

We apologize for the inconvenience...
To ensure we keep this website safe, please can you confirm you are a human by ticking the box below.
If you are unable to complete the above request please contact us using the below link, providing a screenshot of your experience.
https://ioppublishing.org/contacts/
An Overview of Copper Nanoparticles: Synthesis, Characterisation and Anticancer Activity
Affiliations.
- 1 Department of Mathematics and Sciences, College of Arts and Applied Sciences, Dhofar University, Salalah 211, Oman.
- 2 Department of Chemical Engineering, College of Engineering, Dhofar University, Salalah 211, Oman.
- 3 Department of Biochemistry, Aligarh Muslim University, U.P., India.
- 4 College of Engineering, Dhofar University, Salalah 211, Oman.
- 5 Department of Urology, Masonic Cancer Center, University of Minnesota, MN55455,, United States.
- 6 Department of Chemistry, Khalifa University of Science and Technology, Main Campus, Abu Dhabi, PO Box 127788, United Arab Emirates.
- 7 School of Mathe- matics and Physics, College of Science, University of Lincoln, Lincoln, LN6 7TS, United Kingdom.
- 8 Department of Chemistry, Faculty of Sci- ence, King Abdulaziz University, Jeddah 21589, Saudi Arabia.
- 9 Department of Pharmaceutics and Pharmaceutical Technology, Fac- ulty of Pharmacy, Yarmouk University, Irbid 566, Jordan.
- 10 School of Pharmacy and Pharmaceutical Science, Ulster University, Coleraine, County Londonderry, BT52 1SA, Northern Ireland, United Kingdom.
- PMID: 34348615
- DOI: 10.2174/1381612827666210804100303
In this review, we summarised the different methods for copper nanoparticle synthesis, including green methods. We highlighted that the synthesis of the copper nanoparticles from green sources is preferable as they serve as stable and reducing entities. Furthermore, we critically reviewed the effectiveness of copper- based nanoparticles in oncogenic treatments emphasizing breast, lung, colorectal, and skin cancers. Finally, we have summarised the recent progress made in copper-based nanoparticles and their applications to amplify and rectify present cancer treatment options. The synthesis, characterization, stabilization, and functionalization techniques of various copper-based nanoparticles have also been highlighted in each section. In conclusion, the review provides the outlook of copper nanoparticles in cancer diagnostics and therapeutics.
Keywords: Cancer; cancer treatment; copper nanoparticles; diagnostics; nanomaterials.; nanomedicine; therapeutics.
Copyright© Bentham Science Publishers; For any queries, please email at [email protected].
Publication types
- Research Support, Non-U.S. Gov't
- Metal Nanoparticles*
- Nanoparticles*
- Plant Extracts
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 15 June 2021
Green copper oxide nanoparticles for lead, nickel, and cadmium removal from contaminated water
- Alaa El Din Mahmoud 1 , 2 ,
- Khairia M. Al-Qahtani 3 ,
- Sahab O. Alflaij 3 ,
- Salma F. Al-Qahtani 4 &
- Faten A. Alsamhan 3
Scientific Reports volume 11 , Article number: 12547 ( 2021 ) Cite this article
11k Accesses
110 Citations
3 Altmetric
Metrics details
- Environmental sciences
- Nanoscience and technology
Environmentally friendly copper oxide nanoparticles (CuO NPs) were prepared with a green synthesis route without using hazardous chemicals. Hence, the extracts of mint leaves and orange peels were utilized as reducing agents to synthesize CuO NPs-1 and CuO NPs-2, respectively. The synthesized CuO NPs nanoparticles were characterized using scanning electron microscopy (SEM), Energy Dispersive X-ray Analysis (EDX), BET surface area, Ultraviolet–Visible spectroscopy (UV–Vis), and Fourier Transform Infrared Spectroscopy (FT-IR). Various parameters of batch experiments were considered for the removal of Pb(II), Ni(II), and Cd(II) using the CuO NPs such as nanosorbent dose, contact time, pH, and initial metal concentration. The maximum uptake capacity (q m ) of both CuO NPs-1 and CuO NPs-2 followed the order of Pb(II) > Ni(II) > Cd(II). The optimum q m of CuO NPs were 88.80, 54.90, and 15.60 mg g −1 for Pb(II), Ni(II), and Cd(II), respectively and occurred at sorbent dose of 0.33 g L −1 and pH of 6. Furthermore, isotherm and kinetic models were applied to fit the experimental data. Freundlich models (R 2 > 0.97) and pseudo-second-order model (R 2 > 0.96) were fitted well to the experimental data and the equilibrium of metal adsorption occurred within 60 min.
Similar content being viewed by others

Near-complete destruction of PFAS in aqueous film-forming foam by integrated photo-electrochemical processes

Removal of heavy metal ions from wastewater: a comprehensive and critical review

Environmental impact of direct lithium extraction from brines
Introduction.
Nanotechnology has gained more attention since the synthesized materials are on the nanoscale that differ in chemical and physical properties from those of bulk materials. This allows the integration of nanomaterials in environmental 1 , 2 , medicinal 3 , and agricultural 4 applications.
Several synthesis approaches have been used to produce metallic oxide nanoparticles, including physical and chemical routes. In literature, there are many reported techniques for the synthesis of Copper oxide nanoparticles (CuO NPs) via thermal reduction and microwave irradiation 5 , chemical vapor deposition 6 , polyol 7 , photochemical 8 , 9 , and electrochemical methods 10 . Most of these mentioned techniques have potential environmental impacts because they involve the use of harsh, dangerous, and toxic chemicals in addition to being very expensive with costly reaction conditions. Akintelu et al. 11 recommended that more research work should be conducted to minimize the toxicity of CuO NPs synthesis route while maintaining and/or improving their performance in environmental or medical applications. Therefore, green chemistry routes have attracted researchers’ interest for producing environmentally friendly metal nanoparticles that are free from the use of expensive, harsh, and toxic chemicals.
As there is a fast progress in the field of nanotechnology, the green synthesis of metallic oxide nanoparticles using plant extract has presented as an eco-friendly science by which there exists an ability to control the size, shape, and material quality 12 , 13 . The green synthesis technique is dependent on eco-friendly reducing and capping agents so it eliminates the generation of toxic intermediate during chemical reactions 14 . This would prompt researchers to develop non-toxic green synthesis methods for producing CuO NPs.
Water pollution is a global problem with the increasing usage of chemical compounds 15 . This can be due to the progress in industrialization and technological development, and the runoff of household wastes 16 , 17 . It is believed that heavy metal pollution is one of the serious factors affecting water bodies because of their toxic, non-biodegradable, and persistent nature when released into the environment through natural sources (weathering, erosion) and anthropogenic sources (car exhausts, industrial discharges, and mining) 18 .
The burgeoning demand for obtaining high-quality water has become a reason for researchers to develop advanced technology to deliver clean water. There are many conventional wastewater treatment techniques including precipitation, flocculation, electrocoagulation, ion exchange, etc. Ion exchangers are classified into organic and inorganic. However, composite ion exchangers are preferable to be applied in the removal of heavy metals because of their mechanical stability and enhanced ion exchange capacity which are lacked in organic or inorganic resins. For instance, Mohammad et al. 19 found that the poly (3,4-ethylenedioxythiophene): polystyrene sulfonate-Zr(IV) phosphate enhanced the ion exchange capacity of Cd(II) to be 2.34 meq g −1 . Whereas the composite of carbon nanotubes with cerium(IV) phosphate possesses Cd(II) exchange capacity of 1.64 meq g −1 20 . Polyvinyl alcohol Ce(IV) phosphate composite proved its efficiency as ion exchange for the mixture of Cu(II)–Zn(II), Cu(II)–Cd(II), and Cu(II)–Ni(II) 21 . However, the synthesis routes require many chemical reagents which harm the environment and most of the above-mentioned techniques cannot remove heavy metals from wastewater completely.
The most common adsorbents used in removal of a wide range of heavy metals are activated carbon and zeolite, but their costs are still high at large scale applications 22 , 23 . Accordingly, agricultural or plant byproducts can be an alternative for the preparation of adsorbents or nanosorbents. This has led to the integration of various nanomaterials in the adsorption process for the removal of heavy metals from water and/or wastewater.
Nanomaterials possess the potential of heavy metal removal from water over conventional techniques because of their high surface area (surface/volume ratio) 24 . As an example, metallic oxide nanoparticles can be used to provide a long-term solution to/for water quality and make possible water reuse 25 .
Plant extracts assisted CuO NPs have attracted much attentions because green synthesis techniques hold several advantages over chemical ones, which are using non-toxic solvents such as biological extracts in addition to their simplicity. For instance, aqueous black bean extract 26 , fruit extract of Duranta erecta 27 , Eclipta prostrata leaves extract 28 , clove extract 29 , Solanum lycopersicum leaf extract 30 , and Hawthorn berries extract 31 .
Herein, we focus on green preparation of CuO NPs due to the abundance of copper, its cost effective preparation, and its excellent optical, mechanical, thermal, electrical and catalytic properties 11 , 12 . Recently, CuO NPs have been made available for various applications. This led the researchers to apply alternative sustainable synthesis techniques. Singh et al. 32 synthesized CuO NPs synthesized using Psidium guajava leaf extract as reducing agent as well as capping agent. They confirmed its potential for photocatalytic degradation of Nile blue (93%) and reactive yellow 160 (81%) dyes in 120 min. Khani et al. 33 used the fruit extracts of Ziziphus spina-christi (L.) as reducing agents to prepare CuNPs and tested in crystal violet (CV) adsorption. CV removal reached 95% with a high adsorption capacity (37.5 mg g −1 ) in 7.5 min. Currently, CuO NPs were successfully synthesized using the seed extract of Caesalpinia bonducella but evaluated for electrochemical detection of riboflavin 34 .
Most literature are focused on the application of chemically synthesized CuO NPs in heavy metals removal from contaminated water. Fakhri 35 prepared CuO NPs by sol–gel method and its uptake capacity for Hg(II) reached 46.10 mg g −1 at pH of 9 and nanosorbent dose of 0.05 g. Another CuO NPs were prepared by chemical precipitation technique and applied for Ni(II) removal from water 36 . Its adsorption capacity was 15.4 mg g −1 with nanosorbent dos of 0.2 g L −1 , pH of 7.0, and contact time of 90 min.
Consequently, the objective of our work is to synthesize nontoxic CuO NPs by using the extracts of mint leaves and orange peels as green reducing agents. The obtained CuO NPs are tested as an adsorptive nanomaterial to purify polluted water from heavy metals such as Pb(II), Ni(II), and Cd(II) as well as modelling the experimental results with isotherm and kinetic models.
Results and discussions
Characterization of nanoparticles.
The SEM micrographs of the CuO NPs-1 (synthesized using the extract of mint leaves) and CuO NPs-2 (synthesized using the extract of orange peels) are illustrated in Fig. 1 . Figure 1 a,b shows that the prepared CuO NPs-1 were mostly spherical in shape, while CuO NPs-2 appear with more aggregates (Fig. 1 c,d). This can be due to the coating of different surface functional groups from the prepared extracts (see Fig. 3 b). The same issue was observed with Khani et al. 33 . The SEM micrographs revealed that the synthesized CuO NPs were in the nanometer range of ~ 150 nm. Sankar et al. 37 found that the size of CuO NP was 140 nm when it is synthesized with the extract of Carica papaya leaves. On the other hand, Prasad et al. 38 obtained spherical CuO NPs with sizes of 40–70 nm when the leaves extract of Saraca indica was utilized.
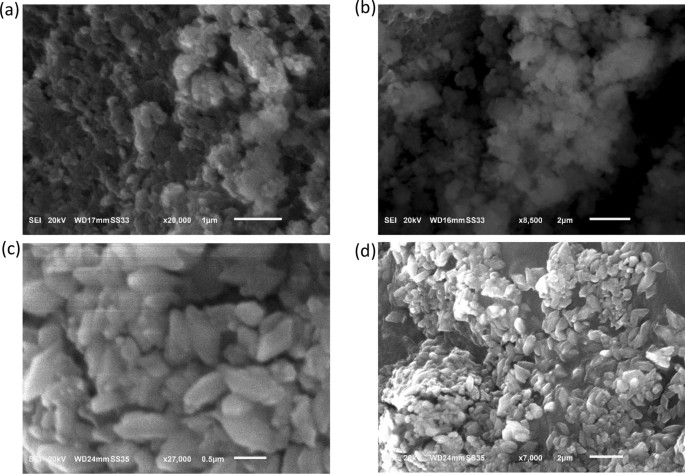
SEM micrographs of ( a,b ) CuO NPS-1 and ( b,c ) CuO NPs-2 at different magnifications.
The BET surface area of the synthesized CuO NPs were found ~20 m 2 g −1 . In literature, the prepared CuO NPs with a precipitation technique has surface area of 34 m 2 g −1 with a size of 196 nm 39 . Another study found that the BET surface area of the prepared CuO NPs with the same technique was 1.7 m 2 g −1 with a size of 140 and 180 nm 40 . On the other hand, Dörner et al. 41 found the BET of sol–gel synthesized CuO NPs was 16 m 2 g −1 with a size of 100–140 nm.
The elemental compositions of the prepared CuO NPs were confirmed using Energy-dispersive X-ray (EDX) and the peaks obtained are illustrated in Fig. 2 a,b. It is observed that the prepared CuO NPs are mainly composed of Cu, O and C without any trace of other materials. In both samples, EDX patterns show a strong signal peak at 1.0 keV representing Cu atoms. The detected carbon and high oxide peaks must be due to the phytochemicals already present in both plant extracts which are added in large volume. There are no other elements were detected even from the extract. The same findings were found with using the leaf extract of Psidium guajava as a reducing agent for the synthesis of CuO NPs 32 .
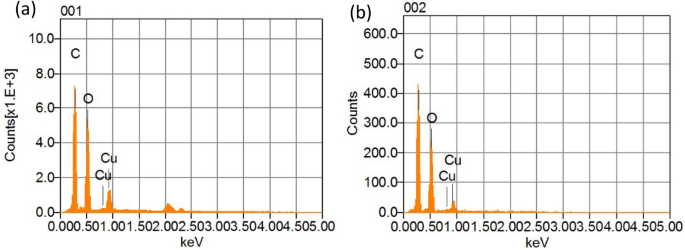
EDX spectra of ( a ) CuO NPs-1 and ( b ) CuO NPs-2.
The phytochemicals in the extracts are responsible for the formation of complexes with the copper salt that is reduced the ions to form nanoparticles. Hence, we observed the color transformation in the prepared Solutions and UV–Vis spectroscopy is used in the range of 200‒600 nm. Figure 3 a indicates a noticeable peak at 325 nm due to the inter band transition of the core electrons of the CuO NPs. Aziz et al. 42 used also mint leaf extract for the synthesis of CuO NPs and detected its absorption peak at 346 nm. Sankar et al. 37 detected a strong absorbance peak between 250 and 300 nm suggesting the formation of CuO NPs.
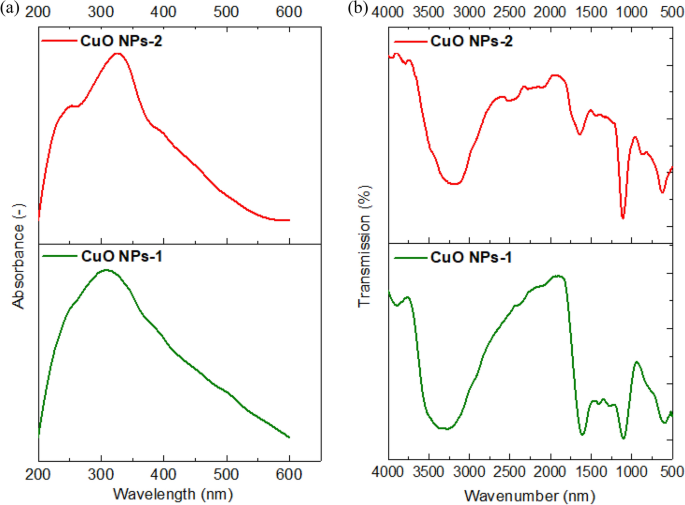
( a ) UV–Vis spectra and ( b ) FT-IR Spectra of CuO NPs using mint extract (CuO NPs-1) and orange peel extract (CuO NPs-2).
The result of FT- IR spectrum of synthesized CuO NPs using the extract of mint and orange leaves were shown in Fig. 3 b. The broad peaks at 3290 cm −1 correspond to the O–H stretching of the Phenols and alcohols. The most intense bands between 1603 and 1627 cm −1 represent C=O stretching band. The peak value at 1273 cm −1 shows the presence of C–O stretching of alcohols. The peak at 1100 cm −1 stands for C–N stretching aliphatic amines, or 1150–1085 cm −1 for strong C–O stretching aliphatic ether. The absorption at 1743 cm −1 was caused by C=O stretching esters, saturated aliphatic, aldehydes. The peaks at ~ 590 cm −1 and ~ 610 cm −1 exhibit the CuO phase. Similar characteristic peaks were observed by Priya et al. 43 with Aerva lanata -mediated CuO NPs at 580 cm −1 and 525 cm −1 . This indicated that the synthetic method conditions reflect the CuO phase.
Metal ions treatment experiments
Effect of nanosorbents dose.
The dose of nanosorbents has a great effect on the adsorption performance. Various dose concentrations (0.17, 0.33, 0.67, 1.00, 1.33, 1.67, and 2.00 g L −1 ) of CuO NP-1 and CuO NP-2 were used to evaluate the efficiency of removing the studied metal ions. Figure 4 Shows an increase in the removal efficiency of Pb(II), Ni(II), and Cd(II) with increasing in the dose of nanosorbents. The reason is due to the availability of more binding sites on the surface of the nanosorbents to the complexity of the metal ions. The selectivity sequence of CuO NPs for the adsorption process was Pb(II) > Ni(II) > Cd(II). Thus, the adsorption of Cd(II) is the least due to its lower electronegativity (1.69) and its bigger radius hydrated radius (0.404 nm) than Ni(II) (1.91 nm) and Pb(II) (0.401 nm).
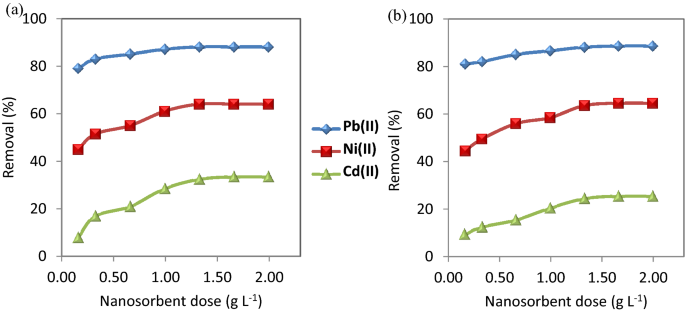
Effect of nanosorbent doses on the removal of the studied metal ions using ( a ) CuO NPs-1 and ( b ) CuO NPs-2 at initial concentration: 20 mg L −1 , pH: 6, and contact time: 60 min.
Our results indicated that 0.33 g L −1 of CuO NPs can be used for further experiments because the nanosorbent dose is a key parameter in the cost analysis of the adsorption process. Therefore, it is recommended to use the lower nanosorbent dose but if have high adsorption performance. This nanosorbent dose is much less than one stated in literature. Sreekala et al. 44 observed that the optimum dose of CuO NPs (synthesized with Simarouba glauca leaf extract) for 10 mg L −1 Pb(II) was 1.00 g L −1 .
Effect of contact time
Figure 5 shows that the removal efficiency of the selected metal ions on CuO NPs required 60 min contact time to reach equilibrium. It is observed that the adsorption rate became almost fixed after 60 min and had a little effect on its rate. This can be attributed to the saturated capacity of the studied nanosorbents.
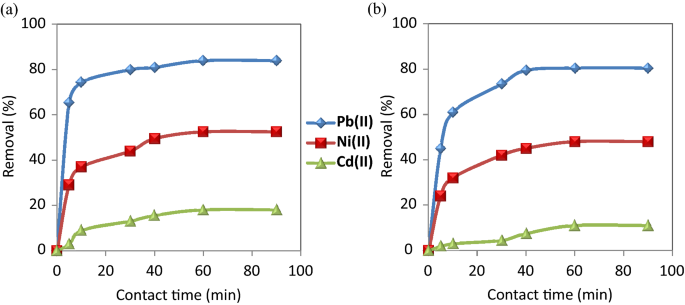
Effect of contact time on the removal of the studied metal ions using ( a ) CuO NPs-1 and ( b ) CuO NPs-2 at initial concentration: 20 mg L −1 , pH: 6, and dose: 0.33 g L −1 .
The removal efficiency of CuO NPs-1 was compared to CuO NPs-2 with the studied metal ions. The removal % of Cd (II), Ni(II) and Pb (II) were 18%, 52.5%, 84% and 11%, 48%, 80.5% when using CuO NPs-1 and CuO NPs-2, respectively. The variation in the removal % of heavy metals is due to the types of the used extracts and their volumes. They can influence the application of CuO NPs in the heavy metals removal. Due to the high intensity of the surface functional groups of CuO NPs-1 as detected in FT-IR (Fig. 3 b), the highest percentage removal of the studied metal ions was obtained using CuO NPs-1. The reason for that can be the richness of mint leaves extract with various phytochemical constituents as reported in Alexa et al. 45 and Thawkar 46 compared to the orange peels extract 47 . Hence, we can conclude that CuO NPs-1 are effective in removing heavy metals.
Effect of pH
Removal of heavy metals from contaminated water depends largely on the pH of the solution. Consequently, the effect of pH on the adsorption of Pb(II), Ni(II),and Cd(II) on CuO NPs was evaluated with pH values, ranging from 3 to 9 at the equilibrium time. The results are shown in Fig. 6 . When pH is increased from 3 to 6, the removal efficiency of Cd(II), Ni(II) and Pb(II) increased from 6.5, 11.5 and 24% to 18, 52.5 and 84%, respectively in the case of CuO NPs-1 which were higher than those values of CuO NPs-2. Subsequent to these values,the adsorption rate decreased.
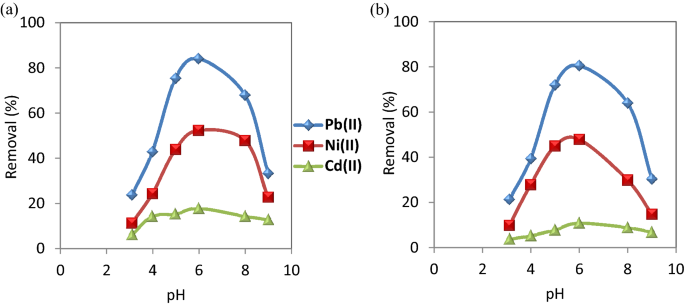
Effect of pH on the removal of the studied metal ions using ( a ) CuO NPs-1 and ( b ) CuO NPs-2 at initial concentration: 20 mg L −1 , contact time: 60 min, pH: 6, and dose: 0.33 g L −1 .
With increasing the pH values till 6, the removal % of the studied metal ions increased because of the decrement of the positive charge of the nanosorbent resulted in a lower electrostatic repulsion between the nanosorbents surface and metal cations as well as the competition decrement between the metal cations and protons of hydrogen ions for the functional groups of the nanosorbents 48 . In addition, pH values beyond 6 resulted in the precipitation of metal ions.
Effect of initial concentration of the selected metal ions
As shown in Figure 7 , the adsorption of the metal ions at different concentrations indicates the removal % decreases with increasing the concentration of the metal ions. Utilizing CuO NP-1 nanosorbent in the removal of Pb(II), Ni(II), and Cd(II) that are ranging from 5 to 40 mg L −1 cause the removal % of Pb(II), Ni(II), and Cd(II) decreased from 92.0, 58.0, and 28.0% to 74.0, 45.7, and 13.0%, respectively. While the nanosorbent of CuO NP-2 led to the decrement of Pb(II), Ni(II), and Cd(II) removal from 89.0, 54.0, and 20.0% to 71.0, 40.0, and 7.0%, respectively. Similar findings were reported with CuO NPs in Jain et al. 36 and Singh et al. 32 . This phenomenon illustrates a significant relationship between the adsorption efficiency and the metal concentration. At low metal concentrations, more absorbable vacant sites are available which lead to an increase in the prevalence of metal ions on the nanosorbent. At a higher concentration of metal ions, the available adsorbed sites become less and thus the removal rate of these ions decreases 23 .
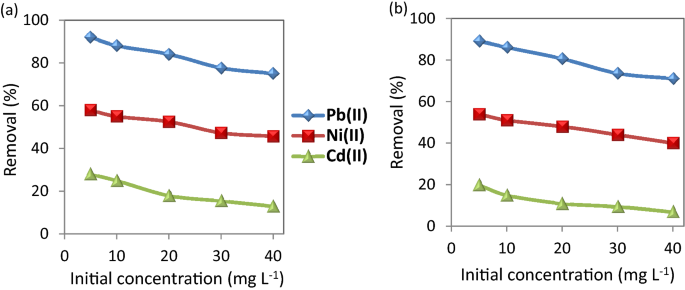
Effect of initial metal concentration on the removal of the studied metal ions using ( a ) CuO NPs-1 and ( b ) CuO NPs-2 at contact time: 60 min, and dose: 0.33 g L −1 .
In this work, the understanding mechanism of the studied metal ions removal could be predicted using FT-IR for the spent CuO NPs. After the metal ions adsorption, the O–H stretching bands get weaker and we observed new peaks as well as shifts in the intensities and positions of FT-IR bands as shown in Fig. 8 . The band of CH 2 and CH 3 groups appeared which might be induced by C–H stretching vibration of CH 2 and CH 3 groups 49 . It became more intense and shifted with each metal ions removal. This band of CuO NPs is shifted to 2960, 2928, and 2956 cm −1 after removal of Pb(II), Ni(II), and Cd(II), respectively. Other shifts were observed in the bands of C–O stretching aliphatic ether to be 1063, 1068, 1070 cm −1 after removal of Pb(II), Ni(II), and Cd(II), respectively. The intense peaks appeared in the range of 1424–1416 cm −1 are attributed to –C–OH deformation. This indicates that the functional groups present on the synthesized CuO NPs were involved in the adsorption process of the studied metal ions.
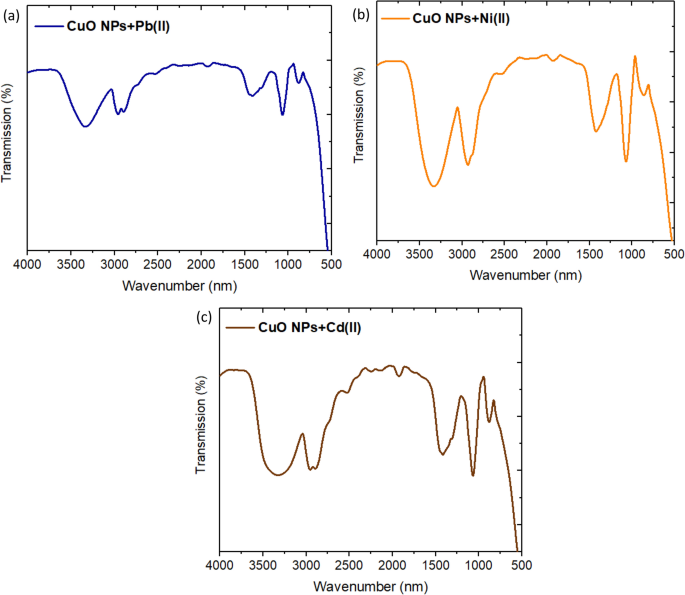
FT-IR Spectra of CuO NPs after adsorption of ( a ) Pb(II), ( b ) Ni(II), and ( c ) Cd(II).
Adsorption models
Adsorption isotherms.
Two isotherm models (Langmuir and Freundlich) were applied to describe the adsorption process. Their linear equations are expressed in Eqs. ( 3 ) and ( 4 ), respectively. The differentiation between the two models is that the Langmuir model suggests homogeneity of the surface of the nanosorbent and no further adsorption occurs once the available adsorption sites are filled, while the Freundlich model proposes heterogeneous of the surface of the nanosorbent.
As shown in Figure 9 , R 2 values of the Freundlich models are higher than the Langmuir models. This indicates that the adsorption of metal ions to the surface of the CuO NPs is carried out by multiple, heterogeneous layers of the nanosorbent surface. Therefore, the adsorption of metal ions using CuO NPs can be described by the Freundlich model. On the other hand, the chemically synthesized CuO NPs (precipitation method) showed monolayer adsorption with Ni(II) 36 .
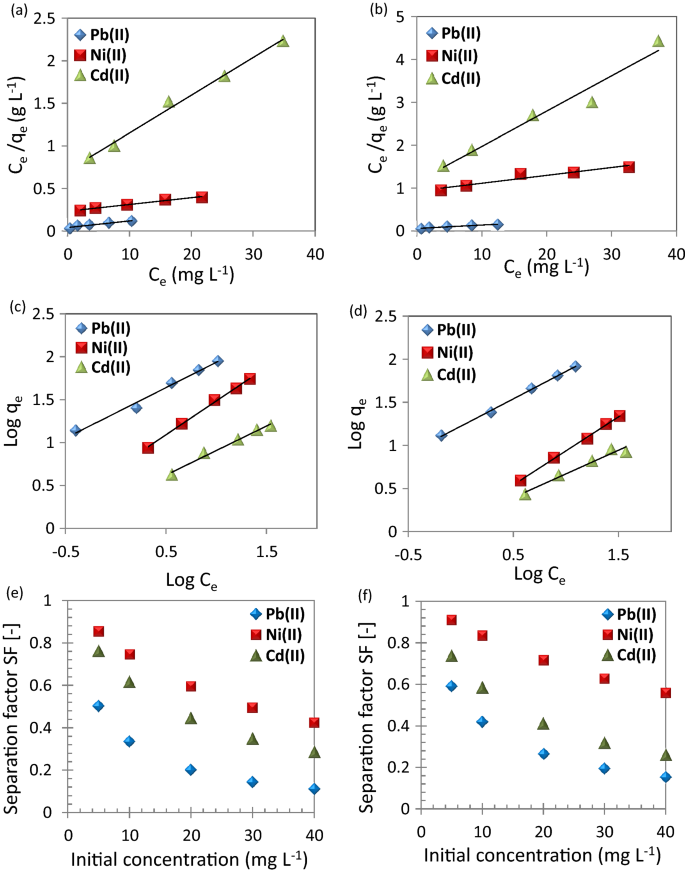
( a,b ) Langmuir plots, ( c,d ) Freundlich plots, and ( e,f ) separation factor for the adsorption of metal ions; Pb(II), Ni(II), and Cd(II) using ( a,c,e ) CuO NPs-1 and ( b,d,f ) CuO NPs-2 at contact time: 60 min, dose: 0.33 g L −1 , and pH: 6.
Table 1 illustrates the isotherm parameters for the adsorption of Pb(II), Ni(II), and Cd(II). The adsorption intensity (n) indicates the sorption driving force’s magnitude. n values are usually in the range of 0–1. The calculations of n values indicated that the adsorption isotherm is favorable because their values are < 1. The adsorption intensity can be also checked using separation factor (SF; Eq. ( 5 )). Their values confirmed the findings of n values as illustrated in Fig. 9 e,f. Furthermore, the SF values of CuO NP-1 were less than CuO NP-2 so the CuO NP-1 adsorption of the studied metal ions is expected to be more as confirmed from the experimental work. Desta 50 found the adsorption of Cr(VI), Cd(II), Pb(II), Ni(II), and Cu(II) is favorable using teff straw waste due to the SF values were in the range of 0.298 to 0.986.
The maximum uptake capacity, q m of Pb(II) was 88.80 and 82.80 mg g −1 using CuO NP-1 and CuO NP-2, respectively. Faisal et al. 22 estimated the q m of Pb(II) using sludge to be 20.41 mg g −1 under similar our experimental conditions except for the dose of 6 g L −1 . The Langmuir affinity constant (K L ) of CuO NPs-1 and CuO NPs-2 for Pb(II) adsorption was higher than K L of Ni(II) and Cd(II). The high K L value estimates the studied metal ions affinity to the available adsorption sites of CuO NPs. Such findings are attributed to the behavior of Pb(II) in the aqueous solutions. For instance, the high electronegativity of Pb(II) which is 2.10 and its small radius hydrated radius 0.401 nm 51 .
Adsorption kinetics
Figure 10 a,b provides a straight line with slope (K 1 ; min −1 ) and intercept equal to log q e . It is worth noting that the values of q e exp are different from the calculated ones obtained from the pseudo-first order which indicates that this model is not valid to represent the adsorption process. On the other hand, Figure 10 c,d represents linear plots of (t/q t ) versus time . Its linear fit gives a straight line with slope of the rate constant (1/q e ) and intercept 1/k 2 q e 2 .
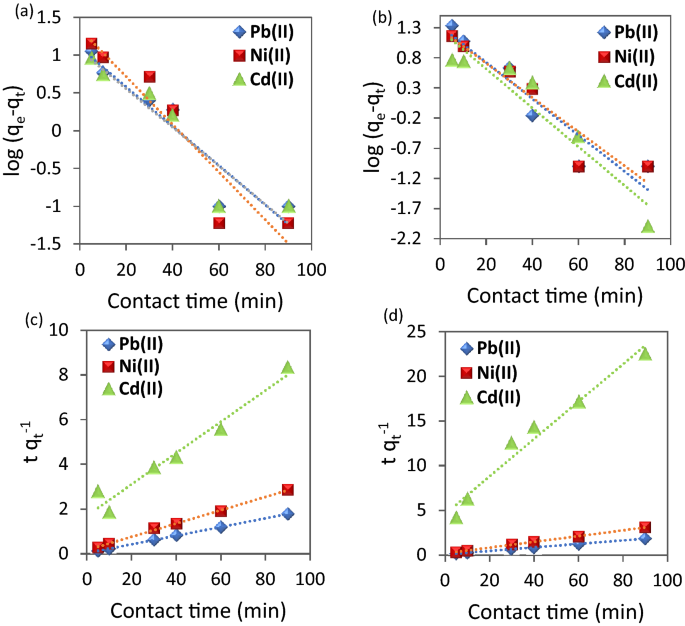
( a,b ) Pseudo-first order and ( c,d ) Pseudo-second order for the adsorption metal ions using ( a,c ) CuO NPs-1 and ( b,d ) CuO NPs-2 at initial concentration: 20 mg L −1 , dose: 0.01 g, and pH 6.
The highest correlation coefficients (R 2 ) were obtained for pseudo-second order kinetic models (Table 2 ). The validation of pseudo-second order indicates that the adsorption capacity is related to the available active sites on nanosorbents 23 . Farghali et al. 52 found the same behavior for Pb(II) kinetics removal by CuO NPs which assume the adsorption process is rate limiting process. However, they estimated the optimum contact time is 240 min for using CuO NPs synthesized from microwave radiation which is more than our reported optimum contact time (60 min). Both models’ parameters are summarized in Table 2 . In addition, the values of initial adsorption rate (h; Eq. ( 8 )) indicated that Pb(II) possesses the high rate compared to Ni(II) and Cd(II).
Conclusions
The preparation of green CuO NPs was successful with the mint leaves and orange peels extracts as reducing agents. This proposed method holds several merits such as easy preparation, cost-effective, and safe compared to the chemical methods as well as the green synthesis method could be applicable for preparing other metal oxide nanoparticles. The EDX and UV–Vis spectroscopy confirmed the preparation of CuO NPs using both extracts. The adsorption application of CuO NPs on the removal of Pb(II), Ni(II), and Cd(II) is found to be dependent on the nanosorbent dose, the metal ions concentration, pH and the contact time. The optimum equilibrium contact time (60 min) and nanosorbent dose (0.33 g L −1 ) are less than those stated in literature for the adsorption of the studied metal ions. The affinity of these metal ions to CuO NPs followed the sequence Pb 2+ > Ni 2+ > Cd 2+ .
The optimum removal efficiency of Pb(II), Ni(II), and Cd(II) were found 84.00, 52.50%, and 18.00%, respectively and obtained at pH 6 for simulating wastewater under normal environmental conditions. The experimental data indicated that the Freundlich isotherm model fitted to the adsorption process as well as pseudo-second order. The maximum adsorption uptakes were 88.80, 54.90, and 15.60 mg g −1 for Pb(II), Ni(II), and Cd(II) with CuO NPs-1. These findings revealed that CuO NPs can be a good nanosorbent to purify water contaminated with heavy metals and its regeneration and reusing should be studied in the future. Furthermore, the environmental application performance of metallic oxide nanoparticles relies on the type of the used extract and its volume for the green synthesis method that influence the morphological properties of the produced nanoparticles and reflect its application performance.
Materials and methods
Preparation of plant extracts.
Orange peels and mint leaves were collected from a local vegetable market. Then, we prepared the orange peel extract and mint leaves extract by washing them with double distilled water and drying at room temperature for 48 h. Each one was grinded, and we added 25 g in a standard beaker filled with 500 mL of double distilled water, the solution is boiled for 5 min (Fig. 10 ). Subsequent to boiling and leaving to the solution to cool down, we filtered and stored each extract at 4 °C and used it within a week as a reducing agent for preparing CuO NPs.
Preparation of CuO NPs
In the green synthesis technique, 20 mL of either orange peel extract or mint leaves extract were added to 80 mL of copper sulfate (CuSO 4 ) at a concentration of 0.01 M in a 250 mL Erlenmeyer flask and placed on a magnetic stirrer and heated to 50 °C, then the extract is slowly added to the solution for 20 min and then left stirred for 2 h where the solution changes when adding the mint leaves extract to the brown color, the obtained nanoparticles denoted as CuO NPs-1, and when adding the orange peel extract, the color changed to the light green color, and the obtained nanoparticles denoted as CuO NPs-2. The two mixtures were left for 24 h at room temperature then separated using a centrifuge (12,000×g cycles) for 15 min and the nanoparticles were obtained after drying in an oven at 45 °C for 24 h. The schematic diagram for both nanoparticles synthesis is shown in Fig. 11 .
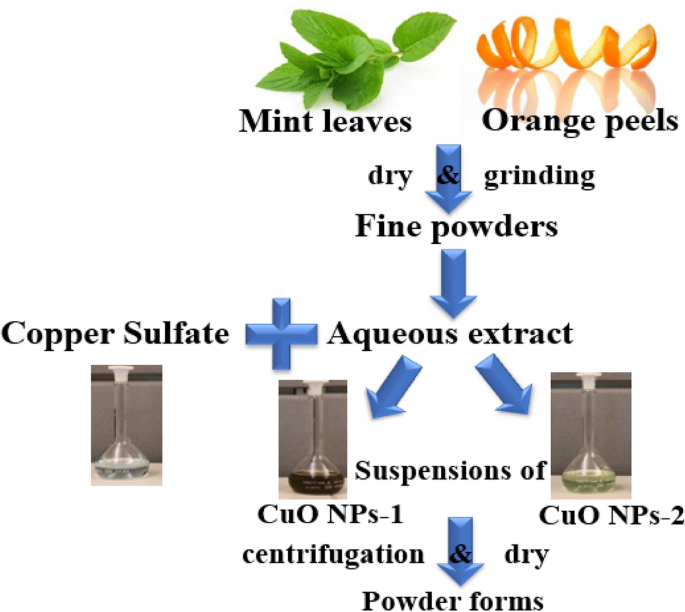
Schematic diagram for the green synthesis of CuO NPs.
Detection and characterization of CuO NPs
The primary detections of CuO NPs were carried out by visual observation of the change in the color of the precursor. The synthesized nanostructures have been characterized by UV–Vis spectroscopy using Shimadzu UV-1700, Japan. The BET surface area of CuO NPs were measured with a Belsorp-miniX (Germany). Prior to this measurement, the samples were degassed at 140 °C. Scanning Electron Microscopy (SEM) coupled with Energy dispersive X-ray (EDX) was used to examine the surface morphology and size of the synthesized CuO NPs as well as its elemental composition. Fourier transform infrared spectroscopy (FT-IR) spectroscopy is used to identify the stretching and bending frequencies of molecular functional groups attached to CuO NPs surface 49 . Its spectra record was conducted in the range of 500–4000 cm −1 .
We have prepared artificial wastewater containing lead, nickel, and cadmium. Several factors were studied. For instance, the doses of CuO NPs-1 and CuO NPs-2 were 0.17, 0.33, 0.67, 1.00, 1.33, 1.67, and 2.00 g L −1 . The optimum dose was fixed at 0.33 g L −1 when studying the other factors. The second factor was contact time at different times (5–90 min) and the time was fixed at 60 min when studying other factors. The third factor was studying the effect of the different metal concentrations (5–40 mg L −1 ) and the concentration was fixed at 20 mg L −1 . The fourth factor was the pH of the solutions. It was adjusted in the range of 3–9 by 0.1 M NaOH or 0.1 M HCl and the pH was fixed at 6.00 when studying the other factors.
The experimental experiments were carried out by shaking 0.33 g L −1 of either CuO NPs-1 or CuO NPs-2 in 30 mL solution of each metal ions, with concentration range from 5 to 40 mg L −1 , onto a bath shaker at 120 rpm. The adsorption capacity and removal percentage of the nanosorbents can be estimated with the following equations 53 , 54 , 55 .
where q e is the equilibrium adsorption capacity (mg g −1 ), C o is the metal ion initial concentration (mg L −1 ), C e is the metal ion concentration (mg L −1 ) at equilibrium, V is the volume of solution (mL) and W is the weight (g) of nanosorbent, R is the removal percentage of the studied metal ions.
Isotherm and kinetics models are investigated to get the optimum conditions of the batch adsorption process. Langmuir and Freundlich models were used as they are most used isotherm models and can be compared to literature based on Eqs. ( 3 ) and ( 4 ). In addition, separation factor (SF; Eq. ( 5 )) is calculated at different initial metal ion concentrations to express the adsorption process feasibility 23 , 56 .
where K L is the Langmuir adsorption equilibrium constant related to the affinity between the metal ions and nanosorbents (L mg −1 ), n is the measure of adsorption intensity and it indicates the relative distribution of energy sites. K f (mg g −1 ) (L mg −1 ) n constant is concerned with the ability of nanosorbent to adsorb. SF is the separation factor (dimensionless).
The kinetics removal of the studied metal ions can be explained using pseudo-first order (Eq. ( 6 )) and pseudo-second order (Eq. ( 7 )). Initial adsorption rate (h) is calculated using Eq. ( 8 ).
where q t is adsorption capacity at contact time (t), K 1 is the pseudo first order rate constant (min −1 ), K 2 the pseudo second order rate constant (g mg −1 min −1 ).
Saratale, R. G. et al. New insights on the green synthesis of metallic nanoparticles using plant and waste biomaterials: Current knowledge, their agricultural and environmental applications. Environ. Sci. Pollut. Res. 25 , 10164–10183. https://doi.org/10.1007/s11356-017-9912-6 (2018).
Article CAS Google Scholar
Mahmoud, A. E. D., Franke, M., Stelter, M. & Braeutigam, P. Mechanochemical versus chemical routes for graphitic precursors and their performance in micropollutants removal in water. Powder Technol. 366 , 629–640. https://doi.org/10.1016/j.powtec.2020.02.073 (2020).
Shaheen, M. N. F., El-hadedy, D. E. & Ali, Z. I. Medical and Microbial applications of controlled shape of silver nanoparticles prepared by ionizing radiation. BioNanoScience 9 , 414–422. https://doi.org/10.1007/s12668-019-00622-2 (2019).
Article Google Scholar
Kole, C. et al. Nanobiotechnology can boost crop production and quality: First evidence from increased plant biomass, fruit yield and phytomedicine content in bitter melon ( Momordica charantia ). BMC Biotechnol. 13 , 37. https://doi.org/10.1186/1472-6750-13-37 (2013).
Article PubMed PubMed Central Google Scholar
Zhao, Y., Zhu, J. J., Hong, J. M., Bian, N. & Chen, H. Y. Microwave-Induced polyol-process synthesis of copper and copper oxide nanocrystals with controllable morphology. Eur. J. Inorg. Chem. 2004 , 4072–4080 (2004).
Wang, S. et al. Synthesis, growth mechanism and thermal stability of copper nanoparticles encapsulated by multi-layer graphene. Carbon 50 , 2119–2125. https://doi.org/10.1016/j.carbon.2011.12.063 (2012).
Article ADS CAS Google Scholar
Ramyadevi, J., Jeyasubramanian, K., Marikani, A., Rajakumar, G. & Rahuman, A. A. Synthesis and antimicrobial activity of copper nanoparticles. Mater. Lett. 71 , 114–116. https://doi.org/10.1016/j.matlet.2011.12.055 (2012).
Lin, S. K. & Cheng, W. T. Fabrication and characterization of colloidal silver nanoparticle via photochemical synthesis. Mater. Lett. 261 , 127077. https://doi.org/10.1016/j.matlet.2019.127077 (2020).
Xiao, X. et al. High-efficient and synergetic antibacterial nanocomposite hydrogel with quaternized chitosan/Ag nanoparticles prepared by one-pot UV photochemical synthesis. Biopolymers 111 , e23354 (2020).
Zhang, Q. & Hua, Y. Electrochemical synthesis of copper nanoparticles using cuprous oxide as a precursor in choline chloride–urea deep eutectic solvent: nucleation and growth mechanism. Phys. Chem. Chem. Phys. 16 , 27088–27095 (2014).
Akintelu, S. A., Folorunso, A. S., Folorunso, F. A. & Oyebamiji, A. K. Green synthesis of copper oxide nanoparticles for biomedical application and environmental remediation. Heliyon 6 , e04508. https://doi.org/10.1016/j.heliyon.2020.e04508 (2020).
AbdelHamid, A. A., Al-Ghobashy, M. A., Fawzy, M., Mohamed, M. B. & Abdel-Mottaleb, M. M. S. A. Phytosynthesis of Au, Ag, and Au–Ag bimetallic nanoparticles using aqueous extract of sago pondweed ( Potamogeton pectinatus L.). ACS Sustain. Chem. Eng. 1 , 1520–1529. https://doi.org/10.1021/sc4000972 (2013).
Salem, S. S. & Fouda, A. Green synthesis of metallic nanoparticles and their prosective biotechnological applications: An overview. Biol. Trace Elem. Res. 199 , 344 (2020).
Mahmoud, A. E. D. Eco-friendly reduction of graphene oxide via agricultural byproducts or aquatic macrophytes. Mater. Chem. Phys. 253 , 123336. https://doi.org/10.1016/j.matchemphys.2020.123336 (2020).
Mahmoud, A. E. D., Umachandran, K., Sawicka, B. & Mtewa, T. K. Water resources security and management for sustainable communities. In Phytochemistry, the Military and Health (eds Mtewa, A. G. & Egbuna, C.) 509–522 (Elsevier, 2021).
Chapter Google Scholar
Badr, N. B. E., Al-Qahtani, K. M. & Mahmoud, A. E. D. Factorial experimental design for optimizing selenium sorption on Cyperus laevigatus biomass and green-synthesized nano-silver. Alex. Eng. J. https://doi.org/10.1016/j.aej.2020.09.051 (2020).
Mahmoud, A. E. D., Fawzy, M. & Radwan, A. Optimization of Cadmium (CD2+) removal from aqueous solutions by novel biosorbent. Int. J. Phytoremediat. 18 , 619–625. https://doi.org/10.1080/15226514.2015.1086305 (2016).
El Din Mahmoud, A. & Fawzy, M. Bio-based methods for wastewater treatment: green sorbents. In Phytoremediation: Management of Environmental Contaminants Vol. 3 (eds Ansari, A. A. et al. ) 209–238 (Springer, 2016).
Mohammad, A. & Hussain, S. Poly (3,4-ethylenedioxythiophene): polystyrene sulfonate (PEDOT:PSS) Zr(IV) phosphate composite cation exchanger: Sol-gel synthesis and physicochemical characterization. Ionics 21 , 1063–1071. https://doi.org/10.1007/s11581-014-1247-4 (2015).
Rangreez, T. A. & Khan, A. J. P. C. Synthesis of single-walled carbon nanotubes cerium (IV) phosphate composite cation exchnager: Ion exchange studies and its application as ion-selective membrane electrode for determination of Cd (II) ions. Polymer Compos. 38 , 1005–1013 (2017).
Mohammad, A. & Hussain, S. Sol–gel synthesis, physicochemical characterization, and analytical applications of copper selective composite cation exchanger: Polyvinyl alcohol Ce (IV) phosphate. Polym. Compos. 38 , 332–340 (2017).
Faisal, A. A. H., Al-Wakel, S. F. A., Assi, H. A., Naji, L. A. & Naushad, M. Waterworks sludge-filter sand permeable reactive barrier for removal of toxic lead ions from contaminated groundwater. J. Water Process Eng. 33 , 101112. https://doi.org/10.1016/j.jwpe.2019.101112 (2020).
Mahmoud, A. E. D. Graphene-based nanomaterials for the removal of organic pollutants: Insights into linear versus nonlinear mathematical models. J. Environ. Manage. 270 , 110911. https://doi.org/10.1016/j.jenvman.2020.110911 (2020).
Article CAS PubMed Google Scholar
Mahmoud, A. E. D., Stolle, A. & Stelter, M. Sustainable synthesis of high-surface-area graphite oxide via dry ball milling. ACS Sustain. Chem. Eng. 6 , 6358–6369. https://doi.org/10.1021/acssuschemeng.8b00147 (2018).
Sun, L., Xin, X., An, X. & Qian, X. Nano-Cu2O-loaded paper: Green preparation and high visible light photocatalytic performance for formaldehyde removal. Paper Biomater. 4 , 1 (2019).
Google Scholar
Nagajyothi, P. C., Muthuraman, P., Sreekanth, T. V. M., Kim, D. H. & Shim, J. Green synthesis: In-vitro anticancer activity of copper oxide nanoparticles against human cervical carcinoma cells. Arab. J. Chem. 10 , 215–225. https://doi.org/10.1016/j.arabjc.2016.01.011 (2017).
Ismail, M. et al. Green synthesis of zerovalent copper nanoparticles for efficient reduction of toxic azo dyes congo red and methyl orange. Green Process. Synth. 8 , 135–143 (2019).
Chung, I. M. et al. Green synthesis of copper nanoparticles using Eclipta prostrata leaves extract and their antioxidant and cytotoxic activities. Exp. Ther. Med. 14 , 18–24. https://doi.org/10.3892/etm.2017.4466 (2017).
Article CAS PubMed PubMed Central Google Scholar
Rajesh, K. M., Ajitha, B., Reddy, Y. A. K., Suneetha, Y. & Reddy, P. S. Assisted green synthesis of copper nanoparticles using Syzygium aromaticum bud extract: Physical, optical and antimicrobial properties. Optik 154 , 593–600. https://doi.org/10.1016/j.ijleo.2017.10.074 (2018).
Vaidehi, D., Bhuvaneshwari, V., Bharathi, D. & Sheetal, B. P. Antibacterial and photocatalytic activity of copper oxide nanoparticles synthesized using Solanum lycopersicum leaf extract. Mater. Res. Express 5 , 085403 (2018).
Article ADS Google Scholar
Długosz, O., Chwastowski, J. & Banach, M. Hawthorn berries extract for the green synthesis of copper and silver nanoparticles. Chem. Pap. 74 , 239–252. https://doi.org/10.1007/s11696-019-00873-z (2020).
Singh, J., Kumar, V., Kim, K.-H. & Rawat, M. Biogenic synthesis of copper oxide nanoparticles using plant extract and its prodigious potential for photocatalytic degradation of dyes. Environ. Res. 177 , 108569. https://doi.org/10.1016/j.envres.2019.108569 (2019).
Khani, R., Roostaei, B., Bagherzade, G. & Moudi, M. Green synthesis of copper nanoparticles by fruit extract of Ziziphus spina -christi (L.) Willd.: Application for adsorption of triphenylmethane dye and antibacterial assay. J. Mol. Liq. 255 , 541–549. https://doi.org/10.1016/j.molliq.2018.02.010 (2018).
Sukumar, S., Rudrasenan, A. & Padmanabhan-Nambiar, D. Green-synthesized rice-shaped copper oxide nanoparticles using Caesalpinia bonducella seed extract and their applications. ACS Omega 5 , 1040–1051. https://doi.org/10.1021/acsomega.9b02857 (2020).
Fakhri, A. Investigation of mercury (II) adsorption from aqueous solution onto copper oxide nanoparticles: Optimization using response surface methodology. Process Saf. Environ. Prot. 93 , 1–8. https://doi.org/10.1016/j.psep.2014.06.003 (2015).
Jain, M., Yadav, M. & Chaudhry, S. Copper oxide nanoparticles for the removal of divalent nickel ions from aqueous solution. Toxin Rev. https://doi.org/10.1080/15569543.2020.1799407 (2020).
Sankar, R. et al. Green synthesis of colloidal copper oxide nanoparticles using Carica papaya and its application in photocatalytic dye degradation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 121 , 746–750. https://doi.org/10.1016/j.saa.2013.12.020 (2014).
Article ADS CAS PubMed Google Scholar
Prasad, K. S., Patra, A., Shruthi, G. & Chandan, S. Aqueous extract of Saraca indica leaves in the synthesis of copper oxide nanoparticles: Finding a way towards going green. J. Nanotechnol. 2017 , 1–6 (2017).
Siddiqui, M. A. et al. Copper oxide nanoparticles induced mitochondria mediated apoptosis in human hepatocarcinoma cells. PLoS ONE 8 , e69534 (2013).
Baylan, N., İlalan, İ & İnci, İ. Copper oxide nanoparticles as a novel adsorbent for separation of acrylic acid from aqueous solution: Synthesis, characterization, and application. Water Air Soil Pollut. 231 , 465. https://doi.org/10.1007/s11270-020-04832-3 (2020).
Dörner, L. et al. Cost-effective sol-gel synthesis of porous CuO nanoparticle aggregates with tunable specific surface area. Sci. Rep. 9 , 11758. https://doi.org/10.1038/s41598-019-48020-8 (2019).
Article ADS CAS PubMed PubMed Central Google Scholar
Aziz, W. J., Abid, M. A. & Hussein, E. H. Biosynthesis of CuO nanoparticles and synergistic antibacterial activity using mint leaf extract. Mater. Technol. 35 , 447–451 (2020).
Priya, D. D. et al. Aerva lanata-mediated bio-treated production of copper oxide nanoparticles, optimization by BBD–RSM method and it behaviour against water related mosquito. Appl. Nanosci. 11 , 207–216. https://doi.org/10.1007/s13204-020-01573-x (2021).
Sreekala, G., Beevi, A. F. & Beena, B. Adsorption of lead (II) Ions by ecofriendly copper oxide nanoparticles. Orient. J. Chem. 35 , 1731 (2019).
Alexa, E. et al. Phytochemical screening and biological activity of Mentha × piperita L and Lavandula angustifolia mill extracts. Anal. Cell. Pathol. 2018 , 1–7 (2018).
Thawkar, B. S. Phytochemical and pharmacological review of Mentha arvensis . International Journal of Green Pharmacy 10 , 2 (2016).
Gotmare, S. & Gade, J. Orange peel: A potential source of phytochemical compounds. Int. J. ChemTech Res. 11 , 240–243 (2018).
CAS Google Scholar
Nasr, M., Mahmoud, A. E. D., Fawzy, M. & Radwan, A. Artificial intelligence modeling of cadmium(II) biosorption using rice straw. Appl. Water Sci. 7 , 823–831. https://doi.org/10.1007/s13201-015-0295-x (2017).
Pasandide, B., Khodaiyan, F., Mousavi, Z. & Hosseini, S. S. Pectin extraction from citron peel: Optimization by Box-Behnken response surface design. Food Sci. Biotechnol. 27 , 997–1005. https://doi.org/10.1007/s10068-018-0365-6 (2018).
Desta, M. B. Batch sorption experiments: Langmuir and Freundlich isotherm studies for the adsorption of textile metal ions onto teff straw ( Eragrostis tef ) agricultural waste. Journal of Thermodynamics 2013 (2013).
Kharissova, O. V., Martínez, L. M. T. & Kharisov, B. I. Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications 1–21 (Springer, 2020).
Book Google Scholar
Farghali, A. A., Bahgat, M., Enaiet Allah, A. & Khedr, M. H. Adsorption of Pb(II) ions from aqueous solutions using copper oxide nanostructures. Beni-Suef Univ. J. Basic Appl. Sci. 2 , 61–71. https://doi.org/10.1016/j.bjbas.2013.01.001 (2013).
Mahmoud, A. E. D., Fawzy, M., Hosny, G. & Obaid, A. Equilibrium, kinetic, and diffusion models of chromium(VI) removal using Phragmites australis and Ziziphus spina -christi biomass. Int. J. Environ. Sci. Technol. https://doi.org/10.1007/s13762-020-02968-7 (2020).
Mahmoud, A. E. D., Franke, M., Stelter, M. & Braeutigam, P. 4 th International Congress on Water, Waste and Energy Management (2018).
Mahmoud, A. E. D. & Fawzy, M. Statistical methodology for Cadmium (Cd (II)) removal from wastewater by different plant biomasses. J. Bioremediat. Biodegr. 6 , 1–7 (2015).
Mahmoud, A., Stolle, A., Stelter, M. & Braeutigam, P. Adsorption Technique for Organic Pollutants Using Different Carbon Materials, Abstracts of Papers of the American Chemical Society (American Chemical Society, 2018).
Download references
Acknowledgements
This research was supported by the Chair of Environmental Pollution Research at Princess Nourah bint Abdulrahman University (Grant No. EPR-013).
Author information
Authors and affiliations.
Environmental Sciences Department, Faculty of Science, Alexandria University, Alexandria, 21511, Egypt
Alaa El Din Mahmoud
Green Technology Group, Faculty of Science, Alexandria University, Alexandria, 21511, Egypt
Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia
Khairia M. Al-Qahtani, Sahab O. Alflaij & Faten A. Alsamhan
Center of Excellence for Advanced Materials and Manufacturing, King Abdulaziz City for Science and Technology, Riyadh, Saudi Arabia
Salma F. Al-Qahtani
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization, A.E.D.M. and K.M.A.-Q.; visualization, A.E.D.M.; project administration, K.M.A.-Q.; writing—original draft preparation, A.E.D.M., S.O.A. and S.F.A.-Q.; writing—review and editing, A.E.D.M., methodology, A.E.D.M. and F.A.A.; Formal analysis, A.E.D.M. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Correspondence to Alaa El Din Mahmoud .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Mahmoud, A.E.D., Al-Qahtani, K.M., Alflaij, S.O. et al. Green copper oxide nanoparticles for lead, nickel, and cadmium removal from contaminated water. Sci Rep 11 , 12547 (2021). https://doi.org/10.1038/s41598-021-91093-7
Download citation
Received : 12 November 2020
Accepted : 18 May 2021
Published : 15 June 2021
DOI : https://doi.org/10.1038/s41598-021-91093-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Inhibitory role of copper and silver nanocomposite on important bacterial and fungal pathogens in rice (oryza sativa).
- Arnab Roy Chowdhury
- Rishikesh Kumar
- Biplab Sarkar
Scientific Reports (2024)
Potential of nano-phytoremediation of heavy metal contaminated soil: emphasizing the role of mycorrhizal fungi in the amelioration process
- D. K. Gupta
International Journal of Environmental Science and Technology (2024)
Biogenic synthesis of copper oxide nanoparticles using a cold tolerant bacterium for RB5/DR23 Azo dye adsorption
- Mahshad Kamalian
- Bahar Shahnavaz
- Mohsen Karrabi
Biomass Conversion and Biorefinery (2024)
Utilizing sequence transformation of selective copper metal as an efficient heterogeneous Fenton-like catalyst for the degradation of aqueous methylene blue
- Harez Rashid Ahmed
- Fryad S. Mustafa
- Steven John Hinder
Reaction Kinetics, Mechanisms and Catalysis (2024)
Penicillium oxalicum-mediated the green synthesis of silica nanoparticles: characterization and environmental applications
- Hazem Elsayed Kaabo
- Ebrahim Saied
- Mahmoud H. Sultan
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
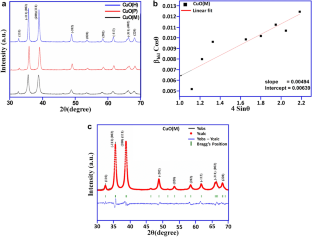
Structural and optical properties of copper oxide nanoparticles: A study of variation in structure and antibiotic activity
- Published: 19 April 2021
- Volume 36 , pages 1496–1509, ( 2021 )
Cite this article

- Ankush Chauhan 1 ,
- Ritesh Verma 1 ,
- Khalid Mujasam Batoo ORCID: orcid.org/0000-0001-8264-8203 2 ,
- Swati Kumari 3 ,
- Rahul Kalia 1 ,
- Rajesh Kumar 1 , 4 ,
- Muhammad Hadi 5 ,
- Emad H. Raslan 5 &
- Ahamad Imran 3
983 Accesses
30 Citations
Explore all metrics
In this paper, we study the synthesis dependence of structural, optical and antimicrobial properties for copper oxide nanoparticles on, synthesized using microwave irradiation CuO(M), co-precipitation CuO(P) and hydrothermal CuO(H) protocols. Structural and morphological properties were studied using XRD, SEM, TEM and SAED techniques. XPS studies confirmed the presence of copper ions in Cu 2+ oxidation state, and Raman spectroscopy confirmed the presence of nanostructured phase in all the samples. The synthesized CuO(M), CuO(P) and CuO(H) nanoparticles were investigated for antimicrobial activity against different pathogenic bacteria including methicillin-resistant Staphylococcus aureus . The result showed that maximum inhibition zone was detected in CuO(M) nanoparticles against Gram-negative bacteria i.e. Klebsiella pneumoniae (20 mm). CuO(H) and CuO(P) nanoparticles have antibacterial inhibition zone of 17 mm and 13 mm against K. pneumoniae and S. aureus , respectively. The CuO(P) and CuO(H) nanoparticles displayed mild antimicrobial activity as compared to the CuO(M) nanoparticles.
Graphic abstract
This is a preview of subscription content, log in via an institution to check access.

Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
Precursor-dependent structural properties and antibacterial activity of copper oxide
Chemical synthesis, characterization and evaluation of antimicrobial properties of cu and its oxide nanoparticles, synthesis, characterization, and antibacterial activity of copper(ii) oxide nanoparticles prepared by thermal decomposition, data availability.
All data generated or analysed during this study are included in this published article and in supplementary file.
Y. Liu, Q. Huang, G. Jiang, D. Liu, W. Yu, Cu 2 O nanoparticles supported on carbon nanofibers as a cost-effective and efficient catalyst for RhB and phenol degradation. J. Mater. Res. 32 , 3605–3615 (2017)
Article CAS Google Scholar
K. Vishveshvar, M.A. Krishnan, K. Haribabu, S. Vishnuprasad, Green synthesis of copper oxide nanoparticles using ixiro coccinea plant leaves and its characterization. BioNanoScience 8 , 554–558 (2018)
Article Google Scholar
M. Khan, M.R. Shaik, S.F. Adil, M. Kuniyil, M. Ashraf, H. Frerichs et al., Facile synthesis of Pd@ graphene nanocomposites with enhanced catalytic activity towards Suzuki coupling reaction. Sci. Rep. 10 , 1–14 (2020)
CAS Google Scholar
K. Gherab, Y. Al-Douri, U. Hashim, M. Ameri, A. Bouhemadou, K.M. Batoo et al., Fabrication and characterizations of Al nanoparticles doped ZnO nanostructures-based integrated electrochemical biosensor. J. Market. Res. 9 , 857–867 (2020)
S.M. Boddapati, J.M.R. Saketi, B.R. Mutchu, H.B. Bollikolla, S.F. Adil, M. Khan, Copper promoted desulfurization and CN cross coupling reactions: Simple approach to the synthesis of substituted 2-aminobenzoxazoles and 2, 5-disubstituted tetrazole amines. Arab. J. Chem. 13 , 4477–4494 (2020)
S.F. Adil, M.E. Assal, M.R. Shaik, M. Kuniyil, N.M. AlOtaibi, M. Khan et al., A facile synthesis of ZrOx-MnCO 3 /graphene oxide (GRO) nanocomposites for the oxidation of alcohols using molecular oxygen under base free conditions. Catalysts 9 , 759 (2019)
S. Kumar, R. Rani, N. Dilbaghi, K. Tankeshwar, K.-H. Kim, Carbon nanotubes: a novel material for multifaceted applications in human healthcare. Chem. Soc. Rev. 46 , 158–196 (2017)
S. Kumar, W. Ahlawat, G. Bhanjana, S. Heydarifard, M.M. Nazhad, N. Dilbaghi, Nanotechnology-based water treatment strategies. J. Nanosci. Nanotechnol. 14 , 1838–1858 (2014)
N.D. Mu’azu, N. Jarrah, M. Zubair, M.S. Manzar, T.S. Kazeem, A. Qureshi et al., Mechanistic aspects of magnetic MgAlNi barium-ferrite nanocomposites enhanced adsorptive removal of an anionic dye from aqueous phase. J. Saudi Chem. Soc. 24 , 715–732 (2020)
S. Saif, A. Tahir, T. Asim, Y. Chen, S.F. Adil, Polymeric nanocomposites of iron-oxide nanoparticles (IONPs) synthesized using terminalia chebula leaf extract for enhanced adsorption of arsenic (V) from water. Colloids Interfaces 3 , 17 (2019)
M. Signoretto, F. Menegazzo, A. Di Michele, E. Fioriniello, Effects of support and synthetic procedure for sol-immobilized Au nanoparticles. Catalysts 6 , 87 (2016)
M.S. Bakshi, How surfactants control crystal growth of nanomaterials. Cryst. Growth Des. 16 , 1104–1133 (2016)
Y. Li, C. Li, B. Wang, W. Li, P. Che, A comparative study on the thermoelectric properties of CoSb3 prepared by hydrothermal and solvothermal route. J. Alloy. Compd. 772 , 770–774 (2019)
J.Y. Cheon, S.J. Kim, Y.H. Rhee, O.H. Kwon, W.H. Park, Shape-dependent antimicrobial activities of silver nanoparticles. Int. J. Nanomed. 14 , 2773 (2019)
J.J. Lv, M.Y. Li, and Q.X. Zeng, Preparation and characterization of copper oxide and copper nanoparticles. in Advanced Materials Research , 2011, pp. 715–721
N. Dhineshbabu, V. Rajendran, N. Nithyavathy, R. Vetumperumal, Study of structural and optical properties of cupric oxide nanoparticles. Appl. Nanosci. 6 , 933–939 (2016)
J.K. Sharma, M.S. Akhtar, S. Ameen, P. Srivastava, G. Singh, Green synthesis of CuO nanoparticles with leaf extract of Calotropis gigantea and its dye-sensitized solar cells applications. J. Alloy. Compd. 632 , 321–325 (2015)
O. Waser, M. Hess, A. Grntner, P. Novßk, S.E. Pratsinis, Size controlled CuO nanoparticles for Li-ion batteries. J. Power Sources 241 , 415–422 (2013)
F. Wang, H. Li, Z. Yuan, Y. Sun, F. Chang, H. Deng et al., A highly sensitive gas sensor based on CuO nanoparticles synthetized via a sol–gel method. RSC Adv. 6 , 79343–79349 (2016)
M. Shahmiri, N.A. Ibrahim, F. Shayesteh, N. Asim, N. Motallebi, Preparation of PVP-coated copper oxide nanosheets as antibacterial and antifungal agents. J. Mater. Res. 28 , 3109 (2013)
P. Sutradhar, M. Saha, D. Maiti, Microwave synthesis of copper oxide nanoparticles using tea leaf and coffee powder extracts and its antibacterial activity. J. Nanostruct. Chem. 4 , 86 (2014)
C. Boruban, E.N. Esenturk, Synthesis of CuO nanostructures on zeolite-Y and investigation of their CO2 adsorption properties. J. Mater. Res. 32 , 3669 (2017)
G. Mustafa, H. Tahir, M. Sultan, N. Akhtar, Synthesis and characterization of cupric oxide (CuO) nanoparticles and their application for the removal of dyes. Afr. J. Biotechnol. 12 , 6650–6660 (2013)
R. Sankar, R. Maheswari, S. Karthik, K.S. Shivashangari, V. Ravikumar, Anticancer activity of Ficus religiosa engineered copper oxide nanoparticles. Mater. Sci. Eng. C 44 , 234–239 (2014)
A. Hussain, M.F. AlAjmi, M.T. Rehman, S. Amir, F.M. Husain, A. Alsalme et al., Copper (II) complexes as potential anticancer and nonsteroidal anti-inflammatory agents: in vitro and in vivo studies. Sci. Rep. 9 , 1–17 (2019)
C.L. Carnes, K.J. Klabunde, The catalytic methanol synthesis over nanoparticle metal oxide catalysts. J. Mol. Catal. A 194 , 227–236 (2003)
L. Sun, Z. Zhang, Z. Wang, Z. Wu, H. Dang, Synthesis and characterization of CuO nanoparticles from liquid ammonia. Mater. Res. Bull. 40 , 1024–1027 (2005)
P. Saravanan, S. Alam, G. Mathur, A liquid−liquid interface technique to form films of CuO nanowhiskers. Thin Solid Films 491 , 168–172 (2005)
T. Ahmad, R. Chopra, K. Ramanujachary, S. Lofland, A. Ganguli, Canted antiferromagnetism in copper oxide nanoparticles synthesized by the reverse-micellar route. Solid State Sci. 7 , 891–895 (2005)
V.V.T. Padil, M. Černík, Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. Int. J. Nanomed. 8 , 889 (2013)
Google Scholar
B.D. Cullity, Elements of X-Ray Diffraction (Addison-Wesley Publishing, Boston, 1956).
E.E. Kaya, S. Gürmen, A straightforward approach for the synthesis of nanostructured Y 2 O 3 particles: synthesis, morphology, microstructure and crystal imperfection. Phys. E. 115 , 113668 (2020)
V. Mote, Y. Purushotham, B. Dole, Williamson-Hall analysis in estimation of lattice strain in nanometer-sized ZnO particles. J. Theoret. Appl. Phys. 6 , 6 (2012)
G. Rajender, P. Giri, Strain induced phase formation, microstructural evolution and bandgap narrowing in strained TiO 2 nanocrystals grown by ball milling. J. Alloy. Compd. 676 , 591–600 (2016)
A. Chauhan, R. Verma, S. Kumari, A. Sharma, P. Shandilya, X. Li et al., Photocatalytic dye degradation and antimicrobial activities of pure and Ag-doped ZnO using Cannabis sativa leaf extract. Sci. Rep. 10 , 1–16 (2020)
P. Bindu, S. Thomas, Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis. J. Theoret. Appl. Phys. 8 , 123–134 (2014)
Y. Asakuma, M. Miura, Effect of microwave radiation on diffusion behavior of anti-solvent during crystallization. J. Cryst. Growth 402 , 32–36 (2014)
Y. Wang, Y. Lü, W. Zhan, Z. Xie, Q. Kuang, L. Zheng, Synthesis of porous Cu 2 O/CuO cages using Cu-based metal–organic frameworks as templates and their gas-sensing properties. J. Mater. Chem. A 3 , 12796–12803 (2015)
R. Mariammal, K. Ramachandran, B. Renganathan, D. Sastikumar, On the enhancement of ethanol sensing by CuO modified SnO 2 nanoparticles using fiber-optic sensor. Sens. Actuators B 169 , 199–207 (2012)
D.D.M. Prabaharan, K. Sadaiyandi, M. Mahendran, S. Sagadevan, Precipitation method and characterization of cobalt oxide nanoparticles. Appl. Phys. A 123 , 264 (2017)
H. Siddiqui, M. Qureshi, F.Z. Haque, Effect of copper precursor salts: facile and sustainable synthesis of controlled shaped copper oxide nanoparticles. Optik 127 , 4726–4730 (2016)
M. Dar, Q. Ahsanulhaq, Y. Kim, J. Sohn, W. Kim, H. Shin, Versatile synthesis of rectangular shaped nanobat-like CuO nanostructures by hydrothermal method; structural properties and growth mechanism. Appl. Surf. Sci. 255 , 6279–6284 (2009)
J.D. Rodney, S. Deepapriya, P.A. Vinosha, S. Krishnan, S.J. Priscilla, R. Daniel et al., Photo-Fenton degradation of nano-structured La doped CuO nanoparticles synthesized by combustion technique. Optik 161 , 204–216 (2018)
H. Siddiqui, M.R. Parra, F.Z. Haque, Optimization of process parameters and its effect on structure and morphology of CuO nanoparticle synthesized via the sol−gel technique. J. Sol-Gel. Sci. Technol. 87 , 125–135 (2018)
A. Bhaumik, A.M. Shearin, R. Patel, K. Ghosh, Significant enhancement of optical absorption through nano-structuring of copper based oxide semiconductors: possible future materials for solar energy applications. Phys. Chem. Chem. Phys. 16 , 11054–11066 (2014)
J. Irwin, T. Wei, Raman scattering investigation of Cu18O. J. Phys. 3 , 299 (1991)
J. Xu, W. Ji, Z. Shen, W. Li, S. Tang, X. Ye et al., Raman spectra of CuO nanocrystals. J. Raman Spectrosc. 30 , 413–415 (1999)
M. Rashad, M. Rüsing, G. Berth, K. Lischka, and A. Pawlis, CuO and Co 3 O 4 nanoparticles: synthesis, characterizations, and Raman spectroscopy. J. Nanomater. 2013 (2013)
Z.N. Kayani, M. Umer, S. Riaz, S. Naseem, Characterization of copper oxide nanoparticles fabricated by the sol–gel method. J. Electron. Mater. 44 , 3704–3709 (2015)
R. Jana, A. Dey, M. Das, J. Datta, P. Das, P.P. Ray, Improving performance of device made up of CuO nanoparticles synthesized by hydrothermal over the reflux method. Appl. Surf. Sci. 452 , 155–164 (2018)
A. Bhattacharjee, M. Ahmaruzzaman, Microwave assisted facile and green route for synthesis of CuO nanoleaves and their efficacy as a catalyst for reduction and degradation of hazardous organic compounds. J. Photochem. Photobiol. A 353 , 215–228 (2018)
X. Wu, L. Ye, K. Liu, W. Wang, J. Wei, F. Chen et al., Antibacterial properties of mesoporous copper-doped silica xerogels. Biomed. Mater. 4 , 045008 (2009)
K.R. Raghupathi, R.T. Koodali, A.C. Manna, Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 27 , 4020–4028 (2011)
J.E. Weckx, H.M. Clijsters, Oxidative damage and defense mechanisms in primary leaves of Phaseolus vulgaris as a result of root assimilation of toxic amounts of copper. Physiol. Plant. 96 , 506–512 (1996)
R. Brayner, R. Ferrari-Iliou, N. Brivois, S. Djediat, M.F. Benedetti, F. Fiévet, Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 6 , 866–870 (2006)
Z. Huang, X. Zheng, D. Yan, G. Yin, X. Liao, Y. Kang et al., Toxicological effect of ZnO nanoparticles based on bacteria. Langmuir 24 , 4140–4144 (2008)
A. Abdal Dayem, M.K. Hossain, S.B. Lee, K. Kim, S.K. Saha, G.-M. Yang et al., The role of reactive oxygen species (ROS) in the biological activities of metallic nanoparticles. Int. J. Mol. Sci. 18 , 120 (2017)
M. Raffi, S. Mehrwan, T.M. Bhatti, J.I. Akhter, A. Hameed, W. Yawar et al., Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli . Ann. Microbiol. 60 , 75–80 (2010)
J.P. Ruparelia, A.K. Chatterjee, S.P. Duttagupta, S. Mukherji, Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 4 , 707–716 (2008)
R. Verma, A. Chauhan, M. Shandilya, X. Li, R. Kumar, S. Kulshrestha, Antimicrobial potential of Ag-doped ZnO nanostructure synthesized by the green method using Moringa oleifera extract. J. Environ. Chem. Eng. 8 , 103730 (2020)
Download references
Acknowledgments
Author K M Batoo is thankful to the Deanship of Scientific Research at King Saud University for financial support through the project Code (RG-1437-030).
Author information
Authors and affiliations.
School of Physics and Materials Science, Shoolini University of Biotechnology & Management Sciences, Bajhol-Solan, HP, 173212, India
Ankush Chauhan, Ritesh Verma, Rahul Kalia & Rajesh Kumar
King Abdullah Institute for Nanotechnology, King Saud University, P.O. Box 2455, Riyadh, 11451, Saudi Arabia
Khalid Mujasam Batoo
School of Applied Science and Biotechnology, Shoolini University of Biotechnology & Management Sciences, Bajhol-Solan, HP, 173212, India
Swati Kumari & Ahamad Imran
Himalayan Centre of Excellence for Nanotechnology, Shoolini University of Biotechnology & Management Sciences, Bajhol-Solan, HP, 173212, India
Rajesh Kumar
Department of Physics and Astronomy, King Saud University, P.O. Box 2455, Riyadh, 11451, Saudi Arabia
Muhammad Hadi & Emad H. Raslan
You can also search for this author in PubMed Google Scholar
Corresponding authors
Correspondence to Khalid Mujasam Batoo or Rajesh Kumar .
Ethics declarations
Conflict of interest.
Authors declare that they have no conflict of interest among them.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 2422 kb)
Rights and permissions.
Reprints and permissions
About this article
Chauhan, A., Verma, R., Batoo, K.M. et al. Structural and optical properties of copper oxide nanoparticles: A study of variation in structure and antibiotic activity. Journal of Materials Research 36 , 1496–1509 (2021). https://doi.org/10.1557/s43578-021-00193-7
Download citation
Received : 13 February 2021
Accepted : 28 March 2021
Published : 19 April 2021
Issue Date : 14 April 2021
DOI : https://doi.org/10.1557/s43578-021-00193-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Nanoparticles
- Antimicrobial
- Find a journal
- Publish with us
- Track your research

Evaluation the thermal conductivity of denture soft liner reinforcement with copper oxide nanoparticles
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Reprints and Permissions
- Cite Icon Cite
- Search Site
Maha Hussain Dewan , Hawraa Khalid Aziz; Evaluation the thermal conductivity of denture soft liner reinforcement with copper oxide nanoparticles. AIP Conf. Proc. 7 May 2024; 3097 (1): 090030. https://doi.org/10.1063/5.0211091
Download citation file:
- Ris (Zotero)
- Reference Manager
The goal of this research was to assess the effect of adding different percentages of copper oxide nanoparticles into soft denture lining materials on thermal conductance. The 30 samples were made of soft liner (The di-ameter is forty millimeters, while the thickness is 2.5 mm) were separated into (3) groups, Group (A) Control without adding Nano filler of (CuO) (10) specimens, Group (B) 0.3%CuO NPs addition (10) specimens, Group (C) 0.5% CuO NPs addition (10) specimens Each sample was measured with the thermal conductivity test machine (HOT DISK) to tested the thermal conductivity. The results showed Statistics that are descriptive for thermal conductivity for all experimental groups as shown in table (1) revealed that the mean value for 0.5% CuONPs groups was highest among all the groups (0.33±0.014), then Group (B) 0.3%CuO NPs. Then Group (A) Control without adding Nano filler.
Citing articles via
Publish with us - request a quote.

Sign up for alerts
- Online ISSN 1551-7616
- Print ISSN 0094-243X
- For Researchers
- For Librarians
- For Advertisers
- Our Publishing Partners
- Physics Today
- Conference Proceedings
- Special Topics
pubs.aip.org
- Privacy Policy
- Terms of Use
Connect with AIP Publishing
This feature is available to subscribers only.
Sign In or Create an Account
RSC Advances
Different morphologies on cu–ce/tio 2 catalysts for the selective catalytic reduction of no x with nh 3 and drifts study on sol–gel nanoparticles †.

* Corresponding authors
a State Power Environmental Protection Research Institute, Nanjing 210031, Jiangsu, China
b School of Environment, Jiangsu Province Engineering Research Center of Environmental Risk Prevention and Emergency Response Technology, Nanjing Normal University, Nanjing 210023, Jiangsu, China E-mail: [email protected]
c College of Chemistry & Environmental Sciences, Yili Normal University, Yining 835000, Xinjiang, China
d Guangzhou HuaKe Environmental Protection Engineering Co Ltd, Guangzhou 510655, Guangdong, China E-mail: [email protected]
e South China Institute of Environmental Science, Ministry of Ecology and Environment, Guangzhou 510655, Guangdong, China
The copper–cerium binary oxide catalysts supported by titanium dioxide with nanosphere core–shell structures, nanotube (TNT) core–shell structures, impregnation (imp) nanoparticles and sol–gel nanoparticles were prepared for NH 3 -SCR of NO x under medium-low temperature conditions. The effect of different morphologies on the Cu–Ce/TiO 2 catalysts was comprehensively studied through physicochemical characterization. The results showed that the sol–gel nanoparticles exhibited 100% NO x reduction efficiency in the temperature range of 180–400 °C. Compared with the other catalysts, the sol–gel nanoparticle catalyst had the highest dispersion and lowest crystallinity, indicating that morphology played an important role in the NH 3 -SCR of the catalyst. The in situ DRIFTS study on the sol–gel nanoparticle catalyst shows that cerium could promote Cu 2+ to produce abundant Lewis acid sites, which would significantly increase the adsorption reaction of ammonia on the catalyst surface, thereby promoting the occurrence of the Eley–Rideal (E–R) mechanism. With the Ce–Ti interaction on the atomic scale, the Ce–O–Ti structure enhanced the redox properties at a medium temperature. In addition, cerium oxide enhances the strong interaction between the catalyst matrix and CuO particles. Therefore, the reducibility of the CuO species was enhanced.

Supplementary files
- Supplementary information PDF (381K)
Article information
Download Citation
Permissions.
Different morphologies on Cu–Ce/TiO 2 catalysts for the selective catalytic reduction of NO x with NH 3 and DRIFTS study on sol–gel nanoparticles
K. Zhuang, P. Jin, L. Yang, J. Yao, L. Yu, Z. Sheng, X. Chu, Z. Zhuang and X. Chen, RSC Adv. , 2023, 13 , 25989 DOI: 10.1039/D3RA03018K
This article is licensed under a Creative Commons Attribution-NonCommercial 3.0 Unported Licence . You can use material from this article in other publications, without requesting further permission from the RSC, provided that the correct acknowledgement is given and it is not used for commercial purposes.
To request permission to reproduce material from this article in a commercial publication , please go to the Copyright Clearance Center request page .
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party commercial publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page .
Read more about how to correctly acknowledge RSC content .
Social activity
Search articles by author, advertisements.
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Green synthesis and characterization of copper nanoparticles for investigating their effect on germination and growth of wheat
Contributed equally to this work with: Humaira Kausar, Ansar Mehmood
Roles Conceptualization, Investigation
Affiliation Department of Botany, University of Poonch Rawalakot, Azad Kashmir, Pakistan
Roles Conceptualization, Supervision, Writing – original draft, Writing – review & editing
* E-mail: [email protected] , [email protected]
Roles Formal analysis
¶ ‡ RTK, KSA, SH, FN, MSI, MN and TSU also contributed equally to this work.
Affiliation Department of Botany, the University of Azad Jammu and Kashmir (UAJK), Muzaffarabad, Pakistan
Roles Methodology, Writing – review & editing
Roles Validation
Roles Software
Current address: Institute of Crop Science (340 h), University of Hohenheim, Stuttgart, Germany
Affiliation Department of Agronomy, MNS University of Agriculture Multan, Punjab, Pakistan
Affiliation Department of Botany, University of Gujrat, Punjab, Pakistan
Roles Resources
Affiliation Department of Botany, University of Kotli, Azad Jammu and Kashmir, Pakistan
- Humaira Kausar,
- Ansar Mehmood,
- Rizwan Taj Khan,
- Khawaja Shafique Ahmad,
- Sajjad Hussain,
- Fahim Nawaz,
- Muhammad Sajjad Iqbal,
- Muhammad Nasir,
- Tariq Saif Ullah

- Published: June 21, 2022
- https://doi.org/10.1371/journal.pone.0269987
- Reader Comments
Today, different types of nanoparticles (NPs) are being synthesized and used for medical and agricultural applications. In this study, copper nanoparticles (CuNPs) were synthesized using the aqueous extract of mint ( Mentha longifolia L.). For the characterization of CuNPs, UV-visible spectroscopy, scanning electron microscopy, X-ray diffraction, and Fourier transform infrared spectrometry were used. The UV-Visible absorption peak at 558 nm confirmed the formation of CuNPs. The XRD pattern confirmed the phase-centered crystalline nature of CuNPs. FTIR analysis showed the O-H, Cu-H and C-C bonds, indicating the active role of these functional groups as reducing agents of Cu ions to CuNPS. The synthesized NPs were found to have an almost spherical shape with an average size of 23 nm. When applied to wheat, a condition dependent effect of CuNPs was found. Variety 18-Elite Line 1, Elite Line 3, and 18-Elite Line 6 showed maximum germination and growth rate at 50 mg CuNPs/L, while variety 18-Elite Line 5 showed that increase at 25 mg CuNPs/L. Beyond these concentrations, the seed germination and growth of wheat declined. In conclusion, the application of CuNPs showed a beneficial effect in improving the growth of wheat at a certain concentration.
Citation: Kausar H, Mehmood A, Khan RT, Ahmad KS, Hussain S, Nawaz F, et al. (2022) Green synthesis and characterization of copper nanoparticles for investigating their effect on germination and growth of wheat. PLoS ONE 17(6): e0269987. https://doi.org/10.1371/journal.pone.0269987
Editor: Mohammad Shahid, Aligarh Muslim University, INDIA
Received: February 18, 2022; Accepted: June 1, 2022; Published: June 21, 2022
Copyright: © 2022 Kausar et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the paper and its Supporting information files.
Funding: The author(s) received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Research in nanotechnology has evolved rapidly over the past few decades. Nanotechnology is a branch of science that deals with the atomic and molecular analysis of material objects [ 1 ]. Nanoparticles (NPs) are classified as particles up to 100 nm in size and consisting of chemical and physical processes, including unique properties [ 2 ]. They have a very significant role in various applications because of their unique properties such as optical, electrical, catalytic, electromagnetic, and mechanical, which enable them to be used in various sectors such as medical diagnostics, therapy, electronics, clothing, and agriculture [ 3 ]. Nano research is having an impact on a variety of fields, including the environment and catalysts [ 4 – 7 ]. Metal NPs production has been given great attention in the last few years, particularly to control and discover their possible applications and specific properties. The increased production of metal NPs has also increased their discharge into our environment, and the effect of their particular physical and chemical properties on the ecosystem is becoming a key concern [ 8 ]. NPs can have both negative and positive effects on the growth and development of a plant, and their influence on plants depends on the size, shape, and properties of both plant species and NPs [ 9 ].
Copper (Cu), an element in block D of the periodic table, is a microelement essential for plant improvement and growth. It is a cofactor of superoxide phenol oxidases, ascorbate oxidase, a part of regulatory proteins, and is involved in the electron transport chain during photosynthesis and respiration [ 10 , 11 ]. Cu in the form of nanomaterial gains exceptional properties like its small size and high surface area, which give it chemical reactivity, physical resistance, magnetism, and optical effects [ 12 ]. Due to these unique properties, copper nanoparticles (CuNPs) are used for a wide variety of applications, including bioactive coatings, air and liquid filtration, sensors, ceramics, films, skin products, lubricant oils, inks, wood protection, and textiles. Recent estimates suggest that the global production of CuNPs, or Cu-based NPs, has come to 200 tons/year [ 13 ]. Their extensive engineering and use have led them to enter the environment and interact with agriculture. They can cause either positive or negative effects following contact with plants, depending upon the concentration of the NPs and the type of plant. It is therefore the need of the hour to investigate the effect of CuNPs on crops, as there are only a few studies available that show the influence of CuNPs on crop plants.
CuNPs can be engineered by way of specific routes like physical, chemical, and biological techniques [ 14 – 16 ]. The main problems with the physical and chemical methods are the time-consuming nature of them and their usage of exclusive and toxic chemicals [ 17 ]. The chemical methods are eco-incompatible, costly, and also have a low yield. In contrast to physical and chemical methods, biological methods (green synthesis) are more advantageous in the sense of being eco-friendly, cost-effective, and high yielding [ 18 ]. Green synthesis is largely motivated by environmental concerns, with the goal of developing a green pathway for NPs synthesis that is also contamination-free [ 19 ].
Green synthesis involves the use of algae [ 20 ], sea cucumbers [ 6 ], marine animals [ 21 ], plants [ 16 , 22 , 23 ], and microorganisms among many others [ 24 , 25 ]. Biological techniques make it easier to reduce dissolved metal ions to a zero-valence state and produce the corresponding nanoparticles because of their inherent capabilities. NPs synthesis with the aid of using plants is beneficial over the use of microorganisms because it gets rid of the complicated procedure of keeping cellular cultures and can also be well scaled up [ 26 ]. Keeping in mind the importance and benefits of green synthesis, CuNPs were synthesized in this study by using a plant extract and their effect was evaluated on the growth and development of wheat ( T . aestivum ), an essential and widely consumed staple food. In Pakistan, wheat crops provide a living for 80 percent of farmers. It is also a very significant single commodity in Pakistan’s rural areas as a source of earnings [ 27 ]. Although some studies have reported the effect of CuNPs on wheat, they have used either very small concentrations (0.2, 0.4, 0.6, 0.8, and 1.0 ppm) of CuNPs [ 28 ] or chemically engineered CuNPs (50 nm) of large size [ 29 ]. In contrast to these, we used biologically synthesized CuNPs of 23 nm in size at a concentration of 0, 25, 50, and 100 mg/L.
Therefore, the first aim of this study is the green synthesis and characterization of CuNPs. In this study, an aqueous extract of Mentha longifolia will be used for the reduction of Cu ions to CuNPs at room temperature. The CuNPs will be characterized by UV-visible spectroscopy, scanning electron microscopy, X-ray diffraction, and Fourier transform infrared spectrometry. The second aim is the investigation of the effect of biosynthesized CuNPs on germination, growth, and biochemical attributes of the widely consumed staple food wheat.
Material and methods
Synthesis and characterization of cunps.
A biological method, i.e., the use of an aqueous extract of M . longifolia was adopted in this study for the synthesis of CuNPs. The plant material was collected from the Rawalakot region of Azad Jammu and Kashmir, Pakistan. The plant was identified with the help of the flora of Pakistan and other available literature, and a voucher specimen was submitted to the herbarium, Department of Botany, University of Poonch Rawalakot. To prepare the aqueous extract, the whole plant was dried in the shade, ground to powder, and 25 g of powder was dissolved in 400 mL of distilled water, then kept at room temperature for 24 hours. After that, it was filtered through Whatman filter paper no. 42 and the filtrate was used for the synthesis of CuNPs. The CuNPs synthesis was attained by treating 80 mL of 1 mM CuSO 4 at room temperature with 20 mL of plant extract, held in the dark, and color changes were observed. When the brown color developed (after 24 hrs), the reacting mixture was analyzed for UV-vis spectroscopy in the range of wavelengths between 300 to 800 nm using Perkin-Elmer lambda 750 spectrophotometers, indicating the formation of CuNPs. After initial indication, the reacting solution was centrifuged for 15 min at 10,000 rpm, the supernatant was discarded, the pellet was re-dispersed in distilled water and the centrifugation cycle was repeated at the same pace and duration. The final round of re-dispersion and centrifugation was accomplished with acetone to obtain cleaned and purified CuNPs. The morphology, phase, and capped functional groups of CuNPs were determined through scanning electron microscopy (MIRA 3 Tescan), x-ray diffraction analysis, and Fourier transform infrared spectrometry (Perkin Elmer Spectrum 100 FTIR).
Application of CuNPs on wheat using Petri plate assay
Seed source, treatments, and germination..
The seeds of four wheat varieties like 18-Elite Line 1, 18-Elite Line 3, 18-Elite Line 5, and 18-Elite Line 6 have been obtained from the Department of Plant Breeding Genetics, University of Poonch Rawalakot. The seeds were sterilized for 1 minute with 75 percent ethanol and 15 minutes with 2.5 percent calcium hypochlorite after being soaked in 0.6 percent HNO 3 for 10 minutes to end seed dormancy for successful germination [ 30 ]. The experiment was conducted in a completely randomized design (CRD) with 5 replicates. The four concentrations of CuNPs (0, 25, 50, and 100 mg/L) were applied in two ways, i.e., seed treatment and foliar spray. For seed treatment, the seeds were soaked in an aqueous solution of CuNPs for 3 h, and then 10 seeds of each variety were evenly placed in each Petri plate (labeled) already containing wet blotting paper. All the Petri plates were placed at room temperature and allowed to germinate with a daily supply of 5 mL of each concentration as a foliar spray. Samples were collected after 10 days of treatment and examined the effects of CuNPs on germination, growth, and biochemical attributes.
Germination indices measurements.
The germinated seeds were counted in each treatment for the measurement of germination percentage (GP). The GP was determined as GP = GN/SN × 100, where GN and SN are the total germinated seeds and tested seeds, respectively. The germination index (GI) was measured as GI = number of seeds germinated seeds/days of the first count + number of germinated seeds/days of the final count.
Growth parameters measurements.
To estimate growth, the primary root and shoot length of 10 days of seedlings were measured. For this, 5 plants were selected randomly from each treatment, the length was measured in cm, and the average was calculated. Similarly, the fresh weight of seedlings was measured in grams with the help of an analytical balance.
Biochemical contents measurements.
For the extraction of chlorophyll and carotenoid pigments, fresh leaves weighing 0.1 g were homogenized in 10 mL of 80% acetone. The homogenate was then centrifuged at 15000 rpm for 10 min, the pellet was discarded, and the absorbance of the supernatant was recorded at 666, 653, and 470 nm on the Shimadzu UV-1601 spectrophotometer. The formulae developed by [ 31 ] were used for the calculation of chlorophyll and carotenoid: Chla (mg/g) = [(12.25 x A663.2)—(2.79 x A646.8)] × ml acetone / mg leaf tissue, Chlb (mg/g) = [(21.50 x A646.8)—(5.10 x A663.2)] × ml acetone / mg leaf tissue. Total Chl = Chla + Chlb and Carotenoids (mg/g) = (1000 A470–1.8Chla– 85. 02 Chlb) /198. Total phenolic content was measured by Folin–Ciocalteu’s phenol reagent assay [ 32 ]. A 0.2 mL of plant extract mixed with 5 mL of 10-fold diluted Folin–Ciocalteu’s phenol reagent was kept for 4 minutes and then it was aided with 4 mL of sodium carbonate (7.5 percent w/v). The whole mixture was diluted up to a volume of 15 mL with distilled water and mixed well. The reaction was permitted to stand for 90 min, and the absorption of each sample was reported at 760 nm using a spectrophotometer Shimadzu UV-1601. The total phenols were measured using the calibration curve for gallic acid (GA) and presented as the equivalent of mg GA / g FW. To extract total soluble sugars, 50 mg of fresh leaves were crushed thoroughly in 5 mL of 80% ethanol and centrifuged for 10 minutes at 3000 rpm. From the supernatant, 0.1 mL was taken in a test tube and kept in a water bath for the evaporation of ethanol. After the ethanol had completely evaporated, 4 mL of 0.2 percent enthrone reagent was added to the test tube, heated in boiling water for 10 minutes, and then allowed to cool at room temperature. The absorption of each sample was reported at 760 nm using a spectrophotometer Shimadzu UV-1601. Total soluble sugars were measured using the calibration curve for glucose and presented as the equivalent of mg Gu/g FW.
Statistical analysis
The experiment was conducted in CRD using three replicates and analysis of variance (ANOVA) was performed in statistix 8.1. The statistical difference between means was evaluated, considering p < 0.05 as a significance level.
Results and discussion
Synthesis, morphology, size, and structure of cunps.
The CuNPs were synthesized from the aqueous extract of M . longifolia . The addition of aqueous extract to the aqueous solution of CuSO 4 resulted in the development of a color change from pale yellow to dark brown over the course of 24 h, the first indication of the formation of CuNPs in the solution ( Fig 1 ). This color change is developed due to the excitation of surface plasmon resonance (SPR) [ 33 ]. Previous research has also suggested that brown color can be established in the reaction mixture of plant extract and CuSO 4 [ 34 ]. This color change was further accomplished by taking a UV-vis spectrum of a brown-colored solution in the range of 400 to 800 nm ( Fig 1 ). The spectrophotometric study revealed an absorption band at 558 nm, a result of interaction between the free electrons presents on the surface of metal NPs and light of specific wavelengths. This absorption band indicates the formation of CuNPs in the colloidal solution because it is a known fact that CuNPs show characteristic absorption peaks in the range of 200–800 nm [ 35 ]. Moreover, it has been analyzed that a single SPR band represents round-shaped nanoparticles, whereas two or more SPR bands correspond to the anisotropy of nanoparticles [ 36 ]. In our study, CuNPs in the solution showed a single SPR band, which reveals the round shape of CuNPs. The morphology and size of the prepared CuNPs are shown in the images of SEM ( Fig 2a ) and their mean size distribution histogram ( Fig 2b ). The CuNPs exhibited a spherical shape, were distributed irregularly, and had an average size of 23 nm. Fig 2c represents the energy dispersive x-ray (EDX) of the powder and shows the presence of Cu in the material. The peaks belonging to elemental Cu, C, and O were clearly detected, and there were no extra peaks, demonstrating the purity of the synthesized NPs and correlating with the XRD analysis. It’s also possible that the existence of C and O is due to bioactive molecules that have been capped. Furthermore, only a few copper atoms on the surface of the NPs are likely to have been oxidized, yielding modest quantities of CuO and Cu 2 O. The XRD pattern of CuNPs is shown in Fig 3 , confirms the face-centered crystalline nature, as all the peaks match Cu with FCC symmetry and are consistent with JCPDS No: 04–0836, which reveals the crystalline nature of CuNPs [ 37 ]. With the help of FTIR ( Fig 3 ), the functional groups of all the possible biocompounds involved in the reduction and capping of CuNPs are identified. The FTIR spectrum showed peaks at 3754.36, 2054.64, 1937.67, 1587.34, 1266.30, and 1043.06 cm -1 . The peak at 3754.36 cm -1 corresponds to O-H stretching frequency of hydroxyl groups of polyphenols. The peaks at 2054.64 and 1937.67`cm -1 correspond to Cu-H (metal-hydrogen) bonds. The peaks at 1587.34, 1266.30, and 1043.06 cm -1 could be due to C-C stretching vibrations of an aromatic compound, C-O stretching of carboxylic acid, and ester bonds of phenolic compounds. These results suggest an interaction between the functional groups present in plant extract and Cu ions that results in bio-reduction, the formation, and stabilization of CuNPs [ 4 , 6 , 38 ]. CuNPs are thought to be formed from copper salts using plant extracts in three steps: production of copper ions, reduction of the ions, and lastly, oxidation of the reduced ions [ 16 , 20 ].
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
a copper sulfate solution. b plant extract. c mixture of copper sulfate solution and plant extract at zero-time. d change in color of solution after 24 h of time lap. e UV-visible spectrograph of CuNPs.
https://doi.org/10.1371/journal.pone.0269987.g001
(a) FESEM micrograph of CuNPs, showing spherical shape of CuNPs; (b) Size distribution histogram of CuNPs, showing average size of 23 nm. c EDX spectrum of CuNPs.
https://doi.org/10.1371/journal.pone.0269987.g002
(a) XRD pattern of CuNPs, predicting crystalline nature of CuNPs; (b) FTIR spectrum of CuNPs, confirming functional groups from plant extract capping the CuNPs.
https://doi.org/10.1371/journal.pone.0269987.g003
Effect of CuNPs on seed germination of wheat
Seed germination is the beginning of a physiological process of a plant that needs water imbibition and is directly linked to the yield of the plant. In this study, seed germination and germination index of the tested wheat varieties exhibited an almost similar trend, except for the variety 18-Elite line-5. Fig 4 shows the GP and GI of wheat varieties after the 10 th day of treatment with different concentrations of an aqueous solution of CuNPs. When exposed to 50 mg CuNPs/L, GP and GI increased considerably in varieties 18-Elite line-1, 18-Elite line-3, and 18-Elite line-6, while the variety 18-Elite line-5 showed increased GP and GI under 25 mg CuNPs/L. After 50 mg CuNPs/L exposure, the GP increased to approximately 67, 31, 33, and 11 percent in 18-Elite line-1, 18-Elite line-3, 18-Elite line-5, and 18-Elite line-6, respectively, of the GP observed in control. However, in 18-Elite line-5, a higher increase was found at 25 mg CuNPs/L (100%) as compared to control. A similar trend was found for GI, it increased to approximately 76, 37, 41, and 17 percent in 18-Elite line-1, 18-Elite line-3, 18-Elite line-5, and 18-Elite line-6, respectively, of the GI, recorded in control after the application of 50 mg CuNPs/L. More than double the increase in GI was apparent in 18-Elite line-5 after treatment with 25 mg CuNPs/L. When varieties were exposed to 100 mg CuNPs/L, no statistical difference was found in GP compared to control, except in variety 18-Elite line-6, which showed a reduced GP and GI as compared to control. Similar results were reported by [ 39 ], where lettuce seeds showed better germination when treated with CuNPs. It is found that NPs tend to come into contact with seed coats, penetrate the seeds, and improve seed germination and seedling growth of the plants [ 40 ]. They found that CuNPs penetrated the cell wall and formed new pores, so water absorption was improved, and as a result, favorable seed germination was started. Another study demonstrated that high seed germination rates might be due to the photo-generation of active oxygen like hydroxide anions and superoxide, which causes re-activation of aged seeds. In the nanocomposite, activated carbon provides moisture and a large surface area for seed germination [ 41 ].
Different letters in the same sub-group of columns indicate significant difference at p < 0.05 level.
https://doi.org/10.1371/journal.pone.0269987.g004
Effect of CuNPs on seedling growth of wheat
RL, SL, and FW were measured for all the tested varieties after 10 days of seedling ( Fig 5 ) to investigate the effect of CuNPs on seedling growth of wheat. All the concentrations of CuNPs posed a positive effect on the RL of all wheat varieties, but the most prominent increase was found at 50 mg CuNPs/L in 18-Elite line-1, 18-Elite line -3, and 18-Elite line-6 and 25 mg CuNPs/L in 18-Elite line-5. When treated with 50 mg CuNPs/L, the RL increased by 50, 153, and 33% in 18-Elite line-1, 18-Elite line-3, and 18-Elite line-6, respectively, and by 134 percent in 18-Elite line-5 when treated with 25 mg CuNPs/L. When compared to the control, the SL in 18-Elite line-1, 18-Elite line-3, and 18-Elite line-6 increased by 19, 20, and 37 percent, respectively, under 50 mg CuNPs/L, and by 54 percent in 18-Elite line-5 under 25 mg CuNPs/L. The FW also displayed the same results as for RL and SL, where a CuNPs concentration of 50 mg/L exhibited the most prominent effect on the FW of three wheat varieties. The maximum increase of 75, 95, and 35 in FW was observed for 18-Elite line-1, 18-Elite line -3, and 18-Elite line-6, respectively, treated with 50 mg CuNPs/L, while a maximum increase of 18% in FW was observed for 18-Elite line-5, treated with 25 mg CuNPs/L.
https://doi.org/10.1371/journal.pone.0269987.g005
After 10 days of exposure, the CuNPs treatments modified the RL, SL, and FW of wheat. RL, SL, and FW were significantly increased, especially for varieties exposed to 50 mg CuNPs/L. These changes in the root and shoot system might be due to hormonal imbalances, like auxin and cytokinin imbalances [ 42 ]. Our findings are supported by the study [ 33 ], where CuNPs, at low concentration, increased the fresh weight of wheat. Moreover, we have observed that at 100 mg/L treatment of CuNPs, the seedling growth is significantly reduced compared to the treatment of 50 mg CuNPs/L, suggesting that a high dose of CuNPs can be lethal to plants. At high concentrations, CuNPs may release cupric ions that change the physiological processes of the plant. These modifications increase the capacity of particles that cross the cell membrane of a plant cell [ 3 ]. Previous studies also supported this fact that a higher concentration of CuNPs produces toxic effects, e.g., in chickpea and soybean, CuONPs increased root and shoot growth at 100 ppm, but beyond this concentration (200 ppm), the root and shoot growth was found to decrease [ 43 ]. Other studies also suggest the same result, e.g., CuNPs reduced the seedling length by 65 and 75% in Phaseolus radiatus and Triticum aestivum , respectively [ 44 ]. In a similar way, CuONPs decreased the RL in Cucurbita pepo when applied at a 1000 mg/L concentration [ 45 ].
Effect of CuNPs on photosynthetic pigments of wheat seedling
The effect of CuNPs on photosynthetic pigments of wheat varieties was measured in terms of total chlorophyll and carotenoid ( Fig 6 ). Chlorophylls are located in the chloroplast where they play a crucial role in the photosynthesis system, which is highly related to plant productivity and biomass [ 46 ]. As a result, the total content of Chl and carotenoid in wheat varieties was measured in all treatments in the current study. The total chl content started increasing upon exposure to CuNPs and a higher level was found at 50 mg CuNPs/L in 18-Elite line-1, 18-Elite line -3, and 18-Elite line-6, while in 18-Elite line-5, 25 mg CuNPs/L evidenced stimulation of chl up to 48% as compared with no application of CuNPs (control). Carotenoid content in test plants also followed the same trend as total Chl. A maximum increase of 61, 40, and 42% in carotenoid content was found in 18-Elite line-1, 18-Elite line -3, and 18-Elite line-6, respectively, at 50 mg CuNPs/L. Some authors also noticed changes in the content of photosynthetic pigments as a result of nanoparticle application. For example, a concentration-dependent effect of CuNPs on chlorophyll and carotenoid pigments was studied by [ 47 ], where CuNPs increased the chlorophyll and carotenoid content. Similarly, Vigna radiata has shown increased chlorophyll and carotenoid content under the treatment of CuNPs [ 48 ]. We also observed a significant inhibitory effect at higher concentrations (100 mg CuNPs/L). This could be attributed directly to oxidative stress or the interaction of RuBP carboxylase, due to copper interaction with SH groups. Additionally, the Chl content decrease might also be due to the lowered shoot biomass upon contact with higher concentrations of CuONPs or to the membrane damage as a result of excess lipid peroxidation of chloroplast membranes under oxidative stress [ 49 ].
https://doi.org/10.1371/journal.pone.0269987.g006
Effect of CuNPs on phenolic and sugar content of wheat seedling
Treatment with CuNPs significantly influenced the content of phenol and soluble sugar in the seedlings of wheat ( Fig 7 ). A maximum increase in total phenols was found at 100 mg CuNPs/L. It has been identified that plants induce the production of phenolic compounds in response to NPs [ 50 ]. In some other studies, an increase in phenolic content was also observed under a high concentration of nanoparticles. For example, fruits of Jalapeno pepper showed an increase in the total phenols under the application of CuNPs + Chitosan-PVA [ 51 ]. Similarly, the application of zinc nano fertilizer also increased the total phenols in pomegranate fruits [ 52 ]. This increase in phenolic content at higher concentrations may be related to the plant’s response to stress, as we observed 100 mg CuNPs/L caused toxic effects on wheat. Phenols are antioxidant compounds that trigger the synthesis of a series of secondary metabolites from the shikimic acid pathway or through phenylpropanoids under conditions of abiotic stress [ 53 ]. Therefore, the observed response may be related to ROS formation due to CuNPs. However, unlike the phenolic content, the soluble sugar content was found to be higher at 50 mg CuNPs/L treatment, as was the case with all other factors investigated. Overall, we found a positive effect of CuNPs on the growth and development of wheat. These results were supported by previous studies that showed Cu plays an important role in plant growth and development, and plant productivity [ 54 ].
https://doi.org/10.1371/journal.pone.0269987.g007
Conclusions
This study used an eco-friendly and appropriate method for the synthesis of CuNPs using Mentha longifolia extract . There were no chemical reagents or surfactant templates required, allowing green bioprocesses for a range of biomedical applications. During the application of CuNPs on wheat, we found in our study that with an increase in the concentration of CuNPs, the seed germination and growth of wheat plants were also increased. However, after a certain concentration (50 mg CuNPs/L), the seed germination and seedling growth were found to decrease. Overall results showed that application of CuNPs influences the seed germination and seedling growth of wheat at different concentrations. The higher germination and growth were found at 50 mg CuNPs/L treatment for 18-Elite line-1, 18-Elite line -3, and 18-Elite line-6 wheat varieties and at 25 mg CuNPs/L for 18-Elite line-5. Beyond this treatment, germination and growth were inhibited. Effective germination and growth at a certain optimum concentration and decreased germination and growth beyond this concentration may be attributed to the varieties of wheat and the uptake and accumulation of CuNPs by the roots, because the germination and growth were dependent on the exposure concentration of CuNPs. In particular, the contact of plants with NPs and the impact of such contact on plant growth could spur new research focus on nanobiotechnology. Further studies are needed to understand the mechanism of accumulation and uptake of CuNPs in plants and the way they act during seed germination and growth.
Supporting information
https://doi.org/10.1371/journal.pone.0269987.s001
Acknowledgments
We are thankful to Department of Plant Breeding and Genetics, University of Poonch Rawalakot for providing seeds of wheat varieties. We are also thankful to IST Islamabad for providing scanning electron microscopy and X-ray diffraction facilities.
- View Article
- PubMed/NCBI
- Google Scholar
- 26. Kim BS, Song JY. Biological synthesis of metal nanoparticles. In: Hou CT, Shaw JF, editors. Biocatalysis and Agricultural Biotechnology. CRC Press, Boca Raton; 2009. pp. 399–407.
- 49. Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. Clarendon Press, Oxford; 1989.

IMAGES
VIDEO
COMMENTS
The applications of copper (Cu) and Cu-based nanoparticles, which are based on the earth-abundant and inexpensive copper metal, have generated a great deal of interest in recent years, especially in the field of catalysis. The possible modification of the chemical and physical properties of these nanoparticles using different synthetic strategies and conditions and/or via postsynthetic ...
1. Introduction. Nanotechnology, a cutting-edge domain situated at the convergence of engineering, medicine, chemistry, biology, and physics, takes advantage of the distinctive characteristics that are manifesting at the nanoparticle level (Contera, 2019).According to research, as the size of nanoparticles decreases, they acquire new properties and functions, which accelerates the development ...
Here, a complete list of the literature on the synthesis of copper nanoparticles, their properties, stabilizing agents, factors affecting the morphology, and their applications is presented. The importance of copper nanoparticles compared to other metal nanoparticles are due to high conductivity.
Research shows that copper and its composites have been used both due to their low-cost and effective methods for sterilizing liquids, surfaces, ... As the current paper focuses on copper nanoparticles, we have looked at how copper nanoparticles can be obtained using green, chemical and physical methods. For each of the methods, based on the ...
The synthesis of copper nanoparticles is a difficult task for a researcher to find the best valuable points, such as pH, temperature, concentration ratio and time. Time plays an important role in the synthesis of nanoparticles. In some cases, a color change occurs within 30 min, while sometimes it takes up to 48 h.
Published 20 May 2014. Copper nanoparticles received much attention due to its high electrical co nductivity, high. melting point, low electrochemical migration behavior and low cost. Top down ...
The microwave-assisted synthesis of copper nanoparticles allowed to increase the efficiency of materials by providing rapid heating and rapid reaction time. 73 It can accelerate volumetric heating and kinetics, rapid volumetric heating and kinetics, short reaction periods and increasing yields of products compared to conventional heating methods.
The controlled fabrication of nanoparticles has propelled nanotechnology into one of today's most promising and popular fields of scientific research [].Potential future advancement requires the ability to prepare nanomaterials in a reproducible and controlled manner [].Currently, there is an increasing interest to synthesize metallic copper and copper (I) oxide nanocrystals not only for the ...
Copper nanoparticles (NPs) with an average particle diameter of 50-60 nm were successfully obtained by reducing an aqueous solution of a copper(II)-nitrilotriacetic acid complex with an aqueous ...
Finally, we have summarised the recent progress made in copper-based nanoparticles and their applications to amplify and rectify present cancer treatment options. The synthesis, characterization, stabilization, and functionalization techniques of various copper-based nanoparticles have also been highlighted in each section. In conclusion, the ...
The objective of the present work is the introduction of a quick and simple literature survey about the green bio-synthesized of copper nanoparticles. The survey revealed that the eco-friendly preparation methods using different plant species, properties and potential applications as alternative promising for silver and gold nanoparticles. The review enumerates the classification of ...
Today, different types of nanoparticles (NPs) are being synthesized and used for medical and agricultural applications. In this study, copper nanoparticles (CuNPs) were synthesized using the aqueous extract of mint (Mentha longifolia L.).For the characterization of CuNPs, UV-visible spectroscopy, scanning electron microscopy, X-ray diffraction, and Fourier transform infrared spectrometry were ...
Although research is still at an early stage, copper nanoparticles can enhance the effectiveness of immunotherapeutic approaches, which requires further studies to optimize (Wang et al. 2019a, b; Li et al. 2023). Generally, the reports on copper-based nanoparticles are also limited to in vitro, in vivo, or ex vivo phases (Kang et al. 2023). The ...
Copper nanoparticles have not received as much attention in biomedical research compared to other metallic nanoparticles such as gold, silver, or platinum. Although there is a steady expansion of the body of scientific literature concerning the biomedical use of copper nanomaterials, it is necessary to overcome significant challenges before ...
Copper nanoparticles are presented as an alternative to rising antibiotic resistance. The basic mechanisms of bacterial, fungal, and viral inactivation, which explain their potential, are presented. ... Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a ...
In this paper we review a few chemical methods for synthesis of Cu nanoparticles and its utilization in plasmonic solar cell. 2. Plasmonic features of copper. Cu, with atomic number 29 and atomic weight 63.54, occupies the first position of subgroup IB in the periodic chart of the elements.
This research aimed to explore the eco-friendly green synthesis ... The prepared extract was filtered using Whatman filter paper with size 11 μm followed by vacuum filtration using cellulose nitrate membrane. ... 2.2.2. Synthesis of nanoparticles. For the synthesis of C. paniculatus copper nanoparticles, 50 mL (5 mM) copper sulfate solution ...
Environmentally friendly copper oxide nanoparticles (CuO NPs) were prepared with a green synthesis route without using hazardous chemicals. Hence, the extracts of mint leaves and orange peels were ...
Well-dispersed and uniform-size preparation of Copper nanoparticles and its X-ray diffraction studies are reported in this paper. A simple and cheapest chemical reduction method with copper sulfate pentahydrate as a main precursor is used for the synthesis of Copper nanoparticles. ... {Asian Journal of Pharmaceutical Research and Development ...
3.2 Optimal biosynthesis system confirmation. It is demonstrated that G. biloba L. leaf extract has the ability to synthesise CuO nanoparticles, and the volume of extract affected their formation. As shown in Figure 2(a), the maximum absorbance at 398 nm (A 398) was measured based on different volumes (10-40 ml) of leaf extract.A 398 decreased with increasing extract volume, and the optimal ...
Semantic Scholar extracted view of "COPPER NANOPARTICLES AS A POTENTIAL EMERGING POLLUTANT: DIVERGENT EFFECTS IN THE AGRICULTURE, RISK-BENEFIT BALANCE AND INTEGRATED STRATEGIES FOR ITS USE" by G. Tortella et al. ... Search 218,239,401 papers from all fields of science. Search. Sign In Create Free Account. DOI: 10.1016/j.emcon.2024.100352 ...
Abstract In this paper, we study the synthesis dependence of structural, optical and antimicrobial properties for copper oxide nanoparticles on, synthesized using microwave irradiation CuO(M), co-precipitation CuO(P) and hydrothermal CuO(H) protocols. Structural and morphological properties were studied using XRD, SEM, TEM and SAED techniques. XPS studies confirmed the presence of copper ions ...
The goal of this research was to assess the effect of adding different percentages of copper oxide nanoparticles into soft denture lining materials on thermal conductance.
Bioactive copper nanomaterials are an emerging class of nano-antimicrobials providing complimentary effects and characteristics, as compared to other nano-sized metals, such as silver or zinc oxide nanoparticles. In this chapter, copper nano-antimicrobials are reviewed and classified firstly as a function of the preparation methods, and ...
An examination using numerical data shows how the addition of nanoparticles to the melting of PCMs in a semi-circular container influences the existence of copper rods with different forms. It was carried out using the ANSYS/FLUENT 16 programme and the enthalpy-porosity combination.
DOI: 10.1016/j.micpath.2024.106672 Corpus ID: 269583847; Evaluation of ALA-Capped Silver, Cooper, and Silver-Copper Nanoparticles for Controlling Fungal Plant Pathogens @article{Lopes2024EvaluationOA, title={Evaluation of ALA-Capped Silver, Cooper, and Silver-Copper Nanoparticles for Controlling Fungal Plant Pathogens}, author={Isabela Santos Lopes and Jullio Kennedy Castro Soares and L{\'i ...
The copper-cerium binary oxide catalysts supported by titanium dioxide with nanosphere core-shell structures, nanotube (TNT) core-shell structures, impregnation (imp) nanoparticles and sol-gel nanoparticles were prepared for NH 3-SCR of NO x under medium-low temperature conditions. The effect of different morphologies on the Cu-Ce/TiO 2 catalysts was comprehensively studied through ...
Today, different types of nanoparticles (NPs) are being synthesized and used for medical and agricultural applications. In this study, copper nanoparticles (CuNPs) were synthesized using the aqueous extract of mint (Mentha longifolia L.). For the characterization of CuNPs, UV-visible spectroscopy, scanning electron microscopy, X-ray diffraction, and Fourier transform infrared spectrometry were ...
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications. ... J. Copper sulfide nanoparticles ...
After that extract is cooled and filtered by whatman filter paper. For synthesis of silver nanoparticles, ... In current research work silver and copper nanoparticles were synthesized from plant extracts such as (Aloe barbedensis, Azadirachta indica and Coriandrum sativum) and their application was done as biosorbents for the decontamination of ...