SVG format .
Download spectrum in JCAMP-DX format.
This IR spectrum is from the Coblentz Society's evaluated infrared reference spectra collection .
Go To: Top , Infrared Spectrum , Notes
No reference data available.
Go To: Top , Infrared Spectrum , References
- Data from NIST Standard Reference Database 69: NIST Chemistry WebBook
- The National Institute of Standards and Technology (NIST) uses its best efforts to deliver a high quality copy of the Database and to verify that the data contained therein have been selected on the basis of sound scientific judgment. However, NIST makes no warranties to that effect, and NIST shall not be liable for any damage that may result from errors or omissions in the Database.
- Customer support for NIST Standard Reference Data products.
- If you believe that this page may contain an error, please fill out the error report form for this page.
Physical Chemistry Chemical Physics
New molecular-scale information on polystyrene dynamics in ps and ps–batio 3 composites from ftir spectroscopy †.
* Corresponding authors
a Departamento de Ciencia e Ingeniería de Materiales e Ingeniería Química and IQMAAB, Universidad Carlos III de Madrid, Av. Universidad 30, 28911 Leganés, Spain E-mail: [email protected] Tel: +34 91 624 8870
A new idea to understand the macromolecular motion occurring along the thermal relaxations of polystyrene (PS) and PS–barium titanate composites is proposed. Detailed analysis of PS infrared bands provides a better knowledge of the factors affecting polymer dynamics. Average spectral positions and integrated absorbance of bands in the region of C–H out-of-plane vibrations showed a continuous decrease with temperature, whereas those in the region of aliphatic and aromatic C–H stretching vibrations showed the sharpest changes with temperature. Relaxation temperatures were determined from the changes observed in the band wavenumber or area with temperature. These results were attributed to changes in the distribution of the phenyl π-electron cloud, causing important dipole moment variations in the different vibration modes when the thermal transitions are taking place. Finally, although the presence of BaTiO 3 particles does not seem to exert any specific effect on the PS dynamics in the glassy state, the Curie transition of these particles might induce a kind of confinement effect observable by FTIR.
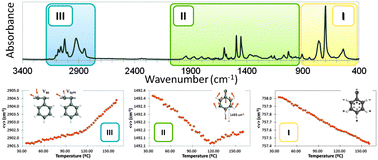
Supplementary files
- Supplementary information PDF (2153K)

Article information
Download citation, author version available, permissions.
New molecular-scale information on polystyrene dynamics in PS and PS–BaTiO 3 composites from FTIR spectroscopy
D. Olmos, E. V. Martín and J. González-Benito, Phys. Chem. Chem. Phys. , 2014, 16 , 24339 DOI: 10.1039/C4CP03516J
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page .
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page .
Read more about how to correctly acknowledge RSC content .
Social activity
Search articles by author, advertisements.
- Pharma and Nutra Labs: Addressing Elemental Analysis Bottlenecks in Samples

- Publications
- Conferences
The Infrared Spectra of Polymers II: Polyethylene
In our second installment of the infrared (IR) spectra of polymers, we finish the discussion of the spectrum of polyethylene (PE) started last time. We explore how different PE syntheses produce materials with different physical and spectroscopic properties, which introduces us to the concept of crystalline splitting and the rest of the story on CH 2 rocking peaks.
In the last installment (1), I showed that the spectra of functional groups in polymers are similar to those of functional groups in small molecules, which means that our analysis of polymer spectra provides us a great opportunity to review the spectra of the functional groups we have covered over the last several years.
It is important to recall from an early installment in this series that hydrocarbons are molecules that contain hydrogen and carbon only (2), thus hydrocarbon polymers are large molecules that contain hydrogen and carbon only. The simplest hydrocarbons are alkanes, which contain CH3 (methyl) and CH 2 (methylene units) (2). However, this does not mean that alkanes and alkane polymers comprise a small family of molecules. In fact, the extended hydrocarbon family is a large one.
The spectra of alkanes are dominated by the C-H stretching and bending peaks from the methyl and methylene groups. Table I lists the group wavenumber peak position ranges for CH 3 and CH 2 stretching vibrations.
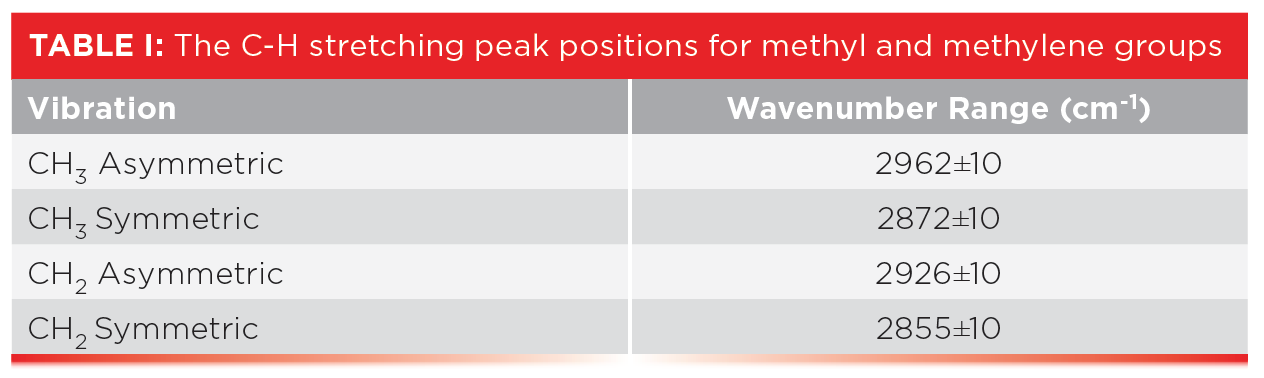
Figure 1: The infrared spectrum of high-density polyethylene (HDPE).
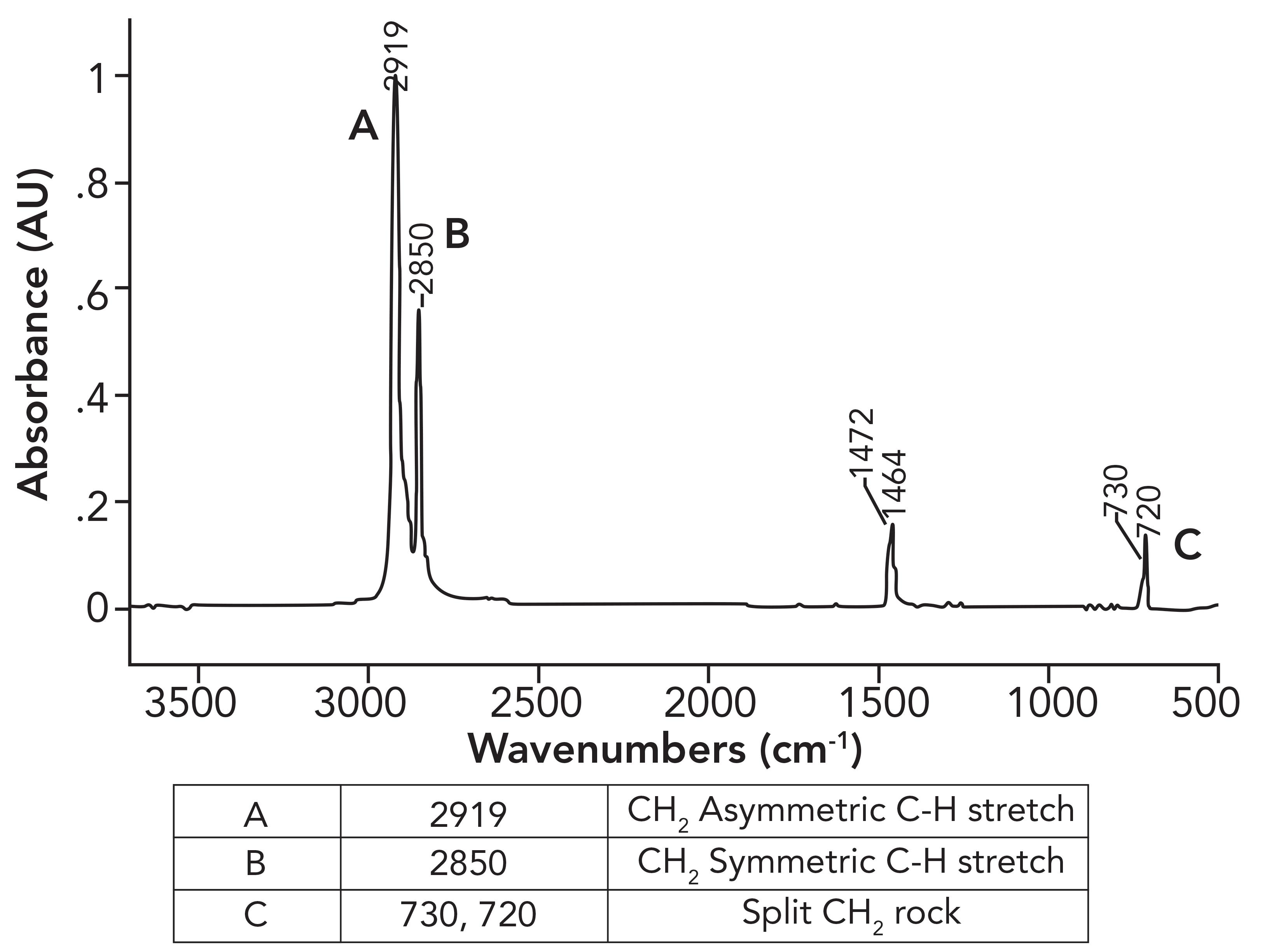
What was not discussed in the previous column (1) was why the CH 2 rocking peak in HDPE is split, with peaks at 730 and 720 rather having one expected peak at 720±10. To understand this splitting, we need to first examine the spectrum of a mixture of long chain but not polymeric alkanes. The spectrum of petroleum jelly will serve as a good example, and its spectrum is seen in Figure 2.
Figure 2: The infrared spectrum of petroleum jelly.
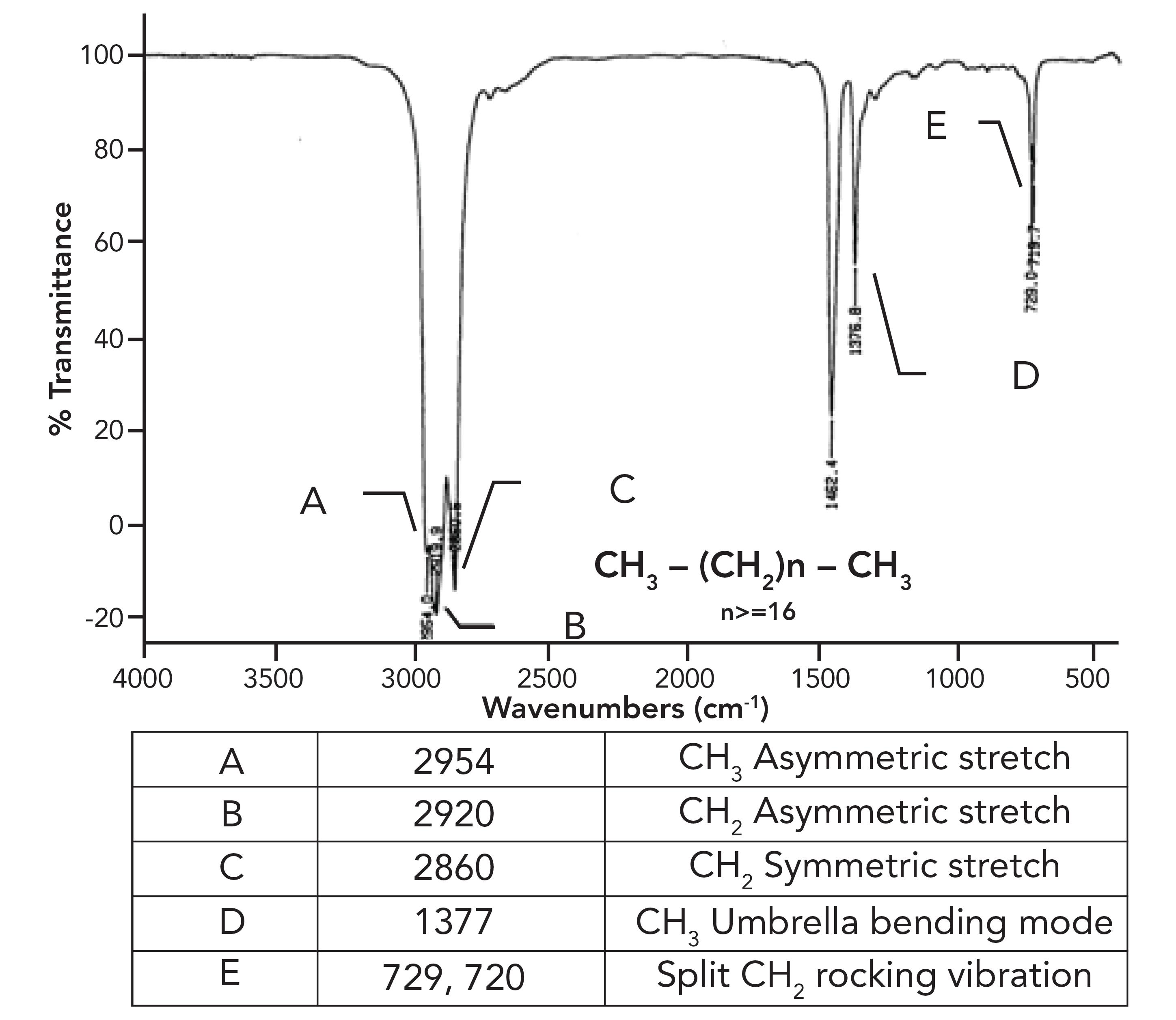
This sample consists of a mixture of straight chain alkanes with at least 18 carbons total and, at minimum, 16 methylenes in a row. As expected, we have three peaks between 3000 and 2850, confirming we have CH 3 and CH 2 groups present. The methyl group umbrella mode is at 1377 as expected, but note that similar to HDPE, the CH 2 rocking peak in Figure 2 is split. What is up with that?
What HDPE and petroleum jelly have in common is that they contain long chains of methylene groups, and that they are solids at room temperature. The CH 2 chains in these molecules can orient parallel to each other forming areas of long range order (that is, they crystallize as labeled b in Figure 3). It may seem hard to imagine that something as squishy as petroleum jelly can crystallize, but remember the definition of crystallinity is long range order, it has nothing to do with how hard a sample is (4). For example, a snowflake is crystalline, but breaks under the gentlest of pressure. Figure 3 also shows, labeled a, what randomly oriented CH 2 chains look like. Randomly oriented CH 2 chains are found in liquid alkanes or amorphous regions of polymers.
Figure 3: An idealized picture of long methylene chains. (a) Random orientation produces amorphous materials, (b) parallel orientation produces long range ordered crystalline materials.
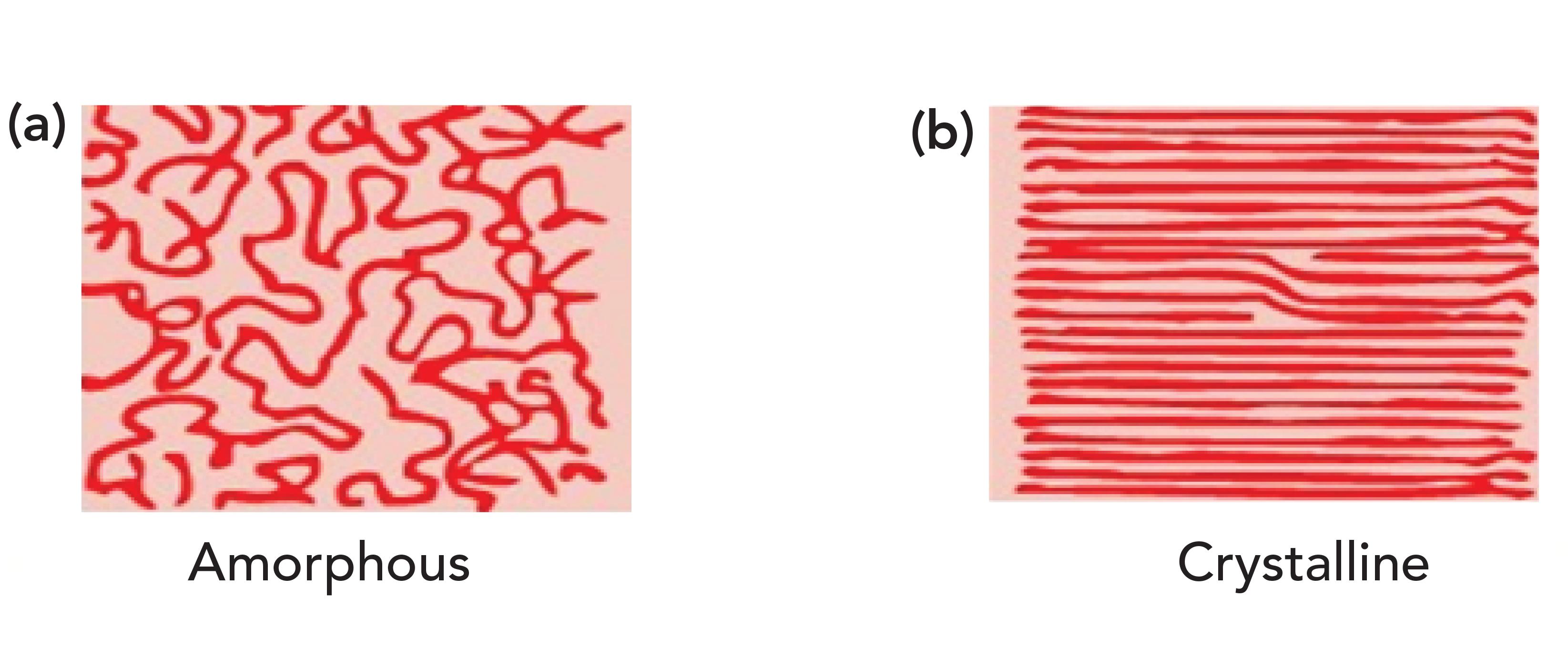
Why then do HDPE and petroleum jelly have a split CH 2 rocking peak? Imagine methylene groups on two parallel CH 2 chains both absorbing photons of the appropriate energy at the same time. They will undergo the CH 2 rocking vibration simultaneously as seen in Figure 4.
Figure 4: Methylene groups on adjacent molecules rocking in-phase and out-of-phase with each other.
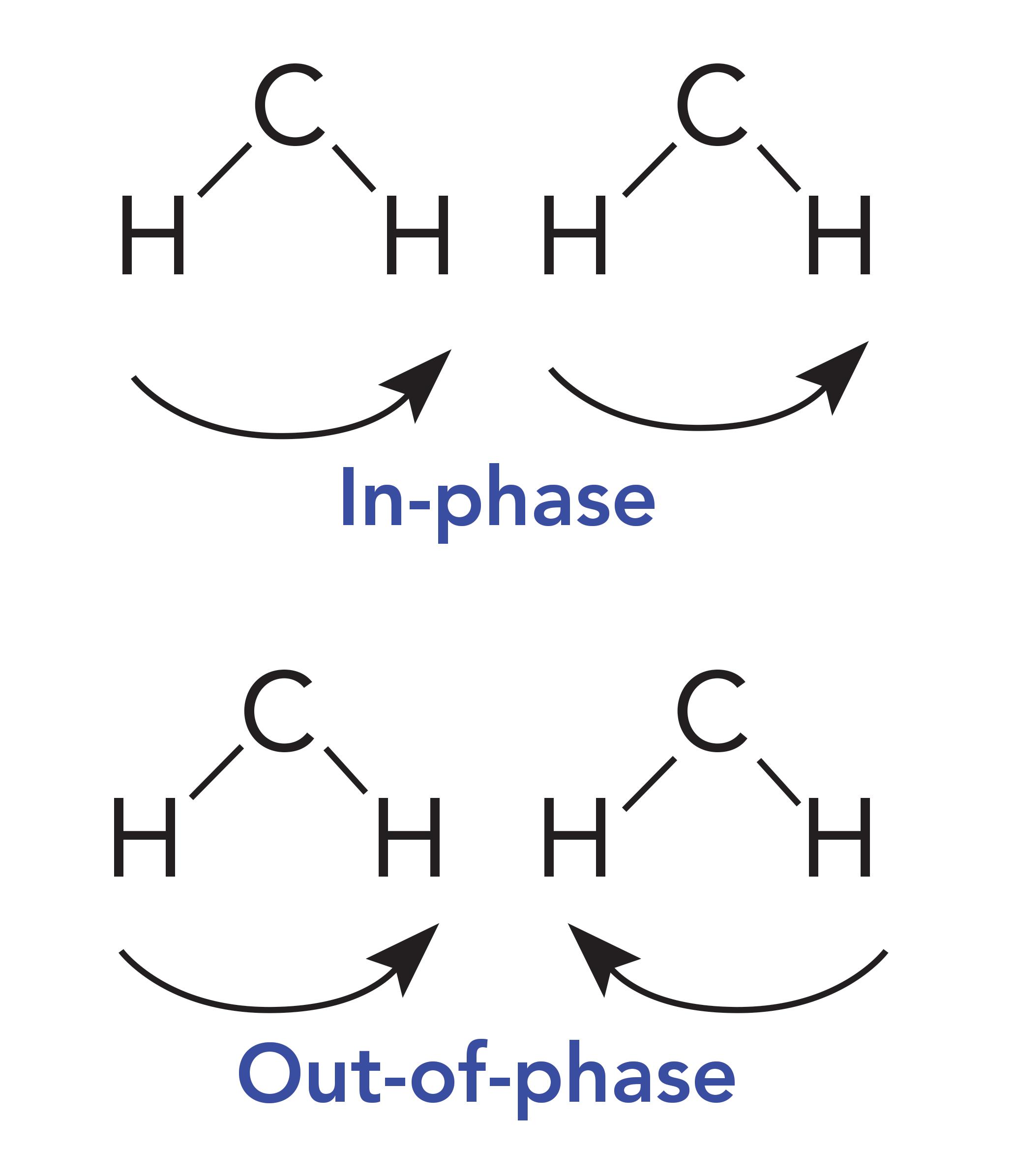
These CH 2 groups can vibrate in two different ways: They can move in-phase as illustrated at the top of Figure 4, or out-of-phase as seen in the bottom of Figure 4. The difference between these two situations is that when the two CH 2 groups rock in-phase, they do not touch, but when they rock out-of-phase, they collide with each other. As a result of this collision, there is an interaction between these methylenes altering the force constant of the vibration and hence its peak position. It was discussed in a previous article that one of the variables that determines peak position is the force constant for a vibration (5). Because the in-phase and out-of-phase rocking vibrations have different force constants, they will have different peak positions and result in two CH 2 rocking peaks instead of one. The rocking vibrations having different force constants is why the spectrum of petroleum jelly in Figure 2 has two CH 2 rocking peaks at 729 and 720, and why the spectrum of HDPE in Figure 1 has two CH 2 rocking peaks at 730 and 720. We will call this pair of peaks a “split CH 2 rocking mode peak.” This splitting is indicative of solid, long chain alkanes, of which both petroleum jelly and HDPE are examples. Liquid alkanes, such as melted petroleum jelly, do not exhibit this splitting because their chains are oriented randomly with respect each other. LDPE does not exhibit this splitting because the side chains force the methylene chains apart, preventing them from crystallizing. For review, the spectrum of LDPE is shown in Figure 5.
Figure 5: The infrared spectrum of low density polyethylene (LDPE).
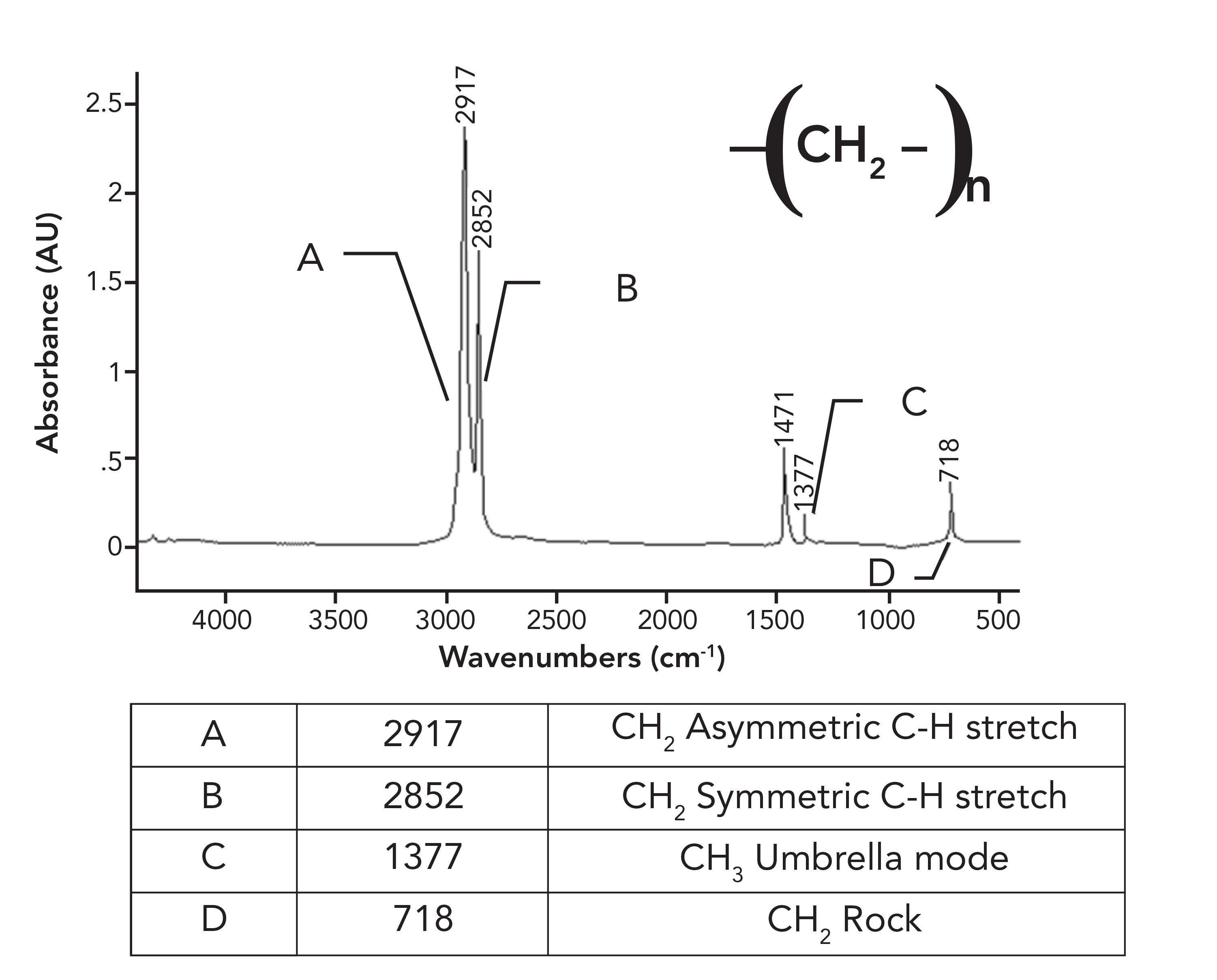
Note in the spectrum of LDPE that there is only one CH 2 rocking peak at 718, as opposed to the pair seen for HDPE in Figure 1. Crystalline samples giving split peaks is a common enough phenomenon in infrared (IR) spectroscopy that it has its own name, crystalline splitting (6). This explains why HDPE has a pair of peaks at 1472 and 1464 and 730 and 720 compared to the unsplit peaks in LDPE. The lack of side chains in HDPE allows the methylene chains to get close enough to orient and crystallize, whereas in LDPE, the side chains prevent this. To summarize this discussion, the differences between the spectra of LDPE and HDPE is that LDPE has a small CH 3 umbrella mode from side chains that HDPE does not have (1), and HDPE has split peaks that LDPE does not have because it has crystalline regions. Because of the differences between LDPE and HDPE, this means that IR spectroscopy can easily distinguish LDPE from HDPE.
But Wait, There’s More!
Polymer chemists are clever people, and in their quest to customize polymer properties, they have developed many more types of polyethylene than just LDPE and HDPE. For example, one can take advantage of the fact that some syntheses of polyethylene produce custom side chain lengths, hence customizing polymer properties. Have a look at the spectrum of what is known as “linear low-density polyethylene,” or LLDPE, in Figure 6.
Figure 6: The infrared spectrum of linear low-density (LLDPE) polyethylene.
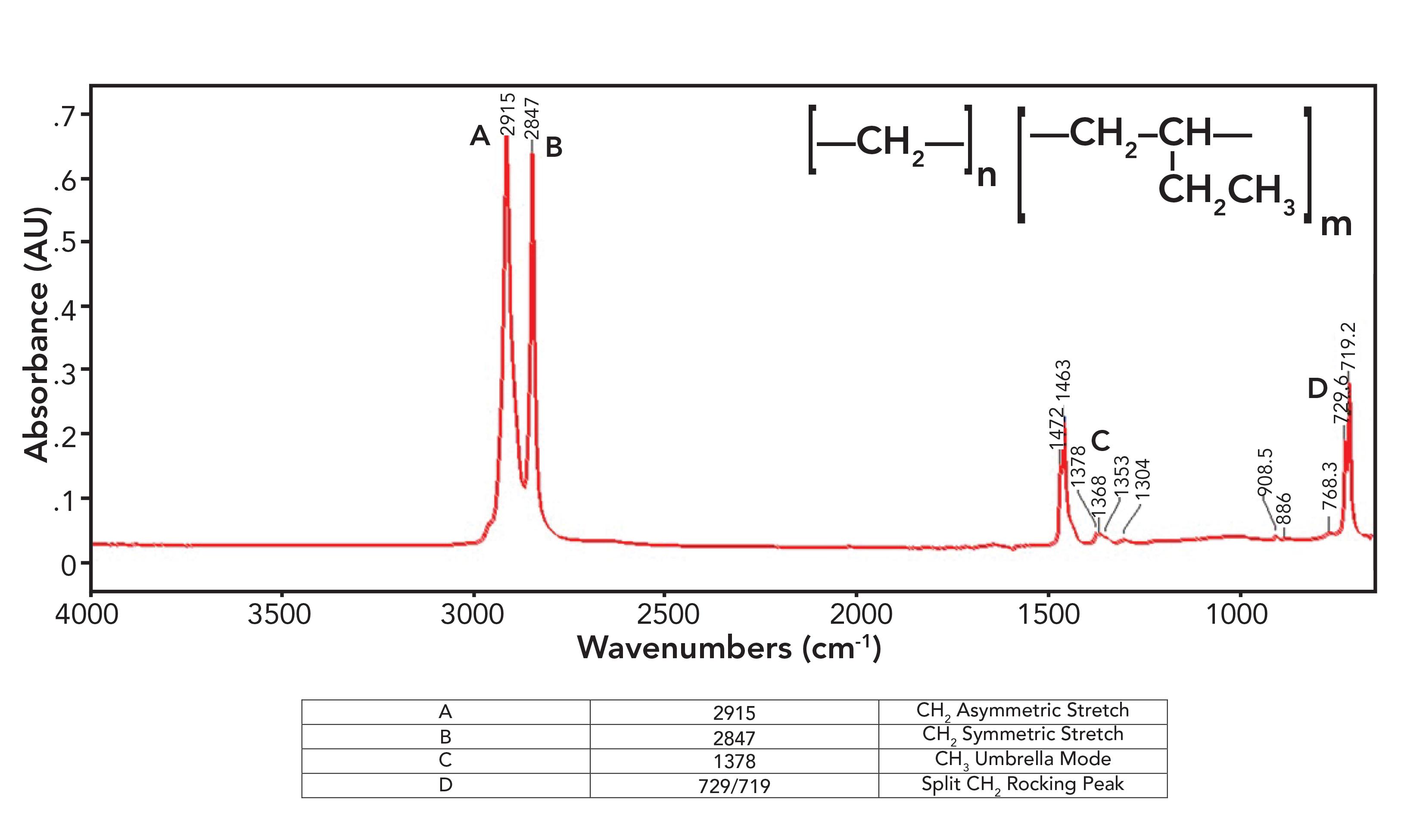
Figure 6 violates everything I just said in the last section about polyethylene spectra as LLDPE has a small methyl umbrella mode peak at 1378 from side chains, which is seen in the spectrum of LDPE, and split peaks at 1472 and 1463 and 729 and 719 which are seen in the spectrum of HDPE. How can a polyethylene (PE) sample be both high-density and low-density at the same time? That is because LLDPE is made with side chains, but they are all short ethyl groups compared to the typical hexyl group side chains in LDPE. These ethyl groups contain CH 3 , hence the presence of the CH 3 umbrella mode peak, but the chains are short enough, allowing them to orient in a parallel fashion to form crystalline regions, hence the split peaks. The spectra of LDPE, HDPE, and LLDPE show that rather than being all one thing or all another, PE comes in a spectrum of forms, and IR spectroscopy can help distinguish between them.
The Secret Life of CH 2 Rocking Vibrations
As we have seen the length of side chains in PE can be customized. How, then, would we determine how long the side chains in a sample are? IR spectroscopy comes to the rescue yet again.
In a previous column (3), I mentioned that the CH 2 rocking mode falls at 720±10, and that this peak only appears if there are four or more methylenes in a row as indicated in Table III. Well, what about alkyl chains with 1, 2, or 3 methylenes in a row? How do we figure out if they are present?
The rest of the story is that ethyl, propyl, and butyl chains have unique CH 2 rocking peaks as seen in Table IV.
Note that an ethyl group with just one CH 2 has a high wavenumber CH 2 rock around 780, that a propyl group with a -CH 2 CH 2 -CH 3 moiety exhibits this peak around 740, and in the spectra of butyl or -CH 2 CH 2 CH 2 -CH 3 groups, this peak appears around 730. Our classic four-or-more-methylenes-in-a-row CH 2 peaks typically appear from 725 to 720. As a result, Table IV can be used to determine the length of short alkyl chains. These CH 2 rocking peaks are illustrated in Figure 7, which shows the spectra of a tertiary butyl group with chains of 1, 2, and 3 methylenes attached.
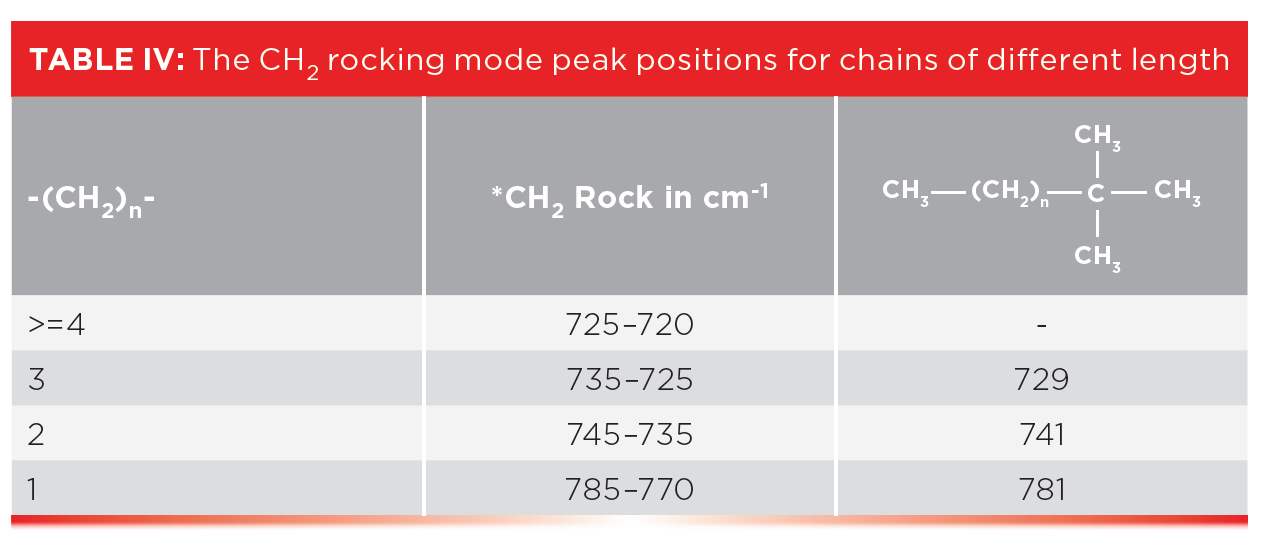
The CH 2 rock in the spectrum of the ethyl group labeled A in Figure 7 falls at 781, for the propyl group labeled B at 741, and for the butyl group labeled C it falls at 729. This information is summarized in the right most column of Table IV, and all these peaks are consistent with the ranges stated in the table.
So, if Table IV is so useful, why didn’t I discuss it back in 2015 (3)? That is because the CH 2 rock is an inherently weak peak (6). For longer chains with many CH 2 groups, it has measurable intensity, but in a molecule with only one short chain, the peaks listed in Table IV can be hard to see. However, in custom-made polyethylene materials where the length of side chain is controlled, we have many side chains of the same length hence these CH 2 rocking peaks may be visible. Table IV then can be used to determine the length of side chains in polyethylene polymers.
We reviewed the spectra of CH 3 and CH 2 stretching peaks and applied them to the spectra of HDPE and LDPE, where we learned how crystallinity causes some HDPE peaks to be split compared to LDPE. We also found that there are many forms of PE with custom length side chains, and that CH 2 rocks can sometimes be used to distinguish these from each other.
- B.C. Smith, Spectroscopy 36 (7), 17–22 (2021).
- B.C. Smith, Spectroscopy 3 0(4), 18–23 (2015).
- B.C. Smith, Spectroscopy 30 (7), 26–31, 48 (2015).
- Crystal - Wikipedia.
- B.C. Smith, Spectroscopy, 30 (1), (2015).
- B.C. Smith, Infrared Spectral Interpretation: A Systematic Approach (CRC Press, Boca Raton, Florida, 1999).
Best of the Week: Recapping Analytica 2024
Here are the top five articles that the editors of Spectroscopy published this week.

Combining Spectroscopic and Chromatographic Techniques
An interview with Charles Wilkins, the winner of the 2013 American Chemical Society Division of Analytical Chemistry Award in Chemical Instrumentation, sponsored by the Dow Chemical Company.

Measurement of Ammonia Leakage by TDLAS in Mid-Infrared Combined with an EMD-SG Filter Method
In this article, tunable diode laser absorption spectroscopy (TDLAS) is used to measure ammonia leakage, where a new denoising method combining empirical mode decomposition with the Savitzky-Golay smoothing algorithm (EMD-SG) is proposed to improve the signal-to-noise ratio (SNR) of absorbance signals.

Bioprocess Monitoring with Ultrasound-Enhanced ATR Mid-IR Spectroscopy
Bernhard Lendl and Cosima Koch of the Vienna University of Technology have developed a new method for on-line monitoring of fermentations using mid-infrared spectroscopy.

Reflectance Spectroscopy Tested for Identifying Fungi Species
Scientists in Poland recently tested how multidimensional discriminant analysis and reflectance spectroscopy can help identify species within fungi strains.

How Hyperspectral Imaging Can Evaluate Asian Soybean Rust Severity
A study from Brazil explored how to use hyperspectral imaging and machine learning to determine the severity of Asian soybean rust.
2 Commerce Drive Cranbury, NJ 08512
609-716-7777


IMAGES
VIDEO
COMMENTS
The infrared absorption spectra of the PSNP spheres are shown in Fig. S1B and Table S2. Polystyrene had only two saturated C -H stretching peaks at 2924 and 2851 cm − 1 , while the unsaturated C ...
The structure, spectrum, and peak assignments for polystyrene are seen in Figure 2. FIGURE 2: The chemical structure, infrared (IR) spectrum, and peak assignments for polystyrene. Note that the structure of polystyrene contains a CH 2 and a mono-substituted benzene ring pendant group. Recall that saturated C-H stretches fall below 3000 as we ...
Peaks at 3060 and 3026 cm −1 , along with other peaks at 1600, 1492, and 1452 cm −1 that confirm the presence of benzene rings in the molecule, were also identified in the study of [63] for PS
FIGURE 1: The chemical structure of polyethylene. Polyethylene is named by appending "poly-" to the name of the monomer "ethylene.". The repeat unit in polyethylene is the CH 2 or methylene group. Note from Figure 1 that the chemical structure of a polymer is drawn with brackets around the repeat unit and a subscript n.
Chapter 16. Polystyrene is readily soluble in a number of common solvents and can easily be cast as a film by pouring the polymer solution onto a glass plate and letting the solvent evaporate. Polystyrene films give a very good IR spectrum, which is often used as a standard. Polystyrene foam, toluene or acetone are used in the experiment.
Go To: Top, Infrared Spectrum, References. Data from NIST Standard Reference Database 69: NIST Chemistry WebBook; The National Institute of Standards and Technology (NIST) uses its best efforts to deliver a high quality copy of the Database and to verify that the data contained therein have been selected on the basis of sound scientific judgment.
FTIR spectr um of PS and SP composites shown in Fig.1, the polystyrene spectrum shows the following peaks: The peaks at range (3082 -2850 cm -1 ) are due to stretching of C-H aliphatic and ...
Although a polystyrene film is useful for routine checking of FT-IR spectrometer performance, variations in film thickness and scattering can cause large variations in the results for the resolution tests described in pharmacopoeias. The test using the peak at 2850 cm -1 is insensitive to resolution but extremely sensitive to film thickness.
The infrared spectrum of polystyrene has been obtained in the region of 70 cm. −1 to 3200 cm. −1. These data, plus Raman scattering data from the literature, are used to make a complete assignment of the normal modes of the polystyrene molecule. ... Assignments of the CH 2, CH, and skeletal modes are made on the basis of previous studies of ...
A new idea to understand the macromolecular motion occurring along the thermal relaxations of polystyrene (PS) and PS-barium titanate composites is proposed. Detailed analysis of PS infrared bands provides a better knowledge of the factors affecting polymer dynamics. Average spectral positions and integrated
IR spectra contains peaks at frequencies where it represent transitions between quantized vibrational energy states, which corresponds to a particular ... (PP) and polystyrene (PS). LDPE sample was taken from a milk container, HDPE sample was taken from 6 pack ring holder, PP sample was taken from CD case and PS sample was taken from coil ...
Turn Knob counter-clockwise = raise Tower. Turn Knob clockwise = lower Tower. Inspect the Pressure Tip by moving. Tower Arm to Cleaning Position. Move Tower Arm to the right until it stops. Clean the Pressure Tip. (remove if necessary) with appropriate solvent. Recommend Water and IPA.
An infrared spectroscopy correlation table (or table of infrared absorption frequencies) is a list of absorption peaks and frequencies, typically reported in wavenumber, for common types of molecular bonds and functional groups. In physical and analytical chemistry, infrared spectroscopy (IR spectroscopy) is a technique used to identify chemical compounds based on the way infrared radiation is ...
Alcohols. Alcohols have characteristic IR absorptions associated with both the O-H and the C-O stretching vibrations. When run as a thin liquid film, or "neat", the O-H stretch of alcohols appears in the region 3500-3200 cm-1 and is a very intense, broad band. The C-O stretch shows up in the region 1260-1050 cm-1.
The 1 H NMR of polystyrene-co-poly(styrene sulfonic acid) (PS-co-PSSA) in dimethyl sulfoxide ... The peak at 1175 cm −1 was also not observed in our NaP4SS FT-IR spectrum, instead a peak at 1185 ... Peak assignments of PtBSS were made based on these HMBC and HMQC 2D NMR spectra.
A detailed careful analysis of the infrared resonance (IR) spectra of polystyrene (PSt), polymethyl methacrylate (PMMA), polyacrylonitrile (PAN) and their co-mixtures were performed. Through this study the absorption peak area to weight ratios as well as working curves were obtained to test for their reliability as well as their suitability.
The characteristic peaks for NaPSS and Pt BSS in FT-IR and NMR spectra were identified. Introduction. Asymmetric ... however, NMR studies of sulfonated PS (sPS) are rare [15], [16]. The 1 H NMR of polystyrene-co-poly(styrene ... and the assignment of resonance in 1 H and 13 C NMR spectra of PtBS was given [17]. The FT-IR technique has also been ...
7.1 Polystyrene The Polystyrene spectrum is acceptable if the following four peaks are within a ±4 cm-1 window of the expected wavenumber. If values lie outside the specified range, align the bench/accessory and re-analyze the polystyrene. If the results are still poor or failing, contact appropriate instrument support personnel. Expected (cm-1
In all the blends, characteristic peaks of PANI due to the quinoid and benzenoid absorption appear approximately at 1414-1453 and 1595-1680 cm −1 , respectively. ... View in full-text Context 4
Esters are characterized by C=O, C-C-O, and O-C-C linkages, and are divided into the families of saturated or aromatic esters depending upon the nature of the carbon attached to the carbonyl carbon. Ester infrared (IR) spectra follow the Rule of Three (1). This means they have three big peaks at approximately 1700, 1200, and 1100 cm -1 (going ...
The peak area method is another one often used. In IR spectroscopy a quantitative peak is sometimes interfered by an adjacent peak, for instance, the peak at 967 cm −1 in SBR and SBS spectra was interfered by the one at 995 cm −1. The contribution of the interference peak to the area must be subtracted, which is a rather tedious and ...
The scaled vibrational frequencies of styrene monomer are presented in Fig. 1, where two main vibration regions (0-1700 cm −1, 3000-3200 cm −1) could be observed.The FT-IR spectra of styrene monomer and polystyrene resin bead were measured and are shown in Fig. 2.A large absorption band located at 3200-3800 cm −1 could be observed in Fig. 2 (b), which is probably attributed to the ...
Figure 1 shows the details of the HDPE spectra. Figure 1: The infrared spectrum of high-density polyethylene (HDPE). What was not discussed in the previous column (1) was why the CH 2 rocking peak in HDPE is split, with peaks at 730 and 720 rather having one expected peak at 720±10. To understand this splitting, we need to first examine the ...