Participating in Health Research Studies
What is health research.
- Is Health Research Safe?
- Is Health Research Right for Me?
- Types of Health Research
The term "health research," sometimes also called "medical research" or "clinical research," refers to research that is done to learn more about human health. Health research also aims to find better ways to prevent and treat disease. Health research is an important way to help improve the care and treatment of people worldwide.
Have you ever wondered how certain drugs can cure or help treat illness? For instance, you might have wondered how aspirin helps reduce pain. Well, health research begins with questions that have not been answered yet such as:
"Does a certain drug improve health?"
To gain more knowledge about illness and how the human body and mind work, volunteers can help researchers answer questions about health in studies of an illness. Studies might involve testing new drugs, vaccines, surgical procedures, or medical devices in clinical trials . For this reason, health research can involve known and unknown risks. To answer questions correctly, safely, and according to the best methods, researchers have detailed plans for the research and procedures that are part of any study. These procedures are called "protocols."
An example of a research protocol includes the process for determining participation in a study. A person might meet certain conditions, called "inclusion criteria," if they have the required characteristics for a study. A study on menopause may require participants to be female. On the other hand, a person might not be able to enroll in a study if they do not meet these criteria based on "exclusion criteria." A male may not be able to enroll in a study on menopause. These criteria are part of all research protocols. Study requirements are listed in the description of the study.

A Brief History
While a few studies of disease were done using a scientific approach as far back as the 14th Century, the era of modern health research started after World War II with early studies of antibiotics. Since then, health research and clinical trials have been essential for the development of more than 1,000 Food and Drug Administration (FDA) approved drugs. These drugs help treat infections, manage long term or chronic illness, and prolong the life of patients with cancer and HIV.
Sound research demands a clear consent process. Public knowledge of the potential abuses of medical research arose after the severe misconduct of research in Germany during World War II. This resulted in rules to ensure that volunteers freely agree, or give "consent," to any study they are involved in. To give consent, one should have clear knowledge about the study process explained by study staff. Additional safeguards for volunteers were also written in the Nuremberg Code and the Declaration of Helsinki .
New rules and regulations to protect research volunteers and to eliminate ethical violations have also been put in to place after the Tuskegee trial . In this unfortunate study, African American patients with syphilis were denied known treatment so that researchers could study the history of the illness. With these added protections, health research has brought new drugs and treatments to patients worldwide. Thus, health research has found cures to many diseases and helped manage many others.
Why is Health Research Important?
The development of new medical treatments and cures would not happen without health research and the active role of research volunteers. Behind every discovery of a new medicine and treatment are thousands of people who were involved in health research. Thanks to the advances in medical care and public health, we now live on average 10 years longer than in the 1960's and 20 years longer than in the 1930's. Without research, many diseases that can now be treated would cripple people or result in early death. New drugs, new ways to treat old and new illnesses, and new ways to prevent diseases in people at risk of developing them, can only result from health research.
Before health research was a part of health care, doctors would choose medical treatments based on their best guesses, and they were often wrong. Now, health research takes the guesswork out. In fact, the Food and Drug Administration (FDA) requires that all new medicines are fully tested before doctors can prescribe them. Many things that we now take for granted are the result of medical studies that have been done in the past. For instance, blood pressure pills, vaccines to prevent infectious diseases, transplant surgery, and chemotherapy are all the result of research.
Medical research often seems much like standard medical care, but it has a distinct goal. Medical care is the way that your doctors treat your illness or injury. Its only purpose is to make you feel better and you receive direct benefits. On the other hand, medical research studies are done to learn about and to improve current treatments. We all benefit from the new knowledge that is gained in the form of new drugs, vaccines, medical devices (such as pacemakers) and surgeries. However, it is crucial to know that volunteers do not always receive any direct benefits from being in a study. It is not known if the treatment or drug being studied is better, the same, or even worse than what is now used. If this was known, there would be no need for any medical studies.
- Next: Is Health Research Safe? >>
- Last Updated: May 27, 2020 3:05 PM
- URL: https://guides.library.harvard.edu/healthresearch
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Impact of nih research.
NIH-funded research generated about $96.84 billion in economic activity in FY22.

NIH works to turn scientific discoveries into better health for all. As the largest public funder of biomedical and behavioral research in the world, NIH is the driving force behind decades of advances that improve health , revolutionize science , and serve society more broadly.
Evidence of the varied, long-term impacts of NIH activities comes from a variety of sources, ranging from studies on specific health topics, to broader analyses of NIH as a whole. Explore the sections below to discover more about how NIH provides value for the public’s investment.

Improving Health
Discoveries emerging from NIH-supported research have led to new ways to prevent, diagnose, and treat illness, ultimately improving the health of the nation and the world.

Revolutionizing Science
NIH fuels the biomedical research enterprise—cultivating world-class scientists and catalyzing new scientific fields, tools, and resources that have changed how science is done.

Serving Society
NIH-supported research leads to improvements in health that can bolster the economy, improve productivity, and reduce the costly burden of illness. NIH funding also supports jobs and innovations that advance the biotechnology sector.
asthma-case-study-spread-screenshot.jpg

Our Stories
The knowledge produced by NIH-supported research can take many years and pass through many stages on its pathway toward improving health. Explore stories of how NIH has contributed to successful health interventions, and how these interventions have made a difference in our lives.
Learn More About NIH
- Research Matters — weekly update of recent NIH research highlights
- NIH RePORT — reports, data, and analyses of NIH research activities
- NIH Clinical Trials — overview of NIH-funded clinical trials and how you can participate
- Celebration of Science: NIH Highlights — spotlighting remarkable advances from NIH-supported scientists
- NIH Home Page — learn how NIH and its 27 institutes and centers work
- NIH Director’s blog — NIH Director Dr. Monica Bertagnolli discusses new discoveries and latest trends in biomedical research and medicine
- NIH Catalyst — bimonthly NIH newsletter that showcases the scientific research conducted at the NIH
- NIH Record — biweekly NIH newsletter that covers issues of significance to NIH staff, contractors, and trainees
- NIH History Office & Stetten Museum — discover the scientific, legislative, and social history of the National Institutes of Health
NIH Research News
20240507-prostate.jpg.

Urine test identifies high-risk prostate cancers
May 7, 2024 — Researchers developed a urine-based test that can distinguish between slow-growing prostate cancers that pose little risk and more aggressive cancers that need treatment.
20240507-mosquito.jpg

Antibody reduces risk of malaria in children
May 7, 2024 — A single dose of a monoclonal antibody given to children 6 to 10 years of age in Mali proved up to 77% effective at preventing malaria disease.
20240507-knee.jpg

Blood biomarkers for knee osteoarthritis
May 7, 2024 — Researchers found biomarkers in blood that may predict knee osteoarthritis up to eight years before clinical diagnosis.
Connect with Us
- More Social Media from NIH
- Interesting MedStuff
- International News
- Medical Devices
- Social Media
- Life Experiences
- Student Articles
- Student Tips
- Clinical Medicine
- Clinical Procedures
- Clinical Surgery
- Guidelines & Protocols
- Publisher Square
- Smartphone Apps
- Aesthetic Medicine
- Beauty & Cosmetics
- Elderly Health
- Kid’s Health
- Medical Tips for Daily Life
- Men’s Health
- Mental Health
- Women’s Health
- Subscription Options
- Partners & Supporters
- Publishing Guidelines
- Medicalopedia Citations
- DMCA Notice
- Privacy Policy

Technology Meets Health: 7 Core Benefits of Telehealth

Virginia Beach Chiropractor Offers Free Consultation Coupon on Website

How To Use Synthetic Urine For A Drug Test

Ease Your Pain With Regenerative Medicine

How to Minimize the Risk of an Injury During a Car…

- Medical News
Why Is Medical Research Important?
Medical research has become an important part of the health care industry, and advances in technology have made it possible for much of it to be done on an outpatient basis, meaning that investigators sometimes don’t need to do extensive studies in a research facility. The field of medical research is one of the most interesting fields in all of science because of its focus on science and medicine in conjunction with a great deal of research.
Medical research covers a wide variety of studies, stretching from ‘baseline’ investigation, through systematic reviews and to the cutting-edge of medical science. It involves the study of all human diseases or may have only disease-specific research. For example, AIDS research includes both studying patients who have AIDS and those who do not, as well as studying children with AIDS and children without AIDS. Similarly, researchers may be investigating the causes of Parkinson’s disease in old age and Parkinson’s disease in young adulthood.
4 Phases of Medical Research Studies
The four phases of Medical Research Studies are experimental, comparative/expository, understudy, and last, analysis and validation/regression.
- Comparative/expository medical research compares experimental and comparative samples from which the study population is developed; compare post hoc comparisons with the initial data; evaluates associations among variables measured.
- Understudy studies consist of data from observational studies and random chance sampling.
- Experimental refers to clinical trials that are done specifically to test a new medical product, device, or technique.
- Finally, validation/ regression Research studies compare new designs or drugs to earlier designs and evaluate their effect on any association found.
There are many reasons why medical research is so valuable. Whether you aim to start a career in this field or to gain more knowledge about health conditions and their treatments, it’s important to understand the benefits that medical research provides. You can learn about medical research at http://hrmdresearch.com/ .
The Importance Of Medical Research
The breakthroughs that people enjoy today are virtually unimaginable without the knowledge gained through medical research. Here are some of the most important reasons medical research is important:
- Generate Valuable Insights
Medical research helps people learn more about themselves and their health. The knowledge gained by medical research is constantly improving.
- With new scientific information coming from medical studies, people will be able to take care of their health and well-being more effectively.
- It also seeks to understand the reasons for diseases, to discover new methods of preventing or controlling diseases, and to develop treatments for these diseases and their effects.
2. Development Of New Drugs
Medical research must be done to find a cure for diseases and illnesses. Without medical research, medicine and other medical innovations as we know it could not exist. Sometimes called pharmaceutical research, medical research encompasses a broad spectrum of scientific studies. It starts with the research and development of drugs, followed by treatments and procedures used in clinical practice.
The process of new drug development may involve the following steps:
- It can be done in several different ways, including doing laboratory experiments in bioresources such as blood and cells, or cell culture, to studying the effects of chronic exposure to toxins, drugs, hormones, and other compounds in the environment.
- Other research is directed toward understanding disease mechanisms, to find better ways of treating or preventing disease, and how disease progression is influenced by environmental factors.
3. Improve The Quality Of Life
Medical researchers don’t just look for ways to manage the symptoms of diseases, they try their best to find a cure for a specific illness or a group of diseases. There are several ways drug research and testing improve the quality of life:
- Medical studies that aid in the development and administration of vaccines allow people to live without worrying about deadly diseases. An example will be the ongoing development and clinical trials for the COVID-19 vaccine which may help the world go back to normal.
- Some people can live with their conditions for years by understanding how to manage them properly. As time goes by, everyone is also learning how to prevent certain illnesses from occurring and even eliminate them.
Why is medical research so important? Medical research saves lives every day. Scientists and researchers work day and night to develop new treatments, drugs, and procedures. Without the help of dedicated scientists, doctors, and other medical professionals, the advances made would be slow and limited.
When a patient participates in a trial, they must undergo several physical tests and provide some information about their lifestyle and diet. The findings and insights derived from these studies and trials are invaluable in coming up with treatments and cures for various health conditions.
RELATED ARTICLES MORE FROM AUTHOR

Liraglutide for Weight Loss: An Overview

The Impact of Consolidation: Healthcare Mergers and Acquisitions Explained

Importance of Peptides In Therapeutics

Now Get Freedom from Snoring With Somnomed Sleep Oral Sleep Apnea...

Anesthesiology: The Myths and Realities

The Role of Science Publishers in Advancing Scientific Knowledge
- Research Note
- Open access
- Published: 13 October 2023
“Publish or Perish”: barriers to research publication in an undergraduate medical research program
- Abdulrahman F Alsulami 1 , 2 ,
- Zeyad O Khaimi 1 , 2 ,
- Mohammed A Hadi 1 , 2 ,
- Yazeed H Aljabri 1 , 2 ,
- Talha S Mayet 1 , 2 &
- Alaa Althubaiti 1 , 2
BMC Research Notes volume 16 , Article number: 269 ( 2023 ) Cite this article
861 Accesses
3 Altmetric
Metrics details
Publication is one of the crucial parameters in research, and the inability to publish has been noted in many medical students’ projects due to different reasons. This cross-sectional study aimed to determine the obstacles that prevented medical students in a health science university from publishing their research from 2018 to 2021. First, an online survey was distributed to assess the obstacles to publication perceived by the medical students. Second, a total of 81 research projects were evaluated by scientific reviewers and their final decision about the publication was recorded.
In total, 162 students filled out the survey. The barriers faced by the students were various. They included an unsupportive research supervisor, a lack of time, an insufficient sample size, and many others. In the reviewer’s evaluation, out of 81 projects, 70 projects (86.4%) were recommended to be published after minor or major modifications, while 11 projects (13.6%) were rejected due to poor writing style, poor results interpretation, and incorrect methodology.
Articulating the barriers to undergraduate medical research publication is important in boosting publication rates and research experience of graduating medical students. Medical research educators and research supervisors should strongly consider creating a framework that tackles existing obstacles and any future matters.
Peer Review reports
Introduction
Research is essential for the advancement of medicine and the improvement of healthcare. It is also crucial to the practice of evidence-based medicine [ 1 , 2 , 3 , 4 ]. Medical students play a vital role in the published output and research productivity of any institution [ 2 , 3 ]. For example, medical students largely contributed to the publications of one German institution, as 28% of the output was authored by students [ 5 ]. In addition, exposing medical students to research activities is strongly linked to developing positive attitudes towards research and a tendency to be involved in academic careers later in life. Such exposure is found to be a valuable method to promote health research in the long-term [ 3 ]. Even if students do not later pursue a career in research, research experience has been shown to provide students with teamwork skills, knowledge about critical appraisal of literature, and writing skills [ 2 , 3 ]. Moreover, after writing a paper, nothing motivates students more than getting their work published. Nowadays, fierce competition among graduating medical students creates challenges in securing a position in a decent residency program [ 3 ]. One approach used for evaluating doctors is assessing their publishing practices and research skills as not all students can publish their research [ 2 , 6 ].
Publication rates
Previous research investigating publication rates and obstacles is limited and shows variable results [ 2 , 6 , 7 , 8 ]. In one study targeting the medical college of Chennai, 17.4% of the students had their research published [ 7 ]. Another British study found that only 14% of students submitted their papers for publication, mainly attributing this small number to a lack of opportunity to conduct a study and a lack of time [ 2 ]. Limited research is done locally. A study performed at a local university targeting medical interns resulted in a low rate of student publication, at 3.2%, ascribing it to a lack of support during data analysis, manuscript writing, and financial support [ 6 ]. This local percentage is low in comparison with another study conducted in Peru, in which 17.6% of theses were published [ 8 ].
Largely, the challenges facing medical students in publishing research remain unexplored, which creates a need for further investigations regarding the rates and obstacles of publication, especially in local institutions. This is likely to increase students’ awareness about the challenges that could decrease their chances of publication.
The medicine program at the College of Medicine at King Saud bin Abdulaziz University for Health Sciences (KSAU-HS) is internationally accredited and aims to enhance the publication trends of undergraduate medical students. As part of the program, students undergo a medical research course for two years where they can select research team members, determine a research topic, and choose a supervisor to provide guidance throughout the course [ 9 ]. All of which is facilitated by a panel of research assistants and coordinators. Monitoring publication results is done to enhance the quality of the program. In the campus, the research program has been implemented for four times and has led to a total of 80 publications (i.e., 15% of the total research projects are published).
This study aims primarily at identifying the barriers influencing the publication of research conducted by medical students and presents potential ways to improve outcomes.
Materials and methods
Sample, setting, and data collection.
The study was conducted in the College of Medicine at KSAU-HS. Secondary and primary data collection methods were utilized. In the secondary data collection approach, the database of students’ research conducted under the medical research course was retrieved from the College. The variables collected were the number of research groups, and the reports of the instructors’ evaluations.
For the survey distribution, a convenience sampling method was used to select participants. All research students that enrolled in the medical research course from 2018 to 2021, (i.e. 4th, 5th, and 6th year medical students) were invited to participate in the study. The survey was developed for this study and can be found in Supplement file 1. Variables in the survey included sex, the topic of the research, and perceived obstacles.
Data analysis
Descriptive analysis was used to describe the data. Percentages were used to present categorical variables. To determine the challenges faced by medical students in publication, a multiple-response analysis was used. The percent of cases was reported and determined by dividing each count by the total number of cases or respondents choosing an obstacle. Statistical analysis was performed using JMP Pro 14 software (JMP, Pro 14; SAS Institute Inc, Cary, NC, 1989–2019).
Participant characteristics
A total of 162 out of 545 students participated in the study reporting the obstacles faced when attempting to publish their research, through the self-administered survey (with a response rate of approximately 30%). Each participant was able to choose multiple answers. The number of male responses was 93 (57.4%), and the number of female responses was 69 (42.6%). The current GPA of the students, which is out of 5, varied with the majority of 133 (82.1%) participants scoring between 4.5 and 5. There were 20 (12.3%) participants whose GPA was between 4 and 4.49. In addition, there were eight (4.9%) participants whose GPA was between 3.5 and 3.99. There was a single (0.6%) participant with a GPA that was less than 2.5. Examples of current research topics include medicine (25.9%), oncology (13.6%), surgery (10.5%), and medical education (4.9%).
Most students used cross-sectional study designs (n = 91, 56.1%), or cohort study designs (n = 55, 34%). In addition, other study designs were also used; these were randomized clinical trials (5.6%), case controls (3.1%), systematic review/meta-analysis (0.6%), and case reports/series (0.6%).
Obstacles faced in research publication
Out of 162 students, a total of 18 students reported no obstacles, and 144 students reported at least one obstacle (the total responses to the obstacles question was 315). Figure 1 shows the distribution of obstacles among the 144 students, expressed as the percent of cases. As shown in Fig. 1, a total of 63 participants (38.9%) perceived that an unsupportive research supervisor was a barrier to publishing their research. Lack of time had a total of 50 responses (30.9%) and the inability to reconcile between research and studying had 37 responses (22.8%), representing a large part of the problem that students face. Insufficient sample size was reported by 41 participants (25.3%) and was the third most significant factor.
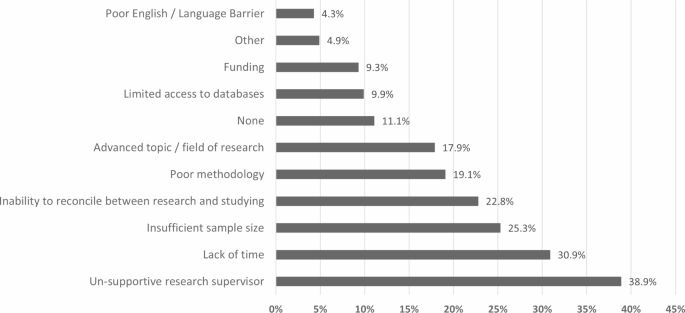
Percentages of responses for obstacles faced in publishing medical research as perceived by students in undergraduate medical research program
Poor methodology and choice of an advanced topic or field of research had a small gap between them with 31 responses (19.1%) and 29 responses (17.9%), respectively. Limited access to data, for example, incomplete or unavailable electronic medical records, was reported by 16 students (9.9%), while funding was reported by 15 students (9.3%) and poor English was reported by 7 students (4.3%). Moreover, 4.9% (n = 8) of participants reported other factors. Students were required to elaborate if “other” was chosen, using an open-question format. Their answers were “uncooperative team members” (n = 2), “having difficulties with journals’ websites”, “problems with the results conducted”, “further editing of manuscript”, “bad documentation within old physical record”, and “wanting to publish their work in higher-impact journals”.
Opinions of reviewers on manuscripts’ publishability
Over the two cohorts that started the medical research program in 2019 and 2020, a total of 93 projects were implemented. All research manuscripts were evaluated by two research reviewers from the College Research Committee. Each research group received feedback from both. To evaluate the research manuscript’s publishability, instructors in the program have added a new evaluation criterion in addition to the graded evaluation of the manuscript. All manuscripts were screened and grouped into three categories of recommendations: recommend publication after minor modification, recommend publication after major modification, and not publishable. This was added to monitor the quality of research and address the reasons. In addition, the program’s instructors considered the final evaluation on publishability to be of the wary reviewer to insure necessary feedback to the students.
The summary results of publishability are shown in Fig. 2 . Out of the 93 projects, 12 projects had no complete evaluations, were published, or were submitted for publication. They were therefore excluded from the analysis. Results showed that out of the 81 projects, 13.6% (n = 11) of the projects were seen as not publishable by a reviewer, 42% (n = 34) could be published after major modifications, and 44.4% (n = 36) were publishable after minor modifications. For the not publishable projects, the main reasons indicated by reviewers were poor writing styles, poor results interpretation, poor English language (n = 9), and insufficient sample size/incomplete data collection (n = 2), indicating an incorrect methodology. In addition, manuscripts with major modifications needed additional time and effort from the investigators. If this was not accomplished, it was likely that upon submission to the journal, the research manuscript would be rejected and would not be publishable [ 10 ]. Addressing the common reasons that prevent manuscripts from publication is important at the early stage of the medical research process.
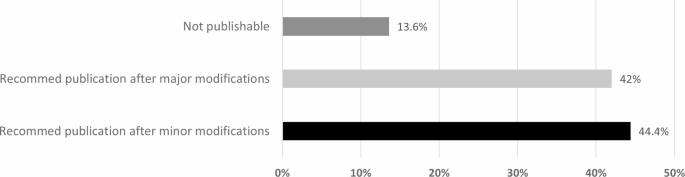
Opinion of reviewers on research manuscripts’ publishability at the end of medical research program (n = 81 research manuscripts)
Identifying the obstacles preventing students from publishing their work is crucial for the educative process and scientific advancement. This study is one of a limited number of research papers discussing and acknowledging these barriers in undergraduate medical curricula and particularly in Saudi Arabian universities [ 4 , 6 ].
The difference in publication rates over the years, and between the different universities, is attributed to different factors. In our study, the most frequent obstacle was having an unsupportive research supervisor. This could be because most supervisors are full-time physicians and university lecturers. This means that research programs are an added responsibility for them. This seems to be more common than expected in previously mentioned studies. Additionally, a study in Government Medical College, Nagpur, India, amongst 156 medical students, suggested that 68% of these students faced a lack of proper guidance as an obstacle [ 11 ].
As anticipated, lack of time and the inability to reconcile research and studying represent a large part of the problem that students face. It was predicted that a significant number of students would report not having enough time to balance medical school and the research publication process. Supporting these findings, a paper conducted by Giri et al. [ 12 ] in India showed that 59.5% of the students of the Pravara Institute of Medical Sciences also reported a lack of time due to the curriculum as a barrier in conducting a study. Lack of time was also reported as the main obstacle in a Malaysian study [ 13 ].
Insufficient sample size, poor methodology, and choice of an advanced topic or field of research were also important factors. This could be the result of starting the research curriculum immediately after joining the college of medicine, meaning that students may not be knowledgeable enough to start the research process. Poor English was also reported as an obstacle, although the students took 24 h of English courses involving reading, communication skills (i.e. writing and oral skills), and grammar before joining the college.
One of the interesting obstacles reported was problems with results preparation after data gathering. This factor is important because it is one of the fundamental skills of a researcher. Furthermore, KSAU-HS has a set mandatory biostatistics course for all students before they enter the college of medicine. In addition, as part of the research program, students undergo descriptive and inferential statistics classes in addition to practical hands-on sessions. Research conducted in similar settings enrolled 327 students to assess attitudes toward statistics in medical research. These findings suggest that although students can see the benefit of statistics in their professional life, they carry neutral to negative attitudes towards this area [ 14 ]. These attitudes may have impacted the willingness of students to develop the results section of their manuscript.
Current recommendations in the literature include providing workshops and courses for research methodology and the publication ethics and process, which is already covered in KSAU-HS as part of the mandatory research curriculum and other activities [ 4 , 10 ]. Therefore, the obstacles that must be tackled depend on students’ implementation of proper perceived solutions after they have been addressed, such as cultivating time management skills, working with a cooperative supervisor, and improving the required biostatistical skills to plan a proper methodology [ 6 , 14 , 15 , 16 ].
Limitations
The study findings presented may be insightful for undergraduate medical students from the two cities Jeddah and Riyadh KSAU-HS campuses, as the same research curriculum is set for both campuses. However, results cannot be generalized to all research programs as every college has its own curriculum and a different approach towards research [ 17 ].
For future work, differences in publication rates and obstacles reported by gender, and other factors related to students’ demographics, could be investigated. In addition, due to the COVID-19 pandemic, the teaching of the medical research course has shifted to an online delivery format for three years. It would be interesting to examine the obstacles faced by students in conducting their research and publishing the final manuscript using online learning.
Overall, medical students reported experiencing numerous obstacles that affect their chances of publishing papers, especially having an unsupportive supervisor, lack of time, and facing issues with sample size. Existing research shows varying and unsatisfying results reflecting the need for deeper exploration. Further research is required to provide a clearer image and solutions that are practical and would aid in overcoming these barriers to improve students’ research conduction and quality.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
King Saud bin Abdulaziz University for Health Sciences
Rosenberg WMC, Sackett DL. On the need for evidence-based medicine. J Public Heal Med. 1995;17:330–4. https://www.jstor.org/stable/45160659 . Accessed 2 Oct 2022.
Griffin MF, Hindocha S. Publication practices of medical students at british medical schools: experience, attitudes and barriers to publish. Med Teach. 2011;33. https://doi.org/10.3109/0142159X.2011.530320 .
Aslam F, Shakir M, Qayyum MA. Why medical students are crucial to the future of Research in South Asia. PLoS Med. 2005;2:1110–1. https://doi.org/10.1371/JOURNAL.PMED.0020322 .
Article Google Scholar
Alsaleem SA, Alkhairi MAY, Alzahrani MAA, Alwadai MI, Alqahtani SSA, Alaseri YFY, et al. Challenges and Barriers toward Medical Research among Medical and Dental students at King Khalid University, Abha, Kingdom of Saudi Arabia. Front Public Heal. 2021;9. https://doi.org/10.3389/FPUBH.2021.706778 .
Cursiefen C, Altunbas A. Contribution of medical student research to the MedlineTM-indexed publications of a german medical faculty. Med Educ. 1998;32:439–40. https://doi.org/10.1046/J.1365-2923.1998.00255.X .
Article CAS PubMed Google Scholar
Alsayed N, Eldeek B, Tayeb S, Ayuob N, Al-Harbi A. Research practices and publication obstacles among interns at King Abdulaziz University Hospital, Jeddah, Saudi Arabia, 2011–2012. J Egypt Public Health Assoc. 2012;87:64–70. https://doi.org/10.1097/01.EPX.0000417978.44502.61 .
Article PubMed Google Scholar
Chellaiyan VG, Manoharan A, Jasmine M, Liaquathali F. Medical research: perception and barriers to its practice among medical school students of Chennai. J Educ Health Promot. 2019;8. https://doi.org/10.4103/JEHP.JEHP_464_18 .
Arriola-Quiroz I, Curioso WH, Cruz-Encarnacion M, Gayoso O. Characteristics and publication patterns of theses from a peruvian medical school. Health Info Libr J. 2010;27:148–54. https://doi.org/10.1111/J.1471-1842.2010.00878.X .
Althubaiti A. Undergraduate Medical Research Programme: a cross-sectional study of students’ Satisfactions, Perceived Challenges, and attitudes. Glob J Health Sci. 2015;7:117. https://doi.org/10.5539/GJHS.V7N5P117 .
Article PubMed PubMed Central Google Scholar
Menon V, Varadharajan N, Praharaj SK, Ameen S. Why do manuscripts get rejected? A content analysis of rejection reports from the Indian Journal of Psychological Medicine. Indian J Psychol Med. 2022;44:59–65. https://doi.org/10.1177/0253717620965845 .
Sharma SK, Thatikonda N, Uttamrao Ukey U, Knowledge. Attitude, practice and barriers for research amongst medical students of GMC, Nagpur. J Res Med Dent Sci. 2021;9. www.jrmds.in. Accessed 2 Oct 2022.
Giri P, Bangal V, Phalke D, Knowledge. Attitude and Practices towards Medical Research amongst the Postgraduate students of Pravara Institute of Medical Sciences University of Central India. J Fam Med Prim care. 2014;3:22. https://doi.org/10.4103/2249-4863.130263 .
Soe HHK, Than NN, Lwin H, Htay MNNN, Phyu KL, Abas AL. Knowledge, attitudes, and barriers toward research: the perspectives of undergraduate medical and dental students. J Educ Health Promot. 2018;7:23. https://doi.org/10.4103/JEHP.JEHP_61_17 .
Althubaiti A. Attitudes of Medical Students toward Statistics in Medical Research: evidence from Saudi Arabia. J Stat Data Sci Educ. 2021;29:115–21. https://doi.org/10.1080/10691898.2020.1850220 .
Althubaiti A, Al Muqbil B, Al Buraikan D. Assessment of Medical Students’ attitudes towards Research and Perceived Barriers. Int J Med Students. 2017;5:95–8. https://doi.org/10.5195/ijms.2017.28 . Accessed 4 Oct 2022.
Althubaiti A, Althubaiti SM. Medical research: what to expect in a student–supervisor relationship. BMC Med Educ. 2022;22:1–8. https://doi.org/10.1186/S12909-022-03851-4 .
Althubaiti A. Sample size determination: a practical guide for health researchers. J Gen Fam Med. 2022;24:72–8. https://doi.org/10.1002/JGF2.600 .
Download references
Acknowledgements
The authors acknowledge the respondents for participating in the study and would like to thank the Research Unit’s assistants at the College of Medicine, KSAU-HS for administrative support and secondary data collection.
Not Applicable.
Author information
Authors and affiliations.
College of Medicine, King Saud bin Abdulaziz University for Health Sciences, P.O. Box 9515, Jeddah, 6656, 21423, Saudi Arabia
Abdulrahman F Alsulami, Zeyad O Khaimi, Mohammed A Hadi, Yazeed H Aljabri, Talha S Mayet & Alaa Althubaiti
King Abdullah International Medical Research Centre, Jeddah, Saudi Arabia
You can also search for this author in PubMed Google Scholar
Contributions
AFA designed and carried out the study, reviewed the statistical analysis, wrote and drafted the manuscript, prepared and distributed the survey. ZOK designed and carried out the study, wrote, reviewed, and edited the manuscript. MAH designed and carried out the study, wrote, reviewed, and edited the manuscript. YHA designed, reviewed, and edited the manuscript. TSM designed, reviewed, edited the manuscript, and distributed the survey. AA performed the statistical analysis, wrote, reviewed, and edited the manuscript. All research authors read and approved the final manuscript.

Corresponding author
Correspondence to Alaa Althubaiti .
Ethics declarations
Ethics approval and consent to participate.
The study was approved by the concerned Ethical committee “Institutional Review Board (IRB)” at King Abdullah International Medical Research Centre (IRB approval number: IRB/0073/22). An informed consent was obtained from participants. All methods were performed in accordance with the relevant guidelines and regulations. The confidentiality and anonymity of participants were completely preserved, and only the research team had access to the data.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Alsulami, A.F., Khaimi, Z.O., Hadi, M.A. et al. “Publish or Perish”: barriers to research publication in an undergraduate medical research program. BMC Res Notes 16 , 269 (2023). https://doi.org/10.1186/s13104-023-06542-5
Download citation
Received : 13 November 2022
Accepted : 27 September 2023
Published : 13 October 2023
DOI : https://doi.org/10.1186/s13104-023-06542-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Medical research
- Undergraduate
- Publication
BMC Research Notes
ISSN: 1756-0500
- Submission enquiries: [email protected]
- General enquiries: [email protected]

Why Medical Research is Important

The high quality of medical care we enjoy today is built upon years of effort by physicians, physician-scientists, PhDs, and other medical professionals investigating the causes of and potential treatments for disease. The tireless effort of these professionals has made many once life-threatening diseases and conditions just a memory.
However, there is still much work to be done. Insights provided by medical research today promise to lessen the impact of our greatest health problems, including diabetes, cancer, and heart disease. As science continues to unravel the molecular workings that underpin disease, we will see profound changes in the approach to treatments.
HHRI researchers are at the forefront in this revolution in medical knowledge. To capitalize on the advances that have been made in our understanding of disease, a majority of our research projects are translational in nature. Translational medical research seeks to take the medical discoveries that have been made in a laboratory setting and move them into medical practices that can be used by physicians to improve the lives of their patients.
When you support medical research, you are helping to build the future of medicine. With the partnership of individual and corporate donors, we are able to make significant headway in advancing medical knowledge and improving patient care.
Our philanthropic partner, the Hennepin Healthcare Foundation (HHF), offers many ways you can support medical research. HHF supports the mission and goals of HHRI and HCMC (the flagship acute care and teaching hospital of the Hennepin Healthcare System) and provides an avenue for individuals and organizations to support our programs and services.
While the predominant source of our research dollars come from federal grants and industry contracts, individual contributions play an important role in allowing HHRI to continue the important work it does to improve patient care. If you would like to support our research programs, contact:
Chad Boysen Director of Development Hennepin Healthcare Foundation
[email protected] 612-873-2217
- R&E Practice
Why should practitioners publish their research in journals?

Annette N. Brown
My team recently encouraged a colleague who is collecting and analyzing data in one of our projects to submit the analysis and results to a journal. His response was “why?” In our first attempt to answer his question, we pointed him to David Evans’ recent post about why people might want to submit short (economics) papers as “letters” to journals that publish letters. David says, “If you believe there is value in expanding the audience, in the quality improvement, and if the citations are useful for you professionally, then it may be worthwhile.”
My colleague argued that his audience is not reading journals, that there are other mechanisms for getting feedback, and that since he is a technical person and not a researcher, he doesn’t need citations. He believes publishing in other ways is equally valued. In other words, he wasn’t convinced.*
This post is my attempt to convince him, and you, that practitioners who are conducting research should publish in journals.
Before giving you my reasons, I think it is important to define what I mean by research. I have in mind a slightly altered version of the US Department for Health and Human Services definition . Mine is: a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to knowledge. My alteration is that I deleted the word “generalizable” before knowledge. To me, a contribution to knowledge means it needs to be more than just information about a specific project useful only to those working on that project. But I’ve found that people can get caught up in what the requirements are for “generalizability” or “externally validity”, so I am avoiding those more loaded terms here. My interest here is when people carefully analyze data or other information to produce new knowledge that can be helpful to others.
So why should practitioners go to the effort of submitting an article to a journal instead of just self-publishing and writing a blog post about it? I see three reasons: because it increases the credibility of the research, it increases the credibility of the practitioner, and it puts the knowledge in the permanent, searchable record. Spoiler alert: I think the third is the most important.
We know that the US Agency for International Development (USAID) and other funders place a premium on evidence from peer-reviewed journals. From my own experience producing evidence products for USAID (see for example the work on STIP ), I know that many at USAID do look for whether evidence is from a peer-reviewed journal. In fact, USAID launched its own peer-reviewed journal, Global Health: Science and Practice to make this medium more accessible to both the producers and users of research. Here is a statement from the Gates Foundation highlighting the importance of peer-reviewed publishing.
It is a reasonable question whether the process of publishing in a peer-reviewed journal indeed improves the quality of the research. Many have debated this question (for example here ). I personally do not believe that peer review guarantees the quality of the research published in journals, which is why I think that replication is so important . But I firmly believe that the average quality of studies published in peer-reviewed journals is higher than the average quality of studies published elsewhere, even if those published elsewhere are subject to some kind of peer review. You can find empirical evidence to that effect by looking at systematic reviews that do risk-of-bias assessments on included studies that come from various publication sources.
My claim about the relationship between journal publication and quality is a claim of correlation not necessarily causality. Submission to a peer-reviewed journal is an important signal, both that the researcher feels the work is of a standard to be published in a journal and that she is willing to undergo rigorous external peer review. So even if the peer review process itself doesn’t improve the quality (although more often than not it does), the work that was done to get the manuscript journal-ready probably did.
The USAID Bureau for Global Health commissioned this guide on peer-reviewed publishing, which encourages its own staff to publish and states, “One way to show and develop technical leadership is by publishing, especially in peer-reviewed journals.”
Many of you who have made it this far in the post are asking (perhaps emphatically) “if USAID cares so much about journal publication, why do they refuse to pay for it??” Good question. Very good question. It is definitely true that many USAID projects—even some with research as a primary component—do not support journal publication. I think the concepts of time inconsistency and public goods help us to understand this, but I’ll leave that discussion for another post. What I’ll say here is, we need to keep gently encouraging USAID to let their projects contribute credible research to the knowledge base upon which they themselves rely.
To make my third point, about the permanence of journal articles, I’ll start with an anecdote. A few weeks ago, we celebrated the milestone of having more than 10 posts in our new R&E Search for Evidence blog. As I opened the web page to post number 11 and then scrolled backward through the earlier posts, I saw that the post number 1 had disappeared! In turns out the blog was initially programmed to include only 10 posts at a time. We have fixed this, but it drove home the point that blog posts can be ephemeral.
I will concede one point to my colleague: citations are not such a big deal to technical specialists. Citations reflect whether other researchers are using your research when they write journal articles. If the objective of your research is to inform policy or practice directly, a journal article summarized in a blog post or policy brief may have a big impact without garnering a lot of citations. I do know that people reviewing CVs pay some attention to the quality of journals in which candidates publish, but I have never heard of a case where someone reviewing CVs for a technical position has looked up the number of citations that the candidate has.
Not all practitioners conduct research, so I am not arguing that all practitioners should be publishing in journals. But there are many times when technical specialists do conduct research as part of their project work. Ideally, that would happen more often than it already does, as the need to learn is infinite and our projects are a better source of data than we realize. It is true that publishing in journals is not easy and takes time (another topic for another post) so it is not always feasible. But I think practitioners can and should be publishing in journals more than they currently do. *In fairness to David, he was not making a case for publishing overall, just for publishing letters, so we misapplied his post a bit in trying to make the initial case to our colleague.
Photo credit: ksandrphoto/Freepik
Sharing is caring!
Related Posts

How to find the journal that is just right
A pathway for sampling success

Turning lemons into lemonade, and then drinking it: Rigorous evaluation under challenging conditions
Stay up to date
Never miss an email
- Monthly summaries only
Our use of cookies
Privacy overview.
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- JME Commentaries
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 44, Issue 10
- Should all medical research be published? The moral responsibility of medical journal editors
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0002-3693-0547 Thomas Ploug
- Centre for Applied Ethics and Philosophy of Science, Department of Communication , Aalborg University Copenhagen , København , Denmark
- Correspondence to Professor Thomas Ploug, Centre for Applied Ethics and Philosophy of Science, Department of Communication, Aalborg University Copenhagen, København S 2450, Denmark; ploug{at}hum.aau.dk
This article reinvigorates a key question in publication ethics: Is there research that it is permissible to conduct but that ought not to be published? The article raises the question in relation to two recent medical studies. It is argued (1) that the publication of these studies may cause significant harm to individuals, (2) that editors of medical journals have a moral responsibility for such harm, (3) that denial of publication is inadequate as an instrument to fulfil this moral responsibility and (4) that internationally acknowledged publication ethics codes should incorporate this aspect of editors’ moral responsibility.
- publication ethics
- research ethics
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/medethics-2018-104785
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Introduction
A recent Danish study found that symptoms of a widespread chronic disease could be alleviated by the use of antibiotics. Getting the study published turned out to be difficult. At least one reviewer from a reputable journal noted that the study could influence the clinical practice in an undesirable way by leading to an increased use of antibiotics and thus ultimately adding to the problem of microbial multiresistance. The BMJ recently published an article suggesting that side-effects of statins may outweigh health benefits in patients at low and intermediate risk of cardiovascular disease. 1 The article sparked intense debate 2 —not least in the mass media 3–7 —and one study suggests that as many as 200 000 people in the UK have stopped taking statins as a consequence of the media coverage, potentially leading to 2000 cardiovascular events in the future. 8–10
The two cases reinvigorate but also add important new aspects to a contested topic in publication ethics, namely whether there is medical research that is permissible to conduct but that ought not to be published.
This question is not a new one. 11 Previous considerations have, however, focused almost exclusively on cases in which medical research may be used for biological warfare and bioterrorism. 11–14 The threat of bioterrorism led a group of journal editors and authors to issue a statement in Science and Nature in 2003. In this statement, they recognised that the potential harm of publication occasionally may outweigh the societal benefits and that in such cases, ‘the paper should be modified, or not be published’. 15 However, the moral responsibility of editors and reviewers to consider the potential harmful effects of publishing research is not included in two of the main publication ethics codes, the Committee on Publication Ethics’ (COPE) Code of Conduct and the International Committee of Medical Journal Editors’ (ICMJE) Recommendations. 16 17 The World Association of Medical Editors’ (WAME) states that medical editors, ‘should also take into account whether studies are ethical and whether their publication might cause harm to readers or to the public interest’, 18 but this requirement only figures in their policy statement entitled ‘Geopolitical Intrusion on Editorial Decisions’ dealing with editorial influence from ‘policies of governments or other agencies outside of the journal itself’ and therefore seems likely to be interpreted as concerning only the threat of biological warfare and bioterrorism.
In this article, it is argued (1) that the publication of the Danish and UK studies may cause significant harm to individuals, (2) that medical journal editors have a moral responsibility for the potential harmful effects of publishing research, (3) that denial of publication is inadequate as an instrument for fulfilling this moral responsibility and (4) that therefore internationally acknowledged publication ethics codes should provide editors with a firm basis for evaluating and mitigating the potential effects of publishing medical research in general. The article deals solely with the publication of medical research simply because this is a field of research that is already regulated by a number of international codes. However, the points made certainly also apply to other fields of research.
Research ethics and publication ethics
Research ethics is concerned with the ethical legitimacy of conducting research. It provides a set of moral reasons for permitting or not permitting the conducting of certain types of research. In practice, the ethical principles and values playing a regulatory role for conducting healthcare research are laid out in the Helsinki and Taipei declarations and the Oviedo Convention. 19–21
Publication ethics is concerned with the ethics of publishing research. It provides a set of moral reasons for permitting or not permitting the publishing of certain types of research. In practice, publication of medical research is guided by international codes such as the COPE Code of Conduct for Journal Editors, ICMJE Recommendations and the WAME Professionalism Code of Conduct with its supplementary recommendations. 16 17 22
The principles of research ethics and the principles of publication ethics may lead to different conclusions in relation to the same research. Table 1 shows the possible combinations of views concerning the ethical legitimacy of conducting and publishing a certain type of research.
- View inline
That there are cases of research that fit in box 1 and box 3 seems straightforward.
Box 2 has received some attention in the literature. It has been argued that although certain types of research are not permissible—because they cause harm to research participants or violate requirements of informed consent—the results of such research may be of such importance for public health that it should be published if it has been conducted. 23 24
Box 4 is the object of this article. Clearly, otherwise valuable research may fit in this category if the reporting of this research violates principles of for example, scientific integrity. Research may also, however, fit in this category because of the potential harmful effects following publication of such research. Research that may be used for bioterrorism could be one such example. The Danish and UK studies raise the question whether research may fit into this category because the publishing of this research may have harmful effects other than those associated with bioterrorism.
The moral responsibility of editors for the harmful effects of publishing research
The harmful effects of publishing research.
If we have moral reasons to consider rejecting publication of research that may be used for bioterrorism, we certainly also have moral reasons to consider rejecting publication of the Danish and UK studies. Thus, they are similar to research posing a security/safety risk in at least two ways:
1. They may lead to significant harmful effects to a significant number of people.
2. Publishing the research significantly increases the likelihood of these harmful effects.
The Danish study may lead to an increased use of antibiotics ultimately increasing the problem of microbial multiresistance. The UK study could lead to increased numbers cardiovascular events due to undertreatment or due to patient incompliance. In both cases, the harmful effects are the potential outcome of changes in clinical practice. In the latter case, the harmful effects could also follow unwarranted changes in patient behaviour.
As in the case of research that may be used for bioterrorism, it is the publishing of these studies in a scientific journal that significantly increases the likelihood of the harmful effects. Isolated research into the effects of antibiotics on a chronic disease and the effects of statins on levels of cholesterol without exposure in scientific journals and mass media seem unlikely to really affect clinical practice and patient behaviour.
It may be objected that publication does not make such effects likely in absolute terms. However, the publishing of an article on the possibility of artificially synthesising a live poliovirus does not make bioterrorism likely in absolute terms. It still requires a number of persons with significant expertise, access to appropriate facilities and funding and arguably also limited morality. Likely or not, there are important differences between this type of study and the Danish and UK studies. Differences that seem to make the harmful effects of publishing the latter studies considerably more likely:
They are of direct relevance to the clinical practice.
They hold promise of novel and significant healthcare benefits.
These potential health benefits are in the interest of large groups of patients/people.
These features will make the publishing of these studies attract the attention of researchers and healthcare professionals. They will also, however, increase the likelihood of exposure in the mass media (the Danish and UK study both received attention in the mass media), which in turn may come to fuel a clinical, political and public demand to further develop and implement these new treatment options and findings into healthcare policy and practice or it may simply lead to changes in patient behaviour, all of which pave the way for the potentially harmful effects suggested above.
The moral responsibility of editors
An adequate account of the moral responsibility of journal editors should make editors morally accountable for the harmful effects of publishing research for at least two reasons:
1. Editors make the decision to publish research that may have harmful effects.
2. Editors are in a position to foresee the potential harmful effects.
For an agent to be morally responsible for certain harmful effects, the agent must be the cause of these effects. Editors make the decision on whether or not to publish research. This means that they influence whether or not the harmful effects of publishing obtain, and in this sense they may be said to be part of the set of conditions causing or not causing the harmful effects.
For an agent to be morally responsible for certain harmful effects, the agent must reasonably be able to foresee these effects. Editors are arguably in a very good position with respect to foreseeing the potential harmful effects of publishing research because:
They understand medical research,
They have insights into the interests of various stakeholders and
They know about the role of different media in the dissemination of research.
Most if not all editors have a background in research and therefore must be expected to be able to understand and critically engage with the content of research publications. And, very importantly, they are aided in these efforts by the reviewers, who may have a firmer grasp of specific areas within a particular field of medical research.
Surely editors also have insight into the interests of researchers and research groups, healthcare professionals and organisations, decision-makers and the wider public as these interests are reflected in the ongoing discussions in medical journals and in the broader healthcare-related dialogue in the mass media. And, most certainly, editors have substantial experience of the role of medical journals in the wider dissemination chain from researcher to the public. They have seen how scientific studies may get picked up—perhaps distorted or one-sidedly represented—by mass media and how this may influence public opinion and debate, individual behaviour as well as political decision-making.
These insights—at least in certain cases —place editor’s in a good position in terms of foreseeing the potential harmful effects of publishing research.
Dismissing the moral responsibility of editors I: benefits of publishing and academic freedom
The moral responsibility of editors for such effects may be considered negligible for at least two reasons:
1. The benefits of publishing always outweigh the harm it can cause.
2. The value of academic freedom outweighs the harm caused.
Certainly most, if not all, research may provide new and useful knowledge—at least in the weak sense of leading to the evolution of science. It is not, however, self-evidently true that these benefits always outweigh the harm caused. The Danish and UK studies both yielded novel insights that in turn may lead to more effective treatments/less overtreatment, but it is not self-evidently true that these potential benefits outweigh the harm of increased microbial multiresistance and the risk of undertreatment of high levels of cholesterol. To determine this requires a cost-benefit analysis on a case-by-case basis—it cannot be established a priori.
It may be objected that providing an adequate cost-benefit analysis is impossible since it requires both an adequate catalogue of benefits and harms and an adequate prediction of the course of the world following publication of research. The former is not readily available and the latter seems unrealistic. However, there are no compelling reasons to believe that international or journal-specific publication ethics codes could not—through open and transparent processes involving relevant stakeholders—be amended to take into account the potential harmful effects of publishing in a way that is adequate for all practical purposes.
The potential benefits of publishing may be claimed to outweigh the harms and so may the right to academic freedom. Academic freedom may tentatively be defined as the right of researchers to freely choose their object and methods of research and to communicate their findings to the wider public without being censored or sanctioned. Certainly academic freedom is valuable. However, it cannot reasonably be thought to be an absolute right. As Mill realised, rights to liberty may only be exercised to the extent that such exercise do not cause harm to others. 25 From this viewpoint, it is the risk of harm that outweighs academic freedom. The fact that harm may outweigh academic freedom is already reflected in international research ethics codes such as the Helsinki Declaration.
Most importantly, however, even if we maintain that 1. and 2. hold for all cases, it still does not follow that the moral responsibility of editors is negligible. It may at best provide editors with a strong case for publishing research, but it does not mean that they are not morally responsible for taking steps to minimise or entirely avoid the harmful effects. If, on balance, we believe that the Danish and UK studies should be published, this does not mean that editors and reviewers should not call attention to the potential harmful effects of publishing these studies and try to avoid or at least mitigate such effects.
Dismissing the moral responsibility of editors II: distribution of moral responsibility
The moral responsibility of editors may also be considered negligible on the grounds that:
3. The moral responsibility for the harmful effects must be distributed between several agents
There is a problem of ‘many hands’ in relation to the potential harmful effects of publishing research. 26 Several other agents—the researchers, the mass media, the healthcare professionals, decision-makers to mention but a few—play a causal role in producing harmful effects that could be foreseen by any of them. Hence, they all have moral responsibility. The cardinal question, therefore, becomes how the moral responsibility should be distributed among the relevant agents. What is a reasonable distribution of moral responsibility for the harmful effects of publishing?
First of all, it should once again be noted that, even if we accept 3., it does not entail that editors are not morally responsible or that their moral responsibility is negligible. Second, there are good grounds for thinking that editors should be assigned responsibility beyond the negligible. Editors are in a good position with respect to foreseeing the potential harm of publishing. But they are in a unique position with respect to acting on their moral responsibility in two ways:
Editors are capable of strongly influencing the reception of research.
They can block publication and can also in and through the publication process influence both the content of research publications and the context of the publication and thereby shape the broader reception of the research among the various healthcare stakeholders including the research community, healthcare professionals and organisations, mass media and decision-makers. More on this below.
Although editors may have various interests in publishing a specific piece of research beside the quality of the study, it seems that they would generally be less biased in making publishing decisions about the specific research than researchers.
Evidently, researchers may also strongly influence what is published—and hence may be argued to have moral responsibility for the harmful effects of publishing 27–29 —but they can hardly be expected to serve as impartial judges of the potential harmful effects of publishing their research and to act accordingly. 11 13 30
Should all medical research be published
The moral responsibility of editors for the harmful effects of publishing research may be exercised in different ways. An obvious instrument for exercising moral responsibility is to deny publication. But is this an adequate instrument? A guiding principle in law-making and policy-making is the principle of proportionality, which requires of a law or policy (1) that it should be an effective way of achieving a desired goal and (2) that the goal should be desirable and (3) that there should be no less invasive, alternative ways of achieving the same goal. 31 The proportionality principle thus suggests three criteria for evaluating the adequacy of any instrument of moral responsibility.
Instruments of moral responsibility I: rejection of publication
Denying the publication of research may at first sight seem an ineffective instrument for avoiding the harmful effects of publishing research. After all, there are numerous both printed and online journals in a competitive market, and a multitude of online platforms providing access to both the wider research community and the public. However, denying publication of a paper may still play a regulatory role by ‘cooling off’ the desire to seek publication of a certain type of research. Researchers have various interests. They have an interest in getting their research published and to get it published in particularly relevant and prestigious scientific journals with rigorous peer review, access to a large research community and so forth. 14 These secondary interests confer on editors the power to influence what researchers seek to publish. Moreover, if the rejection of publication is based on an internationally adopted of publication ethics, then the ‘cooling off’ effect will gain some impetus, and the rejection of publication may be effective in some degree in avoiding the harms of publishing. Criteria (1) of the proportionality principle may thus be satisfied, if only partially.
That effectiveness comes at a price. The rejection of publication by editors may lead to the self-censoring of research by researchers. That is, it seems likely that the stronger the ‘cooling off’ effect on the desire to publish a certain type of research, the stronger the ‘cooling off’ effect on the desire to conduct this research. After all, why conduct research that one may not be able to get published in journals that are considered attractive. This will obviously not pose a problem in those cases where the research seems unlikely to generate any benefit, but there are very few, if any, clear-cut cases of such research. Most medical research may be argued to generate insights that eventually will benefit patients and society. It seems undeniable that the Danish and UK studies provided valuable insights that, if applied in the right way, may come to benefit patients. This ultimately goes to show that the denial of publication fails as a means to achieving what is ultimately the desired goal, namely gaining the benefits from publishing research while avoiding the harms. Criteria (2) of the proportionality principle is thus only partially satisfied.
Instruments of moral responsibility II: influencing content and context of publications
Finally, denying publication fails as an adequate instrument because of the availability of alternative instruments that may achieve the truly desired goal of avoiding the harmful effects while preserving the benefits of publishing research. These alternative instrument may even promote academic freedom.
Arguably, the harmful effects following the publication of medical research are in many cases due to one-sided communication of research findings to the various stakeholders as well as insufficient regulation of the uses of these findings in research and clinical practice. The Danish and UK studies are prototypical examples of this. The potential harmful effects of publishing these studies only obtain if the dissemination of these studies leads to undesirable changes in clinical practice or to unsolicited changes in patient compliance. Clinical practice may, however, be regulated through policy and law, and patient compliance may be influenced through information (as the follow-up study of the effects of publishing the UK study shows). Thus the harmful effects of publishing these studies may to a large extent be avoided if the relevant clinical practices become properly regulated and if the public is provided with balanced and rounded information. If so, then certainly there is a pivotal role for editors to play in avoiding the harmful effects of publishing. They may raise awareness of the potential harmful effects of publishing research and of the need for proper regulation and they may take steps to ensure balanced information to all relevant stakeholders. And they are in unique position to do so, as they may influence both the content and the context of research publications.
Editors may influence the content of publications with the aim of avoiding harmful effects in a variety of ways. They may require authors to explicitly describe and address the potential harmful effects and the need for regulation, and they may ask authors to revise unnecessarily strong, one-sided, unbalanced statements that are likely to be picked up and communicated uncritically by the mass media.
Editors may also influence the context of publications by inviting reviewer or open peer comments or by an editorial comment. They may choose to include such research in special thematic issues with a special emphasis on the wider effects of publishing this research. They may highlight the sensitive issues in their advertising on social media and in potential press releases to the mass media. They could even ask a panel of healthcare organisations and relevant decision-makers to comment in the journal on the potential harmful effects and the need for regulation. The dampening effect on the motivation to conduct valuable research would presumably be non-existent or very small. The wider implication being that the denial of publication also fails to satisfy criteria (3) of the proportionality principle.
Policy implications
The previous arguments have provided a basis for suggesting that publication ethics codes should:
1. Acknowledge moral responsibility for the effects of publishing.
2. Define benefits and harms of publishing.
3. Specify a range of actions an editor may take.
An internationally adopted and enforced code of publication ethics with these elements would serve as a guide for editors and thus secure consistency in the relevant editorial decisions. It would also be an important step towards creating transparency and thus underpinning accountability in such decisions.
Could such a code of publication ethics reasonably be journal-specific? Could each medical journal make up its own standards and sanctions? Needless to say, the preventive effect would be limited, and it would generate uncertainty among researchers about submission requirements. Hence, there are good grounds for seeking an international code of publication ethics with the suggested elements.
- Abramson JD ,
- Rosenberg HG ,
- Jewell N , et al
- ↵ Reporter BDM . Statins do NOT have major side effects, claims study . 2014 http://www.dailymail.co.uk/health/article-2579739/Statins-NOT-major-effects-claims-study-Research-finds-users-likely-suffer-maladies-control-group.html ( cited 16 Oct 2017 ).
- Gallagher J
- Matthews A ,
- Herrett E ,
- Gasparrini A , et al
- Selgelid MJ
- Singleton A
- Campbell P ,
- Cozzarelli NR , et al
- ↵ COPE Committee on Publication Ethics . Code of Conduct and Best Practice Guidelines for Journal Editors . 2011 https://publicationethics.org/files/Code%20of%20Conduct_2.pdf .
- ↵ ICMJE International Committee of Medical Journal Editors . Recommendations for the conduct, reporting, editing, and publication of scholarly work in medical journals . 2016 .
- ↵ WAME World Association of Medical Editors . Geopolitical Intrusion on Editorial Decisions . 2004 http://www.wame.org/about/policy-statements .
- ↵ World Medical Association . WMA Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects . 2013 https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ .
- ↵ World Medical Association . WMA Declaration of Taipei on Ethical Considerations regarding Health Databases and Biobanks . 2016 https://www.wma.net/policies-post/wma-declaration-of-taipei-on-ethical-considerations-regarding-health-databases-and-biobanks/ .
- ↵ Council of Europe . Convention for the Protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine: Convention on Human Rights and Biomedicine . 1997 https://www.coe.int/en/web/conventions/full-list/-/conventions/rms/090000168007cf98 .
- ↵ WAME World Association of Medical Editors . Professionalism Code of Conduct . 2016 http://www.wame.org/wame-professionalism-code-of-conduct .
- Royakkers L ,
- Pittenger DJ
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Read the full text or download the PDF:
Other content recommended for you.
- Non-equivalent stringency of ethical review in the Baltic States: a sign of a systematic problem in Europe? E Gefenas et al., Journal of Medical Ethics, 2010
- Great expectations—ethics, avian flu and the value of progress Nicholas G Evans, Journal of Medical Ethics, 2012
- Legal and ethical considerations in processing patient-identifiable data without patient consent: lessons learnt from developing a disease register Charlotte L Haynes et al., Journal of Medical Ethics, 2007
- The experiences of ethics committee members: contradictions between individuals and committees L Elliott et al., Journal of Medical Ethics, 2008
- In defence of academic freedom: bioethics journals under siege Udo Schüklenk, Journal of Medical Ethics, 2013
- Responsible dissemination of health and medical research: some guidance points Raffaella Ravinetto et al., BMJ Evidence-Based Medicine, 2022
- Research and complicity: the case of Julius Hallervorden Franklin G Miller, Journal of Medical Ethics, 2011
- Perception of electronic cigarettes in the general population: does their usefulness outweigh their risks? Jose M Martínez-Sánchez et al., BMJ Open, 2015
- Why unethical papers should be retracted William Bülow et al., Journal of Medical Ethics, 2020
- Biomedical conflicts of interest: a defence of the sequestration thesis—learning from the cases of Nancy Olivieri and David Healy A Schafer, Journal of Medical Ethics, 2004
Why the world needs more health researchers

Malaria is a disease that has plagued much of the world’s poorer populations for decades. In 2020, the WHO noted an estimated 241 million cases of malaria worldwide , and 627,000 deaths that came out of it. Nearly all of these were deaths in sub-Saharan Africa — but new advances in medical research seem to point to a hopeful end of such tragedies.
At the core of this is the arrival of two new vaccines . Mosquirix — the first of these vaccines — was recently approved by the WHO after 35 years in the making. It may be distributed late next year.
The second is being developed by the team who created AstraZeneca and is thought to be a more powerful version. According to experts, this is only a year or two away — and may provide the formula that eradicates the vicious disease once and for all.
Still, medical researchers point to a lot more complications that exist outside of the simple development of an effective vaccine. Much of this has to do with funding. Mosquirix, for example, costs more than US$200 million to develop over the course of 30 years and its supply is expected to be limited .
The biggest factor, however, has to do with the limited number of medical researchers who are able to work on developing such vaccines.
From blood drives to tissue samples, participation in clinical trials is integral to advancing healthcare research. Source: Ethan Miller/AFP
The importance of medical research
Health research is crucial to our society. It enables doctors and scientists to better prevent and treat common and dangerous illnesses.
When it comes to research, more data means better chances of developing a suitable cure. The COVID-19 vaccine is a clear example of this. Developing a suitable vaccine for the COVID-19 pandemic had been an ongoing struggle, as not enough data was initially available to conduct research.
However, data isn’t the part that forms the research process. Clinical research is usually conducted by thoroughly examining a sample of people as well as tissue samples. This helps find new methods of detection, diagnosis, treatment, and prevention of new and established diseases.
Many of the vaccines for malaria were developed in the same way. According to the New York Times, the vaccine was tested in Kenya, Ghana and Malawi in children younger than two years. “It is more deliverable in rural, remote settings than many other vaccines have been,” said Prashant Yadav, an expert in healthcare supply chains at the Centre for Global Development.
More than that, such efforts require a large amount of funding — which is severely lacking. Compared to the search for an effective COVID-19 vaccine, funding for malaria — and many other diseases — are not high enough to guarantee the generation of research.
For example, the Gates Foundation spends around US$270 million a year to fight malaria. This is a far cry from figures that suggest the US government spent more than US$900 million to support non-clinical studies and research to encourage candidate vaccines into clinical trials at large pharmaceutical companies.
What does this mean? A scarcity of resources — which in turn results in fewer healthcare professionals willing to enter the world of medical research.

Clinical research is important for the development of vaccines and other preventative healthcare measures. Source: Ernesto Benavides/AFP
The need for more medical researchers
Medical researchers are low in number compared to other professions in the field. For example, the WHO records that only 15% of researchers in Africa are health researchers, with this figure decreasing across other regions. In Southeast Asia, only 6% are medical researchers.
This points to a pressing issue: there are just not enough medical researchers available to address the overwhelming demand for healthcare solutions. Most of it is due to a lack of healthcare experts who end up going into research.
The other side of the coin is that funding worldwide is severely lacking. In 2013, for example, the National Institute of Health’s budget was 21.9% lower than in 2003. In this, the proportion of medical doctors who engage in scientific research makes up only 1.5% of the physician workforce — a measly number considering the urgency and necessity of the matter.
The solution? Ensuring medical students are fully aware of the shortcomings and scarcity of medical researchers — and making them aware of the importance of such an endeavour, outside of just practice.
Popular stories
10 powerful body language examples to look and be more confident.

The ultimate chow down: 10 universities with the best cafeteria food

Beyond the boundary: These are India’s most educated cricket players

Birds, bulls, critters and corn? The most iconic university mascots today

Nigeria's ban on Ukrainian medical degrees could fuel brain drain crisis further

How an ER simulation helps medical and engineering students see new points of view

Explore various avenues of opportunity within leading medical schools
Research Makes for Better Doctors, Benefits Patients
Research helps create good doctors because they are better able to critically evaluate new evidence and provide the best patient care with the latest knowledge.
By Wendy Sarubbi | April 2, 2019

Research doesn’t end when students graduate. Conducting research keeps practicing engineers, emergency management operators and doctors current on new techniques and allows them to discover best practices that can impact entire industries.
That’s why medical residents continue to conduct research once they are treating patients in hospitals.
The leaders of the UCF/HCA consortium residency programs in Central and North Florida say research and other scholarly activity create scientific knowledge and help physicians share best practices, which is why they emphasize it as part of their programs.
It’s no surprise those residents conducted more than 300 scholarly research projects in the last year – scholarly work that residency leaders say is making the young doctors better caregivers.
“Scholarly activities and research help our physicians learn how to critically evaluate the scientific literature and medical studies,” says Hale Toklu, director of graduate medical education research for the North Florida Division.
UCF’s associate dean for graduate medical education Diane Davey agrees.
“Research helps you become a better physician because you are able to more critically evaluate new evidence and provide the best patient care,” Davey says. “Physicians experienced in research will be able design high quality patient safety and quality improvement studies in their own practices in the future.”
A recent report about the consortium’s research efforts showed that from 2017 to 2018, UCF-HCA residents in the North Florida Division increased their scientific efforts four-fold. In 2018, those physicians-in-training conducted 324 research projects. Twenty percent of those studies were published in medical journals or books and 43 percent were presented at a scientific meeting. About half of those studies are ongoing.
Some residents conducted case reports highlighting and researching a unique or unusual case they treated. Others conducted quality control studies on ways to improve patient outcomes. Others did research using HCA’s national patient database. With more than 34 million patient encounters a year at 179 HCA hospitals in 21 states, HCA’s database has been the foundation for nationwide studies, including a landmark 2013 study that reduced potentially deadly MRSA infections by 44 percent in hospital intensive care units.
Matthew Calestino, who leads the consortium’s Transitional Year residency at North Florida Regional in Gainesville, organized the first annual North Florida Chapter SHM Scientific Symposium in Jacksonville recently. The SHM represents those who care for hospitalized patients – including physicians, nurses, nurse practitioners and hospital administrators — and is focused on improving the quality of in-patient care.
UCF/HCA residents Hiren Patel and Jake Cho won the top two poster awards. Research posters at the event came from the Internal Medicine residents at the University of Florida’s Jacksonville location, Orange Park Medical Center, Ocala Regional Medical Center, Oak Hill Hospital and the greater Orlando program anchored at Osceola Regional Medical Center as well as the Internal Medicine, Transitional Year and Psychiatry residents from North Florida Regional Medical Center.
In 2018, the recent report showed, North Florida Regional Medical Center led all UCF-HCA consortium locations with its number of research projects, and the internal medicine program headed by Christopher Bray had the highest number in the consortium.
Toklu says research during residency is associated with superior clinical performance. By doing research and participating in scholarly activities, young physicians gain insight into how diseases progress, preventative medicine and differential diagnosis. That means patients will benefit.
The UCF College of Medicine and HCA’s North Florida Division offer residency programs at Osceola, Ocala and North Florida regional medical centers and will soon begin residencies at West Florida Hospital in Pensacola. The Orlando VA Medical Center also participates in several of the programs.
More Topics
Pegasus magazine.

For a decade, UCF-based nonprofit Limbitless Solutions has transformed kids’ lives through bionic limbs.


COMMENTS
The publication of the findings of scientific research is important for two reasons. First, the progression of science depends on the publication of research findings in the peer-reviewed literature. Second, the publication of research is important for career development. The old dictum "publish or perish" suggests the critical role ...
Publishing Medical Research. Getting research published at any stage of your career is vital to a physician's development. Having your research published aids in establishing you as an expert your field of study. Get the latest tips and advice about publishing medical research with the AMA. Catalog of Topics.
It has been argued that although certain types of research are not permissible—because they cause harm to research participants or violate requirements of informed consent—the results of such research may be of such importance for public health that it should be published if it has been conducted. 23 24. Box 4 is the object of this article.
The term "health research," sometimes also called "medical research" or "clinical research," refers to research that is done to learn more about human health. Health research also aims to find better ways to prevent and treat disease. Health research is an important way to help improve the care and treatment of people worldwide.
Critical reading of published literature is the starting point for educated discussions and for finding unsolved problems which should be addressed by the scientific process to improve medical knowledge. Research projects follow a common path including the following steps: step 1, defining the hypothesis/specific research question; step 2 ...
NIH works to turn scientific discoveries into better health for all. As the largest public funder of biomedical and behavioral research in the world, NIH is the driving force behind decades of advances that improve health, revolutionize science, and serve society more broadly. Evidence of the varied, long-term impacts of NIH activities comes from a variety of sources, ranging from studies on ...
Start at the very beginning. Your success in getting published shouldn't start with a complete paper. It should start right when you conceptualize your research. Edward Livingston, MD, deputy editor of clinical content for the Journal of the American Medical Association ( JAMA ), said it all begins with asking the right questions.
Here are some of the most important reasons medical research is important: Generate Valuable Insights. Medical research helps people learn more about themselves and their health. The knowledge gained by medical research is constantly improving. With new scientific information coming from medical studies, people will be able to take care of ...
Research is essential for the advancement of medicine and the improvement of healthcare. It is also crucial to the practice of evidence-based medicine [1,2,3,4].Medical students play a vital role in the published output and research productivity of any institution [2, 3].For example, medical students largely contributed to the publications of one German institution, as 28% of the output was ...
Every clinician is responsible for evaluating their own practice, and to do that in a robust and meaningful way you need to use the tools of research. We all need to be able to critically review research done by others. For example, the guidelines used in everyday clinical practice are based on meta-analyses and systematic reviews.
If you would like to support our research programs, contact: Chad Boysen. Director of Development. Hennepin Healthcare Foundation. [email protected]. 612-873-2217. Our Research Programs. Volunteer for a Study. Medical Research FAQs.
When asked about the purpose of medical research most people would hopefully reply: to advance knowledge for the good of society; to improve the health of people worldwide; or to find better ways to treat and prevent disease. The reality is different. The research environment, with its different players, is now much less conducive to thinking ...
I see three reasons: because it increases the credibility of the research, it increases the credibility of the practitioner, and it puts the knowledge in the permanent, searchable record. Spoiler alert: I think the third is the most important. It is important to recognize that where people want to get their information is not the same thing as ...
It has been argued that although certain types of research are not permissible—because they cause harm to research participants or violate requirements of informed consent—the results of such research may be of such importance for public health that it should be published if it has been conducted.23 24. Box 4 is the object of this article.
The importance of medical research. Health research is crucial to our society. It enables doctors and scientists to better prevent and treat common and dangerous illnesses. When it comes to research, more data means better chances of developing a suitable cure. The COVID-19 vaccine is a clear example of this.
UCF's associate dean for graduate medical education Diane Davey agrees. "Research helps you become a better physician because you are able to more critically evaluate new evidence and provide the best patient care," Davey says. "Physicians experienced in research will be able design high quality patient safety and quality improvement ...
As part of the most recent AMA Research Challenge, Charles Lopresto, DO, offered insight on what it takes to get published during training. An AMA member, Dr. Lopresto's research background includes serving on the editorial board of The Journal of the American Osteopathic Association.. For medical students looking to showcase their research, the deadline for abstract submissions for the 2023 ...
The results are then analyzed and discussed in light of published findings to conclude the study with applications that can help solve the research problem. Thus, through systematic study, critical thinking and problem-solving, undergraduates can develop essential skills that can help prepare them for future academic and professional endeavors. 1
Allowing patient records to be published on medical websites for the general public to learn from. ... Which of the following professions does NOT fall into the biotechnology research and development pathway? Patent lawyer. Published medical research is important because it _____. All of the answer choices are correct.
Peer review is a mutual responsibility among fellow scientists, and scientists are expected, as part of the academic community, to take part in peer review. If one is to expect others to review their work, they should commit to reviewing the work of others as well, and put effort into it. 2) Be pleasant. If the paper is of low quality, suggest ...
In case of a medical emergency, you should take the patient to _____ ... Sometimes research is conducted on large numbers of people who are chosen in a way that reduces biases. ... Published research studies are important because they-promote dialogue among scholars about the subject.-prompt further research to confirm findings.-prompt further ...
AP coordinators are responsible for notifying students when and where to report for the exams. Early testing or testing at times other than those published by College Board is not permitted under any circumstances. Late-testing dates are available if students cannot test during the first two full weeks of May. See the late-testing schedule.
Medicare.gov Care Compare is a new tool that helps you find and compare the quality of Medicare-approved providers near you. You can search for nursing homes, doctors, hospitals, hospice centers, and more. Learn how to use Care Compare and make informed decisions about your health care. Official Medicare site.