Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 16 September 2021

The role of cardiac rehabilitation in improving cardiovascular outcomes
- Rod S. Taylor ORCID: orcid.org/0000-0002-3043-6011 1 , 2 ,
- Hasnain M. Dalal ORCID: orcid.org/0000-0002-7316-7544 3 &
- Sinéad T. J. McDonagh ORCID: orcid.org/0000-0002-0283-3095 3
Nature Reviews Cardiology volume 19 , pages 180–194 ( 2022 ) Cite this article
55k Accesses
151 Citations
221 Altmetric
Metrics details
- Cardiovascular diseases
- Rehabilitation
Cardiac rehabilitation is a complex intervention that seeks to improve the functional capacity, wellbeing and health-related quality of life of patients with heart disease. A substantive evidence base supports cardiac rehabilitation as a clinically effective and cost-effective intervention for patients with acute coronary syndrome or heart failure with reduced ejection fraction and after coronary revascularization. In this Review, we discuss the major contemporary challenges that face cardiac rehabilitation. Despite the strong recommendation in current clinical guidelines for the referral of these patient groups, global access to cardiac rehabilitation remains poor. The COVID-19 pandemic has contributed to a further reduction in access to cardiac rehabilitation. An increasing body of evidence supports home-based and technology-based models of cardiac rehabilitation as alternatives or adjuncts to traditional centre-based programmes, especially in low-income and middle-income countries, in which cardiac rehabilitation services are scarce, and scalable and affordable models are much needed. Future approaches to the delivery of cardiac rehabilitation need to align with the growing multimorbidity of an ageing population and cater to the needs of the increasing numbers of patients with cardiac disease who present with two or more chronic diseases. Future research priorities include strengthening the evidence base for cardiac rehabilitation in other indications, including heart failure with preserved ejection fraction, atrial fibrillation and congenital heart disease and after valve surgery or heart transplantation, and evaluation of the implementation of sustainable and affordable models of delivery that can improve access to cardiac rehabilitation in all income settings.
Cardiac rehabilitation is a complex, multicomponent intervention that includes exercise training and physical activity promotion, health education, cardiovascular risk management and psychological support, personalized to the individual needs of patients with heart disease.
Data from randomized, controlled trials support cardiac rehabilitation as a clinically effective and cost-effective intervention for patients with acute coronary syndrome or heart failure with reduced ejection fraction and after coronary revascularization.
Despite this robust evidence base and strong guideline recommendations, global access to cardiac rehabilitation is persistently poor, with scarce cardiac rehabilitation provision in low-income and middle-income settings.
Home-based and technology-based models of cardiac rehabilitation with appropriate quality assurance as an alternative or adjunct to traditional, centre-based programmes are needed to improve access to cardiac rehabilitation.
Cardiac rehabilitation programmes need to cater for and manage the needs of the increasing number of patients with heart disease who present with two or more chronic diseases.
Further research needs to strengthen the evidence base for cardiac rehabilitation in patients with heart failure with preserved ejection fraction, atrial fibrillation or congenital heart disease and after cardiac valve surgery or heart transplantation.
Similar content being viewed by others
Intensified ambulatory cardiology care: effects on mortality and hospitalisation—a comparative observational study
Combined telemonitoring and telecoaching for heart failure improves outcome

A nationwide survey on participation in cardiac rehabilitation among patients with coronary heart disease using health claims data in Japan
Introduction.
Cardiac rehabilitation is a complex intervention that includes exercise training, physical activity promotion, health education, cardiovascular risk management and psychological support, personalized to the individual needs of patients with diagnosed heart disease 1 (Fig. 1 ). In addition to secondary prevention and improvement in cardiovascular prognosis, a focus of modern cardiac rehabilitation has been the drive to improve patient wellbeing and health-related quality of life 2 , 3 , 4 .
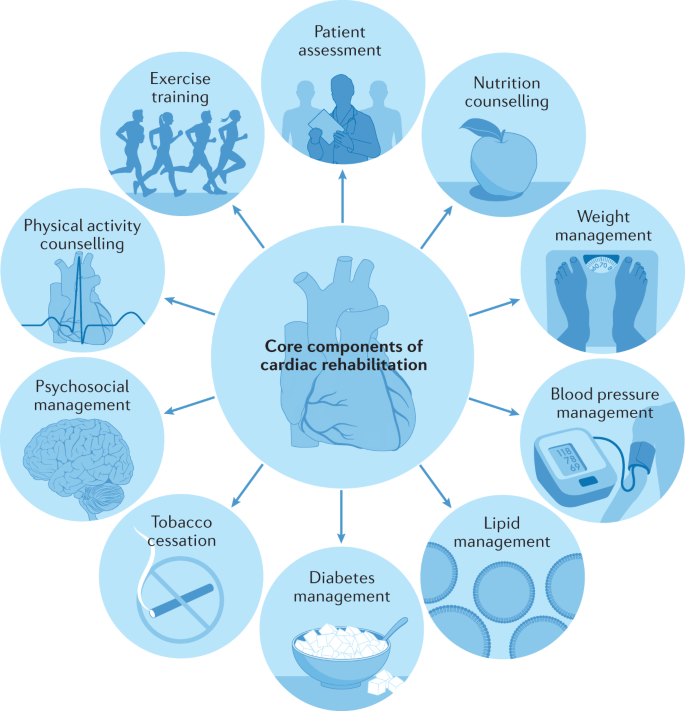
A schematic summary of the major components of comprehensive cardiac rehabilitation. Adapted by permission from BMJ Publishing Group Limited. [Advances in rehabilitation for chronic diseases: improving health outcomes and function. Richardson C.R., Franklin B., Moy M.L., Jackson E.A., 365, l2191, 2019].
Introduced in the late 1960s, the recommendation for the provision of cardiac rehabilitation was, at that time, confined to low-risk patients who had survived an acute myocardial infarction (MI). With the development of an evidence base over the past two decades supporting the benefits of cardiac rehabilitation, contemporary clinical guidelines now routinely recommend the referral to comprehensive cardiac rehabilitation across a wider range of cardiac diagnoses, including acute coronary syndrome, heart failure with reduced ejection fraction (HFrEF) and coronary revascularization (percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) surgery).
An important emphasis of contemporary guidelines, including the 2020 position statement from the European Association of Preventive Cardiology (EAPC) 5 , the 2017 guidance from the British Association for Cardiovascular Prevention and Rehabilitation 6 and the 2020 position statement from the Secondary Prevention and Rehabilitation Section of EAPC, is the importance of quality assurance in cardiac rehabilitation delivery 7 (Box 1 ). Key quality assurance elements include the involvement of a multidisciplinary team (including cardiologists, general practitioners and physicians with special interest, nurse specialists, physiotherapists, dietitians and psychologists) trained in the core competencies and effective delivery of the various core elements of a comprehensive cardiac rehabilitation programme (that is, exercise training and promotion, risk factor and self-management education, and psychological support) 1 , 6 , following a detailed initial assessment of the patient. Initially, cardiac rehabilitation was primarily practised as an exercise training intervention alone 8 . Although exercise training remains a central component of cardiac rehabilitation, the comprehensive model of modern cardiac rehabilitation is central to enabling patients to reduce their cardiovascular risk, foster and maintain their health-promotion behavioural patterns, increase their mental wellbeing, reduce their disability and promote an active lifestyle — with the overall aim of improving wellbeing and health-related quality of life. In response to the continuing evolution of cardiac rehabilitation practice and policy, this Review provides a state-of-the-art contemporary overview.
In this Review, we provide a detailed summary of the current evidence base supporting the use of cardiac rehabilitation, an overview of key international guidelines and position statements for cardiac rehabilitation and a synopsis of four key contemporary issues facing cardiac rehabilitation delivery across the globe: improving poor uptake, the effects of the coronavirus disease 2019 (COVID-19) pandemic, managing patient multimorbidity, and the provision of cardiac rehabilitation in low-income and middle-income countries (LMICs). We conclude with our recommendations for future research.
Box 1 Quality assurance standards according to BACPR 6
The British Association of Cardiopulmonary Rehabilitation (BACPR) has six standards for cardiovascular prevention and rehabilitation.
Standard One. The delivery of six core components by a qualified and competent multidisciplinary team led by a clinical coordinator.
Standard Two. Prompt identification, referral and recruitment of eligible patient populations.
Standard Three. Early initial assessment of individual patient needs, which informs the agreed personalized goals, which are reviewed regularly.
Standard Four. Early provision of a structured cardiovascular prevention and rehabilitation programme, with a defined pathway of care, which meets the individual’s goals and is aligned with patient preference and choice.
Standard Five. Upon programme completion, a final assessment of individual patient needs and demonstration of sustainable health outcomes.
Standard Six. Registration and submission of data to the National Audit for Cardiac Rehabilitation and participation in the National Certification Programme.
Box 1 adapted courtesy of British Association for Cardiovascular Prevention and Rehabilitation.
Overview of the evidence base
Our evidence overview is based on Cochrane systematic reviews and meta-analyses of cardiac rehabilitation. Cochrane reviews, with their rigorous methodological requirements and inclusion of only randomized controlled trials (RCTs), are internationally regarded as providing the highest quality of evidence for interventions. We focus on Cochrane reviews that compare the effects of exercise-based cardiac rehabilitation (exercise interventions alone or a comprehensive programme) with a control group (who did not receive cardiac rehabilitation). Key outcome findings (mortality, cardiovascular events, hospitalizations and health-related quality of life) for each indication are presented in Table 1 and summarized below. Researchers used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to summarize the certainty of the evidence for each outcome 9 (Box 2 ).
Box 2 The GRADE system
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) system 9 is a framework for rating the quality of evidence, applied to each outcome in a Cochrane systematic review, because the quality of evidence often varies between outcomes. GRADE has four levels of evidence (also known as certainty in evidence or quality of evidence).
Very low: the true effect is probably markedly different from the estimated effect.
Low: the true effect might be markedly different from the estimated effect.
Moderate: the authors believe that the true effect is probably close to the estimated effect.
High: the authors have high confidence that the true effect is similar to the estimated effect.
Evidence from randomized controlled trials starts at high quality. The certainty in the evidence is increased or decreased for several reasons. Reasons why certainty can be rated down:
Risk of bias: when the results of a study do not represent the truth because of inherent limitations in the design or conduct of a study
Imprecision: the rating focuses on the 95% confidence interval around the best estimate of the intervention effect
Inconsistency: assessed by similarity of point estimates and the overlap of their confidence intervals (statistical heterogeneity)
Indirectness: if the patients or intervention studied are different from those for whom the recommendation applies or the reported outcome is a surrogate for a different outcome
Publication bias: when the trial outcome influences the decision whether to publish or otherwise distribute the finding
Reasons why certainty can be rated up:
Large magnitude of effect
Dose–response gradient
Coronary heart disease
The 2021 update 10 of the 2016 version 11 of the Cochrane review of cardiac rehabilitation for coronary heart disease included 23,172 patients with MI (40 RCTs) or stable angina pectoris (five RCTs), after revascularization (14 RCTs) or in mixed populations. Meta-analysis of trials with outcomes up to 12 months of follow-up showed no effect of cardiac rehabilitation compared with control on all-cause mortality or the risk of revascularization. Participation in cardiac rehabilitation resulted in reductions in the risk of fatal or non-fatal MI and all-cause hospitalization. Although 29 trials collected health-related quality-of-life data, pooling of data was limited owing to variation in the outcome measures. Pooled analysis across three trials showed that cardiac rehabilitation improved generic health-related quality of life, assessed with the Short-Form 36 or 12 (mental component score), but had weak evidence of an improvement in the physical component score. Twenty of the 29 trials reported higher levels of health-related quality of life in one or more subscales with exercise-based cardiac rehabilitation than with control at follow-up. Outcome evidence assessed by GRADE was judged to be of ‘moderate’ certainty, downgraded owing to poor reporting on the randomization process (selection bias), lack of blinding (detection bias) and wide 95% confidence intervals (imprecision). Meta-regression (trial-level) analyses indicated that the benefits of cardiac rehabilitation seemed to be consistent across types and settings of cardiac rehabilitation (home versus centre, exercise-only versus comprehensive cardiac rehabilitation programmes, aerobic versus aerobic plus resistance training, dose of aerobic exercise) and study characteristics (single-centre versus multicentre).
This Cochrane review has been criticized for the inclusion of older RCTs that might not reflect contemporary practice and studies that might not have used robust quality assurance in terms of the delivery of the cardiac rehabilitation intervention — for example, the UK-based, multicentre RAMIT trial 12 , 13 . Given that trials included in the Cochrane review span the period 1974–2020, the authors sought to address this issue by undertaking an assessment of the change in cardiac rehabilitation outcome over time. Interestingly, weak evidence exists of a reduction (slope 1.005, 95% CI 0.0098–1.0118, P = 0.13) in the all-cause mortality effect (log relative risk) of cardiac rehabilitation over time (Fig. 2 ). The authors interpreted this absence of an improvement in the effect of cardiac rehabilitation on all-cause mortality over the past 2–3 decades as reflecting the evolution of usual care and the introduction of life-saving therapies, including thrombolysis and secondary prevention drugs, such as β-blockers and statins. Interestingly, the 2020 meta-analysis of the CROSII study 14 , which included RCTs and prospective and retrospective cohort studies, reported a reduction in mortality with cardiac rehabilitation in patients with acute coronary syndrome or after revascularization, with an index event in 1995 or later. However, with the inclusion of observational evidence, the prognostic benefit reported by the CROSII study is subject to selection bias and confounding.
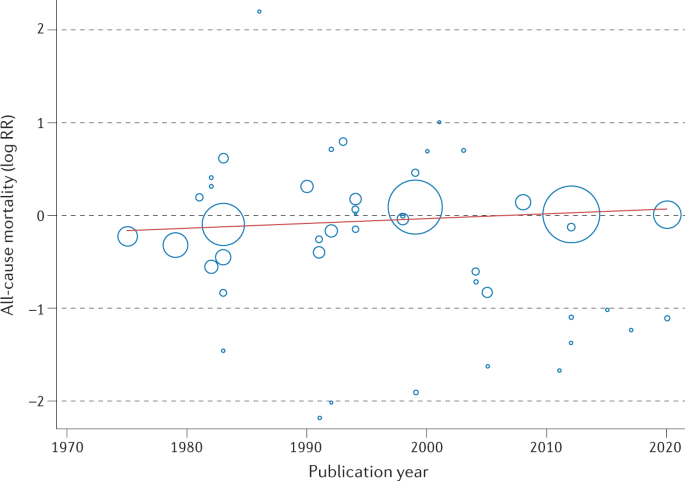
Meta-regression analysis of the treatment effect of cardiac rehabilitation on all-cause mortality over time in patients with coronary heart disease. The area of each data point is inversely related to the standard error of log relative risk (RR). The absence of an improvement in the effect of cardiac rehabilitation on all-cause mortality over the past 2–3 decades might reflect the evolution of usual care and the introduction of life-saving therapies, including thrombolysis and secondary prevention drugs.
Heart failure
A 2019 Cochrane review of cardiac rehabilitation in heart failure included 44 RCTs in 5,783 participants, predominantly with HFrEF 15 . This meta-analysis showed that participation in cardiac rehabilitation was associated with reduced rates of all-cause and heart-failure-specific hospitalization and improved health-related quality of life compared with control, whereas no significant effect of cardiac rehabilitation on all-cause mortality was detected. Pooled data across the 17 trials reporting the Minnesota Living with Heart Failure questionnaire (a disease-specific, health-related quality-of-life measure) showed not only a significant improvement with cardiac rehabilitation (mean difference –7.1, 95% CI −10.5 to –3.7), but also a magnitude of effect that is deemed ‘clinically important’ (an increase in the score by ≥5 points, compared with control) 16 . Certainty of outcomes was judged to be low to moderate, downgraded primarily owing to selection bias, imprecision (wide 95% confidence intervals or lack of events) and detection bias or placebo effects (health-related quality of life). Meta-regression analyses indicated that the benefits of cardiac rehabilitation for heart failure were consistent, irrespective of the nature of the cardiac rehabilitation or the setting.
Atrial fibrillation
The 2017 Cochrane review of cardiac rehabilitation in atrial fibrillation included six RCTs in 421 patients with various types of atrial fibrillation 17 . Given the small number of trials and reported clinical events, the effect of cardiac rehabilitation in this patient population in terms of the key outcomes of mortality, cardiovascular events, hospitalizations and health-related quality of life are all uncertain, with moderate to very low certainty (downgraded primarily owing to imprecision as a result of the small evidence base). Peak oxygen uptake (aerobic exercise capacity) was, on average, 3.76 ml/kg/min (95% CI 1.37–6.15 ml/kg/min) higher with cardiac rehabilitation than with the control (moderate quality of evidence).
Congenital heart disease
The 2020 Cochrane review focused on physical activity interventions across 15 RCTs in 924 adults and children with various forms of congenital heart disease 18 . Owing to the absence of trials reporting events, the authors concluded that there was no basis to determine the effect of cardiac rehabilitation in terms of either mortality or hospitalizations. In addition, evidence supporting the effect of cardiac rehabilitation on health-related quality of life was uncertain (very low quality of evidence owing to a small evidence base). Small improvements in both peak oxygen uptake (mean difference 1.89 ml/kg/min, 95% CI 0.22–3.99 ml/kg/min; 14 trials, 732 patients) and muscle strength (mean difference 17.1 N/m, 95% CI 3.4–30.8 N/m) were reported with cardiac rehabilitation (both moderate quality of evidence).
After cardioverter–defibrillator implantation
The 2019 Cochrane review included eight RCTs in 1,730 individuals with an implanted cardioverter–defibrillator, primarily for an indication of heart failure 19 . Owing to the small number of trials and reported events, the effect of cardiac rehabilitation on mortality, adverse events and health-related quality of life were all uncertain (low to very low quality of evidence). Low-quality evidence indicated that participating in cardiac rehabilitation resulted in a small increase in exercise capacity (determined by peak oxygen uptake) compared with control (mean difference 0.91 ml/kg/min, 95% CI 0.60–1.21 ml/kg/min; seven trials, 1,485 patients).
After heart transplantation
The 2017 Cochrane review included ten RCTs in 300 individuals after heart transplantation 20 . Cardiac rehabilitation increased peak oxygen uptake compared with the no-exercise control group (mean difference 2.5 ml/kg/min, 95% CI 1.63–3.36 ml/kg/min; nine trials, 284 patients, moderate quality of evidence). Although a meta-analysis was not possible owing to the lack of consistency of outcome reporting, the three individual trials that reported health-related quality of life showed no consistent advantage of cardiac rehabilitation over control. Owing to the small number of trials and reported events, a meta-analysis was not undertaken, and the effect of cardiac rehabilitation on all-cause mortality and hospitalizations was uncertain.
After valve surgery
The 2021 Cochrane review included six RCTs in 364 patients who had received either open or percutaneous heart valve surgery 21 . Owing to the lack of trials and outcome data, the authors were unable to conclude definitively the effect of cardiac rehabilitation in this population in terms of mortality, hospitalization or health-related quality of life (all very low quality of evidence). Cardiac rehabilitation increased peak oxygen uptake for all but the submaximal measures (mean difference 2.38 ml/kg/min, 95% CI 0.36–4.40 ml/kg/min; five trials, 294 patients, moderate quality of evidence) compared with no exercise.
General quality of evidence
Although systematic reviews and meta-analyses of RCTs are the gold standard for establishing the effects of intervention, a consistent limitation identified across the Cochrane reviews was the potential risk of bias and lack of consistency of outcomes reported by RCTs on cardiac rehabilitation to date. Therefore, improvement in the certainty of the evidence base for cardiac rehabilitation in the future depends on the conduct and reporting of high-quality RCTs, including the consistent collection and reporting of outcome measures, such as health-related quality of life (Box 3 ). It is important to recognize the limitations of meta-regression analyses and that this analysis can be subject to ecological fallacy, that is, study-level assessment of the relationships between study characteristics and patient outcomes does not necessarily reflect the true (patient-level) association 22 . For example, both meta-regression analyses reported in the Cochrane reviews on coronary heart disease and heart failure indicate that the benefit of cardiac rehabilitation is not affected by the study-level dose of exercise prescription. However, other (patient-level) data show that the dose of exercise is very important and that cardiac rehabilitation might result in no benefits when the prescription of exercise is too low in intensity or is of insufficient duration 23 , 24 . A more detailed review on this topic was published previously 5 .
Although developing areas for the application of cardiac rehabilitation, such as cardio-oncology and patients with left ventricular assist devices or spontaneous coronary artery dissection, have not been the subject of a Cochrane review, reviews of the evidence base for cardiac rehabilitation in these indications have been reviewed previously 25 , 26 , 27 .
Box 3 Future research recommendations
The following key priorities for future cardiac rehabilitation research are drawn from current Cochrane reviews, clinical guidelines and other sources cited in this Review. These priorities apply to the following indications: heart failure with preserved ejection fraction, stable angina pectoris, atrial fibrillation, congenital heart disease and heart transplantation.
Future evidence collection should take the form of well-reported, large, multicentre, randomized, controlled trials, adequately powered and deemed high in quality and low in risk of bias, and should collect data on key outcomes, including mortality, hospitalization, health-related quality of life, health-care and societal costs and cost-effectiveness.
Given the current suboptimal uptake of cardiac rehabilitation, future trials of alternative models of cardiac rehabilitation delivery that can improve patient access and adherence are needed, including home-based and mobile, computer and digital technology-assisted programmes, as an alternative to or alongside traditional, centre-based models of delivery, especially for marginalized groups, for example, elderly individuals, women, and those from ethnic minorities and socioeconomically deprived groups. These trials need to consider assessing the patient-level and system-level outcome, including safety, costs and the quality assurance of programme delivery.
Development and evaluation of rehabilitation programmes that serve the needs of patients with cardiac disease who present with multimorbidity (the presence of two or more long-term conditions).
Development and evaluation of affordable and sustainable cardiac rehabilitation for patients with cardiac disease in low-income and middle-income countries.
Cost-effectiveness
In addition to clinical efficacy (‘effectiveness’) and safety, with the growing cost pressures on health-care systems across the world, the costs and cost-effectiveness of cardiac rehabilitation need to be considered. A 2018 systematic review of the cost-effectiveness of cardiac rehabilitation identified 19 economic studies 28 . Seven of these studies compared cardiac rehabilitation with no cardiac rehabilitation and the remaining studies compared intervention types within cardiac rehabilitation, for example, home-delivered or digitally delivered versus centre-based programmes. To facilitate comparison across studies, the authors converted all costs into 2016 US$, with the use of the consumer price index and purchasing power parity conversion. Most of the studies concluded that cardiac rehabilitation was cost-effective compared with no cardiac rehabilitation (incremental cost-effectiveness ratios (ICERs) ranged from US$1,065 to US$71,755 per quality-adjusted life-year (QALY)). In the UK, an acceptable level of cost-effectiveness is judged to be intervention with an ICER between £20,000 and £30,000 per QALY or lower, that is, ~US$25,000 to ~US$45,000 per QALY or lower 29 . Although generally cost-effective, the authors of the review concluded that further research was required to determine the most cost-effective design of cardiac rehabilitation, for example, a comparison of the cost-effectiveness of different modes of delivery (centre-based, home-based or using mobile technology) and combinations of interventions.
Clinical guideline recommendations
Reflecting the RCT evidence presented above, current clinical guidelines consistently provide a strong recommendation for cardiac rehabilitation referral for patients with MI or heart failure and after revascularization (CABG surgery or PCI). Table 2 summarizes the guideline statements from the ESC 30 , 31 , AHA/ACC 32 , 33 , National Institute for Health and Care Excellence (NICE) 34 , 35 in the UK, and National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand 36 , 37 .
European, US and Australian/New Zealand guidelines all give cardiac rehabilitation their highest recommendation (class I: evidence and/or general agreement that a given treatment or procedure is beneficial, useful and effective and should be recommended) on the basis of an evidence rating of level A (data derived from multiple RCTs or meta-analyses) or level B (data derived from a single RCT or large non-randomized studies). The NICE recommendations are based on both clinical effectiveness and cost-effectiveness. Although these latter recommendations do not use the class and level approach, a strong recommendation for cardiac rehabilitation is made.
Given the small number of RCTs in patients with stable angina or heart failure with preserved ejection fraction, the European and Australian/New Zealand recommendations focus on HFrEF, and a level B rating is given for angina by the AHA/ACC. These guidelines recommend the need to conduct further research in these indications. The importance of a comprehensive nature of modern cardiac rehabilitation delivery is emphasized by the UK NICE guidance, recommending that programmes comprise physical activity, lifestyle advice, stress management and health education components.
Given the current underuse (referral and uptake) of cardiac rehabilitation services, with only a minority of eligible patients participating in cardiac rehabilitation over the past decade, the clinical guidelines emphasize the importance of alternative models of cardiac rehabilitation delivery to the traditional, centre-based programmes. The Australian/New Zealand, UK and US guidance all include a formal recommendation for consideration of home-based delivery to improve access to cardiac rehabilitation. The 2019 American Association of Cardiovascular and Pulmonary Rehabilitation, AHA and ACC joint scientific statement notes that although home-based cardiac rehabilitation is a common model in Canada and Europe, it is less common in the USA and emphasizes the need for quality assurance for the delivery of home-based cardiac rehabilitation programmes in their country 38 .
Given the more limited RCT evidence, current guidelines for the management of other cardiac indications, such as atrial fibrillation and congenital heart disease, provide no strong recommendation for or against the use of cardiac rehabilitation. Future high-quality RCTs of cardiac rehabilitation in these indications are needed to inform future guideline updates and clinical policy and practice. Although not the focus of this Review, a previous comparison of cardiac rehabilitation guidelines provides details of the differences and consensus in recommendations for exercise testing, prescription and monitoring 39 .
Major contemporary issues
Improving poor uptake.
Despite the evidence for benefits of cardiac rehabilitation and strong guideline recommendations, the uptake of cardiac rehabilitation remains poor. Although the availability of cardiac rehabilitation is virtually absent in some global localities, in many areas, including Europe, North America and Australasia, a fairly small proportion of patients with acute coronary syndrome or HFrEF or who have undergone revascularization are currently referred for cardiac rehabilitation.
The latest data from the 2019 UK National Audit of Cardiac Rehabilitation (NACR) reported that 68,074 out of 135,861 (50%) individuals with a main diagnosis of coronary heart disease received cardiac rehabilitation (MI 29%, PCI 51% and CABG surgery 75%) 40 . For heart failure, the national level of cardiac rehabilitation attendance was <10% 40 . Cardiac rehabilitation participation rates in the USA are very low, ranging from 19% to 34% in national analyses, with large state-by-state geographical variations and differences according to cardiac diagnosis 41 . Consistent with the findings of many national and single-centre studies of cardiac rehabilitation, the 2019 UK NACR data show that certain groups are much less likely to attend cardiac rehabilitation than others, that is, older individuals, women, non-white and ethnic minority groups and patients with multimorbidity (defined as the presence of two or more long-term conditions) 40 .
The basis of suboptimal uptake of cardiac rehabilitation is complex and multilayered and reflects potential barriers at the level of the clinician, the patient and the health service (Table 3 ). At the clinician level, the absence of education on cardiac rehabilitation in their general medical and cardiology training might result in the low rate of referral by physicians 41 , 42 , 43 . For patients, a range of factors might influence their individual decision to act on this referral and attend a cardiac rehabilitation programme, such as inconvenience (and costs) of transport to attend a centre-based programme held during the ‘9–5’ working day, especially if these individuals are in employment. At the health service level, barriers can include the capacity and funding of cardiac rehabilitation programmes. For example, the 2019 UK NACR showed that group-based, supervised cardiac rehabilitation was the most common mode of delivery of cardiac rehabilitation, with 75.4% of patients receiving this method of cardiac rehabilitation compared with only 8.8% taking up home-based cardiac rehabilitation 40 . Barriers at these three levels are probably interactive. For example, travelling to centres and a dislike of group-based cardiac rehabilitation sessions are known to be particularly relevant for certain groups of patients, including women, ethnic minorities and people from areas of high deprivation who are elderly, living with complex health conditions or living in rural areas 42 , 43 . Cardiac rehabilitation is a crucial environment to contribute to the optimization of a patient’s cardiovascular risk, with opportunities for screening, education and medical treatment (exercise, nutrition, smoking cessation and medications). Despite these potential benefits of risk-factor reduction, the results from the EUROASPIRE III study 44 indicated the underuse of cardiac rehabilitation, with poor referral and low participation rates and wide variations between European countries. Some of the key proposed solutions to these patient, clinician and health service barriers to accessing cardiac rehabilitation are summarized in Box 4 .
Given that the potential loss to patients of important gains in health-related quality of life and the rise in pressures and costs on health-care systems as a result of increased unplanned hospitalization, poor participation (uptake) of cardiac rehabilitation is an increasingly important policy priority. For example, in the UK, the NHS England Long Term Plan 45 was published in 2019, with the aim to increase the overall national uptake of cardiac rehabilitation to 85% (from the current 50%) of all eligible patients by 2028. In the USA, a road map was proposed to achieve >70% participation in cardiac rehabilitation by 2022, with the aim of saving 25,000 lives and preventing 180,000 hospitalizations per year 46 .
An example of innovative service development is the Rehabilitation Enablement in Chronic Heart Failure (REACH-HF) programme of facilitated cardiac rehabilitation. This comprehensive programme of cardiac rehabilitation for use at home comprises a heart failure manual, a relaxation CD, a choice of exercise (walking programme or a chair-based DVD), a progress tracker for patients, and a family and friends resource for caregivers. A UK-based, multicentre RCT in 216 individuals with HFrEF confirmed that the addition of REACH-HF to usual care compared with usual care alone was effective in improving the primary outcome of health-related quality of life, which was assessed using the Minnesota Living with Heart Failure questionnaire (–5.7 points, 95% CI −10.6 to −0.7 points, P = 0.025) 47 . Subsequent economic modelling on the basis of the results from the trial confirmed the acceptable cost-effectiveness of the REACH-HF programme, with an ICER of £1,720 per QALY 48 . Given its clinical effectiveness and cost-effectiveness, the REACH-HF programme is now being rolled out into routine care across the UK and the Republic of Ireland to improve access to and uptake of cardiac rehabilitation 49 , 50 .
A growing body of research now shows that home-based models of cardiac rehabilitation delivery achieve similar gains in patient efficacy and safety to traditional, centre-based programmes at similar cost-effectiveness and, indeed, might lead to higher levels of patient adherence 51 , 52 . As with many previous trials of cardiac rehabilitation, studies of home-based cardiac rehabilitation have focused on low–moderate risk populations. Several cardiac rehabilitation programmes are now using a hybrid approach to deliver cardiac rehabilitation. For example, this approach initially offers patients centre-based cardiac rehabilitation and then evolves to longer-term maintenance through technology-supported, home-based sessions 53 . The effectiveness of these innovative models is likely to depend on active, ongoing contact between patients and health-care professionals through more traditional methods, such as home visits and telephone consultations, or the use of technology-based solutions, which include web-based video calls and social networking platforms 54 .
Box 4 Strategies to facilitate increased referral to and enrolment and long-term participation in CR programmes
Achieve strong endorsement of cardiac rehabilitation (CR) by referring clinicians (cardiologists, physicians and health-care professionals) by incorporating it into the hospital discharge plan.
Automatically refer all eligible patients for CR at the time of hospital discharge — giving patients a choice to attend a centre-based or home-based hybrid programme.
Provide CR information (printed and web links or videos) and education to inpatients before discharge from hospital.
Ensure good communication with the patient’s primary care physician or general practitioner so they are sent the discharge details with information on CR programmes — giving the option of centre-based CR or home-based CR (for low-to-moderate-risk patients) or a hybrid programme.
Schedule CR enrolment appointments via the patient’s preferred communication mode (telephone call, text message, e-mail or post).
Advise the patient’s primary care physician or general practitioner to refer patients for CR if the patient has not been referred — encouraging enrolment and participation.
Consider system-level, provider-level and patient-level financial incentives for referral to, enrolment in and completion of CR sessions.
Target and identify racial and ethnic minorities, women, older adults, and rural and socioeconomically deprived groups who are least likely to enrol in and complete CR.
Encourage long-term support through trained health-care professionals using face-to-face or web-based applications to track ongoing efforts for cardiovascular risk reduction, including physical activity and fitness, for example, by the primary-care team.
Box 4 adapted with permission from ref. 38 , Elsevier.
Effects of the COVID-19 pandemic
The COVID-19 pandemic has had a major global effect on the use and delivery of health and health-care systems. As we write this Review, the UK has become the first European country to officially record >125,000 deaths associated with COVID-19. Although vaccine rollout has begun, many countries across the world are having to take various public-health measures to suppress virus transmission rates, including lockdown measures, provision of social distancing guidance, track and trace of individuals with COVID-19 and quarantining of travellers between one country and another 55 .
Worse COVID-19 outcomes and increased risk of death are linked to pre-existing cardiovascular disease 56 , 57 , 58 , and these individuals are advised to shield or self-isolate at home to minimize the risk of infection 59 . The pandemic has led to the disruption of many hospital services, including non-urgent outpatient appointments and routine ambulatory care, which have been curtailed or minimized. For cardiac rehabilitation, the pandemic has accentuated existing barriers to access discussed above (Table 3 ). In Canada, the USA and Europe, many cardiac rehabilitation centres have been closed, with some countries observing an overall reduction in cardiac rehabilitation participation 60 , 61 . In addition, cardiac rehabilitation capacity has been reduced because rehabilitation staff are being deployed to the ‘front line’ of intensive medical care for COVID-19. The increased risk of infection can lead to patients with diagnosed heart disease being anxious about travelling to centres to undertake rehabilitation. The dramatic effect of the pandemic on access to cardiac rehabilitation is illustrated by the British Heart Foundation NACR 61 , which has observed more than a two-thirds decrease in cardiac rehabilitation attendance in patients with heart failure from the pre-COVID period (4,969 patients in May 2019 to January 2020) to the COVID period (1,474 patients in February 2020 to August 2020). However, this drop in uptake was associated with a substantial increase in the proportion of patients enrolling in home-based cardiac rehabilitation programmes, which increased from 22.2% to 72.4% in the same time frame.
Conventional cardiac rehabilitation services that have relied on patients attending group-based sessions in hospitals or community centres have been difficult to sustain, and renewed calls have been made for alternatives to centre-based cardiac rehabilitation 59 , 60 . Even before the outbreak of COVID-19, the uptake of cardiac rehabilitation in many countries was suboptimal.
As described above, increasing evidence supports the effectiveness and safety of mobile-technology-supported models of delivery 62 , 63 , 64 , which are recommended in international guidelines 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 and receive reimbursement from external agencies 65 . Endorsement of remote delivery of cardiac rehabilitation in the COVID-19 era has come from various international sources 47 , 48 , 54 , 55 , 60 , 61 , 66 , 67 . EAPC has made an emphatic call for cardiac tele-rehabilitation to maintain the delivery of the core components of cardiac rehabilitation and has provided a practical guide for the set-up of a comprehensive cardiac tele-rehabilitation intervention during the COVID-19 pandemic 60 . However, concerns have been raised about equity in the use of technology to maintain access to outpatient care. Lower rates of technology and Internet use and access to these facilities have been documented in elderly individuals, those of lower socioeconomic status and ethnic minorities, mirroring the groups associated with limited enrolment and low levels of participation in cardiac rehabilitation 68 , 69 . In a cohort study of 2,940 patients scheduled to attend cardiology clinics at one centre in the USA in 2020, those individuals with lower income and who were non-English-speaking, female and/or older had more difficulty in engaging in care via telemedicine, suggesting that its rapid adoption exacerbates existing inequities 70 , 71 .
The pandemic has prompted providers of cardiac rehabilitation to seriously consider remote models of delivery, so that patients with heart disease can follow a self-care rehabilitation programme from their own home, which could also include support from their family and friends. A beacon model of innovative, evidence-based service delivery in the UK during the pandemic has been the REACH-HF programme 72 .
Managing multimorbidity
Cardiac rehabilitation has traditionally been commissioned and delivered as a ‘single disease’ service and focuses on the needs of patients with MI or heart failure or who have undergone revascularization. Although referred for cardiac rehabilitation for a specific indication, patients do not typically present with their single index disease alone, but instead have several long-term comorbidities. For example, the large, US-based, multicentre, randomized, controlled HF-ACTION trial 73 of cardiac rehabilitation reported that in addition to an index diagnosis of heart failure, at entry to the study, a substantial proportion of the 2,331 patients with heart failure had several comorbidities, including 59% with hypertension, 21% with atrial fibrillation or flutter and 32% with diabetes mellitus. The 2019 UK NACR reported that approximately 50% of all 6,502 patients referred for cardiac rehabilitation had two or more comorbidities 40 .
The management of multimorbidity is an important challenge facing health-care systems 74 . Levels of multimorbidity are predicted to grow with population demographic changes and improved survival rates resulting in increased numbers of older individuals 75 , 76 . Importantly, patients with multimorbidity are at higher risk of dying prematurely, being admitted to hospital, having longer stays in hospital and having a reduced health-related quality of life 75 , 76 than patients with only one chronic medical condition. The presence of multimorbidity seems to affect the provision of cardiac rehabilitation services. The 2019 UK NACR data set showed that multimorbidity was a strong risk factor for both non-use of cardiac rehabilitation and programme non-completion 40 . For example, a higher proportion of non-completers have symptoms of anxiety (5% higher) and depression (8% higher) than completers 77 .
The increasing burden and complexity of multimorbidity challenge our traditional model of cardiac rehabilitation. Although a core component of cardiac rehabilitation is a detailed patient assessment that includes the assessment of comorbidity, with its focus on single-disease management instead of individualized or personalized care, the delivery of existing programmes of cardiac rehabilitation might be failing to meet the health needs of patients with cardiac disease and multimorbidity. These patients have a high risk of non-referral to a cardiac rehabilitation programme. Furthermore, even if they are referred to rehabilitation, a high risk exists that the programmes will not fully address the needs of patients with multimorbidity. Instead, we need to adapt to the change in population demographics and look to provide a model of personalized multimorbidity rehabilitation that meets the needs of patients, irrespective of their index diagnosis, cardiovascular or otherwise. Arguments for this multimorbidity rehabilitative model approach are summarized in Box 5 .
Although a move to a model of cardiac rehabilitation that more comprehensively addresses the needs of patients with heart disease and their multimorbidity is appealing, the evidence base for innovation remains limited. At present, only two small, developmental studies have specifically focused on multimorbidity rehabilitation. A pilot RCT evaluated the feasibility of 8 weeks of a ‘generic rehabilitation’ programme of supervised exercise and education (based on the principles of cardiac rehabilitation and pulmonary rehabilitation) or no rehabilitation control in 16 patients with multimorbidity at a single centre in Australia 78 . The researchers reported that 71% of patients completed the rehabilitation intervention and had a higher mean improvement in 6-min walking distance than the control population (44 m versus 23 m) 78 .
The Healthy and Active Rehabilitation Programme (HARP) was established in Ayrshire, Scotland, in 2015 (ref. 79 ). The HARP model was developed to focus specifically on deprived and rural communities and those with high unscheduled care demand (that is, cardiac or pulmonary disease, cancer, stroke, diabetes and/or a high risk of falls). Developed from existing models of cardiac rehabilitation and pulmonary rehabilitation, HARP is based on a comprehensive patient assessment followed by a 10-week exercise and education programme. Interviews with patients with multimorbidity indicated that the HARP programme was well received and was perceived to improve confidence and motivation for physical activity and other healthy behaviours.
In the absence of an established evidence base, an urgent need exists for research into the acceptability, efficacy and cost-effectiveness of personalized models of rehabilitation for multimorbidity. Although we should not abandon our existing cardiac rehabilitation practice, there remains the challenge of more comprehensively meeting the needs of patients with cardiac disease and multimorbidity and developing a robust evidence base around these developments. A 2020 editorial identified key research questions around the future evolution of cardiac rehabilitation services for multimorbidity 80 .
Box 5 Adapting the traditional model of cardiac rehabilitation
Advantages of adapting the traditional (single-index) cardiac rehabilitation model for patients with multimorbidity 80 .
Sustainability
In the current financially challenged health service, health-care commissioners and purchasers are likely to consider the expansion of disease-specific rehabilitation services as inefficient and unsustainable. Instead, they would be more attracted to a programme that caters for patients with multimorbidity as a more appropriate and cost-effective model of care.
The failure to consider the effect of multimorbidity on the wellbeing and functionality of the patient and, for example, ‘just rehabilitate their heart failure’ is likely to diminish greatly the potential benefits of rehabilitation. Given that candidate patients for pulmonary and cardiac rehabilitation commonly have multiple chronic conditions, many of the important clinical problems that these patients face are probably not directly related to their cardiac or respiratory disease. We know from qualitative research that treating one condition at a time is inconvenient and unsatisfactory for patients with chronic conditions.
Inclusivity
Personalized multimorbidity rehabilitation presents an opportunity to develop a model by which to extend services to other important long-term conditions that would be amenable to rehabilitation, such as atrial fibrillation. Furthermore, this model could be extended to include other patient groups with, for example, transient ischaemic attack, mild stroke or peripheral vascular disease.
Box 5 adapted with permission from ref. 80 , Oxford University Press.
Improving access in LMICs
It is estimated that by 2030, more than 80% of cardiovascular-related disability and death will occur in the 139 LMICs owing to increasing prevalence of risk factors, such as hypertension, smoking, diabetes and obesity 81 , 82 . Although secondary prevention strategies are vitally important to stemming this growing epidemic, cardiac rehabilitation programmes remain largely non-existent in the LMIC setting compared with high-income economies.
The global inequality in cardiac rehabilitation provision was quantified by the International Council of Cardiovascular Prevention and Rehabilitation (ICCPR) audit 83 . Published in 2019, this ICCPR study revealed that cardiac rehabilitation is available in only half of the countries of the world, and this geographical distribution of cardiac rehabilitation is negatively correlated with the incidence of ischaemic heart disease, according to the Global Burden of Disease study 84 (Fig. 3 ).
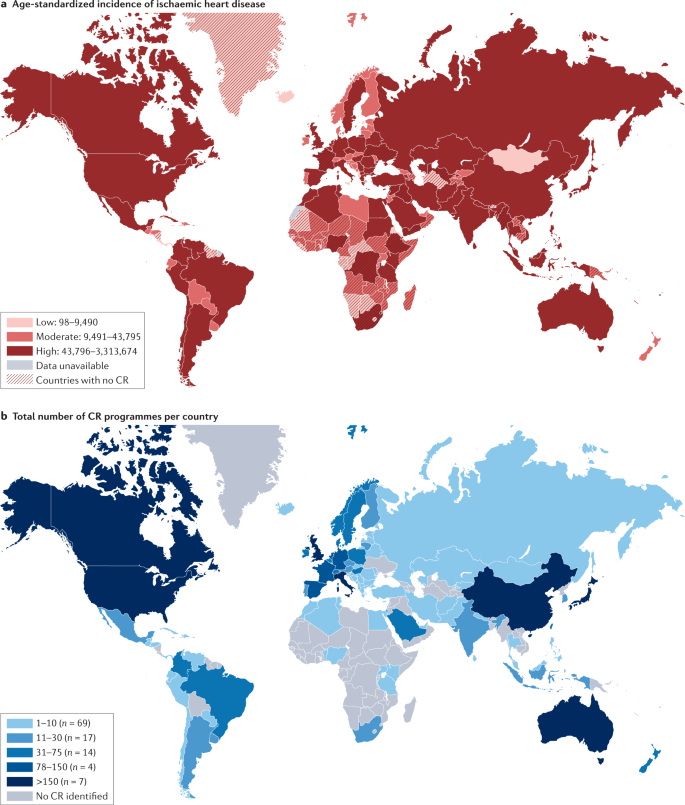
a | Age-standardized incidence of ischaemic heart disease. b | Total number of cardiac rehabilitation (CR) programmes per country. CR is available in only approximately half of the countries of the world and, in broad terms, the geographical distribution of CR is negatively correlated with the incidence of ischaemic heart disease. Data from ref. 83 .
This inequality is put into sharp focus by the contrasting densities in cardiac rehabilitation provision of only one cardiac rehabilitation place available for every 66 patients with ischaemic heart disease in LMICs, compared with one place for every 3.4 patients in high-income counties 85 . For example, Bangladesh has only one cardiac rehabilitation programme across the whole country, whereas England has more than 200 cardiac rehabilitation programmes, despite a similar annual incidence of ischaemic heart disease (409,000 versus 318,284, respectively). Although the barriers to the availability of cardiac rehabilitation are complex (Table 3 ), crucial additional economic constraints include limited health-care system budgets plus the consequent need for patient out-of-pocket payment, for which public funding is not available or is limited 43 .
Although the evidence demonstrating the beneficial effects of cardiac rehabilitation to date has mainly been collected in RCTs conducted in high-income settings, there is now a growing body of literature from developing countries. An ongoing systematic review has identified 26 RCTs of cardiac rehabilitation in 6,380 patients (primarily with ischaemic heart disease or heart failure) conducted across eight LMICs (Bangladesh, Brazil, China, Egypt, India, Iran, Nigeria and Pakistan) 86 . Meta-analysis of these trials conducted in LMICs shows that the increase in exercise capacity with cardiac rehabilitation (mean increase in peak oxygen uptake of 3.1 ml/kg/min, 95% CI 2.6–3.6 ml/kg/min) compared with the control population who received no cardiac rehabilitation was similar to figures reported in trials of cardiac rehabilitation conducted in high-income countries (3.3 ml/kg/min, 95% CI 2.6–4.0 ml/kg/min) 87 .
A systematic review of economic evaluations of cardiac rehabilitation in LMICs found no studies from low-income countries 88 . However, five studies in middle-income settings in Latin America indicated that cardiac rehabilitation could be a cost-effective intervention. In Brazil, the mean cost per patient was US$503 for a 3-month cardiac rehabilitation programme, with a mean monthly saving in health-care costs of US$190 for cardiac rehabilitation, compared with an increase of US$48 in the control group receiving no cardiac rehabilitation. Given the limited health-care budgets in many LMICs, the researchers of this study emphasized the need for affordable cardiac rehabilitation models in this setting 89 .
Box 6 provides a case example of the development of cardiac rehabilitation in the LMIC setting of Bangladesh 90 . Expansion of cardiac rehabilitation services is urgently needed to mitigate the epidemic of cardiovascular diseases in LMICs. Unlike high-resource settings, in which cardiac rehabilitation has traditionally been delivered in the hospital setting, often with a team of highly specialist staff, considerations of affordability, scalability and the needs of the local populations and health-care systems demand alternative approaches for the provision of cardiac rehabilitation services in LMICs. This alternative approach includes home-based and community-based programmes supported by accessible digital technology (such as Internet and mobile phone accessibility) and cost-effective training programmes for health-care staff to ensure the quality of delivery of cardiac rehabilitation practice 91 , 92 . An imperative on the global health community is to incorporate novel cardiac rehabilitation delivery models into efforts directed at the secondary prevention of cardiovascular disease, in line with the WHO’s dual strategic targets of reducing mortality from non-communicable diseases by 25% by 2030 and overcoming the ever-increasing unmet need for rehabilitation worldwide, which is particularly profound in LMICs 93 , 94 .
Box 6 Case example: cardiac rehabilitation in Bangladesh
In the past 10 years, Bangladesh has expanded the number of cardiovascular care facilities and improved service quality throughout the country. These facilities are run by public, private and autonomous sectors and include dedicated cardiac institutions and multi-speciality institutions with cardiac care facilities, with most located in the capital, Dhaka. Although the number of acute cardiac care facilities has increased, currently only one hospital-based cardiac rehabilitation programme is available in Bangladesh, based at the Ibrahim Cardiac Hospital & Research Institute (ICHRI).
From 2010, ICHRI introduced an exercise-based and education-based multidisciplinary cardiac rehabilitation programme for patients after cardiac surgery, consisting of a 30-min group exercise programme supported with a leaflet on sternum protection containing advice that can be followed at home. A single-centre, quasi-randomized controlled trial indicated that this cardiac rehabilitation programme was feasible and had potential benefits in terms of coronary heart disease risk factors, health-related quality of life, mental wellbeing and exercise capacity 90 . Following a 12-month clinical fellowship in Denmark and the UK in 2015, Jamal Uddin (a senior physiotherapist) started a comprehensive cardiac rehabilitation programme at the ICHRI. This programme consists of a group exercise programme from the seventh postoperative day, a risk-factor management educational class, dietary advice from dietitians and a manual to allow participants to maintain home-based cardiac rehabilitation. The manual includes upper-limb and lower-limb exercises, breathing exercises and aerobic exercise (a walking programme). The ICHRI also offers a 1-year cardiac rehabilitation follow-up (three follow-up visits within 1 year). During this follow-up, the patient first visits the cardiology unit and then the physiotherapy and cardiac rehabilitation unit for a cardiac fitness test and receives complete instructions for following an exercise programme.
A stakeholder round-table meeting was held in Dhaka on 30 November 2019: researchers, clinicians, health-care professionals and health-care policy-makers met to discuss affordable, flexible and feasible ways to scale up cardiac rehabilitation provision in Bangladesh and South Asia. This round-table meeting called for three key actions: expand and increase the reach of cardiac rehabilitation services through centre-based and home-based cardiac rehabilitation programmes; emphasize the importance of involving more cardiologists and cardiac surgeons to refer patients to cardiac rehabilitation; and offer inclusive, professional development training for health-care providers to promote the establishment of more cardiac rehabilitation programmes in Bangladesh.
Conclusions
Cardiac rehabilitation is a complex, multicomponent intervention that includes exercise training and physical activity promotion, health education, cardiovascular risk management and psychological support, personalized to the individual needs of patients diagnosed with heart disease. First introduced in the 1960s for low-risk patients who survived an acute MI, a growing body of RCT evidence over the past 3–4 decades now supports contemporary clinical guidelines, which recommend routine referral for cardiac rehabilitation across a range of cardiac diagnoses, including acute coronary syndrome, heart failure and after coronary revascularization (PCI or CABG surgery). As discussed in this Review, despite consistent and strong recommendations for cardiac rehabilitation referral in international clinical guidelines, contemporary cardiac rehabilitation practice faces a number of challenges. Global access to cardiac rehabilitation is persistently poor, with only 5–50% of eligible patients with cardiac disease receiving rehabilitation. Sadly, the ongoing COVID-19 pandemic has substantially added to this challenge: existing centre-based programmes have paused their services, with rehabilitation staff being relocated to critical care settings and patients being anxious about travelling to a centre for their rehabilitation. However, out of this ‘access challenge’ has come the opportunity to expedite the switch to (or combine) accessible home-based and technology-based models of cardiac rehabilitation, with appropriate quality assurance for their delivery. The development and provision of innovative models of delivery are likely to be especially important in LMICs, in which cardiac rehabilitation services are scarce, and scalable and affordable models are much needed. Key areas of research to support the future practice of cardiac rehabilitation are summarized in Box 3 .
Richardson, C. R., Franklin, B., Moy, M. L. & Jackson, E. A. Advances in rehabilitation for chronic diseases: improving health outcomes and function. BMJ 365 , l2191 (2019).
PubMed Google Scholar
Dempster, M. & Donnelly, M. Measuring the health related quality of life of people with ischaemic heart disease. Heart 83 , 641–644 (2000).
CAS PubMed PubMed Central Google Scholar
Taylor, R., Dibben, G., Faulkner, J. & Dalal, H. More evidence of cardiac rehabilitation: need to consider patient quality of life. Can. J. Cardiol. https://doi.org/10.1016/j.cjca.2021.01.012 (2021).
Article PubMed Google Scholar
Cook, R., Davidson, P. & Martin, R. et al. Cardiac rehabilitation for heart failure can improve quality of life and fitness. BMJ 367 , l5456 (2019).
Ambrosetti, M. et al. Secondary prevention through comprehensive cardiovascular rehabilitation: from knowledge to implementation. 2020 update. A position paper from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur. J. Prev. Cardiol. 28 , 460–495 (2021).
Google Scholar
BACPR. The BACPR Standards and Core Components for Cardiovascular Disease Prevention and Rehabilitation 2017 (3rd Edition) https://www.bacpr.com/resources/BACPR_Standards_and_Core_Components_2017.pdf (2017).
Abreu, A. et al. Standardization and quality improvement of secondary prevention through cardiovascular rehabilitation programmes in Europe: the avenue towards EAPC accreditation programme: a position statement of the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology (EAPC). Eur. J. Prev. Cardiol. 28 , 496–509 (2021).
Kavanagh, T. in Cardiac Rehabilitation (eds Jones, D. & West, R.) 4–30 (BMJ Publishing Group, 1995).
Guyatt, G. H. et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336 , 924–926 (2008).
PubMed PubMed Central Google Scholar
Faulkner, J. et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst. Rev. (in press).
Anderson, L. et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst. Rev. 3 , CD001800 (2016).
West, R. R., Jones, D. A. & Henderson, A. H. Rehabilitation after myocardial infarction trial (RAMIT): multi-centre randomised controlled trial of comprehensive cardiac rehabilitation in patients following acute myocardial infarction. Heart 98 , 637–644 (2012).
Rauch, B. et al. The prognostic effect of cardiac rehabilitation in the era of acute revascularisation and statin therapy: a systematic review and meta-analysis of randomized and non-randomized studies - The Cardiac Rehabilitation Outcome Study (CROS). Eur. J. Prev. Cardiol. 23 , 1914–1939 (2016).
Salzwedel, A. et al. Effectiveness of comprehensive cardiac rehabilitation in coronary artery disease patients treated according to contemporary evidence based medicine: update of the Cardiac Rehabilitation Outcome Study (CROS-II). Eur. J. Prev. Cardiol. 27 , 1756–1774 (2020).
Long, L. et al. Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst. Rev. 1 , CD003331 (2019).
American Thoracic Society. Minnesota Living with Heart Failure questionnaire http://qol.thoracic.org/sections/instruments/ko/pages/mlwhfq.html (2004).
Risom, S. S. et al. Exercise-based cardiac rehabilitation for adults with atrial fibrillation. Cochrane Database Syst. Rev. 2 , CD011197 (2017).
Williams, C. A. et al. Physical activity interventions for people with congenital heart disease. Cochrane Database Syst. Rev. 10 , CD013400 (2020).
Nielsen, K. M. et al. Exercise-based cardiac rehabilitation for adult patients with an implantable cardioverter defibrillator. Cochrane Database Syst. Rev. 2 , CD011828 (2019).
Anderson, L. et al. Exercise-based cardiac rehabilitation in heart transplant recipients. Cochrane Database Syst. Rev. 4 , CD012264 (2017).
Abraham, L. N. et al. Exercise-based cardiac rehabilitation for adults after heart valve surgery. Cochrane Database Syst. Rev. 5 , CD010876 (2021).
Higgins, J. P. T. Cochrane handbook for systematic reviews of interventions version 6.2. Cochrane Training https://training.cochrane.org/handbook (2021).
Scherrenberg, M. et al. Is there an optimal dose of cardiac rehabilitation in coronary artery disease patients? Int. J. Cardiol. 330 , 7–11 (2021).
Nichols, S. et al. Routine exercise-based cardiac rehabilitation does not increase aerobic fitness: a CARE CR study. Int. J. Cardiol. 305 , 25–34 (2020).
CAS PubMed Google Scholar
Ganga, H. V. et al. Supervised exercise training versus usual care in ambulatory patients with left ventricular assist devices: a systematic review. PLoS ONE 12 , e0174323 (2017).
Tajrishi, F. Z. et al. Spontaneous coronary artery dissection and associated myocardial bridging: current evidence from cohort study and case reports. Med. Hypotheses 128 , 50–53 (2019).
Wang, H. L. et al. Exercise interventions in cardio-oncology populations: a scoping review of the literature. J. Cardiovasc. Nurs. 36 , 385–404 (2021).
Shields, G. E. et al. Cost-effectiveness of cardiac rehabilitation: a systematic review. Heart 104 , 1403–1410 (2018).
Rawlins, M. D. & Culyer, A. J. National Institute for Clinical Excellence and its value judgments. BMJ 329 , 224–227 (2004).
Knuuti, J. et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 41 , 407–477 (2020).
Ponikowski, P. et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 18 , 891–975 (2016).
Smith, S. C. Jr et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a Guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. J. Am. Coll. Cardiol. 58 , 2432–2446 (2011).
Yancy, C. W. et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 62 , e147–e239 (2013).
NICE. Acute coronary syndromes: NICE guideline [NG185]. https://www.nice.org.uk/guidance/NG185 (2020).
NICE. Chronic heart failure in adults: diagnosis and management: NICE guideline [NG106]. https://www.nice.org.uk/guidance/ng106 (2018).
Chew, D. P. et al. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the management of acute coronary syndromes. Med. J. Aust. 205 , 128–133 (2016).
Atherton, J. J. et al. National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand: guidelines for the prevention, detection, and management of heart failure in Australia 2018. Heart Lung Circ. 27 , 1123–1208 (2018).
Thomas, R. J. et al. Home-based cardiac rehabilitation: a scientific statement from the American association of cardiovascular and pulmonary rehabilitation, the American Heart Association, and the American College of Cardiology. J. Am. Coll. Cardiol. 74 , 133–153 (2019).
Price, K. J. et al. A review of guidelines for cardiac rehabilitation exercise programmes: Is there an international consensus? Eur. J. Prev. Cardiol. 23 , 1715–1733 (2016).
BHF. National Audit of Cardiac Rehabilitation (NACR) Quality and Outcomes Report 2019. https://www.bhf.org.uk/informationsupport/publications/statistics/national-audit-of-cardiac-rehabilitation-quality-and-outcomes-report-2019 (2019).
Peters, A. E. & Keeley, E. C. Trends and predictors of participation in cardiac rehabilitation following acute myocardial infarction: data from the behavioral risk factor surveillance system. J. Am. Heart Assoc. 7 , e007664 (2017).
Ruano-Ravina, A. et al. Participation and adherence to cardiac rehabilitation programs. A systematic review. Int. J. Cardiol. 223 , 436–443 (2016).
Ragupathi, L. et al. Availability, use, and barriers to cardiac rehabilitation in LMIC. Glob. Heart 12 , 323–334.e10 (2017).
Kotseva, K. et al. Use and effects of cardiac rehabilitation in patients with coronary heart disease: results from the EUROASPIRE III survey. Eur. J. Prev. Cardiol. 20 , 817–826 (2013).
NHS. Long-term Plan. Cardiovascular disease. https://www.longtermplan.nhs.uk/online-version/chapter-3-further-progress-on-care-quality-and-outcomes/better-care-for-major-health-conditions/cardiovascular-disease/ (2019).
Ades, P. A. et al. Increasing cardiac rehabilitation participation from 20% to 70%: a road map from the Million Hearts Cardiac Rehabilitation Collaborative. Mayo Clin. Proc. 92 , 234–242 (2017).
Dalal, H. M. et al. The effects and costs of home-based rehabilitation for heart failure with reduced ejection fraction: the REACH-HF multicentre randomized controlled trial. Eur. J. Prev. Cardiol. 26 , 262–272 (2019).
Taylor, R. S. et al. The cost effectiveness of REACH-HF and home-based cardiac rehabilitation compared with the usual medical care for heart failure with reduced ejection fraction: a decision model-based analysis. Eur. J. Prev. Cardiol. 26 , 1252–1261 (2019).
Purcell, C. et al. Protocol for an implementation study of an evidence-based home cardiac rehabilitation programme for people with heart failure and their caregivers in Scotland (SCOT:REACH-HF). BMJ Open 10 , e040771 (2020).
Daw, P. et al. Getting evidence into clinical practice: protocol for evaluation of the implementation of a home-based cardiac rehabilitation programme for patients with heart failure. BMJ Open 10 , e036137 (2020).
Harrison, A. S. & Doherty, P. Does the mode of delivery in routine cardiac rehabilitation have an association with cardiovascular risk factor outcomes? Eur. J. Prev. Cardiol. 25 , 1925–1933 (2018).
Buckingham, S. A. et al. Home-based versus centre-based cardiac rehabilitation: abridged Cochrane systematic review and meta-analysis. Open Heart 3 , e000463 (2016).
Imran, H. M. et al. Home-Based cardiac rehabilitation alone and hybrid with center-based cardiac rehabilitation in heart failure: a systematic review and meta-analysis. J. Am. Heart Assoc. 8 , e012779 (2019).
Thomas, R. J. et al. Home-based cardiac rehabilitation: a scientific statement from the american association of cardiovascular and pulmonary rehabilitation, the American Heart Association, and the American College of Cardiology. J. Am. Coll. Cardiol. 74 , 133–153 (2019).
Hartley, D. M., Reisinger, H. S. & Perencevich, E. N. When infection prevention enters the temple: intergenerational social distancing and COVID-19. Infect. Control Hosp. Epidemiol. 41 , 868–869 (2020).
Guo, T. et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 5 , 811–818 (2020).
Nishiga, M. et al. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 17 , 543–558 (2020).
Schimelpfenig, N. What does a stay-at-home order mean? 11 questions answered. Healthline. https://www.healthline.com/health-news/what-a-stay-at-home-order-means (2020).
Babu, A. S., Arena, R., Ozemek, C. & Lavie, C. J. COVID-19: a time for alternate models in cardiac rehabilitation to take centre stage. Can. J. Cardiol. 36 , 792–794 (2020).
Scherrenberg, M. et al. The future is now: a call for action for cardiac telerehabilitation in the COVID-19 pandemic from the secondary prevention and rehabilitation section of the European Association of Preventive Cardiology. Eur. J. Prev. Cardiol. 28 , 524–540 (2021).
Doherty, P. & Harrison, A. Investigation of the impact of COVID-19 on UK CR: what can we learn from this experience? [abstract]. Presented at the 2020 BACPR Annual Conference (2020).
Rawstorn, J. C. et al. Telehealth exercise-based cardiac rehabilitation: a systematic review and meta-analysis. Heart 102 , 1183–1192 (2016).
Thomas, R. J. et al. 2018 ACC/AHA clinical performance and quality measures for cardiac rehabilitation: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J. Am. Coll. Cardiol. 71 , 1814–1837 (2018).
Vishwanath, V., Beckman, A. L. & Kazi, D. S. Reimagining cardiac rehabilitation in the era of coronavirus disease 2019. JAMA Health Forum 1 , e201346 (2020).
Thomas, E., Gallagher, R. & Grace, S. L. Future-proofing cardiac rehabilitation: transitioning services to telehealth during COVID-19. Eur. J. Prev. Cardiol. 28 , e35–e36 (2021).
Dalal, H. et al. Correspondence to the EJPC in response to position paper by Ambrosetti M et al. 2020 Cardiovascular rehabilitation and COVID-19: the need to maintain access to evidence-based services from the safety of home. Eur. J. Prev. Cardiol. https://doi.org/10.1177/2047487320923053 (2020).
Article PubMed PubMed Central Google Scholar
Anderson, M. & Kumar, M. Digital divide persists even as lower-income Americans make gains in tech adoption. Pew Research Center. https://www.pewresearch.org/fact-tank/2019/05/07/digital-divide-persists-even-as-lower-income-americans-make-gains-in-tech-adoption/ (2021).
Balady, G. J. et al. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: a presidential advisory from the American Heart Association. Circulation 124 , 2951–2960 (2011).
Eberly, L. A. et al. Telemedicine outpatient cardiovascular care during the COVID-19 pandemic: bridging or opening the digital divide? Circulation 142 , 510–512 (2020).
Nouri, S., Khoong, E. C., Lyles, C. R. & Karliner, L. Addressing equity in telemedicine for chronic disease management during the Covid-19 pandemic. NEJM Catalyst https://doi.org/10.1056/CAT.20.0123 (2020).
Article Google Scholar
NICE. COVID-19 ready rehabilitation for heart failure: REACH-HF can deliver. https://www.nice.org.uk/sharedlearning/covid-19-ready-rehabilitation-for-heart-failure-reach-hf-can-deliver (2020).
O’Connor, C. M. et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 301 , 1439–1450 (2009).
Barnett, K. et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 380 , 37–43 (2012).
Jani, B. D. et al. Relationship between multimorbidity, demographic factors and mortality: findings from the UK Biobank cohort. BMC Med. 17 , 74 (2019).
Makovski, T. T. et al. Multimorbidity and quality of life: systematic literature review and meta-analysis. Ageing Res. Rev. 53 , 100903 (2019).
Sumner, J., Böhnke, J. R. & Doherty, P. Does service timing matter for psychological outcomes in cardiac rehabilitation? Insights from the National Audit of Cardiac Rehabilitation. Eur. J. Prev. Cardiol. 25 , 19–28 (2018).
Barker, K. et al. A rehabilitation programme for people with multimorbidity versus usual care: a pilot randomized controlled trial. J. Comorb. 8 , 2235042X18783918 (2018).
Cowie, A., McKay, J. & Keenan, A. Combined generic-specialist multimorbidity rehabilitation post acute cardiac event. Brit. J. Card. Nurs. 13 , 340–347 (2018).
Taylor, R. S. & Singh, S. Personalised rehabilitation for cardiac and pulmonary patients with multimorbidity: time for implementation? Eur. J. Prev. Cardiol. https://doi.org/10.1177/2047487320926058 (2020).
GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392 , 1859–1922 (2018).
PubMed Central Google Scholar
WHO. Global status report on noncommunicable diseases 2010. United Nations Digital Library https://digitallibrary.un.org/record/706319?ln=en (2011).
Turk-Adawi, K. et al. Cardiac rehabilitation availability and density around the globe. EClinicalMedicine 13 , 31–45 (2019).
Global Health Data Exchange. Institute for Health Metrics and Evaluation. GBD results tool. http://ghdx.healthdata.org/gbd-results-tool (2021).
Pesah, E. et al. Cardiac rehabilitation delivery in low/middle-income countries. Heart 105 , 1806–1812 (2019).
Taylor, R. S., Grace, S., Mamataz, T., Uddin, J. & Ibn Alam, S. Effects of cardiac rehabilitation in low- and middle-income countries: a systematic review and meta-analyses of randomised controlled trials. https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=185296 (2020).
Uddin, J. et al. Predictors of exercise capacity following exercise-based rehabilitation in patients with coronary heart disease and heart failure: a meta-regression analysis. Eur. J. Prev. Cardiol. 23 , 683–693 (2016).
Oldridge, N. B., Pakosh, M. T. & Thomas, R. J. Cardiac rehabilitation in low- and middle-income countries: a review on cost and cost-effectiveness. Int. Health 8 , 77–82 (2016).
Salvetti, X. M., Oliveira, J. A., Servantes, D. M. & Vincenzo de Paola, A. A. How much do the benefits cost? Effects of a home-based training programme on cardiovascular fitness, quality of life, programme cost and adherence for patients with coronary disease. Clin. Rehabil. 22 , 987–996 (2008).
Uddin, J. et al. Effect of home-based cardiac rehabilitation in a lower-middle income country: results from a controlled trial. J. Cardiopulm. Rehabil. Prev. 40 , 29–34 (2020).
Grace, S. L. et al. Cardiac rehabilitation delivery model for low-resource settings: an International Council of Cardiovascular Prevention and Rehabilitation consensus statement. Prog. Cardiovasc. Dis. 59 , 303–322 (2016).
Taylor, R., Zwisler, A. D. & Uddin, J. Global health-care systems must prioritise rehabilitation. Lancet 396 , 1946–1947 (2021).
WHO. Global status report on noncommunicable diseases 2014. https://apps.who.int/iris/handle/10665/148114 (2014).
WHO. Rehabilitation 2030: a call for action. https://www.who.int/news-room/events/detail/2017/02/06/default-calendar/rehabilitation-2030-a-call-for-action (2017).
Download references
Acknowledgements
The authors thank J. Uddin (Physiotherapy Unit, Department of Cardiac Surgery Ibrahim Cardiac Hospital & Research Institute, Dhaka, Bangladesh) for drafting the content of Box 6, G. Dibben (MRC/CSO Social and Public Health Sciences Unit, University of Glasgow, UK) for preparing Fig. 2 for initial submission and U. Ahmed (MRC/CSO Social and Public Health Sciences Unit, University of Glasgow, UK) for editorial review of the text.
Author information
Authors and affiliations.
MRC/CSO Social and Public Health Sciences Unit & Robertson Centre for Biostatistics, Institute of Health & Well Being, University of Glasgow, Glasgow, UK
Rod S. Taylor
College of Medicine and Health, University of Exeter, Exeter, UK
University of Exeter Medical School (Primary Care), Smeall Building, St Luke’s Campus, Exeter, UK
Hasnain M. Dalal & Sinéad T. J. McDonagh
You can also search for this author in PubMed Google Scholar
Contributions
All the authors researched data for the article, contributed substantially to discussions of its content, wrote the article, and reviewed and/or edited the manuscript before submission.
Corresponding author
Correspondence to Rod S. Taylor .
Ethics declarations
Competing interests.
R.S.T. is a member of the ESC Association of Cardiovascular Nursing and Allied Professions (ACNAP) Science Committee 2020–2022 and lead investigator for the following ongoing funded projects: ‘Implementation of an evidence-based cardiac rehabilitation home programme for heart failure patients and their caregivers in Scotland: SCOT:REACH-HF project’, funded by Heart Research UK; ‘A randomized controlled trial of a facilitated home-based rehabilitation intervention in patients with heart failure with preserved ejection fraction and their caregivers: the REACH-HFpEF Study’, funded by NIHR HTA Programme (NIHR130487). H.M.D. is a co-opted member of the British Association of Cardiovascular Prevention and Rehabilitation (BACPR) and a co-lead for the ongoing funded research projects: ‘D REACH-HF: Digital Rehabilitation Enablement in Chronic Heart Failure’, funded by the British Heart Foundation, Hope for Hearts fund’; ‘Extending the reach and implementation of the successful REACH-HF programme with a digitally delivered training programme’, funded by NIHR Programme Development Grant (NIHR202040). S.J.D.M. is a researcher on the following ongoing funded research projects: ‘D REACH-HF: Digital Rehabilitation Enablement in Chronic Heart Failure’, funded by the British Heart Foundation, Hope for Hearts fund’; ‘Extending the reach and implementation of the successful REACH-HF programme with a digitally delivered training programme’, funded by NIHR Programme Development Grant (NIHR202040).
Additional information
Peer review information.
Nature Reviews Cardiology thanks D. Forman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Taylor, R.S., Dalal, H.M. & McDonagh, S.T.J. The role of cardiac rehabilitation in improving cardiovascular outcomes. Nat Rev Cardiol 19 , 180–194 (2022). https://doi.org/10.1038/s41569-021-00611-7
Download citation
Accepted : 10 August 2021
Published : 16 September 2021
Issue Date : March 2022
DOI : https://doi.org/10.1038/s41569-021-00611-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Comparing domain- and intensity-specific physical activity in coronary heart disease and non-chd individuals.
- Seon Young Goo
- Mi Kyung Lee
- Justin Y. Jeon
Scientific Reports (2024)
Incremental prognostic value of functional impairment assessed by 6-min walking test for the prediction of mortality in heart failure
- Domenico Scrutinio
- Pietro Guida
- Andrea Passantino
Cardiac telerehabilitation: current status and future perspectives
- Rutger W. M. Brouwers
- Martijn Scherrenberg
- Johan A. Snoek
Netherlands Heart Journal (2024)
Effect of an exercise-based cardiac rehabilitation program on quality of life of patients with chronic Chagas cardiomyopathy: results from the PEACH randomized clinical trial
- Marcelo Carvalho Vieira
- Fernanda de Souza Nogueira Sardinha Mendes
- Mauro Felippe Felix Mediano
Cardiac rehabilitation engagement and associated factors among heart failure patients: a cross-sectional study
BMC Cardiovascular Disorders (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
Cardiac rehabilitation
- Related content
- Peer review
- Hasnain M Dalal , honorary clinical associate professor 1 ,
- Patrick Doherty , chair in cardiovascular health, director of the National Audit of Cardiac Rehabilitation, deputy head of department (research) 2 ,
- Rod S Taylor , chair of health services research, academic lead for Exeter Clinical Trials Support Network, NIHR senior investigator 3
- 1 University of Exeter Medical School (primary care), Truro Campus, Knowledge Spa, Royal Cornwall Hospital, Truro TR1 3HD, UK
- 2 Department of Health Sciences, University of York, York YO10 5DD, UK
- 3 Institute of Health Research, University of Exeter Medical School, Exeter EX1 2LU, UK
- Correspondence to: H M Dalal hmdalal{at}doctors.org.uk
The bottom line
Globally, the prevalence of coronary heart disease and heart failure is increasing, and there is some evidence of the health benefits of cardiac rehabilitation
Effective implementation of cardiac rehabilitation after acute coronary syndrome, coronary revascularisation, and heart failure has remained suboptimal, with overall participation rates <50% over recent decades despite international recommendations
International guidelines now recommend that cardiac rehabilitation programmes include health education and psychological counselling
Patients should be offered a choice of community based and home based cardiac rehabilitation programmes to fit their needs and preferences
Clinicians should endorse cardiac rehabilitation for patients with a recent diagnosis of coronary heart disease or heart failure
Cardiac rehabilitation is a complex intervention offered to patients diagnosed with heart disease, which includes components of health education, advice on cardiovascular risk reduction, physical activity and stress management. Evidence that cardiac rehabilitation reduces mortality, morbidity, unplanned hospital admissions in addition to improvements in exercise capacity, quality of life and psychological well-being is increasing, and it is now recommended in international guidelines. 1 2 3 4 5 6 This review focuses on what cardiac rehabilitation is and the evidence of its benefit and effects on cardiovascular mortality, morbidity and quality of life.
Sources and selection criteria
RST is a member of the Cochrane Heart Group and has led and conducted several systematic reviews of cardiac rehabilitation. We searched the Cochrane database ( www.cochrane.org ) for cardiac rehabilitation and equivalent terms. We identified current national and international clinical guidelines based on systematic reviews and meta-analyses. We referred to the National Audit of Cardiac Rehabilitation annual report, which was led by PD, and the British Heart Foundation’s website for statistics on coronary heart disease in the UK. We also consulted recent review articles from the UK, US, Canada, and Australia. We have included topics that would be of interest to hospital doctors and general practitioners based on a previous review coauthored by HMD and also the level 1 evidence provided by the Cochrane reviews. We also used our personal reference collections.
Why is cardiac rehabilitation important?
Although mortality from coronary heart disease has fallen over recent decades, annually it still claims an estimated 1.8 million lives in Europe, 7 and 785 000 new and 470 000 recurrent myocardial infarctions occur in the US. 8 In the UK, around 110 000 men and 65 000 women have an acute myocardial infarction every year, equivalent to one every three minutes. 9 With improved survival and an aging population, the number of people living with coronary heart disease in the UK has increased to an estimated 2.3 million. 9
What is cardiac rehabilitation and who should get it?
Various organisations and national bodies have defined cardiac rehabilitation, which is encompassed by: “Cardiac rehabilitation (and secondary prevention) services are comprehensive, long term programmes involving medical evaluation, prescribed exercise, cardiac risk factor modification, education, and counselling. These programmes are designed to limit the physiological and psychological effects of cardiac illness, reduce the risk for sudden death or re-infarction, control cardiac symptoms, stabilise or reverse the atherosclerotic process, and enhance the psychosocial and vocational status of selected patients.” Although exercise training is a core component, current practice guidelines consistently recommend “comprehensive rehabilitation” programmes that should include other components to optimise cardiovascular risk reduction, foster healthy behaviours and compliance to these behaviours, reduce disability, and promote an active lifestyle. 5
The National Institute for Health and Care Excellence (NICE), Department of Health, British Association for Cardiovascular Prevention and Rehabilitation (BACPR), and wider European guidelines agree that the patient groups listed in box 1 will benefit from cardiac rehabilitation. 1 2 4 10 11 12 and the core components of cardiac rehabilitation are illustrated in figure 2. 1
Box 1: Patient groups who benefit from cardiac rehabilitation*
Patients with acute coronary syndrome—including ST elevation myocardial infarction, non-ST elevation myocardial infarction, and unstable angina—and all patients undergoing reperfusion (such as coronary artery bypass surgery, primary percutaneous coronary intervention, and percutaneous coronary intervention)
Patients with newly diagnosed chronic heart failure and chronic heart failure with a step change in clinical presentation
Patients with heart transplant and ventricular assist device
Patients who have undergone surgery for implantation of intra-cardiac defibrillator or cardiac resynchronisation therapy for reasons other than acute coronary syndrome and heart failure
Patients with heart valve replacements for reasons other than acute coronary syndrome and heart failure
Patients with a confirmed diagnosis of exertional angina
*According to NICE, Department of Health, BACPR, and European guidelines 1 2 4 10 11 12
Historically, cardiac rehabilitation in the UK, US, and most European countries has been delivered to groups of patients in healthcare or community centres. 13 14 Recent guidance from the UK Department of Health 12 refers to a seven stage pathway of care that begins with diagnosis of a cardiac event and is followed by assessment of eligibility, referral, clinical assessment, and core delivery of cardiac rehabilitation before progressing to long term management (fig 1 ⇓ ).
Fig 1 BACPR standards pathway, showing a patient’s journey through cardiac rehabilitation (reproduced with permission from BACPR 1 ). *CR=cardiac rehabilitation
- Download figure
- Open in new tab
- Download powerpoint
Formal rehabilitation programmes vary in intensity and duration. The European guide for patients with established cardiac disease provides a full review of the impact of the mode and dose of exercise based cardiac rehabilitation. 15 In the UK, formal rehabilitation is predominantly provided to supervised groups in outpatient hospital clinics or community centres, starting 2–4 weeks after percutaneous coronary intervention or myocardial infarction and usually 4–6 weeks after cardiac surgery. 14 The BACPR standard recommends delivery of the seven core components of cardiac rehabilitation after clinical assessment (fig 2 ⇓ ). 1 Programmes are typically delivered by specialist nurses or physiotherapists supported by exercise therapists, although ideally an integrated multidisciplinary team led by an experienced clinician with a special interest in cardiac rehabilitation should deliver the programme (BACPR standard 2, box 2). 1 Most programmes involve weekly attendance at group sessions for an average of 56 (SD 3.6) days or approximately 8 weeks. 16 Centre based sessions involve graduated exercise training, education (covering coronary risk factors and diet), common cardiac misconceptions, preventative medication, and stress management. 14 Ideally, patients should be given information about the cardiac event and lifestyle advice, including the importance of smoking cessation (if appropriate), healthy diet, and physical activity to encourage progressive mobilisation. Prior to discharge, clinicians should ensure that patients are prescribed drugs for secondary prevention and drugs that are beneficial for those with systolic heart failure such as angiotensin-converting enzyme (ACE) inhibitors and beta-blockers. 1 Good communication between secondary and primary care after discharge can improve uptake of cardiac rehabilitation and optimise secondary prevention. 17
Cardiac rehabilitation programmes in the US and Europe tend to be more intensive than those in the UK and are delivered from outpatient departments over 3–6 months. Some European countries offer residential programmes lasting 3–4 weeks. The focus is mainly on “monitored exercise and aggressive risk factor reduction” in medically supervised sessions. 13 18
Fig 2 Core components of cardiac rehabilitation. Reproduced with permission from BACPR 1
Box 2: Core components of cardiac rehabilitation. Adapted from BACPR Standard 2 1
1. Health behaviour change and education
2. Lifestyle risk factor management
- Physical activity and exercise
- Smoking cessation
3. Psychosocial health
4. Medical risk factor management
5. Cardioprotective therapies
6. Long term management
7. Audit and evaluation
Delivery of the core components requires expertise from a range of different professionals. The team may include:
Cardiologist, community cardiologist, physician, or general practitioner with a special interest
Nurse specialist
Physiotherapist
Psychologist
Exercise specialist
Occupational therapist
Clerical administrator
What are the benefits of cardiac rehabilitation?
The benefits of cardiac rehabilitation for individuals after myocardial infarction and revascularisation and for those with heart failure have been reviewed comprehensively in several meta-analyses, including six Cochrane reviews and a recent clinical review from the US. 18 19 20 21 22 23 24
A 2011 Cochrane review and meta-analysis of 47 randomised controlled trials that included 10 794 patients showed that cardiac rehabilitation reduced overall mortality (relative risk 0.87 (95% confidence interval 0.75 to 0.99), absolute risk reduction (ARR) 3.2%, number needed to treat (NNT) 32) and cardiovascular mortality (relative risk 0.74 (0.63 to 0.87), ARR 1.6%, NNT 63), although this benefit was limited to studies with a follow-up of greater than 12 months. 25 With the exception of one large, UK based trial that showed little effect of cardiac rehabilitation on mortality at two years (relative risk 0.98 (0.74 to 1.30)), 26 findings from meta-analyses and observational studies support a mortality benefit. 27 Another systematic review and meta-analysis of 34 randomised controlled trials including 6111 patients after myocardial infarction showed that those who attended cardiac rehabilitation had a lower risk of all-cause mortality than non-attendees (odds ratio 0.74 (0.58 to 0.95)). 28
The latest updated Cochrane review of exercise based cardiac rehabilitation for coronary heart disease reports an absolute risk reduction in cardiovascular mortality from 10.4% to 7.6% (NNT 37) for patients after myocardial infarction and revascularisation who received cardiac rehabilitation compared with those who did not. 19 No significant reduction occurred in overall mortality, 19 which contrasts with results in previous meta-analyses. 25 29 The inclusion of patients from the UK based randomised controlled trial 26 is cited as one reason for this lack of reduction in mortality. 19 The negative findings of this trial have also led to scepticism about the content and delivery of UK based cardiac rehabilitation programmes in the late 1990s, 30 31 and this controversial trial has been the subject of much debate. 27 30 31 32
Reduced hospital admissions
Although the 2015 Cochrane review in coronary heart disease reported no reduction in the risks of fatal or non-fatal myocardial infarction or coronary revascularisation (coronary artery bypass graft or percutaneous coronary intervention), there was a reduced risk of hospital admission (from 30.7% to 26.1%, NNT 22). 19 In another Cochrane review of 33 randomised controlled trials and 4740 patients with heart failure, exercise based cardiac rehabilitation reduced the risk of overall hospitalisation (relative risk 0.75 (0.62 to 0.92), ARR 7.1%, NNT 15) and hospitalisation for heart failure (relative risk 0.61 (0.46 to 0.80), ARR 5.8%, NNT 18). 33
Improvement in psychological wellbeing and quality of life
A US observational study of 635 patients with coronary heart disease reported improvements in depression, anxiety, and hostility scores after cardiac rehabilitation. 34 Early cardiac rehabilitation programmes only offered interventions that focused predominantly on exercise, but significant (P<0.01) improvements in anxiety and depression scores were reported in one randomised controlled trial of 210 men admitted with myocardial infarction undergoing gym based exercise training. 35 Furthermore, a meta-analysis of 23 randomised controlled trials (3180 patients with coronary heart disease) that evaluated the impact of adding psychosocial interventions to standard exercise based cardiac rehabilitation reported a greater reduction in psychological distress (effect size 0.34) and improvements in systolic blood pressure and serum cholesterol (effect sizes −0.24 and −1.54 respectively). 36
Several studies have reported improvement in psychological stress in patients with coronary heart disease who have attended cardiac rehabilitation: one recent US observational study of 189 patients with heart failure (left ventricular ejection fraction <45%) reported a decrease in symptoms of depression by 40% after exercise training cardiac rehabilitation (from 22% to 13%, P<0.0001). 37 Also depressed patients who completed their cardiac rehabilitation had a 59% lower mortality (44% v 18%, P<0.05) compared with depressed dropout patients who did not undergo cardiac rehabilitation. 37
A Cochrane review of exercise based rehabilitation for coronary heart disease showed that seven out of 10 randomised controlled trials that reported quality of life using validated outcome measures found “significant improvement,” but the authors were not able to pool the data to quantify the effect because of the heterogeneity of the outcome measures. 25 Similarly, another Cochrane review of exercise based cardiac rehabilitation for heart failure reported a clinically important improvement in the Minnesota Living with Heart Failure questionnaire (mean difference 5.8 points (95% confidence interval 2.4 to 9.2), P=0.0007) in the 13 randomised controlled trials that used this validated quality of life measure. 33
Cardiovascular risk profile
Before the use of statins for the secondary prevention of coronary heart disease, two observational studies demonstrated the beneficial effects of diet and exercise in improving lipid profiles. 38 39 The findings of a small case series of 18 patients prescribed a low cholesterol diet and daily exercise for 30 minutes on a bicycle ergometer resulted in regression of coronary artery atheroma on angiography in seven of the 18 patients, compared with only one of 18 in the usual care group. 39 Significant reductions in total serum cholesterol concentration (−2%, P=0.05) and low density lipoprotein:high density lipoprotein cholesterol ratios (−9%, P≤0.0001) were reported after 36 sessions of cardiac rehabilitation in another US observational study from the 1990s involving 313 cardiac patients. 38
The prevalence of obesity in those attending cardiac rehabilitation in the US has increased in the past two decades, with >40% having a body mass index >30 and 80% with a body mass index >25. 40 Ades et al conducted a randomised controlled trial of 74 overweight patients with coronary heart disease and showed that a “walk often and walk far” (“high calorie, high expenditure”) exercise protocol of 45-60 minutes per session of lower intensity exercise (70% peak oxygen uptake) resulted in twice the weight loss (8.2 kg v 3.7 kg, P<0.001) compared with the standard cardiac rehabilitation exercise session of 25-40 minutes. This study also reported significant improvements (P<0.05) in systolic blood pressure, body mass index, serum triglycerides, HDL cholesterol, total cholesterol, blood glucose, and peak oxygen uptake in the high calorie, high expenditure exercise group.
What are the risks of cardiac rehabilitation?
A French observational study of more than 25 000 patients undergoing cardiac rehabilitation reported one cardiac event for 50 000 hours of exercise training, equivalent to 1.3 cardiac arrests per million patient-hours. 41 An earlier US study reported one case of ventricular fibrillation per 111 996 patient-hours of exercise and one myocardial infarction per 294 118 patient-hours. 42
Patients with unstable angina, uncontrolled ventricular arrhythmia, and severe heart failure (New York Heart Association (NYHA) level 3 or 4, ejection fraction <35%) have been considered at high risk, with formal risk stratification (to include factors such as a history of arrhythmias and functional capacity) conducted by an experienced clinician before they engage in the exercise component of cardiac rehabilitation. 1 However, the most recent Cochrane review found “no evidence to suggest that exercise training programmes cause harm in terms of an increase in the risk of all cause death in either the short or longer term” in patients with stable chronic heart failure (NYHA level 1–3). 22
Access to cardiac rehabilitation
For those who have difficulty accessing centre based cardiac rehabilitation, or those who dislike groups, home based cardiac rehabilitation programmes are sometimes available. 17 43 The most widely used programme in the UK is the Heart Manual 44 —a six week intervention that uses written material and a relaxation CD and is delivered by a trained healthcare facilitator who makes home visits and provides telephone support—which has been shown to be just as effective as centre based programmes. 45 46
Overcoming barriers to cardiac rehabilitation
Despite robust evidence of clinical and cost effectiveness, uptake of cardiac rehabilitation varies worldwide and by patient group, with participation rates ranging from 20% to 50%. 1 18 47 48 Poor uptake has been attributed to several factors, including physicians’ reluctance to refer some patients, particularly women and those from ethnic minorities or lower socioeconomic classes, and lack of resources, capacity, and funding. 6 18 49 50 51 52 Adherence to cardiac rehabilitation programmes is affected by factors such as psychological wellbeing, geographical location, access to transport, and a dislike of group based rehabilitation sessions (box 3). 13 18 43 The most effective way to increase uptake and optimise adherence and secondary prevention is for clinicians to endorse cardiac rehabilitation by inviting patients still in hospital after a recent diagnosis of coronary heart disease or heart failure to participate and for nurse led prevention clinics to be linked with primary care and cardiac rehabilitation services. 2 53 54 55 56
Novel ways of providing cardiac rehabilitation are emerging using the internet and mobile phones. 57 58 A recent systematic review has evaluated alternative models of delivery 59 that can be provided via secondary prevention clinics. 60 Offering patients a choice of centre based, home, or online programmes on an equitable basis is likely to improve uptake across all groups of cardiac patients. Self management and collaboration with care givers can also improve uptake and outcomes. 61 62 63
Box 3: Barriers to cardiac rehabilitation participation. Adapted from Menezes et al 18
Poor referral rates, especially for certain groups:
- People from ethnic minority groups
- Elderly people
- People living in rural settings
- People in low socioeconomic classes
Poor patient adherence, leading to low enrolment and high dropout rates
Lack of endorsement by a doctor
Obesity (high body mass index)
Multiple morbidities, leading to poor functional capacity
Poor exercise habits
Cigarette smoking
Problems with transport
Poor social support
Lack of leave from work to attend centre-based sessions
Ongoing research and unanswered questions
Ongoing research.
The NIHR has sponsored two UK based studies:
- REACH-HF aims to develop a new self help manual for people with heart failure and their caregivers, which may help them to manage the condition using the principles of cardiac rehabilitation. The team will then evaluate the clinical effectiveness, cost effectiveness, and acceptability of the manual for people with heart failure and their caregivers. www.rcht.nhs.uk/RoyalCornwallHospitalsTrust/WorkingWithUs/TeachingAndResearch/ReachHF/Homepage.aspx . (A protocol paper on REACH-HF has been submitted to BMJ Open.)
- CADENCE is a feasibility study and pilot randomised controlled trial to establish methods to assess the acceptability and the clinical and cost effectiveness of enhanced psychological care in cardiac rehabilitation services for patients with new onset depression. http://medicine.exeter.ac.uk/esmi/workstreams/cochranecardiacrehabilitationreviews/
WREN pilot study of web based cardiac rehabilitation for those declining or dropping out of conventional rehabilitation. http://public.ukcrn.org.uk/search/StudyDetail.aspx?StudyID=19260
Telerehab III, a multicentre randomised controlled trial of 140 patients with coronary heart disease in Belgium, is evaluating the effectiveness of tele-rehabilitation, which has been proposed as an adjunct or alternative to standard, centre based cardiac rehabilitation. The study aims to investigate the long term effectiveness of adding to standard cardiac rehabilitation a patient tailored, internet based, rehabilitation programme that implements multiple core components of cardiac rehabilitation and uses telemonitoring and telecoaching strategies. www.biomedcentral.com/content/pdf/s12872-015-0021-5.pdf
Unanswered questions
What characteristics are associated with uptake and adherence to cardiac rehabilitation after an acute myocardial infarction when rehabilitation is started early?
How can referral and participation rates for eligible patients be increased?
Should referral be the responsibility of the physician or the healthcare team?
How will working and non-working patients afford to pay for these services?
Can advances in information and communication technologies be used to develop novel ways of delivering cardiac rehabilitation to improve uptake and adherence?
How can we improve uptake in hard to reach groups, such as patients living in rural communities, patients from ethnic minority groups, and those from low socioeconomic classes?
Is cardiac rehabilitation, as delivered in routine clinical practice, still effective?
Additional educational resources
Resources for healthcare professionals.
Anderson L, Taylor RS. Cardiac rehabilitation for people with heart disease: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev 2014;(2): CD011273. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD011273.pub2/abstract
This overview describes six Cochrane systematic reviews in cardiac rehabilitation, which included 148 randomised controlled trials in 98 093 participants.
British Heart Foundation. The National Audit of Cardiac Rehabilitation: annual statistical report 2014 . British Heart Foundation, 2014. www.bhf.org.uk/~/media/files/publications/research/nacr_2014.pdf .
Provides information to commissioners and clinicians on the inequalities and insufficiencies in delivery against key service indicators for over 320 cardiac rehabilitation programmes in the UK.
Menezes AR, Lavie CJ, Milani RV, Forman DE, King M, Williams MA. Cardiac rehabilitation in the United States. Prog Cardiovasc Dis 2014;56:522-9.
A clinical review that provides clinicians with information on the benefits of cardiac rehabilitation, risk factors, and factors affecting participation from a US perspective.
National Institute for Health and Care Excellence. Secondary prevention in primary and secondary care for patients following a myocardial infarction (clinical guidance 172). NICE, 2013. www.nice.org.uk/guidance/cg172 .
Provides clinicians and commissioners with new and updated recommendations on cardiac rehabilitation, drug therapy, and communication of diagnosis.
Piepoli M, Corrà U, Adamopoulos S, Benzer W, Bjarnason B, Cupples M, et al. Secondary prevention in the clinical management of patients with cardiovascular diseases. Core components, standards and outcome measures for referral and delivery. Eur J Prev Cardiol 2014;21:664-81.
A policy statement for clinicians and commissioners from the Cardiac Rehabilitation Section of the European Association for Cardiovascular Prevention & Rehabilitation.
Sandesara PB, Lambert CT, Gordon NF, et al. Cardiac rehabilitation and risk reduction: time to “rebrand and reinvigorate.” J Am Coll Cardiol 2015;65:389-95.
A clinical review article that argues that the current model of centre based cardiac rehabilitation is unsustainable and requires a patient centred strategy.
Clark RA, Conway A, Poulsen V, Keech W, Tirimacco R, Tideman P. Alternative models of cardiac rehabilitation: a systematic review. Eur J Prev Cardiol 2015;22:35-74.
Provides evidence on alternatives to the traditional hospital based model of cardiac rehabilitation.
Clark AM, Hartling L, Vandermeer B, McAlister FA. Secondary prevention program for patients with coronary artery disease: a meta-analysis of randomized control trials. Ann Intern Med 2005;143:659-72.
Evaluates clinical evidence to on the effectiveness of secondary cardiac prevention programmes with and without exercise components.
Resources for patients and carers
American Heart Association: What is cardiac rehabilitation? www.heart.org/HEARTORG/Conditions/More/CardiacRehab/What-is-Cardiac-Rehabilitation_UCM_307049_Article.jsp .
Provides answers to frequently asked questions about cardiac rehabilitation, including who needs it and for how long.
Association of Chartered Physiotherapists in Cardiac Rehabilitation. Patient information. http://acpicr.com/patient-information
Information on cardiac rehabilitation, its main components, and when to start it.
British Heart Foundation. www.bhf.org.uk/heart-health/living-with-a-heart-condition/cardiac-rehabilitation .
Information on cardiac rehabilitation programmes and how they can help prevent a heart attack and cardiac surgical interventions. Also has a video clip.
Healthtalkonline. www.healthtalk.org/peoples-experiences/heart-disease/heart-attack/cardiac-rehabilitation-support , www.healthtalk.org/peoples-experiences/heart-disease/heart-attack/topics#ixzz3lzyXycCp .
Text and personal stories on film from UK patients who have had a heart attack. Has stories from 37 people (including four carers) in their own homes.
NHS Choices. CHD Dave’s story: high cholesterol. www.nhs.uk/video/Pages/chd-high-cholesterol.aspx?searchtype=Tag&searchterm=Heart_vascular .
A video in which Dave shares his battle with his cholesterol levels and talks about how he got to where he is now, successfully managing his condition.
NHS Choices. Coronary heart disease-recovery. www.nhs.uk/Conditions/Coronary-heart-disease/Pages/Recovery.aspx .
Information on what to do after having heart surgery or problems such as a heart attack and how it is possible to resume a normal life.
NHS Choices. Heart attack: real story. www.nhs.uk/video/Pages/heart-attack-mike.aspx?searchtype=Tag&searchterm=Heart_vascular .
An account of how a man who is nearly 60 has survived three heart attacks. He explains how the attacks affected him and how his recovery was different for each of them.
NICE information for the public. www.nice.org.uk/guidance/cg172/ifp/chapter/Helping-you-recover-from-a-heart-attack#/your-cardiac-rehabilitation-programme .
Patient information based on the latest NICE guidance on cardiac rehabilitation and includes information on exercise and sessions covering a range of topics including health education and information. Also encourages partners or carers to be involved in cardiac rehabilitation.
Cardiac rehabilitation—a personal view from Philip Boorman
I am a 65 year old retired air traffic controller, and had been treated for hypertension and high cholesterol since 1998. I had experienced mild chest pains in the past, which I could always walk through, but more severe pains in December 2014 led me to seek advice from my general practice, which resulted in a referral to the Fast Track Chest Pain Clinic. Ironically, while I was waiting for my outpatient appointment, I experienced a bout of more severe pain at home and was rushed to hospital, where I was told that I had had a heart attack.
Treatment in hospital was first class, and a single stent was fitted. My first contact with the cardiac rehabilitation team was a home visit by a rehabilitation nurse. She was suitably encouraging, but the cynic in me thought that she was probably encouraging to everyone. However, her advice was sound, and I followed it to the letter. Rehabilitation sessions at the gym started about eight weeks after my heart attack and not only proved to be physically demanding and rewarding (no stopping for at least 50 minutes) but also helped to rebuild my slightly flagging confidence. The programme included “teach-ins” on lifestyle, relaxation, diet, and exercise regimens, and I am extremely grateful for the opportunity to attend.
Will I have another heart attack? I don’t know, but I do know that cardiac rehabilitation has fast-tracked me back to a normal life and given me the knowledge that the chances of another heart attack are greatly reduced. It is also helpful to know that I will have regular follow up by my GP and see the practice nurse in the cardiac clinic at least once a year.
Cite this as: BMJ 2015;351:h5000
We thank Jemma Lough for help with technical editing of the manuscript, and Tony Mourant, retired consultant cardiologist, and Jenny Wingham, senior clinical researcher, for commenting on earlier drafts of this paper.
Contributors: HMD conceived of the article based on a clinical review he co-authored for the BMJ in 2004. He contributed to the literature review, drafting and revising the article, and approval of the final version. RST provided details from the various Cochrane systematic reviews that he has led and conducted. PD and RST also contributed to the literature review and drafting, design, and revision of the article. All authors approved the final manuscript.
Competing interests: We have read and understood the BMJ Group policy on declaration of interests and declare the following interests. HMD and PD have co-authored Cochrane reviews in cardiac rehabilitation with RST. RST is an author on several other Cochrane reviews of cardiac rehabilitation. HMD and RST are co-chief investigators on the REACH-HF programme of research, which is developing and evaluating a home based cardiac rehabilitation intervention for people with heart failure and their carers (NIHR PGfAR RP-PG-0611-12004).
Patient consent: Patient consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
- ↵ British Association for Cardiovascular Prevention and Rehabilitation. BACPR standards and core components for cardiovascular disease prevention and rehabilitation 2012 . 2nd ed. UKBACPR, 2012 . www.bacpr.com/resources/46C_BACPR_Standards_and_Core_Components_2012.pdf .
- ↵ National Institute for Health and Care Excellence. Secondary prevention in primary and secondary care for patients following a myocardial infarction (clinical guidance 172). NICE, 2013. www.nice.org.uk/guidance/cg172 .
- ↵ JBS3 Board. Joint British Societies’ consensus recommendations for the prevention of cardiovascular disease (JBS3). Heart 2014 ; 100 (suppl 2): ii1 -67. OpenUrl FREE Full Text
- ↵ Piepoli MF, Corrà U, Adamopoulos S, Benzer W, Bjarnason-Wehrens B, Cupples M, et al; Endorsed by the Committee for Practice Guidelines of the European Society of Cardiology. Secondary prevention in the clinical management of patients with cardiovascular diseases. Core components, standards and outcome measures for referral and delivery: a policy statement from the cardiac rehabilitation section of the European Association for Cardiovascular Prevention & Rehabilitation. Eur J Prev Cardiol 2014 ; 21 : 664 -81. OpenUrl Abstract / FREE Full Text
- ↵ Balady GJ, Williams MA, Ades PA, Bittner V, Comoss P, Foody JA, et al; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee; Council on Clinical Cardiology; Councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; American Association of Cardiovascular and Pulmonary Rehabilitation. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: a scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; the Councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. J Cardiopulm Rehabil Prev 2007 ; 27 : 121 -9. OpenUrl CrossRef PubMed Web of Science
- ↵ Leon AS, Franklin BA, Costa F, Balady GJ, Berra KA, Stewart KJ, et al; American Heart Association; Council on Clinical Cardiology (Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention); Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity); American association of Cardiovascular and Pulmonary Rehabilitation. Cardiac rehabilitation and secondary prevention of coronary heart disease: an American Heart Association scientific statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity), in collaboration with the American association of Cardiovascular and Pulmonary Rehabilitation. Circulation 2005 ; 111 : 369 -76. OpenUrl Abstract / FREE Full Text
- ↵ British Heart Foundation. European cardiovascular disease statistics 2012. www.bhf.org.uk/publications/statistics/european-cardiovascular-disease-statistics-2012 .
- ↵ Balady GJ, Ades PA, Bittner VA, Franklin BA, Gordon NF, Thomas RJ, et al; American Heart Association Science Advisory and Coordinating Committee. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: a presidential advisory from the American Heart Association. Circulation 2011 ; 124 : 2951 -60. OpenUrl FREE Full Text
- ↵ British Heart Foundation. Heart statistics. www.bhf.org.uk/research/heart-statistics .
- ↵ National Institute for Health and Care Excellence. The early management of unstable angina and non-ST-segment-elevation myocardial infarction (clinical guidance 94). NICE, 2010. www.nice.org.uk/guidance/cg94 .
- ↵ National Institute for Health and Care Excellence. Management of chronic heart failure in adults in primary and secondary care ((clinical guidance 108). NICE, 2010. www.nice.org.uk/guidance/cg108 .
- ↵ Department of Health. Cardiac rehabilitation commissioning pack. DoH, 2010. http://webarchive.nationalarchives.gov.uk/20130107105354/http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/Browsable/DH_117504 .
- ↵ Mampuya WM. Cardiac rehabilitation past, present and future: an overview. Cardiovasc Diagn Ther 2012 ; 2 : 38 -49. OpenUrl PubMed
- ↵ Bethell H, Lewin R, Dalal H. Cardiac rehabilitation in the United Kingdom. Heart 2009 ; 95 : 271 -5. OpenUrl Abstract / FREE Full Text
- ↵ Vanhees L, Rauch B, Piepoli M, van Buuren F, Takken T, Börjesson M, et al; Writing Group, EACPR. Importance of characteristics and modalities of physical activity and exercise in the management of cardiovascular health in individuals with cardiovascular disease (Part III). Eur J Prev Cardiol 2012 ; 19 : 1333 -56. OpenUrl Abstract / FREE Full Text
- ↵ British Heart Foundation. The National Audit of Cardiac Rehabilitation: annual statistical report 2014 . British Heart Foundation, 2014 . www.bhf.org.uk/~/media/files/publications/research/nacr_2014.pdf .
- ↵ Dalal HM, Evans PH. Achieving national service framework standards for cardiac rehabilitation and secondary prevention. BMJ 2003 ; 326 : 481 -4. OpenUrl Abstract / FREE Full Text
- ↵ Menezes AR, Lavie CJ, Milani RV, Forman DE, King M, Williams MA. Cardiac rehabilitation in the United States. Prog Cardiovasc Dis 2014 ; 56 : 522 -9. OpenUrl CrossRef PubMed
- ↵ Anderson L, Thompson DR, Oldridge N, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev (forthcoming).
- ↵ Taylor RS, Dalal H, Jolly K, Zawada A, Dean SG, Cowie A, et al. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev 2015 ; 8 : CD007130 . OpenUrl PubMed
- ↵ Anderson L, Taylor RS. Cardiac rehabilitation for people with heart disease: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev 2014 ; 12 : CD011273 . OpenUrl PubMed
- ↵ Taylor RS, Sagar VA, Davies EJ, Briscoe S, Coats AJ, Dalal H, et al. Exercise-based rehabilitation for heart failure. Cochrane Database Syst Rev 2014 ; 4 : CD003331 . OpenUrl PubMed
- ↵ Brown JP, Clark AM, Dalal H, Welch K, Taylor RS. Patient education in the management of coronary heart disease. Cochrane Database Syst Rev 2011 ;(12):CD008895.
- ↵ Brown JP, Clark AM, Dalal H, Welch K, Taylor RS. Patient education in the contemporary management of coronary heart disease. Cochrane Database Syst Rev 2010 ; 1 : CD008895 . OpenUrl
- ↵ Heran BS, Chen JM, Ebrahim S, Moxham T, Oldridge N, Rees K, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev 2011 ;(7):CD001800.
- ↵ West RR, Jones DA, Henderson AH. Rehabilitation after myocardial infarction trial (RAMIT): multi-centre randomised controlled trial of comprehensive cardiac rehabilitation in patients following acute myocardial infarction. Heart 2012 ; 98 : 637 -44. OpenUrl Abstract / FREE Full Text
- ↵ Doherty P, Lewin R. The RAMIT trial, a pragmatic RCT of cardiac rehabilitation versus usual care: what does it tell us? Heart 2012 ; 98 : 605 -6. OpenUrl FREE Full Text
- ↵ Lawler PR, Filion KB, Eisenberg MJ. Efficacy of exercise-based cardiac rehabilitation post-myocardial infarction: a systematic review and meta-analysis of randomized controlled trials. Am Heart J 2011 ; 162 : 571 -584.e2. OpenUrl CrossRef PubMed Web of Science
- ↵ Oldridge NB, Guyatt GH, Fischer ME, Rimm AA. Cardiac rehabilitation after myocardial infarction. Combined experience of randomized clinical trials. JAMA 1988 ; 260 : 945 -50. OpenUrl CrossRef PubMed Web of Science
- ↵ Wood D. Is cardiac rehabilitation fit for purpose in the NHS: maybe not. Heart 2012 ; 98 : 607 -8. OpenUrl FREE Full Text
- ↵ West R, Jones D. Cardiac rehabilitation and mortality reduction after myocardial infarction: the emperor’s new clothes? Evidence against cardiac rehabilitation. Heart 2013 ; 99 : 911 -3. OpenUrl Abstract / FREE Full Text
- ↵ Lewin B, Doherty P. Cardiac rehabilitation and mortality reduction after myocardial infarction: the emperor’s new clothes? Evidence in favour of cardiac rehabilitation. Heart 2013 ; 99 : 909 -13. OpenUrl Abstract / FREE Full Text
- ↵ Sagar VA, Davies EJ, Briscoe S, Coats AJ, Dalal HM, Lough F, et al. Exercise-based rehabilitation for heart failure: systematic review and meta-analysis. Open Heart 2015 ; 2 : e000163 . OpenUrl Abstract / FREE Full Text
- ↵ Lavie CJ, Milani RV. Adverse psychological and coronary risk profiles in young patients with coronary artery disease and benefits of formal cardiac rehabilitation. Arch Intern Med 2006 ; 166 : 1878 -83. OpenUrl CrossRef PubMed Web of Science
- ↵ Taylor CB, Houston-Miller N, Ahn DK, Haskell W, DeBusk RF. The effects of exercise training programs on psychosocial improvement in uncomplicated postmyocardial infarction patients. J Psychosom Res 1986 ; 30 : 581 -7. OpenUrl CrossRef PubMed Web of Science
- ↵ Linden W, Stossel C, Maurice J. Psychosocial interventions for patients with coronary artery disease: a meta-analysis. Arch Intern Med 1996 ; 156 : 745 -52. OpenUrl CrossRef PubMed Web of Science
- ↵ Milani RV, Lavie CJ, Mehra MR, Ventura HO. Impact of exercise training and depression on survival in heart failure due to coronary heart disease. Am J Cardiol 2011 ; 107 : 64 -8. OpenUrl CrossRef PubMed
- ↵ Lavie CJ, Milani RV. Effects of cardiac rehabilitation and exercise training on low-density lipoprotein cholesterol in patients with hypertriglyceridemia and coronary artery disease. Am J Cardiol 1994 ; 74 : 1192 -5. OpenUrl CrossRef PubMed Web of Science
- ↵ Schuler G, Hambrecht R, Schlierf G, Grunze M, Methfessel S, Hauer K, et al. Myocardial perfusion and regression of coronary artery disease in patients on a regimen of intensive physical exercise and low fat diet. J Am Coll Cardiol 1992 ; 19 : 34 -42. OpenUrl CrossRef PubMed Web of Science
- ↵ Ades PA, Savage PD, Toth MJ, Harvey-Berino J, Schneider DJ, Bunn JY, et al. High-calorie-expenditure exercise: a new approach to cardiac rehabilitation for overweight coronary patients. Circulation 2009 ; 119 : 2671 -8. OpenUrl Abstract / FREE Full Text
- ↵ Pavy B, Iliou MC, Meurin P, Tabet JY, Corone S; Functional Evaluation and Cardiac Rehabilitation Working Group of the French Society of Cardiology. Safety of exercise training for cardiac patients: results of the French registry of complications during cardiac rehabilitation. Arch Intern Med 2006 ; 166 : 2329 -34. OpenUrl CrossRef PubMed Web of Science
- ↵ Van Camp SP, Peterson RA. Cardiovascular complications of outpatient cardiac rehabilitation programs. JAMA 1986 ; 256 : 1160 -3. OpenUrl CrossRef PubMed Web of Science
- ↵ Wingham J, Dalal HM, Sweeney KG, Evans PH. Listening to patients: choice in cardiac rehabilitation. Eur J Cardiovasc Nurs 2006 ; 5 : 289 -94. OpenUrl Abstract / FREE Full Text
- ↵ Lewin B, Robertson IH, Cay EL, Irving JB, Campbell M. Effects of self-help post-myocardial-infarction rehabilitation on psychological adjustment and use of health services. Lancet 1992 ; 339 : 1036 -40. OpenUrl CrossRef PubMed Web of Science
- ↵ Dalal HM, Evans PH, Campbell JL, Taylor RS, Watt A, Read KL, et al. Home-based versus hospital-based rehabilitation after myocardial infarction: A randomized trial with preference arms—Cornwall Heart Attack Rehabilitation Management Study (CHARMS). Int J Cardiol 2007 ; 119 : 202 -11. OpenUrl CrossRef PubMed Web of Science
- ↵ Jolly K, Lip GY, Taylor RS, Raftery J, Mant J, Lane D, et al. The Birmingham Rehabilitation Uptake Maximisation study (BRUM): a randomised controlled trial comparing home-based with centre-based cardiac rehabilitation. Heart 2009 ; 95 : 36 -42. OpenUrl Abstract / FREE Full Text
- ↵ Jelinek MV, Thompson DR, Ski C, Bunker S, Vale MJ. 40 years of cardiac rehabilitation and secondary prevention in post-cardiac ischaemic patients. Are we still in the wilderness? Int J Cardiol 2015 ; 179 : 153 -9. OpenUrl CrossRef PubMed
- ↵ Forman DE, Sanderson BK, Josephson RA, Raikhelkar J, Bittner V; American College of Cardiology’s Prevention of Cardiovascular Disease Section. Heart failure as a newly approved diagnosis for cardiac rehabilitation: challenges and opportunities. J Am Coll Cardiol 2015 ; 65 : 2652 -9. OpenUrl CrossRef PubMed
- ↵ Dalal HM, Wingham J, Palmer J, Taylor R, Petre C, Lewin R; REACH-HF investigators. Why do so few patients with heart failure participate in cardiac rehabilitation? A cross-sectional survey from England, Wales and Northern Ireland. BMJ Open 2012 ; 2 : e000787 . OpenUrl Abstract / FREE Full Text
- ↵ Pack QR, Squires RW, Lopez-Jimenez F, Lichtman SW, Rodriguez-Escudero JP, Zysek VN, et al. The current and potential capacity for cardiac rehabilitation utilization in the United States. J Cardiopulm Rehabil Prev 2014 ; 34 : 318 -26. OpenUrl CrossRef PubMed
- ↵ Bjarnason-Wehrens B, McGee H, Zwisler AD, Piepoli MF, Benzer W, Schmid JP, et al; Cardiac Rehabilitation Section European Association of Cardiovascular Prevention and Rehabilitation. Cardiac rehabilitation in Europe: results from the European Cardiac Rehabilitation Inventory Survey. Eur J Cardiovasc Prev Rehabil 2010 ; 17 : 410 -8. OpenUrl CrossRef PubMed Web of Science
- ↵ Healthcare Commission. Coronary heart disease: survey of patients . Healthcare Commission, 2004 . www.nhssurveys.org/Filestore/CQC/CHD_KF_2004.pdf .
- ↵ Ades PA, Waldmann ML, McCann WJ, Weaver SO. Predictors of cardiac rehabilitation participation in older coronary patients. Arch Intern Med 1992 ; 152 : 1033 -5. OpenUrl CrossRef PubMed Web of Science
- ↵ Grace SL, Gravely-Witte S, Brual J, Monette G, Suskin N, Higginson L, et al. Contribution of patient and physician factors to cardiac rehabilitation enrollment: a prospective multilevel study. Eur J Cardiovasc Prev Rehabil 2008 ; 15 : 548 -56. OpenUrl CrossRef PubMed Web of Science
- ↵ Ghisi GL, Polyzotis P, Oh P, Pakosh M, Grace SL. Physician factors affecting cardiac rehabilitation referral and patient enrollment: a systematic review. Clin Cardiol 2013 ; 36 : 323 -35. OpenUrl CrossRef PubMed
- ↵ Dalal H, Evans PH, Campbell JL. Recent developments in secondary prevention and cardiac rehabilitation after acute myocardial infarction. BMJ 2004 ; 328 : 693 -7. OpenUrl FREE Full Text
- ↵ Varnfield M, Karunanithi M, Lee CK, Honeyman E, Arnold D, Ding H, et al. Smartphone-based home care model improved use of cardiac rehabilitation in postmyocardial infarction patients: results from a randomised controlled trial. Heart 2014 ; 100 : 1770 -9. OpenUrl Abstract / FREE Full Text
- ↵ Beatty AL, Fukuoka Y, Whooley MA. Using mobile technology for cardiac rehabilitation: a review and framework for development and evaluation. J Am Heart Assoc 2013 ; 2 : e000568 . OpenUrl FREE Full Text
- ↵ Clark RA, Conway A, Poulsen V, Keech W, Tirimacco R, Tideman P. Alternative models of cardiac rehabilitation: a systematic review. Eur J Prev Cardiol 2015 ; 22 : 35 -74. OpenUrl Abstract / FREE Full Text
- ↵ Clark AM, Hartling L, Vandermeer B, McAlister FA. Meta-analysis: secondary prevention programs for patients with coronary artery disease. Ann Intern Med 2005 ; 143 : 659 -72. OpenUrl CrossRef PubMed Web of Science
- ↵ Ades PA, Keteyian SJ, Balady GJ, Houston-Miller N, Kitzman DW, Mancini DM, et al. Cardiac rehabilitation exercise and self-care for chronic heart failure. JACC Heart Fail 2013 ; 1 : 540 -7. OpenUrl CrossRef PubMed
- ↵ Buck HG, Harkness K, Wion R, Carroll SL, Cosman T, Kaasalainen S, et al. Caregivers’ contributions to heart failure self-care: a systematic review. Eur J Cardiovasc Nurs 2015 ; 14 : 79 -89. OpenUrl Abstract / FREE Full Text
- ↵ Clark AM, Spaling M, Harkness K, Spiers J, Strachan PH, Thompson DR, et al. Determinants of effective heart failure self-care: a systematic review of patients’ and caregivers’ perceptions. Heart 2014 ; 100 : 716 -21. OpenUrl Abstract / FREE Full Text
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- Browse by collection
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 104, Issue 17
- Cardiac rehabilitation and physical activity: systematic review and meta-analysis
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Grace Olivia Dibben 1 ,
- Hasnain M Dalal 1 , 2 ,
- Rod S Taylor 2 ,
- Patrick Doherty 3 ,
- Lars Hermann Tang 4 ,
- Melvyn Hillsdon 5
- 1 European Centre for Environment and Human Health , Knowledge Spa, Royal Cornwall Hospitals NHS Trust, University of Exeter Medical School , Truro , UK
- 2 Institute of Health Research (Primary Care), University of Exeter Medical School , Exeter , UK
- 3 Department of Health Sciences , University of York , York , UK
- 4 National Knowledge Centre for Rehabilitation and Palliative Care, Odense University Hospital, University of Southern Denmark , Odense , Denmark
- 5 Department of Sport and Health Sciences , University of Exeter , Exeter , UK
- Correspondence to Grace Olivia Dibben, Knowledge Spa, Royal Cornwall Hospitals NHS Trust, University of Exeter Medical School, Truro TR13HD, UK; gd318{at}exeter.ac.uk
Objective To undertake a systematic review and meta-analysis to assess the impact of cardiac rehabilitation (CR) on physical activity (PA) levels of patients with heart disease and the methodological quality of these studies.
Methods Databases (MEDLINE, EMBASE, CENTRAL, CINAHL, PsychINFO and SportDiscus) were searched without language restriction from inception to January 2017 for randomised controlled trials (RCTs) comparing CR to usual care control in adults with heart failure (HF) or coronary heart disease (CHD) and measuring PA subjectively or objectively. The direction of PA difference between CR and control was summarised using vote counting (ie, counting the positive, negative and non-significant results) and meta-analysis.
Results Forty RCTs, (6480 patients: 5825 CHD, 655 HF) were included with 26% (38/145) PA results showing a statistically significant improvement in PA levels with CR compared with control. This pattern of results appeared consistent regardless of type of CR intervention (comprehensive vs exercise-only) or PA measurement (objective vs subjective). Meta-analysis showed PA increases in the metrics of steps/day (1423, 95% CI 757.07 to 2089.43, p<0.0001) and proportion of patients categorised as physically active (relative risk 1.55, 95% CI 1.19 to 2.02, p=0.001). The included trials were at high risk of bias, and the quality of the PA assessment and reporting was relatively poor.
Conclusion Overall, there is moderate evidence of an increase in PA with CR participation compared with control. High-quality trials are required, with robust PA measurement and data analysis methods, to assess if CR definitely leads to important improvements in PA.
- cardiac rehabilitation
- coronary artery disease
- heart failure
- meta-analysis
- systemic review
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
https://doi.org/10.1136/heartjnl-2017-312832
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Introduction
Physical activity (PA) is defined as any bodily movement produced by skeletal muscles resulting in energy expenditure beyond resting expenditure. 1 The current UK recommendation for PA in adults and older adults is ≥150 min of moderate intensity PA per week. 2 This is based on a number of systematic reviews and consensus statement, consistently identifying 150 min/week as providing considerable health benefits, including reduced all-cause mortality, reduced risk factors for chronic diseases, improved cardiovascular fitness and quality of life. 2 3 This is also the standard PA recommendation for patients with cardiac disease by the British Association for Cardiovascular Prevention and Rehabilitation and the Scottish Intercollegiate Guidelines Network. 4 5
The benefits of cardiac rehabilitation (CR) participation for those with coronary heart disease (CHD) and heart failure (HF) are well established and include reduced cardiovascular mortality, reduced risk of hospital admissions, improved exercise capacity and health-related quality of life. 6 7 A key aim of CR is to increase total daily energy expenditure in addition to exercise capacity. 2 However, previous observational studies demonstrated that many patients with heart disease (pre-CR and post-CR) are failing to meet recommended daily PA levels 8 9 and the extent that CR impacts on PA levels of patients remains unclear.
While two systematic reviews to date have indicated inadequate evidence of an impact of CR participation on PA levels of patients with CHD, 10 11 these studies have limitations. Neither included studies involving patients with HF nor attempted meta-analysis due to the heterogeneity of CR interventions. Therefore, an updated systematic review with an improved search strategy and broader population inclusion criteria is justified.
The aim of this systematic review and meta-analysis of randomised controlled trials (RCTs) was twofold. First, to clarify the impact of CR participation on PA levels of patients with CHD and HF. Second, to review the methodological quality of PA outcomes reported in these trials.
The protocol was registered on PROSPERO (CRD42017055137). We conducted and report this systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyse statement. 12
Search strategy and inclusion criteria
Details of the search strategy and inclusion criteria are provided in the online Supplementary file 1 . The full search strategy is provided in the online Supplementary file 2 .
Supplementary file 1
Supplementary file 2, data extraction and risk of bias assessment.
A standardised data extraction form was used to extract study characteristics, patient characteristics, intervention and control details, PA measurement method and outcome data at all follow-up time points. Multiple publications of the same study were assessed for additional data and presented as a single RCT (see online Supplementary file 3 ).
Supplementary file 3
The Cochrane Collaboration’s tool for assessing risk of bias was used to assess the quality of included studies. 13 Data extraction and risk of bias assessment were initially completed by a single reviewer (GD) and then checked for accuracy by one other reviewer (MH, HD or RST). Disagreement was resolved by discussion.
Data synthesis and meta-analysis
Due to the wide range of PA metrics reported across studies, we first summarised the direction of PA results using a vote counting approach 13 (quantifying studies on the basis of their positive, negative or non-significant results). Given the wide range of PA measures, we decided against using standardised effect size for meta-analysis and instead conducted meta-analysis where two or more studies reported the same units of PA measurement. Meta-analysis was completed on all follow-up time points apart from one outcome measure (proportion of patients categorised as physically active) where there was sufficient data to separate into short-term (≤12 months post-CR) and long-term (>12 months post-CR) follow-up.
Given the clinical heterogeneity of the included studies, random-effects models were used to pool data. Statistical heterogeneity was assessed using the I 2 statistic. Binary outcomes for each study were pooled as relative risks (RR) and continuous outcomes as mean differences (MD). Meta-analysis results were reported as means and 95% CIs. A two-tailed p value of ≤0.05 was considered statistically significant. Analyses were performed in Review Manager (RevMan V.5.3, The Cochrane Collaboration) or Stata V.14.
We explored the effect of various potential treatment effect modifiers by stratifying the vote counting results, that is, setting of CR (centre vs home based), patient group (CHD vs HF), publication date (pre-1990, representing the time of major changes in drug and device management of CHD and HF), dose of exercise intervention (dose=number of weeks of exercise training×average sessions/week×average duration of session in minutes. Dose ≥2000 units (median) vs dose <2000 units); objective versus subjective PA measures and method of PA statistical analysis. Studies lacking enough information to calculate dose were omitted from the analysis.
Study selection
Figure 1 summarises the screening process resulting in 47 publications across 40 RCTs included in the review.
- Download figure
- Open in new tab
- Download powerpoint
Preferred Reporting Items for Systematic Reviews and Meta-analyses flow chart of search process. CR, cardiac rehabilitation; PA, physical activity; RCT, randomised controlled trial.
Characteristics of included studies
The 40 RCTs, all published in English, included a total of 6480 patients with cardiac disease (5825 CHD, 655 HF). A summary of study characteristics is shown in table 1 . Individual study characteristics are detailed in the online Supplementary file 4 .
Supplementary file 4
- View inline
Summary of study characteristics
PA measures reported
In total, 28 studies measured PA using subjective approaches, 10 studies used objective methods and two studies used a combination of both. Across all studies, 45 different PA metrics were used (median 1.5, range 1–10). Details of individual study PA measurement methods including a summary is presented in the online Supplementary file 5 .
Supplementary file 5
Risk of bias assessment.
Risk of bias assessments for each study are summarised in figure 2 . All studies were assigned high risk in blinding of participants and personnel due to the nature of CR. The most prevalent methodological issues were non-adequate description of randomisation (25/40, 62.5%), allocation concealment (27/40, 67.5%) and blinding of PA outcome assessment (26/40, 65%). There was high risk of bias in 50% (20/40) trials for incomplete outcome data. Most trials were low risk for selective reporting (33/40, 82.5%), balanced groups at baseline (34/40, 85%) and were free of cointerventions (35/40, 87.5%).
Quality appraisal. + (green), low risk of bias; ? (yellow), unclear risk of bias; − (red), high risk of bias. 40–59
Impact of CR participation on PA levels
Vote counting
A total of 145 CR versus control PA comparisons were reported across all studies (online Supplementary file 6 ). Overall, 26% of results showed a statistically significant improvement in PA with CR ( table 2 ).
Supplementary file 6
Stratified analysis.
The pattern of results was similar whether PA measurement was objective or subjective (online Supplementary file 7 ). The statistical methods used across the studies were varied. The majority reported a p value for between-group differences. Comparing the direction of results by statistical method showed a greater number of positive results reported when the p value for interaction time×group was used (online Supplementary file 8 ). As numbers were small, this is unlikely to be of significance.
Supplementary file 7
Supplementary file 8.
There was a higher proportion of non-significant results (86% vs 63%) and fewer positive results (10% vs 32%) in studies including patients with HF compared with studies with CHD (online Supplementary file 9 ). Removing the results from studies conducted prior to 1990 or those based on exercise frequency did not affect the direction of results (online Supplementary file 10 ).
Supplementary file 9
Supplementary file 10, cr intervention.
Table 3 shows an increased number of positive results with home-based CR interventions compared with centre-based interventions. Studies with a higher exercise dose also produced a slightly increased number of positive results compared with studies with a lower exercise dose (online Supplementary file 11 ). The pattern of results was similar when comparing studies of comprehensive CR to exercise-only CR studies (online Supplementary file 12 ).
Supplementary file 11
Supplementary file 12.
Vote counting—comparing centre-based CR to home-based CR and combined RCTs
Meta-analyses
Five studies used mean steps/day as a measure of PA assessed by either pedometer 14–16 or accelerometer. 17 18 Pooling results across studies showed compared with control, CR participation was associated with an increase in mean steps/day (1423, 95% CI 757.07 to 2089.43, p<0.0001; figure 3 ) at short-term follow-up (median 3, range 1.5–12 months). With no evidence of statistical heterogeneity (I 2 =0%, p=0.845).
Impact of cardiac rehabilitation on mean steps/day at short-term follow-up (median 3 months, range 1.5–12 months). CR, cardiac rehabilitation, PA, physical activity; WMD, weighted mean difference.

Energy expenditure
Energy expenditure (kcal/week) was estimated via questionnaire in three studies (median follow-up time 12 months, range 32 weeks–72 months). 19–21 Meta-analysis showed that CR participation was associated with an increase in energy expenditure compared with control (878.4, 95% CI 433.83 to 1323.01, p=0.0001). Test for statistical heterogeneity was significant (I 2 =70%, p=0.04).
Sedentary time, light PA and moderate–vigorous PA (min/day)
There was no impact on mean min/day spent sedentary or sitting between CR and control (−10.9, 95% CI −39.02 to 17.20, p=0.45; figure 4A ) based on two studies estimating this objectively via accelerometer 14 18 and subjectively via International Physical Activity Questionnaire (IPAQ), 22 at 9 weeks follow-up (median, range 6–12 weeks). There was no evidence of a difference in mean min/day spent in light intensity PA in CR compared with control (−6.6, 95% CI −45.09 to 31.92, p=0.74; figure 4B ) based on two studies reporting this outcome via accelerometer 23 and IPAQ, 22 at 9.5 weeks follow-up (median, range 9–10 weeks). There was no difference in mean min/day spent in moderate–vigorous PA in CR compared with control (8.5, 95% CI −1.44 to 18.44, p=0.09; figure 4C ), measured via accelerometer 18 23 and IPAQ, 22 at 9 weeks follow-up (median, range 6–10 weeks).
Impact of cardiac rehabilitation on (A) min/day spent sedentary or sitting; (B) min/day spent in light intensity PA and (C) min/day spent in moderate–vigorous PA. CR, cardiac rehabilitation.
Proportion of patients categorised as physically active (short-term follow-up ≤12 months)
CR increased the proportion of patients categorised as ‘physically active’, measured at short-term follow-up (median 6 months, range 0–12 months) across nine studies (RR 1.55, 95% CI 1.19 to 2.02, p=0.001; figure 5A ). There was evidence of substantial statistical heterogeneity (I 2 =87%, p<0.00001). The definition of ‘physically active’ varied across studies: that is, exercise frequency ≥3×/week, 24 exercising ≥3×/week for 20 min, 25 26 exercising >100 kcal/day, 27 average daily steps >7500, 15 exercising for >1 hour/week, 28 regularly training (defined as either walking or cycling ≥30 min daily, sport activities once weekly or vigorous physical training) 29 and two studies did not provide any definition. 30 31
Impact of cardiac rehabilitation on proportion of patients categorised as physically active measured at (A) short-term follow-up (≤12 months) and (B) long-term follow-up (>12 months). CR, cardiac rehabilitation.
Proportion of patients categorised as physically active(long-term follow-up >12 months)
CR increased the proportion of patients considered physically active, measured at long-term follow-up (median 5 years, range 2–5 years) in three studies 28 30 31 (RR 1.48, 95% CI 1.19 to 1.83, p=0.0003; figure 5B ) with no evidence of statistical heterogeneity (I 2 =0.0%, p=0.96).
Proportion of patients categorised as sedentary or not physically active
Five studies reported the proportion of patients considered sedentary, 29 exercising <4 hours per week 32 or undertaking no exercise, 24 28 33 at 12 months (median, range 12–24 months) follow-up. There was a reduction in CR participants categorised as sedentary or not physically active(RR 0.76, 95% CI 0.61 to 0.95, p=0.02), with no evidence of statistical heterogeneity (I 2 =36%, p=0.18).
This systematic review of RCTs shows moderate evidence of an increase in PA with CR participation with 26% (38/145) of comparisons reporting a statistically significant result in favour of CR compared with control. This pattern of results appear consistent regardless of whether studies assessed PA using subjective or objective methods, or the CR intervention was comprehensive or exercise only. Studies involving patients with HF appeared less likely to have positive results in favour of CR. There was an increased proportion of positive results with higher doses of CR suggesting that higher doses of exercise training may be more effective in improving PA levels. Similarly, results suggest that home-based interventions may be more effective in improving PA levels.
Meta-analyses showed that CR participation compared with control is associated with an increase in some PA outcomes: steps/day at short-term follow-up, energy expenditure (kcal/week) at short-term follow-up, proportion of patients categorised as physically active both at short-term and long-term follow-up and reduced proportion of patients categorised sedentary or not physically active at short-term follow-up. CR was not shown to have a significant impact on minutes/day spent sedentary or in light or moderate-vigorous PA at short-term follow-up.
It remains uncertain if the mean increase of 1423 steps/day that we observed with CR is clinically meaningful. In patients with chronic obstructive pulmonary disease undergoing rehabilitation, the minimal clinically important difference (MCID) was calculated to lie between 600 and 1100 steps/day and resulted in a reduction in hospital admissions. 34 However, we know of no published MCID for patients with CHD or HF.
We believe there are two potential reasons why we saw improvements in some outcomes, but not others. First, categorising continuous PA data to PA categories (eg, sedentary, light moderate or vigorous) may have resulted in a loss of sensitivity to change. Second, some studies may have been susceptible to measurement bias as they used subjective PA measures.
Comparison of findings to previous studies
Our results build on previous systematic reviews 10 11 that found some evidence to indicate that CR positively impacts on PA in patients with CHD, but little evidence in long term and recommended CR programmes place more emphasis on improving the long-term PA levels of patients. 10 Ter Hoeve et al concluded that centre-based CR was not sufficient to improve and maintain PA levels and suggested home-based CR programmes may be more successful; however, literature is limited in this area. 11 In accord with recent Cochrane systematic reviews of CR, 9 10 the participating patients were relatively young (<60 years), predominantly male, with large differences in the programme location, duration, intensity, modality and length of follow-up.
Strengths and limitations
We believe this to be the first meta-analysis to assess the impact of CR on PA levels of patients with both CHD and HF . Strengths of this review include extensive literature searches, use of RCTs and inclusion of both subjective and objective PA assessment. Compared with the previous systematic reviews, we identified an additional 23 RCTs (2432 additional patients), 10 of which specifically involved patients with HF (655 patients).
However, this review has limitations. With the wide range of PA outcomes reported across the studies, at various follow-up time points, we were limited in the extent of meta-analysis we were able to complete. That only small numbers of studies were suitable for inclusion in the meta-analysis, limits our ability to draw firm conclusions from these pooled results. Vote counting was done to give a quantitative overview of the results. However, this method has limitations: (1) large and small studies carry the same weight, (2) studies reporting multiple PA outcome results contribute more weight and (3) results from multiple outcomes within study may not be independent. Furthermore, judgements by the authors on levels of PA were not based on national recommendations, leading to uncertainty about the clinical meaningfulness of PA improvements.
Key issues raised in risk of bias assessments were insufficiently described randomisation and allocation concealment procedures, leading to difficulty rating the quality of the RCTs. Additionally, 65% of studies had unclear risk of bias with regard to blinding of outcome assessment. This is particularly important in PA measurement since awareness of being assessed may cause both the intervention and control group patients to alter their behaviour and increase their PA on assessment days, potentially introducing bias to results.
There were numerous limitations in approaches studies took to assessing PA. Where questionnaires were used, few had been evidently validated for use in cardiac populations. Self-report commonly considered the frequency of exercise sessions undertaken as opposed to overall PA per se. Self-reported measures of PA are less valid and reliable than direct measures in patients with CR, generally overestimating PA and relying on patient recall. 35 Despite accelerometers being the most commonly used objective PA measurement method, a variety of devices were used, with sensors placed at different body sites, and a wide range of outcome metrics reported across studies, limiting the ability to meta-analyse these data. Additionally, data handling methods were poorly reported; no studies adequately explained the minimum wear time requirement for inclusion in data analysis or data reduction techniques. Where accelerometer thresholds were used to estimate intensity, they were derived from studies in young, healthy adults which may mean the PA level is underestimated in patients with cardiac disease. 36 Resting metabolic rate in patients with cardiac disease has been previously demonstrated to be significantly lower (23%–36%) than the typically utilised value of 3.5 mL/kg/min, 37 which may have implications in underestimating energy expenditure during higher intensity activities. Therefore, researchers should consider using thresholds specifically established for patients with cardiac disease.
There was inconsistency in statistical methods used across the studies. Baseline adjusted regression methods are recommended for analysis of RCTs. 38 However, only 35% reported a p value that took the baseline PA level into account. Although many studies showed between group differences in fitness outcomes, 26% of results demonstrated a statistically significant difference in PA outcomes. This is likely because individual studies were often small and underpowered to detect small differences in PA. Only 13 (32%) of the included studies included formal sample size calculations and of these only 4 (31%) were based on PA outcomes.
Implications for clinical practice and future research
That our results showed no difference in PA outcomes in studies that employed comprehensive CR compared with exercise-only CR suggest that improvements in PA with CR are the result of exercise training rather than components of education and psychosocial interventions. Additionally, improvement in exercise capacity may not be directly related to increases in PA levels. CR programmes should consider supplementing their existing exercise-training intervention with interventions that specifically aim to increase PA level. For example, the ongoing PATHway I trial, where the basis of the CR intervention is PA promotion and the primary outcome is objectively measured PA level. 39
Further research is required to validate interventions that promote PA in cardiac populations. Furthermore, objective measurement of PA requires population-specific calibration studies to establish intensity thresholds. The use of inconsistent PA measures and units made formal pooling of data problematic. We therefore recommend that future studies use objective measures of PA such as accelerometers, be statistically powered to detect small differences in PA, use appropriate data handling and analysis methods, and PA outcomes are reported in relation to national PA recommendations. Studies should assess PA outcomes over the long term.
Conclusions
This systematic review and meta-analysis provides moderate evidence of an increase in PA with CR participation compared with control. However, the included trials were at risk of bias, and the quality of PA assessment and reporting was relatively poor. It is unclear whether increases in PA with CR are clinically meaningful. Further high-quality trials are required to assess if CR leads to important improvements in PA, such as the UK recommended target of 150 min of moderate intensity PA per week, especially in long term.
Acknowledgments
We would like to thank Catriona Organ, librarian at the Royal Cornwall Hospital Library, Knowledge Spa, Truro, and the PenCLARHC Evidence Synthesis Team at University of Exeter Medical School for their assistance throughout the process of this review. We also thank Dr Joe Mills (immediate past president of BACPR) for encouraging us to write this review and facilitating its submission.
- Caspersen CJ ,
- Powell KE ,
- Christenson GM
- ↵ Chief Medical Officers . Start active, stay active. A report on physical activity for health from the four home countries . 2011 https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/216370/dh_128210.pdf ( accessed Oct 2017 ).
- O’Donovan G ,
- Blazevich AJ ,
- Boreham C , et al
- ↵ British Association for Cardiovascular Prevention and Rehabilitation . The BACPR standards and core components for cardiovascular disease prevention and rehabilitation . 2017 http://www.bacpr.com/resources/BACPR_Standards_and_Core_Components_2017.pdf ( accessed Oct 2017 ).
- ↵ Scottish Intercollegiate Guidelines Network . Cardiac rehabilitation . A national clinical guideline http://www.sign.ac.uk/assets/sign150.pdf ( accessed October 2017 ).
- Anderson L ,
- Oldridge N ,
- Thompson DR , et al
- Taylor RS ,
- Davies EJ , et al
- Dontje ML ,
- van der Wal MH ,
- Stolk RP , et al
- Kupzyk K , et al
- Jolliffe J ,
- ter Hoeve N ,
- Huisstede BM ,
- Stam HJ , et al
- Liberati A ,
- Tetzlaff J , et al
- Higgins JP ,
- Borland M ,
- Rosenkvist A ,
- Vadeboncoeur N , et al
- Morrin LI ,
- Beaton LJ , et al
- Granat MH , et al
- Hambrecht R ,
- Niebauer J ,
- Marburger C , et al
- Maloney PM ,
- Mueller L ,
- Kottman W , et al
- Oliveira NL ,
- Ribeiro F ,
- Teixeira M , et al
- Teixeira M ,
- Miranda F , et al
- Engblom E ,
- Hietanen EK ,
- Hämäläinen H , et al
- Hämäläinen H ,
- Luurila OJ ,
- Kallio V , et al
- Higgins HC ,
- Henderson AH
- Otterstad JE
- Carlsson R ,
- Lindberg G ,
- Westin L , et al
- Erdman RA ,
- Duivenvoorden HJ ,
- Verhage F , et al
- Zwisler AD ,
- Rasmussen S , et al
- Bengtsson K
- Demeyer H ,
- Hornikx M , et al
- Alharbi M ,
- Neubeck L , et al
- Freedson PS ,
- Melanson E ,
- Savage PD ,
- Egbewale BE ,
- Woods C , et al
- Astengo M ,
- Karlsson T , et al
- Reed N , et al
- DeBusk RF ,
- Houston N ,
- Haskell W , et al
- Gottlieb SS ,
- Fisher ML ,
- Freudenberger R , et al
- Maddison R ,
- Pfaeffli L ,
- Whittaker R , et al
- Kumar GV , et al
- Oldenburg B ,
- Greenwood J , et al
- Scherwitz LW ,
- Billings JH , et al
- Senden PJ ,
- Sabelis LW ,
- Zonderland ML , et al
- Sivarajan ES ,
- Lindskog BD , et al
- Nordlander R ,
- Rydén L , et al
- Ballantyne D
- Toobert DJ ,
- Glasgow RE ,
- Nettekoven LA , et al
- van den Berg-Emons R ,
- Bussmann H , et al
- Ballard J ,
- Troped P , et al
- He HG , et al
- Willenheimer R ,
- Rydberg E ,
- Cline C , et al
- Witham MD ,
- Johnston DW , et al
- Fulton RL ,
- Greig CA , et al
Contributors GOD, HMD, RST and MH contributed to the conception, design, planning, conduct and reporting of the work described in this article. All authors contributed to the critical revision of the manuscript.
Funding This study was supported by a University of Exeter Postgraduate Studentship Grant.
Competing interests None declared.
Patient consent Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Read the full text or download the PDF:
Advertisement
Cardiac Rehabilitation: Current Review of the Literature and Its Role in Patients with Heart Failure
- Heart Failure (W Tang, Section Editor)
- Published: 24 February 2018
- Volume 20 , article number 12 , ( 2018 )
Cite this article

- Nishant P. Shah MD 1 , 2 ,
- Ahmed AbuHaniyeh MD 3 &
- Haitham Ahmed MD, MPH 1
1256 Accesses
10 Citations
Explore all metrics
Purpose of review
Cardiovascular (CV) disease remains the leading cause of death in the USA despite major advances in its treatment. With time, cardiac rehabilitation (CR) programs have gathered interest to help increase CV health and improve functional status after a CV event. Patients with heart failure have also been shown to benefit. In this review, we will evaluate the current literature showcasing the benefits of CR, particularly in patients with heart failure, discuss current limitations, and avenues for future investigation.
Recent findings
Studies have shown that CR is beneficial in reducing morbidity, mortality, hospitalizations, activity-related symptoms, and increasing quality of life. Similar findings have also been observed in patients with heart failure who underwent CR in addition to optimal medical management.
The positive effects of CR are well established in patients with coronary disease. Recent literature is also showing a trend to benefit in patients with heart failure, though much of the evidence is limited to patients with systolic dysfunction. Despite recommendations by professional societies, the use of CR remains underutilized. Further investigation is needed to better understand the impact of CR in heart failure. Moreover, strategies to increase CR utilization must be explored.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price excludes VAT (USA) Tax calculation will be finalised during checkout.
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
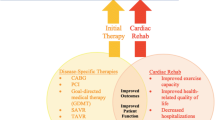
Cardiac Rehab for Functional Improvement

Novel advances in cardiac rehabilitation
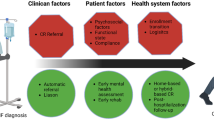
Cardiac rehabilitation utilization, barriers, and outcomes among patients with heart failure
References and recommended reading, papers of particular interest, published recently, have been highlighted as: • of importance.
Pagidipati NJ, Gaziano TA. Estimating deaths from cardiovascular disease: a review of global methodologies of mortality measurement. Circulation . 2013;127(6):749–56. https://doi.org/10.1161/CIRCULATIONAHA.112.128413 .
Article PubMed PubMed Central Google Scholar
Fang J, Ayala C, Luncheon C, Ritchey M, Loustalot F. Use of outpatient cardiac rehabilitation among heart attack survivors—20 States and the District of Columbia, 2013 and Four States, 2015. MMWR Morb Mortal Wkly Rep . 2017;66(33):869–73. https://doi.org/10.15585/mmwr.mm6633a1 .
Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics’2017 update: a report from the American Heart Association. Circulation . 2017;135(10):e146–603. https://doi.org/10.1161/CIR.0000000000000485 .
Taylor RS, Brown A, Ebrahim S, et al. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med . 2004;116(10):682–92. https://doi.org/10.1016/j.amjmed.2004.01.009.
Article PubMed Google Scholar
Goel K, Lennon RJ, Tilbury RT, Squires RW, Thomas RJ. Impact of cardiac rehabilitation on mortality and cardiovascular events after percutaneous coronary intervention in the community. Circulation . 2011;123(21):2344–52. https://doi.org/10.1161/CIRCULATIONAHA.110.983536.
Suaya J a, Stason WB, Ades PA, Normand S-LT, Shepard DS. Cardiac rehabilitation and survival in older coronary patients. J Am Coll Cardiol . 2009;54(1):25–33. https://doi.org/10.1016/j.jacc.2009.01.078 .
Sandesara PB, Lambert CT, Gordon NF, et al. Cardiac rehabilitation and risk reduction: time to “rebrand and reinvigorate.”. J Am Coll Cardiol . 2015;65(4):389–95. https://doi.org/10.1016/j.jacc.2014.10.059 .
Anderson L, Oldridge N, Thompson DR, et al. Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta-analysis. J Am Coll Cardiol . 2016;67(1):1–12. https://doi.org/10.1016/j.jacc.2015.10.044.
Shaw LW. Effects of a prescribed supervised exercise program on mortality and cardiovascular morbidity in patients after myocardial infarction. The National Exercise and Heart Disease Project. Am J Cardiol . 1981;48(1):39–46. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=cctr&AN=CN-00025397%5Cnhttp://sfx.scholarsportal.info/uhn?sid=OVID:cctrdb&id=pmid:6972693&id=doi:&issn=0002-9149&isbn=&volume=48&issue=1&spage=39&pages=39-46&date=1981&title=American+jou
Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship between cardiac rehabilitation and long-term risks of death and myocardial infarction among elderly medicare beneficiaries. Circulation . 2010;121(1):63–70. https://doi.org/10.1161/CIRCULATIONAHA.109.876383 .
• Yancy CW, Jessup M, Bozkurt B, et al. ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American college of cardiology foundation/american heart association task force on practice guidelines. J Am Coll Cardiol 2013. 2013;62(16):1495–539. https://doi.org/10.1016/j.jacc.2013.05.020 . Societal guidelines on recommendations for cardiac rehabilitation in patients with heart failure.
Smith SC, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update. Endorsed by the National Heart, Lung, and Blood Institute. J Am Coll Cardiol . 2006;47(10):2130–9. https://doi.org/10.1016/j.jacc.2006.04.026.
Kachur S, Chongthammakun V, Lavie CJ, et al. Impact of cardiac rehabilitation and exercise training programs in coronary heart disease. Prog Cardiovasc Dis . 2017;60(1):103–14. https://doi.org/10.1016/j.pcad.2017.07.002.
Morris J, Heady J. Mortality in relation to the physical activity of work: a preliminary note on experience in middle age. Br J Intern Med . 1953;10(4):245–54.
CAS Google Scholar
Hellerstein H. Exercise therapy in coronary disease. Bull New York Acad Med J Ubran Heal . 1968;44(8):1028–47.
DHHS. Cardiac Rehabilitation and Intensive Cardiac Rehabilitation .; 2010.
AACPR. Guidelines for Cardiac Rehabilitation and Secondary Prevention Programs .; 2013. http://www.ncbi.nlm.nih.gov/nlmcatalog/101609574 .
van Halewijn G, Deckers J, Tay HY, van Domburg R, Kotseva K, Wood D. Lessons from contemporary trials of cardiovascular prevention and rehabilitation: a systematic review and meta-analysis. Int J Cardiol . 2017;232:294–303. https://doi.org/10.1016/j.ijcard.2016.12.125 .
Heran BS, Chen JM, Ebrahim S, et al. Exercise-based cardiac rehabilitation for coronary heart disease. In: Cochrane Database of Systematic Reviews .; 2011. doi: https://doi.org/10.1002/14651858.CD001800.pub2.
• Dunlay S, Pack Q, Thomas R, Killian J, Roger V. Participation in cardiac rehabilitation, readmissions, and death after acute myocardial infarction. Am J Med . 2014;127(6):538–46. Recent population based study that showed participants who enrolled in cardiac rehabilitation after a myocardial infarction had lower hospital readmission rates and death.
• Rauch B, Davos CH, Doherty P, et al. The prognostic effect of cardiac rehabilitation in the era of acute revascularisation and statin therapy: a systematic review and meta-analysis of randomized and non-randomized studies—the cardiac rehabilitation outcome study (CROS). Eur J Prev Cardiol . 2016;23(18):1914–39. https://doi.org/10.1177/2047487316671181 . Recent systematic review and meta-analysis that evaluated the role of cardiac rehabilitation in patients post acute coronary syndrome or coronary artery bypass grafting. Participants did have an associated reduction in mortality in a modern era of optimal therapy, however there was heterogeneity amongst study designs and rehabilitation programs which suggest a call for standardizations of programs.
Milani RV, Lavie CJ. Impact of cardiac rehabilitation on depression and its associated mortality. Am J Med . 2007;120(9):799–806. https://doi.org/10.1016/j.amjmed.2007.03.026 .
• Ernstsen L, Rangul V, Nauman J, et al. Protective effect of regular physical activity on depression after myocardial infarction: the HUNT study. Am J Med . 2016;129(1):82–8. https://doi.org/10.1016/j.amjmed.2015.08.012 . A small study showing the effect of physical activity in preventing depression post myocardial infarction.
Jolliffe JA, Rees K, Taylor RS, Thompson D, Oldridge N, Ebrahim S. Exercise-based rehabilitation for coronary heart disease...reprinted with permission from the Cochrane Database of Systematic Reviews. Cardiopulm Phys Ther J (American Phys Ther Assoc Cardiopulm Sect . 2001;12(4):131–140 10p. http://search.ebscohost.com/login.aspx?direct=true&db=c8h&AN=106909977&site=ehost-live .
Hunt SA, Hunt SA, Abraham WT, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult. Circulation . 2005;112(12):e154–235. https://doi.org/10.1161/CIRCULATIONAHA.105.167586.
LH C, DJ W, BG H, et al. INcidence and prevalence of heart failure in elderly persons, 1994–2003. Arch Intern Med . 2008;168(4):418–24. https://doi.org/10.1001/archinternmed.2007.80 .
Article Google Scholar
Hobbs FDR, Roalfe AK, Davis RC, Davies MK, Hare R. Prognosis of all-cause heart failure and borderline left ventricular systolic dysfunction: 5 year mortality follow-up of the Echocardiographic Heart of England Screening Study (ECHOES). Eur Heart J . 2007;28(9):1128–34. https://doi.org/10.1093/eurheartj/ehm102 .
Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community. JAMA . 2003;289(2):194. https://doi.org/10.1001/jama.289.2.194 .
Taylor CJ, Ryan R, Nichols L, Gale N, Richard Hobbs FD, Marshall T. Survival following a diagnosis of heart failure in primary care. Fam Pract . 2017;34(2):161–8. https://doi.org/10.1093/fampra/cmw145.
PubMed Google Scholar
Konstam MA, M. G, J.C. BJ, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. J Am Med Assoc . 2007;297(12):1319–31. https://doi.org/10.1001/jama.297.12.1319.
Article CAS Google Scholar
Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L TLCR-HF (CARE-HSI. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539–1549. doi: https://doi.org/10.1056/NEJMoa050496.
Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med . 2004;350(21):2140–50. https://doi.org/10.1056/NEJMoa032423 .
Article CAS PubMed Google Scholar
McKelvie RS. Exercise training in patients with heart failure: clinical outcomes, safety, and indications. Heart Fail Rev 2008;13(1):3–11. doi: https://doi.org/10.1007/s10741-007-9052-z .
Hambrecht R, Niebauer J, Fiehn E, et al. Physical training in patients with stable chronic heart failure: effects on cardiorespiratory fitness and ultrastructural abnormalities of leg muscles. J Am Coll Cardiol . 1995;25(6):1239–49. https://doi.org/10.1016/0735-1097(94)00568-B.
Coats AJ, Adamopoulos S, Radaelli A, et al. Controlled trial of physical training in chronic heart failure. Exercise performance, hemodynamics, ventilation, and autonomic function. Circulation . 1992;85(6):2119–31. https://doi.org/10.1161/01.CIR.85.6.2119.
Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation . 1999;99:1173–82. https://doi.org/10.1161/01.CIR.99.9.1173 .
Smart N, Marwick TH. Exercise training for patients with heart failure: a systematic review of factors that improve mortality and morbidity. Am J Med 2004;116(10):693–706. doi: https://doi.org/10.1016/j.amjmed.2003.11.033 .
Piepoli MF, Davos C, Francis DP, et al. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH). BMJ . 2004;328(7433):189. https://doi.org/10.1136/bmj.37938.645220.EE.
C.M. O, D.J. W, K.L. L, et al. Efficacy and safety of exercise training in patients with chronic heart failure HF-ACTION randomized controlled trial. JAMA - J Am Med Assoc . 2009;301(14):1439–50. http://jama.ama-assn.org/cgi/reprint/301/14/1439%5Cnhttp://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed9&NEWS=N&AN=2009196870.
Flynn KE, Piña IL, Whellan DJ, et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA J Am Med Assoc . 2009;301(14):1451–9. https://doi.org/10.1001/jama.2009.457.
Keteyian SJ, Leifer ES, Houston-Miller N, et al. Relation between volume of exercise and clinical outcomes in patients with heart failure. J Am Coll Cardiol . 2012;60(19):1899–905. https://doi.org/10.1016/j.jacc.2012.08.958 .
Swank AM, Horton J, Fleg JL, et al. Modest increase in peak VO2 is related to better clinical outcomes in chronic heart failure patients: results from heart failure and a controlled trial to investigate outcomes of exercise training. Circ Hear Fail . 2012;5(5):579–85. https://doi.org/10.1161/CIRCHEARTFAILURE.111.965186 .
• Bakker E, Snoek J, Meindersma E, et al. Absence of fitness improvement is associated with outcomes in heart failure patients. Med Sci Sport Exerc . 2017; Epub ahead. A small study that evaluated the clinical impact of baseline cardiorespiratory fitness and improvements of fitness after cardiac rehabilitation in heart failure patients. The authors found that the inability to improve peak VO2, or combination of poor baseline cardiorespiratory fitness and inability to improve peak VO2 resulted in worse clinical outcomes.
Owen A, Croucher L. Effect of an exercise programme for elderly patients with heart failure. Eur J Heart Fail . 2000;2(1):65–70.
Gottlieb S, Fisher M, Freudenberger R, et al. Effects of exercise training on peak performance and quality of life in congestive heart failure patients. J Card Fail 1999;5(3):188–194.
Weilenga R, Huisveld I, Bol E, et al. Exercise training in elderly patients with chronic heart failure. Coron Artery Dis 1998;9:765–770.
Willenheimer R, Erhardt L, Cline C, Rydberg E, Israelsson B. Exercise training in heart failure improves quality of life and exercise capacity. Eur Heart J . 1998;19(5):774–81.
Fleg JL. Exercise therapy for older heart failure patients. Heart Fail Clin . 2017;13(3):607–17. https://doi.org/10.1016/j.hfc.2017.02.012 .
• Chen Y, Li Y. Safety and efficacy of exercise training in elderly heart failure patients: a systematic review and meta-analysis. Int J Clin Pract . 2013;67(11):1192–8. A systematic review and meta-analysis that evaluated the impact of cardiac rehabilitation in elderly patients with heart failure. The authors found that exercise training improved six minute walk distance and quality of life. An impact on mortality or hospital readmission was not seen.
Antonicelli R, Spazzafumo L, Scalvini S. Exercise: a “new drug” for elderly patients with chronic heart failure. Aging (Albany NY) . 2016;8:860–9.
• Reeves GR, Whellan DJ, Duncan P, et al. Rehabilitation therapy in older acute heart failure patients (REHAB-HF) trial: design and rationale. Am Heart J . 2017;185:130–9. https://doi.org/10.1016/j.ahj.2016.12.012 . Design and rational of the first randomized clinical trial of cardiac rehabilitation on older patients hospitalized for acute decompensated heart failure to see if physical interventions can prevent hospital readmissions.
• Acanfora D, Scicchitano P, Casucci G, et al. Exercise training effects on elderly and middle-age patients with chronic heart failure after acute decompensation: a randomized, controlled trial. Int J Cardiol . 2016;225:313–23. https://doi.org/10.1016/j.ijcard.2016.10.026 . A clinical trial looking at the impact of cardiac rehabilitation in both middle and older patients with heart failure who had a recent hospitalization for decompensated heart failure. The authors found that exercise training improved cardiac performance indexes and pulmonary function in both middle and older age groups.
• Pahor M, Guralnik JM, Ambrosius AT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults. The LIFE Study Randomized Clinical Trial. JAMA . 2014;311(23):2387–96. A clinical trial that compared a structured, moderate-intensity physical activity program to a health education program in older adults with the primary outcome being major mobility disability. The authors found that a structured physical activity program resulted in reduced major mobility disability among older adults at risk for disability.
Article CAS PubMed PubMed Central Google Scholar
• Newman AB, Dodson JA, Church TS, et al. Cardiovascular events in a physical activity intervention compared with a successful aging intervention: the LIFE study randomized trial. JAMA Cardiol . 2016;1(5):568–74. An evaluation of the LIFE trial that studied the rates of major adverse cardiovascular events in older individuals who were randomized to either a structured physical activity program or a health education program. The authors found that a structured physical activity program was not associated with reduced cardiovascular events.
Kaila K, Haykowsky M, Thompson R, Paterson I. Heart failure with preserved ejection fraction in the elderly: scope of the problem. Heart Fail Rev . 2012;17(4):555–62.
AA O, Rich J, Shah S. The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep . 2013;10(4):401–10.
• Suchy C, Massen L, Rognmo O, et al. Optimising exercise training in prevention and treatment of diastolic heart failure (OptimEx-CLIN): rationale and design of a prospective, randomised, controlled trial. Eur J Prev Cardiol . 2014;21(2):18–25. Design and rational for a multicentered clinical trial studying the impact of cardiac rehabilitation in patients with heart failure with preserved ejection fraction.
• Koifman E, Grossman E, Elias A, et al. Multidisciplinary rehabilitation program in recently hospitalized patients with heart failure and preserved ejection fraction: rationale and design of a randomized controlled trial. Am Heart J . 2014;168(6):830–7. Another trial evaluating the impact of cardiac rehabilitation in patients with heart failure with preserved ejection fraction.
Palau P, Nunez E, Dominguez E, Sanchis J, Nunez J. Physical therapy in heart failure with preserved ejection fraction: a systematic review. Eur J Prev Cardiol . 2014;23(1):4–13.
Haykowsky M, Kitzman D. Exercise physiology in heart failure and preserved ejection fraction. Heart Fail Clin . 2014;10(3):445–52.
Kitzman D, Nicklas B, Kraus W, et al. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol . 2014;306(9):1364–70.
• Pandey A, Kitzman DW, Brubaker P, et al. Response to endurance exercise training in older adults with heart failure with preserved or reduced ejection fraction. J Am Geriatr Soc . 2017;65(8):1698–704. https://doi.org/10.1111/jgs.14867 . A study that evaluated the role of cardiac rehabilitation in both patients with reduced and preserved systolic function. The authors found that in older patients there was a greater improvement in peak VO2 in those with preserved systolic function.
Forman DE, Sanderson BK, Josephson RA, Raikhelkar J, Bittner V. American College of Cardiology’s Prevention of Cardiovascular Disease Section. Heart Failure as a Newly Approved Diagnosis for Cardiac Rehabilitation: Challenges and Opportunities. J Am Coll Cardiol . 2015;65(24):2652–9. https://doi.org/10.1016/j.jacc.2015.04.052.
Yavari M, Haykowsky M, Savu A, Kaul P, Dyck J, Haennel R. Volume and patterns of physical activity across the health and heart failure continuum. Can J Cardiol . 2017;33(11):1465–71.
Slaughter MS, Pagani FD, Rogers JG, et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Hear Lung Transplant . 2010;29(4 SUPPL.). doi: https://doi.org/10.1016/j.healun.2010.01.011 .
Arena R, Humphrey R, McCall R. Altered exercise pulmonary function after left ventricular assist device implantation. J Cardpulm Rehabil . 1999;19(6):344–6.
• Kerrigan DJ, Williams CT, Ehrman JK, et al. Cardiac rehabilitation improves functional capacity and patient-reported health status in patients with continuous-flow left ventricular assist devices: the rehab-vad randomized controlled trial. JACC Hear Fail . 2014;2(6):653–9. https://doi.org/10.1016/j.jchf.2014.06.011 . A clinical trial which showed that patients with continuous flow left ventricular assist devices had improved functional capacity and health status when attending cardiac rehabilitation programs.
• Haddad M, Saurav A, Smer A, et al. Cardiac rehabilitation in patients with left ventricular assist device: a systematic review and meta-analysis. J Cardiopulm Rehabil Prev . 2017;37(6):390–6. Recent systematic review and meta-analysis showing improved outcomes in patients with left ventricular assist devices who enroll in exercise based cardiac rehabilitation programs.
Suaya J a, Shepard DS, Normand S-LT, Ades P a, Prottas J, Stason WB. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation . 2007;116(15):1653–62. https://doi.org/10.1161/CIRCULATIONAHA.107.701466 .
Doll JA, Hellkamp A, Thomas L, et al. Effectiveness of cardiac rehabilitation among older patients after acute myocardial infarction. Am Heart J . 2015;170(5):855–64. https://doi.org/10.1016/j.ahj.2015.08.001 .
Brown TM, Hernandez AF, Bittner V, et al. Predictors of cardiac rehabilitation referral in coronary artery disease patients. Findings From the American Heart Association’s Get With The Guidelines Program. J Am Coll Cardiol . 2009;54(6):515–21. https://doi.org/10.1016/j.jacc.2009.02.080 .
Aragam KG, Dai D, Neely ML, et al. Gaps in referral to cardiac rehabilitation of patients undergoing percutaneous coronary intervention in the United States. J Am Coll Cardiol . 2015;65(19):2079–88. https://doi.org/10.1016/j.jacc.2015.02.063.
• Park LG, Schopfer DW, Zhang N, Shen H, Whooley MA. Participation in cardiac rehabilitation among patients with heart failure. J Card Fail . 2017;23(5):427–31. https://doi.org/10.1016/j.cardfail.2017.02.003 . A retrospective study that found low participation rates of heart failure patients in cardiac rehabilitation programs.
• Kim M, Kim M-S, Lim S-J, Ahn J-M, Kim J-J, Park S-J. Comparison of supervised hospital-based versus educated home-based exercise training in Korean heart failure patients. Korean Circ J . 2017;47(5):742. https://doi.org/10.4070/kcj.2017.0061 . A small single centered prospective study that compared hospital based exercise therapy to home based exercise therapy in patients with heart failure. The authors found improvements in functional capacity and quality of life in the hospital based exercise group, but no difference in long term cardiac events.
Miller S, Mandrusiak A, Adsett J. Getting to the heart of the matter: what is the landscape of exercise rehabilitation for people with heart failure in Australia? Hear Lung Circ . 2017;17:E pup ahead of print.
Kang Y, McHugh MD, Chittams J, Bowles KH. Risk factors for all-cause rehospitalization among medicare recipients with heart failure receiving telehomecare. Telemed e-Health . 2016;23(4):tmj.2016.0048. https://doi.org/10.1089/tmj.2016.0048 .
Google Scholar
• Ades PA, Keteyian SJ, Wright JS, et al. Increasing cardiac rehabilitation participation from 20% to 70%: a road map from the million hearts cardiac rehabilitation collaborative. Mayo Clin Proc . 2017;92(2):234–42. https://doi.org/10.1016/j.mayocp.2016.10.014 . An initiative aimed at addressing the under utilization of cardiac rehabilitation with a goal to increase participation rates from 20% to at least 70% by year 2022. An increase of this magnitude is calculated to save 25,000 lives and prevent 180,000 hospitalizations annually in the United States.
Download references
Author information
Authors and affiliations.
Department of Cardiovascular Medicine, Heart and Vascular Institute, Cleveland Clinic, Cleveland, OH, USA
Nishant P. Shah MD & Haitham Ahmed MD, MPH
9500 Euclid Avenue J3-6, Cleveland, OH, 44095, USA
Nishant P. Shah MD
Department of Internal Medicine, Cleveland Clinic, Cleveland, OH, USA
Ahmed AbuHaniyeh MD
You can also search for this author in PubMed Google Scholar
Ethics declarations
Conflict of interest.
Nishant P. Shah, Ahmed AbuHaniyeh, and Haitham Ahmed each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Heart Failure
Rights and permissions
Reprints and permissions
About this article
Shah, N.P., AbuHaniyeh, A. & Ahmed, H. Cardiac Rehabilitation: Current Review of the Literature and Its Role in Patients with Heart Failure. Curr Treat Options Cardio Med 20 , 12 (2018). https://doi.org/10.1007/s11936-018-0611-5
Download citation
Published : 24 February 2018
DOI : https://doi.org/10.1007/s11936-018-0611-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Heart failure
- Cardiovascular disease
- Cardiac rehabilitation
- Find a journal
- Publish with us
- Track your research
- Search Menu
- Advance Articles
- Editor's Choice
- Supplements
- Image Library
- ESC Journals App
- ESC Content Collections
- Author Guidelines
- Submission Site
- Open Access Options
- Read & Publish
- Author Resources
- Self-Archiving Policy
- About European Journal of Preventive Cardiology
- Editorial Board
- ESC Publications
- About European Society of Cardiology
- Advertising & Corporate Services
- Developing Countries Initiative
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Lay summary, introduction, conclusions, acknowledgements, author contributions, data availability, psychometric validation of the short version of the information needs in cardiac rehabilitation scale through a first global assessment.
Conflict of interest: none declared.
- Article contents
- Figures & tables
- Supplementary Data
Gabriela Lima de Melo Ghisi, Mayara Moura Alves da Cruz, Luiz Carlos Marques Vanderlei, Xia Liu, Zhimin Xu, Mariya Prakash Jiandani, Lucky Cuenza, Evangelia Kouidi, Francesco Giallauria, Jibril Mohammed, Lela Maskhulia, Patricia Fernandes Trevizan, Ladislav Batalik, Danielle Gomes Pereira, Nidal Tourkmani, Ivana Burazor, Elio Venturini, Gerlene Grudka Lira, Manuella Bennaton Cardoso Vieira Rehfeld, Victor Ribeiro Neves, Geovana de Jesus Borges, Won-Seok Kim, Seungwoo Cha, Ling Zhang, Sherry L Grace, Psychometric validation of the short version of the Information Needs in Cardiac Rehabilitation scale through a first global assessment, European Journal of Preventive Cardiology , 2024;, zwae148, https://doi.org/10.1093/eurjpc/zwae148
- Permissions Icon Permissions
Tailored education is recommended for cardiac patients, yet little is known about information needs in areas of the world where it is most needed. This study aims to assess (i) the measurement properties of the Information Needs in Cardiac Rehabilitation short version (INCR-S) scale and (ii) patient’s information needs globally.
In this cross-sectional study, English, simplified Chinese, Portuguese, or Korean versions of the INCR-S were administered to in- or out-patients via Qualtrics (January 2022–November 2023). Members of the International Council of Cardiovascular Prevention and Rehabilitation community facilitated recruitment. Importance and knowledge sufficiency of 36 items were rated. Links to evidence-based lay education were provided where warranted. A total of 1601 patients from 19 middle- and high-income countries across the world participated. Structural validity was supported upon factor analysis, with five subscales extracted: symptom response/medication, heart diseases/diagnostic tests/treatments, exercise and return-to-life roles/programmes to support, risk factors, and healthy eating/psychosocial management. Cronbach’s alpha was 0.97. Construct validity was supported through significantly higher knowledge sufficiency ratings for all items and information importance ratings for all subscales in cardiac rehabilitation (CR) enrolees vs. non-enrolees (all P < 0.001). All items were rated as very important—particularly regarding cardiac events, nutrition, exercise benefits, medications, symptom response, risk factor control, and CR—but more so in high-income countries in the Americas and Western Pacific. Knowledge sufficiency ranged from 30.0 to 67.4%, varying by region and income class. Ratings were highest for medications and lowest for support groups, resistance training, and alternative medicine.
Identification of information needs using the valid and reliable INCR-S can inform educational approaches to optimize patients’ health outcomes across the globe.
Patients need information to manage their heart diseases, such as what to do if they have chest pain, what a heart attack is, and how to take their medicine to lower the chances they will have another one, so a study of the information needs of over 1600 heart patients from around the globe was undertaken for the first time. Using the Information Needs in Cardiac Rehabilitation short version (INCR-S) scale—which was shown to be a good measurement tool through the study and hence may improve patient education—patients reported they most wanted information about heart events, heart-healthy eating, exercise benefits, their pills, symptom response, risk factor control, and cardiac rehabilitation—but more so in high-income countries in the Americas and Western Pacific. Knowledge sufficiency ratings for each item ranged from 30.0 to 67.4%, also varying by region and income class; perceived knowledge sufficiency ratings were highest for medications and lowest for support groups, resistance training, and alternative medicine.
The prevalence of cardiovascular disease (CVD) is among the highest of all conditions globally—doubling from 2009 to 2019, with 523 million people affected. 1 Cardiovascular disease is also a leading cause of disability and economic burden worldwide. 2 Advances in treatment have resulted in increased survival following an initial cardiac event, but these patients remain at increased risk of subsequent mortality and morbidity. 3
Patients require information about responding to cardiac symptoms and controlling the many CV risk factors through self-managing their disease to reduce this excess risk. 4 Indeed, systematic reviews show that patient education leads to not only increased knowledge but also improved heart health behaviour and quality of life and may decrease cardiovascular events. 5 Accordingly, therapeutic education is recommended for secondary prevention by learned cardiovascular societies. 6 For these reasons, it is considered a core component of cardiac rehabilitation (CR). 7 , 8
To be effective, guidelines assert that education should be tailored to the needs of individual patients. 9 Often however, this does not occur in practice. 10 Given this, Ghisi et al. 11 developed the Information Needs in Cardiac Rehabilitation (INCR) scale, which assesses patients’ top information needs, namely emergency/safety, diagnosis/treatments, medication, and risk factors, among other topics. 12 It is the only available validated and current scale to assess heart patient information needs. 12 The INCR is available in five languages and has been administered in six countries in two World Health Organization (WHO) regions (Americas and Western Pacific), countries of high- and middle-income according to the World Bank. 12 , 13 Despite variation in risk factor burden regionally, 14 there has only been one multicountry study of information needs in CVD patients to enable any comparison. 15
Given the global burden of CVD, much more needs to be known about the information needs of CVD patients around the world, particularly those for whom secondary prevention is most needed. 2 Moreover, through a recent review, INCR items were revisited in light of available evidence, and a short version was proposed (INCR-S). 12 Accordingly, the objectives of this study were to investigate (i) measurement properties of the INCR-S (structural validity, internal reliability, and construct validity) and (ii) CVD patients’ information needs globally. The identification of patients’ greatest information needs can inform educational approaches that optimize their health outcomes.
Design and procedure
This study was cross-sectional in design. York University’s Office of Research Ethics (Toronto, Canada) approved the study (e2021-013). Respondents completed the confidential survey after providing informed written consent.
The study was undertaken through the International Council of Cardiovascular Prevention and Rehabilitation (ICCPR; globalcardiacrehab.com ), a network of ∼45 CR-related societies. An invitation to participate was shared through ICCPR’s programme mailing list, which comprises emails from ∼2000 people that work in cardiac care around the globe. Data were collected from January 2022 to November 2023.
The survey was available in English, Portuguese, simplified Chinese, and Korean. All but the Korean surveys were completed online via Qualtrics; the Korean-speaking respondents completed the survey via paper and pencil. When participants were not proficient in any of the above languages and/or had no internet access, staff at participating centres administered the survey via interview and entered their responses online on participant’s behalf.
Where respondents who completed any non-Korean survey directly online rated any item as 4 or 5 (indicating the information was ‘important’ or ‘very important’ to them) and that they did not yet know enough about the topic (i.e. insufficient knowledge), they were provided with a link to evidence-based lay educational information for that item at the end in the language of the survey. Information responses can be found in the Appendix . In order to facilitate care quality improvement, respondents could elect to provide their institution; this was used to determine respondent’s country. At the end of the study, collaborators were provided anonymous responses for their institution in aggregate to support efforts to address top information gaps.
Participants
This study included adult (i.e. age >18 years old) in- or out-patients with a cardiovascular diagnosis indicated for participation in CR (e.g. acute coronary syndromes ± revascularization, heart failure, and peripheral vascular diseases) and hence having information needs in the domains covered in the INCR-S. Patients were excluded if they had completed CR, given many of their information needs would be fulfilled.
Respondents were first asked to self-report their sociodemographic and other pertinent characteristics through investigator-generated items with forced-choice response options including region of the world (categorized based on WHO region), 13 age, sex, education, social support (on a 5-point Likert scale from 1 = no to 5 = definitely), and financial insecurity (including in relation to healthcare). Respondents were also asked about CR referral and CR participation (yes/no). For respondents reporting their institution, countries were categorized based on World Bank income classification.
The INCR is a scale created by Ghisi et al. 11 to assess information needs of CR patients. It originally comprised 55 items across 10 information areas. Each is scored on a 5-point Likert scale from 1 ( really not important ) to 5 ( very important ); higher scores indicate greater information needs. At the end, there is an open-ended item where respondents can add additional information needs. The internal and re-test reliability, responsiveness, and interpretability as well as criterion, content, construct, structural, and cross-cultural validity of the INCR have been established. 12
In this study, the short version of the INCR-S was administered for the first time. 12 Following a literature review, this version was finalized based on importance ratings in previous studies and the ‘other’ responses of past respondents, as well as input from healthcare providers involved in the INCR translations and cultural adaptations and CR researchers in the patient education field. 12 , 16 The shorter version of the INCR has 36 items covering 9 information areas: the heart (4 items), emergency/safety (2 items), diagnosis/treatment (4 items), risk factors (5 items), medication (5 items), exercise (5 items), nutrition (4 items), psychosocial factors (6 items), and CR (1 item). Furthermore, in addition to the 5-point Likert-type importance rating of each item and the open-ended ‘other’ information needs item, in this revised version, respondents are also asked to denote whether or not they perceive they already know enough about each topic/item (i.e. knowledge sufficiency).
After reviewing information provided to fulfil their greatest needs, respondents were asked to rate helpfulness of the information provided on a Likert-type scale ranging from 1 = very unhelpful to 5 = very helpful.
Statistical analysis
Data were exported from Qualtrics to SPSS version 28 (IBM), where analyses were performed. For the psychometric validation, after suitability of data was confirmed by Bartlett’s test of sphericity and the Kaiser–Meyer–Olkin (KMO) value, exploratory factor analysis (EFA) was undertaken to assess structural validity. Factor extraction was conducted using the principal component method, with varimax rotation. The number of factors extracted was determined by considering those with eigenvalues ≥1.0, percentage of variance accounted for, and examination of the scree plot. Item factor loadings ≥0.3 were considered in finalizing the items for each factor and interpreting them. 17 , 18 Internal consistency of resultant factors was then assessed based on Cronbach’s alpha; a value >0.70 was considered acceptable. 19 To test construct validity, differences in INCR-S item knowledge sufficiency and importance subscale scores were compared by CR enrolment status using χ 2 and Student’s t -tests, respectively.
For the second objective, a descriptive examination of INCR-S scores was performed. Analysis of variance was used to examine differences in INCR-S scores by region and income class. A P < 0.05 was considered statistically significant.
Overall, 1601 surveys were completed, of which 779 (48.7%) were in English, 583 (36.4%) in Portuguese, 200 (12.5%) in simplified Chinese, and 39 (2.4%) in Korean. The 19 countries from which data were collected are shown in Figure 1 . The number of surveys completed per country ranged from 1 to 583. As shown in Table 1 , these responses stem from 6/6 WHO regions, in countries of middle- or high-income class ( Figure 1 ). 13

Greatest informational importance areas by ( A ) region and ( B ) country income classification. ( A ) World Health Organization region (countries): AFR, African (Nigeria); AMR, Americas (Brazil, Mexico, and USA); SEAR, Southeast Asian Region (India); EUR, European (Czech Republic, Greece, Italy, Georgia, Serbia, Poland, and UK); EMR, Eastern Mediterranean Region (Iran); and WPR, Western Pacific Region (Australia, Philippines, China, South Korea, Singapore, and Taiwan). ( B ) World Bank income class (countries): high-income (Australia, Czech Republic, Italy, Greece, Poland, Singapore, South Korea/Republic of Korea, Taiwan, USA, and UK); upper middle-income (Brazil, China, Georgia, Mexico, and Serbia); and lower middle-income countries (India, Iran, Nigeria, and Philippines). *Knowledge sufficiency <50%.
Self-reported characteristics of study participants, overall and by total Information Needs in Cardiac Rehabilitation short version scores ( n = 1601)
Given some missing data, valid percentages are reported.
CR, cardiac rehabilitation; INCR-S, Information Needs in Cardiac Rehabilitation short version.
a Scores range from 1 to 5, with higher scores denoting greater need for information.
b For test of differences in total INCR-S importance scores by characteristic, using Pearson’s correlation, ANOVA, or t -test as applicable.
c n (%) yes.
d For test of differences in total INCR-S knowledge sufficiency scores by characteristic, using t -tests or χ 2 tests as applicable.
Respondent characteristics are shown in Table 1 . Overall, 40.1% had not initiated CR.
Structural validity of the Information Needs in Cardiac Rehabilitation short version and internal reliability
The KMO value of 0.971 indicated a highly acceptable score, with a significant Bartlett’s test of sphericity ( P < 0.0001). Results of the factor analysis are displayed in Table 2 . As shown, five factors were extracted, representing 60.1% of the variance and with few item cross-loadings. Cronbach’s alpha for the total INCR-S was 0.97. Internal consistency of all factors was also considered acceptable (range 0.85–0.90). 19
Mean Information Needs in Cardiac Rehabilitation short version scores by item, as well as exploratory factor analysis, n = 1601
CV, cardiovascular; INCR-S, Information Needs in Cardiac Rehabilitation short version; NA, not applicable.
a Scores range from 1 to 5, with higher scores denoting greater needs for information.
b Factor loadings <0.300 are not shown to support interpretation.
Construct validity
In Table 1 , INCR-S importance scores are shown by respondents’ characteristics. As expected and supporting construct validity, there were significant differences by education. In addition, those that reported having social support, more often worrying about money, and working or previously working rated the information as significantly more important than their counterparts.
Table 1 also displays the association between respondent characteristics and perceived knowledge sufficiency to further consider construct validity. As shown, participants who were older, male, retired or seeking work, and with more education had greater perceived knowledge sufficiency than their counterparts; no other differences were observed.
Finally, to more fully assess construct validity, INCR-S information sufficiency item and importance subscale scores were compared by respondents’ CR enrolment status. Perceived knowledge sufficiency was significantly greater among CR enrolees than non-enrolees for all the 36 items. Among non-CR enrolling respondents, the greatest information needs were ‘What happens when someone has a heart attack or other heart event?’, ‘When should I see the doctor or go to the emergency room?’, and ‘What should I do if I feel angina or chest pain?’. Among enrolling respondents, the greatest information needs were ‘How will exercise help my heart condition?’, ‘What medications do I need for my heart?’, and ‘What should I do if I feel angina or chest pain?’. There were also significant differences in importance ratings for all subscales by CR enrolment status ( Table 3 ), with enrolees perceiving each subscale as more important than non-enrolees, also supporting INCR-S validity.
Ratings of Information Needs in Cardiac Rehabilitation short version item knowledge sufficiency and Information Needs in Cardiac Rehabilitation short version subscale importance, by CR enrolment status
n (%) yes for knowledge sufficiency or mean ± standard deviation for subscale information importance shown.
CR, cardiac rehabilitation; CV, cardiovascular; INCR-S, Information Needs in Cardiac Rehabilitation short version; NA, not applicable.
Cardiovascular disease information needs around the globe
The INCR-S item importance scores are shown in Table 2 ; all items were rated as highly important (i.e. for all but 2 items, the mean scores were above 4/5; the 2 items were sexual activity and complementary and alternative medicine). As shown, respondents perceived as most important the nature of cardiac events, heart-healthy eating, benefits of exercise for the heart, cardiac medications, how to take medications, how to respond to angina and when to seek care, how to control risk factors (particularly blood pressure), and about CR. Total and subscale INCR-S importance scores are shown in Table 3 ; the subscale rated most important was symptom response and medication.
When asked about any other information needs, most respondents left the item blank or stated ‘none’ ( n = 1510), and 16 others responded with a comment related to desired source or mode of information delivery (i.e. not applicable). Of 78 ‘other’ information needs reported, 68 responses related to information were already considered in the scale, such as return to specific activities (e.g. travel, sauna, scuba, bodybuilding, household chores, and social activities), effects of substance abuse on heart health (e.g. marijuana), how to treat dyslipidaemia, information about weight control, and exercise (e.g. using smartwatch to monitor) as well as availability of low-cost CR programmes. Nineteen (1.2%) responses did pertain to topics not included in the scale, such as CPR training, genetics of CVD, activities to avoid after surgery or procedures, tests of lung function, relation to other conditions (e.g. kidney and liver diseases), progression of exercise, and probable life span.
The greatest information needs among women were ‘What happens when someone has a heart attack or other heart event?’, ‘What should I do if I feel angina or chest pain?’, and ‘When should I see the doctor or go to the emergency room?’; among men, the greatest information needs were ‘How will exercise help my heart condition?’, ‘What medications do I need for my heart?’, and ‘What should I do if I feel angina or chest pain?’. Among patients older than 65, the greatest information needs were ‘When should I see the doctor or go to the emergency room?’, ‘What medications do I need for my heart?’, and ‘What happens when someone has a heart attack or other heart event?’; among those younger, the greatest information needs were ‘How will exercise help my heart condition?’, ‘What medications do I need for my heart?’, and ‘What should I do if I feel angina or chest pain?’. Among patients with <12 years of education, the greatest information needs were ‘What should I do if I feel angina or chest pain?’, ‘What medications do I need for my heart?’, and ‘When should I see the doctor or go to the emergency room?’; among those with more education, the greatest information needs were ‘How will exercise help my heart condition?’, ‘What happens when someone has a heart attack or other heart event?’, and ‘What should I do if I feel angina or chest pain?’.
Item knowledge sufficiency scores are shown in Table 3 ; these ranged from 30.0 to 67.4%. Items respondents most often perceived they knew enough about were the following: how to remember to take medications, how to take medications in the right way, and how being around tobacco affects the heart. Items respondents most often perceived they knew least about were as follows: ‘What services, support organizations and groups are available?’, ‘What roles do complementary and alternative therapies play in my heart recovery?’, and ‘Do I need resistance training?’.
The INCR-S total scores are shown by region and income class in Table 1 , with most important information areas by each shown in Figure 1 . As shown in Table 1 , by region, there was significant variation in importance (rated highest in Americas and lowest in Africa) and knowledge sufficiency (rated highest in Africa and lowest in Southeast Asia). Importance subscale scores are shown by region in Table 4 . In Africa, the most important information needs were ‘Do I need resistance training?’, ‘How do alcohol and/or drugs affect my heart?’, and ‘How do I exercise safely?’. In the Americas, the most important information needs were ‘What should I do if I feel angina or chest pain?’, ‘When should I see the doctor or go to the emergency room?’, and ‘How will exercise help my heart condition?’. In Europe, the most important information needs were ‘What medications do I need for my heart?’, ‘What happens when someone has a heart attack or other heart event?’, and ‘What should I do if I feel angina or chest pain?’. In Southeast Asia, the most important information needs were ‘What medications do I need for my heart?’, ‘How do I take my medications in the right way?’, and ‘When should I see the doctor or go to the emergency room?’. Lastly, in the Western Pacific, the most important information needs were ‘What happens when someone has a heart attack or other heart event?’, ‘What should I do if I feel angina or chest pain?’, and ‘When should I see the doctor or go to the emergency room?’.
Information importance by subscale, as well as educational information response helpfulness by WHO region
Eastern Mediterranean not shown due to low sample size ( n = 4). Values in bold are n countries while values in italics are n participants.
NA, not applicable for Africa because the site could not provide education resources in the patients’ first language; WHO, World Health Organization; SD, standard deviation; CR, cardiac rehabilitation; INCR-S, Information Needs in Cardiac Rehabilitation short version.
a Mean scores range from 1 to 5, with higher scores denoting greater information needs or helpfulness.
By income class, there was also significant variation in both, with respondents from lower middle-income countries rating the information as least important and those in high-income countries reporting greatest knowledge sufficiency ( Table 1 ). In lower middle-income countries, the most important information needs were ‘What medications do I need for my heart?’, ‘How will exercise help my heart condition?’, and ‘How can I manage stress?’. In upper middle-income countries, the most important information needs were ‘What should I do if I feel angina or chest pain?’, ‘When should I see the doctor or go to the emergency room?’, and ‘What happens when someone has a heart attack or other heart event?’. In high-income countries, the most important information needs were ‘How will exercise help my heart condition?’, ‘What happens when someone has a heart attack or other heart event?’, and ‘What is cardiac rehabilitation about?’.
Educational information response helpfulness
The mean information helpfulness rating was 4.4 ± 0.7/5, suggesting the education provided in response to high information needs without knowledge sufficiency was highly satisfactory. Scores are shown by region in Table 4 . There was no significant difference in rating by language of survey or CR initiation (both P > 0.05).
In this first ever investigation of cardiac patient information needs around the globe, the INCR-S was established as psychometrically valid. Upon assessment in over 1600 patients from almost 20 countries, patient’s greatest information needs and areas of knowledge sufficiency were identified, with significant variation by region and country income class shown. This brief scale thus should be used to support optimized and individualized CV patient education internationally.
Through assessment of the reliability and validity of the INCR-S in this study, and along with other measurement properties assessed and established as satisfactory in previous research on the INCR, 12 overall results suggest the brief scale is psychometrically valid. Indeed, mean total importance scores of the INCR-S were consistent with the INCR translations, all slightly above 4/5. 12 Exploratory factor analysis revealed five factors, all internally consistent and generally consistent with the INCR 12 : symptom response/medication, heart diseases/diagnostic tests/treatments, exercise/return to life roles/programmes to support, cardiovascular risk factors, and healthy eating/psychosocial management. Construct validity was confirmed by significant differences in information importance and knowledge sufficiency ratings by CR enrolment. Despite reducing the number of items, there should not be great concern about failure to identify information needs, given <2% of respondents reported unique ‘other’ information needs. This brief version will be more usable in clinical settings.
Of interest was the lack of sex difference in ratings of information importance but significant sex difference in knowledge sufficiency. There are few studies of sex differences in cardiac patient information needs, 20 despite that it is known women who develop CVD have lower awareness of their risk 21 and that women with CVD receive less patient education. 22 Therefore, this study adds to our understanding. But given current understanding, the dearth of studies on sex differences in disease management knowledge in cardiac patients is a glaring omission. 23 Whether women cardiac patients generally have lower knowledge than men warrants study, as the higher knowledge sufficiency ratings in men may be explained by their documented over-confidence in many areas. 24
Implications for cardiac rehabilitation programmes
Arguably, patient information needs can best be met within CR, given the multidisciplinary team who have knowledge across all areas of secondary prevention, 25 delivering care over time and hence providing the context to support patient implementation of knowledge gained. 26 Indeed, therapeutic education is a core component of CR. 27 , 28 The ICCPR’s global audit established 97% of programmes deliver education as a standard of care (median 4.5 h per patient). 29
Nevertheless, CR programmes report barriers to providing comprehensive education. 30 For instance, while programmes are generally staffed by a multidisciplinary team 31 as per best practice recommendations, 7 staff desire further training and time to assess knowledge and cover all needed areas for fulsome secondary prevention. 9 Moreover, as demonstrated herein and in previous research, 32 patient information needs change over the course of their disease trajectory.
For these reasons, the INCR-S is a valuable tool to support identification of key information needs in CR participants, enabling prioritization of education content over time to optimize comprehensive delivery, minimizing burden on patients and staff. To support CR programmes, ICCPR hosts the INCR-S in English, Portuguese, and simplified Chinese for patient self-report on its website at https://globalcardiacrehab.com/For-Patients .
It would be prudent for CR programmes to concomitantly assess patient health literacy also using a validated tool 33 to ensure it is sufficient, so education provided can be tailored not only in terms of information needs but also level of difficulty. Moreover, no patient knows what they do not or need to know, and hence, a corresponding assessment of actual knowledge to ensure patients do have all the information needed to successfully self-manage their heart disease would also be prudent, such as with the Coronary Artery Disease Education Questionnaire (CADE-Q). 34 This is particularly true given that some patients seek information about their heart on the internet, and the information may be erroneous or out of date. 35 , 36 In sum, CR programmes are advised to assess patient health literacy, information needs (using potentially the now validated INCR-S), and knowledge a priori to inform delivery of individualized education plans for their patients.
Areas to target for patient education given their high importance combined with low knowledge sufficiency ratings regard understanding their condition (i.e. CVD and angina), managing stress, and treatment options (e.g. bypass surgery). Interestingly, knowledge sufficiency was rated highest for medication adherence tools, yet patient adherence to medications remains grossly suboptimal. 37 Knowledge sufficiency around tobacco was also very high, suggesting awareness campaigns have been successful, 38 but again there was wide regional variation observed in this international study, and hence, this may remain an important education topic in several regions. Moreover, in Southeast Asia, the Western Pacific, and middle-income countries overall, patients reported low knowledge sufficiency in the areas where they most desired information, most commonly related to symptom response and medication ( Figure 1 ).
Limitations
Results of this study should be interpreted with caution. First, generalizability is limited. There were very few responses in the Eastern Mediterranean, so conclusions for that region should not be extrapolated from these findings. Moreover, the survey was not administered in some commonly spoken languages around the world. Information needs of those speaking Spanish, Hindi, and Arabic for example should be assessed in the future, and indeed, a Spanish translation of INCR is available. 15 Additionally, this was a convenience sample, so those that did not complete the survey may have different information needs. However, previously, the INCR had only been administered in Canada, South Korea, Brazil, Costa Rica, Colombia, and Peru, so for the first time, it has been administered in four new WHO regions. Given general consistency in findings, major concerns should be assuaged.
Second, regarding statistical analyses, multiple comparisons were made, inflating the potential for error. Readers are hence cautioned in over-interpreting associations observed without replication research. But given the novel nature of these data, these associations can be considered hypothesis generating. Finally, causal conclusions cannot be drawn due to the design of this study. For example, patients with greater awareness of the importance of disease-related knowledge may advocate for their CR referral.
The identification of patient information needs can guide healthcare providers in the development of tailored therapeutic education to support increases in patient’s disease-related knowledge, which should result in behaviour changes and ultimately improved health outcomes. The INCR-S is established as reliable and valid for this purpose; while more research is needed, overall results confirmed satisfactory measurement properties and supported its administration across many global contexts. Cardiac care providers are urged to assess patient’s health literacy, their perceptions of information importance, and knowledge and/or perceived knowledge sufficiency to inform delivery of prioritized, brief, and individualized education. Based on results of this study, education regarding the nature of their cardiac conditions, managing stress, and treatment options should be prioritized around the globe.
We are grateful to Ms Carley Stewart and Ebone Davis for setting up the surveys on Qualtrics and liaison support with collaborating centres. We are also thankful for the following individuals who supported data collection: Hung-Jui Chuang (Taiwan), Ana Ines Gonzales (Brazil), Mahdieh G. Firoozabadi (Iran), Garyfallia Pepera (Greece), and Iona van der Wiel (Australia).
S.L.G., G.L.M.G., X.L., and W.-S.K. contributed to conceptualization. L.Z. contributed to resources. G.L.M.G. and S.L.G. contributed to data curation, methodology, formal analysis, project administration, and drafted the original manuscript. S.L.G. contributed to supervision. G.L.M.G., M.M.A.d.C., L.C.M.V., Z.X., M.P.J., L.C., E.K., F.G., J.M., L.M., P.F.T., L.B., D.G.P., N.T., I.B., E.V., G.G.L., M.B.C.V.R., V.R.N., G.d.J.B., W.-S.K., S.C., L.Z., and S.L.G. contributed to investigation. Writing—review and editing: M.M.A.d.C., L.C.M.V., X.L., Z.X., M.P.J., L.C., E.K., F.G., J.M., L.M., P.F.T., L.B., D.G.P., N.T., I.B., E.V., G.G.L., M.B.C.V.R., V.R.N., G.d.J.B., W.-S.K., S.C., and L.Z. critically revised the manuscript. All gave final approval and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
None declared.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Links to evidence-based lay educational information as responses for each INCR-S item patients rate highly. Note: translations available at https://cadeq.wordpress.com/related-tools/ .
Roth GA , Mensah GA , Johnson CO , Addolorato G , Ammirati E , Baddour LM , et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study . J Am Coll Cardiol 2020 ; 76 : 2982 – 3021 .
Google Scholar
Vaduganathan M , Mensah GA , Turco JV , Fuster V , Roth GA . The global burden of cardiovascular diseases and risk: a compass for future health . J Am Coll Cardiol 2022 ; 80 : 2361 – 2371 .
Kerr AJ , Broad J , Wells S , Riddell T , Jackson R . Should the first priority in cardiovascular risk management be those with prior cardiovascular disease? Heart 2009 ; 95 : 125 – 129 .
Grady PA , Gough LL . Self-management: a comprehensive approach to management of chronic conditions . Am J Public Health 2014 ; 104 : e25 – e31 .
Anderson L , Brown JP , Clark AM , Dalal H , Rossau HK , Bridges C , et al. Patient education in the management of coronary heart disease . Cochrane Database Syst Rev 2017 ; 6 : CD008895 .
Knuuti J , Wijns W , Saraste A , Capodanno D , Barbato E , Funck-Brentano C , et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes . Eur Heart J 2020 ; 41 : 407 – 477 .
Grace SL , Turk-Adawi KI , Contractor A , Atrey A , Campbell NRC , Derman W , et al. Cardiac rehabilitation delivery model for low-resource settings: an International Council of Cardiovascular Prevention and Rehabilitation consensus statement . Prog Cardiovasc Dis 2016 ; 59 : 303 – 322 .
Smith SC Jr , Benjamin EJ , Bonow RO , Braun LT , Creager MA , Franklin BA , et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation . Circulation 2011 ; 124 : 2458 – 2473 .
National Heart Foundation of Australia . A pathway to cardiac recovery standardised program content for phase II cardiac rehabilitation. 2019. https://www.heartfoundation.org.au/getmedia/c77bac1d-4de5-4000-8e4c0ecb1365f1b2/A_Pathway_to_Phase_II_Cardiac_Recovery_(Full_Resource).pdf (15 December 2023) .
Labrunée M , Pathak A , Loscos M , Coudeyre E , Casillas JM , Gremeaux V . Therapeutic education in cardiovascular diseases: state of the art and perspectives . Ann Phys Rehabil Med 2012 ; 55 : 322 – 341 .
Ghisi GL , Grace SL , Thomas S , Evans MF , Oh P . Development and psychometric validation of a scale to assess information needs in cardiac rehabilitation: the INCR Tool . Patient Educ Couns 2013 ; 91 : 337 – 343 .
Grace SL , Stewart C , Ghisi GLM . Information Needs in Cardiac Rehabilitation (INCR) scale, 2023. In: Krageloh CU Alyami M and Medvedev ON , eds. International Handbook of Behavioral Health Assessment . Cham : Springer ; 2023 . p 1 – 27 .
Google Preview
World Bank . The world by income and region. 2023 https://datatopics.worldbank.org/world-development-indicators/the-world-by-income-and-region.html (15 December 2023) .
Teo K , Lear S , Islam S , Mony P , Dehghan M , Li W , et al. Prevalence of a healthy lifestyle among individuals with cardiovascular disease in high-, middle- and low-income countries: the Prospective Urban Rural Epidemiology (PURE) study . JAMA 2013 ; 309 : 1613 – 1621 .
Ghisi GLM , Anchique CV , Fernandez R , Quesada-Chaves D , Gordillo MX , Acosta S , et al. Validation of a Spanish version of the information needs in cardiac rehabilitation scale to assess information needs and preferences in cardiac rehabilitation . J Cardiovasc Nurs 2018 ; 33 : E29 – E34 .
Christalle E , Zill JM , Frerichs W , Härter M , Nestoriuc Y , Dirmaier J , et al. Assessment of patient information needs: a systematic review of measures . PLoS One 2019 ; 14 : e0209165 .
Schreiber JB . Issues and recommendations for exploratory factor analysis and principal component analysis . Res Social Adm Pharm 2021 ; 17 : 1004 – 1011 .
Alavi M , Visentin DC , Thapa DK , Hunt GE , Watson R , Cleary M . Exploratory factor analysis and principal component analysis in clinical studies: which one should you use? J Adv Nurs 2020 ; 76 : 1886 – 1889 .
Nunnally JC . Psychometric Theory . New York, United States : McGraw-Hill ; 1978 .
Stewart DE , Abbey SE , Shnek ZM , Irvine J , Grace SL . Gender differences in health information needs and decisional preferences in patients recovering from an acute ischemic coronary event . Psychosom Med 2004 ; 66 : 42 – 48 .
Mosca L , Hammond G , Mochari-Greenberger H , Towfighi A , Albert MA ; American Heart Association Cardiovascular Disease and Stroke in Women and Special Populations Committee of the Council on Clinical Cardiology, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on High Bloo . Fifteen-year trends in awareness of heart disease in women: results of a 2012 American Heart Association national survey . Circulation 2013 ; 127 : 1254 – 1263 . e1–e29 .
Hilleary RS , Jabusch SM , Zheng B , Jiroutek MR , Carter CA . Gender disparities in patient education provided during patient visits with a diagnosis of coronary heart disease . Womens Health (Lond) 2019 ; 15 : 1745506519845591 .
Shen T , Teo TY , Yap JJ , Yeo KK . Gender differences in knowledge, attitudes and practices towards cardiovascular disease and its treatment among Asian patients . Ann Acad Med Singap 2017 ; 46 : 20 – 28 .
Pallier G . Gender differences in the self-assessment of accuracy on cognitive tasks . Sex Roles 2023 ; 48 : 265 – 276 .
Visseren FLJ , Mach F , Smulders YM , Carballo D , Koskinas KC , Bäck M , et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice: developed by the task force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies with the special contribution of the European Association of Preventive Cardiology (EAPC) . Rev Esp Cardiol (Engl Ed) 2022 ; 75 : 429 .
Chaves G , Turk-Adawi K , Supervia M , Santiago de Araújo Pio C , Abu-Jeish AH , Mamataz T , et al. Cardiac rehabilitation dose around the world: variation and correlates . Circ Cardiovasc Qual Outcomes 2020 ; 13 : e005453 .
World Health Organization . Package of interventions for rehabilitation: module 4: cardiopulmonary conditions, 2023. https://www.who.int/publications/i/item/9789240071162 (December 15, 2023) .
British Association of Cardiopulmonary Prevention and Rehabilitation . BACPR standards and core components 2023 edition, 2023. https://www.bacpr.org/news/bacpr-standards-and-core-components-2023-edition (December 15, 2023) .
Turk-Adawi K , Supervia M , Lopez-Jimenez F , Pesah E , Ding R , Britto RR , et al. Cardiac rehabilitation availability and density around the globe . EClinicalMedicine 2019 ; 13 : 31 – 45 .
Ghisi GLM , Supervia M , Turk-Adawi K , Beleigoli A , Contractor A , Mampuya WM , et al. Women-focused cardiac rehabilitation delivery around the world and program enablers to support broader implementation . CJC Open 2023 ; 6 : 425 – 435 .
Supervia M , Turk-Adawi K , Lopez-Jimenez F , Pesah E , Ding R , Britto RR , et al. Nature of cardiac rehabilitation around the globe . EClinicalMedicine 2019 ; 13 : 46 – 56 .
de Melo Ghisi GL , Grace SL , Thomas S , Evans MF , Sawula H , Oh P . Healthcare providers’ awareness of the information needs of their cardiac rehabilitation patients throughout the program continuum . Patient Educ Couns 2014 ; 95 : 143 – 150 .
Ghisi GLM , Chaves GSDS , Britto RR , Oh P . Health literacy and coronary artery disease: a systematic review . Patient Educ Couns 2018 ; 101 : 177 – 184 .
de Melo Ghisi GL , Oh P , Thomas S , Benetti M . Development and validation of an English version of the Coronary Artery Disease Education Questionnaire (CADE-Q) . Eur J Prev Cardiol 2013 ; 20 : 291 – 300 .
Daraz L , Morrow AS , Ponce OJ , Beuschel B , Farah MH , Katabi A , et al. Can patients trust online health information? A meta-narrative systematic review addressing the quality of health information on the internet . J Gen Intern Med 2019 ; 34 : 1884 – 1891 .
Lee KS , Cho YM , Oh SH , Jung MS , Yoon JY . Evaluation of the heart failure in internet patient information: descriptive survey study . Int J Environ Res Public Health 2021 ; 18 : 1047 .
Al-Ganmi AH , Perry L , Gholizadeh L , Alotaibi AM . Cardiovascular medication adherence among patients with cardiac disease: a systematic review . J Adv Nurs 2016 ; 72 : 3001 – 3014 .
Nogueira SO , McNeill A , Fu M , Kyriakos CN , Mons U , Fernández E , et al. Impact of anti-smoking advertising on health-risk knowledge and quit attempts across 6 European countries from the EUREST-PLUS ITC Europe survey . Tob Induc Dis 2018 ; 16 : A5 .
Author notes
- cardiac rehabilitation
- heart diseases
- alternative medicine
- patient education
- support groups
- construct validity
- strength training
Email alerts
- Assessing Patient Education Needs in Cardiac Rehabilitation: A Commentary on the INCR-S Validation Study
Related articles in PubMed
Citing articles via.
- Recommend to Your Librarian
- Advertising and Corporate Services
- Journals Career Network
Affiliations
- Online ISSN 2047-4881
- Print ISSN 2047-4873
- Copyright © 2024 European Society of Cardiology
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
National Institute of Environmental Health Sciences
Your environment. your health., climate change and human health literature portal higher fine particulate matter and temperature levels impair exercise capacity in cardiac patients, climate change and human health literature portal.
- Publisher http://dx.doi.org/10.1136/heartjnl-2014-306993
- PubMed https://www.ncbi.nlm.nih.gov/pubmed/26056226
OBJECTIVE: Fine particulate matter (PM2.5) air pollution and variations in ambient temperature have been linked to increased cardiovascular morbidity and mortality. However, no large-scale study has assessed their effects on directly measured aerobic functional capacity among high-risk patients. METHODS: Using a cross-sectional observational design, we evaluated the effects of ambient PM2.5 and temperature levels over 7 days on cardiopulmonary exercise test results performed among 2078 patients enrolling into a cardiac rehabilitation programme at the University of Michigan (from January 2003 to August 2011) using multiple linear regression analyses (controlling for age, sex, body mass index). RESULTS: Peak exercise oxygen consumption was significantly decreased by approximately 14.9% per 10 mug/m(3) increase in ambient PM2.5 levels (median 10.7 mug/m(3), IQR 10.1 mug/m(3)) (lag days 6-7). Elevations in PM2.5 were also related to decreases in ventilatory threshold (lag days 5-7) and peak heart rate (lag days 2-3) and increases in peak systolic blood pressure (lag days 4-5). A 10 degrees C increase in temperature (median 10.5 degrees C, IQR 17.5 degrees C) was associated with reductions in peak exercise oxygen consumption (20.6-27.3%) and ventilatory threshold (22.9-29.2%) during all 7 lag days. In models including both factors, the outcome associations with PM2.5 were attenuated whereas the effects of temperature remained significant. CONCLUSIONS: Short-term elevations in ambient PM2.5, even at low concentrations within current air quality standards, and/or higher temperatures were associated with detrimental changes in aerobic exercise capacity, which can be linked to a worse quality of life and cardiovascular prognosis among cardiac rehabilitation patients.
Resource Description
weather or climate related pathway by which climate change affects health
resource focuses on specific location
specification of health effect or disease related to climate change exposure
format or standard characteristic of resource
related topics that intersect with those captured in other filters
Featured Clinical Reviews
- Screening for Atrial Fibrillation: US Preventive Services Task Force Recommendation Statement JAMA Recommendation Statement January 25, 2022
- Evaluating the Patient With a Pulmonary Nodule: A Review JAMA Review January 18, 2022
Select Your Interests
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
Addressing Health Disparities—The Case for Variant Transthyretin Cardiac Amyloidosis Grows Stronger
- 1 Cardiac Amyloidosis Program, Seymour, Paul, and Gloria Milstein Division of Cardiology, Department of Medicine, Columbia University Irving Medical Center, and NewYork-Presbyterian Hospital, New York
- 2 Section of Cardiovascular Medicine, Yale School of Medicine, New Haven, Connecticut
- 3 Section of Cardiovascular Medicine, Department of Medicine, Boston University Chobanian & Avedisian School of Medicine, Boston Medical Center, Boston, Massachusetts
- 4 Amyloidosis Center, Boston University Chobanian & Avedisian School of Medicine, Boston, Massachusetts
- Editorial Heart Failure in African American Individuals, Version 2.0 Clyde W. Yancy, MD, MSc JAMA
- Review Cardiac Amyloidosis Due to Transthyretin Protein Frederick L. Ruberg, MD; Mathew S. Maurer, MD JAMA
- Original Investigation Cardiovascular Burden of V142I Transthyretin Variant Senthil Selvaraj, MD, MS, MA; Brian Claggett, PhD; Svati H. Shah, MD, MHS; Robert J. Mentz, MD; Michel G. Khouri, MD; Ani W. Manichaikul, PhD; Sadiya S. Khan, MD, MSc; Stephen S. Rich, PhD; Thomas H. Mosley, PhD; Emily B. Levitan, ScD; Pankaj Arora, MD; Parag Goyal, MD, MSc; Bernhard Haring, MD, MPH; Charles B. Eaton, MD, MS; Richard K. Cheng, MD, MSc; Gretchen L. Wells, MD, PhD; JoAnn E. Manson, MD, MPH, DrPH; Marianna Fontana, MD, PhD; Scott D. Solomon, MD JAMA
There has been a transformational change in understanding heart failure due to transthyretin cardiac amyloidosis (ATTR-CA). Previously considered a rare condition, the widespread adaptation of nuclear imaging to establish the diagnosis has led to the recognition that ATTR-CA is commonly encountered in clinical practice. Additionally, the advent of effective disease-modifying therapies that reduce morbidity and mortality among affected patients has afforded hope to those newly diagnosed. 1 Because these disease-modifying therapies are more effective when administered early in the course of the illness, before significant end-organ dysfunction has occurred, it is vital to facilitate early diagnosis.
ATTR-CA results from misfolded transthyretin (TTR) protein and is associated with advancing age (typical onset at age >60 years). Mechanisms underlying misfolding of TTR are incompletely understood, but the process is accelerated by inherited variants of the TTR gene. Accordingly, ATTR-CA is subclassified by the genetic sequence of TTR into (1) normal or wild-type gene sequence (termed ATTRwt-CA , formerly termed senile cardiac amyloidosis ) or (2) variant gene sequence (ATTRv-CA) associated with a single nucleotide change that results in an amino acid substitution that destabilizes TTR and induces misfolding. The most common TTR variant in the US is V142I (also known as Val122Ile or V122I under legacy nomenclature), observed in 3.4% of individuals in the US who self-identify as Black, or approximately 1.5 million people (across all age groups). 1 While larger-scale studies have individually demonstrated an association of the allele with an increased risk for heart failure 2 and reduced survival, 3 analyses examining the association of the cardiac structural changes characteristic of ATTR-CA with observed adverse outcomes have estimated a low clinical penetrance of disease (based on echocardiographic parameters) and no association with mortality. 4 That said, most prior studies were limited by relatively small numbers of V142I carriers, among whom those that survived to advanced age were primarily women. This is an important limitation because observations from large amyloidosis referral programs have demonstrated earlier phenotypic expression of V142I in males and a more severe phenotype. 5 The current study by Selvaraj et al 6 adds meaningfully to knowledge regarding the impact of V142I in the US population.
The authors analyzed 4 large cohorts (Atherosclerosis Risk in Communities, Multi-Ethnic Study of Atherosclerosis, Reasons for Geographic and Racial Differences in Stroke Study, and Women’s Health Initiative) pooling 23 338 self-reported Black participants without prevalent heart failure and found that 754 (3.2%) carried the V142I variant (nearly identical to the known background prevalence). After a mean (SD) follow-up period of 15.5 (8.2) years, V142I carrier status was associated with an increased risk of heart failure and death, confirming findings reported in other publications. 3 But, leveraging the capacity afforded by the larger sample of V142I carriers from the pooled cohorts, the authors were able to demonstrate that the risk of hospitalization for heart failure was predominantly seen in those with an ejection fraction of less than 50% and delineate with precision the age at which the risk for carriers differed from noncarriers for the outcomes of heart failure (63 years) and death (72 years). Notably, while this study was enriched for women derived from the Women’s Health Initiative, there were no sex differences in these estimates. Thus, these data importantly now highlight the impact of the V142I variant among women and men.
Furthermore, the authors demonstrated a continuous reduction in estimated longevity across the spectrum of age, ranging from 1.9 years (95% CI, 0.6-3.1) at age 50 years to 2.8 years (95% CI, 2.0-3.6) at age 81 years. Even at age 50 years, an age previously felt to be before manifestation of clinical penetrance of disease, a 6% reduction (95% CI, 2%-10%) in expected longevity was estimated for V142I carriers. Extrapolating from an estimated US population of V142I allele carriers aged 50 to 95 years (435 851 persons), the authors calculated the total number of years of life lost among untreated carriers to approach 1 million years. It is important to note that carriers were not identified as having ATTR-CA and were untreated with contemporary TTR disease-modifying therapies. Thus, this tremendous gap in years of life lost among those who carry V142I is what can be expected to be ameliorated or even eliminated with early ascertainment and prescription of effective therapies. These data challenge the current approach to counseling V142I carriers as to when to initiate screening for disease penetrance. Previous expert recommendations suggested that evaluation of allele carriers of V142I should commence approximately 10 years before the expected onset of ATTR-CA disease, 7 which is typically at 70 to 75 years in large series. 5 , 8 Because a reduction in longevity was estimated to commence at 50 years, these data suggest that screening should be initiated in at-risk populations at age 50 years.
Efforts to identify at-risk populations for the V142I variant are hindered by numerous barriers to genetic testing in underserved minoritized populations 9 and challenges inherent in the clinical manifestations of the V142I variant. Because V142I is inherited in an autosomal dominant manner (50% risk of inheritance among first-degree relatives), family cascade testing (eg, testing children/first-degree relatives of affected probands) for V142I is a critical but underutilized component of care. Efforts to identify V142I carriers are likely to be of high yield if focused on relatives, especially siblings of affected probands, who are more likely of an age at which risk for ATTR-CA penetrance increases. While there are approximately 130 identified pathological variants in TTR that cause amyloidosis, communication of genetic risk should be less complex to accomplish than in other inherited cardiomyopathies, in part because of the infrequent number of variants of uncertain significance. However, it is challenging to articulate the clinical penetrance of V142I because penetrance varies by the age of ascertainment and by the technique used to diagnose ATTR-CA. Reported studies have cited penetrance as low as 7% based on echocardiographic manifestations 4 to 40% based on nuclear scintigraphy 10 (the current diagnostic standard), with autopsy data suggesting that pathological penetrance is 100% at older than 80 years among V142I heterozygotes. 11 While confirmatory phenotypic data are not available in the current study, we can infer that clinical penetrance likely contributed to the observed excess in risk for morbidity (eg, heart failure) and mortality. Whether more specific diagnostic techniques, such as magnetic resonance imaging or positron emission tomographic imaging with novel amyloid-specific agents, will enhance our understanding of ATTR-CA penetrance is an area of active investigation ( NCT05489549 and NCT05635045 ).
How do we overcome the barriers that impede identification of V142I allele carriers? First, we must develop culturally informed communication strategies to better empower families with information regarding the risks of V142I so that they can make decisions about family cascade testing. Genetic testing for TTR variants is currently available as part of direct-to-consumer genetic tests and recently the American College of Medical Genetics’ Secondary Findings Working Group endorsed the return of results from genomic testing for TTR variants. 12 The working group determined that genes associated with conditions that disproportionately affect racial and ethnic minoritized groups, such as TTR V142I, should have return of results even if they are rare or have lower penetrance in the US population as a whole. Thus, it is imperative that we identify genetic risk variants, such as V142I, that disproportionately affect historically underrepresented populations in an effort to reduce health disparities. Accordingly, because we now have effective treatments for ATTR-CA, it is likewise imperative that we develop communication strategies in a culturally sensitive manner regarding the risks and benefits of family cascade testing for first-degree relatives of V142I carriers. Next, we must ensure adequate representation in clinical trial research of novel ATTR-CA therapeutics to achieve an appropriate diversity of the at-risk population. Indeed, recently completed trials of a TTR stabilizer 13 enrolled a lower percentage of V142I participants than previously conducted clinical trials. 14 The challenge is complex because additional impediments include a lack of knowledgeable clinicians to educate families, poor care coordination, insurance and cost barriers, potential financial impacts of results, cultural norms, low health literacy, concerns about racial profiling and discrimination, concerns about loss of confidentiality, misuse of genetic information, and consideration of family dynamics. We submit that a multidisciplinary approach will be necessary to adequately address these barriers that will require appropriate, multimodal educational materials and policy changes through advocacy that facilitates access for potentially affected patients, many of whom reside in areas remote from centers of excellence in amyloidosis care. 15
In summary, the study by Selvaraj and colleagues 6 provides compelling evidence that the impact of V142I is substantial both to individual carriers and the population as a whole. Now that the problem has been articulated with greater clarity, the steps toward a solution can come into focus. This will require capitalizing on the already strong foundation of collaboration that exists in the amyloidosis community among patients, caregivers, support groups, genetic counselors, clinical experts, basic science and clinical researchers, medical subspecialists, and regulatory agencies.
Corresponding Author: Mathew S. Maurer, MD, Cardiac Amyloidosis Program, Columbia University Irving Medical Center, 622 W 168th St, PH12 Stem Room 134, New York, NY 10032 ( [email protected] ).
Published Online: May 12, 2024. doi:10.1001/jama.2024.2868
Conflict of Interest Disclosures: Dr Maurer reported receiving grants from Pfizer, Attralus, Ionis, Alexion, Prothena, and Ionis and personal fees from Alnylam, AstraZeneca, Akcea, Intellia, and Novo Nordisk. Dr Miller reported receiving grants from Eidos/BioBridge, Pfizer, Alnylam, Argospect, and Siemens and consulting for Pfizer, GE, Roivant, and CSL Behring. Dr Ruberg reported receiving grants from the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI), Pfizer, Akcea Therapeutics/Ionis Pharmaceuticals, and Alnylam Pharmaceuticals and personal fees from AstraZeneca, Attralus, and Alexion.
Funding/Support: This work was supported by NIH/NHLBI (R01-HL139671) to Drs Maurer and Ruberg and NIH/National Institute on Aging (R01-AG081582) to Dr Maurer.
Role of the Funder/Sponsor: The funders had no role in the preparation, review, or approval of the manuscript or the decision to submit the manuscript for publication.
See More About
Maurer MS , Miller EJ , Ruberg FL. Addressing Health Disparities—The Case for Variant Transthyretin Cardiac Amyloidosis Grows Stronger. JAMA. Published online May 12, 2024. doi:10.1001/jama.2024.2868
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Open access
- Published: 05 May 2024
Exoskeleton-based exercises for overground gait and balance rehabilitation in spinal cord injury: a systematic review of dose and dosage parameters
- Patrik Nepomuceno 1 , 2 , 5 ,
- Wagner H. Souza 1 ,
- Maureen Pakosh 1 ,
- Kristin E. Musselman 1 , 3 , 6 &
- B. Catharine Craven 1 , 4 , 5 , 6
Journal of NeuroEngineering and Rehabilitation volume 21 , Article number: 73 ( 2024 ) Cite this article
441 Accesses
2 Altmetric
Metrics details
Exoskeletons are increasingly applied during overground gait and balance rehabilitation following neurological impairment, although optimal parameters for specific indications are yet to be established.
This systematic review aimed to identify dose and dosage of exoskeleton-based therapy protocols for overground locomotor training in spinal cord injury/disease.
A systematic review was conducted in accordance with the Preferred Reporting Items Systematic Reviews and Meta-Analyses guidelines. A literature search was performed using the CINAHL Complete, Embase, Emcare Nursing, Medline ALL, and Web of Science databases. Studies in adults with subacute and/or chronic spinal cord injury/disease were included if they reported (1) dose (e.g., single session duration and total number of sessions) and dosage (e.g., frequency of sessions/week and total duration of intervention) parameters, and (2) at least one gait and/or balance outcome measure.
Of 2,108 studies identified, after removing duplicates and filtering for inclusion, 19 were selected and dose, dosage and efficacy were abstracted. Data revealed a great heterogeneity in dose, dosage, and indications, with overall recommendation of 60-min sessions delivered 3 times a week, for 9 weeks in 27 sessions. Specific protocols were also identified for functional restoration (60-min, 3 times a week, for 8 weeks/24 sessions) and cardiorespiratory rehabilitation (60-min, 3 times a week, for 12 weeks/36 sessions).
This review provides evidence-based best practice recommendations for overground exoskeleton training among individuals with spinal cord injury/disease based on individual therapeutic goals – functional restoration or cardiorespiratory rehabilitation. There is a need for structured exoskeleton clinical translation studies based on standardized methods and common therapeutic outcomes.
Introduction
Over the past decade, lower limb robotic technologies have been increasingly applied in neurorehabilitation [ 1 , 2 ]. Essentially anthropomorphic in concept, these powered mechanical devices are used for locomotor training [ 3 ] and are classified as end-effectors or exoskeletons [ 4 ]. The first one generates movements from the distal segment through a haptic interface [ 5 ], while the latter encompass independent robot joints guided in a pre-programmed trajectory which is further classified as unilateral or bilateral [ 4 ]. Among such technologies, exoskeletons are reportedly useful to promote mobility in individuals with locomotor dysfunction, including those with complete lower extremity paralysis [ 6 ]. Exoskeletons often do so through motorized actuators that assist hip, knee, and ankle motion in dynamic orthoses capable of supporting, stabilizing and reciprocally progressing the lower limbs [ 4 ]. Newer generation devices offer training modes which allow therapists to manually trigger and control steps, in addition to adaptive and variable assistive features for individuals with incomplete injuries and a fair prognosis for voluntary active movement and functional recovery.
More recently, an alternative robotic exoskeleton classification was suggested based on four categories: end-effectors (e.g., Haptic Walker), grounded exoskeletons (e.g., Lokomat), wearable exoskeletons (e.g., Ekso and ReWalk) and soft exoskeletons (e.g., Myosuit) [ 7 ]. These devices seem especially promising as strategies to improve balance and walking abilities [ 8 , 9 ], two of the most frequent goals following subacute or chronic spinal cord injury/disease (SCI/D) [ 7 , 10 ]. The first, characterized by physiologic responses at a cellular level (e.g., glial scars), occurs within a few weeks after the injury [ 11 , 12 , 13 ]. Conversely, the latter is achieved as of 6 months after the injury. In traumatic SCI, the interval between the acute (< 30 days) and chronic (> 6 months) phases has been labelled the intermediate phase [ 14 ].
In terms of motor support, exoskeletons offer different types of assistance including active (equipment performs the movement, partially or totally, through powered assistance to the user); passive (device does not offer powered assistance to the movement, users execute by themselves); active-assisted (offers powered assistance to complete movements initiated by the user); resistive (offers resistance to movements initiated by the user); and interactive (uses feedback to correct movements based on interactions between actuators and control strategies) [ 4 , 7 , 15 ]. Understanding these different levels of assistance is important to account for the variable forms of haptic feedback involved in robotic motor training which can either enhance or degrade motor performance depending on the patient’s impairments and abilities (e.g., novice learners vs. advanced learners, subacute vs. chronic patients, those with autonomic or sensory function, presence or absence of spasticity, etc.) [ 16 ]. Prior publications with these variable assistive devices have shown that gait and balance training with exoskeletons contribute to increased energy expenditure, muscle activation/recruitment and weight bearing [ 17 , 18 , 19 , 20 ], in addition to improved independence and health-related quality of life [ 21 ]. These outcomes are often achieved in response to neurorecovery fostered by functional restoration programs[ 22 ]. Functional Restoration interventions focus on the refinement of sensorimotor function in daily living. That ability is associated with the stimulation of remaining neural connections that even in SCI/D re-enable sensorimotor function following repeated exposure to directed stimuli, hence yielding [ 23 ] greater motor and autonomic recovery [ 23 , 24 ].
Specific to SCI/D, a recent study of exoskeleton-based rehabilitation among individuals with subacute injury reported that exposure to sixteen 30-min sessions of robotic-assisted gait training led to a significant improvement in gait as measured by the Walking Index SCI II (WISCI-II), which translates to more functional gait and activities of daily living [ 25 ]. Moreover, Tamburella et al. [ 26 ] reported that individuals living with SCI/D could walk significantly faster, with longer steps and reduced gait cycles after rehabilitation with a powered exoskeleton. Similarly, Okawara et al. [ 27 ] reported gains in the 10-Meter Walk Test (10MWT), Time Up and Go (TUG) and Berg Balance Scale (BBS) after twenty 60-min sessions of body weight supported treadmill training (BWSTT) with a hybrid-assisted limb system. These results, however, were only observed in SCI/D patients with prior high walking ability as measured by the WISCI-II. In a similar population, Baunsgaard et al. [ 28 ] performed twenty-four 60-min sessions of robotic exoskeleton gait training, which resulted in improvements in the 10MWT, TUG and BBS, however with no treadmill or body weight support. The aforementioned results suggest that individuals living with subacute spinal cord lesions (< 1 year) are most likely to experience therapeutic benefits. However, individuals living with chronic SCI/D may also benefit from these interventions. While neuroplasticity is primarily expected at earlier phases after SCI/D, improvements are still attainable at later stages, specifically in response to coordinated, repeated motor stimuli as fostered by exoskeletons [ 4 , 17 , 28 ].
In response to the growing interest in exoskeletons to enhance the outcomes of neurorehabilitation, particularly in SCI/D, a significant body of literature has been published on associated topics and therapeutic benefits such as cardiovascular function [ 19 ], gait performance and training [ 17 , 19 ], spasticity and pain [ 18 ], device characteristics [ 29 ], cardiorespiratory function and fatigue [ 30 ]. Although the aforementioned evidence is based on structured rehabilitation protocols, little emphasis has been given to discussing dose and dosage parameters of the exercises used in the respective therapeutic protocols beyond feasibility, safety and the specific outcomes observed. Additionally, interventions using powered exoskeleton-based rehabilitation for gait and balance were reportedly delivered under widely variable designs [ 31 , 32 , 33 , 34 ]. Although dose and dosage parameters were reported by previous systematic review authors in adults with SCI/D who underwent lower limb powered exoskeleton rehabilitation for overground gait and balance, most did not discuss these training parameters. Instead, most authors acknowledged the absence of best practice recommendations in the field and endorsed the need to further understand rehabilitation designs aimed to restore or maintain locomotion with powered exoskeletons [ 7 , 15 , 21 , 35 ].
This systematic review addresses two main questions: (1) To what extent are dose (e.g., single session duration, and total number of sessions) and dosage (e.g., frequency of sessions per week, and total duration of the intervention) of exoskeleton-based exercises reported in the literature on overground gait and balance rehabilitation for adults with SCI/D (subacute or chronic, complete or incomplete)?; and, (2) Which outcome measures are used to inform changes in gait and balance following exoskeleton-based rehabilitation in SCI/D? We hypothesized that the investigation of dose and dosage parameters of exoskeleton-based exercises reported from interventions for overground gait and balance rehabilitation interventions among individuals with SCI/D would contribute to: (1) the identification of consistent dose and dosage parameters to inform best practice recommendations related to locomotor rehabilitation strategies; and, (2) informing the development of innovative, clinically robust protocols evaluating exoskeletons for SCI/D rehabilitation; and, (3) to driving implementation of exoskeleton based training programs within tertiary SCI/D rehabilitation settings.
This systematic review was conducted in accordance with the Preferred Reporting Items Systematic Reviews and Meta-Analyses (PRISMA) guidelines [ 36 ] and registered in the International Prospective Register of Systematic Reviews (PROSPERO) under the number CRD42022319271.
Search strategy and data sources
The search strategy was co-developed by the authors in collaboration with a local Medical Librarian and Information Specialist (MP) using the concepts contained in the PICO framework encompassing P opulation, I ntervention, C omparisons, and O utcomes. Valid subject headings for each database were utilized as appropriate, as were free text terms pertinent to each topic or concept (e.g., Spinal Cord Injuries; Paraplegia; Quadriplegia; Exoskeleton Device; Gait; Postural Balance). The search was performed from inception to 31 March 2022 using five electronic databases: CINAHL Complete (EBSCOhost), Embase (Ovid), Emcare Nursing (Ovid), Medline ALL (Ovid; includes PubMed non-Medline records), and the Web of Science Core Collection. Each concept searched was kept as broad as possible to ensure all relevant materials were identified. The Population encompassed adults with Spinal Cord Injuries. The Intervention was the use of Exoskeletons. The Outcomes included any biomechanical and/or clinical measures related to Gait or Balance. No date or language limits were applied. The full Medline search strategy is shown in Additional file 1 .
Study selection criteria
Studies were included according to the following criteria:
Participants: adults regardless of sex/gender identity (≥ 16 years of age) with subacute/chronic (≥ 30 days post injury onset) complete or incomplete SCI/D of traumatic or non-traumatic etiology; and any neurological level of injury (C1-L4 ASIA Impairment Scale A-D).
Intervention/Exposure: overground gait and balance rehabilitation with a lower limb powered exoskeleton – an anthropomorphic device worn by the participants for orthostatic passive or active (facilitated) motor training [ 3 ].
Comparison: no specific rehabilitation strategy was specified for comparison.
Outcomes: studies which included at least 3 of 4 parameters of dose (e.g., single session duration, and total number of sessions) and dosage (e.g., frequency of sessions per week, and total duration of the intervention) of exoskeleton-based exercises; and at least one measure of gait and/or balance (e.g., Mini-Balance Evaluation Systems Test, Community Balance & Mobility Scale, ABC Scale, 6-min walk test (6MWT), 10MWT or other measure of gait speed, BBS, TUG).
Publication type: Experimental studies with more than five participants in randomized clinical trials, quasi-randomized clinical trials, prospective controlled trials, pre-post studies, cross-sectional, crossover and quasi-experimental studies. Studies with mixed populations (e.g., children and adults) or mixed impairments (e. g., SCI/D, stroke, multiple sclerosis), were included when outcome separation was possible. Only peer-reviewed articles were included. Reasons for exclusion included: literature reviews, qualitative studies, case series (n < 5), grey literature (i.e., letters, editorial, white papers), studies with end-effector or grounded systems, equipment design and development studies, and with gait training carried over specialized surfaces (e.g., treadmill). The inclusion and exclusion criteria are listed in the Table 1 .
Screening criteria and study selection
After the initial search, duplicate manuscripts were excluded, and remaining references were imported into the Covidence Systematic Review Manager (Veritas Health Innovation Ltd, Australia). Articles eligible for title and abstract screening were assessed by PN and WHS independently (a third author, KEM, was assigned to resolve eventual conflicts). Prior to working independently, an initial fidelity agreement regarding the article inclusion/exclusion process was established based on the first 10 studies with a 100% agreement between raters. If titles and abstracts did not report enough information to determine article inclusion or exclusion, the full text was screened. Following the title and abstract screening, remaining citations were independently read in full by the same two authors to verify articles met inclusion criteria. Again, disagreements were resolved by the same third author.
Data charting and analysis
The authors created individual versions of a data extraction form. Their forms were compared and merged into a combined form used to abstract data from the included manuscripts. The data extraction form was pilot tested by two authors (PN and WHS), who independently extracted data from two of the included manuscripts. Following a comparison of the outcomes obtained, minor revisions were implemented towards a final, revised version of the abstraction form.
Data were extracted from the selected papers about authors; year of publication; institution and country of the study; participant demographics (age, number of participants, etiology and level of lesion,); dose (e.g., total number of sessions, and duration of each session, in minutes) and dosage (e.g., frequency of sessions per week, and duration of the complete intervention, in weeks); gait and balance outcomes measures (e.g., Mini-Balance Evaluation Systems Test, Community Balance & Mobility Scale, ABC Scale, 6MWT, 10MWT, BBS, TUG, gait speed). The data were synthesised by the authors and reported in tables and graphics. Narrative syntheses were applied.
In the case of articles with missing data (e.g., total duration of intervention), the corresponding author was contacted by e-mail. For some studies included, dose and dosage parameters were not explicitly stated, but could be estimated using available training parameters in the published article. For instance, sessions per week multiplied by the number of intervention weeks informed the total number of sessions; total number of sessions divided by weeks informed weekly frequency; and total number of sessions divided by sessions per week informed the duration of the intervention. For parameters indicated as best practice recommendations, only studies that reported statistically significant improvements (p < 0.05) and/or improvements equal or greater than the minimal clinically important difference (MCID) were considered. The MCID was observed for the 6MWT, 10MWT and TUG, with the following thresholds: 36 m [ 37 ] or 0.1 m/s [ 38 ], 0.13 m/s [ 39 ], and 10.8 s [ 40 ], respectively. For the cardiorespiratory outcomes, no MCID was set, and only statistically significant improvements (p < 0.05) were considered. Conversely, studies with dramatically large variability within the reported protocol (e.g., participants exposed to a different total number of sessions from 12 to 102, duration of intervention from 4 to 34 weeks) were excluded from the average calculation. As for studies with small variability within the protocol, the mean of the total range (e.g., weekly frequency from 4 to 5, was considered as 4.5; duration of each session from 60 to 90-min, was considered 75-min) were computed. Data regarding dose and dosage parameters were reported as mean and standard deviation (normal distribution) or median and interquartile range (non-normal distribution), to determine distribution the Shapiro–Wilk Test was used considering p < 0.05 as non-normal distribution.
The initial electronic database search identified 2,108 references. After removing the duplicates, 977 references were screened for titles and abstracts. At full text screening, 69 articles were revised (Fig. 1 ). Nineteen (n = 19) full text articles were included in the review with a total of 288 participants (214 male) who underwent exoskeleton gait and/or balance training. Five (n = 5) studies had control/comparison groups treated with conventional physical therapy (n = 2) [ 41 , 42 ], Lokomat gait training (n = 1) [ 43 ], BWSTT or no intervention (n = 1) [ 44 ] and BWSTT with overground gait training with functional electrical stimulation (FES) (n = 1) [ 45 ]. One (n = 1) study had a comparison group of individuals with acute SCI/D who underwent the same exoskeleton protocol [ 46 ]. As for the geographical distribution of study sites, five (n = 5) were developed in the United States [ 6 , 33 , 44 , 47 , 48 ], four (n = 4) in Italy [ 43 , 45 , 49 , 50 ], two (n = 2) in Canada [ 51 , 52 ], two (n = 2) in China [ 41 , 53 ] and two (n = 2) in Korea [ 54 , 55 ], one (n = 1) in France [ 56 ], one (n = 1) in Japan [ 46 ], one (n = 1) in South Africa [ 42 ], and one (n = 1) from a 7 site (Denmark, Germany, the Netherlands, Norway, Spain, Sweden and Switzerland) multicenter study in Europe [ 28 ], Fig. 2 displays the countries of origin for 18 studies, except for the multicenter study in Europe, which is the most active region investigating overground exoskeletons training for gait and balance rehabilitation among individuals with SCI/D. Six (n = 6) studies were partially or totally supported by the industry manufacturer, including equipment loan [ 6 , 57 ], trial funding [ 28 , 44 , 56 ] and employees collaborating in manuscript production [ 54 ].
PRISMA flow diagram
Frequency of study per country. Figure represents the country of origin of 18 of the 19 studies included because 1 study was a multicenter study across Europe
The refined dataset included articles describing participants with subacute (1 to 5 months post-injury) or chronic (> 6- or 12-months post-injury) SCI/D. Thirteen (n = 13) studies investigated chronic SCI/D (> 6 months [ 46 , 51 ], > 12 months [ 6 , 42 , 43 , 44 , 48 , 52 , 54 , 55 , 56 , 57 ], stated it is chronic but did not report time since injury[ 50 ]), one study investigated subacute participants (from 1 to 11 months) [ 41 ] and five studies investigated both subacute and chronic participants [ 28 , 45 , 47 , 49 , 53 ]. The participant’s age ranged from 16 to 78 years, although one study included one participant that was 15 years old, however this paper was not included in our best practice recommendation because the authors did not find significant changes. That study, however, had a mean participant age of 41.3 years [ 53 ]. Regarding the etiology of the injury, nine (n = 9) studies included individuals with SCI/D of traumatic and non-traumatic etiology (four chronic [ 6 , 43 , 44 , 51 ], four chronic and subacute [ 28 , 45 , 53 , 57 ], one subacute only [ 41 ]). Five (n = 5) studies only included individuals with traumatic lesions (four chronic [ 42 , 48 , 52 , 56 ] and one subacute and chronic [ 49 ]). One (n = 1) study focused on chronic non-traumatic participants [ 46 ]. Four (n = 4) studies did not report the etiology (three chronic [ 50 , 54 , 55 ], one chronic and one subacute [ 47 ]). As for the extent of injury, twelve (n = 12) studies were conducted in individuals with complete or incomplete SCI/D [ 28 , 41 , 43 , 45 , 47 , 48 , 50 , 51 , 52 , 53 , 54 , 57 ], four (n = 4) studies in complete SCI/D only [ 6 , 49 , 55 , 56 ] and two (n = 2) studies in incomplete SCI/D [ 42 , 44 ]. One study did not report the extent of participant injury [ 46 ]. Relative to the level of injury, one (n = 1) study included individuals with cervical lesions [ 42 ], four (n = 4) included individuals with thoracic lesions [ 6 , 48 , 55 , 56 ], four (n = 4) included individuals with cervical or thoracic lesions [ 43 , 51 , 52 , 57 ], two (n = 2) included cervical, thoracic, or lumbar [ 44 , 54 ], and six (n = 6) studies included thoracic or lumbar injuries [ 41 , 45 , 47 , 49 , 50 , 53 ]. Two (n = 2) studies did not report the level of injury [ 28 , 46 ]. A summary of participants’ characteristics and the exoskeleton device with their respective study protocols are shown in Table 2 .
Exoskeleton training dose, dosage, and outcome measures
The 19 studies included devices from seven different exoskeleton manufacturers. Seven (n = 7) studies used Ekso devices [ 28 , 42 , 43 , 44 , 45 , 50 , 51 ], six (n = 6) used ReWalk [ 6 , 48 , 49 , 52 , 55 , 57 ], two (n = 2) used AIDER [ 41 , 53 ], one (n = 1) used Indego [ 47 ], one (n = 1) H-MEX[ 54 ], one (n = 1) Hybrid Assistive Limb (HAL) [ 46 ] and one (n = 1) Atalante [ 56 ]. In 15 studies, the rehabilitation protocol included only exoskeleton gait and/or balance training [ 6 , 28 , 41 , 42 , 43 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 56 , 57 ]. Four studies included exoskeleton training associated with overground walking without body weight supported (BWS) [ 44 ], FES cycling [ 45 ], BWS [ 46 ], or knee-ankle–foot orthosis (KAFO) gait training [ 55 ]. In respect to the dose and dosage parameters, the total number of sessions reported ranged from 10 to 102 sessions. The number of sessions per week varied from 2 to 5 sessions. The duration of the total intervention ranged from 2 to 34 weeks. The duration of each gait and balance exoskeleton gait training varied from 30 to 90-min (one paper did not report [ 28 ]). The most frequent dose and dosage parameters were: 60-min sessions [ 42 , 43 , 46 , 48 , 49 , 51 , 52 , 54 , 56 ], 3 sessions a week [ 6 , 28 , 42 , 44 , 45 , 47 , 48 , 49 , 51 , 54 , 57 ], over 8 to 12 weeks [ 6 , 28 , 43 , 44 , 47 , 49 , 52 , 54 ], for a total of 20–40 sessions [ 6 , 28 , 44 , 45 , 47 , 49 , 50 , 54 , 55 ].
Overall, considering the dose and dosage parameter averages across all studies included in this review, regardless of clinically relevant change, a protocol with 60-min individual sessions, 3 times a week, for 9 weeks is suggested for a total of 27 sessions. The mean and standard deviation, or median and interquartile range for overall interventions and for protocols focused on specific therapeutic intent (e.g., functional restoration or cardiorespiratory rehabilitation) are described in Table 3 . As for the total number of sessions and the duration of interventions recommended, most studies showed variability within a range of (24–36 sessions) and (8–12 weeks), respectively [ 6 , 28 , 44 , 47 , 49 , 51 , 52 , 54 ]. Also, the duration of each session (60-min) and weekly frequency (3 times a week) were mostly consistent across the reviewed dataset, including studies with clinically relevant changes [ 6 , 28 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 51 , 52 , 54 , 56 ].
The gait and balance outcome measures used include: the 6MWT [ 6 , 41 , 42 , 44 , 45 , 47 , 48 , 49 , 50 , 52 , 53 , 54 , 55 , 57 ], 10MWT [ 6 , 28 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 56 , 57 ], TUG [ 28 , 44 , 45 , 47 , 50 , 57 ], WISCI-II [ 28 , 44 , 45 , 53 ], gait speed [ 43 , 46 , 50 , 52 ], steps taken [ 46 , 51 , 52 ], BBS [ 28 ], step length [ 46 ], stride length [ 50 ], Hoffer Walking Ability [ 53 ], and one paper adapted the 6MWT to 30-min walk test to evaluate gait function during 30-min [ 55 ], the frequency of the gait and balance outcomes across the studies is indicated in the Fig. 3 . Other non-gait related measures reported as main outcomes across different studies were categorized as either cardiorespiratory or physiologic outcomes and are listed in Fig. 3 .
Frequency of clinical outcomes reported. Heat map presenting the frequency of clinical outcomes measures reported, per studies by manufacturer. %HRR percentage of heart rate reserve, CO2 carbon dioxide, FEF forced expiratory flow, FEV1 forced expiratory volume in 1 s; LEMS Lower Extremities Motor Score, MVV maximum voluntary ventilation, NASA-TLX NASA Task Load Index, NBD neurogenic bladder dysfunction, PCI Physiological Cost Index, PEF peak expiratory flow, PGI-I Patient Global Impression of Improvement; Resp. respiratory, RPE rating of perceived exertion, SCATS Spinal Cord Assessment Tool for Spastic Reflexes, SCIM-II Spinal Cord Independence Measure II, UEMS Upper Extremities Motor Score, VAS Visual Analogue Scale, WISCI-II Walking Index for Spinal Cord Injury II
Protocol therapeutic intent
The studies included in this systematic review of overground exoskeleton training dose and dosage were classified in two groups according to the inferred therapeutic intent based on the described study design which addressed: functional restoration [ 6 , 44 , 45 , 46 , 47 , 49 , 50 , 51 , 52 , 53 , 56 , 57 , 58 ] or cardiorespiratory rehabilitation [ 41 , 42 , 43 , 48 , 54 , 55 ]. The therapeutic intent was determined based on each study’s primary research question, aim and main outcome measures in reference to motor (gait or balance) or cardiorespiratory performance, respectively. Although in recent years changes in body composition (e.g., muscle and bone mineral density) have been increasingly associated with exoskeleton training [ 31 , 59 , 60 , 61 ], none of the studies included in this review focused on anatomical adaptations in response to overground exoskeleton training.
Functional restoration
Thirteen (n = 13) studies focused on functional restoration [ 6 , 44 , 45 , 46 , 47 , 49 , 50 , 51 , 52 , 53 , 56 , 57 , 58 ]. Of those, eleven reported statistically significant improvements and/or showed improvements equal or higher than the MCID for gait and/or balance outcome measures [ 6 , 28 , 44 , 45 , 46 , 47 , 49 , 50 , 51 , 52 , 56 ]. Table 4 summarizes the individual studies’ aims and main results. The functional restoration protocols ranged from 10 to 51.5 sessions, 2 to 5 sessions a week, 3 to 12 weeks of duration for 45- to 90-min. Considering the studies with significant motor improvement (n = 11), it is suggested that a protocol aimed towards functional restoration would encompass 60-min individual sessions carried 3 times a week, over 8 weeks for a total of 24 sessions (Table 3 ).
Functional restoration interventions were shorter than cardiorespiratory interventions. They included subacute or chronic SCI/D patients, mostly with complete or incomplete thoracolumbar lesions. In this group analysis, two manuscripts did not report improvements [ 53 , 57 ]. The first one [ 53 ] reported the effects of a new robotic exoskeleton based on ten 30-min sessions over 2 weeks, that is shorter than the period suggested by our recommendation based on studies with significant functional restoration gains. The second study [ 57 ] focused on describing the protocol performed in a rehabilitation research institute, including the process of participant recruitment, fitting, donning, standing, standing balance, walking, mobility training, sitting and doffing. The functional outcomes, however, were measured only after the intervention. Additionally, among the respective study participants, individuals underwent 12 to 102 sessions over 4 to 34 weeks in remarkably variable study designs.
Regarding the therapeutic content, studies on functional restoration mainly focused on sit to stand, and stand to sit transitions, standing balance and walking training for significant changes or improvements above the MCID as per functional restoration outcome measures. The frequency of training, total number of trainings, therapy content (exercise training) and studies with significant changes are shown in Table 5 .A.
Cardiorespiratory rehabilitation
The six studies (n = 6) focused on cardiorespiratory rehabilitation [ 41 , 42 , 43 , 48 , 54 , 55 ] showed significant improvement of cardiorespiratory function. Table 6 summarizes the cardiorespiratory studies’ aims and main results. Cardiorespiratory-centered interventions ranged from 16 to 72 sessions, 2 to 5 sessions weekly, for 4 to 24 weeks. Individual sessions lasted between 55 to 90 min. Because the six protocols yielded significant improvement in cardiorespiratory function, it is suggested that interventions to that end are likely to succeed when based on 60-min sessions carried 3 times a week for 12 weeks in a total of 36 sessions (Table 3 ).
Unexpectedly, protocols focusing on cardiorespiratory outcomes were longer in average than protocols for functional restoration. Conversely, four of the referred articles also reported significant improvements in gait and balance measures [ 42 , 48 , 54 , 55 ], while two manuscripts reported improvements in cardiorespiratory outcomes alone [ 41 , 43 ]. The latter studies were based on 16 sessions over 4 weeks [ 41 ] and 17 sessions over 9 weeks [ 43 ], indicating that shorter interventions could be enough to improve cardiorespiratory function alone, that is uncoupled from significant functional restoration. In this case, the cardiorespiratory recommendation would include 60-min sessions carried out 3 times a week for 6 weeks for a total of 18 sessions.
Regarding the therapeutic content, studies on cardiorespiratory rehabilitation mainly focused on walking training and sit to stand and/or stand to sit transitions for significant improvements in cardiorespiratory outcome measures. The frequency of training, total number of trainings, therapy content and studies with significant changes in cardiorespiratory parameters are shown in Table 5 .B.
This review aimed to identify the dose and dosage parameters of exoskeleton-based exercises for overground gait and balance training in individuals with SCI/D. Although previous studies have discussed this topic in different neurological populations [ 7 , 62 ], to the best of our knowledge, this is the first review to prioritize the investigation and discussion of dose and dosage of overground exoskeleton therapy among individuals with SCI/D – a need repeatedly acknowledged in recent literature [ 7 , 15 , 21 , 35 ] yet widely overlooked as a primary research topic. We have summarized evidence from 19 manuscripts to determine current training parameters for specific therapeutic indications to inform best practice recommendations in exoskeleton-based SCI/D rehabilitation. Of 19 manuscripts, seventeen [ 6 , 28 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 54 , 55 , 56 ] reported statistically significant improvements and/or gains above the MCID in the gait, balance, cardiorespiratory and/or related physiological outcomes they assessed. The evidence gathered supports the assumption that exoskeletons are a promising therapeutic tool in SCI/D, particularly for functional restoration [ 6 , 28 , 44 , 45 , 46 , 47 , 49 , 50 , 51 , 52 , 56 ] and/or cardiorespiratory improvement [ 41 , 42 , 43 , 48 , 54 , 55 ].
Protocol design
Based on strict adherence to the systematic review inclusion criteria, several manuscripts initially screened did not fully report dose (total number of sessions, and duration of the session) and dosage (frequency per week, and duration of the intervention) parameters and were excluded. Consistent with previous reviews [ 35 , 62 ] on exoskeleton-based gait rehabilitation the lack of dose and dosage parameters ultimately limits the replication and generalizability of the outcomes reported. The absence of dose and dosage information also limits the translation of findings to evidence-based clinical practice, whereas the requirement for routine universal reports of dosing parameters in future studies would foster knowledge dissemination and implementation of precision rehabilitation approaches in the field. To support the development of future studies with structured information for better clinical translation, a checklist for reporting exoskeleton therapy is proposed in Table 7 .
We observed considerable inconsistency of protocols for exoskeleton-based gait and balance training, with substantial variability in dose and dosage parameters used. Indeed, the protocols ranged from 10 [ 46 ] to 102 [ 57 ] sessions over 2 to 34 weeks, two [ 53 ] to five [ 55 ] times a week, with individual sessions lasting from 30 [ 53 ] to 90 min [ 47 ]. Further, the systematic review results indicated that the weekly frequency and session duration are the most consistent parameters, with most protocols reporting 3 sessions a week [ 6 , 28 , 42 , 44 , 45 , 47 , 48 , 49 , 51 , 54 , 57 ] at an average of 60 min per session [ 42 , 43 , 46 , 48 , 49 , 51 , 52 , 54 , 56 ]. Another important variable across studies was the device used and the exoskeleton manufacturer, with Ekso (n = 7) [ 28 , 42 , 43 , 44 , 45 , 50 , 51 ] and ReWalk (n = 6) [ 6 , 48 , 49 , 52 , 55 , 57 ] being the most used devices.
Protocol effectiveness
Training effectiveness (e.g., changes in assessment values at or above the MCID) is shaped by multiple factors beyond dose and dosage, including but not limited to device parameters and the extent or intensity of training. Relative to exoskeleton-based gait and balance rehabilitation such factors include device assistance and resistance levels, different walking patterns (e.g., step and stride length, width of base of support, gait speed and step cadence) as well as exercise intensity. The latter is associated with a lack of specific consensus-based measures and definitions universally adopted by experts in the field of neurorehabilitation [ 63 ]. This is particularly true in the SCI/D populations among whom there is substantial heterogeneity in neurological impairment, and associated variability in prognosis and responsiveness to exoskeleton interventions. As a result, variability in prognosis and responsiveness are commonly observed and personalized prescriptions are provided in the absence of consensus-based terminology and practices [ 64 , 65 ]. In this context, the best practice recommendations derived from this systematic review are valid given the reporting of whether the participants achieved a clinically meaningful change in function/assessment parameter based on the dose and dosage reported despite the lack of data specifying exercise intensity.
Despite protocol variability, including that of device choice, therapeutic intent, and training intensity, it is possible that the significant changes reported are associated with the repeated exposure to active standing time versus non-active sitting time [ 65 ]. However, most studies included similar functional therapeutic activities (e.g., sit to stand transitions, standing and balance training and walking training). In fact, exercise intensity in robotic rehabilitation, although not standardized, is often associated with the number of repetitions (e.g., step count), step frequency and total walking distance. The modulation of intensity on a case-by-case basis likely favored the observed performance improvements across the multiple protocol designs reported in this review. Future studies reporting the therapeutic benefits of exoskeleton therapy should include the therapeutic indication, device choice and parameters, exercise intensity, and the dose and dosage parameters as means to improve precision rehabilitation – particularly among people living with a spinal cord impairment and multimorbidity [ 66 ].
Injury characteristics
In addition to exercise parameters, the influence of injury characteristics on exoskeleton-based SCI/D rehabilitation is very likely, yet controversial. Benson et al. [ 67 ] reported that individuals with complete injuries showed greater improvement in walking speed than incomplete injured pairs. That may be because participants with incomplete lesions were functional walkers before the beginning of their training, benefiting mostly from the ability to walk longer distances with exoskeletons as opposed to participants with complete injuries to whom exoskeletons allowed not only orthostatism, but gait initiation and speed improvements. In agreement with those findings, Xiang et al. [ 53 ] reported that individuals with higher spinal lesions and motor complete injuries showed greater improvement in gait and functional outcomes (gait speed and 6MWT) while using exoskeletons compared to people who were functional walkers with lower and or incomplete lesions. Conversely, it has been reported that adults living with lower neurological level of injury (complete versus incomplete) can achieve significantly faster walking speeds following exoskeleton training [ 6 , 68 , 69 ]. The explanation of these findings may be linked to the fact that people with complete SCI/D obtain more remarkable gains with training (e.g., from no standing to walking), although they still walk slower than individuals with incomplete lesions [ 53 , 67 ]. Differences in gait speed is possibly associated with the remaining neural pathways in individuals with incomplete lesions, which foster better neurorecovery in response to functional restoration strategies [ 2 ]. This assumption agrees with Louie et al.’s [ 17 ] report that walking speed with exoskeletons is positively correlated with the level of spinal injury (coded from 0 (cervical) to 17 (lumbar)) and training duration. Thus, lower injuries and longer training could, favor greater locomotor gains for individuals with SCI/D. Nevertheless, Sale et al. [ 50 ] reported that exoskeleton rehabilitation is safe and feasible across a heterogeneous sample of persons with SCI/D provided it is tailored to their personal needs. Further, it is plausible that there may be additional therapeutic benefits of longitudinal training not addressed in this review.
Exoskeleton-based therapeutic intent and physiological considerations
Upon review of the nineteen manuscripts included, consistent similarities across some of the protocols in terms of their therapeutic goals led us to classify the studies in two categories of therapeutic intent (e.g., functional restoration and cardiorespiratory rehabilitation). While the clinical purpose of individual studies seemed distinguishable enough for us to categorize them, that was not explicitly disclosed by the authors.
The current knowledge of the physiological mechanisms involved in exoskeleton-based therapies remains limited. A prior review reported that neurophysiological responses in exoskeleton recovery are linked to the exploitation of neuroplasticity, sensory stimulation, and coordination of limb and muscle activation during the training. The authors purport that functional restoration and neurorecovery are much like a relearning process where preserved sensorimotor and neural circuits are engaged to promote recovery [ 2 ]. For cardiorespiratory function, exoskeleton gait training’s rationale for the observed improvements in function associated with stimulation of the cardiorespiratory system and activation of the lower limbs is due to an increase in metabolic rate indicating this is an effective way of increasing energy expenditure with consequent improvements of cardiorespiratory fitness. Moreover, exoskeleton training contributes to the augmentation of end-systolic and end-diastolic volume, cardiac output, ventricular mass and reduces heart rate following cardiovascular conditioning [ 42 , 54 , 70 ].
Our findings suggest that different exercise exposures are needed to achieve MCID as per therapeutic intent in SCI/D rehabilitation, with cardiorespiratory changes demanding longer protocols compared to functional restoration. Nevertheless, we hypothesized that shorter interventions would be warranted for cardiorespiratory gains due to faster cardiovascular adaptation to structured exercises compared to neurological responses [ 71 , 72 ]. This unexpected outcome may be related to two cardiorespiratory-focused manuscripts in which participants underwent longer interventions (72 [ 42 ] and 60 [ 48 ] sessions) to evaluate changes over the time (early, mid and late changes), justifying the longer experimental designs. Additionally, of the six studies [ 41 , 42 , 43 , 48 , 54 , 55 ] included in cardiorespiratory rehabilitation, two [ 41 , 43 ] had significant improvement in cardiorespiratory function but not in gait, which was achieved with shorter interventions, in line with our initial hypothesis. Supporting our hypothesis, Faulkner et al. [ 73 ] reported that exoskeleton gait training associated with conventional physiotherapy in 5 sessions over a single week improved cardiovascular health, by reducing the augmentation index and mean arterial pressure. Further, Evans et al. [ 42 ] reported statistically significant increases in cardiovascular efficiency as early as 6 weeks after exoskeleton gait training. Interestingly, despite protocol duration variability, the six articles focused on cardiorespiratory training reported significant improvements in cardiorespiratory health as per increased oxygen consumption, heart rate and metabolic equivalent, in addition to reduced perception of effort and oxygen cost [ 41 , 42 , 43 , 48 , 54 , 55 ]. A prior systematic review reported that exoskeleton gait training elevates the energy expenditure, while allowing participants to exercise at moderate intensity, further indicating exoskeletons are beneficial for cardiorespiratory training [ 19 ].
In SCI/D, reduced lower-limb weight bearing and other health complications contribute to the loss of muscle mass and bone mineral density (BMD), specially below the level of injury [ 74 ]. This leads to an increased risk of fragility fractures, which should be accounted for when performing exoskeleton-based gait training. That is important due to previous reports of lower limb fragility fracture after exoskeleton use, mainly induced by the effect of gravity and pressure points created by the resistance of the equipment against the user’s body [ 75 , 76 ]. Thus, people living with SCI/D should be advised of their fracture risk, prior to using wearable exoskeletons for increased safety, regional improvements in bone strength and BMD [ 59 ]. To prevent fragility fractures, Bass et al. [ 59 ] developed a volume and progression algorithm based on BMD thresholds. Accordingly, individuals with osteoporotic profile (T-score ≤ -2.5) should be exposed to a slow-progression program, individuals with osteopenic profile (-2.5 < T-Score < -1.0) should start with moderate-progression and individuals with preserved BMD profile (T-Score ≥ -1.0) should be enrolled in a fast-progression walking program. It is worth noting that as per the position statement 4 in the International Society for Clinical Densitometry, there is no established threshold BMD value below which weight-bearing activities are absolutely contra-indicated, and that BMD and clinical risk factors should be used together on a case-to-case basis to assess risk exposure [ 74 ]. Furthermore, people living with SCI/D are in a higher risk of developing skin abrasions and tissue injury [ 77 ]. Many studies have reported skin abrasions after the use of exoskeleton in SCI/D population [ 44 , 53 , 56 , 58 ]. The reduction of physical activity levels, immobilization, changes in circulation and microcirculation, sensory loss, skin compression due to positioning and impaired venous return are aspects of injury that preclude individuals to lower extremity abrasions [ 77 , 78 ]. Also, participants with sensory impairment are at greater risk of developing skin lesions [ 79 ], and hence warrant ongoing screening for skin integrity. That is particularly true at points of higher pressure caused the interface between the skin and the exoskeleton [ 44 , 58 , 79 ].
Considerations for translation to practice
Recommendations from systematic reviews are extremely helpful at informing new research designs and guiding the translation of optimal evidence-based findings to clinical practice. However, it is also true that best practice recommendations, as identified by this review cannot always be implemented, particularly considering contextual disparities, including different countries (e.g., North America, Europe and Asia, Fig. 2 ), devices and therapeutic intent. Should a clinician find the implementation of the suggested best practice recommendations infeasible, reproducing the observed dose and dosage of therapy with a specific device can be limited to the shortest study with reported clinical effectiveness above the MCID for the outcome of interest (see the reduced dose and dosage but observed MCID with specific interventions on Table 5 ). For instance, ten 60-min sessions at a frequency of 5 sessions per week over two weeks yielded significant improvements in functional restoration [ 46 ]. Alternatively, sixteen 50–60-min sessions at a frequency of 4 times a week over four weeks yielded significant improvements in cardiorespiratory function [ 41 ]. We also suggest that patients be supported to work incrementally with healthcare providers to further implement best practice dose and dosage recommendations.
Study limitations
This study has limitations that include the relatively scarce literature available, which did not allow us to analyze the results according to the participant’s characteristics (sex, ASIA Impairment Scale, neurologic level of injury, etc.). However, the population described in this review are similar to those described in prior reviews among individuals living with SCI/D [ 17 , 62 ]. Also, it is important to state that the implementation of exoskeleton-based interventions is still limited due to the cost, availability of the equipment, equipment specifications and limitations, and the lack of highly trained staff to support exoskeleton-based therapy [ 80 , 81 , 82 ]. As for the limited study sample size, our search was broadened to identify manuscripts applying overground exoskeletons in SCI/D, but many of the identified references did not fully report dose and dosage – that is at least 3 parameters – and were excluded in a strategy that reduced the already restricted sample, but guaranteed data consistency. Additionally, the references included in this systematic review were classified according to their clinical intent by the review authors, which may not reflect the original authors’ intent. Furthermore, the study quality and risk of bias were not assessed as our search aimed to perform a comprehensive overview of dose and dosage in exoskeleton gait and balance training in SCI/D. Nevertheless, this systematic review is consistent with prior reports in the literature that did not report risk of bias in studies involving exoskeleton rehabilitation [ 7 , 21 , 35 , 62 ]. The exoskeleton device donning and doffing times were inconsistently reported across the reviewed studies, with only two of them [ 6 , 44 ] indicating that donning and doffing times were not part of the reported session duration and a single study [ 43 ] indicating that the session duration included donning and doffing. While we believe that some of the other sixteen studies included donning and doffing times in the session duration, we presume that most studies reported the time dedicated to standing/walking training apart from donning and doffing. Altogether, we encourage readers to implement the enclosed practice recommendations and to report device donning and doffing times, device parameters and therapeutic intensity in future reports. We also encourage clinicians and investigators to describe barriers and facilitators to implementation of best practices in different contexts.
Conclusions
In summary, this systematic review advances the understanding of overground exoskeleton-based gait and balance training in SCI/D and its role in facilitating functional recovery and or cardiorespiratory fitness. The review results provide evidence-based clinical practice recommendations, which are tailored to the therapeutic intent of the intervention. However, problems with inconsistent reporting of exoskeleton training dose and dosage and the heterogeneity of study designs among adults with SCI/D preclude fulsome dissemination of data and are acknowledged as important limitations. To advance the field of exoskeleton rehabilitation in SCI/D and increase research quality, there is an urgent need to standardize clinical practice recommendations and guidelines through well-structured studies with clear indications of their therapeutic intent. Finally, we highlight the need for multicentre studies, which could validate the therapeutic effectiveness of specific dose and dosage parameters for optimal gait and balance rehabilitation among adults with SCI/D based on poling of data from multiple sites and contexts.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
10-Meter Walk Test
6-Minute walk test
Berg Balance Scale
Bone mineral density
Body weight supported
Body weight supported treadmill training
Functional electrical stimulation
Hybrid Assistive Limb
Knee-ankle–foot orthosis
Minimal clinically important difference
Preferred Reporting Items Systematic Reviews and Meta-Analyses
International Prospective Register of Systematic Reviews
Spinal cord injury/disease
Time Up and Go
Walking Index SCI II
Reinkensmeyer DJ, Dietz V. Neurorehabilitation technology. New York: Springer; 2016.
Book Google Scholar
Gassert R, Dietz V. Rehabilitation robots for the treatment of sensorimotor deficits: a neurophysiological perspective. J Neuroeng Rehabil. 2018;15(1):46.
Article PubMed PubMed Central Google Scholar
Geonea ID, Tarnita D. Design and evaluation of a new exoskeleton for gait rehabilitation. Mech Sci. 2017;8(2):307–21.
Article Google Scholar
Molteni F, Gasperini G, Cannaviello G, Guanziroli E. Exoskeleton and end-effector robots for upper and lower limbs rehabilitation: narrative review. PM&R. 2018;10(9):S174–88.
Google Scholar
Hesse S, Uhlenbrock D. A mechanized gait trainer for restoration of gait. J Rehabil Res Dev. 2000;37(6):701–8.
CAS PubMed Google Scholar
Esquenazi A, Talaty M, Packel A, Saulino M. The rewalk powered exoskeleton to restore ambulatory function to individuals with thoracic-level motor-complete spinal cord injury. Am J Phys Med Rehabil. 2012;91(11):911–21.
Article PubMed Google Scholar
Rodríguez-Fernández A, Lobo-Prat J, Font-Llagunes JM. Systematic review on wearable lower-limb exoskeletons for gait training in neuromuscular impairments. J Neuroeng Rehabil. 2021;18(1):22.
Russo M, Maggio MG, Naro A, Portaro S, Porcari B, Balletta T, et al. Can powered exoskeletons improve gait and balance in multiple sclerosis? A retrospective study. Int J Rehab Res. 2021;44(2):126–30.
Nam KY, Kim HJ, Kwon BS, Park J, Lee HJ, Yoo A. Robot-assisted gait training (Lokomat) improves walking function and activity in people with spinal cord injury: a systematic review. J Neuroeng Rehabil. 2017;14(1):24.
Scivoletto G, Tamburella F, Laurenza L, Torre M, Molinari M. Who is going to walk? A review of the factors influencing walking recovery after spinal cord injury. Front Human Neurosci. 2014;8:141.
Fehlings MG, Tetreault LA, Wilson JR, Kwon BK, Burns AS, Martin AR, et al. A clinical practice guideline for the management of acute spinal cord injury: introduction, rationale, and scope. Global Spine J. 2017;7(3 Suppl):84S-94S.
Hachem LD, Fehlings MG. Pathophysiology of spinal cord injury. Neurosurg Clin N Am. 2021;32(3):305–13.
Sandean D. Management of acute spinal cord injury: a summary of the evidence pertaining to the acute management, operative and non-operative management. World J Orthop. 2020;11(12):573–83.
Ahuja CS, Wilson JR, Nori S, Kotter M, Druschel C, Curt A, et al. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017;3:17018.
Gandolfi M, Valè N, Posteraro F, Morone G, Dell’orco A, Botticelli A, et al. State of the art and challenges for the classification of studies on electromechanical and robotic devices in neurorehabilitation: a scoping review. Eur J Phys Rehabil Med. 2021;57(5):831–40.
Basalp E, Wolf P, Marchal-Crespo L. Haptic training: which types facilitate (re) learning of which motor task and for whom? Answers by a review. IEEE Trans Haptics. 2021;14(4):722–39.
Louie DR, Eng JJ, Lam T. Gait speed using powered robotic exoskeletons after spinal cord injury: a systematic review and correlational study. J Neuroeng Rehabil. 2015;12:82.
Fang C, Tsai J, Li G, Lien AS, Chang Y. Effects of robot-assisted gait training in individuals with spinal cord injury: a meta-analysis. Biomed Res Int. 2020;2020:2102785.
Duddy D, Doherty R, Connolly J, McNally S, Loughrey J, Faulkner M. The effects of powered exoskeleton gait training on cardiovascular function and gait performance: A systematic review. Sensors. 2021;21(9):3207.
Article CAS PubMed PubMed Central Google Scholar
Cheung EY, Ng TK, Kevin KK, Kwan RL, Cheing GL. Robot-assisted training for people with spinal cord injury: a meta-analysis. Arch Phys Med Rehabil. 2017;98(11):2320-2331.e12.
Mekki M, Delgado AD, Fry A, Putrino D, Huang V. Robotic rehabilitation and spinal cord injury: a narrative review. Neurotherapeutics. 2018;15(3):604–17.
Jacobson PB, Goody R, Lawrence M, Mueller BK, Zhang X, Hooker BA, et al. Elezanumab, a human anti-RGMa monoclonal antibody, promotes neuroprotection, neuroplasticity, and neurorecovery following a thoracic hemicompression spinal cord injury in non-human primates. Neurobiol Dis. 2021;155: 105385.
Article CAS PubMed Google Scholar
Lin A, Shaaya E, Calvert JS, Parker SR, Borton DA, Fridley JS. A review of functional restoration from spinal cord stimulation in patients with spinal cord injury. Neurospine. 2022;19(3):703–34.
James ND, McMahon SB, Field-Fote EC, Bradbury EJ. Neuromodulation in the restoration of function after spinal cord injury. Lancet Neurol. 2018;17(10):905–17.
Yildirim MA, Öneş K, Gökşenoğlu G. Early term effects of robotic assisted gait training on ambulation and functional capacity in patients with spinal cord injury. Turk J Med Sci. 2019;49(3):838–48.
Tamburella F, Tagliamonte NL, Masciullo M, Pisotta I, Arquilla M, van Asseldonk E, et al. Gait training with Achilles ankle exoskeleton in chronic incomplete spinal cord injury subjects. J Biol Regul Homeost Agents. 2020;34(5 Suppl. 3):147–64.
Okawara H, Sawada T, Matsubayashi K, Sugai K, Tsuji O, Nagoshi N, et al. Gait ability required to achieve therapeutic effect in gait and balance function with the voluntary driven exoskeleton in patients with chronic spinal cord injury: a clinical study. Spinal Cord. 2020;58(5):520–7.
Bach Baunsgaard C, Vig Nissen U, Katrin Brust A, Frotzler A, Ribeill C, Kalke Y, et al. Gait training after spinal cord injury: safety, feasibility and gait function following 8 weeks of training with the exoskeletons from Ekso Bionics. Spinal Cord. 2018;56(2):106–16.
Holanda LJ, Silva PM, Amorim TC, Lacerda MO, Simão CR, Morya E. Robotic assisted gait as a tool for rehabilitation of individuals with spinal cord injury: a systematic review. J Neuroeng Rehabil. 2017;14(1):126.
Lefeber N, Swinnen E, Kerckhofs E. The immediate effects of robot-assistance on energy consumption and cardiorespiratory load during walking compared to walking without robot-assistance: a systematic review. Disab Rehabil Assist Technol. 2017;12(7):657–71.
Karelis AD, Carvalho LP, Castillo MJ, Gagnon DH, Aubertin-Leheudre M. Effect on body composition and bone mineral density of walking with a robotic exoskeleton in adults with chronic spinal cord injury. J Rehabil Med. 2017;49(1):84–7.
Choi H, Kim G, Chai JH, Ko C. Effect of gait training program with mechanical exoskeleton on body composition of paraplegics. J Multidiscip Healthc. 2020;13:1879.
Asselin P, Cirnigliaro CM, Kornfeld S, Knezevic S, Lackow R, Elliott M, et al. Effect of Exoskeletal-Assisted Walking on Soft Tissue Body Composition in Persons With Spinal Cord Injury. Arch Phys Med Rehabil. 2021;102(2):196–202.
Gorgey AS, Poarch H, Harnish C, Miller JM, Dolbow D, Gater DR. Acute effects of locomotor training on neuromuscular and metabolic profile after incomplete spinal cord injury. NeuroRehabilitation. 2011;29(1):79–83.
Tan K, Koyama S, Sakurai H, Teranishi T, Kanada Y, Tanabe S. Wearable robotic exoskeleton for gait reconstruction in patients with spinal cord injury: A literature review. J Orthop Translat. 2021;28:55–64.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88: 105906.
Academy of Neurologic Physical Therapy. 6 minute walk test (6MWT). 2019. https://www.neuropt.org/docs/default-source/cpgs/core-outcome-measures/6mwt-pocket-guide-proof9.pdf?sfvrsn=9ee25043_0 . Accessed 12 Mar 2023.
Ryan S. 6 minute walk test. 2013. https://www.sralab.org/rehabilitation-measures/6-minute-walk-test . Accessed 12 Mar 2023.
Physiopedia. 10 metre walk test. 2022. https://www.physio-pedia.com/10_Metre_Walk_Test . Accessed 12 Mar 2023.
Ryan S. Time up and go. 2013. https://www.sralab.org/rehabilitation-measures/timed-and-go . Accessed 12 Mar 2023.
Xiang X, Zong H, Ou Y, Yu X, Cheng H, Du C, et al. Exoskeleton-assisted walking improves pulmonary function and walking parameters among individuals with spinal cord injury: a randomized controlled pilot study. J Neuroeng Rehabil. 2021;18(1):86.
Evans RW, Shackleton CL, West S, Derman W, Laurie Rauch H, Baalbergen E, et al. Robotic locomotor training leads to cardiovascular changes in individuals with incomplete spinal cord injury over a 24-week rehabilitation period: a randomized controlled pilot study. Arch Phys Med Rehabil. 2021;102(8):1447–56.
Corbianco S, Cavallini G, Dini M, Franzoni F, D’Avino C, Gerini A, et al. Energy cost and psychological impact of robotic-assisted gait training in people with spinal cord injury: effect of two different types of devices. Neurol Sci. 2021;42(8):3357–66.
Edwards DJ, Forrest G, Cortes M, Weightman MM, Sadowsky C, Chang S, et al. Walking improvement in chronic incomplete spinal cord injury with exoskeleton robotic training (WISE): a randomized controlled trial. Spinal Cord. 2022;60(6):522–32.
Stampacchia G, Olivieri M, Rustici A, Avino C, Gerini A, Mazzoleni S. Gait rehabilitation in persons with spinal cord injury using innovative technologies: an observational study. Spinal Cord. 2020;58(9):988–97.
Puentes S, Kadone H, Kubota S, Abe T, Shimizu Y, Marushima A, et al. Reshaping of gait coordination by robotic intervention in myelopathy patients after surgery. Front Neurosci. 2018;12:99.
Tefertiller C, Hays K, Jones J, Jayaraman A, Hartigan C, Bushnik T, et al. Initial outcomes from a multicenter study utilizing the indego powered exoskeleton in spinal cord injury. Top Spinal Cord Inj Rehabil. 2018;24(1):78–85.
Knezevic S, Asselin PK, Cirnigliaro CM, Kornfeld S, Emmons RR, Spungen AM. Oxygen uptake during exoskeletal-assisted walking in persons with paraplegia. Arch Phys Med Rehabil. 2021;102(2):185–95.
Guanziroli E, Cazzaniga M, Colombo L, Basilico S, Legnani G, Molteni F. Assistive powered exoskeleton for complete spinal cord injury: correlations between walking ability and exoskeleton control. Eur J Phys Rehabil Med. 2019;55(2):209–16.
Sale P, Russo EF, Scarton A, Calabro RS, Masiero S, Filoni S. Training for mobility with exoskeleton robot in spinal cord injury patients: a pilot study. Eur J Phys Rehabil Med. 2018;54(5):745–51.
Gagnon DH, Escalona MJ, Vermette M, Carvalho LP, Karelis AD, Duclos C, et al. Locomotor training using an overground robotic exoskeleton in long-term manual wheelchair users with a chronic spinal cord injury living in the community: Lessons learned from a feasibility study in terms of recruitment, attendance, learnability, performance and safety. J Neuroeng Rehabil. 2018;15(1):12.
Khan AS, Livingstone DC, Hurd CL, Duchcherer J, Misiaszek JE, Gorassini MA, et al. Retraining walking over ground in a powered exoskeleton after spinal cord injury: a prospective cohort study to examine functional gains and neuroplasticity. J Neuroeng Rehabil. 2019;16:145.
Xiang X, Ding M, Zong H, Liu Y, Cheng H, He C, et al. The safety and feasibility of a new rehabilitation robotic exoskeleton for assisting individuals with lower extremity motor complete lesions following spinal cord injury (SCI): an observational study. Spinal Cord. 2020;58(7):787–94.
Park JH, Kim HS, Jang SH, Hyun DJ, Park SI, Yoon J, et al. Cardiorespiratory responses to 10 weeks of exoskeleton-assisted overground walking training in chronic nonambulatory patients with spinal cord injury. Sensors (Basel, Switzerland). 2021;21(15):5022.
Kwon SH, Lee BS, Lee HJ, Kim EJ, Lee JA, Yang SP, et al. Energy efficiency and patient satisfaction of gait with knee-ankle-foot orthosis and robot (ReWalk)-assisted gait in patients with spinal cord injury. Ann Rehabil Med. 2020;44(2):131–41.
Kerdraon J, Previnaire JG, Tucker M, Coignard P, Allegre W, Knappen E, et al. Evaluation of safety and performance of the self balancing walking system Atalante in patients with complete motor spinal cord injury. Spinal Cord Ser Cases. 2021;7(1):71.
Asselin PK, Avedissian M, Knezevic S, Kornfeld S, Spungen AM. Training persons with spinal cord injury to ambulate using a powered exoskeleton. J Vis Exp. 2016;112:54071.
Baunsgaard CB, Nissen UV, Brust AK, Frotzler A, Ribeill C, Kalke Y, et al. Exoskeleton gait training after spinal cord injury: an exploratory study on secondary health conditions. J Rehabil Med. 2018;50(9):806–13.
Bass A, Aubertin-Leheudre M, Morin SN, Gagnon DH. Preliminary training volume and progression algorithm to tackle fragility fracture risk during exoskeleton-assisted overground walking in individuals with a chronic spinal cord injury. Spinal Cord Ser Cases. 2022;8(1):29.
Bass A, Morin S, Guidea M, Lam J, Hammad R, Aubertin-Leheudre M, et al. Effects of an exoskeleton-assisted walking program on bone strength in wheelchair individuals with spinal cord injury: a preliminary study using imaging and serum biomarkers. Physiology. 2023;38(S1):5796080.
Chen S, Wang Z, Li Y, Tang J, Wang X, Huang L, et al. Safety and feasibility of a novel exoskeleton for locomotor rehabilitation of subjects with spinal cord injury: a prospective, multi-center, and cross-over clinical trial. Front Neurorobot. 2022;16: 848443.
Contreras-Vidal JL, Bhagat NA, Brantley J, Cruz-Garza JG, He Y, Manley Q, et al. Powered exoskeletons for bipedal locomotion after spinal cord injury. J Neural Eng. 2016;13(3): 031001.
Goikoetxea-Sotelo G, van Hedel HJ. Defining, quantifying, and reporting intensity, dose, and dosage of neurorehabilitative interventions focusing on motor outcomes. Front Rehabil Sci. 2023;4:1139251.
Hutchinson MJ, Goosey-Tolfrey VL. Rethinking aerobic exercise intensity prescription in adults with spinal cord injury: time to end the use of “moderate to vigorous” intensity? Spinal Cord. 2022;60(6):484–90.
Craven BC, Souza WH, Jaglal S, Gibbs J, Wiest MJ, Sweet SN et al. Reducing endocrine metabolic disease risk in adults with chronic spinal cord injury: strategic activities conducted by the Ontario-Quebec RIISC team. Disabil Rehabil. 2023:1–13. Ahead of print.
Bierman AS, Tinetti ME. Precision medicine to precision care: managing multimorbidity. Lancet. 2016;388(10061):2721–3.
Benson I, Hart K, Tussler D, van Middendorp JJ. Lower-limb exoskeletons for individuals with chronic spinal cord injury: findings from a feasibility study. Clin Rehabil. 2016;30(1):73–84.
Zeilig G, Weingarden H, Zwecker M, Dudkiewicz I, Bloch A, Esquenazi A. Safety and tolerance of the ReWalk™ exoskeleton suit for ambulation by people with complete spinal cord injury: a pilot study. J Spinal Cord Med. 2012;35(2):96–101.
Hartigan C, Kandilakis C, Dalley S, Clausen M, Wilson E, Morrison S, et al. Mobility outcomes following five training sessions with a powered exoskeleton. Top Spinal Cord Inj Rehab. 2015;21(2):93–9.
Escalona MJ, Brosseau R, Vermette M, Comtois AS, Duclos C, Aubertin-Leheudre M, et al. Cardiorespiratory demand and rate of perceived exertion during overground walking with a robotic exoskeleton in long-term manual wheelchair users with chronic spinal cord injury: a cross-sectional study. Ann Phys Rehabil Med. 2018;61(4):215–23.
Hellsten Y, Nyberg M. Cardiovascular adaptations to exercise training. 2015;6 1:1-32.
Škarabot J, Brownstein CG, Casolo A, Del Vecchio A, Ansdell P. The knowns and unknowns of neural adaptations to resistance training. Eur J Appl Physiol. 2021;121(3):675–85.
Faulkner J, Martinelli L, Cook K, Stoner L, Ryan-Stewart H, Paine E, et al. Effects of robotic-assisted gait training on the central vascular health of individuals with spinal cord injury: a pilot study. J Spinal Cord Med. 2021;44(2):299–305.
Morse LR, Biering-Soerensen F, Carbone LD, Cervinka T, Cirnigliaro CM, Johnston TE, et al. Bone mineral density testing in spinal cord injury: 2019 ISCD official position. J Clin Densitom. 2019;22(4):554–66.
Bass A, Morin SN, Vermette M, Aubertin-Leheudre M, Gagnon DH. Incidental bilateral calcaneal fractures following overground walking with a wearable robotic exoskeleton in a wheelchair user with a chronic spinal cord injury: is zero risk possible? Osteoporosis Int. 2020;31(5):1007–11.
Article CAS Google Scholar
Van Herpen F, Van Dijsseldonk RB, Rijken H, Keijsers N, Louwerens J, van Nes I. Case report: description of two fractures during the use of a powered exoskeleton. Spinal Cord Ser Cases. 2019;5(1):99.
Sunn G. Spinal cord injury pressure ulcer treatment: an experience-based approach. Phys Med Rehabil Clin. 2014;25(3):671–80.
Huiming G, Yuming W, Mingliang Y, Changbin L, Qiuchen H, Jianjun L. Study on the characteristics of microcirculation in the site of pressure ulcer in patients with spinal cord injury. Sci Prog. 2021;104(3):00368504211028726.
Ozdemir RA, Perez MA. Afferent input and sensory function after human spinal cord injury. J Neurophysiol J Neurophysiol. 2018;119(1):134–44.
Hill D, Holloway CS, Ramirez DZM, Smitham P, Pappas Y. What are user perspectives of exoskeleton technology? A literature review. Int J Technol Assess Health Care. 2017;33(2):160–7.
Van Dijsseldonk RB, Van Nes IJ, Geurts AC, Keijsers NL. Exoskeleton home and community use in people with complete spinal cord injury. Sci Rep. 2020;10(1):15600.
Mertz L. The next generation of exoskeletons: lighter, cheaper devices are in the works. IEEE Pulse. 2012;3(4):56–61.
Download references
Acknowledgements
Patrik Nepomuceno acknowledges receipt of a scholarship through the Emerging Leaders in the Americas Program with the support of Global Affairs Canada and Government of Canada. Dr. Wagner H. Souza acknowledges fellowship support from the University Health Network (UHN) Foundation and Spinal Cord Injury Ontario, and inspiration from Mr. Robert MacDonald. Dr. Craven acknowledges support from the UHN Foundation for her UHN/University of Toronto Chair in SCI Rehabilitation.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
KITE Research Institute, University Health Network, Toronto, ON, Canada
Patrik Nepomuceno, Wagner H. Souza, Maureen Pakosh, Kristin E. Musselman & B. Catharine Craven
Graduate Program in Health Promotion, Department of Health Sciences, University of Santa Cruz do Sul, Santa Cruz do Sul, RS, Brazil
Patrik Nepomuceno
Department of Physical Therapy, Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada
Kristin E. Musselman
Department of Medicine, Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada
B. Catharine Craven
Institute of Health Policy Management and Evaluation, University of Toronto, Toronto, Canada
Patrik Nepomuceno & B. Catharine Craven
Rehabilitation Sciences Institute, Temerty Faculty of Medicine, University of Toronto, Toronto, Canada
Kristin E. Musselman & B. Catharine Craven
You can also search for this author in PubMed Google Scholar
Contributions
PN, WHS, KEM and BCC contributed to conceptualization, methodology, design, and development of the study. PN and WHS share first authorship and equally contributed to data curation, acquisition, formal analysis and to the writing of the manuscript. MP contributed to data curation, acquisition and methodology. KEM and BCC contributed to supervision, review and editing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to B. Catharine Craven .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:.
Medline Search Strategy. Contain the Medline Search Strategy.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Nepomuceno, P., Souza, W.H., Pakosh, M. et al. Exoskeleton-based exercises for overground gait and balance rehabilitation in spinal cord injury: a systematic review of dose and dosage parameters. J NeuroEngineering Rehabil 21 , 73 (2024). https://doi.org/10.1186/s12984-024-01365-2
Download citation
Received : 28 November 2023
Accepted : 22 April 2024
Published : 05 May 2024
DOI : https://doi.org/10.1186/s12984-024-01365-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Exoskeleton
- Neurorehabilitation
- Overground training
- Spinal cord injury
Journal of NeuroEngineering and Rehabilitation
ISSN: 1743-0003
- Submission enquiries: [email protected]
Digital Health Interventions for Cardiac Rehabilitation: Systematic Literature Review
Affiliations.
- 1 Johns Hopkins University School of Medicine, Baltimore, MD, United States.
- 2 University of Patras School of Medicine, Patras, Greece.
- 3 Ciccarone Center for the Prevention of Cardiovascular Disease, Division of Cardiology, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, United States.
- 4 UCLA David Geffen School of Medicine, Los Angeles, CA, United States.
- 5 Medstar Franklin Square Hospital, Baltimore, MD, United States.
- 6 Johns Hopkins School of Nursing, Baltimore, MD, United States.
- 7 Department of Medicine, Johns Hopkins Bayview Medical Center, Baltimore, MD, United States.
- 8 Department of Medicine, Division of Cardiology, Stanford University School of Medicine, Stanford, CA, United States.
- 9 Stanford Prevention Research Center, Stanford University School of Medicine, Stanford, CA, United States.
- 10 INTERVENT International, Savannah, GA, United States.
- 11 Centre for Exercise Science and Sports Medicine, School of Therapeutic Sciences, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
- PMID: 33555259
- PMCID: PMC7899799
- DOI: 10.2196/18773
Background: Cardiovascular disease (CVD) is the leading cause of death worldwide. Despite strong evidence supporting the benefits of cardiac rehabilitation (CR), over 80% of eligible patients do not participate in CR. Digital health technologies (ie, the delivery of care using the internet, wearable devices, and mobile apps) have the potential to address the challenges associated with traditional facility-based CR programs, but little is known about the comprehensiveness of these interventions to serve as digital approaches to CR. Overall, there is a lack of a systematic evaluation of the current literature on digital interventions for CR.
Objective: The objective of this systematic literature review is to provide an in-depth analysis of the potential of digital health technologies to address the challenges associated with traditional CR. Through this review, we aim to summarize the current literature on digital interventions for CR, identify the key components of CR that have been successfully addressed through digital interventions, and describe the gaps in research that need to be addressed for sustainable and scalable digital CR interventions.
Methods: Our strategy for identifying the primary literature pertaining to CR with digital solutions (defined as technology employed to deliver remote care beyond the use of the telephone) included a consultation with an expert in the field of digital CR and searches of the PubMed (MEDLINE), Embase, CINAHL, and Cochrane databases for original studies published from January 1990 to October 2018.
Results: Our search returned 31 eligible studies, of which 22 were randomized controlled trials. The reviewed CR interventions primarily targeted physical activity counseling (31/31, 100%), baseline assessment (30/31, 97%), and exercise training (27/31, 87%). The most commonly used modalities were smartphones or mobile devices (20/31, 65%), web-based portals (18/31, 58%), and email-SMS (11/31, 35%). Approximately one-third of the studies addressed the CR core components of nutrition counseling, psychological management, and weight management. In contrast, less than a third of the studies addressed other CR core components, including the management of lipids, diabetes, smoking cessation, and blood pressure.
Conclusions: Digital technologies have the potential to increase access and participation in CR by mitigating the challenges associated with traditional, facility-based CR. However, previously evaluated interventions primarily focused on physical activity counseling and exercise training. Thus, further research is required with more comprehensive CR interventions and long-term follow-up to understand the clinical impact of digital interventions.
Keywords: cardiac rehabilitation; digital technologies; mHealth; mobile phone; telemedicine.
©Shannon Wongvibulsin, Evagelia E Habeos, Pauline P Huynh, Helen Xun, Rongzi Shan, Kori A Porosnicu Rodriguez, Jane Wang, Yousuf K Gandapur, Ngozi Osuji, Lochan M Shah, Erin M Spaulding, George Hung, Kellen Knowles, William E Yang, Francoise A Marvel, Eleanor Levin, David J Maron, Neil F Gordon, Seth S Martin. Originally published in the Journal of Medical Internet Research (http://www.jmir.org), 08.02.2021.
Publication types
- Research Support, N.I.H., Extramural
- Research Support, Non-U.S. Gov't
- Systematic Review
- Cardiac Rehabilitation / methods*
- Mobile Applications / standards*
- Telemedicine / methods*
Grants and funding
- T32 NR012704/NR/NINR NIH HHS/United States
- T32 GM007309/GM/NIGMS NIH HHS/United States
- F31 NR017328/NR/NINR NIH HHS/United States
- TL1 TR003100/TR/NCATS NIH HHS/United States
- F30 HL142131/HL/NHLBI NIH HHS/United States
- P01 HL108800/HL/NHLBI NIH HHS/United States

IMAGES
VIDEO
COMMENTS
A 2019 Cochrane review of cardiac rehabilitation in heart failure included 44 RCTs in 5,783 participants, predominantly with HFrEF 15. This meta-analysis showed that participation in cardiac rehabilitation was associated with reduced rates of all-cause and heart-failure-specific hospitalization and improved health-related quality of life ...
A 2019 Cochrane review of cardiac rehabilitation in heart failure included 44 RCTs in 5,783 participants, ... systematic literature review and meta-analysis. Ageing Res. Rev. 53, 100903 (2019).
Cardiac rehabilitation provides evidence-based, secondary prevention after a cardiovascular event. This review addresses the benefits of cardiac rehabilitation programs and current barriers to part...
Abstract. Cardiac rehabilitation is a comprehensive model of secondary prevention proven to reduce mortality and morbidity. The World Health Organization is developing a Package of Rehabilitation Interventions for implementation by ministries of health as part of universal healthcare across the continuum. Through a systematic review, we sought ...
Cardiac rehabilitation in the United States. Prog Cardiovasc Dis 2014;56:522-9. A clinical review that provides clinicians with information on the benefits of cardiac rehabilitation, risk factors, and factors affecting participation from a US perspective. National Institute for Health and Care Excellence.
Aim: The aim of the project was to conduct a systematic review of meta-analyses of supervised, home-based or telemedicine-based exercise cardiac rehabilitation (CR) published between July 2011 and April 2018.Materials & methods: Evidence on mortality, hospitalization, peak VO 2, exercise capacity, muscle strength and health-related quality of life in patients with coronary heart disease or ...
Cardiac rehabilitation programs must integrate mental health support to address anxiety, depression, and the emotional challenges associated with cardiac events. Neglecting mental health aspects may hinder overall recovery and rehabilitation outcomes.
Cardiac rehabilitation based on exercise therapy is a valuable treatment for patients with a broad spectrum of cardiovascular diseases. Current guidelines support its use in patients with stable ...
Objective To undertake a systematic review and meta-analysis to assess the impact of cardiac rehabilitation (CR) on physical activity (PA) levels of patients with heart disease and the methodological quality of these studies. Methods Databases (MEDLINE, EMBASE, CENTRAL, CINAHL, PsychINFO and SportDiscus) were searched without language restriction from inception to January 2017 for randomised ...
Purpose: Exercise cardiac rehabilitation (CR) represents an evidence-based therapy for patients with heart failure with reduced ejection fraction (HFrEF) and this article provides a concise review of the relevant exercise testing and CR literature, including aspects unique to their care.. Clinical Considerations: A hallmark feature of HFrEF is exercise intolerance (eg, early-onset fatigue).
Purpose of review Cardiovascular (CV) disease remains the leading cause of death in the USA despite major advances in its treatment. With time, cardiac rehabilitation (CR) programs have gathered interest to help increase CV health and improve functional status after a CV event. Patients with heart failure have also been shown to benefit. In this review, we will evaluate the current literature ...
red Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A comprehensive literature search was conducted from database inception dates to July 2022 using the following databases: MEDLINE, EMBASE, APA PsycINFO, Cochrane Database of Systematic Review, CINAHL, Scopus, and Web of Science. The inclusion criteria were studies that examined the barriers and/or facilitators of ...
This review focuses on the progress made in applying EECP to CHD cardiac rehabilitation globally, including a brief history of EECP, the working principle, etc, to help researchers grasp the research outline and gaps in the literature regarding EECP. Enhanced external counterpulsation (EECP) is a non-invasive, outpatient, pulsatile-assisted circulation technique that has been used in many ...
Introduction. Ischemic heart disease (IHD) continues to be a major cause of morbidity and mortality around the globe. 1 Indeed, it is one of the biggest global contributors of disability-adjusted life years. 2, 3 Cardiac patients are at high risk of recurrent events and of having a reduced quality of life, and hence secondary prevention, such as that delivered in cardiac rehabilitation (CR ...
Using the Information Needs in Cardiac Rehabilitation short version (INCR-S) scale—which was shown to be a good measurement tool through the study and hence may improve patient education—patients reported they most wanted information about heart events, heart-healthy eating, exercise benefits, their pills, symptom response, risk factor ...
This literature review aimed to evaluate the effectiveness of cardiac rehabilitation in coronary heart disease patients. Methods: This present study was a literature review discussing cardiac rehabilitation for coronary heart disease patients. Results: The result showed that the functional capacity of the CR group was more increased compared to ...
CONCLUSIONS: Short-term elevations in ambient PM2.5, even at low concentrations within current air quality standards, and/or higher temperatures were associated with detrimental changes in aerobic exercise capacity, which can be linked to a worse quality of life and cardiovascular prognosis among cardiac rehabilitation patients.
The most common TTR variant in the US is V142I (also known as Val122Ile or V122I under legacy nomenclature), observed in 3.4% of individuals in the US who self-identify as Black, or approximately 1.5 million people (across all age groups). 1 While larger-scale studies have individually demonstrated an association of the allele with an increased ...
Background Exoskeletons are increasingly applied during overground gait and balance rehabilitation following neurological impairment, although optimal parameters for specific indications are yet to be established. Objective This systematic review aimed to identify dose and dosage of exoskeleton-based therapy protocols for overground locomotor training in spinal cord injury/disease. Methods A ...
Overall, there is a lack of a systematic evaluation of the current literature on digital interventions for CR. Objective: The objective of this systematic literature review is to provide an in-depth analysis of the potential of digital health technologies to address the challenges associated with traditional CR. Through this review, we aim to ...
1. Introduction. Systemic sclerosis (SSc) is a rare and complex autoimmune disease that manifests itself through vasculopathy, an abnormal immunological response, and fibrosis, leading to progressive dysfunction of multiple organs [ 1, 2 ]. It affects about 2.5 million people worldwide and is more common among women [ 1, 2 ].