

9 Killer Speech Openers to Start a Talk or Presentation.

Danny Riley 8 min read
What you’ll learn:
- The importance of a “killer” speech opening.
- 9 powerful speech openers and how to use them.
- Examples from great speakers you can learn from.

Great speech openers hook your audience.
“ Well begun is half done” – Mary Poppins.
A killer speech opener will make the difference between a presentation that makes you soar or your audience snore .
I’ve researched the whole web to find nine killer speech openers to make your audience lean in and listen rather than tune out and daydream.
You’ll see how masters of the craft have used them, and how you can too.
Number seven takes hutzpah to pull off. Ready for the whole list of killer speech openers?
The Shock Opener
One of the best ways to open your speech with a buzz is to startle or shock them.
You can shock an audience in many ways, but they all rest on the major senses of VAKS:
- Kinesthetic (touch)
We don’t want your audience tasting your talk, but it should leave a good taste in their mouths.
Changing Minds suggests asking if the audience is awake after appearing from a flashbang and a cloud of smoke, and this might work for you if you’re a magician or playing some kind of character for your speech like a genie.
Suppose you aren’t going for the magic angle.
In that case, you can shock them on a psychological level instead, as Conor Neill recommends, and tell your audience a surprising fact or statistic that makes them question their thinking or beliefs.
“Did you know that half the water on earth is older than the sun?”
Questions like these will shake an audience awake and turn on their critical thinking nervous-system.
Don’t take my word for it; you can see an incredible demonstration of the shock opener in Mohammed Qahtani’s speech, The Power of Words .
Qahtani opens by taking out a cigarette and placing it into his mouth before trying to light it. The audience is so shocked that they gasp and tell him to stop.
Remember, if your audience is shocked, they are listening.
Your audience doesn’t always have to be jolted to attention with a shock opener, though you can use a more subtle approach to grab their focus.
Ready to speak with confidence ? Explore our training options...
The story opener.
You can set the tone of your speech instantly with a story .
In Hollywood, filmmakers and directors use an ‘establishing shot’ to set the tone and theme of the entire film.
When creating your speech, think of a short story that sums up your talk.
Maybe you tell half the story to begin with, and then the other half at the end.
The important thing is your tale must be relatable . If your audience can’t imagine themselves in the story, they won’t be engaged.
We all experience very similar things in life:
- We all went to school and had a teacher we loved
- We all have parents who loved us or made mistakes in our upbringing
- We all had a first crush.
We are all cut from the same cloth, so it’s good to be reminded that others are going through what we face or think as we do.
Bryan Stevenson does a stellar job of recounting his mischievous grandmother in his TED talk, We need to talk about an injustice .
The best thing is, you can combine a story-opener with any other speech opener in this list.
It’s truly versatile.
One of my favourite speech openers is next, though.
The Intrigue Opener
I love this speech opener.
What better way to hook your audience than to intrigue them with mystery or a juicy secret?
Take a look at Daniel Pink’s TED Talk The puzzle of motivation . After he begins, Pink, looking like a guilty man sent to the gallows tells his audience:
“I need to confess something, at the outset here. A little over 20 years ago, I did something I regret. Something I’m not particularly proud of”.
Wow. How intriguing, right?
You have to admit; you want to know what he’s about to confess.
Choose every sentence, every word, and every mark of punctuation to increase the tantalisation temperature.
Whether it’s a secret or confession, the Intrigue Opener piques just enough curiosity in your audience to keep them from checking Whatsapp.
As humans, we need closure.
We do not like open loops.
That’s why it is both enthralling and aggravating when someone plays on our need to be sure.
Just as we cannot stand an open loop, we are instantly engaged when someone gives us a puzzle to solve.
You’ll notice the best speeches, books, tv shows, and films do not spoon feed you all of the information.
I’ve always liked the way Malcolm Gladwell writes his non-fiction books because they contain puzzles that you solve as a reader.
This puzzle needs to be related to the speech or presentation you’re delivering, of course. It cannot be a random puzzle and will ideally be impossible or extremely difficult to solve at first.
After the speech begins and the puzzle is revealed, you should slowly drop hints on how to solve the mystery.
Up next, speech openers that use a physical object to create curiosity in the audience’s mind.
The Prop Opener
One of the most potent ways you will captivate your audience is to use a powerful prop in your opening address.
What better way to capture an audience’s imagination than to show them a mysterious or beautiful object?
If you’ve never seen the Prop Opener done well, then take a look at one of the greatest speeches of all time:
Dananjaya Hettiarachchi’s, See Something .
Danajaya enters with a simple rose in his breast pocket, takes it out, gazes at it nostalgically, smells it and then begins to speak.
This same prop appears again right at the end of his speech to end his talk with a flourish.
There are many different props you can use.
JJ Abrams used a Mystery Box to absorb the audience’s attention and used the box as a metaphor for his entire career.
If you think the prop opener is just for TED Talks and Toastmasters Final Speeches, remember that most company product launch centre around one or more props.
Steve Jobs revealed his new products in ever-innovative ways.
Still, while the last two speeches I’ve mentioned opened with physical items, most of Jobs’s presentations built intrigue through the sight of the product.
So remember, you can use an object, or tease your audience with the absence of a prop, but make that prop integral to your talk.
You don’t always have to use a prop, of course.
A more minimalist approach to opening your speech uses the best audience reaction a speaker can receive: laughter.
The Funny Opener
Using laughter to win over your audience is the golden ticket to immediate rapport with your audience.
Jack Schafer, PhD at Psychology Today, said that People Will Like You If You Make Them Laugh , which seems obvious, but at least you know we have scientists on the case.
He also mentions that constructing humour requires and projects a high level of intelligence .
Of course, laughter is subjective, but it is also infectious, and if you get enough members of your audience to titter, it will spread across the whole group.
If you want to see just how quickly you can win an audience over with humour, take a look at Ken Robinson’s subtle but delightful ability to raise a chuckle in his speech Do Schools Kill Creativity?
Ken’s ability to speak conversationally to an audience of thousands is genuinely remarkable.
If you break down his humour, it is easy to see how you could include similar content in your presentations.
Whether you can pull it off as well as Ken is another story.
Not everyone feels like they can be a comedian, though; I get that.
Well, that’s alright because there are other ways to open your talk that play on other strong emotions.
You can inspire your audience too.
The Inspirational Opener
One of my favourite ways to help beginner speakers to open their presentation is with a quote.
A quote acts like a story in that it sets the tone and theme of your speech, but it takes much less effort and even less skill.
An effective quote is usually only one line long and supported by the credibility of the original author who uttered those words.
Watch the way Clint Smith opens his TED Talk The Danger of Silence .
Using Martin Luther King’s voice to start his speech gives Clint what psychologists call the transference effect .
Just by citing someone else, especially someone admired and famous, you redirect the emotions an audience have towards that person onto yourself.
One caveat to using quotes, though:
Fact check them . I cringe whenever I see someone incorrectly quoting someone.
Have you ever heard the quote by Albert Einstein:
“Insanity is doing the same thing over and over, and expecting different results”?
A great quote, isn’t it?
But Albert Einstein never said those words .
A quick check on Reuters will help you add more credibility to your inspirational opener.
Finally, try to use a quote few people have ever heard. Inspiring words have been filling the archives of history for millennia, so seek out something that has been left dusty on the shelf rather than the same recycled iterations.
Next, let’s look at a type of bold speech opener that take real hutzpah to land well.
The Perspective Shift Opener
A powerful speech opener that will take confidence is the perspective shift opener.
This opener will lead the audience in one direction before changing direction and setting a new pace for the speech.
Cameron Russel does a fantastic job of controlling the frame in her TED Talk Looks aren’t everything. Believe me; I’m a model.
Russel takes to the stage dressed in a skimpy dress and begins to tell the audience about her career, but then does a rapid wardrobe change on stage in front of the entire audience.
This change of dress sets a new tone, feel, and direction for the speech.
If you can change the audience’s perspective or frame of reality, you are in the driving seat.
One of the best things you can hope for as a speaker is moving hearts and changing minds.
If you aren’t a confident speaker, start small.
Vanessa Van Edwards suggests never mentioning how nervous you are.
It’s distracting and makes the audience pick up on all the subtle nervous energy and cues you give off. Control the frame instead and act cool and confident: they will buy into it.
Another great way to hold frame control over an audience is by using the power of silence .
The Silence Opener
Silence is a valuable commodity in today’s noisy and distracting digital world.
Creating silence at the beginning of your talk can profoundly affect your audience and their focus.
Did you ever have a teacher at school who used silence effectively?
When my English classmates were noisy, our teacher Mr Rylance would hold up his hand in silence.
Slowly we would settle down and focus on his raised hand.
A few would giggle, but that would peter out until we all wrapt in a hypnotic stillness.
If you want to see an example of how to use silence, then look at Neal Glitterman’s speech The Power of Silence .
You can see how much gravity silence can have , especially as a speech opener.
The final killer opener I want to introduce you to is the big promise opener.
The Big Promise Opener
I believe that all speeches and presentations should contain a big promise as it tells your audience why they should keep on listening.
Ideally, your big promise will be your speech title or phrase that pays which is a recurring foundational phrase you will use throughout your presentation.
A big promise is your way of making a deal with the audience : you listen to me, and you’ll get something in return.
Creating a big promise at the beginning of your speech is like adding a teaser trailer to the beginning of a TV show. It suggests a reason you should stick around.
When Arthur Benjamin introduces his talk Faster than a calculator by announcing:
“I am a human calculator!”
You know that proof is on the way.
Remember the essential rule of the Big Promise Opener: make it big and keep your promise.
WOW your audience with these killer speech openers.
I hope you feel that I kept my promise of sharing nine killer speech openers to start a presentation.
Did you notice any other speech openers at the beginning of this article?
Don’t forget; these openers can be mixed and matched.
You can include a number of these speech openers in the same presentation to create more impact.
Let me know which of these killer openers was your favourite, and let me know if you have any more you’d like to share.
– Danny Riley
Join 350+ leaders getting my weekly tips on confidence and charisma... 👇
Navigation:.
Home About Success Stories Contact Privacy Policy
Work with Ed:
1-to-1 Coaching 1-Day Masterclass Team Training
Follow/Connect:
Get started:, copyright © 2023 project charisma ltd. all rights reserved..
How to Introduce Yourself in a Presentation: Guide to a Killer Opener
Hrideep barot.
- Body Language & Delivery , Speech Writing

Not sure how to introduce yourself in a presentation? Hang on till the end of this article.
Giving a presentation can be unnerving. And introducing yourself can be nerve-wracking.
But, without a fitting introduction, you would just be hitting the dart in a dark room.
The usual “Good Morning! I’m Neil, and I work as a Designer at…” is boring and doesn’t cut the ice anymore.
So, how to Introduce yourself in a presentation or start with a killer opener?
Introducing yourself in a presentation is pitching yourself to the audience so they stick around for the rest of your talk. Include your background, your unique trait, and who you are while sticking to the context in the first 30-60 seconds of your introduction.
Your introduction should be effective and have an interesting hook. You’ve got to nail your introduction in one shot.
A make or break moment indeed.
But, fret not! We’ve outlined what to say before starting a presentation to help get your next presentation right.
Occasions Where you Might Have to Introduce Yourself in a Presentation
Here is what to say to start a presentation on some of the occasions where you would have to introduce yourself before the presentation.
Though the principle focus will be about yourself, tweaking your intro to the context and the place is essential.
The self-introduction should be compelling enough to woo your audience to sit for the next couple of minutes.
1.How to Introduce Yourself in a Business Environment
Introducing yourself in your workplace can be rather common. But, it’s during business meetings and conferences where you need to stand out.
Every time you meet senior managers, introducing yourself with your name and job title doesn’t grab eyeballs anymore.
However, taking the first step matters. Here are certain scenarios where you might be called upon to introduce yourself in your workplace.
How to Introduce Yourself in an Interview Presentation
The “Tell me about yourself” in interviews is intimidating. If you’ve found alibi’s to every presentation in your school and college, it doesn’t work here anymore.
Prepare a short introduction about yourself and be interview-ready. Anytime someone hits you up with that question, you need to be able to answer it with the snap of a finger.
Here is an example of a self-intro during an interview.
“As a skilled designer, with two years of freelance experience, I’ve worked for clients with diverse needs. I’ve also designed brochures, magazines, logo , and packaging materials for my friend’s company. I’m confident that I can leverage my skills and bring in the best for your brand.
How to Introduce Yourself and Your Team in a presentation
Business meetings can be boring. But there are times where you might have to introduce yourself to a new co-worker or a senior leader.
As a team leader yourself, you might have to introduce yourself and your team to present on the performance of the company the previous month.
Presentation introduction ideas if you’re a marketing executive can be,
An increased conversion of 130%, that’s what our marketing team achieved last quarter making our campaign a massive success. The soldiers who made this possible are Ryan, who made sure the User Experience on our website was flawless. Sean who ensured seamless technical functioning, and Abby who is responsible for all the copies on our major assets. I’m John, who heads the marketing team and we want to take you through all the activities we actioned, the metrics we achieved, and the lessons we learned from our recent efforts.
In case you are giving a group presentation , you can check out this video to see how you can introduce different members of your group for seamless transitioning:
How to Introduce Yourself in a Conference Presentation
In a conference presentation, you’re expected to be a little formal. While you can adhere to that school of thought, don’t forget to story tell. That’s what hooks an audience! Here is an example of how to introduce yourself in a business conference:
“Today, I’m going to share a story of how someone with zero marketing skills and training made it to the top by creating massive revenue streams through online campaigns and paid advertising in just 6 months. If you’re passionate about digital marketing, this is for you. Stay tuned till the end for better insights.
If you’re presenting at a business conference, take a look at these 11 tips for presenting at a conference by Brian Campbell.
How to Introduce Yourself in a Business Pitch Presentation
Now, this is for entrepreneurs who are starting out. If you need investors to fund your start-up, you need to have a solid pitch.
Let’s say, your product is AI-driven that alerts drivers who doze off while driving.
Talk about the benefits of it in a single sentence and highlight the downsides of dozing off while driving with stats and figures.
Check out this Crucial Public Speaking Tips for Startup Founders written by us that’ll help you nail your pitch.
Also, have a look at this video below. In this, Josh Light introduces himself in just two simple sentences and moves on to talk about his start-up. It is simple yet effective.
How to Introduce Yourself in Client Presentation
If you’re a freelancer, talking to clients can be a daunting task.
Let’s say you’re an engineer turned copywriter. That’s an interesting combo out there, and if you put it out in a way you write your copy, it would benefit you to a whole another level.
“I’m an experienced travel copywriter and I’ve written ad copies, sales pages, newsletters, landing pages for some of the top travel brands. I have over 5 years of expertise in this niche. One of my landing page copy at XYZ converted 50% of eyeballs into leads thus scaling up revenue drastically and I’m here to do the same if you see me fit after this call.”
2 . How to Introduce Yourself in a Presentation as a Student

Are you that kid/student who always shied away from giving presentations? Did you always come up with excuses and ended up giving barely one or two presentations your whole school life?
Yes? Well, it’s time to come out of your cocoon as it won’t work out that way in college or at work.
Whether it’s a small project presentation or giving a speech in your English class, here is how you can introduce yourself as a student.
How to Introduce Yourself in a Seminar Presentation
We’ve all been there. Hundreds of projects and assignments, be it school or college.
And that’s where you have to introduce yourself before jumping into your project. No matter how good your project, a solid introduction can put you ahead of the game.
“ As a tech enthusiast myself, I was intrigued by blockchain technology for a long time and today I have my project built using that very technology. I’m so excited to share with you all the working of this model and its benefits. Let’s jump right in.
It’s pretty easy and to-the-point. You need to be self-confident while saying those two lines and try to avoid fillers.
3. How to Introduce Yourself as a Trainer
As a trainer or teacher, your audience may be high-school students, undergrads, or even professionals.
Depending on the setting and the audience, you can craft your intro effectively and be of interest to the listeners.
How to Introduce Yourself to Students
As a teacher in a new school or college, introducing yourself is obligatory.
You can go about it this way if you’re a Moral Science teacher or Counselor:
“Hi everyone! I’m Alexandra. Call me Alex for short. We are going to have loads of fun for the next couple of months as I will be handling your Moral Science classes from today. If you are stuck in a dilemma or facing challenges, you can talk to me personally anytime and I’ll help you find a way out.
How to Introduce Yourself in a Workshop
Workshops are where you learn about a subject. What if you’re the one who is conducting the workshop or needs to fill in for your friend for a couple of minutes, you need to introduce yourself.
If you’re an Economics Graduate who is conducting a Calligraphy workshop, your presentation starting words can be something like,
“Back when I was a kid, I used to scribble down letters I saw on posters and fell in love with the notion of lettering and calligraphy. I wanted to get into design, but I thought it was a fleeting moment and took Economics. Little did I know how much it meant to me. I finally figured what to do in life, and here I’m helping and teaching you to do what you love after years of learning and unlearning.”
How to Introduce Yourself in Training Sessions
Whether you’re a corporate trainer or getting into training students after years of experience, introducing yourself never gets old.
You can emphasize your past experiences in the form of a story or start with how it was when you worked with one of the top clients in the industry.
Below is an example to give you a precise picture.
“How excited are you to get your first gig? I’ve been a freelance writer for over a decade now. And freelancing is one of the best jobs as it gives you financial freedom and lets you work from the comforts of your couch or at your favorite café. So, I’m here to teach you to do the exact same thing and help you find your passion.”
5 . How to Introduce Yourself in a Video Presentation

Virtual presentations are a thing right now. If you’re a camera conscious person, you might have a hard time giving a presentation.
Dressing well and looking at the camera and not the screen can help present better. And always, look into the camera and not the screen when it comes to virtual presentations.
No matter how tensed you are, do not reflect it on your face. Have a bottle of water beside you to buy time and calm your nerves.
Here are two possible situations where you might have to introduce yourself virtually.
How to Introduce Yourself in Webinars
Webinars are ever-increasing and if your introduction is not crisp and strong enough, building an online presence can be challenging.
Here is how you can introduce yourself in a webinar:
“ Hi, guys and welcome to this long-awaited session. How excited are you all? I know I am! We’re live and will be having John in a while. I’m so thrilled to see hundreds of you all attending this webinar live. It’s going to be a great session. I’m Patrick and the head of Marketing at XYZ. We started this webinar series two months ago and received phenomenal feedback from you all. And that’s why we’re back again with another one. Thank you and welcome again! Hope you find this session valuable.”
How to Introduce Yourself in a Virtual Presentation
Now, this is for freshers whose onboarding is going virtual. Whether it’s training sessions, virtual presentations, or virtual meetings, you are asked to introduce yourself to every manager and executive multiple times in a day.
Hey everyone! I’ve always loved meeting new people and though this is virtual now, just so thrilled to see you all on screen. If you see a new face popping on your screen during meetings and conferences, that’s me, John the new joinee. Can’t wait to meet you all in-person. Excited to jump-start my career here.
You can also check out this video we made to know certain ninja hacks to engage a virtual audience:
Related Article: All You Need To Know About Presenting Remotely
How to Structure an Intro – How to Start and End
- Add a Compelling Hook
You can begin your speech with a fact or a question to pique curiosity of your audience.
- A Brief Overview about Yourself
In those initial few seconds, greet the audience and talk about your strength or any unique trait in a word or two.
You can mention your achievements or contributions before talking about your background.
- A Quick history or Timeline of your Career/Education
In any context, a brief background or history about yourself should be talked about to let your audience know a little more about you.
It helps them gain trust and reliability.
- Smooth transition to the main topic
You shouldn’t abruptly move to the heart of your speech post introduction. There should be a subtle transition to make it effective.
Here is a presentation introduction example,
“Would you believe if I told you that you could reach 15k+ people on LinkedIn in just 30 days? No? Stick around for the next 7 minutes as I’m going to teach you all about it so you can get started as a rookie with zero connections.” Hi everyone! I’m XYZ – a Linked Growth Hacker. I’ve been helping businesses grow and build a strong personal brand for five years now. If you’re wondering how to generate leads on LinkedIn, take note of the pointers I’ll be sharing with you today.”
Magic ingredients to Introduce Yourself in a Presentation

You’ve got to nail your introduction no matter where you give the presentation.
You need to learn the art of introducing yourself because that’s the one thing you’ll be asked everywhere when you meet new people.
Introducing yourself is like marketing yourself. A stellar introduction can make a difference.
Here are some surefire ways to stand out in a crowd with your introduction.
With practice, your self-introduction will improve over time if you follow these tips.
1 . Brevity is Key
We all know this by now. No matter how many years of experience you have or how much you’ve contributed to the team, your introduction should be short yet powerful.
With an impressive introduction about yourself, your audience will be keen on listening to you more.
2 . Talk about Your Contribution
Instead of starting with your name and your job title, craft a story about the time you have to strive hard to achieve a goal be it personal or professional.
Speak about your contribution subtly without coming off as someone narcissistic. Unfold the little moments and share them with the audience.
Ensure it is related to your speech. Don’t go off course.
3 . Understand Where You Are
The place where you present matters though it is about you. You need to research about the people, the place and craft an introduction aligning with it.
Keep it relatable. Get the audience to be on track with you. Keep your message clear and introduce it in a way it is memorable.
4. Be as Real as Possible
Since you are introducing yourself, be as real as possible.
No, you don’t have to be extremely personal, but you can keep it minimal and include a common ground so that the audience can resonate with you.
5. A Smooth Transition is Essential
Transitioning from your intro to the main speech needs to be done right to keep the flow going.
Craft an intro and shift to the main topic without a pause after the introduction.
6. Create a Hook
Creating a hook is essential no matter the setting you’re introducing yourself in.
You need to grab the attention of the audience with your first sentence. You can quickly introduce yourself in a few sentences without taking much time.
Begin with a question or an interesting fact to hook the listeners every time you introduce yourself.
Want some inspiration? Here is a very practical video we have made on different opening lines from some of the most powerful speeches. Hopefully, it will get your creative juices flowing for what your hook should be:
Level up your public speaking in 15 minutes!
Get the exclusive Masterclass video delivered to your inbox to see immediate speaking results.
The Masterclass video is on its way to your inbox.
Concluding Thoughts
Introducing yourself in a presentation can be stressful. You won’t get it right on your first. Nope. Not on your third attempt.
Heck! Not even on your sixth introduction too.
But, here’s the thing.
You need to keep sailing and believe in yourself. That’s what can make you better.
If you want to evolve as an individual, learning how to introduce yourself can immensely contribute to your professional and personal growth.
Push your boundaries and cross your personal threshold. You will get there one day. And introducing yourself will no longer be a daunting task.
Enroll in our transformative 1:1 Coaching Program
Schedule a call with our expert communication coach to know if this program would be the right fit for you

Lost Voice? Here’s How to Recover Sore Throat and Speak Again

7 Keys to Emcee Like a Pro: Unlock Your Hosting Potential

8 Ways to Rise Above the Noise to Communicate Better

- [email protected]
- +91 98203 57888
Get our latest tips and tricks in your inbox always
Copyright © 2023 Frantically Speaking All rights reserved
Kindly drop your contact details so that we can arrange call back
Select Country Afghanistan Albania Algeria AmericanSamoa Andorra Angola Anguilla Antigua and Barbuda Argentina Armenia Aruba Australia Austria Azerbaijan Bahamas Bahrain Bangladesh Barbados Belarus Belgium Belize Benin Bermuda Bhutan Bosnia and Herzegovina Botswana Brazil British Indian Ocean Territory Bulgaria Burkina Faso Burundi Cambodia Cameroon Canada Cape Verde Cayman Islands Central African Republic Chad Chile China Christmas Island Colombia Comoros Congo Cook Islands Costa Rica Croatia Cuba Cyprus Czech Republic Denmark Djibouti Dominica Dominican Republic Ecuador Egypt El Salvador Equatorial Guinea Eritrea Estonia Ethiopia Faroe Islands Fiji Finland France French Guiana French Polynesia Gabon Gambia Georgia Germany Ghana Gibraltar Greece Greenland Grenada Guadeloupe Guam Guatemala Guinea Guinea-Bissau Guyana Haiti Honduras Hungary Iceland India Indonesia Iraq Ireland Israel Italy Jamaica Japan Jordan Kazakhstan Kenya Kiribati Kuwait Kyrgyzstan Latvia Lebanon Lesotho Liberia Liechtenstein Lithuania Luxembourg Madagascar Malawi Malaysia Maldives Mali Malta Marshall Islands Martinique Mauritania Mauritius Mayotte Mexico Monaco Mongolia Montenegro Montserrat Morocco Myanmar Namibia Nauru Nepal Netherlands Netherlands Antilles New Caledonia New Zealand Nicaragua Niger Nigeria Niue Norfolk Island Northern Mariana Islands Norway Oman Pakistan Palau Panama Papua New Guinea Paraguay Peru Philippines Poland Portugal Puerto Rico Qatar Romania Rwanda Samoa San Marino Saudi Arabia Senegal Serbia Seychelles Sierra Leone Singapore Slovakia Slovenia Solomon Islands South Africa South Georgia and the South Sandwich Islands Spain Sri Lanka Sudan Suriname Swaziland Sweden Switzerland Tajikistan Thailand Togo Tokelau Tonga Trinidad and Tobago Tunisia Turkey Turkmenistan Turks and Caicos Islands Tuvalu Uganda Ukraine United Arab Emirates United Kingdom United States Uruguay Uzbekistan Vanuatu Wallis and Futuna Yemen Zambia Zimbabwe land Islands Antarctica Bolivia, Plurinational State of Brunei Darussalam Cocos (Keeling) Islands Congo, The Democratic Republic of the Cote d'Ivoire Falkland Islands (Malvinas) Guernsey Holy See (Vatican City State) Hong Kong Iran, Islamic Republic of Isle of Man Jersey Korea, Democratic People's Republic of Korea, Republic of Lao People's Democratic Republic Libyan Arab Jamahiriya Macao Macedonia, The Former Yugoslav Republic of Micronesia, Federated States of Moldova, Republic of Mozambique Palestinian Territory, Occupied Pitcairn Réunion Russia Saint Barthélemy Saint Helena, Ascension and Tristan Da Cunha Saint Kitts and Nevis Saint Lucia Saint Martin Saint Pierre and Miquelon Saint Vincent and the Grenadines Sao Tome and Principe Somalia Svalbard and Jan Mayen Syrian Arab Republic Taiwan, Province of China Tanzania, United Republic of Timor-Leste Venezuela, Bolivarian Republic of Viet Nam Virgin Islands, British Virgin Islands, U.S.

How to Give a Killer Presentation: 18 Top Tips

People attend presentations to learn and gain useful insight. But way too often, we see the audience yawn, scroll on their phones or check their watch, wishing that the time would go faster.
Is it that the content of the presentation isn’t interesting enough, or that the speaker doesn’t know how to engage the audience?
Whatever the reason, delivering an engaging presentation is an art that takes some time to master.
Based on my own experience from the stage, and from observing other speakers at industry-leading conferences, I’ve collected these 18 top tips. May they help you give a presentation that will wow your audience:
- Plan your storyline
- Use the rule of three
- Simplify your slides
- Include numbers
- Use the power of visuals
- Practice relentlessly
- Greet the audience in their local language
- Break the ice at the start
- Engage your audience with live polls
- Move around the stage and make gestures
- Smile and make eye contact
- Consider using props
- Go among the audience
- Give rewards for participation
- Prompt a discussion in the audience
- Build in time for Q&A
- Crowdsource questions from the audience
- Gather feedback
1. Plan your storyline
A powerful story can make your whole presentation. Take TED talks, for instance. They’re all based on captivating stories that support the main argument or line of thought of each speech.
Give your presentation a concept. Use a classic narrative structure, from a gripping outset to an impressive end. A presentation designer Nancy Duarte advises presenters to spend twice as much time on framing the storyline than creating the actual slides.
Also, don’t forget to add emotional details and power words. These will make your audience feel much more connected to you. People will eventually forget your slides and your presentation, but they will not forget how you made them feel.
2. Use the rule of three
People can usually remember only three main points from presentations, so take advantage of this psychological phenomenon.
While creating your storyline, think of three key messages that you want your audience to walk out of the room with. To make these three key points stick, you need to make them short, memorable and attention-grabbing.
On the other hand, if your presentation revolves around one main argument, make use of the Aristotelian “triptych” method: “Tell them what you’re going to tell them. Tell them. Then tell them what you told them.” In a nutshell, you should properly introduce the point you will be making, then make your point, and then wrap up with summarizing the main point.
3. Simplify your slides
No matter how rich in content your slides are, if they’re too crowded, nobody is going to read them. Too much text on slides actually takes your audience’s attention away from your presentation, which hinders the learning process.
Make your slides as simple as possible and try to present only one idea per slide. Sometimes, one powerful sentence, a number, or even one word, can say more than a slide full of bullet points.
4. Include numbers
When used sensibly, numbers can strengthen your point and back up your arguments. To make data easy for your audience to digest, you need to make it specific, relevant and contextual.
When Steve Jobs introduced the first iPod , he did not emphasize its 5GB storage and 185g weight. Instead, he repeatedly said that it could hold 1,000 songs and physically manifested that he could fit it into his pocket. This number was easy for the audience to remember, and called even more attention to its tiny size.
5. Use the power of visuals
Videos or images not only engage the audience but also help to evoke emotions that are otherwise super difficult for speakers to elicit by themselves.
Make sure that the visuals you use support the main point of your presentation, or demonstrate what you’re talking about. This works very well in the creative industry, where visual aids are often necessary to complement the main content.
However, if you’re going to use video, be cautious. A too lengthy or unengaging video may put people to sleep rather than keep them attentive.
6. Practice relentlessly
Attending a presentation where the speaker keeps looking down at his notes is painful so don’t underestimate this point. For instance, Winston Churchill rehearsed for hours, even days, to deliver a 10-minute long speech.
Memorize your presentation flow by heart if need be. Do it to the extent that you won’t need the notes.
During your rehearsals, use a video camera to record yourself in order to see where you stutter, where you seem nervous and how you work with your body language. Don’t be afraid to ask a friend to give you feedback.
Tim Ferris , the author of The 4-Hour Workweek, follows a Spartan’s preparation for his public speeches. He splits his presentation into several segments and he goes through each one of them up to ten times.
7. Greet your audience in their local language
As a speaker, you often find yourself addressing an international audience, whether it is at a big conference or an internal company meeting joined by remote teams.
Greeting international participants in their local language gives a nice personal touch to the offset of your presentation. It helps you create a connection and the feeling of intimacy with the people sitting before you.
I always memorize how to say “Hello” and “How are you?” in the local language, and use them as soon as I come onstage. You can even take it a step further and adjust your presentation ad hoc to the audience, by making local references.
For example, Google’s Digital Marketing Evangelist, Avinash Kaushik , started his talk at the Marketing festival by showing pictures from his tour around the hosting city of Brno, Czech Republic. Moreover, he used the Czech websites that the audience was closely familiar with, instead of international ones, to get his point across.
8. Break the ice at the start
Hook your audience right off the bat. Using an effective icebreaker will help you set the stage and energize your attendees.
Here’s an inspiration for you: At the 2018 World Education Congress (WEC) , I asked people to close their eyes and think of a presentation session that had recently impressed them. After 30 seconds, I invited them to share their dream session with their neighbor and describe it using one word, before submitting it to a Slido word cloud poll.

Next, I asked them to picture the usual experience of attending a presentation and describe it again, using a single word. Seeing the differences in the two consequent word cloud polls was very thought-provoking and sparked up a discussion among the attendees.

Other than using technology, you can liven up your audience with a classic show of hands or other brisk icebreaking activities , such as rock, paper, scissors or live barometer.
Related story: The Complete List of 200 Icebreaker Questions and Tips On How to Use Them
9. Engage your audience with live polls
Once you win your audience over, keep up the pace by creating enough interaction points throughout your presentation.
Live polling is your best bet here. When smartly used, live polls will keep your attendees engaged during the whole length of your presentation. They also help you to effectively collect your audience’s insights, which you can then showcase on screen. This multiplies the learning element of your presentation.
In general, I follow the human attention span and use a poll every 8-10 minutes, which is 5-6 polls for a 60-minute talk, maximum.
To give you an example, during the latest webinar I led, I asked the participants a simple rating poll: “How would you rate interaction in the classroom today?”
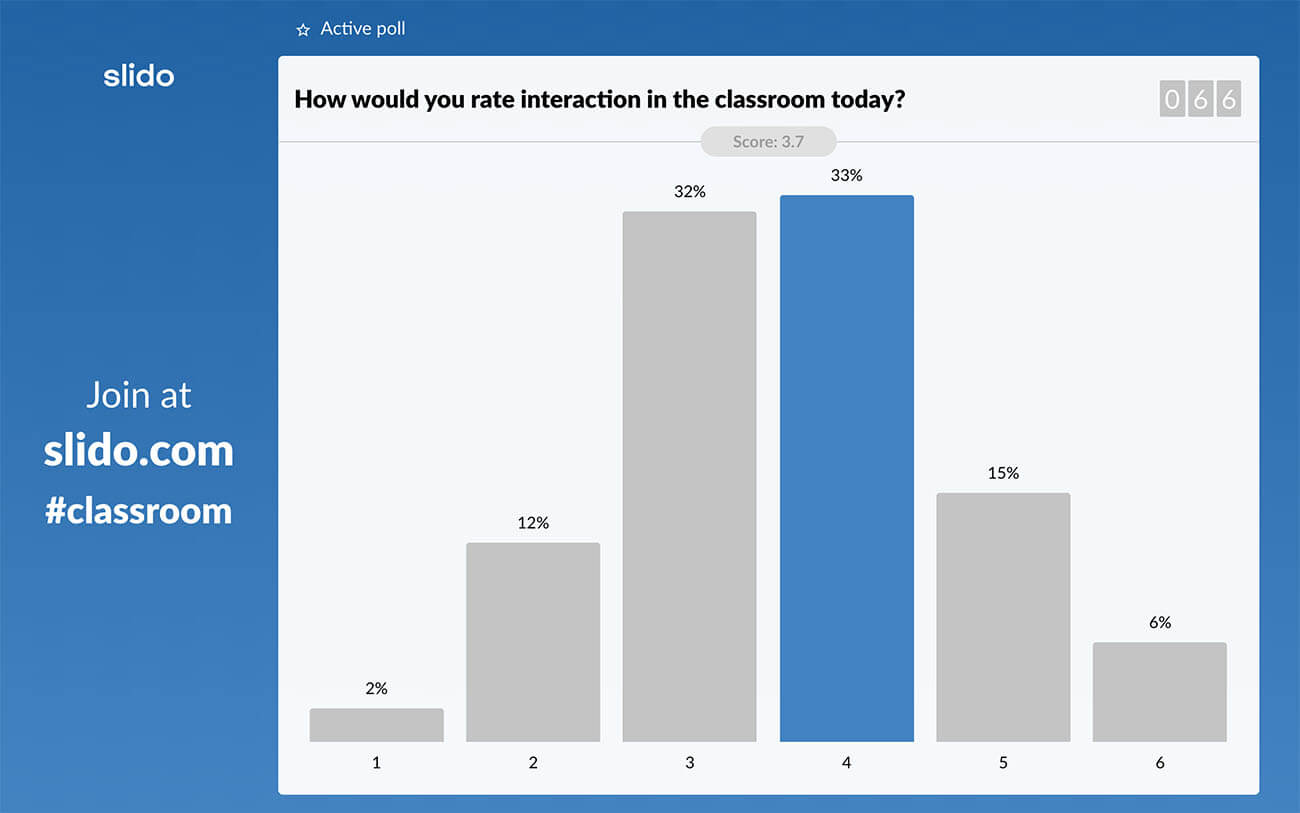
The results set a good ground for the main argument I was going to make about insufficient interactivity in education, and really helped me make my point.
On top of that, this strategy allows me to break the long content deliveries into more digestible chunks, regain the audience’s attention, and ignite conversations based on the results.
The last point is particularly important. Live polls make sense only when you facilitate their use. So make sure to always follow up on the results, share your thoughts on them, or get the audience to share why they voted the way they did.
Related story: The Complete Guide: How to Use and Facilitate Slido Polls in Your Presentation
10. Move around the stage and make gestures
If you stand rigidly in one spot or behind a speaker’s stand, you will only appear unconfident and nervous. Think of yourself as an actor on stage, and your presentation as your performance. Use open, big gestures, point in the direction of the audience, or slowly walk about the stage.
At this year’s Festival of Marketing , Mark Ritson – who was opening the event – reminded me of the importance of using body language. He kept pacing the stage in a natural way and was gesturing throughout the entire length of his speech. It was definitely one of the most engaging sessions I’ve attended this year.
11. Smile and make eye contact
The way you communicate with your audience through your facial expressions makes a huge difference. So don’t look down at your notes, don’t look at your slides, but keep your eyes set on your audience.
Lisa Wentz, a public speaking expert, advises to pick 3 people in the audience that you like, each one at a different corner of the auditorium, and make eye contact with them throughout your presentation. However, avoid staring at one person for too long. Use the selected people only as navigation points that will help you scan the room.
12. Consider using props
Demonstrating the point with the use of props is a powerful way to help the attendees visualize what is being described verbally. Showing a prop at the right moment can help you catch your audience’s attention and enforce your story.
Neuroscientist Jill Bolte Taylor brought a real human brain on stage during her emotional TED talk to explain what had happened to her when she had a stroke. She touched the audience with this demonstration and left them in complete awe.
13. Go among the audience
Asking people questions may feel impersonal if you stand onstage. On top of that, large auditoriums often make it difficult to create intimacy with your audience.
Draw inspiration from rock singers here and “jump” off the stage. Going among the audience will help you build a stronger bond with them and your presentation will feel more personal.
This approach is invaluable if you hope to collect impromptu answers after you have asked your question. Move slowly around the room, and when someone shuffles or raises a hand, approach them with a mic and elicit an answer.
When another hand shoots up, move to that corner of the room, and so on. The point here is to be as close to your audience as possible.
If possible, check the room advance to get used to the space arrangements. This will help you move around more naturally and with more confidence.
14. Give rewards for participation
Despite all your efforts, the audience might need a bit of a nudge. Giving out small rewards can bring another interactive element to your presentation. You can go with the event merchandise or small treats, like chocolates and candy.
For example, at the Eventex conference , one of the speakers, Victor Neyndorff , encouraged people to share their thoughts by handing out chocolate from the Netherlands, his home country.
To give you another idea, at the Jam London conference , the organizers decided to give away books to those attendees who were the most active in asking questions via Slido. This really helped incentivize the audience to participate and improved the dialogue in the room.
15. Prompt a discussion in the audience
You can give audience engagement another spin by giving your attendees an activity that they can participate in.
For example, you can present a statement for the participants to discuss, or give them a task to solve in groups. Where appropriate, walk around the room, join the conversations, and encourage people to talk to each other.
At the Conventa Crossover conference in Slovenia, moderator Jan-Jaap In der Maur put people in small groups and asked them to share the technological trends that they believed will have the biggest impact on the industry in the near future.
Then he collected a few comments from the floor to open a discussion with the whole room.
Simple. Engaging. Useful.
If facilitated properly, activities like these can work equally well with an audience of 20 people as they can with 2,000.
Related story: 5 Essential Pieces of The Audience Engagement Puzzle
16. Build in time for the Q&A
Even if you incorporate interactive elements to your presentation, your audience will surely have additional questions.
For that reason, don’t be scared to allocate as much as 10-20 minutes to the Q&A, depending on the length of your presentation slot.

After I finish my talk, instead of asking, “Are there any questions?” (which typically leads to silence), I like to ask, “What are your questions?”, or say, “Now, let’s get to your questions.” In case I don’t get an instant reaction from the audience, I get off the stage and walk among the audience to encourage the discussion.
In rare moments when no questions come up, I kick off the Q&A by saying: “What people usually ask me is…” and then give an answer. In 9/10 times, the discussion catches on.
17. Crowdsource questions from the audience
Lack of audience questions doesn’t necessarily mean that your audience doesn’t have questions. They may just be uncomfortable with speaking up in public.
Live Q&A tools like Slido allow you to effortlessly crowdsource questions from your audience throughout your presentation via an app.
Compared to passing the mic amongst the people in the audience, you will give everyone an equal chance to ask questions, regardless of their level of shyness.
If you’re using a Q&A app, it’s important that you introduce and facilitate it properly. I often say something like: “Take a minute and think about what you’ve just heard. Come up with a question that you have, and submit it to Slido.” It works every single time.
Then, just take a look at the screen, or a confidence monitor, and address the questions that have the most upvotes.
If you display the crowdsourced questions on the screen, read each question out loud when addressing it. It will help your audience – even the ones sitting at the back – to know which question you are answering.
Extra tip: Sometimes, you get way more questions from your audience than you can answer during your time-limited Q&A slot. Don’t leave them hanging in the air. Here you’ll find 5 tips on what to do with unanswered questions after your Q&A .
18. Gather feedback
Feedback is priceless for improving your presentation skills. There’s never enough of it. You can collect feedback easily via Slido feedback survey . Combine rating polls for quick assessment and open text polls to give your participants space for more in-depth comments.
Your feedback survey could look something like this:
- How would you rate this presentation? (rating poll)
- What is your main takeaway from this session? (open text poll)
- What would you improve? (open text poll)
To boost the response rate, make sure that you ask your attendees to fill out the survey while they’re still in the room.
With the tips I’ve listed above, you’ll be able to turn your presentation or lecture from a one-way content broadcast into an exciting conversation between you and your attendees.
Engage your attendees with Slido live Q&A and polls.
Try Slido now
Get just a single email per month with our best articles.
Presentations
5 ways to use slido in google slides.
In this article, you’ll find examples of poll questions and quizzes that you can create with Slido and use in...

7 Interactive Poll Ideas for Your Next PowerPoint Presentation
Looking for new ways to make your PowerPoint presentation more interactive? Try live polls. With polls, you can collect non-verbal...

How to Give an Interactive PowerPoint Presentation in 2023
Presenting online is tough, yes. You can’t really connect with your audience. You often don’t even know whether those mute...
How to make a great presentation
Stressed about an upcoming presentation? These talks are full of helpful tips on how to get up in front of an audience and make a lasting impression.
The secret structure of great talks

The beauty of data visualization

TED's secret to great public speaking

How to speak so that people want to listen
How great leaders inspire action

Brought to you by:

How to Give a Killer Presentation
By: Chris Anderson
For more than 30 years, the TED conference series has presented enlightening talks that people enjoy watching. In this article, Anderson, TED's curator, shares five keys to great presentations: Frame…
- Length: 3676 word count
- Publication Date: Jun 1, 2013
- Discipline: General Management
- Product #: R1306K-PDF-ENG
What's included:
- Educator Copy
$4.50 per student
degree granting course
$7.95 per student
non-degree granting course
Get access to this material, plus much more with a free Educator Account:
- Access to world-famous HBS cases
- Up to 60% off materials for your students
- Resources for teaching online
- Tips and reviews from other Educators
Already registered? Sign in
- Student Registration
- Non-Academic Registration
- Included Materials
Lessons from TED
Jun 1, 2013
Discipline:
General Management
Harvard Business Review Digital Article
R1306K-PDF-ENG
3676 word count
We use cookies to understand how you use our site and to improve your experience, including personalizing content. Learn More . By continuing to use our site, you accept our use of cookies and revised Privacy Policy .

Researched by Consultants from Top-Tier Management Companies

Powerpoint Templates
Icon Bundle
Kpi Dashboard
Professional
Business Plans
Swot Analysis
Gantt Chart
Business Proposal
Marketing Plan
Project Management
Business Case
Business Model
Cyber Security
Business PPT
Digital Marketing
Digital Transformation
Human Resources
Product Management
Artificial Intelligence
Company Profile
Acknowledgement PPT
PPT Presentation
Reports Brochures
One Page Pitch
Interview PPT
All Categories
Top 10 Introducing Yourself Templates with Examples and Samples

Kavesh Malhotra
"The difference between ordinary and extraordinary is that little extra," Jimmy Johnson, American sports coach.
Jimmy Johnson's words perfectly fit when it comes to introducing yourself. A personal introduction is a part of almost every career option you pick today. How you introduce yourself can open or close the doors of opportunities for you. When you have a killer introduction, people will remember you. It sets the stage for more interactions, showcases your confidence, and helps others understand you better.
Let's say you are pitching for funding for your business. But before you introduce your product in front of the investors, they would love to know where they are putting their money into. In such cases, an outstanding introduction gives you an opportunity to establish a killer rapport from the beginning. It adds credibility from the get-go. Similarly, if you are attending a networking event or attending a job interview, an impactful self-introduction goes a long way in boosting your chances for success.
At Slide Teaml, our experts have prepared self-introduction templates after much analysis and studying human psychology. These 100% content-ready templates are fully editable and give you the ability to present yourself in a much more impactful and creative way. Using these templates, you can transform your routine introduction into a powerful self-endorsement.
Let's take a look at these templates one by one.
Template 1: Introduce Yourself PowerPoint Presentation Slides
This template acts as a powerful tool for creating a powerful personal introduction . It includes a wide range of slides that can help you express your strengths in a much more engaging and effective way. The slides include About Me, Career, SWOT Analysis , Qualifications, and more. Each slide is highlighted by engaging visual icons for milestones, skills, hobbies, and more. The attractive color palate makes the template even more indulging and ensures no one bats an eye when you are introducing yourself. This helps craft a powerful story that talks about your skills and prowesses. It's perfect for interviews and networking and can help create a strong and impactful first impression for yourself.

Download Now
Template 2: Introducing Yourself and Your Capabilities in a PowerPoint Presentation with Slides
This template can be an amazing tool to create a strong first impression in any professional setting. The multi-slide template lets you highlight your personal and professional qualities in a well-structured and powerful way. Its key elements, like a detailed About Me section, a Career Timeline, a Personal SWOT Analysis , and a vivid Personal Profile slide, touch upon every aspect of your self-introduction. The clean and clutter-free design, with its bold color accents and smart graphics, ensures that your strengths and potential are properly highlighted. This template is perfect for job interviews, networking events, and team introductions.

Template 3: 10 Minutes Presentation About Myself PowerPoint Presentation Slides
This template is specifically crafted for professionals who want to make a strong first impression in a brief interaction. It helps you build a powerful narrative about your career path, personal strengths, educational background, and professional accomplishments.
The vibrant green with dark hues helps grab attention, while perfectly organized content blocks ensure the information is digested properly by the audience. Some of the key slides, like the Career Timeline and SWOT Analysis slides, help present a crisp and dynamic view of your professional journey and personal analysis.

Template 4: Introducing Yourself, Employee Achievement, Team Member Candidate Skills
This is a perfect template if you want to shed some light on your individual strengths and team contributions. The sections like "Employee Achievement Timeline in Introducing Yourself" and "Essential Candidate Characteristics in Introducing Yourself" could be a killer way to tell others about your abilities and yourself. The engaging visuals let you present your career progression, key qualifications, and notable achievements. It’s a perfectly balanced mix of imagery and icons that enhance recall and engagement. In short, this template is a great choice for job candidates and team members who want to showcase their skills as an individual and as a team member.

Template 5: Meeting New People and Introducing Yourself PowerPoint Presentation Slides
Meeting someone for the first time and want to nail the first impression? This template can get the job done! It's tailor-made for job seekers and professionals who are looking for growth in their careers. This template offers a structured layout to showcase one's career journey, skillset, and achievements. The 'About Me/Bio' slide allows for a personal touch, integrating an image with key personal details. 'Agenda' and 'Career Objective' slides lay out a crisp outline to showcase the presenter's goals. The template has a soothing color scheme and ensures readability and a professional aesthetic. Download now and create memorable information.

Template 6: SWOT Analysis
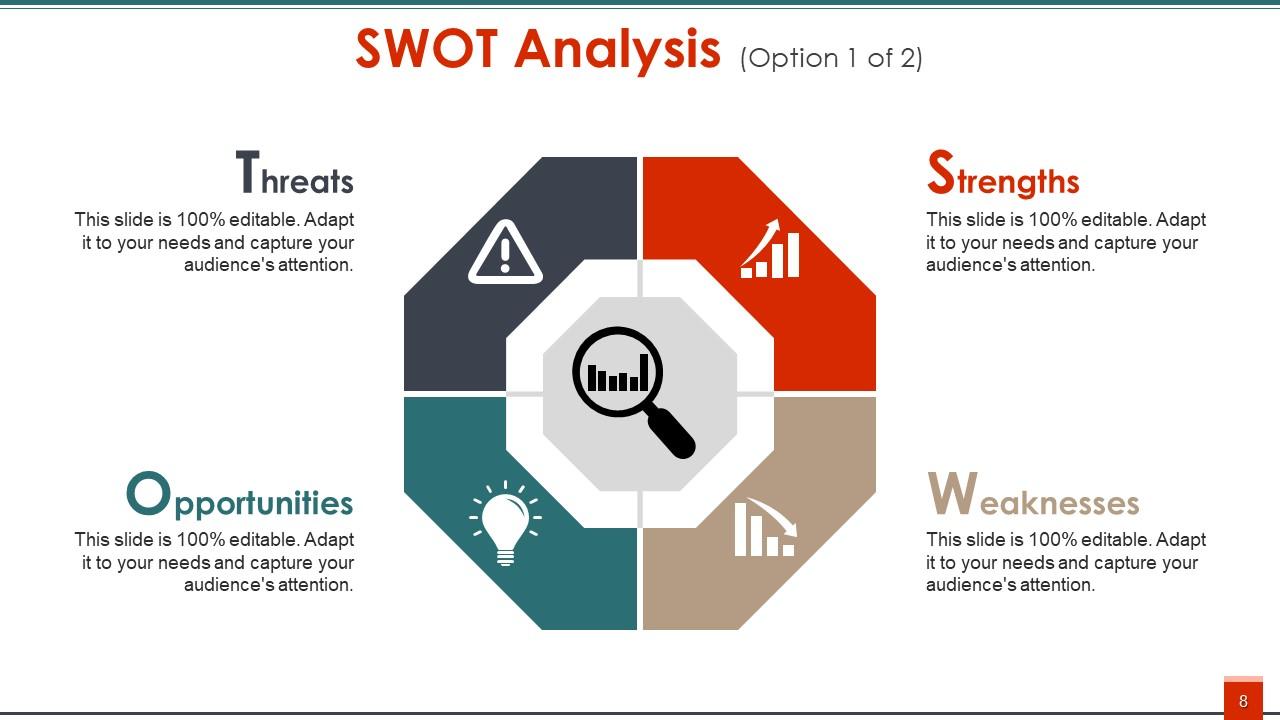
When introducing yourself, you have to present your strengths in front of your audience. But at the same time, you cannot go praising yourself from the start! Instead, a balanced approach goes a long way. And the best way to do so is to present a SWOT analysis of your own! That's where this template comes into play. It has color-coded quadrants that provide intuitive self-assessment for the views. The vibrant red color covers strengths, professional blue for weaknesses, growth-associated greens for opportunities, and cautionary greys for threats. Additionally, the magnifying glass symbolizes the focus and introspection you have done to prepare this analysis.
Template 7: Career Objectives
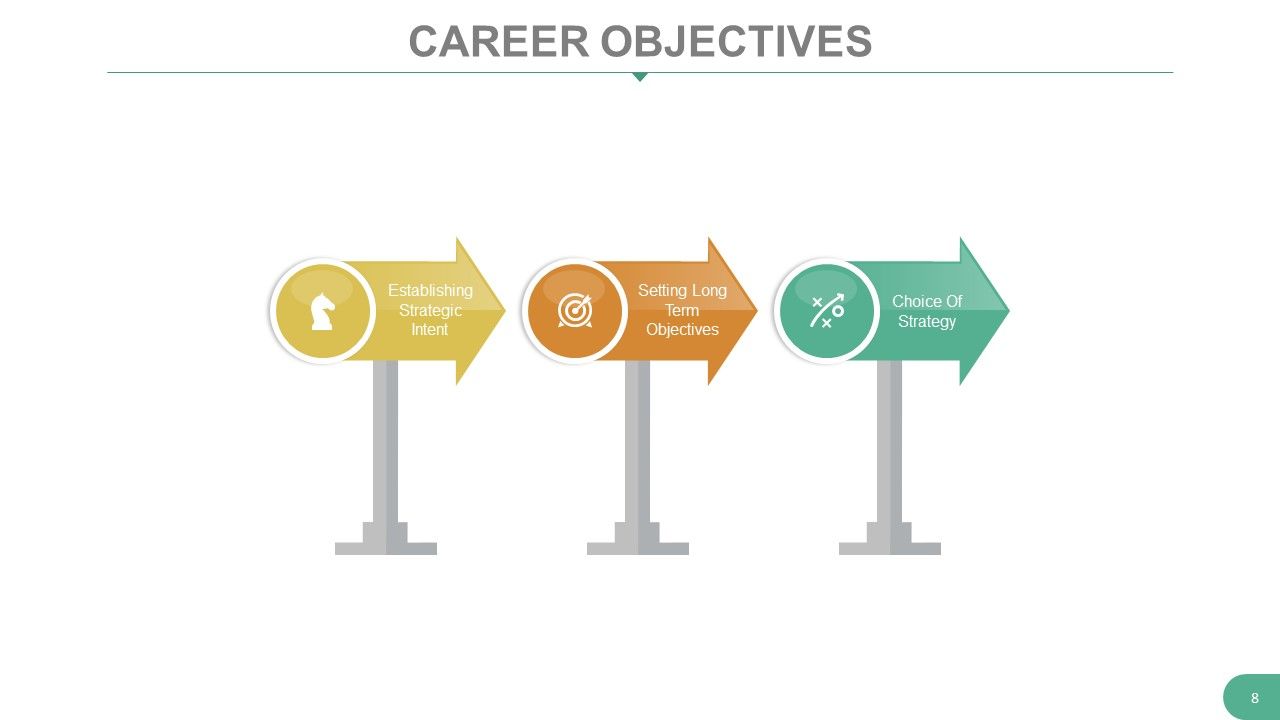
When you are talking about your career objective , it should feel more like a realistic plan instead of daydreams. And this template can help you articulate your objective pretty neatly. Designed with bold colors and direction-pointing arrow signs, this template features a profession over the years and a clear roadmap for the future. It encourages a step-by-step approach to set goals from foundational intentions to long-term objectives and strategic choices. You can effectively express your career vision and align your goals with action. You can demonstrate a forward-thinking mindset and an organized approach to career planning using this template.
Template 8: Case Study
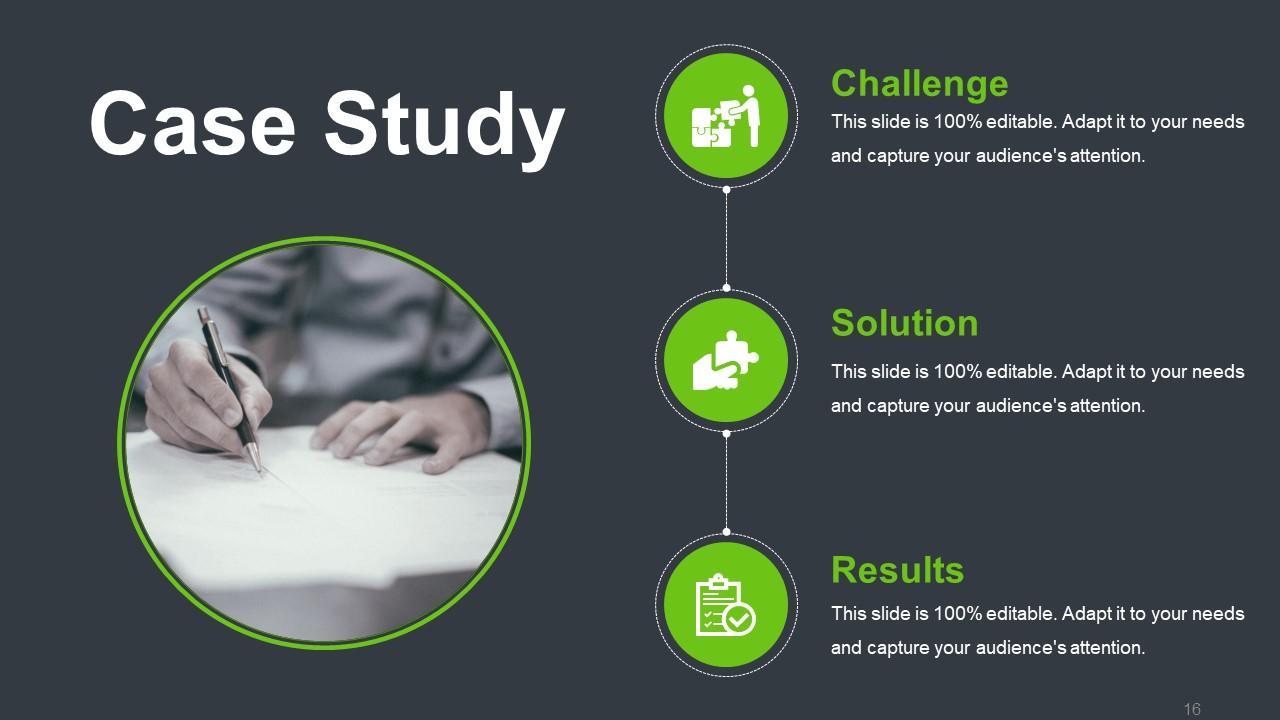
This template is an excellent option if you are presenting a case study to introduce your problem-solving skills. How? Well, this one lets you showcase how you were able to tackle a particular set of challenges that crept up while working on it. When you showcase a real-life example, it gives the viewers more confidence in you. The design is sleek, and a balanced use of space and contrasting colors grab the audience's attention. It helps them focus on the areas that matter the most. The central image acts as an anchor, and the surrounding icons help simplify complex information. Each icon acts as a visual marker with space to add necessary information about the particular case study.
Template 9: Introducing Yourself Depicting Employee Performance
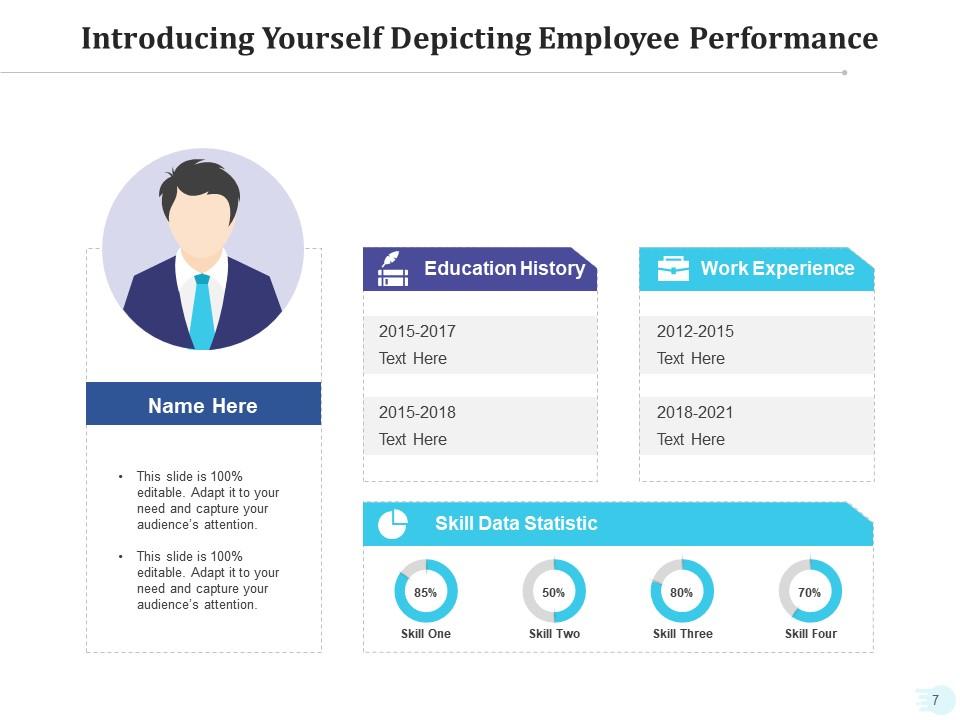
Professional achievements are always a key part of self-introduction, especially for those who are looking to crack an interview. This template is specifically designed for such individuals. This template has a prominent section for the presenter's profile photo, a detailed education history , and work experience. It features a 'Skill Data Statistic' section with customizable gauges. This section can help you visually represent what expertise you have in particular skills. This template is a perfect blend of personal branding and performance metrics that's suitable for interviews, performance reviews, or personal assessments.
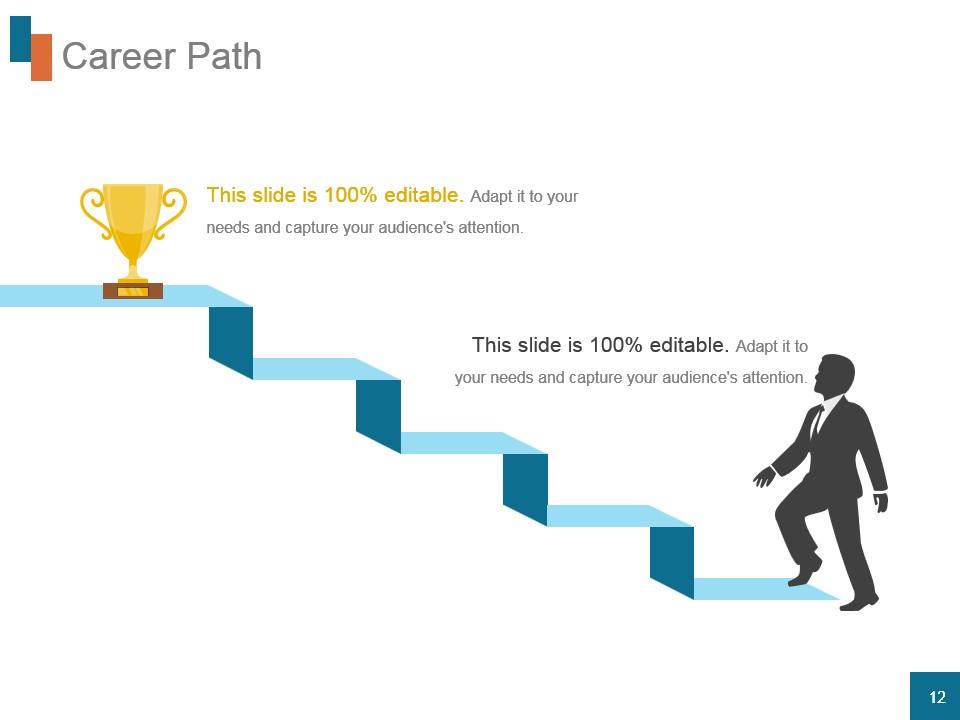
Template 10: Career Path
A career is nothing less than climbing steps, right? This template visualizes the career journey with clarity and motivation, using the same concept. With a staircase graphic that represents a step-by-step progression and a trophy at the end that indicates the ultimate goal, this template is a great career path presentation template. Here, each step of the staircase acts as a distinct phase or accomplishment in your professional career. The silhouette of a person at the base adds a human element to the narrative. This editable slide is perfect for professionals outlining career goals, milestones, or success stories during presentations.
The Final Thought
Your initial introduction can make or break your deal or interview, so it's always better to have a rock-solid first impression whenever you meet someone. These templates could be of great assistance in such cases and help you connect with the audience at professional levels. Download them now and make your introductions more attractive and crystal clear.
Related posts:
- Top 10 Templates for Presentation About Myself with Samples and Examples
- Top 10 Self-Introduction Templates with Samples and Examples
- Must-Have About Me Introduction Samples With Examples and Templates
- Top 5 Professional Profile Templates with Examples and Samples
Liked this blog? Please recommend us

This form is protected by reCAPTCHA - the Google Privacy Policy and Terms of Service apply.

Digital revolution powerpoint presentation slides
Sales funnel results presentation layouts
3d men joinning circular jigsaw puzzles ppt graphics icons

Business Strategic Planning Template For Organizations Powerpoint Presentation Slides
Future plan powerpoint template slide

Project Management Team Powerpoint Presentation Slides

Brand marketing powerpoint presentation slides

Launching a new service powerpoint presentation with slides go to market

Agenda powerpoint slide show
Four key metrics donut chart with percentage
Engineering and technology ppt inspiration example introduction continuous process improvement

Meet our team representing in circular format

We use essential cookies to make Venngage work. By clicking “Accept All Cookies”, you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts.
Manage Cookies
Cookies and similar technologies collect certain information about how you’re using our website. Some of them are essential, and without them you wouldn’t be able to use Venngage. But others are optional, and you get to choose whether we use them or not.
Strictly Necessary Cookies
These cookies are always on, as they’re essential for making Venngage work, and making it safe. Without these cookies, services you’ve asked for can’t be provided.
Show cookie providers
- Google Login
Functionality Cookies
These cookies help us provide enhanced functionality and personalisation, and remember your settings. They may be set by us or by third party providers.
Performance Cookies
These cookies help us analyze how many people are using Venngage, where they come from and how they're using it. If you opt out of these cookies, we can’t get feedback to make Venngage better for you and all our users.
- Google Analytics
Targeting Cookies
These cookies are set by our advertising partners to track your activity and show you relevant Venngage ads on other sites as you browse the internet.
- Google Tag Manager
- Infographics
- Daily Infographics
- Popular Templates
- Accessibility
- Graphic Design
- Graphs and Charts
- Data Visualization
- Human Resources
- Beginner Guides
Blog Beginner Guides How To Make a Good Presentation [A Complete Guide]
How To Make a Good Presentation [A Complete Guide]
Written by: Krystle Wong Jul 20, 2023

A top-notch presentation possesses the power to drive action. From winning stakeholders over and conveying a powerful message to securing funding — your secret weapon lies within the realm of creating an effective presentation .
Being an excellent presenter isn’t confined to the boardroom. Whether you’re delivering a presentation at work, pursuing an academic career, involved in a non-profit organization or even a student, nailing the presentation game is a game-changer.
In this article, I’ll cover the top qualities of compelling presentations and walk you through a step-by-step guide on how to give a good presentation. Here’s a little tip to kick things off: for a headstart, check out Venngage’s collection of free presentation templates . They are fully customizable, and the best part is you don’t need professional design skills to make them shine!
These valuable presentation tips cater to individuals from diverse professional backgrounds, encompassing business professionals, sales and marketing teams, educators, trainers, students, researchers, non-profit organizations, public speakers and presenters.
No matter your field or role, these tips for presenting will equip you with the skills to deliver effective presentations that leave a lasting impression on any audience.
Click to jump ahead:
What are the 10 qualities of a good presentation?
Step-by-step guide on how to prepare an effective presentation, 9 effective techniques to deliver a memorable presentation, faqs on making a good presentation, how to create a presentation with venngage in 5 steps.
When it comes to giving an engaging presentation that leaves a lasting impression, it’s not just about the content — it’s also about how you deliver it. Wondering what makes a good presentation? Well, the best presentations I’ve seen consistently exhibit these 10 qualities:
1. Clear structure
No one likes to get lost in a maze of information. Organize your thoughts into a logical flow, complete with an introduction, main points and a solid conclusion. A structured presentation helps your audience follow along effortlessly, leaving them with a sense of satisfaction at the end.
Regardless of your presentation style , a quality presentation starts with a clear roadmap. Browse through Venngage’s template library and select a presentation template that aligns with your content and presentation goals. Here’s a good presentation example template with a logical layout that includes sections for the introduction, main points, supporting information and a conclusion:

2. Engaging opening
Hook your audience right from the start with an attention-grabbing statement, a fascinating question or maybe even a captivating anecdote. Set the stage for a killer presentation!
The opening moments of your presentation hold immense power – check out these 15 ways to start a presentation to set the stage and captivate your audience.
3. Relevant content
Make sure your content aligns with their interests and needs. Your audience is there for a reason, and that’s to get valuable insights. Avoid fluff and get straight to the point, your audience will be genuinely excited.
4. Effective visual aids
Picture this: a slide with walls of text and tiny charts, yawn! Visual aids should be just that—aiding your presentation. Opt for clear and visually appealing slides, engaging images and informative charts that add value and help reinforce your message.
With Venngage, visualizing data takes no effort at all. You can import data from CSV or Google Sheets seamlessly and create stunning charts, graphs and icon stories effortlessly to showcase your data in a captivating and impactful way.

5. Clear and concise communication
Keep your language simple, and avoid jargon or complicated terms. Communicate your ideas clearly, so your audience can easily grasp and retain the information being conveyed. This can prevent confusion and enhance the overall effectiveness of the message.
6. Engaging delivery
Spice up your presentation with a sprinkle of enthusiasm! Maintain eye contact, use expressive gestures and vary your tone of voice to keep your audience glued to the edge of their seats. A touch of charisma goes a long way!
7. Interaction and audience engagement
Turn your presentation into an interactive experience — encourage questions, foster discussions and maybe even throw in a fun activity. Engaged audiences are more likely to remember and embrace your message.
Transform your slides into an interactive presentation with Venngage’s dynamic features like pop-ups, clickable icons and animated elements. Engage your audience with interactive content that lets them explore and interact with your presentation for a truly immersive experience.

8. Effective storytelling
Who doesn’t love a good story? Weaving relevant anecdotes, case studies or even a personal story into your presentation can captivate your audience and create a lasting impact. Stories build connections and make your message memorable.
A great presentation background is also essential as it sets the tone, creates visual interest and reinforces your message. Enhance the overall aesthetics of your presentation with these 15 presentation background examples and captivate your audience’s attention.
9. Well-timed pacing
Pace your presentation thoughtfully with well-designed presentation slides, neither rushing through nor dragging it out. Respect your audience’s time and ensure you cover all the essential points without losing their interest.
10. Strong conclusion
Last impressions linger! Summarize your main points and leave your audience with a clear takeaway. End your presentation with a bang , a call to action or an inspiring thought that resonates long after the conclusion.
In-person presentations aside, acing a virtual presentation is of paramount importance in today’s digital world. Check out this guide to learn how you can adapt your in-person presentations into virtual presentations .
Preparing an effective presentation starts with laying a strong foundation that goes beyond just creating slides and notes. One of the quickest and best ways to make a presentation would be with the help of a good presentation software .
Otherwise, let me walk you to how to prepare for a presentation step by step and unlock the secrets of crafting a professional presentation that sets you apart.
1. Understand the audience and their needs
Before you dive into preparing your masterpiece, take a moment to get to know your target audience. Tailor your presentation to meet their needs and expectations , and you’ll have them hooked from the start!
2. Conduct thorough research on the topic
Time to hit the books (or the internet)! Don’t skimp on the research with your presentation materials — dive deep into the subject matter and gather valuable insights . The more you know, the more confident you’ll feel in delivering your presentation.
3. Organize the content with a clear structure
No one wants to stumble through a chaotic mess of information. Outline your presentation with a clear and logical flow. Start with a captivating introduction, follow up with main points that build on each other and wrap it up with a powerful conclusion that leaves a lasting impression.
Delivering an effective business presentation hinges on captivating your audience, and Venngage’s professionally designed business presentation templates are tailor-made for this purpose. With thoughtfully structured layouts, these templates enhance your message’s clarity and coherence, ensuring a memorable and engaging experience for your audience members.
Don’t want to build your presentation layout from scratch? pick from these 5 foolproof presentation layout ideas that won’t go wrong.

4. Develop visually appealing and supportive visual aids
Spice up your presentation with eye-catching visuals! Create slides that complement your message, not overshadow it. Remember, a picture is worth a thousand words, but that doesn’t mean you need to overload your slides with text.
Well-chosen designs create a cohesive and professional look, capturing your audience’s attention and enhancing the overall effectiveness of your message. Here’s a list of carefully curated PowerPoint presentation templates and great background graphics that will significantly influence the visual appeal and engagement of your presentation.
5. Practice, practice and practice
Practice makes perfect — rehearse your presentation and arrive early to your presentation to help overcome stage fright. Familiarity with your material will boost your presentation skills and help you handle curveballs with ease.
6. Seek feedback and make necessary adjustments
Don’t be afraid to ask for help and seek feedback from friends and colleagues. Constructive criticism can help you identify blind spots and fine-tune your presentation to perfection.
With Venngage’s real-time collaboration feature , receiving feedback and editing your presentation is a seamless process. Group members can access and work on the presentation simultaneously and edit content side by side in real-time. Changes will be reflected immediately to the entire team, promoting seamless teamwork.
7. Prepare for potential technical or logistical issues
Prepare for the unexpected by checking your equipment, internet connection and any other potential hiccups. If you’re worried that you’ll miss out on any important points, you could always have note cards prepared. Remember to remain focused and rehearse potential answers to anticipated questions.
8. Fine-tune and polish your presentation
As the big day approaches, give your presentation one last shine. Review your talking points, practice how to present a presentation and make any final tweaks. Deep breaths — you’re on the brink of delivering a successful presentation!
In competitive environments, persuasive presentations set individuals and organizations apart. To brush up on your presentation skills, read these guides on how to make a persuasive presentation and tips to presenting effectively .

Whether you’re an experienced presenter or a novice, the right techniques will let your presentation skills soar to new heights!
From public speaking hacks to interactive elements and storytelling prowess, these 9 effective presentation techniques will empower you to leave a lasting impression on your audience and make your presentations unforgettable.
1. Confidence and positive body language
Positive body language instantly captivates your audience, making them believe in your message as much as you do. Strengthen your stage presence and own that stage like it’s your second home! Stand tall, shoulders back and exude confidence.
2. Eye contact with the audience
Break down that invisible barrier and connect with your audience through their eyes. Maintaining eye contact when giving a presentation builds trust and shows that you’re present and engaged with them.
3. Effective use of hand gestures and movement
A little movement goes a long way! Emphasize key points with purposeful gestures and don’t be afraid to walk around the stage. Your energy will be contagious!
4. Utilize storytelling techniques
Weave the magic of storytelling into your presentation. Share relatable anecdotes, inspiring success stories or even personal experiences that tug at the heartstrings of your audience. Adjust your pitch, pace and volume to match the emotions and intensity of the story. Varying your speaking voice adds depth and enhances your stage presence.

5. Incorporate multimedia elements
Spice up your presentation with a dash of visual pizzazz! Use slides, images and video clips to add depth and clarity to your message. Just remember, less is more—don’t overwhelm them with information overload.
Turn your presentations into an interactive party! Involve your audience with questions, polls or group activities. When they actively participate, they become invested in your presentation’s success. Bring your design to life with animated elements. Venngage allows you to apply animations to icons, images and text to create dynamic and engaging visual content.
6. Utilize humor strategically
Laughter is the best medicine—and a fantastic presentation enhancer! A well-placed joke or lighthearted moment can break the ice and create a warm atmosphere , making your audience more receptive to your message.
7. Practice active listening and respond to feedback
Be attentive to your audience’s reactions and feedback. If they have questions or concerns, address them with genuine interest and respect. Your responsiveness builds rapport and shows that you genuinely care about their experience.

8. Apply the 10-20-30 rule
Apply the 10-20-30 presentation rule and keep it short, sweet and impactful! Stick to ten slides, deliver your presentation within 20 minutes and use a 30-point font to ensure clarity and focus. Less is more, and your audience will thank you for it!
9. Implement the 5-5-5 rule
Simplicity is key. Limit each slide to five bullet points, with only five words per bullet point and allow each slide to remain visible for about five seconds. This rule keeps your presentation concise and prevents information overload.
Simple presentations are more engaging because they are easier to follow. Summarize your presentations and keep them simple with Venngage’s gallery of simple presentation templates and ensure that your message is delivered effectively across your audience.

1. How to start a presentation?
To kick off your presentation effectively, begin with an attention-grabbing statement or a powerful quote. Introduce yourself, establish credibility and clearly state the purpose and relevance of your presentation.
2. How to end a presentation?
For a strong conclusion, summarize your talking points and key takeaways. End with a compelling call to action or a thought-provoking question and remember to thank your audience and invite any final questions or interactions.
3. How to make a presentation interactive?
To make your presentation interactive, encourage questions and discussion throughout your talk. Utilize multimedia elements like videos or images and consider including polls, quizzes or group activities to actively involve your audience.
In need of inspiration for your next presentation? I’ve got your back! Pick from these 120+ presentation ideas, topics and examples to get started.
Creating a stunning presentation with Venngage is a breeze with our user-friendly drag-and-drop editor and professionally designed templates for all your communication needs.
Here’s how to make a presentation in just 5 simple steps with the help of Venngage:
Step 1: Sign up for Venngage for free using your email, Gmail or Facebook account or simply log in to access your account.
Step 2: Pick a design from our selection of free presentation templates (they’re all created by our expert in-house designers).
Step 3: Make the template your own by customizing it to fit your content and branding. With Venngage’s intuitive drag-and-drop editor, you can easily modify text, change colors and adjust the layout to create a unique and eye-catching design.
Step 4: Elevate your presentation by incorporating captivating visuals. You can upload your images or choose from Venngage’s vast library of high-quality photos, icons and illustrations.
Step 5: Upgrade to a premium or business account to export your presentation in PDF and print it for in-person presentations or share it digitally for free!
By following these five simple steps, you’ll have a professionally designed and visually engaging presentation ready in no time. With Venngage’s user-friendly platform, your presentation is sure to make a lasting impression. So, let your creativity flow and get ready to shine in your next presentation!
Discover popular designs

Infographic maker

Brochure maker

White paper online

Newsletter creator

Flyer maker
Timeline maker

Letterhead maker
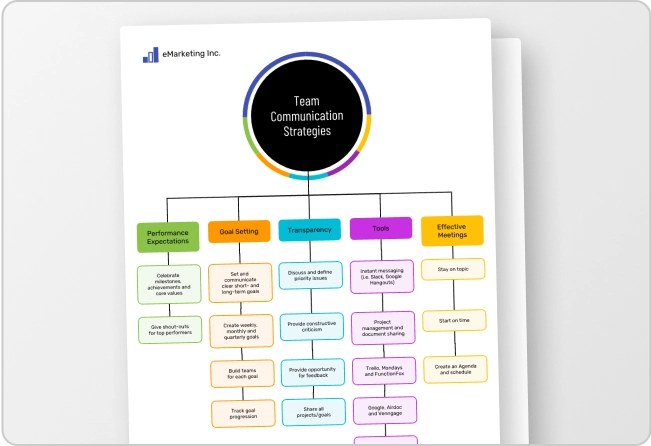
Mind map maker

Ebook maker
- SUGGESTED TOPICS
- The Magazine
- Newsletters
- Managing Yourself
- Managing Teams
- Work-life Balance
- The Big Idea
- Data & Visuals
- Reading Lists
- Case Selections
- HBR Learning
- Topic Feeds
- Account Settings
- Email Preferences
What It Takes to Give a Great Presentation
- Carmine Gallo

Five tips to set yourself apart.
Never underestimate the power of great communication. It can help you land the job of your dreams, attract investors to back your idea, or elevate your stature within your organization. But while there are plenty of good speakers in the world, you can set yourself apart out by being the person who can deliver something great over and over. Here are a few tips for business professionals who want to move from being good speakers to great ones: be concise (the fewer words, the better); never use bullet points (photos and images paired together are more memorable); don’t underestimate the power of your voice (raise and lower it for emphasis); give your audience something extra (unexpected moments will grab their attention); rehearse (the best speakers are the best because they practice — a lot).
I was sitting across the table from a Silicon Valley CEO who had pioneered a technology that touches many of our lives — the flash memory that stores data on smartphones, digital cameras, and computers. He was a frequent guest on CNBC and had been delivering business presentations for at least 20 years before we met. And yet, the CEO wanted to sharpen his public speaking skills.
- Carmine Gallo is a Harvard University instructor, keynote speaker, and author of 10 books translated into 40 languages. Gallo is the author of The Bezos Blueprint: Communication Secrets of the World’s Greatest Salesman (St. Martin’s Press).
Partner Center

Ace the Presentation

Designing a Killer Presentation in 8 Steps
Planning and performing a presentation that meets expectations and involves the public requires a lot of care. The details involved in holding a talk will be super important to ensure her success and approval from those who participated.
Therefore, we have prepared a post with a few crucial steps that you should follow to organize a quality talk; these are simple and easy steps to put into practice that will ensure the success of your presentation. Enjoy!
What is a Presentation?
A presentation is a form of communication that aims to show the content of a given topic before an audience. Unlike many other methods, such as writing or audiovisual, the presentation offers information to an audience in the form of speeches, although multimedia tools often accompany it.
1. The relevant content;
2. Attractive design;
How to Design a Presentation?
There are 4 Key things to a well designed presentation: always keep in mind the message, get to know the audience, plan for a delivery that will share the message in the best way possible, so that the audience buys the idea, and spend time making the simplest yet clear and interesting to look at slides.
- Define the purpose of the presentation
Knowing the goal you want to achieve is the first step in organizing a presentation; the more targeted this goal is, the greater the chances of successful delivery. Remember that it is the goal that will be the basis of all planning, from the definition of the target audience to the presentation of content.
Think about the topic you want to address and study the best way to do it. Will a more in-depth approach be offered, with more recent and specific information? Or is it a new theme or responsive to the public that deserves a lighter approach?
Also, when setting the goal of your talk, don’t forget to take into account the “wins” or “added value” you intend to offer to attendees. For example, will they improve the quality of life, or will they earn more money with the content presented? It is by planning these “wins” that will motivate the public to engage during the presentation and remember you long after you’re done.
- Define your target audience
With the goal in mind, it’s time to define the profile of the participants. Who will be your listener? Check your audience’s gender, age, level of education, interests, and preferences.
These are the different characteristics that will also help you define your talk’s presentation: topics that will be covered, tone of language, and technical level of content.
By having the target audience defined, you will understand how your listener speaks and know the best way to talk to them. What good is unique content if participants don’t see what you want to go through?
- and Meet them!
So, first of all, do the exercise to answer the following questions:
- What problems and needs does my persona have about the subject?
- What are your worldview and your expectations on the subject?
- How valuable is my presentation to them?
- What solution can I offer through my presentation?
Be careful with the language
From the moment you have the definition of an expected audience, it is easier to pay attention to the ideal language for these listeners. For example, it’s very different to give a motivational talk about entrepreneurship to young people and a group of senior entrepreneurs.
Each of these audiences requires not only different language tones but also different forms of approach.
For example, using a meme can yield interest, engagement, and a more significant impact on a group of young people. At the same time, this strategy may not work with a more formal group from another generation.
- Think of the resources needed
Don’t just consider audiovisual resources when organizing a presentation. It is clear that audio and video equipment is paramount to the display, but a speech goes far beyond that. Evaluate whether you’ll be offering notebooks and pens, for example, or a coffee break for attendees.
- Outline the content and order of the presentation
This is the moment when you will punctuate the content of each part of the presentation: a) introduction, b) development, and c) conclusion. If you’re going to use slides, it’s time to score the content of each screen. With the topics of your presentation script set, it’s time to assemble it.
Also, it might be interesting to put a phrase in each to define its content on the use of slides. In this sense, it is necessary to go beyond simple Bullet points.
It is necessary to point out the theme of that slide and talk deeply about the content. Don’t worry about the number of words or information yet.
As you revise the text and control the time, the excesses will naturally be cut.
- Make storytelling your ally.
In practice, it is up to you to find a narrative that can convey the message you want to convey, the one you set your goals on, remember? You can create a character, tell a personal story , fight a battle or even appeal to the drama depending on who you’re presenting to and where you’re going with it. Use your imagination but remember the story has to be related to the content!
We have a great article that goes more in depth about how to master the art of storytelling and how to use it during your presentation, to get higher engagement and feedback from the audience.
- Read your presentation out loud.
Part of the job of building a good presentation script is rehearsing it. What is the point of having everything well structured but without checking if it works during the presentation?
Reading aloud is a great way to make your speech sound more and more natural. The loud voice also allows you to notice at which points your speech is hesitant.
Do this as many times as necessary. Repeat, analyze, and point out your mistakes and hits; the training will make you more confident and sharpen your presentation script more and more.
- Be aware of the time you will spend
This tip ensures that your presentation is not cut before you reach the goals that guided all your work; remember the content you outlined? This is the time when you will need to cut through the excesses.
With the readings out loud, you’ll get a better sense of the time you’re taking to complete the ideas. The goal is to be able to fit it into the time available for your speech.
There’s a good chance that getting too excited about the speech, or nervous, can lead to failure in managing time during your presentation. Let all your rehearsals give you a good sense of how much each slide might take, and stick to that.
Presenting your Content to the Audience
Once your presentation is prepared with all the previous points in mind, it is time to face another situation: presenting your content before an audience.
- Speak with clarity and objectivity
Internalize this mantra from anyone who needs to convey an idea: clarity and objectivity are the best ways to get your message across. People who speak too fast or who are prolific end up losing the interest of the public.
Your message gets lost in the middle of these details, and the talk ends up not reaching your goals.
Remember to speak slowly, even to give your audience time to process all the information, formulate doubts and understand the subject well. Also, train your diction to correctly pronounce words, especially technical terms and in a foreign language.
Don’t stand on one side of the stage or room while performing. This is the recipe for a tedious talk; instead, try to move around constantly as you speak. This sharpens the audience’s attention and stimulates them to maintain greater interest in what you are discussing.
- Set your gaze with the public
Speaking of interest, another exciting strategy to keep public engagement with you is the good old eye-to-eye. Don’t just choose one person to do this during the talk; try to make eye contact with all audience members.
- See them as equals to lessen nervousness.
It can be challenging to present to a more experienced or hierarchically superior audience, such as bosses and investors. These experiences are challenging in themselves, but what you can do to ease the nervousness is treat them as equals.
Remember that giving lectures is part of the routine of any successful person, regardless of the area. It is crucial to treat these people naturally and to forget the credentials in each one’s curriculum.
- Know how to deal with unforeseen events
Unforeseen things happen! From projectors that stop working to interruptions and unexpected questions. None of this is a reason for you to despair or forgets your presentation script.
Instead, learn to deal with the unforeseen naturally, as if they were opportunities for you to gain experience and professional maturity. Depending on the audience, play with the situation and try to recover the direction of the presentation.
- Reveal your personality
Are you outgoing, funny, quiet, or more technical? Go ahead, be yourself! Don’t let your nerves make you hide your true personality.
- Avoid reading
If you are accustomed to reading what is in the presentation, try to avoid this practice as much as possible so as not to lose contact with your audience and seem unsure about what it says.
This is why we recommend the use of highlighted images and keywords in the design of your presentation. Presentations are complementary tools, but you dominate the theme!
- Focus on the important
Sometimes we start talking and end up missing the course of history, right? It can’t happen in a presentation. If you want the public to remember your message, keep the gist of the matter.
Crucial Tips to Keep in Mind on the Before, During, and at the Day of the Presentation
- but not for long so as not to overload it;
- Ask someone to listen to your speech before you go on stage;
- Try to arrive early, and this will help you feel confident with space;
- When you’re in front of the audience, and you’re still nervous, take a deep breath;
- Instead, focus on features like slides that have helped you stay firm in your message, you’ll see how, after a few minutes, you’re building confidence;
- Don’t be afraid to take a few seconds to think before you make the next point during your speech.
Designing a presentation requires time and perspective; when you figure out what message you want to share with the audience, exploring resources that are engaging according to the group you are presenting is crucial.
Check out some recommendations for additional learning below:
8 THINGS YOU CAN DO TO ACE ANY JOB INTERVIEW

The happiness when receiving a call marking the job interview gives rise to endless anxiety. After all, it’s only a few minutes to prove your worth, impress the recruiter and seize the opportunity. However, to do well at the job interview, you need to think about what you will say, how you will present yourself,…
TOP 7 Core Interpersonal Skills in Leadership

At any time, a leader is seen as one who guides one or more people to fulfill something stipulated; today, however, we understand that this journey comprises the achievement of results and the evolution, in some way, of all who participate in the process. Leaders are people with high power to inspire those around them,…
An Easy Guide to All 15 Types of Speech

Reference and Further Reading
9 Tips for Creating Great Slide Presentations. AcethePresentation.
How to Design a Presentation. 10 Essential Tips. Venngage.
How to make a presentation. Lucidpress.
Similar Posts

An Engaging Business presentation? Read This!
You find yourself spending endless hours in tools like Microsoft Teams or Zoom or around a table discussing strategic agendas, busy schedules from beginning to end of the day with conversations about different projects. All this, not infrequently, leaves you exhausted. However, it doesn’t have to be that way! The problem, mind you, is not…

7 Different Types of Presentation
The passionate art of presentation – where you stand in front of an audience, take a deep breath, and talk about something you’ve prepared on. It can be on anything and everything under the sun – the global economic crisis, the history of the Renaissance, a story of how you overcame hardships in life, you…

15 Solid Public Speaking Tips for Women
Women differ from men in a number of ways. One of such ways is the manner each of them makes speeches and conveys information. We will be discussing below, a couple of public speaking tips for women, so that more women can harness and polish their public speaking skills. Men are more of natural orators…

11 Tips for Delivering Entertaining Virtual Presentations
A Virtual Presentation is when the Speaker Delivers the Presentation remotely, and for some reason, it is not necessary to meet in person. For instance, when you want to make a presentation to people from different countries or physical locations. Virtual Presentations cuts the costs by 50% or even more, which is why they are…

5 Disadvantages of Memorized Speech
One of the most compromising situations a person can find themselves in is sitting in front of an audience and realizing that they have suddenly forgotten the speech they were going to give. The most embarrassing thing is perhaps stuttering, struggling to remember the thread of the address, searching for words with difficulty, as if…

120 PERSUASIVE SPEECH TOPICS
In today’s post we will be sharing tips on what you should consider before choosing a persuasive speech topic, and give you over a hundred and twenty persuasive speech topics and ideas you can explore across different niches such as business, health care, college, and high school education, government, politics and policy, religion, technology and…

Home Blog Presentation Ideas About Me Slides: How to Introduce Yourself in a Presentation
About Me Slides: How to Introduce Yourself in a Presentation

From conference talks to client demos, it’s always essential to include an About Me slide in any presentation you are giving. Introducing yourself early into the presentation helps build a better rapport with the audience.
You can start with several fun facts about me slide to break the ice or go for a more formal professional bio to explain your background and what makes you qualified to talk about the topic at hand. At any rate, your goal is to get the audience on your side by revealing some of your personality.
How to Introduce Yourself in a Presentation: 4 Approaches
It’s a good practice to include self-introduction slides at the beginning of your presentation. If you are looking to answer how to introduce yourself professionally, typically somewhere after the title, opening slide , and the main agenda. However, the presentation structure will be somewhat different depending on whether you are presenting to a new audience or a group of people familiar with (e.g., your team, clients, or business partners).
Here are four about me slide ideas you can try out, plus an About me template you can use to present yourself in a presentation.

1. Mention Your Name and Affiliations
Start with the introduction basics. State your name, company, title/position, and several quick facts about who you are and what you do. Even if you present to a familiar audience, a brief recap is always welcome.
To keep things a bit more engaging, consider adding some lesser-known facts about yourself. For example:
- Your interests
- Recent accomplishments
- Testimonial/quote from a team member
- Fun nicknames you got
The above can be nice ice breakers for less formal team presentations, project updates, or catch-ups with clients.
Here are several unique About Me examples you can try out:
For a client case study presentation :
“Hi, I’m Lynda, Chief Customer Success Specialist with Acme Corp. (Also, someone you thought was a chatbot for the first few encounters)
47 NPS | 15% Churn Rate | 40% repeat purchase rate”
For a team after-action review presentation :
Mike, Project Manager at Cool Project
(aka Maximizer)
Personal Project stats:
387 Slack messages answered
56 cups of coffee consumed
Project profit gross margin: $1.2 million
2. Work On Your Elevator Pitch
One of the best ways to introduce yourself in a presentation is to share a punchy elevator pitch. This works extra well if you are presenting to a new audience.
An elevator pitch is a concise statement (1-2 sentences) that summarizes your unique strengths, skills, and abilities and explains how these can benefit your listener.
It’s nice to have one ready for your presentations and networking in general since it helps you immediately connect with new people and communicate your value.
Writing a solid elevator pitch may require several attempts and iterations. But the sooner you start — the faster you’ll arrive at the best formula!
To get your creative juices flowing, here are several elevator pitch ideas you can incorporate in an introduction slide about yourself.
For professionals:
“Certified Salesforce Administrator, data visualization specialist, and analytics for top SaaS brands. I help businesses make more sense of their data to drive better outcomes”.
For a mentor :
“Adjunct professor of creative writing at Columbia University, published author, former lifestyle editor at Esquire, the New York Times. I can teach you how to find, shape, pitch, and publish stories for web & print.”
For a student:
“Third-year Marine Biology student at Denver State Uni. Volunteer at Lake Life Protection NGO, climate change activist, looking to expand my research about water conservation”.
3. Answer Popular Questions or Assumptions
If you are a frequent presenter , chances are you get asked a lot of the same “About Me questions” after your speeches and during the networking bits. So why not address a roaster of these in your About Me slide? Select 4-5 most common questions and list them as quick FAQs on your slide deck.
4. Focus on Telling a Story
Strong introductions are personable. They are meant to offer a sneak-peak into your personality and the passion behind your work. That’s why for less formal presentations, you can (and should!) start with a short personal story.
Remember: reliability is important to “click” with your audience.
For instance, neuroscience research of political ads recently found that ads featuring real people performed better than those with genetic stock footage. Among viewers, emotional engagement and memory encoding (recall) increased dramatically when political ads showed relatable people.
The same holds true for commerce. In 2015, GE launched a viral “What’s the Matter With Owen?” video ad series to attract more young talent to the company. The clips featured a relatable protagonist, struggling to explain what his work at GE entails e.g. that the company isn’t building railroads, but actually does some very innovative pilots. Many engineers related to the promo and work applications to GE shoot up by 800% !
As the above examples show, a good relatable story can go a long way. So think about how you can make a PowerPoint presentation about yourself more representative of who you really are as a person.
How to Give a Presentation About Yourself: 4 Fool-Proof Tips
On other occasions, you may be asked to give a full-length “about me” presentation. Typically, this is the case during a second interview, onboarding , or if you are in attending a training program or workshop where everyone needs to present themselves and their work.
Obviously, you’ll need more than one good about me slide in this case. So here’s how to prepare a superb presentation about me.
What to Put in a Presentation About Yourself?
The audience will expect to learn a mix of personal and professional facts about you. Thus, it’s a good idea to include the following information:
- Your name, contact info, website , social media handles, digital portfolio .
- Short bio or some interesting snippets.
- Career timeline (if applicable).
- Main achievements (preferably quantifiable).
- Education, special training.
- Digital badging awards , accolades, and other types of recognition.
- Something more personal — an interest, hobby, aspiration.
The above mix of items will change a bit, depending on whether you are giving an interview presentation about yourself or introduce yourself post-hiring. For example, in some cases a dedicated bio slide may be useful, but other times focusing on main achievements and goals can be better.
That being said, let’s take a closer look at how to organize the above information in a memorable presentation.
P.S. Grab an about me slide template to make the design process easier!

1. Create a List of “Facts About Me”
The easiest way to answer the “tell me about yourself” question is by having an array of facts you can easily fetch from your brain.
When it comes to a full-length about me presentation , it’s best to have a longer list ready. To keep your brainstorming process productive, organize all your ideas in the following buckets:
- Key skills (soft and hard)
- Educational accolades, training
- Accomplishments and other “bragging rights”
- Personal tidbits (a.k.a. fun facts )
Once you have a list, it gets easier to build a series of slides around it.
2. Think Like Your Audience
Most likely you’d be asked to make a presentation about yourself by a recruiter. There’s a good reason why many ask this — they want to determine if you are a good “cultural fit” for their organization.
After all, 33% of people quit within the first 3 months of accepting a new job. Among these:
- 43% of employees quit because their day-to-day role was different than what they were told it would be during the hiring process.
- 32% cite company culture as a factor for leaving within the first three months.
About me presentations often serve as an extra “filter” helping both parties ensure that they are on the same page expectations- and work style-wise. Thus, when you prepare your slide deck, do some background company research. Then try to align the presentation with it by matching the company tone, communication style, and cultural values.

3. Include Testimonials and Recommendations
Use the voice of others to back up the claims you are making in your presentation. After all, trumping your own horn is what you are expected to do in such a presentation. But the voices of others can strengthen the claims you are personally making.
Depending on your role and industry, try to sprinkle some of the following testimonials:
- LinkedIn recommendations
- Quotes from personal or professional references
- Social media comments
- Data metrics of your performance
- Funny assessments from your colleagues/friends
The above not just strengthen your narrative, but also help the audience learn some extras about you and your background. Testimonial slides can be of help for this purpose.
4. Include a Case Study
One of the best ways to illustrate who you are is to show what you are best in. Remember, an about me presentation often needs to “soft sell” your qualifications, experience, and personality.
One of the best ways to do that is to showcase how you can feel in a specific need and solve issues the business is facing.
So if you have the timeframe, use some of the ending slides to deliver a quick case study. You can present:
- Short retrospective of a past successful project
- Before-after transformations you’ve achieved
- Spotlight of the main accomplishments within the previous role
- Main customer results obtained
- Specific solution delivered by you (or the team you’ve worked with)
Ending your presentation on such a high note will leave the audience positively impressed and wondering what results you could achieve for them.
To Conclude
It’s easy to feel stumped when you are asked to talk about yourself. Because there are so many things you could mention (but not necessarily should). At the same time, you don’t want to make your introduction sound like a bragging context. So always think from the position of your audience. Do the facts you choose to share benefit them in any way? If yes, place them confidently on your About Me slides!
1. Personal Self Introduction PowerPoint Template

Use This Template
2. Self Introduction PowerPoint Template

3. Meet the Team PowerPoint Template Slides

4. Introduce Company Profile PowerPoint Template

5. Modern 1-Page Resume Template for PowerPoint

6. Modern Resume Presentation Template
Like this article? Please share
Introduce Yourself, Introduction, Presentation Ideas Filed under Presentation Ideas
Related Articles
Filed under Design , Presentation Ideas • May 1st, 2024
The Power of Mind Map Note Taking for Presenters
Add a new tool to your repertoire of presentation skills by mastering the art of mind map note taking. An ideal process to facilitate content retention.

Filed under Design • April 23rd, 2024
How to Create the Perfect Handouts for a Presentation
Learn how to create effective handouts for presentations and the recommended structure for handouts with this guide.

Filed under Presentation Ideas • February 15th, 2024
How to Create a 5 Minutes Presentation
Master the art of short-format speeches like the 5 minutes presentation with this article. Insights on content structure, audience engagement and more.
Leave a Reply
👀 Turn any prompt into captivating visuals in seconds with our AI-powered design generator ✨ Try Piktochart AI!
- Piktochart Visual
- Video Editor
- AI Design Generator
- Infographic Maker
- Banner Maker
- Brochure Maker
- Diagram Maker
- Flowchart Maker
- Flyer Maker
- Graph Maker
- Invitation Maker
- Pitch Deck Creator
- Poster Maker
- Presentation Maker
- Report Maker
- Resume Maker
- Social Media Graphic Maker
- Timeline Maker
- Venn Diagram Maker
- Screen Recorder
- Social Media Video Maker
- Video Cropper
- Video to Text Converter
- Video Views Calculator
- AI Brochure Maker
- AI Document Generator
- AI Flyer Generator
- AI Infographic
- AI Instagram Post Generator
- AI Newsletter Generator
- AI Report Generator
- AI Timeline Generator
- For Communications
- For Education
- For eLearning
- For Financial Services
- For Healthcare
- For Human Resources
- For Marketing
- For Nonprofits
- Brochure Templates
- Flyer Templates
- Infographic Templates
- Newsletter Templates
- Presentation Templates
- Resume Templates
- Business Infographics
- Business Proposals
- Education Templates
- Health Posters
- HR Templates
- Sales Presentations
- Community Template
- Explore all free templates on Piktochart
- Course: What is Visual Storytelling?
- The Business Storyteller Podcast
- User Stories
- Video Tutorials
- Need help? Check out our Help Center
- Earn money as a Piktochart Affiliate Partner
- Compare prices and features across Free, Pro, and Enterprise plans.
- For professionals and small teams looking for better brand management.
- For organizations seeking enterprise-grade onboarding, support, and SSO.
- Discounted plan for students, teachers, and education staff.
- Great causes deserve great pricing. Registered nonprofits pay less.
33 Legendary Startup Pitch Decks and What You Can Learn From Them [+10 Free Templates]
A startup pitch deck is a brief presentation that provides investors with an overview of your new business and/or startup idea through presentation slides.
It usually focuses on showcasing your product, sharing your business model, giving a look into your monetization strategy, and introducing your team.
A startup pitch deck is an essential fundraising tool for successful startups, whether you’re looking to raise funding from $50,000, $500,000, or $50 million. However, an investor pitch deck is just one of the best pitch decks and examples we will share below.

Despite the brevity of the successful startup pitch decks, which usually run for 10 slides or less, creating a pitch deck that wins investment is not an easy task.
What Does a Successful Startup Pitch Deck Cover?
A great pitch deck covers key points through visuals and bullet points and usually has a competition slide, a problem slide, and a solution slide to explain your offering and the market.
Additionally, a business model slide and a team slide (if your business is developed enough to present these) can turn a good deck into a great startup pitch deck.
Don’t forget, a simple pitch deck is a good pitch deck—and you’re about to learn how to nail it.

In This Legendary Startup Pitch Deck Article You Will Find:
- Examples of 33 successful pitch decks
- Takeaways that you can apply when creating your own startup pitch deck
- Editable templates of 10 pitch decks that you can use for free
Looking for a winning pitch deck template ASAP to present in front of potential investors? Try our free template created in collaboration with HighSpark – an agency that has helped more than 500 startups raise cumulatively over $80 million in funding.
Here is the list of 33 of the best startup pitch deck examples that we will go through:
- ZenPayRoll (Now Gusto)
- Wealthsimple
- AppVirality
- Shape Integrated Software
- Ooomf (now Crew)
- Sequoia Capital
These startup pitch deck examples were created by top brands in tech. At the time, they were all small startups (seed stage companies) looking to raise money or venture capital through potential investors and grow their businesses. Sound familiar?!
We hope that their business idea and investor pitch decks will inspire you (and of course, potential investors).
If you are more of a visual learner than a reader type, you can watch a video summary of the first 10 startup pitch deck examples mentioned in this blog post:
Alternatively, if you’re ready to create your own pitch deck, we’ve added some startup pitch deck examples and pitch deck templates to the bottom of this article. You can go straight to them by clicking here . Or get access to Piktochart’s online design tool by signing up for a free account and choosing a presentation template to get started easily.
From behemoths like Facebook and YouTube to superstars like Buffer, together these startups have raised millions of dollars and are now worth billions!
It’s time to see how they did it.
33 Legendary Startup Pitch Deck Examples
1. facebook pitch deck.
Here’s a fun fact: Peter Thiel, the billionaire venture capitalist, and entrepreneur, was the first outside investor in Facebook back in 2004. That’s when Mark Zuckerberg first set out to turn his dorm room project into a lasting business. Zuckerberg received $500,000 from Peter Thiel.

Facebook’s pitch deck was more of a media kit of sorts. It was containing the company’s value proposition, key metrics, and marketing services that were used to sell ads to potential clients.
Favorite takeaway : The focus of the startup pitch deck was based on solid numbers such as user engagement, traffic, and growth trajectory.
2. Airbnb pitch deck
Airbnb is a platform that allows people to list, find, and rent lodging.
This company is one of the greatest startup success stories of our time.
The now famous Airbnb pitch deck has become one of the best pitch decks for inspiring entrepreneurs around the world.

Favorite takeaway: The intro. It’s all about hooking your audience. You need to describe your business using as few words as possible. Imagine telling a 5-year-old what your business is about. If you can’t do that, it’s time to put some time into nailing it down.
3. Buffer pitch deck
Buffer is a social media scheduling platform that helps you schedule content for Facebook, Twitter, LinkedIn, and Pinterest.

The almighty startup pitch deck that helped Buffer to raise half a million dollars gained popularity by becoming one of the first pitch decks openly shared online. The founder decided to put it up to help other startups to raise funds.
Favorite takeaway: Similar to Facebook, the deck was based on solid numbers from Buffer’s users (e.g., 800 users, $150,000 annual revenue run rate, etc.)
4. Square pitch deck
Square is a company that allows merchants to accept mobile credit card payments via a dongle.
Favorite takeaway : Social proof! It doesn’t hurt to promote the management team if they’ve been with Twitter, Google, LinkedIn, PayPal, and more. It shows that your management team’s experience is an armor to the company. This detailed startup pitch deck outlines Square’s business model and a simple financial model that portrays its annual revenue and five-year growth rate.
5. LinkedIn pitch deck
Founded in 2002, LinkedIn is the top business-oriented social networking platform.
The company’s pitch talks a great deal about company values, the power of networking, and how it’s different from other social networks out there.
Favorite takeaway : The deck also provides an extensive analogy to showcase to investors what LinkedIn is. For example, it talks about “Web 1.0” vs. “Web 2.0”: Alta Vista was “Search 1.0”, and Google was “Search 2.0”. The deck talks about how LinkedIn is “Networking for Businesses 2.0”.
6. Mint pitch deck
Mint is a personal financial services tool that helps people track their spending and find ways to save money.

This startup pitch deck example was used in a competition and was never used for raising money, but it’s still a powerful deck that startups can learn from.
Favorite takeaway : This simple deck provides a clear value proposition to customers and investors. The creators of this deck also understood that one of the key concerns of an investor is the exit mechanism of his or her investments. I love how the deck highlights a number of exit strategy options.
7. MapMe pitch deck
MapMe allows users to create universally accessible (i.e., on smartphones, tablets, and computers) maps of anywhere they want with no coding required.
This startup deck was used to raise $1 million in seed funding.

Social proof almost always works. The deck showed that the startup had over 20,000 unique visitors, 18,000 monthly alerts, and12 minutes average sessions on the site.
Favorite takeaway : The pitch deck has fewer than 13 slides but provides investors with knowledge of the traction the site got going viral on social media and its go-to-market strategy.
8. LaunchRock pitch deck
LaunchRock allows users to create landing pages and quickly get their startups known through social media, even before the launch of their full site.

Favorite takeaway : As a more creatively designed pitch deck example, this pitch deck had only 15 slides but showed how the product works and the different ways it can be used. They also utilize an analogy similar to what LinkedIn had in their decks.
9. Mixpanel pitch deck
Mixpanel is an advanced analytics platform for mobile and the web. They not only measure page views but also analyze the actions people take. This is the series-B startup pitch deck for Mixpanel that helped them raise over $65 million.
Favorite takeaway : This pitch deck example started off with a problem: people guessing their analytics. It followed up by providing its solution to that problem and, ultimately, its competitive advantage. One of the best pitch decks, this is a great example of showing the problem and solution.
10. Moz pitch deck
Moz started out as an SEO company but has pivoted to support marketers across all inbound marketing strategies.
This is the series-B startup pitch deck for Moz which they used to raise over $18 million. If you’re an established startup, this is a great example of an investor pitch deck, and you can follow this guide. The pitch deck is packed with information about the company since it was founded five years prior to this pitch.

Favorite takeaway : Because the company had already been in operation for five years, they were able to present an accurate estimated revenue, revenue run rate, average customer lifetime value, cost of paid acquisition, etc.
11. Buzzfeed pitch deck
We all have a love-and-hate relationship with Buzzfeed, don’t we? I’m sure you’ve stumbled on their pages or watched their videos before. As of today, BuzzFeed has managed to raise over $240 million in investor capital (another great example of an investor pitch deck).

Favorite takeaway : SOCIAL PROOF! It doesn’t hurt to start a pitch deck with big numbers the company has, like the millions of users visiting the website on a monthly basis and quotations from large organizations such as CNN.
12. YouTube pitch deck
YouTube was acquired by Google in 2006 for $1.6 billion. Like Facebook, this company doesn’t require any introduction. Unfortunately, this is not the original deck. This is YouTube’s pitch deck to Sequoia Capital (one of the most established VC investors who’s often regarded as one of the industry’s best), which was released through a legal proceeding.
Favorite takeaway : The company wanted to be the primary outlet for video content, and it succeeded in doing just that. It goes to show that if you know what your product can do, are able to show its potential, and build on the momentum gained through early investments to create that, then you can achieve its potential. If you’re aiming to build an investor pitch deck to land a VC like Sequoia Capital, this presentation slide deck is a great template for you!
13. Manpacks pitch deck
Manpacks is a platform that delivers men’s essentials such as underwear, razors, grooming, and other products.
The company raised $500,000 with this pitch deck.
Favorite takeaway : This deck stands out! They clearly understand who they are, and they stayed that way throughout the entire presentation. The startup pitch deck is filled with a fun tone that helps explain the product well.
14. Foursquare pitch deck
Foursquare is a mobile platform that helps you find the best places to go in your area.
Favorite takeaway : This pitch deck does a great job using screenshots of social proof that the app already has from its users sharing tweets of them being the ‘mayor’ of a particular area.
15. Flowtab pitch deck
Flowtab was an app that allowed people to order drinks quickly at a crowded bar. Despite shutting down, the founders still made an effort to help other startups.
Favorite takeaway : Simplicity. This pitch deck example does well explaining critical information like the problem, the solution, their business model, and traction. You can’t really go wrong with this pitch deck.
16. Dwolla pitch deck
Dwolla is a payment solution that allows users to send, receive, and request funds from other users. This 18-slide startup pitch deck landed the company $16.5 million.
Favorite takeaway : Most startups are founded because of a problem they faced, but not many people tell their story well through their pitch decks. In their slide deck, Dwolla shared a great story of how the founder paid $50,000 a year in credit card fees and then created a solution for never doing it again.
17. ZenPayRoll (Now Gusto) pitch deck
Gusto (previously ZenPayroll) is a cloud-based solution tool for small businesses to pay employees.
The company raised $6 million with this pitch deck.
Favorite takeaway : This isn’t just a startup pitch deck. It is a template that you can use and replicate easily by filling in the blanks.
18. Bliss pitch deck
Bliss provides metrics for coders and allows them to collaborate easily.
The company raised over $400,000 using Angel List.
Favorite takeaway : The pitch deck was well composed with a clear understanding of the product and the investors they were pitching to. This is one of the best pitch decks to use if you know your target market.
19. Adpushup pitch deck
Adpushup allows companies to maximize ad revenues through advanced A/B testing. They raised more than $632,000 in investments.
Favorite takeaway : This slide deck proves that going back to the basics works. This pitch deck has basic principles like a great introduction, an outline of problems, potential solutions, market opportunities, products, case studies, milestones, traction, and a future plan.
20. Wealthsimple pitch deck
Wealthsimple is Canada’s first online investment manager. They raised more than $2 million in seed funding with this slide deck.
Favorite takeaway : The startup pitch deck is sweet and short but effective. Our favorite part is the transformation of the industry, which is laid out in a table format.
21. AppVirality pitch deck
AppVirality allows app developers to grow their platforms using growth method techniques proven by other startups.

Favorite takeaway : Our favorite takeaway is how the flow of the pitch deck goes through the problem, the proven solution, and how it works within their app to their target market in multiple slides.
22. Shape Integrated Software pitch deck
Shape Integrated Software is budget management software that helps PPC analysts manage various budgets across different channels.

Favorite takeaway : When you have the traction to back your startup, use it. Shape clearly took advantage of it and presented it clearly in their pitch deck.
23. Podozi pitch deck
Podozi is an online e-commerce platform based in Nigeria.

Favorite takeaway : Most startup pitch decks work well when they’re short and sweet, in multiple slides, like Podozi’s. The best takeaway is the working partnership with large brands that this platform already has.
24. Fittr pitch deck
Fittr is a platform that designs custom workouts tailored to equipment, access, time management, and goals.
Favorite takeaway : As a user of this platform, we love the investment goals and the purpose of what the company is planning to use it for.
25. Swipes pitch deck
Swipes is a task manager app to help its users increase their productivity.
Favorite takeaway : One of their pages used social proof of quotations from The Next Web and Lifehacker. You can’t go wrong with that.
26. Canvas pitch deck
Canvas replaces paper-based processes with affordable and easy-to-use mobile apps and forms. They raised $9 million with these decks.

Favorite takeaway : Instead of saying what they do, the second slide in their pitch deck shows how their startup helps businesses. No words are needed.
27. Ooomf (now Crew) pitch deck
Crew (formerly Ooomf & then PickCrew) is a freelancer marketplace that connects mobile and web developers with projects or work. This deck was used to raise over $2 million dollars.
Favorite takeaway : Well-designed with an easy-to-understand flow.
28. Cubeit pitch deck
Cubeit is a mobile application that allows users to aggregate content from anywhere. Cubeit used this 13-slide deck to raise seed funding before they even had a finished product.
Favorite takeaway : A strong introduction will get investors to pay attention. Their deck starts out with a clear message, which was that “owning more devices doesn’t make your life easier”. I can’t help but pay attention to how this company will help.
29. Castle pitch deck
Castle was a startup that let rental owners put their properties on autopilot. This was the deck Castle used to raise $270,000 for their startup.
Favorite takeaway : Great design and easy to digest.
30. Sequoia Capital pitch deck
Sequoia Capital is one of the leading investment firms in Silicon Valley. This deck is a template they recommend following.

Favorite takeaway : It’s like having the keys to the kingdom. You don’t have to guess what this investment giant is looking for. They tell you straight away.
31. Uber pitch deck
When Uber hit the scene, they fundamentally reimagined urban transportation. Their pitch deck tells this audacious story perfectly. Simple yet impactful, it illustrates the problem of expensive taxis and car services, then introduces Uber as the affordable, tech-driven solution.
They use stark data points to highlight the financial opportunity, a vital touch for potential investors.
As you create your pitch, remember Uber’s two key strengths: painting a clear problem-solution scenario and using compelling data to underscore their market potential. This strategy not only shows understanding of their market but also communicates their transformative vision effectively.
Favorite takeaway : The deck is clean and minimalist. The flow is easy to follow, and you get a clear idea of what’s the problem they’re trying to solve, and how they solve it.
32. WeWork pitch deck
WeWork’s pitch deck beautifully encapsulates its community-driven approach to shared workspaces.
They use visually appealing slides that mirror their innovative, modern brand. Their pitch deck deftly articulates the benefits of shared workspaces in today’s economy, setting the stage for their unique value proposition.
Favorite takeaway : what we do, who we do it for, why we do it; the deck walks you through their company vision with smart visuals and copy. We loved how they generated urgency by highlighting how co-working spaces were a fast-growing trend and investors could miss out on a profitable investment if they didn’t act quickly.
33. MatterMark pitch deck
MatterMark’s pitch deck is a testament to the power of storytelling. utilizing clean visuals to aid comprehension and coupled with succinct copy to keep the narrative engaging. The 30-slide deck earned the platform $6.5 million in seed A funding.
Favorite takeaway : Strong visual storytelling through the use of charts and graphs. In just a few moments, you can see where the majority of their revenue comes from compared to their other revenue streams.
Summary of Pitch Deck Template Takeaways
To sum up, a strong startup pitch deck not only serves to reinforce your brand to the target audience or investors, but shows your business plan and unique offering through the slides presented; using a problem slide, a solution slide, and a traction slide including concise bullet points.
The best startup pitch deck also shows off your company’s personality, through the inclusion of a team slide or similar in the next few slides, to be presented after your business plan is clearly outlined.
As your company grows, you’ll probably start thinking about the next stage of growth. You can use these successful pitch decks can as the foundation to make an investor deck for your next round of financing.
Look at the takeaways from these startup pitch decks as a guide to help you in your quest to raise funds and venture capital for your own startup for an investment round.
Here are some of the key takeaways from our pitch deck examples:
- Pitch decks don’t have to be formal or beautiful.
- A great pitch deck will provide an impactful intro or slogan.
- Keep your deck short (less than 20 pages).
- Use analogies to back up the points that you’re making.
After going through so many startup pitch deck examples, we recommend that to make your pitch presentation stand out you should:
- Start with a strong intro/vision.
- Show problems and offer solutions.
- Identify market opportunities.
- Showcase products/services clearly.
- Digest your business model
- Highlight financials
- Add social proof/case studies.
- Differentiate from competition.
- Show an experienced management team.
Designing a strong pitch deck could turn your business idea into a reality after convincing investors to provide support financial support to your project.
If you’re looking for additional information, DocSend shared lessons they got learning from 200 startups who raised $360 million from their first pitch deck.
10 Pitch Deck Templates for You to Try
The following pitch decks are free templates available in Piktochart that you can use. This makes it easy to work on your slides without having to worry about design. We took care of that for you.
If you don’t have an account yet, just sign up for a free Piktochart account here and then click on one of the templates below.
To learn how the online pitch deck creator works, watch this on-demand demo .
1. Investment Pitch Deck Template With HighSpark

2. Finance Pitch Deck Template

3. Business Pitch Deck Template

4. Startup Pitch Deck Examples

5. Tech Pitch Deck Examples

6. Business Keynote Template

7. Product Pitch Deck Template

8. Product Pitch Deck Template

9. App Product Presentation Template

10. Product Website Pitch Deck Template

Other Posts
How to Make a Presentation (2023 Guide With Tips & Templates)
How to Create an Infographic Syllabus With Piktochart (Plus Templates)

5 Studies About Visual Information Processing
- close Subscribe Sign In
James Bay Distillers to Produce "Heart of the Blues" Whiskies

EVERETT, Wash. -- James Bay Distillers, Ltd. has announced their collaboration with Human Tribe LLC of Sammamish, WA to produce and sell Heart of the Blues whiskies nationwide. Human Tribe LLC comprises two of the founding members of the all-time great rock band, Heart: Roger and Michael Fisher. The first release is a 100% rye whiskey, bottled at 100-proof which will initially be available at the distillery in Everett, and online soon at the company’s retail site. Expanded distribution of the Heart of The Blues whiskey will include regional and nationwide sales.
Beginning in 2018, Michael and Roger Fisher, began to explore releasing their own whiskey. In early 2024 in discussion with James Bay Distillers, they discovered a blend that matched their goals for taste, smoothness and super-premium quality.
This release is named after the Fishers’ album release, Heart of The Blues , the music for which was inspired by time spent in the Mississippi Delta, home of the foundation of jazz, blues, rock, and all subsequent related styles of music. In addition to James Bay Distillers’ sales efforts, Roger Fisher and his band will spearhead the introduction of their whiskey during live performances.
“The art of music is like the art of distillation: go to any length to produce the best, most fulfilling and inspiring work. We love the commitment by James Bay Distillers to only produce sipping whiskies,” said Roger Fisher.
Heart of The Blues rye whiskey is bottled in 750-ml bottles at 100 proof (50% alc./vol.). “We are very pleased with this whiskey,” said Ernest Troth, James Bay Distillers’ president. “This is very smooth despite the higher proof, and has a wonderful dark amber color from aging in a heavy char barrel.” The whiskey will also be available in 750ml bottles with autographed labels from the Fisher Brothers, Roger and Michael.
About James Bay Distillers
The company is based at Paine Field Airport in Everett, WA. Their other award-winning spirits include 10 whiskies, gins and vodkas which are rated 90+ points in national and international competitions. Heart of The Blues rye whiskey joins Galloping Goose whiskies (97 points, six gold medals) and their Inner Harbour Scotch whisky (same awards as 18-year Johnnie Walker and international brands). James Bay’s gins are rated “dangerously smooth,” and their Strait Up Killer Vodka is rated as “1 of the top 9 vodkas” in the USA by Wine Enthusiast.
All spirits are available at the distillery and online. Selected spirits are available at liquor stores local to Everett, WA and Washington State. Spirits sales are restricted to consumers 21 years of age and above. Wholesale, off-premise and on-premise pricing are available.
About Human Tribe, LLC
The Fisher brothers established Human Tribe as a company providing music, art and entertainment, products and services; a progressive, innovative, trusted brand based on well-being, and global unity. Roger and Michael Fisher have over 50 years experience in the music business, having sold millions of albums with the group they co-founded, Heart. They envision Human Tribe as a progressive emblem of cooperative well-being and quality products. As a salute to the MS Delta, the rich culture, and its heritage, especially as expressed in Blues, Rock, Jazz, and Spirits, the Fishers have created Heart Of The Blues Whiskey .
For More Information: Learn More
Become an Insider for complete access to BevNET and NOSH news, insights, community.
Featured Insider benefits include:
- Full Access to BevNET & NOSH - Unrestricted access to all written and video content from the leading journalists in food and beverage.
- Daily Briefing Newsletter - Sent Monday-Friday, the Daily Briefing starts your day off with the latest news, trends, and data in an easily digestible format.
- Exclusive Interactive Programs - Have your burning questions answered and find innovative solutions to your toughest challenges through our original programming.
- Educational Video Archive - Video learning at your fingertips from our expansive community of food and beverage experts.
- Insider-only Discounts - Save on BevNET and NOSH events and job board listings.
Login Become an Insider
Attention retailers: get a FREE retailer subscription
- Open access
- Published: 25 May 2024
Spatial transcriptomic brain imaging reveals the effects of immunomodulation therapy on specific regional brain cells in a mouse dementia model
- Eun Ji Lee 1 , 2 ,
- Minseok Suh 1 , 3 , 4 ,
- Hongyoon Choi 1 , 3 ,
- Yoori Choi 1 , 5 ,
- Do Won Hwang 6 ,
- Sungwoo Bae 1 , 3 , 4 &
- Dong Soo Lee 1 , 2 , 3 , 4 , 7
BMC Genomics volume 25 , Article number: 516 ( 2024 ) Cite this article
Metrics details
Increasing evidence of brain-immune crosstalk raises expectations for the efficacy of novel immunotherapies in Alzheimer’s disease (AD), but the lack of methods to examine brain tissues makes it difficult to evaluate therapeutics. Here, we investigated the changes in spatial transcriptomic signatures and brain cell types using the 10x Genomics Visium platform in immune-modulated AD models after various treatments. To proceed with an analysis suitable for barcode-based spatial transcriptomics, we first organized a workflow for segmentation of neuroanatomical regions, establishment of appropriate gene combinations, and comprehensive review of altered brain cell signatures. Ultimately, we investigated spatial transcriptomic changes following administration of immunomodulators, NK cell supplements and an anti-CD4 antibody, which ameliorated behavior impairment, and designated brain cells and regions showing probable associations with behavior changes. We provided the customized analytic pipeline into an application named STquantool. Thus, we anticipate that our approach can help researchers interpret the real action of drug candidates by simultaneously investigating the dynamics of all transcripts for the development of novel AD therapeutics.
Peer Review reports
Introduction
Central nervous system (CNS) and central immune system (bone marrow: BM) interactions, specifically brain-immune cross-talk, can occur by a pathway from the skull BM, meninges and their lymphatics, and cerebrospinal fluid (CSF) to the brain parenchyma [ 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 ] and/or by another pathway from the choroidal plexus (CP) capillary-stroma-epithelium and CSF to brain parenchyma [ 15 , 16 , 17 , 18 , 19 ] in addition to by the classic pathway of crossing the blood‒brain barrier (BBB) [ 20 , 21 , 22 , 23 ]. In explicit neuroinflammatory diseases such as multiple sclerosis in humans or experimental autoimmune encephalomyelitis (EAE) in animal model, immunoglobulins or immune cells have been considered to enter the brain parenchyma via the BBB [ 20 ] of the brain parenchyma or via the brain-CSF barrier of the CP [ 24 , 25 ], or recently via the arachnoid barrier cell (ABC) layer of skull BM-meningeal lymphatics and CSF/perivascular spaces reaching the brain parenchyma [ 3 , 4 , 5 , 17 , 26 , 27 , 28 ].
Novel immunomodulatory therapy in Alzheimer’s disease (AD) transgenic models, such as 5xFAD mice, should be accompanied by the improvement of cognitive decline associated with aging and/or the amelioration of the transgenes’ adverse effects, such as priming brain cells or immune responses during development and aging. When we inadvertently found the effect of the anti-CD4 antibody while investigating the effect of aducanumab [ 29 ] and encountered the probable effect of allogeneic natural killer (NK) cell supplements in AD models [ 30 ], we questioned which cells or transcriptomic markers in the brain areas would be the best to predict the outcome of these novel, currently unaccounted therapeutic candidates. In AD mouse models including 5xFAD mice, the surrogate effect markers of previous findings/trials of systemic or intraventricular administration of CD8 + T cells [ 31 ], anti-CD8 [ 32 ] or anti-CD3 [ 33 ] antibodies, Treg cells [ 34 , 35 , 36 ] (or for stroke model [ 37 ] or DEREG model for traumatic brain injury model [ 38 ]), and amyloid-sensitized Th1 cells [ 39 , 40 , 41 ] were amyloid plaques/Aβ on immunohistochemistry and transcriptional signatures of major brain cells and brain parenchyma [ 32 , 33 , 35 ] or CP infiltrating cells [ 25 , 42 ]. As systemically injected cells and immunoglobulins were not examined for their location or biodistribution, direct CNS effects or systemic actions on immune systems were always the alternative to explain the probable effect of novel immunomodulatory therapies, which inevitably led to the insufficient understanding of the target cells and areas. This resulted in inconsistent findings among the reporting investigators.
Single-cell or single-nucleus RNA sequencing (scRNAseq/snRNAseq) based on tissue dissociation and preparation of a single-cell suspension followed by next-generation sequencing allows comprehensive characterization of cell types in the tissue [ 43 , 44 , 45 , 46 ]. Recently available barcode-based spatial transcriptomics (ST) using the solid-phase capture of RNA on slides, such as Visium® [ 47 , 48 , 49 , 50 ], HDST [ 51 ], slideSeqV2 [ 52 ], Seq-Scope [ 53 ], or stereo-Seq [ 54 , 55 , 56 ], adds a spatial dimension to transcriptomics and enables spatial characterization of genes and cell types through robust regional segmentation of the tissue. Regional and cell-type specific characterization of one or more sections of the mouse brain based on this reliable anatomical segmentation mostly allowed for the comparison of the basal states between groups or even the task-related active states by calcium two-photon imaging and scRNAseq of the visual cortex [ 57 , 58 ]. Given that ST allows us to obtain genome-wide spatial expression profiles, ST can be considered multiplexed molecular imaging of the brain. We can now use spatial transcriptomic brain imaging to investigate whether a probable immunomodulatory therapy yields its effect on major brain cells (and infiltrating or rare cells of the brain) in each segmented brain area after systemic administration [ 30 ]. Biodistribution studies after systemic injection can inform whether immune cells or antibodies enter and directly interact with brain cells; however, if we do not see the immediate presence of cells (usually none) and antibodies (less than 1% of the injected dose), we assumed that they would influence brain cells and pathologic processes. Transcriptomic changes owing to proper novel immunomodulatory therapy will enable us to explore the probable target cells/genes that would have caused or at least be associated with the expected behavioral effects of these therapies [ 30 ]. This is especially helpful early in the pursuit of potential new drugs so that investigators can be confident that they are moving in the right direction to modify and optimize new therapeutic candidates. Transcriptomic changes of a region or regions and a cell type or cells in a group are expected to explain the behavioral results of the mouse model. Finally, the transcriptomic changes might predate the behavioral improvements. In both cases, we expect that transcriptomic profiles at a high spatial resolution would excel for drug screening, in sensitivity and target-cell specificity [ 47 , 54 ], over histological results of immunohistochemistry. Additionally, single-spot ST yields tissue-globally searchable data that can later be reanalyzed repeatedly when the marker gene combination [ 46 , 50 , 59 , 60 , 61 , 62 ] becomes available.
To do this, we needed to advance the single-spot RNA sequencing and its analyses using a customized method to derive cell type/state-specific distribution of the Visium sections. Paying attention to the proper dissociation of cell types/states using the optimum/minimum number of genes and cell‒cell interaction (CCI) and cell‒cell communication (CCC) [ 63 ], marker gene combinations should be established with the existing public database of scRNAseq/snRNAseq [ 46 , 49 , 59 , 64 , 65 , 66 , 67 , 68 ] and our own data [ 30 ]. For both tasks, ready-to-adopt methods are available by the Creative Commons regulations in previous investigations.
Stromal and parenchymal cells of various organs, including the brain, are now known to show common and specific characteristics of cell identity and their ontological characteristics, the best known of which are microglia and perivascular macrophages or resident macrophages [ 67 , 68 ]. Immune cells of monocyte-derived and resident macrophages have distinctive transcriptomic signatures, which predict their immune roles and tissue integrity-preserving roles per characteristic signatures [ 69 ]. This was also the case for T cells, where resident memory T (T RM ) cells for intestines, effector memory T (T EM/EMRA ) cells for blood, liver, and BM, and mixed T RM/EM cells for various organs and BM yield their own characteristic transcriptomic signatures that determine their differentiation of T cells in every tissue of interest, dictating their respective functional roles [ 70 , 71 ]. Unfortunately, neither of these recent cross-tissue, resident immune cell studies [ 69 , 71 ] included the brain, which mandates our own analysis.
In this investigation, we performed segmentation of brain regions on coronal/sagittal sections per dozens of animals using readily available methods and characterized the common pathologic transcriptomic signatures of 7-month-old 5xFAD mice. Immunomodulatory drugs were administered to these mice to confirm behavioral improvement. 99m Tc-hexamethylpropyleneamineoxime (HMPAO)-labeled cell-tracking imaging [ 72 ] ruled out the immediate infiltration of NK cells in the brain. Spatial transcriptomic changes in mice were examined after anti-CD4 immunoglobulin administration and expanded NK cell supplement treatment with a dose schedule, which improved Y-maze alternation behavior impairment at this age range in 5xFAD mice. Transcriptomic changes were dissected across areas and cell types/states using publicly available methods and databases, and the analytical pipelines were organized as an application named STquantool. We found that regional/areal gene set-defined type/state-specific cells showed characteristic differences after each trial treatment in a genetic model of AD, 5xFAD mice, upon spatial transcriptomic Visium analysis. Combined brain major cells including neurons, astrocytes, microglia, and oligodendrocytes with their associated types and states and brain resident/infiltrating rare immune cells per region were explored for their distinctive transcriptomic changes among mouse groups to yield their probable association with behavior improvements.
Spatial transcriptomic characterization of gene-set-defined type/state-specific major brain cells in 7-month-old wild-type and 5xFAD mice
In total, 35 coronal and 28 sagittal brain sections from 63 mice of either wild-type or 5xFAD background were included in the analysis. A spatial barcode was given for every spot, the unit tissue domain of spatial transcriptomics, and at least 50,000 reads were obtained from each of the 4,992 spots in a capture region. The brain tissues were covered by an average of 3,000 spots across all samples. Using the count matrices computed from Space Ranger as inputs to the reciprocal principal component analysis (RPCA) based integration and clustering pipelines supplied by Seurat (Seurat 4.1; https://satijalab.org/seurat/ ), the multiple brain tissues were segmented based on their transcriptome patterns [ 73 ]. By optimizing parameters such as the resolution of spatial clusters and others, we yielded segmented spatial cluster images in every case, including those with various treatments and manipulations. The treatments and manipulations are listed in Supplementary Fig. 1 A and Supplementary Table 1 . These included anti-CD4 antibody treatment and NK cell supplement treatment groups. Others were 3-month-old 5xFAD mice, cervical lymphatic ligation, a P301L model with or without amyloid/tau-rich lysate injection, and fingolimod hydrochloride (FTY720) injection with or without lipopolysaccharide (LPS) pretreatment.
The difference between 10 ST data points from wild-type and 11 from 5xFAD animals was compared for each of 14 spatial clusters using 7 to 8 coronal and 3 sagittal sections. For the integration of slides based on RPCA, the transcriptomes of wild-type animals were used as pivots, and the spots from diseased animals were mapped to the PCA space of the wild-type reference [ 74 ]. The corrected counts derived from the integration were used to cluster the spots. Spots clusters were visualized in all individual sections and verified for their accuracy in designating the areas according to already-known anatomical correlates (Supplementary Fig. 1 B-D). Only the askew sagittal section in a few mice missed the dorsal striatum and instead supplied the septal lobe in a more median position, but the spatial clustering correctly showed the pair of dorsal/ventral striatum in some sections and septal/ventral striatum in others. The spots from each cluster and group were represented by a UMAP plot, a dimensionality reduction method for visualization, and the clusters were well separated in terms of gene expression (Supplementary Fig. 1 E, F).
Using the developed platform, STquantool, the representative genes for each cell type were determined by literature or data-driven methods and validated based on spatial gene expression patterns (Materials and Methods). Cell signature scores for each cell type were calculated by the difference between the average expression of the marker genes and that of the randomly selected control genes. Since the cell scores are derived from the curated marker genes and indirectly measure the abundance of the cell type in each spot, it can be postulated that the spatial distribution of the cell scores reflects the spatial distribution of the corresponding cell type. Regional/areal transcriptomes representing major brain cell types/states were then compared between groups by averaging the major cell scores in wild-type and 5xFAD mice using the Wilcoxon rank-sum test (Supplementary Fig. 2 ). In addition to the major cell types, scores were also calculated in 32 subtypes of neurons [ 75 ], several types of astrocytes [ 76 ], microglia, and oligodendrocytes [ 66 ]. In addition, the reactive state-specific changes and marker genes of astrocytes and microglia were compared between wild-type and 5xFAD animals in 14 brain regions.
To investigate brain regional changes during pathological progression in AD, we obtained ST data from coronal brain sections of the 5xFAD mouse model and age-matched wild-type mice at three and seven months of age. The 5xFAD AD model is known to show amyloid deposition and reactive gliosis from two months of age and synaptic loss and cognitive impairment from four to six months of age (Supplementary Fig. 3 A). Amyloid deposition was observed in the adjacent sections of the samples used for ST analysis (Supplementary Fig. 3 B). In both coronal and sagittal sections, beta-amyloid levels began to increase at three months of age, and dramatic increases were observed remarkably in the deep cortex, thalamus, hippocampus, and amygdala of seven-month-old 5xFAD mice. The major brain cells were classified into neurons, astrocytes, microglia and oligodendrocytes and their associated cell types (Supplementary Fig. 4 A). Neurons were classified according to the reports of Hodge et al. [ 75 ] of the Allen Institute with Aeverman et al.’s [ 59 ] random forest hierarchical clustering method (NSForest) to define the optimal marker gene combination for neuron subtypes, which was verified by the reports of BICCN [ 77 ] and another group’s approach [ 78 , 79 , 80 , 81 , 82 ]. Astrocytes were classified according to the types of white matter-associated and gray matter-associated astrocytes [ 76 ] once and again into region-specific astrocytes for the cortex/hippocampus (telencephalon), thalamus, and other brain regions (diencephalon) [ 49 , 83 ]. Reactive astrocytes and their marker gene combinations were determined by the suggestions of Escartin et al. [ 84 ] and other investigators (Habib et al. [ 85 ], Ioannou et al. [ 86 ], Chamling et al. [ 87 ] etc.). Oligodendrocytes and their associated cell types were designated by following an initial report by Marques et al. [ 66 ] and were verified by other investigators’ suggestions [ 86 , 87 ]. Microglia were classified according to their states but not types considering their homeostatic and reactive (microglia with neurodegeneration: MgND [ 88 ], disease-associated microglia: DAM [ 89 ], lipid-droplet associated microglia: LDAM [ 90 ], etc.) states [ 68 , 91 , 92 ], and thus, no subtypes of microglia were assigned. Instead, the aging-related effect on its own or associated with amyloid pathology was examined to show amyloid pathology excluding the confounding effect of aging [ 93 ].
Using the data by Ximerakis et al. [ 64 ] for defining cell types, the hierarchical clustering method suggested by Hodge et al. [ 75 ] and NSForest (version 2.0) by Aeverman et al. [ 59 ], neurons were typed and subtyped into 20 GABAergic neurons and 12 glutamatergic neurons. Their pattern of expression is displayed for wild-type and 5xFAD mice in Fig. 1 A. The differences between wild-type and 5xFAD mice in coronal and sagittal sections of 9 areas of interest were quantitatively analyzed (Supplementary Fig. 4 B). Subtypes of GABAergic and glutamatergic neurons showed unique patterns in wild-type and 5xFAD mice. The expression patterns of the GABAergic somatostatin (Sst) subtypes in the amygdala differed between wild-type and 5xFAD mice (Fig. 1 B and Supplementary Fig. 4 C). Increased expression in the amygdala was prominent in the 5xFAD mice compared to the wild-type mice. Notably, the individual genes ( Sst, Nr2f2, Tac1 , and Moxd1 ) tended to be expressed at higher levels in the amygdala of 5xFAD mice among the gene combinations (Supplementary Fig. 5 ). Furthermore, increased expression of Sst was identified at the protein level in the amygdala and striatum regions but not in the deep cortex, thalamus, or hippocampal regions of seven-month-old 5xFAD mice (Supplementary Fig. 6 ). The most pronounced increase was found in the amygdala, with only a slight change in the striatum, and the results were consistent with those at the transcript level. Thus, after the accumulation of amyloid plaques in the 5xFAD mice, a marked increase in specific subclasses of inhibitory neuron-associated genes in the amygdala was remarkably identified.
Brain region-specific expression patterns of the signatures of neuronal subclasses and spatial changes in neurons in 5xFAD mice compared to wild-type mice. ( A ) Spatial patterns of diverse neuronal subclass signatures. The left side is a representative slide of a wild-type mouse, and the right side is that of a 5xFAD mouse. Each cell type showed distinct region-specific expression. First, mature neurons were subdivided into GABAergic and glutamatergic neurons, and then the cells were further divided into subclasses to show the regional distribution of subclasses of inhibitory and excitatory neurons. ( B ) Spatial pattern of the neuronal signatures (mature neurons, Sst1, Sst2, Sst3, Sst4, and Sst5; left). Representative images of each group were selected among 10 spatial transcriptome datasets of wild-type mice and 11 of 5xFAD mice. Spatial patterns of the Sst-subclass of inhibitory neuronal signatures of the 5xFAD mice were the most remarkably different compared to those of wild-type mice. The spatial distribution of Sst subclass neurons was similar between wild-type and 5xFAD mice, and the expression of Sst subclasses was higher in 5xFAD exclusively in the amygdala. The boxplot revealed the average module score of Sst-subclass inhibitory neurons, and expression tended to be higher in the amygdala in 5xFAD mice, especially for the Sst4-subclass. Each dot represents a mouse in each group. (mNeur: mature neurons; Sst: somatostatin; WT: wild type; TG: 5xFAD mice; GABAergicCGE: caudal germinal eminence; GABAergicMGE: medial germinal eminence; GlutamateNPCTL6b: near projection, corticothalamic, and layer 6b; GlutamateL5PT: layer 5 and pyramidal tract)
The difference between wild-type and 5xFAD mice was differential according to the definition (by gene combination to define reactivity) of reactive astrocytes and reactive microglia in their density and distribution (Fig. 2 and Supplementary Fig. 7 ). Reactive astrocytes and reactive microglia shared gene signatures and were supposed to collaborate to do the job of waste disposal in situ and out of the brain while promoting the interstitial fluid space (ISF) to perivascular/CSF space to meningeal lymphatics. Astrocytes were classified into deep or upper cortical layer-specific and telencephalon- or diencephalon-origin according to Bayraktar et al. [ 83 ] and Kleshchevnikov et al. [ 49 ]. This classification did not reveal a difference between wild-type and 5xFAD mice (Supplementary Fig. 7 A). However, another two types of astrocytes, white matter-associated and gray matter-associated, according to Werkman et al. [ 76 ], yielded differences in density and distribution between wild-type and 5xFAD mice. The cell score of white matter-associated astrocytes was significantly increased in the white matter and other gray matter regions in the 5xFAD mice, but no differences were observed in the gray matter-associated astrocyte signatures (Fig. 2 A). In addition, reactive astrocytes defined by various ways [ 84 , 85 , 86 , 87 , 94 ] that showed an increase in density in white matter and neighboring gray matter areas (cortex and thalamus) in 5xFAD mice. Their distribution of reactive states was characterized to be diffuse but was prominent around the white matter in coronal and sagittal sections from 7-month-old 5xFAD mice compared with that of wild type mice. Aging astrocytes showed significant but small differences between wild-type and 5xFAD mice in the white matter, deep cortex, thalamus, and striatum (Fig. 2 A). Further analysis with individual transcriptomes used as markers for each state-specific astrocyte revealed the following findings. The expression of individual transcriptomes defining white matter-associated and reactive astrocytes showed similar patterns between wild-type and 5xFAD mice, but the dominant individual transcriptomes were different (Supplementary Fig. 8 A, B). In the gene combination of white matter-associated astrocytes, Lyz2, C1qa, Ctss, C1qb , and C1qc were the top five genes with significant differences. In reactive astrocytes, Gfap, Serpina3n, Vim , and C1qb showed dramatic increases in 5xFAD mice compared to wild-type mice.
Spatial changes in the distribution of the region- or state-specific signatures of microglia and astrocytes in 5xFAD mice compared to wild-type mice. ( A ) Spatial pattern of the region-specific signatures (white matter-associated and gray matter-associated astrocytes) and the state-specific signatures (reactive astrocytes and aging astrocytes; left). Representative images of each group were selected among 10 spatial transcriptome datasets from wild-type mice and 11 from 5xFAD mice. Boxplot showing average module scores (right). Each dot represents a mouse in each group. The average module score of white matter-associated astrocytes was significantly increased in the white matter and other gray matter regions in 5xFAD mice compared to wild-type mice, but no differences were observed in gray matter-associated astrocyte signatures. Moreover, the average module score of reactive astrocytes showed a similar expression pattern to that of white matter-associated astrocytes, while significant but smaller differences were observed in white matter and several areas in the aging astrocyte signatures. ( B ) Spatial pattern of the state-specific signatures (plaque-associated, aging-associated, homeostatic, reactive, and panmicroglia). The average module score of plaque-associated microglia showed a significant increase in 5xFAD mice compared to wild-type mice, whereas aging-associated microglia showed no difference. Interestingly, both homeostatic and reactive microglia signatures showed a dramatic increase in the average module score in 5xFAD mice. Microglia in general (representing all the state-specific and nonspecific signatures) showed increased expression in all the regions without showing any regional distinctiveness. Bonferroni-adj. *p value < 0.05, **p value < 0.01, ****p value < 0.0001. (WM: white matter; GM: gray matter; WT: wild type; TG: 5xFAD mice)
Microglia, classified into homeostatic and reactive states [ 89 ] and aging-related and plaque-related states [ 93 ], showed increased density in wide areas for homeostatic state microglia and reactive microglia (disease-associated microglia, according to Keren-Shaul et al. [ 89 ]) and plaque-related (aging-nonrelated but plaque-related) reactive microglia. Interestingly, both homeostatic and reactive microglia showed a dramatic increase throughout brain regions in 5xFAD mice compared to wild-type mice (Fig. 2 B). Plaque-associated microglia also showed a significant increase in 5xFAD mice, but aging-associated microglia showed no difference (Fig. 2 B). Plaque-associated and reactive microglia shared a similar set of genes (Supplementary Fig. 8 C, D). In particular, Cst7, Spp1, Ccl6 , and Axl showed remarkable increases in 5xFAD mice compared to wild-type mice for both microglial signatures. For homeostatic microglial signatures, other genes, such as Hexb, Cst3, Cx3cr1, Tmem119 , and P2ry12 , showed dramatic increases in 5xFAD mice. Of note, microglial signatures did not show differences by brain region.
Oligodendrocytes and their lineage cells classified by Marques et al. [ 66 ], which comprise mature oligodendrocytes, myelin-forming oligodendrocytes, newly formed oligodendrocytes, committed oligodendrocyte precursors (COP) and oligodendrocyte precursor cells (OPC), showed distinct distribution along the areas, mainly identified in the white matter and faintly in the thalamus and lateral hypothalamus (Supplementary Fig. 7 B). A significant difference in newly formed oligodendrocytes in the deep cortex and thalamus was observed between wild-type and 5xFAD mice, but the expression was very low, and the difference was also small. The classification according to Chamling et al. [ 87 ], consisting of oligodendrocytes, OPCs and cycling progenitors, also showed similar characteristic distributions. The oligodendrocyte signatures showed relatively little difference between wild-type and 5xFAD mice.
Astrocytes and microglia, specifically white matter-associated astrocytes, reactive astrocytes, plaque-associated microglia, and homeostatic and reactive microglia, tended to increase exclusively in the white matter in 3-month-old 5xFAD mice compared to age-matched wild-type mice. This meant that the changes with the signatures started at an earlier age and occurred around white matter, reflecting a similar result to our previous report [ 95 ] (Supplementary Fig. 9 ). The most interesting finding was that homeostatic microglia also revealed increased expression in most gray matter regions at the later stage of amyloid pathology, similar to the expression pattern of reactive microglia. Increased expression of Tmem119 (a marker for homeostatic microglia) and Cst7 (a marker for reactive microglia) in the gray matter regions, especially in the deep cortex, thalamus, hippocampus, and amygdala, was validated at the protein level in 7-month-old 5xFAD mice compared to 3-month-old 5xFAD mice (Supplementary Fig. 10 A). In addition, increased expression of GFAP, S100beta, and Ctss (markers for reactive astrocytes) was confirmed at the protein level in the deep cortex, thalamus, amygdala, and white matter regions (Supplementary Fig. 10 B). Thus, the results of verifying the protein expression level were consistent with the ST analysis results.
Finally, DEGs were explored between the groups using the MAST model [ 96 ] to find regional differences at the gene level. Of note, we considered gene abundance in addition to the log fold change of mean expression in the spots corresponding to the two groups to classify DEGs in each brain region. Based on the properties of barcode-based spatial transcriptomics, adding abundance information for the corresponding gene within one barcode can increase confidence in identifying DEGs. The spatial expression of individual DEGs in 5xFAD mice compared to wild-type mice was visually assessed by STquantool (Supplementary Fig. 11 and Supplementary Table 2 ). Venn diagrams of the significantly different transcripts per region were drawn, and the GO terms associated with the genes were visualized as dot plots to examine the differences between wild-type and 5xFAD mice. The reliability of the applied DE analysis was validated by quantitative reverse-transcription PCR (qRT‒PCR analysis) in the thalamus and hippocampus (Supplementary Fig. 12 ). Among the DEGs from the thalamus, Hexb, Lyz2, Cst7, H2-K1, Ctss , and Gfap were increased in the thalamus of 5xFAD mice compared to wild-type mice. Additionally, the detected DEGs in the hippocampus, Scg5, C1qb, Ctss, Hexb, Cst3, S100a6, Cst7, Gfap , and Lyz2 , were also significantly increased. Thus, we demonstrated that our transcriptomic approach faithfully captured changes in DE analysis. In 5xFAD mice, both the white matter and gray matter regions showed significant increases in gliogenesis- and glial cell activation-related genes. For downregulated genes-associated pathways, none were detected in the white matter, but ATP biosynthetic process and purine nucleoside triphosphate biosynthetic process were significantly decreased in deep cortex of 5xFAD mice compared to wild type. The DEG-related upregulated and downregulated pathways in other regions are listed in Supplementary Table 3 .
Spatial transcriptomic characterization of rare brain-resident or infiltrating cells in 7-month-old wild-type and 5xFAD mice
Spatial transcriptomic characterization of rare brain cells poses problems of finding the proper unique set of gene combinations for determining these rare cells residing among the confounding major cells. Unlike major cells, the distribution of which is already known, rare cells are low in number and do not have any presumed distribution. Information on the propensity (rarity) of these cells is either derived from scRNAseq studies using dissociated samples from various areas of the brain, even collected from a number of animals, or from the zoomed-in small areas observed by histochemistry. Abundance studies of rare immune cells in the brain reported that the abundance of T cells was 4/mm 3 , that of neurons was 90,000/mm 3 and that of microglia was 6,500/mm 3 [ 97 , 98 , 99 ]. Other cells such as B cells, monocytes, infiltrating macrophages, dendritic cells (DCs) either conventional or plasmacytoid, or neutrophils were counted and reported for the brain tissue as a whole because all these studies were from scRNAseq analysis using suspended cells from dissociated brain tissue.
In contrast to the previous studies that disregarded the heterogeneous distribution of rare immune cells in the brain, solid-phase spot RNA sequencing enabled genome-wide quantification and localization of transcripts, as first documented by Ståhl et al. [ 47 ]. In this method and in the now available Visium, a spot has its own count (log1p of the count ratio), which was measured by in situ capture of transcripts in the tissue. However, a spot is composed of a mixture of multiple cells, and it can be difficult to distinguish the transcripts of the rare immune cells from those of the major cell types. In line with this, the problem is to find an appropriate gene (transcriptome) combination to sort out only the specific marker transcriptomes that can discern rare cells from others. Selecting the possible key gene sets defining rare cells with the highest specificity is influenced by the choice of the input data, which are composed of participating cells [ 50 ]. For example, T or B lymphoid cells, quite unique with their high propensity for ribosomal protein genes such as Rpl or Rps , are characterized by any cell-type annotation method to yield candidate marker gene combinations. However, other major brain cells are also equipped with these protein-producing genes expressed in sufficient amounts to appear to be rare brain cells, confounding the presence/density of rare lymphoid cells in any area of the brain. Additionally, since rare immune cells are commonly investigated by combining cell sorting strategies with scRNAseq, the rare cell markers acquired from the subpopulation single-cell dataset may overlap with the major cell type markers. This caused serious overestimation, which was disclosed immediately upon visual assessment. This was also the case despite the use of the recent data available by Schafflick et al. [ 68 ] and NSForest by Aeverman et al. [ 59 ]. We adopted visual curation to exclude the frankly absurd transcriptomes as marker gene candidates and finally sorted out the rare cells with optimal marker gene combinations to compare wild-type and 5xFAD mice (Fig. 3 and Supplementary Fig. 13 ).
Spatial changes in the distribution of myeloid and lymphoid cell signatures in 5xFAD mice compared to wild-type mice. ( A ) Spatial pattern of the signatures of myeloid, B cell, and T-cell and ILC compartments according to the marker gene combination reported by Dominguez Conde et al. [ 71 ] (left) and the boxplot showing the average module scores (right). Each dot represents a mouse in each group. The average module score of the myeloid compartment showed a significant increase in the white matter and gray matter regions adjacent to the white matter, including the thalamus, deep cortex, and striatum. B-cell compartment signatures showed an increase in the deep cortex and white matter. In contrast, T-cell/ILC compartment signatures were low without differences between wild-type and 5xFAD mice. ( B ) Spatial pattern of the subtype signatures of myeloid cells, including CAM, macrophage, monocyte, plasmacytoid DC, and granulocyte according to marker gene combination from Schafflick et al. [ 68 ]. Notably, CAM and macrophage signatures showed the most pronounced increase in 5xFAD mice compared to wild-type mice in most of the regions. Monocytes and plasmacytoid DCs were increased in the deep cortex and white matter, and plasmacytoid DCs were further increased in the thalamus, pyriform area and striatum. ( C ) Spatial pattern of the subtype signatures of lymphoid cells, including NK and T cells, according to marker gene combination from Xiemrakis et al. [ 64 ]. In the case of the NK cell signature, a significant increase was observed in the deep cortex of 5xFAD mice, which is associated with an increase in CD56dim NK cells. Among T-cell signatures, tissue-resident memory T-cell signatures were higher in 5xFAD mice in the deep cortex, white matter, thalamus, pyriform area and striatum. The CD4 signature was explicit in the striatum in both wild-type and 5xFAD mice, but the expression was too low to show a quantitative difference between wild-type and 5xFAD mice. Bonferroni-adj. *p value < 0.05, ***p value < 0.001, ****p value < 0.0001. (WT: wild type; TG: 5xFAD mice; ILC: innate lymphoid cells; CAM: CNS-associated macrophage; DC: dendritic cells; NK: natural killer)
Immune resident cells were classified in three ways (1) using novel data by Eraslan et al. [ 69 ] (Supplementary Fig. 13 A) for tissue-specific monocyte-derived macrophages and data by Dominguez Conde et al. [ 71 ] (Fig. 3 A) for tissue-resident T cells, (2) using the data by Schafflick et al. [ 68 ] (Fig. 3 B) and markers refined using NSForest 2.0 and (3) using the data by Xiemrakis et al. [ 64 ] and refined using NSForest 2.0 by Aeverman et al. [ 59 ] (Fig. 3 C). The first two reports [ 69 , 71 ] were derived by using various tissues excluding the brain, while the other two reports [ 64 , 68 ] were derived by using brain tissues.
Defining marker gene combinations was more intricate for these rare immune resident/infiltrating cells, as they were defined by surface markers in the report of Eraslan et al. [ 69 ], in organs/tissues other than the brain, or by transcriptome signatures suited for each study. Although the data were from body tissues, not the brain, in the first approaches, as the tissue stromal cells are included in DEG analysis and assuming stromal cells might be more similar between tissues including brain, transcriptomes of major parenchymal/stromal cells coexpressing with rare immune cells were to be correctly excluded. We chose NSForest to help exclude confounding stromal tissues. Monocyte-derived macrophages include two types, according to Eraslan et al. [ 69 ]: one for immune function (MHCII+) and the other (LYVE1+) for vascular integrity and repulsion of infiltrating immune cells. For immune function, specifically for the brain, conventional DCs with MHCII + cells were found to be effective for antigen presentation by adaptive immune cells (T cells and B cells), although border-associated macrophages or microglia were not [ 100 ]. We asked whether this surface marker-defined characterization can be translated to mouse (5xFAD) amyloidosis using the signature of MHCII+-related immune-functioning macrophages and LYVE1+-related integrity-charged macrophages described by Eraslan et al. [ 69 ] (Supplementary Fig. 13 A). Integrity-charged macrophage signature scores were not different among the groups of 7-month-old wild-type and 5xFAD mice in all regions of brain. Spot signatures of immune macrophages were higher in 5xFAD mice than in wild-type mice in the white matter.
Signature gene combinations used in the cross-tissue immune cell analysis by Dominguez Conde et al. [ 71 ] revealed no difference between wild type and 5xFAD mice for T cells and innate lymphoid cell T/ILCs. However, significant differences were found among these mice for myeloid compartment cells in the white matter and the gray matter regions adjacent to the white matter, including the thalamus, deep cortex, and striatum and for B cells in the deep cortex and white matter (Fig. 3 A).
This result came from the following stepped analysis including the curation procedure. At the first step of curation, individual transcriptomes belonging to the three compartments described by Dominguez Conde et al. [ 71 ] were examined visually for their distribution/intensity, and several transcriptomes that were already reported in the literature as signatures for major brain cells and their reactive states were removed, which excluded the background effects of abundant brain cells, eventually yielding marker gene combinations for the three compartments and their cell types. Having removed (1) Cx3cr1 and Tyrobp (microglia) from T/ILCs, (2) Ighm (Scheurer et al. [ 101 ] for neurons) and C1qa (microglia) from the B-cell compartment, and (3) Trem2 (microglia), Clu (astrocytes), Selenop (microglia, astrocytes, oligodendrocytes), Igf1 (reactive microglia and reactive astrocytes), C1qa and Cx3cr1 from the myeloid compartment, the scores of T/ILCs were still not different between wild-type and 5xFAD mice, and the scores of B-cell or myeloid compartments revealed a slight but significant increase in 5xFAD mice compared with wild-type mice. Individual variations within 5xFAD mice could also be recognized upon visual assessment. For individual genes for T/ILCs, localization was prominent for Cd4 (striatum) and showed little difference regardless of abundance ( Slc4a4, Spry2, Ncam1 , and Pcdh 9 are abundant), and no difference was observed except for Pdcd1 (smaller cell fraction in various T cells including Trm/em_CD8 according to Dominguez Conde et al. [ 71 ]), which was slightly increased in 5xFAD mice. For the B-cell compartment, the difference between mouse groups, if any, was presumed to be due to Itgax and Fcrls , both of which were related to aging-associated B cells, and Fcrls was related to memory B cells and plasma cells/plasmablasts. For the myeloid compartment, the difference in scores among mouse groups was contributed by Tyrobp, Lyz2, Fcer1g, C1qc , and Apoe , all of which are related to various types of tissue-specific macrophages and classical/nonclassical monocytes (Supplementary Fig. 14 ).
The second one by Schafflick et al.’s data [ 68 ] was tested for either the marker gene combination recommended by Schafflick et al. according to their supplementary table (log fold change: LFC > 0.5) for 12 border cell leukocytes (including microglia) or the marker gene combination curated by NSForest upon inputting their data. Schafflick’s own data yielded obviously too high intensity for CD4 and CD8 T cells among 12 border-associated leukocytes, such as B1, B2, CD4 T, CD8 T and NK cells, microglia, CNS-associated macrophages (CAM), macrophages, monocytes, myeloid DC (mDC), plasmacytoid DC (pDC) and granulocytes. When we surveyed the constituents of the tentative marker transcriptomes for these inappropriate signatures, ribosomal genes (many isoforms of Rpl and Rps ) were presented as false positive markers of CD4 T and CD8 T cells. This misclassification of marker genes is assumed to be caused originally by the fact that the parenchymal and border leukocytes were included after their selection for CD45 positivity, meaning that they could not exclude the differential expression of these cells from the major brain cells, including stromal cells. Individual transcriptomes per spot were easily observed to disclose whether we chose the highest LFC with adjusted p values for determining marker genes, Ighm for b1 cells (also found in the cortex not related to B cells) [ 101 ], H3f3b (histone protein also nonspecific for the brain) for b1 cells, Stmn1 for b2 cells (rather brain-wide expression), Dut (enzyme for nucleotide and ubiquitous, including brain cells) for B cells and many similar examples (Supplementary Fig. 13 B). Although DEG analysis depends upon the input data composition, we tried NSForest on Schafflick’s data and obtained a better marker gene combination. This Schafflick/NSForest analysis yielded improved intensity matching considering the prevalence of cell populations in the brain parenchyma except for b2 cells (still too dense due to Tuba1b (tubulin related)) and CD4 T cells (depending heavily upon one transcriptome Trbc2 (T-cell receptor beta constant 2 but also expressed in the cortex)). The other 10 cell signatures appeared to represent the cell intensity/distribution correctly; however, they also included nonspecific and dense Apoe for CAM, dense Cst3 for macrophages, Mal (Myelin And Lymphocyte Protein, implying its localization both in lymphocytes and myelin of neurons) for monocytes, and Tyrobp (in association with Trem2 , a well-known marker for microglia) for both monocytes and pDCs. Upon the application of NSForest, 6 to 10 marker genes were obtained, and zero to three genes were adjusted (kept or removed, meaning curated by operators’ consensus). The application of curated gene combination to our ST samples revealed that CAM and macrophages showed the most pronounced increase in 5xFAD mice compared to wild-type mice globally throughout the brain regions (Fig. 3 B). Additionally, pDCs showed increases in the white matter and some gray matter regions. However, the transcriptome density of DCs was considered inappropriate, as it yielded much higher intensity along the entire brain, considering that DCs occupy only 0.14% of myeloid/lymphoid cells of the brain and border, including microglia (0.8% among myeloid/lymphoid cells excluding microglia) [ 68 ].
Among lymphoid cell signatures, an increase in tissue-resident memory T cells was inferred in 5xFAD mice compared to wild-type mice (Fig. 3 C and Supplementary Fig. 14 ). However, it is necessary to consider the technical limitations of spot-based transcriptomic analysis for evaluating rare brain cell signatures. It is still unclear which genes specifically define rare cells, while gene combinations may overlap with major brain cells on ST brain imaging.
Using a single gene as a marker would be better and more convenient for designating rare cell types. It was possible to designate infiltrating macrophages derived from circulating monocytes originating from BM (Supplementary Fig. 15 ). The CD11c surface marker and its gene Itgax were used as markers for these cells. Resident T cells were suggested to be CD73 positive, and its gene Nt5e was identified by Fang et al. [ 102 ]. CD56 bright and its gene Ncam1 are considered to be circulating and immature NK cell markers but are also highly expressed in neurons [ 103 , 104 ]. Perivascular macrophages cause a great problem in distinguishing them from microglia, and Lyve1 is the discriminator of pvMϕs and microglia ( Sall1 ) [ 105 ]. Similarly, for brain major cells, Trem2 and Tyrobp were suggested to be conjoint markers for microglia, and Cspg and Olig2 were expected to represent OPCs, not any other cell types. Homeostatic microglia could have been defined by Sall1 ; however, a transgenic mouse study [ 105 ] found that Hexb was the better marker for authentic microglia than Sall1 . The importance of Aif1 (IBA1) as an activated microglial marker and of Gfap as an activated astrocyte marker was disclosed to be nonspecific or at least subtype specific, respectively. Once a marker was well defined for designating rare cell types well discriminated from major brain cells, including microglia and perivascular space (pv) macrophages (and submeningeal macrophages), then that marker in a spot could disclose the fact that the gene signature of that spot might be from the rare cell of interest, but it does not mean that the signature was not from the rare cells if no signal was observed. Genes widely expressed over all cell types but with specific isoforms could be used to define the cell types, and Prdx (for peroxiredoxin) was one of the examples ( Prdx6 and Prdx2 for astrocytes, Prdx4 for microglia and Prdx1 for oligodendrocytes) (Supplementary Fig. 16 ).
To validate the results obtained from the cell signature score based method of measuring cell type abundance, we performed the cell type deconvolution analysis and compared the results between the two methods. The cell type deconvolution method captures the gene expression patterns of cell types from the single-cell reference dataset and predicts the cell type composition in the ST spot, which is a mixture of multiple cells. We performed the analysis mainly for microglia and infiltrating immune cells, which showed significant changes between 5xFAD mice (TG) and wild-type mice (WT) in the signature score-based method. For microglia, the proportions of both homeostatic and reactive microglia were globally increased in the gray and white matter regions of TG mice, which was consistent with the results obtained from the cell signature scores (Supplementary Fig. 17 ). Minor immune cells, including myeloid cells such as macrophages, monocytes, and dendritic cells, were also upregulated in multiple gray and white matter regions of TG, and the results were similar to those obtained using cell signature scores (Supplementary Fig. 18 A, B). However, for lymphoid cell types such as innate lymphoid cells, natural killer cells and T cells, which are rare, the patterns of change between the two methods were inconsistent for a few gray matter regions, while the biological effect may be small due to very low cell abundance (Supplementary Fig. 18 C, D). Overall, the results suggest that cell signature scores derived from curated markers are an accurate and reliable measure of cell abundance for the relatively common cell types, while rare cell types require special attention in interpretation.
Improvement of behaviors with much variation by immunomodulatory therapy of anti-CD4 antibody and NK supplements in the 5xFAD AD mouse model
During a preliminary behavioral study to prove the effect of aducanumab, pretreatment with anti-CD4 antibody caused a larger degree of changes in alternation scores in the control animals (meaning higher improvement in the group of animals treated with anti-CD4 antibody) [ 29 , 30 ]. Three batches of several animals with anti-CD4 antibody treatment reproduced the previous groupwise behavioral improvement with similar variation (67.7% ± 18.4%) at 7 months of age in 5xFAD model mice (Fig. 4 ). We assumed that anti-CD4 antibody treatment modulated the systemic adaptive immune system, as transgenic insertion of five types of mutated human APP/PS1 genes would have caused immune disturbance due to their presence in the mouse chromosome. The presence of human mutated genes would have resulted in brain-immune interaction dysfunction as well as plaque-prone amyloid burden in animals. Spatial transcriptomic analysis was considered to reveal the eventual response of brain cells, either major or rare resident and infiltrating immune cells, if any.
Improved behavior after intravenous administration of NK cell supplement and anti-CD4 antibody in 5xFAD mice. ( A ) Timeline of the experiments for intravenous NK cell supplements (upper) and anti-CD4 antibody (lower) administration in 6.5-month-old wild-type and 5xFAD mice. NK cells (2 × 10 6 cells/injection) were administered once a week for a total of five times, and anti-CD4 antibody (0.5 mg/injection) was administered once as a single injection. After a month, behavior analysis was performed, and brain tissue samples were obtained for spatial transcriptomic brain imaging analysis. ( B ) The behavioral function of exploring new environments was examined using the Y-maze test and expressed as alternating percentages. Each dot represents a mouse in each group. The alternation rate was decreased in 5xFAD mice compared to wild-type mice, with much variation at this age in wild-type and 5xFAD mice. The alternation percentage score of 5xFAD mice increased significantly after injection of NK cell supplements and anti-CD4 antibody treatments compared with that of 5xFAD mice without treatments. Wild-type mice also showed variation; however, their alternation scores were not different between the no treatment and either treatment group. Wilcoxon *p value < 0.05, **p value < 0.01. (aCD4: anti-CD4 antibody; WT: wild type; TG: 5xFAD mice; WT_NK: NK cell-treated wild type; WT_aCD4: anti-CD4 antibody-treated wild type; TG_NK: NK cell-treated 5xFAD; TG_aCD4: anti-CD4 antibody-treated 5xFAD)
In another preliminary study with APP/PS1 model mice using the water maze with expanded NK cell supplements derived from the spleen of wild-type BALB/c mice, anecdotal cases of behavioral improvement were observed (data not shown). Three batches of allogeneic NK cell supplements, as 5xFAD mice are on a B6 background, reproduced the behavioral improvement of alternation scores on Y-maze tests on average, however, with much variation (Fig. 4 ). Much variation in both the anti-CD4 antibody treatment study and NK supplementation study indicated that 5xFAD mice at 7 months of age were undergoing their own course of aggravation of the pathological changes of Aβ oligomer insults and amyloid plaque burden, resulting in later pathological and behavioral dysfunction at approximately 12 months of age or later. Spatial transcriptomic analysis was also considered to reveal the regional and cell-type specific changes of transcriptomes of major and rare brain cells corresponding to each individual mouse’s degree of behavioral dysfunction in the NK supplement-treated group.
Regional cell-type/state-specific transcriptome changes in 5xFAD mice compared with wild-type mice after intravenous administration of NK cell supplements
Three mice with higher alternation scores on the Y-maze test were selected for both the saline-treated and NK cell supplement-treated groups (Supplementary Fig. 19 ). Coronal/sagittal brain sections of these mice were subjected to Visium analysis. Each group was paired to the same plates so that the batch effect of the read per slide would be minimized. Using 30,000 to 50,000 counts per mouse, we retrieved the count data, which were normalized for their total count, and log1p of the ratio data were used for further analysis. Spatial clustering allowed anatomical segmentation to yield 14 regions with almost similar sizes (Supplementary Table 4 ). Cell type- and state-specific marker gene combinations were also used to analyze cell-specific and/or cell state-specific changes after NK cell supplement treatment. For the 5xFAD case with NK cell supplements, one mouse with a very high behavior score was chosen as the ‘behaviorally best’ representative of the group, and another mouse with a very low score was chosen as the ‘behaviorally worst already at 7 months of age’ representative. This essentially allowed us to examine the transcriptomic changes according to the behavioral impairment of the 5xFAD mice at the age of 7 months. NK cell supplements contributed at least to the widening of the distribution of scores of behavioral impairments at this middle age.
GABAergic Sst subtype neurons showed a significant decrease after NK supplementation in the amygdala, which showed an abnormally increased signature in 5xFAD ( n = 11) compared with wild-type mice ( n = 10) (Fig. 5 A). Among the Sst neuronal signatures, Sst, Tac1 , and Nr2f2 showed dramatic decreases in the amygdala after NK cell supplement administration in 5xFAD mice, but there were no distinct differences in the other regions (Supplementary Fig. 20 A, B). The Sst-expressing neurons in the cortex are known to contribute to modulating cortical circuits, synaptic plasticity and maintaining spatial working memory [ 106 ]. Patients with AD exhibited low Sst expression in the cortex and hippocampus. However, the function of Sst-inhibitory neurons in the amygdala remains poorly understood [ 107 ]. No significant difference was found either in the mature neuron score or in any other subtype of neurons other than Sst neurons between 5xFAD mice without NK supplements and those with NK supplement treatment. Thus, normalization of excitatory and inhibitory neuronal imbalances in the amygdala may improve behavior function. Further investigation of the neurons in the amygdala region could play an important role in understanding the pathology of AD and in providing therapeutic directions. Additionally, the NK cell signature tended to increase after administration of NK cell supplements exclusively in the white matter region of 5xFAD mouse brains (Supplementary Fig. 21 A). The change in the module score level was not observed with the anti-CD4 antibody treatment, which was in contrast with the change after NK cell supplement treatment (Supplementary Fig. 21 B). The signatures of astrocytes, microglia, and oligodendrocytes did not show any difference. Additionally, no difference in rare brain cells, either resident or infiltrating, was observed (Supplementary Fig. 22 ). The biodistribution of 99m Tc-HMPAO-labeled NK cells was examined using SPECT/CT to determine how systemically injected NK cells caused changes in the brain (Supplementary Fig. 23 ). Within 1 h after injection, the labeled NK cells were mainly taken up by the liver, and this radioactivity decreased gradually by 16 h. Of note, no definite brain uptake of the labeled NK cells was observed with the resolution of SPECT/CT images. Thus, NK cells may have caused changes in brain cells at the transcriptional level indirectly via cytokines or other secretory factors released by NK cells and/or inherent peripheral immune cells influenced by supplemented NK cells.
Brain region-specific transcriptome changes in cell signatures after NK cell supplementation and anti-CD4 antibody treatment in 5xFAD mice. ( A ) Spatial pattern of the signatures of somatostatin (Sst)-inhibitory neuronal signatures (Sst1, Sst2, Sst3, Sst4, and Sst5; left) and the boxplot showing the average module scores in the amygdala (right). Each dot represents a mouse in each group. The average module scores of Sst neuronal subclasses tended to decrease specifically in the amygdala after administration of NK cell supplement in 5xFAD mice. Interestingly, the Sst neuronal signatures, which were increased in expression in the amygdala of 5xFAD mice, were decreased to the expression level in wild-type mice by NK cell supplements. In contrast, the module score was not different between the no treatment and anti-CD4 antibody treatment groups, while there were differences between the no treatment and NK cell supplement groups. ( B ) Spatial pattern of the signatures of state-specific glial cells (aging astrocytes and reactive microglia; left) and ( C ) immune cells (monocytes and plasmacytoid DCs) and the boxplot showing the average module scores in the white matter (right). The expression of state-specific subtypes of glial cell and immune cell signatures, which showed a significant increase in 5xFAD mice compared to wild-type mice, tended to slightly decrease in the white matter after anti-CD4 antibody treatment. Considering that NK cell supplementation showed no appreciable differences in glial cell and immune cell signatures, anti-CD4 treatment effects on these cell subtypes of state-specifics looked real. Expression, however, did not decrease to the level observed in wild-type mice. In summary, NK cell supplementation and anti-CD4 antibody treatment affected different state/type-cell signatures and brain regions, respectively. (aCD4: anti-CD4 antibody; WT: wild type; TG: 5xFAD mice; WT_NK: NK cell-treated wild type; WT_aCD4: anti-CD4 antibody-treated wild type; TG_NK: NK cell-treated 5xFAD; TG_aCD4: anti-CD4 antibody-treated 5xFAD; Sst: somatostatin; DC: dendritic cells; NK: natural killer)
Regional cell-type/state-specific transcriptome changes in 5xFAD model mice compared with wild-type mice after intravenous anti-CD4 antibody treatment
Three mice in the anti-CD4 antibody treatment group were selected, and their coronal brain sections were plated on Visium slides. The frame of sample distribution on the quadrants of each Visium slide was the same as above for NK cell supplement treatment. Further analysis of transcriptomes per spot and spatial clustering and designation of transcriptomes to the approximately 3,000 spots were also the same.
Among major brain cells, state-specific glial cells, such as aging astrocytes and reactive microglia, which showed a significant increase in 5xFAD mice compared to wild-type mice, showed a slight decrease exclusively in the white matter after administration of anti-CD4 antibody in 5xFAD mice (Fig. 5 B). In the gene combination of aging astrocytes, the expression of Lgmn, Gsn, Mt1, Fcrls , and Hexb was noticeably decreased in the white matter after anti-CD4 antibody administration in 5xFAD mice, and expression decreased slightly in the other regions (Supplementary Fig. 20 C, D). In the reactive microglial signature, Cst7, Spp1 , and Cd9 showed a decreased pattern throughout the region, while other genes, such as Axl , Csf1 , and Ccl6 showed decreased patterns mainly in the white matter (Supplementary Fig. 20 C, D). Interestingly, the CD4 T-cell signature tended to decrease slightly in the deep and upper cortex (striatum) after anti-CD4 antibody treatment (Supplementary Fig. 21 B). However, mature neuronal signatures showed typical and very similar patterns of distribution between 9 major brain regions and within each region irrespective of whether the sample was from wild type, 5xFAD, wild type with anti-CD4 antibody treatment or 5xFAD with anti-CD4 antibody treatment mice (Supplementary Fig. 22 A). No significant differences in other types of glial cells, including white matter-associated astrocytes, reactive astrocytes, plaque-associated microglia, homeostatic microglia, and oligodendrocytes, were found, meaning that all 10 wild-type mice and all 11 5xFAD mice could not be differentiated between no treatment and anti-CD4 antibody treatment (Supplementary Fig. 22 B, C). The difference between wild-type and 5xFAD mice was sustained but did not reveal any dramatic effect of anti-CD4 antibody treatment.
Rare brain cells, resident or infiltrating, were distinguished between the no treatment group and the anti-CD4 antibody treatment group. The expression levels of monocytes and pDCs, which showed a significant increase in 5xFAD mice compared to wild-type mice, tended to slightly decrease only in the white matter after anti-CD4 antibody treatment (Fig. 5 C). In both monocyte and pDC signatures, the expression of Tyrobp was dramatically reduced throughout the brain, whereas S100a4, S100a10 , and Mal in the monocyte signature showed decreased expression patterns exclusively in the white matter after anti-CD4 antibody treatment. A reduction in the expression of individual genes by the anti-CD4 antibody was mainly identified in the white matter region (Supplementary Fig. 20 C, D).
While NK cell supplementation showed no appreciable differences in immune cell signatures, the fact that anti-CD4 antibody treatment showed effects on subtypes of immune cells is noteworthy. However, the expression level was not decreased to that observed in wild-type mice.
Methods to scrutinize spatial cell-type and cell-state specific changes upon the platform of setting norms and characterization of abnormality of a test mouse
As spatial distribution is critical for characterization of a new mouse specimen for their status of normalcy, pathology, and response to therapy, the specimen can be on every section, but on a limited number of coronal sections (per monkey samples in a report by Chen et al. [ 55 ]) or sagittal sections. To acquire representative information regarding mouse groups, we combined multi-individual mouse sections to yield the apparently correct spatial segmentation. Each region was then prepared to present their norms for various scores for cell types, cell states and response to the tentative immunomodulatory drugs. We tried to establish methods to reveal the regional cell-type/state-specific norms and their probable changes by drug intervention. We set up norms for normal mice using wild type mouse data genotype, and then the effect of the age or the influence of drug treatments were characterized. For example, the presence of anomaly were examined for individual mice according to their disease states (5xFAD mice of certain age with diverse behavior/Aβ abnormality, P301L mice with no behavioral/pathological abnormality) and the effect of therapy (anti-CD4 antibody or NK cell treatments).
Cell types should have been annotated to the then-best knowledge of the scientific community based on the resource reports in the literature up to the date of this investigation run by trial-and-evaluation and then the choice; for example, for neurons and neuronal subtypes, Hodge et al.’s report [ 75 ] was adopted as is or after NS Forest [ 59 ] to define 20 GABAergic neurons and 12 glutamatergic neurons. Available data were downloaded from specific sites or supplementary tables of each report. Thus, for example, Scng-VIP neuron subtypes described in a more recent report by Bugeon et al. [ 57 ] were ignored but later can be reanalyzed with the current Visium data by specifying their markers along the regions segmented after integration of slides using RPCA. For astrocytes and microglia, the reactive state signature was surveyed by scrutinizing the counts of transcriptomics at each spot according to the reports by Keren-Shaul et al. [ 89 ], Friedman et al. [ 91 ], Grubman et al. [ 93 ] and others. Coexpression of the same transcriptome by astrocytes and microglia, such as Apoe, Gfap, Tspo and others, was removed from the tentative marker gene combination. The same procedure was performed for astrocytes and oligodendrocytes or microglia and oligodendrocytes. Oligodendrocytes and their lineage cells did not have a ‘reactive’ transcriptome signature. Signature transcriptomes between reactive and homeostatic microglia (and astrocytes) were also surveyed for their conjoint expression between both states. Homeostatic transcriptomes were designated to exclude the signature of reactive transcriptomes and vice versa, referring to literature reports by Prinz et al. [ 108 , 109 , 110 , 111 , 112 , 113 , 114 ], and Kim et al. [ 105 ], so that Sall1 and Hexb were used to measure the abundance of microglia as each microglia express these genes constitutively while assuming that these transcriptomes did not increase in quantity per microglia when reactive [ 105 , 113 ]. As spatial transcriptomic imaging using Visium yielded a linear (semi)quantitative (due to log1p transformation for further processing using Seurat 4.1)) metric, no fractional presentation was adopted to disclose that homeostatic microglia were increased in quantity of signature per spot in 5xFAD mice compared with wild-type or P301L mice.
Quantification was performed for 9 regions (hypothalamus, thalamus, deep cortex, white matter, upper cortex, hippocampus, amygdala, piriform area, and striatum) for major cell types, including neuron subtypes, reactive and homeostatic glial cells, astrocyte subtypes, oligodendrocyte lineage cell subtypes and rare resident/infiltrating immune cells. For regions, for example, white matter-associated astrocytes or white matter-localized microglia were quantified and correlated with white matter-localized oligodendrocyte lineage subtypes. Dense and thus intense quantities of mature oligodendrocytes could be compared among mouse groups. In contrast, diffuse and sparsely distributed rare immune cells were found in three compartments by Dominguez Conde et al. [ 71 ], four types by Xiemerakis/NSForest [ 59 , 64 ], and 12 types by Schafflick et al. [ 68 ]. Tissue resident macrophages by Dogra et al. [ 115 ] and Eraslan et al. [ 69 ] were also quantified for intensity to yield the difference for each region among mouse groups. The Wilcoxon rank-sum test was used to determine the significance between pairs of groups (uncorrected p value or corrected by three for paired group comparisons). Beyond group comparison, an anomaly detection procedure (or confirmation of normalcy meaning no difference found on any regional, cell-type, cell-state specific signature by density per region) was performed for each mouse.
Once a region and cell type and its state were found, we performed DEG analysis to find the transcriptomes of interest in each brain region. Then, the association of discovered genes with biological pathways was examined using an overrepresentation test based on evidence-driven databases. This was to determine the significance of the found transcriptome designating their functional role in pathology (amyloid pathology or tauopathy, glial cell dysfunction, etc.), physiology (aging) and their participation in the response to tentative immunomodulatory therapy. Considering the diversity of behavior improvement after anti-CD4 antibody and NK cell supplement treatments and the unpaired nature of the Visium study, we could only detect the treatment effect (and non-effect) of the transcriptome signature of regional cell types/states upon treatment per individual. Transcriptomes of the marker gene combination that we used were all checked for their individual transcriptomes to elucidate key transcriptomes for specifying type/state characteristics or therapy effects. We also tried to assess their individual contribution to this specification to find one, two or more distinctive transcriptomes to predict their presence in each spot. This means that curation by operator in addition to the readymade Wilcoxon, logistic, or NSForest methods was used in at least two steps, first to choose a seemingly optimal combination ruling out cross-expressing, background, or confounding genes and then at last to find the succinct combination of transcriptomes for cell-type/state annotation or if any, the sole transcriptome (Supplementary Fig. 2 ). The above pipelines for dissecting cell-type- and cell-state-specific regional transcriptomic changes can be readily implemented with our in-house application STquantool, which facilitates the visualization of spatial gene expression and enables quantification across multiple transcriptomic datasets.
In this investigation, we used ST for its superiority over scRNAseq/snRNAseq to localize the specific transcriptomic signature of cell type or cell state in almost 5 thousand spots, among which 3,000 or more spots harbored either coronal or sagittal sections of brain tissue. Before going further to use this transcriptomic signature to disclose the effects of novel but unproven neuromodulatory treatments, we trimmed the method of the use of this Visium-based ST imaging to elucidate regional, cell-type/state-specific changes. The method for choosing one or two optimal transcriptomic marker combinations among so many possible combinations was adjusted to yield the best contrast between cell types/subtypes using the literature resources and our in-house method of curation. A simple and easy method to sort out the candidate transcriptomes was set up to ensure that we found the best cell type/state annotation methods for either abundant brain cells or rare immune cells. The challenge was to separate 4 or more major brain cell types and their subtypes with transcriptome combinations and to define rare immune cells for their exact propensity and distribution/location. Stahl et al.’s [ 47 ] suggestion of counting the transcriptomes per spot using the original ST Visium methods and Tirosh et al.’s [ 44 ] approach to generate cell signature scores based on the curated marker genes and comparing them between mouse groups with genuine or sham treatments worked well for this endeavor. We overcame the problem of high dependence of this endeavor on the choice of tentative marker gene combinations varying upon the diverse input data derived from the preliminary DEG studies using single-cell data of brain tissues [ 67 , 68 , 108 ] and even tissues other than brain tissues. Assessing the sophisticated use of the public database and scrutinizing the individual transcriptomes visually by the operators (neuroimaging experts) were essential. Curation by operators is heuristic at best and is surely subject to operator arbitrariness; however, this was eventually the key step to enhance the authenticity of the observation of large number of cells (2 to 10 per spot) admixed in spots and a dozen specimens from individually different but syngeneic mice. From the neuroimaging perspective, integrated single spot imaging (100 µ x 100 µ x 10 µ) containing an average of 5 cells (2 to 10) in each unit domain did not have a significant batch/individual variation effect to confound further analysis, as we observed dozens/hundreds of spots at the same time, and the batch effect was corrected during sample integration. With this visual investigation, we soon became confident that spatial transcriptomic brain spot imaging with visual assessment and its quantitative analysis using the framework of voxel (spot) imaging of mouse/human brains was suitable for the evaluation of the effect of certain drugs/treatments for disease-course modification in dementia mouse models.
The transcriptomic signature of brain cells could clearly segment every section from the mice, regardless of disease or treatment status, taking advantage of 22,000 or more transcriptomes per cell to identify the cell type/state with thousands of variable transcriptomes. Unlike functional neuroimaging such as functional magnetic resonance imaging (fMRI) or positron emission tomography (PET), which needs coregistration and segmentation considering individual variation for further analysis, the segmentation of neuroanatomical entities on Visium could be performed without any more assumptions, except that functional regional entities could be determined by transcriptomes belonging to spots and their conglomerated spots make explicit functional regions. An eccentric case of regionally remote but similar transcriptome composition was observed in that the cortical amygdala and subcortical septal lobe were categorized as the same cluster on sagittal section, but excluding this exception, all the other spatial clusters were within the expected anatomical border definition (https://connectivity.brain-map.org/3d-viewer?v=1&types=IMAGEPLANE&IMAGEPLANE=imageplanes). Thus, spatial or regional representation of characteristic changes related to pathology and treatment response could then be described and quantified. Finding marker gene combinations to define the spots as belonging to certain functional regions of interest then could be achieved by finding the optimal or best combination, which would be appropriate and succinct.
Determination of the best annotation of neurons and other major brain cells was initially dependent upon previous reports mainly derived from nonspatial scRNAseq/snRNAseq analysis [ 63 , 73 , 80 , 116 ]. In these previous studies, the spatially expected designation of cells was suggested as a success of cell clustering, raising concerns that there was no gold standard information regarding their true location; nevertheless, the cell clustering and annotation allows assignment of regional identity of brain cells based on anatomical region-specific marker genes. ST brain imaging obviated this concern. In ST imaging, however, there remain two major problematic ambiguities for spatial clustering and cell type/state identification per spot. The first one is spatially agnostic annotation by transcriptome signature, which previous researchers tried to solve by sampling regions of brain such as posterior isocortex, hippocampus (or hippocampal formation), striatum, thalamus and hypothalamus, etc., in the reports of Saunders et al. [ 65 ] or Chai et al. [ 117 ]. This problem was easily solved by ST imaging using Visium of 3 to 5 thousand spots, which allows capturing transcriptomic changes across the broad area of the brain. Now, imaging with a resolution of 100 µ x 100 µ on 2D is available, allowing easy segmentation; this differs from fMRI/PET in that the huge multiplexing capability of ST brain imaging allows almost infinite reanalyses using combinatorics. The second is cell/state identification per spot by using the transcriptomic signature of the marker gene combination determined by previous DEG studies using detached and sometimes surface-marker-sorted brain cells. When scRNAseq/snRNAseq was used for detached brain cells to determine the effect of drug/treatment on those brain cells, lack of spatial localization was the major hurdle blocking the understanding of the role of any treatment. In situ hybridization of immunohistochemistry complemented transcriptomic global/regional brain signatures to address this, but without reassuring results to explain the therapy effect. ST brain imaging solved this problem. As shown in this study, ST brain imaging is equipped with the expression profile per spot for the entirety of genes of the individual cells localized on each spot, and the data could be analyzed in an unsupervised fashion without any assumption or in appropriate cases by using a priori knowledge derived from the literature resources of scRNAseq/snRNAseq. Considering the challenges and difficulties in overcoming these problems, we streamlined the use of visual reading by expert operators called curation. The steps required for curation were kept minimal and practical, and it was performed initially to exclude nonspecific and cross-expressing transcriptomes between major cells, and finally to exclude cross-tissue, stromal cell-dependent and confounding background signatures. It would have been better to base curation on individual transcriptomic features of any types of cells for their association with disease states or drug/treatment responsiveness.
To tackle these problems, we asked how we could use individual mouse ST brain imaging data to determine the disease states, which are variable even in syngeneic animals, and the variable treatment responses affecting major and rare brain cells. Taking advantage of the automatic segmentation results for groups of individual mouse specimens, irrespective of section planes and stereotaxic coordinates, we tried to individualize the transcriptomic features of each individual specimen compared with the norms we constructed. Comparing regional, cell type/state-specific transcriptomic signatures using visual and quantitative decisions of an individual mouse with norms was performed. This analysis method allowed for the individuation-based evaluation of animals for their behavior correlates. We were able to obtain and reproduce a wide variety of behavior metrics, which is in this study included the alternation score on the Y-maze; the Y-maze alternation scores of 7-month-old wild-type mice ranged widely as well as those of 5xFAD mice, but those of 8.5-month-old wild-type mice converged with smaller variation to lower values, meaning commonly poorer performance at this age even in wild-type mice. After anti-CD4 antibody treatment, the variation was sustained with a slight improvement in their average scores (Fig. 4 B). After NK cell supplement treatment, variation was also sustained, with slight improvement in their average scores (Fig. 4 B). We assumed that these behavior variabilities are the keystone for proving the feasibility of tentative novel immunomodulatory treatments and that we would find that the mouse behavior scores concord with the transcriptomic signature [ 57 ]. NK cell-treated 5xFAD mice with higher Y-maze alternation scores definitely showed that their amygdala GABAergic Sst neuronal subtypes decreased in intensity (Fig. 5 A). This decrease (or increase, if any) did not prove the efficacy of NK cell supplement treatment on 5xFAD mice but definitely disclosed that transcriptomics of the neuronal subtype of that region were correlated with the degree of behavior impairment. More importantly, this meant that many other neuronal subtypes, other homeostatic or reactive glial cells and their subtypes, did not show any change in intensity over all the regions examined on these sections despite the improved behavior score. Anti-CD4 antibody treatment recapitulated only a slight decrease in specific immune cell signatures in the white matter, but beyond this finding, no other discovery of drug-responsive transcriptomic changes in any region or in any cell type or cell state was found. This was even observed on individual interpretations both visually and quantitatively for each mouse (Fig. 5 B and Supplementary Fig. 21 ). We could say that anti-CD4 immunoglobulins did not affect the transcriptomic signatures of major brain cells (on this single coronal section), and this was also the case with rare immune cells. Due to the lack of Y-maze score measures of the anti-CD4 antibody-treated wild-type and 5xFAD mice, behavior correlation could not be reported here.
The interpretation of rare immune cell signatures for the localization of immune cells presented different challenges from major brain cells. First, due to the intrinsic limitations of Visium, rare cell transcripts may not be well captured compared to the abundant cell type. Additionally, Visium captures a mixture of transcripts from multiple cells and lacks single-cell resolution. Therefore, it relies on cell type abundance estimation tools, which may be less reliable than image-based ST methods that capture transcript expression at the single-cell level. Nevertheless, we attempted to overcome the limitations with several strategies. The first was to remove the background effects of major brain cells. Homeostatic and reactive microglia and their coexpressed transcriptomes between microglia and infiltrating monocytes were the major challenge but were easy to remove, and astrocytes and oligodendrocytes followed by reactive glial cells expressed the same/similar transcriptomes. Double-checking the unique transcriptomes and their combinations was attempted with the data by Ximerakis et al. [ 64 ] and Schafflick et al. [ 68 ]. based on brain tissue studies. The study by Schafflick et al. [ 68 ]. used cells sorted by FACS for CD45 (gene Ptprc ) positivity and thus we were unable to remove the coexpressed transcriptomes of ribosomal protein transcriptomes for lymphoid cells, which if removed, would have enabled correct classification of myeloid and lymphoid cells among major brain cells in terms of intensity and distribution. Nevertheless, visual/manual curation by surveying individual transcriptomes helped to remove absurdly intense and unrealistically distributed transcriptomic signatures. When we used only the data of Schafflick et al. [ 68 ]. , we could not correct the inappropriate signature for B and T-cell compartments even after NSForest application to their data. The data came to look realistic after we adopted cross-tissue data and visual curation upon the two reports by Eraslan et al. [ 69 ] and Dominguez Conde et al. [ 71 ]. DEG data with an arbitrary threshold of 2.0 higher or -2.0 lower log fold change (LFC) for MHC + infiltrating immune macrophages (Mϕs) or LYVE + infiltrating integrity Mϕs produced 200 or more or 100 or more transcriptomes, respectively. We needed to remove, upon visual curation, 30% or 20% of transcriptomes to annotate the infiltrating monocyte-derived Mϕ. Infiltrating Mϕ and border-associated Mϕ [ 67 , 68 ] should have been differentiated but this was not possible due to the lack of clear distinction between the two cell types in the literature and the sparsity of the cells of both types. Tissue-resident and effector memory cells were traced with the transcriptomic signature by Dominguez Conde et al. [ 71 ]. As these authors included a variety of tissues (unfortunately, brain was not included) and stromal tissue specificity was considered a possible confounder in common for every tissue and thus, as expected, they yielded the signature for three compartments of T/ILC, B-cell and myeloid compartments. Of course, the types/subtypes of classically well-known immune cells belonging to these three compartments represented well the rare immune cells that would have originated from the bone marrow. We found differences in the intensity and distribution of the three compartments in the brain sections between 5xFAD mice and wild-type mice (Fig. 3 ). Drug/treatment effects should have been disclosed with this comparison, but we simply state that further investigation is warranted with a larger number of mice to avoid confounding factors which may hide or spuriously render probable false-negative/positive results regarding the effect of any tentative immunomodulatory treatments (Supplementary Tables 5 and 6 ).
The ultimate objective of using ST brain imaging with its visual and quantitative analysis is to convincingly designate the target cells, either major or rare, with regional localization; this can be for either brain parenchymal/stromal or rare immune cells, either resident or infiltrating immune cells and their homeostatic/reactive states, and target genes with significant contributions to pathologic changes in cells/regional tissues and their response to effective or ineffective treatments. More importantly, we could be sure that the unfound cells and transcriptomes were innocent, meaning that they were not affected by the test trial of a novel immunomodulatory therapy. For neuroimmune interactions during the disease process or in response to disease modifying drugs, we now know that the skull BM communicates with the dural sinus and peri-sinus regions, dural lymphatics as well as across ABC and CSF and thus perivascular spaces and ISF; communication can also take a totally different and unique route via the capillary endothelium and stroma of the choroid plexus, and choroid epithelium despite its tight junctions as well as the brain blood vessels’ microvascular endothelium despite its tight junctions. Once immune cells from the three compartments of T/ILCs, B cells and myeloid cells infiltrate the brain parenchyma, dynamically changing along the aging or disease process (in 5xFAD or P301L mice), they can respond to systemic immunomodulatory drug treatment directly or at least indirectly. The abundance of T cells (average 4/mm3) relative to neurons (90,000/mm3) or microglia (6,500/mm3) suggests that a few immune cells could change the response of major brain cells by significantly changing the transcriptomic signature of major brain cells. How the signals are transferred and/or translated from systemically administered anti-CD4 immunoglobulins or NK cell supplements should be investigated further. In this study, the study scheme and analysis methods were proposed to be applied to use ST brain imaging to investigate the impact of novel tentative disease-modifying treatments on neurodegenerative diseases and to elucidate whether regional brain cell-type/state-specific changes in the entire transcriptome per spot/region/cells of the brain or immune system would respond. The comprehensiveness and resolution of the results will be much improved with more novel technology [ 54 , 55 ] that will be available soon in many institutions, such as Visium methods [ 47 ]. Accordingly, methodology for analyzing spatial transcriptomics can be incorporated into high-resolution ST technologies to determine changes in cell types and abundance of rare immune cells with greater confidence.
Materials and methods
Ad models at different ages.
Three-month- and 7.5-month-old male 5xFAD mice (Tg6799; on a C57BL/6-SJL background) containing five FAD mutations in human APP (the Swedish mutation, K670N/M671L; the Florida mutation, I716V; and the London mutation, V717I; and the PS1 mutations M146L/L286V) and wild-type mice were used for spatial transcriptomic brain imaging data. Six- and seventeen-month-old male tau P301L mice (MAPT P301L mutations; on an FVB/N background) were used. Mice of all strains were raised in a laboratory cage with controlled temperature and humidity and on a 12 h light-dark cycle with free access to food and water. All experimental protocols and animal usage were approved (SNU-181018-6, SNU-190221-1-5) by the Institutional Animal Care and Use Committee (IACUC) at Seoul National University. All animals were handled in accordance with the Animal Research: Reporting of in vivo Experiments (ARRIVE) guidelines ( https://arriveguidelines.org ). Details are in Supplementary Notes.
Peripheral CD4 T-cell blockade in the 5xFAD AD model
Anti-CD4 antibody (0.5 mg/mouse; Bio X Cell) was intravenously injected into 6.5-month-old 5xFAD and wild-type mice according to group. Samples of different tissues were obtained after a month. Coronal sections of brain samples were used for spatial transcriptomic data acquisition.
Administration of NK cell supplement in the 5xFAD AD model
NK cells were expanded for 7 days after the isolation of NK cells from BALB/c mouse spleens. NK cells (2 × 10 6 cells/mouse in saline) were intravenously administered once a week for a total of five times to 6.5-month-old 5xFAD and wild-type mice. Brain samples were obtained after five weeks and used for spatial transcriptomic data.
Spatial gene expression library construction
Mice were anesthetized with isoflurane inhalation and perfused intracardially with cold DPBS (Dulbecco’s Phosphate-Buffered Saline; Gibco). Then, whole brains were removed. Brain hemispheres were prepared in frozen blocks using OCT compound (Sakura) and cryosectioned into 10 μm coronal and sagittal sections. According to the manufacturer’s protocols using Visium Spatial Tissue Optimization slides (10X Genomics), the permeabilization time was optimized to 12 min. The brain sections were methanol-fixed, hematoxylin and eosin (H&E)-stained and imaged on a TissueFAXS PLUS (TissueGenostics). The slides were merged into a picture of the whole brain using TissueFAXS imaging software. Then, the sections were permeabilized and processed to obtain cDNA Visium Spatial Gene Expression libraries according to the manufacturer’s protocol. To verify the size of PCR-enriched fragments, the template size distribution was checked using a high-sensitivity DNA assay (Agilent Technologies 2100 Bioanalyzer).
Generation of the count matrix
The libraries were sequenced using HiSeqXten (Illumina) with a read length of 28 bp for read 1 (Spatial Barcode and UMI), 10 bp index read (i7 index), 10 bp index read (i5 index), and 90 bp for read 2 (RNA read). Raw FASTQ data and H&E images were processed by the Space Ranger v1.1.0 (10X Genomics) pipeline for the gene expression analysis of Visium Spatial Gene Expression library data. Illumina base call files from the Illumina sequencing instrument were converted to FASTQ format for each sample using the ‘mkfastq’ command. Visium spatial expression libraries were analyzed with the ‘count’ command. Image alignment to predefined spots was performed using the fiducial alignment grid of the tissue image to determine the orientation and position of the input image. Sequencing reads were aligned to the mm10 reference genome (mm10-2020-A) using STAR (v2.5.1b) aligner. Gene expression profiling in each spot was performed with unique molecular identifier (UMI) and 10X barcode information.
Integration and spot clustering
A total of 63 spatial transcriptomic datasets, including brain tissue from wild-type and 5xFAD mice, with 32,885 genes in common were integrated and analyzed. The generated gene counts were normalized using ‘LogNormalize’ methods with a scale factor of 10,000. The top highly variable genes (HVGs; n = 2,000) in each tissue slide were identified using the variance stabilizing transformation (vst) method. The 2000 integration genes across all slides were then selected by ranking the genes by the number of slides in which they are variable in and their median rank of variability across the slides. For each slide, the log-normalized count matrix for the selected genes was scaled and the total RNA counts in each spot was regressed to remove the influence of the total count in the integration process. Principal component analysis (PCA) was performed for dimensionality reduction. Integration was performed for multiple spatial datasets prior to spot clustering to remove the batch effect. To flexibly integrate a large number of slides with both coronal and sagittal sections, reciprocal PCA (RPCA) was used to discover a set of anchors between the datasets, and normal (wild-type) mice were used as a reference during integration. The anchors were utilized to correct the count matrix in each spatial spot. The corrected counts were then scaled and PCA was performed. For spot clustering, a shared nearest neighbor (SNN) graph was constructed and graph-based clustering was performed using the Louvain algorithm. The resulting spot clusters were visualized using two different approaches: spatially mapped to the tissue based on spatial barcodes, or plotted in 2-dimensional space using Uniform Manifold Approximation and Projection (UMAP). The optimal resolution of the spot clusters was determined by decreasing the resolution value and visually examining the appropriate granularity of the spatial clusters that corresponded well to the anatomical structure. The anatomical location of each cluster was visually determined by comparison with the Allen Mouse Brain Reference Atlas ( https://mouse.brain-map.org/static/atlas ). As a result, the resolution was set to 0.15 for subsequent analysis. All analyses were performed using the R package, Seurat (version 4.1.1) [ 74 ].
Differential gene expression analysis
MAST (Model-based Analysis of Single-cell Transcriptomics) was used to perform differential gene expression analysis [ 96 ]. MAST accounts for the bimodal distribution of counts in the spatial transcriptomics and uses a generalized linear model with the proportion of genes expressed in each spot as a covariate to model the normalized counts. Differentially expressed genes (DEGs) were extracted from the comparison of wild-type and 5xFAD mice in each spot cluster defining the anatomical region in the brain. The cutoff for significantly different genes was false discovery rate (FDR)-adjusted p < 0.05 and log FC > 0.25.
Overrepresentation analysis
Overrepresentation analysis was performed and the Gene Ontology (GO) biological process terms associated with DEGs were identified. The count ratio was defined as the ratio of the proportion of the genes constituting GO terms among the DEGs to the proportion of genes constituting GO terms among total genes. Statistical significance was calculated based on the hypergeometric model, and correction for multiple comparisons was performed using the Benjamini-Hochberg procedure. The dot plots for the significant GO terms were drawn by showing the number of overlapping genes between the DEGs and each GO term, the count ratio, and the adjusted p-values. Overrepresentation tests were performed using clusterProfiler [ 118 ], which supports statistical analysis and visualization of functional profiles for gene sets. The packages ‘enrichplot’ and ‘igraph’ were additionally used to visualize the results.
Marker panel selection and curation
To analyze the spatial patterns of major cell types and immune cell types, the panel of marker genes was constructed and curated for each cell type. For the cell types identified in studies not using scRNAseq, individual genes were determined based on reference papers. For the cell types defined by scRNAseq, Necessary and Sufficient Forest (NSForest) version 2 [ 59 ] was applied and signature genes for the cell type were determined based on the cell type annotation information. The NSForest algorithm scores genes according to binary expression profiles in a specific cell type compared to other cell types. Then, based on the random forest algorithm, the minimum gene set that best describes the given cell type was searched. After selecting signature genes based on NSForest, the gene sets were refined to exclude the genes that are highly expressed in major cell types. This is particularly important when the scRNAseq data represent a subpopulation of the cells in the brain, such as immune cell sorted datasets. As a validation process, the spatial expression of the selected marker genes was examined and the genes were excluded if they showed a non-specific distribution pattern for the cell type. As a final step, genes that were not present in our spatial transcriptomic data were excluded. The curated gene sets are listed in Supplementary Table 7 .
Comparison of cellular signatures across groups
After curation of the marker panel, a gene set that best represents a particular cell type, the signature score of each cell type was computed on the spatial transcriptomic data by utilizing the AddModuleScore function in Seurat [ 44 ] with default parameters. For each gene in the gene set, a fixed number of control genes with the same average expression level as the gene were randomly selected. The difference between the average expression of the gene set and that of the control gene sets was calculated and named the cell signature score. The score in each spot was spatially mapped to the tissue using the SpatialFeaturePlot function in Seurat, and the spatial distribution pattern was identified. The average of the signature scores in a given region of interest was calculated and the values were compared between groups using the Wilcoxon rank-sum test. Correction for multiple comparison was performed using the Bonferroni method. The cutoff for the adjusted p-value was 0.05.
Cell type deconvolution analysis
The cell type distribution represented by the cell signature scores was compared to that derived by the cell type deconvolution method, CellDART [ 46 ]. CellDART first trains a model to extract cell type proportions from the synthetic mixture of cells generated from the reference scRNAseq dataset, and then adapts the model to predict the cell type composition of the spot, which is a mixture of multiple cells. For the major brain cell types, snRNAseq datasets from mouse brain coronal slices [ 49 ] were used as a reference for predicting spatial cell distribution. However, in the case of immune cells, the majority of scRNAseq datasets are obtained after cell sorting strategies such as fluorescence-activated cell sorting (FACS), and there is a mismatch in cell type and composition between spatial and single-cell datasets. Therefore, the cell type deconvolution tool spSeudoMap was used to compensate for this discrepancy [ 50 ]. For lymphoid and myeloid brain cell types, scRNAseq samples from CNS border immune cells were used [ 119 ], and for microglia, scRNAseq samples from brain immune cells were used [ 89 ]. The distribution of representative cell types that showed significant differences between wild-type and 5xFAD mice was evaluated: homeostatic microglia, reactive microglia, macrophages, monocytes, dendritic cells, innate lymphoid cells, natural killer cells, and T cells. The cell type annotation information from the reference single-cell dataset was used for the deconvolution analysis. Default parameter values suggested in the user manual were applied for the analysis.
Statistical analysis
For the spatial transcriptomic data, plots in R were created either with the ggplot2 R package or Seurat modified by custom codes for data visualization. All p-values reported in this study were adjusted by FDR (for DE analysis using MAST) using Benjamini-Hochberg procedure or Bonferroni method (all other analyses). The p-values below 0.05 were considered statistically significant.
Development of an application to visualize and quantify ST datasets
An R shiny-based application named STquantool was developed to comprehensively analyze ST datasets to explore cell type- and cell state-specific regional changes in wild-type, 5xFAD, and treatment mouse models. The application allows users to easily load and integrate the multiple ST datasets and visualize the spatial expression of genes and cell type scores based on Seurat [ 74 ] and shiny running on R (ver. 4.1.1). One of the key features of STquantool is that it facilitates the curation of cell type-specific marker combinations by sorting out key genes based on the NSForest [ 59 ] algorithm and finalizing the markers by visually assessing the spatial expression patterns. As an adjunct, the cell type decomposition method CellDART [ 46 ] can be implemented to find the spatial distribution patterns of major cell types constituting brain tissues. Moreover, the spatial patterns of the cell scores and cell fraction can be quantified and statistically analyzed with STquantool. Finally, the gene-level transcriptomic alterations between the mouse groups can be explored by performing the DEG analysis provided in the application. Then, the functional implications of the selected genes can be represented by gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) terms [ 120 , 121 , 122 ]. The suggested platform was packaged and can be readily installed from GitHub ( https://github.com/bsungwoo/STquantool.git ).
Data availability
All data are available in the main text or the supplementary materials. Additionally, the Visium spatial transcriptomics datasets utilized in this research are accessible on the data repository ( https://zenodo.org/records/10404412 ). The spatial transcriptomics analytical pipeline, STquantool, is available for installation from GitHub at https://github.com/bsungwoo/STquantool.git
Brioschi S, Wang WL, Peng V, Wang M, Shchukina I, Greenberg ZJ et al. Heterogeneity of meningeal B cells reveals a lymphopoietic niche at the CNS borders. Science. 2021;373.
Cugurra A, Mamuladze T, Rustenhoven J, Dykstra T, Beroshvili G, Greenberg ZJ et al. Skull and vertebral bone marrow are myeloid cell reservoirs for the meninges and CNS parenchyma. Science. 2021;373.
Cai R, Pan C, Ghasemigharagoz A, Todorov MI, Förstera B, Zhao S, et al. Panoptic imaging of transparent mice reveals whole-body neuronal projections and skull-meninges connections. Nat Neurosci. 2019;22:317–27.
Article CAS PubMed Google Scholar
Shibata-Germanos S, Goodman JR, Grieg A, Trivedi CA, Benson BC, Foti SC, et al. Structural and functional conservation of non-lumenized lymphatic endothelial cells in the mammalian leptomeninges. Acta Neuropathol. 2020;139:383–401.
Kutomi O, Takeda S. Identification of lymphatic endothelium in cranial arachnoid granulation-like dural gap. Microscopy (Oxf). 2020;69:391–400.
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–41.
Article CAS PubMed PubMed Central Google Scholar
Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212:991–9.
Bedussi B, van der Wel NN, de Vos J, van Veen H, Siebes M, VanBavel E, et al. Paravascular channels, cisterns, and the subarachnoid space in the rat brain: a single compartment with preferential pathways. J Cereb Blood Flow Metab. 2017;37:1374–85.
Article PubMed Google Scholar
Da Mesquita S, Louveau A, Vaccari A, Smirnov I, Cornelison RC, Kingsmore KM, et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature. 2018;560:185–91.
Article PubMed PubMed Central Google Scholar
Ahn JH, Cho H, Kim JH, Kim SH, Ham JS, Park I, et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature. 2019;572:62–6.
Frederick N, Louveau A. Meningeal lymphatics, immunity and neuroinflammation. Curr Opin Neurobiol. 2020;62:41–7.
Papadopoulos Z, Herz J, Kipnis J. Meningeal lymphatics: from anatomy to Central Nervous System Immune Surveillance. J Immunol. 2020;204:286–93.
Alves de Lima K, Rustenhoven J, Kipnis J. Meningeal immunity and its function in maintenance of the Central Nervous System in Health and Disease. Annu Rev Immunol. 2020;38:597–620.
Hsu M, Sandor M, Fabry Z. Current concepts on communication between the central nervous system and peripheral immunity via lymphatics: what roles do lymphatics play in brain and spinal cord disease pathogenesis? Biol Futur. 2021;72:45–60.
Solár P, Zamani A, Kubíčková L, Dubový P, Joukal M. Choroid plexus and the blood-cerebrospinal fluid barrier in disease. Fluids Barriers CNS. 2020;17:35.
Lun MP, Monuki ES, Lehtinen MK. Development and functions of the choroid plexus-cerebrospinal fluid system. Nat Rev Neurosci. 2015;16:445–57.
Damkier H, Praetorius J. Structure of the Mammalian Choroid Plexus. In: Role of the Choroid Plexus in Health and Disease Edited by Praetorius J, Blazer-Yost B, Damkier H. New York, NY: Springer US; 2020: 1–33.
Fame RM, Lehtinen MK. Emergence and developmental roles of the Cerebrospinal Fluid System. Dev Cell. 2020;52:261–75.
Dani N, Herbst RH, McCabe C, Green GS, Kaiser K, Head JP, et al. A cellular and spatial map of the choroid plexus across brain ventricles and ages. Cell. 2021;184:3056–74.
Agarwal N, Carare RO. Cerebral vessels: an overview of anatomy, physiology, and role in the drainage of fluids and solutes. Front Neurol. 2020;11:611485.
Ross JM, Kim C, Allen D, Crouch EE, Narsinh K, Cooke DL, et al. Expanding Cell Divers Brain Vasculature Front Physiol. 2020;11:600767.
Google Scholar
Kalucka J, de Rooij L, Goveia J, Rohlenova K, Dumas SJ, Meta E, et al. Single-cell transcriptome atlas of murine endothelial cells. Cell. 2020;180:764–79.
Vanlandewijck M, He L, Mäe MA, Andrae J, Ando K, Del Gaudio F, et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554:475–80.
Dixon GA, Pérez CA. Multiple sclerosis and the Choroid Plexus: emerging concepts of Disease Immunopathophysiology. Pediatr Neurol. 2020;103:65–75.
Stock AD, Der E, Gelb S, Huang M, Weidenheim K, Ben-Zvi A, et al. Tertiary lymphoid structures in the choroid plexus in neuropsychiatric lupus. JCI Insight. 2019;4:e124203.
Ma Q, Decker Y, Müller A, Ineichen BV, Proulx ST. Clearance of cerebrospinal fluid from the sacral spine through lymphatic vessels. J Exp Med. 2019;216:2492–502.
Jacob L, Boisserand L, Geraldo BS, de Brito Neto LMH. Anatomy and function of the vertebral column lymphatic network in mice. Nat Commun. 2019;10:4594.
Petrova TV, Koh GY. Biological functions of lymphatic vessels. Science. 2020;369:eaax4063.
Lee EJ, Choi Y, Park EJ, Lee DS. Lymphatic dysfunction sustains memory impairment despite Abeta reduction in an Alzheimer’s disease model. Immunity & Ageing; 2022. (in revision).
Lee EJ. Investigation of the spatial transcriptomic signatures and therapeutic mode of action in an Alzheimer’s disease model. Seoul National University (Thesis). 2022.
Huang Q, Belz GT. Parallel worlds of the adaptive and innate immune cell networks. Curr Opin Immunol. 2019;58:53–9.
Unger MS, Li E, Scharnagl L, Poupardin R, Altendorfer B, Mrowetz H, et al. CD8(+) T-cells infiltrate Alzheimer’s disease brains and regulate neuronal- and synapse-related gene expression in APP-PS1 transgenic mice. Brain Behav Immun. 2020;89:67–86.
Laurent C, Dorothée G, Hunot S, Martin E, Monnet Y, Duchamp M, et al. Hippocampal T cell infiltration promotes neuroinflammation and cognitive decline in a mouse model of tauopathy. Brain. 2017;140:184–200.
Faridar A, Thome AD, Zhao W, Thonhoff JR, Beers DR, Pascual B, et al. Restoring regulatory T-cell dysfunction in Alzheimer’s disease through ex vivo expansion. Brain Commun. 2020;2:fcaa112.
Dansokho C, Ait Ahmed D, Aid S, Toly-Ndour C, Chaigneau T, Calle V, et al. Regulatory T cells delay disease progression in Alzheimer-like pathology. Brain. 2016;139:1237–51.
Gingele S, Pul R, Sardari M, Borbor M, Henkel F, Moellenkamp TM, et al. FoxP3 deficiency causes no inflammation or neurodegeneration in the murine brain. J Neuroimmunol. 2020;342:577216.
Ito M, Komai K, Mise-Omata S, Iizuka-Koga M, Noguchi Y, Kondo T, et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature. 2019;565:246–50.
Krämer TJ, Hack N, Brühl TJ, Menzel L, Hummel R, Griemert EV, et al. Depletion of regulatory T cells increases T cell brain infiltration, reactive astrogliosis, and interferon-γ gene expression in acute experimental traumatic brain injury. J Neuroinflammation. 2019;16:163.
Fisher Y, Strominger I, Biton S, Nemirovsky A, Baron R, Monsonego A. Th1 polarization of T cells injected into the cerebrospinal fluid induces brain immunosurveillance. J Immunol. 2014;192:92–102.
Martinez B, Peplow PV. Amelioration of Alzheimer’s disease pathology and cognitive deficits by immunomodulatory agents in animal models of Alzheimer’s disease. Neural Regen Res. 2019;14:1158–76.
Mittal K, Eremenko E, Berner O, Elyahu Y, Strominger I, Apelblat D, et al. CD4 T cells induce a subset of MHCII-Expressing microglia that attenuates Alzheimer Pathology. iScience. 2019;16:298–311.
Raha-Chowdhury R, Henderson JW, Raha AA, Vuono R, Bickerton A, Jones E, et al. Choroid Plexus acts as Gatekeeper for TREM2, abnormal Accumulation of ApoE, and Fibrillary Tau in Alzheimer’s Disease and in Down Syndrome Dementia. J Alzheimers Dis. 2019;69:91–109.
Satija R, Farrell JA, Gennert D, Schier AF, Regev A. Spatial reconstruction of single-cell gene expression data. Nat Biotechnol. 2015;33:495–502.
Tirosh I, Izar B, Prakadan SM, Wadsworth MH 2nd, Treacy D, Trombetta JJ, et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science. 2016;352:189–96.
Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol. 2018;36:411–20.
Bae S, Na KJ, Koh J, Lee DS, Choi H, Kim YT. CellDART: cell type inference by domain adaptation of single-cell and spatial transcriptomic data. Nucleic Acids Res. 2022;50:e57.
Ståhl PL, Salmén F, Vickovic S, Lundmark A, Navarro JF, Magnusson J, et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science. 2016;353:78–82.
Cable DM, Murray E, Zou LS, Goeva A, Macosko EZ, Chen F, et al. Robust decomposition of cell type mixtures in spatial transcriptomics. Nat Biotechnol. 2022;40:517–26.
Kleshchevnikov V, Shmatko A, Dann E, Aivazidis A, King HW, Li T, et al. Cell2location maps fine-grained cell types in spatial transcriptomics. Nat Biotechnol. 2022;40:661–71.
Bae S, Choi H, Lee DS, spSeudoMap. Cell type mapping of spatial transcriptomics using unmatched single-cell RNA-seq data. Genome Med. 2023;15:19.
Vickovic S, Eraslan G, Salmén F, Klughammer J, Stenbeck L, Schapiro D, et al. High-definition spatial transcriptomics for in situ tissue profiling. Nat Methods. 2019;16:987–90.
Stickels RR, Murray E, Kumar P, Li J, Marshall JL, Di Bella DJ, et al. Highly sensitive spatial transcriptomics at near-cellular resolution with Slide-seqV2. Nat Biotechnol. 2021;39:313–9.
Cho CS, Xi J, Si Y, Park SR, Hsu JE, Kim M, et al. Microscopic examination of spatial transcriptome using seq-scope. Cell. 2021;184:3559–72.
Chen A, Liao S, Cheng M, Ma K, Wu L, Lai Y, et al. Spatiotemporal transcriptomic atlas of mouse organogenesis using DNA nanoball-patterned arrays. Cell. 2022;185:1777–92.
Chen A, Sun Y, Lei Y, Li C, Liao S, Liang Z, et al. Single-cell spatial transcriptome reveals cell-type organization in the macaque cortex. Cell. 2023;186:3726–43.
Wei X, Fu S, Li H, Liu Y, Wang S, Feng W, et al. Single-cell stereo-seq reveals induced progenitor cells involved in axolotl brain regeneration. Science. 2022;377:eabp9444.
Bugeon S, Duffield J, Dipoppa M, Ritoux A, Prankerd I, Nicoloutsopoulos D, et al. A transcriptomic axis predicts state modulation of cortical interneurons. Nature. 2022;607:330–8.
Zeng H, de Vries SEJ. A gene-expression axis defines neuron behaviour. Nature. 2022;607:243–4.
Aevermann B, Zhang Y, Novotny M, Keshk M, Bakken T, Miller J, et al. A machine learning method for the discovery of minimum marker gene combinations for cell type identification from single-cell RNA sequencing. Genome Res. 2021;31:1767–80.
Nelson ME, Riva SG, Cvejic A. SMaSH: a scalable, general marker gene identification framework for single-cell RNA-sequencing. BMC Bioinformatics. 2022;23:328.
Vargo AHS, Gilbert AC. A rank-based marker selection method for high throughput scRNA-seq data. BMC Bioinformatics. 2020;21:477.
Delaney C, Schnell A, Cammarata LV, Yao-Smith A, Regev A, Kuchroo VK, et al. Combinatorial prediction of marker panels from single-cell transcriptomic data. Mol Syst Biol. 2019;15:e9005.
Armingol E, Officer A, Harismendy O, Lewis NE. Deciphering cell-cell interactions and communication from gene expression. Nat Rev Genet. 2021;22:71–88.
Ximerakis M, Lipnick SL, Innes BT, Simmons SK, Adiconis X, Dionne D, et al. Single-cell transcriptomic profiling of the aging mouse brain. Nat Neurosci. 2019;22:1696–708.
Saunders A, Macosko EZ, Wysoker A, Goldman M, Krienen FM, de Rivera H, et al. Molecular diversity and specializations among the cells of the adult mouse brain. Cell. 2018;174:1015–30.
Marques S, Zeisel A, Codeluppi S, van Bruggen D, Mendanha Falcao A, Xiao L, et al. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science. 2016;352:1326–9.
Van Hove H, Martens L, Scheyltjens I, De Vlaminck K, Pombo Antunes AR, De Prijck S, et al. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat Neurosci. 2019;22:1021–35.
Schafflick D, Wolbert J, Heming M, Thomas C, Hartlehnert M, Börsch AL, et al. Single-cell profiling of CNS border compartment leukocytes reveals that B cells and their progenitors reside in non-diseased meninges. Nat Neurosci. 2021;24:1225–34.
Eraslan G, Drokhlyansky E, Anand S, Fiskin E, Subramanian A, Slyper M, et al. Single-nucleus cross-tissue molecular reference maps toward understanding disease gene function. Science. 2022;376:eabl4290.
Miragaia RJ, Gomes T, Chomka A, Jardine L, Riedel A, Hegazy AN, et al. Single-Cell Transcriptomics of Regulatory T Cells reveals trajectories of tissue adaptation. Immunity. 2019;50:493–504.
Domínguez Conde C, Xu C, Jarvis LB, Rainbow DB, Wells SB, Gomes T, et al. Cross-tissue immune cell analysis reveals tissue-specific features in humans. Science. 2022;376:eabl5197.
Kim DG, Lee JI, Lee DS, Lee MC, Choi KS, Han DH. 99mTc-HMPAO labeled leukocyte SPECT in intracranial lesions. Surg Neurol. 1995;44:338–45.
Tappan SJ, Eastwood BS, O’Connor N, Wang Q, Ng L, Feng D, et al. Automatic navigation system for the mouse brain. J Comp Neurol. 2019;527:2200–11.
Hao Y, Hao S, Andersen-Nissen E, Mauck WM 3rd, Zheng S, Butler A, et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184:3573–e35873529.
Hodge RD, Bakken TE, Miller JA, Smith KA, Barkan ER, Graybuck LT, et al. Conserved cell types with divergent features in human versus mouse cortex. Nature. 2019;573:61–8.
Werkman IL, Dubbelaar ML, van der Vlies P, de Boer-Bergsma JJ, Eggen BJL, Baron W. Transcriptional heterogeneity between primary adult grey and white matter astrocytes underlie differences in modulation of in vitro myelination. J Neuroinflammation. 2020;17:373.
A multimodal cell. Census and atlas of the mammalian primary motor cortex. Nature. 2021;598:86–102.
Article Google Scholar
Tasic B, Yao Z, Graybuck LT, Smith KA, Nguyen TN, Bertagnolli D, et al. Shared and distinct transcriptomic cell types across neocortical areas. Nature. 2018;563:72–8.
Zeng H, Sanes JR. Neuronal cell-type classification: challenges, opportunities and the path forward. Nat Rev Neurosci. 2017;18:530–46.
Tasic B, Menon V, Nguyen TN, Kim TK, Jarsky T, Yao Z, et al. Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat Neurosci. 2016;19:335–46.
Yuste R, Hawrylycz M, Aalling N, Aguilar-Valles A, Arendt D, Armañanzas R, et al. A community-based transcriptomics classification and nomenclature of neocortical cell types. Nat Neurosci. 2020;23:1456–68.
Yao Z, van Velthoven CTJ, Nguyen TN, Goldy J, Sedeno-Cortes AE, Baftizadeh F, et al. A taxonomy of transcriptomic cell types across the isocortex and hippocampal formation. Cell. 2021;184:3222–e32413226.
Bayraktar OA, Bartels T, Holmqvist S, Kleshchevnikov V, Martirosyan A, Polioudakis D, et al. Astrocyte layers in the mammalian cerebral cortex revealed by a single-cell in situ transcriptomic map. Nat Neurosci. 2020;23:500–9.
Escartin C, Galea E, Lakatos A, O’Callaghan JP, Petzold GC, Serrano-Pozo A, et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci. 2021;24:312–25.
Habib N, McCabe C, Medina S, Varshavsky M, Kitsberg D, Dvir-Szternfeld R, et al. Disease-associated astrocytes in Alzheimer’s disease and aging. Nat Neurosci. 2020;23:701–6.
Ioannou MS, Jackson J, Sheu SH, Chang CL, Weigel AV, Liu H, et al. Neuron-astrocyte metabolic coupling protects against Activity-Induced fatty acid toxicity. Cell. 2019;177:1522–35.
Chamling X, Kallman A, Fang W, Berlinicke CA, Mertz JL, Devkota P, et al. Single-cell transcriptomic reveals molecular diversity and developmental heterogeneity of human stem cell-derived oligodendrocyte lineage cells. Nat Commun. 2021;12:652.
Krasemann S, Madore C, Cialic R, Baufeld C, Calcagno N, El Fatimy R, et al. The TREM2-APOE pathway drives the Transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity. 2017;47:566–81.
Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell. 2017;169:1276–e12901217.
Marschallinger J, Iram T, Zardeneta M, Lee SE, Lehallier B, Haney MS, et al. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat Neurosci. 2020;23:194–208.
Friedman BA, Srinivasan K, Ayalon G, Meilandt WJ, Lin H, Huntley MA, et al. Diverse brain myeloid expression profiles reveal distinct microglial activation States and aspects of Alzheimer’s Disease Not Evident in Mouse models. Cell Rep. 2018;22:832–47.
Uriarte Huarte O, Richart L, Mittelbronn M, Michelucci A. Microglia in Health and Disease: the strength to be diverse and reactive. Front Cell Neurosci. 2021;15:660523.
Grubman A, Choo XY, Chew G, Ouyang JF, Sun G, Croft NP, et al. Transcriptional signature in microglia associated with Aβ plaque phagocytosis. Nat Commun. 2021;12:3015.
Pan J, Ma N, Yu B, Zhang W, Wan J. Transcriptomic profiling of microglia and astrocytes throughout aging. J Neuroinflammation. 2020;17:97.
Choi H, Lee EJ, Shin JS, Kim H, Bae S, Choi Y et al. Spatiotemporal characterization of glial cell activation in an Alzheimer’s disease model by spatially resolved transcriptomics. Exp Mol Med. 2023.
Finak G, McDavid A, Yajima M, Deng J, Gersuk V, Shalek AK, et al. MAST: a flexible statistical framework for assessing transcriptional changes and characterizing heterogeneity in single-cell RNA sequencing data. Genome Biol. 2015;16:278.
Locatelli G, Engelhardt B. Microglia get a little help from Th-eir friends. Immunity. 2020;53:484–6.
Erö C, Gewaltig MO, Keller D, Markram H. A cell atlas for the mouse brain. Front Neuroinform. 2018;12:84.
Keller D, Erö C, Markram H. Cell densities in the mouse brain: a systematic review. Front Neuroanat. 2018;12:83.
Mundt S, Mrdjen D, Utz SG, Greter M, Schreiner B, Becher B. Conventional DCs sample and present myelin antigens in the healthy CNS and allow parenchymal T cell entry to initiate neuroinflammation. Sci Immunol. 2019;4:eaau8380.
Scheurer L, Das Gupta RR, Saebisch A, Grampp T, Benke D, Zeilhofer HU et al. Expression of immunoglobulin constant domain genes in neurons of the mouse central nervous system. Life Sci Alliance. 2021;4.
Fang F, Cao W, Zhu W, Lam N, Li L, Gaddam S, et al. The cell-surface 5′-nucleotidase CD73 defines a functional T memory cell subset that declines with age. Cell Rep. 2021;37:109981.
Yang C, Siebert JR, Burns R, Gerbec ZJ, Bonacci B, Rymaszewski A, et al. Heterogeneity of human bone marrow and blood natural killer cells defined by single-cell transcriptome. Nat Commun. 2019;10:3931.
Crinier A, Milpied P, Escalière B, Piperoglou C, Galluso J, Balsamo A, et al. High-dimensional single-cell analysis identifies organ-specific signatures and conserved NK Cell subsets in humans and mice. Immunity. 2018;49:971–86.
Kim JS, Kolesnikov M, Peled-Hajaj S, Scheyltjens I, Xia Y, Trzebanski S, et al. A binary cre Transgenic Approach dissects Microglia and CNS border-Associated macrophages. Immunity. 2021;54:176–90.
Song YH, Yoon J, Lee SH. The role of neuropeptide somatostatin in the brain and its application in treating neurological disorders. Exp Mol Med. 2021;53:328–38.
Davies P, Katzman R, Terry RD. Reduced somatostatin-like immunoreactivity in cerebral cortex from cases of Alzheimer disease and Alzheimer senile dementa. Nature. 1980;288:279–80.
Jordão MJC, Sankowski R, Brendecke SM, Sagar, Locatelli G, Tai YH, et al. Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science. 2019;363:eaat7554.
Masuda T, Sankowski R, Staszewski O, Bottcher C, Amann L, Sagar, et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature. 2019;566:388–92.
Sankowski R, Böttcher C, Masuda T, Geirsdottir L, Sagar, Sindram E, et al. Mapping microglia states in the human brain through the integration of high-dimensional techniques. Nat Neurosci. 2019;22:2098–110.
Kierdorf K, Masuda T, Jordão MJC, Prinz M. Macrophages at CNS interfaces: ontogeny and function in health and disease. Nat Rev Neurosci. 2019;20:547–62.
Prinz M, Jung S, Priller J. Microglia Biology: one century of evolving concepts. Cell. 2019;179:292–311.
Masuda T, Amann L, Sankowski R, Staszewski O, Lenz M. Author correction: Novel Hexb-based tools for studying microglia in the CNS. Nat Immunol. 2020;21:1302.
Borst K, Prinz M. Deciphering the heterogeneity of myeloid cells during neuroinflammation in the single-cell era. Brain Pathol. 2020;30:1192–207.
Dogra P, Rancan C, Ma W, Toth M, Senda T, Carpenter DJ, et al. Tissue determinants of human NK Cell Development, function, and Residence. Cell. 2020;180:749–63.
Fulcher BD, Arnatkeviciute A, Fornito A. Overcoming false-positive gene-category enrichment in the analysis of spatially resolved transcriptomic brain atlas data. Nat Commun. 2021;12:2669.
Chai H, Diaz-Castro B, Shigetomi E, Monte E, Octeau JC, Yu X, et al. Neural circuit-specialized astrocytes: Transcriptomic, Proteomic, Morphological, and functional evidence. Neuron. 2017;95:531–49.
Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics. 2012;16:284–7.
Posner DA, Lee CY, Portet A, Clatworthy MR. Humoral immunity at the brain borders in homeostasis. Curr Opin Immunol. 2022;76:102188.
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene Ontology: tool for the unification of biology. Nat Genet. 2000;25:25–9.
The Gene Ontology. Resource: enriching a GOld mine. Nucleic Acids Res. 2021;49:D325–34.
Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30.
Download references
Acknowledgements
We thank Joo Young Park for his technical assistance. The work was supported by grants from the National Research Foundation of Korea (NRF-2017M3C7A1048079, NRF- 2020R1A2C2101069, NRF- 2022R1A5A6000840).
This work was supported by National Research Foundation of Korea (NRF) Grants funded by the Korean Government (MSIP) (No. 2017M3C7A1048079, No. 2020R1A2C2101069 and No. 2017R1A5A1015626).
Author information
Authors and affiliations.
Department of Nuclear Medicine, Seoul National University College of Medicine, 101 Daehak-ro, Jongno-gu, Seoul, 03080, Republic of Korea
Eun Ji Lee, Minseok Suh, Hongyoon Choi, Yoori Choi, Sungwoo Bae & Dong Soo Lee
Department of Molecular Medicine and Biopharmaceutical Sciences, Graduate School of Convergence Science and Technology, Seoul National University, Seoul, Republic of Korea
Eun Ji Lee & Dong Soo Lee
Department of Nuclear Medicine, Seoul National University Hospital, Seoul, Republic of Korea
Minseok Suh, Hongyoon Choi, Sungwoo Bae & Dong Soo Lee
Institute of Radiation Medicine, Medical Research Center, Seoul National University, 103 Daehak-ro, Jongno-gu, Seoul, 03080, Republic of Korea
Minseok Suh, Sungwoo Bae & Dong Soo Lee
Cliniclal Research Institute, Seoul National University Hospital, Seoul, Republic of Korea
Research and Development Center, THERABEST Inc., Seocho-daero 40-gil, Seoul, 06657, Republic of Korea
Do Won Hwang
Medical Science and Engineering, School of Convergence Science and Technology, POSTECH, Pohang, Republic of Korea
Dong Soo Lee
You can also search for this author in PubMed Google Scholar
Contributions
EJL: Methodology: Investigation: Visualization: Writing; SB: Methodology: Investigation: Visualization: Revision; MS: Methodology: Investigation: Visualization; HC: Methodology: Investigation: Funding acquisition; YC: Methodology: Investigation; DWH: Methodology: Investigation; DSL: Conceptualization: Methodology: Investigation: Visualization: Funding acquisition: Project administration: Supervision: Writing – original draft: Writing – review & editing.
Corresponding authors
Correspondence to Sungwoo Bae or Dong Soo Lee .
Ethics declarations
Ethics approval and consent to participate.
All experimental protocols and animal usage were approved (SNU-181018-6, SNU-190221-1-5) by the Institutional Animal Care and Use Committee (IACUC) at Seoul National University. All animals were handled in accordance with the Animal Research: Reporting of in vivo Experiments (ARRIVE) guidelines ( https://arriveguidelines.org ). Details are in Supplementary Notes.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary material 2, supplementary material 3, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Lee, E.J., Suh, M., Choi, H. et al. Spatial transcriptomic brain imaging reveals the effects of immunomodulation therapy on specific regional brain cells in a mouse dementia model. BMC Genomics 25 , 516 (2024). https://doi.org/10.1186/s12864-024-10434-8
Download citation
Received : 18 February 2024
Accepted : 20 May 2024
Published : 25 May 2024
DOI : https://doi.org/10.1186/s12864-024-10434-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Spatial transcriptomics
- Brain imaging
- Cell type decomposition
- Cell state annotation
- Major brain cells
- Rare immune cells
- Immunomodulatory therapy
BMC Genomics
ISSN: 1471-2164
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 25 May 2024
Translational Therapeutics
Death receptors 4/5 mediate tumour sensitivitNot applicably to natural killer cell-mediated cytotoxicity in mismatch repair deficient colorectal cancer
- Lin Yang ORCID: orcid.org/0000-0003-4544-8071 1 na1 ,
- Jiahong Yi 2 na1 ,
- Wenzhuo He 2 na1 ,
- Pengfei Kong 3 ,
- Qiankun Xie 1 ,
- Yanan Jin 4 ,
- Zhenchong Xiong ORCID: orcid.org/0000-0002-1129-5663 5 &
- Liangping Xia ORCID: orcid.org/0000-0001-7532-4913 2
British Journal of Cancer ( 2024 ) Cite this article
1 Altmetric
Metrics details
- Prognostic markers
- Risk factors
Identifying the target of natural killer (NK) cells in colorectal cancer (CRC) is critical for optimising the clinical use of NK cell-mediated immunotherapy. Mismatch repair deficiency (dMMR) is associated with high immune cell infiltration and MHC Class I defects. Whether dMMR CRC responses to NK cell therapy remains unclear.
MLH1, DR4, and DR5 knockout cell lines were established using CRISPR-Cas9 system. NK92-MI or NK cell isolated from BABL/C mice were used as effector cells against tumour cells. Inflammatory cytokines secretion by CRC cells was assessed via cytokine analysis. NK-cell-deficient/proficient animal models were used to validate the NK cell sensitivity.
We observed that dMMR CRC cells were more sensitive to NK cell-mediated cytotoxicity than were mismatch-repair-proficient (pMMR) CRC cells. In dMMR CRC, Death receptor (DR)4/5 was upregulated and mediated sensitivity to NK cell-mediated cytotoxicity. DR4/5-mediated secretion of interleukin -12 sustained NK cell viability in dMMR CRC. NK cell depletion induced dMMR CRC tumour growth, and NK cell transfer inhibited lung metastasis of dMMR CRC with DR4/5 expression in vivo. TP53 upregulated DR4/DR5 expression in dMMR CRC.
Conclusions
dMMR associated with increased sensitivity to NK cell-mediated cytotoxicity in CRC. DR4/DR5 sensitise dMMR CRC to NK cell-mediated cytotoxicity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
251,40 € per year
only 10,48 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Data availability
The datasets supporting the conclusions of this article were deposited in the Research Data Deposit system of Sun Yat-sen University Cancer (RDDB2024581025) and can be obtained from the corresponding authors on reasonable request.
Cozar B, Greppi M, Carpentier S, Narni-Mancinelli E, Chiossone L, Vivier E. Tumor-infiltrating natural killer cells. Cancer Discov. 2021;11:34–44.
Article CAS PubMed Google Scholar
Myers JA, Miller JS. Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol. 2021;18:85–100.
Article PubMed Google Scholar
Iliopoulou EG, Kountourakis P, Karamouzis MV, Doufexis D, Ardavanis A, Baxevanis CN, et al. A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer. Cancer Immunol Immunother. 2010;59:1781–9.
Article PubMed PubMed Central Google Scholar
Berrien-Elliott MM, Becker-Hapak M, Cashen AF, Jacobs M, Wong P, Foster M, et al. Systemic IL-15 promotes allogeneic cell rejection in patients treated with natural killer cell adoptive therapy. Blood. 2022;139:1177–83.
Article CAS PubMed PubMed Central Google Scholar
Federico SM, McCarville MB, Shulkin BL, Sondel PM, Hank JA, Hutson P, et al. A pilot trial of humanized Anti-GD2 Monoclonal Antibody (hu14.18K322A) with chemotherapy and natural killer cells in children with recurrent/refractory neuroblastoma. Clin Cancer Res. 2017;23:6441–9.
Jobin G, Rodriguez-Suarez R, Betito K. Association between natural killer cell activity and colorectal cancer in high-risk subjects undergoing colonoscopy. Gastroenterology. 2017;153:980–7.
Ishikawa T, Okayama T, Sakamoto N, Ideno M, Oka K, Enoki T, et al. Phase I clinical trial of adoptive transfer of expanded natural killer cells in combination with IgG1 antibody in patients with gastric or colorectal cancer. Int J Cancer. 2018;142:2599–609.
Li L, Li W, Wang C, Yan X, Wang Y, Niu C, et al. Adoptive transfer of natural killer cells in combination with chemotherapy improves outcomes of patients with locally advanced colon carcinoma. Cytotherapy. 2018;20:134–48.
Shimasaki N, Jain A, Campana D. NK cells for cancer immunotherapy. Nat Rev Drug Discov. 2020;19:200–18.
Prager I, Liesche C, van Ooijen H, Urlaub D, Verron Q, Sandström N, et al. NK cells switch from granzyme B to death receptor-mediated cytotoxicity during serial killing. J Exp Med. 2019;216:2113–27.
Wagner J, Kline CL, Zhou L, Campbell KS, MacFarlane AW, Olszanski AJ, et al. Dose intensification of TRAIL-inducing ONC201 inhibits metastasis and promotes intratumoral NK cell recruitment. J Clin Investig. 2018;128:2325–38.
Deng D, Shah K. TRAIL of hope meeting resistance in cancer. Trends Cancer. 2020;6:989–1001.
Sullivan GP, O’Connor H, Henry CM, Davidovich P, Clancy DM, Albert ML, et al. TRAIL receptors serve as stress-associated molecular patterns to promote ER-stress-induced inflammation. Dev Cell. 2020;52:714–30.e5.
Hallett WH, Ames E, Motarjemi M, Barao I, Shanker A, Tamang DL, et al. Sensitization of tumor cells to NK cell-mediated killing by proteasome inhibition. J Immunol. 2008;180:163–70.
Jin Z, Sinicrope FA. Mismatch repair-deficient colorectal cancer: building on checkpoint blockade. J Clin Oncol. 2022;40:2735–50.
Lanuza PM, Alonso MH, Hidalgo S, Uranga-Murillo I, Garcia-Mulero S, Arnau R, et al. Adoptive NK cell transfer as a treatment in colorectal cancer patients: analyses of tumour cell determinants correlating with efficacy in vitro and in vivo. Front Immunol. 2022;13:890836.
Liu SS, Yang YZ, Jiang C, Quan Q, Xie QK, Wang XP, et al. Comparison of immunological characteristics between paired mismatch repair-proficient and -deficient colorectal cancer patients. J Transl Med. 2018;16:195.
Boyer JC, Umar A, Risinger JI, Lipford JR, Kane M, Yin S, et al. Microsatellite instability, mismatch repair deficiency, and genetic defects in human cancer cell lines. Cancer Res. 1995;55:6063–70.
CAS PubMed Google Scholar
Romanski A, Uherek C, Bug G, Seifried E, Klingemann H, Wels WS, et al. CD19-CAR engineered NK-92 cells are sufficient to overcome NK cell resistance in B-cell malignancies. J Cell Mol Med. 2016;20:1287–94.
Chiorean EG, Dylla SJ, Olsen K, Lenvik T, Soignier Y, Miller JS. BCR/ABL alters the function of NK cells and the acquisition of killer immunoglobulin-like receptors (KIRs). Blood. 2003;101:3527–33.
Campbell AR, Duggan MC, Suarez-Kelly LP, Bhave N, Opheim KS, McMichael EL, et al. MICA-expressing monocytes enhance natural killer cell Fc receptor-mediated antitumor functions. Cancer Immunol Res. 2017;5:778–89.
Shemesh A, Pickering H, Roybal KT, Lanier LL. Differential IL-12 signaling induces human natural killer cell activating receptor-mediated ligand-specific expansion. J Exp Med. 2022;219:e20212434.
Kishi C, Amano H, Suzue K, Ishikawa O. Plasmodium berghei infection ameliorates atopic dermatitis-like skin lesions in NC/Nga mice. Allergy. 2014;69:1412–9.
Qian Q, Chowdhury BP, Sun Z, Lenberg J, Alam R, Vivier E, et al. Maternal diesel particle exposure promotes offspring asthma through NK cell-derived granzyme B. J Clin Investig. 2020;130:4133–51.
CAS PubMed PubMed Central Google Scholar
Xiong Z, Li X, Yang L, Wu L, Xie Y, Xu F, et al. Integrative analysis of gene expression and DNA methylation depicting the impact of obesity on breast cancer. Front Cell Dev Biol. 2022;10:818082.
Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q, et al. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020;48:W509–14.
Barbie DA, Tamayo P, Boehm JS, Kim SY, Moody SE, Dunn IF, et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature. 2009;462:108–12.
Seo H, Jeon I, Kim BS, Park M, Bae EA, Song B, et al. IL-21-mediated reversal of NK cell exhaustion facilitates anti-tumour immunity in MHC class I-deficient tumours. Nat Commun. 2017;8:15776.
Germano G, Lamba S, Rospo G, Barault L, Magri A, Maione F, et al. Inactivation of DNA repair triggers neoantigen generation and impairs tumour growth. Nature. 2017;552:116–20.
Cardoso Alves L, Corazza N, Micheau O, Krebs P. The multifaceted role of TRAIL signaling in cancer and immunity. FEBS J. 2021;288:5530–54.
Willis JA, Reyes-Uribe L, Chang K, Lipkin SM, Vilar E. Immune activation in mismatch repair-deficient carcinogenesis: more than just mutational rate. Clin Cancer Res. 2020;26:11–7.
Garris CS, Arlauckas SP, Kohler RH, Trefny MP, Garren S, Piot C, et al. Successful Anti-PD-1 cancer immunotherapy requires T cell-dendritic cell crosstalk involving the cytokines IFN-gamma and IL-12. Immunity. 2018;49:1148–61.e7.
Pasello G, Urso L, Silic-Benussi M, Schiavon M, Cavallari I, Marulli G, et al. Synergistic antitumor activity of recombinant human Apo2L/tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in combination with carboplatin and pemetrexed in malignant pleural mesothelioma. J Thorac Oncol. 2014;9:1008–17.
Bykov VJN, Eriksson SE, Bianchi J, Wiman KG. Targeting mutant p53 for efficient cancer therapy. Nat Rev Cancer. 2018;18:89–102.
Castro-Mondragon JA, Riudavets-Puig R, Rauluseviciute I, Lemma RB, Turchi L, Blanc-Mathieu R, et al. JASPAR 2022: the 9th release of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2022;50:D165–73.
Zhang L, Zhao Y, Dai Y, Cheng JN, Gong Z, Feng Y, et al. Immune landscape of colorectal cancer tumor microenvironment from different primary tumor location. Front Immunol. 2018;9:1578.
Kim GR, Ha GH, Bae JH, Oh SO, Kim SH, Kang CD. Metastatic colon cancer cell populations contain more cancer stem-like cells with a higher susceptibility to natural killer cell-mediated lysis compared with primary colon cancer cells. Oncol Lett. 2015;9:1641–6.
Song X, Hong SH, Kwon WT, Bailey LM, Basse P, Bartlett DL, et al. Secretory TRAIL-armed natural killer cell-based therapy: in vitro and in vivo colorectal peritoneal carcinomatosis xenograft. Mol Cancer Ther. 2016;15:1591–601.
Bagli DJ, Steele GD Jr, Barlozzari T. Natural killer sensitivity of colorectal carcinoma targets. Correlation with degree of differentiation. Arch Surg. 1989;124:89–93.
Cui C, Wang J, Fagerberg E, Chen PM, Connolly KA, Damo M, et al. Neoantigen-driven B cell and CD4 T follicular helper cell collaboration promotes anti-tumor CD8 T cell responses. Cell. 2021;184:6101–18.e13.
Andre T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. 2020;383:2207–18.
Chan IS, Ewald AJ. The changing role of natural killer cells in cancer metastasis. J Clin Investig. 2022;132:e143762.
Ashkenazi A. Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nat Rev Drug Discov. 2008;7:1001–12.
Rowinsky EK. Curtailing the high rate of late-stage attrition of investigational therapeutics against unprecedented targets in patients with lung and other malignancies. Clin Cancer Res. 2004;10:4220s–6s.
Ciurea SO, Schafer JR, Bassett R, Denman CJ, Cao K, Willis D, et al. Phase 1 clinical trial using mbIL21 ex vivo-expanded donor-derived NK cells after haploidentical transplantation. Blood. 2017;130:1857–68.
Barkholt L, Alici E, Conrad R, Sutlu T, Gilljam M, Stellan B, et al. Safety analysis of ex vivo-expanded NK and NK-like T cells administered to cancer patients: a phase I clinical study. Immunotherapy. 2009;1:753–64.
Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–13.
Gebert J, Gelincik O, Oezcan-Wahlbrink M, Marshall JD, Hernandez-Sanchez A, Urban K, et al. Recurrent frameshift neoantigen vaccine elicits protective immunity with reduced tumor burden and improved overall survival in a lynch syndrome mouse model. Gastroenterology. 2021;161:1288–302.e13.
MacNabb BW, Tumuluru S, Chen X, Godfrey J, Kasal DN, Yu J, et al. Dendritic cells can prime anti-tumor CD8(+) T cell responses through major histocompatibility complex cross-dressing. Immunity. 2022;55:982–97.e8.
Cohen R, Bennouna J, Meurisse A, Tournigand C, De La Fouchardière C, Tougeron D, et al. RECIST and iRECIST criteria for the evaluation of nivolumab plus ipilimumab in patients with microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: the GERCOR NIPICOL phase II study. J Immunother Cancer. 2020;8:e001499.
Download references
Acknowledgements
We would like to thank the professor Xiaojun Xia (State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, China) for the enormous contribution to our manuscript.
This study was supported by the National Natural Science Foundation of China (No. 82002557 to LY; No. 82202850 to ZCX) and the Basic and applied research of science and technology in Guangzhou (2023A04J2392).
Author information
These authors contributed equally: Lin Yang, Jiahong Yi, Wenzhuo He.
Authors and Affiliations
Department of Radiation Oncology, Nanfang Hospital, Southern Medical University, Guangzhou, Guangdong, 510515, China
Lin Yang & Qiankun Xie
Department of Medical Oncology, State Key Laboratory of Oncology in South China, Guangdong Provincial Clinical Research Center for Cancer, Sun Yat-sen University Cancer Center, Guangzhou, 510060, China
Jiahong Yi, Wenzhuo He & Liangping Xia
Department of Gastrointestinal, Fudan University Shanghai Cancer Center, Shang Hai, China
Pengfei Kong
The Cancer Center of the Fifth Affiliated Hospital of Sun Yat-Sen University, ZhuHai, China
Department of Breast Oncology, State Key Laboratory of Oncology in South China, Guangdong Provincial Clinical Research Center for Cancer, Sun Yat-sen University Cancer Center, Guangzhou, 510060, China
Zhenchong Xiong
You can also search for this author in PubMed Google Scholar
Contributions
LY, conceived and designed the experiments; JHY, ZCX designed the experiments; ZCX wrote the manuscript; LY, PFK, and QKX performed the experiments; QKX, WZH and YNJ analyzed the data; PFK and QKX helped in interpretation of the results; ZCX and LPX revised the manuscript based on the comments of reviewers. All authors approved the final version.
Corresponding authors
Correspondence to Zhenchong Xiong or Liangping Xia .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Ethics approval and consent to participate
Ethical approval was obtained from the respective institutional review boards of the Ethics Committee of Sun Yat-sen University Cancer Center and the patients provided signed informed consent to participate in this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplemental material, rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Yang, L., Yi, J., He, W. et al. Death receptors 4/5 mediate tumour sensitivitNot applicably to natural killer cell-mediated cytotoxicity in mismatch repair deficient colorectal cancer. Br J Cancer (2024). https://doi.org/10.1038/s41416-024-02673-z
Download citation
Received : 01 August 2023
Revised : 24 March 2024
Accepted : 26 March 2024
Published : 25 May 2024
DOI : https://doi.org/10.1038/s41416-024-02673-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Open access
- Published: 22 May 2024
Decoding Behcet’s Uveitis: an In-depth review of pathogenesis and therapeutic advances
- Yuxuan Guan 1 , 2 ,
- Fuzhen Li 1 ,
- Na Li 1 &
- Peizeng Yang 1
Journal of Neuroinflammation volume 21 , Article number: 133 ( 2024 ) Cite this article
185 Accesses
Metrics details
Behcet’s disease (BD) is a rare but globally distributed vasculitis that primarily affects populations in the Mediterranean and Asian regions. Behcet’s uveitis (BU) is a common manifestation of BD, occurring in over two-thirds of the patients. BU is characterized by bilateral, chronic, recurrent, non-granulomatous uveitis in association with complications such as retinal ischemia and atrophy, optic atrophy, macular ischemia, macular edema, and further neovascular complications (vitreous hemorrhage, neovascular glaucoma). Although the etiology and pathogenesis of BU remain unclear, numerous studies reveal that genetic factors (such as HLA-B51 ), dysregulated immune responses of both the innate and adaptive immune systems, infections (such as streptococcus), and environmental factors (such as GDP) are all involved in its development. Innate immunity, including hyperactivity of neutrophils and γδT cells and elevated NK1/NK2 ratios, has been shown to play an essential role in this disease. Adaptive immune system disturbance, including homeostatic perturbations, Th1, Th17 overaction, and Treg cell dysfunction, is thought to be involved in BU pathogenesis. Treatment of BU requires a tailored approach based on the location, severity of inflammation, and systemic manifestations. The therapy aims to achieve rapid inflammation suppression, preservation of vision, and prevention of recurrence. Systemic corticosteroids combined with other immunosuppressive agents have been widely used to treat BU, and beneficial effects are observed in most patients. Recently, biologics have been shown to be effective in treating refractory BU cases. Novel therapeutic targets for treating BU include the LCK gene, Th17/Treg balance, JAK pathway inhibition, and cytokines such as IL-17 and RORγt. This article summarizes the recent studies on BU, especially in terms of pathogenesis, diagnostic criteria and classification, auxiliary examination, and treatment options. A better understanding of the significance of microbiome composition, genetic basis, and persistent immune mechanisms, as well as advancements in identifying new biomarkers and implementing objective quantitative detection of BU, may greatly contribute to improving the adequate management of BU patients.
Introduction
Behcet’s disease (BD), also known as Behcet’s syndrome [ 1 ], is a rare chronic recurrent vasculitis with unclear etiology and pathogenesis. Up to date, BD is considered a heterogeneous disease with close association with genetics (e.g., HLA-B51 ), immunity (innate and adaptive immunity), infections (e.g., streptococcus), and the environment (e.g., GDP) [ 2 , 3 , 4 , 5 , 6 ]. More than 60% of BD patients have eye lesions, which can be the primary or only manifestation of BD. The most common eye lesion is uveitis, typically manifesting as recurrent bilateral non-granulomatous uveitis. Behcet’s uveitis (BU) represents an immune-mediated intraocular inflammatory disorder with potential risk of blinding [ 7 , 8 ]. The unique complications of BU are of high concerns, such as retinal ischemia and atrophy, optic atrophy, macular ischemia, macular edema, and further neovascular complications (vitreous hemorrhage, neovascular glaucoma). These are common complications that lead to permanent visual loss. It can develop alone or with systemic manifestations. Although modern immunosuppressive agents have improved BU prognosis, approximately 20.4% of eyes become blind due to recurrent episodes [ 8 , 9 ].
In the therapeutic landscape of BU, the enduring pillars of glucocorticoids and immunosuppressants have been recently complemented by the emergence of biologics, providing new, promising management for this disease. The impetus for these advancements has been catalyzed by fast-paced strides in the fields of genetics, immunology, and technology, thus driving significant breakthroughs in both experimental and clinical research in BU.
Herein, this review article focuses on recent advances in understanding the immunologic etiology and therapeutic advances that contribute to the pathogenesis of BU. In addition to this, some insights are provided on how to improve the diagnosis and management of BU in practice.
BD has its earliest recorded description in the third book of endemic diseases by Hippocrates. He indicated that the Mediterranean region and Asian populations were most affected by BD, which is how BD earned the name old Silk Road disease [ 10 ]. The disease was first recognized by Hulusi Behçet in 1937, and it is characterized by a triad of recurrent clinical symptoms: oral ulcers, genital ulcers, and ocular lesions [ 11 , 12 ]. BD can be diagnosed when oral ulcers are present and at least two of the following criteria are met: distinctive ocular lesions, typical skin lesions, recurrent genital ulcers, or a positive skin pathology test [ 13 , 14 ]. BD symptoms can be erratic, with symptomatic or remission periods lasting months, years, or decades. This condition has been given various names (Table 1 ).
Diagnostic and classification criteria
In clinical practice, there are no specific diagnostic tests or histological features that can definitively identify BD. Current diagnostic criteria rely on clinical symptoms and imaging findings.
The International Study Group for Behcet’s Disease (ISG), established in 1990, is widely used as the first truly international standard with high specificity. Recurrent oral ulcers plus 2 other criteria, including recurrent genital ulcers, eye lesions, skin lesions, and positive pathological tests, are sufficient for diagnosis [ 14 ]. Recurrent oral ulcers are necessary for diagnosis, but the oral manifestations of patients in the early stages of the disease are not completely consistent. Moreover, the differences in the prevalence of diseases in different regions were not considered in the formulation of the criteria, so some regions with higher prevalence were ignored. In contrast, the International Criteria for Behcet’s Disease (ICBD), developed in 2014, incorporates neurological and vascular manifestations, improving diagnostic sensitivity but reducing specificity [ 15 ]. Although useful for diagnostic guidance, all criteria ignore the baseline probability of disease in patients and may be more beneficial for differential diagnosis in non-endemic areas. The Standardization of Uveitis Nomenclature (SUN) working group proposed in 2021 provides a unique framework for the identification of BU and other non-infectious uveitis. In particular, this classification standard includes focal retinal infiltration in the definition of ocular lesions, which improves diagnostic accuracy and is suitable for clinical and translational studies [ 16 ]. It is limited by specificity and may inadvertently exclude cases with atypical manifestations or overlapping with other uveitis entities. Therefore, clinicians should exercise caution when evaluating suspected BU patients and consider the broader clinical context. In addition, Tugal-Tutkun et al. ‘s algorithm offers a promising way to diagnose adult BU based solely on ocular manifestations, providing a solution to clinicians’ bedside challenges [ 17 ].
The absence of universally acknowledged scoring criteria for BD poses a challenge. However, the Ocular Behcet Disease Research Group of Japan introduced the Behcet’s disease ocular attack score 24 (BOS24) scoring system in 2014, which can evaluate the clinical inflammatory activity.
BOS24 serves as a comprehensive measure for assessing ocular inflammation. This scoring system encompasses six distinct parameters, all of which are evaluated on a per-ocular episode basis. The parameters encompass various aspects related to the eye, such as abnormalities in the vitreous, lesions located at the subfoveal area, lesions found in the peripheral region of the fundus, presence of cells in the anterior chamber, lesions affecting the posterior pole, and lesions affecting the optic disc. This particular system of classification divides the retinal field into two main areas: the posterior pole and the peripheral retina. The peripheral retina is then further divided into quadrants for a more detailed analysis and understanding. This innovative system incorporates a set of specific parameters that are carefully chosen to ensure consistency and accuracy in quantifying the severity of ocular inflammation. Through the implementation of these parameters, the BOS24 establishes a standardized approach to assess the level of ocular inflammation in BD patients [ 18 ].
Notably, BOS24 incorporates the grading scales developed by the SUN working group for scoring anterior chamber cells, and follows the system proposed by Nussenblatt et al. for evaluating vitreous opacity [ 19 , 20 ].
An important attribute of BOS24 is its reliance solely on objective ocular findings per episode, explicitly excluding patient-reported symptoms or subjective examination outcomes such as visual acuity. Moreover, the scoring system focuses specifically on new inflammatory manifestations, excluding chronic inflammatory signs. This clear-cut delineation ensures that BOS24 accurately captures the acute inflammatory burden in each episode of BU.
In addition to diagnostic criteria, the differential diagnosis of BU is equally important (Table 2 ).
Epidemiology
BU exhibits a distinct geographical distribution, primarily observed along the ancient Silk Road, extending across the vast expanse from East Asia to the Mediterranean region [ 21 ]. Turkey records the highest incidence rate, with an estimated 420 cases per 100,000 individuals [ 22 ]. Noteworthy prevalence is also observed in Iran, Korea, Japan, Greece, Israel, and Saudi Arabia [ 15 ]. Although the incidence in North America and Europe is lower, cases have been identified on all continents [ 23 ]. This distribution pattern suggests a rational association with genetic factors, potentially linked to the HLA-B51 gene [ 24 ].
BD was the leading cause of uveitis in a multicenter registry in Turkey [ 25 ]. Subsequent studies carried out by various international institutions have consistently reaffirmed these findings and underscored the prevalent occurrence of uveitis as a manifestation of BD [ 26 , 27 , 28 , 29 ]. This ocular complication not only damages the patient’s overall well-being, but also poses a significant risk of permanent vision impairment.
Notably, there are complex connections that can be observed between BU and other BD symptoms. We can see a clear positive correlation between BU and both arthralgia and parenchymal neurological involvement. On the other hand, there is an opposite association when it comes to genital ulcers, gastrointestinal symptoms, and other systemic symptoms [ 30 , 31 , 32 , 33 ]. These complex interactions highlight the need for further investigation to better understand the multifaceted pathogenesis of BD.
Demographically, BU does not exhibit a specific age limitation, but it most frequently emerges in individuals between 25 and 44 years [ 34 ]. Pediatric presentations may deviate from the typical and display a more aggressive disease course [ 35 ]. The elderly may have milder symptoms, but they are susceptible to complications related to treatment due to existing comorbidities. Consequently, medications should be administered with caution in this population [ 36 ]. The interplay between BU and pregnancy remains enigmatic, necessitating cautious therapeutic considerations to protect fetal health [ 37 ].
Clinically, BU typically exhibits as a chronic, recurrent, bilateral non-granulomatous uveitis. The inflammation primarily affects either the anterior (11.1%) or posterior (28.8%) segment of the eye, although panuveitis, which involves inflammation in both segments concurrently, occurs more frequently (60.2%) [ 11 , 26 , 38 , 39 ].
BD exhibits a significant predominance in males, with notable differences in prognosis based on gender. Specifically, male patients with BU experience a more rapid decline in visual prognosis, as indicated by a substantially 5-year and 10-year risk of losing useful visual acuity [ 8 , 38 ]. These differences tied to gender may be attributed to the influence of testosterone on the regulation of neutrophils and T helper 1 cells (Th1), potentially shedding light on the increased morbidity observed in male BD patients [ 40 ].
Clinical features and complications
BU is recurrent and presents as anterior uveitis, posterior uveitis or panuveitis (Table 3 ). It usually involves the whole eye, with bilateral involvement in 4 out of 5 patients. Men predominate and are at higher risk of losing useful vision than women. Although rare, isolated cases of anterior uveitis are predominantly reported among females [ 9 , 38 ]. Smooth layered shifting hypopyon, diffuse vitritis, transient superficial retinal infiltrates, full-thickness retinal infiltrates, diffuse gliotic sheathing of retinal veins, peripheral occlusive periphlebitis, retinal hemorrhages, and fluorescein angiography revealing diffuse retinal capillary leakage, retinal capillary nonperfusion, and optic disc hyperfluorescence/leakage, are suggestive of BU [ 17 , 41 ]. One of the concerning aspects of BU is its potential to cause irreversible vision loss, as well as damage to other organs, and even death. Younger males tend to have the poorest prognosis in these cases.
Auxiliary examinations
The diagnostic and therapeutic options for BU are enhanced by a wide range of retina imaging techniques that effectively outline its pathophysiological changes. Contemporary ophthalmological evaluations significantly rely on imaging methods such as color photography, B-scan ultrasonography, fundus fluorescein angiography, laser flare-cell photometry, and optical coherence tomography (OCT). These technologies introduce fast and accurate diagnostic possibilities, supported by the use of multiple imaging techniques [ 42 , 43 ].
This suite of diagnostic tools ranges from classical color photography, enabling vitreous opacity and retinal infiltration documentation, to the technologically advanced OCT, illuminating macular afflictions and nuanced retinal layer alterations [ 44 ]. Furthermore, projection-resolved optical coherence tomography angiography (PR-OCTA) has illuminated the existence of macular circulatory anomalies in both eyes, irrespective of the BU is unilateral or bilateral [ 45 ]. Despite its ability to provide a detailed examination of retinal circulation, OCTA is limited in its ability to identify vascular leaks, which are typically detected through invasive methods that require the use of dyes [ 46 ].
The major component of BU is retinal inflammation, rather than choroidal inflammation. Indocyanine green angiography (ICGA) offers insights into choroidal inflammation and aids in distinguishing BU with predominant retinal affliction from conditions primarily impacting the choroid [ 47 ].
Fundus fluorescein angiography (FFA) provides valuable insights into both vascular and extravascular retinal that may not be apparent through fundus microscopy. These include vascular leakage, cystoid macular edema, and retinal vascular occlusion, among others [ 8 ]. FFA can detect fundus changes caused by posterior uveitis and panuveitis, determine the site and size of the lesion, and dynamically observe and evaluate the treatment effect. FFA is an indispensable tool in diagnosing and monitoring BU. FFA is particularly useful in identifying diffuse retinal capillary leakage, which presents as a fern-like pattern, indicating suboptimal response to therapy even during asymptomatic periods [ 42 ]. FFA is the gold standard for detecting and monitoring the leakage and occlusion of retinal vasculitis in BU patients [ 42 , 48 , 49 , 50 ]. FFA findings also have prognostic significance. In active BU patients, disc neovascularization, macular window defect and macular ischemia indicate poor visual prognosis [ 49 ]. It not only provides important information on the vascular and optic disc leaks, but also provides crucial clues for clinical judgment. For example, FFA can distinguish the underlying cause (ischemia or pure inflammation) in the presence of neovascularization of optic disc or whether abnormal vessel clumps are shunt vessels or neovascularization in the scenario of retinal vascular occlusions. Although FFA has limitations, such as its invasive nature, potential allergic reactions, and a lack of quantitative measurements. It remains the golden standard among multimodal imaging in BU.
Laser flare-cell photometry (LFCM) has emerged as an effective and non-invasive tool for quantitatively evaluating intraocular inflammation in BU. It allows for precise detection and measurement of cells and proteins in the front part of the eye. Compared to traditional slit-lamp examinations, LFCM offers greater objectivity and accuracy, especially in identifying moderate to severe inflammation in the front part of the eye, which is a characteristic feature of BU [ 8 ]. Furthermore, the utilization of LFCM proves to be advantageous when it comes to the surveillance of continuous retinal vascular leakage in patients who are experiencing clinical remission. The reason for this correlation lies in the fact that the degree of flare observed through LFCM analysis is directly linked to the level of fluorescein angiographic leakage. Consequently, this connection reduces the necessity for frequent invasive procedures such as FFA [ 51 ].
Among them, fundus photography, FFA, and OCT continue to serve as the primary imaging modalities in BU.
Furthermore, thorough examination is being conducted on potential biomarkers such as HLA-B51 、tumor necrosis factor-α (TNF-α), microRNAs, and specific sweat metabolites such as l-citrulline [ 52 , 53 ]. In the case of untreated active BD, an increased risk of uveitis has been significantly associated with elevated serum IgA levels and antibodies against cardiolipin, β2-glycoprotein I, and prothrombin [ 54 ]. Although various autoantibodies and biomarkers associated with BU have been identified, their clinical significance remains to be further validated.
Etiology and pathogenesis
Genetic factors.
The genetic composition of BD, situated within its epidemiological framework, deviates from the majority of systemic diseases. Interestingly, its worldwide presence intersects with the historical Silk Road. Present discussions on genetics firmly establish the significance of host genetic factors in determining susceptibility to BD (Table 4 ).
Although HLA-B51 is not currently used as a diagnostic marker, it plays a significant role as a genetic contributor in BU. HLA-B51 is positively correlated with ocular lesions but negatively correlated with gastrointestinal lesions [ 4 , 55 ]. The precise reasoning behind the association of HLA-B51 with BU is still a subject of scholarly discussion, particularly considering linkage disequilibrium. However, it is important to acknowledge that HLA-B51 contributes to only a small portion (less than 20%) of the genetic risk, suggesting that there may be other genetic factors yet to be identified [ 56 ].
The endoplasmic reticulum aminopeptidase 1 (ERAP1) is pivotal in modulating peptide configurations within the endoplasmic reticulum (ER), thereby influencing the peptides presented by human leukocyte antigen (HLA) class I molecules. Certain ERAP1 variants have shown a significant association with BD, especially in the context of HLA class I. A noteworthy revelation was the linkage of the ERAP1 haplotype, Hap10, with BD. Remarkably, individuals carrying Hap10 and being homozygous for HLA-B51 demonstrated an approximate elevenfold surge in disease susceptibility. Though Hap10’s strong linkage with BD is evident, its low prevalence ensures its limited influence on global risk. However, the combined impact of HLA-B51 and Hap10 insinuates a profound genetic mechanism underpinning BD susceptibility. Recent studies utilizing genome-wide association studies (GWAS) and related functional annotations have brought attention to various susceptibility loci, such as HLA-B51, HLA-A26, HLA-C0704 [ 5 , 57 ], CCR1 [ 58 ], CCR1-CCR3, ERAP1, KLRC4, STAT4 [ 59 , 60 ], FUT2 [ 61 ], IL12A [ 62 , 63 ], IL10, IL23R-IL12RB2 [ 24 , 64 , 65 , 66 ], TRAF5, TRAF3IP2 [ 67 ], ADO-EGR2, CEBPB-PTPN1, IL1A-IL1B, IRF8, LACC1, RIPK2 [ 68 , 69 ], PTPN2 [ 70 ], STAT3 [ 71 ], IL23R [ 72 , 73 ], miR-146a [ 74 ], and miR-182 [ 75 ] (Table 5 ). However, the genetic aspects of BU are not yet fully understood. Despite GWAS identifying HLA-B51 and other non-leukocyte antigen risk factors, a comprehensive genetic understanding of BU is still lacking, necessitating further investigation.
Innate immune system
Neutrophils hyperactivity.
The majority of leukocytes, known as neutrophils, serve as the foremost defense against infections. Although they perform a vital function in innate immunity, there is a potential for unintended harm to tissues, particularly in the presence of inflammation. This damage primarily occurs through phagocytosis, degranulation, and the release of neutrophil extracellular traps (NETs). Inflammation often leads to an increase in the number and lifespan of neutrophils [ 76 , 77 ]. In the context of BD, there is a significant increase in neutrophil activity. This heightened activity may be associated with the HLA-B51 gene and elevated levels of interleukin-17 (IL-17). The activated neutrophils have a propensity to aggregate in close proximity to blood vessels, whereby they release reactive oxygen species (ROS) and proteases. This process eventually culminates in the impairment of the vascular endothelium [ 2 , 11 , 78 , 79 ]. The neutrophil-lymphocyte ratio (NLR) has been identified as a reliable biomarker to assess the extent of inflammation in BD and to evaluate disease severity [ 80 , 81 ]. Further studies on BD patients have shown an enhancement in the oxidative burst and NADPH oxidase activities of neutrophils, resulting in increased production of ROS [ 82 , 83 ]. Neutrophils in these patients also release various components such as NETs, DNA, extracellular reticulated DNA structures, histones, and myeloperoxidase (MPO) [ 84 ]. Notably, the histones from NETs play a dual role. They activate Th17 cells through intermediary cells like monocytes, and they directly induce STAT3 phosphorylation in T cells. This process results in the secretion of substances like IL-6, transforming growth factor-beta (TGF-β), and retinoid acid-related orphan receptor gamma t (RORγt). These substances further promote IL-17 production and the differentiation of Th17 cells, thereby amplifying NET formation (Fig. 1 ) [ 83 , 85 , 86 , 87 ]. Additionally, elevated levels of NETs have been linked to increased differentiation of Th1 cells, specifically IFN-γ-producing CD4 + T cells. The subsequent increase in histone H4 and oxidized DNA within Th1 cells appears to trigger macrophage activation, resulting in enhanced production of IL-8 [ 88 ].
BU immunopathogenesis: Current understanding
Hyperactivity of γδT cells
During the past 2 decades, there was a notable increase observed in the number of γδT cells in peripheral blood mononuclear cells (PBMCs) of BD patients. Normally, these γδT cells make up a small portion of the total T cells, ranging from 0.5 to 5%. It is worth mentioning that γδT cells possess characteristics of both innate and adaptive immunity, undergoing maturation through interactions with dendritic cells and pattern recognition receptors. They express molecules such as the inducible co-stimulator (ICOS) and CD40, and secrete various cytokines including IL-2, IL-4, IL-10, IL-17, IFN-γ, TNF-α, and granzyme (Fig. 1 ). These secreted molecules play a role in Th1/Th2 responses, link innate and adaptive immunity, and participate in autoimmune diseases.
Of particular interest, when it comes to oral pathogens, γδT cells may recognize these microbes through Heat Shock Proteins (HSP) and their T cell receptors. When neutrophils engulf these pathogens, γδT cells detect the resulting pathogenic compounds (such as (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate) and release chemokines, notably CXCL8 (IL-8). The subsequent recruitment of neutrophils and monocytes, along with the induction of Th1 and Th17 responses, may contribute to the persistent inflammation observed in BD patients [ 89 , 90 , 91 , 92 ].
Elevated NK1/NK2 ratios
Natural killer (NK) cells, acting as cytotoxic lymphocytes in the innate immune system, exhibit their functionality independent of the constraints imposed by the major histocompatibility complex (MHC). Their primary function revolves around immunosurveillance while also exhibiting the ability to produce a diverse range of cytokines, including IFN-γ, IL-5, IL-13, GM-CSF, CCL3, and CCL4. In BD patients, abnormalities in the functionality of NK cells have been attributed to the presence of cytokines such as IL-10 and IL-15 [ 93 , 94 , 95 ].
What is particularly intriguing is that NK cells can be classified into two distinct types based on the cytokines they produce: NK1 and NK2 (Fig. 1 ). NK1 cells are primarily responsible to produce IFN-γ, whereas NK2 cells exhibit a broader role in immune modulation, producing cytokines such as IL-5 and IL-13. In active cases of BD, there is a prevalence of NK1 cells, whereas during periods of disease remission, NK2 cells dominate. This ratio between NK1 and NK2 cells provides valuable insights into the activity of the disease, as a higher NK1/NK2 ratio correlates with polarization towards a Th1 immune response and an increase in BD activity [ 96 , 97 , 98 ].
Dendritic cells: gatekeepers of Immune Response
Dendritic cells (DCs) are important antigen-presenting cells (APCs) that play a critical role in activating naïve T lymphocytes and contributing to both cellular and humoral immune responses. These cells play a crucial role in maintaining the balance of the immune system by connecting the innate and adaptive immunity. In normal physiological conditions, DCs can be found in various ocular tissues, including the central and limbal epithelia, basal lamina, and sub-basal nerve plexus layer [ 99 , 100 , 101 ]. However, the precise mechanism behind the inhibition of DC maturation in these conditions requires further investigation. DCs possess a distinctive capability in comparison to other APCs, as they can effectively initiate the activation of naïve T cells and facilitate their differentiation into Th1 and Th17 cells throughout the progression of a disease (Fig. 1 ). When exposed to chemokines or cytokines, immature DCs undergo maturation and migrate to lymph nodes, leading to increased expression of costimulatory molecules and MHC class II molecules [ 101 , 102 , 103 , 104 , 105 ]. The immature state of DCs is associated with the maintenance of immune tolerance. BU patients showed elevated expression of MHC class II and costimulatory molecules in the maturation profiles of peripheral blood DCs, even during periods of non-inflammatory activity. This finding suggests that the transition from an immature to a mature DC state may contribute to the chronic inflammation and relapse observed in BU. Moreover, BD patients exhibited a lower number of plasma cell-like DCs compared to healthy individuals, indicating that these cells may contribute to inflammation by migrating to target organs [ 106 , 107 ]. During inflammation, DCs secrete IL-6, which influences the biological function of DCs and facilitates their activation [ 108 ]. The expression of programmed death ligand-1 (PDL1) and its transcription factor interferon regulatory factor I (IRF1) in DCs from active BU patients has been found to be decreased in recent studies. This decrease in expression is observed to correlate with the level of disease activity [ 109 ]. Confocal imaging studies consistently demonstrate an increased density of DCs in the corneas of BU patients, regardless of disease severity [ 110 ].
Adaptive immune system
Role of t cells.
T cells, central to adaptive immunity, have increasingly been a focus in the study of BU pathogenesis (Fig. 1 ). This increased interest can be traced back to the discovery in 2000 of clonally aggregated T cells in the anterior chamber of BD patients [ 111 , 112 ]. The importance of T cell-mediated immune imbalances in BU has been underscored by recent transcriptome analyses conducted on iris samples. Specifically, the involvement of the T cell receptor signaling pathway and the prominence of helper T cell differentiation pathways highlight this connection. The lymphocyte-specific protein tyrosine kinase (LCK), which plays a critical role in T cell functions, has been identified as a key player in BU. The activated LCK signaling pathway and elevated active LCK expressions observed in BU indicate the potential of the LCK gene for therapeutic developments in BU treatment [ 113 ].
The increased expression of Th1/Th17-associated cytokines has led to the activation of the JAK/STAT signaling pathway, which has been observed in monocytes and CD4 + T cells [ 114 , 115 , 116 ]. The upregulation of gene expression leads to the activation of CD4 + T cells, resulting in their transformation into Th17 cells. This process is influenced by the release of inflammatory cytokines from monocytes. Subsequently, Th17 cells attract neutrophils and intensify the inflammatory reaction. Within the scope of BD, the signaling of serum amyloid-A is recognized as a pivotal element in guiding the differentiation of Th17 cells [ 117 , 118 ]. RNA-seq studies of CD8 + T cells from BD patients have emphasized the importance of the cAMP-mediated signaling pathway in T cell activation. Interestingly, sustained elevation in cAMP levels tends to have an immunosuppressive effect [ 119 , 120 ].
It has been shown that the affected regions are primarily infiltrated by CD8 + T cells. When comparing BU patients to those with other uveitis subtypes such as idiopathic recurrent acute anterior uveitis and Vogt-Koyanagi-Harada syndrome, it is observed that the aqueous humor of BU patients contains a higher concentration of CD8 + T cells, whereas CD4 + T cells dominate in the other subtypes. Conversely, skin samples from BD patients typically exhibit a higher presence of CD4 + T cells, along with fewer CD8 + T and CD56 + cells. This indicates a distinct intraocular immunomodulatory environment in BU that is characterized by a more aggressive inflammatory response. Furthermore, during active BU phases, CD8 bright CD56 + T cells secrete cytotoxic molecules such as dissolved protein perforin and surface FasL. These cells not only possess conventional CD8 + CTL cytolytic functions but also demonstrate NK-like cytotoxic activities. Another notable feature of BU is the significant increase in NKT cells in both the aqueous humor and peripheral blood. The primary subtype of NKT cells, CD8 + CD56 + cells, have the capability to exert strong cytotoxic effects, which can lead to the lysis of vascular endothelial cells through FasL- and perforin-dependent mechanisms, posing serious risks to vision. In contrast, patients with type 1 diabetes, a standard immune-mediated inflammatory disease, do not exhibit these characteristics. This emphasizes the uniqueness of CD8 + CD56 + cells as immune effectors, which may play a crucial role in the visual impairment observed in BU [ 121 , 122 ]. The severe clinical manifestations of BU, in comparison to other types of uveitis, may be attributed to this factor.
The Th1/Th2 balance responses holds significant importance in the development of BU, with the Th1 response exerting a particularly strong influence. Th2 cells exhibit the capability to secrete cytokines with anti-inflammatory properties, namely IL-4, IL-5, IL-10, and IL-13. On the other hand, Th1 cells are distinguished by their ability to generate pro-inflammatory agents such as IL-2, IL-12, interferon, and tumor necrosis factor. It is noteworthy that BD patients typically exhibit elevated levels of Th1-related cytokines in their bloodstream [ 123 , 124 ].
In the aqueous humor of BU patients, a notable increase in the concentrations of multiple cytokines is observed, which encompass IL-1ra, IL-2, IL-6, IL-8, IP-10, IFN-γ, and TNF-α. Conversely, the levels of GM-CSF are diminished. These cytokine levels correspond to the presence of inflammatory cells, particularly monocytes and neutrophils, emphasizing the potential role of the innate immune system in the development of BU. Interestingly, BU patients exhibit elevated concentrations of IL-15 in their aqueous humor, a characteristic not observed in individuals with other uveitis types such as human leukocyte antigen B27-associated uveitis, Vogt-Koyanagi-Harada syndrome, juvenile idiopathic arthritis, and idiopathic uveitis. IL-15, which possesses immunomodulatory properties, enhances the proliferation and activation of specific immune cells like NK cells, NKT cells, and CD8 + T cells. The prominence of CD8 + CD56 + NKT cells in BU suggests their potentially detrimental role in the progression of the disease. Furthermore, active BU patients demonstrate a lack of the anti-inflammatory cytokine, IL-10, highlighting the unique immune characteristics of BU. The distinct presence of pro-inflammatory cytokines like IFN-γ and TNF-α in BU, compared to other forms of uveitis, suggests the potential for novel therapeutic strategies. By identifying the specific immune players and cytokines involved in the pathogenesis of BU, scientific advancements may lead to tailored treatments that alleviate symptoms and address the underlying cause of this immune disorder [ 125 , 126 , 127 , 128 ].
The significant presence of Th1 and Th17 cells in BD patients highlights the critical involvement of the adaptive immune system in both the onset and advancement of the disease. Transcriptomic studies have revealed an active NF-κB pathway in peripheral Th17 cells. Additionally, analysis techniques such as WGCNA and pathway enrichment have highlighted the activation of APCs in BD [ 11 , 129 ]. The expression of IL-27, both at the mRNA and protein levels, is found to be reduced in the PBMCs and serum of active BD patients. IL-27 is known for its ability to suppress Th1 and Th17 cellular responses by inhibiting the expression of certain pro-inflammatory cytokines, including IL-1β, IL-6, and IL-23. Recent findings suggest that IL-27 can inhibit the differentiation of Th17 cells through the IRF-8 pathway [ 104 ], indicating that increasing IL-27 levels may help alleviate the inflammatory responses observed in BD patients.
Another crucial pathway involved in the development of immune-mediated disorders, including BU, is the IL-23/IL-17 axis [ 130 , 131 ]. Increased levels of IL-23 prompt the transformation of naïve T cells into pathogenic Th17 cells. These cells then release pro-inflammatory cytokines like IL-17 A, IL-17 F, and IL-22, with the help of the intracellular JAK/STAT signaling cascade. Additionally, IL-23 contributes to the ongoing inflammatory response by upregulating its receptor, IL-23R [ 96 , 132 ].
With regards to another cytokine, IL-33, a member of the IL-1 family, interacts with the ST2 receptor, resulting in the activation of MAP kinase and NF-κB. This interaction induces the production of pro-inflammatory cytokines, facilitates the differentiation of Th1 and Th17 cells, and is associated with the dysfunction of regulatory T cells (Tregs) [ 133 , 134 ]. BD patients in the active phase display heightened levels of IL-33 and its soluble receptor ST2 (sST2). An intriguing observation is the correlation between ST2 levels and inflammatory markers such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) in BD patients. A potential reduction in serum ST2 levels has been observed following treatment with colchicine [ 135 ]. Furthermore, the investigation of single nucleotide polymorphisms within the IL-33 gene has revealed a correlation between the variants rs7044343 and rs2210463 and the occurrence of BU [ 136 , 137 ].
Role of B cells
B cells are only a small part of the immune cells in BD, but the function of regulatory B cells (Bregs) is increasingly recognized. The primary function of Bregs is to produce anti-inflammatory cytokines, which are crucial for the proper functioning of regulatory T cells. By inhibiting T cell differentiation and suppressing autoimmune reactions, Bregs play a vital role in maintaining immune homeostasis. A noteworthy observation in BD patients is the substantial decrease in IL-10 mRNA levels within B cells. This finding opens up possibilities for developing novel therapeutic approaches for uveitis [ 138 ].
A notable characteristic of BD is the pronounced depletion of B cells, particularly Bregs. This depletion is primarily associated with a decline in CD27 + memory B cells expressing different immunoglobulin subsets, most notably IgM, IgG, and IgA, with a specific focus on CD27 + IgA + B cells. It is speculated that these cells may migrate from the bloodstream to sites of inflammation. Considering their correlation with disease activity, these cells hold promise as potential biomarkers [ 139 ]. Furthermore, Breg counts have been found to be correlated with the severity of BD and ESR values. Interestingly, there appears to be a positive relationship between the number of Bregs and the dosage of corticosteroids administered to patients. However, recent studies suggest that the overall count of B cells and the number of Bregs remain consistent among BD patients, regardless of whether they exhibit symptoms of BU [ 140 ]. This indicates that further investigation is required to fully understand the precise role and impact of B cells in the pathogenesis of BU.
Microbiological factors
Although there is no direct evidence linking BD to microbial infections such as viruses or bacteria, studies suggest that infectious pathogens may play a role in triggering the immune response associated with BD [ 2 , 141 ]. Notably, studies have found an increased presence of Th17 cells in the peripheral blood of BD patients. It is hypothesized that alterations in bacterial composition and metabolism contribute to immune system disruptions, particularly in the balance between Th17 and Treg cells [ 142 , 143 , 144 , 145 ].
BD patients have shown a decrease in fecal concentrations of both Barnesiellaceae and Lachnospira, indicating a shift in gut microbial composition that may be connected to immune irregularities [ 146 ]. In an interesting study conducted by Shimizu et al. in 2018, fecal samples from 13 BD patients and 27 healthy individuals were analyzed. The findings revealed a significant increase in the relative abundance of Eggerthella lenta, along with six other bacterial species, in BD patients. The authors suggested that these gut microbes in BD patients could potentially induce immune anomalies by influencing nucleic acid and fatty acid synthesis, as indicated by the results of PICRUSt functional annotation analysis [ 147 ].
Stool samples from active BD patients have shown a decreased presence of bacteria that produce butyrate [ 148 ]. In another intriguing study, gut microbes from BD patients were transplanted into mice, resulting in weakened intestinal barrier strength and a reduction in three short-chain fatty acids (SCFAs) - butyric acid, propionic acid, and valeric acid. These SCFAs are known to stimulate Treg cells in the intestines and feces. Single-cell sequencing performed on these mice revealed evidence of activated neutrophils promoting the differentiation of Th1 and Th17 cells in specific lymph nodes and spleen cells [ 149 , 150 ].
Other factors to consider in relation to BD include a history of tuberculosis (TB) infection and certain genetic predispositions associated with susceptibility to TB, which have been recognized as potential contributors to the onset of BD [ 151 ]. Additionally, elevated levels of antibodies against specific heat shock protein epitopes from Mycobacteria and Streptococci have been observed in BD patients. It is interesting to note that human heat shock proteins exhibit similarities to these epitopes, potentially leading to cross-reactive immune responses and subsequent autoimmune reactions [ 152 , 153 ]. In another study, specific streptococcal strains were isolated from BD patients with extraocular myopathy [ 154 ].
A fascinating study conducted in mainland China investigated the relationship between air quality and the occurrence of uveitis. It was found that there was a strong association between exposure to particulate matters less than 2.5 μm (PM2.5) and the development of non-infectious uveitis and uveitis associated with systemic diseases, particularly in males aged 20 to 50. Interestingly, this association appeared to weaken over time, possibly due to increased biological adaptation or the implementation of individual protective measures [ 155 ].
Further investigation discovered that the positive association between increased PM2.5 levels and the occurrence of BU was exclusively observed in areas where the Per capita gross domestic product (GDP) exceeded the national average [ 156 ]. Japanese reports showing decreased incidence and severity of BD over decades, presumably associated with improved socioeconomic conditions [ 157 , 158 ]. Also, a recent report from Turkey comparing BU patients with other noninfectious uveitis showed that BU patients were from GDP regions and had lower income [ 159 ]. Previous studies have emphasized the connections between economic development and the prevalence of immune and inflammatory diseases [ 160 , 161 ]. Notably, economic growth itself showed an inverse relationship with the incidence of uveitis, particularly in male patients aged 20–50 years and markedly so in cases of BU [ 162 ]. The underlying reasons for this are not yet fully understood but could be associated with enhancements in mental and overall health stemming from a rise in GDP.
It raises contemplation that regions with a per capita GDP surpassing the national average might indeed experience a spike in uveitis incidence. A prevailing hypothesis attributes this to the concurrent rise in exposure to PM2.5 [ 156 ]. The integration of these findings has significant ramifications, providing insights that could aid the formulation of preventive measures and treatment strategies for uveitis in nations undergoing swift economic progression, especially those in the developing world.
Furthermore, the association between vitamin D and BU is gradually recognized. In individuals of the Chinese Han demographic, the DHCR7 gene, which is associated with the vitamin D pathway, has emerged as a potential genetic predisposition for BD [ 163 ]. Recent research has emphasized the protective role of 1,25-dihydroxy vitamin D3 against BD. Interestingly, Vitamin D3 directly inhibits the differentiation of Th17 cells through the IRF-8 pathway [ 164 ]. A comprehensive study using Mendelian randomization, which included Chinese and Turkish samples with a total of 7,909 participants, demonstrated a correlation between elevated levels of 25-hydroxyvitamin D and an increased risk for BD. This suggests that caution should be exercised by clinicians when considering prolonged or high-dose vitamin D supplementation [ 6 ].
To summarize this section, an important characteristic of BD pathogenesis is the dysregulation of immune responses and the abnormal release of cytokines (Table 6 ). The available data provides substantial evidence to suggest that bacterial factors might have a substantial impact on the initiation of BD, thereby emphasizing the complex interaction between genetic predispositions and environmental factors in the advancement of the disease. These findings underscore the necessity for comprehensive risk assessment strategies in clinical settings to identify individuals with an elevated risk for BD. It is essential to further investigate this area to unravel the complex biological processes underlying these associations. This knowledge could potentially lead to tailored interventions for susceptible populations.
Advances in therapies
Recent developments in therapeutic approaches for uveitis have highlighted the importance of a collaborative effort among ophthalmologists, rheumatologists, and internists, as emphasized by the 2018 European League Against Rheumatism (EULAR) guidelines. The primary goal is to effectively manage uveitis by reducing recurrent episodes and controlling inflammation. Timely intervention is crucial in cases of BU, as complications such as retinal ischemia and atrophy, optic atrophy, macular ischemia, macular edema, and further neovascular complications (vitreous hemorrhage, neovascular glaucoma) can lead to severe visual impairment or even blindness, and severely impacts the quality of life. Various factors, including medication adjustments, disruptions in circadian rhythm, fluctuations in emotions, and excessive consumption of tobacco and alcohol, have been associated with the recurrence of BU [ 165 , 166 , 167 , 168 , 169 ].
To avoid unnecessary complications, it is essential to closely monitor the outcomes of treatment and potential side effects during the management of BU. The choice of treatment strategies depends on the site of inflammation (e.g., anterior, posterior, or pan-uveitis), its severity, and underlying systemic conditions. The primary goals of therapy involve promptly suppressing inflammation, minimizing leakage of FFA, preserving vision, and preventing recurrence [ 170 ]. This section provides an overview of the latest advancements in pharmacological options for the treatment of BU (Table 7 ).
Conventional therapies
Glucocorticoids (gcs).
have a pivotal role in the management of BU. In milder cases of BU with isolated uveitis and no systemic manifestations, oral GCs are suitable. For isolated anterior uveitis, topical GCs such as dexamethasone or betamethasone, along with ciliary muscle-relaxing agents, are beneficial. In cases where immediate inflammation reduction is needed at affected sites, pars plana or retrobulbar GC injections can be used. However, severe cases, particularly in younger males with early-onset disease, may experience anterior uveitis progressing to posterior forms, requiring systemic immunosuppression. These cases may need relatively high doses of systemic GCs to quickly control inflammation, followed by tapering to maintenance doses, ideally combined with immunosuppressants like azathioprine for posterior uveitis management. However, we found that inflammation was usually effectively controlled with relatively low doses of GCs in Chinese patients [ 1 , 8 , 171 ]. Intravenous high-dose methylprednisolone (IVPM) can improve visual clarity, reduce ocular inflammation, and prevent recurrences, often being more cost-effective than biologics [ 172 ]. In the severe cases, drugs like cyclosporine A or TNF-α inhibitors may be necessary, with interferon-alpha as an alternative for those who cannot tolerate TNF-α treatments. Topical steroid administration can be enhanced by the use of an intravitreal dexamethasone implant (Ozurdex), either alone or in combination with other treatments [ 173 ]. Another promising option for uveitis treatment is the intravitreal fludrocortisone implant (Iluvien, 0.19 mg). However, caution should be exercised when using topical steroids in individuals with glaucoma [ 174 ]. Local depot steroid injections should be avoided in patients with glaucoma or with a tendency to develop ocular hypertension with any steroid treatment.
Careful monitoring is essential, especially for growth effects in young patients, since long-term steroid use produces systemic side effects, including infections, hypertension, osteoporosis, and peptic ulcers. A collaborative approach involving ophthalmologists, rheumatologists, and internists is necessary for comprehensive patient evaluations, weighing the pros and cons of different therapies, and ensuring patient compliance.
Immunosuppressants
Medications have emerged as reliable and cost-effective therapeutic options for the treatment of BU by suppressing the proliferation and function of immune cells. However, their tolerability and effectiveness have limitations, despite often being co-administered with steroids. Among these medications, antimetabolites such as azathioprine and methotrexate, as well as T-cell inhibitors like cyclosporine A, are currently used in BU for systemic immunosuppression with the goals of preserving vision and preventing recurrence [ 175 , 176 ].
Azathioprine (AZA)
has been found to be effective in slowing down the progression of BU and reducing complications related to oral and genital ulcers and arthritis at a dose of 2.5 mg/kg/day [ 175 ]. A study involving 157 BU patients suffering from active posterior uveitis or panuveitis showed that a combination of corticosteroids (0.5 to 1 mg/kg/day) and AZA (2.5 mg/kg/day) led to total or partial remission in 93% of the patients, while also improving visual acuity. This allowed for a lower average dose of oral prednisone, which can lessen the risk of steroid side effects. In general, AZA has less severe side effects and is well tolerated in most patients, making it a reliable and efficient BU treatment. Its efficacy correlates with the severity of retinal vasculitis or vision loss and is enhanced with early administration [ 177 ]. It is also considered compatible for use in adolescent BU populations, often in combination with long-term steroid therapy [ 174 ].
Cyclosporine A (CsA)
is one of the most effective immunosuppressants for treating refractory BU and oral ulcers, skin lesions, and genital ulcers with long-term stable efficacy [ 171 , 178 , 179 ]. The daily dose is usually 3 to 5 mg/kg. Administration of 5 mg/kg/day CsA to active BU patients may significantly improve their vision within six months [ 8 , 176 , 180 ]. However, the application of CsA is restricted by its side effects, which include nephrotoxicity, elevated blood pressure, increased levels of liver enzymes, and gastrointestinal issues. Also, treatment of CsA increases the probability of parenchymal nerve involvement and elevates ALT/AST in BU patients, and the risk is greater when used alone than in combination with other drugs [ 181 ]. Other side effects of CsA are elevated uric acid, hyperlipidemia, and hypertension [ 179 ]. Therefore, careful dose adjustments tailored to individual patients are necessary.
Methotrexate (MTX)
is recognized as the least toxic immunosuppressive agent utilized in the management of posterior uveitis. A research study was conducted to assess the effects of a treatment regimen comprising prednisolone (0.5 mg/kg/day) and MTX (7.5 to 15 mg/week) on BU patients. Notable improvements were observed across posterior uveitis (PU), visual acuity (VA), and retinal vasculitis (RV), with PU displaying the most significant effectiveness. The total adjusted disease activity index (TADAI) diminished in 80% of the subjects [ 182 ].
Chlorambucil
, administered at a daily dose of 2 to 6 mg, has demonstrated potential in reducing BU relapses, controlling ocular inflammation, and improving systemic symptoms. Its benefits can persist even after discontinuation of the drug, and some patients may be able to reduce or stop steroid therapy. A retrospective study found that most BU patients responded to chlorambucil, and its use early in the disease resulted in better visual preservation. However, its dose-related side effects, like malignancy and myelosuppression, severely limit its application. Other side effects include nephrotoxicity, gastrointestinal reactions, leukopenia, infections, and temporary amenorrhea in women [ 183 , 184 ]. One study reported that a short-term high-dose (mean duration: 23 weeks; mean total dose: 2.2 g) chlorambucil treatment was safer than a long-term application, with guaranteed efficacy. During the follow-up period, no malignancies were discovered [ 185 ].
In conclusion, while immunosuppressive agents offer a promising way to the management of BU, a cautious and individualized approach that balances efficacy and potential side effects is crucial. Collaboration among specialists is essential to tailor treatment plans to the specific needs of each patient.
Medications are monoclonal antibodies produced through genetic engineering that can rapidly improve disease and should be administered on the clinical characteristics of the patients [ 186 ].
TNF-alpha antagonists
The introduction of TNF-alpha (TNF-α) antagonists has brought about a significant change in the treatment of BU, leading to new therapeutic possibilities and a deeper understanding of the disease’s pathogenesis. Over the last two decades, these inhibitors have become the primary approach in managing severe uveitis manifestations in BD. Interestingly, BU patients tend to respond better to anti-TNF-α agents compared to those with idiopathic uveitis [ 187 , 188 ].
In a groundbreaking development in 2017, adalimumab received approvals from both the US Food and Drug Administration (FDA) and the European Medicines Evaluation Agency (EMEA) for treating non-infectious uveitis. This approval was supported by numerous clinical studies consistently demonstrating the safety and effectiveness of TNF-α monoclonal antibodies in the long-term treatment of BU [ 189 , 190 , 191 , 192 ]. Furthermore, adalimumab and infliximab have comparable efficacy in the treatment of refractory BU. This remains true whether they are used as standalone treatments or in combination with other therapeutic agents like azathioprine (AZA) and methotrexate (MTX). These findings highlight the exceptional potential of anti-TNF-α agents in the treatment of BU [ 192 , 193 ].
Adalimumab (ADA)
, is a recombinant IgG1 monoclonal antibody that is specially designed to target TNF-α. It is a completely humanized antibody that exhibits a strong binding affinity for p55 and p75 TNF receptors. Through its binding to these receptors, ADA effectively suppresses the activity of both the membrane-bound and soluble forms of TNF-α [ 194 ]. This inhibitory action is crucial in the treatment of non-infectious intermediate, posterior, or panuveitis, especially in cases where conventional therapeutic strategies have proven to be ineffective.
To initiate treatment, a subcutaneous dose of 80 mg is usually administered, followed by a maintenance dose of 40 mg every other week. Clinical outcomes have shown positive results with ADA therapy. Notable improvements include a significant reduction in intraocular inflammation, an enhancement in best-corrected visual acuity (BCVA), and a decrease in macular thickness as measured by OCT. Additionally, recurrence rates have been observed to decrease, indicating the safety and efficacy of ADA in the management of BU. The efficacy of ADA is not limited to patients who are newly introduced to the drug. Even patients who have failed primary anti-TNF-α treatments have experienced benefits when switched to ADA or other alternative anti-TNF-α agents [ 193 , 195 ]. It is important to note that both ADA and infliximab can be used for the long-term treatment of BU, as their efficacy does not change even when used concurrently with DMARDs (Disease-Modifying Antirheumatic Drugs). ADA has also demonstrated efficacy in severe refractory BU patients, even in the presence of adverse prognostic indicators [ 196 , 197 ]. While ADA is generally well-tolerated, some patients may experience localized reactions at the injection site [ 198 ]. Recent research highlights the potential of combining ADA with conventional therapies, particularly in the treatment of refractory BU-induced retinal vasculitis (RV). These combination treatments have shown superior outcomes compared to traditional therapies alone. Although patients on ADA often achieve stable long-term results, there may be a slightly increased risk of adverse events. Therefore, an individualized and flexible approach is recommended when administering ADA to ensure optimal outcomes [ 199 , 200 ].
Infliximab (IFX)
is a chimeric monoclonal antibody made up of both human and mouse components. It is engineered to have a high binding affinity for both soluble and membrane-bound forms of TNF-α. In 2001, Sfikakis et al. pioneered the use of a single infusion of IFX (5 mg/kg) to treat five patients with recurrent BU, all experiencing rapid and sustained remission with no notable side effects during the observation period [ 201 ]. Subsequent clinical trials have consistently emphasized the potential effectiveness of IFX in the management of patients with refractory BU. It has been identified as a primary treatment for refractory retinitis caused by BD. The administration of IFX requires careful optimization, especially for patients who have achieved remission. For those who experience a relapse, the recommended treatment regimen involves the continuation of intravenous IFX at a dose of 5 mg/kg every eight weeks [ 202 ]. Infusion intervals are shortened in patients who experience relapse during IFX treatment and higher doses (more than 5 mg/kg) can be administered as well. A notable feature of IFX is its rapid therapeutic effect. Just a single infusion at a dose of 5 mg/kg has been observed to almost resolve all ocular manifestations of BU entirely within 28 days. This encompasses the resolution of retinal vasculitis, the disappearance of persistent symptoms such as macular cystoid edema, and significant improvements in visual acuity. The overall recurrence rate also drops substantially. While some patients do experience relapses, administering IFX again post-relapse has shown to be effective [ 203 , 204 ]. The efficacy of IFX as a monotherapy might be slightly inferior compared to when it’s combined with CsA. Interestingly, after discontinuation of IFX, approximately 40% of patients maintained remission of their ocular inflammation for up to three years. This suggests that IFX offers a prolonged therapeutic effect, and discontinuation might be feasible for patients who demonstrate stable inflammatory control over an extended period [ 205 ]. A long-term (decade-long) clinical study further attested to the efficacy of IFX in managing BU. Patients showed significant visual function improvements and had a reduced incidence of ocular complications, such as glaucoma, during their follow-up. IFX also exhibited potential in managing overall BD symptoms, beyond just the ocular manifestations [ 206 ]. For patients where Interferon-alpha (IFN-α) therapy proves ineffective, IFX emerges as a viable alternative [ 207 ]. An important observation was that patients with uveitis symptoms for less than 18 months derived more benefits from IFX treatment. This underscores the potential advantages of initiating IFX therapy early in the disease course [ 208 , 209 ]. However, caution is warranted when considering discontinuation of IFX. Even in patients who achieved long-term remission, extraocular manifestations, such as recurrent oral ulcers, were prevalent a year post-IFX discontinuation [ 210 ]. While IFX is generally well-tolerated, mild infusion reactions are common adverse events. However, clinicians should be wary of the potential for more serious complications, including severe infections (like reactivation of latent TB) and malignancies [ 198 , 202 ].
A comparative analysis between IFX and ADA in the treatment of refractory BU has demonstrated the effectiveness of both drugs. Both IFX and ADA have shown positive therapeutic effects, but a multicenter study with one-year follow-up showed that ADA had better results in terms of improvement in anterior chamber inflammation, improvement in vitritis, and BCVA [ 198 ].
Golimumab (GOL) , a recent addition to the anti-TNF armamentarium, stands out due to its lower likelihood of inducing neutralizing antibodies compared to IFX and ADA [ 211 ]. Five refractory BU cases (8 eyes) treated with standard doses of GOL (50 mg every four weeks) were followed up for 12 months, and 7/8 (87.5%) eyes were found to have complete control of intraocular inflammation, demonstrating that GOL treatment significantly reduces the number of BU recurrences and rapidly regresses active retinal vasculitis (RV) [ 212 ]. GOL also demonstrated significant reductions in macular center thickness, vitreous opacity grading, and anterior chamber cell grading. The results of these studies suggest that GOL has the potential to become a mainstay for the treatment of refractory BU, especially in patients who have not received prior TNF therapy. Mild adverse effects included elevated liver enzymes, fatigue, and a rash [ 213 ].
Certolizumab pegol (CZP)
has a therapeutic effect on refractory BU that is outside the drug indications. There was a significant reduction in relapses after initiating GOL or CZP, with no discernible difference in the two drugs’ efficacy or survival. When other anti-TNF-α drug treatments are ineffective, GOL and CZP are alternative treatment options that can significantly reduce the frequency of relapses and preserve visual function [ 214 ]. CZP distinguishes itself from other anti-TNF-α drugs by lacking an Fc region, which interacts with the neonatal Fc receptor (FnRn). This structural difference results in a lower rate of placental transfer, making CZP a safer therapeutic option during pregnancy [ 215 ]. Studies have shown that CZP effectively reduces intraocular inflammation and preserves vision during gestation without causing harm to the newborn [ 216 ].
There is growing interest in the localized management of uveitis through intravitreal injections of anti-TNF agents. Early investigations suggest that this mode of administration allows for the rapid attainment of therapeutic drug levels in the eye. Furthermore, intravitreal injections of both IFX and ADA have demonstrated a favorable safety profile, exhibiting neither toxicity nor immunogenicity. Despite these advantages, the short duration of action of intravitreal IFX necessitates repeated injections. As BD is a systemic condition, a comprehensive assessment of the efficacy and safety of intraocular versus systemic medications is needed. It is possible that future treatments may incorporate both local and systemic anti-TNF medications [ 194 ].
BU patients who have achieved remission with repeated anti-TNF therapy may have their dose gradually reduced or their injection interval extended. TNF-α inhibitors can reduce the oral dose of GC, a phenomenon known as the steroid-sparing effect [ 193 ]. The steroid-sparing effect is an additional benefit of anti-TNF therapy. Patients who achieve remission with repeated anti-TNF doses may be able to reduce the dosage or extend the dosing interval. By effectively reducing inflammation, TNF-α inhibitors can decrease or eliminate the need for corticosteroids, thereby reducing the risk of steroid-related side effects. This potential to decrease BU recurrence compared to traditional treatments offers hope for preventing irreversible vision loss [ 217 ]. However, anti-TNF therapy is not without its risks. Systemic inhibition of TNF can lead to severe infections, including the activation of TB or reactivation of hepatitis B virus [ 218 ]. There are other potential side effects associated with the use of TNF-α, including exacerbation of heart failure, neuro-demyelinating lesions and dyslipidemia [ 219 , 220 ]. Therefore, it is crucial for clinicians to use TNF drugs judiciously and regularly monitor their patients. The mechanism by which TNF-α functions likely involves the activation of macrophages, interactions with T cells, and T-cell-driven B cell responses.
Interferon-alpha
Interferon serves as a powerful immunomodulatory agent that has significantly changed the therapeutic landscape for BU [ 221 , 222 ]. Its efficacy may be related to the decrease in dysfunctional Treg cells, Th17 cells, CD4 + T lymphocytes, and increase in IL-10 [ 223 , 224 , 225 ]. In recent guidelines, the EULAR advocates using high-dose corticosteroids, infliximab, or IFN-α for BU patients presenting with severe ocular manifestations. Based on treatment response following conventional therapy, Eser-Ozturk et al. split 25 BU patients receiving IFN-α into three groups: non-responsive group, complete remission group, and partial remission group. IFN-α was delivered at a dose of 6 million units (MU) daily over one week, then 3 MU per day. After clinical remission, IFN-α 3 MU was administered as a maintenance dose every other day. Assessing BCVA, central macular thickness (CMT), and FFA, 21 patients in total, accounting for 84% of the study population, exhibited enhancements after IFN-α therapy administration. Satisfactory results were obtained within a month, with rapid resolution of active inflammation and improvement of mean BCVA and CMT in all patients. Inflammatory episodes were never observed in the group with full remission, whereas increasing the IFN-α dose was effective in the partial remission group [ 226 ]. Besides being effective in treating ocular manifestations, IFN-α has proven to be particularly effective for BU patients with concomitant macular edema [ 227 ]. One of the noteworthy attributes of IFN-α therapy is the potential to achieve long-term remission post-drug withdrawal, indicating its potential for sustained therapeutic effects. Initiating IFN-α therapy early in the disease trajectory appears to yield better outcomes [ 226 ].
A recent study has shown that the co-inhibitory molecule PDL1 is upregulated by IFNα-2a in dendritic cells of BU patients in an IRF1-dependent manner and that PDL1 mRNA expression levels are linked to the treatment efficacy. Treatment with IFNα-2a led to CD4 + T cell apoptosis, without any significant changes in Treg frequency, and resulted in decreased Th1 and Th17 frequency and reduced levels of IFN-γ and IL-17. Suppression of the Th1/Th17 immune response corresponded to uveitis remission. Moreover, IFNα stimulated IL-10 secretion by CD4 + T cells in BU patients, which then hindered IL-17 secretion by PBMCs. In conclusion, the therapeutic benefits of IFNα-2a in BU are mediated by dendritic cells and CD4 + T cells [ 109 ].
In a monocentric retrospective investigation, Shi et al. incorporated 30 patients afflicted by refractory BU, who underwent IFN-α2a therapy at Peking Union Medical College Hospital between February 2015 and June 2018. Utilized as an adjuvant to traditional treatment in patients with poor prognosis led to treatment success in 26 individuals, representing 86.7% of the cohort. Throughout the follow-up period, most patients could achieve a reduction in steroid hormone and immunosuppressant dosage, or even complete discontinuation of immunosuppressant use, with a significant decrease in ocular inflammatory recurrence. No unresolved adverse drug reactions were observed [ 228 ].
Another study involving 36 patients with severe BU manifestations showed the efficacy of IFN-α in alleviating vasculitis, papillitis, and macular edema. There was also a notable decline in the mean annual recurrence rate per patient, even post-discontinuation of the interferon therapy [ 229 , 230 ]. Pegylated interferon is a derivative of IFN-α that improves the solubility of IFN protein and prolongs its half-life. Therefore, in cases where IFN-α needs to be used three times a week, peg-IFN-α only needs to be used once a week at a frequency sufficient to ensure the therapeutic effect. A small case series involving four patients with severe refractory BU found that peg-IFN-α has a potential long-term effect for the treatment of severe uveitis, reducing the number of injections, improving the quality of life of patients, and improving treatment adherence [ 231 ]. There were notable variations in the management of ocular inflammation and good patient tolerability comparing the average number of episodes, visual acuity, ocular inflammation, FA score, disease activity, and side effects between IFX and IFN-α for treating refractory BU. When comparing IFX and IFN-α for the treatment of refractory BU, it was observed that IFN-α is a favorable therapeutic choice for BU patients who do not respond to conventional therapies, even considering its elevated risk of side effects [ 207 , 227 ].
A direct comparison between IFNα-2a and corticosteroids versus CsA and corticosteroids over a 12-month period showed superior outcomes with IFN-α treatment, with significantly lower BOS24 scores, greater rates of BCVA, full remission, and more stable remission of intraocular inflammation. The advantages of IFN-α surpass those of CsA, which had a short-lived effect, a greater incidence of side effects, and a notable absence of significant steroid-sparing effect [ 179 ].
While IFN-α is typically well-tolerated, certain patients may encounter side effects like fever, fatigue, muscle pain, headache, and other flu-like symptoms. Other rare side effects include mild bone marrow suppression and elevated liver enzymes. However, with a multidisciplinary approach, most side effects are reversible [ 226 ].
Collectively, IFN-α has emerged as a reliable and effective treatment option for refractory BU. Its ability to reduce the need for steroids and other immunosuppressants, combined with its superior outcomes when used in the initial stages of the disease, solidifies its position in the therapeutic arsenal against BU.
CD20 antagonists
has been explored as a therapeutic option for B cell-mediated diseases by targeting the CD20 antigen found on B cell surfaces. In the case of refractory BU, rituximab has shown promising treatment outcomes. Specifically, two doses of 1000 mg each of rituximab, administered 15 days apart, have significantly reduced uveitis activity and associated symptoms in patients with retinal vasculitis and edema [ 232 ]. However, the current data on the utility of rituximab for BU is still insufficient to definitively address the side effects associated with uveitis treatment [ 233 ].
CD52 antagonists
Alemtuzumab has also been investigated for its potential in BU treatment. Alemtuzumab is directed against CD52, a protein found on the surface of lymphocytes and macrophages. This targeting results in the depletion of T cells and, ultimately, the reconstitution of immune function within the CD4 + cell subset [ 234 ]. Studies have shown that alemtuzumab can induce remission, reduce steroid dependency, and generally be well-tolerated in BU patients. However, careful assessment is required before its administration due to potential side effects like lymphopenia and thyroid function abnormalities [ 235 ]. Additionally, alemtuzumab has demonstrated efficacy in treating non-infectious uveitis associated with other conditions like multiple sclerosis [ 236 ].
IL-1 antagonists
Anakinra (ana) and canakinumab (can).
, as IL-1 antagonists, may serve as a treatment option for BU patients exhibiting resistance to traditional therapy and/or presenting contraindications for TNF-α inhibitors, such as latent TB or chronic/active infectious disease. A retrospective examination of 36 BD patients treated with ANA or CAN, conducted by Fabiani et al., revealed that IL-1 blockade demonstrated favorable therapeutic efficacy in BU and BD patients with extended disease duration. The therapeutic impact of ANA (100 mg/day) or CAN (150 mg/8 weeks) proved to be both rapid and enduring [ 237 ]. The results of observational studies have shown that IL-1 inhibitors can treat refractory BU and have excellent safety [ 238 ]. ANA is an interleukin-1 receptor antagonist, whereas CAN operates as an anti-interleukin-1 beta antibody. Fabiani et al. explored the roles of ANA and CAN in 19 patients with refractory BU (involving 31 affected eyes). They found that IL-1 inhibition therapy contributed to a considerable reduction in recurrence rates 12 months post-treatment initiation compared to the same duration before treatment initiation. Additionally, it significantly ameliorated retinal vasculitis in both short and long-term contexts, as well as reduced the average steroid dosage. However, the combination of IL-1 inhibitors and immunosuppressants did not enhance efficacy. Patients receiving concomitant DMARDs exhibited a higher rate of BU relapse relative to those undergoing monotherapy. In conclusion, ANA and CAN are effective and safe treatment options for BU, significantly reducing ocular inflammatory response activity, alleviating retinal vasculitis, preventing visual impairment, and significantly reducing steroid dose [ 239 ].
Gevokizumab
, a recombinant humanized variant monoclonal antibody, impedes IL-1 receptor activation by binding to human interleukin (IL)-1β. A phase II investigation involving the administration of 30 or 60 mg of gevokizumab intravenously or subcutaneously every four weeks to BU patients experiencing recent acute ocular deterioration or at risk thereof, yielded rapid control of intraocular inflammation within one week, accompanied by favorable steroid-sparing effects [ 240 ]. Despite demonstrating a good safety profile in the expanded study, gevokizumab failed to substantially reduce the risk of visual deterioration, leading to the study’s primary endpoint not being met. Consequently, it is not advised as a BU treatment based on the current, somewhat promising results [ 241 , 242 ]. As such, further exploration of IL-1β pathway regulation in BU patients is warranted.
Furthermore, the administration of IL-1 inhibitors has the advantage of reducing the dosage of GC, leading to steroid-sparing effect. This reduction is beneficial as it minimizes the systemic and ocular-related side effects associated with prolonged GC use.
IL-6 antagonists
Tocilizumab (tcz).
, a fully-humanized monoclonal antibody, acts on both membrane-bound and soluble IL-6 receptors, presenting a promising approach for treating BU, especially in instances where the condition is refractory. Inhibiting IL-6 can suppress the production of autoantibodies and rectify imbalances between autoantigen-specific Th17 and/or Th1-Treg [ 243 ]. TCZ has produced rapid and long-term improvements in ocular manifestations of BU, including anterior chamber cells, vitreous inflammation, chorioretinitis, and retinitis, but has limited efficacy in treating extraocular manifestations. Tocilizumab application may reduce the dose of GC and produce a steroid-sparing effect [ 244 ]. Macular cystoid edema, the most common complication of BU, can resolve rapidly after the first TCZ infusion, indicating that TCZ has great therapeutic potential for patients with refractory uveitis macular edema. Mild and rare treatment-related side effects include fatigue, chest tightness, transient elevation of serum LDL cholesterol (low-density lipoprotein) levels, and leukopenia [ 245 ]. In a recent multicenter retrospective observational research, tocilizumab showed higher efficacy against BU than IFX and ADA at six months of treatment and induced complete remission of macular edema in uveitis patients [ 246 ]. It demonstrates that TCZ is a secure and successful therapy for BU. TCZ also has a therapeutic effect on arthritis and phlebitis in BD but is ineffective in treating oral/genital ulcers and skin mucosal manifestations [ 247 ].
IL-17 a antagonists
Secukinumab.
, a human monoclonal antibody with a high affinity for interleukin-17 A, has been determined to be ineffective in the treatment of BU. In a phase III randomized controlled trial involving 118 BU patients, administering subcutaneous injections of secukinumab–initiated with 300 mg dose every two weeks, followed by a maintenance dose of 300 mg every four weeks—did not succeed in reducing the recurrence rate of uveitis or improving BCVA. The primary treatment endpoint was not fulfilled, and it was also shown that the treatment group experienced more non-ocular adverse events than the control group did [ 248 ]. A proof-of-concept study found that compared with 300 mg subcutaneous injection 4 times every two weeks, secukinumab 30 mg/kg twice intravenously every four weeks may be necessary to deliver secukinumab in therapeutic concentrations. High-dose intravenous secukinumab has shown positive efficacy in patients with active chronic noninfectious uveitis who required corticosteroid-sparing immunosuppressive therapy [ 249 ]. However, there are also cases of new-onset BD reported in ankylosing spondylitis patients treated with secukinumab [ 250 ]. Secukinumab is currently not used to treat uveitis in BD.
IL-23 antagonists
Ustekinumab.
is a fully-humanized monoclonal antibody designed to target the shared p40 subunit of IL-23 and IL-12. It has been observed that patients with active BU present higher serum levels of IL-23 compared to those with the inactive form of the disease [ 132 ]. Although there are limited studies and reports available on the efficacy of ustekinumab for treating BU, there exists a case report that details a successful instance of treating a BU patient. In this case, the patient received subcutaneous injections of ustekinumab, administered at 45 mg at weeks 0 and 4, and subsequently every 12 weeks, demonstrating effectiveness over a 3-month duration [ 251 ]. Typical side effects encompass nasopharyngitis, headache, abdominal pain, and joint pain [ 252 ].
Janus kinase inhibitors (JAKi)
Tofacitinib.
functions as a JAK1/3 inhibitor, influencing both innate and adaptive components of the immune system. This mechanism is achieved by blocking the signaling pathways of multiple cytokines and interferons, such as IL-2, IL-4, IL-6, IL-23, IFN-γ, and IFN-α, leading to the regulation of immune responses. As a small molecule, tofacitinib possesses the potential to traverse the blood-retinal barrier with greater efficacy compared to conventional drugs. Tofacitinib 5 mg given twice daily significantly improves BCVA by reducing retinal leakage and decreasing recurrence, is well tolerated, and is therefore expected to be the first choice for treating BU in the future [ 253 ].
Upadacitinib
, a selective inhibitor targeting JAK-1, has garnered attention for its therapeutic potential in BU. In a recent study, the efficacy of upadacitinib was investigated in BU patients who exhibited inadequate responses to conventional therapies and anti-TNF-α treatments. Following the administration of upadacitinib to one adult and one pediatric patient, notable improvements were observed. Both patients experienced enhancements in visual acuity, effective control of intraocular inflammation, and resolution of macular edema. Importantly, no severe adverse events were reported during the follow-up period, underscoring the promising safety profile of upadacitinib in the management of BU [ 254 ].
JAKi offers a new option for BU patients, particularly those whose uveitis has not responded well to conventional and biological DMARDs [ 255 ].
Chinese medicines
BD belongs to the “fox confusion disease” category in Chinese medicine. According to Chinese medicine, the formation of BD is internally related to the deficiency of spleen qi caused by factors such as physical constitution, diet, and emotion. Externally, the disease develops due to the invasion of the body by the evil of dampness and heat.
Longdan Xiegan Decoction (Lobelia, Gardenia, Scutellaria, Mouton, Zedoary, Plantago, Bupleurum, Glycyrrhiza, Angelica, Radix et Rhizoma) can regulate CD4/CD8 and Th17/Treg balance, thus effectively alleviating inflammation in experimental autoimmune uveitis (EAU) eyes and regulating systemic immune status [ 256 ].
Berberine, an isoquinoline alkaloid with a unique tetracyclic structure isolated from Chinese herbal medicine, has been found to have immunomodulatory effects in several inflammatory models [ 257 , 258 ]. In uveitis, it has been confirmed that it can significantly ameliorate the BU and EAU, and there are two possible pathways. One is to affect genes belonging to chromatin remodeling and immune-related pathways, directly acting on T cells or indirectly through DC to regulate Treg/Th17 balance. The second is to increase the number of immunomodulatory bacteria in the gut microbiome [ 259 , 260 , 261 ].
These studies open new avenues of thought. Chinese medicine or natural products possess unique inherent principles for treating the disease and other T cell-related conditions. It is anticipated that a unified standard for Chinese medicine treatment of BU will emerge, potentially offering unforeseen therapeutic benefits in managing BU.
Progranulin (PGRN), an immunomodulatory molecule, has been observed to be downregulated during active disease phases in BD patients. Preliminary studies in animal models suggest that PGRN has the potential to alleviate EAU by reducing Th1 and Th17 cell populations, while simultaneously promoting the polarization of Treg cells. These findings suggest that PGRN may become a potential therapeutic target for BU in future investigations [ 262 ].
In addition to pharmaceutical treatments, dietary modifications are emerging as potential therapeutic strategies for BD. More specifically, diets rich in butyrate have shown promising effects by reducing the production of ROS in lymphocytes, monocytes, and neutrophils among BD patients. Furthermore, these dietary adjustments have been associated with decreased levels of CRP and increased overall plasma antioxidant capacity. These modifications contribute to a balanced inflammatory response, decreased disease activity, and reduced reliance on steroids [ 263 ]. The “hygiene hypothesis” holds that BD patients are more likely to live in poor sanitary conditions, characterized by lower monthly income, a history of parasites, use of dried cow dung as fuel, less bathing or brushing, and close contact with pigs and pork [ 264 , 265 ]. The oral health status of BD patients is often worrisome, such as oral infection, need for tooth extraction, caries, loss of teeth, and an elevated plaque index score, which may be potential mediators of disease severity [ 266 , 267 , 268 ]. Hence, improving oral and personal hygiene may be beneficial.
These findings emphasize the importance of a comprehensive approach to managing BD, which should integrate dietary, lifestyle, and pharmacological interventions to optimize patient outcomes.
Conclusions and future work
Taken together, uveitis is one of the primary clinical signs of BD. This manifestation is attributed to a combination of immune dysregulation, genetic predispositions, and the involvement of microbial elements. These elements have the potential to trigger aberrant immune responses that ultimately lead to the onset of the disease.
The treatment of BU extends beyond conventional pharmacological interventions. Lifestyle choices, environmental factors, and dietary habits have been acknowledged for their ability to influence disease activity, presenting a multi-faceted approach to managing the condition. Various molecular and cellular targets, such as the LCK gene, ERAP1 , the balance of Th17 and Treg cells, the JAK signaling pathway, PGRN, and key cytokines like IL-17 and RORγt, have been identified as potential avenues for innovative therapeutic interventions.
Biologics have revolutionized the management of refractory BU due to their targeted mechanisms of action. However, there is still ongoing work to optimize BU treatment. Some current medications, while effective, have limitations, ranging from incomplete disease control to the occurrence of unwanted side effects. This emphasizes the urgent need for therapeutic options that not only offer improved efficacy but also greater tolerability for patients.
The future of BU treatment looks promising. With continued research and a deeper understanding of the disease’s pathophysiology, the medical community is well-positioned to develop novel therapeutic strategies. These advancements, combined with a comprehensive approach to patient care, aim to not only regulate disease activity but also enhance the overall quality of life for BU patients. As we move forward, the integration of state-of-the-art research, traditional wisdom, and patient-centered care will pave the way for a brighter future for those battling BU.
Data availability
No datasets were generated or analysed during the current study.
Hatemi G, Christensen R, Bang D, Bodaghi B, Celik AF, Fortune F, et al. 2018 update of the EULAR recommendations for the management of Behcet’s syndrome. Ann Rheum Dis. 2018;77(6):808–18. https://doi.org/10.1136/annrheumdis-2018-213225 .
Article PubMed Google Scholar
Leccese P, Alpsoy E. Behcet’s Disease: an overview of Etiopathogenesis. Front Immunol. 2019;10:1067. https://doi.org/10.3389/fimmu.2019.01067 .
Article CAS PubMed PubMed Central Google Scholar
Bodis G, Toth V, Schwarting A. Role of human leukocyte antigens (HLA) in Autoimmune diseases. Methods Mol Biol. 2018;1802:11–29. https://doi.org/10.1007/978-1-4939-8546-3_2 .
Article CAS PubMed Google Scholar
Maldini C, Lavalley MP, Cheminant M, de Menthon M, Mahr A. Relationships of HLA-B51 or B5 genotype with Behcet’s disease clinical characteristics: systematic review and meta-analyses of observational studies. Rheumatology (Oxford). 2012;51(5):887–900. https://doi.org/10.1093/rheumatology/ker428 .
Su G, Zhong Z, Zhou Q, Du L, Ye Z, Li F, et al. Identification of novel risk loci for Behcet’s Disease-Related Uveitis in a Chinese Population in a genome-wide Association study. Arthritis Rheumatol. 2022;74(4):671–81. https://doi.org/10.1002/art.41998 .
Zhong Z, Su G, Du L, Zhou Q, Li F, Chi W, et al. Higher 25-hydroxyvitamin D level is associated with increased risk for Behcet’s disease. Clin Nutr. 2021;40(2):518–24. https://doi.org/10.1016/j.clnu.2020.05.049 .
Cunningham ET Jr., Tugal-Tutkun I, Khairallah M, Okada AA, Bodaghi B, Zierhut M. Behcet Uveitis. Ocul Immunol Inflamm. 2017;25(1):2–6. https://doi.org/10.1080/09273948.2017.1279840 .
Yang P, Fang W, Meng Q, Ren Y, Xing L, Kijlstra A. Clinical features of Chinese patients with Behcet’s disease. Ophthalmology. 2008;115(2):312. https://doi.org/10.1016/j.ophtha.2007.04.056 . 8 e4.
Yang P, Zhong Z, Du L, Li F, Chen Z, Zhu Y, et al. Prevalence and clinical features of systemic diseases in Chinese patients with uveitis. Br J Ophthalmol. 2021;105(1):75–82. https://doi.org/10.1136/bjophthalmol-2020-315960 .
Feigenbaum A. Description of Behcet’s syndrome in the hippocratic third book of endemic diseases. Br J Ophthalmol. 1956;40(6):355–7. https://doi.org/10.1136/bjo.40.6.355 .
Greco A, De Virgilio A, Ralli M, Ciofalo A, Mancini P, Attanasio G, et al. Behcet’s disease: new insights into pathophysiology, clinical features and treatment options. Autoimmun Rev. 2018;17(6):567–75. https://doi.org/10.1016/j.autrev.2017.12.006 .
Behcet H, Matteson EL. On relapsing, aphthous ulcers of the mouth, eye and genitalia caused by a virus. 1937. Clin Exp Rheumatol. 2010;28(4 Suppl 60):S2–5.
PubMed Google Scholar
Alpsoy E, Donmez L, Onder M, Gunasti S, Usta A, Karincaoglu Y, et al. Clinical features and natural course of Behcet’s disease in 661 cases: a multicentre study. Br J Dermatol. 2007;157(5):901–6. https://doi.org/10.1111/j.1365-2133.2007.08116.x .
Criteria for diagnosis of Behcet’s disease. International Study Group for Behcet’s Disease. Lancet. 1990;335(8697):1078–80.
Google Scholar
International Team for the Revision of the International Criteria for Behcet’s D. The International Criteria for Behcet’s Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Dermatol Venereol. 2014;28(3):338–47. https://doi.org/10.1111/jdv.12107 .
Article Google Scholar
Standardization of Uveitis Nomenclature Working G. Classification criteria for Behcet Disease Uveitis. Am J Ophthalmol. 2021;228:80–8. https://doi.org/10.1016/j.ajo.2021.03.058 .
Tugal-Tutkun I, Onal S, Stanford M, Akman M, Twisk JWR, Boers M, et al. An algorithm for the diagnosis of Behcet Disease Uveitis in adults. Ocul Immunol Inflamm. 2021;29(6):1154–63. https://doi.org/10.1080/09273948.2020.1736310 .
Keino H. Evaluation of disease activity in uveoretinitis associated with Behcet’s disease. Immunol Med. 2021;44(2):86–97. https://doi.org/10.1080/25785826.2020.1800244 .
Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature Working G. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140(3):509–16. https://doi.org/10.1016/j.ajo.2005.03.057 .
Nussenblatt RB, Palestine AG, Chan CC, Roberge F. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology. 1985;92(4):467–71. https://doi.org/10.1016/s0161-6420(85)34001-0 .
Sakane T, Takeno M, Suzuki N, Inaba G. Behcet’s disease. N Engl J Med. 1999;341(17):1284–91. https://doi.org/10.1056/NEJM199910213411707 .
Azizlerli G, Kose AA, Sarica R, Gul A, Tutkun IT, Kulac M, et al. Prevalence of Behcet’s disease in Istanbul, Turkey. Int J Dermatol. 2003;42(10):803–6. https://doi.org/10.1046/j.1365-4362.2003.01893.x .
Zouboulis CC, Kotter I, Djawari D, Kirch W, Kohl PK, Ochsendorf FR, et al. Epidemiological features of adamantiades-Behcet’s disease in Germany and in Europe. Yonsei Med J. 1997;38(6):411–22. https://doi.org/10.3349/ymj.1997.38.6.411 .
Mizuki N, Meguro A, Ota M, Ohno S, Shiota T, Kawagoe T, et al. Genome-wide association studies identify IL23R-IL12RB2 and IL10 as Behcet’s disease susceptibility loci. Nat Genet. 2010;42(8):703–6. https://doi.org/10.1038/ng.624 .
Yalcindag FN, Ozdal PC, Ozyazgan Y, Batioglu F, Tugal-Tutkun I, Group BS. Demographic and clinical characteristics of Uveitis in Turkey: the First National Registry Report. Ocul Immunol Inflamm. 2018;26(1):17–26. https://doi.org/10.1080/09273948.2016.1196714 .
Saadouli D, Lahmar A, Ben Mansour K, El Afrit N, Yahyaoui S, El Afrit MA. [Ocular manifestations of Behcet’s disease]. J Fr Ophtalmol. 2021;44(2):196–202. https://doi.org/10.1016/j.jfo.2020.04.058 .
Davatchi F, Shahram F, Chams-Davatchi C, Shams H, Abdolahi BS, Nadji A, et al. Behcet’s disease in Iran: analysis of 7641 cases. Mod Rheumatol. 2019;29(6):1023–30. https://doi.org/10.1080/14397595.2018.1558752 .
Abdelwareth Mohammed A, Soliman MM, Osman AA, El-Zanaty RT. Patterns of Uveitis in Egypt. Ocul Immunol Inflamm. 2021;29(5):1007–16. https://doi.org/10.1080/09273948.2020.1714060 .
Suzuki T, Kaburaki T, Tanaka R, Shirahama S, Komae K, Nakahara H, et al. Incidence and changing patterns of uveitis in Central Tokyo. Int Ophthalmol. 2021;41(7):2377–88. https://doi.org/10.1007/s10792-021-01791-4 .
Bitik B, Tufan A, Sahin K, Sucullu Karadag Y, Can Sandikci S, Mercan R, et al. The association between the parenchymal neurological involvement and posterior uveitis in Behcet’s syndrome. Clin Exp Rheumatol. 2016;34(6 Suppl 102):82–5.
Suwa A, Horita N, Ishido T, Takeuchi M, Kawagoe T, Shibuya E, et al. The ocular involvement did not accompany with the genital ulcer or the gastrointestinal symptoms at the early stage of Behcet’s disease. Mod Rheumatol. 2019;29(2):357–62. https://doi.org/10.1080/14397595.2018.1457424 .
Hussein MA, Eissa IM, Dahab AA. Vision-Threatening Behcet’s Disease: severity of ocular involvement predictors. J Ophthalmol. 2018;2018:9518065. https://doi.org/10.1155/2018/9518065 .
Article PubMed PubMed Central Google Scholar
Hou CC, Luo D, Bao HF, Ye JF, Ma HF, Shen Y, et al. Clinical heterogeneity of ocular Behcet’s syndrome versus intestinal Behcet’s syndrome: a cross-sectional study from Shanghai Behcet’s syndrome database. Arthritis Res Ther. 2022;24(1):98. https://doi.org/10.1186/s13075-022-02782-1 .
Yang P, Zhang Z, Zhou H, Li B, Huang X, Gao Y, et al. Clinical patterns and characteristics of uveitis in a tertiary center for uveitis in China. Curr Eye Res. 2005;30(11):943–8. https://doi.org/10.1080/02713680500263606 .
Kone-Paut I. Behcet’s disease in children, an overview. Pediatr Rheumatol Online J. 2016;14(1):10. https://doi.org/10.1186/s12969-016-0070-z .
Ostrovsky M, Rosenblatt A, Iriqat S, Shteiwi A, Sharon Y, Kramer M, et al. Ocular Behcet Disease-Clinical manifestations, treatments and outcomes according to Age at Disease Onset. Biomedicines. 2023;11(2). https://doi.org/10.3390/biomedicines11020624 .
Rabiah PK, Vitale AT. Noninfectious uveitis and pregnancy. Am J Ophthalmol. 2003;136(1):91–8. https://doi.org/10.1016/s0002-9394(03)00110-7 .
Tugal-Tutkun I, Onal S, Altan-Yaycioglu R, Huseyin Altunbas H, Urgancioglu M. Uveitis in Behcet disease: an analysis of 880 patients. Am J Ophthalmol. 2004;138(3):373–80. https://doi.org/10.1016/j.ajo.2004.03.022 .
Yazici H, Tuzun Y, Pazarli H, Yurdakul S, Ozyazgan Y, Ozdogan H, et al. Influence of age of onset and patient’s sex on the prevalence and severity of manifestations of Behcet’s syndrome. Ann Rheum Dis. 1984;43(6):783–9. https://doi.org/10.1136/ard.43.6.783 .
Yavuz S, Akdeniz T, Hancer V, Bicakcigil M, Can M, Yanikkaya-Demirel G. Dual effects of testosterone in Behcet’s disease: implications for a role in disease pathogenesis. Genes Immun. 2016;17(6):335–41. https://doi.org/10.1038/gene.2016.28 .
Tugal-Tutkun I, Gupta V, Cunningham ET. Differential diagnosis of behcet uveitis. Ocul Immunol Inflamm. 2013;21(5):337–50. https://doi.org/10.3109/09273948.2013.795228 .
Tugal-Tutkun I. Imaging in the diagnosis and management of Behcet disease. Int Ophthalmol Clin. 2012;52(4):183–90. https://doi.org/10.1097/IIO.0b013e318265d56a .
Ksiaa I, Ben Aoun S, Zina S, Nefzi D, Khochtali S, Khairallah M. Sequential bilateral Behcet’s neuroretinitis associated with prepapillary vitreous exudate: case report. J Ophthalmic Inflamm Infect. 2020;10(1):33. https://doi.org/10.1186/s12348-020-00226-y .
Tugal-Tutkun I, Ozdal PC, Oray M, Onal S. Review for Diagnostics of the year: Multimodal Imaging in Behcet Uveitis. Ocul Immunol Inflamm. 2017;25(1):7–19. https://doi.org/10.1080/09273948.2016.1205100 .
Pei M, Zhao C, Gao F, Qu Y, Liang A, Xiao J, et al. Analysis of Parafoveal Microvascular Abnormalities in Behcet’s Uveitis using projection-resolved Optical Coherence Tomographic Angiography. Ocul Immunol Inflamm. 2021;29(3):524–9. https://doi.org/10.1080/09273948.2019.1685108 .
Herbort CP Jr., Papasavvas I, Tugal-Tutkun I. Benefits and limitations of OCT-A in the diagnosis and Follow-Up of posterior intraocular inflammation in current clinical practice: a Valuable Tool or a Deceiver? Diagnostics (Basel). 2022;12(10). https://doi.org/10.3390/diagnostics12102384 .
Gedik S, Akova Y, Yilmaz G, Bozbeyoglu S. Indocyanine green and fundus fluorescein angiographic findings in patients with active ocular Behcet’s disease. Ocul Immunol Inflamm. 2005;13(1):51–8. https://doi.org/10.1080/09273940490518757 .
Tugal-Tutkun I. Behcet’s Uveitis. Middle East Afr J Ophthalmol. 2009;16(4):219–24. https://doi.org/10.4103/0974-9233.58425 .
Yu HG, Kim MJ, Oh FS. Fluorescein angiography and visual acuity in active uveitis with Behcet disease. Ocul Immunol Inflamm. 2009;17(1):41–6. https://doi.org/10.1080/09273940802553279 .
Kim M, Kwon HJ, Choi EY, Kim SS, Koh HJ, Lee SC. Correlation between Fluorescein Angiographic findings and Visual Acuity in Behcet Retinal Vasculitis. Yonsei Med J. 2015;56(4):1087–96. https://doi.org/10.3349/ymj.2015.56.4.1087 .
Tugal-Tutkun I, Cingu K, Kir N, Yeniad B, Urgancioglu M, Gul A. Use of laser flare-cell photometry to quantify intraocular inflammation in patients with Behcet uveitis. Graefes Arch Clin Exp Ophthalmol. 2008;246(8):1169–77. https://doi.org/10.1007/s00417-008-0823-6 .
Ahmadi M, Yousefi M, Abbaspour-Aghdam S, Dolati S, Aghebati-Maleki L, Eghbal-Fard S, et al. Disturbed Th17/Treg balance, cytokines, and miRNAs in peripheral blood of patients with Behcet’s disease. J Cell Physiol. 2019;234(4):3985–94. https://doi.org/10.1002/jcp.27207 .
Cui X, Zhang L, Su G, Kijlstra A, Yang P. Specific sweat metabolite profile in ocular Behcet’s disease. Int Immunopharmacol. 2021;97:107812. https://doi.org/10.1016/j.intimp.2021.107812 .
Djaballah-Ider F, Djaballah A, Djeraba Z, Chaib S, Touil-Boukoffa C. Auto-immunity profile evaluation during different clinical manifestations of Behcet disease in Algerian patients: effect of corticosteroid treatment. Inflammopharmacology. 2019;27(6):1113–22. https://doi.org/10.1007/s10787-019-00567-8 .
Soejima Y, Kirino Y, Takeno M, Kurosawa M, Takeuchi M, Yoshimi R, et al. Changes in the proportion of clinical clusters contribute to the phenotypic evolution of Behcet’s disease in Japan. Arthritis Res Ther. 2021;23(1):49. https://doi.org/10.1186/s13075-020-02406-6 .
de Menthon M, Lavalley MP, Maldini C, Guillevin L, Mahr A. HLA-B51/B5 and the risk of Behcet’s disease: a systematic review and meta-analysis of case-control genetic association studies. Arthritis Rheum. 2009;61(10):1287–96. https://doi.org/10.1002/art.24642 .
Ombrello MJ, Kirino Y, de Bakker PI, Gul A, Kastner DL, Remmers EF. Behcet disease-associated MHC class I residues implicate antigen binding and regulation of cell-mediated cytotoxicity. Proc Natl Acad Sci U S A. 2014;111(24):8867–72. https://doi.org/10.1073/pnas.1406575111 .
Sousa I, Shahram F, Francisco D, Davatchi F, Abdollahi BS, Ghaderibarmi F, et al. Brief report: association of CCR1, KLRC4, IL12A-AS1, STAT4, and ERAP1 with Behcet’s disease in iranians. Arthritis Rheumatol. 2015;67(10):2742–8. https://doi.org/10.1002/art.39240 .
Kirino Y, Bertsias G, Ishigatsubo Y, Mizuki N, Tugal-Tutkun I, Seyahi E, et al. Genome-wide association analysis identifies new susceptibility loci for Behcet’s disease and epistasis between HLA-B*51 and ERAP1. Nat Genet. 2013;45(2):202–7. https://doi.org/10.1038/ng.2520 .
Hou S, Yang Z, Du L, Jiang Z, Shu Q, Chen Y, et al. Identification of a susceptibility locus in STAT4 for Behcet’s disease in Han Chinese in a genome-wide association study. Arthritis Rheum. 2012;64(12):4104–13. https://doi.org/10.1002/art.37708 .
Xavier JM, Shahram F, Sousa I, Davatchi F, Matos M, Abdollahi BS, et al. FUT2: filling the gap between genes and environment in Behcet’s disease? Ann Rheum Dis. 2015;74(3):618–24. https://doi.org/10.1136/annrheumdis-2013-204475 .
Kappen JH, Medina-Gomez C, van Hagen PM, Stolk L, Estrada K, Rivadeneira F, et al. Genome-wide association study in an admixed case series reveals IL12A as a new candidate in Behcet disease. PLoS ONE. 2015;10(3):e0119085. https://doi.org/10.1371/journal.pone.0119085 .
Ortiz-Fernandez L, Carmona FD, Montes-Cano MA, Garcia-Lozano JR, Conde-Jaldon M, Ortego-Centeno N, et al. Genetic Analysis with the Immunochip platform in Behcet Disease. Identification of residues Associated in the HLA Class I Region and New susceptibility loci. PLoS ONE. 2016;11(8):e0161305. https://doi.org/10.1371/journal.pone.0161305 .
Remmers EF, Cosan F, Kirino Y, Ombrello MJ, Abaci N, Satorius C, et al. Genome-wide association study identifies variants in the MHC class I, IL10, and IL23R-IL12RB2 regions associated with Behcet’s disease. Nat Genet. 2010;42(8):698–702. https://doi.org/10.1038/ng.625 .
Huang XF, Brown MA. Progress in the genetics of uveitis. Genes Immun. 2022;23(2):57–65. https://doi.org/10.1038/s41435-022-00168-6 .
Yu H, Zheng M, Zhang L, Li H, Zhu Y, Cheng L, et al. Identification of susceptibility SNPs in IL10 and IL23R-IL12RB2 for Behcet’s disease in Han Chinese. J Allergy Clin Immunol. 2017;139(2):621–7. https://doi.org/10.1016/j.jaci.2016.05.024 .
Xiang Q, Chen L, Hou S, Fang J, Zhou Y, Bai L, et al. TRAF5 and TRAF3IP2 gene polymorphisms are associated with Behcet’s disease and vogt-koyanagi-Harada syndrome: a case-control study. PLoS ONE. 2014;9(1):e84214. https://doi.org/10.1371/journal.pone.0084214 .
Takeuchi M, Mizuki N, Meguro A, Ombrello MJ, Kirino Y, Satorius C, et al. Dense genotyping of immune-related loci implicates host responses to microbial exposure in Behcet’s disease susceptibility. Nat Genet. 2017;49(3):438–43. https://doi.org/10.1038/ng.3786 .
Wu P, Du L, Hou S, Su G, Yang L, Hu J, et al. Association of LACC1, CEBPB-PTPN1, RIPK2 and ADO-EGR2 with ocular Behcet’s disease in a Chinese Han population. Br J Ophthalmol. 2018;102(9):1308–14. https://doi.org/10.1136/bjophthalmol-2017-311753 .
Zhang Q, Li H, Hou S, Yu H, Su G, Deng B, et al. Association of genetic variations in PTPN2 and CD122 with ocular Behcet’s disease. Br J Ophthalmol. 2018;102(7):996–1002. https://doi.org/10.1136/bjophthalmol-2017-310820 .
Hu K, Hou S, Jiang Z, Kijlstra A, Yang P. JAK2 and STAT3 polymorphisms in a Han Chinese population with Behcet’s disease. Invest Ophthalmol Vis Sci. 2012;53(1):538–41. https://doi.org/10.1167/iovs.11-8440 .
Montes-Cano MA, Conde-Jaldon M, Garcia-Lozano JR, Ortiz-Fernandez L, Ortego-Centeno N, Castillo-Palma MJ, et al. HLA and non-HLA genes in Behcet’s disease: a multicentric study in the Spanish population. Arthritis Res Ther. 2013;15(5):R145. https://doi.org/10.1186/ar4328 .
Jiang Z, Yang P, Hou S, Du L, Xie L, Zhou H, et al. IL-23R gene confers susceptibility to Behcet’s disease in a Chinese Han population. Ann Rheum Dis. 2010;69(7):1325–8. https://doi.org/10.1136/ard.2009.119420 .
Zhou Q, Hou S, Liang L, Li X, Tan X, Wei L, et al. MicroRNA-146a and Ets-1 gene polymorphisms in ocular Behcet’s disease and vogt-koyanagi-Harada syndrome. Ann Rheum Dis. 2014;73(1):170–6. https://doi.org/10.1136/annrheumdis-2012-201627 .
Yu H, Liu Y, Bai L, Kijlstra A, Yang P. Predisposition to Behcet’s disease and VKH syndrome by genetic variants of miR-182. J Mol Med (Berl). 2014;92(9):961–7. https://doi.org/10.1007/s00109-014-1159-9 .
Thieblemont N, Wright HL, Edwards SW, Witko-Sarsat V. Human neutrophils in auto-immunity. Semin Immunol. 2016;28(2):159–. https://doi.org/10.1016/j.smim.2016.03.004 . 73.
Ley K, Hoffman HM, Kubes P, Cassatella MA, Zychlinsky A, Hedrick CC, et al. Neutrophils: new insights and open questions. Sci Immunol. 2018;3(30). https://doi.org/10.1126/sciimmunol.aat4579 .
Glennon-Alty L, Hackett AP, Chapman EA, Wright HL. Neutrophils and redox stress in the pathogenesis of autoimmune disease. Free Radic Biol Med. 2018;125:25–35. https://doi.org/10.1016/j.freeradbiomed.2018.03.049 .
Nelson CA, Stephen S, Ashchyan HJ, James WD, Micheletti RG, Rosenbach M. Neutrophilic dermatoses: Pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behcet disease. J Am Acad Dermatol. 2018;79(6):987–1006. https://doi.org/10.1016/j.jaad.2017.11.064 .
Hammad M, Shehata OZ, Abdel-Latif SM, El-Din AMM. Neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in Behcet’s disease: which and when to use? Clin Rheumatol. 2018;37(10):2811–7. https://doi.org/10.1007/s10067-018-4194-z .
Lee YH, Song GG. Neutrophil-to-lymphocyte ratio, mean platelet volume and platelet-to-lymphocyte ratio in Behcet’s disease and their correlation with disease activity: a meta-analysis. Int J Rheum Dis. 2018;21(12):2180–7. https://doi.org/10.1111/1756-185X.13404 .
Becatti M, Emmi G, Silvestri E, Bruschi G, Ciucciarelli L, Squatrito D, et al. Neutrophil activation promotes fibrinogen oxidation and Thrombus formation in Behcet Disease. Circulation. 2016;133(3):302–11. https://doi.org/10.1161/CIRCULATIONAHA.115.017738 .
Le Joncour A, Martos R, Loyau S, Lelay N, Dossier A, Cazes A, et al. Critical role of neutrophil extracellular traps (NETs) in patients with Behcet’s disease. Ann Rheum Dis. 2019;78(9):1274–82. https://doi.org/10.1136/annrheumdis-2018-214335 .
Le Joncour A, Cacoub P, Boulaftali Y, Saadoun D, Neutrophil. NETs and Behcet’s disease: a review. Clin Immunol. 2023;250:109318. https://doi.org/10.1016/j.clim.2023.109318 .
Tohme S, Yazdani HO, Sud V, Loughran P, Huang H, Zamora R, et al. Computational analysis supports IL-17A as a central driver of Neutrophil Extracellular trap-mediated Injury in Liver Ischemia Reperfusion. J Immunol. 2019;202(1):268–77. https://doi.org/10.4049/jimmunol.1800454 .
Wilson AS, Randall KL, Pettitt JA, Ellyard JI, Blumenthal A, Enders A, et al. Neutrophil extracellular traps and their histones promote Th17 cell differentiation directly via TLR2. Nat Commun. 2022;13(1):528. https://doi.org/10.1038/s41467-022-28172-4 .
Tan J, Liu H, Huang M, Li N, Tang S, Meng J, et al. Small molecules targeting RORgammat inhibit autoimmune disease by suppressing Th17 cell differentiation. Cell Death Dis. 2020;11(8):697. https://doi.org/10.1038/s41419-020-02891-2 .
Li L, Yu X, Liu J, Wang Z, Li C, Shi J, et al. Neutrophil Extracellular traps promote aberrant macrophages activation in Behcet’s Disease. Front Immunol. 2020;11:590622. https://doi.org/10.3389/fimmu.2020.590622 .
Fortune F, Walker J, Lehner T. The expression of gamma delta T cell receptor and the prevalence of primed, activated and IgA-bound T cells in Behcet’s syndrome. Clin Exp Immunol. 1990;82(2):326–32. https://doi.org/10.1111/j.1365-2249.1990.tb05447.x .
Brandes M, Willimann K, Lang AB, Nam KH, Jin C, Brenner MB, et al. Flexible migration program regulates gamma delta T-cell involvement in humoral immunity. Blood. 2003;102(10):3693–701. https://doi.org/10.1182/blood-2003-04-1016 .
Pineton de Chambrun M, Wechsler B, Geri G, Cacoub P, Saadoun D. New insights into the pathogenesis of Behcet’s disease. Autoimmun Rev. 2012;11(10):687–98. https://doi.org/10.1016/j.autrev.2011.11.026 .
Hasan MS, Bergmeier LA, Petrushkin H, Fortune F. Gamma Delta (gammadelta) T cells and their involvement in Behcet’s Disease. J Immunol Res. 2015;2015:705831. https://doi.org/10.1155/2015/705831 .
Petrushkin H, Hasan MS, Stanford MR, Fortune F, Wallace GR. Behcet’s Disease: do natural killer cells play a significant role? Front Immunol. 2015;6:134. https://doi.org/10.3389/fimmu.2015.00134 .
Hasan MS, Ryan PL, Bergmeier LA, Fortune F. Circulating NK cells and their subsets in Behcet’s disease. Clin Exp Immunol. 2017;188(2):311–22. https://doi.org/10.1111/cei.12939 .
Veneziani I, Alicata C, Pelosi A, Landolina N, Ricci B, D’Oria V, et al. Toll-like receptor 8 agonists improve NK-cell function primarily targeting CD56(bright)CD16(-) subset. J Immunother Cancer. 2022;10(1). https://doi.org/10.1136/jitc-2021-003385 .
van der Houwen TB, van Hagen PM, van Laar JAM. Immunopathogenesis of Behcet’s disease and treatment modalities. Semin Arthritis Rheum. 2022;52:151956. https://doi.org/10.1016/j.semarthrit.2022.151956 .
Kimura MY, Nakayama T. Differentiation of NK1 and NK2 cells. Crit Rev Immunol. 2005;25(5):361–74. https://doi.org/10.1615/critrevimmunol.v25.i5.20 .
Yamaguchi Y, Takahashi H, Satoh T, Okazaki Y, Mizuki N, Takahashi K, et al. Natural killer cells control a T-helper 1 response in patients with Behcet’s disease. Arthritis Res Ther. 2010;12(3):R80. https://doi.org/10.1186/ar3005 .
Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449(7161):419–26. https://doi.org/10.1038/nature06175 .
Postole AS, Knoll AB, Auffarth GU, Mackensen F. In vivo confocal microscopy of inflammatory cells in the corneal subbasal nerve plexus in patients with different subtypes of anterior uveitis. Br J Ophthalmol. 2016;100(11):1551–6. https://doi.org/10.1136/bjophthalmol-2015-307429 .
Lin W, Liu T, Wang B, Bi H. The role of ocular dendritic cells in uveitis. Immunol Lett. 2019;209:4–10. https://doi.org/10.1016/j.imlet.2019.03.016 .
Heuss ND, Lehmann U, Norbury CC, McPherson SW, Gregerson DS. Local activation of dendritic cells alters the pathogenesis of autoimmune disease in the retina. J Immunol. 2012;188(3):1191–200. https://doi.org/10.4049/jimmunol.1101621 .
Xiao Q, Li X, Sun D, Yi H, Lu X, Nian H. TLR7 Engagement on dendritic cells enhances autoreactive Th17 responses via activation of ERK. J Immunol. 2016;197(10):3820–30. https://doi.org/10.4049/jimmunol.1600333 .
Wang C, Tian Y, Ye Z, Kijlstra A, Zhou Y, Yang P. Decreased interleukin 27 expression is associated with active uveitis in Behcet’s disease. Arthritis Res Ther. 2014;16(3):R117. https://doi.org/10.1186/ar4570 .
Ye Z, Deng B, Wang C, Zhang D, Kijlstra A, Yang P. Decreased B and T lymphocyte attenuator in Behcet’s disease may trigger abnormal Th17 and Th1 immune responses. Sci Rep. 2016;6:20401. https://doi.org/10.1038/srep20401 .
Pay S, Simsek I, Erdem H, Pekel A, Musabak U, Sengul A, et al. Dendritic cell subsets and type I interferon system in Behcet’s disease: does functional abnormality in plasmacytoid dendritic cells contribute to Th1 polarization? Clin Exp Rheumatol. 2007;25(4 Suppl 45):S34–40.
CAS PubMed Google Scholar
Kim TW, Kang JS, Kong JM, Bae S, Yu Y, Chung H, et al. Maturation profiles of peripheral blood dendritic cells in patients with endogenous uveitis. Immunol Lett. 2012;142(1–2):14–9. https://doi.org/10.1016/j.imlet.2011.10.012 .
Xu YD, Cheng M, Shang PP, Yang YQ. Role of IL-6 in dendritic cell functions. J Leukoc Biol. 2022;111(3):695–709. https://doi.org/10.1002/JLB.3MR0621-616RR .
Zhu Y, Yu Q, Su G, Shao N, Feng J, Luo X, et al. Interferon-alpha2a induces CD4(+) T cell apoptosis and suppresses Th1/Th17 responses via upregulating IRF1-mediated PDL1 expression in dendritic cells from Behcet’s uveitis. Clin Immunol. 2023;250:109303. https://doi.org/10.1016/j.clim.2023.109303 .
Bitirgen G, Tinkir Kayitmazbatir E, Satirtav G, Malik RA, Ozkagnici A. In Vivo Confocal Microscopic Evaluation of Corneal Nerve Fibers and dendritic cells in patients with Behcet’s Disease. Front Neurol. 2018;9:204. https://doi.org/10.3389/fneur.2018.00204 .
Keino H, Sakai J, Nishioka K, Sumida T, Usui M. Clonally accumulating T cells in the anterior chamber of Behcet disease. Am J Ophthalmol. 2000;130(2):243–5. https://doi.org/10.1016/s0002-9394(00)00498-0 .
Al-Obeidi AF, Nowatzky J. Immunopathogenesis of Behcet’s disease. Clin Immunol. 2023;253:109661. https://doi.org/10.1016/j.clim.2023.109661 .
Deng Y, Zhang Y, Cai T, Wang Q, Zhang W, Chen Z, et al. Transcriptomic profiling of iris tissue highlights LCK signaling and T cell-mediated immunity in Behcet’s uveitis. J Autoimmun. 2022;133:102920. https://doi.org/10.1016/j.jaut.2022.102920 .
O’Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36(4):542–. https://doi.org/10.1016/j.immuni.2012.03.014 . 50.
Tulunay A, Dozmorov MG, Ture-Ozdemir F, Yilmaz V, Eksioglu-Demiralp E, Alibaz-Oner F, et al. Activation of the JAK/STAT pathway in Behcet’s disease. Genes Immun. 2015;16(2):176. https://doi.org/10.1038/gene.2014.80 .
Puccetti A, Fiore PF, Pelosi A, Tinazzi E, Patuzzo G, Argentino G, et al. Gene expression profiling in Behcet’s Disease indicates an autoimmune component in the pathogenesis of the Disease and opens New avenues for targeted therapy. J Immunol Res. 2018;2018:4246965. https://doi.org/10.1155/2018/4246965 .
Lucherini OM, Lopalco G, Cantarini L, Emmi G, Lopalco A, Venerito V, et al. Critical regulation of Th17 cell differentiation by serum amyloid-A signalling in Behcet’s disease. Immunol Lett. 2018;201:38–44. https://doi.org/10.1016/j.imlet.2018.10.013 .
Mao L, Dong H, Yang P, Zhou H, Huang X, Lin X, et al. MALDI-TOF/TOF-MS reveals elevated serum haptoglobin and amyloid A in Behcet’s disease. J Proteome Res. 2008;7(10):4500–7. https://doi.org/10.1021/pr800279m .
Arumugham VB, Baldari CT. cAMP: a multifaceted modulator of immune synapse assembly and T cell activation. J Leukoc Biol. 2017;101(6):1301–16. https://doi.org/10.1189/jlb.2RU1116-474R .
Kim SM, Park MJ, Park S, Cheng JY, Lee ES. Differential expression of novel genes and signalling pathways of senescent CD8 + T cell subsets in Behcet’s disease. Clin Exp Rheumatol. 2020;38(Suppl 127):17–25.
Yu HG, Lee DS, Seo JM, Ahn JK, Yu YS, Lee WJ, et al. The number of CD8 + T cells and NKT cells increases in the aqueous humor of patients with Behcet’s uveitis. Clin Exp Immunol. 2004;137(2):437–43. https://doi.org/10.1111/j.1365-2249.2004.02536.x .
Ahn JK, Chung H, Lee DS, Yu YS, Yu HG. CD8brightCD56 + T cells are cytotoxic effectors in patients with active Behcet’s uveitis. J Immunol. 2005;175(9):6133–42. https://doi.org/10.4049/jimmunol.175.9.6133 .
Ilhan F, Demir T, Turkcuoglu P, Turgut B, Demir N, Godekmerdan A. Th1 polarization of the immune response in uveitis in Behcet’s disease. Can J Ophthalmol. 2008;43(1):105–8. https://doi.org/10.3129/i07-179 .
Akkurt ZM, Bozkurt M, Ucmak D, Yuksel H, Ucak H, Sula B, et al. Serum cytokine levels in Behcet’s Disease. J Clin Lab Anal. 2015;29(4):317–20. https://doi.org/10.1002/jcla.21772 .
Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191(5):771–80. https://doi.org/10.1084/jem.191.5.771 .
Ahn JK, Yu HG, Chung H, Park YG. Intraocular cytokine environment in active Behcet uveitis. Am J Ophthalmol. 2006;142(3):429–34. https://doi.org/10.1016/j.ajo.2006.04.016 .
El-Asrar AM, Struyf S, Kangave D, Al-Obeidan SS, Opdenakker G, Geboes K, et al. Cytokine profiles in aqueous humor of patients with different clinical entities of endogenous uveitis. Clin Immunol. 2011;139(2):177–84. https://doi.org/10.1016/j.clim.2011.01.014 .
Bonacini M, Soriano A, Cimino L, De Simone L, Bolletta E, Gozzi F, et al. Cytokine profiling in aqueous humor samples from patients with non-infectious Uveitis Associated with systemic inflammatory diseases. Front Immunol. 2020;11:358. https://doi.org/10.3389/fimmu.2020.00358 .
Okubo M, Sumitomo S, Tsuchida Y, Nagafuchi Y, Takeshima Y, Yanaoka H, et al. Transcriptome analysis of immune cells from Behcet’s syndrome patients: the importance of IL-17-producing cells and antigen-presenting cells in the pathogenesis of Behcet’s syndrome. Arthritis Res Ther. 2022;24(1):186. https://doi.org/10.1186/s13075-022-02867-x .
Zhong Z, Su G, Yang P. Risk factors, clinical features and treatment of Behcet’s disease uveitis. Prog Retin Eye Res. 2023;97:101216. https://doi.org/10.1016/j.preteyeres.2023.101216 .
Zhong Z, Su G, Kijlstra A, Yang P. Activation of the interleukin-23/interleukin-17 signalling pathway in autoinflammatory and autoimmune uveitis. Prog Retin Eye Res. 2021;80:100866. https://doi.org/10.1016/j.preteyeres.2020.100866 .
Pepple KL, Lin P. Targeting Interleukin-23 in the Treatment of Noninfectious Uveitis. Ophthalmology. 2018;125(12):1977-83. https://doi.org/10.1016/j.ophtha.2018.05.014 .
Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23(5):479–90. https://doi.org/10.1016/j.immuni.2005.09.015 .
Liu X, Xiao Y, Pan Y, Li H, Zheng SG, Su W. The role of the IL-33/ST2 axis in autoimmune disorders: friend or foe? Cytokine Growth Factor Rev. 2019;50:60–74. https://doi.org/10.1016/j.cytogfr.2019.04.004 .
Kim DJ, Baek SY, Park MK, Park KS, Lee JH, Park SH, et al. Serum level of interleukin-33 and soluble ST2 and their association with disease activity in patients with Behcet’s disease. J Korean Med Sci. 2013;28(8):1145–53. https://doi.org/10.3346/jkms.2013.28.8.1145 .
Koca SS, Kara M, Deniz F, Ozgen M, Demir CF, Ilhan N, et al. Serum IL-33 level and IL-33 gene polymorphisms in Behcet’s disease. Rheumatol Int. 2015;35(3):471–7. https://doi.org/10.1007/s00296-014-3111-2 .
Pei M, Liu X, Yang P, Zhao C, Gao F, Qu Y, et al. Genetic Association of Interleukin 33/ST2 polymorphisms with Behcet’s Uveitis. Front Immunol. 2021;12:589639. https://doi.org/10.3389/fimmu.2021.589639 .
Yoon JY, Lee Y, Yu SL, Yoon HK, Park HY, Joung CI, et al. Aberrant expression of interleukin-10 and activation-induced cytidine deaminase in B cells from patients with Behcet’s disease. Biomed Rep. 2017;7(6):520–6. https://doi.org/10.3892/br.2017.996 .
van der Houwen TB, van Hagen PM, Timmermans WM, Bartol SJ, Lam KH, Kappen JH, et al. Chronic signs of memory B cell activation in patients with Behcet’s disease are partially restored by anti-tumour necrosis factor treatment. Rheumatology (Oxford). 2017;56(1):134–44. https://doi.org/10.1093/rheumatology/kew366 .
Hetta HF, Mohamed AAA, Zahran AM, My Sayed SAM, Ga Saleh M. Possible role of Regulatory B cells in different Behcet’s Disease Phenotypes and therapies: First Report from Egypt. J Inflamm Res. 2021;14:737–44. https://doi.org/10.2147/JIR.S279912 .
Bettiol A, Emmi G, Low L, Sofi F, Wallace GR. Microbiome in Behcet’s syndrome. Clin Immunol. 2023;250:109304. https://doi.org/10.1016/j.clim.2023.109304 .
Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–98. https://doi.org/10.1016/j.cell.2009.09.033 .
Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9(5):313–23. https://doi.org/10.1038/nri2515 .
Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500(7461):232–6. https://doi.org/10.1038/nature12331 .
Shimizu J, Takai K, Takada E, Fujiwara N, Arimitsu N, Ueda Y, et al. Possible association of proinflammatory cytokines including IL1beta and TNFalpha with enhanced Th17 cell differentiation in patients with Behcet’s disease. Clin Rheumatol. 2016;35(7):1857–63. https://doi.org/10.1007/s10067-015-2966-2 .
van der Houwen TB, van Laar JAM, Kappen JH, van Hagen PM, de Zoete MR, van Muijlwijk GH, et al. Behcet’s Disease under Microbiotic Surveillance? A combined analysis of two cohorts of Behcet’s Disease patients. Front Immunol. 2020;11:1192. https://doi.org/10.3389/fimmu.2020.01192 .
Shimizu J, Kubota T, Takada E, Takai K, Fujiwara N, Arimitsu N, et al. Relative abundance of Megamonas hypermegale and Butyrivibrio species decreased in the intestine and its possible association with the T cell aberration by metabolite alteration in patients with Behcet’s disease (210 characters). Clin Rheumatol. 2019;38(5):1437–45. https://doi.org/10.1007/s10067-018-04419-8 .
Ye Z, Zhang N, Wu C, Zhang X, Wang Q, Huang X, et al. A metagenomic study of the gut microbiome in Behcet’s disease. Microbiome. 2018;6(1):135. https://doi.org/10.1186/s40168-018-0520-6 .
Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–73. https://doi.org/10.1126/science.1241165 .
Wang Q, Yi S, Su G, Du Z, Pan S, Huang X, et al. Changes in the gut Microbiome Contribute to the development of Behcet’s Disease via Adjuvant effects. Front Cell Dev Biol. 2021;9:716760. https://doi.org/10.3389/fcell.2021.716760 .
Zhong Z, Su G, Zhou Q, Meguro A, Takeuchi M, Mizuki N, et al. Tuberculosis exposure with risk of Behcet Disease among patients with Uveitis. JAMA Ophthalmol. 2021;139(4):415–22. https://doi.org/10.1001/jamaophthalmol.2020.6985 .
Direskeneli H, Hasan A, Shinnick T, Mizushima R, van der Zee R, Fortune F, et al. Recognition of B-cell epitopes of the 65 kDa HSP in Behcet’s disease. Scand J Immunol. 1996;43(4):464–71. https://doi.org/10.1046/j.1365-3083.1996.d01-53.x .
Tanaka T, Yamakawa N, Koike N, Suzuki J, Mizuno F, Usui M. Behcet’s disease and antibody titers to various heat-shock protein 60s. Ocul Immunol Inflamm. 1999;7(2):69–74. https://doi.org/10.1076/ocii.7.2.69.4018 .
Aboul Naga SH, Hassan LM, El Zanaty RT, Refaat M, Amin RH, Ragab G, et al. Behcet uveitis: current practice and future perspectives. Front Med (Lausanne). 2022;9:968345. https://doi.org/10.3389/fmed.2022.968345 .
Tan H, Pan S, Zhong Z, Su G, Kijlstra A, Yang P. Association between Fine Particulate Air Pollution and the onset of Uveitis in Mainland China. Ocul Immunol Inflamm. 2022;30(7–8):1810–5. https://doi.org/10.1080/09273948.2021.1960381 .
Tan H, Feng X, Yang P. Association between uveitis onset and economic development in mainland China. BMC Public Health. 2023;23(1):1711. https://doi.org/10.1186/s12889-023-16591-x .
Namba K, Goto H, Kaburaki T, Kitaichi N, Mizuki N, Asukata Y, et al. A major review: current aspects of ocular Behcet’s Disease in Japan. Ocul Immunol Inflamm. 2015;23(Suppl 1):S1–23. https://doi.org/10.3109/09273948.2014.981547 .
Ideguchi H, Suda A, Takeno M, Ueda A, Ohno S, Ishigatsubo Y. Behcet disease: evolution of clinical manifestations. Med (Baltim). 2011;90(2):125–32. https://doi.org/10.1097/MD.0b013e318211bf28 .
Yalcindag FN, Cakar Ozdal P, Ozyazgan Y, Batioglu F, Tugal-Tutkun I. Comparison of Sociodemographic Features between Behcet Uveitis and other non-infectious Uveitis. Turk J Ophthalmol. 2021;51(4):206–11. https://doi.org/10.4274/tjo.galenos.2020.28485 .
Dang J, Shi D, Li X, Ma N, Liu Y, Zhong P, et al. Artificial Light-at-night exposure and overweight and obesity across GDP levels among Chinese children and adolescents. Nutrients. 2023;15(4). https://doi.org/10.3390/nu15040939 .
Consolaro A, Giancane G, Alongi A, van Dijkhuizen EHP, Aggarwal A, Al-Mayouf SM, et al. Phenotypic variability and disparities in treatment and outcomes of childhood arthritis throughout the world: an observational cohort study. Lancet Child Adolesc Health. 2019;3(4):255–63. https://doi.org/10.1016/S2352-4642(19)30027-6 .
Soria V, Uribe J, Salvat-Pujol N, Palao D, Menchon JM, Labad J. Psychoneuroimmunology of mental disorders. Rev Psiquiatr Salud Ment (Engl Ed). 2018;11(2):115–24. https://doi.org/10.1016/j.rpsm.2017.07.006 .
Fang J, Hou S, Xiang Q, Qi J, Yu H, Shi Y, et al. Polymorphisms in genetics of vitamin D metabolism confer susceptibility to ocular Behcet disease in a Chinese Han population. Am J Ophthalmol. 2014;157(2):488–. https://doi.org/10.1016/j.ajo.2013.10.010 . 94 e6.
Tian Y, Wang C, Ye Z, Xiao X, Kijlstra A, Yang P. Effect of 1,25-dihydroxyvitamin D3 on Th17 and Th1 response in patients with Behcet’s disease. Invest Ophthalmol Vis Sci. 2012;53(10):6434–41. https://doi.org/10.1167/iovs.12-10398 .
Lin P, Loh AR, Margolis TP, Acharya NR. Cigarette smoking as a risk factor for uveitis. Ophthalmology. 2010;117(3):585–. https://doi.org/10.1016/j.ophtha.2009.08.011 . 90.
Neti N, Pimsri A, Boonsopon S, Tesavibul N, Choopong P. Triggering factors associated with a new episode of recurrent acute anterior uveitis. Sci Rep. 2021;11(1):12156. https://doi.org/10.1038/s41598-021-91701-6 .
Berlinberg EJ, Gonzales JA, Doan T, Acharya NR. Association between Noninfectious Uveitis and psychological stress. JAMA Ophthalmol. 2019;137(2):199–205. https://doi.org/10.1001/jamaophthalmol.2018.5893 .
Gomez-Gomez A, Garcia-Gonzalez J, Peiteado D, Borrego-Sanz L, Arriola-Villalobos P, Esteban-Ortega M, et al. Inflammatory relapses after immunosuppressive drug discontinuation in Uveitis patients: a survival analysis. Ocul Immunol Inflamm. 2021;29(2):376–87. https://doi.org/10.1080/09273948.2019.1681469 .
Malherbe DC, Messaoudi I. Transcriptional and epigenetic regulation of Monocyte and Macrophage Dysfunction by Chronic Alcohol Consumption. Front Immunol. 2022;13:911951. https://doi.org/10.3389/fimmu.2022.911951 .
Hatemi G. A treat-to-target approach is needed for Behcet’s syndrome. Curr Opin Rheumatol. 2022;34(1):39–45. https://doi.org/10.1097/BOR.0000000000000854 .
Yang P. Atlas of Uveitis: diagnosis and treatment. Springer; 2021.
Mohammadi M, Shahram F, Shams H, Akhlaghi M, Ashofteh F, Davatchi F. High-dose intravenous steroid pulse therapy in ocular involvement of Behcet’s disease: a pilot double-blind controlled study. Int J Rheum Dis. 2017;20(9):1269–76. https://doi.org/10.1111/1756-185X.13095 .
Tao T, Yang S, He D, Li Z, Chen B, Zhu L, et al. Intravitreal dexamethasone implants facilitate the management of refractory Behcet’s uveitis with vasculitis. Clin Immunol. 2023;251:109633. https://doi.org/10.1016/j.clim.2023.109633 .
Touhami S, Diwo E, Seve P, Trad S, Bielefeld P, Sene D, et al. Expert opinion on the use of biological therapy in non-infectious uveitis. Expert Opin Biol Ther. 2019;19(5):477–90. https://doi.org/10.1080/14712598.2019.1595578 .
Yazici H, Pazarli H, Barnes CG, Tuzun Y, Ozyazgan Y, Silman A, et al. A controlled trial of azathioprine in Behcet’s syndrome. N Engl J Med. 1990;322(5):281–5. https://doi.org/10.1056/NEJM199002013220501 .
Ozyazgan Y, Yurdakul S, Yazici H, Tuzun B, Iscimen A, Tuzun Y, et al. Low dose cyclosporin a versus pulsed cyclophosphamide in Behcet’s syndrome: a single masked trial. Br J Ophthalmol. 1992;76(4):241–3. https://doi.org/10.1136/bjo.76.4.241 .
Saadoun D, Wechsler B, Terrada C, Hajage D, Le Thi Huong D, Resche-Rigon M, et al. Azathioprine in severe uveitis of Behcet’s disease. Arthritis Care Res (Hoboken). 2010;62(12):1733–8. https://doi.org/10.1002/acr.20308 .
Masuda K, Nakajima A, Urayama A, Nakae K, Kogure M, Inaba G. Double-masked trial of cyclosporin versus colchicine and long-term open study of cyclosporin in Behcet’s disease. Lancet. 1989;1(8647):1093–6. https://doi.org/10.1016/s0140-6736(89)92381-7 .
Qian Y, Qu Y, Gao F, Pei M, Liang A, Xiao J, et al. Comparison of the Safety and Efficacy of Interferon Alpha-2a and Cyclosporine-A when combined with glucocorticoid in the Treatment of Refractory Behcet’s Uveitis: a randomized controlled prospective study. Front Pharmacol. 2021;12:699903. https://doi.org/10.3389/fphar.2021.699903 .
Chi W, Yang P, Zhu X, Wang Y, Chen L, Huang X, et al. Production of interleukin-17 in Behcet’s disease is inhibited by cyclosporin A. Mol Vis. 2010;16:880–6.
CAS PubMed PubMed Central Google Scholar
Akman-Demir G, Ayranci O, Kurtuncu M, Vanli EN, Mutlu M, Tugal-Tutkun I. Cyclosporine for Behcet’s uveitis: is it associated with an increased risk of neurological involvement? Clin Exp Rheumatol. 2008;26(4 Suppl 50):S84–90.
Davatchi F, Shams H, Shahram F, Nadji A, Chams-Davatchi C, Sadeghi Abdollahi B, et al. Methotrexate in ocular manifestations of Behcet’s disease: a longitudinal study up to 15 years. Int J Rheum Dis. 2013;16(5):568–77. https://doi.org/10.1111/1756-185X.12139 .
Miserocchi E, Baltatzis S, Ekong A, Roque M, Foster CS. Efficacy and safety of chlorambucil in intractable noninfectious uveitis: the Massachusetts Eye and ear infirmary experience. Ophthalmology. 2002;109(1):137–42. https://doi.org/10.1016/s0161-6420(01)00864-8 .
Zaghetto JM, Yamamoto MM, Souza MB, Silva FT, Hirata CE, Olivalves E, et al. Chlorambucil and cyclosporine A in Brazilian patients with Behcet’s disease uveitis: a retrospective study. Arq Bras Oftalmol. 2010;73(1):40–6. https://doi.org/10.1590/s0004-27492010000100007 .
Tessler HH, Jennings T. High-dose short-term chlorambucil for intractable sympathetic ophthalmia and Behcet’s disease. Br J Ophthalmol. 1990;74(6):353–7. https://doi.org/10.1136/bjo.74.6.353 .
Lu A, Liu Z, Su G, Yang P. Global Research Status regarding Uveitis in the last decade. Ocul Immunol Inflamm 2023:1–10. https://doi.org/10.1080/09273948.2023.2170251 .
Levy-Clarke G, Jabs DA, Read RW, Rosenbaum JT, Vitale A, Van Gelder RN. Expert panel recommendations for the use of anti-tumor necrosis factor biologic agents in patients with ocular inflammatory disorders. Ophthalmology. 2014;121(3):785–. https://doi.org/10.1016/j.ophtha.2013.09.048 . 96 e3.
Maalouf G, Andrillon A, Leclercq M, Seve P, Bielefeld P, Gueudry J, et al. Lower relapses Rate with Infliximab Versus Adalimumab in Sight-threatening Uveitis: a Multicenter Study of 330 patients. Am J Ophthalmol. 2022;238:173–80. https://doi.org/10.1016/j.ajo.2022.02.002 .
Ohno S, Nakamura S, Hori S, Shimakawa M, Kawashima H, Mochizuki M, et al. Efficacy, safety, and pharmacokinetics of multiple administration of infliximab in Behcet’s disease with refractory uveoretinitis. J Rheumatol. 2004;31(7):1362–8.
Takase K, Ohno S, Ideguchi H, Uchio E, Takeno M, Ishigatsubo Y. Successful switching to adalimumab in an infliximab-allergic patient with severe Behcet disease-related uveitis. Rheumatol Int. 2011;31(2):243–5. https://doi.org/10.1007/s00296-009-1178-y .
Diaz-Llopis M, Salom D, Garcia-de-Vicuna C, Cordero-Coma M, Ortega G, Ortego N, et al. Treatment of refractory uveitis with adalimumab: a prospective multicenter study of 131 patients. Ophthalmology. 2012;119(8):1575–81. https://doi.org/10.1016/j.ophtha.2012.02.018 .
Vallet H, Riviere S, Sanna A, Deroux A, Moulis G, Addimanda O, et al. Efficacy of anti-TNF alpha in severe and/or refractory Behcet’s disease: Multicenter study of 124 patients. J Autoimmun. 2015;62:67–74. https://doi.org/10.1016/j.jaut.2015.06.005 .
Kunimi K, Usui Y, Asakage M, Maehara C, Tsubota K, Mitsuhashi R, et al. Anti-TNF-alpha Therapy for Refractory Uveitis Associated with Behcet’s syndrome and sarcoidosis: a single Center Study of 131 patients. Ocul Immunol Inflamm. 2022;30(1):223–30. https://doi.org/10.1080/09273948.2020.1791346 .
Leal I, Rodrigues FB, Sousa DC, Romao VC, Duarte GS, Carreno E, et al. Efficacy and safety of intravitreal anti-tumour necrosis factor drugs in adults with non-infectious uveitis - a systematic review. Acta Ophthalmol. 2018;96(6):e665–75. https://doi.org/10.1111/aos.13699 .
van der Houwen TB, Humer B, Missotten TO, Thiadens A, van Hagen PM, van Laar JAM. Long-term data on efficacy and safety of adalimumab in Behcet’s disease. Clin Immunol. 2023;247:109242. https://doi.org/10.1016/j.clim.2023.109242 .
Fabiani C, Sota J, Vitale A, Rigante D, Emmi G, Vannozzi L, et al. Cumulative retention rate of adalimumab in patients with Behcet’s disease-related uveitis: a four-year follow-up study. Br J Ophthalmol. 2018;102(5):637–41. https://doi.org/10.1136/bjophthalmol-2017-310733 .
Fabiani C, Vitale A, Emmi G, Bitossi A, Lopalco G, Sota J, et al. Long-term retention rates of adalimumab and infliximab in non-infectious intermediate, posterior, and panuveitis. Clin Rheumatol. 2019;38(1):63–70. https://doi.org/10.1007/s10067-018-4069-3 .
Atienza-Mateo B, Martin-Varillas JL, Calvo-Rio V, Demetrio-Pablo R, Beltran E, Sanchez-Burson J, et al. Comparative study of Infliximab Versus Adalimumab in Refractory Uveitis due to Behcet’s Disease: National Multicenter Study of 177 cases. Arthritis Rheumatol. 2019;71(12):2081–9. https://doi.org/10.1002/art.41026 .
Jaffe GJ, Dick AD, Brezin AP, Nguyen QD, Thorne JE, Kestelyn P, et al. Adalimumab in patients with active noninfectious uveitis. N Engl J Med. 2016;375(10):932–43. https://doi.org/10.1056/NEJMoa1509852 .
Yang S, Huang Z, Liu X, Li H, Xie L, Chen X, et al. Comparative study of adalimumab versus conventional therapy in sight-threatening refractory Behcet’s uveitis with vasculitis. Int Immunopharmacol. 2021;93:107430. https://doi.org/10.1016/j.intimp.2021.107430 .
Sfikakis PP, Theodossiadis PG, Katsiari CG, Kaklamanis P, Markomichelakis NN. Effect of infliximab on sight-threatening panuveitis in Behcet’s disease. Lancet. 2001;358(9278):295–6. https://doi.org/10.1016/s0140-6736(01)05497-6 .
Martin-Varillas JL, Atienza-Mateo B, Calvo-Rio V, Beltran E, Sanchez-Burson J, Adan A, et al. Long-term follow-up and optimization of Infliximab in Refractory Uveitis due to Behcet Disease: National Study of 103 White patients. J Rheumatol. 2021;48(5):741–50. https://doi.org/10.3899/jrheum.200300 .
Sfikakis PP, Kaklamanis PH, Elezoglou A, Katsilambros N, Theodossiadis PG, Papaefthimiou S, et al. Infliximab for recurrent, sight-threatening ocular inflammation in Adamantiades-Behcet disease. Ann Intern Med. 2004;140(5):404–6. https://doi.org/10.7326/0003-4819-140-5-200403020-00025 .
Theodossiadis PG, Markomichelakis NN, Sfikakis PP. Tumor necrosis factor antagonists: preliminary evidence for an emerging approach in the treatment of ocular inflammation. Retina. 2007;27(4):399–413. https://doi.org/10.1097/MAJ.0b013e3180318fbc .
Arida A, Fragiadaki K, Giavri E, Sfikakis PP. Anti-TNF agents for Behcet’s disease: analysis of published data on 369 patients. Semin Arthritis Rheum. 2011;41(1):61–70. https://doi.org/10.1016/j.semarthrit.2010.09.002 .
Fabiani C, Sota J, Vitale A, Emmi G, Vannozzi L, Bacherini D, et al. Ten-year Retention Rate of Infliximab in patients with Behcet’s Disease-Related Uveitis. Ocul Immunol Inflamm. 2019;27(1):34–9. https://doi.org/10.1080/09273948.2017.1391297 .
Yalcindag N, Kose HC. Comparison of the treatment results for Behcet Uveitis in patients treated with Infliximab and Interferon. Ocul Immunol Inflamm. 2020;28(2):305–14. https://doi.org/10.1080/09273948.2019.1606256 .
Guzelant G, Ucar D, Esatoglu SN, Hatemi G, Ozyazgan Y, Yurdakul S, et al. Infliximab for uveitis of Behcet’s syndrome: a trend for earlier initiation. Clin Exp Rheumatol. 2017;35(Suppl 108):86–9.
Bozkurt T, Karabacak M, Karatas H, Kutlug Agackiran S, Ergun T, Direskeneli H, et al. Earlier and more aggressive treatment with biologics may prevent relapses and further new organ involvement in Behcet’s disease. Clin Immunol. 2023;248:109263. https://doi.org/10.1016/j.clim.2023.109263 .
Ida Y, Takeuchi M, Ishihara M, Shibuya E, Yamane T, Hasumi Y, et al. An open-label, prospective, single-arm study of switching from infliximab to cyclosporine for refractory uveitis in patients with Behcet’s disease in long-term remission. Jpn J Ophthalmol. 2021;65(6):843–8. https://doi.org/10.1007/s10384-021-00872-2 .
Okada K, Zhou Y, Hashida N, Takagi T, Tian YS. The efficacy of Golimumab against non-infectious uveitis: a PRISMA-compliant systematic review and meta-analysis. Ocul Immunol Inflamm 2022:1–11. https://doi.org/10.1080/09273948.2022.2081584 .
Fabiani C, Sota J, Rigante D, Vitale A, Emmi G, Vannozzi L, et al. Rapid and Sustained Efficacy of Golimumab in the Treatment of Multirefractory Uveitis Associated with Behcet’s Disease. Ocul Immunol Inflamm. 2019;27(1):58–63. https://doi.org/10.1080/09273948.2017.1351573 .
Jin Y, Lu S, Lin Y, Mou X. The efficacy and safety of TNF inhibitor (golimumab) as salvage treatment in patients with refractory noninfectious uveitis. Inflammopharmacology. 2022;30(4):1363–8. https://doi.org/10.1007/s10787-022-01019-6 .
Tosi GM, Sota J, Vitale A, Rigante D, Emmi G, Lopalco G, et al. Efficacy and safety of certolizumab pegol and golimumab in the treatment of non-infectious uveitis. Clin Exp Rheumatol. 2019;37(4):680–3.
Simister NE, Story CM. Human placental fc receptors and the transmission of antibodies from mother to fetus. J Reprod Immunol. 1997;37(1):1–23. https://doi.org/10.1016/s0165-0378(97)00068-5 .
Prieto-Pena D, Calderon-Goercke M, Adan A, Chamorro-Lopez L, Maiz-Alonso O, De Dios-Jimenez Aberasturi JR, et al. Efficacy and safety of certolizumab pegol in pregnant women with uveitis. Recommendations on the management with immunosuppressive and biologic therapies in uveitis during pregnancy. Clin Exp Rheumatol. 2021;39(1):105–14. https://doi.org/10.55563/clinexprheumatol/j9ysbm .
Zhou X, Shi X, Ren Y, Yan T, Ye Q. Anti-tumour necrosis factor-alpha agent therapy, compared with conventional therapy, reduces the relapse of uveitis in patients with behcet’s disease: a systematic review of controlled trials. Front Pharmacol. 2022;13:912906. https://doi.org/10.3389/fphar.2022.912906 .
Nathan DM, Angus PW, Gibson PR. Hepatitis B and C virus infections and anti-tumor necrosis factor-alpha therapy: guidelines for clinical approach. J Gastroenterol Hepatol. 2006;21(9):1366–71. https://doi.org/10.1111/j.1440-1746.2006.04559.x .
Kunchok A, Aksamit AJ Jr., Davis JM 3rd, Kantarci OH, Keegan BM, Pittock SJ, et al. Association between Tumor Necrosis Factor Inhibitor exposure and inflammatory central nervous system events. JAMA Neurol. 2020;77(8):937–46. https://doi.org/10.1001/jamaneurol.2020.1162 .
Monti S, Klersy C, Gorla R, Sarzi-Puttini P, Atzeni F, Pellerito R, et al. Factors influencing the choice of first- and second-line biologic therapy for the treatment of rheumatoid arthritis: real-life data from the Italian LORHEN Registry. Clin Rheumatol. 2017;36(4):753–61. https://doi.org/10.1007/s10067-016-3528-y .
Kotter I, Zierhut M, Eckstein AK, Vonthein R, Ness T, Gunaydin I, et al. Human recombinant interferon alfa-2a for the treatment of Behcet’s disease with sight threatening posterior or panuveitis. Br J Ophthalmol. 2003;87(4):423–31. https://doi.org/10.1136/bjo.87.4.423 .
Kotter I, Eckstein AK, Stubiger N, Zierhut M. Treatment of ocular symptoms of Behcet’s disease with interferon alpha 2a: a pilot study. Br J Ophthalmol. 1998;82(5):488–94. https://doi.org/10.1136/bjo.82.5.488 .
Albayrak O, Oray M, Can F, Uludag Kirimli G, Gul A, Tugal-Tutkun I, et al. Effect of Interferon alfa-2a treatment on adaptive and innate Immune systems in patients with Behcet Disease Uveitis. Invest Ophthalmol Vis Sci. 2019;60(1):52–63. https://doi.org/10.1167/iovs.18-25548 .
Liu X, Yang P, Wang C, Li F, Kijlstra A. IFN-alpha blocks IL-17 production by peripheral blood mononuclear cells in Behcet’s disease. Rheumatology (Oxford). 2011;50(2):293–8. https://doi.org/10.1093/rheumatology/keq330 .
Dick AD, Rosenbaum JT, Al-Dhibi HA, Belfort R Jr., Brezin AP, Chee SP, et al. Guidance on Noncorticosteroid systemic immunomodulatory therapy in noninfectious uveitis: fundamentals of Care for UveitiS (FOCUS). Initiative Ophthalmol. 2018;125(5):757–73. https://doi.org/10.1016/j.ophtha.2017.11.017 .
Eser-Ozturk H, Sullu Y. The results of Interferon-Alpha treatment in Behcet Uveitis. Ocul Immunol Inflamm. 2020;28(3):498–504. https://doi.org/10.1080/09273948.2019.1587473 .
De Simone L, Invernizzi A, Aldigeri R, Mastrofilippo V, Marvisi C, Gozzi F, et al. Effectiveness of Infliximab and Interferon Alpha-2a for the Treatment of Behcet’s Uveitis: customizing therapy according to the clinical features. Ocul Immunol Inflamm. 2022;30(2):506–14. https://doi.org/10.1080/09273948.2020.1815797 .
Shi J, Zhao C, Zhou J, Liu J, Wang L, Gao F, et al. Effectiveness and safety of interferon alpha2a as an add-on treatment for refractory Behcet’s uveitis. Ther Adv Chronic Dis. 2019;10:2040622319847881. https://doi.org/10.1177/2040622319847881 .
Diwo E, Gueudry J, Saadoun D, Weschler B, LeHoang P, Bodaghi B. Long-term efficacy of Interferon in severe Uveitis Associated with Behcet Disease. Ocul Immunol Inflamm. 2017;25(1):76–84. https://doi.org/10.1080/09273948.2016.1206204 .
Deuter CM, Zierhut M, Mohle A, Vonthein R, Stobiger N, Kotter I. Long-term remission after cessation of interferon-alpha treatment in patients with severe uveitis due to Behcet’s disease. Arthritis Rheum. 2010;62(9):2796–805. https://doi.org/10.1002/art.27581 .
Celiker H, Kazokoglu H, Direskeneli H. Long-term efficacy of Pegylated Interferon Alpha-2b in Behcet’s Uveitis: a small Case Series. Ocul Immunol Inflamm. 2019;27(1):15–22. https://doi.org/10.1080/09273948.2017.1332768 .
Davatchi F, Shams H, Rezaipoor M, Sadeghi-Abdollahi B, Shahram F, Nadji A, et al. Rituximab in intractable ocular lesions of Behcet’s disease; randomized single-blind control study (pilot study). Int J Rheum Dis. 2010;13(3):246–52. https://doi.org/10.1111/j.1756-185X.2010.01546.x .
Ng CC, Sy A, Cunningham ET. Jr. Rituximab for non-infectious Uveitis and Scleritis. J Ophthalmic Inflamm Infect. 2021;11(1):23. https://doi.org/10.1186/s12348-021-00252-4 .
Zhang X, Tao Y, Chopra M, Ahn M, Marcus KL, Choudhary N, et al. Differential reconstitution of T cell subsets following immunodepleting treatment with alemtuzumab (anti-CD52 monoclonal antibody) in patients with relapsing-remitting multiple sclerosis. J Immunol. 2013;191(12):5867–74. https://doi.org/10.4049/jimmunol.1301926 .
Mohammad AJ, Smith RM, Chow YW, Chaudhry AN, Jayne DR. Alemtuzumab as Remission induction therapy in Behcet Disease: a 20-year experience. J Rheumatol. 2015;42(10):1906–13. https://doi.org/10.3899/jrheum.141344 .
Willis MD, Pickersgill TP, Robertson NP, Lee RWJ, Dick AD, Carreno E. Alemtuzumab-induced remission of multiple sclerosis-associated uveitis. Int Ophthalmol. 2017;37(5):1229–33. https://doi.org/10.1007/s10792-016-0370-9 .
Fabiani C, Vitale A, Rigante D, Emmi G, Lopalco G, Di Scala G, et al. The Presence of Uveitis is Associated with a sustained response to the interleukin (IL)-1 inhibitors Anakinra and Canakinumab in Behcet’s Disease. Ocul Immunol Inflamm. 2020;28(2):298–304. https://doi.org/10.1080/09273948.2018.1511810 .
Sota J, Vitale A, Insalaco A, Sfriso P, Lopalco G, Emmi G, et al. Safety profile of the interleukin-1 inhibitors anakinra and canakinumab in real-life clinical practice: a nationwide multicenter retrospective observational study. Clin Rheumatol. 2018;37(8):2233–40. https://doi.org/10.1007/s10067-018-4119-x .
Fabiani C, Vitale A, Emmi G, Lopalco G, Vannozzi L, Guerriero S, et al. Interleukin (IL)-1 inhibition with anakinra and canakinumab in Behcet’s disease-related uveitis: a multicenter retrospective observational study. Clin Rheumatol. 2017;36(1):191–7. https://doi.org/10.1007/s10067-016-3506-4 .
Tugal-Tutkun IM, Kadayifcilar SM, Khairallah MM, Lee SCMP, Ozdal P, Ozyazgan Y, et al. Safety and Efficacy of Gevokizumab in patients with Behcet’s Disease Uveitis: results of an exploratory phase 2 study. Ocul Immunol Inflamm. 2017;25(1):62–70. https://doi.org/10.3109/09273948.2015.1092558 .
Tugal-Tutkun I, Pavesio C, De Cordoue A, Bernard-Poenaru O, Gul A. Use of Gevokizumab in patients with Behcet’s Disease Uveitis: An International, Randomized, Double-Masked, placebo-controlled study and open-label extension study. Ocul Immunol Inflamm. 2018;26(7):1023–33. https://doi.org/10.1080/09273948.2017.1421233 .
Arnold DD, Yalamanoglu A, Boyman O. Systematic review of Safety and Efficacy of IL-1-Targeted Biologics in Treating Immune-mediated disorders. Front Immunol. 2022;13:888392. https://doi.org/10.3389/fimmu.2022.888392 .
Ogata A, Tanaka T. Tocilizumab for the treatment of rheumatoid arthritis and other systemic autoimmune diseases: current perspectives and future directions. Int J Rheumatol. 2012;2012:946048. https://doi.org/10.1155/2012/946048 .
Atienza-Mateo B, Calvo-Rio V, Beltran E, Martinez-Costa L, Valls-Pascual E, Hernandez-Garfella M, et al. Anti-interleukin 6 receptor tocilizumab in refractory uveitis associated with Behcet’s disease: multicentre retrospective study. Rheumatology (Oxford). 2018;57(5):856–64. https://doi.org/10.1093/rheumatology/kex480 .
Eser Ozturk H, Oray M, Tugal-Tutkun I. Tocilizumab for the Treatment of Behcet Uveitis that failed Interferon Alpha and Anti-tumor Necrosis factor-alpha therapy. Ocul Immunol Inflamm. 2018;26(7):1005–14. https://doi.org/10.1080/09273948.2017.1355471 .
Leclercq M, Andrillon A, Maalouf G, Seve P, Bielefeld P, Gueudry J, et al. Anti-tumor Necrosis factor alpha versus Tocilizumab in the treatment of Refractory Uveitic Macular Edema: a Multicenter Study from the French Uveitis Network. Ophthalmology. 2022;129(5):520–9. https://doi.org/10.1016/j.ophtha.2021.11.013 .
Atienza-Mateo B, Beltran E, Hernandez-Garfella M, Valls Pascual E, Martinez-Costa L, Atanes A, et al. Tocilizumab in Behcet’s disease with refractory ocular and/or neurological involvement: response according to different clinical phenotypes. Clin Exp Rheumatol. 2021;39(Suppl 132):37–42. https://doi.org/10.55563/clinexprheumatol/9ipkcs .
Dick AD, Tugal-Tutkun I, Foster S, Zierhut M, Melissa Liew SH, Bezlyak V, et al. Secukinumab in the treatment of noninfectious uveitis: results of three randomized, controlled clinical trials. Ophthalmology. 2013;120(4):777–87. https://doi.org/10.1016/j.ophtha.2012.09.040 .
Letko E, Yeh S, Foster CS, Pleyer U, Brigell M, Grosskreutz CL, et al. Efficacy and safety of intravenous secukinumab in noninfectious uveitis requiring steroid-sparing immunosuppressive therapy. Ophthalmology. 2015;122(5):939–48. https://doi.org/10.1016/j.ophtha.2014.12.033 .
Dincses E, Yurttas B, Esatoglu SN, Melikoglu M, Hamuryudan V, Seyahi E. Secukinumab induced Behcet’s syndrome: a report of two cases. Oxf Med Case Rep. 2019;2019(5):omz041. https://doi.org/10.1093/omcr/omz041 .
Baerveldt EM, Kappen JH, Thio HB, van Laar JA, van Hagen PM, Prens EP. Successful long-term triple disease control by ustekinumab in a patient with Behcet’s disease, psoriasis and hidradenitis suppurativa. Ann Rheum Dis. 2013;72(4):626–7. https://doi.org/10.1136/annrheumdis-2012-202392 .
Danese S, Vermeire S, D’Haens G, Panes J, Dignass A, Magro F, et al. Treat to target versus standard of care for patients with Crohn’s disease treated with ustekinumab (STARDUST): an open-label, multicentre, randomised phase 3b trial. Lancet Gastroenterol Hepatol. 2022;7(4):294–306. https://doi.org/10.1016/S2468-1253(21)00474-X .
Zou J, Lin CH, Wang Y, Shen Y, Guan JL. Correspondence on ‘A pilot study of tofacitinib for refractory Behcet’s syndrome’. Ann Rheum Dis 2021. https://doi.org/10.1136/annrheumdis-2020-219810 .
Tao T, He D, Peng X, Huang Z, Su W. Successful Remission with Upadacitinib in Two Patients with Anti-TNF-Refractory Macular Edema Associated with Behcet’s Uveitis. Ocul Immunol Inflamm 2023:1–4. https://doi.org/10.1080/09273948.2023.2263557 .
El-Shabrawi Y, Rath T, Heiligenhaus A. Janus kinase inhibitors: next-generation treatment for Uveitis. Klin Monbl Augenheilkd. 2022;239(5):695–701. https://doi.org/10.1055/a-1741-8104 .
Yin X, Qiu Y, Li Z, Guo L, Wei H, Liu B, et al. Longdan Xiegan Decoction alleviates experimental autoimmune uveitis in rats by inhibiting notch signaling pathway activation and Th17 cell differentiation. Biomed Pharmacother. 2021;136:111291. https://doi.org/10.1016/j.biopha.2021.111291 .
Gravina AG, Pellegrino R, Palladino G, Coppola A, Brandimarte G, Tuccillo C, et al. Hericium erinaceus, in combination with natural flavonoid/alkaloid and B(3)/B(8) vitamins, can improve inflammatory burden in inflammatory bowel diseases tissue: an ex vivo study. Front Immunol. 2023;14:1215329. https://doi.org/10.3389/fimmu.2023.1215329 .
Qin X, Guo BT, Wan B, Fang L, Lu L, Wu L, et al. Regulation of Th1 and Th17 cell differentiation and amelioration of experimental autoimmune encephalomyelitis by natural product compound berberine. J Immunol. 2010;185(3):1855–63. https://doi.org/10.4049/jimmunol.0903853 .
Yang Y, Qi J, Wang Q, Du L, Zhou Y, Yu H, et al. Berberine suppresses Th17 and dendritic cell responses. Invest Ophthalmol Vis Sci. 2013;54(4):2516–22. https://doi.org/10.1167/iovs.12-11217 .
Du Z, Wang Q, Huang X, Yi S, Mei S, Yuan G, et al. Effect of berberine on spleen transcriptome and gut microbiota composition in experimental autoimmune uveitis. Int Immunopharmacol. 2020;81:106270. https://doi.org/10.1016/j.intimp.2020.106270 .
Yang Y, Wang Q, Xie M, Liu P, Qi X, Liu X, et al. Berberine exerts an anti-inflammatory role in ocular Behcet’s disease. Mol Med Rep. 2017;15(1):97–102. https://doi.org/10.3892/mmr.2016.5980 .
Wang C, Zhou W, Su G, Hu J, Yang P. Progranulin suppressed Autoimmune Uveitis and Autoimmune Neuroinflammation by inhibiting Th1/Th17 cells and promoting Treg Cells and M2 macrophages. Neurol Neuroimmunol Neuroinflamm. 2022;9(2). https://doi.org/10.1212/NXI.0000000000001133 .
Emmi G, Bettiol A, Niccolai E, Ramazzotti M, Amedei A, Pagliai G, et al. Butyrate-Rich diets improve redox status and fibrin lysis in Behcet’s syndrome. Circ Res. 2021;128(2):278–80. https://doi.org/10.1161/CIRCRESAHA.120.317789 .
Pehlivan M, Kurtuncu M, Tuzun E, Shugaiv E, Mutlu M, Eraksoy M, et al. The comparison of socio-economic conditions and personal hygiene habits of neuro-Behcet’s disease and multiple sclerosis patients. Int J Hyg Environ Health. 2011;214(4):335–7. https://doi.org/10.1016/j.ijheh.2011.04.001 .
Larsson H, Bengtsson-Stigmar E. Behcet’s disease and close contact with pigs. Acta Med Scand. 1984;216(5):541–3. https://doi.org/10.1111/j.0954-6820.1984.tb05044.x .
Mumcu G, Alibaz Oner F, Ergun T, Direskeneli RH. Decreasing incidence and severity of Behcet’s disease: a changing trend in epidemiological spectrum possibly associated with oral health. Turk J Med Sci. 2020;50(SI–2):1587–90. https://doi.org/10.3906/sag-2003-147 .
Direskeneli H, Mumcu G. A possible decline in the incidence and severity of Behcet’s disease: implications for an infectious etiology and oral health. Clin Exp Rheumatol. 2010;28(4 Suppl 60):S86–90.
Mumcu G, Ergun T, Inanc N, Fresko I, Atalay T, Hayran O, et al. Oral health is impaired in Behcet’s disease and is associated with disease severity. Rheumatology (Oxford). 2004;43(8):1028–33. https://doi.org/10.1093/rheumatology/keh236 .
Chen F, Hou S, Jiang Z, Chen Y, Kijlstra A, Rosenbaum JT, et al. CD40 gene polymorphisms confer risk of Behcet’s disease but not of Vogt-Koyanagi-Harada syndrome in a Han Chinese population. Rheumatology (Oxford). 2012;51(1):47–51. https://doi.org/10.1093/rheumatology/ker345 .
Hou S, Xiao X, Li F, Jiang Z, Kijlstra A, Yang P. Two-stage association study in Chinese Han identifies two independent associations in CCR1/CCR3 locus as candidate for Behcet’s disease susceptibility. Hum Genet. 2012;131(12):1841–50. https://doi.org/10.1007/s00439-012-1200-4 .
Chen Y, Yang P, Li F, Hou S, Jiang Z, Shu Q, et al. Association analysis of TGFBR3 gene with Vogt-Koyanagi-Harada disease and Behcet’s disease in the Chinese Han population. Curr Eye Res. 2012;37(4):312–7. https://doi.org/10.3109/02713683.2011.635398 .
Hou S, Yang P, Du L, Zhou H, Lin X, Liu X, et al. SUMO4 gene polymorphisms in Chinese Han patients with Behcet’s disease. Clin Immunol. 2008;129(1):170–5. https://doi.org/10.1016/j.clim.2008.06.006 .
Fang J, Hu R, Hou S, Ye Z, Xiang Q, Qi J, et al. Association of TLR2 gene polymorphisms with ocular Behcet’s disease in a Chinese Han population. Invest Ophthalmol Vis Sci. 2013;54(13):8384–92. https://doi.org/10.1167/iovs.13-12878 .
Hou S, Shu Q, Jiang Z, Chen Y, Li F, Chen F, et al. Replication study confirms the association between UBAC2 and Behcet’s disease in two independent Chinese sets of patients and controls. Arthritis Res Ther. 2012;14(2):R70. https://doi.org/10.1186/ar3789 .
Novak T, Hamedi M, Bergmeier LA, Fortune F, Hagi-Pavli E. Saliva and serum cytokine profiles during oral Ulceration in Behcet’s Disease. Front Immunol. 2021;12:724900. https://doi.org/10.3389/fimmu.2021.724900 .
Na SY, Park MJ, Park S, Lee ES. Up-regulation of Th17 and related cytokines in Behcet’s disease corresponding to disease activity. Clin Exp Rheumatol. 2013;31(3 Suppl 77):32–40.
Ruiz de Morales JMG, Puig L, Dauden E, Canete JD, Pablos JL, Martin AO, et al. Critical role of interleukin (IL)-17 in inflammatory and immune disorders: an updated review of the evidence focusing in controversies. Autoimmun Rev. 2020;19(1):102429. https://doi.org/10.1016/j.autrev.2019.102429 .
Chi W, Zhu X, Yang P, Liu X, Lin X, Zhou H, et al. Upregulated IL-23 and IL-17 in Behcet patients with active uveitis. Invest Ophthalmol Vis Sci. 2008;49(7):3058–64. https://doi.org/10.1167/iovs.07-1390 .
Download references
The work was supported by Major Program of Medical Science and Technology Project of Health Commission of Henan Province (SBGJ202101011), Medical Science and Technology Project of Health Commission of Henan Province (SBGJ2020003031), National Natural Science Foundation Key Program (82230032 and 81930023), and National Natural Science Foundation Project (81970792 and 82101108).
Author information
Authors and affiliations.
Department of Ophthalmology, Henan International Joint Research Laboratory for Ocular Immunology and Retinal Injury Repair, The First Affiliated Hospital of Zhengzhou University, Henan Province Eye Hospital, Zhengzhou, 450052, People’s Republic of China
Yuxuan Guan, Fuzhen Li, Na Li & Peizeng Yang
The Academy of Medical Sciences, Zhengzhou University, Zhengzhou, 450052, People’s Republic of China
Yuxuan Guan
You can also search for this author in PubMed Google Scholar
Contributions
YG conceived the idea, analyzed the data, and drafted the manuscript. FL, NL, and PY completed the final editing and revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Peizeng Yang .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Take-home messages
Much progress has been made in the study of BU, but there are still gaps in the understanding of its pathogenesis and the optimization of treatment options.
Recent studies have identified the interaction of genetic, immune, and environmental factors, emphasizing the important influence of microbial elements. In addition, the therapeutic potential of biologics and natural products has provided a new dimension to the treatment paradigm of BU.
These advances not only fill the gaps in existing research, but also herald the future of subtle and effective BU treatments, emphasizing the value of integrative therapeutic strategies.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Guan, Y., Li, F., Li, N. et al. Decoding Behcet’s Uveitis: an In-depth review of pathogenesis and therapeutic advances. J Neuroinflammation 21 , 133 (2024). https://doi.org/10.1186/s12974-024-03123-6
Download citation
Received : 05 January 2024
Accepted : 04 May 2024
Published : 22 May 2024
DOI : https://doi.org/10.1186/s12974-024-03123-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Behcet’s disease
- Behcet’s uveitis
- Adaptive immunity
- Innate immunity
Journal of Neuroinflammation
ISSN: 1742-2094
- Submission enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Frame your story (figure out where to start and where to end). Plan your delivery (decide whether to memorize your speech word for word or develop bullet points and then rehearse it—over and ...
One of the best ways to open your speech with a buzz is to startle or shock them. You can shock an audience in many ways, but they all rest on the major senses of VAKS: Visual. Auditory. Kinesthetic (touch) and Smell. We don't want your audience tasting your talk, but it should leave a good taste in their mouths.
Have a presentation coming up? Want to hook you audience from the start? Then watch this Lighthouse Communications video that gives you a step by step formul...
Idea 1: Introduction. There is no better way to get the audience to remember you than putting a giant photo of yourself on the screen and going, this is me, - an extremely edited version of me, but still, me. Buddy. No. That was an attempt at being the funny - clever person. Clearly it didn't work.
There are several ways to achieve this. The choice will depend on your topic, the circumstances, and your presentation style. The techniques below guide us on how to start a presentation strong. 1. Make a Bold Claim. Everyone knows the "I Have a Dream" speech of Martin Luther King, Jr.
Introducing yourself in a presentation is pitching yourself to the audience so they stick around for the rest of your talk. Include your background, your unique trait, and who you are while sticking to the context in the first 30-60 seconds of your introduction. Your introduction should be effective and have an interesting hook.
CREATE THIS PRESENTATION How to start a presentation introduction. Presentations can be scary, I know. But even if stage fright hits, you can always fall back on a simple strategy. Just take a deep breath, introduce yourself and briefly explain the topic of your presentation. To grab attention at the start, try this opening line: Hello everyone.
This video distills 12 killer strategies to start your presentation and keep the audience's attention throughout. Quick Read. ... This introduction by game designer Jane McGonigal, for example, achieves a level of surprise by making a seemingly improbable assertion. After hearing this kind of statement, most people will want to listen to your ...
Introducing yourself in a presentation is pitching yourself to the audience so they stick around for the rest of your talk. Include your background, your uni...
Give rewards for participation. Prompt a discussion in the audience. Build in time for Q&A. Crowdsource questions from the audience. Gather feedback. 1. Plan your storyline. A powerful story can make your whole presentation. Take TED talks, for instance.
5 Steps to dazzle your audience. https://ruletheroompublicspeaking.com/public-speaking-video-library/Be better by tomorrow. Discover the secrets to giving a ...
Let's make the beginning of your presentation count. Here are some ways to start strong: Open with a question or ask the audience a question. Entertain with a great story, prop, or other visual to capture the audience's attention. Use humor, tell a joke, and show vulnerability. Showcase your passion for the topic and/or audience.
The process to create a killer presentation starts six to nine months before the event. That's right! A real killer presentation requires lots of planning, devising, rehearsing, and lots of fine tuning along the way. The actual task of transforming a presentation from muddled to mesmerizing is a matter of hours…spread over a longer period ...
The secret structure of great talks. From the "I have a dream" speech to Steve Jobs' iPhone launch, many great talks have a common structure that helps their message resonate with listeners. In this talk, presentation expert Nancy Duarte shares practical lessons on how to make a powerful call-to-action. 18:00.
How to create an engaging introduction. Consider using the tips below to engage your audience before your next presentation: 1. Tell your audience who you are. Introduce yourself, and then once your audience knows your name, tell them why they should listen to you. Example: "Good morning. My name is Miranda Booker, and I'm here today to ...
For more than 30 years, the TED conference series has presented enlightening talks that people enjoy watching. In this article, Anderson, TED's curator, shares five keys to great presentations: Frame your story (figure out where to start and where to end). Plan your delivery (decide whether to memorize your speech word for word or develop bullet points and then rehearse it-over and over). Work ...
Using these templates, you can transform your routine introduction into a powerful self-endorsement. Let's take a look at these templates one by one. Template 1: Introduce Yourself PowerPoint Presentation Slides. This template acts as a powerful tool for creating a powerful personal introduction. It includes a wide range of slides that can help ...
Apply the 10-20-30 rule. Apply the 10-20-30 presentation rule and keep it short, sweet and impactful! Stick to ten slides, deliver your presentation within 20 minutes and use a 30-point font to ensure clarity and focus. Less is more, and your audience will thank you for it! 9. Implement the 5-5-5 rule. Simplicity is key.
Here are a few tips for business professionals who want to move from being good speakers to great ones: be concise (the fewer words, the better); never use bullet points (photos and images paired ...
Outline the content and order of the presentation; This is the moment when you will punctuate the content of each part of the presentation: a) introduction, b) development, and c) conclusion. If you're going to use slides, it's time to score the content of each screen. With the topics of your presentation script set, it's time to assemble it.
How to craft a killer intro? how to start a presentation? or How to begin your presentation speech? How to sell a vision or sell a story to your prospective ...
Self Introduction PowerPoint Template by SlideModel. 1. Create a List of "Facts About Me". The easiest way to answer the "tell me about yourself" question is by having an array of facts you can easily fetch from your brain. When it comes to a full-length about me presentation, it's best to have a longer list ready.
A startup pitch deck is an essential fundraising tool for successful startups, whether you're looking to raise funding from $50,000, $500,000, or $50 million. However, an investor pitch deck is just one of the best pitch decks and examples we will share below. Despite the brevity of the successful startup pitch decks, which usually run for 10 ...
Premise. Interview with the Vampire centers on the life story of vampire Louis de Pointe du Lac, as told to veteran journalist Daniel Molloy, to whom he previously gave an unpublished interview in 1973.An affluent black man in the 1910s New Orleans, Louis is romanced and later made a vampire by the charismatic Lestat de Lioncourt.But Louis struggles with his humanity, and the introduction of ...
James Bay's gins are rated "dangerously smooth," and their Strait Up Killer Vodka is rated as "1 of the top 9 vodkas" in the USA by Wine Enthusiast. All spirits are available at the ...
Increasing evidence of brain-immune crosstalk raises expectations for the efficacy of novel immunotherapies in Alzheimer's disease (AD), but the lack of methods to examine brain tissues makes it difficult to evaluate therapeutics. Here, we investigated the changes in spatial transcriptomic signatures and brain cell types using the 10x Genomics Visium platform in immune-modulated AD models ...
Natural killer (NK) cells are cytotoxic innate immune cells that act as the first line of defence against tumours and microbial infections [].NK cells mediate anti-tumour immune responses via cell ...
The US Navy's AUTEC base gathers together world-leading experts and technology next to a natural wonder. The ranges off Andros Island - south-west of Nassau - are centred on a natural phenomenon, the Tongue of the Ocean, a huge deep-water bowl carved out of coral reef. It's 20 miles wide, 150 miles long, some 6,000ft deep in places and ...
3 Tips for How to Give a Killer PresentationWe'll start with the good news - fantastic presenters aren't born, they're made. So anyone can become a top publi...
The introduction of TNF-alpha (TNF-α) antagonists has brought about a significant change in the treatment of BU, leading to new therapeutic possibilities and a deeper understanding of the disease's pathogenesis. Over the last two decades, these inhibitors have become the primary approach in managing severe uveitis manifestations in BD.