Research & Innovation Office
Institutional Animal Care and Use Committee
- Regulatory Charge
- Governing Principles
- Regulatory Documents
- Institutional Official
- Committee Membership
- Member Sign In
- Submit & Maintain Protocols
- Criteria of Review
- Help Submitting Protocols
- Finding Models & Alternatives
- Research with Client-Owned Animals
- Research Involving Surgery
- Responding to Committee Stipulations
- Requesting Changes
- Inspections
- Animal Use Guidelines & Exceptions
- Mandatory Training
- Optional Training
- Recordkeeping
- Ethics of Animal Use in Research
Jump to:

Laws & Regulations
Reporting concerns, other guidelines.
- Pain & Distress
The use of animals in research, teaching and testing is a controversial ethical and political issue. Much of the discussion about this issue revolves around the relative value, often referred to as 'moral value', of humans and animals. When the needs of animals and humans come into conflict, which takes precedence? Today there exists a wide spectrum of views on this subject, ranging from those concerned with animal 'rights' to those who view animals only as a resource to be exploited. All of these viewpoints have contributed to the development of ethical principles of animal use. These in turn have shaped animal use regulations promulgated by the USDA and the Public Health Service, and reinforced by organizations such as AAALAC , AALAS and the AVMA .
Current legislation also recognizes that there are diverse viewpoints about the moral value of animals. Thus, all live animal use in research, teaching or testing must be reviewed by a committee (the IACUC) with diverse membership. This evaluation includes an emphasis on minimizing the overall use of animals.
Proposals for animal use are reviewed based on the potential for learning new information, or for teaching skills or concepts that cannot be obtained using an alternative. There are provisions for ensuring that animal use is performed in as humane a manner as possible, minimizing pain, distress or discomfort. These provisions include a requirement for a veterinarian to be employed at each institution, so that the needs of the animals are looked after by someone trained in, and sympathetic toward animals' needs. It is also required that all personnel with animal contact be trained in appropriate handling techniques and that they be skilled in any experimental procedures that will be performed. Finally, basic husbandry requirements are specified, ensuring that an animal's food, water and shelter will be provided for in an optimal manner. Deviations from the numerous requirements are granted by the IACUC only if adequate scientific justification is given that the proposed experiment is scientifically and socially important, and that any methods to alleviate pain or distress would frustrate the experimental objectives.
Animals have been used throughout history for anatomical and physiological research as well as for testing new medications and toxic substances. Many medical advances, including vaccines for polio and rabies, the development of certain antibiotics, cancer treating agents and transplant medicines, have been developed thanks to the use of animals in research.
The use of animals in research is a privilege and not a right. A research institution that receives money and support from the public is responsible for conducting research humanely and responsibly according to the limits set by society and regulatory bodies.
Animal Welfare Act
The Animal Welfare Act (AWA) was passed in 1966. This act licenses dealers, exhibitors and breeders of animals, regulates research facilities that use animals, sets standards for the humane care and treatment of animals, and regulates the transportation of animals. The Act has been amended multiple times adding further protections for animals covered by the Act. The AWA specifically exempts birds, mice, rats, amphibians and reptiles used in research as well as agricultural animals that are used for agricultural production.
The United States Department of Agriculture is the government agency that is responsible for the enforcement of this act. Facilities must submit an annual report to the USDA. The USDA conducts unannounced inspections of research facilities at least once a year. If violations of the Act are found, fines can be imposed or research activities can be stopped.
Public Health Service Policy
The Public Health Service (PHS) Policy on the Humane Care and Use of Laboratory Animals is based on the United States Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research and Training. This policy covers all research that is funded by the National Institutes of Health (NIH) using vertebrate species of animals including birds, mice, and rats.
Institutions covered by this policy must follow the Guide for the Care and Use of Laboratory Animals (see below) and annually submit a written document called an Animal Welfare Assurance to NIH, which documents how the institution is complying with all the regulations covering animals used in research. The Office of Laboratory Animal Welfare (OLAW) at NIH is the agency that is responsible for enforcement of the PHS policy.
Guide for the Care and Use of Laboratory Animals
The Guide for the Care and Use of Laboratory Animals ("The Guide"), first developed in 1963, is a manual for research facilities receiving public funding for research using animals. The latest (2011) version of the Guide , sets specific standards for the care and use of laboratory animals. It addresses institutional responsibilities, husbandry and housing standards, veterinary care and physical plant specifications. It is written by experts in laboratory animal care and is published by the National Research Council.
AAALAC stands for the Association for the Assessment and Accreditation of Laboratory Animal Care. This is an independent (non-government) and voluntary accreditation organization. AAALAC accredits laboratory animal facilities through a process of intensive inspections (every 3 years) and reports (yearly). AAALAC follows the high standards put forth in the Guide. Accreditation, while voluntary, represents commitment to excellence in animal care and is an important factor to many funding agencies.
University of Minnesota Policy
The Regents Policy on Animal Care and Use addresses the use of all animals in research, teaching or display at the University of Minnesota. This policy follows from the federal and other laws and regulations. It addresses the roles and responsibilities of the Institutional Official, the Institutional Animal Care and Use Committee (IACUC), Research Animal Resources, and the University Community.
The Institutional Official (IO) is appointed by the University President and reports directly to him/her as well as to the federal authorities regarding compliance with all laws and regulations governing the use of laboratory animals in research and teaching. The President has formally delegated responsibility to appoint IACUC members to the Institutional Official.
The IACUC, which is a committee mandated by the AWA and the PHS policy, reviews and approves all activities involving animals at the University of Minnesota. The AWA and PHS policy have specific membership requirements for the committee. There must be at least:
- one veterinarian (with laboratory animal background and programmatic responsibility at the institution),
- one member of the community (non-affiliated member to represent the public interest),
- one scientist who uses animals in research, and
- one non-scientist member.
University policy states that the committee should have at least 5 members, but the committee has many members, including several student members and ex-officio representatives from Occupational Health & Safety and the Department of Environmental Health and Safety.
The committee reviews all animal care and use protocols to ensure:
- that the use of animals is necessary to achieve the stated objectives,
- that pain and distress is minimized, and
- that all the laws and policies for the use of animals are followed.
The committee also ensures the humane care of animals through the inspection of animal housing and use facilities twice a year and by investigating any complaints made regarding animal use. The committee is also responsible for reporting any instances of non-compliance and recommending corrective action.
Research Animal Resources (RAR) is designated by University policy as the program that provides the housing and husbandry as well as the veterinary care for the laboratory animals on the Twin Cities campus. They are also designated to serve as a consultation resource for the care and use of animals in research and teaching.
University policy also lists the roles and responsibilities of the University community. The University researchers and staff are to be appropriately trained and qualified to conduct activities with animals and are to abide by the decisions of the University and the IACUC.
For serious questions or concerns about animal welfare, the process of review, or about committee decisions, contact:
Shashank Priya Institutional Official (612) 624-5054 [email protected]
Joanne Billings Deputy Institutional Official (612) 624-0999 [email protected]
You may also report animal welfare concerns or policy violations via the University of Minnesota's reporting system. The UReport provides a way for University community members to report violations of rules, regulations and policies. The report can be made anonymously.
Note that, by federal law, no facility employee, Committee member, or laboratory personnel shall be discriminated against or be subject to any reprisal for reporting violations of any regulation or standards.
Agricultural
The Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching is a text published by the Federation of Animal Sciences Societies. This Guide addresses standards for agricultural animal husbandry, housing and veterinary care. It does not apply to agricultural animals used for biomedical type research or teaching.
The standards are slightly different than those listed in the Guide for the Care and Use of Laboratory Animals. For example, cage space requirements may differ slightly between the two texts. Although this text is not regulatory, the University uses its provisions and principles as the basis for its care and use programs involving animals used for production or agricultural research.
There are several references available for the use of fish , amphibians and mammals in wildlife research . Again, these documents are not regulatory documents but are excellent references for the care and handling of these animals.
Pain & Distress
The AWA defines a painful procedure in an animal as: "any procedure that would reasonably be expected to cause more than slight or momentary pain or distress in a human being to which that procedure was applied, that is, pain in excess of that caused by injections or other minor procedures." Pain can be acute, short lived - or chronic, lasting a long time. The signs manifesting acute or chronic pain may differ and may be different in different species. Prey species of animals can be adept at hiding signs of pain or illness and may be more difficult to assess discomfort.
Signs of Acute Pain in Animals
- vocalization
- attempts to escape the stimulus
- aggressive responses
- increased heart and respiratory rates
- anorexia or shaking
Signs of Chronic Pain
- weight loss
- poor or unkempt hair coats
- depression or lethargy
- general debilitation
Distress is currently defined as "a state in which an animal cannot escape from or adapt to the external or internal stressors or conditions it experiences resulting in negative effects upon its well being…" Distress differs from stress, which is a physiological reaction that can lead to an adaptive response.
Principle IV of the US Government Principles states that unless the contrary is established, the assumption must be made that a procedure that causes pain or distress in a human being will cause pain and distress in an animal.
Alternatives
Current regulations stress the need to search for and utilize alternatives to procedures on animals that cause more than momentary pain or distress. The concept of the three "R"s has been used when thinking about alternatives to animal use. This concept was developed in 1959 by Russell and Burch in their book: The Principles of Humane Animal Experimental Techniques.
The three "R"s are Replacement, Reduction, and Refinement. Investigators at the University of Minnesota, who use animals that may undergo more than momentary pain or distress, should consider the three “R”s when conducting procedures which may be painful or distressful.
Replacement of animals with other systems may be an option. Computer modeling or in vitro testing may be a substitute for animal models. "Lower" or non-vertebrate animals, such as the fruit fly may be used in some situations rather than a higher order animal.
Reduction of the number of animals used for research is also an important concept. This is done mostly through experimental design and the use of statistics. The use of too few animals could result in statistically invalid results, which could necessitate the use of even more animals in subsequent experiments. Pilot studies to help determine statistical parameters can sometimes assist in determination of group sizes. Reduction of pain and distress may also actually require the use of more animals so that repeated procedures are not conducted on the same animal.
Refinement refers to methods that decrease the amount of pain and distress experienced by the animals that are actually needed to perform an experiment. This is not only done through the use of pain relieving measures such as anesthetics and analgesics whenever possible, but also through environmental enrichment.
The use of early endpoints can also be a form of refinement. For instance if an animal were to suffer from an early indicator of disease or a tumor reaches a certain measurable size, this could be used as the endpoint. The animals should be humanely euthanized at this point rather than waiting until the death of the animal.
For more examples of replacement/reduction/refinement and searches for alternatives, see IACUC's web page, “Finding Models and Alternatives”.
The Emergence and Development of Animal Research Ethics: A Review with a Focus on Nonhuman Primates
- Original Research/Scholarship
- Open access
- Published: 29 April 2020
- Volume 26 , pages 2277–2293, ( 2020 )
Cite this article
You have full access to this open access article

- Gardar Arnason ORCID: orcid.org/0000-0002-9667-7096 1
9201 Accesses
15 Citations
42 Altmetric
Explore all metrics
The ethics of using nonhuman animals in biomedical research is usually seen as a subfield of animal ethics. In recent years, however, the ethics of animal research has increasingly become a subfield within research ethics under the term “animal research ethics”. Consequently, ethical issues have become prominent that are familiar in the context of human research ethics, such as autonomy or self-determination, harms and benefits, justice, and vulnerability. After a brief overview of the development of the field and a discussion of relevant theoretical ethical frameworks, I consider two of these issues, namely autonomy and self-determination on the one hand, and harms and benefits on the other hand. My concern is with philosophical and ethical issues, rather than animal research oversight. I focus my discussion on nonhuman primates, as the most plausible nonhuman candidates for this approach. I conclude that the approach, although promising, depends strongly on the moral status of nonhuman research subjects.
Similar content being viewed by others

Research: Animals

Ethics and Evidence Regarding Animal Subjects Research: Splitting Hares–or Swallowing Camels?
Avoid common mistakes on your manuscript.
Introduction
Within the field of philosophical ethics, the ethics of using nonhuman animals in biomedical research is usually seen as a subfield of animal ethics. In that context the central problem is the general justifiability of nonhuman animal research. In contrast to the majority of public views and public policies, most major works in animal ethics have been strongly opposed to the current use of nonhuman animals for research, varying from arguing for restrictive positions to advocating abolition. Footnote 1 In recent years, however, problems in the ethics of animal research have increasingly been discussed within a framework similar to human research ethics, sometimes under the term “animal research ethics” (Gilbert 2012 ; Beauchamp et al. 2014 ; DeGrazia and Beauchamp 2015 ).
When the ethics of animal research is placed in the context of research ethics, questions and problems become prominent that are familiar in the context of human research ethics but often less so in the context of animal ethics; and those problems that are prominent in animal ethics, such as animal harm, are to some extent approached differently. Four of the main issues that come to the fore in animal research ethics are autonomy or self-determination, harms and benefits, justice, and vulnerability (Ferdowsian and Choe 2013 ). When considering these four issues, it may seem that autonomy is an issue that is central to human research ethics but not applicable to animal research ethics, while the issue of harms and benefits is already a central issue in animal ethics, and hence not novel in the context of animal research ethics. I will therefore pay particular attention to these two issues.
The question of the general justifiability of animal research barely matters in animal research ethics, but that does not mean that animal research ethics supports the status quo in animal research or even that it is more permissive than most animal rights positions. On the contrary, the growing literature in animal research ethics tends to be very critical of current practices in nonhuman animal research, but its concerns are much wider and more differentiated than merely the question whether nonhuman animal research can be justified or must be abolished.
This is not to say that animal research ethics, in the sense described here, is fully independent of animal ethics. Many of the concerns that give rise to discussions about animal self-determination and agency, harms and benefits, justice, and vulnerability, are motivated by and seek support from classical works in animal ethics. Scientific research on animal cognition and behavior has also influenced and driven debates both within animal ethics about the justification of using animals in biomedical research and discussions in animal research ethics about specific problems and principles in animal research. The two fields are closely connected.
The extent to which human research ethics concepts and concerns apply to nonhuman animals is closely related to the animals’ moral status. At the extremes, if sentient nonhuman animals (animals that can feel pain) have the same moral status as paradigmatic humans, namely personhood or full moral status, the concepts and concerns of human research ethics will apply to them in the same way as to humans; if sentient nonhuman animals have no moral status and do not matter morally at all, neither the concepts nor concerns of human research ethics will apply to them. If nonhuman animals are considered to have a moral status, but lower than humans, then the consequences for the applicability of both concepts and concerns need to be worked out. By moral status I mean the extent to which something matters morally for its own sake. There is no agreement on what features determine moral status, but according to many accounts moral status depends significantly on cognitive capacities and sentience (Jaworska and Tannenbaum 2018 ). Nonhuman primates, and in particular nonhuman great apes, are in evolutionary terms our closest relatives. They have the highest cognitive capacities of all nonhuman animals and therefore, presumably, a greater capacity for suffering. Some species of nonhuman primates may even have some features of personhood. Footnote 2 If the concerns of human research ethics apply to nonhuman animals at all, they will apply to the greatest extent to nonhuman primates. This makes nonhuman primates an interesting test-case for animal research ethics.
As animal research ethics establishes itself as a subfield of research ethics and the literature on various topics in animal research ethics grows, it is useful to reflect on the development of the field and what it means for the ethics of animal research to be placed in the context of research ethics. In what follows I will attempt to describe the current state of animal research ethics with a focus on nonhuman primates as subjects of biomedical research. After a brief overview of the development of the field and a discussion of relevant theoretical ethical frameworks, I consider two of the main issues that, perhaps unexpectedly, set animal research ethics apart from the traditional animal ethics approach, namely agency and self-determination on the one hand, and harms and benefits on the other hand.
My aim here is to review and reflect on the discussion of the ethics of animal research within the field of research ethics, with nonhuman primates as my main example and a focus on the two issues of agency/self-determination, and harms and benefits. I make three central claims regarding animal research ethics: (1) the ethics of animal research is increasingly being discussed in the context of research ethics; (2) this has led to a richer and more fruitful discourse on animal research, as is exemplified by the issues of agency/self-determination and benefits/harms; and (3) the moral status of nonhuman animals is fundamental to the applicability of the research ethics framework to the ethics of animal research. I hope to advance the discussion of the use of nonhuman sentient animals, in particular nonhuman primates, in biomedical research, by providing an overview of how the debate is increasingly taking place within the framework of research ethics, and what the assumptions and implications of using that framework are. My aim is not to argue for or against the use of animals in research, but I do assume that some animal research can be justified, at the very least if it is conducted under the same ethical restrictions as research on humans. My focus is on philosophical issues in animal research ethics, but I am aware that the term “animal research ethics” is also used to refer to animal research oversight and regulatory frameworks, including laws and regulations, professional codes, ethical guidelines, and institutional rules and procedures. A parallel division exists in the field of human research ethics.
The Emergence of Animal Research Ethics
In his contribution to The Routledge Companion to Bioethics , Tom L. Beauchamp ( 2014 , p. 262) calls animal research ethics “a recently coined term”. It is, indeed, only in the last decade, that animal research has been discussed extensively within the framework of philosophical research ethics, but the term “animal research ethics” goes back at least to the 1980s. Footnote 3 Jerrold Tannenbaum and Andrew N. Rowan conclude in an article published in 1985 that “animal research ethics is still at a rudimentary state of development” (Tannenbaum and Rowan 1985 , p. 42) and compare it to the state of human research ethics in 1966 when Henry Beecher published his influential article on the ethics of biomedical research, revealing 22 examples of research protocols where human subjects were treated unethically (Beecher 1966 ). I note that the lesson drawn from Beecher’s terrifying list of human rights abuses in biomedical research was not that human biomedical research should be abolished, but that the conditions for justifiable research had to be clarified within a better research ethics. Similarly, examples of animal abuse in biomedical research should not lead to calls to abolish animal research, but to improvements in animal research ethics, in regulation and practice. Discussing the use of animals in biomedical research within the context of research ethics signifies exactly such a change of emphasis. It moves the focus of the discussion away from the question of the justifiability of animal research and places a stronger emphasis on the ethical frameworks within which it should be regulated and practiced.
Like Tannenbaum and Rowan before them, Hope R. Ferdowsian and John P. Gluck also discuss Beecher’s work in a recent article and claim that “that there are significant parallels between Beecher’s observations about human research in 1966 and contemporary problems with animal research” (Ferdowsian and Gluck 2015 , p. 391). One might get the impression that not much has happened in animal research ethics during the almost 30 years between the publication dates of these two articles, except that examples of ethically questionable animal research keep accumulating. That impression is not accurate. Since the 1980s, animal research proposals must increasingly be approved by animal ethics committees, which may be institutional, regional or national. In the U.S., federally funded animal research has to be reviewed by an institutional animal care and use committee (IACUC). It is parallel to institutional review boards (IRBs) in human research. In Europe, recent legislation on animal research, Directive 2010/63/EU on the protection of animals used for scientific purposes, stipulates that animal research must receive ethical approval by a competent authority, meeting a specific list of ethical and scientific requirements (European Parliament and the Council of the European Union 2010 ). The Directive also puts the use of nonhuman primates into the spotlight. The former Directive it replaces had only one paragraph mentioning nonhuman primates (European Parliament and the Council of the European Union 1986 ), but the current Directive refers 48 times to them, including three preambles devoted solely to nonhuman primates. The 3R framework proposed by Russell and Burch in 1959 is increasingly being applied, requiring scientists to replace animals with alternative methods (or lower species) if possible, to reduce the number of animals to the minimum required for statistically valid results, and to refine the use of animals by minimizing their pain and suffering as well as improving husbandry, housing, and welfare (Russell and Burch 1959 ). All three requirements can be recognized in Directive 2010/63/EU and corresponding national legislation in Europe. The directive also prohibits the use of great apes except for very specific circumstances, but great apes have not been used for research in Europe since 1999 (UK Home Office 2014 , p. 23). In the United States, the use of chimpanzees is being phased out by the National Institutes of Health, effectively ending the use of great apes in research in the U.S. (Beauchamp et al. 2014 ; Reardon 2015 ). These developments undermine the claim that the situation in animal research is comparable to the one in research involving human beings in the 1960s.
In the academic literature, one possible starting point for the emergence of animal research ethics is the publication in 2006 of a special issue of Theoretical Medicine and Bioethics focusing on animals (DeGrazia 2006 ). This special issue includes two articles that draw a comparison between the use of humans and the use of animals in biomedical research (Walker 2006 ; Pluhar 2006 ). In 2012 the Hastings Center published a Special Report entitled “Animal Research Ethics: Evolving Views and Practices” (Gilbert et al. 2012 ). The report includes a section about chimpanzee research in the United States, but it does not explicitly place the issues in the context of research ethics. The report was followed by another special issue of Theoretical Medicine and Bioethics in 2014, which is primarily concerned with chimpanzee research in the United States (Beauchamp et al. 2014 ). Here questions about autonomy, consent, vulnerability, and harm are directly addressed. In 2015 Cambridge Quarterly of Healthcare Ethics published a special section entitled “Moving Forward in Animal Research Ethics” (DeGrazia and Beauchamp 2015 ) and a year earlier the same journal published a special section on “Neuroethics and Animals” (Buller et al. 2014 ). Both special sections contain articles that discuss the question of autonomy of research animals in terms of the possibility to assent or dissent (Fenton 2014 ; Kantin and Wendler 2015 ). The impetus for this discussion about animal autonomy came from a US Institute of Medicine report on the use of chimpanzees for biomedical and behavioral research, released in 2011, which led to the discontinuation of chimpanzee research in the U.S. (IOM 2011 ). I will consider the issue of autonomy in greater detail below.
Although the literature on animal research in the context of research ethics has been growing rapidly, it does not mean that animal research is no longer discussed in the context of animal ethics. Scholarly articles and books discussing animal research in the familiar animal ethics context, mostly calling for the abolition of animal research (e.g., Herrmann and Jayne 2019 ), continue to be published. Those who have published on animal research in the research ethics context, or those who use the human research ethics framework to some extent when discussing animal research, may not consider themselves moving away from animal ethics. It is nonetheless possible to discern a growing interest in the framework of human research ethics, which has given rise to a body of work large enough and with enough in common to describe it as a distinct field of research, regardless of the intention of the individual authors who have or are contributing to it. One of the main features of animal research ethics, as a subfield of research ethics, is in the ethical approach, with less emphasis on the standard utilitarian (e.g., Singer) or deontological (e.g., Regan) ethical frameworks to an increasing interest in the principlism of Beauchamp and Childress ( 2013 ). Footnote 4 To draw out this difference, let me take a closer look at the ethical frameworks that are usually applied to the issue of animal research.
Ethical Frameworks for Animal Research Ethics
An overview of the main ethical positions concerning the use of nonhuman animals in research reveals a variety of conflicting positions even within the same general theoretical approach. A characteristic trait of medical ethics can also be observed in animal ethics: the limited direct benefit of the “classical” moral theories. No general theoretical approach in ethics can be applied directly and conclusively to determine the moral status of animals, the justifiability of animal research, nor what sort of animal research ethics is appropriate. That is, defendable positions for abolishing animal research, for restricting animal research, and for the current practice, can and have been developed within each of the general theoretical approaches. In this section I will briefly consider some of the main approaches to animal ethics and what they mean for animal research ethics.
Utilitarian arguments have been applied to support a near abolitionist position about animal research in general (Singer 1975 ), but also positions in favor of animal research generally (Brody 2012 ; Cohen 1990 ; Frey 1997 ) and a position in favor of nonhuman primate research specifically (Weatherall 2006 ). The best known utilitarian position in animal ethics is Peter Singer’s preference utilitarianism as developed in his books Animal Liberation (Singer 1975 ) and Practical Ethics (Singer 1979 ). According to that position, we should do what best satisfies our well-considered preferences or objective interests, which in the case of animals amounts to matters of pleasure and pain. Singer also emphasizes the importance of the principle of “equal consideration of interests”. Singer’s position does not rule out animal research, provided its utility (in terms of satisfying interests) is greater than the harm caused. This, Singer claims, is very rarely the case in animal research (Singer 1975 ).
Current regulatory frameworks for animal research are almost exclusively utilitarian in nature. They aim to minimize the suffering of animals and, in Europe at least, the suffering has to be justified by the potential scientific or medical benefits of the research. This is in stark contrast to human research ethics, where deontological concerns dominate. To use a well-known phrase from Robert Nozick, we have “utilitarianism for animals, Kantianism for people” (Nozick 1974 , p. 39).
Although Singer’s utilitarian animal ethics has been very influential, animal ethicists and animal advocates have overwhelmingly relied on deontological approaches, in particular animal rights, when arguing against animal research. The animal rights approach has produced some of the strongest positions against the use of animals in research, for example in Tom Regan’s work (Regan 2004 [1983]) and more recently in the Kantian ethics of Christine Korsgaard ( 2018 ). Some animal rights advocates argue against the use of animals for any human purpose (Francione 2009 ). On the other hand, the classical Kantian deontological approach recognizes only limited, indirect duties towards animals, and some social contract theories put animals squarely outside the moral realm altogether, allowing at most that we have “duties of compassion and humanity” to animals (Rawls 1999 , p. 448). Rather than focusing on rights and duties, some deontological theories are based on the concepts of dignity and justice, such as Nussbaum’s capabilities approach to animal ethics (Nussbaum 2004 ). Nussbaum’s theory would vastly limit human uses of animals, but with the notable exception of allowing the use of animals for biomedical research.
Just as arguments can be made for a wide range of positions on the use of animals for biomedical research within both utilitarian and deontological moral frameworks, virtue ethics provides little guidance in itself. Rosalind Hursthouse ( 2006 ), for example, argues that what we can and cannot do with nonhuman animals depends on the circumstances and our relationship to the animals. The basic moral prescription is to act virtuously, in this case to treat animals with compassion and justice. She assumes that animal research is mostly (but not necessarily always) cruel and useless, and that the just and courageous individual should support actions and organizations that aim to stop cruel and useless animal research. Garret Merriam ( 2012 ) similarly argues that from the standpoint of virtue ethics much of animal research is unjustified, very little of it is clearly justified, and the rest is in between, requiring careful moral judgment. Walker ( 2018 ) has applied virtue ethics specifically to nonhuman primate research, focusing on the virtues and character development of primate researchers and the social and institutional structures involved in the development of the moral character of researchers. These virtue ethics approaches promote moral reflection, the development of moral character of those involved in animal research, and the construction of caring relationships between researchers and their animal subjects, but none of them suggests an absolutist position with regard to the practice of animal research.
Some of the most militant opposition to the use of animals in research is skeptical or even openly hostile to any theoretical basis for its views. This opposition appeals to persons’ empathy with animals and applies a strategy of “exposure and persuasion.” The starting point is that animals are brutally abused and exploited and that bringing animal suffering and injustice into the public eye is the most effective strategy to persuade people to oppose the use of animals in research or in other areas (Aaltola 2011 ). On this view, any theoretical approach just gets in the way or distracts from the political-activist agenda. Organizations supporting the use of animals in research often appeal to the benefits of animal research for children and pets, rather than engage in philosophical debates, but I am not aware of any academic discussion by supporters of animal research about the usefulness of a comparable anti-theoretical position in favor of animal research.
As the discussion of ethical issues in animal research in the context of research ethics has been growing, the animal ethics discussion has independently been moving from applied ethics to political theory in what has been termed “the political turn”. Much of that discussion is concerned with issues of justice and rights, viewing at least some animals as members of our societies and examining the implications of that (Donaldson and Kymlicka 2011 ; Garner and O’Sullivan 2016 ; Cochrane 2018 ). The political turn in animal ethics has significant implications for animal research ethics, in particular if animals are accorded the same basic rights as humans. This would not result in the abolition of animal research, but it would put severe limits on it, requiring animal research to adopt research ethics that are very similar to current human research ethics (Arnason 2017 , 2018a ).
This brief survey of how major ethical frameworks have been applied to animal research suggests that broad moral theories do not provide clear answers or a single approach to the question of the justification of animal research. Rather than looking for general answers from moral theories about whether animal research is generally justifiable, recent work on animal research ethics has considered topics that are closer to human research ethics than animal ethics. This work includes, to give a few examples, Walker ( 2006 ), Pluhar ( 2006 ), and Ferdowsian and Gluck ( 2015 ) comparing the ethics of human and nonhuman research, Fenton ( 2014 ), Beauchamp and Wobber ( 2014 ), and Wendler ( 2014 ) on consent and autonomy of nonhuman primates, Kantin and Wendler ( 2015 ) on assent and dissent in animal research, Johnson and Barnard ( 2014 ) on chimpanzees as vulnerable subjects, Ferdowsian and Fuentes ( 2014 ) and Beauchamp and Morton ( 2015 ) on harms and benefits in human and nonhuman research, and Choe Smith ( 2014 ) on justice in subject selection in animal research. In the following sections, I will focus on two of these topics, autonomy and harm. They are of particular interest for almost opposite reasons: autonomy may seem to be a topic in human research ethics that does not apply in animal research ethics and harm may seem to be a topic that has already been extensively discussed in animal ethics.
Autonomy, Self-Determination, and Agency
Respect for autonomy, or the person, has been one of the cornerstones of research ethics at least since the publication of the Belmont Report (National Commission 1979 ). The principle can be found even earlier, in terms of respecting the voluntariness of the subject, in the publication of the Nuremberg Code in 1949. The very first sentence of the Nuremberg Code states simply: “The voluntary consent of the human subject is absolutely essential” (Shuster 1997 ). It ensures that the potential research subject can protect her own interests, by making an informed and voluntary decision about participating in research. It also ensures respect for autonomy and respect for the person.
It is obvious that nonhuman primates and nonhuman animals generally cannot give informed consent for their participation in research, but it is far from obvious what follows from that fact. One might insist that if a research subject cannot give informed consent, whether the subject is human or not, then she cannot be used in research at all. This position requires a complete abolition of animal research, since no nonhuman animal could be used ethically in research if informed consent is an absolute requirement. When one considers the justification of the requirement for informed consent, this position quickly collapses. If the justification is based on respect for autonomy or respect for persons, then the requirement does not hold in the case of nonhuman animals, since they are neither autonomous nor persons (with the possible exception of great apes), so there is neither autonomy nor personhood to be respected. Footnote 5 If the justification of the requirement for informed consent is to protect the interests of research subjects, then one might simply seek other ways to protect the interests of subjects who are not able to give informed consent. In either case, the requirement for respect of autonomy or the person in the form of informed consent is not only impossible but also irrelevant for nonhuman animal research (Weatherall 2006 , p. 129).
Even for nonhuman primates that cannot be considered autonomous, one might still argue that we should consider some sort of a parallel to respect for autonomy, such as respect for agency or self-determination. There is hardly any doubt that nonhuman primates, and other animals, have volition and preferences, and act on those, although they can reflect neither on their preferences nor the reasons they may have for or against acting on them. In that sense they are agents even if they are not autonomous. Such agency, or self-determination, may require that we take it into our moral consideration. The justification of such a requirement could be that they can protect their own welfare interests, to some extent at least, by avoiding activities that cause them pain or distress. Following the terminology of Kantin and Wendler ( 2015 ), we can term these welfare-based reasons. One might also attempt to justify a principle of respect for animal agency or self-determination by arguing that agency or self-determination is itself morally relevant independently of welfare. These can be termed agency-based reasons. In this case, the nonhuman animal in question would have a morally relevant interest in exercising its capacity of agency or self-determination, as opposed to merely having an interest in avoiding suffering. The difficult theoretical question here is whether agency, or self-determination, has any moral relevance (or even meaning) in the absence of self-consciousness or the ability to reflect on one’s actions, reasons, values, and beliefs, that is, the sort of features that are usually thought to constitute autonomy. I will not argue for an answer to that question, neither affirmative nor negative, but I do suggest that agency or self-determination is morally relevant at least for welfare-based reasons and as such is a parallel in animal research ethics to the principle of respect for autonomy in human research ethics.
If we accept a principle of respect for agency with regard to research on nonhuman animals, the question remains what form the principle would take in practice. Nonhuman animals cannot consent nor even assent, in the sense that children may be able to assent to research when they are not competent to give informed consent (Fenton 2014 , pp. 133–134). Assent requires some grasp of what is going on or going to happen during a specific procedure, which is outside the cognitive capacity even of nonhuman primates (Diekema 2006 , p. S9). Footnote 6 Dissent may be possible; however, since it does not require any information about procedures or actions, it consists simply in a wish to be removed from a painful or stressful situation. The opposite of dissent, in this sense, is acquiescence rather than assent. A principle of respect for agency could then take the form of requiring acquiescence from the nonhuman animal during research and conversely respecting animal dissent (Fenton 2014 ). Such a requirement would clearly put severe limits on nonhuman animal research.
A related but less demanding requirement might consist in seeking voluntary participation from nonhuman animals, in particular nonhuman primates, for research. This is clearly a lower requirement than assent, because no understanding of the procedures and their purpose is required of the research subject. It is similar to acquiescence, but can be considered less demanding as it is induced through training which itself may be considerably more coercive. This sort of voluntariness is increasingly required in some nonhuman primate research, where the primates are trained through positive reinforcement to do such things as offer their arm for injections or the taking of blood samples, entering a primate chair, and performing various actions or solving tasks for rewards. The reasons for respecting voluntariness are in part welfare-based, as applying physical force may cause pain or distress, and in part they are practical, where applying force is not effective. Some common research procedures, for example in primate neuroscience, require the voluntary participation of the primates, since they cannot be physically forced to solve tasks, perform complex actions or make decisions. If they suffer from pain, fear or distress, they might either stop their activities or lose their concentration or interest with the result that the data obtained would be useless. One objection to the practice of seeking voluntariness is that it is mere manipulation and without any moral benefit (Beauchamp and Wobber 2014 ). This may be the case when one considers agency based reasons, but in so far as the practice of seeking voluntariness reduces suffering, it has a moral benefit for welfare-based reasons.
I hope to have shown in this section that although respect for autonomy does not apply to nonhuman primates (except perhaps great apes), animal agency can have moral significance, at the very least for welfare-based reasons, and such concerns can be compared to the principle of respect for autonomy in human research ethics. Although animals cannot give informed consent, they can often communicate their preferences and express their dissent (or acquiescence) to participating in a research procedure. Nonhuman animals are unlikely to be able to assent to anything, with the possible exception of chimpanzees and other great apes. The moral importance of nonhuman dissent depends in the end strongly on the moral status of the dissenting nonhuman animal and in particular on whether we can be ethically justified in sacrificing its welfare for human benefits.
Harms and Benefits
Human research ethics limits in practice the level of harm or risk that human research subjects can be exposed to, even if there are research subjects willing to give informed consent to harmful research. Research ethics codes and regulations generally do not specify any limits to risks or harms to human participants, but rather require that risks are minimized (WMA 2013 , art. 17; CIOMS 2016 , pp. 2, 9–13; US Department of Health and Human Services 2018 , §46.111(a)(1)), that benefits outweigh risks (WMA 2013 , art. 16; CIOMS 2016 , pp. 9–13; US Department of Health and Human Services 2018 , §46.111(a)(2)), and, in the case of the Declaration of Helsinki, that the welfare, rights, and interests of the research subject have priority over scientific or social interests (WMA 2013 , art. 8). The Nuremberg Code explicitly prohibits research that is likely to result in injury, disability or death of the research subject (art. 5 and art. 10) (Shuster 1997 ). In the case of human research subjects, who are not capable of giving informed consent, such as children, a common requirement is that the risk of participating in the research is minimal. “Minimal risk” is often defined as those risks that are comparable to the risks of daily life.
Animal research allows and often requires procedures that result in injury, disability or death of the animals. Our scientific and social interests have, in current practice, a clear priority over the welfare, rights (if applicable at all), and interests of the animals used for research. This difference in human and animal research ethics is only justified if animals have a lower moral status than humans. The issue of moral status is however highly contested; there is no agreement on the moral status of nonhuman animals or what exactly determines it, not even whether the concept is of any use. Two things, however, are uncontroversial. First, some nonhuman animals are capable of experiencing pain. Footnote 7 This includes probably all vertebrates and perhaps some invertebrates (e.g. cephalopods). They have, in virtue of their sentience, morally relevant interests and possibly moral rights, and therefore they have a moral status. In other words, nonhuman animals have interests, in particular welfare interests, that have to be taken into account in our moral judgments. Second, most people intuitively accord animals a lower moral status than humans. For example, most of us would think that one should save a human child from a fire rather than a dog, if only one can be saved and we had to choose between the two. A more mundane example is the fact that most of us use animal products, including in food and clothing, knowing that animals most likely suffer and surely die in their production. This does not mean that we do not care about the death and suffering of animals, but rather that human interests are generally taken to have a priority over animal interests. Most of us would presumably agree that it is wrong to torture an animal for fun, but that we can use animals (and take their lives) as means to our own ends, if that use has reasonable benefits (which does not include sadistic pleasure). I am not arguing that current social norms or attitudes are sufficient to justify the lower moral status of animals, but rather that moral status is a fundamental issue that needs to be further analyzed and debated. This is particularly important in the case of nonhuman primates, since animals with higher cognitive capacities may have stronger welfare interests in virtue of having a stronger sense of self.
In Europe, at least, animal research is, by law, only to be approved if the researchers make a reasonable case that the expected benefits justify the harms caused to the animals. In that respect, animal research ethics overlaps here with human research ethics. The difference is, as noted above, that human research has the constraint that fundamental rights, welfare, and interests of human research subjects cannot be sacrificed for scientific or social benefits. In contrast, animal research lets scientific and social benefits justify setbacks to animal welfare and interests. There are at least two significant concerns here regarding this weighing of harms and benefits. One concern is the claim that in many animal research proposals the expected benefits only justify the suffering to the animals if their interests are massively discounted (for this concern in the context of nonhuman primates see Arnason 2018b and Faria 2018 ). Peter Singer has argued that if we applied a principle of equal consideration of interests, much of our animal research would not be justified (Singer 1975 ).
The second concern is that we cannot assess benefits or harms, whether in research on nonhuman primates or more generally in animal research, in any non-arbitrary way that would allow us to meaningfully weigh one against the other. Footnote 8 We can, however, evaluate the expected benefits and the likely harms and make an informed, moral judgment about whether it is an acceptable trade-off. This approach acknowledges the incommensurability of harms and benefits, as well as the impossibility of accurate, objective, quantifiable measurements, without making the moral judgment trivial or arbitrary. Providing a more detailed argument for this approach is outside the scope of this paper, but this case has been made extensively elsewhere in the context of research on nonhuman primates (Arnason and Clausen 2016 ; Nordgren 2010 ).
At the beginning of this section I noted that human research ethics codes generally do not specify any upper limits of risk or harm, with the exception of the case of research subjects who are not capable of giving informed consent, in particular children. In this case, research is often considered justifiable only if it poses no more than minimal risk and minimal burden to the research subject. If we want to draw on human research ethics in our discussion of animal research ethics, this is the sort of issue that may be seen to apply directly to animals, since they cannot give informed consent either (Wendler 2014 ). Some animal advocates argue indeed that animal research, like nontherapeutic pediatric research, is only justifiable if it poses no more than minimal risk and minimal burden to the research subjects, at least in the case of nonhuman primates (Ferdowsian and Fuentes 2014 ) and great apes (Gagneux et al. 2005 , p. 28). Others, such as Beauchamp and Morton ( 2015 ), have argued for upper-severity limits for animal research in general, excluding all research that causes significant suffering.
In any case, it is intellectually interesting and useful to draw comparisons between the ethical requirements for research involving incompetent humans and research involving animals with regard to upper limits of risk and harm. It is also worth emphasizing, that the justification for having different limits for humans and animals, and more generally treating human and animal interests differently, relies on an argument about the moral status of both humans and animals (Wendler 2014 ; Walker 2016 ). Placing the upper limit of risk and harm in animal research at “minimal risk and minimal harm,” as equal moral status with human would suggest, would surely amount to an abolishment of most animal research, but a higher limit of severe pain or distress over prolonged time, as is the case in EU law, would have considerably less impact on biomedical research (European Parliament and the Council of the European Union 2010 ).
The recent move to address ethical questions in animal research within the framework of human research ethics, rather than within the more traditional framework of animal ethics, has moved the focus away from the basic question of the general, ethical justifiability of animal research and towards specific issues familiar from human research ethics, such as autonomy or agency, and harms and benefits. This move to research ethics is advocated mostly by people who are committed to some sort of an equality principle with regard to the rights or interests of human and nonhuman animals, but not necessarily from a commitment to any particular moral framework. The concern with the two topics of autonomy and harm in animal research ethics is no more and no less tied to any particular moral framework than the concern with the same topics within human research ethics. The concern with autonomy leans towards a deontological framework, the concern with harms leans towards a utilitarian framework. For both topics the Beauchamp and Childress ( 2013 ) principles of biomedical ethics loom large. Still, both topics are regularly discussed within human research ethics without a commitment to any of those moral frameworks.
How far human research ethics applies to animals depends in the end significantly on the moral status accorded to animals. If animals, or some higher mammals such as nonhuman primates, are accorded the same moral status as humans, it would be difficult to avoid the conclusion that human research ethics would apply to them, giving them the same protections as human research subjects who are not competent to give informed consent. This would be the end of much of animal research as it is practiced now. Justifying current levels of risk and harm exposure in animal research depends conversely on animals having a lower moral status than humans. This difference in moral status implies not only unequal consideration of interests, but more importantly that animal welfare and interests cannot give rise to limits based on rights or dignity that trump utilitarian considerations of harms and benefits, as is the case in human research ethics. As fruitful as recent work on animal research ethics has been, its plausibility ultimately requires a defensible account of the moral status of nonhuman animals.
Two obvious examples are Singer‘s ( 1975 ) Animal Liberation and Regan‘s ( 2004 [1983]) The Case for Animal Rights , who argue for the utilitarian and deontological positions, respectively, against animal research. For further elaboration of this contrast between public views and the restrictive views within animal ethics see DeGrazia‘s ( 1999 ) “The ethics of animal research: What are the prospects for agreement?”.
The relationship between cognitive capacities, sentience, personhood, and moral status generally is complicated and the analysis of that relationship is outside the scope of this paper. I merely acknowledge the common assumption that nonhuman primates matter more morally than other nonhuman animals, without having the cognitive capacities necessary for moral personhood. “Personhood” (in the moral sense, as opposed to legal personhood) is a technical term indicating a full moral status, for example having a moral right to life, and was often considered coextensive with humanity (Chan and Harris 2011 ; Tooley 2011 ). Nonhuman primates are certainly sentient, where sentience, the capacity to experience pain and pleasure, is understood either as a specific cognitive capacity or a combination of cognitive capacities underlying awareness and sensation. Contrary to the common assumption in regulation and praxis, lower cognitive capacities do not necessarily imply less suffering, see for example Akhtar ( 2011 ). There remains the problem of knowing animal suffering; there is no way for us humans to know what it is like to be a mouse in pain or a severely depressed monkey, just like we cannot know what it is like to be a bat (Nagel 1974 ). We can merely imagine what it is like to be a human in the same situation. In terms of understanding animal suffering, the best we can do is to infer from behavioral, cognitive and neurological similarities, to what extent the experience of animal suffering may be similar to human suffering.
An early example is Blackmore’s ( 1982 ) paper “Animal Research Ethics at the University of Southern California.”
In biomedical ethics, the principlism of Beauchamp and Childress ( 2013 ) grounds moral decisions on four principles: Respect for autonomy, non-maleficence, beneficence, and justice. It combines aspects of both utilitarianism and the duty-based ethics of deontological moral theories. Within research ethics, the principle of respect for autonomy requires that research subjects can make their own informed decisions about research participation, in particular by giving their informed consent before participating in research. The principle of non-maleficence formulates an obligation to “do no harm”. The principle of beneficence formulates an obligation to benefit others. The principle of justice requires that burdens and benefits are justly distributed, which includes such concerns as the just selection of research subjects.
Various accounts of the concept of autonomy can be found in research ethics, but at its core is the ability to act on one’s own reasons, or to act on one’s considered judgment. Compare the following passage from the Belmont Report (National Commission 1979 , B1): “To show lack of respect for an autonomous agent is to repudiate that person’s considered judgments, to deny an individual the freedom to act on those considered judgments, or to withhold information necessary to make a considered judgment, when there are no compelling reasons to do so”. Nonhuman animals, and indeed some humans, are not capable of making considered judgments and cannot be shown lack of respect as autonomous agents in the sense of this passage.
Here I follow Diekema’s ( 2006 , p. S9) analysis of assent in pediatrics research, where assent “requires only that the child possess the capacity to understand that the research is not being done for his or her benefit, to understand what will happen to him or her in the research project, and to agree or disagree regarding participation”. Diekema expects most children to have the capability to assent by 7 years of age and in some cases earlier.
For a discussion of animal pain, see for example Allen ( 2004 ) and Rollin ( 2011 ). The view that nonhuman animals cannot experience pain, sometimes incorrectly associated with Descartes (Cottingham 1978 ; Harrison 1992 ; Thomas 2006 ), does currently not find any defenders of note (with the often cited exception of Harrison 1991 ). Although Peter Carruthers admits that (some) nonhuman animals can experience pain and other suffering, he has argued that it has no moral significance because their pain is non-conscious (Carruthers 1992 , p. 192).
This point is often made by critics of utilitarianism, see for example Regan’s “Empty Cages: Animal Rights and Vivisection” (Regan 2005 , p. 79). For a spirited attempt to provide a framework for balancing harms and benefits in animal research, see Bateson’s “When to Experiment on Animals” (Bateson 1986 ).
Aaltola, E. (2011). The philosophy behind the movement: Animal studies versus animal rights. Society & Animals, 19, 393–406.
Google Scholar
Akhtar, S. (2011). Animal pain and welfare: Can pain sometimes be worse for them than for us? In T. L. Beauchamp & R. G. Frey (Eds.), The Oxford handbook of animal ethics (pp. 495–518). Oxford: Oxford University Press.
Allen, C. (2004). Animal pain. Nôus, 38 (4), 617–643.
Arnason, G. (2017). Animal research and the political theory of animal rights. In G. G. da Trindade & A. Woodhall (Eds.), Ethical and political approaches to nonhuman animal issues (pp. 327–345). Cham: Palgrave Macmillan.
Arnason, G. (2018a). Human-animal parallels in clinical ethics and research ethics. American Journal of Bioethics, 18 (2), 64–65.
Arnason, G. (2018b). The ethical justification for the use of non-human primates in research: The weather all report revisited. Journal of Medical Ethics, 44, 328–331.
Arnason, G., & Clausen, J. (2016). On balance: Weighing harms and benefits in fundamental neurological research using nonhuman primates. Medicine, Health Care and Philosophy, 19, 229–237.
Bateson, P. (1986). When to experiment on animals. New Scientist, 109 (1496), 30–32.
Beauchamp, T. L. (2014). The ethics of biomedical research involving animals. In J. D. Arras, E. Fenton, & R. Kukla (Eds.), The Routledge companion to bioethics (pp. 261–273). New York, NY: Routledge.
Beauchamp, T. L., & Childress, J. F. (2013). Principles of biomedical ethics (7th ed.). Oxford: Oxford University Press.
Beauchamp, T. L., Ferdowsian, H. R., & Gluck, J. P. (2014). Rethinking the ethics of research involving nonhuman animals: Introduction. Theoretical Medicine and Bioethics, 35, 91–96.
Beauchamp, T. L., & Morton, D. B. (2015). The upper limits of pain and suffering in animal research: A moral assessment of the European Union’s legislative framework. Cambridge Quarterly of Healthcare Ethics, 24, 431–447.
Beauchamp, T. L., & Wobber, V. (2014). Autonomy in chimpanzees. Theoretical Medicine and Bioethics, 35, 117–132.
Beecher, H. K. (1966). Ethics and clinical research. New England Journal of Medicine, 272, 1354–1360.
Blackmore, W. M. (1982). Animal research ethics at the University of Southern California. LabAnimal, 11, 41–47.
Brody, B. (2012). Defending animal research: An international perspective. In J. R. Garrett (Ed.), The ethics of animal research: Exploring the controversy (pp. 53–66). Cambridge, MA: The MIT Press.
Buller, T., Shriver, A., & Farah, M. (2014). Guest editorial: Broadening the focus. Cambridge Quarterly of Healthcare Ethics, 23, 124–128.
Carruthers, P. (1992). The animal issue: Moral theory in practice . Cambridge: Cambridge University Press.
Chan, S., & Harris, J. (2011). Human animals and nonhuman persons. In T. L. Beauchamp & R. G. Frey (Eds.), The Oxford Handbook of Animal Ethics (pp. 304–327). Oxford: Oxford University Press.
Choe Smith, C. U. (2014). Confronting ethical permissibility in animal research: Rejecting a common assumption and extending a principle of justice. Theoretical Medicine and Bioethics, 35, 175–185.
CIOMS, Council for International Organizations of Medical Sciences. (2016). International ethical guidelines for health-related research involving humans (4th ed.). Geneva: CIOMS.
Cochrane, A. (2018). Sentientist politics: A theory of global inter-species justice . Oxford: Oxford University Press.
Cohen, C. (1990). Animal experimentation defended. In S. Garattini & D. W. van Bekkum (Eds.), The importance of animal experimentation for safety and biomedical research (pp. 7–16). Dordrecht: Kluwer Academic Publishers.
Cottingham, J. (1978). ‘A brute to the brutes?’: Descartes’ treatment of animals. Philosophy, 53 (206), 551–559.
DeGrazia, D. (1999). The ethics of animal research: What are the prospects for agreement? Cambridge Quarterly of Healthcare Ethics, 8, 23–34.
DeGrazia, D. (2006). Regarding animals: Mental life, moral status, and use in biomedical research: An introduction to the special issue. Theoretical Medicine and Bioethics, 27, 277–284.
DeGrazia, D., & Beauchamp, T. L. (2015). Guest editorial: Reassessing animal research ethics. Cambridge Quarterly of Healthcare Ethics, 24, 385–389.
Diekema, D. S. (2006). Conducting ethical research in pediatrics: A brief historical overview and review of pediatric regulations. The Journal of Pediatrics, 149 (1), S3–S11.
Donaldson, S., & Kymlicka, W. (2011). Zoopolis: A political theory of animal rights . Oxford: Oxford University Press.
European Parliament and the Council of the European Union. (1986). Directive 86/609/EEC of 24 November 1986 on the approximation of laws, regulations and administrative provisions of the member states regarding the protection of animals used for experimental and other scientific purposes. Official Journal of the European Union L 358, 18/12. Retrieved August 28, 2019, from http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A31986L0609 .
European Parliament and the Council of the European Union. (2010). Directive 2010/63/EU on the protection of animals used for scientific purposes. Official Journal of the European Union L 276/33; adopted 2010 Sept 22. Retrieved August 28, 2019, from http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32010L0063 .
Faria, C. (2018). A flimsy case for the use of non-human primates in research: A reply to Arnason. Journal of Medical Ethics, 44, 332–333.
Fenton, A. (2014). Can a Chimp say “no”? Cambridge Quarterly of Healthcare Ethics, 23, 130–139.
Ferdowsian, H., & Choe, C. (2013). Extending human research protections to non-human animals. In R. Corbey & A. Lanjouw (Eds.), The politics of species: Reshaping our relationships with other animals (pp. 232–240). Cambridge: Cambridge University Press.
Ferdowsian, H., & Fuentes, A. (2014). Harms and deprivation of benefits for nonhuman primates in research. Theoretical Medicine and Bioethics, 35, 143–156.
Ferdowsian, H. R., & Gluck, J. P. (2015). The ethical challenges of animal research: Honoring Henry Beecher’s approach to moral problems. Cambridge Quarterly of Healthcare Ethics, 24, 391–406.
Francione, G. L. (2009). Animals as persons: Essays on the abolition of animal exploitation . New York, NY: Columbia University Press.
Frey, R. G. (1997). Moral community and animal research in medicine. Ethics and Behavior, 7 (2), 123–136.
Gagneux, P., Moore, J. J., & Varki, A. (2005). The ethics of research on great apes. Nature, 437 (1 September), 27–29.
Garner, R., & O’Sullivan, S. (Eds.). (2016). The political turn in animal ethics . London: Rowman & Littlefield International.
Gilbert, S. (2012). Progress in the animal research war. Animal Research Ethics: Evolving Views and Practices, Hastings Center Report Special Report, 42 (6), S2–S3.
Gilbert, S., Kaebnick, G. E., & Murray, T. H. (Eds). (2012). Animal research ethics: Evolving views and practices. Hastings Center Special Report , 42(6). Retrieved August 29, 2019, from http://animalresearch.thehastingscenter.org/special-report/ .
Harrison, P. (1991). Do animals feel pain? Philosophy, 66 (255), 25–40.
Harrison, P. (1992). Descartes on animals. The Philosophical Quarterly, 42 (167), 219–227.
Herrmann, K., & Jayne, K. (Eds.). (2019). Animal experimentation: Working towards a paradigm change . Leiden: Brill.
Hursthouse, R. (2006). Applying virtue ethics to our treatment of the other animals. In J. Welchman (Ed.), The practice of virtue: Classic and contemporary readings in virtue ethics (pp. 136–155). Indianapolis, IN: Hackett Publishing Company.
IOM, Institute of Medicine. (2011). Chimpanzees in biomedical and behavioral research: Assessing the necessity . Washington, DC: The National Academies Press.
Jaworska, A., & Tannenbaum, J. (2018). The grounds of moral status. In E. N. Zalta (Ed.), The Stanford encyclopedia of philosophy . Retrieved August 29, 2019, from https://plato.stanford.edu/archives/spr2018/entries/grounds-moral-status/ .
Johnson, J., & Barnard, N. D. (2014). Chimpanzees as vulnerable subjects in research. Theoretical Medicine and Bioethics, 35, 133–141.
Kantin, H., & Wendler, D. (2015). Is there a role for assent or dissent in animal research? Cambridge Quarterly of Healthcare Ethics, 24, 459–472.
Korsgaard, C. M. (2018). Fellow creatures: Our obligations to the other animals . Oxford: Oxford University Press.
Merriam, G. (2012). Virtue, vice, and vivisection. In J. R. Garrett (Ed.), The ethics of animal research: Exploring the controversy (pp. 125–146). Cambridge, MA: The MIT Press.
Nagel, T. (1974). What is it like to be a bat? The Philosophical Review, 83 (4), 435–450.
National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. (1979). The Belmont report: Ethical principles and guidelines for the protection of human subjects of research. Washington, DC: Department of Health, Education and Welfare. DHEW publications (OS) 78-0013 and (OS) 78-0014.
Nordgren, A. (2010). For our children: The ethics of animal experimentation in the age of genetic engineering . Amsterdam: Rodopi.
Nozick, R. (1974). Anarchy, state, and utopia . New York, NY: Basic Books.
Nussbaum, M. C. (2004). Beyond ‘compassion and humanity’: Justice for nonhuman animals. In C. R. Sunstein & M. C. Nussbaum (Eds.), Animal rights: Current debates and new directions (pp. 299–320). Oxford: Oxford University Press.
Pluhar, E. B. (2006). Experimentation on humans and nonhumans. Theoretical Medicine and Bioethics, 27, 333–355.
Rawls, J. (1999). A theory of justice (Revised ed.). Cambridge, MA: Harvard University Press.
Reardon, S. (2015). NIH to retire all research chimpanzees. Nature News , 18 November. Retrieved August 29, 2019, from https://www.nature.com/news/nih-to-retire-all-research-chimpanzees-1.18817 .
Regan, T. (2004 [1983]). The case for animal rights. Berkeley, CA: University of California Press.
Regan, T. (2005). Empty cages: Animal rights and vivisection. In A. I. Cohen & C. H. Wellman (Eds.), Contemporary debates in applied ethics (pp. 77–90). Oxford: Blackwell Publishing.
Rollin, B. E. (2011). Animal pain: What it is and why it matters. The Journal of Ethics, 15 (4), 425–437.
Russell, W. S., & Burch, R. L. (1959). The principles of humane experimental technique . London: Methuen.
Shuster, E. (1997). Fifty years later: The significance of the Nuremberg Code. New England Journal of Medicine, 337, 1436–1440.
Singer, P. (1975). Animal liberation . New York, NY: New York Review. (distributed by Random House) .
Singer, P. (1979). Practical ethics . Cambridge: Cambridge University Press.
Tannenbaum, J., & Rowan, A. N. (1985). Rethinking the morality of animal research. Hastings Center Report, 15 (5), 32–43.
Thomas, J. (2006). Does Descartes deny consciousness to animals? Ratio, 19 (3), 336–363.
Tooley, M. (2011). Are nonhuman animals persons? In T. L. Beauchamp & R. G. Frey (Eds.), The Oxford handbook of animal ethics (pp. 332–370). Oxford: Oxford University Press.
UK Home Office. (2014). Annual statistics of scientific procedures on living animals; Great Britain 2013 . London: House of Commons.
US Department of Health and Human Services. (2018). 45 CFR 46. Code of federal regulations. Title 45. Retrieved August 29, 2019, from https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/index.html .
Walker, R. L. (2006). Human and animal subjects of research: The moral significance of respect versus welfare. Theoretical Medicine and Bioethics, 27, 305–331.
Walker, R. L. (2016). Beyond primates: Research protections and animal moral value. Hastings Center Report, 46 (4), 28–30.
Walker, R. L. (2018). Virtue, vice, and “voracious” science: How should we approach the ethics of primate research? Perspectives in Biology and Medicine, 61 (1), 130–146.
Weatherall, D. (2006). The use of nonhuman primates in research . London: Academy of Medical Sciences.
Wendler, D. (2014). Should protections for research with humans who cannot consent apply to research with nonhuman primates? Theoretical Medicine and Bioethics, 35, 157–173.
WMA, World Medical Association. (2013). Declaration of Helsinki. Ethical principles for medical research involving human subjects . Retrieved August 29, 2019, from https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ .
Download references
Acknowledgements
Open Access funding provided by Projekt DEAL. My thanks to Urban Wiesing for his help and his comments on previous drafts. I am also grateful to the reviewers for constructive and helpful comments.
This research was supported by the German Research Foundation (DFG) research unit Grant FOR 1847.
Author information
Authors and affiliations.
Institute of Ethics and History of Medicine, University of Tübingen, Gartenstrasse 47, 72074, Tübingen, Germany
Gardar Arnason
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Gardar Arnason .
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Arnason, G. The Emergence and Development of Animal Research Ethics: A Review with a Focus on Nonhuman Primates. Sci Eng Ethics 26 , 2277–2293 (2020). https://doi.org/10.1007/s11948-020-00219-z
Download citation
Received : 31 August 2019
Accepted : 16 April 2020
Published : 29 April 2020
Issue Date : August 2020
DOI : https://doi.org/10.1007/s11948-020-00219-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Research ethics
- Nonhuman primates
- Animal research
- Animal research ethics
- Find a journal
- Publish with us
- Track your research
Ethical Guidelines for the Use of Animals in Research
Given by the National Committee for Research Ethics in Science and Technology (NENT), 2018.

Download as pdf
About the guidelines
These guidelines have been prepared by the National Committee for Research Ethics in Science and Technology (NENT). Their purpose is to provide ethical guidelines for researchers and other people who are considering experiments on animals. The guidelines will be useful when planning projects, assessing them, and when reporting and publishing findings and results. They are also intended to contribute to reflection on research ethics and the use of animals in research in both research communities and in the public debate.
The overarching framework for these guidelines is provided by the Guidelines for Research Ethics in Science and Technology (2016), particularly guidelines 12 and 13. A consultation process and a subsequent workshop organised by NENT in the autumn of 2016 found that relevant players see a need for a set of guidelines that can systematise and elaborate on the ethical responsibility inherent in the use of animals in research. This is the background for the current guidelines.
In Norway, the use of laboratory animals is governed by the Regulations Relating to the Use of Animals in Research, which follow from the Animal Welfare Act. The EEA Agreement obliges Norway to implement EU Directive 2010/63/EU on the Protection of Animals used for Scientific Purposes. These rules provide a zero vision for research using animals. In Norway, the Gene Technology Act provides the legal framework for research on such organisms.
Many of the ethical obligations stipulated in these guidelines are also laid down in applicable legislation. Researchers who violate the guidelines can face legal sanctions. In that case, it is because they have broken the law, not primarily because they have violated the guidelines for research ethics. NENT does not have access to any sanctions of its own. NENT's role in following up the guidelines is to provide advice and recommendations, help increase awareness of animal welfare, and to stimulate continued discussion about research that involves animals.
Ethics and Experiments on Animals
The ethical assessments related to the use of animals in research are wide-ranging. It is generally thought that it may be necessary to use laboratory animals in some cases in order to create improvements for people, animals or the environment. At the same time, the general opinion is that animals have a moral status, and that our treatment of them should be subject to ethical considerations. Such views are reflected in the following positions:
(i) Animals have an intrinsic value which must be respected.
(ii) Animals are sentient creatures with the capacity to feel pain, and the interests of animals must therefore be taken into consideration.
(iii) Our treatment of animals, including the use of animals in research, is an expression of our attitudes and influences us as moral actors.
The guidelines reflect all these positions, and stipulate principles and considerations that can be used as tools when balancing between harm and benefit. The three Rs (Replace, Reduce, Refine) are established principles that are also enshrined in legislation. These principles can establish absolute limits for experiments on animals, even when there are great benefits. These principles also state what can reasonably be considered harm and benefit, and the principles thus facilitate good assessments. Assessments of harm and benefit associated with experiments on animals are particularly demanding, because experiments may result in researchers intentionally causing actual harm to animals, while the future benefits are often uncertain.
The guidelines are dynamic and must be reviewed in line with technological developments and the appearance of new ethical issues. New gene technology methods create new opportunities for the use of genetically modified animals in research, which is a growing trend. Genetically modifying laboratory animals, i.e. changing the genetic material of laboratory animals using gene technology, gives rise to a special responsibility in that this method entails a double intervention: first, intervention in the animal's genetic material and second, use of the animal as a research object. This practice has the potential to change our view of humans and our attitudes towards generating or eliminating genetic characteristics in ourselves.
These guidelines provide a framework that also covers ethical questions associated with the use of genetically modified animals in research.
Definitions
In these guidelines, the term «research» must be understood broadly, and include planning, execution and dissemination. The guidelines primarily address the «researcher» but apply to any person involved when animals are used for research, including funding and approval bodies, which are also responsible for making ethical assessments of projects involving experiments on animals.
The guidelines cover «laboratory animals», as defined in the Regulations Relating to the Use of Animals in Research, but also cover all animals that are otherwise impacted by research activities.
1. Respect for animals' dignity
Researchers must have respect for animals' worth, regardless of their utility value, and for animals' interests as living, sentient creatures. Researchers must be respectful when choosing their topic and methods, and when disseminating their research. Researchers must provide care that is adapted to the needs of each laboratory animal.
2. Responsibility for considering options ( Replace )
Researchers are responsible for studying whether there are alternatives to experiments on animals. Alternative options must be prioritised if the same knowledge can be acquired without using laboratory animals. If no good options are available, researchers should consider whether the research can be postponed until alternative methods have been developed. When justifying experiments on animals, researchers therefore must be able to account for the absence of options and the need to acquire knowledge immediately.
3. The principle of proportionality: responsibility for considering and balancing suffering and benefit
Researchers must consider the risk that laboratory animals experience pain and other suffering (see guideline 5) and assess them in relation to the value of the research for animals, people or the environment. Researchers are responsible for considering whether the experiment may result in improvements for animals, people or the environment. The possible benefits of the study must be considered, substantiated and specified in both the short and the long term. The responsibility also entails an obligation to consider the scientific quality of the experiments and whether the experiments will have relevant scientific benefits.
Suffering can only be caused to animals if this is counterbalanced by a substantial and probable benefit for animals, people or the environment.
There are many different methods for analysing harm and benefit. Research institutions should provide training on suitable models, and researchers are responsible for using such methods of analysis when planning experiments on animals.
4. Responsibility for considering reducing the number of animals ( Reduce )
Researchers are responsible for considering whether it is possible to reduce the number of animals the experiment plans to use and must only include the number necessary to maintain the scientific quality of the experiments and the relevance of the results. This means, among other things, that researchers must conduct literature studies, consider alternative experiment designs and perform design calculations before beginning experiments.
5. Responsibility for minimising the risk of suffering and improving animal welfare ( Refine )
Researchers are responsible for assessing the expected effect on laboratory animals. Researchers must minimise the risk of suffering and provide good animal welfare. Suffering includes pain, hunger, thirst, malnutrition, abnormal cold or heat, fear, stress, injury, illness and restrictions on the ability to behave normally/naturally.
A researcher's assessment of what is considered acceptable suffering should be based on the animals that suffer the most. If there are any doubts regarding perceived suffering, consideration of the animals must be the deciding factor.
Researchers must not only consider the direct suffering that may be endured during the experiment itself, but also the risk of suffering before and after the experiment, including trapping, labelling, anaesthetising, breeding, transportation, stabling and euthanising. This means that researchers must also take account of the need for periods of adaptation before and after the experiment.
6. Responsibility for maintaining biological diversity
Researchers are responsible for ensuring that the use of laboratory animals does not endanger biological diversity. This means that researchers must consider the consequences to the stock and to the ecosystem as a whole. The use of endangered and vulnerable species must be reduced to an absolute minimum. When there is credible, but uncertain, knowledge that the inclusion of animals in research or the use of certain methods may have ethically unacceptable consequences for the stock and the ecosystem as a whole, researchers must observe the precautionary principle.[ 1 ]
7. Responsibility when intervening in a habitat
Researchers are responsible for reducing disruption and any impact on the natural behaviour of individual animals, including those that are not direct subjects of research, as well as of populations and their surroundings. Certain research and technology-related projects, like those regarding environmental technology and environmental surveillance, may impact on animals and their living conditions, for example as a result of installing radar masts, antennas or other measurement instruments. In such cases, researchers must seek to observe the principle of proportionality (see guideline 3) and minimise the possible negative impact.
8. Responsibility for openness and sharing of data and material
Researchers are responsible for ensuring that there is transparency about research findings and facilitating the sharing of data and material from experiments on animals. Such transparency and sharing are important in order to avoid unnecessary repetition of experiments. Transparency is also important in order to ensure that the public are informed and is part of researchers' responsibility for dissemination.
In general, the negative results of experiments on animals should be public knowledge. Disclosing negative results may give other researchers information about which experiments are not worth pursuing, shine a light on unfortunate research design, and help reduce the use of animals in research.
9. Requirement of expertise on animals
Researchers and other parties who handle live animals must have adequately updated and documented expertise on animals. This includes specific knowledge about the biology of the animal species in question, and a willingness and ability to take care of animals properly.
10. Requirement of due care
There are national laws and rules and international conventions and agreements regarding the use of laboratory animals, and both researchers and research managers must comply with these. Any person who plans to use animals in experiments must familiarise themselves with the current rules.
References and useful resources
[ 1 ] The Norwegian National Committee for Research Ethics in Science and Technology (NENT). Guidelines for research ethics in science and technology (2007) 2016. Oslo.
Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32010L0063
Regulation on the capture and collection of wild animals for scientific or other special purposes (Forskrift om innfanging og innsamling av vilt for vitenskapelige eller andre særlige formål). 2003. https://lovdata.no/dokument/LTI/forskrift/2003-03-14-349
Regulation on Animal Experimentation (Forskrift om bruk av dyr i forsøk). 2015. https://lovdata.no/dokument/SF/forskrift/2015-06-18-761
Act of 2 April 1993 No. 38 Relating to the Production and Use of Genetically Modified Organisms, etc. (Gene Technology Act) (Lov om framstilling og bruk av genmodifiserte organismer m.m. 1993). https://www.regjeringen.no/en/ dokumenter/gene-technology-act/id173031/
The Animal Welfare Act. 2009. https://www.regjeringen.no/en/dokumenter/animal-welfare-act/id571188/
The ARRIVE Guidelines (Animal Research: Reporting of In Vivo Experiments). 2010. https://www.nc3rs.org.uk/sites/default/files/documents/Guidelines/NC3Rs%20ARRIVE%20Guidelines%202013.pdf
The Norwegian Food Safety Authority's instructions on the management of the Regulation on Animal Experimentation (Mattilsynets instruks om forvaltningen av Forsøksdyrforskriften): https://www.mattilsynet.no/dyr_og_dyrehold/dyrevelferd/forsoksdyr/ instruks_om_mattilsynets_forvaltning_av_forsoksdyrforskriften.21015/binary/Instruks%20om%20Mattilsynets%20forvaltning%20av%20forsøksdyrforskriften
PREPARE (Planning Research and Experimental Procedures on Animals: Recommendations for Excellence) guidelines. 2017. https://norecopa.no/prepare
SAHMRI's BRIGHT Walk is back!
Research With Integrity
Animal research ethics, animal ethics refers to the moral principles and considerations that guide how we treat and interact with animals..
It involves evaluating the rights, well-being, and interests of animals, and making ethical decisions about how we use and interact with them in various contexts, including research and teaching-based activities.
SAHMRI prides itself on promoting best practices for animal welfare by following the governing principles of the Australian code for the care and use of animals for scientific purposes (8th Ed. 2013). The SAHMRI Animal Ethics Committee (AEC) adheres to the South Australian Animal Welfare Act 1985. The Act sets the legal requirements for the use of animals in research and teaching and regulates the institution’s Animal Ethics Committee function and reporting of animal-related activities.
SAHMRI’s AEC Operating Guidelines outline the institutional policies and procedures for the use of animals for teaching and research purposes. These guidelines extend to any personnel who utilise the SAHMRI AEC for the purpose of animal research or teaching.
Do I need AEC Approval?
All scientific research and teaching involving animals* requires AEC approval. All SAHMRI and partner researchers who wish to use animals for teaching, research, or experimentation within the SAHMRI Bioresources or PIRL facilities must obtain approval from the SAHMRI Animal Ethics Committee (AEC). AEC approval must be obtained before the use or involvement of animals in research projects or experiments, irrespective of the site involved, the ownership of the animal, or the source of funding.
If an institution or individual plans to use animals for research or teaching purposes, they must obtain a license. Please see “Do I need a License?” for more information. In addition, Researchers working with animals at SAHMRI are not permitted to begin any animal work until they have completed all relevant training associated with their project. Finally, any changes to an approved project are also subject to ethical review and must be approved prior to including them within the project. Please see “Submitting an Amendment”.
For any general inquiries regarding the Animal Ethics Committee, please contact [email protected] .
*Animal: any live non-human vertebrate. The definition of an animal includes embryos, foetuses, and larval forms that have progressed beyond half the gestation or incubation period of the relevant species or have become capable of independent feeding.
Examples of animals as defined by the Australian Code:
• Vertebrates: (animals with backbones) e.g., monotremes, marsupials, placental mammals, domestic animals, companion animals (cats, dogs), laboratory animals, wildlife, pest animals, fish, rats, mice, guinea pigs, rabbits, non-human primates. • Cephalopods: e.g., octopus, squid, cuttlefish, nautilus. • Fish, including bony fish and cartilaginous fish (sharks, skates, and rays). • Not insects, millipedes, annelids (worms), gastropods (slugs & snails), or spiders. • Not shellfish (bivalves, mussels, oysters, scallops). • Not humans
Do I need a License?
If an institution or individual plans to use animals for research or teaching purposes, they must obtain a license from the Minister in accordance with the Animal Welfare Act 1985 (SA). It's important to note that each state has its own act and specific requirements.
To apply for a license, the interested party must submit an application to the Department for Environment and Water (DEW) Animal Welfare Unit at least 28 days before the proposed start date. The cost of these licenses is $90 for a duration of 2 years. For more details and the application process, visit DEW .
Furthermore, individuals can contact DEW to verify the validity of their existing license or to check if their institution holds a valid license.
In cases where an entire project has received approval from an interstate committee, and this project includes work within SAHMRI, the ethics approval must be delegated from the interstate committee to SAHMRI.
How to Apply for AEC Approval
Approval is obtained through a detailed written submission to the SAHMRI AEC outlining the project activities, justifying the use of animals requested, discussing the principle of the 3Rs (Replacement, Reduction, and Refinement), and recognising and accepting responsibility for the care and use of animals.
all animal ethics applications and amendments are required to be submitted via SAHMRI’s online database Tick@lab.
For guidelines on how to submit a new application or amendment within this database, please read the guide to applications and amendments for researchers .
Please allow ample time for submitting changes and requests to prevent potential delays. Ethical review is necessary for applications and modifications, and initial approval is not always guaranteed. The AEC may provide suggestions before granting final approval. To prevent any inconvenience, kindly submit applications and amendments well before the project's expected start date.
Additionally, all new applications must be submitted for pre-screening, and late submissions will NOT be accepted.
2024 Deadlines and Scheduled Meetings
If you have any difficulties with the database, please contact [email protected] .
AEC Applications Checklist
Animal Ethics Committee terms of reference
Ethics and Compliance Training
Researchers working with animals at SAHMRI are not permitted to begin any animal work until they have completed Animal Ethics Training and must repeat a training course every three years (or when the training has expired). People excluded from this requirement are those who have provided intellectual input to a project but who are not involved in the project and statisticians who will complete the post-processing of data. SAHMRI uses the ANZCCART ComPass Core (Phase 1) Online Animal Ethics Training Course.
If you have any questions or concerns, please do not hesitate to reach out to the Animal Ethics Officer (AEO) or the Animal Welfare Officer (AWO).
Submitting an Amendment
Any changes to existing approvals need to be submitted as an amendment to the Animal Ethics Committee (AEC). Amendments to a project must be approved by the AEC before the amendment is proceeded with.
The AEC emphasises the importance of submitting amendments at least 2-3 weeks in advance of ordering or utilising animals affected by the proposed changes.
All amendment applications need to be submitted online via Tick@lab . Please see the Create an Application Amendment guide .
All applications for amendments are considered at scheduled AEC meetings. However, the AEC may deem it appropriate for the Executive Committee (AEC Chair and Category C and D Members) to review out of session if the amendment is considered to be a minor change to the protocol.
A Minor amendment of a Protocol does not involve a change in the main aims of the project or the asking of a new scientific question. A Minor amendment would be a change that, in comparison to what has already been approved, has a minor or positive impact on:
- Animal Welfare
- The anticipated scientific or educational value
- The likelihood of meeting the project's objectives
Some examples of a minor amendment include:
- Extension of time of an existing approved project.
- Modification of procedures in previously approved projects.
- Minor increase in animal numbers.
- Where they are appropriately qualified or supervised.
- Additional tissues or samples collected post-mortem.
- Collection amount does not change.
- Additional of a new research location but using the same project methodology.
Amendments fitting the above classification of ‘Minor’ will be reviewed by the executive committee. The Executive Committee will aim to finalise this review within 5-10 business days, however, during busy times this may be longer, and the AEC Secretariat will communicate this with you directly. Any amendments outside of this scope are ‘Major’ amendments and require a full committee review. The AEC will aim to finalise their response following the major amendment review within 5-10 business days.
If the amendment is to be reviewed by the AEC, please submit it by the published deadlines.
Scavenging and Animal Transfers
Scavenging is the collection of already available animal tissues and substances from discarded dead animals. These animals have been killed for other purposes, not for the collection of such tissues and substances. "Scavenging" tissue from carcasses is highly recommended by the AEC as it is an alternative to killing animals and greatly reduces the number of animals being used in research and teaching.
The opportunity for scavenging must not influence the decision to kill the animal, nor the time when this occurs, if this comprises animal welfare. Killing an animal specifically to collect tissues or substances for scientific or teaching purposes is also a scientific procedure, is not considered to be a case of “scavenging”, and therefore requires prior approval from the AEC. Collection of organs, tissues, materials or substances from a living animal for scientific or teaching purposes is a scientific procedure and requires prior approval from the AEC.
Prior approval by the Animal Ethics Committee (AEC) is not a legislative requirement. However, the AEC should be informed when an investigator or teacher is "scavenging", this can be done via Tick@lab under “Scavenged Tissue” in an active protocol or by using SAHMRI's Scavenged Tissue Form and forwarding it to the Animal Ethics Officer.
Animal Transfers between approved projects can significantly reduce overall animal counts across various projects. These transfers are authorised solely by the AEC and approval is subject to AEC discretion, with a focus on preventing undue stress on the animals and maintaining ethical standards throughout the process.
The Animal Transfer Form is intended for transfers between SAHMRI experimental projects only and does not apply to breeding applications or transferring animals between different institutions.
Once completed, this form must be promptly sent to the AEC Secretary for submission to the next scheduled AEC meeting for official ratification.
Annual Reporting to the AEC
Annual Progress Reports are required to be submitted to the AEC and are due on the date of approval of the project each year the project is active. Final Reports are required to be submitted soon after project completion. Once the AEC has reviewed the Final Report, the application will be closed on the researcher’s behalf.
These forms will be available in your application on Tick@lab under Annual Review. The continuation of a project is subject to the fulfilment of annual progress reporting requirements for ongoing projects. Please refer to the application guide .
Alternatively, Annual Progress and Final Reports can be requested and submitted via email to the Animal Ethics Officer .
Reporting an Unexpected Adverse Event
An Unexpected Adverse Event (UAE) is an unanticipated and undesirable incident or outcome that occurs while conducting a study or experiment involving animals. This event is not part of the expected or predicted results and may potentially harm the animal's health, welfare, or overall condition. These events could include unexpected health issues, reactions to treatments or procedures, injuries, or any other negative occurrences that were not foreseen. Proper documentation, analysis, and reporting of such events are essential to ensure animal welfare, ethical considerations, and the validity of the research findings.
- Act: Immediately ensure the adverse impacts on the well-being of the animal are addressed. Alleviate any pain or undue distress to the animal by emergency treatment or humane killing.
- The AWO Within the first 24 hours of the incident via phone or email
- The Manager of the facility in which the event occurred ( Bioresources or PIRL )
- The Primary Investigator names on the application
- Report : Please submit a formal report within 72 hours of the UAE via Tick@lab and notify the AEO and AWO when this has been done. Please refer to this User Guide for step-by-step instructions.
The report must include the following information: - Ethics Number - Animal ID, Strain, Age and Gender - Location of event: Bioresources or PIRL - Summary of events leading up to UAE - Actions taken to alleviate pain or distress - Attach Clinical Records Sheets, or other monitoring sheets. - If applicable, post-mortem examination results - Considerations for the prevention of future UAE and interventions/corrective actions that may be taken. When an animal dies unexpectedly, or is humanely killed due to unforeseen complications, a post-mortem should be performed by the AWO, Facility Veterinarian, or a person with appropriate experience or qualifications. Please place the body in the fridge to maintain anatomical structures if the post-mortem cannot be done immediately.
The AWO will investigate and advise of any interim arrangements required. This may involve modifications to procedures, urgent medical treatment, husbandry intervention, or, in exceptional cases, suspension of the project.
Deadline Dates
SAHMRI is committed to conducting and supporting...
Research A to Z
Explore the wide variety or research programs underway at...
Core Facilities
SAHMRI is located on Kaurna Country. We pay respects to the Kaurna people of the Adelaide Plains and to all Aboriginal and Torres Strait Islander people. We are committed to embracing knowledge and culture as we continue our working journey to incorporate Aboriginal health research across all of our themes and further reconciliation.
Quick links
- Find funding
- Find publications
- News centre
- Outcomes of funding rounds
- Sapphire Login
- Research policy
- Animal ethics
The 3Rs – Replacement, Reduction and Refinement
Ensuring the ethical, humane and responsible use of animals in health and medical research forms part of the sector’s social licence to operate in Australia.
The 3Rs (Replacement, Reduction and Refinement) are accepted internationally as critical components of the ethical, humane and responsible care and use of animals for scientific purposes.
The 3Rs underpin the requirements in the Australian code for the care and use of animals for scientific purposes . All institutions, animal ethics committees (AECs), investigators and animal carers need to be aware of their responsibilities:
- All those involved have a responsibility to ensure the consideration and implementation of alternative approaches that do not use animals (Replacement).
- involve the smallest number of animals necessary to achieve the study’s aims and to satisfy good statistical design (Reduction)
- support and safeguard animal wellbeing (Refinement).
NHMRC’s Expectations
Under NHMRC's Funding Agreement, and as outlined in the Code , NHMRC requires:
- Institutions to ensure, through the AEC, that the 3Rs are applied at all stages of animal care and use
- Researchers to apply the 3Rs throughout all stages of the research cycle including the development of the research question and the design, conduct, analysis and reporting of research.
Research Funding
Applications to NHMRC schemes can focus on the development and validation of models, methods, tools and methodologies to replace, reduce or refine animal use. Proposals for funding must meet the specific NHMRC’s Grant Program scheme criteria, and must demonstrate a direct relationship with, or significance for, improvement in human health.
The 3Rs in Australia
NHMRC’s Information Paper: Implementation of the 3Rs in Australia (2019) identified practices, strengths and opportunities for improvements in the implementation of the 3Rs in Australia.
NHMRC’s 3Rs survey (2018) reported on how the 3Rs are understood, considered and implemented in Australia. The survey report and appendices are available under ‘Downloads’ below.
CSIRO Report: Non-animal models: A strategy for maturing Australia’s medical product development capabilities . Non-animal models use human-derived or humanised cells, tissues, or data. These models are becoming increasingly sophisticated and are surpassing the performance of traditional animal models at anticipating the safety and efficacy of novel medical products. In collaboration with industry, research and government partners, CSIRO produced the report: Non-animal models: A strategy for maturing Australia’s medical product development capabilities (2023) . NHMRC sponsored and contributed to the development of this report, which assesses the potential of non-animal models to complement or replace traditional animal models for medical product development.
International 3Rs Centres
International 3Rs Centres are a source of information about current and emerging 3Rs methods and techniques that can be used by Australian researchers:
- Canadian Council on Animal Care: Three Rs
- Danish 3R-Centre
- EU Reference Laboratory for alternatives to animal testing (EURL ECVAM)
- Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM)
- Johns Hopkins University Center for Alternatives to Animal Testing (CAAT)
- National Centre for the Replacement Refinement and Reduction of Animals in Research (NC3Rs) (UK)
- Norwegian Consensus-Platform for Alternatives (NORECOPA)
For further information, please contact [email protected] .
Related resources

Principles and guidelines for the care and use of non-human primates for scientific purposes
Guidelines to promote the wellbeing of animals used for scientific purposes

A guide to the care and use of Australian native mammals in research and teaching
- Ideas Grants 2022 Peer reviewer briefing webinar introductions and presentation
- Indigenous Research Excellence Criteria video transcript
- Funding agreement and deed of agreement
- Institution approvals
- Institutional Annual Compliance Report
- Vary your grant
- Financial reporting
- Progress, final and additional reporting
- Direct Research Cost Guidelines
- Previous Personnel Support Package rates
- Previous Salary Support Package rates
- Institutional approvals and grant conditions
- Funding Agreement
- Financial Reports
- Scientific reporting and milestones
- Direct Research Costs and Personnel and Salary Support Packages
- Eligibility
- Peer review
- Research funding data
- Analysis of Australian health and medical research publications
- Medical Research Future Fund
- International Collaborative Health Research Funding
- Funding calendar
- Working together to support research
- Statements of Expectations
- Targeted Calls for Research
- Capacity and capability building and strengthening
- Health Advice and Publications
- Health effects of water fluoridation
- Preventing infection
- How NHMRC develops public health guidelines
- Complementary medicines
- Electronic cigarettes
- Myalgic Encephalomyelitis and Chronic Fatigue Syndrome
- Lead blood levels
- Recreational Water Quality Advisory Committee
- Australian Guidelines to Reduce Health Risks from Drinking Alcohol - Public submissions
- National Statement on Ethical Conduct in Human Research
- Genomics resources for clinicians and researchers
- Vitamin K for newborns
- Guidelines for Guidelines
- COVID-19 impacts
- Clinical trials reform
- Determining whether an embryo model is regulated by the ERLC
- Information for applicants
- Information for Licence Holders
- Mitochondrial Donation Licensing Scheme
- Training and Quality Assurance activities
- Import and export of cell lines
- Database of Licences issued
- Training and Quality Assurance
- Use of animals in NHMRC funded research
- The Human Research Ethics Applications (HREA)
- Ethical issues and further resources
- National Certification Scheme
- Human research ethics committees
- Clinical ethics
- Ethical guidelines for Assisted Reproductive Technology
- Ethical guidelines for research with Aboriginal and Torres Strait Islander Peoples
- NHMRC ethical guidelines on organ and tissue donation and transplantation
- Guideline development
- International engagement
- Research quality
- Research translation
- Research impact
- Dementia research
- NHMRC Special Initiative in Mental Health
- NHMRC’s role in addressing health implications of environmental change
- Submission of Targeted Calls for Research online pathway
- NHMRC health priorities 2021–2024
- Framework for Identifying and Prioritising Targeted Calls for Research
- Targeted Calls for Research Prioritisation Criteria Rubric
- Guide for Proposing Targeted Calls for Research
- Administering Institution Policy
- NHMRC Gender Equity Strategy 2022-2025
- Statement on sex and gender in health and medical research
- Structural priority funding and gender equity
- Accountability and reporting
- Fifteenth edition
- Fourteenth edition
- Ten of the Best Archive
- Ten of the Best
- 2022 Research Excellence Awards
- 2023 Research Excellence Awards
- Health Innovation Advisory Committee 2015-2018
- Legislative basis to NHMRC
- Senior executive and leadership team
- Indigenous Research Ethics Guidelines Review Working Committee
- Natural Therapies Working Committee
- Publications
- Probity Event - Additional Guidance
- NHMRC Complaints Policy
- Temporary Employment Register
- Working at NHMRC
- How we select our people
- Indigenous internship program
- Freedom of information
- Child Safe Policy
- Annual reports and corporate plans
- Consumer and community engagement
- About the review
- Consumer Statement review
- Consumer and community representative involvement in the peer review process of Targeted Calls for Research
Faculty of Health Sciences
- Staff Online Resources
- Faculty of Health Sciences (FHS) COVID-19 Updates
- Faculty Vaccination Updates
- FHS COVID-19 Research Summary
- UCT COVID-19 Response
- Mayosi Impilo Bursary Fund
- Body Donation Programme
- Unveiling of Centenary Wall
- About the Centenary
- First Year Students
- Returning Undergraduates
- Orientation
- Eligibility & Process
- Funding your Undergraduate Studies
- Student Housing
- Current Undergraduates
- Undergraduate Programmes
- FHS Student Development & Wellness Services
- Campus Life
- Health Sciences Students' Council (HSSC)
- Undergraduate FAQ's
- Registration
- Eligibility and Process
- Funding your Postgraduate Studies
- International Applicants and Foreign-qualified Doctors
- Postgraduate Registration
- Postgraduate degrees and diplomas
- Specialist & Sub-specialist training
- Postgraduate FAQ's
- Faculty Deanery
- Executive Committees
- Faculty Organogram
- Transformation
- Achievements
- Anaesthesia & Perioperative Medicine
- Family, Community and Emergency Care
- Health Sciences Education
- Paediatrics & Child Health
- Health & Rehabilitation Sciences
- Human Biology
- Integrative Biomedical Sciences
- Obstetrics & Gynaecology
- Psychiatry & Mental Health
- Public Health
- BMedSc(Hons) in Radiobiology
- Clinical Staff
- Clinical Activities
- Educational Activities
- Medical Physics
- Otorhinolaryngology (ENT)
- Plastic, Reconstructive & Maxillo-Facial Surgery
- Communications & Marketing
- Faculty Management Accounting
- Faculty Procurement
- Research Finance
- Continuing Education FHS
- Health and Safety
- Off-Campus Resource Centres
- Student Computing Labs
- Faculty Operations
- Bioethics Centre
- Research Intelligence
- Research Diligence
- Research Development
- UCT Clinical Research Centre
- Faculty Research Committee
- Human Research Ethics Committee
- Animal Ethics Committee
- Faculty Biosafety Committee (FBC)
- Research Facts & Figures
- Research Approach in Time of COVID-19
- Research Noticeboard
- Human Research Ethics
Animal Research Ethics
- Faculty Biosafety
- Research Integrity and Responsible Conduct of Research
- Regulatory Compliance
- Internal Funding Opportunities
- National Funding Opportunities
- International Funding Opportunities
- Nominations & awards
- Research Professional Africa
- Current Funding Opportunities
- Accessing grant writing support
- Initiating an eRA proposal approval request
- Submitting a grant application
- Initiating an eRA Contract Approval Request
- Reviewing and signing the agreement
- Manage an awarded grant
- Disseminate research findings
- Core Facilities
- Accredited Institutes
- Accredited Centres
- Accredited Units
- Cancer Research Initiative Overview
- Quarterly Newsletter
- Cancer Research Groups
- Cancer Projects
- Publications
- CRI Postgraduate Group
- Training Opportunities
- Cancer Databases
- Training Resources
- Basic Science Infrastructure
- Clinical Infrastructure
- Transformation Roles & Structures
- FHS Transformation Focus Areas
- FHS Transformation Initiatives
- Transformation Resources
- News & Workshops
- Jeffrey Dumo Baqwa Room
- Frances Ames Room
- Aadil Moerat Room
- About the Alumni Office
- Alumni Support Offering
- UCT Alumni News
- UCT Fund Inc (USA)
- UCT Trust (UK)
- UCT Australian Trust
- MBChB Graduation Photo Album
- Reunions Overview
- Class of 2000 - 15th Reunion | 13 - 15 November 2015
- Class of 1990 - 25th Reunion | 4 - 6 December 2015
- Class of 1975 - 40th Reunion | 20 - 22 November 2015
- Class of 1965 - 50th Reunion | 27 - 29 November 2015
- Class of 1964
- Cape Town Reunion
- Sydney Reunion
- Class of 1999
- Class of 1988 | 22 November - 24 November
- Class of 1988 | 13 December - 15 December 2013
- Class of 1973
- Class of 1963
- Class of 2002
- Class of 1997
- Class of 1987
- Class of 1962
- Class of 1996
- Class of 1986
- Class of 1981
- Class of 1971
- Class of 1961
- Class of 1970
- Class of 1985
- Class of 1995
- Class of 1960
- Class of 1984
- Class of 1969
- Class of 1959
- Class of 1983
- Class of 1968
- Class of 1958
- Class of 1992
- Class of 1982
- Class of 1967
- Class of 1957
- Physiotherapy 50th Anniversary
- Class of 1976
- Class of 1966
- Class of 1956
- Class of 1980
- Class of 1975
- Class of 1965
- Class of 1955
- Class of 1994
- Class of 1979
- Class of 1974
- Class of 1954
- Class of 1978
- Class of 1953
- Class of 1977
- Class of 1952
- Memorabilia
- Oath-taking 2022
- 2023 at a glance
- From the Dean's Desk
- Faculty Highlights & Announcements
- National Health Insurance (NHI)
- Publication Archive
- Faculty Focus
- 16 days of activism 2020
- Celebrating FHS Womxn for the month of August 2020
- Centenary News
- Centenary diary
- Department of Medicine dinner
- Frances Ames Room renaming ceremony
- Centenary History Tour
- Centenary concert
- Wolfson Memorial Collquium
- Centenary Debate
- Centenary Dinner
- Faculty Noticeboard
- Courses & Workshops
- Terms & Vacations
- Departmental contacts
All Faculty of Health Sciences research staff, postdoctoral fellows and students who wish to use live animals and/or animal tissue for teaching, research or scientific experimentation must obtain ethical approval from the Faculty Animal Ethics Committee (AEC) prior to any use or involvement with animals.
The FHS AEC helps to ensure that the highest ethical and welfare standards are maintained whenever animals are involved in research in the Faculty. At a minimum, the use of animals in research, teaching and testing at should be guided by the principles of the 3R’s (Replacement, Refinement, Reduction) as outlined in the South African National Standard 10386:2021.
Application forms and templates
- Use this form for requesting ethical approval for research or teaching involving the use of live vertebrate animals Animal Research Ethics Committee - AEC Application form (FHS003)
- Use this form for requesting ethical approval for using animal material only: Use of Animal Material (FHS029)
- Use this form (external researchers) to apply for reciprocal acknowledgement from FHS AEC Reciprocal acknowledgement (FHS030)
- How to submit your AEC Amendment Application Form (FHS005)
- FHS005 Signature Page
- Use this form to submit annual progress reports or final report Annual Progress or Final Report Form (FHS004)
- Use this form to provide feedback on a pilot study involving animals Pilot Feedback Report Form (FHS032)
- Use this form to report any adverse event or unanticipated problem affecting animal welfare, any animal deaths and euthanasia due to animals found sick or in distress, protocol deviations or violations Protocol Violations, Deviations, Unanticipated Problems and Adverse Events Form (FHS031)
Guidelines and Policies
- UCT research ethics code for use of animals in research and teaching
- UCT policy and standard operating procedures regarding scientific use of non-human primates
- Faculty of Health Sciences use of animals in teaching and research: standard operating procedures
- SANS (10386:2008) The care and use of animals for scientific purposes (Edition 1) .
Provides the minimum benchmark to ensure ethical and humane care of animals used for scientific and teaching activities, in line with the fundamental principles of Replace, Reduce and Refine animal use. AECs and researchers are expected to familiarise themselves with these guidelines, as appropriate
- Department of Health: Ethics in Health Research: Principles, Structures and Processes, 2015
AECs and researchers that review and conduct health research using animals must adhere to the Department of Health (2015) guidelines/ NHREC requirements for animal research ethics.
- US Office of Laboratory Animal Welfare
- US Public Health Service Policy on Humane Care and Use of Laboratory Animals
- Australian Code for the Care and Use of Animials for Scientific Purposes (8th Edition 2013)
- EUs Directive 2010/63/EU on the protection of animals used for scientific purposes
- Guide for the Care and Use of Laboratory Animals (8th Edition)
- Guide for the Care and Use of Agricultural Animals in Research and Teaching
- NC3Rs ARRIVE Guidelines - The ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments) are a checklist of information to include in publications describing animal research
- Scientists Center for Animal Welfare
- Database on Refinement of Housing and Handling Conditions and Environmental Enrichment for Laboratory Animals (Rodents, Rabbits, Cats, Dogs, Ferrets, Farm Animals, Horses, Birds, Fishes, Amphibians, and Reptiles.
Animal welfare training and resources
Introductory Course in Laboratory Animal Science the ethics, care and use of animals for scientific purposes
All faculty, staff, and students involved with the use of vertebrate animals in research and teaching purposes are required to complete the Introductory Course in Laboratory Animal Science the ethics, care and use of animals for scientific purposes before protocols are submitted for AEC approval. Please contact UCT Research Animal Facility and Veterinary Scientific Services for more information on the course .
Meeting and Submission dates
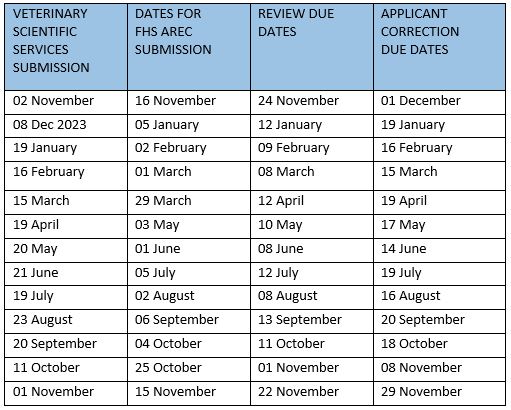
- Protocols should be submitted as early as possible BEFORE the submission date. This is essential to facilitate assessment by the reviewers prior to the full committee meeting. Submissions received late will be held until the next scheduled meeting. Please only use the latest forms available here as they will always be the most current version. Older versions of the forms will not be accepted
- Kindly note that the VSS (Veterinary Support Services) deadline date includes the timeline and time taken for any changes recommended by VSS and PI application revisions of protocols. Kindly ensure corrections are submitted timeously to VSS (at least 1 week prior to formal FHS AEC submission deadline via email to [email protected] )
Email [email protected] for the latest fee structure
The fee structure is developed purely to cover costs associated with animal research ethics review administration. The decision of the Animal Ethics Committee whether to approve a protocol is NOT in any way linked to payment of the ethics fee. If you have questions, please contact the Animal Ethics Administrative staff for information regarding the fee schedule.
Animal Research Ethics FAQs
Frequently Asked Questions Related to Research Involving Animals
Please refer to the Application forms and templates section of this webpage for all latest application forms.
AEC approval is valid for 3 years, after which an application for renewal or extension has to be submitted for review and approval .
Please send all questions to [email protected] .
Yes, you are not allowed to commence any research or teaching activities using dead animals or animal materiaL unless approval is obtained from the AEC.
Yes, you are not permitted to order or house any animals for research or teaching activities before AEC approval is granted.
Yes, please submit application for review and approval before commencement of study.
Yes, no teaching or any animal activities can commence without AEC approval
If you want to make any changes in the approved protocol before the expiry date.
Please complete and submit the final report form .
Explore UCD
- (opens in a new window) University Strategy
- University Governance
- President's Office
- Equality, Diversity & Inclusion
- Campus Development
- Study at UCD
- Current Students
- Campus Accommodation
- International Student Experience
- Access & Lifelong Learning
- Careers Network
- Sports Clubs
- (opens in a new window) Student Societies

Research & Innovation
- Innovation at NovaUCD
- Graduate Studies
- Support for Researchers
- (opens in a new window) Find a UCD Researcher
- UCD College of Arts and Humanities
- UCD College of Business
- UCD College of Engineering and Architecture
- UCD College of Health and Agricultural Sciences
- UCD College of Science
- UCD College of Social Sciences and Law
- All Colleges and Schools
- News & Opinion
- Work at UCD
- UCD in the Community
- Global Partnerships
- (opens in a new window) UCD Foundation
- University Relations
Key Services
- Staff Directory
- Sport & Fitness
- IT Services
- (opens in a new window) Commuting
- (opens in a new window) UCD Map
- (opens in a new window)
Animal Research Ethics Committe
Animal research ethics committee (arec).
The Animal Research Ethics Committee ensures that all ethical issues arising in connection with UCD research and teaching activities involving animals are identified and reviewed and that the use of animals can be ethically justified.
Appropriate decisions are taken to ensure that all procedures are conducted to the standards of international regulation and best practice and in accordance with the principals of the 3Rs: Replacement , Reduction and Refinement.
In order to support UCD in the delivery of the highest quality animal science and animal welfare, the Animal Research Ethics Committee review process includes protocols of work, accommodation and care of animals, and the training of those who use animals.
All AREC application forms and guidelines can be obtained via the (opens in a new window) AREC Intranet (PIs only).
Contact UCD Office of Research Ethics
- www.mandela.ac.za

Change the world
Office of Research Development
- Message from the Director
- Thematic Analysis
- Content Analysis
- Qualitative and Quantitative Data Collection and Analyses
- MathWorks_Elevate Your Workflows
- MathWorks_Research and Innovation
- MathWorks_Teaching and Learning
- FindFunding@RD
- Postgraduate Research Scholarships
- Internal Research Bursaries (IRB's)
- Student Account Refunds
- Postdoctoral and Research Fellowships
- Research Ethics Committee: Human (REC-H)
Research Ethics Committee: Animal (REC-A)
- Unit for Statistical Consultation
- RD Facebook Page
The Nelson Mandela University Research Ethics Committee (Animal) (REC-A) is charged with monitoring the treatment of animals used in both research and teaching at the institution. It has the responsibility of reviewing all protocols involving animal use in order to ensure that they are in accordance with acceptable ethical and scientific standards as described in the South African National Standard for the Care and Use of Animals for Scientific Purposes 10386:2021. The REC-A assists the institution in complying with legislation and creating an awareness of ethical conduct in researchers and teachers.
Respect for animals must underpin all decisions and actions of the people involved in the care and use of animals for scientific purposes. This respect is demonstrated by
- using animals only when it is justified,
- supporting the well-being of the animals involved,
- avoiding or minimizing harm, including pain, suffering, distress and lasting harm, to those animals,
- applying high standards of scientific integrity,
- the Replacement of animals with alternatives;
- the Reductio n in the number of animals used;
- the Refinement of techniques used to minimize the adverse impact on animals; and
- the knowledge and acceptance of one’s Responsibilities .
SANS 10386:2021_Ed 2: The care and use of animals for scientific purposes (Please note: This document is subject to copyright and is only available to staff and students at Nelson Mandela University. It may reside on a LAN, WAN, intranet, internet or ECM server and is available in accordance with copyright exploitation agreement no. 014/009/21-006, valid until 2023-03-31. Only students, lecturers and staff members of the Nelson Mandela University may make paper copies of the standard. No paper copy may be photocopied or reproduced in any way.’)
Applying for Ethics Approval from REC-A:
All applications for approval by Mandela staff and/or registered students must be submitted online on MEOS (Mandela Ethics Online System). This includes the following:
- Research studies: Any activity involving the acquisition, keeping or use of live animals for a research study
- Practicals: Any activity involving the acquisition, keeping or use of live animals for practical work
- Use of an External Animal Sample Collection: Any activity that requires the acquisition of already existing animal samples
- Use of Data from an Animal Sample Collection: Any activity where there there is a need for previously collected/secondary data to be used in a study
- Extension Applications
- Amendments to an Approved Protocol
- Reporting of an Unexpected Mortality/Adverse Event
- Reporting the Closure of a Research Study
Reporting on protocols not approved on MEOS:
Progress reports, amendments, extensions, etc. for protocols that were not approved on MEOS cannot be submitted on MEOS. The processes to be followed can be found on the previous REC-A webpage .
Reporting Research Misconduct:
Any member of the public or Nelson Mandela fraternity who is concerned that Mandela staff or student research might be contravening ethical research principles is asked to report their concerns. The reporting process as well as further information on what constitutes research misconduct is available on the Report Research Misconduct page.

Meeting Dates
Reference documentation, mandela university animal ethics reference documentation:.
- REC-A Standard Operating Procedures (January 2024): The Nelson Mandela University Research Ethics Committee - Animal is charged with monitoring the treatment of animals used in research and teaching at the University. This work-in-progress document outlines the standard operating procedures of REC-A.
- REC-A Terms of Reference : This can be found on the REC-A Terms of Reference and Standard Operating Procedures document of 2016.
- Guidelines for Ethical Conduct in the Care and Handling of Animals used for Research and Education at the Nelson Mandela University
- Nelson Mandela University Code of Conduct for Researchers: This policy gives expression to the values that apply at the Nelson Mandela University and to which all researchers (students, academic and support staff) commit themselves in their research activities.
- Nelson Mandela University Research Ethics Policy : This policy aims to promote awareness of fundamental ethical standards, principles and practices when conducting research with human participants.
Recommended Reading:
- South African Medical Research Council Research Ethics Policy
- South African Medical Research Council Guidelines on the Responsible Conduct of Research
- Department of Health Research Ethics Guidelines (2015) : This document provides the minimum national benchmark of norms and standards for conducting responsible and ethical research.
- World Organisation for Animal Health: Terrestrial Animal Health Code (2019)
How-To Users Guides / Videos
Meos applicant/supervisor user guides:.
- Applicant User Guide (ver 1.5, updated 19 Jun '23)
- Update Project (ver 1.0, published Aug '23)
- Responding to a Signature Request (ver 1.0, published 2 Jun '23)
MEOS How-to Video:
- Starting an ethics application (4.30 min)
For Enquiries about the REC-A Ethics Application Process:
Imtiaz Khan ( [email protected] ) Terry Adams ( [email protected] )
For Enquiries about Animal Ethics Principles:
Shaun Welman ( [email protected] ) Pierre Pistorius ( [email protected] )
For Technical Enquiries about the Online System:
Work@Mandela
FindIt@Mandela

Commercial Services@Mandela
Tel: +27 (0) 41 504 1111
Fax: +27 (0) 41 504 2574 / 2731
Email: [email protected]
PO Box 77000, Nelson Mandela University
Gqeberha, 6031, South Africa
Connect@Mandela
- A - Z Index
- Privacy statement

Privacy statement Mail & Portals --> BEE & Tax Certificate PAIA ISPA FAQ Sitemap A - Z Index --> WCMS
- MyExperience
- Research Ethics & Safety
- Human research ethics
Frequently Asked Questions (FAQs)
Below is a list of questions or common misconceptions from the REB survey conducted in 2022 to early 2023, and some frequently asked questions that the Ethics Office receives.
REB = Research Ethics Board
TCPS2 = Tri-Council Policy Statement
UAlberta REO = University of Alberta Research Ethics Office
HPRC = Human Participant Research Committee
Questions relating to the online ARISE system
How do i access the arise system for the first time.
The “Getting Started” document (linked here: Getting Started - University of Lethbridge applicants ) lists the steps you need to follow. You will first complete the CCID request form (linked here: CCID Request Form ), following which the UAlberta Research Ethics Office will send you an email containing a temporary password. Once you set your permanent password, it may take 24-48 hours for the ARISE system to be updated, and then you will be able to sign in and use the ARISE system.
Who do I contact if I have issues receiving or using my UAlberta CCID?
Contact the UAlberta Research Ethics Office at [email protected] to troubleshoot your CCID. Do NOT contact the general IT help desk through UAlberta, as they will just direct you to the ethics office.
How do I log into the ARISE system?
You can find a link to the ARISE login page on the main page of our human research ethics website: Human Research Ethics | University of Lethbridge (ulethbridge.ca) . Click on the link, then sign in using your UAlberta CCID.
Why am I not receiving emails or notifications from the ARISE system about my ethics applications?
You may have forgotten to change your preferred email address in ARISE to your ULethbridge email. Please check the “Getting Started” document (linked here: Getting Started - University of Lethbridge applicants ) for steps on how to do this.
Where can I get help learning to use the ARISE system?
The UAlberta Research Ethics Office has some great tutorial videos on their website that can be helpful for learning to complete a variety of functions in the ARISE system (linked here: Alberta Research Information Services (ARISE) System | Research + Innovation (ualberta.ca) ). They are also able to provide ARISE training workshops on request (contact [email protected] if interested), and our Ethics and Grant Coordinator is happy to meet with any ULethbridge researchers (including faculty, students, post-docs, and external researchers) to provide one-on-one or small group assistance either in-person or virtually. If you require assistance, please reach out to us at [email protected] and we would be happy to assist you!
Where can I find tutorials for the ARISE system?
Tutorial videos for using the ARISE system have been created by the UAlberta Research Ethics Office and are linked here: Alberta Research Information Services (ARISE) System | Research + Innovation (ualberta.ca) .
How long is the ARISE ethics application form?
The ARISE ethics application is a “smart form”, meaning that relevant sections of the form will appear based on what you select in previous sections. Thus, the length of your ARISE application form will depend on the type of research you are planning to conduct. A typical application is approximately 10 pages.
For students and postdoctoral fellows: Why is my supervisor’s name not showing up in the “supervisor” section of the ARISE form?
Your supervisor must also have access to the ARISE system (i.e. have a UAlberta CCID and be able to log in) AND have selected the “REB Supervisor” role to appear in the “supervisor” section of the form. Students must enter their supervisor before the ARISE application form will let them proceed beyond the first page . You will want to ensure that your supervisor has completed the first two steps in the “Getting Started” document at least one week before you plan to fill out your ethics application form to avoid delays.
Will ULethbridge continue to use the ARISE system in the future?
Yes, the change from the old paper-based system to the new online ARISE system was permanent and ongoing. Whether ULethbridge continues in our current agreement with the UAlberta Research Ethics Office or gets our own REB back, we will use the online ARISE system for the foreseeable future.
Do I need human research ethics approval for ___________?
Do i still need ethics approval if my project is unfunded.
Yes , ethics approval is required for all research involving human participants, their data, or their biological materials. The requirement for ethics approval rests on whether or not the project meets the definition of human participant research, regardless of whether the project is funded or not.
Do I need ethics approval for a student project?
Yes, all research or activities that meet the criteria for human participant research, including student projects, require ethics approval prior to participant recruitment, data collection, or working with human biological materials.
Do I need ethics approval for a student research project that is part of an independent study or undergraduate honour's thesis?
Yes, all research or activities that meet the criteria for human participant research (including student projects that are part of courses such as independent studies or honour's theses) require ethics approval prior to participant recruitment, data collection, or working with human biological materials.
Do I need ethics approval for students completing “mini” research projects as part of a course I am teaching?
Yes, all research or activities that meet the criteria for human participant research require ethics approval prior to participant recruitment, data collection, or working with human biological materials. However, for student “mini” projects there is an abbreviated “Instructor/Course-Based” research project application form in the ARISE system that can be filled out by the instructor prior to the beginning course and/or prior to the students conducting their mini research projects. To access this abbreviated form, log into ARISE, create a “New Human Study”, and on the first page for “Type of research/study” in Section 1.1 (6.0), select “ Instructor Course-based (where all students in a class, individually or in groups, conduct the same or similar MINIMAL risk research assignments, following project guidelines provided by instructor)”. For more information, please refer to the Course Based Guidelines . If you have additional questions about this process or would like assistance, please reach out to us at [email protected] .
Do I need ethics approval to conduct quality assurance / quality improvement work?
Please consult the following guidance Differences between Research, Quality Improvement and Quality Assurance for information on what qualifies as quality assurance/quality improvement. It is advisable that you complete a Request a Determination of Ethics Review Form to receive an official determination from the REB. As per the TCPS2, the REB has the final say as to the requirement for ethics approval. Erroneous self-determinations can lead to academic or other legal consequences.
I am the instructor of a course and want my students to conduct mini “research projects” with human participants to become familiar with research methods (i.e. conducting interviews). Do I need ethics approval for this?
Yes, these types of projects usually require human research ethics review and there is a specific short form tailored to these types of projects. In an ARISE ethics application, select “Instructor Course-Based Research” in Section 1.1 (6.0) and the ARISE “smart form” will appear on the following page for you to fill out once you click “Continue” from the first page. Once approved, this type of ethics application provides “blanket approval” for each of the mini research projects within a single course under the supervision of the instructor.
I’m planning a project with human research participants, but don’t know if I need ethics approval or not. How do I find out?
Please fill out the Request a Determination of Ethics Review form (linked here: Request a Determination of Ethics Review (google.com) ) and the UAlberta Research Ethics Office will get back to you with an official determination of whether or not your proposed project would require ethics approval.
Transition from ULethbridge HPRC to UAlberta REBs
Have all ulethbridge human research ethics functions been moved to ualberta.
No. We have made a decision to transition human ethics applications to the ARISE online platform hosted by UAlberta.
When and how will a decision be made about human research ethics functions at ULethbridge?
We view the current environment as a pilot test for what future options we have for supporting safe and ethical research with humans. Feedback will be sought throughout 2022 and 2023 to learn more about the benefits and challenges of sharing human ethics review and approval functions with UAlberta vs. hosting all functions solely at ULethbridge (while continuing to use the ARISE online platform). Many options exist for a hybrid UAlberta-ULethbridge REB and we want to consider and discuss all options before making a decision.
Decisions will not be made without an extensive pilot period, consultation and feedback options, and consideration of both researcher workload and TCPS2 requirements.
Why did ULethbridge lose our own independent Research Ethics Board (REB), called the Human Participant Research Committee (HPRC)?
At the beginning of November 2021, multiple issues suddenly arose that made it impossible for our REB (called the HPRC) to continue to function in accordance with the mandatory requirements from the Tri-Council Policy Statement (TCPS2). Since health/biomedical research at ULethbridge had been reviewed by the UAlberta Research Ethics Office (REO) since 2014, we arranged for the UAlberta REO to support all of our human ethics protocol revisions and approvals so as not to disrupt and halt research falling under these requirements. At this time, we have an ongoing temporary agreement with the UAlberta REO for them to review and approve all our human research ethics protocols, and we are strategizing a long-term solution.
Will we ever get our own Research Ethics Board (REB) back at ULethbridge?
This is certainly a possibility! Our office is diligently working towards seeing if this would be feasible in the future. Our current arrangement with the UAlberta Research Ethics Office extends until Spring 2025, at which time our current arrangement would need to be renewed or our own REB would need to be fully up and running.
What would be required for us to get our own Research Ethics Board (REB) back at ULethbridge?
The most important requirement for getting our own REB back is having enough ULethbridge faculty members volunteer to serve on the REB. According to the TCPS2-2022 (linked here: Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans – TCPS 2 (2022) (ethics.gc.ca) ), all REBs in Canada are required to have a minimum of 5 members with various types of expertise (in research, ethics, and relevant laws), and to have additional members in proportion to the number and types of ethics applications received. Here at ULethbridge, we would require a minimum of 10 members with various areas of research ethics expertise to meet the TCPCS2-2022 minimum requirements and to support the number and diversity of ethics applications we receive.
*Note that health and biomedical research applications would continue to be reviewed by the UAlberta REB as mandated by the Minister of Health and the Health Information Act Designation Regulation.
What can I do to get involved and/or learn more?
We are always looking for additional ULethbridge faculty members to serve on the current UAlberta/ULethbridge Shared REB! This is an excellent way to fulfill the service requirements of your faculty position. Faculty serving on the shared REB can generally expect to spend 4-6 hours per month reviewing ethics applications and attending regular meetings (depending on the specific REB they are serving on). For more information, or to volunteer, please contact our Ethics and Grant Coordinator at [email protected] .
General Queries
How long will it take to get ethics approval for my project.
The current average time from initial submission of a minimal risk ULethbridge ethics application to approval is around two weeks, with most ethics applications receiving approval in approximately one month. The REB specialist(s) and delegated reviewer(s) may request revisions as part of this process, so some of this timeframe depends on how quickly you are able to revise and submit the requested revisions. Review times are also impacted by submission volume and reviewer availability.
Why do the minimal risk social sciences and humanities ethics applications go through the same “medicalized” ethics model as biomedical and health-based research?
One of the main purposes of human research ethics review is to prevent the harm of participants or society as a result of research. Many of the past harms to human participants from research activities were within the realm of medical research, and these atrocities led to international guidelines for the treatment of human participants in research. Here in Canada, our national guideline is the Tri-Council Policy Statement for Ethical Conduct for Research Involving Humans ( TCPS2-2022 ), and this policy takes a proactive approach to preventing the harm of human participants in research by upholding the three core principles: Respect for Persons, Concern for Welfare, and Justice. In upholding these principles as defined in the TCPS2-2022, issues such as informed consent, voluntary participation in research, data confidentiality, assessment of the risks versus the benefits of participating, addressing real or potential conflicts of interest, and others are applicable to both medical research and non-medical research (such as interviews or surveys). While some of the requirements of the TCPS2-2022 may seem overly strict for human participant research in the social sciences and humanities, the same three core principles apply and must be upheld to protect human participants, society, and researchers.
While some of the questions in the ethics application form may seem daunting, it is important to keep in mind that the process requires us to consider these important and complex questions for good reasons that will ultimately improve the outcomes of our research, the validity of our data, and the safety of everyone involved.
It is also important to recognize that our current human research ethics process reflects a colonized, Eurocentric point of view. Going forward, we are hoping to incorporate more Indigenous values such as knowledge sharing, but we acknowledge that we have a long way to go.
How can I contact a human research ethics person at ULethbridge? (Do we have one?)
The ULethbridge contact for human research ethics questions is the Ethics and Grant Coordinator, Haley Dennis. Haley can be reached at [email protected] or 403-329-2464. Her office is B633, which is inside the Office of Research & Innovation Services (B610).
How can I contact the UAlberta Research Ethics Office?
The UAlberta Research Ethics Office can be reached by emailing [email protected] , or if you have questions about your submitted ARISE application, you can send an email to your REB Specialist within ARISE or find their email address through the ARISE system by clicking on their name.
Can someone from the ethics office come to my class to do a presentation on human research ethics?
Yes , we would be happy to arrange this! Please contact our office at [email protected] to discuss what type of presentation you would like to have.
This webpage was updated May 27, 2024
House Ethics Committee will investigate Rep. Henry Cuellar after his federal indictment
by: Associated Press
Posted: May 29, 2024 / 03:37 PM EDT
Updated: May 29, 2024 / 03:42 PM EDT
WASHINGTON (AP) — The House Ethics Committee is opening an investigation into Rep. Henry Cuellar, D-Texas, after his indictment earlier this month on allegations of bribery, money laundering and working on behalf of a foreign government.
The committee said Wednesday that it voted unanimously to take the rare step of pursuing an investigation into Cuellar, 68, while he and his wife remain under investigation by the Department of Justice over the couple’s ties to the former Soviet republic of Azerbaijan.
“The Committee is aware of the risks associated with dual investigations and is in communication with the Department of Justice to mitigate the potential risks while still meeting the Committee’s obligations to safeguard the integrity of the House,” said the chair, Rep. Michael Guest, R-Miss., and ranking member, Rep. Susan Wild, D-Pa., said in a statement.
Cuellar, in a statement to The Associated Press, said he respects the committee’s work. He said he is “innocent of these allegations, and everything I have done in Congress has been to serve the people of South Texas.”
House and committee rules require that within 30 days of a member being indicted or formally charged, the committee shall either establish an investigative subcommittee to look into the allegations or publicly report its reasons for not doing so.
Guest and Rep. Glenn Ivy, D-Md., will lead the review of Cuellar. The committee cautioned in its statement that opening an investigation does not mean that the lawmaker has violated House rules.
Culler is the third lawmaker to face a federal indictment since the current congressional session began in January. He has said that he and his wife, Imelda Cuellar, 67, “are innocent of these allegations.”
In a statement in early May, Cuellar said that before he took action, “I proactively sought legal advice from the House Ethics Committee, who gave me more than one written opinion, along with an additional opinion from a national law firm.”
The indictment against the couple states that from 2014 to 2021, they accepted nearly $600,000 in bribes from an Azerbaijan-controlled energy company and a bank in Mexico, and in exchange, Cuellar agreed to advance the interests of the country and the bank in the United States.
Among other things, Cuellar agreed to influence legislation favorable to Azerbaijan and deliver a pro-Azerbaijan speech on the floor of the House, according to the indictment.
In addition to bribery and conspiracy, Cuellar and his wife face charges including wire fraud conspiracy, acting as agents of foreign principals and money laundering. If convicted, they face up to decades in prison and forfeiture of any property linked to proceeds from the alleged scheme.
Thanks for signing up!
Watch for us in your inbox.
Subscribe Now
News 8 Breaking News Alerts
Trending stories, multi-million dollar movie to film in connecticut …, paid sick day requirement signed into connecticut …, rain with a few storms tonight and tomorrow morning, man charged with murder of 2 teens in hartford, stop & shop set to close underperforming stores, 4 shot on mitchell avenue in waterbury, man faces animal cruelty charges after video shows …, ag tong sues naugatuck car dealership for deceptive ….
Notable Places in the Area

Elektrostal Satellite Map
Popular Destinations in Moscow Oblast
Escape to a random place.
An official website of the United States government Here's how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
May 2024 Air Travel Consumer Report
About this document.
The Air Travel Consumer Report is a monthly product of the Department of Transportation's Office of Aviation Consumer Protection. The report is designed to assist consumers with information on the quality of services provided by the airlines.
Data Included in this Report
Flight Delays: March 2024 / January- March 2024 Mishandled Baggage / Wheelchairs and Scooters: March 2024 /January - March 2024 Oversales: 1st Quarter 2024 (January- March 2024) Consumer Complaints: See Report for Details Customer Service Reports to the Dept. of Homeland Security: March 2024 Airline Animal Incident Reports: March 2024
Individual redacted animal incident reports may be seen by clicking the airline’s name in the report.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Korean Med Sci
- v.38(25); 2023 Jun 26
- PMC10293659

Ethics Committees: Structure, Roles, and Issues
Pankti mehta.
1 Department of Clinical Immunology and Rheumatology, King George’s Medical University, Lucknow, India.
Olena Zimba
2 Department of Clinical Rheumatology and Immunology, University Hospital in Krakow, Krakow, Poland.
3 National Institute of Geriatrics, Rheumatology and Rehabilitation, Warsaw, Poland.
4 Department of Internal Medicine N2, Danylo Halytsky Lviv National Medical University, Lviv, Ukraine.
Armen Yuri Gasparyan
5 Departments of Rheumatology and Research and Development, Dudley Group NHS Foundation Trust (Teaching Trust of the University of Birmingham, UK), Russells Hall Hospital, Dudley, UK.
Birzhan Seiil
6 Department of Biology and Biochemistry, South Kazakhstan Medical Academy, Shymkent, Kazakhstan.
Marlen Yessirkepov
An Ethics Committee (EC) is an independent body composed of members with expertise in both scientific and nonscientific arenas which functions to ensure the protection of human rights and the well-being of research subjects based on six basic principles of autonomy, justice, beneficence, nonmaleficence, confidentiality, and honesty. MEDLINE, Scopus, and Directory of Open Access Journals were searched for studies relevant to this topic. This review is focused on the types of research articles that need EC approval, the submission process, and exemptions. It further highlights the constitution of ECs, their duties, the review process, and the assessment of the risk-benefit of the proposed research including privacy issues. It’s pertinent for academicians and researchers to abide by the rules and regulations put forth by ECs for upholding of human rights and protecting research subjects primarily, as well as avoiding other issues like retraction of publications. Despite various issues of cost, backlogs, lack of expertise, lesser representation of laypersons, need for multiple approvals for multisite projects, conflicts of interest, and monitoring of ongoing research for the continued safety of participants, the ECs form the central force in regulating research and participant safety. Data safety and monitoring boards complement the ECs for carrying out continuous monitoring for better protection of research subjects. The establishment of ECs has ensured safe study designs, the safety of human subjects along with the protection of researchers from before the initiation until the completion of a study.
Graphical Abstract

INTRODUCTION
The journey of the role of ethics in biomedical research began with “The Doctor’s Trial” post-World War II in which 23 doctors and administrators were tried for war crimes, crimes against humanity, and conducting research without informed consent. This judgment, known as the “Nuremberg Code” was one of the first international ethical standards which gave a ten-point rule with respect to the protection of human research participants. The core principle was the requirement of voluntary consent of human subjects and respecting human autonomy. 1 , 2
However, some researchers continued to ignore the code and violations like the Willow Brook Hepatitis Study (1956), Jewish Chronic Disease Study (1963), and 22 others were highlighted by Beecher in 1966. 3 , 4 This led to the composition of the Declaration of Helsinki by the World Medical Association in Finland in 1964 with revisions at regular intervals. 5 This affirmed the principles highlighted in the Nuremberg Code stating that research should be conducted upholding the interests and rights of the human subjects. It proposed for the first time, the submission of a research protocol to an ethics committee (EC) before the initiation of the study ( Fig. 1 ). 6

EC refers to “Committees established by professional societies, health facilities, or other institutions to consider decisions that have bioethical implications. The role of these committees may include consultation, education, mediation, and/or review of policies and practices.” Committees that consider the ethical dimensions of patient care are Clinical ECs whereas committees established to protect the welfare of research subjects are Research ECs. 7 In this review, we will be using the terms ECs and Research ECs interchangeably.
It was in the 1960s that most nations developed guidelines regarding the formation of ECs with the main task of protection of human subjects. 8 EC is an independent body composed of members with expertise in both scientific and nonscientific arenas which functions to ensure the protection of human rights and the well-being of research subjects. ECs can be of two types—Institutional Review Boards (IRBs) or Institutional ECs (IECs) (referred to IRB or IEC by different countries) that are formally constituted by an institution to review research projects for that institute. An independent EC is an autonomous EC that is not part of any institute and performs the same functions independently. It is helpful for institutes that don’t have an IRB.
Despite these regulations, the unethical standards of the Tuskegee Syphilis study emerged in 1972 in which treatment was denied to the participants in order to study the natural course of the disease. This was followed by the conversion of the National Research Act in the USA into law (1974) and the setting up of the national commission of ‘International Ethical Guidelines for Biomedical Research Involving Human Subjects’ that submitted the Belmont report in 1979. The Belmont report described the role of assessment of risk-benefit of research involving human subjects, appropriate guidelines for selection of human subjects, and definition of informed consent. It was based on the three pillars of ethics- respect, beneficence, and justice. 9 , 10 It stressed the need for the approval of studies by an EC in accordance with the 1975 revision of the World Medical Association in Tokyo. Subsequently, countries like China, India, and South Korea adopted and legalized the need for submission of protocols to ECs from the 1980s onwards. 11 , 12 , 13 , 14
ECs function on six basic principles 15 :
- 1. Autonomy: respect the patient’s right to act on his/her own value and choice.
- 2. Justice: fair treatment of the research subjects.
- 3. Beneficence: work for the benefit of the patient.
- 4. Nonmaleficence: primum non-nocere or first do no harm to the patient.
- 5. Confidentiality: privacy protection.
- 6. Honesty: truthfulness in terms of the study.
Ethics approval is required for most research studies to uphold the above-mentioned principles, and protect the participants as well as the researcher. 16
In this narrative review, we aim to study the structure and function of ECs or IRBs with a focus on the composition, role, violations, and development perspectives of ECs.
Searches through MEDLINE (PubMed) and Scopus were performed in line with previously published recommendations. 17
Articles published till March 15, 2023 were reviewed using the following keywords: ("Ethics Committees, Clinical/classification"[Mesh] OR "Ethics Committees, Clinical/economics"[Mesh] OR "Ethics Committees, Clinical/ethics"[Mesh] OR "Ethics Committees, Clinical/history"[Mesh] OR "Ethics Committees, Clinical/legislation and jurisprudence"[Mesh] OR "Ethics Committees, Clinical/organization AND administration"[Mesh] OR "Ethics Committees, Clinical/standards"[Mesh] OR "Ethics Committees, Clinical/statistics and numerical data"[Mesh] OR "Ethics Committees, Clinical/trends"[Mesh]). Additional searches about subtopics were also carried out (“Data Safety Monitoring Boards” OR “Independent Data Review Committees”, “Institutional Review Boards” OR “Ethics Committees” and “Problems” OR “Issues”).
Articles in languages other than English, and reviews, conference proceedings, and editorials were excluded. Relevant articles searchable at the Directory of Open Access Journals and references of included articles were also processed for eligibility and inclusion for this narrative review. 18 , 19 , 20 , 21
RESEARCH, SUBMISSION PROCESS, AND EXEMPTIONS
IRB approval is required for most research to protect human rights and assess the scientific soundness of the research. For this, we first need to understand what research is. Research is defined as “a systematic investigation, including research development, testing, and evaluation, designed to develop or contribute to generalizable knowledge” ( Table 1 ). 8
IRB = Institutional Review Board.
An EC approval is required for studies with more than minimal risk to the subjects where the intention is to publish findings or contribute to the scientific knowledge, studies involving the compilation or analysis of data containing patient identifying information, studies with any risk of physical or mental discomfort to participants or their families, and studies on vulnerable groups. 22 Minimal risk refers to the probability of discomfort posed by the research is not greater than that ordinarily encountered in routine daily life activities of an average healthy individual. 5 , 8 , 22
Thus, even surveys and archived data that contain patient identifying information (name, age, address) and sensitive information (illicit drug use, comorbidities, communicable diseases, e.g., HIV AIDS) need ethical approval to uphold the privacy and anonymity of the participants as well as protection the possibility of psychological discomfort to them. 10 , 23 , 24 , 25
Some studies may be exempted from ethical approval including most educational research, case reports on one to three patients (without any hypothesis testing), those that pose no risk to the participants, involve information freely available in the open domain for the community, analysis of open-source datasets or anonymized datasets obtained from other researchers with due informed consent taken at the time of primary data collection, research evaluating the public health programs or government public schemes. 26 , 27 However, a formal exemption is to be decided by the IRB and not the investigator. 8 , 28
For projects requiring an EC approval, the type of reviews includes expedited and a full board review. Expedited review is for research involving no more than minimal risk to the subjects, minor revisions of an already approved study, and is usually conducted by an experienced person or the chair of the IRB. A full board review on the other hand is for research with greater than minimal risk to the subjects or those involving vulnerable populations. This is reviewed extensively by a full IRB meeting.
The documents usually required for a full ethics review include the name of the applicant with designation, approval of the head of the department, research/trial protocol, ethical issues if any, and plans to address them, written informed consent form (and assent forms) in the language the participant understands, data collection tools, patient information sheet, regulatory clearances (e.g., Drug Controller General of India in India for drug trials), finance and funding details, Insurance, statement of conflicts of interest, information about payment or compensation to the subjects, scientific or departmental review board permission, Curriculum Vitae of the investigators, declaration of interests and any other relevant information. 29 , 30 Waivers of consent may be provided for no more than minimal risk to the subjects when the waiver will not endanger the rights and welfare of the subjects like retrospective studies, secondary analysis of data wherein consent had been taken previously, use of open access databases with anonymized data, and emergency research as seen fit by the EC. 31 , 32 In emergencies like the coronavirus disease 2019 pandemic, waivers may be provided if the patient is incapacitated or in life-threatening situations where there is no time for informed consent. Pandemics like these may even call for common documents for risk disclosure and audio/video/electronic consent. 33 , 34
EC PERSONNEL, THEIR EXPERIENCE, AND DUTIES
ECs have the primary responsibility of reviewing research and its alignment with the Good Clinical Practice (GCP) guidelines. 35 The research design must be scientifically sound and conducted in an ethical way to include human subjects with voluntary informed consent.
The composition of ECs varies depending on the country, center, volume, and nature of the research reviewed. However, there are some basic recommendations laid down by national authorities and GCP. 30
- a) Most countries in Europe, the USA, and South Korea have a requirement of at least five members whereas recommendations in China and India need a minimum of seven members and a maximum of 12–15 members. 14 , 36 , 37 , 38 , 39
- b) At least one member who is autonomous, independent of the institution or trial site. It is mandatory that the chairperson of the EC is not part of the institution where the research is to be conducted.

Others include a member secretary from within the institution and members from the scientific field. The composition should have an adequate gender and age representation with a blend of basic scientists, clinician scientists, one legal expert, one social scientist, one philosopher, and one layperson. Review of research involving vulnerable populations like children, pregnant women, handicapped, prisoners, etc., must involve one member with expertise in dealing with that population. 40 , 41 It is also desirable to have a member or expert advisor for special areas of research who has proficiency in that field.
Responsibilities
The chairperson has the primary responsibility of independent and smooth functioning of the EC, ensuring the participation of all members, seeking Conflict of Interests from all members, and handling complaints against the researchers and EC members. 39 It’s the responsibility of the member secretary to schedule EC meetings, handle documentation, organize an effective review of proposals, define and maintain adherence to standard operating procedures (SOPs), train EC members, and assess the need for expedited reviews/exemption from review. 39 The members of the scientific community have the primary responsibility of reviewing the research protocols and their scientific soundness. The non-scientist member is crucial to safeguard the human subjects and practical issues of the research. 40 , 41 However, studies have shown lesser participation by laypersons as compared to scientific members. A study conducted across 10 academic centers across the USA with 20 IRB meetings recorded noted that 29 community members were present in 17 of those meetings. They were primary reviewers in only two of the 93 submitted protocols due to refusal on grounds of lack of knowledge regarding medical research. Even as secondary and tertiary reviewers, they were less active and were more likely to focus on issues related to confidentiality. However, they played a greater role when they were not designated reviewers. 42
The EC or IRBs function to review and approve research protocols, monitor ongoing research involving human subjects with the aims of continual protection of human volunteers, advancement of research, and protecting the institute from litigation. Its main role is the protection of the human rights, autonomy, confidentiality, and welfare of the research subjects especially vulnerable populations. The GCP recommends the following for duties of the IRB ( Table 2 , Fig. 3 ) 35 :
e.g., An immunosuppressive drug “X” being evaluated for patients with Lupus Nephritis.
IRB = Institutional Review Board, SOC = standard of care, GCP = Good Clinical Practice, MMF = mycophenolate mofetil, CYC = cyclophosphamide, ICU = intensive care unit, SOP = standard operating procedure, RCT = randomized controlled trial.

- • The IRB should obtain and review all the necessary documents for the research/trial within a reasonable time and document its views following standardized operating procedures with clear identification of the dates for approval, modifications, disapproval, or termination of an ongoing trial that was initially approved in writing.
- • Qualification of the investigators should be considered for the proposed research.
- • Reviewing of ongoing research as appropriate to the risks involved (at least once a year).
- • Protocols indicating exemption of prior consent of the subject or their legally acceptable representative (e.g., emergency situations) should be assessed in detail for all the regulatory needs.
- • Review the sum and method of compensatory payment to subjects if required.
- • Functions should be performed as per written SOPs which should comply with the GCP guideline.
Most IRBs conduct meetings regularly (one–two per month depending on the number of protocols) and SOPs are followed as per the national governing authority.
An EC review is a continuous process and is needed before the initiation of research, before the extension of the approval period, prior to modifications to an already approved study, for monitoring of any adverse events, and until all the data collection and analysis is complete. 8 An oversight to the monitoring of trials (usually single center, early phase, less risky) is provided by the IRBs through annual reviews, adverse event monitoring, and reporting of undue events by the principal investigator (PI). However, complex clinical trials and/or multicenter, randomized controlled trials, interventional studies with pre-existing concerns about safety, or study participants who might need additional protection through an additional committee referred to as the Data Safety Monitoring Board (DSMB). 43 , 44
RISKS, BENEFITS, CONFIDENTIALITY, AND PRIVACY ISSUES IN RESEARCH PROTOCOLS
The role of the EC is not only to provide direct protection to human subjects from physical or mental harm but also to weigh the risks and benefits involved in the research. It must be assessed if the study is designed to add to the current scientific knowledge base and help society. 8
The research protocol is the document that includes the research question, aims and objectives, a critical literature review, methodology, and statistical plan. It is pertinent that the IRB reviews the protocol with respect to the clarity and focus of the research question; and whether the study design is suitable to answer the same. This is decided by the chair or a special departmental committee ( Table 2 , Fig. 3 ).
Privacy and confidentiality are a part and parcel of every physician-patient relationship. Needless to say, this must be maintained in a researcher-human subject relationship as well. It helps build trust, curbs participant anxiety, maintains their dignity, and above all their autonomy. 10 The International Committee of Medical Journal Editors recommends that authors must ensure that nonessential information like names, initials hospital record numbers, etc., are omitted during data collection, storage, and publication whenever possible. 45 However, there’s an extent to which this confidentiality can be maintained. Information required for scientific purposes (e.g., clinical photographs) or those with mandated legal reporting may breach participant privacy. This needs to be explained to the participant and recorded in written informed consent ( Table 2 ).
The role of the IRB with respect to privacy and confidentiality is to:
- • Review the consent document and assess the sensitivity of the information, the duration for which it will be held, the usefulness of the information, and the ability to protect it.
- • For multicenter projects, review the measures taken by the research team to maintain the privacy of the research subjects including the number of personnel with access to the information, data storage, and transfer.
- • An ongoing review of the research must include monitoring of confidentiality issues to check for maintenance of the same and the need for a revised privacy protection plan.
- • Educate researchers and IRB members regarding the data privacy and protection process. 46
Review of informed consent by IRBs is especially important in low-middle-income countries. There are various issues related to the lack of understanding of the information provided, maintaining privacy due to interference by family members, and the inability to assess risk and benefit by the research participant. IRBs have an additional responsibility to ensure that studies have minimal/no risk to the participant, the consent forms are clear and simple to understand and ensure the proper process of obtaining informed consent is being followed without undue pressure or coercion to participate in the study. 47
VIOLATIONS OF ETHICS APPROVAL RULES AND REGULATIONS
Violations of IRB approval rules like lack of approval, lack of approval of modifications to the protocol, and lack of informed consent can result in dire aftermaths for the authors. It can result in the withdrawal of the article if it’s still in press, retraction if it’s already published, and even removal if it has legal consequences. The number of papers retracted as searched on the retraction database 48 is steadily increasing by the decade from 474 in the 1990s to 6120 in the 2010s. The most common reason for retraction is plagiarism whereas violation of IRB rules accounts for 4–5% of all retractions. 49 , 50 When consultations for ethical inquiries to the Korean Association of Medical Journal Editor were analyzed, the most common reason was duplicate publications (12 of 80) with issues with IRB approval (5 of 80) and informed consent (6 of 80). 51 Some of the examples of types of studies and their reasons for retractions have been summarized in Table 3 .
Violations can be assessed before the studies are published for those with IRB approval. It is the responsibility of the IRBs to monitor whether ongoing studies are abiding by the ethical regulations and whether the approved protocol is being followed. A study conducted in India by an IRB at a tertiary care hospital in Mumbai monitored 12 clinical trials from 2011–2017. The most common violations were related to informed consent, followed by a lack of understanding of protocol and protocol deviations. This was corrected by re-taking of the informed consent and retraining in GCP by the IRB. 52 A similar study in Uganda done from 2007–2010 with monitoring of 40 research projects also found a similar frequency and reasons for violations. 53
Journal editors routinely check if a statement mentioning whether ethics approval was sought has been mentioned in the manuscript. Depending on the journal and type of article, further details of the EC approval can be sought by the journal editorial board. 54
ISSUES AND ONGOING DEVELOPMENTS
ECs were developed to provide ethical oversight to clinical research. But here are various issues associated with the functioning of IRBs.
- • Composition: Most studies indicate a skewed gender representation in the structure IRBs. Further, the participation of laypersons on the board is minimal. 14 , 42 , 55 , 56 , 57
- • Overburdened IRBs, delays, and operational costs: The IRB reviews have been associated with delays from over 4 to 7 months on average from surveys conducted across the USA. 58 , 59 A delay in biomedical research can translate into more than monetary loss as biomedical research saves lives and a delay in the approvals can result in greater loss of life. 60 An older survey conducted across 63 institutions (with 20 being low volume, 24 intermediate volume, and 19 being high volume centers) in the USA in 2005 reported the median amount spent by academic medical centers on IRB was $750,000/year with an average of $559 per review. The main costs are divided across staff salary, board salary, space, outsourcing of the reviews, travel, supplies, and equipment. 61 Over the years, there is a definite increase in the number of ongoing research projects thus increasing these costs further. Furthermore, documentation of Food and Drug Administration (FDA) warning letters to IRBs was predominantly related to paperwork stressing on documentation of reviews and meetings rather than ethical issues. 62 Increasing paperwork further results in delays and added costs. These deficiencies are more marked in developing nations like India and China dealing with issues like lack of regulation, informal ethics reviews, lack of supervision, and insufficient ethics review capacity. 63 , 64
- • Multi-site projects: With multicenter projects on the rise, a single protocol is often reviewed by multiple IRBs. In a review of 17 articles reported from UK, USA and Europe, which underwent multiple IRB reviews of the same protocol there were discrepancies in the judgment. Five of 26 reported rejection at some and acceptance by some IRBs. However, there were great differences in the protocol revisions, consent, patient information sheets, risk-benefit assessment, and compensation arrangements. 65 Keeping these issues in mind, the Common Rule in the USA was revised in 2017 with IRB approval required only from one center for multisite projects. 66 This may be extrapolated to other nations or consideration of an expedited review at other sites when fully reviewed at one IRB can be considered.
- • Independent EC and IEC: Independent ECs have inherent tissues of limitation of knowledge about the local community and use of these may promote IRB shopping. Whereas, local IRBs can have conflicts of interest as colleagues of investigators may be on the review board. Thus, a central IRB can alleviate some of these concerns by avoiding repetitive reviews, minimizing conflicts, and establishing a centralized adverse event reporting system. 67 , 68 , 69 A central IRB can be formed by experts on a particular subject or by a group of institutes like the National Cancer Institute’s Central IRB and the Biomedical Research Alliance of New York respectively. 70 , 71
- • Scientific expertise of the IRB reviewers: The IRB reviewers may lack the scientific expertise to review sophisticated research projects that may affect the quality of the research. 11 , 14 , 57 , 72 Regular training in research ethics and GCP along with adequate consultations with external experts is needed. This can be done at a national, regional, and international level. First, by identifying core issues and then solutions for them by focused training. 73 Training of EC members is conducted across Central Asia and Eastern Europe under the framework of Forum for Ethics Committees in the Confederation of Independent States and Strategic Initiative for Developing Capacity in Ethical Review program that train members regarding GCP, bioethics, the establishment of an EC, review processes and SOPs, choosing independent consultants, and confidentiality agreements. 74
- • Review of studies involving complementary and integrative medicine (CIM) is a challenge due to the lack of quality evidence to support the basis for their use. Moreover, most international regulatory bodies and research regulations do not address CIM, thus leaving the review process and decision-making to the IRBs. However, it is to be emphasized here studies irrespective of the type (modern or CIM) must be reviewed using the same principles of respect, beneficence, and justice. Well-designed studies on CIM are essential to ascertain the health and safety of patients. 75
DSMB is defined by the FDA, USA as “a group of individuals with pertinent scientific expertise that review research data of an ongoing trial on a regular basis, advises the sponsor/or researcher regarding the continuing safety of research subjects and those yet to be recruited into the research trial, and advises as to the continuing validity and scientific merit of the trial.” 76 It’s an autonomous entity independent of the researchers, sponsors, and the IRB so as to control data sharing and protect the authenticity of the clinical trial from unfavorable impact. 35 It was first developed in the USA in the 1960s as the NIH began sponsoring multicenter trials, the first trial was the Coronary Drug Project which used a DSMB for monitoring. 77 Over time, it became a common practice for the sponsors to have experienced scientific personnel serving on these committees. Although the FDA does not mandate DSMB for all trials, DSMBs are generally recommended for large, multi-site studies evaluating treatments that intend to reduce mortality and morbidity.
DSMBs are usually constituted for:
- • The study outcome is such that a highly encouraging or detrimental result is a possibility in an interim analysis that may require an early termination of the study on ethical grounds.
- • When the safety concerns are high, e.g., invasive therapy is administered.
- • Previous data suggesting serious toxicity with the study treatment.
- • Studies involving vulnerable populations.
- • Studies including subjects at an increased risk of death or serious outcomes.
- • Large, multisite, long-duration studies.
In India, it is recommended by the Indian GCP guidelines that the sponsor may establish a DSMB to assess the progress of the trial, and in 2006 Indian Council of Medical Research (ICMR) mandated a DSMB to review data emerging from research on interventions in the emergency setting. 39 These were updated in 2012 by the ICMR to include all stem cell research involving human subjects. The SOPs for the constitution and responsibilities of the DSMB are laid down by the World Health Organization and are similar across USA, Europe, and South Korea. 78 , 79
DSMBs are constituted by scientific members and are appointed by the funding agency, before the recruitment of the first subject in the trial. It can consist of as few as three members and is typically constituted of clinicians and at least one biostatistician. Others that may be included are medical ethicists, other scientists, etc. The most important requisite is that the members should be independent of the sponsors, investigators IRBs, regulatory authorities, and site or study staff. They should have no conflicts of interest with the sponsors, researchers, or study staff.
The functions of the DSMB are:
- • To uphold participant safety.
- • Ensure credibility and integrity of the trial for future subjects.
- • Ensure the timely conclusion of the study so that the results can be disseminated.
- • Identify protocol violations if any.
- • Identify unexpectedly high dropouts and evaluate for the same.
- • Ensure the validity of the results.
The above functions are carried out by an initial organizational meeting to understand the protocol and safety monitoring plan followed by an early safety review meeting to review early safety information. Continuing periodic reviews to assess safety, efficacy, and the progress of the trial are then carried out with reporting of serious adverse events. 44 A final meeting is to be held at the termination of a study. DSMBs function independently of the IRBS but the PIs must submit DSMB reports or minutes to the IRB.
Dramatic instances in which trials have been stopped prematurely on the recommendation of the DSMB include the withdrawal of rofecoxib and celecoxib in two trials on the prevention of colonic polyps due to increased cardiovascular events. 80 , 81
We have come a long way from the horrific ethical compromises in clinical studies in history to establishing adequate safety for the human subjects participating in clinical research today. The establishment of the IRB or EC has ensured safe study designs and the safety of human subjects right from before the study initiation until its completion. This is further supplanted by additional boards like DSMBs. However, we still need studies assessing the outcomes of the ECs on a global basis and addressing various issues that are still pertinent to the working of the ECs. 82
Disclosures: The authors have no potential conflicts of interest to disclose.
Author Contributions:
- Conceptualization: Mehta P, Zimba O, Gasparyan AY, Seiil B, Yessirkepov M.
- Data curation: Mehta P.
- Writing - original draft: Mehta P.
- Writing - review & editing: Mehta P, Zimba O, Gasparyan AY, Seiil B, Yessirkepov M.
Current time by city
For example, New York
Current time by country
For example, Japan
Time difference
For example, London
For example, Dubai
Coordinates
For example, Hong Kong
For example, Delhi
For example, Sydney
Geographic coordinates of Elektrostal, Moscow Oblast, Russia
City coordinates
Coordinates of Elektrostal in decimal degrees
Coordinates of elektrostal in degrees and decimal minutes, utm coordinates of elektrostal, geographic coordinate systems.
WGS 84 coordinate reference system is the latest revision of the World Geodetic System, which is used in mapping and navigation, including GPS satellite navigation system (the Global Positioning System).
Geographic coordinates (latitude and longitude) define a position on the Earth’s surface. Coordinates are angular units. The canonical form of latitude and longitude representation uses degrees (°), minutes (′), and seconds (″). GPS systems widely use coordinates in degrees and decimal minutes, or in decimal degrees.
Latitude varies from −90° to 90°. The latitude of the Equator is 0°; the latitude of the South Pole is −90°; the latitude of the North Pole is 90°. Positive latitude values correspond to the geographic locations north of the Equator (abbrev. N). Negative latitude values correspond to the geographic locations south of the Equator (abbrev. S).
Longitude is counted from the prime meridian ( IERS Reference Meridian for WGS 84) and varies from −180° to 180°. Positive longitude values correspond to the geographic locations east of the prime meridian (abbrev. E). Negative longitude values correspond to the geographic locations west of the prime meridian (abbrev. W).
UTM or Universal Transverse Mercator coordinate system divides the Earth’s surface into 60 longitudinal zones. The coordinates of a location within each zone are defined as a planar coordinate pair related to the intersection of the equator and the zone’s central meridian, and measured in meters.
Elevation above sea level is a measure of a geographic location’s height. We are using the global digital elevation model GTOPO30 .
Elektrostal , Moscow Oblast, Russia
Leading Womanist Ethicist and Theologian Named New MLK Professor of Religion and Black Studies at STH
Emilie townes says she hopes to foster conversations in an increasingly polarized culture.

Emilie Townes comes to BU from Vanderbilt University’s Divinity School, where she was dean emerita and the Distinguished Professor of Womanist Ethics and Society. She previously taught at Yale University and Union Theological Seminary. Photo courtesy of Vanderbilt Divinity School
Emilie Townes was all set to retire in two years from the Divinity School at Vanderbilt University and return to teaching full-time. Then she accepted an invitation to meet with a search committee member from Boston University’s School of Theology regarding a renowned—and recently vacated—professorship bearing the name of civil rights icon and BU alum Martin Luther King, Jr. (GRS’55, Hon.’59).
The committee member advised Townes , Vanderbilt’s E. Rhodes and Leona B. Carpenter Chair in Ethics and Society and University Distinguished Professor of Womanist Ethics and Society, to view the potential role—newly renamed the Martin Luther King, Jr. Professor of Religion and Black Studies—as a capstone to a career in theological education that dates back more than four decades.
“I hate that kind of language,” Townes says with a smile. “You don’t have a capstone, you have a job to do, and you do it.”
Townes says she took the meeting “as a courtesy.” It didn’t take long in the interview process, however, for Townes to feel STH was where she needed to be.
“As soon as Emilie Townes applied and expressed interest in the MLK professorship, we knew we need not look any further,” says G. Sujin Pak , dean of STH. “She was not just our top candidate, she is our dream candidate! So, the search process became crystal clear from STH’s view. The possibility of attracting the Emilie Townes is an unparalleled, transformative opportunity.”
Townes will become STH’s new Martin Luther King, Jr. Professor of Religion and Black Studies effective July 1. According to STH, the professorship honors King “by modeling the moral authority, prophetic vision of justice, peace, and love, ethical leadership, and global consciousness that he advocated for and embodied.” The chair, previously named the Martin Luther King, Jr. Professor of Ethical Leadership, had been held by longtime STH faculty member Rev. Walter E. Fluker (GRS’88, STH’88, Hon.’24), with whom Townes spoke before accepting the position. Fluker retired from the position in 2020. “He spoke [about the professorship] with a certain amount of affection, which you never really know if that’s what you’re going to hear when you say, ‘Tell me about it,’” Townes says. “He, more than anyone outside of BU, helped convince me this will be a good place.”
Townes’ scholarship has delved into many corners of womanist and Black theology, as well as social issues such as racial health disparities and environmental racism. Before becoming the first Black dean at Vanderbilt Divinity School in 2013, she was the first Black woman president of the American Academy of Religion, the first African American and first woman to serve as associate dean for academic affairs at the Yale Divinity School, and the first Black woman president of the American Academy of Religion. She currently serves as the president of the Society of Christian Ethics, the first Black woman to hold the office.
Pak calls Townes a “trailblazing scholar of remarkable interdisciplinary range,” who has left a mark on every field she touches.
“Emilie Townes is a towering figure and recognizably the leading living womanist ethicist today,” Pak says. “She is not only an astonishing scholar; the incredible gift is this: she is a passionate teacher, a prophetic advocate, an innovative institutional leader, and a beautiful human being. The STH community will reap immeasurable blessings from her experiential wisdom, gifts of discernment, and compassionate counsel.”
Emilie Townes is a towering figure and recognizably the leading living womanist ethicist today. She is not only an astonishing scholar; the incredible gift is this: she is a passionate teacher, a prophetic advocate, an innovative institutional leader, and a beautiful human being. G. Sujin Pak, STH Dean
Townes will not teach any courses this fall, instead using the time to learn about the School of Theology and its curriculum and meet faculty and students. When she does return to the classroom in spring 2025, she says, she’s eager to pull from current events to teach about how the world is structured and humanity’s capacity to create change. “I’ll try to help students get a better sense of the structures we’re dealing with and not just personal opinion or sound bites or all those other things that are not quite what you should be using to build a society that is sustainable, but also where are each of us individually in that structure,” Townes says. “And, how can we be better people in light of it?”
Townes says a main vision she has for her role is that of unifier.
“The polarizations we’re living with now are heartbreaking to me,” she says. “I’m feeling like this [professorship] is a great challenge, and more than that, a great possibility to see if there are ways in which the prominence of the chair can help start conversations that lead people to working together as opposed to being at each other’s throats or not listening.”
Explore Related Topics:
- Martin Luther King
- Share this story
- 7 Comments Add
Senior Editor/Writer Twitter Profile

Steve Holt is a senior editor and writer responsible for print alumni magazines at the Wheelock College of Education and Human Development, School of Theology, and the Sargent College of Health and Rehabilitation Sciences. He came to BU in 2022 from Appalachian Mountain Club, where he was a senior editor at the nonprofit’s award-winning member magazine. For more than a decade before that, Steve built a prolific freelance journalism career, collecting bylines in numerous print and online publications, such as The Boston Globe , Boston magazine, Civil Eats , Business Insider , and Bloomberg CityLab . His Edible Boston story about sustainable hamburgers in Boston was selected for inclusion in the Best Food Writing 2011 anthology. Steve holds a bachelor’s degree in journalism and a master’s in theology from Abilene Christian University. Profile
Comments & Discussion
Boston University moderates comments to facilitate an informed, substantive, civil conversation. Abusive, profane, self-promotional, misleading, incoherent or off-topic comments will be rejected. Moderators are staffed during regular business hours (EST) and can only accept comments written in English. Statistics or facts must include a citation or a link to the citation.
There are 7 comments on Leading Womanist Ethicist and Theologian Named New MLK Professor of Religion and Black Studies at STH
Congratulations Emilie!!! How blessed we are that you’re still leading the way and sharing your insights and lighting the passion in new theologians. How honored I am that I had the privilege of studying under you. Hope you’re still loving golf.
Congrats! Welcome to STH and the BU community overall. We look forward to all that you have to offer our students, faculty, staff and beyond.
Ray Joyce Asst. Dean of Development STH & SSW
Her title alone, with its focus on Religion and Race, then calling herself a Womanist, all seem counterproductive for someone who hopes to be a unifier. I like the former title better, because we need ethics that transcend labels. Let’s focus on our common thread of humanity under the Deity who inspires us to be better than we are!
I read through the story three times and still don’t know what STH is.
Hi, thanks, in the story’s first paragraph, it refers to BU’s School of Theology, which is shortened to STH: “Emilie Townes was all set to retire in two years from the Divinity School at Vanderbilt University and return to teaching full-time. Then she accepted an invitation to meet with a search committee member from Boston University’s School of Theology regarding a renowned—and recently vacated—professorship bearing the name of civil rights icon and BU alum Martin Luther King, Jr. (GRS’55, Hon.’59).”
Congratulations and welcome to the BU Community!
Hurray! STH and Boston University have found a pearl of great price in the embodied wisdom and grace of Professor Emilie Townes! May her tenure with my beloved alma mater increase the legacy of the school of the prophets and be filled with “the singing of angels”!
Post a comment. Cancel reply
Your email address will not be published. Required fields are marked *
Latest from BU Today
Bu hub turns six—and it’s more important than ever, to do today: free dunder-mifflin paint night, can animal organ transplants save thousands of dying people, to do today: knit & crochet night at fenway community center, boston university removes the myles standish name from dorm, pov: remembering charlie pohonich, a final look back at bu’s 151st commencement, how to make the most of memorial day weekend in boston, did you win the free furiosa: a mad max saga tickets for tonight, karen antman stepping down as bu’s medical school dean and medical campus provost, a bu class tackles the massachusetts housing crisis, zesty potato salad, my reflections on the last four years of college: why small life events matter, exploring bu’s vibrant asian student communities for aapi heritage month, photo essay: behind the scenes at a bu commencement, how three class of 2024 grads landed jobs before graduating, protests unfold in and around nickerson field during bu’s 151st commencement, quotes, comments, and cheers from bu’s 151st commencement, sights and sounds from boston university’s 151st commencement.

IMAGES
VIDEO
COMMENTS
The charge of the Committee on Animal Research and Ethics is: To safeguard responsible research with animals, other than humans, and to establish and maintain cooperative relations with organizations sharing common interests. To disseminate in cooperation with other organizations accurate information about such research. To review the ethics of ...
Thus, animal ethics committees commonly review research projects with reference to the 4 Rs principles . The first "R", Reduction means that the experimental design is examined to ensure that researchers have reduced the number of experimental animals in a research project to the minimum required for reliable data .
The Regents Policy on Animal Care and Use addresses the use of all animals in research, teaching or display at the University of Minnesota. This policy follows from the federal and other laws and regulations. It addresses the roles and responsibilities of the Institutional Official, the Institutional Animal Care and Use Committee (IACUC ...
Hereinafter, by "animal ethics committees" or "AECs," I am referring to the bodies entrusted with the task of the ethical evaluation of research involving living animals. The need for such evaluation is usually restricted to vertebrate animals only, which, according to scientific evidence, are believed to be sentient.
The Emergence of Animal Research Ethics. In his contribution to The Routledge Companion to Bioethics, Tom L. Beauchamp (2014, p. 262) calls animal research ethics "a recently coined term".It is, indeed, only in the last decade, that animal research has been discussed extensively within the framework of philosophical research ethics, but the term "animal research ethics" goes back at ...
The ethics of using nonhuman animals in biomedical research is usually seen as a subfield of animal ethics. In recent years, however, the ethics of animal research has increasingly become a subfield within research ethics under the term "animal research ethics". Consequently, ethical issues have become prominent that are familiar in the context of human research ethics, such as autonomy or ...
The Norwegian National Committee for Research Ethics in Science and Technology (NENT). Guidelines for research ethics in science and technology (2007) 2016. Oslo. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes.
research in animal ethics and food ethics. She is also a member of the committee for ethics and education in animal research at the Swedish Board of Agriculture since its establishment in 2008. Mickey Gjerris is a bioethicist at The University of Copenhagen where he for more than 15 years has been doing research in (among other subjects) ani-
The SAHMRI Animal Ethics Committee (AEC) adheres to the South Australian Animal Welfare Act 1985. The Act sets the legal requirements for the use of animals in research and teaching and regulates the institution's Animal Ethics Committee function and reporting of animal-related activities. SAHMRI's AEC Operating Guidelines outline the ...
Ensuring the ethical, humane and responsible use of animals in health and medical research forms part of the sector's social licence to operate in Australia. The 3Rs (Replacement, Reduction and Refinement) are accepted internationally as critical components of the ethical, humane and responsible care and use of animals for scientific purposes.
The decision of the Animal Ethics Committee whether to approve a protocol is NOT in any way linked to payment of the ethics fee. If you have questions, please contact the Animal Ethics Administrative staff for information regarding the fee schedule. Animal Research Ethics FAQs. Frequently Asked Questions Related to Research Involving Animals
The Animal Research Ethics Committee ensures that all ethical issues arising in connection with UCD research and teaching activities involving animals are identified and reviewed and that the use of animals can be ethically justified. Appropriate decisions are taken to ensure that all procedures are conducted to the standards of international ...
The Nelson Mandela University Research Ethics Committee (Animal) (REC-A) is charged with monitoring the treatment of animals used in both research and teaching at the institution. It has the responsibility of reviewing all protocols involving animal use in order to ensure that they are in accordance with acceptable ethical and scientific ...
The LTU AEC is the institutional animal ethics committee for all LTU campuses, national and international. The LTU AEC reports to the Senior Deputy Vice-Chancellor (Research & Industry Engagement) (SDVCRIE) through the Research and Graduate Studies Committee (RGSC) and the Licence Nominee. Mechanisms of Reporting Internal and External Reporting
supplier, the animal care and ethics committee for the corporation has both the functions set out in subsection (1) and (1A). (2) An animal care and ethics committee appointed by the Secretary has the following functions— (a) the making of recommendations concerning the granting of animal research authorities by the Secretary,
1. Introduction. In response to growing public concerns about the welfare of animals in laboratories stemming from exposés of abuse at several high-profile federally-funded research facilities, in 1985 U.S. Congress and the Public Health Service (PHS) mandated the institutional animal care and use committee (IACUC) system to oversee vertebrate animal use and ensure compliance with federal ...
Frequently Asked Questions (FAQs) Below is a list of questions or common misconceptions from the REB survey conducted in 2022 to early 2023, and some frequently asked questions that the Ethics Office receives. Acronyms: REB = Research Ethics Board. TCPS2 = Tri-Council Policy Statement. UAlberta REO = University of Alberta Research Ethics Office.
News 8 Breaking News Alerts. WASHINGTON (AP) — The House Ethics Committee is opening an investigation into Rep. Henry Cuellar, D-Texas, after his indictment this month on allegations of bribery ...
Elektrostal is a city in Moscow Oblast, Russia, located 58 kilometers east of Moscow. Elektrostal has about 158,000 residents. Mapcarta, the open map.
Animals and Pets Anime Art Cars and Motor Vehicles Crafts and DIY Culture, Race, and Ethnicity Ethics and Philosophy Fashion Food and Drink History Hobbies Law Learning and Education Military Movies Music Place Podcasts and Streamers Politics Programming Reading, Writing, ...
The Air Travel Consumer Report is a monthly product of the Department of Transportation's Office of Aviation Consumer Protection. The report is designed to assist consumers with information on the quality of services provided by the airlines. Data Included in this Report. Flight Delays: March 2024 / January- March 2024.
For artists, writers, gamemasters, musicians, programmers, philosophers and scientists alike! The creation of new worlds and new universes has long been a key element of speculative fiction, from the fantasy works of Tolkien and Le Guin, to the science-fiction universes of Delany and Asimov, to the tabletop realm of Gygax and Barker, and beyond.
Abstract. An Ethics Committee (EC) is an independent body composed of members with expertise in both scientific and nonscientific arenas which functions to ensure the protection of human rights and the well-being of research subjects based on six basic principles of autonomy, justice, beneficence, nonmaleficence, confidentiality, and honesty.
Geographic coordinates of Elektrostal, Moscow Oblast, Russia in WGS 84 coordinate system which is a standard in cartography, geodesy, and navigation, including Global Positioning System (GPS). Latitude of Elektrostal, longitude of Elektrostal, elevation above sea level of Elektrostal.
The committee member advised Townes, Vanderbilt's E. Rhodes and Leona B. Carpenter Chair in Ethics and Society and University Distinguished Professor of Womanist Ethics and Society, to view the potential role—newly renamed the Martin Luther King, Jr. Professor of Religion and Black Studies—as a capstone to a career in theological ...
Martonosi, the Hugh Trumbull Adams '35 Professor of Computer Science at Princeton University, is a leader in the design, modelling, and verification of power efficient computer architecture. The ACM Frances E. Allen Award is presented biennially to an individual who has exemplified excellence and/or innovation in mentoring, with particular ...